User login
Dry cough and dyspnea
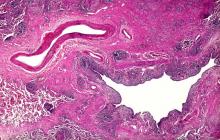
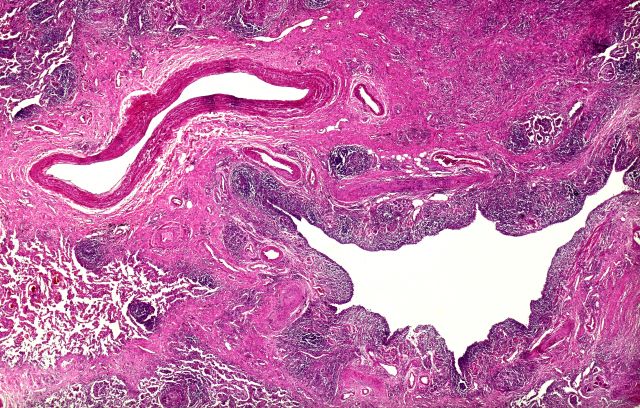
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man with a 20-year–pack history of smoking initially presented with a persistent dry cough and dyspnea. Clubbing was noted on physical examination and breath sounds in the right upper lung were weak. Other than hypertension, which the patient manages with angiotensin-converting enzyme (ACE) inhibitors, medical history is unremarkable. The patient notes that this medication has always made him cough, but dyspnea has only developed over the past 6 weeks. Respiratory symptoms prompted a chest radiograph which revealed a mass in the upper lobe of the right lung. Transbronchial lung biopsy of the right lung reveals components of adenocarcinoma; the specimen is otherwise squamous.
Drugging the undruggable
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Aggression toward health care providers common during pandemic
After an aggressive event or abuse occurred, 56% of providers considered changing their care tasks, and more than a third considered quitting their profession.
“Aggression of any sort against health care providers is not a new social phenomenon, and it has existed as far as medicine and health care is reported in literature. However, the phenomenon of aggression against health care providers during the pandemic grew worse,” senior study author Adrian Baranchuk, MD, a professor of medicine at Queen’s University, Kingston, Ont., told this news organization.
The study was published online in Current Problems in Cardiology
Survey snapshot
Dr. Baranchuk and colleagues, with the support of the Inter-American Society of Cardiology, developed a survey to characterize the frequency and types of abuse that frontline health professionals faced. They invited health care professionals from Latin America who had provided care since March 2020 to participate.
Between January and February 2022, 3,544 participants from 19 countries took the survey. Among them, 70.8% were physicians, 16% were nurses, and 13.2% were other health team members, such as administrative staff and technicians. About 58.5% were women, and 74.7% provided direct care to patients with COVID-19.
Overall, 54.8% of respondents reported acts of aggression. Of this group, 95.6% reported verbal abuse, 11.1% reported physical abuse, and 19.9% reported other types of abuse, including microaggressions.
About 13% of respondents reported experiencing some form of aggression daily, 26.4% experienced abuse weekly, and 38.8% reported violence a few times per month. Typically, the incidents involved patients’ relatives or both the patients and their relatives.
Nearly half of those who reported abuse experienced psychosomatic symptoms after the event, and 12% sought psychological care.
Administrative staff were 3.5 times more likely to experience abuse than other health care workers. Doctors and nurses were about twice as likely to experience abuse.
In addition, women, younger staff, and those who worked directly with COVID-19 patients were more likely to report abuse.
‘Shocking results’
Dr. Baranchuk, a native of Argentina, said people initially celebrated doctors and nurses for keeping communities safe. In several countries across Latin America, for instance, people lit candles, applauded at certain hours, and posted support on social media. As pandemic-related policies changed, however, health care providers faced unrest as people grew tired of wearing masks, maintaining social distance, and obeying restrictions at public spaces such as clubs and restaurants.
“This fatigue toward the social changes grew, but people didn’t have a specific target, and slowly and gradually, health care providers became the target of frustration and hate,” said Dr. Baranchuk. “In areas of the world where legislation is more flexible and less strict in charging individuals with poor or unacceptable behavior toward members of the health care team, aggression and microaggression became more frequent.”
“The results we obtained were more shocking than we expected,” Sebastián García-Zamora, MD, the lead study author and head of the coronary care unit at the Delta Clinic, Buenos Aires, said in an interview.
Dr. García-Zamora, also the coordinator of the International Society of Electrocardiology Young Community, noted the particularly high numbers of reports among young health care workers and women.
“Unfortunately, young women seem to be the most vulnerable staff to suffering violence, regardless of the work they perform in the health system,” he said. “Notably, less than one in four health team members that suffered workplace violence pursued legal action based on the events.”
The research team is now conducting additional analyses on the different types of aggression based on gender, region, and task performed by the health care team. They’re trying to understand who is most vulnerable to physical attacks, as well as the consequences.
“The most important thing to highlight is that this problem exists, it is more frequent than we think, and we can only solve it if we all get involved in it,” Dr. García-Zamora said.
‘Complete systematic failure’
Health care workers in certain communities faced more aggression as well. In a CMAJ Open study published in November 2021, Asian Canadian and Asian American health care workers experienced discrimination, racial microaggressions, threats of violence, and violent acts during the pandemic. Women and frontline workers with direct patient contact were more likely to face verbal and physical abuse.
“This highlights that we need to continue the fight against misogyny, racism, and health care worker discrimination,” lead study author Zhida Shang, a medical student at McGill University, Montreal, told this news organization.
“As we are managing to live with the COVID-19 pandemic, it is important to study our successes and shortcomings. I sincerely believe that during the pandemic, the treatment of various racialized communities, including Asian Americans and Asian Canadians, was a complete systematic failure,” he said. “It is crucial to continue to examine, reflect, and learn from these lessons so that there will be equitable outcomes during the next public health emergency.”
The study was conducted without funding support. Dr. Baranchuk, Dr. García-Zamora, and Ms. Shang report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After an aggressive event or abuse occurred, 56% of providers considered changing their care tasks, and more than a third considered quitting their profession.
“Aggression of any sort against health care providers is not a new social phenomenon, and it has existed as far as medicine and health care is reported in literature. However, the phenomenon of aggression against health care providers during the pandemic grew worse,” senior study author Adrian Baranchuk, MD, a professor of medicine at Queen’s University, Kingston, Ont., told this news organization.
The study was published online in Current Problems in Cardiology
Survey snapshot
Dr. Baranchuk and colleagues, with the support of the Inter-American Society of Cardiology, developed a survey to characterize the frequency and types of abuse that frontline health professionals faced. They invited health care professionals from Latin America who had provided care since March 2020 to participate.
Between January and February 2022, 3,544 participants from 19 countries took the survey. Among them, 70.8% were physicians, 16% were nurses, and 13.2% were other health team members, such as administrative staff and technicians. About 58.5% were women, and 74.7% provided direct care to patients with COVID-19.
Overall, 54.8% of respondents reported acts of aggression. Of this group, 95.6% reported verbal abuse, 11.1% reported physical abuse, and 19.9% reported other types of abuse, including microaggressions.
About 13% of respondents reported experiencing some form of aggression daily, 26.4% experienced abuse weekly, and 38.8% reported violence a few times per month. Typically, the incidents involved patients’ relatives or both the patients and their relatives.
Nearly half of those who reported abuse experienced psychosomatic symptoms after the event, and 12% sought psychological care.
Administrative staff were 3.5 times more likely to experience abuse than other health care workers. Doctors and nurses were about twice as likely to experience abuse.
In addition, women, younger staff, and those who worked directly with COVID-19 patients were more likely to report abuse.
‘Shocking results’
Dr. Baranchuk, a native of Argentina, said people initially celebrated doctors and nurses for keeping communities safe. In several countries across Latin America, for instance, people lit candles, applauded at certain hours, and posted support on social media. As pandemic-related policies changed, however, health care providers faced unrest as people grew tired of wearing masks, maintaining social distance, and obeying restrictions at public spaces such as clubs and restaurants.
“This fatigue toward the social changes grew, but people didn’t have a specific target, and slowly and gradually, health care providers became the target of frustration and hate,” said Dr. Baranchuk. “In areas of the world where legislation is more flexible and less strict in charging individuals with poor or unacceptable behavior toward members of the health care team, aggression and microaggression became more frequent.”
“The results we obtained were more shocking than we expected,” Sebastián García-Zamora, MD, the lead study author and head of the coronary care unit at the Delta Clinic, Buenos Aires, said in an interview.
Dr. García-Zamora, also the coordinator of the International Society of Electrocardiology Young Community, noted the particularly high numbers of reports among young health care workers and women.
“Unfortunately, young women seem to be the most vulnerable staff to suffering violence, regardless of the work they perform in the health system,” he said. “Notably, less than one in four health team members that suffered workplace violence pursued legal action based on the events.”
The research team is now conducting additional analyses on the different types of aggression based on gender, region, and task performed by the health care team. They’re trying to understand who is most vulnerable to physical attacks, as well as the consequences.
“The most important thing to highlight is that this problem exists, it is more frequent than we think, and we can only solve it if we all get involved in it,” Dr. García-Zamora said.
‘Complete systematic failure’
Health care workers in certain communities faced more aggression as well. In a CMAJ Open study published in November 2021, Asian Canadian and Asian American health care workers experienced discrimination, racial microaggressions, threats of violence, and violent acts during the pandemic. Women and frontline workers with direct patient contact were more likely to face verbal and physical abuse.
“This highlights that we need to continue the fight against misogyny, racism, and health care worker discrimination,” lead study author Zhida Shang, a medical student at McGill University, Montreal, told this news organization.
“As we are managing to live with the COVID-19 pandemic, it is important to study our successes and shortcomings. I sincerely believe that during the pandemic, the treatment of various racialized communities, including Asian Americans and Asian Canadians, was a complete systematic failure,” he said. “It is crucial to continue to examine, reflect, and learn from these lessons so that there will be equitable outcomes during the next public health emergency.”
The study was conducted without funding support. Dr. Baranchuk, Dr. García-Zamora, and Ms. Shang report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After an aggressive event or abuse occurred, 56% of providers considered changing their care tasks, and more than a third considered quitting their profession.
“Aggression of any sort against health care providers is not a new social phenomenon, and it has existed as far as medicine and health care is reported in literature. However, the phenomenon of aggression against health care providers during the pandemic grew worse,” senior study author Adrian Baranchuk, MD, a professor of medicine at Queen’s University, Kingston, Ont., told this news organization.
The study was published online in Current Problems in Cardiology
Survey snapshot
Dr. Baranchuk and colleagues, with the support of the Inter-American Society of Cardiology, developed a survey to characterize the frequency and types of abuse that frontline health professionals faced. They invited health care professionals from Latin America who had provided care since March 2020 to participate.
Between January and February 2022, 3,544 participants from 19 countries took the survey. Among them, 70.8% were physicians, 16% were nurses, and 13.2% were other health team members, such as administrative staff and technicians. About 58.5% were women, and 74.7% provided direct care to patients with COVID-19.
Overall, 54.8% of respondents reported acts of aggression. Of this group, 95.6% reported verbal abuse, 11.1% reported physical abuse, and 19.9% reported other types of abuse, including microaggressions.
About 13% of respondents reported experiencing some form of aggression daily, 26.4% experienced abuse weekly, and 38.8% reported violence a few times per month. Typically, the incidents involved patients’ relatives or both the patients and their relatives.
Nearly half of those who reported abuse experienced psychosomatic symptoms after the event, and 12% sought psychological care.
Administrative staff were 3.5 times more likely to experience abuse than other health care workers. Doctors and nurses were about twice as likely to experience abuse.
In addition, women, younger staff, and those who worked directly with COVID-19 patients were more likely to report abuse.
‘Shocking results’
Dr. Baranchuk, a native of Argentina, said people initially celebrated doctors and nurses for keeping communities safe. In several countries across Latin America, for instance, people lit candles, applauded at certain hours, and posted support on social media. As pandemic-related policies changed, however, health care providers faced unrest as people grew tired of wearing masks, maintaining social distance, and obeying restrictions at public spaces such as clubs and restaurants.
“This fatigue toward the social changes grew, but people didn’t have a specific target, and slowly and gradually, health care providers became the target of frustration and hate,” said Dr. Baranchuk. “In areas of the world where legislation is more flexible and less strict in charging individuals with poor or unacceptable behavior toward members of the health care team, aggression and microaggression became more frequent.”
“The results we obtained were more shocking than we expected,” Sebastián García-Zamora, MD, the lead study author and head of the coronary care unit at the Delta Clinic, Buenos Aires, said in an interview.
Dr. García-Zamora, also the coordinator of the International Society of Electrocardiology Young Community, noted the particularly high numbers of reports among young health care workers and women.
“Unfortunately, young women seem to be the most vulnerable staff to suffering violence, regardless of the work they perform in the health system,” he said. “Notably, less than one in four health team members that suffered workplace violence pursued legal action based on the events.”
The research team is now conducting additional analyses on the different types of aggression based on gender, region, and task performed by the health care team. They’re trying to understand who is most vulnerable to physical attacks, as well as the consequences.
“The most important thing to highlight is that this problem exists, it is more frequent than we think, and we can only solve it if we all get involved in it,” Dr. García-Zamora said.
‘Complete systematic failure’
Health care workers in certain communities faced more aggression as well. In a CMAJ Open study published in November 2021, Asian Canadian and Asian American health care workers experienced discrimination, racial microaggressions, threats of violence, and violent acts during the pandemic. Women and frontline workers with direct patient contact were more likely to face verbal and physical abuse.
“This highlights that we need to continue the fight against misogyny, racism, and health care worker discrimination,” lead study author Zhida Shang, a medical student at McGill University, Montreal, told this news organization.
“As we are managing to live with the COVID-19 pandemic, it is important to study our successes and shortcomings. I sincerely believe that during the pandemic, the treatment of various racialized communities, including Asian Americans and Asian Canadians, was a complete systematic failure,” he said. “It is crucial to continue to examine, reflect, and learn from these lessons so that there will be equitable outcomes during the next public health emergency.”
The study was conducted without funding support. Dr. Baranchuk, Dr. García-Zamora, and Ms. Shang report no relevant disclosures.
A version of this article first appeared on Medscape.com.
Inflation and health care: The prognosis for doctors
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Do behavioral interventions improve nighttime sleep in children < 1 year old?
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
EVIDENCE-BASED ANSWER:
YES. Infants respond to behavioral interventions, although objective data are limited. Behavioral interventions include establishing regular daytime and sleep routines for the infant, reducing environmental noises or distractions, and allowing for self-soothing at bedtime (strength of recommendation: B, based on multiple randomized and nonrandomized studies).
Are antipsychotics effective adjunctive Tx for patients with moderate-to-severe depression?
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
EVIDENCE-BASED ANSWER:
YES. Augmentation with second- generation antipsychotics, especially aripiprazole and quetiapine, appears to be effective in patients with moderate-to-severe depression who have had a suboptimal response to a selective serotonin reuptake inhibitor or a serotonin-norepinephrine reuptake inhibitor (strength of recommendation [SOR]: A, based on a systematic review of randomized controlled trials [RCTs] and an individual RCT). Augmenting antidepressant therapy with cariprazine, ziprasidone, or olanzapine also appears to improve depressive symptoms over the short term. All antipsychotics studied carried an increased likelihood of adverse effects that could lead to discontinuation (SOR: A, based on a systematic review of RCTs).
BA.4 and BA.5 subvariants are more evasive of antibodies, but not of cellular immunity
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
The picture around the BA.4 and BA.5 subvariants of Omicron has been really confusing in that the pair is driving up cases but global COVID-19 deaths remain at their lowest level since the beginning of the pandemic. Explaining the two components of the immune response – antibodies versus cellular immune responses – can help us understand where we are in the pandemic and future booster options.
These two subvariants of Omicron, as of July 5, make up more than half of the COVID-19 strains in the United States and are expected to keep increasing. One of two reasons can lead to a variant or subvariant becoming dominant strain: increased transmissibility or evasion of antibodies.
Although BA.4 and BA.5 could be more transmissible than other subvariants of Omicron (which is already very transmissible), this has not yet been established in experiments showing increased affinity for the human receptor or in animal models. What we do know is that BA.4 and BA.5 seem to evade neutralizing antibodies conferred by the vaccines or even prior BA.1 infection (an earlier subvariant of Omicron), which could be the reason we are seeing so many reinfections now. Of note, BA.1 infection conferred antibodies that protected against subsequent BA.2 infection, so we did not see the same spike in cases in the United States with BA.2 (after a large BA.1 spike over the winter) earlier this spring.
Okay, so isn’t evasion of antibodies a bad thing? Of course it is but, luckily, our immune system is “redundant” and doesn›t just rely on antibodies to protect us from infection. In fact, antibodies (such as IgA, which is the mucosal antibody most prevalent in the nose and mouth, and IgG, which is the most prevalent antibody in the bloodstream) are our first line of COVID-19 defense in the nasal mucosa. Therefore, mild upper respiratory infections will be common as BA.4/BA.5 evade our nasal antibodies. Luckily, the rate of severe disease is remaining low throughout the world, probably because of the high amounts of cellular immunity to the virus. B and T cells are our protectors from severe disease.
For instance, two-dose vaccines are still conferring high rates of protection from severe disease with the BA.4 and BA.5 variants, with 87% protection against hospitalization per South Africa data. This is probably attributable to the fact that T-cell immunity from the vaccines remains protective across variants “from Alpha to Omicron,” as described by a recent and elegant paper.
Data from Qatar show that natural infection (even occurring up to 14 months ago) remains very protective (97.3%) against severe disease with the current circulating subvariants, including BA.4 and BA.5. Again, this is probably attributable to T cells which specifically amplify in response to a piece of the virus and help recruit cells to attack the pathogen directly.
The original BA.1 subvariant of Omicron has 26-32 mutations along its spike protein that differ from the “ancestral strain,” and BA.4 and BA.5 variants have a few more. Our T-cell response, even across a mutated spike protein, is so robust that we have not seen Omicron yet able to evade the many T cells (which we produce from the vaccines or infection) that descend upon the mutated virus to fight severe disease. Antibody-producing memory B cells, generated by the vaccines (or prior infection), have been shown to actually adapt their immune response to the variant to which they are exposed.
Therefore, the story of the BA.4 and BA.5 subvariants seems to remain about antibodies vs. cellular immunity. Our immunity in the United States is growing and is from both vaccination and natural infection, with 78.3% of the population having had at least one dose of the vaccine and at least 60% of adults (and 75% of children 0-18) having been exposed to the virus by February 2022, per the Centers for Disease Control and Prevention (with exposure probably much higher now in July 2022 after subsequent Omicron subvariants waves).
So, what about Omicron-specific boosters? A booster shot will just raise antibodies temporarily, but their effectiveness wanes several months later. Moreover, a booster shot against the ancestral strain is not very effective in neutralizing BA.4 and BA.5 (with a prior BA.1 Omicron infection being more effective than a booster). Luckily, Pfizer has promised a BA.4/BA.5-specific mRNA vaccine by October, and Moderna has promised a bivalent vaccine containing BA.4/BA.5 mRNA sequences around the same time. A vaccine that specifically increases antibodies against the most prevalent circulating strain should be important as a booster for those who are predisposed to severe breakthrough infections (for example, those with immunocompromise or older individuals with multiple comorbidities). Moreover, BA.4/BA.5–specific booster vaccines may help prevent mild infections for many individuals. Finally, any booster (or exposure) should diversify and broaden T-cell responses to the virus, and a booster shot will also expand the potency of B cells, making them better able to respond to the newest subvariants as we continue to live with COVID-19.
Monica Gandhi, MD, MPH, is an infectious diseases doctor, professor of medicine, and associate chief in the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco.
A version of this article first appeared on Medscape.com.
High residual liver cancer risk in HCV-cured cirrhosis
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study confirms the very high risk of hepatocellular carcinoma faced by patients with cirrhosis who have been cured of hepatitis C, a finding the researchers hope will encourage clinicians to communicate risk information to patients and encourage regular HCC screening.
On average, the predicted probability of HCC in cirrhosis patients was 410 times greater than the equivalent probability in the general population, the study team found.
Hamish Innes, PhD, with Public Health Scotland, Glasgow, and colleagues wrote.
“Central to this is ensuring that cured cirrhosis patients understand the risk of HCC and are provided with appropriate surveillance,” they added.
“Most patients with cirrhosis do not adhere to HCC screening guidelines,” Nina Beri, MD, medical oncologist with New York University Perlmutter Cancer Center, who wasn’t involved in the study, said in an interview.
The “important” finding in this study “should be conveyed to patients, as this may help improve screening adherence rates,” Dr. Beri said.
The study was published online in the American Journal of Gastroenterology.
Findings may help promote screening uptake
Dr. Innes and colleagues compared the predicted probability of HCC in 1,803 Scottish adults (mean age, 50 years; 74% male) with cirrhosis and cured hepatitis C to the background risk in the general population of Scotland.
The mean predicted 3-year probability of HCC at the time of sustained viral response (SVR), determined using the aMAP prognostic model, was 3.64% (range, 0.012%-36.12%).
This contrasts with a 3-year HCC probability in the general population ranging from less than 0.0001% to 0.25% depending on demographics.
All patients with cirrhosis – even those at lowest risk – had a higher probability of HCC than the general population, but there was considerable heterogeneity from one patient to the next.
For example, the mean 3-year predicted probability was 18 times higher in the top quintile (9.8%) versus the lowest quintile (0.5%) of risk, the researchers found.
They could not identify a patient subgroup who exhibited a similar HCC risk profile to the general population, as was their hope going into the study.
Dr. Innes and colleagues have developed an online tool that allows clinicians to frame a patient›s 3-year HCC probability against the equivalent probability in the general population.
In the future, they said the scope of the tool could be extended by incorporating general population data from countries beyond Scotland.
“Our hope is that this tool will springboard patient-clinician discussions about HCC risk, and could mitigate low screening uptake,” Dr. Innes and colleagues wrote.
Curing HCV doesn’t eliminate risk
Commenting on the study, Nancy Reau, MD, section chief of hepatology at Rush University Medical Center, Chicago, said curing HCV is “very important and significantly reduces risk for complications, but it doesn’t return you to the normal population.”
Dr. Reau’s advice to cirrhosis patients: “Get screened twice a year.”
Dr. Beri said, in addition to conveying this risk to patients, “it is also important to disseminate this information to the community and to primary care practices, particularly as some patients may not currently follow in a specialized liver disease clinic.”
Also weighing in, Amit Singal, MD, chief of hepatology at the University of Texas Southwestern Medical Center, Dallas, said this study highlights that underlying cirrhosis is “the strongest risk factor for the development of HCC.”
In contrast to other cancers, such as breast and colorectal cancer, in which high risk populations can be identified by readily available information such as age and sex, implementation of HCC screening programs requires identification of patients with cirrhosis, Dr. Singal noted.
“Underuse of HCC screening in clinical practice is often related to providers having difficulty at this step in the process and contributes to the high proportion of HCC detected at late stages,” he told this news organization.
“Availability of accurate noninvasive markers of fibrosis will hopefully help with better identification of patients with cirrhosis moving forward,” Dr. Singal said, “although we as hepatologists need to work closely with our primary care colleagues to ensure these tools are used routinely in at-risk patients, such as those with nonalcoholic fatty liver disease, alcohol-associated liver disease, or history of cured (post-SVR) hepatitis C infection.”
The study was supported by the Medical Research Foundation and Public Health Scotland. Dr. Innes, Dr. Beri, Dr. Reau, and Dr. Singal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Obesity links to faster fading of COVID vaccine protection
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Researchers published the study covered in this summary on medRxiv.org as a preprint that has not yet been peer reviewed.
Key takeaways
- The study results suggest that
- The findings documented evidence of reduced neutralizing antibody capacity 6 months after primary vaccination in people with severe obesity.
- This was a large study involving about more than 3.5 million people who had received at least two doses of COVID-19 vaccine, including more than 650,000 with obesity.
Why this matters
- Obesity is associated with comorbidities that independently increase the risk for severe COVID-19, including type 2 diabetes, chronic kidney disease, and heart failure.
- The authors concluded that additional or more frequent booster doses are likely to be required to maintain protection among people with obesity against COVID-19.
Study design
- Prospective longitudinal study of the incidence and severity of COVID-19 infections and immune responses in a cohort of more than 3.5 million adults from a Scottish healthcare database who received two or three doses of COVID-19 vaccine. The data came from the study, centered at the University of Edinburgh.
- About 16% had obesity with a body mass index of 30-39.9 kg/m2, and an additional 3% had severe obesity with a BMI of 40 or greater.
- Although not specified in this preprint, another said that the vaccines administered in Scotland have been the Pfizer-BioNTech and Oxford-AstraZeneca formulations.
Key results
- Between Sept. 14, 2020, and March 19, 2022, 10,983 people (0.3% of the total cohort; 6.0 events per 1,000 person-years) had severe COVID-19, consisting of 9,733 who were hospitalized and 2,207 who died (957 of those hospitalized also died).
- People with obesity or severe obesity were at higher risk of hospitalization or death from COVID-19 after both a second and third (booster) dose of vaccine.
- Compared with those with normal weight, those with severe obesity (BMI higher than 40) were at significantly increased risk for severe COVID-19 after a second vaccine dose, with an adjusted rate ratio 1.76, whereas those with standard obesity (BMI, 30-40) were at a modestly but significantly increased risk with an adjusted rate ratio of 1.11.
- Breakthrough infections after the second dose for those with severe obesity, obesity, and normal weight occurred on average at 10 weeks, 15 weeks, and 20 weeks, respectively.
- Interaction testing showed that vaccine effectiveness significantly diminished over time across BMI groups, and protection waned more rapidly as BMI increased.
- Results from immunophenotyping studies run in a subgroup of several dozen subjects with severe obesity or normal weight showed significant decrements in the robustness of antibody responses in those with severe obesity 6 months after a second or third vaccine dose.
Limitations
- The authors did not specify any limitations.
Disclosures
- The study received no commercial funding.
- One author received funding from Wellcome.
This is a summary of a preprint research study , “Accelerated waning of the humoral response to SARS-CoV-2 vaccines in obesity,” published by researchers primarily at the University of Cambridge (England), on medRxiv. This study has not yet been peer reviewed. The full text of the study can be found on medRxiv.org.
A version of this article first appeared on Medscape.com.
Liver disease and death rates fall after hepatitis C treatment barriers are dismantled
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.
As obstacles to hepatitis C treatment uptake were removed, rates of hepatitis-related liver disease in marginalized groups plummeted, according to a new study in Baltimore, published in Annals of Internal Medicine.
A community-based cohort study that follows current and former people who inject drugs (PWID) with hepatitis C documented drastic reductions in liver disease and death as effective oral antivirals became more readily accessible there between 2015 and 2019.
The researchers concluded that hepatitis C elimination targets are achievable. But, they warned, uptake is uneven, and more needs to be done to facilitate treatment.
“[The study] gives us a real-world perspective on what’s happening on the ground, in terms of people getting treated,” said first author Javier Cepeda, PhD, MPH, an assistant professor at the Johns Hopkins Bloomberg School of Public Health, Baltimore, in an interview. “Changing policy, reducing barriers, [and] getting them access to treatment really does have this really important public health benefit.”
” said Maria Corcorran, MD, MPH. Dr. Corcorran, an acting assistant professor for the department of medicine at the University of Washington, was not involved with the study. “It’s just further evidence that we need to really be linking people to care and getting people treated and cured.”
The World Health Organization has called for the disease’s global elimination by 2030. Cure rates top 95%. But, there are so many new cases and so many barriers to detection and treatment that how to develop and roll out a public health response is the most important question in the field, wrote study co-author David L. Thomas, MD, MPH, in a review article in The New England Journal of Medicine.
“Folks who inject drugs ... do well on hep C treatment and have similar rates of sustained virologic response or cure,” said Dr. Corcorran, who runs a low-barrier clinic for people experiencing homelessness.
But, she added, “there are barriers that are still put up to treatment in terms of who can treat and what insurance is going to cover.”
A look at a vulnerable population
The authors studied adults enrolled in ALIVE (AIDS Linked to the Intravenous Experience), a cohort study that has recruited current and former PWID in the Baltimore area since 1988.
Participants visit the clinic twice a year for health-related interviews and blood testing, including HIV serology, hepatitis C virus (HCV) antibody and RNA testing, and liver function tests. They are counseled about HCV testing and treatment but do not receive treatment through the study.
Beginning in 2006, researchers added liver stiffness measures (LSMs), a noninvasive measure conducted with transient elastography.
From 2006 to 2019, the authors followed 1,323 ALIVE participants with chronic HCV infection. The primary outcome was LSMs.
Less liver disease, fewer deaths
At baseline, participants’ median age was 49 years; 82% of participants were Black individuals, 71% percent were male, and two-thirds were HIV-negative.
Three percent reported receiving hepatitis C treatment in 2014, which increased to 39% in 2019.
Among 10,350 LSMs, 15% showed cirrhosis at baseline in 2006. In 2015, that rose to 19%, but by 2019, it had fallen to 8%.
By definition, 100% had detectable HCV RNA at baseline. In 2015, 91% still did. By 2019, that rate had fallen to 48%.
Undetectable HCV RNA correlated with lower log LSM in adjusted models (P < .001). It also correlated strongly with lower odds of liver cirrhosis, with an adjusted odds ratio of 0.28 (95% confidence interval, 0.17-0.45; P < .001). In addition, it correlated with lower risk for all-cause mortality, with an adjusted hazard ratio of 0.54 (95% CI, 0.38-0.77; P < .001).
Limitations include the fact that, although transient elastography is considered the most valid way to detect cirrhosis in people with hepatitis C, liver stiffness has not been validated as a measure of fibrosis among people with a sustained virologic response.
In addition, ALIVE participants are older and more likely to be African American individuals, compared with the general population of PWID in Baltimore, wrote co-author Shruti H. Mehta, PhD, a professor of epidemiology at Johns Hopkins University, Baltimore, in an email exchange with this news organization. That could affect generalizability.
Treatment is crucial
The first direct-acting antiviral (DAA) for hepatitis C was approved in 2011, and an oral fixed-dose combination antiviral was approved in 2014, ushering in treatments with cure rates far exceeding those with interferon-based therapy.
But until recently, Medicaid patients in Maryland seeking DAA therapy for hepatitis C required prior authorization, with initial restrictions related to disease stage, substance use, and provider type, according to Dr. Mehta.
Gradually, those restrictions were lifted, Dr. Mehta added, and all were eliminated by 2019.
Dr. Cepeda urges clinicians to treat patients infected with hepatitis C immediately.
“There are really important implications on both reducing liver disease progression and all-cause mortality,” he said.
“Hep C is just one part of a whole constellation of health care delivery [and] of treating all of the other potential problems that might need to be addressed – especially with people who inject drugs,” Dr. Cepeda added. “Getting them into care is really, really important.”
The study was funded by the National Institutes of Health. Dr. Cepeda and Dr. Corcorran report no relevant financial relationships. Dr. Mehta reports receiving payments or honoraria and travel support from Gilead Sciences, the makers of the oral hepatitis C medication ledipasvir/sofosbuvir (Harvoni), as well as equipment, materials, drugs, medical writing, gifts, or other services from Abbott, which sells hepatitis C diagnostics. Dr. Thomas reports ties to Excision Bio and to Merck DSMB.
A version of this article first appeared on Medscape.com.