User login
New KRAS inhibitor shows promise in NSCLC
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
FDA approves combination pegloticase and methotrexate for refractory gout
Pegloticase, which has been available for 12 years, is a pegylated uric acid specific enzyme that lowers sUA by converting it to allantoin.
Though pegloticase is effective in treating chronic gout in patients refractory to conventional treatment, approximately 92% of patients develop antibodies against the drug, resulting in reduced efficacy.
Based on the immunomodulatory effects of methotrexate, researchers of the randomized, placebo-controlled MIRROR trial sought to determine whether combination treatment of pegloticase with methotrexate (multiple brands) would prevent the development of anti-drug antibodies.
Findings from the phase 4 trial found that co-administration of pegloticase and methotrexate reduced the formation of new anti-PEG antibodies. In the group receiving methotrexate and pegloticase, 23.2% (22 out of 95) of patients had an increase in anti-PEG antibodies, compared with 50% (24 of 48) in the pegloticase plus placebo group, according to a recent company press release.
Nearly three-quarters (71%) of participants in the group pretreated with methotrexate, followed by combination pegloticase-methotrexate, had sUA levels that dopped to below 6 mg/dL during the 52-week study. By comparison, 38.5% of participants in the pegloticase and placebo group reached the endpoint. Though gout flare occurred in both groups, methotrexate did not appear to increase the risk for adverse events or gout flare.
The study, led by John Botson, MD, RPh, CCD, a rheumatologist in Anchorage, Alaska, concluded that these measurements demonstrated a significant improvement from traditional pegloticase-only treatment of gout. “This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” Dr. Botson said last month in an interview with this news organization.
The study was funded by Horizon. Dr. Botson reports receiving research support from Horizon and Radius Health and speaker fees from AbbVie, Amgen, Aurinia, ChemoCentryx, Horizon, Eli Lilly, and Novartis.
A version of this article first appeared on Medscape.com.
Pegloticase, which has been available for 12 years, is a pegylated uric acid specific enzyme that lowers sUA by converting it to allantoin.
Though pegloticase is effective in treating chronic gout in patients refractory to conventional treatment, approximately 92% of patients develop antibodies against the drug, resulting in reduced efficacy.
Based on the immunomodulatory effects of methotrexate, researchers of the randomized, placebo-controlled MIRROR trial sought to determine whether combination treatment of pegloticase with methotrexate (multiple brands) would prevent the development of anti-drug antibodies.
Findings from the phase 4 trial found that co-administration of pegloticase and methotrexate reduced the formation of new anti-PEG antibodies. In the group receiving methotrexate and pegloticase, 23.2% (22 out of 95) of patients had an increase in anti-PEG antibodies, compared with 50% (24 of 48) in the pegloticase plus placebo group, according to a recent company press release.
Nearly three-quarters (71%) of participants in the group pretreated with methotrexate, followed by combination pegloticase-methotrexate, had sUA levels that dopped to below 6 mg/dL during the 52-week study. By comparison, 38.5% of participants in the pegloticase and placebo group reached the endpoint. Though gout flare occurred in both groups, methotrexate did not appear to increase the risk for adverse events or gout flare.
The study, led by John Botson, MD, RPh, CCD, a rheumatologist in Anchorage, Alaska, concluded that these measurements demonstrated a significant improvement from traditional pegloticase-only treatment of gout. “This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” Dr. Botson said last month in an interview with this news organization.
The study was funded by Horizon. Dr. Botson reports receiving research support from Horizon and Radius Health and speaker fees from AbbVie, Amgen, Aurinia, ChemoCentryx, Horizon, Eli Lilly, and Novartis.
A version of this article first appeared on Medscape.com.
Pegloticase, which has been available for 12 years, is a pegylated uric acid specific enzyme that lowers sUA by converting it to allantoin.
Though pegloticase is effective in treating chronic gout in patients refractory to conventional treatment, approximately 92% of patients develop antibodies against the drug, resulting in reduced efficacy.
Based on the immunomodulatory effects of methotrexate, researchers of the randomized, placebo-controlled MIRROR trial sought to determine whether combination treatment of pegloticase with methotrexate (multiple brands) would prevent the development of anti-drug antibodies.
Findings from the phase 4 trial found that co-administration of pegloticase and methotrexate reduced the formation of new anti-PEG antibodies. In the group receiving methotrexate and pegloticase, 23.2% (22 out of 95) of patients had an increase in anti-PEG antibodies, compared with 50% (24 of 48) in the pegloticase plus placebo group, according to a recent company press release.
Nearly three-quarters (71%) of participants in the group pretreated with methotrexate, followed by combination pegloticase-methotrexate, had sUA levels that dopped to below 6 mg/dL during the 52-week study. By comparison, 38.5% of participants in the pegloticase and placebo group reached the endpoint. Though gout flare occurred in both groups, methotrexate did not appear to increase the risk for adverse events or gout flare.
The study, led by John Botson, MD, RPh, CCD, a rheumatologist in Anchorage, Alaska, concluded that these measurements demonstrated a significant improvement from traditional pegloticase-only treatment of gout. “This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” Dr. Botson said last month in an interview with this news organization.
The study was funded by Horizon. Dr. Botson reports receiving research support from Horizon and Radius Health and speaker fees from AbbVie, Amgen, Aurinia, ChemoCentryx, Horizon, Eli Lilly, and Novartis.
A version of this article first appeared on Medscape.com.
Topical gel for epidermolysis bullosa shows ongoing benefit
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
GLASGOW, Scotland – the phase 3 safety and efficacy study of the treatment.
Over 200 patients from the trial, including 105 who began treatment with a control gel, continued taking oleogel-S10 after 90 days. The current interim analysis at 12 months indicates there was a 55% reduction in the proportion of the body affected, compared with baseline.
Moreover, reductions in skin activity scores seen in the double-blind phase of the trial were maintained during the open-label extension. About 6% of patients experienced adverse events that led to withdrawal from the study.
The results show that oleogel-S10 was associated with “accelerated wound healing,” said study presenter Tracey Cunningham, MD, chief medical officer, Amryt Pharmaceuticals DAC, Dublin, which is developing the topical agent. “There were no new safety signals with this longer exposure to oleogel-S10, and patients had sustained improvement in wound burden,” she added.
The research was presented at the British Association of Dermatologists (BAD) 2022 Annual Meeting on July 6.
In April, European Medicines Agency recommended approval of oleogel-S10 for the treatment of partial-thickness skin wounds associated with dystrophic and junctional EB for patients aged 6 months and older.
However, just a month earlier, the U.S. Food and Drug Administration declined to approve the topical agent for use in EB, even after it extended its review by 3 months to include additional analyses of data previously submitted by the company.
In the post-presentation discussion, Dr. Cunningham said that the FDA had “not been satisfied at this point with the information that we have given them,” adding, “We don’t agree with the decision, and we will be appealing.”
Raman K. Madan, MD, a dermatologist at Northwell Health, Huntington, New York, who was not involved in the study, said that the reductions in wound healing seen in the study are “meaningful” and that the numbers represent a “big breakthrough.”
He told this news organization that there are “very few products on the market” for EB and that having an option for patients “would be amazing.”
“The big issue here would be cost and coverage for patients,” he said. If approved, “hopefully” it will be affordable, he added.
Dr. Madan noted that from his perspective, the majority of the reactions to the topical gel were “mild,” and there are “a lot of confounding factors” underlying the number of serious adverse events. “These patients with epidermolysis are prone to some of these issues regardless of treatment,” he said.
During her presentation, Dr. Cunningham noted that EB is a rare, debilitating condition that is characterized by varying degrees of skin fragility, blisters, and impaired wound healing that in turn lead to serious complications that affect quality of life.
While wound management is a “fundamental priority” for patients living with EB, she said, there is a “high, unmet” clinical need.
To those ends, EASE was the largest randomized controlled phase 3 efficacy and safety study in EB. In the study, 252 patients were allocated to receive oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing.
The double-blind phase of the trial met its primary endpoint: A higher proportion of patients who were given oleogel-S10 achieved first complete closure of the EB target wound by day 45, compared with patients who were given control gel, at 41.3% versus 28.9%. This equated to a relative risk of wound closure by day 45 of 1.44, or an odds ratio of 1.84 (P = .013).
However, as reported at the time by this news organization, the difference in time to wound healing by day 90 between the two patient groups was not statistically significant (P = .302), with 50.5% of oleogel-S10 patients achieving wound closure, versus 43.9% of those in the control group.
Dr. Cunningham discussed the open-label extension, which involved 205 patients from the double-blind phase (mean age, of 16.3 years) treated with oleogel-S10 or control gel plus standard-of-care nonadhesive wound dressing for 24 months.
In presenting the results of the first 12 months of the open-label extension, she said that oleogel-S10 led to “consistent” reductions in the body surface area percentage (BSAP) affected by EB. The overall reduction from baseline was 55% after receiving treatment for 15 months.
Between day 90 and month 12 of the open-label extension, the absolute BSAP was reduced from 7.4% to 5.4% for patients who had received oleogel-S10 from the start of the study. For those who started in the control group and then switched to the oleogel-S10 arm during the open-label extension, the reduction was from 8.3% to 6.4%.
Dr. Cunningham pointed out that a 1% reduction in BSAP equates approximately to the palmar surface of the hand.
Scores on the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI) Skin activity subscale indicated that the reductions achieved in the double-blind phase of the trial were maintained.
Among patients who received oleogel-S10 from the start of the trial, EBDASI Skin scores were reduced from 19.6 at baseline to 13.5 at 12 months’ follow-up in the open-label extension. The reduction was from 19.6 to 13.5 for those who began the trial taking control gel.
Dr. Cunningham showed that adverse events of any grade were seen in 72.0% of patients who began taking oleogel-S10 at the start of the trial and in 69.5% of those who began the trial taking control gel.
Serious adverse events were recorded in 23.0% and 20.0% of patients, respectively, while 6.0% of those who initially received oleogel-S10 and 6.7% of those initially assigned to control gel experienced adverse events that led to study withdrawal during the open-label phase.
The most frequently reported adverse events in the open-label extension were wound complications, seen in 39.5% of patients; anemia, seen in 14.1%; wound infection, seen in 9.3%; pyrexia, seen in 8.3%; and pruritus, seen in 5.9%. No more details regarding adverse events were provided.
The study was funded by Amryt Pharmaceuticals DAC. Dr. Cunningham is an employee of Amryt Pharmaceuticals. No other relevant financial relationships have been disclosed.
A version of this article first appeared on Medscape.com.
Children and COVID: Vaccination a harder sell in the summer
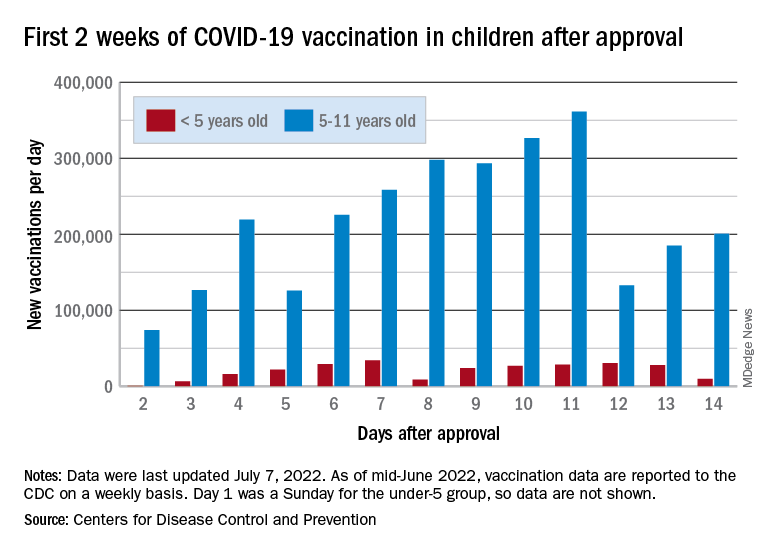
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.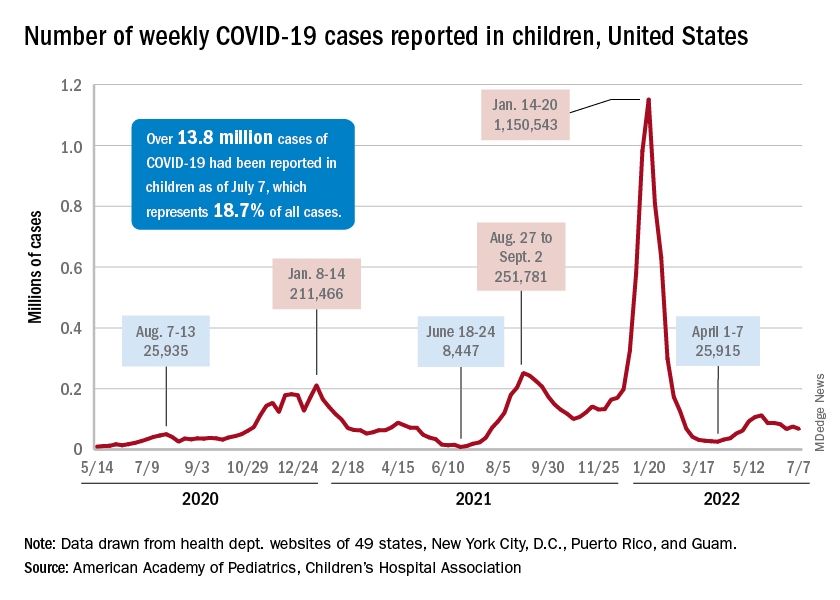
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
Medical management of miscarriage curbs costs and maintains quality of care
Medical management of early pregnancy loss costs less and offers similar quality of life to uterine aspiration, based on data from an analytical model.
Early pregnancy loss (EPL) occurs in more than 1 million women in the United States each year, and many patients are diagnosed before they show symptoms, wrote Divyah Nagendra, MD, of Cambridge Health Alliance, Mass., and colleagues.
A 2018 study showed that medical management of EPL with mifepristone added to misoprostol increased effectiveness and reduced the need for additional medication or subsequent uterine procedures, but the cost of mifepristone is perceived as a barrier, and the cost-effectiveness of its use, compared with surgical or expectant management, has not been well studied, the researchers noted.
“We already know that adding mifepristone to the medical management of early pregnancy loss increases the effectiveness of the regimen,” coauthor Courtney A. Schreiber, MD, of the University of Pennsylvania, Philadelphia, said in an interview. “Procedural uterine aspiration is highly effective as well, so patients and providers may consider the cost when deciding on a treatment strategy,” she added.
“If medication management is preferred by many patients, decreases the need to access in-person clinical care during a pandemic, and is found to be cost-effective, clinicians and policymakers should increase efforts to improve mifepristone availability and reduce access burdens,” the researchers wrote.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers created a decision-analytic model using data from published literature and the Pregnancy Failure Regiments Trial (PreFaiR) to compare office-based uterine aspiration to medical management with mifepristone pretreatment followed by misoprostol for EPL.
The PrFaiR study randomized 300 women who experienced EPL before 12 weeks’ gestation to medication management with 800 mcg misoprostol vaginally, with or without pretreatment of 200 mg mifeprestone orally. The average age of the participants was 30.7 years, and demographics were similar between the groups.
The researchers used the PrFaiR data for medical management and patient-level data from published literature for uterine aspiration.
The primary outcome was the cost per quality-adjusted life year (QALY) gained. QALY was based on a modified utility score from the published literature. Effectiveness was based on QALY gained and the rate of complete expulsion of the gestational sac without additional intervention.
Overall, the mean costs per person were significantly higher for uterine aspiration, compared with medical management ($828 vs. $661, P = .004). Uterine aspiration was significantly more effective for complete gestational sac expulsion (97.3% vs. 83.8%, P = .0001). However, the QALYs were significantly higher for medical management, compared with uterine aspiration (0.082 vs. 0.079, P < .0001).
Cost-effectiveness was greater for medical management from a health care sector perspective, with lower costs and higher QALYs than uterine aspiration, the researchers noted.
They also evaluated the effect of mifepristone pretreatment on cost-effectiveness and found that medical management would remain cost effective, compared with uterine aspiration even if uterine aspiration procedures decreased in cost and mifepristone increased in cost, and even if medication management had a decreased completion rate and utility score, compared with uterine aspiration.
“Our analysis demonstrates that the incremental cost-effectiveness ratio (ICER) for medical management is well below the maximum willingness-to-pay threshold of approximately $100,000 per QALY gained,” the researchers wrote in their discussion of the findings.
Potential savings, uncertain access
Despite the potential savings and patient benefits, access to mifepristone remains a barrier, the researchers said.
Although the FDA lifted some restrictions on mifepristone in 2021 in the wake of the ongoing COVID-19 pandemic, the effect of new abortion-related restrictions remains to be seen.
The study findings were limited by several factors, including the use of 2018 National Medicare reimbursement rates to calculate costs, though actual costs vary by region and payer, the researchers noted. Other limitations include variations in cost of mifepristone by region and time and the differences in data sources between the uterine aspiration and medical management groups. More research is needed to assess QALYs for early pregnancy loss to establish standard measures and to assess the societal perspective of ESL as well as the health care perspective, they added.
However, the current results support medical management of EPL with mifepristone pretreatment followed by misoprostol as a “high-value care alternative” to office-based uterine aspiration, they said. “Increasing access to mifepristone and eliminating unnecessary restrictions will improve early pregnancy care,” they concluded.
“Given how effective procedural management is, we were slightly surprised that medical management remains cost effective,” Dr. Schreiber said in an interview.
Looking ahead in the wake of new restrictions on use for abortion, “patients may have difficulty accessing either medical or procedural management for early pregnancy loss,” Dr. Schreiber noted. “We support the accessibility of all evidence-based care and hope that our data will help overcome perceived financial barriers,” she said. Additional research needs include improved implementation and access to evidence-based early pregnancy loss care, she added.
Reasons to lift regulations
“Given the recent overturning of Roe v. Wade, any medications that are associated with abortion have increased scrutiny, especially mifepristone and misoprostol, even though these medications are also used for managing early pregnancy loss,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Demonstrating that medication management of EPL with mifepristone/misoprostol is less expensive and has increased QALYs associated with it is yet another reason to deregulate mifepristone so it can also be more accessible for management of EPL,” said Dr. Prager, who was not involved in the study.
Dr. Prager said she was not surprised by the findings, as effective medication should be less expensive than a procedure. “I would caution that the increased QALYs found in this study should not be interpreted as a reason to restrict surgical management of EPL but to increase access also to medication management, even though medication has a slightly lower rate of complete gestational sac expulsion,” she noted. “Mode of management should be up to the patient, unless there is a clear medical reason for one or the other.”
Going forward, “the FDA has it in its power to remove the REMS, which would immediately make mifepristone a medication that can be prescribed through a pharmacy and therefore much more available,” said Dr. Prager. “Restrictions for both medication and surgical management of EPL will likely increase in states where abortion is illegal, and it could possibly lead to patients having less choice as to mode of management,” she explained.
“There are many studies showing that all modes of EPL management are safe and effective and should be supported with respect to patient choice,” Dr. Prager noted. “There are also substantial data supporting the overall safety of mifepristone, and there are no scientific or medical data suggesting the REMS increases safety in any way. Frankly, there are no good, evidence-based reasons to continue to keep the REMS in place,” she said.
The study was supported by the National Institute of Child Health and Human Development of the National Institutes of Health and a Society of Family Planning Research Fund Midcareer Mentor Award. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Medical management of early pregnancy loss costs less and offers similar quality of life to uterine aspiration, based on data from an analytical model.
Early pregnancy loss (EPL) occurs in more than 1 million women in the United States each year, and many patients are diagnosed before they show symptoms, wrote Divyah Nagendra, MD, of Cambridge Health Alliance, Mass., and colleagues.
A 2018 study showed that medical management of EPL with mifepristone added to misoprostol increased effectiveness and reduced the need for additional medication or subsequent uterine procedures, but the cost of mifepristone is perceived as a barrier, and the cost-effectiveness of its use, compared with surgical or expectant management, has not been well studied, the researchers noted.
“We already know that adding mifepristone to the medical management of early pregnancy loss increases the effectiveness of the regimen,” coauthor Courtney A. Schreiber, MD, of the University of Pennsylvania, Philadelphia, said in an interview. “Procedural uterine aspiration is highly effective as well, so patients and providers may consider the cost when deciding on a treatment strategy,” she added.
“If medication management is preferred by many patients, decreases the need to access in-person clinical care during a pandemic, and is found to be cost-effective, clinicians and policymakers should increase efforts to improve mifepristone availability and reduce access burdens,” the researchers wrote.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers created a decision-analytic model using data from published literature and the Pregnancy Failure Regiments Trial (PreFaiR) to compare office-based uterine aspiration to medical management with mifepristone pretreatment followed by misoprostol for EPL.
The PrFaiR study randomized 300 women who experienced EPL before 12 weeks’ gestation to medication management with 800 mcg misoprostol vaginally, with or without pretreatment of 200 mg mifeprestone orally. The average age of the participants was 30.7 years, and demographics were similar between the groups.
The researchers used the PrFaiR data for medical management and patient-level data from published literature for uterine aspiration.
The primary outcome was the cost per quality-adjusted life year (QALY) gained. QALY was based on a modified utility score from the published literature. Effectiveness was based on QALY gained and the rate of complete expulsion of the gestational sac without additional intervention.
Overall, the mean costs per person were significantly higher for uterine aspiration, compared with medical management ($828 vs. $661, P = .004). Uterine aspiration was significantly more effective for complete gestational sac expulsion (97.3% vs. 83.8%, P = .0001). However, the QALYs were significantly higher for medical management, compared with uterine aspiration (0.082 vs. 0.079, P < .0001).
Cost-effectiveness was greater for medical management from a health care sector perspective, with lower costs and higher QALYs than uterine aspiration, the researchers noted.
They also evaluated the effect of mifepristone pretreatment on cost-effectiveness and found that medical management would remain cost effective, compared with uterine aspiration even if uterine aspiration procedures decreased in cost and mifepristone increased in cost, and even if medication management had a decreased completion rate and utility score, compared with uterine aspiration.
“Our analysis demonstrates that the incremental cost-effectiveness ratio (ICER) for medical management is well below the maximum willingness-to-pay threshold of approximately $100,000 per QALY gained,” the researchers wrote in their discussion of the findings.
Potential savings, uncertain access
Despite the potential savings and patient benefits, access to mifepristone remains a barrier, the researchers said.
Although the FDA lifted some restrictions on mifepristone in 2021 in the wake of the ongoing COVID-19 pandemic, the effect of new abortion-related restrictions remains to be seen.
The study findings were limited by several factors, including the use of 2018 National Medicare reimbursement rates to calculate costs, though actual costs vary by region and payer, the researchers noted. Other limitations include variations in cost of mifepristone by region and time and the differences in data sources between the uterine aspiration and medical management groups. More research is needed to assess QALYs for early pregnancy loss to establish standard measures and to assess the societal perspective of ESL as well as the health care perspective, they added.
However, the current results support medical management of EPL with mifepristone pretreatment followed by misoprostol as a “high-value care alternative” to office-based uterine aspiration, they said. “Increasing access to mifepristone and eliminating unnecessary restrictions will improve early pregnancy care,” they concluded.
“Given how effective procedural management is, we were slightly surprised that medical management remains cost effective,” Dr. Schreiber said in an interview.
Looking ahead in the wake of new restrictions on use for abortion, “patients may have difficulty accessing either medical or procedural management for early pregnancy loss,” Dr. Schreiber noted. “We support the accessibility of all evidence-based care and hope that our data will help overcome perceived financial barriers,” she said. Additional research needs include improved implementation and access to evidence-based early pregnancy loss care, she added.
Reasons to lift regulations
“Given the recent overturning of Roe v. Wade, any medications that are associated with abortion have increased scrutiny, especially mifepristone and misoprostol, even though these medications are also used for managing early pregnancy loss,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Demonstrating that medication management of EPL with mifepristone/misoprostol is less expensive and has increased QALYs associated with it is yet another reason to deregulate mifepristone so it can also be more accessible for management of EPL,” said Dr. Prager, who was not involved in the study.
Dr. Prager said she was not surprised by the findings, as effective medication should be less expensive than a procedure. “I would caution that the increased QALYs found in this study should not be interpreted as a reason to restrict surgical management of EPL but to increase access also to medication management, even though medication has a slightly lower rate of complete gestational sac expulsion,” she noted. “Mode of management should be up to the patient, unless there is a clear medical reason for one or the other.”
Going forward, “the FDA has it in its power to remove the REMS, which would immediately make mifepristone a medication that can be prescribed through a pharmacy and therefore much more available,” said Dr. Prager. “Restrictions for both medication and surgical management of EPL will likely increase in states where abortion is illegal, and it could possibly lead to patients having less choice as to mode of management,” she explained.
“There are many studies showing that all modes of EPL management are safe and effective and should be supported with respect to patient choice,” Dr. Prager noted. “There are also substantial data supporting the overall safety of mifepristone, and there are no scientific or medical data suggesting the REMS increases safety in any way. Frankly, there are no good, evidence-based reasons to continue to keep the REMS in place,” she said.
The study was supported by the National Institute of Child Health and Human Development of the National Institutes of Health and a Society of Family Planning Research Fund Midcareer Mentor Award. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
Medical management of early pregnancy loss costs less and offers similar quality of life to uterine aspiration, based on data from an analytical model.
Early pregnancy loss (EPL) occurs in more than 1 million women in the United States each year, and many patients are diagnosed before they show symptoms, wrote Divyah Nagendra, MD, of Cambridge Health Alliance, Mass., and colleagues.
A 2018 study showed that medical management of EPL with mifepristone added to misoprostol increased effectiveness and reduced the need for additional medication or subsequent uterine procedures, but the cost of mifepristone is perceived as a barrier, and the cost-effectiveness of its use, compared with surgical or expectant management, has not been well studied, the researchers noted.
“We already know that adding mifepristone to the medical management of early pregnancy loss increases the effectiveness of the regimen,” coauthor Courtney A. Schreiber, MD, of the University of Pennsylvania, Philadelphia, said in an interview. “Procedural uterine aspiration is highly effective as well, so patients and providers may consider the cost when deciding on a treatment strategy,” she added.
“If medication management is preferred by many patients, decreases the need to access in-person clinical care during a pandemic, and is found to be cost-effective, clinicians and policymakers should increase efforts to improve mifepristone availability and reduce access burdens,” the researchers wrote.
In a study published in the American Journal of Obstetrics & Gynecology, the researchers created a decision-analytic model using data from published literature and the Pregnancy Failure Regiments Trial (PreFaiR) to compare office-based uterine aspiration to medical management with mifepristone pretreatment followed by misoprostol for EPL.
The PrFaiR study randomized 300 women who experienced EPL before 12 weeks’ gestation to medication management with 800 mcg misoprostol vaginally, with or without pretreatment of 200 mg mifeprestone orally. The average age of the participants was 30.7 years, and demographics were similar between the groups.
The researchers used the PrFaiR data for medical management and patient-level data from published literature for uterine aspiration.
The primary outcome was the cost per quality-adjusted life year (QALY) gained. QALY was based on a modified utility score from the published literature. Effectiveness was based on QALY gained and the rate of complete expulsion of the gestational sac without additional intervention.
Overall, the mean costs per person were significantly higher for uterine aspiration, compared with medical management ($828 vs. $661, P = .004). Uterine aspiration was significantly more effective for complete gestational sac expulsion (97.3% vs. 83.8%, P = .0001). However, the QALYs were significantly higher for medical management, compared with uterine aspiration (0.082 vs. 0.079, P < .0001).
Cost-effectiveness was greater for medical management from a health care sector perspective, with lower costs and higher QALYs than uterine aspiration, the researchers noted.
They also evaluated the effect of mifepristone pretreatment on cost-effectiveness and found that medical management would remain cost effective, compared with uterine aspiration even if uterine aspiration procedures decreased in cost and mifepristone increased in cost, and even if medication management had a decreased completion rate and utility score, compared with uterine aspiration.
“Our analysis demonstrates that the incremental cost-effectiveness ratio (ICER) for medical management is well below the maximum willingness-to-pay threshold of approximately $100,000 per QALY gained,” the researchers wrote in their discussion of the findings.
Potential savings, uncertain access
Despite the potential savings and patient benefits, access to mifepristone remains a barrier, the researchers said.
Although the FDA lifted some restrictions on mifepristone in 2021 in the wake of the ongoing COVID-19 pandemic, the effect of new abortion-related restrictions remains to be seen.
The study findings were limited by several factors, including the use of 2018 National Medicare reimbursement rates to calculate costs, though actual costs vary by region and payer, the researchers noted. Other limitations include variations in cost of mifepristone by region and time and the differences in data sources between the uterine aspiration and medical management groups. More research is needed to assess QALYs for early pregnancy loss to establish standard measures and to assess the societal perspective of ESL as well as the health care perspective, they added.
However, the current results support medical management of EPL with mifepristone pretreatment followed by misoprostol as a “high-value care alternative” to office-based uterine aspiration, they said. “Increasing access to mifepristone and eliminating unnecessary restrictions will improve early pregnancy care,” they concluded.
“Given how effective procedural management is, we were slightly surprised that medical management remains cost effective,” Dr. Schreiber said in an interview.
Looking ahead in the wake of new restrictions on use for abortion, “patients may have difficulty accessing either medical or procedural management for early pregnancy loss,” Dr. Schreiber noted. “We support the accessibility of all evidence-based care and hope that our data will help overcome perceived financial barriers,” she said. Additional research needs include improved implementation and access to evidence-based early pregnancy loss care, she added.
Reasons to lift regulations
“Given the recent overturning of Roe v. Wade, any medications that are associated with abortion have increased scrutiny, especially mifepristone and misoprostol, even though these medications are also used for managing early pregnancy loss,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Demonstrating that medication management of EPL with mifepristone/misoprostol is less expensive and has increased QALYs associated with it is yet another reason to deregulate mifepristone so it can also be more accessible for management of EPL,” said Dr. Prager, who was not involved in the study.
Dr. Prager said she was not surprised by the findings, as effective medication should be less expensive than a procedure. “I would caution that the increased QALYs found in this study should not be interpreted as a reason to restrict surgical management of EPL but to increase access also to medication management, even though medication has a slightly lower rate of complete gestational sac expulsion,” she noted. “Mode of management should be up to the patient, unless there is a clear medical reason for one or the other.”
Going forward, “the FDA has it in its power to remove the REMS, which would immediately make mifepristone a medication that can be prescribed through a pharmacy and therefore much more available,” said Dr. Prager. “Restrictions for both medication and surgical management of EPL will likely increase in states where abortion is illegal, and it could possibly lead to patients having less choice as to mode of management,” she explained.
“There are many studies showing that all modes of EPL management are safe and effective and should be supported with respect to patient choice,” Dr. Prager noted. “There are also substantial data supporting the overall safety of mifepristone, and there are no scientific or medical data suggesting the REMS increases safety in any way. Frankly, there are no good, evidence-based reasons to continue to keep the REMS in place,” she said.
The study was supported by the National Institute of Child Health and Human Development of the National Institutes of Health and a Society of Family Planning Research Fund Midcareer Mentor Award. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the Editorial Advisory Board of Ob.Gyn. News.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
New insights into worldwide biliary tract cancer incidence, mortality
Incidence and mortality for biliary tract cancer (BTC) are both on the rise worldwide, according to a new analysis of data from the International Agency for Research on Cancer and the World Health Organization.
This diverse group of hepatic and perihepatic cancers include gallbladder cancer (GBC), intrahepatic and extrahepatic cholangiocarcinoma (ICC and ECC), and ampulla of Vater cancer. Although BTC is considered rare, incidence of its subtypes can vary significantly by geographic region. Because BTC is typically asymptomatic in its early stage, diagnosis is often made after tumors have spread, when there are few therapeutic options available. In the United States and Europe, 5-year survival is less than 20%.
Although previous studies have examined worldwide BTC incidence, few looked at multiple global regions or at all subtypes. Instead, subtypes may be grouped together and reported as composites, or BTC is lumped together with primary liver cancer. “To our knowledge, this is the first report combining data on worldwide incidence and mortality of all BTC subtypes per the International Classification of Diseases, Tenth Revision,” the authors wrote in the study, published online in Gastro Hep Advances.
The researchers pointed out that classification coding systems have improved at defining BTC subtypes, so that studies using older coding subtypes could cause misinterpretation of incidence rates.
BTC subtypes also have unique sets of risk factors and different prognoses and treatment outcomes. “Thus, there is a need to define accurate epidemiologic trends that will allow specific risk factors to be identified, guiding experts in implementing policies to improve diagnosis and survival,” the authors wrote.
The study included data from 22 countries. BTC incidence ranged from 1.12 cases per 100,000 person-years in Vietnam to 12.42 in Chile. As expected, incidence rates were higher in the Asia-Pacific region (1.12-9.00) and South America (2.73-12.42), compared with Europe (2.00-3.59) and North America (2.33-2.35). Within the United States, Asian Americans had a higher BTC incidence than the general population (2.99 vs. 2.33).
In most countries, new cases were dominated by GBC, while ICC was the most common cause of death.
In each country, older patients were 5-10 times more likely to die than BTC patients generally. The sixth and seventh decades of life are the most common time of diagnosis, and treatment options may be limited in older patients.
Risk factors for BTC may include common comorbidities like obesity, nonalcoholic fatty liver disease, and diabetes. Each is increasing individually, which may in turn contribute to rising BTC incidence. Observational analyses suggest that obesity may contribute to risk of ECC and gallbladder cancer, while diabetes and obesity may raise the risk of ICC. Smoking is associated with increased risk of all BTC subtypes except GBC, and alcohol consumption is associated with ICC.
“This study highlights how each subtype may be vulnerable to specific risk factors and emphasizes the value of separating epidemiologic data by subtype in order to better understand disease etiology,” the researchers wrote.
Risk factors associated with incidence and mortality from BTC aren’t limited to clinical characteristics. Genetic susceptibility may also play a role in incidence and mortality of different subtypes. There is also a relationship between gallstones and BTC risk. In Chile, about 50% of women have gallstones versus 17% of women in the United States. The cancer incidence is 27 per 100,000 person-years in Chile and 2 per 100,000 person-years in the United States. BTC is also the leading cause of cancer death among women in Chile.
The authors also highlighted the high rates of gallbladder cancer in India, despite a low prevalence of gallstones. Incidences can vary with geography along the flow of the Ganges River, which might reflect varying risks from contamination caused by agricultural runoff or industrial or human waste.
Worldwide BTC incidence and mortality was generally higher among women than men, with the exception of ampulla of Vater cancer, which was more common in men.
The study is limited by quality of data, which varied significantly between countries. Mortality data was missing from some countries know to have high BTC incidence. The databases had little survival data, which could have provided insights into treatment efficacy.
The study was funded by AstraZeneca. The authors have extensive financial relationships with pharmaceutical companies.
Biliary tract cancers (BTCs) are understudied malignancies with poor prognoses. A major impediment to a deeper understanding of BTC epidemiology is that the term BTC encompasses a heterogeneous group of cancers including cholangiocarcinoma (both intrahepatic and extrahepatic), as well as ampullary and gallbladder cancer. Studies have often lumped all BTC subgroups together despite differences in their geographic distribution, risk factors, and underlying pathogenesis. Furthermore, epidemiological reporting has often grouped “intrahepatic liver and bile duct cancers” which include hepatocellular carcinoma, a biologically different entity requiring a separate management strategy.
The study highlights the importance of future policy work to address the risk factors for BTCs that vary by region and that will likely evolve over time. It also stresses the urgent need for both early diagnostic strategies and improved biomarker-driven medical therapy, areas of ongoing research requiring accelerated development.
Irun Bhan, MD, is a transplant hepatologist at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. He has no relevant conflicts.
Biliary tract cancers (BTCs) are understudied malignancies with poor prognoses. A major impediment to a deeper understanding of BTC epidemiology is that the term BTC encompasses a heterogeneous group of cancers including cholangiocarcinoma (both intrahepatic and extrahepatic), as well as ampullary and gallbladder cancer. Studies have often lumped all BTC subgroups together despite differences in their geographic distribution, risk factors, and underlying pathogenesis. Furthermore, epidemiological reporting has often grouped “intrahepatic liver and bile duct cancers” which include hepatocellular carcinoma, a biologically different entity requiring a separate management strategy.
The study highlights the importance of future policy work to address the risk factors for BTCs that vary by region and that will likely evolve over time. It also stresses the urgent need for both early diagnostic strategies and improved biomarker-driven medical therapy, areas of ongoing research requiring accelerated development.
Irun Bhan, MD, is a transplant hepatologist at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. He has no relevant conflicts.
Biliary tract cancers (BTCs) are understudied malignancies with poor prognoses. A major impediment to a deeper understanding of BTC epidemiology is that the term BTC encompasses a heterogeneous group of cancers including cholangiocarcinoma (both intrahepatic and extrahepatic), as well as ampullary and gallbladder cancer. Studies have often lumped all BTC subgroups together despite differences in their geographic distribution, risk factors, and underlying pathogenesis. Furthermore, epidemiological reporting has often grouped “intrahepatic liver and bile duct cancers” which include hepatocellular carcinoma, a biologically different entity requiring a separate management strategy.
The study highlights the importance of future policy work to address the risk factors for BTCs that vary by region and that will likely evolve over time. It also stresses the urgent need for both early diagnostic strategies and improved biomarker-driven medical therapy, areas of ongoing research requiring accelerated development.
Irun Bhan, MD, is a transplant hepatologist at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. He has no relevant conflicts.
Incidence and mortality for biliary tract cancer (BTC) are both on the rise worldwide, according to a new analysis of data from the International Agency for Research on Cancer and the World Health Organization.
This diverse group of hepatic and perihepatic cancers include gallbladder cancer (GBC), intrahepatic and extrahepatic cholangiocarcinoma (ICC and ECC), and ampulla of Vater cancer. Although BTC is considered rare, incidence of its subtypes can vary significantly by geographic region. Because BTC is typically asymptomatic in its early stage, diagnosis is often made after tumors have spread, when there are few therapeutic options available. In the United States and Europe, 5-year survival is less than 20%.
Although previous studies have examined worldwide BTC incidence, few looked at multiple global regions or at all subtypes. Instead, subtypes may be grouped together and reported as composites, or BTC is lumped together with primary liver cancer. “To our knowledge, this is the first report combining data on worldwide incidence and mortality of all BTC subtypes per the International Classification of Diseases, Tenth Revision,” the authors wrote in the study, published online in Gastro Hep Advances.
The researchers pointed out that classification coding systems have improved at defining BTC subtypes, so that studies using older coding subtypes could cause misinterpretation of incidence rates.
BTC subtypes also have unique sets of risk factors and different prognoses and treatment outcomes. “Thus, there is a need to define accurate epidemiologic trends that will allow specific risk factors to be identified, guiding experts in implementing policies to improve diagnosis and survival,” the authors wrote.
The study included data from 22 countries. BTC incidence ranged from 1.12 cases per 100,000 person-years in Vietnam to 12.42 in Chile. As expected, incidence rates were higher in the Asia-Pacific region (1.12-9.00) and South America (2.73-12.42), compared with Europe (2.00-3.59) and North America (2.33-2.35). Within the United States, Asian Americans had a higher BTC incidence than the general population (2.99 vs. 2.33).
In most countries, new cases were dominated by GBC, while ICC was the most common cause of death.
In each country, older patients were 5-10 times more likely to die than BTC patients generally. The sixth and seventh decades of life are the most common time of diagnosis, and treatment options may be limited in older patients.
Risk factors for BTC may include common comorbidities like obesity, nonalcoholic fatty liver disease, and diabetes. Each is increasing individually, which may in turn contribute to rising BTC incidence. Observational analyses suggest that obesity may contribute to risk of ECC and gallbladder cancer, while diabetes and obesity may raise the risk of ICC. Smoking is associated with increased risk of all BTC subtypes except GBC, and alcohol consumption is associated with ICC.
“This study highlights how each subtype may be vulnerable to specific risk factors and emphasizes the value of separating epidemiologic data by subtype in order to better understand disease etiology,” the researchers wrote.
Risk factors associated with incidence and mortality from BTC aren’t limited to clinical characteristics. Genetic susceptibility may also play a role in incidence and mortality of different subtypes. There is also a relationship between gallstones and BTC risk. In Chile, about 50% of women have gallstones versus 17% of women in the United States. The cancer incidence is 27 per 100,000 person-years in Chile and 2 per 100,000 person-years in the United States. BTC is also the leading cause of cancer death among women in Chile.
The authors also highlighted the high rates of gallbladder cancer in India, despite a low prevalence of gallstones. Incidences can vary with geography along the flow of the Ganges River, which might reflect varying risks from contamination caused by agricultural runoff or industrial or human waste.
Worldwide BTC incidence and mortality was generally higher among women than men, with the exception of ampulla of Vater cancer, which was more common in men.
The study is limited by quality of data, which varied significantly between countries. Mortality data was missing from some countries know to have high BTC incidence. The databases had little survival data, which could have provided insights into treatment efficacy.
The study was funded by AstraZeneca. The authors have extensive financial relationships with pharmaceutical companies.
Incidence and mortality for biliary tract cancer (BTC) are both on the rise worldwide, according to a new analysis of data from the International Agency for Research on Cancer and the World Health Organization.
This diverse group of hepatic and perihepatic cancers include gallbladder cancer (GBC), intrahepatic and extrahepatic cholangiocarcinoma (ICC and ECC), and ampulla of Vater cancer. Although BTC is considered rare, incidence of its subtypes can vary significantly by geographic region. Because BTC is typically asymptomatic in its early stage, diagnosis is often made after tumors have spread, when there are few therapeutic options available. In the United States and Europe, 5-year survival is less than 20%.
Although previous studies have examined worldwide BTC incidence, few looked at multiple global regions or at all subtypes. Instead, subtypes may be grouped together and reported as composites, or BTC is lumped together with primary liver cancer. “To our knowledge, this is the first report combining data on worldwide incidence and mortality of all BTC subtypes per the International Classification of Diseases, Tenth Revision,” the authors wrote in the study, published online in Gastro Hep Advances.
The researchers pointed out that classification coding systems have improved at defining BTC subtypes, so that studies using older coding subtypes could cause misinterpretation of incidence rates.
BTC subtypes also have unique sets of risk factors and different prognoses and treatment outcomes. “Thus, there is a need to define accurate epidemiologic trends that will allow specific risk factors to be identified, guiding experts in implementing policies to improve diagnosis and survival,” the authors wrote.
The study included data from 22 countries. BTC incidence ranged from 1.12 cases per 100,000 person-years in Vietnam to 12.42 in Chile. As expected, incidence rates were higher in the Asia-Pacific region (1.12-9.00) and South America (2.73-12.42), compared with Europe (2.00-3.59) and North America (2.33-2.35). Within the United States, Asian Americans had a higher BTC incidence than the general population (2.99 vs. 2.33).
In most countries, new cases were dominated by GBC, while ICC was the most common cause of death.
In each country, older patients were 5-10 times more likely to die than BTC patients generally. The sixth and seventh decades of life are the most common time of diagnosis, and treatment options may be limited in older patients.
Risk factors for BTC may include common comorbidities like obesity, nonalcoholic fatty liver disease, and diabetes. Each is increasing individually, which may in turn contribute to rising BTC incidence. Observational analyses suggest that obesity may contribute to risk of ECC and gallbladder cancer, while diabetes and obesity may raise the risk of ICC. Smoking is associated with increased risk of all BTC subtypes except GBC, and alcohol consumption is associated with ICC.
“This study highlights how each subtype may be vulnerable to specific risk factors and emphasizes the value of separating epidemiologic data by subtype in order to better understand disease etiology,” the researchers wrote.
Risk factors associated with incidence and mortality from BTC aren’t limited to clinical characteristics. Genetic susceptibility may also play a role in incidence and mortality of different subtypes. There is also a relationship between gallstones and BTC risk. In Chile, about 50% of women have gallstones versus 17% of women in the United States. The cancer incidence is 27 per 100,000 person-years in Chile and 2 per 100,000 person-years in the United States. BTC is also the leading cause of cancer death among women in Chile.
The authors also highlighted the high rates of gallbladder cancer in India, despite a low prevalence of gallstones. Incidences can vary with geography along the flow of the Ganges River, which might reflect varying risks from contamination caused by agricultural runoff or industrial or human waste.
Worldwide BTC incidence and mortality was generally higher among women than men, with the exception of ampulla of Vater cancer, which was more common in men.
The study is limited by quality of data, which varied significantly between countries. Mortality data was missing from some countries know to have high BTC incidence. The databases had little survival data, which could have provided insights into treatment efficacy.
The study was funded by AstraZeneca. The authors have extensive financial relationships with pharmaceutical companies.
FROM GASTRO HEP ADVANCES
Childhood type 1 diabetes tests suggested at ages 2 and 6
, new data suggest.
Both genetic screening and islet-cell autoantibody screening for type 1 diabetes risk have become less expensive in recent years. Nonetheless, as of now, most children who receive such screening do so through programs that screen relatives of people who already have the condition, such as the global TrialNet program.
Some in the type 1 diabetes field have urged wider screening, with the rationale that knowledge of increased risk can prepare families to recognize the early signs of hyperglycemia and seek medical help to prevent the onset of diabetic ketoacidosis.
Moreover, potential therapies to prevent or delay type 1 diabetes are currently in development, including the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio).
However, given that the incidence of type 1 diabetes is about 1 in 300 children, any population-wide screening program would need to be implemented in the most efficient and cost-effective way possible with limited numbers of tests, say Mohamed Ghalwash, PhD, of the Center for Computational Health, IBM Research, Yorktown Heights, N.Y., and colleagues.
Results from their analysis of nearly 25,000 children from five prospective cohorts in Europe and the United States were published online in Lancet Diabetes & Endocrinology.
Screening in kids feasible, but may need geographic tweaking
“Our results show that initial screening for islet autoantibodies at two ages (2 years and 6 years) is sensitive and efficient for public health translation but might require adjustment by country on the basis of population-specific disease characteristics,” Dr. Ghalwash and colleagues write.
In an accompanying editorial, pediatric endocrinologist Maria J. Redondo, MD, PhD, writes: “This study is timely because recent successes in preventing type 1 diabetes highlight the need to identify the best candidates for intervention ... This paper constitutes an important contribution to the literature.”
However, Dr. Redondo, of Baylor College of Medicine and Texas Children’s Hospital, Houston, also cautioned: “It remains to be seen whether Dr. Ghalwash and colleagues’ strategy could work in the general population, because all the participants in the combined dataset had genetic risk factors for the disease or a relative with type 1 diabetes, in whom performance is expected to be higher.”
She also noted that most participants were of northern European ancestry and that it is unknown whether the same or a similar screening strategy could be applied to individuals older than 15 years, in whom preclinical type 1 diabetes progresses more slowly.
Two-time childhood screening yielded high sensitivity, specificity
The data from a total of 24,662 participants were pooled from five prospective cohorts from Finland (DIPP), Germany (BABYDIAB), Sweden (DiPiS), and the United States (DAISY and DEW-IT).
All were at elevated risk for type 1 diabetes based on human leukocyte antigen (HLA) genotyping, and some had first-degree relatives with the condition. Participants were screened annually for three type 1 diabetes–associated autoantibodies up to age 15 years or the onset of type 1 diabetes.
During follow-up, 672 children developed type 1 diabetes by age 15 years and 6,050 did not. (The rest hadn’t yet reached age 15 years or type 1 diabetes onset.) The median age at first appearance of islet autoantibodies was 4.5 years.
A two-age screening strategy at 2 years and 6 years was more sensitive than screening at just one age, with a sensitivity of 82% and a positive predictive value of 79% for the development of type 1 diabetes by age 15 years.
The predictive value increased with the number of autoantibodies tested. For example, a single islet autoantibody at age 2 years indicated a 4-year risk of developing type 1 diabetes by age 5.99 years of 31%, while multiple antibody positivity at age 2 years carried a 4-year risk of 55%.
By age 6 years, the risk over the next 9 years was 39% if the test had been negative at age 2 years and 70% if the test had been positive at 2 years. But overall, a 6-year-old with multiple autoantibodies had an overall 83% risk of type 1 diabetes regardless of the test result at 2 years.
The predictive performance of sensitivity by age differed by country, suggesting that the optimal ages for autoantibody testing might differ by geographic region, Dr. Ghalwash and colleagues say.
Dr. Redondo commented, “The model might require adaptation to local factors that affect the progression and prevalence of type 1 diabetes.” And, she added, “important aspects, such as screening cost, global access, acceptability, and follow-up support will need to be addressed for this strategy to be a viable public health option.”
The study was funded by JDRF. Dr. Ghalwash and another author are employees of IBM. A third author was a JDRF employee when the research was done and is now an employee of Janssen Research and Development. Dr. Redondo has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data suggest.
Both genetic screening and islet-cell autoantibody screening for type 1 diabetes risk have become less expensive in recent years. Nonetheless, as of now, most children who receive such screening do so through programs that screen relatives of people who already have the condition, such as the global TrialNet program.
Some in the type 1 diabetes field have urged wider screening, with the rationale that knowledge of increased risk can prepare families to recognize the early signs of hyperglycemia and seek medical help to prevent the onset of diabetic ketoacidosis.
Moreover, potential therapies to prevent or delay type 1 diabetes are currently in development, including the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio).
However, given that the incidence of type 1 diabetes is about 1 in 300 children, any population-wide screening program would need to be implemented in the most efficient and cost-effective way possible with limited numbers of tests, say Mohamed Ghalwash, PhD, of the Center for Computational Health, IBM Research, Yorktown Heights, N.Y., and colleagues.
Results from their analysis of nearly 25,000 children from five prospective cohorts in Europe and the United States were published online in Lancet Diabetes & Endocrinology.
Screening in kids feasible, but may need geographic tweaking
“Our results show that initial screening for islet autoantibodies at two ages (2 years and 6 years) is sensitive and efficient for public health translation but might require adjustment by country on the basis of population-specific disease characteristics,” Dr. Ghalwash and colleagues write.
In an accompanying editorial, pediatric endocrinologist Maria J. Redondo, MD, PhD, writes: “This study is timely because recent successes in preventing type 1 diabetes highlight the need to identify the best candidates for intervention ... This paper constitutes an important contribution to the literature.”
However, Dr. Redondo, of Baylor College of Medicine and Texas Children’s Hospital, Houston, also cautioned: “It remains to be seen whether Dr. Ghalwash and colleagues’ strategy could work in the general population, because all the participants in the combined dataset had genetic risk factors for the disease or a relative with type 1 diabetes, in whom performance is expected to be higher.”
She also noted that most participants were of northern European ancestry and that it is unknown whether the same or a similar screening strategy could be applied to individuals older than 15 years, in whom preclinical type 1 diabetes progresses more slowly.
Two-time childhood screening yielded high sensitivity, specificity
The data from a total of 24,662 participants were pooled from five prospective cohorts from Finland (DIPP), Germany (BABYDIAB), Sweden (DiPiS), and the United States (DAISY and DEW-IT).
All were at elevated risk for type 1 diabetes based on human leukocyte antigen (HLA) genotyping, and some had first-degree relatives with the condition. Participants were screened annually for three type 1 diabetes–associated autoantibodies up to age 15 years or the onset of type 1 diabetes.
During follow-up, 672 children developed type 1 diabetes by age 15 years and 6,050 did not. (The rest hadn’t yet reached age 15 years or type 1 diabetes onset.) The median age at first appearance of islet autoantibodies was 4.5 years.
A two-age screening strategy at 2 years and 6 years was more sensitive than screening at just one age, with a sensitivity of 82% and a positive predictive value of 79% for the development of type 1 diabetes by age 15 years.
The predictive value increased with the number of autoantibodies tested. For example, a single islet autoantibody at age 2 years indicated a 4-year risk of developing type 1 diabetes by age 5.99 years of 31%, while multiple antibody positivity at age 2 years carried a 4-year risk of 55%.
By age 6 years, the risk over the next 9 years was 39% if the test had been negative at age 2 years and 70% if the test had been positive at 2 years. But overall, a 6-year-old with multiple autoantibodies had an overall 83% risk of type 1 diabetes regardless of the test result at 2 years.
The predictive performance of sensitivity by age differed by country, suggesting that the optimal ages for autoantibody testing might differ by geographic region, Dr. Ghalwash and colleagues say.
Dr. Redondo commented, “The model might require adaptation to local factors that affect the progression and prevalence of type 1 diabetes.” And, she added, “important aspects, such as screening cost, global access, acceptability, and follow-up support will need to be addressed for this strategy to be a viable public health option.”
The study was funded by JDRF. Dr. Ghalwash and another author are employees of IBM. A third author was a JDRF employee when the research was done and is now an employee of Janssen Research and Development. Dr. Redondo has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data suggest.
Both genetic screening and islet-cell autoantibody screening for type 1 diabetes risk have become less expensive in recent years. Nonetheless, as of now, most children who receive such screening do so through programs that screen relatives of people who already have the condition, such as the global TrialNet program.
Some in the type 1 diabetes field have urged wider screening, with the rationale that knowledge of increased risk can prepare families to recognize the early signs of hyperglycemia and seek medical help to prevent the onset of diabetic ketoacidosis.
Moreover, potential therapies to prevent or delay type 1 diabetes are currently in development, including the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio).
However, given that the incidence of type 1 diabetes is about 1 in 300 children, any population-wide screening program would need to be implemented in the most efficient and cost-effective way possible with limited numbers of tests, say Mohamed Ghalwash, PhD, of the Center for Computational Health, IBM Research, Yorktown Heights, N.Y., and colleagues.
Results from their analysis of nearly 25,000 children from five prospective cohorts in Europe and the United States were published online in Lancet Diabetes & Endocrinology.
Screening in kids feasible, but may need geographic tweaking
“Our results show that initial screening for islet autoantibodies at two ages (2 years and 6 years) is sensitive and efficient for public health translation but might require adjustment by country on the basis of population-specific disease characteristics,” Dr. Ghalwash and colleagues write.
In an accompanying editorial, pediatric endocrinologist Maria J. Redondo, MD, PhD, writes: “This study is timely because recent successes in preventing type 1 diabetes highlight the need to identify the best candidates for intervention ... This paper constitutes an important contribution to the literature.”
However, Dr. Redondo, of Baylor College of Medicine and Texas Children’s Hospital, Houston, also cautioned: “It remains to be seen whether Dr. Ghalwash and colleagues’ strategy could work in the general population, because all the participants in the combined dataset had genetic risk factors for the disease or a relative with type 1 diabetes, in whom performance is expected to be higher.”
She also noted that most participants were of northern European ancestry and that it is unknown whether the same or a similar screening strategy could be applied to individuals older than 15 years, in whom preclinical type 1 diabetes progresses more slowly.
Two-time childhood screening yielded high sensitivity, specificity
The data from a total of 24,662 participants were pooled from five prospective cohorts from Finland (DIPP), Germany (BABYDIAB), Sweden (DiPiS), and the United States (DAISY and DEW-IT).
All were at elevated risk for type 1 diabetes based on human leukocyte antigen (HLA) genotyping, and some had first-degree relatives with the condition. Participants were screened annually for three type 1 diabetes–associated autoantibodies up to age 15 years or the onset of type 1 diabetes.
During follow-up, 672 children developed type 1 diabetes by age 15 years and 6,050 did not. (The rest hadn’t yet reached age 15 years or type 1 diabetes onset.) The median age at first appearance of islet autoantibodies was 4.5 years.
A two-age screening strategy at 2 years and 6 years was more sensitive than screening at just one age, with a sensitivity of 82% and a positive predictive value of 79% for the development of type 1 diabetes by age 15 years.
The predictive value increased with the number of autoantibodies tested. For example, a single islet autoantibody at age 2 years indicated a 4-year risk of developing type 1 diabetes by age 5.99 years of 31%, while multiple antibody positivity at age 2 years carried a 4-year risk of 55%.
By age 6 years, the risk over the next 9 years was 39% if the test had been negative at age 2 years and 70% if the test had been positive at 2 years. But overall, a 6-year-old with multiple autoantibodies had an overall 83% risk of type 1 diabetes regardless of the test result at 2 years.
The predictive performance of sensitivity by age differed by country, suggesting that the optimal ages for autoantibody testing might differ by geographic region, Dr. Ghalwash and colleagues say.
Dr. Redondo commented, “The model might require adaptation to local factors that affect the progression and prevalence of type 1 diabetes.” And, she added, “important aspects, such as screening cost, global access, acceptability, and follow-up support will need to be addressed for this strategy to be a viable public health option.”
The study was funded by JDRF. Dr. Ghalwash and another author are employees of IBM. A third author was a JDRF employee when the research was done and is now an employee of Janssen Research and Development. Dr. Redondo has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM LANCET DIABETES & ENDOCRINOLOGY
Social media in the lives of adolescents
Adolescence is a time of growing autonomy fueled by puberty, intellectual development, and identity formation. Social media engages adolescents by giving them easy access to (semi) private communication with peers, the ability to safely explore their sexuality, and easily investigate issues of intellectual curiosity, as they move from childhood to older adolescence. Social media facilitates the creation of a teenager’s own world, separate and distinct from adult concern or scrutiny. It is clearly compelling for adolescents, but we are in the early days of understanding the effect of various types of digital activities on the health and well-being of youth. There is evidence that for some, the addictive potential of these applications is potent, exacerbating or triggering mood, anxiety, and eating disorder symptoms. Their drive to explore their identity and relationships and their immature capacity to regulate emotions and behaviors make the risks of overuse substantial. But it would be impossible (and probably socially very costly) to simply avoid social media. So how to discuss its healthy use with your patients and their parents?
The data
Social media are digital communication platforms that allow users to build a public profile and then accumulate a network of followers, and follow other users, based on shared interests. They include FaceBook, Instagram, Snapchat, YouTube, and Twitter. Surveys demonstrated that 90% of U.S. adolescents use social media, with 75% having at least one social media profile and over half visiting social media sites at least once daily. Adolescents spend over 7 hours daily on their phones, not including time devoted to online schoolwork, and 8- to 12-year-olds are not far behind at almost 5 hours of daily phone use. On average, 39% of adolescent screen time is spent on passive consumption, 26% on social media, 25% on interactive activities (browsing the web, interactive video gaming) and 3% on content creation (coding, etc). There was considerable variability in survey results, and differences between genders, with boys engaged in video games almost eight times as often as girls, and girls in social media nearly twice as often as boys.1
The research
There is a growing body of research devoted to understanding the effects of all of this digital activity on youth health and well-being.
A large, longitudinal study of Canadian 13- to 17-year-olds found that time spent on social media or watching television was strongly associated with depressive and anxiety symptoms, with a robust dose-response relationship.2 However, causality is not clear, as anxious, shy, and depressed adolescents may use more social media as a consequence of their mood. Interestingly, there was no such relationship with mood and anxiety symptoms and time spent on video games.3 For youth with depression and anxiety, time spent on social media has been strongly associated with increased levels of self-reported distress, self-injury and suicidality, but again, causality is hard to prove.
One very large study from the United Kingdom (including more than 10,000 participants), demonstrated a strong relationship between time spent on social media and severity of depressive symptoms, with a more pronounced effect in girls than in boys.4 Many more nuanced studies have demonstrated that excessive time spent on social media, the presence of an addictive pattern of use, and the degree to which an adolescent’s sense of well-being is connected to social media are the variables that strongly predict an association with worsening depressive or anxiety symptoms.5
Several studies have demonstrated that low to moderate use of social media, and use to gather information and make plans were associated with better scores of emotional self-regulation and lower rates of depressive symptoms in teens.6 It seems safe to say that social media can be useful and fun, but that too much can be bad for you. So help your adolescent patients to expand their perspective on its use by discussing it with them.
Make them curious about quantity
Most teens feel they do not have enough time for all of the things they need to do, so invite them to play detective by using their phone’s applications that can track their time spent online and in different apps.
Remind them that these apps were designed to be so engaging that for some addiction is a real problem. As with tobacco, addiction is the business model by which these companies earn advertising dollars. Indeed, adolescents are the target demographic, as they are most sensitive to social rewards and are the most valuable audience for advertisers. Engage their natural suspicion of authority by pointing out that with every hour on Insta, someone else is making a lot of money. They get to choose how they want to relax, connect with friends, and explore the world, so help them to be aware of how these apps are designed to keep them from choosing.
Raise awareness of vulnerability
Adolescents who have attention-deficit/hyperactivity disorder already have difficulty with impulse control and with shifting their attention to less engaging activities. Adolescents with anxiety are prone to avoid stressful situations, but still hunger for knowledge and connections. Adolescents with depression are managing low motivation and self-esteem, and the rewards of social media may keep them from exercise and actual social engagement that are critical to their treatment. Youth with eating disorders are especially prone to critical comparison of themselves to others, feeding their distorted body images. Help your patients with these common illnesses to be aware of how social media may make their treatment harder, rather than being the source of relief it may feel like.
Protect their health
For all young people, too much time spent in virtual activities and passive media consumption may not leave enough time to explore potential interests, talents, or relationships. These are important activities throughout life, but they are the central developmental tasks of adolescence. They also need 8-10 hours of sleep nightly and regular exercise. And of course, they have homework! Help them to think about how to use their time wisely to support satisfying relationships and activities, with time for relaxation and good health.
Keep parents in the room for these discussions
State that most of us have difficulty putting down our phones. Children and teens need adults who model striving for balance in all areas of choice. Just as we try to teach them to make good choices about food, getting excellent nutrition while still valuing taste and pleasure, we can talk about how to balance virtual activities with actual activities, work with play, and effort with relaxation. You can help expand your young patients’ self-awareness, acknowledge the fun and utility of their digital time, and enhance their sense of how we must all learn how to put screens down sometimes. In so doing, you can help families to ensure that they are engaging with the digital tools and toys available to all of us in ways that can support their health and well-being.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
References
1. Geena Davis Institute on Gender and Media. The Common Sense Census: Media Use by Teens and Tweens, 2015.
2. Abi-Jaoude E et al. CMAJ 2020;192(6):E136-41.
3. Boers E et al. Can J Psychiatry. 2020 Mar;65(3):206-8.
4. Kelly Y et al. EClinicalMedicine. 2019 Jan 4;6:59-68.
5. Vidal C et al. Int Rev Psychiatry. 2020 May;32(3):235-53.
6. Coyne SM et al. J Res Adolescence. 2019;29(4):897-907.
Adolescence is a time of growing autonomy fueled by puberty, intellectual development, and identity formation. Social media engages adolescents by giving them easy access to (semi) private communication with peers, the ability to safely explore their sexuality, and easily investigate issues of intellectual curiosity, as they move from childhood to older adolescence. Social media facilitates the creation of a teenager’s own world, separate and distinct from adult concern or scrutiny. It is clearly compelling for adolescents, but we are in the early days of understanding the effect of various types of digital activities on the health and well-being of youth. There is evidence that for some, the addictive potential of these applications is potent, exacerbating or triggering mood, anxiety, and eating disorder symptoms. Their drive to explore their identity and relationships and their immature capacity to regulate emotions and behaviors make the risks of overuse substantial. But it would be impossible (and probably socially very costly) to simply avoid social media. So how to discuss its healthy use with your patients and their parents?
The data
Social media are digital communication platforms that allow users to build a public profile and then accumulate a network of followers, and follow other users, based on shared interests. They include FaceBook, Instagram, Snapchat, YouTube, and Twitter. Surveys demonstrated that 90% of U.S. adolescents use social media, with 75% having at least one social media profile and over half visiting social media sites at least once daily. Adolescents spend over 7 hours daily on their phones, not including time devoted to online schoolwork, and 8- to 12-year-olds are not far behind at almost 5 hours of daily phone use. On average, 39% of adolescent screen time is spent on passive consumption, 26% on social media, 25% on interactive activities (browsing the web, interactive video gaming) and 3% on content creation (coding, etc). There was considerable variability in survey results, and differences between genders, with boys engaged in video games almost eight times as often as girls, and girls in social media nearly twice as often as boys.1
The research
There is a growing body of research devoted to understanding the effects of all of this digital activity on youth health and well-being.
A large, longitudinal study of Canadian 13- to 17-year-olds found that time spent on social media or watching television was strongly associated with depressive and anxiety symptoms, with a robust dose-response relationship.2 However, causality is not clear, as anxious, shy, and depressed adolescents may use more social media as a consequence of their mood. Interestingly, there was no such relationship with mood and anxiety symptoms and time spent on video games.3 For youth with depression and anxiety, time spent on social media has been strongly associated with increased levels of self-reported distress, self-injury and suicidality, but again, causality is hard to prove.
One very large study from the United Kingdom (including more than 10,000 participants), demonstrated a strong relationship between time spent on social media and severity of depressive symptoms, with a more pronounced effect in girls than in boys.4 Many more nuanced studies have demonstrated that excessive time spent on social media, the presence of an addictive pattern of use, and the degree to which an adolescent’s sense of well-being is connected to social media are the variables that strongly predict an association with worsening depressive or anxiety symptoms.5
Several studies have demonstrated that low to moderate use of social media, and use to gather information and make plans were associated with better scores of emotional self-regulation and lower rates of depressive symptoms in teens.6 It seems safe to say that social media can be useful and fun, but that too much can be bad for you. So help your adolescent patients to expand their perspective on its use by discussing it with them.
Make them curious about quantity
Most teens feel they do not have enough time for all of the things they need to do, so invite them to play detective by using their phone’s applications that can track their time spent online and in different apps.
Remind them that these apps were designed to be so engaging that for some addiction is a real problem. As with tobacco, addiction is the business model by which these companies earn advertising dollars. Indeed, adolescents are the target demographic, as they are most sensitive to social rewards and are the most valuable audience for advertisers. Engage their natural suspicion of authority by pointing out that with every hour on Insta, someone else is making a lot of money. They get to choose how they want to relax, connect with friends, and explore the world, so help them to be aware of how these apps are designed to keep them from choosing.
Raise awareness of vulnerability
Adolescents who have attention-deficit/hyperactivity disorder already have difficulty with impulse control and with shifting their attention to less engaging activities. Adolescents with anxiety are prone to avoid stressful situations, but still hunger for knowledge and connections. Adolescents with depression are managing low motivation and self-esteem, and the rewards of social media may keep them from exercise and actual social engagement that are critical to their treatment. Youth with eating disorders are especially prone to critical comparison of themselves to others, feeding their distorted body images. Help your patients with these common illnesses to be aware of how social media may make their treatment harder, rather than being the source of relief it may feel like.
Protect their health
For all young people, too much time spent in virtual activities and passive media consumption may not leave enough time to explore potential interests, talents, or relationships. These are important activities throughout life, but they are the central developmental tasks of adolescence. They also need 8-10 hours of sleep nightly and regular exercise. And of course, they have homework! Help them to think about how to use their time wisely to support satisfying relationships and activities, with time for relaxation and good health.
Keep parents in the room for these discussions
State that most of us have difficulty putting down our phones. Children and teens need adults who model striving for balance in all areas of choice. Just as we try to teach them to make good choices about food, getting excellent nutrition while still valuing taste and pleasure, we can talk about how to balance virtual activities with actual activities, work with play, and effort with relaxation. You can help expand your young patients’ self-awareness, acknowledge the fun and utility of their digital time, and enhance their sense of how we must all learn how to put screens down sometimes. In so doing, you can help families to ensure that they are engaging with the digital tools and toys available to all of us in ways that can support their health and well-being.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
References
1. Geena Davis Institute on Gender and Media. The Common Sense Census: Media Use by Teens and Tweens, 2015.
2. Abi-Jaoude E et al. CMAJ 2020;192(6):E136-41.
3. Boers E et al. Can J Psychiatry. 2020 Mar;65(3):206-8.
4. Kelly Y et al. EClinicalMedicine. 2019 Jan 4;6:59-68.
5. Vidal C et al. Int Rev Psychiatry. 2020 May;32(3):235-53.
6. Coyne SM et al. J Res Adolescence. 2019;29(4):897-907.
Adolescence is a time of growing autonomy fueled by puberty, intellectual development, and identity formation. Social media engages adolescents by giving them easy access to (semi) private communication with peers, the ability to safely explore their sexuality, and easily investigate issues of intellectual curiosity, as they move from childhood to older adolescence. Social media facilitates the creation of a teenager’s own world, separate and distinct from adult concern or scrutiny. It is clearly compelling for adolescents, but we are in the early days of understanding the effect of various types of digital activities on the health and well-being of youth. There is evidence that for some, the addictive potential of these applications is potent, exacerbating or triggering mood, anxiety, and eating disorder symptoms. Their drive to explore their identity and relationships and their immature capacity to regulate emotions and behaviors make the risks of overuse substantial. But it would be impossible (and probably socially very costly) to simply avoid social media. So how to discuss its healthy use with your patients and their parents?
The data
Social media are digital communication platforms that allow users to build a public profile and then accumulate a network of followers, and follow other users, based on shared interests. They include FaceBook, Instagram, Snapchat, YouTube, and Twitter. Surveys demonstrated that 90% of U.S. adolescents use social media, with 75% having at least one social media profile and over half visiting social media sites at least once daily. Adolescents spend over 7 hours daily on their phones, not including time devoted to online schoolwork, and 8- to 12-year-olds are not far behind at almost 5 hours of daily phone use. On average, 39% of adolescent screen time is spent on passive consumption, 26% on social media, 25% on interactive activities (browsing the web, interactive video gaming) and 3% on content creation (coding, etc). There was considerable variability in survey results, and differences between genders, with boys engaged in video games almost eight times as often as girls, and girls in social media nearly twice as often as boys.1
The research
There is a growing body of research devoted to understanding the effects of all of this digital activity on youth health and well-being.
A large, longitudinal study of Canadian 13- to 17-year-olds found that time spent on social media or watching television was strongly associated with depressive and anxiety symptoms, with a robust dose-response relationship.2 However, causality is not clear, as anxious, shy, and depressed adolescents may use more social media as a consequence of their mood. Interestingly, there was no such relationship with mood and anxiety symptoms and time spent on video games.3 For youth with depression and anxiety, time spent on social media has been strongly associated with increased levels of self-reported distress, self-injury and suicidality, but again, causality is hard to prove.
One very large study from the United Kingdom (including more than 10,000 participants), demonstrated a strong relationship between time spent on social media and severity of depressive symptoms, with a more pronounced effect in girls than in boys.4 Many more nuanced studies have demonstrated that excessive time spent on social media, the presence of an addictive pattern of use, and the degree to which an adolescent’s sense of well-being is connected to social media are the variables that strongly predict an association with worsening depressive or anxiety symptoms.5
Several studies have demonstrated that low to moderate use of social media, and use to gather information and make plans were associated with better scores of emotional self-regulation and lower rates of depressive symptoms in teens.6 It seems safe to say that social media can be useful and fun, but that too much can be bad for you. So help your adolescent patients to expand their perspective on its use by discussing it with them.
Make them curious about quantity
Most teens feel they do not have enough time for all of the things they need to do, so invite them to play detective by using their phone’s applications that can track their time spent online and in different apps.
Remind them that these apps were designed to be so engaging that for some addiction is a real problem. As with tobacco, addiction is the business model by which these companies earn advertising dollars. Indeed, adolescents are the target demographic, as they are most sensitive to social rewards and are the most valuable audience for advertisers. Engage their natural suspicion of authority by pointing out that with every hour on Insta, someone else is making a lot of money. They get to choose how they want to relax, connect with friends, and explore the world, so help them to be aware of how these apps are designed to keep them from choosing.
Raise awareness of vulnerability
Adolescents who have attention-deficit/hyperactivity disorder already have difficulty with impulse control and with shifting their attention to less engaging activities. Adolescents with anxiety are prone to avoid stressful situations, but still hunger for knowledge and connections. Adolescents with depression are managing low motivation and self-esteem, and the rewards of social media may keep them from exercise and actual social engagement that are critical to their treatment. Youth with eating disorders are especially prone to critical comparison of themselves to others, feeding their distorted body images. Help your patients with these common illnesses to be aware of how social media may make their treatment harder, rather than being the source of relief it may feel like.
Protect their health
For all young people, too much time spent in virtual activities and passive media consumption may not leave enough time to explore potential interests, talents, or relationships. These are important activities throughout life, but they are the central developmental tasks of adolescence. They also need 8-10 hours of sleep nightly and regular exercise. And of course, they have homework! Help them to think about how to use their time wisely to support satisfying relationships and activities, with time for relaxation and good health.
Keep parents in the room for these discussions
State that most of us have difficulty putting down our phones. Children and teens need adults who model striving for balance in all areas of choice. Just as we try to teach them to make good choices about food, getting excellent nutrition while still valuing taste and pleasure, we can talk about how to balance virtual activities with actual activities, work with play, and effort with relaxation. You can help expand your young patients’ self-awareness, acknowledge the fun and utility of their digital time, and enhance their sense of how we must all learn how to put screens down sometimes. In so doing, you can help families to ensure that they are engaging with the digital tools and toys available to all of us in ways that can support their health and well-being.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
References
1. Geena Davis Institute on Gender and Media. The Common Sense Census: Media Use by Teens and Tweens, 2015.
2. Abi-Jaoude E et al. CMAJ 2020;192(6):E136-41.
3. Boers E et al. Can J Psychiatry. 2020 Mar;65(3):206-8.
4. Kelly Y et al. EClinicalMedicine. 2019 Jan 4;6:59-68.
5. Vidal C et al. Int Rev Psychiatry. 2020 May;32(3):235-53.
6. Coyne SM et al. J Res Adolescence. 2019;29(4):897-907.
Transplanted pig hearts functioned normally in deceased persons on ventilator support
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
Pain and photophobia
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 48-year-old male patient presents with blurred vision, pain, and photophobia. He recently had a routine visit with an ophthalmologist, which was normal. The affected pupil appears irregular in shape. The anterior chamber appears foggy. Local ciliary flush is observed on slit lamp exam. The physical examination is also notable for axial arthropathy. The patient has an 11-year history of moderate to severe psoriatic arthritis (PsA) which he typically manages with methotrexate (MTX) therapy, to which he has had a partial response. He was initially diagnosed when he presented with worsening psoriasis and enthesitis on the insertion sites of the plantar fascia, as well as dactylitis.