User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
FDA okays Tidepool Loop app to help guide insulin delivery
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the Tidepool Loop, a mobile application for use with compatible continuous glucose monitors (CGMs) and insulin pumps to enable automated insulin delivery.
Indicated for people with type 1 diabetes ages 6 years and up, the app algorithm was developed by the diabetes startup Tidepool, which already hosts a cloud-based platform for users to download and review data from different glucose meters, insulin pumps, and CGM systems. The Tidepool Loop project arose from patient-led, open-source initiatives to enable interoperability between the devices.
“The [FDA] authorization of the Tidepool Loop is a huge win for the type 1 diabetes (T1D) community and is a vital step towards a world where people with T1D can choose the pump, CGM, and algorithm that are best for them – and have all three work together seamlessly,” Aaron Kowalski, PhD, CEO of the advocacy organization JDRF, said in a statement.
JDRF helped support preclinical and clinical research in the development of the Loop algorithm, along with The Leona M. and Harry B. Helmsley Charitable Trust, the Tullman Foundation, and partnerships with device makers and donations from the T1D community.
Available by prescription only, the Tidepool app is for single patient use. It works with designated “integrated CGMs” and “alternate controller enabled pumps” to automatically increase, decrease, or suspend insulin delivery, based on the glucose readings and predicted values. The app can also recommend correction doses, which the user can confirm.
According to an FDA statement:“Tidepool Loop’s algorithm technology is designed to be compatible with other individual interoperable devices that meet prespecified acceptance criteria set forth in a validation and integration plan provided by the sponsor and cleared by the FDA as part of the premarket submission.”
Tidepool is finalizing agreements with the various device manufacturers “to create a seamless experience for both physicians prescribing Tidepool Loop and the patients using it,” according to a company statement.
Tidepool’s initial launch device partners have not yet been announced, but the company “has a development partnership with Dexcom and other yet-to-be-named medical device companies for future inclusion of their components with the Tidepool Loop platform,” the statement says.
A version of this article first appeared on Medscape.com.
Clarity on torsemide vs. furosemide in HF: TRANSFORM-HF published
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival and readmission risk were similar whether patients hospitalized with heart failure (HF) were discharged on furosemide or torsemide in a randomized trial.
The study, TRANSFORM-HF, helps fill a major gap in the sparse evidence base guiding diuretic therapy in patients with a history of HF hospitalization. In that setting, for example, results suggest that discharge on any appropriate loop diuretic is more important than which loop diuretic is chosen.
TRANSFORM-HF is no ordinary randomized trial. Designed as a pragmatic comparative effectiveness study, it featured a streamlined protocol and other adaptations that made it easier and cheaper to conduct but that have also complicated its interpretation, the trialists and some observers acknowledge.
Perceived torsemide advantages
Furosemide may be the most-prescribed loop diuretic in HF, but in practice – based on some limited evidence – clinicians often prefer torsemide for its perceived advantages that include greater bioavailability, potassium sparing, and potentially helpful pleiotropic effects.
TRANSFORM-HF, however, provides no evidence to support such a preference. The primary endpoint of all-cause mortality was about 26% over a median 17 months whether patients were assigned to an initial furosemide or torsemide-first strategy, regardless of ejection fraction. Composite rates of death or hospitalization at 12 months also weren’t significantly different, at about 49% and 47%, respectively.
The findings suggest that clinicians may safely continue to prescribe either loop diuretic at their discretion, now with the support of data from a randomized trial.
TRANSFORM-HF was published in the Journal of the American Medical Association, with lead author Robert J. Mentz, MD, Duke University School of Medicine, Durham, N.C.
Dr. Mentz had also presented the trial’s preliminary results at the November American Heart Association Scientific Sessions in Chicago. The findings unveiled at the meeting and those published in the journal are essentially the same.
Reflections of standard practice
With its pragmatic design, TRANSFORM-HF entered a diverse HF population broadly representative of actual clinical practice. Patients were managed with few restrictions in a protocol that allowed, for example, loop-diuretic crossovers and other discretionary diuretic changes.
Diuretic dosing also varied significantly between the groups, and there was an unexpectedly high prevalence of diuretic withdrawal, the published report notes. Those factors, it states, may have “diminished” the trial’s ability “to distinguish the hypothesized between-group differences.”
Still, the trial “should be celebrated for dispelling a long-standing myth, based on surrogate markers and small trials, of the superiority of torsemide over furosemide,” writes Michelle M. Kittleson, MD, PhD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, in an accompanying editorial .
Now, she continues, “when faced with a patient with heart failure and congestive symptoms, clinicians can focus their energy on what really matters: Not the relative merits of different loop diuretics, but rather the initiation and optimization of evidence and guideline-based therapies to help their patients feel better and live longer.”
Trial design caveats
But that pragmatic design raises cautions, the editorial notes. “Pragmatic trials are more flexible and nimbler in design and execution, but this agility comes at a cost. An overly heterogeneous patient population can impact the trial’s ability to assess efficacy of therapies while minimally intensive follow-up precludes comprehensive outcome assessment.”
The study’s 2,859 patients hospitalized with HF were assigned to open-label treatment with furosemide or torsemide at more than 60 U.S. centers. Of the 1,428 and 1,431 patients, respectively, about 37% were women and 34% were African American.
The hazard ratio for all cause mortality across the 17.4-month follow-up, torsemide versus furosemide, was 1.02 (95% confidence interval, 0.89-1.18). The HR for death or hospitalization for any cause at 12 months was 0.92 (95% CI, 0.83-1.02). And the rate ratio for 12-month all-cause hospitalization was 0.94 (95% CI, 0.84-1.07).
“TRANSFORM-HF joins a catalog of cautionary tales in cardiology, whereby carefully executed negative trials have refuted the misleading promise of plausible surrogate end points and preliminary data,” Dr. Kittleson writes.
“The lesson: Clinicians should have a healthy suspicion for plausible pathophysiology, surrogate end points, and nonrandomized data as the sole basis of defining superiority of an intervention.”
TRANSFORM-HF was funded by the National Institutes of Health. Dr. Mentz reports receiving grants from American Regent and Novartis; personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Cytokinetics, Bayer, Merck, and Pharmacosmos; and research support from Abbott, Amgen, Bayer, Boston Scientific, Fast BioMedical, Gilead, Innolife, Medtronic, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Disclosures for the other authors can be found with the original article. Dr. Kittleson reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
EMR screening in emergency department tags undiagnosed diabetes
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
A diabetes screening program built into an electronic medical records system identified diabetes or prediabetes in 52% of individuals flagged for abnormal hemoglobin A1c, based on data from more than 2,000 adults.
“Despite the best efforts of clinicians, researchers, and educators, the number of patients living with undiagnosed diabetes is still rising and is currently at approximately 8.5 million, and the number of people unaware of their prediabetes is approximately 77 million,” lead investigator Kristie K. Danielson, PhD, said in an interview. Screening for diabetes is critical to start treatment early, to potentially reverse prediabetes, and to prevent the long-term complications of diabetes and reduced life expectancy.
In a pilot study published in JAMA Network Open, Dr. Danielson and colleagues reviewed data from 8,441 adults who visited a single emergency department in Chicago during February–April 2021.
The EMR at the hospital contained a built-in best practice alert (BPA) that flagged patients as being at risk for type 2 diabetes based the American Diabetes Association recommendations; the identification algorithm included age 45 years and older, or those aged 18-44 years with a body mass index of 25 kg/m2 or higher, no previous history of diabetes, and no A1c measure in the last 3 years, according to the EMR.
A total of 8,441 adult patients visited the ED during the study period; 2,576 triggered BPA tests, and 2,074 had A1c results for review. Among the patients with A1c results, 52% had elevated values of 5.7% or higher. Of these, a total of 758 individuals were identified with prediabetes (A1c, 5.7%-6.4%), 265 with diabetes (A1c, 6.5%-9.9%), and 62 with severe diabetes (A1c, 10% or higher).
After testing, 352 patients with elevated A1c were contacted by the researchers. The mean age of this group was 52.2 years, 54.5% were women, and nearly two-thirds (64.8%) were non-Hispanic Black. The median income of those contacted was in the 44th percentile, and 50% had public insurance.
Most of those contacted (264 patients) were not aware of a previous diagnosis of prediabetes or diabetes; the remaining 88 had a previous diagnosis, but only 51 self-reported receiving treatment, the researchers noted.
Although the screening program successfully identified a significant number of previously undiagnosed individuals with diabetes, prediabetes, or poorly controlled diabetes, its feasibility in routine practice requires further study, the researchers wrote.
The findings were limited by several factors including the identification of patients previously diagnosed with diabetes but who were not being treated, and the potential bias toward individuals of higher socioeconomic status, the researchers noted. However, the results support further exploration of the program as a way to identify undiagnosed diabetes, especially in underserved populations.
Diabetes in underserved groups goes undetected
“We were surprised by the sheer number of people newly diagnosed with diabetes or prediabetes,” which was far greater than expected, commented Dr. Danielson of the University of Illinois at Chicago. “Clearly, we tapped into a new population that has not often been seen by primary care providers or endocrinologists, as is often the case for underserved and vulnerable individuals who visit the emergency department as a first line for health care.”
The screening alert system is straightforward to build into an existing EMR, with technical support, Dr. Danielson said. “In theory, it should be able to be incorporated into other clinical centers and emergency departments. One of the current limitations that we are seeing is that the EMR is still flagging some people already diagnosed with diabetes to be screened for diabetes.” However, “because of this, we also see this as an opportunity to identify and reach out to those with diabetes who are still underserved and not receiving the appropriate diabetes care they need.”
The study results have broader public health implications, Dr. Danielson added. “We have identified a new, large population of people with diabetes who need medical care and diabetes education. This will further add to the burden of health care and costs, and it raises the ethical question of screening and not having full resources readily available to help.
“In my opinion, the study sheds light on a significant issue that will hopefully help drive change at both a health systems and public health level locally and nationally,” she added.
“One of the significant research gaps that has emerged now is how to link these new patients to health care and diabetes education at our institution after they leave the emergency department,” said Dr. Danielson. Diabetes screening in the ED setting is “a very novel area for health system scientists, social workers, and others to now come to the table and collaborate on next steps to help our patients.”
The study was initiated by the investigators, but was supported by a grant from Novo Nordisk to two coauthors. Dr. Danielson also disclosed grant funding from Novo Nordisk during the conduct of the study.
FROM JAMA NETWORK OPEN
Severe health diagnoses drive suicide risk
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals diagnosed with a severe physical health condition were significantly more likely to commit suicide at 6 months and at 1 year later, based on data from more than 47 million individuals in a national database.
Previous smaller studies have shown a link between increased risk for suicide and a range of health conditions including cancer, coronary heart disease, neurologic conditions, diabetes, and osteoporosis, Vahé Nafilyan, PhD, of the Office for National Statistics, Newport, England, and colleagues wrote.
However, large-scale population-level studies of the association between specific diagnoses and suicide are lacking, they said.
In a study published in The Lancet Regional Health–Europe, the researchers reviewed a dataset that combined the 2011 Census, death registration records, and the Hospital Episode Statistics. The study population included 47,354,696 individuals aged 6 years and older living in England in 2017. The mean age of the study population was 39.6 years, and 52% were female. The researchers examined deaths that occurred between Jan. 1, 2017, and Dec. 31, 2021.
The health conditions included in the analysis were low-survival cancers, chronic ischemic heart disease, chronic obstructive pulmonary disease, and degenerative neurological disease.
The diagnosis of any of these conditions significantly increased the risk for suicide compared with controls. The highest risk appeared within 6 months of a diagnosis or first treatment, but the increased risk persisted at 1 year.
The suicide rate among low-survival cancer patients was 16.6 per 100,000 patients, compared with 5.7 per 100,000 controls; at 1 year, these rates were 21.6 and 9.5 per 100,000 patients and controls, respectively.
For COPD patients, the suicide rate at 6 months after diagnosis was 13.7 per 100,000 patients versus 5.6 per 100,000 matched controls; the suicide rates at 1 year were 22.4 per 100,000 patients and 10.6 per 100,000 matched controls.
The suicide rate at 6 months for individuals diagnosed with chronic ischemic heart disease was 11.0 per 100,000 patients and 4.2 per 100,000 matched controls; at 1 year, the suicide rates were 16.1 per 100,000 patients and 8.8 per 100,000 matched controls.
The 1-year suicide rate was especially high among patients with degenerative neurological conditions (114.5 per 100,000 patients); however, the estimate was considered imprecise because of the rarity of these diseases and subsequent low number of suicides, the researchers noted.
The results support data from previous studies showing links between increased risk of suicide and severe physical conditions, the researchers wrote. Patterns of suicide were similar between men and women and after adjusting for sociodemographic factors.
The findings were limited by the inability to fully control for a history of depression or self-harm, and by the imprecise estimates given the rare occurrence of suicide overall, the researchers noted. Other limitations included the late registration of deaths from external causes and the focus only on suicides that occurred in England and Wales, meaning that individuals who traveled abroad for assisted suicide were not captured in the dataset.
“Further research is needed to understand the mechanisms driving the elevated risk of suicide and help provide the best support to these patients,” the researchers concluded.
However, the current results enhance the literature with a large, population-based review of the elevated suicide risk among individuals newly diagnosed with severe health conditions, and reflect the need for better support for these patients to help with coping, they said.
The study was funded by the Office for National Statistics. The researchers reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH–EUROPE
The longevity gene: Healthy mutant reverses heart aging
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Everybody wants a younger heart
As more people live well past 90, scientists have been taking a closer look at how they’ve been doing it. Mostly it boiled down to genetics. You either had it or you didn’t. Well, a recent study suggests that doesn’t have to be true anymore, at least for the heart.
Scientists from the United Kingdom and Italy found an antiaging gene in some centenarians that has shown possible antiaging effects in mice and in human heart cells. A single administration of the mutant antiaging gene, they found, stopped heart function decay in middle-aged mice and even reversed the biological clock by the human equivalent of 10 years in elderly mice.
When the researchers applied the antiaging gene to samples of human heart cells from elderly people with heart problems, the cells “resumed functioning properly, proving to be more efficient in building new blood vessels,” they said in a written statement. It all kind of sounds like something out of Dr. Frankenstein’s lab.
I want to believe … in better sleep
The “X-Files” theme song plays. Mulder and Scully are sitting in a diner, breakfast laid out around them. The diner is quiet, with only a few people inside.
Mulder: I’m telling you, Scully, there’s something spooky going on here.
Scully: You mean other than the fact that this town in Georgia looks suspiciously like Vancouver?
Mulder: Not one person we spoke to yesterday has gotten a full night’s sleep since the UFO sighting last month. I’m telling you, they’re here, they’re experimenting.
Scully: Do you really want me to do this to you again?
Mulder: Do what again?
Scully: There’s nothing going on here that can’t be explained by the current research. Why, in January 2023 a study was published revealing a link between poor sleep and belief in paranormal phenomena like UFOS, demons, or ghosts. Which probably explains why you’re on your third cup of coffee for the morning.
Mulder: Scully, you’ve literally been abducted by aliens. Do we have to play this game every time?
Scully: Look, it’s simple. In a sample of nearly 9,000 people, nearly two-thirds of those who reported experiencing sleep paralysis or exploding head syndrome reported believing in UFOs and aliens walking amongst humanity, despite making up just 3% of the overall sample.
Furthermore, about 60% of those reporting sleep paralysis also reported believing near-death experiences prove the soul lingers on after death, and those with stronger insomnia symptoms were more likely to believe in the devil.
Mulder: Aha!
Scully: Aha what?
Mulder: You’re a devout Christian. You believe in the devil and the soul.
Scully: Yes, but I don’t let it interfere with a good night’s sleep, Mulder. These people saw something strange, convinced themselves it was a UFO, and now they can’t sleep. It’s a vicious cycle. The study authors even said that people experiencing strange nighttime phenomena could interpret this as evidence of aliens or other paranormal beings, thus making them even more susceptible to further sleep disruption and deepening beliefs. Look who I’m talking to.
Mulder: Always with the facts, eh?
Scully: I am a doctor, after all. And if you want more research into how paranormal belief and poor sleep quality are linked, I’d be happy to dig out the literature, because the truth is out there, Mulder.
Mulder: I hate you sometimes.
It’s ChatGPT’s world. We’re just living in it
Have you heard about ChatGPT? The artificial intelligence chatbot was just launched in November and it’s already more important to the Internet than either Vladimir Putin or “Rick and Morty.”
What’s that? You’re wondering why you should care? Well, excuuuuuse us, but we thought you might want to know that ChatGPT is in the process of taking over the world. Let’s take a quick look at what it’s been up to.
“ChatGPT bot passes law school exam”
“ChatGPT passes MBA exam given by a Wharton professor”
“A freelance writer says ChatGPT wrote a $600 article in just 30 seconds”
And here’s one that might be of interest to those of the health care persuasion: “ChatGPT can pass part of the U.S. Medical Licensing Exam.” See? It’s coming for you, too.
The artificial intelligence known as ChatGPT “performed at >50% accuracy across [the three USMLE] examinations, exceeding 60% in most analyses,” a group of researchers wrote on the preprint server medRxiv, noting that 60% is usually the pass threshold for humans taking the exam in any given year.
ChatGPT was not given any special medical training before the exam, but the investigators pointed out that another AI, PubMedGPT, which is trained exclusively on biomedical domain literature, was only 50.8% accurate on the USMLE. Its reliance on “ongoing academic discourse that tends to be inconclusive, contradictory, or highly conservative or noncommittal in its language” was its undoing, the team suggested.
To top it off, ChatGPT is listed as one of the authors at the top of the medRxiv report, with an acknowledgment at the end saying that “ChatGPT contributed to the writing of several sections of this manuscript.”
We’ve said it before, and no doubt we’ll say it again: We’re doomed.
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
High-deductible health plans detrimental for those with diabetes
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors for severe COVID? Pilot trial signals of benefit
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves new type 2 diabetes drug bexagliflozin
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved bexagliflozin (Brenzavvy, TheracosBio) for the treatment of adults with type 2 diabetes.
The once-daily 20-mg oral sodium-glucose cotransporter 2 (SGLT2) inhibitor is indicated as an adjunct to diet and exercise to improve glycemic control for those with type 2 diabetes, but not type 1 diabetes. It can be used in adults with an estimated glomerular filtration rate (eGFR) > 30 mL/min per 1.73 m2.
Approval was based on results from 23 clinical trials with more than 5,000 participants, including more than 300 patients with stage 3 kidney disease (eGFR < 60 and > 30 mL/min per 1.73 m2).
In the phase 3 studies, bexagliflozin significantly reduced hemoglobin A1c and fasting blood glucose at 24 weeks as monotherapy or as add-on to metformin and other glucose-lowering drugs and combinations. It also produced modest reductions in body weight and systolic blood pressure.
In the phase 3 Bexagliflozin Efficacy and Safety Trial (BEST) cardiovascular outcomes trial, the drug met its efficacy and safety objectives in patients at high cardiovascular risk. Noninferiority was demonstrated for the composite outcome of cardiovascular death, myocardial infarction, stroke, or unstable angina.
“As a class of drugs, SGLT2 inhibitors have shown tremendous benefit in treating adults with type 2 diabetes,” said Mason Freeman, MD, director of the Translational Research Center at Massachusetts General Hospital, Boston, in a press release from TheracosBio.
“Being involved in all of the clinical trials for Brenzavvy, I am greatly impressed with the efficacy of the drug in reducing blood glucose levels and I believe it is an important addition to the SGLT2 inhibitor class of drugs.”
As with other SGLT2 inhibitors, adverse events seen in the trials include ketoacidosis, lower limb amputation, volume depletion, urosepsis, pyelonephritis, Fournier’s gangrene, genital mycotic infections, and hypoglycemia when used with insulin or insulin secretagogues.
Bexagliflozin joins an already crowded field of SGLT2 inhibitors, some of which have been approved for additional cardiovascular and kidney indications.
Of interest, bexagliflozin was approved by the FDA for diabetes in cats in December 2022, as the first oral new animal drug to improve glycemic control in otherwise healthy cats with diabetes not previously treated with insulin.
A version of this article first appeared on Medscape.com.
AHA scientific statement on rapid evaluation for suspected TIA
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.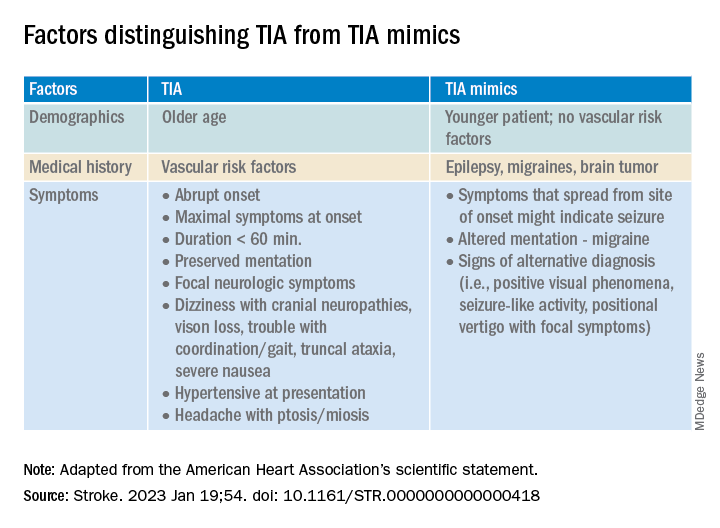
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
FROM STROKE











