User login
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis in Pediatric Patients
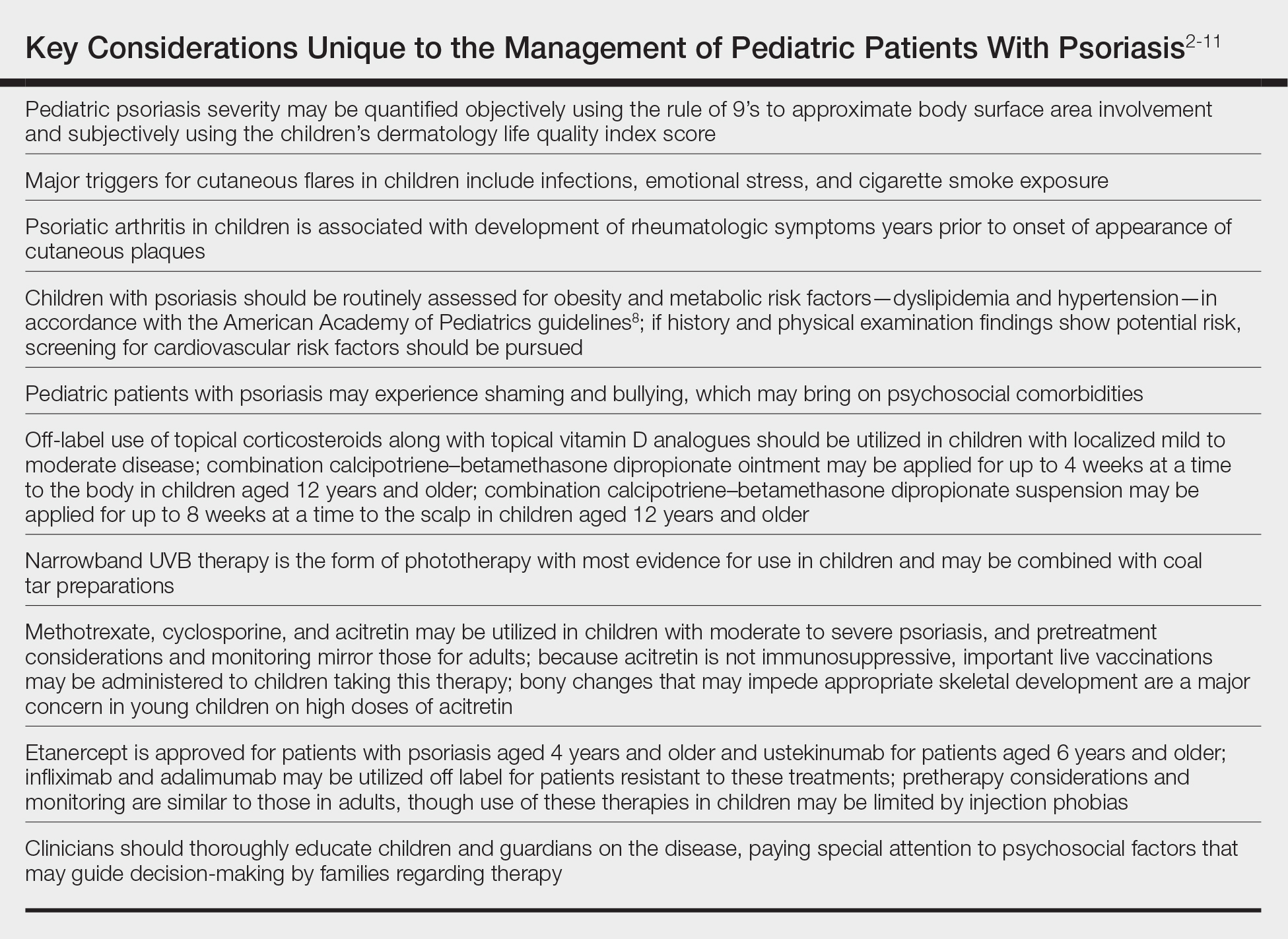
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
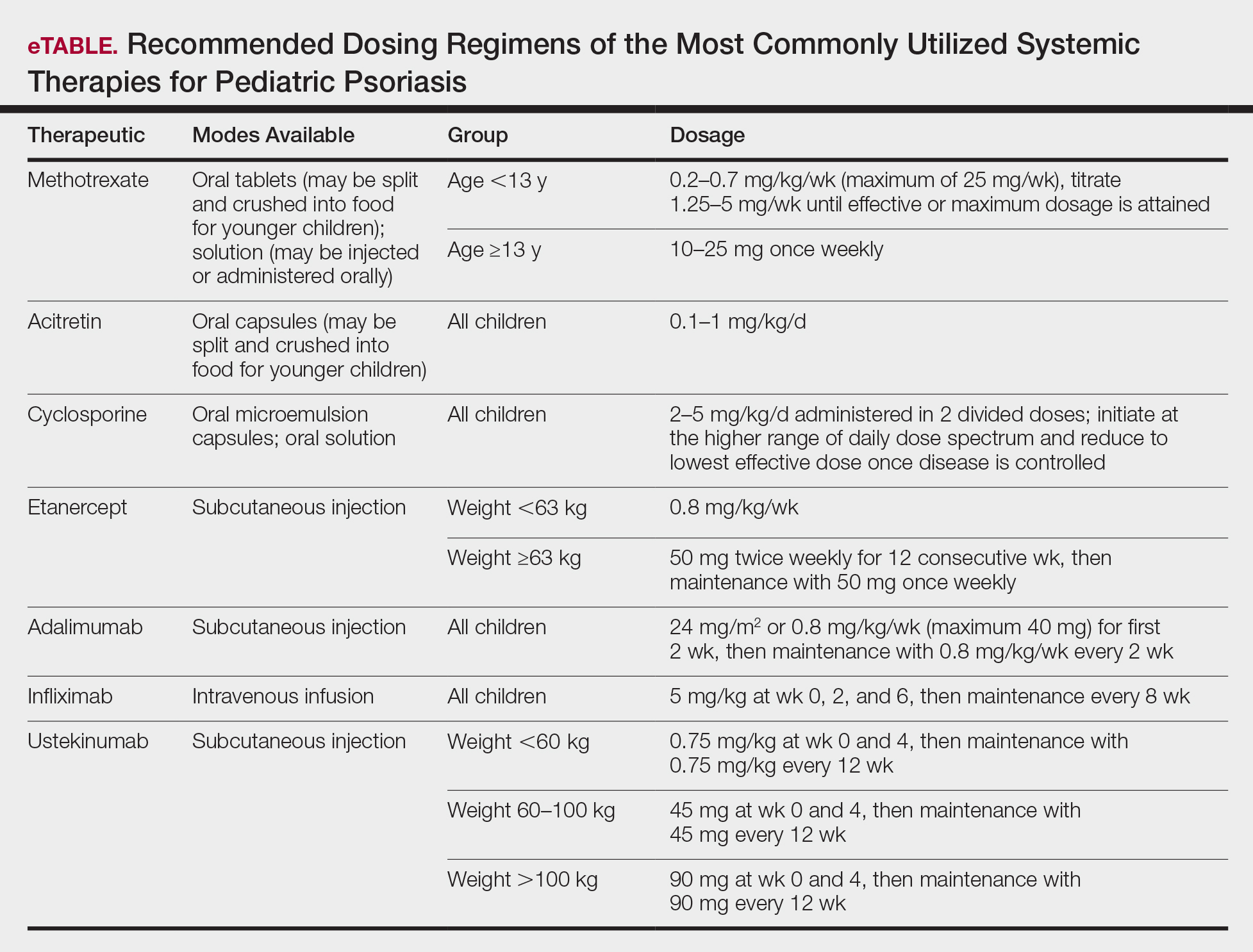
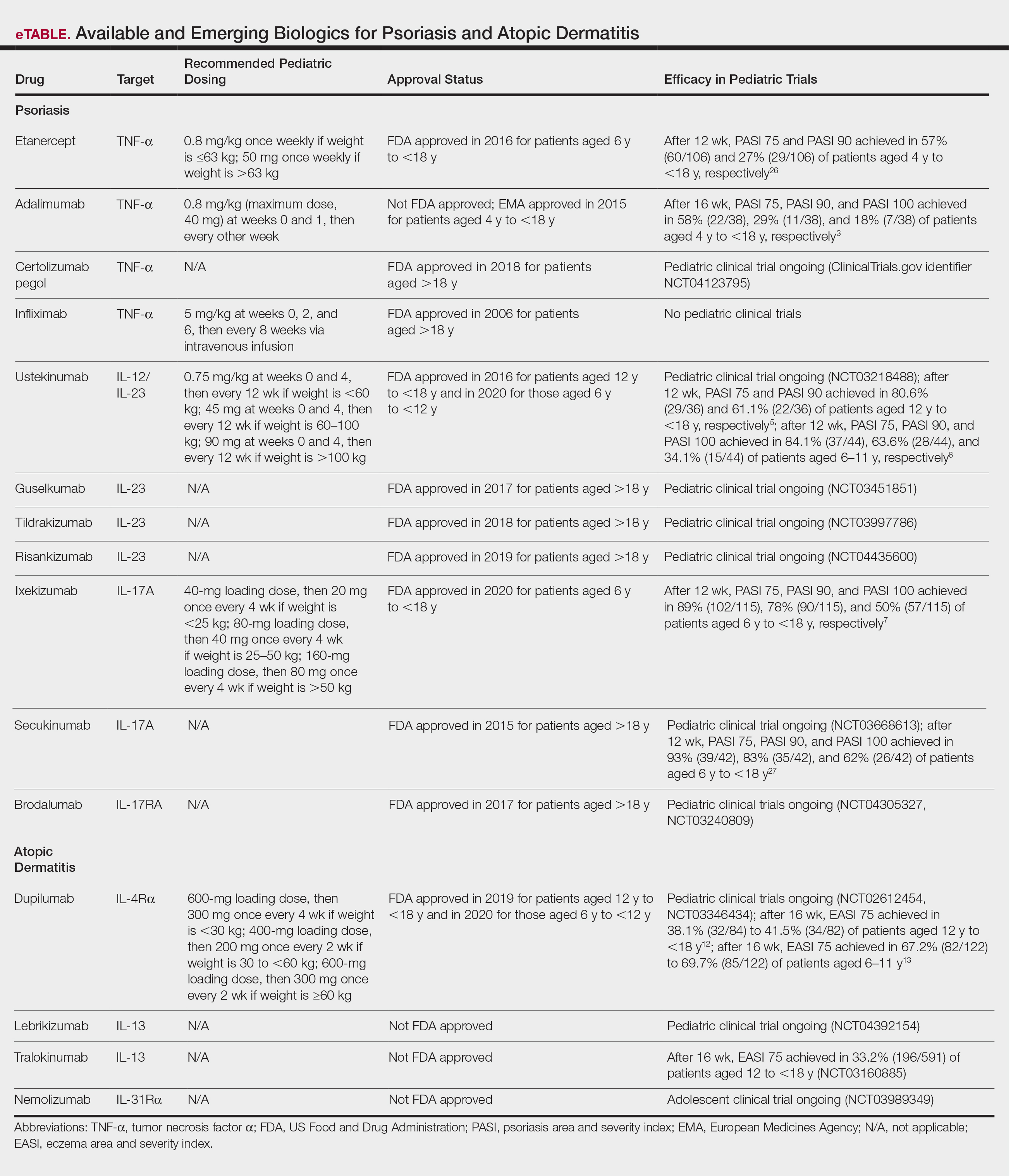
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
Practice Points
- For children, several environmental factors may prompt psoriasis flares, and it is critical to identify and eliminate these triggers.
- Although the use of biologics may be limited by cost and injection phobias in children, they may be an appropriate option for children with moderate to severe psoriasis when other therapies have failed. A growing body of literature is establishing the safety and effectiveness of biologics in children.
- Clinicians should thoroughly educate parents/ guardians on the course of psoriasis and treatment options as well as pay special attention to treatment goals and psychosocial factors that may guide decision-making regarding therapy.
Palmoplantar Eruption in a Patient With Mercury Poisoning
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
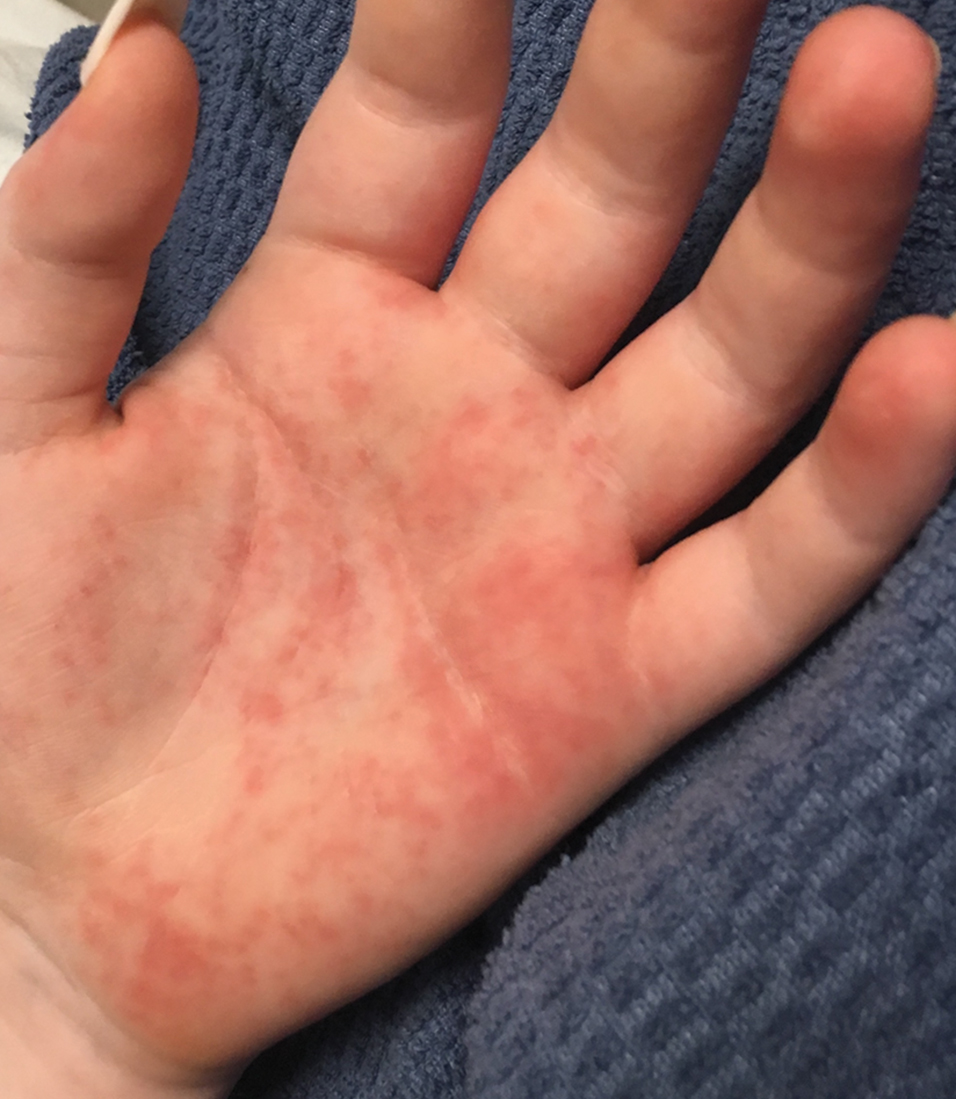
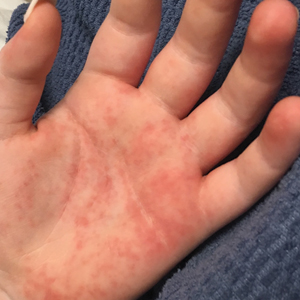
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.
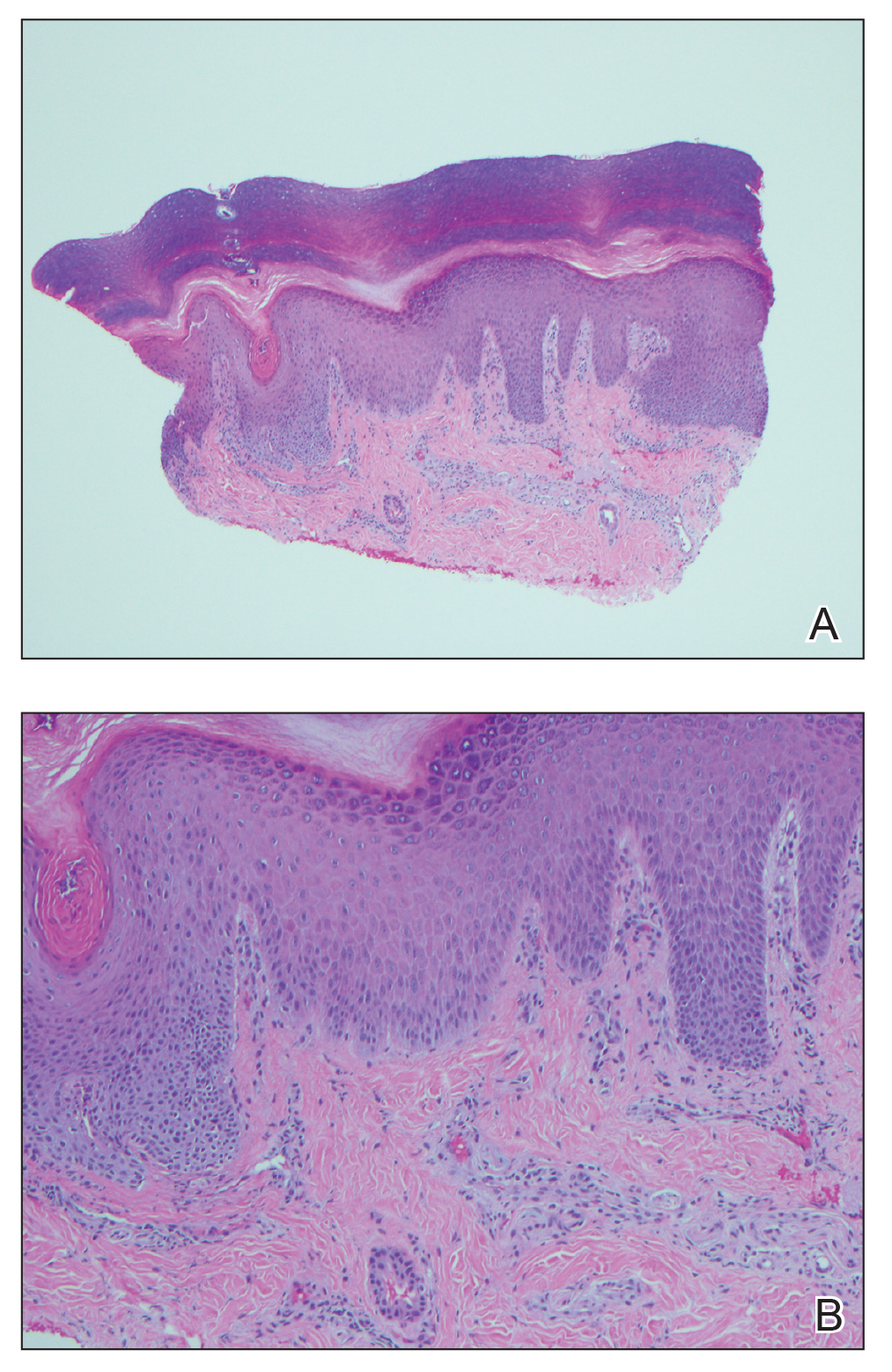
An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.
Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.
An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.
Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
Mercury poisoning affects multiple body systems, leading to variable clinical presentations. Mercury intoxication at low levels frequently presents with weakness, fatigue, weight loss, and abdominal pain. At higher levels of mercury intoxication, tremors and neurologic dysfunction are more prevalent.1 Dermatologic manifestations of mercury exposure vary and include pink disease (acrodynia), mercury exanthem, contact dermatitis, and cutaneous granulomas. Untreated mercury poisoning may result in severe complications, including renal tubular necrosis, pneumonitis, persistent neurologic dysfunction, and fatality in some cases.1,2
Pink disease is a rare disease that typically arises in infants and young children from chronic mercury exposure.3 We report a unique presentation of pink disease occurring in an 18-year-old woman following mercury exposure.
Case Report
An 18-year-old woman who was previously healthy presented to the hospital for evaluation of body aches and back pain. She reported a transient rash on the torso 2 weeks prior, but at the current presentation, only the distal upper and lower extremities were involved. A review of systems revealed myalgia, most severe in the lower back; muscle spasms; stiffness in the fingers; abdominal pain; constipation; paresthesia in the hands and feet; hyperhidrosis; and generalized weakness.
Vitals on admission revealed tachycardia (112 beats per minute). Physical examination revealed the patient was pale and fatigued; she appeared to be in pain, with observable facial grimacing and muscle spasms in the legs. She had poorly demarcated pink macules and papules scattered on the left palm (Figure 1), right forearm, right wrist, and dorsal aspects of the feet including the soles. A few pinpoint pustules were present on the left fifth digit.
An extensive workup was initiated to rule out infectious, autoimmune, or toxic etiologies. Two 4-mm punch biopsies of the left palm were performed for hematoxylin and eosin staining and tissue culture. Findings on hematoxylin and eosin stain were nonspecific, showing acanthosis, orthokeratosis, and a mild interface and perivascular lymphocytic infiltrate (Figure 2); superficial bacterial colonization was present, but the tissue culture was negative.
Laboratory studies showed mild transaminitis, and stool was positive for Campylobacter antigen. Electromyography showed myokymia (fascicular muscle contractions). A heavy metal serum panel and urine screen were positive for elevated mercury levels, with a serum mercury level of 23 µg/L (reference range, 0.0–14.9 µg/L) and a urine mercury level of 76 µg/L (reference range, 0–19 µg/L).
Upon further questioning, it was discovered that the patient’s brother and neighbor found a glass bottle containing mercury in their house 10 days prior. They played with the mercury beads with their hands, throwing them around the room and spilling them around the house, which led to mercury exposure in multiple individuals, including our patient. Of note, her brother and neighbor also were hospitalized at the same time as our patient with similar symptoms.
A diagnosis of mercury poisoning was made along with a component of postinfectious reactive arthropathy due to Campylobacter. The myokymia and skin eruption were believed to be secondary to mercury poisoning. The patient was started on ciprofloxacin (750 mg twice daily), intravenous immunoglobulin for Campylobacter, a 2-week treatment regimen with the chelating agent succimer (500 mg twice daily) for mercury poisoning, and a 3-day regimen of pulse intravenous steroids (intravenous methylprednisolone 500 mg once daily) to reduce inflammation. Repeat mercury levels showed a downward trend, and the rash improved with time. All family members were advised to undergo testing for mercury exposure.
Comment
Manifestations of Mercury Poisoning
Dermatologic manifestations of mercury exposure are varied. The most common—allergic contact dermatitis—presents after repeat systemic or topical exposure.4 Mercury exanthem is an acute systemic contact dermatitis most commonly triggered by mercury vapor inhalation. It manifests as an erythematous maculopapular eruption predominantly involving the flexural areas and the anterior thighs in a V-shaped distribution.5 Purpura may be seen in severe cases. Cutaneous granulomas after direct injection of mercury also have been reported as well as cutaneous hyperpigmentation after chronic mercury absorption.6
Presentation of Pink Disease
Pink disease occurs in children after chronic mercury exposure. It was a common pediatric disorder in the 19th century due to the presence of mercury in certain anthelmintics and teething powders.7 However, prevalence drastically decreased after the removal of mercury from these products.3 Although pink disease classically was associated with mercury ingestion, cases also occurred secondary to external application of mercury.7 Additionally, in 1988 a case was reported in a 14-month-old girl after inhalation of mercury vapor from a spilled bottle of mercury.3
Pink disease begins with pink discoloration of the fingertips, nose, and toes, and later progresses to involvement of the hands and feet. Erythema, edema, and desquamation of the hands and feet are seen, along with irritability and autonomic dysfunction that manifests as profuse perspiration, tachycardia, and hypertension.3
Diagnosis of Pink Disease
The differential diagnosis of palmoplantar rash is broad and includes rickettsial disease; syphilis; scabies; toxic shock syndrome; infective endocarditis; meningococcal infection; hand-foot-and-mouth disease; dermatophytosis; and palmoplantar keratodermas. The involvement of the hands and feet in our patient, along with hyperhidrosis, tachycardia, and paresthesia, led us to believe that her condition was a variation of pink disease. The patient’s age at presentation (18 years) was unique, as it is atypical for pink disease. Although the polyarthropathy was attributed to Campylobacter, it is important to note that high levels of mercury exposure also have been associated with polyarthritis,8 polyneuropathy,4 and neuromuscular abnormalities on electromyography.4 Therefore, it is possible that the presence of these symptoms in our patient was either secondary to or compounded by mercury exposure.
Mercury Poisoning
Diagnosis of mercury poisoning can be made by assessing blood, urine, hair, or nail concentrations. However, as mercury deposits in multiple organs, individual concentrations do not correlate with total-body mercury levels.1 Currently, no universal diagnostic criteria for mercury toxicity exist, though a provocation test with the chelating agent 2,
Elemental mercury, as found in some thermometers, dental amalgams, and electrical appliances (eg, certain switches, fluorescent light bulbs), can be converted to inorganic mercury in the body.9 Elemental mercury is vaporized at room temperature; the predominant route of exposure is by subsequent inhalation and lung absorbtion.10 Cutaneous absorption of high concentrations of elementary mercury in either liquid or vapor form may occur, though the rate is slow and absorption is poor. In cases of accidental exposure, contaminated clothing should be removed and immediately decontaminated or disposed. Exposed skin should be washed with a mild soap and water and rinsed thoroughly.10
The treatment of inorganic mercury poisoning is accomplished with the chelating agents succimer, dimercaptopropanesulfonate, dimercaprol, or D-penicillamine.1 In symptomatic cases with high clinical suspicion, the first dose of chelation treatment should be initiated early without delay for laboratory confirmation, as treatment efficacy decreases with an increased interim between exposure and onset of chelation.11 Combination chelation therapy also may be used in treatment. Plasma exchange or hemodialysis are treatment options for extreme, life-threatening cases.1
Conclusion
Mercury exposure should be included in the differential diagnosis of patients presenting with a rash on the palms and soles, especially in young patients with systemic symptoms. A high level of suspicion and a thorough history can prevent a delay in treatment and an unnecessarily extensive and expensive workup. An emphasis on early diagnosis and treatment is important for optimal outcomes and can prevent the severe and potentially devastating consequences of mercury toxicity.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508.
- Kamensky OL, Horton D, Kingsley DP, et al. A case of accidental mercury intoxication. J Emerg Med. 2019;56:275-278.
- Dinehart SM, Dillard R, Raimer SS, et al. Cutaneous manifestations of acrodynia (pink disease). Arch Dermatol. 1988;124:107-109.
- Malek A, Aouad K, El Khoury R, et al. Chronic mercury intoxication masquerading as systemic disease: a case report and review of the literature. Eur J Case Rep Intern Med. 2017;4:000632.
- Nakayama H, Niki F, Shono M, et al. Mercury exanthem. Contact Dermatitis. 1983;9:411-417.
- Boyd AS, Seger D, Vannucci S, et al. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000;43:81-90.
- Warkany J. Acrodynia—postmortem of a disease. Am J Dis Child. 1966;112:147-156.
- Karatas¸ GK, Tosun AK, Karacehennem E, et al. Mercury poisoning: an unusual cause of polyarthritis. Clin Rheumatol. 2002;21:73-75.
- Mercury Factsheet. Centers for Disease Control and Prevention website. https://www.cdc.gov/biomonitoring/Mercury_FactSheet.html. Reviewed April 7, 2017. Accessed October 21, 2020.
- Medical management guidelines for mercury. Agency for Toxic Substances & Disease Registry website. https://www.atsdr.cdc .gov/MMG/MMG.asp?id=106&tid=24. Update October 21, 2014. Accessed September 11, 2020.
- Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9:347-354.
Practice Points
- The dermatologic and histologic presentation of mercury exposure may be nonspecific, requiring a high degree of clinical suspicion to make a diagnosis.
- Mercury exposure should be included in the differential diagnosis in patients presenting with a rash of the palms and soles, especially in young patients with systemic symptoms.
Cystic fibrosis treatment: Triple combination benefits patients with advanced disease
New CFTR [cystic fibrosis transmembrane conductance regulator] modulator therapies can offer life-altering benefits to some patients with cystic fibrosis, even those with advanced disease.
, according to a multicenter analysis of patients taking elexacaftor, tezacaftor, and ivacaftor.
The study participants had a percent predicted forced expiratory volume in 1 second (ppFEV1) of 40% or below, or other high-risk factors. Researchers compared them to control patients who were genetically ineligible for triple combination therapy.
Previous studies of such patients on individual drugs or previous combinations showed increases in lung function in patients with advanced disease, though the magnitude of improvement varied across regimens. “With this improvement, it’s unclear how CFTR modulators should affect lung transplant referral timing,” Brent Bermingham, MD, said during a presentation of the study at the virtual North American Cystic Fibrosis Conference.
“The rationale for our study was that despite patients with advanced lung disease being excluded from phase III trials (of elexacaftor, tezacaftor, and ivacaftor), they are receiving a therapy with an unknown clinical efficacy and safety profile,” said Dr. Bermingham, a pulmonary and critical care fellow at the Medical University of South Carolina, Charleston.
Lung transplant referral guidelines recommend that physicians initiate discussions about the potential benefit of lung transplant when FEV1 drops below 50% of the predicted value. Patients should be referred for a transplant when the value is below 50% and rapidly declining (>20% decline in the past 12 months), when it drops below 40% with accompanying predictors of shortened survival, or when it drops below 30%. The guidelines were published before approval of triple combination therapy.
The researchers conducted an open-label retrospective analysis of 60 patients started on triple combination therapy between September 2019 and February 2020 at three centers in the Southeast. They compared percent predicted ppFEV1 values prior to initiation of therapy to ppFEV1 values obtained 2-12 weeks after the start of therapy. Patients on therapy were compared with 10 genetically ineligible controls. The two groups were generally similar aside from genetic status, though 100% of the therapy group had pancreatic insufficiency, compared with 90% of controls (P = .013).
The therapeutic group experienced a 7.8% increase in ppFEV1 after starting therapy (P < .001), compared with a 0.5% decrease in controls (P = .65). Before initiation of therapy, 33% of the therapy group met the criteria for initiating a transplant discussion, while 67% had been recommended for transplant. After therapy, 55% met the criteria for discussion, 33% were recommended for transplant, and 12% no longer met the criteria for discussion of transplantation. Fifty percent of controls were in discussion, and this dropped to 40%, while 50% were referred for transplantation, and this increased to 60%. On therapy, transplant referral candidates had an increase of forced vital capacity from 48.9 to 59.16 (P < .001).
Adverse events were rare, with only one discontinuation that occurred following a lung transplant and was not believed to be treatment related.
“Our study had a large number of patients taken from multiple centers, which suggests generalizabilty and real-world experience,” said Dr. Bermingham.
The results are encouraging, said Robert J. Giusti, MD, clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center.
“We’re all remarking how wonderful patients feel these days. It’s really a disease-altering treatment. But for the high-risk group, whose FEV1 is less than 40%, those are the patients we’re more concerned about because we thought maybe they had too much lung disease, and that they wouldn’t benefit from triple combination. But they seem to be improving, so that’s very reassuring,” said Dr. Giusti, who was not involved in the study.
The study received funding from the Cystic Fibrosis Foundation and Dartmouth College. Dr. Bermingham and Dr. Giusti have no relevant financial disclosures.
SOURCE: Bermingham B et al. NACFC 2020, Abstract 645.
New CFTR [cystic fibrosis transmembrane conductance regulator] modulator therapies can offer life-altering benefits to some patients with cystic fibrosis, even those with advanced disease.
, according to a multicenter analysis of patients taking elexacaftor, tezacaftor, and ivacaftor.
The study participants had a percent predicted forced expiratory volume in 1 second (ppFEV1) of 40% or below, or other high-risk factors. Researchers compared them to control patients who were genetically ineligible for triple combination therapy.
Previous studies of such patients on individual drugs or previous combinations showed increases in lung function in patients with advanced disease, though the magnitude of improvement varied across regimens. “With this improvement, it’s unclear how CFTR modulators should affect lung transplant referral timing,” Brent Bermingham, MD, said during a presentation of the study at the virtual North American Cystic Fibrosis Conference.
“The rationale for our study was that despite patients with advanced lung disease being excluded from phase III trials (of elexacaftor, tezacaftor, and ivacaftor), they are receiving a therapy with an unknown clinical efficacy and safety profile,” said Dr. Bermingham, a pulmonary and critical care fellow at the Medical University of South Carolina, Charleston.
Lung transplant referral guidelines recommend that physicians initiate discussions about the potential benefit of lung transplant when FEV1 drops below 50% of the predicted value. Patients should be referred for a transplant when the value is below 50% and rapidly declining (>20% decline in the past 12 months), when it drops below 40% with accompanying predictors of shortened survival, or when it drops below 30%. The guidelines were published before approval of triple combination therapy.
The researchers conducted an open-label retrospective analysis of 60 patients started on triple combination therapy between September 2019 and February 2020 at three centers in the Southeast. They compared percent predicted ppFEV1 values prior to initiation of therapy to ppFEV1 values obtained 2-12 weeks after the start of therapy. Patients on therapy were compared with 10 genetically ineligible controls. The two groups were generally similar aside from genetic status, though 100% of the therapy group had pancreatic insufficiency, compared with 90% of controls (P = .013).
The therapeutic group experienced a 7.8% increase in ppFEV1 after starting therapy (P < .001), compared with a 0.5% decrease in controls (P = .65). Before initiation of therapy, 33% of the therapy group met the criteria for initiating a transplant discussion, while 67% had been recommended for transplant. After therapy, 55% met the criteria for discussion, 33% were recommended for transplant, and 12% no longer met the criteria for discussion of transplantation. Fifty percent of controls were in discussion, and this dropped to 40%, while 50% were referred for transplantation, and this increased to 60%. On therapy, transplant referral candidates had an increase of forced vital capacity from 48.9 to 59.16 (P < .001).
Adverse events were rare, with only one discontinuation that occurred following a lung transplant and was not believed to be treatment related.
“Our study had a large number of patients taken from multiple centers, which suggests generalizabilty and real-world experience,” said Dr. Bermingham.
The results are encouraging, said Robert J. Giusti, MD, clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center.
“We’re all remarking how wonderful patients feel these days. It’s really a disease-altering treatment. But for the high-risk group, whose FEV1 is less than 40%, those are the patients we’re more concerned about because we thought maybe they had too much lung disease, and that they wouldn’t benefit from triple combination. But they seem to be improving, so that’s very reassuring,” said Dr. Giusti, who was not involved in the study.
The study received funding from the Cystic Fibrosis Foundation and Dartmouth College. Dr. Bermingham and Dr. Giusti have no relevant financial disclosures.
SOURCE: Bermingham B et al. NACFC 2020, Abstract 645.
New CFTR [cystic fibrosis transmembrane conductance regulator] modulator therapies can offer life-altering benefits to some patients with cystic fibrosis, even those with advanced disease.
, according to a multicenter analysis of patients taking elexacaftor, tezacaftor, and ivacaftor.
The study participants had a percent predicted forced expiratory volume in 1 second (ppFEV1) of 40% or below, or other high-risk factors. Researchers compared them to control patients who were genetically ineligible for triple combination therapy.
Previous studies of such patients on individual drugs or previous combinations showed increases in lung function in patients with advanced disease, though the magnitude of improvement varied across regimens. “With this improvement, it’s unclear how CFTR modulators should affect lung transplant referral timing,” Brent Bermingham, MD, said during a presentation of the study at the virtual North American Cystic Fibrosis Conference.
“The rationale for our study was that despite patients with advanced lung disease being excluded from phase III trials (of elexacaftor, tezacaftor, and ivacaftor), they are receiving a therapy with an unknown clinical efficacy and safety profile,” said Dr. Bermingham, a pulmonary and critical care fellow at the Medical University of South Carolina, Charleston.
Lung transplant referral guidelines recommend that physicians initiate discussions about the potential benefit of lung transplant when FEV1 drops below 50% of the predicted value. Patients should be referred for a transplant when the value is below 50% and rapidly declining (>20% decline in the past 12 months), when it drops below 40% with accompanying predictors of shortened survival, or when it drops below 30%. The guidelines were published before approval of triple combination therapy.
The researchers conducted an open-label retrospective analysis of 60 patients started on triple combination therapy between September 2019 and February 2020 at three centers in the Southeast. They compared percent predicted ppFEV1 values prior to initiation of therapy to ppFEV1 values obtained 2-12 weeks after the start of therapy. Patients on therapy were compared with 10 genetically ineligible controls. The two groups were generally similar aside from genetic status, though 100% of the therapy group had pancreatic insufficiency, compared with 90% of controls (P = .013).
The therapeutic group experienced a 7.8% increase in ppFEV1 after starting therapy (P < .001), compared with a 0.5% decrease in controls (P = .65). Before initiation of therapy, 33% of the therapy group met the criteria for initiating a transplant discussion, while 67% had been recommended for transplant. After therapy, 55% met the criteria for discussion, 33% were recommended for transplant, and 12% no longer met the criteria for discussion of transplantation. Fifty percent of controls were in discussion, and this dropped to 40%, while 50% were referred for transplantation, and this increased to 60%. On therapy, transplant referral candidates had an increase of forced vital capacity from 48.9 to 59.16 (P < .001).
Adverse events were rare, with only one discontinuation that occurred following a lung transplant and was not believed to be treatment related.
“Our study had a large number of patients taken from multiple centers, which suggests generalizabilty and real-world experience,” said Dr. Bermingham.
The results are encouraging, said Robert J. Giusti, MD, clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center.
“We’re all remarking how wonderful patients feel these days. It’s really a disease-altering treatment. But for the high-risk group, whose FEV1 is less than 40%, those are the patients we’re more concerned about because we thought maybe they had too much lung disease, and that they wouldn’t benefit from triple combination. But they seem to be improving, so that’s very reassuring,” said Dr. Giusti, who was not involved in the study.
The study received funding from the Cystic Fibrosis Foundation and Dartmouth College. Dr. Bermingham and Dr. Giusti have no relevant financial disclosures.
SOURCE: Bermingham B et al. NACFC 2020, Abstract 645.
FROM NACFC 2020
What’s Eating You? Human Flea (Pulex irritans)
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Practice Points
- The human flea, Pulex irritans, is a vector for various human diseases including the bubonic plague, bartonellosis, and rickettsioses.
- Presenting symptoms of flea bites include intensely pruritic, urticarial to vesicular papules on exposed areas of skin.
- The primary method of flea control includes a combination of insecticidal products and insect growth regulators.
Intraoperative Tissue Expansion to Allow Primary Linear Closure of 2 Large Adjacent Surgical Defects
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
Practice Gap
Nonmelanoma skin cancers most commonly are found on the head and neck. In these locations, many of these malignancies will meet criteria to undergo treatment with Mohs micrographic surgery. It is becoming increasingly common for patients to have multiple lesions treated at the same time, and sometimes these lesions can be in close proximity to one another. The final size of the adjacent defects, along with the amount of normal tissue remaining between them, will determine how to best repair both defects.1 Many times, repair options are limited to the use of a larger and more extensive repair such as a flap or graft. We present a novel option to increase the options for surgical repair.
The Technique
We present a case of 2 large adjacent postsurgical defects where intraoperative tissue relaxation allowed for successful primary linear closure of both defects under notably decreased tension from baseline. A 70-year-old man presented for treatment of 2 adjacent invasive squamous cell carcinomas on the left temple and left frontal scalp. The initial lesion sizes were 2.0×1.0 and 2.0×2.0 cm, respectively. Mohs micrographic surgery was performed on both lesions, and the final defect sizes measured 2.0×1.4 and 3.0×1.6 cm, respectively. The island of normal tissue between the defects measured 2.3-cm wide. Different repair options were discussed with the patient, including allowing 1 or both lesions to heal via secondary intention, creating 1 large wound to repair with a full-thickness skin graft, using a large skin flap to cover both wounds, or utilizing a 2-to-Z flap.2 We also discussed using an intraoperative skin relaxation device to stretch the skin around 1 or both defects and close both defects in a linear fashion; the patient opted for the latter treatment option.
The left temple had adequate mobility to perform a primary closure oriented horizontally along the long axis of the defect. Although it would have been a simple repair for this lesion, the superior defect on the frontal scalp would have been subjected to increased downward tension. The scalp defect was already under considerable tension with limited tissue mobility, so closing the temple defect horizontally would have required repair of the scalp defect using a skin graft or leaving it open to heal on its own. Similarly, the force necessary to close the frontal scalp wound first would have prevented primary closure of the temple defect.
A SUTUREGARD ISR device (Sutureguard Medical Inc) was secured centrally over both defects at a 90° angle to one another to provide intraoperative tissue relaxation without undermining. The devices were held in place by a US Pharmacopeia 2-0 nylon suture and allowed to sit for 60 minutes (Figure 1).3
After 60 minutes, the temple defect had adequate relaxion to allow a standard layered intermediate closure in a vertical orientation along the hairline using 3-0 polyglactin 910 and 3-0 nylon. Although the scalp defect was not completely approximated, it was more than 60% smaller and able to be closed at both wound edges using the same layered approach. There was a central defect area approximately 4-mm wide that was left to heal by secondary intention (Figure 2). Undermining was not used to close either defect.
The patient tolerated the procedure well with minimal pain or discomfort. He followed standard postoperative care instructions and returned for suture removal after 14 days of healing. At the time of suture removal there were no complications. At 1-month follow-up the patient presented with excellent cosmetic results (Figure 3).
Practice Implications
The methods of repairing 2 adjacent postsurgical defects are numerous and vary depending on the size of the individual defects, the location of the defects, and the amount of normal skin remaining between them. Various methods of closure for the adjacent defects include healing by secondary intention, primary linear closure, skin grafts, skin flaps, creating 1 larger wound to be repaired, or a combination of these approaches.1,2,4,5
In our patient, closing the high-tension wound of the scalp would have prevented both wounds from being closed in a linear fashion without first stretching the tissue. Although Zitelli5 has cited that many wounds will heal well on their own despite a large size, many patients prefer the cosmetic appearance and shorter healing time of wounds that have been closed with sutures, particularly if those defects are greater than 8-mm wide. In contrast, patients preferred the cosmetic appearance of 4-mm wounds that healed via secondary intention.6 In our case, we closed the majority of the wound and left a small 4-mm-wide portion to heal on its own. The overall outcome was excellent and healed much quicker than leaving the entire scalp defect to heal by secondary intention.
The other methods of closure, such as a 2-to-Z flap, would have been difficult given the orientation of the lesions and the island between them.2 To create this flap, an extensive amount of undermining would have been necessary, leading to serious disruption of the blood and nerve supply and an increased risk for flap necrosis. Creating 1 large wound and repairing with a flap would have similar requirements and complications.
Intraoperative tissue relaxation can be used to allow primary closure of adjacent wounds without the need for undermining. Prior research has shown that 30 minutes of stress relaxation with 20 Newtons of applied tension yields a 65% reduction in wound-closure tension.7 Orienting the devices between 45° to 90° angles to one another creates opposing tension vectors so that the closure of one defect does not prevent the closure of the other defect. Even in cases in which the defects cannot be completely approximated, closing the wound edges to create a smaller central defect can decrease healing time and lead to an excellent cosmetic outcome without the need for a flap or graft.
The SUTUREGARD ISR suture retention bridge also is cost-effective for the surgeon and the patient. The device and suture-guide washer are included in a set that retails for $35 each or $300 for a box of 12.8 The suture most commonly used to secure the device in our practice is 2-0 nylon and retails for approximately $34 for a box of 12,9 which brings the total cost with the device to around $38 per use. The updated Current Procedural Terminology guidelines from the Centers for Medicare & Medicaid Services define that an intermediate repair requires a layered closure and may include, but does not require, limited undermining. A complex linear closure must meet criteria for an intermediate closure plus at least 1 additional criterion, such as exposure of cartilage, bone, or tendons within the defect; extensive undermining; wound-edge debridement; involvement of free margins; or use of a retention suture.10 Use of a suture retention bridge such as the SUTUREGARD ISR device and therefore a retention suture qualifies the repair as a complex linear closure. Overall, use of the device expands the surgeon’s choices for surgical closures and helps to limit the need for larger, more invasive repair procedures.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
- McGinness JL, Parlette HL. A novel technique using a rotation flap for repairing adjacent surgical defects. Dermatol Surg. 2006;32:272-275.
- Blattner CM, Perry B, Young J, et al. 2-to-Z flap for reconstruction of adjacent skin defects. J Am Acad Dermatol. 2019;80:E77-E78.
- Blattner CM, Perry B, Young J, et al. The use of a suture retention device to enhance tissue expansion and healing in the repair of scalp and lower leg wounds. JAAD Case Rep. 2018;4:655-661.
- Zivony D, Siegle RJ. Burrow’s wedge advancement flaps for reconstruction of adjacent surgical defects. Dermatol Surg. 2002;28:1162-1164.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2:92-106.
- Christenson LJ, Phillips PK, Weaver AL, et al. Primary closure vs second-intention treatment of skin punch biopsy sites: a randomized trial. Arch Dermatol. 2005;141:1093-1099.
- Lear W, Blattner CM, Mustoe TA, et al. In vivo stress relaxation of human scalp. J Mech Behav Biomed Mater. 2019;97:85-89.
- SUTUREGARD purchasing facts. SUTUREGARD® Medical Inc website. https://suturegard.com/SUTUREGARD-Purchasing-Facts. Accessed October 15, 2020.
- Shop products: suture with needle McKesson nonabsorbable uncoated black suture monofilament nylon size 2-0 18 inch suture 1-needle 26 mm length 3/8 circle reverse cutting needle. McKesson website. https://mms.mckesson.com/catalog?query=1034509. Accessed October 15, 2020.
- Norris S. 2020 CPT updates to wound repair guidelines. Zotec Partners website. http://zotecpartners.com/resources/2020-cpt-updates-to-wound-repair-guidelines/. Published June 4, 2020. Accessed October 21, 2020.
The Gips Procedure for Pilonidal Disease: A Retrospective Review of Adolescent Patients
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
Pilonidal disease (PD) is common in Turkey. In a study in Turkey, 19,013 young patients aged 17 to 28 years were examined; PD was detected in 6.6% of patients (0.37% of females in the cohort and 6.23% of males).1 The incidence of PD in military personnel (women 18 years and older; men 22 years and older) is remarkably higher, with an incidence of 9% reported in Turkish soldiers.2
Pilonidal disease has become common in Turkish adolescents, who now experience an increase in desk time because of computer use and a long duration of preparation for high school and university entrance examinations. In adolescent and adult population studies, Yildiz et al3 and Harlak et al4 reported that sitting for 6 hours or more per day was found to significantly increase the risk for PD compared to the control group (P=.028 and P<.001, respectively).
Surgery for PD often is followed by a considerable and unpleasant postoperative course, with a long period of limited physical activity, loss of school time, and reduced social relationships. The recurrence rate of PD is reported to be as high as 40% to 50% after incision and drainage, 40% to 55% with rigorous hygiene and weekly shaving, and as high as 30% following operative intervention. Drawbacks of operative intervention include associated morbidity; lost work and school time; and prolonged wound healing, which can take days to months.5-7
For these reasons, minimally invasive surgical techniques have become popular for treating PD in adolescents, as surgery can cause less disruption of the school and examination schedule and provide an earlier return to normal activities. Gips et al8—who operated on 1358 adults using skin trephines to extirpate pilonidal pits and the underlying fistulous tract and hair debris—reported a low recurrence rate and good postoperative functional outcomes with this technique. Herein, we present our short-duration experience with the Gips procedure of minimally invasive sinusectomy in adolescent PD.
Methods
Patients
We performed a retrospective medical record review of patients with symptomatic PD who were treated in our clinic between January 2018 and February 2019 using the Gips procedure of minimally invasive sinusectomy. We identified 19 patients younger than 17 years. Patients with acute inflammation and an acute undrained collection of pits were treated with incision and drainage, with close clinical follow-up until inflammation resolved. We also recommended that patients take a warm sitz bath at least once daily and chemically epilate the hair in the affected area if they were hirsute.
Gips Procedure
For all patients, the Gips procedure was performed in the left lateral position under general anesthesia using a laryngeal mask airway for anesthesia. Patients were closely shaved (if hirsute) then prepared with povidone-iodine solution. First, each fistulous opening was probed to assess depth and direction of underlying tracts using a thin (0.5–1.0 mm), round-tipped probe. Next, a trephine—comprising a cylindrical blade on a handle—was used to remove cylindrical cores of tissue. All visible median pits and lateral fistulous skin openings were excised using skin trephines of various diameters (Figure, A and B). Once the pilonidal cavity was reached, attention was directed to removing all residual underlying tissue—granulation tissue, debris, and hair—through all available accesses. The cavity was cleaned with hydrogen peroxide and normal saline. Then, all trephine-made openings were left unpacked or were packed for only a few hours and were not sutured (Figure, C and D); a light gauze bandage was eventually applied with a minimum of tape and skin traction. Patients were kept supine during a 1- or 2-hour clinical observation period before they were discharged.
Postoperatively, no regular medications other than analgesics were recommended; routine daily activities were allowed. Patients were encouraged to sleep supine and wash the sacrococcygeal region with running water several times a day after the second postoperative day. Frequent showering, application of povidone-iodine to the wound after defecation, and regular epilation of the sacrococcygeal area also were recommended to all patients.
All patients were routinely followed by the same surgical group weekly until wound healing was complete (Figure, E).
Medical Record Review
Patients’ electronic medical records were reviewed retrospectively, and parameters including age at surgery, surgical history, symptoms, duration of operation and hospital stay, time to return to activity, wound healing time, and recurrence were recorded.
Results
Of the 19 patients who underwent the Gips procedure, 17 (90%) were male; 2 (10%) were female. The mean (standard deviation [SD]) body mass index was 25 (3.7). (Body mass index was calculated as weight in kilograms divided by height in meters squared.) The mean age (SD) of patients was 15 (1.1) years (range, 12–17 years). The most common symptom at presentation was purulent discharge (11/19 [58%]). Other common symptoms included pain (8/19 [42%]), pilonidal abscess (6/19 [32%]), and bleeding (4/19 [21%]). Nine patients (47%) had prior abscess drainage at presentation; 1 (5%) had previously undergone surgery, and 5 (26%) previously had phenol injections.
The median (SD) length of stay in the hospital was 15 (3.2) hours (range, 11–22 hours). The mean (SD) time before returning to daily activities and school was 2 (0.6) days (range, 1–3 days). In our patients, the Gips procedure was performed on either a Thursday or more often a Friday; therefore, patients could be scheduled to be discharged from the hospital and return to home the next day, and then return to school on Monday. All patients were advised to take an oral analgesic for 2 days following the procedure.
The mean (SD) duration of the operative procedure was 14 (3) minutes (range, 10–20 minutes). One patient (5%) developed bleeding that ceased spontaneously. The mean (SD) complete wound healing time was 3 (0.6) weeks (range, 2–4 weeks).
Postoperative clinical examination and telephone interviews were performed for follow-up. The mean follow-up period was 5 months (range, 1–13 months); 17 of 19 patients (89%) made a complete recovery. Two patients (11%) reported recurrence in the third and fourth months following the procedure and were treated with a repeat Gips procedure 6 months after the first treatment. Improvement was noted after a second Gips procedure in 1 of 2 patients who had recurrence, leaving the success rate of the procedure in our practice at 95% (18/19).
Comment
Various treatment methods for PD have been postulated,5-7 including incision and drainage, hair removal and hygiene alone, excision and primary wound closure, excision and secondary wound closure, and various flap techniques. More recently, there has been a dramatic shift to management of patients with PD in an outpatient setting. The Gips procedure, an innovative minimally surgical technique for PD, was introduced in 2008 based on a large consecutive series of more than 1300 patients.8 Studies have shown promising results and minimal recovery time for the Gips procedure in adult and pediatric patients.8-10
Nevertheless, conventional excision down to the sacral fascia, with or without midline or asymmetrical closure, is still the procedure performed most often for PD worldwide.
Advantages of the Gips Procedure
Advantages of the Gips procedure are numerous. It is easily applicable, inexpensive, well tolerated, and requires minimal postoperative care. Placing the patient in the lateral position for the procedure—rather than the prone position that is required for more extensive surgical procedures—is highly feasible, permitting the easy application of a laryngeal mask for anesthesia. The Gips procedure can be performed on patients with severe PD after a period of improved hygiene and hair control and allows for less morbidity than older surgical techniques. Overall, results are satisfactory.
Health services and the hospital admissions process are less costly in university hospitals in Turkey. This procedure costs an average of 400 Turkish liras (<US $50). For that reason, patients in our review were discharged the next day; however, patients could be discharged within a few hours. In the future, it is possible for appropriate cases to be managed in an outpatient setting with sedation and local anesthesia only. Because their postoperative courses are eventless, these patients can be managed without hospitalization.
Recovery is quick and allows for early return to school and other physical activities. Because the procedure was most often performed on the last school day of the week, we did not see any restriction of physical or social activities in our patients.
Lastly, this procedure can be applied to PD patients who have previously undergone extensive surgery or phenol injection, as was the case in our patients.
Conclusion
The Gips procedure is an easy-to-use technique in children and adolescents with PD. It has a high success rate and places fewer restrictions on school and social activities than traditional surgical therapies.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
- Duman K, Gırgın M, Harlak A. Prevalence of sacrococcygeal pilonidal disease in Turkey. Asian J Surg. 2017;40:434-437.
- Akinci OF, Bozer M, Uzunköy A, et al. Incidence and aetiological factors in pilonidal sinus among Turkish soldiers. Eur J Surg. 1999;165:339-342.
- Yildiz T, Elmas B, Yucak A, et al. Risk factors for pilonidal sinus disease in teenagers. Indian J Pediatr. 2017;84:134-138.
- Harlak A, Mentes O, Kilic S, et al. Sacrococcygeal pilonidal disease: analysis of previously proposed risk factors. Clinics (Sao Paulo). 2010;65:125-131.
- Delshad HR, Dawson M, Melvin P, et al. Pit-picking resolves pilonidal disease in adolescents. J Pediatr Surg. 2019;54:174-176.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am. 2010;90:113-124.
- Bascom J. Pilonidal disease: origin from follicles of hairs and results of follicle removal as treatment. Surgery. 1980;87:567-572.
- Gips M, Melki Y, Salem L, et al. Minimal surgery for pilonidal disease using trephines: description of a new technique and long-term outcomes in 1,358 patients. Dis Colon Rectum. 2008;51:1656-1662; discussion, 1662-1663.
- Speter C, Zmora O, Nadler R, et al. Minimal incision as a promising technique for resection of pilonidal sinus in children. J Pediatr Surg. 2017;52:1484-1487.
- Di Castro A, Guerra F, Levi Sandri GB, et al. Minimally invasive surgery for the treatment of pilonidal disease. the Gips procedure on 2347 patients. Int J Surg. 2016;36:201-205.
- Guerra F, Giuliani G, Amore Bonapasta S, et al. Cleft lift versus standard excision with primary midline closure for the treatment of pilonidal disease. a snapshot of worldwide current practice. Eur Surg. 2016;48:269-272.
Practice Points
- The Gips procedure is an easy-to-use outpatient procedure for adolescents with pilonidal disease.
- This procedure has a high success rate and does not restrict school or social activities.
FIT unfit for inpatient, emergency settings
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Most fecal immunochemical tests (FIT) in the hospital setting or the ED are performed for inappropriate indications, according to new data.
“This is the largest study that focuses exclusively on the use of FIT in the ED, inpatient wards, and in the ICU, and it shows significant misuse,” said investigator Umer Bhatti, MD, from Indiana University, Indianapolis.
The only “validated indication” for FIT is to screen for colorectal cancer. However, “99.5% of the FIT tests done in our study were for inappropriate indications,” he reported at the annual meeting of the American College of Gastroenterology, where the study was honored with an ACG Presidential Poster Award.
And the inappropriate use of FIT in these settings had no positive effect on clinical decision-making, he added.
For their study, Dr. Bhatti and colleagues looked at all instances of FIT use in their hospital’s electronic medical records from November 2017 to October 2019 to assess how often FIT was being used, the indications for which it was being used, and the impact of its use on clinical care.
They identified 550 patients, 48% of whom were women, who underwent at least one FIT test. Mean age of the study cohort was 54 years. Only three of the tests, or 0.5%, were performed to screen for colorectal cancer (95% confidence interval, 0.09%-1.52%).
Among the indications documented for FIT were anemia in 242 (44.0%) patients, suspected GI bleeding in 225 (40.9%), abdominal pain in 31 (5.6%), and change in bowel habits in 19 (3.5%).
The tests were performed most often in the ED (45.3%) and on the hospital floor (42.2%), but were also performed in the ICU (10.5%) and burn unit (2.0%).
Overall, 297 of the tests, or 54%, were negative, and 253, or 46%, were positive.
“GI consults were obtained in 46.2% of the FIT-positive group, compared with 13.1% of the FIT-negative patients” (odds ratio, 5.93; 95% CI, 3.88-9.04, P < .0001), Dr. Bhatti reported.
Among FIT-positive patients, those with overt bleeding were more likely to receive a GI consultation than those without (OR, 3.3; 95% CI, 1.9-5.5; P < .0001).
Of the 117 FIT-positive patients who underwent a GI consultation, upper endoscopy was a more common outcome than colonoscopy (51.3% vs. 23.1%; P < .0001). Of the 34 patients who underwent colonoscopy or sigmoidoscopy, one was diagnosed with colorectal cancer and one with advanced adenoma.
Overt GI bleeding was a better predictor of a GI consultation than a positive FIT result. In fact, use of FIT for patients with overt GI bleeding indicates a poor understanding of the test’s utility, the investigators reported.
“For patients with overt GI bleeding, having a positive FIT made no difference on how often a bleeding source was identified on endoscopy, suggesting that FIT should not be used to guide decisions about endoscopy or hospitalization,” Dr. Bhatti said.
In light of these findings, the team urges their peers to consider measures to reduce FIT tests for unnecessary indications.
“We feel that FIT is unfit for use in the inpatient and emergency settings, and measures should be taken to curb its use,” Dr. Bhatti concluded. “We presented our data to our hospital leadership and a decision was made to remove the FIT as an orderable test from the EMR.”
These results are “striking,” said Jennifer Christie, MD, from the University, Atlanta.
“We should be educating our ER providers and inpatient providers about the proper use of FIT,” she said in an interview. “Another option – and this has been done in many settings with the fecal occult blood test – is just take FIT off the units or out of the ER, so providers won’t be tempted to use it as an assessment of these patients. Because often times, as this study showed, it doesn’t really impact outcomes.”
In fact, unnecessary FI testing could put patients at risk for unnecessary procedures. “We also know that calling for an inpatient or ER consult from a gastroenterologist may increase both length of stay and costs,” she added.
Dr. Bhatti and Dr. Christie disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Biologics in Pediatric Psoriasis and Atopic Dermatitis: Revolutionizing the Treatment Landscape
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Aging with HIV adds to comorbidity burden
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
The age of antiretroviral therapy (ART) for HIV is in its third decade, and many of the patients who live in areas of the world fortunate enough to have had early access to therapy have now lived for several decades with complications of HIV and viral suppressive therapy.
But while the life-expectancy of persons with HIV has approached that of noninfected persons over the last 20 years, the higher burden of comorbidities for aging patients with HIV has remained largely the same, according to an epidemiologist who specializes in HIV/AIDS research and aging.
“The pathways from HIV and its treatments to comorbidities are very long and winding, spanning a life course. Social determinants of health and individual risk factors also play an important role, and must be considered,” said Keri N. Althoff, PhD, MPH, of Johns Hopkins University, Baltimore.
Dr. Althoff discussed long-term complications of HIV and its treatment in a virtual symposium during an annual scientific meeting on infectious diseases.
“Many urban HIV providers have an increased proportion of patients who are older long-term survivors of the epidemic. Many, but not all of the comorbidities (including cardiovascular, neurocognitive, renal, and malignancies) have been associated with age, long-term HIV infection, especially uncontrolled HIV infection, and low CD4 nadirs,” commented Harry Lampiris, MD, professor of clinical medicine at the University of California, San Francisco.
“An increasing number of patients are experiencing geriatric syndromes (especially problems with mobility, cognitive decline, food insecurity, polypharmacy, and social isolation) at younger ages than HIV-negative populations,” he added.
Dr. Lampiris, who moderated the session where Dr. Althoff presented her findings, commented on it in an interview, but was not involved in her research.
Pathways to comorbidity
The three primary pathways to comorbidities in people with HIV infections are as follows, according to Dr. Athloff:
- The virus itself, with its associated inflammation, immunosuppression, immune activation, and AIDS.
- HIV therapies, beginning with the notoriously toxic dideoxynucleoside analogues or “d-drugs,” and following with subsequent generations of newer, less toxic agents.
- Individual risk factors, including smoking, stress, diet, exercise, and environment.
Cardiovascular and renal complications
Persons with HIV have an approximately twofold higher risk for major adverse cardiovascular events (myocardial infarction, stroke) compared with persons without HIV. Conditions contributing to cardiovascular disease including hypertension, diabetes, and hyperlipidemia are also significantly higher among persons with HIV, Dr. Althoff said.
Hypertension among persons with HIV from the ages 60-69 years is especially high for Black men and to a lesser degree non-Black men, compared with either White or Black women, she noted.
Pathways to renal disease in persons with HIV include diabetes and hypertension, as well as therapies to treat them, hepatitis B and C coinfection, HIV-associated nephropathy, and immune complex kidney disease, as well as chronic kidney disease resulting from acute kidney injury related to therapy.
“Cardiovascular disease and kidney disease are excellent examples of why the life-course perspective is essential when caring for people with HIV. For those diagnosed with HIV at younger ages, there are points of intervention along the decades-long path, and the timing and implementation of the most effective intervention may preserve comorbidity-free years,” Dr. Althoff said.
Prevention and screening interventions to lower risk for future heart- and kidney-related comorbidities include smoking cessation and lifestyle optimization (diet, exercise, mental health), as well as lipid-lowering medications to lower risk for cardiovascular events.
Liver comorbidities
“Primary drivers of liver disease are social determinants of health and individual lifestyle risk factors that share the same pathways as HIV, resulting in this increased burden of liver disease in people with HIV,” she said.
Risk factors include alcoholic liver disease, nonalcoholic fatty liver disease, hepatitis B and C coinfection, drug use, autoimmune disease, and aging. These risk factors contribute to oxidative stress, mitochondrial injury, lipotoxicity, cytotoxicity, and other mechanisms that can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and end-stage liver disease.
“I want to be sure to acknowledge the importance of liver disease as a comorbidity among people with HIV. Liver disease accounts for nearly 20% of mortality in persons with HIV,” she said.
Neurocognitive problems
HIV has been linked to neurocognitive decline since the beginning of the epidemic, Dr. Althoff noted. The term HIV-associated neurocognitive disorders encompasses the broad spectrum of cognitive effects of HIV, from asymptomatic illness to AIDS-related dementia. Estimates of cognitive impairment in people with HIV range from 14% to 64% across various study populations, but diagnosing and treating it in the community can be challenging.
“Routine monitoring of cognition is often just out of reach in the clinical setting, due to the time it takes to use validated tools. We need a deeper toolbox of quick and validated tools calibrated to people with HIV in order to accurately monitor cognition,” she said.
She noted that the average age of onset of Alzheimer’s disease in the general population is 80 years, and that relatively few people with HIV infection have reached that age.
“But before the population age distribution shifts to the older ages, we can do more to monitor cognition in people with HIV,” she added.
In addition to HIV, factors that can contribute to worse neurocognitive outcomes include major depressive disorder, occurring in and estimated 20%-40% of adults with HIV versus 8% of the U.S. population, generalized anxiety disorder (10%-25% vs. 3%), bipolar disorder (3%-9% vs. 3%), schizophrenia (4%-15% vs. 1%), and posttraumatic stress disorder (10%-30% vs. 8%).
Substance use and polypharmacy, common among adults with HIV, can also contribute to cognitive decline, she said.
Decreased mobility
The Multicenter AIDS Cohort Study (MACS) showed that decreased mobility, defined as a gait speed less than 1 m/sec, occurred earlier in life among HIV-positive men than in HIV-negative men.
In the general aging population, slow gait speed is a predictor for lower extremity limitations, hospitalization, and death, and in more recent MACS studies was associated with increased hemoglobin A1C levels, as well as neurocognitive impairment.
“Hemoglobin A1C is an intervenable target, and perhaps it will help to slow the decline in gait speed,” Dr. Althoff said.
Reduce ‘healthspan’ disparities
The goal for treating aging adults with HIV “is to reduce the disparity in healthspan between people with HIV compared to people without HIV by delaying or eliminating the onset of comorbidities among people with HIV,” she said.
The gerontological concept of extending “healthspan” – the duration of life without significant comorbidities – is to target common mechanisms of aging, thereby delaying the onset of more than one age-related disease at the same time.
“Crude translation of this concept to the population of aging with HIV includes reducing that gap in comorbidity-free survival in people with versus without HIV,” she said.
Modification of care models from geriatrics may help infectious disease specialists manage adults with HIV who have increasingly complex needs.
For example, the geriatric “5 M” model emphasizes focusing on issues of mind (mentation, dementia, delirium, depression), mobility (impaired gait and balance, as well as fall prevention), medications (reducing polypharmacy, optimal prescribing), multicomplexity (multiple morbidities and complex bio-psycho-social situations), and “matters most” (each patient’s individual meaningful health outcome goals and care preferences).
Changing exposures that may influence the pattern of comorbidities for patients with HIV in the future include earlier start on ART, shorter duration of uncontrolled viremia, compared with older populations, newer and less toxic ARTs, long-term viral suppression, and risk factor interventions, Dr. Althoff concluded.
Dr. Lampiris noted that “patients who have initiated therapy in the last 5-10 years are more likely to initiate antiretroviral therapy at higher CD4 counts, and less likely to experience long-term toxicities of antiretroviral therapy. However, African Americans, Hispanics and HIV-positive women continue to lag behind others with regard to timely initiation of treatment.
“In addition there are toxicities associated with the newer agents, particularly weight gain, which disproportionately affect African Americans and women and which may be made worse by poverty, food insecurity, and other health-related behaviors.”
Dr. Athloff’s work is supported by grants from the National Institutes for Health. She disclosed serving as a consultant to the NIH-funded All of US study and to MediQ, and as an adviser to TrioHealth. Dr. Lampiris reported having no disclosures.
FROM IDWEEK 2020
Biometric changes on fitness trackers, smartwatches detect COVID-19
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.
A smartphone app that combines passively collected physiologic data from wearable devices, such as fitness trackers, and self-reported symptoms can discriminate between COVID-19–positive and –negative individuals among those who report symptoms, new data suggest.
After analyzing data from more than 30,000 participants, researchers from the Digital Engagement and Tracking for Early Control and Treatment (DETECT) study concluded that adding individual changes in sensor data improves models based on symptoms alone for differentiating symptomatic persons who are COVID-19 positive and symptomatic persons who are COVID-19 negative.
The combination can potentially identify infection clusters before wider community spread occurs, Giorgio Quer, PhD, and colleagues report in an article published online Oct. 29 in Nature Medicine. DETECT investigators note that marrying participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and resting heart rate, resulted in an area under the curve (AUC) of 0.80 (interquartile range [IQR], 0.73-0.86) for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
“By better characterizing each individual’s unique baseline, you can then identify changes that may indicate that someone has a viral illness,” said Dr. Quer, director of artificial intelligence at Scripps Research Translational Institute in La Jolla, Calif. “In previous research, we found that the proportion of individuals with elevated resting heart rate and sleep duration compared with their normal could significantly improve real-time detection of influenza-like illness rates at the state level,” he said in an interview.
Thus, continuous passively captured data may be a useful adjunct to bricks-and-mortar site testing, which is generally a one-off or infrequent sampling assay and is not always easily accessible, he added. Furthermore, traditional screening with temperature and symptom reporting is inadequate. An elevation in temperature is not as common as frequently believed for people who test positive for COVID-19, Dr. Quer continued. “Early identification via sensor variables of those who are presymptomatic or even asymptomatic would be especially valuable, as people may potentially be infectious during this period, and early detection is the ultimate goal,” Dr. Quer said.
According to his group, adding these physiologic changes from baseline values significantly outperformed detection (P < .01) using a British model described in an earlier study by by Cristina Menni, PhD, and associates. That method, in which symptoms were considered alone, yielded an AUC of 0.71 (IQR, 0.63-0.79).
According to Dr. Quer, one in five Americans currently wear an electronic device. “If we could enroll even a small percentage of these individuals, we’d be able to potentially identify clusters before they have the opportunity to spread,” he said.
DETECT study details
During the period March 15 to June 7, 2020, the study enrolled 30,529 participants from all 50 states. They ranged in age from younger than 35 years (23.1%) to older than 65 years (12.8%); the majority (63.5%) were aged 35-65 years, and 62% were women. Sensor devices in use by the cohort included Fitbit activity trackers (78.4%) and Apple HealthKit (31.2%).
Participants downloaded an app called MyDataHelps, which collects smartwatch and activity tracker information, including self-reported symptoms and diagnostic testing results. The app also monitors changes from baseline in resting heart rate, sleep duration, and physical activity, as measured by steps.
Overall, 3,811 participants reported having at least one symptom of some kind (e.g., fatigue, cough, dyspnea, loss of taste or smell). Of these, 54 reported testing positive for COVID-19, and 279 reported testing negative.
Sleep and activity were significantly different for the positive and negative groups, with an AUC of 0.68 (IQR, 0.57-0.79) for the sleep metric and 0.69 (IQR, 0.61-0.77) for the activity metric, suggesting that these parameters were more affected in COVID-19–positive participants.
When the investigators combined resting heart rate, sleep, and activity into a single metric, predictive performance improved to an AUC of 0.72 (IQR, 0.64-0.80).
The next step, Dr. Quer said, is to include an alert to notify users of possible infection.
Alerting users to possible COVID-19 infection
In a similar study, an alert feature was already incorporated. The study, led by Michael P. Snyder, PhD, director of the Center for Genomics and Personalized Medicine at Stanford (Calif.) University, will soon be published online in Nature Biomedical Engineering. In that study, presymptomatic detection of COVID-19 was achieved in more than 80% of participants using resting heart rate.
“The median is 4 days prior to symptom formation,” Dr. Snyder said in an interview. “We have an alarm system to notify people when their heart rate is elevated. So a positive signal from a smartwatch can be used to follow up by polymerase chain reaction [testing].”
Dr. Snyder said these approaches offer a roadmap to containing widespread infections. “Public health authorities need to be open to these technologies and begin incorporating them into their tracking,” he said. “Right now, people do temperature checks, which are of limited value. Resting heart rate is much better information.”
Although the DETECT researchers have not yet received feedback on their results, they believe public health authorities could recommend the use of such apps. “These are devices that people routinely wear for tracking their fitness and sleep, so it would be relatively easy to use the data for viral illness tracking,” said co–lead author Jennifer Radin, PhD, an epidemiologist at Scripps. “Testing resources are still limited and don’t allow for routine serial testing of individuals who may be asymptomatic or presymptomatic. Wearables can offer a different way to routinely monitor and screen people for changes in their data that may indicate COVID-19.”
The marshaling of data through consumer digital platforms to fight the coronavirus is gaining ground. New York State and New Jersey are already embracing smartphone apps to alert individuals to possible exposure to the virus.
More than 710,000 New Yorkers have downloaded the COVID NY Alert app, launched in October to help protect individuals and communities from COVID-19 by sending alerts without compromising privacy or personal information. “Upon receiving a notification about a potential exposure, users are then able to self-quarantine, get tested, and reduce the potential exposure risk to family, friends, coworkers, and others,” Jonah Bruno, a spokesperson for the New York State Department of Health, said in an interview.
And recently the Mayo Clinic and Safe Health Systems launched a platform to store COVID-19 testing and vaccination data.
Both the Scripps and Stanford platforms are part of a global technologic response to the COVID-19 pandemic. Prospective studies, led by device manufacturers and academic institutions, allow individuals to voluntarily share sensor and clinical data to address the crisis. Similar approaches have been used to track COVID-19 in large populations in Germany via the Corona Data Donation app.
The study by Dr. Quer and colleagues was funded by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. One coauthor reported grants from Janssen and personal fees from Otsuka and Livongo outside of the submitted work. The other authors have disclosed no relevant financial relationships. Dr. Snyder has ties to Personalis, Qbio, January, SensOmics, Protos, Mirvie, and Oralome.
A version of this article originally appeared on Medscape.com.