User login
Nodule on the Neck
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
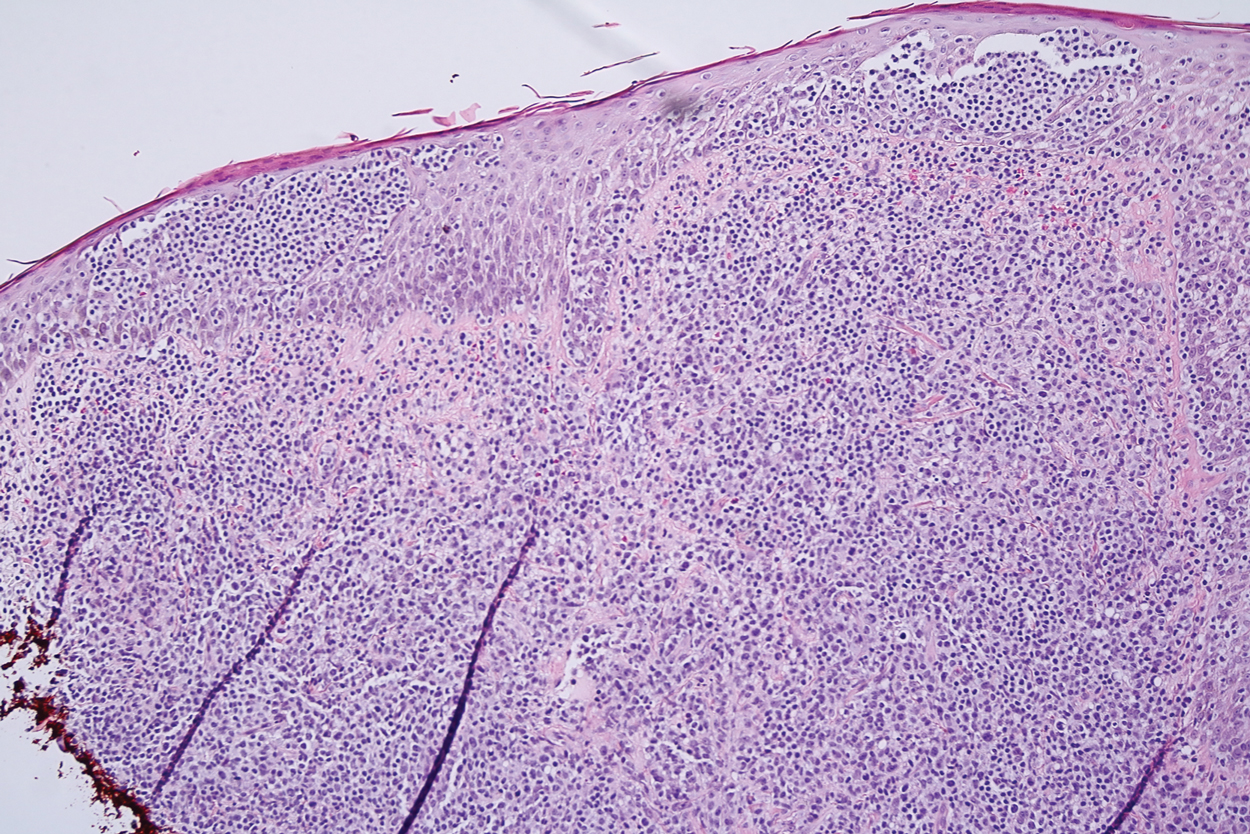
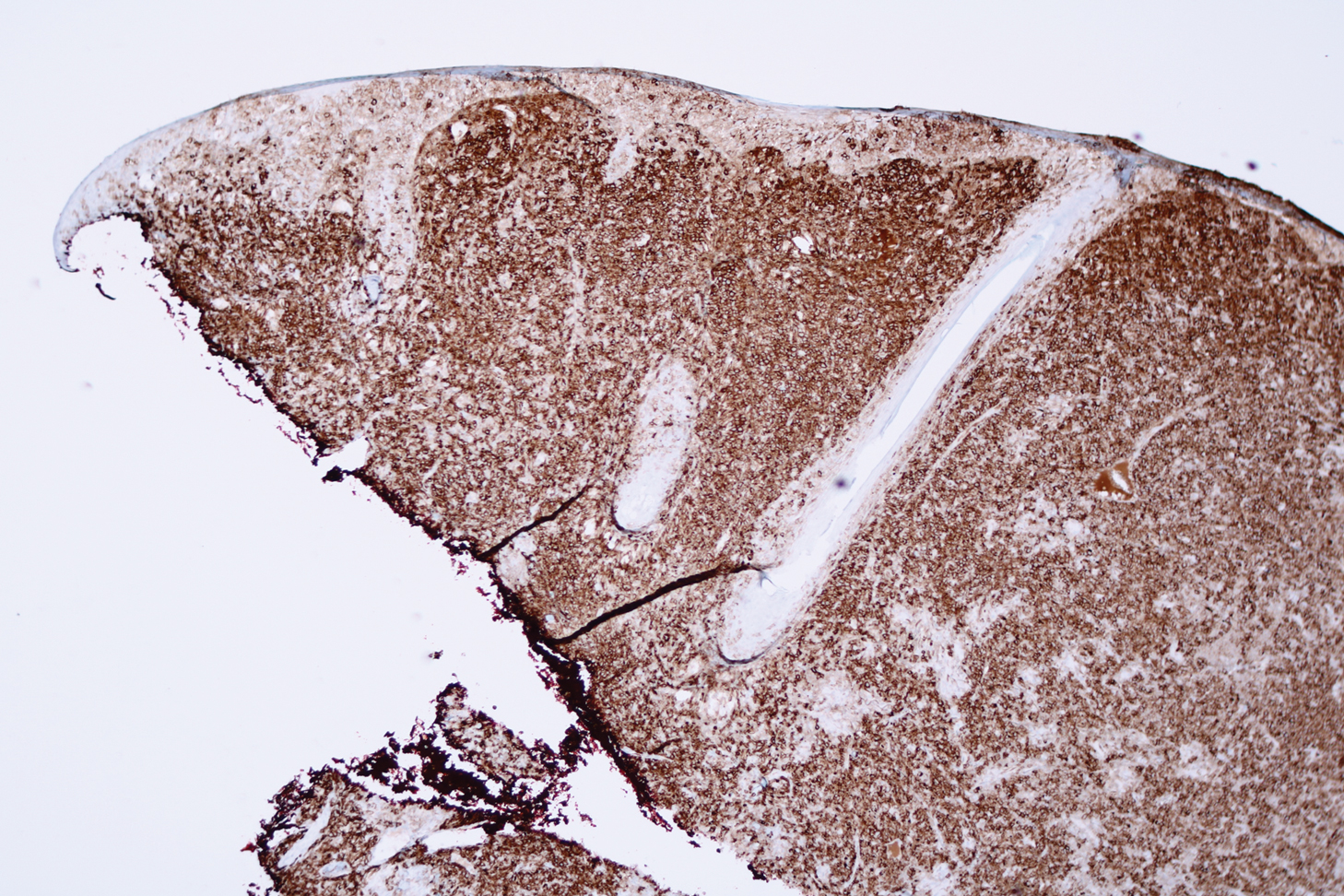
Microscopic analysis showed a dense proliferation of mononuclear cells filling and expanding the dermis with focal epidermotropism (Figure 1). Immunohistochemistry demonstrated strong and diffuse staining for CD3, CD4, and CD30 (Figure 2) and lack of staining for anaplastic lymphoma kinase (ALK). Workup to exclude systemic disease was initiated and included unremarkable computed tomography (CT) of the neck, chest, abdomen, and pelvis along with no abnormal cells on bone marrow biopsy. Complete blood cell count, basic metabolic panel, and lactate dehydrogenase were within reference range. Given the lack of evidence for systemic involvement, a diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) was made. The treatment plan for our patient with a solitary lesion was localized radiation therapy.
Primary cutaneous CD30+ lymphoproliferative disorders encompass a spectrum of conditions, with premalignant lymphomatoid papulosis (LyP) at one extreme and the malignant PC-ALCL on the other.1 The diagnosis of PC-ALCL is made by clinicopathologic correlation, and lesions typically present abruptly as solitary or grouped nodules with a tendency to ulcerate over time. Spontaneous regression has been reported, but relapse in the skin is frequent.2
A representative, typically excisional, biopsy should be performed if the clinician suspects PC-ALCL. Histologic criteria include a dense dermal infiltrate of large pleomorphic cells and the expression of CD30 in at least 75% of tumor cells.3 Primary cutaneous anaplastic large cell lymphoma typically lacks the ALK gene translocation with the nucleophosmin gene, NPM, that is common in systemic disease; however, a small subset of PC-ALCL may be ALK positive and indicate a higher chance of transformation into systemic disease.2
The extent of the lymphoma should be staged to exclude the possibility of systemic disease. This assessment includes a complete physical examination; laboratory investigation, including complete blood cell count with differential and blood chemistries; and radiography. A positron emission tomography-CT scan of the neck, chest, abdomen, and pelvis, or a whole-body integrated positron emission tomography-CT are sufficient for the radiographic examination.3
The initial choice of treatment for solitary or localized PC-ALCL is localized radiation therapy or low-dose methotrexate. Targeted therapy such as brentuximab has been shown to be effective for those with multifocal systemic involvement or refractory disease.2 Cure rates from radiation therapy alone approach 95%.3 It is important to highlight radiation therapy as the initial management plan to increase awareness and to avoid inappropriate treatment of PC-ALCL with traditional chemotherapy.
Large lesions of LyP may appear similar to PC-ALCL on histopathology, making the two entities difficult to distinguish. However, in contrast to PC-ALCL, LyP classically has a different clinical course characterized by waxing and waning crops of lesions that typically are smaller (<1 cm) than those of PC-ALCL.2 Large cell transformation of mycosis fungoides is another entity to consider, but these patients usually have a known history of mycosis fungoides.4
Keratoacanthomas, considered to be a variant of a well-differentiated squamous cell carcinoma, present as rapidly enlarging crateriform nodules with a keratotic core. They usually are found on the head and neck or sun-exposed areas of the extremities and may regress spontaneously.5 Histology will show atypical, highly differentiated squamous epithelia. Merkel cell carcinoma also has a predilection for the head and neck in older patients and may present as a rapidly growing nodule. However, histology will show an aggressive tumor with small round blue cells, and immunohistochemistry will show the characteristic paranuclear dot staining for CK20 along with staining for various neuroendocrine markers. Similarly, atypical fibroxanthoma is a low-grade sarcoma that also presents on the head and neck of elderly sun-damaged patients.5 Histology will show dermal proliferation of spindle cells that often extend up against the epidermis along with pleomorphism and atypical mitoses. Basal cell carcinoma is a common tumor that can present on the head and neck in sun-damaged patients. Nodular basal cell carcinomas can enlarge and ulcerate, but growth is seen over years rather than weeks.5 Histology characteristically will show tumor islands composed of basaloid cells with peripheral palisading and clefting between the tumor islands and the stroma.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44:570-577.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Saunders Elsevier; 2015:475-489.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Microscopic analysis showed a dense proliferation of mononuclear cells filling and expanding the dermis with focal epidermotropism (Figure 1). Immunohistochemistry demonstrated strong and diffuse staining for CD3, CD4, and CD30 (Figure 2) and lack of staining for anaplastic lymphoma kinase (ALK). Workup to exclude systemic disease was initiated and included unremarkable computed tomography (CT) of the neck, chest, abdomen, and pelvis along with no abnormal cells on bone marrow biopsy. Complete blood cell count, basic metabolic panel, and lactate dehydrogenase were within reference range. Given the lack of evidence for systemic involvement, a diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) was made. The treatment plan for our patient with a solitary lesion was localized radiation therapy.
Primary cutaneous CD30+ lymphoproliferative disorders encompass a spectrum of conditions, with premalignant lymphomatoid papulosis (LyP) at one extreme and the malignant PC-ALCL on the other.1 The diagnosis of PC-ALCL is made by clinicopathologic correlation, and lesions typically present abruptly as solitary or grouped nodules with a tendency to ulcerate over time. Spontaneous regression has been reported, but relapse in the skin is frequent.2
A representative, typically excisional, biopsy should be performed if the clinician suspects PC-ALCL. Histologic criteria include a dense dermal infiltrate of large pleomorphic cells and the expression of CD30 in at least 75% of tumor cells.3 Primary cutaneous anaplastic large cell lymphoma typically lacks the ALK gene translocation with the nucleophosmin gene, NPM, that is common in systemic disease; however, a small subset of PC-ALCL may be ALK positive and indicate a higher chance of transformation into systemic disease.2
The extent of the lymphoma should be staged to exclude the possibility of systemic disease. This assessment includes a complete physical examination; laboratory investigation, including complete blood cell count with differential and blood chemistries; and radiography. A positron emission tomography-CT scan of the neck, chest, abdomen, and pelvis, or a whole-body integrated positron emission tomography-CT are sufficient for the radiographic examination.3
The initial choice of treatment for solitary or localized PC-ALCL is localized radiation therapy or low-dose methotrexate. Targeted therapy such as brentuximab has been shown to be effective for those with multifocal systemic involvement or refractory disease.2 Cure rates from radiation therapy alone approach 95%.3 It is important to highlight radiation therapy as the initial management plan to increase awareness and to avoid inappropriate treatment of PC-ALCL with traditional chemotherapy.
Large lesions of LyP may appear similar to PC-ALCL on histopathology, making the two entities difficult to distinguish. However, in contrast to PC-ALCL, LyP classically has a different clinical course characterized by waxing and waning crops of lesions that typically are smaller (<1 cm) than those of PC-ALCL.2 Large cell transformation of mycosis fungoides is another entity to consider, but these patients usually have a known history of mycosis fungoides.4
Keratoacanthomas, considered to be a variant of a well-differentiated squamous cell carcinoma, present as rapidly enlarging crateriform nodules with a keratotic core. They usually are found on the head and neck or sun-exposed areas of the extremities and may regress spontaneously.5 Histology will show atypical, highly differentiated squamous epithelia. Merkel cell carcinoma also has a predilection for the head and neck in older patients and may present as a rapidly growing nodule. However, histology will show an aggressive tumor with small round blue cells, and immunohistochemistry will show the characteristic paranuclear dot staining for CK20 along with staining for various neuroendocrine markers. Similarly, atypical fibroxanthoma is a low-grade sarcoma that also presents on the head and neck of elderly sun-damaged patients.5 Histology will show dermal proliferation of spindle cells that often extend up against the epidermis along with pleomorphism and atypical mitoses. Basal cell carcinoma is a common tumor that can present on the head and neck in sun-damaged patients. Nodular basal cell carcinomas can enlarge and ulcerate, but growth is seen over years rather than weeks.5 Histology characteristically will show tumor islands composed of basaloid cells with peripheral palisading and clefting between the tumor islands and the stroma.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Microscopic analysis showed a dense proliferation of mononuclear cells filling and expanding the dermis with focal epidermotropism (Figure 1). Immunohistochemistry demonstrated strong and diffuse staining for CD3, CD4, and CD30 (Figure 2) and lack of staining for anaplastic lymphoma kinase (ALK). Workup to exclude systemic disease was initiated and included unremarkable computed tomography (CT) of the neck, chest, abdomen, and pelvis along with no abnormal cells on bone marrow biopsy. Complete blood cell count, basic metabolic panel, and lactate dehydrogenase were within reference range. Given the lack of evidence for systemic involvement, a diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) was made. The treatment plan for our patient with a solitary lesion was localized radiation therapy.
Primary cutaneous CD30+ lymphoproliferative disorders encompass a spectrum of conditions, with premalignant lymphomatoid papulosis (LyP) at one extreme and the malignant PC-ALCL on the other.1 The diagnosis of PC-ALCL is made by clinicopathologic correlation, and lesions typically present abruptly as solitary or grouped nodules with a tendency to ulcerate over time. Spontaneous regression has been reported, but relapse in the skin is frequent.2
A representative, typically excisional, biopsy should be performed if the clinician suspects PC-ALCL. Histologic criteria include a dense dermal infiltrate of large pleomorphic cells and the expression of CD30 in at least 75% of tumor cells.3 Primary cutaneous anaplastic large cell lymphoma typically lacks the ALK gene translocation with the nucleophosmin gene, NPM, that is common in systemic disease; however, a small subset of PC-ALCL may be ALK positive and indicate a higher chance of transformation into systemic disease.2
The extent of the lymphoma should be staged to exclude the possibility of systemic disease. This assessment includes a complete physical examination; laboratory investigation, including complete blood cell count with differential and blood chemistries; and radiography. A positron emission tomography-CT scan of the neck, chest, abdomen, and pelvis, or a whole-body integrated positron emission tomography-CT are sufficient for the radiographic examination.3
The initial choice of treatment for solitary or localized PC-ALCL is localized radiation therapy or low-dose methotrexate. Targeted therapy such as brentuximab has been shown to be effective for those with multifocal systemic involvement or refractory disease.2 Cure rates from radiation therapy alone approach 95%.3 It is important to highlight radiation therapy as the initial management plan to increase awareness and to avoid inappropriate treatment of PC-ALCL with traditional chemotherapy.
Large lesions of LyP may appear similar to PC-ALCL on histopathology, making the two entities difficult to distinguish. However, in contrast to PC-ALCL, LyP classically has a different clinical course characterized by waxing and waning crops of lesions that typically are smaller (<1 cm) than those of PC-ALCL.2 Large cell transformation of mycosis fungoides is another entity to consider, but these patients usually have a known history of mycosis fungoides.4
Keratoacanthomas, considered to be a variant of a well-differentiated squamous cell carcinoma, present as rapidly enlarging crateriform nodules with a keratotic core. They usually are found on the head and neck or sun-exposed areas of the extremities and may regress spontaneously.5 Histology will show atypical, highly differentiated squamous epithelia. Merkel cell carcinoma also has a predilection for the head and neck in older patients and may present as a rapidly growing nodule. However, histology will show an aggressive tumor with small round blue cells, and immunohistochemistry will show the characteristic paranuclear dot staining for CK20 along with staining for various neuroendocrine markers. Similarly, atypical fibroxanthoma is a low-grade sarcoma that also presents on the head and neck of elderly sun-damaged patients.5 Histology will show dermal proliferation of spindle cells that often extend up against the epidermis along with pleomorphism and atypical mitoses. Basal cell carcinoma is a common tumor that can present on the head and neck in sun-damaged patients. Nodular basal cell carcinomas can enlarge and ulcerate, but growth is seen over years rather than weeks.5 Histology characteristically will show tumor islands composed of basaloid cells with peripheral palisading and clefting between the tumor islands and the stroma.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44:570-577.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Saunders Elsevier; 2015:475-489.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44:570-577.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Saunders Elsevier; 2015:475-489.
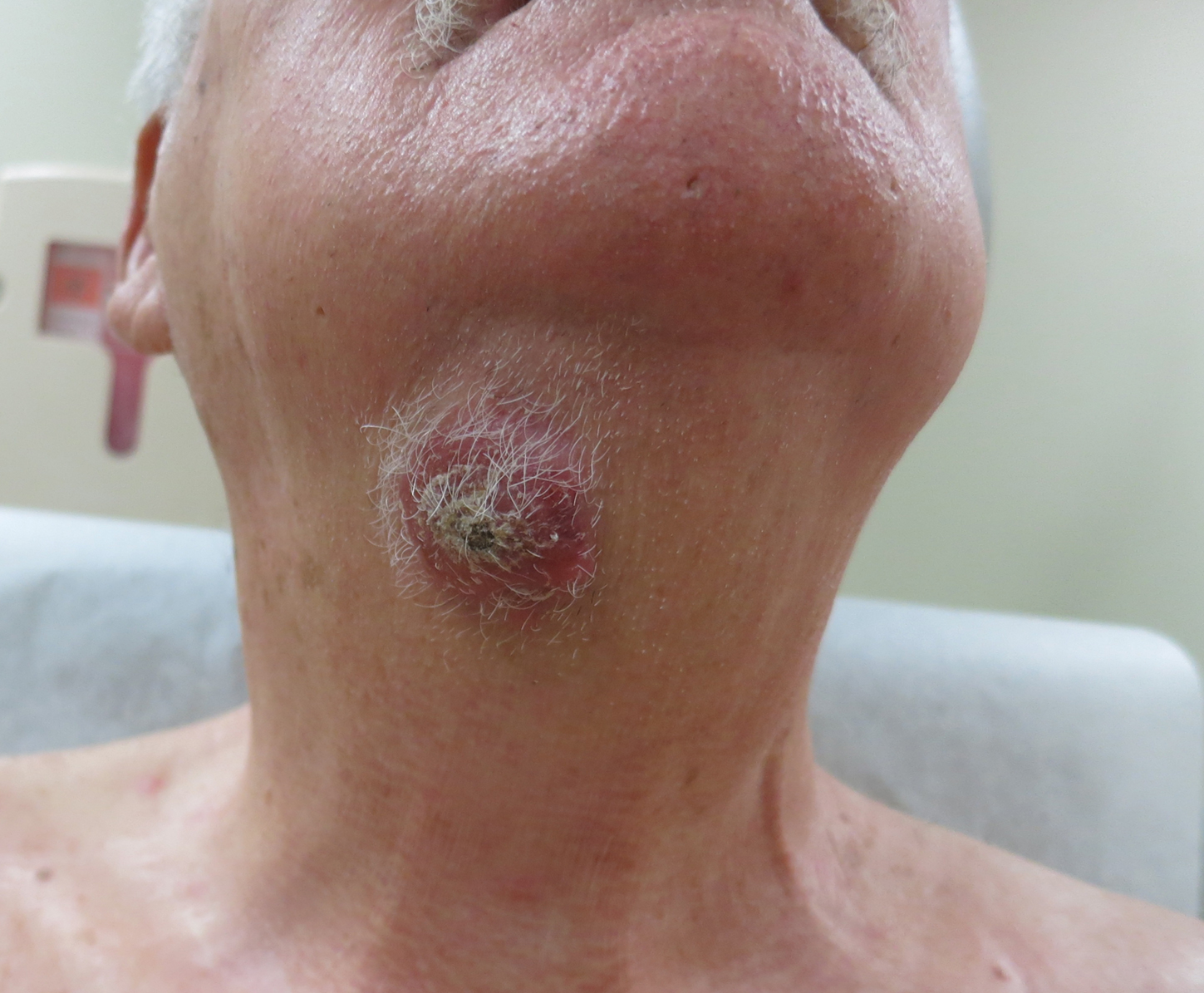
An 80-year-old man presented to our clinic with a large lesion on the right upper neck of approximately 4 weeks’ duration. He reported that it was rapidly increasing in size and had bled on several occasions. No treatments were attempted prior to the initial visit. He denied any constitutional symptoms. The patient had a history of nonmelanoma skin cancers but no other chronic medical problems. Physical examination revealed a large, 35×40-mm, erythematous nodule with central ulceration and overlying hyperkeratosis on the right upper neck. No palpable cervical, supraclavicular, or axillary lymphadenopathy was observed. An excisional biopsy of the lesion was obtained.
A tumultuous and unforgettable year
SHM president bids farewell
As my SHM presidency wraps up, it is a good time to reflect on the past year in hospital medicine. Dominated by COVID-19 preparedness, mitigation, and (now) recovery efforts, the impacts of COVID-19 throughout the medical industry have been profound. For hospital medicine, although we have endured work and home stress unlike anything in recent memory, fortunately a few notably good changes have come about as a result of COVID-19.
Hospitalists have proven that we are extremely capable of adapting to rapidly changing evidence-based practice. The old adage of evidence taking 7 years to become mainstream clinical practice certainly has not been the paradigm during COVID-19. In many cases, clinical care pathways were changing by the week, or even by the day. Usage of SHM’s website, HMX, and educational platforms rose exponentially to keep pace with the changing landscape. Information exchange between and among hospital medicine groups was efficient and effective. This is exactly how it should be, with SHM serving as the catalyst for such information exchange.
Hospitalists were able to shift to telehealth care as the need arose. The use of telehealth is now becoming a core competency for hospitalists around the country, and we are leading the way for other specialists in adoption. COVID-19 enabled not only rapid transformation, but also better payer coverage for the use of all types of telehealth services. SHM will remain a source of training and education in telehealth best practices going forward.
Related, hospitalists also found their programs were being asked to become purveyors for remote monitoring and hospital-at-home programs. Because CMS has allowed some reimbursement for these programs, at least during the public health emergency, hospital medicine programs can more feasibly pursue building and sustaining such programs, and SHM can serve as the hub for best practice exchanges in the field.
The pandemic also created a sizable shift in the mindset of the need and enthusiasm for mainstream maintenance of certification. Although there were already questions about the value of high-stakes exams before the pandemic, both within and outside the medical industry, the pandemic created an immediate need to shift away from such exams. Now, the entire pipeline is questioning the value of these high-stakes exams, such as SATs and ACTs for college admissions, Step 1 exams for medical students, and certification exams for physicians. The pandemic has made us question these milestone exams with more scrutiny and has created a sense of urgency for a change to more adult-learner–focused alternatives. SHM will continue to be at the centerpiece of the discussion, as well as the leader in cultivating educational venues for continuous learning.
So where do we go from here?
I am confident that SHM will continue to pay deep attention to the activities that bring value to hospitalists and support changing practice patterns such as telehealth and hospital-at-home work. Not only will SHM serve as a center for best practices and a conduit for networking and information sharing at the national level – there will be significantly more focus on the support and growth of local chapters. SHM realizes that local chapters are a vital source of networking, education, and pipeline development and will continue to increase the resources to make the chapter programs dynamic and inviting for everyone interested in hospital medicine.
While this presidency year was far different than expected, I have continuously been amazed and delighted with the resiliency and endurance of our hospitalists around the country. We stood out at the front lines of the pandemic, with a mission toward service and a relentless commitment to our patients. Although we still have a long way to go before the pandemic is behind us, I firmly believe we are emerging from the haze stronger and more agile than ever. Thank you for allowing me to serve this incredible organization during such a tumultuous and unforgettable year.
Yours in service.
Dr. Scheurer is a hospitalist and chief quality officer, MUSC Health System, Medical University of South Carolina, Charleston. She is the outgoing president of SHM.
SHM president bids farewell
SHM president bids farewell
As my SHM presidency wraps up, it is a good time to reflect on the past year in hospital medicine. Dominated by COVID-19 preparedness, mitigation, and (now) recovery efforts, the impacts of COVID-19 throughout the medical industry have been profound. For hospital medicine, although we have endured work and home stress unlike anything in recent memory, fortunately a few notably good changes have come about as a result of COVID-19.
Hospitalists have proven that we are extremely capable of adapting to rapidly changing evidence-based practice. The old adage of evidence taking 7 years to become mainstream clinical practice certainly has not been the paradigm during COVID-19. In many cases, clinical care pathways were changing by the week, or even by the day. Usage of SHM’s website, HMX, and educational platforms rose exponentially to keep pace with the changing landscape. Information exchange between and among hospital medicine groups was efficient and effective. This is exactly how it should be, with SHM serving as the catalyst for such information exchange.
Hospitalists were able to shift to telehealth care as the need arose. The use of telehealth is now becoming a core competency for hospitalists around the country, and we are leading the way for other specialists in adoption. COVID-19 enabled not only rapid transformation, but also better payer coverage for the use of all types of telehealth services. SHM will remain a source of training and education in telehealth best practices going forward.
Related, hospitalists also found their programs were being asked to become purveyors for remote monitoring and hospital-at-home programs. Because CMS has allowed some reimbursement for these programs, at least during the public health emergency, hospital medicine programs can more feasibly pursue building and sustaining such programs, and SHM can serve as the hub for best practice exchanges in the field.
The pandemic also created a sizable shift in the mindset of the need and enthusiasm for mainstream maintenance of certification. Although there were already questions about the value of high-stakes exams before the pandemic, both within and outside the medical industry, the pandemic created an immediate need to shift away from such exams. Now, the entire pipeline is questioning the value of these high-stakes exams, such as SATs and ACTs for college admissions, Step 1 exams for medical students, and certification exams for physicians. The pandemic has made us question these milestone exams with more scrutiny and has created a sense of urgency for a change to more adult-learner–focused alternatives. SHM will continue to be at the centerpiece of the discussion, as well as the leader in cultivating educational venues for continuous learning.
So where do we go from here?
I am confident that SHM will continue to pay deep attention to the activities that bring value to hospitalists and support changing practice patterns such as telehealth and hospital-at-home work. Not only will SHM serve as a center for best practices and a conduit for networking and information sharing at the national level – there will be significantly more focus on the support and growth of local chapters. SHM realizes that local chapters are a vital source of networking, education, and pipeline development and will continue to increase the resources to make the chapter programs dynamic and inviting for everyone interested in hospital medicine.
While this presidency year was far different than expected, I have continuously been amazed and delighted with the resiliency and endurance of our hospitalists around the country. We stood out at the front lines of the pandemic, with a mission toward service and a relentless commitment to our patients. Although we still have a long way to go before the pandemic is behind us, I firmly believe we are emerging from the haze stronger and more agile than ever. Thank you for allowing me to serve this incredible organization during such a tumultuous and unforgettable year.
Yours in service.
Dr. Scheurer is a hospitalist and chief quality officer, MUSC Health System, Medical University of South Carolina, Charleston. She is the outgoing president of SHM.
As my SHM presidency wraps up, it is a good time to reflect on the past year in hospital medicine. Dominated by COVID-19 preparedness, mitigation, and (now) recovery efforts, the impacts of COVID-19 throughout the medical industry have been profound. For hospital medicine, although we have endured work and home stress unlike anything in recent memory, fortunately a few notably good changes have come about as a result of COVID-19.
Hospitalists have proven that we are extremely capable of adapting to rapidly changing evidence-based practice. The old adage of evidence taking 7 years to become mainstream clinical practice certainly has not been the paradigm during COVID-19. In many cases, clinical care pathways were changing by the week, or even by the day. Usage of SHM’s website, HMX, and educational platforms rose exponentially to keep pace with the changing landscape. Information exchange between and among hospital medicine groups was efficient and effective. This is exactly how it should be, with SHM serving as the catalyst for such information exchange.
Hospitalists were able to shift to telehealth care as the need arose. The use of telehealth is now becoming a core competency for hospitalists around the country, and we are leading the way for other specialists in adoption. COVID-19 enabled not only rapid transformation, but also better payer coverage for the use of all types of telehealth services. SHM will remain a source of training and education in telehealth best practices going forward.
Related, hospitalists also found their programs were being asked to become purveyors for remote monitoring and hospital-at-home programs. Because CMS has allowed some reimbursement for these programs, at least during the public health emergency, hospital medicine programs can more feasibly pursue building and sustaining such programs, and SHM can serve as the hub for best practice exchanges in the field.
The pandemic also created a sizable shift in the mindset of the need and enthusiasm for mainstream maintenance of certification. Although there were already questions about the value of high-stakes exams before the pandemic, both within and outside the medical industry, the pandemic created an immediate need to shift away from such exams. Now, the entire pipeline is questioning the value of these high-stakes exams, such as SATs and ACTs for college admissions, Step 1 exams for medical students, and certification exams for physicians. The pandemic has made us question these milestone exams with more scrutiny and has created a sense of urgency for a change to more adult-learner–focused alternatives. SHM will continue to be at the centerpiece of the discussion, as well as the leader in cultivating educational venues for continuous learning.
So where do we go from here?
I am confident that SHM will continue to pay deep attention to the activities that bring value to hospitalists and support changing practice patterns such as telehealth and hospital-at-home work. Not only will SHM serve as a center for best practices and a conduit for networking and information sharing at the national level – there will be significantly more focus on the support and growth of local chapters. SHM realizes that local chapters are a vital source of networking, education, and pipeline development and will continue to increase the resources to make the chapter programs dynamic and inviting for everyone interested in hospital medicine.
While this presidency year was far different than expected, I have continuously been amazed and delighted with the resiliency and endurance of our hospitalists around the country. We stood out at the front lines of the pandemic, with a mission toward service and a relentless commitment to our patients. Although we still have a long way to go before the pandemic is behind us, I firmly believe we are emerging from the haze stronger and more agile than ever. Thank you for allowing me to serve this incredible organization during such a tumultuous and unforgettable year.
Yours in service.
Dr. Scheurer is a hospitalist and chief quality officer, MUSC Health System, Medical University of South Carolina, Charleston. She is the outgoing president of SHM.
A ‘mess’ of a diagnosis: Is it type 2 MI or a nonischemic imposter?
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Survival gains in the management of acute myocardial infarction in recent decades don’t apply to one increasingly common category of MI.
Type 2 MI, triggered by a surge in myocardial oxygen demand or a drop in its supply, is on the rise and might be more prognostically serious than the “classic” atherothrombotic type 1 form, for which there have been such impressive strides in therapy.
Strategies for assessing and treating type 2 MI and another condition it can resemble clinically – nonischemic myocardial injury – have been less rigorously explored and are far less settled.
That could be partly because recent iterations of the consensus-based universal definition of MI define type 1 MI primarily by the atherothrombotic process, whereas “demand” type 2 MI is characterized as secondary to other disorders. The list of potential primary conditions, cardiac and noncardiac, is long.
As a result, patients with type 1 MI are clinically well defined, but those with type 2 MI have so far defied efforts to be clinically characterized in a consistent way. However, recent efforts might change that, given growing appreciation that all-cause and cardiovascular (CV) mortality outcomes are actually worse for patients with type 2 MI.
“That’s because we have lots of treatments for type 1 MI. Type 2 and myocardial injury? We don’t know how to treat them,” David E. Newby, MD, PhD, University of Edinburgh, said in an interview.
Dr. Newby pointed to a widely cited 2018 publication, of which he is a coauthor, documenting 5-year outcomes of 2,122 patients with type 1 MI, type 2 MI, or nonischemic myocardial injury per the newly minted fourth universal definition.
Risk-factor profiles for patients with the latter two conditions contrasted with those of patients with type 1 MI, he observed. They were “a lot older,” were less likely to be smokers, had more hypertension and previous stroke, and a less prominent CV family history.
“So they’re a different beast,” Dr. Newby said. And their prognosis tended to be worse: all-cause mortality was about 62% for patients with type 2 MI and 72% with nonischemic myocardial injury, but only 37% for patients with type 1 MI. The difference between the two types of infarction was driven by an excess of noncardiovascular death after type 2 MI.
Mortality in patients with type 2 MI is “quite high, but it may well be a marker of the fact that you’ve got other serious diseases on board that are associated with poorer outcome,” he said.
Risk varies
The degree of risk in type 2 MI seems to vary with the underlying condition, a recent cohort study suggests. In about 3,800 patients with cardiac troponin (cTn) elevations qualifying as MI – a younger group; most were in their 30s and 40s – mortality at 10 years was 12% for those with type 1 MI, but 34% for those with type 2 MI and 46% for the remainder with nonischemic myocardial injury.
Underlying precipitating conditions varied widely among the patients with type 2 MI or nonischemic myocardial injury, and there was broad variation in mortality by etiology among those with type 2 MI. Sepsis and anemia entailed some of the highest risk, and hypertension and arrhythmias some of the lowest.
A prospective, community-based study of 5,460 patients with type 1 MI or type 2 MI reached a similar conclusion, but with a twist. Five-year all-cause mortality contrasted significantly between types of MI at 31% and 52%, respectively, but CV mortality rates were similar in this study.
Mortality in type 2 MI again varied by the precipitating etiology, suggesting that patients can be risk stratified according to pathophysiological mechanism behind their demand infarction, the authors concluded, “underscoring that type 2 MI is not a single entity, rather a group of phenotypic clusters.”
The usually high comorbidity burden and CV risk in patients with type 2 MI, one of those authors said in an interview, suggest there are “opportunities to see whether we can reduce that risk.”
Formal recommendations consistently say that, in patients with type 2 MI, “your first and foremost target should be to treat the underlying trigger and cause,” said Yader Sandoval, MD, Mayo Clinic, Rochester, Minn. That means such opportunities for further CV risk reduction tend to be “underappreciated.”
“In principle, treating the inciting cause of type 2 MI or the injury is important,” said James L. Januzzi, MD, Massachusetts General Hospital, Boston, in an interview, “but I feel quite strongly that there must be more that we can do for these folks.”
Dr. Januzzi is senior author on a recent analysis based on more than 200,000 admissions across the United States that saw a 43% lower risk for in-hospital death and 54% lower risk for 30-day MI readmission for patients with type 2 MI than those with type 1, adjusted for risk factors and comorbidities.
But, “it is important to emphasize that type 2 MI patients had a substantial risk for adverse outcome, nonetheless, and lack a clear management approach,” Dr. Januzzi and colleagues stated in their publication, as reported by this news organization.
“Due to the high rates of long-term cardiovascular events experienced by the frequently encountered type 2 MI patients,” they wrote, “identifying evidence-based therapies represents a major unmet need.”
That such patients tend to be sick with multiple comorbidities and have not yet been clinically well characterized, Dr. Januzzi said, “has stymied our ability to develop a treatment strategy.”
Role of the universal definitions
That challenge might in some ways be complicated by the universal definition, especially version 4, in which the definitions for type 1 MI, type 2 MI, and nonischemic myocardial injury are unified biochemically.
This version, published in 2018 in the European Heart Journal and Circulation, introduced a formal definition of myocardial injury, which was hailed as an innovation: cTn elevation to the 99th percentile of the upper limit of normal in a reference population.
It differentiates type 1 MI from type 2 MI by the separate pathophysiology of the ischemia – plaque rupture with intracoronary thrombosis and myocardial oxygen supply–demand mismatch, respectively. In both cases, however, there must be symptoms or objective evidence of ischemia. Absent signs of ischemia, the determination would be nonischemic myocardial injury.
Yet clinically and prognostically, type 2 MI and nonischemic myocardial injury in some ways are more similar to each other than either is to type 1 MI. Both occur secondary to other conditions across diverse clinical settings and can be a challenge to tell apart.
The universal definition’s perspective of the three events – so heavily dependent on cTn levels and myocardial ischemia – fails to account for the myriad complexities of individual patients in practice, some say, and so can muddle the process of risk assessment and therapy.
“Abnormal troponin identifies injury, but it doesn’t identify mechanism. Type 2 MI is highly prevalent, but there are other things that cause abnormal troponins,” Dr. Januzzi said. That’s why it’s important to explore and map out the clinical variables associated with the two conditions, to “understand who has a type 2 MI and who has cardiac injury. And believe it or not, it’s actually harder than it sounds to sort that out.”
“Practically speaking, the differentiation between these events is clinical,” Dr. Sandoval agreed. “There’s not always perfect agreement on what we’re calling what.”
Consequently, the universal definitions might categorize some events in ways that seem inconsistent from a management perspective. For example, they make a sharp distinction between coronary atherothrombotic and coronary nonatherothrombotic MI etiologies. Some clinicians would group MI caused by coronary spasm, coronary embolism, or spontaneous coronary artery dissection along with MI from coronary plaque rupture and thrombosis. But, Dr. Sandoval said, “even though these are coronary issues, they would fall into the type 2 bin.”
Also, about half of cases identified as type 2 MI are caused by tachyarrhythmias, which can elevate troponin and cause ECG changes and possibly symptoms resembling angina, Dr. Newby observed. “But that is completely different from other types of myocardial infarction, which are much more serious.”
So, “it’s a real mess of a diagnosis – acute myocardial injury, type 2 and type 1 MI – and it can be quite difficult to disentangle,” he said. “I think that the definition certainly has let us down.”
The diversity of type 2 MI clinical settings might also be a challenge. Myocardial injury according to cTn, with or without ischemia, occurs widely during critical illnesses and acute conditions, including respiratory distress, sepsis, internal bleeding, stroke, and pulmonary embolism.
Early in the COVID-19 pandemic, much was made of elevated troponin levels and myocarditis as an apparently frequent complication among hospitalized patients. “I raised my hand and said, we’ve been seeing abnormal troponins in people with influenza for 20 years,” Dr. Januzzi said. “Critical illness, infection, toxicity from drugs, from chemotherapy, from alcohol – there are all sorts of potential triggers of myocardial injury.”
Troponin ‘overdependence’
With many clinical settings in common and the presence or absence of myocardial ischemia to primarily distinguish them, type 2 MI and nonischemic myocardial injury both can be mistaken for the other. That can send management decisions in inappropriate directions.
A 2019 study looked at 633 cases that had been coded as type 2 MI at a major center and readjudicated them according to the fourth universal definition. Only 57% met all the type 2 criteria, 42% were reclassified as nonischemic myocardial injury, and a few were determined to have unstable angina.
“There’s overdependence on the easiest tool in the universal definition,” said Dr. Januzzi, a coauthor on that study. “Frequently people get seduced by the rise in a troponin value and immediately call it a myocardial infarction, lacking the other components of the universal definition that require evidence for coronary ischemia. That happens every day, where someone with an abnormal troponin is incorrectly branded as having an MI.”
It may not help that the current ICD-10-CM system features a diagnostic code for type 2 MI but not for myocardial injury.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” wrote Kristian Thygesen, MD, DSc, and Allan S. Jaffe, MD, in a recent editorial. They caution against “using this code for myocardial injury because it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading,” thereby worsening the potential for miscoding and “misattribution of MI diagnoses.”
That potential suggests there could be a growing population of patients who have been told they had an MI, which then becomes part of their medical record, when, actually, they experienced nonischemic myocardial injury.
“Having seen this occur,” Dr. Januzzi explained, “it affects people emotionally to think they’ve had an MI. Precision in diagnosis is important, which is why the universal definition is so valuable. If people would adhere to it more assiduously, we could reduce the frequency of people getting a misdiagnosis of MI when in fact they had injury.”
Still, he added, “if someone has an illness severe enough to cause myocardial injury, they’re at risk for a bad outcome regardless of whether they did or didn’t have an MI.”
The uncertain role of angiography
Angiography isn’t ordered nearly as often for patients ultimately diagnosed with type 2 MI or myocardial injury as for those with type 1 MI. Type 2 MI can hit some patients who have remained symptom free despite possibly unrecognized obstructive coronary artery disease (CAD) when myocardial demand is pushed past supply by a critical illness, tachyarrhythmia, or other acute conditions.
In such cases, “it’s reasonable to hypothesize that revascularization, something that really is not done in the vast majority of patients with type 2 MI, might actually be of benefit,” Dr. Januzzi said.
Whether these patients should routinely have angiography remains an open question. Without intervention, any newly identified obstructive CAD would continue to lurk in the background as a potential threat.
In efforts to differentiate type 2 MI from nonischemic injury, it can be “incredibly hard to know whether or not there’s actual ischemia in the mix. And that’s the only thing that defines the difference before taking an angiogram,” Derek P. Chew, MBBS, MPH, Flinders Medical Centre, Bedford Park, Australia, said in an interview.
Dr. Chew is principal investigator for the ongoing ACT-2 trial that is enrolling hospitalized, hemodynamically stable patients with cTn elevations but no suspicion of type 1 MI and “an unequivocal acute intercurrent diagnosis.” Qualifying diagnoses are prespecified on a list that includes sepsis, pneumonia, septicemia, a systemic inflammatory response, anemia, atrial tachycardia, acute kidney injury, and recent noncardiac surgery.
The patients are randomly assigned to a strategy of routine, usually invasive coronary angiography with discretionary revascularization, or to conservative care with noninvasive functional testing as appropriate. The sicker the patient, the greater the competing risk from other conditions and the less revascularization is likely to improve outcomes, Dr. Chew observed. Importantly, therefore, outcomes in the trial will be stratified by patient risk from comorbidities, measured with baseline GRACE and APACHE III scores.
Dr. Chew said the study aims to determine whether routine angiography is of benefit in patients at some identifiable level of risk, if not the whole range. One possible result, he said, is that there could be a risk-profile “sweet spot” associated with better outcomes in those assigned to angiography.
Enrollment in the trial started about 3 years ago, but “the process has been slow,” he said, because many potentially referring clinicians have a “bias on one side or another,” with about half of them preferring the angiography approach and the other half conservative management.
The unsettled role of drug therapy
With their often-complicated clinical profile, patients with type 2 MI or nonischemic myocardial injury tend to be medically undertreated, yet there is observational evidence they can benefit from familiar drug therapies.
In the previously noted cohort study of 3,800 younger patients with one of the three forms of myocardial injury, less than half of patients with type 2 MI received any form of CAD secondary prevention therapy at discharge, the researchers, with first author Avinainder Singh, MD, from Yale University, New Haven, Conn, wrote.
The finding, consistent with Dr. Newby’s study from 2018, suggests that “categorizing the type of MI in young subjects might inform long-term cardiovascular prognosis,” and “emphasizes the need to identify and implement secondary prevention strategies to mitigate the high rate of cardiovascular death in patients with type 2 MI,” they concluded.
Further, outcomes varied with the number of discharge CV meds in an older cohort of patients with myocardial injury. Those with type 2 MI or acute or chronic nonischemic myocardial injury were far less likely than patients with type 1 MI to be prescribed guideline-based drugs. Survival was greater for those on two or three classes of CV medications, compared with one or none, in patients with acute or chronic nonischemic injury.
The investigators urged that patients with nonischemic myocardial injury or type 2 MI “be treated with cardiovascular medication to a larger degree than what is done today.”
When there is documented CAD in patients with type 2 MI, “it would be reasonable to suggest that preventative secondary prevention approaches, such as such lipid-reduction therapy or aspirin, would be beneficial,” Dr. Sandoval said. “But the reality is, there are no randomized trials, there are no prospective studies. ACT-2 is one of the few and early studies that’s really trying to address this.”
“The great majority of these people are not going to the cath lab, but when they do, there seems to be a signal of potential benefit,” Dr. Januzzi said. “For someone with a type 2 MI, it’s quite possible revascularization might help. Then more long-term treatment with medications that are proven in randomized trials to reduce risk would be a very plausible intervention.”
“We’ve actually proposed a number of potential therapeutic interventions to explore, both in people with type 2 MI and in people with injury without MI,” he said. “They might include sodium glucose cotransporter 2 inhibitors. They might include antithrombotic therapy or more aggressive lipid lowering, possibly for the pleiotropic effects rather than the effects on atherosclerosis.”
Any such therapies that prove successful in well-designed trials could well earn both type 2 MI and nonischemic myocardial injury, neglected as disorders in their own right, the kind of respect in clinical care pathways that they likely deserve.
Dr. Newby has disclosed receiving consulting fees or honoraria from Eli Lilly, Roche, Toshiba, Jansen, Reckitt Benckiser Pharmaceuticals, Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, CellProthera, and Oncoarendi; and conducting research or receiving grants from Pfizer, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Boehringer Ingelheim, and Inositec. Sandoval reports serving on an advisory board and as a speaker for Abbott Diagnostics and on an advisory board for Roche Diagnostics. Dr. Januzzi has disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on endpoint committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda. Dr. Chew has reported receiving grants from AstraZeneca and Edwards Life Sciences. ACT-2 is sponsored by the National Medical and Health Research Council of Australia.
A version of this article first appeared on Medscape.com.
Quinolones and tendon health: Third-generation drugs may be safer
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
Finerenone scores second pivotal-trial success in patients with diabetic kidney disease
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Finerenone, an investigational agent from a new drug class, just scored a second pivotal trial win after showing significant benefit for slowing progression of diabetic kidney disease in patients with type 2 diabetes in the FIDELIO-DKD pivotal trial with more than 5,700 patients.
Top-line results from FIGARO-DKD showed significant benefit for the primary endpoint of cardiovascular death and nonfatal cardiovascular disease endpoints in a placebo-controlled trial with about 7,400 patients with type 2 diabetes, reported Bayer, the company developing finerenone in statement released on May 10, 2021.
Based on the FIDELIO-DKD results, finerenone is currently under review by the U.S. Food and Drug Administration for marketing approval as a treatment for patients with type 2 diabetes and chronic kidney disease. FIDELIO-DKD, in addition to the primary endpoint that focused on slowing progression of diabetic kidney disease, had a secondary endpoint that assessed the combined incidence on treatment of cardiovascular death, or nonfatal episodes of stroke, MI, or hospitalization for heart failure. Results from the study published in 2020 in the New England Journal of Medicine showed that finerenone was safe and effective for both endpoints.
In the current study, FIGARO-DKD, run at more than 1,000 sites in 47 countries, these endpoints flipped. The primary outcome was a composite of cardiovascular death or nonfatal cardiovascular disease events, and the secondary outcome was prevention of DKD progression.
Other than stating the results significantly fulfilled FIGARO-DKD’s primary endpoint of reducing the incidence of combined cardiovascular disease endpoints, the release gave no further outcome details. The release noted that the enrolled patient cohort in FIGARO-DKD included more patients with earlier-stage chronic kidney disease, compared with FIDELIO-DKD.
Finerenone is a first-in-class investigational nonsteroidal, selective mineralocorticoid receptor antagonist (MRA). As an MRA it shares certain activities with the steroidal MRAs spironolactone and eplerenone. But the absence of a steroidal structure means that finerenone does not cause steroidal adverse effects such as gynecomastia. Results in FIDELIO-DKD showed that finerenone caused more hyperkalemia than placebo, but the level of hyperkalemia that it causes relative to spironolactone or eplerenone remains uncertain.
Low-fat diet upped quality of life in ulcerative colitis
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
For patients with mild or remitted ulcerative colitis, a catered, low-fat, high-fiber diet improved quality of life and stool markers of dysbiosis and inflammation, according to the findings of a small crossover trial.
Patients with inflammatory bowel disease often ask what they should eat, but few studies have addressed that question, Julia Fritsch, of the University of Miami and her associates wrote in Clinical Gastroenterology and Hepatology. Building on previous findings that a high-fat diet may contribute to inflammatory bowel disease, they randomly assigned 38 adults whose ulcerative colitis was in remission or mild (with a flare within the past 18 months) to receive either a low-fat diet (with 10% of daily calories from fat and high amounts of fruit and vegetables) or an “improved American standard diet” (with 35%-40% of daily calories from fat but more fruit and vegetables than Americans typically eat). Each diet was catered, delivered to patients’ homes, and lasted 4 weeks, followed by a 2-week washout period, after which each participant switched to the other diet.
Of the 38 patients, 17 completed the study. Food recall surveys over 24 hours showed that both diets were healthier than what participants ate at baseline, and daily web-based food diaries (such as www.nutrihand.com/Static/index.html) confirmed that more than 94% of patients adhered to the amount of fat in each diet. Even though participants in both groups ate only about half of the provided fruits and vegetables, the primary outcome of quality of life based on the short inflammatory bowel disease questionnaire (SIBDQ) significantly improved from a median of 4.98 (interquartile range, 4.1-6.0) at baseline to 5.77 (IQR, 5-6.4) with the low-fat diet and 5.55 (IQR, 4.75-6.25) with the improved American standard diet. Both diets also produced significant improvements in quality of life as measured by the 36-Item Short Form Survey and in disease activity as measured by the partial Mayo score.
Notably, however, only the low-fat diet significantly reduced serum amyloid A, which is a marker of mucosal inflammation, and intestinal dysbiosis, which was quantified by 16S RNA ribosomal sequencing. “Of note, there were several variables that were associated with changes in the microbiota composition,” the researchers wrote. These included the SIBDQ, C-reactive protein, interleukin-6, interleukin-1 beta, and 32 dietary components such as protein, potassium, iron, and zinc.
“These data suggest that even patients in remission [from ulcerative colitis] could benefit from a healthier diet,” the investigators concluded. “Just as importantly, neither diet exacerbated symptoms, which is notable given the higher fiber in both catered diets.” They called catering “a feasible way to perform a diet intervention study with high adherence,” noting that “catering a diet for a patient with IBD for a year costs between $19,000 and $21,000 per patient. The cost of a patient on a biologic such as ustekinumab is approximately $130,752 to $261,504.”
The study was supported by the Crohn’s and Colitis Foundation Broad Medical Research Program, Micky and Madeleine Arison Family Foundation Crohn’s and Colitis Discovery Laboratory, and the Martin Kalser Chair. The senior author disclosed ties to Boehringer Ingelheim, Gilead, AbbVie, Seres Therapeutics, Shire, Landos, Pfizer, and several other pharmaceutical companies. The other researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
In-hospital glucose management program gives dramatic savings
Initiatives targeting hypoglycemia and insulin pen wastage could lead to dramatic cost savings in small community hospitals, new data suggest.
The two projects are part of a dedicated inpatient glucose management service led by Mihail (“Misha”) Zilbermint, MD, one of the few full-time endocrine hospitalists in the United States and one of even fewer who work at a small community hospital.
In 2019, Dr. Zilbermint and colleagues reported that their inpatient glucose management program resulted in a 27% reduction in length of stay and a 10.7% lower 30-day readmission rate. The projected cost savings for the period January 2016 to May 2017 was $953,578.
Dr. Zilbermint’s team has written two new articles that document cost savings for specific elements of the program; namely, a set of hospital-wide hypoglycemia prevention measures, and an initiative that reduced duplicate inpatient insulin pen dispensing.
About 1 in 4 people in U.S. hospitals have diabetes or hyperglycemia. Large academic hospitals have endocrine divisions and training programs, but 85% of people receive care at small community hospitals.
“There are management guidelines, but they’re not always followed ... That’s why I’ve been advocating for endocrine hospitalists to be deployed nationally,” Dr. Zilbermint said. He is chief and director of endocrinology, diabetes, and metabolism at Johns Hopkins Community Physicians at Suburban Hospital, Bethesda, Maryland.
Asked to comment on behalf of the Society of Hospital Medicine (SHM), Greg Maynard, MD, program lead for SHM’s Electronic Quality Improvement Programs, said that Suburban’s overall program goals align with those of the SHM.
“Dedicated inpatient glycemic control teams are very important and desirable to improve the quality and safety of care for inpatients with hyperglycemia and diabetes,” he said.
Regarding specific initiatives, such as those aimed at reducing hypoglycemia and insulin pen wastage, Dr. Maynard said, “All of these are feasible in a wide variety of institutions. The main barrier is getting the institutional support for people to work on these interventions. This series of studies can help spread the word about the positive return on investment.”
Another barrier – the current lack of publicly reported measures or pay-for-performance programs for hypoglycemia prevention and glycemic control – may soon change, added Dr. Maynard, who is also chief quality officer at the University of California, Davis, Medical Center.
“The National Quality Forum has endorsed new measures, and the CDC’s National Healthcare Safety Network is working on ways to augment those measures and embed them into their infrastructure,” he said.
Although SHM doesn’t specifically endorse full-time glycemic control hospitalists over endocrinology-trained glycemic control experts, “certainly hospitalists who accrue added training are very well positioned to be an important part of these interdisciplinary teams,” Dr. Maynard said.
‘The nurses were so afraid of hypoglycemia’
Tackling hypoglycemia was Dr. Zilbermint’s first priority when he started the glycemic management program at Suburban in late 2015.
“One of the most common complaints from the nurses was that a lot of their patients had hypoglycemia, especially in the ICU, when patients were placed on insulin infusion protocols ... Every time, the nurse would have to call the attending and ask what to do,” he explains.
In addition, Dr. Zilbermint says, there was no standard for treating hypoglycemia. A nurse in one unit would give two cups of juice, another a 50% dextrose infusion, or another, milk. Even more concerning, “the nurses were so afraid of hypoglycemia they would reflexively discontinue all insulin, including basal.”
So one of the new initiatives, led by Carter Shelton, MSHCM, an administrative fellow at the Medical University of South Carolina, Charleston, was to implement a set of hospital-wide hypoglycemia prevention measures, as described in an article published online April 21 in the Journal of Diabetes Science and Technology.
Inpatient hypoglycemia rate was cut nearly in half
This began in 2016, when the multidisciplinary Suburban Hospital Glucose Steering Committee identified four main causes of insulin-induced hypoglycemia (defined as a blood glucose level of ≤70 mg/dL in a patient who had received at least one dose of insulin in the past 24 hours) and devised solutions for each:
1. Lack of a unified hypoglycemia protocol. A formal, evidence-based, nurse-driven treatment protocol with clinical decision support in the electronic medical record was developed. The Suburban team adapted much of the protocol from one that had been recently implemented at the flagship Johns Hopkins Hospital, in Baltimore, Maryland.
According to that protocol, if patients are able to swallow, they are given 15 g or 30 g of carbohydrates in order to achieve a blood glucose level of 50 to 70 mg/dL and <50 mg/dL, respectively. Levels are checked 15 minutes later. Intravenous D50 or glucagon is reserved for patients who can’t swallow.
2. For patients in critical care, the insulin infusion protocol that had been in use set blood glucose targets of 80 to 110 mg/dL, which resulted in hypoglycemia in nearly every patient who received an insulin infusion. This protocol was changed to the currently recommended 140 to 180 mg/dL.
3. Most patients were managed with sliding-scale insulin, an outdated yet still widely used regimen whereby insulin is given based only on current blood glucose without accounting for carbohydrates consumed with meals and not corrected until the subsequent meal. This was changed so that nurses give insulin after the patient has consumed at least 50% of their meal carbohydrates.
4. Lack of hypoglycemia reporting. A glucometrics dashboard – now used throughout the Johns Hopkins system – was adopted to produce daily hypoglycemia reports in the EMR system that could be reviewed by the inpatient glucose management service to track quality metrics and plan further interventions.
Between Jan. 1, 2016, and Sept. 30, 2019, out of a total 49,315 patient-days, there were 2,682 days on which any hypoglycemia occurred and 874 days on which moderate hypoglycemia occurred (≤54 mg/dL). Type 2 diabetes accounted for 84.4% of the total patient-days; type 1 accounted for 4.4%.
The overall frequency of any hypoglycemia patient-days per month decreased from 7.5% to 3.9% during the study period (P = .001). This was significant for the patients with type 2 diabetes (7.4% to 3.8%; P < .0001) but not for those with type 1 diabetes (18.5% to 18.0%; P = .08).
Rates of moderate hypoglycemia also decreased significantly among the patients with type 2 diabetes (1.9% to 1.0%; P = .03) but not for those with type 1 diabetes (7.4% to 6.0%; P = .14).
On the basis of these rates in reducing hypoglycemia, in which the inpatient hypoglycemia rate was cut nearly in half, the estimated savings in cost of care to the hospital was $98,635 during the period of January 2016 to September 2019.
Reducing insulin pen waste by minimizing duplicate prescriptions
Suburban Hospital had been using insulin vials and syringes when Dr. Zilbermint first arrived there. He lobbied the administration to allow use of pens, because they’re easier to use and they reduce the risk for needlestick injuries. Nurses were educated and retrained monthly in their use.
The switch to pens – aspart (Novolog Flexpen) for bolus insulin and glargine (Lantus SoloSTAR) – took place in 2018. The cost of the aspart pen was $16.19, and the cost of glargine was $25.08. Each holds 300 units of insulin.
After the first month, the team noticed a large increase in expenses. A quality improvement project was devised to address the issue.
“We were dispensing sometimes three or four pens per person. That’s a lot. Each pen holds 300 units, so one pen should last the entire hospital stay of an average 4- or 5-day stay,” Dr. Zilbermint explained. “We had to figure out where we were bleeding the money and where the pens were going.”
When pens disappeared, the pharmacy would have to dispense new ones. One problem was that when patients were transferred from one unit to another, the pen would be left behind and the room would be cleaned. Sometimes the pens weren’t stored properly or were misplaced. Often, they’d end up in a nurse’s pocket.
The second intervention was led by Urooj Najmi, MD, of the American International School of Medicine, Atlanta, Georgia. A program was instituted to reduce duplicate inpatient insulin pen dispensing, as detailed in an article published in the same issue of the Journal of Diabetes Science and Technology.
Solutions to reduce duplicate pen dispensing included having pharmacy track daily insulin pen reports and monitor duplicate orders, with “do not dispense” instructions conveyed via the EMR system. All multidose medications, including insulin pens, were to be placed in patients’ bins at the nursing station, and nurses were instructed to look for patients’ insulin pens prior to their being transferred to another unit, rather than ask for a replacement pen.
From July 2018 to July 2019, 3,121 patients received insulin, of whom 95% received aspart and 47% received glargine. Of the 9,516 pens dispensed, 68% were for aspart and 32% were for glargine. During the study period, the number of pens dispensed per patient dropped from 2.2 to 1.2 for aspart and from 2.1 to 1.3 for glargine; differences were highly significant (P = .0002 and P = .0005, respectively).
The total amount of unnecessary dispensing during the first 4 months after initiating the pen implementation program was 58%. The average monthly cost was $11,820.68; the projected cost per year was $141,848.
Six months after the waste reduction strategies were implemented, monthly waste had dropped to 42%, translating to an estimated potential cost savings of $66,261 over 12 months.
Because Suburban Hospital doesn’t have an outpatient dispensing license, there is still wastage when patients are discharged, because they can’t take their pens home with them. That remains a challenge, Dr. Zilbermint noted.
The team is working on implementing automatic A1c testing for patients admitted with hyperglycemia who either have a history of diabetes or whose blood glucose level is >140 mg/dL. Dr. Zilbermint said, “it’s in the guidelines, but it’s not always done.”
Dr. Zilbermint is a consultant for Guidepoint. Dr. Maynard, Mr. Shelton, and Dr. Najmi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initiatives targeting hypoglycemia and insulin pen wastage could lead to dramatic cost savings in small community hospitals, new data suggest.
The two projects are part of a dedicated inpatient glucose management service led by Mihail (“Misha”) Zilbermint, MD, one of the few full-time endocrine hospitalists in the United States and one of even fewer who work at a small community hospital.
In 2019, Dr. Zilbermint and colleagues reported that their inpatient glucose management program resulted in a 27% reduction in length of stay and a 10.7% lower 30-day readmission rate. The projected cost savings for the period January 2016 to May 2017 was $953,578.
Dr. Zilbermint’s team has written two new articles that document cost savings for specific elements of the program; namely, a set of hospital-wide hypoglycemia prevention measures, and an initiative that reduced duplicate inpatient insulin pen dispensing.
About 1 in 4 people in U.S. hospitals have diabetes or hyperglycemia. Large academic hospitals have endocrine divisions and training programs, but 85% of people receive care at small community hospitals.
“There are management guidelines, but they’re not always followed ... That’s why I’ve been advocating for endocrine hospitalists to be deployed nationally,” Dr. Zilbermint said. He is chief and director of endocrinology, diabetes, and metabolism at Johns Hopkins Community Physicians at Suburban Hospital, Bethesda, Maryland.
Asked to comment on behalf of the Society of Hospital Medicine (SHM), Greg Maynard, MD, program lead for SHM’s Electronic Quality Improvement Programs, said that Suburban’s overall program goals align with those of the SHM.
“Dedicated inpatient glycemic control teams are very important and desirable to improve the quality and safety of care for inpatients with hyperglycemia and diabetes,” he said.
Regarding specific initiatives, such as those aimed at reducing hypoglycemia and insulin pen wastage, Dr. Maynard said, “All of these are feasible in a wide variety of institutions. The main barrier is getting the institutional support for people to work on these interventions. This series of studies can help spread the word about the positive return on investment.”
Another barrier – the current lack of publicly reported measures or pay-for-performance programs for hypoglycemia prevention and glycemic control – may soon change, added Dr. Maynard, who is also chief quality officer at the University of California, Davis, Medical Center.
“The National Quality Forum has endorsed new measures, and the CDC’s National Healthcare Safety Network is working on ways to augment those measures and embed them into their infrastructure,” he said.
Although SHM doesn’t specifically endorse full-time glycemic control hospitalists over endocrinology-trained glycemic control experts, “certainly hospitalists who accrue added training are very well positioned to be an important part of these interdisciplinary teams,” Dr. Maynard said.
‘The nurses were so afraid of hypoglycemia’
Tackling hypoglycemia was Dr. Zilbermint’s first priority when he started the glycemic management program at Suburban in late 2015.
“One of the most common complaints from the nurses was that a lot of their patients had hypoglycemia, especially in the ICU, when patients were placed on insulin infusion protocols ... Every time, the nurse would have to call the attending and ask what to do,” he explains.
In addition, Dr. Zilbermint says, there was no standard for treating hypoglycemia. A nurse in one unit would give two cups of juice, another a 50% dextrose infusion, or another, milk. Even more concerning, “the nurses were so afraid of hypoglycemia they would reflexively discontinue all insulin, including basal.”
So one of the new initiatives, led by Carter Shelton, MSHCM, an administrative fellow at the Medical University of South Carolina, Charleston, was to implement a set of hospital-wide hypoglycemia prevention measures, as described in an article published online April 21 in the Journal of Diabetes Science and Technology.
Inpatient hypoglycemia rate was cut nearly in half
This began in 2016, when the multidisciplinary Suburban Hospital Glucose Steering Committee identified four main causes of insulin-induced hypoglycemia (defined as a blood glucose level of ≤70 mg/dL in a patient who had received at least one dose of insulin in the past 24 hours) and devised solutions for each:
1. Lack of a unified hypoglycemia protocol. A formal, evidence-based, nurse-driven treatment protocol with clinical decision support in the electronic medical record was developed. The Suburban team adapted much of the protocol from one that had been recently implemented at the flagship Johns Hopkins Hospital, in Baltimore, Maryland.
According to that protocol, if patients are able to swallow, they are given 15 g or 30 g of carbohydrates in order to achieve a blood glucose level of 50 to 70 mg/dL and <50 mg/dL, respectively. Levels are checked 15 minutes later. Intravenous D50 or glucagon is reserved for patients who can’t swallow.
2. For patients in critical care, the insulin infusion protocol that had been in use set blood glucose targets of 80 to 110 mg/dL, which resulted in hypoglycemia in nearly every patient who received an insulin infusion. This protocol was changed to the currently recommended 140 to 180 mg/dL.
3. Most patients were managed with sliding-scale insulin, an outdated yet still widely used regimen whereby insulin is given based only on current blood glucose without accounting for carbohydrates consumed with meals and not corrected until the subsequent meal. This was changed so that nurses give insulin after the patient has consumed at least 50% of their meal carbohydrates.
4. Lack of hypoglycemia reporting. A glucometrics dashboard – now used throughout the Johns Hopkins system – was adopted to produce daily hypoglycemia reports in the EMR system that could be reviewed by the inpatient glucose management service to track quality metrics and plan further interventions.
Between Jan. 1, 2016, and Sept. 30, 2019, out of a total 49,315 patient-days, there were 2,682 days on which any hypoglycemia occurred and 874 days on which moderate hypoglycemia occurred (≤54 mg/dL). Type 2 diabetes accounted for 84.4% of the total patient-days; type 1 accounted for 4.4%.
The overall frequency of any hypoglycemia patient-days per month decreased from 7.5% to 3.9% during the study period (P = .001). This was significant for the patients with type 2 diabetes (7.4% to 3.8%; P < .0001) but not for those with type 1 diabetes (18.5% to 18.0%; P = .08).
Rates of moderate hypoglycemia also decreased significantly among the patients with type 2 diabetes (1.9% to 1.0%; P = .03) but not for those with type 1 diabetes (7.4% to 6.0%; P = .14).
On the basis of these rates in reducing hypoglycemia, in which the inpatient hypoglycemia rate was cut nearly in half, the estimated savings in cost of care to the hospital was $98,635 during the period of January 2016 to September 2019.
Reducing insulin pen waste by minimizing duplicate prescriptions
Suburban Hospital had been using insulin vials and syringes when Dr. Zilbermint first arrived there. He lobbied the administration to allow use of pens, because they’re easier to use and they reduce the risk for needlestick injuries. Nurses were educated and retrained monthly in their use.
The switch to pens – aspart (Novolog Flexpen) for bolus insulin and glargine (Lantus SoloSTAR) – took place in 2018. The cost of the aspart pen was $16.19, and the cost of glargine was $25.08. Each holds 300 units of insulin.
After the first month, the team noticed a large increase in expenses. A quality improvement project was devised to address the issue.
“We were dispensing sometimes three or four pens per person. That’s a lot. Each pen holds 300 units, so one pen should last the entire hospital stay of an average 4- or 5-day stay,” Dr. Zilbermint explained. “We had to figure out where we were bleeding the money and where the pens were going.”
When pens disappeared, the pharmacy would have to dispense new ones. One problem was that when patients were transferred from one unit to another, the pen would be left behind and the room would be cleaned. Sometimes the pens weren’t stored properly or were misplaced. Often, they’d end up in a nurse’s pocket.
The second intervention was led by Urooj Najmi, MD, of the American International School of Medicine, Atlanta, Georgia. A program was instituted to reduce duplicate inpatient insulin pen dispensing, as detailed in an article published in the same issue of the Journal of Diabetes Science and Technology.
Solutions to reduce duplicate pen dispensing included having pharmacy track daily insulin pen reports and monitor duplicate orders, with “do not dispense” instructions conveyed via the EMR system. All multidose medications, including insulin pens, were to be placed in patients’ bins at the nursing station, and nurses were instructed to look for patients’ insulin pens prior to their being transferred to another unit, rather than ask for a replacement pen.
From July 2018 to July 2019, 3,121 patients received insulin, of whom 95% received aspart and 47% received glargine. Of the 9,516 pens dispensed, 68% were for aspart and 32% were for glargine. During the study period, the number of pens dispensed per patient dropped from 2.2 to 1.2 for aspart and from 2.1 to 1.3 for glargine; differences were highly significant (P = .0002 and P = .0005, respectively).
The total amount of unnecessary dispensing during the first 4 months after initiating the pen implementation program was 58%. The average monthly cost was $11,820.68; the projected cost per year was $141,848.
Six months after the waste reduction strategies were implemented, monthly waste had dropped to 42%, translating to an estimated potential cost savings of $66,261 over 12 months.
Because Suburban Hospital doesn’t have an outpatient dispensing license, there is still wastage when patients are discharged, because they can’t take their pens home with them. That remains a challenge, Dr. Zilbermint noted.
The team is working on implementing automatic A1c testing for patients admitted with hyperglycemia who either have a history of diabetes or whose blood glucose level is >140 mg/dL. Dr. Zilbermint said, “it’s in the guidelines, but it’s not always done.”
Dr. Zilbermint is a consultant for Guidepoint. Dr. Maynard, Mr. Shelton, and Dr. Najmi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Initiatives targeting hypoglycemia and insulin pen wastage could lead to dramatic cost savings in small community hospitals, new data suggest.
The two projects are part of a dedicated inpatient glucose management service led by Mihail (“Misha”) Zilbermint, MD, one of the few full-time endocrine hospitalists in the United States and one of even fewer who work at a small community hospital.
In 2019, Dr. Zilbermint and colleagues reported that their inpatient glucose management program resulted in a 27% reduction in length of stay and a 10.7% lower 30-day readmission rate. The projected cost savings for the period January 2016 to May 2017 was $953,578.
Dr. Zilbermint’s team has written two new articles that document cost savings for specific elements of the program; namely, a set of hospital-wide hypoglycemia prevention measures, and an initiative that reduced duplicate inpatient insulin pen dispensing.
About 1 in 4 people in U.S. hospitals have diabetes or hyperglycemia. Large academic hospitals have endocrine divisions and training programs, but 85% of people receive care at small community hospitals.
“There are management guidelines, but they’re not always followed ... That’s why I’ve been advocating for endocrine hospitalists to be deployed nationally,” Dr. Zilbermint said. He is chief and director of endocrinology, diabetes, and metabolism at Johns Hopkins Community Physicians at Suburban Hospital, Bethesda, Maryland.
Asked to comment on behalf of the Society of Hospital Medicine (SHM), Greg Maynard, MD, program lead for SHM’s Electronic Quality Improvement Programs, said that Suburban’s overall program goals align with those of the SHM.
“Dedicated inpatient glycemic control teams are very important and desirable to improve the quality and safety of care for inpatients with hyperglycemia and diabetes,” he said.
Regarding specific initiatives, such as those aimed at reducing hypoglycemia and insulin pen wastage, Dr. Maynard said, “All of these are feasible in a wide variety of institutions. The main barrier is getting the institutional support for people to work on these interventions. This series of studies can help spread the word about the positive return on investment.”
Another barrier – the current lack of publicly reported measures or pay-for-performance programs for hypoglycemia prevention and glycemic control – may soon change, added Dr. Maynard, who is also chief quality officer at the University of California, Davis, Medical Center.
“The National Quality Forum has endorsed new measures, and the CDC’s National Healthcare Safety Network is working on ways to augment those measures and embed them into their infrastructure,” he said.
Although SHM doesn’t specifically endorse full-time glycemic control hospitalists over endocrinology-trained glycemic control experts, “certainly hospitalists who accrue added training are very well positioned to be an important part of these interdisciplinary teams,” Dr. Maynard said.
‘The nurses were so afraid of hypoglycemia’
Tackling hypoglycemia was Dr. Zilbermint’s first priority when he started the glycemic management program at Suburban in late 2015.
“One of the most common complaints from the nurses was that a lot of their patients had hypoglycemia, especially in the ICU, when patients were placed on insulin infusion protocols ... Every time, the nurse would have to call the attending and ask what to do,” he explains.
In addition, Dr. Zilbermint says, there was no standard for treating hypoglycemia. A nurse in one unit would give two cups of juice, another a 50% dextrose infusion, or another, milk. Even more concerning, “the nurses were so afraid of hypoglycemia they would reflexively discontinue all insulin, including basal.”
So one of the new initiatives, led by Carter Shelton, MSHCM, an administrative fellow at the Medical University of South Carolina, Charleston, was to implement a set of hospital-wide hypoglycemia prevention measures, as described in an article published online April 21 in the Journal of Diabetes Science and Technology.
Inpatient hypoglycemia rate was cut nearly in half
This began in 2016, when the multidisciplinary Suburban Hospital Glucose Steering Committee identified four main causes of insulin-induced hypoglycemia (defined as a blood glucose level of ≤70 mg/dL in a patient who had received at least one dose of insulin in the past 24 hours) and devised solutions for each:
1. Lack of a unified hypoglycemia protocol. A formal, evidence-based, nurse-driven treatment protocol with clinical decision support in the electronic medical record was developed. The Suburban team adapted much of the protocol from one that had been recently implemented at the flagship Johns Hopkins Hospital, in Baltimore, Maryland.
According to that protocol, if patients are able to swallow, they are given 15 g or 30 g of carbohydrates in order to achieve a blood glucose level of 50 to 70 mg/dL and <50 mg/dL, respectively. Levels are checked 15 minutes later. Intravenous D50 or glucagon is reserved for patients who can’t swallow.
2. For patients in critical care, the insulin infusion protocol that had been in use set blood glucose targets of 80 to 110 mg/dL, which resulted in hypoglycemia in nearly every patient who received an insulin infusion. This protocol was changed to the currently recommended 140 to 180 mg/dL.
3. Most patients were managed with sliding-scale insulin, an outdated yet still widely used regimen whereby insulin is given based only on current blood glucose without accounting for carbohydrates consumed with meals and not corrected until the subsequent meal. This was changed so that nurses give insulin after the patient has consumed at least 50% of their meal carbohydrates.
4. Lack of hypoglycemia reporting. A glucometrics dashboard – now used throughout the Johns Hopkins system – was adopted to produce daily hypoglycemia reports in the EMR system that could be reviewed by the inpatient glucose management service to track quality metrics and plan further interventions.
Between Jan. 1, 2016, and Sept. 30, 2019, out of a total 49,315 patient-days, there were 2,682 days on which any hypoglycemia occurred and 874 days on which moderate hypoglycemia occurred (≤54 mg/dL). Type 2 diabetes accounted for 84.4% of the total patient-days; type 1 accounted for 4.4%.
The overall frequency of any hypoglycemia patient-days per month decreased from 7.5% to 3.9% during the study period (P = .001). This was significant for the patients with type 2 diabetes (7.4% to 3.8%; P < .0001) but not for those with type 1 diabetes (18.5% to 18.0%; P = .08).
Rates of moderate hypoglycemia also decreased significantly among the patients with type 2 diabetes (1.9% to 1.0%; P = .03) but not for those with type 1 diabetes (7.4% to 6.0%; P = .14).
On the basis of these rates in reducing hypoglycemia, in which the inpatient hypoglycemia rate was cut nearly in half, the estimated savings in cost of care to the hospital was $98,635 during the period of January 2016 to September 2019.
Reducing insulin pen waste by minimizing duplicate prescriptions
Suburban Hospital had been using insulin vials and syringes when Dr. Zilbermint first arrived there. He lobbied the administration to allow use of pens, because they’re easier to use and they reduce the risk for needlestick injuries. Nurses were educated and retrained monthly in their use.
The switch to pens – aspart (Novolog Flexpen) for bolus insulin and glargine (Lantus SoloSTAR) – took place in 2018. The cost of the aspart pen was $16.19, and the cost of glargine was $25.08. Each holds 300 units of insulin.
After the first month, the team noticed a large increase in expenses. A quality improvement project was devised to address the issue.
“We were dispensing sometimes three or four pens per person. That’s a lot. Each pen holds 300 units, so one pen should last the entire hospital stay of an average 4- or 5-day stay,” Dr. Zilbermint explained. “We had to figure out where we were bleeding the money and where the pens were going.”
When pens disappeared, the pharmacy would have to dispense new ones. One problem was that when patients were transferred from one unit to another, the pen would be left behind and the room would be cleaned. Sometimes the pens weren’t stored properly or were misplaced. Often, they’d end up in a nurse’s pocket.
The second intervention was led by Urooj Najmi, MD, of the American International School of Medicine, Atlanta, Georgia. A program was instituted to reduce duplicate inpatient insulin pen dispensing, as detailed in an article published in the same issue of the Journal of Diabetes Science and Technology.
Solutions to reduce duplicate pen dispensing included having pharmacy track daily insulin pen reports and monitor duplicate orders, with “do not dispense” instructions conveyed via the EMR system. All multidose medications, including insulin pens, were to be placed in patients’ bins at the nursing station, and nurses were instructed to look for patients’ insulin pens prior to their being transferred to another unit, rather than ask for a replacement pen.
From July 2018 to July 2019, 3,121 patients received insulin, of whom 95% received aspart and 47% received glargine. Of the 9,516 pens dispensed, 68% were for aspart and 32% were for glargine. During the study period, the number of pens dispensed per patient dropped from 2.2 to 1.2 for aspart and from 2.1 to 1.3 for glargine; differences were highly significant (P = .0002 and P = .0005, respectively).
The total amount of unnecessary dispensing during the first 4 months after initiating the pen implementation program was 58%. The average monthly cost was $11,820.68; the projected cost per year was $141,848.
Six months after the waste reduction strategies were implemented, monthly waste had dropped to 42%, translating to an estimated potential cost savings of $66,261 over 12 months.
Because Suburban Hospital doesn’t have an outpatient dispensing license, there is still wastage when patients are discharged, because they can’t take their pens home with them. That remains a challenge, Dr. Zilbermint noted.
The team is working on implementing automatic A1c testing for patients admitted with hyperglycemia who either have a history of diabetes or whose blood glucose level is >140 mg/dL. Dr. Zilbermint said, “it’s in the guidelines, but it’s not always done.”
Dr. Zilbermint is a consultant for Guidepoint. Dr. Maynard, Mr. Shelton, and Dr. Najmi have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
IL-6 trans-signaling targeted by olamkicept in IBD
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
The selective interleukin-6 (IL-6) trans-signaling inhibitor olamkicept was well tolerated and induced clinical remissions in 3 of 16 adults with moderately to severely active inflammatory bowel disease (IBD), and remission was associated with clear alterations in levels of phospho-STAT3 (pSTAT3) in the intestinal mucosa, researchers reported.
In a 12-week, open-label, prospective phase 2a trial, patients received up to seven infusions of 600-mg olamkicept (sgp130Fc) every 2 weeks. Clinical remissions occurred in two of nine patients with ulcerative colitis and one of seven patients with Crohn’s disease. The overall rate of clinical response was 44%, which included five patients with ulcerative colitis and two patients with Crohn’s disease. Transcriptome isolation and high-throughput RNA sequencing of mucosal tissue specimens showed that clinical remitters had a decrease from baseline to week 14 in the expression of TNF, IL-1A, REG1A, IL-8, IL-1B, and LILRA, a known composite molecular surrogate for mucosal inflammation. In addition, exposing whole-blood samples to a recombinant IL-6/IL-6R fusion protein mimicked physiologic IL-6 activity and demonstrated that pSTAT3 levels dropped within 4 hours of the first olamkicept infusion and throughout treatment. “Our overall finding of decreased pSTAT3-positive cells in remission patients indicates that STAT3 is crucially involved in the mechanism of action of olamkicept,” wrote Stefan Schreiber, MD, of University Medical Center Schleswig-Holstein, Campus Kiel (Germany) together with his associates. The study is published in Gastroenterology.
Blocking the IL-6/ILR receptor can induce IBD remissions but causes “profound immunosuppression,” the investigators noted. Building on prior findings that chronic proinflammatory IL-6 activity is primarily mediated by trans-signaling of a complex of IL-6 and soluble IL6R that engages the gp130 receptor, the researchers developed a “decoy protein,” sgp130Fc (now known as olamkicept), which “exclusively blocks” IL-6 proinflammatory trans-signaling. This decoy protein showed promise in preclinical studies, with no evidence of immunosuppression, they wrote. To further evaluate olamkicept, they recruited adults with moderately to severely active ulcerative colitis or Crohn’s disease from two centers in Germany. The primary clinical assessment was remission, defined as a Mayo score under 2, with a bleeding score of 0 and an endoscopy score of less than 1 for patients with ulcerative colitis, and a Crohn’s Disease Activity Index (CDAI) of less than 150 for patients with Crohn’s disease. The primary molecular outcome was change in the composite molecular surrogate score.
Of the 16 patients, 10 completed the trial. At week 14, endoscopic responses were observed in six patients, all of whom also had a clinical response, and all three patients with clinical remissions also had endoscopic remissions. “The drug was well tolerated in all 16 treated individuals, similar to the results of the [two prior] phase 1 trials,” the researchers wrote. Although significant immunosuppression and intestinal perforations were not seen, 13 patients developed adverse events, most commonly seasonal upper respiratory tract infections, recurrence of herpes labialis, and eczema or erythema. There were five serious adverse events, two of which were cardiac in nature. A larger placebo-controlled trial is underway to further evaluate safety. For now, the researchers wrote, it appears that IL-6 trans-signaling inhibition “might open up novel therapeutic avenues for the treatment of IBD.”
University Hospital Schleswig-Holstein sponsored the study. Ferring AG provided funding and donated the olamkicept. Analyses were funded by EU H2020 SYSCID and EU H2020 Innovative Medicines Initiative 2 Joint Undertaking. Dr. Schreiber reported having coinvented IP and having ties to Pfizer, Bristol Myers Squibb, and Roche. Four coinvestigators disclosed ties to Ferring, AbbVie, Chugai, Roche, Regeneron, Pfizer, Sanofi, Conaris, and Genentech Roche. The other researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Two treatments show early promise for hypothalamic obesity
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
Two different agents showed potential for safely treating patients with hypothalamic obesity in two pilot studies with small numbers of patients.
One study prospectively randomized 21 adults with acquired hypothalamic obesity to treatment with placebo or Tesomet, a compound that combines the novel monoamine reuptake inhibitor tesofensine with metoprolol, a beta-blocker added to protect against adverse effects from tesofensine on heart rate and cardiac contractility. After 24 weeks of treatment, people on tesofensine/metoprolol had significant weight loss, compared with controls, while showing good tolerance with no significant effects on heart rate, blood pressure, or heart rhythm, Ulla Feldt-Rasmussen, MD, DMSc, reported at the annual meeting of the Endocrine Society.
The second report reviewed 18 children and adolescents with either acquired or genetic hypothalamic obesity who received open-label treatment with dextroamphetamine for an average of 20 months, and overall patients safely lost an average of 0.43 in their body mass index (BMI) standard deviation score, reported Jiska van Schaik, MD, in a separate talk at the meeting.
‘A supplement for lost satiety’
Patients with hypothalamic obesity face a dual problem from hypothalamic dysfunction that’s addressed by tesofensine, the weight-loss agent in Tesomet that increases hypothalamic levels of dopamine, serotonin, and noradrenaline by blocking reuptake, and thereby dulls appetite and food craving while also increasing fat metabolism, explained Dr. Feldt-Rasmussen, a professor of medical endocrinology at the University of Denmark and Rigshospitalet in Copenhagen. No treatment currently has regulatory approval for treating any form of hypothalamic obesity.
Tesofensine works as “a supplement for lost satiety, and satiety is what is lost” in patients with hypothalamic obesity as well in patients as Prader-Willi syndrome, the two disorders for which tesofensine/metoprolol is currently undergoing testing. “That’s the rationale, and it seems to work,” she declared during her talk. The formulation contains 0.5 mg tesofensine and 50 mg metoprolol administered orally once daily.
The study, run at Rigshospitalet, randomized 21 patients aged 18-75 years and with a BMI of at least 27 kg/m2who all had acquired hypothalamic obesity secondary to hypothalamic damage following cancer treatment. Patients averaged about 45 years of age, three-quarters were women, and their average BMI was about 37, with 90% having a BMI of at least 30.
The study’s design calls for 48-week follow-up; Dr. Feldt-Rasmussen presented the interim results after 24 weeks, with 18 of the 21 enrolled patients remaining in the study through 24 weeks. Three patients dropped out because of adverse events: one in the placebo arm, and two who received tesofensine/metoprolol.
Weight dropped by an average of 6.6 kg from baseline among the 11 patients who completed 24 weeks on tesofensine/metoprolol treatment, compared with no average change from baseline among the seven patients who completed the study on placebo, a significant difference. The researchers measured a validated, composite satiety score every 4 weeks, and found significantly more improvement among patients on tesofensine/metoprolol than in those on placebo during the study’s first half, but subsequently average scores among the actively treated patients fell to the same level of modest improvement as in the placebo patients.
Despite this, average weight loss in the patients on tesofensine/metoprolol steadily increased throughout the full 24 weeks.
Safety measures of diastolic blood pressure, heart rate, and corrected QT interval showed no significant between-group difference. Systolic pressure showed a transient average rise of 4 mm Hg above baseline in the tesofensine/metoprolol group, compared with a small dip in the control patients, but by 24 weeks average systolic blood pressure had reverted closer to baseline levels in both subgroups and showed no significant between-group difference. Two patients on tesofensine/metoprolol developed serious adverse events. In one patient these were not treatment related. The other patient developed anxiety after 8 weeks that was possibly treatment related but remained on treatment. Other adverse effects on tesofensine/metoprolol included dizziness, sleep disorder, and dry mouth, but all of these were mild and patients were willing to tolerate them to achieve their weight loss, Dr. Feldt-Rasmussen said.
Repurposing an ADHD treatment
Dextroamphetamine increases satiety and boosts resting energy expenditure, and is a common treatment for attention deficit hyperactivity disorder. Dr. van Schaik and coauthors reviewed 13 children and adolescents with acquired hypothalamic obesity and 5 with genetic hypothalamic obesity who received the treatment at either of two Dutch hospitals during 2014-2020. All 18 patients went on dextroamphetamine after other interventions had failed to produce improvement, said Dr. van Schaik, a researcher at University Medical Center and Wilhelmina Children’s Hospital in Utrecht, the Netherlands. The patients averaged about 13 years of age.
In addition to an overall effect on weight across all 18 subjects, the researchers found they could subdivide the full cohort into 10 responders (56%), 4 (22%) with weight stabilization on treatment, and 4 nonresponders (22%) who continued to gain weight despite treatment. The 10 responding patients had an average drop in their BMI standard deviation score of 0.91. All 10 responders had acquired hypothalamic obesity, and they averaged a 12.5 percentage point rise in their resting energy expenditure level, compared with baseline, while on treatment. The four whose weight stabilized on treatment included three patients with genetic hypothalamic obesity. The four nonresponders split into two with acquired hypothalamic obesity and two with the genetic form.
Thirteen patients (72%) had improvements in hyperphagia, energy, and behavior, and no patient had a serious adverse effect. One patient stopped treatment after 1 month because of elevated blood pressure.
“Dextroamphetamine may be promising, especially for acquired hypothalamic obesity,” Dr. van Schaik concluded, adding that prospective, controlled assessments are needed, and that a healthy lifestyle is the foundation of hypothalamic obesity treatment.
The Tesomet study was sponsored by Saniona, the company developing Tesomet. Dr Feldt-Rasmussen is an advisor to Saniona, and some of the coauthors on the study are Saniona employees. Dr. van Schaik had no disclosures.
FROM ENDO 2021
TIPP the scales in managing stress
The past year presented unprecedented challenges for many. In addition, mental health services have also been stretched to capacity. Anecdotally, some hospitals and emergency departments note that more youth have been presenting in mental health crises, and the severity of symptoms has also been higher. Safety planning is important, including working with patients to identify skills they can use in distress. Even those who do not experience suicidal thoughts may struggle with dysregulation or may use coping strategies that may not be the healthiest in the long term.
Within my practice, I see some families who are still waiting for an available therapist, or some may not wish to participate in therapy despite its being recommended. For these families, supporting them in using strategies that they may be willing and able to use in the moment to help them get through the moment of crisis can been helpful:
Case example (identifying details have been changed)
Emily is a 17-year-old girl who has a history of generalized anxiety disorder and obsessive-compulsive disorder. She has had multiple medication trials and a course of cognitive behavioral therapy when younger, with significant improvement in symptoms. She returns to clinic because of increased anxiety related to stressors of the pandemic. She wishes to not return to therapy because of feeling that she received maximal benefit and that further sessions would not be fruitful. However, she struggles with identifying what skills she can use, and her anxiety heightens significantly to near-panic and hyperventilating with even cursory exploration of triggers for her symptoms. Medications are also discussed during this appointment, and it is noted that it may take some time to see therapeutic effect. Of note, she reports no acute safety concerns. She has engaged in skin picking. No reported substance use. As she hyperventilates, she was asked to identify items in the room matching the colors of the rainbow in order. She was able to quickly do this, and then was asked to do it again. Afterward, she noted feeling much less anxious because it distracted her from her thoughts.
Distress tolerance skills can be very helpful to navigate getting through a crisis. When under stress, some may be more likely to engage in behaviors that are not helpful in the long term such as using avoidance; procrastinating; consuming tobacco, alcohol, or other substances; spending too much time on screens; or engaging in self-harm behaviors. While some of these activities may be okay in moderation, others are always harmful. At times, when encouraging patients to use skills with which they may be more familiar, e.g., deep breathing, progressive muscle relaxation, the response may be, “these don’t work!” It can be important to distinguish that the function of these skills is not to make someone feel good or to eliminate the stressor, but to “take some of the edge off” so they are less likely to slide into problematic behaviors. It can be beneficial to have multiple tools at one’s disposal because not all skills will always be effective or available.
TIPP skills (temperature, intense exercise, paced breathing, progressive muscle relaxation) are distress tolerance skills from dialectical behavioral therapy (DBT),1 which was initially developed to treat individuals with borderline personality disorder. More recently, the therapy modality has been applied to individuals who may struggle with emotion regulation for a variety of reasons. TIPP skills work quickly (within seconds to minutes) with the aim to decrease physiological arousal. They do not require a lot of thinking, and many are portable or easy to use. Given the speed of effect, these skills can also be used in lieu of p.r.n. medications or patients can be counseled about trying these instead of turning to substance use. The effect is brief (5-20 minutes), although this may lower the affective temperature sufficiently for someone to get through the intense moment or to be able to then utilize other skills that may require more cognitive reserves.
T – Temperature
Holding one’s breath and placing one’s face in cold water (above 50°) for 10-20 seconds to stimulate the diving response and decrease heart rate. Patients can repeat this up to 3 times. Alternatively, cold compresses or gel eye masks can be used.
I – Intense exercise
Aerobic exercise for 10-20 minutes. This can include running, jumping jacks, dancing to loud music in a way that feels intense. The parasympathetic nervous system (PNS) is activated for approximately 20 minutes after cessation of intense exercise.
P – Paced breathing
Decreasing rate of breathing, with each inhalation/exhalation cycle lasting 10-12 seconds and the exhale being longer than the inhale also activates the PNS.
P – Progressive muscle relaxation (PMR)
Sequentially tensing and relaxing muscles from head to toes. Having at least 5-10 minutes to perform this exercise is preferred.2 Children’s Hospital of Philadelphia offerssample PMR recordings.
Body scans can also be helpful. This practice differs from PMR in that it is a mindfulness practice noting body sensations without trying to change them. The University of Vermont offers some sample exercises.3
These skills were described to Emily. She noted that dunking her face in cold water was effective and it was reassuring knowing she had a tool to help her anxiety. She started to push herself to go outside to exercise. We additionally incorporated other distraction techniques such as identifying items from colors of the rainbow that were around her. She appreciated that she could even do this discreetly while at school. At times she had to do a couple of rounds, but this could help stop her repetitive thoughts so she could use other skills.
Helping patients identify skills that can help in the moment can help them feel supported and gain traction in other areas.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures
References
1. Rathus JH, Miller AL. DBT® Skills manual for adolescents. 2015. Guilford Press.
2. Guided Relaxation Exercises, Children’s Hospital of Philadelphia.
3. Vermont Center for Children, Youth, and Families: Staying Close While Keeping Your Distance.
The past year presented unprecedented challenges for many. In addition, mental health services have also been stretched to capacity. Anecdotally, some hospitals and emergency departments note that more youth have been presenting in mental health crises, and the severity of symptoms has also been higher. Safety planning is important, including working with patients to identify skills they can use in distress. Even those who do not experience suicidal thoughts may struggle with dysregulation or may use coping strategies that may not be the healthiest in the long term.
Within my practice, I see some families who are still waiting for an available therapist, or some may not wish to participate in therapy despite its being recommended. For these families, supporting them in using strategies that they may be willing and able to use in the moment to help them get through the moment of crisis can been helpful:
Case example (identifying details have been changed)
Emily is a 17-year-old girl who has a history of generalized anxiety disorder and obsessive-compulsive disorder. She has had multiple medication trials and a course of cognitive behavioral therapy when younger, with significant improvement in symptoms. She returns to clinic because of increased anxiety related to stressors of the pandemic. She wishes to not return to therapy because of feeling that she received maximal benefit and that further sessions would not be fruitful. However, she struggles with identifying what skills she can use, and her anxiety heightens significantly to near-panic and hyperventilating with even cursory exploration of triggers for her symptoms. Medications are also discussed during this appointment, and it is noted that it may take some time to see therapeutic effect. Of note, she reports no acute safety concerns. She has engaged in skin picking. No reported substance use. As she hyperventilates, she was asked to identify items in the room matching the colors of the rainbow in order. She was able to quickly do this, and then was asked to do it again. Afterward, she noted feeling much less anxious because it distracted her from her thoughts.
Distress tolerance skills can be very helpful to navigate getting through a crisis. When under stress, some may be more likely to engage in behaviors that are not helpful in the long term such as using avoidance; procrastinating; consuming tobacco, alcohol, or other substances; spending too much time on screens; or engaging in self-harm behaviors. While some of these activities may be okay in moderation, others are always harmful. At times, when encouraging patients to use skills with which they may be more familiar, e.g., deep breathing, progressive muscle relaxation, the response may be, “these don’t work!” It can be important to distinguish that the function of these skills is not to make someone feel good or to eliminate the stressor, but to “take some of the edge off” so they are less likely to slide into problematic behaviors. It can be beneficial to have multiple tools at one’s disposal because not all skills will always be effective or available.
TIPP skills (temperature, intense exercise, paced breathing, progressive muscle relaxation) are distress tolerance skills from dialectical behavioral therapy (DBT),1 which was initially developed to treat individuals with borderline personality disorder. More recently, the therapy modality has been applied to individuals who may struggle with emotion regulation for a variety of reasons. TIPP skills work quickly (within seconds to minutes) with the aim to decrease physiological arousal. They do not require a lot of thinking, and many are portable or easy to use. Given the speed of effect, these skills can also be used in lieu of p.r.n. medications or patients can be counseled about trying these instead of turning to substance use. The effect is brief (5-20 minutes), although this may lower the affective temperature sufficiently for someone to get through the intense moment or to be able to then utilize other skills that may require more cognitive reserves.
T – Temperature
Holding one’s breath and placing one’s face in cold water (above 50°) for 10-20 seconds to stimulate the diving response and decrease heart rate. Patients can repeat this up to 3 times. Alternatively, cold compresses or gel eye masks can be used.
I – Intense exercise
Aerobic exercise for 10-20 minutes. This can include running, jumping jacks, dancing to loud music in a way that feels intense. The parasympathetic nervous system (PNS) is activated for approximately 20 minutes after cessation of intense exercise.
P – Paced breathing
Decreasing rate of breathing, with each inhalation/exhalation cycle lasting 10-12 seconds and the exhale being longer than the inhale also activates the PNS.
P – Progressive muscle relaxation (PMR)
Sequentially tensing and relaxing muscles from head to toes. Having at least 5-10 minutes to perform this exercise is preferred.2 Children’s Hospital of Philadelphia offerssample PMR recordings.
Body scans can also be helpful. This practice differs from PMR in that it is a mindfulness practice noting body sensations without trying to change them. The University of Vermont offers some sample exercises.3
These skills were described to Emily. She noted that dunking her face in cold water was effective and it was reassuring knowing she had a tool to help her anxiety. She started to push herself to go outside to exercise. We additionally incorporated other distraction techniques such as identifying items from colors of the rainbow that were around her. She appreciated that she could even do this discreetly while at school. At times she had to do a couple of rounds, but this could help stop her repetitive thoughts so she could use other skills.
Helping patients identify skills that can help in the moment can help them feel supported and gain traction in other areas.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures
References
1. Rathus JH, Miller AL. DBT® Skills manual for adolescents. 2015. Guilford Press.
2. Guided Relaxation Exercises, Children’s Hospital of Philadelphia.
3. Vermont Center for Children, Youth, and Families: Staying Close While Keeping Your Distance.
The past year presented unprecedented challenges for many. In addition, mental health services have also been stretched to capacity. Anecdotally, some hospitals and emergency departments note that more youth have been presenting in mental health crises, and the severity of symptoms has also been higher. Safety planning is important, including working with patients to identify skills they can use in distress. Even those who do not experience suicidal thoughts may struggle with dysregulation or may use coping strategies that may not be the healthiest in the long term.
Within my practice, I see some families who are still waiting for an available therapist, or some may not wish to participate in therapy despite its being recommended. For these families, supporting them in using strategies that they may be willing and able to use in the moment to help them get through the moment of crisis can been helpful:
Case example (identifying details have been changed)
Emily is a 17-year-old girl who has a history of generalized anxiety disorder and obsessive-compulsive disorder. She has had multiple medication trials and a course of cognitive behavioral therapy when younger, with significant improvement in symptoms. She returns to clinic because of increased anxiety related to stressors of the pandemic. She wishes to not return to therapy because of feeling that she received maximal benefit and that further sessions would not be fruitful. However, she struggles with identifying what skills she can use, and her anxiety heightens significantly to near-panic and hyperventilating with even cursory exploration of triggers for her symptoms. Medications are also discussed during this appointment, and it is noted that it may take some time to see therapeutic effect. Of note, she reports no acute safety concerns. She has engaged in skin picking. No reported substance use. As she hyperventilates, she was asked to identify items in the room matching the colors of the rainbow in order. She was able to quickly do this, and then was asked to do it again. Afterward, she noted feeling much less anxious because it distracted her from her thoughts.
Distress tolerance skills can be very helpful to navigate getting through a crisis. When under stress, some may be more likely to engage in behaviors that are not helpful in the long term such as using avoidance; procrastinating; consuming tobacco, alcohol, or other substances; spending too much time on screens; or engaging in self-harm behaviors. While some of these activities may be okay in moderation, others are always harmful. At times, when encouraging patients to use skills with which they may be more familiar, e.g., deep breathing, progressive muscle relaxation, the response may be, “these don’t work!” It can be important to distinguish that the function of these skills is not to make someone feel good or to eliminate the stressor, but to “take some of the edge off” so they are less likely to slide into problematic behaviors. It can be beneficial to have multiple tools at one’s disposal because not all skills will always be effective or available.
TIPP skills (temperature, intense exercise, paced breathing, progressive muscle relaxation) are distress tolerance skills from dialectical behavioral therapy (DBT),1 which was initially developed to treat individuals with borderline personality disorder. More recently, the therapy modality has been applied to individuals who may struggle with emotion regulation for a variety of reasons. TIPP skills work quickly (within seconds to minutes) with the aim to decrease physiological arousal. They do not require a lot of thinking, and many are portable or easy to use. Given the speed of effect, these skills can also be used in lieu of p.r.n. medications or patients can be counseled about trying these instead of turning to substance use. The effect is brief (5-20 minutes), although this may lower the affective temperature sufficiently for someone to get through the intense moment or to be able to then utilize other skills that may require more cognitive reserves.
T – Temperature
Holding one’s breath and placing one’s face in cold water (above 50°) for 10-20 seconds to stimulate the diving response and decrease heart rate. Patients can repeat this up to 3 times. Alternatively, cold compresses or gel eye masks can be used.
I – Intense exercise
Aerobic exercise for 10-20 minutes. This can include running, jumping jacks, dancing to loud music in a way that feels intense. The parasympathetic nervous system (PNS) is activated for approximately 20 minutes after cessation of intense exercise.
P – Paced breathing
Decreasing rate of breathing, with each inhalation/exhalation cycle lasting 10-12 seconds and the exhale being longer than the inhale also activates the PNS.
P – Progressive muscle relaxation (PMR)
Sequentially tensing and relaxing muscles from head to toes. Having at least 5-10 minutes to perform this exercise is preferred.2 Children’s Hospital of Philadelphia offerssample PMR recordings.
Body scans can also be helpful. This practice differs from PMR in that it is a mindfulness practice noting body sensations without trying to change them. The University of Vermont offers some sample exercises.3
These skills were described to Emily. She noted that dunking her face in cold water was effective and it was reassuring knowing she had a tool to help her anxiety. She started to push herself to go outside to exercise. We additionally incorporated other distraction techniques such as identifying items from colors of the rainbow that were around her. She appreciated that she could even do this discreetly while at school. At times she had to do a couple of rounds, but this could help stop her repetitive thoughts so she could use other skills.
Helping patients identify skills that can help in the moment can help them feel supported and gain traction in other areas.
Dr. Strange is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and University of Vermont Robert Larner College of Medicine, both in Burlington. She works with children and adolescents. She has no relevant financial disclosures
References
1. Rathus JH, Miller AL. DBT® Skills manual for adolescents. 2015. Guilford Press.
2. Guided Relaxation Exercises, Children’s Hospital of Philadelphia.
3. Vermont Center for Children, Youth, and Families: Staying Close While Keeping Your Distance.