User login
FDA approves first targeted treatment for rare DMD mutation
, the agency has announced.
This particular mutation of the DMD gene “is amenable to exon 45 skipping,” the FDA noted in a press release. The agency added that this is its first approval of a targeted treatment for patients with the mutation.
“Developing drugs designed for patients with specific mutations is a critical part of personalized medicine,” Eric Bastings, MD, deputy director of the Office of Neuroscience at the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval was based on results from a 43-person randomized controlled trial. Patients who received casimersen had a greater increase in production of the muscle-fiber protein dystrophin compared with their counterparts who received placebo.
Approved – with cautions
The FDA noted that DMD prevalence worldwide is about 1 in 3,600 boys – although it can also affect girls in rare cases. Symptoms of the disorder are commonly first observed around age 3 years but worsen steadily over time. DMD gene mutations lead to a decrease in dystrophin.
As reported by Medscape Medical News in August, the FDA approved viltolarsen (Viltepso, NS Pharma) for the treatment of DMD in patients with a confirmed mutation amenable to exon 53 skipping, following approval of golodirsen injection (Vyondys 53, Sarepta Therapeutics) for the same indication in December 2019.
The DMD gene mutation that is amenable to exon 45 skipping is present in about 8% of patients with DMD.
The trial that carried weight with the FDA included 43 male participants with DMD aged 7-20 years. All were confirmed to have the exon 45-skipping gene mutation and all were randomly assigned 2:1 to received IV casimersen 30 mg/kg or matching placebo.
Results showed that, between baseline and 48 weeks post treatment, the casimersen group showed a significantly higher increase in levels of dystrophin protein than in the placebo group.
Upper respiratory tract infections, fever, joint and throat pain, headache, and cough were the most common adverse events experienced by the active-treatment group.
Although the clinical studies assessing casimersen did not show any reports of kidney toxicity, the adverse event was observed in some nonclinical studies. Therefore, clinicians should monitor kidney function in any patient receiving this treatment, the FDA recommended.
Overall, “the FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit” in this patient population, the agency said in its press release.
However, it noted that definitive clinical benefits such as improved motor function were not “established.”
“In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of [other] available therapy,” the agency said.
It added that the manufacturer is currently conducting a multicenter study focused on the safety and efficacy of the drug in ambulatory patients with DMD.
The FDA approved casimersen using its Accelerated Approval pathway, granted Fast Track and Priority Review designations to its applications, and gave the treatment Orphan Drug designation.
A version of this article first appeared on Medscape.com.
, the agency has announced.
This particular mutation of the DMD gene “is amenable to exon 45 skipping,” the FDA noted in a press release. The agency added that this is its first approval of a targeted treatment for patients with the mutation.
“Developing drugs designed for patients with specific mutations is a critical part of personalized medicine,” Eric Bastings, MD, deputy director of the Office of Neuroscience at the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval was based on results from a 43-person randomized controlled trial. Patients who received casimersen had a greater increase in production of the muscle-fiber protein dystrophin compared with their counterparts who received placebo.
Approved – with cautions
The FDA noted that DMD prevalence worldwide is about 1 in 3,600 boys – although it can also affect girls in rare cases. Symptoms of the disorder are commonly first observed around age 3 years but worsen steadily over time. DMD gene mutations lead to a decrease in dystrophin.
As reported by Medscape Medical News in August, the FDA approved viltolarsen (Viltepso, NS Pharma) for the treatment of DMD in patients with a confirmed mutation amenable to exon 53 skipping, following approval of golodirsen injection (Vyondys 53, Sarepta Therapeutics) for the same indication in December 2019.
The DMD gene mutation that is amenable to exon 45 skipping is present in about 8% of patients with DMD.
The trial that carried weight with the FDA included 43 male participants with DMD aged 7-20 years. All were confirmed to have the exon 45-skipping gene mutation and all were randomly assigned 2:1 to received IV casimersen 30 mg/kg or matching placebo.
Results showed that, between baseline and 48 weeks post treatment, the casimersen group showed a significantly higher increase in levels of dystrophin protein than in the placebo group.
Upper respiratory tract infections, fever, joint and throat pain, headache, and cough were the most common adverse events experienced by the active-treatment group.
Although the clinical studies assessing casimersen did not show any reports of kidney toxicity, the adverse event was observed in some nonclinical studies. Therefore, clinicians should monitor kidney function in any patient receiving this treatment, the FDA recommended.
Overall, “the FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit” in this patient population, the agency said in its press release.
However, it noted that definitive clinical benefits such as improved motor function were not “established.”
“In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of [other] available therapy,” the agency said.
It added that the manufacturer is currently conducting a multicenter study focused on the safety and efficacy of the drug in ambulatory patients with DMD.
The FDA approved casimersen using its Accelerated Approval pathway, granted Fast Track and Priority Review designations to its applications, and gave the treatment Orphan Drug designation.
A version of this article first appeared on Medscape.com.
, the agency has announced.
This particular mutation of the DMD gene “is amenable to exon 45 skipping,” the FDA noted in a press release. The agency added that this is its first approval of a targeted treatment for patients with the mutation.
“Developing drugs designed for patients with specific mutations is a critical part of personalized medicine,” Eric Bastings, MD, deputy director of the Office of Neuroscience at the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval was based on results from a 43-person randomized controlled trial. Patients who received casimersen had a greater increase in production of the muscle-fiber protein dystrophin compared with their counterparts who received placebo.
Approved – with cautions
The FDA noted that DMD prevalence worldwide is about 1 in 3,600 boys – although it can also affect girls in rare cases. Symptoms of the disorder are commonly first observed around age 3 years but worsen steadily over time. DMD gene mutations lead to a decrease in dystrophin.
As reported by Medscape Medical News in August, the FDA approved viltolarsen (Viltepso, NS Pharma) for the treatment of DMD in patients with a confirmed mutation amenable to exon 53 skipping, following approval of golodirsen injection (Vyondys 53, Sarepta Therapeutics) for the same indication in December 2019.
The DMD gene mutation that is amenable to exon 45 skipping is present in about 8% of patients with DMD.
The trial that carried weight with the FDA included 43 male participants with DMD aged 7-20 years. All were confirmed to have the exon 45-skipping gene mutation and all were randomly assigned 2:1 to received IV casimersen 30 mg/kg or matching placebo.
Results showed that, between baseline and 48 weeks post treatment, the casimersen group showed a significantly higher increase in levels of dystrophin protein than in the placebo group.
Upper respiratory tract infections, fever, joint and throat pain, headache, and cough were the most common adverse events experienced by the active-treatment group.
Although the clinical studies assessing casimersen did not show any reports of kidney toxicity, the adverse event was observed in some nonclinical studies. Therefore, clinicians should monitor kidney function in any patient receiving this treatment, the FDA recommended.
Overall, “the FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit” in this patient population, the agency said in its press release.
However, it noted that definitive clinical benefits such as improved motor function were not “established.”
“In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of [other] available therapy,” the agency said.
It added that the manufacturer is currently conducting a multicenter study focused on the safety and efficacy of the drug in ambulatory patients with DMD.
The FDA approved casimersen using its Accelerated Approval pathway, granted Fast Track and Priority Review designations to its applications, and gave the treatment Orphan Drug designation.
A version of this article first appeared on Medscape.com.
New-onset arrhythmias low in COVID-19 and flu
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Enfortumab vedotin offers hope to poor-prognosis patients with advanced urothelial cancer
Approximately half of all patients with locally advanced or metastatic urothelial cancer (la/mUC) are ineligible to receive cisplatin-based chemotherapy. They face poor outlooks and extremely limited treatment options.
A new study indicates that enfortumab vedotin (EV) can cause major, prolonged responses in most patients in that unfortunate setting.
EV is an antibody-drug conjugate directed against nectin-4, an immunoglobulin-like cell adhesion molecule that is highly expressed in UC, obviating the need for testing prior to treatment. It is internalized in malignant cells, with release of the active moiety (monomethyl auristatin E; MMAE). MMAE causes microtubule disruption, with resultant cell-cycle arrest and apoptosis.
EV received accelerated approval from the Food and Drug Administration in December 2019 after publication of the results from cohort 1 of the open-label, single-arm, phase 2 EV-201 study.
Arjun V. Balar, MD, of the Perlmutter Cancer Center at New York University Langone Health, presented results from cohort 2 of EV-201 – the cisplatin-ineligible cohort – at the 2021 Genitourinary Cancer Symposium (Abstract 394).
EV in patients ineligible for platinum-based therapy
Patients in cohort 2 of EV-201 had received immune checkpoint inhibitor therapy for la/mUC. They received EV in the FDA-approved dose for cohort 1: 1.25 mg/kg EV on days 1, 8, and 15 of a 28-day cycle.
Patients experienced disease progression during or following their most recent treatment. Patients with more than two neuropathies, active central nervous system metastases, and uncontrolled diabetes mellitus were excluded.
“Platinum ineligible” was defined as a creatinine clearance between 30-59 cm3/min, Eastern Cooperative Oncology Group performance status (ECOG PS) 2, or hearing loss of grade 2 or greater.
The primary endpoint for cohort 2 was confirmed overall response rate (ORR) per RECIST 1.1 by blinded independent central review. Secondary endpoints were duration of response, progression-free survival, overall survival, and safety.
There were 91 patients enrolled. Two patients never received EV treatment because of deterioration after registration. The median treatment duration among the remaining 89 patients was 6.0 months (range, 0.3-24.6).
Impressive results in poor-risk patients
The patients in EV-201 cohort 2 were elderly (median age, 75 years; range, 49-90) with comorbidities. The primary reasons for platinum-ineligibility were creatinine clearance less than 60 mL/min (66%), grade 2 or greater hearing loss (15%), and ECOG PS 2 (7%); 12% of patients met more than one criterion for platinum ineligibility.
The primary tumor site was in the upper urinary tract in 43% of patients, and 79% had visceral metastases, including 24% with liver involvement.
The confirmed ORR was 52% (95% confidence interval, 40.8-62.4), with 20% complete responses. There were responses in all subgroups, including patients with primary tumor sites in the upper tract (ORR, 61%), those with liver metastasis (ORR, 48%), and patients who had not responded to immune checkpoint inhibitors (ORR, 48%).
A total of 88% of patients had some decrease in measurable tumor diameters, generally within a few weeks of treatment initiation (median time to response, 1.8 months). The rapid response to treatment was especially important to patients having cancer-associated pain.
The median progression-free and overall survival durations were 5.8 months (95% CI, 5.0-8.3) and 14.7 months (95% CI, 10.5-18.2), respectively. The median response duration was 10.9 months (95% CI, 5.78-NR). More than 25% of responses extended beyond 12 months.
About 82% of patients in cohort 2 discontinued treatment, most commonly because of disease progression (51%). The second most common reason was the development of treatment-related adverse events (TRAE; 24%).
Drilling down on treatment-related adverse events
As might be expected for cisplatin-ineligible patients, adverse events were higher for patients in cohort 2 than for cohort 1 and led to treatment discontinuation in 16% of patients overall.
TRAEs over grade 3 occurred in 55% of patients. TRAEs of special interest included rash (61% overall; 17% ≥ grade 3), peripheral neuropathy (54% overall; 8% ≥ grade 3), and hyperglycemia (10% overall; 6% ≥ grade 3). Dose reductions, interruptions, and physical therapy were helpful.
Twenty percent of patients with TRAE hyperglycemia had hyperglycemia at baseline, and 30% of TRAEs were in patients with high body mass index (BMI).
There were four treatment-related deaths, all in patients 75 years or older with multiple comorbidities. Three of the four deaths occurred within 30 days of first EV dose in patients with BMI of 30 or greater (acute kidney injury, metabolic acidosis, and multiple organ dysfunction syndrome). The remaining death occurred more than 30 days after the last dose (pneumonitis).
Context and caution
The authors concluded that EV produced durable responses in platinum-ineligible patients with la/mUC, including 20% complete responses. Safety was felt to be as expected, given the known toxicities of the agent and the compromised medical condition of the patients studied.
The study discussant, Arlene O. Siefker-Radtke, MD, of the University of Texas MD Anderson Cancer Center, Houston, agreed that EV fills an unmet need, showing impressive responses in patients with visceral, liver, and bone metastases. She agreed that EV should be investigated across the spectrum of urothelial cancer.
Dr. Siefker-Radtke reminded attendees that the FDA package insert for EV described a 48% increase in the area under the concentration-time curve concentration of the MMAE active moiety in patients with mild hepatic impairment and that EV use should be avoided entirely in patients with moderate to severe liver disease.
She speculated whether augmented toxicity in patients with a high BMI could be attributable to clinically occult impaired hepatic function from fatty liver infiltration.
She indicated that clinicians should monitor closely patients with higher BMI and grade 3-4 hyperglycemia or elevated hemoglobin A1c levels and advised holding EV in patients who develop:
- Glucose levels above 250 mg/dL
- Peeling skin or bullous skin lesions. These lesions can be heralded by a diffuse erythematous or papillary rash in the preceding weeks.
- Grade 3 diarrhea or mucosal membrane toxicity of other types.
Notwithstanding concerns about toxicity and the need for monitoring, EV merits continued study in combination with other agents and in additional settings in the clinical spectrum of urothelial cancer. It is an important new option for oncologists caring for patients with urothelial cancer.
The EV-201 study was funded by Seagen. Dr. Balar and Dr. Siefker-Radtke disclosed relationships with Seagen and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Approximately half of all patients with locally advanced or metastatic urothelial cancer (la/mUC) are ineligible to receive cisplatin-based chemotherapy. They face poor outlooks and extremely limited treatment options.
A new study indicates that enfortumab vedotin (EV) can cause major, prolonged responses in most patients in that unfortunate setting.
EV is an antibody-drug conjugate directed against nectin-4, an immunoglobulin-like cell adhesion molecule that is highly expressed in UC, obviating the need for testing prior to treatment. It is internalized in malignant cells, with release of the active moiety (monomethyl auristatin E; MMAE). MMAE causes microtubule disruption, with resultant cell-cycle arrest and apoptosis.
EV received accelerated approval from the Food and Drug Administration in December 2019 after publication of the results from cohort 1 of the open-label, single-arm, phase 2 EV-201 study.
Arjun V. Balar, MD, of the Perlmutter Cancer Center at New York University Langone Health, presented results from cohort 2 of EV-201 – the cisplatin-ineligible cohort – at the 2021 Genitourinary Cancer Symposium (Abstract 394).
EV in patients ineligible for platinum-based therapy
Patients in cohort 2 of EV-201 had received immune checkpoint inhibitor therapy for la/mUC. They received EV in the FDA-approved dose for cohort 1: 1.25 mg/kg EV on days 1, 8, and 15 of a 28-day cycle.
Patients experienced disease progression during or following their most recent treatment. Patients with more than two neuropathies, active central nervous system metastases, and uncontrolled diabetes mellitus were excluded.
“Platinum ineligible” was defined as a creatinine clearance between 30-59 cm3/min, Eastern Cooperative Oncology Group performance status (ECOG PS) 2, or hearing loss of grade 2 or greater.
The primary endpoint for cohort 2 was confirmed overall response rate (ORR) per RECIST 1.1 by blinded independent central review. Secondary endpoints were duration of response, progression-free survival, overall survival, and safety.
There were 91 patients enrolled. Two patients never received EV treatment because of deterioration after registration. The median treatment duration among the remaining 89 patients was 6.0 months (range, 0.3-24.6).
Impressive results in poor-risk patients
The patients in EV-201 cohort 2 were elderly (median age, 75 years; range, 49-90) with comorbidities. The primary reasons for platinum-ineligibility were creatinine clearance less than 60 mL/min (66%), grade 2 or greater hearing loss (15%), and ECOG PS 2 (7%); 12% of patients met more than one criterion for platinum ineligibility.
The primary tumor site was in the upper urinary tract in 43% of patients, and 79% had visceral metastases, including 24% with liver involvement.
The confirmed ORR was 52% (95% confidence interval, 40.8-62.4), with 20% complete responses. There were responses in all subgroups, including patients with primary tumor sites in the upper tract (ORR, 61%), those with liver metastasis (ORR, 48%), and patients who had not responded to immune checkpoint inhibitors (ORR, 48%).
A total of 88% of patients had some decrease in measurable tumor diameters, generally within a few weeks of treatment initiation (median time to response, 1.8 months). The rapid response to treatment was especially important to patients having cancer-associated pain.
The median progression-free and overall survival durations were 5.8 months (95% CI, 5.0-8.3) and 14.7 months (95% CI, 10.5-18.2), respectively. The median response duration was 10.9 months (95% CI, 5.78-NR). More than 25% of responses extended beyond 12 months.
About 82% of patients in cohort 2 discontinued treatment, most commonly because of disease progression (51%). The second most common reason was the development of treatment-related adverse events (TRAE; 24%).
Drilling down on treatment-related adverse events
As might be expected for cisplatin-ineligible patients, adverse events were higher for patients in cohort 2 than for cohort 1 and led to treatment discontinuation in 16% of patients overall.
TRAEs over grade 3 occurred in 55% of patients. TRAEs of special interest included rash (61% overall; 17% ≥ grade 3), peripheral neuropathy (54% overall; 8% ≥ grade 3), and hyperglycemia (10% overall; 6% ≥ grade 3). Dose reductions, interruptions, and physical therapy were helpful.
Twenty percent of patients with TRAE hyperglycemia had hyperglycemia at baseline, and 30% of TRAEs were in patients with high body mass index (BMI).
There were four treatment-related deaths, all in patients 75 years or older with multiple comorbidities. Three of the four deaths occurred within 30 days of first EV dose in patients with BMI of 30 or greater (acute kidney injury, metabolic acidosis, and multiple organ dysfunction syndrome). The remaining death occurred more than 30 days after the last dose (pneumonitis).
Context and caution
The authors concluded that EV produced durable responses in platinum-ineligible patients with la/mUC, including 20% complete responses. Safety was felt to be as expected, given the known toxicities of the agent and the compromised medical condition of the patients studied.
The study discussant, Arlene O. Siefker-Radtke, MD, of the University of Texas MD Anderson Cancer Center, Houston, agreed that EV fills an unmet need, showing impressive responses in patients with visceral, liver, and bone metastases. She agreed that EV should be investigated across the spectrum of urothelial cancer.
Dr. Siefker-Radtke reminded attendees that the FDA package insert for EV described a 48% increase in the area under the concentration-time curve concentration of the MMAE active moiety in patients with mild hepatic impairment and that EV use should be avoided entirely in patients with moderate to severe liver disease.
She speculated whether augmented toxicity in patients with a high BMI could be attributable to clinically occult impaired hepatic function from fatty liver infiltration.
She indicated that clinicians should monitor closely patients with higher BMI and grade 3-4 hyperglycemia or elevated hemoglobin A1c levels and advised holding EV in patients who develop:
- Glucose levels above 250 mg/dL
- Peeling skin or bullous skin lesions. These lesions can be heralded by a diffuse erythematous or papillary rash in the preceding weeks.
- Grade 3 diarrhea or mucosal membrane toxicity of other types.
Notwithstanding concerns about toxicity and the need for monitoring, EV merits continued study in combination with other agents and in additional settings in the clinical spectrum of urothelial cancer. It is an important new option for oncologists caring for patients with urothelial cancer.
The EV-201 study was funded by Seagen. Dr. Balar and Dr. Siefker-Radtke disclosed relationships with Seagen and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Approximately half of all patients with locally advanced or metastatic urothelial cancer (la/mUC) are ineligible to receive cisplatin-based chemotherapy. They face poor outlooks and extremely limited treatment options.
A new study indicates that enfortumab vedotin (EV) can cause major, prolonged responses in most patients in that unfortunate setting.
EV is an antibody-drug conjugate directed against nectin-4, an immunoglobulin-like cell adhesion molecule that is highly expressed in UC, obviating the need for testing prior to treatment. It is internalized in malignant cells, with release of the active moiety (monomethyl auristatin E; MMAE). MMAE causes microtubule disruption, with resultant cell-cycle arrest and apoptosis.
EV received accelerated approval from the Food and Drug Administration in December 2019 after publication of the results from cohort 1 of the open-label, single-arm, phase 2 EV-201 study.
Arjun V. Balar, MD, of the Perlmutter Cancer Center at New York University Langone Health, presented results from cohort 2 of EV-201 – the cisplatin-ineligible cohort – at the 2021 Genitourinary Cancer Symposium (Abstract 394).
EV in patients ineligible for platinum-based therapy
Patients in cohort 2 of EV-201 had received immune checkpoint inhibitor therapy for la/mUC. They received EV in the FDA-approved dose for cohort 1: 1.25 mg/kg EV on days 1, 8, and 15 of a 28-day cycle.
Patients experienced disease progression during or following their most recent treatment. Patients with more than two neuropathies, active central nervous system metastases, and uncontrolled diabetes mellitus were excluded.
“Platinum ineligible” was defined as a creatinine clearance between 30-59 cm3/min, Eastern Cooperative Oncology Group performance status (ECOG PS) 2, or hearing loss of grade 2 or greater.
The primary endpoint for cohort 2 was confirmed overall response rate (ORR) per RECIST 1.1 by blinded independent central review. Secondary endpoints were duration of response, progression-free survival, overall survival, and safety.
There were 91 patients enrolled. Two patients never received EV treatment because of deterioration after registration. The median treatment duration among the remaining 89 patients was 6.0 months (range, 0.3-24.6).
Impressive results in poor-risk patients
The patients in EV-201 cohort 2 were elderly (median age, 75 years; range, 49-90) with comorbidities. The primary reasons for platinum-ineligibility were creatinine clearance less than 60 mL/min (66%), grade 2 or greater hearing loss (15%), and ECOG PS 2 (7%); 12% of patients met more than one criterion for platinum ineligibility.
The primary tumor site was in the upper urinary tract in 43% of patients, and 79% had visceral metastases, including 24% with liver involvement.
The confirmed ORR was 52% (95% confidence interval, 40.8-62.4), with 20% complete responses. There were responses in all subgroups, including patients with primary tumor sites in the upper tract (ORR, 61%), those with liver metastasis (ORR, 48%), and patients who had not responded to immune checkpoint inhibitors (ORR, 48%).
A total of 88% of patients had some decrease in measurable tumor diameters, generally within a few weeks of treatment initiation (median time to response, 1.8 months). The rapid response to treatment was especially important to patients having cancer-associated pain.
The median progression-free and overall survival durations were 5.8 months (95% CI, 5.0-8.3) and 14.7 months (95% CI, 10.5-18.2), respectively. The median response duration was 10.9 months (95% CI, 5.78-NR). More than 25% of responses extended beyond 12 months.
About 82% of patients in cohort 2 discontinued treatment, most commonly because of disease progression (51%). The second most common reason was the development of treatment-related adverse events (TRAE; 24%).
Drilling down on treatment-related adverse events
As might be expected for cisplatin-ineligible patients, adverse events were higher for patients in cohort 2 than for cohort 1 and led to treatment discontinuation in 16% of patients overall.
TRAEs over grade 3 occurred in 55% of patients. TRAEs of special interest included rash (61% overall; 17% ≥ grade 3), peripheral neuropathy (54% overall; 8% ≥ grade 3), and hyperglycemia (10% overall; 6% ≥ grade 3). Dose reductions, interruptions, and physical therapy were helpful.
Twenty percent of patients with TRAE hyperglycemia had hyperglycemia at baseline, and 30% of TRAEs were in patients with high body mass index (BMI).
There were four treatment-related deaths, all in patients 75 years or older with multiple comorbidities. Three of the four deaths occurred within 30 days of first EV dose in patients with BMI of 30 or greater (acute kidney injury, metabolic acidosis, and multiple organ dysfunction syndrome). The remaining death occurred more than 30 days after the last dose (pneumonitis).
Context and caution
The authors concluded that EV produced durable responses in platinum-ineligible patients with la/mUC, including 20% complete responses. Safety was felt to be as expected, given the known toxicities of the agent and the compromised medical condition of the patients studied.
The study discussant, Arlene O. Siefker-Radtke, MD, of the University of Texas MD Anderson Cancer Center, Houston, agreed that EV fills an unmet need, showing impressive responses in patients with visceral, liver, and bone metastases. She agreed that EV should be investigated across the spectrum of urothelial cancer.
Dr. Siefker-Radtke reminded attendees that the FDA package insert for EV described a 48% increase in the area under the concentration-time curve concentration of the MMAE active moiety in patients with mild hepatic impairment and that EV use should be avoided entirely in patients with moderate to severe liver disease.
She speculated whether augmented toxicity in patients with a high BMI could be attributable to clinically occult impaired hepatic function from fatty liver infiltration.
She indicated that clinicians should monitor closely patients with higher BMI and grade 3-4 hyperglycemia or elevated hemoglobin A1c levels and advised holding EV in patients who develop:
- Glucose levels above 250 mg/dL
- Peeling skin or bullous skin lesions. These lesions can be heralded by a diffuse erythematous or papillary rash in the preceding weeks.
- Grade 3 diarrhea or mucosal membrane toxicity of other types.
Notwithstanding concerns about toxicity and the need for monitoring, EV merits continued study in combination with other agents and in additional settings in the clinical spectrum of urothelial cancer. It is an important new option for oncologists caring for patients with urothelial cancer.
The EV-201 study was funded by Seagen. Dr. Balar and Dr. Siefker-Radtke disclosed relationships with Seagen and many other companies.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM GUCS 2021
Data on atopic dermatitis risk factors are accumulating
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
FROM REVOLUTIONIZING AD 2020
March 2021 - What's your diagnosis?
Answer: esophageal Crohn’s disease.
The esophageal biopsies demonstrate severe chronic inflammation of the subepithelial tissue with marked lymphocytic infiltration and the presence of granulomas containing multinucleate giant cells (Figure B, arrow). Given his immunosuppression with azathioprine, stains for cytomegalovirus, herpes simplex virus, and mycobacterial and fungal organisms were performed and returned negative.
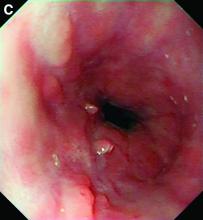
A diagnosis of esophageal Crohn’s disease was made, and adalimumab was recommenced. A rapid and dramatic clinical improvement was observed, with complete resolution of his symptoms. Adalimumab trough levels were checked and found to be therapeutic (9 mcg/mL). Repeat esophagogastroduodenoscopy at 6 months showed healing of the esophageal ulceration, with residual scarring and the presence of two postinflammatory polyps (Figure C). The histopathology was consistent with quiescent Crohn’s disease.
Recognition of this very rare manifestation of Crohn’s is challenging but important so that appropriate treatment is not delayed. It is both unexplained and unusual for Crohn’s disease to flare in a new gastrointestinal location. Moreover, although accurate adult prevalence data for esophageal Crohn’s are scarce, retrospective data suggest it is present in just 0.2% of Crohn’s disease patients.1 By contrast, gastroesophageal reflux disease prevalence is between 18% and 28% of the total population in North America. Esophageal Crohn’s commonly leads to nonspecific symptoms that resemble gastroesophageal reflux disease, and as for acid reflux, the mid and distal esophagus are the most common sites of involvement. In keeping with the behavior of luminal Crohn’s disease, progression from inflammation to stenosis (causing marked dysphagia) or perforation (leading to fistula formation) may occur.2 Histopathology typically demonstrates chronic inflammation, although noncaseating granulomas are seen in the minority (7%-39%) of patients.3 Multiple deep biopsies are recommended to improve diagnostic yield,3 and our case demonstrates the value of repeat endoscopic evaluation.
Unsurprisingly given its rarity, there are no systematic data on optimal treatment. Acid suppression therapy may provide symptomatic benefit but does not treat the underlying inflammatory process. Oral prednisolone, topical budesonide, and immunomodulators including thiopurines have been used in case series, but biological therapy (typically anti–tumor necrosis factor therapy) is likely to be required for severe disease.2,3 There are no data on the use of more novel biologics. Critically, almost all reported cases of esophageal Crohn’s disease have concomitant intestinal disease, and the presence of upper gastrointestinal Crohn’s predicts a more severe disease phenotype, supporting the use of more aggressive medical therapy in this instance.3
References
1. Decker GA et al. Inflamm Bowel Dis. 2001 May;7(2):113-9.
2. De Felice KM et al. Inflamm Bowel Dis. 2015 Sep;21(9):2106-13.
3. Laube R et al. J Gastroenterol Hepatol. 2018 Feb;33(2):355-64.
Answer: esophageal Crohn’s disease.
The esophageal biopsies demonstrate severe chronic inflammation of the subepithelial tissue with marked lymphocytic infiltration and the presence of granulomas containing multinucleate giant cells (Figure B, arrow). Given his immunosuppression with azathioprine, stains for cytomegalovirus, herpes simplex virus, and mycobacterial and fungal organisms were performed and returned negative.
A diagnosis of esophageal Crohn’s disease was made, and adalimumab was recommenced. A rapid and dramatic clinical improvement was observed, with complete resolution of his symptoms. Adalimumab trough levels were checked and found to be therapeutic (9 mcg/mL). Repeat esophagogastroduodenoscopy at 6 months showed healing of the esophageal ulceration, with residual scarring and the presence of two postinflammatory polyps (Figure C). The histopathology was consistent with quiescent Crohn’s disease.
Recognition of this very rare manifestation of Crohn’s is challenging but important so that appropriate treatment is not delayed. It is both unexplained and unusual for Crohn’s disease to flare in a new gastrointestinal location. Moreover, although accurate adult prevalence data for esophageal Crohn’s are scarce, retrospective data suggest it is present in just 0.2% of Crohn’s disease patients.1 By contrast, gastroesophageal reflux disease prevalence is between 18% and 28% of the total population in North America. Esophageal Crohn’s commonly leads to nonspecific symptoms that resemble gastroesophageal reflux disease, and as for acid reflux, the mid and distal esophagus are the most common sites of involvement. In keeping with the behavior of luminal Crohn’s disease, progression from inflammation to stenosis (causing marked dysphagia) or perforation (leading to fistula formation) may occur.2 Histopathology typically demonstrates chronic inflammation, although noncaseating granulomas are seen in the minority (7%-39%) of patients.3 Multiple deep biopsies are recommended to improve diagnostic yield,3 and our case demonstrates the value of repeat endoscopic evaluation.
Unsurprisingly given its rarity, there are no systematic data on optimal treatment. Acid suppression therapy may provide symptomatic benefit but does not treat the underlying inflammatory process. Oral prednisolone, topical budesonide, and immunomodulators including thiopurines have been used in case series, but biological therapy (typically anti–tumor necrosis factor therapy) is likely to be required for severe disease.2,3 There are no data on the use of more novel biologics. Critically, almost all reported cases of esophageal Crohn’s disease have concomitant intestinal disease, and the presence of upper gastrointestinal Crohn’s predicts a more severe disease phenotype, supporting the use of more aggressive medical therapy in this instance.3
References
1. Decker GA et al. Inflamm Bowel Dis. 2001 May;7(2):113-9.
2. De Felice KM et al. Inflamm Bowel Dis. 2015 Sep;21(9):2106-13.
3. Laube R et al. J Gastroenterol Hepatol. 2018 Feb;33(2):355-64.
Answer: esophageal Crohn’s disease.
The esophageal biopsies demonstrate severe chronic inflammation of the subepithelial tissue with marked lymphocytic infiltration and the presence of granulomas containing multinucleate giant cells (Figure B, arrow). Given his immunosuppression with azathioprine, stains for cytomegalovirus, herpes simplex virus, and mycobacterial and fungal organisms were performed and returned negative.
A diagnosis of esophageal Crohn’s disease was made, and adalimumab was recommenced. A rapid and dramatic clinical improvement was observed, with complete resolution of his symptoms. Adalimumab trough levels were checked and found to be therapeutic (9 mcg/mL). Repeat esophagogastroduodenoscopy at 6 months showed healing of the esophageal ulceration, with residual scarring and the presence of two postinflammatory polyps (Figure C). The histopathology was consistent with quiescent Crohn’s disease.
Recognition of this very rare manifestation of Crohn’s is challenging but important so that appropriate treatment is not delayed. It is both unexplained and unusual for Crohn’s disease to flare in a new gastrointestinal location. Moreover, although accurate adult prevalence data for esophageal Crohn’s are scarce, retrospective data suggest it is present in just 0.2% of Crohn’s disease patients.1 By contrast, gastroesophageal reflux disease prevalence is between 18% and 28% of the total population in North America. Esophageal Crohn’s commonly leads to nonspecific symptoms that resemble gastroesophageal reflux disease, and as for acid reflux, the mid and distal esophagus are the most common sites of involvement. In keeping with the behavior of luminal Crohn’s disease, progression from inflammation to stenosis (causing marked dysphagia) or perforation (leading to fistula formation) may occur.2 Histopathology typically demonstrates chronic inflammation, although noncaseating granulomas are seen in the minority (7%-39%) of patients.3 Multiple deep biopsies are recommended to improve diagnostic yield,3 and our case demonstrates the value of repeat endoscopic evaluation.
Unsurprisingly given its rarity, there are no systematic data on optimal treatment. Acid suppression therapy may provide symptomatic benefit but does not treat the underlying inflammatory process. Oral prednisolone, topical budesonide, and immunomodulators including thiopurines have been used in case series, but biological therapy (typically anti–tumor necrosis factor therapy) is likely to be required for severe disease.2,3 There are no data on the use of more novel biologics. Critically, almost all reported cases of esophageal Crohn’s disease have concomitant intestinal disease, and the presence of upper gastrointestinal Crohn’s predicts a more severe disease phenotype, supporting the use of more aggressive medical therapy in this instance.3
References
1. Decker GA et al. Inflamm Bowel Dis. 2001 May;7(2):113-9.
2. De Felice KM et al. Inflamm Bowel Dis. 2015 Sep;21(9):2106-13.
3. Laube R et al. J Gastroenterol Hepatol. 2018 Feb;33(2):355-64.
A 49-year-old man presented with symptoms of retrosternal discomfort and mild dysphagia to solids. He had a 30-year history of ileocolonic Crohn's disease requiring previous resections of the ileum and sigmoid colon. Clinical remission had been achieved with adalimumab and azathioprine combination therapy, with the subsequent decision to de-escalate to maintenance with azathioprine monotherapy after consideration of the risks and benefits of dual immunosuppression. After 5 years of azathioprine monotherapy, complete endoscopic remission was reconfirmed at a recent ileocolonoscopy.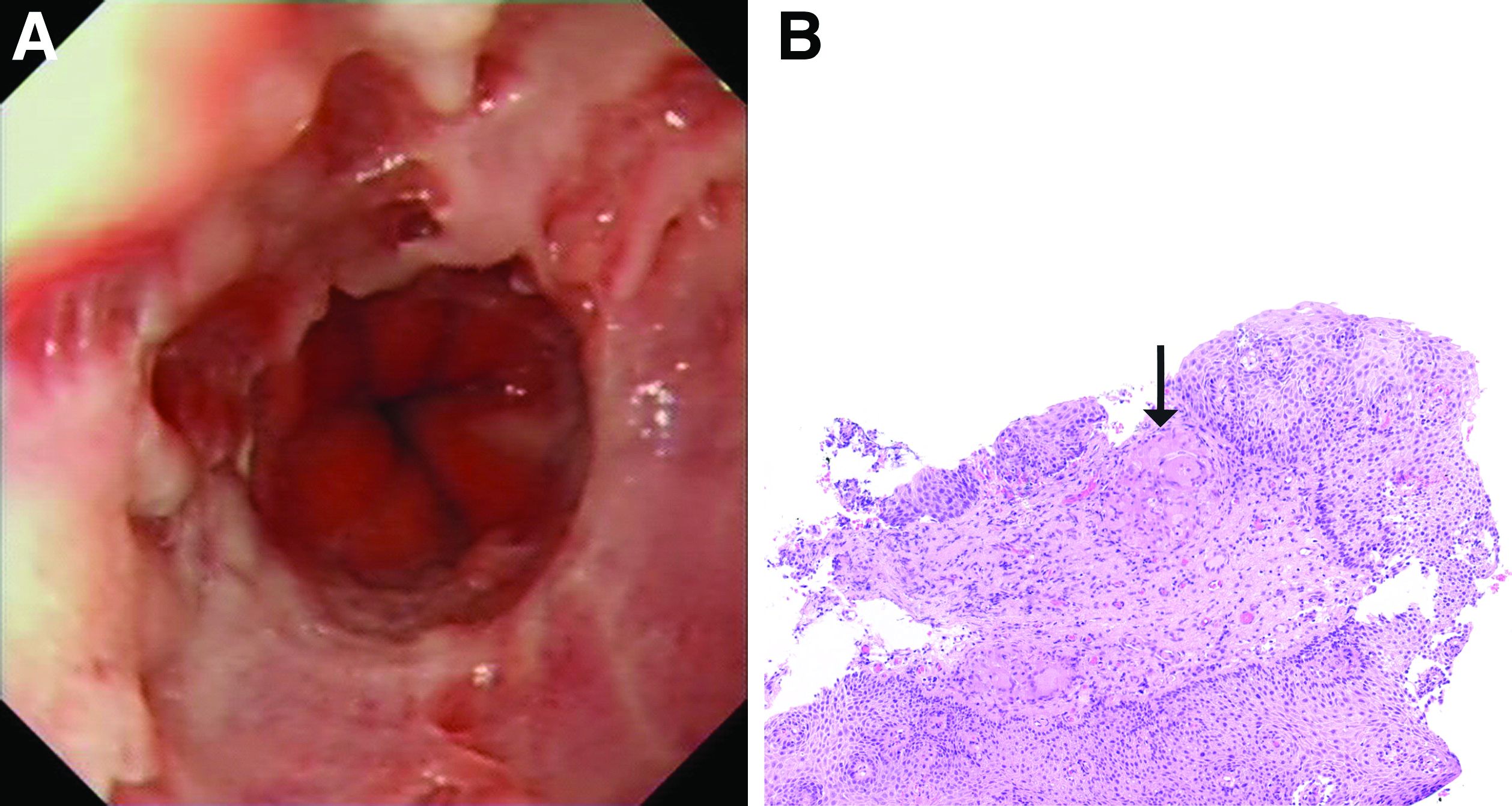
To investigate his upper gastrointestinal symptoms he underwent esophagogastroduodenoscopy that demonstrated severe esophagitis (Los Angeles grade D) of the lower esophagus with biopsies confirming apparent reflux esophagitis. However, his symptoms worsened despite a course of high dose proton pump inhibitor, and a repeat esophagogastroduodenoscopy was performed. This demonstrated deep longitudinal ulcers and inflammation of the lower two-thirds of the esophagus (Figure A). Biopsies were sent for histopathologic analysis (Figure B).
OA risk-reduction program targets injured knees
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
A novel educational and personalized physical therapy program is showing signs that it may help people to mitigate their risk of developing knee osteoarthritis after an injury.
Speaking at the Canadian Arthritis Research Conference: Research with Impact, Jackie Whittaker, PhD, observed that initial work from the Stop Osteoarthritis (SOAR) program showed that meaningful improvements in knee-related quality of life and improvement in participants’ perceived self-management could be achieved.
Further feasibility work is ongoing and a proof-of-concept and phase 3 study need to follow, but the research suggests the approach could potentially help to reduce the substantial burden of managing people who develop posttraumatic OA (PTOA) of the knee.
Understanding the post–knee injury period
“Despite the progress that we’ve made in preventing injuries, and reducing disability in people with osteoarthritis, we lack good evidence about what should be done in the period between joint injury and the onset of osteoarthritis to delay or halt that onset,” Dr. Whittaker said at the virtual meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis.
That’s where the SOAR program comes in. For the past 8 years, Dr. Whittaker, an assistant professor in the department of physical therapy at the University of British Columbia in Vancouver and affiliated to Arthritis Research Canada, and collaborators have been looking into the post–knee injury period with the aim of developing an intervention that could potentially reduce the risk of OA further down the line.
Much work has gone into understanding the burden and risk factors for PTOA of the knee in order to know who exactly to target with the intervention and what the risk factors may be for the subsequent development of OA .
This research suggests that knee injuries are most commonly seen in people aged between 15 and 35 years who participated in sporting or other physical activities, so this is the target population for the SOAR intervention.
Broadly speaking, sustaining any knee injury is associated with a sixfold increased risk for subsequent PTOA, Dr. Whittaker observed.
“Despite the fact that ACL [anterior cruciate ligament] and meniscal tears get all the press, collateral ligament injury are still associated with about a fivefold increased risk of osteoarthritis, and therefore maybe shouldn’t be so easily dismissed as an important target,” Dr. Whittaker said.
Postinjury risk factors for OA
“Basically, what all prevention comes down to is our understanding of risk factors and our ability to be able to modify them,” she said.
Previous joint injury is one of the strongest and most established modifiable risk factors for developing knee OA, and Dr. Whittaker and associates have performed two small but “mighty” cohort studies comparing people who have and have not had a knee injury. These two studies have looked at different time periods following injury to see if they could first identify the risk factors for developing OA some 3-10 years later, and then to look more closely at some of those risk factors in first 2 years after injury with a view to targeting these with an intervention.
Data analysis of the latter study is still ongoing but have shown that, among injured subjects, there is a fear of movement and reinjury, knee strength is weaker in both injured and uninjured knees, and they are perhaps less physically active than those who have not been injured.
“Going into those two studies, we knew that this group of people already [had an] increased risk for osteoarthritis because they had an injury. However, what we found is that it looks like this risk may be compounded through adiposity [and] deficits in muscle strength and physical inactivity, which are associated with pain, stiffness, lack of confidence, and at times, unrealistic expectations and poor pacing,” Dr. Whittaker said.
She added: “It also looks like some of these additional factors and particular adiposity or fat gain may develop after injury, which would then give us a concrete target for delaying or halting the onset of osteoarthritis in the segment of the population.”
SOAR program components
The SOAR program intervention is an 8-week, physiotherapist-led program that targets people aged 15-35 years who have had a sport-related knee injury and received formal care. All of this is conducted via videoconferencing software and starts off with a 2-hour group education session or “knee camp.” This is followed by a one-on-one assessment with a physiotherapist and setting exercise and physical activity goals for the week.
Participants then undertake their personalized exercise and physical activity programs at home and track their progress using an activity monitor. They can participate in an optional weekly group exercise class and receive weekly one-on-one physiotherapy counseling where goals can be modified and any issues participants might be experiencing solved.
According to Dr. Whittaker, “this program really aims to increase participants capacity to manage their elevated risk for osteoarthritis, and we’re doing this by also optimizing their knee muscle function and their physical activity participation.”
While the knee camp enables a therapeutic alliance to be formed between participants and their physiotherapists, the weekly group classes provide social support and an opportunity to interact with others.
“Brief action planning builds self-efficacy [and] promotes autonomous health behaviors, while goal setting and tracking provide accountability, feedback about progress, and facilitated adherence,” she said.
And finally, regular communication with a physiotherapist in the program ensures timely support to learn how to navigate obstacles and helps participants to learn how to deal with their own knee health.
Testing the feasibility of the SOAR program intervention
“Currently we are smack in the middle of our feasibility study,” Dr. Whittaker said. So far, four physiotherapists have been trained to deliver an abridged, 4-week version of the program, and 25 of a planned 30 participants have been enrolled.
Results seem promising so far. No participants have dropped out of the program to date and attendance is at 100%.
“Based on data from the first 12 participants who completed the program, we are meeting all of our ‘a priori’ program benchmarks,” Dr. Whittaker said.
“It is very early days,” she emphasized, but “we are excited to see clinically important improvements in both knee-related quality of life and perceived self-management.
“This gives us some confidence that maybe all this time that we’ve put into developing our intervention is paying off, but obviously time will tell if we’re headed in the right direction,” she said. “Perhaps in time, we may be able to look at whether or not the individuals that participated in that program have fewer symptoms of OA disease. But that will obviously take us a few years before we’ll be able to get to that point.”
Dr. Whittaker acknowledged receiving funding for the SOAR program from the Arthritis Society, the Michael Smith Foundation for Health Research, BC SUPPORT Unit, and the Canadian Musculoskeletal Rehab Network.
FROM CARC 2021
AI detects ugly-duckling skin lesions for melanoma follow-up
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
Variant found in NYC, Northeast
, according to CNN.
The variant, called B.1.526, has appeared in diverse neighborhoods in New York City and is “scattered in the Northeast,” the researchers said.
“We observed a steady increase in the detection rate from late December to mid-February, with an alarming rise to 12.7% in the past two weeks,” researchers from Columbia University Medical Center wrote in a report, which was published as a preprint Feb. 25.
On Feb. 22, the team released another preprint about the B.1.1.7 and B.1.351 variants first identified in the United Kingdom and South Africa, respectively, which also mentions the B.1.526 variant in the U.S. Neither report has been peer reviewed.
Viruses mutate often, and several coronavirus variants have been identified and followed during the pandemic. Not all mutations are significant or are necessarily more contagious or dangerous. Researchers have been tracking the B.1.526 variant in the U.S. to find out if there are significant mutations that could be a cause for concern.
In the most recent preprints, the variant appears to have the same mutation found in B.1.351, called E484K, which may allow the virus to evade vaccines and the body’s natural immune response. The E484K mutation has shown up in at least 59 lines of the coronavirus, the research team said. That means the virus is evolving independently across the country and world, which could give the virus an advantage.
“A concern is that it might be beginning to overtake other strains, just like the U.K. and South African variants,” David Ho, MD, the lead study author and director of the Aaron Diamond AIDS Research Center at Columbia, told CNN.
“However, we don’t have enough data to firm up this point now,” he said.
In a separate preprint posted Feb. 23, a research team at the California Institute of Technology developed a software tool that noticed the rise of B.1.526 in the New York region. The preprint hasn’t yet been peer reviewed.
“It appears that the frequency of lineage B.1.526 has increased rapidly in New York,” they wrote.
Both teams also reported on another variant, called B.1.427/B.1.429, which appears to be increasing in California. The variant could be more contagious and cause more severe disease, they said, but the research is still in the early stages.
Researchers at the University of California, San Francisco, have tested virus samples from recent outbreaks in California and also found that the variant is becoming more common. The variant didn’t appear in samples from September but was in half of the samples by late January. It has a different pattern of mutations than other variants, and one called L452R may affect the spike protein on the virus and allow it attach to cells more easily.
“Our data shows that this is likely the key mutation that makes this variant more infectious,” Charles Chiu, MD, associate director of the clinical microbiology lab at UCSF, told CNN.
The team also noticed that patients with a B.1.427/B.1.429 infection had more severe COVID-19 cases and needed more oxygen, CNN reported. The team plans to post a preprint once public health officials in San Francisco review the report.
Right now, the CDC provides public data for three variants: B.1.1.7, B.1.351, and P.1, which was first identified in Brazil. The U.S. has reported 1,881 B.1.1.7 cases across 45 states, 46 B.1.351 cases in 14 states, and five P.1 cases in four states, according to a CDC tally as of Feb. 23.
At the moment, lab officials aren’t able to tell patients or doctors whether someone has been infected by a variant, according to Kaiser Health News. High-level labs conduct genomic sequencing on samples and aren’t able to communicate information back to individual people.
But the Association of Public Health Laboratories and public health officials in several states are pushing for federal authorization of a test that could sequence the full genome and notify doctors. The test could be available in coming weeks, the news outlet reported.
A version of this article first appeared on WebMD.com.
, according to CNN.
The variant, called B.1.526, has appeared in diverse neighborhoods in New York City and is “scattered in the Northeast,” the researchers said.
“We observed a steady increase in the detection rate from late December to mid-February, with an alarming rise to 12.7% in the past two weeks,” researchers from Columbia University Medical Center wrote in a report, which was published as a preprint Feb. 25.
On Feb. 22, the team released another preprint about the B.1.1.7 and B.1.351 variants first identified in the United Kingdom and South Africa, respectively, which also mentions the B.1.526 variant in the U.S. Neither report has been peer reviewed.
Viruses mutate often, and several coronavirus variants have been identified and followed during the pandemic. Not all mutations are significant or are necessarily more contagious or dangerous. Researchers have been tracking the B.1.526 variant in the U.S. to find out if there are significant mutations that could be a cause for concern.
In the most recent preprints, the variant appears to have the same mutation found in B.1.351, called E484K, which may allow the virus to evade vaccines and the body’s natural immune response. The E484K mutation has shown up in at least 59 lines of the coronavirus, the research team said. That means the virus is evolving independently across the country and world, which could give the virus an advantage.
“A concern is that it might be beginning to overtake other strains, just like the U.K. and South African variants,” David Ho, MD, the lead study author and director of the Aaron Diamond AIDS Research Center at Columbia, told CNN.
“However, we don’t have enough data to firm up this point now,” he said.
In a separate preprint posted Feb. 23, a research team at the California Institute of Technology developed a software tool that noticed the rise of B.1.526 in the New York region. The preprint hasn’t yet been peer reviewed.
“It appears that the frequency of lineage B.1.526 has increased rapidly in New York,” they wrote.
Both teams also reported on another variant, called B.1.427/B.1.429, which appears to be increasing in California. The variant could be more contagious and cause more severe disease, they said, but the research is still in the early stages.
Researchers at the University of California, San Francisco, have tested virus samples from recent outbreaks in California and also found that the variant is becoming more common. The variant didn’t appear in samples from September but was in half of the samples by late January. It has a different pattern of mutations than other variants, and one called L452R may affect the spike protein on the virus and allow it attach to cells more easily.
“Our data shows that this is likely the key mutation that makes this variant more infectious,” Charles Chiu, MD, associate director of the clinical microbiology lab at UCSF, told CNN.
The team also noticed that patients with a B.1.427/B.1.429 infection had more severe COVID-19 cases and needed more oxygen, CNN reported. The team plans to post a preprint once public health officials in San Francisco review the report.
Right now, the CDC provides public data for three variants: B.1.1.7, B.1.351, and P.1, which was first identified in Brazil. The U.S. has reported 1,881 B.1.1.7 cases across 45 states, 46 B.1.351 cases in 14 states, and five P.1 cases in four states, according to a CDC tally as of Feb. 23.
At the moment, lab officials aren’t able to tell patients or doctors whether someone has been infected by a variant, according to Kaiser Health News. High-level labs conduct genomic sequencing on samples and aren’t able to communicate information back to individual people.
But the Association of Public Health Laboratories and public health officials in several states are pushing for federal authorization of a test that could sequence the full genome and notify doctors. The test could be available in coming weeks, the news outlet reported.
A version of this article first appeared on WebMD.com.
, according to CNN.
The variant, called B.1.526, has appeared in diverse neighborhoods in New York City and is “scattered in the Northeast,” the researchers said.
“We observed a steady increase in the detection rate from late December to mid-February, with an alarming rise to 12.7% in the past two weeks,” researchers from Columbia University Medical Center wrote in a report, which was published as a preprint Feb. 25.
On Feb. 22, the team released another preprint about the B.1.1.7 and B.1.351 variants first identified in the United Kingdom and South Africa, respectively, which also mentions the B.1.526 variant in the U.S. Neither report has been peer reviewed.
Viruses mutate often, and several coronavirus variants have been identified and followed during the pandemic. Not all mutations are significant or are necessarily more contagious or dangerous. Researchers have been tracking the B.1.526 variant in the U.S. to find out if there are significant mutations that could be a cause for concern.
In the most recent preprints, the variant appears to have the same mutation found in B.1.351, called E484K, which may allow the virus to evade vaccines and the body’s natural immune response. The E484K mutation has shown up in at least 59 lines of the coronavirus, the research team said. That means the virus is evolving independently across the country and world, which could give the virus an advantage.
“A concern is that it might be beginning to overtake other strains, just like the U.K. and South African variants,” David Ho, MD, the lead study author and director of the Aaron Diamond AIDS Research Center at Columbia, told CNN.
“However, we don’t have enough data to firm up this point now,” he said.
In a separate preprint posted Feb. 23, a research team at the California Institute of Technology developed a software tool that noticed the rise of B.1.526 in the New York region. The preprint hasn’t yet been peer reviewed.
“It appears that the frequency of lineage B.1.526 has increased rapidly in New York,” they wrote.
Both teams also reported on another variant, called B.1.427/B.1.429, which appears to be increasing in California. The variant could be more contagious and cause more severe disease, they said, but the research is still in the early stages.
Researchers at the University of California, San Francisco, have tested virus samples from recent outbreaks in California and also found that the variant is becoming more common. The variant didn’t appear in samples from September but was in half of the samples by late January. It has a different pattern of mutations than other variants, and one called L452R may affect the spike protein on the virus and allow it attach to cells more easily.
“Our data shows that this is likely the key mutation that makes this variant more infectious,” Charles Chiu, MD, associate director of the clinical microbiology lab at UCSF, told CNN.
The team also noticed that patients with a B.1.427/B.1.429 infection had more severe COVID-19 cases and needed more oxygen, CNN reported. The team plans to post a preprint once public health officials in San Francisco review the report.
Right now, the CDC provides public data for three variants: B.1.1.7, B.1.351, and P.1, which was first identified in Brazil. The U.S. has reported 1,881 B.1.1.7 cases across 45 states, 46 B.1.351 cases in 14 states, and five P.1 cases in four states, according to a CDC tally as of Feb. 23.
At the moment, lab officials aren’t able to tell patients or doctors whether someone has been infected by a variant, according to Kaiser Health News. High-level labs conduct genomic sequencing on samples and aren’t able to communicate information back to individual people.
But the Association of Public Health Laboratories and public health officials in several states are pushing for federal authorization of a test that could sequence the full genome and notify doctors. The test could be available in coming weeks, the news outlet reported.
A version of this article first appeared on WebMD.com.
Researchers identify four small cell lung cancer subtypes and their best therapies
Researchers studying a large set of small cell lung cancer (SCLC) tumor samples have identified four SCLC subtypes, and they propose that matching baseline tumor subtypes to SCLC therapy may enhance the depth and duration of response.
Carl M. Gay, MD, PhD, of University of Texas MD Anderson Cancer Center in Houston, and colleagues conducted this research and described their findings in Cancer Cell.
The authors noted that survival rates in SCLC remain dismal despite recent modest gains in progression-free survival and overall survival achieved through adding immunotherapy to platinum-based frontline chemotherapy.
Based on transcription factors indicating which genes are activated, prior research had already identified three possible SCLC subtypes. Many SCLC tumors, however, do not fit into one of these three groups, the authors said.
Inflamed gene signature
The four groups were identified using tumor expression data and nonnegative matrix factorization from published sources on 81 SCLC patients, and then validated via the largest SCLC data set available (276 SCLC patients enrolled in the phase 3 IMpower133 trial).
The SCLC subtypes were defined largely by differential expression of transcription factors – subtype SCLC-A by ASCL1, subtype SCLC-N by NEUROD1, and subtype SCLC-P by POU2F3. The fourth subtype, SCLC-I, is characterized by low expression of all three transcription factor signatures and an inflamed gene signature with a high expression of multiple immune genes, including significantly greater levels of genes indicating the presence of CD8-positive cytotoxic T cells.
Because each subtype demonstrates unique vulnerability to investigational therapies, this subtype classification has significant clinical implications.
“We propose that matching baseline tumor subtype to therapy, as well as manipulating subtype switching on therapy, may enhance depth and duration of response for SCLC patients,” the authors stated.
“Our paper shows that the inflamed group has a distinct biology and environment and tends to be more responsive to immunotherapy,” study author Lauren Averett Byers, MD, also of the University of Texas MD Anderson Cancer Center, stated in a press release. “Identifying the inflamed group is very important because, so far, there have not been any validated biomarkers for small cell lung cancer that predict which patients get the most benefit from immunotherapy.”
In samples from the other three subtypes, SCLC-A was most responsive to BCL2 inhibitors, SCLC-N to Aurora kinase inhibitors, and SCLC-P to PARP inhibitors.
Treatment resistance
The tendency of SCLC to develop treatment resistance, even after an initial response, is a known challenge. Using single-cell RNA sequencing to evaluate tumor evolution, the authors observed a tendency of SCLC-A to switch to SCLC-I after chemotherapy treatment, a possible contributor to treatment resistance.
It will be necessary to verify the study findings through further investigations, particularly regarding the therapeutic vulnerabilities for each group.
“Now we can develop more effective strategies for each group in clinical trials, taking into account that they each have different biology and optimal drug targets,” Dr. Byers said. “As a field, small cell lung cancer is about 15 years behind non–small cell lung cancer’s renaissance of biomarkers and personalized therapies. This represents a huge step in understanding which drugs work best for which patients and gives us a path forward for personalized approaches for small cell lung cancer.”
“Dr. Gay’s work is the latest in a growing series of exciting studies demonstrating the utility of defining subtypes of small cell lung cancer based on expression of master transcriptional regulators,” commented Charles Rudin, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, in an interview.
He added, “While tumors can evolve between some of these categories, the dominant subtype assignment influences therapeutic vulnerabilities. It is an exciting time for those of us engaged in small cell research. Subtyping should help guide more focused and successful clinical trials for patients with small cell lung cancer.”
The authors disclosed multiple relationships with companies. The study was supported by the National Institutes of Health/National Cancer Institute, the University of Texas Southwestern and MD Anderson Cancer Center, and a variety of other governmental and nonprofit groups. Dr. Rudin is principal investigator of the NCI small cell lung cancer research consortium.
Researchers studying a large set of small cell lung cancer (SCLC) tumor samples have identified four SCLC subtypes, and they propose that matching baseline tumor subtypes to SCLC therapy may enhance the depth and duration of response.
Carl M. Gay, MD, PhD, of University of Texas MD Anderson Cancer Center in Houston, and colleagues conducted this research and described their findings in Cancer Cell.
The authors noted that survival rates in SCLC remain dismal despite recent modest gains in progression-free survival and overall survival achieved through adding immunotherapy to platinum-based frontline chemotherapy.
Based on transcription factors indicating which genes are activated, prior research had already identified three possible SCLC subtypes. Many SCLC tumors, however, do not fit into one of these three groups, the authors said.
Inflamed gene signature
The four groups were identified using tumor expression data and nonnegative matrix factorization from published sources on 81 SCLC patients, and then validated via the largest SCLC data set available (276 SCLC patients enrolled in the phase 3 IMpower133 trial).
The SCLC subtypes were defined largely by differential expression of transcription factors – subtype SCLC-A by ASCL1, subtype SCLC-N by NEUROD1, and subtype SCLC-P by POU2F3. The fourth subtype, SCLC-I, is characterized by low expression of all three transcription factor signatures and an inflamed gene signature with a high expression of multiple immune genes, including significantly greater levels of genes indicating the presence of CD8-positive cytotoxic T cells.
Because each subtype demonstrates unique vulnerability to investigational therapies, this subtype classification has significant clinical implications.
“We propose that matching baseline tumor subtype to therapy, as well as manipulating subtype switching on therapy, may enhance depth and duration of response for SCLC patients,” the authors stated.
“Our paper shows that the inflamed group has a distinct biology and environment and tends to be more responsive to immunotherapy,” study author Lauren Averett Byers, MD, also of the University of Texas MD Anderson Cancer Center, stated in a press release. “Identifying the inflamed group is very important because, so far, there have not been any validated biomarkers for small cell lung cancer that predict which patients get the most benefit from immunotherapy.”
In samples from the other three subtypes, SCLC-A was most responsive to BCL2 inhibitors, SCLC-N to Aurora kinase inhibitors, and SCLC-P to PARP inhibitors.
Treatment resistance
The tendency of SCLC to develop treatment resistance, even after an initial response, is a known challenge. Using single-cell RNA sequencing to evaluate tumor evolution, the authors observed a tendency of SCLC-A to switch to SCLC-I after chemotherapy treatment, a possible contributor to treatment resistance.
It will be necessary to verify the study findings through further investigations, particularly regarding the therapeutic vulnerabilities for each group.
“Now we can develop more effective strategies for each group in clinical trials, taking into account that they each have different biology and optimal drug targets,” Dr. Byers said. “As a field, small cell lung cancer is about 15 years behind non–small cell lung cancer’s renaissance of biomarkers and personalized therapies. This represents a huge step in understanding which drugs work best for which patients and gives us a path forward for personalized approaches for small cell lung cancer.”
“Dr. Gay’s work is the latest in a growing series of exciting studies demonstrating the utility of defining subtypes of small cell lung cancer based on expression of master transcriptional regulators,” commented Charles Rudin, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, in an interview.
He added, “While tumors can evolve between some of these categories, the dominant subtype assignment influences therapeutic vulnerabilities. It is an exciting time for those of us engaged in small cell research. Subtyping should help guide more focused and successful clinical trials for patients with small cell lung cancer.”
The authors disclosed multiple relationships with companies. The study was supported by the National Institutes of Health/National Cancer Institute, the University of Texas Southwestern and MD Anderson Cancer Center, and a variety of other governmental and nonprofit groups. Dr. Rudin is principal investigator of the NCI small cell lung cancer research consortium.
Researchers studying a large set of small cell lung cancer (SCLC) tumor samples have identified four SCLC subtypes, and they propose that matching baseline tumor subtypes to SCLC therapy may enhance the depth and duration of response.
Carl M. Gay, MD, PhD, of University of Texas MD Anderson Cancer Center in Houston, and colleagues conducted this research and described their findings in Cancer Cell.
The authors noted that survival rates in SCLC remain dismal despite recent modest gains in progression-free survival and overall survival achieved through adding immunotherapy to platinum-based frontline chemotherapy.
Based on transcription factors indicating which genes are activated, prior research had already identified three possible SCLC subtypes. Many SCLC tumors, however, do not fit into one of these three groups, the authors said.
Inflamed gene signature
The four groups were identified using tumor expression data and nonnegative matrix factorization from published sources on 81 SCLC patients, and then validated via the largest SCLC data set available (276 SCLC patients enrolled in the phase 3 IMpower133 trial).
The SCLC subtypes were defined largely by differential expression of transcription factors – subtype SCLC-A by ASCL1, subtype SCLC-N by NEUROD1, and subtype SCLC-P by POU2F3. The fourth subtype, SCLC-I, is characterized by low expression of all three transcription factor signatures and an inflamed gene signature with a high expression of multiple immune genes, including significantly greater levels of genes indicating the presence of CD8-positive cytotoxic T cells.
Because each subtype demonstrates unique vulnerability to investigational therapies, this subtype classification has significant clinical implications.
“We propose that matching baseline tumor subtype to therapy, as well as manipulating subtype switching on therapy, may enhance depth and duration of response for SCLC patients,” the authors stated.
“Our paper shows that the inflamed group has a distinct biology and environment and tends to be more responsive to immunotherapy,” study author Lauren Averett Byers, MD, also of the University of Texas MD Anderson Cancer Center, stated in a press release. “Identifying the inflamed group is very important because, so far, there have not been any validated biomarkers for small cell lung cancer that predict which patients get the most benefit from immunotherapy.”
In samples from the other three subtypes, SCLC-A was most responsive to BCL2 inhibitors, SCLC-N to Aurora kinase inhibitors, and SCLC-P to PARP inhibitors.
Treatment resistance
The tendency of SCLC to develop treatment resistance, even after an initial response, is a known challenge. Using single-cell RNA sequencing to evaluate tumor evolution, the authors observed a tendency of SCLC-A to switch to SCLC-I after chemotherapy treatment, a possible contributor to treatment resistance.
It will be necessary to verify the study findings through further investigations, particularly regarding the therapeutic vulnerabilities for each group.
“Now we can develop more effective strategies for each group in clinical trials, taking into account that they each have different biology and optimal drug targets,” Dr. Byers said. “As a field, small cell lung cancer is about 15 years behind non–small cell lung cancer’s renaissance of biomarkers and personalized therapies. This represents a huge step in understanding which drugs work best for which patients and gives us a path forward for personalized approaches for small cell lung cancer.”
“Dr. Gay’s work is the latest in a growing series of exciting studies demonstrating the utility of defining subtypes of small cell lung cancer based on expression of master transcriptional regulators,” commented Charles Rudin, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, in an interview.
He added, “While tumors can evolve between some of these categories, the dominant subtype assignment influences therapeutic vulnerabilities. It is an exciting time for those of us engaged in small cell research. Subtyping should help guide more focused and successful clinical trials for patients with small cell lung cancer.”
The authors disclosed multiple relationships with companies. The study was supported by the National Institutes of Health/National Cancer Institute, the University of Texas Southwestern and MD Anderson Cancer Center, and a variety of other governmental and nonprofit groups. Dr. Rudin is principal investigator of the NCI small cell lung cancer research consortium.
FROM CANCER CELL
How to convince patients muscle pain isn’t a statin Achilles heel: StatinWISE
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.