User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Patients With Refractory Systemic Sclerosis Have Early Success With CAR T-Cell Therapy
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
CD19-targeting chimeric antigen receptor (CAR) T-cell therapy shows potential to intercept fibrotic organ manifestations and improve disease measures in patients with diffuse cutaneous systemic sclerosis (SSc) who had disease progression despite multiple previous treatments.
METHODOLOGY:
- Researchers conducted a case series to examine the effect of CD19-targeting CAR T-cell therapy on fibrotic and vascular organ manifestations in six patients with diffuse cutaneous SSc (median age, 42 years; four men and two women) who had an insufficient response to at least two previous treatments.
- Participants received CD19-targeting CAR T-cell treatment at a dose of 1 × 106 CAR T cells per kilogram of body weight after lymphodepletion with fludarabine and cyclophosphamide.
- The primary outcome was event-free time or treatment intensification after study entry, with events defined as the progression of interstitial lung disease, onset of congestive heart or renal failure or arterial hypertension, or initiation of new therapy.
- The secondary outcomes included changes in the modified Rodnan skin score (mRSS), imaging and laboratory assessments, patient-reported outcomes, and the modified American College of Rheumatology Composite Response Index in Systemic Sclerosis (ACR-CRISS), assessed at baseline and 3, 6, 9, and 12 months after treatment.
TAKEAWAY:
- No progression of organ manifestations or new lung, cardiac, or renal events occurred within the median follow-up period of 487 days.
- The probability of improvement in the ACR-CRISS score increased to a median value of 100% within 6 and 12 months of CAR T-cell treatment compared with baseline.
- Skin involvement improved in all the patients after CAR T-cell treatment, with a median mRSS decrease of 8 points within 100 days; the improvements were maintained throughout the 1-year follow-up period.
- This treatment also led to a depletion of antinuclear antibodies and SSc-specific autoantibodies.
IN PRACTICE:
“This case series highlights the potential of CAR T-cell therapy to address a crucial unmet need in refractory systemic sclerosis treatment. The study’s most significant contribution is the demonstration that CD19-targeting CAR T-cell therapy can halt or reverse aspects of fibrosis in systemic sclerosis,” Jérôme Avouac, Service de Rhumatologie, Hôpital Cochin, AP-HP Centre-Université Paris Cité, Paris, France, wrote in an accompanying editorial.
SOURCE:
The study was led by Janina Auth, MD, Deutsches Zentrum Immuntherapie, Friedrich-Alexander-Universität Erlangen-Nürnberg and Universitätsklinikum Erlangen in Germany, and was published online on November 11, 2024, in The Lancet Rheumatology.
LIMITATIONS:
The study lacked a control group, which limited the ability to draw definitive conclusions about the efficacy of CD19-targeting CAR T-cell therapy compared with standard treatments. The unpredictable nature of SSc, in which periods of stability can occur spontaneously, makes it difficult to attribute the improvements merely to the intervention. Moreover, the effect of CAR T-cell therapy on other disease manifestations, such as pulmonary hypertension, myocardial involvement, and scleroderma renal crisis, remains unclear.
DISCLOSURES:
The study was funded by Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, ELAN Foundation Erlangen, Interdisziplinäres Zentrum für Klinische Forschung Erlangen, Bundesministerium für Bildung und Forschung, and the European Union. Some authors reported receiving research grants, consulting fees, speaker fees, honoraria, or travel grants from Boehringer Ingelheim, Novartis, Almirall, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Topical Tapinarof Approved for Treating Atopic Dermatitis, Ages 2 and Up
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
An aryl hydrocarbon receptor agonist, tapinarof cream, 1% was first approved in May 2022 for the topical treatment of plaque psoriasis in adults.
According to a press release from the manufacturer, Organon — which markets tapinarof cream, 1%, under the brand name VTAMA — the new indication for AD is based on results from the ADORING pivotal studies. In ADORING 1, the proportion of patients in the tapinarof cream, 1% treatment group who achieved a score of clear (0) or almost clear (1) and a minimum 2-grade improvement from baseline at week 8 on the Validated Investigator Global Assessment for AD was 45.4%, compared with 13.9% of patients who received vehicle alone. ADORING 2 yielded similar results (46.4% vs 18.0%, respectively; P < .0001 for both associations).
Secondary endpoints measured at week 8 also significantly favored the treatment group over the vehicle group, including the Eczema Area and Severity Index score improvement of at least 75% from baseline and achievement of a ≥ 4-point improvement in the patient-reported Peak Pruritus Numerical Rating Scale from baseline.
The most common adverse reactions (incidence ≥ 1%) were upper respiratory tract infection (12%), folliculitis (9%), lower respiratory tract infection (5%), headache (4%), asthma (2%), vomiting (2%), ear infection (2%), pain in extremity (2%), and abdominal pain (1%), according to the release.
Among 728 patients in the ADORING studies who enrolled in an open-label 48-week extension trial (ADORING 3), 378 entered with or achieved complete disease clearance and discontinued treatment. In this subset of patients, the mean duration of the first treatment-free interval was approximately 80 consecutive days, according to the release.
A version of this article first appeared on Medscape.com.
FDA Approves Cosibelimab for Cutaneous SCC
The programmed death ligand-1 (PD-L1)–blocking antibody is the first and only treatment of its kind approved for advanced CSCC, according to a Checkpoint Therapeutics press release. The FDA approval was based on findings from the multicenter, open-label Study CK-301-101 trial of 109 patients.
In that trial, the objective response rate (ORR) was 47% in 78 patients with metastatic CSCC and 48% in 31 patients with locally advanced CSCC. Median duration of response (DOR) in treated patients was not reached in those with metastatic disease and was 17.7 months in those with locally advanced disease, according to the FDA approval notice.
Adverse reactions occurring in at least 10% of patients included fatigue, musculoskeletal pain, rash, diarrhea, hypothyroidism, constipation, nausea, headache, pruritus, edema, localized infection, and urinary tract infection.
The recommended treatment dose, according to the prescribing information, is 1200 mg given as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
The agent offers “a differentiated treatment option versus available therapies by binding to PD-L1, rather than programmed death receptor-1 (PD-1), to release the inhibitory effects of PD-L1 on the anti-tumor immune response,” Checkpoint Therapeutics president and chief executive officer James Oliviero stated in the company press release.
The agent has also “demonstrated the ability to induce antibody-dependent cell-mediated cytotoxicity, another potential differentiating feature of the drug compared to existing marketing therapies for CSCC,” Oliviero noted.
“CSCC is the second most common form of skin cancer, and those diagnosed with advanced disease that has recurred or metastasized face a poor prognosis,” stated Emily Ruiz, MD, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital and director of the High-Risk Skin Cancer Clinic at Dana-Farber Brigham Cancer Center.
“With its dual mechanisms of action and compelling safety profile, this promising drug will provide US oncologists with an important new immunotherapy option for the treatment of CSCC,” she added.
A version of this article appeared on Medscape.com.
The programmed death ligand-1 (PD-L1)–blocking antibody is the first and only treatment of its kind approved for advanced CSCC, according to a Checkpoint Therapeutics press release. The FDA approval was based on findings from the multicenter, open-label Study CK-301-101 trial of 109 patients.
In that trial, the objective response rate (ORR) was 47% in 78 patients with metastatic CSCC and 48% in 31 patients with locally advanced CSCC. Median duration of response (DOR) in treated patients was not reached in those with metastatic disease and was 17.7 months in those with locally advanced disease, according to the FDA approval notice.
Adverse reactions occurring in at least 10% of patients included fatigue, musculoskeletal pain, rash, diarrhea, hypothyroidism, constipation, nausea, headache, pruritus, edema, localized infection, and urinary tract infection.
The recommended treatment dose, according to the prescribing information, is 1200 mg given as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
The agent offers “a differentiated treatment option versus available therapies by binding to PD-L1, rather than programmed death receptor-1 (PD-1), to release the inhibitory effects of PD-L1 on the anti-tumor immune response,” Checkpoint Therapeutics president and chief executive officer James Oliviero stated in the company press release.
The agent has also “demonstrated the ability to induce antibody-dependent cell-mediated cytotoxicity, another potential differentiating feature of the drug compared to existing marketing therapies for CSCC,” Oliviero noted.
“CSCC is the second most common form of skin cancer, and those diagnosed with advanced disease that has recurred or metastasized face a poor prognosis,” stated Emily Ruiz, MD, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital and director of the High-Risk Skin Cancer Clinic at Dana-Farber Brigham Cancer Center.
“With its dual mechanisms of action and compelling safety profile, this promising drug will provide US oncologists with an important new immunotherapy option for the treatment of CSCC,” she added.
A version of this article appeared on Medscape.com.
The programmed death ligand-1 (PD-L1)–blocking antibody is the first and only treatment of its kind approved for advanced CSCC, according to a Checkpoint Therapeutics press release. The FDA approval was based on findings from the multicenter, open-label Study CK-301-101 trial of 109 patients.
In that trial, the objective response rate (ORR) was 47% in 78 patients with metastatic CSCC and 48% in 31 patients with locally advanced CSCC. Median duration of response (DOR) in treated patients was not reached in those with metastatic disease and was 17.7 months in those with locally advanced disease, according to the FDA approval notice.
Adverse reactions occurring in at least 10% of patients included fatigue, musculoskeletal pain, rash, diarrhea, hypothyroidism, constipation, nausea, headache, pruritus, edema, localized infection, and urinary tract infection.
The recommended treatment dose, according to the prescribing information, is 1200 mg given as an intravenous infusion over 60 minutes every 3 weeks until disease progression or unacceptable toxicity.
The agent offers “a differentiated treatment option versus available therapies by binding to PD-L1, rather than programmed death receptor-1 (PD-1), to release the inhibitory effects of PD-L1 on the anti-tumor immune response,” Checkpoint Therapeutics president and chief executive officer James Oliviero stated in the company press release.
The agent has also “demonstrated the ability to induce antibody-dependent cell-mediated cytotoxicity, another potential differentiating feature of the drug compared to existing marketing therapies for CSCC,” Oliviero noted.
“CSCC is the second most common form of skin cancer, and those diagnosed with advanced disease that has recurred or metastasized face a poor prognosis,” stated Emily Ruiz, MD, academic director of the Mohs and Dermatologic Surgery Center at Brigham and Women’s Hospital and director of the High-Risk Skin Cancer Clinic at Dana-Farber Brigham Cancer Center.
“With its dual mechanisms of action and compelling safety profile, this promising drug will provide US oncologists with an important new immunotherapy option for the treatment of CSCC,” she added.
A version of this article appeared on Medscape.com.
A 16-Year-Old Hispanic Male with a History of Hyperlipidemia Reports a Pruritic Rash on His Neck and Chest
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
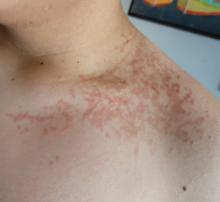
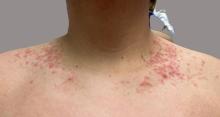
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Discussion
Given the patient’s recent dietary changes, particularly his switch to a ketogenic diet, he was diagnosed with prurigo pigmentosa and treated with doxycycline, which cleared the rash. Prurigo pigmentosa is a rare inflammatory dermatosis characterized by net-like or reticulated pink, and later hyperpigmented, papules and plaques. Although the condition predominantly affects young women of East Asian descent, cases have been reported worldwide, highlighting the importance of considering this diagnosis in diverse populations, including children. Here, we describe a case of prurigo pigmentosa in a young male who had recently adopted a ketogenic diet for weight loss.
The association between prurigo pigmentosa and dietary changes, particularly ketosis, is becoming increasingly recognized. This condition is strongly linked to ketosis, a metabolic state marked by the production of ketone bodies (e.g., beta-hydroxybutyrate and acetoacetate) during carbohydrate restriction, fasting, or ketogenic diets, as seen in our patient. These ketone bodies may act as irritants or trigger oxidative stress and inflammatory cascades in the skin.
Ketoacidosis, particularly in prolonged or intense ketosis, is thought to alter the local skin microenvironment, promoting activation of inflammatory cytokines and immune cells. The ketogenic state is believed to generate oxidative stress through increased free fatty acid oxidation, leading to the production of reactive oxygen species (ROS). ROS can induce apoptosis of keratinocytes and inflammation in the epidermis, which is predominantly mediated by neutrophilic infiltration, as seen in histopathological findings. Elevated levels of pro-inflammatory cytokines, such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), have been implicated in neutrophil recruitment and activation. IL-8 is particularly important for guiding neutrophils to areas of injury.
Secondary hyperpigmentation, a hallmark of this condition, is thought to result from melanin-laden macrophages and persistent melanocyte activation in response to inflammation at the dermo-epidermal junction.
The condition progresses in three stages. In the early stage, lesions appear as pruritic, urticarial plaques. These evolve into crusted erythematous papules and papulovesicles in the middle stage, as observed in our patient. Finally, in the late stage, the lesions mature into smooth, hyperpigmented plaques. Each stage of prurigo pigmentosa has distinct histopathological features.
Differential Diagnosis
The differential diagnosis for prurigo pigmentosa includes several conditions that may present similarly. Allergic contact dermatitis (ACD) can initially mimic the erythematous papules of prurigo pigmentosa, but the absence of a clear allergen exposure and failure to improve with avoidance measures makes ACD less likely. Psoriasis is another possibility, as its erythematous plaques may overlap with prurigo pigmentosa. However, the lack of silvery scales and chronicity makes psoriasis less likely in this case. Eczema, or atopic dermatitis, typically presents with pruritic, ill-defined plaques, often in flexural areas, which were not observed in this patient. Flagellate dermatitis, often caused by exposure to bleomycin or consumption of shiitake mushrooms, can present with linear erythematous lesions resembling prurigo pigmentosa. However, the absence of relevant exposures and a flagellate pattern in this patient rules out this diagnosis.
This case highlights the growing recognition of prurigo pigmentosa in the context of dietary trends, especially ketogenic diets, which have become popular for weight loss and other health benefits. Pediatric populations, in particular, may adopt such diets for various reasons and require careful monitoring, as their physiological responses may differ from those in adults. Prurigo pigmentosa has also been reported in a teenager girl with a history of anorexia nervosa, who was in a ketotic state.
Treatment options for prurigo pigmentosa include antibiotics such as minocycline or doxycycline, or macrolides for 4–10 weeks. Other treatment modalities include dapsone, Q-switch Nd:YAG laser, narrow-band ultraviolet B (UVB) phototherapy, and topical treatments like crisaborole and tacrolimus.
Early recognition of this condition is crucial to avoid unnecessary interventions and to allow for resolution through dietary modification. Dermatologists and pediatricians should maintain a high index of suspicion for prurigo pigmentosa in patients presenting with characteristic eruptions and a history of dietary ketosis.
Catalina Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested Reading
1. Mufti A et al. Clinical Manifestations and Treatment Outcomes in Prurigo Pigmentosa (Nagashima Disease): A Systematic Review of the Literature. JAAD Int. 2021 Apr 10:3:79-87. doi: 10.1016/j.jdin.2021.03.003.
2. Yang J et al. Use of Minocycline for the Treatment of Prurigo Pigmentosa with Intraepidermal Vesiculation: A Case Report. J Int Med Res. 2021 May;49(5):3000605211015593. doi: 10.1177/03000605211015593.
3. Capucilli P et al. Prurigo Pigmentosa: An Itchy, Urticarial Eruption Confused for Food Allergy. J Allergy Clin Immunol Pract. 2018 Jul-Aug;6(4):1381-1382. doi: 10.1016/j.jaip.2018.02.033.
Case Report
A 16-year-old Hispanic male with a history of hyperlipidemia presents to his pediatrician's office for a routine well-child check-up. He reports a pruritic rash on his neck and chest that has been present for the past 1.5 weeks. The rash is itchy, and although a cream from Mexico initially helped, it has not been effective recently. The patient mentions that he has increased his gym workouts and has been training for basketball. He has a history of obesity but has lost almost 100 pounds in the last 6 months. Most recently, he has stopped consuming carbohydrates and has been fasting in the mornings.
There is no history of eczema or psoriasis, either in the patient or his family.
Physical Examination
The patient weighs 147 pounds, a significant decrease from his previous weight of 270 pounds 6 months ago. Other vital signs are within normal limits.
On physical examination, the patient presents with net-like, pink, scaly plaques on his neck, with no other rashes on the body (see Pictures 1 and 2).
Skin Cancer Screening: Biopsy-Free Technology Advancing
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — now in routine use at his own institution.
For skin cancer screening, existing and coming technologies represent “the future of dermatology,” but “we can and should be [already] trying to incorporate these into routine practice,” said Jonathan Ungar, MD, assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York City.
Technologies such as electrical impedance spectroscopy (EIS), optical coherence tomography (OCT), and reflectance confocal microscopy (RCM) have immediate utility for improving skin cancer detection with fewer biopsies, but this is just the beginning, according to Ungar, who is also medical director of the Kimberly and Eric J. Waldman Melanoma and Skin Cancer Center at Mount Sinai, New York City.
“There is going to be a day when we are not cutting to make a diagnosis,” he said during a presentation at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024.
Four Noninvasive Tools Are in Routine Use
Each of these technologies, along with total body photography (TBP), is currently in use at Mount Sinai as well as other tertiary centers to improve diagnostic accuracy at the same time they reduce invasive tests. The initial excitement about these technologies was based on their potential to avoid biopsy in cosmetically sensitive areas, but Ungar suggested that wider application is being driven by better rates of detection, less morbidity, and improved patient satisfaction.
Patients are happy to avoid invasive procedures whenever they can, Ungar noted. In addition to concern about pain or discomfort and a small but measurable risk for infection, patients face a wound that requires healing and the potential for an enduring scar whether the histology is positive for a malignancy.
While none of the four technologies Ungar outlined typically provide a yes or no answer regarding the presence of a malignancy, they do improve diagnostic accuracy with a lower rate of biopsy.
Each Noninvasive Tool Reduces Biopsy Rates
In the case of EIS, for example, the impedance of a painless and harmless electrical current directed into the skin with a handheld probe differentiates normal from abnormal skin through an EIS algorithm. Ungar said it does not require training. A result negative for an abnormality has about a 90% predictive value, and it means that a biopsy can be avoided if there are no other reasons for suspicion.
With a price estimated in the thousands of dollars, the device and software are “not so expensive,” particularly when the tool results in fewer biopsies, Ungar noted.
OCT has a similar profile. Again, used as an adjunct to other types of evaluations, including a history and visual inspection, this helps in modulating suspicion of malignancy. In published studies, OCT has proven superior to dermatoscopy for cancer detection. Citing a 14-study meta-analysis, Ungar said that the sensitivity of OCT for melanoma exceeds, and the specificity approaches, 90%. For basal cell cancers, it is even better.
RCM involves directing a laser into the skin to detect abnormal cells that reflect light. It enables visualization of the skin by layers to the papillary dermis in a detail that is comparable with histology, according to Ungar. Imaging performed with the device used at Mount Sinai (VivaScope 1500, Caliber Imaging & Diagnostics) is reimbursed by Medicare.
Once comfortable with the technology, scanning and interpretation take slightly more time than that required of EIS or OCT, but, like the others, it is painless and helpful for determining whether further evaluation is needed, according to Ungar.
“It is extremely useful in reducing the number of biopsies,” whether melanoma or basal cell malignancies, he said.
Total Body Photograph Helps With Serial Screens
While not specifically a diagnostic tool, TBP can also play a role in reducing biopsies through its highly efficient ability to document the evolution of lesions over time.
As its name implies, essentially the entire body surface is captured by multiple cameras mounted in a circle around the patient. Unlike sequential photos that require far more time to take and store and are challenging to organize and retrieve, the device used at Mount Sinai (Vectra Wb180 1360, Canfield Scientific) can complete the photos in about 2 minutes.
Software for organizing and storing the photos, to which dermatoscope images of individual lesions can be attached if helpful, results in efficient retrieval of photos at sequential visits for evaluating change in any specific lesion.
“It is very easy to use,” according to Ungar, who noted that although the underlying idea is not, the technology of taking, storing, and retrieving photographs has been “perhaps perfected” with this approach.
Noninvasive Screening Training Is Appropriate
Year after year, dermatology residents undergo intensive instruction to master the traditional methods of skin examination with the naked eye and the help of a dermatoscope, but Ungar considers the noninvasive tools to be another step forward. They lower miss rates while reducing the need for histopathology.
Adding these new technologies to routine patient care resonates for many experts, even if the protocols of when to use with the tool are not well established.
Angela J. Lamb, MD, an associate professor of dermatology at Mount Sinai, who has been following the work of Ungar with interest, sees merit in his argument. Not surprisingly, she thinks that any approach shown to boost skin cancer detection is something that deserves attention, but she thinks the effort to safely eliminate biopsies with a low likelihood of a positive finding cannot be ignored.
“Patients want to avoid biopsies when they can,” Lamb told this news organization, and she does not think this is limited to biopsies on the face or other cosmetically sensitive areas.
As a result, she said that she does see the rationale for incorporating the newer technologies into routine care and called this an “important” effort to improve the patient experience as well as reduce missed lesions.
Ungar reported financial relationships with AbbVie, Bristol-Myers Squibb, Castle Biosciences, Dermavant, Janssen Pharmaceuticals, Menlo Therapeutics, Mitsubishi Tanabe Pharma America, and UCB. Lamb reported no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM MSWS 2024
What to Know About Sexually Transmitted Ringworm
Ringworm (also known as tinea, jock itch, or athlete’s foot) is a common infection caused by dermatophyte fungi, known to affect skin, hair, or nails. It causes skin infections that are typically mild and are often treated with topical antifungals.
However, in recent years, newly emerging dermatophyte strains have been causing more severe and harder-to-treat ringworm. Notably, one emerging strain, Trichophyton mentagrophytes genotype VII(TMVII), is associated with sexual contact. In recent years, TMVII infections linked to sexual contact have been reported among men who have sex with men in Europe and in travelers returning from Southeast Asia. The first US case of TMVII was reported in June 2024, after which public health authorities were alerted to additional cases; all were associated with recent sexual contact. Other dermatophyte species have also been reported to cause ringworm transmitted through sexual contact.
Here are some key points to know about sexually transmitted ringworm.
Tell me more about sexually transmitted ringworm: What is causing it?
Skin-to-skin contact is a common mode of ringworm transmission. Infections with sexually transmitted TMVII commonly cause lesions on anatomical sites that may be exposed during intimate or sexual contact, such as the face, genitals, and perianal region. Sexual transmission of TMVII has been reported in Europe, predominantly among men who have sex with men, for several years. Other dermatophyte strains have been reported in association with sexual contact, including the emerging strain Trichophyton indotineae. However, sexual transmission is not the main mode of transmission for T indotineae and other dermatophyte strains.
When should clinicians suspect a potential case of sexually transmitted ringworm?
Providers should consider sexually transmitted ringworm when seeing ringworm in locations associated with intimate contact (for example, a rash on or around the genitals, perianal area, or mouth).
The typical appearance of ringworm is a raised, ring-like, erythematous rash with a scaly border that grows over time. The rash may appear pink, brown, or gray on different types of skin. Patients may note itching and flaking of the rash. In areas with hair such as the beard area, ringworm can present as pustules and be associated with hair loss.
Emerging ringworm infections can present in atypical or more severe ways, including a highly inflammatory (painful, scarring, or otherwise severe) rash, a rash affecting a large area or multiple sites, nodules, and pustules.
Sexually transmitted ringworm may be considered based on sexual history and recent sexual contact with someone with known TMVII. Recent history of travel to a region with reported sexually transmitted ringworm may increase suspicion of TMVII. In patients with a travel history to South Asia, T indotineae should be considered, especially if the rash does not improve with oral terbinafine.
How can testing help guide the diagnosis of sexually transmitted ringworm infection?
When evaluating a rash that may represent ringworm, providers should use a confirmatory test such as potassium hydroxide (KOH) preparation when possible. KOH prep can confirm the presence of a fungus that causes ringworm, but it does not identify the species or type of ringworm. Testing such as fungal culture and molecular testing can help identify specific types of ringworm, but these tests are not often performed and may take a long time to yield results.
Routine fungal cultures cannot identify TMVII and T indotineae; these tests may identify the genus Trichophyton, but only advanced molecular testing, which is available at selected US laboratories, can identify TMVII and T indotineae.
We recommend confirmatory testing because ringworm can easily be misdiagnosed as skin conditions such as psoriasis or eczema. The use of topical steroids can worsen a ringworm infection, so clinicians should be cautious about treating a rash with topical steroids if the etiology is unclear. Treatment should not be delayed if testing is not available.
Clinicians who suspect a case of TMVII infection or infection with another emerging type of severe or antifungal-resistant ringworm can contact the Centers for Disease Control and Prevention (CDC) at fungaloutbreaks@cdc.gov. More details on how clinicians can pursue testing to identify emerging strains of ringworm can be found on the American Academy of Dermatology (AAD) emerging diseases task force website.
How should clinicians treat and manage sexually transmitted ringworm?
If TMVII infection is suspected, providers can consider starting empirical treatment with oral terbinafine. Although data are limited, experience from case series suggests that TMVII may require oral antifungal treatment because it can cause severe skin infections and often does not improve with topical antifungals. Clinicians should advise patients that they may need prolonged treatment courses until the rash resolves, with possible need for treatment courses of 6-8 weeks or longer.
Any diagnosis of a sexually transmitted infection is an opportunity to engage patients in comprehensive sexual health services. Patients with suspected sexually transmitted ringworm should be evaluated for HIV and other sexually transmitted infections, including syphilis, chlamydia, and gonorrhea; clinicians should discuss and facilitate access to other preventive services, such as HIV pre-exposure prophylaxis if the patient is HIV negative and at risk for HIV. Patients should also notify their partner(s) about the diagnosis.
Is sexually transmitted ringworm a public health concern?
It is important to know that very few cases of TMVII have been reported in the United States thus far. CDC continues to monitor emerging dermatophyte strains because these types of ringworm can cause more severe or difficult-to-treat infections. Clinicians should be aware of the potential severity of sexually transmitted ringworm infections and of how diagnosis and treatment of these infections may differ from typical management of ringworm.
So far, TMVII, the dermatophyte strain most associated with spread through sexual contact, has not been documented to have antifungal resistance. More rarely, sexually transmitted ringworm may be caused by other emerging dermatophyte strains that are antifungal resistant, such as T indotineae. Itraconazole is the recommended first-line treatment for T indotineae infections.
How can clinicians counsel patients with sexually transmitted ringworm?
Ringworm can spread with skin-to-skin contact, so patients should avoid such contact with others while they have a rash. They should also avoid sharing personal items (such as razors or towels) and clothing, and launder their clothing, towels, and bedding in a high heat cycle.
People can reduce their risk of getting all types of ringworm infection by keeping their skin clean and dry, changing their socks and underwear daily, and wearing sandals in public locker rooms and other public spaces. People should avoid skin-to-skin contact with anyone with ringworm or an unexplained rash. Before having sex, people can check in with their partners and be aware of unexplained rashes on their partners’ bodies.
Where can clinicians go to learn more about sexually transmitted and other emerging types of ringworm?
CDC has partnered with the AAD to create set of online resources for clinicians for diagnosing and managing emerging dermatophyte infections. Clinicians who suspect or confirm antimicrobial resistant ringworm infection are also encouraged to submit cases to the AAD’s Emerging Diseases Registry. Clinicians wanting further guidance on how to manage suspected or confirmed ringworm infection with an emerging dermatophyte strain can also contact the CDC at fungaloutbreaks@cdc.gov. Useful information on emerging dermatophyte infections for providers and patients is also available on CDC’s website.
Relevant Reading
Zucker J et al. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.Spivack S et al. Emerg Infect Dis. 2024;30:807-809.Jabet A et al. Emerg Infect Dis. 2023;29:1411-1414.
A version of this article appeared on Medscape.com.
Dr Anand is Epidemic Intelligence Service Officer, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia. Dr Gold is Medical Officer, Mycotic Diseases Branch, Centers for Disease Control and Prevention. Dr Quilter is Medical Officer, Division of STD Prevention, Centers for Disease Control and Prevention. None reported any relevant conflicts of interest.
Ringworm (also known as tinea, jock itch, or athlete’s foot) is a common infection caused by dermatophyte fungi, known to affect skin, hair, or nails. It causes skin infections that are typically mild and are often treated with topical antifungals.
However, in recent years, newly emerging dermatophyte strains have been causing more severe and harder-to-treat ringworm. Notably, one emerging strain, Trichophyton mentagrophytes genotype VII(TMVII), is associated with sexual contact. In recent years, TMVII infections linked to sexual contact have been reported among men who have sex with men in Europe and in travelers returning from Southeast Asia. The first US case of TMVII was reported in June 2024, after which public health authorities were alerted to additional cases; all were associated with recent sexual contact. Other dermatophyte species have also been reported to cause ringworm transmitted through sexual contact.
Here are some key points to know about sexually transmitted ringworm.
Tell me more about sexually transmitted ringworm: What is causing it?
Skin-to-skin contact is a common mode of ringworm transmission. Infections with sexually transmitted TMVII commonly cause lesions on anatomical sites that may be exposed during intimate or sexual contact, such as the face, genitals, and perianal region. Sexual transmission of TMVII has been reported in Europe, predominantly among men who have sex with men, for several years. Other dermatophyte strains have been reported in association with sexual contact, including the emerging strain Trichophyton indotineae. However, sexual transmission is not the main mode of transmission for T indotineae and other dermatophyte strains.
When should clinicians suspect a potential case of sexually transmitted ringworm?
Providers should consider sexually transmitted ringworm when seeing ringworm in locations associated with intimate contact (for example, a rash on or around the genitals, perianal area, or mouth).
The typical appearance of ringworm is a raised, ring-like, erythematous rash with a scaly border that grows over time. The rash may appear pink, brown, or gray on different types of skin. Patients may note itching and flaking of the rash. In areas with hair such as the beard area, ringworm can present as pustules and be associated with hair loss.
Emerging ringworm infections can present in atypical or more severe ways, including a highly inflammatory (painful, scarring, or otherwise severe) rash, a rash affecting a large area or multiple sites, nodules, and pustules.
Sexually transmitted ringworm may be considered based on sexual history and recent sexual contact with someone with known TMVII. Recent history of travel to a region with reported sexually transmitted ringworm may increase suspicion of TMVII. In patients with a travel history to South Asia, T indotineae should be considered, especially if the rash does not improve with oral terbinafine.
How can testing help guide the diagnosis of sexually transmitted ringworm infection?
When evaluating a rash that may represent ringworm, providers should use a confirmatory test such as potassium hydroxide (KOH) preparation when possible. KOH prep can confirm the presence of a fungus that causes ringworm, but it does not identify the species or type of ringworm. Testing such as fungal culture and molecular testing can help identify specific types of ringworm, but these tests are not often performed and may take a long time to yield results.
Routine fungal cultures cannot identify TMVII and T indotineae; these tests may identify the genus Trichophyton, but only advanced molecular testing, which is available at selected US laboratories, can identify TMVII and T indotineae.
We recommend confirmatory testing because ringworm can easily be misdiagnosed as skin conditions such as psoriasis or eczema. The use of topical steroids can worsen a ringworm infection, so clinicians should be cautious about treating a rash with topical steroids if the etiology is unclear. Treatment should not be delayed if testing is not available.
Clinicians who suspect a case of TMVII infection or infection with another emerging type of severe or antifungal-resistant ringworm can contact the Centers for Disease Control and Prevention (CDC) at fungaloutbreaks@cdc.gov. More details on how clinicians can pursue testing to identify emerging strains of ringworm can be found on the American Academy of Dermatology (AAD) emerging diseases task force website.
How should clinicians treat and manage sexually transmitted ringworm?
If TMVII infection is suspected, providers can consider starting empirical treatment with oral terbinafine. Although data are limited, experience from case series suggests that TMVII may require oral antifungal treatment because it can cause severe skin infections and often does not improve with topical antifungals. Clinicians should advise patients that they may need prolonged treatment courses until the rash resolves, with possible need for treatment courses of 6-8 weeks or longer.
Any diagnosis of a sexually transmitted infection is an opportunity to engage patients in comprehensive sexual health services. Patients with suspected sexually transmitted ringworm should be evaluated for HIV and other sexually transmitted infections, including syphilis, chlamydia, and gonorrhea; clinicians should discuss and facilitate access to other preventive services, such as HIV pre-exposure prophylaxis if the patient is HIV negative and at risk for HIV. Patients should also notify their partner(s) about the diagnosis.
Is sexually transmitted ringworm a public health concern?
It is important to know that very few cases of TMVII have been reported in the United States thus far. CDC continues to monitor emerging dermatophyte strains because these types of ringworm can cause more severe or difficult-to-treat infections. Clinicians should be aware of the potential severity of sexually transmitted ringworm infections and of how diagnosis and treatment of these infections may differ from typical management of ringworm.
So far, TMVII, the dermatophyte strain most associated with spread through sexual contact, has not been documented to have antifungal resistance. More rarely, sexually transmitted ringworm may be caused by other emerging dermatophyte strains that are antifungal resistant, such as T indotineae. Itraconazole is the recommended first-line treatment for T indotineae infections.
How can clinicians counsel patients with sexually transmitted ringworm?
Ringworm can spread with skin-to-skin contact, so patients should avoid such contact with others while they have a rash. They should also avoid sharing personal items (such as razors or towels) and clothing, and launder their clothing, towels, and bedding in a high heat cycle.
People can reduce their risk of getting all types of ringworm infection by keeping their skin clean and dry, changing their socks and underwear daily, and wearing sandals in public locker rooms and other public spaces. People should avoid skin-to-skin contact with anyone with ringworm or an unexplained rash. Before having sex, people can check in with their partners and be aware of unexplained rashes on their partners’ bodies.
Where can clinicians go to learn more about sexually transmitted and other emerging types of ringworm?
CDC has partnered with the AAD to create set of online resources for clinicians for diagnosing and managing emerging dermatophyte infections. Clinicians who suspect or confirm antimicrobial resistant ringworm infection are also encouraged to submit cases to the AAD’s Emerging Diseases Registry. Clinicians wanting further guidance on how to manage suspected or confirmed ringworm infection with an emerging dermatophyte strain can also contact the CDC at fungaloutbreaks@cdc.gov. Useful information on emerging dermatophyte infections for providers and patients is also available on CDC’s website.
Relevant Reading
Zucker J et al. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.Spivack S et al. Emerg Infect Dis. 2024;30:807-809.Jabet A et al. Emerg Infect Dis. 2023;29:1411-1414.
A version of this article appeared on Medscape.com.
Dr Anand is Epidemic Intelligence Service Officer, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia. Dr Gold is Medical Officer, Mycotic Diseases Branch, Centers for Disease Control and Prevention. Dr Quilter is Medical Officer, Division of STD Prevention, Centers for Disease Control and Prevention. None reported any relevant conflicts of interest.
Ringworm (also known as tinea, jock itch, or athlete’s foot) is a common infection caused by dermatophyte fungi, known to affect skin, hair, or nails. It causes skin infections that are typically mild and are often treated with topical antifungals.
However, in recent years, newly emerging dermatophyte strains have been causing more severe and harder-to-treat ringworm. Notably, one emerging strain, Trichophyton mentagrophytes genotype VII(TMVII), is associated with sexual contact. In recent years, TMVII infections linked to sexual contact have been reported among men who have sex with men in Europe and in travelers returning from Southeast Asia. The first US case of TMVII was reported in June 2024, after which public health authorities were alerted to additional cases; all were associated with recent sexual contact. Other dermatophyte species have also been reported to cause ringworm transmitted through sexual contact.
Here are some key points to know about sexually transmitted ringworm.
Tell me more about sexually transmitted ringworm: What is causing it?
Skin-to-skin contact is a common mode of ringworm transmission. Infections with sexually transmitted TMVII commonly cause lesions on anatomical sites that may be exposed during intimate or sexual contact, such as the face, genitals, and perianal region. Sexual transmission of TMVII has been reported in Europe, predominantly among men who have sex with men, for several years. Other dermatophyte strains have been reported in association with sexual contact, including the emerging strain Trichophyton indotineae. However, sexual transmission is not the main mode of transmission for T indotineae and other dermatophyte strains.
When should clinicians suspect a potential case of sexually transmitted ringworm?
Providers should consider sexually transmitted ringworm when seeing ringworm in locations associated with intimate contact (for example, a rash on or around the genitals, perianal area, or mouth).
The typical appearance of ringworm is a raised, ring-like, erythematous rash with a scaly border that grows over time. The rash may appear pink, brown, or gray on different types of skin. Patients may note itching and flaking of the rash. In areas with hair such as the beard area, ringworm can present as pustules and be associated with hair loss.
Emerging ringworm infections can present in atypical or more severe ways, including a highly inflammatory (painful, scarring, or otherwise severe) rash, a rash affecting a large area or multiple sites, nodules, and pustules.
Sexually transmitted ringworm may be considered based on sexual history and recent sexual contact with someone with known TMVII. Recent history of travel to a region with reported sexually transmitted ringworm may increase suspicion of TMVII. In patients with a travel history to South Asia, T indotineae should be considered, especially if the rash does not improve with oral terbinafine.
How can testing help guide the diagnosis of sexually transmitted ringworm infection?
When evaluating a rash that may represent ringworm, providers should use a confirmatory test such as potassium hydroxide (KOH) preparation when possible. KOH prep can confirm the presence of a fungus that causes ringworm, but it does not identify the species or type of ringworm. Testing such as fungal culture and molecular testing can help identify specific types of ringworm, but these tests are not often performed and may take a long time to yield results.
Routine fungal cultures cannot identify TMVII and T indotineae; these tests may identify the genus Trichophyton, but only advanced molecular testing, which is available at selected US laboratories, can identify TMVII and T indotineae.
We recommend confirmatory testing because ringworm can easily be misdiagnosed as skin conditions such as psoriasis or eczema. The use of topical steroids can worsen a ringworm infection, so clinicians should be cautious about treating a rash with topical steroids if the etiology is unclear. Treatment should not be delayed if testing is not available.
Clinicians who suspect a case of TMVII infection or infection with another emerging type of severe or antifungal-resistant ringworm can contact the Centers for Disease Control and Prevention (CDC) at fungaloutbreaks@cdc.gov. More details on how clinicians can pursue testing to identify emerging strains of ringworm can be found on the American Academy of Dermatology (AAD) emerging diseases task force website.
How should clinicians treat and manage sexually transmitted ringworm?
If TMVII infection is suspected, providers can consider starting empirical treatment with oral terbinafine. Although data are limited, experience from case series suggests that TMVII may require oral antifungal treatment because it can cause severe skin infections and often does not improve with topical antifungals. Clinicians should advise patients that they may need prolonged treatment courses until the rash resolves, with possible need for treatment courses of 6-8 weeks or longer.
Any diagnosis of a sexually transmitted infection is an opportunity to engage patients in comprehensive sexual health services. Patients with suspected sexually transmitted ringworm should be evaluated for HIV and other sexually transmitted infections, including syphilis, chlamydia, and gonorrhea; clinicians should discuss and facilitate access to other preventive services, such as HIV pre-exposure prophylaxis if the patient is HIV negative and at risk for HIV. Patients should also notify their partner(s) about the diagnosis.
Is sexually transmitted ringworm a public health concern?
It is important to know that very few cases of TMVII have been reported in the United States thus far. CDC continues to monitor emerging dermatophyte strains because these types of ringworm can cause more severe or difficult-to-treat infections. Clinicians should be aware of the potential severity of sexually transmitted ringworm infections and of how diagnosis and treatment of these infections may differ from typical management of ringworm.
So far, TMVII, the dermatophyte strain most associated with spread through sexual contact, has not been documented to have antifungal resistance. More rarely, sexually transmitted ringworm may be caused by other emerging dermatophyte strains that are antifungal resistant, such as T indotineae. Itraconazole is the recommended first-line treatment for T indotineae infections.
How can clinicians counsel patients with sexually transmitted ringworm?
Ringworm can spread with skin-to-skin contact, so patients should avoid such contact with others while they have a rash. They should also avoid sharing personal items (such as razors or towels) and clothing, and launder their clothing, towels, and bedding in a high heat cycle.
People can reduce their risk of getting all types of ringworm infection by keeping their skin clean and dry, changing their socks and underwear daily, and wearing sandals in public locker rooms and other public spaces. People should avoid skin-to-skin contact with anyone with ringworm or an unexplained rash. Before having sex, people can check in with their partners and be aware of unexplained rashes on their partners’ bodies.
Where can clinicians go to learn more about sexually transmitted and other emerging types of ringworm?
CDC has partnered with the AAD to create set of online resources for clinicians for diagnosing and managing emerging dermatophyte infections. Clinicians who suspect or confirm antimicrobial resistant ringworm infection are also encouraged to submit cases to the AAD’s Emerging Diseases Registry. Clinicians wanting further guidance on how to manage suspected or confirmed ringworm infection with an emerging dermatophyte strain can also contact the CDC at fungaloutbreaks@cdc.gov. Useful information on emerging dermatophyte infections for providers and patients is also available on CDC’s website.
Relevant Reading
Zucker J et al. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.Spivack S et al. Emerg Infect Dis. 2024;30:807-809.Jabet A et al. Emerg Infect Dis. 2023;29:1411-1414.
A version of this article appeared on Medscape.com.
Dr Anand is Epidemic Intelligence Service Officer, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia. Dr Gold is Medical Officer, Mycotic Diseases Branch, Centers for Disease Control and Prevention. Dr Quilter is Medical Officer, Division of STD Prevention, Centers for Disease Control and Prevention. None reported any relevant conflicts of interest.
Survey Highlights Trends in Pediatric Cosmetic Dermatology Procedures
TOPLINE:
METHODOLOGY:
- An anonymous online survey conducted with SurveyMonkey targeted healthcare providers who routinely used lasers to treat cutaneous conditions in pediatric patients.
- The survey included members of the Society for Pediatric Dermatology and the American Society for Laser Medicine and Surgery and Surgery, as well as fellowship directors and current fellows of the American Society for Dermatologic Surgery.
- A total of 85 practitioners responded to the survey, with 86% answering all questions; respondents primarily included pediatric dermatologists (77.65%), general dermatologists (18.82%), cosmetic dermatologists (8.24%), and dermatologic/Mohs surgeons (1.18%).
TAKEAWAY:
- Hypertrophic or traumatic scars ranked as the most frequently treated pediatric cosmetic condition (95.29%), followed by acne (89.41%), axillary and facial hyperhidrosis (77.65%), hypertrichosis/hirsutism (67.06%), and pigmented lesion removal (64.71%).
- The most common procedures performed were vascular lasers (77.65%), laser hair removal (50.59%), lasers for pigmentation (28.24%), neuromodulators (25.88%), and laser skin resurfacing (22.35%).
- Additional treatments respondents performed included chemical peels (20.00%), radiofrequency microneedling (16.47%), soft tissue fillers (4.71%), and cryolipolysis/body contouring (4.17%).
- About 50% of respondents said they would start cosmetic treatment of acne, and about 66% said they would start laser hair removal treatment between the ages of 12 and 15 years.
IN PRACTICE:
Noting that the survey results provided insight into the types of cosmetic procedures being performed for pediatric patients, the authors wrote, “These interventions can play a significant role in addressing the emotional and social challenges faced by pediatric patients with cosmetic concerns, allowing them to navigate social interactions more confidently and positively.” Before any procedure, they added, “It is important that any comorbid conditions be addressed,” they added, and “ethical considerations regarding informed consent, patient autonomy, and long-term consequences should be carefully weighed, given the vulnerable nature of pediatric patients.”
SOURCE:
The study was led by Lauren Hoffman, MD, who practices dermatology in Great Neck, New York. It was published online in December 2024 in Dermatologic Surgery.
LIMITATIONS:
The study was subjective in nature and had a small sample size, and the exact number of survey recipients was unclear, hindering an accurate calculation of the response rate. The absolute number of responses accounted for a small portion of the total memberships of the participating societies. Also, the data collection periods varied among the three academic societies, and dermatologists’ practice types may have influenced the range and nature of treated conditions.
DISCLOSURES:
The authors did not disclose funding information. They declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- An anonymous online survey conducted with SurveyMonkey targeted healthcare providers who routinely used lasers to treat cutaneous conditions in pediatric patients.
- The survey included members of the Society for Pediatric Dermatology and the American Society for Laser Medicine and Surgery and Surgery, as well as fellowship directors and current fellows of the American Society for Dermatologic Surgery.
- A total of 85 practitioners responded to the survey, with 86% answering all questions; respondents primarily included pediatric dermatologists (77.65%), general dermatologists (18.82%), cosmetic dermatologists (8.24%), and dermatologic/Mohs surgeons (1.18%).
TAKEAWAY:
- Hypertrophic or traumatic scars ranked as the most frequently treated pediatric cosmetic condition (95.29%), followed by acne (89.41%), axillary and facial hyperhidrosis (77.65%), hypertrichosis/hirsutism (67.06%), and pigmented lesion removal (64.71%).
- The most common procedures performed were vascular lasers (77.65%), laser hair removal (50.59%), lasers for pigmentation (28.24%), neuromodulators (25.88%), and laser skin resurfacing (22.35%).
- Additional treatments respondents performed included chemical peels (20.00%), radiofrequency microneedling (16.47%), soft tissue fillers (4.71%), and cryolipolysis/body contouring (4.17%).
- About 50% of respondents said they would start cosmetic treatment of acne, and about 66% said they would start laser hair removal treatment between the ages of 12 and 15 years.
IN PRACTICE:
Noting that the survey results provided insight into the types of cosmetic procedures being performed for pediatric patients, the authors wrote, “These interventions can play a significant role in addressing the emotional and social challenges faced by pediatric patients with cosmetic concerns, allowing them to navigate social interactions more confidently and positively.” Before any procedure, they added, “It is important that any comorbid conditions be addressed,” they added, and “ethical considerations regarding informed consent, patient autonomy, and long-term consequences should be carefully weighed, given the vulnerable nature of pediatric patients.”
SOURCE:
The study was led by Lauren Hoffman, MD, who practices dermatology in Great Neck, New York. It was published online in December 2024 in Dermatologic Surgery.
LIMITATIONS:
The study was subjective in nature and had a small sample size, and the exact number of survey recipients was unclear, hindering an accurate calculation of the response rate. The absolute number of responses accounted for a small portion of the total memberships of the participating societies. Also, the data collection periods varied among the three academic societies, and dermatologists’ practice types may have influenced the range and nature of treated conditions.
DISCLOSURES:
The authors did not disclose funding information. They declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- An anonymous online survey conducted with SurveyMonkey targeted healthcare providers who routinely used lasers to treat cutaneous conditions in pediatric patients.
- The survey included members of the Society for Pediatric Dermatology and the American Society for Laser Medicine and Surgery and Surgery, as well as fellowship directors and current fellows of the American Society for Dermatologic Surgery.
- A total of 85 practitioners responded to the survey, with 86% answering all questions; respondents primarily included pediatric dermatologists (77.65%), general dermatologists (18.82%), cosmetic dermatologists (8.24%), and dermatologic/Mohs surgeons (1.18%).
TAKEAWAY:
- Hypertrophic or traumatic scars ranked as the most frequently treated pediatric cosmetic condition (95.29%), followed by acne (89.41%), axillary and facial hyperhidrosis (77.65%), hypertrichosis/hirsutism (67.06%), and pigmented lesion removal (64.71%).
- The most common procedures performed were vascular lasers (77.65%), laser hair removal (50.59%), lasers for pigmentation (28.24%), neuromodulators (25.88%), and laser skin resurfacing (22.35%).
- Additional treatments respondents performed included chemical peels (20.00%), radiofrequency microneedling (16.47%), soft tissue fillers (4.71%), and cryolipolysis/body contouring (4.17%).
- About 50% of respondents said they would start cosmetic treatment of acne, and about 66% said they would start laser hair removal treatment between the ages of 12 and 15 years.
IN PRACTICE:
Noting that the survey results provided insight into the types of cosmetic procedures being performed for pediatric patients, the authors wrote, “These interventions can play a significant role in addressing the emotional and social challenges faced by pediatric patients with cosmetic concerns, allowing them to navigate social interactions more confidently and positively.” Before any procedure, they added, “It is important that any comorbid conditions be addressed,” they added, and “ethical considerations regarding informed consent, patient autonomy, and long-term consequences should be carefully weighed, given the vulnerable nature of pediatric patients.”
SOURCE:
The study was led by Lauren Hoffman, MD, who practices dermatology in Great Neck, New York. It was published online in December 2024 in Dermatologic Surgery.
LIMITATIONS:
The study was subjective in nature and had a small sample size, and the exact number of survey recipients was unclear, hindering an accurate calculation of the response rate. The absolute number of responses accounted for a small portion of the total memberships of the participating societies. Also, the data collection periods varied among the three academic societies, and dermatologists’ practice types may have influenced the range and nature of treated conditions.
DISCLOSURES:
The authors did not disclose funding information. They declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves IL-31 Inhibitor for Atopic Dermatitis
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
Wound Healing: Dermatologist’s Toolbox Requires Frequent Updates
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
FROM MSWS 2024
An 81-Year-Old White Woman Presented With a 2-Week History of a Painful Lesion on Her Left Calf
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
An 81-year-old White woman with a medical history significant for end stage renal disease (ESRD) on dialysis, diabetes, and a cerebrovascular accident presented with a 2-week history of a very painful lesion on her left calf. Upon physical exam, she was also noted to have tender subcutaneous nodules on her left anterolateral thigh that had been present for several weeks.
What's your diagnosis?