User login
Risk Factors and Antipsychotic Usage Patterns Associated With Terminal Delirium in a Veteran Long-Term Care Hospice Population
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
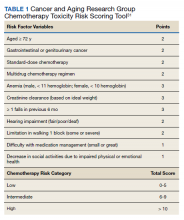
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
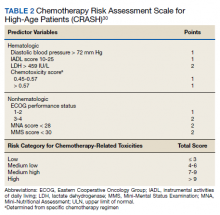
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Photographic Confirmation of Biopsy Sites Saves Lives
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
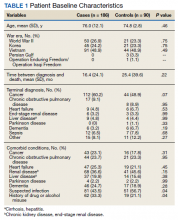
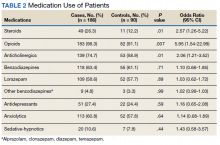
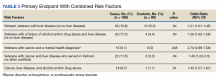
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
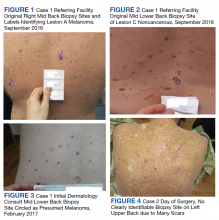
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Quality photographic documentation of lesions prior to biopsy can decrease the risk of wrong site surgery, improve patient care, and save lives.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
Preventable errors by health care workers are widespread and cause significant morbidity and mortality. Wrong site surgery (WSS) is a preventable error that causes harm through both the direct insult of surgery and propagation of the untreated initial problem. WSS also can cause poor patient outcomes, low morale, malpractice claims, and increased costs to the health care system. The estimated median prevalence of WSS across all specialties is 9 events per 1,000,000 surgical procedures, and an institutional study of 112,500 surgical procedures reported 1 wrong-site event, which involved removing the incorrect skin lesion and not removing the intended lesion.1,2
Though the prevalence is low when examining all specialties together, dermatology is also susceptible to WSS.3 Watson and colleagues demonstrated that 31% of intervention errors were due to WSS and suggested that prebiopsy photography helps decrease errors.4 Thus, the American Academy of Dermatology has emphasized the importance of reducing WSS.5 A study by Nijhawan and colleagues found that 25% of patients receiving Mohs surgery at a private single cancer center could not identify their biopsy location because the duration between biopsy and surgery allowed biopsy sites to heal well, which made finding the lesion difficult.6
Risk factors for WSS include having multiple health care providers (HCPs) living remote from the surgery location involved in the case, being a traveling veteran, receiving care at multiple facilities inside and outside the US Department of Veterans Affairs (VA) system, mislabeling photographs or specimens, and photographs not taken at time of biopsy and too close with no frame of reference to assist in finding the correct site. The VA electronic health record (EHR) is not integrated with outside facility EHRs, and the Office of Community Care (OCC) at the VA is responsible for obtaining copies of outside records. If unsuccessful, the HCP and/or patient must provide the records. Frequently, records are not received or require multiple attempts to be obtained. This mostly affects veterans receiving care at multiple facilities inside and outside the VA system as the lack of or timely receipt of past health records could increase the risk for WSS.
To combat WSS, some institutions have implemented standardized protocols requiring photographic documentation of lesions before biopsy so that the surgeon can properly identify the correct site prior to operating.7 Fortunately, recent advances in technology have made it easier to provide photographic documentation of skin lesions. Highsmith and colleagues highlighted use of smartphones to avoid WSS in dermatology.7 Despite these advances, photographic documentation of lesions is not universal. A study by Rossy and colleagues found that less than half of patients referred for Mohs surgery had clear documentation of the biopsy site with photography, diagram, or measurements, and of those documented, only a small fraction used photographs.8
Photographic documentation is not currently required by the VA, increasing the risk of WSS. About 20% of the ~150 VA dermatology departments nationwide are associated with a dermatology residency program and have implemented photographic documentation of lesions before biopsy. The other 80% of departments may not be using photographic documentation. The following 3 cases experienced by the authors highlight instances of how quality photographic documentation of lesions prior to biopsy can improve patient care and save lives. Then, we propose a photographic documentation protocol for VA dermatology departments to follow based on the photographic standards outlined by the American Society for Dermatologic Surgery.9
Case 1 Presentation
A 36-year-old traveling veteran who relocates frequently and receives care at multiple VA medical centers (VAMCs) presented for excision of a melanoma. The patient had been managed at another VAMC where the lesion was biopsied in September 2016. He presented to the Orlando, Florida, VAMC dermatology clinic 5 months later with the photographs of his biopsy sites along with the biopsy reports. The patient had 6 biopsies labeled A through F. Lesion A at the right mid back was positive for melanoma (Figure 1), whereas lesion C on the mid lower back was not cancerous (Figure 2). On examination of the patient’s back, he had numerous moles and scars. The initial receiving HCP circled and photographed the scar presumed to be the melanoma on the mid lower back (Figure 3).
On the day of surgery, the surgeon routinely checked the biopsy report as well as the photograph from the patient’s most recent HCP visit. The surgeon noted that biopsy A (right mid back) on the pathology report had been identified as the melanoma; however, biopsy C (mid lower back) was circled and presumed to be the melanoma in the recent photograph by the receiving HCP—a nurse practitioner. The surgeon compared the initial photos from the referring VAMC with those from the receiving HCP and subsequently matched biopsy A (melanoma) with the correct location on the right mid back.
This discrepancy was explained to the patient with photographic confirmation, allowing for agreement on the correct site before the surgery. The pathology results of the surgical excision confirmed melanoma in the specimen and clear margins. Thus, the correct site was operated on.
Case 2 Presentation
A veteran aged 86 years with a medical history of a double transplant and long-term immunosuppression leading to numerous skin cancers was referred for surgical excision of a confirmed squamous cell carcinoma (SCC) on the left upper back. On the day of surgery, the biopsy site could not be identified clearly due to numerous preexisting scars (Figure 4). No photograph of the original biopsy site was available. The referring HCP was called to the bedside to assist in identifying the biopsy site but also was unable to clearly identify the site. This was explained to the patient. As 2-person confirmation was unsuccessful, conservative treatment was used with patient consent. The patient has since had subsequent close follow-up to monitor for recurrence, as SCC in transplant patients can display aggressive growth and potential for metastasis.
Case 3 Presentation
A veteran was referred for surgical excision of a nonmelanoma skin cancer. The biopsy was completed well in advance of the anticipated surgery day. On the day of surgery, the site could not be detected as it healed well after the biopsy. Although a clinical photograph was available, it was taken too close-up to find a frame of reference for identifying the location of the biopsy site. The referring HCP was called to the bedside to assist in identification of the biopsy site, but 2-person confirmation was unsuccessful. This was explained to the patient, and with his consent, the HCPs agreed on conservative treatment and close follow-up.
Discussion
To prevent and minimize poor outcomes associated with WSS, the health care team should routinely document the lesion location in detail before the biopsy. Many HCPs believe a preoperative photograph is the best method for documentation. As demonstrated in the third case presentation, photographs must be taken at a distance that includes nearby anatomic landmarks for reference. It is suggested that the providers obtain 2 images, one that is far enough to include landmarks, and one that is close enough to clearly differentiate the targeted lesion from others.10
Although high-resolution digital cameras are preferred, mobile phones also can be used if they provide quality images. As phones with built-in cameras are ubiquitous, they offer a quick and easy method of photographic documentation. St John and colleagues also presented the possibility of having patients keep pictures of the lesion on their phones, as this removes potential privacy concerns and facilitates easy transportation of information between HCPs.10 If it is discovered that a photograph was not taken at the time of biopsy, our practice contacts the patient and asks them to photograph and circle the biopsy site using their mobile phone or camera and bring it to the surgery appointment. We propose a VA protocol for photographic documentation of biopsy sites (Table).
HCPs who are not comfortable with technology may be hesitant to use photographic documentation using a smartphone or camera. Further, HCPs often face time constraints, and taking photographs and uploading them to the EHR could decrease patient contact time. Therefore, photographic documentation presents an opportunity for a team approach to patient-centered care: Nursing and other medical staff can assist with these duties and learn the proper photographic documentation of biopsy sites. Using phone or tablet applications that provide rapid photographic documentation and uploading to the EHR also would facilitate universal use of photographic documentation.
If a HCP is uncomfortable or unable to use photography to document lesions, alternative strategies for documenting lesions exist, including diagrams, anatomic landmarks, ultraviolet (UV) fluorescent tattoos, and patient identification of lesions.10 In the diagram method, a HCP marks the lesion location on a diagram of the body preferably with a short description of the lesion’s location and/or characteristics.11 The diagram should be uploaded into the EHR. There are other methods for documenting lesion location relative to anatomic landmarks. Triangulation involves documenting distance between the lesion and 3 distinct anatomic locations.10 UV fluorescent tattooing involves putting UV tattoo dye in the biopsy site and locating the dye using a Wood lamp at the time of surgery. The lamp was used in a single case report of a patient with recurrent basal cell carcinoma.12 Patient identification of lesions by phone applications that allow patients to track their lesion, a phone selfie of the biopsy site, or a direct account of a lesion can be used to confirm lesion location based on the other methods mentioned.10
Patients often are poorly adherent to instructions aimed at reducing the risk of WSS. In a study that asked patients undergoing elective foot or ankle surgery to mark the foot not being operated on, 41% of patients were either partially or nonadherent with this request.13 Educating patients on the importance of lesion self-identification has the potential to improve identification of biopsy location and prevent WSS. Nursing and medical staff can provide patient education while photographing the biopsy site including taking a photograph with the patient’s cell phone for their records.
Due to subsequent morbidity and mortality that can result from WSS, photographic confirmation of biopsy sites is a step that surgeons can take to ensure identification of the correct site prior to surgery. Case 1 provides an example of how photographs taken prior to biopsy can prevent WSS. In a disease such as melanoma, photographs are particularly important, as insufficient treatment can lead to fatal metastases. To increase quality of care, all available photographs should be reviewed, especially in cases where the pathology report does not match the clinical presentation.
If WSS occurs, HCPs may be hesitant to disclose their mistakes due to potential lawsuits, the possibility that disclosure may inadvertently harm the patient, and their relative inexperience in and training regarding disclosure skills.14 Surgeons who perform WSS may receive severe penalties from state licensing boards, including suspension of medical license. Financially, many insurers will not compensate providers for WSS. Also, many incidents of WSS result in a malpractice claim, with about 80% of those cases resulting in a malpractice award.15 However, it is important that HCPs are open with their patients regarding WSS.
As demonstrated in case presentations 2 and 3, having 2-person confirmation and patient confirmation before to surgery is important in preventing WSS for patients who have poor documentation of biopsy sites. In cases where agreement is not achieved, HCPs can consider several other options to help identify lesions. Dermabrasion and alcohol wipes are options.10 Dermabrasion uses friction to expose surgical sights that have healed, scarred, or been hidden by sun damage.10 Alcohol wipes remove surface scale and crust, creating a glisten with tangential lighting that highlights surface irregularities. Anesthesia injection prior to surgery creates a blister at the location of the cancer. This is because skin cancer weakens the attachments between keratinocytes, and as a result, the hydrostatic pressure from the anesthesia favorably blisters the malignancy location.10,16
Dermoscopy is another strategy shown to help identify scar margins.10,17 Under dermoscopy, a scar demonstrates a white-pink homogenous patch with underlying vessels, whereas basal cell carcinoma remnants include blue-gray ovoid nests and globules, telangiectasias, spoke wheel and leaflike structures.17 As a final option, HCPs can perform an additional biopsy of potential cancer locations to find the lesion again.10 If the lesions cannot be identified, HCPs should consider conservative measures or less invasive treatments with close and frequent follow-up.
Conclusions
The cases described here highlight how the lack of proper photographic documentation can prevent the use of curative surgical treatment. In order to reduce WSS and improve quality care, HCPs must continue to take steps and create safeguards to minimize risk. Proper documentation of lesions prior to biopsy provides an effective route to reduce incidence of WSS. If the biopsy site cannot be found, various strategies to properly identify the site can be employed. If WSS occurs, it is important that HCPs provide full disclosure to patients. With a growing emphasis on patient safety measures and advances in technology, HCPs are becoming increasingly cognizant about the most effective ways to optimize patient care, and it is anticipated that this will result in a decrease in morbidity and mortality.
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
1. Hempel S, Maggard-Gibbons M, Nguyen DK, et al. Wrong-site surgery, retained surgical items, and surgical fires: a systematic review of surgical never events. JAMA Surg. 2015;150(8):796-805. doi:10.1001/jamasurg.2015.0301
2. Knight N, Aucar J. Use of an anatomic marking form as an alternative to the Universal Protocol for Preventing Wrong Site, Wrong Procedure and Wrong Person Surgery. Am J Surg. 2010;200(6):803-809. doi:10.1016/j.amjsurg.2010.06.010
3. Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases [published correction appears in J Am Acad Dermatol. 2016 Oct;75(4):854]. J Am Acad Dermatol. 2016;74(1):1-18. doi:10.1016/j.jaad.2015.06.033
4. Watson AJ, Redbord K, Taylor JS, Shippy A, Kostecki J, Swerlick R. Medical error in dermatology practice: development of a classification system to drive priority setting in patient safety efforts. J Am Acad Dermatol. 2013;68(5):729-737. doi:10.1016/j.jaad.2012.10.058
5. Elston DM, Taylor JS, Coldiron B, et al. Patient safety: Part I. Patient safety and the dermatologist. J Am Acad Dermatol. 2009;61(2):179-191. doi:10.1016/j.jaad.2009.04.056
6. Nijhawan RI, Lee EH, Nehal KS. Biopsy site selfies--a quality improvement pilot study to assist with correct surgical site identification. Dermatol Surg. 2015;41(4):499-504. doi:10.1097/DSS.0000000000000305
7. Highsmith JT, Weinstein DA, Highsmith MJ, Etzkorn JR. BIOPSY 1-2-3 in dermatologic surgery: improving smartphone use to avoid wrong-site surgery. Technol Innov. 2016;18(2-3):203-206. doi:10.21300/18.2-3.2016.203
8. Rossy KM, Lawrence N. Difficulty with surgical site identification: what role does it play in dermatology? J Am Acad Dermatol. 2012;67(2):257-261. doi:10.1016/j.jaad.2012.02.034
9. American Society for Dermatologic Surgery. Photographic standards in dermatologic surgery poster. Accessed April 12, 2021. https://www.asds.net/medical-professionals/members-resources/product-details/productname/photographic-standards-poster
10. St John J, Walker J, Goldberg D, Maloney ME. Avoiding Medical Errors in Cutaneous Site Identification: A Best Practices Review. Dermatol Surg. 2016;42(4):477-484. doi:10.1097/DSS.0000000000000683
11. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. doi:10.1001/jamadermatol.2013.9804
12. Chuang GS, Gilchrest BA. Ultraviolet-fluorescent tattoo location of cutaneous biopsy site. Dermatol Surg. 2012;38(3):479-483. doi:10.1111/j.1524-4725.2011.02238.x
13. DiGiovanni CW, Kang L, Manuel J. Patient compliance in avoiding wrong-site surgery. J Bone Joint Surg Am. 2003;85(5):815-819. doi:10.2106/00004623-200305000-00007
14. Gallagher TH. A 62-year-old woman with skin cancer who experienced wrong-site surgery: review of medical error. JAMA. 2009;302(6):669-677. doi:10.1001/jama.2009.1011
15. Mulloy DF, Hughes RG. Wrong-site surgery: a preventable medical error. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008:chap 36. Accessed April 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK2678
16. Zaiac M, Tongdee E, Porges L, Touloei K, Prodanovich S. Anesthetic blister induction to identify biopsy site prior to Mohs surgery. J Drugs Dermatol. 2015;14(5):446-447.
17. Jawed SI, Goldberg LH, Wang SQ. Dermoscopy to identify biopsy sites before Mohs surgery. Dermatol Surg. 2014;40(3):334-337. doi:10.1111/dsu.12422
Use of Comprehensive Geriatric Assessment in Oncology Patients to Guide Treatment Decisions and Predict Chemotherapy Toxicity
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
Age is a well recognized risk factor for cancer development. The population of older Americans is growing, and by 2030, 20% of the US population will be aged ≥ 65 years.1 While 25% of all new cancer cases are diagnosed in people aged 65 to 74 years, more than half of cancers occur in individuals aged ≥ 70 years, with even higher rates in those aged ≥ 75 years.2 Although cancer rates have declined slightly overall among people aged ≥ 65 years, this population still has an 11-fold increased incidence of cancer compared with that of younger individuals.3 With a rapidly growing older population, there will be increasing demand for cancer care.
Treatment of cancer in older individuals often is complicated by medical comorbidities, frailty, and poor functional status. Distinguishing patients who can tolerate aggressive therapy from those who require less intensive therapy can be challenging. Age-related physiologic changes predispose older adults to an increased risk of therapy-related toxicities, resulting in suboptimal therapeutic benefit and substantial morbidity. For example, cardiovascular changes can lead to reduction of the cardiac functional reserve, which can increase the risk of congestive heart failure. Similarly, decline in renal function leads to an increased potential for nephrotoxicity.4 Although patients may be of the same chronologic age, their performance, functional, and biologic status may be quite variable; thus, tolerance to aggressive treatment is not easily predicted. The comprehensive geriatric assessment (CGA) may be used as a global assessment tool to risk stratify older patients prior to oncologic treatment decisions.
Health care providers (HCPs), including physician assistants, nurse practitioners, clinical nurse specialists, nurses, and physicians, routinely participate in every aspect of cancer care by ordering and interpreting diagnostic tests, addressing comorbidities, managing symptoms, and discussing cancer treatment recommendations. HCPs in oncology will continue to play a vital role in the coordination and management of older patients with cancer. However, in general, CGA has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools.
What Is Geriatric Assessment?
Geriatric assessment is a multidisciplinary, multidimensional process aimed at detecting medical, psychosocial, and functional issues of older adults that are not identified by traditional performance status measures alone. It provides guidance for management of identified problems and improvement in quality of life.6 CGA was developed by geriatricians and multidisciplinary care teams to evaluate the domains of functional, nutritional, cognitive, psychosocial, and economic status; comorbidities; geriatric syndromes; and mood, and it has been tested in both clinics and hospitals.7 Although such assessment requires additional time and resources, its goals are to identify areas of vulnerability, assist in clinical decisions of treatable health problems, and guide therapeutic interventions.6 In oncology practice, the assessment not only addresses these global issues, but also is critical in predicting toxicity and survival outcomes in older oncology patients.
Components of CGA
Advancing age brings many physiologic, psychosocial, and functional challenges, and a cancer diagnosis only adds to these issues. CGA provides a system of assessing older and/or frail patients with cancer through specific domains to identify issues that are not apparent on routine evaluation in a clinic setting before and during chemotherapy treatments. These domains include comorbidity, polypharmacy, functional status, cognition, psychological and social status, and nutrition.8
Comorbidity
The prevalence of multiple medical problems and comorbidities, including cancer, among people aged > 65 years is increasing.9 Studies have shown that two-thirds of patients with cancer had ≥ 2 medical conditions, and nearly one quarter had ≥ 4 medical conditions.10 In older adults, common comorbidities include cardiovascular disease, hypertension, diabetes mellitus, and dementia. These comorbidities can impact treatment decisions, increase the risk of disease, impact treatment-related complications, and affect a patient’s life expectancy.11 Assessing comorbidities is essential to CGA and is done using the Charlson Comorbidity Index and/or the Cumulative Illness Rating Scale.12
The Charlson Comorbidity Index was originally designed to predict 1-year mortality on the basis of a weighted composite score for the following categories: cardiovascular, endocrine, pulmonary, neurologic, renal, hepatic, gastrointestinal, and neoplastic disease.13 It is now the most widely used comorbidity index and has been adapted and verified as applicable and valid for predicting the outcomes and risk of death from many comorbid diseases.14 The Cumulative Illness Rating Scale has been validated as a predictor for readmission for hospitalized older adults, hospitalization within 1 year in a residential setting, and long-term mortality when assessed in inpatient and residential settings.15
Polypharmacy
Polypharmacy (use of ≥ 5 medications) is common in older patients regardless of cancer diagnosis and is often instead defined as “the use of multiple drugs or more than are medically necessary.”16 The use of multiple medications, including those not indicated for existing medical conditions (such as over‐the‐counter, herbal, and complementary/alternative medicines, which patients often fail to declare to their specialist, doctor, or pharmacist) adds to the potential negative aspects of polypharmacy that affect older patients.17
Patients with cancer usually are prescribed an extensive number of medicines, both for the disease and for supportive care, which can increase the chance of drug-drug interactions and adverse reactions.18 While these issues certainly affect quality of life, they also may influence chemotherapy treatment and potentially impact survival. Studies have shown that the presence of polypharmacy has been associated with higher numbers of comorbidities, increased use of inappropriate medications, poor performance status, decline in functional status, and poor survival.18
Functional Status
Although Eastern Cooperative Oncology Group (ECOG) performance status and Karnofsky Performance Status are commonly used by oncologists, these guidelines are limited in focus and do not reliably measure functional status in older patients. Functional status is determined by the ability to perform daily acts of self-care, which includes assessment of activities of daily living (ADLs) and instrumental activities of daily living (IADLs). ADLs refer to such tasks as bathing, dressing, eating, mobility, balance, and toileting.19 IADLs include the ability to perform activities required to live within a community and include shopping, transportation, managing finances, medication management, cooking, and cleaning.11
Physical functionality also can be assessed by measures such as gait speed, grip strength, balance, and lower extremity strength. These are more sensitive and shown to be associated with worse clinical outcomes.20 Grip strength and gait speed, as assessed by the Timed Up and Go test or the Short Physical Performance Battery measure strength and balance.12 Reduction in gait speed and/or grip strength are associated with adverse clinical outcomes and increased risk of mortality.21 Patients with cancer who have difficulty with ADLs are at increased risk for falls, which can limit their functional independence, compromise cancer therapy, and increase the risk of chemotherapy toxicities.11 Impaired hearing and poor vision are added factors that can be barriers to cancer treatment.
Cognition
Cognitive impairment in patients with cancer is becoming more of an issue for oncology HCPs as both cancer and cognitive decline are more common with advancing age. Cognition in cancer patients is important for understanding their diagnosis, prognosis, treatment options, and adherence. Impaired cognition can affect decision making regarding treatment options and administration. Cognition can be assessed through validated screening tools such as the Mini-Mental State Examination and Mini-Cog.11
Psychological and Social Status
A cancer diagnosis has a major impact on the mental and emotional state of patients and family members. Clinically significant anxiety has been reported in approximately 21% of older patients with cancer, and the incidence of depression ranges from 17 to 26%.22 In older patients with, psychologic distress can impact cancer treatment, resulting in less definitive therapy and poorer outcomes.23 All patients with cancer should be screened for psychologic distress using standardized methods, such as the Geriatric Depression Scale or the General Anxiety Disorder-7 scale.24 A positive screen should lead to additional assessments that evaluate the severity of depression and other comorbid psychological problems and medical conditions.
Social isolation and loneliness are factors that can affect both depression and anxiety. Older patients with cancer are at risk for decreased social activities and are already challenged with issues related to home care, comorbidities, functional status, and caregiver support.23 Therefore, it is important to assess the social interactions of an older and/or frail patient with cancer and use social work assistance to address needs for supportive services.
Nutrition
Nutrition is important in any patient with cancer undergoing chemotherapy treatment. However, it is of greater importance in older adults, as malnutrition and weight loss are negative prognostic factors that correlate with poor tolerance to chemotherapy treatment, decline in quality of life, and increased mortality.25 The Mini-Nutritional Assessment is a widely used validated tool to assess nutritional status and risk of malnutrition.11 This tool can help identify those older and/or frail patients with cancer with impaired nutritional status and aid in instituting corrective measures to treat or prevent malnutrition.
Effectiveness of CGA
Multiple randomized controlled clinical trials assessing the effectiveness of CGA have been conducted over the past 3 decades with overall positive outcomes related to its value.26 Benefits of CGA can include overall improved medical care, avoidance of hospitalization or nursing home placement, identification of cognitive impairment, and prevention of geriatric syndrome (a range of conditions representing multiple organ impairment in older adults).27
In oncology, CGA is particularly beneficial, as it can identify issues in nearly 70% of patients that may not be apparent through traditional oncology assessment.28 A systematic review of 36 studies assessing the prognostic value of CGA in elderly patients with cancer receiving chemotherapy concluded that impaired performance and functional status as well as a frail and vulnerable profile are important predictors of severe chemotherapy-related toxicity and are associated with a higher risk of mortality.29 Therefore, CGA should be an integral part of the evaluation of older and/or frail patients with cancer prior to chemotherapy consideration.
Several screening tools have been developed using information from CGA to assess the risk of severe toxicities. The most commonly used tools for predicting toxicity include the Cancer and Aging Research Group (CARG) chemotoxicity calculator and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH).30,31 Although these tools are readily available to facilitate CGA, and despite their proven beneficial outcome and recommended usage by national guidelines, implementation of these tools in routine oncology practice has been challenging and slow to spread. Unless these recommended interventions are effectively implemented, the benefits of CGA cannot be realized. With the expected surge in the number of older patients with cancer, hopefully this will change.
Geriatric Assessment Screening Tools
A screening tool recommended for use in older and/or frail patients with cancer allows for a brief assessment to help clinicians identify patients in need of further evaluation by CGA and to provides information on treatment-related toxicities, functional decline, and survival.32 The predictive value and utility of geriatric assessment screening tools have been repeatedly proven to identify older and/or frail adults at risk for treatment-related toxicities.12 The CARG and the CRASH are validated screening tools used in identifying patients at higher risk for chemotherapy toxicity. These screening tools are intended to provide guidance to the clinical oncology practitioner on risk stratification of chemotherapy toxicity in older patients with cancer.33
Both of these screening tools provide similar predictive performance for chemotherapy toxicity in older patients with cancer.34 However, the CARG tool seems to have the advantage of using more data that had already been obtained during regular office visits and is clear and easy to use clinically. The CRASH tool is slightly more involved, as it uses multiple geriatric instruments to determine the predictive risk of both hematologic and nonhematologic toxicities of chemotherapy.
CARG Chemotoxicity Calculator
Hurria and colleagues originally developed the CARG tool from data obtained through a prospective multicenter study involving 500 patients with cancer aged ≥ 65 years.35 They concluded that chemotherapy-related toxicity is common in older adults, with 53% of patients sustaining grade 3 or 4 treatment-related toxicities and 2% treatment-related mortality.12 This predictive model for chemotherapy-related toxicity used 11 variables, both objective (obtained during a regular clinical encounter: age, tumor type, chemotherapy dosing, number of drugs, creatinine, and hemoglobin) and subjective (completed by patient: number of falls, social support, the ability to take medications, hearing impairment, and physical performance), to determine at-risk patients (Table 1).31
Compared with standard performance status measures in oncology practice, the CARG model was better able to predict chemotherapy-related toxicities. In 2016, Hurria and colleagues published the results of an updated external validation study with a cohort of 250 older patients with cancer receiving chemotherapy that confirmed the prediction of chemotherapy toxicity using the CARG screening tool in this population.31 An appealing feature of this tool is the free online accessibility and the expedited manner in which screening can be conducted.
CRASH Score
The CRASH score was derived from the results of a prospective, multicenter study of 518 patients aged ≥ 70 years who were assessed on 24 parameters prior to starting chemotherapy.30 A total of 64% of patients experienced significant toxicities, including 32% with grade 4 hematologic toxicity and 56% with grade 3 or 4 nonhematologic toxicity. The hematologic and nonhematologic toxicity risks are the 2 categories that comprise the CRASH score. Both baseline patient variables and chemotherapy regimen are incorporated into an 8-item assessment profile that determines the risk categories (Table 2).30
Increased risk of hematologic toxicities was associated with increased diastolic blood pressure, increased lactate dehydrogenase, need for assistance with IADL, and increased toxicity potential of the chemotherapy regimen. Nonhematologic toxicities were associated with ECOG performance score, Mini Mental Status Examination and Mini-Nutritional Assessment, and increased toxicity of the chemotherapy regimen.12 Patient scores are stratified into 4 risk categories: low, medium-low, medium-high, and high.30 Like the CARG tool, the CRASH screening tool also is available as a free online resource and can be used in everyday clinical practice to assess older and/or frail adults with cancer.
Conclusions
In older adults, cancer may significantly impact the natural course of concurrent comorbidities due to physiologic and functional changes. These vulnerabilities predispose older patients with cancer to an increased risk of adverse outcomes, including treatment-related toxicities.36 Given the rapidly aging population, it is critical for oncology clinical teams to be prepared to assess for, prevent, and manage issues for older adults that could impact outcomes, including complications and toxicities from chemotherapy.35 Studies have reported that 78 to 93% of older oncology patients have at least 1 geriatric impairment that could potentially impact oncology treatment plans.37,38 This supports the utility of CGA as a global assessment tool to risk stratify older and/or frail patients prior to deciding on subsequent oncologic treatment approaches.5 In fact, major cooperative groups sponsored by the National Cancer Institute, such as the Alliance for Clinical Trials in Oncology, are including CGA as part of some of their treatment trials. CGA was conducted as part of a multicenter cooperative group study in older patients with acute myeloid leukemia prior to inpatient intensive induction chemotherapy and was determined to be feasible and useful in clinical trials and practice.39
Despite the increasing evidence for benefits of CGA, it has not been a consistent part of oncology practices, and few HCPs are familiar with the benefits of CGA screening tools. Although oncology providers routinely participate in every aspect of cancer care and play a vital role in the coordination and management of older patients with cancer, CGA implementation into routine clinical practice has been slow in part due to lack of knowledge and training regarding the use of GA tools.
Oncology providers can easily incorporate CGA screening tools into the history and physical examination process for older patients with cancer, which will add an important dimension to these patient evaluations. Oncology providers are not only well positioned to administer these screening tools, but also can lead the field in developing innovative ways for effective implementation in busy routine oncology clinics. However, to be successful, oncology providers must be knowledgeable about these tools and understand their utility in guiding treatment decisions and improving quality of care in older patients with cancer.
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
1. Sharless NE. The challenging landscape of cancer and aging: charting a way forward. Published January 24, 2018. Accessed April 16, 2021. https://www.cancer.gov/news-events/cancer-currents-blog/2018/sharpless-aging-cancer-research
2. National Cancer Institute. Age and cancer risk. Updated March 5, 2021. Accessed April 16, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551 4. Sawhney R, Sehl M, Naeim A. Physiologic aspects of aging: impact on cancer management and decision making, part I. Cancer J. 2005;11(6):449-460. doi:10.1097/00130404-200511000-00004
5. Kenis C, Bron D, Libert Y, et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann Oncol. 2013;24(5):1306-1312. doi:10.1093/annonc/mds619
6. Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract. 2018;14(2):85-94. doi:10.1200/JOP.2017.026435
7. Cohen HJ. Evolution of geriatric assessment in oncology. J Oncol Pract. 2018;14(2):95-96. doi:10.1200/JOP.18.00017
8. Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. doi:10.1200/JCO.2013.54.8347
9. American Cancer Society. Cancer facts & figures 2019. Accessed April 16, 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html
10. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249-257. doi:10.1016/j.jgo.2015.12.002
11. Korc-Grodzicki B, Holmes HM, Shahrokni A. Geriatric assessment for oncologists. Cancer Biol Med. 2015;12(4):261-274. doi:10.7497/j.issn.2095-3941.2015.0082
12. Li D, Soto-Perez-de-Celis E, Hurria A. Geriatric assessment and tools for predicting treatment toxicity in older adults with cancer. Cancer J. 2017;23(4):206-210. doi:10.1097/PPO.0000000000000269
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
14. Huang Y, Gou R, Diao Y, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66. doi:10.1631/jzus.B1300109
15. Osborn KP IV, Nothelle S, Slaven JE, Montz K, Hui S, Torke AM. Cumulative Illness Rating Scale (CIRS) can be used to predict hospital outcomes in older adults. J Geriatric Med Gerontol. 2017;3(2). doi:10.23937/2469-5858/1510030
16. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57-65. doi:10.1517/14740338.2013.827660
17. Shrestha S, Shrestha S, Khanal S. Polypharmacy in elderly cancer patients: challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019;2(1):42-49. doi:10.1002/agm2.12051
18. Sharma M, Loh KP, Nightingale G, Mohile SG, Holmes HM. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J Geriatr Oncol. 2016;7(5):346-353. doi:10.1016/j.jgo.2016.07.010
19. Norburn JE, Bernard SL, Konrad TR, et al. Self-care and assistance from others in coping with functional status limitations among a national sample of older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(2):S101-S109. doi:10.1093/geronb/50b.2.s101
20. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. doi:10.1111/jgs.13871
21. Owusu C, Berger NA. Comprehensive geriatric assessment in the older cancer patient: coming of age in clinical cancer care. Clin Pract (Lond). 2014;11(6):749-762. doi:10.2217/cpr.14.72
22. Weiss Wiesel TR, Nelson CJ, Tew WP, et al. The relationship between age, anxiety, and depression in older adults with cancer. Psychooncology. 2015;24(6):712-717. doi:10.1002/pon.3638
23. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305-e316. doi:10.1016/S1470-2045(18)30348-6
24. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619. doi:10.1200/JCO.2013.52.4611
25. Muscaritoli M, Lucia S, Farcomeni A, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79886. doi:10.18632/oncotarget.20168
26. Ekdahl AW, Axmon A, Sandberg M, Steen Carlsson K. Is care based on comprehensive geriatric assessment with mobile teams better than usual care? A study protocol of a randomised controlled trial (the GerMoT study). BMJ Open. 2018;8(10)e23969. doi:10.1136/bmjopen-2018-023969
27. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
28. Hernandez Torres C, Hsu T. Comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4-5):330-339. doi:10.1016/j.euf.2017.10.010
29. Janssens K, Specenier P. The prognostic value of the comprehensive geriatric assessment (CGA) in elderly cancer patients (ECP) treated with chemotherapy (CT): a systematic review. Eur J Cancer. 2017;72(1):S164-S165. doi:10.1016/S0959-8049(17)30611-1
30. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. doi:10.1002/cncr.26646
31. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
32. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288-300. doi:10.1093/annonc/mdu210
33. Schiefen JK, Madsen LT, Dains JE. Instruments that predict oncology treatment risk in the senior population. J Adv Pract Oncol. 2017;8(5):528-533.
34. Ortland I, Mendel Ott M, Kowar M, et al. Comparing the performance of the CARG and the CRASH score for predicting toxicity in older patients with cancer. J Geriatr Oncol. 2020;11(6):997-1005. doi:10.1016/j.jgo.2019.12.016
35. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. doi:10.1200/JCO.2011.34.7625
36. Mohile SG, Velarde C, Hurria A, et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J Natl Compr Canc Netw. 2015;13(9):1120-1130. doi:10.6004/jnccn.2015.0137
37. Schiphorst AHW, Ten Bokkel Huinink D, Breumelhof R, Burgmans JPJ, Pronk A, Hamaker ME. Geriatric consultation can aid in complex treatment decisions for elderly cancer patients. Eur J Cancer Care (Engl). 2016;25(3):365-370. doi:10.1111/ecc.12349
38. Schulkes KJG, Souwer ETD, Hamaker ME, et al. The effect of a geriatric assessment on treatment decisions for patients with lung cancer. Lung. 2017;195(2):225-231. doi:10.1007/s00408-017-9983-7
39. Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: report of CALGB 361006 (Alliance). J Geriatr Oncol. 2020;11(1):107-113. doi:10.1016/j.jgo.2019.10.002
States ready plans to get Pfizer COVID vaccine to younger teens
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
Adulterants in street drugs could increase susceptibility to COVID
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
The composition of street drugs like heroin and cocaine are changing. According to a new analysis, almost all contain at least one toxic adulterant, and many contain a plethora. Most adulterants have pharmacologic activities and toxicities. Their presence has added impact in the context of the COVID-19 pandemic, since some may cause a drastic drop in white blood cells that could leave drug users more vulnerable to infection.
“It’s remarkable that we just forgot to notice, in the horrendous transition from prescription opioid epidemic to the illicit opioid and psychostimulant epidemics, that we would have to pay special attention to what the medications are in the drugs that the person was exposed to – and for how long,” said Mark S. Gold, MD, a coauthor of the review.
The analysis showed that adulterants include new psychoactive substances, industrial compounds, fungicides, veterinary medications, and various impurities. In addition, other various medications are being found in street drugs, such as antipsychotics, antidepressants, anxiolytics, antihistamines, anthelmintics, anesthetics, anti-inflammatory agents, antipyretics, analgesics, antispasmodics, antiarrhythmics, antimalarials, bronchodilators, decongestants, expectorants, muscle relaxers, natural/synthetic hallucinogens, and sedatives.
Illicit drugs are by nature manufactured without Food and Drug Administration oversight, and it is becoming increasingly common that substances like leftover medicines and other active drugs are added to illicit drug batches to add weight, said Dr. Gold, a professor at Washington University,St. Louis. The study appeared in Current Psychopharmacology.
Effects of adulterants ‘terrifying’
The findings of adulterants and their consequences are concerning, according to Jean Lud Cadet, MD, who was asked to comment on the findings. “The blood dysplasia, the pulmonary problems that some of those adulterants can cause – it’s actually terrifying, to put it bluntly,” said Dr. Cadet, who is a senior investigator and chief of the Molecular Neuropsychiatry Research Branch at the National Institute on Drug Abuse.
Before 2000, street drugs were generally diluted with comparatively benign substances such as caffeine, sugars, or lidocaine. Drugs like phenacetin, levamisole, acetaminophen, and diltiazem began to appear in heroin and cocaine in the late 1990s, and by 2010, more powerful adulterants like fentanyl, ketamine, and quetiapine became common.
In 2015, the U.S. Department of State partnered with the Colombo Plan, an international organization based in Sri Lanka, to use field spectroscopy to detect toxins directly in cocaine and heroin samples found in Argentina, Brazil, Ecuador, Peru, Sri Lanka, Thailand, Honduras, Guatemala, Mexico, Colombia, and South Africa. They found a range of adulterants such as aminopyrine, diltiazem, metamizole, levamisole, and phenacetin.
A similar project with 431 heroin and cocaine samples from Vermont and Kentucky found that 69% of samples had five or more controlled drugs, toxic adulterants, or impurities. About 15% had nine or more, and 95% of samples had at least one toxic adulterant.
In the midst of the COVID-19 pandemic, these adulterants take on even greater significance. Individuals with substance use disorders often have other health conditions that can make them more vulnerable to viral infections, and this could be exacerbated by the effects of adulterants on white blood cells or other systems. The pandemic has also had an indirect effect by causing a shortage of street drugs. During production shortages, traffickers might boost potency by adding more cutting agents and adulterants. As a result, COVID-19 and opioid addiction tend to reinforce each other.
“The clinical message would be that our [substance use] patients will contract infectious disease and need to be prioritized for [COVID-19] vaccination,” said Dr. Gold.
The findings came as a surprise to Dr. Cadet, and that illustrates a need to publicize the presence of adulterants in street drugs.
“If I wasn’t aware of many of these, then the general public is also not going to be aware of them,” Dr. Cadet said. “Scientists, including myself, and government agencies need to do a better job [of communicating this issue].”
The study references individuals with substance use disorder, but Dr. Cadet cautioned that anyone who uses street drugs, even once or twice, could be a victim of adulterants. “You don’t need to have met criteria for diagnosis in order to suffer the consequences.”
The study had no funding. Dr. Gold and Dr. Cadet have no relevant financial disclosures.
FROM CURRENT PSYCHOPHARMACOLOGY
ADHD in preschool kids: Adrenergic agonists may be a better fit
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study finds that alpha2-adrenergic agonists may be of benefit and have fewer side effects than stimulant medications for the treatment of attention-deficit/hyperactivity disorder in preschool-age children.
The study was published online May 4 in JAMA.
As part of a retrospective analysis, Elizabeth Harstad, MD, MPH, of Boston Children’s Hospital and colleagues evaluated health record data from 497 preschool-age children with ADHD across seven developmental-behavioral pediatric practices in the United States. Children included in the evaluation were younger than 6 years and were treated for ADHD between Jan. 1, 2013, and July 1, 2017, with either an alpha2-adrenergic agonist or a stimulant.
Overall, 175 children (35%) were prescribed an alpha2-adrenergic agonist (most often guanfacine) as first-line ADHD medication, and 322 children (65%) were prescribed a stimulant (most often a methylphenidate-based preparation). Before any medication regimens were initiated, 62% of children received behavioral therapy.
“These findings suggest that for some children there may be a concern about either how well a stimulant will work or how well a stimulant will be tolerated that is leading clinicians to instead prescribe an alpha2-adrenergic agonist as the first medication tried,” Dr. Harstad said in an interview.
Clinical improvement was noted in 66% of children treated with alpha2-adrenergic agonists (95% confidence interval, 57.5%-73.9%) and in 78% of children treated with stimulants (95% CI, 72.4%-83.4%).
Most adverse effects were more common among children who received stimulants than among those who received alpha2-adrenergic agonists. These adverse effects included difficulty falling asleep (21% vs. 11%), decreased appetite (38% vs. 7%), increased stomachaches (13% vs. 5%), and increased skin picking/repetitive behaviors (11% vs. 5%). Only daytime sleepiness was more frequent among children who received an alpha2-adrenergic agonist rather than a stimulant (38% vs. 3%).
“We also found that for the youngest children (<4 years old), those initiated on alpha2-adrenergic agonists stayed on these medications longer than those initiated on stimulants, which may indicate that they are better tolerated, although more research is needed to confirm this,” Dr. Harstad said.
“While our study focused on how well medications work and how well they are tolerated when used to treat preschool-age children with ADHD, it is important to remember that behavioral therapy is recommended as first-line treatment for ADHD in preschool-age children, not medication,” Dr. Harstad added.
Mark Wolraich, MD, of the University of Oklahoma, echoed that sentiment. “The article mentions that behavioral interventions, in the form of parent training in behavior management, is an effective first-line treatment” and, per the American Academy of Pediatrics guidelines, “is the first line of treatment recommended for preschool-age children before medication should be considered.”
Dr. Wolraich also noted that “neither drug has official FDA [U.S. Food and Drug Administration] approval in this age group” but that “methylphenidate comes the closest to having met the FDA requirements for approval in this age group, which is why the AAP guidelines recommended its use if parent training in behavior management is not sufficient.”
Although Dr. Harstad and colleagues note that the study included a large and diverse sample size from across the United States, they acknowledge that “further research, including from randomized clinical trials, is needed to assess comparative effectiveness of alpha2-adrenergic agonists versus stimulants.”
Funding for the study was provided through a cooperative agreement with the Maternal and Child Health Bureau, the Health Resources and Services Administration, and the U.S. Department of Health & Human Services. Dr. Harstad has reported receiving reported receiving compensation for serving as a medical reviewer for Understood.org and grant funding from the Palmer Family Fund for Autism Research to conduct research related to autism spectrum disorder at Boston Children’s Hospital. Disclosures for the other authors are listed in the original article. Dr. Wolraich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Keep antibiotics unchanged in breakthrough UTIs
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Attending a patient’s funeral: How psychiatrists decide
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychiatrists often develop long-term relationships with their patients, but what happens when a patient dies? Should the psychiatrist attend the patient’s funeral?
It’s a question Ashley Pettaway, MD, faced as a medical resident at the University of Alabama School of Medicine.
For 2 months, Dr. Pettaway was involved in the day-to-day care of a woman in her 40s who ultimately died. As part of that care, Dr. Pettaway had regular meetings with the patient’s husband and family members.
“The patient was about my mother’s age, so I naturally was kind of attached to her,” Dr. Pettaway told this news organization. After she died, her family invited Dr. Pettaway to the funeral.
“While I couldn’t make it to the funeral, it got me thinking. Should I go? If I go, what do I say? Who do I sit with? How do I introduce myself?” wondered Dr. Pettaway, now a resident in the department of psychiatry and neurobehavioral sciences, University of Virginia, Charlottesville.
She turned to the literature but found very little regarding psychiatrists attending their patients’ funerals. “This was surprising to me because in psychiatry, you can get so engrossed in patients’ lives,” Dr. Pettaway said.
Given the lack of rules or formal guidance on psychiatrists attending patients’ funerals, Dr. Pettaway and her mentor, Gabrielle Marzani, MD, conducted an informal survey of 12 supervising psychiatrists at the University of Virginia.
The survey results were presented at the virtual American Psychiatric Association 2021 Annual Meeting.
Ten of the 12 psychiatrists who were surveyed were caring for a patient who died while under their care. Five of those psychiatrists reported going to at least one patient’s funeral over the course of their career.
Among the psychiatrists who attended a patient’s funeral, their attendance was often based on their clinical intuition, their relationship with the family, or whether the patient was an established presence in the community. In the latter case, the psychiatrist attended as a community member.
The number of years in practice also mattered. Fewer senior faculty reported that they would be hesitant to attend and that they would not attend without a formal invitation from the family. Senior career psychiatrists were more likely to attend and felt that an invitation was not required.
None of the psychiatrists surveyed had received training or guidance on attending patients’ funerals at any point in their career.
Given the absence of formal recommendations, Dr. Pettaway believes increased conversation on this topic as part of residency training programs would help psychiatrists navigate these complex situations.
A complex issue
Commenting on the topic for an interview, Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, said this is an “interesting and important topic that is underdiscussed.”
“I don’t think there’s a right answer that applies to every situation,” said Dr. Appelbaum, a past president of the APA.
There will be times, he said, when psychiatrists or other mental health professionals have worked closely with a patient for many years and may have interacted with the family over that period.
“When that patient passes away, they may feel, and the family may feel, that it would be comforting and appropriate for them to be at the funeral,” said Dr. Appelbaum.
However, he added,
“There are obviously a number of complexities involved. One is how the family feels about the relationship with the psychiatrist – whether they were accepting of the reality that the patient had a mental disorder and was in treatment,” he said.
There is also the question of confidentiality, said Dr. Appelbaum.
“If it’s a large funeral and the psychiatrist is just one face in the crowd, that’s not likely to be an issue. But if it’s a relatively small group of mourners, all of whom know each other, and an unknown figure pops up, that could raise questions and perhaps inadvertently reveal to family members or friends that the deceased had a psychiatric condition and was in treatment. That needs to be taken into account as well,” he added.
In cases in which the family invites the psychiatrist, confidentiality is not a concern, and attendance by the psychiatrist is something the patient would have wanted, said Dr. Appelbaum.
How the patient died may also be factor. When a patient dies by suicide, it’s an “emotionally charged situation for both sides,” said Dr. Appelbaum.
In the case of a suicide, he noted, the deceased was often an active patient, and both the psychiatrist and the family are dealing with strong emotions – the psychiatrist with regret over loss of the patient and perhaps with questions as to what could have been done differently, and the family with sorrow but “also sometimes with suspicion or anger in that the psychiatrist somehow failed to keep the patient alive,” Dr. Appelbaum noted.
“In this situation, it’s even more crucial for the psychiatrist or other mental health professionals to take the lead from the family – perhaps to initiate contact to express condolences and inquire delicately about the funeral arrangements and whether their presence would be welcomed,” he said.
The research had no specific funding. Dr. Pettaway and Dr. Appelbaum have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA clears cap device for ‘smart’ insulin pens for diabetes
The U.S. Food and Drug Administration has cleared the Bigfoot Unity Diabetes Management System, a cap device that connects to insulin pens and translates continuous glucose data into dosing recommendations, for use in individuals aged 12 and older.
The Bigfoot Unity System has three primary components – proprietary smart pen caps for both rapid- and long-acting insulin, a mobile app, and an integrated FreeStyle Libre 2 continuous glucose monitor (iCGM) sensor, which was FDA-cleared in June 2020 – that fit into the person’s dose-decision process when they need it throughout the day.
It allows the user to scan the FreeStyle Libre 2 sensor, displaying the user’s current glucose value, trend arrow, and recommended correction dose. The smart pen cap also directly displays the health care provider’s suggested meal insulin doses with the correction dose. In just a few steps the system gives the person with diabetes support to make real-time treatment decisions.
It also includes hypoglycemia alerts and is compatible with all major U.S. brands of rapid- and long-acting disposable insulin pens.
Health care providers can monitor the patient’s data through a secure web portal called the Bigfoot Clinic Hub.
JDRF said in a statement it “applauds the U.S. FDA on its decision to provide clearance for the Bigfoot Unity Diabetes Management by Bigfoot Biomedical.”
The new system “fills a critical gap and brings benefits of automation and device interconnectedness to people with diabetes who rely on multiple daily injections to manage their blood sugar levels.” It is a “win for both the type 1 and type 2 diabetes communities as it broadens the options of treatment to alleviate daily burdens.”
Growing market for smart insulin pens
The device is the latest advance in the “smart pen” field of semiautomated insulin delivery in which pen and compatible devices, software, and platforms are teamed up in various combinations to provide easier insulin dosing for patients with diabetes who require multiple daily injections but don’t wear insulin pumps.
On May 6, 2021, Eli Lilly announced it had signed “strategic international agreements” with Dexcom, Glooko, MyDiabby Healthcare, and Roche to provide platforms or devices compatible with Lilly’s prefilled Tempo Pen, which is already available in several global markets, and the Tempo Smart Button, currently in late-stage development and pending CE mark.
And in November 2020, Medtronic launched a new version of its smart insulin pen with integrated CGM called the InPen. The reusable insulin injector pen uses a smartphone app to calculate dosing of short-acting insulin based on CGM readings and allows users to view glucose readings and insulin dose information. It was originally launched in 2017 by Companion Medical, and the company was acquired by Medtronic in September 2020.
Novo Nordisk and Sanofi are also developing products in the smart pen space.
More information about the Bigfoot Unity Program is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has cleared the Bigfoot Unity Diabetes Management System, a cap device that connects to insulin pens and translates continuous glucose data into dosing recommendations, for use in individuals aged 12 and older.
The Bigfoot Unity System has three primary components – proprietary smart pen caps for both rapid- and long-acting insulin, a mobile app, and an integrated FreeStyle Libre 2 continuous glucose monitor (iCGM) sensor, which was FDA-cleared in June 2020 – that fit into the person’s dose-decision process when they need it throughout the day.
It allows the user to scan the FreeStyle Libre 2 sensor, displaying the user’s current glucose value, trend arrow, and recommended correction dose. The smart pen cap also directly displays the health care provider’s suggested meal insulin doses with the correction dose. In just a few steps the system gives the person with diabetes support to make real-time treatment decisions.
It also includes hypoglycemia alerts and is compatible with all major U.S. brands of rapid- and long-acting disposable insulin pens.
Health care providers can monitor the patient’s data through a secure web portal called the Bigfoot Clinic Hub.
JDRF said in a statement it “applauds the U.S. FDA on its decision to provide clearance for the Bigfoot Unity Diabetes Management by Bigfoot Biomedical.”
The new system “fills a critical gap and brings benefits of automation and device interconnectedness to people with diabetes who rely on multiple daily injections to manage their blood sugar levels.” It is a “win for both the type 1 and type 2 diabetes communities as it broadens the options of treatment to alleviate daily burdens.”
Growing market for smart insulin pens
The device is the latest advance in the “smart pen” field of semiautomated insulin delivery in which pen and compatible devices, software, and platforms are teamed up in various combinations to provide easier insulin dosing for patients with diabetes who require multiple daily injections but don’t wear insulin pumps.
On May 6, 2021, Eli Lilly announced it had signed “strategic international agreements” with Dexcom, Glooko, MyDiabby Healthcare, and Roche to provide platforms or devices compatible with Lilly’s prefilled Tempo Pen, which is already available in several global markets, and the Tempo Smart Button, currently in late-stage development and pending CE mark.
And in November 2020, Medtronic launched a new version of its smart insulin pen with integrated CGM called the InPen. The reusable insulin injector pen uses a smartphone app to calculate dosing of short-acting insulin based on CGM readings and allows users to view glucose readings and insulin dose information. It was originally launched in 2017 by Companion Medical, and the company was acquired by Medtronic in September 2020.
Novo Nordisk and Sanofi are also developing products in the smart pen space.
More information about the Bigfoot Unity Program is available here.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has cleared the Bigfoot Unity Diabetes Management System, a cap device that connects to insulin pens and translates continuous glucose data into dosing recommendations, for use in individuals aged 12 and older.
The Bigfoot Unity System has three primary components – proprietary smart pen caps for both rapid- and long-acting insulin, a mobile app, and an integrated FreeStyle Libre 2 continuous glucose monitor (iCGM) sensor, which was FDA-cleared in June 2020 – that fit into the person’s dose-decision process when they need it throughout the day.
It allows the user to scan the FreeStyle Libre 2 sensor, displaying the user’s current glucose value, trend arrow, and recommended correction dose. The smart pen cap also directly displays the health care provider’s suggested meal insulin doses with the correction dose. In just a few steps the system gives the person with diabetes support to make real-time treatment decisions.
It also includes hypoglycemia alerts and is compatible with all major U.S. brands of rapid- and long-acting disposable insulin pens.
Health care providers can monitor the patient’s data through a secure web portal called the Bigfoot Clinic Hub.
JDRF said in a statement it “applauds the U.S. FDA on its decision to provide clearance for the Bigfoot Unity Diabetes Management by Bigfoot Biomedical.”
The new system “fills a critical gap and brings benefits of automation and device interconnectedness to people with diabetes who rely on multiple daily injections to manage their blood sugar levels.” It is a “win for both the type 1 and type 2 diabetes communities as it broadens the options of treatment to alleviate daily burdens.”
Growing market for smart insulin pens
The device is the latest advance in the “smart pen” field of semiautomated insulin delivery in which pen and compatible devices, software, and platforms are teamed up in various combinations to provide easier insulin dosing for patients with diabetes who require multiple daily injections but don’t wear insulin pumps.
On May 6, 2021, Eli Lilly announced it had signed “strategic international agreements” with Dexcom, Glooko, MyDiabby Healthcare, and Roche to provide platforms or devices compatible with Lilly’s prefilled Tempo Pen, which is already available in several global markets, and the Tempo Smart Button, currently in late-stage development and pending CE mark.
And in November 2020, Medtronic launched a new version of its smart insulin pen with integrated CGM called the InPen. The reusable insulin injector pen uses a smartphone app to calculate dosing of short-acting insulin based on CGM readings and allows users to view glucose readings and insulin dose information. It was originally launched in 2017 by Companion Medical, and the company was acquired by Medtronic in September 2020.
Novo Nordisk and Sanofi are also developing products in the smart pen space.
More information about the Bigfoot Unity Program is available here.
A version of this article first appeared on Medscape.com.
Support group for Asian Americans uses theater to cope with COVID
An online, culturally based peer support group that uses theater and other creative outlets is helping Asian Americans cope with the COVID-19 pandemic, new research shows.
The findings of the qualitative study suggest that the program could be a model to support the mental health of other minority community groups during the COVID pandemic and beyond, say investigators from the Yale University Child Study Center, New Haven, Conn.
The Yale Compassionate Home, Action Together (CHATogether) group was created to promote emotional wellness among Asian American youth, young adults, and their families.
Early in the pandemic, it expanded its purpose to serve as a COVID-19 support group. Through social media outreach, CHATogether encourages members to cope with COVID-19 by using productive and creative outlets.
“We are a community education program serving Asian American families,” said Eunice Yuen, MD, PhD, the program’s founder and director, who is with the Yale University Child Study Center.
“ such as family conflict and xenophobic attacks,” said Dr. Yuen.
She discussed the program at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Skits, role playing
CHATogether groups consist of people with similar experiences and challenges who support each other through weekly online group meetings, she explained.
Group members work together to create family conflict scenarios and role-play dialogues on topics amplified during the COVID-19 pandemic, such as cross-cultural challenges among Asian Americans, academic expectations in home schooling, and Black Lives Matter and LGBTQ conflicts within Asian families.
Group members create skits that are based on their personal experiences and that allow them to work through their own internal conflicts and gain a sense of agency, said Dr. Yuen.
“CHATogether is really the interface of mental health, art, and theater, and we’re trying to create a vehicle that can be a lighthearted way for people to talk about mental health, especially for Asian American families,” said Dr. Yuen.
Preliminary results from a focus group with 10 CHATogether members who joined the program since the pandemic started identified four major ways in which the program has had a positive impact on the mental health and well-being of participants:
- It provides a safe and supportive environment, strengthens bonds between members, and increases the sense of belonging, thus encouraging engagement.
- It provides structural consistency/stability through regular meetings and consistent group functions. Weekly meetings provide a sense of control and hope in the midst of uncertainty during periods of sheltering in place.
- Through adapting the group to virtual platforms, group members experience the inherent strengths of a growth mindset and cognitive flexibility when facing challenges.
- It supports healthy coping skills through sublimation and altruism.
Looking ahead, Dr. Yuen said, the team plans to investigate the validity and effectiveness of this model and to expand the group to include other minorities, school educators, and medical education for trainees and medical students.
Commenting on the program, briefing moderator Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, described the initiative as a “great project that serves as a model that can be used not only for Asian Americans but for other groups.
“I think the key to it is that cultural sensitivity that we need to really take into account and cultural differences among people in order to best engage them and help support them. I think this program does that beautifully,” said Dr. Borenstein.
The work was supported by the APA’s Substance Abuse and Mental Health Services Administration Minority Fellowship, which provides a 1-year fellowship to psychiatry residents committed to addressing minority psychiatric mental health issues. Dr. Yuen and Dr. Borenstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online, culturally based peer support group that uses theater and other creative outlets is helping Asian Americans cope with the COVID-19 pandemic, new research shows.
The findings of the qualitative study suggest that the program could be a model to support the mental health of other minority community groups during the COVID pandemic and beyond, say investigators from the Yale University Child Study Center, New Haven, Conn.
The Yale Compassionate Home, Action Together (CHATogether) group was created to promote emotional wellness among Asian American youth, young adults, and their families.
Early in the pandemic, it expanded its purpose to serve as a COVID-19 support group. Through social media outreach, CHATogether encourages members to cope with COVID-19 by using productive and creative outlets.
“We are a community education program serving Asian American families,” said Eunice Yuen, MD, PhD, the program’s founder and director, who is with the Yale University Child Study Center.
“ such as family conflict and xenophobic attacks,” said Dr. Yuen.
She discussed the program at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Skits, role playing
CHATogether groups consist of people with similar experiences and challenges who support each other through weekly online group meetings, she explained.
Group members work together to create family conflict scenarios and role-play dialogues on topics amplified during the COVID-19 pandemic, such as cross-cultural challenges among Asian Americans, academic expectations in home schooling, and Black Lives Matter and LGBTQ conflicts within Asian families.
Group members create skits that are based on their personal experiences and that allow them to work through their own internal conflicts and gain a sense of agency, said Dr. Yuen.
“CHATogether is really the interface of mental health, art, and theater, and we’re trying to create a vehicle that can be a lighthearted way for people to talk about mental health, especially for Asian American families,” said Dr. Yuen.
Preliminary results from a focus group with 10 CHATogether members who joined the program since the pandemic started identified four major ways in which the program has had a positive impact on the mental health and well-being of participants:
- It provides a safe and supportive environment, strengthens bonds between members, and increases the sense of belonging, thus encouraging engagement.
- It provides structural consistency/stability through regular meetings and consistent group functions. Weekly meetings provide a sense of control and hope in the midst of uncertainty during periods of sheltering in place.
- Through adapting the group to virtual platforms, group members experience the inherent strengths of a growth mindset and cognitive flexibility when facing challenges.
- It supports healthy coping skills through sublimation and altruism.
Looking ahead, Dr. Yuen said, the team plans to investigate the validity and effectiveness of this model and to expand the group to include other minorities, school educators, and medical education for trainees and medical students.
Commenting on the program, briefing moderator Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, described the initiative as a “great project that serves as a model that can be used not only for Asian Americans but for other groups.
“I think the key to it is that cultural sensitivity that we need to really take into account and cultural differences among people in order to best engage them and help support them. I think this program does that beautifully,” said Dr. Borenstein.
The work was supported by the APA’s Substance Abuse and Mental Health Services Administration Minority Fellowship, which provides a 1-year fellowship to psychiatry residents committed to addressing minority psychiatric mental health issues. Dr. Yuen and Dr. Borenstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An online, culturally based peer support group that uses theater and other creative outlets is helping Asian Americans cope with the COVID-19 pandemic, new research shows.
The findings of the qualitative study suggest that the program could be a model to support the mental health of other minority community groups during the COVID pandemic and beyond, say investigators from the Yale University Child Study Center, New Haven, Conn.
The Yale Compassionate Home, Action Together (CHATogether) group was created to promote emotional wellness among Asian American youth, young adults, and their families.
Early in the pandemic, it expanded its purpose to serve as a COVID-19 support group. Through social media outreach, CHATogether encourages members to cope with COVID-19 by using productive and creative outlets.
“We are a community education program serving Asian American families,” said Eunice Yuen, MD, PhD, the program’s founder and director, who is with the Yale University Child Study Center.
“ such as family conflict and xenophobic attacks,” said Dr. Yuen.
She discussed the program at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Skits, role playing
CHATogether groups consist of people with similar experiences and challenges who support each other through weekly online group meetings, she explained.
Group members work together to create family conflict scenarios and role-play dialogues on topics amplified during the COVID-19 pandemic, such as cross-cultural challenges among Asian Americans, academic expectations in home schooling, and Black Lives Matter and LGBTQ conflicts within Asian families.
Group members create skits that are based on their personal experiences and that allow them to work through their own internal conflicts and gain a sense of agency, said Dr. Yuen.
“CHATogether is really the interface of mental health, art, and theater, and we’re trying to create a vehicle that can be a lighthearted way for people to talk about mental health, especially for Asian American families,” said Dr. Yuen.
Preliminary results from a focus group with 10 CHATogether members who joined the program since the pandemic started identified four major ways in which the program has had a positive impact on the mental health and well-being of participants:
- It provides a safe and supportive environment, strengthens bonds between members, and increases the sense of belonging, thus encouraging engagement.
- It provides structural consistency/stability through regular meetings and consistent group functions. Weekly meetings provide a sense of control and hope in the midst of uncertainty during periods of sheltering in place.
- Through adapting the group to virtual platforms, group members experience the inherent strengths of a growth mindset and cognitive flexibility when facing challenges.
- It supports healthy coping skills through sublimation and altruism.
Looking ahead, Dr. Yuen said, the team plans to investigate the validity and effectiveness of this model and to expand the group to include other minorities, school educators, and medical education for trainees and medical students.
Commenting on the program, briefing moderator Jeffrey Borenstein, MD, president and CEO of the Brain and Behavior Research Foundation and editor-in-chief of Psychiatric News, described the initiative as a “great project that serves as a model that can be used not only for Asian Americans but for other groups.
“I think the key to it is that cultural sensitivity that we need to really take into account and cultural differences among people in order to best engage them and help support them. I think this program does that beautifully,” said Dr. Borenstein.
The work was supported by the APA’s Substance Abuse and Mental Health Services Administration Minority Fellowship, which provides a 1-year fellowship to psychiatry residents committed to addressing minority psychiatric mental health issues. Dr. Yuen and Dr. Borenstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.