User login
Transplantation palliative care: The time is ripe
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
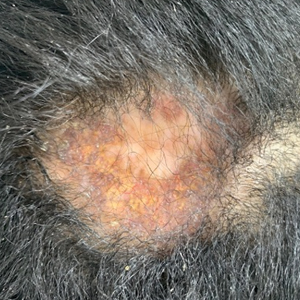
Best Practices: Protecting Dry Vulnerable Skin with CeraVe® Healing Ointment
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
American Hunger Games: Food Insecurity Among the Military and Veterans
American Hunger Games: Food Insecurity Among the Military and Veterans
The requisites of government are that there be sufficiency of food, sufficiency of military equipment, and the confidence of the people in their ruler.
Analects by Confucius1
From ancient festivals to modern holidays, autumn has long been associated with the gathering of the harvest. Friends and families come together around tables laden with delicious food to enjoy the pleasures of peace and plenty. During these celebrations, we must never forget that without the strength of the nation’s military and the service of its veterans, this freedom and abundance would not be possible. Our debt of gratitude to the current and former members of the armed services makes the fact that a substantial minority experiences food insecurity not only a human tragedy, but a travesty of the nation’s promise to support those who wear or have worn the uniform.
The National Defense Authorization Act for Fiscal Year 2020 charged the Secretary of Defense to investigate food insecurity among active-duty service members and their dependents.2 The RAND Corporation conducted the assessment and, based on the results of its analysis, made recommendations to reduce hunger among armed forces members and their families.3
The RAND study found that 10% of active-duty military met US Department of Agriculture (USDA) criteria for very low food security; another 15% were classified as having low food security. The USDA defines food insecurity with hunger as “reports of multiple indications of disrupted eating patterns and reduced food intake.” USDA defines low food security as “reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake.”4
As someone who grew up on an Army base with the commissary a short trip from military housing, I was unpleasantly surprised that food insecurity was more common among in-service members living on post. I was even more dismayed to read that a variety of factors constrained 14% of active-duty military experiencing food insecurity to seek public assistance to feed themselves and their families. As with so many health care and social services, (eg, mental health care), those wearing the uniform were concerned that participating in a food assistance program would damage their career or stigmatize them. Others did not seek help, perhaps because they believed they were not eligible, and in many cases were correct: they did not qualify for food banks or food stamps due to receiving other benefits. A variety of factors contribute to periods of food insecurity among military families, including remote or rural bases that lack access to grocery stores or jobs for partners or other family members, and low base military pay.5
Food insecurity is an even more serious concern among veterans who are frequently older and have more comorbidities, often leading to unemployment and homelessness. Feeding America, the nation’s largest organization of community food banks, estimates that 1 in 9 working-age veterans are food insecure.5 US Department of Veterans Affairs (VA) statistics indicate that veterans are 7% more likely to experience food insecurity than other sectors of the population.6 The Veterans Health Administration has recognized that food insecurity is directly related to medical problems already common among veterans, including diabetes, obesity, and depression. Women and minority veterans are the most at risk of food insecurity.7
Recognizing that many veterans are at risk of food insecurity, the US Department of Defense and VA have taken steps to try and reduce hunger among those who serve. In response to the shocking statistic that food insecurity was found in 27% of Iraq and Afghanistan veterans, the VA and Rockefeller Foundation are partnering on the Food as Medicine initiative to improve veteran nutrition as a means of improving nutrition-related health consequences of food insecurity.8
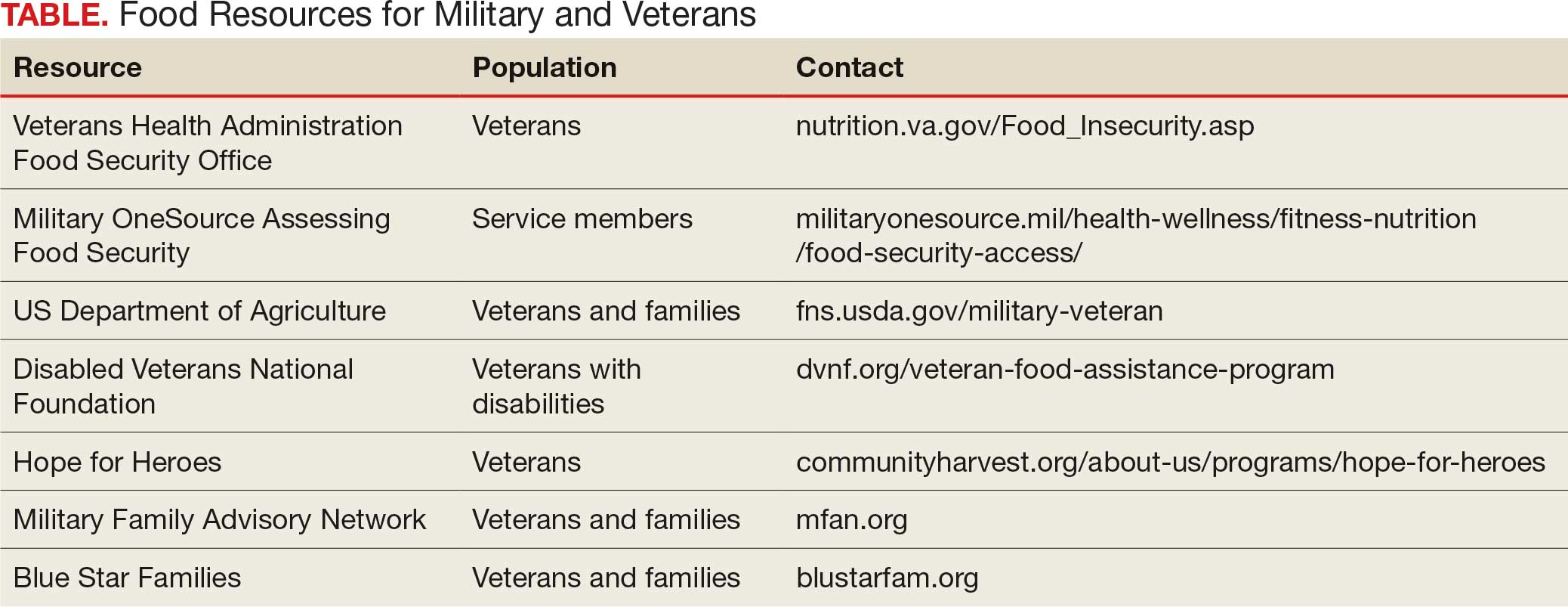
Like many federal practitioners, I was unaware of the food insecurity assistance available to active-duty service members or veterans, or how to help individuals access it. In addition to the resources outlined in the Table, there are many community-based options open to anyone, including veterans and service members.
I have written columns on many difficult issues in my years as the Editor-in-Chief of Federal Practitioner, but personally this is one of the most distressing editorials I have ever published. That individuals dedicated to defending our rights and protecting our safety should be compelled to go hungry or not know if they have enough money at the end of the month to buy food is manifestly unjust. It is challenging when faced with such a large-scale injustice to think we cannot make a difference, but that resignation or abdication only magnifies this inequity. I have a friend who kept giving back even after they retired from federal service: they volunteered at a community garden and brought produce to the local food bank and helped distribute it. That may seem too much for those still working yet almost anyone can pick up a few items on their weekly shopping trip and donate them to a food drive.
As we approach Veterans Day, let’s not just express our gratitude to our military and veterans in words but in deeds like feeding the hungry and urging elected representatives to fulfill their commitment to ensure that service members and veterans and their families do not experience food insecurity. Confucian wisdom written in a very distant time and vastly dissimilar context still rings true: there are direct and critical links between food and trust and between hunger and the military.1
Dawson MM. The Wisdom of Confucius: A Collection of the Ethical Sayings of Confucius and of his disciples. International Pocket Library; 1932.
National Defense Authorization Act for Fiscal Year 2020. 116th Cong (2019), Public Law 116-92. U.S. Government Printing Office. https://www.govinfo.gov/content/pkg/PLAW-116publ92/html/PLAW-116publ92.htm
Asch BJ, Rennane S, Trail TE, et al. Food insecurity among members of the armed forces and their dependents. RAND Corporation. January 3, 2023. Accessed September 22, 2025. https://www.rand.org/pubs/research_reports/RRA1230-1.html
US Department of Agriculture Economic Research Service. Food Security in the U.S.—Definitions of Food Security. US Department of Agriculture Economic Research Service. January 10, 2025. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security
Active military and veteran food insecurity. Feeding America. Accessed September 22, 2025. https://www.feedingamerica.org/hunger-in-america/food-insecurity-in-veterans
Pradun S. Find access to stop food insecurity in your community. VA News. September 19, 2025. Accessed September 22, 2025. https://news.va.gov/142733/find-access-stop-food-insecurity-your-community/
Cohen AJ, Dosa DM, Rudolph JL, et al. Risk factors for veteran food insecurity: findings from a National US Department of Veterans Affairs Food Insecurity Screener. Public Health Nutr. 2022;25:819-828. doi:10.1017/S1368980021004584
Chen C. VA and Rockefeller Foundation collaborate to access food for Veterans. VA News. September 5, 2023. Accessed September 22, 2025. https://news.va.gov/123228/va-rockefeller-foundation-expand-access-to-food/
The requisites of government are that there be sufficiency of food, sufficiency of military equipment, and the confidence of the people in their ruler.
Analects by Confucius1
From ancient festivals to modern holidays, autumn has long been associated with the gathering of the harvest. Friends and families come together around tables laden with delicious food to enjoy the pleasures of peace and plenty. During these celebrations, we must never forget that without the strength of the nation’s military and the service of its veterans, this freedom and abundance would not be possible. Our debt of gratitude to the current and former members of the armed services makes the fact that a substantial minority experiences food insecurity not only a human tragedy, but a travesty of the nation’s promise to support those who wear or have worn the uniform.
The National Defense Authorization Act for Fiscal Year 2020 charged the Secretary of Defense to investigate food insecurity among active-duty service members and their dependents.2 The RAND Corporation conducted the assessment and, based on the results of its analysis, made recommendations to reduce hunger among armed forces members and their families.3
The RAND study found that 10% of active-duty military met US Department of Agriculture (USDA) criteria for very low food security; another 15% were classified as having low food security. The USDA defines food insecurity with hunger as “reports of multiple indications of disrupted eating patterns and reduced food intake.” USDA defines low food security as “reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake.”4
As someone who grew up on an Army base with the commissary a short trip from military housing, I was unpleasantly surprised that food insecurity was more common among in-service members living on post. I was even more dismayed to read that a variety of factors constrained 14% of active-duty military experiencing food insecurity to seek public assistance to feed themselves and their families. As with so many health care and social services, (eg, mental health care), those wearing the uniform were concerned that participating in a food assistance program would damage their career or stigmatize them. Others did not seek help, perhaps because they believed they were not eligible, and in many cases were correct: they did not qualify for food banks or food stamps due to receiving other benefits. A variety of factors contribute to periods of food insecurity among military families, including remote or rural bases that lack access to grocery stores or jobs for partners or other family members, and low base military pay.5
Food insecurity is an even more serious concern among veterans who are frequently older and have more comorbidities, often leading to unemployment and homelessness. Feeding America, the nation’s largest organization of community food banks, estimates that 1 in 9 working-age veterans are food insecure.5 US Department of Veterans Affairs (VA) statistics indicate that veterans are 7% more likely to experience food insecurity than other sectors of the population.6 The Veterans Health Administration has recognized that food insecurity is directly related to medical problems already common among veterans, including diabetes, obesity, and depression. Women and minority veterans are the most at risk of food insecurity.7
Recognizing that many veterans are at risk of food insecurity, the US Department of Defense and VA have taken steps to try and reduce hunger among those who serve. In response to the shocking statistic that food insecurity was found in 27% of Iraq and Afghanistan veterans, the VA and Rockefeller Foundation are partnering on the Food as Medicine initiative to improve veteran nutrition as a means of improving nutrition-related health consequences of food insecurity.8

Like many federal practitioners, I was unaware of the food insecurity assistance available to active-duty service members or veterans, or how to help individuals access it. In addition to the resources outlined in the Table, there are many community-based options open to anyone, including veterans and service members.
I have written columns on many difficult issues in my years as the Editor-in-Chief of Federal Practitioner, but personally this is one of the most distressing editorials I have ever published. That individuals dedicated to defending our rights and protecting our safety should be compelled to go hungry or not know if they have enough money at the end of the month to buy food is manifestly unjust. It is challenging when faced with such a large-scale injustice to think we cannot make a difference, but that resignation or abdication only magnifies this inequity. I have a friend who kept giving back even after they retired from federal service: they volunteered at a community garden and brought produce to the local food bank and helped distribute it. That may seem too much for those still working yet almost anyone can pick up a few items on their weekly shopping trip and donate them to a food drive.
As we approach Veterans Day, let’s not just express our gratitude to our military and veterans in words but in deeds like feeding the hungry and urging elected representatives to fulfill their commitment to ensure that service members and veterans and their families do not experience food insecurity. Confucian wisdom written in a very distant time and vastly dissimilar context still rings true: there are direct and critical links between food and trust and between hunger and the military.1
The requisites of government are that there be sufficiency of food, sufficiency of military equipment, and the confidence of the people in their ruler.
Analects by Confucius1
From ancient festivals to modern holidays, autumn has long been associated with the gathering of the harvest. Friends and families come together around tables laden with delicious food to enjoy the pleasures of peace and plenty. During these celebrations, we must never forget that without the strength of the nation’s military and the service of its veterans, this freedom and abundance would not be possible. Our debt of gratitude to the current and former members of the armed services makes the fact that a substantial minority experiences food insecurity not only a human tragedy, but a travesty of the nation’s promise to support those who wear or have worn the uniform.
The National Defense Authorization Act for Fiscal Year 2020 charged the Secretary of Defense to investigate food insecurity among active-duty service members and their dependents.2 The RAND Corporation conducted the assessment and, based on the results of its analysis, made recommendations to reduce hunger among armed forces members and their families.3
The RAND study found that 10% of active-duty military met US Department of Agriculture (USDA) criteria for very low food security; another 15% were classified as having low food security. The USDA defines food insecurity with hunger as “reports of multiple indications of disrupted eating patterns and reduced food intake.” USDA defines low food security as “reports of reduced quality, variety, or desirability of diet. Little or no indication of reduced food intake.”4
As someone who grew up on an Army base with the commissary a short trip from military housing, I was unpleasantly surprised that food insecurity was more common among in-service members living on post. I was even more dismayed to read that a variety of factors constrained 14% of active-duty military experiencing food insecurity to seek public assistance to feed themselves and their families. As with so many health care and social services, (eg, mental health care), those wearing the uniform were concerned that participating in a food assistance program would damage their career or stigmatize them. Others did not seek help, perhaps because they believed they were not eligible, and in many cases were correct: they did not qualify for food banks or food stamps due to receiving other benefits. A variety of factors contribute to periods of food insecurity among military families, including remote or rural bases that lack access to grocery stores or jobs for partners or other family members, and low base military pay.5
Food insecurity is an even more serious concern among veterans who are frequently older and have more comorbidities, often leading to unemployment and homelessness. Feeding America, the nation’s largest organization of community food banks, estimates that 1 in 9 working-age veterans are food insecure.5 US Department of Veterans Affairs (VA) statistics indicate that veterans are 7% more likely to experience food insecurity than other sectors of the population.6 The Veterans Health Administration has recognized that food insecurity is directly related to medical problems already common among veterans, including diabetes, obesity, and depression. Women and minority veterans are the most at risk of food insecurity.7
Recognizing that many veterans are at risk of food insecurity, the US Department of Defense and VA have taken steps to try and reduce hunger among those who serve. In response to the shocking statistic that food insecurity was found in 27% of Iraq and Afghanistan veterans, the VA and Rockefeller Foundation are partnering on the Food as Medicine initiative to improve veteran nutrition as a means of improving nutrition-related health consequences of food insecurity.8

Like many federal practitioners, I was unaware of the food insecurity assistance available to active-duty service members or veterans, or how to help individuals access it. In addition to the resources outlined in the Table, there are many community-based options open to anyone, including veterans and service members.
I have written columns on many difficult issues in my years as the Editor-in-Chief of Federal Practitioner, but personally this is one of the most distressing editorials I have ever published. That individuals dedicated to defending our rights and protecting our safety should be compelled to go hungry or not know if they have enough money at the end of the month to buy food is manifestly unjust. It is challenging when faced with such a large-scale injustice to think we cannot make a difference, but that resignation or abdication only magnifies this inequity. I have a friend who kept giving back even after they retired from federal service: they volunteered at a community garden and brought produce to the local food bank and helped distribute it. That may seem too much for those still working yet almost anyone can pick up a few items on their weekly shopping trip and donate them to a food drive.
As we approach Veterans Day, let’s not just express our gratitude to our military and veterans in words but in deeds like feeding the hungry and urging elected representatives to fulfill their commitment to ensure that service members and veterans and their families do not experience food insecurity. Confucian wisdom written in a very distant time and vastly dissimilar context still rings true: there are direct and critical links between food and trust and between hunger and the military.1
Dawson MM. The Wisdom of Confucius: A Collection of the Ethical Sayings of Confucius and of his disciples. International Pocket Library; 1932.
National Defense Authorization Act for Fiscal Year 2020. 116th Cong (2019), Public Law 116-92. U.S. Government Printing Office. https://www.govinfo.gov/content/pkg/PLAW-116publ92/html/PLAW-116publ92.htm
Asch BJ, Rennane S, Trail TE, et al. Food insecurity among members of the armed forces and their dependents. RAND Corporation. January 3, 2023. Accessed September 22, 2025. https://www.rand.org/pubs/research_reports/RRA1230-1.html
US Department of Agriculture Economic Research Service. Food Security in the U.S.—Definitions of Food Security. US Department of Agriculture Economic Research Service. January 10, 2025. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security
Active military and veteran food insecurity. Feeding America. Accessed September 22, 2025. https://www.feedingamerica.org/hunger-in-america/food-insecurity-in-veterans
Pradun S. Find access to stop food insecurity in your community. VA News. September 19, 2025. Accessed September 22, 2025. https://news.va.gov/142733/find-access-stop-food-insecurity-your-community/
Cohen AJ, Dosa DM, Rudolph JL, et al. Risk factors for veteran food insecurity: findings from a National US Department of Veterans Affairs Food Insecurity Screener. Public Health Nutr. 2022;25:819-828. doi:10.1017/S1368980021004584
Chen C. VA and Rockefeller Foundation collaborate to access food for Veterans. VA News. September 5, 2023. Accessed September 22, 2025. https://news.va.gov/123228/va-rockefeller-foundation-expand-access-to-food/
Dawson MM. The Wisdom of Confucius: A Collection of the Ethical Sayings of Confucius and of his disciples. International Pocket Library; 1932.
National Defense Authorization Act for Fiscal Year 2020. 116th Cong (2019), Public Law 116-92. U.S. Government Printing Office. https://www.govinfo.gov/content/pkg/PLAW-116publ92/html/PLAW-116publ92.htm
Asch BJ, Rennane S, Trail TE, et al. Food insecurity among members of the armed forces and their dependents. RAND Corporation. January 3, 2023. Accessed September 22, 2025. https://www.rand.org/pubs/research_reports/RRA1230-1.html
US Department of Agriculture Economic Research Service. Food Security in the U.S.—Definitions of Food Security. US Department of Agriculture Economic Research Service. January 10, 2025. https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security
Active military and veteran food insecurity. Feeding America. Accessed September 22, 2025. https://www.feedingamerica.org/hunger-in-america/food-insecurity-in-veterans
Pradun S. Find access to stop food insecurity in your community. VA News. September 19, 2025. Accessed September 22, 2025. https://news.va.gov/142733/find-access-stop-food-insecurity-your-community/
Cohen AJ, Dosa DM, Rudolph JL, et al. Risk factors for veteran food insecurity: findings from a National US Department of Veterans Affairs Food Insecurity Screener. Public Health Nutr. 2022;25:819-828. doi:10.1017/S1368980021004584
Chen C. VA and Rockefeller Foundation collaborate to access food for Veterans. VA News. September 5, 2023. Accessed September 22, 2025. https://news.va.gov/123228/va-rockefeller-foundation-expand-access-to-food/
American Hunger Games: Food Insecurity Among the Military and Veterans
American Hunger Games: Food Insecurity Among the Military and Veterans
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
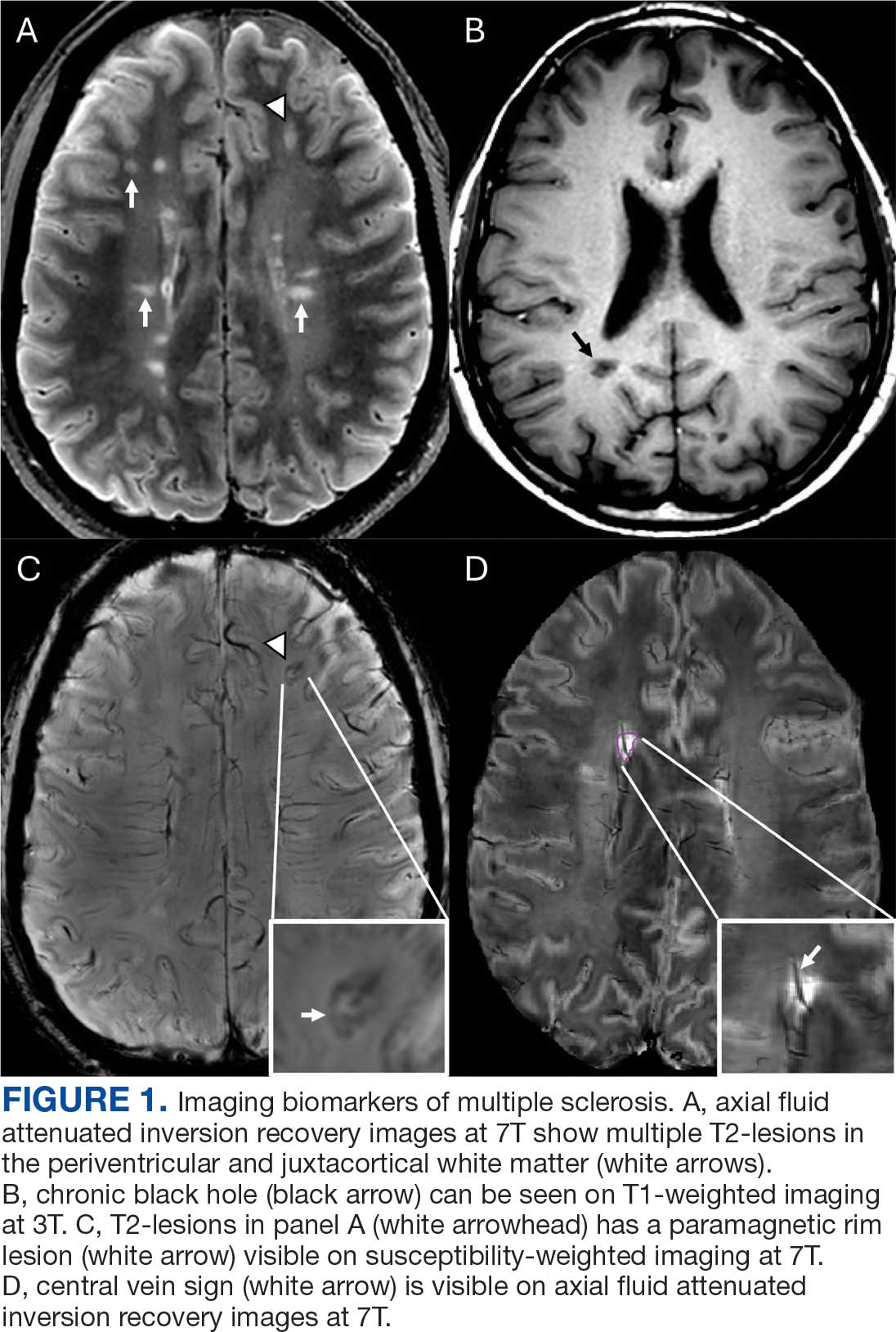
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
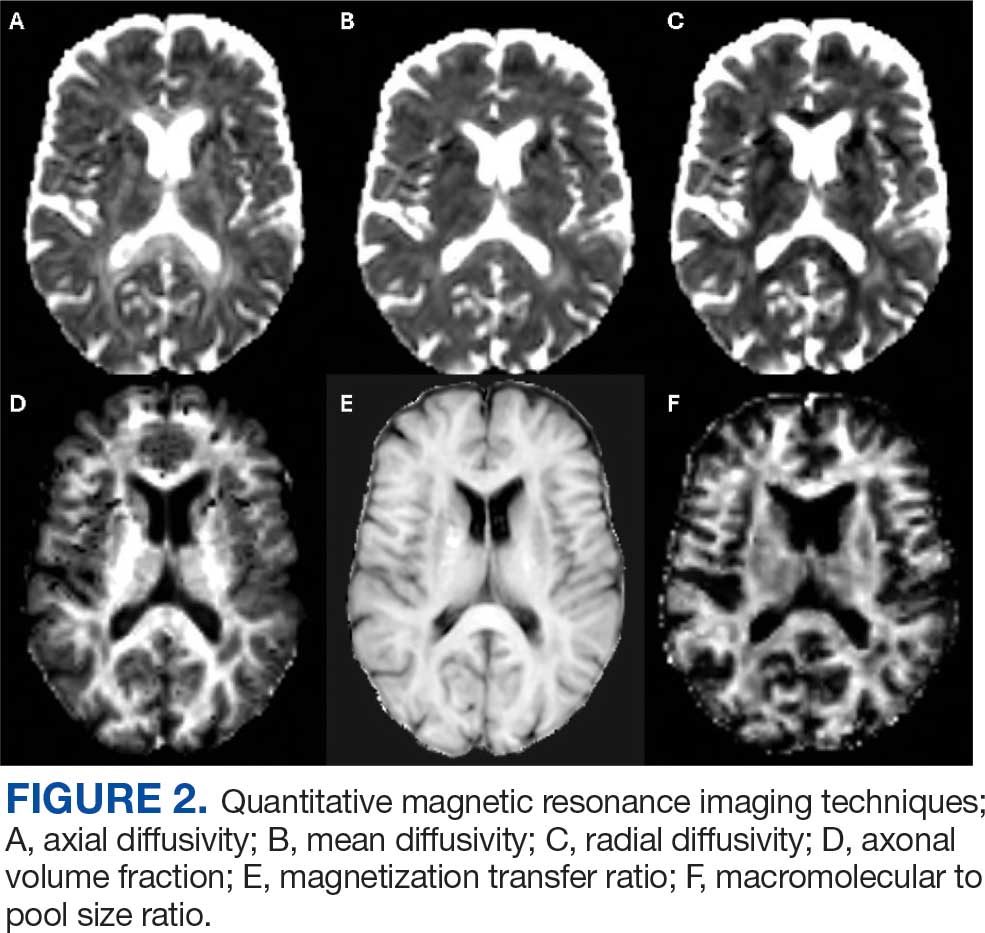
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5

Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33

CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5

Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33

CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
In 2020, the US Multi-Society Task Force (USMSTF) on Colorectal Cancer (CRC) increased the recommended colon polyp surveillance interval for 1 to 2 subcentimeter tubular adenomas from 5 to 10 years to 7 to 10 years.1 This change was prompted by emerging research indicating that rates of CRC and advanced neoplasia among patients with a history of only 1 to 2 subcentimeter tubular adenomas are lower than initially estimated.2,3 This extension provides an opportunity to increase endoscopy capacity and improve access to colonoscopies by retroactively applying the 2020 guidelines to surveillance interval recommendations made before their introduction. For example, based on the updated guidelines, patients previously recommended to undergo colon polyp surveillance colonoscopy 5 years after an index colonoscopy could extend their surveillance interval by 2 to 5 years. Increasing endoscopic capacity could address the growing demand for colonoscopies from new screening guidelines that reduced the age of initial CRC screening from 50 years to 45 years and the backlog of procedures due to COVID-19 restrictions.4
As part of a project to increase endoscopic capacity at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS), this study assessed the potential impact of retroactively applying the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity. These results may be informative for other VA and private-sector health care systems seeking to identify strategies to improve endoscopy capacity.
Methods
VAPHS is an integrated health care system in the Veterans Health Administration (VHA) serving 85,000 patients across 8 health care institutions in Pennsylvania, Ohio, and West Virginia. VAPHS manages colorectal screening recommendations for patients receiving medical care in the health care system regardless of whether their prior colonoscopy was performed at VAPHS or external facilities. The VA maintains a national CRC screening and surveillance electronic medical record reminder that prompts health care practitioners to order colon polyp surveillance based on interval recommendations from the index colonoscopy. This study reviewed all patients from the VAPHS panel with a reminder to undergo colonoscopy for screening for CRC or surveillance of colon polyps within 12 months from September 1, 2022.
Among patients with a reminder, 3 investigators reviewed index colonoscopy and pathology reports to identify CRC risk category, colonoscopy indication, procedural quality, and recommended repeat colonoscopy interval. Per the USMSTF guidelines, patients with incomplete colonoscopy or pathology records, high-risk indications (ie, personal history of inflammatory bowel disease, personal history of CRC, or family history of CRC), or inadequate bowel preparation (Boston Bowel Preparation Score < 6) were excluded. Additionally, patients who had CRC screening or surveillance discontinued due to age or comorbidities, had completed a subsequent follow-up colonoscopy, or were deceased at the time of review were excluded.
Retroactive Interval Reclassification
Among eligible patients, this study compared the repeat colonoscopy interval recommended by the prior endoscopist with those from the 2020 USMSTF guidelines. In cases where the interval was documented as a range of years, the lower end was considered the recommendation. Similarly, the lower end of the range from the 2020 USMSTF guidelines was used for the reclassified surveillance interval. Years extended per patient were quantified relative to September 1, 2023 (ie, 1 year after the review date). For example, if the index colonoscopy was completed on September 1, 2016, the initial surveillance recommendation was 5 years, and the reclassified recommendation was 7 years, the interval extension beyond September 1, 2023, was 0 years.
Furthermore, because index surveillance recommendations are not always guideline concordant, the years extended per patient were calculated by harmonizing the index endoscopist’s recommendations with the guidelines at the time of the index colonoscopy.5 For example, if the index colonoscopy was completed on September 1, 2018, and the endoscopist recommended a 5-year follow-up for a patient with average risk for CRC, adequate bowel preparation, and no colorectal polyps, that patient is eligible to extend their colonoscopy to September 1, 2028, based on guideline recommendations at the time of index endoscopy recommending that the next colonoscopy occur in 10 years. In this analysis the 2012 USMSTF guidelines were applied to all index colonoscopies completed in 2021 or earlier to allow time for adoption of the 2020 guidelines.
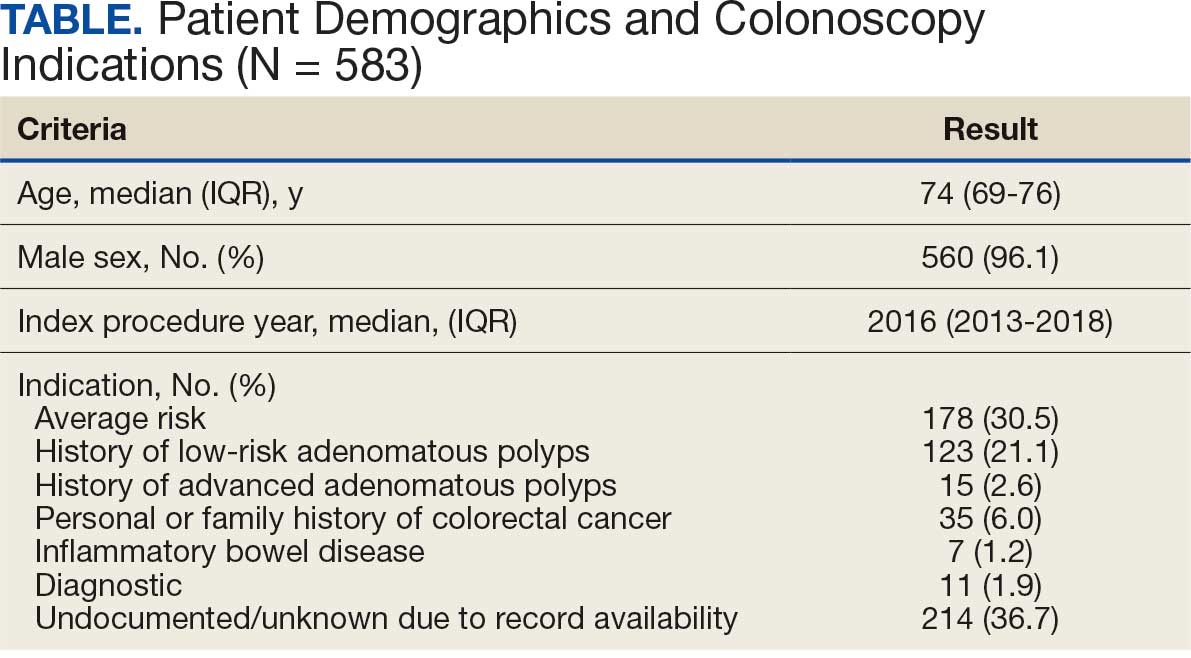
This project fulfilled a facility mandate to increase capacity to conduct endoscopic procedures. Institutional review board approval was not required by VAPHS policy relating to clinical operations projects. Approval for publication of clinical operations activity was obtained from the VAPHS facility director.
Results
Within 1 year of the September 1, 2022, review date, 637 patients receiving care at VAPHS had clinical reminders for an upcoming colonoscopy. Of these, 54 (8.4%) were already up to date or were deceased at the time of review. Of the 583 eligible patients, 96% were male, the median age was 74 years, the median index colonoscopy year was 2016, and 178 (30.5%) had an average-risk CRC screening indication at the index colonoscopy (Table).
Of the 583 patients due for colonoscopy, 331 (56.7%) had both colonoscopy and pathology reports available. The majority of those with incomplete records had the index colonoscopy completed outside VAPHS. Among these patients, 222 (67.0%) had adequate bowel preparation. Of those with adequate bowel preparation, 43 were not eligible for interval extension because of high-risk conditions and 13 were not eligible because there was no index surveillance interval recommendation from the index endoscopist. Of the patients due for colonoscopy, 166 (28.4%) were potentially eligible for surveillance interval extension (Figure).
Sixty-five (39.2%) of the 166 patients had 1 to 2 subcentimeter tubular adenomas on their index colonoscopy. Sixty-two patients were eligible for interval extension to 7 years, but this only resulted in ≥ 1 year of extension beyond the review date for 36 (6% of all 583 patients due for colonoscopy). The 36 patients were extended 63 years. By harmonizing the index endoscopists’ surveillance interval recommendation with the guideline at the time of the index colonoscopy, 29 additional patients could have their colonoscopy extended by ≥ 1 year. Harmonization extended colonoscopy intervals by 93 years. Retroactively applying the 2020 USMSTF polyp surveillance guidelines and harmonizing recommendations to guidelines extended the time of index colonoscopy by 153 years.
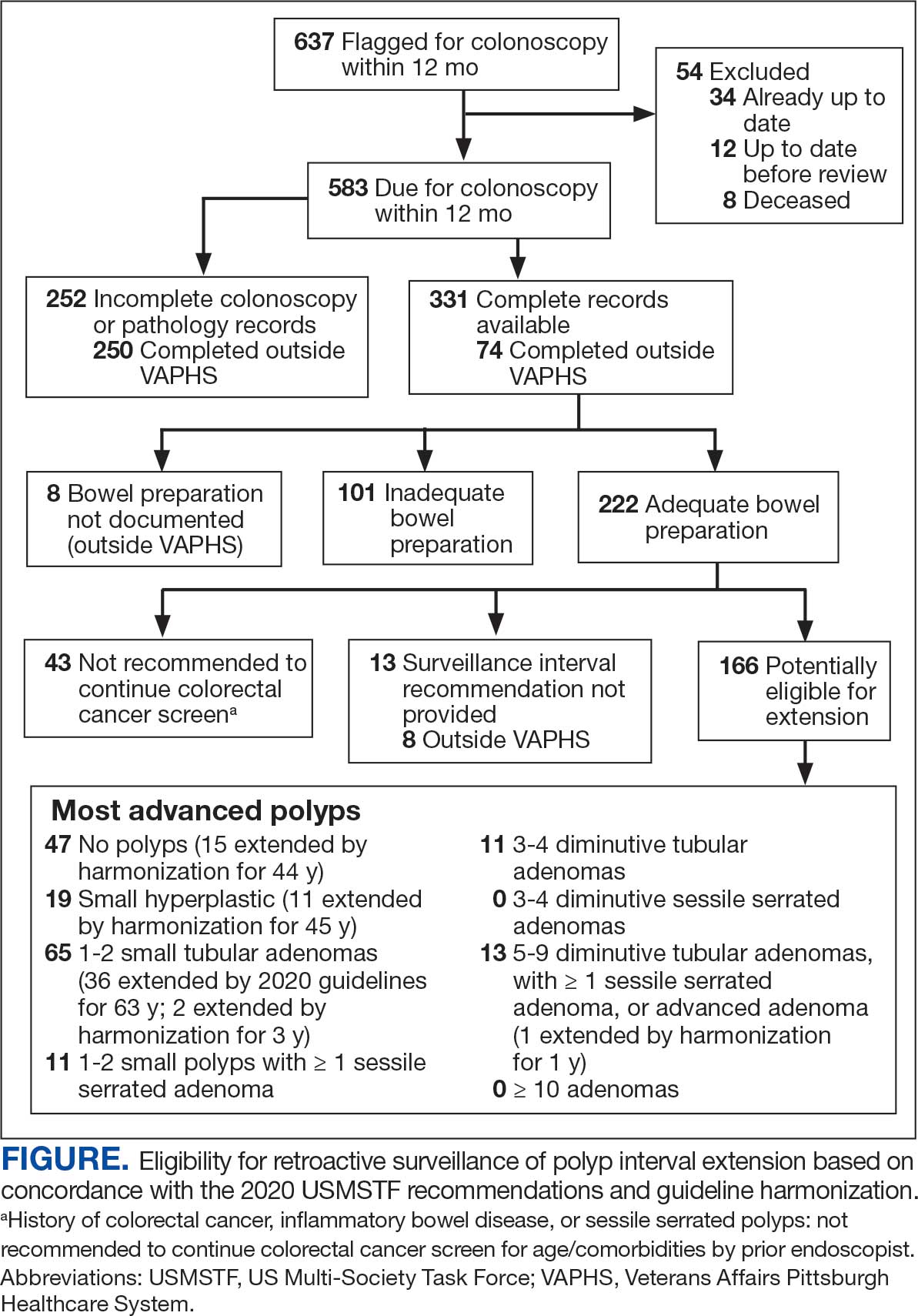
Discussion
With retroactive application of the 2020 USMSTF polyp surveillance guidelines, 6% of patients due for an upcoming colonoscopy could extend their follow-up by ≥ 1 year by extending the surveillance interval for 1 to 2 subcentimeter tubular adenomas to 7 years. An additional 5% of patients could extend their interval by harmonizing the index endoscopist’s interval recommendation with polyp surveillance guidelines at the time of the index colonoscopy. These findings are consistent with the results of 2 studies that demonstrated that about 14% of patients due for colonoscopy could have their interval extended.6,7 The current study enhances those insights by separating the contribution of 2020 USMSTF polyp surveillance guidelines from the contribution of harmonizing surveillance intervals with guidelines for other polyp histologies. This study found that there is an opportunity to improve endoscopic capacity by harmonizing recommendations with guidelines. This complements a 2023 study showing that even when knowledgeable about guidelines, clinicians do not necessarily follow recommendations.8 While this and previous research have identified that 11% to 14% of patients are eligible for extension, these individuals would also have to be willing to have their polyp surveillance intervals extended for there to be a real-world impact on endoscopic capacity. A 2024 study found that only 19% to 37% of patients with 1 to 2 small tubular adenomas were willing to have polyps surveillance interval extension.9 This suggests the actual effect on capacity may be even lower than reported.
Limitations
The overall impact of the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity was blunted by the high prevalence of incomplete index colonoscopy records among the study population. Without data on bowel preparation quality or procedure indications, this study could not assess whether 43% of patients were eligible for surveillance interval extension. Most index colonoscopies with incomplete documentation were completed at community-care gastroenterology facilities. This high rate of incomplete documentation is likely generalizable to other VA health care systems—especially in the era of the Veterans Access, Choice, and Accountability Act of 2014, which increased veteran access to non-VA community care.10 Veterans due for colon polyp surveillance colonoscopies are more likely to have had their prior colonoscopy in community care compared with prior eras.11 Furthermore, because the VHA is among the most established integrated health care systems offering primary and subspecialty care in the US, private sector health care systems may have even greater rates of care fragmentation for longitudinal CRC screening and colon polyp surveillance, as these systems have only begun to regionally integrate recently.12,13
Another limitation is that nearly one-third of the individuals with documentation had inadequate bowel preparation for surveillance recommendations. This results in shorter surveillance follow-up colonoscopies and increases downstream demand for future colonoscopies. The low yield of extending colon polyp surveillance interval in this study emphasizes that improved efforts to obtain colonoscopy and pathology reports from community care, right-sizing the colon polyp surveillance intervals recommended by endoscopists, and improving quality of bowel preparation could have downstream health care system benefits in the future. These efforts could increase colonoscopy capacity at VA health care systems, thereby shortening colonoscopy wait times, decreasing fragmentation of care, and increasing the number of veterans who receive high-quality colonoscopies at VA health care systems.14
Conclusions
Eleven percent of patients in this study due for a colonoscopy could extend their follow-up by ≥ 1 year. About half of these extensions were directly due to the 2020 USMSTF polyp surveillance interval extension for 1 to 2 subcentimeter tubular adenomas. The rest resulted from harmonizing recommendations with guidelines at the time of the procedure. To determine whether retroactively applying polyp surveillance guidelines to follow-up interval recommendations will result in improved endoscopic capacity, health care system administrators should consider the degree of CRC screening care fragmentation in their patient population. Greater long-term gains in endoscopic capacity may be achieved by proactively supporting endoscopists in making guideline-concordant screening recommendations at the time of colonoscopy.
Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485. doi:10.1016/j.gie.2020.01.014
Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790-1801. doi:10.1038/ajg.2017.360
Click B, Pinsky PF, Hickey T, Doroudi M, Shoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319:2021-2031. doi:10.1001/jama.2018.5809
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi:10.1001/jama.2021.6238
Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. doi:10.1055/a-0865-2082
Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216-1220.e1. doi:10.1053/j.gastro.2020.07.033
Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66:2578-2584. doi:10.1007/s10620-020-06539-1
Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 U.S. Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97:350-356.e3. doi:10.1016/j.gie.2022.08.020
Lee JK, Koripella PC, Jensen CD, et al. Randomized trial of patient outreach approaches to de-implement outdated colonoscopy surveillance intervals. Clin Gastroenterol Hepatol. 2024;22:1315-1322.e7. doi:10.1016/j.cgh.2023.12.027
Veterans Access, Choice, and Accountability Act of 2014, HR 3230, 113th Cong (2014). Accessed September 8, 2025. https://www.congress.gov/bill/113th-congress/house-bill/3230
Dueker JM, Khalid A. Performance of the Veterans Choice Program for improving access to colonoscopy at a tertiary VA facility. Fed Pract. 2020;37:224-228.
Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85:5-35. doi:10.1111/j.1468-0009.2007.00475.x
Furukawa MF, Machta RM, Barrett KA, et al. Landscape of health systems in the United States. Med Care Res Rev. 2020;77:357-366. doi:10.1177/1077558718823130
Petros V, Tsambikos E, Madhoun M, Tierney WM. Impact of community referral on colonoscopy quality metrics in a Veterans Affairs Medical Center. Clin Transl Gastroenterol. 2022;13:e00460. doi:10.14309/ctg.0000000000000460
In 2020, the US Multi-Society Task Force (USMSTF) on Colorectal Cancer (CRC) increased the recommended colon polyp surveillance interval for 1 to 2 subcentimeter tubular adenomas from 5 to 10 years to 7 to 10 years.1 This change was prompted by emerging research indicating that rates of CRC and advanced neoplasia among patients with a history of only 1 to 2 subcentimeter tubular adenomas are lower than initially estimated.2,3 This extension provides an opportunity to increase endoscopy capacity and improve access to colonoscopies by retroactively applying the 2020 guidelines to surveillance interval recommendations made before their introduction. For example, based on the updated guidelines, patients previously recommended to undergo colon polyp surveillance colonoscopy 5 years after an index colonoscopy could extend their surveillance interval by 2 to 5 years. Increasing endoscopic capacity could address the growing demand for colonoscopies from new screening guidelines that reduced the age of initial CRC screening from 50 years to 45 years and the backlog of procedures due to COVID-19 restrictions.4
As part of a project to increase endoscopic capacity at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS), this study assessed the potential impact of retroactively applying the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity. These results may be informative for other VA and private-sector health care systems seeking to identify strategies to improve endoscopy capacity.
Methods
VAPHS is an integrated health care system in the Veterans Health Administration (VHA) serving 85,000 patients across 8 health care institutions in Pennsylvania, Ohio, and West Virginia. VAPHS manages colorectal screening recommendations for patients receiving medical care in the health care system regardless of whether their prior colonoscopy was performed at VAPHS or external facilities. The VA maintains a national CRC screening and surveillance electronic medical record reminder that prompts health care practitioners to order colon polyp surveillance based on interval recommendations from the index colonoscopy. This study reviewed all patients from the VAPHS panel with a reminder to undergo colonoscopy for screening for CRC or surveillance of colon polyps within 12 months from September 1, 2022.
Among patients with a reminder, 3 investigators reviewed index colonoscopy and pathology reports to identify CRC risk category, colonoscopy indication, procedural quality, and recommended repeat colonoscopy interval. Per the USMSTF guidelines, patients with incomplete colonoscopy or pathology records, high-risk indications (ie, personal history of inflammatory bowel disease, personal history of CRC, or family history of CRC), or inadequate bowel preparation (Boston Bowel Preparation Score < 6) were excluded. Additionally, patients who had CRC screening or surveillance discontinued due to age or comorbidities, had completed a subsequent follow-up colonoscopy, or were deceased at the time of review were excluded.
Retroactive Interval Reclassification
Among eligible patients, this study compared the repeat colonoscopy interval recommended by the prior endoscopist with those from the 2020 USMSTF guidelines. In cases where the interval was documented as a range of years, the lower end was considered the recommendation. Similarly, the lower end of the range from the 2020 USMSTF guidelines was used for the reclassified surveillance interval. Years extended per patient were quantified relative to September 1, 2023 (ie, 1 year after the review date). For example, if the index colonoscopy was completed on September 1, 2016, the initial surveillance recommendation was 5 years, and the reclassified recommendation was 7 years, the interval extension beyond September 1, 2023, was 0 years.
Furthermore, because index surveillance recommendations are not always guideline concordant, the years extended per patient were calculated by harmonizing the index endoscopist’s recommendations with the guidelines at the time of the index colonoscopy.5 For example, if the index colonoscopy was completed on September 1, 2018, and the endoscopist recommended a 5-year follow-up for a patient with average risk for CRC, adequate bowel preparation, and no colorectal polyps, that patient is eligible to extend their colonoscopy to September 1, 2028, based on guideline recommendations at the time of index endoscopy recommending that the next colonoscopy occur in 10 years. In this analysis the 2012 USMSTF guidelines were applied to all index colonoscopies completed in 2021 or earlier to allow time for adoption of the 2020 guidelines.

This project fulfilled a facility mandate to increase capacity to conduct endoscopic procedures. Institutional review board approval was not required by VAPHS policy relating to clinical operations projects. Approval for publication of clinical operations activity was obtained from the VAPHS facility director.
Results
Within 1 year of the September 1, 2022, review date, 637 patients receiving care at VAPHS had clinical reminders for an upcoming colonoscopy. Of these, 54 (8.4%) were already up to date or were deceased at the time of review. Of the 583 eligible patients, 96% were male, the median age was 74 years, the median index colonoscopy year was 2016, and 178 (30.5%) had an average-risk CRC screening indication at the index colonoscopy (Table).
Of the 583 patients due for colonoscopy, 331 (56.7%) had both colonoscopy and pathology reports available. The majority of those with incomplete records had the index colonoscopy completed outside VAPHS. Among these patients, 222 (67.0%) had adequate bowel preparation. Of those with adequate bowel preparation, 43 were not eligible for interval extension because of high-risk conditions and 13 were not eligible because there was no index surveillance interval recommendation from the index endoscopist. Of the patients due for colonoscopy, 166 (28.4%) were potentially eligible for surveillance interval extension (Figure).
Sixty-five (39.2%) of the 166 patients had 1 to 2 subcentimeter tubular adenomas on their index colonoscopy. Sixty-two patients were eligible for interval extension to 7 years, but this only resulted in ≥ 1 year of extension beyond the review date for 36 (6% of all 583 patients due for colonoscopy). The 36 patients were extended 63 years. By harmonizing the index endoscopists’ surveillance interval recommendation with the guideline at the time of the index colonoscopy, 29 additional patients could have their colonoscopy extended by ≥ 1 year. Harmonization extended colonoscopy intervals by 93 years. Retroactively applying the 2020 USMSTF polyp surveillance guidelines and harmonizing recommendations to guidelines extended the time of index colonoscopy by 153 years.

Discussion
With retroactive application of the 2020 USMSTF polyp surveillance guidelines, 6% of patients due for an upcoming colonoscopy could extend their follow-up by ≥ 1 year by extending the surveillance interval for 1 to 2 subcentimeter tubular adenomas to 7 years. An additional 5% of patients could extend their interval by harmonizing the index endoscopist’s interval recommendation with polyp surveillance guidelines at the time of the index colonoscopy. These findings are consistent with the results of 2 studies that demonstrated that about 14% of patients due for colonoscopy could have their interval extended.6,7 The current study enhances those insights by separating the contribution of 2020 USMSTF polyp surveillance guidelines from the contribution of harmonizing surveillance intervals with guidelines for other polyp histologies. This study found that there is an opportunity to improve endoscopic capacity by harmonizing recommendations with guidelines. This complements a 2023 study showing that even when knowledgeable about guidelines, clinicians do not necessarily follow recommendations.8 While this and previous research have identified that 11% to 14% of patients are eligible for extension, these individuals would also have to be willing to have their polyp surveillance intervals extended for there to be a real-world impact on endoscopic capacity. A 2024 study found that only 19% to 37% of patients with 1 to 2 small tubular adenomas were willing to have polyps surveillance interval extension.9 This suggests the actual effect on capacity may be even lower than reported.
Limitations
The overall impact of the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity was blunted by the high prevalence of incomplete index colonoscopy records among the study population. Without data on bowel preparation quality or procedure indications, this study could not assess whether 43% of patients were eligible for surveillance interval extension. Most index colonoscopies with incomplete documentation were completed at community-care gastroenterology facilities. This high rate of incomplete documentation is likely generalizable to other VA health care systems—especially in the era of the Veterans Access, Choice, and Accountability Act of 2014, which increased veteran access to non-VA community care.10 Veterans due for colon polyp surveillance colonoscopies are more likely to have had their prior colonoscopy in community care compared with prior eras.11 Furthermore, because the VHA is among the most established integrated health care systems offering primary and subspecialty care in the US, private sector health care systems may have even greater rates of care fragmentation for longitudinal CRC screening and colon polyp surveillance, as these systems have only begun to regionally integrate recently.12,13
Another limitation is that nearly one-third of the individuals with documentation had inadequate bowel preparation for surveillance recommendations. This results in shorter surveillance follow-up colonoscopies and increases downstream demand for future colonoscopies. The low yield of extending colon polyp surveillance interval in this study emphasizes that improved efforts to obtain colonoscopy and pathology reports from community care, right-sizing the colon polyp surveillance intervals recommended by endoscopists, and improving quality of bowel preparation could have downstream health care system benefits in the future. These efforts could increase colonoscopy capacity at VA health care systems, thereby shortening colonoscopy wait times, decreasing fragmentation of care, and increasing the number of veterans who receive high-quality colonoscopies at VA health care systems.14
Conclusions
Eleven percent of patients in this study due for a colonoscopy could extend their follow-up by ≥ 1 year. About half of these extensions were directly due to the 2020 USMSTF polyp surveillance interval extension for 1 to 2 subcentimeter tubular adenomas. The rest resulted from harmonizing recommendations with guidelines at the time of the procedure. To determine whether retroactively applying polyp surveillance guidelines to follow-up interval recommendations will result in improved endoscopic capacity, health care system administrators should consider the degree of CRC screening care fragmentation in their patient population. Greater long-term gains in endoscopic capacity may be achieved by proactively supporting endoscopists in making guideline-concordant screening recommendations at the time of colonoscopy.
In 2020, the US Multi-Society Task Force (USMSTF) on Colorectal Cancer (CRC) increased the recommended colon polyp surveillance interval for 1 to 2 subcentimeter tubular adenomas from 5 to 10 years to 7 to 10 years.1 This change was prompted by emerging research indicating that rates of CRC and advanced neoplasia among patients with a history of only 1 to 2 subcentimeter tubular adenomas are lower than initially estimated.2,3 This extension provides an opportunity to increase endoscopy capacity and improve access to colonoscopies by retroactively applying the 2020 guidelines to surveillance interval recommendations made before their introduction. For example, based on the updated guidelines, patients previously recommended to undergo colon polyp surveillance colonoscopy 5 years after an index colonoscopy could extend their surveillance interval by 2 to 5 years. Increasing endoscopic capacity could address the growing demand for colonoscopies from new screening guidelines that reduced the age of initial CRC screening from 50 years to 45 years and the backlog of procedures due to COVID-19 restrictions.4
As part of a project to increase endoscopic capacity at the US Department of Veterans Affairs (VA) Pittsburgh Healthcare System (VAPHS), this study assessed the potential impact of retroactively applying the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity. These results may be informative for other VA and private-sector health care systems seeking to identify strategies to improve endoscopy capacity.
Methods
VAPHS is an integrated health care system in the Veterans Health Administration (VHA) serving 85,000 patients across 8 health care institutions in Pennsylvania, Ohio, and West Virginia. VAPHS manages colorectal screening recommendations for patients receiving medical care in the health care system regardless of whether their prior colonoscopy was performed at VAPHS or external facilities. The VA maintains a national CRC screening and surveillance electronic medical record reminder that prompts health care practitioners to order colon polyp surveillance based on interval recommendations from the index colonoscopy. This study reviewed all patients from the VAPHS panel with a reminder to undergo colonoscopy for screening for CRC or surveillance of colon polyps within 12 months from September 1, 2022.
Among patients with a reminder, 3 investigators reviewed index colonoscopy and pathology reports to identify CRC risk category, colonoscopy indication, procedural quality, and recommended repeat colonoscopy interval. Per the USMSTF guidelines, patients with incomplete colonoscopy or pathology records, high-risk indications (ie, personal history of inflammatory bowel disease, personal history of CRC, or family history of CRC), or inadequate bowel preparation (Boston Bowel Preparation Score < 6) were excluded. Additionally, patients who had CRC screening or surveillance discontinued due to age or comorbidities, had completed a subsequent follow-up colonoscopy, or were deceased at the time of review were excluded.
Retroactive Interval Reclassification
Among eligible patients, this study compared the repeat colonoscopy interval recommended by the prior endoscopist with those from the 2020 USMSTF guidelines. In cases where the interval was documented as a range of years, the lower end was considered the recommendation. Similarly, the lower end of the range from the 2020 USMSTF guidelines was used for the reclassified surveillance interval. Years extended per patient were quantified relative to September 1, 2023 (ie, 1 year after the review date). For example, if the index colonoscopy was completed on September 1, 2016, the initial surveillance recommendation was 5 years, and the reclassified recommendation was 7 years, the interval extension beyond September 1, 2023, was 0 years.
Furthermore, because index surveillance recommendations are not always guideline concordant, the years extended per patient were calculated by harmonizing the index endoscopist’s recommendations with the guidelines at the time of the index colonoscopy.5 For example, if the index colonoscopy was completed on September 1, 2018, and the endoscopist recommended a 5-year follow-up for a patient with average risk for CRC, adequate bowel preparation, and no colorectal polyps, that patient is eligible to extend their colonoscopy to September 1, 2028, based on guideline recommendations at the time of index endoscopy recommending that the next colonoscopy occur in 10 years. In this analysis the 2012 USMSTF guidelines were applied to all index colonoscopies completed in 2021 or earlier to allow time for adoption of the 2020 guidelines.

This project fulfilled a facility mandate to increase capacity to conduct endoscopic procedures. Institutional review board approval was not required by VAPHS policy relating to clinical operations projects. Approval for publication of clinical operations activity was obtained from the VAPHS facility director.
Results
Within 1 year of the September 1, 2022, review date, 637 patients receiving care at VAPHS had clinical reminders for an upcoming colonoscopy. Of these, 54 (8.4%) were already up to date or were deceased at the time of review. Of the 583 eligible patients, 96% were male, the median age was 74 years, the median index colonoscopy year was 2016, and 178 (30.5%) had an average-risk CRC screening indication at the index colonoscopy (Table).
Of the 583 patients due for colonoscopy, 331 (56.7%) had both colonoscopy and pathology reports available. The majority of those with incomplete records had the index colonoscopy completed outside VAPHS. Among these patients, 222 (67.0%) had adequate bowel preparation. Of those with adequate bowel preparation, 43 were not eligible for interval extension because of high-risk conditions and 13 were not eligible because there was no index surveillance interval recommendation from the index endoscopist. Of the patients due for colonoscopy, 166 (28.4%) were potentially eligible for surveillance interval extension (Figure).
Sixty-five (39.2%) of the 166 patients had 1 to 2 subcentimeter tubular adenomas on their index colonoscopy. Sixty-two patients were eligible for interval extension to 7 years, but this only resulted in ≥ 1 year of extension beyond the review date for 36 (6% of all 583 patients due for colonoscopy). The 36 patients were extended 63 years. By harmonizing the index endoscopists’ surveillance interval recommendation with the guideline at the time of the index colonoscopy, 29 additional patients could have their colonoscopy extended by ≥ 1 year. Harmonization extended colonoscopy intervals by 93 years. Retroactively applying the 2020 USMSTF polyp surveillance guidelines and harmonizing recommendations to guidelines extended the time of index colonoscopy by 153 years.

Discussion
With retroactive application of the 2020 USMSTF polyp surveillance guidelines, 6% of patients due for an upcoming colonoscopy could extend their follow-up by ≥ 1 year by extending the surveillance interval for 1 to 2 subcentimeter tubular adenomas to 7 years. An additional 5% of patients could extend their interval by harmonizing the index endoscopist’s interval recommendation with polyp surveillance guidelines at the time of the index colonoscopy. These findings are consistent with the results of 2 studies that demonstrated that about 14% of patients due for colonoscopy could have their interval extended.6,7 The current study enhances those insights by separating the contribution of 2020 USMSTF polyp surveillance guidelines from the contribution of harmonizing surveillance intervals with guidelines for other polyp histologies. This study found that there is an opportunity to improve endoscopic capacity by harmonizing recommendations with guidelines. This complements a 2023 study showing that even when knowledgeable about guidelines, clinicians do not necessarily follow recommendations.8 While this and previous research have identified that 11% to 14% of patients are eligible for extension, these individuals would also have to be willing to have their polyp surveillance intervals extended for there to be a real-world impact on endoscopic capacity. A 2024 study found that only 19% to 37% of patients with 1 to 2 small tubular adenomas were willing to have polyps surveillance interval extension.9 This suggests the actual effect on capacity may be even lower than reported.
Limitations
The overall impact of the 2020 USMSTF polyp surveillance guidelines on endoscopic capacity was blunted by the high prevalence of incomplete index colonoscopy records among the study population. Without data on bowel preparation quality or procedure indications, this study could not assess whether 43% of patients were eligible for surveillance interval extension. Most index colonoscopies with incomplete documentation were completed at community-care gastroenterology facilities. This high rate of incomplete documentation is likely generalizable to other VA health care systems—especially in the era of the Veterans Access, Choice, and Accountability Act of 2014, which increased veteran access to non-VA community care.10 Veterans due for colon polyp surveillance colonoscopies are more likely to have had their prior colonoscopy in community care compared with prior eras.11 Furthermore, because the VHA is among the most established integrated health care systems offering primary and subspecialty care in the US, private sector health care systems may have even greater rates of care fragmentation for longitudinal CRC screening and colon polyp surveillance, as these systems have only begun to regionally integrate recently.12,13
Another limitation is that nearly one-third of the individuals with documentation had inadequate bowel preparation for surveillance recommendations. This results in shorter surveillance follow-up colonoscopies and increases downstream demand for future colonoscopies. The low yield of extending colon polyp surveillance interval in this study emphasizes that improved efforts to obtain colonoscopy and pathology reports from community care, right-sizing the colon polyp surveillance intervals recommended by endoscopists, and improving quality of bowel preparation could have downstream health care system benefits in the future. These efforts could increase colonoscopy capacity at VA health care systems, thereby shortening colonoscopy wait times, decreasing fragmentation of care, and increasing the number of veterans who receive high-quality colonoscopies at VA health care systems.14
Conclusions
Eleven percent of patients in this study due for a colonoscopy could extend their follow-up by ≥ 1 year. About half of these extensions were directly due to the 2020 USMSTF polyp surveillance interval extension for 1 to 2 subcentimeter tubular adenomas. The rest resulted from harmonizing recommendations with guidelines at the time of the procedure. To determine whether retroactively applying polyp surveillance guidelines to follow-up interval recommendations will result in improved endoscopic capacity, health care system administrators should consider the degree of CRC screening care fragmentation in their patient population. Greater long-term gains in endoscopic capacity may be achieved by proactively supporting endoscopists in making guideline-concordant screening recommendations at the time of colonoscopy.
Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485. doi:10.1016/j.gie.2020.01.014
Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790-1801. doi:10.1038/ajg.2017.360
Click B, Pinsky PF, Hickey T, Doroudi M, Shoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319:2021-2031. doi:10.1001/jama.2018.5809
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi:10.1001/jama.2021.6238
Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. doi:10.1055/a-0865-2082
Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216-1220.e1. doi:10.1053/j.gastro.2020.07.033
Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66:2578-2584. doi:10.1007/s10620-020-06539-1
Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 U.S. Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97:350-356.e3. doi:10.1016/j.gie.2022.08.020
Lee JK, Koripella PC, Jensen CD, et al. Randomized trial of patient outreach approaches to de-implement outdated colonoscopy surveillance intervals. Clin Gastroenterol Hepatol. 2024;22:1315-1322.e7. doi:10.1016/j.cgh.2023.12.027
Veterans Access, Choice, and Accountability Act of 2014, HR 3230, 113th Cong (2014). Accessed September 8, 2025. https://www.congress.gov/bill/113th-congress/house-bill/3230
Dueker JM, Khalid A. Performance of the Veterans Choice Program for improving access to colonoscopy at a tertiary VA facility. Fed Pract. 2020;37:224-228.
Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85:5-35. doi:10.1111/j.1468-0009.2007.00475.x
Furukawa MF, Machta RM, Barrett KA, et al. Landscape of health systems in the United States. Med Care Res Rev. 2020;77:357-366. doi:10.1177/1077558718823130
Petros V, Tsambikos E, Madhoun M, Tierney WM. Impact of community referral on colonoscopy quality metrics in a Veterans Affairs Medical Center. Clin Transl Gastroenterol. 2022;13:e00460. doi:10.14309/ctg.0000000000000460
Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:463-485. doi:10.1016/j.gie.2020.01.014
Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low-risk adenomas at baseline colonoscopy: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1790-1801. doi:10.1038/ajg.2017.360
Click B, Pinsky PF, Hickey T, Doroudi M, Shoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA. 2018;319:2021-2031. doi:10.1001/jama.2018.5809
US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi:10.1001/jama.2021.6238
Djinbachian R, Dubé AJ, Durand M, et al. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. doi:10.1055/a-0865-2082
Gawron AJ, Kaltenbach T, Dominitz JA. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159:1216-1220.e1. doi:10.1053/j.gastro.2020.07.033
Xiao AH, Chang SY, Stevoff CG, Komanduri S, Pandolfino JE, Keswani RN. Adoption of multi-society guidelines facilitates value-based reduction in screening and surveillance colonoscopy volume during COVID-19 pandemic. Dig Dis Sci. 2021;66:2578-2584. doi:10.1007/s10620-020-06539-1
Dong J, Wang LF, Ardolino E, Feuerstein JD. Real-world compliance with the 2020 U.S. Multi-Society Task Force on Colorectal Cancer polypectomy surveillance guidelines: an observational study. Gastrointest Endosc. 2023;97:350-356.e3. doi:10.1016/j.gie.2022.08.020
Lee JK, Koripella PC, Jensen CD, et al. Randomized trial of patient outreach approaches to de-implement outdated colonoscopy surveillance intervals. Clin Gastroenterol Hepatol. 2024;22:1315-1322.e7. doi:10.1016/j.cgh.2023.12.027
Veterans Access, Choice, and Accountability Act of 2014, HR 3230, 113th Cong (2014). Accessed September 8, 2025. https://www.congress.gov/bill/113th-congress/house-bill/3230
Dueker JM, Khalid A. Performance of the Veterans Choice Program for improving access to colonoscopy at a tertiary VA facility. Fed Pract. 2020;37:224-228.
Oliver A. The Veterans Health Administration: an American success story? Milbank Q. 2007;85:5-35. doi:10.1111/j.1468-0009.2007.00475.x
Furukawa MF, Machta RM, Barrett KA, et al. Landscape of health systems in the United States. Med Care Res Rev. 2020;77:357-366. doi:10.1177/1077558718823130
Petros V, Tsambikos E, Madhoun M, Tierney WM. Impact of community referral on colonoscopy quality metrics in a Veterans Affairs Medical Center. Clin Transl Gastroenterol. 2022;13:e00460. doi:10.14309/ctg.0000000000000460
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
Impact of Retroactive Application of Updated Surveillance Guidelines on Endoscopy Center Capacity at a Large VA Health Care System
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
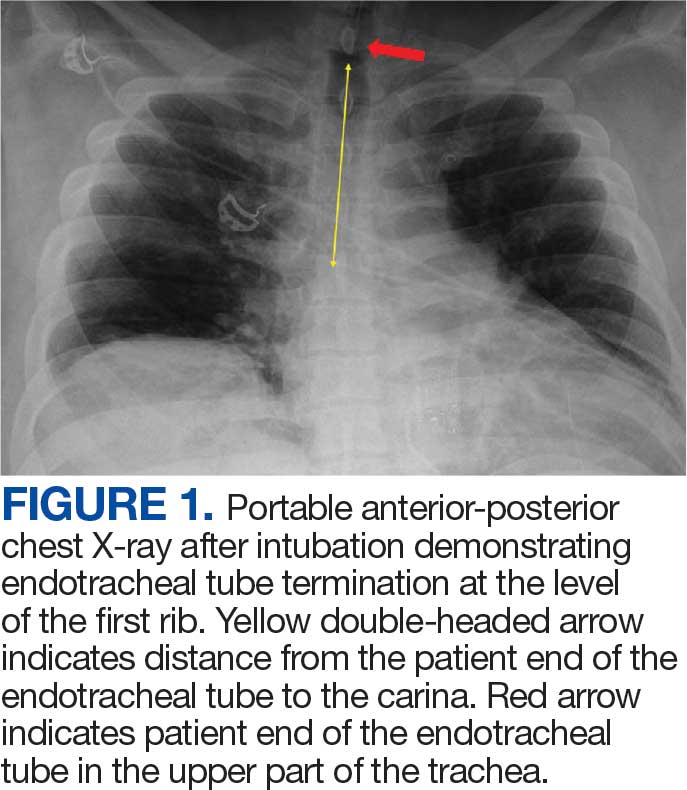
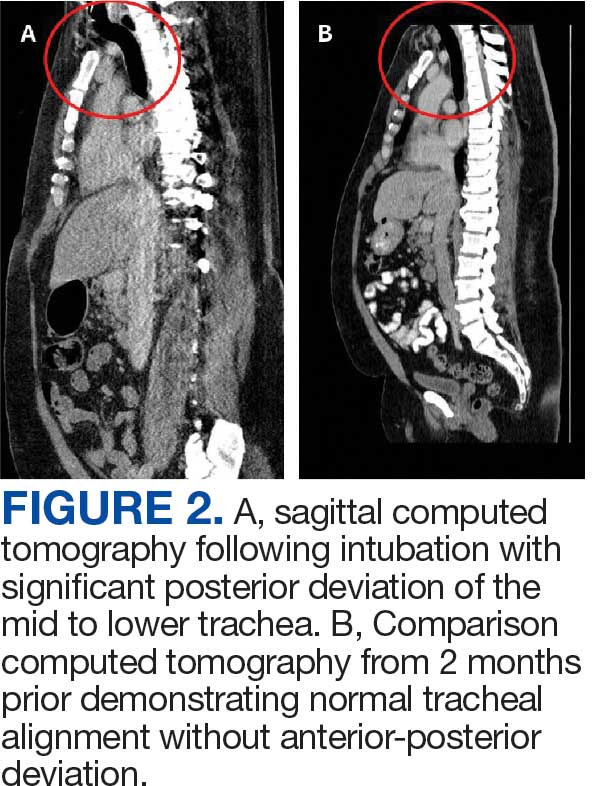
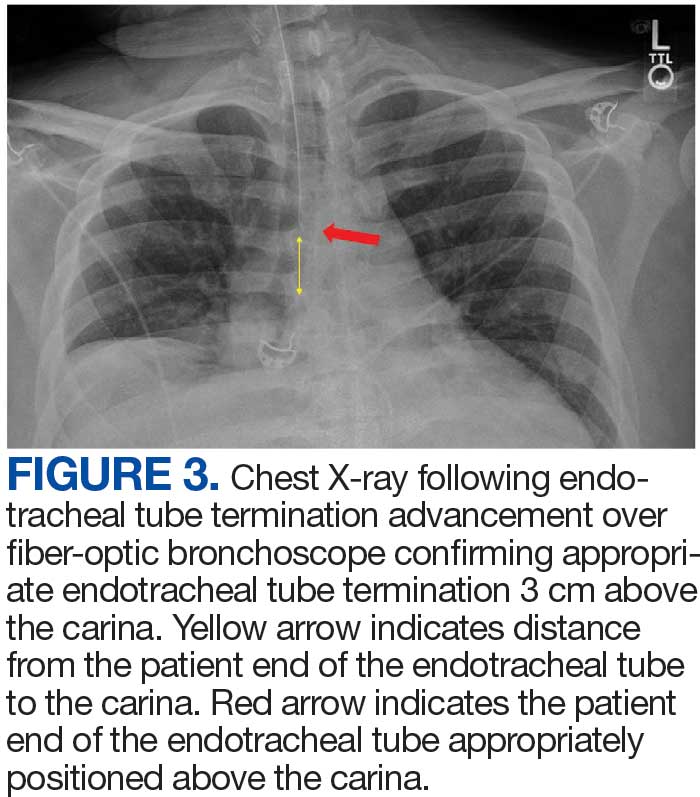
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).



Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).



Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.
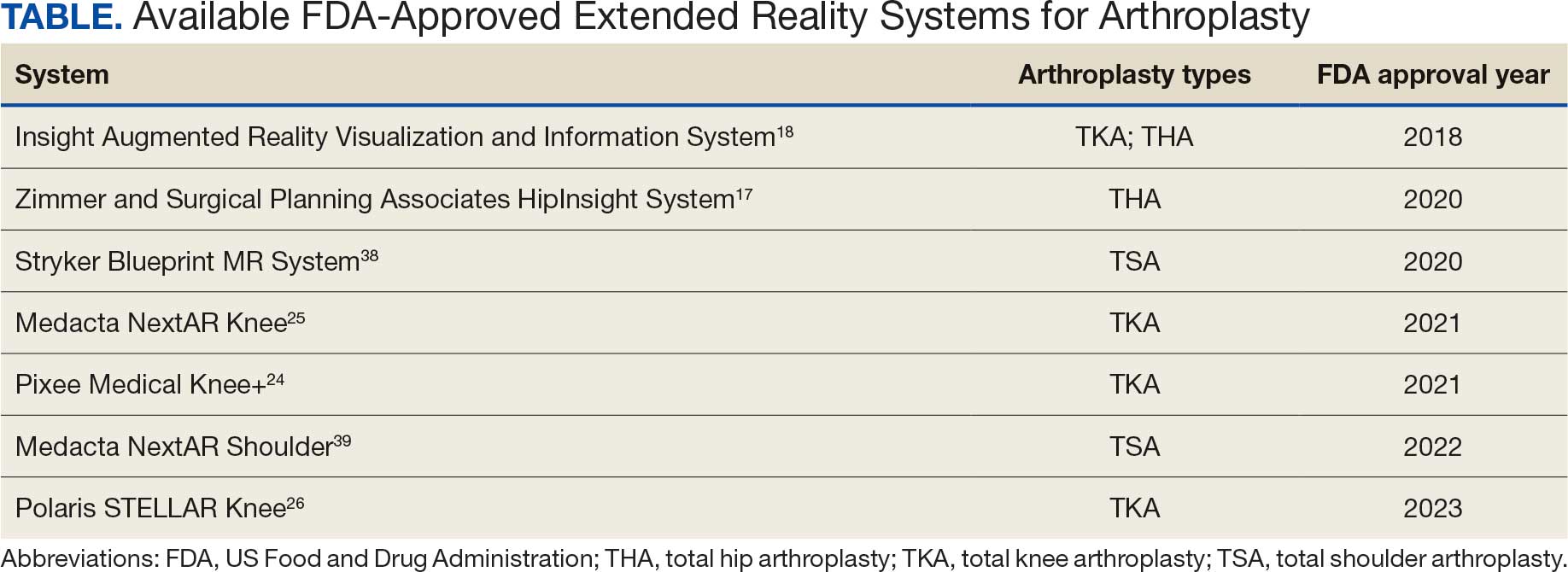
Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23
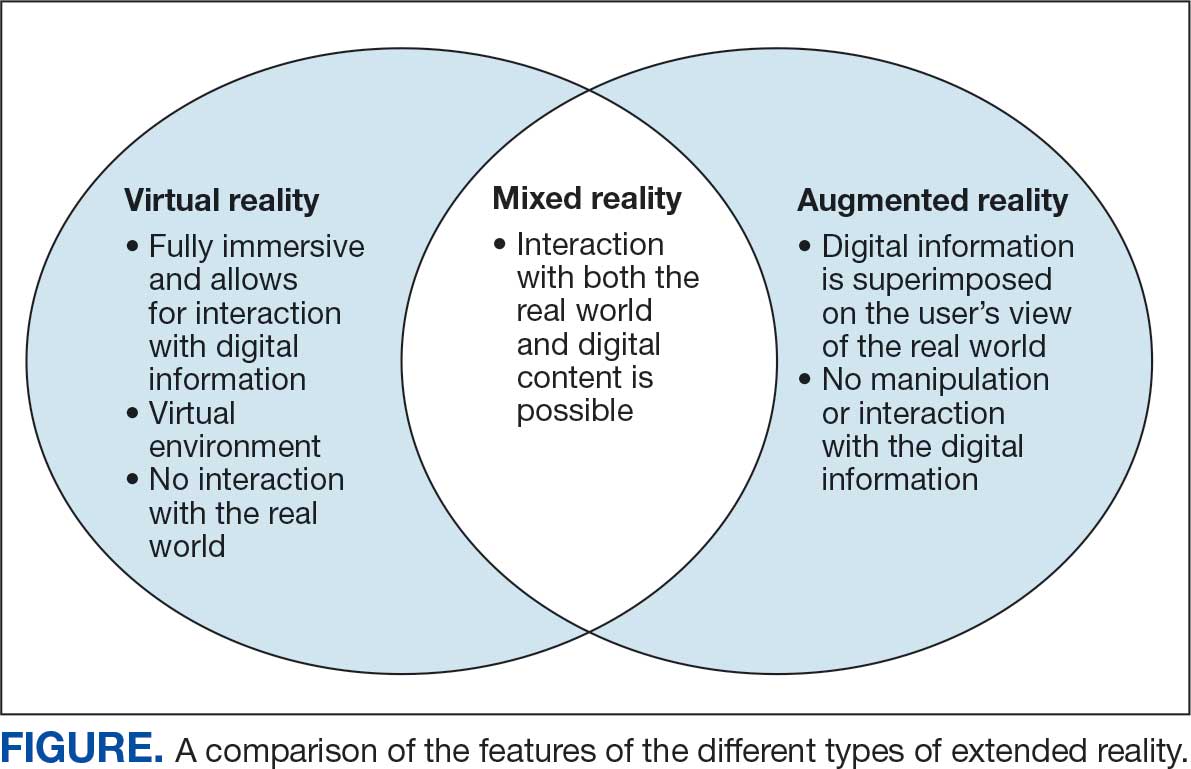
Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.

Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23

Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
The introduction of extended reality (XR) to the operating room (OR) has proved promising for enhancing surgical precision and improving patient outcomes. In the field of orthopedic surgery, precise alignment of implants is integral to maintaining functional range of motion and preventing impingement of adjacent neurovascular structures. XR systems have shown promise in arthroplasty including by improving precision and streamlining surgery by allowing surgeons to create 3D preoperative plans that are accessible intraoperatively. This article explores the current applications of XR in arthroplasty, highlights recent advancements and benefits, and describes limitations in comparison to traditional techniques.
Methods
A literature search identified studies involving the use of XR in arthroplasty and current US Food and Drug Administration (FDA)-approved XR systems. Multiple electronic databases were used, including PubMed, Google Scholar, and IEEE Xplore. Search terms included: extended reality, augmented reality, virtual reality, arthroplasty, joint replacement, total knee arthroplasty, total shoulder arthroplasty, and total hip arthroplasty. The study design, intervention details, outcomes, and comparisons with traditional surgical techniques were thematically analyzed, with identification of common ideas associated with XR use in arthroplasty. This narrative report highlights the integration of XR in arthroplasty.
Extended Reality Fundamentals
XR encompasses augmented reality (AR), virtual reality (VR), and mixed reality (MR). AR involves superimposing digitally rendered information and images onto the surgeon’s view of the real world, typically through the use of a headset and smart glasses.1 AR allows the surgeon to move and interact freely within the OR, removing the need for additional screens or devices to display patient information or imaging. VR is a fully immersive simulation using a headset that obstructs the view of the real world but allows the user to move freely within this virtual setting, often with audio or other sensory stimuli. MR combines AR and VR to create a digital model that allows for real-world interaction, with the advantage of adapting information and models in real time.2 Whereas in AR the surgeon can view the data projected from the headset, MR provides the ability to interact with and manipulate the digital content (Figure). Both AR and MR have been adapted for use in the OR, while VR has been adapted for use in surgical planning and training.

Extended Reality Use in Orthopedics
The HipNav system was introduced in 1995 to create preoperative plans that assist surgeons in accurately implanting the acetabular cup during total hip arthroplasty (THA).3 Although not commercially successful, this system spurred surgeons to experiment with XR to improve the accuracy and alignment of orthopedic implants. Systems capable of displaying the desired intraoperative implant placement have flourished, with applications in fracture reduction, arthroplasty, solid tumor resection, and hardware placement.4-7 Accurate alignment has been linked to improvements in patient outcomes.8-10 XR has great potential within the field of arthroplasty, with multiple new systems approved by the FDA and currently available in the US (Table).
Hip Arthroplasty
Orientation of the acetabular cup is a technically challenging part of THA. Accuracy in the anteversion and inclination angles of the acetabular cup is required to maintain implant stability, preserve functional range of motion (ROM), and prevent precocious wear.11,12 Despite preoperative planning, surgeons often overestimate the inclination angle and underestimate anteversion.13 Improper implantation of the acetabular cup can lead to joint instability caused by aseptic loosening, increasing the risk of dislocation and the need for revision surgery.14,15 Dislocations typically present to the emergency department, but primary care practitioners may encounter patients with pain or diminished sensation due to impingement or instability.16
The introduction of XR into the OR has provided the opportunity for real-time navigation and adjustment of the acetabular cup to maximize anteversion and inclination angles. Currently, 2 FDA-approved systems are available for THA: the Zimmer and Surgical Planning Associates HipInsight system, and the Insight Augmented Reality Visualization and Information System (ARVIS). The HipInsight system consists of a hologram projection using the Microsoft HoloLens2 device and optimizes preoperative planning, producing accuracy of anteversion and inclination angles within 3°.17 ARVIS employs existing surgical helmets and 2 mounted tracking cameras to provide navigation intraoperatively. ARVIS has also been approved for use in total knee arthroplasty (TKA) and unicompartmental knee arthroplasty.18
HipInsight has shown utility in increasing the accuracy of acetabular cup placement along with the use of biplanar radiographic scans.19 However, there are no studies validating the efficacy of ARVIS and HipInsight and assessing long-term disease-oriented or patient-oriented outcomes.
Knee Arthroplasty
In the setting of TKA, XR is most effective in ensuring accurate resection of the tibial and femoral components. Achieving the planned femoral coronal, axial, and sagittal angles allows the prosthesis to be on the femoral axis of rotation, improving functional outcomes. XR systems for TKA have been shown to increase the accuracy of distal femoral resection with a limited increase in surgery duration.20,21 For TKA in particular, patients are often less satisfied with the result than surgeons expect.22 Accurate alignment can improve patient satisfaction and reduce return-to-clinic rates for postoperative pain management, a factor that primary care practitioners should consider when recommending a patient for TKA.23

Along with ARVIS, 3 additional XR systems are FDA-approved for use in TKA. The Pixee Medical Knee+ system uses smart glasses and trackers to aid in the positioning of instruments for improved accuracy while allowing real-time navigation.24 The Medacta NextAR Knee’s single-use tracking system allows for intraoperative navigation with the use of AR glasses.25 The Polaris STELLAR Knee uses MR and avoids the need for preoperative imaging by capturing real-time anatomic data.26
The Pixee Medical Knee+ system was commercially available in Europe for several years prior to FDA approval, so more research exists on its efficacy. One study found that the Pixee Medical Knee+ system initially demonstrated an inferior clinical outcome, attributed to the learning curve associated with using the system.27 However, more recent studies have shown its utility in improving alignment, regardless of implant specifications.28,29 The Medacta NextAR Knee system has been shown to improve accuracy of tibial rotation and soft tissue balance and even increase OR efficiency.30,31 The Polaris STELLAR Knee system received FDA approval in 2023; no published research exists on its accuracy and outcomes.26
Shoulder Arthroplasty
Minimally invasive techniques are favored in total shoulder arthroplasty (TSA) due to the vitality of maintaining the surrounding soft tissue to maximize preservation of motility and strength.32 However, this complicates the procedure by decreasing the ability to effectively access and visualize key structures of the shoulder. Accordingly, issues with implant positioning and alignment are more common with TSA than other joint arthroplasties, making XR particularly promising.33 Some studies report that up to 67% of patients experience glenohumeral instability, which can clinically present as weakness, decreased range of motion, and persistent shoulder pain.34,35 The use of preoperative computed tomography to improve understanding of glenoid anatomy and glenohumeral subluxation is becoming increasingly common, and it can be combined with XR to improve accuracy.36,37
Two FDA-approved systems are available. The Stryker Blueprint MR system is used for intraoperative guidance and integration for patient imaging used for preoperative planning. The Medacta NextAR Shoulder system is a parallel of the company’s TKA system. The Stryker Blueprint MR system combines the Microsoft HoloLens 2 headset to display preoperative plans with a secondary display for coordination with the rest of the surgical team.38 Similar to the Medacta NextAR Knee, the Medacta NextAR Shoulder system uses the same single-use tracking system and AR glasses for intraoperative guidance.39
Data on the long-term outcomes of using these systems are still limited, but the Stryker Blueprint MR system has not been shown to accurately predict postoperative ROM.40 Cadaveric studies have demonstrated that the Medacta NextAR Shoulder system can provide accurate inclination, retroversion, entry point, depth, and rotation values based on the preoperative planned values.41,42 However, this accuracy has yet to be confirmed in vivo, and the impact of using XR in TSA on long-term outcomes is still unknown.
Challenges and Limitations
Though XR has proven to be promising in arthroplasty, several limitations regarding widespread implementation exist. In particular, there is a steep learning curve associated with the use of XR systems, which can cause increased operative time and even initial inferior outcomes, as demonstrated with the Pixee Medical Knee+ system. The need for extensive practice and training prior to use could delay widespread adoption and may cause discrepancies in surgical outcomes. Unfamiliarity with the system and technological difficulties that may require troubleshooting can also increase operative time, particularly for surgeons new to using the XR system. Though intraoperative navigation is expected to improve accuracy of implant alignment, its added complexity may also result in longer surgeries.
In addition to the steep learning curve and increased operative time, there is a high upfront cost associated with XR systems. Exact costs of XR systems are not typically disclosed, but available estimates suggest an average sales price of about $1000 per case. Given the proprietary nature of these technologies, publicly available cost data are limited, making it challenging to fully assess the financial burden on health care institutions. Though some systems, such as ARVIS, can be integrated with existing surgical helmets, many require the purchase of AR glasses and secondary displays. This can cause further variation in the total expense for each system. In low-resource settings, this represents a significant challenge to widespread implementation. To justify this cost, additional research on long-term patient outcomes is needed to ensure the benefits of XR systems outweigh the cost.
Although early studies on XR systems in arthroplasty have shown improvements in precision and short-term outcomes, long-term data regarding effectiveness remains. Even systems such as ARVIS and HipInsight have limited long-term follow-up, making it difficult to assess whether the improved accuracy with these XR systems translates into improved patient outcomes compared with traditional arthroplasty.
CONCLUSIONS
XR technologies have shown significant potential in enhancing precision and patient outcomes. Through the integration of XR in the OR, surgeons can visualize preoperative plans and even make intraoperative changes, with the benefit of improving implant alignment.
There are some disadvantages to its use, however, including high cost and increased operative time. Despite this, the integration of XR into surgical practice can deliver more precise implant alignment and address other challenges faced with conventional techniques. As these technologies evolve and studies on long-term outcomes validate their utility, XR has the potential to transform the field of arthroplasty.
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
Azuma RT. A survey of augmented reality. Presence-Teleop Virt. 1997;6:355-385. doi:10.1162/pres.1997.6.4.355
Speicher M, Hall BD, Nebeling M. What is Mixed Reality? In: Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems. Association for Computing Machinery; 2019:1-15. doi:10.1145/3290605.3300767
Digioia AM, Jaramaz B, Nikou C, et al. Surgical navigation for total hip replacement with the use of hipnav. Oper Tech Orthop. 2000;10:3-8. doi:10.1016/S1048-6666(00)80036-1
Ogawa H, Hasegawa S, Tsukada S, et al. A pilot study of augmented reality technology applied to the acetabular cup placement during total hip arthroplasty. J Arthroplasty. 2018;33:1833-1837. doi:10.1016/j.arth.2018.01.067
Shen F, Chen B, Guo Q, et al. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int J CARS. 2013;8:169-179. doi:10.1007/s11548-012-0775-5
Cho HS, Park YK, Gupta S, et al. Augmented reality in bone tumour resection: an experimental study. Bone Joint Res. 2017;6:137-143. doi:10.1302/2046-3758.63.bjr-2016-0289.r1
Wu X, Liu R, Yu J, et al. Mixed reality technology launches in orthopedic surgery for comprehensive preoperative management of complicated cervical fractures. Surg Innov. 2018;25:421-422. doi:10.1177/1553350618761758
Dossett HG, Arthur JR, Makovicka JL, et al. A randomized controlled trial of kinematically and mechanically aligned total knee arthroplasties: long-term follow-up. J Arthroplasty. 2023;38:S209-S214. doi:10.1016/j.arth.2023.03.065
Kazarian GS, Haddad FS, Donaldson MJ, et al. Implant malalignment may be a risk factor for poor patient-reported outcomes measures (PROMs) following total knee arthroplasty (TKA). J Arthroplasty. 2022;37:S129-S133. doi:10.1016/j.arth.2022.02.087
Peng Y, Arauz P, An S, et al. Does component alignment affect patient reported outcomes following bicruciate retaining total knee arthroplasty? An in vivo three-dimensional analysis. J Knee Surg. 2020;33:798-803. doi:10.1055/s-0039-1688500
D’Lima DD, Urquhart AG, Buehler KO, et al. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315-321. doi:10.2106/00004623-200003000-00003
Yamaguchi M, Akisue T, Bauer TW, et al. The spatial location of impingement in total hip arthroplasty. J Arthroplasty. 2000;15:305-313. doi:10.1016/s0883-5403(00)90601-6
Grammatopoulos G, Alvand A, Monk AP, et al. Surgeons’ accuracy in achieving their desired acetabular component orientation. J Bone Joint Surg. 2016;98:e72. doi:10.2106/JBJS.15.01080
Kennedy JG, Rogers WB, Soffe KE, et al. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530-534. doi:10.1016/S0883-5403(98)90052-3
Del Schutte H, Lipman AJ, Bannar SM, et al. Effects of acetabular abduction on cup wear rates in total hip arthroplasty. J Arthroplasty. 1998;13:621-626. doi:10.1016/S0883-5403(98)80003-X
Aresti N, Kassam J, Bartlett D, et al. Primary care management of postoperative shoulder, hip, and knee arthroplasty. BMJ. 2017;359:j4431. doi:10.1136/bmj.j4431
HipInsightTM System. Zimmer Biomet. Accessed September 3, 2025. https://www.zimmerbiomet.com/en/products-and-solutions/zb-edge/mixed-reality-portfolio/hipinsight-system.html
ARVIS. Insight Medical Systems. Accessed September 3, 2025. https://www.insightmedsys.com/arvis
Sun DC, Murphy WS, Amundson AJ, et al. Validation of a novel method of measuring cup orientation using biplanar simultaneous radiographic images. J Arthroplasty. 2023;38:S252-S256. doi:10.1016/j.arth.2023.04.011
Tsukada S, Ogawa H, Nishino M, et al. Augmented reality-assisted femoral bone resection in total knee arthroplasty. JBJS Open Access. 2021;6:e21.00001. doi:10.2106/JBJS.OA.21.00001
Castellarin G, Bori E, Barbieux E, et al. Is total knee arthroplasty surgical performance enhanced using augmented reality? A single-center study on 76 consecutive patients. J Arthroplasty. 2024;39:332-335. doi:10.1016/j.arth.2023.08.013
Choi YJ, Ra HJ. Patient satisfaction after total knee arthroplasty. Knee Surg Relat Res. 2016;28:1. doi:10.5792/ksrr.2016.28.1.1
Hazratwala K, Gouk C, Wilkinson MPR, et al. Navigated functional alignment total knee arthroplasty achieves reliable, reproducible and accurate results with high patient satisfaction. Knee Surg Sports Traumatol Arthrosc. 2023;31:3861-3870. doi:10.1007/s00167-023-07327-w
Knee+. Pixee Medical. Accessed September 3, 2025. https://www.pixee-medical.com/en/products/knee-nexsight/
KNEE | NEXTAR. Nextar. Accessed September 3, 2025. https://nextar.medacta.com/knee
POLARIS AR receives clearance from the U.S. Food and Drug Administration for STELLAR Knee. News release. PRNewswire. November 3, 2023. Accessed September 3, 2025. https://www.prnewswire.com/news-releases/polarisar-receives-clearance-from-the-us-food-and-drug-administration-for-stellar-knee-301976747.html
van Overschelde P, Vansintjan P, Byn P, Lapierre C, van Lysebettens W. Does augmented reality improve clinical outcome in TKA? A prospective observational report. In: The 20th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. 2022:170-174.
Sakellariou E, Alevrogiannis P, Alevrogianni F, et al. Single-center experience with Knee+TM augmented reality navigation system in primary total knee arthroplasty. World J Orthop. 2024;15:247-256. doi:10.5312/wjo.v15.i3.247
León-Muñoz VJ, Moya-Angeler J, López-López M, et al. Integration of square fiducial markers in patient-specific instrumentation and their applicability in knee surgery. J Pers Med. 2023;13:727. doi:10.3390/jpm13050727
Fucentese SF, Koch PP. A novel augmented reality-based surgical guidance system for total knee arthroplasty. Arch Orthop Trauma Surg. 2021;141:2227-2233. doi:10.1007/s00402-021-04204-4
Sabatini L, Ascani D, Vezza D, et al. Novel surgical technique for total knee arthroplasty integrating kinematic alignment and real-time elongation of the ligaments using the NextAR system. J Pers Med. 2024;14:794. doi:10.3390/jpm14080794
Daher M, Ghanimeh J, Otayek J, et al. Augmented reality and shoulder replacement: a state-of-the-art review article. JSES Rev Rep Tech. 2023;3:274-278. doi:10.1016/j.xrrt.2023.01.008
Atmani H, Merienne F, Fofi D, et al. Computer aided surgery system for shoulder prosthesis placement. Comput Aided Surg. 2007;12:60-70. doi:10.3109/10929080701210832
Eichinger JK, Galvin JW. Management of complications after total shoulder arthroplasty. Curr Rev Musculoskelet Med. 2015;8:83-91. doi:10.1007/s12178-014-9251-x
Bonnevialle N, Melis B, Neyton L, et al. Aseptic glenoid loosening or failure in total shoulder arthroplasty: revision with glenoid reimplantation. J Shoulder Elbow Surg. 2013;22:745-751. doi:10.1016/j.jse.2012.08.009
Erickson BJ, Chalmers PN, Denard P, et al. Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg. 2021;30:413-420. doi:10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, et al. The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26:1477-1483. doi:10.1016/j.jse.2017.01.006
Blueprint. Stryker. Updated August 2025. Accessed September 3, 2025. https://www.stryker.com/us/en/trauma-and-extremities/products/blueprint.html
NextAR Shoulder. Medacta. Accessed September 3, 2025. https://nextar.medacta.com/shoulder
Baumgarten KM. Accuracy of Blueprint software in predicting range of motion 1 year after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2023;32:1088-1094. doi:10.1016/j.jse.2022.12.009
Rojas JT, Jost B, Zipeto C, et al. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J Shoulder Elbow Surg. 2023;32:e587-e596. doi:10.1016/j.jse.2023.05.002
Dey Hazra RO, Paksoy A, Imiolczyk JP, et al. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2025;34(2):577-583. doi:10.1016/j.jse.2024.05.039
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
The Integration of Extended Reality in Arthroplasty: Reviewing Technological Progress and Clinical Benefits
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.
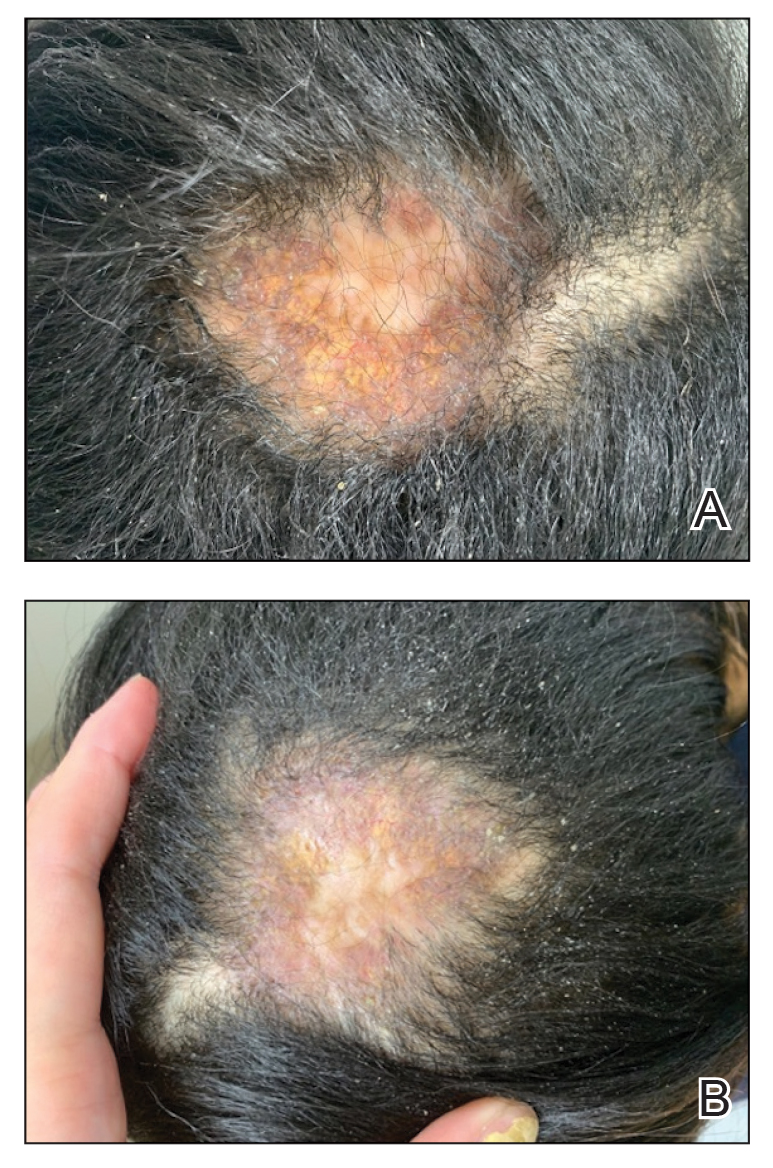
We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
To the Editor:
Necrobiotic xanthogranuloma (NXG) is classified as a cutaneous non–Langerhans cell histiocytosis, often seen with monoclonal gammopathy of undetermined significance or multiple myeloma.1 Clinically, it appears as a red or yellow plaque with occasional ulceration and telangiectasias, most commonly seen periorbitally and on the trunk. On pathology, NXG appears as necrobiosis, giant cells, and various inflammatory cells extending into the subcutaneous tissue.2 In this article, we describe a rare presentation of NXG in location and skin type.
A 52-year-old woman with a history of systemic lupus erythematosus (SLE) presented with alopecia and a tender lesion on the scalp of 5 years’ duration (Figure 1). The patient had no history of a similar lesion, and no other lesions were present. A biopsy performed at an outside clinic a few weeks to months prior to the initial presentation to our clinic showed NXG (Figure 2). Evaluation at our clinic revealed a 4x4-cm orange-brown annular plaque on the left parietal scalp. Serum and urine protein electrophoresis studies were negative. The patient reported she was up to date with recommended screenings such as mammography and colonoscopy.


We started the patient on topical triamcinolone and topical ruxolitinib and administered intralesional triamcinolone. She was already taking hydroxychloroquine and leflunomide for SLE. Three weeks later, she returned with improved symptoms and appearance (Figure 1). She remained on intralesional triamcinolone and ruxolitinib and continues to experience improvement.
Necrobiotic xanthogranuloma is rare and typically is associated with monoclonal gammopathy.2 In one study, 83 of 100 of patients with NXG presented with or were found to have a monoclonal gammopathy.2 In another study, paraproteinemia was detected in 82.1% of patients.3 The majority of case reports and systematic reviews detail periorbital or thoracic lesions.4 The location on the scalp and lack of association with paraproteinemia make this a rare presentation of NXG. Studies may be warranted to explore any association of SLE with NXG if more cases present.
In a multicenter cross-sectional study and systematic review of 235 patients with NXG, 87% were White, 12% were Asian, and only 1% were Black or African American.3 The limited representation of skin of color raises concern for the possibility of missed diagnoses and delays in care.
Treatment of NXG often is multimodal with use of intravenous immunoglobulin, oral steroids, chlorambucil, melphalan, and other alkylating agents, and response is variable.3-6 Recent studies show treatment effectiveness with Janus kinase inhibitors in granulomatous dermatitides.7-9 As our patient was not responding to prior treatments, we decided to try ruxolitinib, and she has continued to improve with it.10,11 Interestingly, the patient experienced continued improvement with intralesional triamcinolone, which is not often reported in the literature.2-6 Overall, NXG is an extremely rare condition that requires special care in workup to rule out paraproteinemia and a thoughtful approach to treatment modalities.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681.
- Spicknall KE, Mehregan DA. Necrobiotic xanthogranuloma. Int J Dermatol. 2009;48:1-10.
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter cross-sectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279.
- Huynh KN, Nguyen BD. Histiocytosis and neoplasms of macrophagedendritic cell lineages: multimodality imaging with emphasis on PET/CT. Radiographics. 2021;41:576-594. doi: 10.1148/rg.2021200096
- Hilal T, DiCaudo DJ, Connolly SM, et al. Necrobiotic xanthogranuloma: a 30-year single-center experience. Ann Hematol. 2018;97:1471-1479.
- Oumeish OY, Oumeish I, Tarawneh M, et al. Necrobiotic xanthogranuloma associated with paraproteinemia and non- Hodgkin’s lymphoma developing into chronic lymphocytic leukemia: the first case reported in the literature and review of the literature. Int J Dermatol. 2006;45:306-310.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-621. doi:10.1016 /j.jaad.2019.05.098
- Wang A, Rahman NT, McGeary MK, et al. Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-1809. doi:10.1016 /j.jaci.2020.10.012
- Stratman S, Amara S, Tan KJ, et al. Systemic Janus kinase inhibitors in the management of granuloma annulare. Arch Dermatol Res. 2025;317:743. doi:10.1007/s00403-025-04248-1
- McPhie ML, Swales WC, Gooderham MJ. Improvement of granulomatous skin conditions with tofacitinib in three patients: a case report. SAGE Open Med Case Rep. 2021;9:2050313X211039477. doi: 10.1177/2050313X211039477
- Sood S, Heung M, Georgakopoulos JR, et al. Use of Janus kinase inhibitors for granulomatous dermatoses: a systematic review. J Am Acad Dermatol. 2023;89:357-359. doi: 10.1016/j.jaad.2023.03.024
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
Rare Case of Necrobiotic Xanthogranuloma on the Scalp
PRACTICE POINTS
- In skin of color, necrobiotic xanthogranuloma can appear orange or brown compared to its yellow appearance in lighter skin types.
- When necrobiotic xanthogranuloma is suspected, a thorough malignancy workup should be conducted.
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
As the health care landscape continues to shift, direct care (also known as direct pay) models have emerged as attractive alternatives to traditional insurance-based practice. For dermatology residents poised to enter the workforce, the direct care model offers potential advantages in autonomy, patient relationships, and work-life balance, but not without considerable risks and operational challenges. This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
The transition from dermatology residency to clinical practice allows for a variety of paths, from large academic institutions to private practice to corporate entities (private equity–owned groups). In recent years, the direct care model has gained traction, particularly among physicians seeking greater autonomy and a more sustainable pace of practice.
Direct care dermatology practices operate outside the constraints of third-party payers, offering patients transparent pricing and direct access to care in exchange for fees paid out of pocket. By eliminating insurance companies as the middleman, it allows for less overhead, longer visits with patients, and increased access to care; however, though this model may seem appealing, direct care practices are not without their own set of challenges, especially amid rising concerns over physician burnout and administrative burden.
This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
Despite its appeal, starting a direct care practice is not without substantial risks and hurdles—particularly for residents just out of training. These challenges include financial risks and startup costs, market uncertainty, lack of mentorship or support, and limitations in treating complex dermatologic conditions.
Before committing to practicing via a direct care model, dermatology residents should reflect on the following:
- Risk tolerance: Are you comfortable navigating the business and financial risk?
- Location: Does your target community have patients willing and able to pay out of pocket?
- Scope of interest: Will a direct care practice align with your clinical passions?
- Support systems: Do you have access to mentors, legal and financial advisors, and operational support?
- Long-term goals: Are you building a lifestyle practice, a scalable business, or a stepping stone to a future opportunity?
Ultimately, the decision to pursue a direct care model requires careful reflection on personal values, financial preparedness, and the unique needs of the community one intends to serve.
The direct care dermatology model offers an appealing alternative to traditional practice, especially for those prioritizing autonomy, patient connection, and work-life balance; however, it demands an entrepreneurial spirit as well as careful planning and an acceptance of financial uncertainty—factors that may pose challenges for new graduates. For dermatology residents, the decision to pursue direct care should be grounded in personal values, practical considerations, and a clear understanding of both the opportunities and limitations of this evolving practice model.
- Sinsky CA, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med.
- Dorrell DN, Feldman S, Wei-ting Huang W. The most common causes of burnout among US academic dermatologists based on a survey study. J Am Acad of Dermatol. 2019;81:269-270.
- Carlasare LE. Defining the place of direct primary care in a value-based care system. WMJ. 2018;117:106-110.
As the health care landscape continues to shift, direct care (also known as direct pay) models have emerged as attractive alternatives to traditional insurance-based practice. For dermatology residents poised to enter the workforce, the direct care model offers potential advantages in autonomy, patient relationships, and work-life balance, but not without considerable risks and operational challenges. This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
The transition from dermatology residency to clinical practice allows for a variety of paths, from large academic institutions to private practice to corporate entities (private equity–owned groups). In recent years, the direct care model has gained traction, particularly among physicians seeking greater autonomy and a more sustainable pace of practice.
Direct care dermatology practices operate outside the constraints of third-party payers, offering patients transparent pricing and direct access to care in exchange for fees paid out of pocket. By eliminating insurance companies as the middleman, it allows for less overhead, longer visits with patients, and increased access to care; however, though this model may seem appealing, direct care practices are not without their own set of challenges, especially amid rising concerns over physician burnout and administrative burden.
This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
Despite its appeal, starting a direct care practice is not without substantial risks and hurdles—particularly for residents just out of training. These challenges include financial risks and startup costs, market uncertainty, lack of mentorship or support, and limitations in treating complex dermatologic conditions.
Before committing to practicing via a direct care model, dermatology residents should reflect on the following:
- Risk tolerance: Are you comfortable navigating the business and financial risk?
- Location: Does your target community have patients willing and able to pay out of pocket?
- Scope of interest: Will a direct care practice align with your clinical passions?
- Support systems: Do you have access to mentors, legal and financial advisors, and operational support?
- Long-term goals: Are you building a lifestyle practice, a scalable business, or a stepping stone to a future opportunity?
Ultimately, the decision to pursue a direct care model requires careful reflection on personal values, financial preparedness, and the unique needs of the community one intends to serve.
The direct care dermatology model offers an appealing alternative to traditional practice, especially for those prioritizing autonomy, patient connection, and work-life balance; however, it demands an entrepreneurial spirit as well as careful planning and an acceptance of financial uncertainty—factors that may pose challenges for new graduates. For dermatology residents, the decision to pursue direct care should be grounded in personal values, practical considerations, and a clear understanding of both the opportunities and limitations of this evolving practice model.
As the health care landscape continues to shift, direct care (also known as direct pay) models have emerged as attractive alternatives to traditional insurance-based practice. For dermatology residents poised to enter the workforce, the direct care model offers potential advantages in autonomy, patient relationships, and work-life balance, but not without considerable risks and operational challenges. This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
The transition from dermatology residency to clinical practice allows for a variety of paths, from large academic institutions to private practice to corporate entities (private equity–owned groups). In recent years, the direct care model has gained traction, particularly among physicians seeking greater autonomy and a more sustainable pace of practice.
Direct care dermatology practices operate outside the constraints of third-party payers, offering patients transparent pricing and direct access to care in exchange for fees paid out of pocket. By eliminating insurance companies as the middleman, it allows for less overhead, longer visits with patients, and increased access to care; however, though this model may seem appealing, direct care practices are not without their own set of challenges, especially amid rising concerns over physician burnout and administrative burden.
This article explores the key benefits and drawbacks of starting a direct care dermatology practice, providing a framework to help early-career dermatologists determine whether this path aligns with their personal and professional goals.
Despite its appeal, starting a direct care practice is not without substantial risks and hurdles—particularly for residents just out of training. These challenges include financial risks and startup costs, market uncertainty, lack of mentorship or support, and limitations in treating complex dermatologic conditions.
Before committing to practicing via a direct care model, dermatology residents should reflect on the following:
- Risk tolerance: Are you comfortable navigating the business and financial risk?
- Location: Does your target community have patients willing and able to pay out of pocket?
- Scope of interest: Will a direct care practice align with your clinical passions?
- Support systems: Do you have access to mentors, legal and financial advisors, and operational support?
- Long-term goals: Are you building a lifestyle practice, a scalable business, or a stepping stone to a future opportunity?
Ultimately, the decision to pursue a direct care model requires careful reflection on personal values, financial preparedness, and the unique needs of the community one intends to serve.
The direct care dermatology model offers an appealing alternative to traditional practice, especially for those prioritizing autonomy, patient connection, and work-life balance; however, it demands an entrepreneurial spirit as well as careful planning and an acceptance of financial uncertainty—factors that may pose challenges for new graduates. For dermatology residents, the decision to pursue direct care should be grounded in personal values, practical considerations, and a clear understanding of both the opportunities and limitations of this evolving practice model.
- Sinsky CA, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med.
- Dorrell DN, Feldman S, Wei-ting Huang W. The most common causes of burnout among US academic dermatologists based on a survey study. J Am Acad of Dermatol. 2019;81:269-270.
- Carlasare LE. Defining the place of direct primary care in a value-based care system. WMJ. 2018;117:106-110.
- Sinsky CA, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med.
- Dorrell DN, Feldman S, Wei-ting Huang W. The most common causes of burnout among US academic dermatologists based on a survey study. J Am Acad of Dermatol. 2019;81:269-270.
- Carlasare LE. Defining the place of direct primary care in a value-based care system. WMJ. 2018;117:106-110.
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
Direct Care Dermatology: Weighing the Pros and Cons for the Early-Career Physician
PRACTICE POINTS
- Direct care practices may be the new horizon of health care.
- Starting a direct care practice offers autonomy but demands entrepreneurial readiness.
- New dermatologists can enjoy control over scheduling, pricing, and patient care, but success requires business acumen, financial planning, and comfort with risk.