User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Artificial intelligence in your office
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Patisiran (Onpattro) for ATTR cardiomyopathy gets FDA panel thumbs up
The Cardiovascular and Renal Drugs Advisory Committee of the Food and Drug Administration has voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy – although many panel members questioned whether the benefits are clinically meaningful.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, and fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of the polyneuropathy of hereditary ATTR amyloidosis in adults.
In the APOLLO-B trial, patisiran showed a statistically significant and clinically meaningful benefit on functional capacity, as measured by the 6-minute walk test, compared with placebo, in patients with ATTR amyloidosis with cardiomyopathy.
The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life.
But in explaining her “no” vote, committee member C. Noel Bairey Merz, MD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said she “did not feel like there was benefit” using existing clinically relevant thresholds typically used in cardiology.
Committee chair Javed Butler, MD, MPH, Baylor Scott & White Research Institute, Dallas, who also voted no, said he “struggled” with this vote and emphasized that it “absolutely does not reflect that there is not a potential with the therapy.”
Dr. Butler said he voted no largely because he wasn’t sure whether the benefits are clinically meaningful in the context of the study design and how it was conducted. He did not have any safety concerns, which was the general feeling of the committee.
Edward Kasper, MD, Johns Hopkins University, Baltimore, who voted in favor of patisiran for ATTR amyloidosis with cardiomyopathy, said there is a “light wind for benefit and no wind for risk. So, if you’re asking do benefits outweigh the risks, the answer is yes.”
But Dr. Kasper also noted: “It would have been a more difficult question to answer: Is there clinically meaningful benefit versus risk? But that’s not what the question asked.”
In explaining his “yes” vote, Ravi Thadhani, MD, MPH, Emory University, Atlanta, said: “We’re dealing with a rare disease with few options and devastating consequences. We heard from clinicians loud and clear, and from patients for that matter, that options and alternatives are critical, and that there is a continuous decline of cardiac function and worsening of disease in a number of patients that have received the current standard of care. For me, the benefits outweigh the risks.”
Dr. Thadhani also noted that from the data provided, no benefit was shown – ”disappointingly” he lamented – for women, for Black persons, and among individuals who were receiving tafamidis, and he urged the FDA and sponsor to consider this.
The FDA has set a target action date for patisiran for ATTR amyloidosis cardiomyopathy of Oct. 8.
A version of this article first appeared on Medscape.com.
The Cardiovascular and Renal Drugs Advisory Committee of the Food and Drug Administration has voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy – although many panel members questioned whether the benefits are clinically meaningful.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, and fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of the polyneuropathy of hereditary ATTR amyloidosis in adults.
In the APOLLO-B trial, patisiran showed a statistically significant and clinically meaningful benefit on functional capacity, as measured by the 6-minute walk test, compared with placebo, in patients with ATTR amyloidosis with cardiomyopathy.
The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life.
But in explaining her “no” vote, committee member C. Noel Bairey Merz, MD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said she “did not feel like there was benefit” using existing clinically relevant thresholds typically used in cardiology.
Committee chair Javed Butler, MD, MPH, Baylor Scott & White Research Institute, Dallas, who also voted no, said he “struggled” with this vote and emphasized that it “absolutely does not reflect that there is not a potential with the therapy.”
Dr. Butler said he voted no largely because he wasn’t sure whether the benefits are clinically meaningful in the context of the study design and how it was conducted. He did not have any safety concerns, which was the general feeling of the committee.
Edward Kasper, MD, Johns Hopkins University, Baltimore, who voted in favor of patisiran for ATTR amyloidosis with cardiomyopathy, said there is a “light wind for benefit and no wind for risk. So, if you’re asking do benefits outweigh the risks, the answer is yes.”
But Dr. Kasper also noted: “It would have been a more difficult question to answer: Is there clinically meaningful benefit versus risk? But that’s not what the question asked.”
In explaining his “yes” vote, Ravi Thadhani, MD, MPH, Emory University, Atlanta, said: “We’re dealing with a rare disease with few options and devastating consequences. We heard from clinicians loud and clear, and from patients for that matter, that options and alternatives are critical, and that there is a continuous decline of cardiac function and worsening of disease in a number of patients that have received the current standard of care. For me, the benefits outweigh the risks.”
Dr. Thadhani also noted that from the data provided, no benefit was shown – ”disappointingly” he lamented – for women, for Black persons, and among individuals who were receiving tafamidis, and he urged the FDA and sponsor to consider this.
The FDA has set a target action date for patisiran for ATTR amyloidosis cardiomyopathy of Oct. 8.
A version of this article first appeared on Medscape.com.
The Cardiovascular and Renal Drugs Advisory Committee of the Food and Drug Administration has voted 9 to 3 that the benefits of patisiran outweigh the risks for the treatment of ATTR amyloidosis cardiomyopathy – although many panel members questioned whether the benefits are clinically meaningful.
ATTR amyloidosis is an underdiagnosed, rapidly progressive, debilitating, and fatal disease caused by misfolded TTR proteins, which accumulate as amyloid deposits in various parts of the body, including the heart.
Intravenously administered patisiran is already approved in the United States and Canada for the treatment of the polyneuropathy of hereditary ATTR amyloidosis in adults.
In the APOLLO-B trial, patisiran showed a statistically significant and clinically meaningful benefit on functional capacity, as measured by the 6-minute walk test, compared with placebo, in patients with ATTR amyloidosis with cardiomyopathy.
The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life.
But in explaining her “no” vote, committee member C. Noel Bairey Merz, MD, Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said she “did not feel like there was benefit” using existing clinically relevant thresholds typically used in cardiology.
Committee chair Javed Butler, MD, MPH, Baylor Scott & White Research Institute, Dallas, who also voted no, said he “struggled” with this vote and emphasized that it “absolutely does not reflect that there is not a potential with the therapy.”
Dr. Butler said he voted no largely because he wasn’t sure whether the benefits are clinically meaningful in the context of the study design and how it was conducted. He did not have any safety concerns, which was the general feeling of the committee.
Edward Kasper, MD, Johns Hopkins University, Baltimore, who voted in favor of patisiran for ATTR amyloidosis with cardiomyopathy, said there is a “light wind for benefit and no wind for risk. So, if you’re asking do benefits outweigh the risks, the answer is yes.”
But Dr. Kasper also noted: “It would have been a more difficult question to answer: Is there clinically meaningful benefit versus risk? But that’s not what the question asked.”
In explaining his “yes” vote, Ravi Thadhani, MD, MPH, Emory University, Atlanta, said: “We’re dealing with a rare disease with few options and devastating consequences. We heard from clinicians loud and clear, and from patients for that matter, that options and alternatives are critical, and that there is a continuous decline of cardiac function and worsening of disease in a number of patients that have received the current standard of care. For me, the benefits outweigh the risks.”
Dr. Thadhani also noted that from the data provided, no benefit was shown – ”disappointingly” he lamented – for women, for Black persons, and among individuals who were receiving tafamidis, and he urged the FDA and sponsor to consider this.
The FDA has set a target action date for patisiran for ATTR amyloidosis cardiomyopathy of Oct. 8.
A version of this article first appeared on Medscape.com.
SGLT2 inhibitors: No benefit or harm in hospitalized COVID-19
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Should intravascular imaging be almost routine in PCI?
A routine role for intravascular imaging (IVI) guidance for percutaneous coronary intervention (PCI) has long been favored by many of the technology’s researchers and enthusiasts. Now evidence from large, randomized trials may be catching up with such aspirations, though not without caveats.
One way IVI guidance may achieve that, the research suggests, albeit more speculatively, is by cutting risk for stent thrombosis, compared with the risk associated with angiography-only PCI.
The new studies, two large randomized IVI trials plus a meta-analysis of 20 such studies, were presented at the annual congress of the European Society of Cardiology.
In one, called ILUMIEN-4, PCI guided by optical coherence tomography (OCT) was associated with fewer procedural complications and better acute results – that is, larger post-PCI minimum stent area (MSA) – than in angiography-only procedures (P < .001). Poststenting MSA, an established predictor of clinical outcomes, was the primary imaging endpoint of the trial with almost 2,500 patients.
Yet the OCT group’s greater post-PCI MSA did not translate to reduced risk for the primary clinical endpoint of 2-year target-vessel failure. Among secondary endpoints, however, stent thrombosis at some point during the follow-up was 64% less likely (P = .02) with OCT guidance than angiography-only PCI.
ILUMIEN-4, despite its neutral clinical result, still “strongly advocates” for PCI guidance by OCT, at least among patients like those in the trial, said principal investigator Ziad Ali, MD, DPhil. He based that largely on the strategy’s greater postprocedure lumen areas in the trials, which are among “the strongest independent predictors for long term outcomes,” said Dr. Ali, of St. Francis Hospital & Heart Center, Roslyn, N.Y., at a press conference on IVI trials during the ESC Congress.
Selected complex lesion type
In contrast, the OCTOBER trial, presented at the sessions back to back with ILUMIEN-4, saw OCT guidance lead to better clinical outcomes than angiography alone after PCI of bifurcation lesions, which normally can be a special challenge for operators.
In the trial, which entered about 1,200 patients with such complex lesions, the 2-year risk for major adverse cardiac events (MACE) fell 60% after OCT-guided PCI, compared with angiography-only procedures (P = .035).
The finding is novel for showing that OCT guidance in bifurcation PCI can make a significant clinical difference, said OCTOBER investigator Niels R. Holm, MD, at the same media presentation on IVI trials.
“Multiple studies have shown that OCT allows for optimization of bifurcation PCI, and our results confirm that such optimization may improve the patient’s prognosis,” said Dr. Holm of Aarhus (Denmark) University Hospital.
ILUMIEN-4 and OCTOBER, both of which prespecified the Xience (Abbott) everolimus-eluting stent for the procedures, were published in the New England Journal of Medicine in tandem with their respective presentations at the ESC sessions.
Covering the spectrum
A meta-analysis presented at the same ESC session compared IVI using either OCT or intravascular ultrasound (IVUS) with angiography-only PCI across 20 randomized trials with a total of more than 12,000 patients.
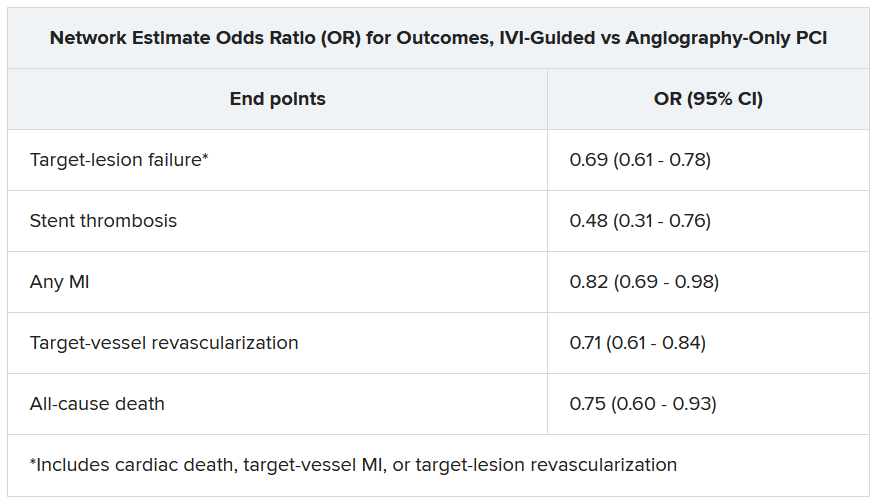
Significant outcomes for IVI guidance versus angiography alone included a 31% drop in risk for target-lesion failure, the primary endpoint. And this study, as well, showed a steep 52% reduction in risk for in-stent thrombosis with the IVI-guided approach.
And “for the first time” in IVI studies, “we demonstrated reductions in all-myocardial-infarction and all-cause death, the latter by 25%,” Gregg Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in presenting the meta-analysis. Dr. Stone is also the ILUMIEN-4 study chairperson.
“The routine use of OCT or IVUS to guide most PCI procedures will substantially improve patient event-free survival,” he predicted, “enhancing both the long-term safety and effectiveness of the procedure.”
Dr. Stone said that IVI guidance “should be standard of care, if not in all patients, then in most patients.” Part of the rationale: PCI is unlikely to be improved much further by incremental gains in drug-eluting stent design. “That technology has almost plateaued.” But there’s yet room for “substantially improved outcomes” from adjunctive treatments and techniques such as IVI guidance.
The 20 studies in the meta-analysis encompassed an array of patients and lesions both complex and noncomplex, Dr. Stone observed, including bifurcation lesions, chronic total occlusions, left-main coronary stenoses, and MI culprit lesions.
“They really covered the spectrum of PCI,” he said. “I’m not recommending that intravascular imaging be used in every single case. But I do think it should be used in the majority of patients” and be standard of care for PCI in left-main lesions and “complex coronary disease, high-risk patients, and high-risk lesions.”
Unique advantage
The IVI-guidance groups in both ILUMIEN-4 and the meta-analysis showed a significant drop in risk for stent thrombosis – that is, abrupt thrombotic vessel closure, which typically occurs in 1% or fewer PCI cases but can trigger an MI and pose a mortality risk up to 45%.
Those risk reductions are consistent with a unique IVI advantage: the ability to guide optimization of stent deployments. When formally presenting ILUMIEN-4 at the ESC sessions, Ali observed that IVUS and OCT imaging allows operators to identify and often correct less-than-ideal results of an initial stent delivery – such as residual gaps between stent struts and vessel wall – that may encroach on the lumen, with possible clinical consequences.
Such imaging, said Dr. Ali, “lets you identify tissue protrusions, malappositions, dissections, and untreated reference-segment disease” that may potentially trigger thrombosis. That makes a strong argument for giving IVI guidance a more common, perhaps even routine role in PCI procedures.
Selling routine IVI-guided PCI in practice
“I think the study results are quite clear,” said Deepak L. Bhatt, MD, MPH, as session comoderator following the OCTOBER presentation. “The challenge, though, will be convincing the average interventional cardiologist worldwide that it was specifically the imaging and not the extra care that the patient getting OCT also inherently receives.”
Did OCT’s better trial outcomes stem from IVI itself or from greater operator attentiveness to procedural results – such as, for example, more high-pressure expansions to optimize stent placement, “the sort of thing that tends to occur when invasive imaging is added on to just plain old angiography?” Dr. Bhatt asked of Lene N. Andreasen, MD, who had just presented the OCTOBER trial. “There’s no way of uncoupling the two things.”
What can be said, “at this point, to convince interventional cardiologists that the extra time, energy, expense, is truly indicated,” that the data are “sufficient to change global practice?” asked Dr. Bhatt, Mount Sinai Hospital and Icahn School of Medicine at Mount Sinai.
That remains an open question,” acknowledged Dr. Andreasen of Aarhus University Hospital. The best argument in favor of selective IVI-guided PCI is that “we actually see a clinical benefit” in the trials. “But of course, it comes with a cost. It comes with longer procedures and more contrast.” How clinical practice responds to the new data remains to be seen, she proposed.
ILUMIEN-4 and OCTOBER in detail
Conducted at 80 centers in 18 countries, ILUMIEN-4 randomly assigned patients with diabetes or complex coronary lesions to undergo PCI guided by OCT or using standard angiography only, 1,233 and 1,254 patients, respectively.
Post-PCI MSA averaged 5.72 mm2 with OCT guidance and 5.36 mm2 in the angiography-only group (P < .001).
Their rates of target-vessel failure at 2 years were not significantly different at 7.4% and 8.2%, respectively. The 2-year composite endpoint included cardiac death, target vessel–related MI, or ischemia-driven target-vessel revascularization.
Definite or probable stent thrombosis was observed over 2 years in 0.5% of the OCT group and 1.4% of those with angiography-only PCI (hazard ratio, 0.36; 95% confidence interval, 0.14-0.91; P = .02) favoring OCT.
The OCTOBER trial, conducted at 38 centers in Europe, entered 1201 patients with stable angina or acute coronary syndromes and angiographically identified complex bifurcation lesions. They involved the left-main coronary artery in about one-fifth of cases.
Patients were randomly assigned to bifurcation PCI guided by OCT or under standard angiography, 600 and 601 patients, respectively. Rates for procedure-related complications were similar at 6.8% and 5.7%, respectively.
Over a median of 2 years, 10.1% of the OCT group and 14.1% of angiography-only patients developed a MACE event, including cardiac death, target-lesion MI, or ischemia-driven target-lesion revascularization. The adjusted HR was 0.71 (95% CI, 0.51-0.98; P = .035) in favor of OCT.
Meta-analysis, trials to date
The meta-analysis presented by Dr. Stone included ILUMIEN-4, OCTOBER, and 18 earlier outcomes trials comparing PCI guided by IVI, either OCT or IVUS, and angiography-only PCI. It covered 12,428 patients with chronic or acute coronary disease and followed them a mean of 26 months; the longest follow-up was 5 years. They were assigned to IVI-guided or angiography-only PCI, 7,038 and 5,390 patients, respectively.
Dr. Stone and colleagues conducted a network meta-analysis of the 20 studies, that is, a combined analysis that allowed both direct and indirect comparisons of standard angiography-only procedures to each of the other studied comparator interventions including OCT, IVUS, and either OCT or IVUS. They then derived network-estimate odds ratios for IVI-guided PCI vs angiography-only procedures.
“Hopefully, this will impact the guidelines,” Dr. Stone said of the meta-analysis. Procedures guided by IVI might become more common in clinical practice if they were to garner a Class-I guideline recommendation, the strongest recommendation category.
“That would make a difference, but we’d also need to work to remove impediments to increasing intravascular imaging guidance” for most patients undergoing PCI, he said, referring to challenges in obtaining reimbursement for IVI-guided PCI and training enough operators to handle the projected demand.
ILUMIEN-4 was funded by Abbott. OCTOBER was supported by grants from Abbott Vascular, St. Jude Medical, and Aarhus University. The network meta-analysis received statistical support from Abbott. Dr. Ali disclosed institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems, Medtronic, the National Institutes of Health, Opsens Medical, Philips, and Teleflex; consulting fees from Astra Zeneca, Philips, Shockwave; and holding equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr. Holm and Dr. Bhatt reported numerous conflicts of interest. Dr. Andreasen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A routine role for intravascular imaging (IVI) guidance for percutaneous coronary intervention (PCI) has long been favored by many of the technology’s researchers and enthusiasts. Now evidence from large, randomized trials may be catching up with such aspirations, though not without caveats.
One way IVI guidance may achieve that, the research suggests, albeit more speculatively, is by cutting risk for stent thrombosis, compared with the risk associated with angiography-only PCI.
The new studies, two large randomized IVI trials plus a meta-analysis of 20 such studies, were presented at the annual congress of the European Society of Cardiology.
In one, called ILUMIEN-4, PCI guided by optical coherence tomography (OCT) was associated with fewer procedural complications and better acute results – that is, larger post-PCI minimum stent area (MSA) – than in angiography-only procedures (P < .001). Poststenting MSA, an established predictor of clinical outcomes, was the primary imaging endpoint of the trial with almost 2,500 patients.
Yet the OCT group’s greater post-PCI MSA did not translate to reduced risk for the primary clinical endpoint of 2-year target-vessel failure. Among secondary endpoints, however, stent thrombosis at some point during the follow-up was 64% less likely (P = .02) with OCT guidance than angiography-only PCI.
ILUMIEN-4, despite its neutral clinical result, still “strongly advocates” for PCI guidance by OCT, at least among patients like those in the trial, said principal investigator Ziad Ali, MD, DPhil. He based that largely on the strategy’s greater postprocedure lumen areas in the trials, which are among “the strongest independent predictors for long term outcomes,” said Dr. Ali, of St. Francis Hospital & Heart Center, Roslyn, N.Y., at a press conference on IVI trials during the ESC Congress.
Selected complex lesion type
In contrast, the OCTOBER trial, presented at the sessions back to back with ILUMIEN-4, saw OCT guidance lead to better clinical outcomes than angiography alone after PCI of bifurcation lesions, which normally can be a special challenge for operators.
In the trial, which entered about 1,200 patients with such complex lesions, the 2-year risk for major adverse cardiac events (MACE) fell 60% after OCT-guided PCI, compared with angiography-only procedures (P = .035).
The finding is novel for showing that OCT guidance in bifurcation PCI can make a significant clinical difference, said OCTOBER investigator Niels R. Holm, MD, at the same media presentation on IVI trials.
“Multiple studies have shown that OCT allows for optimization of bifurcation PCI, and our results confirm that such optimization may improve the patient’s prognosis,” said Dr. Holm of Aarhus (Denmark) University Hospital.
ILUMIEN-4 and OCTOBER, both of which prespecified the Xience (Abbott) everolimus-eluting stent for the procedures, were published in the New England Journal of Medicine in tandem with their respective presentations at the ESC sessions.
Covering the spectrum
A meta-analysis presented at the same ESC session compared IVI using either OCT or intravascular ultrasound (IVUS) with angiography-only PCI across 20 randomized trials with a total of more than 12,000 patients.
Significant outcomes for IVI guidance versus angiography alone included a 31% drop in risk for target-lesion failure, the primary endpoint. And this study, as well, showed a steep 52% reduction in risk for in-stent thrombosis with the IVI-guided approach.
And “for the first time” in IVI studies, “we demonstrated reductions in all-myocardial-infarction and all-cause death, the latter by 25%,” Gregg Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in presenting the meta-analysis. Dr. Stone is also the ILUMIEN-4 study chairperson.
“The routine use of OCT or IVUS to guide most PCI procedures will substantially improve patient event-free survival,” he predicted, “enhancing both the long-term safety and effectiveness of the procedure.”
Dr. Stone said that IVI guidance “should be standard of care, if not in all patients, then in most patients.” Part of the rationale: PCI is unlikely to be improved much further by incremental gains in drug-eluting stent design. “That technology has almost plateaued.” But there’s yet room for “substantially improved outcomes” from adjunctive treatments and techniques such as IVI guidance.
The 20 studies in the meta-analysis encompassed an array of patients and lesions both complex and noncomplex, Dr. Stone observed, including bifurcation lesions, chronic total occlusions, left-main coronary stenoses, and MI culprit lesions.
“They really covered the spectrum of PCI,” he said. “I’m not recommending that intravascular imaging be used in every single case. But I do think it should be used in the majority of patients” and be standard of care for PCI in left-main lesions and “complex coronary disease, high-risk patients, and high-risk lesions.”
Unique advantage
The IVI-guidance groups in both ILUMIEN-4 and the meta-analysis showed a significant drop in risk for stent thrombosis – that is, abrupt thrombotic vessel closure, which typically occurs in 1% or fewer PCI cases but can trigger an MI and pose a mortality risk up to 45%.
Those risk reductions are consistent with a unique IVI advantage: the ability to guide optimization of stent deployments. When formally presenting ILUMIEN-4 at the ESC sessions, Ali observed that IVUS and OCT imaging allows operators to identify and often correct less-than-ideal results of an initial stent delivery – such as residual gaps between stent struts and vessel wall – that may encroach on the lumen, with possible clinical consequences.
Such imaging, said Dr. Ali, “lets you identify tissue protrusions, malappositions, dissections, and untreated reference-segment disease” that may potentially trigger thrombosis. That makes a strong argument for giving IVI guidance a more common, perhaps even routine role in PCI procedures.
Selling routine IVI-guided PCI in practice
“I think the study results are quite clear,” said Deepak L. Bhatt, MD, MPH, as session comoderator following the OCTOBER presentation. “The challenge, though, will be convincing the average interventional cardiologist worldwide that it was specifically the imaging and not the extra care that the patient getting OCT also inherently receives.”
Did OCT’s better trial outcomes stem from IVI itself or from greater operator attentiveness to procedural results – such as, for example, more high-pressure expansions to optimize stent placement, “the sort of thing that tends to occur when invasive imaging is added on to just plain old angiography?” Dr. Bhatt asked of Lene N. Andreasen, MD, who had just presented the OCTOBER trial. “There’s no way of uncoupling the two things.”
What can be said, “at this point, to convince interventional cardiologists that the extra time, energy, expense, is truly indicated,” that the data are “sufficient to change global practice?” asked Dr. Bhatt, Mount Sinai Hospital and Icahn School of Medicine at Mount Sinai.
That remains an open question,” acknowledged Dr. Andreasen of Aarhus University Hospital. The best argument in favor of selective IVI-guided PCI is that “we actually see a clinical benefit” in the trials. “But of course, it comes with a cost. It comes with longer procedures and more contrast.” How clinical practice responds to the new data remains to be seen, she proposed.
ILUMIEN-4 and OCTOBER in detail
Conducted at 80 centers in 18 countries, ILUMIEN-4 randomly assigned patients with diabetes or complex coronary lesions to undergo PCI guided by OCT or using standard angiography only, 1,233 and 1,254 patients, respectively.
Post-PCI MSA averaged 5.72 mm2 with OCT guidance and 5.36 mm2 in the angiography-only group (P < .001).
Their rates of target-vessel failure at 2 years were not significantly different at 7.4% and 8.2%, respectively. The 2-year composite endpoint included cardiac death, target vessel–related MI, or ischemia-driven target-vessel revascularization.
Definite or probable stent thrombosis was observed over 2 years in 0.5% of the OCT group and 1.4% of those with angiography-only PCI (hazard ratio, 0.36; 95% confidence interval, 0.14-0.91; P = .02) favoring OCT.
The OCTOBER trial, conducted at 38 centers in Europe, entered 1201 patients with stable angina or acute coronary syndromes and angiographically identified complex bifurcation lesions. They involved the left-main coronary artery in about one-fifth of cases.
Patients were randomly assigned to bifurcation PCI guided by OCT or under standard angiography, 600 and 601 patients, respectively. Rates for procedure-related complications were similar at 6.8% and 5.7%, respectively.
Over a median of 2 years, 10.1% of the OCT group and 14.1% of angiography-only patients developed a MACE event, including cardiac death, target-lesion MI, or ischemia-driven target-lesion revascularization. The adjusted HR was 0.71 (95% CI, 0.51-0.98; P = .035) in favor of OCT.
Meta-analysis, trials to date
The meta-analysis presented by Dr. Stone included ILUMIEN-4, OCTOBER, and 18 earlier outcomes trials comparing PCI guided by IVI, either OCT or IVUS, and angiography-only PCI. It covered 12,428 patients with chronic or acute coronary disease and followed them a mean of 26 months; the longest follow-up was 5 years. They were assigned to IVI-guided or angiography-only PCI, 7,038 and 5,390 patients, respectively.
Dr. Stone and colleagues conducted a network meta-analysis of the 20 studies, that is, a combined analysis that allowed both direct and indirect comparisons of standard angiography-only procedures to each of the other studied comparator interventions including OCT, IVUS, and either OCT or IVUS. They then derived network-estimate odds ratios for IVI-guided PCI vs angiography-only procedures.
“Hopefully, this will impact the guidelines,” Dr. Stone said of the meta-analysis. Procedures guided by IVI might become more common in clinical practice if they were to garner a Class-I guideline recommendation, the strongest recommendation category.
“That would make a difference, but we’d also need to work to remove impediments to increasing intravascular imaging guidance” for most patients undergoing PCI, he said, referring to challenges in obtaining reimbursement for IVI-guided PCI and training enough operators to handle the projected demand.
ILUMIEN-4 was funded by Abbott. OCTOBER was supported by grants from Abbott Vascular, St. Jude Medical, and Aarhus University. The network meta-analysis received statistical support from Abbott. Dr. Ali disclosed institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems, Medtronic, the National Institutes of Health, Opsens Medical, Philips, and Teleflex; consulting fees from Astra Zeneca, Philips, Shockwave; and holding equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr. Holm and Dr. Bhatt reported numerous conflicts of interest. Dr. Andreasen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A routine role for intravascular imaging (IVI) guidance for percutaneous coronary intervention (PCI) has long been favored by many of the technology’s researchers and enthusiasts. Now evidence from large, randomized trials may be catching up with such aspirations, though not without caveats.
One way IVI guidance may achieve that, the research suggests, albeit more speculatively, is by cutting risk for stent thrombosis, compared with the risk associated with angiography-only PCI.
The new studies, two large randomized IVI trials plus a meta-analysis of 20 such studies, were presented at the annual congress of the European Society of Cardiology.
In one, called ILUMIEN-4, PCI guided by optical coherence tomography (OCT) was associated with fewer procedural complications and better acute results – that is, larger post-PCI minimum stent area (MSA) – than in angiography-only procedures (P < .001). Poststenting MSA, an established predictor of clinical outcomes, was the primary imaging endpoint of the trial with almost 2,500 patients.
Yet the OCT group’s greater post-PCI MSA did not translate to reduced risk for the primary clinical endpoint of 2-year target-vessel failure. Among secondary endpoints, however, stent thrombosis at some point during the follow-up was 64% less likely (P = .02) with OCT guidance than angiography-only PCI.
ILUMIEN-4, despite its neutral clinical result, still “strongly advocates” for PCI guidance by OCT, at least among patients like those in the trial, said principal investigator Ziad Ali, MD, DPhil. He based that largely on the strategy’s greater postprocedure lumen areas in the trials, which are among “the strongest independent predictors for long term outcomes,” said Dr. Ali, of St. Francis Hospital & Heart Center, Roslyn, N.Y., at a press conference on IVI trials during the ESC Congress.
Selected complex lesion type
In contrast, the OCTOBER trial, presented at the sessions back to back with ILUMIEN-4, saw OCT guidance lead to better clinical outcomes than angiography alone after PCI of bifurcation lesions, which normally can be a special challenge for operators.
In the trial, which entered about 1,200 patients with such complex lesions, the 2-year risk for major adverse cardiac events (MACE) fell 60% after OCT-guided PCI, compared with angiography-only procedures (P = .035).
The finding is novel for showing that OCT guidance in bifurcation PCI can make a significant clinical difference, said OCTOBER investigator Niels R. Holm, MD, at the same media presentation on IVI trials.
“Multiple studies have shown that OCT allows for optimization of bifurcation PCI, and our results confirm that such optimization may improve the patient’s prognosis,” said Dr. Holm of Aarhus (Denmark) University Hospital.
ILUMIEN-4 and OCTOBER, both of which prespecified the Xience (Abbott) everolimus-eluting stent for the procedures, were published in the New England Journal of Medicine in tandem with their respective presentations at the ESC sessions.
Covering the spectrum
A meta-analysis presented at the same ESC session compared IVI using either OCT or intravascular ultrasound (IVUS) with angiography-only PCI across 20 randomized trials with a total of more than 12,000 patients.
Significant outcomes for IVI guidance versus angiography alone included a 31% drop in risk for target-lesion failure, the primary endpoint. And this study, as well, showed a steep 52% reduction in risk for in-stent thrombosis with the IVI-guided approach.
And “for the first time” in IVI studies, “we demonstrated reductions in all-myocardial-infarction and all-cause death, the latter by 25%,” Gregg Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in presenting the meta-analysis. Dr. Stone is also the ILUMIEN-4 study chairperson.
“The routine use of OCT or IVUS to guide most PCI procedures will substantially improve patient event-free survival,” he predicted, “enhancing both the long-term safety and effectiveness of the procedure.”
Dr. Stone said that IVI guidance “should be standard of care, if not in all patients, then in most patients.” Part of the rationale: PCI is unlikely to be improved much further by incremental gains in drug-eluting stent design. “That technology has almost plateaued.” But there’s yet room for “substantially improved outcomes” from adjunctive treatments and techniques such as IVI guidance.
The 20 studies in the meta-analysis encompassed an array of patients and lesions both complex and noncomplex, Dr. Stone observed, including bifurcation lesions, chronic total occlusions, left-main coronary stenoses, and MI culprit lesions.
“They really covered the spectrum of PCI,” he said. “I’m not recommending that intravascular imaging be used in every single case. But I do think it should be used in the majority of patients” and be standard of care for PCI in left-main lesions and “complex coronary disease, high-risk patients, and high-risk lesions.”
Unique advantage
The IVI-guidance groups in both ILUMIEN-4 and the meta-analysis showed a significant drop in risk for stent thrombosis – that is, abrupt thrombotic vessel closure, which typically occurs in 1% or fewer PCI cases but can trigger an MI and pose a mortality risk up to 45%.
Those risk reductions are consistent with a unique IVI advantage: the ability to guide optimization of stent deployments. When formally presenting ILUMIEN-4 at the ESC sessions, Ali observed that IVUS and OCT imaging allows operators to identify and often correct less-than-ideal results of an initial stent delivery – such as residual gaps between stent struts and vessel wall – that may encroach on the lumen, with possible clinical consequences.
Such imaging, said Dr. Ali, “lets you identify tissue protrusions, malappositions, dissections, and untreated reference-segment disease” that may potentially trigger thrombosis. That makes a strong argument for giving IVI guidance a more common, perhaps even routine role in PCI procedures.
Selling routine IVI-guided PCI in practice
“I think the study results are quite clear,” said Deepak L. Bhatt, MD, MPH, as session comoderator following the OCTOBER presentation. “The challenge, though, will be convincing the average interventional cardiologist worldwide that it was specifically the imaging and not the extra care that the patient getting OCT also inherently receives.”
Did OCT’s better trial outcomes stem from IVI itself or from greater operator attentiveness to procedural results – such as, for example, more high-pressure expansions to optimize stent placement, “the sort of thing that tends to occur when invasive imaging is added on to just plain old angiography?” Dr. Bhatt asked of Lene N. Andreasen, MD, who had just presented the OCTOBER trial. “There’s no way of uncoupling the two things.”
What can be said, “at this point, to convince interventional cardiologists that the extra time, energy, expense, is truly indicated,” that the data are “sufficient to change global practice?” asked Dr. Bhatt, Mount Sinai Hospital and Icahn School of Medicine at Mount Sinai.
That remains an open question,” acknowledged Dr. Andreasen of Aarhus University Hospital. The best argument in favor of selective IVI-guided PCI is that “we actually see a clinical benefit” in the trials. “But of course, it comes with a cost. It comes with longer procedures and more contrast.” How clinical practice responds to the new data remains to be seen, she proposed.
ILUMIEN-4 and OCTOBER in detail
Conducted at 80 centers in 18 countries, ILUMIEN-4 randomly assigned patients with diabetes or complex coronary lesions to undergo PCI guided by OCT or using standard angiography only, 1,233 and 1,254 patients, respectively.
Post-PCI MSA averaged 5.72 mm2 with OCT guidance and 5.36 mm2 in the angiography-only group (P < .001).
Their rates of target-vessel failure at 2 years were not significantly different at 7.4% and 8.2%, respectively. The 2-year composite endpoint included cardiac death, target vessel–related MI, or ischemia-driven target-vessel revascularization.
Definite or probable stent thrombosis was observed over 2 years in 0.5% of the OCT group and 1.4% of those with angiography-only PCI (hazard ratio, 0.36; 95% confidence interval, 0.14-0.91; P = .02) favoring OCT.
The OCTOBER trial, conducted at 38 centers in Europe, entered 1201 patients with stable angina or acute coronary syndromes and angiographically identified complex bifurcation lesions. They involved the left-main coronary artery in about one-fifth of cases.
Patients were randomly assigned to bifurcation PCI guided by OCT or under standard angiography, 600 and 601 patients, respectively. Rates for procedure-related complications were similar at 6.8% and 5.7%, respectively.
Over a median of 2 years, 10.1% of the OCT group and 14.1% of angiography-only patients developed a MACE event, including cardiac death, target-lesion MI, or ischemia-driven target-lesion revascularization. The adjusted HR was 0.71 (95% CI, 0.51-0.98; P = .035) in favor of OCT.
Meta-analysis, trials to date
The meta-analysis presented by Dr. Stone included ILUMIEN-4, OCTOBER, and 18 earlier outcomes trials comparing PCI guided by IVI, either OCT or IVUS, and angiography-only PCI. It covered 12,428 patients with chronic or acute coronary disease and followed them a mean of 26 months; the longest follow-up was 5 years. They were assigned to IVI-guided or angiography-only PCI, 7,038 and 5,390 patients, respectively.
Dr. Stone and colleagues conducted a network meta-analysis of the 20 studies, that is, a combined analysis that allowed both direct and indirect comparisons of standard angiography-only procedures to each of the other studied comparator interventions including OCT, IVUS, and either OCT or IVUS. They then derived network-estimate odds ratios for IVI-guided PCI vs angiography-only procedures.
“Hopefully, this will impact the guidelines,” Dr. Stone said of the meta-analysis. Procedures guided by IVI might become more common in clinical practice if they were to garner a Class-I guideline recommendation, the strongest recommendation category.
“That would make a difference, but we’d also need to work to remove impediments to increasing intravascular imaging guidance” for most patients undergoing PCI, he said, referring to challenges in obtaining reimbursement for IVI-guided PCI and training enough operators to handle the projected demand.
ILUMIEN-4 was funded by Abbott. OCTOBER was supported by grants from Abbott Vascular, St. Jude Medical, and Aarhus University. The network meta-analysis received statistical support from Abbott. Dr. Ali disclosed institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems, Medtronic, the National Institutes of Health, Opsens Medical, Philips, and Teleflex; consulting fees from Astra Zeneca, Philips, Shockwave; and holding equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr. Holm and Dr. Bhatt reported numerous conflicts of interest. Dr. Andreasen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Recent leaps in heart failure therapy spur ESC guideline–focused update
Two years is a long time in the world of heart failure (HF) management, enough to see publication of more than a dozen studies with insights that would supplant and expand key sections of a far-reaching European Society of Cardiology (ESC) clinical practice guideline on HF unveiled in 2021.
“Back in 2021, we had three and a half decades of data to consider,” but recent years have seen “an amazing amount of progress” that has necessitated some adjustments and key additions, including several Class I recommendations, observed Roy S. Gardner, MBChB, MD, Golden Jubilee National Hospital, Clydebank, United Kingdom.
, which Dr. Gardner helped unveil over several days at the annual congress of the European Society of Cardiology, held in Amsterdam.
The new document was also published in the European Heart Journal during the ESC sessions. Dr. Gardner is a co-author on both the 2021 and 2023 documents.
The task force that was charged with the focused update’s development “considered a large number of trials across the spectrum of acute chronic heart failure and the comorbidities associated with it,” Ultimately, it considered only those with “results that would lead to new or changed Class I or Class IIa recommendations,” noted Theresa A. McDonagh, MD, during the ESC sessions.
Dr. McDonagh, of King’s College Hospital, London, chaired the task force and led the document’s list of authors along with Marco Metra, MD, University of Brescia (Italy).
Chronic HF management
The 2021 document’s “beautiful algorithm” on managing HF with reduced ejection fraction, that is HF with an LVEF less than 40%, had helped enshrine the expeditious uptitration of the “four pillars” of drug therapy as a top management goal. That remains unchanged in the focused update, Dr. Gardner noted.
But the new document gives a boost to recommendations for HF with mildly reduced ejection fraction (HFmrEF), characterized by an LVEF greater than 40% to less than 50%. For that, the 2021 document recommended three of the four pillars of HF medical therapy: beta blockers, mineralocorticoid receptor antagonists (MRA), renin-angiotensin system (RAS) inhibitors.
The focused update, however, adds the fourth pillar – SGLT2 inhibitors – to core therapy for both HFmrEF and HF with preserved ejection fraction (HFpEF), the latter defined by an LVEF greater than 50%. Publication of trials supporting those new recommendations had narrowly missed availability for the 2021 document.
EMPEROR-Preserved, for example, was published during the same ESC 2021 sessions that introduced the 2021 guidelines. Its patients with HFpEF (which at the time included patients meeting the current definition of HFmrEF) assigned to the SGLT2 inhibitor empagliflozin (Jardiance) showed a 21% reduction in risk for a composite primary endpoint that was driven by the HF-hospitalization component.
“This wasn’t a fluke finding,” Dr. Gardner said, as the following year saw publication of the DELIVER trial, which resembled EMPEROR-Preserved in design and outcomes using the SGLT2 inhibitor dapagliflozin (Farxiga).
The two trials, backed up by meta-analyses that also included DAPA-HF and other studies, suggested as well that the two SGLT2 inhibitors “work across the spectrum of ejection fraction,” Dr. Gardner said.
The 2023 focused update indicates an SGLT2 inhibitor, either empagliflozin or dapagliflozin, for patients with either HFmrEF or HFpEF to reduce the risk of HF hospitalization or cardiovascular death. Both recommendations are of Class I, level of evidence A.
The new indications make SGLT2 inhibitors and diuretics (as needed for fluid retention) the only drugs for HFmrEF or HFpEF with a Class I recommendation. Previously established “rather weaker” Class IIb recommendation for RAS inhibitors, MRAs, and beta blockers that had been “based on subgroup analyses of neutral trials” remained unchanged in the focused update, Dr. McDonagh noted.
Patients hospitalized with HF
The 2021 guidelines had recommended that patients hospitalized with acute HF be started on evidence-based meds before discharge and that they return for evaluation 1 to 2 weeks after discharge. But the recommendation was unsupported by randomized trials.
That changed with the 2022 publication of STRONG-HF, in which a strategy of early and rapid uptitration of guideline-directed meds, initiated predischarge regardless of LVEF, led to a one-third reduced 6-month risk for death or HF readmission.
Based primarily on STRONG-HF, the focused update recommends “an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks after hospitalization” to reduce readmission and mortality: Class I, level of evidence B.
“There was a large consensus around this recommendation,” said STRONG-HF principal investigator Alexandre Mebazaa, MD, PhD, a co-author of both the 2021 and 2023 documents. Conducted before the advent of the four pillars of drug therapy, sometimes called quartet therapy, the trial’s requirement for evidence-based meds didn’t include SGLT2 inhibitors.
The new focused update considers the new status of those agents, especially with regard to their benefits independent of LVEF. So, it completed the quartet by adding empagliflozin or dapagliflozin to the agents that should be initiated predischarge, observed Dr. Mebazaa, University Hospitals Saint Louis‐Lariboisière, Paris, at the focused-update’s ESC 2023 sessions.
The new document also follows STRONG-HF with its emphasis on “frequent and careful follow-up” by recommending certain clinical and laboratory evaluations known to be prognostic in HF. They include congestion status, blood pressure, heart rate, natriuretic peptide (NT-proBNP) and potassium levels and estimated glomerular filtration rate.
Dr. Mebazaa stressed the importance of monitoring NT-proBNP after discharge. “What we saw in STRONG-HF is that sometimes the clinical signs do not necessarily tell you that the patient is still congested.”
After discharge, he said, NT-proBNP levels “should only go down.” So, knowing whether NT-proBNP levels “are stable or increasing” during the med optimization process can help guide diuretic dosing.
HF with comorbidities
The new document includes two new Class I recommendations for patients with HF and both type 2 diabetes and chronic kidney disease based on several recent randomized trials and meta-analyses.
The focused update recommends SGLT2 inhibitors as well as the selective, non-steroidal MRA finerenone (Kerendia) in HF patients with CKD and type-2 diabetes. Both Class I recommendations are supported by a level of evidence A.
The SGLT2 indication is based on DAPA-CKD and EMPA-KIDNEY plus meta-analyses that included those trials along with others. The recommendation for finerenone derives from the FIDELIO-DKD and FIGARO-DKD trials and a pooled analysis of the two studies.
The 2023 focused update also accounts for new clinical-trial insights for patients with HF and iron deficiency. The 2021 document featured recommendations for the diagnosis and iron-repletion therapy in such cases, but only as Class IIa or at lower low levels of evidence. The focused update considers more recent studies, especially IRONMAN and some meta-analyses.
The 2023 document indicates intravenous iron supplementation for symptomatic patients with iron deficiency and either HFrEF or HFmrEF to improve symptoms and quality of life (Class I, level of evidence A), and says it should be considered (Class IIa, level of evidence A) to reduce risk for HF hospitalization.
When the task force assembled to plan the 2023 focused update, Dr. Gardner observed, “the first thing we thought about was the nomenclature around the phenotyping of heart failure.”
Although the 2021 guidelines relied fundamentally on the distinctions between HFrEF, HFmrEF, and HFpEF, it had become apparent to some in the field that some meds, especially the SGLT2 inhibitors, were obscuring their LVEF-based boundaries, at least with respect to drug therapy.
The 2023 document’s developers, Dr. Gardner said, seriously considered changing the three categories to two, that is HFrEF and – to account for all other heart failure – HF with normal ejection fraction (HFnEF).
That didn’t happen, although the proposal was popular within the task force. Any changes to the 2021 document would require a 75% consensus on the matter, Dr. Gardner explained. When the task force took a vote on whether to change the nomenclature, he said, 71% favored the proposal.
Disclosures for members of the task force can be found in a supplement to the published 2023 Focused Update.
A version of this article first appeared on Medscape.com.
Two years is a long time in the world of heart failure (HF) management, enough to see publication of more than a dozen studies with insights that would supplant and expand key sections of a far-reaching European Society of Cardiology (ESC) clinical practice guideline on HF unveiled in 2021.
“Back in 2021, we had three and a half decades of data to consider,” but recent years have seen “an amazing amount of progress” that has necessitated some adjustments and key additions, including several Class I recommendations, observed Roy S. Gardner, MBChB, MD, Golden Jubilee National Hospital, Clydebank, United Kingdom.
, which Dr. Gardner helped unveil over several days at the annual congress of the European Society of Cardiology, held in Amsterdam.
The new document was also published in the European Heart Journal during the ESC sessions. Dr. Gardner is a co-author on both the 2021 and 2023 documents.
The task force that was charged with the focused update’s development “considered a large number of trials across the spectrum of acute chronic heart failure and the comorbidities associated with it,” Ultimately, it considered only those with “results that would lead to new or changed Class I or Class IIa recommendations,” noted Theresa A. McDonagh, MD, during the ESC sessions.
Dr. McDonagh, of King’s College Hospital, London, chaired the task force and led the document’s list of authors along with Marco Metra, MD, University of Brescia (Italy).
Chronic HF management
The 2021 document’s “beautiful algorithm” on managing HF with reduced ejection fraction, that is HF with an LVEF less than 40%, had helped enshrine the expeditious uptitration of the “four pillars” of drug therapy as a top management goal. That remains unchanged in the focused update, Dr. Gardner noted.
But the new document gives a boost to recommendations for HF with mildly reduced ejection fraction (HFmrEF), characterized by an LVEF greater than 40% to less than 50%. For that, the 2021 document recommended three of the four pillars of HF medical therapy: beta blockers, mineralocorticoid receptor antagonists (MRA), renin-angiotensin system (RAS) inhibitors.
The focused update, however, adds the fourth pillar – SGLT2 inhibitors – to core therapy for both HFmrEF and HF with preserved ejection fraction (HFpEF), the latter defined by an LVEF greater than 50%. Publication of trials supporting those new recommendations had narrowly missed availability for the 2021 document.
EMPEROR-Preserved, for example, was published during the same ESC 2021 sessions that introduced the 2021 guidelines. Its patients with HFpEF (which at the time included patients meeting the current definition of HFmrEF) assigned to the SGLT2 inhibitor empagliflozin (Jardiance) showed a 21% reduction in risk for a composite primary endpoint that was driven by the HF-hospitalization component.
“This wasn’t a fluke finding,” Dr. Gardner said, as the following year saw publication of the DELIVER trial, which resembled EMPEROR-Preserved in design and outcomes using the SGLT2 inhibitor dapagliflozin (Farxiga).
The two trials, backed up by meta-analyses that also included DAPA-HF and other studies, suggested as well that the two SGLT2 inhibitors “work across the spectrum of ejection fraction,” Dr. Gardner said.
The 2023 focused update indicates an SGLT2 inhibitor, either empagliflozin or dapagliflozin, for patients with either HFmrEF or HFpEF to reduce the risk of HF hospitalization or cardiovascular death. Both recommendations are of Class I, level of evidence A.
The new indications make SGLT2 inhibitors and diuretics (as needed for fluid retention) the only drugs for HFmrEF or HFpEF with a Class I recommendation. Previously established “rather weaker” Class IIb recommendation for RAS inhibitors, MRAs, and beta blockers that had been “based on subgroup analyses of neutral trials” remained unchanged in the focused update, Dr. McDonagh noted.
Patients hospitalized with HF
The 2021 guidelines had recommended that patients hospitalized with acute HF be started on evidence-based meds before discharge and that they return for evaluation 1 to 2 weeks after discharge. But the recommendation was unsupported by randomized trials.
That changed with the 2022 publication of STRONG-HF, in which a strategy of early and rapid uptitration of guideline-directed meds, initiated predischarge regardless of LVEF, led to a one-third reduced 6-month risk for death or HF readmission.
Based primarily on STRONG-HF, the focused update recommends “an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks after hospitalization” to reduce readmission and mortality: Class I, level of evidence B.
“There was a large consensus around this recommendation,” said STRONG-HF principal investigator Alexandre Mebazaa, MD, PhD, a co-author of both the 2021 and 2023 documents. Conducted before the advent of the four pillars of drug therapy, sometimes called quartet therapy, the trial’s requirement for evidence-based meds didn’t include SGLT2 inhibitors.
The new focused update considers the new status of those agents, especially with regard to their benefits independent of LVEF. So, it completed the quartet by adding empagliflozin or dapagliflozin to the agents that should be initiated predischarge, observed Dr. Mebazaa, University Hospitals Saint Louis‐Lariboisière, Paris, at the focused-update’s ESC 2023 sessions.
The new document also follows STRONG-HF with its emphasis on “frequent and careful follow-up” by recommending certain clinical and laboratory evaluations known to be prognostic in HF. They include congestion status, blood pressure, heart rate, natriuretic peptide (NT-proBNP) and potassium levels and estimated glomerular filtration rate.
Dr. Mebazaa stressed the importance of monitoring NT-proBNP after discharge. “What we saw in STRONG-HF is that sometimes the clinical signs do not necessarily tell you that the patient is still congested.”
After discharge, he said, NT-proBNP levels “should only go down.” So, knowing whether NT-proBNP levels “are stable or increasing” during the med optimization process can help guide diuretic dosing.
HF with comorbidities
The new document includes two new Class I recommendations for patients with HF and both type 2 diabetes and chronic kidney disease based on several recent randomized trials and meta-analyses.
The focused update recommends SGLT2 inhibitors as well as the selective, non-steroidal MRA finerenone (Kerendia) in HF patients with CKD and type-2 diabetes. Both Class I recommendations are supported by a level of evidence A.
The SGLT2 indication is based on DAPA-CKD and EMPA-KIDNEY plus meta-analyses that included those trials along with others. The recommendation for finerenone derives from the FIDELIO-DKD and FIGARO-DKD trials and a pooled analysis of the two studies.
The 2023 focused update also accounts for new clinical-trial insights for patients with HF and iron deficiency. The 2021 document featured recommendations for the diagnosis and iron-repletion therapy in such cases, but only as Class IIa or at lower low levels of evidence. The focused update considers more recent studies, especially IRONMAN and some meta-analyses.
The 2023 document indicates intravenous iron supplementation for symptomatic patients with iron deficiency and either HFrEF or HFmrEF to improve symptoms and quality of life (Class I, level of evidence A), and says it should be considered (Class IIa, level of evidence A) to reduce risk for HF hospitalization.
When the task force assembled to plan the 2023 focused update, Dr. Gardner observed, “the first thing we thought about was the nomenclature around the phenotyping of heart failure.”
Although the 2021 guidelines relied fundamentally on the distinctions between HFrEF, HFmrEF, and HFpEF, it had become apparent to some in the field that some meds, especially the SGLT2 inhibitors, were obscuring their LVEF-based boundaries, at least with respect to drug therapy.
The 2023 document’s developers, Dr. Gardner said, seriously considered changing the three categories to two, that is HFrEF and – to account for all other heart failure – HF with normal ejection fraction (HFnEF).
That didn’t happen, although the proposal was popular within the task force. Any changes to the 2021 document would require a 75% consensus on the matter, Dr. Gardner explained. When the task force took a vote on whether to change the nomenclature, he said, 71% favored the proposal.
Disclosures for members of the task force can be found in a supplement to the published 2023 Focused Update.
A version of this article first appeared on Medscape.com.
Two years is a long time in the world of heart failure (HF) management, enough to see publication of more than a dozen studies with insights that would supplant and expand key sections of a far-reaching European Society of Cardiology (ESC) clinical practice guideline on HF unveiled in 2021.
“Back in 2021, we had three and a half decades of data to consider,” but recent years have seen “an amazing amount of progress” that has necessitated some adjustments and key additions, including several Class I recommendations, observed Roy S. Gardner, MBChB, MD, Golden Jubilee National Hospital, Clydebank, United Kingdom.
, which Dr. Gardner helped unveil over several days at the annual congress of the European Society of Cardiology, held in Amsterdam.
The new document was also published in the European Heart Journal during the ESC sessions. Dr. Gardner is a co-author on both the 2021 and 2023 documents.
The task force that was charged with the focused update’s development “considered a large number of trials across the spectrum of acute chronic heart failure and the comorbidities associated with it,” Ultimately, it considered only those with “results that would lead to new or changed Class I or Class IIa recommendations,” noted Theresa A. McDonagh, MD, during the ESC sessions.
Dr. McDonagh, of King’s College Hospital, London, chaired the task force and led the document’s list of authors along with Marco Metra, MD, University of Brescia (Italy).
Chronic HF management
The 2021 document’s “beautiful algorithm” on managing HF with reduced ejection fraction, that is HF with an LVEF less than 40%, had helped enshrine the expeditious uptitration of the “four pillars” of drug therapy as a top management goal. That remains unchanged in the focused update, Dr. Gardner noted.
But the new document gives a boost to recommendations for HF with mildly reduced ejection fraction (HFmrEF), characterized by an LVEF greater than 40% to less than 50%. For that, the 2021 document recommended three of the four pillars of HF medical therapy: beta blockers, mineralocorticoid receptor antagonists (MRA), renin-angiotensin system (RAS) inhibitors.
The focused update, however, adds the fourth pillar – SGLT2 inhibitors – to core therapy for both HFmrEF and HF with preserved ejection fraction (HFpEF), the latter defined by an LVEF greater than 50%. Publication of trials supporting those new recommendations had narrowly missed availability for the 2021 document.
EMPEROR-Preserved, for example, was published during the same ESC 2021 sessions that introduced the 2021 guidelines. Its patients with HFpEF (which at the time included patients meeting the current definition of HFmrEF) assigned to the SGLT2 inhibitor empagliflozin (Jardiance) showed a 21% reduction in risk for a composite primary endpoint that was driven by the HF-hospitalization component.
“This wasn’t a fluke finding,” Dr. Gardner said, as the following year saw publication of the DELIVER trial, which resembled EMPEROR-Preserved in design and outcomes using the SGLT2 inhibitor dapagliflozin (Farxiga).
The two trials, backed up by meta-analyses that also included DAPA-HF and other studies, suggested as well that the two SGLT2 inhibitors “work across the spectrum of ejection fraction,” Dr. Gardner said.
The 2023 focused update indicates an SGLT2 inhibitor, either empagliflozin or dapagliflozin, for patients with either HFmrEF or HFpEF to reduce the risk of HF hospitalization or cardiovascular death. Both recommendations are of Class I, level of evidence A.
The new indications make SGLT2 inhibitors and diuretics (as needed for fluid retention) the only drugs for HFmrEF or HFpEF with a Class I recommendation. Previously established “rather weaker” Class IIb recommendation for RAS inhibitors, MRAs, and beta blockers that had been “based on subgroup analyses of neutral trials” remained unchanged in the focused update, Dr. McDonagh noted.
Patients hospitalized with HF
The 2021 guidelines had recommended that patients hospitalized with acute HF be started on evidence-based meds before discharge and that they return for evaluation 1 to 2 weeks after discharge. But the recommendation was unsupported by randomized trials.
That changed with the 2022 publication of STRONG-HF, in which a strategy of early and rapid uptitration of guideline-directed meds, initiated predischarge regardless of LVEF, led to a one-third reduced 6-month risk for death or HF readmission.
Based primarily on STRONG-HF, the focused update recommends “an intensive strategy of initiation and rapid up-titration of evidence-based treatment before discharge and during frequent and careful follow-up visits in the first 6 weeks after hospitalization” to reduce readmission and mortality: Class I, level of evidence B.
“There was a large consensus around this recommendation,” said STRONG-HF principal investigator Alexandre Mebazaa, MD, PhD, a co-author of both the 2021 and 2023 documents. Conducted before the advent of the four pillars of drug therapy, sometimes called quartet therapy, the trial’s requirement for evidence-based meds didn’t include SGLT2 inhibitors.
The new focused update considers the new status of those agents, especially with regard to their benefits independent of LVEF. So, it completed the quartet by adding empagliflozin or dapagliflozin to the agents that should be initiated predischarge, observed Dr. Mebazaa, University Hospitals Saint Louis‐Lariboisière, Paris, at the focused-update’s ESC 2023 sessions.
The new document also follows STRONG-HF with its emphasis on “frequent and careful follow-up” by recommending certain clinical and laboratory evaluations known to be prognostic in HF. They include congestion status, blood pressure, heart rate, natriuretic peptide (NT-proBNP) and potassium levels and estimated glomerular filtration rate.
Dr. Mebazaa stressed the importance of monitoring NT-proBNP after discharge. “What we saw in STRONG-HF is that sometimes the clinical signs do not necessarily tell you that the patient is still congested.”
After discharge, he said, NT-proBNP levels “should only go down.” So, knowing whether NT-proBNP levels “are stable or increasing” during the med optimization process can help guide diuretic dosing.
HF with comorbidities
The new document includes two new Class I recommendations for patients with HF and both type 2 diabetes and chronic kidney disease based on several recent randomized trials and meta-analyses.
The focused update recommends SGLT2 inhibitors as well as the selective, non-steroidal MRA finerenone (Kerendia) in HF patients with CKD and type-2 diabetes. Both Class I recommendations are supported by a level of evidence A.
The SGLT2 indication is based on DAPA-CKD and EMPA-KIDNEY plus meta-analyses that included those trials along with others. The recommendation for finerenone derives from the FIDELIO-DKD and FIGARO-DKD trials and a pooled analysis of the two studies.
The 2023 focused update also accounts for new clinical-trial insights for patients with HF and iron deficiency. The 2021 document featured recommendations for the diagnosis and iron-repletion therapy in such cases, but only as Class IIa or at lower low levels of evidence. The focused update considers more recent studies, especially IRONMAN and some meta-analyses.
The 2023 document indicates intravenous iron supplementation for symptomatic patients with iron deficiency and either HFrEF or HFmrEF to improve symptoms and quality of life (Class I, level of evidence A), and says it should be considered (Class IIa, level of evidence A) to reduce risk for HF hospitalization.
When the task force assembled to plan the 2023 focused update, Dr. Gardner observed, “the first thing we thought about was the nomenclature around the phenotyping of heart failure.”
Although the 2021 guidelines relied fundamentally on the distinctions between HFrEF, HFmrEF, and HFpEF, it had become apparent to some in the field that some meds, especially the SGLT2 inhibitors, were obscuring their LVEF-based boundaries, at least with respect to drug therapy.
The 2023 document’s developers, Dr. Gardner said, seriously considered changing the three categories to two, that is HFrEF and – to account for all other heart failure – HF with normal ejection fraction (HFnEF).
That didn’t happen, although the proposal was popular within the task force. Any changes to the 2021 document would require a 75% consensus on the matter, Dr. Gardner explained. When the task force took a vote on whether to change the nomenclature, he said, 71% favored the proposal.
Disclosures for members of the task force can be found in a supplement to the published 2023 Focused Update.
A version of this article first appeared on Medscape.com.
FROM THE ESC CONGRESS 2023
Lead exposure still a global health burden
TOPLINE:
Globally, lead exposure is linked to more than 5.5 million adult cardiovascular deaths in 2019, as well as loss of 765 million intelligence quotient (IQ) points in children younger than 5 years, which cost U.S. $6 trillion in lost productivity, new research suggests.
METHODOLOGY:
- Global lead exposure has declined substantially since leaded gasoline was phased out, but several sources of lead remain, resulting in adverse health and economic effects, particularly in low- and middle-income countries (LMICs).
- Estimates of cardiovascular disease (CVD) deaths from lead exposure have been limited to effects of increased blood pressure, but studies show that lead exposure has cardiovascular impacts through mechanisms other than hypertension.
- Drawing from various sources and studies, researchers estimated global blood lead levels and the impact of lead exposure on CVD mortality in 2019 among adults aged 25 years or older, IQ loss in children younger than 5 years, and the related economic costs.
TAKEAWAY:
- Researchers estimated that there were 5,545,000 (95% confidence interval, 2,305,000-8,271,000) cardiovascular deaths in adults from lead exposure in 2019, with as many as 90.2% of these deaths in LMICs; however, this estimate may be incomplete because it does not include the effect of lead exposure on CVD mortality mediated through hypertension.
- The estimated global IQ loss in children younger than 5 years due to lead exposure was 765 million (95% CI, 443 million-1,098 million) IQ points in 2019, 95.3% of which occurred in LMICs.
- These estimates place lead exposure on a par with ambient particulate matter and household air pollution combined, and ahead of unsafe household drinking water, sanitation, and handwashing, as an environmental risk factor.
- The estimated global cost of lead exposure from CVD mortality and IQ loss combined is U.S. $6.0 trillion (range, $2.6 trillion-9.0 trillion) in 2019, equivalent to 6.9% of the 2019 global gross domestic product.
IN PRACTICE:
Given the magnitude of the estimated health effects of lead exposure, particularly in LMICs, “it is imperative that nationally representative periodic blood lead level measurements be institutionalized,” write the authors, adding that these measurements could be incorporated into existing household surveys.
STUDY DETAILS:
The study was conducted by Bjorn Larsen, PhD, environmental economist and consultant to the World Bank, and Ernesto Sánchez-Triana. It was published online in The Lancet Planetary Health.
LIMITATIONS:
- Global blood lead level estimates may be inaccurate, given that measurements are absent for many countries.
- Certain income projections and income losses are uncertain.
- Because the study does not capture the detrimental effects of lead exposure other than IQ loss and CVD mortality, the estimates of global costs are conservative.
DISCLOSURES:
The study received support from the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Globally, lead exposure is linked to more than 5.5 million adult cardiovascular deaths in 2019, as well as loss of 765 million intelligence quotient (IQ) points in children younger than 5 years, which cost U.S. $6 trillion in lost productivity, new research suggests.
METHODOLOGY:
- Global lead exposure has declined substantially since leaded gasoline was phased out, but several sources of lead remain, resulting in adverse health and economic effects, particularly in low- and middle-income countries (LMICs).
- Estimates of cardiovascular disease (CVD) deaths from lead exposure have been limited to effects of increased blood pressure, but studies show that lead exposure has cardiovascular impacts through mechanisms other than hypertension.
- Drawing from various sources and studies, researchers estimated global blood lead levels and the impact of lead exposure on CVD mortality in 2019 among adults aged 25 years or older, IQ loss in children younger than 5 years, and the related economic costs.
TAKEAWAY:
- Researchers estimated that there were 5,545,000 (95% confidence interval, 2,305,000-8,271,000) cardiovascular deaths in adults from lead exposure in 2019, with as many as 90.2% of these deaths in LMICs; however, this estimate may be incomplete because it does not include the effect of lead exposure on CVD mortality mediated through hypertension.
- The estimated global IQ loss in children younger than 5 years due to lead exposure was 765 million (95% CI, 443 million-1,098 million) IQ points in 2019, 95.3% of which occurred in LMICs.
- These estimates place lead exposure on a par with ambient particulate matter and household air pollution combined, and ahead of unsafe household drinking water, sanitation, and handwashing, as an environmental risk factor.
- The estimated global cost of lead exposure from CVD mortality and IQ loss combined is U.S. $6.0 trillion (range, $2.6 trillion-9.0 trillion) in 2019, equivalent to 6.9% of the 2019 global gross domestic product.
IN PRACTICE:
Given the magnitude of the estimated health effects of lead exposure, particularly in LMICs, “it is imperative that nationally representative periodic blood lead level measurements be institutionalized,” write the authors, adding that these measurements could be incorporated into existing household surveys.
STUDY DETAILS:
The study was conducted by Bjorn Larsen, PhD, environmental economist and consultant to the World Bank, and Ernesto Sánchez-Triana. It was published online in The Lancet Planetary Health.
LIMITATIONS:
- Global blood lead level estimates may be inaccurate, given that measurements are absent for many countries.
- Certain income projections and income losses are uncertain.
- Because the study does not capture the detrimental effects of lead exposure other than IQ loss and CVD mortality, the estimates of global costs are conservative.
DISCLOSURES:
The study received support from the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
Globally, lead exposure is linked to more than 5.5 million adult cardiovascular deaths in 2019, as well as loss of 765 million intelligence quotient (IQ) points in children younger than 5 years, which cost U.S. $6 trillion in lost productivity, new research suggests.
METHODOLOGY:
- Global lead exposure has declined substantially since leaded gasoline was phased out, but several sources of lead remain, resulting in adverse health and economic effects, particularly in low- and middle-income countries (LMICs).
- Estimates of cardiovascular disease (CVD) deaths from lead exposure have been limited to effects of increased blood pressure, but studies show that lead exposure has cardiovascular impacts through mechanisms other than hypertension.
- Drawing from various sources and studies, researchers estimated global blood lead levels and the impact of lead exposure on CVD mortality in 2019 among adults aged 25 years or older, IQ loss in children younger than 5 years, and the related economic costs.
TAKEAWAY:
- Researchers estimated that there were 5,545,000 (95% confidence interval, 2,305,000-8,271,000) cardiovascular deaths in adults from lead exposure in 2019, with as many as 90.2% of these deaths in LMICs; however, this estimate may be incomplete because it does not include the effect of lead exposure on CVD mortality mediated through hypertension.
- The estimated global IQ loss in children younger than 5 years due to lead exposure was 765 million (95% CI, 443 million-1,098 million) IQ points in 2019, 95.3% of which occurred in LMICs.
- These estimates place lead exposure on a par with ambient particulate matter and household air pollution combined, and ahead of unsafe household drinking water, sanitation, and handwashing, as an environmental risk factor.
- The estimated global cost of lead exposure from CVD mortality and IQ loss combined is U.S. $6.0 trillion (range, $2.6 trillion-9.0 trillion) in 2019, equivalent to 6.9% of the 2019 global gross domestic product.
IN PRACTICE:
Given the magnitude of the estimated health effects of lead exposure, particularly in LMICs, “it is imperative that nationally representative periodic blood lead level measurements be institutionalized,” write the authors, adding that these measurements could be incorporated into existing household surveys.
STUDY DETAILS:
The study was conducted by Bjorn Larsen, PhD, environmental economist and consultant to the World Bank, and Ernesto Sánchez-Triana. It was published online in The Lancet Planetary Health.
LIMITATIONS:
- Global blood lead level estimates may be inaccurate, given that measurements are absent for many countries.
- Certain income projections and income losses are uncertain.
- Because the study does not capture the detrimental effects of lead exposure other than IQ loss and CVD mortality, the estimates of global costs are conservative.
DISCLOSURES:
The study received support from the Korea Green Growth Trust Fund and the World Bank’s Pollution Management and Environmental Health Program. The authors have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Your workplace is toxic: Can you make it better?
A physician in your office is hot-tempered, critical, and upsets both the physicians and staff. Two of your partners are arguing over a software vendor and refuse to compromise. One doctor’s spouse is the office manager and snipes at everyone; the lead partner micromanages and second-guesses other doctors’ treatment plans, and no one will stand up to her.
If your practice has similar scenarios, you’re likely dealing with your own anger, irritation, and dread at work. You’re struggling with a toxic practice atmosphere, and you must make changes – fast.
However, this isn’t easy, given that what goes on in a doctor’s office is “high consequence,” says Leonard J. Marcus, PhD, founding director of the program for health care negotiation and conflict resolution at the Harvard School of Public Health in Boston.
The two things that tend to plague medical practices most: A culture of fear and someone who is letting ego run the day-to-day, he says.
“Fear overwhelms any chance for good morale among colleagues,” says Dr. Marcus, who is also the coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration.” “In a work environment where the fear is overwhelming, the ego can take over, and someone at the practice becomes overly concerned about getting credit, taking control, ordering other people around, and deciding who is on top and who is on the bottom.”
Tension, stress, back-biting, and rudeness are also symptoms of a more significant problem, says Jes Montgomery, MD, a psychiatrist and medical director of APN Dallas, a mental health–focused practice.
“If you don’t get toxicity under control, it will blow the office apart,” Dr. Montgomery says.
1. Recognize the signs
Part of the problem with a toxic medical practice is that, culturally, we don’t treat mental health and burnout as real illnesses. “A physician who is depressed is not going to be melancholy or bursting into tears with patients,” Dr. Montgomery says. “They’ll get behind on paperwork, skip meals, or find that it’s difficult to sleep at night. Next, they’ll yell at the partners and staff, always be in a foul mood, and gripe about inconsequential things. Their behavior affects everyone.”
Dr. Montgomery says that physicians aren’t taught to ask for help, making it difficult to see what’s really going on when someone displays toxic behavior in the practice. If it’s a partner, take time to ask what’s going on. If it’s yourself, step back and see if you can ask someone for the help you need.
2. Have difficult conversations
This is tough for most of us, says Jeremy Pollack, PhD, CEO and founder of Pollack Peacebuilding Systems, a conflict resolution consulting firm. If a team member is hot-tempered, disrespectful, or talking to patients in an unproductive manner, see if you can have an effective conversation with that person. The tricky part is critiquing in a way that doesn’t make them feel defensive – and wanting to push back.
For a micromanaging office manager, for example, you could say something like,”You’re doing a great job with the inventory, but I need you to let the staff have some autonomy and not hover over every supply they use in the break room, so that people won’t feel resentful toward us.” Make it clear you’re a team, and this is a team challenge. “However, if a doctor feels like they’ve tried to communicate to that colleague and are still walking on eggshells, it’s time to try to get help from someone – perhaps a practice management organization,” says Dr. Pollack.
3. Open lines of communication
It’s critical to create a comfortable space to speak with your colleagues, says Marisa Garshick, MD, a dermatologist in private practice in New York. “Creating an environment where there is an open line of communication, whether it’s directly to somebody in charge or having a system where you can give feedback more privately or anonymously, is important so that tension doesn’t build.”
“Being a doctor is a social enterprise,” Dr. Marcus says. “The science of medicine is critically important, but patients and the other health care workers on your team are also critically important. In the long run, the most successful physicians pay attention to both. It’s a full package.”
4. Emphasize the positive
Instead of discussing things only when they go wrong, try optimism, Dr. Garshick said. When positive things happen, whether it’s an excellent patient encounter or the office did something really well together, highlight it so everyone has a sense of accomplishment. If a patient compliments a medical assistant or raves about a nurse, share those compliments with the employees so that not every encounter you have calls out problems and staff missteps.
Suppose partners have a conflict with one another or are arguing over something. In that case, you may need to mediate or invest in a meaningful intervention so people can reflect on the narrative they’re contributing to the culture.
5. Practice self-care
Finally, the work of a physician is exhausting, so it’s crucial to practice personal TLC. That may mean taking micro breaks, getting adequate sleep, maintaining a healthy diet, and exercising well and managing stress to maintain energy levels and patience.
“Sometimes, when I’m fed up with the office, I need to get away,” Dr. Montgomery says. “I’ll take a day to go fishing, golfing, and not think about the office.” Just a small break can shift the lens that you see through when you return to the office and put problems in perspective.
A version of this article first appeared on Medscape.com.
A physician in your office is hot-tempered, critical, and upsets both the physicians and staff. Two of your partners are arguing over a software vendor and refuse to compromise. One doctor’s spouse is the office manager and snipes at everyone; the lead partner micromanages and second-guesses other doctors’ treatment plans, and no one will stand up to her.
If your practice has similar scenarios, you’re likely dealing with your own anger, irritation, and dread at work. You’re struggling with a toxic practice atmosphere, and you must make changes – fast.
However, this isn’t easy, given that what goes on in a doctor’s office is “high consequence,” says Leonard J. Marcus, PhD, founding director of the program for health care negotiation and conflict resolution at the Harvard School of Public Health in Boston.
The two things that tend to plague medical practices most: A culture of fear and someone who is letting ego run the day-to-day, he says.
“Fear overwhelms any chance for good morale among colleagues,” says Dr. Marcus, who is also the coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration.” “In a work environment where the fear is overwhelming, the ego can take over, and someone at the practice becomes overly concerned about getting credit, taking control, ordering other people around, and deciding who is on top and who is on the bottom.”
Tension, stress, back-biting, and rudeness are also symptoms of a more significant problem, says Jes Montgomery, MD, a psychiatrist and medical director of APN Dallas, a mental health–focused practice.
“If you don’t get toxicity under control, it will blow the office apart,” Dr. Montgomery says.
1. Recognize the signs
Part of the problem with a toxic medical practice is that, culturally, we don’t treat mental health and burnout as real illnesses. “A physician who is depressed is not going to be melancholy or bursting into tears with patients,” Dr. Montgomery says. “They’ll get behind on paperwork, skip meals, or find that it’s difficult to sleep at night. Next, they’ll yell at the partners and staff, always be in a foul mood, and gripe about inconsequential things. Their behavior affects everyone.”
Dr. Montgomery says that physicians aren’t taught to ask for help, making it difficult to see what’s really going on when someone displays toxic behavior in the practice. If it’s a partner, take time to ask what’s going on. If it’s yourself, step back and see if you can ask someone for the help you need.
2. Have difficult conversations
This is tough for most of us, says Jeremy Pollack, PhD, CEO and founder of Pollack Peacebuilding Systems, a conflict resolution consulting firm. If a team member is hot-tempered, disrespectful, or talking to patients in an unproductive manner, see if you can have an effective conversation with that person. The tricky part is critiquing in a way that doesn’t make them feel defensive – and wanting to push back.
For a micromanaging office manager, for example, you could say something like,”You’re doing a great job with the inventory, but I need you to let the staff have some autonomy and not hover over every supply they use in the break room, so that people won’t feel resentful toward us.” Make it clear you’re a team, and this is a team challenge. “However, if a doctor feels like they’ve tried to communicate to that colleague and are still walking on eggshells, it’s time to try to get help from someone – perhaps a practice management organization,” says Dr. Pollack.
3. Open lines of communication
It’s critical to create a comfortable space to speak with your colleagues, says Marisa Garshick, MD, a dermatologist in private practice in New York. “Creating an environment where there is an open line of communication, whether it’s directly to somebody in charge or having a system where you can give feedback more privately or anonymously, is important so that tension doesn’t build.”
“Being a doctor is a social enterprise,” Dr. Marcus says. “The science of medicine is critically important, but patients and the other health care workers on your team are also critically important. In the long run, the most successful physicians pay attention to both. It’s a full package.”
4. Emphasize the positive
Instead of discussing things only when they go wrong, try optimism, Dr. Garshick said. When positive things happen, whether it’s an excellent patient encounter or the office did something really well together, highlight it so everyone has a sense of accomplishment. If a patient compliments a medical assistant or raves about a nurse, share those compliments with the employees so that not every encounter you have calls out problems and staff missteps.
Suppose partners have a conflict with one another or are arguing over something. In that case, you may need to mediate or invest in a meaningful intervention so people can reflect on the narrative they’re contributing to the culture.
5. Practice self-care
Finally, the work of a physician is exhausting, so it’s crucial to practice personal TLC. That may mean taking micro breaks, getting adequate sleep, maintaining a healthy diet, and exercising well and managing stress to maintain energy levels and patience.
“Sometimes, when I’m fed up with the office, I need to get away,” Dr. Montgomery says. “I’ll take a day to go fishing, golfing, and not think about the office.” Just a small break can shift the lens that you see through when you return to the office and put problems in perspective.
A version of this article first appeared on Medscape.com.
A physician in your office is hot-tempered, critical, and upsets both the physicians and staff. Two of your partners are arguing over a software vendor and refuse to compromise. One doctor’s spouse is the office manager and snipes at everyone; the lead partner micromanages and second-guesses other doctors’ treatment plans, and no one will stand up to her.
If your practice has similar scenarios, you’re likely dealing with your own anger, irritation, and dread at work. You’re struggling with a toxic practice atmosphere, and you must make changes – fast.
However, this isn’t easy, given that what goes on in a doctor’s office is “high consequence,” says Leonard J. Marcus, PhD, founding director of the program for health care negotiation and conflict resolution at the Harvard School of Public Health in Boston.
The two things that tend to plague medical practices most: A culture of fear and someone who is letting ego run the day-to-day, he says.
“Fear overwhelms any chance for good morale among colleagues,” says Dr. Marcus, who is also the coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration.” “In a work environment where the fear is overwhelming, the ego can take over, and someone at the practice becomes overly concerned about getting credit, taking control, ordering other people around, and deciding who is on top and who is on the bottom.”
Tension, stress, back-biting, and rudeness are also symptoms of a more significant problem, says Jes Montgomery, MD, a psychiatrist and medical director of APN Dallas, a mental health–focused practice.
“If you don’t get toxicity under control, it will blow the office apart,” Dr. Montgomery says.
1. Recognize the signs
Part of the problem with a toxic medical practice is that, culturally, we don’t treat mental health and burnout as real illnesses. “A physician who is depressed is not going to be melancholy or bursting into tears with patients,” Dr. Montgomery says. “They’ll get behind on paperwork, skip meals, or find that it’s difficult to sleep at night. Next, they’ll yell at the partners and staff, always be in a foul mood, and gripe about inconsequential things. Their behavior affects everyone.”
Dr. Montgomery says that physicians aren’t taught to ask for help, making it difficult to see what’s really going on when someone displays toxic behavior in the practice. If it’s a partner, take time to ask what’s going on. If it’s yourself, step back and see if you can ask someone for the help you need.
2. Have difficult conversations
This is tough for most of us, says Jeremy Pollack, PhD, CEO and founder of Pollack Peacebuilding Systems, a conflict resolution consulting firm. If a team member is hot-tempered, disrespectful, or talking to patients in an unproductive manner, see if you can have an effective conversation with that person. The tricky part is critiquing in a way that doesn’t make them feel defensive – and wanting to push back.
For a micromanaging office manager, for example, you could say something like,”You’re doing a great job with the inventory, but I need you to let the staff have some autonomy and not hover over every supply they use in the break room, so that people won’t feel resentful toward us.” Make it clear you’re a team, and this is a team challenge. “However, if a doctor feels like they’ve tried to communicate to that colleague and are still walking on eggshells, it’s time to try to get help from someone – perhaps a practice management organization,” says Dr. Pollack.
3. Open lines of communication
It’s critical to create a comfortable space to speak with your colleagues, says Marisa Garshick, MD, a dermatologist in private practice in New York. “Creating an environment where there is an open line of communication, whether it’s directly to somebody in charge or having a system where you can give feedback more privately or anonymously, is important so that tension doesn’t build.”
“Being a doctor is a social enterprise,” Dr. Marcus says. “The science of medicine is critically important, but patients and the other health care workers on your team are also critically important. In the long run, the most successful physicians pay attention to both. It’s a full package.”
4. Emphasize the positive
Instead of discussing things only when they go wrong, try optimism, Dr. Garshick said. When positive things happen, whether it’s an excellent patient encounter or the office did something really well together, highlight it so everyone has a sense of accomplishment. If a patient compliments a medical assistant or raves about a nurse, share those compliments with the employees so that not every encounter you have calls out problems and staff missteps.
Suppose partners have a conflict with one another or are arguing over something. In that case, you may need to mediate or invest in a meaningful intervention so people can reflect on the narrative they’re contributing to the culture.
5. Practice self-care
Finally, the work of a physician is exhausting, so it’s crucial to practice personal TLC. That may mean taking micro breaks, getting adequate sleep, maintaining a healthy diet, and exercising well and managing stress to maintain energy levels and patience.
“Sometimes, when I’m fed up with the office, I need to get away,” Dr. Montgomery says. “I’ll take a day to go fishing, golfing, and not think about the office.” Just a small break can shift the lens that you see through when you return to the office and put problems in perspective.
A version of this article first appeared on Medscape.com.
Heart attack deaths static in those with type 1 diabetes
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
FROM EASD 2023
12 steps to closing your practice without problems
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Cold weather may challenge blood pressure control
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2023