User login
Adding cetuximab to afatinib provides no benefit in EGFR-mutant NSCLC
In addition, toxicity was greater with the afatinib/cetuximab combination, according to study author Sarah S. Goldberg, MD, of Yale University, New Haven, Conn.
Dr. Goldberg and colleagues reported these results, from the SWOG S1403 trial, in the Journal of Clinical Oncology.
The authors noted that activating EGFR mutations are present in about 15% of patients with lung adenocarcinomas in Western populations and the mutations confer heightened sensitivity to EGFR tyrosine kinase inhibitors (TKIs). EGFR-TKIs have been shown to improve clinical outcomes, quality of life, and toxicity when compared with chemotherapy.
Based on better outcomes over chemotherapy, the third-generation EGFR-TKI osimertinib is now the standard treatment for patients with T790M-mediated resistance, but osimertinib is not effective in TKI-resistant T790M-negative disease, the authors pointed out.
In a phase 1b trial of patients with EGFR-mutant NSCLC with acquired resistance to first-generation agents, afatinib/cetuximab produced a response rate of 29% and comparable activity regardless of T790M status.
The aim of the SWOG S1403 study was to test whether adding cetuximab to afatinib would improve PFS over afatinib alone in patients with treatment-naive, EGFR-mutant NSCLC by preventing or delaying resistance.
Trial details
The phase 2, multicenter trial included 168 eligible patients with EGFR-mutant NSCLC without prior treatment of advanced disease. The patients’ median age was 66 years (range, 27-93 years), and 66% were women.
The most common histology was adenocarcinoma (96%). EGFR exon 19 deletions were detected in 64% of patients and L858R point mutations in 36%.
Patients were randomly assigned 2:1 to receive afatinib at 40 mg orally daily plus cetuximab at 500 mg/m2 intravenously every 2 weeks or afatinib at 40 mg alone. Patients received diphenhydramine at 50 mg intravenously before the first dose of cetuximab to prevent hypersensitivity reaction, and it was recommended before subsequent doses.
Patients continued on treatment until disease progression, symptomatic deterioration, unacceptable toxicity, pregnancy, treatment delay greater than 28 days, or patient decision. The study’s primary endpoint was PFS.
Further accrual not supported
At the interim analysis, the SWOG data safety and monitoring committee decided there was insufficient evidence to support further accrual, and the trial was closed.
The primary endpoint analysis revealed a median PFS of 11.9 months in the afatinib/cetuximab group and 13.4 months in the afatinib-alone group (hazard ratio, 1.01; 95% confidence interval, 0.72-1.43; P = .94). A subset analysis showed no PFS differences based on clinical or tumor characteristics.
Overall survival, time to response, and overall response rate were not improved in the afatinib/cetuximab arm.
PFS and overall survival were longer in patients with tumors harboring exon 19 deletions than in patients with L858R mutations. However, there were no mutation subtype–based PFS and overall survival differences between the treatment arms.
Grade 3 or higher treatment-related adverse events were more common in the combination arm than in the monotherapy arm (72% and 40%, respectively; P < .0001).
The most common grade 3 or higher treatment-related adverse events (in the combination and monotherapy arms, respectively) were acneiform rash (27% and 2%), maculopapular rash (13% and 0%), and diarrhea (15% and 20%).
Patients receiving afatinib plus cetuximab required dose reductions more often (56.7% vs. 26.2%), and treatment discontinuation because of an adverse event was more frequent in the combination arm (14% vs. 11%).
Turn toward third-generation drug
“Treatment with a single-agent EGFR-TKI remains the standard of care for patients with EGFR-mutant NSCLC,” Dr. Goldberg and colleagues wrote.
Why the combination of afatinib and cetuximab, which has demonstrated activity in the resistance setting, failed in the first-line setting remains unclear, Dr. Goldberg observed in an interview.
She noted that about one-quarter of patients receiving the afatinib/cetuximab combination discontinued cetuximab because of toxicity.
“That’s a good amount. It could be part of the explanation,” Dr. Goldberg said.
Investigators are currently analyzing collected tissue and blood samples in an effort to identify biomarkers that could potentially predict subgroups receiving benefit.
“That’s our next step: looking at specific EGFR mutation types, comutation types, and amplification of other genes and of EGFR,” Dr. Goldberg said.
She noted that, because of a superior side-effect profile and possibly greater efficacy, osimertinib is being used in the first-line setting.
“So now the idea of combining an EGFR-TKI with an EGFR antibody is still an active area of research, but with osimertinib rather than a first- or second-generation drug,” she said.
This research was supported by Boehringer Ingelheim, Eli Lilly, grants from the National Institutes of Health/National Cancer Institute, The Hope Foundation Career Development Award, and the SWOG and NIH Yale SPORE in Lung Cancer Grant. Dr. Goldberg and colleagues disclosed relationships with many pharmaceutical companies.
SOURCE: Goldberg SB et al. J Clin Oncol. 2020 Oct 6. doi: 10.1200/JCO.20.01149.
In addition, toxicity was greater with the afatinib/cetuximab combination, according to study author Sarah S. Goldberg, MD, of Yale University, New Haven, Conn.
Dr. Goldberg and colleagues reported these results, from the SWOG S1403 trial, in the Journal of Clinical Oncology.
The authors noted that activating EGFR mutations are present in about 15% of patients with lung adenocarcinomas in Western populations and the mutations confer heightened sensitivity to EGFR tyrosine kinase inhibitors (TKIs). EGFR-TKIs have been shown to improve clinical outcomes, quality of life, and toxicity when compared with chemotherapy.
Based on better outcomes over chemotherapy, the third-generation EGFR-TKI osimertinib is now the standard treatment for patients with T790M-mediated resistance, but osimertinib is not effective in TKI-resistant T790M-negative disease, the authors pointed out.
In a phase 1b trial of patients with EGFR-mutant NSCLC with acquired resistance to first-generation agents, afatinib/cetuximab produced a response rate of 29% and comparable activity regardless of T790M status.
The aim of the SWOG S1403 study was to test whether adding cetuximab to afatinib would improve PFS over afatinib alone in patients with treatment-naive, EGFR-mutant NSCLC by preventing or delaying resistance.
Trial details
The phase 2, multicenter trial included 168 eligible patients with EGFR-mutant NSCLC without prior treatment of advanced disease. The patients’ median age was 66 years (range, 27-93 years), and 66% were women.
The most common histology was adenocarcinoma (96%). EGFR exon 19 deletions were detected in 64% of patients and L858R point mutations in 36%.
Patients were randomly assigned 2:1 to receive afatinib at 40 mg orally daily plus cetuximab at 500 mg/m2 intravenously every 2 weeks or afatinib at 40 mg alone. Patients received diphenhydramine at 50 mg intravenously before the first dose of cetuximab to prevent hypersensitivity reaction, and it was recommended before subsequent doses.
Patients continued on treatment until disease progression, symptomatic deterioration, unacceptable toxicity, pregnancy, treatment delay greater than 28 days, or patient decision. The study’s primary endpoint was PFS.
Further accrual not supported
At the interim analysis, the SWOG data safety and monitoring committee decided there was insufficient evidence to support further accrual, and the trial was closed.
The primary endpoint analysis revealed a median PFS of 11.9 months in the afatinib/cetuximab group and 13.4 months in the afatinib-alone group (hazard ratio, 1.01; 95% confidence interval, 0.72-1.43; P = .94). A subset analysis showed no PFS differences based on clinical or tumor characteristics.
Overall survival, time to response, and overall response rate were not improved in the afatinib/cetuximab arm.
PFS and overall survival were longer in patients with tumors harboring exon 19 deletions than in patients with L858R mutations. However, there were no mutation subtype–based PFS and overall survival differences between the treatment arms.
Grade 3 or higher treatment-related adverse events were more common in the combination arm than in the monotherapy arm (72% and 40%, respectively; P < .0001).
The most common grade 3 or higher treatment-related adverse events (in the combination and monotherapy arms, respectively) were acneiform rash (27% and 2%), maculopapular rash (13% and 0%), and diarrhea (15% and 20%).
Patients receiving afatinib plus cetuximab required dose reductions more often (56.7% vs. 26.2%), and treatment discontinuation because of an adverse event was more frequent in the combination arm (14% vs. 11%).
Turn toward third-generation drug
“Treatment with a single-agent EGFR-TKI remains the standard of care for patients with EGFR-mutant NSCLC,” Dr. Goldberg and colleagues wrote.
Why the combination of afatinib and cetuximab, which has demonstrated activity in the resistance setting, failed in the first-line setting remains unclear, Dr. Goldberg observed in an interview.
She noted that about one-quarter of patients receiving the afatinib/cetuximab combination discontinued cetuximab because of toxicity.
“That’s a good amount. It could be part of the explanation,” Dr. Goldberg said.
Investigators are currently analyzing collected tissue and blood samples in an effort to identify biomarkers that could potentially predict subgroups receiving benefit.
“That’s our next step: looking at specific EGFR mutation types, comutation types, and amplification of other genes and of EGFR,” Dr. Goldberg said.
She noted that, because of a superior side-effect profile and possibly greater efficacy, osimertinib is being used in the first-line setting.
“So now the idea of combining an EGFR-TKI with an EGFR antibody is still an active area of research, but with osimertinib rather than a first- or second-generation drug,” she said.
This research was supported by Boehringer Ingelheim, Eli Lilly, grants from the National Institutes of Health/National Cancer Institute, The Hope Foundation Career Development Award, and the SWOG and NIH Yale SPORE in Lung Cancer Grant. Dr. Goldberg and colleagues disclosed relationships with many pharmaceutical companies.
SOURCE: Goldberg SB et al. J Clin Oncol. 2020 Oct 6. doi: 10.1200/JCO.20.01149.
In addition, toxicity was greater with the afatinib/cetuximab combination, according to study author Sarah S. Goldberg, MD, of Yale University, New Haven, Conn.
Dr. Goldberg and colleagues reported these results, from the SWOG S1403 trial, in the Journal of Clinical Oncology.
The authors noted that activating EGFR mutations are present in about 15% of patients with lung adenocarcinomas in Western populations and the mutations confer heightened sensitivity to EGFR tyrosine kinase inhibitors (TKIs). EGFR-TKIs have been shown to improve clinical outcomes, quality of life, and toxicity when compared with chemotherapy.
Based on better outcomes over chemotherapy, the third-generation EGFR-TKI osimertinib is now the standard treatment for patients with T790M-mediated resistance, but osimertinib is not effective in TKI-resistant T790M-negative disease, the authors pointed out.
In a phase 1b trial of patients with EGFR-mutant NSCLC with acquired resistance to first-generation agents, afatinib/cetuximab produced a response rate of 29% and comparable activity regardless of T790M status.
The aim of the SWOG S1403 study was to test whether adding cetuximab to afatinib would improve PFS over afatinib alone in patients with treatment-naive, EGFR-mutant NSCLC by preventing or delaying resistance.
Trial details
The phase 2, multicenter trial included 168 eligible patients with EGFR-mutant NSCLC without prior treatment of advanced disease. The patients’ median age was 66 years (range, 27-93 years), and 66% were women.
The most common histology was adenocarcinoma (96%). EGFR exon 19 deletions were detected in 64% of patients and L858R point mutations in 36%.
Patients were randomly assigned 2:1 to receive afatinib at 40 mg orally daily plus cetuximab at 500 mg/m2 intravenously every 2 weeks or afatinib at 40 mg alone. Patients received diphenhydramine at 50 mg intravenously before the first dose of cetuximab to prevent hypersensitivity reaction, and it was recommended before subsequent doses.
Patients continued on treatment until disease progression, symptomatic deterioration, unacceptable toxicity, pregnancy, treatment delay greater than 28 days, or patient decision. The study’s primary endpoint was PFS.
Further accrual not supported
At the interim analysis, the SWOG data safety and monitoring committee decided there was insufficient evidence to support further accrual, and the trial was closed.
The primary endpoint analysis revealed a median PFS of 11.9 months in the afatinib/cetuximab group and 13.4 months in the afatinib-alone group (hazard ratio, 1.01; 95% confidence interval, 0.72-1.43; P = .94). A subset analysis showed no PFS differences based on clinical or tumor characteristics.
Overall survival, time to response, and overall response rate were not improved in the afatinib/cetuximab arm.
PFS and overall survival were longer in patients with tumors harboring exon 19 deletions than in patients with L858R mutations. However, there were no mutation subtype–based PFS and overall survival differences between the treatment arms.
Grade 3 or higher treatment-related adverse events were more common in the combination arm than in the monotherapy arm (72% and 40%, respectively; P < .0001).
The most common grade 3 or higher treatment-related adverse events (in the combination and monotherapy arms, respectively) were acneiform rash (27% and 2%), maculopapular rash (13% and 0%), and diarrhea (15% and 20%).
Patients receiving afatinib plus cetuximab required dose reductions more often (56.7% vs. 26.2%), and treatment discontinuation because of an adverse event was more frequent in the combination arm (14% vs. 11%).
Turn toward third-generation drug
“Treatment with a single-agent EGFR-TKI remains the standard of care for patients with EGFR-mutant NSCLC,” Dr. Goldberg and colleagues wrote.
Why the combination of afatinib and cetuximab, which has demonstrated activity in the resistance setting, failed in the first-line setting remains unclear, Dr. Goldberg observed in an interview.
She noted that about one-quarter of patients receiving the afatinib/cetuximab combination discontinued cetuximab because of toxicity.
“That’s a good amount. It could be part of the explanation,” Dr. Goldberg said.
Investigators are currently analyzing collected tissue and blood samples in an effort to identify biomarkers that could potentially predict subgroups receiving benefit.
“That’s our next step: looking at specific EGFR mutation types, comutation types, and amplification of other genes and of EGFR,” Dr. Goldberg said.
She noted that, because of a superior side-effect profile and possibly greater efficacy, osimertinib is being used in the first-line setting.
“So now the idea of combining an EGFR-TKI with an EGFR antibody is still an active area of research, but with osimertinib rather than a first- or second-generation drug,” she said.
This research was supported by Boehringer Ingelheim, Eli Lilly, grants from the National Institutes of Health/National Cancer Institute, The Hope Foundation Career Development Award, and the SWOG and NIH Yale SPORE in Lung Cancer Grant. Dr. Goldberg and colleagues disclosed relationships with many pharmaceutical companies.
SOURCE: Goldberg SB et al. J Clin Oncol. 2020 Oct 6. doi: 10.1200/JCO.20.01149.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Primary care workforce expanding, but mostly in cities
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
researchers say.
The finding may provide some reassurance for those who have worried about a shortage of health care workers and whether they will be able to meet the nation’s growing burden of chronic diseases.
“Access to primary care doctors is critical to population health and to reduce health care disparities in this country,” said Donglan Zhang, PhD, an assistant professor of public health at the University of Georgia, Athens.
However, many counties remain underserved, Dr. Zhang said in an interview. The need for primary care in the United States is increasing not only with population growth but because the population is aging.
Dr. Zhang and colleagues published the finding in JAMA Network Open.
Many previous reports have warned of a shortage in primary care providers. To examine recent trends in the primary care workforce, Dr. Zhang and colleagues obtained data on all the primary care clinicians registered with the Centers for Medicare & Medicaid Services from 2009 to 2017.
For the study, the researchers included general practitioners, family physicians and internists without subspecialties, nurse practitioners, and physician assistants. They then compared the number of providers with the number of residents in each county as recorded by the US Census, using urban or rural classifications for each county from the Centers for Disease Control and Prevention.
Because the U.S. Health Resources and Services Administration defines a primary care “shortage” as fewer than 1 primary care practitioner per 3,500 people, the researchers focused on this ratio. They found that the number of nurse practitioners and physician assistants was increasing much faster than the number of primary care physicians. This was true especially in rural areas, but the percentage increase for both nurse practitioners and physician assistants was lower in rural areas versus urban.
The researchers also found that there were more primary care physicians per capita in counties with higher household incomes, a higher proportion of Asian residents, and a higher proportion of college graduates.
They didn’t find a significant association between the median household income and per capita number of nurse practitioners.
They found that counties with a higher proportion of Black and Asian residents had a higher number of nurse practitioners per capita. But they found an opposite association between the proportion of Black residents and the number of physician assistants per capita.
The authors hypothesized that health care reform, particularly the passage of the Affordable Care Act in 2010, may explain the recent increase in the primary care workforce. The legislation expanded the number of people with health insurance and provided incentives for primary and preventive care.
Another factor behind the increase in the primary care workforce could be state laws that have expanded the scope of practice for nurse practitioners and primary care providers, she said.
Numbers may overestimate available care
The gap between rural and urban areas could be even wider than this study suggests, Ada D. Stewart, MD, president of the American Academy of Family Physicians, said in an interview. Many nurse practitioners and physician assistants don’t actually practice primary care, but instead assist physicians in other specialties such as orthopedics or general surgery.

“They are part of a team and I don’t want to diminish that at all, but especially when we talk about infant and maternal mortality, family physicians need to be there themselves providing primary care,” she said. “We’re there in hospitals and emergency rooms, and not just taking care of diabetes and hypertension.”
In addition, the primary care workforce may have been reduced since the conclusion of the study period (Dec. 31, 2017) as a result of the COVID-19 pandemic forcing some primary care physicians into retirement, Dr. Stewart said.
Measures that could help reduce the disparity include a more robust system of teaching health centers in rural counties, higher reimbursement for primary care, a lower cost of medical education, and recruiting more people from rural areas to become physicians, Dr. Stewart said.
Telehealth can enhance health care in rural areas, but many people in rural areas lack internet or cellular service, or don’t have access to computers. “We don’t want to create another healthcare disparity,” she said.
And physicians can get to know their patients’ needs better in a face-to-face visit, she said. “Telehealth does have a place, but it does not replace that person-to-person visit.”
This study was funded by National Institute on Minority Health and Health Disparities. Dr. Zhang and Dr. Stewart disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
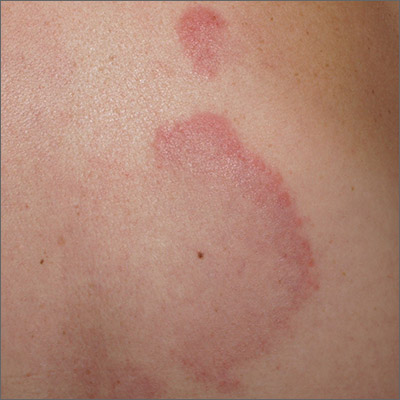
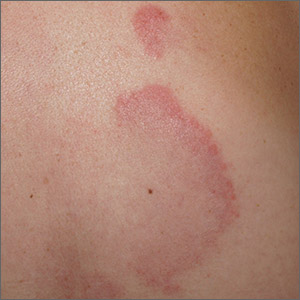
Arcuate eruption on the back
A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
A punch biopsy of the markedly erythematous lateral edge helped to confirm this as tumid lupus erythematosus (TLE), a rare subtype of chronic cutaneous lupus erythematosus. TLE occurs in men and women of all ages. Annular or arcuate patches and plaques most often arise on the face, trunk, extremities, and V of the neck after sun exposure. However, as in this case, plaques may appear in areas covered by clothing. Plaques generally do not itch or hurt, but their presence can be alarming.
Annular and arcuate plaques raise a complex differential diagnosis including common conditions such as urticaria and tinea corporis, as well as more uncommon disorders such as erythema annulare centrifugum and lymphoma cutis. Unlike tinea corporis and erythema annulare centrifugum, there is very little, if any, scaling of the superficial epidermis. Plaques heal without scarring or changes to skin pigmentation.
Multiple punch biopsies of affected areas are key to a proper diagnosis. Patients with confirmed TLE should undergo antinuclear antibody testing to rule out systemic lupus erythematosus, although the vast majority will have normal results.
Treatment includes potent or ultrapotent topical steroids for the trunk and extremities, and mid- to low-potency steroids for intertriginous areas or the face. Systemic immunomodulators with hydroxychloroquine are used as first-line treatment for more extensive disease.
In this case, the patient had a normal antinuclear antibody titer and was treated with topical betamethasone dipropionate augmented 0.05% cream bid for 2 weeks, which led to complete clearance. She experienced a flare-up a year later and was retreated with the same results.
Text and photos courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. (Photo copyright retained.)
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033–1041.
Black patients less likely to receive H. pylori eradication testing
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
Black patients may be significantly less likely to receive eradication testing after treatment for Helicobacter pylori infection than patients of other races/ethnic groups, based on a retrospective analysis of more than 1,700 individuals.
This disparity may exacerbate the already increased burden of H. pylori infection and gastric cancer among Black individuals, according to principal author David A. Leiman, MD, MSHP, of Duke University Medical Center in Durham, N.C.
“H. pylori infection disproportionately affects racial/ethnic minorities and those of lower socioeconomic status,” Dr. Leiman, coauthor Julius Wilder, MD, PhD, of Duke University in Durham, and colleagues wrote in their abstract presented at the annual meeting of the American College of Gastroenterology. “ACG guidelines recommend treatment for H. pylori infection followed by confirmation of cure. Adherence to these recommendations varies and its impact on practice patterns is unclear. This study characterizes the management of H. pylori infection and predictors of guideline adherence.”
The investigators analyzed electronic medical records from 1,711 patients diagnosed with H. pylori infection through the Duke University Health System between June 2016 and June 2018, most often (71%) via serum antibody test. Approximately two-thirds of those diagnosed were non-White (66%) and female (63%). Out of 1,711 patients, 622 (36%) underwent eradication testing, of whom 559 (90%) were cured.
Despite publication of the ACG H. pylori guideline midway through the study (February 2017), testing rates dropped significantly from 43.1% in 2016 to 35.9% in 2017, and finally 25.5% in 2018 (P < .0001).
“These findings are consistent with other work that has shown low rates of testing to confirm cure in patients treated for H. pylori,” Dr. Leiman said. “There remains a disappointingly low number of patients who are tested for cure.”
Across the entire study period, patients were significantly more likely to undergo eradication testing if they were treated in the gastroenterology department (52.4%), compared with rates ranging from 33% to 34.6% for internal medicine, family medicine, and other departments (P < .001).
Across all departments, Black patients underwent eradication testing significantly less often than patients of other races/ethnicities, at a rate of 30.5% versus 32.2% for White patients, 35.1% for Asian patients, and 36.7% for patients who were of other backgrounds (P < .001). Compared with White patients, Black patients were 38% less likely to undergo eradication testing (odds ratio, 0.62; 95% confidence interval, 0.48-0.79).
Dr. Leiman noted that these findings contrast with a study by Dr. Shria Kumar and colleagues from earlier this year, which found no racial disparity in eradication testing within a Veterans Health Affairs cohort.
“Black patients are significantly less likely to undergo testing for eradication than [patients of other races/ethnicities],” Dr. Leiman said. “More work is needed to understand the mechanisms driving this disparity.” He suggested a number of possible contributing factors, including provider knowledge gaps, fragmented care, and social determinants of health.
“It is clear that a greater emphasis on characterizing and addressing the social determinants of health, including poverty, education, and location, are needed,” Dr. Leiman said. “Although health systems are not solely responsible for the known and ongoing observations of disparities in care, interventions must be identified and implemented to mitigate these issues.” Such interventions would likely require broad participation, he said, including policy makers, health systems, and individual practitioners.
“We plan to perform a prospective mixed methods study to contextualize which social determinants are associated with a decreased likelihood of receiving appropriate eradication testing by exploring barriers at patient, practitioner, and health-system levels,” Dr. Leiman said. “Ultimately, we aim to leverage these findings to develop an evidence-based intervention to circumnavigate those identified barriers, thereby eliminating the observed disparities in H. pylori care.”
According to Gregory L. Hall, MD, of Northeast Ohio Medical University, Rootstown, and Case Western Reserve University, Cleveland, and codirector of the Partnership for Urban Health Research, Atlanta, the higher rate of H. pylori infection in Black individuals may stem partly from genetic factors.
“Studies have shown that African Americans with a higher proportion of African ancestry have higher rates of H. pylori, suggesting a genetic component to this increased risk,” he said.
Still, Dr. Hall, who is the author of the book Patient-Centered Clinical Care for African Americans, went on to emphasize appropriate H. pylori management and recognition of racial disparities in medicine.
“The ability to test for, treat, and confirm eradication of H. pylori infections represents a great opportunity to improve quality of life through decreased gastritis, gastric ulcers, and gastric cancer,” he said. “[The present findings] show yet another disparity in our clinical care of African Americans that needs increased awareness among providers to these communities.”
Rotonya Carr, MD, of the Hospital of the University of Pennsylvania, Philadelphia, and lead author of a recent publication addressing racism and health disparities in gastroenterology, said the findings of the present study add weight to a known equity gap.
“These data are concerning in view of the twofold higher prevalence of H. pylori seropositivity and twofold higher incidence of gastric cancer in Black patients, compared with White patients,” Dr. Carr said. “These and other data support a comprehensive approach to reduce GI disparities that includes targeted education of both GI specialists and referring providers.”
According to Dr. Leiman, individual practitioners may work toward more equitable outcomes through a comprehensive clinical approach, regardless of patient race or ethnicity.
“Clinicians should consider H. pylori therapy an episode of care that spans diagnosis, treatment, and confirmation of cure,” he said. “Closing the loop in that episode by ensuring eradication is vital to conforming with best practices, and to reduce patients’ long-term risks.”The investigators disclosed relationships with Exact Sciences, Guardant Health, and Phathom Pharmaceuticals. Dr. Hall and Dr. Carr reported no relevant conflicts of interest.
SOURCE: Reichstein J et al. ACG 2020. Abstract S1332.
FROM ACG 2020
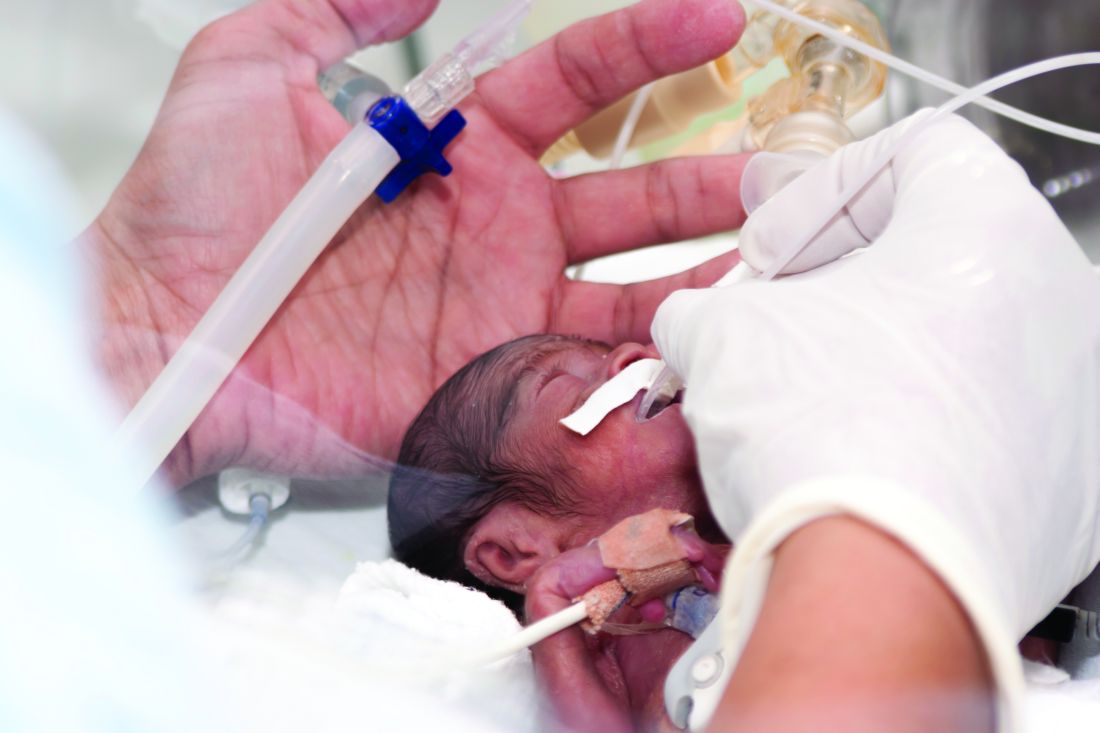
Proposed withdrawal of approval of preterm drug: Two opposing views
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Primary care journals address systemic racism in medicine
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
Sumi Sexton, MD, editor in chief of American Family Physician (AFP), said in an interview she had been working on changes at her journal that would answer the need for action that was made clear by this summer’s Black Lives Matter protests and realized the issue was much bigger than one journal. She proposed the collaboration with the other editors.
The editors wrote a joint statement explaining what they plan to do collectively. It was published online Oct. 15 ahead of print and will be published in all 10 journals at the beginning of the year.
Following the action by family medicine editors, the American College of Physicians issued a statement expressing commitment to being an antiracist organization. It calls on all doctors to speak out against hate and discrimination and to act against institutional and systemic racism. The statement also apologizes for the organization’s own past actions: “ACP acknowledges and regrets its own historical organizational injustices and inequities, and past racism, discrimination and exclusionary practices throughout its history, whether intentional or unintentional, by act or omission.”
Family medicine journals plan changes
Changes will differ at each family medicine publication, according to Sexton and other interviewees. Some specific changes at AFP, for example, include creating a medical editor role dedicated to diversity, equity, and inclusion to ensure that content is not only accurate but also that more content addresses racism, Dr. Sexton said.
AFP is creating a Web page dedicated to diversity and will now capitalize the word “Black” in racial and cultural references. Recent calls for papers have included emphasis on finding authors from underrepresented groups and on mentoring new authors.
“We really need to enable our colleagues,” Dr. Sexton said.
The journals are also pooling their published research on topics of racism and inclusion and have established a joint bibliography.
The steps are important, Dr. Sexton said, because reform in research will start a “cascade of action” that will result in better patient care.
“Our mission is to care for the individual as a whole person,” Dr. Sexton said. “This is part of that mission.”
Increasing diversity on editorial boards
Family physician Kameron Leigh Matthews, MD, chief medical officer for the Veterans Health Administration, praised the journals’ plan.
She noted that the groups are addressing diversity on their editorial boards, as well as evaluating content. Effective change must also happen regarding the people reviewing the content, she said in an interview. “It has to be both.
“I’m very proud as a family physician that our editors came together and are giving the right response. It’s not enough to say we stand against racism. They’re actually offering concrete actions that they will take as editors, and that will influence health care,” she said.
Dr. Matthews pointed to an example of what can happen when the editorial process fails and racism is introduced in research.
She cited the retraction of an article in the Journal of the American Heart Association entitled, “Evolution of Race and Ethnicity Considerations for the Cardiology Workforce.” The article advocated for ending racial and ethnic preferences in undergraduate and medical school admissions.
The American Heart Association said the article concluded “incorrectly that Black and Hispanic trainees in medicine are less qualified than White and Asian trainees.” The article had “rightfully drawn criticism for its misrepresentations and conclusions,” the AHA said, adding that it would launch an investigation into how the article came to be published.
Dr. Matthews says that’s why it’s so important that, in their statement, the family medicine editors vow to address not only the content but also the editing process to avoid similar systemic lapses.
Dr. Matthews added that, because the proportion of physicians from underrepresented groups is small – only 5% of physicians are Black and 6% are Hispanic – it is vital, as recommended in the editors’ statement, to mentor researchers from underrepresented groups and to reach out to students and residents to be coauthors.
“To sit back and say there’s not enough to recruit from is not sufficient,” Dr. Matthews said. “You need to recognize that you need to assist with expanding the pool.”
She also said she would like to see the journals focus more heavily on solutions to racial disparities in health care rather than on pointing them out.
At the Journal of Family Practice (JFP), Editor in Chief John Hickner, MD, said adding diversity to the editorial board is a top priority. He also reiterated that diversity in top leadership is a concern across all the journals, inasmuch as only 1 of the 10 editors in chief is a person of color.
As an editor, he said, he will personally, as well as through family medicine department chairs, be seeking authors who are members of underrepresented groups and that he will be assisting those who need help.
“I’m committed to giving them special attention in the editorial process,” he said.
Dr. Hickner said the 10 journals have also committed to periodically evaluate whether their approaches are making substantial changes. He said the editors have vowed to meet at least once a year to review progress “and hold each other accountable.”
Statement authors, in addition to Dr. Sexton and Dr. Hickner, include these editors in chief: Caroline R. Richardson, MD, Annals of Family Medicine; Sarina B. Schrager, MD, FPM; Marjorie A. Bowman, MD, The Journal of the American Board of Family Medicine; Christopher P. Morley, PhD, PRiMER; Nicholas Pimlott, MD, PhD, Canadian Family Physician; John W. Saultz, MD, Family Medicine; and Barry D. Weiss, MD, FP Essentials.
The authors have disclosed no relevant financial relationships. The Journal of Family Practice is owned by the same news organization as this publication.
A version of this article originally appeared on Medscape.com.
How to help families get through climate-related disasters
Wildfires burned millions of acres in California, Oregon, and Washington this year. Record numbers of tropical storms and hurricanes formed in the Atlantic. “Climate change is here. Disasters are here. They are going to be increasing, which is why we want to talk about this and talk about how pediatricians can help and respond to these events,” Scott Needle, MD, said at the annual meeting American Academy of Pediatrics, held virtually this year.

said Dr. Needle, chief medical officer of Elica Health Centers in Sacramento, California. “We can be a positive influence in terms of getting out proactive messaging and keeping people informed.”
The Federal Emergency Management Agency (FEMA) 2019 National Household Survey found that about half of households had an emergency plan. A theme across surveys is that, although households take some steps to get ready for disasters, the public generally “is not as prepared for these events as they really need to be,” Dr. Needle said.
The AAP, the Red Cross, and FEMA are among the organizations that offer planning guides, most of which emphasize three simple things: have a kit, have a plan, and be informed, he said.
To prepare for a disaster, parents might refill a child’s medications ahead of time if possible, Dr. Needle suggested. And during the COVID-19 pandemic, families should add masks, sanitizers, and wipes to their go-bags.
Physicians also can help families by asking how they are coping.
Wildfire smoke
“Smoke from wildfires can blanket large, large areas,” Mark Miller, MD, MPH, said during the presentation at the AAP meeting. “This year, we have seen wildfire smoke from the western states reach all the way to the East Coast. So this impacts your patients and your own families sometimes, regardless of wherever you live.”
Children may be more vulnerable to wildfire smoke because they often spend more time outdoors and tend to be more active. In addition, their ongoing development means exposure to air pollutants could have lifelong consequences, said Dr. Miller, who recently reviewed the effects of wildfire smoke on children.
“Children with asthma should have some information about wildfires built into their asthma management plan,” said Dr. Miller, who is affiliated with Western States Pediatric Environmental Health Specialty Unit (PEHSU) and University of California, San Francisco. Pollutants are associated with respiratory visits and admissions, asthma exacerbations, decreased lung function, and neurocognitive effects. They also may be carcinogenic.
A study in monkeys found that smoke exposure during California wildfires in 2008 was associated with immune dysregulation and compromised lung function in adolescence.
Another study of three cohorts of children in southern California found that air pollutant levels were associated with children’s lung function.
Organizations have provided resources on creating cleaner air spaces during wildfires, including guides to build DIY air filter fans. AirNow.gov provides air quality and fire maps that can inform decisions about school closures and outdoor activities. Communities should prioritize establishing schools as clean air shelters, Dr. Miller suggested.
Studies have found that respirators and medical masks may decrease children’s exposure to smoke. Children should not use face coverings, however, if they are younger than 2 years, if they are not able to remove the face covering on their own or tell an adult that they need help, or if they have difficulty breathing with a face covering. Younger children should be observed by an adult.
During the pandemic, families should be aware that some types of masks are sold only for health care use, many foreign respirators are counterfeit, and cloth masks used for COVID-19 are not suitable for reducing wildfire smoke exposure, Dr. Miller said.
Hazards may linger
Long-term mental health issues may be the disaster consequence that pediatricians encounter most often, Dr. Needle said.
Eighteen months after a major wildfire in Canada, more than one-third of middle and high school students in one community had probable posttraumatic stress disorder (that is, intrusive thoughts, avoidance, and increased arousal). In addition, 31% of students had probable depression. Rates were elevated relative to a control group of students in another community that was not affected by the fire.
Findings indicate that a patient’s degree of exposure to a disaster affects the likelihood of adverse outcomes. On the other hand, resiliency may help mitigate adverse effects. “The hope is that if we can find ways to encourage resiliency before or in the aftermath of an event, we may be able to, in a sense, reduce some of these mental health sequelae,” Dr. Needle said.
Posttraumatic reactions in kids are likely after a disaster. “They may not rise to the level of a diagnosable condition, but they are very common in kids,” he said. “It is important to at least be able to counsel parents to recognize some of the common reactions,” such as acting withdrawn or aggressive, somatic complaints, and having trouble sleeping.
The AAP has a policy statement that encourages talking to children about their concerns with honest and age-appropriate responses, he noted.
When returning to an area after a disaster, many hazards may remain, such as floodwaters, ash pits, mold, and carbon monoxide from generators. “Generally speaking, you don’t want to have kids return to these areas until it is safe,” Dr. Needle said.
Exacerbation of existing conditions – perhaps because of lost medications, smoke exposure, or stress – may be another common problem. Other problems after a disaster could include domestic violence (direct or witnessed) and substance abuse.
“We have a responsibility to take care of our own health as well,” Dr. Needle added. “You can’t take care of others if you’re not taking care of yourself. It’s not being selfish. As a matter of fact, it’s being prudent. It’s survival.”
Dr. Needle and Dr. Miller had no relevant financial disclosures. Dr. Miller’s presentation was supported by the AAP and funded in part by the Agency for Toxic Substances and Disease Registry. The U.S. Environmental Protection Agency (EPA) provides funding support for the Pediatric Environmental Health Specialty Unit.
Wildfires burned millions of acres in California, Oregon, and Washington this year. Record numbers of tropical storms and hurricanes formed in the Atlantic. “Climate change is here. Disasters are here. They are going to be increasing, which is why we want to talk about this and talk about how pediatricians can help and respond to these events,” Scott Needle, MD, said at the annual meeting American Academy of Pediatrics, held virtually this year.

said Dr. Needle, chief medical officer of Elica Health Centers in Sacramento, California. “We can be a positive influence in terms of getting out proactive messaging and keeping people informed.”
The Federal Emergency Management Agency (FEMA) 2019 National Household Survey found that about half of households had an emergency plan. A theme across surveys is that, although households take some steps to get ready for disasters, the public generally “is not as prepared for these events as they really need to be,” Dr. Needle said.
The AAP, the Red Cross, and FEMA are among the organizations that offer planning guides, most of which emphasize three simple things: have a kit, have a plan, and be informed, he said.
To prepare for a disaster, parents might refill a child’s medications ahead of time if possible, Dr. Needle suggested. And during the COVID-19 pandemic, families should add masks, sanitizers, and wipes to their go-bags.
Physicians also can help families by asking how they are coping.
Wildfire smoke
“Smoke from wildfires can blanket large, large areas,” Mark Miller, MD, MPH, said during the presentation at the AAP meeting. “This year, we have seen wildfire smoke from the western states reach all the way to the East Coast. So this impacts your patients and your own families sometimes, regardless of wherever you live.”
Children may be more vulnerable to wildfire smoke because they often spend more time outdoors and tend to be more active. In addition, their ongoing development means exposure to air pollutants could have lifelong consequences, said Dr. Miller, who recently reviewed the effects of wildfire smoke on children.
“Children with asthma should have some information about wildfires built into their asthma management plan,” said Dr. Miller, who is affiliated with Western States Pediatric Environmental Health Specialty Unit (PEHSU) and University of California, San Francisco. Pollutants are associated with respiratory visits and admissions, asthma exacerbations, decreased lung function, and neurocognitive effects. They also may be carcinogenic.
A study in monkeys found that smoke exposure during California wildfires in 2008 was associated with immune dysregulation and compromised lung function in adolescence.
Another study of three cohorts of children in southern California found that air pollutant levels were associated with children’s lung function.
Organizations have provided resources on creating cleaner air spaces during wildfires, including guides to build DIY air filter fans. AirNow.gov provides air quality and fire maps that can inform decisions about school closures and outdoor activities. Communities should prioritize establishing schools as clean air shelters, Dr. Miller suggested.
Studies have found that respirators and medical masks may decrease children’s exposure to smoke. Children should not use face coverings, however, if they are younger than 2 years, if they are not able to remove the face covering on their own or tell an adult that they need help, or if they have difficulty breathing with a face covering. Younger children should be observed by an adult.
During the pandemic, families should be aware that some types of masks are sold only for health care use, many foreign respirators are counterfeit, and cloth masks used for COVID-19 are not suitable for reducing wildfire smoke exposure, Dr. Miller said.
Hazards may linger
Long-term mental health issues may be the disaster consequence that pediatricians encounter most often, Dr. Needle said.
Eighteen months after a major wildfire in Canada, more than one-third of middle and high school students in one community had probable posttraumatic stress disorder (that is, intrusive thoughts, avoidance, and increased arousal). In addition, 31% of students had probable depression. Rates were elevated relative to a control group of students in another community that was not affected by the fire.
Findings indicate that a patient’s degree of exposure to a disaster affects the likelihood of adverse outcomes. On the other hand, resiliency may help mitigate adverse effects. “The hope is that if we can find ways to encourage resiliency before or in the aftermath of an event, we may be able to, in a sense, reduce some of these mental health sequelae,” Dr. Needle said.
Posttraumatic reactions in kids are likely after a disaster. “They may not rise to the level of a diagnosable condition, but they are very common in kids,” he said. “It is important to at least be able to counsel parents to recognize some of the common reactions,” such as acting withdrawn or aggressive, somatic complaints, and having trouble sleeping.
The AAP has a policy statement that encourages talking to children about their concerns with honest and age-appropriate responses, he noted.
When returning to an area after a disaster, many hazards may remain, such as floodwaters, ash pits, mold, and carbon monoxide from generators. “Generally speaking, you don’t want to have kids return to these areas until it is safe,” Dr. Needle said.
Exacerbation of existing conditions – perhaps because of lost medications, smoke exposure, or stress – may be another common problem. Other problems after a disaster could include domestic violence (direct or witnessed) and substance abuse.
“We have a responsibility to take care of our own health as well,” Dr. Needle added. “You can’t take care of others if you’re not taking care of yourself. It’s not being selfish. As a matter of fact, it’s being prudent. It’s survival.”
Dr. Needle and Dr. Miller had no relevant financial disclosures. Dr. Miller’s presentation was supported by the AAP and funded in part by the Agency for Toxic Substances and Disease Registry. The U.S. Environmental Protection Agency (EPA) provides funding support for the Pediatric Environmental Health Specialty Unit.
Wildfires burned millions of acres in California, Oregon, and Washington this year. Record numbers of tropical storms and hurricanes formed in the Atlantic. “Climate change is here. Disasters are here. They are going to be increasing, which is why we want to talk about this and talk about how pediatricians can help and respond to these events,” Scott Needle, MD, said at the annual meeting American Academy of Pediatrics, held virtually this year.

said Dr. Needle, chief medical officer of Elica Health Centers in Sacramento, California. “We can be a positive influence in terms of getting out proactive messaging and keeping people informed.”
The Federal Emergency Management Agency (FEMA) 2019 National Household Survey found that about half of households had an emergency plan. A theme across surveys is that, although households take some steps to get ready for disasters, the public generally “is not as prepared for these events as they really need to be,” Dr. Needle said.
The AAP, the Red Cross, and FEMA are among the organizations that offer planning guides, most of which emphasize three simple things: have a kit, have a plan, and be informed, he said.
To prepare for a disaster, parents might refill a child’s medications ahead of time if possible, Dr. Needle suggested. And during the COVID-19 pandemic, families should add masks, sanitizers, and wipes to their go-bags.
Physicians also can help families by asking how they are coping.
Wildfire smoke
“Smoke from wildfires can blanket large, large areas,” Mark Miller, MD, MPH, said during the presentation at the AAP meeting. “This year, we have seen wildfire smoke from the western states reach all the way to the East Coast. So this impacts your patients and your own families sometimes, regardless of wherever you live.”
Children may be more vulnerable to wildfire smoke because they often spend more time outdoors and tend to be more active. In addition, their ongoing development means exposure to air pollutants could have lifelong consequences, said Dr. Miller, who recently reviewed the effects of wildfire smoke on children.
“Children with asthma should have some information about wildfires built into their asthma management plan,” said Dr. Miller, who is affiliated with Western States Pediatric Environmental Health Specialty Unit (PEHSU) and University of California, San Francisco. Pollutants are associated with respiratory visits and admissions, asthma exacerbations, decreased lung function, and neurocognitive effects. They also may be carcinogenic.
A study in monkeys found that smoke exposure during California wildfires in 2008 was associated with immune dysregulation and compromised lung function in adolescence.
Another study of three cohorts of children in southern California found that air pollutant levels were associated with children’s lung function.
Organizations have provided resources on creating cleaner air spaces during wildfires, including guides to build DIY air filter fans. AirNow.gov provides air quality and fire maps that can inform decisions about school closures and outdoor activities. Communities should prioritize establishing schools as clean air shelters, Dr. Miller suggested.
Studies have found that respirators and medical masks may decrease children’s exposure to smoke. Children should not use face coverings, however, if they are younger than 2 years, if they are not able to remove the face covering on their own or tell an adult that they need help, or if they have difficulty breathing with a face covering. Younger children should be observed by an adult.
During the pandemic, families should be aware that some types of masks are sold only for health care use, many foreign respirators are counterfeit, and cloth masks used for COVID-19 are not suitable for reducing wildfire smoke exposure, Dr. Miller said.
Hazards may linger
Long-term mental health issues may be the disaster consequence that pediatricians encounter most often, Dr. Needle said.
Eighteen months after a major wildfire in Canada, more than one-third of middle and high school students in one community had probable posttraumatic stress disorder (that is, intrusive thoughts, avoidance, and increased arousal). In addition, 31% of students had probable depression. Rates were elevated relative to a control group of students in another community that was not affected by the fire.
Findings indicate that a patient’s degree of exposure to a disaster affects the likelihood of adverse outcomes. On the other hand, resiliency may help mitigate adverse effects. “The hope is that if we can find ways to encourage resiliency before or in the aftermath of an event, we may be able to, in a sense, reduce some of these mental health sequelae,” Dr. Needle said.
Posttraumatic reactions in kids are likely after a disaster. “They may not rise to the level of a diagnosable condition, but they are very common in kids,” he said. “It is important to at least be able to counsel parents to recognize some of the common reactions,” such as acting withdrawn or aggressive, somatic complaints, and having trouble sleeping.
The AAP has a policy statement that encourages talking to children about their concerns with honest and age-appropriate responses, he noted.
When returning to an area after a disaster, many hazards may remain, such as floodwaters, ash pits, mold, and carbon monoxide from generators. “Generally speaking, you don’t want to have kids return to these areas until it is safe,” Dr. Needle said.
Exacerbation of existing conditions – perhaps because of lost medications, smoke exposure, or stress – may be another common problem. Other problems after a disaster could include domestic violence (direct or witnessed) and substance abuse.
“We have a responsibility to take care of our own health as well,” Dr. Needle added. “You can’t take care of others if you’re not taking care of yourself. It’s not being selfish. As a matter of fact, it’s being prudent. It’s survival.”
Dr. Needle and Dr. Miller had no relevant financial disclosures. Dr. Miller’s presentation was supported by the AAP and funded in part by the Agency for Toxic Substances and Disease Registry. The U.S. Environmental Protection Agency (EPA) provides funding support for the Pediatric Environmental Health Specialty Unit.
FROM AAP 2020
Choosing pharmacotherapy for bipolar disorder requires a risk-benefit analysis
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
Dermatologists as Social Media Contributors During the COVID-19 Pandemic
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
Practice Points
- With the coronavirus disease 2019 (COVID-19) pandemic, strict physical distancing measures have made patients and providers alike reliant on global digital social networks such as Instagram, Twitter, and Facebook to facilitate information sharing about COVID-19.
- Dermatologists should utilize social media as a platform to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during the global pandemic.
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Overlap Syndrome in a Patient With Relapsing Polychondritis
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.
Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.
Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
To the Editor:
Relapsing polychondritis (RP) is a chronic, progressive, and episodic systemic inflammatory disease that primarily affects the cartilaginous structures of the ears and nose. Involvement of other proteoglycan-rich structures such as the joints, eyes, inner ears, blood vessels, heart, and kidneys also may be seen. Dermatologic manifestations occur in 35% to 50% of patients and may be the presenting sign in up to 12% of cases.1 The most commonly reported dermatologic findings include oral aphthosis, erythema nodosum, and purpura with vasculitic changes. Less commonly reported associations include Sweet syndrome, pyoderma gangrenosum, panniculitis, erythema elevatum diutinum, erythema annulare centrifugum, and erythema multiforme.1
A 43-year-old woman who was otherwise healthy developed new-onset tenderness and swelling of the left pinna while on vacation. She was treated with trimethoprim-sulfamethoxazole, clindamycin, and levofloxacin for presumed auricular cellulitis. The patient developed a fever; sore throat; and a progressive, pruritic, blistering rash on the face, torso, bilateral extremities, palms, and soles 1 day after completing the antibiotic course. After 5 days of unremitting symptoms despite oral, intramuscular, and topical steroids, the patient presented to the emergency department. Physical examination revealed diffuse, tender, erythematous to violaceous macules with varying degrees of coalescence on the chest, back, and extremities. Scattered flaccid bullae and erosions of the oral and genital mucosa also were seen. Laboratory analysis was notable only for a urinary tract infection with Klebsiella pneumoniae. A punch biopsy demonstrated full-thickness necrosis of the epidermis with subepidermal bullae and a mild to moderate lymphocytic infiltrate with rare eosinophils, consistent with a diagnosis of Stevens-Johnson syndrome (SJS). Because of the body surface area involved (20%) and the recent history of trimethoprim-sulfamethoxazole use, a diagnosis of SJS/toxic epidermal necrolysis (TEN) overlap syndrome was made. The patient was successfully treated with subcutaneous etanercept (50 mg), supportive care, and cephalexin for the urinary tract infection.
Approximately 5 weeks after discharge from the hospital, the patient was evaluated for new-onset pain and swelling of the right ear (Figure) in conjunction with recent tenderness and depression of the superior septal structure of the nose. A punch biopsy of the ear revealed mild perichondral inflammation without vasculitic changes and a superficial, deep perivascular, and periadnexal lymphoplasmacytic inflammatory infiltrate with scattered eosinophils. A diagnosis of RP was made, as the patient met Damiani and Levine’s2 criteria with bilateral auricular inflammation, ocular inflammation, and nasal chondritis.
Although the exact pathogenesis of RP remains unclear, there is strong evidence to suggest an underlying autoimmune etiology.3 Autoantibodies against type II collagen, in addition to other minor collagen and cartilage proteins, such as cartilage oligomeric matrix proteins and matrilin-1, are seen in a subset of patients. Titers have been reported to correlate with disease activity.3,4 Direct immunofluorescence also has demonstrated plentiful CD4+ T cells, as well as IgM, IgA, IgG, and C3 deposits in the inflamed cartilage of patients with RP.3 Additionally, approximately 30% of patients with RP will have another autoimmune disease, and more than 50% of patients with RP carry the HLA-DR4 antigen.3 Alternatively, SJS and TEN are not reported in association with autoimmune diseases and are believed to be CD8+ T-cell driven. Some HLA-B subtypes have been found in strong association with SJS and TEN, suggesting the role of a potential genetic susceptibility.5
We report a unique case of SJS/TEN overlap syndrome occurring in a patient with RP.1 Although the association may be coincidental, it is well known that patients with lupus erythematosus are predisposed to the development of SJS and TEN. Therefore, a shared underlying genetic predisposition or immune system hyperactivity secondary to active RP is a possible explanation for our patient’s unique presentation.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
- Watkins S, Magill JM Jr, Ramos-Caro FA. Annular eruption preceding relapsing polychondritis: case report and review of the literature. Int J Dermatol. 2009;48:356-362.
- Damiani JM, Levine HL. Relapsing polychondritis—report of ten cases. Laryngoscope. 1979;89:929-46.
- Puéchal X, Terrier B, Mouthon L, et al. Relapsing polychondritis. Joint Bone Spine. 2014;81:118-24.
- Arnaud L, Mathian A, Haroche J, et al. Pathogenesis of relapsing polychondritis. Autoimmun Rev. 2014;13:90-95.
- Harr T, French L. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16;5:39.
Practice Points
- The clinical presentation of relapsing polychondritis (RP) may demonstrate cutaneous manifestations other than the typical inflammation of cartilage-rich structures.
- Approximately 30% of patients with RP will have another autoimmune disease.