User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FDA clears new biomarker assays for early Alzheimer’s detection
The Elecsys beta-amyloid (1-42) CSF II (Abeta42) and Elecsys total-tau CSF assays (tTau) (used as a tTau/Abeta42 ratio) are for use in adults ages 55 and older being evaluated for AD.
They join the Elecsys beta-amyloid (1-42) CSF II (Abeta42) and Elecsys phospho-tau (181P) CSF (pTau181) assays (used as a pTau181/Abeta42 ratio) that received FDA 510(k) clearance in 2022.
“An early and accurate diagnosis can help patients, caregivers and physicians determine a path forward, and the Elecsys CSF assays support diagnosis at early disease stages, when treatment is most effective,” Brad Moore, president and CEO of Roche Diagnostics North America, said in a statement.
Appropriate use recommendations for new and emerging AD drugs call for confirmation of amyloid pathology. Currently, the only FDA-cleared methods to confirm amyloid pathology are CSF tests and PET scans.
“The Elecsys AD CSF assays are concordant with amyloid PET scan imaging and have the potential to provide a more affordable and accessible routine option to confirm the presence of amyloid pathology in the brain,” Roche said.
“They also offer detection of both amyloid and tau biomarkers from one draw, with no radiation and potential to detect Alzheimer’s pathology in early stages of disease,” the company added.
The previously approved Elecsys pTau181/Abeta42 ratio is currently available and the newly approved Elecsys tTau/Abeta42 ratio will be available in the fourth quarter of 2023.
A version of this article first appeared on Medscape.com.
The Elecsys beta-amyloid (1-42) CSF II (Abeta42) and Elecsys total-tau CSF assays (tTau) (used as a tTau/Abeta42 ratio) are for use in adults ages 55 and older being evaluated for AD.
They join the Elecsys beta-amyloid (1-42) CSF II (Abeta42) and Elecsys phospho-tau (181P) CSF (pTau181) assays (used as a pTau181/Abeta42 ratio) that received FDA 510(k) clearance in 2022.
“An early and accurate diagnosis can help patients, caregivers and physicians determine a path forward, and the Elecsys CSF assays support diagnosis at early disease stages, when treatment is most effective,” Brad Moore, president and CEO of Roche Diagnostics North America, said in a statement.
Appropriate use recommendations for new and emerging AD drugs call for confirmation of amyloid pathology. Currently, the only FDA-cleared methods to confirm amyloid pathology are CSF tests and PET scans.
“The Elecsys AD CSF assays are concordant with amyloid PET scan imaging and have the potential to provide a more affordable and accessible routine option to confirm the presence of amyloid pathology in the brain,” Roche said.
“They also offer detection of both amyloid and tau biomarkers from one draw, with no radiation and potential to detect Alzheimer’s pathology in early stages of disease,” the company added.
The previously approved Elecsys pTau181/Abeta42 ratio is currently available and the newly approved Elecsys tTau/Abeta42 ratio will be available in the fourth quarter of 2023.
A version of this article first appeared on Medscape.com.
The Elecsys beta-amyloid (1-42) CSF II (Abeta42) and Elecsys total-tau CSF assays (tTau) (used as a tTau/Abeta42 ratio) are for use in adults ages 55 and older being evaluated for AD.
They join the Elecsys beta-amyloid (1-42) CSF II (Abeta42) and Elecsys phospho-tau (181P) CSF (pTau181) assays (used as a pTau181/Abeta42 ratio) that received FDA 510(k) clearance in 2022.
“An early and accurate diagnosis can help patients, caregivers and physicians determine a path forward, and the Elecsys CSF assays support diagnosis at early disease stages, when treatment is most effective,” Brad Moore, president and CEO of Roche Diagnostics North America, said in a statement.
Appropriate use recommendations for new and emerging AD drugs call for confirmation of amyloid pathology. Currently, the only FDA-cleared methods to confirm amyloid pathology are CSF tests and PET scans.
“The Elecsys AD CSF assays are concordant with amyloid PET scan imaging and have the potential to provide a more affordable and accessible routine option to confirm the presence of amyloid pathology in the brain,” Roche said.
“They also offer detection of both amyloid and tau biomarkers from one draw, with no radiation and potential to detect Alzheimer’s pathology in early stages of disease,” the company added.
The previously approved Elecsys pTau181/Abeta42 ratio is currently available and the newly approved Elecsys tTau/Abeta42 ratio will be available in the fourth quarter of 2023.
A version of this article first appeared on Medscape.com.
New DEA CME mandate affects 2 million prescribers
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.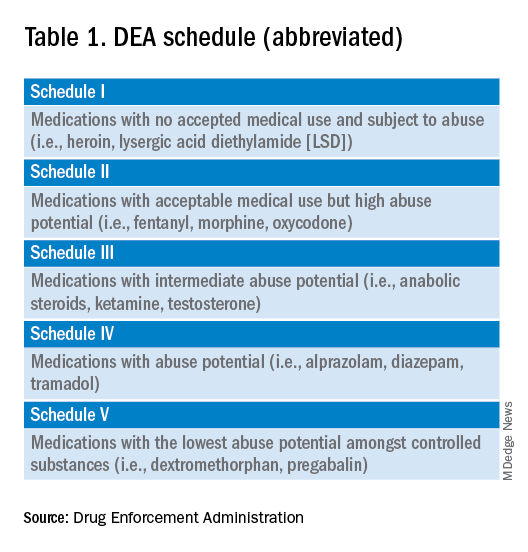
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 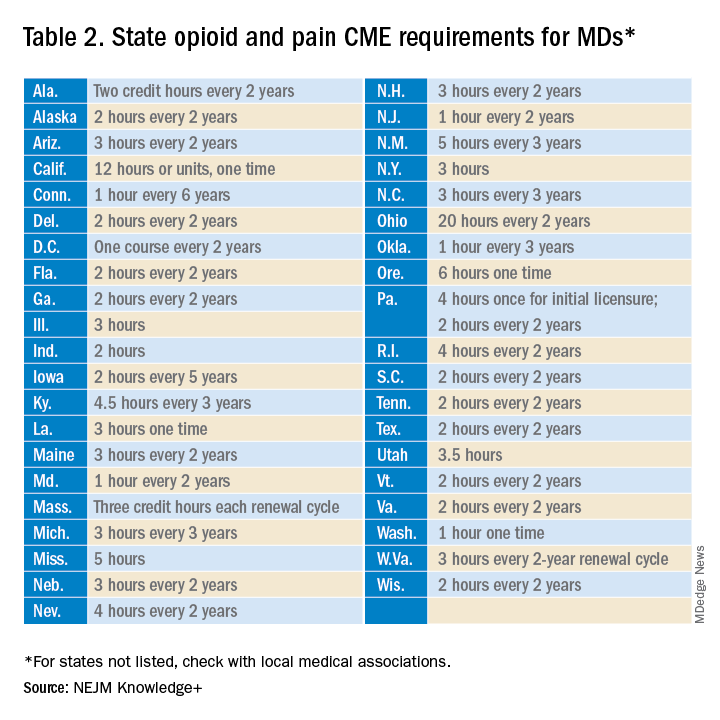
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
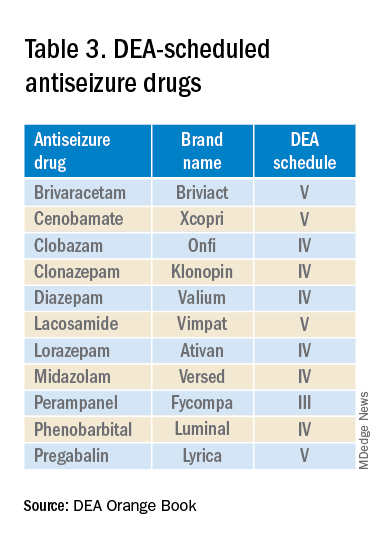
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).
Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The Consolidated Appropriations Act of 2023 mandates that all Drug Enforcement Administration–registered physicians and health care providers complete a one-time, 8-hour CME training on managing and treating opioid and other substance abuse disorders. This requirement goes into effect on June 27, 2023. New DEA registrants must also comply. Veterinarians are exempt.
A DEA registration is required to prescribe any controlled substance. The DEA categorizes these as Schedule I-V, with V being the least likely to be abused (Table 1). For example, opioids like fentanyl, oxycodone, and morphine are Schedule II. Medications without abuse potential are not scheduled.
Will 16 million hours of opioid education save lives?
One should not underestimate the sweeping scope of this new federal requirement. DEA registrants include physicians and other health care providers such as nurse practitioners, physician assistants, and dentists. That is 8 hours per provider x 2 million providers: 16 million hours of CME!
Many states already require 1 or more hours of opioid training and pain management as part of their relicensure requirements (Table 2). To avoid redundancy, the DEA-mandated 8-hour training satisfies the various states’ requirements. 
An uncompensated mandate
Physicians are no strangers to lifelong learning and most eagerly pursue educational opportunities. Though some physicians may have CME time and stipends allocated by their employers, many others, such as the approximately 50,000 locum tenens doctors, do not. However, as enthusiastic as these physicians may be about this new CME course, they will likely lose a day of seeing patients (and income) to comply with this new obligation.
Not just pain doctors
The mandate’s broad brush includes many health care providers who hold DEA certificates but do not prescribe opioids. For example, as a general neurologist and epileptologist, I do not treat patients with chronic pain and cannot remember the last time I wrote an opioid prescription. However, I frequently prescribe lacosamide, a Schedule V drug. A surprisingly large number of antiseizure drugs are Schedule III, IV, or V drugs (Table 3).

Real-world abuse?
How often scheduled antiseizure drugs are diverted or abused in an epilepsy population is unknown but appears to be infrequent. For example, perampanel abuse has not been reported despite its classification as a Schedule III drug. Anecdotally, in more than 40 years of clinical practice, I have never known a patient with epilepsy to abuse their antiseizure medications.
Take the course
Many organizations are happy to charge for the new 8-hour course. For example, the Tennessee Medical Association offers the training for $299 online or $400 in person. Materials from Elite Learning satisfy the 8-hour requirement for $80. However, NEJM Knowledge+ provides a complimentary 10-hour DEA-compliant course.
I recently completed the NEJM course. The information was thorough and took the whole 10 hours to finish. As excellent as it was, the content was only tangentially relevant to my clinical practice.
Conclusions
To obtain or renew a DEA certificate, neurologists, epilepsy specialists, and many other health care providers must comply with the new 8-hour CME opioid training mandate. Because the course requires 1 day to complete, health care providers would be prudent to obtain their CME well before their DEA certificate expires.
Though efforts to control the morbidity and mortality of the opioid epidemic are laudatory, perhaps the training should be more targeted to physicians who actually prescribe opioids rather than every DEA registrant. In the meantime, whether 16 million CME hours will save lives remains to be seen.
Dr. Wilner is professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
No link between heartburn meds and dementia
A new study provides reassurance about the safety of long-term proton pump inhibitor (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
It was published online in Gastroenterology.
The post hoc observational study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston.
The researchers analyzed results from the Aspirin in Reducing Events in the Elderly clinical trial. The randomized trial of aspirin included 18,934 adults aged 65 and older from the United States and Australia. Patients’ use of PPI and H2RA was tracked, along with dementia incidence and cognitive changes.
The results showed that there was no link to new dementia diagnoses in patients who used PPIs (25%) and H2RA (2%) at baseline, versus those who did not use either heartburn medication.
Limitations of prior studies are referenced, including the potential for residual confounding and underestimation of PPI and H2RA use, the lack of data on medication dose and duration, and the absence of apo E4 allele status.
The study was funded by grants from the National Institute on Aging, the National Cancer Institute, and other institutions. Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
A new study provides reassurance about the safety of long-term proton pump inhibitor (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
It was published online in Gastroenterology.
The post hoc observational study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston.
The researchers analyzed results from the Aspirin in Reducing Events in the Elderly clinical trial. The randomized trial of aspirin included 18,934 adults aged 65 and older from the United States and Australia. Patients’ use of PPI and H2RA was tracked, along with dementia incidence and cognitive changes.
The results showed that there was no link to new dementia diagnoses in patients who used PPIs (25%) and H2RA (2%) at baseline, versus those who did not use either heartburn medication.
Limitations of prior studies are referenced, including the potential for residual confounding and underestimation of PPI and H2RA use, the lack of data on medication dose and duration, and the absence of apo E4 allele status.
The study was funded by grants from the National Institute on Aging, the National Cancer Institute, and other institutions. Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
A new study provides reassurance about the safety of long-term proton pump inhibitor (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
It was published online in Gastroenterology.
The post hoc observational study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston.
The researchers analyzed results from the Aspirin in Reducing Events in the Elderly clinical trial. The randomized trial of aspirin included 18,934 adults aged 65 and older from the United States and Australia. Patients’ use of PPI and H2RA was tracked, along with dementia incidence and cognitive changes.
The results showed that there was no link to new dementia diagnoses in patients who used PPIs (25%) and H2RA (2%) at baseline, versus those who did not use either heartburn medication.
Limitations of prior studies are referenced, including the potential for residual confounding and underestimation of PPI and H2RA use, the lack of data on medication dose and duration, and the absence of apo E4 allele status.
The study was funded by grants from the National Institute on Aging, the National Cancer Institute, and other institutions. Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM GASTROENTEROLOGY
Women with atrial fibrillation more likely to develop dementia
New data suggest a significantly stronger link in women compared with men between atrial fibrillation (AF) and mild cognitive impairment (MCI) and dementia.
“Our findings imply that women with AF may be at higher risk for MCI and dementia with potentially more rapid disease progression from normal cognition to MCI or dementia than women without AF or men with and without AF,” wrote authors of a new study led by Kathryn A. Wood, PhD, RN, Neil Hodgson Woodruff School of Nursing at Emory University in Atlanta.
The findings were published online in Alzheimer’s & Dementia.
Researchers used the National Alzheimer’s Coordinating Center data with 43,630 patients and analyzed sex differences between men and women with AF and their performance on neuropsychological tests and cognitive disease progression.
Higher odds of dementia, MCI in women
According to the paper, AF is associated with higher odds of dementia (odds ratio [OR], 3.00; 95% confidence interval [CI], 1.22-7.37) in women and MCI in women (OR, 3.43; 95% CI, 1.55-7.55) compared with men.
Women with AF and normal cognition at baseline had a higher risk of disease progression (hazard ratio [HR], 1.26; 95% CI, 1.06-1.50) from normal to MCI and from MCI to vascular dementia (HR, 3.27; 95% CI, 1.89-5.65) than that of men with AF or men and women without AF.
AF is a major public health problem linked with stroke and heart failure, and is an independent risk factor of increased mortality. It is associated with higher risk of cognitive impairment and dementia independent of stroke history.
Cognitive screening for AF patients
The authors wrote that cognitive screening, especially in women, should be part of yearly cardiology visits for patients with AF to help identify early those at highest risk for cognitive disease.
T. Jared Bunch, MD, professor of medicine in the division of cardiovascular medicine at University of Utah in Salt Lake City, said in an interview, “We have learned that how we treat atrial fibrillation can influence risk.”
First, he said, outcomes, including brain health, are better when rhythm control approaches are used within the first year of diagnosis.
“Restoring a normal heart rhythm improves brain perfusion and cognitive function. Next, aggressive rhythm control – such as catheter ablation – is associated with much lower long-term risks of dementia in the [patients]. Finally, early and effective use of anticoagulation in patients with atrial fibrillation lowers risk of stroke, dementia, and cognitive decline.”
Several factors unknown
Dr. Bunch said there are some unknowns in the study, such as how long patients were in atrial fibrillation.
He said one way to address the inequities is to refer women earlier as women are often referred later in disease to specialty care, which can have consequences.
He said it is not known how many people underwent early and effective rhythm control.
“Women also are less likely to receive catheter ablation, a cardioversion, or be placed on antiarrhythmic drugs,” said Dr. Bunch, who was not part of the study. “These also represent potential opportunities to improve outcomes by treating the rhythm in a similar and aggressive manner in both men and women.”
Also unknown is how many people were on effective oral anticoagulation, Dr. Bunch noted.
The study importantly highlights a significant problem surrounding the care of women with AF, he said, but there are strategies to improve outcomes.
In addition to earlier screening and referral for women, providers should recognize that men and women may present differently with different AF symptoms. He added that physicians should offer catheter ablation, the most effective treatment, equally to men and women who are candidates.
In all people, he said, it’s important “to start anticoagulation very early in the disease to lower the risk of micro- and macrothrombotic events that lead to poor brain health and function.”
The study authors and Dr. Bunch declared no relevant financial relationships.
New data suggest a significantly stronger link in women compared with men between atrial fibrillation (AF) and mild cognitive impairment (MCI) and dementia.
“Our findings imply that women with AF may be at higher risk for MCI and dementia with potentially more rapid disease progression from normal cognition to MCI or dementia than women without AF or men with and without AF,” wrote authors of a new study led by Kathryn A. Wood, PhD, RN, Neil Hodgson Woodruff School of Nursing at Emory University in Atlanta.
The findings were published online in Alzheimer’s & Dementia.
Researchers used the National Alzheimer’s Coordinating Center data with 43,630 patients and analyzed sex differences between men and women with AF and their performance on neuropsychological tests and cognitive disease progression.
Higher odds of dementia, MCI in women
According to the paper, AF is associated with higher odds of dementia (odds ratio [OR], 3.00; 95% confidence interval [CI], 1.22-7.37) in women and MCI in women (OR, 3.43; 95% CI, 1.55-7.55) compared with men.
Women with AF and normal cognition at baseline had a higher risk of disease progression (hazard ratio [HR], 1.26; 95% CI, 1.06-1.50) from normal to MCI and from MCI to vascular dementia (HR, 3.27; 95% CI, 1.89-5.65) than that of men with AF or men and women without AF.
AF is a major public health problem linked with stroke and heart failure, and is an independent risk factor of increased mortality. It is associated with higher risk of cognitive impairment and dementia independent of stroke history.
Cognitive screening for AF patients
The authors wrote that cognitive screening, especially in women, should be part of yearly cardiology visits for patients with AF to help identify early those at highest risk for cognitive disease.
T. Jared Bunch, MD, professor of medicine in the division of cardiovascular medicine at University of Utah in Salt Lake City, said in an interview, “We have learned that how we treat atrial fibrillation can influence risk.”
First, he said, outcomes, including brain health, are better when rhythm control approaches are used within the first year of diagnosis.
“Restoring a normal heart rhythm improves brain perfusion and cognitive function. Next, aggressive rhythm control – such as catheter ablation – is associated with much lower long-term risks of dementia in the [patients]. Finally, early and effective use of anticoagulation in patients with atrial fibrillation lowers risk of stroke, dementia, and cognitive decline.”
Several factors unknown
Dr. Bunch said there are some unknowns in the study, such as how long patients were in atrial fibrillation.
He said one way to address the inequities is to refer women earlier as women are often referred later in disease to specialty care, which can have consequences.
He said it is not known how many people underwent early and effective rhythm control.
“Women also are less likely to receive catheter ablation, a cardioversion, or be placed on antiarrhythmic drugs,” said Dr. Bunch, who was not part of the study. “These also represent potential opportunities to improve outcomes by treating the rhythm in a similar and aggressive manner in both men and women.”
Also unknown is how many people were on effective oral anticoagulation, Dr. Bunch noted.
The study importantly highlights a significant problem surrounding the care of women with AF, he said, but there are strategies to improve outcomes.
In addition to earlier screening and referral for women, providers should recognize that men and women may present differently with different AF symptoms. He added that physicians should offer catheter ablation, the most effective treatment, equally to men and women who are candidates.
In all people, he said, it’s important “to start anticoagulation very early in the disease to lower the risk of micro- and macrothrombotic events that lead to poor brain health and function.”
The study authors and Dr. Bunch declared no relevant financial relationships.
New data suggest a significantly stronger link in women compared with men between atrial fibrillation (AF) and mild cognitive impairment (MCI) and dementia.
“Our findings imply that women with AF may be at higher risk for MCI and dementia with potentially more rapid disease progression from normal cognition to MCI or dementia than women without AF or men with and without AF,” wrote authors of a new study led by Kathryn A. Wood, PhD, RN, Neil Hodgson Woodruff School of Nursing at Emory University in Atlanta.
The findings were published online in Alzheimer’s & Dementia.
Researchers used the National Alzheimer’s Coordinating Center data with 43,630 patients and analyzed sex differences between men and women with AF and their performance on neuropsychological tests and cognitive disease progression.
Higher odds of dementia, MCI in women
According to the paper, AF is associated with higher odds of dementia (odds ratio [OR], 3.00; 95% confidence interval [CI], 1.22-7.37) in women and MCI in women (OR, 3.43; 95% CI, 1.55-7.55) compared with men.
Women with AF and normal cognition at baseline had a higher risk of disease progression (hazard ratio [HR], 1.26; 95% CI, 1.06-1.50) from normal to MCI and from MCI to vascular dementia (HR, 3.27; 95% CI, 1.89-5.65) than that of men with AF or men and women without AF.
AF is a major public health problem linked with stroke and heart failure, and is an independent risk factor of increased mortality. It is associated with higher risk of cognitive impairment and dementia independent of stroke history.
Cognitive screening for AF patients
The authors wrote that cognitive screening, especially in women, should be part of yearly cardiology visits for patients with AF to help identify early those at highest risk for cognitive disease.
T. Jared Bunch, MD, professor of medicine in the division of cardiovascular medicine at University of Utah in Salt Lake City, said in an interview, “We have learned that how we treat atrial fibrillation can influence risk.”
First, he said, outcomes, including brain health, are better when rhythm control approaches are used within the first year of diagnosis.
“Restoring a normal heart rhythm improves brain perfusion and cognitive function. Next, aggressive rhythm control – such as catheter ablation – is associated with much lower long-term risks of dementia in the [patients]. Finally, early and effective use of anticoagulation in patients with atrial fibrillation lowers risk of stroke, dementia, and cognitive decline.”
Several factors unknown
Dr. Bunch said there are some unknowns in the study, such as how long patients were in atrial fibrillation.
He said one way to address the inequities is to refer women earlier as women are often referred later in disease to specialty care, which can have consequences.
He said it is not known how many people underwent early and effective rhythm control.
“Women also are less likely to receive catheter ablation, a cardioversion, or be placed on antiarrhythmic drugs,” said Dr. Bunch, who was not part of the study. “These also represent potential opportunities to improve outcomes by treating the rhythm in a similar and aggressive manner in both men and women.”
Also unknown is how many people were on effective oral anticoagulation, Dr. Bunch noted.
The study importantly highlights a significant problem surrounding the care of women with AF, he said, but there are strategies to improve outcomes.
In addition to earlier screening and referral for women, providers should recognize that men and women may present differently with different AF symptoms. He added that physicians should offer catheter ablation, the most effective treatment, equally to men and women who are candidates.
In all people, he said, it’s important “to start anticoagulation very early in the disease to lower the risk of micro- and macrothrombotic events that lead to poor brain health and function.”
The study authors and Dr. Bunch declared no relevant financial relationships.
FROM ALZHEIMER’S & DEMENTIA
New law allows international medical graduates to bypass U.S. residency
Pediatric nephrologist Bryan Carmody, MD, recalls working alongside an extremely experienced neonatologist during his residency. She had managed a neonatal intensive care unit in her home country of Lithuania, but because she wanted to practice in the United States, it took years of repeat training before she was eligible for a medical license.
“She was very accomplished, and she was wonderful to have as a coresident at the time,” Dr. Carmody said in an interview.
The neonatologist now practices at a U.S. academic medical center, but to obtain that position, she had to complete 3 years of pediatric residency and 3 years of fellowship in the United States, Dr. Carmody said.
Such training for international medical graduates (IMGs) is a routine part of obtaining a U.S. medical license, but
The American Medical Association took similar measures at its recent annual meeting, making it easier for IMGs to gain licensure. Because the pandemic and Russia’s invasion of Ukraine disrupted the process by which some IMGs had their licenses verified, the AMA is now encouraging state licensing boards and other credentialing institutions to accept certification from the Educational Commission for Foreign Medical Graduates as verification, rather than requiring documents directly from international medical schools.
When it comes to Tennessee’s new law, signed by Gov. Bill Lee in April, experienced IMGs who have received medical training abroad can skip U.S. residency requirements and obtain a temporary license to practice medicine in Tennessee if they meet certain qualifications.
The international doctors must demonstrate competency, as determined by the state medical board. In addition, they must have completed a 3-year postgraduate training program in the graduate’s licensing country or otherwise have practiced as a medical professional in which they performed the duties of a physician for at least 3 of the past 5 years outside the United States, according to the new law.
To be approved, IMGs must also have received an employment offer from a Tennessee health care provider that has a residency program accredited by the Accreditation Council for Graduate Medical Education.
If physicians remain in good standing for 2 years, the board will grant them a full and unrestricted license to practice in Tennessee.
“The new legislation opens up a lot of doors for international medical graduates and is also a lifeline for a lot of underserved areas in Tennessee,” said Asim Ansari, MD, a Canadian who attended medical school in the Caribbean and is an advocate for IMGs.
Dr. Ansari is participating in a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City, until he can apply for the sixth time to a residency program. “This could possibly be a model that other states may want to implement in a few years.”
What’s behind the law?
A predicted physician shortage in Tennessee drove the legislation, said Rep. Sabi “Doc” Kumar, MD, vice chair for the Tennessee House Health Committee and a cosponsor of the legislation. Legislators hope the law will mitigate that shortage and boost the number of physicians practicing in underserved areas of the state.
“Considering that one in four physicians in the U.S. are international medical gradates, it was important for us to be able to attract those physicians to Tennessee,” he said.
The Tennessee Board of Medical Examiners will develop administrative rules for the law, which may take up to a year, Rep. Kumar said. He expects the program to be available to IMGs beginning in mid-2024.
Upon completion of the program, IMGs will be able to practice general medicine in Tennessee, not a specialty. Requirements for specialty certification would have to be met through the specialties’ respective boards.
Dr. Carmody, who blogs about medical education, including the new legislation, said in an interview the law will greatly benefit experienced IMGs, who often are bypassed as residency candidates because they graduated years ago. Hospitals also win because they can fill positions that otherwise might sit vacant, he said.
Family physician Sahil Bawa, MD, an IMG from India who recently matched into his specialty, said the Tennessee legislation will help fellow IMGs find U.S. medical jobs.
“It’s very difficult for IMGs to get into residency in the U.S.,” he said. “I’ve seen people with medical degrees from other countries drive Uber or do odd jobs to sustain themselves here. I’ve known a few people who have left and gone back to their home country because they were not accepted into a residency.”
Who benefits most?
Dr. Bawa noted that the legislation would not have helped him, as he needed a visa to practice in the United States and the law does not include the sponsoring of visas. The legislation requires IMGs to show evidence of citizenship or evidence that they are legally entitled to live or work in the United States.
U.S. citizen IMGs who haven’t completed residency or who practiced in another country also are left out of the law, Dr. Carmody said.
“This law is designed to take the most accomplished cream of the crop international medical graduates with the most experience and the most sophisticated skill set and send them to Tennessee. I think that’s the intent,” he said. “But many international medical graduates are U.S. citizens who don’t have the opportunity to practice in countries other than United States or do residencies. A lot of these people are sitting on the sidelines, unable to secure residency positions. I’m sure they would be desperate for a program like this.”
Questions remain
“Just because the doctor can get a [temporary] license without the training doesn’t mean employers are going to be interested in sponsoring those doctors,” said Adam Cohen, an immigration attorney who practices in Memphis. “What is the inclination of these employers to hire these physicians who have undergone training outside the U.S.? And will there be skepticism on the part of employers about the competence of these doctors?”
“Hospital systems will be able to hire experienced practitioners for a very low cost,” Dr. Ansari said. “So now you have these additional bodies who can do the work of a physician, but you don’t have to pay them as much as a physician for 2 years. And because some are desperate to work, they will take lower pay as long as they have a pathway to full licensure in Tennessee. What are the protections for these physicians? Who will cover their insurance? Who will be responsible for them, the attendees? And will the attendees be willing to put their license on the line for them?”
In addition, Dr. Carmody questions what, if anything, will encourage IMGs to work in underserved areas in Tennessee after their 2 years are up and whether there will be any incentives to guide them. He wonders, too, whether the physicians will be stuck practicing in Tennessee following completion of the program.
“Will these physicians only be able to work in Tennessee?” he asked. “I think that’s probably going to be the case, because they’ll be licensed in Tennessee, but to go to another state, they would be missing the required residency training. So it might be these folks are stuck in Tennessee unless other states develop reciprocal arrangements.”
Other states would have to decide whether to recognize the Tennessee license acquired through this pathway, Rep. Kumar said.
He explained that the sponsoring sites would be responsible for providing work-hour restrictions and liability protections. There are currently no incentives in the legislation for IMGs to practice in rural, underserved areas, but the hospitals and communities there generally offer incentives when recruiting, Rep. Kumar said.
“The law definitely has the potential to be helpful,” Mr. Cohen said, “because there’s an ability to place providers in the state without having to go through the bottleneck of limited residency slots. If other states see a positive effect on Tennessee or are exploring ways to alleviate their own shortages, it’s possible [they] might follow suit.”
Rep. Kumar agreed that other states will be watching Tennessee to weigh the law’s success.
“I think the law will have to prove itself and show that Tennessee has benefited from it and that the results have been good,” he said. “We are providing a pioneering way for attracting medical graduates and making it easier for them to obtain a license. I would think other states would want to do that.”
A version of this article first appeared on Medscape.com.
Pediatric nephrologist Bryan Carmody, MD, recalls working alongside an extremely experienced neonatologist during his residency. She had managed a neonatal intensive care unit in her home country of Lithuania, but because she wanted to practice in the United States, it took years of repeat training before she was eligible for a medical license.
“She was very accomplished, and she was wonderful to have as a coresident at the time,” Dr. Carmody said in an interview.
The neonatologist now practices at a U.S. academic medical center, but to obtain that position, she had to complete 3 years of pediatric residency and 3 years of fellowship in the United States, Dr. Carmody said.
Such training for international medical graduates (IMGs) is a routine part of obtaining a U.S. medical license, but
The American Medical Association took similar measures at its recent annual meeting, making it easier for IMGs to gain licensure. Because the pandemic and Russia’s invasion of Ukraine disrupted the process by which some IMGs had their licenses verified, the AMA is now encouraging state licensing boards and other credentialing institutions to accept certification from the Educational Commission for Foreign Medical Graduates as verification, rather than requiring documents directly from international medical schools.
When it comes to Tennessee’s new law, signed by Gov. Bill Lee in April, experienced IMGs who have received medical training abroad can skip U.S. residency requirements and obtain a temporary license to practice medicine in Tennessee if they meet certain qualifications.
The international doctors must demonstrate competency, as determined by the state medical board. In addition, they must have completed a 3-year postgraduate training program in the graduate’s licensing country or otherwise have practiced as a medical professional in which they performed the duties of a physician for at least 3 of the past 5 years outside the United States, according to the new law.
To be approved, IMGs must also have received an employment offer from a Tennessee health care provider that has a residency program accredited by the Accreditation Council for Graduate Medical Education.
If physicians remain in good standing for 2 years, the board will grant them a full and unrestricted license to practice in Tennessee.
“The new legislation opens up a lot of doors for international medical graduates and is also a lifeline for a lot of underserved areas in Tennessee,” said Asim Ansari, MD, a Canadian who attended medical school in the Caribbean and is an advocate for IMGs.
Dr. Ansari is participating in a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City, until he can apply for the sixth time to a residency program. “This could possibly be a model that other states may want to implement in a few years.”
What’s behind the law?
A predicted physician shortage in Tennessee drove the legislation, said Rep. Sabi “Doc” Kumar, MD, vice chair for the Tennessee House Health Committee and a cosponsor of the legislation. Legislators hope the law will mitigate that shortage and boost the number of physicians practicing in underserved areas of the state.
“Considering that one in four physicians in the U.S. are international medical gradates, it was important for us to be able to attract those physicians to Tennessee,” he said.
The Tennessee Board of Medical Examiners will develop administrative rules for the law, which may take up to a year, Rep. Kumar said. He expects the program to be available to IMGs beginning in mid-2024.
Upon completion of the program, IMGs will be able to practice general medicine in Tennessee, not a specialty. Requirements for specialty certification would have to be met through the specialties’ respective boards.
Dr. Carmody, who blogs about medical education, including the new legislation, said in an interview the law will greatly benefit experienced IMGs, who often are bypassed as residency candidates because they graduated years ago. Hospitals also win because they can fill positions that otherwise might sit vacant, he said.
Family physician Sahil Bawa, MD, an IMG from India who recently matched into his specialty, said the Tennessee legislation will help fellow IMGs find U.S. medical jobs.
“It’s very difficult for IMGs to get into residency in the U.S.,” he said. “I’ve seen people with medical degrees from other countries drive Uber or do odd jobs to sustain themselves here. I’ve known a few people who have left and gone back to their home country because they were not accepted into a residency.”
Who benefits most?
Dr. Bawa noted that the legislation would not have helped him, as he needed a visa to practice in the United States and the law does not include the sponsoring of visas. The legislation requires IMGs to show evidence of citizenship or evidence that they are legally entitled to live or work in the United States.
U.S. citizen IMGs who haven’t completed residency or who practiced in another country also are left out of the law, Dr. Carmody said.
“This law is designed to take the most accomplished cream of the crop international medical graduates with the most experience and the most sophisticated skill set and send them to Tennessee. I think that’s the intent,” he said. “But many international medical graduates are U.S. citizens who don’t have the opportunity to practice in countries other than United States or do residencies. A lot of these people are sitting on the sidelines, unable to secure residency positions. I’m sure they would be desperate for a program like this.”
Questions remain
“Just because the doctor can get a [temporary] license without the training doesn’t mean employers are going to be interested in sponsoring those doctors,” said Adam Cohen, an immigration attorney who practices in Memphis. “What is the inclination of these employers to hire these physicians who have undergone training outside the U.S.? And will there be skepticism on the part of employers about the competence of these doctors?”
“Hospital systems will be able to hire experienced practitioners for a very low cost,” Dr. Ansari said. “So now you have these additional bodies who can do the work of a physician, but you don’t have to pay them as much as a physician for 2 years. And because some are desperate to work, they will take lower pay as long as they have a pathway to full licensure in Tennessee. What are the protections for these physicians? Who will cover their insurance? Who will be responsible for them, the attendees? And will the attendees be willing to put their license on the line for them?”
In addition, Dr. Carmody questions what, if anything, will encourage IMGs to work in underserved areas in Tennessee after their 2 years are up and whether there will be any incentives to guide them. He wonders, too, whether the physicians will be stuck practicing in Tennessee following completion of the program.
“Will these physicians only be able to work in Tennessee?” he asked. “I think that’s probably going to be the case, because they’ll be licensed in Tennessee, but to go to another state, they would be missing the required residency training. So it might be these folks are stuck in Tennessee unless other states develop reciprocal arrangements.”
Other states would have to decide whether to recognize the Tennessee license acquired through this pathway, Rep. Kumar said.
He explained that the sponsoring sites would be responsible for providing work-hour restrictions and liability protections. There are currently no incentives in the legislation for IMGs to practice in rural, underserved areas, but the hospitals and communities there generally offer incentives when recruiting, Rep. Kumar said.
“The law definitely has the potential to be helpful,” Mr. Cohen said, “because there’s an ability to place providers in the state without having to go through the bottleneck of limited residency slots. If other states see a positive effect on Tennessee or are exploring ways to alleviate their own shortages, it’s possible [they] might follow suit.”
Rep. Kumar agreed that other states will be watching Tennessee to weigh the law’s success.
“I think the law will have to prove itself and show that Tennessee has benefited from it and that the results have been good,” he said. “We are providing a pioneering way for attracting medical graduates and making it easier for them to obtain a license. I would think other states would want to do that.”
A version of this article first appeared on Medscape.com.
Pediatric nephrologist Bryan Carmody, MD, recalls working alongside an extremely experienced neonatologist during his residency. She had managed a neonatal intensive care unit in her home country of Lithuania, but because she wanted to practice in the United States, it took years of repeat training before she was eligible for a medical license.
“She was very accomplished, and she was wonderful to have as a coresident at the time,” Dr. Carmody said in an interview.
The neonatologist now practices at a U.S. academic medical center, but to obtain that position, she had to complete 3 years of pediatric residency and 3 years of fellowship in the United States, Dr. Carmody said.
Such training for international medical graduates (IMGs) is a routine part of obtaining a U.S. medical license, but
The American Medical Association took similar measures at its recent annual meeting, making it easier for IMGs to gain licensure. Because the pandemic and Russia’s invasion of Ukraine disrupted the process by which some IMGs had their licenses verified, the AMA is now encouraging state licensing boards and other credentialing institutions to accept certification from the Educational Commission for Foreign Medical Graduates as verification, rather than requiring documents directly from international medical schools.
When it comes to Tennessee’s new law, signed by Gov. Bill Lee in April, experienced IMGs who have received medical training abroad can skip U.S. residency requirements and obtain a temporary license to practice medicine in Tennessee if they meet certain qualifications.
The international doctors must demonstrate competency, as determined by the state medical board. In addition, they must have completed a 3-year postgraduate training program in the graduate’s licensing country or otherwise have practiced as a medical professional in which they performed the duties of a physician for at least 3 of the past 5 years outside the United States, according to the new law.
To be approved, IMGs must also have received an employment offer from a Tennessee health care provider that has a residency program accredited by the Accreditation Council for Graduate Medical Education.
If physicians remain in good standing for 2 years, the board will grant them a full and unrestricted license to practice in Tennessee.
“The new legislation opens up a lot of doors for international medical graduates and is also a lifeline for a lot of underserved areas in Tennessee,” said Asim Ansari, MD, a Canadian who attended medical school in the Caribbean and is an advocate for IMGs.
Dr. Ansari is participating in a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City, until he can apply for the sixth time to a residency program. “This could possibly be a model that other states may want to implement in a few years.”
What’s behind the law?
A predicted physician shortage in Tennessee drove the legislation, said Rep. Sabi “Doc” Kumar, MD, vice chair for the Tennessee House Health Committee and a cosponsor of the legislation. Legislators hope the law will mitigate that shortage and boost the number of physicians practicing in underserved areas of the state.
“Considering that one in four physicians in the U.S. are international medical gradates, it was important for us to be able to attract those physicians to Tennessee,” he said.
The Tennessee Board of Medical Examiners will develop administrative rules for the law, which may take up to a year, Rep. Kumar said. He expects the program to be available to IMGs beginning in mid-2024.
Upon completion of the program, IMGs will be able to practice general medicine in Tennessee, not a specialty. Requirements for specialty certification would have to be met through the specialties’ respective boards.
Dr. Carmody, who blogs about medical education, including the new legislation, said in an interview the law will greatly benefit experienced IMGs, who often are bypassed as residency candidates because they graduated years ago. Hospitals also win because they can fill positions that otherwise might sit vacant, he said.
Family physician Sahil Bawa, MD, an IMG from India who recently matched into his specialty, said the Tennessee legislation will help fellow IMGs find U.S. medical jobs.
“It’s very difficult for IMGs to get into residency in the U.S.,” he said. “I’ve seen people with medical degrees from other countries drive Uber or do odd jobs to sustain themselves here. I’ve known a few people who have left and gone back to their home country because they were not accepted into a residency.”
Who benefits most?
Dr. Bawa noted that the legislation would not have helped him, as he needed a visa to practice in the United States and the law does not include the sponsoring of visas. The legislation requires IMGs to show evidence of citizenship or evidence that they are legally entitled to live or work in the United States.
U.S. citizen IMGs who haven’t completed residency or who practiced in another country also are left out of the law, Dr. Carmody said.
“This law is designed to take the most accomplished cream of the crop international medical graduates with the most experience and the most sophisticated skill set and send them to Tennessee. I think that’s the intent,” he said. “But many international medical graduates are U.S. citizens who don’t have the opportunity to practice in countries other than United States or do residencies. A lot of these people are sitting on the sidelines, unable to secure residency positions. I’m sure they would be desperate for a program like this.”
Questions remain
“Just because the doctor can get a [temporary] license without the training doesn’t mean employers are going to be interested in sponsoring those doctors,” said Adam Cohen, an immigration attorney who practices in Memphis. “What is the inclination of these employers to hire these physicians who have undergone training outside the U.S.? And will there be skepticism on the part of employers about the competence of these doctors?”
“Hospital systems will be able to hire experienced practitioners for a very low cost,” Dr. Ansari said. “So now you have these additional bodies who can do the work of a physician, but you don’t have to pay them as much as a physician for 2 years. And because some are desperate to work, they will take lower pay as long as they have a pathway to full licensure in Tennessee. What are the protections for these physicians? Who will cover their insurance? Who will be responsible for them, the attendees? And will the attendees be willing to put their license on the line for them?”
In addition, Dr. Carmody questions what, if anything, will encourage IMGs to work in underserved areas in Tennessee after their 2 years are up and whether there will be any incentives to guide them. He wonders, too, whether the physicians will be stuck practicing in Tennessee following completion of the program.
“Will these physicians only be able to work in Tennessee?” he asked. “I think that’s probably going to be the case, because they’ll be licensed in Tennessee, but to go to another state, they would be missing the required residency training. So it might be these folks are stuck in Tennessee unless other states develop reciprocal arrangements.”
Other states would have to decide whether to recognize the Tennessee license acquired through this pathway, Rep. Kumar said.
He explained that the sponsoring sites would be responsible for providing work-hour restrictions and liability protections. There are currently no incentives in the legislation for IMGs to practice in rural, underserved areas, but the hospitals and communities there generally offer incentives when recruiting, Rep. Kumar said.
“The law definitely has the potential to be helpful,” Mr. Cohen said, “because there’s an ability to place providers in the state without having to go through the bottleneck of limited residency slots. If other states see a positive effect on Tennessee or are exploring ways to alleviate their own shortages, it’s possible [they] might follow suit.”
Rep. Kumar agreed that other states will be watching Tennessee to weigh the law’s success.
“I think the law will have to prove itself and show that Tennessee has benefited from it and that the results have been good,” he said. “We are providing a pioneering way for attracting medical graduates and making it easier for them to obtain a license. I would think other states would want to do that.”
A version of this article first appeared on Medscape.com.
Harvard medical school sued over stolen body part scandal
Plaintiffs include relatives of people whose remains were allegedly stolen and sold. The lawsuit alleges that as many as 400 cadavers may have been trafficked in a multi-year scheme. Details were revealed in a June 13 indictment by the U.S. attorney for the Middle District of Pennsylvania.
“Medical schools like Harvard have a duty to ensure [donated remains] are handled properly and with decency and to ensure they are used for their intended purpose of scientific study,” attorney Jeff Catalano said in a statement.
“I do think Harvard has that duty,” said Arthur Caplan, PhD, director, Division of Medical Ethics, New York University. But, he added, “I will say there’s not much they can do when employees set out to systematically undermine them.”
The indictment alleges that from 2018 through August 2022, Harvard morgue manager Cedric Lodge stole dissected portions of donated cadavers, including heads, brains, skin, and bones, which were then sold by him and his wife, Denise Lodge, to Katrina Maclean, owner of Kat’s Creepy Creations, Peabody, Mass. Ms. Maclean allegedly sold human remains to Joshua Taylor and Jeremy Pauley, both Pennsylvania residents.
On occasion, Mr. Lodge allowed Ms. Maclean, Mr. Taylor, and others into the morgue to choose which parts they wanted, according to the indictment. Mr. Taylor, Ms. Maclean, and Denise Lodge are all named in the indictment. Mr. Pauley was charged separately.
They each face a maximum of 15 years in prison.
Ms. Maclean allegedly bought two dissected faces for $600 and shipped human skin to Mr. Pauley to be made into leather; Mr. Pauley then eventually shipped the “tanned human skin” back to Ms. Maclean, according to the indictment. Over a 3-year period, Mr. Taylor paid the Lodges some $37,000 for stolen human remains, the indictment charges.
Mr. Pauley also purchased human remains from Candace Chapman Scott, who stole them from her employer, a mortuary in Little Rock, Ark. The mortuary received remains for cremation from an area medical school, according to the indictment.
After being notified of the investigation in March, Harvard cooperated fully, the school said in a statement from George Q. Daley, MD, PhD, dean of the Faculty of Medicine.
“We are appalled to learn that something so disturbing could happen on our campus – a community dedicated to healing and serving others,” the statement said. “The reported incidents are a betrayal of HMS and, most importantly, each of the individuals who altruistically chose to will their bodies to HMS through the Anatomical Gift Program to advance medical education and research.”
The U.S. attorney thanked Harvard for its cooperation, saying that it “is also a victim here.”
Dr. Caplan, who also writes an ethics column for this news organization, agrees. The school was betrayed, he said.
“You expect professionalism, integrity on the part of your doctors, on the part of your technicians, on the part of your workforce,” he said. He noted that those expectations are explained in institutions’ codes of ethics and policies.
Harvard said Mr. Lodge had worked in the morgue since 1995 and that he took several leaves: from September 2021 to February 2022, and again starting February 14. The school terminated his employment on May 6.
His duties included intake of anatomic donors’ bodies. He coordinated embalming and oversaw the storage and movement of cadavers to and from teaching labs. When studies were complete, he prepared remains to be transported to and from the external crematorium and, when appropriate, for burial, according to a Harvard fact sheet for families.
The medical school has convened an outside expert panel to evaluate the Anatomical Gift Program and morgue policies and practices. The panel is expected to make its findings public at the end of the summer.
Dr. Caplan said he hoped the committee recommends unannounced audits of cadaver donation programs and medical tissue and bone suppliers, which could help expose illicit diversions. “You need to keep an eye, which no one seems to do because it’s a state issue and it’s not a priority, on that trade in body parts,” he said.
He believes other medical schools will reexamine their donation programs, especially given Harvard’s status.
“With a prominent place like that having this kind of problem, I can’t imagine there’s not a little bit of a scramble at a lot of the body programs to make sure that they know that things are as they should be,” Dr. Caplan said.
A version of this article first appeared on Medscape.com.
Plaintiffs include relatives of people whose remains were allegedly stolen and sold. The lawsuit alleges that as many as 400 cadavers may have been trafficked in a multi-year scheme. Details were revealed in a June 13 indictment by the U.S. attorney for the Middle District of Pennsylvania.
“Medical schools like Harvard have a duty to ensure [donated remains] are handled properly and with decency and to ensure they are used for their intended purpose of scientific study,” attorney Jeff Catalano said in a statement.
“I do think Harvard has that duty,” said Arthur Caplan, PhD, director, Division of Medical Ethics, New York University. But, he added, “I will say there’s not much they can do when employees set out to systematically undermine them.”
The indictment alleges that from 2018 through August 2022, Harvard morgue manager Cedric Lodge stole dissected portions of donated cadavers, including heads, brains, skin, and bones, which were then sold by him and his wife, Denise Lodge, to Katrina Maclean, owner of Kat’s Creepy Creations, Peabody, Mass. Ms. Maclean allegedly sold human remains to Joshua Taylor and Jeremy Pauley, both Pennsylvania residents.
On occasion, Mr. Lodge allowed Ms. Maclean, Mr. Taylor, and others into the morgue to choose which parts they wanted, according to the indictment. Mr. Taylor, Ms. Maclean, and Denise Lodge are all named in the indictment. Mr. Pauley was charged separately.
They each face a maximum of 15 years in prison.
Ms. Maclean allegedly bought two dissected faces for $600 and shipped human skin to Mr. Pauley to be made into leather; Mr. Pauley then eventually shipped the “tanned human skin” back to Ms. Maclean, according to the indictment. Over a 3-year period, Mr. Taylor paid the Lodges some $37,000 for stolen human remains, the indictment charges.
Mr. Pauley also purchased human remains from Candace Chapman Scott, who stole them from her employer, a mortuary in Little Rock, Ark. The mortuary received remains for cremation from an area medical school, according to the indictment.
After being notified of the investigation in March, Harvard cooperated fully, the school said in a statement from George Q. Daley, MD, PhD, dean of the Faculty of Medicine.
“We are appalled to learn that something so disturbing could happen on our campus – a community dedicated to healing and serving others,” the statement said. “The reported incidents are a betrayal of HMS and, most importantly, each of the individuals who altruistically chose to will their bodies to HMS through the Anatomical Gift Program to advance medical education and research.”
The U.S. attorney thanked Harvard for its cooperation, saying that it “is also a victim here.”
Dr. Caplan, who also writes an ethics column for this news organization, agrees. The school was betrayed, he said.
“You expect professionalism, integrity on the part of your doctors, on the part of your technicians, on the part of your workforce,” he said. He noted that those expectations are explained in institutions’ codes of ethics and policies.
Harvard said Mr. Lodge had worked in the morgue since 1995 and that he took several leaves: from September 2021 to February 2022, and again starting February 14. The school terminated his employment on May 6.
His duties included intake of anatomic donors’ bodies. He coordinated embalming and oversaw the storage and movement of cadavers to and from teaching labs. When studies were complete, he prepared remains to be transported to and from the external crematorium and, when appropriate, for burial, according to a Harvard fact sheet for families.
The medical school has convened an outside expert panel to evaluate the Anatomical Gift Program and morgue policies and practices. The panel is expected to make its findings public at the end of the summer.
Dr. Caplan said he hoped the committee recommends unannounced audits of cadaver donation programs and medical tissue and bone suppliers, which could help expose illicit diversions. “You need to keep an eye, which no one seems to do because it’s a state issue and it’s not a priority, on that trade in body parts,” he said.
He believes other medical schools will reexamine their donation programs, especially given Harvard’s status.
“With a prominent place like that having this kind of problem, I can’t imagine there’s not a little bit of a scramble at a lot of the body programs to make sure that they know that things are as they should be,” Dr. Caplan said.
A version of this article first appeared on Medscape.com.
Plaintiffs include relatives of people whose remains were allegedly stolen and sold. The lawsuit alleges that as many as 400 cadavers may have been trafficked in a multi-year scheme. Details were revealed in a June 13 indictment by the U.S. attorney for the Middle District of Pennsylvania.
“Medical schools like Harvard have a duty to ensure [donated remains] are handled properly and with decency and to ensure they are used for their intended purpose of scientific study,” attorney Jeff Catalano said in a statement.
“I do think Harvard has that duty,” said Arthur Caplan, PhD, director, Division of Medical Ethics, New York University. But, he added, “I will say there’s not much they can do when employees set out to systematically undermine them.”
The indictment alleges that from 2018 through August 2022, Harvard morgue manager Cedric Lodge stole dissected portions of donated cadavers, including heads, brains, skin, and bones, which were then sold by him and his wife, Denise Lodge, to Katrina Maclean, owner of Kat’s Creepy Creations, Peabody, Mass. Ms. Maclean allegedly sold human remains to Joshua Taylor and Jeremy Pauley, both Pennsylvania residents.
On occasion, Mr. Lodge allowed Ms. Maclean, Mr. Taylor, and others into the morgue to choose which parts they wanted, according to the indictment. Mr. Taylor, Ms. Maclean, and Denise Lodge are all named in the indictment. Mr. Pauley was charged separately.
They each face a maximum of 15 years in prison.
Ms. Maclean allegedly bought two dissected faces for $600 and shipped human skin to Mr. Pauley to be made into leather; Mr. Pauley then eventually shipped the “tanned human skin” back to Ms. Maclean, according to the indictment. Over a 3-year period, Mr. Taylor paid the Lodges some $37,000 for stolen human remains, the indictment charges.
Mr. Pauley also purchased human remains from Candace Chapman Scott, who stole them from her employer, a mortuary in Little Rock, Ark. The mortuary received remains for cremation from an area medical school, according to the indictment.
After being notified of the investigation in March, Harvard cooperated fully, the school said in a statement from George Q. Daley, MD, PhD, dean of the Faculty of Medicine.
“We are appalled to learn that something so disturbing could happen on our campus – a community dedicated to healing and serving others,” the statement said. “The reported incidents are a betrayal of HMS and, most importantly, each of the individuals who altruistically chose to will their bodies to HMS through the Anatomical Gift Program to advance medical education and research.”
The U.S. attorney thanked Harvard for its cooperation, saying that it “is also a victim here.”
Dr. Caplan, who also writes an ethics column for this news organization, agrees. The school was betrayed, he said.
“You expect professionalism, integrity on the part of your doctors, on the part of your technicians, on the part of your workforce,” he said. He noted that those expectations are explained in institutions’ codes of ethics and policies.
Harvard said Mr. Lodge had worked in the morgue since 1995 and that he took several leaves: from September 2021 to February 2022, and again starting February 14. The school terminated his employment on May 6.
His duties included intake of anatomic donors’ bodies. He coordinated embalming and oversaw the storage and movement of cadavers to and from teaching labs. When studies were complete, he prepared remains to be transported to and from the external crematorium and, when appropriate, for burial, according to a Harvard fact sheet for families.
The medical school has convened an outside expert panel to evaluate the Anatomical Gift Program and morgue policies and practices. The panel is expected to make its findings public at the end of the summer.
Dr. Caplan said he hoped the committee recommends unannounced audits of cadaver donation programs and medical tissue and bone suppliers, which could help expose illicit diversions. “You need to keep an eye, which no one seems to do because it’s a state issue and it’s not a priority, on that trade in body parts,” he said.
He believes other medical schools will reexamine their donation programs, especially given Harvard’s status.
“With a prominent place like that having this kind of problem, I can’t imagine there’s not a little bit of a scramble at a lot of the body programs to make sure that they know that things are as they should be,” Dr. Caplan said.
A version of this article first appeared on Medscape.com.
Migraine device expands treatment possibilities
AUSTIN, TEX – Migraine treatment and prevention is challenging in any population, but some present even more difficulties. Pregnant women and pediatric patients are two such groups where physicians and patients may be hesitant to use drugs.
Neuromodulation devices are proven alternatives to medical interventions, and the remote electrical neuromodulation device Nerivio (Theranica) was cleared by the Food and Drug Administration for acute treatment of migraine patients aged 12 and over in 2021. In March 2023, the agency expanded the clearance to include prevention of migration in adolescents aged 12 and over as well as adults.
Two studies presented at the annual meeting of the American Headache Society showed The latter study yielded similar findings to adults and was used by FDA in its decision to expand the device’s indication in adolescents in 2023, according to Teshamae Monteith, MD, who presented the study at a poster session.
The device, worn on the arm, allows the user to modulate the intensity of the stimulation so that it activates nociceptive pain receptors, but not in a painful way. “Each [patient] raises the intensity until it feels strong, yet comfortable, and when that happens, they activate the nociceptive receptors and the arm sends a signal all the way back up to the brainstem, where the pain control area is. Activating it causes the release of neurotransmitters that inhibit pain. That inhibition is a global pain inhibition mechanism, which causes inhibition of the migraine pain, and also the symptoms associated with migraine like photophobia and vomiting,” said Alit Stark-Inbar, PhD, who presented the study of treatment of pregnant women during a poster session.
Declining treatment days over time in adolescents
Dr. Monteith’s team studied high-frequency remote electrical neuromodulation device use in adolescents who had migraine on 10 days or more per month. They also required at least three treatment days in months 2 and 3 to control for the possibility that patients might stop using the device because they couldn’t afford it or for some reason other than efficacy or because their migraines went away.
The study included 83 adolescents aged 12-17 (mean, 15.9 years, 89% female). In the first month of use, the mean number of migraine treatment days was 12.6, which dropped to 9.0 in month 2 (P < .001), and 7.4 in month 3 (P < .001 from month 2). At 2 hours after treatment, 61.9% had pain relief, 24.5% had freedom from pain, 67.4% had functional disability relief, and 41.3% had functional disability freedom.
“It parallels the findings of the randomized, sham-controlled study in adults. The safety profile was excellent with just one person complaining of minor discomfort of the arm that resolved after treatment. The combination of the exceedingly safe profile and the likelihood of efficacy based on using monthly migraine treatment days as a proxy, the FDA decided to clear this for an adolescent indication,” said Dr. Monteith, associate professor of clinical neurology and chief of the headache division at the University of Miami.
The device design is convenient, according to Dr. Monteith. “The arm is just an easy place to stimulate. It’s a wearable device, and it’s 45 minutes [of treatment] and it’s app controlled. You know adolescents like their technology. They can track their symptoms here, and there’s some biobehavioral power to this because they can do biobehavioral exercises in addition to receiving the simulation,” she said.
The fact that the device is discrete is also an advantage for adolescents in school. “You have to go to the nurse to get your medication versus a device, you can just put it on, it’s easy, no one sees it, and no one’s making fun of you,” said Dr. Monteith.
Advantages for adolescents
The device offers a useful alternative to medication, according to Alan M. Rapoport, MD, who was asked for comment on the adolescent study. “I’d rather not give medication and certainly not preventive medication to an adolescent,” he said. He noted that over-the-counter acute care migraine medications such as aspirin or acetaminophen and combination medications with caffeine, as well as prescription medications such as triptans, “all have possible side effects, and when used to an increased extent can even cause medication overuse headache, increasing the severity and frequency of headache and migraine days per month,” Dr. Rapoport said. Using an effective device with almost no side effects is preferable to any of these acute care medications, especially if there are several headaches a month,” he said. Some newer medications that block calcitonin gene-related peptide might be quite effective when they are approved for adolescents, and should have few adverse events, he added.
In the past, Dr. Rapoport has favored biofeedback training for acute and especially preventive treatment of migraine in adolescents. “[Remote electrical neuromodulation] seems to do just as well, children enjoy it, and it’s easier for a patient to do at home,” said Dr. Rapoport.
Biofeedback training is usually taught to patients by a PhD psychologist. Once the patients have been on the biofeedback equipment and learn the techniques, they can practice on their own at home without equipment. “This new device treatment using Nerivio for acute care and prevention of migraine in adults and children 12 and older, where they can easily apply the device in almost any situation, whether they are at home or possibly even in school or out and about, looks very promising,” said Dr. Rapoport. It is quite effective and has almost no adverse events, which is what you really want, especially for adolescents,” he said.
Also asked to comment on the study of remote electrical neuromodulation use in adolescents, Abraham Avi Ashkenazi, MD, director of the Headache Clinic at Shaare Zedek Medical Center in Jerusalem, who attended the session, was enthusiastic, and said he has begun using it in his own practice. “It shows that remote electrical neuromodulation can not only be effective for the acute migraine attack, but also has a potential preventive effect on future migraine attacks. [This] actually makes sense, because we know that the more migraine attacks a person has, the more likely they are to progress to a more chronic form of the disease,” he said in an interview.
Asked what distinguishes REN from other neuromodulation therapies such as vagus nerve stimulation or transcranial magnetic stimulation (TMS), Dr. Ashkenazi said: “It’s just a different way of modulating the brain system via a different mechanism. In both ways, though, the advantage is that there are literally no adverse effects, as opposed to drug treatment.”
An alternative during pregnancy
Adolescents aren’t the only population where there is reluctance to use medication. Physicians have been prescribing the device for pregnant women, who are reluctant to take medication due to concerns effects on the fetus. However, pregnant women were not included in the pivotal studies. “They expect it to be safe. This study was done in order to validate that assumption. We reached out to women who either used the device during pregnancy or women from the same database who started it using afterwards, but did not use it during the pregnancy,” said Dr. Stark-Inbar, vice president of medical information at Theranica.
The study included 140 women, 59 in the remote electrical neuromodulation device group and 81 controls. The primary endpoint was gestational age, which was 38 weeks and 5 days in the remote electrical neuromodulation device group and 39 weeks among controls (P = .150). There were no significant between-group differences with respect to newborn birth weight, miscarriage rate, preterm birth rate, birth defect rate, developmental milestone rate, or emergency department visit rate.
Dr. Monteith and Dr. Ashkenazi have no relevant financial disclosures. Dr. Rapoport advises AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica. He is on the speakers bureau of AbbVie, Dr. Reddy’s, Impel, Pfizer and Teva Pharmaceutical Industries. Dr. Rapoport is the editor-in-chief of Neurology Reviews and on the editorial board of CNS Drugs.
AUSTIN, TEX – Migraine treatment and prevention is challenging in any population, but some present even more difficulties. Pregnant women and pediatric patients are two such groups where physicians and patients may be hesitant to use drugs.
Neuromodulation devices are proven alternatives to medical interventions, and the remote electrical neuromodulation device Nerivio (Theranica) was cleared by the Food and Drug Administration for acute treatment of migraine patients aged 12 and over in 2021. In March 2023, the agency expanded the clearance to include prevention of migration in adolescents aged 12 and over as well as adults.
Two studies presented at the annual meeting of the American Headache Society showed The latter study yielded similar findings to adults and was used by FDA in its decision to expand the device’s indication in adolescents in 2023, according to Teshamae Monteith, MD, who presented the study at a poster session.
The device, worn on the arm, allows the user to modulate the intensity of the stimulation so that it activates nociceptive pain receptors, but not in a painful way. “Each [patient] raises the intensity until it feels strong, yet comfortable, and when that happens, they activate the nociceptive receptors and the arm sends a signal all the way back up to the brainstem, where the pain control area is. Activating it causes the release of neurotransmitters that inhibit pain. That inhibition is a global pain inhibition mechanism, which causes inhibition of the migraine pain, and also the symptoms associated with migraine like photophobia and vomiting,” said Alit Stark-Inbar, PhD, who presented the study of treatment of pregnant women during a poster session.
Declining treatment days over time in adolescents
Dr. Monteith’s team studied high-frequency remote electrical neuromodulation device use in adolescents who had migraine on 10 days or more per month. They also required at least three treatment days in months 2 and 3 to control for the possibility that patients might stop using the device because they couldn’t afford it or for some reason other than efficacy or because their migraines went away.
The study included 83 adolescents aged 12-17 (mean, 15.9 years, 89% female). In the first month of use, the mean number of migraine treatment days was 12.6, which dropped to 9.0 in month 2 (P < .001), and 7.4 in month 3 (P < .001 from month 2). At 2 hours after treatment, 61.9% had pain relief, 24.5% had freedom from pain, 67.4% had functional disability relief, and 41.3% had functional disability freedom.
“It parallels the findings of the randomized, sham-controlled study in adults. The safety profile was excellent with just one person complaining of minor discomfort of the arm that resolved after treatment. The combination of the exceedingly safe profile and the likelihood of efficacy based on using monthly migraine treatment days as a proxy, the FDA decided to clear this for an adolescent indication,” said Dr. Monteith, associate professor of clinical neurology and chief of the headache division at the University of Miami.
The device design is convenient, according to Dr. Monteith. “The arm is just an easy place to stimulate. It’s a wearable device, and it’s 45 minutes [of treatment] and it’s app controlled. You know adolescents like their technology. They can track their symptoms here, and there’s some biobehavioral power to this because they can do biobehavioral exercises in addition to receiving the simulation,” she said.
The fact that the device is discrete is also an advantage for adolescents in school. “You have to go to the nurse to get your medication versus a device, you can just put it on, it’s easy, no one sees it, and no one’s making fun of you,” said Dr. Monteith.
Advantages for adolescents
The device offers a useful alternative to medication, according to Alan M. Rapoport, MD, who was asked for comment on the adolescent study. “I’d rather not give medication and certainly not preventive medication to an adolescent,” he said. He noted that over-the-counter acute care migraine medications such as aspirin or acetaminophen and combination medications with caffeine, as well as prescription medications such as triptans, “all have possible side effects, and when used to an increased extent can even cause medication overuse headache, increasing the severity and frequency of headache and migraine days per month,” Dr. Rapoport said. Using an effective device with almost no side effects is preferable to any of these acute care medications, especially if there are several headaches a month,” he said. Some newer medications that block calcitonin gene-related peptide might be quite effective when they are approved for adolescents, and should have few adverse events, he added.
In the past, Dr. Rapoport has favored biofeedback training for acute and especially preventive treatment of migraine in adolescents. “[Remote electrical neuromodulation] seems to do just as well, children enjoy it, and it’s easier for a patient to do at home,” said Dr. Rapoport.
Biofeedback training is usually taught to patients by a PhD psychologist. Once the patients have been on the biofeedback equipment and learn the techniques, they can practice on their own at home without equipment. “This new device treatment using Nerivio for acute care and prevention of migraine in adults and children 12 and older, where they can easily apply the device in almost any situation, whether they are at home or possibly even in school or out and about, looks very promising,” said Dr. Rapoport. It is quite effective and has almost no adverse events, which is what you really want, especially for adolescents,” he said.
Also asked to comment on the study of remote electrical neuromodulation use in adolescents, Abraham Avi Ashkenazi, MD, director of the Headache Clinic at Shaare Zedek Medical Center in Jerusalem, who attended the session, was enthusiastic, and said he has begun using it in his own practice. “It shows that remote electrical neuromodulation can not only be effective for the acute migraine attack, but also has a potential preventive effect on future migraine attacks. [This] actually makes sense, because we know that the more migraine attacks a person has, the more likely they are to progress to a more chronic form of the disease,” he said in an interview.
Asked what distinguishes REN from other neuromodulation therapies such as vagus nerve stimulation or transcranial magnetic stimulation (TMS), Dr. Ashkenazi said: “It’s just a different way of modulating the brain system via a different mechanism. In both ways, though, the advantage is that there are literally no adverse effects, as opposed to drug treatment.”
An alternative during pregnancy
Adolescents aren’t the only population where there is reluctance to use medication. Physicians have been prescribing the device for pregnant women, who are reluctant to take medication due to concerns effects on the fetus. However, pregnant women were not included in the pivotal studies. “They expect it to be safe. This study was done in order to validate that assumption. We reached out to women who either used the device during pregnancy or women from the same database who started it using afterwards, but did not use it during the pregnancy,” said Dr. Stark-Inbar, vice president of medical information at Theranica.
The study included 140 women, 59 in the remote electrical neuromodulation device group and 81 controls. The primary endpoint was gestational age, which was 38 weeks and 5 days in the remote electrical neuromodulation device group and 39 weeks among controls (P = .150). There were no significant between-group differences with respect to newborn birth weight, miscarriage rate, preterm birth rate, birth defect rate, developmental milestone rate, or emergency department visit rate.
Dr. Monteith and Dr. Ashkenazi have no relevant financial disclosures. Dr. Rapoport advises AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica. He is on the speakers bureau of AbbVie, Dr. Reddy’s, Impel, Pfizer and Teva Pharmaceutical Industries. Dr. Rapoport is the editor-in-chief of Neurology Reviews and on the editorial board of CNS Drugs.
AUSTIN, TEX – Migraine treatment and prevention is challenging in any population, but some present even more difficulties. Pregnant women and pediatric patients are two such groups where physicians and patients may be hesitant to use drugs.
Neuromodulation devices are proven alternatives to medical interventions, and the remote electrical neuromodulation device Nerivio (Theranica) was cleared by the Food and Drug Administration for acute treatment of migraine patients aged 12 and over in 2021. In March 2023, the agency expanded the clearance to include prevention of migration in adolescents aged 12 and over as well as adults.
Two studies presented at the annual meeting of the American Headache Society showed The latter study yielded similar findings to adults and was used by FDA in its decision to expand the device’s indication in adolescents in 2023, according to Teshamae Monteith, MD, who presented the study at a poster session.
The device, worn on the arm, allows the user to modulate the intensity of the stimulation so that it activates nociceptive pain receptors, but not in a painful way. “Each [patient] raises the intensity until it feels strong, yet comfortable, and when that happens, they activate the nociceptive receptors and the arm sends a signal all the way back up to the brainstem, where the pain control area is. Activating it causes the release of neurotransmitters that inhibit pain. That inhibition is a global pain inhibition mechanism, which causes inhibition of the migraine pain, and also the symptoms associated with migraine like photophobia and vomiting,” said Alit Stark-Inbar, PhD, who presented the study of treatment of pregnant women during a poster session.
Declining treatment days over time in adolescents
Dr. Monteith’s team studied high-frequency remote electrical neuromodulation device use in adolescents who had migraine on 10 days or more per month. They also required at least three treatment days in months 2 and 3 to control for the possibility that patients might stop using the device because they couldn’t afford it or for some reason other than efficacy or because their migraines went away.
The study included 83 adolescents aged 12-17 (mean, 15.9 years, 89% female). In the first month of use, the mean number of migraine treatment days was 12.6, which dropped to 9.0 in month 2 (P < .001), and 7.4 in month 3 (P < .001 from month 2). At 2 hours after treatment, 61.9% had pain relief, 24.5% had freedom from pain, 67.4% had functional disability relief, and 41.3% had functional disability freedom.
“It parallels the findings of the randomized, sham-controlled study in adults. The safety profile was excellent with just one person complaining of minor discomfort of the arm that resolved after treatment. The combination of the exceedingly safe profile and the likelihood of efficacy based on using monthly migraine treatment days as a proxy, the FDA decided to clear this for an adolescent indication,” said Dr. Monteith, associate professor of clinical neurology and chief of the headache division at the University of Miami.
The device design is convenient, according to Dr. Monteith. “The arm is just an easy place to stimulate. It’s a wearable device, and it’s 45 minutes [of treatment] and it’s app controlled. You know adolescents like their technology. They can track their symptoms here, and there’s some biobehavioral power to this because they can do biobehavioral exercises in addition to receiving the simulation,” she said.
The fact that the device is discrete is also an advantage for adolescents in school. “You have to go to the nurse to get your medication versus a device, you can just put it on, it’s easy, no one sees it, and no one’s making fun of you,” said Dr. Monteith.
Advantages for adolescents
The device offers a useful alternative to medication, according to Alan M. Rapoport, MD, who was asked for comment on the adolescent study. “I’d rather not give medication and certainly not preventive medication to an adolescent,” he said. He noted that over-the-counter acute care migraine medications such as aspirin or acetaminophen and combination medications with caffeine, as well as prescription medications such as triptans, “all have possible side effects, and when used to an increased extent can even cause medication overuse headache, increasing the severity and frequency of headache and migraine days per month,” Dr. Rapoport said. Using an effective device with almost no side effects is preferable to any of these acute care medications, especially if there are several headaches a month,” he said. Some newer medications that block calcitonin gene-related peptide might be quite effective when they are approved for adolescents, and should have few adverse events, he added.
In the past, Dr. Rapoport has favored biofeedback training for acute and especially preventive treatment of migraine in adolescents. “[Remote electrical neuromodulation] seems to do just as well, children enjoy it, and it’s easier for a patient to do at home,” said Dr. Rapoport.
Biofeedback training is usually taught to patients by a PhD psychologist. Once the patients have been on the biofeedback equipment and learn the techniques, they can practice on their own at home without equipment. “This new device treatment using Nerivio for acute care and prevention of migraine in adults and children 12 and older, where they can easily apply the device in almost any situation, whether they are at home or possibly even in school or out and about, looks very promising,” said Dr. Rapoport. It is quite effective and has almost no adverse events, which is what you really want, especially for adolescents,” he said.
Also asked to comment on the study of remote electrical neuromodulation use in adolescents, Abraham Avi Ashkenazi, MD, director of the Headache Clinic at Shaare Zedek Medical Center in Jerusalem, who attended the session, was enthusiastic, and said he has begun using it in his own practice. “It shows that remote electrical neuromodulation can not only be effective for the acute migraine attack, but also has a potential preventive effect on future migraine attacks. [This] actually makes sense, because we know that the more migraine attacks a person has, the more likely they are to progress to a more chronic form of the disease,” he said in an interview.
Asked what distinguishes REN from other neuromodulation therapies such as vagus nerve stimulation or transcranial magnetic stimulation (TMS), Dr. Ashkenazi said: “It’s just a different way of modulating the brain system via a different mechanism. In both ways, though, the advantage is that there are literally no adverse effects, as opposed to drug treatment.”
An alternative during pregnancy
Adolescents aren’t the only population where there is reluctance to use medication. Physicians have been prescribing the device for pregnant women, who are reluctant to take medication due to concerns effects on the fetus. However, pregnant women were not included in the pivotal studies. “They expect it to be safe. This study was done in order to validate that assumption. We reached out to women who either used the device during pregnancy or women from the same database who started it using afterwards, but did not use it during the pregnancy,” said Dr. Stark-Inbar, vice president of medical information at Theranica.
The study included 140 women, 59 in the remote electrical neuromodulation device group and 81 controls. The primary endpoint was gestational age, which was 38 weeks and 5 days in the remote electrical neuromodulation device group and 39 weeks among controls (P = .150). There were no significant between-group differences with respect to newborn birth weight, miscarriage rate, preterm birth rate, birth defect rate, developmental milestone rate, or emergency department visit rate.
Dr. Monteith and Dr. Ashkenazi have no relevant financial disclosures. Dr. Rapoport advises AbbVie, Biohaven, Cala Health, Dr. Reddy’s, Pfizer, Satsuma, Teva Pharmaceutical Industries, and Theranica. He is on the speakers bureau of AbbVie, Dr. Reddy’s, Impel, Pfizer and Teva Pharmaceutical Industries. Dr. Rapoport is the editor-in-chief of Neurology Reviews and on the editorial board of CNS Drugs.
AT AHS 2023
Can a repurposed Parkinson’s drug slow ALS progression?
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.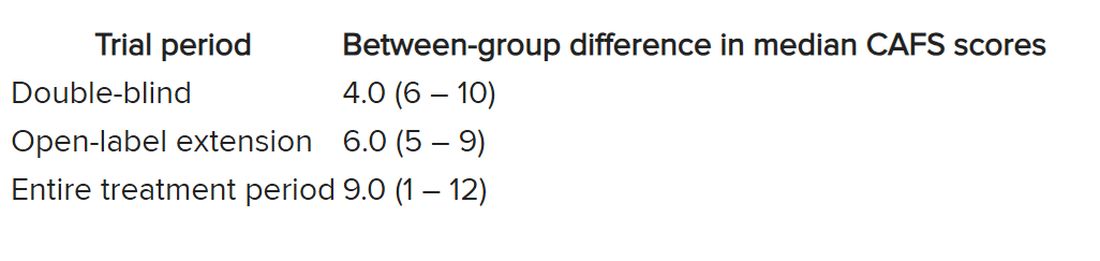
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
However, at least one expert believes the study has “significant flaws.”
Investigators randomly assigned 20 individuals with sporadic ALS to receive either ropinirole or placebo for 24 weeks. During the double-blind period, there was no difference between the groups in terms of decline in functional status.
However, during a further open-label extension period, the ropinirole group showed significant suppression of functional decline and an average of an additional 7 months of progression-free survival.
The researchers were able to predict clinical responsiveness to ropinirole in vitro by analyzing motor neurons derived from participants’ stem cells.
“We found that ropinirole is safe and tolerable for ALS patients and shows therapeutic promise at helping them sustain daily activity and muscle strength,” first author Satoru Morimoto, MD, of the department of physiology, Keio University School of Medicine, Tokyo, said in a news release.
The study was published online in Cell Stem Cell.
Feasibility study
“ALS is totally incurable and it’s a very difficult disease to treat,” senior author Hideyuki Okano, MD, PhD, professor, department of physiology, Keio University, said in the news release.
Preclinical animal models have “limited translational potential” for identifying drug candidates, but induced pluripotent stem cell (iPSC)–derived motor neurons (MNs) from ALS patients can “overcome these limitations for drug screening,” the authors write.
“We previously identified ropinirole [a dopamine D2 receptor agonist] as a potential anti-ALS drug in vitro by iPSC drug discovery,” Dr. Okano said.
The current trial was a randomized, placebo-controlled phase 1/2a feasibility trial that evaluated the safety, tolerability, and efficacy of ropinirole in patients with ALS, using several parameters:
- The revised ALS functional rating scale (ALSFRS-R) score.
- Composite functional endpoints.
- Event-free survival.
- Time to ≤ 50% forced vital capacity (FVC).
The trial consisted of a 12-week run-in period, a 24-week double-blind period, an open-label extension period that lasted from 4 to 24 weeks, and a 4-week follow-up period after administration.
Thirteen patients were assigned to receive ropinirole (23.1% women; mean age, 65.2 ± 12.6 years; 7.7% with clinically definite and 76.9% with clinically probable ALS); seven were assigned to receive placebo (57.1% women; mean age, 66.3 ± 7.5 years; 14.3% with clinically definite and 85.7% with clinically probable ALS).
Of the treatment group, 30.8% had a bulbar onset lesion vs. 57.1% in the placebo group. At baseline, the mean FVC was 94.4% ± 14.9 and 81.5% ± 23.2 in the ropinirole and placebo groups, respectively. The mean body mass index (BMI) was 22.91 ± 3.82 and 19.69 ± 2.63, respectively.
Of the participants,12 in the ropinirole and six in the control group completed the full 24-week treatment protocol; 12 in the ropinirole and five in the placebo group completed the open-label extension (participants who had received placebo were switched to the active drug).
However only seven participants in the ropinirole group and one participant in the placebo group completed the full 1-year trial.
‘Striking correlation’
“During the double-blind period, muscle strength and daily activity were maintained, but a decline in the ALSFRS-R … was not different from that in the placebo group,” the researchers write.
In the open-label extension period, the ropinirole group showed “significant suppression of ALSFRS-R decline,” with an ALSFRS-R score change of only 7.75 (95% confidence interval, 10.66-4.63) for the treatment group vs. 17.51 (95% CI, 22.46-12.56) for the placebo group.
The researchers used the assessment of function and survival (CAFS) score, which adjusts the ALSFRS-R score against mortality, to see whether functional benefits translated into improved survival.
The score “favored ropinirole” in the open-extension period and the entire treatment period but not in the double-blind period.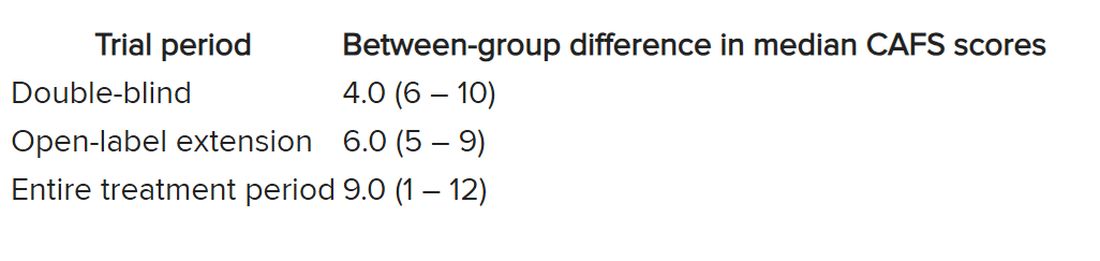
Disease progression events occurred in 7 of 7 (100%) participants in the placebo group and 7 of 13 (54%) in the ropinirole group, “suggesting a twofold decrease in disease progression” in the treatment group.
The ropinirole group experienced an additional 27.9 weeks of disease progression–free survival, compared with the placebo group.
“No participant discontinued treatment because of adverse experiences in either treatment group,” the authors report.
The analysis of iPSC-derived motor neurons from participants showed dopamine D2 receptor expression, as well as the potential involvement of the cholesterol pathway SREBP2 in the therapeutic effects of ropinirole. Lipid peroxide was also identified as a good “surrogate clinical marker to assess disease progression and drug efficacy.”
“We found a very striking correlation between a patient’s clinical response and the response of their motor neurons in vitro,” said Dr. Morimoto. “Patients whose motor neurons responded robustly to ropinirole in vitro had a much slower clinical disease progression with ropinirole treatment, while suboptimal responders showed much more rapid disease progression, despite taking ropinirole.”
Limitations include “small sample sizes and high attrition rates in the open-label extension period,” so “further validation” is required, the authors state.
Significant flaws
Commenting for this article, Carmel Armon, MD, MHS, professor of neurology, Loma Linda (Calif.) University, said the study “falls short of being a credible 1/2a clinical trial.”
Although the “intentions were good and the design not unusual,” the two groups were not “balanced on risk factors for faster progressing disease.” Rather, the placebo group was “tilted towards faster progressing disease” because there were more clinically definite and probable ALS patients in the placebo group than the treatment group, and there were more patients with bulbar onset.
Participants in the placebo group also had shorter median disease duration, lower BMI, and lower FVC, noted Dr. Armon, who was not involved with the study.
And only 1 in 7 control patients completed the open-label extension, compared with 7 of 13 patients in the intervention group.
“With these limitations, I would be disinclined to rely on the findings to justify a larger clinical trial,” Dr. Armon concluded.
The trial was sponsored by K Pharma. The study drug, active drugs, and placebo were supplied free of charge by GlaxoSmithKline K.K. Dr. Okano received grants from JSPS and AMED and grants and personal fees from K Pharma during the conduct of the study and personal fees from Sanbio, outside the submitted work. Dr. Okano has a patent on a therapeutic agent for ALS and composition for treatment licensed to K Pharma. The other authors’ disclosures and additional information are available in the original article. Dr. Armon reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL STEM CELL
Regular napping linked to greater brain volume
Investigators at University College London, and the University of the Republic of Uruguay, Montevideo, found individuals genetically predisposed to regular napping had larger total brain volume, a surrogate of better cognitive health.
“Our results suggest that napping may improve brain health,” first author Valentina Paz, MSc, a PhD candidate at the University of the Republic of Uruguay said in an interview. “Specifically, our work revealed a 15.8 cubic cm increase in total brain volume with more frequent daytime napping,” she said.
The findings were published online in Sleep Health.
Higher brain volume
Previous studies examining the potential link between napping and cognition in older adults have yielded conflicting results.
To clarify this association, Ms. Paz and colleagues used Mendelian randomization to study DNA samples, cognitive outcomes, and functional magnetic resonance imaging data in participants from the ongoing UK Biobank Study.
Starting with data from 378,932 study participants (mean age 57), investigators compared measures of brain health and cognition of those who are more genetically programmed to nap with people who did not have these genetic variations.
More specifically, the investigators examined 97 sections of genetic code previously linked to the likelihood of regular napping and correlated these results with fMRI and cognitive outcomes between those genetically predisposed to take regular naps and those who weren’t.
Study outcomes included total brain volume, hippocampal volume, reaction time, and visual memory.
The final study sample included 35,080 with neuroimaging, cognitive assessment, and genotype data.
The researchers estimated that the average difference in brain volume between individuals genetically programmed to be habitual nappers and those who were not was equivalent to 15.8 cubic cm, or 2.6-6.5 years of aging.
However, there was no difference in the other three outcomes – hippocampal volume, reaction time, and visual processing – between the two study groups.
Since investigators did not have information on the length of time participants napped, Ms. Paz suggested that “taking a short nap in the early afternoon may help cognition in those needing it.”
However, she added, the study’s findings need to be replicated before any firm conclusions can be made.
“More work is needed to examine the associations between napping and cognition, and the replication of these findings using other datasets and methods,” she said.
The investigators note that the study’s findings augment the knowledge of the “impact of habitual daytime napping on brain health, which is essential to understanding cognitive impairment in the aging population. The lack of evidence for an association between napping and hippocampal volume and cognitive outcomes (for example, alertness) may be affected by habitual daytime napping and should be studied in the future.”
Strengths, limitations
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, said, “the study shows a small but significant increase in brain volume in people who have a genetic signature associated with taking daytime naps.”
Dr. Spires-Jones, who was not involved in the research, noted that while the study is well-conducted, it has limitations. Because Mendelian randomization uses a genetic signature, she noted, outcomes depend on the accuracy of the signature.
“The napping habits of UK Biobank participants were self-reported, which might not be entirely accurate, and the ‘napping’ signature overlapped substantially with the signature for cognitive outcomes in the study, which makes the causal link weaker,” she said.
“Even with those limitations, this study is interesting because it adds to the data indicating that sleep is important for brain health,” said Dr. Spires-Jones.
The study was supported by Diabetes UK, the British Heart Foundation, and the Diabetes Research and Wellness Foundation. In Uruguay, it was supported by Programa de Desarrollo de las Ciencias Básicas, Agencia Nacional de Investigación e Innovación, Comisión Sectorial de Investigación Científica, and Comisión Académica de Posgrado. In the United States it was supported by the National Heart, Lung, and Blood Institute. There were no disclosures reported.
A version of this article first appeared on Medscape.com.
Investigators at University College London, and the University of the Republic of Uruguay, Montevideo, found individuals genetically predisposed to regular napping had larger total brain volume, a surrogate of better cognitive health.
“Our results suggest that napping may improve brain health,” first author Valentina Paz, MSc, a PhD candidate at the University of the Republic of Uruguay said in an interview. “Specifically, our work revealed a 15.8 cubic cm increase in total brain volume with more frequent daytime napping,” she said.
The findings were published online in Sleep Health.
Higher brain volume
Previous studies examining the potential link between napping and cognition in older adults have yielded conflicting results.
To clarify this association, Ms. Paz and colleagues used Mendelian randomization to study DNA samples, cognitive outcomes, and functional magnetic resonance imaging data in participants from the ongoing UK Biobank Study.
Starting with data from 378,932 study participants (mean age 57), investigators compared measures of brain health and cognition of those who are more genetically programmed to nap with people who did not have these genetic variations.
More specifically, the investigators examined 97 sections of genetic code previously linked to the likelihood of regular napping and correlated these results with fMRI and cognitive outcomes between those genetically predisposed to take regular naps and those who weren’t.
Study outcomes included total brain volume, hippocampal volume, reaction time, and visual memory.
The final study sample included 35,080 with neuroimaging, cognitive assessment, and genotype data.
The researchers estimated that the average difference in brain volume between individuals genetically programmed to be habitual nappers and those who were not was equivalent to 15.8 cubic cm, or 2.6-6.5 years of aging.
However, there was no difference in the other three outcomes – hippocampal volume, reaction time, and visual processing – between the two study groups.
Since investigators did not have information on the length of time participants napped, Ms. Paz suggested that “taking a short nap in the early afternoon may help cognition in those needing it.”
However, she added, the study’s findings need to be replicated before any firm conclusions can be made.
“More work is needed to examine the associations between napping and cognition, and the replication of these findings using other datasets and methods,” she said.
The investigators note that the study’s findings augment the knowledge of the “impact of habitual daytime napping on brain health, which is essential to understanding cognitive impairment in the aging population. The lack of evidence for an association between napping and hippocampal volume and cognitive outcomes (for example, alertness) may be affected by habitual daytime napping and should be studied in the future.”
Strengths, limitations
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, said, “the study shows a small but significant increase in brain volume in people who have a genetic signature associated with taking daytime naps.”
Dr. Spires-Jones, who was not involved in the research, noted that while the study is well-conducted, it has limitations. Because Mendelian randomization uses a genetic signature, she noted, outcomes depend on the accuracy of the signature.
“The napping habits of UK Biobank participants were self-reported, which might not be entirely accurate, and the ‘napping’ signature overlapped substantially with the signature for cognitive outcomes in the study, which makes the causal link weaker,” she said.
“Even with those limitations, this study is interesting because it adds to the data indicating that sleep is important for brain health,” said Dr. Spires-Jones.
The study was supported by Diabetes UK, the British Heart Foundation, and the Diabetes Research and Wellness Foundation. In Uruguay, it was supported by Programa de Desarrollo de las Ciencias Básicas, Agencia Nacional de Investigación e Innovación, Comisión Sectorial de Investigación Científica, and Comisión Académica de Posgrado. In the United States it was supported by the National Heart, Lung, and Blood Institute. There were no disclosures reported.
A version of this article first appeared on Medscape.com.
Investigators at University College London, and the University of the Republic of Uruguay, Montevideo, found individuals genetically predisposed to regular napping had larger total brain volume, a surrogate of better cognitive health.
“Our results suggest that napping may improve brain health,” first author Valentina Paz, MSc, a PhD candidate at the University of the Republic of Uruguay said in an interview. “Specifically, our work revealed a 15.8 cubic cm increase in total brain volume with more frequent daytime napping,” she said.
The findings were published online in Sleep Health.
Higher brain volume
Previous studies examining the potential link between napping and cognition in older adults have yielded conflicting results.
To clarify this association, Ms. Paz and colleagues used Mendelian randomization to study DNA samples, cognitive outcomes, and functional magnetic resonance imaging data in participants from the ongoing UK Biobank Study.
Starting with data from 378,932 study participants (mean age 57), investigators compared measures of brain health and cognition of those who are more genetically programmed to nap with people who did not have these genetic variations.
More specifically, the investigators examined 97 sections of genetic code previously linked to the likelihood of regular napping and correlated these results with fMRI and cognitive outcomes between those genetically predisposed to take regular naps and those who weren’t.
Study outcomes included total brain volume, hippocampal volume, reaction time, and visual memory.
The final study sample included 35,080 with neuroimaging, cognitive assessment, and genotype data.
The researchers estimated that the average difference in brain volume between individuals genetically programmed to be habitual nappers and those who were not was equivalent to 15.8 cubic cm, or 2.6-6.5 years of aging.
However, there was no difference in the other three outcomes – hippocampal volume, reaction time, and visual processing – between the two study groups.
Since investigators did not have information on the length of time participants napped, Ms. Paz suggested that “taking a short nap in the early afternoon may help cognition in those needing it.”
However, she added, the study’s findings need to be replicated before any firm conclusions can be made.
“More work is needed to examine the associations between napping and cognition, and the replication of these findings using other datasets and methods,” she said.
The investigators note that the study’s findings augment the knowledge of the “impact of habitual daytime napping on brain health, which is essential to understanding cognitive impairment in the aging population. The lack of evidence for an association between napping and hippocampal volume and cognitive outcomes (for example, alertness) may be affected by habitual daytime napping and should be studied in the future.”
Strengths, limitations
Tara Spires-Jones, PhD, president of the British Neuroscience Association and group leader at the UK Dementia Research Institute, said, “the study shows a small but significant increase in brain volume in people who have a genetic signature associated with taking daytime naps.”
Dr. Spires-Jones, who was not involved in the research, noted that while the study is well-conducted, it has limitations. Because Mendelian randomization uses a genetic signature, she noted, outcomes depend on the accuracy of the signature.
“The napping habits of UK Biobank participants were self-reported, which might not be entirely accurate, and the ‘napping’ signature overlapped substantially with the signature for cognitive outcomes in the study, which makes the causal link weaker,” she said.
“Even with those limitations, this study is interesting because it adds to the data indicating that sleep is important for brain health,” said Dr. Spires-Jones.
The study was supported by Diabetes UK, the British Heart Foundation, and the Diabetes Research and Wellness Foundation. In Uruguay, it was supported by Programa de Desarrollo de las Ciencias Básicas, Agencia Nacional de Investigación e Innovación, Comisión Sectorial de Investigación Científica, and Comisión Académica de Posgrado. In the United States it was supported by the National Heart, Lung, and Blood Institute. There were no disclosures reported.
A version of this article first appeared on Medscape.com.
FROM SLEEP HEALTH
No link between PPIs and dementia in new study
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.

