User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Expelled from high school, Alister Martin became a Harvard doc
It’s not often that a high school brawl with gang members sets you down a path to becoming a Harvard-trained doctor. But that’s exactly how Alister Martin’s life unfolded.
In retrospect, he should have seen the whole thing coming. That night at the party, his best friend was attacked by a gang member from a nearby high school. Martin was not in a gang but he jumped into the fray to defend his friend.
“I wanted to save the day, but that’s not what happened,” he says. “There were just too many of them.”
When his mother rushed to the hospital, he was so bruised and bloody that she couldn’t recognize him at first. Ever since he was a baby, she had done her best to shield him from the neighborhood where gang violence was a regular disruption. But it hadn’t worked.
“My high school had a zero-tolerance policy for gang violence,” Martin says, “so even though I wasn’t in a gang, I was kicked out.”
Now expelled from high school, his mother wanted him out of town, fearing gang retaliation, or that Martin might seek vengeance on the boy who had brutally beaten him. So, the biology teacher and single mom who worked numerous jobs to keep them afloat, came up with a plan to get him far away from any temptations.
Martin had loved tennis since middle school, when his 8th-grade math teacher, Billie Weise, also a tennis pro, got him a job as a court sweeper at an upscale tennis club nearby. He knew nothing then about tennis but would come to fall in love with the sport. To get her son out of town, Martin’s mother took out loans for $30,000 and sent him to a Florida tennis training camp.
After 6 months of training, Martin, who earned a GED degree while attending the camp, was offered a scholarship to play tennis at Rutgers University in New Brunswick, N.J. The transition to college was tough, however. He was nervous and felt out of place. “I could have died that first day. It became so obvious how poorly my high school education had prepared me for this.”
But the unease he felt was also motivating in a way. Worried about failure, “he locked himself in a room with another student and they studied day and night,” recalls Kamal Khan, director of the office for diversity and academic success at Rutgers. “I’ve never seen anything like it.”
And Martin displayed other attributes that would draw others to him – and later prove important in his career as a doctor. His ability to display empathy and interact with students and teachers separated him from his peers, Mr. Khan says. “There’re a lot of really smart students out there,” he says, “but not many who understand people like Martin.”
After graduating, he decided to pursue his dream of becoming a doctor. He’d wanted to be a doctor since he was 10 years old after his mom was diagnosed with metastatic breast cancer. He remembers overhearing a conversation she was having with a family friend about where he would go if she died.
“That’s when I knew it was serious,” he says.
Doctors saved her life, and it’s something he’ll never forget. But it wasn’t until his time at Rutgers that he finally had the confidence to think he could succeed in medical school.
Martin went on to attend Harvard Medical School and Harvard Kennedy School of Government as well as serving as chief resident at Brigham and Women’s Hospital. He was also a fellow at the White House in the Office of the Vice President and today, he’s an assistant professor at Harvard Medical School in Boston..
He is most at home in the emergency room at Massachusetts General Hospital, where he works as an emergency medical specialist. For him, the ER is the first line of defense for meeting the community’s health needs. Growing up in Neptune, the ER “was where poor folks got their care,” he says. His mom worked two jobs and when she got off work at 8 p.m. there was no pediatrician open. “When I was sick as a kid we always went to the emergency room,” he says.
While at Harvard, he also pursued a degree from the Kennedy School of Government, because of the huge role he feels that politics play in our health care system and especially in bringing care to impoverished communities. And since then he’s taken numerous steps to bridge the gap.
Addiction, for example, became an important issue for Martin, ever since a patient he encountered in his first week as an internist. She was a mom of two who had recently gotten surgery because she broke her ankle falling down the stairs at her child’s daycare, he says. Prescribed oxycodone, she feared she was becoming addicted and needed help. But at the time, there was nothing the ER could do.
“I remember that look in her eyes when we had to turn her away,” he says.
Martin has worked to change protocol at his hospital and others throughout the nation so they can be better set up to treat opioid addiction. He’s the founder of GetWaivered, an organization that trains doctors throughout the country to use evidence-based medicine to manage opioid addiction. In the U.S. doctors need what’s called a DEA X waiver to be able to prescribe buprenorphine to opioid-addicted patients. That means that currently only about 1% of all emergency room doctors nationwide have the waiver and without it, it’s impossible to help patients when they need it the most.
Shuhan He, MD, an internist with Martin at Massachusetts General Hospital who also works on the GetWaivered program, says Martin has a particular trait that helps him be successful.
“He’s a doer and when he sees a problem, he’s gonna try and fix it.”
A version of this article first appeared on Medscape.com.
It’s not often that a high school brawl with gang members sets you down a path to becoming a Harvard-trained doctor. But that’s exactly how Alister Martin’s life unfolded.
In retrospect, he should have seen the whole thing coming. That night at the party, his best friend was attacked by a gang member from a nearby high school. Martin was not in a gang but he jumped into the fray to defend his friend.
“I wanted to save the day, but that’s not what happened,” he says. “There were just too many of them.”
When his mother rushed to the hospital, he was so bruised and bloody that she couldn’t recognize him at first. Ever since he was a baby, she had done her best to shield him from the neighborhood where gang violence was a regular disruption. But it hadn’t worked.
“My high school had a zero-tolerance policy for gang violence,” Martin says, “so even though I wasn’t in a gang, I was kicked out.”
Now expelled from high school, his mother wanted him out of town, fearing gang retaliation, or that Martin might seek vengeance on the boy who had brutally beaten him. So, the biology teacher and single mom who worked numerous jobs to keep them afloat, came up with a plan to get him far away from any temptations.
Martin had loved tennis since middle school, when his 8th-grade math teacher, Billie Weise, also a tennis pro, got him a job as a court sweeper at an upscale tennis club nearby. He knew nothing then about tennis but would come to fall in love with the sport. To get her son out of town, Martin’s mother took out loans for $30,000 and sent him to a Florida tennis training camp.
After 6 months of training, Martin, who earned a GED degree while attending the camp, was offered a scholarship to play tennis at Rutgers University in New Brunswick, N.J. The transition to college was tough, however. He was nervous and felt out of place. “I could have died that first day. It became so obvious how poorly my high school education had prepared me for this.”
But the unease he felt was also motivating in a way. Worried about failure, “he locked himself in a room with another student and they studied day and night,” recalls Kamal Khan, director of the office for diversity and academic success at Rutgers. “I’ve never seen anything like it.”
And Martin displayed other attributes that would draw others to him – and later prove important in his career as a doctor. His ability to display empathy and interact with students and teachers separated him from his peers, Mr. Khan says. “There’re a lot of really smart students out there,” he says, “but not many who understand people like Martin.”
After graduating, he decided to pursue his dream of becoming a doctor. He’d wanted to be a doctor since he was 10 years old after his mom was diagnosed with metastatic breast cancer. He remembers overhearing a conversation she was having with a family friend about where he would go if she died.
“That’s when I knew it was serious,” he says.
Doctors saved her life, and it’s something he’ll never forget. But it wasn’t until his time at Rutgers that he finally had the confidence to think he could succeed in medical school.
Martin went on to attend Harvard Medical School and Harvard Kennedy School of Government as well as serving as chief resident at Brigham and Women’s Hospital. He was also a fellow at the White House in the Office of the Vice President and today, he’s an assistant professor at Harvard Medical School in Boston..
He is most at home in the emergency room at Massachusetts General Hospital, where he works as an emergency medical specialist. For him, the ER is the first line of defense for meeting the community’s health needs. Growing up in Neptune, the ER “was where poor folks got their care,” he says. His mom worked two jobs and when she got off work at 8 p.m. there was no pediatrician open. “When I was sick as a kid we always went to the emergency room,” he says.
While at Harvard, he also pursued a degree from the Kennedy School of Government, because of the huge role he feels that politics play in our health care system and especially in bringing care to impoverished communities. And since then he’s taken numerous steps to bridge the gap.
Addiction, for example, became an important issue for Martin, ever since a patient he encountered in his first week as an internist. She was a mom of two who had recently gotten surgery because she broke her ankle falling down the stairs at her child’s daycare, he says. Prescribed oxycodone, she feared she was becoming addicted and needed help. But at the time, there was nothing the ER could do.
“I remember that look in her eyes when we had to turn her away,” he says.
Martin has worked to change protocol at his hospital and others throughout the nation so they can be better set up to treat opioid addiction. He’s the founder of GetWaivered, an organization that trains doctors throughout the country to use evidence-based medicine to manage opioid addiction. In the U.S. doctors need what’s called a DEA X waiver to be able to prescribe buprenorphine to opioid-addicted patients. That means that currently only about 1% of all emergency room doctors nationwide have the waiver and without it, it’s impossible to help patients when they need it the most.
Shuhan He, MD, an internist with Martin at Massachusetts General Hospital who also works on the GetWaivered program, says Martin has a particular trait that helps him be successful.
“He’s a doer and when he sees a problem, he’s gonna try and fix it.”
A version of this article first appeared on Medscape.com.
It’s not often that a high school brawl with gang members sets you down a path to becoming a Harvard-trained doctor. But that’s exactly how Alister Martin’s life unfolded.
In retrospect, he should have seen the whole thing coming. That night at the party, his best friend was attacked by a gang member from a nearby high school. Martin was not in a gang but he jumped into the fray to defend his friend.
“I wanted to save the day, but that’s not what happened,” he says. “There were just too many of them.”
When his mother rushed to the hospital, he was so bruised and bloody that she couldn’t recognize him at first. Ever since he was a baby, she had done her best to shield him from the neighborhood where gang violence was a regular disruption. But it hadn’t worked.
“My high school had a zero-tolerance policy for gang violence,” Martin says, “so even though I wasn’t in a gang, I was kicked out.”
Now expelled from high school, his mother wanted him out of town, fearing gang retaliation, or that Martin might seek vengeance on the boy who had brutally beaten him. So, the biology teacher and single mom who worked numerous jobs to keep them afloat, came up with a plan to get him far away from any temptations.
Martin had loved tennis since middle school, when his 8th-grade math teacher, Billie Weise, also a tennis pro, got him a job as a court sweeper at an upscale tennis club nearby. He knew nothing then about tennis but would come to fall in love with the sport. To get her son out of town, Martin’s mother took out loans for $30,000 and sent him to a Florida tennis training camp.
After 6 months of training, Martin, who earned a GED degree while attending the camp, was offered a scholarship to play tennis at Rutgers University in New Brunswick, N.J. The transition to college was tough, however. He was nervous and felt out of place. “I could have died that first day. It became so obvious how poorly my high school education had prepared me for this.”
But the unease he felt was also motivating in a way. Worried about failure, “he locked himself in a room with another student and they studied day and night,” recalls Kamal Khan, director of the office for diversity and academic success at Rutgers. “I’ve never seen anything like it.”
And Martin displayed other attributes that would draw others to him – and later prove important in his career as a doctor. His ability to display empathy and interact with students and teachers separated him from his peers, Mr. Khan says. “There’re a lot of really smart students out there,” he says, “but not many who understand people like Martin.”
After graduating, he decided to pursue his dream of becoming a doctor. He’d wanted to be a doctor since he was 10 years old after his mom was diagnosed with metastatic breast cancer. He remembers overhearing a conversation she was having with a family friend about where he would go if she died.
“That’s when I knew it was serious,” he says.
Doctors saved her life, and it’s something he’ll never forget. But it wasn’t until his time at Rutgers that he finally had the confidence to think he could succeed in medical school.
Martin went on to attend Harvard Medical School and Harvard Kennedy School of Government as well as serving as chief resident at Brigham and Women’s Hospital. He was also a fellow at the White House in the Office of the Vice President and today, he’s an assistant professor at Harvard Medical School in Boston..
He is most at home in the emergency room at Massachusetts General Hospital, where he works as an emergency medical specialist. For him, the ER is the first line of defense for meeting the community’s health needs. Growing up in Neptune, the ER “was where poor folks got their care,” he says. His mom worked two jobs and when she got off work at 8 p.m. there was no pediatrician open. “When I was sick as a kid we always went to the emergency room,” he says.
While at Harvard, he also pursued a degree from the Kennedy School of Government, because of the huge role he feels that politics play in our health care system and especially in bringing care to impoverished communities. And since then he’s taken numerous steps to bridge the gap.
Addiction, for example, became an important issue for Martin, ever since a patient he encountered in his first week as an internist. She was a mom of two who had recently gotten surgery because she broke her ankle falling down the stairs at her child’s daycare, he says. Prescribed oxycodone, she feared she was becoming addicted and needed help. But at the time, there was nothing the ER could do.
“I remember that look in her eyes when we had to turn her away,” he says.
Martin has worked to change protocol at his hospital and others throughout the nation so they can be better set up to treat opioid addiction. He’s the founder of GetWaivered, an organization that trains doctors throughout the country to use evidence-based medicine to manage opioid addiction. In the U.S. doctors need what’s called a DEA X waiver to be able to prescribe buprenorphine to opioid-addicted patients. That means that currently only about 1% of all emergency room doctors nationwide have the waiver and without it, it’s impossible to help patients when they need it the most.
Shuhan He, MD, an internist with Martin at Massachusetts General Hospital who also works on the GetWaivered program, says Martin has a particular trait that helps him be successful.
“He’s a doer and when he sees a problem, he’s gonna try and fix it.”
A version of this article first appeared on Medscape.com.
Joint effort: CBD not just innocent bystander in weed
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.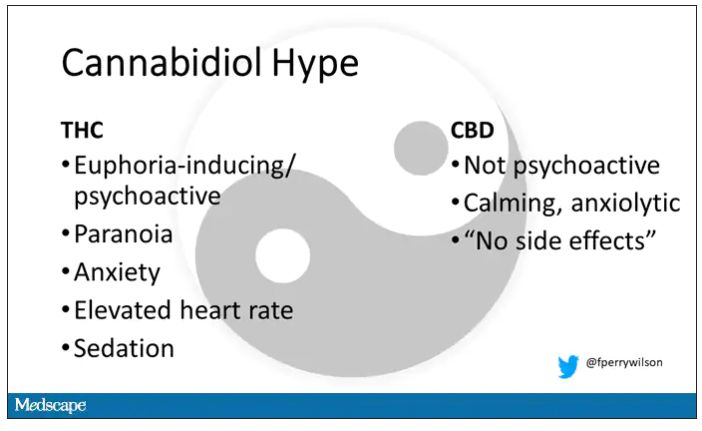
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.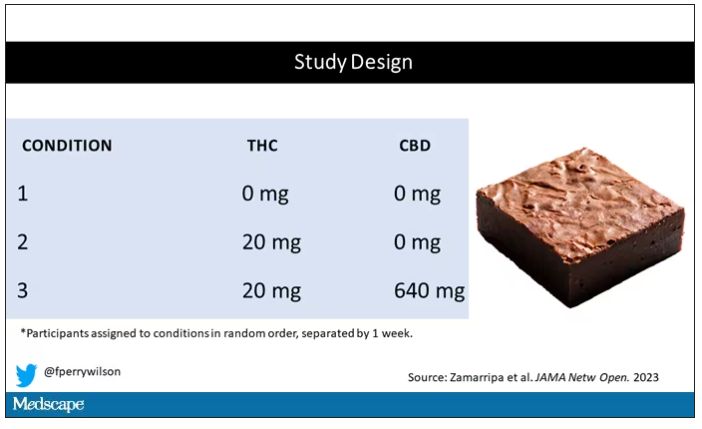
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.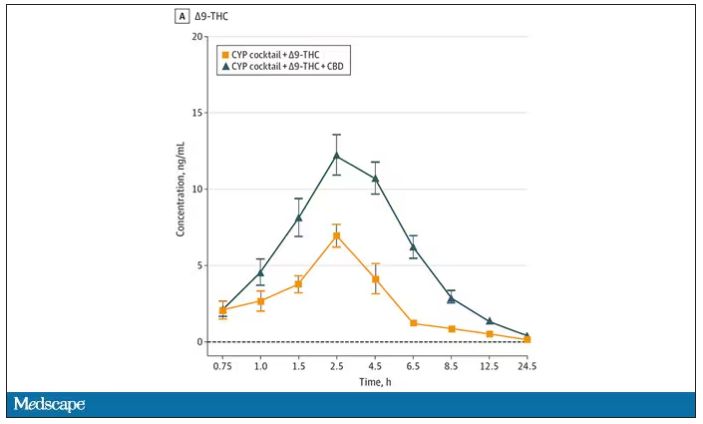
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.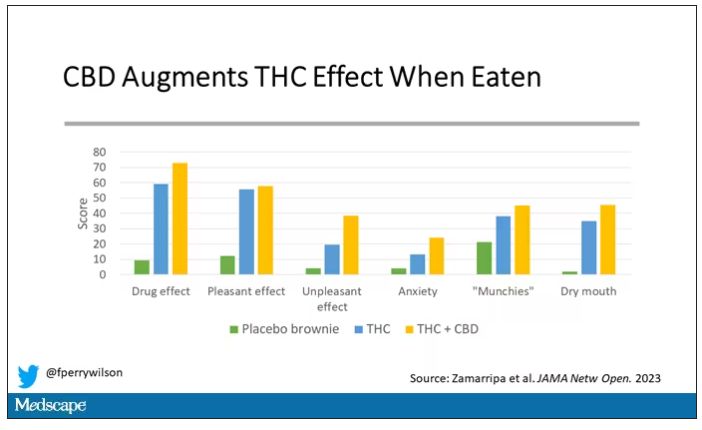
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.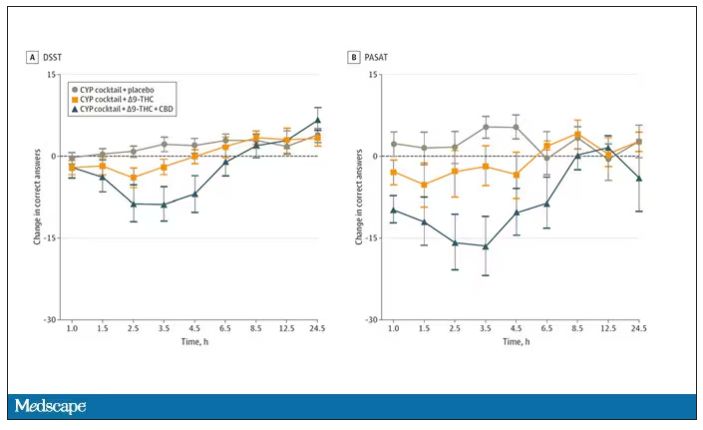
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.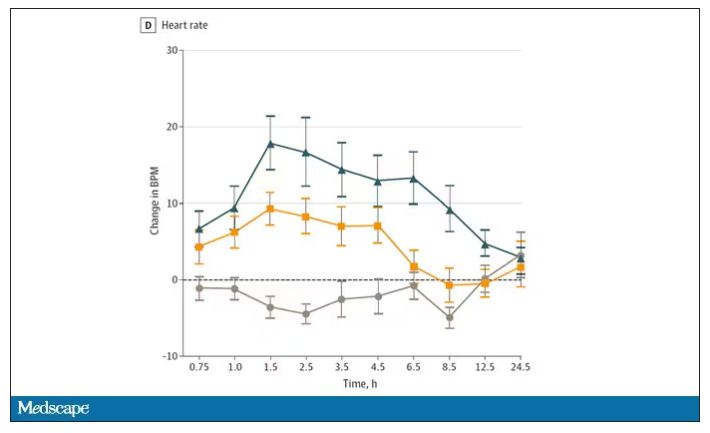
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
Doctors are disappearing from emergency departments as hospitals look to cut costs
She didn’t know much about miscarriage, but this seemed like one.
In the emergency department, she was examined then sent home, she said. She went back when her cramping became excruciating. Then home again. It ultimately took three trips to the ED on 3 consecutive days, generating three separate bills, before she saw a doctor who looked at her blood work and confirmed her fears.
“At the time I wasn’t thinking, ‘Oh, I need to see a doctor,’ ” Ms. Valle recalled. “But when you think about it, it’s like, ‘Well, dang – why didn’t I see a doctor?’ ” It’s unclear whether the repeat visits were due to delays in seeing a physician, but the experience worried her. And she’s still paying the bills.
The hospital declined to discuss Ms. Valle’s care, citing patient privacy. But 17 months before her 3-day ordeal, Tennova had outsourced its emergency departments to American Physician Partners, a medical staffing company owned by private equity investors. APP employs fewer doctors in its EDs as one of its cost-saving initiatives to increase earnings, according to a confidential company document obtained by KHN and NPR.
This staffing strategy has permeated hospitals, and particularly emergency departments, that seek to reduce their top expense: physician labor. While diagnosing and treating patients was once their domain, doctors are increasingly being replaced by nurse practitioners and physician assistants, collectively known as “midlevel practitioners,” who can perform many of the same duties and generate much of the same revenue for less than half of the pay.
“APP has numerous cost saving initiatives underway as part of the Company’s continual focus on cost optimization,” the document says, including a “shift of staffing” between doctors and midlevel practitioners.
In a statement to KHN, American Physician Partners said this strategy is a way to ensure all EDs remain fully staffed, calling it a “blended model” that allows doctors, nurse practitioners, and physician assistants “to provide care to their fullest potential.”
Critics of this strategy say the quest to save money results in treatment meted out by someone with far less training than a physician, leaving patients vulnerable to misdiagnoses, higher medical bills, and inadequate care. And these fears are bolstered by evidence that suggests dropping doctors from EDs may not be good for patients.
A working paper, published in October by the National Bureau of Economic Research, analyzed roughly 1.1 million visits to 44 EDs throughout the Veterans Health Administration, where nurse practitioners can treat patients without oversight from doctors.
Researchers found that treatment by a nurse practitioner resulted on average in a 7% increase in cost of care and an 11% increase in length of stay, extending patients’ time in the ED by minutes for minor visits and hours for longer ones. These gaps widened among patients with more severe diagnoses, the study said, but could be somewhat mitigated by nurse practitioners with more experience.
The study also found that ED patients treated by a nurse practitioner were 20% more likely to be readmitted to the hospital for a preventable reason within 30 days, although the overall risk of readmission remained very small.
Yiqun Chen, PhD, who is an assistant professor of economics at the University of Illinois at Chicago and coauthored the study, said these findings are not an indictment of nurse practitioners in the ED. Instead, she said, she hopes the study will guide how to best deploy nurse practitioners: in treatment of simpler patients or circumstances when no doctor is available.
“It’s not just a simple question of if we can substitute physicians with nurse practitioners or not,” Dr. Chen said. “It depends on how we use them. If we just use them as independent providers, especially ... for relatively complicated patients, it doesn’t seem to be a very good use.”
Dr. Chen’s research echoes smaller studies, like one from The Harvey L. Neiman Health Policy Institute that found nonphysician practitioners in EDs were associated with a 5.3% increase in imaging, which could unnecessarily increase bills for patients. Separately, a study at the Hattiesburg Clinic in Mississippi found that midlevel practitioners in primary care – not in the emergency department – increased the out-of-pocket costs to patients while also leading to worse performance on 9 of 10 quality-of-care metrics, including cancer screenings and vaccination rates.
But definitive evidence remains elusive that replacing ER doctors with nonphysicians has a negative impact on patients, said Cameron Gettel, MD, an assistant professor of emergency medicine at Yale University, New Haven, Conn. Private equity investment and the use of midlevel practitioners rose in lockstep in the ED, Dr. Gettel said, and in the absence of game-changing research, the pattern will likely continue.
“Worse patient outcomes haven’t really been shown across the board,” he said. “And I think until that is shown, then they will continue to play an increasing role.”
For private equity, dropping ED docs is a “simple equation”
Private equity companies pool money from wealthy investors to buy their way into various industries, often slashing spending and seeking to flip businesses in 3 to 7 years. While this business model is a proven moneymaker on Wall Street, it raises concerns in health care, where critics worry the pressure to turn big profits will influence life-or-death decisions that were once left solely to medical professionals.
Nearly $1 trillion in private equity funds have gone into almost 8,000 health care transactions over the past decade, according to industry tracker PitchBook, including buying into medical staffing companies that many hospitals hire to manage their emergency departments.
Two firms dominate the ED staffing industry: TeamHealth, bought by private equity firm Blackstone in 2016, and Envision Healthcare, bought by KKR in 2018. Trying to undercut these staffing giants is American Physician Partners, a rapidly expanding company that runs EDs in at least 17 states and is 50% owned by private equity firm BBH Capital Partners.
These staffing companies have been among the most aggressive in replacing doctors to cut costs, said Robert McNamara, MD, a founder of the American Academy of Emergency Medicine and chair of emergency medicine at Temple University, Philadelphia.
“It’s a relatively simple equation,” Dr. McNamara said. “Their No. 1 expense is the board-certified emergency physician. So they are going to want to keep that expense as low as possible.”
Not everyone sees the trend of private equity in ED staffing in a negative light. Jennifer Orozco, president of the American Academy of Physician Associates, which represents physician assistants, said even if the change – to use more nonphysician providers – is driven by the staffing firms’ desire to make more money, patients are still well served by a team approach that includes nurse practitioners and physician assistants.
“Though I see that shift, it’s not about profits at the end of the day,” Ms. Orozco said. “It’s about the patient.”
The “shift” is nearly invisible to patients because hospitals rarely promote branding from their ED staffing firms and there is little public documentation of private equity investments.
Arthur Smolensky, MD, a Tennessee emergency medicine specialist attempting to measure private equity’s intrusion into EDs, said his review of hospital job postings and employment contracts in 14 major metropolitan areas found that 43% of ED patients were seen in EDs staffed by companies with nonphysician owners, nearly all of whom are private equity investors.
Dr. Smolensky hopes to publish his full study, expanding to 55 metro areas, later this year. But this research will merely quantify what many doctors already know: The ED has changed. Demoralized by an increased focus on profit, and wary of a looming surplus of emergency medicine residents because there are fewer jobs to fill, many experienced doctors are leaving the ED on their own, he said.
“Most of us didn’t go into medicine to supervise an army of people that are not as well trained as we are,” Dr. Smolensky said. “We want to take care of patients.”
“I guess we’re the first guinea pigs for our ER”
Joshua Allen, a nurse practitioner at a small Kentucky hospital, snaked a rubber hose through a rack of pork ribs to practice inserting a chest tube to fix a collapsed lung.
It was 2020, and American Physician Partners was restructuring the ED where Mr. Allen worked, reducing shifts from two doctors to one. Once Mr. Allen had placed 10 tubes under a doctor’s supervision, he would be allowed to do it on his own.
“I guess we’re the first guinea pigs for our ER,” he said. “If we do have a major trauma and multiple victims come in, there’s only one doctor there. ... We need to be prepared.”
Mr. Allen is one of many midlevel practitioners finding work in emergency departments. Nurse practitioners and physician assistants are among the fastest-growing occupations in the nation, according to the U.S. Bureau of Labor Statistics.
Generally, they have master’s degrees and receive several years of specialized schooling but have significantly less training than doctors. Many are permitted to diagnose patients and prescribe medication with little or no supervision from a doctor, although limitations vary by state.
The Neiman Institute found that the share of ED visits in which a midlevel practitioner was the main clinician increased by more than 172% between 2005 and 2020. Another study, in the Journal of Emergency Medicine, reported that if trends continue there may be equal numbers of midlevel practitioners and doctors in EDs by 2030.
There is little mystery as to why. Federal data shows emergency medicine doctors are paid about $310,000 a year on average, while nurse practitioners and physician assistants earn less than $120,000. Generally, hospitals can bill for care by a midlevel practitioner at 85% the rate of a doctor while paying them less than half as much.
Private equity can make millions in the gap.
For example, Envision once encouraged EDs to employ “the least expensive resource” and treat up to 35% of patients with midlevel practitioners, according to a 2017 PowerPoint presentation. The presentation drew scorn on social media and disappeared from Envision’s website.
Envision declined a request for a phone interview. In a written statement to KHN, spokesperson Aliese Polk said the company does not direct its physician leaders on how to care for patients and called the presentation a “concept guide” that does not represent current views.
American Physician Partners touted roughly the same staffing strategy in 2021 in response to the No Surprises Act, which threatened the company’s profits by outlawing surprise medical bills. In its confidential pitch to lenders, the company estimated it could cut almost $6 million by shifting more staffing from physicians to midlevel practitioners.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
She didn’t know much about miscarriage, but this seemed like one.
In the emergency department, she was examined then sent home, she said. She went back when her cramping became excruciating. Then home again. It ultimately took three trips to the ED on 3 consecutive days, generating three separate bills, before she saw a doctor who looked at her blood work and confirmed her fears.
“At the time I wasn’t thinking, ‘Oh, I need to see a doctor,’ ” Ms. Valle recalled. “But when you think about it, it’s like, ‘Well, dang – why didn’t I see a doctor?’ ” It’s unclear whether the repeat visits were due to delays in seeing a physician, but the experience worried her. And she’s still paying the bills.
The hospital declined to discuss Ms. Valle’s care, citing patient privacy. But 17 months before her 3-day ordeal, Tennova had outsourced its emergency departments to American Physician Partners, a medical staffing company owned by private equity investors. APP employs fewer doctors in its EDs as one of its cost-saving initiatives to increase earnings, according to a confidential company document obtained by KHN and NPR.
This staffing strategy has permeated hospitals, and particularly emergency departments, that seek to reduce their top expense: physician labor. While diagnosing and treating patients was once their domain, doctors are increasingly being replaced by nurse practitioners and physician assistants, collectively known as “midlevel practitioners,” who can perform many of the same duties and generate much of the same revenue for less than half of the pay.
“APP has numerous cost saving initiatives underway as part of the Company’s continual focus on cost optimization,” the document says, including a “shift of staffing” between doctors and midlevel practitioners.
In a statement to KHN, American Physician Partners said this strategy is a way to ensure all EDs remain fully staffed, calling it a “blended model” that allows doctors, nurse practitioners, and physician assistants “to provide care to their fullest potential.”
Critics of this strategy say the quest to save money results in treatment meted out by someone with far less training than a physician, leaving patients vulnerable to misdiagnoses, higher medical bills, and inadequate care. And these fears are bolstered by evidence that suggests dropping doctors from EDs may not be good for patients.
A working paper, published in October by the National Bureau of Economic Research, analyzed roughly 1.1 million visits to 44 EDs throughout the Veterans Health Administration, where nurse practitioners can treat patients without oversight from doctors.
Researchers found that treatment by a nurse practitioner resulted on average in a 7% increase in cost of care and an 11% increase in length of stay, extending patients’ time in the ED by minutes for minor visits and hours for longer ones. These gaps widened among patients with more severe diagnoses, the study said, but could be somewhat mitigated by nurse practitioners with more experience.
The study also found that ED patients treated by a nurse practitioner were 20% more likely to be readmitted to the hospital for a preventable reason within 30 days, although the overall risk of readmission remained very small.
Yiqun Chen, PhD, who is an assistant professor of economics at the University of Illinois at Chicago and coauthored the study, said these findings are not an indictment of nurse practitioners in the ED. Instead, she said, she hopes the study will guide how to best deploy nurse practitioners: in treatment of simpler patients or circumstances when no doctor is available.
“It’s not just a simple question of if we can substitute physicians with nurse practitioners or not,” Dr. Chen said. “It depends on how we use them. If we just use them as independent providers, especially ... for relatively complicated patients, it doesn’t seem to be a very good use.”
Dr. Chen’s research echoes smaller studies, like one from The Harvey L. Neiman Health Policy Institute that found nonphysician practitioners in EDs were associated with a 5.3% increase in imaging, which could unnecessarily increase bills for patients. Separately, a study at the Hattiesburg Clinic in Mississippi found that midlevel practitioners in primary care – not in the emergency department – increased the out-of-pocket costs to patients while also leading to worse performance on 9 of 10 quality-of-care metrics, including cancer screenings and vaccination rates.
But definitive evidence remains elusive that replacing ER doctors with nonphysicians has a negative impact on patients, said Cameron Gettel, MD, an assistant professor of emergency medicine at Yale University, New Haven, Conn. Private equity investment and the use of midlevel practitioners rose in lockstep in the ED, Dr. Gettel said, and in the absence of game-changing research, the pattern will likely continue.
“Worse patient outcomes haven’t really been shown across the board,” he said. “And I think until that is shown, then they will continue to play an increasing role.”
For private equity, dropping ED docs is a “simple equation”
Private equity companies pool money from wealthy investors to buy their way into various industries, often slashing spending and seeking to flip businesses in 3 to 7 years. While this business model is a proven moneymaker on Wall Street, it raises concerns in health care, where critics worry the pressure to turn big profits will influence life-or-death decisions that were once left solely to medical professionals.
Nearly $1 trillion in private equity funds have gone into almost 8,000 health care transactions over the past decade, according to industry tracker PitchBook, including buying into medical staffing companies that many hospitals hire to manage their emergency departments.
Two firms dominate the ED staffing industry: TeamHealth, bought by private equity firm Blackstone in 2016, and Envision Healthcare, bought by KKR in 2018. Trying to undercut these staffing giants is American Physician Partners, a rapidly expanding company that runs EDs in at least 17 states and is 50% owned by private equity firm BBH Capital Partners.
These staffing companies have been among the most aggressive in replacing doctors to cut costs, said Robert McNamara, MD, a founder of the American Academy of Emergency Medicine and chair of emergency medicine at Temple University, Philadelphia.
“It’s a relatively simple equation,” Dr. McNamara said. “Their No. 1 expense is the board-certified emergency physician. So they are going to want to keep that expense as low as possible.”
Not everyone sees the trend of private equity in ED staffing in a negative light. Jennifer Orozco, president of the American Academy of Physician Associates, which represents physician assistants, said even if the change – to use more nonphysician providers – is driven by the staffing firms’ desire to make more money, patients are still well served by a team approach that includes nurse practitioners and physician assistants.
“Though I see that shift, it’s not about profits at the end of the day,” Ms. Orozco said. “It’s about the patient.”
The “shift” is nearly invisible to patients because hospitals rarely promote branding from their ED staffing firms and there is little public documentation of private equity investments.
Arthur Smolensky, MD, a Tennessee emergency medicine specialist attempting to measure private equity’s intrusion into EDs, said his review of hospital job postings and employment contracts in 14 major metropolitan areas found that 43% of ED patients were seen in EDs staffed by companies with nonphysician owners, nearly all of whom are private equity investors.
Dr. Smolensky hopes to publish his full study, expanding to 55 metro areas, later this year. But this research will merely quantify what many doctors already know: The ED has changed. Demoralized by an increased focus on profit, and wary of a looming surplus of emergency medicine residents because there are fewer jobs to fill, many experienced doctors are leaving the ED on their own, he said.
“Most of us didn’t go into medicine to supervise an army of people that are not as well trained as we are,” Dr. Smolensky said. “We want to take care of patients.”
“I guess we’re the first guinea pigs for our ER”
Joshua Allen, a nurse practitioner at a small Kentucky hospital, snaked a rubber hose through a rack of pork ribs to practice inserting a chest tube to fix a collapsed lung.
It was 2020, and American Physician Partners was restructuring the ED where Mr. Allen worked, reducing shifts from two doctors to one. Once Mr. Allen had placed 10 tubes under a doctor’s supervision, he would be allowed to do it on his own.
“I guess we’re the first guinea pigs for our ER,” he said. “If we do have a major trauma and multiple victims come in, there’s only one doctor there. ... We need to be prepared.”
Mr. Allen is one of many midlevel practitioners finding work in emergency departments. Nurse practitioners and physician assistants are among the fastest-growing occupations in the nation, according to the U.S. Bureau of Labor Statistics.
Generally, they have master’s degrees and receive several years of specialized schooling but have significantly less training than doctors. Many are permitted to diagnose patients and prescribe medication with little or no supervision from a doctor, although limitations vary by state.
The Neiman Institute found that the share of ED visits in which a midlevel practitioner was the main clinician increased by more than 172% between 2005 and 2020. Another study, in the Journal of Emergency Medicine, reported that if trends continue there may be equal numbers of midlevel practitioners and doctors in EDs by 2030.
There is little mystery as to why. Federal data shows emergency medicine doctors are paid about $310,000 a year on average, while nurse practitioners and physician assistants earn less than $120,000. Generally, hospitals can bill for care by a midlevel practitioner at 85% the rate of a doctor while paying them less than half as much.
Private equity can make millions in the gap.
For example, Envision once encouraged EDs to employ “the least expensive resource” and treat up to 35% of patients with midlevel practitioners, according to a 2017 PowerPoint presentation. The presentation drew scorn on social media and disappeared from Envision’s website.
Envision declined a request for a phone interview. In a written statement to KHN, spokesperson Aliese Polk said the company does not direct its physician leaders on how to care for patients and called the presentation a “concept guide” that does not represent current views.
American Physician Partners touted roughly the same staffing strategy in 2021 in response to the No Surprises Act, which threatened the company’s profits by outlawing surprise medical bills. In its confidential pitch to lenders, the company estimated it could cut almost $6 million by shifting more staffing from physicians to midlevel practitioners.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
She didn’t know much about miscarriage, but this seemed like one.
In the emergency department, she was examined then sent home, she said. She went back when her cramping became excruciating. Then home again. It ultimately took three trips to the ED on 3 consecutive days, generating three separate bills, before she saw a doctor who looked at her blood work and confirmed her fears.
“At the time I wasn’t thinking, ‘Oh, I need to see a doctor,’ ” Ms. Valle recalled. “But when you think about it, it’s like, ‘Well, dang – why didn’t I see a doctor?’ ” It’s unclear whether the repeat visits were due to delays in seeing a physician, but the experience worried her. And she’s still paying the bills.
The hospital declined to discuss Ms. Valle’s care, citing patient privacy. But 17 months before her 3-day ordeal, Tennova had outsourced its emergency departments to American Physician Partners, a medical staffing company owned by private equity investors. APP employs fewer doctors in its EDs as one of its cost-saving initiatives to increase earnings, according to a confidential company document obtained by KHN and NPR.
This staffing strategy has permeated hospitals, and particularly emergency departments, that seek to reduce their top expense: physician labor. While diagnosing and treating patients was once their domain, doctors are increasingly being replaced by nurse practitioners and physician assistants, collectively known as “midlevel practitioners,” who can perform many of the same duties and generate much of the same revenue for less than half of the pay.
“APP has numerous cost saving initiatives underway as part of the Company’s continual focus on cost optimization,” the document says, including a “shift of staffing” between doctors and midlevel practitioners.
In a statement to KHN, American Physician Partners said this strategy is a way to ensure all EDs remain fully staffed, calling it a “blended model” that allows doctors, nurse practitioners, and physician assistants “to provide care to their fullest potential.”
Critics of this strategy say the quest to save money results in treatment meted out by someone with far less training than a physician, leaving patients vulnerable to misdiagnoses, higher medical bills, and inadequate care. And these fears are bolstered by evidence that suggests dropping doctors from EDs may not be good for patients.
A working paper, published in October by the National Bureau of Economic Research, analyzed roughly 1.1 million visits to 44 EDs throughout the Veterans Health Administration, where nurse practitioners can treat patients without oversight from doctors.
Researchers found that treatment by a nurse practitioner resulted on average in a 7% increase in cost of care and an 11% increase in length of stay, extending patients’ time in the ED by minutes for minor visits and hours for longer ones. These gaps widened among patients with more severe diagnoses, the study said, but could be somewhat mitigated by nurse practitioners with more experience.
The study also found that ED patients treated by a nurse practitioner were 20% more likely to be readmitted to the hospital for a preventable reason within 30 days, although the overall risk of readmission remained very small.
Yiqun Chen, PhD, who is an assistant professor of economics at the University of Illinois at Chicago and coauthored the study, said these findings are not an indictment of nurse practitioners in the ED. Instead, she said, she hopes the study will guide how to best deploy nurse practitioners: in treatment of simpler patients or circumstances when no doctor is available.
“It’s not just a simple question of if we can substitute physicians with nurse practitioners or not,” Dr. Chen said. “It depends on how we use them. If we just use them as independent providers, especially ... for relatively complicated patients, it doesn’t seem to be a very good use.”
Dr. Chen’s research echoes smaller studies, like one from The Harvey L. Neiman Health Policy Institute that found nonphysician practitioners in EDs were associated with a 5.3% increase in imaging, which could unnecessarily increase bills for patients. Separately, a study at the Hattiesburg Clinic in Mississippi found that midlevel practitioners in primary care – not in the emergency department – increased the out-of-pocket costs to patients while also leading to worse performance on 9 of 10 quality-of-care metrics, including cancer screenings and vaccination rates.
But definitive evidence remains elusive that replacing ER doctors with nonphysicians has a negative impact on patients, said Cameron Gettel, MD, an assistant professor of emergency medicine at Yale University, New Haven, Conn. Private equity investment and the use of midlevel practitioners rose in lockstep in the ED, Dr. Gettel said, and in the absence of game-changing research, the pattern will likely continue.
“Worse patient outcomes haven’t really been shown across the board,” he said. “And I think until that is shown, then they will continue to play an increasing role.”
For private equity, dropping ED docs is a “simple equation”
Private equity companies pool money from wealthy investors to buy their way into various industries, often slashing spending and seeking to flip businesses in 3 to 7 years. While this business model is a proven moneymaker on Wall Street, it raises concerns in health care, where critics worry the pressure to turn big profits will influence life-or-death decisions that were once left solely to medical professionals.
Nearly $1 trillion in private equity funds have gone into almost 8,000 health care transactions over the past decade, according to industry tracker PitchBook, including buying into medical staffing companies that many hospitals hire to manage their emergency departments.
Two firms dominate the ED staffing industry: TeamHealth, bought by private equity firm Blackstone in 2016, and Envision Healthcare, bought by KKR in 2018. Trying to undercut these staffing giants is American Physician Partners, a rapidly expanding company that runs EDs in at least 17 states and is 50% owned by private equity firm BBH Capital Partners.
These staffing companies have been among the most aggressive in replacing doctors to cut costs, said Robert McNamara, MD, a founder of the American Academy of Emergency Medicine and chair of emergency medicine at Temple University, Philadelphia.
“It’s a relatively simple equation,” Dr. McNamara said. “Their No. 1 expense is the board-certified emergency physician. So they are going to want to keep that expense as low as possible.”
Not everyone sees the trend of private equity in ED staffing in a negative light. Jennifer Orozco, president of the American Academy of Physician Associates, which represents physician assistants, said even if the change – to use more nonphysician providers – is driven by the staffing firms’ desire to make more money, patients are still well served by a team approach that includes nurse practitioners and physician assistants.
“Though I see that shift, it’s not about profits at the end of the day,” Ms. Orozco said. “It’s about the patient.”
The “shift” is nearly invisible to patients because hospitals rarely promote branding from their ED staffing firms and there is little public documentation of private equity investments.
Arthur Smolensky, MD, a Tennessee emergency medicine specialist attempting to measure private equity’s intrusion into EDs, said his review of hospital job postings and employment contracts in 14 major metropolitan areas found that 43% of ED patients were seen in EDs staffed by companies with nonphysician owners, nearly all of whom are private equity investors.
Dr. Smolensky hopes to publish his full study, expanding to 55 metro areas, later this year. But this research will merely quantify what many doctors already know: The ED has changed. Demoralized by an increased focus on profit, and wary of a looming surplus of emergency medicine residents because there are fewer jobs to fill, many experienced doctors are leaving the ED on their own, he said.
“Most of us didn’t go into medicine to supervise an army of people that are not as well trained as we are,” Dr. Smolensky said. “We want to take care of patients.”
“I guess we’re the first guinea pigs for our ER”
Joshua Allen, a nurse practitioner at a small Kentucky hospital, snaked a rubber hose through a rack of pork ribs to practice inserting a chest tube to fix a collapsed lung.
It was 2020, and American Physician Partners was restructuring the ED where Mr. Allen worked, reducing shifts from two doctors to one. Once Mr. Allen had placed 10 tubes under a doctor’s supervision, he would be allowed to do it on his own.
“I guess we’re the first guinea pigs for our ER,” he said. “If we do have a major trauma and multiple victims come in, there’s only one doctor there. ... We need to be prepared.”
Mr. Allen is one of many midlevel practitioners finding work in emergency departments. Nurse practitioners and physician assistants are among the fastest-growing occupations in the nation, according to the U.S. Bureau of Labor Statistics.
Generally, they have master’s degrees and receive several years of specialized schooling but have significantly less training than doctors. Many are permitted to diagnose patients and prescribe medication with little or no supervision from a doctor, although limitations vary by state.
The Neiman Institute found that the share of ED visits in which a midlevel practitioner was the main clinician increased by more than 172% between 2005 and 2020. Another study, in the Journal of Emergency Medicine, reported that if trends continue there may be equal numbers of midlevel practitioners and doctors in EDs by 2030.
There is little mystery as to why. Federal data shows emergency medicine doctors are paid about $310,000 a year on average, while nurse practitioners and physician assistants earn less than $120,000. Generally, hospitals can bill for care by a midlevel practitioner at 85% the rate of a doctor while paying them less than half as much.
Private equity can make millions in the gap.
For example, Envision once encouraged EDs to employ “the least expensive resource” and treat up to 35% of patients with midlevel practitioners, according to a 2017 PowerPoint presentation. The presentation drew scorn on social media and disappeared from Envision’s website.
Envision declined a request for a phone interview. In a written statement to KHN, spokesperson Aliese Polk said the company does not direct its physician leaders on how to care for patients and called the presentation a “concept guide” that does not represent current views.
American Physician Partners touted roughly the same staffing strategy in 2021 in response to the No Surprises Act, which threatened the company’s profits by outlawing surprise medical bills. In its confidential pitch to lenders, the company estimated it could cut almost $6 million by shifting more staffing from physicians to midlevel practitioners.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Thrombectomy benefits stroke with large core volumes: SELECT2 trial results
in a major international trial, which is expected to lead to a change in clinical practice and the way in which systems of stroke care are organized.
The results of the SELECT2 trial, which was conducted in sites in the United States, Canada, Europe, Australia, and New Zealand, showed that endovascular thrombectomy plus medical care resulted in better clinical outcomes than medical care alone in patients with a large ischemic core who presented within 24 hours after the time they were last known to be well.
The results of the SELECT2 trial were presented at the International Stroke Conference by Amrou Sarraj, MD. Dr. Sarraj is professor of neurology at University Hospitals Cleveland Medical Center–Case Western Reserve University in Ohio. The study was also simultaneously published online in the New England Journal of Medicine.
A similar trial conducted in China, the ANGEL-ASPECT trial, was also presented at the same ISC session and showed very similar results.
These two trials add to another Japanese study reported last year, the RESCUE-JAPAN LIMIT trial, also showing benefit of thrombectomy in patients with large core strokes.
Dr. Sarraj concluded that the results of these three trials together “unequivocally demonstrate the benefit of endovascular thrombectomy in patients with large ischemic core.”
A clear benefit
Approximately 20% of large-vessel occlusion strokes have a large core, but these patients have not been considered candidates for endovascular thrombectomy because of concerns about potential reperfusion injury in necrotic brain tissue, resulting in an increased risk of hemorrhage, edema, disability, and death.
This has resulted in uncertainty about how to manage these patients with a core infarct, Dr. Sarraj noted at the conference presented by the American Stroke Association, a division of the American Heart Association.
The SELECT2 trial involved patients with stroke as a result of occlusion of the internal carotid artery or the first segment of the middle cerebral artery. Patients had a large ischemic core volume, defined as an ASPECTS (Alberta Stroke Program Early Computed Tomography Score) of 3-5, or a core volume of at least 50 mL on imaging. They were randomly assigned to endovascular thrombectomy plus medical care or to medical care alone.
The trial was aiming to enroll 560 patients but was stopped early for efficacy after 178 patients had been assigned to the thrombectomy group and 174 to the medical-care group.
The primary outcome – the generalized odds ratio for a shift in the distribution of modified Rankin scale scores toward better outcomes in favor of thrombectomy was 1.51 (P < .001).
“This translates into a 60% probability of achieving a better functional outcome in patients receiving thrombectomy, with a number needed to treat of five. That means five patients need to be treated with thrombectomy for one to achieve a better functional outcome,” Dr. Sarraj stated.
The secondary outcome of functional independence at 90 days (a score on the modified Rankin scale of 0-2) occurred in 20% of the patients in the thrombectomy group and 7% in the medical-care group (relative risk, 2.97), with a number needed to treat of seven.
Independent ambulation (a score on the modified Rankin Scale of 0-3) at 90 days occurred in 37.9% of the patients in the thrombectomy group and in 18.7% of the patients in the medical-care group (relative risk, 2.06), with a number needed to treat of five.
Mortality was similar in the two groups.
The results for other secondary outcomes were generally in the same direction as those of the primary analysis, with the possible exception of early neurologic improvement, the authors reported.
The incidence of symptomatic intracranial hemorrhage was low in both trial groups, occurring in one patient in the thrombectomy group and two in the medical care group.
The investigators pointed out that previous studies have reported rates of symptomatic intracranial hemorrhage in patients with large ischemic core lesions that are higher than those in this trial. “Therefore, the low percentage of patients with symptomatic intracranial hemorrhage observed in both trial groups was unexpected.”
Approximately 20% of the patients in the thrombectomy group had complications associated with the procedure. In the thrombectomy group, arterial access-site complications occurred in 5 patients, dissection in 10, cerebral vessel perforation in 7, and transient vasospasm in 11.
Early neurologic worsening, defined as an increase of 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), occurred in 24.7% in the thrombectomy group and in 15.5% in the medical-care group (relative risk, 1.59).
In a post-hoc analysis, “from which no conclusions can be drawn,” the authors reported, early neurologic worsening was associated with worse functional outcomes at 90 days, and patients who had neurologic worsening had larger ischemic core lesions at baseline (median volume, 107 mL) versus 77 mL among patients without neurologic worsening.
They noted that a potential cause of deterioration in some of these patients was brain edema associated with reperfusion. However, they emphasize that overall, endovascular thrombectomy was associated with better outcomes than medical care alone.
“Two-thirds of patients had core infarct sizes more than 70 mL, and one-third of patients had core infarct sizes of more than 100 mL, but even in patients with large and very large core volumes, thrombectomy was superior to medical care alone,” Dr. Sarraj said.
This will ‘change practice’
In a comment, ISC 2023 chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This trial shows that even patients with a large core infarct who we would not have treated with thrombectomy in the past, actually do benefit from this procedure. And the surprise is that the benefit is nearly to the same extent as that in patients with smaller core infarcts. That is going to change practice.”
Dr. Jovin said that these results should not only change the selection of patients for thrombectomy, but they should also change systems of care. “Because the systems of care now are based around excluding these patients with large infarcts. We won’t need to do that in future.”
He elaborated: “I think imaging has held us back to be honest. We can exclude hemorrhage with a plain CT scan. Then after this, the biggest piece of information we need from imaging is the size of the infarct. We were concerned that we might hurt the patient if the infarct was large. Outside hospitals had to do advanced imaging before deciding whether to transfer patients for thrombectomy. These are all sources of delays.
“I am very pleased to see these results, and I hope to see a much more simplified triage of patients that will be more liberal to patients with the large infarcts,” he added.
Also commenting, Joseph Broderick, MD, professor of neurology and director of the Neuroscience Institute at the University of Cincinnati, said the results were “robust and important.”
He said the results of the SELECT2 trial, along with the other two similar trials, “will change practice and extend endovascular therapy to more patients with severe strokes.”
But Dr. Broderick believes imaging will still be necessary to exclude patients with ASPECTS scores of 0-2, who were not included in these trials. “These are patients who have very large areas of clear hypodensity on the baseline image (brain already dying or dead). These patients do not benefit from reperfusion with lytic drugs or endovascular therapy,” he noted.
‘Welcome news’
In an editorial accompanying the print publication of the two new studies, Pierre Fayad, MD, University of Nebraska Medical Center, Omaha, points out that all three trials of thrombectomy in patients with large core infarct strokes “showed remarkably similar results” despite differences in design, patient selection, thrombolytic treatment and dose, geographic location, and imaging criteria.
“Together, the trials provide reassuring information from more than a thousand patients with large ischemic strokes in different medical systems that will probably lead to changes in patterns of care delivery.”
Dr. Fayad said it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes if they arrive in a timely fashion at a center that is capable of performing the procedure, and if the patients have an ASPECTS value of 3-5 or an ischemic core volume of 50 mL or greater.
Higher rates of good outcomes may be anticipated if this treatment is performed, despite increased risks of symptomatic hemorrhage, edema, neurologic worsening, and hemicraniectomy, he noted.
“Patients and families should be made aware of the limitations of treatment and the anticipated residual neurologic deficits resulting from the large infarction. The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment,” he concluded.
The SELECT2 trial was supported by an investigator-initiated grant from Stryker Neurovascular to University Hospitals Cleveland Medical Center and the University of Texas McGovern Medical School.
A version of this article first appeared on Medscape.com.
in a major international trial, which is expected to lead to a change in clinical practice and the way in which systems of stroke care are organized.
The results of the SELECT2 trial, which was conducted in sites in the United States, Canada, Europe, Australia, and New Zealand, showed that endovascular thrombectomy plus medical care resulted in better clinical outcomes than medical care alone in patients with a large ischemic core who presented within 24 hours after the time they were last known to be well.
The results of the SELECT2 trial were presented at the International Stroke Conference by Amrou Sarraj, MD. Dr. Sarraj is professor of neurology at University Hospitals Cleveland Medical Center–Case Western Reserve University in Ohio. The study was also simultaneously published online in the New England Journal of Medicine.
A similar trial conducted in China, the ANGEL-ASPECT trial, was also presented at the same ISC session and showed very similar results.
These two trials add to another Japanese study reported last year, the RESCUE-JAPAN LIMIT trial, also showing benefit of thrombectomy in patients with large core strokes.
Dr. Sarraj concluded that the results of these three trials together “unequivocally demonstrate the benefit of endovascular thrombectomy in patients with large ischemic core.”
A clear benefit
Approximately 20% of large-vessel occlusion strokes have a large core, but these patients have not been considered candidates for endovascular thrombectomy because of concerns about potential reperfusion injury in necrotic brain tissue, resulting in an increased risk of hemorrhage, edema, disability, and death.
This has resulted in uncertainty about how to manage these patients with a core infarct, Dr. Sarraj noted at the conference presented by the American Stroke Association, a division of the American Heart Association.
The SELECT2 trial involved patients with stroke as a result of occlusion of the internal carotid artery or the first segment of the middle cerebral artery. Patients had a large ischemic core volume, defined as an ASPECTS (Alberta Stroke Program Early Computed Tomography Score) of 3-5, or a core volume of at least 50 mL on imaging. They were randomly assigned to endovascular thrombectomy plus medical care or to medical care alone.
The trial was aiming to enroll 560 patients but was stopped early for efficacy after 178 patients had been assigned to the thrombectomy group and 174 to the medical-care group.
The primary outcome – the generalized odds ratio for a shift in the distribution of modified Rankin scale scores toward better outcomes in favor of thrombectomy was 1.51 (P < .001).
“This translates into a 60% probability of achieving a better functional outcome in patients receiving thrombectomy, with a number needed to treat of five. That means five patients need to be treated with thrombectomy for one to achieve a better functional outcome,” Dr. Sarraj stated.
The secondary outcome of functional independence at 90 days (a score on the modified Rankin scale of 0-2) occurred in 20% of the patients in the thrombectomy group and 7% in the medical-care group (relative risk, 2.97), with a number needed to treat of seven.
Independent ambulation (a score on the modified Rankin Scale of 0-3) at 90 days occurred in 37.9% of the patients in the thrombectomy group and in 18.7% of the patients in the medical-care group (relative risk, 2.06), with a number needed to treat of five.
Mortality was similar in the two groups.
The results for other secondary outcomes were generally in the same direction as those of the primary analysis, with the possible exception of early neurologic improvement, the authors reported.
The incidence of symptomatic intracranial hemorrhage was low in both trial groups, occurring in one patient in the thrombectomy group and two in the medical care group.
The investigators pointed out that previous studies have reported rates of symptomatic intracranial hemorrhage in patients with large ischemic core lesions that are higher than those in this trial. “Therefore, the low percentage of patients with symptomatic intracranial hemorrhage observed in both trial groups was unexpected.”
Approximately 20% of the patients in the thrombectomy group had complications associated with the procedure. In the thrombectomy group, arterial access-site complications occurred in 5 patients, dissection in 10, cerebral vessel perforation in 7, and transient vasospasm in 11.
Early neurologic worsening, defined as an increase of 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), occurred in 24.7% in the thrombectomy group and in 15.5% in the medical-care group (relative risk, 1.59).
In a post-hoc analysis, “from which no conclusions can be drawn,” the authors reported, early neurologic worsening was associated with worse functional outcomes at 90 days, and patients who had neurologic worsening had larger ischemic core lesions at baseline (median volume, 107 mL) versus 77 mL among patients without neurologic worsening.
They noted that a potential cause of deterioration in some of these patients was brain edema associated with reperfusion. However, they emphasize that overall, endovascular thrombectomy was associated with better outcomes than medical care alone.
“Two-thirds of patients had core infarct sizes more than 70 mL, and one-third of patients had core infarct sizes of more than 100 mL, but even in patients with large and very large core volumes, thrombectomy was superior to medical care alone,” Dr. Sarraj said.
This will ‘change practice’
In a comment, ISC 2023 chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This trial shows that even patients with a large core infarct who we would not have treated with thrombectomy in the past, actually do benefit from this procedure. And the surprise is that the benefit is nearly to the same extent as that in patients with smaller core infarcts. That is going to change practice.”
Dr. Jovin said that these results should not only change the selection of patients for thrombectomy, but they should also change systems of care. “Because the systems of care now are based around excluding these patients with large infarcts. We won’t need to do that in future.”
He elaborated: “I think imaging has held us back to be honest. We can exclude hemorrhage with a plain CT scan. Then after this, the biggest piece of information we need from imaging is the size of the infarct. We were concerned that we might hurt the patient if the infarct was large. Outside hospitals had to do advanced imaging before deciding whether to transfer patients for thrombectomy. These are all sources of delays.
“I am very pleased to see these results, and I hope to see a much more simplified triage of patients that will be more liberal to patients with the large infarcts,” he added.
Also commenting, Joseph Broderick, MD, professor of neurology and director of the Neuroscience Institute at the University of Cincinnati, said the results were “robust and important.”
He said the results of the SELECT2 trial, along with the other two similar trials, “will change practice and extend endovascular therapy to more patients with severe strokes.”
But Dr. Broderick believes imaging will still be necessary to exclude patients with ASPECTS scores of 0-2, who were not included in these trials. “These are patients who have very large areas of clear hypodensity on the baseline image (brain already dying or dead). These patients do not benefit from reperfusion with lytic drugs or endovascular therapy,” he noted.
‘Welcome news’
In an editorial accompanying the print publication of the two new studies, Pierre Fayad, MD, University of Nebraska Medical Center, Omaha, points out that all three trials of thrombectomy in patients with large core infarct strokes “showed remarkably similar results” despite differences in design, patient selection, thrombolytic treatment and dose, geographic location, and imaging criteria.
“Together, the trials provide reassuring information from more than a thousand patients with large ischemic strokes in different medical systems that will probably lead to changes in patterns of care delivery.”
Dr. Fayad said it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes if they arrive in a timely fashion at a center that is capable of performing the procedure, and if the patients have an ASPECTS value of 3-5 or an ischemic core volume of 50 mL or greater.
Higher rates of good outcomes may be anticipated if this treatment is performed, despite increased risks of symptomatic hemorrhage, edema, neurologic worsening, and hemicraniectomy, he noted.
“Patients and families should be made aware of the limitations of treatment and the anticipated residual neurologic deficits resulting from the large infarction. The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment,” he concluded.
The SELECT2 trial was supported by an investigator-initiated grant from Stryker Neurovascular to University Hospitals Cleveland Medical Center and the University of Texas McGovern Medical School.
A version of this article first appeared on Medscape.com.
in a major international trial, which is expected to lead to a change in clinical practice and the way in which systems of stroke care are organized.
The results of the SELECT2 trial, which was conducted in sites in the United States, Canada, Europe, Australia, and New Zealand, showed that endovascular thrombectomy plus medical care resulted in better clinical outcomes than medical care alone in patients with a large ischemic core who presented within 24 hours after the time they were last known to be well.
The results of the SELECT2 trial were presented at the International Stroke Conference by Amrou Sarraj, MD. Dr. Sarraj is professor of neurology at University Hospitals Cleveland Medical Center–Case Western Reserve University in Ohio. The study was also simultaneously published online in the New England Journal of Medicine.
A similar trial conducted in China, the ANGEL-ASPECT trial, was also presented at the same ISC session and showed very similar results.
These two trials add to another Japanese study reported last year, the RESCUE-JAPAN LIMIT trial, also showing benefit of thrombectomy in patients with large core strokes.
Dr. Sarraj concluded that the results of these three trials together “unequivocally demonstrate the benefit of endovascular thrombectomy in patients with large ischemic core.”
A clear benefit
Approximately 20% of large-vessel occlusion strokes have a large core, but these patients have not been considered candidates for endovascular thrombectomy because of concerns about potential reperfusion injury in necrotic brain tissue, resulting in an increased risk of hemorrhage, edema, disability, and death.
This has resulted in uncertainty about how to manage these patients with a core infarct, Dr. Sarraj noted at the conference presented by the American Stroke Association, a division of the American Heart Association.
The SELECT2 trial involved patients with stroke as a result of occlusion of the internal carotid artery or the first segment of the middle cerebral artery. Patients had a large ischemic core volume, defined as an ASPECTS (Alberta Stroke Program Early Computed Tomography Score) of 3-5, or a core volume of at least 50 mL on imaging. They were randomly assigned to endovascular thrombectomy plus medical care or to medical care alone.
The trial was aiming to enroll 560 patients but was stopped early for efficacy after 178 patients had been assigned to the thrombectomy group and 174 to the medical-care group.
The primary outcome – the generalized odds ratio for a shift in the distribution of modified Rankin scale scores toward better outcomes in favor of thrombectomy was 1.51 (P < .001).
“This translates into a 60% probability of achieving a better functional outcome in patients receiving thrombectomy, with a number needed to treat of five. That means five patients need to be treated with thrombectomy for one to achieve a better functional outcome,” Dr. Sarraj stated.
The secondary outcome of functional independence at 90 days (a score on the modified Rankin scale of 0-2) occurred in 20% of the patients in the thrombectomy group and 7% in the medical-care group (relative risk, 2.97), with a number needed to treat of seven.
Independent ambulation (a score on the modified Rankin Scale of 0-3) at 90 days occurred in 37.9% of the patients in the thrombectomy group and in 18.7% of the patients in the medical-care group (relative risk, 2.06), with a number needed to treat of five.
Mortality was similar in the two groups.
The results for other secondary outcomes were generally in the same direction as those of the primary analysis, with the possible exception of early neurologic improvement, the authors reported.
The incidence of symptomatic intracranial hemorrhage was low in both trial groups, occurring in one patient in the thrombectomy group and two in the medical care group.
The investigators pointed out that previous studies have reported rates of symptomatic intracranial hemorrhage in patients with large ischemic core lesions that are higher than those in this trial. “Therefore, the low percentage of patients with symptomatic intracranial hemorrhage observed in both trial groups was unexpected.”
Approximately 20% of the patients in the thrombectomy group had complications associated with the procedure. In the thrombectomy group, arterial access-site complications occurred in 5 patients, dissection in 10, cerebral vessel perforation in 7, and transient vasospasm in 11.
Early neurologic worsening, defined as an increase of 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), occurred in 24.7% in the thrombectomy group and in 15.5% in the medical-care group (relative risk, 1.59).
In a post-hoc analysis, “from which no conclusions can be drawn,” the authors reported, early neurologic worsening was associated with worse functional outcomes at 90 days, and patients who had neurologic worsening had larger ischemic core lesions at baseline (median volume, 107 mL) versus 77 mL among patients without neurologic worsening.
They noted that a potential cause of deterioration in some of these patients was brain edema associated with reperfusion. However, they emphasize that overall, endovascular thrombectomy was associated with better outcomes than medical care alone.
“Two-thirds of patients had core infarct sizes more than 70 mL, and one-third of patients had core infarct sizes of more than 100 mL, but even in patients with large and very large core volumes, thrombectomy was superior to medical care alone,” Dr. Sarraj said.
This will ‘change practice’
In a comment, ISC 2023 chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This trial shows that even patients with a large core infarct who we would not have treated with thrombectomy in the past, actually do benefit from this procedure. And the surprise is that the benefit is nearly to the same extent as that in patients with smaller core infarcts. That is going to change practice.”
Dr. Jovin said that these results should not only change the selection of patients for thrombectomy, but they should also change systems of care. “Because the systems of care now are based around excluding these patients with large infarcts. We won’t need to do that in future.”
He elaborated: “I think imaging has held us back to be honest. We can exclude hemorrhage with a plain CT scan. Then after this, the biggest piece of information we need from imaging is the size of the infarct. We were concerned that we might hurt the patient if the infarct was large. Outside hospitals had to do advanced imaging before deciding whether to transfer patients for thrombectomy. These are all sources of delays.
“I am very pleased to see these results, and I hope to see a much more simplified triage of patients that will be more liberal to patients with the large infarcts,” he added.
Also commenting, Joseph Broderick, MD, professor of neurology and director of the Neuroscience Institute at the University of Cincinnati, said the results were “robust and important.”
He said the results of the SELECT2 trial, along with the other two similar trials, “will change practice and extend endovascular therapy to more patients with severe strokes.”
But Dr. Broderick believes imaging will still be necessary to exclude patients with ASPECTS scores of 0-2, who were not included in these trials. “These are patients who have very large areas of clear hypodensity on the baseline image (brain already dying or dead). These patients do not benefit from reperfusion with lytic drugs or endovascular therapy,” he noted.
‘Welcome news’
In an editorial accompanying the print publication of the two new studies, Pierre Fayad, MD, University of Nebraska Medical Center, Omaha, points out that all three trials of thrombectomy in patients with large core infarct strokes “showed remarkably similar results” despite differences in design, patient selection, thrombolytic treatment and dose, geographic location, and imaging criteria.
“Together, the trials provide reassuring information from more than a thousand patients with large ischemic strokes in different medical systems that will probably lead to changes in patterns of care delivery.”
Dr. Fayad said it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes if they arrive in a timely fashion at a center that is capable of performing the procedure, and if the patients have an ASPECTS value of 3-5 or an ischemic core volume of 50 mL or greater.
Higher rates of good outcomes may be anticipated if this treatment is performed, despite increased risks of symptomatic hemorrhage, edema, neurologic worsening, and hemicraniectomy, he noted.
“Patients and families should be made aware of the limitations of treatment and the anticipated residual neurologic deficits resulting from the large infarction. The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment,” he concluded.
The SELECT2 trial was supported by an investigator-initiated grant from Stryker Neurovascular to University Hospitals Cleveland Medical Center and the University of Texas McGovern Medical School.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
UnitedHealthcare tried to deny coverage to a chronically ill patient. He fought back, exposing the insurer’s inner workings.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
Doctors and dating: There’s an app (or three) for that
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Pounding heart, sweating, insomnia. Surges of dopamine, norepinephrine, and adrenaline. All symptoms of a very common yet frustrating condition: Falling in love.
The prognosis is vague. A prescription pad and knowledge of biochemistry aren’t helpful when it comes to relationships.
Medical training can consume decades when others are exploring relationships and starting families. There are few recent data on this, but
But there is hope! By age 36, the number of doctors in long-term relationships had overtaken everyone else by more than 10% for women and 20% for men. The Medscape 2022 Physician Happiness & Lifestyle Report found that 83% were in committed relationships, and even better, happy ones. At least three-quarters of doctors in every specialty described their partnerships as “very good” or “good.”
How should a single medical student, resident, or attending physician find happiness ever after in 2023? Sometimes Mr./Ms. Right can be found in the anatomy lab or hospital, with sparks flying between students or colleagues. But for many in health care, along with millions of others looking for love, the solution is dating apps.
When ‘MD’ is a turnoff
Dr. M, a psychiatry resident in California who prefers not to give her name, hadn’t found a life partner during college, grad school, or medical school. When she passed her final Step 3 board exam, she decided it was time to take the plunge. She signed up for popular dating apps like Hinge, Bumble, and Coffee Meets Bagel, but her dates seemed to follow a disappointing pattern.
“I met lots of guys, but it was incredibly rare to find another physician,” said Dr. M. “I found myself always wanting to talk about my life as a resident. More often than not, the guys would give me this blank stare as I complained about being on call or spoke about spending 12 hours a day studying for a board exam, or even the process of The Match and how I ended up in California.”
Both of Dr. M’s parents are physicians, and she grew up watching how they supported each other through residency, exams, and exhausting schedules. A relationship with another physician, her parents told her, would give both partners the best chance to understand each other’s lives. The problem was how to find one.
That was when Dr. M saw an ad for a dating app with a cute medical name: DownToDate, a play on the clinical evidence resource UpToDate. “I thought it was a meme,” she said. “It was this doctors-only app. I remember thinking, ‘this has to be a joke,’ but then it was very real.”
She signed up and was required to provide a photo of her ID and her NPI number. Immediately, men began “requesting a consult,” the app’s form of “liking” her profile, and sending her “pages” (messages).
DownToDate was created by another physician, Robin Boyer, MD, MBA, a pediatrics resident in Loma Linda, Calif. The inspiration came in 2020 during the initial COVID crisis. Exhausted from long and often heartbreaking shifts, Dr. Boyer was grateful for her husband’s unwavering support. But many of her coresidents weren’t so lucky. The women in particular talked about their dating struggles, and there was a recurring theme. They didn’t feel confident putting “physician” on a dating site profile.
“If you’re male and you tell people you’re a doctor, it seems like it really attracts people,” Dr. Boyer said. “But if you’re female, it brings up a lot of stereotypes where you’re perceived as too intimidating either as the breadwinner, being more educated, or having a [demanding] career. It does make it more difficult.”
Dr. Boyer met her husband in high school, and she had never used a dating app. She convinced a coresident, Celestine Odigwe, MD, to pursue the idea as partners. They began researching the market within their network and heard from over a thousand interested physicians, both men and women, heterosexual and LGBTQ+. They even created fake accounts on other sites to gauge how easy it is to falsify a profile. From these insights, the app took shape. It launched in 2021 and currently has more than 5000 verified users.
Branches from the same tree
Around the same time that DownToDate began, Shivani Shah, DO, a pediatric neurology resident at Duke University, Durham, N.C., and her brother, Sagar Shah, an entrepreneur, had a similar idea.
At the time, Dr. Shah was a fourth-year medical student about to move from New Jersey to North Carolina. Friends who were internal medicine residents described the grueling reality of the early COVID pandemic.
“It was just horrible,” said Dr. Shah. “You were isolated from your family, your support system, everything. ... I think the pandemic really pushed us into realizing that this is a very important need, and sometimes it feels like community is lacking in the health care field.”
The sibling duo developed ForeverX, an app for health care workers to find meaningful and long-term romantic connections. It launched in 2021.
Concerned that the medical field was “siloed,” the Shahs chose to open the app to physicians, dentists, nurses, physical therapists, and other health care professionals. “Opening up the doors to more communication” between the health care branches was a priority.
To prevent catfishing, the app uses a twofold vetting system. Each user submits a photo of their driver’s license and a selfie that must match. There is also health care verification through an NPI number, nurse’s ID, or a manual process for those without either. None of the information is stored.
Through personal experience with dating apps, Dr. Shah hopes ForeverX can improve on some of their flaws, particularly the problem of matches being overly filtered by preferences. The “natural way” of meeting people is not filtered. And while most people have a dating checklist in mind, meeting someone face to face might send some of those prerequisites “out the window.”
“You can’t really put into words how you feel with someone ... the vibe,” Dr. Shah said. That is why her goal is to get people off the app and on an actual date IRL. “Something we’ve discussed internally is, how do we make this experience that’s virtual more human?”
She acknowledged that certain requirements, like a desire for children, might be crucial to some users. Many female doctors in their 30’s feel the “time crunch” of a ticking biological clock.
Optimize your date-ability
“I think people either love or hate dating apps, and I love them,” said Kevin Jubbal, MD. “I get to meet cool people and schedule dates from the comfort of my home.”
Dr. Jubbal, a former plastic surgery resident who left medicine to become an entrepreneur, is the founder of Med School Insiders, a tutoring and advising resource for premeds, medical students, and residents. His YouTube channel has more than 1.5 million subscribers, and he often receives questions about whether dating is feasible in medical school and how to balance a personal and academic/professional life.
Those who hate dating apps or receive few matches would do well to look inward instead of blaming the process, he said. It helps to view the experience as a learning tool that provides feedback very quickly.
“If you want to find a really amazing person, then you need to be what you want to find,” said Dr. Jubbal. “If you want to find someone who’s fit and intelligent and well read and well traveled, you need to be that. Otherwise, you’re probably not going to attract that person.”
An app designed to help single female MDs
Ifie Williams, MD, a psychiatrist in Washington, D.C., believes a wider dating pool is key – provided everyone understands the situation up front. When Dr. Williams started residency in 2014, she was “as single as can be.” She tried many dating apps, but they were extremely time consuming. Even when she set specific preferences, she found herself sifting through “matches” that didn’t fit her criteria.
“Dating nowadays has become almost like a second job,” said Dr. Williams. “Just the amount of time that people are having to spend on apps, swiping left and right and then meeting people. You think they’re interested and then you deal with all these games.”
By 2017, Dr. Williams had invented Miss Doctor, a dating app that would connect female physicians and other doctoral-level professionals with men or women on a similar achievement level.
By definition, these people would not be intimidated by ambitious, busy women. They would be heavily screened and vetted. And one other proviso: they would have to pay for “likes.”
Most dating apps charge a subscription fee. Users are allowed to “like” numerous profiles and perhaps not bother responding to many matches. By contrast, Miss Doctor accounts are free and include a limited number of “likes” to indicate interest. Beyond that, there’s a price.
“We wanted to find a way to make people a little more intentional with how they like people on the app, so they give a little more thought to it,” Dr. Williams said. “So, we monetize it and use that to change behavior.”
After an initial launch in 2017, the app had to take a back seat while Dr. Williams started her psychiatry practice and got married herself. She plans to relaunch it in spring 2023.
Male or female, there is general agreement that finding time to date as a young physician isn’t easy. While DownToDate has had “doctor meets doctor” success stories, many users are still searching for “the one.”
Dr. Boyer believes that career challenges are not a reason to give up. “There are so many single and available people out there,” she said. “And everyone’s deserving of love. Even if you only have an hour a week.”
A version of this article first appeared on Medscape.com.
Blood pressure lowering after thrombectomy may be harmful
, new research suggests. Preliminary results of a new study showed that using an antihypertensive drug to target systolic blood pressure to below 160 mm Hg or 140 mm Hg in these patients may not be beneficial, and may even be harmful.
“This line of inquiry is probably not worth pursuing,” said stroke neurologist Eva A. Mistry, MBBS, MSCI, assistant professor of clinical neurology and rehabilitation medicine, University of Cincinnati.
Following current blood pressure guidelines in these patients (so targeting blood pressure under 180/105 mm Hg) “is probably reasonable,” unless the patient’s systolic blood pressure goes above 180, Dr. Mistry said. “Artificially trying to lower it may result in harm, at least in terms of the disability outcome.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Endovascular therapy has become standard of care for patients with large vessel occlusion after studies showed “massive benefit,” yet about 50% of patients remain disabled or die at 90 days, Dr. Mistry said.
“We have been on the quest to understand if there’s something we can do to improve these outcomes.”
One approach could be optimizing medical management. Previous observational studies showed that higher blood pressure values after thrombectomy are associated with worse outcomes.
Taking it forward
“We wanted to take that forward in a randomized inquiry to see first with this trial if [artificially] lowering blood pressure using medications is safe, and preliminarily understand if it could be efficacious in a larger trial,” she said.
This blood pressure–lowering strategy is already practiced in some centers. A nationwide survey conducted by Dr. Mistry and her colleagues showed a wide range of targets, with some institutions aiming it as low as under 120 mm Hg after thrombectomy, which she found “surprising.”
The Blood pressure after Endovascular Stroke Treatment-II (BEST-II) study included 120 ischemic stroke patients at three stroke centers, mean age 70 years and 57% female, who had undergone endovascular treatment. They were randomized to one of three target blood pressure groups: 180 mm Hg or under, less than 160 mm Hg, or under 140 mm Hg.
To lower blood pressure, researchers used intravenous nicardipine, a calcium channel blocker, as a first line. This was started within 1 hour of the endovascular treatment and given for 24 hours if the patient’s systolic blood pressure was above the target of their group.
In the highest target group (≤180 mm Hg), the average systolic blood pressure reached 129 mm Hg. In the middle target group (<160 mm Hg), the average systolic blood pressure was 131 mm Hg, and in the lowest target group (<140 mm Hg), systolic blood pressure was lowered to an average of 123 mm Hg.
Mean infarct volumes
At 36 hours, the mean adjusted infarct volume was slightly lower in the lowest blood pressure target group (32.4), compared with the other groups (46.4 for the 180 mm Hg group and 50.7 for the under-160 mm Hg group).
“Based on a model or a slope that would be associated with serial lowering of blood pressure targets, we found the point estimate of the effect size was slightly in the direction of benefit of lower blood pressure targets in terms of lower infarct volume,” Dr. Mistry said.
But this was not conclusive. While the point estimate was in the direction of benefit, Dr. Mistry stressed that the trial design doesn’t “definitely rule out” the possibility of harm.
Researchers also measured functional status at 90 days with the modified Rankin Scale (mRS). They found that the utility-weighted mRS was slightly lower in the lowest blood pressure target group (0.507), compared with the higher target groups (0.584 and 0.475, respectively, for the 180 mm Hg and under-160 mm Hg groups).
“The effect size was slightly in the direction of harm,” Dr. Mistry said. “To me, that means there might be safety issues associated with the lower blood pressure target.”
Probably futile
The results suggest that studying this issue further is probably futile. “If lowering blood pressure improves outcomes, that improvement is fairly marginal, and there are trends that suggest that, in fact, it might be harmful,” Dr. Mistry said. Her researcher team “believes it would not be the wisest decision” to pursue this strategy any further in a phase 3 study, she said.
“We wanted to understand whether or not we should spend millions of dollars to do a thousand-patient or two thousand-patient trial, and the answer to that is probably not.”
And there are other therapeutics “we can test that might be more promising than this approach,” she added.
In the meantime, Dr. Mistry stressed that clinicians should be cautious about automatically lowering blood pressure in this patient population and that decisions to target lower levels should be done on an individual basis.
Timely and important
In a comment, Karen Furie, MD, MPH, chair of neurology, Brown University, Providence, R.I., said that the study is “timely and important,” given the uncertainty about management of blood pressure after opening the vessel again using endovascular treatment.
“We already knew that letting the blood pressure go very high after reperfusion was bad, and this study shows that lowering it may also pose a risk, and I think that’s an important message for the community.”
The results send a cautionary message to clinicians but do not provide definitive evidence, she added. “Perhaps in the future we will have a better understanding of what the optimal range is.”
Dr. Furie stressed that this was a small pilot study and conclusions are “guarded.”
“I think the authors didn’t want to overinterpret the results so they ended up concluding that because the final disability might have been worse in the patients who had their blood pressure significantly lowered, recommending that as an approach across the board is sort of discouraged.”
Instead, the authors indicated that there may be factors such as degree of recanalization, size of the infarct, or other patient-specific factors “that would dictate where you target blood pressures,” Dr. Furie said.
The study was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Mistry receives funding from the Patient-Centered Outcomes Research Institute and compensation from the American Heart Association for editorial activities, and is a consultant for RapidAI. Dr. Furie has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Preliminary results of a new study showed that using an antihypertensive drug to target systolic blood pressure to below 160 mm Hg or 140 mm Hg in these patients may not be beneficial, and may even be harmful.
“This line of inquiry is probably not worth pursuing,” said stroke neurologist Eva A. Mistry, MBBS, MSCI, assistant professor of clinical neurology and rehabilitation medicine, University of Cincinnati.
Following current blood pressure guidelines in these patients (so targeting blood pressure under 180/105 mm Hg) “is probably reasonable,” unless the patient’s systolic blood pressure goes above 180, Dr. Mistry said. “Artificially trying to lower it may result in harm, at least in terms of the disability outcome.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Endovascular therapy has become standard of care for patients with large vessel occlusion after studies showed “massive benefit,” yet about 50% of patients remain disabled or die at 90 days, Dr. Mistry said.
“We have been on the quest to understand if there’s something we can do to improve these outcomes.”
One approach could be optimizing medical management. Previous observational studies showed that higher blood pressure values after thrombectomy are associated with worse outcomes.
Taking it forward
“We wanted to take that forward in a randomized inquiry to see first with this trial if [artificially] lowering blood pressure using medications is safe, and preliminarily understand if it could be efficacious in a larger trial,” she said.
This blood pressure–lowering strategy is already practiced in some centers. A nationwide survey conducted by Dr. Mistry and her colleagues showed a wide range of targets, with some institutions aiming it as low as under 120 mm Hg after thrombectomy, which she found “surprising.”
The Blood pressure after Endovascular Stroke Treatment-II (BEST-II) study included 120 ischemic stroke patients at three stroke centers, mean age 70 years and 57% female, who had undergone endovascular treatment. They were randomized to one of three target blood pressure groups: 180 mm Hg or under, less than 160 mm Hg, or under 140 mm Hg.
To lower blood pressure, researchers used intravenous nicardipine, a calcium channel blocker, as a first line. This was started within 1 hour of the endovascular treatment and given for 24 hours if the patient’s systolic blood pressure was above the target of their group.
In the highest target group (≤180 mm Hg), the average systolic blood pressure reached 129 mm Hg. In the middle target group (<160 mm Hg), the average systolic blood pressure was 131 mm Hg, and in the lowest target group (<140 mm Hg), systolic blood pressure was lowered to an average of 123 mm Hg.
Mean infarct volumes
At 36 hours, the mean adjusted infarct volume was slightly lower in the lowest blood pressure target group (32.4), compared with the other groups (46.4 for the 180 mm Hg group and 50.7 for the under-160 mm Hg group).
“Based on a model or a slope that would be associated with serial lowering of blood pressure targets, we found the point estimate of the effect size was slightly in the direction of benefit of lower blood pressure targets in terms of lower infarct volume,” Dr. Mistry said.
But this was not conclusive. While the point estimate was in the direction of benefit, Dr. Mistry stressed that the trial design doesn’t “definitely rule out” the possibility of harm.
Researchers also measured functional status at 90 days with the modified Rankin Scale (mRS). They found that the utility-weighted mRS was slightly lower in the lowest blood pressure target group (0.507), compared with the higher target groups (0.584 and 0.475, respectively, for the 180 mm Hg and under-160 mm Hg groups).
“The effect size was slightly in the direction of harm,” Dr. Mistry said. “To me, that means there might be safety issues associated with the lower blood pressure target.”
Probably futile
The results suggest that studying this issue further is probably futile. “If lowering blood pressure improves outcomes, that improvement is fairly marginal, and there are trends that suggest that, in fact, it might be harmful,” Dr. Mistry said. Her researcher team “believes it would not be the wisest decision” to pursue this strategy any further in a phase 3 study, she said.
“We wanted to understand whether or not we should spend millions of dollars to do a thousand-patient or two thousand-patient trial, and the answer to that is probably not.”
And there are other therapeutics “we can test that might be more promising than this approach,” she added.
In the meantime, Dr. Mistry stressed that clinicians should be cautious about automatically lowering blood pressure in this patient population and that decisions to target lower levels should be done on an individual basis.
Timely and important
In a comment, Karen Furie, MD, MPH, chair of neurology, Brown University, Providence, R.I., said that the study is “timely and important,” given the uncertainty about management of blood pressure after opening the vessel again using endovascular treatment.
“We already knew that letting the blood pressure go very high after reperfusion was bad, and this study shows that lowering it may also pose a risk, and I think that’s an important message for the community.”
The results send a cautionary message to clinicians but do not provide definitive evidence, she added. “Perhaps in the future we will have a better understanding of what the optimal range is.”
Dr. Furie stressed that this was a small pilot study and conclusions are “guarded.”
“I think the authors didn’t want to overinterpret the results so they ended up concluding that because the final disability might have been worse in the patients who had their blood pressure significantly lowered, recommending that as an approach across the board is sort of discouraged.”
Instead, the authors indicated that there may be factors such as degree of recanalization, size of the infarct, or other patient-specific factors “that would dictate where you target blood pressures,” Dr. Furie said.
The study was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Mistry receives funding from the Patient-Centered Outcomes Research Institute and compensation from the American Heart Association for editorial activities, and is a consultant for RapidAI. Dr. Furie has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Preliminary results of a new study showed that using an antihypertensive drug to target systolic blood pressure to below 160 mm Hg or 140 mm Hg in these patients may not be beneficial, and may even be harmful.
“This line of inquiry is probably not worth pursuing,” said stroke neurologist Eva A. Mistry, MBBS, MSCI, assistant professor of clinical neurology and rehabilitation medicine, University of Cincinnati.
Following current blood pressure guidelines in these patients (so targeting blood pressure under 180/105 mm Hg) “is probably reasonable,” unless the patient’s systolic blood pressure goes above 180, Dr. Mistry said. “Artificially trying to lower it may result in harm, at least in terms of the disability outcome.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Endovascular therapy has become standard of care for patients with large vessel occlusion after studies showed “massive benefit,” yet about 50% of patients remain disabled or die at 90 days, Dr. Mistry said.
“We have been on the quest to understand if there’s something we can do to improve these outcomes.”
One approach could be optimizing medical management. Previous observational studies showed that higher blood pressure values after thrombectomy are associated with worse outcomes.
Taking it forward
“We wanted to take that forward in a randomized inquiry to see first with this trial if [artificially] lowering blood pressure using medications is safe, and preliminarily understand if it could be efficacious in a larger trial,” she said.
This blood pressure–lowering strategy is already practiced in some centers. A nationwide survey conducted by Dr. Mistry and her colleagues showed a wide range of targets, with some institutions aiming it as low as under 120 mm Hg after thrombectomy, which she found “surprising.”
The Blood pressure after Endovascular Stroke Treatment-II (BEST-II) study included 120 ischemic stroke patients at three stroke centers, mean age 70 years and 57% female, who had undergone endovascular treatment. They were randomized to one of three target blood pressure groups: 180 mm Hg or under, less than 160 mm Hg, or under 140 mm Hg.
To lower blood pressure, researchers used intravenous nicardipine, a calcium channel blocker, as a first line. This was started within 1 hour of the endovascular treatment and given for 24 hours if the patient’s systolic blood pressure was above the target of their group.
In the highest target group (≤180 mm Hg), the average systolic blood pressure reached 129 mm Hg. In the middle target group (<160 mm Hg), the average systolic blood pressure was 131 mm Hg, and in the lowest target group (<140 mm Hg), systolic blood pressure was lowered to an average of 123 mm Hg.
Mean infarct volumes
At 36 hours, the mean adjusted infarct volume was slightly lower in the lowest blood pressure target group (32.4), compared with the other groups (46.4 for the 180 mm Hg group and 50.7 for the under-160 mm Hg group).
“Based on a model or a slope that would be associated with serial lowering of blood pressure targets, we found the point estimate of the effect size was slightly in the direction of benefit of lower blood pressure targets in terms of lower infarct volume,” Dr. Mistry said.
But this was not conclusive. While the point estimate was in the direction of benefit, Dr. Mistry stressed that the trial design doesn’t “definitely rule out” the possibility of harm.
Researchers also measured functional status at 90 days with the modified Rankin Scale (mRS). They found that the utility-weighted mRS was slightly lower in the lowest blood pressure target group (0.507), compared with the higher target groups (0.584 and 0.475, respectively, for the 180 mm Hg and under-160 mm Hg groups).
“The effect size was slightly in the direction of harm,” Dr. Mistry said. “To me, that means there might be safety issues associated with the lower blood pressure target.”
Probably futile
The results suggest that studying this issue further is probably futile. “If lowering blood pressure improves outcomes, that improvement is fairly marginal, and there are trends that suggest that, in fact, it might be harmful,” Dr. Mistry said. Her researcher team “believes it would not be the wisest decision” to pursue this strategy any further in a phase 3 study, she said.
“We wanted to understand whether or not we should spend millions of dollars to do a thousand-patient or two thousand-patient trial, and the answer to that is probably not.”
And there are other therapeutics “we can test that might be more promising than this approach,” she added.
In the meantime, Dr. Mistry stressed that clinicians should be cautious about automatically lowering blood pressure in this patient population and that decisions to target lower levels should be done on an individual basis.
Timely and important
In a comment, Karen Furie, MD, MPH, chair of neurology, Brown University, Providence, R.I., said that the study is “timely and important,” given the uncertainty about management of blood pressure after opening the vessel again using endovascular treatment.
“We already knew that letting the blood pressure go very high after reperfusion was bad, and this study shows that lowering it may also pose a risk, and I think that’s an important message for the community.”
The results send a cautionary message to clinicians but do not provide definitive evidence, she added. “Perhaps in the future we will have a better understanding of what the optimal range is.”
Dr. Furie stressed that this was a small pilot study and conclusions are “guarded.”
“I think the authors didn’t want to overinterpret the results so they ended up concluding that because the final disability might have been worse in the patients who had their blood pressure significantly lowered, recommending that as an approach across the board is sort of discouraged.”
Instead, the authors indicated that there may be factors such as degree of recanalization, size of the infarct, or other patient-specific factors “that would dictate where you target blood pressures,” Dr. Furie said.
The study was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Mistry receives funding from the Patient-Centered Outcomes Research Institute and compensation from the American Heart Association for editorial activities, and is a consultant for RapidAI. Dr. Furie has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
STROKE AF at 3 years: High AFib rate after atherosclerotic stroke
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
FROM ISC 2023
Novel neuroprotective agent promising in stroke
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Must-read acute care medicine articles from 2022
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When 2022 began, we started seeing some light at the end of the COVID-19 tunnel. Vaccines were widely available, and even with new variants of the virus still occasionally emerging, the rates of severe morbidity and mortality appeared to be decreasing.
Expectedly, journals appeared to start moving more toward mainstream topics and publications rather than what seemed like a major focus on COVID-19 publications. The resulting literature was fantastic.
Several of those topics were discussed in a prior Emergency Medicine Viewpoint from this news organization, and many more of the research advances of 2022 will be discussed in the near future. However, in this Viewpoint, I would like to present my annual review of my three “must-read” articles of the past year.
As in past years, I am choosing reviews of the literature rather than original research articles (which, all too often, become outdated or debunked within a few years). I choose these articles in the hopes that readers will not simply settle for my brief reviews of the key points but instead will feel compelled to download and read the entire articles. These publications address common conditions and quandaries we face in the daily practice of emergency medicine and are practice-changing.
Myocardial dysfunction after cardiac arrest: Tips and pitfalls
The management of post–cardiac arrest patients remains a hot topic in the resuscitation literature as we continue to understand that the immediate post-arrest period is critical to patient outcome.
Ortuno and colleagues reviewed the current literature on post-arrest care and wrote an outstanding summary of how to optimally care for these patients. More specifically, they focused on post-arrest patients who demonstrate continued shock, or “post–cardiac arrest myocardial dysfunction” (PCAMD).
They propose three mechanisms for the pathogenesis of PCAMD: ischemia reperfusion phenomenon, systemic inflammatory response, and increased catecholamine release
I will skip through the details of the pathophysiology that they describe in the article, but I certainly do recommend that everyone review their descriptions.
Management of these patients begins with a good hemodynamic assessment, which includes clinical markers of perfusion (blood pressure, capillary refill), ECG, and point-of-care ultrasound (POCUS). If the initial assessment reveals an obvious cause of the cardiac arrest (e.g., massive pulmonary embolism, myocardial infarction, pericardial tamponade), then the underlying cause should be treated expeditiously.
In the absence of an obvious treatable cause of the shock, the fluid status and cardiac function should be addressed with POCUS. If the patient is hypovolemic, intravenous fluids should be administered. If the fluid status is adequate, POCUS should be used to estimate the patient’s ventricular function. If the ventricle appears to be hyperdynamic with good contractility, shock should be treated with norepinephrine. On the other hand, if the ventricle is hypodynamic, dobutamine should be substituted for norepinephrine or, more often, added to norepinephrine.
The above represents a simplified summary of the critical points, but the authors do delve into further detail and also discuss some other options for therapies, including steroids, coronary revascularization, extracorporeal membrane oxygenation, and so on. The review is very thoughtful, thorough, and definitely worth a full read.
Top myths of diagnosis and management of infectious diseases in hospital medicine
Most, if not all of us in medicine, have heard the saying that 50% of what we learn in medical school (or residency) will turn out to be wrong. I certainly believe in this concept and consequently, like many of you, I enjoy reading about myths and misconceptions that we have been taught. With that in mind, I have to say that I love this article because it seems to have been written specifically to address what I was taught!
This author group, consisting mostly of clinical PharmDs who are experts in antibiotic use, provide us with an evidence-based discussion of myths and pitfalls in how antibiotics are often used in current clinical practice. The authors review their top 10 myths involving the use of antibiotics in treating infections in the hospital setting. A few of these relate more to the inpatient setting, but here are my favorite emergency department (ED)–related myths that they address:
- “Antibiotics do no harm.” The authors address the risk-benefit of antibiotics based on assumed vs. confirmed infections, including a brief discussion of adverse drug effects.
- “Antibiotic durations of 7, 14, or 21 days are typically necessary.” The authors address appropriate duration of antibiotic use and the fact that unnecessarily long durations of use can lead to resistance. They also provide reassurance that some infections can be treated with quite short durations of antibiotics.
- “If one drug is good, two (or more!) is better.” The use of multiple antibiotics, often with overlapping bacterial coverage, is rampant in medicine and further increases the risk for adverse drug effects and resistance.
- “Oral antibiotics are not as good as intravenous antibiotics for hospitalized patients.” This is definitely a myth that I learned. I recall being taught by many senior physicians that anyone sick enough for admission should be treated with intravenous antibiotics. As it turns out, absorption and effectiveness of most oral antibiotics is just as good as intravenous antibiotics, and the oral formulations are often safer.
- “A history of a penicillin allergy means the patient can never receive a beta-lactam antibiotic.” This is a myth that was debunked quite a few years ago, but it seems that many clinicians still need a reminder.
The authors included five more myths that are worth the read. This is an article that needs to be disseminated among all hospital clinicians.
Guidelines for low-risk, recurrent abdominal pain in the emergency department
The Society for Academic Emergency Medicine (SAEM) recently initiated a program focused on creating evidence-based approaches to challenging chief complaints and presentations in the emergency department (ED). In 2021, they published an approach to managing patients with recurrent, low-risk chest pain in the ED. This past year, they published their second guideline, focused on the management of patients with low-risk, recurrent abdominal pain in the ED.
Recurrent low-risk abdominal pain is a common and vexing presentation to EDs around the world, and there is little prior published guidance. Do all of these patients need repeat imaging? How do we manage their pain? Are there nonabdominal conditions that should be considered?
Broder and colleagues did a fantastic review of the current literature and, on behalf of SAEM, have provided a rational approach to optimal management of these patients. The four major questions they addressed, with brief summaries of their recommendations, are:
- Should adult ED patients with low-risk, recurrent and previously undifferentiated abdominal pain receive a repeat CT abdomen-pelvis (CTAP) after a negative CTAP within the past 12 months? This is a typical question that we all ponder when managing these patients. Unfortunately, the writing group found insufficient evidence to definitively identify populations in whom CTAP was recommended vs could be safely withheld. It is a bit disappointing that there is no definite answer to the question. On the other hand, it is reassuring to know that the world’s best evidence essentially says that it is perfectly appropriate to use your own good clinical judgment.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain with a negative CTAP receive additional imaging with abdominal ultrasound? In this case, the writing group found enough evidence, though low-level, to suggest against routine ultrasound in the absence of concern specifically for pelvic or hepatobiliary pathology. Like most tests, ultrasound is best used when there are specific concerns rather than being used in an undifferentiated fashion.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive screening for depression/anxiety? The writing group found enough evidence, though low-level again, to suggest that screening for depression and/or anxiety be performed during the ED evaluation. This could lead to successful therapy for the abdominal pain.
- Should adult ED patients with low-risk, recurrent, and previously undifferentiated abdominal pain receive nonopioid and/or nonpharmacologic analgesics? The writing group found little evidence to suggest for or against these analgesics, but they made a consensus recommendation suggesting an opioid-minimizing strategy for pain control.
Although the final recommendations of the writing group were not definitive or based on the strongest level of evidence, I find it helpful to have this guidance, nevertheless, on behalf of a major national organization. I also find it helpful to know that even with the best evidence available, optimal patient care will often boil down to physician experience and gestalt. I should also add that the overall article is chock-full of pearls and helpful information that will further inform the readers’ decisions, and so the full version is definitely worth the read.
In summary
There you have it – my three favorite practice-changing articles of 2022. Although I have tried to provide key points here, the full discussions of those key points in the published articles will provide a great deal more education than I can offer in this brief write-up, and so I strongly encourage everyone to read the full versions. Please be sure to include in the comments section your own pick for favorite or must-read articles from the past year.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.