User login
FDA approves first twice-yearly antipsychotic for schizophrenia
The U.S. Food and Drug Administration has approved a 6-month injection form of the long-acting atypical antipsychotic paliperidone palmitate (Invega Hafyera, Janssen Pharmaceuticals) for the treatment of schizophrenia in adults, the company has announced.
This marks the “first-and-only twice-yearly injectable” approved for treating schizophrenia, the company added in a press release.
Before transitioning to the 6-month form, patients must be adequately treated for a minimum of 4 months with the company’s 1-month formulation of paliperidone (Invega Sustenna), or with the 3-month version (Invega Trinza) for at least one 3-month injection cycle.
The FDA approved the twice-yearly formulation on the basis of results from a 12-month, randomized, double-blind, phase 3 study that enrolled 702 adults with schizophrenia from 20 countries.
“The phase 3 trial results provide compelling evidence that 6-month paliperidone palmitate offers longer-term symptom control with the fewest doses per year, which may support greater patient adherence,” Gustavo Alva, MD, medical director at ATP Clinical Research, Costa Mesa, Calif., and 6-month paliperidone palmitate clinical trial investigator, said in the release.
Noninferiority results
In the phase 3 trial, the twice-yearly version of the drug proved noninferior to the 3-month version on the primary endpoint of time to first relapse at the end of 12 months, with 92.5% and 95% of patients, respectively, relapse-free at 12 months.
Relapse was defined as psychiatric hospitalization, increase in Positive and Negative Syndrome Scale (PANSS) total score, increase in individual PANSS item scores, self-injury, violent behavior, or suicidal/homicidal ideation.
The safety profile observed in the trial was in line with prior studies of the 1-month and 3-month versions, with no new safety signals, the researchers note.
The most common adverse reactions affecting at least 5% of participants in the clinical trial receiving twice-year paliperidone were upper respiratory tract infection (12%), injection site reaction (11%), weight gain (9%), headache (7%), and parkinsonism (5%).
Relapse is common in adults with schizophrenia, often because of missed doses of medication, the company said in the news release.
, while research continues to demonstrate that stronger medication adherence means better patient outcomes,” Dr. Alva said.
Recently updated evidence-based guidelines from the American Psychiatric Association recommend consideration of long-acting injectables for appropriate adults living with schizophrenia.
“Long-acting injectable treatments offer a number of advantages, compared to oral medication for schizophrenia, including relief from needing to remember to take medication daily, lower discontinuation rates, and sustained treatment over longer periods,” Bill Martin, PhD, with Janssen Research & Development, said in the release.
“Today’s approval enables us to rethink how we manage this chronic disease by offering patients and caregivers the potential for a life less defined by schizophrenia medication,” Dr. Martin added.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a 6-month injection form of the long-acting atypical antipsychotic paliperidone palmitate (Invega Hafyera, Janssen Pharmaceuticals) for the treatment of schizophrenia in adults, the company has announced.
This marks the “first-and-only twice-yearly injectable” approved for treating schizophrenia, the company added in a press release.
Before transitioning to the 6-month form, patients must be adequately treated for a minimum of 4 months with the company’s 1-month formulation of paliperidone (Invega Sustenna), or with the 3-month version (Invega Trinza) for at least one 3-month injection cycle.
The FDA approved the twice-yearly formulation on the basis of results from a 12-month, randomized, double-blind, phase 3 study that enrolled 702 adults with schizophrenia from 20 countries.
“The phase 3 trial results provide compelling evidence that 6-month paliperidone palmitate offers longer-term symptom control with the fewest doses per year, which may support greater patient adherence,” Gustavo Alva, MD, medical director at ATP Clinical Research, Costa Mesa, Calif., and 6-month paliperidone palmitate clinical trial investigator, said in the release.
Noninferiority results
In the phase 3 trial, the twice-yearly version of the drug proved noninferior to the 3-month version on the primary endpoint of time to first relapse at the end of 12 months, with 92.5% and 95% of patients, respectively, relapse-free at 12 months.
Relapse was defined as psychiatric hospitalization, increase in Positive and Negative Syndrome Scale (PANSS) total score, increase in individual PANSS item scores, self-injury, violent behavior, or suicidal/homicidal ideation.
The safety profile observed in the trial was in line with prior studies of the 1-month and 3-month versions, with no new safety signals, the researchers note.
The most common adverse reactions affecting at least 5% of participants in the clinical trial receiving twice-year paliperidone were upper respiratory tract infection (12%), injection site reaction (11%), weight gain (9%), headache (7%), and parkinsonism (5%).
Relapse is common in adults with schizophrenia, often because of missed doses of medication, the company said in the news release.
, while research continues to demonstrate that stronger medication adherence means better patient outcomes,” Dr. Alva said.
Recently updated evidence-based guidelines from the American Psychiatric Association recommend consideration of long-acting injectables for appropriate adults living with schizophrenia.
“Long-acting injectable treatments offer a number of advantages, compared to oral medication for schizophrenia, including relief from needing to remember to take medication daily, lower discontinuation rates, and sustained treatment over longer periods,” Bill Martin, PhD, with Janssen Research & Development, said in the release.
“Today’s approval enables us to rethink how we manage this chronic disease by offering patients and caregivers the potential for a life less defined by schizophrenia medication,” Dr. Martin added.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a 6-month injection form of the long-acting atypical antipsychotic paliperidone palmitate (Invega Hafyera, Janssen Pharmaceuticals) for the treatment of schizophrenia in adults, the company has announced.
This marks the “first-and-only twice-yearly injectable” approved for treating schizophrenia, the company added in a press release.
Before transitioning to the 6-month form, patients must be adequately treated for a minimum of 4 months with the company’s 1-month formulation of paliperidone (Invega Sustenna), or with the 3-month version (Invega Trinza) for at least one 3-month injection cycle.
The FDA approved the twice-yearly formulation on the basis of results from a 12-month, randomized, double-blind, phase 3 study that enrolled 702 adults with schizophrenia from 20 countries.
“The phase 3 trial results provide compelling evidence that 6-month paliperidone palmitate offers longer-term symptom control with the fewest doses per year, which may support greater patient adherence,” Gustavo Alva, MD, medical director at ATP Clinical Research, Costa Mesa, Calif., and 6-month paliperidone palmitate clinical trial investigator, said in the release.
Noninferiority results
In the phase 3 trial, the twice-yearly version of the drug proved noninferior to the 3-month version on the primary endpoint of time to first relapse at the end of 12 months, with 92.5% and 95% of patients, respectively, relapse-free at 12 months.
Relapse was defined as psychiatric hospitalization, increase in Positive and Negative Syndrome Scale (PANSS) total score, increase in individual PANSS item scores, self-injury, violent behavior, or suicidal/homicidal ideation.
The safety profile observed in the trial was in line with prior studies of the 1-month and 3-month versions, with no new safety signals, the researchers note.
The most common adverse reactions affecting at least 5% of participants in the clinical trial receiving twice-year paliperidone were upper respiratory tract infection (12%), injection site reaction (11%), weight gain (9%), headache (7%), and parkinsonism (5%).
Relapse is common in adults with schizophrenia, often because of missed doses of medication, the company said in the news release.
, while research continues to demonstrate that stronger medication adherence means better patient outcomes,” Dr. Alva said.
Recently updated evidence-based guidelines from the American Psychiatric Association recommend consideration of long-acting injectables for appropriate adults living with schizophrenia.
“Long-acting injectable treatments offer a number of advantages, compared to oral medication for schizophrenia, including relief from needing to remember to take medication daily, lower discontinuation rates, and sustained treatment over longer periods,” Bill Martin, PhD, with Janssen Research & Development, said in the release.
“Today’s approval enables us to rethink how we manage this chronic disease by offering patients and caregivers the potential for a life less defined by schizophrenia medication,” Dr. Martin added.
A version of this article first appeared on Medscape.com.
Office-based pediatricians unprepared for emergencies
Emergency preparedness in U.S. pediatric offices is variable and less than ideal, especially in smaller independent practices, a 15-month multicenter study has found.
Researchers led by Kamal Abulebda, MD, associate professor of clinical pediatrics in the division of pediatric critical care medicine at Indiana University and Riley Hospital for Children in Indianapolis, report that adherence to the 2007 policy statement of the American Academy of Pediatrics on emergency preparedness in pediatric primary care offices was suboptimal across 42 offices in 9 states. They suggest that academic and community partnerships use in-situ simulation exercises to address preparedness gaps and implement standard procedures for contacting emergency medical services.
The group’s findings were published online in Pediatrics. “These data can be used to guide the development of interventions to improve emergency preparedness and care delivery in pediatric offices, Dr. Abulebda and coauthors wrote, noting that theirs is the first multicenter study to directly measure preparedness and quality of care in pediatric offices.
According to the authors, the incidence of a child’s requiring emergent stabilization in an individual office ranges from weekly to monthly, with seizures and respiratory distress being the most common events.
The study was conducted from 2018 to 2020 by 48 national teams participating in in-situ simulated sessions in the ambulatory setting. Office teams, recruited from practices by members of regional academic medical centers, included two patients – a child with respiratory distress and a child with a seizure. Almost 40% were from Indiana.
The scenarios and checklists for the mock exercises were created by content experts in pediatric emergency medicine and critical care using evidence-based guidelines and best practices.
Previous research has relied on self-reported surveys rather than direct measurement to assess adherence to the AAP guidelines, the authors say. In-person surveys assessed adherence to AAP recommendations for emergency preparedness. In-person surveys were, however, used to gauge adherence to AAP recommendations for emergency preparedness.
Findings
The overall mean emergency preparedness score was 74.7% (standard deviation [SD] 12.9), with an unweighted percentage of adherence to checklists calculated for each case. By emergency type, the median asthma case performance score was 63.6% (interquartile range [IQR] 43.2-81.2), and the median seizure case score was 69.2% (IQR 46.2-80.8).
On the measure of essential equipment and supplies, the mean subscore (relating to availability of such items as oxygen sources, suction devices, and epinephrine, for example) was 82.2% (SD 15.1).
As for recommended policies and protocols (e.g., regular assessment of the office, maintenance of emergency equipment and medications) the mean subscore fell to 57.1% (SD 25.6).
In multivariable analyses, offices with a standardized procedure for contacting EMS had a higher rate of activating that service during the simulations.
Independent practices and smaller total staff size were associated with lower preparedness compared with larger groups: beta = –11.89, 95% confidence interval [CI], 19.33-4.45).
Higher annual patient volume and larger total staff size were slightly associated with higher scores (beta = .001, 95% CI, .00-001, P = .017; and beta = .51, 95% CI, .19-.83, P = .002, respectively).
Affiliation with an academic medical center and the presence of learners were not associated with higher scores. And in multivariable regression, a higher annual patient volume lost its significant association with greater preparedness.
So why the lag in preparedness despite the long-standing AAP recommendations? “It’s most likely due to the rare occurrence of these emergencies in the office setting, in addition to most offices’ dependence on EMS when they encounter pediatric emergencies in their setting,” Dr. Abulebda said in an interview. “A 2018 study published by Yuknis and associates demonstrated that the average time from EMS notification to arrival on scene was just 6 minutes.”
In other study findings, 82% of offices did not have an infant bag valve mask and would therefore need to wait for EMS to administer lifesaving ventilation. “This highlights the need to have this equipment available and maintain the skills necessary to care for patients in respiratory distress, the most common emergency encountered in the office setting,” Dr. Abulebda and associates wrote.
A cardiac arrest board is another example of a potentially lifesaving piece of equipment that was not available in the majority of offices, likely because of the rarity of this event in the office setting, but lack of this item may result in poor cardiopulmonary resuscitation quality before the arrival of EMS.
In an accompanying editorial, Jesse Hackell, MD, a pediatrician at Boston Children’s Health Physicians and New York Medical College in Pomona, N.Y., noted that data from 2 decades ago suggested that many pediatric offices saw multiple children requiring emergency intervention each week. More recent figures, however, indicate the situation has evolved, with fewer than 1% of current pediatric EMS transports originating from the office setting.
Dr. Hackell agrees that implementation of AAP recommendations has been far from universal and cites the cost of equipment and supplies as well as a lack of access to training and evaluation as significant barriers to implementation. “In addition, the infrequent occurrence of these emergencies makes maintenance of resuscitation skills even more difficult without frequent practice,” he wrote.
Further complicating the issue, preparedness needs vary with practice location, the response time of local EMS, and proximity to an emergency department. “Pediatric offices in more rural areas, which are farther from these services, will require more equipment and more skills to provide optimal emergency care to children living in these underresourced areas,” he wrote.
He called for equitable distribution of preparedness training, equipment, and staffing, with guidance designed to meet patient needs and ensure optimal outcomes. “In discussion of recommendations, one should consider the likely conditions requiring this response, availability of resources beyond the pediatric office, and ongoing training and support needed to maintain provider skills at the level needed for a successful response to any pediatric emergency,” Dr. Hackell wrote.
This study was supported by grants from Indiana University Health Values and the RBaby Foundation. One study coauthor is a board observer of a medical device company. No other authors disclosed financial relationships relevant to this work. Dr. Hackell has disclosed having no competing interests.
Emergency preparedness in U.S. pediatric offices is variable and less than ideal, especially in smaller independent practices, a 15-month multicenter study has found.
Researchers led by Kamal Abulebda, MD, associate professor of clinical pediatrics in the division of pediatric critical care medicine at Indiana University and Riley Hospital for Children in Indianapolis, report that adherence to the 2007 policy statement of the American Academy of Pediatrics on emergency preparedness in pediatric primary care offices was suboptimal across 42 offices in 9 states. They suggest that academic and community partnerships use in-situ simulation exercises to address preparedness gaps and implement standard procedures for contacting emergency medical services.
The group’s findings were published online in Pediatrics. “These data can be used to guide the development of interventions to improve emergency preparedness and care delivery in pediatric offices, Dr. Abulebda and coauthors wrote, noting that theirs is the first multicenter study to directly measure preparedness and quality of care in pediatric offices.
According to the authors, the incidence of a child’s requiring emergent stabilization in an individual office ranges from weekly to monthly, with seizures and respiratory distress being the most common events.
The study was conducted from 2018 to 2020 by 48 national teams participating in in-situ simulated sessions in the ambulatory setting. Office teams, recruited from practices by members of regional academic medical centers, included two patients – a child with respiratory distress and a child with a seizure. Almost 40% were from Indiana.
The scenarios and checklists for the mock exercises were created by content experts in pediatric emergency medicine and critical care using evidence-based guidelines and best practices.
Previous research has relied on self-reported surveys rather than direct measurement to assess adherence to the AAP guidelines, the authors say. In-person surveys assessed adherence to AAP recommendations for emergency preparedness. In-person surveys were, however, used to gauge adherence to AAP recommendations for emergency preparedness.
Findings
The overall mean emergency preparedness score was 74.7% (standard deviation [SD] 12.9), with an unweighted percentage of adherence to checklists calculated for each case. By emergency type, the median asthma case performance score was 63.6% (interquartile range [IQR] 43.2-81.2), and the median seizure case score was 69.2% (IQR 46.2-80.8).
On the measure of essential equipment and supplies, the mean subscore (relating to availability of such items as oxygen sources, suction devices, and epinephrine, for example) was 82.2% (SD 15.1).
As for recommended policies and protocols (e.g., regular assessment of the office, maintenance of emergency equipment and medications) the mean subscore fell to 57.1% (SD 25.6).
In multivariable analyses, offices with a standardized procedure for contacting EMS had a higher rate of activating that service during the simulations.
Independent practices and smaller total staff size were associated with lower preparedness compared with larger groups: beta = –11.89, 95% confidence interval [CI], 19.33-4.45).
Higher annual patient volume and larger total staff size were slightly associated with higher scores (beta = .001, 95% CI, .00-001, P = .017; and beta = .51, 95% CI, .19-.83, P = .002, respectively).
Affiliation with an academic medical center and the presence of learners were not associated with higher scores. And in multivariable regression, a higher annual patient volume lost its significant association with greater preparedness.
So why the lag in preparedness despite the long-standing AAP recommendations? “It’s most likely due to the rare occurrence of these emergencies in the office setting, in addition to most offices’ dependence on EMS when they encounter pediatric emergencies in their setting,” Dr. Abulebda said in an interview. “A 2018 study published by Yuknis and associates demonstrated that the average time from EMS notification to arrival on scene was just 6 minutes.”
In other study findings, 82% of offices did not have an infant bag valve mask and would therefore need to wait for EMS to administer lifesaving ventilation. “This highlights the need to have this equipment available and maintain the skills necessary to care for patients in respiratory distress, the most common emergency encountered in the office setting,” Dr. Abulebda and associates wrote.
A cardiac arrest board is another example of a potentially lifesaving piece of equipment that was not available in the majority of offices, likely because of the rarity of this event in the office setting, but lack of this item may result in poor cardiopulmonary resuscitation quality before the arrival of EMS.
In an accompanying editorial, Jesse Hackell, MD, a pediatrician at Boston Children’s Health Physicians and New York Medical College in Pomona, N.Y., noted that data from 2 decades ago suggested that many pediatric offices saw multiple children requiring emergency intervention each week. More recent figures, however, indicate the situation has evolved, with fewer than 1% of current pediatric EMS transports originating from the office setting.
Dr. Hackell agrees that implementation of AAP recommendations has been far from universal and cites the cost of equipment and supplies as well as a lack of access to training and evaluation as significant barriers to implementation. “In addition, the infrequent occurrence of these emergencies makes maintenance of resuscitation skills even more difficult without frequent practice,” he wrote.
Further complicating the issue, preparedness needs vary with practice location, the response time of local EMS, and proximity to an emergency department. “Pediatric offices in more rural areas, which are farther from these services, will require more equipment and more skills to provide optimal emergency care to children living in these underresourced areas,” he wrote.
He called for equitable distribution of preparedness training, equipment, and staffing, with guidance designed to meet patient needs and ensure optimal outcomes. “In discussion of recommendations, one should consider the likely conditions requiring this response, availability of resources beyond the pediatric office, and ongoing training and support needed to maintain provider skills at the level needed for a successful response to any pediatric emergency,” Dr. Hackell wrote.
This study was supported by grants from Indiana University Health Values and the RBaby Foundation. One study coauthor is a board observer of a medical device company. No other authors disclosed financial relationships relevant to this work. Dr. Hackell has disclosed having no competing interests.
Emergency preparedness in U.S. pediatric offices is variable and less than ideal, especially in smaller independent practices, a 15-month multicenter study has found.
Researchers led by Kamal Abulebda, MD, associate professor of clinical pediatrics in the division of pediatric critical care medicine at Indiana University and Riley Hospital for Children in Indianapolis, report that adherence to the 2007 policy statement of the American Academy of Pediatrics on emergency preparedness in pediatric primary care offices was suboptimal across 42 offices in 9 states. They suggest that academic and community partnerships use in-situ simulation exercises to address preparedness gaps and implement standard procedures for contacting emergency medical services.
The group’s findings were published online in Pediatrics. “These data can be used to guide the development of interventions to improve emergency preparedness and care delivery in pediatric offices, Dr. Abulebda and coauthors wrote, noting that theirs is the first multicenter study to directly measure preparedness and quality of care in pediatric offices.
According to the authors, the incidence of a child’s requiring emergent stabilization in an individual office ranges from weekly to monthly, with seizures and respiratory distress being the most common events.
The study was conducted from 2018 to 2020 by 48 national teams participating in in-situ simulated sessions in the ambulatory setting. Office teams, recruited from practices by members of regional academic medical centers, included two patients – a child with respiratory distress and a child with a seizure. Almost 40% were from Indiana.
The scenarios and checklists for the mock exercises were created by content experts in pediatric emergency medicine and critical care using evidence-based guidelines and best practices.
Previous research has relied on self-reported surveys rather than direct measurement to assess adherence to the AAP guidelines, the authors say. In-person surveys assessed adherence to AAP recommendations for emergency preparedness. In-person surveys were, however, used to gauge adherence to AAP recommendations for emergency preparedness.
Findings
The overall mean emergency preparedness score was 74.7% (standard deviation [SD] 12.9), with an unweighted percentage of adherence to checklists calculated for each case. By emergency type, the median asthma case performance score was 63.6% (interquartile range [IQR] 43.2-81.2), and the median seizure case score was 69.2% (IQR 46.2-80.8).
On the measure of essential equipment and supplies, the mean subscore (relating to availability of such items as oxygen sources, suction devices, and epinephrine, for example) was 82.2% (SD 15.1).
As for recommended policies and protocols (e.g., regular assessment of the office, maintenance of emergency equipment and medications) the mean subscore fell to 57.1% (SD 25.6).
In multivariable analyses, offices with a standardized procedure for contacting EMS had a higher rate of activating that service during the simulations.
Independent practices and smaller total staff size were associated with lower preparedness compared with larger groups: beta = –11.89, 95% confidence interval [CI], 19.33-4.45).
Higher annual patient volume and larger total staff size were slightly associated with higher scores (beta = .001, 95% CI, .00-001, P = .017; and beta = .51, 95% CI, .19-.83, P = .002, respectively).
Affiliation with an academic medical center and the presence of learners were not associated with higher scores. And in multivariable regression, a higher annual patient volume lost its significant association with greater preparedness.
So why the lag in preparedness despite the long-standing AAP recommendations? “It’s most likely due to the rare occurrence of these emergencies in the office setting, in addition to most offices’ dependence on EMS when they encounter pediatric emergencies in their setting,” Dr. Abulebda said in an interview. “A 2018 study published by Yuknis and associates demonstrated that the average time from EMS notification to arrival on scene was just 6 minutes.”
In other study findings, 82% of offices did not have an infant bag valve mask and would therefore need to wait for EMS to administer lifesaving ventilation. “This highlights the need to have this equipment available and maintain the skills necessary to care for patients in respiratory distress, the most common emergency encountered in the office setting,” Dr. Abulebda and associates wrote.
A cardiac arrest board is another example of a potentially lifesaving piece of equipment that was not available in the majority of offices, likely because of the rarity of this event in the office setting, but lack of this item may result in poor cardiopulmonary resuscitation quality before the arrival of EMS.
In an accompanying editorial, Jesse Hackell, MD, a pediatrician at Boston Children’s Health Physicians and New York Medical College in Pomona, N.Y., noted that data from 2 decades ago suggested that many pediatric offices saw multiple children requiring emergency intervention each week. More recent figures, however, indicate the situation has evolved, with fewer than 1% of current pediatric EMS transports originating from the office setting.
Dr. Hackell agrees that implementation of AAP recommendations has been far from universal and cites the cost of equipment and supplies as well as a lack of access to training and evaluation as significant barriers to implementation. “In addition, the infrequent occurrence of these emergencies makes maintenance of resuscitation skills even more difficult without frequent practice,” he wrote.
Further complicating the issue, preparedness needs vary with practice location, the response time of local EMS, and proximity to an emergency department. “Pediatric offices in more rural areas, which are farther from these services, will require more equipment and more skills to provide optimal emergency care to children living in these underresourced areas,” he wrote.
He called for equitable distribution of preparedness training, equipment, and staffing, with guidance designed to meet patient needs and ensure optimal outcomes. “In discussion of recommendations, one should consider the likely conditions requiring this response, availability of resources beyond the pediatric office, and ongoing training and support needed to maintain provider skills at the level needed for a successful response to any pediatric emergency,” Dr. Hackell wrote.
This study was supported by grants from Indiana University Health Values and the RBaby Foundation. One study coauthor is a board observer of a medical device company. No other authors disclosed financial relationships relevant to this work. Dr. Hackell has disclosed having no competing interests.
FROM PEDIATRICS
Another COVID-19 patient to get ivermectin after court order
Another case, another state, another judge ordering a hospital to give a patient a controversial horse deworming drug to treat a severe case of COVID-19.
, according to the Ohio Capital Journal. Judge Gregory Howard’s ruling comes after Mr. Smith’s wife sued to force the hospital to provide the controversial drug to her husband, who has been hospitalized since July 15.
Julie Smith has gotten Fred Wagshul, MD, to agree to administer ivermectin to her husband. Dr. Wagshul is known as a member of a group of doctors who say the Centers for Disease Control and Prevention and the Food and Drug Administration are lying about ivermectin’s usefulness in fighting COVID-19. Both agencies have warned against using the drug to treat COVID-19, saying there is no evidence it works and that it can be dangerous in large amounts.
According to the Ohio Capital Journal, Dr. Wagshul accused the CDC and FDA of engaging in a “conspiracy” to prevent ivermectin’s use.
But Arthur L. Caplan, MD, professor of bioethics at New York University’s Langone Medical Center, said, “it is absurd that this order was issued,” according to an interview in Ars Technica. “If I were these doctors, I simply wouldn’t do it.”
It is not the first time a judge has ordered ivermectin’s use against a hospital’s wishes.
A 68-year-old woman with COVID-19 in an Illinois hospital started receiving the controversial drug in May after her family sued the hospital to have someone administer it.
Nurije Fype’s daughter, Desareta, filed suit against Elmhurst Hospital, part of Edward-Elmhurst Health, asking that her mother receive the treatment, which is approved as an antiparasitic drug but not approved for the treatment of COVID-19. Desareta Fype was granted temporary guardianship of her mother.
The FDA has published guidance titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19” on its website. The National Institutes of Health said there is not enough data to recommend either for or against its use in treating COVID-19.
But DuPage County Judge James Orel ruled Ms. Fype should be allowed to get the treatment.
Three days later, according to the Daily Herald, the lawyer for the hospital, Joseph Monahan, argued the hospital could not find a hospital-affiliated doctor to administer the ivermectin.
The Herald reported the judge told the hospital to “get out of the way” and allow any board-certified doctor to administer the drug.
When Ms. Fype’s doctor was unable to administer it, the legal team found another doctor, Alan Bain, DO, to do it. Mr. Monahan said Dr. Bain was granted credentials to work at the hospital so he could administer it.
Judge Orel denied a request from Desareta Fype’s lawyer to order the hospital’s nurses to administer further doses. The judge also denied a request to hold the hospital in contempt of court.
A version of this article first appeared on WebMD.com.
Another case, another state, another judge ordering a hospital to give a patient a controversial horse deworming drug to treat a severe case of COVID-19.
, according to the Ohio Capital Journal. Judge Gregory Howard’s ruling comes after Mr. Smith’s wife sued to force the hospital to provide the controversial drug to her husband, who has been hospitalized since July 15.
Julie Smith has gotten Fred Wagshul, MD, to agree to administer ivermectin to her husband. Dr. Wagshul is known as a member of a group of doctors who say the Centers for Disease Control and Prevention and the Food and Drug Administration are lying about ivermectin’s usefulness in fighting COVID-19. Both agencies have warned against using the drug to treat COVID-19, saying there is no evidence it works and that it can be dangerous in large amounts.
According to the Ohio Capital Journal, Dr. Wagshul accused the CDC and FDA of engaging in a “conspiracy” to prevent ivermectin’s use.
But Arthur L. Caplan, MD, professor of bioethics at New York University’s Langone Medical Center, said, “it is absurd that this order was issued,” according to an interview in Ars Technica. “If I were these doctors, I simply wouldn’t do it.”
It is not the first time a judge has ordered ivermectin’s use against a hospital’s wishes.
A 68-year-old woman with COVID-19 in an Illinois hospital started receiving the controversial drug in May after her family sued the hospital to have someone administer it.
Nurije Fype’s daughter, Desareta, filed suit against Elmhurst Hospital, part of Edward-Elmhurst Health, asking that her mother receive the treatment, which is approved as an antiparasitic drug but not approved for the treatment of COVID-19. Desareta Fype was granted temporary guardianship of her mother.
The FDA has published guidance titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19” on its website. The National Institutes of Health said there is not enough data to recommend either for or against its use in treating COVID-19.
But DuPage County Judge James Orel ruled Ms. Fype should be allowed to get the treatment.
Three days later, according to the Daily Herald, the lawyer for the hospital, Joseph Monahan, argued the hospital could not find a hospital-affiliated doctor to administer the ivermectin.
The Herald reported the judge told the hospital to “get out of the way” and allow any board-certified doctor to administer the drug.
When Ms. Fype’s doctor was unable to administer it, the legal team found another doctor, Alan Bain, DO, to do it. Mr. Monahan said Dr. Bain was granted credentials to work at the hospital so he could administer it.
Judge Orel denied a request from Desareta Fype’s lawyer to order the hospital’s nurses to administer further doses. The judge also denied a request to hold the hospital in contempt of court.
A version of this article first appeared on WebMD.com.
Another case, another state, another judge ordering a hospital to give a patient a controversial horse deworming drug to treat a severe case of COVID-19.
, according to the Ohio Capital Journal. Judge Gregory Howard’s ruling comes after Mr. Smith’s wife sued to force the hospital to provide the controversial drug to her husband, who has been hospitalized since July 15.
Julie Smith has gotten Fred Wagshul, MD, to agree to administer ivermectin to her husband. Dr. Wagshul is known as a member of a group of doctors who say the Centers for Disease Control and Prevention and the Food and Drug Administration are lying about ivermectin’s usefulness in fighting COVID-19. Both agencies have warned against using the drug to treat COVID-19, saying there is no evidence it works and that it can be dangerous in large amounts.
According to the Ohio Capital Journal, Dr. Wagshul accused the CDC and FDA of engaging in a “conspiracy” to prevent ivermectin’s use.
But Arthur L. Caplan, MD, professor of bioethics at New York University’s Langone Medical Center, said, “it is absurd that this order was issued,” according to an interview in Ars Technica. “If I were these doctors, I simply wouldn’t do it.”
It is not the first time a judge has ordered ivermectin’s use against a hospital’s wishes.
A 68-year-old woman with COVID-19 in an Illinois hospital started receiving the controversial drug in May after her family sued the hospital to have someone administer it.
Nurije Fype’s daughter, Desareta, filed suit against Elmhurst Hospital, part of Edward-Elmhurst Health, asking that her mother receive the treatment, which is approved as an antiparasitic drug but not approved for the treatment of COVID-19. Desareta Fype was granted temporary guardianship of her mother.
The FDA has published guidance titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19” on its website. The National Institutes of Health said there is not enough data to recommend either for or against its use in treating COVID-19.
But DuPage County Judge James Orel ruled Ms. Fype should be allowed to get the treatment.
Three days later, according to the Daily Herald, the lawyer for the hospital, Joseph Monahan, argued the hospital could not find a hospital-affiliated doctor to administer the ivermectin.
The Herald reported the judge told the hospital to “get out of the way” and allow any board-certified doctor to administer the drug.
When Ms. Fype’s doctor was unable to administer it, the legal team found another doctor, Alan Bain, DO, to do it. Mr. Monahan said Dr. Bain was granted credentials to work at the hospital so he could administer it.
Judge Orel denied a request from Desareta Fype’s lawyer to order the hospital’s nurses to administer further doses. The judge also denied a request to hold the hospital in contempt of court.
A version of this article first appeared on WebMD.com.
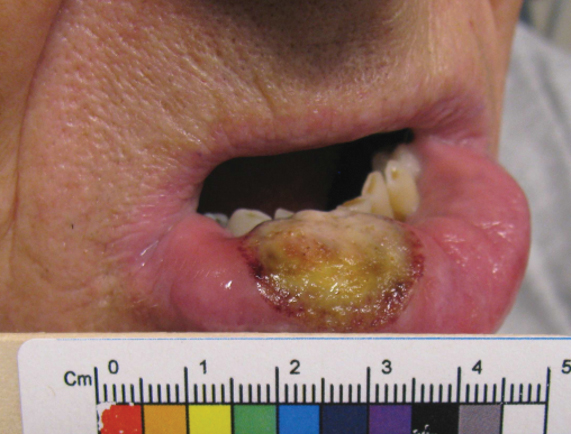
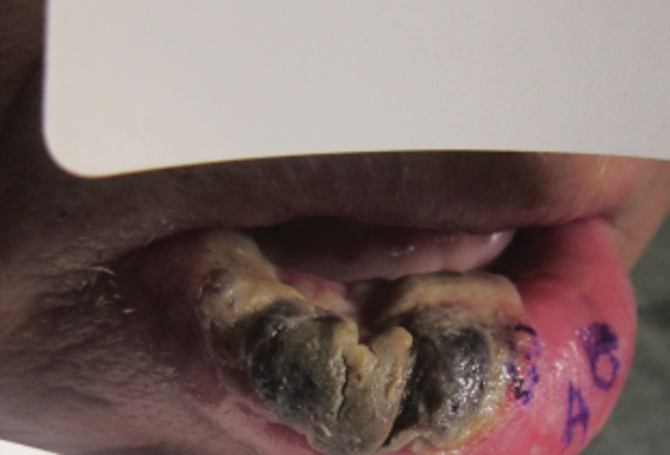
What’s your diagnosis?
Choledocopyloric fistula secondary to peptic ulcer disease.
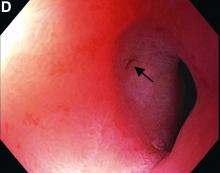
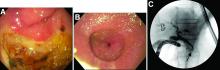
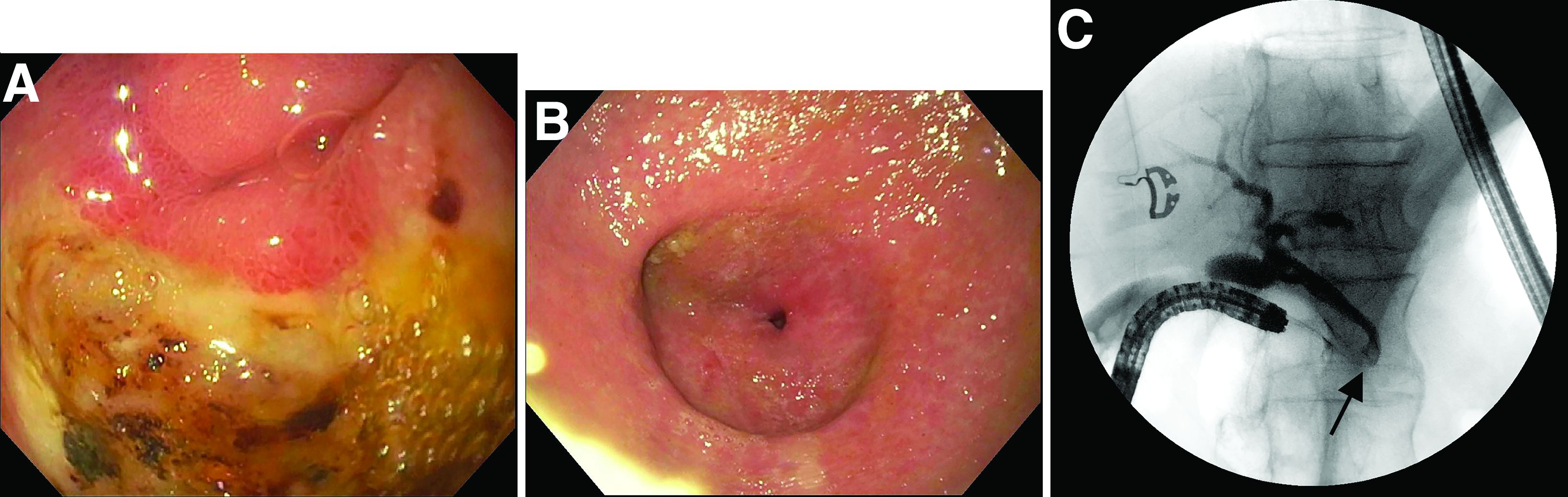
This patient has a known history of prepyloric peptic ulcer disease and related gastric outlet obstruction requiring two previous dilations. Upon endoscopic examination, we observed high-grade obstruction at the pylorus similar to previous examinations. During the initial positioning of the balloon for dilation, we inadvertently cannulated the fistula located in the pyloric channel using the guidewire (arrow in Figure D) and were able to characterize its anatomy upon contrast administration (Figure C). However, after repositioning the guidewire into the duodenal lumen beyond pyloric stricture, the balloon was inflated to a maximal diameter of 15 mm under fluoroscopic guidance. Discounting other common causes, our patient presented with an infrequent occurrence of choledocopyloric fistula secondary to peptic ulcer disease.
The most common cause of choledocoenteric fistula is bile duct inflammation due to gallstone formation, while other minor causes include neoplasms, ulcers, and inflammation of neighboring organs.1 Additionally, in recent years, fistula formation is a relatively rare complication of peptic ulcer disease due to the increased effectiveness of ulcer drugs.2 Similar to this patient's condition, cholangitis, jaundice, or anomaly of biological liver examinations are rarely observed. Consequently, diagnosis is mainly incidental with pneumobilia being the most helpful marker present in 50% of cases.3 Because cholangitis and biliary sequelae remain rare, choledocoenteric fistulas do not warrant prophylactic surgical treatment. As a result, treatment is generally focused on the underlying ulcer disease.
The quiz authors disclose no conflicts.
References
1. Stagnitti F et al. G Chir. 2000 Mar;21(3):110-7.
2. Wu MB et al. Ann Surg Treat Res. 2015 Nov;89(5):240-6.
3. Dewulf E et al. J Chir (Paris). 1987 Jan;124(1):19-23.
Previously published in Gastroenterology (2019 Oct;157[4]:936-7).
Choledocopyloric fistula secondary to peptic ulcer disease.
This patient has a known history of prepyloric peptic ulcer disease and related gastric outlet obstruction requiring two previous dilations. Upon endoscopic examination, we observed high-grade obstruction at the pylorus similar to previous examinations. During the initial positioning of the balloon for dilation, we inadvertently cannulated the fistula located in the pyloric channel using the guidewire (arrow in Figure D) and were able to characterize its anatomy upon contrast administration (Figure C). However, after repositioning the guidewire into the duodenal lumen beyond pyloric stricture, the balloon was inflated to a maximal diameter of 15 mm under fluoroscopic guidance. Discounting other common causes, our patient presented with an infrequent occurrence of choledocopyloric fistula secondary to peptic ulcer disease.
The most common cause of choledocoenteric fistula is bile duct inflammation due to gallstone formation, while other minor causes include neoplasms, ulcers, and inflammation of neighboring organs.1 Additionally, in recent years, fistula formation is a relatively rare complication of peptic ulcer disease due to the increased effectiveness of ulcer drugs.2 Similar to this patient's condition, cholangitis, jaundice, or anomaly of biological liver examinations are rarely observed. Consequently, diagnosis is mainly incidental with pneumobilia being the most helpful marker present in 50% of cases.3 Because cholangitis and biliary sequelae remain rare, choledocoenteric fistulas do not warrant prophylactic surgical treatment. As a result, treatment is generally focused on the underlying ulcer disease.
The quiz authors disclose no conflicts.
References
1. Stagnitti F et al. G Chir. 2000 Mar;21(3):110-7.
2. Wu MB et al. Ann Surg Treat Res. 2015 Nov;89(5):240-6.
3. Dewulf E et al. J Chir (Paris). 1987 Jan;124(1):19-23.
Previously published in Gastroenterology (2019 Oct;157[4]:936-7).
Choledocopyloric fistula secondary to peptic ulcer disease.
This patient has a known history of prepyloric peptic ulcer disease and related gastric outlet obstruction requiring two previous dilations. Upon endoscopic examination, we observed high-grade obstruction at the pylorus similar to previous examinations. During the initial positioning of the balloon for dilation, we inadvertently cannulated the fistula located in the pyloric channel using the guidewire (arrow in Figure D) and were able to characterize its anatomy upon contrast administration (Figure C). However, after repositioning the guidewire into the duodenal lumen beyond pyloric stricture, the balloon was inflated to a maximal diameter of 15 mm under fluoroscopic guidance. Discounting other common causes, our patient presented with an infrequent occurrence of choledocopyloric fistula secondary to peptic ulcer disease.
The most common cause of choledocoenteric fistula is bile duct inflammation due to gallstone formation, while other minor causes include neoplasms, ulcers, and inflammation of neighboring organs.1 Additionally, in recent years, fistula formation is a relatively rare complication of peptic ulcer disease due to the increased effectiveness of ulcer drugs.2 Similar to this patient's condition, cholangitis, jaundice, or anomaly of biological liver examinations are rarely observed. Consequently, diagnosis is mainly incidental with pneumobilia being the most helpful marker present in 50% of cases.3 Because cholangitis and biliary sequelae remain rare, choledocoenteric fistulas do not warrant prophylactic surgical treatment. As a result, treatment is generally focused on the underlying ulcer disease.
The quiz authors disclose no conflicts.
References
1. Stagnitti F et al. G Chir. 2000 Mar;21(3):110-7.
2. Wu MB et al. Ann Surg Treat Res. 2015 Nov;89(5):240-6.
3. Dewulf E et al. J Chir (Paris). 1987 Jan;124(1):19-23.
Previously published in Gastroenterology (2019 Oct;157[4]:936-7).
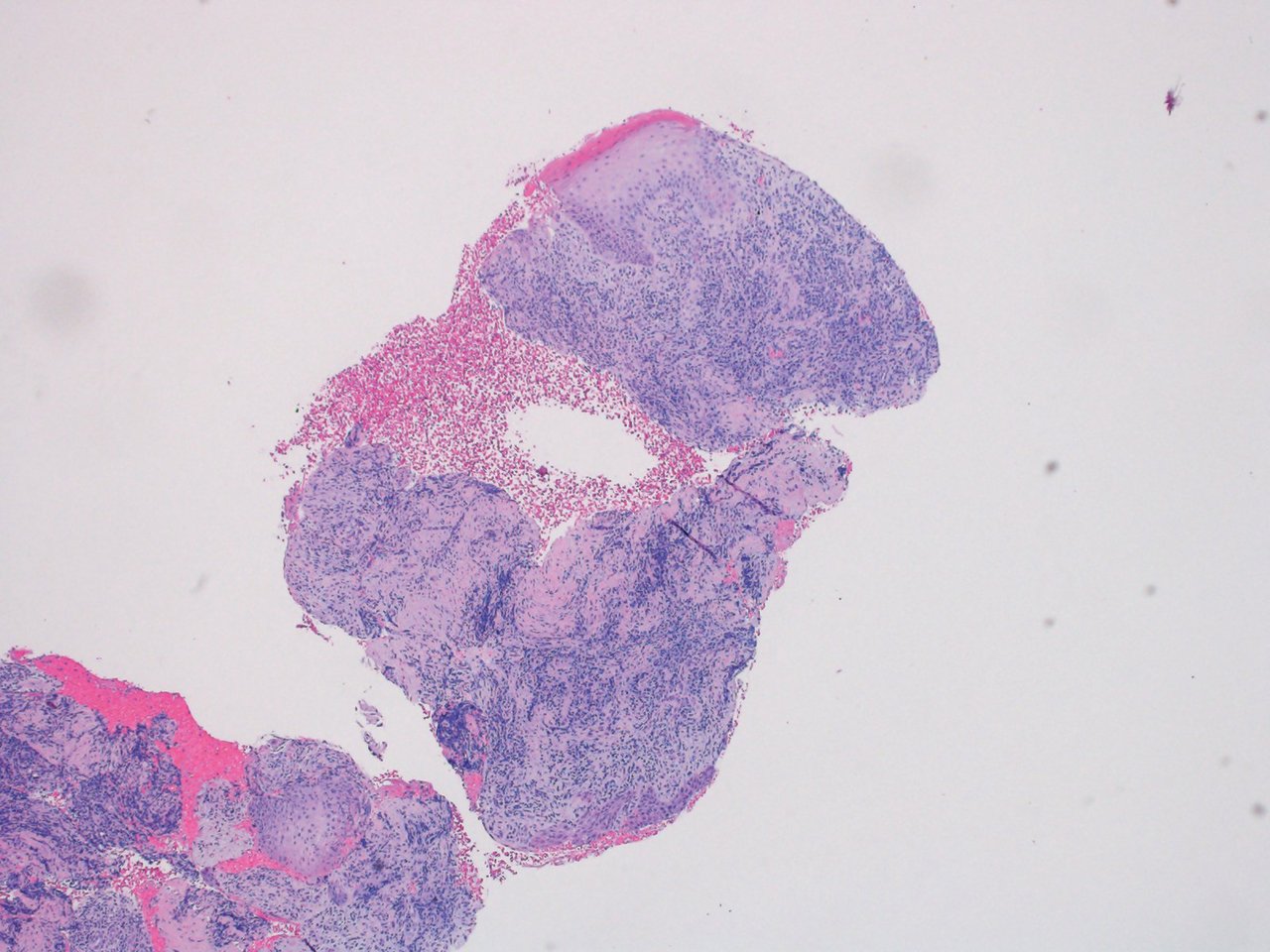
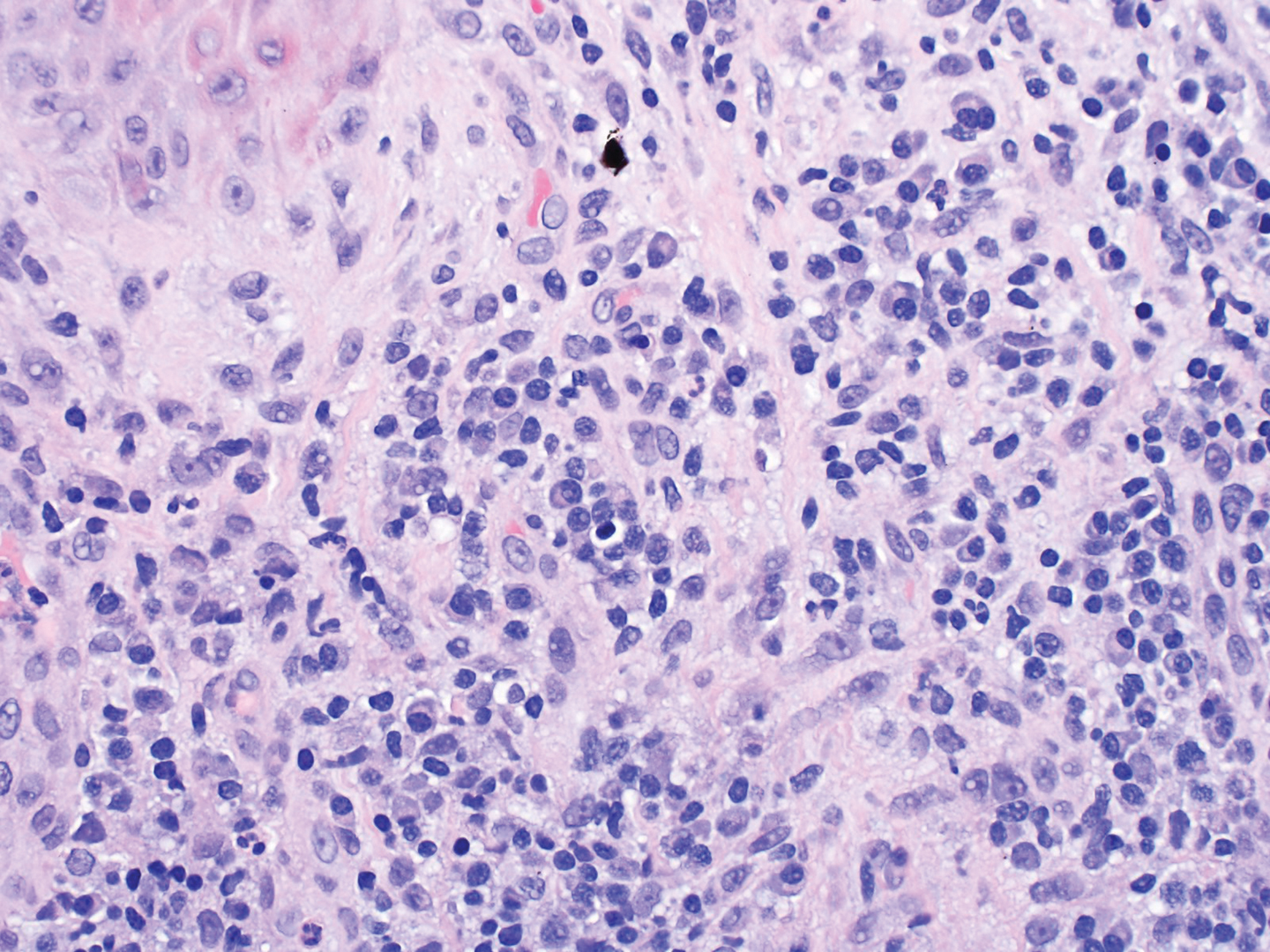
What’s the diagnosis?
CMS’s proposed 22% cut to radiation oncology is ‘tone deaf’
The changes, proposed by the Centers for Medicare & Medicaid Services to their Radiation Oncology (RO) Model, have been challenged primarily by the American Society for Radiation Oncology (ASTRO), but by other groups as well.
These excessive reductions will jeopardize access to lifesaving radiation therapy services for Medicare beneficiaries at a time when the U.S. health care system needs “all doors open” to treat patients with cancer, says ASTRO.
The proposed cuts are scheduled to take effect Jan. 1 and will be mandatory for the 30% of providers that will be randomly selected to participate.
The timing could not be worse, says the Community Oncology Alliance. “How can payment be ratcheted down on a vital aspect of cancer in the middle of a pandemic?” said Ted Okon, MBA, executive director of COA. “What was CMS thinking? These are extreme circumstances, and this is so ill-timed and so tone deaf that it just takes my breath away.”
He pointed out that with hospitals being overrun with COVID-19 patients, community practices have to keep their doors open to treat patients. “This is an extended public health emergency, and a variant can reignite the surge,” said Mr. Okon. “CMS should be asking practices what they can do to help them – and not trying to make drastic cuts.”
Mr. Okon is also concerned that as a result of the pandemic, “we are going to be seeing more advanced cancers, which are more difficult and expensive to treat, and radiation therapy is going to come into play,” he said. “These are serious and unintended consequences, and CMS needs to come out of their [Washington] D.C. bubble and see what’s really going on.”
The timing of the rollout is particularly precarious, given the financial upheaval caused by the COVID-19 pandemic, agrees Constantine Mantz, MD, ASTRO’s Health Policy Council vice-chair.
“Medicare’s proposed cuts, unfortunately, compound the enormous financial challenges imposed by the COVID-19 pandemic on physicians and their practices,” said Dr. Mantz. “Radiation oncology is particularly at risk given its dependence on expensive treatment equipment to deliver cancer care.”
The high costs of maintaining this equipment remain the same whether the equipment is used or not. “This means that fewer patients being seen during the pandemic combined with these steep reimbursement cuts in the near future risk the continued viability of many centers and their ability to provide lifesaving cancer treatment,” he said.
ASTRO calls on Congress to intervene
ASTRO has asked President Biden and Congress to intervene immediately on not only the Radiation Oncology model but the severe cuts that were proposed for the 2022 Medicare Physician Fee Schedule.
“The RO Model, which was last updated in the 2022 Medicare Hospital Outpatient Prospective Payment System Proposed Rule, would cut payments for radiation therapy services by 3.75% for physicians and 4.75% for facilities,” said Dr. Mantz. “This cut would be in addition to an 8.75% cut to radiation oncology in the 2022 Medicare Physician Fee Schedule Proposed Rule.”
As a result, the physicians and facilities that are required to participate in the RO Model are facing steep declines in Medicare reimbursement. “This amounts to well over 10% for their patients covered by Medicare next year,” Dr. Mantz told this news organization.
The radiation oncology model
The goal of the RO Model is to test a change in the way radiation therapy services are paid – from the current “fee-for-service” payments” to prospective, site-neutral, modality-agnostic, episode-based payments that incentivize physicians to deliver higher-value care. It requires mandatory participation of practices.
The Center for Medicare and Medicaid Innovation published a final rule in September 2020 that established the RO Model, which was to begin on Jan. 1, 2021. However, because of the ongoing COVID-19 pandemic, the start of the RO Model was delayed until July 1, 2021, and subsequently, the Consolidated Appropriations Act, 2021, included a provision that prohibits implementation of the RO Model before Jan. 1, 2022.
Further changes to the RO model were proposed last month, which included some slight revisions to the discount factor. But ASTRO points out that these revisions did not address numerous concerns raised by both the radiation oncology community and a broad coalition of medical provider groups, patients, hospitals, health systems, and bipartisan members of Congress.
The new model would provide an alternative to the traditional fee-for-service payments. Instead, the payments are prospective and episode-based, and based on the cancer diagnosis. It would cover radiation therapy that is administered during a 90-day episode, and would meet the included cancer type criteria described in the final rule.
The RO model would use “site-neutral payment” by establishing a common, adjusted national base payment amount for the episode, regardless of the setting where it is administered.
The episode payments would be divided into professional and technical components to allow the current claims systems for the Physician Fee Schedule (PFS) and the Outpatient Prospective Payment System (OPPS) to be used to adjudicate claims as well as to maintain consistency with existing business relationships.
Another aspect is that the model links “payment” to “quality” using reporting and performance on quality measures, clinical data reporting, and patient experience as factors when determining payment to RO participants. Finally, providers will be randomly selected and participation is mandatory.
Mr. Okon feels that the idea of a mandatory model is wrong. “They are going to be taking 30% of practices to participate in an experiment,” he said. “Mandatory means that you can’t get enough people to participate so it is mandated to force them into it.”
Dr. Mantz also noted that the model is going to have a widespread impact on a wide range of issues. “Sharply reduced reimbursement for radiation therapy services under the RO Model is expected to delay, if not prevent, the equipment upgrades and other improvements that are necessary for practices to continue to provide high-quality, high-value cancer care.
“These problematic barriers to access advanced treatment technology also come at a critical time for radiation oncology, in that we already are seeing more difficult-to-treat cancers and caring for patients with more advanced-stage diseases due to delayed diagnoses during the peak of the pandemic last year.”
In addition, the RO model, in its current inception, is expected to further widen existing gaps in access to cancer care, disproportionately harming patients from marginalized groups, such as poor patients and medically underserved patients.
“For example, ASTRO analyses have demonstrated that patients in rural areas currently face significantly reduced access to stereotactic radiotherapy and lifesaving brachytherapy treatments, compared to patients in urban areas,” said Dr. Mantz. “It’s difficult to imagine that these serious health inequities could even begin to be addressed with the aggregate payment cuts imposed by the RO model.”
A version of this article first appeared on Medscape.com.
The changes, proposed by the Centers for Medicare & Medicaid Services to their Radiation Oncology (RO) Model, have been challenged primarily by the American Society for Radiation Oncology (ASTRO), but by other groups as well.
These excessive reductions will jeopardize access to lifesaving radiation therapy services for Medicare beneficiaries at a time when the U.S. health care system needs “all doors open” to treat patients with cancer, says ASTRO.
The proposed cuts are scheduled to take effect Jan. 1 and will be mandatory for the 30% of providers that will be randomly selected to participate.
The timing could not be worse, says the Community Oncology Alliance. “How can payment be ratcheted down on a vital aspect of cancer in the middle of a pandemic?” said Ted Okon, MBA, executive director of COA. “What was CMS thinking? These are extreme circumstances, and this is so ill-timed and so tone deaf that it just takes my breath away.”
He pointed out that with hospitals being overrun with COVID-19 patients, community practices have to keep their doors open to treat patients. “This is an extended public health emergency, and a variant can reignite the surge,” said Mr. Okon. “CMS should be asking practices what they can do to help them – and not trying to make drastic cuts.”
Mr. Okon is also concerned that as a result of the pandemic, “we are going to be seeing more advanced cancers, which are more difficult and expensive to treat, and radiation therapy is going to come into play,” he said. “These are serious and unintended consequences, and CMS needs to come out of their [Washington] D.C. bubble and see what’s really going on.”
The timing of the rollout is particularly precarious, given the financial upheaval caused by the COVID-19 pandemic, agrees Constantine Mantz, MD, ASTRO’s Health Policy Council vice-chair.
“Medicare’s proposed cuts, unfortunately, compound the enormous financial challenges imposed by the COVID-19 pandemic on physicians and their practices,” said Dr. Mantz. “Radiation oncology is particularly at risk given its dependence on expensive treatment equipment to deliver cancer care.”
The high costs of maintaining this equipment remain the same whether the equipment is used or not. “This means that fewer patients being seen during the pandemic combined with these steep reimbursement cuts in the near future risk the continued viability of many centers and their ability to provide lifesaving cancer treatment,” he said.
ASTRO calls on Congress to intervene
ASTRO has asked President Biden and Congress to intervene immediately on not only the Radiation Oncology model but the severe cuts that were proposed for the 2022 Medicare Physician Fee Schedule.
“The RO Model, which was last updated in the 2022 Medicare Hospital Outpatient Prospective Payment System Proposed Rule, would cut payments for radiation therapy services by 3.75% for physicians and 4.75% for facilities,” said Dr. Mantz. “This cut would be in addition to an 8.75% cut to radiation oncology in the 2022 Medicare Physician Fee Schedule Proposed Rule.”
As a result, the physicians and facilities that are required to participate in the RO Model are facing steep declines in Medicare reimbursement. “This amounts to well over 10% for their patients covered by Medicare next year,” Dr. Mantz told this news organization.
The radiation oncology model
The goal of the RO Model is to test a change in the way radiation therapy services are paid – from the current “fee-for-service” payments” to prospective, site-neutral, modality-agnostic, episode-based payments that incentivize physicians to deliver higher-value care. It requires mandatory participation of practices.
The Center for Medicare and Medicaid Innovation published a final rule in September 2020 that established the RO Model, which was to begin on Jan. 1, 2021. However, because of the ongoing COVID-19 pandemic, the start of the RO Model was delayed until July 1, 2021, and subsequently, the Consolidated Appropriations Act, 2021, included a provision that prohibits implementation of the RO Model before Jan. 1, 2022.
Further changes to the RO model were proposed last month, which included some slight revisions to the discount factor. But ASTRO points out that these revisions did not address numerous concerns raised by both the radiation oncology community and a broad coalition of medical provider groups, patients, hospitals, health systems, and bipartisan members of Congress.
The new model would provide an alternative to the traditional fee-for-service payments. Instead, the payments are prospective and episode-based, and based on the cancer diagnosis. It would cover radiation therapy that is administered during a 90-day episode, and would meet the included cancer type criteria described in the final rule.
The RO model would use “site-neutral payment” by establishing a common, adjusted national base payment amount for the episode, regardless of the setting where it is administered.
The episode payments would be divided into professional and technical components to allow the current claims systems for the Physician Fee Schedule (PFS) and the Outpatient Prospective Payment System (OPPS) to be used to adjudicate claims as well as to maintain consistency with existing business relationships.
Another aspect is that the model links “payment” to “quality” using reporting and performance on quality measures, clinical data reporting, and patient experience as factors when determining payment to RO participants. Finally, providers will be randomly selected and participation is mandatory.
Mr. Okon feels that the idea of a mandatory model is wrong. “They are going to be taking 30% of practices to participate in an experiment,” he said. “Mandatory means that you can’t get enough people to participate so it is mandated to force them into it.”
Dr. Mantz also noted that the model is going to have a widespread impact on a wide range of issues. “Sharply reduced reimbursement for radiation therapy services under the RO Model is expected to delay, if not prevent, the equipment upgrades and other improvements that are necessary for practices to continue to provide high-quality, high-value cancer care.
“These problematic barriers to access advanced treatment technology also come at a critical time for radiation oncology, in that we already are seeing more difficult-to-treat cancers and caring for patients with more advanced-stage diseases due to delayed diagnoses during the peak of the pandemic last year.”
In addition, the RO model, in its current inception, is expected to further widen existing gaps in access to cancer care, disproportionately harming patients from marginalized groups, such as poor patients and medically underserved patients.
“For example, ASTRO analyses have demonstrated that patients in rural areas currently face significantly reduced access to stereotactic radiotherapy and lifesaving brachytherapy treatments, compared to patients in urban areas,” said Dr. Mantz. “It’s difficult to imagine that these serious health inequities could even begin to be addressed with the aggregate payment cuts imposed by the RO model.”
A version of this article first appeared on Medscape.com.
The changes, proposed by the Centers for Medicare & Medicaid Services to their Radiation Oncology (RO) Model, have been challenged primarily by the American Society for Radiation Oncology (ASTRO), but by other groups as well.
These excessive reductions will jeopardize access to lifesaving radiation therapy services for Medicare beneficiaries at a time when the U.S. health care system needs “all doors open” to treat patients with cancer, says ASTRO.
The proposed cuts are scheduled to take effect Jan. 1 and will be mandatory for the 30% of providers that will be randomly selected to participate.
The timing could not be worse, says the Community Oncology Alliance. “How can payment be ratcheted down on a vital aspect of cancer in the middle of a pandemic?” said Ted Okon, MBA, executive director of COA. “What was CMS thinking? These are extreme circumstances, and this is so ill-timed and so tone deaf that it just takes my breath away.”
He pointed out that with hospitals being overrun with COVID-19 patients, community practices have to keep their doors open to treat patients. “This is an extended public health emergency, and a variant can reignite the surge,” said Mr. Okon. “CMS should be asking practices what they can do to help them – and not trying to make drastic cuts.”
Mr. Okon is also concerned that as a result of the pandemic, “we are going to be seeing more advanced cancers, which are more difficult and expensive to treat, and radiation therapy is going to come into play,” he said. “These are serious and unintended consequences, and CMS needs to come out of their [Washington] D.C. bubble and see what’s really going on.”
The timing of the rollout is particularly precarious, given the financial upheaval caused by the COVID-19 pandemic, agrees Constantine Mantz, MD, ASTRO’s Health Policy Council vice-chair.
“Medicare’s proposed cuts, unfortunately, compound the enormous financial challenges imposed by the COVID-19 pandemic on physicians and their practices,” said Dr. Mantz. “Radiation oncology is particularly at risk given its dependence on expensive treatment equipment to deliver cancer care.”
The high costs of maintaining this equipment remain the same whether the equipment is used or not. “This means that fewer patients being seen during the pandemic combined with these steep reimbursement cuts in the near future risk the continued viability of many centers and their ability to provide lifesaving cancer treatment,” he said.
ASTRO calls on Congress to intervene
ASTRO has asked President Biden and Congress to intervene immediately on not only the Radiation Oncology model but the severe cuts that were proposed for the 2022 Medicare Physician Fee Schedule.
“The RO Model, which was last updated in the 2022 Medicare Hospital Outpatient Prospective Payment System Proposed Rule, would cut payments for radiation therapy services by 3.75% for physicians and 4.75% for facilities,” said Dr. Mantz. “This cut would be in addition to an 8.75% cut to radiation oncology in the 2022 Medicare Physician Fee Schedule Proposed Rule.”
As a result, the physicians and facilities that are required to participate in the RO Model are facing steep declines in Medicare reimbursement. “This amounts to well over 10% for their patients covered by Medicare next year,” Dr. Mantz told this news organization.
The radiation oncology model
The goal of the RO Model is to test a change in the way radiation therapy services are paid – from the current “fee-for-service” payments” to prospective, site-neutral, modality-agnostic, episode-based payments that incentivize physicians to deliver higher-value care. It requires mandatory participation of practices.
The Center for Medicare and Medicaid Innovation published a final rule in September 2020 that established the RO Model, which was to begin on Jan. 1, 2021. However, because of the ongoing COVID-19 pandemic, the start of the RO Model was delayed until July 1, 2021, and subsequently, the Consolidated Appropriations Act, 2021, included a provision that prohibits implementation of the RO Model before Jan. 1, 2022.
Further changes to the RO model were proposed last month, which included some slight revisions to the discount factor. But ASTRO points out that these revisions did not address numerous concerns raised by both the radiation oncology community and a broad coalition of medical provider groups, patients, hospitals, health systems, and bipartisan members of Congress.
The new model would provide an alternative to the traditional fee-for-service payments. Instead, the payments are prospective and episode-based, and based on the cancer diagnosis. It would cover radiation therapy that is administered during a 90-day episode, and would meet the included cancer type criteria described in the final rule.
The RO model would use “site-neutral payment” by establishing a common, adjusted national base payment amount for the episode, regardless of the setting where it is administered.
The episode payments would be divided into professional and technical components to allow the current claims systems for the Physician Fee Schedule (PFS) and the Outpatient Prospective Payment System (OPPS) to be used to adjudicate claims as well as to maintain consistency with existing business relationships.
Another aspect is that the model links “payment” to “quality” using reporting and performance on quality measures, clinical data reporting, and patient experience as factors when determining payment to RO participants. Finally, providers will be randomly selected and participation is mandatory.
Mr. Okon feels that the idea of a mandatory model is wrong. “They are going to be taking 30% of practices to participate in an experiment,” he said. “Mandatory means that you can’t get enough people to participate so it is mandated to force them into it.”
Dr. Mantz also noted that the model is going to have a widespread impact on a wide range of issues. “Sharply reduced reimbursement for radiation therapy services under the RO Model is expected to delay, if not prevent, the equipment upgrades and other improvements that are necessary for practices to continue to provide high-quality, high-value cancer care.
“These problematic barriers to access advanced treatment technology also come at a critical time for radiation oncology, in that we already are seeing more difficult-to-treat cancers and caring for patients with more advanced-stage diseases due to delayed diagnoses during the peak of the pandemic last year.”
In addition, the RO model, in its current inception, is expected to further widen existing gaps in access to cancer care, disproportionately harming patients from marginalized groups, such as poor patients and medically underserved patients.
“For example, ASTRO analyses have demonstrated that patients in rural areas currently face significantly reduced access to stereotactic radiotherapy and lifesaving brachytherapy treatments, compared to patients in urban areas,” said Dr. Mantz. “It’s difficult to imagine that these serious health inequities could even begin to be addressed with the aggregate payment cuts imposed by the RO model.”
A version of this article first appeared on Medscape.com.
COVID-clogged ICUs ‘terrify’ those with chronic or emergency illness
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data sharing to improve AI used in breast-imaging research
The curated dataset, which consists of 22,032 DBT volumes associated with 5,610 studies from 5,060 patients, was published online in JAMA Network Open. The studies were divided into types: normal studies (91.4%), actionable studies that required additional imaging but no biopsy (5.0%), benign biopsied studies (2.0%), and studies that detected cancer (1.6%).
To develop and evaluate their deep-learning model for the detection of architectural distortions and masses, the researchers used a test set of 460 studies from 418 patients with cancer. Their algorithm reached a breast-based sensitivity of two false positives per DBT volume, or 65%.
“The main focus of this publication is on the dataset, rather than on a specific hypothesis,” said principal researcher Maciej A. Mazurowski, PhD, scientific director of the Duke Center for Artificial Intelligence in Radiology in Durham, N.C.
“We have publicly shared a large dataset of digital breast tomosynthesis images, which are sometimes referred to as 3D mammograms, for more than 5,000 patients. There are two purposes for sharing data like these. One is to improve research and development of machine-learning algorithms. You can train models with these data. The other reason, maybe even more important, is to provide a benchmark to test algorithms,” he said in an interview.
The large-scale sharing of data is a key step toward transparency in science, said Dr. Mazurowski. “It is about making sure results can be easily reproduced and setting benchmarks.”
The dataset includes masses and architectural distortions that were annotated by two experienced radiologists, but does not include annotations for calcifications and/or microcalcifications.
This lack of calcifications is a limitation of the study, said Jean Seely, MD, professor of radiology at the University of Ottawa, who is president of the Canadian Society of Breast Imaging and regional lead for the Ontario Breast Screening Program.
“About 45% of invasive breast cancers are diagnosed based on calcifications,” she explained.
Still, although the sensitivity of the AI algorithm was not high (65%) – the average sensitivity of 2D mammography is 85% – the researchers should be commended for releasing such a large dataset, said Dr. Seely.
“The fact that they have made it publicly available is very, very useful,” she said, adding that the dataset can be leveraged in future breast-imaging research.
Although DBT is much better at identifying breast cancers than mammography, DBT exams take about 30% more time to read.
“There’s a lot of work being done in artificial intelligence in breast imaging to not only improve the workflow for breast radiologists, but also to help with the diagnosis and detection,” she noted. “Anything that helps improve the confidence and the accuracy of the radiologist is really what we’re aiming for right now.”
The size and the content of this dataset will contribute to breast-imaging research, said Jaron Chong, MD, of the department of medical imaging at Western University in London, Ontario, who is chair of the AI Standing Committee at the Canadian Association of Radiologists.
“The contribution could be valuable in the long term because DBT is a rare dataset in comparison to conventional 2D mammography,” said Dr. Chong. “Most existing datasets have focused on two-dimensional imaging. We might see more research papers reference this dataset in the future, iterating and improving upon this article’s algorithm performance.”
Dr. Mazurowski reports serving as an adviser to Gradient Health. Dr. Seely is an unpaid principal investigator for the Ottawa site of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST). Dr. Chong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The curated dataset, which consists of 22,032 DBT volumes associated with 5,610 studies from 5,060 patients, was published online in JAMA Network Open. The studies were divided into types: normal studies (91.4%), actionable studies that required additional imaging but no biopsy (5.0%), benign biopsied studies (2.0%), and studies that detected cancer (1.6%).
To develop and evaluate their deep-learning model for the detection of architectural distortions and masses, the researchers used a test set of 460 studies from 418 patients with cancer. Their algorithm reached a breast-based sensitivity of two false positives per DBT volume, or 65%.
“The main focus of this publication is on the dataset, rather than on a specific hypothesis,” said principal researcher Maciej A. Mazurowski, PhD, scientific director of the Duke Center for Artificial Intelligence in Radiology in Durham, N.C.
“We have publicly shared a large dataset of digital breast tomosynthesis images, which are sometimes referred to as 3D mammograms, for more than 5,000 patients. There are two purposes for sharing data like these. One is to improve research and development of machine-learning algorithms. You can train models with these data. The other reason, maybe even more important, is to provide a benchmark to test algorithms,” he said in an interview.
The large-scale sharing of data is a key step toward transparency in science, said Dr. Mazurowski. “It is about making sure results can be easily reproduced and setting benchmarks.”
The dataset includes masses and architectural distortions that were annotated by two experienced radiologists, but does not include annotations for calcifications and/or microcalcifications.
This lack of calcifications is a limitation of the study, said Jean Seely, MD, professor of radiology at the University of Ottawa, who is president of the Canadian Society of Breast Imaging and regional lead for the Ontario Breast Screening Program.
“About 45% of invasive breast cancers are diagnosed based on calcifications,” she explained.
Still, although the sensitivity of the AI algorithm was not high (65%) – the average sensitivity of 2D mammography is 85% – the researchers should be commended for releasing such a large dataset, said Dr. Seely.
“The fact that they have made it publicly available is very, very useful,” she said, adding that the dataset can be leveraged in future breast-imaging research.
Although DBT is much better at identifying breast cancers than mammography, DBT exams take about 30% more time to read.
“There’s a lot of work being done in artificial intelligence in breast imaging to not only improve the workflow for breast radiologists, but also to help with the diagnosis and detection,” she noted. “Anything that helps improve the confidence and the accuracy of the radiologist is really what we’re aiming for right now.”
The size and the content of this dataset will contribute to breast-imaging research, said Jaron Chong, MD, of the department of medical imaging at Western University in London, Ontario, who is chair of the AI Standing Committee at the Canadian Association of Radiologists.
“The contribution could be valuable in the long term because DBT is a rare dataset in comparison to conventional 2D mammography,” said Dr. Chong. “Most existing datasets have focused on two-dimensional imaging. We might see more research papers reference this dataset in the future, iterating and improving upon this article’s algorithm performance.”
Dr. Mazurowski reports serving as an adviser to Gradient Health. Dr. Seely is an unpaid principal investigator for the Ottawa site of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST). Dr. Chong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The curated dataset, which consists of 22,032 DBT volumes associated with 5,610 studies from 5,060 patients, was published online in JAMA Network Open. The studies were divided into types: normal studies (91.4%), actionable studies that required additional imaging but no biopsy (5.0%), benign biopsied studies (2.0%), and studies that detected cancer (1.6%).
To develop and evaluate their deep-learning model for the detection of architectural distortions and masses, the researchers used a test set of 460 studies from 418 patients with cancer. Their algorithm reached a breast-based sensitivity of two false positives per DBT volume, or 65%.
“The main focus of this publication is on the dataset, rather than on a specific hypothesis,” said principal researcher Maciej A. Mazurowski, PhD, scientific director of the Duke Center for Artificial Intelligence in Radiology in Durham, N.C.
“We have publicly shared a large dataset of digital breast tomosynthesis images, which are sometimes referred to as 3D mammograms, for more than 5,000 patients. There are two purposes for sharing data like these. One is to improve research and development of machine-learning algorithms. You can train models with these data. The other reason, maybe even more important, is to provide a benchmark to test algorithms,” he said in an interview.
The large-scale sharing of data is a key step toward transparency in science, said Dr. Mazurowski. “It is about making sure results can be easily reproduced and setting benchmarks.”
The dataset includes masses and architectural distortions that were annotated by two experienced radiologists, but does not include annotations for calcifications and/or microcalcifications.
This lack of calcifications is a limitation of the study, said Jean Seely, MD, professor of radiology at the University of Ottawa, who is president of the Canadian Society of Breast Imaging and regional lead for the Ontario Breast Screening Program.
“About 45% of invasive breast cancers are diagnosed based on calcifications,” she explained.
Still, although the sensitivity of the AI algorithm was not high (65%) – the average sensitivity of 2D mammography is 85% – the researchers should be commended for releasing such a large dataset, said Dr. Seely.
“The fact that they have made it publicly available is very, very useful,” she said, adding that the dataset can be leveraged in future breast-imaging research.
Although DBT is much better at identifying breast cancers than mammography, DBT exams take about 30% more time to read.
“There’s a lot of work being done in artificial intelligence in breast imaging to not only improve the workflow for breast radiologists, but also to help with the diagnosis and detection,” she noted. “Anything that helps improve the confidence and the accuracy of the radiologist is really what we’re aiming for right now.”
The size and the content of this dataset will contribute to breast-imaging research, said Jaron Chong, MD, of the department of medical imaging at Western University in London, Ontario, who is chair of the AI Standing Committee at the Canadian Association of Radiologists.
“The contribution could be valuable in the long term because DBT is a rare dataset in comparison to conventional 2D mammography,” said Dr. Chong. “Most existing datasets have focused on two-dimensional imaging. We might see more research papers reference this dataset in the future, iterating and improving upon this article’s algorithm performance.”
Dr. Mazurowski reports serving as an adviser to Gradient Health. Dr. Seely is an unpaid principal investigator for the Ottawa site of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST). Dr. Chong has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Severe Presentation of Plasma Cell Cheilitis
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
PRACTICE POINTS
- Plasma cell cheilitis (PCC) is a benign condition that affects the lower lip in older individuals, presenting as a nonspecific, red-brown patch or plaque that can progress slowly to erosions and edema.
- Our patient with PCC experienced full resolution of symptoms with application of a class I topical corticosteroid.
Verruca Vulgaris Arising Within the Red Portion of a Multicolored Tattoo
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
Practice Points
- Various adverse reactions and infectious agents may involve tattoos.
- Verruca vulgaris may affect tattoos in a color-restricted manner and demonstrate latency of many years after tattoo placement.
- Timely diagnosis of the tattoo-involving process, confirmed by biopsy, allows for appropriate management.
Study calls higher surgery costs at NCI centers into question
according to a recent report in JAMA Network Open.
“While acceptable to pay higher prices for care that is expected to be of higher quality, we found no differences in short-term postsurgical outcomes,” said authors led by Samuel Takvorian, MD, a medical oncologist at the University of Pennsylvania, Philadelphia.
The team looked at what insurance companies paid for incident breast, colon, and lung cancer surgeries, which together account for most cancer surgeries, among 66,878 patients treated from 2011 to 2014 at almost 3,000 U.S. hospitals.
Three-quarters had surgery at a community hospital, and 8.3% were treated at one of the nation’s 71 NCI centers, which are recognized by the NCI as meeting rigorous standards in cancer care. The remaining patients were treated at non-NCI academic hospitals.
The mean surgery-specific insurer prices paid at NCI centers was $18,526 versus $14,772 at community hospitals, a difference of $3,755 (P < .001) that was driven primarily by higher facility payments at NCI centers, a mean of $17,704 versus $14,120 at community hospitals.
Mean 90-day postdischarge payments were also $5,744 higher at NCI centers, $47,035 versus $41,291 at community hospitals (P = .006).
The team used postsurgical acute care utilization as a marker of quality but found no differences between the two settings. Mean length of stay was 5.1 days and the probability of ED utilization just over 13% in both, and both had a 90-day readmission rate of just over 10%.
Who should be treated at an NCI center?
The data didn’t allow for direct comparison of surgical quality, such as margin status, number of lymph nodes assessed, or postoperative complications, but the postsurgery utilization outcomes “suggest that quality may have been similar,” said Nancy Keating, MD, a health care policy and medicine professor at Harvard Medical School, Boston, in an invited commentary.
The price differences are probably because NCI centers, with their comprehensive offerings, market share, and prestige, can negotiate higher reimbursement rates from insurers, the researchers said.
There is also evidence of better outcomes at NCI centers, particularly for more advanced and complex cases. However, “this study focused on common cancer surgical procedures ... revealing that there is a premium associated with receipt of surgical cancer care at NCI centers.” Further research “is necessary to judge whether and under what circumstances the premium price of NCI centers is justified,” the investigators said.
Dr. Keating noted that “it is likely that some patients benefit from the highly specialized care available at NCI-designated cancer centers ... but it is also likely that many other patients will do equally well regardless of where they receive their care.”
Amid ever-increasing cancer care costs and the need to strategically allocate financial resources, more research is needed to “identify subgroups of patients for whom highly specialized care is particularly necessary to achieve better outcomes. Such data could also be used by payers considering tiered networks and by physician organizations participating in risk contracts for decisions about where to refer patients with cancer for treatment,” she said.
Rectifying a ‘misalignment’
The researchers also said the findings reveal competing incentives, with commercial payers wanting to steer patients away from high-cost hospitals but health systems hoping to maximize surgical volume at lucrative referral centers.
“Value-based or bundled payment reimbursement for surgical episodes, particularly when paired with mandatory reporting on surgical outcomes, could help to rectify this misalignment,” they said.
Out-of-pocket spending wasn’t analyzed in the study, so it’s unknown how the higher prices at NCI centers hit patients in the pocketbook.
Meanwhile, non-NCI academic hospitals also had higher insurer prices paid than community hospitals, but the differences were not statistically significant, nor were differences in the study’s utilization outcomes.
Over half the patients had breast cancer, about one-third had colon cancer, and the rest had lung tumors. Patients treated at NCI centers tended to be younger than those treated at community hospitals and more likely to be women, but comorbidity scores were similar between the groups.
NCI centers, compared with community hospitals, were larger with higher surgical volumes and in more populated areas. They also had higher rates of laparoscopic partial colectomies and pneumonectomies.
Data came from the Health Care Cost Institute’s national commercial claims data set, which includes claims from three of the country’s five largest commercial insurers: Aetna, Humana, and UnitedHealthcare.
The work was funded by the Commonwealth of Pennsylvania and the National Cancer Institute. Dr. Takvorian and Dr. Keating didn’t have any disclosures. One of Dr. Takvorian’s coauthors reported grants and/or personal fees from several sources, including Pfizer, UnitedHealthcare, and Blue Cross Blue Shield of North Carolina.
according to a recent report in JAMA Network Open.
“While acceptable to pay higher prices for care that is expected to be of higher quality, we found no differences in short-term postsurgical outcomes,” said authors led by Samuel Takvorian, MD, a medical oncologist at the University of Pennsylvania, Philadelphia.
The team looked at what insurance companies paid for incident breast, colon, and lung cancer surgeries, which together account for most cancer surgeries, among 66,878 patients treated from 2011 to 2014 at almost 3,000 U.S. hospitals.
Three-quarters had surgery at a community hospital, and 8.3% were treated at one of the nation’s 71 NCI centers, which are recognized by the NCI as meeting rigorous standards in cancer care. The remaining patients were treated at non-NCI academic hospitals.
The mean surgery-specific insurer prices paid at NCI centers was $18,526 versus $14,772 at community hospitals, a difference of $3,755 (P < .001) that was driven primarily by higher facility payments at NCI centers, a mean of $17,704 versus $14,120 at community hospitals.
Mean 90-day postdischarge payments were also $5,744 higher at NCI centers, $47,035 versus $41,291 at community hospitals (P = .006).
The team used postsurgical acute care utilization as a marker of quality but found no differences between the two settings. Mean length of stay was 5.1 days and the probability of ED utilization just over 13% in both, and both had a 90-day readmission rate of just over 10%.
Who should be treated at an NCI center?
The data didn’t allow for direct comparison of surgical quality, such as margin status, number of lymph nodes assessed, or postoperative complications, but the postsurgery utilization outcomes “suggest that quality may have been similar,” said Nancy Keating, MD, a health care policy and medicine professor at Harvard Medical School, Boston, in an invited commentary.
The price differences are probably because NCI centers, with their comprehensive offerings, market share, and prestige, can negotiate higher reimbursement rates from insurers, the researchers said.
There is also evidence of better outcomes at NCI centers, particularly for more advanced and complex cases. However, “this study focused on common cancer surgical procedures ... revealing that there is a premium associated with receipt of surgical cancer care at NCI centers.” Further research “is necessary to judge whether and under what circumstances the premium price of NCI centers is justified,” the investigators said.
Dr. Keating noted that “it is likely that some patients benefit from the highly specialized care available at NCI-designated cancer centers ... but it is also likely that many other patients will do equally well regardless of where they receive their care.”
Amid ever-increasing cancer care costs and the need to strategically allocate financial resources, more research is needed to “identify subgroups of patients for whom highly specialized care is particularly necessary to achieve better outcomes. Such data could also be used by payers considering tiered networks and by physician organizations participating in risk contracts for decisions about where to refer patients with cancer for treatment,” she said.
Rectifying a ‘misalignment’
The researchers also said the findings reveal competing incentives, with commercial payers wanting to steer patients away from high-cost hospitals but health systems hoping to maximize surgical volume at lucrative referral centers.
“Value-based or bundled payment reimbursement for surgical episodes, particularly when paired with mandatory reporting on surgical outcomes, could help to rectify this misalignment,” they said.
Out-of-pocket spending wasn’t analyzed in the study, so it’s unknown how the higher prices at NCI centers hit patients in the pocketbook.
Meanwhile, non-NCI academic hospitals also had higher insurer prices paid than community hospitals, but the differences were not statistically significant, nor were differences in the study’s utilization outcomes.
Over half the patients had breast cancer, about one-third had colon cancer, and the rest had lung tumors. Patients treated at NCI centers tended to be younger than those treated at community hospitals and more likely to be women, but comorbidity scores were similar between the groups.
NCI centers, compared with community hospitals, were larger with higher surgical volumes and in more populated areas. They also had higher rates of laparoscopic partial colectomies and pneumonectomies.
Data came from the Health Care Cost Institute’s national commercial claims data set, which includes claims from three of the country’s five largest commercial insurers: Aetna, Humana, and UnitedHealthcare.
The work was funded by the Commonwealth of Pennsylvania and the National Cancer Institute. Dr. Takvorian and Dr. Keating didn’t have any disclosures. One of Dr. Takvorian’s coauthors reported grants and/or personal fees from several sources, including Pfizer, UnitedHealthcare, and Blue Cross Blue Shield of North Carolina.
according to a recent report in JAMA Network Open.
“While acceptable to pay higher prices for care that is expected to be of higher quality, we found no differences in short-term postsurgical outcomes,” said authors led by Samuel Takvorian, MD, a medical oncologist at the University of Pennsylvania, Philadelphia.
The team looked at what insurance companies paid for incident breast, colon, and lung cancer surgeries, which together account for most cancer surgeries, among 66,878 patients treated from 2011 to 2014 at almost 3,000 U.S. hospitals.
Three-quarters had surgery at a community hospital, and 8.3% were treated at one of the nation’s 71 NCI centers, which are recognized by the NCI as meeting rigorous standards in cancer care. The remaining patients were treated at non-NCI academic hospitals.
The mean surgery-specific insurer prices paid at NCI centers was $18,526 versus $14,772 at community hospitals, a difference of $3,755 (P < .001) that was driven primarily by higher facility payments at NCI centers, a mean of $17,704 versus $14,120 at community hospitals.
Mean 90-day postdischarge payments were also $5,744 higher at NCI centers, $47,035 versus $41,291 at community hospitals (P = .006).
The team used postsurgical acute care utilization as a marker of quality but found no differences between the two settings. Mean length of stay was 5.1 days and the probability of ED utilization just over 13% in both, and both had a 90-day readmission rate of just over 10%.
Who should be treated at an NCI center?
The data didn’t allow for direct comparison of surgical quality, such as margin status, number of lymph nodes assessed, or postoperative complications, but the postsurgery utilization outcomes “suggest that quality may have been similar,” said Nancy Keating, MD, a health care policy and medicine professor at Harvard Medical School, Boston, in an invited commentary.
The price differences are probably because NCI centers, with their comprehensive offerings, market share, and prestige, can negotiate higher reimbursement rates from insurers, the researchers said.
There is also evidence of better outcomes at NCI centers, particularly for more advanced and complex cases. However, “this study focused on common cancer surgical procedures ... revealing that there is a premium associated with receipt of surgical cancer care at NCI centers.” Further research “is necessary to judge whether and under what circumstances the premium price of NCI centers is justified,” the investigators said.
Dr. Keating noted that “it is likely that some patients benefit from the highly specialized care available at NCI-designated cancer centers ... but it is also likely that many other patients will do equally well regardless of where they receive their care.”
Amid ever-increasing cancer care costs and the need to strategically allocate financial resources, more research is needed to “identify subgroups of patients for whom highly specialized care is particularly necessary to achieve better outcomes. Such data could also be used by payers considering tiered networks and by physician organizations participating in risk contracts for decisions about where to refer patients with cancer for treatment,” she said.
Rectifying a ‘misalignment’
The researchers also said the findings reveal competing incentives, with commercial payers wanting to steer patients away from high-cost hospitals but health systems hoping to maximize surgical volume at lucrative referral centers.
“Value-based or bundled payment reimbursement for surgical episodes, particularly when paired with mandatory reporting on surgical outcomes, could help to rectify this misalignment,” they said.
Out-of-pocket spending wasn’t analyzed in the study, so it’s unknown how the higher prices at NCI centers hit patients in the pocketbook.
Meanwhile, non-NCI academic hospitals also had higher insurer prices paid than community hospitals, but the differences were not statistically significant, nor were differences in the study’s utilization outcomes.
Over half the patients had breast cancer, about one-third had colon cancer, and the rest had lung tumors. Patients treated at NCI centers tended to be younger than those treated at community hospitals and more likely to be women, but comorbidity scores were similar between the groups.
NCI centers, compared with community hospitals, were larger with higher surgical volumes and in more populated areas. They also had higher rates of laparoscopic partial colectomies and pneumonectomies.
Data came from the Health Care Cost Institute’s national commercial claims data set, which includes claims from three of the country’s five largest commercial insurers: Aetna, Humana, and UnitedHealthcare.
The work was funded by the Commonwealth of Pennsylvania and the National Cancer Institute. Dr. Takvorian and Dr. Keating didn’t have any disclosures. One of Dr. Takvorian’s coauthors reported grants and/or personal fees from several sources, including Pfizer, UnitedHealthcare, and Blue Cross Blue Shield of North Carolina.
FROM JAMA NETWORK OPEN