User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Administrative hassle hacks: Strategies to curb physician stress
The American Medical Association estimates that physician burnout costs the country $4.6 billion annually, and that doesn’t include the cost for nurses and other clinicians. In addition, physicians note too many bureaucratic tasks as a main contributor to their daily stress.
Such revelations have prompted many in the health care industry to focus on clinician burnout, including a panel at the recent American Telemedicine Association annual conference in Boston.
Not surprisingly, the discussion quickly turned to the COVID-19 pandemic, commonly cited as an event that has exacerbated existing clinician burnout and caused what has become known as the “great resignation.”
Peter Yellowlees, MBBS, MD, professor of psychiatry and chief wellness officer at the University of California, Davis, said his health system has experienced a lot of its nursing staff resigning or moving to other employment, particularly from intensive care units and the emergency department.
“We actually haven’t had too many physicians go, but I have a funny feeling we’re going to see that over the next year or so because I think a lot of people have just put their head down during the pandemic and they’ve worked themselves hard,” he said. “They’re now sort of putting their heads up above the wall,” and could realize that they want a change.
In his role as the wellness officer at the academic medical center, Dr. Yellowlees is proactively addressing burnout among the organization’s 14,000 employees. For example, during the pandemic, he developed a peer responder program. Under this initiative, 600 staff members received training in “psychological first aid,” essentially utilizing staff to become therapists for peers.
For example, if a clinician is struggling emotionally while dealing with a patient who has had significant trauma, a peer responder could talk with the clinician, helping him or her to better deal with the situation.
Marlene McDermott, senior director of therapy services at Array Behavioral Care, a national telepsychiatry provider with offices in New Jersey and Illinois, noted that her organization also addresses burnout by creating opportunities for peer-to-peer support.
“We’ve got hundreds of clinicians and we’ll take 10 to 15 of them, put them in small treatment teams and they have a live chat, a one-off virtual meeting with each other to vent and to ask clinical questions. It’s all clinicians, there’s no administrative staff in there,” Ms. McDermott said. The clinicians have found value in these meetings, as they can share their concerns as well as “silly images or quotes, just to keep things light at times. That’s made a big difference.”
Retraining, technology can help curb administrative burdens
In addition to providing peer support, both Dr. Yellowlees and Ms. McDermott are addressing the significant administrative burden that plagues physicians.
This burden is especially onerous for physicians in the United States, according to a study that compared the number of keystrokes required to produce clinical notes among physicians in several countries.
“What [the study] discovered was that the American notes were three to five times longer than the notes of the Australian or U.K. physicians. I’ve worked in all three countries and I can promise you there’s no difference in the quality of the doctors across those places,” Dr. Yellowlees said.
To address this issue, Dr. Yellowlees is training physicians to reduce the length of their clinical documentation.
“I am trying to retrain physicians who for many years have been trained to be defensive in their documentation – to write absurd amounts just to justify billing,” Dr. Yellowlees said. “We are trying to go back in some respects to the way that we used to write notes 20 years ago ... so much shorter. This is a huge retraining exercise but it’s an exercise that is essential.”
Ms. McDermott also is tackling the administrative burden at her organization.
“We are trying to make the workflow as efficient as possible, doing some asynchronous work where consumers are completing information before a session ... so clinicians are essentially reconciling information instead of gathering all nonpertinent information. They can just work at the top of the license and not be burdened by some of the questions that don’t directly affect treatment,” Ms. McDermott noted.
Encouraging and training physicians in concurrent documentation also can help reduce administrative burden.
“Being proficient at remaining in session and documenting as much as you can during a session can help. So that at the end, you’re pressing the button, closing the encounter and you’ve finished documenting,” Ms. McDermott said. “It’s definitely possible to do that without losing the connection with the patient.”
To accomplish this, physicians need to leverage touch-typing – the practice of typing without looking at the keyboard. Fortunately, telehealth makes this mode of documentation easily achievable. Consider the following: During an online session, clinicians can place the patient’s picture “right underneath the camera and make it small. And then you type with the note floating behind it. So you’re actually staring at the note and the person all at the same time,” Ms. McDermott said.
The continued uptake of telehealth in general could also reduce stress for physicians, added Dr. Yellowlees.
“One of the interesting things about that is just how much time we save the physicians because it actually takes quite a lot of time to room patients,” Dr. Yellowlees concluded. “We are now doing about 20% of all our outpatient visits in all disciplines by video. We were higher than that midway through COVID. I’m hoping we’ll go back to being higher than that.”
A version of this article first appeared on Medscape.com.
The American Medical Association estimates that physician burnout costs the country $4.6 billion annually, and that doesn’t include the cost for nurses and other clinicians. In addition, physicians note too many bureaucratic tasks as a main contributor to their daily stress.
Such revelations have prompted many in the health care industry to focus on clinician burnout, including a panel at the recent American Telemedicine Association annual conference in Boston.
Not surprisingly, the discussion quickly turned to the COVID-19 pandemic, commonly cited as an event that has exacerbated existing clinician burnout and caused what has become known as the “great resignation.”
Peter Yellowlees, MBBS, MD, professor of psychiatry and chief wellness officer at the University of California, Davis, said his health system has experienced a lot of its nursing staff resigning or moving to other employment, particularly from intensive care units and the emergency department.
“We actually haven’t had too many physicians go, but I have a funny feeling we’re going to see that over the next year or so because I think a lot of people have just put their head down during the pandemic and they’ve worked themselves hard,” he said. “They’re now sort of putting their heads up above the wall,” and could realize that they want a change.
In his role as the wellness officer at the academic medical center, Dr. Yellowlees is proactively addressing burnout among the organization’s 14,000 employees. For example, during the pandemic, he developed a peer responder program. Under this initiative, 600 staff members received training in “psychological first aid,” essentially utilizing staff to become therapists for peers.
For example, if a clinician is struggling emotionally while dealing with a patient who has had significant trauma, a peer responder could talk with the clinician, helping him or her to better deal with the situation.
Marlene McDermott, senior director of therapy services at Array Behavioral Care, a national telepsychiatry provider with offices in New Jersey and Illinois, noted that her organization also addresses burnout by creating opportunities for peer-to-peer support.
“We’ve got hundreds of clinicians and we’ll take 10 to 15 of them, put them in small treatment teams and they have a live chat, a one-off virtual meeting with each other to vent and to ask clinical questions. It’s all clinicians, there’s no administrative staff in there,” Ms. McDermott said. The clinicians have found value in these meetings, as they can share their concerns as well as “silly images or quotes, just to keep things light at times. That’s made a big difference.”
Retraining, technology can help curb administrative burdens
In addition to providing peer support, both Dr. Yellowlees and Ms. McDermott are addressing the significant administrative burden that plagues physicians.
This burden is especially onerous for physicians in the United States, according to a study that compared the number of keystrokes required to produce clinical notes among physicians in several countries.
“What [the study] discovered was that the American notes were three to five times longer than the notes of the Australian or U.K. physicians. I’ve worked in all three countries and I can promise you there’s no difference in the quality of the doctors across those places,” Dr. Yellowlees said.
To address this issue, Dr. Yellowlees is training physicians to reduce the length of their clinical documentation.
“I am trying to retrain physicians who for many years have been trained to be defensive in their documentation – to write absurd amounts just to justify billing,” Dr. Yellowlees said. “We are trying to go back in some respects to the way that we used to write notes 20 years ago ... so much shorter. This is a huge retraining exercise but it’s an exercise that is essential.”
Ms. McDermott also is tackling the administrative burden at her organization.
“We are trying to make the workflow as efficient as possible, doing some asynchronous work where consumers are completing information before a session ... so clinicians are essentially reconciling information instead of gathering all nonpertinent information. They can just work at the top of the license and not be burdened by some of the questions that don’t directly affect treatment,” Ms. McDermott noted.
Encouraging and training physicians in concurrent documentation also can help reduce administrative burden.
“Being proficient at remaining in session and documenting as much as you can during a session can help. So that at the end, you’re pressing the button, closing the encounter and you’ve finished documenting,” Ms. McDermott said. “It’s definitely possible to do that without losing the connection with the patient.”
To accomplish this, physicians need to leverage touch-typing – the practice of typing without looking at the keyboard. Fortunately, telehealth makes this mode of documentation easily achievable. Consider the following: During an online session, clinicians can place the patient’s picture “right underneath the camera and make it small. And then you type with the note floating behind it. So you’re actually staring at the note and the person all at the same time,” Ms. McDermott said.
The continued uptake of telehealth in general could also reduce stress for physicians, added Dr. Yellowlees.
“One of the interesting things about that is just how much time we save the physicians because it actually takes quite a lot of time to room patients,” Dr. Yellowlees concluded. “We are now doing about 20% of all our outpatient visits in all disciplines by video. We were higher than that midway through COVID. I’m hoping we’ll go back to being higher than that.”
A version of this article first appeared on Medscape.com.
The American Medical Association estimates that physician burnout costs the country $4.6 billion annually, and that doesn’t include the cost for nurses and other clinicians. In addition, physicians note too many bureaucratic tasks as a main contributor to their daily stress.
Such revelations have prompted many in the health care industry to focus on clinician burnout, including a panel at the recent American Telemedicine Association annual conference in Boston.
Not surprisingly, the discussion quickly turned to the COVID-19 pandemic, commonly cited as an event that has exacerbated existing clinician burnout and caused what has become known as the “great resignation.”
Peter Yellowlees, MBBS, MD, professor of psychiatry and chief wellness officer at the University of California, Davis, said his health system has experienced a lot of its nursing staff resigning or moving to other employment, particularly from intensive care units and the emergency department.
“We actually haven’t had too many physicians go, but I have a funny feeling we’re going to see that over the next year or so because I think a lot of people have just put their head down during the pandemic and they’ve worked themselves hard,” he said. “They’re now sort of putting their heads up above the wall,” and could realize that they want a change.
In his role as the wellness officer at the academic medical center, Dr. Yellowlees is proactively addressing burnout among the organization’s 14,000 employees. For example, during the pandemic, he developed a peer responder program. Under this initiative, 600 staff members received training in “psychological first aid,” essentially utilizing staff to become therapists for peers.
For example, if a clinician is struggling emotionally while dealing with a patient who has had significant trauma, a peer responder could talk with the clinician, helping him or her to better deal with the situation.
Marlene McDermott, senior director of therapy services at Array Behavioral Care, a national telepsychiatry provider with offices in New Jersey and Illinois, noted that her organization also addresses burnout by creating opportunities for peer-to-peer support.
“We’ve got hundreds of clinicians and we’ll take 10 to 15 of them, put them in small treatment teams and they have a live chat, a one-off virtual meeting with each other to vent and to ask clinical questions. It’s all clinicians, there’s no administrative staff in there,” Ms. McDermott said. The clinicians have found value in these meetings, as they can share their concerns as well as “silly images or quotes, just to keep things light at times. That’s made a big difference.”
Retraining, technology can help curb administrative burdens
In addition to providing peer support, both Dr. Yellowlees and Ms. McDermott are addressing the significant administrative burden that plagues physicians.
This burden is especially onerous for physicians in the United States, according to a study that compared the number of keystrokes required to produce clinical notes among physicians in several countries.
“What [the study] discovered was that the American notes were three to five times longer than the notes of the Australian or U.K. physicians. I’ve worked in all three countries and I can promise you there’s no difference in the quality of the doctors across those places,” Dr. Yellowlees said.
To address this issue, Dr. Yellowlees is training physicians to reduce the length of their clinical documentation.
“I am trying to retrain physicians who for many years have been trained to be defensive in their documentation – to write absurd amounts just to justify billing,” Dr. Yellowlees said. “We are trying to go back in some respects to the way that we used to write notes 20 years ago ... so much shorter. This is a huge retraining exercise but it’s an exercise that is essential.”
Ms. McDermott also is tackling the administrative burden at her organization.
“We are trying to make the workflow as efficient as possible, doing some asynchronous work where consumers are completing information before a session ... so clinicians are essentially reconciling information instead of gathering all nonpertinent information. They can just work at the top of the license and not be burdened by some of the questions that don’t directly affect treatment,” Ms. McDermott noted.
Encouraging and training physicians in concurrent documentation also can help reduce administrative burden.
“Being proficient at remaining in session and documenting as much as you can during a session can help. So that at the end, you’re pressing the button, closing the encounter and you’ve finished documenting,” Ms. McDermott said. “It’s definitely possible to do that without losing the connection with the patient.”
To accomplish this, physicians need to leverage touch-typing – the practice of typing without looking at the keyboard. Fortunately, telehealth makes this mode of documentation easily achievable. Consider the following: During an online session, clinicians can place the patient’s picture “right underneath the camera and make it small. And then you type with the note floating behind it. So you’re actually staring at the note and the person all at the same time,” Ms. McDermott said.
The continued uptake of telehealth in general could also reduce stress for physicians, added Dr. Yellowlees.
“One of the interesting things about that is just how much time we save the physicians because it actually takes quite a lot of time to room patients,” Dr. Yellowlees concluded. “We are now doing about 20% of all our outpatient visits in all disciplines by video. We were higher than that midway through COVID. I’m hoping we’ll go back to being higher than that.”
A version of this article first appeared on Medscape.com.
Spell it out: Writing out common medical terms boosts patient understanding, says study
MI. HTN. hx. Although these abbreviations might make it easier for physicians and other health care professionals to create and consume clinical documentation, the shorthand confuses patients, according to a study published in JAMA Network Open.
Researchers, who conducted clinical trials at three hospitals, found that expansion of 10 common medical abbreviations and acronyms in patient health records significantly increased overall comprehension.
Corresponding author Lisa Grossman Liu, PhD, MD, of Columbia University, New York, told this news organization that “comprehension of abbreviations was much lower than we expected and much lower than the clinicians who participated in this study expected.”
This discovery is particularly relevant in this era of digital care, where providers are now communicating with patients electronically more than ever before – and are required by rules emanating from the 21st Century Cures Act to provide online access to electronic health records.
Using elongated terms
Although the study found that expansion of medical abbreviations and acronyms can improve patient understanding, identifying all of the medical abbreviations that exist is difficult because the terms vary by specialty and geography. The fact that many abbreviations and acronyms have multiple meanings complicates matters even more. For example, the abbreviation PA has 128 possible meanings, Dr. Grossman Liu pointed out.
Technology, fortunately, has advanced in the last few years and is on the cusp of providing a solution. Artificial intelligence systems could help to develop large compendiums of abbreviations and acronyms and then machine learning could elongate the words.
“We’re almost to the point where we have these automated systems that can actually expand abbreviations pretty well and with a great degree of accuracy and ... where those can actually be used in medicine to help with patient communication,” Dr. Grossman Liu said.
Such intervention, however, is not a cure-all.
“There are abbreviations that are really hard to understand even after you expand them, such as MI for myocardial infarction, which is really a tough term all around. It means heart attack. So even if you tell patients, MI means myocardial infarction, they’re still not going to understand it,” Dr. Grossman Liu said.
On the flip side, patients are likely to understand some abbreviations such as hrs, which stands for hours, without elongating the words.
Moving from in-person to online communication
A look at the evolution of clinical documentation explains how this abbreviation problem came to fruition. Prior to this digital age where providers communicate with patients through portals, secure messaging, and other electronic methods, patients and providers would talk face to face. Now, however, electronic written communication is becoming the norm.
“We are not only seeing direct written communication through things like messaging systems or email, but also patients are now reading their medical records online and you can consider that as a form of communication,” Dr. Grossman Liu said. “It’s really interesting that the electronic health record itself has essentially become a medium for communication between patients and providers when previously it was only a way for providers to communicate with themselves and document patient care. So, clinicians use abbreviations because they aren’t intending for patients to see the records.”
Requiring physicians to use complete words in clinical documentation now that electronic records are relied on for patient communication, however, is not a practical solution.
“Abbreviations are so commonly used because they are more efficient to read and more efficient to write. We really shouldn’t be putting the onus on providers to spell out all the abbreviations in their notes. That’s realistically not going to work, because it compromises clinical efficiency,” Dr. Grossman Liu said.
While physicians should not be forced to use complete words in documentation, they should be wary of patients’ unfamiliarity with abbreviations as they communicate in person.
“I use terms like ED constantly when I talk to patients, and it turns out that only 67% of patients understand what you’re talking about when you say ED in reference to the emergency department. So it’s important to be mindful of that,” Dr. Grossman Liu concluded.
A version of this article first appeared on Medscape.com.
MI. HTN. hx. Although these abbreviations might make it easier for physicians and other health care professionals to create and consume clinical documentation, the shorthand confuses patients, according to a study published in JAMA Network Open.
Researchers, who conducted clinical trials at three hospitals, found that expansion of 10 common medical abbreviations and acronyms in patient health records significantly increased overall comprehension.
Corresponding author Lisa Grossman Liu, PhD, MD, of Columbia University, New York, told this news organization that “comprehension of abbreviations was much lower than we expected and much lower than the clinicians who participated in this study expected.”
This discovery is particularly relevant in this era of digital care, where providers are now communicating with patients electronically more than ever before – and are required by rules emanating from the 21st Century Cures Act to provide online access to electronic health records.
Using elongated terms
Although the study found that expansion of medical abbreviations and acronyms can improve patient understanding, identifying all of the medical abbreviations that exist is difficult because the terms vary by specialty and geography. The fact that many abbreviations and acronyms have multiple meanings complicates matters even more. For example, the abbreviation PA has 128 possible meanings, Dr. Grossman Liu pointed out.
Technology, fortunately, has advanced in the last few years and is on the cusp of providing a solution. Artificial intelligence systems could help to develop large compendiums of abbreviations and acronyms and then machine learning could elongate the words.
“We’re almost to the point where we have these automated systems that can actually expand abbreviations pretty well and with a great degree of accuracy and ... where those can actually be used in medicine to help with patient communication,” Dr. Grossman Liu said.
Such intervention, however, is not a cure-all.
“There are abbreviations that are really hard to understand even after you expand them, such as MI for myocardial infarction, which is really a tough term all around. It means heart attack. So even if you tell patients, MI means myocardial infarction, they’re still not going to understand it,” Dr. Grossman Liu said.
On the flip side, patients are likely to understand some abbreviations such as hrs, which stands for hours, without elongating the words.
Moving from in-person to online communication
A look at the evolution of clinical documentation explains how this abbreviation problem came to fruition. Prior to this digital age where providers communicate with patients through portals, secure messaging, and other electronic methods, patients and providers would talk face to face. Now, however, electronic written communication is becoming the norm.
“We are not only seeing direct written communication through things like messaging systems or email, but also patients are now reading their medical records online and you can consider that as a form of communication,” Dr. Grossman Liu said. “It’s really interesting that the electronic health record itself has essentially become a medium for communication between patients and providers when previously it was only a way for providers to communicate with themselves and document patient care. So, clinicians use abbreviations because they aren’t intending for patients to see the records.”
Requiring physicians to use complete words in clinical documentation now that electronic records are relied on for patient communication, however, is not a practical solution.
“Abbreviations are so commonly used because they are more efficient to read and more efficient to write. We really shouldn’t be putting the onus on providers to spell out all the abbreviations in their notes. That’s realistically not going to work, because it compromises clinical efficiency,” Dr. Grossman Liu said.
While physicians should not be forced to use complete words in documentation, they should be wary of patients’ unfamiliarity with abbreviations as they communicate in person.
“I use terms like ED constantly when I talk to patients, and it turns out that only 67% of patients understand what you’re talking about when you say ED in reference to the emergency department. So it’s important to be mindful of that,” Dr. Grossman Liu concluded.
A version of this article first appeared on Medscape.com.
MI. HTN. hx. Although these abbreviations might make it easier for physicians and other health care professionals to create and consume clinical documentation, the shorthand confuses patients, according to a study published in JAMA Network Open.
Researchers, who conducted clinical trials at three hospitals, found that expansion of 10 common medical abbreviations and acronyms in patient health records significantly increased overall comprehension.
Corresponding author Lisa Grossman Liu, PhD, MD, of Columbia University, New York, told this news organization that “comprehension of abbreviations was much lower than we expected and much lower than the clinicians who participated in this study expected.”
This discovery is particularly relevant in this era of digital care, where providers are now communicating with patients electronically more than ever before – and are required by rules emanating from the 21st Century Cures Act to provide online access to electronic health records.
Using elongated terms
Although the study found that expansion of medical abbreviations and acronyms can improve patient understanding, identifying all of the medical abbreviations that exist is difficult because the terms vary by specialty and geography. The fact that many abbreviations and acronyms have multiple meanings complicates matters even more. For example, the abbreviation PA has 128 possible meanings, Dr. Grossman Liu pointed out.
Technology, fortunately, has advanced in the last few years and is on the cusp of providing a solution. Artificial intelligence systems could help to develop large compendiums of abbreviations and acronyms and then machine learning could elongate the words.
“We’re almost to the point where we have these automated systems that can actually expand abbreviations pretty well and with a great degree of accuracy and ... where those can actually be used in medicine to help with patient communication,” Dr. Grossman Liu said.
Such intervention, however, is not a cure-all.
“There are abbreviations that are really hard to understand even after you expand them, such as MI for myocardial infarction, which is really a tough term all around. It means heart attack. So even if you tell patients, MI means myocardial infarction, they’re still not going to understand it,” Dr. Grossman Liu said.
On the flip side, patients are likely to understand some abbreviations such as hrs, which stands for hours, without elongating the words.
Moving from in-person to online communication
A look at the evolution of clinical documentation explains how this abbreviation problem came to fruition. Prior to this digital age where providers communicate with patients through portals, secure messaging, and other electronic methods, patients and providers would talk face to face. Now, however, electronic written communication is becoming the norm.
“We are not only seeing direct written communication through things like messaging systems or email, but also patients are now reading their medical records online and you can consider that as a form of communication,” Dr. Grossman Liu said. “It’s really interesting that the electronic health record itself has essentially become a medium for communication between patients and providers when previously it was only a way for providers to communicate with themselves and document patient care. So, clinicians use abbreviations because they aren’t intending for patients to see the records.”
Requiring physicians to use complete words in clinical documentation now that electronic records are relied on for patient communication, however, is not a practical solution.
“Abbreviations are so commonly used because they are more efficient to read and more efficient to write. We really shouldn’t be putting the onus on providers to spell out all the abbreviations in their notes. That’s realistically not going to work, because it compromises clinical efficiency,” Dr. Grossman Liu said.
While physicians should not be forced to use complete words in documentation, they should be wary of patients’ unfamiliarity with abbreviations as they communicate in person.
“I use terms like ED constantly when I talk to patients, and it turns out that only 67% of patients understand what you’re talking about when you say ED in reference to the emergency department. So it’s important to be mindful of that,” Dr. Grossman Liu concluded.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
More practice merger options
The continuing than larger ones. While there are some smaller offices offering unique services that may be able to remain small, most small general practices will be forced to at least consider a larger alternative. Recently, I discussed one option – merging individual practices into a larger one – but others are available.
One alternate strategy is to form a cooperative group. If you look around your area of practice, you will likely find other small practices in similar situations that might be willing to collaborate with you for the purpose of pooling your billing and purchasing resources. This allows each participant to maintain independence, yet share office overhead expenses and employee salaries for mutual benefit. If that arrangement works, and remains satisfactory for all participants, you can consider expanding your sharing of expenditures, such as collective purchasing of supplies and equipment, and centralizing appointment scheduling. Such an arrangement might be particularly attractive to physicians in later stages of their careers who need to alleviate financial burdens but don’t wish to close up shop just yet.
After more time has passed, if everyone remains happy with the arrangement, an outright merger can be considered, allowing the group to negotiate higher insurance remunerations and even lower overhead costs. Obviously, projects of this size and scope require careful planning and implementation, and should not be undertaken without the help of competent legal counsel and an experienced business consultant.
Another option is to join an independent practice association (IPA), if one is operating in your area. IPAs are physician-directed legal entities, formed to provide the same advantages enjoyed by large group practices while allowing individual members to remain independent. IPAs have greater purchasing power, allowing members to cut costs on medical and office supplies. They can also negotiate more favorable contracts with insurance companies and other payers.
Before joining such an organization, examine its legal status carefully. Some IPAs have been charged with antitrust violations because their member practices are, in reality, competitors. Make certain that any IPA you consider joining abides by antitrust and price fixing laws. Look carefully at its financial solvency as well, as IPAs have also been known to fail, leaving former members to pick up the tab.
An alternative to the IPA is the accountable care organization (ACO), a relatively new entity created as part of the Affordable Care Act. Like an IPA, an ACO’s basic purpose is to limit unnecessary spending; but ACOs are typically limited to Medicare and Medicaid recipients, and involve a larger network of doctors and hospitals sharing financial and medical responsibility for patient care. Criteria for limits on spending are established by the Centers for Medicare & Medicaid Services (CMS).
ACOs offer financial incentives to cooperate, and to save money by avoiding unnecessary tests and procedures. A key component is the sharing of information. Providers who save money while also meeting quality targets are theoretically entitled to a portion of the savings. According to federal data, ACOs saved Medicare $4.1 billion in 2020). As of January 2022, 483 ACOs were participating in the Medicare Shared Savings Program. A similar entity designed for private-sector patients is the clinically integrated network (CIN), created by the Federal Trade Commission to serve the commercial or self-insured market, while ACOs treat Medicare and Medicaid patients. Like ACOs, the idea is to work together to improve care and reduce costs by sharing records and tracking data.
When joining any group, read the agreement carefully for any clauses that might infringe on your clinical judgment. In particular, be sure that there are no restrictions on patient treatment or physician referral options for your patients. You should also negotiate an escape clause, allowing you to opt out if you become unhappy with the arrangement.
Clearly, the price of remaining autonomous is significant, and many private practitioners are unwilling to pay it. In 2019, the American Medical Association reported that for the first time, there were fewer physician owners (45.9%) than employees (47.4%).
But as I have written many times, those of us who remain committed to independence will find ways to preserve it. In medicine, as in life, those most responsive to change will survive and flourish.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
The continuing than larger ones. While there are some smaller offices offering unique services that may be able to remain small, most small general practices will be forced to at least consider a larger alternative. Recently, I discussed one option – merging individual practices into a larger one – but others are available.
One alternate strategy is to form a cooperative group. If you look around your area of practice, you will likely find other small practices in similar situations that might be willing to collaborate with you for the purpose of pooling your billing and purchasing resources. This allows each participant to maintain independence, yet share office overhead expenses and employee salaries for mutual benefit. If that arrangement works, and remains satisfactory for all participants, you can consider expanding your sharing of expenditures, such as collective purchasing of supplies and equipment, and centralizing appointment scheduling. Such an arrangement might be particularly attractive to physicians in later stages of their careers who need to alleviate financial burdens but don’t wish to close up shop just yet.
After more time has passed, if everyone remains happy with the arrangement, an outright merger can be considered, allowing the group to negotiate higher insurance remunerations and even lower overhead costs. Obviously, projects of this size and scope require careful planning and implementation, and should not be undertaken without the help of competent legal counsel and an experienced business consultant.
Another option is to join an independent practice association (IPA), if one is operating in your area. IPAs are physician-directed legal entities, formed to provide the same advantages enjoyed by large group practices while allowing individual members to remain independent. IPAs have greater purchasing power, allowing members to cut costs on medical and office supplies. They can also negotiate more favorable contracts with insurance companies and other payers.
Before joining such an organization, examine its legal status carefully. Some IPAs have been charged with antitrust violations because their member practices are, in reality, competitors. Make certain that any IPA you consider joining abides by antitrust and price fixing laws. Look carefully at its financial solvency as well, as IPAs have also been known to fail, leaving former members to pick up the tab.
An alternative to the IPA is the accountable care organization (ACO), a relatively new entity created as part of the Affordable Care Act. Like an IPA, an ACO’s basic purpose is to limit unnecessary spending; but ACOs are typically limited to Medicare and Medicaid recipients, and involve a larger network of doctors and hospitals sharing financial and medical responsibility for patient care. Criteria for limits on spending are established by the Centers for Medicare & Medicaid Services (CMS).
ACOs offer financial incentives to cooperate, and to save money by avoiding unnecessary tests and procedures. A key component is the sharing of information. Providers who save money while also meeting quality targets are theoretically entitled to a portion of the savings. According to federal data, ACOs saved Medicare $4.1 billion in 2020). As of January 2022, 483 ACOs were participating in the Medicare Shared Savings Program. A similar entity designed for private-sector patients is the clinically integrated network (CIN), created by the Federal Trade Commission to serve the commercial or self-insured market, while ACOs treat Medicare and Medicaid patients. Like ACOs, the idea is to work together to improve care and reduce costs by sharing records and tracking data.
When joining any group, read the agreement carefully for any clauses that might infringe on your clinical judgment. In particular, be sure that there are no restrictions on patient treatment or physician referral options for your patients. You should also negotiate an escape clause, allowing you to opt out if you become unhappy with the arrangement.
Clearly, the price of remaining autonomous is significant, and many private practitioners are unwilling to pay it. In 2019, the American Medical Association reported that for the first time, there were fewer physician owners (45.9%) than employees (47.4%).
But as I have written many times, those of us who remain committed to independence will find ways to preserve it. In medicine, as in life, those most responsive to change will survive and flourish.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
The continuing than larger ones. While there are some smaller offices offering unique services that may be able to remain small, most small general practices will be forced to at least consider a larger alternative. Recently, I discussed one option – merging individual practices into a larger one – but others are available.
One alternate strategy is to form a cooperative group. If you look around your area of practice, you will likely find other small practices in similar situations that might be willing to collaborate with you for the purpose of pooling your billing and purchasing resources. This allows each participant to maintain independence, yet share office overhead expenses and employee salaries for mutual benefit. If that arrangement works, and remains satisfactory for all participants, you can consider expanding your sharing of expenditures, such as collective purchasing of supplies and equipment, and centralizing appointment scheduling. Such an arrangement might be particularly attractive to physicians in later stages of their careers who need to alleviate financial burdens but don’t wish to close up shop just yet.
After more time has passed, if everyone remains happy with the arrangement, an outright merger can be considered, allowing the group to negotiate higher insurance remunerations and even lower overhead costs. Obviously, projects of this size and scope require careful planning and implementation, and should not be undertaken without the help of competent legal counsel and an experienced business consultant.
Another option is to join an independent practice association (IPA), if one is operating in your area. IPAs are physician-directed legal entities, formed to provide the same advantages enjoyed by large group practices while allowing individual members to remain independent. IPAs have greater purchasing power, allowing members to cut costs on medical and office supplies. They can also negotiate more favorable contracts with insurance companies and other payers.
Before joining such an organization, examine its legal status carefully. Some IPAs have been charged with antitrust violations because their member practices are, in reality, competitors. Make certain that any IPA you consider joining abides by antitrust and price fixing laws. Look carefully at its financial solvency as well, as IPAs have also been known to fail, leaving former members to pick up the tab.
An alternative to the IPA is the accountable care organization (ACO), a relatively new entity created as part of the Affordable Care Act. Like an IPA, an ACO’s basic purpose is to limit unnecessary spending; but ACOs are typically limited to Medicare and Medicaid recipients, and involve a larger network of doctors and hospitals sharing financial and medical responsibility for patient care. Criteria for limits on spending are established by the Centers for Medicare & Medicaid Services (CMS).
ACOs offer financial incentives to cooperate, and to save money by avoiding unnecessary tests and procedures. A key component is the sharing of information. Providers who save money while also meeting quality targets are theoretically entitled to a portion of the savings. According to federal data, ACOs saved Medicare $4.1 billion in 2020). As of January 2022, 483 ACOs were participating in the Medicare Shared Savings Program. A similar entity designed for private-sector patients is the clinically integrated network (CIN), created by the Federal Trade Commission to serve the commercial or self-insured market, while ACOs treat Medicare and Medicaid patients. Like ACOs, the idea is to work together to improve care and reduce costs by sharing records and tracking data.
When joining any group, read the agreement carefully for any clauses that might infringe on your clinical judgment. In particular, be sure that there are no restrictions on patient treatment or physician referral options for your patients. You should also negotiate an escape clause, allowing you to opt out if you become unhappy with the arrangement.
Clearly, the price of remaining autonomous is significant, and many private practitioners are unwilling to pay it. In 2019, the American Medical Association reported that for the first time, there were fewer physician owners (45.9%) than employees (47.4%).
But as I have written many times, those of us who remain committed to independence will find ways to preserve it. In medicine, as in life, those most responsive to change will survive and flourish.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
COVID drove telehealth forward in high gear: Now what?
Before the pandemic hit in 2019, Pooja Aysola, MD, considered herself lucky because she could tap into telehealth for neurology consults in her work as an emergency department physician.
“We would wheel in a computer screen with a neurologist on board every time we had a suspected stroke patient. And I was able to talk directly to the neurologist about my patient’s symptoms. And it was great,” Dr. Aysola said.
The pandemic, however, prompted the need for telehealth in many situations beyond specialty care. As such, investment exploded over the past few years.
“We’re seeing telehealth across all specialties ... more than half of clinicians are now saying that they do believe that virtual visits will surpass in-person visits for primary care needs,” said Dr. Aysola, who also serves as senior director, clinical operations at Wheel, a Texas-based telehealth company.
Dr. Aysola spoke during an American Telemedicine Association conference panel addressing how COVID prompted an uptick in telehealth investment and utilization and how such virtual care is likely to evolve moving forward.
Nathaniel Lacktman, a partner at law firm Foley & Lardner, agreed with Dr. Aysola’s assessment of the market.
“The appetite for virtual care has become voracious,” said Mr. Lacktman, who chairs the firm’s telemedicine and digital health team. “It reminds me in some ways of taking my kids out to dinner and saying, ‘Try this new food.’ They’re like, ‘No, I won’t like it.’ They finally get a little taste and they’re like, ‘This is amazing.’”
While there is no doubt that stakeholders – from innovators to investors to providers to patients – will want more than just a taste of telehealth in the future, panelists addressed if this undeniable demand for virtual care was simply a short-term response to the pandemic or if there is a long-term desire to fundamentally change how care is delivered.
Expanding on the pandemic-driven ‘sandbox’
While the uptick in telehealth investment and utilization is not expected to continue at such jarring rates in the future, the panelists pointed out that innovation will proceed but perhaps at a different pace.
“The last 3 years have been a sandbox during which the industry was able to experiment,” said Mr. Lacktman. “What we’re going to see more of even post pandemic is building upon that experimental sandbox and creating models that aren’t just high growth and really quick but that are sustainable and meaningful.”
As such, patients and providers won’t be looking for telehealth to simply provide access to care but to provide a full scope of services while also improving quality.
Rachel Stillman, vice president of 7wireVentures, a Chicago-based venture capital firm, also expects interest in telehealth to continue but at a less frenetic pace. In 2021, the industry witnessed nearly $31 billion of venture financing directed towards digital health companies, she said.
“Now, Q1 2022 has had a little bit of a slower start. But with that said, we still have invested $6 billion in early stage companies. So ... we’re seeing some initial signs perhaps of – I don’t want to call it a slowdown – but increased discipline,” Ms. Stillman said.
Start-up companies will need to carefully position themselves for success in this post pandemic environment. “Ultimately, it really goes down to making sure your fundamentals are strong ... and having a really compelling [return on investment] case for your health plan, your self-insured employer, your health system, or your ultimate buyer,” Ms. Stillman said.
Two models are coming into play as innovation continues, she added. One is a traditional care delivery model whereby a start-up organization is building their own provider network specialized for the conditions or patient populations they are serving.
“Conversely, there are new entrants that are thinking about how they can leverage their insightful and strong technology foundations and platforms for existing provider networks that could benefit from a telemedicine partner,” Ms. Stillman pointed out.
Dr. Aysola added that companies are moving forward strategically to achieve post pandemic success. Some telehealth start-ups, for instance, are “capturing some of the low-hanging fruit, the simple UTIs, the really easy things to treat,” Dr. Aysola said.
Others are addressing the clinician’s experience. “Over 50% of clinicians have thought about leaving their jobs at some point during the pandemic. And so it’s becoming really clear that focusing on the clinician and the clinician’s needs are just imperative to [creating a] winning model post-pandemic,” Dr. Aysola said.
Adapting to the new normal
Health care provider organizations also need to adjust to post pandemic realities. “We work with a number of hospital systems, and it’s astounding how slow they are compared to the start-ups because there’s a lot more constituents; there’s bureaucracy,” Mr. Lacktman said. As a result, “the hospitals are in a more uncomfortable position post pandemic than the start-ups.”
To move forward successfully, these organizations, which are typically risk averse, need to create alignment among legal, compliance, and clinical leaders, Mr. Lacktman advised.
One of the first decisions that these teams need to make is whether they should proceed on their own or enter into a partnership with a start-up or pursue a merger and acquisition. In addition, some health systems, hospitals, and health plans are even opting to establish their own venture funds.
“Building your own venture fund or even investing ... in companies directly or in other venture funds [are strategies] that health systems might be able to leverage both to accelerate partnerships and also really be on top of key trends,” Ms. Stillman said.
No matter how health care systems invest in and implement telemedicine technologies, though, the need to move quickly is paramount.
Traditional health care systems “don’t always have the luxury of time. Things have to be done pretty quickly in order to remain competitive,” Dr. Aysola concluded. “We’ve found that companies can launch a virtual care offering in a matter of weeks. When in reality, if a traditional health care system were to try to launch it on their own, it could take upwards of 15 months.”
A version of this article first appeared on Medscape.com.
Before the pandemic hit in 2019, Pooja Aysola, MD, considered herself lucky because she could tap into telehealth for neurology consults in her work as an emergency department physician.
“We would wheel in a computer screen with a neurologist on board every time we had a suspected stroke patient. And I was able to talk directly to the neurologist about my patient’s symptoms. And it was great,” Dr. Aysola said.
The pandemic, however, prompted the need for telehealth in many situations beyond specialty care. As such, investment exploded over the past few years.
“We’re seeing telehealth across all specialties ... more than half of clinicians are now saying that they do believe that virtual visits will surpass in-person visits for primary care needs,” said Dr. Aysola, who also serves as senior director, clinical operations at Wheel, a Texas-based telehealth company.
Dr. Aysola spoke during an American Telemedicine Association conference panel addressing how COVID prompted an uptick in telehealth investment and utilization and how such virtual care is likely to evolve moving forward.
Nathaniel Lacktman, a partner at law firm Foley & Lardner, agreed with Dr. Aysola’s assessment of the market.
“The appetite for virtual care has become voracious,” said Mr. Lacktman, who chairs the firm’s telemedicine and digital health team. “It reminds me in some ways of taking my kids out to dinner and saying, ‘Try this new food.’ They’re like, ‘No, I won’t like it.’ They finally get a little taste and they’re like, ‘This is amazing.’”
While there is no doubt that stakeholders – from innovators to investors to providers to patients – will want more than just a taste of telehealth in the future, panelists addressed if this undeniable demand for virtual care was simply a short-term response to the pandemic or if there is a long-term desire to fundamentally change how care is delivered.
Expanding on the pandemic-driven ‘sandbox’
While the uptick in telehealth investment and utilization is not expected to continue at such jarring rates in the future, the panelists pointed out that innovation will proceed but perhaps at a different pace.
“The last 3 years have been a sandbox during which the industry was able to experiment,” said Mr. Lacktman. “What we’re going to see more of even post pandemic is building upon that experimental sandbox and creating models that aren’t just high growth and really quick but that are sustainable and meaningful.”
As such, patients and providers won’t be looking for telehealth to simply provide access to care but to provide a full scope of services while also improving quality.
Rachel Stillman, vice president of 7wireVentures, a Chicago-based venture capital firm, also expects interest in telehealth to continue but at a less frenetic pace. In 2021, the industry witnessed nearly $31 billion of venture financing directed towards digital health companies, she said.
“Now, Q1 2022 has had a little bit of a slower start. But with that said, we still have invested $6 billion in early stage companies. So ... we’re seeing some initial signs perhaps of – I don’t want to call it a slowdown – but increased discipline,” Ms. Stillman said.
Start-up companies will need to carefully position themselves for success in this post pandemic environment. “Ultimately, it really goes down to making sure your fundamentals are strong ... and having a really compelling [return on investment] case for your health plan, your self-insured employer, your health system, or your ultimate buyer,” Ms. Stillman said.
Two models are coming into play as innovation continues, she added. One is a traditional care delivery model whereby a start-up organization is building their own provider network specialized for the conditions or patient populations they are serving.
“Conversely, there are new entrants that are thinking about how they can leverage their insightful and strong technology foundations and platforms for existing provider networks that could benefit from a telemedicine partner,” Ms. Stillman pointed out.
Dr. Aysola added that companies are moving forward strategically to achieve post pandemic success. Some telehealth start-ups, for instance, are “capturing some of the low-hanging fruit, the simple UTIs, the really easy things to treat,” Dr. Aysola said.
Others are addressing the clinician’s experience. “Over 50% of clinicians have thought about leaving their jobs at some point during the pandemic. And so it’s becoming really clear that focusing on the clinician and the clinician’s needs are just imperative to [creating a] winning model post-pandemic,” Dr. Aysola said.
Adapting to the new normal
Health care provider organizations also need to adjust to post pandemic realities. “We work with a number of hospital systems, and it’s astounding how slow they are compared to the start-ups because there’s a lot more constituents; there’s bureaucracy,” Mr. Lacktman said. As a result, “the hospitals are in a more uncomfortable position post pandemic than the start-ups.”
To move forward successfully, these organizations, which are typically risk averse, need to create alignment among legal, compliance, and clinical leaders, Mr. Lacktman advised.
One of the first decisions that these teams need to make is whether they should proceed on their own or enter into a partnership with a start-up or pursue a merger and acquisition. In addition, some health systems, hospitals, and health plans are even opting to establish their own venture funds.
“Building your own venture fund or even investing ... in companies directly or in other venture funds [are strategies] that health systems might be able to leverage both to accelerate partnerships and also really be on top of key trends,” Ms. Stillman said.
No matter how health care systems invest in and implement telemedicine technologies, though, the need to move quickly is paramount.
Traditional health care systems “don’t always have the luxury of time. Things have to be done pretty quickly in order to remain competitive,” Dr. Aysola concluded. “We’ve found that companies can launch a virtual care offering in a matter of weeks. When in reality, if a traditional health care system were to try to launch it on their own, it could take upwards of 15 months.”
A version of this article first appeared on Medscape.com.
Before the pandemic hit in 2019, Pooja Aysola, MD, considered herself lucky because she could tap into telehealth for neurology consults in her work as an emergency department physician.
“We would wheel in a computer screen with a neurologist on board every time we had a suspected stroke patient. And I was able to talk directly to the neurologist about my patient’s symptoms. And it was great,” Dr. Aysola said.
The pandemic, however, prompted the need for telehealth in many situations beyond specialty care. As such, investment exploded over the past few years.
“We’re seeing telehealth across all specialties ... more than half of clinicians are now saying that they do believe that virtual visits will surpass in-person visits for primary care needs,” said Dr. Aysola, who also serves as senior director, clinical operations at Wheel, a Texas-based telehealth company.
Dr. Aysola spoke during an American Telemedicine Association conference panel addressing how COVID prompted an uptick in telehealth investment and utilization and how such virtual care is likely to evolve moving forward.
Nathaniel Lacktman, a partner at law firm Foley & Lardner, agreed with Dr. Aysola’s assessment of the market.
“The appetite for virtual care has become voracious,” said Mr. Lacktman, who chairs the firm’s telemedicine and digital health team. “It reminds me in some ways of taking my kids out to dinner and saying, ‘Try this new food.’ They’re like, ‘No, I won’t like it.’ They finally get a little taste and they’re like, ‘This is amazing.’”
While there is no doubt that stakeholders – from innovators to investors to providers to patients – will want more than just a taste of telehealth in the future, panelists addressed if this undeniable demand for virtual care was simply a short-term response to the pandemic or if there is a long-term desire to fundamentally change how care is delivered.
Expanding on the pandemic-driven ‘sandbox’
While the uptick in telehealth investment and utilization is not expected to continue at such jarring rates in the future, the panelists pointed out that innovation will proceed but perhaps at a different pace.
“The last 3 years have been a sandbox during which the industry was able to experiment,” said Mr. Lacktman. “What we’re going to see more of even post pandemic is building upon that experimental sandbox and creating models that aren’t just high growth and really quick but that are sustainable and meaningful.”
As such, patients and providers won’t be looking for telehealth to simply provide access to care but to provide a full scope of services while also improving quality.
Rachel Stillman, vice president of 7wireVentures, a Chicago-based venture capital firm, also expects interest in telehealth to continue but at a less frenetic pace. In 2021, the industry witnessed nearly $31 billion of venture financing directed towards digital health companies, she said.
“Now, Q1 2022 has had a little bit of a slower start. But with that said, we still have invested $6 billion in early stage companies. So ... we’re seeing some initial signs perhaps of – I don’t want to call it a slowdown – but increased discipline,” Ms. Stillman said.
Start-up companies will need to carefully position themselves for success in this post pandemic environment. “Ultimately, it really goes down to making sure your fundamentals are strong ... and having a really compelling [return on investment] case for your health plan, your self-insured employer, your health system, or your ultimate buyer,” Ms. Stillman said.
Two models are coming into play as innovation continues, she added. One is a traditional care delivery model whereby a start-up organization is building their own provider network specialized for the conditions or patient populations they are serving.
“Conversely, there are new entrants that are thinking about how they can leverage their insightful and strong technology foundations and platforms for existing provider networks that could benefit from a telemedicine partner,” Ms. Stillman pointed out.
Dr. Aysola added that companies are moving forward strategically to achieve post pandemic success. Some telehealth start-ups, for instance, are “capturing some of the low-hanging fruit, the simple UTIs, the really easy things to treat,” Dr. Aysola said.
Others are addressing the clinician’s experience. “Over 50% of clinicians have thought about leaving their jobs at some point during the pandemic. And so it’s becoming really clear that focusing on the clinician and the clinician’s needs are just imperative to [creating a] winning model post-pandemic,” Dr. Aysola said.
Adapting to the new normal
Health care provider organizations also need to adjust to post pandemic realities. “We work with a number of hospital systems, and it’s astounding how slow they are compared to the start-ups because there’s a lot more constituents; there’s bureaucracy,” Mr. Lacktman said. As a result, “the hospitals are in a more uncomfortable position post pandemic than the start-ups.”
To move forward successfully, these organizations, which are typically risk averse, need to create alignment among legal, compliance, and clinical leaders, Mr. Lacktman advised.
One of the first decisions that these teams need to make is whether they should proceed on their own or enter into a partnership with a start-up or pursue a merger and acquisition. In addition, some health systems, hospitals, and health plans are even opting to establish their own venture funds.
“Building your own venture fund or even investing ... in companies directly or in other venture funds [are strategies] that health systems might be able to leverage both to accelerate partnerships and also really be on top of key trends,” Ms. Stillman said.
No matter how health care systems invest in and implement telemedicine technologies, though, the need to move quickly is paramount.
Traditional health care systems “don’t always have the luxury of time. Things have to be done pretty quickly in order to remain competitive,” Dr. Aysola concluded. “We’ve found that companies can launch a virtual care offering in a matter of weeks. When in reality, if a traditional health care system were to try to launch it on their own, it could take upwards of 15 months.”
A version of this article first appeared on Medscape.com.
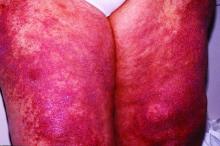
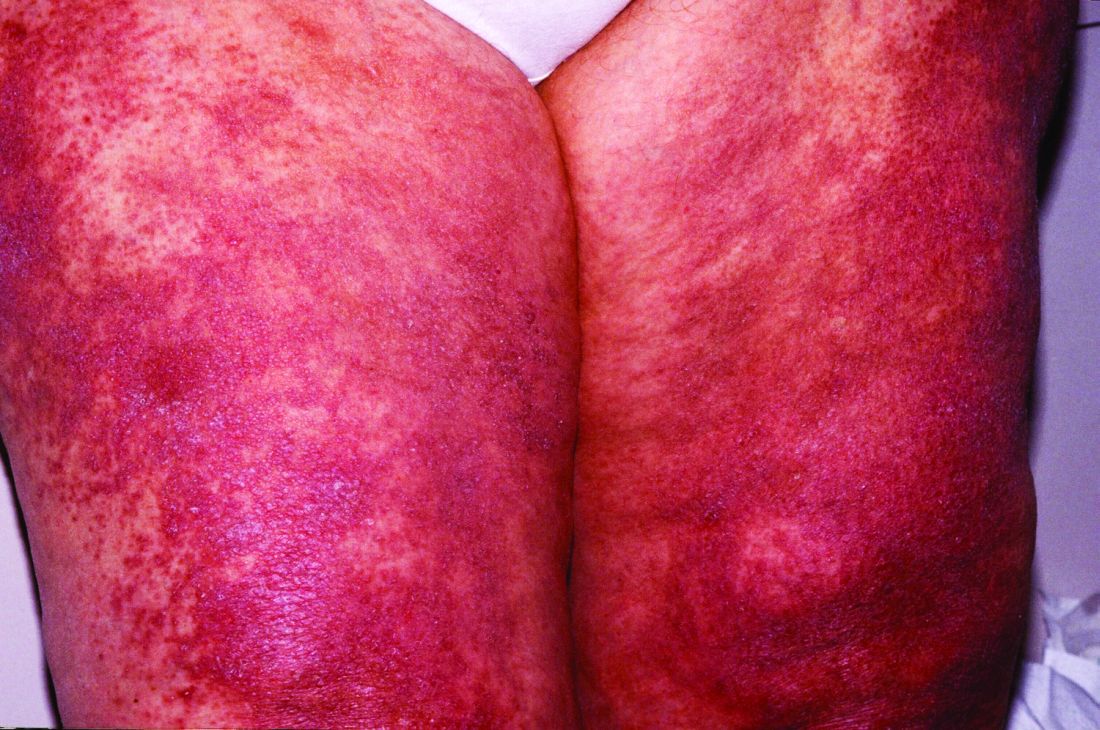
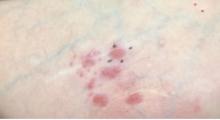
Lenabasum improved skin symptoms in dermatomyositis, but future is uncertain
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Severe infections often accompany severe psoriasis
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
of nearly 95,000 patients.
Although previous studies have shown a higher risk for comorbid conditions in people with psoriasis, compared with those without psoriasis, data on the occurrence of severe and rare infections in patients with psoriasis are limited, wrote Nikolai Loft, MD, of the department of dermatology and allergy, Copenhagen University Hospital, Gentofte, and colleagues.
Psoriasis patients are often treated with immunosuppressive therapies that may promote or aggravate infections; therefore, a better understanding of psoriasis and risk of infections is needed, they said. In a study published in the British Journal of Dermatology, Dr. Loft and his coinvestigators reviewed data on adults aged 18 years and older from the Danish National Patient Register between Jan. 1, 1997 and Dec. 31, 2018. The study population included 94,450 adults with psoriasis and 566,700 matched controls. Patients with any type of psoriasis and any degree of severity were included.
The primary outcome was the occurrence of severe infections, defined as those requiring assessment at a hospital, and rare infections, defined as HIV, TB, HBV, and HCV. The median age of the participants was 52.3 years, and slightly more than half were women.
Overall, the incidence rate of severe and rare infections among patients with any type of psoriasis was 3,104.9 per 100,000 person-years, compared with 2,381.1 for controls, with a hazard ratio, adjusted for gender, age, ethnicity, socioeconomic status, alcohol-related conditions, and Charlson comorbidity index (aHR) of 1.29.
For any infections resulting in hospitalization, the incidence rate was 2,005.1 vs. 1,531.8 per 100,000 person-years for patients with any type of psoriasis and controls, respectively.
The results were similar when severe infections and rare infections were analyzed separately. The incidence rate of severe infections was 3,080.6 and 2,364.4 per 100,000 person-years for patients with any psoriasis, compared with controls; the incidence rate for rare infections was 42.9 and 31.8 for all psoriasis patients and controls, respectively.
When the data were examined by psoriasis severity, the incidence rate of severe and rare infections among patients with severe psoriasis was 3,847.7 per 100,000 person-years, compared with 2,351.9 per 100,000 person years among controls (aHR, 1.58) and also higher than in patients with mild psoriasis. The incidence rate of severe and rare infections in patients with mild psoriasis (2,979.1 per 100,000 person-years) also was higher than in controls (aHR, 1.26).
Factors that might explain the increased infection risk with severe psoriasis include the altered immune environment in these patients, the researchers wrote in their discussion of the findings. Also, “patients with severe psoriasis are defined by their eligibility for systemics, either conventional or biologic,” and their increased infection risk may stem from these treatments, rather than disease severity itself, they noted.
The study findings were limited by several factors including the lack of data on such confounders as weight, body mass index, and smoking status, they added. Other limitations included potential surveillance bias because of greater TB screening, and the use of prescriptions, rather than the Psoriasis Area Severity Index, to define severity. However, the results were strengthened by the large sample size, and suggest that patients with any type of psoriasis have higher rates of any infection, severe or rare, than the general population, the researchers concluded.
Data show need for clinician vigilance
Based on the 2020 Census data, an estimated 7.55 million adults in the United States have psoriasis, David Robles, MD, said in an interview. “Patients with psoriasis have a high risk for multiple comorbid conditions including metabolic syndrome, which is characterized by obesity, hypertension, and dyslipidemia,” said Dr. Robles, a dermatologist in private practice in Pomona, Calif., who was not involved in the study. “Although these complications were previously attributed to diet and obesity, it has become clear that the proinflammatory cytokines associated with psoriasis may be playing an important role underlying the pathologic basis of these other comorbidities.”
There is an emerging body of literature “indicating that psoriasis is associated with an increased risk of infections,” he added. Research in this area is particularly important because of the increased risk of infections associated with many biologic and immune-modulating treatments for psoriasis, Dr. Robles noted.
The study findings “indicate that, as the severity of psoriasis increases, so does the risk of severe and rare infections,” he said. “This makes it imperative for clinicians to be alert to the possibility of severe or rare infections in patients with psoriasis, especially those with severe psoriasis, so that early intervention can be initiated.”
As for additional research, “as an immunologist and dermatologist, I cannot help but think about the possible role the genetic and cytokine pathways involved in psoriasis may be playing in modulating the immune system and/or microbiome, and whether this contributes to a higher risk of infections,” Dr. Robles said. “Just as it was discovered that patients with atopic dermatitis have decreased levels of antimicrobial peptides in their skin, making them susceptible to recurrent bacterial skin infections, we may find that the genetic and immunological changes associated with psoriasis may independently contribute to infection susceptibility,” he noted. “More basic immunology and virology research may one day shed light on this observation.”
The study was supported by Novartis. Lead author Dr. Loft disclosed serving as a speaker for Eli Lilly and Janssen Cilag, other authors disclosed relationships with multiple companies including Novartis, and two authors are Novartis employees. Dr. Robles had no relevant financial disclosures.
FROM BRITISH JOURNAL OF DERMATOLOGY
A 64-year-old woman presents with a history of asymptomatic erythematous grouped papules on the right breast
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
Experts urge stopping melanoma trial because of failure and harm
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
New
The approach seemed promising, given the efficacy of PD-1 and PD-L1 inhibitors in metastatic melanoma, and the relatively short response times to BRAF and MEK inhibitors could potentially be supplemented by longer response times associated with PD-1 and PD-L1 inhibitors. The two categories also have different mechanisms of action and nonoverlapping toxicities, which led to an expectation that the combination would be well tolerated.
But the new study joins two previous randomized, controlled trials that also failed to show much clinical benefit. IMspire150 assigned BRAF V600–mutated melanoma patients to vemurafenib and cobimetinib plus the anti–PD-L1 antibody atezolizumab or placebo. The treatment arm had a small benefit in progression-free survival (hazard ratio, 0.78), which led to Food and Drug Administration approval of the combination, though there was no significant difference when the two cohorts were assessed by an independent review committee. The KEYNOTE-022 trial examined dabrafenib plus trametinib with or without the anti–PD-1 antibody pembrolizumab, and found no difference in investigator-assessed progression free survival.
The new study was published in the Journal of Clinical Oncology. In an accompanying editorial, Margaret K. Callahan, MD, PhD, of Memorial Sloan Kettering Cancer Center, and Paul B. Chapman, MD, of Weill Cornell Medicine, both in New York, speculated that the toxicity of the triplet combination might explain the latest failure, since patients in the triplet arm had more treatment interruptions and dose reductions than the doublet arm (32% received full-dose dabrafenib vs. 54% in the doublet arm), which may have undermined efficacy.
Citing the fact that there are now three randomized, controlled trials with discouraging results, “we believe that there are sufficient data now to be confident that the addition of anti–PD-1 or anti–PD-L1 antibodies to combination RAFi [RAF inhibitors] plus MEKi [MEK inhibitors] is not associated with a significant clinical benefit and should not be studied further in melanoma.
Moreover, “there is some evidence of harm,” the editorial authors wrote. “As the additional toxicity of triplet combination limited the delivery of combination RAFi plus MEKi therapy in COMBI-I. Focus should turn instead to optimizing doses and schedules of combination RAFi plus MEKi and checkpoint inhibitors, developing treatment strategies to overcome resistance to these therapies, and determining how best to sequence combination RAFi plus MEKi therapy and checkpoint inhibitors. Regarding the latter point, there are several sequential therapy trials currently underway in previously untreated patients with BRAF V600–mutated melanoma.”
In the study, patients were randomized to receive dabrafenib and trametinib plus the anti–PD receptor–1 antibody spartalizumab or placebo. After a median follow-up of 27.2 months, mean progression-free survival was 16.2 months in the spartalizumab arm and 12.0 months in the placebo arm (HR, 0.82; P = .042). The spartalizumab group had a 69% objective response rate versus 64% in the placebo group. 55% of the spartalizumab group experienced grade 3 or higher treatment-related adverse events, compared with 33% in the placebo group.
“These results do not support broad use of first-line immunotherapy plus targeted therapy combination, but they provide additional data toward understanding the optimal application of these therapeutic classes in patients with BRAF V600–mutant metastatic melanoma,” the authors of the study wrote.
The study was funded by F Hoffmann–La Roche and Genentech. Dr. Callahan has been employed at Bristol-Myers Squibb, Celgene, and Kleo Pharmaceuticals. Dr. Callahan has consulted for or advised AstraZeneca, Moderna Therapeutics, Merck, and Immunocore. Dr. Chapman has stock or ownership interest in Rgenix; has consulted for or advised Merck, Pfizer, and Black Diamond Therapeutics; and has received research funding from Genentech.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Are physician white coats becoming obsolete? How docs dress for work now
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Early in the COVID-19 pandemic, Trisha Pasricha, MD, a gastroenterologist and research fellow at Massachusetts General Hospital in Boston, was talking to a patient who had been hospitalized for a peptic ulcer.
Like other physicians in her institution, Dr. Pasricha was wearing scrubs instead of a white coat, out of concern that the white coat might be more prone to accumulating or transmitting COVID-19 pathogens. Her badge identified her as a physician, and she introduced herself clearly as “Dr. Pasricha.”
The patient “required an emergent procedure, which I discussed with him,” Dr. Pasricha told this news organization. “I went over what the procedure entailed, the risks and benefits, and the need for informed consent. The patient nodded and seemed to understand, but at the end of the discussion he said: ‘That all sounds fine, but I need to speak to the doctor first.’ ”
Dr. Pasricha was taken aback. She wondered: “Who did he think I was the whole time that I was reviewing medical concerns, explaining medical concepts, and describing a procedure in a way that a physician would describe it?”
She realized the reason he didn’t correctly identify her was that, clad only in scrubs, she was less easily recognizable as a physician. And to be misidentified as technicians, nurses, physician assistants, or other health care professionals, according to Dr. Pasricha.
Dr. Pasricha said she has been the recipient of this “implicit bias” not only from patients but also from members of the health care team, and added that other female colleagues have told her that they’ve had similar experiences, especially when they’re not wearing a white coat.
Changing times, changing trends
When COVID-19 began to spread, “there was an initial concern that COVID-19 was passed through surfaces, and concerns about whether white coats could carry viral particles,” according to Jordan Steinberg, MD, PhD, surgical director of the craniofacial program at Nicklaus Children’s Pediatric Specialists/Nicklaus Children’s Health System, Miami. “Hospitals didn’t want to launder the white coats as frequently as scrubs, due to cost concerns. There was also a concern raised that a necktie might dangle in patients’ faces, coming in closer contact with pathogens, so more physicians were wearing scrubs.”
Yet even before the pandemic, physician attire in hospital and outpatient settings had started to change. Dr. Steinberg, who is also a clinical associate professor at Florida International University, Miami, told this news organization that, in his previous appointment at Johns Hopkins University, Baltimore, he and his colleagues “had noticed in our institution, as well as other facilities, an increasing trend that moved from white coats worn over professional attire toward more casual dress among medical staff – increased wearing of casual fleece or softshell jackets with the institutional logo.”
This was especially true with trainees and the “younger generation,” who were preferring “what I would almost call ‘warm-up clothes,’ gym clothes, and less shirt-tie-white-coat attire for men or white-coats-and-business attire for women.” Dr. Steinberg thinks that some physicians prefer the fleece with the institutional logo “because it’s like wearing your favorite sports team jersey. It gives a sense of belonging.”
Todd Shaffer, MD, MBA, a family physician at University Physicians Associates, Truman Medical Centers and the Lakewood Medical Pavilion, Kansas City, Mo., has been at his institution for 30 years and has seen a similar trend. “At one point, things were very formal,” he told this news organization. But attire was already becoming less formal before the pandemic, and new changes took place during the pandemic, as physicians began wearing scrubs instead of white coats because of fears of viral contamination.
Now, there is less concern about potential viral contamination with the white coat. Yet many physicians continue to wear scrubs – especially those who interact with patients with COVID – and it has become more acceptable to do so, or to wear personal protective equipment (PPE) over ordinary clothing, but it is less common in routine clinical practice, said Dr. Shaffer, a member of the board of directors of the American Academy of Family Physicians.
“The world has changed since COVID. People feel more comfortable dressing more casually during professional Zoom calls, when they have the convenience of working from home,” said Dr. Shaffer, who is also a professor of family medicine at University of Missouri–Kansas City.
Dr. Shaffer himself hasn’t worn a white coat for years. “I’m more likely to wear medium casual pants. I’ve bought some nicer shirts, so I still look professional and upbeat. I don’t always tuck in my shirt, and I don’t dress as formally.” He wears PPE and a mask and/or face shield when treating patients with COVID-19. And he wears a white coat “when someone wants a photograph taken with the doctors – with the stethoscope draped around my neck.”
Traditional symbol of medicine
Because of the changing mores, Dr. Steinberg and colleagues at Johns Hopkins wondered if there might still be a role for professional attire and white coats and what patients prefer. To investigate the question, they surveyed 487 U.S. adults in the spring of 2020.
Respondents were asked where and how frequently they see health care professionals wearing white coats, scrubs, and fleece or softshell jackets. They were also shown photographs depicting models wearing various types of attire commonly seen in health care settings and were asked to rank the “health care provider’s” level of experience, professionalism, and friendliness.
The majority of participants said they had seen health care practitioners in white coats “most of the time,” in scrubs “sometimes,” and in fleece or softshell jackets “rarely.” Models in white coats were regarded by respondents as more experienced and professional, although those in softshell jackets were perceived as friendlier.
There were age as well as regional differences in the responses, Dr. Steinberg said. Older respondents were significantly more likely than their younger counterparts to perceive a model wearing a white coat over business attire as being more experienced, and – in all regions of the United States except the West coast – respondents gave lower professionalism scores to providers wearing fleece jackets with scrubs underneath.
Respondents tended to prefer surgeons wearing a white coat with scrubs underneath, while a white coat over business attire was the preferred dress code for family physicians and dermatologists.
“People tended to respond as if there was a more professional element in the white coat. The age-old symbol of the white coat still marked something important,” Dr. Steinberg said. “Our data suggest that the white coat isn’t ready to die just yet. People still see an air of authority and a traditional symbol of medicine. Nevertheless, I do think it will become less common than it used to be, especially in certain regions of the country.”
Organic, subtle changes
Christopher Petrilli, MD, assistant professor at New York University, conducted research in 2018 regarding physician attire by surveying over 4,000 patients in 10 U.S. academic hospitals. His team found that most patients continued to prefer physicians to wear formal attire under a white coat, especially older respondents.
Dr. Petrilli and colleagues have been studying the issue of physician attire since 2015. “The big issue when we did our initial study – which might not be accurate anymore – is that few hospitals actually had a uniform dress code,” said Dr. Petrilli, the medical director of clinical documentation improvement and the clinical lead of value-based medicine at NYU Langone Hospitals. “When we looked at ‘honor roll hospitals’ during our study, we cold-called these hospitals and also looked online for their dress code policies. Except for the Mayo Clinic, hospitals that had dress code policies were more generic.”
For example, the American Medical Association guidance merely states that attire should be “clean, unsoiled, and appropriate to the setting of care” and recommends weighing research findings regarding textile transmission of health care–associated infections when individual institutions determine their dress code policies. The AMA’s last policy discussion took place in 2015 and its guidance has not changed since the pandemic.
Regardless of what institutions and patients prefer, some research suggests that many physicians would prefer to stay with wearing scrubs rather than reverting to the white coat. One study of 151 hospitalists, conducted in Ireland, found that three-quarters wanted scrubs to remain standard attire, despite the fact that close to half had experienced changes in patients› perception in the absence of their white coat and “professional attire.”
Jennifer Workman, MD, assistant professor of pediatrics, division of pediatric critical care, University of Utah, Salt Lake City, said in an interview that, as the pandemic has “waxed and waned, some trends have reverted to what they were prepandemic, but other physicians have stayed with wearing scrubs.”
Much depends on practice setting, said Dr. Workman, who is also the medical director of pediatric sepsis at Intermountain Care. In pediatrics, for example, many physicians prefer not to wear white coats when they are interacting with young children or adolescents.
Like Dr. Shaffer, Dr. Workman has seen changes in physicians’ attire during video meetings, where they often dress more casually, perhaps wearing sweatshirts. And in the hospital, more are continuing to wear scrubs. “But I don’t see it as people trying to consciously experiment or push boundaries,” she said. “I see it as a more organic, subtle shift.”
Dr. Petrilli thinks that, at this juncture, it’s “pretty heterogeneous as to who is going to return to formal attire and a white coat and who won’t.” Further research needs to be done into currently evolving trends. “We need a more thorough survey looking at changes. We need to ask [physician respondents]: ‘What is your current attire, and how has it changed?’ ”
Navigating the gender divide
In their study, Dr. Steinberg and colleagues found that respondents perceived a male model wearing business attire underneath any type of outerwear (white coat or fleece) to be significantly more professional than a female model wearing the same attire. Respondents also perceived males wearing scrubs to be more professional than females wearing scrubs.
Male models in white coats over business attire were also more likely to be identified as physicians, compared with female models in the same attire. Females were also more likely to be misidentified as nonphysician health care professionals.
Shikha Jain, MD, assistant professor of medicine at the University of Illinois Cancer Center in Chicago, said that Dr. Steinberg’s study confirmed experiences that she and other female physicians have had. Wearing a white coat makes it more likely that a patient will identify you as a physician, but women are less likely to be identified as physicians, regardless of what they wear.
“I think that individuals of color and especially people with intersectional identities – such as women of color – are even more frequently targeted and stereotyped. Numerous studies have shown that a person of color is less likely to be seen as an authority figure, and studies have shown that physicians of color are less likely to be identified as ‘physicians,’ compared to a Caucasian individual,” she said.
Does that mean that female physicians should revert back to prepandemic white coats rather than scrubs or more casual attire? Not necessarily, according to Dr. Jain.
“The typical dress code guidance is that physicians should dress ‘professionally,’ but what that means is a question that needs to be addressed,” Dr. Jain said. “Medicine has evolved from the days of house calls, in which one’s patient population is a very small, intimate group of people in the physician’s community. Yet now, we’ve given rebirth to the ‘house call’ when we do telemedicine with a patient in his or her home. And in the old days, doctors often had offices their homes and now, with telemedicine, patients often see the interior of their physician’s home.” As the delivery of medicine evolves, concepts of “professionalism” – what is defined as “casual” and what is defined as “formal” – is also evolving.
The more important issue, according to Dr. Jain, is to “continue the conversation” about the discrepancies between how men and women are treated in medicine. Attire is one arena in which this issue plays out, and it’s a “bigger picture” that goes beyond the white coat.
Dr. Jain has been “told by patients that a particular outfit doesn’t make me look like a doctor or that scrubs make me look younger. I don’t think my male colleagues have been subjected to these types of remarks, but my female colleagues have heard them as well.”
Even fellow health care providers have commented on Dr. Jain’s clothing. She was presenting at a major medical conference via video and was wearing a similar outfit to the one she wore for her headshot. “Thirty seconds before beginning my talk, one of the male physicians said: ‘Are you wearing the same outfit you wore for your headshot?’ I can’t imagine a man commenting that another man was wearing the same jacket or tie that he wore in the photograph. I found it odd that this was something that someone felt the need to comment on right before I was about to address a large group of people in a professional capacity.”
Addressing these systemic issues “needs to be done and amplified not only by women but also by men in medicine,” said Dr. Jain, founder and director of Women in Medicine, an organization consisting of women physicians whose goal is to “find and implement solutions to gender inequity.”
Dr. Jain said the organization offers an Inclusive Leadership Development Lab – a course specifically for men in health care leadership positions to learn how to be more equitable, inclusive leaders.
A personal decision
Dr. Pasricha hopes she “handled the patient’s misidentification graciously.” She explained to him that she would be the physician conducting the procedure. The patient was initially “a little embarrassed” that he had misidentified her, but she put him at ease and “we moved forward quickly.”
At this point, although some of her colleagues have continued to wear scrubs or have returned to wearing fleeces with hospital logos, Dr. Pasricha prefers to wear a white coat in both inpatient and outpatient settings because it reduces the likelihood of misidentification.
And white coats can be more convenient – for example, Dr. Jain likes the fact that the white coat has pockets where she can put her stethoscope and other items, while some of her professional clothes don’t always have pockets.
Dr. Jain noted that there are some institutions where everyone seems to wear white coats, not only the physician – “from the chaplain to the phlebotomist to the social worker.” In those settings, the white coat no longer distinguishes physicians from nonphysicians, and so wearing a white coat may not confer additional credibility as a physician.
Nevertheless, “if you want to wear a white coat, if you feel it gives you that added level of authority, if you feel it tells people more clearly that you’re a physician, by all means go ahead and do so,” she said. “There’s no ‘one-size-fits-all’ strategy or solution. What’s more important than your clothing is your professionalism.”
A version of this article first appeared on Medscape.com.
Myositis guidelines aim to standardize adult and pediatric care
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
All patients with idiopathic inflammatory myopathies (IIM) should be screened for swallowing difficulties, according to the first evidence-based guideline to be produced.
The guideline, which has been developed by a working group of the British Society for Rheumatology (BSR), also advises that all diagnosed patients should have their myositis antibody levels checked and have their overall well-being assessed. Other recommendations for all patients include the use of glucocorticoids to reduce muscle inflammation and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) for long-term treatment.
“Finally, now, we’re able to standardize the way we treat adults and children with IIM,” senior guideline author Hector Chinoy, PhD, said at the society’s annual meeting.
It has been a long labor of love, however, taking 4 years to get the guideline published, said Dr. Chinoy, professor of rheumatology and neuromuscular disease at the University of Manchester (England), and a consultant at Salford (England) Royal Hospital.
“We’re not covering diagnosis, classification, or the investigation of suspected IIM,” said Dr. Chinoy. Inclusion body myositis also is not included.
Altogether, there are 13 recommendations that have been developed using a PICO (patient or population, intervention, comparison, outcome) format, graded based on the quality of the available evidence, and then voted on by the working group members to give a score of the strength of agreement. Dr. Chinoy noted that there was a checklist included in the Supplementary Data section of the guideline to help follow the recommendations.
“The target audience for the guideline reflects the variety of clinicians caring for patients with IIM,” Dr. Chinoy said. So that is not just pediatric and adult rheumatologists, but also neurologists, dermatologists, respiratory physicians, oncologists, gastroenterologists, cardiologists, and of course other health care professionals. This includes rheumatology and neurology nurses, psychologists, speech and language therapists, and podiatrists, as well as rheumatology specialist pharmacists, physiotherapists, and occupational therapists.
With reference to the latter, Liza McCann, MBBS, who co-led the development of the guideline, said in a statement released by the BSR that the guideline “highlights the importance of exercise, led and monitored by specialist physiotherapists and occupational therapists.”
Dr. McCann, a consultant pediatric rheumatologist at Alder Hey Hospital, Liverpool, England, and Honorary Clinical Lecturer at the University of Liverpool, added that the guidelines also cover “the need to address psychological wellbeing as an integral part of treatment, in parallel with pharmacological therapies.”
Recommendation highlights
Some of the highlights of the recommendations include the use of high-dose glucocorticoids to manage skeletal muscle inflammation at the time of treatment induction, with specific guidance on the different doses to use in adults and in children. There also is guidance on the use of csDMARDs in both populations and what to use if there is refractory disease – with the strongest evidence supporting the use of intravenous immunoglobulin (IVIG) or cyclophosphamide, and possibly rituximab and abatacept.
“There is insufficient evidence to recommend JAK inhibition,” Dr. Chinoy said. The data search used to develop the guideline had a cutoff of October 2020, but even now there is only anecdotal evidence from case studies, he added.
Importantly, the guidelines recognize that childhood IIM differs from adult disease and call for children to be managed by pediatric specialists.
“Routine assessment of dysphagia should be considered in all patients,” Dr. Chinoy said, “so ask the question.” The recommendation is that a swallowing assessment should involve a speech and language therapist or gastroenterologist, and that IVIG be considered for active disease and dysphagia that is resistant to other treatments.
There also are recommendations to screen adult patients for interstitial lung disease, consider fracture risk, and screen adult patients for cancer if they have specific risk factors that include older age at onset, male gender, dysphagia, and rapid disease onset, among others.
Separate cancer screening guidelines on cards
“Around one in four patients with myositis will develop cancer within the 3 years either before or after myositis onset,” Alexander Oldroyd, MBChB, PhD, said in a separate presentation at the BSR annual meeting.
“It’s a hugely increased risk compared to the general population, and a great worry for patients,” he added. Exactly why there is an increased risk is not known, but “there’s a big link between the biological onset of cancer and myositis.”
Dr. Oldroyd, who is an NIHR Academic Clinical Lecturer at the University of Manchester in England and a coauthor of the BSR myositis guideline, is part of a special interest group set up by the International Myositis Assessment and Clinical Studies Group (IMACS) that is in the process of developing separate guidelines for cancer screening in people newly diagnosed with IIM.
The aim was to produce evidence-based recommendations that were both “pragmatic and practical,” that could help clinicians answer patient’s questions on their risk and how best and how often to screen them, Dr. Oldroyd explained. Importantly, IMACS has endeavored to create recommendations that should be applicable across different countries and health care systems.
“We had to acknowledge that there’s not a lot of evidence base there,” Dr. Oldroyd said, noting that he and colleagues conducted a systematic literature review and meta-analysis and used a Delphi process to draft 20 recommendations. These cover identifying risk factors for cancer in people with myositis and categorizing people into low, medium, and high-risk categories. The recommendations also cover what should constitute basic and enhanced screening, and how often someone should be screened.
Moreover, the authors make recommendations on the use of imaging modalities such as PET and CT scans, as well as upper and lower gastrointestinal endoscopy and naso-endoscopy.
“As rheumatologists, we don’t talk about cancer a lot,” Dr. Oldroyd said. “We pick up a lot of incidental cancers, but we don’t usually talk about cancer screening with patients.” That’s something that needs to change, he said.
“It’s important – just get it out in the open, talk to people about it,” Dr. Oldroyd said.
“Tell them what you’re wanting to do, how you’re wanting to investigate for it, clearly communicate their risk,” he said. “But also acknowledge the limited evidence as well, and clearly communicate the results.”
Dr. Chinoy acknowledged he had received fees for presentations (UCB, Biogen), consultancy (Alexion, Novartis, Eli Lilly, Orphazyme, AstraZeneca), or grant support (Eli Lilly, UCB) that had been paid via his institution for the purpose of furthering myositis research. Dr. Oldroyd had no conflicts of interest to disclose.
FROM BSR 2022