User login
Navigating Moonlighting Opportunities During Dermatology Training
Navigating Moonlighting Opportunities During Dermatology Training
Residents and fellows in training have to navigate time management to balance reading, hands-on training, family responsibilities, exercise, diet, and sleep requirements. In addition, they grapple with the stress of financial commitments for food, housing, clothing, family members, transportation, and student loans. A brilliant friend of mine once said that she struggled throughout residency and her early career to find balance until it finally occurred to her that, while balance was aspirational, resilience was key. All that said, residents in training may find it appealing to earn a little extra money and gain additional clinical experience through moonlighting. This article discusses some key considerations when embarking on such a decision, including the effects of moonlighting on other commitments and some logistical factors to consider.
Will Moonlighting Adversely Affect My Other Commitments?
Residency and fellowship are precious opportunities to gain medical knowledge, hone your ability to make diagnoses through complex pattern recognition, and refine the necessary surgical and interpersonal skills to carry you through a successful career. Dermatology encompasses a vast array of conditions related only by their manifestation in skin. Dermatology residents and fellows may spend fewer sleepless hours on call, but the reading requirements are massive. Our treatment armamentarium has expanded rapidly with highly effective treatments for chronic conditions that have a dramatic impact on quality of life. With so many effective agents available, the choice often relates as much to comorbidities as to disease severity and location. There is so much to learn.
While making a full commitment to acquiring the skills of an expert clinician, it is important for residents to remain aware of those who depend on you—in particular, the fleeting time you have with your growing children. They grow up fast, and your interactions with them determine who they will grow up to be. In the past, salt, silk, gold, and jewels were the world’s greatest luxuries. Now, it’s time—time with family, time for self-care, time to reflect, and time to rest and renew. Be careful how you squander time in exchange for material possessions.
What Logistical Factors Should You Consider When Embarking on Moonlighting?
There are clearly stated policies from the Accreditation Council for Graduate Medical Education for when moonlighting can occur during training.1 It should not occur during typical residency or fellowship work hours, and the individual must be in good standing academically and progressing well on their journey to becoming a competent dermatologist. They must also have the appropriate skills to practice in the field of medicine chosen for moonlighting.
Moonlighting opportunities may exist in the form of emergency department or “quick clinic” coverage, especially for the evaluation and treatment of acute minor illnesses. Fellows who have completed a dermatology residency may supervise dermatology residents in afterhours or weekend clinics, offering enhanced opportunities for autonomy, additional clinical experience, and some welcome cash. To make such clinics viable, the office space must be available; the building must be open; and the costs of the space, scheduling, reception, and security services must be covered as well as nursing support (which should be voluntary and likely will require overtime pay scales). After all of these—as well as supplies—have been paid for, what is left is what is available to distribute as pay for service. Working through these factors provides valuable experience in resource management and helps prepare trainees for the economic realities of private practice. Large organizations may be able to provide the space and support, but all of that needs to be paid for through the proceeds that come from the patient care provided. No-show rates often are quite high for after-hours and weekend clinics, but the expenses for those unfilled appointment slots remain and must be paid in full. Be sure the demand exists and that you plan appropriately with strategic overbooking based on historical data on patient mix, procedural needs, and no-show rates.
My department has supported resident and fellow requests for moonlighting opportunities in the past. The most successful model was to have a limited number of early morning appointment slots prior to the start of morning didactics. Security typically already exists, rooms are available, and patients can be seen and still get to work or get their kids to school. No-show rates remained very low for morning appointments, and strategic overbooking was unnecessary.
In contrast, evening and weekend clinics start out strong with high patient satisfaction and deteriorate fairly quickly with accelerating no-show rates. People are busy at the end of the day, and unforeseen circumstances often affect their ability to keep an appointment. Weekends are precious; potential patients may be less schedule minded in the evenings and on weekends, and the residents and fellows themselves often find it stressful to commit to giving up a chunk of weekend time on a scheduled basis.
Before you commit to a moonlighting job, be sure to weigh all of the above factors and be sure the juice is worth the squeeze.
Final Thoughts
Moonlighting opportunities are a way to acquire both clinical and management skills and can provide a welcome extra bit of cash to ease financial burdens, but these benefits should be balanced with other time commitments and overall quality of life. Time is precious—choose wisely and be sure you spend it well.
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated September 17, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
Residents and fellows in training have to navigate time management to balance reading, hands-on training, family responsibilities, exercise, diet, and sleep requirements. In addition, they grapple with the stress of financial commitments for food, housing, clothing, family members, transportation, and student loans. A brilliant friend of mine once said that she struggled throughout residency and her early career to find balance until it finally occurred to her that, while balance was aspirational, resilience was key. All that said, residents in training may find it appealing to earn a little extra money and gain additional clinical experience through moonlighting. This article discusses some key considerations when embarking on such a decision, including the effects of moonlighting on other commitments and some logistical factors to consider.
Will Moonlighting Adversely Affect My Other Commitments?
Residency and fellowship are precious opportunities to gain medical knowledge, hone your ability to make diagnoses through complex pattern recognition, and refine the necessary surgical and interpersonal skills to carry you through a successful career. Dermatology encompasses a vast array of conditions related only by their manifestation in skin. Dermatology residents and fellows may spend fewer sleepless hours on call, but the reading requirements are massive. Our treatment armamentarium has expanded rapidly with highly effective treatments for chronic conditions that have a dramatic impact on quality of life. With so many effective agents available, the choice often relates as much to comorbidities as to disease severity and location. There is so much to learn.
While making a full commitment to acquiring the skills of an expert clinician, it is important for residents to remain aware of those who depend on you—in particular, the fleeting time you have with your growing children. They grow up fast, and your interactions with them determine who they will grow up to be. In the past, salt, silk, gold, and jewels were the world’s greatest luxuries. Now, it’s time—time with family, time for self-care, time to reflect, and time to rest and renew. Be careful how you squander time in exchange for material possessions.
What Logistical Factors Should You Consider When Embarking on Moonlighting?
There are clearly stated policies from the Accreditation Council for Graduate Medical Education for when moonlighting can occur during training.1 It should not occur during typical residency or fellowship work hours, and the individual must be in good standing academically and progressing well on their journey to becoming a competent dermatologist. They must also have the appropriate skills to practice in the field of medicine chosen for moonlighting.
Moonlighting opportunities may exist in the form of emergency department or “quick clinic” coverage, especially for the evaluation and treatment of acute minor illnesses. Fellows who have completed a dermatology residency may supervise dermatology residents in afterhours or weekend clinics, offering enhanced opportunities for autonomy, additional clinical experience, and some welcome cash. To make such clinics viable, the office space must be available; the building must be open; and the costs of the space, scheduling, reception, and security services must be covered as well as nursing support (which should be voluntary and likely will require overtime pay scales). After all of these—as well as supplies—have been paid for, what is left is what is available to distribute as pay for service. Working through these factors provides valuable experience in resource management and helps prepare trainees for the economic realities of private practice. Large organizations may be able to provide the space and support, but all of that needs to be paid for through the proceeds that come from the patient care provided. No-show rates often are quite high for after-hours and weekend clinics, but the expenses for those unfilled appointment slots remain and must be paid in full. Be sure the demand exists and that you plan appropriately with strategic overbooking based on historical data on patient mix, procedural needs, and no-show rates.
My department has supported resident and fellow requests for moonlighting opportunities in the past. The most successful model was to have a limited number of early morning appointment slots prior to the start of morning didactics. Security typically already exists, rooms are available, and patients can be seen and still get to work or get their kids to school. No-show rates remained very low for morning appointments, and strategic overbooking was unnecessary.
In contrast, evening and weekend clinics start out strong with high patient satisfaction and deteriorate fairly quickly with accelerating no-show rates. People are busy at the end of the day, and unforeseen circumstances often affect their ability to keep an appointment. Weekends are precious; potential patients may be less schedule minded in the evenings and on weekends, and the residents and fellows themselves often find it stressful to commit to giving up a chunk of weekend time on a scheduled basis.
Before you commit to a moonlighting job, be sure to weigh all of the above factors and be sure the juice is worth the squeeze.
Final Thoughts
Moonlighting opportunities are a way to acquire both clinical and management skills and can provide a welcome extra bit of cash to ease financial burdens, but these benefits should be balanced with other time commitments and overall quality of life. Time is precious—choose wisely and be sure you spend it well.
Residents and fellows in training have to navigate time management to balance reading, hands-on training, family responsibilities, exercise, diet, and sleep requirements. In addition, they grapple with the stress of financial commitments for food, housing, clothing, family members, transportation, and student loans. A brilliant friend of mine once said that she struggled throughout residency and her early career to find balance until it finally occurred to her that, while balance was aspirational, resilience was key. All that said, residents in training may find it appealing to earn a little extra money and gain additional clinical experience through moonlighting. This article discusses some key considerations when embarking on such a decision, including the effects of moonlighting on other commitments and some logistical factors to consider.
Will Moonlighting Adversely Affect My Other Commitments?
Residency and fellowship are precious opportunities to gain medical knowledge, hone your ability to make diagnoses through complex pattern recognition, and refine the necessary surgical and interpersonal skills to carry you through a successful career. Dermatology encompasses a vast array of conditions related only by their manifestation in skin. Dermatology residents and fellows may spend fewer sleepless hours on call, but the reading requirements are massive. Our treatment armamentarium has expanded rapidly with highly effective treatments for chronic conditions that have a dramatic impact on quality of life. With so many effective agents available, the choice often relates as much to comorbidities as to disease severity and location. There is so much to learn.
While making a full commitment to acquiring the skills of an expert clinician, it is important for residents to remain aware of those who depend on you—in particular, the fleeting time you have with your growing children. They grow up fast, and your interactions with them determine who they will grow up to be. In the past, salt, silk, gold, and jewels were the world’s greatest luxuries. Now, it’s time—time with family, time for self-care, time to reflect, and time to rest and renew. Be careful how you squander time in exchange for material possessions.
What Logistical Factors Should You Consider When Embarking on Moonlighting?
There are clearly stated policies from the Accreditation Council for Graduate Medical Education for when moonlighting can occur during training.1 It should not occur during typical residency or fellowship work hours, and the individual must be in good standing academically and progressing well on their journey to becoming a competent dermatologist. They must also have the appropriate skills to practice in the field of medicine chosen for moonlighting.
Moonlighting opportunities may exist in the form of emergency department or “quick clinic” coverage, especially for the evaluation and treatment of acute minor illnesses. Fellows who have completed a dermatology residency may supervise dermatology residents in afterhours or weekend clinics, offering enhanced opportunities for autonomy, additional clinical experience, and some welcome cash. To make such clinics viable, the office space must be available; the building must be open; and the costs of the space, scheduling, reception, and security services must be covered as well as nursing support (which should be voluntary and likely will require overtime pay scales). After all of these—as well as supplies—have been paid for, what is left is what is available to distribute as pay for service. Working through these factors provides valuable experience in resource management and helps prepare trainees for the economic realities of private practice. Large organizations may be able to provide the space and support, but all of that needs to be paid for through the proceeds that come from the patient care provided. No-show rates often are quite high for after-hours and weekend clinics, but the expenses for those unfilled appointment slots remain and must be paid in full. Be sure the demand exists and that you plan appropriately with strategic overbooking based on historical data on patient mix, procedural needs, and no-show rates.
My department has supported resident and fellow requests for moonlighting opportunities in the past. The most successful model was to have a limited number of early morning appointment slots prior to the start of morning didactics. Security typically already exists, rooms are available, and patients can be seen and still get to work or get their kids to school. No-show rates remained very low for morning appointments, and strategic overbooking was unnecessary.
In contrast, evening and weekend clinics start out strong with high patient satisfaction and deteriorate fairly quickly with accelerating no-show rates. People are busy at the end of the day, and unforeseen circumstances often affect their ability to keep an appointment. Weekends are precious; potential patients may be less schedule minded in the evenings and on weekends, and the residents and fellows themselves often find it stressful to commit to giving up a chunk of weekend time on a scheduled basis.
Before you commit to a moonlighting job, be sure to weigh all of the above factors and be sure the juice is worth the squeeze.
Final Thoughts
Moonlighting opportunities are a way to acquire both clinical and management skills and can provide a welcome extra bit of cash to ease financial burdens, but these benefits should be balanced with other time commitments and overall quality of life. Time is precious—choose wisely and be sure you spend it well.
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated September 17, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
- Accreditation Council for Graduate Medical Education. Common Program Requirements (Residency). Updated September 17, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/cprresidency_2023v3.pdf
Navigating Moonlighting Opportunities During Dermatology Training
Navigating Moonlighting Opportunities During Dermatology Training
PRACTICE POINTS
- Dermatology training demands extensive study and hands-on skill development, which need to be balanced with family time, finances, and self-care.
- Before moonlighting, ensure it will not compromise your family’s quality of life or your core residency/fellowship commitments and that your program’s policies permit it.
- Carefully assess logistics to determine if an afterhours or weekend clinic can be a financially viable moonlighting opportunity.
Multiple Fungating Plaques on the Face, Arms, and Legs
Multiple Fungating Plaques on the Face, Arms, and Legs
THE DIAGNOSIS: Mpox
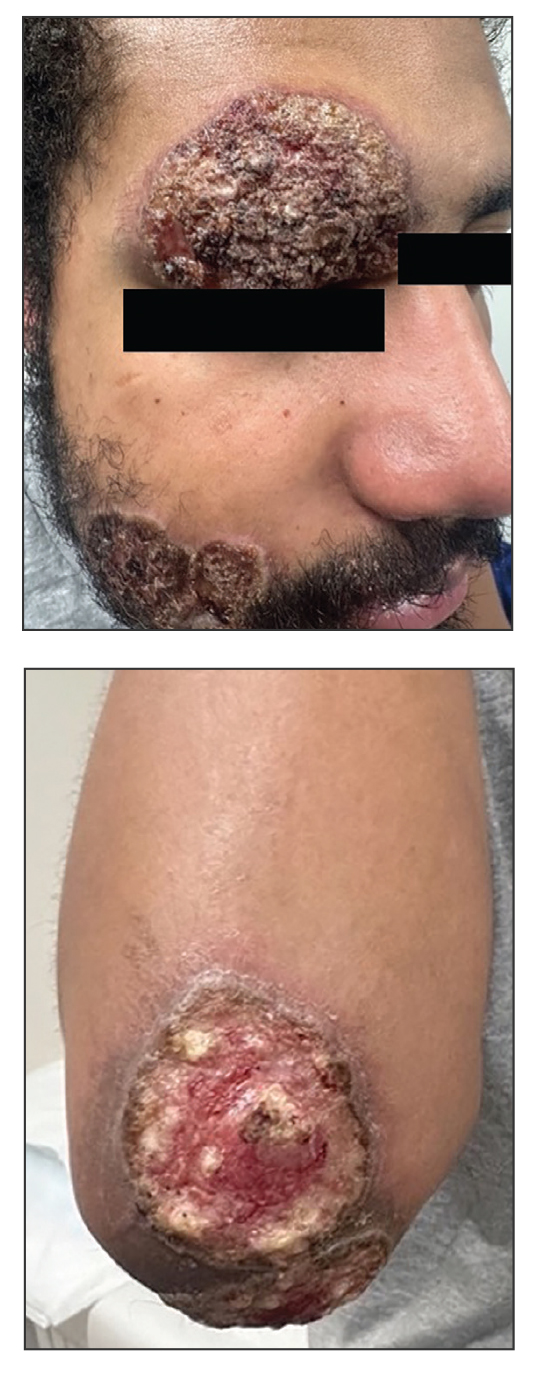
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.
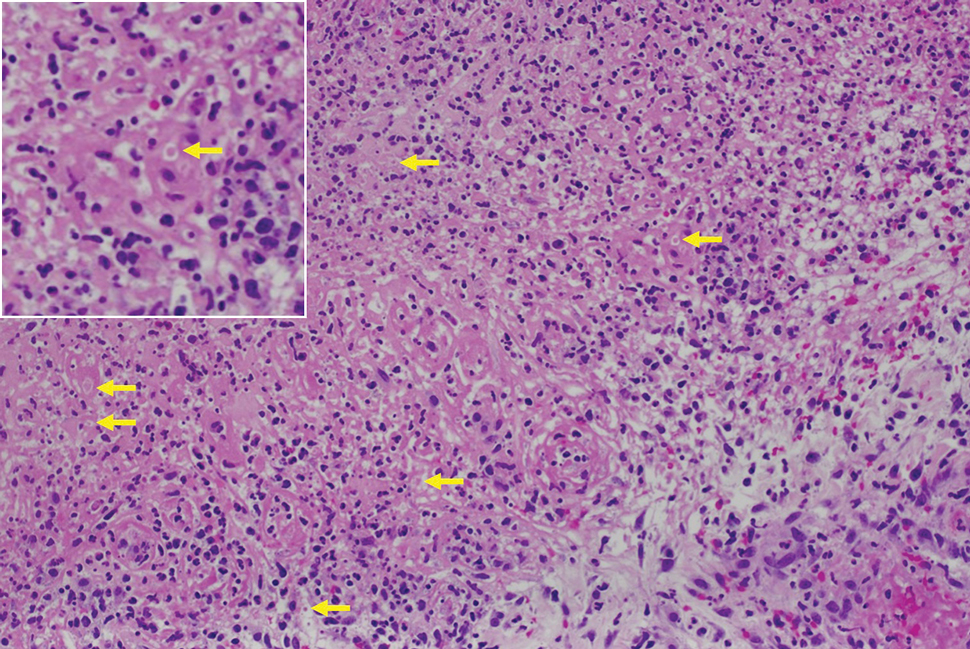
shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).
Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.

shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).

Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
THE DIAGNOSIS: Mpox
Histologic examination demonstrated dense aggregates of necrotic cellular debris composed of karyorrhectic nuclear fragments intermixed with neutrophils, lymphocytes, and histiocytes. Eosinophilic intracytoplasmic inclusions also were observed (Figure 1). The bacterial, fungal, and mycobacterial histologic special stains and cultures were negative. Three weeks after the initial visit with dermatology, the patient was admitted to the hospital for worsening symptoms of fever, chills, and painful erythema surrounding the skin lesions. Serology and viral workup revealed a positive mpox polymerase chain reaction test, suggesting a diagnosis of mpox. Following the Centers for Disease Control and Prevention protocol, the patient was started on oral tecovirimat 200 mg twice daily for 3 weeks and intravenous infusions of cidofovir 345 mg once weekly for 2 weeks. After treatment was initiated, the skin lesions showed rapid improvement (Figure 2), and he was discharged from the hospital after finishing the second dose of cidofovir. Four months after the initial dermatology consultation, the lesions had resolved completely with residual scarring. At that time, the patient had full movement of the right eye.

shows higher digital magnification of eosinophilic inclusions observed throughout the biopsy specimen (original magnification ×400).

Mpox virus is a member of the Poxviridae family of zoonotic viruses, which are transmitted from animals to humans. The mpox virus is brick-shaped (rectangular) and has a genome of linear double-stranded DNA encoding 180 proteins.1 Primates and rodents are the typical host reservoirs for viral circulation of mpox.2 Animal-to-human transmission occurs through direct contact with mucous membranes, bodily fluids, or tissues of an infected animal. Human-to-human transmission occurs through direct contact with infected mucous membranes, bodily fluids, respiratory droplets, and contaminated fomites.2
Symptoms typically occur within 1 week of exposure to the mpox virus. Prodromal symptoms of fever, sore throat, body aches, and headaches last for 3 days.1 Many patients experience a facial rash that spreads to the arms and legs over a period of 2 to 4 weeks. The rash initially manifests as small papules that progress to painful pustules and vesicles measuring 0.5 to 1.0 cm in diameter.3 The mpox virus is transmitted through these skin lesions until they crust over and re-epithelialize.1 The case fatality rate for mpox infection remains low (0.18%).4
Mpox outbreaks mainly were limited to central and western Africa prior to 2022. From May 17, 2022, through October 6, 2022, 26,384 cases of mpox were reported in the United States.5 During this outbreak, immunocompromised patients diagnosed with HIV and men who have sex with men were disproportionately affected.5
Due to the similarities between the smallpox virus and other orthopoxviruses, certain smallpox vaccines have been indicated for pre-exposure prophylaxis.6 The efficacy of prophylactic vaccination is believed to stem from the production of neutralizing antibodies that are cross-protective against other orthopoxviruses, including mpox.7 The 2 vaccines approved in the United States for mpox prophylaxis are JYNNEOS and ACAM2000, which are both live attenuated vaccines. Pre-exposure prophylaxis is indicated for patients at risk for severe disease, including men who have sex with men, individuals diagnosed with HIV or other immunosuppressive disorders, and individuals with recent diagnoses of one or more sexually transmitted diseases.8
Most mpox cases resolve within 2 to 4 weeks and only require supportive care (eg, nonsteroidal anti-inflammatory drugs, topical steroids, topical anesthetics) to treat pain.8 For patients at risk for severe disease, antiviral medications are warranted. Tecovirimat, brincidofovir, and cidofovir are antiviral medications used to treat smallpox that are thought to be effective against mpox.8,9 Tecovirimat and cidofovir have been shown to be effective against mpox in animal trials, but randomized or nonrandomized trials have not been performed in humans.9-11 Tecovirimat currently is available for the treatment of severe mpox in patients who meet the Centers for Disease Control and Prevention’s Investigational New Drug protocol; for these patients, a 200-mg course is administered orally or intravenously every 12 hours for 2 weeks.8
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
- Lu J, Xing H, Wang C, et al. Mpox (formerly monkeypox): pathogenesis, prevention, and treatment. Signal Transduct Target Ther. 2023;8:458. doi:10.1038/s41392-023-01675-
- Lim CK, Roberts J, Moso M, et al. Mpox diagnostics: review of current and emerging technologies. J Med Virol. 2023;95:e28429. doi:10.1002/jmv.28429
- Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1:8. doi:10.3390/tropicalmed1010008
- World Health Organization. Mpox (monkeypox) World Health Organization. Published April 18, 2023. Accessed May 28, 2025. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response—United States, May 17–October 6, 2022. 2022:1449-1456. https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a4.htm?s_cid=mm7145a4_w
- Rizk JG, Lippi G, Henry BM, et al. Prevention and treatment of monkeypox. Drugs. 2022;82:957-963. doi:10.1007/s40265-022-01742-y
- Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740-747. doi:10.1038 /nm1261
- Centers for Disease Control and Prevention. Mpox treatment information for healthcare professionals. Updated June 18, 2024. Accessed May 28, 2025. https://www.cdc.gov/mpox/hcp/clinical-care/?CDC_AAref_Val=https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html
- Mitja O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401:60-74. doi:10.1016/S0140-6736(22)02075-X
- Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620-2625. doi:10.1128/aac.00021-09
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44-53. doi:10.1056 /nejmoa1705688
Multiple Fungating Plaques on the Face, Arms, and Legs
Multiple Fungating Plaques on the Face, Arms, and Legs
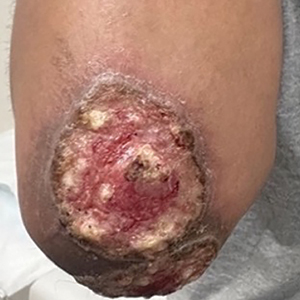
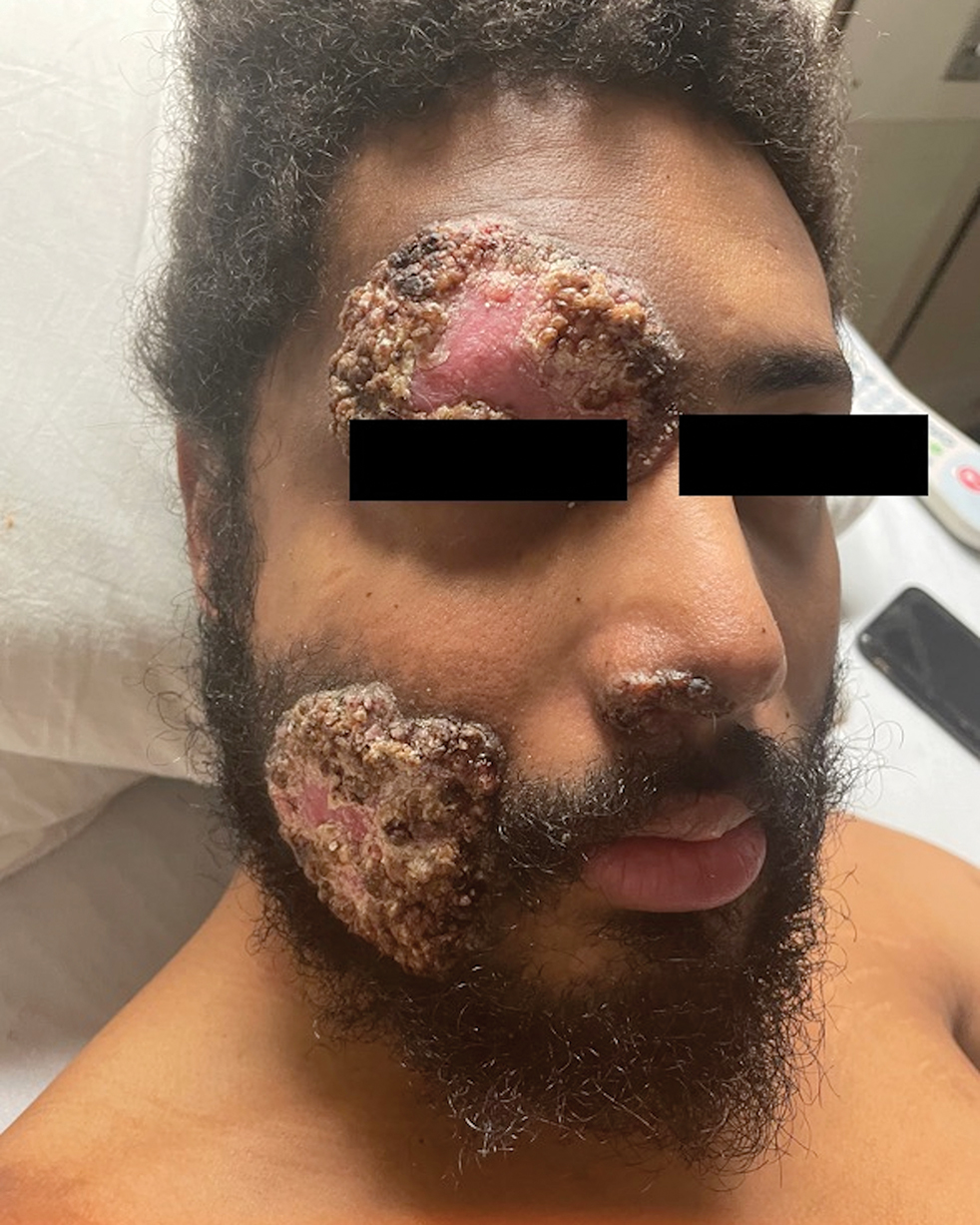
A 27-year-old man presented to his primary care physician after he was struck in the head by a tree branch while working outside. The next day, ulcerating lesions emerged on the right supraorbital ridge, along with subjective fevers, chills, fatigue, and shortness of breath. The patient reported a history of unprotected sexual intercourse with a male partner who was HIV positive. His medical history included syphilis status posttreatment with a course of 5 penicillin injections, hepatitis C, and HIV diagnosed one month prior to presentation (CD4 count, 169 cells/mm3 [reference range, 500-1500 cells/mm3]). A punch biopsy performed by the primary care physician revealed suppurative granulomatous inflammation, and the patient was prescribed antibiotics with mild improvement. He then was referred to dermatology for further evaluation of the ulcerating lesions.
Three months after the initial trauma, the patient presented to the dermatology clinic for evaluation of multiple large fungating plaques affecting multiple sites on the face (top), arms (bottom), and legs. Physical examination revealed large circinate verrucous plaques involving the right supraorbital ridge and eyelid. The patient was unable to fully open the right eye. Similar plaques also were observed on the right malar cheek, arms, and feet. Four 5-mm punch biopsies from lesions on the right elbow and left ankle were obtained with fungal and bacterial cultures.
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic scarring inflammatory skin condition of the follicular epithelium that impacts 1% to 4% of the general population (eFigure).1-3 This statistic likely is an underrepresentation of the affected population due to missed and delayed diagnoses.1 Hidradenitis suppurativa has been identified as having one of the strongest negative impacts on patients’ lives based on studied skin diseases.4 Its recurrent nature can negatively impact both the patient’s physical and mental state.3 Due to the debilitating effects of HS, we aimed to create updated recommendations for empiric antibotics based on affected anatomic locations in an effort to improve patient quality of life.
Methods
An institutional review board–approved retrospective medical chart review of 485 patients diagnosed with HS and evaluated at the University of Texas Medical Branch in Galveston from January 2006 to December 2021 was conducted. Males and females of all ages (including pregnant and pediatric patients) were included. Only patients for whom anatomic locations of HS lesions or culture sites were not documented were excluded from the analysis. Locations of cultures were categorized into 5 groups: axilla; groin; buttocks; inframammary; and multiple sites of involvement, which included any combination of 2 or more sites. Types of bacteria collected from cultures and recorded included Escherichia coli, Enterococcus species, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and other Gram-negative species. Sensitivity profiles also were analyzed for the most commonly cultured bacteria to create recommendations on antibiotic use based on the anatomic location of the lesions. Data analysis was conducted using descriptive statistics and bivariate analysis.
Results
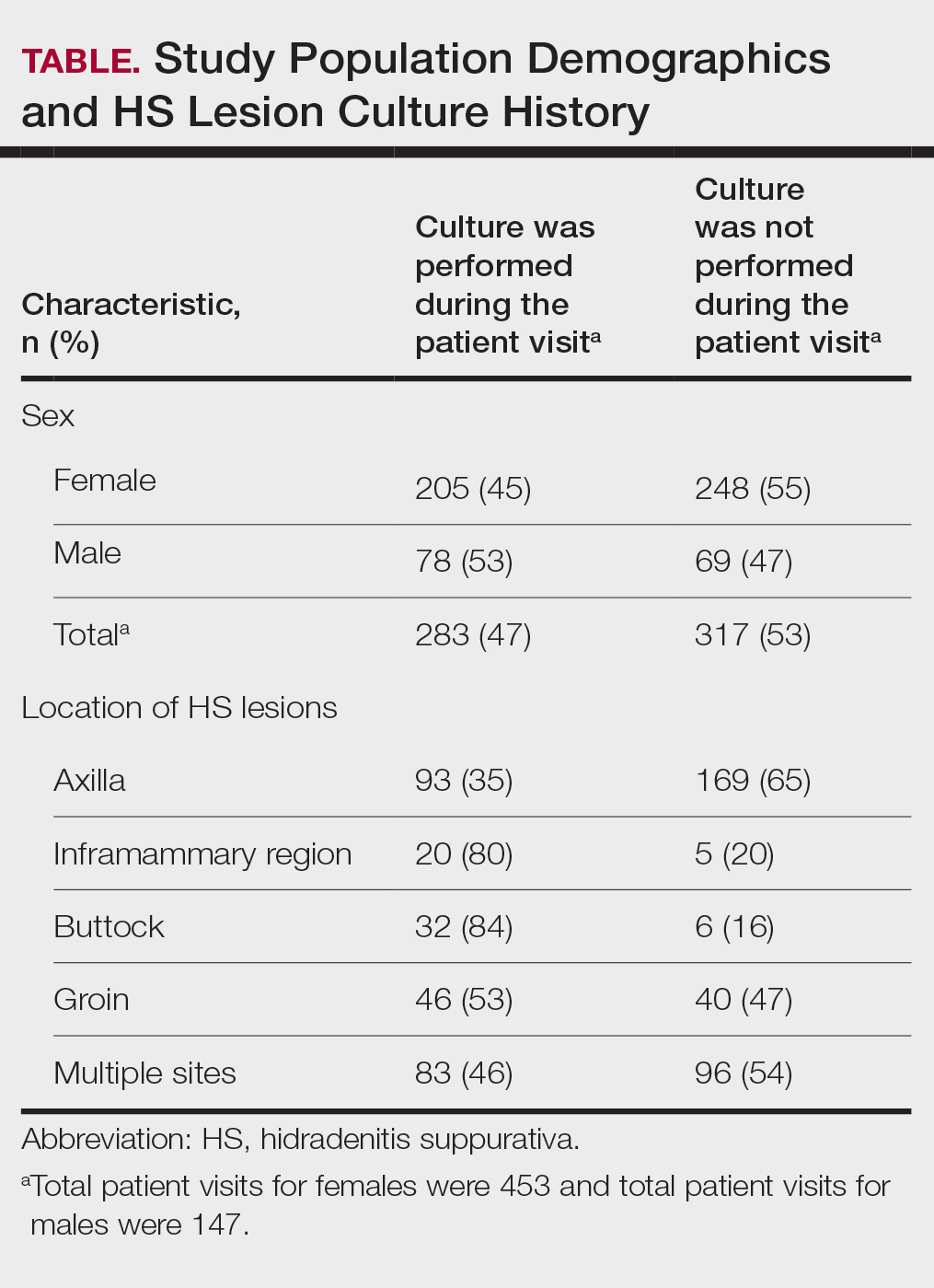
The analysis included 485 patients comprising 600 visits. Seventy-five percent (363/485) of the study population was female. The axilla was the most common anatomic location for HS lesions followed by multiple sites of involvement. In total, 283 cultures were performed; males were 1.1 times more likely than females to be cultured. While cultures were most frequently obtained in patients with axillary lesions only (93/262 [35%]) or from multiple sites of involvement (83/179 [46%]) as this was the most common presentation of HS in our patient population, cultures were more likely to be obtained when patients presented with only buttock (32/38 [84%]) and inframammary (20/25 [80%]) lesions (Table).
Staphylococcus aureus was the most commonly cultured bacteria in general (53/283 [19%]) as well as for HS located the axilla (24/56 [43%]) and in multiple sites (16/51 [31%]). Proteus mirabilis (29/283 [10%]) was the second most commonly cultured bacteria overall and was cultured most often in the axilla (15/56 [27%]) and inframammary region (6/14 [43%]). These were followed by beta-hemolytic Streptococcus species (26/283 [9%]) and Enterococcus species (21/283 [7%]), which was second to P mirabilis as the most commonly cultured bacteria in the inframammary region (6/14 [43%])(eTable 1).
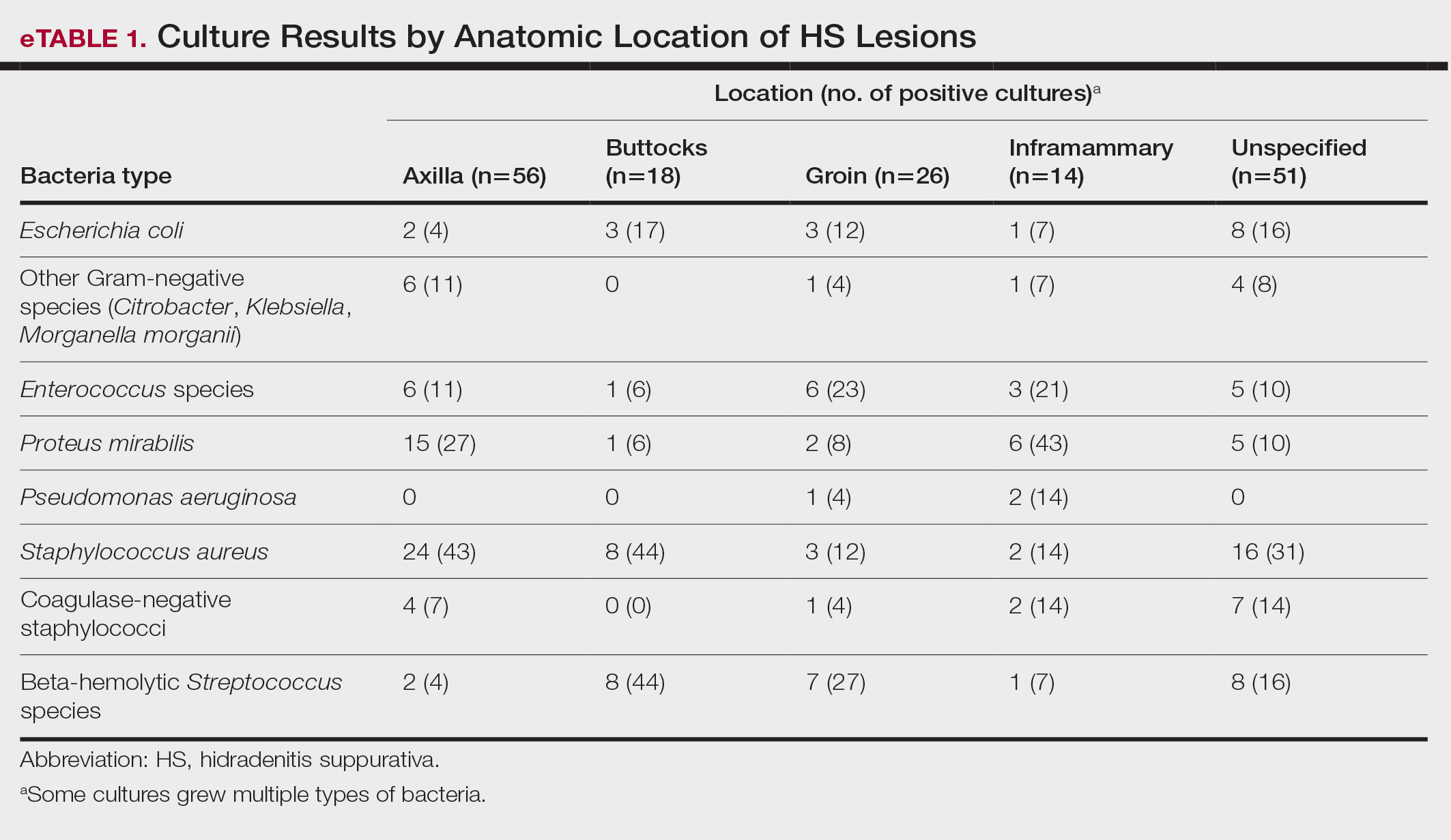
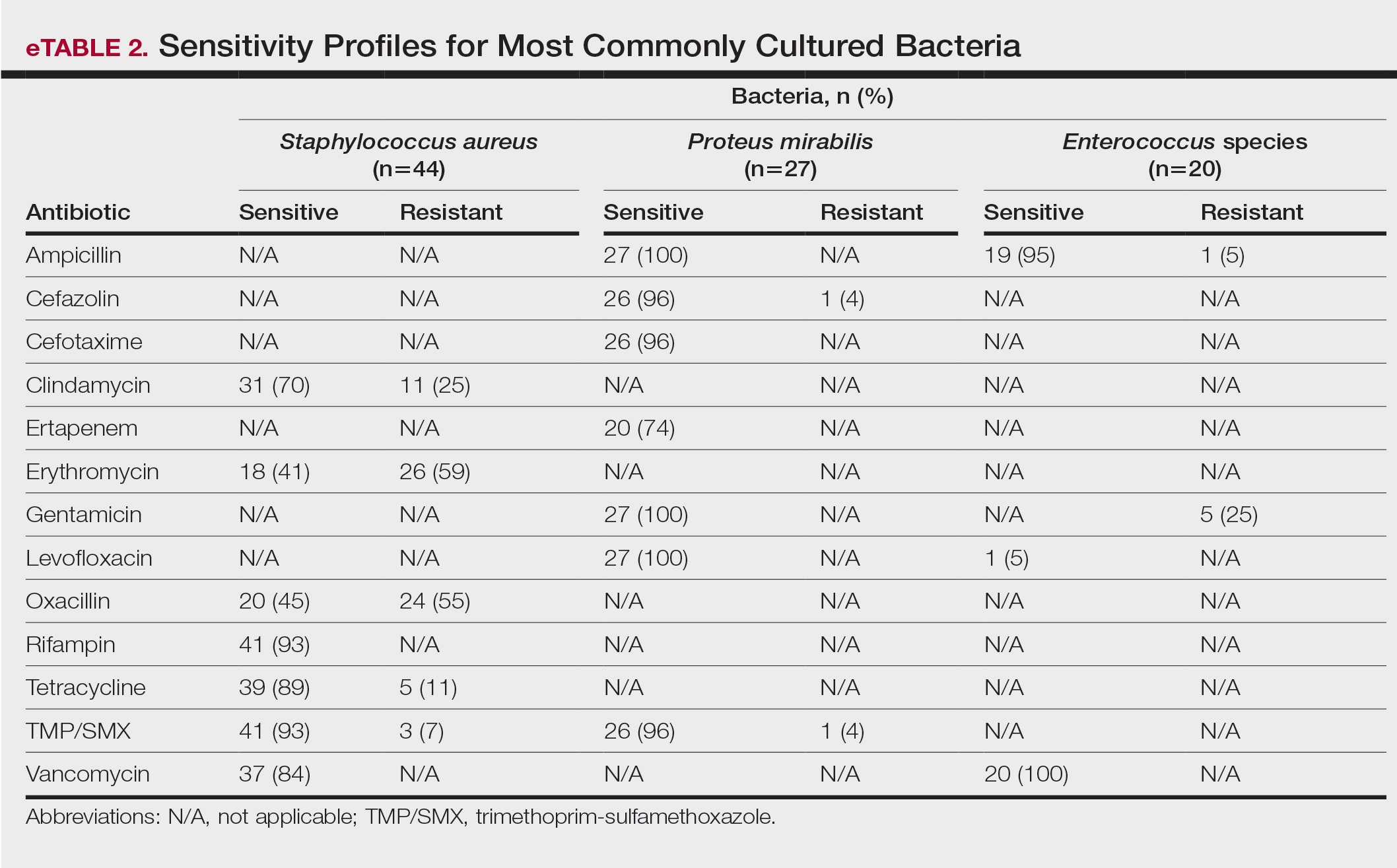
eTable 2 shows the sensitivity profiles for the most commonly cultured bacteria: S aureus, P mirabilis, and Enterococcus species. Staphylococcus aureus located in the axilla, buttocks, and groin was most sensitive to rifampin (41/44 [93%]), TMP/SMX (41/44 [93%]), and tetracycline (39/44 [89%]) and most resistant to erythromycin (26/44 [59%]) and oxacillin (24/44 [55%]). Proteus mirabilis in the inframammary region was most sensitive to ampicillin (27/27 [100%]), gentamicin (27/27 [100%]), levofloxacin (27/27 [100%]), and TMP/SMX (26/27 [96%]). Enterococcus species were most sensitive to vancomycin (20/20 [100%]) and ampicillin (19/20 [95%]) and most resistant to gentamicin (5/20 [25%]).
Comment
To treat HS, it is important to understand the cause of the condition. Although the pathogenesis of HS has many unknowns, bacterial colonization and biofilms are thought to play a role. Lipopolysaccharides found in the outer membrane of Gram-negative bacteria are pathogen-associated molecular patterns that present to the toll-like receptors of the human immune system. Once the toll-like receptors recognize the pathogen-associated molecular patterns, macrophages and keratinocytes are activated and release proinflammatory and anti-inflammatory cytokines and chemokines. Persistent presentation of bacteria to the immune system increases immune-cell recruitment and worsens chronic inflammation in patients with HS. Evidence has revealed that bacteria initiate and sustain the inflammation seen in patients with HS; therefore, reducing the amount of bacteria could alleviate some of the symptoms of HS.5 It is important to continue learning about the pathophysiology of this disease as well as formulating tailored treatments to minimize patient discomfort and improve quality of life.
Based on the findings of the current study and the safety profile of the medication, tetracyclines may be considered for first-line empiric therapy in patients with HS involving the axilla only, buttocks only, or multiple sites. For additional coverage of P mirabilis in the axilla or inframammary region, TMP/SMX monotherapy or tetracycline plus ampicillin may be considered. For inframammary lesions only, empiric treatment with ampicillin or TMP/ SMX is recommended. For HS lesions in the groin area, coverage of Enterococcus species with ampicillin should be considered. Patients with multiple sites of involvement that include the inframammary or groin regions similarly should receive empiric antibiotics that cover both S aureus and Gram-negative bacteria, such as TMP/SMX or tetracycline and ampicillin, respectively; if the multiple sites do not include the inframammary or groin regions, Gram-negative coverage may not be indicated. Based on our findings, standardization of treatment for patients with HS can allow for earlier and potentially more effective treatment.
In a similar study conducted in 2016, bacteria species were isolated from the axilla, groin, and gluteus/perineum in patients with HS.5 In that study, the most prominent bacteria in the axilla was CoNS; in the groin, P mirabilis and E coli; and in the gluteus/perineum, E coli and CoNS. These results differed from ours, which found S aureus as the abundant bacteria in these areas. In the 2016 study, the highest rates of resistance were found for penicillin G, erythromycin, clindamycin, and ampicillin.5 In contrast, the current study found high sensitivities for clindamycin and ampicillin, but our results support the finding of high resistance for erythromycin. These differences could be accounted for by the lower sample size of patients in the 2016 study: 68 patients were analyzed for sensitivity results, and 171 patients were analyzed for frequency of bacterial species in patients with HS.5
Our study is limited by its relatively small sample size. Additionally, all patients were seen at 1 of 2 clinic sites, located in League City and Galveston, Texas, and the data from this geographic area may not be applicable to patients seen in different climates.
Conclusion
Outcomes for patients with HS improve with early intervention; however, HS treatment may be delayed by selection of ineffective antibiotic therapy. Our study provides clinicians with recommendations for empiric antibiotic treatment based on anatomic location of HS lesions and culture sensitivity profiles. Utilizing tailored antibiotic therapy on initial clinical evaluation may increase early disease control and improve morbidity and disease outcomes, thereby increasing patient quality of life.
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563.
- Yazdanyar S, Jemec GBE. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Hessam S, Sand M, Georgas D, et al. Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol. 2016; 29:161-167.
Hidradenitis suppurativa (HS) is a chronic scarring inflammatory skin condition of the follicular epithelium that impacts 1% to 4% of the general population (eFigure).1-3 This statistic likely is an underrepresentation of the affected population due to missed and delayed diagnoses.1 Hidradenitis suppurativa has been identified as having one of the strongest negative impacts on patients’ lives based on studied skin diseases.4 Its recurrent nature can negatively impact both the patient’s physical and mental state.3 Due to the debilitating effects of HS, we aimed to create updated recommendations for empiric antibotics based on affected anatomic locations in an effort to improve patient quality of life.

Methods
An institutional review board–approved retrospective medical chart review of 485 patients diagnosed with HS and evaluated at the University of Texas Medical Branch in Galveston from January 2006 to December 2021 was conducted. Males and females of all ages (including pregnant and pediatric patients) were included. Only patients for whom anatomic locations of HS lesions or culture sites were not documented were excluded from the analysis. Locations of cultures were categorized into 5 groups: axilla; groin; buttocks; inframammary; and multiple sites of involvement, which included any combination of 2 or more sites. Types of bacteria collected from cultures and recorded included Escherichia coli, Enterococcus species, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and other Gram-negative species. Sensitivity profiles also were analyzed for the most commonly cultured bacteria to create recommendations on antibiotic use based on the anatomic location of the lesions. Data analysis was conducted using descriptive statistics and bivariate analysis.
Results
The analysis included 485 patients comprising 600 visits. Seventy-five percent (363/485) of the study population was female. The axilla was the most common anatomic location for HS lesions followed by multiple sites of involvement. In total, 283 cultures were performed; males were 1.1 times more likely than females to be cultured. While cultures were most frequently obtained in patients with axillary lesions only (93/262 [35%]) or from multiple sites of involvement (83/179 [46%]) as this was the most common presentation of HS in our patient population, cultures were more likely to be obtained when patients presented with only buttock (32/38 [84%]) and inframammary (20/25 [80%]) lesions (Table).

Staphylococcus aureus was the most commonly cultured bacteria in general (53/283 [19%]) as well as for HS located the axilla (24/56 [43%]) and in multiple sites (16/51 [31%]). Proteus mirabilis (29/283 [10%]) was the second most commonly cultured bacteria overall and was cultured most often in the axilla (15/56 [27%]) and inframammary region (6/14 [43%]). These were followed by beta-hemolytic Streptococcus species (26/283 [9%]) and Enterococcus species (21/283 [7%]), which was second to P mirabilis as the most commonly cultured bacteria in the inframammary region (6/14 [43%])(eTable 1).

eTable 2 shows the sensitivity profiles for the most commonly cultured bacteria: S aureus, P mirabilis, and Enterococcus species. Staphylococcus aureus located in the axilla, buttocks, and groin was most sensitive to rifampin (41/44 [93%]), TMP/SMX (41/44 [93%]), and tetracycline (39/44 [89%]) and most resistant to erythromycin (26/44 [59%]) and oxacillin (24/44 [55%]). Proteus mirabilis in the inframammary region was most sensitive to ampicillin (27/27 [100%]), gentamicin (27/27 [100%]), levofloxacin (27/27 [100%]), and TMP/SMX (26/27 [96%]). Enterococcus species were most sensitive to vancomycin (20/20 [100%]) and ampicillin (19/20 [95%]) and most resistant to gentamicin (5/20 [25%]).

Comment
To treat HS, it is important to understand the cause of the condition. Although the pathogenesis of HS has many unknowns, bacterial colonization and biofilms are thought to play a role. Lipopolysaccharides found in the outer membrane of Gram-negative bacteria are pathogen-associated molecular patterns that present to the toll-like receptors of the human immune system. Once the toll-like receptors recognize the pathogen-associated molecular patterns, macrophages and keratinocytes are activated and release proinflammatory and anti-inflammatory cytokines and chemokines. Persistent presentation of bacteria to the immune system increases immune-cell recruitment and worsens chronic inflammation in patients with HS. Evidence has revealed that bacteria initiate and sustain the inflammation seen in patients with HS; therefore, reducing the amount of bacteria could alleviate some of the symptoms of HS.5 It is important to continue learning about the pathophysiology of this disease as well as formulating tailored treatments to minimize patient discomfort and improve quality of life.
Based on the findings of the current study and the safety profile of the medication, tetracyclines may be considered for first-line empiric therapy in patients with HS involving the axilla only, buttocks only, or multiple sites. For additional coverage of P mirabilis in the axilla or inframammary region, TMP/SMX monotherapy or tetracycline plus ampicillin may be considered. For inframammary lesions only, empiric treatment with ampicillin or TMP/ SMX is recommended. For HS lesions in the groin area, coverage of Enterococcus species with ampicillin should be considered. Patients with multiple sites of involvement that include the inframammary or groin regions similarly should receive empiric antibiotics that cover both S aureus and Gram-negative bacteria, such as TMP/SMX or tetracycline and ampicillin, respectively; if the multiple sites do not include the inframammary or groin regions, Gram-negative coverage may not be indicated. Based on our findings, standardization of treatment for patients with HS can allow for earlier and potentially more effective treatment.
In a similar study conducted in 2016, bacteria species were isolated from the axilla, groin, and gluteus/perineum in patients with HS.5 In that study, the most prominent bacteria in the axilla was CoNS; in the groin, P mirabilis and E coli; and in the gluteus/perineum, E coli and CoNS. These results differed from ours, which found S aureus as the abundant bacteria in these areas. In the 2016 study, the highest rates of resistance were found for penicillin G, erythromycin, clindamycin, and ampicillin.5 In contrast, the current study found high sensitivities for clindamycin and ampicillin, but our results support the finding of high resistance for erythromycin. These differences could be accounted for by the lower sample size of patients in the 2016 study: 68 patients were analyzed for sensitivity results, and 171 patients were analyzed for frequency of bacterial species in patients with HS.5
Our study is limited by its relatively small sample size. Additionally, all patients were seen at 1 of 2 clinic sites, located in League City and Galveston, Texas, and the data from this geographic area may not be applicable to patients seen in different climates.
Conclusion
Outcomes for patients with HS improve with early intervention; however, HS treatment may be delayed by selection of ineffective antibiotic therapy. Our study provides clinicians with recommendations for empiric antibiotic treatment based on anatomic location of HS lesions and culture sensitivity profiles. Utilizing tailored antibiotic therapy on initial clinical evaluation may increase early disease control and improve morbidity and disease outcomes, thereby increasing patient quality of life.
Hidradenitis suppurativa (HS) is a chronic scarring inflammatory skin condition of the follicular epithelium that impacts 1% to 4% of the general population (eFigure).1-3 This statistic likely is an underrepresentation of the affected population due to missed and delayed diagnoses.1 Hidradenitis suppurativa has been identified as having one of the strongest negative impacts on patients’ lives based on studied skin diseases.4 Its recurrent nature can negatively impact both the patient’s physical and mental state.3 Due to the debilitating effects of HS, we aimed to create updated recommendations for empiric antibotics based on affected anatomic locations in an effort to improve patient quality of life.

Methods
An institutional review board–approved retrospective medical chart review of 485 patients diagnosed with HS and evaluated at the University of Texas Medical Branch in Galveston from January 2006 to December 2021 was conducted. Males and females of all ages (including pregnant and pediatric patients) were included. Only patients for whom anatomic locations of HS lesions or culture sites were not documented were excluded from the analysis. Locations of cultures were categorized into 5 groups: axilla; groin; buttocks; inframammary; and multiple sites of involvement, which included any combination of 2 or more sites. Types of bacteria collected from cultures and recorded included Escherichia coli, Enterococcus species, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, coagulase-negative staphylococci (CoNS), and other Gram-negative species. Sensitivity profiles also were analyzed for the most commonly cultured bacteria to create recommendations on antibiotic use based on the anatomic location of the lesions. Data analysis was conducted using descriptive statistics and bivariate analysis.
Results
The analysis included 485 patients comprising 600 visits. Seventy-five percent (363/485) of the study population was female. The axilla was the most common anatomic location for HS lesions followed by multiple sites of involvement. In total, 283 cultures were performed; males were 1.1 times more likely than females to be cultured. While cultures were most frequently obtained in patients with axillary lesions only (93/262 [35%]) or from multiple sites of involvement (83/179 [46%]) as this was the most common presentation of HS in our patient population, cultures were more likely to be obtained when patients presented with only buttock (32/38 [84%]) and inframammary (20/25 [80%]) lesions (Table).

Staphylococcus aureus was the most commonly cultured bacteria in general (53/283 [19%]) as well as for HS located the axilla (24/56 [43%]) and in multiple sites (16/51 [31%]). Proteus mirabilis (29/283 [10%]) was the second most commonly cultured bacteria overall and was cultured most often in the axilla (15/56 [27%]) and inframammary region (6/14 [43%]). These were followed by beta-hemolytic Streptococcus species (26/283 [9%]) and Enterococcus species (21/283 [7%]), which was second to P mirabilis as the most commonly cultured bacteria in the inframammary region (6/14 [43%])(eTable 1).

eTable 2 shows the sensitivity profiles for the most commonly cultured bacteria: S aureus, P mirabilis, and Enterococcus species. Staphylococcus aureus located in the axilla, buttocks, and groin was most sensitive to rifampin (41/44 [93%]), TMP/SMX (41/44 [93%]), and tetracycline (39/44 [89%]) and most resistant to erythromycin (26/44 [59%]) and oxacillin (24/44 [55%]). Proteus mirabilis in the inframammary region was most sensitive to ampicillin (27/27 [100%]), gentamicin (27/27 [100%]), levofloxacin (27/27 [100%]), and TMP/SMX (26/27 [96%]). Enterococcus species were most sensitive to vancomycin (20/20 [100%]) and ampicillin (19/20 [95%]) and most resistant to gentamicin (5/20 [25%]).

Comment
To treat HS, it is important to understand the cause of the condition. Although the pathogenesis of HS has many unknowns, bacterial colonization and biofilms are thought to play a role. Lipopolysaccharides found in the outer membrane of Gram-negative bacteria are pathogen-associated molecular patterns that present to the toll-like receptors of the human immune system. Once the toll-like receptors recognize the pathogen-associated molecular patterns, macrophages and keratinocytes are activated and release proinflammatory and anti-inflammatory cytokines and chemokines. Persistent presentation of bacteria to the immune system increases immune-cell recruitment and worsens chronic inflammation in patients with HS. Evidence has revealed that bacteria initiate and sustain the inflammation seen in patients with HS; therefore, reducing the amount of bacteria could alleviate some of the symptoms of HS.5 It is important to continue learning about the pathophysiology of this disease as well as formulating tailored treatments to minimize patient discomfort and improve quality of life.
Based on the findings of the current study and the safety profile of the medication, tetracyclines may be considered for first-line empiric therapy in patients with HS involving the axilla only, buttocks only, or multiple sites. For additional coverage of P mirabilis in the axilla or inframammary region, TMP/SMX monotherapy or tetracycline plus ampicillin may be considered. For inframammary lesions only, empiric treatment with ampicillin or TMP/ SMX is recommended. For HS lesions in the groin area, coverage of Enterococcus species with ampicillin should be considered. Patients with multiple sites of involvement that include the inframammary or groin regions similarly should receive empiric antibiotics that cover both S aureus and Gram-negative bacteria, such as TMP/SMX or tetracycline and ampicillin, respectively; if the multiple sites do not include the inframammary or groin regions, Gram-negative coverage may not be indicated. Based on our findings, standardization of treatment for patients with HS can allow for earlier and potentially more effective treatment.
In a similar study conducted in 2016, bacteria species were isolated from the axilla, groin, and gluteus/perineum in patients with HS.5 In that study, the most prominent bacteria in the axilla was CoNS; in the groin, P mirabilis and E coli; and in the gluteus/perineum, E coli and CoNS. These results differed from ours, which found S aureus as the abundant bacteria in these areas. In the 2016 study, the highest rates of resistance were found for penicillin G, erythromycin, clindamycin, and ampicillin.5 In contrast, the current study found high sensitivities for clindamycin and ampicillin, but our results support the finding of high resistance for erythromycin. These differences could be accounted for by the lower sample size of patients in the 2016 study: 68 patients were analyzed for sensitivity results, and 171 patients were analyzed for frequency of bacterial species in patients with HS.5
Our study is limited by its relatively small sample size. Additionally, all patients were seen at 1 of 2 clinic sites, located in League City and Galveston, Texas, and the data from this geographic area may not be applicable to patients seen in different climates.
Conclusion
Outcomes for patients with HS improve with early intervention; however, HS treatment may be delayed by selection of ineffective antibiotic therapy. Our study provides clinicians with recommendations for empiric antibiotic treatment based on anatomic location of HS lesions and culture sensitivity profiles. Utilizing tailored antibiotic therapy on initial clinical evaluation may increase early disease control and improve morbidity and disease outcomes, thereby increasing patient quality of life.
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563.
- Yazdanyar S, Jemec GBE. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Hessam S, Sand M, Georgas D, et al. Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol. 2016; 29:161-167.
- Vinkel C, Thomsen SF. Hidradenitis suppurativa: causes, features, and current treatments. J Clin Aesthet Dermatol. 2018;11:17-23.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-563.
- Yazdanyar S, Jemec GBE. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Hessam S, Sand M, Georgas D, et al. Microbial profile and antimicrobial susceptibility of bacteria found in inflammatory hidradenitis suppurativa lesions. Skin Pharmacol Physiol. 2016; 29:161-167.
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
Recommendations for Empiric Antibiotic Therapy in Hidradenitis Suppurativa
PRACTICE POINTS
- The inflammation seen in patients with hidradenitis suppurativa (HS) is initiated and sustained by bacteria; therefore, reducing the number of bacteria may alleviate some of the symptoms of HS.
- For HS involving the axillae or buttocks, tetracyclines should be recommended as first-line empiric therapy.
- Patients with HS with multiple sites affected that include the inframammary or groin regions should receive empiric antibiotics that cover both Staphylococcus aureus and Gram-negative bacteria, such as trimethoprim-sulfamethoxazole or tetracycline plus ampicillin.
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Oritavancin and dalbavancin are long acting lipoglycopeptides indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI).1,2 Largely due to their long half-lives, prolonged tissue concentrations at sites of infection, tolerability, and minimal requirement for therapeutic drug monitoring, these agents are attractive options in outpatient settings.3,4 A 1- or 2-dose treatment of oritavancin and dalbavancin may be sufficient for conditions traditionally treated with outpatient parenteral antimicrobial therapy (OPAT) via peripherally inserted central catheter (PICC).
Limited research supports the use of dalbavancin and oritavancin for bone and joint infections, infective endocarditis, and bloodstream infections (BSIs). However, the US Food and Drug Administration has approved an indication for the treatment of ABSSSI.3-9 Dosing for these off-label indications varies but typically consists of an initial intravenous (IV) dose (1000 mg, 1200 mg, or 1500 mg), with a subsequent dose 1 to 2 weeks later or administered once weekly.6-10
Due in part to the recent availability of oritavancin and dalbavancin relative to the publication of practice guidelines, their appropriate place in therapy continues to evolve based on emerging literature.11,12 One potential barrier of use for these medications is their cost. Based on the number of doses administered, the 2022 estimated total acquisition cost of therapy for oritavancin and dalbavancin was $1014 to $4397 and $3046 to $7150, respectively (eAppendix). Despite the high acquisition costs, these agents do not require the placement of an indwelling central line, can be administered in outpatient settings, and require minimal therapeutic dose monitoring compared to vancomycin.13-15 This medication use evaluation (MUE) compared the total cost of treatment with oritavancin and dalbavancin vs therapies traditionally used for OPAT or prolonged IV inpatient therapy.
METHODS
This retrospective MUE was conducted at the Boise Veterans Affairs Medical Center (BVAMC), a level 2 facility with an extensive rural catchment area. BVAMC provides many OPAT services, including medications, supplies, and dressing changes after initial clinic or inpatient education. Contracted vendors may also assist with at-home nursing care using supplies provided by the BVAMC. Cases were identified using an internal database of OPAT patients and those who received oritavancin or dalbavancin between September 1, 2017, and November 1, 2022. Patients aged ≥ 18 years who received ≥ 1 dose of oritavancin or dalbavancin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were included. Comparator treatments consisting of ≥ 1 week of vancomycin or daptomycin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were identified through review of OPAT and Infectious Diseases service consults during the same timeframe. Patients were excluded if any antibiotic was prescribed by a non- VA clinician, if medications were not provided by OPAT, or if chart review did not identify an ABSSSI, osteomyelitis/ joint infection, or BSI diagnosis.
Electronic medical record review was conducted using a standardized data collection form (eAppendix). Data collected included demographics, infectious diagnosis, treatment administered, administration procedures and related visits and treatment locations, outcomes including clinical failure, adverse events (AEs), and hospital readmission.
Clinical failure was defined as readmission or death due to worsening infection or readmission secondary to a documented potential AE to the evaluated antibiotics within 90 days after initiation. Clinical failures excluded readmissions not associated with infection including comorbidities or elective procedures. AEs included new onset renal failure (serum creatinine ≥ 0.5 mg/dL), neutropenia (neutrophils ≤ 500), thrombocytopenia (platelets < 100,000), eosinophilia (> 15% eosinophils), or creatine phosphokinase > 10 times the upper limit of normal, and Clostridioides difficile (C. difficile) infection. Line complications included thrombophlebitis, local inflammation, or infection requiring line replacement (eAppendix).
A cost-minimization approach was used to assess the total cost of treatment.16 Patients who received oritavancin or dalbavancin were matched with patients that received vancomycin and daptomycin for the same indication and about 1 month of initiation through the randomization function in Microsoft Excel. This accounted for changes in personnel, nonformulary drug approvals, cost, and changes in practice during the pandemic. Costs were calculated using a decision tree as a base model (Figure 1). In this model, each treatment dyad was assessed for the presence or absence of clinical failure, adverse event (medication and line complications), and treatment setting endpoints. Cost estimates were tabulated for each patient that received treatment using published VA data, literature, pharmacoeconomist guidance, or best faith effort based on workflow. 17-20 All cost estimates were based on 2022 figures or adjusted for inflation if obtained prior to 2022. Secondary endpoints of this analysis included estimated total cost of medication acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home-health services.
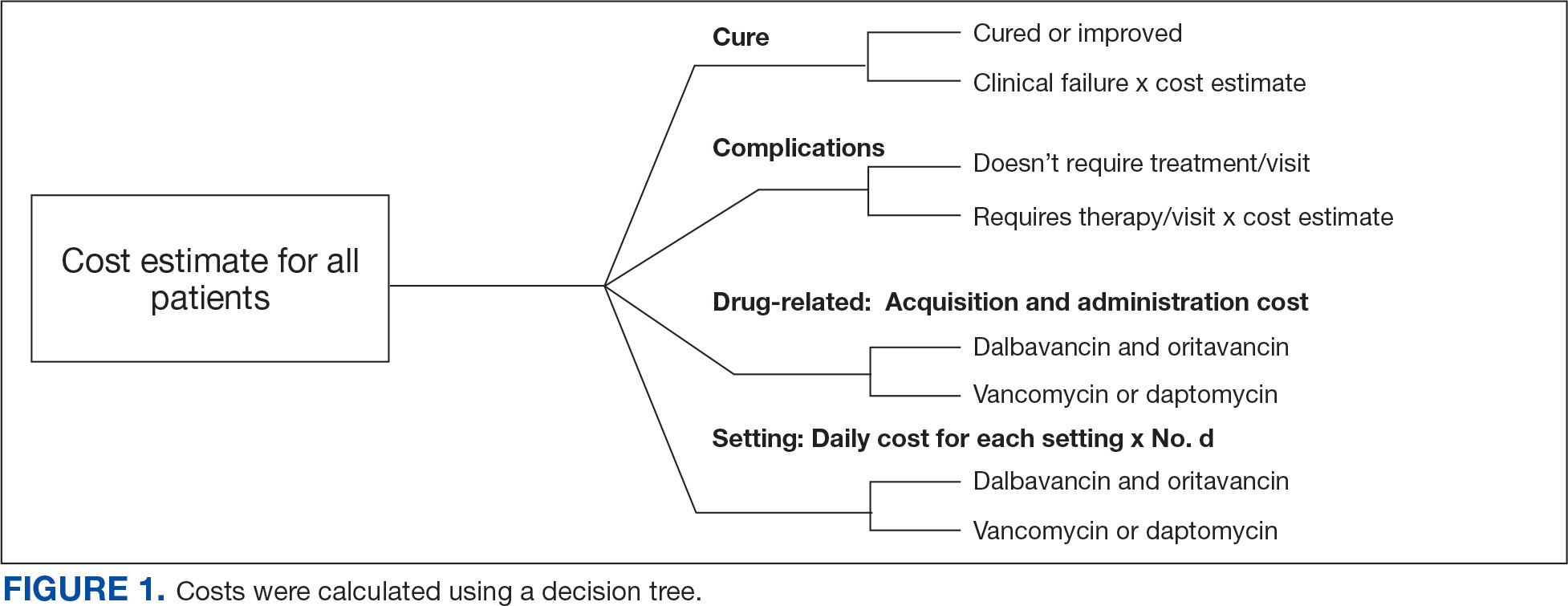
This evaluation was classified by the BVAMC Medication Use Evaluation research determination subcommittee as a quality improvement project and was considered exempt from VA Human Subjects Research requirements based on the VA Policy Handbook guideline 1058.05.
RESULTS
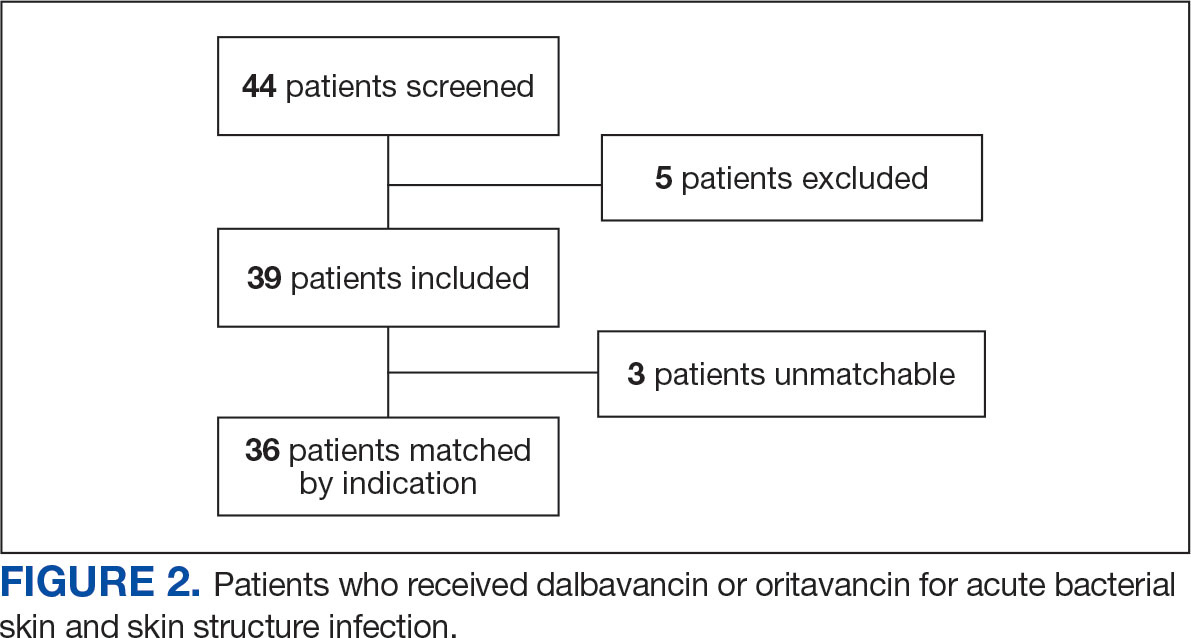
The study identified 44 patients who received dalbavancin or oritavancin between September 1, 2017, and October 31, 2022. Thirty-nine patients were included in the analysis: 24 received oritavancin and 15 received dalbavancin and were matched by indication to 10 patients who received vancomycin and 8 patients who received daptomycin. Three patients could not be matched by indication of ABSSSI (Figure 2). Most patients were male, aged > 65 years, and were treated for osteomyelitis (Table 1). No patients were treated for infective endocarditis. A myriad of concomitant antibiotics were used to treat patients and culture results indicated that most infections treated with oritavancin and dalbavancin were polymicrobial.
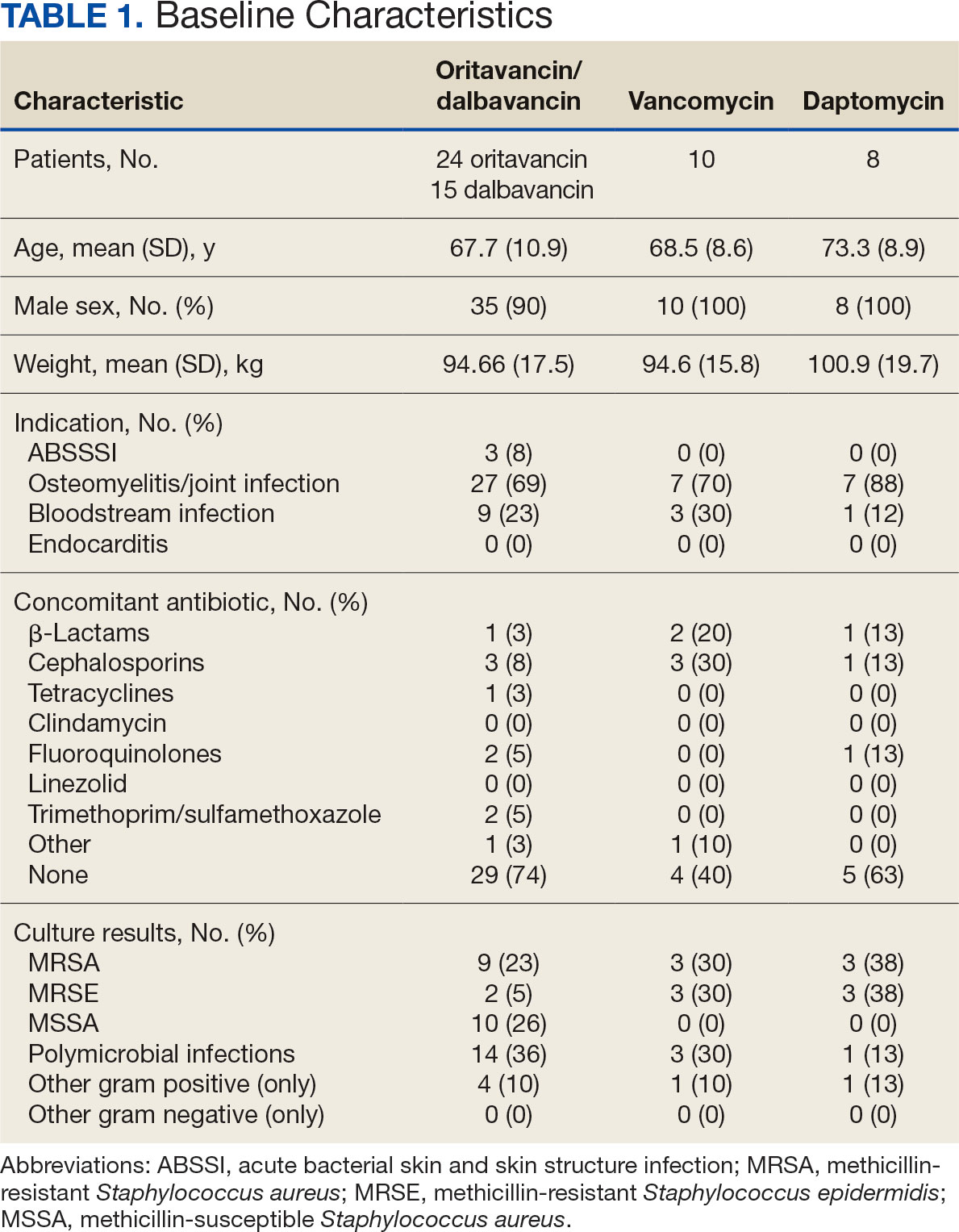
The mean total cost of therapy per patient receiving oritavancin, dalbavancin, vancomycin, and daptomycin was $35,630, $59,612, $73,333, and $73,708, respectively (Figure 3). When stratified by indication, 27 patients (69%) in the oritavancin/dalbavancin group were treated for osteomyelitis/ joint infections (16 oritavancin, 11 dalbavancin), 9 patients (23%) were treated for BSI (6 oritavancin, 3 dalbavancin), and 3 patients (8%) were treated for ABSSSI (2 oritavancin, 1 dalbavancin). The mean cost per patient for osteomyelitis/joint infections with oritavancin, dalbavancin, vancomycin, and daptomycin was $34,678, $54,224, $87,488, and $85,044, respectively. The mean cost per patient for BSI for oritavancin, dalbavancin, vancomycin, and daptomycin was $35,048, $75,349, $40,305, and $68,068, respectively. The mean cost per patient for ABSSSI for oritavancin and dalbavancin was $44,771 and $71,672.51.
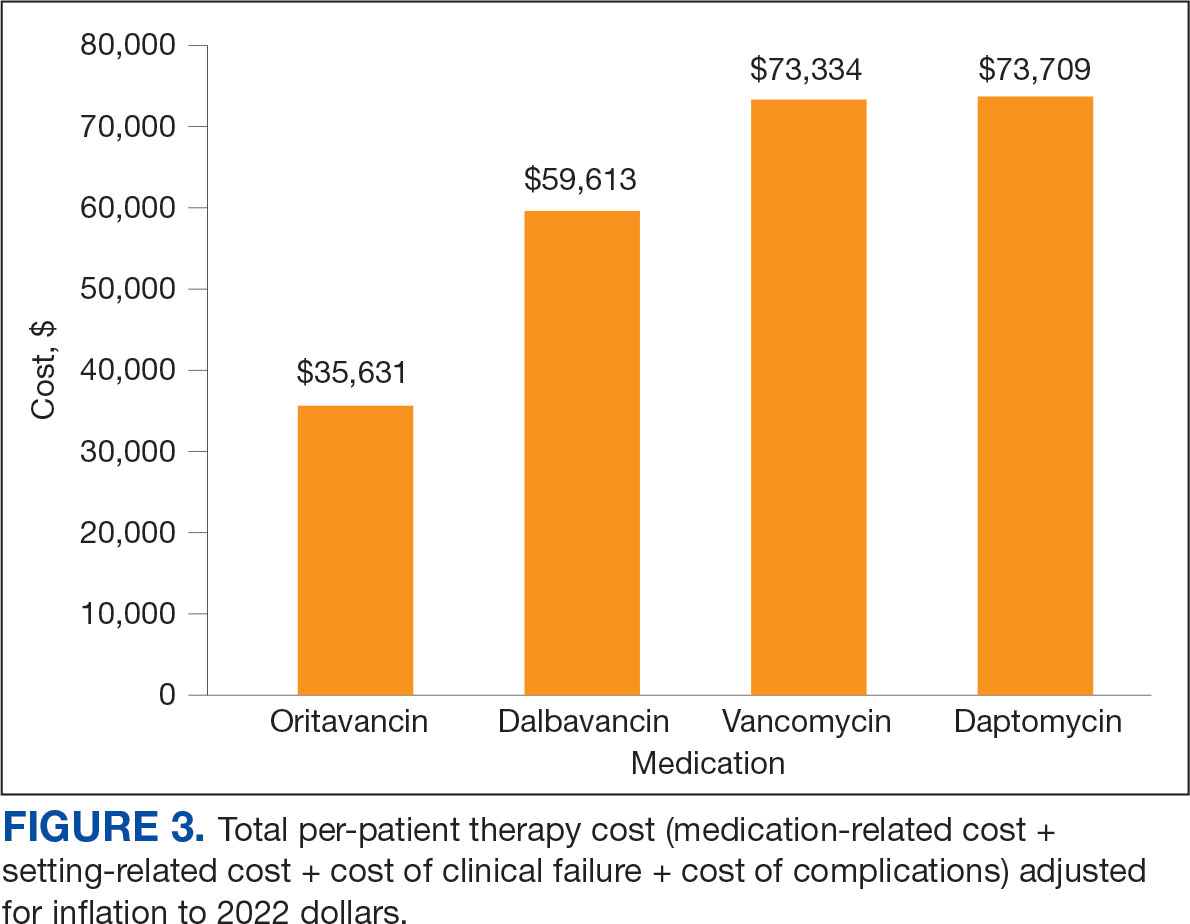
Estimated total drug cost represents the cost of drug acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home health services. The mean cost per patient of drug-related therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2203, $5924, $3637, and $7146, respectively (Table 2).
The mean cost per patient for osteomyelitis therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2375, $6775, $4164, $8152, respectively. The mean cost of per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $1737, $3475, $2409, and $1016, respectively. The mean cost per patient for oritavancin and dalbavancin for ABSSSI treatment, was $1553 and $3910, respectively.
Setting-related costs include expenses from inpatient admissions and postdischarge stays at community living centers (CLCs), skilled nursing facilities (SNFs), or rehabilitation facilities (RFs) for the duration of antimicrobial therapy. The mean setting-related therapy cost for osteomyelitis treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $27,852, $17,815, $83,324, and $72,856, respectively. The mean setting-related therapy cost per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $33,310, $60,668, $37,734, and $67,074, respectively. The mean setting-related therapy cost per patient for ABSSSI treatment for oritavancin and dalbavancin was $43,218 and $67,762.00, respectively.
Six of 39 patients (15%) had clinical failure: 2 patients with oritavancin and 4 patients with dalbavancin. Four patients were readmitted for worsening infection and 2 for AEs. One patient (13%) in the daptomycin group had clinical failure due to readmission for worsening infection. There was no clinical failure with vancomycin. The costs associated with clinical failure per patient for oritavancin, dalbavancin, vancomycin, and daptomycin were $2925, $23,972, $0, and $3601, respectively (Table 3).
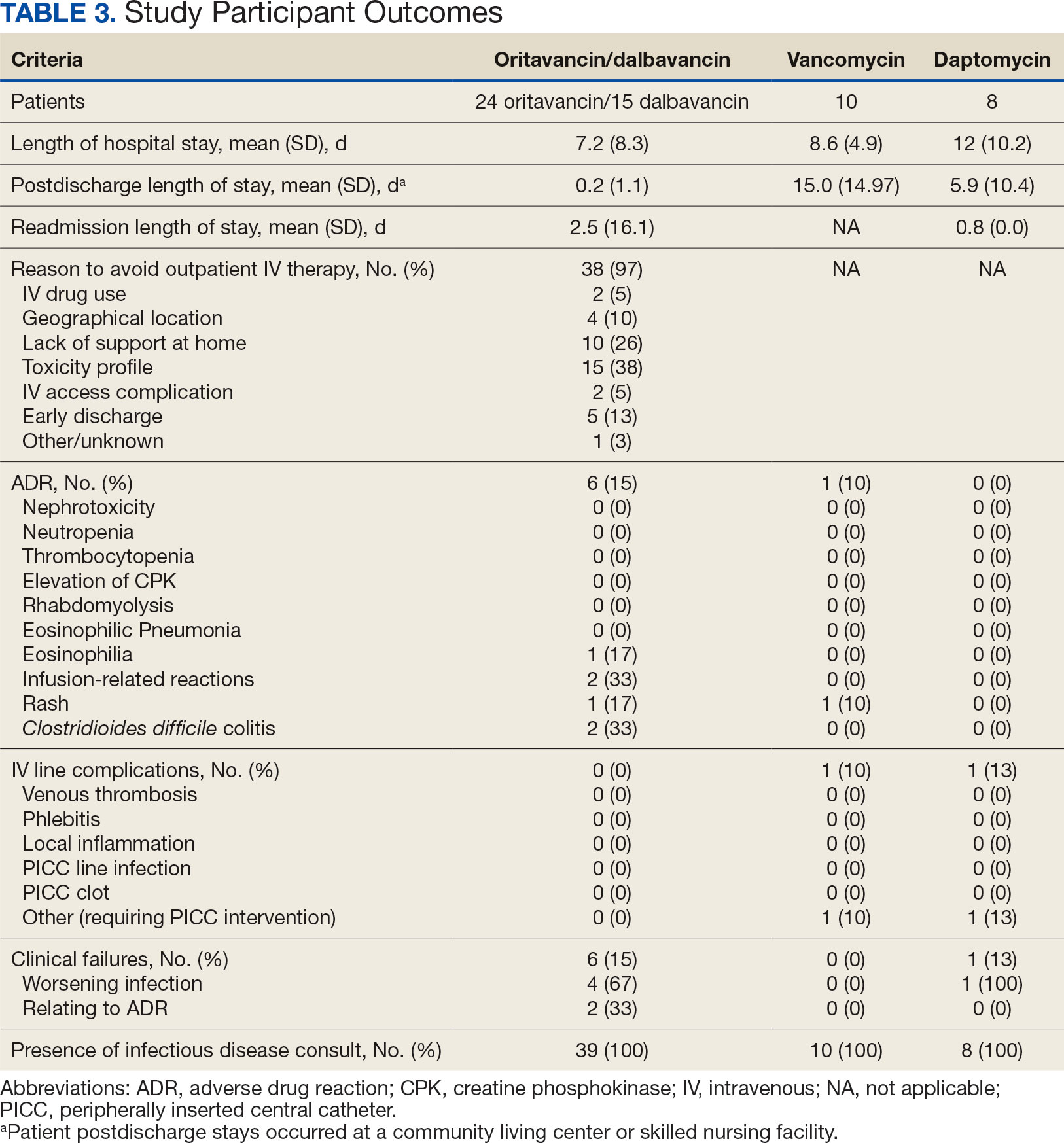
Thirty-eight patients (97%) who received oritavancin or dalbavancin had difficulty adhering to vancomycin or daptomycin OPAT. Oritavancin or dalbavancin was used in 10 patients (26%) who lacked support at home and 15 patients (38%) who had either a contraindication or previous failure with other antimicrobials, which were the most common explanations.
DISCUSSION
Long-acting lipoglycopeptides represent a potential alternative to home IV therapy that can avoid prolonged IV access with traditional OPAT. This offers significant advantages, allowing patients to be discharged from the hospital early, especially in rural areas with little OPAT infrastructure or those with logistic challenges. In this analysis, treatment with oritavancin for osteomyelitis, BSI, or ABSSSI, yielded an estimated cost savings of about $37,000 per patient, compared to treatment of matched indications with vancomycin and daptomycin. For every patient treated with dalbavancin for osteomyelitis, BSI, or ABSSSI, the cost savings was about $13,000 per patient, compared to treatment of matched indications for daptomycin and vancomycin. The estimated cost savings per patient for oritavancin was similar to previously published projections ($30,500 to $55,831).15
Cost savings were primarily driven by setting-related costs. The greatest contrast between the oritavancin and dalbavancin group compared to the vancomycin and daptomycin group was the length of stay in a postdischarge CLC, SNF, or RF setting. This analysis estimated that for every patient treated with oritavancin for osteomyelitis, the setting-related cost savings per patient was about $55,000 compared with vancomycin, and about $45,000 per patient compared with daptomycin. Furthermore, the estimated setting-related cost savings for osteomyelitis treatment with dalbavancin was about $65,000 compared with vancomycin and about $55,000 compared with daptomycin.
Clinical failure occurred with greater frequency in the oritavancin and dalbavancin groups (15%), compared with the vancomycin (0%) and daptomycin (13%) groups. Although the clinical failure rates in patients with osteomyelitis treated with oritavancin and dalbavancin compared with daptomycin were like those in previously published research (10%-30%), the rates of clinical failure for vancomycin in this analysis were lower than those in the oritavancin and dalbavancin group.8,21,22 The discrepancy in clinical failure rates between this analysis and previous research is likely due to selection bias. Based on the percentages of clinical failure found in the analysis, it is not surprising to note that the total clinical failure-related cost per patient was higher for oritavancin and dalbavancin compared to vancomycin, but similar between oritavancin and daptomycin.
This analysis also found that 15% of patients in the oritavancin and dalbavancin group experienced an AE compared to 10% of patients in the vancomycin group and none in the daptomycin group. In the oritavancin and dalbavancin group, the 2 most common AEs were infusion-related reactions and C. difficile colitis. Although infusion related reactions are easier to correspond to oritavancin and dalbavancin, it becomes difficult to definitively attribute the occurrence of C. difficile to these drugs as many patients were receiving concomitant antibiotics. Although not a primary or secondary objective, the rate of IV-line AEs were more prevalent in the vancomycin (10%), and daptomycin (13%) groups, compared to none in the oritavancin and dalbavancin group. This finding was expected; oritavancin and dalbavancin do not require a central IV line for administration.
Pharmacoeconomic literature continues to emerge with long-acting lipoglycopeptides. A 2024 Italian retrospective single-center analysis of 62 patients reported mean cost reductions > €3200 per patient (> $3400) given dalbavancin compared with the standard of care for ABSSSI or more deep-seeded infections such as osteomyelitis.23 A 2023 Spanish observational multicenter analysis of 124 patients with infective endocarditis demonstrated high efficacy, safety and cost-effectiveness with dalbavancin vs conventional treatments, with a mean savings of > €5548 per patient (> $6200).24 An analysis of the implementation of a dalbavancin order pathway for ABSSSI to avert inpatient admissions at 11 US emergency departments found a mean cost savings of $5133 per patient and $1211 per hospitalization day avoided, compared with inpatient usual care.25
Conversely, a multicenter, retrospective study of 209 patients in a community-based health care system failed to show a financial benefit for dalbavancin use when compared to standard of care for ABSSSI with higher readmission rates.26 Turco et al also reported increased cost results for 64 patients who received dalbavancin vs standard of care for ABSSSI.27 These discordant findings in ABSSSI studies may be impacted by the authors' patient selection choices and cost assumptions, especially with significantly cheaper oral alternatives. More data are needed to best identify the optimal therapeutic use for the long-acting lipoglycopeptides.
Limitations
The most significant limitation in this analysis was selection bias: 38 of 39 patients (97%) who received dalbavancin or oritavancin had a documented reason that described why OPAT therapy with traditional medications would not be optimal, including logistics, AEs, or clinical failures. Most patients treated with vancomycin and daptomycin were admitted into a SNF, RF, or CLC for the remainder of their treatment, allowing for closer monitoring and care compared to patients treated with oritavancin and dalbavancin, but at a greater cost. For patients sent to a community based SNF or RF, laboratory data were not available unless internally drawn or documented in the electronic medical record.
Additionally, not all cost data were available from VA sources; some were applied from literature, pharmacoeconomist, or best faith effort based on workflow. The cost data from third party contractors providing OPAT services to some BVAMC patients during the time frame of this analysis were not available. Due to its small sample size, outliers had the potential to affect averages reported and accuracy of the cost analysis. Emerging evidence suggests that daptomycin doses higher than the manufacturer-recommended regimen may be required for select indications, a factor that could affect cost, AEs, and efficacy outcomes.28 The acquisition cost of oritavancin and dalbavancin may vary by institution (ie, VA contract prices vs non- VA contract prices) and change over time. A current assessment of cost is needed to best visualize institutional benefit.
Finally, while the patient demographic of this MUE was highly representative of the demographic treated at the BVAMC (males aged >65 years), it may not be applicable to external patient populations. This analysis evaluated off-label indications for these medications. Consequently, this analysis would likely not be applicable to non-VA institution, as third-party payers (eg, insurance) are unlikely to cover medications for off-label indications.
CONCLUSIONS
This study found cost savings associated with the use of oritavancin and dalbavancin compared with vancomycin and daptomycin, particularly for the treatment of osteomyelitis. As safety and efficacy data continues to emerge, the use of long-acting lipoglycopeptides appears to be an increasingly attractive alternative option compared to traditional outpatient antimicrobial therapy, depending on the structure of the program. Larger, multicenter cost-effectiveness studies are needed to further establish the impact of these novel agents.
- Dalvance. Package insert. AbbVie Inc.; 2025.
- Orbactiv. Package insert. Melinta Therapeutics; 2022.
- Cooper CC, Stein GE, Mitra S, Abubaker A, Havlichek DH. Long-acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt). 2021;22(8):771- 779. doi:10.1089/sur.2020.413
- Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and offlabel utilization. Ther Clin Risk Manag. 2021;17:223-232. doi:10.2147/TCRM.S271445
- Bloem A, Bax HI, Yusuf E, Verkaik NJ. New-generation antibiotics for treatment of gram-positive infections: a review with focus on endocarditis and osteomyelitis. J Clin Med. 2021;10(8):1743. doi:10.3390/jcm10081743
- Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. doi:10.1093/ofid/ofy331
- Cain AR, Bremmer DN, Carr DR, et al. Effectiveness of dalbavancin compared with standard of care for the treatment of osteomyelitis: a real-world analysis. Open Forum Infect Dis. 2021;9(2):ofab589. doi:10.1093/ofid/ofab589
- Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes. 2020;7(Suppl 1):41-45. doi:10.1007/s40801-020-00195-7
- Cooper MM, Preslaski CR, Shihadeh KC, Hawkins KL, Jenkins TC. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: a scoping review. Open Forum Infect Dis. 2021;8(11):ofab486. doi:10.1093/ofid/ofab486
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-46. doi:10.1093/cid/civ482
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, López- Medrano F, San Juan-Garrido R, Ferrari-Piquero JM. Effectiveness, safety, and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022;29(1):55-58. doi:10.1136/ejhpharm-2020-002315
- Pascale R, Maccaro A, Mikus E, et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT study. J Glob Antimicrob Resist. 2022;30:390-394. doi:10.1016/j.jgar.2022.07.018
- Antosz K, Al-Hasan MN, Lu ZK, et at. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep seated infections among patients with social and economic barriers to care. Pharmacy (Basel). 2021;10(1):1. doi:10.3390/pharmacy10010001
- Roberts MS. Economic aspects of evaluation. In: Friedman CP, Wyatt JC, eds. Evaluation Methods in Biomedical Informatics. 2nd ed. Springer; 2006:301-337.
- US Department of Veterans Affairs. HERC inpatient average cost data. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=inpatient
- US Department of Veterans Affairs. HERC Outpatient average cost dataset. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=outpatient
- Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. doi:10.3111/13696998.2015.1078339
- Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther. 2015;37(11):2527-2535. doi:10.1016/j.clinthera.2015.09.009
- Nakrani M, Yu D, Skikka M, et al. Comparison of vancomycin and daptomycin complications and interventions in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2020;7(Suppl 1):S361-S362. doi:10.1093/ofid/ofaa439.791
- Scoble PJ, Reilly J, Tilloston GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46-54. doi:10.1007/s40801-020-00194-8
- Segala D, Barbieri M, Di Nuzzo M, et al. Clinical, organizational, and pharmacoeconomic perspectives of dalbavancin vs standard of care in the infectious disease network. Glob Reg Health Technol Assess. 2024;11(Suppl 2):5-12. doi:10.33393/grhta.2024.3094
- Gómez A, et al. EN-DALBACEN 2.0 Cohort: real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int J Antimicrob Agents. 2023;62(3):106918. doi:10.1016/j.ijantimicag.2023.106918
- LoVecchio F, McCarthy MW, Ye X, et al. Single intravenous dose dalbavancin pathway for the treatment of acute bacterial skin and skin structure infections: considerations for emergency department implementation and cost savings. J Emerg Med. 2024;67(2):e217-e229. doi:10.1016/j.jemermed.2024.03.003
- Gonzalez J, Andrade DC, Niu J. Cost-consequence analysis of single-dose dalbavancin versus standard of care for the treatment of acute bacterial skin and skin structure infections in a multisite healthcare system. Clin Infect Dis. 2021;73(7):e1436-e1442. doi:10.1093/cid/ciaa1732
- Turco NJ, Kane-Gill SL, Hernandez I, Oleksiuk LM, D’Amico F, Pickering AJ. A cost-minimization analysis of dalbavancin compared to conventional therapy for the outpatient treatment of acute bacterial skin and skin-structure infections. Expert Opin Pharmacother. 2018;19(4):319-325. doi:10.1080/14656566.2018.1442439
- Jones TW, Jun AH, Michal JL, Olney WJ. High-dose daptomycin and clinical applications. Ann Pharmacother. 2021;55(11):1363-1378. doi:10.1177/1060028021991943
Oritavancin and dalbavancin are long acting lipoglycopeptides indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI).1,2 Largely due to their long half-lives, prolonged tissue concentrations at sites of infection, tolerability, and minimal requirement for therapeutic drug monitoring, these agents are attractive options in outpatient settings.3,4 A 1- or 2-dose treatment of oritavancin and dalbavancin may be sufficient for conditions traditionally treated with outpatient parenteral antimicrobial therapy (OPAT) via peripherally inserted central catheter (PICC).
Limited research supports the use of dalbavancin and oritavancin for bone and joint infections, infective endocarditis, and bloodstream infections (BSIs). However, the US Food and Drug Administration has approved an indication for the treatment of ABSSSI.3-9 Dosing for these off-label indications varies but typically consists of an initial intravenous (IV) dose (1000 mg, 1200 mg, or 1500 mg), with a subsequent dose 1 to 2 weeks later or administered once weekly.6-10
Due in part to the recent availability of oritavancin and dalbavancin relative to the publication of practice guidelines, their appropriate place in therapy continues to evolve based on emerging literature.11,12 One potential barrier of use for these medications is their cost. Based on the number of doses administered, the 2022 estimated total acquisition cost of therapy for oritavancin and dalbavancin was $1014 to $4397 and $3046 to $7150, respectively (eAppendix). Despite the high acquisition costs, these agents do not require the placement of an indwelling central line, can be administered in outpatient settings, and require minimal therapeutic dose monitoring compared to vancomycin.13-15 This medication use evaluation (MUE) compared the total cost of treatment with oritavancin and dalbavancin vs therapies traditionally used for OPAT or prolonged IV inpatient therapy.
METHODS
This retrospective MUE was conducted at the Boise Veterans Affairs Medical Center (BVAMC), a level 2 facility with an extensive rural catchment area. BVAMC provides many OPAT services, including medications, supplies, and dressing changes after initial clinic or inpatient education. Contracted vendors may also assist with at-home nursing care using supplies provided by the BVAMC. Cases were identified using an internal database of OPAT patients and those who received oritavancin or dalbavancin between September 1, 2017, and November 1, 2022. Patients aged ≥ 18 years who received ≥ 1 dose of oritavancin or dalbavancin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were included. Comparator treatments consisting of ≥ 1 week of vancomycin or daptomycin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were identified through review of OPAT and Infectious Diseases service consults during the same timeframe. Patients were excluded if any antibiotic was prescribed by a non- VA clinician, if medications were not provided by OPAT, or if chart review did not identify an ABSSSI, osteomyelitis/ joint infection, or BSI diagnosis.
Electronic medical record review was conducted using a standardized data collection form (eAppendix). Data collected included demographics, infectious diagnosis, treatment administered, administration procedures and related visits and treatment locations, outcomes including clinical failure, adverse events (AEs), and hospital readmission.
Clinical failure was defined as readmission or death due to worsening infection or readmission secondary to a documented potential AE to the evaluated antibiotics within 90 days after initiation. Clinical failures excluded readmissions not associated with infection including comorbidities or elective procedures. AEs included new onset renal failure (serum creatinine ≥ 0.5 mg/dL), neutropenia (neutrophils ≤ 500), thrombocytopenia (platelets < 100,000), eosinophilia (> 15% eosinophils), or creatine phosphokinase > 10 times the upper limit of normal, and Clostridioides difficile (C. difficile) infection. Line complications included thrombophlebitis, local inflammation, or infection requiring line replacement (eAppendix).
A cost-minimization approach was used to assess the total cost of treatment.16 Patients who received oritavancin or dalbavancin were matched with patients that received vancomycin and daptomycin for the same indication and about 1 month of initiation through the randomization function in Microsoft Excel. This accounted for changes in personnel, nonformulary drug approvals, cost, and changes in practice during the pandemic. Costs were calculated using a decision tree as a base model (Figure 1). In this model, each treatment dyad was assessed for the presence or absence of clinical failure, adverse event (medication and line complications), and treatment setting endpoints. Cost estimates were tabulated for each patient that received treatment using published VA data, literature, pharmacoeconomist guidance, or best faith effort based on workflow. 17-20 All cost estimates were based on 2022 figures or adjusted for inflation if obtained prior to 2022. Secondary endpoints of this analysis included estimated total cost of medication acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home-health services.

This evaluation was classified by the BVAMC Medication Use Evaluation research determination subcommittee as a quality improvement project and was considered exempt from VA Human Subjects Research requirements based on the VA Policy Handbook guideline 1058.05.
RESULTS
The study identified 44 patients who received dalbavancin or oritavancin between September 1, 2017, and October 31, 2022. Thirty-nine patients were included in the analysis: 24 received oritavancin and 15 received dalbavancin and were matched by indication to 10 patients who received vancomycin and 8 patients who received daptomycin. Three patients could not be matched by indication of ABSSSI (Figure 2). Most patients were male, aged > 65 years, and were treated for osteomyelitis (Table 1). No patients were treated for infective endocarditis. A myriad of concomitant antibiotics were used to treat patients and culture results indicated that most infections treated with oritavancin and dalbavancin were polymicrobial.


The mean total cost of therapy per patient receiving oritavancin, dalbavancin, vancomycin, and daptomycin was $35,630, $59,612, $73,333, and $73,708, respectively (Figure 3). When stratified by indication, 27 patients (69%) in the oritavancin/dalbavancin group were treated for osteomyelitis/ joint infections (16 oritavancin, 11 dalbavancin), 9 patients (23%) were treated for BSI (6 oritavancin, 3 dalbavancin), and 3 patients (8%) were treated for ABSSSI (2 oritavancin, 1 dalbavancin). The mean cost per patient for osteomyelitis/joint infections with oritavancin, dalbavancin, vancomycin, and daptomycin was $34,678, $54,224, $87,488, and $85,044, respectively. The mean cost per patient for BSI for oritavancin, dalbavancin, vancomycin, and daptomycin was $35,048, $75,349, $40,305, and $68,068, respectively. The mean cost per patient for ABSSSI for oritavancin and dalbavancin was $44,771 and $71,672.51.

Estimated total drug cost represents the cost of drug acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home health services. The mean cost per patient of drug-related therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2203, $5924, $3637, and $7146, respectively (Table 2).

The mean cost per patient for osteomyelitis therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2375, $6775, $4164, $8152, respectively. The mean cost of per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $1737, $3475, $2409, and $1016, respectively. The mean cost per patient for oritavancin and dalbavancin for ABSSSI treatment, was $1553 and $3910, respectively.
Setting-related costs include expenses from inpatient admissions and postdischarge stays at community living centers (CLCs), skilled nursing facilities (SNFs), or rehabilitation facilities (RFs) for the duration of antimicrobial therapy. The mean setting-related therapy cost for osteomyelitis treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $27,852, $17,815, $83,324, and $72,856, respectively. The mean setting-related therapy cost per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $33,310, $60,668, $37,734, and $67,074, respectively. The mean setting-related therapy cost per patient for ABSSSI treatment for oritavancin and dalbavancin was $43,218 and $67,762.00, respectively.
Six of 39 patients (15%) had clinical failure: 2 patients with oritavancin and 4 patients with dalbavancin. Four patients were readmitted for worsening infection and 2 for AEs. One patient (13%) in the daptomycin group had clinical failure due to readmission for worsening infection. There was no clinical failure with vancomycin. The costs associated with clinical failure per patient for oritavancin, dalbavancin, vancomycin, and daptomycin were $2925, $23,972, $0, and $3601, respectively (Table 3).

Thirty-eight patients (97%) who received oritavancin or dalbavancin had difficulty adhering to vancomycin or daptomycin OPAT. Oritavancin or dalbavancin was used in 10 patients (26%) who lacked support at home and 15 patients (38%) who had either a contraindication or previous failure with other antimicrobials, which were the most common explanations.
DISCUSSION
Long-acting lipoglycopeptides represent a potential alternative to home IV therapy that can avoid prolonged IV access with traditional OPAT. This offers significant advantages, allowing patients to be discharged from the hospital early, especially in rural areas with little OPAT infrastructure or those with logistic challenges. In this analysis, treatment with oritavancin for osteomyelitis, BSI, or ABSSSI, yielded an estimated cost savings of about $37,000 per patient, compared to treatment of matched indications with vancomycin and daptomycin. For every patient treated with dalbavancin for osteomyelitis, BSI, or ABSSSI, the cost savings was about $13,000 per patient, compared to treatment of matched indications for daptomycin and vancomycin. The estimated cost savings per patient for oritavancin was similar to previously published projections ($30,500 to $55,831).15
Cost savings were primarily driven by setting-related costs. The greatest contrast between the oritavancin and dalbavancin group compared to the vancomycin and daptomycin group was the length of stay in a postdischarge CLC, SNF, or RF setting. This analysis estimated that for every patient treated with oritavancin for osteomyelitis, the setting-related cost savings per patient was about $55,000 compared with vancomycin, and about $45,000 per patient compared with daptomycin. Furthermore, the estimated setting-related cost savings for osteomyelitis treatment with dalbavancin was about $65,000 compared with vancomycin and about $55,000 compared with daptomycin.
Clinical failure occurred with greater frequency in the oritavancin and dalbavancin groups (15%), compared with the vancomycin (0%) and daptomycin (13%) groups. Although the clinical failure rates in patients with osteomyelitis treated with oritavancin and dalbavancin compared with daptomycin were like those in previously published research (10%-30%), the rates of clinical failure for vancomycin in this analysis were lower than those in the oritavancin and dalbavancin group.8,21,22 The discrepancy in clinical failure rates between this analysis and previous research is likely due to selection bias. Based on the percentages of clinical failure found in the analysis, it is not surprising to note that the total clinical failure-related cost per patient was higher for oritavancin and dalbavancin compared to vancomycin, but similar between oritavancin and daptomycin.
This analysis also found that 15% of patients in the oritavancin and dalbavancin group experienced an AE compared to 10% of patients in the vancomycin group and none in the daptomycin group. In the oritavancin and dalbavancin group, the 2 most common AEs were infusion-related reactions and C. difficile colitis. Although infusion related reactions are easier to correspond to oritavancin and dalbavancin, it becomes difficult to definitively attribute the occurrence of C. difficile to these drugs as many patients were receiving concomitant antibiotics. Although not a primary or secondary objective, the rate of IV-line AEs were more prevalent in the vancomycin (10%), and daptomycin (13%) groups, compared to none in the oritavancin and dalbavancin group. This finding was expected; oritavancin and dalbavancin do not require a central IV line for administration.
Pharmacoeconomic literature continues to emerge with long-acting lipoglycopeptides. A 2024 Italian retrospective single-center analysis of 62 patients reported mean cost reductions > €3200 per patient (> $3400) given dalbavancin compared with the standard of care for ABSSSI or more deep-seeded infections such as osteomyelitis.23 A 2023 Spanish observational multicenter analysis of 124 patients with infective endocarditis demonstrated high efficacy, safety and cost-effectiveness with dalbavancin vs conventional treatments, with a mean savings of > €5548 per patient (> $6200).24 An analysis of the implementation of a dalbavancin order pathway for ABSSSI to avert inpatient admissions at 11 US emergency departments found a mean cost savings of $5133 per patient and $1211 per hospitalization day avoided, compared with inpatient usual care.25
Conversely, a multicenter, retrospective study of 209 patients in a community-based health care system failed to show a financial benefit for dalbavancin use when compared to standard of care for ABSSSI with higher readmission rates.26 Turco et al also reported increased cost results for 64 patients who received dalbavancin vs standard of care for ABSSSI.27 These discordant findings in ABSSSI studies may be impacted by the authors' patient selection choices and cost assumptions, especially with significantly cheaper oral alternatives. More data are needed to best identify the optimal therapeutic use for the long-acting lipoglycopeptides.
Limitations
The most significant limitation in this analysis was selection bias: 38 of 39 patients (97%) who received dalbavancin or oritavancin had a documented reason that described why OPAT therapy with traditional medications would not be optimal, including logistics, AEs, or clinical failures. Most patients treated with vancomycin and daptomycin were admitted into a SNF, RF, or CLC for the remainder of their treatment, allowing for closer monitoring and care compared to patients treated with oritavancin and dalbavancin, but at a greater cost. For patients sent to a community based SNF or RF, laboratory data were not available unless internally drawn or documented in the electronic medical record.
Additionally, not all cost data were available from VA sources; some were applied from literature, pharmacoeconomist, or best faith effort based on workflow. The cost data from third party contractors providing OPAT services to some BVAMC patients during the time frame of this analysis were not available. Due to its small sample size, outliers had the potential to affect averages reported and accuracy of the cost analysis. Emerging evidence suggests that daptomycin doses higher than the manufacturer-recommended regimen may be required for select indications, a factor that could affect cost, AEs, and efficacy outcomes.28 The acquisition cost of oritavancin and dalbavancin may vary by institution (ie, VA contract prices vs non- VA contract prices) and change over time. A current assessment of cost is needed to best visualize institutional benefit.
Finally, while the patient demographic of this MUE was highly representative of the demographic treated at the BVAMC (males aged >65 years), it may not be applicable to external patient populations. This analysis evaluated off-label indications for these medications. Consequently, this analysis would likely not be applicable to non-VA institution, as third-party payers (eg, insurance) are unlikely to cover medications for off-label indications.
CONCLUSIONS
This study found cost savings associated with the use of oritavancin and dalbavancin compared with vancomycin and daptomycin, particularly for the treatment of osteomyelitis. As safety and efficacy data continues to emerge, the use of long-acting lipoglycopeptides appears to be an increasingly attractive alternative option compared to traditional outpatient antimicrobial therapy, depending on the structure of the program. Larger, multicenter cost-effectiveness studies are needed to further establish the impact of these novel agents.
Oritavancin and dalbavancin are long acting lipoglycopeptides indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI).1,2 Largely due to their long half-lives, prolonged tissue concentrations at sites of infection, tolerability, and minimal requirement for therapeutic drug monitoring, these agents are attractive options in outpatient settings.3,4 A 1- or 2-dose treatment of oritavancin and dalbavancin may be sufficient for conditions traditionally treated with outpatient parenteral antimicrobial therapy (OPAT) via peripherally inserted central catheter (PICC).
Limited research supports the use of dalbavancin and oritavancin for bone and joint infections, infective endocarditis, and bloodstream infections (BSIs). However, the US Food and Drug Administration has approved an indication for the treatment of ABSSSI.3-9 Dosing for these off-label indications varies but typically consists of an initial intravenous (IV) dose (1000 mg, 1200 mg, or 1500 mg), with a subsequent dose 1 to 2 weeks later or administered once weekly.6-10
Due in part to the recent availability of oritavancin and dalbavancin relative to the publication of practice guidelines, their appropriate place in therapy continues to evolve based on emerging literature.11,12 One potential barrier of use for these medications is their cost. Based on the number of doses administered, the 2022 estimated total acquisition cost of therapy for oritavancin and dalbavancin was $1014 to $4397 and $3046 to $7150, respectively (eAppendix). Despite the high acquisition costs, these agents do not require the placement of an indwelling central line, can be administered in outpatient settings, and require minimal therapeutic dose monitoring compared to vancomycin.13-15 This medication use evaluation (MUE) compared the total cost of treatment with oritavancin and dalbavancin vs therapies traditionally used for OPAT or prolonged IV inpatient therapy.
METHODS
This retrospective MUE was conducted at the Boise Veterans Affairs Medical Center (BVAMC), a level 2 facility with an extensive rural catchment area. BVAMC provides many OPAT services, including medications, supplies, and dressing changes after initial clinic or inpatient education. Contracted vendors may also assist with at-home nursing care using supplies provided by the BVAMC. Cases were identified using an internal database of OPAT patients and those who received oritavancin or dalbavancin between September 1, 2017, and November 1, 2022. Patients aged ≥ 18 years who received ≥ 1 dose of oritavancin or dalbavancin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were included. Comparator treatments consisting of ≥ 1 week of vancomycin or daptomycin for ABSSSI, osteomyelitis/joint infections, endocarditis, and BSI were identified through review of OPAT and Infectious Diseases service consults during the same timeframe. Patients were excluded if any antibiotic was prescribed by a non- VA clinician, if medications were not provided by OPAT, or if chart review did not identify an ABSSSI, osteomyelitis/ joint infection, or BSI diagnosis.
Electronic medical record review was conducted using a standardized data collection form (eAppendix). Data collected included demographics, infectious diagnosis, treatment administered, administration procedures and related visits and treatment locations, outcomes including clinical failure, adverse events (AEs), and hospital readmission.
Clinical failure was defined as readmission or death due to worsening infection or readmission secondary to a documented potential AE to the evaluated antibiotics within 90 days after initiation. Clinical failures excluded readmissions not associated with infection including comorbidities or elective procedures. AEs included new onset renal failure (serum creatinine ≥ 0.5 mg/dL), neutropenia (neutrophils ≤ 500), thrombocytopenia (platelets < 100,000), eosinophilia (> 15% eosinophils), or creatine phosphokinase > 10 times the upper limit of normal, and Clostridioides difficile (C. difficile) infection. Line complications included thrombophlebitis, local inflammation, or infection requiring line replacement (eAppendix).
A cost-minimization approach was used to assess the total cost of treatment.16 Patients who received oritavancin or dalbavancin were matched with patients that received vancomycin and daptomycin for the same indication and about 1 month of initiation through the randomization function in Microsoft Excel. This accounted for changes in personnel, nonformulary drug approvals, cost, and changes in practice during the pandemic. Costs were calculated using a decision tree as a base model (Figure 1). In this model, each treatment dyad was assessed for the presence or absence of clinical failure, adverse event (medication and line complications), and treatment setting endpoints. Cost estimates were tabulated for each patient that received treatment using published VA data, literature, pharmacoeconomist guidance, or best faith effort based on workflow. 17-20 All cost estimates were based on 2022 figures or adjusted for inflation if obtained prior to 2022. Secondary endpoints of this analysis included estimated total cost of medication acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home-health services.

This evaluation was classified by the BVAMC Medication Use Evaluation research determination subcommittee as a quality improvement project and was considered exempt from VA Human Subjects Research requirements based on the VA Policy Handbook guideline 1058.05.
RESULTS
The study identified 44 patients who received dalbavancin or oritavancin between September 1, 2017, and October 31, 2022. Thirty-nine patients were included in the analysis: 24 received oritavancin and 15 received dalbavancin and were matched by indication to 10 patients who received vancomycin and 8 patients who received daptomycin. Three patients could not be matched by indication of ABSSSI (Figure 2). Most patients were male, aged > 65 years, and were treated for osteomyelitis (Table 1). No patients were treated for infective endocarditis. A myriad of concomitant antibiotics were used to treat patients and culture results indicated that most infections treated with oritavancin and dalbavancin were polymicrobial.


The mean total cost of therapy per patient receiving oritavancin, dalbavancin, vancomycin, and daptomycin was $35,630, $59,612, $73,333, and $73,708, respectively (Figure 3). When stratified by indication, 27 patients (69%) in the oritavancin/dalbavancin group were treated for osteomyelitis/ joint infections (16 oritavancin, 11 dalbavancin), 9 patients (23%) were treated for BSI (6 oritavancin, 3 dalbavancin), and 3 patients (8%) were treated for ABSSSI (2 oritavancin, 1 dalbavancin). The mean cost per patient for osteomyelitis/joint infections with oritavancin, dalbavancin, vancomycin, and daptomycin was $34,678, $54,224, $87,488, and $85,044, respectively. The mean cost per patient for BSI for oritavancin, dalbavancin, vancomycin, and daptomycin was $35,048, $75,349, $40,305, and $68,068, respectively. The mean cost per patient for ABSSSI for oritavancin and dalbavancin was $44,771 and $71,672.51.

Estimated total drug cost represents the cost of drug acquisition, administration supplies, laboratory monitoring, and human resources for OPAT visits or receiving home health services. The mean cost per patient of drug-related therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2203, $5924, $3637, and $7146, respectively (Table 2).

The mean cost per patient for osteomyelitis therapy for oritavancin, dalbavancin, vancomycin, and daptomycin was $2375, $6775, $4164, $8152, respectively. The mean cost of per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $1737, $3475, $2409, and $1016, respectively. The mean cost per patient for oritavancin and dalbavancin for ABSSSI treatment, was $1553 and $3910, respectively.
Setting-related costs include expenses from inpatient admissions and postdischarge stays at community living centers (CLCs), skilled nursing facilities (SNFs), or rehabilitation facilities (RFs) for the duration of antimicrobial therapy. The mean setting-related therapy cost for osteomyelitis treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $27,852, $17,815, $83,324, and $72,856, respectively. The mean setting-related therapy cost per patient for BSI treatment with oritavancin, dalbavancin, vancomycin, and daptomycin was $33,310, $60,668, $37,734, and $67,074, respectively. The mean setting-related therapy cost per patient for ABSSSI treatment for oritavancin and dalbavancin was $43,218 and $67,762.00, respectively.
Six of 39 patients (15%) had clinical failure: 2 patients with oritavancin and 4 patients with dalbavancin. Four patients were readmitted for worsening infection and 2 for AEs. One patient (13%) in the daptomycin group had clinical failure due to readmission for worsening infection. There was no clinical failure with vancomycin. The costs associated with clinical failure per patient for oritavancin, dalbavancin, vancomycin, and daptomycin were $2925, $23,972, $0, and $3601, respectively (Table 3).

Thirty-eight patients (97%) who received oritavancin or dalbavancin had difficulty adhering to vancomycin or daptomycin OPAT. Oritavancin or dalbavancin was used in 10 patients (26%) who lacked support at home and 15 patients (38%) who had either a contraindication or previous failure with other antimicrobials, which were the most common explanations.
DISCUSSION
Long-acting lipoglycopeptides represent a potential alternative to home IV therapy that can avoid prolonged IV access with traditional OPAT. This offers significant advantages, allowing patients to be discharged from the hospital early, especially in rural areas with little OPAT infrastructure or those with logistic challenges. In this analysis, treatment with oritavancin for osteomyelitis, BSI, or ABSSSI, yielded an estimated cost savings of about $37,000 per patient, compared to treatment of matched indications with vancomycin and daptomycin. For every patient treated with dalbavancin for osteomyelitis, BSI, or ABSSSI, the cost savings was about $13,000 per patient, compared to treatment of matched indications for daptomycin and vancomycin. The estimated cost savings per patient for oritavancin was similar to previously published projections ($30,500 to $55,831).15
Cost savings were primarily driven by setting-related costs. The greatest contrast between the oritavancin and dalbavancin group compared to the vancomycin and daptomycin group was the length of stay in a postdischarge CLC, SNF, or RF setting. This analysis estimated that for every patient treated with oritavancin for osteomyelitis, the setting-related cost savings per patient was about $55,000 compared with vancomycin, and about $45,000 per patient compared with daptomycin. Furthermore, the estimated setting-related cost savings for osteomyelitis treatment with dalbavancin was about $65,000 compared with vancomycin and about $55,000 compared with daptomycin.
Clinical failure occurred with greater frequency in the oritavancin and dalbavancin groups (15%), compared with the vancomycin (0%) and daptomycin (13%) groups. Although the clinical failure rates in patients with osteomyelitis treated with oritavancin and dalbavancin compared with daptomycin were like those in previously published research (10%-30%), the rates of clinical failure for vancomycin in this analysis were lower than those in the oritavancin and dalbavancin group.8,21,22 The discrepancy in clinical failure rates between this analysis and previous research is likely due to selection bias. Based on the percentages of clinical failure found in the analysis, it is not surprising to note that the total clinical failure-related cost per patient was higher for oritavancin and dalbavancin compared to vancomycin, but similar between oritavancin and daptomycin.
This analysis also found that 15% of patients in the oritavancin and dalbavancin group experienced an AE compared to 10% of patients in the vancomycin group and none in the daptomycin group. In the oritavancin and dalbavancin group, the 2 most common AEs were infusion-related reactions and C. difficile colitis. Although infusion related reactions are easier to correspond to oritavancin and dalbavancin, it becomes difficult to definitively attribute the occurrence of C. difficile to these drugs as many patients were receiving concomitant antibiotics. Although not a primary or secondary objective, the rate of IV-line AEs were more prevalent in the vancomycin (10%), and daptomycin (13%) groups, compared to none in the oritavancin and dalbavancin group. This finding was expected; oritavancin and dalbavancin do not require a central IV line for administration.
Pharmacoeconomic literature continues to emerge with long-acting lipoglycopeptides. A 2024 Italian retrospective single-center analysis of 62 patients reported mean cost reductions > €3200 per patient (> $3400) given dalbavancin compared with the standard of care for ABSSSI or more deep-seeded infections such as osteomyelitis.23 A 2023 Spanish observational multicenter analysis of 124 patients with infective endocarditis demonstrated high efficacy, safety and cost-effectiveness with dalbavancin vs conventional treatments, with a mean savings of > €5548 per patient (> $6200).24 An analysis of the implementation of a dalbavancin order pathway for ABSSSI to avert inpatient admissions at 11 US emergency departments found a mean cost savings of $5133 per patient and $1211 per hospitalization day avoided, compared with inpatient usual care.25
Conversely, a multicenter, retrospective study of 209 patients in a community-based health care system failed to show a financial benefit for dalbavancin use when compared to standard of care for ABSSSI with higher readmission rates.26 Turco et al also reported increased cost results for 64 patients who received dalbavancin vs standard of care for ABSSSI.27 These discordant findings in ABSSSI studies may be impacted by the authors' patient selection choices and cost assumptions, especially with significantly cheaper oral alternatives. More data are needed to best identify the optimal therapeutic use for the long-acting lipoglycopeptides.
Limitations
The most significant limitation in this analysis was selection bias: 38 of 39 patients (97%) who received dalbavancin or oritavancin had a documented reason that described why OPAT therapy with traditional medications would not be optimal, including logistics, AEs, or clinical failures. Most patients treated with vancomycin and daptomycin were admitted into a SNF, RF, or CLC for the remainder of their treatment, allowing for closer monitoring and care compared to patients treated with oritavancin and dalbavancin, but at a greater cost. For patients sent to a community based SNF or RF, laboratory data were not available unless internally drawn or documented in the electronic medical record.
Additionally, not all cost data were available from VA sources; some were applied from literature, pharmacoeconomist, or best faith effort based on workflow. The cost data from third party contractors providing OPAT services to some BVAMC patients during the time frame of this analysis were not available. Due to its small sample size, outliers had the potential to affect averages reported and accuracy of the cost analysis. Emerging evidence suggests that daptomycin doses higher than the manufacturer-recommended regimen may be required for select indications, a factor that could affect cost, AEs, and efficacy outcomes.28 The acquisition cost of oritavancin and dalbavancin may vary by institution (ie, VA contract prices vs non- VA contract prices) and change over time. A current assessment of cost is needed to best visualize institutional benefit.
Finally, while the patient demographic of this MUE was highly representative of the demographic treated at the BVAMC (males aged >65 years), it may not be applicable to external patient populations. This analysis evaluated off-label indications for these medications. Consequently, this analysis would likely not be applicable to non-VA institution, as third-party payers (eg, insurance) are unlikely to cover medications for off-label indications.
CONCLUSIONS
This study found cost savings associated with the use of oritavancin and dalbavancin compared with vancomycin and daptomycin, particularly for the treatment of osteomyelitis. As safety and efficacy data continues to emerge, the use of long-acting lipoglycopeptides appears to be an increasingly attractive alternative option compared to traditional outpatient antimicrobial therapy, depending on the structure of the program. Larger, multicenter cost-effectiveness studies are needed to further establish the impact of these novel agents.
- Dalvance. Package insert. AbbVie Inc.; 2025.
- Orbactiv. Package insert. Melinta Therapeutics; 2022.
- Cooper CC, Stein GE, Mitra S, Abubaker A, Havlichek DH. Long-acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt). 2021;22(8):771- 779. doi:10.1089/sur.2020.413
- Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and offlabel utilization. Ther Clin Risk Manag. 2021;17:223-232. doi:10.2147/TCRM.S271445
- Bloem A, Bax HI, Yusuf E, Verkaik NJ. New-generation antibiotics for treatment of gram-positive infections: a review with focus on endocarditis and osteomyelitis. J Clin Med. 2021;10(8):1743. doi:10.3390/jcm10081743
- Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. doi:10.1093/ofid/ofy331
- Cain AR, Bremmer DN, Carr DR, et al. Effectiveness of dalbavancin compared with standard of care for the treatment of osteomyelitis: a real-world analysis. Open Forum Infect Dis. 2021;9(2):ofab589. doi:10.1093/ofid/ofab589
- Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes. 2020;7(Suppl 1):41-45. doi:10.1007/s40801-020-00195-7
- Cooper MM, Preslaski CR, Shihadeh KC, Hawkins KL, Jenkins TC. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: a scoping review. Open Forum Infect Dis. 2021;8(11):ofab486. doi:10.1093/ofid/ofab486
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-46. doi:10.1093/cid/civ482
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, López- Medrano F, San Juan-Garrido R, Ferrari-Piquero JM. Effectiveness, safety, and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022;29(1):55-58. doi:10.1136/ejhpharm-2020-002315
- Pascale R, Maccaro A, Mikus E, et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT study. J Glob Antimicrob Resist. 2022;30:390-394. doi:10.1016/j.jgar.2022.07.018
- Antosz K, Al-Hasan MN, Lu ZK, et at. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep seated infections among patients with social and economic barriers to care. Pharmacy (Basel). 2021;10(1):1. doi:10.3390/pharmacy10010001
- Roberts MS. Economic aspects of evaluation. In: Friedman CP, Wyatt JC, eds. Evaluation Methods in Biomedical Informatics. 2nd ed. Springer; 2006:301-337.
- US Department of Veterans Affairs. HERC inpatient average cost data. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=inpatient
- US Department of Veterans Affairs. HERC Outpatient average cost dataset. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=outpatient
- Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. doi:10.3111/13696998.2015.1078339
- Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther. 2015;37(11):2527-2535. doi:10.1016/j.clinthera.2015.09.009
- Nakrani M, Yu D, Skikka M, et al. Comparison of vancomycin and daptomycin complications and interventions in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2020;7(Suppl 1):S361-S362. doi:10.1093/ofid/ofaa439.791
- Scoble PJ, Reilly J, Tilloston GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46-54. doi:10.1007/s40801-020-00194-8
- Segala D, Barbieri M, Di Nuzzo M, et al. Clinical, organizational, and pharmacoeconomic perspectives of dalbavancin vs standard of care in the infectious disease network. Glob Reg Health Technol Assess. 2024;11(Suppl 2):5-12. doi:10.33393/grhta.2024.3094
- Gómez A, et al. EN-DALBACEN 2.0 Cohort: real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int J Antimicrob Agents. 2023;62(3):106918. doi:10.1016/j.ijantimicag.2023.106918
- LoVecchio F, McCarthy MW, Ye X, et al. Single intravenous dose dalbavancin pathway for the treatment of acute bacterial skin and skin structure infections: considerations for emergency department implementation and cost savings. J Emerg Med. 2024;67(2):e217-e229. doi:10.1016/j.jemermed.2024.03.003
- Gonzalez J, Andrade DC, Niu J. Cost-consequence analysis of single-dose dalbavancin versus standard of care for the treatment of acute bacterial skin and skin structure infections in a multisite healthcare system. Clin Infect Dis. 2021;73(7):e1436-e1442. doi:10.1093/cid/ciaa1732
- Turco NJ, Kane-Gill SL, Hernandez I, Oleksiuk LM, D’Amico F, Pickering AJ. A cost-minimization analysis of dalbavancin compared to conventional therapy for the outpatient treatment of acute bacterial skin and skin-structure infections. Expert Opin Pharmacother. 2018;19(4):319-325. doi:10.1080/14656566.2018.1442439
- Jones TW, Jun AH, Michal JL, Olney WJ. High-dose daptomycin and clinical applications. Ann Pharmacother. 2021;55(11):1363-1378. doi:10.1177/1060028021991943
- Dalvance. Package insert. AbbVie Inc.; 2025.
- Orbactiv. Package insert. Melinta Therapeutics; 2022.
- Cooper CC, Stein GE, Mitra S, Abubaker A, Havlichek DH. Long-acting lipoglycopeptides for the treatment of bone and joint infections. Surg Infect (Larchmt). 2021;22(8):771- 779. doi:10.1089/sur.2020.413
- Simonetti O, Rizzetto G, Molinelli E, Cirioni O, Offidani A. Review: a safety profile of dalbavancin for on- and offlabel utilization. Ther Clin Risk Manag. 2021;17:223-232. doi:10.2147/TCRM.S271445
- Bloem A, Bax HI, Yusuf E, Verkaik NJ. New-generation antibiotics for treatment of gram-positive infections: a review with focus on endocarditis and osteomyelitis. J Clin Med. 2021;10(8):1743. doi:10.3390/jcm10081743
- Thomas G, Henao-Martínez AF, Franco-Paredes C, Chastain DB. Treatment of osteoarticular, cardiovascular, intravascular-catheter-related and other complicated infections with dalbavancin and oritavancin: a systematic review. Int J Antimicrob Agents. 2020;56(3):106069. doi:10.1016/j.ijantimicag.2020.106069
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2018;6(1):ofy331. doi:10.1093/ofid/ofy331
- Cain AR, Bremmer DN, Carr DR, et al. Effectiveness of dalbavancin compared with standard of care for the treatment of osteomyelitis: a real-world analysis. Open Forum Infect Dis. 2021;9(2):ofab589. doi:10.1093/ofid/ofab589
- Van Hise NW, Chundi V, Didwania V, et al. Treatment of acute osteomyelitis with once-weekly oritavancin: a two-year, multicenter, retrospective study. Drugs Real World Outcomes. 2020;7(Suppl 1):41-45. doi:10.1007/s40801-020-00195-7
- Cooper MM, Preslaski CR, Shihadeh KC, Hawkins KL, Jenkins TC. Multiple-dose dalbavancin regimens as the predominant treatment of deep-seated or endovascular infections: a scoping review. Open Forum Infect Dis. 2021;8(11):ofab486. doi:10.1093/ofid/ofab486
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/CIR.0000000000000296
- Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61(6):e26-46. doi:10.1093/cid/civ482
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, López- Medrano F, San Juan-Garrido R, Ferrari-Piquero JM. Effectiveness, safety, and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022;29(1):55-58. doi:10.1136/ejhpharm-2020-002315
- Pascale R, Maccaro A, Mikus E, et al. A retrospective multicentre study on dalbavancin effectiveness and cost-evaluation in sternotomic wound infection treatment: DALBA SWIT study. J Glob Antimicrob Resist. 2022;30:390-394. doi:10.1016/j.jgar.2022.07.018
- Antosz K, Al-Hasan MN, Lu ZK, et at. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep seated infections among patients with social and economic barriers to care. Pharmacy (Basel). 2021;10(1):1. doi:10.3390/pharmacy10010001
- Roberts MS. Economic aspects of evaluation. In: Friedman CP, Wyatt JC, eds. Evaluation Methods in Biomedical Informatics. 2nd ed. Springer; 2006:301-337.
- US Department of Veterans Affairs. HERC inpatient average cost data. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=inpatient
- US Department of Veterans Affairs. HERC Outpatient average cost dataset. Updated May 1, 2025. Accessed May 9, 2025. https://www.herc.research.va.gov/include/page.asp?id=outpatient
- Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram + acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092-1101. doi:10.3111/13696998.2015.1078339
- Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther. 2015;37(11):2527-2535. doi:10.1016/j.clinthera.2015.09.009
- Nakrani M, Yu D, Skikka M, et al. Comparison of vancomycin and daptomycin complications and interventions in outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2020;7(Suppl 1):S361-S362. doi:10.1093/ofid/ofaa439.791
- Scoble PJ, Reilly J, Tilloston GS. Real-world use of oritavancin for the treatment of osteomyelitis. Drugs Real World Outcomes. 2020;7(Suppl 1):46-54. doi:10.1007/s40801-020-00194-8
- Segala D, Barbieri M, Di Nuzzo M, et al. Clinical, organizational, and pharmacoeconomic perspectives of dalbavancin vs standard of care in the infectious disease network. Glob Reg Health Technol Assess. 2024;11(Suppl 2):5-12. doi:10.33393/grhta.2024.3094
- Gómez A, et al. EN-DALBACEN 2.0 Cohort: real-life study of dalbavancin as sequential/consolidation therapy in patients with infective endocarditis due to Gram-positive cocci. Int J Antimicrob Agents. 2023;62(3):106918. doi:10.1016/j.ijantimicag.2023.106918
- LoVecchio F, McCarthy MW, Ye X, et al. Single intravenous dose dalbavancin pathway for the treatment of acute bacterial skin and skin structure infections: considerations for emergency department implementation and cost savings. J Emerg Med. 2024;67(2):e217-e229. doi:10.1016/j.jemermed.2024.03.003
- Gonzalez J, Andrade DC, Niu J. Cost-consequence analysis of single-dose dalbavancin versus standard of care for the treatment of acute bacterial skin and skin structure infections in a multisite healthcare system. Clin Infect Dis. 2021;73(7):e1436-e1442. doi:10.1093/cid/ciaa1732
- Turco NJ, Kane-Gill SL, Hernandez I, Oleksiuk LM, D’Amico F, Pickering AJ. A cost-minimization analysis of dalbavancin compared to conventional therapy for the outpatient treatment of acute bacterial skin and skin-structure infections. Expert Opin Pharmacother. 2018;19(4):319-325. doi:10.1080/14656566.2018.1442439
- Jones TW, Jun AH, Michal JL, Olney WJ. High-dose daptomycin and clinical applications. Ann Pharmacother. 2021;55(11):1363-1378. doi:10.1177/1060028021991943
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Are Oritavancin and Dalbavancin More Cost Effective for Outpatient Parenteral Antimicrobial Therapy at a Veterans Affairs Medical Center?
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
There is a shortage of pediatric dermatologists in the United States, with fewer than 2% of practicing dermatologists specializing in pediatrics.1 Pediatric dermatology has the third highest referral rate by pediatricians but also is the third most challenging specialty to access, with an average appointment wait time of 92 days.2,3 Another factor leading to increased appointment wait times is the specificity of care required for pediatric patients. Frequently, pediatric patients evaluated by a general dermatologist will be referred to their pediatric dermatology colleagues. As medical students, we were introduced to the field of pediatric dermatology through different avenues—personal experience, research mentorship, or a clinical rotation in medical school. We found ourselves curious about the discrepancy between the supply of and demand for pediatric dermatologists and wondered what could be done to increase awareness of this subspecialty among medical students. We believe this workforce shortage can be ameliorated by improving early exposure to pediatric dermatology. In this article, we explore the existing framework surrounding pediatric dermatology in medical education and offer feasible recommendations and solutions to realistically combat this problem.
Pediatric dermatologists are essential to the greater dermatology community. Pediatric dermatologists receive advanced training in complex pediatric skin conditions that often is lacking in general dermatology residency. A large percentage of pediatric dermatology patients seen in academic medical centers have already been seen by general dermatologists who subsequently referred them to specialty care. In one study, 9.6% (10/108) of practicing pediatric dermatologists noted that their referrals were from general dermatologists.4 In another study, 42% (19/45) of referrals to a multidisciplinary pediatric dermatology-genetics were from general dermatologists.5 Given the shortage of pediatric dermatologists, these referrals undoubtedly overwhelm the system, and the results of these studies underscore the reality that general dermatologists do not necessarily feel adequately trained in complex pediatric conditions, creating an intrinsic need for pediatric dermatologists.
Admani et al6 reported that early mentorship was the single most important factor to 84% (91/109) of survey respondents who pursued pediatric dermatology. Forty percent (40/100) of survey respondents chose their specialty of pediatric dermatology during pediatrics residency, 34% (34/100) during medical school, 17% (17/100) during dermatology residency, and 5% (5/100) during internship, indicating that medical school is a crucial time for recruitment.6 It has been noted in the literature that more medical students matched to dermatology residency from schools with dermatology clerkships built into the curriculum than from schools without dedicated dermatology rotations, suggesting that early clinical exposure to dermatology fields has a predictable influence in matching.7 Currently, only about 10% (15/155) of allopathic medical schools in the United States offer a formal elective in pediatric dermatology via the Association of American Medical College’s Visiting Student Learning Opportunities program.8 When this information was cross-referenced with the most recently matched pediatric dermatology fellowship class (2023-2024), provided by the Fellowship Directors Chair of the Society for Pediatric Dermatology, we found that 17% (4/24) of the matched fellows attended one of these 15 medical schools. We also found that the 2023-2024 pediatric dermatology fellowship class had 12 unmatched spots out of 36 total positions nationwide (33%), highlighting a gap in pediatric dermatology care and placing further strain on an already underserved subspecialty. These data suggest that, while dermatologists may decide to pursue pediatric dermatology fellowships during residency, there is an opportunity to foster interest during medical school training and improve the fellowship match rate.
Several medical schools in the United States incorporate pediatric dermatology into their curricula, including lectures in preclinical courses and career panels to pediatric dermatology electives in the third and fourth years. These institutions can serve as models for other medical schools. Within preclinical content, we recommend creating a designated dermatology unit that can incorporate common pediatric dermatology pathologies also seen by general practitioners, such as common childhood rashes, atopic dermatitis, alopecia areata, seborrheic dermatitis, and acne. Rare pediatric diseases such as epidermolysis bullosa, tuberous sclerosis, and Ehlers-Danlos syndrome also may be included in the unit. If schools are not able to offer a stand-alone dermatology preclinical course, this content can be added to the immunology, musculoskeletal, infectious diseases, or genetics courses to account for the multisystemic effects of some of these conditions. Ideally, schools would offer elective exposure to pediatric dermatology during the clinical years of medical school to increase knowledge of the field; for example, pediatric dermatology materials could be included in core clerkships, as much of this content is applicable to the general pediatrics rotation. In particular, a lecture on common rashes in pediatric patients could be given before starting the core pediatric rotation. Additionally, problem-based pediatric dermatology cases could be implemented during the core pediatrics rotation. If students are offered an independent dermatology clinical elective, the already formatted 2- and 4-week basic dermatology courses designed by the American Academy of Dermatology could serve as suggested teaching guides or as self-teaching resources that could complement the dermatology rotation.9,10 Pediatric topics (eg, pediatric cutaneous fungal infections) are included within the American Academy of Dermatology basic dermatology curriculum.8,9
Increasing access to pediatric dermatology resources such as lecture series and mentorship opportunities could further broaden the pediatric dermatology knowledge base of medical students. Within medical school dermatology interest groups, there is an opportunity to have a pediatric dermatology lead to help coordinate lecture series and journal club sessions for interested students. The Society for Pediatric Dermatology and the Pediatric Dermatology Research Alliance have created programs to support students, and we encourage schools to raise awareness of these organizations as well as conference and grant opportunities. These initiatives foster meaningful mentor-mentee relationships, and more medical students may be interested if they are aware of these support networks.
There also may be opportunities to create residency tracks that increase the number of dermatology residency applicants. Programs such as the newly implemented pediatric dermatology track at the University of Pennsylvania and New York University allow medical students who are interested in pursuing pediatric dermatology to have a more focused and linear training path.11,12 Due to the inherent competition in matching into dermatology, we surmise that many students with interest in pediatric dermatology are lost to pediatric residencies. Given the large percentage of pediatric residents who ultimately develop an interest in pediatric dermatology, holding a spot for pediatric dermatology applicants—akin to the combined medical-dermatology spots—may be an avenue to increase the pool of pediatric dermatology fellows.1,6 Another avenue is to encourage the development of first-year pediatric internship tracks that lead directly into dermatology residency, such as newly established programs at the University of Pennsylvania and New York University.11,12
As a group of both aspiring and practicing pediatric dermatologists, we have identified opportunities for formalized education in and early exposure to this subspecialty during medical training instead of leaving the discovery of the field to chance. The gaps in medical education that we have identified have already led us to present potential curricular changes to the medical education committee at our home institution. We hope to inspire the development of strong pediatric dermatology education at the medical school level.
While the solution to the pediatric dermatology workforce shortage is complex and multifaceted, there is a unique opportunity to target medical students through mentorship, access to education, and clinical experiences. We recommend that medical schools implement these educational methods and track the efficacy of these interventions to quantify the predicted association between an increased workforce and early exposure to pediatric dermatology. Addressing a lack of exposure to the field and increasing support of students pursuing pediatric dermatology can help to alleviate the shortage at the earliest point in training.
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
- Prindaville B, Antaya RJ, Siegfried EC. Pediatric dermatology: past, present, and future. Pediatr Dermatol. 2015;32:1-12. doi:10.1111/pde.12362
- Wright TS. Update on the pediatric dermatology workforce shortage. Cutis. 2021;108:237-238. doi:10.12788/cutis.0379
- Stephens MR, Murthy AS, McMahon PJ. Wait times, health care touchpoints, and nonattendance in an academic pediatric dermatology clinic. ediatr Dermatol. 2019;36:893-897. doi:10.1111/pde.13943
- Fogel AL, Teng JM. A survey to assess perceived differences in referral pathways to board-certified pediatric dermatologists. Pediatr Dermatol. 2015;32:e314-e315. doi:10.1111/pde.12703
- Parker JC, Rangu S, Grand KL, et al. Genetic skin disorders: the value of a multidisciplinary clinic. Am J Med Genet A. 2021;185:1159-1167. doi:10.1002/ajmg.a.62095
- Admani S, Caufield M, Kim SS, et al. Understanding the pediatric dermatology workforce shortage: mentoring matters. J Pediatr. 2014;164:372-5.e1. doi:10.1016/j.jpeds.2013.10.004
- Ogidi P, Ahmed F, Cahn BA, et al. Medical schools as gatekeepers: a survey and analysis of factors predicting dermatology residency placement. J Am Acad Dermatol. 2022;86:490-492. doi:10.1016 /j.jaad.2021.09.027
- Visiting Student Learning Opportunities (VSLO). Accessed May 30, 2025. https://students-residents.aamc.org/visiting-student-learning-opportunities/visiting-student-learning-opportunities-vslo
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (2-week rotation). Accessed May 12, 2025. https://learning.aad.org/Listing/Basic-Dermatology-Curriculum-2-Week-Rotation-5395
- American Academy of Dermatology Association. AAD Learning Center. Basic dermatology curriculum (4-week rotation). Accessed May 12, 2025. https://learning.aad.org/Public/Catalog/Details.aspx?id=YPssTVIbBO3Zb%2bOuf%2fM7Kg%3d%3d&returnurl=%2fUsers%2fUserOnlineCourse.aspx%3fLearningActivityID%3dYPssTVIbBO3Zb%252bOuf%252fM7Kg%253d%253d
- Penn Medicine Dermatology Residency Training Program. Residency tracks. Accessed May 12, 2025. https://dermatology.upenn.edu/residents/residency-tracks/
- Pediatric Dermatology Residency Track at NYU Grossman School of Medicine. Pediatric Track. Accessed May 30, 2025. https://med.nyu.edu/departments-institutes/dermatology/education/residency/pediatric-track
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
Workforce Shortage of Pediatric Dermatologists: A Medical Student’s Perspective
PRACTICE POINTS
- Addressing a lack of exposure to pediatric dermatology in medical school and increasing support for students who are interested in the field can help alleviate the shortage of physicians at the earliest point in training.
- Increasing access to pediatric dermatology resources, such as lecture series and mentorship opportunities, could further broaden the medical student knowledge base.
- There is an opportunity to create residency tracks that increase the number of dermatology residency applicants who are medical students interested in pursuing pediatric dermatology.
Eruptive Erythematous Papules on the Forearms
Eruptive Erythematous Papules on the Forearms
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.
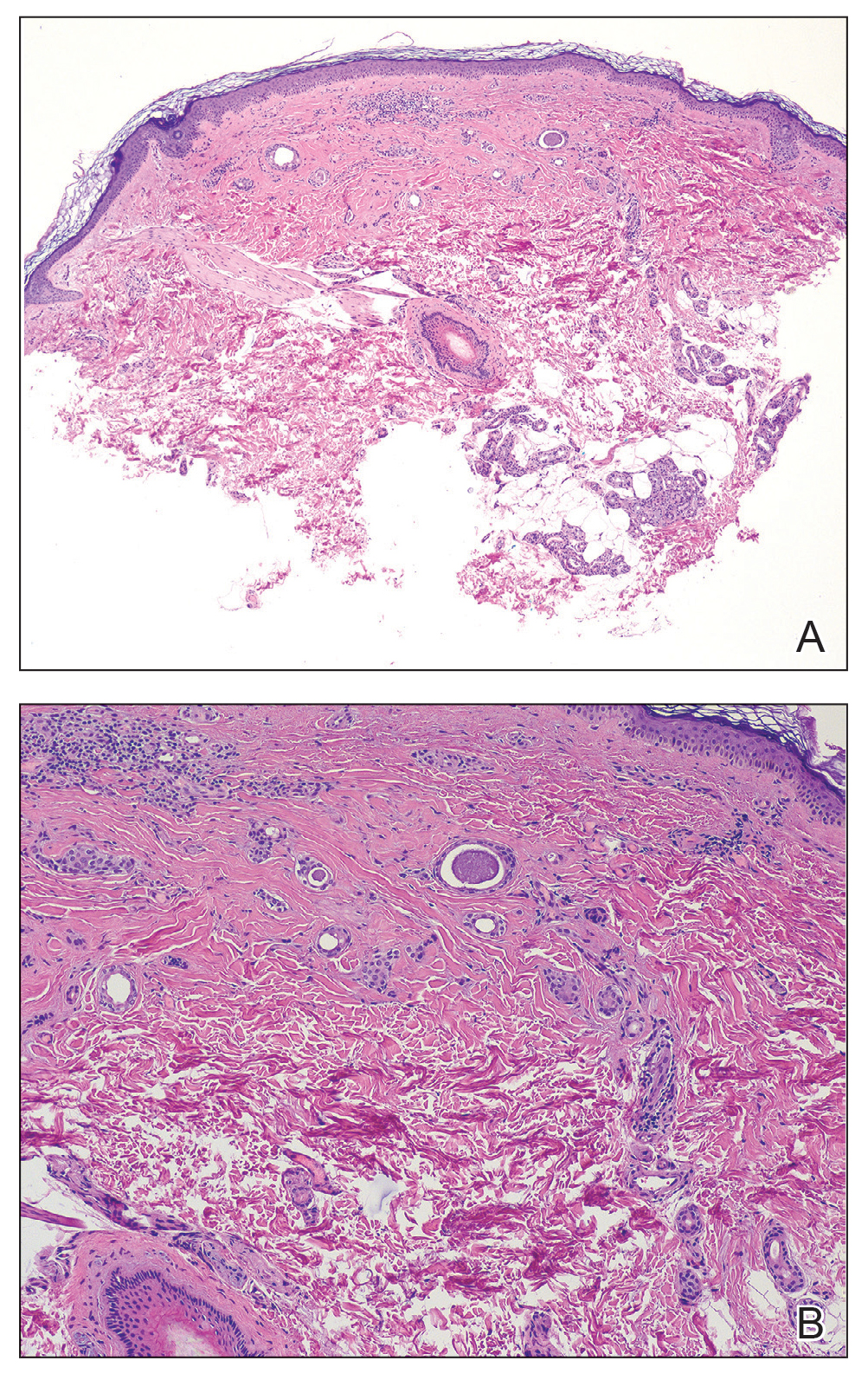
Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.

Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
THE DIAGNOSIS: Acral Eruptive Syringoma
Syringomas are small, benign, often asymptomatic eccrine tumors that originate in the intraepidermal portion of eccrine sweat ducts.1 Clinically, they present as multiple symmetric white-to-yellow or discrete flesh-colored papules measuring 1 to 3 mm in diameter, often located on the face (most commonly on the eyelids), with a greater prevalence in middle-aged women. Occasionally, they manifest in other locations such as the cheeks, chest, axillae, abdomen, and groin.2
In 1987, Friedman and Butler3 developed a classification system categorizing syringomas into 4 clinical subtypes: familial syringoma, localized syringoma, Down syndrome–related syringoma, and generalized syringoma. The fourth subtype includes the variant of eruptive syringoma,3 a rare clinical manifestation that often develops before or during puberty with several flesh-colored or lightly pigmented papules on the neck, anterior chest, upper abdomen, axillae, periumbilical region, and/or genital region.1,4,5 The etiology of eruptive syringomas is unclear, although it has been linked to abnormal proliferation of sweat glands due to an underlying local inflammatory process.6
Acral distribution of syringomas is a rare variant that can manifest as part of generalized eruptive syringoma with consequent involvement of the arms and other areas.5,7 There are limited case reports on eruptive syringomas with predominant acral distribution.8 Compared to classic syringomas, the acral variant is associated with an older age of onset as well as a similar prevalence between men and women.9 Acral eruptive syringoma (AES) usually is isolated to the distal arms and legs. The most commonly affected region is the anterior surface of the forearms, although involvement of the dorsal hands, wrists, and feet also has been reported.10-16
The first known case of AES, which was reported in 1977, described eruptive syringomas on the dorsal hands of a healthy 31-year-old man.17 Several cases have been reported since then, mostly in patients aged 30 to 60 years, with predominant involvement of the dorsal hands and forearms.18-24 A review of Embase as well as PubMed articles indexed for MEDLINE using the search terms syringoma OR eccrine ductal tumor and eruptive OR acral OR arms OR forearms OR extremities identified 19 reported cases of AES between 1977 and 2023. For the reported AES cases, the mean (SD) age at diagnosis was 45.1 years (15.96 years), with patient ages ranging from 19 to 76 years. Notably, most cases occurred in individuals aged between 30 and 60 years, which deviates from the typical age of onset of localized syringomas, commonly seen during puberty or early adulthood.
Currently, AES is categorized within the clinical presentation of eruptive syringoma. Nevertheless, some authors have proposed classifying it as a distinct fifth clinical group due to specific features that distinguish it from generalized eruptive syringoma.9 This reclassification has considerable implications for the differential diagnosis, particularly because exclusive acral involvement poses a substantial diagnostic challenge and often requires histologic confirmation.
As shown in the Figure, histopathologic examination revealed tubular structures in the upper dermis with characteristic comma-shaped extensions. Some of these structures were lined with cuboidal cells and contained eosinophilic material within the lumen. There was no involvement of the epidermis or deeper dermis. The histologic features were consistent with syringoma, which is distinguished by its predominant involvement of the upper dermis and the presence of enlarged, dilated eccrine ducts, as observed in our case.

Treatment of syringomas often is challenging due to the high rate of recurrence and the risk for postinflammatory hyperpigmentation. Since the condition is benign, treatment typically is pursued for aesthetic reasons. Various therapeutic approaches have been reported, each with diverse response rates. The most common method involves surgical intervention, either with electrodesiccation or CO2 laser—both of which have shown satisfactory resolution of lesions without recurrence at 1-year follow-up, with no major scarring reported.25,26 Alternatively, topical management with retinoids daily over a 4-month period leads to flattening of the tumors with no further appearance of new lesions.27 Despite the availability of numerous management options, establishing a first-line treatment remains controversial due to the high risk for recurrence and the variability in the number and location of lesions among individual patients. In our case, given the benign nature of syringomas, the asymptomatic nature of the lesions, the involvement of noncritical aesthetic areas, and the limited response to noninvasive therapeutic options, the patient was informed of the diagnosis, and no further pharmacologic or surgical intervention was pursued.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.E9. doi:10.1016 /j.jaad.2015.12.006
- Resende C, Araújo C, Santos R, et al. Late-onset of eruptive syringomas: a diagnostic challenge. An Bras Dermatol. 2015;90(3 suppl 1):239-241. doi:10.1590/abd1806-4841.20153899
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Avhad G, Ghuge P, Jerajani HR. Generalized eruptive syringoma. Indian J Dermatol. 2015;60:214. doi:10.4103/0019-5154.152586
- Ning WV, Bashey S, Cole C, et al. Multiple eruptive syringomas on the penis. Cutis. 2019;103:E15-E16.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Jamalipour M, Heidarpour M, Rajabi P. Generalized eruptive syringomas. Indian J Dermatol. 2009;54:65-67. doi:10.4103/0019-5154.48992
- Mohaghegh F, Amiri A, Fatemi Naeini F, et al. Acral eruptive syringoma: an unusual presentation with misdiagnosis. Case Rep Dermatol Med. 2020;2020:5416285. doi:10.1155/2020/5416285
- Valdivielso-Ramos M, de la Cueva P, Gimeno M, et al. Acral syringomas. Actas Dermosifiliogr. 2010;101:458-460.
- Patel K, Lundgren AD, Ahmed AM, et al. Disseminated syringomas of the upper extremities in a young woman. Cureus. 2018;10:E3619. doi:10.7759/cureus.3619
- Balci DD, Atik E, Altintas S. Coexistence of acral syringomas and multiple trichoepitheliomas on the face. J Cutan Med Surg. 2009;13:169-171. doi:10.2310/7750.2008.08011
- Martín-García RF, Muñoz CM. Acral syringomas presenting as a photosensitive papular eruption. Cutis. 2006;77:33-36.
- Varas-Meis E, Prada-García C, Samaniego-González E, et al. Acral syringomas associated with hematological neoplasm. Indian J Dermatol Venereol Leprol. 2017;83:136. doi:10.4103/0378-6323.192961
- Berbis P, Fabre JF, Jancovici E, et al. Late-onset syringomas of the upper extremities associated with a carcinoid tumor. Arch Dermatol. 1989;125:848-849.
- Metze D, Jurecka W, Gebhart W. Disseminated syringomas of the upper extremities. case history and immunohistochemical and ultrastructural study. Dermatologica. 1990;180:228-235. doi:10.1159/000248036
- Gómez-de Castro C, Vivanco Allende B, García-García B. Multiple acral syringomas. siringomas acrales múltiples. Actas Dermosifiliogr (Engl Ed). 2018;109:834-836. doi:10.1016/j.ad.2017.10.014
- Hughes PS, Apisarnthanarax P. Acral syringoma. Arch Dermatol. 1977;113:1435-1436.
- Asai Y, Ishii M, Hamada T. Acral syringoma: electron microscopic studies on its origin. Acta Derm Venereol. 1982;62:64-68.
- van den Broek H, Lundquist CD. Syringomas of the upper extremities with onset in the sixth decade. J Am Acad Dermatol. 1982,6:534-536. doi:10.1016/S0190-9622(82)80368-X
- Garcia C, Krunic AL, Grichnik J, et al. Multiple acral syringomata with uniform involvement of the hands and feet. Cutis. 1997;59:213-214, 216.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Iglesias Sancho M, Serra Llobet J, Salleras Redonnet M, et al. Siringomas disem- inados de inicio acral, aparecidos en la octava década. Actas Dermosifiliofr. 1999;90:253-257.
- Muniesa C, Fortuño Y, Moreno A, et al. Papules on the dorsum of the fingers. Actas Dermosifiliogr. 2008;99:812-813. doi:10.1016 /S1578-2190(08)70371-8
- Koh MJ. Multiple acral syringomas involving the hands. Clin Exp Dermatol. 2009;34:E438. doi:10.1111/j.1365-2230.2009.03462.x
- Karam P, Benedetto AV. Syringomas: new approach to an old technique. Int J Dermatol. 1996;35:219-220. doi:10.1111/j.1365-4362 .1996.tb01647.x
- Wang JI, Roenigk HH. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139. doi:10.1046/j.1524-4725.1999.08111.x
- Gómez MI, Pérez B, Azaña JM, et al. Eruptive syringoma: treatment with topical tretinoin. Dermatology. 2009;189:105-106. doi:10.1159/000246803
Eruptive Erythematous Papules on the Forearms
Eruptive Erythematous Papules on the Forearms
A 44-year-old man presented to the dermatology department with multiple eruptive, nonconfluent, erythematous papules on the anterior forearms of 2 years’ duration. The patient’s medical history was notable for right-sided testicular cancer diagnosed in childhood and 3 excised basal cell carcinomas, the most recent of which was concurrent with the present case. The patient denied any recent pruritus, exposure to irritants, or use of over-the-counter medications. Physical examination was remarkable for numerous monomorphic, symmetric, nonconfluent, flesh-colored to slightly pigmented papules on the dorsal aspect of the forearms. No involvement of the fingers or lower extremities was observed. Two punch biopsies of representative lesions on the right and left forearms were taken. Histopathologic examination revealed eccrine ductal proliferations lined by cuboidal cells embedded within bundles of sclerotic collagen.
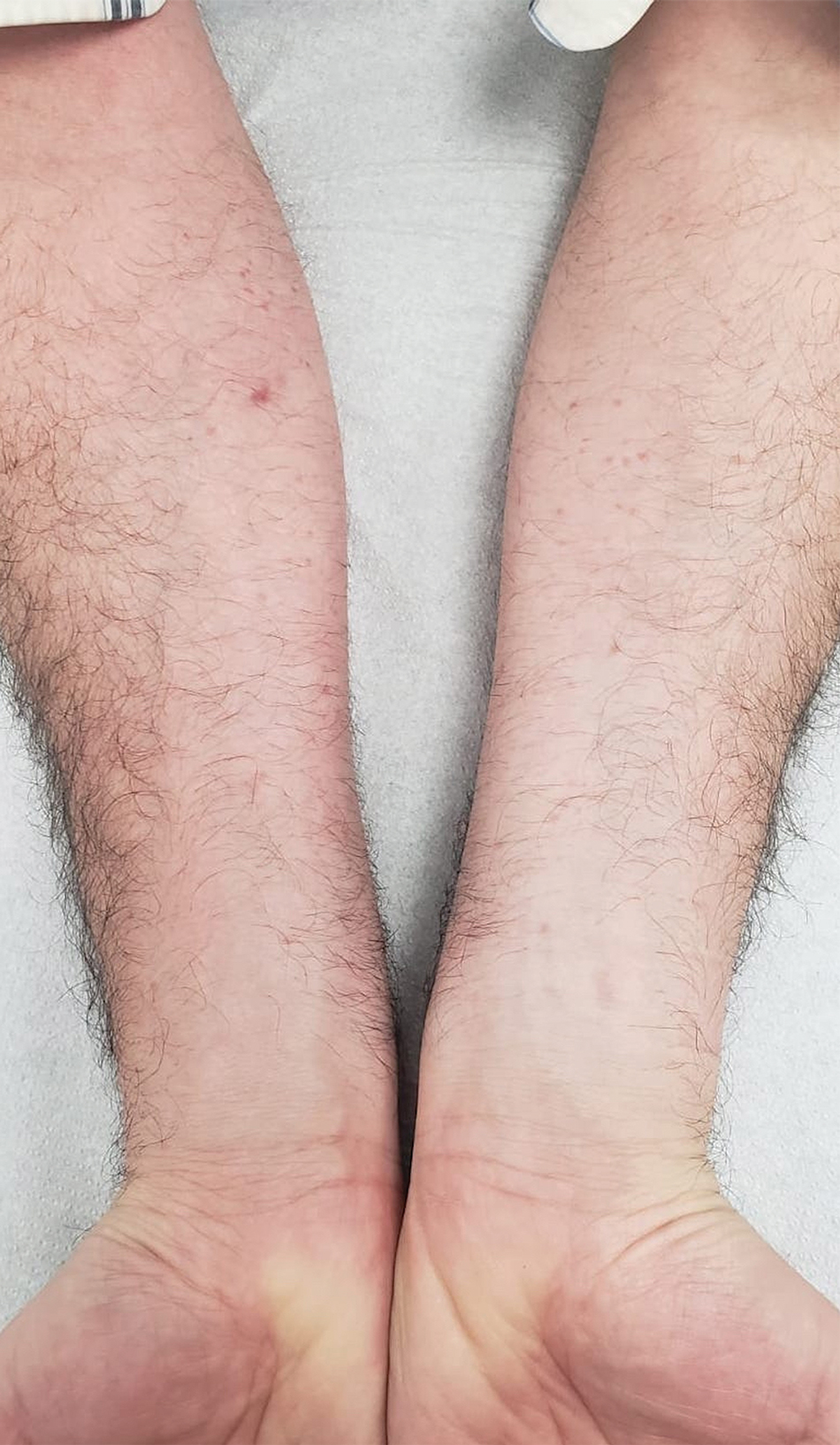
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.
For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
To the Editor:
Actinic keratoses (AKs) are keratinocyte neoplasms that manifest as rough, scaly, erythematous papules with ill-defined borders (commonly known as precancers) and develop due to long-term UV light exposure.1 They must be treated promptly due to the risk for progression to squamous cell carcinoma (SCC). One US Department of Veterans Affairs study reported that 0.6% of AKs progress to SCC in 1 year and 2.6% progressed to SCC in 4 years.2 In 10% of AKs that will progress to SCC, one study reported progression in approximately 2 years.3
The risk for progression also increases in patients with multiple AKs; the risk is 4-fold higher in patients with 6 to 20 AKs and 11-fold higher in patients with more than 20 AKs.4 Common treatment options include lesion-directed therapies such as cryotherapy, laser therapy, surgery, and curettage, as well as field-directed therapies such as topical 5-fluorouracil (5-FU), diclofenac gel 3%, chemical peeling, topical imiquimod, and photodynamic therapy (PDT).4 When diclofenac gel is chosen as a treatment modality, it is commonly prescribed in the 3% formulation. Diclofenac gel 3% has been shown to be effective in the treatment of AKs,5,6 but diclofenac gel 1% has not been well described in the literature. We report the case of a patient with AKs on the lower legs who was treated with diclofenac gel after other therapies failed.
A 55-year-old woman presented for a routine skin check due to a history of nonmelanoma skin cancer. Her medical history also included palmar hyperhidrosis, disseminated superficial actinic porokeratosis, and extensive actinic damage, as well as numerous biopsy-proven AKs. She had been evaluated every 3 months up to presentation due to the frequency of AK development over the past 5 years. The lesions were mainly localized to both lower legs, where the patient had acquired considerable lifetime sun exposure from tanning beds and sunbathing while boating. She also noted exposure to well water as a child, but none of her family members had a similar issue with AKs.
Prior to this visit, the patient had undergone 5 years of therapy for AKs. She initially was treated with multiple courses of topical 5-FU, but she consequently developed severe allergic contact dermatitis. Subsequent treatments included cryotherapy as well as application of tretinoin cream nightly for 2 weeks followed by PDT. She was unable to tolerate the tretinoin, which she reported led to dryness and irritation. She reported mild improvement after her first session of PDT but only minimal improvement after the next session. Ingenol mebutate was then prescribed for topical use on the legs for 2 days, which did not result in improvement. The patient continued to follow up for unresolved AKs on the legs and was prescribed acitretin to help reduce the risk for progression to SCC. At follow-up 3 months later, she reported decreased soreness from AKs after starting the acitretin and, aside from mild dryness, she tolerated the medication well; however, with continued use of acitretin, she began to experience adverse effects 6 months later, including thyroid suppression and hair loss, leading to discontinuation. Instead, 3 months later, she was recommended to start nicotinamide supplementation for prevention of SCC.
Due to continued AK development (Figure, A), we eventually prescribed diclofenac gel 3% twice daily for both legs 9 months after prescribing nicotinamide. This regimen was cost prohibitive, as the medication was not covered by her insurance and the cost was $300 for one tube. We recommended the patient instead apply the 3% gel to the right leg only due to greater severity of AKs on this leg and over-the-counter diclofenac gel 1% twice daily to the left leg. Approximately 5 months later, she reported a reduction in the discomfort from AKs as well as a reduction in the total number of AKs. She applied the 2 different products as instructed for the first month but did not notice a difference between them. She then continued to apply only the 1% gel on both legs for a total of 8 months with excellent response (Figure, B). At subsequent follow-up visits over a 2-year period, she has only required cryotherapy as spot treatment for AKs.

For 1 to a few discrete AKs, liquid nitrogen cryotherapy is considered first-line therapy.7 However, if multiple AKs are present, surrounding photodamaged skin also should be treated with field-directed therapy due to surrounding keratinocytes bearing a high mutational burden and risk of cancerization.8 Common field-directed therapies include topical 5-FU, topical imiquimod, topical tirbanibulin, PDT, retinoids, and topical diclofenac 3%.
One challenge in field-directed treatment of AKs is the side-effect profile seen in some patients, causing them to prematurely discontinue treatment. In our patient, 5-FU cream, tretinoin cream, and oral acitretin were not well tolerated. Topical diclofenac generally is well tolerated, with mostly mild local skin reactions and low risk for systemic adverse events. Adverse effects mainly consist of mild local skin reactions including pruritus (reported in 31%-52% of patients who used topical diclofenac), dryness (25%-27%), and irritation (less than 1%).9,10 Although diclofenac carries a black-box warning for serious cardiovascular thrombotic events and serious gastrointestinal tract bleeding, systemic absorption of topical diclofenac has been proven to be substantially lower (5- to 17-fold) compared to the oral formulation, and resulting serious adverse effects have been found to be largely reduced compared to the oral formulation.11,12 If allergic contact dermatitis develops, diclofenac should be discontinued.9,13
Diclofenac’s antineoplastic mechanism of action of cyclooxygenase-2 inhibition involves induction of apoptosis as well as reduction in tumor cell proliferation and tumor angiogenesis.14,15 Topical diclofenac may result in decreased levels of lactate and amino acid in AK lesions, particularly in lesions responding to treatment.16 Topical diclofenac may alter immune infiltration by inducing infiltration of dermal CD8+ T cells along with high IFN-γ messenger RNA expression, suggesting improvement of T-cell function after topical diclofenac treatment.16
Although diclofenac gel 3% has been shown to be effective in treatment of AKs,5,6 diclofenac gel 1% has not yet been well studied. Use of the 1% gel is indicated for osteoarthritis and musculoskeletal pain by the US Food and Drug Administration.10,17 Efficacy of the 1% gel has been documented for these and other conditions including seborrheic keratoses.18-20
Because the 1% diclofenac formulation is available over-the-counter, it is more accessible to patients compared to the 3% formulation and often substantially decreases the cost of the medication for the patient. The cost of diclofenac gel 1% in the United States ranges from $0.04 to $0.31 per gram compared to $1.07 to $11.79 per gram for the 3% gel prescription formulation.17 Efficacy of the 1% formulation compared to the 3% formulation could represent an avenue to increase accessibility to field-directed therapy in the population for the treatment of AKs with a potentially well-tolerated, effective, and low-cost medication formulation.
This case represents the effectiveness of diclofenac gel 1% in treating AKs. Several treatment modalities failed in our case, but she experienced improvement with use of over-the-counter diclofenac gel 1%. She also noted no difference in response between the prescription 3% diclofenac formulation and the over-the-counter 1% formulation. Diclofenac gel 1% may represent an excellent therapeutic option in treatment-refractory cases of AKs. Larger randomized trials should be considered to assess safety and efficacy.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
- FEisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:e209-e233.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33: 1099-1101.
- Dianzani C, Conforti C, Giuffrida R, et al. Current therapies for actinic keratosis. Int J Dermatol. 2020;59:677-684.
- Javor S, Cozzani E, Parodi A. Topical treatment of actinic keratosis with 3.0% diclofenac in 2.5% hyaluronan gel: review of the literature about the cumulative evidence of its efficacy and safety. G Ital Dermatol Venereol. 2016;151:275-280.
- Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11:600-608.
- Arisi M, Guasco Pisani E, et al. Cryotherapy for actinic keratosis: basic principles and literature review. Clin Cosmet Investig Dermatol. 2022;15:357-365.
- Calzavara-Pinton P, Calzavara-Pinton I, Rovati C, et al. Topical pharmacotherapy for actinic keratoses in older adults. Drugs Aging. 2022;39:143-152.
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance. Contact Dermatitis. 2022;86:41-43.
- Voltaren gel (diclofenac sodium topical gel). Prescribing information. Novartis Consumer Health, Inc; 2009. Accessed May 21, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2009/022122s006lbl.pdf
- Moreira SA, Liu DJ. Diclofenac systemic bioavailability of a topical 1% diclofenac + 3% menthol combination gel vs. an oral diclofenac tablet in healthy volunteers: a randomized, open-label, crossover study. Int J Clin Pharmacol Ther. 2017;55:368-372.
- Kienzler JL, Gold M, Nollevaux F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J Clin Pharmacol. 2010;50:50-61.
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15.
- Fecker LF, Stockfleth E, Nindl I, et al. The role of apoptosis in therapy and prophylaxis of epithelial tumours by nonsteroidal antiinflammatory drugs (NSAIDs). Br J Dermatol. 2007;156(Suppl 3):25-33.
- Thomas GJ, Herranz P, Cruz SB, et al. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: potential mechanism of action of diclofenac sodium 3% in hyaluronic acid 2.5. Dermatol Ther. 2019;32:e12800.
- Singer K, Dettmer K, Unger P, et al. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front Oncol. 2019;9:605.
- Diclofenac (topical). Drug information. UpToDate. https://www-uptodate-com.libraryaccess.elpaso.ttuhsc.edu/contents/diclofenac-topical-drug-information?source=auto_suggest&selectedTitle=1~3---3~4---diclofenac&search=diclofenac%20topical#F8017265
- Afify AA, Hana MR. Comparative evaluation of topical diclofenac sodium versus topical ibuprofen in the treatment of seborrheic keratosis. Dermatol Ther. 2020;33:e14370.
- Yin F, Ma J, Xiao H, et al. Randomized, double-blind, noninferiority study of diclofenac diethylamine 2.32% gel applied twice daily versus diclofenac diethylamine 1.16% gel applied four times daily in patients with acute ankle sprain. BMC Musculoskelet Disord. 2022;23:1125.
- van Herwaarden N, van den Elsen GAH, de Jong ICA, et al. Topical NSAIDs: ineffective or undervalued? [in Dutch]. Ned Tijdschr Geneeskd. 2021;165:D5317.
Actinic Keratosis Treatment With Diclofenac Gel 1%
Actinic Keratosis Treatment With Diclofenac Gel 1%
PRACTICE POINTS
- There are numerous field-directed therapies for actinic keratoses (AKs); however, efficacy and tolerability vary among the available treatments.
- Diclofenac gel 1% is an affordable option that could potentially increase accessibility and decrease cost of field therapy for the treatment of AKs, while maintaining therapeutic efficacy.
Behavioral Health Trainee Satisfaction at the US Department of Veterans Affairs During the COVID-19 Pandemic
Behavioral Health Trainee Satisfaction at the US Department of Veterans Affairs During the COVID-19 Pandemic
The COVID-19 pandemic changed the education and training experiences of health care students and those set to comprise the future workforce. Apart from general training disruptions or delays due to the pandemic, behavioral health trainees such as psychologists and social workers faced limited opportunities to provide in-person services.1-5 Trainees also experienced fewer referrals to mental health services from primary care and more disrupted, no-show, or cancelled appointments.4-6 Behavioral health trainees experienced a limited ability to establish rapport and more difficulty providing effective services because of the limited in-person interaction presented by telehealth.6 The pandemic also resulted in feelings of increased isolation and decreased teamwork.1,7 The virtual or remote setting made it more difficult for trainees to feel as if they were a member of a team or community of behavioral health professionals.1,7
Behavioral health trainees had to adapt to conducting patient visits and educational didactics through virtual platforms.1,3-7 Challenges included access or technological problems with online platforms and a lack of telehealth training use.3,4,6 One study found that while both behavioral health trainees and licensed practitioners reported similar rates of telehealth use for mental health services by early April 2020, trainees had more difficulties implementing telehealth compared with licensed practitioners. This study found that US Department of Veteran Affairs (VA) facilities reported higher use of telehealth in February 2020.5
A mission of the VA is to provide education and training to health care professionals through partnerships with affiliated academic institutions. The VA is the largest education and training supplier for health care professions in the US. As many as 50% of psychologists in the US received some training at the VA.8 Additionally, more graduate-level social work students are trained at the VA than at any other organization.9 The VA is a major contributor to not only its own behavioral health workforce, but that of the entire country.
The VA is also the largest employer of psychologists and social workers in the US.10,11 The VA Office of Academic Affiliations (OAA) oversees health care profession education and training at all VA facilities. In 2012, OAA began the Mental Health Education Expansion program to increase training for behavioral health professionals, including psychologists and social workers. 12 The OAA initiative was aligned with VA training and workforce priorities.8,12 To gauge the effectiveness of VA education and training, OAA encourages VA trainees to complete the Trainee Satisfaction Survey (TSS), which measures trainee satisfaction and the likelihood of a trainee to consider the VA for future employment.
Researchers at the Veterans Emergency Management Evaluation Center sought to understand the impact the COVID-19 pandemic had on behavioral health trainees’ experiences by examining TSS data from before and after the onset of the pandemic. This study expands on prior research among physician residents and fellows which found associations between VA training experiences and the COVID- 19 pandemic. The previous study found declines in trainee satisfaction and a decreased likelihood to consider the VA for future employment.13
It is important to understand the effects the pandemic had on the professional development and wellness for both physician and behavioral health professional trainees. Identifying how the pandemic impacted trainee satisfaction may help improve education programs and mitigate the impact of future public health emergencies. This is particularly important due to the shortage of behavioral health professionals in the VA and the US.12,14
METHODS
This study used TSS data collected from August 2018 to July 2021 from 153 VA facilities. A behavioral health trainee was defined as any psychology or social work trainee who completed 1 rotation at a VA facility. Psychiatric trainees were excluded because as physicians their training programs differ markedly from those for psychology and social work. Excluding psychiatry, psychology and social work comprise the 2 largest mental health care training groups.
This study was reviewed and approved as a quality improvement project by the VA Greater Los Angeles Healthcare System (VAGLAHS) Institutional Review Board, which waived informed consent requirements. The OAA granted access to data using a process open to all VA researchers. At the time of data collection, respondents were assured their anonymity; participation was voluntary.
Measures
Any response provided before February 29, 2020, was defined as the prepandemic period. The pandemic period included any response from April 1, 2020, to July 31, 2021. Responses collected in March 2020 were excluded as it would be unclear from the survey whether the training period occurred before or after the onset of the pandemic.
To measure overall trainee satisfaction with the VA training experience, responses were grouped as satisfied (satisfied/ very satisfied) and dissatisfied (dissatisfied/ very dissatisfied). To measure a trainee’s likelihood to consider the VA for future employment as a result of their training experience, responses were grouped as likely (likely/very likely) and unlikely (unlikely/very unlikely).
Other components of satisfaction were also collected including onboarding, clinical faculty/preceptors, clinical learning environment, physical environment, working environment, and respect felt at work. If a respondent chose very dissatisfied or dissatisfied, they were subsequently asked to specify the reason for their dissatisfaction with an open-ended response. Open-ended responses were not permitted if a respondent indicated a satisfied or very satisfied response.
Statistical Analyses
Stata SE 17 was used for statistical analyses. To test the relationship between the pandemic group and the 2 separate outcome variables, logistic regressions were conducted to measure overall satisfaction and likelihood of future VA employment. Margin commands were used to calculate the difference in the probability of reporting satisfied/very satisfied and likely/very likely for the prepandemic and pandemic groups. The association of the COVID-19 group with each outcome variable was expressed as the difference in the percentage of the outcome between the prepandemic and pandemic groups. Preliminary analyses demonstrated similar effects of the pandemic on psychology and social work trainees; therefore, the groups were combined.
Rapid Coding and Thematic Analyses
Qualitative data were based on open-ended responses from behavioral health trainees when they were asked to specify the cause of dissatisfaction in the aforementioned areas of satisfaction. Methods for qualitative data included rapid coding and thematic content analyses.15,16 Additional general information regarding the qualitative data analyses is described elsewhere.13 A keyword search was completed to identify all open-ended responses related to COVID-19 pandemic causes of dissatisfaction. Keywords included: virus, COVID, corona, pandemic, PPE, N95, mask, social distance, and safety. All open-ended responses were reviewed to ensure keywords were appropriately identifying pandemic-related causes of dissatisfaction and did not overlook other references to the pandemic, and to identify initial themes and corresponding definitions based on survey questions. After review, additional keywords were included in the content analyses that were related to providing mental health services using remote or telehealth options. This included the following keywords: remote, video, VVC (VA Video Connect), and tele. The research team completed a review of the initial themes and definitions and created a final coding construct with definitions before completing an independent coding of all identified pandemic-related responses. Frequency counts of each code were provided to identify which pandemic-related causes of dissatisfaction were mentioned most.
RESULTS
A total of 3950 behavioral health trainees responded to the TSS, including 2715 psychology trainees and 1235 social work trainees who indicated they received training at the VA in academic years 2018/2019, 2019/2020, or 2020/2021. The academic year 2018/2019 was considered in an effort to provide a larger sample of prepandemic trainees.
The percentage of trainees reporting satisfaction with their training decreased across prepandemic to pandemic groups. In the pandemic group, 2166 of 2324 respondents (93.2%) reported satisfaction compared to 1474 of 1555 (94.8%) in the prepandemic trainee group (P = .04; 95% CI, -3.10 to -0.08). There was no association between the pandemic group and behavioral health trainees’ reported willingness to consider the VA for future employment (Table 1). Preliminary analyses demonstrated similar effects of the pandemic on psychology and social work trainees, therefore the groups were combined, and overall effects were reported.
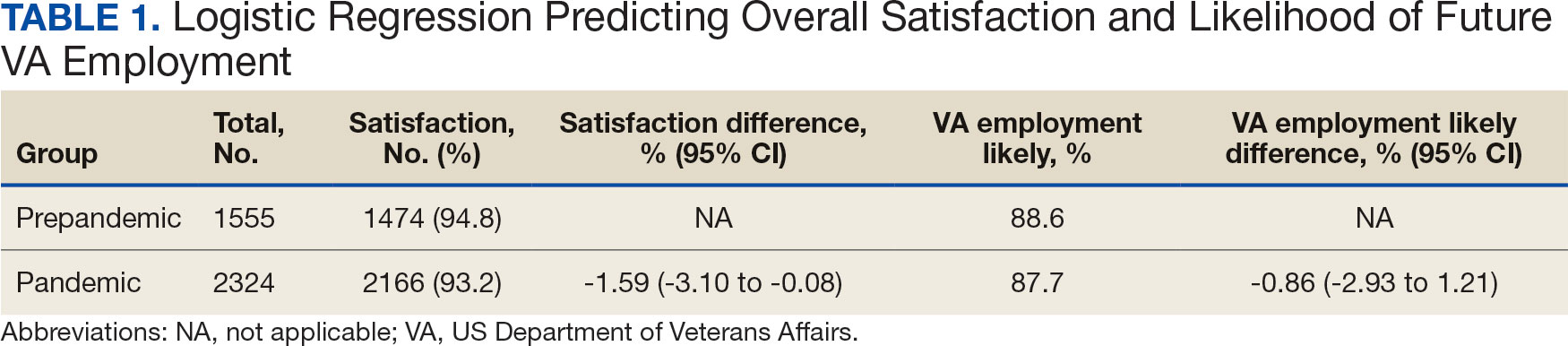
Pandemic-Related Dissatisfaction
Of the 3950 psychology and social work trainees who responded to the survey, 75 (1.9%) indicated dissatisfaction with their VA training experience using pandemic-related keywords. Open-ended responses were generally short (range, 1-32 words; median, 19 words). Qualitative analyses revealed 7 themes (Table 2).
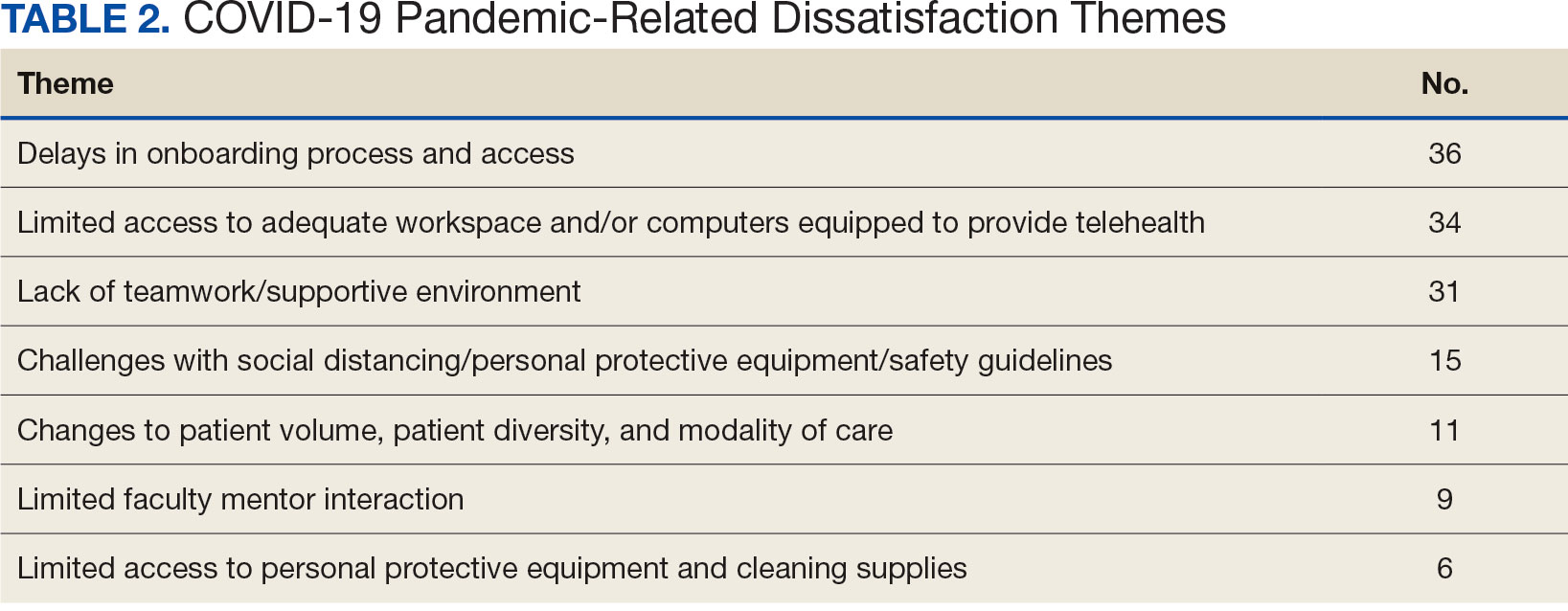
The most frequently identified theme was challenges with onboarding. One respondent indicated the modified onboarding procedures in place due to the pandemic were difficult to understand and resulted in delays. Another frequently mentioned cause of dissatisfaction was limited work or office space and insufficient computer availability. This was often noted to relate to a lack of private space to conduct telehealth visits or computers that were not equipped to provide telehealth. Several respondents also noted technological issues when attempting to use VVC to provide telehealth.
Another common theme was that the pandemic diminished teamwork, generated feelings of isolation, and created unsupportive environments for trainees. For instance, some trainees indicated that COVID-19 decreased the inclusion of trainees as part of the regular staff groups and accordingly resulted in limited networking opportunities. Other causes of dissatisfaction included the pandemic’s impacts on the learning environment, such as decreases in patient volume, decreased diversity of patient cases, and a limited presence of faculty mentors. Several respondents indicated that the pandemic limited their caseloads and indicated that most patients were seen virtually. Open-ended responses from a few respondents indicated their training environments were noncompliant with social distancing, personal protective equipment requirements, or other safety guidelines.
DISCUSSION
This study illustrates the impact of the COVID-19 pandemic on the behavioral health trainee experience, which was expressed through decreased satisfaction with their clinical training at the VA. The narrative data indicated that the observed pandemic-related dissatisfaction was linked specifically to onboarding, a lack of safe and private workspaces and computers, as well as a lack of a supportive work environment.
Although the reported decrease in satisfaction was statistically significant, the effect size was not large. Additionally, while satisfaction did decrease, the trainees’ reported likelihood to consider the VA for future employment was not impacted. This may suggest psychologist and social work trainees’ perseverance and dedication to their chosen profession persisted despite the challenges presented by the pandemic. Furthermore, the qualitative data suggest potential ways to mitigate health care profession trainee challenges that can follow a crisis like the COVID-19 pandemic, although further study is warranted.
While narrative responses with pandemic-related keywords did indicate challenges specific to COVID-19 (ie, limited access to workspaces and/or computers equipped for telehealth), the overall frequency of pandemic-related responses was low. This may indicate these are institutional challenges trainees face independent of the pandemic. These findings warrant longterm attention to the satisfaction of psychology and social worker trainees’ during the pandemic. For example, additional training for the use of telehealth could be provided. One study indicated that < 61% of psychology postdoctoral fellows received telepsychology training during the pandemic, and of those who did receive training, less than half were satisfied with it.3
Similarly, strategies could be developed to ensure a more supportive learning and work environment, and provide additional networking opportunities for trainees, despite social distancing. Education specific to disaster response should be incorporated into behavioral health care professionals’ training, especially because behavioral health care professionals provided major contributions during the pandemic due to reported increases in mental health concerns (eg, anxiety and depression) during the period.7,17,18 As the pandemic progressed, policies and procedures were established or modified to address some of these concerns because they were not necessarily limited to trainees. For example, additional training resources were developed to support the use of various telehealth technologies, virtual resources were used more often for meetings, and supervisors developed more comfort and familiarity with how to manage in a virtual or hybrid environment.
Limitations
Although the TSS data provide a large national sample of behavioral health care trainees, it only includes VA trainees, and therefore may not be completely generalizable across health care. However, because many psychologists and social workers throughout the US train at the VA, and because the VA is the largest employer of practicing psychologists and social workers, understanding the impacts felt at the VA informs institutions nationally.8-11 The TSS has limited demographic data (eg, age, race, ethnicity, and sex), so it is unclear whether the respondent groups before and during the pandemic differed in ways that could relate to outcomes. The data also do not specify exact training dates; however, anecdotal evidence suggests respondents generally complete the survey close to the end of their training.
Additionally, open-ended narrative responses were only asked for replies that indicated dissatisfaction, precluding a more nuanced understanding of potential positive outcomes. Furthermore, the TSS is limited to questions about the trainees’ clinical experiences, but because the pandemic created many stressors, there may have been personal issues that affected their work. It is possible that changes in overall satisfaction may have been rooted in something outside of their clinical experience. Finally, the response rate for the TSS is consistently low both before and during the pandemic and includes a limited number of narrative responses.
CONCLUSIONS
The VA is an important contributor to the education, training, and composition of the behavioral health care workforce. A deeper understanding of the VA trainee experience is important to identify how to improve behavioral health care professional education and training. This is especially true as behavioral health care faces shortages within the VA and nationwide.8,12,19
This study reinforces research that found health care trainees experienced decreased learning opportunities and telehealth-related challenges during the COVID-19 pandemic. 13,20 Despite the observed decline in trainee satisfaction, the lack of a corresponding change in likelihood to seek employment with the VA is encouraging for VA efforts to maintain and grow its behavioral health care workforce and for similar efforts outside VA. This resilience may relate to the substantial prepandemic time invested in their professional development. Future studies should examine long term impacts of the pandemic on trainee’s clinical experience and whether the pipeline of behavioral health care workers declines over time as students that are earlier in their career paths instead chose other professions. Future research should also explore ways to improve professional development and wellness of behavioral health care trainees during disasters (eg, telehealth training, additional networking, and social support).
- Muddle S, Rettie H, Harris O, Lawes A, Robinson R. Trainee life under COVID-19: a systemic case report. J Fam Ther. 2022;44(2):239-249. doi:10.1111/1467-6427.12354
- Valenzuela J, Crosby LE, Harrison RR. Commentary: reflections on the COVID-19 pandemic and health disparities in pediatric psychology. J Pediatr Psychol. 2020;45(8):839- 841. doi:10.1093/jpepsy/jsaa063
- Frye WS, Feldman M, Katzenstein J, Gardner L. Modified training experiences for psychology interns and fellows during COVID-19: use of telepsychology and telesupervision by child and adolescent training programs. J Clin Psychol Med Settings. 2022;29(4):840- 848. doi:10.1007/s10880-021-09839-4
- Perrin PB, Rybarczyk BD, Pierce BS, Jones HA, Shaffer C, Islam L. Rapid telepsychology deployment during the COVID-19 pandemic: a special issue commentary and lessons from primary care psychology training. J Clin Psychol. 2020;76(6):1173-1185. doi:10.1002/jclp.22969
- Reilly SE, Zane KL, McCuddy WT, et al. Mental health practitioners’ immediate practical response during the COVID-19 pandemic: observational questionnaire study. JMIR Ment Health. 2020;7(9):e21237. doi:10.2196/21237
- Sadicario JS, Parlier-Ahmad AB, Brechbiel JK, Islam LZ, Martin CE. Caring for women with substance use disorders through pregnancy and postpartum during the COVID-19 pandemic: lessons learned from psychology trainees in an integrated OBGYN/substance use disorder outpatient treatment program. J Subst Abuse Treat. 2021;122:108200. doi:10.1016/j.jsat.2020.108200
- Schneider NM, Steinberg DM, Garcia AM, et al. Pediatric consultation-liaison psychology: insights and lessons learned during the COVID-19 pandemic. J Clin Psychol Med Settings. 2023;30(1):51-60. doi:10.1007/s10880-022-09887-4
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee to Evaluate the Department of Veterans Affairs Mental Health. Mental Health Workforce and Facilities Infrastructure. In: Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press (US); 2018. https://www.ncbi.nlm.nih.gov/books/NBK499512/
- U.S. Department of Veterans Affairs Veterans Health Administration. Career as a VA social worker. Updated March 3, 2025. Accessed May 6, 2025. https://www.socialwork.va.gov/VA_Employment.asp
- United States Senate Committee on Veterans Affairs hearing on “Making the VA the Workplace of Choice for Health Care Providers.” News release. American Psychological Association. April 9, 2008. Accessed April 9, 2025. https:// www.apa.org/news/press/releases/2008/04/testimony
- VA National Professional Social Work Month Planning Committee. The diverse, far-reaching VA social worker profession. March 17, 2023. Accessed April 9, 2025. https://news.va.gov/116804/diverse-far-reaching-social-worker-profession/
- Patel EL, Bates JM, Holguin JK, et al. Program profile: the expansion of associated health training in the VA. Fed Pract. 2021;38(8):374-380. doi:10.12788/fp.0163
- Northcraft H, Bai J, Griffin AR, Hovsepian S, Dobalian A. Association of the COVID-19 pandemic on VA resident and fellow training satisfaction and future VA employment: a mixed methods study. J Grad Med Educ. 2022;14(5):593- 598. doi:10.4300/JGME-D-22-00168.1
- Health Resources and Services Administration. Health workforce shortage areas. Accessed April 9, 2025. https://data.hrsa.gov/topics/health-workforce/shortage-areas
- Gale RC, Wu J, Erhardt T, et al. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement Sci. 2019;14(1):11. doi:10.1186/s13012-019-0853-y
- Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis. BMJ Open. 2018;8(10):e019993. doi:10.1136/bmjopen-2017-019993
- Kranke D, Der-Martirosian C, Hovsepian S, et al. Social workers being effective in disaster settings. Soc Work Public Health. 2020;35(8):664-668. doi:10.1080/19371918.20 20.1820928
- Kranke D, Gin JL, Der-Martirosian C, Weiss EL, Dobalian A. VA social work leadership and compassion fatigue during the 2017 hurricane season. Soc Work Ment Health. 2020;18:188-199. doi:10.1080/15332985.2019.1700873
- Health Resources and Services Administration. Workforce projections. Accessed April 9, 2025. https://data.hrsa.gov/topics/health-workforce/workforce-projections
- Der-Martirosian C, Wyte-Lake T, Balut M, et al. Implementation of telehealth services at the US Department of Veterans Affairs during the COVID-19 pandemic: mixed methods study. JMIR Form Res. 2021;5(9):e29429. doi:10.2196/29429
The COVID-19 pandemic changed the education and training experiences of health care students and those set to comprise the future workforce. Apart from general training disruptions or delays due to the pandemic, behavioral health trainees such as psychologists and social workers faced limited opportunities to provide in-person services.1-5 Trainees also experienced fewer referrals to mental health services from primary care and more disrupted, no-show, or cancelled appointments.4-6 Behavioral health trainees experienced a limited ability to establish rapport and more difficulty providing effective services because of the limited in-person interaction presented by telehealth.6 The pandemic also resulted in feelings of increased isolation and decreased teamwork.1,7 The virtual or remote setting made it more difficult for trainees to feel as if they were a member of a team or community of behavioral health professionals.1,7
Behavioral health trainees had to adapt to conducting patient visits and educational didactics through virtual platforms.1,3-7 Challenges included access or technological problems with online platforms and a lack of telehealth training use.3,4,6 One study found that while both behavioral health trainees and licensed practitioners reported similar rates of telehealth use for mental health services by early April 2020, trainees had more difficulties implementing telehealth compared with licensed practitioners. This study found that US Department of Veteran Affairs (VA) facilities reported higher use of telehealth in February 2020.5
A mission of the VA is to provide education and training to health care professionals through partnerships with affiliated academic institutions. The VA is the largest education and training supplier for health care professions in the US. As many as 50% of psychologists in the US received some training at the VA.8 Additionally, more graduate-level social work students are trained at the VA than at any other organization.9 The VA is a major contributor to not only its own behavioral health workforce, but that of the entire country.
The VA is also the largest employer of psychologists and social workers in the US.10,11 The VA Office of Academic Affiliations (OAA) oversees health care profession education and training at all VA facilities. In 2012, OAA began the Mental Health Education Expansion program to increase training for behavioral health professionals, including psychologists and social workers. 12 The OAA initiative was aligned with VA training and workforce priorities.8,12 To gauge the effectiveness of VA education and training, OAA encourages VA trainees to complete the Trainee Satisfaction Survey (TSS), which measures trainee satisfaction and the likelihood of a trainee to consider the VA for future employment.
Researchers at the Veterans Emergency Management Evaluation Center sought to understand the impact the COVID-19 pandemic had on behavioral health trainees’ experiences by examining TSS data from before and after the onset of the pandemic. This study expands on prior research among physician residents and fellows which found associations between VA training experiences and the COVID- 19 pandemic. The previous study found declines in trainee satisfaction and a decreased likelihood to consider the VA for future employment.13
It is important to understand the effects the pandemic had on the professional development and wellness for both physician and behavioral health professional trainees. Identifying how the pandemic impacted trainee satisfaction may help improve education programs and mitigate the impact of future public health emergencies. This is particularly important due to the shortage of behavioral health professionals in the VA and the US.12,14
METHODS
This study used TSS data collected from August 2018 to July 2021 from 153 VA facilities. A behavioral health trainee was defined as any psychology or social work trainee who completed 1 rotation at a VA facility. Psychiatric trainees were excluded because as physicians their training programs differ markedly from those for psychology and social work. Excluding psychiatry, psychology and social work comprise the 2 largest mental health care training groups.
This study was reviewed and approved as a quality improvement project by the VA Greater Los Angeles Healthcare System (VAGLAHS) Institutional Review Board, which waived informed consent requirements. The OAA granted access to data using a process open to all VA researchers. At the time of data collection, respondents were assured their anonymity; participation was voluntary.
Measures
Any response provided before February 29, 2020, was defined as the prepandemic period. The pandemic period included any response from April 1, 2020, to July 31, 2021. Responses collected in March 2020 were excluded as it would be unclear from the survey whether the training period occurred before or after the onset of the pandemic.
To measure overall trainee satisfaction with the VA training experience, responses were grouped as satisfied (satisfied/ very satisfied) and dissatisfied (dissatisfied/ very dissatisfied). To measure a trainee’s likelihood to consider the VA for future employment as a result of their training experience, responses were grouped as likely (likely/very likely) and unlikely (unlikely/very unlikely).
Other components of satisfaction were also collected including onboarding, clinical faculty/preceptors, clinical learning environment, physical environment, working environment, and respect felt at work. If a respondent chose very dissatisfied or dissatisfied, they were subsequently asked to specify the reason for their dissatisfaction with an open-ended response. Open-ended responses were not permitted if a respondent indicated a satisfied or very satisfied response.
Statistical Analyses
Stata SE 17 was used for statistical analyses. To test the relationship between the pandemic group and the 2 separate outcome variables, logistic regressions were conducted to measure overall satisfaction and likelihood of future VA employment. Margin commands were used to calculate the difference in the probability of reporting satisfied/very satisfied and likely/very likely for the prepandemic and pandemic groups. The association of the COVID-19 group with each outcome variable was expressed as the difference in the percentage of the outcome between the prepandemic and pandemic groups. Preliminary analyses demonstrated similar effects of the pandemic on psychology and social work trainees; therefore, the groups were combined.
Rapid Coding and Thematic Analyses
Qualitative data were based on open-ended responses from behavioral health trainees when they were asked to specify the cause of dissatisfaction in the aforementioned areas of satisfaction. Methods for qualitative data included rapid coding and thematic content analyses.15,16 Additional general information regarding the qualitative data analyses is described elsewhere.13 A keyword search was completed to identify all open-ended responses related to COVID-19 pandemic causes of dissatisfaction. Keywords included: virus, COVID, corona, pandemic, PPE, N95, mask, social distance, and safety. All open-ended responses were reviewed to ensure keywords were appropriately identifying pandemic-related causes of dissatisfaction and did not overlook other references to the pandemic, and to identify initial themes and corresponding definitions based on survey questions. After review, additional keywords were included in the content analyses that were related to providing mental health services using remote or telehealth options. This included the following keywords: remote, video, VVC (VA Video Connect), and tele. The research team completed a review of the initial themes and definitions and created a final coding construct with definitions before completing an independent coding of all identified pandemic-related responses. Frequency counts of each code were provided to identify which pandemic-related causes of dissatisfaction were mentioned most.
RESULTS
A total of 3950 behavioral health trainees responded to the TSS, including 2715 psychology trainees and 1235 social work trainees who indicated they received training at the VA in academic years 2018/2019, 2019/2020, or 2020/2021. The academic year 2018/2019 was considered in an effort to provide a larger sample of prepandemic trainees.
The percentage of trainees reporting satisfaction with their training decreased across prepandemic to pandemic groups. In the pandemic group, 2166 of 2324 respondents (93.2%) reported satisfaction compared to 1474 of 1555 (94.8%) in the prepandemic trainee group (P = .04; 95% CI, -3.10 to -0.08). There was no association between the pandemic group and behavioral health trainees’ reported willingness to consider the VA for future employment (Table 1). Preliminary analyses demonstrated similar effects of the pandemic on psychology and social work trainees, therefore the groups were combined, and overall effects were reported.

Pandemic-Related Dissatisfaction
Of the 3950 psychology and social work trainees who responded to the survey, 75 (1.9%) indicated dissatisfaction with their VA training experience using pandemic-related keywords. Open-ended responses were generally short (range, 1-32 words; median, 19 words). Qualitative analyses revealed 7 themes (Table 2).

The most frequently identified theme was challenges with onboarding. One respondent indicated the modified onboarding procedures in place due to the pandemic were difficult to understand and resulted in delays. Another frequently mentioned cause of dissatisfaction was limited work or office space and insufficient computer availability. This was often noted to relate to a lack of private space to conduct telehealth visits or computers that were not equipped to provide telehealth. Several respondents also noted technological issues when attempting to use VVC to provide telehealth.
Another common theme was that the pandemic diminished teamwork, generated feelings of isolation, and created unsupportive environments for trainees. For instance, some trainees indicated that COVID-19 decreased the inclusion of trainees as part of the regular staff groups and accordingly resulted in limited networking opportunities. Other causes of dissatisfaction included the pandemic’s impacts on the learning environment, such as decreases in patient volume, decreased diversity of patient cases, and a limited presence of faculty mentors. Several respondents indicated that the pandemic limited their caseloads and indicated that most patients were seen virtually. Open-ended responses from a few respondents indicated their training environments were noncompliant with social distancing, personal protective equipment requirements, or other safety guidelines.
DISCUSSION
This study illustrates the impact of the COVID-19 pandemic on the behavioral health trainee experience, which was expressed through decreased satisfaction with their clinical training at the VA. The narrative data indicated that the observed pandemic-related dissatisfaction was linked specifically to onboarding, a lack of safe and private workspaces and computers, as well as a lack of a supportive work environment.
Although the reported decrease in satisfaction was statistically significant, the effect size was not large. Additionally, while satisfaction did decrease, the trainees’ reported likelihood to consider the VA for future employment was not impacted. This may suggest psychologist and social work trainees’ perseverance and dedication to their chosen profession persisted despite the challenges presented by the pandemic. Furthermore, the qualitative data suggest potential ways to mitigate health care profession trainee challenges that can follow a crisis like the COVID-19 pandemic, although further study is warranted.
While narrative responses with pandemic-related keywords did indicate challenges specific to COVID-19 (ie, limited access to workspaces and/or computers equipped for telehealth), the overall frequency of pandemic-related responses was low. This may indicate these are institutional challenges trainees face independent of the pandemic. These findings warrant longterm attention to the satisfaction of psychology and social worker trainees’ during the pandemic. For example, additional training for the use of telehealth could be provided. One study indicated that < 61% of psychology postdoctoral fellows received telepsychology training during the pandemic, and of those who did receive training, less than half were satisfied with it.3
Similarly, strategies could be developed to ensure a more supportive learning and work environment, and provide additional networking opportunities for trainees, despite social distancing. Education specific to disaster response should be incorporated into behavioral health care professionals’ training, especially because behavioral health care professionals provided major contributions during the pandemic due to reported increases in mental health concerns (eg, anxiety and depression) during the period.7,17,18 As the pandemic progressed, policies and procedures were established or modified to address some of these concerns because they were not necessarily limited to trainees. For example, additional training resources were developed to support the use of various telehealth technologies, virtual resources were used more often for meetings, and supervisors developed more comfort and familiarity with how to manage in a virtual or hybrid environment.
Limitations
Although the TSS data provide a large national sample of behavioral health care trainees, it only includes VA trainees, and therefore may not be completely generalizable across health care. However, because many psychologists and social workers throughout the US train at the VA, and because the VA is the largest employer of practicing psychologists and social workers, understanding the impacts felt at the VA informs institutions nationally.8-11 The TSS has limited demographic data (eg, age, race, ethnicity, and sex), so it is unclear whether the respondent groups before and during the pandemic differed in ways that could relate to outcomes. The data also do not specify exact training dates; however, anecdotal evidence suggests respondents generally complete the survey close to the end of their training.
Additionally, open-ended narrative responses were only asked for replies that indicated dissatisfaction, precluding a more nuanced understanding of potential positive outcomes. Furthermore, the TSS is limited to questions about the trainees’ clinical experiences, but because the pandemic created many stressors, there may have been personal issues that affected their work. It is possible that changes in overall satisfaction may have been rooted in something outside of their clinical experience. Finally, the response rate for the TSS is consistently low both before and during the pandemic and includes a limited number of narrative responses.
CONCLUSIONS
The VA is an important contributor to the education, training, and composition of the behavioral health care workforce. A deeper understanding of the VA trainee experience is important to identify how to improve behavioral health care professional education and training. This is especially true as behavioral health care faces shortages within the VA and nationwide.8,12,19
This study reinforces research that found health care trainees experienced decreased learning opportunities and telehealth-related challenges during the COVID-19 pandemic. 13,20 Despite the observed decline in trainee satisfaction, the lack of a corresponding change in likelihood to seek employment with the VA is encouraging for VA efforts to maintain and grow its behavioral health care workforce and for similar efforts outside VA. This resilience may relate to the substantial prepandemic time invested in their professional development. Future studies should examine long term impacts of the pandemic on trainee’s clinical experience and whether the pipeline of behavioral health care workers declines over time as students that are earlier in their career paths instead chose other professions. Future research should also explore ways to improve professional development and wellness of behavioral health care trainees during disasters (eg, telehealth training, additional networking, and social support).
The COVID-19 pandemic changed the education and training experiences of health care students and those set to comprise the future workforce. Apart from general training disruptions or delays due to the pandemic, behavioral health trainees such as psychologists and social workers faced limited opportunities to provide in-person services.1-5 Trainees also experienced fewer referrals to mental health services from primary care and more disrupted, no-show, or cancelled appointments.4-6 Behavioral health trainees experienced a limited ability to establish rapport and more difficulty providing effective services because of the limited in-person interaction presented by telehealth.6 The pandemic also resulted in feelings of increased isolation and decreased teamwork.1,7 The virtual or remote setting made it more difficult for trainees to feel as if they were a member of a team or community of behavioral health professionals.1,7
Behavioral health trainees had to adapt to conducting patient visits and educational didactics through virtual platforms.1,3-7 Challenges included access or technological problems with online platforms and a lack of telehealth training use.3,4,6 One study found that while both behavioral health trainees and licensed practitioners reported similar rates of telehealth use for mental health services by early April 2020, trainees had more difficulties implementing telehealth compared with licensed practitioners. This study found that US Department of Veteran Affairs (VA) facilities reported higher use of telehealth in February 2020.5
A mission of the VA is to provide education and training to health care professionals through partnerships with affiliated academic institutions. The VA is the largest education and training supplier for health care professions in the US. As many as 50% of psychologists in the US received some training at the VA.8 Additionally, more graduate-level social work students are trained at the VA than at any other organization.9 The VA is a major contributor to not only its own behavioral health workforce, but that of the entire country.
The VA is also the largest employer of psychologists and social workers in the US.10,11 The VA Office of Academic Affiliations (OAA) oversees health care profession education and training at all VA facilities. In 2012, OAA began the Mental Health Education Expansion program to increase training for behavioral health professionals, including psychologists and social workers. 12 The OAA initiative was aligned with VA training and workforce priorities.8,12 To gauge the effectiveness of VA education and training, OAA encourages VA trainees to complete the Trainee Satisfaction Survey (TSS), which measures trainee satisfaction and the likelihood of a trainee to consider the VA for future employment.
Researchers at the Veterans Emergency Management Evaluation Center sought to understand the impact the COVID-19 pandemic had on behavioral health trainees’ experiences by examining TSS data from before and after the onset of the pandemic. This study expands on prior research among physician residents and fellows which found associations between VA training experiences and the COVID- 19 pandemic. The previous study found declines in trainee satisfaction and a decreased likelihood to consider the VA for future employment.13
It is important to understand the effects the pandemic had on the professional development and wellness for both physician and behavioral health professional trainees. Identifying how the pandemic impacted trainee satisfaction may help improve education programs and mitigate the impact of future public health emergencies. This is particularly important due to the shortage of behavioral health professionals in the VA and the US.12,14
METHODS
This study used TSS data collected from August 2018 to July 2021 from 153 VA facilities. A behavioral health trainee was defined as any psychology or social work trainee who completed 1 rotation at a VA facility. Psychiatric trainees were excluded because as physicians their training programs differ markedly from those for psychology and social work. Excluding psychiatry, psychology and social work comprise the 2 largest mental health care training groups.
This study was reviewed and approved as a quality improvement project by the VA Greater Los Angeles Healthcare System (VAGLAHS) Institutional Review Board, which waived informed consent requirements. The OAA granted access to data using a process open to all VA researchers. At the time of data collection, respondents were assured their anonymity; participation was voluntary.
Measures
Any response provided before February 29, 2020, was defined as the prepandemic period. The pandemic period included any response from April 1, 2020, to July 31, 2021. Responses collected in March 2020 were excluded as it would be unclear from the survey whether the training period occurred before or after the onset of the pandemic.
To measure overall trainee satisfaction with the VA training experience, responses were grouped as satisfied (satisfied/ very satisfied) and dissatisfied (dissatisfied/ very dissatisfied). To measure a trainee’s likelihood to consider the VA for future employment as a result of their training experience, responses were grouped as likely (likely/very likely) and unlikely (unlikely/very unlikely).
Other components of satisfaction were also collected including onboarding, clinical faculty/preceptors, clinical learning environment, physical environment, working environment, and respect felt at work. If a respondent chose very dissatisfied or dissatisfied, they were subsequently asked to specify the reason for their dissatisfaction with an open-ended response. Open-ended responses were not permitted if a respondent indicated a satisfied or very satisfied response.
Statistical Analyses
Stata SE 17 was used for statistical analyses. To test the relationship between the pandemic group and the 2 separate outcome variables, logistic regressions were conducted to measure overall satisfaction and likelihood of future VA employment. Margin commands were used to calculate the difference in the probability of reporting satisfied/very satisfied and likely/very likely for the prepandemic and pandemic groups. The association of the COVID-19 group with each outcome variable was expressed as the difference in the percentage of the outcome between the prepandemic and pandemic groups. Preliminary analyses demonstrated similar effects of the pandemic on psychology and social work trainees; therefore, the groups were combined.
Rapid Coding and Thematic Analyses
Qualitative data were based on open-ended responses from behavioral health trainees when they were asked to specify the cause of dissatisfaction in the aforementioned areas of satisfaction. Methods for qualitative data included rapid coding and thematic content analyses.15,16 Additional general information regarding the qualitative data analyses is described elsewhere.13 A keyword search was completed to identify all open-ended responses related to COVID-19 pandemic causes of dissatisfaction. Keywords included: virus, COVID, corona, pandemic, PPE, N95, mask, social distance, and safety. All open-ended responses were reviewed to ensure keywords were appropriately identifying pandemic-related causes of dissatisfaction and did not overlook other references to the pandemic, and to identify initial themes and corresponding definitions based on survey questions. After review, additional keywords were included in the content analyses that were related to providing mental health services using remote or telehealth options. This included the following keywords: remote, video, VVC (VA Video Connect), and tele. The research team completed a review of the initial themes and definitions and created a final coding construct with definitions before completing an independent coding of all identified pandemic-related responses. Frequency counts of each code were provided to identify which pandemic-related causes of dissatisfaction were mentioned most.
RESULTS
A total of 3950 behavioral health trainees responded to the TSS, including 2715 psychology trainees and 1235 social work trainees who indicated they received training at the VA in academic years 2018/2019, 2019/2020, or 2020/2021. The academic year 2018/2019 was considered in an effort to provide a larger sample of prepandemic trainees.
The percentage of trainees reporting satisfaction with their training decreased across prepandemic to pandemic groups. In the pandemic group, 2166 of 2324 respondents (93.2%) reported satisfaction compared to 1474 of 1555 (94.8%) in the prepandemic trainee group (P = .04; 95% CI, -3.10 to -0.08). There was no association between the pandemic group and behavioral health trainees’ reported willingness to consider the VA for future employment (Table 1). Preliminary analyses demonstrated similar effects of the pandemic on psychology and social work trainees, therefore the groups were combined, and overall effects were reported.

Pandemic-Related Dissatisfaction
Of the 3950 psychology and social work trainees who responded to the survey, 75 (1.9%) indicated dissatisfaction with their VA training experience using pandemic-related keywords. Open-ended responses were generally short (range, 1-32 words; median, 19 words). Qualitative analyses revealed 7 themes (Table 2).

The most frequently identified theme was challenges with onboarding. One respondent indicated the modified onboarding procedures in place due to the pandemic were difficult to understand and resulted in delays. Another frequently mentioned cause of dissatisfaction was limited work or office space and insufficient computer availability. This was often noted to relate to a lack of private space to conduct telehealth visits or computers that were not equipped to provide telehealth. Several respondents also noted technological issues when attempting to use VVC to provide telehealth.
Another common theme was that the pandemic diminished teamwork, generated feelings of isolation, and created unsupportive environments for trainees. For instance, some trainees indicated that COVID-19 decreased the inclusion of trainees as part of the regular staff groups and accordingly resulted in limited networking opportunities. Other causes of dissatisfaction included the pandemic’s impacts on the learning environment, such as decreases in patient volume, decreased diversity of patient cases, and a limited presence of faculty mentors. Several respondents indicated that the pandemic limited their caseloads and indicated that most patients were seen virtually. Open-ended responses from a few respondents indicated their training environments were noncompliant with social distancing, personal protective equipment requirements, or other safety guidelines.
DISCUSSION
This study illustrates the impact of the COVID-19 pandemic on the behavioral health trainee experience, which was expressed through decreased satisfaction with their clinical training at the VA. The narrative data indicated that the observed pandemic-related dissatisfaction was linked specifically to onboarding, a lack of safe and private workspaces and computers, as well as a lack of a supportive work environment.
Although the reported decrease in satisfaction was statistically significant, the effect size was not large. Additionally, while satisfaction did decrease, the trainees’ reported likelihood to consider the VA for future employment was not impacted. This may suggest psychologist and social work trainees’ perseverance and dedication to their chosen profession persisted despite the challenges presented by the pandemic. Furthermore, the qualitative data suggest potential ways to mitigate health care profession trainee challenges that can follow a crisis like the COVID-19 pandemic, although further study is warranted.
While narrative responses with pandemic-related keywords did indicate challenges specific to COVID-19 (ie, limited access to workspaces and/or computers equipped for telehealth), the overall frequency of pandemic-related responses was low. This may indicate these are institutional challenges trainees face independent of the pandemic. These findings warrant longterm attention to the satisfaction of psychology and social worker trainees’ during the pandemic. For example, additional training for the use of telehealth could be provided. One study indicated that < 61% of psychology postdoctoral fellows received telepsychology training during the pandemic, and of those who did receive training, less than half were satisfied with it.3
Similarly, strategies could be developed to ensure a more supportive learning and work environment, and provide additional networking opportunities for trainees, despite social distancing. Education specific to disaster response should be incorporated into behavioral health care professionals’ training, especially because behavioral health care professionals provided major contributions during the pandemic due to reported increases in mental health concerns (eg, anxiety and depression) during the period.7,17,18 As the pandemic progressed, policies and procedures were established or modified to address some of these concerns because they were not necessarily limited to trainees. For example, additional training resources were developed to support the use of various telehealth technologies, virtual resources were used more often for meetings, and supervisors developed more comfort and familiarity with how to manage in a virtual or hybrid environment.
Limitations
Although the TSS data provide a large national sample of behavioral health care trainees, it only includes VA trainees, and therefore may not be completely generalizable across health care. However, because many psychologists and social workers throughout the US train at the VA, and because the VA is the largest employer of practicing psychologists and social workers, understanding the impacts felt at the VA informs institutions nationally.8-11 The TSS has limited demographic data (eg, age, race, ethnicity, and sex), so it is unclear whether the respondent groups before and during the pandemic differed in ways that could relate to outcomes. The data also do not specify exact training dates; however, anecdotal evidence suggests respondents generally complete the survey close to the end of their training.
Additionally, open-ended narrative responses were only asked for replies that indicated dissatisfaction, precluding a more nuanced understanding of potential positive outcomes. Furthermore, the TSS is limited to questions about the trainees’ clinical experiences, but because the pandemic created many stressors, there may have been personal issues that affected their work. It is possible that changes in overall satisfaction may have been rooted in something outside of their clinical experience. Finally, the response rate for the TSS is consistently low both before and during the pandemic and includes a limited number of narrative responses.
CONCLUSIONS
The VA is an important contributor to the education, training, and composition of the behavioral health care workforce. A deeper understanding of the VA trainee experience is important to identify how to improve behavioral health care professional education and training. This is especially true as behavioral health care faces shortages within the VA and nationwide.8,12,19
This study reinforces research that found health care trainees experienced decreased learning opportunities and telehealth-related challenges during the COVID-19 pandemic. 13,20 Despite the observed decline in trainee satisfaction, the lack of a corresponding change in likelihood to seek employment with the VA is encouraging for VA efforts to maintain and grow its behavioral health care workforce and for similar efforts outside VA. This resilience may relate to the substantial prepandemic time invested in their professional development. Future studies should examine long term impacts of the pandemic on trainee’s clinical experience and whether the pipeline of behavioral health care workers declines over time as students that are earlier in their career paths instead chose other professions. Future research should also explore ways to improve professional development and wellness of behavioral health care trainees during disasters (eg, telehealth training, additional networking, and social support).
- Muddle S, Rettie H, Harris O, Lawes A, Robinson R. Trainee life under COVID-19: a systemic case report. J Fam Ther. 2022;44(2):239-249. doi:10.1111/1467-6427.12354
- Valenzuela J, Crosby LE, Harrison RR. Commentary: reflections on the COVID-19 pandemic and health disparities in pediatric psychology. J Pediatr Psychol. 2020;45(8):839- 841. doi:10.1093/jpepsy/jsaa063
- Frye WS, Feldman M, Katzenstein J, Gardner L. Modified training experiences for psychology interns and fellows during COVID-19: use of telepsychology and telesupervision by child and adolescent training programs. J Clin Psychol Med Settings. 2022;29(4):840- 848. doi:10.1007/s10880-021-09839-4
- Perrin PB, Rybarczyk BD, Pierce BS, Jones HA, Shaffer C, Islam L. Rapid telepsychology deployment during the COVID-19 pandemic: a special issue commentary and lessons from primary care psychology training. J Clin Psychol. 2020;76(6):1173-1185. doi:10.1002/jclp.22969
- Reilly SE, Zane KL, McCuddy WT, et al. Mental health practitioners’ immediate practical response during the COVID-19 pandemic: observational questionnaire study. JMIR Ment Health. 2020;7(9):e21237. doi:10.2196/21237
- Sadicario JS, Parlier-Ahmad AB, Brechbiel JK, Islam LZ, Martin CE. Caring for women with substance use disorders through pregnancy and postpartum during the COVID-19 pandemic: lessons learned from psychology trainees in an integrated OBGYN/substance use disorder outpatient treatment program. J Subst Abuse Treat. 2021;122:108200. doi:10.1016/j.jsat.2020.108200
- Schneider NM, Steinberg DM, Garcia AM, et al. Pediatric consultation-liaison psychology: insights and lessons learned during the COVID-19 pandemic. J Clin Psychol Med Settings. 2023;30(1):51-60. doi:10.1007/s10880-022-09887-4
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee to Evaluate the Department of Veterans Affairs Mental Health. Mental Health Workforce and Facilities Infrastructure. In: Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press (US); 2018. https://www.ncbi.nlm.nih.gov/books/NBK499512/
- U.S. Department of Veterans Affairs Veterans Health Administration. Career as a VA social worker. Updated March 3, 2025. Accessed May 6, 2025. https://www.socialwork.va.gov/VA_Employment.asp
- United States Senate Committee on Veterans Affairs hearing on “Making the VA the Workplace of Choice for Health Care Providers.” News release. American Psychological Association. April 9, 2008. Accessed April 9, 2025. https:// www.apa.org/news/press/releases/2008/04/testimony
- VA National Professional Social Work Month Planning Committee. The diverse, far-reaching VA social worker profession. March 17, 2023. Accessed April 9, 2025. https://news.va.gov/116804/diverse-far-reaching-social-worker-profession/
- Patel EL, Bates JM, Holguin JK, et al. Program profile: the expansion of associated health training in the VA. Fed Pract. 2021;38(8):374-380. doi:10.12788/fp.0163
- Northcraft H, Bai J, Griffin AR, Hovsepian S, Dobalian A. Association of the COVID-19 pandemic on VA resident and fellow training satisfaction and future VA employment: a mixed methods study. J Grad Med Educ. 2022;14(5):593- 598. doi:10.4300/JGME-D-22-00168.1
- Health Resources and Services Administration. Health workforce shortage areas. Accessed April 9, 2025. https://data.hrsa.gov/topics/health-workforce/shortage-areas
- Gale RC, Wu J, Erhardt T, et al. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement Sci. 2019;14(1):11. doi:10.1186/s13012-019-0853-y
- Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis. BMJ Open. 2018;8(10):e019993. doi:10.1136/bmjopen-2017-019993
- Kranke D, Der-Martirosian C, Hovsepian S, et al. Social workers being effective in disaster settings. Soc Work Public Health. 2020;35(8):664-668. doi:10.1080/19371918.20 20.1820928
- Kranke D, Gin JL, Der-Martirosian C, Weiss EL, Dobalian A. VA social work leadership and compassion fatigue during the 2017 hurricane season. Soc Work Ment Health. 2020;18:188-199. doi:10.1080/15332985.2019.1700873
- Health Resources and Services Administration. Workforce projections. Accessed April 9, 2025. https://data.hrsa.gov/topics/health-workforce/workforce-projections
- Der-Martirosian C, Wyte-Lake T, Balut M, et al. Implementation of telehealth services at the US Department of Veterans Affairs during the COVID-19 pandemic: mixed methods study. JMIR Form Res. 2021;5(9):e29429. doi:10.2196/29429
- Muddle S, Rettie H, Harris O, Lawes A, Robinson R. Trainee life under COVID-19: a systemic case report. J Fam Ther. 2022;44(2):239-249. doi:10.1111/1467-6427.12354
- Valenzuela J, Crosby LE, Harrison RR. Commentary: reflections on the COVID-19 pandemic and health disparities in pediatric psychology. J Pediatr Psychol. 2020;45(8):839- 841. doi:10.1093/jpepsy/jsaa063
- Frye WS, Feldman M, Katzenstein J, Gardner L. Modified training experiences for psychology interns and fellows during COVID-19: use of telepsychology and telesupervision by child and adolescent training programs. J Clin Psychol Med Settings. 2022;29(4):840- 848. doi:10.1007/s10880-021-09839-4
- Perrin PB, Rybarczyk BD, Pierce BS, Jones HA, Shaffer C, Islam L. Rapid telepsychology deployment during the COVID-19 pandemic: a special issue commentary and lessons from primary care psychology training. J Clin Psychol. 2020;76(6):1173-1185. doi:10.1002/jclp.22969
- Reilly SE, Zane KL, McCuddy WT, et al. Mental health practitioners’ immediate practical response during the COVID-19 pandemic: observational questionnaire study. JMIR Ment Health. 2020;7(9):e21237. doi:10.2196/21237
- Sadicario JS, Parlier-Ahmad AB, Brechbiel JK, Islam LZ, Martin CE. Caring for women with substance use disorders through pregnancy and postpartum during the COVID-19 pandemic: lessons learned from psychology trainees in an integrated OBGYN/substance use disorder outpatient treatment program. J Subst Abuse Treat. 2021;122:108200. doi:10.1016/j.jsat.2020.108200
- Schneider NM, Steinberg DM, Garcia AM, et al. Pediatric consultation-liaison psychology: insights and lessons learned during the COVID-19 pandemic. J Clin Psychol Med Settings. 2023;30(1):51-60. doi:10.1007/s10880-022-09887-4
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee to Evaluate the Department of Veterans Affairs Mental Health. Mental Health Workforce and Facilities Infrastructure. In: Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press (US); 2018. https://www.ncbi.nlm.nih.gov/books/NBK499512/
- U.S. Department of Veterans Affairs Veterans Health Administration. Career as a VA social worker. Updated March 3, 2025. Accessed May 6, 2025. https://www.socialwork.va.gov/VA_Employment.asp
- United States Senate Committee on Veterans Affairs hearing on “Making the VA the Workplace of Choice for Health Care Providers.” News release. American Psychological Association. April 9, 2008. Accessed April 9, 2025. https:// www.apa.org/news/press/releases/2008/04/testimony
- VA National Professional Social Work Month Planning Committee. The diverse, far-reaching VA social worker profession. March 17, 2023. Accessed April 9, 2025. https://news.va.gov/116804/diverse-far-reaching-social-worker-profession/
- Patel EL, Bates JM, Holguin JK, et al. Program profile: the expansion of associated health training in the VA. Fed Pract. 2021;38(8):374-380. doi:10.12788/fp.0163
- Northcraft H, Bai J, Griffin AR, Hovsepian S, Dobalian A. Association of the COVID-19 pandemic on VA resident and fellow training satisfaction and future VA employment: a mixed methods study. J Grad Med Educ. 2022;14(5):593- 598. doi:10.4300/JGME-D-22-00168.1
- Health Resources and Services Administration. Health workforce shortage areas. Accessed April 9, 2025. https://data.hrsa.gov/topics/health-workforce/shortage-areas
- Gale RC, Wu J, Erhardt T, et al. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement Sci. 2019;14(1):11. doi:10.1186/s13012-019-0853-y
- Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis. BMJ Open. 2018;8(10):e019993. doi:10.1136/bmjopen-2017-019993
- Kranke D, Der-Martirosian C, Hovsepian S, et al. Social workers being effective in disaster settings. Soc Work Public Health. 2020;35(8):664-668. doi:10.1080/19371918.20 20.1820928
- Kranke D, Gin JL, Der-Martirosian C, Weiss EL, Dobalian A. VA social work leadership and compassion fatigue during the 2017 hurricane season. Soc Work Ment Health. 2020;18:188-199. doi:10.1080/15332985.2019.1700873
- Health Resources and Services Administration. Workforce projections. Accessed April 9, 2025. https://data.hrsa.gov/topics/health-workforce/workforce-projections
- Der-Martirosian C, Wyte-Lake T, Balut M, et al. Implementation of telehealth services at the US Department of Veterans Affairs during the COVID-19 pandemic: mixed methods study. JMIR Form Res. 2021;5(9):e29429. doi:10.2196/29429
Behavioral Health Trainee Satisfaction at the US Department of Veterans Affairs During the COVID-19 Pandemic
Behavioral Health Trainee Satisfaction at the US Department of Veterans Affairs During the COVID-19 Pandemic
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Blood cultures provide crucial evidence for diagnostic medicine, specifically aimed at identifying the presence of microbial infections in the bloodstream. Blood culturing is instrumental in diagnosing conditions such as sepsis, bacteremia, or fungemia, where the identification of the causative agent is necessary for targeted and effective treatment.1
The process involves aseptically drawing blood into sterile culture bottles, minimizing the risk of contamination with well-established guidelines. These culture bottles contain specific growth media that support the replication of microorganisms if they are present. Once the blood specimen is collected, it incubates, allowing any potential pathogens to grow. Subsequent analysis and identification of these microorganisms enable health care professionals (HCPs) to prescribe appropriate antimicrobial therapies to treat specific infections, contributing to more effective and targeted patient care.2
The reliability of blood culture results depends on minimizing contamination risk, a challenge inherent in the procedure. Contamination can lead to false-positive results, potentially misguiding treatment.3 HCPs must adhere to strict aseptic techniques during blood draws, ensuring proper skin preparation with antiseptic solutions. The use of sterile equipment and avoiding prolonged tourniquet application helps maintain the integrity of the blood specimen. Timely inoculation of blood into culture bottles and careful handling are essential to mitigate contamination risk.2 Regular training and reinforcement of proper techniques is important to uphold the accuracy of blood culture results and enhance the reliability of diagnoses and treatment decisions.3 Despite diligent contamination prevention efforts, health care systems struggle to maintain contamination rates below the 3.0% national benchmark set by the Clinical & Laboratory Standards Institute (CLSI).4
Blood culture contamination is a critical concern in clinical practice; it can lead to misdiagnosis, prolonged hospital stays, unnecessary antibiotic use, and increased health care costs.5 Monitoring blood culture contamination is integral to patient safety, avoiding inappropriate and potentially harmful treatment, providing efficient care, contributing to antibiotic stewardship, supporting cost efficiency, and maintaining quality assurance and clinical research practices for public health.6
The initial specimen diversion technique (ISDT) recently emerged as a potential strategy to reduce blood culture contamination rates. This technique involves diverting a small portion of the initial blood plus the skin plug from the hollow needle away from the primary collection site before filling the culture bottles. This process minimizes skin surface contaminants, providing a cleaner blood specimen for culturing.7
The ISDT was introduced as a result of historically elevated contamination rates.8 Despite implementing various mitigation methods, the US Department of Veterans Affairs (VA) Central Texas Healthcare System (VACTHCS) has struggled to meet the national benchmark of maintaining blood culture contamination < 3.0%. The VACTHCS is a 146-bed teaching hospital with about 30,000 annual visits at the Olin E. Teague Veterans Affairs Medical Center (OETVMC) emergency department (ED). VACTHCS conducted a 16-month pilot study using 2 commercially available ISDT devices and published the findings.8
The Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022 (MilCon-VA Act) committee report prioritized the reduction of blood culture contamination to < 1% to prevent health risks and harm to veterans undergoing blood testing for the diagnosis of sepsis.9 Because it had been 5 years since OETVMC began using an ISDT in the ED, the ISDT adaptation strategy for mitigating blood culture contamination was revisited per institution policy.
The objective of this quality improvement project was to analyze retrospective data to understand the long-term impact of ISDT use on blood culture contamination rates. We hypothesized that ISDT use would contribute to efforts to maintain OETVMC ED blood culture contamination rate below the national (3.0%) and VACTHCS (2.5%) thresholds. This project assessed the progress for reducing blood culture contamination compared with the pre-ISDT era.8
METHODS
This retrospective analysis compared the blood culture contamination rates 36 months before and after the introduction of the ISDT device at the OETVMC ED. The preimplementation period was from December 2014 through November 2017 (36 months) and the postimplementation period was December 2017 through November 2020 (36 months). Data were collected from the Department of Pathology and Microbiology blood culture records of all adult patients admitted to the hospital through the ED and required blood cultures for suspicion of infection. Protected health information and VA sensitive information were not collected: all data were deidentified. A total of 18,541 blood cultures were collected 36 months preimplementation and 14,865 blood cultures were collected up to 36 months postimplementation. For comparison purposes, a similar dataset was collected from patients’ blood samples drawn by phlebotomists in the laboratory, where there had been no previous issues with overcontamination; no ISDT devices were used in the collection of these samples.
Blood Culture Contamination Variable
Blood cultures were monitored using the BACT/ALERT 3D (bioMérieux) and subsequently BACT/ALERT VIRTUO (bioMérieux), with positive bottles characterized by VITEK MS Matrix Assisted Laser Desorption Ionization Time-of-Flight technology (bioMérieux) and automated susceptibility testing (VITEK 2 [bioMérieux]).10 In an updated review of blood culture contamination, the American Society for Microbiology used the College of American Pathologists' Q-Probes quality improvement studies as a guideline for classifying contamination. A sample was determined to be contaminated if ≥ 1 of the following organisms were found in only 1 bottle in a series of blood culture sets: coagulase-negative staphylococci, Micrococcus species, α-hemolytic viridans group streptococci, Corynebacterium species, Propionibacterium acnes, and Bacillus species.11 The contamination assessment criteria remained unchanged, except for use of an ISDT device in blood culture collection at the ED.
The VACTHCS Infection Prevention Department ensured that the ISDT device was available and that ED nurses were trained annually on its use to collect blood cultures. Monthly reports of contamination were sent to the nursing supervisor for corrective action and retraining. The initial performance improvement project was slated for 16 months but was expanded to a 6-year period of retrospective data to obtain strong correlation.
Statistical Analysis
Contamination rates were recorded monthly from the hospital laboratory information management system for 36 months both before and after ISDT adoption. Statistical analysis was performed using a 2-tailed unpaired t-test to compare monthly contamination rates for the 2 periods with GraphPad Prism version 10.0.0 for Windows.
RESULTS
Prior to 2017, the ED reported contamination rates above the national (3.0%) and OETVMC thresholds (2.5%), with a mean of 4.5% (95% CI, 3.90-4.90).8 After ISDT implementation, the ED showed significant improvement with a reduction to mean 2.6% (95% CI, 2.10-3.20) (P < .001) (Figure 1). Figure 2 shows monthly blood culture contamination rates at the ED from December 2014 through November 2020. Month 36 (November 2017) shows a clear dip in contamination rate when the ISDT was introduced and month 37 to month 44 show remarkably low contamination rates. During this time, the institute experimented with 2 ISDT devices, and closer scrutiny may reveal this period as an outlier due to the monitoring of ISDT application, as previously reported.8
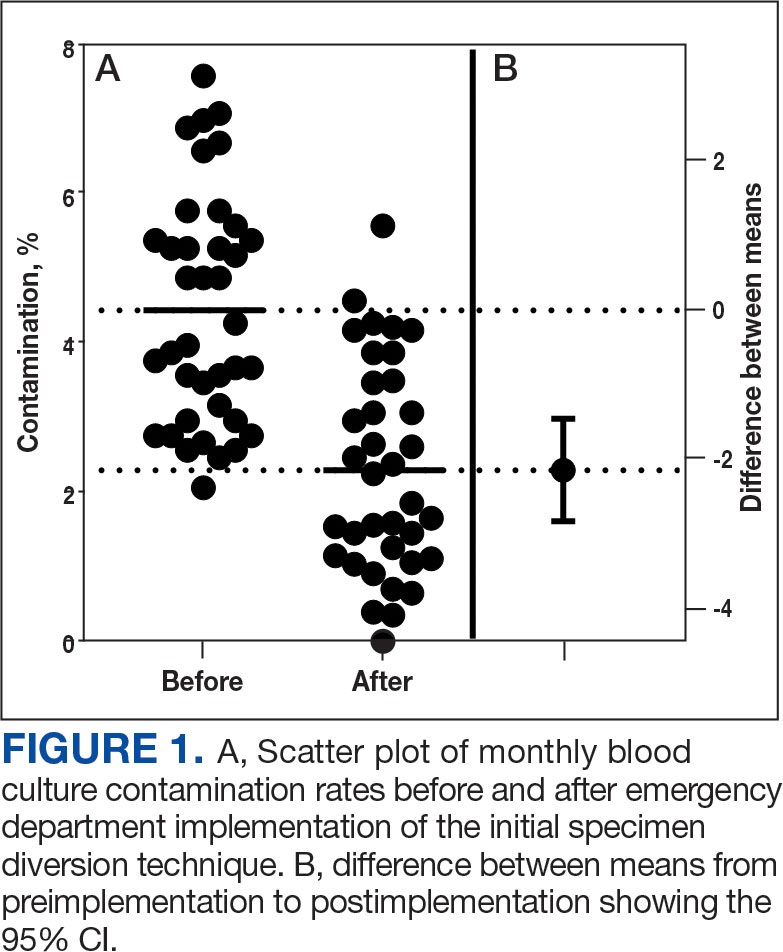
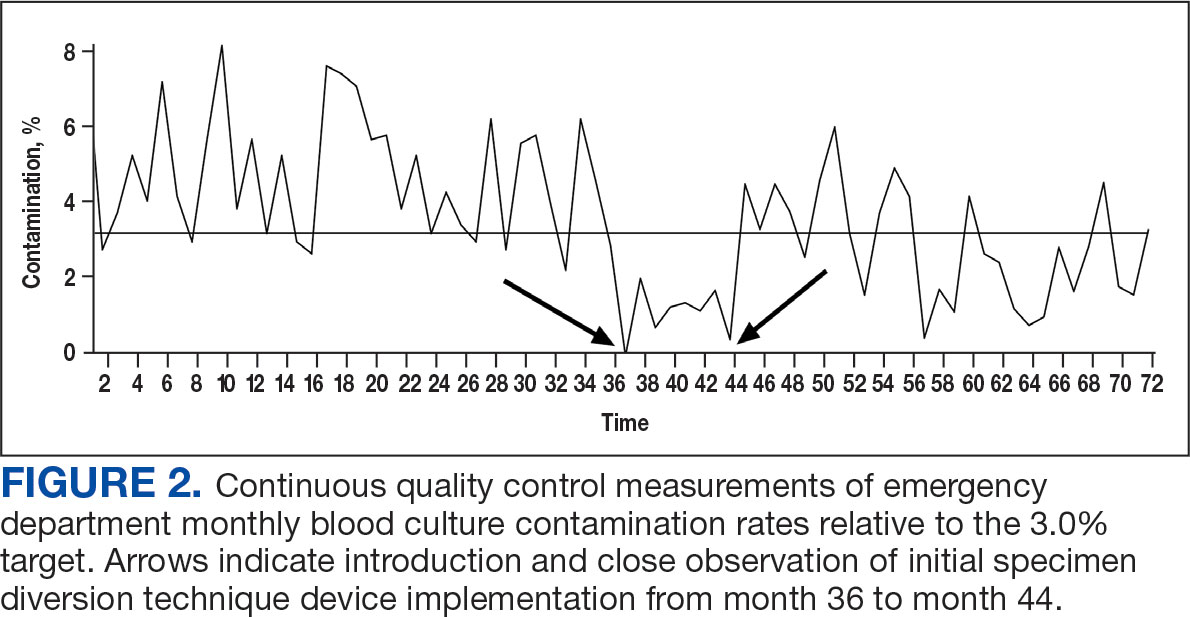
The blood culture contamination rate for samples drawn by the phlebotomists in the laboratory (excluding the ED) was calculated during the same time period (Figure 3). Non-ED contamination rates remained below 2.5% for 69 of 72 months.
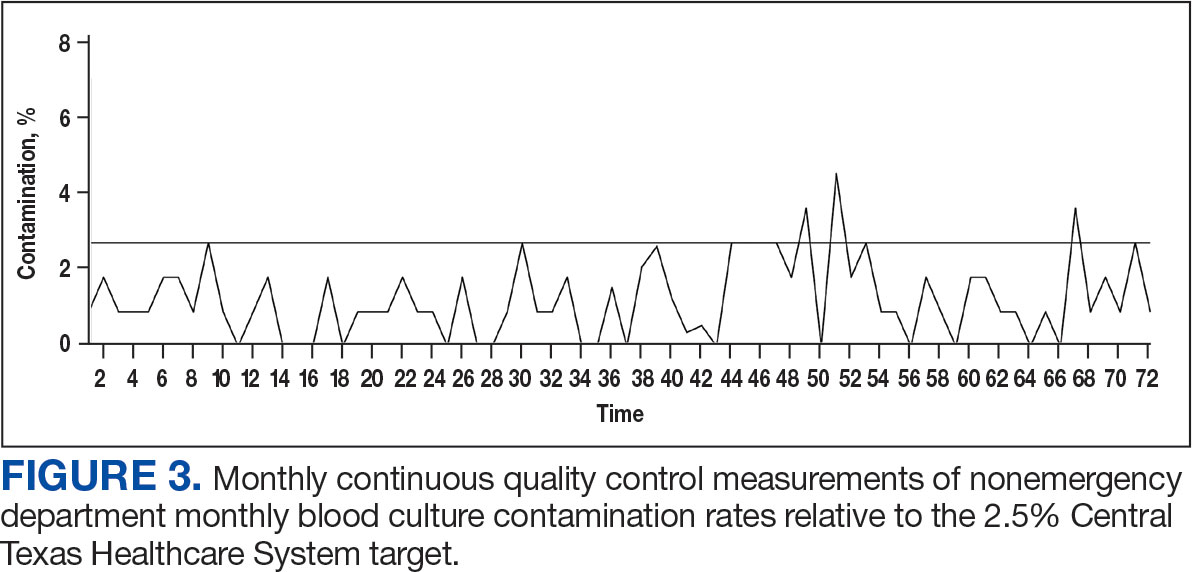
DISCUSSION
The blood culture contamination rate in the OETVMC ED dropped following ISDT implementation and continued to show long-term benefits. For the 36-month period following ISDT implementation, the mean contamination rate was 2.6%, which was below the national target threshold of 3.0% and close to the OETVMC target of 2.5%. These results suggest that ISDT can have a positive impact on patient care and laboratory efficiency. Improvements in the blood contamination rates in the ED can have a positive impact on the overall hospital contamination rates.
Blood drawn by phlebotomists in the hospital laboratory infrequently had contamination rates that exceeded the 2.5% target threshold. Because the non-ED contamination rates did not change throughout the comparison period, other factors were likely not involved in the improvements seen in the ED. The decision to implement ISDT exclusively in the ED was based on its historically elevated contamination rate.8 Issues with blood culture contamination in EDs across various hospital systems are well documented and not unique to VACTHCS.12
Contamination in blood cultures can be a significant issue in the hospital. It occurs when microorganisms from the skin or environment enter the blood culture sample during collection. Moreover, it can contribute to antibiotic resistance when patients are prescribed inappropriate antibiotics. It is also important to ensure HCPs are well-trained and consistently follow standardized protocols and understand the implications of false-positive results.13
ISDT helps reduce false-positive results and is a significant advancement in the field of blood culture collection.8,14 By discarding the initial blood, it ensures that only the true bloodstream sample is cultured, leading to more accurate results.15 It also may minimize the risk of contamination-related delays in diagnosis and treatment and benefits patients and health care institutions by potentially reducing hospital stays, unnecessary antibiotic use, and health care costs.
One of the ISDT device manufacturers estimated the financial impact on OETVMC based on the pilot project.8 While this study did not calculate the direct and indirect cost savings associated with this process improvement, the manufacturer’s website suggests that VACTHCS could annually save about $486,000.16 Furthermore, implementation of ISDT may improve laboratory efficiency, as they reduce the workload associated with identifying and reporting false-positive cultures. 6 ISDT devices represent a valuable tool in the efforts to reduce blood culture contamination and its wide-ranging implications in clinical settings. While ISDT alone will not be sufficient in achieving a lower threshold (< 1%) of blood culture contamination, it can be part of a multiprong effort that optimizes best practices in the collection, handling, and management of blood cultures.
Continuous quality improvement efforts and monitoring of blood culture contamination rates can help health care institutions identify problem areas and implement necessary changes. Addressing blood culture contamination can improve patient care, reduce costs, and address antibiotic resistance.
Limitations
This study was limited by its study design, which did not use a side-by-side comparison of blood cultures from groups with and without ISDT. All blood cultures from patients in the region were processed at OETVMC, which may not be representative of non-VA EDs. Part of this study took place during the COVID-19 pandemic, which may have skewed data. Additionally, hospital data were collected from a veteran population in Central Texas, and the lack of demographic diversity may not be generalizable to the greater population.
CONCLUSIONS
The findings of this study suggest ISDT may be effective in reducing blood culture contamination rates in the high-risk ED environment, which aligns with previous research. 5,14 The ISDT may help reduce blood culture contamination rates, improving the quality of patient care and reducing health care costs. MilCon-VA mandated that all VA facilities have blood culture contamination as a metric with a goal of blood culture contamination rates < 1%.8 However, achieving this goal remains a challenge. Further research and continuous quality improvement efforts are necessary to achieve it. Consistently achieving a contamination threshold of < 1% may require minimizing human error. An automated robotic venipuncture device, as recently designed and reported, may be necessary to reduce human error in blood draw and contamination.16
- Chela HK, Vasudevan A, Rojas-Moreno C, Naqvi SH. Approach to positive blood cultures in the hospitalized patient: a review. Mo Med. 2019;116(4):313-317.
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009-19. doi:10.1128/CMR.00009-19
- Wilson ML, Kirn Jr TJ, Antonara S, et al. Clinical and Laboratory Standards Institute Guideline M47—Principles and Procedures for Blood Cultures. Clinical and Laboratory Standards Institute. April 22, 2022. Accessed May 21, 2025. https://clsi.org/shop/standards/m47/
- Hancock JA, Campbell S, Jones MM, Wang-Rodriguez J, VHA Microbiology SME Workgroup, Klutts JS. Development and validation of a standardized blood culture contamination definition and metric dashboard for a large health care system. Am J Clin Pathol. 2023;160(3):255-260. doi:10.1093/ajcp/aqad044
- Shinozaki T, Deane RS, Mazuzan JE Jr, Hamel AJ, Hazelton D. Bacterial contamination of arterial lines. A prospective study. JAMA. 1983;249(2):223-225.
- Al Mohajer M, Lasco T. The impact of initial specimen diversion systems on blood culture contamination. Open Forum Infect Dis. 2023;10:ofad182. doi:10.1093/ofid/ofad182
- Arenas M, Boseman GM, Coppin JD, Lukey J, Jinadatha C, Navarathna DH. Asynchronous testing of 2 specimen-diversion devices to reduce blood culture contamination: a single-site product supply quality improvement project. J Emerg Nurs. 2021;47(2):256-264. e6. doi:10.1016/j.jen.2020.11.008
- Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022, HR 4355, 117th Cong (2021-2022). Accessed May 12, 2025. https://www.congress.gov/bill/117th-congress/house-bill/4355?
- Altun O, Almuhayawi M, Lüthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the New BacT/ Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol. 2016;54(4):1148-1151. doi:10.1128/JCM.03362-15
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. doi:10.1128/CMR.00062-05
- Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):1021-1024. doi:10.1128/JCM.02162-08
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central lineassociated bloodstream infections. Am J Infect Control. 2015;43(11):1222-1237. doi:10.1016/j.ajic.2015.06.030
- Callado GY, Lin V, Thottacherry E, et al. Diagnostic stewardship: a systematic review and meta-analysis of blood collection diversion devices used to reduce blood culture contamination and improve the accuracy of diagnosis in clinical settings. Open Forum Infect Dis. 2023;10(9):ofad433. doi:10.1093/ofid/ofad433
- Patton RG, Schmitt T. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol. 2010;48:4501-4503. doi:10.1128/JCM.00910-10
- Kurin. Clinical evidence: published Kurin studies. 2024. Accessed May 12, 2025. https://www.kurin.com/studies
- Leipheimer JM, Balter ML, Chen AI, et al. First-in-human evaluation of a hand-held automated venipuncture device for rapid venous blood draws. Technology (Singap World Sci). 2019;7(3-4):98-107. doi:10.1142/S2339547819500067?
Blood cultures provide crucial evidence for diagnostic medicine, specifically aimed at identifying the presence of microbial infections in the bloodstream. Blood culturing is instrumental in diagnosing conditions such as sepsis, bacteremia, or fungemia, where the identification of the causative agent is necessary for targeted and effective treatment.1
The process involves aseptically drawing blood into sterile culture bottles, minimizing the risk of contamination with well-established guidelines. These culture bottles contain specific growth media that support the replication of microorganisms if they are present. Once the blood specimen is collected, it incubates, allowing any potential pathogens to grow. Subsequent analysis and identification of these microorganisms enable health care professionals (HCPs) to prescribe appropriate antimicrobial therapies to treat specific infections, contributing to more effective and targeted patient care.2
The reliability of blood culture results depends on minimizing contamination risk, a challenge inherent in the procedure. Contamination can lead to false-positive results, potentially misguiding treatment.3 HCPs must adhere to strict aseptic techniques during blood draws, ensuring proper skin preparation with antiseptic solutions. The use of sterile equipment and avoiding prolonged tourniquet application helps maintain the integrity of the blood specimen. Timely inoculation of blood into culture bottles and careful handling are essential to mitigate contamination risk.2 Regular training and reinforcement of proper techniques is important to uphold the accuracy of blood culture results and enhance the reliability of diagnoses and treatment decisions.3 Despite diligent contamination prevention efforts, health care systems struggle to maintain contamination rates below the 3.0% national benchmark set by the Clinical & Laboratory Standards Institute (CLSI).4
Blood culture contamination is a critical concern in clinical practice; it can lead to misdiagnosis, prolonged hospital stays, unnecessary antibiotic use, and increased health care costs.5 Monitoring blood culture contamination is integral to patient safety, avoiding inappropriate and potentially harmful treatment, providing efficient care, contributing to antibiotic stewardship, supporting cost efficiency, and maintaining quality assurance and clinical research practices for public health.6
The initial specimen diversion technique (ISDT) recently emerged as a potential strategy to reduce blood culture contamination rates. This technique involves diverting a small portion of the initial blood plus the skin plug from the hollow needle away from the primary collection site before filling the culture bottles. This process minimizes skin surface contaminants, providing a cleaner blood specimen for culturing.7
The ISDT was introduced as a result of historically elevated contamination rates.8 Despite implementing various mitigation methods, the US Department of Veterans Affairs (VA) Central Texas Healthcare System (VACTHCS) has struggled to meet the national benchmark of maintaining blood culture contamination < 3.0%. The VACTHCS is a 146-bed teaching hospital with about 30,000 annual visits at the Olin E. Teague Veterans Affairs Medical Center (OETVMC) emergency department (ED). VACTHCS conducted a 16-month pilot study using 2 commercially available ISDT devices and published the findings.8
The Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022 (MilCon-VA Act) committee report prioritized the reduction of blood culture contamination to < 1% to prevent health risks and harm to veterans undergoing blood testing for the diagnosis of sepsis.9 Because it had been 5 years since OETVMC began using an ISDT in the ED, the ISDT adaptation strategy for mitigating blood culture contamination was revisited per institution policy.
The objective of this quality improvement project was to analyze retrospective data to understand the long-term impact of ISDT use on blood culture contamination rates. We hypothesized that ISDT use would contribute to efforts to maintain OETVMC ED blood culture contamination rate below the national (3.0%) and VACTHCS (2.5%) thresholds. This project assessed the progress for reducing blood culture contamination compared with the pre-ISDT era.8
METHODS
This retrospective analysis compared the blood culture contamination rates 36 months before and after the introduction of the ISDT device at the OETVMC ED. The preimplementation period was from December 2014 through November 2017 (36 months) and the postimplementation period was December 2017 through November 2020 (36 months). Data were collected from the Department of Pathology and Microbiology blood culture records of all adult patients admitted to the hospital through the ED and required blood cultures for suspicion of infection. Protected health information and VA sensitive information were not collected: all data were deidentified. A total of 18,541 blood cultures were collected 36 months preimplementation and 14,865 blood cultures were collected up to 36 months postimplementation. For comparison purposes, a similar dataset was collected from patients’ blood samples drawn by phlebotomists in the laboratory, where there had been no previous issues with overcontamination; no ISDT devices were used in the collection of these samples.
Blood Culture Contamination Variable
Blood cultures were monitored using the BACT/ALERT 3D (bioMérieux) and subsequently BACT/ALERT VIRTUO (bioMérieux), with positive bottles characterized by VITEK MS Matrix Assisted Laser Desorption Ionization Time-of-Flight technology (bioMérieux) and automated susceptibility testing (VITEK 2 [bioMérieux]).10 In an updated review of blood culture contamination, the American Society for Microbiology used the College of American Pathologists' Q-Probes quality improvement studies as a guideline for classifying contamination. A sample was determined to be contaminated if ≥ 1 of the following organisms were found in only 1 bottle in a series of blood culture sets: coagulase-negative staphylococci, Micrococcus species, α-hemolytic viridans group streptococci, Corynebacterium species, Propionibacterium acnes, and Bacillus species.11 The contamination assessment criteria remained unchanged, except for use of an ISDT device in blood culture collection at the ED.
The VACTHCS Infection Prevention Department ensured that the ISDT device was available and that ED nurses were trained annually on its use to collect blood cultures. Monthly reports of contamination were sent to the nursing supervisor for corrective action and retraining. The initial performance improvement project was slated for 16 months but was expanded to a 6-year period of retrospective data to obtain strong correlation.
Statistical Analysis
Contamination rates were recorded monthly from the hospital laboratory information management system for 36 months both before and after ISDT adoption. Statistical analysis was performed using a 2-tailed unpaired t-test to compare monthly contamination rates for the 2 periods with GraphPad Prism version 10.0.0 for Windows.
RESULTS
Prior to 2017, the ED reported contamination rates above the national (3.0%) and OETVMC thresholds (2.5%), with a mean of 4.5% (95% CI, 3.90-4.90).8 After ISDT implementation, the ED showed significant improvement with a reduction to mean 2.6% (95% CI, 2.10-3.20) (P < .001) (Figure 1). Figure 2 shows monthly blood culture contamination rates at the ED from December 2014 through November 2020. Month 36 (November 2017) shows a clear dip in contamination rate when the ISDT was introduced and month 37 to month 44 show remarkably low contamination rates. During this time, the institute experimented with 2 ISDT devices, and closer scrutiny may reveal this period as an outlier due to the monitoring of ISDT application, as previously reported.8


The blood culture contamination rate for samples drawn by the phlebotomists in the laboratory (excluding the ED) was calculated during the same time period (Figure 3). Non-ED contamination rates remained below 2.5% for 69 of 72 months.

DISCUSSION
The blood culture contamination rate in the OETVMC ED dropped following ISDT implementation and continued to show long-term benefits. For the 36-month period following ISDT implementation, the mean contamination rate was 2.6%, which was below the national target threshold of 3.0% and close to the OETVMC target of 2.5%. These results suggest that ISDT can have a positive impact on patient care and laboratory efficiency. Improvements in the blood contamination rates in the ED can have a positive impact on the overall hospital contamination rates.
Blood drawn by phlebotomists in the hospital laboratory infrequently had contamination rates that exceeded the 2.5% target threshold. Because the non-ED contamination rates did not change throughout the comparison period, other factors were likely not involved in the improvements seen in the ED. The decision to implement ISDT exclusively in the ED was based on its historically elevated contamination rate.8 Issues with blood culture contamination in EDs across various hospital systems are well documented and not unique to VACTHCS.12
Contamination in blood cultures can be a significant issue in the hospital. It occurs when microorganisms from the skin or environment enter the blood culture sample during collection. Moreover, it can contribute to antibiotic resistance when patients are prescribed inappropriate antibiotics. It is also important to ensure HCPs are well-trained and consistently follow standardized protocols and understand the implications of false-positive results.13
ISDT helps reduce false-positive results and is a significant advancement in the field of blood culture collection.8,14 By discarding the initial blood, it ensures that only the true bloodstream sample is cultured, leading to more accurate results.15 It also may minimize the risk of contamination-related delays in diagnosis and treatment and benefits patients and health care institutions by potentially reducing hospital stays, unnecessary antibiotic use, and health care costs.
One of the ISDT device manufacturers estimated the financial impact on OETVMC based on the pilot project.8 While this study did not calculate the direct and indirect cost savings associated with this process improvement, the manufacturer’s website suggests that VACTHCS could annually save about $486,000.16 Furthermore, implementation of ISDT may improve laboratory efficiency, as they reduce the workload associated with identifying and reporting false-positive cultures. 6 ISDT devices represent a valuable tool in the efforts to reduce blood culture contamination and its wide-ranging implications in clinical settings. While ISDT alone will not be sufficient in achieving a lower threshold (< 1%) of blood culture contamination, it can be part of a multiprong effort that optimizes best practices in the collection, handling, and management of blood cultures.
Continuous quality improvement efforts and monitoring of blood culture contamination rates can help health care institutions identify problem areas and implement necessary changes. Addressing blood culture contamination can improve patient care, reduce costs, and address antibiotic resistance.
Limitations
This study was limited by its study design, which did not use a side-by-side comparison of blood cultures from groups with and without ISDT. All blood cultures from patients in the region were processed at OETVMC, which may not be representative of non-VA EDs. Part of this study took place during the COVID-19 pandemic, which may have skewed data. Additionally, hospital data were collected from a veteran population in Central Texas, and the lack of demographic diversity may not be generalizable to the greater population.
CONCLUSIONS
The findings of this study suggest ISDT may be effective in reducing blood culture contamination rates in the high-risk ED environment, which aligns with previous research. 5,14 The ISDT may help reduce blood culture contamination rates, improving the quality of patient care and reducing health care costs. MilCon-VA mandated that all VA facilities have blood culture contamination as a metric with a goal of blood culture contamination rates < 1%.8 However, achieving this goal remains a challenge. Further research and continuous quality improvement efforts are necessary to achieve it. Consistently achieving a contamination threshold of < 1% may require minimizing human error. An automated robotic venipuncture device, as recently designed and reported, may be necessary to reduce human error in blood draw and contamination.16
Blood cultures provide crucial evidence for diagnostic medicine, specifically aimed at identifying the presence of microbial infections in the bloodstream. Blood culturing is instrumental in diagnosing conditions such as sepsis, bacteremia, or fungemia, where the identification of the causative agent is necessary for targeted and effective treatment.1
The process involves aseptically drawing blood into sterile culture bottles, minimizing the risk of contamination with well-established guidelines. These culture bottles contain specific growth media that support the replication of microorganisms if they are present. Once the blood specimen is collected, it incubates, allowing any potential pathogens to grow. Subsequent analysis and identification of these microorganisms enable health care professionals (HCPs) to prescribe appropriate antimicrobial therapies to treat specific infections, contributing to more effective and targeted patient care.2
The reliability of blood culture results depends on minimizing contamination risk, a challenge inherent in the procedure. Contamination can lead to false-positive results, potentially misguiding treatment.3 HCPs must adhere to strict aseptic techniques during blood draws, ensuring proper skin preparation with antiseptic solutions. The use of sterile equipment and avoiding prolonged tourniquet application helps maintain the integrity of the blood specimen. Timely inoculation of blood into culture bottles and careful handling are essential to mitigate contamination risk.2 Regular training and reinforcement of proper techniques is important to uphold the accuracy of blood culture results and enhance the reliability of diagnoses and treatment decisions.3 Despite diligent contamination prevention efforts, health care systems struggle to maintain contamination rates below the 3.0% national benchmark set by the Clinical & Laboratory Standards Institute (CLSI).4
Blood culture contamination is a critical concern in clinical practice; it can lead to misdiagnosis, prolonged hospital stays, unnecessary antibiotic use, and increased health care costs.5 Monitoring blood culture contamination is integral to patient safety, avoiding inappropriate and potentially harmful treatment, providing efficient care, contributing to antibiotic stewardship, supporting cost efficiency, and maintaining quality assurance and clinical research practices for public health.6
The initial specimen diversion technique (ISDT) recently emerged as a potential strategy to reduce blood culture contamination rates. This technique involves diverting a small portion of the initial blood plus the skin plug from the hollow needle away from the primary collection site before filling the culture bottles. This process minimizes skin surface contaminants, providing a cleaner blood specimen for culturing.7
The ISDT was introduced as a result of historically elevated contamination rates.8 Despite implementing various mitigation methods, the US Department of Veterans Affairs (VA) Central Texas Healthcare System (VACTHCS) has struggled to meet the national benchmark of maintaining blood culture contamination < 3.0%. The VACTHCS is a 146-bed teaching hospital with about 30,000 annual visits at the Olin E. Teague Veterans Affairs Medical Center (OETVMC) emergency department (ED). VACTHCS conducted a 16-month pilot study using 2 commercially available ISDT devices and published the findings.8
The Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022 (MilCon-VA Act) committee report prioritized the reduction of blood culture contamination to < 1% to prevent health risks and harm to veterans undergoing blood testing for the diagnosis of sepsis.9 Because it had been 5 years since OETVMC began using an ISDT in the ED, the ISDT adaptation strategy for mitigating blood culture contamination was revisited per institution policy.
The objective of this quality improvement project was to analyze retrospective data to understand the long-term impact of ISDT use on blood culture contamination rates. We hypothesized that ISDT use would contribute to efforts to maintain OETVMC ED blood culture contamination rate below the national (3.0%) and VACTHCS (2.5%) thresholds. This project assessed the progress for reducing blood culture contamination compared with the pre-ISDT era.8
METHODS
This retrospective analysis compared the blood culture contamination rates 36 months before and after the introduction of the ISDT device at the OETVMC ED. The preimplementation period was from December 2014 through November 2017 (36 months) and the postimplementation period was December 2017 through November 2020 (36 months). Data were collected from the Department of Pathology and Microbiology blood culture records of all adult patients admitted to the hospital through the ED and required blood cultures for suspicion of infection. Protected health information and VA sensitive information were not collected: all data were deidentified. A total of 18,541 blood cultures were collected 36 months preimplementation and 14,865 blood cultures were collected up to 36 months postimplementation. For comparison purposes, a similar dataset was collected from patients’ blood samples drawn by phlebotomists in the laboratory, where there had been no previous issues with overcontamination; no ISDT devices were used in the collection of these samples.
Blood Culture Contamination Variable
Blood cultures were monitored using the BACT/ALERT 3D (bioMérieux) and subsequently BACT/ALERT VIRTUO (bioMérieux), with positive bottles characterized by VITEK MS Matrix Assisted Laser Desorption Ionization Time-of-Flight technology (bioMérieux) and automated susceptibility testing (VITEK 2 [bioMérieux]).10 In an updated review of blood culture contamination, the American Society for Microbiology used the College of American Pathologists' Q-Probes quality improvement studies as a guideline for classifying contamination. A sample was determined to be contaminated if ≥ 1 of the following organisms were found in only 1 bottle in a series of blood culture sets: coagulase-negative staphylococci, Micrococcus species, α-hemolytic viridans group streptococci, Corynebacterium species, Propionibacterium acnes, and Bacillus species.11 The contamination assessment criteria remained unchanged, except for use of an ISDT device in blood culture collection at the ED.
The VACTHCS Infection Prevention Department ensured that the ISDT device was available and that ED nurses were trained annually on its use to collect blood cultures. Monthly reports of contamination were sent to the nursing supervisor for corrective action and retraining. The initial performance improvement project was slated for 16 months but was expanded to a 6-year period of retrospective data to obtain strong correlation.
Statistical Analysis
Contamination rates were recorded monthly from the hospital laboratory information management system for 36 months both before and after ISDT adoption. Statistical analysis was performed using a 2-tailed unpaired t-test to compare monthly contamination rates for the 2 periods with GraphPad Prism version 10.0.0 for Windows.
RESULTS
Prior to 2017, the ED reported contamination rates above the national (3.0%) and OETVMC thresholds (2.5%), with a mean of 4.5% (95% CI, 3.90-4.90).8 After ISDT implementation, the ED showed significant improvement with a reduction to mean 2.6% (95% CI, 2.10-3.20) (P < .001) (Figure 1). Figure 2 shows monthly blood culture contamination rates at the ED from December 2014 through November 2020. Month 36 (November 2017) shows a clear dip in contamination rate when the ISDT was introduced and month 37 to month 44 show remarkably low contamination rates. During this time, the institute experimented with 2 ISDT devices, and closer scrutiny may reveal this period as an outlier due to the monitoring of ISDT application, as previously reported.8


The blood culture contamination rate for samples drawn by the phlebotomists in the laboratory (excluding the ED) was calculated during the same time period (Figure 3). Non-ED contamination rates remained below 2.5% for 69 of 72 months.

DISCUSSION
The blood culture contamination rate in the OETVMC ED dropped following ISDT implementation and continued to show long-term benefits. For the 36-month period following ISDT implementation, the mean contamination rate was 2.6%, which was below the national target threshold of 3.0% and close to the OETVMC target of 2.5%. These results suggest that ISDT can have a positive impact on patient care and laboratory efficiency. Improvements in the blood contamination rates in the ED can have a positive impact on the overall hospital contamination rates.
Blood drawn by phlebotomists in the hospital laboratory infrequently had contamination rates that exceeded the 2.5% target threshold. Because the non-ED contamination rates did not change throughout the comparison period, other factors were likely not involved in the improvements seen in the ED. The decision to implement ISDT exclusively in the ED was based on its historically elevated contamination rate.8 Issues with blood culture contamination in EDs across various hospital systems are well documented and not unique to VACTHCS.12
Contamination in blood cultures can be a significant issue in the hospital. It occurs when microorganisms from the skin or environment enter the blood culture sample during collection. Moreover, it can contribute to antibiotic resistance when patients are prescribed inappropriate antibiotics. It is also important to ensure HCPs are well-trained and consistently follow standardized protocols and understand the implications of false-positive results.13
ISDT helps reduce false-positive results and is a significant advancement in the field of blood culture collection.8,14 By discarding the initial blood, it ensures that only the true bloodstream sample is cultured, leading to more accurate results.15 It also may minimize the risk of contamination-related delays in diagnosis and treatment and benefits patients and health care institutions by potentially reducing hospital stays, unnecessary antibiotic use, and health care costs.
One of the ISDT device manufacturers estimated the financial impact on OETVMC based on the pilot project.8 While this study did not calculate the direct and indirect cost savings associated with this process improvement, the manufacturer’s website suggests that VACTHCS could annually save about $486,000.16 Furthermore, implementation of ISDT may improve laboratory efficiency, as they reduce the workload associated with identifying and reporting false-positive cultures. 6 ISDT devices represent a valuable tool in the efforts to reduce blood culture contamination and its wide-ranging implications in clinical settings. While ISDT alone will not be sufficient in achieving a lower threshold (< 1%) of blood culture contamination, it can be part of a multiprong effort that optimizes best practices in the collection, handling, and management of blood cultures.
Continuous quality improvement efforts and monitoring of blood culture contamination rates can help health care institutions identify problem areas and implement necessary changes. Addressing blood culture contamination can improve patient care, reduce costs, and address antibiotic resistance.
Limitations
This study was limited by its study design, which did not use a side-by-side comparison of blood cultures from groups with and without ISDT. All blood cultures from patients in the region were processed at OETVMC, which may not be representative of non-VA EDs. Part of this study took place during the COVID-19 pandemic, which may have skewed data. Additionally, hospital data were collected from a veteran population in Central Texas, and the lack of demographic diversity may not be generalizable to the greater population.
CONCLUSIONS
The findings of this study suggest ISDT may be effective in reducing blood culture contamination rates in the high-risk ED environment, which aligns with previous research. 5,14 The ISDT may help reduce blood culture contamination rates, improving the quality of patient care and reducing health care costs. MilCon-VA mandated that all VA facilities have blood culture contamination as a metric with a goal of blood culture contamination rates < 1%.8 However, achieving this goal remains a challenge. Further research and continuous quality improvement efforts are necessary to achieve it. Consistently achieving a contamination threshold of < 1% may require minimizing human error. An automated robotic venipuncture device, as recently designed and reported, may be necessary to reduce human error in blood draw and contamination.16
- Chela HK, Vasudevan A, Rojas-Moreno C, Naqvi SH. Approach to positive blood cultures in the hospitalized patient: a review. Mo Med. 2019;116(4):313-317.
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009-19. doi:10.1128/CMR.00009-19
- Wilson ML, Kirn Jr TJ, Antonara S, et al. Clinical and Laboratory Standards Institute Guideline M47—Principles and Procedures for Blood Cultures. Clinical and Laboratory Standards Institute. April 22, 2022. Accessed May 21, 2025. https://clsi.org/shop/standards/m47/
- Hancock JA, Campbell S, Jones MM, Wang-Rodriguez J, VHA Microbiology SME Workgroup, Klutts JS. Development and validation of a standardized blood culture contamination definition and metric dashboard for a large health care system. Am J Clin Pathol. 2023;160(3):255-260. doi:10.1093/ajcp/aqad044
- Shinozaki T, Deane RS, Mazuzan JE Jr, Hamel AJ, Hazelton D. Bacterial contamination of arterial lines. A prospective study. JAMA. 1983;249(2):223-225.
- Al Mohajer M, Lasco T. The impact of initial specimen diversion systems on blood culture contamination. Open Forum Infect Dis. 2023;10:ofad182. doi:10.1093/ofid/ofad182
- Arenas M, Boseman GM, Coppin JD, Lukey J, Jinadatha C, Navarathna DH. Asynchronous testing of 2 specimen-diversion devices to reduce blood culture contamination: a single-site product supply quality improvement project. J Emerg Nurs. 2021;47(2):256-264. e6. doi:10.1016/j.jen.2020.11.008
- Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022, HR 4355, 117th Cong (2021-2022). Accessed May 12, 2025. https://www.congress.gov/bill/117th-congress/house-bill/4355?
- Altun O, Almuhayawi M, Lüthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the New BacT/ Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol. 2016;54(4):1148-1151. doi:10.1128/JCM.03362-15
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. doi:10.1128/CMR.00062-05
- Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):1021-1024. doi:10.1128/JCM.02162-08
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central lineassociated bloodstream infections. Am J Infect Control. 2015;43(11):1222-1237. doi:10.1016/j.ajic.2015.06.030
- Callado GY, Lin V, Thottacherry E, et al. Diagnostic stewardship: a systematic review and meta-analysis of blood collection diversion devices used to reduce blood culture contamination and improve the accuracy of diagnosis in clinical settings. Open Forum Infect Dis. 2023;10(9):ofad433. doi:10.1093/ofid/ofad433
- Patton RG, Schmitt T. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol. 2010;48:4501-4503. doi:10.1128/JCM.00910-10
- Kurin. Clinical evidence: published Kurin studies. 2024. Accessed May 12, 2025. https://www.kurin.com/studies
- Leipheimer JM, Balter ML, Chen AI, et al. First-in-human evaluation of a hand-held automated venipuncture device for rapid venous blood draws. Technology (Singap World Sci). 2019;7(3-4):98-107. doi:10.1142/S2339547819500067?
- Chela HK, Vasudevan A, Rojas-Moreno C, Naqvi SH. Approach to positive blood cultures in the hospitalized patient: a review. Mo Med. 2019;116(4):313-317.
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697. doi:10.3389/fmicb.2016.00697
- Doern GV, Carroll KC, Diekema DJ, et al. Practical guidance for clinical microbiology laboratories: a comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clin Microbiol Rev. 2019;33:e00009-19. doi:10.1128/CMR.00009-19
- Wilson ML, Kirn Jr TJ, Antonara S, et al. Clinical and Laboratory Standards Institute Guideline M47—Principles and Procedures for Blood Cultures. Clinical and Laboratory Standards Institute. April 22, 2022. Accessed May 21, 2025. https://clsi.org/shop/standards/m47/
- Hancock JA, Campbell S, Jones MM, Wang-Rodriguez J, VHA Microbiology SME Workgroup, Klutts JS. Development and validation of a standardized blood culture contamination definition and metric dashboard for a large health care system. Am J Clin Pathol. 2023;160(3):255-260. doi:10.1093/ajcp/aqad044
- Shinozaki T, Deane RS, Mazuzan JE Jr, Hamel AJ, Hazelton D. Bacterial contamination of arterial lines. A prospective study. JAMA. 1983;249(2):223-225.
- Al Mohajer M, Lasco T. The impact of initial specimen diversion systems on blood culture contamination. Open Forum Infect Dis. 2023;10:ofad182. doi:10.1093/ofid/ofad182
- Arenas M, Boseman GM, Coppin JD, Lukey J, Jinadatha C, Navarathna DH. Asynchronous testing of 2 specimen-diversion devices to reduce blood culture contamination: a single-site product supply quality improvement project. J Emerg Nurs. 2021;47(2):256-264. e6. doi:10.1016/j.jen.2020.11.008
- Military Construction, Veterans Affairs, and Related Agencies Appropriations Act, 2022, HR 4355, 117th Cong (2021-2022). Accessed May 12, 2025. https://www.congress.gov/bill/117th-congress/house-bill/4355?
- Altun O, Almuhayawi M, Lüthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the New BacT/ Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol. 2016;54(4):1148-1151. doi:10.1128/JCM.03362-15
- Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. doi:10.1128/CMR.00062-05
- Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):1021-1024. doi:10.1128/JCM.02162-08
- Garcia RA, Spitzer ED, Beaudry J, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central lineassociated bloodstream infections. Am J Infect Control. 2015;43(11):1222-1237. doi:10.1016/j.ajic.2015.06.030
- Callado GY, Lin V, Thottacherry E, et al. Diagnostic stewardship: a systematic review and meta-analysis of blood collection diversion devices used to reduce blood culture contamination and improve the accuracy of diagnosis in clinical settings. Open Forum Infect Dis. 2023;10(9):ofad433. doi:10.1093/ofid/ofad433
- Patton RG, Schmitt T. Innovation for reducing blood culture contamination: initial specimen diversion technique. J Clin Microbiol. 2010;48:4501-4503. doi:10.1128/JCM.00910-10
- Kurin. Clinical evidence: published Kurin studies. 2024. Accessed May 12, 2025. https://www.kurin.com/studies
- Leipheimer JM, Balter ML, Chen AI, et al. First-in-human evaluation of a hand-held automated venipuncture device for rapid venous blood draws. Technology (Singap World Sci). 2019;7(3-4):98-107. doi:10.1142/S2339547819500067?
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Impact of Initial Specimen Diversion Technique on Blood Culture Contamination Rates
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis