User login
To scan or not to scan: Routine ultrasonography is not necessary after medication management of early pregnancy loss
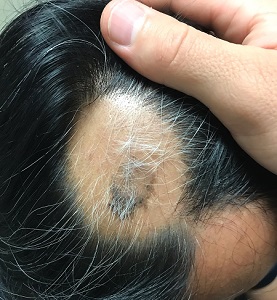
CASE Patient finds that follow-up ultrasonography is burdensome
Ms. MB presents to the clinic for dating ultrasonography and is diagnosed with a missed abortion measuring 7 weeks. After reviewing her management options, she elects for medication management. She receives mifepristone 200 mg and misoprostol 800 µg, with a plan to follow-up in clinic for repeat ultrasonography in a week. The day of her follow-up appointment, there is a large snowstorm. She calls her care team to ask if she needs to have a follow-up visit, as she is certain she has passed tissue and her bleeding is now minimal. She is told, however, that a follow-up ultrasonography is required, per clinic policy, to ensure successful management. Despite Ms. MB’s grief and the difficult travel conditions, she makes the arduous journey back to the clinic to complete the ultrasound.
Do all patients need an ultrasound after medication management of early pregnancy loss? Or is there an alternative follow-up option?
Early pregnancy loss (EPL) is a common pregnancy complication, and its management is a routine part of reproductive health care. In the clinically stable patient, EPL may be managed expectantly, surgically, or medically, based on the patient’s preference. For patients who select medication management, clear evidence supports that a combination regimen of mifepristone and misoprostol is more effective than treatment with misoprostol alone.1,2 The data suggest that 91% of patients will experience expulsion of the gestational sac by 30 days with medication management.1 Because a minority of patients will have a retained gestational sac despite medication therapy, follow-up ensures complete expulsion of pregnancy tissue.
In the United States, most follow-up protocols include an ultrasound examination, which often entails transvaginal ultrasonography. Returning to clinic for an additional ultrasound may be costly and inconvenient—and during a global pandemic medically risky. Further, it may undermine a fundamental principle in management of EPL: autonomy. Many patients who select medication management do so out of a desire to minimize interventions or procedures. Follow-up protocols that align with patient preferences for fewer interventions are critically important to the provision of patient-centered care. Additionally, the COVID-19 pandemic highlights the value of offering an alternative follow-up strategy that minimizes the need for additional visits to a clinic or hospital.
Lessons from medication abortion management
In many ways, follow-up after medication management of EPL is analogous to follow-up after medication abortion. In both cases, the goal of follow-up is to ensure that complete expulsion has occurred without complication and to identify patients with incomplete expulsion of pregnancy tissue who may benefit from further treatment with additional medication or uterine aspiration. A key difference in the management of EPL is that there is no concern for ongoing pregnancy.
Historically, follow-up transvaginal ultrasonography was routinely performed after medication abortion to ensure complete expulsion of pregnancy.3 However, requiring patients to return to a health care facility for ultrasonography after abortion can be burdensome, both for patients and clinicians. To provide more accessible, patient-centered care, researchers have investigated alternative follow-up strategies for medication abortion that remove the necessity for ultrasonography. Guidelines from both the National Abortion Federation and the American College of Obstetricians and Gynecologists state that routine ultrasonography is not necessary after medication abortion.4,5
Quantitative serum human chorionic gonadotropin (hCG) testing before treatment and at a follow-up visit is one reasonable strategy to ensure successful treatment. In one study of medication abortion patients, an 80% decrease in serum hCG was predictive of complete expulsion in 98.5% of patients.6 While this strategy avoids ultrasonography, it still necessitates a visit to a health care facility for a blood draw. As an alternative, substantial evidence now demonstrates the safety and feasibility of using a combination of clinical symptoms and urine pregnancy testing to confirm completed medication abortion. The evidence for follow-up using a combination of clinical symptoms and urine pregnancy testing is discussed below.
Continue to: Symptoms...
Symptoms. An assessment of symptoms alone, by the patient or clinician, is an important indicator of treatment success and can be completed easily via telephone. In one study of medication abortion with mifepristone and misoprostol, patients correctly predicted passage of a gestational sac 85% of the time based on symptoms alone.7 In another study, the combined clinical assessment from the patient and the clinician had a sensitivity of 96% and a specificity of 67% for predicting complete pregnancy expulsion.8 Finally, in an analysis of 931 patients after medication abortion, when both the patient and clinician believed that the gestational sac had passed, ultrasonography demonstrated complete expulsion 99% of the time.9
Urine pregnancy testing. Several studies have demonstrated that the addition of urine pregnancy testing to a clinical assessment of symptoms is a safe and effective follow-up strategy in medication abortion. Contemporary over-the-counter pregnancy tests are high-sensitivity tests that have an hCG detection threshold of 25 to 50 mIU/mL. As these tests are widely and commercially available in the United States, they can be a useful tool in follow-up strategies.
In a study by Chen and colleagues, patients seeking medication abortion were offered a choice of follow-up with ultrasonography at 1 week versus a combination of a 1-week phone call and a 4-week high-sensitivity urine pregnancy test. In this study, approximately 40% of patients opted for phone follow-up. The rates of incomplete abortion and loss to follow-up were similar between the 2 groups, highlighting the significant number of individuals interested in alternative models of follow-up and the efficacy of phone and urine testing specifically.10
In another study that evaluated the feasibility of a telephone and urine testing follow-up strategy, 97% of patients completed follow-up and all continuing pregnancies were diagnosed prior to the 4-week urine pregnancy test.8
In a hospital in Edinburgh, where a telephone-based symptom assessment in combination with a 2-week low-sensitivity pregnancy test (hCG detection threshold of 2,000 mIU/mL; not commercially available in the United States) is the standard of care for medication abortion follow-up, Michie and Cameron reported a sensitivity of 100% and a specificity of 88% to detect ongoing pregnancies.11
Taken together, these data demonstrate that a combination of symptom assessment via telephone and home urine pregnancy testing (in addition to standard patient instructions and return precautions) is an appropriate strategy for medication abortion follow-up, and they suggest that similar strategies can be employed in the medication management of EPL.
To scan or not to scan?
Many published studies of EPL have used ultrasonography to confirm complete expulsion of pregnancy tissue; however, others have relied on either clinical evaluation or urine pregnancy testing to determine treatment success, using ultrasonography only as clinically indicated.12-14 In their evaluation of medication management versus surgical management of miscarriage, Niinimäki and colleagues performed urine hCG testing at a 5- to 6-week follow-up visit to determine treatment success; ultrasonography was obtained only if the urine hCG test was positive. They demonstrated a treatment success rate of 90% with mifepristone and misoprostol treatment,12 congruent with previously published results.
While a follow-up ultrasound scan may be helpful to accurately assess treatment efficacy in research protocols, it should not be considered necessary in clinical practice. Posttreatment imaging in an asymptomatic patient may place additional burden on the patient and health care system and may result in unnecessary intervention. Although treatment success is reliably defined by the absence of a gestational sac,15,16 the finding of a thickened endometrium or presence of vascularity may result in the patient receiving an unnecessary aspiration or other intervention.
The evidence from the medication abortion literature suggests that a combination of a 1-week telephone call to assess patient symptoms in addition to a 4-week high-sensitivity pregnancy test is a reasonable alternative follow-up strategy. A similar strategy is already used in the United Kingdom, where current National Institute for Health and Care Excellence guidelines for follow-up after medication management of EPL recommend home pregnancy testing in 3 weeks unless the patient experiences worsening pain or bleeding symptoms.17
Time to rethink follow-up care
Follow-up care for EPL should be provided in a way that is sensitive to the needs and preferences of the patient and, if desired, minimizes additional health care visits, testing, or procedures. While some patients may prefer ultrasonography follow-up, it is important for the clinician to recognize that there are safe and effective alternatives. Patient preference guides the choice of EPL management; this logic extends to follow-up strategies. As we strive to provide evidence-based, patient-centered EPL care, there is no need for universal follow-up ultrasonography. ●
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LF, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Benson J, Clark KA, Gerhardt A, et al. Early abortion services in the United States: a provider survey. Contraception. 2003;67:287-294.
- Medication abortion up to 70 days of gestation: ACOG practice bulletin summary, No. 225. Obstet Gynecol. 2020;136:855-858.
- National Abortion Federation. 2020 Clinical Policy Guidelines for Abortion Care. Washington, DC; 2020. https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna -ssl.com/wp-content/uploads/2020_CPGs.pdf. Accessed July 19, 2021.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190-195.
- Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception. 2001;64:87-92.
- Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143-149.
- Rossi B, Creinin MD, Meyn LA. Ability of the clinician and patient to predict the outcome of mifepristone and misoprostol medical abortion. Contraception. 2004;70:313-317.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89:440-445.
- Niinimäki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367- 372.
- Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol. 2005;106:540-547.
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563-566.
- Reeves MF, Lohr PA, Harwood B, et al. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstet Gynecol. 2008;111:106-112.
- Reeves MF, Fox MC, Lohr PA, et al. Endometrial thickness following medical abortion is not predictive of subsequent surgical intervention. Ultrasound Obstet Gynecol. 2009;34:104-109.
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guideline NG126. April 17, 2019. ttps:// www.nice.org.uk/guidance/ng126/chapter/Recommen dations#management-of-miscarriage. Accessed July 19, 2021.
CASE Patient finds that follow-up ultrasonography is burdensome
Ms. MB presents to the clinic for dating ultrasonography and is diagnosed with a missed abortion measuring 7 weeks. After reviewing her management options, she elects for medication management. She receives mifepristone 200 mg and misoprostol 800 µg, with a plan to follow-up in clinic for repeat ultrasonography in a week. The day of her follow-up appointment, there is a large snowstorm. She calls her care team to ask if she needs to have a follow-up visit, as she is certain she has passed tissue and her bleeding is now minimal. She is told, however, that a follow-up ultrasonography is required, per clinic policy, to ensure successful management. Despite Ms. MB’s grief and the difficult travel conditions, she makes the arduous journey back to the clinic to complete the ultrasound.
Do all patients need an ultrasound after medication management of early pregnancy loss? Or is there an alternative follow-up option?
Early pregnancy loss (EPL) is a common pregnancy complication, and its management is a routine part of reproductive health care. In the clinically stable patient, EPL may be managed expectantly, surgically, or medically, based on the patient’s preference. For patients who select medication management, clear evidence supports that a combination regimen of mifepristone and misoprostol is more effective than treatment with misoprostol alone.1,2 The data suggest that 91% of patients will experience expulsion of the gestational sac by 30 days with medication management.1 Because a minority of patients will have a retained gestational sac despite medication therapy, follow-up ensures complete expulsion of pregnancy tissue.
In the United States, most follow-up protocols include an ultrasound examination, which often entails transvaginal ultrasonography. Returning to clinic for an additional ultrasound may be costly and inconvenient—and during a global pandemic medically risky. Further, it may undermine a fundamental principle in management of EPL: autonomy. Many patients who select medication management do so out of a desire to minimize interventions or procedures. Follow-up protocols that align with patient preferences for fewer interventions are critically important to the provision of patient-centered care. Additionally, the COVID-19 pandemic highlights the value of offering an alternative follow-up strategy that minimizes the need for additional visits to a clinic or hospital.
Lessons from medication abortion management
In many ways, follow-up after medication management of EPL is analogous to follow-up after medication abortion. In both cases, the goal of follow-up is to ensure that complete expulsion has occurred without complication and to identify patients with incomplete expulsion of pregnancy tissue who may benefit from further treatment with additional medication or uterine aspiration. A key difference in the management of EPL is that there is no concern for ongoing pregnancy.
Historically, follow-up transvaginal ultrasonography was routinely performed after medication abortion to ensure complete expulsion of pregnancy.3 However, requiring patients to return to a health care facility for ultrasonography after abortion can be burdensome, both for patients and clinicians. To provide more accessible, patient-centered care, researchers have investigated alternative follow-up strategies for medication abortion that remove the necessity for ultrasonography. Guidelines from both the National Abortion Federation and the American College of Obstetricians and Gynecologists state that routine ultrasonography is not necessary after medication abortion.4,5
Quantitative serum human chorionic gonadotropin (hCG) testing before treatment and at a follow-up visit is one reasonable strategy to ensure successful treatment. In one study of medication abortion patients, an 80% decrease in serum hCG was predictive of complete expulsion in 98.5% of patients.6 While this strategy avoids ultrasonography, it still necessitates a visit to a health care facility for a blood draw. As an alternative, substantial evidence now demonstrates the safety and feasibility of using a combination of clinical symptoms and urine pregnancy testing to confirm completed medication abortion. The evidence for follow-up using a combination of clinical symptoms and urine pregnancy testing is discussed below.
Continue to: Symptoms...
Symptoms. An assessment of symptoms alone, by the patient or clinician, is an important indicator of treatment success and can be completed easily via telephone. In one study of medication abortion with mifepristone and misoprostol, patients correctly predicted passage of a gestational sac 85% of the time based on symptoms alone.7 In another study, the combined clinical assessment from the patient and the clinician had a sensitivity of 96% and a specificity of 67% for predicting complete pregnancy expulsion.8 Finally, in an analysis of 931 patients after medication abortion, when both the patient and clinician believed that the gestational sac had passed, ultrasonography demonstrated complete expulsion 99% of the time.9
Urine pregnancy testing. Several studies have demonstrated that the addition of urine pregnancy testing to a clinical assessment of symptoms is a safe and effective follow-up strategy in medication abortion. Contemporary over-the-counter pregnancy tests are high-sensitivity tests that have an hCG detection threshold of 25 to 50 mIU/mL. As these tests are widely and commercially available in the United States, they can be a useful tool in follow-up strategies.
In a study by Chen and colleagues, patients seeking medication abortion were offered a choice of follow-up with ultrasonography at 1 week versus a combination of a 1-week phone call and a 4-week high-sensitivity urine pregnancy test. In this study, approximately 40% of patients opted for phone follow-up. The rates of incomplete abortion and loss to follow-up were similar between the 2 groups, highlighting the significant number of individuals interested in alternative models of follow-up and the efficacy of phone and urine testing specifically.10
In another study that evaluated the feasibility of a telephone and urine testing follow-up strategy, 97% of patients completed follow-up and all continuing pregnancies were diagnosed prior to the 4-week urine pregnancy test.8
In a hospital in Edinburgh, where a telephone-based symptom assessment in combination with a 2-week low-sensitivity pregnancy test (hCG detection threshold of 2,000 mIU/mL; not commercially available in the United States) is the standard of care for medication abortion follow-up, Michie and Cameron reported a sensitivity of 100% and a specificity of 88% to detect ongoing pregnancies.11
Taken together, these data demonstrate that a combination of symptom assessment via telephone and home urine pregnancy testing (in addition to standard patient instructions and return precautions) is an appropriate strategy for medication abortion follow-up, and they suggest that similar strategies can be employed in the medication management of EPL.
To scan or not to scan?
Many published studies of EPL have used ultrasonography to confirm complete expulsion of pregnancy tissue; however, others have relied on either clinical evaluation or urine pregnancy testing to determine treatment success, using ultrasonography only as clinically indicated.12-14 In their evaluation of medication management versus surgical management of miscarriage, Niinimäki and colleagues performed urine hCG testing at a 5- to 6-week follow-up visit to determine treatment success; ultrasonography was obtained only if the urine hCG test was positive. They demonstrated a treatment success rate of 90% with mifepristone and misoprostol treatment,12 congruent with previously published results.
While a follow-up ultrasound scan may be helpful to accurately assess treatment efficacy in research protocols, it should not be considered necessary in clinical practice. Posttreatment imaging in an asymptomatic patient may place additional burden on the patient and health care system and may result in unnecessary intervention. Although treatment success is reliably defined by the absence of a gestational sac,15,16 the finding of a thickened endometrium or presence of vascularity may result in the patient receiving an unnecessary aspiration or other intervention.
The evidence from the medication abortion literature suggests that a combination of a 1-week telephone call to assess patient symptoms in addition to a 4-week high-sensitivity pregnancy test is a reasonable alternative follow-up strategy. A similar strategy is already used in the United Kingdom, where current National Institute for Health and Care Excellence guidelines for follow-up after medication management of EPL recommend home pregnancy testing in 3 weeks unless the patient experiences worsening pain or bleeding symptoms.17
Time to rethink follow-up care
Follow-up care for EPL should be provided in a way that is sensitive to the needs and preferences of the patient and, if desired, minimizes additional health care visits, testing, or procedures. While some patients may prefer ultrasonography follow-up, it is important for the clinician to recognize that there are safe and effective alternatives. Patient preference guides the choice of EPL management; this logic extends to follow-up strategies. As we strive to provide evidence-based, patient-centered EPL care, there is no need for universal follow-up ultrasonography. ●
CASE Patient finds that follow-up ultrasonography is burdensome
Ms. MB presents to the clinic for dating ultrasonography and is diagnosed with a missed abortion measuring 7 weeks. After reviewing her management options, she elects for medication management. She receives mifepristone 200 mg and misoprostol 800 µg, with a plan to follow-up in clinic for repeat ultrasonography in a week. The day of her follow-up appointment, there is a large snowstorm. She calls her care team to ask if she needs to have a follow-up visit, as she is certain she has passed tissue and her bleeding is now minimal. She is told, however, that a follow-up ultrasonography is required, per clinic policy, to ensure successful management. Despite Ms. MB’s grief and the difficult travel conditions, she makes the arduous journey back to the clinic to complete the ultrasound.
Do all patients need an ultrasound after medication management of early pregnancy loss? Or is there an alternative follow-up option?
Early pregnancy loss (EPL) is a common pregnancy complication, and its management is a routine part of reproductive health care. In the clinically stable patient, EPL may be managed expectantly, surgically, or medically, based on the patient’s preference. For patients who select medication management, clear evidence supports that a combination regimen of mifepristone and misoprostol is more effective than treatment with misoprostol alone.1,2 The data suggest that 91% of patients will experience expulsion of the gestational sac by 30 days with medication management.1 Because a minority of patients will have a retained gestational sac despite medication therapy, follow-up ensures complete expulsion of pregnancy tissue.
In the United States, most follow-up protocols include an ultrasound examination, which often entails transvaginal ultrasonography. Returning to clinic for an additional ultrasound may be costly and inconvenient—and during a global pandemic medically risky. Further, it may undermine a fundamental principle in management of EPL: autonomy. Many patients who select medication management do so out of a desire to minimize interventions or procedures. Follow-up protocols that align with patient preferences for fewer interventions are critically important to the provision of patient-centered care. Additionally, the COVID-19 pandemic highlights the value of offering an alternative follow-up strategy that minimizes the need for additional visits to a clinic or hospital.
Lessons from medication abortion management
In many ways, follow-up after medication management of EPL is analogous to follow-up after medication abortion. In both cases, the goal of follow-up is to ensure that complete expulsion has occurred without complication and to identify patients with incomplete expulsion of pregnancy tissue who may benefit from further treatment with additional medication or uterine aspiration. A key difference in the management of EPL is that there is no concern for ongoing pregnancy.
Historically, follow-up transvaginal ultrasonography was routinely performed after medication abortion to ensure complete expulsion of pregnancy.3 However, requiring patients to return to a health care facility for ultrasonography after abortion can be burdensome, both for patients and clinicians. To provide more accessible, patient-centered care, researchers have investigated alternative follow-up strategies for medication abortion that remove the necessity for ultrasonography. Guidelines from both the National Abortion Federation and the American College of Obstetricians and Gynecologists state that routine ultrasonography is not necessary after medication abortion.4,5
Quantitative serum human chorionic gonadotropin (hCG) testing before treatment and at a follow-up visit is one reasonable strategy to ensure successful treatment. In one study of medication abortion patients, an 80% decrease in serum hCG was predictive of complete expulsion in 98.5% of patients.6 While this strategy avoids ultrasonography, it still necessitates a visit to a health care facility for a blood draw. As an alternative, substantial evidence now demonstrates the safety and feasibility of using a combination of clinical symptoms and urine pregnancy testing to confirm completed medication abortion. The evidence for follow-up using a combination of clinical symptoms and urine pregnancy testing is discussed below.
Continue to: Symptoms...
Symptoms. An assessment of symptoms alone, by the patient or clinician, is an important indicator of treatment success and can be completed easily via telephone. In one study of medication abortion with mifepristone and misoprostol, patients correctly predicted passage of a gestational sac 85% of the time based on symptoms alone.7 In another study, the combined clinical assessment from the patient and the clinician had a sensitivity of 96% and a specificity of 67% for predicting complete pregnancy expulsion.8 Finally, in an analysis of 931 patients after medication abortion, when both the patient and clinician believed that the gestational sac had passed, ultrasonography demonstrated complete expulsion 99% of the time.9
Urine pregnancy testing. Several studies have demonstrated that the addition of urine pregnancy testing to a clinical assessment of symptoms is a safe and effective follow-up strategy in medication abortion. Contemporary over-the-counter pregnancy tests are high-sensitivity tests that have an hCG detection threshold of 25 to 50 mIU/mL. As these tests are widely and commercially available in the United States, they can be a useful tool in follow-up strategies.
In a study by Chen and colleagues, patients seeking medication abortion were offered a choice of follow-up with ultrasonography at 1 week versus a combination of a 1-week phone call and a 4-week high-sensitivity urine pregnancy test. In this study, approximately 40% of patients opted for phone follow-up. The rates of incomplete abortion and loss to follow-up were similar between the 2 groups, highlighting the significant number of individuals interested in alternative models of follow-up and the efficacy of phone and urine testing specifically.10
In another study that evaluated the feasibility of a telephone and urine testing follow-up strategy, 97% of patients completed follow-up and all continuing pregnancies were diagnosed prior to the 4-week urine pregnancy test.8
In a hospital in Edinburgh, where a telephone-based symptom assessment in combination with a 2-week low-sensitivity pregnancy test (hCG detection threshold of 2,000 mIU/mL; not commercially available in the United States) is the standard of care for medication abortion follow-up, Michie and Cameron reported a sensitivity of 100% and a specificity of 88% to detect ongoing pregnancies.11
Taken together, these data demonstrate that a combination of symptom assessment via telephone and home urine pregnancy testing (in addition to standard patient instructions and return precautions) is an appropriate strategy for medication abortion follow-up, and they suggest that similar strategies can be employed in the medication management of EPL.
To scan or not to scan?
Many published studies of EPL have used ultrasonography to confirm complete expulsion of pregnancy tissue; however, others have relied on either clinical evaluation or urine pregnancy testing to determine treatment success, using ultrasonography only as clinically indicated.12-14 In their evaluation of medication management versus surgical management of miscarriage, Niinimäki and colleagues performed urine hCG testing at a 5- to 6-week follow-up visit to determine treatment success; ultrasonography was obtained only if the urine hCG test was positive. They demonstrated a treatment success rate of 90% with mifepristone and misoprostol treatment,12 congruent with previously published results.
While a follow-up ultrasound scan may be helpful to accurately assess treatment efficacy in research protocols, it should not be considered necessary in clinical practice. Posttreatment imaging in an asymptomatic patient may place additional burden on the patient and health care system and may result in unnecessary intervention. Although treatment success is reliably defined by the absence of a gestational sac,15,16 the finding of a thickened endometrium or presence of vascularity may result in the patient receiving an unnecessary aspiration or other intervention.
The evidence from the medication abortion literature suggests that a combination of a 1-week telephone call to assess patient symptoms in addition to a 4-week high-sensitivity pregnancy test is a reasonable alternative follow-up strategy. A similar strategy is already used in the United Kingdom, where current National Institute for Health and Care Excellence guidelines for follow-up after medication management of EPL recommend home pregnancy testing in 3 weeks unless the patient experiences worsening pain or bleeding symptoms.17
Time to rethink follow-up care
Follow-up care for EPL should be provided in a way that is sensitive to the needs and preferences of the patient and, if desired, minimizes additional health care visits, testing, or procedures. While some patients may prefer ultrasonography follow-up, it is important for the clinician to recognize that there are safe and effective alternatives. Patient preference guides the choice of EPL management; this logic extends to follow-up strategies. As we strive to provide evidence-based, patient-centered EPL care, there is no need for universal follow-up ultrasonography. ●
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LF, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Benson J, Clark KA, Gerhardt A, et al. Early abortion services in the United States: a provider survey. Contraception. 2003;67:287-294.
- Medication abortion up to 70 days of gestation: ACOG practice bulletin summary, No. 225. Obstet Gynecol. 2020;136:855-858.
- National Abortion Federation. 2020 Clinical Policy Guidelines for Abortion Care. Washington, DC; 2020. https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna -ssl.com/wp-content/uploads/2020_CPGs.pdf. Accessed July 19, 2021.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190-195.
- Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception. 2001;64:87-92.
- Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143-149.
- Rossi B, Creinin MD, Meyn LA. Ability of the clinician and patient to predict the outcome of mifepristone and misoprostol medical abortion. Contraception. 2004;70:313-317.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89:440-445.
- Niinimäki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367- 372.
- Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol. 2005;106:540-547.
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563-566.
- Reeves MF, Lohr PA, Harwood B, et al. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstet Gynecol. 2008;111:106-112.
- Reeves MF, Fox MC, Lohr PA, et al. Endometrial thickness following medical abortion is not predictive of subsequent surgical intervention. Ultrasound Obstet Gynecol. 2009;34:104-109.
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guideline NG126. April 17, 2019. ttps:// www.nice.org.uk/guidance/ng126/chapter/Recommen dations#management-of-miscarriage. Accessed July 19, 2021.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Chu JJ, Devall AJ, Beeson LF, et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;396:770-778.
- Benson J, Clark KA, Gerhardt A, et al. Early abortion services in the United States: a provider survey. Contraception. 2003;67:287-294.
- Medication abortion up to 70 days of gestation: ACOG practice bulletin summary, No. 225. Obstet Gynecol. 2020;136:855-858.
- National Abortion Federation. 2020 Clinical Policy Guidelines for Abortion Care. Washington, DC; 2020. https://5aa1b2xfmfh2e2mk03kk8rsx-wpengine.netdna -ssl.com/wp-content/uploads/2020_CPGs.pdf. Accessed July 19, 2021.
- Fiala C, Safar P, Bygdeman M, et al. Verifying the effectiveness of medical abortion; ultrasound versus hCG testing. Eur J Obstet Gynecol Reprod Biol. 2003;109:190-195.
- Pymar HC, Creinin MD, Schwartz JL. Mifepristone followed on the same day by vaginal misoprostol for early abortion. Contraception. 2001;64:87-92.
- Perriera LK, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010;81:143-149.
- Rossi B, Creinin MD, Meyn LA. Ability of the clinician and patient to predict the outcome of mifepristone and misoprostol medical abortion. Contraception. 2004;70:313-317.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Michie L, Cameron ST. Simplified follow-up after early medical abortion: 12-month experience of a telephone call and self-performed low-sensitivity urine pregnancy test. Contraception. 2014;89:440-445.
- Niinimäki M, Jouppila P, Martikainen H, et al. A randomized study comparing efficacy and patient satisfaction in medical or surgical treatment of miscarriage. Fertil Steril. 2006;86:367- 372.
- Weeks A, Alia G, Blum J, et al. A randomized trial of misoprostol compared with manual vacuum aspiration for incomplete abortion. Obstet Gynecol. 2005;106:540-547.
- Wood SL, Brain PH. Medical management of missed abortion: a randomized clinical trial. Obstet Gynecol. 2002;99:563-566.
- Reeves MF, Lohr PA, Harwood B, et al. Ultrasonographic endometrial thickness after medical and surgical management of early pregnancy failure. Obstet Gynecol. 2008;111:106-112.
- Reeves MF, Fox MC, Lohr PA, et al. Endometrial thickness following medical abortion is not predictive of subsequent surgical intervention. Ultrasound Obstet Gynecol. 2009;34:104-109.
- National Institute for Health and Care Excellence. Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guideline NG126. April 17, 2019. ttps:// www.nice.org.uk/guidance/ng126/chapter/Recommen dations#management-of-miscarriage. Accessed July 19, 2021.
COVID-19 linked to rise in suicide-related ED visits among youth
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
FROM JAMA PSYCHIATRY
COVID vaccine response in patients with solid tumors
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among cancer patients with solid tumors, the response to the COVID-19 vaccine 6 months after receiving a second dose was as good as that of the general population, according to new findings from a case-control study.
The BNT162b2, or Pfizer-BioNTech, vaccine (Comirnaty) was previously shown to have good short-term efficacy, immunogenicity, and safety in cancer patients with solid tumors, but little is known about longer-term efficacy in this population, say the investigators.
They assessed responses 6 months after a second dose of the vaccine.
Serologic tests showed that 122 of 154 patients with solid tumors who were actively undergoing cancer treatment (79%) and 114 of 135 age-matched health care workers who served as control persons (84%) were seropositive at 6 months (P = .32).
Most (81%) of the patients with cancer who were seronegative were receiving chemotherapy, the researchers report.
One case of severe COVID-19 that required hospitalization occurred among the solid-tumor group; none occurred among the control persons, they also note.
The findings were published online on Sept. 2 in Cancer Discovery.
“In our study we saw that in all outcomes, including immunogenicity, infectivity rate throughout the 6-month period, and safety, patients with solid tumors depicted a similar trend as the general population,” commented lead author Irit Ben-Aharon, MD, PhD, director of the division of oncology at Rambam Health Care Campus, Haifa, Israel, in an American Association for Cancer Research press statement.
However, she and her coauthors stressed the importance of continuing to follow guidance, such as social distancing and mask wearing, for reducing COVID-19 transmission. “Due to uncertainty of the extended efficacy of the vaccine in the general population and recent reports on rising infection rates among vaccinated individuals, adherence to health care risk reduction recommendations is cardinal,” they write.
The mean age of the control persons in the study was 63 years, and the mean age of the case patients was 66 years. The most common cancers were gastrointestinal (36%), lung (23%), breast (17%), and genitourinary (11%). Treatment protocols included chemotherapy (62%), biological agents (36%), and immunotherapy (30%). Some patients received more than one type of treatment.
All of the reported adverse effects associated with vaccination had resolved at the time of follow-up.
These data can “help inform recommendations surrounding the prioritization of different groups for booster vaccines,” Dr. Ben-Aharon adds.
In fact, recently updated guidelines from the National Comprehensive Cancer Network state that cancer patients with solid tumors who are receiving treatment within 1 year of their initial vaccine dose should be prioritized for a booster vaccine.
The study was partially supported by the Israel Cancer Research Fund. Serologic testing of the control cohort was supported by the Ministry of Health, Israel. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID could spell kidney troubles down the line
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians caring for COVID-19 survivors should routinely check kidney function, which is often damaged by the SARS-CoV-2 virus months after both severe and milder cases, new research indicates.
The largest study to date with the longest follow-up of COVID-19-related kidney outcomes also found that every type of kidney problem, including end-stage kidney disease (ESKD), was far more common in COVID-19 survivors who were admitted to the ICU or experienced acute kidney injury (AKI) while hospitalized.
Researchers analyzed U.S. Veterans Health Administration data from more than 1.7 million patients, including more than 89,000 who tested positive for COVID-19, for the study, which was published online Sept. 1, 2021, in the Journal of the American Society of Nephrology.
The risk of kidney problems “is more robust or pronounced in people who have had severe infection, but present in even asymptomatic and mild disease, which shouldn’t be discounted. Those people represent the majority of those with COVID-19,” said senior author Ziyad Al-Aly, MD, of the Veteran Affairs St. Louis Health Care System.
“That’s why the results are important, because even in people with mild disease to start with, the risk of kidney problems is not trivial,” he told this news organization. “It’s smaller than in people who were in the ICU, but it’s not ... zero.”
Experts aren’t yet certain how COVID-19 can damage the kidneys, hypothesizing that several factors may be at play. The virus may directly infect kidney cells rich in ACE2 receptors, which are key to infection, said nephrologist F. Perry Wilson, MD, of Yale University, New Haven, Conn., and a member of Medscape’s advisory board.
Kidneys might also be particularly vulnerable to the inflammatory cascade or blood clotting often seen in COVID-19, Dr. Al-Aly and Wilson both suggested.
COVID-19 survivors more likely to have kidney damage than controls
“A lot of health systems either have or are establishing post-COVID care clinics, which we think should definitely incorporate a kidney component,” Dr. Al-Aly advised. “They should check patients’ blood and urine for kidney problems.”
This is particularly important because “kidney problems, for the most part, are painless and silent,” he added.
“Realizing 2 years down the road that someone has ESKD, where they need dialysis or a kidney transplant, is what we don’t want. We don’t want this to be unrecognized, uncared for, unattended to,” he said.
Dr. Al-Aly and colleagues evaluated VA health system records, including data from 89,216 patients who tested positive for COVID-19 between March 2020 and March 2021, as well as 1.7 million controls who did not have COVID-19. Over a median follow-up of about 5.5 months, participants’ estimated glomerular filtration rate and serum creatinine levels were tracked to assess kidney health and outcomes according to infection severity.
Results were striking, with COVID-19 survivors about one-third more likely than controls to have kidney damage or significant declines in kidney function between 1 and 6 months after infection. More than 4,700 COVID-19 survivors had lost at least 30% of their kidney function within a year, and these patients were 25% more likely to reach that level of decline than controls.
Additionally, COVID-19 survivors were nearly twice as likely to experience AKI and almost three times as likely to be diagnosed with ESKD as controls.
If your patient had COVID-19, ‘it’s reasonable to check kidney function’
“This information tells us that if your patient was sick with COVID-19 and comes for follow-up visits, it’s reasonable to check their kidney function,” Dr. Wilson, who was not involved with the research, told this news organization.
“Even for patients who were not hospitalized, if they were laid low or dehydrated ... it should be part of the post-COVID care package,” he said.
If just a fraction of the millions of COVID-19 survivors in the United States develop long-term kidney problems, the ripple effect on American health care could be substantial, Dr. Wilson and Dr. Al-Aly agreed.
“We’re still living in a pandemic, so it’s hard to tell the total impact,” Dr. Al-Aly said. “But this ultimately will contribute to a rise in burden of kidney disease. This and other long COVID manifestations are going to alter the landscape of clinical care and health care in the United States for a decade or more.”
Because renal problems can limit a patient’s treatment options for other major diseases, including diabetes and cancer, COVID-related kidney damage can ultimately impact survivability.
“There are a lot of medications you can’t use in people with advanced kidney problems,” Dr. Al-Aly said.
The main study limitation was that patients were mostly older White men (median age, 68 years), although more than 9,000 women were included in the VA data, Dr. Al-Aly noted. Additionally, controls were more likely to be younger, Black, living in long-term care, and have higher rates of chronic health conditions and medication use.
The experts agreed that ongoing research tracking kidney outcomes is crucial for years to come.
“We also need to be following a cohort of these patients as part of a research protocol where they come in every 6 months for a standard set of lab tests to really understand what’s going on with their kidneys,” Dr. Wilson said.
“Lastly – and a much tougher sell – is we need biopsies. It’s very hard to infer what’s going on in complex disease with the kidneys without biopsy tissue,” he added.
The study was funded by the American Society of Nephrology and the Department of Veterans Affairs. Dr. Al-Aly and Dr. Wilson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOP-DAPT 2 ACS: 1 month of DAPT proves inadequate for patients with recent ACS
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Explaining Away Those Shades of Gray
ANSWER
The correct answer is that new hairs growing in to replace those lost from alopecia areata tend to be white (choice “b”). They usually regain their normal color, eventually.
DISCUSSION
Alopecia areata is an autoimmune phenomenon implying an increased tendency to develop other autoimmune diseases (eg, vitiligo [choice “a”], which can appear initially in the scalp).
This case turned out to be simple but had the potential to be far more serious. The biopsy of the dark patch showed benign seborrheic keratosis, but it was possible that another section could have demonstrated features of melanoma (choice “c”). When present, melanoma can occasionally trigger an immune response that destroys pigment cells in hair follicles, causing the hairs to lose their pigment. This is why the entire dark patch was later excised. Fortunately, the pathology report ruled out melanoma.
While it has been reported that stress can cause hair to turn gray (choice “d”), there were better (and more accurate) explanations for this patient’s presentation.
This case, though fairly straightforward, serves as a reminder that it is our job as clinicians to connect the dots to rule out worst-case scenarios.
Outcome
This patient’s hair all grew back, regaining its normal color, without any treatment.
ANSWER
The correct answer is that new hairs growing in to replace those lost from alopecia areata tend to be white (choice “b”). They usually regain their normal color, eventually.
DISCUSSION
Alopecia areata is an autoimmune phenomenon implying an increased tendency to develop other autoimmune diseases (eg, vitiligo [choice “a”], which can appear initially in the scalp).
This case turned out to be simple but had the potential to be far more serious. The biopsy of the dark patch showed benign seborrheic keratosis, but it was possible that another section could have demonstrated features of melanoma (choice “c”). When present, melanoma can occasionally trigger an immune response that destroys pigment cells in hair follicles, causing the hairs to lose their pigment. This is why the entire dark patch was later excised. Fortunately, the pathology report ruled out melanoma.
While it has been reported that stress can cause hair to turn gray (choice “d”), there were better (and more accurate) explanations for this patient’s presentation.
This case, though fairly straightforward, serves as a reminder that it is our job as clinicians to connect the dots to rule out worst-case scenarios.
Outcome
This patient’s hair all grew back, regaining its normal color, without any treatment.
ANSWER
The correct answer is that new hairs growing in to replace those lost from alopecia areata tend to be white (choice “b”). They usually regain their normal color, eventually.
DISCUSSION
Alopecia areata is an autoimmune phenomenon implying an increased tendency to develop other autoimmune diseases (eg, vitiligo [choice “a”], which can appear initially in the scalp).
This case turned out to be simple but had the potential to be far more serious. The biopsy of the dark patch showed benign seborrheic keratosis, but it was possible that another section could have demonstrated features of melanoma (choice “c”). When present, melanoma can occasionally trigger an immune response that destroys pigment cells in hair follicles, causing the hairs to lose their pigment. This is why the entire dark patch was later excised. Fortunately, the pathology report ruled out melanoma.
While it has been reported that stress can cause hair to turn gray (choice “d”), there were better (and more accurate) explanations for this patient’s presentation.
This case, though fairly straightforward, serves as a reminder that it is our job as clinicians to connect the dots to rule out worst-case scenarios.
Outcome
This patient’s hair all grew back, regaining its normal color, without any treatment.
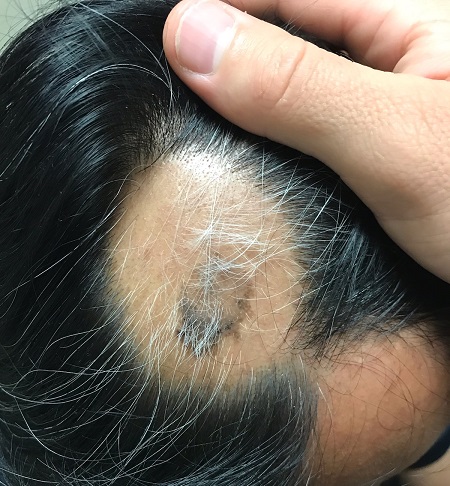
About 2 months ago, a 55-year-old man suddenly experienced complete hair loss in 1 confined area of his scalp. There were no accompanying symptoms. Some of the hair subsequently grew back, but it was partially gray—a phenomenon that greatly disturbed the patient.
In general, the patient’s health was quite good, although he reported that the initial hair loss occurred about 1 month after he lost his job and got divorced.
Most of the hair was missing from a roughly round, 5-cm, ill-defined area of the left parietal scalp. The few hairs left were gray. More disturbing, though, was a dark (brown, tan, and black), oddly shaped, 2.8-cm patch in the center of the alopecic area.
Punch biopsy from the bald area showed clear evidence of alopecia areata (T-cells surrounding hair follicles, and lack of features that would support other items in the differential). Shave biopsy of the dark patch showed seborrheic keratosis, with no atypia.
FDA inaction on hair loss drug’s suicide, depression, erectile dysfunction risk sparks lawsuit
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Consumer advocacy group 4 years ago.
The September 2017 petition requested that the FDA take the popular hair-loss drug (1 mg finasteride, Propecia) off the market because of evidence of serious risk of patient injury, including depression and suicidal ideation.
As an alternative, PFSF requested that the FDA require the drug’s manufacturers revise the safety information on the labeling and add boxed warnings to disclose the potential for side effects, another of which is erectile dysfunction.
Public Citizen points to a recent analysis of the VigiBase global database, which tracks adverse effects from global pharmacovigilance agencies, lists 356 reports of suicidality and 2,926 reports of psychological adverse events in finasteride users. Yet, 4 years after submitting the petition, the FDA has neither granted nor denied it.
The lawsuit claims that FDA has acted unlawfully in failing to act on PFSF’s petition, and further cites “88 cases of completed suicide associated with finasteride use” per data from the VigiBase database.
“On the same day that PFSF submitted the petition, FDA’s docket management division acknowledged receipt and assigned the petition a docket number,” Michael Kirkpatrick, the Public Citizen attorney serving as lead counsel for PFSF, told this news organization.
Yet, to date, “there has been no substantive response to the petition. The lawsuit filed today seeks to force FDA to issue a decision on PFSF’s petition,” Mr. Kirkpatrick said.
“The FDA needs to act in a timely way to protect the public from the risks associated with use of Propecia. The FDA’s failure to act exposes consumers to potentially life-threatening harm,” he added in a statement.
The complaint filed today by Public Citizen in the U.S. District Court for the District of Columbia is available online.
This news organization reached out to the FDA for comment but did not receive a response by press time.
A version of this article first appeared on Medscape.com.
Will interchangeable insulin be more affordable in the U.S.?
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration approved Semglee, the first interchangeable biosimilar insulin, the agency pitched it as having the potential to be less costly than insulins currently on the market, but lack of transparency in pharmaceutical pricing has left analysts and advocates guessing whether it will indeed be a source of relief.
Semglee (Mylan Pharmaceuticals), first approved as a biosimilar in June 2020, costs about $100 a vial.
But receiving the “interchangeable designation” in July 2021, the first for any insulin, now allows Semglee to be substituted for the branded Lantus (insulin glargine, Sanofi) at the pharmacy without the need for a separate prescription, the same way as generic medicines.
A spokesperson for Viatris – Mylan’s parent company told this news organization that the interchangeable, with its new labeling, will be “introduced before the end of the year,” but it would not give any more details.
“Additional information, including pricing information, for interchangeable biosimilar Semglee will be provided at the time of product launch,” said the spokesperson.
Even at $100 a vial, it is not cheap
Ian Devaney, a spokesman for the advocacy group T1 International, said the organization is optimistic, given that “another player has been able to enter into a space that has for so long been dominated by Eli Lilly, Novo Nordisk, and Sanofi.” Increased competition “will help drive down the overall costs of insulin,” Mr. Devaney said in an interview. But, he added, for many people, especially in low-income countries, Semglee’s launch will have little to no impact on price.
Even at $100 a vial in the United States, “this is not an insignificant amount of money and presents a very difficult financial challenge for those dependent on insulin to survive,” he said.
A current Semglee user agreed, sharing her story with this news organization via T1 International. “My son uses three to five vials of long-acting insulin per month, and I use one to three vials per month,” said the woman, who prefers to remain anonymous. “If we were to lose Medicaid, we would still be paying up to $800 out of pocket monthly to survive, and that’s not even counting fast-acting insulin or other supplies. While $100 a vial may be cheaper, these costs are still outrageous.”
The woman also noted that, while new competitors are welcome, they also have been disruptive. After her doctor switched her to Semglee, she was notified that it was on back order. “It took a week to get it filled, and when it finally came in, it was in short supply,” she said, noting that she and her son received one Semglee pen each, “well short of the three and five each we were expecting.”
U.S. pricing is all ‘smoke and mirrors’
Sara W. Koblitz, a food and drug law attorney with Hyman Phelps in Washington, D.C., noted in a blog post that interchangeable Semglee will likely be awarded a year of marketing exclusivity, which will block other interchangeable competitors from entering the market during that time.
With no competition, “Mylan can price Semglee only slightly less than Lantus and still take market share, only marginally reducing costs to consumers,” she wrote.
Jing Luo, MD, MPH, an assistant professor of medicine at the University of Pittsburgh, who has studied insulin access and costs, said that having just one interchangeable on the market might not be enough to drive insulin costs down.
And, he told this news organization, “there’s even a possibility that Semglee prices will go up, but hopefully that will not be the case.”
Manufacturers like Mylan can also offer confidential discounts and rebates to pharmacy benefit managers (PBMs), health plans, and health plan sponsors (usually large companies that are self-insured) that make it difficult to assess the true cost, said David Steinberg, PharmD, director of pharmacy insights at Scripta Insights. The Wellesley, Mass.–based company advises self-insured employers on how to optimize pharmacy benefits.
When it comes to pricing, “it’s a lot of smoke and mirrors,” Dr. Steinberg said in an interview.
Dr. Steinberg also noted that some PBMs might choose to continue contracts with Sanofi that offer rebates for Lantus, leaving Semglee in a less-preferred position on a formulary, which could increase how much the patient pays at the pharmacy counter.
Medicare and Medicaid, however, can put Semglee in the top-tier preferred formulary position. Most Medicaid plans cover Semglee, but it appears that Medicare has not added coverage yet.
Does current pricing predict the future?
The currently marketed Semglee has an average wholesale price (AWP) that is one third of Lantus’, and about half of what is published for Basaglar (insulin glargine, Eli Lilly), a “follow-on biologic” approved in 2015 that is similar to Lantus, Dr. Steinberg said.
The AWP is often cited by analysts when talking about costs. The AWP of the current Semglee 10-mL vial is $118.38; the Lantus 10-mL vial is $340.27, said Steinberg.
Five prefilled Semglee pens (each 3 mL) are $177.58; for Lantus, the AWP for five 3-mL pens is $510.37.
Dr. Luo said he has seen a box of Semglee pens retail between $177 and $195, compared with about $500 retail for the Lantus pens.
Currently, people with commercial insurance can get Semglee for $0-75 a month, for up to a year, using the company’s savings program.
Steinberg said it’s possible that Mylan could increase the list price for the interchangeable Semglee, but that move could backfire. “I think their goal initially is to get market share,” he said.
After Basaglar came on the market – in late 2016 – the price of Lantus came down significantly over the next few years, according to a 2019 study by Dr. Luo’s colleagues at the University of Pittsburgh.
But Basaglar has not hung on to market share, according to Scott Strumello, a person with autoimmune type 1 diabetes who tweets and blogs about insulin and other issues.
In early August, Mr. Strumello tweeted some Lilly data that showed U.S. sales of Basaglar declined 42% in the first two quarters of 2021, compared with the same period in 2020.
Dr. Steinberg noted that the decline may have to do with rebates being given to PBMs by competitors Sanofi and Novo Nordisk. Sanofi “is very aggressive when it comes to pricing with their PBM partners,” he said.
While Mr. Devaney said people with diabetes are hopeful that Semglee can break the big three manufacturers’ monopoly, he added: “We don’t see Semglee as something that is solving the root cause of the insulin price crisis, which is high list prices and pharmaceutical industry greed.”
A version of this article first appeared on Medscape.com.
VARSITY: Better histologic outcomes with vedolizumab than adalimumab in UC
In patients with moderate to severe ulcerative colitis (UC), treatment with vedolizumab leads to better histologic outcomes than treatment with adalimumab, according to findings from the VARSITY trial.
The findings come from an analysis in Gastroenterology of prespecified histologic exploratory endpoints from the phase 3, multicenter, randomized, controlled VARSITY trial, which was the first head-to-head comparison of two biologics in the treatment of UC. VARSITY demonstrated improved rates of clinical remission and endoscopic improvement at week 52 with vedolizumab.
The authors, led by Laurent Peyrin-Biroulet of the department of gastroenterology at Nancy (France) University Hospital, noted that there is general consensus that endoscopic improvement is considered the best endpoint for demonstrating effective maintenance therapy in UC. However, they added that “endoscopic changes do not necessarily reflect quiescent microscopic disease, and complete resolution of mucosal inflammation can only be confirmed by histologic assessment.” Still, histologic outcomes are not currently recommended as a goal of therapy in clinical practice, possibly due to a lack of standardized and validated scoring systems suitable for routine clinical use. Nevertheless, histologic outcomes have been shown to predict hospitalization, corticosteroid use, exacerbation, and the risk of advanced colorectal neoplasia.
To assess histologic outcomes in the two treatment regimens, the researchers included the Geboes Index score and the Robarts Histopathology Index (RHI) as two validated scoring systems.
During the 52-week study, 769 patients were assigned to vedolizumab (300 mg IV) or adalimumab (40 mg subcutaneously).
At week 14 and week 52, more patients in the vedolizumab group achieved histologic remission as determined by Geboes Index score less than 2 (week 52, 29.2% vs. 8.3%; difference, 20.9%; 95% confidence interval, 15.6%-26.2%; P < .0001) and RHI score of 2 or less (week 52, 37.6% vs. 19.9%; difference, 17.6%; 95% CI, 11.3%-23.8%; P < .0001).
At week 52, more patients in the vedolizumab group than in the adalimumab group achieved minimum histologic disease activity as determined by Geboes Index score of 3.1 or less (45.7% vs. 30.8%; difference, 14.8%; 95% CI, 8.0%-21.5%; P < .0001) and RHI score of 4 or less(42.3% vs. 25.6%; difference, 16.6%; 95% CI, 10.0%-23.1%; P < .0001).
The investigators performed post hoc analyses of mucosal healing, defined as a composite of the histologic and endoscopic outcomes, with the latter defined as Mayo endoscopic subscore of 1 or less. A greater proportion of patients treated with vedolizumab than with adalimumab met the composite of histologic remission on each score plus endoscopic improvement (Geboes, 35.0% vs. 20.2%; RHI, 33.7% vs. 18.1%), with similar findings for minimal histologic disease activity plus endoscopic improvement (Geboes, 35.0% vs. 20.2%; RHI, 33.7% vs. 18.1%).
The authors noted that the RHI scoring system revealed greater associations between histologic outcomes and endoscopic improvement than did the Geboes Index score, which is an important finding considering the European Crohn’s and Colitis Organisation’s stance recommending consideration of mucosal healing based on findings from endoscopy and histology.
Some study limitations included how the study design precluded dose escalation and a lack of long-term follow-up among these patients.
The researchers believe that the RHI score may be a better choice than the Geboes score for comparing efficacy in clinical trials because RHI is more reproducible, more sensitive to change, and is comparatively easy to interpret.
The study was funded by Takeda, which makes vedolizumab. The authors disclosed several relationships with industry, including some having stock options with or being employed by Takeda.
Another important element of this study was the exploration of association between endoscopic and histologic outcomes using two validated histologic indices (the Geboes Index score and the Robarts Histopathology Index). While both indices showed moderate agreement overall between histologic activity and endoscopic improvement, the Robarts score correlated better with endoscopic improvement. Therefore, the authors propose that the Robarts scoring system may be the better index for assessing histologic outcomes. This is important because standardized scoring systems would be needed to translate histologic outcomes as a goal in real clinical practice.
The landscape continues to evolve for treatment goals in UC. Symptom control is the tip of the iceberg and endoscopic along with histologic control may lead to a more durable remission.
Robin Dalal, MD, is an assistant professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn. She has nothing to disclose.
Another important element of this study was the exploration of association between endoscopic and histologic outcomes using two validated histologic indices (the Geboes Index score and the Robarts Histopathology Index). While both indices showed moderate agreement overall between histologic activity and endoscopic improvement, the Robarts score correlated better with endoscopic improvement. Therefore, the authors propose that the Robarts scoring system may be the better index for assessing histologic outcomes. This is important because standardized scoring systems would be needed to translate histologic outcomes as a goal in real clinical practice.
The landscape continues to evolve for treatment goals in UC. Symptom control is the tip of the iceberg and endoscopic along with histologic control may lead to a more durable remission.
Robin Dalal, MD, is an assistant professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn. She has nothing to disclose.
Another important element of this study was the exploration of association between endoscopic and histologic outcomes using two validated histologic indices (the Geboes Index score and the Robarts Histopathology Index). While both indices showed moderate agreement overall between histologic activity and endoscopic improvement, the Robarts score correlated better with endoscopic improvement. Therefore, the authors propose that the Robarts scoring system may be the better index for assessing histologic outcomes. This is important because standardized scoring systems would be needed to translate histologic outcomes as a goal in real clinical practice.
The landscape continues to evolve for treatment goals in UC. Symptom control is the tip of the iceberg and endoscopic along with histologic control may lead to a more durable remission.
Robin Dalal, MD, is an assistant professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn. She has nothing to disclose.
In patients with moderate to severe ulcerative colitis (UC), treatment with vedolizumab leads to better histologic outcomes than treatment with adalimumab, according to findings from the VARSITY trial.
The findings come from an analysis in Gastroenterology of prespecified histologic exploratory endpoints from the phase 3, multicenter, randomized, controlled VARSITY trial, which was the first head-to-head comparison of two biologics in the treatment of UC. VARSITY demonstrated improved rates of clinical remission and endoscopic improvement at week 52 with vedolizumab.
The authors, led by Laurent Peyrin-Biroulet of the department of gastroenterology at Nancy (France) University Hospital, noted that there is general consensus that endoscopic improvement is considered the best endpoint for demonstrating effective maintenance therapy in UC. However, they added that “endoscopic changes do not necessarily reflect quiescent microscopic disease, and complete resolution of mucosal inflammation can only be confirmed by histologic assessment.” Still, histologic outcomes are not currently recommended as a goal of therapy in clinical practice, possibly due to a lack of standardized and validated scoring systems suitable for routine clinical use. Nevertheless, histologic outcomes have been shown to predict hospitalization, corticosteroid use, exacerbation, and the risk of advanced colorectal neoplasia.
To assess histologic outcomes in the two treatment regimens, the researchers included the Geboes Index score and the Robarts Histopathology Index (RHI) as two validated scoring systems.
During the 52-week study, 769 patients were assigned to vedolizumab (300 mg IV) or adalimumab (40 mg subcutaneously).
At week 14 and week 52, more patients in the vedolizumab group achieved histologic remission as determined by Geboes Index score less than 2 (week 52, 29.2% vs. 8.3%; difference, 20.9%; 95% confidence interval, 15.6%-26.2%; P < .0001) and RHI score of 2 or less (week 52, 37.6% vs. 19.9%; difference, 17.6%; 95% CI, 11.3%-23.8%; P < .0001).
At week 52, more patients in the vedolizumab group than in the adalimumab group achieved minimum histologic disease activity as determined by Geboes Index score of 3.1 or less (45.7% vs. 30.8%; difference, 14.8%; 95% CI, 8.0%-21.5%; P < .0001) and RHI score of 4 or less(42.3% vs. 25.6%; difference, 16.6%; 95% CI, 10.0%-23.1%; P < .0001).
The investigators performed post hoc analyses of mucosal healing, defined as a composite of the histologic and endoscopic outcomes, with the latter defined as Mayo endoscopic subscore of 1 or less. A greater proportion of patients treated with vedolizumab than with adalimumab met the composite of histologic remission on each score plus endoscopic improvement (Geboes, 35.0% vs. 20.2%; RHI, 33.7% vs. 18.1%), with similar findings for minimal histologic disease activity plus endoscopic improvement (Geboes, 35.0% vs. 20.2%; RHI, 33.7% vs. 18.1%).
The authors noted that the RHI scoring system revealed greater associations between histologic outcomes and endoscopic improvement than did the Geboes Index score, which is an important finding considering the European Crohn’s and Colitis Organisation’s stance recommending consideration of mucosal healing based on findings from endoscopy and histology.
Some study limitations included how the study design precluded dose escalation and a lack of long-term follow-up among these patients.
The researchers believe that the RHI score may be a better choice than the Geboes score for comparing efficacy in clinical trials because RHI is more reproducible, more sensitive to change, and is comparatively easy to interpret.
The study was funded by Takeda, which makes vedolizumab. The authors disclosed several relationships with industry, including some having stock options with or being employed by Takeda.
In patients with moderate to severe ulcerative colitis (UC), treatment with vedolizumab leads to better histologic outcomes than treatment with adalimumab, according to findings from the VARSITY trial.
The findings come from an analysis in Gastroenterology of prespecified histologic exploratory endpoints from the phase 3, multicenter, randomized, controlled VARSITY trial, which was the first head-to-head comparison of two biologics in the treatment of UC. VARSITY demonstrated improved rates of clinical remission and endoscopic improvement at week 52 with vedolizumab.
The authors, led by Laurent Peyrin-Biroulet of the department of gastroenterology at Nancy (France) University Hospital, noted that there is general consensus that endoscopic improvement is considered the best endpoint for demonstrating effective maintenance therapy in UC. However, they added that “endoscopic changes do not necessarily reflect quiescent microscopic disease, and complete resolution of mucosal inflammation can only be confirmed by histologic assessment.” Still, histologic outcomes are not currently recommended as a goal of therapy in clinical practice, possibly due to a lack of standardized and validated scoring systems suitable for routine clinical use. Nevertheless, histologic outcomes have been shown to predict hospitalization, corticosteroid use, exacerbation, and the risk of advanced colorectal neoplasia.
To assess histologic outcomes in the two treatment regimens, the researchers included the Geboes Index score and the Robarts Histopathology Index (RHI) as two validated scoring systems.
During the 52-week study, 769 patients were assigned to vedolizumab (300 mg IV) or adalimumab (40 mg subcutaneously).
At week 14 and week 52, more patients in the vedolizumab group achieved histologic remission as determined by Geboes Index score less than 2 (week 52, 29.2% vs. 8.3%; difference, 20.9%; 95% confidence interval, 15.6%-26.2%; P < .0001) and RHI score of 2 or less (week 52, 37.6% vs. 19.9%; difference, 17.6%; 95% CI, 11.3%-23.8%; P < .0001).
At week 52, more patients in the vedolizumab group than in the adalimumab group achieved minimum histologic disease activity as determined by Geboes Index score of 3.1 or less (45.7% vs. 30.8%; difference, 14.8%; 95% CI, 8.0%-21.5%; P < .0001) and RHI score of 4 or less(42.3% vs. 25.6%; difference, 16.6%; 95% CI, 10.0%-23.1%; P < .0001).
The investigators performed post hoc analyses of mucosal healing, defined as a composite of the histologic and endoscopic outcomes, with the latter defined as Mayo endoscopic subscore of 1 or less. A greater proportion of patients treated with vedolizumab than with adalimumab met the composite of histologic remission on each score plus endoscopic improvement (Geboes, 35.0% vs. 20.2%; RHI, 33.7% vs. 18.1%), with similar findings for minimal histologic disease activity plus endoscopic improvement (Geboes, 35.0% vs. 20.2%; RHI, 33.7% vs. 18.1%).
The authors noted that the RHI scoring system revealed greater associations between histologic outcomes and endoscopic improvement than did the Geboes Index score, which is an important finding considering the European Crohn’s and Colitis Organisation’s stance recommending consideration of mucosal healing based on findings from endoscopy and histology.
Some study limitations included how the study design precluded dose escalation and a lack of long-term follow-up among these patients.
The researchers believe that the RHI score may be a better choice than the Geboes score for comparing efficacy in clinical trials because RHI is more reproducible, more sensitive to change, and is comparatively easy to interpret.
The study was funded by Takeda, which makes vedolizumab. The authors disclosed several relationships with industry, including some having stock options with or being employed by Takeda.
FROM GASTROENTEROLOGY
Elderly mice receive the gift of warmth
Steal from the warm, give to the cold
If there’s one constant in life other than taxes, it’s elderly people moving to Florida. The Sunshine State’s reputation as a giant retirement home needs no elaboration, but why do senior citizens gravitate there? Well, many reasons, but a big one is that, the older you get, the more susceptible and sensitive you are to the cold. And now, according to a new study, we may have identified a culprit.
Researchers from Yale University examined a group of mice and found that the older ones lacked ICL2 cells in their fatty tissue. These cells, at least in younger mice, help restore body heat when exposed to cold temperatures. Lacking these cells meant that older mice had a limited ability to burn their fat and raise their temperature in response to cold.
Well, job done, all we need to do now is stimulate production of ICL2 cells in elderly people, and they’ll be able to go outside in 80-degree weather without a sweater again. Except there’s a problem. In a cruel twist of fate, when the elderly mice were given a molecule to boost ICL2 cell production, they actually became less tolerant of the cold than at baseline. Oops.
The scientists didn’t give up though, and gave their elderly mice ICL2 cells from young mice. This finally did the trick, though we have to admit, if that treatment does eventually scale up to humans, the prospect of a bunch of senior citizens taking ICL2 cells from young people to stay warm does sound a bit like a bad vampire movie premise. “I vant to suck your immune cell group 2 innate lymphoid cells!” Not the most pithy catch phrase in the world.
Grocery store tapping your subconscious? It’s a good thing
We all know there’s marketing and functionality elements to grocery stores and how they’re set up for your shopping pleasure. But what if I told you that the good old supermarket subconscious trick works on how healthy food decisions are?
In a recent study, researchers at the University of Southampton in England found that if you placed a wider selection of fruits and vegetables near the entrances and more nonfood items near checkouts, sales decreased on the sweets and increased on the produce. “The findings of our study suggest that a healthier store layout could lead to nearly 10,000 extra portions of fruit and vegetables and approximately 1,500 fewer portions of confectionery being sold on a weekly basis in each store,” lead author Dr. Christina Vogel explained.
You’re probably thinking that food placement studies aren’t new. That’s true, but this one went above and beyond. Instead of just looking at the influence placement has on purchase, this one took it further by trying to reduce the consumers’ “calorie opportunities” and examining the effect on sales. Also, customer loyalty, patterns, and diets were taken into account across multiple household members.
The researchers think shifting the layouts in grocery stores could shift people’s food choices, producing a domino effect on the population’s overall diet. With obesity, diabetes, and cardiology concerns always looming, swaying consumers toward healthier food choices makes for better public health overall.
So if you feel like you’re being subconsciously assaulted by veggies every time you walk into Trader Joe’s, just know it’s for your own good.
TikTokers take on tics
We know TikTok is what makes a lot of teens and young adults tick, but what if TikTokers are actually catching tic disorders from other TikTokers?
TikTok blew up during the pandemic. Many people were stuck at home and had nothing better to do than make and watch TikTok videos. The pandemic brought isolation, uncertainty, and anxiety. The stress that followed may have caused many people, mostly women and young girls, to develop tic disorders.
There’s a TikTok for everything, whether it’s a new dance or a recipe. Many people even use TikTok to speak out about their illnesses. Several TikTokers have Tourette’s syndrome and show their tics on their videos. It appears that some audience members actually “catch” the tics from watching the videos and are then unable to stop certain jerking movements or saying specific words.
Neurologists at the University of Calgary (Alta.), who were hearing from colleagues and getting referrals of such patients, called it “an epidemic within the pandemic.” The behavior is not actually Tourette’s, they told Vice, but the patients “cannot stop, and we have absolutely witnessed that.”
There is, of course, controversy over the issue. One individual with the condition said, “I feel like there’s a lot of really weird, backwards stigma on TikTok about tic disorders. Like, you aren’t allowed to have one unless it’s this one.”
Who would have guessed that people would disagree over stuff on the Internet?
Look on the bright side: Obesity edition
The pandemic may have postponed “Top Gun: Maverick” and “The Marvelous Mrs. Maisel” until who-knows-when, but we here at LOTME are happy to announce the nearly-as-anticipated return of Bacteria vs. the World.
As you may recall from our last edition of BVTW, bacteria battled the ghost of Charles Darwin, who had taken the earthly form of antibiotics capable of stopping bacterial evolution. Tonight, our prokaryotic protagonists take on an equally relentless and ubiquitous challenger: obesity.
Specifically, we’re putting bacteria up against the obesity survival paradox, that phenomenon in which obesity and overweight seem to protect against – yes, you guessed it – bacterial infections.
A Swedish research team observed a group of 2,196 individual adults who received care for suspected severe bacterial infection at Skaraborg Hospital in Skövde. One year after hospitalization, 26% of normal-weight (body mass index, 18.5-24.99) patients were dead, compared with 17% of overweight (BMI, 25.0-29.99), 16% of obese (BMI, 30.0-34.99), and 9% of very obese (BMI >35) patients.
These results confirm the obesity survival paradox, but “what we don’t know is how being overweight can benefit the patient with a bacterial infection, or whether it’s connected with functions in the immune system and how they’re regulated,” lead author Dr. Åsa Alsiö said in a written statement.
A spokes-cell for the bacteria disputed the results and challenged the legitimacy of the investigators. When asked if there should be some sort of reexamination of the findings, he/she/it replied: “You bet your flagella.” We then pointed out that humans don’t have flagellum, and the representative raised his/her/its flagella in what could only be considered an obscene gesture.
Steal from the warm, give to the cold
If there’s one constant in life other than taxes, it’s elderly people moving to Florida. The Sunshine State’s reputation as a giant retirement home needs no elaboration, but why do senior citizens gravitate there? Well, many reasons, but a big one is that, the older you get, the more susceptible and sensitive you are to the cold. And now, according to a new study, we may have identified a culprit.
Researchers from Yale University examined a group of mice and found that the older ones lacked ICL2 cells in their fatty tissue. These cells, at least in younger mice, help restore body heat when exposed to cold temperatures. Lacking these cells meant that older mice had a limited ability to burn their fat and raise their temperature in response to cold.
Well, job done, all we need to do now is stimulate production of ICL2 cells in elderly people, and they’ll be able to go outside in 80-degree weather without a sweater again. Except there’s a problem. In a cruel twist of fate, when the elderly mice were given a molecule to boost ICL2 cell production, they actually became less tolerant of the cold than at baseline. Oops.
The scientists didn’t give up though, and gave their elderly mice ICL2 cells from young mice. This finally did the trick, though we have to admit, if that treatment does eventually scale up to humans, the prospect of a bunch of senior citizens taking ICL2 cells from young people to stay warm does sound a bit like a bad vampire movie premise. “I vant to suck your immune cell group 2 innate lymphoid cells!” Not the most pithy catch phrase in the world.
Grocery store tapping your subconscious? It’s a good thing
We all know there’s marketing and functionality elements to grocery stores and how they’re set up for your shopping pleasure. But what if I told you that the good old supermarket subconscious trick works on how healthy food decisions are?
In a recent study, researchers at the University of Southampton in England found that if you placed a wider selection of fruits and vegetables near the entrances and more nonfood items near checkouts, sales decreased on the sweets and increased on the produce. “The findings of our study suggest that a healthier store layout could lead to nearly 10,000 extra portions of fruit and vegetables and approximately 1,500 fewer portions of confectionery being sold on a weekly basis in each store,” lead author Dr. Christina Vogel explained.
You’re probably thinking that food placement studies aren’t new. That’s true, but this one went above and beyond. Instead of just looking at the influence placement has on purchase, this one took it further by trying to reduce the consumers’ “calorie opportunities” and examining the effect on sales. Also, customer loyalty, patterns, and diets were taken into account across multiple household members.
The researchers think shifting the layouts in grocery stores could shift people’s food choices, producing a domino effect on the population’s overall diet. With obesity, diabetes, and cardiology concerns always looming, swaying consumers toward healthier food choices makes for better public health overall.
So if you feel like you’re being subconsciously assaulted by veggies every time you walk into Trader Joe’s, just know it’s for your own good.
TikTokers take on tics
We know TikTok is what makes a lot of teens and young adults tick, but what if TikTokers are actually catching tic disorders from other TikTokers?
TikTok blew up during the pandemic. Many people were stuck at home and had nothing better to do than make and watch TikTok videos. The pandemic brought isolation, uncertainty, and anxiety. The stress that followed may have caused many people, mostly women and young girls, to develop tic disorders.
There’s a TikTok for everything, whether it’s a new dance or a recipe. Many people even use TikTok to speak out about their illnesses. Several TikTokers have Tourette’s syndrome and show their tics on their videos. It appears that some audience members actually “catch” the tics from watching the videos and are then unable to stop certain jerking movements or saying specific words.
Neurologists at the University of Calgary (Alta.), who were hearing from colleagues and getting referrals of such patients, called it “an epidemic within the pandemic.” The behavior is not actually Tourette’s, they told Vice, but the patients “cannot stop, and we have absolutely witnessed that.”
There is, of course, controversy over the issue. One individual with the condition said, “I feel like there’s a lot of really weird, backwards stigma on TikTok about tic disorders. Like, you aren’t allowed to have one unless it’s this one.”
Who would have guessed that people would disagree over stuff on the Internet?
Look on the bright side: Obesity edition
The pandemic may have postponed “Top Gun: Maverick” and “The Marvelous Mrs. Maisel” until who-knows-when, but we here at LOTME are happy to announce the nearly-as-anticipated return of Bacteria vs. the World.
As you may recall from our last edition of BVTW, bacteria battled the ghost of Charles Darwin, who had taken the earthly form of antibiotics capable of stopping bacterial evolution. Tonight, our prokaryotic protagonists take on an equally relentless and ubiquitous challenger: obesity.
Specifically, we’re putting bacteria up against the obesity survival paradox, that phenomenon in which obesity and overweight seem to protect against – yes, you guessed it – bacterial infections.
A Swedish research team observed a group of 2,196 individual adults who received care for suspected severe bacterial infection at Skaraborg Hospital in Skövde. One year after hospitalization, 26% of normal-weight (body mass index, 18.5-24.99) patients were dead, compared with 17% of overweight (BMI, 25.0-29.99), 16% of obese (BMI, 30.0-34.99), and 9% of very obese (BMI >35) patients.
These results confirm the obesity survival paradox, but “what we don’t know is how being overweight can benefit the patient with a bacterial infection, or whether it’s connected with functions in the immune system and how they’re regulated,” lead author Dr. Åsa Alsiö said in a written statement.
A spokes-cell for the bacteria disputed the results and challenged the legitimacy of the investigators. When asked if there should be some sort of reexamination of the findings, he/she/it replied: “You bet your flagella.” We then pointed out that humans don’t have flagellum, and the representative raised his/her/its flagella in what could only be considered an obscene gesture.
Steal from the warm, give to the cold
If there’s one constant in life other than taxes, it’s elderly people moving to Florida. The Sunshine State’s reputation as a giant retirement home needs no elaboration, but why do senior citizens gravitate there? Well, many reasons, but a big one is that, the older you get, the more susceptible and sensitive you are to the cold. And now, according to a new study, we may have identified a culprit.
Researchers from Yale University examined a group of mice and found that the older ones lacked ICL2 cells in their fatty tissue. These cells, at least in younger mice, help restore body heat when exposed to cold temperatures. Lacking these cells meant that older mice had a limited ability to burn their fat and raise their temperature in response to cold.
Well, job done, all we need to do now is stimulate production of ICL2 cells in elderly people, and they’ll be able to go outside in 80-degree weather without a sweater again. Except there’s a problem. In a cruel twist of fate, when the elderly mice were given a molecule to boost ICL2 cell production, they actually became less tolerant of the cold than at baseline. Oops.
The scientists didn’t give up though, and gave their elderly mice ICL2 cells from young mice. This finally did the trick, though we have to admit, if that treatment does eventually scale up to humans, the prospect of a bunch of senior citizens taking ICL2 cells from young people to stay warm does sound a bit like a bad vampire movie premise. “I vant to suck your immune cell group 2 innate lymphoid cells!” Not the most pithy catch phrase in the world.
Grocery store tapping your subconscious? It’s a good thing
We all know there’s marketing and functionality elements to grocery stores and how they’re set up for your shopping pleasure. But what if I told you that the good old supermarket subconscious trick works on how healthy food decisions are?
In a recent study, researchers at the University of Southampton in England found that if you placed a wider selection of fruits and vegetables near the entrances and more nonfood items near checkouts, sales decreased on the sweets and increased on the produce. “The findings of our study suggest that a healthier store layout could lead to nearly 10,000 extra portions of fruit and vegetables and approximately 1,500 fewer portions of confectionery being sold on a weekly basis in each store,” lead author Dr. Christina Vogel explained.
You’re probably thinking that food placement studies aren’t new. That’s true, but this one went above and beyond. Instead of just looking at the influence placement has on purchase, this one took it further by trying to reduce the consumers’ “calorie opportunities” and examining the effect on sales. Also, customer loyalty, patterns, and diets were taken into account across multiple household members.
The researchers think shifting the layouts in grocery stores could shift people’s food choices, producing a domino effect on the population’s overall diet. With obesity, diabetes, and cardiology concerns always looming, swaying consumers toward healthier food choices makes for better public health overall.
So if you feel like you’re being subconsciously assaulted by veggies every time you walk into Trader Joe’s, just know it’s for your own good.
TikTokers take on tics
We know TikTok is what makes a lot of teens and young adults tick, but what if TikTokers are actually catching tic disorders from other TikTokers?
TikTok blew up during the pandemic. Many people were stuck at home and had nothing better to do than make and watch TikTok videos. The pandemic brought isolation, uncertainty, and anxiety. The stress that followed may have caused many people, mostly women and young girls, to develop tic disorders.
There’s a TikTok for everything, whether it’s a new dance or a recipe. Many people even use TikTok to speak out about their illnesses. Several TikTokers have Tourette’s syndrome and show their tics on their videos. It appears that some audience members actually “catch” the tics from watching the videos and are then unable to stop certain jerking movements or saying specific words.
Neurologists at the University of Calgary (Alta.), who were hearing from colleagues and getting referrals of such patients, called it “an epidemic within the pandemic.” The behavior is not actually Tourette’s, they told Vice, but the patients “cannot stop, and we have absolutely witnessed that.”
There is, of course, controversy over the issue. One individual with the condition said, “I feel like there’s a lot of really weird, backwards stigma on TikTok about tic disorders. Like, you aren’t allowed to have one unless it’s this one.”
Who would have guessed that people would disagree over stuff on the Internet?
Look on the bright side: Obesity edition
The pandemic may have postponed “Top Gun: Maverick” and “The Marvelous Mrs. Maisel” until who-knows-when, but we here at LOTME are happy to announce the nearly-as-anticipated return of Bacteria vs. the World.
As you may recall from our last edition of BVTW, bacteria battled the ghost of Charles Darwin, who had taken the earthly form of antibiotics capable of stopping bacterial evolution. Tonight, our prokaryotic protagonists take on an equally relentless and ubiquitous challenger: obesity.
Specifically, we’re putting bacteria up against the obesity survival paradox, that phenomenon in which obesity and overweight seem to protect against – yes, you guessed it – bacterial infections.
A Swedish research team observed a group of 2,196 individual adults who received care for suspected severe bacterial infection at Skaraborg Hospital in Skövde. One year after hospitalization, 26% of normal-weight (body mass index, 18.5-24.99) patients were dead, compared with 17% of overweight (BMI, 25.0-29.99), 16% of obese (BMI, 30.0-34.99), and 9% of very obese (BMI >35) patients.
These results confirm the obesity survival paradox, but “what we don’t know is how being overweight can benefit the patient with a bacterial infection, or whether it’s connected with functions in the immune system and how they’re regulated,” lead author Dr. Åsa Alsiö said in a written statement.
A spokes-cell for the bacteria disputed the results and challenged the legitimacy of the investigators. When asked if there should be some sort of reexamination of the findings, he/she/it replied: “You bet your flagella.” We then pointed out that humans don’t have flagellum, and the representative raised his/her/its flagella in what could only be considered an obscene gesture.