User login
Inpatient sleep medicine: An invaluable service for hospital medicine
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.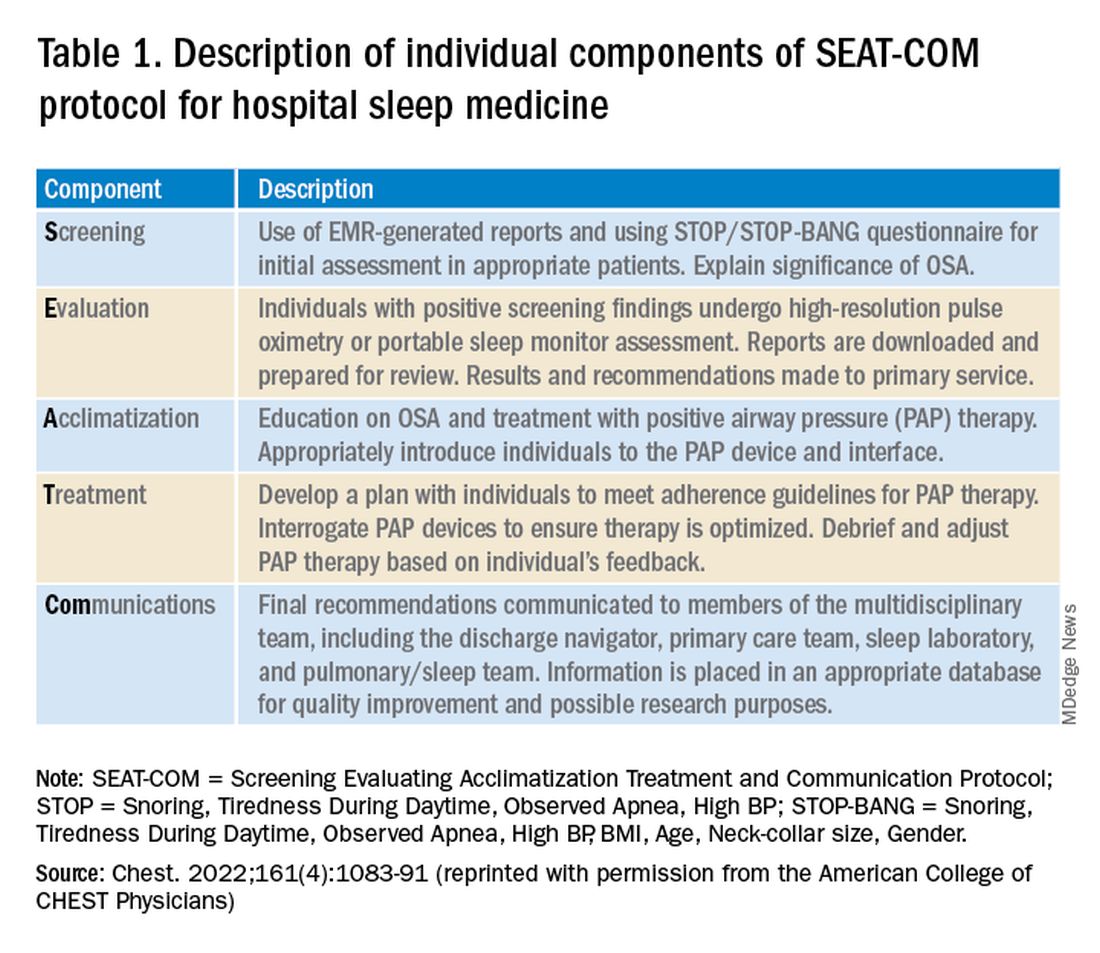
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Study sheds new light on RAS inhibitors’ role for advanced CKD
ORLANDO – Treatment with a renin-angiotensin system (RAS) inhibitor is widely accepted as standard practice for slowing progression of chronic kidney disease (CKD), but data have been inconsistent as to whether there is benefit to continuing RAS inhibition when patients develop advanced CKD, defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1.73 m2.
Now, in STOP ACEi, a new multicenter, randomized trial of 411 patients, , for 3 years.
People who continued RAS inhibitor treatment did not develop a significant or clinically relevant decrease in eGFR, the study’s primary outcome, both overall as well as in several prespecified subgroups compared with those who discontinued treatment, said Sunil Bhandari, MBChB, PhD, and associates, who presented the research in a poster at the annual meeting of the American Society of Nephrology.
“I hope these results will reassure clinicians to continue ACE inhibitors or ARBs” in patients with advanced CKD, “with their known beneficial cardiovascular effects,” Dr. Bhandari said in an interview.
The results were simultaneously published in the New England Journal of Medicine.
Similar eGFR levels after 3 years
While it’s clear that in patients with mild or moderate CKD, treatment with a RAS inhibitor, which includes angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), reduces blood pressure, slows decline in eGFR, reduces proteinuria, and delays progression to advanced CKD, there has been little evidence that the use of RAS inhibitors benefits patients with advanced CKD.
Data from previous trials have been inconsistent regarding whether the use of RAS inhibitors is nephroprotective in patients with advanced CKD, say Dr. Bhandari, a nephrologist and professor at Hull York Medical School, Hull, England, and colleagues.
“Current guidelines do not provide specific advice on whether to continue or stop ACE inhibitors or ARBs for advanced chronic kidney disease,” they also note.
And so they decided to assess whether discontinuation of ACE inhibitors/ARBs could slow progression of CKD in patients with advanced CKD.
Three years after 206 study participants stopped RAS inhibitor treatment, the least-squares mean eGFR was 12.6 mL/min per 1.73m2 in the discontinuation group and 13.3 mL/min per 1.73 m2 in the 205 patients in the continuation group, a difference that was not significant.
In addition to the primary outcome, 62% of patients who stopped RAS inhibitor treatment and 56% of those who continued developed end-stage kidney disease or required renal-replacement therapy, which translated into an adjusted hazard ratio of 1.28 for this outcome among those who discontinued compared with those who continued, which was just short of significance (95% CI, 0.99-1.65).
The two study groups also showed no significant differences in the 3-year incidence of hospitalization for any reason, cardiovascular events, or deaths. The two groups also showed no meaningful differences in various domains of quality of life and no differences in serious adverse effects.
Participants had an eGFR less than 30 mL/min per 1.73 m2
The study ran at 39 United Kingdom centers in 2014-2019. Investigators enrolled adults with an eGFR of less than 30 mL/min per 1.73 m2 who were not on dialysis and had not received a kidney transplant. In addition, all enrolled patients had to have an annual drop in eGFR of more than 2 mL/min per 1.73 m2 during the prior 2 years and had to have been on treatment with at least one RAS inhibitor for more than 6 months.
The randomization protocol insured balanced distribution of subjects between the two study arms by age, eGFR, presence of diabetes, and level of proteinuria, among other factors. The study design also mandated that participants maintain a blood pressure of no more than 140/85 mm Hg.
Those who discontinued RAS-inhibitor treatment could receive any guideline-recommended antihypertensive agent that was not a RAS inhibitor, although adding a RAS inhibitor was permitted as a last treatment resort.
People in the maintenance group could receive whichever additional antihypertensive agents their treating clinicians deemed necessary for maintaining the target blood pressure.
The enrolled population was a median age of 63 years old and 68% were men. Their average eGFR at baseline was 18 mL/min per 1.73 m2, and 118 (29%) had an eGFR of less than 15 mL/min per 1.73 m2. Their median level of proteinuria was 115 mg/mmol (about 1,018 mg/g). Diabetes was prevalent in 37%, and 58% of participants were taking at least three antihypertensive medications at entry.
Among the study’s limitations, the researchers cited the open-label design, which may have affected clinical care and the tally of subjective endpoints, including quality of life and exercise capacity. Also, because the study enrolled people who were on a RAS inhibitor at the time of randomization, it did not include anyone who had already discontinued these agents.
Continue RAS inhibitors in advanced CKD for best outcomes
Dr. Bhandari and colleagues note that in a large observational trial published in January 2021, Swedish researchers found an increase in the incidence of major cardiovascular events and death among patients with advanced CKD who had discontinued RAS inhibitors.
But they observe, “Our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on cardiovascular events or mortality. However, because our findings are consistent with a lack of advantage for such discontinuation with respect to kidney function, there is little rationale to conduct a larger randomized trial to investigate cardiovascular safety.”
“Our findings do not support the hypothesis that the discontinuation of RAS inhibitors in patients with advanced and progressive chronic kidney disease would improve kidney function, quality of life, or exercise capacity.”
“The results of this trial will inform future clinical practice worldwide and guideline recommendations,” they conclude.
STOP ACEi received no commercial funding. Dr. Bhandari has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – Treatment with a renin-angiotensin system (RAS) inhibitor is widely accepted as standard practice for slowing progression of chronic kidney disease (CKD), but data have been inconsistent as to whether there is benefit to continuing RAS inhibition when patients develop advanced CKD, defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1.73 m2.
Now, in STOP ACEi, a new multicenter, randomized trial of 411 patients, , for 3 years.
People who continued RAS inhibitor treatment did not develop a significant or clinically relevant decrease in eGFR, the study’s primary outcome, both overall as well as in several prespecified subgroups compared with those who discontinued treatment, said Sunil Bhandari, MBChB, PhD, and associates, who presented the research in a poster at the annual meeting of the American Society of Nephrology.
“I hope these results will reassure clinicians to continue ACE inhibitors or ARBs” in patients with advanced CKD, “with their known beneficial cardiovascular effects,” Dr. Bhandari said in an interview.
The results were simultaneously published in the New England Journal of Medicine.
Similar eGFR levels after 3 years
While it’s clear that in patients with mild or moderate CKD, treatment with a RAS inhibitor, which includes angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), reduces blood pressure, slows decline in eGFR, reduces proteinuria, and delays progression to advanced CKD, there has been little evidence that the use of RAS inhibitors benefits patients with advanced CKD.
Data from previous trials have been inconsistent regarding whether the use of RAS inhibitors is nephroprotective in patients with advanced CKD, say Dr. Bhandari, a nephrologist and professor at Hull York Medical School, Hull, England, and colleagues.
“Current guidelines do not provide specific advice on whether to continue or stop ACE inhibitors or ARBs for advanced chronic kidney disease,” they also note.
And so they decided to assess whether discontinuation of ACE inhibitors/ARBs could slow progression of CKD in patients with advanced CKD.
Three years after 206 study participants stopped RAS inhibitor treatment, the least-squares mean eGFR was 12.6 mL/min per 1.73m2 in the discontinuation group and 13.3 mL/min per 1.73 m2 in the 205 patients in the continuation group, a difference that was not significant.
In addition to the primary outcome, 62% of patients who stopped RAS inhibitor treatment and 56% of those who continued developed end-stage kidney disease or required renal-replacement therapy, which translated into an adjusted hazard ratio of 1.28 for this outcome among those who discontinued compared with those who continued, which was just short of significance (95% CI, 0.99-1.65).
The two study groups also showed no significant differences in the 3-year incidence of hospitalization for any reason, cardiovascular events, or deaths. The two groups also showed no meaningful differences in various domains of quality of life and no differences in serious adverse effects.
Participants had an eGFR less than 30 mL/min per 1.73 m2
The study ran at 39 United Kingdom centers in 2014-2019. Investigators enrolled adults with an eGFR of less than 30 mL/min per 1.73 m2 who were not on dialysis and had not received a kidney transplant. In addition, all enrolled patients had to have an annual drop in eGFR of more than 2 mL/min per 1.73 m2 during the prior 2 years and had to have been on treatment with at least one RAS inhibitor for more than 6 months.
The randomization protocol insured balanced distribution of subjects between the two study arms by age, eGFR, presence of diabetes, and level of proteinuria, among other factors. The study design also mandated that participants maintain a blood pressure of no more than 140/85 mm Hg.
Those who discontinued RAS-inhibitor treatment could receive any guideline-recommended antihypertensive agent that was not a RAS inhibitor, although adding a RAS inhibitor was permitted as a last treatment resort.
People in the maintenance group could receive whichever additional antihypertensive agents their treating clinicians deemed necessary for maintaining the target blood pressure.
The enrolled population was a median age of 63 years old and 68% were men. Their average eGFR at baseline was 18 mL/min per 1.73 m2, and 118 (29%) had an eGFR of less than 15 mL/min per 1.73 m2. Their median level of proteinuria was 115 mg/mmol (about 1,018 mg/g). Diabetes was prevalent in 37%, and 58% of participants were taking at least three antihypertensive medications at entry.
Among the study’s limitations, the researchers cited the open-label design, which may have affected clinical care and the tally of subjective endpoints, including quality of life and exercise capacity. Also, because the study enrolled people who were on a RAS inhibitor at the time of randomization, it did not include anyone who had already discontinued these agents.
Continue RAS inhibitors in advanced CKD for best outcomes
Dr. Bhandari and colleagues note that in a large observational trial published in January 2021, Swedish researchers found an increase in the incidence of major cardiovascular events and death among patients with advanced CKD who had discontinued RAS inhibitors.
But they observe, “Our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on cardiovascular events or mortality. However, because our findings are consistent with a lack of advantage for such discontinuation with respect to kidney function, there is little rationale to conduct a larger randomized trial to investigate cardiovascular safety.”
“Our findings do not support the hypothesis that the discontinuation of RAS inhibitors in patients with advanced and progressive chronic kidney disease would improve kidney function, quality of life, or exercise capacity.”
“The results of this trial will inform future clinical practice worldwide and guideline recommendations,” they conclude.
STOP ACEi received no commercial funding. Dr. Bhandari has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – Treatment with a renin-angiotensin system (RAS) inhibitor is widely accepted as standard practice for slowing progression of chronic kidney disease (CKD), but data have been inconsistent as to whether there is benefit to continuing RAS inhibition when patients develop advanced CKD, defined as an estimated glomerular filtration rate (eGFR) of less than 30 mL/min per 1.73 m2.
Now, in STOP ACEi, a new multicenter, randomized trial of 411 patients, , for 3 years.
People who continued RAS inhibitor treatment did not develop a significant or clinically relevant decrease in eGFR, the study’s primary outcome, both overall as well as in several prespecified subgroups compared with those who discontinued treatment, said Sunil Bhandari, MBChB, PhD, and associates, who presented the research in a poster at the annual meeting of the American Society of Nephrology.
“I hope these results will reassure clinicians to continue ACE inhibitors or ARBs” in patients with advanced CKD, “with their known beneficial cardiovascular effects,” Dr. Bhandari said in an interview.
The results were simultaneously published in the New England Journal of Medicine.
Similar eGFR levels after 3 years
While it’s clear that in patients with mild or moderate CKD, treatment with a RAS inhibitor, which includes angiotensin-converting enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs), reduces blood pressure, slows decline in eGFR, reduces proteinuria, and delays progression to advanced CKD, there has been little evidence that the use of RAS inhibitors benefits patients with advanced CKD.
Data from previous trials have been inconsistent regarding whether the use of RAS inhibitors is nephroprotective in patients with advanced CKD, say Dr. Bhandari, a nephrologist and professor at Hull York Medical School, Hull, England, and colleagues.
“Current guidelines do not provide specific advice on whether to continue or stop ACE inhibitors or ARBs for advanced chronic kidney disease,” they also note.
And so they decided to assess whether discontinuation of ACE inhibitors/ARBs could slow progression of CKD in patients with advanced CKD.
Three years after 206 study participants stopped RAS inhibitor treatment, the least-squares mean eGFR was 12.6 mL/min per 1.73m2 in the discontinuation group and 13.3 mL/min per 1.73 m2 in the 205 patients in the continuation group, a difference that was not significant.
In addition to the primary outcome, 62% of patients who stopped RAS inhibitor treatment and 56% of those who continued developed end-stage kidney disease or required renal-replacement therapy, which translated into an adjusted hazard ratio of 1.28 for this outcome among those who discontinued compared with those who continued, which was just short of significance (95% CI, 0.99-1.65).
The two study groups also showed no significant differences in the 3-year incidence of hospitalization for any reason, cardiovascular events, or deaths. The two groups also showed no meaningful differences in various domains of quality of life and no differences in serious adverse effects.
Participants had an eGFR less than 30 mL/min per 1.73 m2
The study ran at 39 United Kingdom centers in 2014-2019. Investigators enrolled adults with an eGFR of less than 30 mL/min per 1.73 m2 who were not on dialysis and had not received a kidney transplant. In addition, all enrolled patients had to have an annual drop in eGFR of more than 2 mL/min per 1.73 m2 during the prior 2 years and had to have been on treatment with at least one RAS inhibitor for more than 6 months.
The randomization protocol insured balanced distribution of subjects between the two study arms by age, eGFR, presence of diabetes, and level of proteinuria, among other factors. The study design also mandated that participants maintain a blood pressure of no more than 140/85 mm Hg.
Those who discontinued RAS-inhibitor treatment could receive any guideline-recommended antihypertensive agent that was not a RAS inhibitor, although adding a RAS inhibitor was permitted as a last treatment resort.
People in the maintenance group could receive whichever additional antihypertensive agents their treating clinicians deemed necessary for maintaining the target blood pressure.
The enrolled population was a median age of 63 years old and 68% were men. Their average eGFR at baseline was 18 mL/min per 1.73 m2, and 118 (29%) had an eGFR of less than 15 mL/min per 1.73 m2. Their median level of proteinuria was 115 mg/mmol (about 1,018 mg/g). Diabetes was prevalent in 37%, and 58% of participants were taking at least three antihypertensive medications at entry.
Among the study’s limitations, the researchers cited the open-label design, which may have affected clinical care and the tally of subjective endpoints, including quality of life and exercise capacity. Also, because the study enrolled people who were on a RAS inhibitor at the time of randomization, it did not include anyone who had already discontinued these agents.
Continue RAS inhibitors in advanced CKD for best outcomes
Dr. Bhandari and colleagues note that in a large observational trial published in January 2021, Swedish researchers found an increase in the incidence of major cardiovascular events and death among patients with advanced CKD who had discontinued RAS inhibitors.
But they observe, “Our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on cardiovascular events or mortality. However, because our findings are consistent with a lack of advantage for such discontinuation with respect to kidney function, there is little rationale to conduct a larger randomized trial to investigate cardiovascular safety.”
“Our findings do not support the hypothesis that the discontinuation of RAS inhibitors in patients with advanced and progressive chronic kidney disease would improve kidney function, quality of life, or exercise capacity.”
“The results of this trial will inform future clinical practice worldwide and guideline recommendations,” they conclude.
STOP ACEi received no commercial funding. Dr. Bhandari has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT KIDNEY WEEK 2022
Therapeutic drug monitoring pays off for arthritis patients
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
Therapeutic drug monitoring allowed patients with rheumatoid arthritis, psoriatic arthritis, and spondyloarthritis to reduce their dosage of tumor necrosis factor–alpha (TNF) inhibitors, based on data from 239 individuals.
Use of TNF-alpha inhibitors improves treatment response for many arthritis patients but dosage is rarely adjusted on an individual level, which may lead to unnecessary overdosing in some patients, Mogens Pfeiffer-Jensen, MD, of Aarhus (Denmark) University Hospital, and colleagues wrote.
Data from previous studies suggest that therapeutic drug monitoring (TDM) based on serum trough levels may allow for dose optimization and dose reduction in inflammatory bowel disease patients, but data in patients with arthritis are lacking, they wrote.
In a study published in the Scandinavian Journal of Rheumatology, the researchers recruited 99 patients with RA, 48 with psoriatic arthritis (PsA), and 92 with spondyloarthritis (SpA). The participants were randomized to standard care or standard care plus TDM. Serum trough levels were assessed at baseline and at every 4 months, and prescription changes or drug switches were implemented based on these levels. At baseline, 81 patients were being treated with infliximab (Remicade and biosimilars), 79 with etanercept (Enbrel), and 79 with adalimumab (Humira).
The primary endpoint was reduced drug prescription after 48 weeks.
Overall, TDM significantly reduced prescription of infliximab by 12% (P = .001) and prescription of etanercept by 15% (P = .01), compared with standard care. TDM also prolonged the interdosing intervals of etanercept by 235% (P = .02) and of adalimumab by 28% (P = .04), compared with standard care.
TDM patients taking infliximab had more frequent dose reduction and less frequent dose increases during and after the study when compared with patients who stayed with standard care; similar trends were seen with adalimumab. TDM also accelerated the switch to other biologics for patients on all three medications.
No significant differences occurred in adverse events or hospitalizations between the TDM and standard care patients.
Clinical composite scores (Disease Activity Score based on 28 joints with C-reactive protein) were reduced in patients with RA and PsA who were taking adalimumab and randomized to TDM, but no other clinical outcome differences were noted. Scores on the Health Assessment Questionnaire and global Visual Analog Scale for pain were significantly lower in patients in the TDM group who were taking infliximab and adalimumab, “indicating equally or superior sustained remission across diagnoses,” the researchers emphasized.
The findings were limited by several factors, including the variations in pathophysiology and open-label design. “However, since the TDM was based on an objective serum value and decision procedures were clear, we do not consider the potential of unconscious bias to outweigh the benefits of dose-changing abilities,” they wrote.
The researchers expressed surprise that the reduced use of TNF-alpha inhibitors did not significantly reduce adverse events or serious adverse events, compared with standard care, but they proposed that standard of care may have taken adverse events into account, because all patients had received prescriptions at least 3 months before the study.
As for clinical implications, the current costs of the biochemical assays necessary for TDM may be a barrier to implementing TDM as a standard part of daily clinical practice, the researchers added. However, the study was strengthened by the inclusion of patients with RA, PsA, and SpA, and is the first known to include patients receiving etanercept or adalimumab in an examination of TDM.
“Our data support TDM based solely on serum trough levels in [TNF-alpha inhibitors] with different pharmacokinetics as a future key player in personalized medicine for chronic rheumatoid diseases treated with biologics,” they concluded.
The study was supported by Spydspidspuljen, Region Midt, Denmark, and Department of Rheumatology, Aarhus University Hospital. The researchers had no financial conflicts to disclose.
FROM THE SCANDINAVIAN JOURNAL OF RHEUMATOLOGY
Moving the needle: SGLT2 inhibitor role for isolated kidney disease
ORLANDO – in a pivotal trial with more than 6,600 patients.
This confirms the efficacy for this population that was previously seen with dapagliflozin, another agent from the same class, in the DAPA-CKD trial.
In the new trial, EMPA-Kidney, treatment with empagliflozin 10 mg daily for a median of 2.0 years led to a significant 28% relative risk reduction in the primary combined endpoint in comparison with placebo, William G. Herrington, MD, reported at the annual meeting of the American Society of Nephrology.
The results were simultaneously published in the New England Journal of Medicine.
In 2020, a different team of researchers running DAPA-CKD reported that during a median of 2.4 years, treatment of 4,304 patients with dapagliflozin 10 mg daily resulted in a significant 39% relative risk reduction, compared with placebo for an identical combined primary endpoint. Enrollment criteria for the DAPA-CKD trial were mostly similar to that of the current trial.
‘Remarkably similar’ findings
Results from EMPA-Kidney and DAPA-CKD are “remarkably similar,” said Dr. Herrington during a press briefing at the meeting.
He also noted that when the EMPA-Kidney study began – before results from DAPA-CKD were known – “we never imagined such a large effect” on important endpoints in people with CKD.
In addition to cardiovascular death, the combined primary endpoint included the incidence of renal death, incident end-stage kidney disease, a sustained decrease in estimated glomerular filtration rate to less than 10 mL/min per 1.73m2, or a sustained decrease in eGFR of at least 40% from baseline.
Having similar evidence from both trials “will hopefully provide people with the confidence to start to use SGLT2 inhibitors as standard care in people with CKD” who match enrollment criteria of the two trials, added Dr. Herrington, a nephrologist at the University of Oxford (England).
The analyses he reported also showed that empagliflozin had similar efficacy for the primary endpoint regardless of whether patients had type 2 diabetes at the time of enrollment and regardless of their eGFR at entry.
To enter EMPA-Kidney, people needed to have either an eGFR of 20-44 mL/min per 1.73m2 with no minimum level of albuminuria or an eGFR of 45-89 mL/min per 1.73m2 with a urine albumin-to-creatinine ratio (UACR) of at least 200 mg/g.
In contrast, to enroll in DAPA-CKD, patients had to have a UACR of at least 200 mg/g. This means that for the first time, EMPA-Kidney produced data on the relationship between albuminuria severity and the impact of treatment with an SGLT2 inhibitor in the enrolled population.
A signal of greater efficacy with higher UACR
A total of 6,609 patients underwent randomization in EMPA-Kidney. During a median of 2.0 years of follow-up, the primary endpoint – progression of kidney disease or death from cardiovascular causes – occurred in 432 of 3,304 patients (13.1%) in the empagliflozin group and in 558 of 3,305 patients (16.9%) in the placebo group (hazard ratio, 0.72; P < .001).
The results “suggested that the effects [of empagliflozin] are greater in patients with higher levels of albuminuria, with statistically significant heterogeneity between this subgroup and those with a UACR of less than 200 mg/g (P = .02),” Dr. Herrington said.
Of the study population, 54% had no evidence of diabetes at enrollment.
Having data from a second large trial of an SGLT2 inhibitor that included people with isolated CKD who did not have diabetes or heart failure “will start to move the needle” on using this class of drugs in these types of patients, commented F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn.
On the basis of the DAPA-CKD results, in April 2021 the Food and Drug Administration expanded dapagliflozin’s indications to include CKD, yet, “a lot of nephrologists consider SGLT2 inhibitors to be agents for people with diabetes or heart failure, and they defer prescribing them to endocrinologists and cardiologists,” Dr. Wilson said in an interview.
‘Flozinators’ rising
But Pascale H. Lane, MD, a pediatric nephrologist at the University of Oklahoma Health Sciences Center, Oklahoma City, commented that many nephrologists she knows have been prescribing dapagliflozin “widely” to their patients with CKD.
“I know many adult nephrologists who use it almost universally now,” Dr. Lane said. “They call themselves ‘flozinators.’ ”
EMPA-Kidney was sponsored by Boehringer Ingelheim, the company that along with Lilly markets empagliflozin (Jardiance). Dr. Herrington, Dr. Wilson, and Dr. Lane disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – in a pivotal trial with more than 6,600 patients.
This confirms the efficacy for this population that was previously seen with dapagliflozin, another agent from the same class, in the DAPA-CKD trial.
In the new trial, EMPA-Kidney, treatment with empagliflozin 10 mg daily for a median of 2.0 years led to a significant 28% relative risk reduction in the primary combined endpoint in comparison with placebo, William G. Herrington, MD, reported at the annual meeting of the American Society of Nephrology.
The results were simultaneously published in the New England Journal of Medicine.
In 2020, a different team of researchers running DAPA-CKD reported that during a median of 2.4 years, treatment of 4,304 patients with dapagliflozin 10 mg daily resulted in a significant 39% relative risk reduction, compared with placebo for an identical combined primary endpoint. Enrollment criteria for the DAPA-CKD trial were mostly similar to that of the current trial.
‘Remarkably similar’ findings
Results from EMPA-Kidney and DAPA-CKD are “remarkably similar,” said Dr. Herrington during a press briefing at the meeting.
He also noted that when the EMPA-Kidney study began – before results from DAPA-CKD were known – “we never imagined such a large effect” on important endpoints in people with CKD.
In addition to cardiovascular death, the combined primary endpoint included the incidence of renal death, incident end-stage kidney disease, a sustained decrease in estimated glomerular filtration rate to less than 10 mL/min per 1.73m2, or a sustained decrease in eGFR of at least 40% from baseline.
Having similar evidence from both trials “will hopefully provide people with the confidence to start to use SGLT2 inhibitors as standard care in people with CKD” who match enrollment criteria of the two trials, added Dr. Herrington, a nephrologist at the University of Oxford (England).
The analyses he reported also showed that empagliflozin had similar efficacy for the primary endpoint regardless of whether patients had type 2 diabetes at the time of enrollment and regardless of their eGFR at entry.
To enter EMPA-Kidney, people needed to have either an eGFR of 20-44 mL/min per 1.73m2 with no minimum level of albuminuria or an eGFR of 45-89 mL/min per 1.73m2 with a urine albumin-to-creatinine ratio (UACR) of at least 200 mg/g.
In contrast, to enroll in DAPA-CKD, patients had to have a UACR of at least 200 mg/g. This means that for the first time, EMPA-Kidney produced data on the relationship between albuminuria severity and the impact of treatment with an SGLT2 inhibitor in the enrolled population.
A signal of greater efficacy with higher UACR
A total of 6,609 patients underwent randomization in EMPA-Kidney. During a median of 2.0 years of follow-up, the primary endpoint – progression of kidney disease or death from cardiovascular causes – occurred in 432 of 3,304 patients (13.1%) in the empagliflozin group and in 558 of 3,305 patients (16.9%) in the placebo group (hazard ratio, 0.72; P < .001).
The results “suggested that the effects [of empagliflozin] are greater in patients with higher levels of albuminuria, with statistically significant heterogeneity between this subgroup and those with a UACR of less than 200 mg/g (P = .02),” Dr. Herrington said.
Of the study population, 54% had no evidence of diabetes at enrollment.
Having data from a second large trial of an SGLT2 inhibitor that included people with isolated CKD who did not have diabetes or heart failure “will start to move the needle” on using this class of drugs in these types of patients, commented F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn.
On the basis of the DAPA-CKD results, in April 2021 the Food and Drug Administration expanded dapagliflozin’s indications to include CKD, yet, “a lot of nephrologists consider SGLT2 inhibitors to be agents for people with diabetes or heart failure, and they defer prescribing them to endocrinologists and cardiologists,” Dr. Wilson said in an interview.
‘Flozinators’ rising
But Pascale H. Lane, MD, a pediatric nephrologist at the University of Oklahoma Health Sciences Center, Oklahoma City, commented that many nephrologists she knows have been prescribing dapagliflozin “widely” to their patients with CKD.
“I know many adult nephrologists who use it almost universally now,” Dr. Lane said. “They call themselves ‘flozinators.’ ”
EMPA-Kidney was sponsored by Boehringer Ingelheim, the company that along with Lilly markets empagliflozin (Jardiance). Dr. Herrington, Dr. Wilson, and Dr. Lane disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ORLANDO – in a pivotal trial with more than 6,600 patients.
This confirms the efficacy for this population that was previously seen with dapagliflozin, another agent from the same class, in the DAPA-CKD trial.
In the new trial, EMPA-Kidney, treatment with empagliflozin 10 mg daily for a median of 2.0 years led to a significant 28% relative risk reduction in the primary combined endpoint in comparison with placebo, William G. Herrington, MD, reported at the annual meeting of the American Society of Nephrology.
The results were simultaneously published in the New England Journal of Medicine.
In 2020, a different team of researchers running DAPA-CKD reported that during a median of 2.4 years, treatment of 4,304 patients with dapagliflozin 10 mg daily resulted in a significant 39% relative risk reduction, compared with placebo for an identical combined primary endpoint. Enrollment criteria for the DAPA-CKD trial were mostly similar to that of the current trial.
‘Remarkably similar’ findings
Results from EMPA-Kidney and DAPA-CKD are “remarkably similar,” said Dr. Herrington during a press briefing at the meeting.
He also noted that when the EMPA-Kidney study began – before results from DAPA-CKD were known – “we never imagined such a large effect” on important endpoints in people with CKD.
In addition to cardiovascular death, the combined primary endpoint included the incidence of renal death, incident end-stage kidney disease, a sustained decrease in estimated glomerular filtration rate to less than 10 mL/min per 1.73m2, or a sustained decrease in eGFR of at least 40% from baseline.
Having similar evidence from both trials “will hopefully provide people with the confidence to start to use SGLT2 inhibitors as standard care in people with CKD” who match enrollment criteria of the two trials, added Dr. Herrington, a nephrologist at the University of Oxford (England).
The analyses he reported also showed that empagliflozin had similar efficacy for the primary endpoint regardless of whether patients had type 2 diabetes at the time of enrollment and regardless of their eGFR at entry.
To enter EMPA-Kidney, people needed to have either an eGFR of 20-44 mL/min per 1.73m2 with no minimum level of albuminuria or an eGFR of 45-89 mL/min per 1.73m2 with a urine albumin-to-creatinine ratio (UACR) of at least 200 mg/g.
In contrast, to enroll in DAPA-CKD, patients had to have a UACR of at least 200 mg/g. This means that for the first time, EMPA-Kidney produced data on the relationship between albuminuria severity and the impact of treatment with an SGLT2 inhibitor in the enrolled population.
A signal of greater efficacy with higher UACR
A total of 6,609 patients underwent randomization in EMPA-Kidney. During a median of 2.0 years of follow-up, the primary endpoint – progression of kidney disease or death from cardiovascular causes – occurred in 432 of 3,304 patients (13.1%) in the empagliflozin group and in 558 of 3,305 patients (16.9%) in the placebo group (hazard ratio, 0.72; P < .001).
The results “suggested that the effects [of empagliflozin] are greater in patients with higher levels of albuminuria, with statistically significant heterogeneity between this subgroup and those with a UACR of less than 200 mg/g (P = .02),” Dr. Herrington said.
Of the study population, 54% had no evidence of diabetes at enrollment.
Having data from a second large trial of an SGLT2 inhibitor that included people with isolated CKD who did not have diabetes or heart failure “will start to move the needle” on using this class of drugs in these types of patients, commented F. Perry Wilson, MD, a nephrologist at Yale University, New Haven, Conn.
On the basis of the DAPA-CKD results, in April 2021 the Food and Drug Administration expanded dapagliflozin’s indications to include CKD, yet, “a lot of nephrologists consider SGLT2 inhibitors to be agents for people with diabetes or heart failure, and they defer prescribing them to endocrinologists and cardiologists,” Dr. Wilson said in an interview.
‘Flozinators’ rising
But Pascale H. Lane, MD, a pediatric nephrologist at the University of Oklahoma Health Sciences Center, Oklahoma City, commented that many nephrologists she knows have been prescribing dapagliflozin “widely” to their patients with CKD.
“I know many adult nephrologists who use it almost universally now,” Dr. Lane said. “They call themselves ‘flozinators.’ ”
EMPA-Kidney was sponsored by Boehringer Ingelheim, the company that along with Lilly markets empagliflozin (Jardiance). Dr. Herrington, Dr. Wilson, and Dr. Lane disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT KIDNEY WEEK 2022
Low-carb diet aids weight loss in liver transplant recipients with obesity
A low-carbohydrate diet appears to be an effective weight-loss intervention in liver transplant recipients with obesity as compared with a calorie-restrictive diet, according to interim findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
In particular, the intervention showed significant improvements in the metabophenotype profile, including visceral adipose tissue and abdominal subcutaneous adipose tissue, said Mohammad Siddiqui, MD, a gastroenterologist and liver transplant specialist at Virginia Commonwealth University, Richmond.
“Weight gain and obesity after liver transplantation is common,” he said. “Posttransplant obesity is associated with increased cardiometabolic risk burden, increased risk of cardiovascular disease and mortality, and overall mortality.”
Previously, Dr. Siddiqui and colleagues have shown that posttransplant weight loss is difficult because of metabolic inflexibility and mitochondrial inefficiency. By specifically targeting carbohydrate utilization, metabolic flexibility could be restored in liver transplant recipients, he noted.
Dr. Siddiqui and colleagues conducted a randomized controlled trial of 27 adult liver transplant recipients with obesity for 24 weeks. The primary endpoint was change in weight, and the secondary endpoints involved metabophenotype, metabolic flexibility, mitochondrial function, and metabolic risk. The research team excluded patients with end-stage disease, terminal disease, use of weight-loss medications, pregnancy, or uncontrolled psychiatric illness that could interfere with adherence.
Among the participants, 13 were randomized to a calorie restrictive diet of less than 1,200-1,500 calories per day, and 14 were randomized to a low-carbohydrate diet of 20 grams or less per day. At enrollment, the participants underwent dietary, activity, skeletal muscle, and body composition assessments, as well as metabophenotype measurements of visceral adipose tissue, abdominal subcutaneous adipose tissue, muscle fat infiltration, fat-free muscle volume, and proton density fat fraction.
All participants were advised to maintain the same level of physical activity, which was measured through 7-day accelerometry. In addition, the patients were contacted every 2 weeks throughout the 24-week study period.
“We wanted to reinforce the dietary advice. We wanted to identify factors that may lead to compliance,” Dr. Siddiqui said. “Multiple studies have documented that the more contact that patients have during weight-loss studies with medical personnel, the more effective those strategies are.”
Overall, the dietary interventions were well tolerated, and neither group showed a significant change in renal function.
The average weight loss was –7.6 kg over 6 months in the low-carbohydrate group, as compared with –0.6 kg in the calorie-restrictive group.
The low carbohydrate diet also positively affected participants’ metabophenotype profile, particularly fat deposits. As compared with the calorie-restrictive group, the low-carbohydrate group showed statistically significant improvements in visceral adipose tissue, abdominal subcutaneous adipose tissue, and muscle fat infiltration.
The liver proton density fat fraction, which is associated with fatty liver disease, decreased by 0.53% in the low-carbohydrate group and increased by 0.46% in the calorie-restrictive group, but the difference didn’t reach statistical significance.
The fat-free muscle volume decreased by about 5% in the low-carbohydrate group. Dr. Siddiqui noted that the researchers don’t know yet whether this translates to a decrease in muscle function.
In terms of metabolic risk, the low-carbohydrate diet did not affect serum lipids (such as triglycerides or cholesterol measures), renal function (such as serum creatinine, glomerular filtration rate, or blood urea nitrogen), or insulin resistance (through glucose or hemoglobin A1c). At the same time, among patients taking insulin at the time of enrollment, about 90% of patients randomized to the low-carbohydrate group were able to reduce insulin to zero during the study.
Upon completion of the current study, Dr. Siddiqui and colleagues hope to provide foundational safety and efficacy data for carbohydrate restriction in liver transplant recipients. In the ongoing study, the researchers are further investigating the dietary intervention impacts on metabolic flexibility, skeletal muscle mitochondrial function, atherogenic lipoproteins, and vascular function.
“Are we actually, on a molecular level, fixing the fundamental problem that liver transplant recipients have to improve outcomes?” he said. “We’re doing very detailed profiling of these patients, so we will have data that shows how this actually affects them.”
Dr. Siddiqui was asked about the sustainability of the low-carbohydrate diet, particularly with a restrictive parameter of 20 grams per day. During the COVID-19 pandemic, Dr. Siddiqui noted, the study was slowed and the research team was able to collect follow-up data.
“Surprisingly, we have a high rate of compliance, even after 6 months of therapy, and I think this has to do with a patient population that’s been through cirrhosis and has almost died,” he said. “They’re far more compliant, and we’re seeing that. We’re also changing the physiology and improving mitochondrial function, which improves the weight loss and weight maintenance, though I don’t know how long that’s going to last.”
The study sponsorship was not disclosed. Dr. Siddiqui reported no relevant conflicts of interest.
A low-carbohydrate diet appears to be an effective weight-loss intervention in liver transplant recipients with obesity as compared with a calorie-restrictive diet, according to interim findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
In particular, the intervention showed significant improvements in the metabophenotype profile, including visceral adipose tissue and abdominal subcutaneous adipose tissue, said Mohammad Siddiqui, MD, a gastroenterologist and liver transplant specialist at Virginia Commonwealth University, Richmond.
“Weight gain and obesity after liver transplantation is common,” he said. “Posttransplant obesity is associated with increased cardiometabolic risk burden, increased risk of cardiovascular disease and mortality, and overall mortality.”
Previously, Dr. Siddiqui and colleagues have shown that posttransplant weight loss is difficult because of metabolic inflexibility and mitochondrial inefficiency. By specifically targeting carbohydrate utilization, metabolic flexibility could be restored in liver transplant recipients, he noted.
Dr. Siddiqui and colleagues conducted a randomized controlled trial of 27 adult liver transplant recipients with obesity for 24 weeks. The primary endpoint was change in weight, and the secondary endpoints involved metabophenotype, metabolic flexibility, mitochondrial function, and metabolic risk. The research team excluded patients with end-stage disease, terminal disease, use of weight-loss medications, pregnancy, or uncontrolled psychiatric illness that could interfere with adherence.
Among the participants, 13 were randomized to a calorie restrictive diet of less than 1,200-1,500 calories per day, and 14 were randomized to a low-carbohydrate diet of 20 grams or less per day. At enrollment, the participants underwent dietary, activity, skeletal muscle, and body composition assessments, as well as metabophenotype measurements of visceral adipose tissue, abdominal subcutaneous adipose tissue, muscle fat infiltration, fat-free muscle volume, and proton density fat fraction.
All participants were advised to maintain the same level of physical activity, which was measured through 7-day accelerometry. In addition, the patients were contacted every 2 weeks throughout the 24-week study period.
“We wanted to reinforce the dietary advice. We wanted to identify factors that may lead to compliance,” Dr. Siddiqui said. “Multiple studies have documented that the more contact that patients have during weight-loss studies with medical personnel, the more effective those strategies are.”
Overall, the dietary interventions were well tolerated, and neither group showed a significant change in renal function.
The average weight loss was –7.6 kg over 6 months in the low-carbohydrate group, as compared with –0.6 kg in the calorie-restrictive group.
The low carbohydrate diet also positively affected participants’ metabophenotype profile, particularly fat deposits. As compared with the calorie-restrictive group, the low-carbohydrate group showed statistically significant improvements in visceral adipose tissue, abdominal subcutaneous adipose tissue, and muscle fat infiltration.
The liver proton density fat fraction, which is associated with fatty liver disease, decreased by 0.53% in the low-carbohydrate group and increased by 0.46% in the calorie-restrictive group, but the difference didn’t reach statistical significance.
The fat-free muscle volume decreased by about 5% in the low-carbohydrate group. Dr. Siddiqui noted that the researchers don’t know yet whether this translates to a decrease in muscle function.
In terms of metabolic risk, the low-carbohydrate diet did not affect serum lipids (such as triglycerides or cholesterol measures), renal function (such as serum creatinine, glomerular filtration rate, or blood urea nitrogen), or insulin resistance (through glucose or hemoglobin A1c). At the same time, among patients taking insulin at the time of enrollment, about 90% of patients randomized to the low-carbohydrate group were able to reduce insulin to zero during the study.
Upon completion of the current study, Dr. Siddiqui and colleagues hope to provide foundational safety and efficacy data for carbohydrate restriction in liver transplant recipients. In the ongoing study, the researchers are further investigating the dietary intervention impacts on metabolic flexibility, skeletal muscle mitochondrial function, atherogenic lipoproteins, and vascular function.
“Are we actually, on a molecular level, fixing the fundamental problem that liver transplant recipients have to improve outcomes?” he said. “We’re doing very detailed profiling of these patients, so we will have data that shows how this actually affects them.”
Dr. Siddiqui was asked about the sustainability of the low-carbohydrate diet, particularly with a restrictive parameter of 20 grams per day. During the COVID-19 pandemic, Dr. Siddiqui noted, the study was slowed and the research team was able to collect follow-up data.
“Surprisingly, we have a high rate of compliance, even after 6 months of therapy, and I think this has to do with a patient population that’s been through cirrhosis and has almost died,” he said. “They’re far more compliant, and we’re seeing that. We’re also changing the physiology and improving mitochondrial function, which improves the weight loss and weight maintenance, though I don’t know how long that’s going to last.”
The study sponsorship was not disclosed. Dr. Siddiqui reported no relevant conflicts of interest.
A low-carbohydrate diet appears to be an effective weight-loss intervention in liver transplant recipients with obesity as compared with a calorie-restrictive diet, according to interim findings presented at the annual meeting of the American Association for the Study of Liver Diseases.
In particular, the intervention showed significant improvements in the metabophenotype profile, including visceral adipose tissue and abdominal subcutaneous adipose tissue, said Mohammad Siddiqui, MD, a gastroenterologist and liver transplant specialist at Virginia Commonwealth University, Richmond.
“Weight gain and obesity after liver transplantation is common,” he said. “Posttransplant obesity is associated with increased cardiometabolic risk burden, increased risk of cardiovascular disease and mortality, and overall mortality.”
Previously, Dr. Siddiqui and colleagues have shown that posttransplant weight loss is difficult because of metabolic inflexibility and mitochondrial inefficiency. By specifically targeting carbohydrate utilization, metabolic flexibility could be restored in liver transplant recipients, he noted.
Dr. Siddiqui and colleagues conducted a randomized controlled trial of 27 adult liver transplant recipients with obesity for 24 weeks. The primary endpoint was change in weight, and the secondary endpoints involved metabophenotype, metabolic flexibility, mitochondrial function, and metabolic risk. The research team excluded patients with end-stage disease, terminal disease, use of weight-loss medications, pregnancy, or uncontrolled psychiatric illness that could interfere with adherence.
Among the participants, 13 were randomized to a calorie restrictive diet of less than 1,200-1,500 calories per day, and 14 were randomized to a low-carbohydrate diet of 20 grams or less per day. At enrollment, the participants underwent dietary, activity, skeletal muscle, and body composition assessments, as well as metabophenotype measurements of visceral adipose tissue, abdominal subcutaneous adipose tissue, muscle fat infiltration, fat-free muscle volume, and proton density fat fraction.
All participants were advised to maintain the same level of physical activity, which was measured through 7-day accelerometry. In addition, the patients were contacted every 2 weeks throughout the 24-week study period.
“We wanted to reinforce the dietary advice. We wanted to identify factors that may lead to compliance,” Dr. Siddiqui said. “Multiple studies have documented that the more contact that patients have during weight-loss studies with medical personnel, the more effective those strategies are.”
Overall, the dietary interventions were well tolerated, and neither group showed a significant change in renal function.
The average weight loss was –7.6 kg over 6 months in the low-carbohydrate group, as compared with –0.6 kg in the calorie-restrictive group.
The low carbohydrate diet also positively affected participants’ metabophenotype profile, particularly fat deposits. As compared with the calorie-restrictive group, the low-carbohydrate group showed statistically significant improvements in visceral adipose tissue, abdominal subcutaneous adipose tissue, and muscle fat infiltration.
The liver proton density fat fraction, which is associated with fatty liver disease, decreased by 0.53% in the low-carbohydrate group and increased by 0.46% in the calorie-restrictive group, but the difference didn’t reach statistical significance.
The fat-free muscle volume decreased by about 5% in the low-carbohydrate group. Dr. Siddiqui noted that the researchers don’t know yet whether this translates to a decrease in muscle function.
In terms of metabolic risk, the low-carbohydrate diet did not affect serum lipids (such as triglycerides or cholesterol measures), renal function (such as serum creatinine, glomerular filtration rate, or blood urea nitrogen), or insulin resistance (through glucose or hemoglobin A1c). At the same time, among patients taking insulin at the time of enrollment, about 90% of patients randomized to the low-carbohydrate group were able to reduce insulin to zero during the study.
Upon completion of the current study, Dr. Siddiqui and colleagues hope to provide foundational safety and efficacy data for carbohydrate restriction in liver transplant recipients. In the ongoing study, the researchers are further investigating the dietary intervention impacts on metabolic flexibility, skeletal muscle mitochondrial function, atherogenic lipoproteins, and vascular function.
“Are we actually, on a molecular level, fixing the fundamental problem that liver transplant recipients have to improve outcomes?” he said. “We’re doing very detailed profiling of these patients, so we will have data that shows how this actually affects them.”
Dr. Siddiqui was asked about the sustainability of the low-carbohydrate diet, particularly with a restrictive parameter of 20 grams per day. During the COVID-19 pandemic, Dr. Siddiqui noted, the study was slowed and the research team was able to collect follow-up data.
“Surprisingly, we have a high rate of compliance, even after 6 months of therapy, and I think this has to do with a patient population that’s been through cirrhosis and has almost died,” he said. “They’re far more compliant, and we’re seeing that. We’re also changing the physiology and improving mitochondrial function, which improves the weight loss and weight maintenance, though I don’t know how long that’s going to last.”
The study sponsorship was not disclosed. Dr. Siddiqui reported no relevant conflicts of interest.
FROM THE LIVER MEETING
FDA expands tenofovir alafenamide (Vemlidy) use to adolescents with chronic HBV
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
Education about OTC tools key for patients with acne and rosacea
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – , Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical Center, New Brunswick, N.J., said in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
In some cases, the use of good-quality over-the -counter skin care products can improve acne without prescription treatment, said Dr. Baldwin, who is medical director of the Acne Treatment and Research Center, New York. Good skin care can enhance the effects of prescription medication by decreasing side effects such as inflammation, pain, and erythema, and improving compliance; and use of OTC products has not been shown to interfere with the efficacy of prescription products, she noted.
However, patient education about OTC products is key, she said. In particular, “cleansers are a double-edged sword,” Dr. Baldwin emphasized.
Cleansing is important to preserve barrier function, but “there is a risk of skin damage” if cleansers are too harsh, she said. The goal is to remove dirt, oils, and bacteria without disrupting the lipids, proteins, and normal flora that keep skin healthy, and to avoid altering pH, she added.
Key considerations for OTC cleansers include surfactants, pH, and patient preferences, Dr. Baldwin said.
Surfactants, the main components of OTC cleansers, can do more harm than good in some cases. Surfactants break down impurities on the skin surface, but not all are created equal, and some may cause skin irritation, she explained.
Surfactants fall into four categories: nonionic (no charge), anionic (negative charge), cationic (positive charge), and amphoteric (dual charge). Of these, cationic surfactants have the highest level of antimicrobial activity.
Many patients with acne seek out antibacterial cleansers, but many of these products have a high pH, which can inhibit healthy skin function and promote inflammation, Dr. Baldwin noted.
The right OTC skin care products can normalize pH, which promotes repair of the skin barrier and reduces inflammation, she said. While some products are labeled as “gentle,” they may have a high pH, and many products don’t list a pH, Dr. Baldwin pointed out. Many antibacterial products have pH levels in the 10-12 range, while true soaps fall in the 9-10 range, and hydrating liquid cleansers often land in the 5-7 range, she said.
“Most of our patients don’t know what ingredients to look for” in a cleanser, she noted. However, data show that a majority of patients prefer a foaming cleanser, enjoy the face-washing experience – and wash their faces at least twice a day, with a range of products including bath soap, said Dr. Baldwin. Consequently, “educate your patient about moisturizing,” she advised.
For patients with greasy or oily skin, Dr. Baldwin recommends lipid-free foaming cleansers, such as those with ceramides or glycerin. For patients with dry, irritated acne, she advises once-daily washing only, without cleansing devices, which includes washcloths, she said. Look for hydrating cleansers that are nonfoaming or slightly foaming for these patients, she added.
Another tip for patients is to remind them that “sebum is not a moisturizer,” said Dr. Baldwin. Acne patients may still need moisturizers, especially if they experience dry skin as a side effect of their acne medication, but finding the right fit can be a challenge requiring some trial and error, she noted.
OTC products for rosacea
Dr. Baldwin also addressed the use of OTC products for patients with rosacea. For cleansers, she recommends the same hydrating, nonfoaming categories as for her acne patients, with a once-daily, no-device regimen. She advises rosacea patients to avoid pure humectants for moisturizing and noted that silicone-based products are often the least irritating.
Seek moisturizers with ceramides, hyaluronic acid, glycerin, or niacinamide, she said. Data have shown that effective moisturization improves the ability of patients with rosacea to use and adhere to their prescription medications, Dr. Baldwin emphasized. Moisturizers also can make the medication more effective by enhancing the penetration of products such as azelaic acid, she added.
No acne or rosacea visit is complete until overall skin care has been discussed, Dr. Baldwin said.
Dr. Baldwin disclosed serving as a consultant or adviser for Almirall, EPI Health, Galderma, La Roche Posay, Ortho Dermatologics, Sun, and Vyne; and serving as a speaker or member of the speakers’ bureau for Almirall, Galderma, La Roche Posay, Ortho Dermatologics, and Sun. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Numbers of adolescents who vape within 5 minutes of waking jumps
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
CRISPR gene editing takes next step in TTR amyloidosis
CHICAGO – Treatment with the investigational CRISPR-Cas9 gene-editing therapy, NTLA-2001, led to rapid responses in patients with transthyretin (TTR) amyloidosis with cardiomyopathy (ATTR-CM), interim phase 1 results show.
Serum levels of the disease-causing TTR protein were reduced by at least 90% at day 28 with a single infusion of NTLA-2001 at two different doses, with reductions sustained across 4-6 months’ follow-up.
NTLA-2001 was generally well-tolerated, and the results were similar in patients with New York Heart Association (NYHA) class I-III heart failure.
“These data further support and extend the early findings demonstrating the promise of CRISPR-based in vivo genome editing in humans,” said Julian Gillmore, MD, PhD, MBBS, who is leading the study at University College London.
“More specifically, the deep TTR reductions observed in patients with ATTR amyloidosis in this study provide a real possibility of genuine clinical improvement in a condition that has hitherto been ultimately progressive and invariably fatal,” he said.
The results were reported in a late-breaking session at the American Heart Association scientific sessions.
Mutations in the TTR gene and age-related changes in the stability of the TTR protein can cause misfolding of the TTR protein, resulting in amyloid deposits in skin and myocardial tissues.
An estimated 50,000 people worldwide are thought to have hereditary ATTR and up to 500,000 to have wild-type ATTR amyloidosis. Amyloid cardiomyopathy is underdiagnosed and fatal in 3-10 years without treatment. Current treatment options only slow progression and require lifelong administration, he said.
Results reported last year from the polyneuropathy arm of the study were hailed as a breakthrough and further proof-of-concept that CRISPR could be used to treat other diseases
CRISPR gene editing has shown success, for example, in beta-thalassemia and sickle cell disease but involved stem cells extracted from patients’ bone marrow, edited in the lab, and then replaced.
NTLA-2001 (Intellia Therapeutics/Regeneron) is an in vivo treatment that uses lipid nanoparticles containing messenger RNA for Cas9 and a single-guide RNA targeting TTR in the liver, where it’s almost exclusively produced.
The new analysis included 12 patients with heart failure: 3 in NYHA class I-II and 6 in NYHA class III who received a single dose of NTLA-2001 at 0.7 mg/kg, while the remaining 3 patients in NYHA class I-II received a single dose of 1.0 mg/kg.
During follow-up out to 6 months, TTR reductions averaged:
- 93% in the 0.7 mg/kg NYHA I-II group at 6 months.
- 94% in the 0.7 mg/kg NYHA III group at 4 months.
- 92% in the 1.0 mg/kg NYHA I-II group at 4 months.
Eight patients reported mild or moderate adverse events, and two patients experienced transient infusion reactions, including one grade 3 reaction in the 0.7 mg/kg NYHA class III group that resolved without clinical consequence. This group was expanded to six patients per study protocol. No additional treatment-related adverse events higher than grade 1 were reported, and no further dose escalation was undertaken, Dr. Gillmore reported.
There were no clinically relevant laboratory findings; one patient had a transient grade 1 liver enzyme elevation.
One disadvantage of CRISPR is the potential for off-target effects, but Dr. Gillmore said in an interview that the drug developers went through a “very rigorous process when selecting the guide RNA, which is what really targets the specificity of the TTR gene.”
“That’s a really, really important point,” he said. “When they did various studies using, for example, primary human hepatocytes, they found no evidence of off-target editing at concentrations of NTLA-2001 threefold greater than the EC90, the concentration at which one knocks down the protein by 90%. So, what we can say at the moment, is the specificity of NTLA-2001 for the TTR gene seems to be absolute.”
In terms of other challenges going forward, Dr. Gillmore added, “I think that it’s really to see whether the knockdown that is being achieved is going to translate into greater clinical benefit.”
Invited discussant Kevin M. Alexander, MD, of Stanford (Calif.) University, said therapies that stabilize or reduce TTR have recently emerged that have improved ATTR amyloidosis outcomes, including tafamidis and patisiran.
Nevertheless, there has been an unmet need to develop therapies that can halt or reverse disease, are effective in advanced ATTR, and have an improved route or frequency of administration, given that this is a chronic disease, he said.
Dr. Alexander noted that the reductions of greater than 90% were achieved with higher doses than used in the polyneuropathy arm reported last year but were well tolerated in patients that for the most part had wild-type ATTR (83%) and reflect the wild-type ATTR population in practice. “The data support consideration for subsequent efficacy trials for this compound.”
Unanswered questions in ongoing ATTR trials are whether TTR reductions translate into improved clinical outcomes, the long-term safety of TTR lowering, and the efficacy of NTLA-2001, particularly in higher-risk patients, such as those in NYHA class III and those with hereditary ATTR, Dr. Alexander said.
During a media briefing earlier in the day, invited discussant Kiran Musunuru, MD, University of Pennsylvania, Philadelphia, pointed out that, in the recent APOLLO-B trial of patisiran, patients with ATTR amyloidosis with cardiomyopathy had an average 87% TTR reduction but need intravenous infusions every 3 weeks for the rest of their lives.
“In contrast, gene editing is a one-and-done proposition,” he said. “You receive a single treatment that turns off the TTR gene permanently and the effects are durable and likely last a lifetime.”
Dr. Musunuru noted that patients who received patisiran also had significantly and substantially better functional capacity and quality of life, compared with those who received placebo. “Based on today’s results, we can expect future clinical trials for gene editing to have the same beneficial effects and possibly a mortality benefit as well.”
Today’s study is also important because it is part of the first wave of putting CRISPR into the body for an array of diseases, he commented.
“TTR gene editing stands out because it’s the very first CRISPR trial to show unequivocal success – you see that with a greater than 90% reduction in TTR,” Dr. Musunuru said. “So, in my view that makes it a milestone for modern medicine.”
Dosing at 55 mg, corresponding to a fixed 0.7 mg/kg dose, is ongoing in the dose-expansion portion of the trial, with enrollment across both arms expected to be completed by the end of 2022, Intellia Therapeutics reported.
The study was funded by Intellia Therapeutics and Regeneron Pharmaceuticals. Dr. Gillmore reports receiving consultancy fees from Alnylam, Ionis, AstraZeneca, Pfizer, Intellia, ATTRalus, and Novo Nordisk and has received grant support from Alnylam Pharmaceuticals. Dr. Alexander reports serving on advisory boards for Almylam and Arbor Biotechnologies; has consulted for Eidos, Ionis, Novo Nordisk, and Pfizer; and has received grants from AHA, Alnylam, Eidos, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
CHICAGO – Treatment with the investigational CRISPR-Cas9 gene-editing therapy, NTLA-2001, led to rapid responses in patients with transthyretin (TTR) amyloidosis with cardiomyopathy (ATTR-CM), interim phase 1 results show.
Serum levels of the disease-causing TTR protein were reduced by at least 90% at day 28 with a single infusion of NTLA-2001 at two different doses, with reductions sustained across 4-6 months’ follow-up.
NTLA-2001 was generally well-tolerated, and the results were similar in patients with New York Heart Association (NYHA) class I-III heart failure.
“These data further support and extend the early findings demonstrating the promise of CRISPR-based in vivo genome editing in humans,” said Julian Gillmore, MD, PhD, MBBS, who is leading the study at University College London.
“More specifically, the deep TTR reductions observed in patients with ATTR amyloidosis in this study provide a real possibility of genuine clinical improvement in a condition that has hitherto been ultimately progressive and invariably fatal,” he said.
The results were reported in a late-breaking session at the American Heart Association scientific sessions.
Mutations in the TTR gene and age-related changes in the stability of the TTR protein can cause misfolding of the TTR protein, resulting in amyloid deposits in skin and myocardial tissues.
An estimated 50,000 people worldwide are thought to have hereditary ATTR and up to 500,000 to have wild-type ATTR amyloidosis. Amyloid cardiomyopathy is underdiagnosed and fatal in 3-10 years without treatment. Current treatment options only slow progression and require lifelong administration, he said.
Results reported last year from the polyneuropathy arm of the study were hailed as a breakthrough and further proof-of-concept that CRISPR could be used to treat other diseases
CRISPR gene editing has shown success, for example, in beta-thalassemia and sickle cell disease but involved stem cells extracted from patients’ bone marrow, edited in the lab, and then replaced.
NTLA-2001 (Intellia Therapeutics/Regeneron) is an in vivo treatment that uses lipid nanoparticles containing messenger RNA for Cas9 and a single-guide RNA targeting TTR in the liver, where it’s almost exclusively produced.
The new analysis included 12 patients with heart failure: 3 in NYHA class I-II and 6 in NYHA class III who received a single dose of NTLA-2001 at 0.7 mg/kg, while the remaining 3 patients in NYHA class I-II received a single dose of 1.0 mg/kg.
During follow-up out to 6 months, TTR reductions averaged:
- 93% in the 0.7 mg/kg NYHA I-II group at 6 months.
- 94% in the 0.7 mg/kg NYHA III group at 4 months.
- 92% in the 1.0 mg/kg NYHA I-II group at 4 months.
Eight patients reported mild or moderate adverse events, and two patients experienced transient infusion reactions, including one grade 3 reaction in the 0.7 mg/kg NYHA class III group that resolved without clinical consequence. This group was expanded to six patients per study protocol. No additional treatment-related adverse events higher than grade 1 were reported, and no further dose escalation was undertaken, Dr. Gillmore reported.
There were no clinically relevant laboratory findings; one patient had a transient grade 1 liver enzyme elevation.
One disadvantage of CRISPR is the potential for off-target effects, but Dr. Gillmore said in an interview that the drug developers went through a “very rigorous process when selecting the guide RNA, which is what really targets the specificity of the TTR gene.”
“That’s a really, really important point,” he said. “When they did various studies using, for example, primary human hepatocytes, they found no evidence of off-target editing at concentrations of NTLA-2001 threefold greater than the EC90, the concentration at which one knocks down the protein by 90%. So, what we can say at the moment, is the specificity of NTLA-2001 for the TTR gene seems to be absolute.”
In terms of other challenges going forward, Dr. Gillmore added, “I think that it’s really to see whether the knockdown that is being achieved is going to translate into greater clinical benefit.”
Invited discussant Kevin M. Alexander, MD, of Stanford (Calif.) University, said therapies that stabilize or reduce TTR have recently emerged that have improved ATTR amyloidosis outcomes, including tafamidis and patisiran.
Nevertheless, there has been an unmet need to develop therapies that can halt or reverse disease, are effective in advanced ATTR, and have an improved route or frequency of administration, given that this is a chronic disease, he said.
Dr. Alexander noted that the reductions of greater than 90% were achieved with higher doses than used in the polyneuropathy arm reported last year but were well tolerated in patients that for the most part had wild-type ATTR (83%) and reflect the wild-type ATTR population in practice. “The data support consideration for subsequent efficacy trials for this compound.”
Unanswered questions in ongoing ATTR trials are whether TTR reductions translate into improved clinical outcomes, the long-term safety of TTR lowering, and the efficacy of NTLA-2001, particularly in higher-risk patients, such as those in NYHA class III and those with hereditary ATTR, Dr. Alexander said.
During a media briefing earlier in the day, invited discussant Kiran Musunuru, MD, University of Pennsylvania, Philadelphia, pointed out that, in the recent APOLLO-B trial of patisiran, patients with ATTR amyloidosis with cardiomyopathy had an average 87% TTR reduction but need intravenous infusions every 3 weeks for the rest of their lives.
“In contrast, gene editing is a one-and-done proposition,” he said. “You receive a single treatment that turns off the TTR gene permanently and the effects are durable and likely last a lifetime.”
Dr. Musunuru noted that patients who received patisiran also had significantly and substantially better functional capacity and quality of life, compared with those who received placebo. “Based on today’s results, we can expect future clinical trials for gene editing to have the same beneficial effects and possibly a mortality benefit as well.”
Today’s study is also important because it is part of the first wave of putting CRISPR into the body for an array of diseases, he commented.
“TTR gene editing stands out because it’s the very first CRISPR trial to show unequivocal success – you see that with a greater than 90% reduction in TTR,” Dr. Musunuru said. “So, in my view that makes it a milestone for modern medicine.”
Dosing at 55 mg, corresponding to a fixed 0.7 mg/kg dose, is ongoing in the dose-expansion portion of the trial, with enrollment across both arms expected to be completed by the end of 2022, Intellia Therapeutics reported.
The study was funded by Intellia Therapeutics and Regeneron Pharmaceuticals. Dr. Gillmore reports receiving consultancy fees from Alnylam, Ionis, AstraZeneca, Pfizer, Intellia, ATTRalus, and Novo Nordisk and has received grant support from Alnylam Pharmaceuticals. Dr. Alexander reports serving on advisory boards for Almylam and Arbor Biotechnologies; has consulted for Eidos, Ionis, Novo Nordisk, and Pfizer; and has received grants from AHA, Alnylam, Eidos, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
CHICAGO – Treatment with the investigational CRISPR-Cas9 gene-editing therapy, NTLA-2001, led to rapid responses in patients with transthyretin (TTR) amyloidosis with cardiomyopathy (ATTR-CM), interim phase 1 results show.
Serum levels of the disease-causing TTR protein were reduced by at least 90% at day 28 with a single infusion of NTLA-2001 at two different doses, with reductions sustained across 4-6 months’ follow-up.
NTLA-2001 was generally well-tolerated, and the results were similar in patients with New York Heart Association (NYHA) class I-III heart failure.
“These data further support and extend the early findings demonstrating the promise of CRISPR-based in vivo genome editing in humans,” said Julian Gillmore, MD, PhD, MBBS, who is leading the study at University College London.
“More specifically, the deep TTR reductions observed in patients with ATTR amyloidosis in this study provide a real possibility of genuine clinical improvement in a condition that has hitherto been ultimately progressive and invariably fatal,” he said.
The results were reported in a late-breaking session at the American Heart Association scientific sessions.
Mutations in the TTR gene and age-related changes in the stability of the TTR protein can cause misfolding of the TTR protein, resulting in amyloid deposits in skin and myocardial tissues.
An estimated 50,000 people worldwide are thought to have hereditary ATTR and up to 500,000 to have wild-type ATTR amyloidosis. Amyloid cardiomyopathy is underdiagnosed and fatal in 3-10 years without treatment. Current treatment options only slow progression and require lifelong administration, he said.
Results reported last year from the polyneuropathy arm of the study were hailed as a breakthrough and further proof-of-concept that CRISPR could be used to treat other diseases
CRISPR gene editing has shown success, for example, in beta-thalassemia and sickle cell disease but involved stem cells extracted from patients’ bone marrow, edited in the lab, and then replaced.
NTLA-2001 (Intellia Therapeutics/Regeneron) is an in vivo treatment that uses lipid nanoparticles containing messenger RNA for Cas9 and a single-guide RNA targeting TTR in the liver, where it’s almost exclusively produced.
The new analysis included 12 patients with heart failure: 3 in NYHA class I-II and 6 in NYHA class III who received a single dose of NTLA-2001 at 0.7 mg/kg, while the remaining 3 patients in NYHA class I-II received a single dose of 1.0 mg/kg.
During follow-up out to 6 months, TTR reductions averaged:
- 93% in the 0.7 mg/kg NYHA I-II group at 6 months.
- 94% in the 0.7 mg/kg NYHA III group at 4 months.
- 92% in the 1.0 mg/kg NYHA I-II group at 4 months.
Eight patients reported mild or moderate adverse events, and two patients experienced transient infusion reactions, including one grade 3 reaction in the 0.7 mg/kg NYHA class III group that resolved without clinical consequence. This group was expanded to six patients per study protocol. No additional treatment-related adverse events higher than grade 1 were reported, and no further dose escalation was undertaken, Dr. Gillmore reported.
There were no clinically relevant laboratory findings; one patient had a transient grade 1 liver enzyme elevation.
One disadvantage of CRISPR is the potential for off-target effects, but Dr. Gillmore said in an interview that the drug developers went through a “very rigorous process when selecting the guide RNA, which is what really targets the specificity of the TTR gene.”
“That’s a really, really important point,” he said. “When they did various studies using, for example, primary human hepatocytes, they found no evidence of off-target editing at concentrations of NTLA-2001 threefold greater than the EC90, the concentration at which one knocks down the protein by 90%. So, what we can say at the moment, is the specificity of NTLA-2001 for the TTR gene seems to be absolute.”
In terms of other challenges going forward, Dr. Gillmore added, “I think that it’s really to see whether the knockdown that is being achieved is going to translate into greater clinical benefit.”
Invited discussant Kevin M. Alexander, MD, of Stanford (Calif.) University, said therapies that stabilize or reduce TTR have recently emerged that have improved ATTR amyloidosis outcomes, including tafamidis and patisiran.
Nevertheless, there has been an unmet need to develop therapies that can halt or reverse disease, are effective in advanced ATTR, and have an improved route or frequency of administration, given that this is a chronic disease, he said.
Dr. Alexander noted that the reductions of greater than 90% were achieved with higher doses than used in the polyneuropathy arm reported last year but were well tolerated in patients that for the most part had wild-type ATTR (83%) and reflect the wild-type ATTR population in practice. “The data support consideration for subsequent efficacy trials for this compound.”
Unanswered questions in ongoing ATTR trials are whether TTR reductions translate into improved clinical outcomes, the long-term safety of TTR lowering, and the efficacy of NTLA-2001, particularly in higher-risk patients, such as those in NYHA class III and those with hereditary ATTR, Dr. Alexander said.
During a media briefing earlier in the day, invited discussant Kiran Musunuru, MD, University of Pennsylvania, Philadelphia, pointed out that, in the recent APOLLO-B trial of patisiran, patients with ATTR amyloidosis with cardiomyopathy had an average 87% TTR reduction but need intravenous infusions every 3 weeks for the rest of their lives.
“In contrast, gene editing is a one-and-done proposition,” he said. “You receive a single treatment that turns off the TTR gene permanently and the effects are durable and likely last a lifetime.”
Dr. Musunuru noted that patients who received patisiran also had significantly and substantially better functional capacity and quality of life, compared with those who received placebo. “Based on today’s results, we can expect future clinical trials for gene editing to have the same beneficial effects and possibly a mortality benefit as well.”
Today’s study is also important because it is part of the first wave of putting CRISPR into the body for an array of diseases, he commented.
“TTR gene editing stands out because it’s the very first CRISPR trial to show unequivocal success – you see that with a greater than 90% reduction in TTR,” Dr. Musunuru said. “So, in my view that makes it a milestone for modern medicine.”
Dosing at 55 mg, corresponding to a fixed 0.7 mg/kg dose, is ongoing in the dose-expansion portion of the trial, with enrollment across both arms expected to be completed by the end of 2022, Intellia Therapeutics reported.
The study was funded by Intellia Therapeutics and Regeneron Pharmaceuticals. Dr. Gillmore reports receiving consultancy fees from Alnylam, Ionis, AstraZeneca, Pfizer, Intellia, ATTRalus, and Novo Nordisk and has received grant support from Alnylam Pharmaceuticals. Dr. Alexander reports serving on advisory boards for Almylam and Arbor Biotechnologies; has consulted for Eidos, Ionis, Novo Nordisk, and Pfizer; and has received grants from AHA, Alnylam, Eidos, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
AT AHA 2022
No survival advantage for either torsemide or furosemide in HF: TRANSFORM-HF
CHICAGO – The choice of loop diuretic for decongestion in patients hospitalized with heart failure (HF) may make little difference to survival or readmission risk over the next year, at least when deciding between furosemide or torsemide, a randomized trial suggests.
Both drugs are old and widely used, but differences between the two loop diuretics in bioavailability, effects on potassium levels, and other features have led some clinicians to sometimes prefer torsemide. Until now, however, no randomized HF trials have compared the two drugs.
The new findings suggest clinicians can continue starting such patients with HF on either agent, at their discretion, without concern that the choice may compromise outcomes, say researchers from the TRANSFORM-HF trial, which compared furosemide-first and torsemide-first diuretic strategies in a diverse population of patients with HF.
Given that the two strategies were similarly effective for survival and rehospitalization, clinicians caring for patients with HF can focus more on “getting patients on the right dose for their loop diuretic, and prioritizing those therapies proven to improve clinical outcomes,” said Robert J. Mentz, MD, of Duke University Clinical Research Institute, Durham, N.C.
Dr. Mentz, a TRANSFORM-HF principal investigator, presented the primary results November 5 at the American Heart Association scientific sessions.
The trial had randomly assigned 2,859 patients hospitalized with HF and with a plan for oral loop diuretic therapy to initiate treatment with furosemide or torsemide. Clinicians were encouraged to maintain patients on the assigned diuretic, but crossovers to the other drug or other diuretic changes were allowed.
Rates of death from any cause, the primary endpoint, were about 26% in both groups over a median 17-month follow-up, regardless of ejection fraction (EF).
The composite rates of all-cause death or hospitalization at 12 months were also not significantly different, about 49% for those started on furosemide and about 47% for patients initially prescribed torsemide.
As a pragmatic comparative effectiveness trial, TRANSFORM-HF entered diverse patients with HF, broadly representative of actual clinical practice, who were managed according to routine practice and a streamlined study protocol at more than 60 U.S. centers, Dr. Mentz observed.
One of the pragmatic design’s advantages, he told this news organization, was “how efficient it was” as a randomized comparison of treatment strategies for clinical outcomes. It was “relatively low cost” and recruited patients quickly, compared with conventional randomized trials, “and we answered the question clearly.” The trial’s results, Dr. Mentz said, reflect “what happens in the real world.”
When might torsemide have the edge?
Although furosemide is the most commonly used loop diuretic in HF, and there are others besides it and torsemide, the latter has both known and theoretical advantages that set it apart. Torsemide is more than twice as potent as furosemide and more bioavailable, and its treatment effect lasts longer, the TRANSFORM-HF investigators have noted.
In addition, preclinical and small clinical studies suggest torsemide may have pleiotropic effects that might be theoretical advantages for patients with HF. For example, it appears to downregulate the renin-angiotensin-aldosterone system (RAAS) and reduce myocardial fibrosis and promote reverse ventricular remodeling, the group writes.
In practice, therefore, torsemide may be preferred in patients with furosemide resistance or “challenges with bioavailability, especially those with very advanced heart failure with congestion who may have gut edema, where oral furosemide and other loop diuretics are not effectively absorbed,” Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
In such patients, she said, torsemide “is considered to be a better choice for individuals who have diuretic resistance with advanced congestion.”
The drug’s apparent pleiotropic effects, such as RAAS inhibition, may have less relevance to the TRANSFORM-HF primary endpoint of all-cause mortality than to clinical outcomes more likely associated with successful decongestion, such as HF hospitalization, Dr. Bozkurt proposed.
The trial’s pragmatic design, however, made it more feasible to focus on all-cause mortality and less practical to use measures of successful decongestion, such as volume loss or reduction in natriuretic peptide levels, she observed. Those are endpoints of special interest when diuretics are compared, “especially for the subgroup of patients who are diuretic resistant.”
Over the last 20 years or so, “we’ve learned that hospitalized heart failure is a very different disease process with a different natural history,” observed Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, who was not part of the current study.
“So, the idea that something as nuanced as choice of one loop diuretic over the other, in that setting, would be sufficient to change the natural history, may be still a high bar for us,” he said in an interview.
“Based on these data, one would have to argue that whichever loop diuretic you select for the hospitalized patient – and a lot of that is driven by market exigencies right now – it turns out that the response is indistinguishable,” Dr. Yancy said. “That means if your hospital happens to have furosemide on the formulary, use it. If furosemide is not available but torsemide is available, use it.”
Dr. Yancy said he’d like to see a trial similar to TRANSFORM-HF but in outpatients receiving today’s guideline-directed medical therapy, which includes the sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs that increase the fractional excretion of sodium and have a “diureticlike” effect.
Such a trial, he said, would explore “the combination of not one, or two, but three agents with a diuretic effect – a loop diuretic, a mineralocorticoid antagonist, and an SGLT2 inhibitor – in ambulatory, optimized patients. It might make a difference.”
HF regardless of EF
The trial enrolled patients hospitalized with worsening or new-onset HF with a plan for long-term loop diuretic therapy who had either an EF of 40% or lower or, regardless of EF, elevated natriuretic peptide levels when hospitalized.
Of the 2,859 participants, whose mean age was about 65 years, about 36% were women and 34% African American. Overall, 1,428 were assigned to receive furosemide as their initial oral diuretic and 1,431 patients were assigned to the torsemide-first strategy.
The rate of death from any cause in both groups was 17 per 100 patient-years at a median of 17.4 months. The hazard ratio for torsemide vs. furosemide was 1.02 (95% confidence interval, 0.89-1.18; P = .77).
The corresponding HR at 12 months for all-cause death or hospitalization was 0.92 (95% CI, 0.83-1.02; P = .11). The relative risk for any hospitalization was 0.94 (95% CI, 0.84-1.07).
Pragmatic design: Other implications
Dosing was left to clinician discretion in the open-label study, as was whether patients maintained their assigned drug or switched over to the other agent. Indeed, 5.4% of patients crossed over to the other loop diuretic, and 2.8% went off loop diuretics entirely between in-hospital randomization and discharge, Dr. Mentz reported. By day 30, 6.7% had crossed over, and 7% had stopped taking loop diuretics.
The diuretic crossovers and discontinuations, Dr. Mentz said, likely biased the trial’s outcomes, such that the two strategies performed about equally well. Efforts were made, however, to at least partially overcome that limitation.
“We put measures in place to support adherence – sending letters to their primary doctors, giving them a wallet card so they would know which therapy they were on, having conversations about the importance of trying to stay on the randomized therapy,” Dr. Mentz said in an interview. Still, some clinicians saw differences between the two agents that prompted them, at some point, to switch patients from one loop diuretic to the other.
But interestingly, Dr. Mentz reported, the two strategies did not significantly differ in all-cause mortality or the composite of all-cause mortality or hospitalization in analysis by intention to treat.
Dr. Mentz discloses receiving honoraria from AstraZeneca, Bayer/Merck, Boehringer Ingelheim/Lilly, Cytokinetics, Pharmacosmos, Respicardia, Windtree Therapeutics, and Zoll; and research grants from American Regent and Novartis. Dr. Bozkurt discloses receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion. Dr. Yancy discloses a modest relationship with Abbott.
A version of this article first appeared on Medscape.com.
CHICAGO – The choice of loop diuretic for decongestion in patients hospitalized with heart failure (HF) may make little difference to survival or readmission risk over the next year, at least when deciding between furosemide or torsemide, a randomized trial suggests.
Both drugs are old and widely used, but differences between the two loop diuretics in bioavailability, effects on potassium levels, and other features have led some clinicians to sometimes prefer torsemide. Until now, however, no randomized HF trials have compared the two drugs.
The new findings suggest clinicians can continue starting such patients with HF on either agent, at their discretion, without concern that the choice may compromise outcomes, say researchers from the TRANSFORM-HF trial, which compared furosemide-first and torsemide-first diuretic strategies in a diverse population of patients with HF.
Given that the two strategies were similarly effective for survival and rehospitalization, clinicians caring for patients with HF can focus more on “getting patients on the right dose for their loop diuretic, and prioritizing those therapies proven to improve clinical outcomes,” said Robert J. Mentz, MD, of Duke University Clinical Research Institute, Durham, N.C.
Dr. Mentz, a TRANSFORM-HF principal investigator, presented the primary results November 5 at the American Heart Association scientific sessions.
The trial had randomly assigned 2,859 patients hospitalized with HF and with a plan for oral loop diuretic therapy to initiate treatment with furosemide or torsemide. Clinicians were encouraged to maintain patients on the assigned diuretic, but crossovers to the other drug or other diuretic changes were allowed.
Rates of death from any cause, the primary endpoint, were about 26% in both groups over a median 17-month follow-up, regardless of ejection fraction (EF).
The composite rates of all-cause death or hospitalization at 12 months were also not significantly different, about 49% for those started on furosemide and about 47% for patients initially prescribed torsemide.
As a pragmatic comparative effectiveness trial, TRANSFORM-HF entered diverse patients with HF, broadly representative of actual clinical practice, who were managed according to routine practice and a streamlined study protocol at more than 60 U.S. centers, Dr. Mentz observed.
One of the pragmatic design’s advantages, he told this news organization, was “how efficient it was” as a randomized comparison of treatment strategies for clinical outcomes. It was “relatively low cost” and recruited patients quickly, compared with conventional randomized trials, “and we answered the question clearly.” The trial’s results, Dr. Mentz said, reflect “what happens in the real world.”
When might torsemide have the edge?
Although furosemide is the most commonly used loop diuretic in HF, and there are others besides it and torsemide, the latter has both known and theoretical advantages that set it apart. Torsemide is more than twice as potent as furosemide and more bioavailable, and its treatment effect lasts longer, the TRANSFORM-HF investigators have noted.
In addition, preclinical and small clinical studies suggest torsemide may have pleiotropic effects that might be theoretical advantages for patients with HF. For example, it appears to downregulate the renin-angiotensin-aldosterone system (RAAS) and reduce myocardial fibrosis and promote reverse ventricular remodeling, the group writes.
In practice, therefore, torsemide may be preferred in patients with furosemide resistance or “challenges with bioavailability, especially those with very advanced heart failure with congestion who may have gut edema, where oral furosemide and other loop diuretics are not effectively absorbed,” Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
In such patients, she said, torsemide “is considered to be a better choice for individuals who have diuretic resistance with advanced congestion.”
The drug’s apparent pleiotropic effects, such as RAAS inhibition, may have less relevance to the TRANSFORM-HF primary endpoint of all-cause mortality than to clinical outcomes more likely associated with successful decongestion, such as HF hospitalization, Dr. Bozkurt proposed.
The trial’s pragmatic design, however, made it more feasible to focus on all-cause mortality and less practical to use measures of successful decongestion, such as volume loss or reduction in natriuretic peptide levels, she observed. Those are endpoints of special interest when diuretics are compared, “especially for the subgroup of patients who are diuretic resistant.”
Over the last 20 years or so, “we’ve learned that hospitalized heart failure is a very different disease process with a different natural history,” observed Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, who was not part of the current study.
“So, the idea that something as nuanced as choice of one loop diuretic over the other, in that setting, would be sufficient to change the natural history, may be still a high bar for us,” he said in an interview.
“Based on these data, one would have to argue that whichever loop diuretic you select for the hospitalized patient – and a lot of that is driven by market exigencies right now – it turns out that the response is indistinguishable,” Dr. Yancy said. “That means if your hospital happens to have furosemide on the formulary, use it. If furosemide is not available but torsemide is available, use it.”
Dr. Yancy said he’d like to see a trial similar to TRANSFORM-HF but in outpatients receiving today’s guideline-directed medical therapy, which includes the sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs that increase the fractional excretion of sodium and have a “diureticlike” effect.
Such a trial, he said, would explore “the combination of not one, or two, but three agents with a diuretic effect – a loop diuretic, a mineralocorticoid antagonist, and an SGLT2 inhibitor – in ambulatory, optimized patients. It might make a difference.”
HF regardless of EF
The trial enrolled patients hospitalized with worsening or new-onset HF with a plan for long-term loop diuretic therapy who had either an EF of 40% or lower or, regardless of EF, elevated natriuretic peptide levels when hospitalized.
Of the 2,859 participants, whose mean age was about 65 years, about 36% were women and 34% African American. Overall, 1,428 were assigned to receive furosemide as their initial oral diuretic and 1,431 patients were assigned to the torsemide-first strategy.
The rate of death from any cause in both groups was 17 per 100 patient-years at a median of 17.4 months. The hazard ratio for torsemide vs. furosemide was 1.02 (95% confidence interval, 0.89-1.18; P = .77).
The corresponding HR at 12 months for all-cause death or hospitalization was 0.92 (95% CI, 0.83-1.02; P = .11). The relative risk for any hospitalization was 0.94 (95% CI, 0.84-1.07).
Pragmatic design: Other implications
Dosing was left to clinician discretion in the open-label study, as was whether patients maintained their assigned drug or switched over to the other agent. Indeed, 5.4% of patients crossed over to the other loop diuretic, and 2.8% went off loop diuretics entirely between in-hospital randomization and discharge, Dr. Mentz reported. By day 30, 6.7% had crossed over, and 7% had stopped taking loop diuretics.
The diuretic crossovers and discontinuations, Dr. Mentz said, likely biased the trial’s outcomes, such that the two strategies performed about equally well. Efforts were made, however, to at least partially overcome that limitation.
“We put measures in place to support adherence – sending letters to their primary doctors, giving them a wallet card so they would know which therapy they were on, having conversations about the importance of trying to stay on the randomized therapy,” Dr. Mentz said in an interview. Still, some clinicians saw differences between the two agents that prompted them, at some point, to switch patients from one loop diuretic to the other.
But interestingly, Dr. Mentz reported, the two strategies did not significantly differ in all-cause mortality or the composite of all-cause mortality or hospitalization in analysis by intention to treat.
Dr. Mentz discloses receiving honoraria from AstraZeneca, Bayer/Merck, Boehringer Ingelheim/Lilly, Cytokinetics, Pharmacosmos, Respicardia, Windtree Therapeutics, and Zoll; and research grants from American Regent and Novartis. Dr. Bozkurt discloses receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion. Dr. Yancy discloses a modest relationship with Abbott.
A version of this article first appeared on Medscape.com.
CHICAGO – The choice of loop diuretic for decongestion in patients hospitalized with heart failure (HF) may make little difference to survival or readmission risk over the next year, at least when deciding between furosemide or torsemide, a randomized trial suggests.
Both drugs are old and widely used, but differences between the two loop diuretics in bioavailability, effects on potassium levels, and other features have led some clinicians to sometimes prefer torsemide. Until now, however, no randomized HF trials have compared the two drugs.
The new findings suggest clinicians can continue starting such patients with HF on either agent, at their discretion, without concern that the choice may compromise outcomes, say researchers from the TRANSFORM-HF trial, which compared furosemide-first and torsemide-first diuretic strategies in a diverse population of patients with HF.
Given that the two strategies were similarly effective for survival and rehospitalization, clinicians caring for patients with HF can focus more on “getting patients on the right dose for their loop diuretic, and prioritizing those therapies proven to improve clinical outcomes,” said Robert J. Mentz, MD, of Duke University Clinical Research Institute, Durham, N.C.
Dr. Mentz, a TRANSFORM-HF principal investigator, presented the primary results November 5 at the American Heart Association scientific sessions.
The trial had randomly assigned 2,859 patients hospitalized with HF and with a plan for oral loop diuretic therapy to initiate treatment with furosemide or torsemide. Clinicians were encouraged to maintain patients on the assigned diuretic, but crossovers to the other drug or other diuretic changes were allowed.
Rates of death from any cause, the primary endpoint, were about 26% in both groups over a median 17-month follow-up, regardless of ejection fraction (EF).
The composite rates of all-cause death or hospitalization at 12 months were also not significantly different, about 49% for those started on furosemide and about 47% for patients initially prescribed torsemide.
As a pragmatic comparative effectiveness trial, TRANSFORM-HF entered diverse patients with HF, broadly representative of actual clinical practice, who were managed according to routine practice and a streamlined study protocol at more than 60 U.S. centers, Dr. Mentz observed.
One of the pragmatic design’s advantages, he told this news organization, was “how efficient it was” as a randomized comparison of treatment strategies for clinical outcomes. It was “relatively low cost” and recruited patients quickly, compared with conventional randomized trials, “and we answered the question clearly.” The trial’s results, Dr. Mentz said, reflect “what happens in the real world.”
When might torsemide have the edge?
Although furosemide is the most commonly used loop diuretic in HF, and there are others besides it and torsemide, the latter has both known and theoretical advantages that set it apart. Torsemide is more than twice as potent as furosemide and more bioavailable, and its treatment effect lasts longer, the TRANSFORM-HF investigators have noted.
In addition, preclinical and small clinical studies suggest torsemide may have pleiotropic effects that might be theoretical advantages for patients with HF. For example, it appears to downregulate the renin-angiotensin-aldosterone system (RAAS) and reduce myocardial fibrosis and promote reverse ventricular remodeling, the group writes.
In practice, therefore, torsemide may be preferred in patients with furosemide resistance or “challenges with bioavailability, especially those with very advanced heart failure with congestion who may have gut edema, where oral furosemide and other loop diuretics are not effectively absorbed,” Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
In such patients, she said, torsemide “is considered to be a better choice for individuals who have diuretic resistance with advanced congestion.”
The drug’s apparent pleiotropic effects, such as RAAS inhibition, may have less relevance to the TRANSFORM-HF primary endpoint of all-cause mortality than to clinical outcomes more likely associated with successful decongestion, such as HF hospitalization, Dr. Bozkurt proposed.
The trial’s pragmatic design, however, made it more feasible to focus on all-cause mortality and less practical to use measures of successful decongestion, such as volume loss or reduction in natriuretic peptide levels, she observed. Those are endpoints of special interest when diuretics are compared, “especially for the subgroup of patients who are diuretic resistant.”
Over the last 20 years or so, “we’ve learned that hospitalized heart failure is a very different disease process with a different natural history,” observed Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, who was not part of the current study.
“So, the idea that something as nuanced as choice of one loop diuretic over the other, in that setting, would be sufficient to change the natural history, may be still a high bar for us,” he said in an interview.
“Based on these data, one would have to argue that whichever loop diuretic you select for the hospitalized patient – and a lot of that is driven by market exigencies right now – it turns out that the response is indistinguishable,” Dr. Yancy said. “That means if your hospital happens to have furosemide on the formulary, use it. If furosemide is not available but torsemide is available, use it.”
Dr. Yancy said he’d like to see a trial similar to TRANSFORM-HF but in outpatients receiving today’s guideline-directed medical therapy, which includes the sodium-glucose cotransporter 2 (SGLT2) inhibitors, drugs that increase the fractional excretion of sodium and have a “diureticlike” effect.
Such a trial, he said, would explore “the combination of not one, or two, but three agents with a diuretic effect – a loop diuretic, a mineralocorticoid antagonist, and an SGLT2 inhibitor – in ambulatory, optimized patients. It might make a difference.”
HF regardless of EF
The trial enrolled patients hospitalized with worsening or new-onset HF with a plan for long-term loop diuretic therapy who had either an EF of 40% or lower or, regardless of EF, elevated natriuretic peptide levels when hospitalized.
Of the 2,859 participants, whose mean age was about 65 years, about 36% were women and 34% African American. Overall, 1,428 were assigned to receive furosemide as their initial oral diuretic and 1,431 patients were assigned to the torsemide-first strategy.
The rate of death from any cause in both groups was 17 per 100 patient-years at a median of 17.4 months. The hazard ratio for torsemide vs. furosemide was 1.02 (95% confidence interval, 0.89-1.18; P = .77).
The corresponding HR at 12 months for all-cause death or hospitalization was 0.92 (95% CI, 0.83-1.02; P = .11). The relative risk for any hospitalization was 0.94 (95% CI, 0.84-1.07).
Pragmatic design: Other implications
Dosing was left to clinician discretion in the open-label study, as was whether patients maintained their assigned drug or switched over to the other agent. Indeed, 5.4% of patients crossed over to the other loop diuretic, and 2.8% went off loop diuretics entirely between in-hospital randomization and discharge, Dr. Mentz reported. By day 30, 6.7% had crossed over, and 7% had stopped taking loop diuretics.
The diuretic crossovers and discontinuations, Dr. Mentz said, likely biased the trial’s outcomes, such that the two strategies performed about equally well. Efforts were made, however, to at least partially overcome that limitation.
“We put measures in place to support adherence – sending letters to their primary doctors, giving them a wallet card so they would know which therapy they were on, having conversations about the importance of trying to stay on the randomized therapy,” Dr. Mentz said in an interview. Still, some clinicians saw differences between the two agents that prompted them, at some point, to switch patients from one loop diuretic to the other.
But interestingly, Dr. Mentz reported, the two strategies did not significantly differ in all-cause mortality or the composite of all-cause mortality or hospitalization in analysis by intention to treat.
Dr. Mentz discloses receiving honoraria from AstraZeneca, Bayer/Merck, Boehringer Ingelheim/Lilly, Cytokinetics, Pharmacosmos, Respicardia, Windtree Therapeutics, and Zoll; and research grants from American Regent and Novartis. Dr. Bozkurt discloses receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion. Dr. Yancy discloses a modest relationship with Abbott.
A version of this article first appeared on Medscape.com.
AT AHA 2022