User login
MD-IQ only
Occurrence of pulmonary embolisms in hospitalized patients nearly doubled during 2004-2015
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
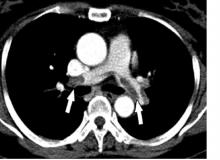
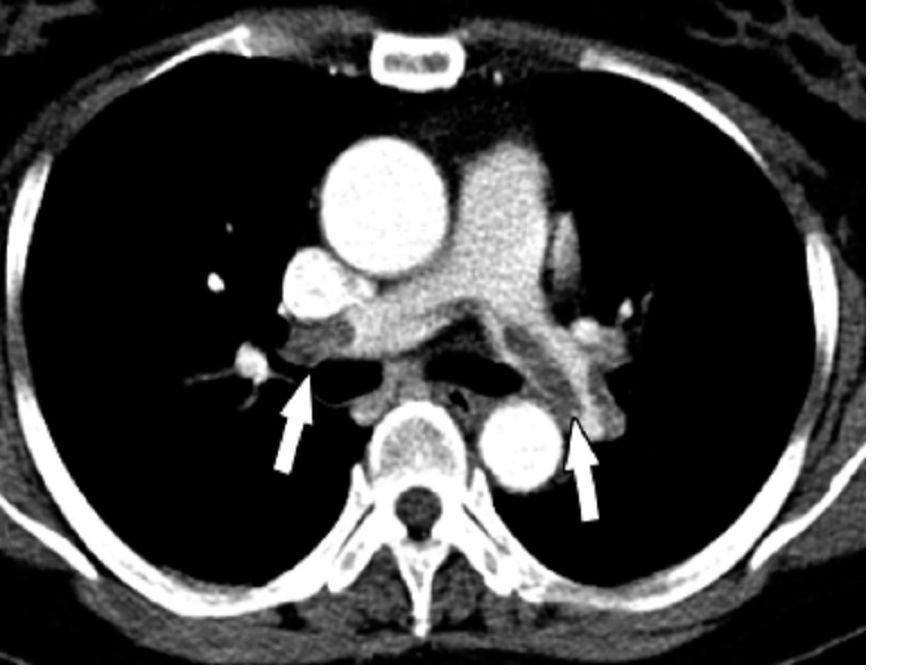
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
REPORTING FROM ACC 2019
Algorithm ruled out PE, averts radiation exposure in pregnant women
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
FROM The New England Journal of Medicine
AUGUSTUS: Dual surpasses triple therapy when AFib patients have PCI or ACS
NEW ORLEANS – For patients with atrial fibrillation and either a recent acute coronary syndrome or percutaneous coronary intervention, combined treatment for 6 months with the anticoagulant apixaban and a P2Y12 inhibitor antiplatelet drug, but without aspirin, was safer than and as effective as a regimen that either also included aspirin or that substituted a vitamin K antagonist, such as warfarin, for the direct-acting oral anticoagulant, based on results from a multicenter, randomized trial with more than 4,600 patients.
The apixaban plus P2Y12 inhibitor (typically, clopidogrel) combination “resulted in less bleeding and fewer hospitalizations without significant differences in ischemic events than regimens that included a vitamin K antagonist, aspirin, or both,” Renato D. Lopes, MD, said at the annual meeting of the American College of Cardiology. Concurrently, his report of the results also appeared in an online article.
This finding in the AUGUSTUS trial gives clinicians more guidance for the long-standing dilemma of how to best prevent arterial thrombus formation in patients with atrial fibrillation (AFib). To prevent a stroke, these patients routinely receive treatment with an anticoagulant when they have an acute coronary syndrome (ACS) event or undergo percutaneous coronary intervention (PCI). Typically, they receive several months of dual antiplatelet therapy with aspirin plus a P2Y12 inhibitor to prevent a clot from forming in the stented or unstable region of a coronary artery.
These patients are not uncommon; this circumstance occurs for about 20% of all AFib patients, and poses the question of what is the safest and most effective way to treat them. Should they get triple therapy with an anticoagulant, aspirin, and a P2Y12 inhibitor, an option that could cause excess bleeding; or should one of the three drugs drop out with the potential for an increased rate of ischemic events? The AUGUSTUS findings suggest that one solution is treatment with a combination of the direct-acting oral anticoagulant apixaban (Eliquis) and the P2Y12 inhibitor clopidogrel (Plavix) but without aspirin.
For the majority of patients like the ones enrolled, “less is more.” By dropping aspirin from the treatment mix, patients did better, said Dr. Lopes, a professor of medicine at Duke University in Durham, N.C.
Dr. Lopes and his associates designed AUGUSTUS (A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis [Blood Clots] Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart) as a two-by-two factorial study to address two different questions: During 6 months of treatment, how did apixaban compare with a vitamin K antagonist (usually warfarin) in these patients for safety and efficacy, and how did aspirin compare with placebo in this setting for the same endpoints?
The trial enrolled 4,614 patients at 492 sites in 33 countries. All patients in the study received a P2Y12 inhibitor, with 93% treated with clopidogrel. The study had roughly as many patients as the combined total of patients enrolled in two smaller, prior studies that had looked at roughly the same questions in similar patients.
“The aspirin part is the more interesting, and probably more unique and important finding,” John H. Alexander, MD, a coinvestigator on the study, said in a video interview. Regardless of the anticoagulant used, patients who received aspirin had a 16% rate of major bleeds or clinically relevant non-major bleeds, compared with a 9% rate among those on placebo, a statistically significant result that underscored the bleeding risk posed by adding aspirin to an anticoagulant and a P2Y12 inhibitor.
The results also showed no statistically significant difference in any efficacy measure with or without aspirin, including the rate of death or hospitalization, or of any individual ischemic endpoint. However the results showed a signal of a small increase in the rates of each of three types of ischemic events – stent thrombosis, MI, and need for urgent revascularization, each of which showed a numerical increase when aspirin was dropped. But the increase was small.
Dr. Lopes calculated that, for example, to prevent one episode of stent thrombosis by treating with aspirin also would cause 15 major or clinically relevant non-major bleeds, which makes inclusion of aspirin something of a judgment call for each patient, said Dr. Alexander, a professor of medicine at Duke. An AFib patient with a high risk for thrombosis but a low risk for bleeding following PCI or an ACS event might be a reasonable patient to treat with aspirin along with apixaban and a P2Y12 inhibitor, he explained.
The rate of major or clinically relevant bleeds was 11% with apixaban and 15% with a vitamin K antagonist, a statistically significant difference. Patients treated with apixaban also had a significantly reduced rate of death or hospitalization, 24%, compared with 27% among those on the vitamin K antagonist, as well as a significantly lower rate of stroke.
Overall the lowest bleeding rate was in patients on apixaban but no aspirin, a 7% rate, while the highest rate was in patients on a vitamin K antagonist plus aspirin, a 19% rate.
Dr. Alexander said that it would be an overreach to extrapolate these findings to other direct-acting oral anticoagulants, compared with a vitamin K antagonist, but he believed that the findings the study generated about aspirin were probably relevant regardless of the anticoagulant used.
mzoler@mdedge.com
On Twitter @mitchelzoler
It’s very reassuring to see that you can use a direct-acting oral anticoagulant like apixaban along with a P2Y12 inhibitor, but with no aspirin, and have no statistically significant increase in ischemic events. This is a fantastic finding. The finding shows once again that warfarin is a problematic drug. As the cost for direct-acting oral anticoagulants has decreased, their use has increased.
These results were not unexpected and also are probably the final nail in the coffin for using a combination of warfarin and aspirin. Prior findings from the PIONEER AF-PCI study that used rivaroxaban (N Engl J Med. 2016 Dec 22;375[25]:2423-34) and from the RE-DUAL PCI study that used dabigatran (N Engl J Med. 2017 Oct 19;377[16]:1513-24) also showed the advantages of using a direct-acting oral anticoagulant when compared with a vitamin K antagonist in this setting, The AUGUSTUS trial, with just over 4,600 patients, had nearly as many patients as the roughly 4,850 enrolled in these two prior studies put together.
Dhanunjaya Lakkireddy, MD , is medical director of the Kansas City Heart Rhythm Institute in Overland Park. He had no disclosures. He made these comments as the designated discussant during a press briefing.
It’s very reassuring to see that you can use a direct-acting oral anticoagulant like apixaban along with a P2Y12 inhibitor, but with no aspirin, and have no statistically significant increase in ischemic events. This is a fantastic finding. The finding shows once again that warfarin is a problematic drug. As the cost for direct-acting oral anticoagulants has decreased, their use has increased.
These results were not unexpected and also are probably the final nail in the coffin for using a combination of warfarin and aspirin. Prior findings from the PIONEER AF-PCI study that used rivaroxaban (N Engl J Med. 2016 Dec 22;375[25]:2423-34) and from the RE-DUAL PCI study that used dabigatran (N Engl J Med. 2017 Oct 19;377[16]:1513-24) also showed the advantages of using a direct-acting oral anticoagulant when compared with a vitamin K antagonist in this setting, The AUGUSTUS trial, with just over 4,600 patients, had nearly as many patients as the roughly 4,850 enrolled in these two prior studies put together.
Dhanunjaya Lakkireddy, MD , is medical director of the Kansas City Heart Rhythm Institute in Overland Park. He had no disclosures. He made these comments as the designated discussant during a press briefing.
It’s very reassuring to see that you can use a direct-acting oral anticoagulant like apixaban along with a P2Y12 inhibitor, but with no aspirin, and have no statistically significant increase in ischemic events. This is a fantastic finding. The finding shows once again that warfarin is a problematic drug. As the cost for direct-acting oral anticoagulants has decreased, their use has increased.
These results were not unexpected and also are probably the final nail in the coffin for using a combination of warfarin and aspirin. Prior findings from the PIONEER AF-PCI study that used rivaroxaban (N Engl J Med. 2016 Dec 22;375[25]:2423-34) and from the RE-DUAL PCI study that used dabigatran (N Engl J Med. 2017 Oct 19;377[16]:1513-24) also showed the advantages of using a direct-acting oral anticoagulant when compared with a vitamin K antagonist in this setting, The AUGUSTUS trial, with just over 4,600 patients, had nearly as many patients as the roughly 4,850 enrolled in these two prior studies put together.
Dhanunjaya Lakkireddy, MD , is medical director of the Kansas City Heart Rhythm Institute in Overland Park. He had no disclosures. He made these comments as the designated discussant during a press briefing.
NEW ORLEANS – For patients with atrial fibrillation and either a recent acute coronary syndrome or percutaneous coronary intervention, combined treatment for 6 months with the anticoagulant apixaban and a P2Y12 inhibitor antiplatelet drug, but without aspirin, was safer than and as effective as a regimen that either also included aspirin or that substituted a vitamin K antagonist, such as warfarin, for the direct-acting oral anticoagulant, based on results from a multicenter, randomized trial with more than 4,600 patients.
The apixaban plus P2Y12 inhibitor (typically, clopidogrel) combination “resulted in less bleeding and fewer hospitalizations without significant differences in ischemic events than regimens that included a vitamin K antagonist, aspirin, or both,” Renato D. Lopes, MD, said at the annual meeting of the American College of Cardiology. Concurrently, his report of the results also appeared in an online article.
This finding in the AUGUSTUS trial gives clinicians more guidance for the long-standing dilemma of how to best prevent arterial thrombus formation in patients with atrial fibrillation (AFib). To prevent a stroke, these patients routinely receive treatment with an anticoagulant when they have an acute coronary syndrome (ACS) event or undergo percutaneous coronary intervention (PCI). Typically, they receive several months of dual antiplatelet therapy with aspirin plus a P2Y12 inhibitor to prevent a clot from forming in the stented or unstable region of a coronary artery.
These patients are not uncommon; this circumstance occurs for about 20% of all AFib patients, and poses the question of what is the safest and most effective way to treat them. Should they get triple therapy with an anticoagulant, aspirin, and a P2Y12 inhibitor, an option that could cause excess bleeding; or should one of the three drugs drop out with the potential for an increased rate of ischemic events? The AUGUSTUS findings suggest that one solution is treatment with a combination of the direct-acting oral anticoagulant apixaban (Eliquis) and the P2Y12 inhibitor clopidogrel (Plavix) but without aspirin.
For the majority of patients like the ones enrolled, “less is more.” By dropping aspirin from the treatment mix, patients did better, said Dr. Lopes, a professor of medicine at Duke University in Durham, N.C.
Dr. Lopes and his associates designed AUGUSTUS (A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis [Blood Clots] Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart) as a two-by-two factorial study to address two different questions: During 6 months of treatment, how did apixaban compare with a vitamin K antagonist (usually warfarin) in these patients for safety and efficacy, and how did aspirin compare with placebo in this setting for the same endpoints?
The trial enrolled 4,614 patients at 492 sites in 33 countries. All patients in the study received a P2Y12 inhibitor, with 93% treated with clopidogrel. The study had roughly as many patients as the combined total of patients enrolled in two smaller, prior studies that had looked at roughly the same questions in similar patients.
“The aspirin part is the more interesting, and probably more unique and important finding,” John H. Alexander, MD, a coinvestigator on the study, said in a video interview. Regardless of the anticoagulant used, patients who received aspirin had a 16% rate of major bleeds or clinically relevant non-major bleeds, compared with a 9% rate among those on placebo, a statistically significant result that underscored the bleeding risk posed by adding aspirin to an anticoagulant and a P2Y12 inhibitor.
The results also showed no statistically significant difference in any efficacy measure with or without aspirin, including the rate of death or hospitalization, or of any individual ischemic endpoint. However the results showed a signal of a small increase in the rates of each of three types of ischemic events – stent thrombosis, MI, and need for urgent revascularization, each of which showed a numerical increase when aspirin was dropped. But the increase was small.
Dr. Lopes calculated that, for example, to prevent one episode of stent thrombosis by treating with aspirin also would cause 15 major or clinically relevant non-major bleeds, which makes inclusion of aspirin something of a judgment call for each patient, said Dr. Alexander, a professor of medicine at Duke. An AFib patient with a high risk for thrombosis but a low risk for bleeding following PCI or an ACS event might be a reasonable patient to treat with aspirin along with apixaban and a P2Y12 inhibitor, he explained.
The rate of major or clinically relevant bleeds was 11% with apixaban and 15% with a vitamin K antagonist, a statistically significant difference. Patients treated with apixaban also had a significantly reduced rate of death or hospitalization, 24%, compared with 27% among those on the vitamin K antagonist, as well as a significantly lower rate of stroke.
Overall the lowest bleeding rate was in patients on apixaban but no aspirin, a 7% rate, while the highest rate was in patients on a vitamin K antagonist plus aspirin, a 19% rate.
Dr. Alexander said that it would be an overreach to extrapolate these findings to other direct-acting oral anticoagulants, compared with a vitamin K antagonist, but he believed that the findings the study generated about aspirin were probably relevant regardless of the anticoagulant used.
mzoler@mdedge.com
On Twitter @mitchelzoler
NEW ORLEANS – For patients with atrial fibrillation and either a recent acute coronary syndrome or percutaneous coronary intervention, combined treatment for 6 months with the anticoagulant apixaban and a P2Y12 inhibitor antiplatelet drug, but without aspirin, was safer than and as effective as a regimen that either also included aspirin or that substituted a vitamin K antagonist, such as warfarin, for the direct-acting oral anticoagulant, based on results from a multicenter, randomized trial with more than 4,600 patients.
The apixaban plus P2Y12 inhibitor (typically, clopidogrel) combination “resulted in less bleeding and fewer hospitalizations without significant differences in ischemic events than regimens that included a vitamin K antagonist, aspirin, or both,” Renato D. Lopes, MD, said at the annual meeting of the American College of Cardiology. Concurrently, his report of the results also appeared in an online article.
This finding in the AUGUSTUS trial gives clinicians more guidance for the long-standing dilemma of how to best prevent arterial thrombus formation in patients with atrial fibrillation (AFib). To prevent a stroke, these patients routinely receive treatment with an anticoagulant when they have an acute coronary syndrome (ACS) event or undergo percutaneous coronary intervention (PCI). Typically, they receive several months of dual antiplatelet therapy with aspirin plus a P2Y12 inhibitor to prevent a clot from forming in the stented or unstable region of a coronary artery.
These patients are not uncommon; this circumstance occurs for about 20% of all AFib patients, and poses the question of what is the safest and most effective way to treat them. Should they get triple therapy with an anticoagulant, aspirin, and a P2Y12 inhibitor, an option that could cause excess bleeding; or should one of the three drugs drop out with the potential for an increased rate of ischemic events? The AUGUSTUS findings suggest that one solution is treatment with a combination of the direct-acting oral anticoagulant apixaban (Eliquis) and the P2Y12 inhibitor clopidogrel (Plavix) but without aspirin.
For the majority of patients like the ones enrolled, “less is more.” By dropping aspirin from the treatment mix, patients did better, said Dr. Lopes, a professor of medicine at Duke University in Durham, N.C.
Dr. Lopes and his associates designed AUGUSTUS (A Study of Apixaban in Patients With Atrial Fibrillation, Not Caused by a Heart Valve Problem, Who Are at Risk for Thrombosis [Blood Clots] Due to Having Had a Recent Coronary Event, Such as a Heart Attack or a Procedure to Open the Vessels of the Heart) as a two-by-two factorial study to address two different questions: During 6 months of treatment, how did apixaban compare with a vitamin K antagonist (usually warfarin) in these patients for safety and efficacy, and how did aspirin compare with placebo in this setting for the same endpoints?
The trial enrolled 4,614 patients at 492 sites in 33 countries. All patients in the study received a P2Y12 inhibitor, with 93% treated with clopidogrel. The study had roughly as many patients as the combined total of patients enrolled in two smaller, prior studies that had looked at roughly the same questions in similar patients.
“The aspirin part is the more interesting, and probably more unique and important finding,” John H. Alexander, MD, a coinvestigator on the study, said in a video interview. Regardless of the anticoagulant used, patients who received aspirin had a 16% rate of major bleeds or clinically relevant non-major bleeds, compared with a 9% rate among those on placebo, a statistically significant result that underscored the bleeding risk posed by adding aspirin to an anticoagulant and a P2Y12 inhibitor.
The results also showed no statistically significant difference in any efficacy measure with or without aspirin, including the rate of death or hospitalization, or of any individual ischemic endpoint. However the results showed a signal of a small increase in the rates of each of three types of ischemic events – stent thrombosis, MI, and need for urgent revascularization, each of which showed a numerical increase when aspirin was dropped. But the increase was small.
Dr. Lopes calculated that, for example, to prevent one episode of stent thrombosis by treating with aspirin also would cause 15 major or clinically relevant non-major bleeds, which makes inclusion of aspirin something of a judgment call for each patient, said Dr. Alexander, a professor of medicine at Duke. An AFib patient with a high risk for thrombosis but a low risk for bleeding following PCI or an ACS event might be a reasonable patient to treat with aspirin along with apixaban and a P2Y12 inhibitor, he explained.
The rate of major or clinically relevant bleeds was 11% with apixaban and 15% with a vitamin K antagonist, a statistically significant difference. Patients treated with apixaban also had a significantly reduced rate of death or hospitalization, 24%, compared with 27% among those on the vitamin K antagonist, as well as a significantly lower rate of stroke.
Overall the lowest bleeding rate was in patients on apixaban but no aspirin, a 7% rate, while the highest rate was in patients on a vitamin K antagonist plus aspirin, a 19% rate.
Dr. Alexander said that it would be an overreach to extrapolate these findings to other direct-acting oral anticoagulants, compared with a vitamin K antagonist, but he believed that the findings the study generated about aspirin were probably relevant regardless of the anticoagulant used.
mzoler@mdedge.com
On Twitter @mitchelzoler
REPORTING FROM ACC 19
Repeat VTE risk heightened in HIV patients
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
REPORTING FROM CROI 2019
Myeloma therapies raise cardiovascular risks
WASHINGTON – Proteasome inhibitors are essential components of therapeutic regimens for multiple myeloma, but at least one member of this class of life-extending agents, carfilzomib (Kyprolis), is also associated with a significant increase in risk of heart failure, cautioned a specialist in plasma cell disorders.
In addition, immunomodulating agents such as lenalidomide (Revlimid) and pomalidomide (Pomalyst) are associated with increased risk for thromboembolic events, said R. Frank Cornell, MD, clinical director of plasma cell disorders at Vanderbilt University Medical Center in Nashville, Tenn.
In an ongoing, prospective study comparing rates of cardiac adverse events in patients receiving carfilzomib or another proteasome inhibitor, bortezomib (Velcade), Dr. Cornell and his colleagues found that while there were no significant differences in progression-free survival (PFS) or overall survival (OS) between the treatments, “patients who experienced a cardiovascular event had significantly worse progression-free and overall survival compared to those that did not have a cardiovascular event,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
The Prospective Observation of Cardiac Safety With Proteasome Inhibition (PROTECT) trial, scheduled for completion in August 2019, enrolled 95 patients with relapsed multiple myeloma and randomly assigned them on a 2:1 basis to receive carfilzomib or bortezomib.
The investigators found that cardiovascular adverse events occurred in 33 of the 65 patients (51%) randomized to carfilzomib, compared with 5 of 30 patients (17%) assigned to bortezomib.
The events included grade 1 or 2 heart failure (HF) in 12 patients on carfilzomib vs. 2 on bortezomib, and grade 3 or 4 HF in 11 vs. 1, respectively. Hypertension was significantly more frequent among patients on carfilzomib, and one patient on carfilzomib died from the acute coronary syndrome 24 hours after receiving carfilzomib in the second week of treatment.
The investigators found that both B-type natriuretic peptide (BNP) and N-terminal pro b-type natriuretic peptide (NT-proBNP) were highly predictive of cardiovascular adverse events. Patients on carfilzomib who had levels of the markers above normal at baseline had an odds ratio (OR) for cardiovascular events of 7.39 (P less than .0001), and those with BNP or NT-proBNP increases at week 2 or 3 during cycle 1 had an OR for a cardiovascular adverse event of 63.5 (P less than .001).
In multivariate analysis, the risk for cardiovascular events for patients treated with carfilzomib was significantly lower for patients with one or no traditional cardiovascular risk factors, compared with patients with two or more.
“Prospective monitoring with natriuretic peptides should be considered, particularly early in treatment,” Dr. Cornell said.
IMiDs and thromboembolism
In early clinical trials of immunomodulators (IMiDs) for multiple myeloma, investigators saw that the incidence of thromboembolic events was lower among patients who received thromboprophylaxis than among those who did not, Dr. Cornell noted.
“From this, certain guidelines have been developed such that all patients considered to be at risk should at least receive an aspirin, 81-325 mg, and patients at higher risk for thromboembolism should receive low-molecular-weight heparin or therapeutic-dose warfarin,” he said.
There is little guidance, however, about the use of direct oral anticoagulants in this population, he added, a fact that prompted him and his colleagues in oncology and cardiology to perform a pilot study of apixaban (Eliquis) for primary prevention of venous thromboembolism (VTE) in patients with multiple myeloma who were receiving immunodulatory drugs.
Results of the pilot study, reported in a poster session at the 2018 annual meeting of the American Society of Hematology, showed that among 50 patients who received apixaban 2.5 mg twice daily for 6 months during IMiD therapy, there were no VTEs, stroke, or myocardial infarction, and no episodes of major bleeding. There were just three nonmajor bleeding events, and one early withdrawal from apixaban due to an allergic reaction manifesting as generalized edema.
“Further study is needed to validate this as a potential primary prophylaxis in patients receiving IMiDs for multiple myeloma,” Dr. Cornell said.
He reported having no financial disclosures. Millennium Pharmaceuticals is a sponsor of the PROTECT trial.
WASHINGTON – Proteasome inhibitors are essential components of therapeutic regimens for multiple myeloma, but at least one member of this class of life-extending agents, carfilzomib (Kyprolis), is also associated with a significant increase in risk of heart failure, cautioned a specialist in plasma cell disorders.
In addition, immunomodulating agents such as lenalidomide (Revlimid) and pomalidomide (Pomalyst) are associated with increased risk for thromboembolic events, said R. Frank Cornell, MD, clinical director of plasma cell disorders at Vanderbilt University Medical Center in Nashville, Tenn.
In an ongoing, prospective study comparing rates of cardiac adverse events in patients receiving carfilzomib or another proteasome inhibitor, bortezomib (Velcade), Dr. Cornell and his colleagues found that while there were no significant differences in progression-free survival (PFS) or overall survival (OS) between the treatments, “patients who experienced a cardiovascular event had significantly worse progression-free and overall survival compared to those that did not have a cardiovascular event,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
The Prospective Observation of Cardiac Safety With Proteasome Inhibition (PROTECT) trial, scheduled for completion in August 2019, enrolled 95 patients with relapsed multiple myeloma and randomly assigned them on a 2:1 basis to receive carfilzomib or bortezomib.
The investigators found that cardiovascular adverse events occurred in 33 of the 65 patients (51%) randomized to carfilzomib, compared with 5 of 30 patients (17%) assigned to bortezomib.
The events included grade 1 or 2 heart failure (HF) in 12 patients on carfilzomib vs. 2 on bortezomib, and grade 3 or 4 HF in 11 vs. 1, respectively. Hypertension was significantly more frequent among patients on carfilzomib, and one patient on carfilzomib died from the acute coronary syndrome 24 hours after receiving carfilzomib in the second week of treatment.
The investigators found that both B-type natriuretic peptide (BNP) and N-terminal pro b-type natriuretic peptide (NT-proBNP) were highly predictive of cardiovascular adverse events. Patients on carfilzomib who had levels of the markers above normal at baseline had an odds ratio (OR) for cardiovascular events of 7.39 (P less than .0001), and those with BNP or NT-proBNP increases at week 2 or 3 during cycle 1 had an OR for a cardiovascular adverse event of 63.5 (P less than .001).
In multivariate analysis, the risk for cardiovascular events for patients treated with carfilzomib was significantly lower for patients with one or no traditional cardiovascular risk factors, compared with patients with two or more.
“Prospective monitoring with natriuretic peptides should be considered, particularly early in treatment,” Dr. Cornell said.
IMiDs and thromboembolism
In early clinical trials of immunomodulators (IMiDs) for multiple myeloma, investigators saw that the incidence of thromboembolic events was lower among patients who received thromboprophylaxis than among those who did not, Dr. Cornell noted.
“From this, certain guidelines have been developed such that all patients considered to be at risk should at least receive an aspirin, 81-325 mg, and patients at higher risk for thromboembolism should receive low-molecular-weight heparin or therapeutic-dose warfarin,” he said.
There is little guidance, however, about the use of direct oral anticoagulants in this population, he added, a fact that prompted him and his colleagues in oncology and cardiology to perform a pilot study of apixaban (Eliquis) for primary prevention of venous thromboembolism (VTE) in patients with multiple myeloma who were receiving immunodulatory drugs.
Results of the pilot study, reported in a poster session at the 2018 annual meeting of the American Society of Hematology, showed that among 50 patients who received apixaban 2.5 mg twice daily for 6 months during IMiD therapy, there were no VTEs, stroke, or myocardial infarction, and no episodes of major bleeding. There were just three nonmajor bleeding events, and one early withdrawal from apixaban due to an allergic reaction manifesting as generalized edema.
“Further study is needed to validate this as a potential primary prophylaxis in patients receiving IMiDs for multiple myeloma,” Dr. Cornell said.
He reported having no financial disclosures. Millennium Pharmaceuticals is a sponsor of the PROTECT trial.
WASHINGTON – Proteasome inhibitors are essential components of therapeutic regimens for multiple myeloma, but at least one member of this class of life-extending agents, carfilzomib (Kyprolis), is also associated with a significant increase in risk of heart failure, cautioned a specialist in plasma cell disorders.
In addition, immunomodulating agents such as lenalidomide (Revlimid) and pomalidomide (Pomalyst) are associated with increased risk for thromboembolic events, said R. Frank Cornell, MD, clinical director of plasma cell disorders at Vanderbilt University Medical Center in Nashville, Tenn.
In an ongoing, prospective study comparing rates of cardiac adverse events in patients receiving carfilzomib or another proteasome inhibitor, bortezomib (Velcade), Dr. Cornell and his colleagues found that while there were no significant differences in progression-free survival (PFS) or overall survival (OS) between the treatments, “patients who experienced a cardiovascular event had significantly worse progression-free and overall survival compared to those that did not have a cardiovascular event,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
The Prospective Observation of Cardiac Safety With Proteasome Inhibition (PROTECT) trial, scheduled for completion in August 2019, enrolled 95 patients with relapsed multiple myeloma and randomly assigned them on a 2:1 basis to receive carfilzomib or bortezomib.
The investigators found that cardiovascular adverse events occurred in 33 of the 65 patients (51%) randomized to carfilzomib, compared with 5 of 30 patients (17%) assigned to bortezomib.
The events included grade 1 or 2 heart failure (HF) in 12 patients on carfilzomib vs. 2 on bortezomib, and grade 3 or 4 HF in 11 vs. 1, respectively. Hypertension was significantly more frequent among patients on carfilzomib, and one patient on carfilzomib died from the acute coronary syndrome 24 hours after receiving carfilzomib in the second week of treatment.
The investigators found that both B-type natriuretic peptide (BNP) and N-terminal pro b-type natriuretic peptide (NT-proBNP) were highly predictive of cardiovascular adverse events. Patients on carfilzomib who had levels of the markers above normal at baseline had an odds ratio (OR) for cardiovascular events of 7.39 (P less than .0001), and those with BNP or NT-proBNP increases at week 2 or 3 during cycle 1 had an OR for a cardiovascular adverse event of 63.5 (P less than .001).
In multivariate analysis, the risk for cardiovascular events for patients treated with carfilzomib was significantly lower for patients with one or no traditional cardiovascular risk factors, compared with patients with two or more.
“Prospective monitoring with natriuretic peptides should be considered, particularly early in treatment,” Dr. Cornell said.
IMiDs and thromboembolism
In early clinical trials of immunomodulators (IMiDs) for multiple myeloma, investigators saw that the incidence of thromboembolic events was lower among patients who received thromboprophylaxis than among those who did not, Dr. Cornell noted.
“From this, certain guidelines have been developed such that all patients considered to be at risk should at least receive an aspirin, 81-325 mg, and patients at higher risk for thromboembolism should receive low-molecular-weight heparin or therapeutic-dose warfarin,” he said.
There is little guidance, however, about the use of direct oral anticoagulants in this population, he added, a fact that prompted him and his colleagues in oncology and cardiology to perform a pilot study of apixaban (Eliquis) for primary prevention of venous thromboembolism (VTE) in patients with multiple myeloma who were receiving immunodulatory drugs.
Results of the pilot study, reported in a poster session at the 2018 annual meeting of the American Society of Hematology, showed that among 50 patients who received apixaban 2.5 mg twice daily for 6 months during IMiD therapy, there were no VTEs, stroke, or myocardial infarction, and no episodes of major bleeding. There were just three nonmajor bleeding events, and one early withdrawal from apixaban due to an allergic reaction manifesting as generalized edema.
“Further study is needed to validate this as a potential primary prophylaxis in patients receiving IMiDs for multiple myeloma,” Dr. Cornell said.
He reported having no financial disclosures. Millennium Pharmaceuticals is a sponsor of the PROTECT trial.
REPORTING FROM ACC CARDIO-ONCOLOGY
FDA: Safety signal emerged with higher dose of tofacitinib in RA study
the Food and Drug Administration reported.
The trial’s Data Safety and Monitoring Board identified the signal in patients taking a 10-mg dose of tofacitinib twice daily, the FDA said in a safety announcement.
Pfizer, the trial’s sponsor, took “immediate action” to transition patients in the ongoing trial from the 10-mg, twice-daily dose to 5 mg twice daily, which is the approved dose for adult patients with moderate to severe rheumatoid arthritis, the agency said. The 10-mg, twice-daily dose is approved only in the dosing regimen for patients with ulcerative colitis. Xeljanz is also approved to treat psoriatic arthritis. The 11-mg, once-daily dose of Xeljanz XR that is approved to treat rheumatoid arthritis and psoriatic arthritis was not tested in the trial.
The ongoing study was designed to assess risks of cardiovascular events, cancer, and opportunistic infections with tofacitinib 10 mg twice daily or 5 mg twice daily versus the risks in a control group treated with a tumor necrosis factor (TNF) inhibitor, according to the statement.
Patients had to be 50 years of age or older and have at least one cardiovascular risk factor to be eligible for the study, which was required by the agency in 2012 when it approved tofacitinib, the statement says.
The FDA is reviewing trial data and working with Pfizer to better understand the safety signal, its effect on patients, and how tofacitinib should be used, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a news release. The trial will continue and is expected to be completed by the end of 2019.
“The agency will take appropriate action, as warranted, to ensure patients enrolled in this and other trials are protected and that health care professionals and clinical trial researchers understand the risks associated with this use,” she added.
Health care professionals should follow tofacitinib prescribing information, monitor patients for the signs and symptoms of pulmonary embolism, and advise patients to seek medical attention immediately if they experience those signs and symptoms, according to the statement.
“We are communicating now, given the serious nature of the safety issue, to ensure that patients taking tofacitinib are aware that the FDA still believes the benefits of taking tofacitinib for its approved uses continue to outweigh the risks,” Dr. Woodcock said in the release.
While not approved in rheumatoid arthritis, the 10-mg, twice-daily dose of tofacitinib is approved in the dosing regimen for patients with ulcerative colitis, the release says.
the Food and Drug Administration reported.
The trial’s Data Safety and Monitoring Board identified the signal in patients taking a 10-mg dose of tofacitinib twice daily, the FDA said in a safety announcement.
Pfizer, the trial’s sponsor, took “immediate action” to transition patients in the ongoing trial from the 10-mg, twice-daily dose to 5 mg twice daily, which is the approved dose for adult patients with moderate to severe rheumatoid arthritis, the agency said. The 10-mg, twice-daily dose is approved only in the dosing regimen for patients with ulcerative colitis. Xeljanz is also approved to treat psoriatic arthritis. The 11-mg, once-daily dose of Xeljanz XR that is approved to treat rheumatoid arthritis and psoriatic arthritis was not tested in the trial.
The ongoing study was designed to assess risks of cardiovascular events, cancer, and opportunistic infections with tofacitinib 10 mg twice daily or 5 mg twice daily versus the risks in a control group treated with a tumor necrosis factor (TNF) inhibitor, according to the statement.
Patients had to be 50 years of age or older and have at least one cardiovascular risk factor to be eligible for the study, which was required by the agency in 2012 when it approved tofacitinib, the statement says.
The FDA is reviewing trial data and working with Pfizer to better understand the safety signal, its effect on patients, and how tofacitinib should be used, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a news release. The trial will continue and is expected to be completed by the end of 2019.
“The agency will take appropriate action, as warranted, to ensure patients enrolled in this and other trials are protected and that health care professionals and clinical trial researchers understand the risks associated with this use,” she added.
Health care professionals should follow tofacitinib prescribing information, monitor patients for the signs and symptoms of pulmonary embolism, and advise patients to seek medical attention immediately if they experience those signs and symptoms, according to the statement.
“We are communicating now, given the serious nature of the safety issue, to ensure that patients taking tofacitinib are aware that the FDA still believes the benefits of taking tofacitinib for its approved uses continue to outweigh the risks,” Dr. Woodcock said in the release.
While not approved in rheumatoid arthritis, the 10-mg, twice-daily dose of tofacitinib is approved in the dosing regimen for patients with ulcerative colitis, the release says.
the Food and Drug Administration reported.
The trial’s Data Safety and Monitoring Board identified the signal in patients taking a 10-mg dose of tofacitinib twice daily, the FDA said in a safety announcement.
Pfizer, the trial’s sponsor, took “immediate action” to transition patients in the ongoing trial from the 10-mg, twice-daily dose to 5 mg twice daily, which is the approved dose for adult patients with moderate to severe rheumatoid arthritis, the agency said. The 10-mg, twice-daily dose is approved only in the dosing regimen for patients with ulcerative colitis. Xeljanz is also approved to treat psoriatic arthritis. The 11-mg, once-daily dose of Xeljanz XR that is approved to treat rheumatoid arthritis and psoriatic arthritis was not tested in the trial.
The ongoing study was designed to assess risks of cardiovascular events, cancer, and opportunistic infections with tofacitinib 10 mg twice daily or 5 mg twice daily versus the risks in a control group treated with a tumor necrosis factor (TNF) inhibitor, according to the statement.
Patients had to be 50 years of age or older and have at least one cardiovascular risk factor to be eligible for the study, which was required by the agency in 2012 when it approved tofacitinib, the statement says.
The FDA is reviewing trial data and working with Pfizer to better understand the safety signal, its effect on patients, and how tofacitinib should be used, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in a news release. The trial will continue and is expected to be completed by the end of 2019.
“The agency will take appropriate action, as warranted, to ensure patients enrolled in this and other trials are protected and that health care professionals and clinical trial researchers understand the risks associated with this use,” she added.
Health care professionals should follow tofacitinib prescribing information, monitor patients for the signs and symptoms of pulmonary embolism, and advise patients to seek medical attention immediately if they experience those signs and symptoms, according to the statement.
“We are communicating now, given the serious nature of the safety issue, to ensure that patients taking tofacitinib are aware that the FDA still believes the benefits of taking tofacitinib for its approved uses continue to outweigh the risks,” Dr. Woodcock said in the release.
While not approved in rheumatoid arthritis, the 10-mg, twice-daily dose of tofacitinib is approved in the dosing regimen for patients with ulcerative colitis, the release says.
ICYMI: Rivaroxaban reduces VTE incidence in ambulatory cancer patients
While treatment with rivaroxaban did not significantly reduce venous thromboembolism incidence in high-risk ambulatory patients with cancer over the entire course of a 180-day intervention period (6.0% vs. 8.8% in controls; hazard ratio, 0.66; 95% confidence interval, 0.40-1.09), it did reduce major bleeding incidence while patients were on treatment (2.0% vs. 6.4%; HR, 0.40; 95% CI, 0.20 0.80), according to results from the multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3b CASSINI trial published in the New England Journal of Medicine (2019 Feb 20. doi: 10.1056/NEJMoa1814630).
We reported this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
While treatment with rivaroxaban did not significantly reduce venous thromboembolism incidence in high-risk ambulatory patients with cancer over the entire course of a 180-day intervention period (6.0% vs. 8.8% in controls; hazard ratio, 0.66; 95% confidence interval, 0.40-1.09), it did reduce major bleeding incidence while patients were on treatment (2.0% vs. 6.4%; HR, 0.40; 95% CI, 0.20 0.80), according to results from the multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3b CASSINI trial published in the New England Journal of Medicine (2019 Feb 20. doi: 10.1056/NEJMoa1814630).
We reported this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
While treatment with rivaroxaban did not significantly reduce venous thromboembolism incidence in high-risk ambulatory patients with cancer over the entire course of a 180-day intervention period (6.0% vs. 8.8% in controls; hazard ratio, 0.66; 95% confidence interval, 0.40-1.09), it did reduce major bleeding incidence while patients were on treatment (2.0% vs. 6.4%; HR, 0.40; 95% CI, 0.20 0.80), according to results from the multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3b CASSINI trial published in the New England Journal of Medicine (2019 Feb 20. doi: 10.1056/NEJMoa1814630).
We reported this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Supplementary compression doesn’t improve DVT odds in critically ill
SAN DIEGO – In critically ill patients receiving pharmacologic thromboprophylaxis, (DVT), according to a new trial.
“I was surprised. My hypothesis was that it would work,” said lead author Yaseen M. Arabi, MD, chairman of the intensive care department at King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
Many physicians routinely carry out the practice on the assumption that IPC should lead to better blood flow and further cut DVT risk. The procedure carries few risks, aside from patient discomfort. “The main issue is that it’s not needed. It might be useful in patients who are not receiving heparin or low-molecular-weight heparin,” said Dr. Arabi, who presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published online in the New England Journal of Medicine.
Unfractionated or low-molecular-weight heparin reduces the risk of DVT by about 50%, but about 5%-20% of critically ill patients will develop DVT in spite of treatment, and mechanical thromboprophylaxis reduces DVT risk, compared with no prophylaxis. Some researchers have attempted to address whether adjunct intermittent pneumatic compression could further reduce DVT risk, but their studies were marked by a lack of controls, unoptimized pharmacologic regimens, and other limitations.
The trial included 2,003 adults from 20 sites in Saudi Arabia, Canada, Australia, and India, who were expected to have an intensive care unit stay of at least 72 hours. They were randomized to receive IPC combined with pharmacologic thromboprophylaxis (pneumatic compression group) or pharmacologic thromboprophylaxis alone (control).
The proportion of patients receiving unfractionated heparin versus low-molecular-weight heparin was similar between the two groups, with about 58% treated with unfractionated heparin.
A total of 3.9% of patients in the pneumatic compression group experienced incident proximal DVT, compared with 4.2% of controls (relative risk, 0.93; P =.74). A total of 3.4% experienced prevalent proximal DVT, compared with 2.7% of controls (RR, 1.29; 95% confidence interval, 0.78-2.12). There was no significant difference in the incidence of any lower-limb DVT (9.6% vs. 8.4%; RR, 1.14; 95% CI, 0.86-1.51).
There was no difference between the two groups in a composite outcome that included pulmonary embolism or all prevalent and incident lower-limb DVT (RR, 1.11; 95% CI, 0.85-1.44), and there were no between-group differences with respect to lower-limb skin injury or ischemia.
The results should change practice among those who still provide adjunct intermittent pneumatic compression, however surprising physicians may find these new results to be, according to Dr. Arabi: “People believed strongly that (adjunct IPC) should work, but you need to be evidence based, and here it showed no difference. But that’s why we do studies, right?”
The study was funded by King Abdulaziz City for Science and Technology and King Abdullah International Medical Research Center. Dr. Arabi has no relevant financial conflicts.
SOURCE: Arabi Y et al. CCC48, Abstract 142. N Engl J Med Feb 18. doi: 10.1056/NEJMoa1816150.
SAN DIEGO – In critically ill patients receiving pharmacologic thromboprophylaxis, (DVT), according to a new trial.
“I was surprised. My hypothesis was that it would work,” said lead author Yaseen M. Arabi, MD, chairman of the intensive care department at King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
Many physicians routinely carry out the practice on the assumption that IPC should lead to better blood flow and further cut DVT risk. The procedure carries few risks, aside from patient discomfort. “The main issue is that it’s not needed. It might be useful in patients who are not receiving heparin or low-molecular-weight heparin,” said Dr. Arabi, who presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published online in the New England Journal of Medicine.
Unfractionated or low-molecular-weight heparin reduces the risk of DVT by about 50%, but about 5%-20% of critically ill patients will develop DVT in spite of treatment, and mechanical thromboprophylaxis reduces DVT risk, compared with no prophylaxis. Some researchers have attempted to address whether adjunct intermittent pneumatic compression could further reduce DVT risk, but their studies were marked by a lack of controls, unoptimized pharmacologic regimens, and other limitations.
The trial included 2,003 adults from 20 sites in Saudi Arabia, Canada, Australia, and India, who were expected to have an intensive care unit stay of at least 72 hours. They were randomized to receive IPC combined with pharmacologic thromboprophylaxis (pneumatic compression group) or pharmacologic thromboprophylaxis alone (control).
The proportion of patients receiving unfractionated heparin versus low-molecular-weight heparin was similar between the two groups, with about 58% treated with unfractionated heparin.
A total of 3.9% of patients in the pneumatic compression group experienced incident proximal DVT, compared with 4.2% of controls (relative risk, 0.93; P =.74). A total of 3.4% experienced prevalent proximal DVT, compared with 2.7% of controls (RR, 1.29; 95% confidence interval, 0.78-2.12). There was no significant difference in the incidence of any lower-limb DVT (9.6% vs. 8.4%; RR, 1.14; 95% CI, 0.86-1.51).
There was no difference between the two groups in a composite outcome that included pulmonary embolism or all prevalent and incident lower-limb DVT (RR, 1.11; 95% CI, 0.85-1.44), and there were no between-group differences with respect to lower-limb skin injury or ischemia.
The results should change practice among those who still provide adjunct intermittent pneumatic compression, however surprising physicians may find these new results to be, according to Dr. Arabi: “People believed strongly that (adjunct IPC) should work, but you need to be evidence based, and here it showed no difference. But that’s why we do studies, right?”
The study was funded by King Abdulaziz City for Science and Technology and King Abdullah International Medical Research Center. Dr. Arabi has no relevant financial conflicts.
SOURCE: Arabi Y et al. CCC48, Abstract 142. N Engl J Med Feb 18. doi: 10.1056/NEJMoa1816150.
SAN DIEGO – In critically ill patients receiving pharmacologic thromboprophylaxis, (DVT), according to a new trial.
“I was surprised. My hypothesis was that it would work,” said lead author Yaseen M. Arabi, MD, chairman of the intensive care department at King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia.
Many physicians routinely carry out the practice on the assumption that IPC should lead to better blood flow and further cut DVT risk. The procedure carries few risks, aside from patient discomfort. “The main issue is that it’s not needed. It might be useful in patients who are not receiving heparin or low-molecular-weight heparin,” said Dr. Arabi, who presented the results of the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine. The study was simultaneously published online in the New England Journal of Medicine.
Unfractionated or low-molecular-weight heparin reduces the risk of DVT by about 50%, but about 5%-20% of critically ill patients will develop DVT in spite of treatment, and mechanical thromboprophylaxis reduces DVT risk, compared with no prophylaxis. Some researchers have attempted to address whether adjunct intermittent pneumatic compression could further reduce DVT risk, but their studies were marked by a lack of controls, unoptimized pharmacologic regimens, and other limitations.
The trial included 2,003 adults from 20 sites in Saudi Arabia, Canada, Australia, and India, who were expected to have an intensive care unit stay of at least 72 hours. They were randomized to receive IPC combined with pharmacologic thromboprophylaxis (pneumatic compression group) or pharmacologic thromboprophylaxis alone (control).
The proportion of patients receiving unfractionated heparin versus low-molecular-weight heparin was similar between the two groups, with about 58% treated with unfractionated heparin.
A total of 3.9% of patients in the pneumatic compression group experienced incident proximal DVT, compared with 4.2% of controls (relative risk, 0.93; P =.74). A total of 3.4% experienced prevalent proximal DVT, compared with 2.7% of controls (RR, 1.29; 95% confidence interval, 0.78-2.12). There was no significant difference in the incidence of any lower-limb DVT (9.6% vs. 8.4%; RR, 1.14; 95% CI, 0.86-1.51).
There was no difference between the two groups in a composite outcome that included pulmonary embolism or all prevalent and incident lower-limb DVT (RR, 1.11; 95% CI, 0.85-1.44), and there were no between-group differences with respect to lower-limb skin injury or ischemia.
The results should change practice among those who still provide adjunct intermittent pneumatic compression, however surprising physicians may find these new results to be, according to Dr. Arabi: “People believed strongly that (adjunct IPC) should work, but you need to be evidence based, and here it showed no difference. But that’s why we do studies, right?”
The study was funded by King Abdulaziz City for Science and Technology and King Abdullah International Medical Research Center. Dr. Arabi has no relevant financial conflicts.
SOURCE: Arabi Y et al. CCC48, Abstract 142. N Engl J Med Feb 18. doi: 10.1056/NEJMoa1816150.
REPORTING FROM CCC48
ICYMI: Andexanet alfa reduces anti–factor Xa activity from apixaban, rivaroxaban
Patients with acute major bleeding associated with factor Xa inhibitor usage who received andexanet alfa experienced a significant decrease in anti–factor Xa activity, with more than three-quarters of patients experiencing good or excellent hemostatic efficiency after 12 hours. That finding emerged from the multicenter, prospective, open-label, single-group ANNEXA-4 trial published in the New England Journal of Medicine (2019 Feb 11. doi: 10.1056/NEJMoa1814051).
We reported this story at the annual meeting of the American College of Cardiology before it was published in the journal. Find our coverage at the link below.
Patients with acute major bleeding associated with factor Xa inhibitor usage who received andexanet alfa experienced a significant decrease in anti–factor Xa activity, with more than three-quarters of patients experiencing good or excellent hemostatic efficiency after 12 hours. That finding emerged from the multicenter, prospective, open-label, single-group ANNEXA-4 trial published in the New England Journal of Medicine (2019 Feb 11. doi: 10.1056/NEJMoa1814051).
We reported this story at the annual meeting of the American College of Cardiology before it was published in the journal. Find our coverage at the link below.
Patients with acute major bleeding associated with factor Xa inhibitor usage who received andexanet alfa experienced a significant decrease in anti–factor Xa activity, with more than three-quarters of patients experiencing good or excellent hemostatic efficiency after 12 hours. That finding emerged from the multicenter, prospective, open-label, single-group ANNEXA-4 trial published in the New England Journal of Medicine (2019 Feb 11. doi: 10.1056/NEJMoa1814051).
We reported this story at the annual meeting of the American College of Cardiology before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Venous thromboembolism risk elevated in ankylosing spondylitis patients
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
Newly diagnosed ankylosing spondylitis (AS) patients are at increased risk for venous thromboembolism (VTE), especially during the first year after diagnosis, according to a population-based study of 7,190 cases.
In a study published in Annals of the Rheumatic Diseases, the researchers identified 7,190 incident cases of AS among adults using a health care database of residents of British Columbia and matched them for age, sex, and entry time into the cohort with 71,900 healthy individuals from the general population over a mean follow-up time of 6.2 years.
The incidence rate of VTE overall per 1,000 person-years was 1.56 among AS patients, compared with 0.77 in a control cohort from the general population. The incidence rates for DVT were 1.06 in AS patients and 0.50 in controls; incidence rates for PE were 0.79 in AS patients and 0.40 in controls.
The adjusted hazard ratios for VTE overall and DVT were similar and statistically significant in AS patients at 1.53 and 1.62, respectively, versus controls. But the adjusted hazard ratio of 1.36 for PE did not reach statistical significance. The adjusted risks of VTE overall, PE, and DVT were highest in the first year of diagnosis, reaching twofold greater risk for all, but none of the risks were statistically significant.
More research is needed to better identify subsets of AS patients at increased risk for VTE, and to assess whether treatment of inflammation can mitigate this risk, but in the meantime clinicians should be alert to the possibility of life-threatening complications from DVT and PE in their AS patients, especially soon after diagnosis, the researchers said.
The findings are supported by the study’s large sample size but are also limited by several factors, including the observational nature of the study and an inability to account for use of NSAIDs, the researchers noted.
“These results call for awareness of this complication, increased vigilance, and preventive intervention by controlling the inflammatory process or by anticoagulation in a high-risk AS population,” they concluded.
The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
SOURCE: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Newly diagnosed AS patients demonstrated increased risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism, compared with controls.
Major finding: The relative risk for deep vein thrombosis was 63% higher for AS patients versus controls, but a 39% higher risk of pulmonary embolism did not reach statistical significance.
Study details: A population-based study including 7,190 incident AS cases and 71,900 matched controls from a health care database of residents of British Columbia.
Disclosures: The study was supported in part by grants from the Canadian Arthritis Network, the Arthritis Society of Canada, the British Columbia Lupus Society, and the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
Source: Aviña-Zubieta JA et al. Ann Rheum Dis. 2019 Feb 8. doi: 10.1136/annrheumdis-2018-214388.