User login
MD-IQ only
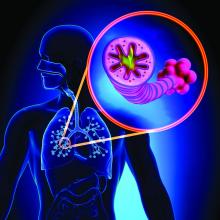
Biomarkers predict VTE risk with menopausal oral hormone therapy
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
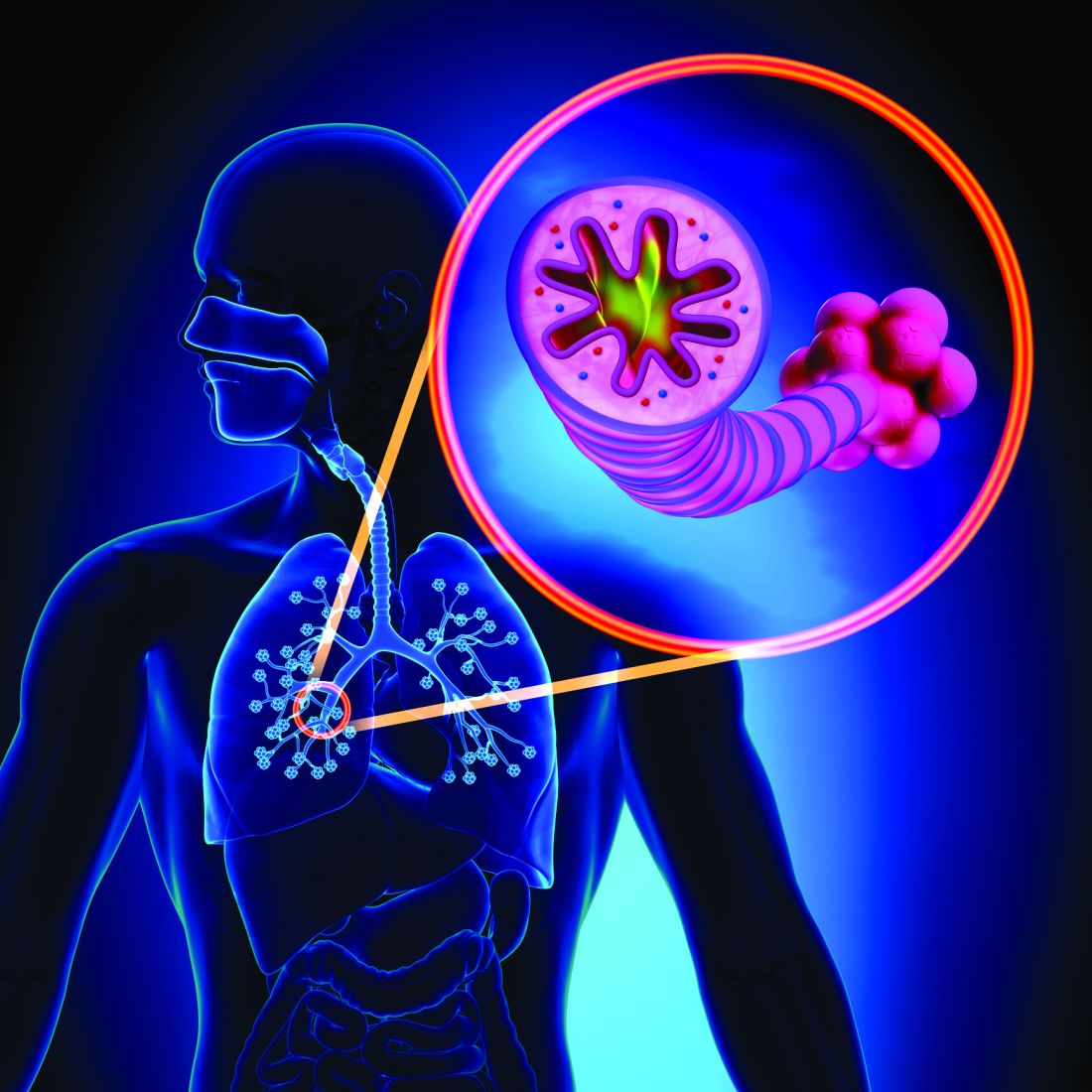
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
CHICAGO – An elevated baseline D-dimer level is helpful to women and their physicians in clarifying decision making about oral hormone therapy for troublesome menopausal symptoms, Mary Cushman, MD, said at the American Heart Association scientific sessions.
She was lead investigator in a nested case-control study embedded in the landmark Women’s Health Initiative (WHI), which showed that participants who had a baseline D-dimer greater than 0.54 mg/L – putting them in the top 25% – and were randomized to oral menopausal hormone therapy had a 5-year incidence of venous thromboembolism (VTE) of 6%. That’s 500% higher than in women with a lower D-dimer randomized to placebo.
“The number needed to test for D-dimer in advance of prescribing in order to prevent one VTE over 5 years of hormone therapy was only 33. So this is potentially something in the toolbox you can use in counseling women about oral hormone therapy,” said Dr. Cushman, professor of medicine and pathology and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
The biomarker study included 1,082 WHI participants aged 50-79 years randomized to oral conjugated equine estrogen with or without medroxyprogesterone acetate or to placebo, 215 of whom experienced VTE during a mean 4.1 years of follow-up. Levels of a variety of biomarkers obtained at baseline were assessed in terms of their associated risk of future VTE. The biomarkers included C-reactive protein and procoagulant, anticoagulant, and fibrinolytic factors.
In a logistic regression analysis adjusted for age, race, body mass index, and hysterectomy, the strongest association with VTE was a high D-dimer. That 500% increased risk of VTE with hormone therapy in women with a D-dimer greater than 0.54 mg/L was comparable in magnitude with the risk Dr. Cushman and her coinvestigators previously reported for the combination of factor V Leiden and hormone therapy.
Dr. Cushman and her associates also took a first step towards developing a multibiomarker risk score. They found that WHI participants randomized to hormone therapy who had abnormal baseline values for any three or more of eight biomarkers had a 1,450% greater risk of future VTE than women with zero or one abnormal biomarker who were assigned to placebo. The eight-biomarker panel described in the recently published study comprised D-dimer, factor V Leiden, protein C, total protein S, free protein S, antithrombin, plasmin-antiplasmin complex, and fragment 1.2. However, the investigators indicated the risk score needs further study before it’s ready for adoption in clinical practice (Res Pract Thromb Haemost. 2018 Apr 17;2[2]:310-9).
Dr. Cushman noted that, although the main findings of the WHI have largely resulted in abandonment of menopausal hormone therapy for disease prevention, many women still want to take oral hormone therapy for relief of bothersome menopausal symptoms. She tries to steer them instead to safer nonoral formulations. Transdermal estrogen replacement has no associated risk of VTE and doesn’t activate anticoagulation. Neither does vaginal estradiol.
In offering what she called “the 30,000-foot view of the impact of venous thrombosis on women’s health,” Dr. Cushman noted that VTE is the third-most common vascular disease in the United States, with up to 900,000 cases per year. The lifetime risk in women after age 45 is 8.4%. Half of VTEs are provoked and therefore potentially preventable, with common triggers being surgery, cancer, pregnancy, trauma, and immobilization, especially during travel.
In addition, a retrospective study conducted in the Worcester, Mass., area showed that 1-month mortality after VTE remained static in the 5%-10% range during 1999-2009.
“This is a fatal disease, even though we treat it as an outpatient quite a lot,” Dr. Cushman observed.
Common nonfatal complications of VTE include major bleeding in 5%-10% of cases, a recurrence rate of 5%-10% annually, a 20%-40% of the burdensome and not infrequently disabling condition known as postthrombotic syndrome, and a 3%-4% incidence of chronic thromboembolic pulmonary hypertension. Yet despite the seriousness of VTE, awareness about VTE is poor among both patients and physicians, and appropriate prophylaxis is underutilized, she said.
The key to improved primary prevention of VTE, Dr. Cushman continued, is greater attention to modifiable behavioral risk factors, along with more use of prophylactic medication when needed.
The traditional cardiovascular risk factors, like hypertension, smoking, and hyperlipidemia, aren’t relevant to VTE risk. But obesity and sedentary lifestyle have come to be recognized as important modifiable risk factors. In one study of more than 30,000 Americans, the risk of VTE was shown to be reduced by 40% in individuals who exercised at least four times per week, compared with the physically inactive.
And in an analysis led by Dr. Cushman of nearly 21,000 participants over age 45 years with 12.6 years of follow-up in the Longitudinal Investigation of Thromboembolism Etiology (LITE), the investigators found that greater levels of all body size measures – not just body mass index, but calf circumference, waist-hip ratio, hip circumference, and others – were associated with increased VTE risk. These associations weren’t affected by levels of circulating biomarkers for inflammation or hypercoagulability, suggesting that it’s obesity per se, with its associated adverse impact on blood flow caused by physical factors, that explains the mechanism underlying obesity as a risk factor for VTE (Thromb Res. 2016 Aug;144:127-32).
At the meeting’s opening ceremonies, AHA President Ivor Benjamin, MD, of the Medical College of Wisconsin, Milwaukee, presented Dr. Cushman with the AHA Population Research Prize. She was honored for her “critically acclaimed research utilizing biomarker assessments in population studies to elucidate pathways of disease etiology for the three most common vascular diseases – coronary heart disease, stroke, and venous thromboembolism – as well as their risk factors,” said Dr. Benjamin.
Dr. Cushman reported having no financial conflicts regarding her D-dimer study, which was funded by the National Institutes of Health.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Women in the top 25% for D-dimer level before going on menopausal hormone therapy had a 6% incidence of venous thromboembolism over 5 years.
Study details: This was a nested case-control study focused on identifying biomarkers for venous thromboembolism risk which included 1,082 participants in the Women’s Health Initiative randomized to menopausal hormone therapy or placebo.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by the National Institutes of Health.
Immunotherapy’s cardiac effects require early monitoring, management
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
WASHINGTON – Unquestionably, immunotherapy is revolutionizing the care of patients with various solid tumors and hematologic malignancies.
But it’s equally true that there’s no such thing as either a free lunch or a cancer therapy free of side effects, whether it’s increased risk for heart failure associated with anthracycline-based chemotherapy, or inflammatory conditions, arrhythmias, and thromboembolic events associated with immune checkpoint inhibitors, said R. Frank Cornell, MD, of Vanderbilt University Medical Center in Nashville, Tenn.
“Early awareness and intervention is critical for improved outcomes, and a multidisciplinary approach between oncology, cardiology, the clinic nurse, and other health care providers is critical in managing these patients with these complicated therapies,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Checkpoint inhibitors and the heart
Toxicities associated with immune checkpoint inhibitors such as the programmed death 1/ligand 1 (PD-1/PD-L1) inhibitors nivolumab (Opdivo) and pembrolizumab (Keytruda) and the cytotoxic T-lymphocyte antigen 4 antibody ipilimumab (Yervoy) tend to mimic autoimmune conditions, Dr. Cornell said.
Cardiovascular events associated with these agents, while uncommon, include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, vasculitis, and venous thromboembolism, he said, citing an American Society of Clinical Oncology (ASCO) clinical practice guideline (J Clin Oncol 2018;36[17]:1714-68).
Dr. Cornell described the case of a 63-year-old woman with disseminated metastatic melanoma who presented to the emergency department 10 days after starting on combination therapy with ipilimumab and nivolumab. She had developed shortness of breath, pleuritic chest pain, and a mild cough for 1 or 2 days.
Her cardiac laboratory markers had been normal at baseline, but were markedly elevated on presentation, and electrocardiograms showed complete heart block and subsequent ventricular tachycardia.
The patient was started on high-dose prednisone, but she died in hospital, and an autopsy showed that the cause of death was infiltration into the myocardium of CD3-positive and CD8-positive T lymphocytes.
“So how do we manage this? This is a good opportunity, I think, for further cardiology and oncology collaboration to develop more robust guidelines for what we can do to best prevent this,” Dr. Cornell said.
Patients started on the ipilimumab/nivolumab combination should be tested weekly for cardiac troponin, creatine kinase (CK) and CK-muscle/brain (CK-MB) weekly for the first 3-4 weeks of therapy. Therapy should be stopped if troponin levels continue to rise, and the patient should be started on high-dose steroids, he said.
The role of other anti-inflammatory agents such as infliximab (Remicade and biosimilars) is unclear and needs further study, he added.
Dr. Cornell cited a 2018 letter to The Lancet by Javid J. Moslehi, MD, and colleagues from Vanderbilt describing an increase in reports of fatal myocarditis among patients treated with checkpoint inhibitors.
“We highlight the high mortality rate with severe immune checkpoint inhibitor–related myocarditis, which is more frequent with combination PD-1 and CTLA-4 blockade, but can also occur with monotherapy. Myocarditis was observed across immune checkpoint inhibitor regimens, although it remains too early to determine whether the incidence differs between use of anti-PD1 and anti-PD-L1 drugs. Furthermore, this condition occurs early on during therapy and across cancer types,” they wrote.
Most of the patients had no preexisting cardiovascular disease, and most were not taking medications for hypertension, cardiovascular disease, or diabetes.
CAR-T cells and cardiac disease
The primary cardiac complications associated with CAR-T cell therapy are related to the cytokine release syndrome (CRS), a condition marked by progressive elevation in inflammatory cytokines that in turn leads to marked elevations in C-reactive protein (CRP), interferon gamma, tumor necrosis factor al, and release of pro-inflammatory cytokines including interleukin (IL) 6, IL-10, IL-12, and IL-1 beta.
In rare instances, CRS can lead to disseminated intravascular coagulation (DIC), capillary leak syndrome, and a hemophagocytic lymphohistiocytosis-like (HLH) syndrome, Dr. Cornell said.
Package inserts for the two Food and Drug Administration–approved CAR-T cell products, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) show that each was associated in clinical trials with a high incidence of CRS.
Among patients treated with axicabtagene ciloleucel, 94% developed CRS, which was grade 3 or greater in severity in 13%. The median time to onset was 2 days, and the median duration was 7 days. Cardiovascular adverse events included grade 3 or greater tachycardia in 2%, arrhythmias in 7%, edema in 1%, dyspnea in 3%, pleural effusion in 2%, hypotension in 15%, hypertension in 6%, and thrombosis in 1%.
Among patients treated with tisagenlecleucel, 79% treated for B-cell acute lymphoblastic leukemia (B-ALL) and 74% treated for diffuse large B cell lymphoma (DLBCL) developed CRS, which was grade 3 or greater in 49% and 23% of patients, respectively. The median time to onset was 3 days, and the median duration of CRS was 8 days.
Cardiovascular adverse events of grade 3 or greater among these patients included tachycardia in 4%, fluid overload in 7%, edema in 1%, dyspnea in 12%, pulmonary edema in 4%, hypotension in 22%, and hypertension in 6%.
Risk factors for CRS include high pre-infusion tumor burden, active infections, and concurrent inflammatory processes, Dr. Cornell said.
Prevention of cardiovascular complications of CAR-T cell therapy requires management of CRS. Patients with grade 2 or greater CRS should receive the anti-IL-6 agent tocilizumab (Actemra) 8 mg/kg intravenously over 1 hour to a maximum dose of 800 mg. Tocilizumab infusions can be repeated every 8 hours as needed if the patient is not responsive to intravenous fluids or increasing supplement oxygen, but should be limited to a maximum of three doses over 24 hours, and a maximum total of four doses.
Patients with grade 3 CRS should also receive intravenous methylprednisolone 1 mg/kg twice daily or the equivalent amount of dexamethasone, with corticosteroids continued until the severity of CRS is grade 1 or less, then tapered over 3 days,
Patients with grade 4 CRS should also receive IV methylprednisolone 1,000 mg per day for 3 days, and if symptoms improve, continue management as per grade 3, Dr. Cornell said.
Dr. Cornell reported having nothing to disclose.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Monitor for cardiac symptoms and treat or interrupt immunotherapy as needed.
Major finding: Immune checkpoint inhibitors and CAR T-cell therapies are associated with distinct cardiovascular adverse events.
Study details: Review of strategies for managing the cardiovascular consequences of cancer immunotherapies.
Disclosures: Dr. Cornell reported having nothing to disclose.
New recall for CoaguChek test strips issued
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
According to a release, the Food and Drug Administration has identified this recall as Class I, which is the most serious type of recall and indicates that “use of these devices may cause serious injuries or death.”
These strips are used by patients taking warfarin to help determine the patients’ international normalized ratio, which doctors and patients then use to decide whether the dose is appropriate. Roche Diagnostics, the strips’ manufacturer, issued a recall in September 2018; the test strips distributed by Terrific Care and Medex, however, were not labeled or authorized for sale in the United States and were therefore not included in that original recall. According to the release, the strips in this recall, which was initiated Dec. 21, 2018, were purchased by Terrific Care and Medex from an unknown source and then distributed in the United States. On Jan. 28, 2019, Terrific Care sent an Urgent Medical Device Recall Notification Letter to customers.
The full recall is described on the FDA website.
COPD linked to higher in-hospital death rates in patients with PAD
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
A growing body of evidence suggests that, along with other vascular beds, smoking and chronic obstructive pulmonary disease (COPD) affect the arteries of the lower limbs in terms of the development of peripheral arterial disease (PAD), reported Karsten Keller, MD, of the Johannes Gutenberg-University Mainz (Germany) and his colleagues.
This provided the rationale for their large database analysis of inpatients with concomitant COPD and PAD. They found that the additional presence of COPD was associated with increased in-hospital mortality in patients with PAD.
“Our data suggest that COPD increased the mortality of PAD patients by the factor 1.2-fold,” they wrote in Respiratory Medicine. “Unexpectedly, this increase was not driven by [myocardial infarction] as the life-threatening acute presentation of [coronary artery disease], but rather related to an increased risk for [pulmonary embolism] and a higher coprevalence of cancer.”
Dr. Keller and his colleagues inspected the German inpatient national database based on ICD codes. They identified 5,611,827 adult inpatients (64.8% men) diagnosed with PAD between January 2005 and December 2015, and of those, 13.6% also were coded for COPD. Overall, 277,894 PAD patients (5.0%) died in the hospital, Dr. Keller and his colleagues wrote.
The all-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without COPD (6.5% vs. 4.7%, respectively; P less than .001), and cardiovascular events comprising pulmonary embolism (PE), deep vein thrombosis (DVT), and myocardial infarction (MI) occurred more often in coprevalence with PAD and COPD than in PAD without COPD.
In PAD patients, COPD was an independent predictor of in-hospital death (odds ratio, 1.16; 95% confidence interval, 1.15-1.17; P less than .001) as well as an independent predictor for PE (OR, 1.44; 95% CI, 1.40-1.49; P less than .001).
Overall, PAD patients with COPD were of similar age as (73 years), but stayed slightly longer in the hospital than (9 vs. 8 days), those without COPD. PAD patients without COPD revealed more often cardiovascular risk factors like essential arterial hypertension and diabetes, but the prevalence of cardiovascular diseases such as coronary artery disease and heart failure were more often found in PAD patients with COPD. In addition, cancer and renal insufficiency also were more common in PAD patients with COPD, according to the authors.
“Remarkably, PAD patients with COPD showed more frequently lower PAD stages than those without COPD. Especially, PAD stage IV was more prevalent in PAD patients without COPD (19.6% vs. 13.8%; P less than 0.001),” the authors stated. In addition, amputations were more often performed in PAD patients without COPD.
Dr. Keller and his colleagues had the following conclusions regarding the clinical implications of their study: “I) PAD patients with long-standing tobacco use might benefit from COPD screening and treatment. II) PAD patients with additional COPD should be monitored more intensively, and the treatment for COPD should be optimized. III) COPD increases the risk for PE, and it is critical not to overlook this life-threatening disease. IV) MI and PE are important causes of in-hospital death in PAD patients with and without COPD.”
The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
SOURCE: Keller K et al. Respir Med. 2019 Feb;147:1-6.
FROM RESPIRATORY MEDICINE
Key clinical point:
Major finding: All-cause, in-hospital mortality was significantly higher in PAD patients with COPD, compared with those without (6.5% vs. 4.7%; P less than 0.001).
Study details: Database analysis of 5.6 million German PAD inpatients stratified for COPD.
Disclosures: The German Federal Ministry of Education and Research funded the study, and the authors reported having no conflicts.
Source: Keller K et al. Respir Med. 2019 Feb;147:1-6.
Treprostinil improves function for complex PAH patients
Treatment with , a study based on data from 105 adults has shown.
Data on the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) with treprostinil are limited, although alternatives to surgery are needed for many patients with the condition, wrote Roela Sadushi-Koliçi, MD, of the Medical University of Vienna, and her colleagues.
The researchers conducted a phase 3 randomized, controlled trial of the safety and efficacy of subcutaneous treprostinil for nonoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy; the findings were published online in the Lancet Respiratory Medicine. The patients received continuous subcutaneous treprostinil at either 30 ng/kg per min (high dose) or 3 ng/kg per min (low dose) and all patients were assessed at weeks 6, 12, 18, and 24.
Overall, 6-minute walk distance, hemodynamics, and functional status significantly improved in the high-dose patients, compared with the low-dose patients.
The primary outcome of 6-minute walk distance increased by 44.98 m from baseline in the high-dose group, compared with an increase of 4.29 m from baseline in the low-dose group.
In addition, “changes in pulmonary vascular resistance, one of the most important prognostic indicators of CTEPH, were significant in favour of high-dose subcutaneous treprostinil, as were improvements of WHO functional class and N-terminal probrain natriuretic peptide,” the researchers noted.
Rates of serious adverse events were similar between the groups; a total of 12 serious adverse events were reported in 10 of 52 patients in the low-dose group (19%) and 16 serious adverse events were reported in 9 of 53 patients in the high-dose group (17%). In both groups, the most common treatment-related adverse events were infusion site pain and other infusion site reactions.
The findings were limited by the small sample size and the possibility that the 6-minute walk test might not translate to long-term outcomes for PAH and CTEPH, the researchers wrote. However, the data support the safety and efficacy of subcutaneous treprostinil for CTEPH patients who do not tolerate riociguat, the other approved option for nonoperable CTEPH, or those who need combination therapy, they said.
The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
SOURCE: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
Treatment with , a study based on data from 105 adults has shown.
Data on the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) with treprostinil are limited, although alternatives to surgery are needed for many patients with the condition, wrote Roela Sadushi-Koliçi, MD, of the Medical University of Vienna, and her colleagues.
The researchers conducted a phase 3 randomized, controlled trial of the safety and efficacy of subcutaneous treprostinil for nonoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy; the findings were published online in the Lancet Respiratory Medicine. The patients received continuous subcutaneous treprostinil at either 30 ng/kg per min (high dose) or 3 ng/kg per min (low dose) and all patients were assessed at weeks 6, 12, 18, and 24.
Overall, 6-minute walk distance, hemodynamics, and functional status significantly improved in the high-dose patients, compared with the low-dose patients.
The primary outcome of 6-minute walk distance increased by 44.98 m from baseline in the high-dose group, compared with an increase of 4.29 m from baseline in the low-dose group.
In addition, “changes in pulmonary vascular resistance, one of the most important prognostic indicators of CTEPH, were significant in favour of high-dose subcutaneous treprostinil, as were improvements of WHO functional class and N-terminal probrain natriuretic peptide,” the researchers noted.
Rates of serious adverse events were similar between the groups; a total of 12 serious adverse events were reported in 10 of 52 patients in the low-dose group (19%) and 16 serious adverse events were reported in 9 of 53 patients in the high-dose group (17%). In both groups, the most common treatment-related adverse events were infusion site pain and other infusion site reactions.
The findings were limited by the small sample size and the possibility that the 6-minute walk test might not translate to long-term outcomes for PAH and CTEPH, the researchers wrote. However, the data support the safety and efficacy of subcutaneous treprostinil for CTEPH patients who do not tolerate riociguat, the other approved option for nonoperable CTEPH, or those who need combination therapy, they said.
The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
SOURCE: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
Treatment with , a study based on data from 105 adults has shown.
Data on the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) with treprostinil are limited, although alternatives to surgery are needed for many patients with the condition, wrote Roela Sadushi-Koliçi, MD, of the Medical University of Vienna, and her colleagues.
The researchers conducted a phase 3 randomized, controlled trial of the safety and efficacy of subcutaneous treprostinil for nonoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy; the findings were published online in the Lancet Respiratory Medicine. The patients received continuous subcutaneous treprostinil at either 30 ng/kg per min (high dose) or 3 ng/kg per min (low dose) and all patients were assessed at weeks 6, 12, 18, and 24.
Overall, 6-minute walk distance, hemodynamics, and functional status significantly improved in the high-dose patients, compared with the low-dose patients.
The primary outcome of 6-minute walk distance increased by 44.98 m from baseline in the high-dose group, compared with an increase of 4.29 m from baseline in the low-dose group.
In addition, “changes in pulmonary vascular resistance, one of the most important prognostic indicators of CTEPH, were significant in favour of high-dose subcutaneous treprostinil, as were improvements of WHO functional class and N-terminal probrain natriuretic peptide,” the researchers noted.
Rates of serious adverse events were similar between the groups; a total of 12 serious adverse events were reported in 10 of 52 patients in the low-dose group (19%) and 16 serious adverse events were reported in 9 of 53 patients in the high-dose group (17%). In both groups, the most common treatment-related adverse events were infusion site pain and other infusion site reactions.
The findings were limited by the small sample size and the possibility that the 6-minute walk test might not translate to long-term outcomes for PAH and CTEPH, the researchers wrote. However, the data support the safety and efficacy of subcutaneous treprostinil for CTEPH patients who do not tolerate riociguat, the other approved option for nonoperable CTEPH, or those who need combination therapy, they said.
The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
SOURCE: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: Treprostinil is a safe and effective nonsurgical treatment option for severe CTEPH patients.
Major finding: After 24 weeks, 6-minute walk distance improved by 44.98 m from baseline in the high-dose group compared with an increase of 4.29 m from baseline in the low-dose group.
Study details: The data come from a randomized trial of 105 adults with confirmed CTEPH.
Disclosures: The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
Source: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
Most oral HRT linked to increased VTE risk
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
FROM THE BMJ
Key clinical point: Transdermal HRT is not associated with any increase in VTE risk.
Major finding: Conjugated equine estrogen with medroxyprogesterone shows a twofold increase in VTE risk.
Study details: Nested case-control study in 80,396 women and 391,494 female controls.
Disclosures: One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
Source: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810
Phase 3 data support apixaban for cancer-associated VTE
SAN DIEGO – according to the Phase 3 ADAM VTE trial.
The rates of major bleeding and clinically relevant nonmajor bleeding in patients who received apixaban were similar to those in patients who received dalteparin. However, the rate of VTE recurrence was significantly lower with apixaban than it was with dalteparin.
“[A]pixaban was associated with very low bleeding rates and venous thrombosis recurrence rates compared to dalteparin,” said Robert D. McBane II, MD, of the Mayo Clinic in Rochester, Minn., at the annual meeting of the American Society of Hematology.
The trial included 300 adults (aged 18 years and older) with active cancer and acute VTE who were randomized to receive apixaban (n = 150) or dalteparin (n = 150). The dose and schedule for oral apixaban was 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months. Dalteparin was given subcutaneously at 200 IU/kg per day for 1 month followed by 150 IU/kg daily for 6 months. Among the patients in the study, 145 patients in the apixaban arm and 142 in the dalteparin arm ultimately received their assigned treatment.
Every month, patients completed an anticoagulation satisfaction survey and bruise survey (a modification of the Duke Anticoagulation Satisfaction Scale). They also underwent lab testing (complete blood count, liver and renal function testing) and were assessed for outcomes, medication reconciliation, drug compliance, and ECOG status on a monthly basis.
Patient characteristics
Baseline characteristics were similar between the treatment arms. The mean age was 64 years in both arms, and roughly half of patients in both arms were female. Hematologic malignancies were present in 9% of patients in the apixaban arm and 11% in the dalteparin arm. Others included lung, colorectal,
pancreatic/hepatobiliary, gynecologic, breast, genitourinary, upper gastrointestinal, and brain cancers.
Of patients in the study, 65% of those in the apixaban arm and 66% in the dalteparin arm had distant metastasis, and 74% of patients in both arms were receiving chemotherapy while on study.
Patients had the following qualifying thrombotic events:
- Any pulmonary embolism (PE) – 55% of patients in the apixaban arm and 51% in the dalteparin arm
- Any deep vein thrombosis (DVT) – 48% and 47%, respectively
- PE only – 44% and 39%, respectively
- PE with DVT – 12% in both arms
- DVT only – 37% and 35%, respectively
- Lower extremity DVT – 31% and 34%, respectively
- Upper extremity DVT – 17% and 14%, respectively
- Cerebral venous thrombosis (VT) – 1% and 0%, respectively
- Splanchnic VT – 8% and 18%, respectively.
Bleeding, thrombosis, and death
The study’s primary endpoint was major bleeding, which did not occur in any of the apixaban-treated patients. However, major bleeding did occur in two (1.4%) patients in the dalteparin arm (P = .14).
A secondary endpoint was major bleeding plus clinically relevant nonmajor bleeding. This occurred in nine (6.2%) patients in the apixaban arm and nine (6.3%) in the dalteparin arm (P = .88).
The researchers also assessed VTE recurrence. One patient in the apixaban arm (0.7%) and nine in the dalteparin arm (6.3%) had VTE recurrence (P = .03).
The patient in the apixaban arm experienced cerebral VT, and the patients with recurrence in the dalteparin arm had leg (n = 4) or arm (n = 2) VTE, PE (n = 1), or splanchnic VT (n = 2).
One patient in each arm (0.7%) had arterial thrombosis.
There was no significant difference in cumulative mortality between the treatment arms (hazard ratio, 1.40; P = .3078).
Satisfaction and discontinuation
Overall, apixaban fared better than dalteparin in the monthly patient satisfaction surveys. At various time points, apixaban-treated patients were significantly less likely to be concerned about excessive bruising, find anticoagulant treatment a burden or difficult to carry out, or say anticoagulant treatment added stress to their lives, negatively impacted their quality of life, or caused them “a great deal” of worry, irritation, or frustration.
However, apixaban-treated patients were less likely than dalteparin recipients to have confidence that their drug protected them from VTE recurrence, while the apixaban recipients were more likely than the dalteparin group to report overall satisfaction with their treatment.
In addition, premature treatment discontinuation was more common in the dalteparin group than in the apixaban group – 15% and 4%, respectively (P = .0012).
“Apixaban was well tolerated with superior patient safety satisfaction, as well as significantly fewer study drug discontinuations compared to dalteparin,” Dr. McBane said. “I believe that these data support the use of apixaban for the acute treatment of cancer-associated venous thromboembolism.”
This study was funded by BMS/Pfizer Alliance. Dr. McBane declared no other conflicts of interest.
jensmith@mdedge.com
SOURCE: McBane RD et al. ASH 2018, Abstract 421.
SAN DIEGO – according to the Phase 3 ADAM VTE trial.
The rates of major bleeding and clinically relevant nonmajor bleeding in patients who received apixaban were similar to those in patients who received dalteparin. However, the rate of VTE recurrence was significantly lower with apixaban than it was with dalteparin.
“[A]pixaban was associated with very low bleeding rates and venous thrombosis recurrence rates compared to dalteparin,” said Robert D. McBane II, MD, of the Mayo Clinic in Rochester, Minn., at the annual meeting of the American Society of Hematology.
The trial included 300 adults (aged 18 years and older) with active cancer and acute VTE who were randomized to receive apixaban (n = 150) or dalteparin (n = 150). The dose and schedule for oral apixaban was 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months. Dalteparin was given subcutaneously at 200 IU/kg per day for 1 month followed by 150 IU/kg daily for 6 months. Among the patients in the study, 145 patients in the apixaban arm and 142 in the dalteparin arm ultimately received their assigned treatment.
Every month, patients completed an anticoagulation satisfaction survey and bruise survey (a modification of the Duke Anticoagulation Satisfaction Scale). They also underwent lab testing (complete blood count, liver and renal function testing) and were assessed for outcomes, medication reconciliation, drug compliance, and ECOG status on a monthly basis.
Patient characteristics
Baseline characteristics were similar between the treatment arms. The mean age was 64 years in both arms, and roughly half of patients in both arms were female. Hematologic malignancies were present in 9% of patients in the apixaban arm and 11% in the dalteparin arm. Others included lung, colorectal,
pancreatic/hepatobiliary, gynecologic, breast, genitourinary, upper gastrointestinal, and brain cancers.
Of patients in the study, 65% of those in the apixaban arm and 66% in the dalteparin arm had distant metastasis, and 74% of patients in both arms were receiving chemotherapy while on study.
Patients had the following qualifying thrombotic events:
- Any pulmonary embolism (PE) – 55% of patients in the apixaban arm and 51% in the dalteparin arm
- Any deep vein thrombosis (DVT) – 48% and 47%, respectively
- PE only – 44% and 39%, respectively
- PE with DVT – 12% in both arms
- DVT only – 37% and 35%, respectively
- Lower extremity DVT – 31% and 34%, respectively
- Upper extremity DVT – 17% and 14%, respectively
- Cerebral venous thrombosis (VT) – 1% and 0%, respectively
- Splanchnic VT – 8% and 18%, respectively.
Bleeding, thrombosis, and death
The study’s primary endpoint was major bleeding, which did not occur in any of the apixaban-treated patients. However, major bleeding did occur in two (1.4%) patients in the dalteparin arm (P = .14).
A secondary endpoint was major bleeding plus clinically relevant nonmajor bleeding. This occurred in nine (6.2%) patients in the apixaban arm and nine (6.3%) in the dalteparin arm (P = .88).
The researchers also assessed VTE recurrence. One patient in the apixaban arm (0.7%) and nine in the dalteparin arm (6.3%) had VTE recurrence (P = .03).
The patient in the apixaban arm experienced cerebral VT, and the patients with recurrence in the dalteparin arm had leg (n = 4) or arm (n = 2) VTE, PE (n = 1), or splanchnic VT (n = 2).
One patient in each arm (0.7%) had arterial thrombosis.
There was no significant difference in cumulative mortality between the treatment arms (hazard ratio, 1.40; P = .3078).
Satisfaction and discontinuation
Overall, apixaban fared better than dalteparin in the monthly patient satisfaction surveys. At various time points, apixaban-treated patients were significantly less likely to be concerned about excessive bruising, find anticoagulant treatment a burden or difficult to carry out, or say anticoagulant treatment added stress to their lives, negatively impacted their quality of life, or caused them “a great deal” of worry, irritation, or frustration.
However, apixaban-treated patients were less likely than dalteparin recipients to have confidence that their drug protected them from VTE recurrence, while the apixaban recipients were more likely than the dalteparin group to report overall satisfaction with their treatment.
In addition, premature treatment discontinuation was more common in the dalteparin group than in the apixaban group – 15% and 4%, respectively (P = .0012).
“Apixaban was well tolerated with superior patient safety satisfaction, as well as significantly fewer study drug discontinuations compared to dalteparin,” Dr. McBane said. “I believe that these data support the use of apixaban for the acute treatment of cancer-associated venous thromboembolism.”
This study was funded by BMS/Pfizer Alliance. Dr. McBane declared no other conflicts of interest.
jensmith@mdedge.com
SOURCE: McBane RD et al. ASH 2018, Abstract 421.
SAN DIEGO – according to the Phase 3 ADAM VTE trial.
The rates of major bleeding and clinically relevant nonmajor bleeding in patients who received apixaban were similar to those in patients who received dalteparin. However, the rate of VTE recurrence was significantly lower with apixaban than it was with dalteparin.
“[A]pixaban was associated with very low bleeding rates and venous thrombosis recurrence rates compared to dalteparin,” said Robert D. McBane II, MD, of the Mayo Clinic in Rochester, Minn., at the annual meeting of the American Society of Hematology.
The trial included 300 adults (aged 18 years and older) with active cancer and acute VTE who were randomized to receive apixaban (n = 150) or dalteparin (n = 150). The dose and schedule for oral apixaban was 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months. Dalteparin was given subcutaneously at 200 IU/kg per day for 1 month followed by 150 IU/kg daily for 6 months. Among the patients in the study, 145 patients in the apixaban arm and 142 in the dalteparin arm ultimately received their assigned treatment.
Every month, patients completed an anticoagulation satisfaction survey and bruise survey (a modification of the Duke Anticoagulation Satisfaction Scale). They also underwent lab testing (complete blood count, liver and renal function testing) and were assessed for outcomes, medication reconciliation, drug compliance, and ECOG status on a monthly basis.
Patient characteristics
Baseline characteristics were similar between the treatment arms. The mean age was 64 years in both arms, and roughly half of patients in both arms were female. Hematologic malignancies were present in 9% of patients in the apixaban arm and 11% in the dalteparin arm. Others included lung, colorectal,
pancreatic/hepatobiliary, gynecologic, breast, genitourinary, upper gastrointestinal, and brain cancers.
Of patients in the study, 65% of those in the apixaban arm and 66% in the dalteparin arm had distant metastasis, and 74% of patients in both arms were receiving chemotherapy while on study.
Patients had the following qualifying thrombotic events:
- Any pulmonary embolism (PE) – 55% of patients in the apixaban arm and 51% in the dalteparin arm
- Any deep vein thrombosis (DVT) – 48% and 47%, respectively
- PE only – 44% and 39%, respectively
- PE with DVT – 12% in both arms
- DVT only – 37% and 35%, respectively
- Lower extremity DVT – 31% and 34%, respectively
- Upper extremity DVT – 17% and 14%, respectively
- Cerebral venous thrombosis (VT) – 1% and 0%, respectively
- Splanchnic VT – 8% and 18%, respectively.
Bleeding, thrombosis, and death
The study’s primary endpoint was major bleeding, which did not occur in any of the apixaban-treated patients. However, major bleeding did occur in two (1.4%) patients in the dalteparin arm (P = .14).
A secondary endpoint was major bleeding plus clinically relevant nonmajor bleeding. This occurred in nine (6.2%) patients in the apixaban arm and nine (6.3%) in the dalteparin arm (P = .88).
The researchers also assessed VTE recurrence. One patient in the apixaban arm (0.7%) and nine in the dalteparin arm (6.3%) had VTE recurrence (P = .03).
The patient in the apixaban arm experienced cerebral VT, and the patients with recurrence in the dalteparin arm had leg (n = 4) or arm (n = 2) VTE, PE (n = 1), or splanchnic VT (n = 2).
One patient in each arm (0.7%) had arterial thrombosis.
There was no significant difference in cumulative mortality between the treatment arms (hazard ratio, 1.40; P = .3078).
Satisfaction and discontinuation
Overall, apixaban fared better than dalteparin in the monthly patient satisfaction surveys. At various time points, apixaban-treated patients were significantly less likely to be concerned about excessive bruising, find anticoagulant treatment a burden or difficult to carry out, or say anticoagulant treatment added stress to their lives, negatively impacted their quality of life, or caused them “a great deal” of worry, irritation, or frustration.
However, apixaban-treated patients were less likely than dalteparin recipients to have confidence that their drug protected them from VTE recurrence, while the apixaban recipients were more likely than the dalteparin group to report overall satisfaction with their treatment.
In addition, premature treatment discontinuation was more common in the dalteparin group than in the apixaban group – 15% and 4%, respectively (P = .0012).
“Apixaban was well tolerated with superior patient safety satisfaction, as well as significantly fewer study drug discontinuations compared to dalteparin,” Dr. McBane said. “I believe that these data support the use of apixaban for the acute treatment of cancer-associated venous thromboembolism.”
This study was funded by BMS/Pfizer Alliance. Dr. McBane declared no other conflicts of interest.
jensmith@mdedge.com
SOURCE: McBane RD et al. ASH 2018, Abstract 421.
REPORTING FROM ASH 2018
Key clinical point: Apixaban is associated with a similar risk of major bleeding and a lower risk of VTE recurrence when compared with dalteparin in patients with cancer-associated VTE.
Major finding: There were no major bleeding events in the apixaban arm and two in the dalteparin arm (P = .14).
Study details: Phase 3 study of 300 patients.
Disclosures: This study was funded by BMS/Pfizer Alliance.
Source: McBane RD et al. ASH 2018, Abstract 421.
Large cohort study IDs prognostic factors in thromboangiitis obliterans
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
CHICAGO – Nonwhite ethnicity and limb infection at diagnosis predict vascular events in patients with thromboangiitis obliterans (TAO), and the latter also predicts amputation, which occurs within 10 years of diagnosis in nearly a third of patients, according to findings from a large retrospective French cohort study.
After a mean follow-up of 5.7 years, 58.9% of 224 patients with TAO – also known as Buerger’s disease – experienced a vascular event, 21.4% experienced at least one amputation, and 1.3% died, Alexandre Le Joncour, MD, reported at the annual meeting of the American College of Rheumatology.
The 5- and 15-year vascular event-free survival rates were 45% and 28%, respectively, and the 10- and 15-year amputation-free survival rates were 74%, and 66%, respectively, said Dr. Le Joncour of Sorbonne University, Paris.
Of note, no significant difference was seen in the vascular event-free survival rates based on tobacco use levels (more than 22 pack-years vs. 22 or fewer pack-years; HR, 1.2), he said.
Patient characteristics and clinical factors found to independently predict vascular events included nonwhite ethnicity (hazard ratio, 2.35; P = .005) and limb infection at diagnosis (HR, 3.29; P = .045). Limb infection at diagnosis also independently predicted amputation (HR, 12.1; P less than .001), he said.
“But there was no significant [association with amputation] in patients who had claudication, critical ischemia, or ischemic ulcers/necrosis,” he noted, adding that a comparison of white and nonwhite patients showed that the groups were similar with respect to epidemiologic and cardiovascular factors, clinical symptom distribution, and rates of addiction to tobacco, alcohol, and illicit drugs.
It was also clear that patients who quit using tobacco had a significantly lower risk of amputation than did those who continued using tobacco (P = .001), he said, explaining that 43 of the 48 patients who experienced amputation were current smokers, and 5 were ex-smokers at the time of amputation.
Dr. Le Joncour and his colleagues included TAO patients diagnosed between 1967 and 2016 at a median age of 36 years at the time of first symptoms, with a median of 12 months from symptom onset until diagnosis. About 76% were men, and about 83% were white. Patients with diabetes, atherosclerosis, arterial emboli, connective tissue disease, and/or thrombophilia were excluded.
Vascular events in this study were defined as “an acute worsening of the disease course requiring treatment modifications,” and included critical ischemia (35% of cases), ulcers/necrosis (33%), claudication worsening (16%), deep vein thrombosis (3%), superficial phlebitis (7%), limb infection (4%), and “other” events (2%).
Major amputation was defined as “an amputation involving the tibio-tarsian articulation for lower limbs and the metacarpophalangeal articulation for upper limbs,” he explained.
The median time to amputation was 4 years, and patients who experienced amputation had a median age of 39 years. Half of the 48 patients who experienced amputation had one amputation, nearly a third had two amputations, and 19% had three amputations. About two-thirds had minor amputations and a third had major amputations.
The findings provide important prognostic information regarding TAO, Dr. Le Joncour said, noting that long-term data on outcomes in TAO patients have been lacking.
“We found specific characteristics that identified those at highest risk for subsequent vascular complications, and these factors are not only important predictors of vascular complications or relapse, but may also serve to adjust more aggressive management and close follow-up of these patients,” he concluded.
Dr. Le Joncour reported having no disclosures.
SOURCE: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Nonwhite ethnicity and limb infection predict poor prognosis in TAO.
Major finding: Ethnicity predicts vascular events (HR, 2.35); limb infection at diagnosis predicts vascular events and amputation (HR, 3.29 and 12.1, respectively).
Study details: A retrospective cohort study of 224 patients.
Disclosures: Dr. Le Joncour reported having no disclosures.
Source: Le Joncour A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1885.
ASH releases new VTE guidelines
The new guidelines, released on Nov. 27, contain more than 150 individual recommendations, including sections devoted to managing venous thromboembolism (VTE) during pregnancy and in pediatric patients. Guideline highlights cited by some of the writing-panel participants included a high reliance on low-molecular-weight heparin (LMWH) agents as the preferred treatment for many patients, reliance on the D-dimer test to rule out VTE in patients with a low pretest probability of disease, and reliance on the 4Ts score to identify patients with heparin-induced thrombocytopenia.
The guidelines took more than 3 years to develop, an effort that began in 2015.
An updated set of VTE guidelines were needed because clinicians now have a “greater understanding of risk factors” for VTE as well as having “more options available for treating VTE, including new medications,” Adam C. Cuker, MD, cochair of the guideline-writing group and a hematologist and thrombosis specialist at the University of Pennsylvania, Philadelphia, said during a webcast to unveil the new guidelines.
Prevention
For preventing VTE in hospitalized medical patients the guidelines recommended initial assessment of the patient’s risk for both VTE and bleeding. Patients with a high bleeding risk who need VTE prevention should preferentially receive mechanical prophylaxis, either compression stockings or pneumatic sleeves. But in patients with a high VTE risk and an “acceptable” bleeding risk, prophylaxis with an anticoagulant is preferred over mechanical measures, said Mary Cushman, MD, professor and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
For prevention of VTE in medical inpatients, LMWH is preferred over unfractionated heparin because of its once-daily dosing and fewer complications, said Dr. Cushman, a member of the writing group. The panel also endorsed LMWH over a direct-acting oral anticoagulant, both during hospitalization and following discharge. The guidelines for prevention in medical patients explicitly “recommended against” using a direct-acting oral anticoagulant “over other treatments” both for hospitalized medical patients and after discharge, and the guidelines further recommend against extended prophylaxis after discharge with any other anticoagulant.
Another important takeaway from the prevention section was a statement that combining both mechanical and medical prophylaxis was not needed for medical inpatients. And once patients are discharged, if they take a long air trip they have no need for compression stockings or aspirin if their risk for thrombosis is not elevated. People with a “substantially increased” thrombosis risk “may benefit” from compression stockings or treatment with LMWH, Dr. Cushman said.
Diagnosis
For diagnosis, Wendy Lim, MD, highlighted the need for first categorizing patients as having a low or high probability for VTE, a judgment that can aid the accuracy of the diagnosis and helps avoid unnecessary testing.
For patients with low pretest probability, the guidelines recommended the D-dimer test as the best first step. Further testing isn’t needed when the D-dimer is negative, noted Dr. Lim, a hematologist and professor at McMaster University, Hamilton, Ont.
The guidelines also recommended using ventilation-perfusion scintigraphy (V/Q scan) for imaging a pulmonary embolism over a CT scan, which uses more radiation. But V/Q scans are not ideal for assessing older patients or patients with lung disease, Dr. Lim cautioned.
Management
Management of VTE should occur, when feasible, through a specialized anticoagulation management service center, which can provide care that is best suited to the complexities of anticoagulation therapy. But it’s a level of care that many U.S. patients don’t currently receive and hence is an area ripe for growth, said Daniel M. Witt, PharmD, professor and vice-chair of pharmacotherapy at the University of Utah, Salt Lake City.
The guidelines recommended against bridging therapy with LMWH for most patients who need to stop warfarin when undergoing an invasive procedure. The guidelines also called for “thoughtful” use of anticoagulant reversal agents and advised that patients who survive a major bleed while on anticoagulation should often resume the anticoagulant once they are stabilized.
For patients who develop heparin-induced thrombocytopenia, the 4Ts score is the best way to make a more accurate diagnosis and boost the prospects for recovery, said Dr. Cuker (Blood. 2012 Nov 15;120[20]:4160-7). The guidelines cite several agents now available to treat this common complication, which affects about 1% of the 12 million Americans treated with heparin annually: argatroban, bivalirudin, danaparoid, fondaparinux, apixaban, dabigatran, edoxaban, and rivaroxaban.
ASH has a VTE website with links to detailed information for each of the guideline subcategories: prophylaxis in medical patients, diagnosis, therapy, heparin-induced thrombocytopenia, VTE in pregnancy, and VTE in children. The website indicates that additional guidelines will soon be released on managing VTE in patients with cancer, in patients with thrombophilia, and for prophylaxis in surgical patients, as well as further information on treatment. A spokesperson for ASH said that these additional documents will post sometime in 2019.
At the time of the release, the guidelines panel published six articles in the journal Blood Advances that detailed the guidelines and their documentation.
The articles include prophylaxis of medical patients (Blood Advances. 2018 Nov 27;2[22]:3198-225), diagnosis (Blood Advances. 2018 Nov 27;2[22]:3226-56), anticoagulation therapy (Blood Advances. 2018 Nov 27;2[22]:3257-91), pediatrics (Blood Advances. 2018 Nov 27;2[22]:3292-316), pregnancy (Blood Advances. 2018 Nov 27;2[22]:3317-59), and heparin-induced thrombocytopenia (Blood Advances. 2018 Nov 27;2[22]:3360-92).
Dr. Cushman, Dr. Lim, and Dr. Witt reported having no relevant disclosures. Dr. Cuker reported receiving research support from T2 Biosystems.
The new guidelines, released on Nov. 27, contain more than 150 individual recommendations, including sections devoted to managing venous thromboembolism (VTE) during pregnancy and in pediatric patients. Guideline highlights cited by some of the writing-panel participants included a high reliance on low-molecular-weight heparin (LMWH) agents as the preferred treatment for many patients, reliance on the D-dimer test to rule out VTE in patients with a low pretest probability of disease, and reliance on the 4Ts score to identify patients with heparin-induced thrombocytopenia.
The guidelines took more than 3 years to develop, an effort that began in 2015.
An updated set of VTE guidelines were needed because clinicians now have a “greater understanding of risk factors” for VTE as well as having “more options available for treating VTE, including new medications,” Adam C. Cuker, MD, cochair of the guideline-writing group and a hematologist and thrombosis specialist at the University of Pennsylvania, Philadelphia, said during a webcast to unveil the new guidelines.
Prevention
For preventing VTE in hospitalized medical patients the guidelines recommended initial assessment of the patient’s risk for both VTE and bleeding. Patients with a high bleeding risk who need VTE prevention should preferentially receive mechanical prophylaxis, either compression stockings or pneumatic sleeves. But in patients with a high VTE risk and an “acceptable” bleeding risk, prophylaxis with an anticoagulant is preferred over mechanical measures, said Mary Cushman, MD, professor and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
For prevention of VTE in medical inpatients, LMWH is preferred over unfractionated heparin because of its once-daily dosing and fewer complications, said Dr. Cushman, a member of the writing group. The panel also endorsed LMWH over a direct-acting oral anticoagulant, both during hospitalization and following discharge. The guidelines for prevention in medical patients explicitly “recommended against” using a direct-acting oral anticoagulant “over other treatments” both for hospitalized medical patients and after discharge, and the guidelines further recommend against extended prophylaxis after discharge with any other anticoagulant.
Another important takeaway from the prevention section was a statement that combining both mechanical and medical prophylaxis was not needed for medical inpatients. And once patients are discharged, if they take a long air trip they have no need for compression stockings or aspirin if their risk for thrombosis is not elevated. People with a “substantially increased” thrombosis risk “may benefit” from compression stockings or treatment with LMWH, Dr. Cushman said.
Diagnosis
For diagnosis, Wendy Lim, MD, highlighted the need for first categorizing patients as having a low or high probability for VTE, a judgment that can aid the accuracy of the diagnosis and helps avoid unnecessary testing.
For patients with low pretest probability, the guidelines recommended the D-dimer test as the best first step. Further testing isn’t needed when the D-dimer is negative, noted Dr. Lim, a hematologist and professor at McMaster University, Hamilton, Ont.
The guidelines also recommended using ventilation-perfusion scintigraphy (V/Q scan) for imaging a pulmonary embolism over a CT scan, which uses more radiation. But V/Q scans are not ideal for assessing older patients or patients with lung disease, Dr. Lim cautioned.
Management
Management of VTE should occur, when feasible, through a specialized anticoagulation management service center, which can provide care that is best suited to the complexities of anticoagulation therapy. But it’s a level of care that many U.S. patients don’t currently receive and hence is an area ripe for growth, said Daniel M. Witt, PharmD, professor and vice-chair of pharmacotherapy at the University of Utah, Salt Lake City.
The guidelines recommended against bridging therapy with LMWH for most patients who need to stop warfarin when undergoing an invasive procedure. The guidelines also called for “thoughtful” use of anticoagulant reversal agents and advised that patients who survive a major bleed while on anticoagulation should often resume the anticoagulant once they are stabilized.
For patients who develop heparin-induced thrombocytopenia, the 4Ts score is the best way to make a more accurate diagnosis and boost the prospects for recovery, said Dr. Cuker (Blood. 2012 Nov 15;120[20]:4160-7). The guidelines cite several agents now available to treat this common complication, which affects about 1% of the 12 million Americans treated with heparin annually: argatroban, bivalirudin, danaparoid, fondaparinux, apixaban, dabigatran, edoxaban, and rivaroxaban.
ASH has a VTE website with links to detailed information for each of the guideline subcategories: prophylaxis in medical patients, diagnosis, therapy, heparin-induced thrombocytopenia, VTE in pregnancy, and VTE in children. The website indicates that additional guidelines will soon be released on managing VTE in patients with cancer, in patients with thrombophilia, and for prophylaxis in surgical patients, as well as further information on treatment. A spokesperson for ASH said that these additional documents will post sometime in 2019.
At the time of the release, the guidelines panel published six articles in the journal Blood Advances that detailed the guidelines and their documentation.
The articles include prophylaxis of medical patients (Blood Advances. 2018 Nov 27;2[22]:3198-225), diagnosis (Blood Advances. 2018 Nov 27;2[22]:3226-56), anticoagulation therapy (Blood Advances. 2018 Nov 27;2[22]:3257-91), pediatrics (Blood Advances. 2018 Nov 27;2[22]:3292-316), pregnancy (Blood Advances. 2018 Nov 27;2[22]:3317-59), and heparin-induced thrombocytopenia (Blood Advances. 2018 Nov 27;2[22]:3360-92).
Dr. Cushman, Dr. Lim, and Dr. Witt reported having no relevant disclosures. Dr. Cuker reported receiving research support from T2 Biosystems.
The new guidelines, released on Nov. 27, contain more than 150 individual recommendations, including sections devoted to managing venous thromboembolism (VTE) during pregnancy and in pediatric patients. Guideline highlights cited by some of the writing-panel participants included a high reliance on low-molecular-weight heparin (LMWH) agents as the preferred treatment for many patients, reliance on the D-dimer test to rule out VTE in patients with a low pretest probability of disease, and reliance on the 4Ts score to identify patients with heparin-induced thrombocytopenia.
The guidelines took more than 3 years to develop, an effort that began in 2015.
An updated set of VTE guidelines were needed because clinicians now have a “greater understanding of risk factors” for VTE as well as having “more options available for treating VTE, including new medications,” Adam C. Cuker, MD, cochair of the guideline-writing group and a hematologist and thrombosis specialist at the University of Pennsylvania, Philadelphia, said during a webcast to unveil the new guidelines.
Prevention
For preventing VTE in hospitalized medical patients the guidelines recommended initial assessment of the patient’s risk for both VTE and bleeding. Patients with a high bleeding risk who need VTE prevention should preferentially receive mechanical prophylaxis, either compression stockings or pneumatic sleeves. But in patients with a high VTE risk and an “acceptable” bleeding risk, prophylaxis with an anticoagulant is preferred over mechanical measures, said Mary Cushman, MD, professor and medical director of the thrombosis and hemostasis program at the University of Vermont, Burlington.
For prevention of VTE in medical inpatients, LMWH is preferred over unfractionated heparin because of its once-daily dosing and fewer complications, said Dr. Cushman, a member of the writing group. The panel also endorsed LMWH over a direct-acting oral anticoagulant, both during hospitalization and following discharge. The guidelines for prevention in medical patients explicitly “recommended against” using a direct-acting oral anticoagulant “over other treatments” both for hospitalized medical patients and after discharge, and the guidelines further recommend against extended prophylaxis after discharge with any other anticoagulant.
Another important takeaway from the prevention section was a statement that combining both mechanical and medical prophylaxis was not needed for medical inpatients. And once patients are discharged, if they take a long air trip they have no need for compression stockings or aspirin if their risk for thrombosis is not elevated. People with a “substantially increased” thrombosis risk “may benefit” from compression stockings or treatment with LMWH, Dr. Cushman said.
Diagnosis
For diagnosis, Wendy Lim, MD, highlighted the need for first categorizing patients as having a low or high probability for VTE, a judgment that can aid the accuracy of the diagnosis and helps avoid unnecessary testing.
For patients with low pretest probability, the guidelines recommended the D-dimer test as the best first step. Further testing isn’t needed when the D-dimer is negative, noted Dr. Lim, a hematologist and professor at McMaster University, Hamilton, Ont.
The guidelines also recommended using ventilation-perfusion scintigraphy (V/Q scan) for imaging a pulmonary embolism over a CT scan, which uses more radiation. But V/Q scans are not ideal for assessing older patients or patients with lung disease, Dr. Lim cautioned.
Management
Management of VTE should occur, when feasible, through a specialized anticoagulation management service center, which can provide care that is best suited to the complexities of anticoagulation therapy. But it’s a level of care that many U.S. patients don’t currently receive and hence is an area ripe for growth, said Daniel M. Witt, PharmD, professor and vice-chair of pharmacotherapy at the University of Utah, Salt Lake City.
The guidelines recommended against bridging therapy with LMWH for most patients who need to stop warfarin when undergoing an invasive procedure. The guidelines also called for “thoughtful” use of anticoagulant reversal agents and advised that patients who survive a major bleed while on anticoagulation should often resume the anticoagulant once they are stabilized.
For patients who develop heparin-induced thrombocytopenia, the 4Ts score is the best way to make a more accurate diagnosis and boost the prospects for recovery, said Dr. Cuker (Blood. 2012 Nov 15;120[20]:4160-7). The guidelines cite several agents now available to treat this common complication, which affects about 1% of the 12 million Americans treated with heparin annually: argatroban, bivalirudin, danaparoid, fondaparinux, apixaban, dabigatran, edoxaban, and rivaroxaban.
ASH has a VTE website with links to detailed information for each of the guideline subcategories: prophylaxis in medical patients, diagnosis, therapy, heparin-induced thrombocytopenia, VTE in pregnancy, and VTE in children. The website indicates that additional guidelines will soon be released on managing VTE in patients with cancer, in patients with thrombophilia, and for prophylaxis in surgical patients, as well as further information on treatment. A spokesperson for ASH said that these additional documents will post sometime in 2019.
At the time of the release, the guidelines panel published six articles in the journal Blood Advances that detailed the guidelines and their documentation.
The articles include prophylaxis of medical patients (Blood Advances. 2018 Nov 27;2[22]:3198-225), diagnosis (Blood Advances. 2018 Nov 27;2[22]:3226-56), anticoagulation therapy (Blood Advances. 2018 Nov 27;2[22]:3257-91), pediatrics (Blood Advances. 2018 Nov 27;2[22]:3292-316), pregnancy (Blood Advances. 2018 Nov 27;2[22]:3317-59), and heparin-induced thrombocytopenia (Blood Advances. 2018 Nov 27;2[22]:3360-92).
Dr. Cushman, Dr. Lim, and Dr. Witt reported having no relevant disclosures. Dr. Cuker reported receiving research support from T2 Biosystems.
Tofacitinib and TNF inhibitors show similar VTE rates
CHICAGO – Rheumatoid arthritis patients treated with tofacitinib did not have a significantly increased incidence of hospitalization for venous thromboembolism, compared with patients treated with a tumor necrosis factor (TNF) inhibitor, in a study of more than 50,000 U.S. patients culled from a pair of health insurance databases.
The Janus kinase inhibitor class of agents, including tofacitinib (Xeljanz), has acquired a reputation for causing an excess of venous thromboembolic events (VTE) (Drug Saf. 2018 Jul;41[7]:645-53). To assess this in a real-world setting Seoyoung C. Kim, MD, and her associates took data from Medicare patients during 2012-2015 and from Truven MarketScan for commercially insured patients during 2012-2016 and derived a database of 16,091 RA patients on newly begun treatment with a TNF inhibitor and 995 newly begun on tofacitinib in the Medicare data, and 32,164 RA patients newly started on a TNF inhibitor and 1,910 on tofacitinib in the Truven database. The analysis excluded patients with a history of VTE.
Using propensity score–adjusted matching of patients in the two treatment arms in both of these databases, and using a VTE event – either a pulmonary embolism or deep-vein thrombosis that resulted in hospitalization – as the primary endpoint, the results showed statistically nonsignificant excesses of VTE in the patients treated with tofacitinib, compared with a TNF inhibitor, Dr. Kim reported in a poster she presented at the annual meeting of the American College of Rheumatology.
In the adjusted comparison, the Medicare data showed a nonsignificant 12% higher VTE rate in the tofacitinib-treated patients, while the Truven data showed a nonsignificant 55% higher rate of VTE during tofacitinib treatment. When the data were pooled, the result was a 33% higher rate of VTE while on tofacitinib treatment, which was not statistically significant.
Dr. Kim cautioned that the low rate of VTE events, especially among the patients on tofacitinib, limited the precision of the results. The combined data included 2,905 patients on tofacitinib treatment who had 15 VTE events, a rate of 0.77 events/100 person-years of follow-up. This compared with a rate of 0.52/100 person-years among patients on a TNF inhibitor. Thus, in both treatment groups the absolute VTE rate was low.
The most reliable finding from the analysis is that it “rules out a large increase in the risk for VTE events with tofacitinib,” said Dr. Kim, a rheumatologist at Brigham and Women’s Hospital in Boston.
The researchers also ran an analysis that included not only VTE events that resulted in hospitalization but also VTE events managed on an outpatient basis. Dr. Kim did not report the specific numbers involved in this calculation, but she reported that, when her group included both types of VTE events, the patients treated with tofacitinib had a nonsignificant 12% lower rate of events, compared with patients treated with a TNF inhibitor.
Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
CHICAGO – Rheumatoid arthritis patients treated with tofacitinib did not have a significantly increased incidence of hospitalization for venous thromboembolism, compared with patients treated with a tumor necrosis factor (TNF) inhibitor, in a study of more than 50,000 U.S. patients culled from a pair of health insurance databases.
The Janus kinase inhibitor class of agents, including tofacitinib (Xeljanz), has acquired a reputation for causing an excess of venous thromboembolic events (VTE) (Drug Saf. 2018 Jul;41[7]:645-53). To assess this in a real-world setting Seoyoung C. Kim, MD, and her associates took data from Medicare patients during 2012-2015 and from Truven MarketScan for commercially insured patients during 2012-2016 and derived a database of 16,091 RA patients on newly begun treatment with a TNF inhibitor and 995 newly begun on tofacitinib in the Medicare data, and 32,164 RA patients newly started on a TNF inhibitor and 1,910 on tofacitinib in the Truven database. The analysis excluded patients with a history of VTE.
Using propensity score–adjusted matching of patients in the two treatment arms in both of these databases, and using a VTE event – either a pulmonary embolism or deep-vein thrombosis that resulted in hospitalization – as the primary endpoint, the results showed statistically nonsignificant excesses of VTE in the patients treated with tofacitinib, compared with a TNF inhibitor, Dr. Kim reported in a poster she presented at the annual meeting of the American College of Rheumatology.
In the adjusted comparison, the Medicare data showed a nonsignificant 12% higher VTE rate in the tofacitinib-treated patients, while the Truven data showed a nonsignificant 55% higher rate of VTE during tofacitinib treatment. When the data were pooled, the result was a 33% higher rate of VTE while on tofacitinib treatment, which was not statistically significant.
Dr. Kim cautioned that the low rate of VTE events, especially among the patients on tofacitinib, limited the precision of the results. The combined data included 2,905 patients on tofacitinib treatment who had 15 VTE events, a rate of 0.77 events/100 person-years of follow-up. This compared with a rate of 0.52/100 person-years among patients on a TNF inhibitor. Thus, in both treatment groups the absolute VTE rate was low.
The most reliable finding from the analysis is that it “rules out a large increase in the risk for VTE events with tofacitinib,” said Dr. Kim, a rheumatologist at Brigham and Women’s Hospital in Boston.
The researchers also ran an analysis that included not only VTE events that resulted in hospitalization but also VTE events managed on an outpatient basis. Dr. Kim did not report the specific numbers involved in this calculation, but she reported that, when her group included both types of VTE events, the patients treated with tofacitinib had a nonsignificant 12% lower rate of events, compared with patients treated with a TNF inhibitor.
Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
CHICAGO – Rheumatoid arthritis patients treated with tofacitinib did not have a significantly increased incidence of hospitalization for venous thromboembolism, compared with patients treated with a tumor necrosis factor (TNF) inhibitor, in a study of more than 50,000 U.S. patients culled from a pair of health insurance databases.
The Janus kinase inhibitor class of agents, including tofacitinib (Xeljanz), has acquired a reputation for causing an excess of venous thromboembolic events (VTE) (Drug Saf. 2018 Jul;41[7]:645-53). To assess this in a real-world setting Seoyoung C. Kim, MD, and her associates took data from Medicare patients during 2012-2015 and from Truven MarketScan for commercially insured patients during 2012-2016 and derived a database of 16,091 RA patients on newly begun treatment with a TNF inhibitor and 995 newly begun on tofacitinib in the Medicare data, and 32,164 RA patients newly started on a TNF inhibitor and 1,910 on tofacitinib in the Truven database. The analysis excluded patients with a history of VTE.
Using propensity score–adjusted matching of patients in the two treatment arms in both of these databases, and using a VTE event – either a pulmonary embolism or deep-vein thrombosis that resulted in hospitalization – as the primary endpoint, the results showed statistically nonsignificant excesses of VTE in the patients treated with tofacitinib, compared with a TNF inhibitor, Dr. Kim reported in a poster she presented at the annual meeting of the American College of Rheumatology.
In the adjusted comparison, the Medicare data showed a nonsignificant 12% higher VTE rate in the tofacitinib-treated patients, while the Truven data showed a nonsignificant 55% higher rate of VTE during tofacitinib treatment. When the data were pooled, the result was a 33% higher rate of VTE while on tofacitinib treatment, which was not statistically significant.
Dr. Kim cautioned that the low rate of VTE events, especially among the patients on tofacitinib, limited the precision of the results. The combined data included 2,905 patients on tofacitinib treatment who had 15 VTE events, a rate of 0.77 events/100 person-years of follow-up. This compared with a rate of 0.52/100 person-years among patients on a TNF inhibitor. Thus, in both treatment groups the absolute VTE rate was low.
The most reliable finding from the analysis is that it “rules out a large increase in the risk for VTE events with tofacitinib,” said Dr. Kim, a rheumatologist at Brigham and Women’s Hospital in Boston.
The researchers also ran an analysis that included not only VTE events that resulted in hospitalization but also VTE events managed on an outpatient basis. Dr. Kim did not report the specific numbers involved in this calculation, but she reported that, when her group included both types of VTE events, the patients treated with tofacitinib had a nonsignificant 12% lower rate of events, compared with patients treated with a TNF inhibitor.
Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Rheumatoid arthritis patients treated with tofacitinib showed no excess incidence of venous thromboembolism, compared with patients on a tumor necrosis factor inhibitor.
Major finding: Propensity score–adjusted rates of VTE were 33% higher with tofacitinib, compared with TNF inhibition, which was not a statistically significant difference.
Study details: Review of 51,160 rheumatoid arthritis patients from U.S. health insurance databases.
Disclosures: Dr. Kim has received research support from Bristol-Myers Squibb, Pfizer, and Roche.
Source: Desai RJ et al. Arthritis Rheumatol. 2018;70(suppl 10), Abstract L09.