User login
Vaginal and bilateral thigh removal of a transobturator sling

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.

Additional videos from SGS are available here, including these recent offerings:
- Morcellation at the time of vaginal hysterectomy
- Surgical management of non-tubal ectopic pregnancies
- Size can matter: Laparoscopic hysterectomy for the very large uterus
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This video is brought to you by
Myomectomy of a large cervical fibroid in a patient desiring future fertility
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
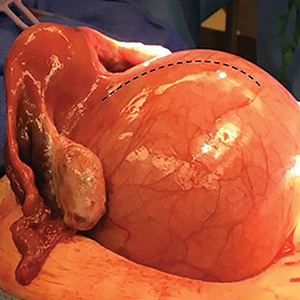
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).
What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
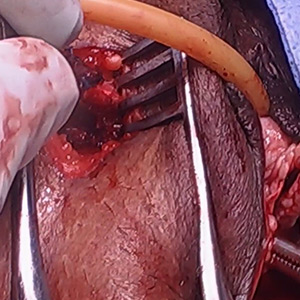
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
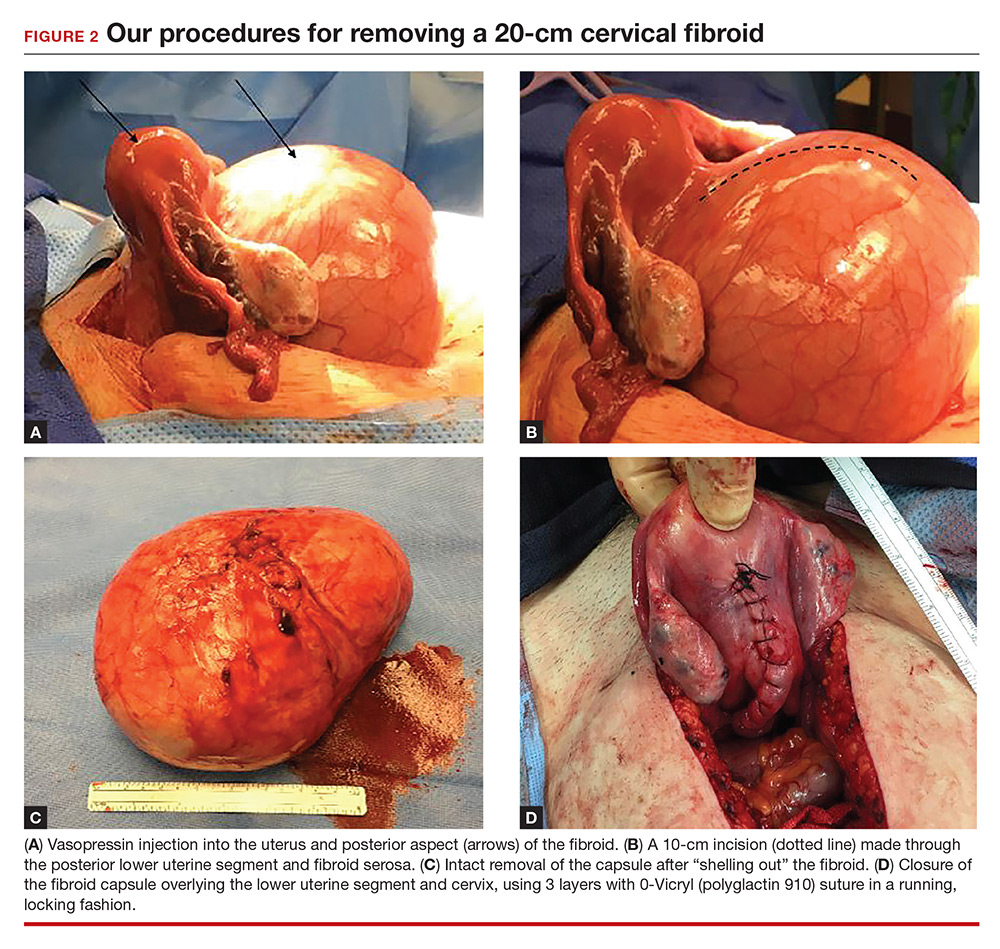
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).
Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).
What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).
Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Uterine fibroids are the most common tumors of the uterus. Clinically significant fibroids that arise from the cervix are less common.1 Removing large cervical fibroids when a patient desires future fertility is a surgical challenge because of the risks of significant blood loss, bladder and ureteral injury, and unplanned hysterectomy. For women who desire future fertility, myomectomy can improve the chances of pregnancy by restoring normal anatomy.2 In this article, we describe a technique for myomectomy with uterine preservation in a patient with a 20-cm cervical fibroid.
CASE Woman with increasing girth and urinary symptoms is unable to conceive
A 33-year-old white woman with a history of 1 prior vaginal delivery presents with symptoms of increasing abdominal girth, intermittent urinary retention and urgency, and inability to become pregnant. She reports normal monthly menstrual periods. On pelvic examination, the ObGyn notes a large fibroid partially protruding through a dilated cervix. Abdominal examination reveals a fundal height at the level of the umbilicus.
Transvaginal ultrasonography shows a uterus that measures 4.5 x 6.1 x 13.6 cm. Arising from the posterior aspect of the uterine fundus, body, and lower uterine segment is a fibroid that measures 9.7 x 15.5 x 18.9 cm. Magnetic resonance imaging is performed and confirms a fibroid measuring 10 x 16 x 20 cm. The inferior-most aspect of the fibroid appears to be within the endometrial cavity and cervical canal. Most of the fibroid, however, is posterior to the uterus, pressing on and anteriorly displacing the endometrial cavity (FIGURE 1).
What is your surgical approach?
Comprehensive preoperative planning
In this case, the patient should receive extensive preoperative counseling about the significantly increased risk for hysterectomy with an attempted myomectomy. Prior to being scheduled for surgery, she also should have a consultation with a gynecologic oncologist. To optimize visualization during the procedure, we recommend to plan for a midline vertical skin incision. Because of the potential bleeding risks, blood products should be made available in the operating room at the time of surgery.
Techniques for surgery
Intraoperatively, a vertical midline incision exteriorizes the uterus from the peritoneal cavity. Opening of the retroperitoneal spaces allows for identification of the ureters. Perform dissection in the midline away from the ureters. Inject vasopressin (5 U) into the uterine fundus. Incise the uterine serosa over the myoma posteriorly in the midline.
Perform a myomectomy, with gentle “shelling out” of the myoma; in this way the specimen can be removed intact. Reapproximate the fibroid cavity in 3 layers with 0-Vicryl (polyglactin 910) suture in a running fashion (FIGURE 2).
Continue to: CASE Resolved
CASE Resolved
The estimated blood loss during surgery was 50 mL. Final pathology reported a 1,660-g intact myoma. The patient’s postoperative course was uncomplicated and she was discharged home on postoperative day 1.
Her postoperative evaluation was 1 month later. Her abdominal incision was well healed. Her fibroid-related symptoms had resolved, and she planned to attempt pregnancy. Cesarean delivery for future pregnancies was recommended.
Increase the chances of a good outcome
Advanced planning for attempted myomectomy of a large cervical fibroid can increase the probability of a successful outcome. We suggest the following:
Counsel the patient on risks. Our preoperative strategy includes extensive counseling on the significantly increased surgical risks and the possibility of unavoidable hysterectomy. Given the anatomic distortion with respect to the ureters, bladder, and major blood vessels, involving gynecologic oncology is beneficial to the surgery planning process.
Prepare for possible transfusion. Ensure blood products are made available in the operating room in case transfusion is needed.
Control bleeding. Randomized studies have shown that intrauterine injection of vasopressin, through its action as a vasoconstrictor, decreases surgical bleeding.3,4 While little data are available on vasopressin’s most effective dosage and dilution, 5 U at a very dilute concentration (0.1–0.2 U/mL) has been recommended.5 A midline cervical incision away from lateral structures and gentle shelling out of the cervical fibroid help to avoid intraoperative damage to the bladder, ureters, and vascular supply.
Close in multiple layers. This approach can prevent a potential space for hematoma accumulation.6 Further, a multiple-layer closure of a myometrial incision may decrease the risk for uterine rupture in subsequent pregnancies.7
Advise abstinence postsurgery. There are no consistent data to guide patient counseling regarding recommendations for the timing of conception following myomectomy. We counseled our patient to abstain from vaginal intercourse for 4 weeks, after which time she soon should attempt to conceive. Although there are no published data regarding when it is best to resume sexual relations following such a surgery, we advise a 1-month period primarily to allow healing of the skin incision. Any further delay in attempting to become pregnant may allow for the growth of additional fibroids.
Plan for future deliveries. When the myomais extensively involved, such as in this case, we recommend cesarean delivery for future pregnancies to avoid the known risk of uterine rupture.8 In general, we recommend cesarean delivery in future pregnancies if an incision larger than 50% of the myometrial thickness is made in the contractile portion of the uterus.
Final takeaway. Despite increased surgical risks, myomectomy of a large cervical fibroid is possible and can alleviate symptoms and improve future fertility.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Ryan GL, Syrop CH, Van Voorhis BJ. Role, epidemiology, and natural history of benign uterine mass lesions. Clin Obstet Gynecol. 2005;48(2):312–324.
- Milazzo GN, Catalano A, Badia V, Mallozzi M, Caserta D. Myoma and myomectomy: poor evidence concern in pregnancy. J Obstet Gynaecol Res. 2017;43(12):1789–1804.
- Okin CR, Guido RS, Meyn LA, Ramanathan S. Vasopressin during abdominal hysterectomy: a randomized controlled trial. Obstet Gynecol. 2001;97(6):867–872.
- Kongnyuy EJ, van den Broek N, Wiysonge CS. A systematic review of randomized controlled trials to reduce hemorrhage during myomectomy for uterine fibroids. Int J Gynaecol Obstet. 2008;100(1):4–9.
- Barbieri RL. Give vasopressin to reduce bleeding in gynecologic surgery. OBG Manage. 2010;22(3):12–15.
- Tian YC, Long TF, Dai YN. Pregnancy outcomes following different surgical approaches of myomectomy. J Obstet Gynaecol Res. 2015;41(3):350–357.
- Bujold E, Bujold C, Hamilton EF, Harel F, Gauthier RJ. The impact of a single-layer or double-layer closure on uterine rupture. Am J Obstet Gynecol. 2002;186(6):1326–1330.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
Laparoscopic suturing is an option
Laparoscopic suturing is an option
Dr. Lum presented a nicely produced video demonstrating various strategies aimed at facilitating total laparoscopic hysterectomy (TLH) of the very large uterus. Her patient’s evaluation included magnetic resonance imaging. In the video, she demonstrates a variety of interventions, including the use of a preoperative gonadotropin–releasing hormone (GNRH) agonist and immediate perioperative radial artery–uterine artery embolization. Intraoperative techniques include use of ureteral stents and securing the uterine arteries at their origins.
Clearly, TLH of a huge uterus is a technical challenge. However, I’d like to suggest that a relatively basic and important skill would greatly assist in such procedures and likely obviate the need for a GNRH agonist and/or uterine artery embolization. The vessel-sealing devices shown in the video are generally not capable of sealing such large vessels adequately, and this is what leads to the massive hemorrhaging that often occurs.
Laparoscopic suturing with extracorporeal knot tying can be used effectively to control the extremely large vessels associated with a huge uterus. The judicious placement of sutures can completely control such vessels and prevent bleeding from both proximal and distal ends when 2 sutures are placed and the vessels are transected between the stitches. Many laparoscopic surgeons have come to rely on bipolar energy or ultrasonic devices to coagulate vessels. But when dealing with huge vessels, a return to basics using laparoscopic suturing will greatly benefit the patient and the surgeon by reducing blood loss and operative time.
David L. Zisow, MD
Baltimore, Maryland
Dr. Lum responds
I thank Dr. Zisow for his thoughtful comments. I agree that laparoscopic suturing is an essential skill that can be utilized to suture ligate vessels. If we consider the basics of an open hysterectomy, the uterine artery is clamped first, then suture ligated. When approaching a very large vessel during TLH, I would be concerned that a simple suture around a large vessel might tear through and cause more bleeding. To mitigate this risk, the vessel can be clamped with a grasper first, similar to the approach in an open hysterectomy. However, once a vessel is compressed, a sealing device can usually work just as well as a suture. It becomes a matter of preference and cost.
During hysterectomy of a very large uterus, a big challenge is managing bleeding of the uterus itself during manipulation from above. Bleeding from the vascular sinuses of the myometrium can be brisk and obscure visualization, potentially leading to laparotomy conversion. A common misconception is that uterine artery embolization is equivalent to suturing the uterine arteries. In actuality, the goal of a uterine artery embolization is to embolize the distal branches of the uterine arteries, which can help with any potential bleeding from the uterus itself during hysterectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Laparoscopic suturing is an option
Dr. Lum presented a nicely produced video demonstrating various strategies aimed at facilitating total laparoscopic hysterectomy (TLH) of the very large uterus. Her patient’s evaluation included magnetic resonance imaging. In the video, she demonstrates a variety of interventions, including the use of a preoperative gonadotropin–releasing hormone (GNRH) agonist and immediate perioperative radial artery–uterine artery embolization. Intraoperative techniques include use of ureteral stents and securing the uterine arteries at their origins.
Clearly, TLH of a huge uterus is a technical challenge. However, I’d like to suggest that a relatively basic and important skill would greatly assist in such procedures and likely obviate the need for a GNRH agonist and/or uterine artery embolization. The vessel-sealing devices shown in the video are generally not capable of sealing such large vessels adequately, and this is what leads to the massive hemorrhaging that often occurs.
Laparoscopic suturing with extracorporeal knot tying can be used effectively to control the extremely large vessels associated with a huge uterus. The judicious placement of sutures can completely control such vessels and prevent bleeding from both proximal and distal ends when 2 sutures are placed and the vessels are transected between the stitches. Many laparoscopic surgeons have come to rely on bipolar energy or ultrasonic devices to coagulate vessels. But when dealing with huge vessels, a return to basics using laparoscopic suturing will greatly benefit the patient and the surgeon by reducing blood loss and operative time.
David L. Zisow, MD
Baltimore, Maryland
Dr. Lum responds
I thank Dr. Zisow for his thoughtful comments. I agree that laparoscopic suturing is an essential skill that can be utilized to suture ligate vessels. If we consider the basics of an open hysterectomy, the uterine artery is clamped first, then suture ligated. When approaching a very large vessel during TLH, I would be concerned that a simple suture around a large vessel might tear through and cause more bleeding. To mitigate this risk, the vessel can be clamped with a grasper first, similar to the approach in an open hysterectomy. However, once a vessel is compressed, a sealing device can usually work just as well as a suture. It becomes a matter of preference and cost.
During hysterectomy of a very large uterus, a big challenge is managing bleeding of the uterus itself during manipulation from above. Bleeding from the vascular sinuses of the myometrium can be brisk and obscure visualization, potentially leading to laparotomy conversion. A common misconception is that uterine artery embolization is equivalent to suturing the uterine arteries. In actuality, the goal of a uterine artery embolization is to embolize the distal branches of the uterine arteries, which can help with any potential bleeding from the uterus itself during hysterectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Laparoscopic suturing is an option
Dr. Lum presented a nicely produced video demonstrating various strategies aimed at facilitating total laparoscopic hysterectomy (TLH) of the very large uterus. Her patient’s evaluation included magnetic resonance imaging. In the video, she demonstrates a variety of interventions, including the use of a preoperative gonadotropin–releasing hormone (GNRH) agonist and immediate perioperative radial artery–uterine artery embolization. Intraoperative techniques include use of ureteral stents and securing the uterine arteries at their origins.
Clearly, TLH of a huge uterus is a technical challenge. However, I’d like to suggest that a relatively basic and important skill would greatly assist in such procedures and likely obviate the need for a GNRH agonist and/or uterine artery embolization. The vessel-sealing devices shown in the video are generally not capable of sealing such large vessels adequately, and this is what leads to the massive hemorrhaging that often occurs.
Laparoscopic suturing with extracorporeal knot tying can be used effectively to control the extremely large vessels associated with a huge uterus. The judicious placement of sutures can completely control such vessels and prevent bleeding from both proximal and distal ends when 2 sutures are placed and the vessels are transected between the stitches. Many laparoscopic surgeons have come to rely on bipolar energy or ultrasonic devices to coagulate vessels. But when dealing with huge vessels, a return to basics using laparoscopic suturing will greatly benefit the patient and the surgeon by reducing blood loss and operative time.
David L. Zisow, MD
Baltimore, Maryland
Dr. Lum responds
I thank Dr. Zisow for his thoughtful comments. I agree that laparoscopic suturing is an essential skill that can be utilized to suture ligate vessels. If we consider the basics of an open hysterectomy, the uterine artery is clamped first, then suture ligated. When approaching a very large vessel during TLH, I would be concerned that a simple suture around a large vessel might tear through and cause more bleeding. To mitigate this risk, the vessel can be clamped with a grasper first, similar to the approach in an open hysterectomy. However, once a vessel is compressed, a sealing device can usually work just as well as a suture. It becomes a matter of preference and cost.
During hysterectomy of a very large uterus, a big challenge is managing bleeding of the uterus itself during manipulation from above. Bleeding from the vascular sinuses of the myometrium can be brisk and obscure visualization, potentially leading to laparotomy conversion. A common misconception is that uterine artery embolization is equivalent to suturing the uterine arteries. In actuality, the goal of a uterine artery embolization is to embolize the distal branches of the uterine arteries, which can help with any potential bleeding from the uterus itself during hysterectomy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Admission eosinopenia predicted severe CDI outcomes
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
FROM JAMA SURGERY
Key clinical point: Undetectable peripheral eosinophils predicted severe outcomes in patients admitted with Clostridium difficile infection.
Major finding: In the training and validation datasets, odds of in-hospital mortality were 2.01 (95% CI, 1.08-3.73) and 2.26 (95% CI, 1.33-3.83), respectively.
Study details: Two-hospital cohort study of 2,065 patients admitted with C. difficile infection.
Disclosures: The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
Source: Kulaylat A et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
Five-year follow-up confirms safety of antibiotics for uncomplicated appendicitis
Longer-term outcomes of treating uncomplicated acute appendicitis with antibiotics suggest it is a feasible alternative to appendectomy.
Researchers have presented the 5-year follow-up data from the Appendicitis Acuta (APPAC) multicenter randomized clinical trial comparing appendectomy with antibiotic therapy in 530 patients.
They found that 39.1% (100) of the 257 patients randomized to antibiotic therapy – 3 days of intravenous ertapenem followed by 7 days of oral levofloxacin and metronidazole – experienced a recurrence of appendicitis within 5 years and subsequently had surgery.
However the authors noted that seven of these patients were later found not to have appendicitis, so the true success rate for antibiotic treatment was actually 63.7%.
Seventy of the patients who experienced a recurrence underwent surgery in the first year after randomization, 17 in the second year, 3 in the third year, 5 in the fourth year, and the remaining 5 patients in the fifth year, the authors wrote in an article published in JAMA.
However the overall complication rate was similar in patients who were randomized to undergo appendectomy and in those who were initially randomized to the antibiotic group but later experienced a recurrence and underwent surgery.
“No patient initially treated with antibiotics, who ultimately developed recurrent appendicitis, had any complications related to the delay in surgery,” wrote Paulina Salminen, MD, from Turku (Finland) University Hospital and coauthors. “Nearly two-thirds of all patients who initially presented with uncomplicated appendicitis were successfully treated with antibiotics alone, and those who ultimately developed recurrent disease did not experience any adverse outcomes related to the delay in appendectomy.”
Of the 100 patients randomized to antibiotics who underwent appendectomy after a recurrence, 15 were operated on when they were first hospitalized at study admission.
The authors commented that the study design allowed for surgeons to exercise their clinical judgment in choosing when to perform an appendectomy on patients in the antibiotic group, because antibiotics alone was not considered acceptable treatment for appendicitis.
“This led to some patients undergoing appendectomy who did not have appendicitis or who might have been successfully treated with antibiotics or an another course of antibiotics,” they wrote. “Future studies should investigate protocols for further imaging or antibiotic treatment for patients who develop recurrent appendicitis after they were initially treated with antibiotics.”
In the recurrence group, the majority were found to have uncomplicated appendicitis, but complicated appendicitis was seen in two patients between 2 and 5 years after the index admission.
There was a significant 17.9% higher complication rate in the appendectomy group, compared with the antibiotic group – 24.4% versus 6.5% – at 5 years and two patients in the appendectomy group had severe complications requiring reoperation.
They suggested that the higher complication rate with surgery, which was mostly attributable to infections, could be reduced by the use of laparoscopic appendectomy, which is also associated with faster recovery.
The median length of hospital stay was 3 days for both the appendectomy group and the antibiotics-only group, but patients randomized to appendectomy took a median of 22 days of sick leave, compared with 11 days for those randomized to antibiotics (P less than .001).
In the absence of standard protocol on treating appendicitis with antibiotics, the authors noted that they took a conservative approach, using broad-spectrum antibiotics and keeping patients in hospital for 3 days for observation.
“The success of antibiotic treatment for appendicitis calls into question prior beliefs that appendicitis inevitably results in serious intra-abdominal infection if appendectomy is not performed.”
The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
SOURCE: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
“When the earlier results of the APPAC trial were published, showing that 73% of patients with uncomplicated acute appendicitis did not require surgery at 1 year of follow-up, critics raised concerns that many more of these patients would eventually require surgery,” wrote Edward H. Livingston, MD, deputy editor of JAMA in an accompanying editorial (JAMA 2018;320:1245-46).Surgeons have since been waiting until longer-term outcomes were known. These 5-year results are supportive of the antibiotics approach. “They show no increase in major complications in patients who experienced a recurrence and underwent appendectomy after initially being randomized to antibiotic therapy. They challenge the notion that uncomplicated acute appendicitis is a surgical emergency and show that nonsurgical treatment is a reasonable option,” Dr Livingston wrote.
He declared no conflicts of interest.
“When the earlier results of the APPAC trial were published, showing that 73% of patients with uncomplicated acute appendicitis did not require surgery at 1 year of follow-up, critics raised concerns that many more of these patients would eventually require surgery,” wrote Edward H. Livingston, MD, deputy editor of JAMA in an accompanying editorial (JAMA 2018;320:1245-46).Surgeons have since been waiting until longer-term outcomes were known. These 5-year results are supportive of the antibiotics approach. “They show no increase in major complications in patients who experienced a recurrence and underwent appendectomy after initially being randomized to antibiotic therapy. They challenge the notion that uncomplicated acute appendicitis is a surgical emergency and show that nonsurgical treatment is a reasonable option,” Dr Livingston wrote.
He declared no conflicts of interest.
“When the earlier results of the APPAC trial were published, showing that 73% of patients with uncomplicated acute appendicitis did not require surgery at 1 year of follow-up, critics raised concerns that many more of these patients would eventually require surgery,” wrote Edward H. Livingston, MD, deputy editor of JAMA in an accompanying editorial (JAMA 2018;320:1245-46).Surgeons have since been waiting until longer-term outcomes were known. These 5-year results are supportive of the antibiotics approach. “They show no increase in major complications in patients who experienced a recurrence and underwent appendectomy after initially being randomized to antibiotic therapy. They challenge the notion that uncomplicated acute appendicitis is a surgical emergency and show that nonsurgical treatment is a reasonable option,” Dr Livingston wrote.
He declared no conflicts of interest.
Longer-term outcomes of treating uncomplicated acute appendicitis with antibiotics suggest it is a feasible alternative to appendectomy.
Researchers have presented the 5-year follow-up data from the Appendicitis Acuta (APPAC) multicenter randomized clinical trial comparing appendectomy with antibiotic therapy in 530 patients.
They found that 39.1% (100) of the 257 patients randomized to antibiotic therapy – 3 days of intravenous ertapenem followed by 7 days of oral levofloxacin and metronidazole – experienced a recurrence of appendicitis within 5 years and subsequently had surgery.
However the authors noted that seven of these patients were later found not to have appendicitis, so the true success rate for antibiotic treatment was actually 63.7%.
Seventy of the patients who experienced a recurrence underwent surgery in the first year after randomization, 17 in the second year, 3 in the third year, 5 in the fourth year, and the remaining 5 patients in the fifth year, the authors wrote in an article published in JAMA.
However the overall complication rate was similar in patients who were randomized to undergo appendectomy and in those who were initially randomized to the antibiotic group but later experienced a recurrence and underwent surgery.
“No patient initially treated with antibiotics, who ultimately developed recurrent appendicitis, had any complications related to the delay in surgery,” wrote Paulina Salminen, MD, from Turku (Finland) University Hospital and coauthors. “Nearly two-thirds of all patients who initially presented with uncomplicated appendicitis were successfully treated with antibiotics alone, and those who ultimately developed recurrent disease did not experience any adverse outcomes related to the delay in appendectomy.”
Of the 100 patients randomized to antibiotics who underwent appendectomy after a recurrence, 15 were operated on when they were first hospitalized at study admission.
The authors commented that the study design allowed for surgeons to exercise their clinical judgment in choosing when to perform an appendectomy on patients in the antibiotic group, because antibiotics alone was not considered acceptable treatment for appendicitis.
“This led to some patients undergoing appendectomy who did not have appendicitis or who might have been successfully treated with antibiotics or an another course of antibiotics,” they wrote. “Future studies should investigate protocols for further imaging or antibiotic treatment for patients who develop recurrent appendicitis after they were initially treated with antibiotics.”
In the recurrence group, the majority were found to have uncomplicated appendicitis, but complicated appendicitis was seen in two patients between 2 and 5 years after the index admission.
There was a significant 17.9% higher complication rate in the appendectomy group, compared with the antibiotic group – 24.4% versus 6.5% – at 5 years and two patients in the appendectomy group had severe complications requiring reoperation.
They suggested that the higher complication rate with surgery, which was mostly attributable to infections, could be reduced by the use of laparoscopic appendectomy, which is also associated with faster recovery.
The median length of hospital stay was 3 days for both the appendectomy group and the antibiotics-only group, but patients randomized to appendectomy took a median of 22 days of sick leave, compared with 11 days for those randomized to antibiotics (P less than .001).
In the absence of standard protocol on treating appendicitis with antibiotics, the authors noted that they took a conservative approach, using broad-spectrum antibiotics and keeping patients in hospital for 3 days for observation.
“The success of antibiotic treatment for appendicitis calls into question prior beliefs that appendicitis inevitably results in serious intra-abdominal infection if appendectomy is not performed.”
The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
SOURCE: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
Longer-term outcomes of treating uncomplicated acute appendicitis with antibiotics suggest it is a feasible alternative to appendectomy.
Researchers have presented the 5-year follow-up data from the Appendicitis Acuta (APPAC) multicenter randomized clinical trial comparing appendectomy with antibiotic therapy in 530 patients.
They found that 39.1% (100) of the 257 patients randomized to antibiotic therapy – 3 days of intravenous ertapenem followed by 7 days of oral levofloxacin and metronidazole – experienced a recurrence of appendicitis within 5 years and subsequently had surgery.
However the authors noted that seven of these patients were later found not to have appendicitis, so the true success rate for antibiotic treatment was actually 63.7%.
Seventy of the patients who experienced a recurrence underwent surgery in the first year after randomization, 17 in the second year, 3 in the third year, 5 in the fourth year, and the remaining 5 patients in the fifth year, the authors wrote in an article published in JAMA.
However the overall complication rate was similar in patients who were randomized to undergo appendectomy and in those who were initially randomized to the antibiotic group but later experienced a recurrence and underwent surgery.
“No patient initially treated with antibiotics, who ultimately developed recurrent appendicitis, had any complications related to the delay in surgery,” wrote Paulina Salminen, MD, from Turku (Finland) University Hospital and coauthors. “Nearly two-thirds of all patients who initially presented with uncomplicated appendicitis were successfully treated with antibiotics alone, and those who ultimately developed recurrent disease did not experience any adverse outcomes related to the delay in appendectomy.”
Of the 100 patients randomized to antibiotics who underwent appendectomy after a recurrence, 15 were operated on when they were first hospitalized at study admission.
The authors commented that the study design allowed for surgeons to exercise their clinical judgment in choosing when to perform an appendectomy on patients in the antibiotic group, because antibiotics alone was not considered acceptable treatment for appendicitis.
“This led to some patients undergoing appendectomy who did not have appendicitis or who might have been successfully treated with antibiotics or an another course of antibiotics,” they wrote. “Future studies should investigate protocols for further imaging or antibiotic treatment for patients who develop recurrent appendicitis after they were initially treated with antibiotics.”
In the recurrence group, the majority were found to have uncomplicated appendicitis, but complicated appendicitis was seen in two patients between 2 and 5 years after the index admission.
There was a significant 17.9% higher complication rate in the appendectomy group, compared with the antibiotic group – 24.4% versus 6.5% – at 5 years and two patients in the appendectomy group had severe complications requiring reoperation.
They suggested that the higher complication rate with surgery, which was mostly attributable to infections, could be reduced by the use of laparoscopic appendectomy, which is also associated with faster recovery.
The median length of hospital stay was 3 days for both the appendectomy group and the antibiotics-only group, but patients randomized to appendectomy took a median of 22 days of sick leave, compared with 11 days for those randomized to antibiotics (P less than .001).
In the absence of standard protocol on treating appendicitis with antibiotics, the authors noted that they took a conservative approach, using broad-spectrum antibiotics and keeping patients in hospital for 3 days for observation.
“The success of antibiotic treatment for appendicitis calls into question prior beliefs that appendicitis inevitably results in serious intra-abdominal infection if appendectomy is not performed.”
The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
SOURCE: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
FROM JAMA
Key clinical point: Antibiotics could be used as an alternative to surgery for uncomplicated acute appendicitis.
Major finding: Around two-thirds of patients with acute appendicitis treated with antibiotics only did not experience a recurrence in 5 years.
Study details: Randomized controlled study in 530 patients with uncomplicated acute appendicitis.
Disclosures: The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
Source: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
Sustainability of Ambulatory Safety Event Reporting Improvement After Intervention Discontinuation
From Novant Health and Novant Health Medical Group, Winston-Salem, NC (Dr. C
Abstract
- Objective: An educational intervention stressing anonymous, voluntary safety event reporting together with monthly regular audit and feedback led to significantly increased reporting of safety events in a nonacademic, community practice setting during a 15-month intervention period. We assessed whether these increased reporting rates would be sustained during the 30-month period after the intervention was discontinued.
- Methods: We reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016, and selected 6 clinics that comprised the intervention collaborative and 18 specialty- and size-matched clinics (1:3 match) that comprised the comparator group. To test the changes in safety event reporting (SER) rates between the intervention and postintervention periods for the intervention collaborative, interrupted time series analysis with a control group was performed.
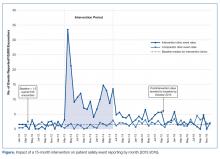
- Results: The SER rate peaked in the first month following the start of the intervention. Following discontinuation of regular auditing and feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention.
- Conclusion: It is likely that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Keywords: patient safety; safety event reporting; voluntary reporting system; risk management; ambulatory clinic.
We have previously shown that patient safety reporting rates for a 6-practice collaborative group in our non-academic community clinics increased 10-fold after we implemented an improvement initiative consisting of an initial education session followed by provision of monthly audit and written and in-person feedback [1]. The intervention was implemented for 15 months, and after discontinuation of the intervention we have continued to monitor reporting rates. Our objective was to assess whether the increased reporting rates observed in this collaborative during the intervention period would be sustained for 30 months following the intervention.
Methods
This study’s methods have been described in detail previously [1]. For this improvement initiative, we reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016. We identified 6 clinics, the intervention collaborative, in family medicine (n = 3), pediatrics (n = 2), and general surgery (n = 1), and 18 specialty- and size-matched clinics (1:3 match), the comparator group [1]. For the intervention collaborative only, we provided an initial 1-hour educational session on safety events with a listing of all safety event types, along with a 1-page reporting form for voluntary, anonymous submission, with use of the term “safety event” rather than “ error,” to support a nonpunitive culture. After the educational session, we provided monthly audit and written and in-person feedback with peer comparison data by clinic. Monthly audit and feedback continued throughout the intervention and was discontinued postintervention. For event reporting, in our inpatient and outpatient facilities we used VIncident (Verge Solutions, Mt. Pleasant, SC) for the period 2012–2015 and RL6: Risk (RL Solutions, Toronto, ON) for 2016.
The baseline period was 15 months (January 2012–March 2013), the intervention period was 15 months (April 2013–June 2014), and the postintervention period was 30 months (July 2014–December 2016). All 24 clinics were monitored for the 60-month period.
To test the changes in the rate of safety event reporting (SER) between the pre-intervention and postintervention periods and between the intervention and the postintervention periods, interrupted time series (ITS) analysis with a control group was performed using PROC AUTOREG in SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC). Because SER rates are reported monthly, ITS analysis was used to control for autocorrelation, nonstationary variance, seasonality, and trends [2,3].
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.
The few studies of ambulatory SER do not adequately address the effect of intervention cessation, but researchers who implemented other ambulatory quality improvement efforts have reported that gains often deteriorate or revert to baseline without consistent, ongoing feedback [4]. Likewise, in hospital-based residency programs, a multifaceted approach that includes feedback can increase SER rates, but it is uncertain if the success of this approach can be maintained long-term without continuing feedback of some type [5–7].
There are likely many factors influencing SER in ambulatory clinics, many of which are also applicable in the hospital setting. These include ease of reporting, knowing what events to report, confidentiality of reporting, and the belief that reporting makes a difference in enhancing patient safety [8]. A strong culture of safety in ambulatory clinics may lead to enhanced voluntary SER [9], and a nonpunitive, team-based approach has been advocated to promote reporting and improve ambulatory safety [10]. Historically, our ambulatory medical group clinics have had a strong culture of safety and, with patient safety coaches present in all of our clinics, we have supported a nonpunitive, team-based approach to SER [11].
In our intervention, we made reporting safety events easy, reporters knew which events to report, events could be reported anonymously, and reporters were rewarded, at least with data feedback, for reporting. The only factor known to have changed was discontinuation of monthly feedback. Which factors are most important could not be determined by our work, but we strongly suspect that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Corresponding author: Herbert Clegg, MD, 108 Providence Road, Charlotte NC, 28207, hwclegg@novanthealth.org.
Financial disclosures: None.
1. Clegg HW, Cardwell T, West AM, Ferrell F. Improved safety event reporting in outpatient, nonacademic practices with an anonymous, nonpunitive approach. J Clin Outcomes Manag 2015;22:66–72.
2. Newland JG, Stach LM, De Lurgio SA, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc 2012; 1:179–86.
3. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13 (6 Suppl):S38–44.
4. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014;312:2569–70.
5. Steen S, Jaeger C, Price L, Griffen D. Increasing patient safety event reporting in an emergency medicine residency. BMJ Qual Improv Rep 2017;6(1).
6. Fox M, Bump G, Butler G, et al. Making residents part of the safety culture: improving error reporting and reducing harms. J Patient Saf 2017. [Epub ahead of print]
7. Dunbar AE 3rd, Cupit M, Vath RJ, et al. An improvement approach to integrate teaching teams in the reporting of safety events. Pediatrics 2017;139:e20153807.
8. Institute of Medicine. To err is human: Building a safer health system. National Academies. www.nationalacademies.org/hmd/~/media/Files/Report%20Files/1999/To-Err-is-Human/To%20Err%20is%20Human%201999%20%20report%20brief.pdf Published November 1999. Accessed August 22, 2018.
9. Miller N, Bhowmik S, Ezinwa M, et al. The relationship between safety culture and voluntary event reporting in a large regional ambulatory care group. J Patient Saf 2017. [Epub ahead of print]
10. Neuspiel DR, Stubbs EH. Patient safety in ambulatory care. Pediatr Clin North Am 2012;59:1341–54.
11. West AM, Cardwell T, Clegg HW. Improving patient safety culture through patient safety coaches in the ambulatory setting. Presented at: Institute for Healthcare Improvement Annual Summit on Improving Patient Care in the Office Practice and the Community; March 2015; Dallas, Texas.
From Novant Health and Novant Health Medical Group, Winston-Salem, NC (Dr. C
Abstract
- Objective: An educational intervention stressing anonymous, voluntary safety event reporting together with monthly regular audit and feedback led to significantly increased reporting of safety events in a nonacademic, community practice setting during a 15-month intervention period. We assessed whether these increased reporting rates would be sustained during the 30-month period after the intervention was discontinued.
- Methods: We reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016, and selected 6 clinics that comprised the intervention collaborative and 18 specialty- and size-matched clinics (1:3 match) that comprised the comparator group. To test the changes in safety event reporting (SER) rates between the intervention and postintervention periods for the intervention collaborative, interrupted time series analysis with a control group was performed.
- Results: The SER rate peaked in the first month following the start of the intervention. Following discontinuation of regular auditing and feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention.
- Conclusion: It is likely that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Keywords: patient safety; safety event reporting; voluntary reporting system; risk management; ambulatory clinic.
We have previously shown that patient safety reporting rates for a 6-practice collaborative group in our non-academic community clinics increased 10-fold after we implemented an improvement initiative consisting of an initial education session followed by provision of monthly audit and written and in-person feedback [1]. The intervention was implemented for 15 months, and after discontinuation of the intervention we have continued to monitor reporting rates. Our objective was to assess whether the increased reporting rates observed in this collaborative during the intervention period would be sustained for 30 months following the intervention.
Methods
This study’s methods have been described in detail previously [1]. For this improvement initiative, we reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016. We identified 6 clinics, the intervention collaborative, in family medicine (n = 3), pediatrics (n = 2), and general surgery (n = 1), and 18 specialty- and size-matched clinics (1:3 match), the comparator group [1]. For the intervention collaborative only, we provided an initial 1-hour educational session on safety events with a listing of all safety event types, along with a 1-page reporting form for voluntary, anonymous submission, with use of the term “safety event” rather than “ error,” to support a nonpunitive culture. After the educational session, we provided monthly audit and written and in-person feedback with peer comparison data by clinic. Monthly audit and feedback continued throughout the intervention and was discontinued postintervention. For event reporting, in our inpatient and outpatient facilities we used VIncident (Verge Solutions, Mt. Pleasant, SC) for the period 2012–2015 and RL6: Risk (RL Solutions, Toronto, ON) for 2016.
The baseline period was 15 months (January 2012–March 2013), the intervention period was 15 months (April 2013–June 2014), and the postintervention period was 30 months (July 2014–December 2016). All 24 clinics were monitored for the 60-month period.
To test the changes in the rate of safety event reporting (SER) between the pre-intervention and postintervention periods and between the intervention and the postintervention periods, interrupted time series (ITS) analysis with a control group was performed using PROC AUTOREG in SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC). Because SER rates are reported monthly, ITS analysis was used to control for autocorrelation, nonstationary variance, seasonality, and trends [2,3].
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.
The few studies of ambulatory SER do not adequately address the effect of intervention cessation, but researchers who implemented other ambulatory quality improvement efforts have reported that gains often deteriorate or revert to baseline without consistent, ongoing feedback [4]. Likewise, in hospital-based residency programs, a multifaceted approach that includes feedback can increase SER rates, but it is uncertain if the success of this approach can be maintained long-term without continuing feedback of some type [5–7].
There are likely many factors influencing SER in ambulatory clinics, many of which are also applicable in the hospital setting. These include ease of reporting, knowing what events to report, confidentiality of reporting, and the belief that reporting makes a difference in enhancing patient safety [8]. A strong culture of safety in ambulatory clinics may lead to enhanced voluntary SER [9], and a nonpunitive, team-based approach has been advocated to promote reporting and improve ambulatory safety [10]. Historically, our ambulatory medical group clinics have had a strong culture of safety and, with patient safety coaches present in all of our clinics, we have supported a nonpunitive, team-based approach to SER [11].
In our intervention, we made reporting safety events easy, reporters knew which events to report, events could be reported anonymously, and reporters were rewarded, at least with data feedback, for reporting. The only factor known to have changed was discontinuation of monthly feedback. Which factors are most important could not be determined by our work, but we strongly suspect that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Corresponding author: Herbert Clegg, MD, 108 Providence Road, Charlotte NC, 28207, hwclegg@novanthealth.org.
Financial disclosures: None.
From Novant Health and Novant Health Medical Group, Winston-Salem, NC (Dr. C
Abstract
- Objective: An educational intervention stressing anonymous, voluntary safety event reporting together with monthly regular audit and feedback led to significantly increased reporting of safety events in a nonacademic, community practice setting during a 15-month intervention period. We assessed whether these increased reporting rates would be sustained during the 30-month period after the intervention was discontinued.
- Methods: We reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016, and selected 6 clinics that comprised the intervention collaborative and 18 specialty- and size-matched clinics (1:3 match) that comprised the comparator group. To test the changes in safety event reporting (SER) rates between the intervention and postintervention periods for the intervention collaborative, interrupted time series analysis with a control group was performed.
- Results: The SER rate peaked in the first month following the start of the intervention. Following discontinuation of regular auditing and feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention.
- Conclusion: It is likely that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Keywords: patient safety; safety event reporting; voluntary reporting system; risk management; ambulatory clinic.
We have previously shown that patient safety reporting rates for a 6-practice collaborative group in our non-academic community clinics increased 10-fold after we implemented an improvement initiative consisting of an initial education session followed by provision of monthly audit and written and in-person feedback [1]. The intervention was implemented for 15 months, and after discontinuation of the intervention we have continued to monitor reporting rates. Our objective was to assess whether the increased reporting rates observed in this collaborative during the intervention period would be sustained for 30 months following the intervention.
Methods
This study’s methods have been described in detail previously [1]. For this improvement initiative, we reviewed all patient safety events reported in our ambulatory clinics for the period 2012–2016. We identified 6 clinics, the intervention collaborative, in family medicine (n = 3), pediatrics (n = 2), and general surgery (n = 1), and 18 specialty- and size-matched clinics (1:3 match), the comparator group [1]. For the intervention collaborative only, we provided an initial 1-hour educational session on safety events with a listing of all safety event types, along with a 1-page reporting form for voluntary, anonymous submission, with use of the term “safety event” rather than “ error,” to support a nonpunitive culture. After the educational session, we provided monthly audit and written and in-person feedback with peer comparison data by clinic. Monthly audit and feedback continued throughout the intervention and was discontinued postintervention. For event reporting, in our inpatient and outpatient facilities we used VIncident (Verge Solutions, Mt. Pleasant, SC) for the period 2012–2015 and RL6: Risk (RL Solutions, Toronto, ON) for 2016.
The baseline period was 15 months (January 2012–March 2013), the intervention period was 15 months (April 2013–June 2014), and the postintervention period was 30 months (July 2014–December 2016). All 24 clinics were monitored for the 60-month period.
To test the changes in the rate of safety event reporting (SER) between the pre-intervention and postintervention periods and between the intervention and the postintervention periods, interrupted time series (ITS) analysis with a control group was performed using PROC AUTOREG in SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC). Because SER rates are reported monthly, ITS analysis was used to control for autocorrelation, nonstationary variance, seasonality, and trends [2,3].
Results
The SER rate was assessed monthly, so the number of SER rates for each group (intervention and comparator) was 15 during the pre-intervention and intervention periods, respectively, and 30 during the postintervention period. During the pre-intervention period, the intervention collaborative’s baseline median rate of safety events reported was 1.5 per 10,000 patient encounters (Figure). Also, for the intervention collaborative, the pre-intervention baseline mean (standard deviation, SD) SER rate (per 10,000 patient encounters by month) was 1.3 (1.2), the intervention mean SER rate was 12.0 (7.3), and the postintervention rate was 3.2 (1.8). Based on the ITS analysis, there was a significant change in the SER rate between the intervention and postintervention periods for the intervention collaborative (P = 0.01).
The SER rate peaked in the first month following the start of the intervention. After discontinuation of feedback, reporting rates declined abruptly and reverted to baseline by 16 months post intervention (Figure). The postintervention SER rate was also significantly higher than the pre-intervention rate (P = 0.001).
For the comparator clinics, no significant change in SER rates occurred for the 3 time periods.
Discussion
In this initiative with a 5-year reporting window, we had previously shown that with education and prospective audit and feedback, we could achieve a 10-fold increase in patient SER rates among a multi-practice collaborative while the intervention was maintained [1]. Even though there was a modest but significant increase in the SER rate in the postintervention period for the 6-clinic intervention collaborative compared to baseline, the substantial gains seen during the course of the intervention were not maintained when monthly audit and feedback ceased and monitoring continued for 30 months.
Limitations of this study include possible selection bias resulting from including clinics felt likely to participate rather than identifying clinics in a random fashion. In addition, we did not attempt to determine the specific reasons for the decrease in reporting among these clinics.
The few studies of ambulatory SER do not adequately address the effect of intervention cessation, but researchers who implemented other ambulatory quality improvement efforts have reported that gains often deteriorate or revert to baseline without consistent, ongoing feedback [4]. Likewise, in hospital-based residency programs, a multifaceted approach that includes feedback can increase SER rates, but it is uncertain if the success of this approach can be maintained long-term without continuing feedback of some type [5–7].
There are likely many factors influencing SER in ambulatory clinics, many of which are also applicable in the hospital setting. These include ease of reporting, knowing what events to report, confidentiality of reporting, and the belief that reporting makes a difference in enhancing patient safety [8]. A strong culture of safety in ambulatory clinics may lead to enhanced voluntary SER [9], and a nonpunitive, team-based approach has been advocated to promote reporting and improve ambulatory safety [10]. Historically, our ambulatory medical group clinics have had a strong culture of safety and, with patient safety coaches present in all of our clinics, we have supported a nonpunitive, team-based approach to SER [11].
In our intervention, we made reporting safety events easy, reporters knew which events to report, events could be reported anonymously, and reporters were rewarded, at least with data feedback, for reporting. The only factor known to have changed was discontinuation of monthly feedback. Which factors are most important could not be determined by our work, but we strongly suspect that sustaining enhanced reporting rates requires ongoing audit and feedback to maintain a focus on event reporting.
Corresponding author: Herbert Clegg, MD, 108 Providence Road, Charlotte NC, 28207, hwclegg@novanthealth.org.
Financial disclosures: None.
1. Clegg HW, Cardwell T, West AM, Ferrell F. Improved safety event reporting in outpatient, nonacademic practices with an anonymous, nonpunitive approach. J Clin Outcomes Manag 2015;22:66–72.
2. Newland JG, Stach LM, De Lurgio SA, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc 2012; 1:179–86.
3. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13 (6 Suppl):S38–44.
4. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014;312:2569–70.
5. Steen S, Jaeger C, Price L, Griffen D. Increasing patient safety event reporting in an emergency medicine residency. BMJ Qual Improv Rep 2017;6(1).
6. Fox M, Bump G, Butler G, et al. Making residents part of the safety culture: improving error reporting and reducing harms. J Patient Saf 2017. [Epub ahead of print]
7. Dunbar AE 3rd, Cupit M, Vath RJ, et al. An improvement approach to integrate teaching teams in the reporting of safety events. Pediatrics 2017;139:e20153807.
8. Institute of Medicine. To err is human: Building a safer health system. National Academies. www.nationalacademies.org/hmd/~/media/Files/Report%20Files/1999/To-Err-is-Human/To%20Err%20is%20Human%201999%20%20report%20brief.pdf Published November 1999. Accessed August 22, 2018.
9. Miller N, Bhowmik S, Ezinwa M, et al. The relationship between safety culture and voluntary event reporting in a large regional ambulatory care group. J Patient Saf 2017. [Epub ahead of print]
10. Neuspiel DR, Stubbs EH. Patient safety in ambulatory care. Pediatr Clin North Am 2012;59:1341–54.
11. West AM, Cardwell T, Clegg HW. Improving patient safety culture through patient safety coaches in the ambulatory setting. Presented at: Institute for Healthcare Improvement Annual Summit on Improving Patient Care in the Office Practice and the Community; March 2015; Dallas, Texas.
1. Clegg HW, Cardwell T, West AM, Ferrell F. Improved safety event reporting in outpatient, nonacademic practices with an anonymous, nonpunitive approach. J Clin Outcomes Manag 2015;22:66–72.
2. Newland JG, Stach LM, De Lurgio SA, et al. Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc 2012; 1:179–86.
3. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr 2013;13 (6 Suppl):S38–44.
4. Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA 2014;312:2569–70.
5. Steen S, Jaeger C, Price L, Griffen D. Increasing patient safety event reporting in an emergency medicine residency. BMJ Qual Improv Rep 2017;6(1).
6. Fox M, Bump G, Butler G, et al. Making residents part of the safety culture: improving error reporting and reducing harms. J Patient Saf 2017. [Epub ahead of print]
7. Dunbar AE 3rd, Cupit M, Vath RJ, et al. An improvement approach to integrate teaching teams in the reporting of safety events. Pediatrics 2017;139:e20153807.
8. Institute of Medicine. To err is human: Building a safer health system. National Academies. www.nationalacademies.org/hmd/~/media/Files/Report%20Files/1999/To-Err-is-Human/To%20Err%20is%20Human%201999%20%20report%20brief.pdf Published November 1999. Accessed August 22, 2018.
9. Miller N, Bhowmik S, Ezinwa M, et al. The relationship between safety culture and voluntary event reporting in a large regional ambulatory care group. J Patient Saf 2017. [Epub ahead of print]
10. Neuspiel DR, Stubbs EH. Patient safety in ambulatory care. Pediatr Clin North Am 2012;59:1341–54.
11. West AM, Cardwell T, Clegg HW. Improving patient safety culture through patient safety coaches in the ambulatory setting. Presented at: Institute for Healthcare Improvement Annual Summit on Improving Patient Care in the Office Practice and the Community; March 2015; Dallas, Texas.
Minilaparoscopy is the next step in minimally invasive surgery
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minimally invasive surgeons have been intrigued for more than 2 decades by the clinical aspects and benefits of minilaparoscopy. Miniature instruments (2-3.5 mm) were introduced starting in the late 1980s, and through the 1990s minilaparoscopic procedures were performed across multiple specialties. However, the instrumentation available at the time had limited durability and functionality (for example, a lack of electrosurgical capability), and clinical experience and resulting data were sparse. The minilaparoscopic approach failed to gain momentum and was never widely adopted.
In the past 5-10 years, with new innovations in technology and improved instrumentation, minilaparoscopy is undergoing a renaissance in surgical circles. Medical device companies have developed numerous electrosurgical and other advanced energy options as well as a variety of needle holders, graspers, and other instruments – all with diameters of 3.5 mm or less and with significantly more durability than the earlier generation of mini-instruments. While surgeons oftentimes still use larger telescopes for better visualization, 2- to 3.5-mm telescopes are available in various lengths and angles, and optic quality is continually improving.
The minilaparoscopic approach is more similar to conventional laparoscopy than laparoendoscopic single-site surgery, which has not met early expectations. It is a more logical next step in the evolution of minimally invasive surgery and its goals of further reducing surgical trauma and improving cosmesis. I am performing hysterectomies in which I place two 5-mm nonbladed trocars through incisions inside the umbilicus and a minilaparoscopic percutaneous cannula below the bikini line; it is a “hybrid” procedure, in essence, that incorporates the use of mini-instrumentation.
In addition to diagnostic laparoscopy, I also use minilaparoscopy for some of my patients who need ovarian cystectomy, oophorectomy, appendectomy, treatment of early-stage endometriosis or adhesiolysis. Throughout the world and across multiple specialties, it is being adopted for a wide range of adult and pediatric procedures, from abdominopelvic adhesions and inguinal hernia repair to cholecystectomy, and even to enhance diagnosis in the ED or ICU.1
The importance of surgical scars
The resurgence of interest in minilaparoscopy has been driven largely by its clinical advantages. From a clinical standpoint, less intrusion through the abdominal wall with the use of smaller instruments and fewer insertion points generally means less surgical trauma, and less analgesic medication and postoperative pain, for instance, as well as fewer vascular injuries and a more minimal risk of adhesions. Scar cosmesis also has been viewed as an advantage, just as it was when the abdominal hysterectomy was being replaced by laparoscopic hysterectomy starting in 1989. Still, for me, the clinical aspects have long been at the forefront.
My interest in providing my patients the very best cosmetic results changed after we surveyed patients who were scheduled for a hysterectomy in my practice over the span of 1 year. All patients seen during that time (from November 2012 to November 2013) were asked to complete a questionnaire on their knowledge of hysterectomy incisional scars, their perceptions, and their desires. Almost all of the 200 women who completed the survey – 93% – indicated that cosmetic issues such as scars are important to them (“slightly,” “moderately,” “quite,” or “extremely” important), and of these, 24% chose “extremely important.”
Asked how they feel about the appearance of their scars from prior abdominal surgery, 58% indicated the appearance bothered them to some extent, and 11% said they were “extremely” bothered. Almost all of the 200 patients – 92% – said they would be interested in a surgery that would leave no scars, and 45% said they were “extremely” interested.2
The findings juxtaposed the clinical benefits of more minimally invasive surgery – what had been foremost on my mind – with patients’ attention to and concern about scars. The study demonstrated that patient preferences are just as compelling, if not more, than what the surgeon wants. It showed, moreover, how important it is to discuss hysterectomy incision options – and patient preferences regarding incision location, size, and number – prior to surgery.
When asked about their familiarity with the locations of skin incisions in different hysterectomy procedures (abdominal, vaginal, laparoscopic, robotic, and mini), between 25% and 56% indicated they were not at all familiar with them. Familiarity was greatest with incisions in traditional laparoscopic hysterectomy. Yet patients want to have that knowledge: Almost all of the survey participants – 93% – indicated it is important to discuss the location, number, and size of incisions prior to surgery, and 59% said it is “extremely” important.
Patients also were asked to rank a short list of incision locations (above or below the belly button, and above or below the bikini line) from the least desirable to most desirable, and the results suggest just how different personal preferences can be. The most-desirable incision location was below the bikini line for 68% of patients, followed by above the belly button for 16%. The least-desirable location was above the belly button for 69%, followed by below the bikini line for 15%. Asked whether it is cosmetically superior for one’s incisions to be low (below the bikini line), 86% said they agreed.
Other research has similarly shown that cosmesis is important for women undergoing gynecologic surgery. For instance, women in another single-practice study were more likely to prefer single-site and traditional laparoscopic incisions over robotic ones when they were shown photos of an abdomen marked up with the incision lengths and locations typical for each of these three approaches.3 And notably, there has been research looking at the psychological impact of incisional scars specifically in patients who are morbidly obese.
While we may not be accustomed to discussing incisions and scars, it behooves us as surgeons to consider initiating a conversation about incisions with all our patients – regardless of their body mass index and prior surgical history – during the preoperative evaluation.
My hysterectomy approach
I have utilized one of the most recent developments in minilaparoscopy instrumentation – the MiniLap percutaneous surgical system (Teleflex) – to develop a mini technique for hysterectomy I’ve trademarked as the Cosmetic Hysterectomy. The percutaneous system has an outer diameter of 2.3 mm, integrated needle tips that facilitate insertion without a trocar, and a selection of integrated graspers (e.g., a miniature clutch or alligator) that open up to 12.5 mm and can be advanced and retracted through the cannula. The graspers can be locked onto the tissue, and the system itself can be stabilized extracorporeally so that it can be hands free.
For the hysterectomy, I make two 5-mm vertical incisions within the umbilicus – one for a nonbladed 5-mm trocar at 12 o’clock and the other for a second nonbladed 5-mm trocar at 6 o’clock, penetrating the fascia. The trocars house a 30-degree extra-long laparoscope with camera attached, and an advanced bipolar electrosurgery device.
The minilaparoscopic cannula is inserted in the lower-abdominal area through a single 1-mm stab incision, and one or two instruments can be placed as needed. Tissues can be removed vaginally once dissection is completed, and the vaginal cuff can be closed laparoscopically or vaginally. The edges of the minilaparoscopic cannula are approximated together and held with surgical glue or a sterile skin-closure strip. There is no need to close the fascia.4
The percutaneous system opens new windows for minimally invasive surgery. It can be moved and used in several locations throughout a surgical procedure such that we can achieve more patient-specific “incisional mapping,” as I’m now calling it, rather than uniformly utilizing standard trocar placement sites.
Even without use of this particular innovation, the use of smaller instruments is proving both feasible and advantageous. A study that randomized 75 women scheduled for a hysterectomy to traditional laparoscopy (with a 5- to 10-mm port size) or minilaparoscopy (with a 3-mm port size) found no statistically significant differences in blood loss, hemoglobin drop, pain scores, or analgesic use. The authors concluded that the smaller port sizes did not affect the ability to perform the procedure. Moreover, they noted, the minilaparoscopy group had consistently smaller scars and better cosmesis.5
Another retrospective study of perioperative outcomes with standard laparoscopic, minilaparoscopic, and laparoendoscopic single-site hysterectomy found that postoperative pain control and the need for analgesic medication was significantly less with minilaparoscopy and laparoendoscopic single-site (LESS) hysterectomy, compared with traditional laparoscopy. Pain and medication in patients undergoing minilaparoscopy was reduced by more than 50%, compared with the traditional laparoscopy group, which suggests less operative trauma.6
In my practice, postoperative analgesia is simply intranasal ketorolac tromethamine (Sprix) and/or long-acting tramadol (Conzip); opioids have been eliminated in all minilaparoscopic procedures. We have had no complications, including no trocar-site bleeding, nerve entrapments, trocar-site herniations, or infections. Not every patient is a candidate for consideration of a minilaparoscopic hysterectomy, of course. The patient who has extensive adhesions from multiple previous surgeries or a large uterus with fibroids, for instance, should be treated with traditional laparoscopy regardless of her concerns regarding cosmesis.
No two surgeons are alike; each has his/her own ideas, skill sets, and approaches. Minilaparoscopy may not be for everyone, but given the number of durable miniature instruments now available, it’s an approach to consider integrating into a variety of gynecologic procedures.
For a right salpingo-oophorectomy, for instance, a 3-mm trocar placed at 12 o’clock through the umbilicus can accommodate a 3-mm scope with a high-definition camera, and an 11-mm trocar placed at 6 o’clock can house an energy device. In the right and left lower quadrants, two additional 3-mm trocars can be placed – one to accommodate a grasping instrument and the other to house the scope after the fallopian tube has been transected. A specimen bag can be passed through the 11-mm trocar in the umbilicus for removal of the ovary and tube. With the umbilicus hiding the largest of scars, the procedure is less invasive with better cosmetic results.
Dr. McCarus disclosed that he is a consultant for Ethicon.
References
1. Surg Technol Int. 2015 Nov;27:19-30.
2. Surg Technol Int. 2014 Nov;25:150-6.
3. J Minim Invasive Gynecol. 2011 Sep-Oct;18(5):640-3.
4. Surg Technol Int 2013 Sep;23:129-32.
5. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
6. Surg Endosc. 2012 Dec;26(12):3592-6.
Minilaparoscopy is a relevant surgical technique
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
With the wax and wane in the popularity of single-port surgery and with the advent of improved instrumentation, minilaparoscopy would appear to be the next long-lasting surgical technique to enhance postsurgical cosmetic appearance. For this reason, it is surprising that the use of minilaparoscopy has not been acknowledged and evaluated as a viable option more often in general surgery and urology. This, despite the fact that the use of this technique in hysterectomy was described nearly 20 years ago.1
Our minimally invasive gynecologic surgery (MIGS) team has utilized minilaparoscopy for diagnostic laparoscopy, lysis of adhesions, treatment of stage I, II, and occasionally stage III endometriosis, ovarian cystectomy, ureterolysis, presacral neurectomy, and total laparoscopic hysterectomy – as has our guest author Steven McCarus, MD. When performing hysterectomy via minilaparoscopy, our team closes the vaginal cuff laparoscopically, placing the suture transvaginally.
By removing the fibroid via a colpotomy incision, the Italian MIGS surgeon Fabio Ghezzi, MD, is able to perform myomectomy and hysterectomy routinely via minilaparoscopy.2 Articles have been published regarding the feasibility of performing minilaparoscopic surgery for both the treatment of benign adnexal mases3 and endometriosis.4
Dr. McCarus presents compelling evidence regarding the cosmetic advantage of minilaparoscopy, but the reported impact on pain has been variable: As Alyssa Small Layne et al. states, “Some studies associate minilaparoscopy with decreased pain, whereas others did not find a difference.”5 In part, this is attributable to the fact that no matter what technique is performed, the pathology must be excised. However, it is my belief that with improvements in instrumentation – as noted by Dr. McCarus and our collected added experience – the postoperative pain profile for the patient undergoing minilaparoscopy will change dramatically.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Dr. McCarus, who is the chief of gynecological surgery at Florida Hospital Celebration Health, Celebration. With over 25 years of experience, Dr. McCarus is nationally known as a leader in the practice of minimally invasive gynecologic surgery.
It is a pleasure to welcome Dr. McCarus to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class.
References
1. J Am Assoc Gynecol Laparosc. 1999 Feb;6(1):97-100.
2. J Minim Invasive Gynecol. 2011 Jul-Aug;18(4):455-61.
3. J Clin Med Res. 2017 Jul;9(7):613-7.
4. Gynecol Minim Invasive Ther. 2013 Aug;2(3):85-8.
5. Curr Opin Obstet Gynecol. 2016 Aug;28(4):255-60.
Vaginal intraepithelial neoplasia: What to do when dysplasia persists after hysterectomy
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Vaginal intraepithelial neoplasia (VAIN) is a condition that frequently poses therapeutic dilemmas for gynecologists. VAIN represents dysplastic changes to the epithelium of the vaginal mucosa, and like cervical neoplasia, the extent of disease is characterized as levels I, II, or III dependent upon the depth of involvement in the epithelial layer by dysplastic cells. While VAIN itself typically is asymptomatic and not a harmful condition, it carries a 12% risk of progression to invasive vaginal carcinoma, so accurate identification, thorough treatment, and ongoing surveillance are essential.1
VAIN is associated with high-risk human papillomavirus (HPV) infection, tobacco use, and prior cervical dysplasia. Of women with VAIN, 65% have undergone a prior hysterectomy for cervical dysplasia, which emphasizes the nondefinitive nature of such an intervention.2 These women should be very closely followed for at least 20 years with vaginal cytologic and/or HPV surveillance. High-risk HPV infection is present in 85% of women with VAIN, and the presence of high-risk HPV is a predictor for recurrent VAIN. Recurrent and persistent VAIN also is more common in postmenopausal women and those with multifocal disease.
The most common location for VAIN is at the upper third of the vagina (including the vaginal cuff). It commonly arises within the vaginal fornices, which may be difficult to fully visualize because of their puckered appearance, redundant vaginal tissues, and extensive vaginal rogation.
A diagnosis of VAIN is typically obtained from vaginal cytology which reveals atypical or dysplastic cells. Such a result should prompt the physician to perform vaginal colposcopy and directed biopsies. Comprehensive visualization of the vaginal cuff can be limited in cases where the vaginal fornices are tethered, deeply puckered, or when there is significant mucosal rogation.
The application of 4% acetic acid or Lugol’s iodine are techniques that can enhance the detection of dysplastic vaginal mucosa. Lugol’s iodine selectively stains normal, glycogenated cells, and spares dysplastic glycogen-free cells. The sharp contrast between the brown iodine-stained tissues and the white dysplastic tissues aids in detection of dysplastic areas.
If colposcopic biopsy reveals low grade dysplasia (VAIN I) it does not require intervention, and has a very low rate of conversion to invasive vaginal carcinoma. However moderate- and high-grade vaginal dysplastic lesions should be treated because of the potential for malignant transformation.
Options for treatment of VAIN include topical, ablative, and excisional procedures. Observation also is an option but should be reserved for patients who are closely monitored with repeated colposcopic examinations, and probably should best be reserved for patients with VAIN I or II lesions.
Excisional procedures
The most common excisional procedure employed for VAIN is upper vaginectomy. In this procedure, the surgeon grasps and tents up the vaginal mucosa, incises the mucosa without penetrating the subepithelial tissue layers such as bladder and rectum. The vaginal mucosa then is carefully separated from the underlying endopelvic fascial plane. The specimen should be oriented, ideally on a cork board, with pins or sutures to ascribe margins and borders. Excision is best utilized for women with unifocal disease, or those who fail or do not tolerate ablative or topical interventions.
The most significant risks of excision include the potential for damage to underlying pelvic visceral structures, which is particularly concerning in postmenopausal women with thin vaginal epithelium. Vaginectomy is commonly associated with vaginal shortening or narrowing, which can be deleterious for quality of life. Retrospective series have described a 30% incidence of recurrence after vaginectomy, likely secondary to incomplete excision of all affected tissue.3
Ablation
Ablation of dysplastic foci with a carbon dioxide (CO2) laser is a common method for treatment of VAIN. CO2 laser should ablate tissue to a 1.5 mm minimum depth.3 The benefit of using CO2 laser is its ability to treat multifocal disease in situ without an extensive excisional procedure.
It is technically more straightforward than upper vaginectomy with less blood loss and shorter surgical times, and it can be easily accomplished in an outpatient surgical or office setting. However, one of its greatest limitations is the difficulty in visualizing all lesions and therefore adequately treating all sites. The vaginal rogations also make adequate laser ablation challenging because laser only is able to effectively ablate tissue that is oriented perpendicular to the laser beam.
In addition, there is no pathologic confirmation of adequacy of excision or margin status. These features may contribute to the modestly higher rates of recurrence of dysplasia following laser ablation, compared with vaginectomy.3 It also has been associated with more vaginal scarring than vaginectomy, which can have a negative effect on sexual health.
Topical agents
The most commonly utilized topical therapy for VAIN is the antimetabolite chemotherapeutic agent 5-fluorouracil (5FU). A typical schedule for 5FU treatment is to apply vaginally, at night, once a week for 8 weeks.4 Because it can cause extensive irritation to the vulvar and urethral epithelium, patients are recommended to apply barrier creams or ointments before and following the use of 5FU for several days, wash hands thoroughly after application, and to rinse and shower in the morning after rising. Severe irritation occurs in up to 16% of patients, but in general it is very well tolerated.

Its virtue is that it is able to conform and travel to all parts of the vaginal mucosa, including those that are poorly visualized within the fornices or vaginal folds. 5FU does not require a hospitalization or surgical procedure, can be applied by the patient at home, and preserves vaginal length and function. In recent reports, 5FU is associated with the lowest rates of recurrence (10%-30%), compared with excision or ablation, and therefore is a very attractive option for primary therapy.3 However, it requires patients to have a degree of comfort with vaginal application of drug and adherence with perineal care strategies to minimize the likelihood of toxicity.
The immune response modifier, imiquimod, that is commonly used in the treatment of vulvar dysplasia also has been described in the treatment of VAIN. It appears to have high rates of clearance (greater than 75%) and be most effective in the treatment of VAIN I.5 It requires application under colposcopic guidance three times a week for 8 weeks, which is a laborious undertaking for both patient and physician. Like 5FU, imiquimod is associated with vulvar and perineal irritation.
Vaginal estrogens are an alternative topical therapy for moderate- and high-grade VAIN and particularly useful for postmenopausal patients. They have been associated with a high rate (up to 90%) of resolution on follow-up vaginal cytology testing and are not associated with toxicities of the above stated therapies.6 Vaginal estrogen can be used alone or in addition to other therapeutic strategies. For example, it can be added to the nontreatment days of 5FU or postoperatively prescribed following laser or excisional procedures.
Radiation
Intracavitary brachytherapy is a technique in which a radiation source is placed within a cylinder or ovoids and placed within the vagina.7 Typically 45 Gy is delivered to a depth 0.5mm below the vaginal mucosal surface (“point z”). Recurrence occurs is approximately 10%-15% of patients, and toxicities can be severe, including vaginal stenosis and ulceration. This aggressive therapy typically is best reserved for cases that are refractory to other therapies. Following radiation, subsequent treatments are more difficult because of radiation-induced changes to the vaginal mucosa that can affect healing.
Vaginal dysplasia is a relatively common sequelae of high-risk HPV, particularly among women who have had a prior hysterectomy for cervical dysplasia. Because of anatomic changes following hysterectomy, adequate visualization and comprehensive vaginal treatment is difficult. Therefore, surgeons should avoid utilization of hysterectomy as a routine strategy to “cure” dysplasia as it may fail to achieve this cure and make subsequent evaluations and treatments of persistent dysplasia more difficult. Women who have had a hysterectomy for dysplasia should be closely followed for several decades, and they should be counseled that they have a persistent risk for vaginal disease. When VAIN develops, clinicians should consider topical therapies as primary treatment options because they may minimize toxicity and have high rates of enduring response.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant conflicts of interest.
References
1. Gynecol Oncol. 2016 Jun;141(3):507-10.
2. Arch Gynecol Obstet. 2016 Feb;293(2):415-9.
3. Anticancer Res. 2013 Jan;33(1):29-38.
4. Obstet Gynecol. 2017 Dec;130(6):1237-43.
5. Eur J Obstet Gynecol Reprod Biol. 2017 Nov;218:129-36.
6. J Low Genit Tract Dis. 2014 Apr;18(2):115-21.
7. Gynecol Oncol. 2007 Jul;106(1):105-11.
Risk factors for postop cardiac events differ between vascular and general surgery
Predictive risk factors for cardiac events (CEs) after general and vascular surgery differed significantly, according to a large retrospective study. However, there was no significant difference seen in the overall incidence of CEs between the two types of surgery, reported Derrick Acheampong, MD, and his colleagues at the Icahn School of Medicine at Mount Sinai, New York.
They performed a retrospective data analysis of 8,441 adult patients at their large urban teaching hospital; these patients had undergone general or vascular surgery during 2013-2016 and, in the analysis, were grouped by whether they experienced postoperative CEs.
Univariate and multivariate analyses identified predictors of postoperative CE and the association of CEs with adverse postoperative outcomes. CEs were defined as myocardial infarction or cardiac arrest within the 30-day postoperative period.
A total of 157 patients (1.9%) experienced CEs after major general and vascular surgery, with no significant difference in incidence between the two types of surgery (P = .44), according to their report, published online in the Annals of Medicine and Surgery. CE-associated mortality among this group was high, at 55.4%.
The occurrence of a CE following surgery in both groups was significantly associated with increased mortality, as well as pulmonary, renal, and neurological complications, in addition to systemic sepsis, postoperative red blood cell transfusion, unplanned return to the operating room, and prolonged hospitalization, according to the researchers.
However, predictors of CEs risk between vascular and general surgery were significantly different.
For general surgery, American Society of Anesthesiologists (ASA) status greater than 3, dependent functional status, acute renal failure or dialysis, weight loss, creatinine greater than 1.2 mg/dL, international normalized ratio (INR) greater than 1.5, and partial thromboplastin time (PTT) less than 35 seconds were all unique independent predictors of postoperative CEs.
For vascular surgery, the unique significant predictors of postoperative CEs were age greater than 65 years, emergency surgery, diabetes, congestive heart failure, systemic sepsis, and operative time greater than 240 minutes.
The only common predictive risk factors for postoperative CEs for the two forms of surgery were hematocrit less than 34% and ventilator dependence.
“The present study corroborates reported studies that recommend separate predictive CE risk indices and risk stratification among different surgical specialties. Predictors for CE greatly differed between general and vascular surgery patients in our patient population,” the authors stated.
They concluded with the hope that their study “provides useful information to surgeons and allows for the necessary resources to be focused on identified at-risk patients to improve surgical outcomes.”
Dr. Acheampong and his colleagues reported having no disclosures.
SOURCE: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
Predictive risk factors for cardiac events (CEs) after general and vascular surgery differed significantly, according to a large retrospective study. However, there was no significant difference seen in the overall incidence of CEs between the two types of surgery, reported Derrick Acheampong, MD, and his colleagues at the Icahn School of Medicine at Mount Sinai, New York.
They performed a retrospective data analysis of 8,441 adult patients at their large urban teaching hospital; these patients had undergone general or vascular surgery during 2013-2016 and, in the analysis, were grouped by whether they experienced postoperative CEs.
Univariate and multivariate analyses identified predictors of postoperative CE and the association of CEs with adverse postoperative outcomes. CEs were defined as myocardial infarction or cardiac arrest within the 30-day postoperative period.
A total of 157 patients (1.9%) experienced CEs after major general and vascular surgery, with no significant difference in incidence between the two types of surgery (P = .44), according to their report, published online in the Annals of Medicine and Surgery. CE-associated mortality among this group was high, at 55.4%.
The occurrence of a CE following surgery in both groups was significantly associated with increased mortality, as well as pulmonary, renal, and neurological complications, in addition to systemic sepsis, postoperative red blood cell transfusion, unplanned return to the operating room, and prolonged hospitalization, according to the researchers.
However, predictors of CEs risk between vascular and general surgery were significantly different.
For general surgery, American Society of Anesthesiologists (ASA) status greater than 3, dependent functional status, acute renal failure or dialysis, weight loss, creatinine greater than 1.2 mg/dL, international normalized ratio (INR) greater than 1.5, and partial thromboplastin time (PTT) less than 35 seconds were all unique independent predictors of postoperative CEs.
For vascular surgery, the unique significant predictors of postoperative CEs were age greater than 65 years, emergency surgery, diabetes, congestive heart failure, systemic sepsis, and operative time greater than 240 minutes.
The only common predictive risk factors for postoperative CEs for the two forms of surgery were hematocrit less than 34% and ventilator dependence.
“The present study corroborates reported studies that recommend separate predictive CE risk indices and risk stratification among different surgical specialties. Predictors for CE greatly differed between general and vascular surgery patients in our patient population,” the authors stated.
They concluded with the hope that their study “provides useful information to surgeons and allows for the necessary resources to be focused on identified at-risk patients to improve surgical outcomes.”
Dr. Acheampong and his colleagues reported having no disclosures.
SOURCE: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
Predictive risk factors for cardiac events (CEs) after general and vascular surgery differed significantly, according to a large retrospective study. However, there was no significant difference seen in the overall incidence of CEs between the two types of surgery, reported Derrick Acheampong, MD, and his colleagues at the Icahn School of Medicine at Mount Sinai, New York.
They performed a retrospective data analysis of 8,441 adult patients at their large urban teaching hospital; these patients had undergone general or vascular surgery during 2013-2016 and, in the analysis, were grouped by whether they experienced postoperative CEs.
Univariate and multivariate analyses identified predictors of postoperative CE and the association of CEs with adverse postoperative outcomes. CEs were defined as myocardial infarction or cardiac arrest within the 30-day postoperative period.
A total of 157 patients (1.9%) experienced CEs after major general and vascular surgery, with no significant difference in incidence between the two types of surgery (P = .44), according to their report, published online in the Annals of Medicine and Surgery. CE-associated mortality among this group was high, at 55.4%.
The occurrence of a CE following surgery in both groups was significantly associated with increased mortality, as well as pulmonary, renal, and neurological complications, in addition to systemic sepsis, postoperative red blood cell transfusion, unplanned return to the operating room, and prolonged hospitalization, according to the researchers.
However, predictors of CEs risk between vascular and general surgery were significantly different.
For general surgery, American Society of Anesthesiologists (ASA) status greater than 3, dependent functional status, acute renal failure or dialysis, weight loss, creatinine greater than 1.2 mg/dL, international normalized ratio (INR) greater than 1.5, and partial thromboplastin time (PTT) less than 35 seconds were all unique independent predictors of postoperative CEs.
For vascular surgery, the unique significant predictors of postoperative CEs were age greater than 65 years, emergency surgery, diabetes, congestive heart failure, systemic sepsis, and operative time greater than 240 minutes.
The only common predictive risk factors for postoperative CEs for the two forms of surgery were hematocrit less than 34% and ventilator dependence.
“The present study corroborates reported studies that recommend separate predictive CE risk indices and risk stratification among different surgical specialties. Predictors for CE greatly differed between general and vascular surgery patients in our patient population,” the authors stated.
They concluded with the hope that their study “provides useful information to surgeons and allows for the necessary resources to be focused on identified at-risk patients to improve surgical outcomes.”
Dr. Acheampong and his colleagues reported having no disclosures.
SOURCE: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.
FROM ANNALS OF MEDICINE AND SURGERY
Key clinical point: There was a significant difference in predictive risk factors for postoperative cardiac events between vascular and general surgery.
Major finding: The 1.9% incidence of cardiac events following general or vascular surgery was associated with a mortality rate of 55%.
Study details: Retrospective study of 8,441 patients who underwent vascular or general surgery during 2013-2015.
Disclosures: The authors reported having no disclosures.
Source: Acheampong D et al. Ann Med Surg. 2018. doi: 10.1016/j.amsu.2018.08.001.