User login
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
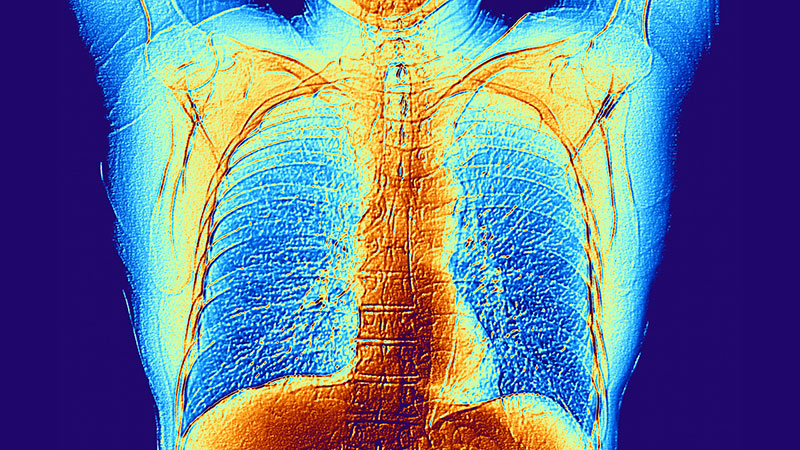
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
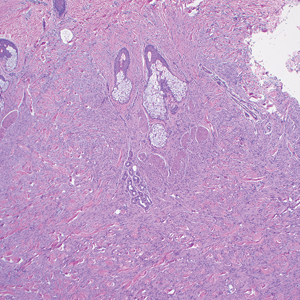
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
What About Stolen Valor is Actually Illegal?
What About Stolen Valor is Actually Illegal?
Memorial Day is the most solemn of all American military commemorations. It is the day when we honor those who sacrificed their lives so that their fellow citizens could flourish in freedom. At 3 PM, a grateful nation is called to observe 2 minutes of silence in remembrance of the heroes who died in battle or of the wounds they sustained in combat. Communities across the country will carry out ceremonies, lining national cemeteries with flags, holding patriotic parades, and conducting spiritual observances.1
Sadly, almost as long as there has been a United States, there has been a parallel practice dishonoring the uniform and deceiving veterans and the public alike known as stolen valor. Stolen valor is a persistent, yet strange, psychological behavior: individuals who never served in the US Armed Forces claim they have done heroic deeds for which they often sustained serious injuries in the line of duty and almost always won medals for their heroism.2 This editorial will trace the US legal history of stolen valor cases to provide the background for next month’s editorial examining its clinical and ethical aspects.
While many cases of stolen valor do not receive media attention, the experience of Sarah Cavanaugh, a former VA social worker who claimed to be a marine veteran who served in Iraq and Afghanistan, was the subject of the Deep Cover podcast series.3 Cavanaugh had claimed that an improvised explosive device blew up her Humvee, crushing her hip. Still she somehow was able to help her fellow Marines and earned the Bronze Star among other decorations for her heroism. That was not the only lie Cavanaugh told: she also told her friends and wife that she had advanced lung cancer due to burn pit exposure. In line with the best-worst of those who have stolen valor, her mastery of manipulation enabled her to become the commander of a local Veterans of Foreign Wars post. Using stolen identities and fraudulent documents, Cavanaugh was able to purloin veteran benefits, donated leave from other VA employees and money, and stole goods and services from various charitable organizations whose mission was to help wounded veterans and those struggling to adjust to civilian life. Before law enforcement unraveled her sordid tale, she misappropriated hundreds of thousands of dollars in VA benefits and donations and exploited dozens of generous veterans and compassionate civilians.4
Cavanaugh’s story was so sordidly compelling that I kept saying out loud to myself (and my spouse), “This has to be illegal.” The truth about stolen valor law is far more ambivalent and frustrating than I had anticipated or wanted. The first insult to my sense of justice was that lying about military service is not in itself illegal: you can pad your military resume with unearned decorations or impress a future partner or employer with your combat exploits without much fear of legal repercussions. The legal history of attempting to make stealing valor a crime has almost as many twists and turns as the fallacious narratives of military imposters and illustrates the uniquely American experiment in balancing freedom and fairness.
The Stolen Valor Act of 2005 made it a federal misdemeanor to wear, manufacture, or sell military decorations, or medals (Cavanaugh bought her medals online) without legal authorization. It also made it a crime to falsely represent oneself as having been the recipient of a decoration, medical, or service badge that Congress or the Armed Forces authorized. There were even stiffer penalties if the medal was a Silver Star, Distinguished Service Cross, US Air Force or US Navy Cross, or Purple Heart. Punishments include fines and imprisonment. The stated legislative purpose was to prohibit fraud that devalued military awards and the dignity of those who legitimately earned them.5
Next comes a distinctly American reaction to the initial Congressional attempt to protect the legacy of those who served—a lawsuit. Xavier Alvarez was an official on a California district water board claimed to be a 25-year veteran of the US Marine Corps wounded in combat and received the Congressional Medal of Honor. The Federal Bureau of Investigation exposed the lie and instead of the nation’s highest honor, Alvarez was the first to be convicted under the Stolen Valor Act of 2005. Alvarez appealed the decision, ironically claiming the law violated his free speech rights. The case landed in the Supreme Court, which ruled that the Stolen Valor Act did indeed violate the Free Speech Clause of the First Amendment. The majority opinion found the Act as passed was too encompassing of all speech and needed to target only cases in which false statements resulted in actual harm.6
The Stolen Valor Act of 2013 amends the criminal code regarding fraudulent claims about military service to include those who don’t only lie but also profit from it, as Cavanaugh did. The revised act specifically focuses on individuals who claim to have earned military honors for the intended purpose of obtaining money, property, or any other tangible benefit.7
Despite the complicated nature of Stolen Valor Law, it did prevail in Cavanaugh’s case. A US District Court Judge in Rhode Island found her guilty of stolen valor in all its permutations, along with identity theft of other veterans’ military and medical records and fraud in obtaining benefits and services intended for real veterans. Cavanaugh was sentenced to 70 months in federal prison, 3 years of supervised release, ordered to pay $284,796.82 in restitution, and to restore 261 hours of donated leave to the federal government, charitable organizations, and good Samaritans she duped and swindled.8
The revised law under which Cavanaugh was punished lasted 10 years until another classically American ethical concern—privacy—motivated additional legislative effort. A 2023/2024 US House of Representatives proposal to amend the Stolen Valor Act would have strengthened the privacy protections afforded military records. It would have required the information to only be accessed with the permission of the individual who served or their family or through a Freedom of Information Act request. This would make the kind of journalistic and law enforcement investigations that eventually caught Cavanaugh in her lies far more laborious for false valor hunters while at the same time preventing unscrupulous inquiries into service members’ personal information. Advocates for free speech and defenders of military honor are both lobbying Congress; as of this writing the legislation has not been passed.9
As we close part 1 of this review of stolen valor, we return to Memorial Day. This day provides the somber recognition that without the brave men and women of integrity who died in defense of a democracy that promotes the political activity of its citizens, we would not even be able to have this debate over justice, freedom, and truth.
- US Department of Veterans Affairs. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed May 27, 2025. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- Home of Heroes. Stolen valor. Accessed May 27, 2025. https://homeofheroes.com/stolen-valor
- Halpern J. Deep cover: the truth about Sarah. May 2025. Accessed May 27, 2025. https://www.pushkin.fm/podcasts/deep-cover
- Stillwell B. The latest season of the ‘deep cover’ podcast dives into one of the biggest stolen valor cases ever. Military. com. May 22, 2025. Accessed May 27, 2025. https:// www.military.com/off-duty/2025/05/22/latest-season-of-deep-cover-podcast-dives-one-of-biggest-stolen-valor-cases-ever.html
- The Stolen Valor Act of 2005. Pub L No: 109-437. 120 Stat 3266
- Alvarez v United States. 567 US 2012.
- The Stolen Valor Act of 2013. 18 USC § 704(b)
- US Attorney’s Office, District of Rhode Island. Rhode Island woman sentenced to federal prison for falsifying military service; false use of military medals; identify theft, and fraudulently collecting more than $250,000, in veteran benefits and charitable contributions. March 14, 2023. Accessed May 27, 2025. https://www.justice.gov/usao-ri/pr/rhode-island-woman-sentenced-federal-prison-falsifying-military-service-false-use
- Armed Forces Benefit Association. Stolen Valor Act: all you need to know. February 21, 2024. Accessed May 27, 2025. https://www.afba.com/military-life/active-duty-and-veterans/stolen-valor-act-all-you-need-to-know/
Memorial Day is the most solemn of all American military commemorations. It is the day when we honor those who sacrificed their lives so that their fellow citizens could flourish in freedom. At 3 PM, a grateful nation is called to observe 2 minutes of silence in remembrance of the heroes who died in battle or of the wounds they sustained in combat. Communities across the country will carry out ceremonies, lining national cemeteries with flags, holding patriotic parades, and conducting spiritual observances.1
Sadly, almost as long as there has been a United States, there has been a parallel practice dishonoring the uniform and deceiving veterans and the public alike known as stolen valor. Stolen valor is a persistent, yet strange, psychological behavior: individuals who never served in the US Armed Forces claim they have done heroic deeds for which they often sustained serious injuries in the line of duty and almost always won medals for their heroism.2 This editorial will trace the US legal history of stolen valor cases to provide the background for next month’s editorial examining its clinical and ethical aspects.
While many cases of stolen valor do not receive media attention, the experience of Sarah Cavanaugh, a former VA social worker who claimed to be a marine veteran who served in Iraq and Afghanistan, was the subject of the Deep Cover podcast series.3 Cavanaugh had claimed that an improvised explosive device blew up her Humvee, crushing her hip. Still she somehow was able to help her fellow Marines and earned the Bronze Star among other decorations for her heroism. That was not the only lie Cavanaugh told: she also told her friends and wife that she had advanced lung cancer due to burn pit exposure. In line with the best-worst of those who have stolen valor, her mastery of manipulation enabled her to become the commander of a local Veterans of Foreign Wars post. Using stolen identities and fraudulent documents, Cavanaugh was able to purloin veteran benefits, donated leave from other VA employees and money, and stole goods and services from various charitable organizations whose mission was to help wounded veterans and those struggling to adjust to civilian life. Before law enforcement unraveled her sordid tale, she misappropriated hundreds of thousands of dollars in VA benefits and donations and exploited dozens of generous veterans and compassionate civilians.4
Cavanaugh’s story was so sordidly compelling that I kept saying out loud to myself (and my spouse), “This has to be illegal.” The truth about stolen valor law is far more ambivalent and frustrating than I had anticipated or wanted. The first insult to my sense of justice was that lying about military service is not in itself illegal: you can pad your military resume with unearned decorations or impress a future partner or employer with your combat exploits without much fear of legal repercussions. The legal history of attempting to make stealing valor a crime has almost as many twists and turns as the fallacious narratives of military imposters and illustrates the uniquely American experiment in balancing freedom and fairness.
The Stolen Valor Act of 2005 made it a federal misdemeanor to wear, manufacture, or sell military decorations, or medals (Cavanaugh bought her medals online) without legal authorization. It also made it a crime to falsely represent oneself as having been the recipient of a decoration, medical, or service badge that Congress or the Armed Forces authorized. There were even stiffer penalties if the medal was a Silver Star, Distinguished Service Cross, US Air Force or US Navy Cross, or Purple Heart. Punishments include fines and imprisonment. The stated legislative purpose was to prohibit fraud that devalued military awards and the dignity of those who legitimately earned them.5
Next comes a distinctly American reaction to the initial Congressional attempt to protect the legacy of those who served—a lawsuit. Xavier Alvarez was an official on a California district water board claimed to be a 25-year veteran of the US Marine Corps wounded in combat and received the Congressional Medal of Honor. The Federal Bureau of Investigation exposed the lie and instead of the nation’s highest honor, Alvarez was the first to be convicted under the Stolen Valor Act of 2005. Alvarez appealed the decision, ironically claiming the law violated his free speech rights. The case landed in the Supreme Court, which ruled that the Stolen Valor Act did indeed violate the Free Speech Clause of the First Amendment. The majority opinion found the Act as passed was too encompassing of all speech and needed to target only cases in which false statements resulted in actual harm.6
The Stolen Valor Act of 2013 amends the criminal code regarding fraudulent claims about military service to include those who don’t only lie but also profit from it, as Cavanaugh did. The revised act specifically focuses on individuals who claim to have earned military honors for the intended purpose of obtaining money, property, or any other tangible benefit.7
Despite the complicated nature of Stolen Valor Law, it did prevail in Cavanaugh’s case. A US District Court Judge in Rhode Island found her guilty of stolen valor in all its permutations, along with identity theft of other veterans’ military and medical records and fraud in obtaining benefits and services intended for real veterans. Cavanaugh was sentenced to 70 months in federal prison, 3 years of supervised release, ordered to pay $284,796.82 in restitution, and to restore 261 hours of donated leave to the federal government, charitable organizations, and good Samaritans she duped and swindled.8
The revised law under which Cavanaugh was punished lasted 10 years until another classically American ethical concern—privacy—motivated additional legislative effort. A 2023/2024 US House of Representatives proposal to amend the Stolen Valor Act would have strengthened the privacy protections afforded military records. It would have required the information to only be accessed with the permission of the individual who served or their family or through a Freedom of Information Act request. This would make the kind of journalistic and law enforcement investigations that eventually caught Cavanaugh in her lies far more laborious for false valor hunters while at the same time preventing unscrupulous inquiries into service members’ personal information. Advocates for free speech and defenders of military honor are both lobbying Congress; as of this writing the legislation has not been passed.9
As we close part 1 of this review of stolen valor, we return to Memorial Day. This day provides the somber recognition that without the brave men and women of integrity who died in defense of a democracy that promotes the political activity of its citizens, we would not even be able to have this debate over justice, freedom, and truth.
Memorial Day is the most solemn of all American military commemorations. It is the day when we honor those who sacrificed their lives so that their fellow citizens could flourish in freedom. At 3 PM, a grateful nation is called to observe 2 minutes of silence in remembrance of the heroes who died in battle or of the wounds they sustained in combat. Communities across the country will carry out ceremonies, lining national cemeteries with flags, holding patriotic parades, and conducting spiritual observances.1
Sadly, almost as long as there has been a United States, there has been a parallel practice dishonoring the uniform and deceiving veterans and the public alike known as stolen valor. Stolen valor is a persistent, yet strange, psychological behavior: individuals who never served in the US Armed Forces claim they have done heroic deeds for which they often sustained serious injuries in the line of duty and almost always won medals for their heroism.2 This editorial will trace the US legal history of stolen valor cases to provide the background for next month’s editorial examining its clinical and ethical aspects.
While many cases of stolen valor do not receive media attention, the experience of Sarah Cavanaugh, a former VA social worker who claimed to be a marine veteran who served in Iraq and Afghanistan, was the subject of the Deep Cover podcast series.3 Cavanaugh had claimed that an improvised explosive device blew up her Humvee, crushing her hip. Still she somehow was able to help her fellow Marines and earned the Bronze Star among other decorations for her heroism. That was not the only lie Cavanaugh told: she also told her friends and wife that she had advanced lung cancer due to burn pit exposure. In line with the best-worst of those who have stolen valor, her mastery of manipulation enabled her to become the commander of a local Veterans of Foreign Wars post. Using stolen identities and fraudulent documents, Cavanaugh was able to purloin veteran benefits, donated leave from other VA employees and money, and stole goods and services from various charitable organizations whose mission was to help wounded veterans and those struggling to adjust to civilian life. Before law enforcement unraveled her sordid tale, she misappropriated hundreds of thousands of dollars in VA benefits and donations and exploited dozens of generous veterans and compassionate civilians.4
Cavanaugh’s story was so sordidly compelling that I kept saying out loud to myself (and my spouse), “This has to be illegal.” The truth about stolen valor law is far more ambivalent and frustrating than I had anticipated or wanted. The first insult to my sense of justice was that lying about military service is not in itself illegal: you can pad your military resume with unearned decorations or impress a future partner or employer with your combat exploits without much fear of legal repercussions. The legal history of attempting to make stealing valor a crime has almost as many twists and turns as the fallacious narratives of military imposters and illustrates the uniquely American experiment in balancing freedom and fairness.
The Stolen Valor Act of 2005 made it a federal misdemeanor to wear, manufacture, or sell military decorations, or medals (Cavanaugh bought her medals online) without legal authorization. It also made it a crime to falsely represent oneself as having been the recipient of a decoration, medical, or service badge that Congress or the Armed Forces authorized. There were even stiffer penalties if the medal was a Silver Star, Distinguished Service Cross, US Air Force or US Navy Cross, or Purple Heart. Punishments include fines and imprisonment. The stated legislative purpose was to prohibit fraud that devalued military awards and the dignity of those who legitimately earned them.5
Next comes a distinctly American reaction to the initial Congressional attempt to protect the legacy of those who served—a lawsuit. Xavier Alvarez was an official on a California district water board claimed to be a 25-year veteran of the US Marine Corps wounded in combat and received the Congressional Medal of Honor. The Federal Bureau of Investigation exposed the lie and instead of the nation’s highest honor, Alvarez was the first to be convicted under the Stolen Valor Act of 2005. Alvarez appealed the decision, ironically claiming the law violated his free speech rights. The case landed in the Supreme Court, which ruled that the Stolen Valor Act did indeed violate the Free Speech Clause of the First Amendment. The majority opinion found the Act as passed was too encompassing of all speech and needed to target only cases in which false statements resulted in actual harm.6
The Stolen Valor Act of 2013 amends the criminal code regarding fraudulent claims about military service to include those who don’t only lie but also profit from it, as Cavanaugh did. The revised act specifically focuses on individuals who claim to have earned military honors for the intended purpose of obtaining money, property, or any other tangible benefit.7
Despite the complicated nature of Stolen Valor Law, it did prevail in Cavanaugh’s case. A US District Court Judge in Rhode Island found her guilty of stolen valor in all its permutations, along with identity theft of other veterans’ military and medical records and fraud in obtaining benefits and services intended for real veterans. Cavanaugh was sentenced to 70 months in federal prison, 3 years of supervised release, ordered to pay $284,796.82 in restitution, and to restore 261 hours of donated leave to the federal government, charitable organizations, and good Samaritans she duped and swindled.8
The revised law under which Cavanaugh was punished lasted 10 years until another classically American ethical concern—privacy—motivated additional legislative effort. A 2023/2024 US House of Representatives proposal to amend the Stolen Valor Act would have strengthened the privacy protections afforded military records. It would have required the information to only be accessed with the permission of the individual who served or their family or through a Freedom of Information Act request. This would make the kind of journalistic and law enforcement investigations that eventually caught Cavanaugh in her lies far more laborious for false valor hunters while at the same time preventing unscrupulous inquiries into service members’ personal information. Advocates for free speech and defenders of military honor are both lobbying Congress; as of this writing the legislation has not been passed.9
As we close part 1 of this review of stolen valor, we return to Memorial Day. This day provides the somber recognition that without the brave men and women of integrity who died in defense of a democracy that promotes the political activity of its citizens, we would not even be able to have this debate over justice, freedom, and truth.
- US Department of Veterans Affairs. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed May 27, 2025. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- Home of Heroes. Stolen valor. Accessed May 27, 2025. https://homeofheroes.com/stolen-valor
- Halpern J. Deep cover: the truth about Sarah. May 2025. Accessed May 27, 2025. https://www.pushkin.fm/podcasts/deep-cover
- Stillwell B. The latest season of the ‘deep cover’ podcast dives into one of the biggest stolen valor cases ever. Military. com. May 22, 2025. Accessed May 27, 2025. https:// www.military.com/off-duty/2025/05/22/latest-season-of-deep-cover-podcast-dives-one-of-biggest-stolen-valor-cases-ever.html
- The Stolen Valor Act of 2005. Pub L No: 109-437. 120 Stat 3266
- Alvarez v United States. 567 US 2012.
- The Stolen Valor Act of 2013. 18 USC § 704(b)
- US Attorney’s Office, District of Rhode Island. Rhode Island woman sentenced to federal prison for falsifying military service; false use of military medals; identify theft, and fraudulently collecting more than $250,000, in veteran benefits and charitable contributions. March 14, 2023. Accessed May 27, 2025. https://www.justice.gov/usao-ri/pr/rhode-island-woman-sentenced-federal-prison-falsifying-military-service-false-use
- Armed Forces Benefit Association. Stolen Valor Act: all you need to know. February 21, 2024. Accessed May 27, 2025. https://www.afba.com/military-life/active-duty-and-veterans/stolen-valor-act-all-you-need-to-know/
- US Department of Veterans Affairs. The difference between Veterans Day and Memorial Day. October 30, 2023. Accessed May 27, 2025. https://news.va.gov/125549/difference-between-veterans-day-memorial-day/
- Home of Heroes. Stolen valor. Accessed May 27, 2025. https://homeofheroes.com/stolen-valor
- Halpern J. Deep cover: the truth about Sarah. May 2025. Accessed May 27, 2025. https://www.pushkin.fm/podcasts/deep-cover
- Stillwell B. The latest season of the ‘deep cover’ podcast dives into one of the biggest stolen valor cases ever. Military. com. May 22, 2025. Accessed May 27, 2025. https:// www.military.com/off-duty/2025/05/22/latest-season-of-deep-cover-podcast-dives-one-of-biggest-stolen-valor-cases-ever.html
- The Stolen Valor Act of 2005. Pub L No: 109-437. 120 Stat 3266
- Alvarez v United States. 567 US 2012.
- The Stolen Valor Act of 2013. 18 USC § 704(b)
- US Attorney’s Office, District of Rhode Island. Rhode Island woman sentenced to federal prison for falsifying military service; false use of military medals; identify theft, and fraudulently collecting more than $250,000, in veteran benefits and charitable contributions. March 14, 2023. Accessed May 27, 2025. https://www.justice.gov/usao-ri/pr/rhode-island-woman-sentenced-federal-prison-falsifying-military-service-false-use
- Armed Forces Benefit Association. Stolen Valor Act: all you need to know. February 21, 2024. Accessed May 27, 2025. https://www.afba.com/military-life/active-duty-and-veterans/stolen-valor-act-all-you-need-to-know/
What About Stolen Valor is Actually Illegal?
What About Stolen Valor is Actually Illegal?
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.

STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
The landmark Crossing the Quality Chasm report from the National Academy of Medicine identified patient- centered care as essential to health care quality. The report defines patientcentered care as “respectful of and responsive to individual patient preferences, needs, and values.”1 Many health care systems, including the Veterans Health Administration, are transforming to a patient-centered model of care.2 The US Department of Veterans Affairs (VA) Whole Health System of Care initiative is a system-wide, cultural transformation. Within whole health, what matters most to the patient—including their preferences, needs, and values—is foundational to health care and meant to be essential in every clinical encounter. Whole health implementation includes a progressive rollout with health care practitioner (HCP) trainings across the VA.2
Shared decision-making (SDM) is a different but aligned patient-centered care concept. SDM is a process through which a decision or care plan, based on patients’ preferences, needs, and values, is made or developed.3-5 SDM is ideal in situations with equipoise (decisions with equivalent choices), individualized risks, and/or greater uncertainty of the net benefit, such as with lung cancer screening (LCS).3 SDM for LCS is required by the US Centers for Medicare and Medicaid Services and has been adopted by many US health care systems, including the VA.6,7 Early detection of lung cancer can reduce death by 20% at the population level.8 However, at the patient level there is wide variation in the risk of developing lung cancer and a range of potential harms.8 LCS follow-up procedures may be more invasive than with other cancer screenings. Thus, there is concern about the risk of false-positive results leading to unnecessary care or complications.8 Given this balance between benefit and harm and the differing patient value on the trade-offs of LCS, an individualized, patient-centered approach is essential when deciding whether LCS is the right choice for a specific patient.
Despite the importance of LCS SDM, observational studies have shown poor implementation in clinical encounters.9,10 HCP barriers include competing demands, limited time, lack of familiarity with and training in SDM, and beliefs biasing screening over no screening.11-13 Additionally, HCPs may assume that patients want them to make the decision. However, research has shown that patients actually want to be more involved in their health care decisions.14 One suggested strategy to overcome these barriers is aligning SDM for LCS within an organization’s broader patient-centered initiatives.15
This project sought to align the need for SDM for LCS and the broader VA whole health initiative as part of a multilevel strategy to implement SDM for LCS across Veterans Integrated Service Network (VISN) 1.16
This article addresses HCP-level barriers. HCPs targeted are those typically involved in LCS. The VA utilizes LCS coordinators (LCSCs) in both centralized or consult models (in which LCSCs are involved in all aspects of screening) and hybrid models (in which primary care practitioners and LCSCs are both engaged in LCS tasks). The goal of this program was to generate areas of conceptual alignment between SDM and whole health as a first step in integrating these VA initiatives. This work was conducted as a foundation for an SDM for lung cancer HCP training and consultation initiative.
ALIGNMENT PROCESS
We reviewed relevant literature and resources for SDM and whole health. In reviewing the SDM literature, we included a sample of the most widely cited literature on the topic, and focused primarily on the systematic review by Bomhof-Roordink et al.4,5,17,18 This review provided a synthesis of SDM elements across SDM models and identified 53 different elements clustered into 24 components.4 The most common components were present in at least half of all SDM published models, including: make the decision, patient preferences, tailor information, deliberate, create choice awareness, and learn about the patient. Bomhof-Roordink et al provided the guiding framework for this conceptualization of SDM because that study included the available recent published SDM models.4
Second, published literature on VA whole health along with supplemental promotional and training materials were reviewed. The whole health materials included 2 sets of training slides developed for VA HCPs (available to VA employees): Implementing Whole Health in Clinical Care, which is focused on HCPs’ work with patients, and Whole Health for You and Me, which is about HCPs’ personal well-being.19 We also reviewed a publication describing the history of whole health and patient-facing online whole health tools.2,19
Each document was reviewed for key elements related to SDM, patient-centered care, and whole health. Using the 53 elements identified by Bomhof-Roordink et al, we reviewed and compared each element to the whole health materials to create the integrated model of SDM and whole health. We iteratively discussed and organized the elements until we reached consensus.
SDM and Whole Health Alignment
We created an integrated model of SDM for LCS within the context of the VA whole health initiative. This integrated model is directed at HCPs who would likely engage patients in discussions of LCS, including primary care practitioners and nurse coordinators. The model includes 3 steps for HCPs to follow that align SDM within whole health: (1) frame the conversation and partner with the patient; (2) share clinical perspective and elicit patient values; and (3) deliberate and decide together. For each step, the SDM elements, whole health elements, and integration of SDM and whole health are provided. Table 1 provides an overview of the similarities and differences between SDM and whole health. Example phrases that merge SDM and whole health for HCPs to use in patient conversations about LCS are included in Table 2.


STEP 1. FRAME THE CONVERSATION AND PARTNER WITH THE PATIENT
Shared decision-making. Traditional SDM literature includes an initial step of letting patients know that there is a choice to be made between ≥ 2 clinical options.4 Ancillary elements of this first step include asking patients their preferences about the degree to which they want to be involved in SDM and about how they like to receive information (eg, verbal, written, video). These steps open the SDM conversation and ensure the patient and HCP are on the same page before moving forward. For example, the US Agency for Healthcare Research and Quality SHARE model’s first step is for HCPs to communicate that choices exist and to invite the patient to be involved in decisions.20 Similarly, Elwyn’s 3-step SDM model begins with establishing that a choice exists and inviting patient input on making that choice.17
Whole health. Patients are encouraged to play an active role in their health care. Through whole health programs such as Taking Charge of My Life and Health, patients explore their values and set self-care goals.21 HCP whole health trainings teach and reinforce communication skills, including SDM, listening skills, and motivational interviewing.19
Shared decision-making/whole health integration. SDM and whole health both prioritize respect, compassion, and patients’ expertise. They focus on the patient-HCP relationship with an emphasis on fostering egalitarian interactions. HCPs frame the SDM conversation and partner with the patient so they know what to expect and who will be involved. This conversation is framed from the outset as a collaborative discussion. HCPs empower the patient to play an active role in decision-making and help them understand why their engagement is critical.
STEP 2. SHARE CLINICAL PERSPECTIVE AND ELICIT PATIENT VALUES
Shared decision-making. HCPs share clinical perspective on LCS tailored to individual patients while explicitly inviting the patient to share their preferences and values when thinking about whether to undergo LCS. HCPs give a balanced description of LCS, including the benefits and harms, tailored to the patient’s unique information needs and questions. Sharing clinical perspective also includes describing treatment options, the most common element across SDM models.4 Decision aids, which provide unbiased information and include a values clarification exercise, may be helpful in sharing clinical perspectives and clarifying patient values related to the trade-offs of LCS.22 For example, the VA National Center for Health Promotion and Disease Prevention developed a LCS decision aid to be used for SDM for LCS.
Whole health. The conversation shifts from “What is the matter with you?” to “What matters to you?” starting with the patient’s goals and priorities rather than disease prevention, diagnosis, and treatment.2 Several whole health tools exist, including the Personal Health Inventory, used to identify what matters most to patients and understand their current well-being and self-care.23 Using the inventory, the patient and their health care team develop the patient’s personal health plan.24 Additionally, whole health trains HCPs to reflect on their own attitudes and biases when providing clinical care.
Shared decision-making/whole health integration. The LCS conversation can build on other whole health-related conversations with a HCP or other team members. HCPs can reference the patient’s personal health plan for documentation of the patient’s preferences, values, and goals in the electronic medical record. During this process, HCPs can give space for patients to discuss factors in their life and experiences that impact their perspective and decision-making. For example, patient concerns could be explored here, including fear of a cancer diagnosis, stigma around smoking, and fears around the screening and/or treatment process. HCPs may ask, “What matters most to you when making this decision?” Finally, by sharing clinical information, HCPs will focus on patient values to help overcome their own biases toward a desire for LCS. HCPs, similar to the rest of the US public, tend to hold highly favorable attitudes toward cancer screening as well as misconceptions about the magnitude of benefits from screening.13
STEP 3. DELIBERATE AND DECIDE TOGETHER
Shared decision-making. Decision-making is almost always considered the last SDM step.4 In the final step, the patient and HCP discuss the options (ie, to screen or not to screen) considering the patient’s values and preferences, and patients decide with their HCP whether they will undergo LCS. Patients may decide they need more time to think about these options. As part of deliberation, HCPs assess what other information patients may need to arrive at a decision. Family members, friends, or peers may be included in making the final decision.
Whole health. In Whole health, decisions also may include the entire health care team and other individuals important to the patient (eg, family, friends). Integration across different health care settings is also considered a key whole health element. Finally, whole health focuses on long-term relationships with patients; thus, the LCS SDM process is situated within longer term relationship building and patient empowerment, both of which will facilitate partnering with the patient in future conversations about other decisions.
Shared decision-making/whole health integration. Both SDM and whole health emphasize partnership with the patient in making a final decision. There is also focus on decision-making as an ongoing process. Deciding whether LCS is the best choice might include naming and addressing emotions, voicing questions not raised, and exploring whether screening fits the patient’s goals, values, and life context. HCPs may give guidance, but patients retain the authority to make decisions. The goal is to empower patients to know that the only right decision is the one right for them and they will be supported.
Limitations
This article describes a VA practice program and was not a formal research study. Further work is needed to evaluate the presented strategies. Additionally, we did not conduct a systematic literature review and thus elements of SDM and whole health may not be exhaustive.
CONCLUSIONS
This article describes the alignment of 2 distinct VA initiatives, whole health and SDM for LCS. The goal was to reduce known barriers to SDM, such as competing demands, limited time, and lack of familiarity with and training in SDM.11-13 These concepts are well aligned. This integrated model is the first step in informing the development of a HCP training program and materials as part of a multilevel strategy that our team is using to implement SDM for LCS in VISN 1.16 The final training and materials resulting from this work were delivered to LCSCs in 3 ways: (1) a series of 3 interactive group training sessions, including didactic elements, role play, and time for open discussion; (2) 1-on-1 academic detailing; and (3) educational handouts. In academic detailing, a member of the research team trained in academic detailing met virtually with each nurse coordinator, identified that individual’s barriers to SDM, and used the training materials to highlight messages to overcome those barriers; follow-up calls provided a forum for discussing progress and overcoming additional challenges. Although this article focused specifically on whole health and SDM, the conceptual alignment process strategy can be applied to other implementations of multiple initiatives.
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
- Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. The National Academies Press; 2001. doi:10.17226/10027
- Bokhour BG, Haun JN, Hyde J, Charns M, Kligler B. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295- 300. doi:10.1097/MLR.0000000000001316
- Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4:75. doi:7510.1186/1748-5908-4-75
- Bomhof-Roordink H, Gärtner FR, Stiggelbout AM, Pieterse AH. Key components of shared decision making models: a systematic review. BMJ Open. 2019;9:e031763. doi:10.1136/bmjopen-2019-031763
- Charles C, Gafni A, Whelan T. Decision-making in the physician- patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651-661. doi:10.1016/s0277-9536(99)00145-8
- Moyer VA; US Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330- 338. doi:10.7326/m13-2771
- Centers for Medicare & Medicaid Services. Screening for lung cancer with low dose computed tomography (LDCT). February 10, 2022. Accessed February 7, 2025. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=304
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. doi:10.1056/NEJMoa1102873
- Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153:1004-1015. doi:10.1016/j.chest.2017.10.013
- Nishi SPE, Lowenstein LM, Mendoza TR, et al. Shared decision-making for lung cancer screening: how well are we “sharing”? Chest. 2021;160:330-340. doi:10.1016/j.chest.2021.01.041
- Wiener RS, Koppelman E, Bolton R, et al. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33:1035-1042. doi:10.1007/s11606-018-4350-9
- Melzer AC, Golden SE, Ono SS, Datta S, Triplette M, Slatore CG. “We just never have enough time”: clinician views of lung cancer screening processes and implementation. Ann Am Thorac Soc. 2020. doi:10.1513/AnnalsATS.202003-262OC
- Schwartz LM, Woloshin S, Fowler FJ Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291:71-78. doi:10.1001/jama.291.1.71
- Lown BA, Rosen J, Marttila J. An agenda for improving compassionate care: a survey shows about half of patients say such care is missing. Health Aff (Millwood). 2011;30:1772-1778. doi:10.1377/hlthaff.2011.0539
- Scholl I, LaRussa A, Hahlweg P, Kobrin S, Elwyn G. Organizational- and system-level characteristics that influence implementation of shared decision-making and strategies to address them - a scoping review. Implement Sci. 2018;13:40. doi:10.1186/s13012-018-0731-z
- Khanna A, Fix GM, Anderson E, et al. Towards a framework for patient-centred care coordination: a scoping review protocol. BMJ Open. 2022;12:e066808. doi:10.1136/bmjopen-2022-066808
- Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. doi:10.1136/bmj.j4891
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301-312. doi:10.1016/j.pec.2005.06.010
- Whole Health. US Department of Veterans Affairs. Accessed April 14, 2025. https://www.va.gov/wholehealth/
- Agency for Healthcare Research and Quality. The SHARE approach. Accessed April 14, 2025. https://www.ahrq.gov/health-literacy/professional-training/shared-decision/index.html
- Abadi MH, Barker AM, Rao SR, Orner M, Rychener D, Bokhour BG. Examining the impact of a peer-led group program for veteran engagement and well-being. J Altern Complement Med. 2021;27:S37-S44. doi:10.1089/acm.2020.0124
- Stacey D, Lewis KB, Smith M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2024;1:CD001431. doi:10.1002/14651858.CD001431.pub6
- US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Personal health inventory. Revised April 2019. Accessed April 14, 2025. https://www.va.gov/wholehealth/docs/10-773_PHI_July2019_508.pdf
- US Department of Veterans Affairs. Build your personal health plan. Updated July 24, 2024. Accessed April 14, 2025. https://www.va.gov/wholehealth/phi.asp
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
When Patient-Centered Care Initiatives Align: Integrating VA Whole Health and Shared Decision-Making for Lung Cancer Screening
Painful Flesh-Colored Nodule on the Shoulder
Painful Flesh-Colored Nodule on the Shoulder
THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6
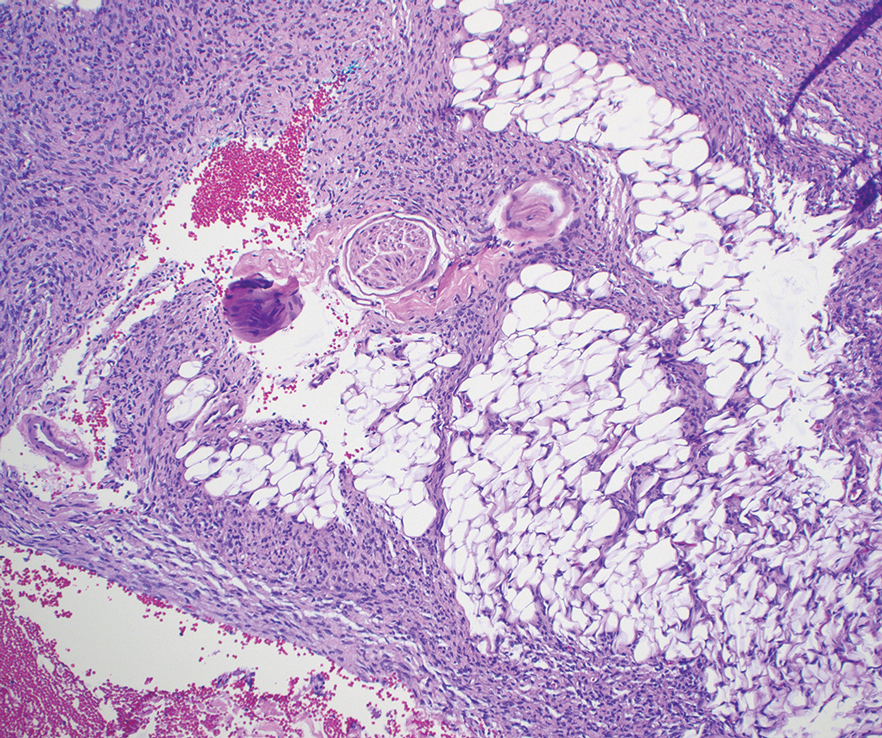
Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.
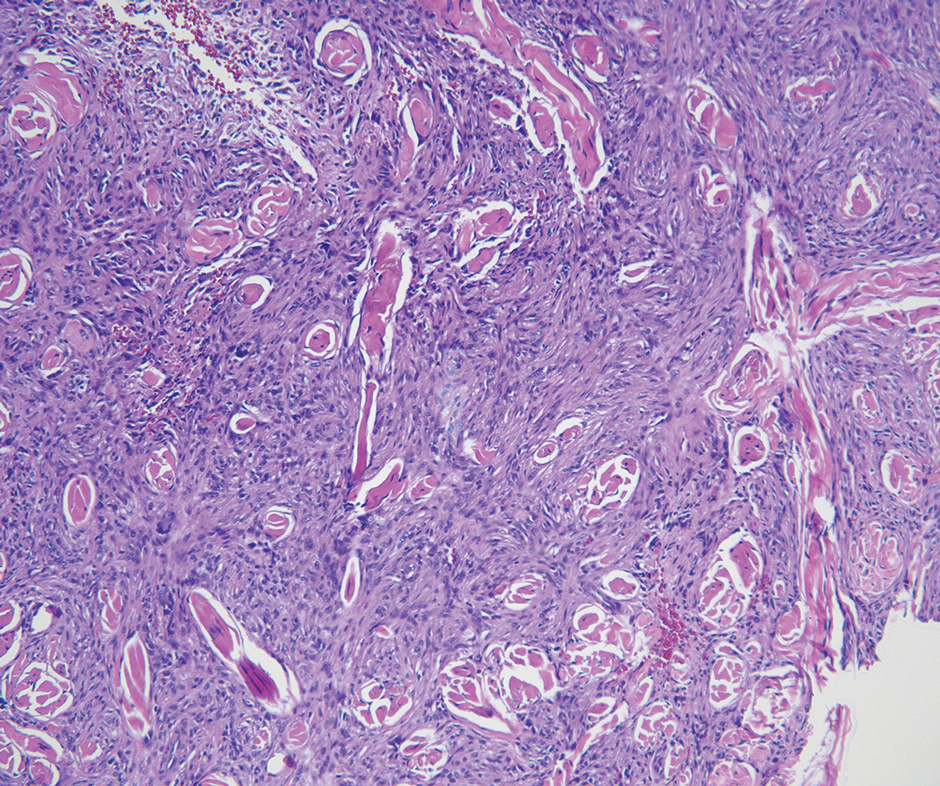
Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11
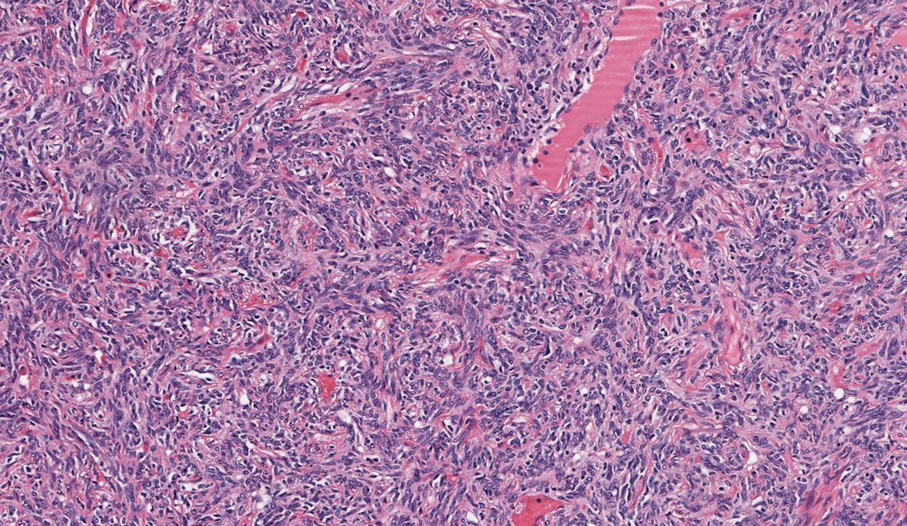
Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13
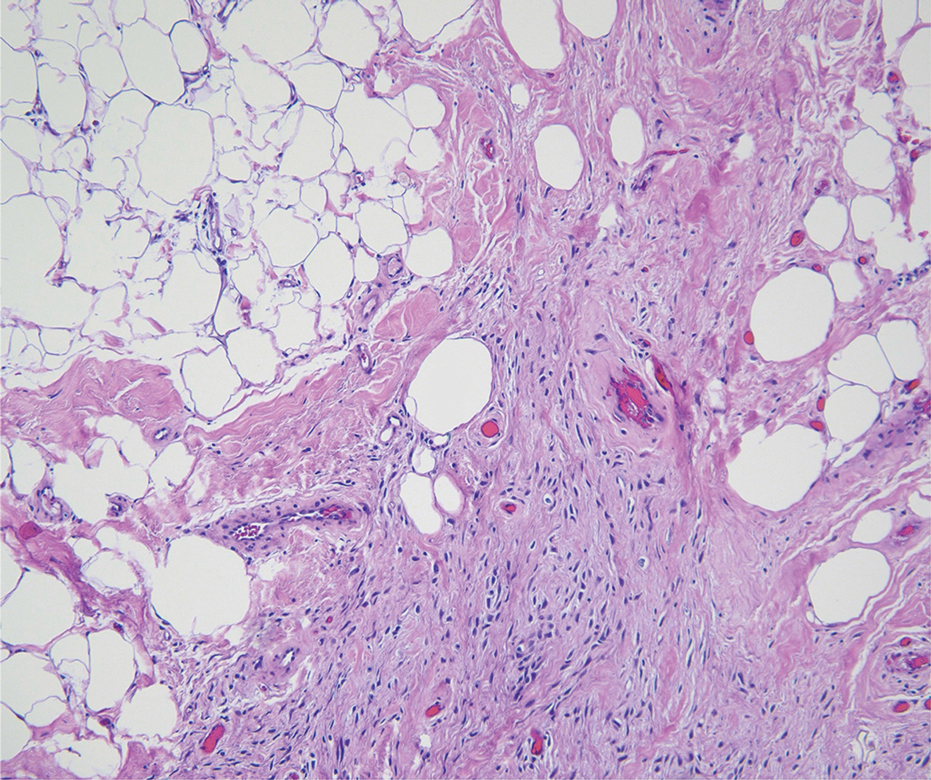
Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16
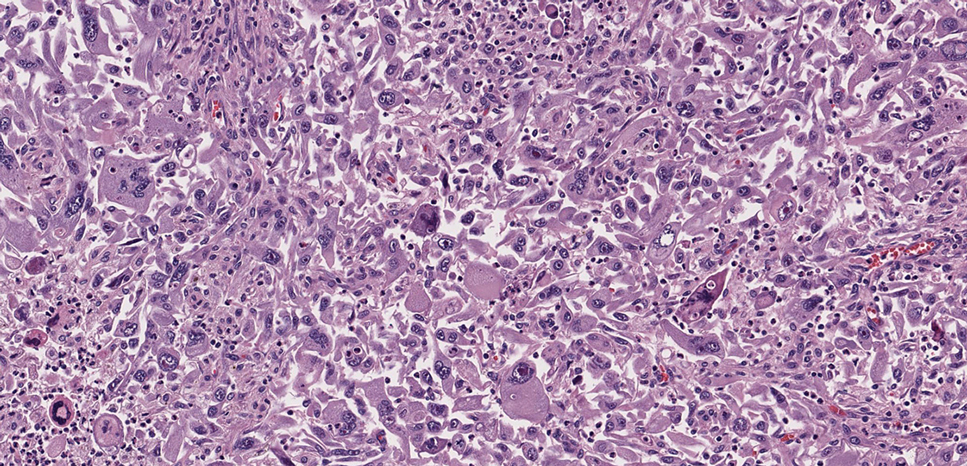
- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6

Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.

Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11

Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13

Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16

THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6

Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.

Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11

Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13

Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16

- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
Painful Flesh-Colored Nodule on the Shoulder
Painful Flesh-Colored Nodule on the Shoulder
A 26-year-old man with no notable medical history presented to the dermatology clinic with an inconspicuous, painful, raised lesion on the right posterior shoulder of 6 months’ duration. The patient reported that the lesion was tender to light palpation and bothersome in his daily activities. Physical examination revealed a firm, flesh-colored, 1.8-cm nodule with no erythema or pigmentation on the right shoulder. An elliptical excisional biopsy was performed and submitted for histologic evaluation.
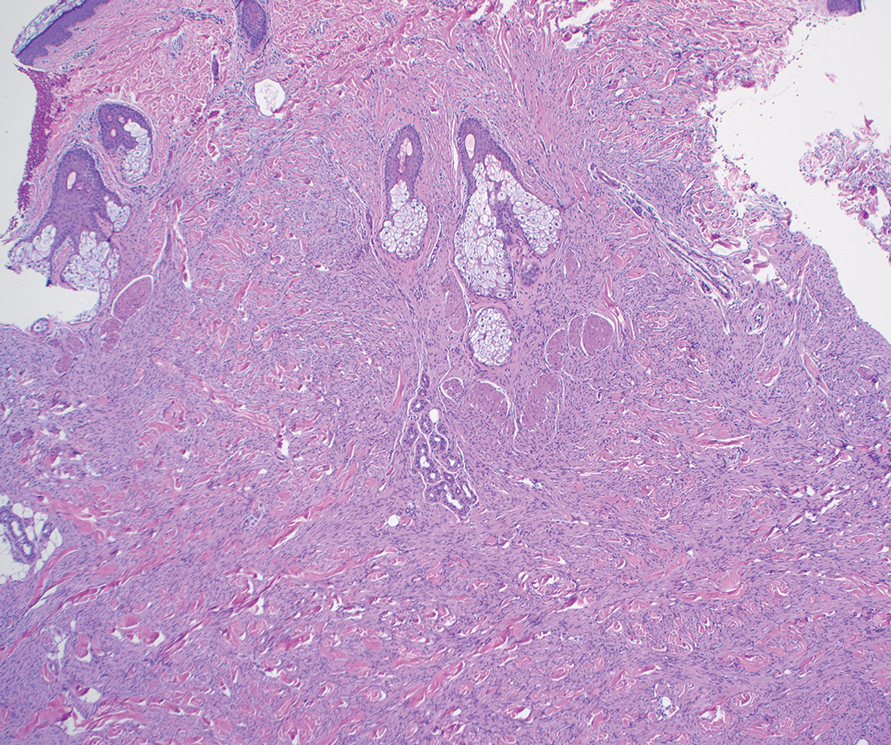
Measles Resurgence: A Dermatologist’s Guide
Measles Resurgence: A Dermatologist’s Guide
Measles, also known as rubeola, is a highly contagious paramyxovirus that has neared elimination in the United States since 2000 due to widespread adoption of the measles vaccine; however, measles recently has made a comeback, with outbreaks reported in more than 60 countries. In the United States, vaccine hesitancy coupled with decreasing vaccination rates, international travel to endemic areas, and decreased funding and resources for monitoring and immunization programs likely led to a re-emergence of measles cases.1,2 The resurgence of measles is troubling given its infectiousness and potential severity in at-risk populations. Since measles has a basic reproduction number of 12 to 18 (ie, 1 infected individual will on average infect 12 to 18 others3), it has the capacity to spread quickly. This is why, prior to the development of the measles vaccine in the 1960s, it was responsible for millions of deaths across the globe.
Prior to the introduction of the measles vaccine, both physicians and the public generally were aware of the signs and symptoms of measles due to its prevalence; however, since there have been so few cases in recent decades, images and descriptions of patients presenting with measles can be found only in textbooks, and many physicians are ill-prepared to diagnose the disease.4 In response to the recent surge in measles cases, dermatologists—who often are among the first medical professionals to encounter febrile patients with rashes—must be prepared to bridge this divide. Herein, we review the clinical signs, diagnostic approach, operational precautions, and public health responsibilities that dermatologists must relearn amid the current measles outbreak.
Background
Measles is primarily transmitted via respiratory droplets and may remain airborne for up to 2 hours.5 It also can be transmitted through direct contact with secretions such as mucus. Indirect transmission via fomites, while certainly plausible, is thought to be the least effective mechanism of transmission.6 Following exposure, the incubation period ranges from 7 to 21 days, during which the virus replicates asymptomatically before causing clinical disease.7 Herd immunity for measles requires 93% immunity in the population; public health agencies typically target greater than 95% immunity.8 Humans are the only reservoir for the measles virus, making eradication possible.
The road to eradication began with the introduction of the measles vaccine in 1963 and subsequent development of the combined measles-mumps-rubella (MMR) vaccine in 1971. As MMR is a live vaccine, 2 doses confer approximately 97% protection.9 The first dose is given at 12 to 15 months of age, and the second dose is given at 4 to 6 years of age. Immunity is considered lifelong, and the Centers for Disease Control and Prevention and the World Health Organization do not recommend routine measles boosters for individuals who have completed the primary 2-dose series.10,11
Widespread vaccination led to a dramatic reduction in incidence, with many countries eliminating measles infections.7 The United States declared measles eliminated in 2000, with confirmed cases between 2000 and 2020 ranging from 37 to 1282.12 Vaccination progress stalled in the late 1990s due to vaccine hesitancy resulting from (subsequently debunked) reports of an association between the MMR vaccine and autism.13 Despite efforts to correct this misinformation, many patients continue to espouse these concerns.
Recognizing Measles: Clinical Presentation
Measles, which most often manifests in childhood but also can occur in adults, follows a distinctive clinical course. The prodromal phase is characterized by high fever, cough, coryza (nasal congestion), and conjunctivitis— conjunctivitis—the 3 “Cs” that serve as early warning signs of the disease. Patients may develop small white macules on the buccal mucosa known as Koplik spots (phonetically the fourth “C”), which appear just before the rash. Three to 5 days after the onset of systemic symptoms, patients will develop a classic morbilliform exanthem. In some cases, the exanthem manifests on the head and neck (Figure 1)—first behind the ears and along the hairline, then spreading caudally to the trunk and extremities. The lesions may become confluent, with patients presenting with diffuse erythema. The exanthem fades over several days to weeks, often accompanied by superficial desquamation.14
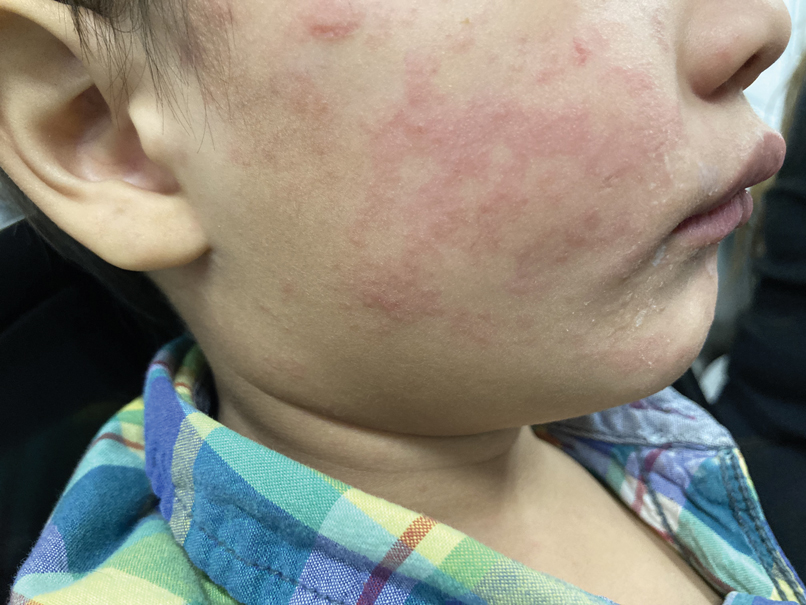
Given the nonspecificity of the early symptoms of measles, a high index of suspicion is needed for patients presenting with a febrile illness and a morbilliform eruption (Figure 2). Consideration of MMR vaccination status, exposure history, and local outbreak patterns can help guide risk stratification and the need for testing. Immunocompromised individuals, including those receiving immunosuppressive therapies for dermatologic conditions, may present atypically, lacking the prototypical exanthem or displaying milder signs and further complicating the diagnosis.15 The differential diagnosis for measles includes a drug reaction or other viral exanthem, and a detailed history may help elucidate the culprit.
Evaluation and Diagnosis
Definitive diagnosis of measles relies on both molecular and serologic testing. Nasopharyngeal swabs for measles polymerase chain reaction testing are obtained using synthetic (noncotton) swabs placed in a viral transport medium. Serum samples also should be collected for measles IgM and IgG antibody testing. Importantly, measles is a reportable illness, and testing may be coordinated with local departments of health.
Determining a patient’s immune status may be important for certain populations. Patients with documented 2-dose MMR vaccination, positive measles IgG serology, or a prior confirmed measles infection are considered immune. While a positive measles IgG indicates immunity, a negative result in an exposed patient should prompt consideration of postexposure prophylaxis with intravenous immunoglobulin.
Many patients, specifically those presenting to dermatology, are taking immunomodulatory or immunosuppressive medications—a contraindication for vaccination with the live MMR vaccine. At the time of publication, there was a single reported case of a patient taking a tumor necrosis factor α inhibitor for rheumatoid arthritis who had acquired measles.16 While the benefits of titer assessment in patients who are starting or continuing immunomodulatory therapy are not known and currently it is not recommended by the Centers for Disease Control and Prevention, dermatologists might consider checking MMR titers and vaccinating (or referring for vaccination) nonimmune patients.17
Infection Control
Early identification of a suspected measles case is paramount. Patients in whom measles is a possibility should be isolated as quickly as possible, and the patient and accompanying caregivers should be masked. Clinical staff should don appropriate personal protective equipment, including an N95 mask. Coordination with the local department of health must occur as soon as measles is suspected.
If testing is an option in the outpatient setting, a nasopharyngeal viral swab and serologic titers can be obtained. If testing is not available on site, patients should be sent to appropriate care facilities; prenotification is critical to prevent nosocomial outbreaks. Patients should be encouraged to isolate and avoid public spaces and/or public transport for 4 days following development of an exanthem.18 Offices should develop clinical protocols for suspected measles cases with training for clinical and office staff.
Final Thoughts
As measles outbreaks become more prevalent, it is incumbent upon physicians to remind ourselves of the signs and symptoms of this largely eliminated disease so that we may pursue early detection and intervention strategies. The primary cutaneous manifestations of measles make dermatologists critical to early recognition and containment efforts. Dermatologists should prepare for the arrival of patients with measles by maintaining vigilance for the classic signs of the disease, implementing stringent isolation protocols, verifying patient immunity when appropriate, and partnering closely with public health authorities.
More broadly, efforts to contain and re-establish a paradigm for eliminating measles outbreaks must be pursued. Encouraging vaccination and developing programs to help combat misinformation surrounding vaccines are critical to this effort. In an era of vaccine hesitancy, measles is a multidisciplinary public health emergency. Dermatologists must remain ready.
- Bedford H, Elliman D. Measles rates are rising again. BMJ. 2024;384.
- Harris E. Measles outbreaks grow amid declining vaccination rates. JAMA. 2023;330:2242.
- Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:E420-E428.
- Swartz MK. Measles: public and professional education. J Pediatr Health Care. 2019;33:367-368.
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for measles in healthcare settings. Accessed April 27, 2025. https://www.cdc.gov/infection-control/hcp/measles/
- Moss WJ, Griffin DE, Feinstone WH. Measles. In: Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2009: 551-565.
- Moss WJ. Measles. Lancet. 2017;390:2490-2502.
- Maintain the vaccination coverage level of 2 doses of the MMR vaccine for children in kindergarten— IID04. Healthy People 2030 website. Accessed May 6, 2025. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/maintain-vaccination-coverage-level-2-doses-mmr-vaccine-children-kindergarten-iid-04
- Franconeri L, Antona D, Cauchemez S, et al. Two-dose measles vaccine effectiveness remains high over time: a French observational study, 2017–2019. Vaccine. 2023;41:5797-5804.
- World Health Organization. Measles. Accessed May 8, 2025. https:// www.who.int/news-room/fact-sheets/detail/measles
- Centers for Disease Control and Prevention. Measles vaccine recommendations. Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Accessed May 6, 2025. https://www.cdc.gov/measles/cases-outbreaks.html
- Dyer C. Lancet retracts Wakefield’s MMR paper. BMJ. 2010;340.
- Alves Graber EM, Andrade FJ, Bost W, et al. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610-615.
- Kaplan LJ, Daum RS, Smaron M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237-1241.
- Takahashi E, Kurosaka D, Yoshida K, et al. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Japanese J Clin Immunol. 2010;33:37-41.
- Worth A, Waldman RA, Dieckhaus K, et al. Art of prevention: our approach to the measles-mumps-rubella vaccine in adult patients vaccinated against measles before 1968 on biologic therapy for the treatment of psoriasis. Int J Womens Dermatol. 2019;6:94.
- Centers for Disease Control and Prevention. Clinical overview of measles (rubeola). Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/clinical-overview/index.html
Measles, also known as rubeola, is a highly contagious paramyxovirus that has neared elimination in the United States since 2000 due to widespread adoption of the measles vaccine; however, measles recently has made a comeback, with outbreaks reported in more than 60 countries. In the United States, vaccine hesitancy coupled with decreasing vaccination rates, international travel to endemic areas, and decreased funding and resources for monitoring and immunization programs likely led to a re-emergence of measles cases.1,2 The resurgence of measles is troubling given its infectiousness and potential severity in at-risk populations. Since measles has a basic reproduction number of 12 to 18 (ie, 1 infected individual will on average infect 12 to 18 others3), it has the capacity to spread quickly. This is why, prior to the development of the measles vaccine in the 1960s, it was responsible for millions of deaths across the globe.
Prior to the introduction of the measles vaccine, both physicians and the public generally were aware of the signs and symptoms of measles due to its prevalence; however, since there have been so few cases in recent decades, images and descriptions of patients presenting with measles can be found only in textbooks, and many physicians are ill-prepared to diagnose the disease.4 In response to the recent surge in measles cases, dermatologists—who often are among the first medical professionals to encounter febrile patients with rashes—must be prepared to bridge this divide. Herein, we review the clinical signs, diagnostic approach, operational precautions, and public health responsibilities that dermatologists must relearn amid the current measles outbreak.
Background
Measles is primarily transmitted via respiratory droplets and may remain airborne for up to 2 hours.5 It also can be transmitted through direct contact with secretions such as mucus. Indirect transmission via fomites, while certainly plausible, is thought to be the least effective mechanism of transmission.6 Following exposure, the incubation period ranges from 7 to 21 days, during which the virus replicates asymptomatically before causing clinical disease.7 Herd immunity for measles requires 93% immunity in the population; public health agencies typically target greater than 95% immunity.8 Humans are the only reservoir for the measles virus, making eradication possible.
The road to eradication began with the introduction of the measles vaccine in 1963 and subsequent development of the combined measles-mumps-rubella (MMR) vaccine in 1971. As MMR is a live vaccine, 2 doses confer approximately 97% protection.9 The first dose is given at 12 to 15 months of age, and the second dose is given at 4 to 6 years of age. Immunity is considered lifelong, and the Centers for Disease Control and Prevention and the World Health Organization do not recommend routine measles boosters for individuals who have completed the primary 2-dose series.10,11
Widespread vaccination led to a dramatic reduction in incidence, with many countries eliminating measles infections.7 The United States declared measles eliminated in 2000, with confirmed cases between 2000 and 2020 ranging from 37 to 1282.12 Vaccination progress stalled in the late 1990s due to vaccine hesitancy resulting from (subsequently debunked) reports of an association between the MMR vaccine and autism.13 Despite efforts to correct this misinformation, many patients continue to espouse these concerns.
Recognizing Measles: Clinical Presentation
Measles, which most often manifests in childhood but also can occur in adults, follows a distinctive clinical course. The prodromal phase is characterized by high fever, cough, coryza (nasal congestion), and conjunctivitis— conjunctivitis—the 3 “Cs” that serve as early warning signs of the disease. Patients may develop small white macules on the buccal mucosa known as Koplik spots (phonetically the fourth “C”), which appear just before the rash. Three to 5 days after the onset of systemic symptoms, patients will develop a classic morbilliform exanthem. In some cases, the exanthem manifests on the head and neck (Figure 1)—first behind the ears and along the hairline, then spreading caudally to the trunk and extremities. The lesions may become confluent, with patients presenting with diffuse erythema. The exanthem fades over several days to weeks, often accompanied by superficial desquamation.14

Given the nonspecificity of the early symptoms of measles, a high index of suspicion is needed for patients presenting with a febrile illness and a morbilliform eruption (Figure 2). Consideration of MMR vaccination status, exposure history, and local outbreak patterns can help guide risk stratification and the need for testing. Immunocompromised individuals, including those receiving immunosuppressive therapies for dermatologic conditions, may present atypically, lacking the prototypical exanthem or displaying milder signs and further complicating the diagnosis.15 The differential diagnosis for measles includes a drug reaction or other viral exanthem, and a detailed history may help elucidate the culprit.

Evaluation and Diagnosis
Definitive diagnosis of measles relies on both molecular and serologic testing. Nasopharyngeal swabs for measles polymerase chain reaction testing are obtained using synthetic (noncotton) swabs placed in a viral transport medium. Serum samples also should be collected for measles IgM and IgG antibody testing. Importantly, measles is a reportable illness, and testing may be coordinated with local departments of health.
Determining a patient’s immune status may be important for certain populations. Patients with documented 2-dose MMR vaccination, positive measles IgG serology, or a prior confirmed measles infection are considered immune. While a positive measles IgG indicates immunity, a negative result in an exposed patient should prompt consideration of postexposure prophylaxis with intravenous immunoglobulin.
Many patients, specifically those presenting to dermatology, are taking immunomodulatory or immunosuppressive medications—a contraindication for vaccination with the live MMR vaccine. At the time of publication, there was a single reported case of a patient taking a tumor necrosis factor α inhibitor for rheumatoid arthritis who had acquired measles.16 While the benefits of titer assessment in patients who are starting or continuing immunomodulatory therapy are not known and currently it is not recommended by the Centers for Disease Control and Prevention, dermatologists might consider checking MMR titers and vaccinating (or referring for vaccination) nonimmune patients.17
Infection Control
Early identification of a suspected measles case is paramount. Patients in whom measles is a possibility should be isolated as quickly as possible, and the patient and accompanying caregivers should be masked. Clinical staff should don appropriate personal protective equipment, including an N95 mask. Coordination with the local department of health must occur as soon as measles is suspected.
If testing is an option in the outpatient setting, a nasopharyngeal viral swab and serologic titers can be obtained. If testing is not available on site, patients should be sent to appropriate care facilities; prenotification is critical to prevent nosocomial outbreaks. Patients should be encouraged to isolate and avoid public spaces and/or public transport for 4 days following development of an exanthem.18 Offices should develop clinical protocols for suspected measles cases with training for clinical and office staff.
Final Thoughts
As measles outbreaks become more prevalent, it is incumbent upon physicians to remind ourselves of the signs and symptoms of this largely eliminated disease so that we may pursue early detection and intervention strategies. The primary cutaneous manifestations of measles make dermatologists critical to early recognition and containment efforts. Dermatologists should prepare for the arrival of patients with measles by maintaining vigilance for the classic signs of the disease, implementing stringent isolation protocols, verifying patient immunity when appropriate, and partnering closely with public health authorities.
More broadly, efforts to contain and re-establish a paradigm for eliminating measles outbreaks must be pursued. Encouraging vaccination and developing programs to help combat misinformation surrounding vaccines are critical to this effort. In an era of vaccine hesitancy, measles is a multidisciplinary public health emergency. Dermatologists must remain ready.
Measles, also known as rubeola, is a highly contagious paramyxovirus that has neared elimination in the United States since 2000 due to widespread adoption of the measles vaccine; however, measles recently has made a comeback, with outbreaks reported in more than 60 countries. In the United States, vaccine hesitancy coupled with decreasing vaccination rates, international travel to endemic areas, and decreased funding and resources for monitoring and immunization programs likely led to a re-emergence of measles cases.1,2 The resurgence of measles is troubling given its infectiousness and potential severity in at-risk populations. Since measles has a basic reproduction number of 12 to 18 (ie, 1 infected individual will on average infect 12 to 18 others3), it has the capacity to spread quickly. This is why, prior to the development of the measles vaccine in the 1960s, it was responsible for millions of deaths across the globe.
Prior to the introduction of the measles vaccine, both physicians and the public generally were aware of the signs and symptoms of measles due to its prevalence; however, since there have been so few cases in recent decades, images and descriptions of patients presenting with measles can be found only in textbooks, and many physicians are ill-prepared to diagnose the disease.4 In response to the recent surge in measles cases, dermatologists—who often are among the first medical professionals to encounter febrile patients with rashes—must be prepared to bridge this divide. Herein, we review the clinical signs, diagnostic approach, operational precautions, and public health responsibilities that dermatologists must relearn amid the current measles outbreak.
Background
Measles is primarily transmitted via respiratory droplets and may remain airborne for up to 2 hours.5 It also can be transmitted through direct contact with secretions such as mucus. Indirect transmission via fomites, while certainly plausible, is thought to be the least effective mechanism of transmission.6 Following exposure, the incubation period ranges from 7 to 21 days, during which the virus replicates asymptomatically before causing clinical disease.7 Herd immunity for measles requires 93% immunity in the population; public health agencies typically target greater than 95% immunity.8 Humans are the only reservoir for the measles virus, making eradication possible.
The road to eradication began with the introduction of the measles vaccine in 1963 and subsequent development of the combined measles-mumps-rubella (MMR) vaccine in 1971. As MMR is a live vaccine, 2 doses confer approximately 97% protection.9 The first dose is given at 12 to 15 months of age, and the second dose is given at 4 to 6 years of age. Immunity is considered lifelong, and the Centers for Disease Control and Prevention and the World Health Organization do not recommend routine measles boosters for individuals who have completed the primary 2-dose series.10,11
Widespread vaccination led to a dramatic reduction in incidence, with many countries eliminating measles infections.7 The United States declared measles eliminated in 2000, with confirmed cases between 2000 and 2020 ranging from 37 to 1282.12 Vaccination progress stalled in the late 1990s due to vaccine hesitancy resulting from (subsequently debunked) reports of an association between the MMR vaccine and autism.13 Despite efforts to correct this misinformation, many patients continue to espouse these concerns.
Recognizing Measles: Clinical Presentation
Measles, which most often manifests in childhood but also can occur in adults, follows a distinctive clinical course. The prodromal phase is characterized by high fever, cough, coryza (nasal congestion), and conjunctivitis— conjunctivitis—the 3 “Cs” that serve as early warning signs of the disease. Patients may develop small white macules on the buccal mucosa known as Koplik spots (phonetically the fourth “C”), which appear just before the rash. Three to 5 days after the onset of systemic symptoms, patients will develop a classic morbilliform exanthem. In some cases, the exanthem manifests on the head and neck (Figure 1)—first behind the ears and along the hairline, then spreading caudally to the trunk and extremities. The lesions may become confluent, with patients presenting with diffuse erythema. The exanthem fades over several days to weeks, often accompanied by superficial desquamation.14

Given the nonspecificity of the early symptoms of measles, a high index of suspicion is needed for patients presenting with a febrile illness and a morbilliform eruption (Figure 2). Consideration of MMR vaccination status, exposure history, and local outbreak patterns can help guide risk stratification and the need for testing. Immunocompromised individuals, including those receiving immunosuppressive therapies for dermatologic conditions, may present atypically, lacking the prototypical exanthem or displaying milder signs and further complicating the diagnosis.15 The differential diagnosis for measles includes a drug reaction or other viral exanthem, and a detailed history may help elucidate the culprit.

Evaluation and Diagnosis
Definitive diagnosis of measles relies on both molecular and serologic testing. Nasopharyngeal swabs for measles polymerase chain reaction testing are obtained using synthetic (noncotton) swabs placed in a viral transport medium. Serum samples also should be collected for measles IgM and IgG antibody testing. Importantly, measles is a reportable illness, and testing may be coordinated with local departments of health.
Determining a patient’s immune status may be important for certain populations. Patients with documented 2-dose MMR vaccination, positive measles IgG serology, or a prior confirmed measles infection are considered immune. While a positive measles IgG indicates immunity, a negative result in an exposed patient should prompt consideration of postexposure prophylaxis with intravenous immunoglobulin.
Many patients, specifically those presenting to dermatology, are taking immunomodulatory or immunosuppressive medications—a contraindication for vaccination with the live MMR vaccine. At the time of publication, there was a single reported case of a patient taking a tumor necrosis factor α inhibitor for rheumatoid arthritis who had acquired measles.16 While the benefits of titer assessment in patients who are starting or continuing immunomodulatory therapy are not known and currently it is not recommended by the Centers for Disease Control and Prevention, dermatologists might consider checking MMR titers and vaccinating (or referring for vaccination) nonimmune patients.17
Infection Control
Early identification of a suspected measles case is paramount. Patients in whom measles is a possibility should be isolated as quickly as possible, and the patient and accompanying caregivers should be masked. Clinical staff should don appropriate personal protective equipment, including an N95 mask. Coordination with the local department of health must occur as soon as measles is suspected.
If testing is an option in the outpatient setting, a nasopharyngeal viral swab and serologic titers can be obtained. If testing is not available on site, patients should be sent to appropriate care facilities; prenotification is critical to prevent nosocomial outbreaks. Patients should be encouraged to isolate and avoid public spaces and/or public transport for 4 days following development of an exanthem.18 Offices should develop clinical protocols for suspected measles cases with training for clinical and office staff.
Final Thoughts
As measles outbreaks become more prevalent, it is incumbent upon physicians to remind ourselves of the signs and symptoms of this largely eliminated disease so that we may pursue early detection and intervention strategies. The primary cutaneous manifestations of measles make dermatologists critical to early recognition and containment efforts. Dermatologists should prepare for the arrival of patients with measles by maintaining vigilance for the classic signs of the disease, implementing stringent isolation protocols, verifying patient immunity when appropriate, and partnering closely with public health authorities.
More broadly, efforts to contain and re-establish a paradigm for eliminating measles outbreaks must be pursued. Encouraging vaccination and developing programs to help combat misinformation surrounding vaccines are critical to this effort. In an era of vaccine hesitancy, measles is a multidisciplinary public health emergency. Dermatologists must remain ready.
- Bedford H, Elliman D. Measles rates are rising again. BMJ. 2024;384.
- Harris E. Measles outbreaks grow amid declining vaccination rates. JAMA. 2023;330:2242.
- Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:E420-E428.
- Swartz MK. Measles: public and professional education. J Pediatr Health Care. 2019;33:367-368.
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for measles in healthcare settings. Accessed April 27, 2025. https://www.cdc.gov/infection-control/hcp/measles/
- Moss WJ, Griffin DE, Feinstone WH. Measles. In: Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2009: 551-565.
- Moss WJ. Measles. Lancet. 2017;390:2490-2502.
- Maintain the vaccination coverage level of 2 doses of the MMR vaccine for children in kindergarten— IID04. Healthy People 2030 website. Accessed May 6, 2025. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/maintain-vaccination-coverage-level-2-doses-mmr-vaccine-children-kindergarten-iid-04
- Franconeri L, Antona D, Cauchemez S, et al. Two-dose measles vaccine effectiveness remains high over time: a French observational study, 2017–2019. Vaccine. 2023;41:5797-5804.
- World Health Organization. Measles. Accessed May 8, 2025. https:// www.who.int/news-room/fact-sheets/detail/measles
- Centers for Disease Control and Prevention. Measles vaccine recommendations. Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Accessed May 6, 2025. https://www.cdc.gov/measles/cases-outbreaks.html
- Dyer C. Lancet retracts Wakefield’s MMR paper. BMJ. 2010;340.
- Alves Graber EM, Andrade FJ, Bost W, et al. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610-615.
- Kaplan LJ, Daum RS, Smaron M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237-1241.
- Takahashi E, Kurosaka D, Yoshida K, et al. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Japanese J Clin Immunol. 2010;33:37-41.
- Worth A, Waldman RA, Dieckhaus K, et al. Art of prevention: our approach to the measles-mumps-rubella vaccine in adult patients vaccinated against measles before 1968 on biologic therapy for the treatment of psoriasis. Int J Womens Dermatol. 2019;6:94.
- Centers for Disease Control and Prevention. Clinical overview of measles (rubeola). Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/clinical-overview/index.html
- Bedford H, Elliman D. Measles rates are rising again. BMJ. 2024;384.
- Harris E. Measles outbreaks grow amid declining vaccination rates. JAMA. 2023;330:2242.
- Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:E420-E428.
- Swartz MK. Measles: public and professional education. J Pediatr Health Care. 2019;33:367-368.
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for measles in healthcare settings. Accessed April 27, 2025. https://www.cdc.gov/infection-control/hcp/measles/
- Moss WJ, Griffin DE, Feinstone WH. Measles. In: Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2009: 551-565.
- Moss WJ. Measles. Lancet. 2017;390:2490-2502.
- Maintain the vaccination coverage level of 2 doses of the MMR vaccine for children in kindergarten— IID04. Healthy People 2030 website. Accessed May 6, 2025. https://odphp.health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/maintain-vaccination-coverage-level-2-doses-mmr-vaccine-children-kindergarten-iid-04
- Franconeri L, Antona D, Cauchemez S, et al. Two-dose measles vaccine effectiveness remains high over time: a French observational study, 2017–2019. Vaccine. 2023;41:5797-5804.
- World Health Organization. Measles. Accessed May 8, 2025. https:// www.who.int/news-room/fact-sheets/detail/measles
- Centers for Disease Control and Prevention. Measles vaccine recommendations. Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/vaccine-considerations/index.html
- Centers for Disease Control and Prevention. Measles cases and outbreaks. Accessed May 6, 2025. https://www.cdc.gov/measles/cases-outbreaks.html
- Dyer C. Lancet retracts Wakefield’s MMR paper. BMJ. 2010;340.
- Alves Graber EM, Andrade FJ, Bost W, et al. An update and review of measles for emergency physicians. J Emerg Med. 2020;58:610-615.
- Kaplan LJ, Daum RS, Smaron M, et al. Severe measles in immunocompromised patients. JAMA. 1992;267:1237-1241.
- Takahashi E, Kurosaka D, Yoshida K, et al. Onset of modified measles after etanercept treatment in rheumatoid arthritis. Japanese J Clin Immunol. 2010;33:37-41.
- Worth A, Waldman RA, Dieckhaus K, et al. Art of prevention: our approach to the measles-mumps-rubella vaccine in adult patients vaccinated against measles before 1968 on biologic therapy for the treatment of psoriasis. Int J Womens Dermatol. 2019;6:94.
- Centers for Disease Control and Prevention. Clinical overview of measles (rubeola). Accessed May 8, 2025. https://www.cdc.gov/measles/hcp/clinical-overview/index.html
Measles Resurgence: A Dermatologist’s Guide
Measles Resurgence: A Dermatologist’s Guide
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
The scabies mite, originally known as Acarus scabiei,1 now is considered an arthropod of the class Arachnida, order Astigmata, and family Sarcoptidae.2 Scabies mites are able to adhere to the surface of human skin.3 The mites burrow and lay eggs in the top layer of the epidermis; most patients have 10 to 15 mites.3 The patient’s immune system incites an allergic reaction to the mite protein and feces in the skin, causing itching and rash.4
Scabies is common in indigenous populations and in low-income areas of developing countries.5 It is most prevalent in Africa, South America, Australia, and Southeast Asia, in part due to poverty, poor nutritional status, homelessness, and inadequate hygiene.2 In 2009, the World Health Organization declared scabies a neglected skin disease2; however, in 2010, 1.5 million disability adjusted life-years were attributed to scabies,6 and it is estimated that 200 million people worldwide have scabies at any given time. Children and elderly individuals in resource-poor communities are the most at risk. In fact, 5% to 50% of children in low-income areas have scabies.4
The purpose of this article is to provide background on scabies and its effect on the human immune system. We also discuss manipulation of the immune response for the purposes of creating a potential scabies vaccine.
Life Cycle and Transmission
The life cycle of Sarcoptes scabiei consists of 4 stages. The first is the egg. As female scabies mites burrow under the skin, they lay 2 to 3 ovular eggs per day.3 The second stage is the larva. When the egg hatches, the larva has 3 pairs of legs and travels to the surface of the skin where it burrows into the stratum corneum, creating short, nearly invisible burrows called molting pouches. After 3 to 4 days, the larva molts into a nymph, which is the third stage. The nymph has 4 pairs of legs and will continue to grow before molting into an adult, which is the fourth stage. Both the larva and nymph may be found in hair follicles or molting pouches. The fourth stage is the adult, which is round and saclike and does not have eyes. Adult females are 0.30 mm to 0.45 mm long and 0.25 mm to 0.35 mm wide, which is half the size of adult males.3 On warm skin, the female mite can crawl at a rate of 2.5 cm per minute.7
Scabies mites mate via an active male penetrating the molting pouch of a female. This only occurs once but leaves the female fertile for the rest of her life. Once a female is pregnant, she leaves her molting pouch and travels along the surface of the skin looking for a place to make her permanent burrow.3 The most common sites for scabies burrows are the axillae, umbilicus, interdigital spaces, beltline, buttocks, flexor surfaces of the wrists, female nipples, and male penile shaft.5 Once she finds an acceptable location, the female scabies mite will create a serpentine burrow and lay her eggs. Once she burrows, she will stay there and continue to lay eggs for the rest of her life, lengthening the burrow as needed.3 Female mites lay their eggs in the superficial epidermis, and the eggs take approximately 2 to 3 weeks to hatch. Female mites die 30 to 60 days later.2
Scabies infestations typically spread via the transfer of pregnant adult females during skin-to-skin contact, but they also can spread via fomites.3 During all stages of their life cycle, scabies mites can secrete enzymes that allow them to penetrate the intact epidermis in less than 30 minutes; in fact, an otherwise healthy patient with scabies must have 15 to 20 minutes of close skin-to-skin contact with an infected individual for the disease to be transmitted.7 Because scabies mites can survive for more than 3 days outside the human body, it is thought that fomites also may be involved in transmission. Scabies mites also have been collected from clothing, bedding, and furniture, which further supports the idea that fomites are involved in disease transmission.7
Clinical Manifestation of Scabies
Scabies symptoms include severe pruritus as well as linear burrows and vesicles in the interdigital spaces on the hands, wrists, arms and legs, and lower abdomen. Infants and young children also can develop a rash on the palms, soles, ankles, and scalp. Men can develop inflammatory scabies nodules on the penis and scrotum, while women can develop these nodules on the nipple.4 Type I and type IV hypersensitivity reactions contribute to the rash and itching associated with scabies infestation via host allergic and inflammatory reactions to the mites and their byproducts. Patients with scabies typically are infested with fewer than 15 mites,6 but just a few can cause substantial pruritus and scratching, leading to hyperkeratosis.8
Additionally, when patients with scabies scratch the skin, they become vulnerable to bacterial infections.4 Scabies lesions can be coinfected with group A streptococci and Staphylococcus aureus,8 potentially leading to abscesses and septicemia. These secondary infections also can cause renal and cardiac complications; in fact, in tropical areas, scabies infections are considered a risk factor for kidney disease and rheumatic heart disease.4
The 2 main forms of scabies infestations are ordinary and crusted. The most common form is ordinary scabies, which typically manifests with fewer than 15 mites per patient; crusted scabies (CS) is the more rare and extreme form.6 Cases of CS present with thousands to millions of mites per patient, leading to more widespread and severe symptoms.4 Because of the large increase in the number of mites, CS is more contagious than ordinary scabies.6
Patients with CS typically present with hyperkeratotic skin disease, as evidenced by thick scaly crusts with large numbers of mites, which can lead to permanent skin disfiguration. Patients with CS also can develop deep fissuring of the crusts, within which other microbes can gain entry to the body and lead to secondary infection and possibly sepsis and death. Also, because of the increased number of mites as well as the crusted skin, patients with CS are contagious for longer. As it is more difficult to eradicate, reinfestation is common with CS.6
Patients with compromised immune systems are predisposed to CS. Specifically, patients with HIV or human T-lymphotropic virus 1 or those undergoing organ transplantation are thought to be the most at risk for CS.6 Crusted scabies also has been identified in large numbers in patients with Down syndrome and in Aboriginal Australians; however, the reasoning for this is poorly understood.6
Immune Response
The inflammatory reaction associated with scabies infestations occurs 4 to 6 weeks after initial exposure. It is hypothesized that scabies can alter parts of the host immune system, which contributes to the delayed onset of symptoms. Scabies mites also produce inactivated protease paralogues and serpins, which help to protect the mites from the host immune system by inhibiting the complement system.6
The complement system is part of the innate immune response and is the first line of defense against pathogens. Specifically with scabies infestations, C3 and C4 complement components have been found in skin lesions.6 C3a and C4a fragments cause local inflammation, while C3a and C5a activate mast cells to release histamine and tumor necrosis factor (TNF) α, further amplifying the inflammatory response; however, CS lesions show low C3 and C4, which can indicate immunodeficiency in patients with CS. It also can be due to the sheer number of mites in a CS infection causing the host immune system to be overloaded.6
Innate effector immune cells also are an important part of the innate immune response to scabies; for example, eosinophilia is seen in scabies infections. Specifically, in CS, eosinophils help modulate and sustain the T-helper (Th) 2 inflammatory response. One cytokine secreted by Th2 cells is IL-5, which is closely associated with the attraction, maturation, and survival of eosinophils.6 Eosinophils also can influence the Th1 inflammatory response in that they produce IL-12, interferon (IFN) γ, and several Toll-like receptors. Furthermore, eosinophilic expression of IL-2 can lead to expansion of regulatory T cells, while eosinophilic expression of IL-10 and transforming growth factor (TGF) Β also can suppress local inflammation by influencing regulatory T cells.6
Additionally, mast cells and basophils are important in the IgE-mediated allergic reaction as well as the host immune response to parasites. When activated, basophils and mast cells produce TNF-α, IL-6, Il-4, IL-5, and IL-13, which contribute to the Th2 inflammatory response; however, the role of mast cells and basophils in scabies infections still is poorly understood.6
Macrophages, neutrophils, and dendritic cells (DCs) contribute to phagocytosis, antigen presentation, and differentiation of T cells, which also contribute to the inflammatory and allergic reactions associated with parasitic infections.6 Macrophages have been found in low numbers in scabies infestation, possibly due to immune-modulating molecules secreted by scabies mites. Early in an infestation, the mites secrete immune-modulating molecules, which inhibit macrophage migration to the site of inflammation, allowing the mites to grow.6 Neutrophils and DCs also are involved in the host immune response to scabies. Neutrophils are the predominant inflammatory cell infiltrate in scabies lesions. The scabies protein SMSB4 inhibits neutrophil opsonization and phagocytosis, thus suppressing bacterial killing.6 Some of the first antigen-presenting cells encountered by the antigen are DCs. They are involved in preparing the antigens for presentation to effector T cells, which leads to T-cell differentiation and activation.6
Cytokines are another important factor in the innate immune response. The host immune response to ordinary scabies is Th1-cell mediated, during which CD4+ and CD8+ T cells secrete IFN-γ, TNF-α, and IL-2.6 Therefore, IFN- γ and TNF-α are elevated in the serum of patients with ordinary scabies. Conversely, the host immune response to CS is Th2-cell mediated. T-helper 2 cells are needed in IgE-mediated hypersensitivity reactions, and they secrete IL-4, IL-5, and IL-13. In the serum of patients with CS, IL-l4, IL-5, and IL-13 are elevated while IFN-γ is decreased.6 Additionally, IL-6, TGF-Β, IL-23, IL-1Β, or IL-18 can induce Th17 cells to generate and secrete IL-17, which enhances the inflammatory response by inducing further expression of TNF-α, IL-1Β, IL-6, keratinocytes, and fibroblasts. T-helper 17 and IL-17 also are involved in psoriasis and atopic dermatitis, as well as Leishmania major and Schistosoma japonicum.6
Regulatory T cells Tregs secrete TGF-Β and IL-10, which suppress pathologic inflammation, and IL-10 is substantially reduced in patients with CS compared to those with ordinary scabies and uninfected control patients. Additionally, IL-10 can inhibit the synthesis of TNF-γ and IFN-α. Reduced IL-10 expression can lead to proliferation of IL-17 secretion, resulting in a regulatory T cell/Th17 dysfunctional immune response.6
Immunoglobulins are antibodies that are involved in the host’s adaptive immune response. The first antibody to appear in response to an antigen is IgM, and IgM bound to scabies antigens is present in 74%6 of patients with ordinary scabies. Because IgM is the first antibody to appear in response to a scabies infection, detection of serum IgM may allow for earlier detection of scabies; however, IgM has a high cross-reactivity between scabies mites and dust mites, which can hinder scabies diagnosis via IgM detection.6
Both patients with ordinary scabies and CS also show an increased circulatory IgG concentration compared to control groups; patients with CS have higher concentrations. Increased IgG also can be in part due to concurrent bacterial infections.6 When IgG or IgM antibodies bind to a pathogen, they activate the complement cascade, which further enhances the activity of these antibodies.9
Additionally, IgA is important in mucosal immune function. In both patients with ordinary scabies and CS, there is increased IgA binding to recombinant scabies mite antigens.6Sarcoptes scabiei proteases that are localized in the mite’s gut and scybala suggest their involvement in mite digestion and burrowing. The increased secretion of these proteases into the host skin may contribute to the increased IgA,9 and these increased IgA levels have been shown to be positively correlated with severity of scabies infection.6
Also essential in allergic and parasitic inflammation, IgE is observed at higher levels in secondary infections of scabies compared to primary infections.6 Additionally, T-cell infiltrates are implicated in adaptive immune response to scabies. CD4+ T cells are the most prevalent T cells in ordinary scabies skin lesions; however, CD4+ T cells are minimal and CD8+ T cells are elevated in CS skin lesions. The increased CD8+ T cells may cause apoptosis of keratinocytes, leading to epidermal hyperproliferation. The apoptotic keratinocytes can secrete cytokines, which can lead to tissue damage.6 These T cells also may be involved in the failure of the skin’s immune system to mount an effective response to the parasite infestation, leading to uncontrolled parasitic growth. Because patients with AIDS who are infected with scabies mites often develop CS, it is also thought that CD4+ T cells are essential in the immune response to scabies.6
Diagnosis and Current Treatment Options
Current diagnosis of scabies is based on mites, eggs, and fecal matter from the host’s skin. Dermoscopy and fluorescent dermoscopy can be helpful in identifying the mites, eggs, and feces on the patient’s skin. Scabies treatment sometimes may be based solely on symptoms without any positive tests.8
Acaricides are the current method of treatment for scabies infestations.5 Acaricides can be expensive and toxic to the environment and food sources,10 and some agents have been associated with neurotoxicity5 in children or the development of certain cancers.11 Although topical acaricides are the standard form of treatment, oral ivermectin also can be used. Ivermectin is not associated with selective fetal toxicity, but there are limited safety data in pregnant women and in children weighing less than 15 kg (33 lb). Additionally, because symptoms typically are not present during an early infection, treating everyone in the household and those who had close contact with the patient can help prevent reinfection.4
Although these drugs have been shown to be effective at treating scabies, scabies mites are becoming increasingly resistant to acaricides.5 There are 4 main proposed mechanisms for why this occurs.12 The first is through voltage-gated sodium channels, which are involved in the normal functioning of neurons and myocytes. Permethrin, a type of acaricide, binds to voltage-gated sodium channels when it is in an open or active state and prevents it from closing. This creates repetitive neuron firing and hyperactivity, which ultimately kills the scabies mite. Some mites have mutated to close this channel, which reduces the binding potential of permethrin. Glutathione S-transferase is another mechanism of resistance. It catalyzes a bond that tags drugs for elimination. Increased activity or expressivity of glutathione S-transferase by scabies mites can lead to drug resistance.12 Adenosine triphosphate– binding cassette (ABC) transporters also may contribute to this resistance. The ABC transporters use adenosine triphosphate to facilitate the import or export of molecules. Scabies mites express a protein called the multidrug-resistant protein, which is an ABC transporter that is associated with drug resistance and is present in scabies mites.12 Lastly, ligand-gated chloride channels have been implicated in scabies resistance to acaricides. Ligand-gated chloride channels also are important in normal functioning of neurons and myocytes. Some antiparasitic drugs act on these channels, leading to a continuous influx of chloride, but some scabies mites have mutated this pathway.12
Pesticides and the Risk for Cancer
Pesticides commonly are used to treat scabies; however, a link between pesticide exposure and leukemia and lymphoma has been seen through epidemiologic studies, and there also is increasing biological evidence to suggest this.11 For example, the pesticide permethrin, which works by paralyzing the nervous system of insects,13 has been associated with an increased risk for leukemia and lymphoma in humans. Permethrin is a pyrethroid and, compared to control patients, children with leukemia had higher levels of pyrethroid metabolites in their blood.14 Numerical and structural chromosomal aberrations that give rise to gene fusions are the most common abnormalities seen in leukemia, and permethrin has been shown to induce DNA breaks, chromosome aberrations, and sister chromatid exchanges.14 Permethrin also has been associated with an increased risk for multiple myeloma.13
Furthermore, in utero exposure to pesticides has been associated with an increased risk for childhood leukemia.15 Pesticide exposure shortly before conception, during pregnancy, and after birth is associated with an increased risk for acute lymphocytic leukemia.16 In fact, the children of mothers who were exposed to pesticides 3 months before conception have been found to be at least twice as likely to be diagnosed with acute lymphocytic leukemia within the first year of life compared with children whose mothers were not exposed to pesticides.17 It is hypothesized that permethrin can cross the placenta and alter the hematopoietic precursor cells in the fetus, resulting in leukemogenesis.18 Pyrethroid metabolites also have been detected in umbilical cord blood samples and breast milk.15
In contrast to the research demonstrating a link between permethrin and cancer, other studies have found no association between permethrin19 and leukemia20; non-Hodgkin lymphoma19; or cancers of the colon, rectum, pancreas, lungs, skin, female breast, prostate, and urinary bladder.20 Because of conflicting research on the link between permethrin and cancer, more research is needed.,20
Importance of a Scabies Vaccine
Because scabies mites are developing increasing treatment resistance, more radical approaches such as vaccines are becoming important. While a scabies vaccine is still aspirational, animals that have been infected for a second time with scabies demonstrate a milder response to the second infection compared to the first infection, which could mean there is a potential for disease prevention through a vaccine.21 While educating patients and physicians, reporting cases of infection, and improving drug supply and access can help decrease scabies infestations, these are costly and difficult to implement. Scabies already is most prevalent in low-income areas, so costly interventions are even less feasible. An effective, one-dose vaccine would cost less than these efforts and therefore could be implemented more easily.9
In older adults, scabies more often manifests atypically and is more likely to progress to CS. Aged care centers are prone to institutional outbreaks, even in developed countries, so a vaccine also would greatly help this population. Additionally, the number of children attending day care centers, which also are prone to scabies outbreaks, is increasing. When a child contracts scabies, all close contacts need to be treated, so a preventive vaccine can be useful.9
One potential candidate for a scabies vaccine is total mite extract. Studies show that rabbits immunized with a total mite extract induce antibodies to more antigens than rabbits naturally infested with scabies mites; however, the mites cannot be cultured in vitro, which makes obtaining a large amount of their total extract difficult. Therefore, recombinant vaccines also have been proposed, as they are more easily available.22 One recombinant vaccine candidate is recombinant S scabiei serpin (rSs-serpin). Immunization with rSs-serpin has strong immunogenicity and produced immune protection in rabbits.22
Two other recombinant vaccine candidates are the rSs chitinaselike protein (CLP) 12 and the rSsCLP5. Chitinaselike proteins are very similar to chitinases; however, they are unable to degrade chitin. They are involved in immune reactions to infections, and CLPs from scabies mites have been shown to induce the host immune response.22 For example, in a particular rabbit study, rSsCLP5 demonstrated high immunoreactivity and immunogenicity. In fact, after exposure to S scabiei, 74.3% of rabbits who were vaccinated with rSsCLP5 had no detectable lesions.5 Also, after immunization with rSsCLP5 and rSsCLP12, there were increased levels of specific IgG and IgE antibodies produced and decreased numbers of infesting mites.22 Weight loss also is associated with severe scabies infection. Rabbits vaccinated with rSsCLP5 and exposed to the parasite gained weight, indicating protection via rSsCLP5. Even rabbits who did develop symptoms of scabies after immunization with rSsCLP5 and exposure to S scabiei showed less serious manifestations.5
A combination vaccine cocktail of rSs-serpin, rSsCLP12, and rSsCLP5 also has been proposed by Shen et al.22 Four test groups and a control group (n=12 per group) were included in a vaccine trial. Between 83.33% and 91.67% of rabbits vaccinated with this mixed recombinant cocktail vaccine had no detectable skin lesions from scabies. After immunization with the cocktail vaccine, the specific serum IgG and IgE antibodies also increased. For both IgG and IgE, increased levels were first detected at 1 week postimmunization and peaked at 2 weeks postimmunization.22 A multiepitope vaccine derived from these 3 recombinant proteins also was explored by Shen et al22; fewer rabbits vaccinated with it had no detectable scabies skin lesions compared to those treated with the vaccine cocktail. Although the multiepitope vaccine yielded less immume protection, it was associated with a slower disease course and milder symptoms compared with no vaccination.22
Two more proposed scabies recombinant vaccine candidates are derived from the antigens Ssag1 and Ssag2; however, rabbits vaccinated with Ssag1 or Ssag2 showed no immune protection or mite burden reduction.22 The lack of protection could be due to denaturation or degradation of the protective antigens. It also can be due to the low abundance of these antigens, meaning they may not be vital for the mite’s survival—survival—a potential avenue for future research. The antigens also could have lost their native structure and immunogenic properties during the purification and production process. Therefore, more research is needed to investigate how to purify these vaccines to keep the peptides more structurally similar to their native makeups.10 More research also is needed to better understand the antigen or antigens and their mechanisms that elicit a protective immune response.9
Final Thoughts
Scabies causes severe pruritus in mild cases but also can lead to severe disfigurement, sepsis, and even death. Scabies infestations are seen disproportionately more often in low-income and resource-poor communities, and the current treatment options are less accessible to these populations. Scabies infestations induce a complex immune response that involves multiple aspects of both the innate and adaptive immune systems and can be targeted to create a scabies vaccine. Development of a scabies vaccine is crucial considering the growing resistance to current standard treatments. Acaricides potentially are associated with an increased risk for malignancy, which further amplifies the need for a scabies vaccine. There currently are multiple promising scabies vaccine candidates; however, more research is needed to better understand the host’s immune response to scabies as well as how to more accurately and efficiently produce the vaccine. The development of a safe, effective, economical vaccine that can be mass distributed would be beneficial in the treatment of scabies, especially in resource-poor communities.
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Murray RL, Crane JS. Scabies. In: StatPearls. StatPearls Publishing. Updated July 31, 2023.
- Centers for Disease Control and Prevention. CDC—scabies—biology. November 2, 2010. https://www.cdc.gov/dpdx/scabies/index.html
- World Health Organization. Scabies. May 31, 2023. Accessed May 8, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Shen N, Zhang H, Ren Y, et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors. 2018;11:599. doi:10.1186/s13071- 018-3184-y
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Hicks MI, Elston DM. Scabies. Dermatolog Ther. 2009;22:279-292. doi:10.1111/j.1529-8019.2009.01243.x
- Morgan MS, Arlian LG, Rider SD, et al. A proteomic analysis of Sarcoptes scabiei (acari: Sarcoptidae). J Med Entomol. 2016;53:553-561. doi:10.1093/jme/tjv247
- Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725-732. doi:10.1017 /s0031182013002047
- Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors. 2016;9:435. doi:10.1186 /s13071-016-1717-9?
- Navarrete-Meneses MP, Pedraza-Meléndez AI, Salas-Labadía C, et al. Low concentrations of permethrin and malathion induce numerical and structural abnormalities in KMT2A and IGH genes in vitro. J Appl Toxicol. 2018;38:1262-1270. doi:10.1002/jat.3638
- Khalil S, Abbas O, Kibbi AG, et al. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11:E0005920. doi:10.1371 /journal.pntd.0005920
- Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581-586. doi:10.1289 /ehp.11318
- Navarrete-Meneses MP, Salas-Labadía C, Sanabrais-Jiménez M, et al. Exposure to the insecticides permethrin and malathion induces leukemia and lymphoma-associated gene aberrations in vitro. Toxicol In Vitro. 2017;44:17-26. doi:10.1016/j.tiv.2017.06.013
- Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. 2019;34:197-210. doi:10.1515 /reveh-2018-0070
- Madrigal JM, Jones RR, Gunier RB, et al. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2021;201:111501. doi:10.1016/j.envres.2021.111501
- Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children <2 years of age. Environ Health Perspect. 2013;121:269-275. doi:10.1289/ehp.1103942
- Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88:F436-F437. doi:10.1136/fn.88.5.f436
- De Roos AJ, Schinasi LH, Miligi L, et al. Occupational insecticide exposure and risk of non]Hodgkin lymphoma: a pooled case]control study from the InterLymph consortium. Int J Cancer. 2021;149:1768-1786. doi:10.1002/ijc.33740
- Boffett, P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48:433-442. doi:10.1080/1040 8444.2018.1439449
- Adji A, Rumokoy LJM, Salaki CL. Scabies vaccine as a new breakthrough for the challenge of acaricides resistance. Adv Biolog Sci Res. 2020;8:208-213. doi:10.2991/absr.k.200513.036
- Shen N, Wei W, Chen Y, et al. Vaccination with a cocktail vaccine elicits significant protection against Sarcoptes scabiei in rabbits, whereas the multi-epitope vaccine offers limited protection. Exp Parasitol. 2023;245:108442. doi:10.1016/j.exppara.2022.108442
The scabies mite, originally known as Acarus scabiei,1 now is considered an arthropod of the class Arachnida, order Astigmata, and family Sarcoptidae.2 Scabies mites are able to adhere to the surface of human skin.3 The mites burrow and lay eggs in the top layer of the epidermis; most patients have 10 to 15 mites.3 The patient’s immune system incites an allergic reaction to the mite protein and feces in the skin, causing itching and rash.4
Scabies is common in indigenous populations and in low-income areas of developing countries.5 It is most prevalent in Africa, South America, Australia, and Southeast Asia, in part due to poverty, poor nutritional status, homelessness, and inadequate hygiene.2 In 2009, the World Health Organization declared scabies a neglected skin disease2; however, in 2010, 1.5 million disability adjusted life-years were attributed to scabies,6 and it is estimated that 200 million people worldwide have scabies at any given time. Children and elderly individuals in resource-poor communities are the most at risk. In fact, 5% to 50% of children in low-income areas have scabies.4
The purpose of this article is to provide background on scabies and its effect on the human immune system. We also discuss manipulation of the immune response for the purposes of creating a potential scabies vaccine.
Life Cycle and Transmission
The life cycle of Sarcoptes scabiei consists of 4 stages. The first is the egg. As female scabies mites burrow under the skin, they lay 2 to 3 ovular eggs per day.3 The second stage is the larva. When the egg hatches, the larva has 3 pairs of legs and travels to the surface of the skin where it burrows into the stratum corneum, creating short, nearly invisible burrows called molting pouches. After 3 to 4 days, the larva molts into a nymph, which is the third stage. The nymph has 4 pairs of legs and will continue to grow before molting into an adult, which is the fourth stage. Both the larva and nymph may be found in hair follicles or molting pouches. The fourth stage is the adult, which is round and saclike and does not have eyes. Adult females are 0.30 mm to 0.45 mm long and 0.25 mm to 0.35 mm wide, which is half the size of adult males.3 On warm skin, the female mite can crawl at a rate of 2.5 cm per minute.7
Scabies mites mate via an active male penetrating the molting pouch of a female. This only occurs once but leaves the female fertile for the rest of her life. Once a female is pregnant, she leaves her molting pouch and travels along the surface of the skin looking for a place to make her permanent burrow.3 The most common sites for scabies burrows are the axillae, umbilicus, interdigital spaces, beltline, buttocks, flexor surfaces of the wrists, female nipples, and male penile shaft.5 Once she finds an acceptable location, the female scabies mite will create a serpentine burrow and lay her eggs. Once she burrows, she will stay there and continue to lay eggs for the rest of her life, lengthening the burrow as needed.3 Female mites lay their eggs in the superficial epidermis, and the eggs take approximately 2 to 3 weeks to hatch. Female mites die 30 to 60 days later.2
Scabies infestations typically spread via the transfer of pregnant adult females during skin-to-skin contact, but they also can spread via fomites.3 During all stages of their life cycle, scabies mites can secrete enzymes that allow them to penetrate the intact epidermis in less than 30 minutes; in fact, an otherwise healthy patient with scabies must have 15 to 20 minutes of close skin-to-skin contact with an infected individual for the disease to be transmitted.7 Because scabies mites can survive for more than 3 days outside the human body, it is thought that fomites also may be involved in transmission. Scabies mites also have been collected from clothing, bedding, and furniture, which further supports the idea that fomites are involved in disease transmission.7
Clinical Manifestation of Scabies
Scabies symptoms include severe pruritus as well as linear burrows and vesicles in the interdigital spaces on the hands, wrists, arms and legs, and lower abdomen. Infants and young children also can develop a rash on the palms, soles, ankles, and scalp. Men can develop inflammatory scabies nodules on the penis and scrotum, while women can develop these nodules on the nipple.4 Type I and type IV hypersensitivity reactions contribute to the rash and itching associated with scabies infestation via host allergic and inflammatory reactions to the mites and their byproducts. Patients with scabies typically are infested with fewer than 15 mites,6 but just a few can cause substantial pruritus and scratching, leading to hyperkeratosis.8
Additionally, when patients with scabies scratch the skin, they become vulnerable to bacterial infections.4 Scabies lesions can be coinfected with group A streptococci and Staphylococcus aureus,8 potentially leading to abscesses and septicemia. These secondary infections also can cause renal and cardiac complications; in fact, in tropical areas, scabies infections are considered a risk factor for kidney disease and rheumatic heart disease.4
The 2 main forms of scabies infestations are ordinary and crusted. The most common form is ordinary scabies, which typically manifests with fewer than 15 mites per patient; crusted scabies (CS) is the more rare and extreme form.6 Cases of CS present with thousands to millions of mites per patient, leading to more widespread and severe symptoms.4 Because of the large increase in the number of mites, CS is more contagious than ordinary scabies.6
Patients with CS typically present with hyperkeratotic skin disease, as evidenced by thick scaly crusts with large numbers of mites, which can lead to permanent skin disfiguration. Patients with CS also can develop deep fissuring of the crusts, within which other microbes can gain entry to the body and lead to secondary infection and possibly sepsis and death. Also, because of the increased number of mites as well as the crusted skin, patients with CS are contagious for longer. As it is more difficult to eradicate, reinfestation is common with CS.6
Patients with compromised immune systems are predisposed to CS. Specifically, patients with HIV or human T-lymphotropic virus 1 or those undergoing organ transplantation are thought to be the most at risk for CS.6 Crusted scabies also has been identified in large numbers in patients with Down syndrome and in Aboriginal Australians; however, the reasoning for this is poorly understood.6
Immune Response
The inflammatory reaction associated with scabies infestations occurs 4 to 6 weeks after initial exposure. It is hypothesized that scabies can alter parts of the host immune system, which contributes to the delayed onset of symptoms. Scabies mites also produce inactivated protease paralogues and serpins, which help to protect the mites from the host immune system by inhibiting the complement system.6
The complement system is part of the innate immune response and is the first line of defense against pathogens. Specifically with scabies infestations, C3 and C4 complement components have been found in skin lesions.6 C3a and C4a fragments cause local inflammation, while C3a and C5a activate mast cells to release histamine and tumor necrosis factor (TNF) α, further amplifying the inflammatory response; however, CS lesions show low C3 and C4, which can indicate immunodeficiency in patients with CS. It also can be due to the sheer number of mites in a CS infection causing the host immune system to be overloaded.6
Innate effector immune cells also are an important part of the innate immune response to scabies; for example, eosinophilia is seen in scabies infections. Specifically, in CS, eosinophils help modulate and sustain the T-helper (Th) 2 inflammatory response. One cytokine secreted by Th2 cells is IL-5, which is closely associated with the attraction, maturation, and survival of eosinophils.6 Eosinophils also can influence the Th1 inflammatory response in that they produce IL-12, interferon (IFN) γ, and several Toll-like receptors. Furthermore, eosinophilic expression of IL-2 can lead to expansion of regulatory T cells, while eosinophilic expression of IL-10 and transforming growth factor (TGF) Β also can suppress local inflammation by influencing regulatory T cells.6
Additionally, mast cells and basophils are important in the IgE-mediated allergic reaction as well as the host immune response to parasites. When activated, basophils and mast cells produce TNF-α, IL-6, Il-4, IL-5, and IL-13, which contribute to the Th2 inflammatory response; however, the role of mast cells and basophils in scabies infections still is poorly understood.6
Macrophages, neutrophils, and dendritic cells (DCs) contribute to phagocytosis, antigen presentation, and differentiation of T cells, which also contribute to the inflammatory and allergic reactions associated with parasitic infections.6 Macrophages have been found in low numbers in scabies infestation, possibly due to immune-modulating molecules secreted by scabies mites. Early in an infestation, the mites secrete immune-modulating molecules, which inhibit macrophage migration to the site of inflammation, allowing the mites to grow.6 Neutrophils and DCs also are involved in the host immune response to scabies. Neutrophils are the predominant inflammatory cell infiltrate in scabies lesions. The scabies protein SMSB4 inhibits neutrophil opsonization and phagocytosis, thus suppressing bacterial killing.6 Some of the first antigen-presenting cells encountered by the antigen are DCs. They are involved in preparing the antigens for presentation to effector T cells, which leads to T-cell differentiation and activation.6
Cytokines are another important factor in the innate immune response. The host immune response to ordinary scabies is Th1-cell mediated, during which CD4+ and CD8+ T cells secrete IFN-γ, TNF-α, and IL-2.6 Therefore, IFN- γ and TNF-α are elevated in the serum of patients with ordinary scabies. Conversely, the host immune response to CS is Th2-cell mediated. T-helper 2 cells are needed in IgE-mediated hypersensitivity reactions, and they secrete IL-4, IL-5, and IL-13. In the serum of patients with CS, IL-l4, IL-5, and IL-13 are elevated while IFN-γ is decreased.6 Additionally, IL-6, TGF-Β, IL-23, IL-1Β, or IL-18 can induce Th17 cells to generate and secrete IL-17, which enhances the inflammatory response by inducing further expression of TNF-α, IL-1Β, IL-6, keratinocytes, and fibroblasts. T-helper 17 and IL-17 also are involved in psoriasis and atopic dermatitis, as well as Leishmania major and Schistosoma japonicum.6
Regulatory T cells Tregs secrete TGF-Β and IL-10, which suppress pathologic inflammation, and IL-10 is substantially reduced in patients with CS compared to those with ordinary scabies and uninfected control patients. Additionally, IL-10 can inhibit the synthesis of TNF-γ and IFN-α. Reduced IL-10 expression can lead to proliferation of IL-17 secretion, resulting in a regulatory T cell/Th17 dysfunctional immune response.6
Immunoglobulins are antibodies that are involved in the host’s adaptive immune response. The first antibody to appear in response to an antigen is IgM, and IgM bound to scabies antigens is present in 74%6 of patients with ordinary scabies. Because IgM is the first antibody to appear in response to a scabies infection, detection of serum IgM may allow for earlier detection of scabies; however, IgM has a high cross-reactivity between scabies mites and dust mites, which can hinder scabies diagnosis via IgM detection.6
Both patients with ordinary scabies and CS also show an increased circulatory IgG concentration compared to control groups; patients with CS have higher concentrations. Increased IgG also can be in part due to concurrent bacterial infections.6 When IgG or IgM antibodies bind to a pathogen, they activate the complement cascade, which further enhances the activity of these antibodies.9
Additionally, IgA is important in mucosal immune function. In both patients with ordinary scabies and CS, there is increased IgA binding to recombinant scabies mite antigens.6Sarcoptes scabiei proteases that are localized in the mite’s gut and scybala suggest their involvement in mite digestion and burrowing. The increased secretion of these proteases into the host skin may contribute to the increased IgA,9 and these increased IgA levels have been shown to be positively correlated with severity of scabies infection.6
Also essential in allergic and parasitic inflammation, IgE is observed at higher levels in secondary infections of scabies compared to primary infections.6 Additionally, T-cell infiltrates are implicated in adaptive immune response to scabies. CD4+ T cells are the most prevalent T cells in ordinary scabies skin lesions; however, CD4+ T cells are minimal and CD8+ T cells are elevated in CS skin lesions. The increased CD8+ T cells may cause apoptosis of keratinocytes, leading to epidermal hyperproliferation. The apoptotic keratinocytes can secrete cytokines, which can lead to tissue damage.6 These T cells also may be involved in the failure of the skin’s immune system to mount an effective response to the parasite infestation, leading to uncontrolled parasitic growth. Because patients with AIDS who are infected with scabies mites often develop CS, it is also thought that CD4+ T cells are essential in the immune response to scabies.6
Diagnosis and Current Treatment Options
Current diagnosis of scabies is based on mites, eggs, and fecal matter from the host’s skin. Dermoscopy and fluorescent dermoscopy can be helpful in identifying the mites, eggs, and feces on the patient’s skin. Scabies treatment sometimes may be based solely on symptoms without any positive tests.8
Acaricides are the current method of treatment for scabies infestations.5 Acaricides can be expensive and toxic to the environment and food sources,10 and some agents have been associated with neurotoxicity5 in children or the development of certain cancers.11 Although topical acaricides are the standard form of treatment, oral ivermectin also can be used. Ivermectin is not associated with selective fetal toxicity, but there are limited safety data in pregnant women and in children weighing less than 15 kg (33 lb). Additionally, because symptoms typically are not present during an early infection, treating everyone in the household and those who had close contact with the patient can help prevent reinfection.4
Although these drugs have been shown to be effective at treating scabies, scabies mites are becoming increasingly resistant to acaricides.5 There are 4 main proposed mechanisms for why this occurs.12 The first is through voltage-gated sodium channels, which are involved in the normal functioning of neurons and myocytes. Permethrin, a type of acaricide, binds to voltage-gated sodium channels when it is in an open or active state and prevents it from closing. This creates repetitive neuron firing and hyperactivity, which ultimately kills the scabies mite. Some mites have mutated to close this channel, which reduces the binding potential of permethrin. Glutathione S-transferase is another mechanism of resistance. It catalyzes a bond that tags drugs for elimination. Increased activity or expressivity of glutathione S-transferase by scabies mites can lead to drug resistance.12 Adenosine triphosphate– binding cassette (ABC) transporters also may contribute to this resistance. The ABC transporters use adenosine triphosphate to facilitate the import or export of molecules. Scabies mites express a protein called the multidrug-resistant protein, which is an ABC transporter that is associated with drug resistance and is present in scabies mites.12 Lastly, ligand-gated chloride channels have been implicated in scabies resistance to acaricides. Ligand-gated chloride channels also are important in normal functioning of neurons and myocytes. Some antiparasitic drugs act on these channels, leading to a continuous influx of chloride, but some scabies mites have mutated this pathway.12
Pesticides and the Risk for Cancer
Pesticides commonly are used to treat scabies; however, a link between pesticide exposure and leukemia and lymphoma has been seen through epidemiologic studies, and there also is increasing biological evidence to suggest this.11 For example, the pesticide permethrin, which works by paralyzing the nervous system of insects,13 has been associated with an increased risk for leukemia and lymphoma in humans. Permethrin is a pyrethroid and, compared to control patients, children with leukemia had higher levels of pyrethroid metabolites in their blood.14 Numerical and structural chromosomal aberrations that give rise to gene fusions are the most common abnormalities seen in leukemia, and permethrin has been shown to induce DNA breaks, chromosome aberrations, and sister chromatid exchanges.14 Permethrin also has been associated with an increased risk for multiple myeloma.13
Furthermore, in utero exposure to pesticides has been associated with an increased risk for childhood leukemia.15 Pesticide exposure shortly before conception, during pregnancy, and after birth is associated with an increased risk for acute lymphocytic leukemia.16 In fact, the children of mothers who were exposed to pesticides 3 months before conception have been found to be at least twice as likely to be diagnosed with acute lymphocytic leukemia within the first year of life compared with children whose mothers were not exposed to pesticides.17 It is hypothesized that permethrin can cross the placenta and alter the hematopoietic precursor cells in the fetus, resulting in leukemogenesis.18 Pyrethroid metabolites also have been detected in umbilical cord blood samples and breast milk.15
In contrast to the research demonstrating a link between permethrin and cancer, other studies have found no association between permethrin19 and leukemia20; non-Hodgkin lymphoma19; or cancers of the colon, rectum, pancreas, lungs, skin, female breast, prostate, and urinary bladder.20 Because of conflicting research on the link between permethrin and cancer, more research is needed.,20
Importance of a Scabies Vaccine
Because scabies mites are developing increasing treatment resistance, more radical approaches such as vaccines are becoming important. While a scabies vaccine is still aspirational, animals that have been infected for a second time with scabies demonstrate a milder response to the second infection compared to the first infection, which could mean there is a potential for disease prevention through a vaccine.21 While educating patients and physicians, reporting cases of infection, and improving drug supply and access can help decrease scabies infestations, these are costly and difficult to implement. Scabies already is most prevalent in low-income areas, so costly interventions are even less feasible. An effective, one-dose vaccine would cost less than these efforts and therefore could be implemented more easily.9
In older adults, scabies more often manifests atypically and is more likely to progress to CS. Aged care centers are prone to institutional outbreaks, even in developed countries, so a vaccine also would greatly help this population. Additionally, the number of children attending day care centers, which also are prone to scabies outbreaks, is increasing. When a child contracts scabies, all close contacts need to be treated, so a preventive vaccine can be useful.9
One potential candidate for a scabies vaccine is total mite extract. Studies show that rabbits immunized with a total mite extract induce antibodies to more antigens than rabbits naturally infested with scabies mites; however, the mites cannot be cultured in vitro, which makes obtaining a large amount of their total extract difficult. Therefore, recombinant vaccines also have been proposed, as they are more easily available.22 One recombinant vaccine candidate is recombinant S scabiei serpin (rSs-serpin). Immunization with rSs-serpin has strong immunogenicity and produced immune protection in rabbits.22
Two other recombinant vaccine candidates are the rSs chitinaselike protein (CLP) 12 and the rSsCLP5. Chitinaselike proteins are very similar to chitinases; however, they are unable to degrade chitin. They are involved in immune reactions to infections, and CLPs from scabies mites have been shown to induce the host immune response.22 For example, in a particular rabbit study, rSsCLP5 demonstrated high immunoreactivity and immunogenicity. In fact, after exposure to S scabiei, 74.3% of rabbits who were vaccinated with rSsCLP5 had no detectable lesions.5 Also, after immunization with rSsCLP5 and rSsCLP12, there were increased levels of specific IgG and IgE antibodies produced and decreased numbers of infesting mites.22 Weight loss also is associated with severe scabies infection. Rabbits vaccinated with rSsCLP5 and exposed to the parasite gained weight, indicating protection via rSsCLP5. Even rabbits who did develop symptoms of scabies after immunization with rSsCLP5 and exposure to S scabiei showed less serious manifestations.5
A combination vaccine cocktail of rSs-serpin, rSsCLP12, and rSsCLP5 also has been proposed by Shen et al.22 Four test groups and a control group (n=12 per group) were included in a vaccine trial. Between 83.33% and 91.67% of rabbits vaccinated with this mixed recombinant cocktail vaccine had no detectable skin lesions from scabies. After immunization with the cocktail vaccine, the specific serum IgG and IgE antibodies also increased. For both IgG and IgE, increased levels were first detected at 1 week postimmunization and peaked at 2 weeks postimmunization.22 A multiepitope vaccine derived from these 3 recombinant proteins also was explored by Shen et al22; fewer rabbits vaccinated with it had no detectable scabies skin lesions compared to those treated with the vaccine cocktail. Although the multiepitope vaccine yielded less immume protection, it was associated with a slower disease course and milder symptoms compared with no vaccination.22
Two more proposed scabies recombinant vaccine candidates are derived from the antigens Ssag1 and Ssag2; however, rabbits vaccinated with Ssag1 or Ssag2 showed no immune protection or mite burden reduction.22 The lack of protection could be due to denaturation or degradation of the protective antigens. It also can be due to the low abundance of these antigens, meaning they may not be vital for the mite’s survival—survival—a potential avenue for future research. The antigens also could have lost their native structure and immunogenic properties during the purification and production process. Therefore, more research is needed to investigate how to purify these vaccines to keep the peptides more structurally similar to their native makeups.10 More research also is needed to better understand the antigen or antigens and their mechanisms that elicit a protective immune response.9
Final Thoughts
Scabies causes severe pruritus in mild cases but also can lead to severe disfigurement, sepsis, and even death. Scabies infestations are seen disproportionately more often in low-income and resource-poor communities, and the current treatment options are less accessible to these populations. Scabies infestations induce a complex immune response that involves multiple aspects of both the innate and adaptive immune systems and can be targeted to create a scabies vaccine. Development of a scabies vaccine is crucial considering the growing resistance to current standard treatments. Acaricides potentially are associated with an increased risk for malignancy, which further amplifies the need for a scabies vaccine. There currently are multiple promising scabies vaccine candidates; however, more research is needed to better understand the host’s immune response to scabies as well as how to more accurately and efficiently produce the vaccine. The development of a safe, effective, economical vaccine that can be mass distributed would be beneficial in the treatment of scabies, especially in resource-poor communities.
The scabies mite, originally known as Acarus scabiei,1 now is considered an arthropod of the class Arachnida, order Astigmata, and family Sarcoptidae.2 Scabies mites are able to adhere to the surface of human skin.3 The mites burrow and lay eggs in the top layer of the epidermis; most patients have 10 to 15 mites.3 The patient’s immune system incites an allergic reaction to the mite protein and feces in the skin, causing itching and rash.4
Scabies is common in indigenous populations and in low-income areas of developing countries.5 It is most prevalent in Africa, South America, Australia, and Southeast Asia, in part due to poverty, poor nutritional status, homelessness, and inadequate hygiene.2 In 2009, the World Health Organization declared scabies a neglected skin disease2; however, in 2010, 1.5 million disability adjusted life-years were attributed to scabies,6 and it is estimated that 200 million people worldwide have scabies at any given time. Children and elderly individuals in resource-poor communities are the most at risk. In fact, 5% to 50% of children in low-income areas have scabies.4
The purpose of this article is to provide background on scabies and its effect on the human immune system. We also discuss manipulation of the immune response for the purposes of creating a potential scabies vaccine.
Life Cycle and Transmission
The life cycle of Sarcoptes scabiei consists of 4 stages. The first is the egg. As female scabies mites burrow under the skin, they lay 2 to 3 ovular eggs per day.3 The second stage is the larva. When the egg hatches, the larva has 3 pairs of legs and travels to the surface of the skin where it burrows into the stratum corneum, creating short, nearly invisible burrows called molting pouches. After 3 to 4 days, the larva molts into a nymph, which is the third stage. The nymph has 4 pairs of legs and will continue to grow before molting into an adult, which is the fourth stage. Both the larva and nymph may be found in hair follicles or molting pouches. The fourth stage is the adult, which is round and saclike and does not have eyes. Adult females are 0.30 mm to 0.45 mm long and 0.25 mm to 0.35 mm wide, which is half the size of adult males.3 On warm skin, the female mite can crawl at a rate of 2.5 cm per minute.7
Scabies mites mate via an active male penetrating the molting pouch of a female. This only occurs once but leaves the female fertile for the rest of her life. Once a female is pregnant, she leaves her molting pouch and travels along the surface of the skin looking for a place to make her permanent burrow.3 The most common sites for scabies burrows are the axillae, umbilicus, interdigital spaces, beltline, buttocks, flexor surfaces of the wrists, female nipples, and male penile shaft.5 Once she finds an acceptable location, the female scabies mite will create a serpentine burrow and lay her eggs. Once she burrows, she will stay there and continue to lay eggs for the rest of her life, lengthening the burrow as needed.3 Female mites lay their eggs in the superficial epidermis, and the eggs take approximately 2 to 3 weeks to hatch. Female mites die 30 to 60 days later.2
Scabies infestations typically spread via the transfer of pregnant adult females during skin-to-skin contact, but they also can spread via fomites.3 During all stages of their life cycle, scabies mites can secrete enzymes that allow them to penetrate the intact epidermis in less than 30 minutes; in fact, an otherwise healthy patient with scabies must have 15 to 20 minutes of close skin-to-skin contact with an infected individual for the disease to be transmitted.7 Because scabies mites can survive for more than 3 days outside the human body, it is thought that fomites also may be involved in transmission. Scabies mites also have been collected from clothing, bedding, and furniture, which further supports the idea that fomites are involved in disease transmission.7
Clinical Manifestation of Scabies
Scabies symptoms include severe pruritus as well as linear burrows and vesicles in the interdigital spaces on the hands, wrists, arms and legs, and lower abdomen. Infants and young children also can develop a rash on the palms, soles, ankles, and scalp. Men can develop inflammatory scabies nodules on the penis and scrotum, while women can develop these nodules on the nipple.4 Type I and type IV hypersensitivity reactions contribute to the rash and itching associated with scabies infestation via host allergic and inflammatory reactions to the mites and their byproducts. Patients with scabies typically are infested with fewer than 15 mites,6 but just a few can cause substantial pruritus and scratching, leading to hyperkeratosis.8
Additionally, when patients with scabies scratch the skin, they become vulnerable to bacterial infections.4 Scabies lesions can be coinfected with group A streptococci and Staphylococcus aureus,8 potentially leading to abscesses and septicemia. These secondary infections also can cause renal and cardiac complications; in fact, in tropical areas, scabies infections are considered a risk factor for kidney disease and rheumatic heart disease.4
The 2 main forms of scabies infestations are ordinary and crusted. The most common form is ordinary scabies, which typically manifests with fewer than 15 mites per patient; crusted scabies (CS) is the more rare and extreme form.6 Cases of CS present with thousands to millions of mites per patient, leading to more widespread and severe symptoms.4 Because of the large increase in the number of mites, CS is more contagious than ordinary scabies.6
Patients with CS typically present with hyperkeratotic skin disease, as evidenced by thick scaly crusts with large numbers of mites, which can lead to permanent skin disfiguration. Patients with CS also can develop deep fissuring of the crusts, within which other microbes can gain entry to the body and lead to secondary infection and possibly sepsis and death. Also, because of the increased number of mites as well as the crusted skin, patients with CS are contagious for longer. As it is more difficult to eradicate, reinfestation is common with CS.6
Patients with compromised immune systems are predisposed to CS. Specifically, patients with HIV or human T-lymphotropic virus 1 or those undergoing organ transplantation are thought to be the most at risk for CS.6 Crusted scabies also has been identified in large numbers in patients with Down syndrome and in Aboriginal Australians; however, the reasoning for this is poorly understood.6
Immune Response
The inflammatory reaction associated with scabies infestations occurs 4 to 6 weeks after initial exposure. It is hypothesized that scabies can alter parts of the host immune system, which contributes to the delayed onset of symptoms. Scabies mites also produce inactivated protease paralogues and serpins, which help to protect the mites from the host immune system by inhibiting the complement system.6
The complement system is part of the innate immune response and is the first line of defense against pathogens. Specifically with scabies infestations, C3 and C4 complement components have been found in skin lesions.6 C3a and C4a fragments cause local inflammation, while C3a and C5a activate mast cells to release histamine and tumor necrosis factor (TNF) α, further amplifying the inflammatory response; however, CS lesions show low C3 and C4, which can indicate immunodeficiency in patients with CS. It also can be due to the sheer number of mites in a CS infection causing the host immune system to be overloaded.6
Innate effector immune cells also are an important part of the innate immune response to scabies; for example, eosinophilia is seen in scabies infections. Specifically, in CS, eosinophils help modulate and sustain the T-helper (Th) 2 inflammatory response. One cytokine secreted by Th2 cells is IL-5, which is closely associated with the attraction, maturation, and survival of eosinophils.6 Eosinophils also can influence the Th1 inflammatory response in that they produce IL-12, interferon (IFN) γ, and several Toll-like receptors. Furthermore, eosinophilic expression of IL-2 can lead to expansion of regulatory T cells, while eosinophilic expression of IL-10 and transforming growth factor (TGF) Β also can suppress local inflammation by influencing regulatory T cells.6
Additionally, mast cells and basophils are important in the IgE-mediated allergic reaction as well as the host immune response to parasites. When activated, basophils and mast cells produce TNF-α, IL-6, Il-4, IL-5, and IL-13, which contribute to the Th2 inflammatory response; however, the role of mast cells and basophils in scabies infections still is poorly understood.6
Macrophages, neutrophils, and dendritic cells (DCs) contribute to phagocytosis, antigen presentation, and differentiation of T cells, which also contribute to the inflammatory and allergic reactions associated with parasitic infections.6 Macrophages have been found in low numbers in scabies infestation, possibly due to immune-modulating molecules secreted by scabies mites. Early in an infestation, the mites secrete immune-modulating molecules, which inhibit macrophage migration to the site of inflammation, allowing the mites to grow.6 Neutrophils and DCs also are involved in the host immune response to scabies. Neutrophils are the predominant inflammatory cell infiltrate in scabies lesions. The scabies protein SMSB4 inhibits neutrophil opsonization and phagocytosis, thus suppressing bacterial killing.6 Some of the first antigen-presenting cells encountered by the antigen are DCs. They are involved in preparing the antigens for presentation to effector T cells, which leads to T-cell differentiation and activation.6
Cytokines are another important factor in the innate immune response. The host immune response to ordinary scabies is Th1-cell mediated, during which CD4+ and CD8+ T cells secrete IFN-γ, TNF-α, and IL-2.6 Therefore, IFN- γ and TNF-α are elevated in the serum of patients with ordinary scabies. Conversely, the host immune response to CS is Th2-cell mediated. T-helper 2 cells are needed in IgE-mediated hypersensitivity reactions, and they secrete IL-4, IL-5, and IL-13. In the serum of patients with CS, IL-l4, IL-5, and IL-13 are elevated while IFN-γ is decreased.6 Additionally, IL-6, TGF-Β, IL-23, IL-1Β, or IL-18 can induce Th17 cells to generate and secrete IL-17, which enhances the inflammatory response by inducing further expression of TNF-α, IL-1Β, IL-6, keratinocytes, and fibroblasts. T-helper 17 and IL-17 also are involved in psoriasis and atopic dermatitis, as well as Leishmania major and Schistosoma japonicum.6
Regulatory T cells Tregs secrete TGF-Β and IL-10, which suppress pathologic inflammation, and IL-10 is substantially reduced in patients with CS compared to those with ordinary scabies and uninfected control patients. Additionally, IL-10 can inhibit the synthesis of TNF-γ and IFN-α. Reduced IL-10 expression can lead to proliferation of IL-17 secretion, resulting in a regulatory T cell/Th17 dysfunctional immune response.6
Immunoglobulins are antibodies that are involved in the host’s adaptive immune response. The first antibody to appear in response to an antigen is IgM, and IgM bound to scabies antigens is present in 74%6 of patients with ordinary scabies. Because IgM is the first antibody to appear in response to a scabies infection, detection of serum IgM may allow for earlier detection of scabies; however, IgM has a high cross-reactivity between scabies mites and dust mites, which can hinder scabies diagnosis via IgM detection.6
Both patients with ordinary scabies and CS also show an increased circulatory IgG concentration compared to control groups; patients with CS have higher concentrations. Increased IgG also can be in part due to concurrent bacterial infections.6 When IgG or IgM antibodies bind to a pathogen, they activate the complement cascade, which further enhances the activity of these antibodies.9
Additionally, IgA is important in mucosal immune function. In both patients with ordinary scabies and CS, there is increased IgA binding to recombinant scabies mite antigens.6Sarcoptes scabiei proteases that are localized in the mite’s gut and scybala suggest their involvement in mite digestion and burrowing. The increased secretion of these proteases into the host skin may contribute to the increased IgA,9 and these increased IgA levels have been shown to be positively correlated with severity of scabies infection.6
Also essential in allergic and parasitic inflammation, IgE is observed at higher levels in secondary infections of scabies compared to primary infections.6 Additionally, T-cell infiltrates are implicated in adaptive immune response to scabies. CD4+ T cells are the most prevalent T cells in ordinary scabies skin lesions; however, CD4+ T cells are minimal and CD8+ T cells are elevated in CS skin lesions. The increased CD8+ T cells may cause apoptosis of keratinocytes, leading to epidermal hyperproliferation. The apoptotic keratinocytes can secrete cytokines, which can lead to tissue damage.6 These T cells also may be involved in the failure of the skin’s immune system to mount an effective response to the parasite infestation, leading to uncontrolled parasitic growth. Because patients with AIDS who are infected with scabies mites often develop CS, it is also thought that CD4+ T cells are essential in the immune response to scabies.6
Diagnosis and Current Treatment Options
Current diagnosis of scabies is based on mites, eggs, and fecal matter from the host’s skin. Dermoscopy and fluorescent dermoscopy can be helpful in identifying the mites, eggs, and feces on the patient’s skin. Scabies treatment sometimes may be based solely on symptoms without any positive tests.8
Acaricides are the current method of treatment for scabies infestations.5 Acaricides can be expensive and toxic to the environment and food sources,10 and some agents have been associated with neurotoxicity5 in children or the development of certain cancers.11 Although topical acaricides are the standard form of treatment, oral ivermectin also can be used. Ivermectin is not associated with selective fetal toxicity, but there are limited safety data in pregnant women and in children weighing less than 15 kg (33 lb). Additionally, because symptoms typically are not present during an early infection, treating everyone in the household and those who had close contact with the patient can help prevent reinfection.4
Although these drugs have been shown to be effective at treating scabies, scabies mites are becoming increasingly resistant to acaricides.5 There are 4 main proposed mechanisms for why this occurs.12 The first is through voltage-gated sodium channels, which are involved in the normal functioning of neurons and myocytes. Permethrin, a type of acaricide, binds to voltage-gated sodium channels when it is in an open or active state and prevents it from closing. This creates repetitive neuron firing and hyperactivity, which ultimately kills the scabies mite. Some mites have mutated to close this channel, which reduces the binding potential of permethrin. Glutathione S-transferase is another mechanism of resistance. It catalyzes a bond that tags drugs for elimination. Increased activity or expressivity of glutathione S-transferase by scabies mites can lead to drug resistance.12 Adenosine triphosphate– binding cassette (ABC) transporters also may contribute to this resistance. The ABC transporters use adenosine triphosphate to facilitate the import or export of molecules. Scabies mites express a protein called the multidrug-resistant protein, which is an ABC transporter that is associated with drug resistance and is present in scabies mites.12 Lastly, ligand-gated chloride channels have been implicated in scabies resistance to acaricides. Ligand-gated chloride channels also are important in normal functioning of neurons and myocytes. Some antiparasitic drugs act on these channels, leading to a continuous influx of chloride, but some scabies mites have mutated this pathway.12
Pesticides and the Risk for Cancer
Pesticides commonly are used to treat scabies; however, a link between pesticide exposure and leukemia and lymphoma has been seen through epidemiologic studies, and there also is increasing biological evidence to suggest this.11 For example, the pesticide permethrin, which works by paralyzing the nervous system of insects,13 has been associated with an increased risk for leukemia and lymphoma in humans. Permethrin is a pyrethroid and, compared to control patients, children with leukemia had higher levels of pyrethroid metabolites in their blood.14 Numerical and structural chromosomal aberrations that give rise to gene fusions are the most common abnormalities seen in leukemia, and permethrin has been shown to induce DNA breaks, chromosome aberrations, and sister chromatid exchanges.14 Permethrin also has been associated with an increased risk for multiple myeloma.13
Furthermore, in utero exposure to pesticides has been associated with an increased risk for childhood leukemia.15 Pesticide exposure shortly before conception, during pregnancy, and after birth is associated with an increased risk for acute lymphocytic leukemia.16 In fact, the children of mothers who were exposed to pesticides 3 months before conception have been found to be at least twice as likely to be diagnosed with acute lymphocytic leukemia within the first year of life compared with children whose mothers were not exposed to pesticides.17 It is hypothesized that permethrin can cross the placenta and alter the hematopoietic precursor cells in the fetus, resulting in leukemogenesis.18 Pyrethroid metabolites also have been detected in umbilical cord blood samples and breast milk.15
In contrast to the research demonstrating a link between permethrin and cancer, other studies have found no association between permethrin19 and leukemia20; non-Hodgkin lymphoma19; or cancers of the colon, rectum, pancreas, lungs, skin, female breast, prostate, and urinary bladder.20 Because of conflicting research on the link between permethrin and cancer, more research is needed.,20
Importance of a Scabies Vaccine
Because scabies mites are developing increasing treatment resistance, more radical approaches such as vaccines are becoming important. While a scabies vaccine is still aspirational, animals that have been infected for a second time with scabies demonstrate a milder response to the second infection compared to the first infection, which could mean there is a potential for disease prevention through a vaccine.21 While educating patients and physicians, reporting cases of infection, and improving drug supply and access can help decrease scabies infestations, these are costly and difficult to implement. Scabies already is most prevalent in low-income areas, so costly interventions are even less feasible. An effective, one-dose vaccine would cost less than these efforts and therefore could be implemented more easily.9
In older adults, scabies more often manifests atypically and is more likely to progress to CS. Aged care centers are prone to institutional outbreaks, even in developed countries, so a vaccine also would greatly help this population. Additionally, the number of children attending day care centers, which also are prone to scabies outbreaks, is increasing. When a child contracts scabies, all close contacts need to be treated, so a preventive vaccine can be useful.9
One potential candidate for a scabies vaccine is total mite extract. Studies show that rabbits immunized with a total mite extract induce antibodies to more antigens than rabbits naturally infested with scabies mites; however, the mites cannot be cultured in vitro, which makes obtaining a large amount of their total extract difficult. Therefore, recombinant vaccines also have been proposed, as they are more easily available.22 One recombinant vaccine candidate is recombinant S scabiei serpin (rSs-serpin). Immunization with rSs-serpin has strong immunogenicity and produced immune protection in rabbits.22
Two other recombinant vaccine candidates are the rSs chitinaselike protein (CLP) 12 and the rSsCLP5. Chitinaselike proteins are very similar to chitinases; however, they are unable to degrade chitin. They are involved in immune reactions to infections, and CLPs from scabies mites have been shown to induce the host immune response.22 For example, in a particular rabbit study, rSsCLP5 demonstrated high immunoreactivity and immunogenicity. In fact, after exposure to S scabiei, 74.3% of rabbits who were vaccinated with rSsCLP5 had no detectable lesions.5 Also, after immunization with rSsCLP5 and rSsCLP12, there were increased levels of specific IgG and IgE antibodies produced and decreased numbers of infesting mites.22 Weight loss also is associated with severe scabies infection. Rabbits vaccinated with rSsCLP5 and exposed to the parasite gained weight, indicating protection via rSsCLP5. Even rabbits who did develop symptoms of scabies after immunization with rSsCLP5 and exposure to S scabiei showed less serious manifestations.5
A combination vaccine cocktail of rSs-serpin, rSsCLP12, and rSsCLP5 also has been proposed by Shen et al.22 Four test groups and a control group (n=12 per group) were included in a vaccine trial. Between 83.33% and 91.67% of rabbits vaccinated with this mixed recombinant cocktail vaccine had no detectable skin lesions from scabies. After immunization with the cocktail vaccine, the specific serum IgG and IgE antibodies also increased. For both IgG and IgE, increased levels were first detected at 1 week postimmunization and peaked at 2 weeks postimmunization.22 A multiepitope vaccine derived from these 3 recombinant proteins also was explored by Shen et al22; fewer rabbits vaccinated with it had no detectable scabies skin lesions compared to those treated with the vaccine cocktail. Although the multiepitope vaccine yielded less immume protection, it was associated with a slower disease course and milder symptoms compared with no vaccination.22
Two more proposed scabies recombinant vaccine candidates are derived from the antigens Ssag1 and Ssag2; however, rabbits vaccinated with Ssag1 or Ssag2 showed no immune protection or mite burden reduction.22 The lack of protection could be due to denaturation or degradation of the protective antigens. It also can be due to the low abundance of these antigens, meaning they may not be vital for the mite’s survival—survival—a potential avenue for future research. The antigens also could have lost their native structure and immunogenic properties during the purification and production process. Therefore, more research is needed to investigate how to purify these vaccines to keep the peptides more structurally similar to their native makeups.10 More research also is needed to better understand the antigen or antigens and their mechanisms that elicit a protective immune response.9
Final Thoughts
Scabies causes severe pruritus in mild cases but also can lead to severe disfigurement, sepsis, and even death. Scabies infestations are seen disproportionately more often in low-income and resource-poor communities, and the current treatment options are less accessible to these populations. Scabies infestations induce a complex immune response that involves multiple aspects of both the innate and adaptive immune systems and can be targeted to create a scabies vaccine. Development of a scabies vaccine is crucial considering the growing resistance to current standard treatments. Acaricides potentially are associated with an increased risk for malignancy, which further amplifies the need for a scabies vaccine. There currently are multiple promising scabies vaccine candidates; however, more research is needed to better understand the host’s immune response to scabies as well as how to more accurately and efficiently produce the vaccine. The development of a safe, effective, economical vaccine that can be mass distributed would be beneficial in the treatment of scabies, especially in resource-poor communities.
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Murray RL, Crane JS. Scabies. In: StatPearls. StatPearls Publishing. Updated July 31, 2023.
- Centers for Disease Control and Prevention. CDC—scabies—biology. November 2, 2010. https://www.cdc.gov/dpdx/scabies/index.html
- World Health Organization. Scabies. May 31, 2023. Accessed May 8, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Shen N, Zhang H, Ren Y, et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors. 2018;11:599. doi:10.1186/s13071- 018-3184-y
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Hicks MI, Elston DM. Scabies. Dermatolog Ther. 2009;22:279-292. doi:10.1111/j.1529-8019.2009.01243.x
- Morgan MS, Arlian LG, Rider SD, et al. A proteomic analysis of Sarcoptes scabiei (acari: Sarcoptidae). J Med Entomol. 2016;53:553-561. doi:10.1093/jme/tjv247
- Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725-732. doi:10.1017 /s0031182013002047
- Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors. 2016;9:435. doi:10.1186 /s13071-016-1717-9?
- Navarrete-Meneses MP, Pedraza-Meléndez AI, Salas-Labadía C, et al. Low concentrations of permethrin and malathion induce numerical and structural abnormalities in KMT2A and IGH genes in vitro. J Appl Toxicol. 2018;38:1262-1270. doi:10.1002/jat.3638
- Khalil S, Abbas O, Kibbi AG, et al. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11:E0005920. doi:10.1371 /journal.pntd.0005920
- Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581-586. doi:10.1289 /ehp.11318
- Navarrete-Meneses MP, Salas-Labadía C, Sanabrais-Jiménez M, et al. Exposure to the insecticides permethrin and malathion induces leukemia and lymphoma-associated gene aberrations in vitro. Toxicol In Vitro. 2017;44:17-26. doi:10.1016/j.tiv.2017.06.013
- Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. 2019;34:197-210. doi:10.1515 /reveh-2018-0070
- Madrigal JM, Jones RR, Gunier RB, et al. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2021;201:111501. doi:10.1016/j.envres.2021.111501
- Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children <2 years of age. Environ Health Perspect. 2013;121:269-275. doi:10.1289/ehp.1103942
- Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88:F436-F437. doi:10.1136/fn.88.5.f436
- De Roos AJ, Schinasi LH, Miligi L, et al. Occupational insecticide exposure and risk of non]Hodgkin lymphoma: a pooled case]control study from the InterLymph consortium. Int J Cancer. 2021;149:1768-1786. doi:10.1002/ijc.33740
- Boffett, P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48:433-442. doi:10.1080/1040 8444.2018.1439449
- Adji A, Rumokoy LJM, Salaki CL. Scabies vaccine as a new breakthrough for the challenge of acaricides resistance. Adv Biolog Sci Res. 2020;8:208-213. doi:10.2991/absr.k.200513.036
- Shen N, Wei W, Chen Y, et al. Vaccination with a cocktail vaccine elicits significant protection against Sarcoptes scabiei in rabbits, whereas the multi-epitope vaccine offers limited protection. Exp Parasitol. 2023;245:108442. doi:10.1016/j.exppara.2022.108442
- Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017;10:297. doi:10.1186/s13071-017-2234-1
- Murray RL, Crane JS. Scabies. In: StatPearls. StatPearls Publishing. Updated July 31, 2023.
- Centers for Disease Control and Prevention. CDC—scabies—biology. November 2, 2010. https://www.cdc.gov/dpdx/scabies/index.html
- World Health Organization. Scabies. May 31, 2023. Accessed May 8, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Shen N, Zhang H, Ren Y, et al. A chitinase-like protein from Sarcoptes scabiei as a candidate anti-mite vaccine that contributes to immune protection in rabbits. Parasit Vectors. 2018;11:599. doi:10.1186/s13071- 018-3184-y
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Hicks MI, Elston DM. Scabies. Dermatolog Ther. 2009;22:279-292. doi:10.1111/j.1529-8019.2009.01243.x
- Morgan MS, Arlian LG, Rider SD, et al. A proteomic analysis of Sarcoptes scabiei (acari: Sarcoptidae). J Med Entomol. 2016;53:553-561. doi:10.1093/jme/tjv247
- Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology. 2014;141:725-732. doi:10.1017 /s0031182013002047
- Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors. 2016;9:435. doi:10.1186 /s13071-016-1717-9?
- Navarrete-Meneses MP, Pedraza-Meléndez AI, Salas-Labadía C, et al. Low concentrations of permethrin and malathion induce numerical and structural abnormalities in KMT2A and IGH genes in vitro. J Appl Toxicol. 2018;38:1262-1270. doi:10.1002/jat.3638
- Khalil S, Abbas O, Kibbi AG, et al. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis. 2017;11:E0005920. doi:10.1371 /journal.pntd.0005920
- Rusiecki JA, Patel R, Koutros S, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581-586. doi:10.1289 /ehp.11318
- Navarrete-Meneses MP, Salas-Labadía C, Sanabrais-Jiménez M, et al. Exposure to the insecticides permethrin and malathion induces leukemia and lymphoma-associated gene aberrations in vitro. Toxicol In Vitro. 2017;44:17-26. doi:10.1016/j.tiv.2017.06.013
- Navarrete-Meneses MDP, Pérez-Vera P. Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev Environ Health. 2019;34:197-210. doi:10.1515 /reveh-2018-0070
- Madrigal JM, Jones RR, Gunier RB, et al. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ Res. 2021;201:111501. doi:10.1016/j.envres.2021.111501
- Ferreira JD, Couto AC, Pombo-de-Oliveira MS, et al. In utero pesticide exposure and leukemia in Brazilian children <2 years of age. Environ Health Perspect. 2013;121:269-275. doi:10.1289/ehp.1103942
- Borkhardt A, Wilda M, Fuchs U, et al. Congenital leukaemia after heavy abuse of permethrin during pregnancy. Arch Dis Child Fetal Neonatal Ed. 2003;88:F436-F437. doi:10.1136/fn.88.5.f436
- De Roos AJ, Schinasi LH, Miligi L, et al. Occupational insecticide exposure and risk of non]Hodgkin lymphoma: a pooled case]control study from the InterLymph consortium. Int J Cancer. 2021;149:1768-1786. doi:10.1002/ijc.33740
- Boffett, P, Desai V. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 2018;48:433-442. doi:10.1080/1040 8444.2018.1439449
- Adji A, Rumokoy LJM, Salaki CL. Scabies vaccine as a new breakthrough for the challenge of acaricides resistance. Adv Biolog Sci Res. 2020;8:208-213. doi:10.2991/absr.k.200513.036
- Shen N, Wei W, Chen Y, et al. Vaccination with a cocktail vaccine elicits significant protection against Sarcoptes scabiei in rabbits, whereas the multi-epitope vaccine offers limited protection. Exp Parasitol. 2023;245:108442. doi:10.1016/j.exppara.2022.108442
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
Immune Responses and Health Disparities Warrant Scabies Vaccine Development
PRACTICE POINTS
- Dermatologists should be aware of the impact scabies has on patients, especially on those in lower socioeconomic groups.
- Physicians and patients should be educated on scabies prevention and treatment to help decrease the spread of scabies infections.
Managing Seromas Following Skin Graft Placement in Dermatologic Surgery
Managing Seromas Following Skin Graft Placement in Dermatologic Surgery
A seroma is a collection of serous lymphatic fluid that forms in an anatomic or surgically created dead space—a void left between tissue layers, such as between the skin and underlying tissue, where fluid can accumulate. Seromas represent possible postoperative complications in many types of procedures, including general, oncologic, reconstructive, and dermatologic surgeries.1-3 While seroma formation following dermatologic surgery generally is uncommon, associated procedures include placement of split- or full-thickness skin grafts or liposuction.4,5 Many seromas follow a self-limited course. In some cases, seromas may cause discomfort, recur, or possibly become infected. Surgical techniques for prevention of seroma formation have been described in the dermatologic literature, but discussion of seroma management, particularly in dermatology, is not well documented. In this article, we describe a management approach for primary, recurrent, or late-stage seromas following placement of split- and full-thickness skin grafts in dermatologic surgery.
Practice Gap
To minimize the risk for seroma formation, attention should be paid to reducing dead space during graft placement. Small slits may be created in the skin graft after placement if the graft is larger than 2 to 3 cm in diameter to facilitate fluid drainage.6 Additionally, a tie-over bolster dressing that provides sustained even pressure over the entire graft should be applied and left in place for 1 week.7 Adjunctive measures, such as the use of fibrin sealants or quilting sutures, may further reduce the likelihood of fluid accumulation.7,8 Factors such as obesity, smoking, limited mobility, and inadequate elevation of the extremities undergoing surgery also should be addressed preoperatively to optimize outcomes of skin grafts.
Although these preventive strategies can be used during skin graft placement, seromas still can occur. Seromas typically manifest during the postoperative period after the removal of the protective dressings, including the bolster. The characteristic finding is the formation of a fluid-filled bulla under the graft. The associated serous lymphatic collection usually is yellow-tinged but may appear violaceous if bleeding has occurred beneath the graft. If the patient presents within 24 to 48 hours of seroma formation, the bulla may be tense or slightly tense; however, if days to weeks have passed since the seroma formed, the lesion may undergo fibrosis with thickening of the overlying tissue. If untreated, fibrosis may progress for several weeks, eventually resulting in nodule formation. Chronic seromas with retained fluid will persist for months. Seromas are more likely to develop under larger skin grafts (typically those exceeding 5-10 cm in diameter) or grafts placed in dependent positions, such as areas below the level of the heart where fluid pooling is more likely, especially on the arms and legs with associated movement.
The Technique
Our approach to seroma management is based on the timeline at presentation and whether the seroma is primary or recurrent or demonstrates late fibrosis. Successful management of primary seromas is centered on prompt drainage. Complete drainage using a #11 surgical blade may be accomplished with a single puncture to create a 2- to 3-mm opening for smaller seromas. Larger or multiple seromas under larger skin grafts may require creating multiple small punctures or small slits (ie, 5-10 mm) to allow for adequate drainage and reduce the incidence of seroma reaccumulation. Once successful drainage has occurred, a pressure dressing consisting of a thin layer of petroleum based ointment, a nonadherent dressing, gauze, and secure tape can help reduce the risk for reaccumulation.
Infrequently, seromas will reaccumulate under a skin graft. If this occurs, the graft may appear fibrous with lumps and loculations of seroma fluid separated by intact graft tissue, resulting in a “bound down” appearance (eFigure 1). This may require creating adequate slits for drainage in the graft. Multiple slits should be created if the seroma is larger (typically more than 3-4 cm in diameter) or loculations are present. If the fluid continues to reaccumulate and the drainage slits reseal, the next step is to cut a small hole in the graft to allow for uninterrupted drainage (eFigure 2). Manual digital pressure with moist gauze can assist in decompressing the seroma and removing residual fluid and gelatinous contents, promoting continuous drainage and preventing further fluid buildup (eFigure 3). These openings heal by secondary intention (eFigure 4). Local care during this time also is achieved with a thin layer of ointment, a nonstick pad, gauze, and secure tape. Dressings should be changed every 1 to 2 days until healing is complete.
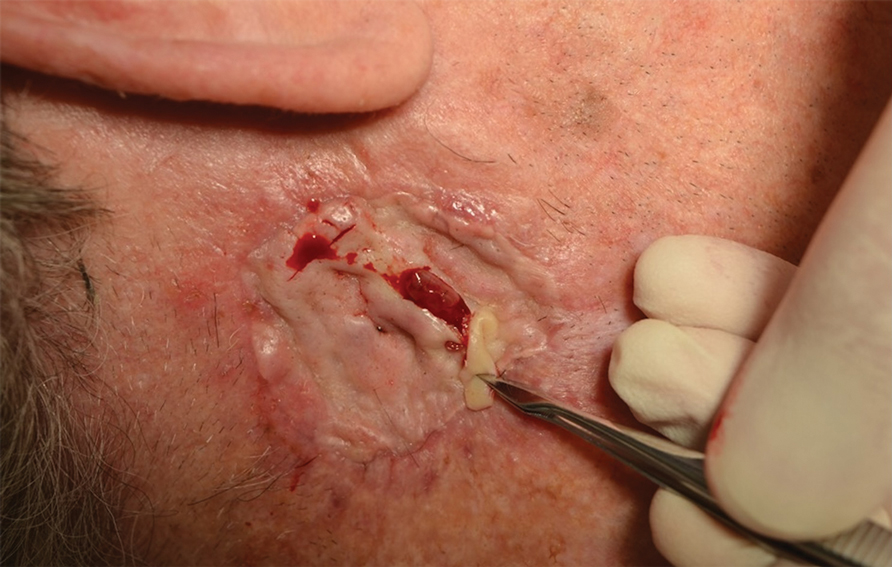
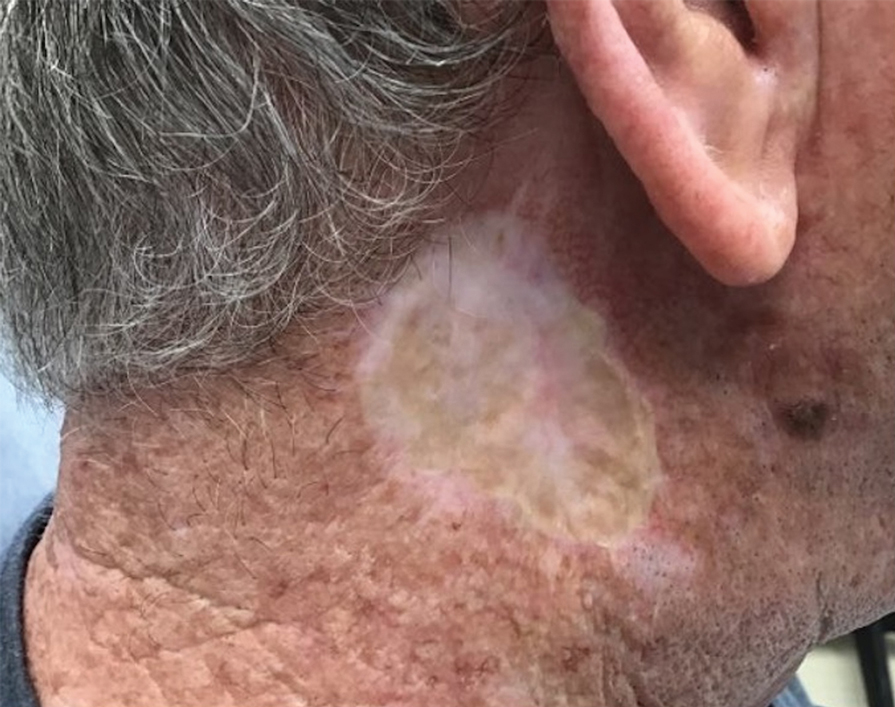
Seromas that lead to fibrotic nodule formation—typically occurring within several weeks to months if untreated—require additional steps for resolution. Once fibrosis occurs, these nodules can be managed by (1) placing adequate local anesthesia, (2) tangentially excising the nodules using either a skin biopsy blade or a #10 or #15 surgical blade, (3) using a handheld heat cautery or electrocautery device to achieve hemostasis, and (4) performing local care, as with any shave or tangential biopsy, until healing is complete. Typically, this requires a single treatment.
Practice Implications
While conservative management with continued compression dressings can be considered for postoperative seroma formation, interventional management sometimes is required. The size and duration of the seroma often guide management. For small seromas (typically less than 2-3 cm in diameter), a small slit incision with a #11 surgical blade may be performed at the dependent point of the seroma. Gentle pressure with a cotton-tipped applicator or moist gauze can be useful to express serous fluid; however, care should be taken not to disrupt adherence of the graft. Recurrent seromas or those with late fibrosis benefit from creation of a surgical window to allow uninterrupted drainage and removal of fibrous components, then can be left to heal by secondary intention with conservative local care.
- DeWitt C, Norris I, Fischer A, et al. A dermatologic approach to a recurrent auricular seroma. Dermatol Surg. 2018;44:1033-1035. doi:10.1097/DSS.0000000000001390
- Woodworth PA, McBoyle MF, Helmer SD, et al. Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg. 2000;66:444-451.
- Salari N, Fatahi B, Bartina Y, et al. The global prevalence of seroma after abdominoplasty: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45:2821-2836. doi:10.1007/s00266-021-02365-6
- Bolognia J, Cerroni L, Schaffer JV. Dermatology. Elsevier; 2018.
- Taha AA, Wahba MM, Tahseen H. Liposuction: drains, are they adequate? Plast Reconstr Surg Glob Open. 2020;8:E2677. doi:10.1097/ GOX.0000000000002677
- Ishii N, Sakai S, Kishi K. A simple and safe method to create a drainage hole for thick skin grafts. Eplasty. 2017;17:ic27.
- Davis M, Baird D, Hill D, et al. Management of full-thickness skin grafts. Proc (Bayl Univ Med Cent). 2021;34:683-686. doi:10.1080 /08998280.2021.1953867
- Mittermayr R, Wassermann E, Thurnher M, et al. Skin graft fixation by slow clotting fibrin sealant applied as a thin layer. Burns. 2006; 32:305-311. doi:10.1016/j.burns.2005.10.010
A seroma is a collection of serous lymphatic fluid that forms in an anatomic or surgically created dead space—a void left between tissue layers, such as between the skin and underlying tissue, where fluid can accumulate. Seromas represent possible postoperative complications in many types of procedures, including general, oncologic, reconstructive, and dermatologic surgeries.1-3 While seroma formation following dermatologic surgery generally is uncommon, associated procedures include placement of split- or full-thickness skin grafts or liposuction.4,5 Many seromas follow a self-limited course. In some cases, seromas may cause discomfort, recur, or possibly become infected. Surgical techniques for prevention of seroma formation have been described in the dermatologic literature, but discussion of seroma management, particularly in dermatology, is not well documented. In this article, we describe a management approach for primary, recurrent, or late-stage seromas following placement of split- and full-thickness skin grafts in dermatologic surgery.
Practice Gap
To minimize the risk for seroma formation, attention should be paid to reducing dead space during graft placement. Small slits may be created in the skin graft after placement if the graft is larger than 2 to 3 cm in diameter to facilitate fluid drainage.6 Additionally, a tie-over bolster dressing that provides sustained even pressure over the entire graft should be applied and left in place for 1 week.7 Adjunctive measures, such as the use of fibrin sealants or quilting sutures, may further reduce the likelihood of fluid accumulation.7,8 Factors such as obesity, smoking, limited mobility, and inadequate elevation of the extremities undergoing surgery also should be addressed preoperatively to optimize outcomes of skin grafts.
Although these preventive strategies can be used during skin graft placement, seromas still can occur. Seromas typically manifest during the postoperative period after the removal of the protective dressings, including the bolster. The characteristic finding is the formation of a fluid-filled bulla under the graft. The associated serous lymphatic collection usually is yellow-tinged but may appear violaceous if bleeding has occurred beneath the graft. If the patient presents within 24 to 48 hours of seroma formation, the bulla may be tense or slightly tense; however, if days to weeks have passed since the seroma formed, the lesion may undergo fibrosis with thickening of the overlying tissue. If untreated, fibrosis may progress for several weeks, eventually resulting in nodule formation. Chronic seromas with retained fluid will persist for months. Seromas are more likely to develop under larger skin grafts (typically those exceeding 5-10 cm in diameter) or grafts placed in dependent positions, such as areas below the level of the heart where fluid pooling is more likely, especially on the arms and legs with associated movement.
The Technique
Our approach to seroma management is based on the timeline at presentation and whether the seroma is primary or recurrent or demonstrates late fibrosis. Successful management of primary seromas is centered on prompt drainage. Complete drainage using a #11 surgical blade may be accomplished with a single puncture to create a 2- to 3-mm opening for smaller seromas. Larger or multiple seromas under larger skin grafts may require creating multiple small punctures or small slits (ie, 5-10 mm) to allow for adequate drainage and reduce the incidence of seroma reaccumulation. Once successful drainage has occurred, a pressure dressing consisting of a thin layer of petroleum based ointment, a nonadherent dressing, gauze, and secure tape can help reduce the risk for reaccumulation.
Infrequently, seromas will reaccumulate under a skin graft. If this occurs, the graft may appear fibrous with lumps and loculations of seroma fluid separated by intact graft tissue, resulting in a “bound down” appearance (eFigure 1). This may require creating adequate slits for drainage in the graft. Multiple slits should be created if the seroma is larger (typically more than 3-4 cm in diameter) or loculations are present. If the fluid continues to reaccumulate and the drainage slits reseal, the next step is to cut a small hole in the graft to allow for uninterrupted drainage (eFigure 2). Manual digital pressure with moist gauze can assist in decompressing the seroma and removing residual fluid and gelatinous contents, promoting continuous drainage and preventing further fluid buildup (eFigure 3). These openings heal by secondary intention (eFigure 4). Local care during this time also is achieved with a thin layer of ointment, a nonstick pad, gauze, and secure tape. Dressings should be changed every 1 to 2 days until healing is complete.




Seromas that lead to fibrotic nodule formation—typically occurring within several weeks to months if untreated—require additional steps for resolution. Once fibrosis occurs, these nodules can be managed by (1) placing adequate local anesthesia, (2) tangentially excising the nodules using either a skin biopsy blade or a #10 or #15 surgical blade, (3) using a handheld heat cautery or electrocautery device to achieve hemostasis, and (4) performing local care, as with any shave or tangential biopsy, until healing is complete. Typically, this requires a single treatment.
Practice Implications
While conservative management with continued compression dressings can be considered for postoperative seroma formation, interventional management sometimes is required. The size and duration of the seroma often guide management. For small seromas (typically less than 2-3 cm in diameter), a small slit incision with a #11 surgical blade may be performed at the dependent point of the seroma. Gentle pressure with a cotton-tipped applicator or moist gauze can be useful to express serous fluid; however, care should be taken not to disrupt adherence of the graft. Recurrent seromas or those with late fibrosis benefit from creation of a surgical window to allow uninterrupted drainage and removal of fibrous components, then can be left to heal by secondary intention with conservative local care.
A seroma is a collection of serous lymphatic fluid that forms in an anatomic or surgically created dead space—a void left between tissue layers, such as between the skin and underlying tissue, where fluid can accumulate. Seromas represent possible postoperative complications in many types of procedures, including general, oncologic, reconstructive, and dermatologic surgeries.1-3 While seroma formation following dermatologic surgery generally is uncommon, associated procedures include placement of split- or full-thickness skin grafts or liposuction.4,5 Many seromas follow a self-limited course. In some cases, seromas may cause discomfort, recur, or possibly become infected. Surgical techniques for prevention of seroma formation have been described in the dermatologic literature, but discussion of seroma management, particularly in dermatology, is not well documented. In this article, we describe a management approach for primary, recurrent, or late-stage seromas following placement of split- and full-thickness skin grafts in dermatologic surgery.
Practice Gap
To minimize the risk for seroma formation, attention should be paid to reducing dead space during graft placement. Small slits may be created in the skin graft after placement if the graft is larger than 2 to 3 cm in diameter to facilitate fluid drainage.6 Additionally, a tie-over bolster dressing that provides sustained even pressure over the entire graft should be applied and left in place for 1 week.7 Adjunctive measures, such as the use of fibrin sealants or quilting sutures, may further reduce the likelihood of fluid accumulation.7,8 Factors such as obesity, smoking, limited mobility, and inadequate elevation of the extremities undergoing surgery also should be addressed preoperatively to optimize outcomes of skin grafts.
Although these preventive strategies can be used during skin graft placement, seromas still can occur. Seromas typically manifest during the postoperative period after the removal of the protective dressings, including the bolster. The characteristic finding is the formation of a fluid-filled bulla under the graft. The associated serous lymphatic collection usually is yellow-tinged but may appear violaceous if bleeding has occurred beneath the graft. If the patient presents within 24 to 48 hours of seroma formation, the bulla may be tense or slightly tense; however, if days to weeks have passed since the seroma formed, the lesion may undergo fibrosis with thickening of the overlying tissue. If untreated, fibrosis may progress for several weeks, eventually resulting in nodule formation. Chronic seromas with retained fluid will persist for months. Seromas are more likely to develop under larger skin grafts (typically those exceeding 5-10 cm in diameter) or grafts placed in dependent positions, such as areas below the level of the heart where fluid pooling is more likely, especially on the arms and legs with associated movement.
The Technique
Our approach to seroma management is based on the timeline at presentation and whether the seroma is primary or recurrent or demonstrates late fibrosis. Successful management of primary seromas is centered on prompt drainage. Complete drainage using a #11 surgical blade may be accomplished with a single puncture to create a 2- to 3-mm opening for smaller seromas. Larger or multiple seromas under larger skin grafts may require creating multiple small punctures or small slits (ie, 5-10 mm) to allow for adequate drainage and reduce the incidence of seroma reaccumulation. Once successful drainage has occurred, a pressure dressing consisting of a thin layer of petroleum based ointment, a nonadherent dressing, gauze, and secure tape can help reduce the risk for reaccumulation.
Infrequently, seromas will reaccumulate under a skin graft. If this occurs, the graft may appear fibrous with lumps and loculations of seroma fluid separated by intact graft tissue, resulting in a “bound down” appearance (eFigure 1). This may require creating adequate slits for drainage in the graft. Multiple slits should be created if the seroma is larger (typically more than 3-4 cm in diameter) or loculations are present. If the fluid continues to reaccumulate and the drainage slits reseal, the next step is to cut a small hole in the graft to allow for uninterrupted drainage (eFigure 2). Manual digital pressure with moist gauze can assist in decompressing the seroma and removing residual fluid and gelatinous contents, promoting continuous drainage and preventing further fluid buildup (eFigure 3). These openings heal by secondary intention (eFigure 4). Local care during this time also is achieved with a thin layer of ointment, a nonstick pad, gauze, and secure tape. Dressings should be changed every 1 to 2 days until healing is complete.




Seromas that lead to fibrotic nodule formation—typically occurring within several weeks to months if untreated—require additional steps for resolution. Once fibrosis occurs, these nodules can be managed by (1) placing adequate local anesthesia, (2) tangentially excising the nodules using either a skin biopsy blade or a #10 or #15 surgical blade, (3) using a handheld heat cautery or electrocautery device to achieve hemostasis, and (4) performing local care, as with any shave or tangential biopsy, until healing is complete. Typically, this requires a single treatment.
Practice Implications
While conservative management with continued compression dressings can be considered for postoperative seroma formation, interventional management sometimes is required. The size and duration of the seroma often guide management. For small seromas (typically less than 2-3 cm in diameter), a small slit incision with a #11 surgical blade may be performed at the dependent point of the seroma. Gentle pressure with a cotton-tipped applicator or moist gauze can be useful to express serous fluid; however, care should be taken not to disrupt adherence of the graft. Recurrent seromas or those with late fibrosis benefit from creation of a surgical window to allow uninterrupted drainage and removal of fibrous components, then can be left to heal by secondary intention with conservative local care.
- DeWitt C, Norris I, Fischer A, et al. A dermatologic approach to a recurrent auricular seroma. Dermatol Surg. 2018;44:1033-1035. doi:10.1097/DSS.0000000000001390
- Woodworth PA, McBoyle MF, Helmer SD, et al. Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg. 2000;66:444-451.
- Salari N, Fatahi B, Bartina Y, et al. The global prevalence of seroma after abdominoplasty: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45:2821-2836. doi:10.1007/s00266-021-02365-6
- Bolognia J, Cerroni L, Schaffer JV. Dermatology. Elsevier; 2018.
- Taha AA, Wahba MM, Tahseen H. Liposuction: drains, are they adequate? Plast Reconstr Surg Glob Open. 2020;8:E2677. doi:10.1097/ GOX.0000000000002677
- Ishii N, Sakai S, Kishi K. A simple and safe method to create a drainage hole for thick skin grafts. Eplasty. 2017;17:ic27.
- Davis M, Baird D, Hill D, et al. Management of full-thickness skin grafts. Proc (Bayl Univ Med Cent). 2021;34:683-686. doi:10.1080 /08998280.2021.1953867
- Mittermayr R, Wassermann E, Thurnher M, et al. Skin graft fixation by slow clotting fibrin sealant applied as a thin layer. Burns. 2006; 32:305-311. doi:10.1016/j.burns.2005.10.010
- DeWitt C, Norris I, Fischer A, et al. A dermatologic approach to a recurrent auricular seroma. Dermatol Surg. 2018;44:1033-1035. doi:10.1097/DSS.0000000000001390
- Woodworth PA, McBoyle MF, Helmer SD, et al. Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg. 2000;66:444-451.
- Salari N, Fatahi B, Bartina Y, et al. The global prevalence of seroma after abdominoplasty: a systematic review and meta-analysis. Aesthetic Plast Surg. 2021;45:2821-2836. doi:10.1007/s00266-021-02365-6
- Bolognia J, Cerroni L, Schaffer JV. Dermatology. Elsevier; 2018.
- Taha AA, Wahba MM, Tahseen H. Liposuction: drains, are they adequate? Plast Reconstr Surg Glob Open. 2020;8:E2677. doi:10.1097/ GOX.0000000000002677
- Ishii N, Sakai S, Kishi K. A simple and safe method to create a drainage hole for thick skin grafts. Eplasty. 2017;17:ic27.
- Davis M, Baird D, Hill D, et al. Management of full-thickness skin grafts. Proc (Bayl Univ Med Cent). 2021;34:683-686. doi:10.1080 /08998280.2021.1953867
- Mittermayr R, Wassermann E, Thurnher M, et al. Skin graft fixation by slow clotting fibrin sealant applied as a thin layer. Burns. 2006; 32:305-311. doi:10.1016/j.burns.2005.10.010
Managing Seromas Following Skin Graft Placement in Dermatologic Surgery
Managing Seromas Following Skin Graft Placement in Dermatologic Surgery
PRACTICE POINTS
- If seromas are identified early (within 24 to 48 hours postoperatively), prompt drainage with a small incision can prevent complications, such as fibrosis or nodule formation, and improve patient comfort.
- For larger or recurrent seromas, multiple small slits or a surgical window should be created to ensure continuous drainage and prevent reaccumulation. Manual compression with moist gauze also can aid in fluid removal.
- If fibrosis develops and leads to nodule formation, early excision of the fibrotic tissue with local anesthesia is essential for resolution. This approach typically requires a single treatment, with secondary intention healing.
Advocacy and Compliance Issues Impacting Dermatology in 2025
Advocacy and Compliance Issues Impacting Dermatology in 2025
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6
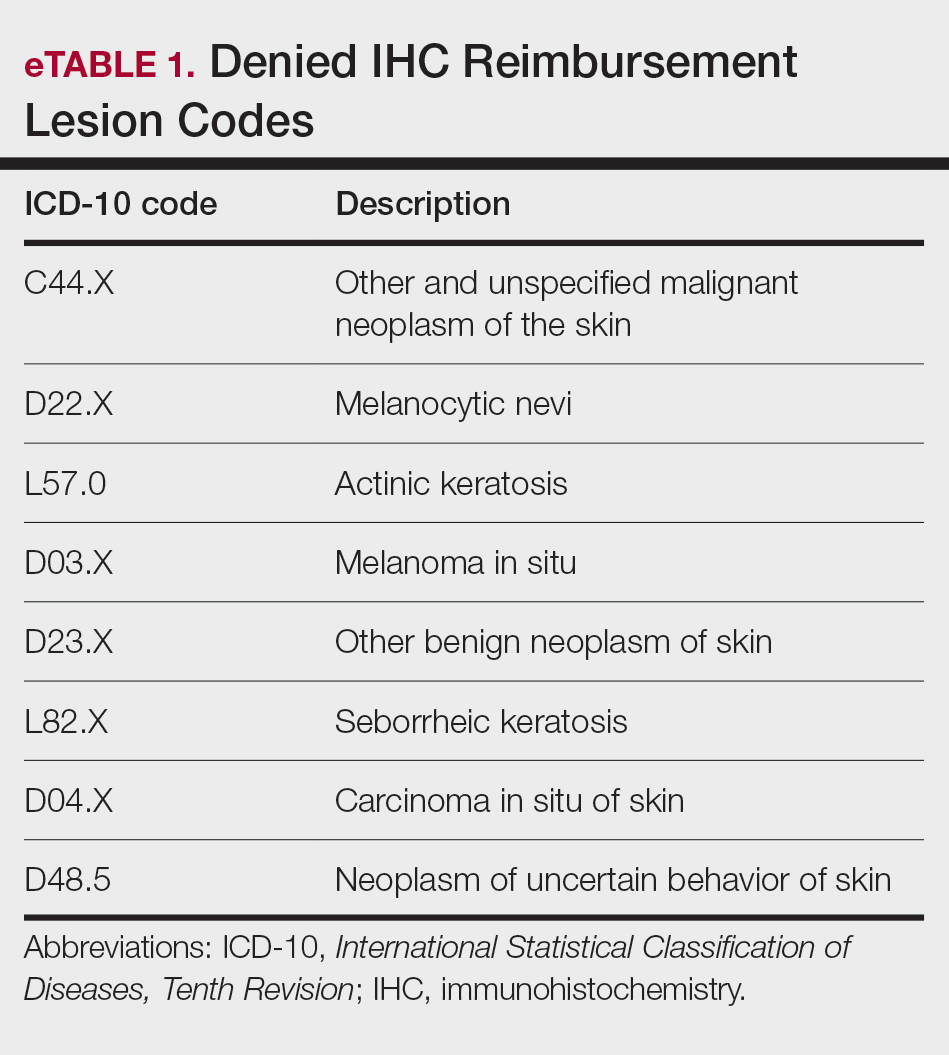

Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6


Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
The US health care system presents major administrative burdens—particularly in coding, billing, and reimbursement—that impact clinical efficiency and patient access. Dermatologists have experienced disproportionate reimbursement declines. A longitudinal review of 20 dermatologic service codes found a 10% average decline in Medicare reimbursement between 2000 and 2020.1 A recent cross-sectional study showed a 4.7% average decline in reimbursement rates from 2007 to 2021 for commonly performed dermatologic procedures, with variation across procedure categories.2 These reductions threaten practice sustainability and highlight the urgent need for comprehensive, long-term payment reform to preserve access to high-quality dermatologic care.
In dermatopathology, policy changes to reimbursement and laboratory oversight directly impact practice operations. Specialty-specific advocacy remains vital in driving policy changes. In this article, we highlight a recent advocacy win—the reversal of immunohistochemistry (IHC) stain denials—and provide updates on a new position statement on IHC guidance. We also outline regulatory changes to the Clinical Laboratory Improvement Amendments (CLIA) of 1988 and College of American Pathologists (CAP) laboratory director requirements and emphasize the importance of continued legislative advocacy.
Reversal of Reimbursement Denials for IHC Stains
EviCore, a medical benefits management company serving over one-third of insured individuals in the United States, is hired by an extensive network of insurance companies to develop clinical and laboratory guidelines and utilization and payment integrity programs.3 EviCore’s laboratory management guidelines for 2024 denied IHC stains (Current Procedural Terminology codes 88341 and 88342) as not medically necessary when associated with specific International Statistical Classification of Diseases, Tenth Revision, skin lesion codes (eTable 1).3-5 These policies caused major disruption to dermatopathology services nationwide, impacting both academic and private laboratories (eTable 2).5 The implementation of such blanket denials interferes with clinical decision-making, compromising diagnostic quality by restricting medically necessary and essential laboratory and pathology services. The American Academy of Dermatology Association (AADA) and CAP leadership formally objected to the policy, citing how these reimbursement denials fail to account for the importance of clinical judgment and diagnostic nuance.6


Thanks to broad advocacy efforts, EviCore updated its guidelines effective January 1, 2025. The skin-related International Statistical Classification of Diseases, Tenth Revision, codes were removed from IHC coverage restrictions, with automatic payment reinstated retroactive to March 15, 2024. EviCore also rescinded language denying reimbursement if a diagnosis could be made without the use of IHC stains.7 While this reversal is a notable achievement, ongoing monitoring of emerging trends in claim denials remains crucial. Continued advocacy, proper documentation, and adherence to American Society of Dermatopathology (ASDP) Appropriate Use Criteria is essential to protecting clinical autonomy.
The AADA’s Dermatopathology Committee developed a new position statement on IHC utilization supporting the advocacy efforts with payers, who recently have tried to implement restrictive limitations.8 Immunohistochemistry is considered a valuable tool for dermatopathology diagnosis, and its utility aids in the confirmation, exclusion, or change in diagnosis.9 By clearly outlining the clinical value of IHC in dermatopathology, this statement reinforces the need to advocate against restrictive payer policies to preserve physician autonomy and promote appropriate, evidence-based use of IHC stains.8
In addition, the ASDP Standards of Practice Committee is working with the Johns Hopkins–Global Appropriateness Measures data-powered analytics platform to develop physician-led IHC benchmarks. The ASDP Appropriate Use Criteria mobile application is a valuable clinical tool for dermatopathologists, general pathologists, dermatologists, and other providers, offering case-based recommendations for test utilization grounded in current evidence.9
Legislative Advocacy: Support for H.R. 879
Physician payment cuts have reached a critical tipping point. Since 2001, physicians have experienced a 33% average reduction in Medicare reimbursement, unadjusted for inflation or rising overhead.10 In January 2025, the Centers for Medicare & Medicaid Services (CMS) imposed a further 2.83% cut, despite projecting a 3.5% increase in the Medicare Economic Index.11,12 Dermatologists and other physician groups cannot continue to absorb these reductions, as they have several consequences, including the inability to maintain practices, forcing some physicians out of business, driving health care consolidation, and limiting patient access.
The Medicare Patient Access and Practice Stabilization Act (H.R. 879)13 is bipartisan legislation that seeks to stop the 2.8% Medicare physician payment cut that went into effect in January 2025, provide physicians with an additional 2% inflation-adjusted payment increase for 2025, and help stabilize Medicare reimbursement rates.13,14 As the impact of continued cuts threatens both patient access and practice viability, member engagement is essential to advancing federal physician payment reform. To support sustainable payment reform and protect access to care, visit the AADA Advocacy Action Center online.14
2025 CLIA and CAP Laboratory Director Requirements: What’s Changing?
As of December 28, 2024, updated CLIA regulations took effect for all laboratories performing moderate- or high-complexity testing. These revisions aim to modernize outdated requirements and update regulations to incorporate technological advancements such as automation and artificial intelligence.15 New CLIA standards require laboratory directors with Doctor of Medicine or Doctor of Osteopathy degrees to be certified in anatomic and/or clinical pathology by the American Board of Pathology or the American Osteopathic Board of Pathology.15 For physicians who do not hold these board-certified qualifications, there are alternative pathways to becoming a laboratory director based on experience and education for physicians licensed to practice in the jurisdiction where the laboratory is located. For high-complexity laboratories, individuals need at least 2 years of experience directing or supervising high-complexity testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities. For moderate-complexity laboratories, individuals need at least 1 year of experience supervising nonwaived laboratory testing and at least 20 continuing education credit hours in laboratory practice that cover director responsibilities.16
If the current laboratory director is not board certified in pathology, the new regulation will permit the grandfathering of current laboratory directors if existing laboratory directors have remained continuously employed in their current role since December 28, 2024.16 Therefore, individuals who were already employed in qualifying positions as of December 28, 2024, will be grandfathered in and will not need to meet the new educational requirements if they remain employed without interruption. All individuals qualifying after December 28, 2024, will be required to do so under the new provisions stated earlier.
The CMS updated laboratory personnel requirements, thereby impacting all CLIA-certified laboratories and those seeking CLIA certification. Likewise, laboratories seeking accreditation by the CAP must meet the new laboratory personnel requirements.17 In some cases, CAP requirements are more stringent than the CLIA regulations (CAP accreditation is more stringent in areas of quality control, personnel qualifications, proficiency testing, and in oversight of laboratory developed tests).15-17 If more stringent state or local regulations are in place for personnel qualifications, including requirements for state licensure, they must be followed.
The AADA formed an ad hoc workgroup to address the CLIA laboratory director requirements and is actively engaging CMS to amend these requirements immediately. Formal objections have been submitted, and direct dialogue with CMS leadership is under way in collaboration with the American Board of Dermatology and leading dermatology and pathology societies.
Final Thoughts
Advocacy remains essential to the future of dermatology. From payer policy reversals to laboratory compliance reforms and federal payment advocacy, physicians must remain engaged. Whether it is safeguarding diagnostic autonomy or securing financial sustainability, we must continue to put “skin in the game.”
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358.
- Miller TC, Rucker P, Armstrong D. “Not medically necessary”: inside the company helping America’s biggest health insurers deny coverage for care. ProPublica. October 23, 2024. Accessed April 23, 2025. https://www.propublica.org/article/evicore-health-insurance-denials-cigna-unitedhealthcare-aetna-prior-authorizations
- EviCore healthcare. Immunohistochemistry (IHC). Lab Management Guidelines v2.0.2024. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/MOL.CS_.104.A_Immunohistochemistry%20%28IHC%29_V2.0.2024_eff11.01.2024_pub12.31.2024.pdf
- EviCore. Laboratory management. Accessed April 23, 2025. https://www.evicore.com/provider/clinical-guidelines-details?solution=laboratory%20management
- Saad AJ. College of American Pathologists. December 12, 2023. Accessed April 23, 2025. https://documents.cap.org/documents /Wellmark-Letter- https://documents.cap.org/documents/wellmarkcap-letter2023.pdf
- EviCore healthcare. Clinical Guidelines: Lab Management Program. Accessed April 23, 2025. https://www.evicore.com/sites/default/files/clinical-guidelines/2024-08/Cigna_LabMgmt_V1.0.2025_eff01.01.2025_pub08.22.2024_0.pdf
- American Academy of Dermatology Association. Position statement on immunohistochemistry utilization. Accessed May 9, 2024. https://server.aad.org/forms/policies/Uploads/PS/PS-Immunohistochemistry%20Utilization.pdf
- Naert KA, Trotter MJ. Utilization and utility of immunohistochemistry in dermatopathology. Am J Dermatopathol. 2013;35:74-77.
- American Medical Association. Medicare physician payment continues to fall further behind practice cost inflation. Accessed April 23, 2025. https:// www.ama-assn.org/system/files/2025-medicare-updates-inflation-chart.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule final rule. Accessed April 23, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rule
- American Medical Association. The Medicare Economic Index. Accessed April 23 2025. https://www.ama-assn.org/system/files/medicare-basics-medicare-economic-index.pdf
- Medicare Patient Access and Practice Stabilization Act, HR 879, 119th Cong (2025). Accessed April 23, 2025. https://www.congress.gov/bill/119th-congress/house-bill/879
- American Academy of Dermatology Association. AADA advocacy action center. Accessed April 23, 2025. https://www.aad.org/member/advocacy/take-action
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Clinical Laboratory Improvement Amendments of 1988 (CLIA) fees; histocompatibility, personnel, and alternative sanctions for certificate of waiver laboratories. Fed Regist. 2023;88:89976-90044.
- College of American Pathologists. CAP accreditation checklists – 2024 edition. Accessed April 23, 2025. https://documents.cap.org/documents/2024-Checklist-Summary.pdf?_gl=1*1b4rei9*_ga*NDc0NjYwNjM5LjE3NDQ3NTI4NjA.*_ga_97ZFJSQQ0X*MTc0NDc2OTc3My40LjEuMTc0NDc2OTgyOC4wLjAuMA
- Bennett SA, Conn CM, Gill HE, et al. Regulatory requirements for laboratory developed tests in the United States. J Immunol Methods. 2025;537:113813.
Advocacy and Compliance Issues Impacting Dermatology in 2025
Advocacy and Compliance Issues Impacting Dermatology in 2025
PRACTICE POINTS
- Recent advocacy efforts have led to the reversal of widespread insurer denials for immunohistochemistry stains; however, continued vigilance is necessary, as restrictive coverage policies may re-emerge.
- Laboratory directors must comply with updated Clinical Laboratory Improvement Amendments of 1988 and College of American Pathologists personnel requirements effective December 28, 2024, including stricter board certification and 2 years of laboratory training or experience and 20 hours of continuing education requirements.
- The American Society of Dermatopathology Appropriate Use Criteria mobile application provides physicians with evidence-based guidance for test selection in dermatopathology.
Scurvy in Hospitalized Patients
Scurvy in Hospitalized Patients
Scurvy, caused by vitamin C or ascorbic acid deficiency, historically has been associated primarily with developing nations and famine; however, specific populations in industrialized nations remain at an increased risk, particularly individuals with a history of smoking, alcohol use, restrictive diet, poor oral intake, psychiatric disorders, dementia, bone marrow transplantation, gastroesophageal reflux disease, end-stage renal disease, and hospitalization.1 Micronutrient deficiency– associated dermatoses have been linked to poor clinical outcomes in hospitalized patients.2 In this case series, we report 4 hospitalized patients with scurvy, each presenting with unique comorbidities and risk factors for vitamin C deficiency (eTable).
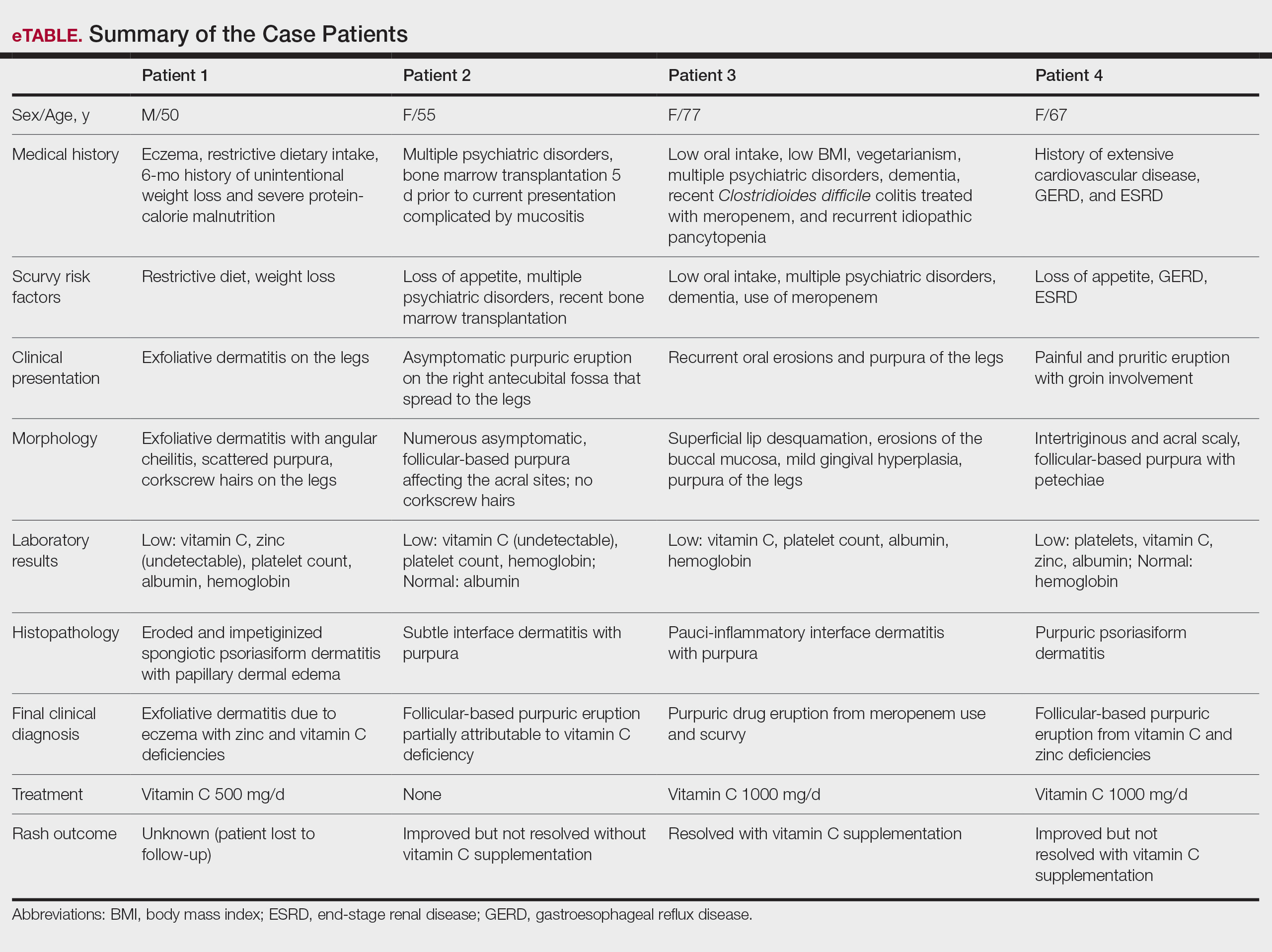
Case Reports
Patient 1—A 50-year-old man with a 6-month history of eczema and restrictive dietary intake was admitted to the hospital for septic shock attributed to a left foot infection of 5-days’ duration. The patient had experienced unintentional weight loss with severe protein-calorie malnutrition. His dietary history was notable for selective eating behaviors, intermittent meal skipping, and vegetarianism. Mucocutaneous examination by the dermatology consult team showed exfoliative dermatitis with angular cheilitis, corkscrew hairs on the legs (eFigure 1), and scattered purpura throughout the body. The differential diagnosis included eczema exacerbation, cutaneous T-cell lymphoma/Sézary syndrome, and malnutrition-related dermatosis. Punch biopsies of the left medial knee and right lateral arm revealed impetiginized, spongiotic, psoriasiform dermatitis with papillary dermal edema. The histologic changes were consistent with malnutrition-related dermatosis. Laboratory results included low vitamin C levels (0.1 mg/dL [reference range, 0.2-2.1 mg/dL]), undetectable zinc levels (<10 μg/dL [reference range ,60-130 μg/dL]), a low platelet count (21 kμ/L [reference range, 150-400 k/μL]),low albumin levels (0.9 mg/dL (13.0 g/dL [reference range, 14.0-17.4 g/dL]). The final diagnosis was exfoliative dermatitis due to eczema and multiple nutrient deficiencies (vitamin C and zinc). The patient was treated with vitamin C 500 mg/d and was started on mirtazapine to improve his appetite. Following a 3-month hospitalization, the patient was lost to follow-up after discharge.
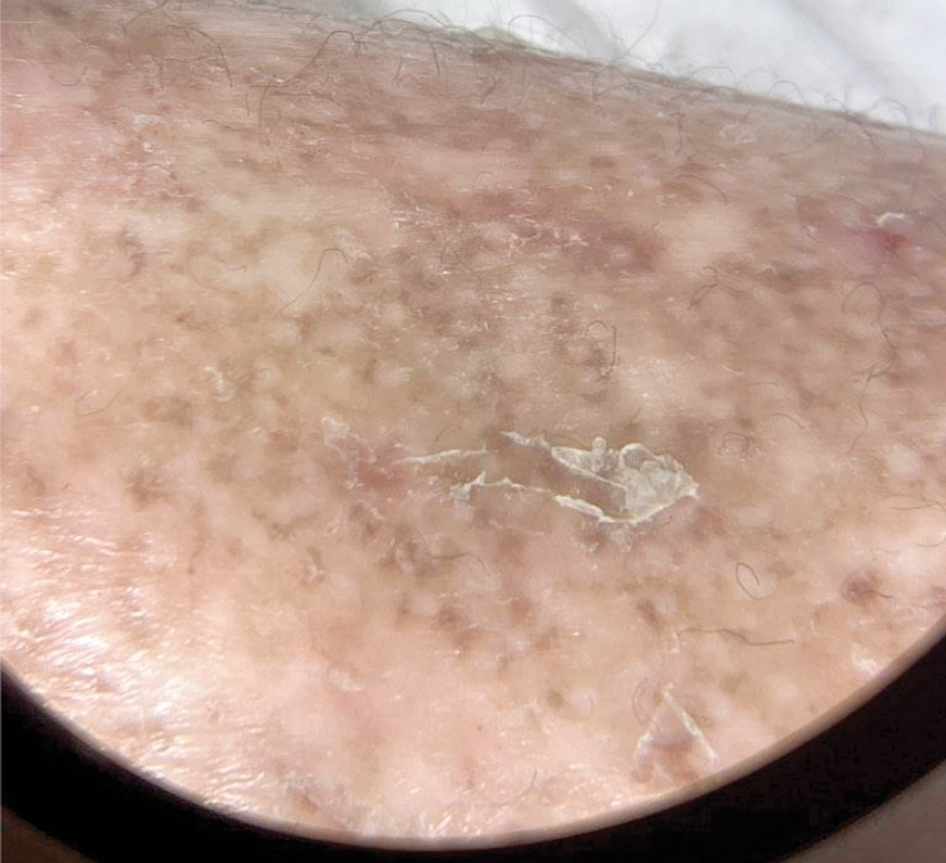
Patient 2—A 55-year-old woman with a history of multiple psychiatric disorders presented to the dermatology consult service with an asymptomatic purpuric eruption on the right antecubital fossa of 2 days’ duration that spread to the proximal thighs. Five days prior to presentation, she had received an allogeneic bone marrow transplant complicated by mucositis. She also reported a 4-month history of decreased appetite. At the current presentation, numerous acral, follicular based, purpuric macules and papules without associated corkscrew hairs were observed (eFigure 2). The differential diagnosis included a purpuric drug reaction, viral exanthem, acute graft-vs-host disease, neutrophilic dermatoses, and vitamin C deficiency–related dermatosis. Laboratory results revealed undetectable vitamin C levels (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (8 k/μL [reference range, 150-400 k/μL]), normal albumin levels (3.7 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (7.8 g/dL [reference range, 14.0-17.4 g/dL]). Based on the histopathologic finding of subtle interface dermatitis with purpura from a punch biopsy of the right forearm, the eruption was attributed to scurvy. Although dermatology recommended supplementation with vitamin C 1000 mg/d, the decision was deferred by the primary team and the purpura improved without it—suggesting the purpura was only partly attributable to low vitamin C.
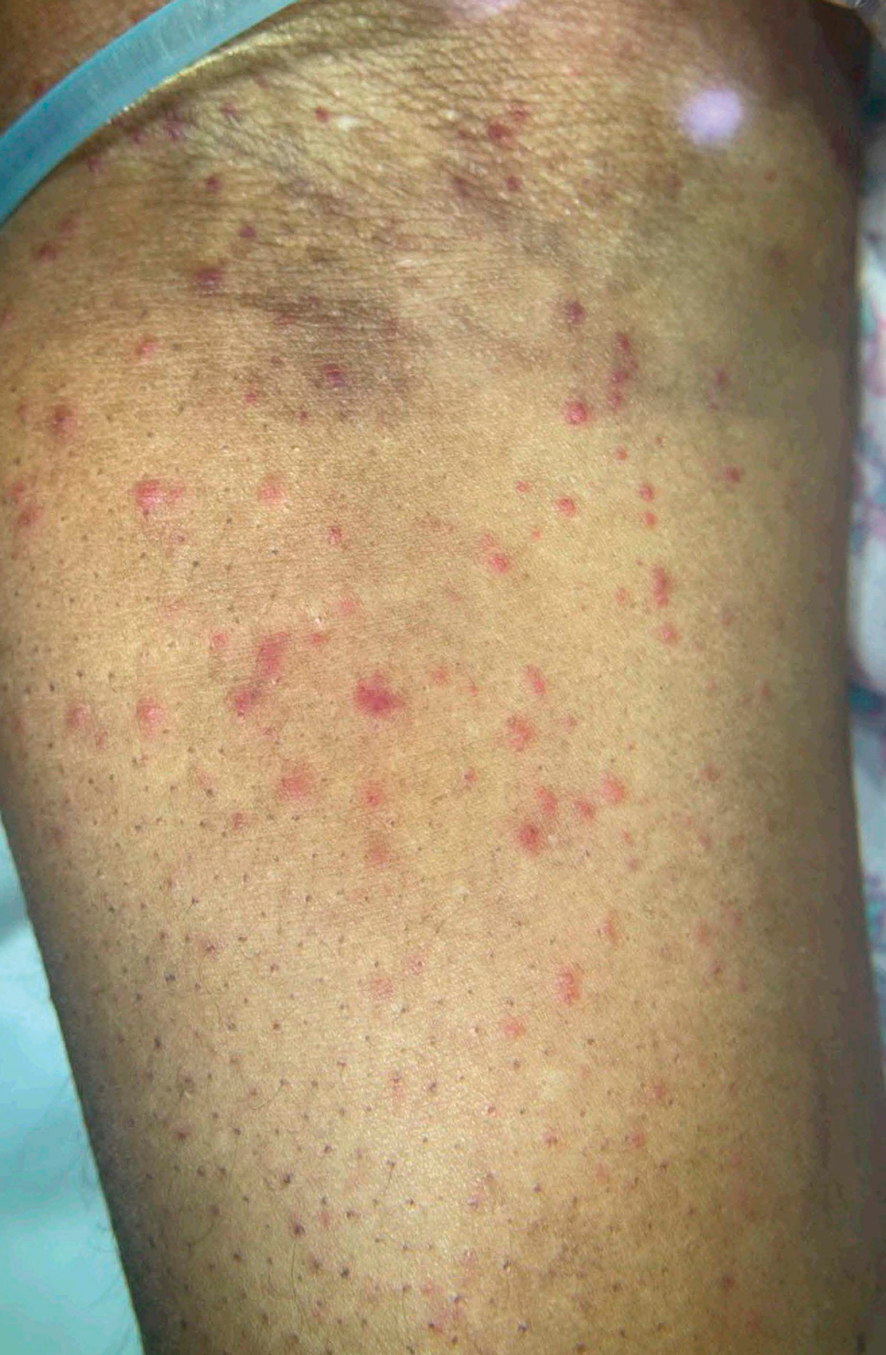
Patient 3—A 77-year-old woman with a history of low oral intake, a low body mass index (18.15 kg/m2 [reference range, 18.5-24.9]), vegetarianism, multiple psychiatric disorders, dementia, recent Clostridioides difficile colitis treated with meropenem, and recurrent idiopathic pancytopenia presented to the hospital with recurrent oral erosions and purpura of the legs for an unknown period. Physical examination by the dermatology consult team revealed superficial lip desquamation; erosions of the buccal mucosa with no involvement of the inner lip or gingiva; mild gingival hyperplasia (eFigure 3); and scaly, purpuric, follicular macules and papules on the legs. The arms and legs were devoid of hair. Laboratory results were notable for low vitamin C levels (0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (28 k/μL [reference range, 150-500 k/μL]), low albumin levels (2.9 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (8.8 g/dL [reference range, 12.0-16.0 g/ dL]). A punch biopsy from the left thigh revealed pauci-inflammatory interface dermatitis with purpura. Based on the clinical and histologic findings, a final diagnosis of purpuric drug eruption (from the meropenem) and scurvy was made. Nutritional support included supplementation with vitamin C 1000 mg/d. The patient’s oral erosions and purpura gradually resolved with treatment throughout her 1.5-month hospitalization.
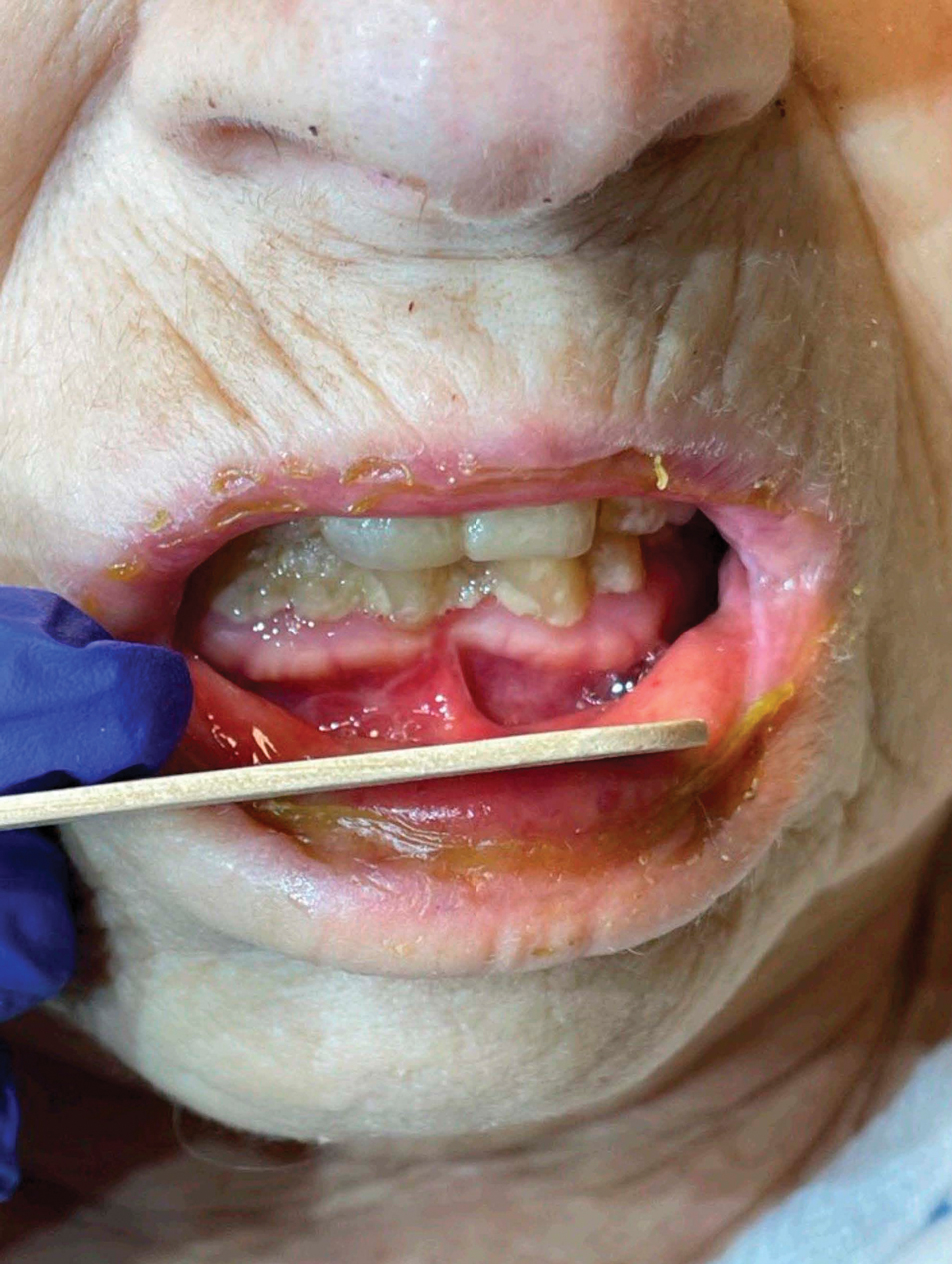
Patient 4—A 67-year-old woman with a history of extensive cardiovascular disease, gastroesophageal reflux disease without esophagitis, end-stage renal disease not requiring hemodialysis, and loss of appetite presented with a painful pruritic eruption on the legs with groin involvement of 2 months’ duration. The patient was admitted to the hospital for worsening mental status and weakness accompanied by dark stools, hematuria, and a productive cough with red-tinged sputum. Physical examination by the dermatology consult team showed a scaly, follicular, purpuric eruption affecting the acral and intertriginous sites (eFigure 4). The patient had sparse leg hair, making it difficult to assess for hair tortuosity. A punch biopsy of the left posterior knee revealed purpuric psoriasiform dermatitis, which was consistent with nutritional deficiency– associated dermatosis. Laboratory results included low vitamin C (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), zinc, (58 μg/dL [reference range, 60-130 μg/dL]), and albumin levels (3.3 g/dL [reference range, 3.5-5.0 g/dL]) and a low platelet count (67 k/μL [reference range, 150- 500 k/μL]). The patient was started on supplementation with vitamin C 1000 mg/d with improvement of the purpura.
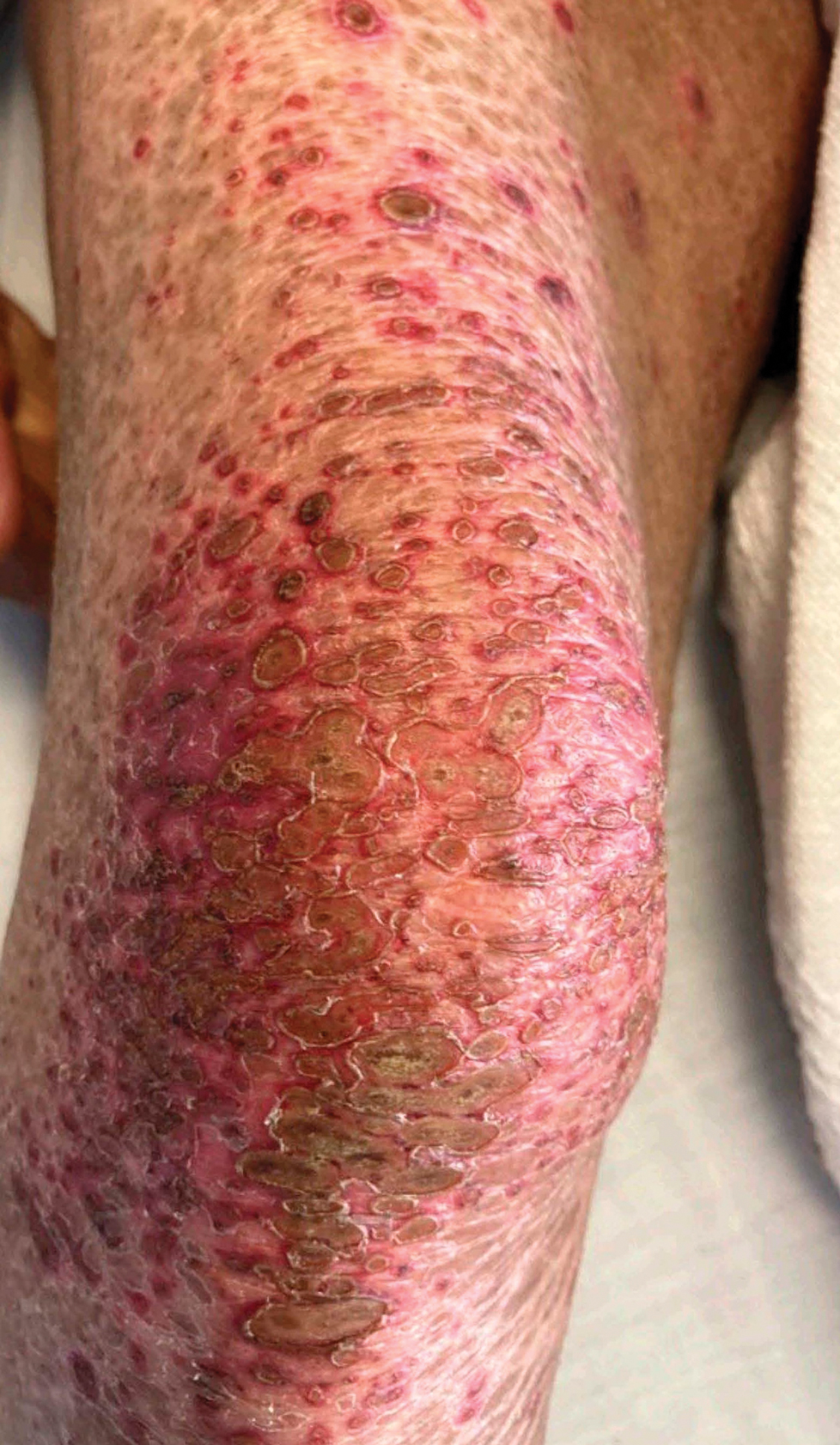
Comment
Micronutrient deficiencies may be common in hospitalized patients due to an increased prevalence of predisposing risk factors including infection, malnutrition, malabsorptive conditions, psychiatric diseases, and chronic illnesses.3 Acute-phase response in hospitalized patients also has been strongly associated with decreased plasma vitamin C levels.4 This phenomenon is postulated to be due to the increase in ascorbic acid uptake by circulating granulocytes in acute disease5; however because low vitamin C levels during the acute-phase response may not always accurately reflect total body stores, other clinical features should be assessed. Previously reported social history risk factors include smoking, alcohol consumption, marijuana use, restrictive diets, vegetarianism, and living alone.6,7
The unifying clinical clues for scurvy in our 4 patients were a history of poor oral intake and purpura. While purpura is nonspecific and can appear after traumatic injury to the skin in elderly patients with photodamage and coagulation disorders, it also is associated with vitamin C deficiency, even with a normal platelet count, circulating von Willebrand factor levels, and prothrombin time/partial thromboplastin time.8 This is because vitamin C is vital in forming the collagen and extracellular matrix. Specifically, it is a cofactor for lysine and proline hydroxylase enzymes needed for the á-helix crosslinks in collagen, which are essential for its structural integrity.9 Collagen is a structural protein that maintains the blood vessel walls, skin, and the basement membrane. A deficiency in vitamin C leads to impairment in collagen synthesis, and insufficient collagen results in compromised connective tissue, blood vessels, and hair strength, which may lead to purpura. All of our patients had thrombocytopenia, and similarly, consideration for scurvy in hospitalized patients with risk factors for micronutrient deficiency is a must. Additional findings such as a follicular-based pattern of the purpura, hair tortuosity, restrictive dietary history, histopathology reports consistent with nutritional dermatoses, serum vitamin C levels, and improvement with vitamin C supplementation are more specific for scurvy. All of these factors can assist the clinician in detecting and confirming these micronutrient deficiencies.
Although there are no established therapeutic guidelines for scurvy, the mainstay of treatment is vitamin C repletion, either orally or parenterally. In hospitalized patients, one suggested regimen is 1000 mg of intravenous ascorbic acid daily for 3 days, followed by further supplementation with a dose of 250 to 500 mg twice daily for 1 month as needed after discharge.10 Symptom improvement occurs about 72 hours after vitamin replacement.8 We recommended 500 to 1000 mg of daily vitamin C supplementation for our patients.
Final Thoughts
This case series highlights the importance of maintaining a high index of suspicion for scurvy in hospitalized patients presenting with purpura, especially in a follicular-based pattern, who have multiple medical comorbidities and risk factors for vitamin C deficiency. The manifestations of scurvy are heterogeneous, necessitating a comprehensive mucocutaneous examination. The diagnosis of scurvy requires correlation of the findings from the patient history, clinical examination, laboratory results, and histopathology.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999; 41:895-910.
- Marsh RL, Trinidad J, Shearer S, et al. Association between micronutrient deficiency dermatoses and clinical outcomes in hospitalized patients. J Am Acad Dermatol. 2020;82:1226-1228.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Moser U, Weber F. Uptake of ascorbic acid by human granulocytes. Int J Vitam Nutr Res. 1984;54:47-53.
- Swanson AM, Hughey LC. Acute inpatient presentation of scurvy. Cutis. 2010;86:205-207.
- Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis. 2019;103:E21-E23.
- Antonelli M, Burzo ML, Pecorini G, et al. Scurvy as cause of purpura in the XXI century: a review on this “ancient” disease. Eur Rev Med Pharmacol Sci. 2018;22:4355-4358.
- Maxfield L, Daley SF, Crane JS. Vitamin C deficiency. StatPearls [Internet]. Updated November 12, 2023. Accessed September 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493187/
- Gandhi M, Elfeky O, Ertugrul H, et al. Scurvy: rediscovering a forgotten disease. Diseases. 2023;11:78.
Scurvy, caused by vitamin C or ascorbic acid deficiency, historically has been associated primarily with developing nations and famine; however, specific populations in industrialized nations remain at an increased risk, particularly individuals with a history of smoking, alcohol use, restrictive diet, poor oral intake, psychiatric disorders, dementia, bone marrow transplantation, gastroesophageal reflux disease, end-stage renal disease, and hospitalization.1 Micronutrient deficiency– associated dermatoses have been linked to poor clinical outcomes in hospitalized patients.2 In this case series, we report 4 hospitalized patients with scurvy, each presenting with unique comorbidities and risk factors for vitamin C deficiency (eTable).

Case Reports
Patient 1—A 50-year-old man with a 6-month history of eczema and restrictive dietary intake was admitted to the hospital for septic shock attributed to a left foot infection of 5-days’ duration. The patient had experienced unintentional weight loss with severe protein-calorie malnutrition. His dietary history was notable for selective eating behaviors, intermittent meal skipping, and vegetarianism. Mucocutaneous examination by the dermatology consult team showed exfoliative dermatitis with angular cheilitis, corkscrew hairs on the legs (eFigure 1), and scattered purpura throughout the body. The differential diagnosis included eczema exacerbation, cutaneous T-cell lymphoma/Sézary syndrome, and malnutrition-related dermatosis. Punch biopsies of the left medial knee and right lateral arm revealed impetiginized, spongiotic, psoriasiform dermatitis with papillary dermal edema. The histologic changes were consistent with malnutrition-related dermatosis. Laboratory results included low vitamin C levels (0.1 mg/dL [reference range, 0.2-2.1 mg/dL]), undetectable zinc levels (<10 μg/dL [reference range ,60-130 μg/dL]), a low platelet count (21 kμ/L [reference range, 150-400 k/μL]),low albumin levels (0.9 mg/dL (13.0 g/dL [reference range, 14.0-17.4 g/dL]). The final diagnosis was exfoliative dermatitis due to eczema and multiple nutrient deficiencies (vitamin C and zinc). The patient was treated with vitamin C 500 mg/d and was started on mirtazapine to improve his appetite. Following a 3-month hospitalization, the patient was lost to follow-up after discharge.

Patient 2—A 55-year-old woman with a history of multiple psychiatric disorders presented to the dermatology consult service with an asymptomatic purpuric eruption on the right antecubital fossa of 2 days’ duration that spread to the proximal thighs. Five days prior to presentation, she had received an allogeneic bone marrow transplant complicated by mucositis. She also reported a 4-month history of decreased appetite. At the current presentation, numerous acral, follicular based, purpuric macules and papules without associated corkscrew hairs were observed (eFigure 2). The differential diagnosis included a purpuric drug reaction, viral exanthem, acute graft-vs-host disease, neutrophilic dermatoses, and vitamin C deficiency–related dermatosis. Laboratory results revealed undetectable vitamin C levels (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (8 k/μL [reference range, 150-400 k/μL]), normal albumin levels (3.7 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (7.8 g/dL [reference range, 14.0-17.4 g/dL]). Based on the histopathologic finding of subtle interface dermatitis with purpura from a punch biopsy of the right forearm, the eruption was attributed to scurvy. Although dermatology recommended supplementation with vitamin C 1000 mg/d, the decision was deferred by the primary team and the purpura improved without it—suggesting the purpura was only partly attributable to low vitamin C.

Patient 3—A 77-year-old woman with a history of low oral intake, a low body mass index (18.15 kg/m2 [reference range, 18.5-24.9]), vegetarianism, multiple psychiatric disorders, dementia, recent Clostridioides difficile colitis treated with meropenem, and recurrent idiopathic pancytopenia presented to the hospital with recurrent oral erosions and purpura of the legs for an unknown period. Physical examination by the dermatology consult team revealed superficial lip desquamation; erosions of the buccal mucosa with no involvement of the inner lip or gingiva; mild gingival hyperplasia (eFigure 3); and scaly, purpuric, follicular macules and papules on the legs. The arms and legs were devoid of hair. Laboratory results were notable for low vitamin C levels (0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (28 k/μL [reference range, 150-500 k/μL]), low albumin levels (2.9 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (8.8 g/dL [reference range, 12.0-16.0 g/ dL]). A punch biopsy from the left thigh revealed pauci-inflammatory interface dermatitis with purpura. Based on the clinical and histologic findings, a final diagnosis of purpuric drug eruption (from the meropenem) and scurvy was made. Nutritional support included supplementation with vitamin C 1000 mg/d. The patient’s oral erosions and purpura gradually resolved with treatment throughout her 1.5-month hospitalization.

Patient 4—A 67-year-old woman with a history of extensive cardiovascular disease, gastroesophageal reflux disease without esophagitis, end-stage renal disease not requiring hemodialysis, and loss of appetite presented with a painful pruritic eruption on the legs with groin involvement of 2 months’ duration. The patient was admitted to the hospital for worsening mental status and weakness accompanied by dark stools, hematuria, and a productive cough with red-tinged sputum. Physical examination by the dermatology consult team showed a scaly, follicular, purpuric eruption affecting the acral and intertriginous sites (eFigure 4). The patient had sparse leg hair, making it difficult to assess for hair tortuosity. A punch biopsy of the left posterior knee revealed purpuric psoriasiform dermatitis, which was consistent with nutritional deficiency– associated dermatosis. Laboratory results included low vitamin C (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), zinc, (58 μg/dL [reference range, 60-130 μg/dL]), and albumin levels (3.3 g/dL [reference range, 3.5-5.0 g/dL]) and a low platelet count (67 k/μL [reference range, 150- 500 k/μL]). The patient was started on supplementation with vitamin C 1000 mg/d with improvement of the purpura.

Comment
Micronutrient deficiencies may be common in hospitalized patients due to an increased prevalence of predisposing risk factors including infection, malnutrition, malabsorptive conditions, psychiatric diseases, and chronic illnesses.3 Acute-phase response in hospitalized patients also has been strongly associated with decreased plasma vitamin C levels.4 This phenomenon is postulated to be due to the increase in ascorbic acid uptake by circulating granulocytes in acute disease5; however because low vitamin C levels during the acute-phase response may not always accurately reflect total body stores, other clinical features should be assessed. Previously reported social history risk factors include smoking, alcohol consumption, marijuana use, restrictive diets, vegetarianism, and living alone.6,7
The unifying clinical clues for scurvy in our 4 patients were a history of poor oral intake and purpura. While purpura is nonspecific and can appear after traumatic injury to the skin in elderly patients with photodamage and coagulation disorders, it also is associated with vitamin C deficiency, even with a normal platelet count, circulating von Willebrand factor levels, and prothrombin time/partial thromboplastin time.8 This is because vitamin C is vital in forming the collagen and extracellular matrix. Specifically, it is a cofactor for lysine and proline hydroxylase enzymes needed for the á-helix crosslinks in collagen, which are essential for its structural integrity.9 Collagen is a structural protein that maintains the blood vessel walls, skin, and the basement membrane. A deficiency in vitamin C leads to impairment in collagen synthesis, and insufficient collagen results in compromised connective tissue, blood vessels, and hair strength, which may lead to purpura. All of our patients had thrombocytopenia, and similarly, consideration for scurvy in hospitalized patients with risk factors for micronutrient deficiency is a must. Additional findings such as a follicular-based pattern of the purpura, hair tortuosity, restrictive dietary history, histopathology reports consistent with nutritional dermatoses, serum vitamin C levels, and improvement with vitamin C supplementation are more specific for scurvy. All of these factors can assist the clinician in detecting and confirming these micronutrient deficiencies.
Although there are no established therapeutic guidelines for scurvy, the mainstay of treatment is vitamin C repletion, either orally or parenterally. In hospitalized patients, one suggested regimen is 1000 mg of intravenous ascorbic acid daily for 3 days, followed by further supplementation with a dose of 250 to 500 mg twice daily for 1 month as needed after discharge.10 Symptom improvement occurs about 72 hours after vitamin replacement.8 We recommended 500 to 1000 mg of daily vitamin C supplementation for our patients.
Final Thoughts
This case series highlights the importance of maintaining a high index of suspicion for scurvy in hospitalized patients presenting with purpura, especially in a follicular-based pattern, who have multiple medical comorbidities and risk factors for vitamin C deficiency. The manifestations of scurvy are heterogeneous, necessitating a comprehensive mucocutaneous examination. The diagnosis of scurvy requires correlation of the findings from the patient history, clinical examination, laboratory results, and histopathology.
Scurvy, caused by vitamin C or ascorbic acid deficiency, historically has been associated primarily with developing nations and famine; however, specific populations in industrialized nations remain at an increased risk, particularly individuals with a history of smoking, alcohol use, restrictive diet, poor oral intake, psychiatric disorders, dementia, bone marrow transplantation, gastroesophageal reflux disease, end-stage renal disease, and hospitalization.1 Micronutrient deficiency– associated dermatoses have been linked to poor clinical outcomes in hospitalized patients.2 In this case series, we report 4 hospitalized patients with scurvy, each presenting with unique comorbidities and risk factors for vitamin C deficiency (eTable).

Case Reports
Patient 1—A 50-year-old man with a 6-month history of eczema and restrictive dietary intake was admitted to the hospital for septic shock attributed to a left foot infection of 5-days’ duration. The patient had experienced unintentional weight loss with severe protein-calorie malnutrition. His dietary history was notable for selective eating behaviors, intermittent meal skipping, and vegetarianism. Mucocutaneous examination by the dermatology consult team showed exfoliative dermatitis with angular cheilitis, corkscrew hairs on the legs (eFigure 1), and scattered purpura throughout the body. The differential diagnosis included eczema exacerbation, cutaneous T-cell lymphoma/Sézary syndrome, and malnutrition-related dermatosis. Punch biopsies of the left medial knee and right lateral arm revealed impetiginized, spongiotic, psoriasiform dermatitis with papillary dermal edema. The histologic changes were consistent with malnutrition-related dermatosis. Laboratory results included low vitamin C levels (0.1 mg/dL [reference range, 0.2-2.1 mg/dL]), undetectable zinc levels (<10 μg/dL [reference range ,60-130 μg/dL]), a low platelet count (21 kμ/L [reference range, 150-400 k/μL]),low albumin levels (0.9 mg/dL (13.0 g/dL [reference range, 14.0-17.4 g/dL]). The final diagnosis was exfoliative dermatitis due to eczema and multiple nutrient deficiencies (vitamin C and zinc). The patient was treated with vitamin C 500 mg/d and was started on mirtazapine to improve his appetite. Following a 3-month hospitalization, the patient was lost to follow-up after discharge.

Patient 2—A 55-year-old woman with a history of multiple psychiatric disorders presented to the dermatology consult service with an asymptomatic purpuric eruption on the right antecubital fossa of 2 days’ duration that spread to the proximal thighs. Five days prior to presentation, she had received an allogeneic bone marrow transplant complicated by mucositis. She also reported a 4-month history of decreased appetite. At the current presentation, numerous acral, follicular based, purpuric macules and papules without associated corkscrew hairs were observed (eFigure 2). The differential diagnosis included a purpuric drug reaction, viral exanthem, acute graft-vs-host disease, neutrophilic dermatoses, and vitamin C deficiency–related dermatosis. Laboratory results revealed undetectable vitamin C levels (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (8 k/μL [reference range, 150-400 k/μL]), normal albumin levels (3.7 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (7.8 g/dL [reference range, 14.0-17.4 g/dL]). Based on the histopathologic finding of subtle interface dermatitis with purpura from a punch biopsy of the right forearm, the eruption was attributed to scurvy. Although dermatology recommended supplementation with vitamin C 1000 mg/d, the decision was deferred by the primary team and the purpura improved without it—suggesting the purpura was only partly attributable to low vitamin C.

Patient 3—A 77-year-old woman with a history of low oral intake, a low body mass index (18.15 kg/m2 [reference range, 18.5-24.9]), vegetarianism, multiple psychiatric disorders, dementia, recent Clostridioides difficile colitis treated with meropenem, and recurrent idiopathic pancytopenia presented to the hospital with recurrent oral erosions and purpura of the legs for an unknown period. Physical examination by the dermatology consult team revealed superficial lip desquamation; erosions of the buccal mucosa with no involvement of the inner lip or gingiva; mild gingival hyperplasia (eFigure 3); and scaly, purpuric, follicular macules and papules on the legs. The arms and legs were devoid of hair. Laboratory results were notable for low vitamin C levels (0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (28 k/μL [reference range, 150-500 k/μL]), low albumin levels (2.9 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (8.8 g/dL [reference range, 12.0-16.0 g/ dL]). A punch biopsy from the left thigh revealed pauci-inflammatory interface dermatitis with purpura. Based on the clinical and histologic findings, a final diagnosis of purpuric drug eruption (from the meropenem) and scurvy was made. Nutritional support included supplementation with vitamin C 1000 mg/d. The patient’s oral erosions and purpura gradually resolved with treatment throughout her 1.5-month hospitalization.

Patient 4—A 67-year-old woman with a history of extensive cardiovascular disease, gastroesophageal reflux disease without esophagitis, end-stage renal disease not requiring hemodialysis, and loss of appetite presented with a painful pruritic eruption on the legs with groin involvement of 2 months’ duration. The patient was admitted to the hospital for worsening mental status and weakness accompanied by dark stools, hematuria, and a productive cough with red-tinged sputum. Physical examination by the dermatology consult team showed a scaly, follicular, purpuric eruption affecting the acral and intertriginous sites (eFigure 4). The patient had sparse leg hair, making it difficult to assess for hair tortuosity. A punch biopsy of the left posterior knee revealed purpuric psoriasiform dermatitis, which was consistent with nutritional deficiency– associated dermatosis. Laboratory results included low vitamin C (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), zinc, (58 μg/dL [reference range, 60-130 μg/dL]), and albumin levels (3.3 g/dL [reference range, 3.5-5.0 g/dL]) and a low platelet count (67 k/μL [reference range, 150- 500 k/μL]). The patient was started on supplementation with vitamin C 1000 mg/d with improvement of the purpura.

Comment
Micronutrient deficiencies may be common in hospitalized patients due to an increased prevalence of predisposing risk factors including infection, malnutrition, malabsorptive conditions, psychiatric diseases, and chronic illnesses.3 Acute-phase response in hospitalized patients also has been strongly associated with decreased plasma vitamin C levels.4 This phenomenon is postulated to be due to the increase in ascorbic acid uptake by circulating granulocytes in acute disease5; however because low vitamin C levels during the acute-phase response may not always accurately reflect total body stores, other clinical features should be assessed. Previously reported social history risk factors include smoking, alcohol consumption, marijuana use, restrictive diets, vegetarianism, and living alone.6,7
The unifying clinical clues for scurvy in our 4 patients were a history of poor oral intake and purpura. While purpura is nonspecific and can appear after traumatic injury to the skin in elderly patients with photodamage and coagulation disorders, it also is associated with vitamin C deficiency, even with a normal platelet count, circulating von Willebrand factor levels, and prothrombin time/partial thromboplastin time.8 This is because vitamin C is vital in forming the collagen and extracellular matrix. Specifically, it is a cofactor for lysine and proline hydroxylase enzymes needed for the á-helix crosslinks in collagen, which are essential for its structural integrity.9 Collagen is a structural protein that maintains the blood vessel walls, skin, and the basement membrane. A deficiency in vitamin C leads to impairment in collagen synthesis, and insufficient collagen results in compromised connective tissue, blood vessels, and hair strength, which may lead to purpura. All of our patients had thrombocytopenia, and similarly, consideration for scurvy in hospitalized patients with risk factors for micronutrient deficiency is a must. Additional findings such as a follicular-based pattern of the purpura, hair tortuosity, restrictive dietary history, histopathology reports consistent with nutritional dermatoses, serum vitamin C levels, and improvement with vitamin C supplementation are more specific for scurvy. All of these factors can assist the clinician in detecting and confirming these micronutrient deficiencies.
Although there are no established therapeutic guidelines for scurvy, the mainstay of treatment is vitamin C repletion, either orally or parenterally. In hospitalized patients, one suggested regimen is 1000 mg of intravenous ascorbic acid daily for 3 days, followed by further supplementation with a dose of 250 to 500 mg twice daily for 1 month as needed after discharge.10 Symptom improvement occurs about 72 hours after vitamin replacement.8 We recommended 500 to 1000 mg of daily vitamin C supplementation for our patients.
Final Thoughts
This case series highlights the importance of maintaining a high index of suspicion for scurvy in hospitalized patients presenting with purpura, especially in a follicular-based pattern, who have multiple medical comorbidities and risk factors for vitamin C deficiency. The manifestations of scurvy are heterogeneous, necessitating a comprehensive mucocutaneous examination. The diagnosis of scurvy requires correlation of the findings from the patient history, clinical examination, laboratory results, and histopathology.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999; 41:895-910.
- Marsh RL, Trinidad J, Shearer S, et al. Association between micronutrient deficiency dermatoses and clinical outcomes in hospitalized patients. J Am Acad Dermatol. 2020;82:1226-1228.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Moser U, Weber F. Uptake of ascorbic acid by human granulocytes. Int J Vitam Nutr Res. 1984;54:47-53.
- Swanson AM, Hughey LC. Acute inpatient presentation of scurvy. Cutis. 2010;86:205-207.
- Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis. 2019;103:E21-E23.
- Antonelli M, Burzo ML, Pecorini G, et al. Scurvy as cause of purpura in the XXI century: a review on this “ancient” disease. Eur Rev Med Pharmacol Sci. 2018;22:4355-4358.
- Maxfield L, Daley SF, Crane JS. Vitamin C deficiency. StatPearls [Internet]. Updated November 12, 2023. Accessed September 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493187/
- Gandhi M, Elfeky O, Ertugrul H, et al. Scurvy: rediscovering a forgotten disease. Diseases. 2023;11:78.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999; 41:895-910.
- Marsh RL, Trinidad J, Shearer S, et al. Association between micronutrient deficiency dermatoses and clinical outcomes in hospitalized patients. J Am Acad Dermatol. 2020;82:1226-1228.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Moser U, Weber F. Uptake of ascorbic acid by human granulocytes. Int J Vitam Nutr Res. 1984;54:47-53.
- Swanson AM, Hughey LC. Acute inpatient presentation of scurvy. Cutis. 2010;86:205-207.
- Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis. 2019;103:E21-E23.
- Antonelli M, Burzo ML, Pecorini G, et al. Scurvy as cause of purpura in the XXI century: a review on this “ancient” disease. Eur Rev Med Pharmacol Sci. 2018;22:4355-4358.
- Maxfield L, Daley SF, Crane JS. Vitamin C deficiency. StatPearls [Internet]. Updated November 12, 2023. Accessed September 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493187/
- Gandhi M, Elfeky O, Ertugrul H, et al. Scurvy: rediscovering a forgotten disease. Diseases. 2023;11:78.
Scurvy in Hospitalized Patients
Scurvy in Hospitalized Patients
PRACTICE POINTS
- Clinicians should maintain a high index of suspicion for vitamin C deficiency/scurvy in hospitalized patients with purpura who have multiple medical comorbidities and risk factors.
- A low platelet count may mask underlying vitamin C deficiency, and patients may have concurrent deficiencies in other nutrients such as zinc.