User login
Urethral bulking agents for SUI: Rethinking their indications
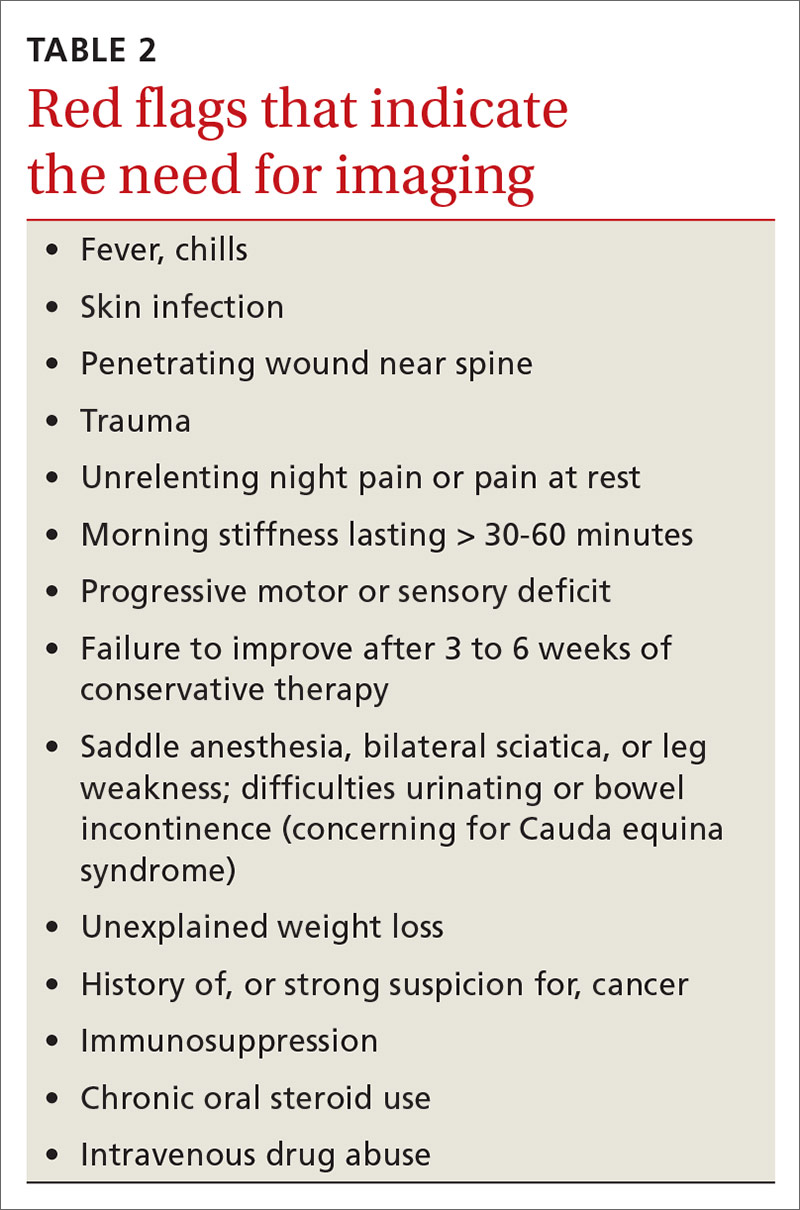
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
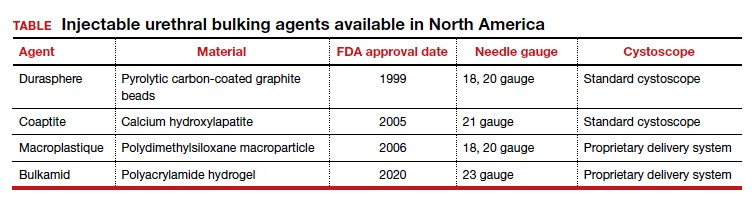
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.
Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
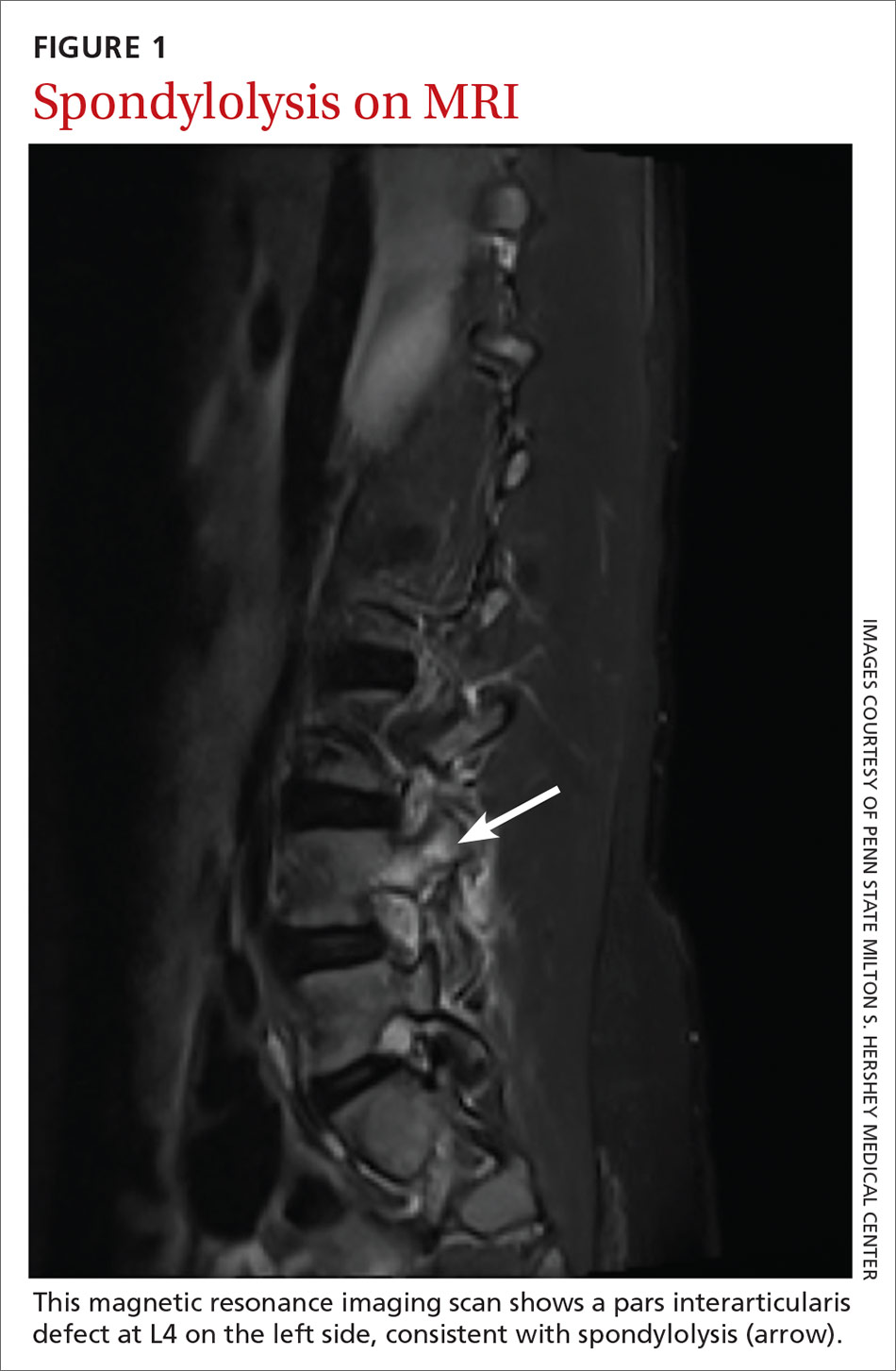
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4

After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
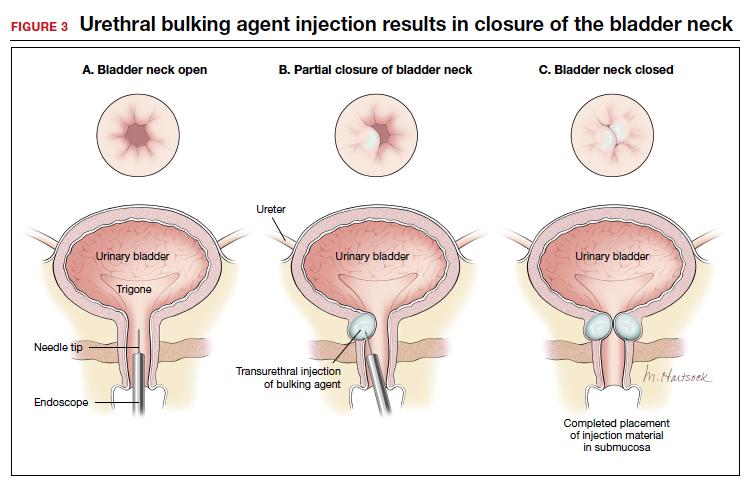
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).
Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
Stress urinary incontinence (SUI) is the involuntary loss of urine with increased intra-abdominal pressure, such as with physical exertion, sneezing, or coughing.1 Currently, the gold standard treatment for SUI is surgical repair with the use of a synthetic midurethral sling (MUS), based on long-term data that support its excellent efficacy and durability. The risk-benefit balance of MUS continues to be scrutinized, however, with erosions and pain poorly studied and apparently underreported.
The medical-legal risks associated with the MUS are a significant concern and have led many patients to reconsider this option for their condition. Many other countries (United Kingdom, Australia, New Zealand, and European Union) are now re-evaluating the use of the MUS.2 In the United Kingdom, for example, the National Institute for Health and Care Excellence (NICE) Guideline advises considering the MUS only when another surgical intervention is not suitable for the patient.3
In light of the heightened skepticism surrounding the MUS, interest has increased in the use of urethral bulking agents. These agents consist of a material injected into the wall of the urethra to improve urethral coaptation in women with SUI.4
A brief history of bulking agents
In 1938, Murless first reported the injection of sodium morrhuate for the management of urinary incontinence.4 Other early bulking agents introduced in the 1950s and 1960s included paraffin wax and sclerosing agents. Subsequently, Teflon, collagen, and autologous fat, among other agents, were found to be efficacious for augmenting urethral coaptation; however, only collagen initially demonstrated acceptable safety.5
Contigen (bovine dermal collagen cross-linked with gluteraldehyde) was approved as a bulking agent by the US Food and Drug Administration (FDA) in 1993; however, the manufacturing of bovine collagen was halted in 2011. Contigen was the only nonpermanent biodegradable urethral bulking agent, and its use required skin testing prior to use, as 2% to 5% of women experienced allergic reaction.4
Presently, 3 particle-based urethral bulking agents are FDA approved for marketing in the United States: Macroplastique (Laborie Medical Technologies), Coaptite (Boston Scientific), and Durasphere (Coloplast). In addition, Bulkamid (Contura), which was approved earlier this year, is a nonparticulate agent composed of a nonresorbable polyacrylamide hydrogel.5
Continue to: Indications for use...
Indications for use
According to the FDA premarket approvals (PMAs) for the particle-based urethral bulking agents, their use is indicated for adult women with SUI primarily due to intrinsic sphincter deficiency (ISD).6 The PMA indication for the nonparticulate agent, however, allows it to be used for SUI as well as SUI-predominant mixed urinary incontinence (MUI) due to ISD.7 Traditionally, ISD is defined by urodynamic criteria that includes a maximal urethral closure pressure less than 20 to 25 cm of water and/or a Valsalva leak point pressure of less than 60 cm of water.4
The American Urological Association (AUA) guideline lists bulking agents as an option for women who do not wish to pursue invasive surgical intervention for SUI, are concerned about lengthier recovery after surgery, or have previously undergone anti-incontinence procedures with suboptimal results.8 In general, most urologists and urogynecologists who perform urethral bulking agree with the AUA guideline.
Perceptions of bulking agents have shifted
Urethral bulking agents traditionally have been thought of as a "salvage therapy." Perceived indications for these agents include use in women with persistent SUI after more invasive treatment options or in women who were medically fragile and thus could not undergo a more invasive procedure.9 As mentioned, however, circumstances related to mesh use have shifted the current perception of indications for urethral bulking agents from salvage therapy only to use as a possible first-line treatment in the appropriately selected patient.9
Recent data that note improved durability and patient satisfaction, as well as better appreciation of the fact that, if the bulking agent fails, a synthetic sling procedure still can be performed without significant concerns, have contributed to this shift in intervention strategy.10,11 There also has been the perception that urethral bulking agents should not be considered in women who have urethral mobility. However, studies have shown that outcomes are not significantly different in patients with urethral mobility compared with those with a fixed urethra.11
Types of bulking agents
The ideal bulking agent should be made of a material that is biocompatible--with low host reactivity, low carcinogenic potential, low risk of migration--and easy to administer.5 Currently available bulking agents are classified as particulate and nonparticulate agents. The TABLE provides summary details of the available agents FDA approved for use.

Particulate bulking agents
Durasphere, approved by the FDA in 1999, is composed of carbon-coated zirconium oxide in a water-based and beta-glucan carrier. The first generation of this agent had particles that ranged in size from 212 to 500 µm and required an 18-gauge needle for injection.4 The second-generation preparation has a smaller particle size, ranging from 90 to 212 µm, which permits injection with a smaller needle, typically 20 gauge.4 Theoretically, the larger bead size reduces the risk of migration as particles larger than 80 µm cannot be engulfed by macrophages.4
Coaptite is a calcium hydroxylapatite-based product approved by the FDA in 2005. The carrier media is composed of sodium carboxymethylcellulose, sterile water, and glycerin. The particle size ranges from 75 to 125 µm, with an average of 100 µm.5 This synthetic material historically has been used in orthopedics and dental applications. The aqueous gel carrier dissipates over months, resulting in tissue growth; thereafter, the particulate beads slowly degrade.12
Macroplastique, a polydimethylsiloxane compound, was approved by the FDA in 2006. It has a long history of use primarily in Europe where it has been used since 1991. It is composed of a nonbiodegradable silicone (polydimethylsiloxane) elastomer suspended in a water-soluble gel. The initial composition was of particles that ranged in size from 5 to 400 µm, with 25% of the particles smaller than 50 µm. Because of the large number of particles smaller than 50 µm, there were concerns for migration.5 The agent's current composition contains particles that range from 120 to 600 µm, with an average particle size of 140 µm.4
Nonparticulate bulking agent
Bulkamid has been available in Europe since 2003 and was FDA approved in January 2020. It is the only available nonparticulate urethral bulking agent; it is composed uniquely of a nonresorbable polyacrylamide hydrogel made of cross-linked 2.5% polyacrylamide and water. Its bulking effect is achieved through the actual volume of hydrogel injected, which integrates with host tissue by vessel ingrowth, suggestive of a persistent durable effect. Because Bulkamid contains no particles or crystals, the theoretical risk of migration is mitigated.4
Continue to: The urethral bulking technique...
The urethral bulking technique
The basic technique for urethral bulking is similar for all agents, with nuances in technique for each agent.
The procedure typically begins with placement of 2% lidocaine gel in the urethra for 5 to 10 minutes. The disposable needle is primed with the agent.4 For Durasphere, an 18- or 21-gauge rigid needle is used; for Coaptite, a 21-gauge rigid side injecting needle called the SideKick is used; and for Macroplastique, an 18- or 20-gauge rigid needle is used.4 Bulkamid administration requires the use of a special 23-gauge needle. Durasphere and Coaptite are delivered via a standard cystoscope.4 Macroplastique requires a proprietary delivery system4 (FIGURE 1). Bulkamid has a proprietary urethroscope and rotatable sheath to guide accuracy of injection (FIGURE 2).4


After the needle is primed and the delivery device placed into the urethra, the injection site is selected, approximately 1.5 to 2 cm from the bladder neck. The needle is introduced into the suburethral tissue at a 30- to 45-degree angle.
The injection site varies by agent. The 4 and 8 o'clock positions are recommended for Coaptite and Durasphere, while the 2, 6, and 10 o'clock positions are recommended for Macroplastique. For Bulkamid, the recommendation is to create 3 cushions at the 2, 6, and 10 o'clock positions.13 Regardless of the agent used, the bulking is easily visualized and should result in the various sites meeting in the midline (FIGURE 3).

Continue to: Evidence-based outcomes...
Evidence-based outcomes
The published data on outcomes of urethral bulking treatments have used inconsistent measures of efficacy. Most of the FDA trials used subjective success calculated with use of the Stamey Urinary Incontinence Scale (Stamey Grade) and validated questionnaires as well as objective data collected via voiding diaries and pad tests.4
In 2007, a multicenter prospective randomized controlled trial (RCT) compared Coaptite with Contigen treatment and found that 63.4% versus 57.0% of patients, respectively, experienced an improvement on the Stamey Urinary Incontinence Scale at 12-month follow-up.14
A prospective multicenter RCT in 2009 was conducted to test the durability and efficacy of Macroplastique treatment at 12-month follow-up.15 The authors noted that at 12 months, 62% of treated women reported significant improvement.15 Further, a systematic review and meta-analysis of the literature (1990-2010) on Macroplastique use was published in 2013.16 Data from 958 patients from 23 cohorts were analyzed in a random-effects model for 3 time periods: short term (less than 6 months), mid term (6-12 months), and long term (>18 months). Cure/dry rates were reported for short, mid, and long-term follow-up as 43% (95% confidence interval [CI], 33%-54%), 37% (95% CI, 28%-46%), and 36% (95% CI, 27%-46%), respectively.16
The newest bulking product in the United States, Bulkamid, has been available for use in Europe since 2003.17 In a 3-year follow-up of a prospective nonrandomized single-site study, 212 of 256 (82.8%) participants were subjectively cured or had significant improvement in SUI or MUI, and this result was maintained until the end of the study period (a median of 38 months).10 In 2014, an 8-year follow-up of 24 women was published.18 Subjectively, 44% of the women reported cure or significant improvement, and 11 women who presented for objective evaluation all had polyacrylamide hydrogel visible on vaginal ultrasound.18
In addition, an RCT published in 2020 compared surgery with tension-free vaginal tape (TVT) and Bulkamid use in 224 women with SUI. At the 12-month follow-up, TVT was found to be more effective than Bulkamid; the median visual analog scale score for satisfaction was 99 for the TVT-treated group and 85 for the Bulkamid-treated patients.11 Additionally, a cough stress test was negative in 95.0% and 66.4% of participants, respectively, but reoperations occurred only in patients who received the TVT procedure (n = 6). The authors concluded that while TVT treatment provided higher satisfaction rates than did Bulkamid, all major perioperative and follow-up complications were associated with TVT use. The study is ongoing and will eventually report 3-year outcomes.11
According to a 2017 Cochrane Review on urethral bulking, treatments with all 3 of the particulate bulking agents resulted in improvements that were no more or less effective than Contigen treatment. The review failed to include publications on Bulkamid treatment.19
Continue to: Complications and safety issues...
Complications and safety issues
Adverse events. Reported adverse effects associated with urethral bulking include mild pain, transient urinary retention (typically resolving within 1-2 days after injection), dysuria, hematuria, and urinary tract infection (UTI).4,12
In a 12-month RCT involving 355 women treated with Durasphere or bovine collagen, adverse events were reported in 178 Durasphere-treated women; dysuria (24.7%) and temporary urinary retention (16.9%) were the most commonly reported adverse events.20
An RCT of Coaptite injection (n = 296) found that temporary urinary retention (41%) was the most common adverse event.14
In a 12-month comparative study of Macroplastique versus Contigen (n = 122), UTI was reported as the most common adverse event (23.8%), followed by dysuria (9%) and urgency (9%).15 In addition, in a meta-analysis involving 958 patients in 23 cohorts, Ghoniem and Miller reported that the median rates for adverse events were temporary dysuria, 50%; hematuria, 45%; urge incontinence, 7%; temporary urinary retention, 7%; and UTI, 3%.16
A 3-year summary outcome of 256 patients who received Bulkamid injection reported that only 1 patient developed infection, abscess, or allergic reaction at the injection site and 1 patient had a UTI.10 In an 8-year follow-up of patients who received Bulkamid injection, 1 patient experienced stranguria and 7 patients had recurrent cystitis.18
It appears that transient dysuria, urgency, and urinary retention occur more frequently after urethral bulking with particulate agents.12
Complications. Few delayed but serious complications after urethral bulking have been reported, including suburethral abscess, urethral prolapse, and particle migration.4 Cases of urethral prolapse have been reported with both Coaptite and Durasphere. Notably, all cases of urethral prolapse occurred in patients with a history of pelvic surgery and/or previous urethral bulking.21,22 Cases also have been reported of Durasphere carbon bead particles migrating to regional and distant lymph nodes, and pseudoabscess also has been reported.12,23 A single case of periurethral abscess was reported after Bulkamid injection in a patient who had prior vaginal hysterectomy and a transobturator tape procedure after a total vaginal mesh repair.24
Bulking agent use: Time to go mainstream?
Historically, urethral bulking agents have had limited utility, largely due to the inaccurate and unsubstantiated perceptions of them being indicated only in women with ISD and a well-supported urethra. More recently, urethral bulking agents are commonly being used in patients who: have recurrent SUI after a surgical intervention, have infrequent but bothersome SUI symptoms, are not ideal candidates to undergo anesthesia, or wish to avoid mesh.
Some data suggest that objective and subjective success rates are lower with bulking agent treatment compared with the gold standard MUS procedure. However, in the appropriately selected patient, urethral bulking agents may be considered primary treatment due to their associated low morbidity and, as recently reported with newer nonparticulate agents, high subjective success rates. If the patient is not satisfied with the results of bulking treatment, surgical repair with any type of sling remains a subsequent option. This feature adds to the potential viability and appropriateness of considering a bulking agent as a primary treatment. ●
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37-49.
- NHS Improvement and NHS England website. Provider bulletin, July 11, 2018. Vaginal mesh: high vigilance restriction period: immediate action required, all cases should be postponed if it is clinically safe to do so. https://www.england .nhs.uk/2018/07/provider-bulletin-11-july-2018/#vaginal -mesh-restriction. Accessed September 17, 2020.
- National Institute for Health and Care Excellence (UK) website. NICE guideline (NG123). Urinary incontinence and pelvic organ prolapse in women: management. April 2019. https://www.nice.org.uk/guidance/ng123. Accessed September 17, 2020.
- Vaccaro CM, Clemons J. Urethral injection of bulking agents for intrinsic sphincter deficiency. In: Walters M, Karram M, eds. Urognecology and Reconstructive Pelvic Surgery. 4th ed. Philadelphia, PA: Elsevier Saunders; 2015:317-324.
- Zoorob D, Karram M. Bulking agents: a urogynecology perspective. Urol Clin North Am. 2012;39:273-277.
- US Food and Drug Administration. Premarket approval (PMA): Macroplastique implants. https://www.accessdata. fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P040050. Updated September 14, 2020. Accessed September 17, 2020.
- US Food and Drug Administration. Premarket approval (PMA): Bulkamid urethral bulking system. https://www .accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma .cfm?id=P170023. Updated September 14, 2020. Accessed September 17, 2020.
- Kobashi KC, Albo ME, Dmochowski RR, et al. Surgical treatment of female stress urinary incontinence (SUI): AUA/ SUFU guideline (2017). J Urol. 2017;198:875-883.
- Hartigan SM, Dmochowski RR. Which procedure for stress urinary incontinence? Injectable. Curr Opin Urol. 2020;30:272-274.
- Pai A, Al-Singary W. Durability, safety and efficacy of polyacrylamide hydrogel (Bulkamid) in the management of stress and mixed urinary incontinence: three year follow up outcomes. Cent Eur J Urol. 2015;68:428-433.
- Itkonen Freitas AM, Mentula M, Rahkola-Soisalo P, et al. Tension-free vaginal tape surgery versus polyacrylamide hydrogel injection for primary stress urinary incontinence: a randomized clinical trial. J Urol. 2020;203:372-378.
- Chapple C, Dmochowski R. Particulate versus nonparticulate bulking agents in the treatment of stress urinary incontinence. Res Reports Urol. 2019;11:299-310.
- Contura website. Bulkamid standard operating procedure. January 2018. https://bulkamid.com/wp-content /uploads/2019/03/BULK_2018_041.2_SOP_12.04.18.pdf. Accessed September 17, 2020.
- Mayer RD, Dmochowski RR, Appell RA, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876-880.
- Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204-210.
- Ghoniem GM, Miller CJ. A systematic review and metaanalysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24:27-36.
- Lose G, Sørensen HC, Axelsen SM, et al. An open multicenter study of polyacrylamide hydrogel (Bulkamid) for female stress and mixed urinary incontinence. Int Urogynecol J. 2010;21:1471-1477.
- Mouritsen L, Lose G, Møller-Bek K. Long-term follow-up after urethral injection with polyacrylamide hydrogel for female stress incontinence. Acta Obstet Gynecol Scand. 2014;93:209- 212.
- Kirchin V, Page T, Keegan PE, et al. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD003881.
- Lightner D, Calvosa C, Andersen R, et al. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled double-blind study of Durasphere. Urology. 2001;58:12-15.
- Ghoniem GM, Khater U. Urethral prolapse after Durasphere injection. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:297-298.
- Ko EY, Williams BF, Petrou SP. Bulking agent induced early urethral prolapse after distal urethrectomy. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1511-1513.
- Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;1661350-1353.
- Gopinath D, Smith ARB, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid) for stress urinary incontinence. Int Urogynecol J. 2012;23:1645-1648.
Does early introduction of peanuts to an infant’s diet reduce the risk for peanut allergy?
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE-BASED ANSWER:
Probably not, unless the child has severe eczema or egg allergy. In a general pediatric population, introducing peanuts early (at age 3 to 6 months) doesn’t appear to alter rates of subsequent peanut allergy compared with introduction after age 6 months (strength of recommendation [SOR]: B, randomized clinical trial [RCT] using multiple potential food allergens).
In children with severe eczema, egg allergy, or both, however, the risk for a peanut allergy is 12% to 24% lower when peanut-containing foods are introduced at age 4 to 11 months than after age 1 year. Early introduction of peanuts is associated with about 1 additional mild virus-associated syndrome (upper respiratory infection [URI], exanthem, conjunctivitis, or gastroenteritis) per patient (SOR: B, RCT).
Introducing peanuts before age 1 year is recommended for atopic children without evidence of pre-existing peanut allergy; an earlier start, at age 4 to 6 months, is advised for infants with severe eczema or egg allergy (SOR: C, expert opinion).
Dual therapy serves as well as triple for most HIV patients
based on a meta-analysis including data from more than 5,000 patients.
Although triple therapy remains the standard of care, the availability of more potent drugs has revived interest in dual and mono therapies, wrote Pisaturo Mariantonietta, MD, of the University of Campania Luigi Vanvitelli, Naples, Italy, and colleagues.
In a study published in Clinical Microbiology and Infection, the researchers identified 14 articles including 5,205 treatment-naive HIV adults. The studies were published between 2008 and 2020; 13 were randomized, controlled trials.
The dual therapies used in the studies included atazanavir/r plus maraviroc; lopinavir/r plus lamivudine; raltegravir plus darunavir/r; lopinavir/r plus tenofovir, raltegravir, efavirenz, or maraviroc; atazanavir/r plus raltegravir and darunavir/r plus maraviroc; and dolutegravir plus lamivudine.
Overall, no significant differences occurred in the primary endpoint of treatment failure across 10 studies between dual therapy and triple therapy patients based on data at 48 weeks (relative risk 1.20). “The rate of treatment failure did not differ among the two groups when stratifying the patients according to the drug used in the dual regimen,” the researchers said.
Low viral load’s link to treatment failure
Among 2,398 patients with a low HIV viral load (less than 100,000 copies/mL), dual therapy patients were significantly more likely to experience treatment failure than were triple therapy patients (RR, 1.47, P = .007). No differences were noted between dual and triple therapy failure among patients with high HIV viral loads at baseline. Patterns were similar at 96 weeks, but only three studies included 96-week data, the researchers said.
The rate of discontinuation because of adverse events was not significantly different between the groups at 48 weeks.
The study findings were limited by several factors, including the use of different regimens in the dual strategies, some of which are no longer in use, as well as there being insufficient data to fully compare outcomes at 96 weeks, and lack of information on cerebrospinal fluid viral load, the researchers noted.
However, the results suggest that dual therapy might be considered for HIV-naive patients with a low viral load, they said.
“Further RCTs that will evaluate the efficacy of antiretroviral regimens in use today among difficult-to-treat populations, such as patients with high viral load, including both intention-to-treat and per-protocol analysis, are needed to address this topic,” they concluded.
Consider range of patient factors when choosing therapies
Conducting the study at this time was important because of the expanding options for treating HIV patients, Donna E. Sweet, MD, an HIV specialist and professor of medicine at the University of Kansas, Wichita, said in an interview.
“We now have two single tablet formulations that are dual rather than triple therapy, and as treaters we are all trying to know when to use them,” she explained.
Dr. Sweet said she was not surprised by the study findings, given that well-conducted, randomized, controlled trials allowed the combination therapies to be approved.
Some of the key challenges to identifying the optimal treatment for HIV patients include factoring in the use of concomitant medications that could lead to drug-drug interactions, noted Dr. Sweet, who serves an editorial advisory board member of Internal Medicine News.
The take-home message for clinicians, in her opinion, is that “less drugs may mean less toxicity, but we don’t want to sacrifice efficacy,” she said. “There may be patients who are better suited than others for two vs. three drugs,” Dr. Sweet emphasized.
The next steps for research on the value of dual vs. triple therapy should include longer term efficacy studies, especially in those with lower CD4 counts and higher viral loads, said Dr. Sweet. In addition to factors such as CD4 counts and viral load, the food requirements of certain ART regimens could affect adherence and therefore a clinician decision to use two drugs rather than three, she noted.
Dr. Sweet disclosed past relationships with ViiV, Gilead, Merck, and Janssen on their speakers bureaus, and current advisory roles with Gilead and ViiV.
The study received no outside funding. Lead author Dr. Mariantonietta and several coauthors disclosed relationships with companies including ViiV Healthcare, AbbVie, Janssen-Cilag and Gilead Science, and Merck Sharp & Dohme, but no conflicts in connection with this study.
SOURCE: Mariantonietta P et al. Clin Microbiol Infect. 2020 Oct 5. doi: 10.1016/j.cmi.2020.09.048.
based on a meta-analysis including data from more than 5,000 patients.
Although triple therapy remains the standard of care, the availability of more potent drugs has revived interest in dual and mono therapies, wrote Pisaturo Mariantonietta, MD, of the University of Campania Luigi Vanvitelli, Naples, Italy, and colleagues.
In a study published in Clinical Microbiology and Infection, the researchers identified 14 articles including 5,205 treatment-naive HIV adults. The studies were published between 2008 and 2020; 13 were randomized, controlled trials.
The dual therapies used in the studies included atazanavir/r plus maraviroc; lopinavir/r plus lamivudine; raltegravir plus darunavir/r; lopinavir/r plus tenofovir, raltegravir, efavirenz, or maraviroc; atazanavir/r plus raltegravir and darunavir/r plus maraviroc; and dolutegravir plus lamivudine.
Overall, no significant differences occurred in the primary endpoint of treatment failure across 10 studies between dual therapy and triple therapy patients based on data at 48 weeks (relative risk 1.20). “The rate of treatment failure did not differ among the two groups when stratifying the patients according to the drug used in the dual regimen,” the researchers said.
Low viral load’s link to treatment failure
Among 2,398 patients with a low HIV viral load (less than 100,000 copies/mL), dual therapy patients were significantly more likely to experience treatment failure than were triple therapy patients (RR, 1.47, P = .007). No differences were noted between dual and triple therapy failure among patients with high HIV viral loads at baseline. Patterns were similar at 96 weeks, but only three studies included 96-week data, the researchers said.
The rate of discontinuation because of adverse events was not significantly different between the groups at 48 weeks.
The study findings were limited by several factors, including the use of different regimens in the dual strategies, some of which are no longer in use, as well as there being insufficient data to fully compare outcomes at 96 weeks, and lack of information on cerebrospinal fluid viral load, the researchers noted.
However, the results suggest that dual therapy might be considered for HIV-naive patients with a low viral load, they said.
“Further RCTs that will evaluate the efficacy of antiretroviral regimens in use today among difficult-to-treat populations, such as patients with high viral load, including both intention-to-treat and per-protocol analysis, are needed to address this topic,” they concluded.
Consider range of patient factors when choosing therapies
Conducting the study at this time was important because of the expanding options for treating HIV patients, Donna E. Sweet, MD, an HIV specialist and professor of medicine at the University of Kansas, Wichita, said in an interview.
“We now have two single tablet formulations that are dual rather than triple therapy, and as treaters we are all trying to know when to use them,” she explained.
Dr. Sweet said she was not surprised by the study findings, given that well-conducted, randomized, controlled trials allowed the combination therapies to be approved.
Some of the key challenges to identifying the optimal treatment for HIV patients include factoring in the use of concomitant medications that could lead to drug-drug interactions, noted Dr. Sweet, who serves an editorial advisory board member of Internal Medicine News.
The take-home message for clinicians, in her opinion, is that “less drugs may mean less toxicity, but we don’t want to sacrifice efficacy,” she said. “There may be patients who are better suited than others for two vs. three drugs,” Dr. Sweet emphasized.
The next steps for research on the value of dual vs. triple therapy should include longer term efficacy studies, especially in those with lower CD4 counts and higher viral loads, said Dr. Sweet. In addition to factors such as CD4 counts and viral load, the food requirements of certain ART regimens could affect adherence and therefore a clinician decision to use two drugs rather than three, she noted.
Dr. Sweet disclosed past relationships with ViiV, Gilead, Merck, and Janssen on their speakers bureaus, and current advisory roles with Gilead and ViiV.
The study received no outside funding. Lead author Dr. Mariantonietta and several coauthors disclosed relationships with companies including ViiV Healthcare, AbbVie, Janssen-Cilag and Gilead Science, and Merck Sharp & Dohme, but no conflicts in connection with this study.
SOURCE: Mariantonietta P et al. Clin Microbiol Infect. 2020 Oct 5. doi: 10.1016/j.cmi.2020.09.048.
based on a meta-analysis including data from more than 5,000 patients.
Although triple therapy remains the standard of care, the availability of more potent drugs has revived interest in dual and mono therapies, wrote Pisaturo Mariantonietta, MD, of the University of Campania Luigi Vanvitelli, Naples, Italy, and colleagues.
In a study published in Clinical Microbiology and Infection, the researchers identified 14 articles including 5,205 treatment-naive HIV adults. The studies were published between 2008 and 2020; 13 were randomized, controlled trials.
The dual therapies used in the studies included atazanavir/r plus maraviroc; lopinavir/r plus lamivudine; raltegravir plus darunavir/r; lopinavir/r plus tenofovir, raltegravir, efavirenz, or maraviroc; atazanavir/r plus raltegravir and darunavir/r plus maraviroc; and dolutegravir plus lamivudine.
Overall, no significant differences occurred in the primary endpoint of treatment failure across 10 studies between dual therapy and triple therapy patients based on data at 48 weeks (relative risk 1.20). “The rate of treatment failure did not differ among the two groups when stratifying the patients according to the drug used in the dual regimen,” the researchers said.
Low viral load’s link to treatment failure
Among 2,398 patients with a low HIV viral load (less than 100,000 copies/mL), dual therapy patients were significantly more likely to experience treatment failure than were triple therapy patients (RR, 1.47, P = .007). No differences were noted between dual and triple therapy failure among patients with high HIV viral loads at baseline. Patterns were similar at 96 weeks, but only three studies included 96-week data, the researchers said.
The rate of discontinuation because of adverse events was not significantly different between the groups at 48 weeks.
The study findings were limited by several factors, including the use of different regimens in the dual strategies, some of which are no longer in use, as well as there being insufficient data to fully compare outcomes at 96 weeks, and lack of information on cerebrospinal fluid viral load, the researchers noted.
However, the results suggest that dual therapy might be considered for HIV-naive patients with a low viral load, they said.
“Further RCTs that will evaluate the efficacy of antiretroviral regimens in use today among difficult-to-treat populations, such as patients with high viral load, including both intention-to-treat and per-protocol analysis, are needed to address this topic,” they concluded.
Consider range of patient factors when choosing therapies
Conducting the study at this time was important because of the expanding options for treating HIV patients, Donna E. Sweet, MD, an HIV specialist and professor of medicine at the University of Kansas, Wichita, said in an interview.
“We now have two single tablet formulations that are dual rather than triple therapy, and as treaters we are all trying to know when to use them,” she explained.
Dr. Sweet said she was not surprised by the study findings, given that well-conducted, randomized, controlled trials allowed the combination therapies to be approved.
Some of the key challenges to identifying the optimal treatment for HIV patients include factoring in the use of concomitant medications that could lead to drug-drug interactions, noted Dr. Sweet, who serves an editorial advisory board member of Internal Medicine News.
The take-home message for clinicians, in her opinion, is that “less drugs may mean less toxicity, but we don’t want to sacrifice efficacy,” she said. “There may be patients who are better suited than others for two vs. three drugs,” Dr. Sweet emphasized.
The next steps for research on the value of dual vs. triple therapy should include longer term efficacy studies, especially in those with lower CD4 counts and higher viral loads, said Dr. Sweet. In addition to factors such as CD4 counts and viral load, the food requirements of certain ART regimens could affect adherence and therefore a clinician decision to use two drugs rather than three, she noted.
Dr. Sweet disclosed past relationships with ViiV, Gilead, Merck, and Janssen on their speakers bureaus, and current advisory roles with Gilead and ViiV.
The study received no outside funding. Lead author Dr. Mariantonietta and several coauthors disclosed relationships with companies including ViiV Healthcare, AbbVie, Janssen-Cilag and Gilead Science, and Merck Sharp & Dohme, but no conflicts in connection with this study.
SOURCE: Mariantonietta P et al. Clin Microbiol Infect. 2020 Oct 5. doi: 10.1016/j.cmi.2020.09.048.
FROM CLINICAL MICROBIOLOGY AND INFECTION
Early hearing impairment interventions key to kindergarten readiness
Starting early intervention (EI) enrollment before age 6 months in children who are deaf or hard of hearing may have a lasting influence on ensuring kindergarten readiness, Jareen Meinzen-Derr, PhD, MPH of Cincinnati Children’s Hospital Medical Center and colleagues reported in Pediatrics.
The researchers created a comprehensive, longitudinal, population-based database, which linked hearing screening and diagnostic data to that of early intervention data and educational records for 1,746 infants identified with permanent hearing loss who were born between Jan. 1, 2008 and Dec. 31, 2014 The database was established in partnership with the Ohio Departments of Health, Developmental Disabilities and Education, and with the support of the Centers for Disease Control and Prevention and the National Center on Birth Defects and Developmental Disabilities.
Of those, 784 children ranging from preschool to fourth grade were evaluated based on education data available for the 2017 and 2018 school year that had been linked by way of an identifier that flagged students enrolled in EI.
All together, 417 students had kindergarten assessment records, and of those, 385 had Kindergarten Readiness Assessments (KRAs) between 2014 and 2018; 222 (58%) had been enrolled in EI before the age of 6 months. Of those who were enrolled early, the median age of EI enrollment was 3.4 months (2.4-4.3 months) and in those enrolled later, the median age was 9.2 months (7.5-15.4 months).
The importance of EI prior to 6 months
A total of 109 children (28%) receiving services as part of Ohio’s early intervention programs demonstrated kindergarten readiness on their overall KRA scores. The scores revealed that children receiving EI early (34%, n = 75) were more likely to be ready for kindergarten than were those who entered later (21%, n = 34; P = .005). They also were more likely to have on track language and literacy scores (60% vs. 42%, respectively; P = .0006).
Dr. Meinzen-Derr and colleagues noted that factors identified with “an increased odds of being on track included having private insurance and some college education for the mother.” Conversely, factors identified with a decreased likelihood included having a diagnosed disability and bilateral hearing loss.
The researchers cautioned that children transitioned from EI to academic settings will face challenges that may go underrecognized because a school’s focus often is largely on social and academic performance. Thus, working with linked data systems can provide the data to track outcomes that might otherwise be missed, the researchers noted.
Furthermore, they cautioned that even though kindergarten readiness offers some glimpse into future academic success, these measures alone may not be sufficient predictors for children who are deaf or hard of hearing. Risk for communication, social, and academic delays persist throughout school so it is important to employ alternative methods of reading instruction in order to “achieve more complex skills (e.g., complex syntax and advanced vocabulary) necessary for reading proficiency,” the researchers said.
Collecting data from public health and education systems posed limitations for the study. In addition, the absence of kindergarten language assessments prevented Dr. Meinzen-Derr and colleagues from better elucidating reasons for kindergarten readiness. Also beyond the scope of the study was the ability to evaluate the effect service types may have had on outcomes.
The next step in the research process is to evaluate the link between outcomes and specific EI parameters, they said. “Our study demonstrates that an integrated data system can address relevant and important topics regarding early academic outcomes (kindergarten readiness and reading levels) among children who received EI. The current findings provide a new context by evaluating later outcomes among children who are deaf or hard of hearing,” they added, noting that more research is needed to grasp how various EI services impact outcomes since enrollment age is a marker of EI exposure.
Early intervention is everyone’s business
In a separate interview, Amy Hardy M.S. CCC-SLP, speech language pathologist and clinical professor at Idaho State University, emphasized the importance of early intervention, citing reports from the National Center for Hearing Assessment and Management, which credits detection and treatment of hearing loss at birth per child to saving $400,000 in special education costs by the time they graduate from high school (https://www.ncsl.org/research/health/newborn-hearing-screening-state-laws.aspx).
Earliest possible hearing detection is and should be a standard of care for infants and children, and the importance of follow up appointments also cannot be understated,” Ms. Hardy said. Perhaps the biggest challenge for professionals involved with early learning is that many children are delayed in receiving follow up appointments for hearing detection, she added. When families fail to receive a follow-up notice or opt not attend the follow-up appointment, this leaves infants that may be deaf or hard of hearing unidentified, she explained, noting that in some states, lack of consistent and stable state funding needed for effective follow-up with these children and families is a factor.
Ms. Hardy urged that anyone who knows an expectant family can tout the importance of early screenings. Even daycare workers have a responsibility to play a role in early hearing detection, she noted.
Although speech language pathologists routinely advocate for early intervention, “it is never too late to work on skills that will assist children in their everyday lives,” she advised.
The authors had no relevant financial disclosures. The study was funded in part by the Disability Research and Dissemination Center via cooperative agreements with the Centers for Disease Control and Prevention.
SOURCE: Meinzen-Derr J et al. Pediatrics. 2020 October. doi: 10.1542/peds.2020-0557.
Starting early intervention (EI) enrollment before age 6 months in children who are deaf or hard of hearing may have a lasting influence on ensuring kindergarten readiness, Jareen Meinzen-Derr, PhD, MPH of Cincinnati Children’s Hospital Medical Center and colleagues reported in Pediatrics.
The researchers created a comprehensive, longitudinal, population-based database, which linked hearing screening and diagnostic data to that of early intervention data and educational records for 1,746 infants identified with permanent hearing loss who were born between Jan. 1, 2008 and Dec. 31, 2014 The database was established in partnership with the Ohio Departments of Health, Developmental Disabilities and Education, and with the support of the Centers for Disease Control and Prevention and the National Center on Birth Defects and Developmental Disabilities.
Of those, 784 children ranging from preschool to fourth grade were evaluated based on education data available for the 2017 and 2018 school year that had been linked by way of an identifier that flagged students enrolled in EI.
All together, 417 students had kindergarten assessment records, and of those, 385 had Kindergarten Readiness Assessments (KRAs) between 2014 and 2018; 222 (58%) had been enrolled in EI before the age of 6 months. Of those who were enrolled early, the median age of EI enrollment was 3.4 months (2.4-4.3 months) and in those enrolled later, the median age was 9.2 months (7.5-15.4 months).
The importance of EI prior to 6 months
A total of 109 children (28%) receiving services as part of Ohio’s early intervention programs demonstrated kindergarten readiness on their overall KRA scores. The scores revealed that children receiving EI early (34%, n = 75) were more likely to be ready for kindergarten than were those who entered later (21%, n = 34; P = .005). They also were more likely to have on track language and literacy scores (60% vs. 42%, respectively; P = .0006).
Dr. Meinzen-Derr and colleagues noted that factors identified with “an increased odds of being on track included having private insurance and some college education for the mother.” Conversely, factors identified with a decreased likelihood included having a diagnosed disability and bilateral hearing loss.
The researchers cautioned that children transitioned from EI to academic settings will face challenges that may go underrecognized because a school’s focus often is largely on social and academic performance. Thus, working with linked data systems can provide the data to track outcomes that might otherwise be missed, the researchers noted.
Furthermore, they cautioned that even though kindergarten readiness offers some glimpse into future academic success, these measures alone may not be sufficient predictors for children who are deaf or hard of hearing. Risk for communication, social, and academic delays persist throughout school so it is important to employ alternative methods of reading instruction in order to “achieve more complex skills (e.g., complex syntax and advanced vocabulary) necessary for reading proficiency,” the researchers said.
Collecting data from public health and education systems posed limitations for the study. In addition, the absence of kindergarten language assessments prevented Dr. Meinzen-Derr and colleagues from better elucidating reasons for kindergarten readiness. Also beyond the scope of the study was the ability to evaluate the effect service types may have had on outcomes.
The next step in the research process is to evaluate the link between outcomes and specific EI parameters, they said. “Our study demonstrates that an integrated data system can address relevant and important topics regarding early academic outcomes (kindergarten readiness and reading levels) among children who received EI. The current findings provide a new context by evaluating later outcomes among children who are deaf or hard of hearing,” they added, noting that more research is needed to grasp how various EI services impact outcomes since enrollment age is a marker of EI exposure.
Early intervention is everyone’s business
In a separate interview, Amy Hardy M.S. CCC-SLP, speech language pathologist and clinical professor at Idaho State University, emphasized the importance of early intervention, citing reports from the National Center for Hearing Assessment and Management, which credits detection and treatment of hearing loss at birth per child to saving $400,000 in special education costs by the time they graduate from high school (https://www.ncsl.org/research/health/newborn-hearing-screening-state-laws.aspx).
Earliest possible hearing detection is and should be a standard of care for infants and children, and the importance of follow up appointments also cannot be understated,” Ms. Hardy said. Perhaps the biggest challenge for professionals involved with early learning is that many children are delayed in receiving follow up appointments for hearing detection, she added. When families fail to receive a follow-up notice or opt not attend the follow-up appointment, this leaves infants that may be deaf or hard of hearing unidentified, she explained, noting that in some states, lack of consistent and stable state funding needed for effective follow-up with these children and families is a factor.
Ms. Hardy urged that anyone who knows an expectant family can tout the importance of early screenings. Even daycare workers have a responsibility to play a role in early hearing detection, she noted.
Although speech language pathologists routinely advocate for early intervention, “it is never too late to work on skills that will assist children in their everyday lives,” she advised.
The authors had no relevant financial disclosures. The study was funded in part by the Disability Research and Dissemination Center via cooperative agreements with the Centers for Disease Control and Prevention.
SOURCE: Meinzen-Derr J et al. Pediatrics. 2020 October. doi: 10.1542/peds.2020-0557.
Starting early intervention (EI) enrollment before age 6 months in children who are deaf or hard of hearing may have a lasting influence on ensuring kindergarten readiness, Jareen Meinzen-Derr, PhD, MPH of Cincinnati Children’s Hospital Medical Center and colleagues reported in Pediatrics.
The researchers created a comprehensive, longitudinal, population-based database, which linked hearing screening and diagnostic data to that of early intervention data and educational records for 1,746 infants identified with permanent hearing loss who were born between Jan. 1, 2008 and Dec. 31, 2014 The database was established in partnership with the Ohio Departments of Health, Developmental Disabilities and Education, and with the support of the Centers for Disease Control and Prevention and the National Center on Birth Defects and Developmental Disabilities.
Of those, 784 children ranging from preschool to fourth grade were evaluated based on education data available for the 2017 and 2018 school year that had been linked by way of an identifier that flagged students enrolled in EI.
All together, 417 students had kindergarten assessment records, and of those, 385 had Kindergarten Readiness Assessments (KRAs) between 2014 and 2018; 222 (58%) had been enrolled in EI before the age of 6 months. Of those who were enrolled early, the median age of EI enrollment was 3.4 months (2.4-4.3 months) and in those enrolled later, the median age was 9.2 months (7.5-15.4 months).
The importance of EI prior to 6 months
A total of 109 children (28%) receiving services as part of Ohio’s early intervention programs demonstrated kindergarten readiness on their overall KRA scores. The scores revealed that children receiving EI early (34%, n = 75) were more likely to be ready for kindergarten than were those who entered later (21%, n = 34; P = .005). They also were more likely to have on track language and literacy scores (60% vs. 42%, respectively; P = .0006).
Dr. Meinzen-Derr and colleagues noted that factors identified with “an increased odds of being on track included having private insurance and some college education for the mother.” Conversely, factors identified with a decreased likelihood included having a diagnosed disability and bilateral hearing loss.
The researchers cautioned that children transitioned from EI to academic settings will face challenges that may go underrecognized because a school’s focus often is largely on social and academic performance. Thus, working with linked data systems can provide the data to track outcomes that might otherwise be missed, the researchers noted.
Furthermore, they cautioned that even though kindergarten readiness offers some glimpse into future academic success, these measures alone may not be sufficient predictors for children who are deaf or hard of hearing. Risk for communication, social, and academic delays persist throughout school so it is important to employ alternative methods of reading instruction in order to “achieve more complex skills (e.g., complex syntax and advanced vocabulary) necessary for reading proficiency,” the researchers said.
Collecting data from public health and education systems posed limitations for the study. In addition, the absence of kindergarten language assessments prevented Dr. Meinzen-Derr and colleagues from better elucidating reasons for kindergarten readiness. Also beyond the scope of the study was the ability to evaluate the effect service types may have had on outcomes.
The next step in the research process is to evaluate the link between outcomes and specific EI parameters, they said. “Our study demonstrates that an integrated data system can address relevant and important topics regarding early academic outcomes (kindergarten readiness and reading levels) among children who received EI. The current findings provide a new context by evaluating later outcomes among children who are deaf or hard of hearing,” they added, noting that more research is needed to grasp how various EI services impact outcomes since enrollment age is a marker of EI exposure.
Early intervention is everyone’s business
In a separate interview, Amy Hardy M.S. CCC-SLP, speech language pathologist and clinical professor at Idaho State University, emphasized the importance of early intervention, citing reports from the National Center for Hearing Assessment and Management, which credits detection and treatment of hearing loss at birth per child to saving $400,000 in special education costs by the time they graduate from high school (https://www.ncsl.org/research/health/newborn-hearing-screening-state-laws.aspx).
Earliest possible hearing detection is and should be a standard of care for infants and children, and the importance of follow up appointments also cannot be understated,” Ms. Hardy said. Perhaps the biggest challenge for professionals involved with early learning is that many children are delayed in receiving follow up appointments for hearing detection, she added. When families fail to receive a follow-up notice or opt not attend the follow-up appointment, this leaves infants that may be deaf or hard of hearing unidentified, she explained, noting that in some states, lack of consistent and stable state funding needed for effective follow-up with these children and families is a factor.
Ms. Hardy urged that anyone who knows an expectant family can tout the importance of early screenings. Even daycare workers have a responsibility to play a role in early hearing detection, she noted.
Although speech language pathologists routinely advocate for early intervention, “it is never too late to work on skills that will assist children in their everyday lives,” she advised.
The authors had no relevant financial disclosures. The study was funded in part by the Disability Research and Dissemination Center via cooperative agreements with the Centers for Disease Control and Prevention.
SOURCE: Meinzen-Derr J et al. Pediatrics. 2020 October. doi: 10.1542/peds.2020-0557.
FROM PEDIATRICS
Migraine nerve stimulation device now available over the counter
The Food and Drug Administration has cleared Cefaly Dual (Cefaly Technology) which was previously available only by prescription.
Most migraines involve the trigeminal nerve, which can be accessed through the skin on the forehead. Cefaly Dual stimulates the trigeminal nerve using a reusable self-adhesive electrode placed on the forehead.
The device has two settings, ACUTE and PREVENT. In the ACUTE setting, the individual wears the device for 60 minutes at headache onset or during a migraine attack. In the PREVENT setting, the individual wears the device for 20 minutes daily to help prevent future episodes.
At the start of a session, the wearer may feel a slight tingling sensation, which gradually increases and spreads throughout the forehead and the front part of the head. After about 14 minutes, the intensity stabilizes and remains constant until the treatment session is over, according to the company. The device automatically shuts off at the end of each session. It can be used as a stand-alone option or with existing treatment, the company noted.
“For millions of people across the U.S., living with migraine pain and coping with debilitating symptoms are daily realities. It is our mission to provide consumers with increased access to an effective and safe dual modality migraine treatment that is scientifically proven to reduce the number of monthly migraine days by almost half,” Jennifer Trainor McDermott, CEO of Cefaly Technology, said in a news release.
The FDA’s over-the-counter clearance of Cefaly Dual was based on several randomized, controlled clinical trials supporting the efficacy and safety of the device, the company said.
An earlier version of the Cefaly device was approved in the United States in March 2014 to help prevent migraine headache in adults aged 18 or older. The next-generation Cefaly Dual device is “small and sleek in comparison to its older model, which uses bands along the sides to create room for batteries. The newest device is palm-sized, more portable, and uses a battery that is rechargeable via USB,” the company said.
Last spring, the company announced a buyback program where customers in the United States may return their original device and receive a discount of the purchase of the Cefaly Dual device.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has cleared Cefaly Dual (Cefaly Technology) which was previously available only by prescription.
Most migraines involve the trigeminal nerve, which can be accessed through the skin on the forehead. Cefaly Dual stimulates the trigeminal nerve using a reusable self-adhesive electrode placed on the forehead.
The device has two settings, ACUTE and PREVENT. In the ACUTE setting, the individual wears the device for 60 minutes at headache onset or during a migraine attack. In the PREVENT setting, the individual wears the device for 20 minutes daily to help prevent future episodes.
At the start of a session, the wearer may feel a slight tingling sensation, which gradually increases and spreads throughout the forehead and the front part of the head. After about 14 minutes, the intensity stabilizes and remains constant until the treatment session is over, according to the company. The device automatically shuts off at the end of each session. It can be used as a stand-alone option or with existing treatment, the company noted.
“For millions of people across the U.S., living with migraine pain and coping with debilitating symptoms are daily realities. It is our mission to provide consumers with increased access to an effective and safe dual modality migraine treatment that is scientifically proven to reduce the number of monthly migraine days by almost half,” Jennifer Trainor McDermott, CEO of Cefaly Technology, said in a news release.
The FDA’s over-the-counter clearance of Cefaly Dual was based on several randomized, controlled clinical trials supporting the efficacy and safety of the device, the company said.
An earlier version of the Cefaly device was approved in the United States in March 2014 to help prevent migraine headache in adults aged 18 or older. The next-generation Cefaly Dual device is “small and sleek in comparison to its older model, which uses bands along the sides to create room for batteries. The newest device is palm-sized, more portable, and uses a battery that is rechargeable via USB,” the company said.
Last spring, the company announced a buyback program where customers in the United States may return their original device and receive a discount of the purchase of the Cefaly Dual device.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has cleared Cefaly Dual (Cefaly Technology) which was previously available only by prescription.
Most migraines involve the trigeminal nerve, which can be accessed through the skin on the forehead. Cefaly Dual stimulates the trigeminal nerve using a reusable self-adhesive electrode placed on the forehead.
The device has two settings, ACUTE and PREVENT. In the ACUTE setting, the individual wears the device for 60 minutes at headache onset or during a migraine attack. In the PREVENT setting, the individual wears the device for 20 minutes daily to help prevent future episodes.
At the start of a session, the wearer may feel a slight tingling sensation, which gradually increases and spreads throughout the forehead and the front part of the head. After about 14 minutes, the intensity stabilizes and remains constant until the treatment session is over, according to the company. The device automatically shuts off at the end of each session. It can be used as a stand-alone option or with existing treatment, the company noted.
“For millions of people across the U.S., living with migraine pain and coping with debilitating symptoms are daily realities. It is our mission to provide consumers with increased access to an effective and safe dual modality migraine treatment that is scientifically proven to reduce the number of monthly migraine days by almost half,” Jennifer Trainor McDermott, CEO of Cefaly Technology, said in a news release.
The FDA’s over-the-counter clearance of Cefaly Dual was based on several randomized, controlled clinical trials supporting the efficacy and safety of the device, the company said.
An earlier version of the Cefaly device was approved in the United States in March 2014 to help prevent migraine headache in adults aged 18 or older. The next-generation Cefaly Dual device is “small and sleek in comparison to its older model, which uses bands along the sides to create room for batteries. The newest device is palm-sized, more portable, and uses a battery that is rechargeable via USB,” the company said.
Last spring, the company announced a buyback program where customers in the United States may return their original device and receive a discount of the purchase of the Cefaly Dual device.
A version of this article originally appeared on Medscape.com.
Being HIV positive increases risk of death from COVID-19
compared with people without HIV.
A comparison of outcomes of people with HIV to people without HIV who were hospitalized in the United Kingdom with COVID-19 from Jan. 17 to June 4 showed that HIV-positive status was associated with a 63% increased risk of day 28 mortality.
This was especially true for HIV+ patients younger than 70 years of age, said Anna Maria Geretti, MD, PhD, professor of virology and infectious diseases, University of Liverpool, England.
The results are from an analysis of data from the ISARIC World Health Organization (WHO) Clinical Characterisation Protocol (UK) study, and were presented at the HIV Glasgow annual meeting, held virtually this year because of the pandemic.
“We investigated whether HIV status could be important in COVID-19 outcomes because there was anxiety on the part of our patients, and we wanted to gather some evidence-based information in order to help guide them,” Dr. Geretti said in an interview.
“ISARIC is an international protocol and the UK is one of the nations participating. We applied for access to its very large database, which connects data from all patients who are hospitalized with either known or suspected COVID-19. We wanted to see specifically how the presentation and outcomes of patients with HIV compared with the rest of the population without HIV. It afforded us an ideal opportunity to start to answer this question, and this is our first analysis in what will be an ongoing process. Importantly, we showed that there is a need to really look more carefully at the population with HIV,” she said.
Out of a total of 47,539 patients in the database, 115 (0.24%) had confirmed HIV-positive status, and 103 of those 115, or 89.6%, had a record of being on antiretroviral therapy.
On admission, the patients with HIV were younger, with a median age of 55 compared with 74 for patients without HIV (P < .001). They also had a higher prevalence of obesity, moderate to severe liver disease, higher lymphocyte counts and C-reactive protein, as well as more systemic symptoms.
There were no differences in respiratory rate, need for oxygen, or prevalence of chest infiltrates.
The cumulative incidence of mortality at day 28 was 25.2% in HIV-positive patients compared with 32.1% in HIV-negative patients (P = .12).
But when the researchers looked more closely, they noticed that the mortality rate was actually higher in younger HIV+ patients compared with HIV-negative patients.
Stratified by age, 28-day mortality was significantly higher in HIV+ patients aged <50 years (P =.004); and those aged 50 to 59 years (P = .05).
“So below the age of 70, the risk of mortality was double in people with HIV. The people with HIV who died often had diabetes with complications and also more frequent obesity, but this was not the only explanation,” Dr. Geretti said. “There is something to do with the HIV status per se.”
Next steps will be to expand the data set and repeat the analysis with an additional 100 patients “at least” she said.
The researchers also hope to zero in on what about being HIV+ is increasing the mortality risk from COVID-19.
“Right now we need greater numbers and we hope that the research community will be stimulated to take a closer look at this information, and merge other data so that we can strengthen confidence in the data and tease out what factors are causing this increased risk for mortality,” Dr. Geretti said.
She also emphasized that all patients admitted to hospital with COVID-19 should be asked about their HIV status.
“It is important that the HIV status be recorded if we want to increase our ability to understand how HIV impacts survival,” she stressed. “In our experience we found that most of the hospital records were not doing that. Since HIV+ patients seem to be at increased risk, HIV status should be factored into the clinical management. Ask patients if they are HIV+, and if it is not known, then do a test. That would be good practice.”
Dr. Geretti reported no relevant financial relationships. The work was supported by grants from the National Institute of Health Research, the Medical Research Council, the Wellcome Trust, the Department for International Development, and the Bill and Melinda Gates Foundation.
A version of this article originally appeared on Medscape.com.
compared with people without HIV.
A comparison of outcomes of people with HIV to people without HIV who were hospitalized in the United Kingdom with COVID-19 from Jan. 17 to June 4 showed that HIV-positive status was associated with a 63% increased risk of day 28 mortality.
This was especially true for HIV+ patients younger than 70 years of age, said Anna Maria Geretti, MD, PhD, professor of virology and infectious diseases, University of Liverpool, England.
The results are from an analysis of data from the ISARIC World Health Organization (WHO) Clinical Characterisation Protocol (UK) study, and were presented at the HIV Glasgow annual meeting, held virtually this year because of the pandemic.
“We investigated whether HIV status could be important in COVID-19 outcomes because there was anxiety on the part of our patients, and we wanted to gather some evidence-based information in order to help guide them,” Dr. Geretti said in an interview.
“ISARIC is an international protocol and the UK is one of the nations participating. We applied for access to its very large database, which connects data from all patients who are hospitalized with either known or suspected COVID-19. We wanted to see specifically how the presentation and outcomes of patients with HIV compared with the rest of the population without HIV. It afforded us an ideal opportunity to start to answer this question, and this is our first analysis in what will be an ongoing process. Importantly, we showed that there is a need to really look more carefully at the population with HIV,” she said.
Out of a total of 47,539 patients in the database, 115 (0.24%) had confirmed HIV-positive status, and 103 of those 115, or 89.6%, had a record of being on antiretroviral therapy.
On admission, the patients with HIV were younger, with a median age of 55 compared with 74 for patients without HIV (P < .001). They also had a higher prevalence of obesity, moderate to severe liver disease, higher lymphocyte counts and C-reactive protein, as well as more systemic symptoms.
There were no differences in respiratory rate, need for oxygen, or prevalence of chest infiltrates.
The cumulative incidence of mortality at day 28 was 25.2% in HIV-positive patients compared with 32.1% in HIV-negative patients (P = .12).
But when the researchers looked more closely, they noticed that the mortality rate was actually higher in younger HIV+ patients compared with HIV-negative patients.
Stratified by age, 28-day mortality was significantly higher in HIV+ patients aged <50 years (P =.004); and those aged 50 to 59 years (P = .05).
“So below the age of 70, the risk of mortality was double in people with HIV. The people with HIV who died often had diabetes with complications and also more frequent obesity, but this was not the only explanation,” Dr. Geretti said. “There is something to do with the HIV status per se.”
Next steps will be to expand the data set and repeat the analysis with an additional 100 patients “at least” she said.
The researchers also hope to zero in on what about being HIV+ is increasing the mortality risk from COVID-19.
“Right now we need greater numbers and we hope that the research community will be stimulated to take a closer look at this information, and merge other data so that we can strengthen confidence in the data and tease out what factors are causing this increased risk for mortality,” Dr. Geretti said.
She also emphasized that all patients admitted to hospital with COVID-19 should be asked about their HIV status.
“It is important that the HIV status be recorded if we want to increase our ability to understand how HIV impacts survival,” she stressed. “In our experience we found that most of the hospital records were not doing that. Since HIV+ patients seem to be at increased risk, HIV status should be factored into the clinical management. Ask patients if they are HIV+, and if it is not known, then do a test. That would be good practice.”
Dr. Geretti reported no relevant financial relationships. The work was supported by grants from the National Institute of Health Research, the Medical Research Council, the Wellcome Trust, the Department for International Development, and the Bill and Melinda Gates Foundation.
A version of this article originally appeared on Medscape.com.
compared with people without HIV.
A comparison of outcomes of people with HIV to people without HIV who were hospitalized in the United Kingdom with COVID-19 from Jan. 17 to June 4 showed that HIV-positive status was associated with a 63% increased risk of day 28 mortality.
This was especially true for HIV+ patients younger than 70 years of age, said Anna Maria Geretti, MD, PhD, professor of virology and infectious diseases, University of Liverpool, England.
The results are from an analysis of data from the ISARIC World Health Organization (WHO) Clinical Characterisation Protocol (UK) study, and were presented at the HIV Glasgow annual meeting, held virtually this year because of the pandemic.
“We investigated whether HIV status could be important in COVID-19 outcomes because there was anxiety on the part of our patients, and we wanted to gather some evidence-based information in order to help guide them,” Dr. Geretti said in an interview.
“ISARIC is an international protocol and the UK is one of the nations participating. We applied for access to its very large database, which connects data from all patients who are hospitalized with either known or suspected COVID-19. We wanted to see specifically how the presentation and outcomes of patients with HIV compared with the rest of the population without HIV. It afforded us an ideal opportunity to start to answer this question, and this is our first analysis in what will be an ongoing process. Importantly, we showed that there is a need to really look more carefully at the population with HIV,” she said.
Out of a total of 47,539 patients in the database, 115 (0.24%) had confirmed HIV-positive status, and 103 of those 115, or 89.6%, had a record of being on antiretroviral therapy.
On admission, the patients with HIV were younger, with a median age of 55 compared with 74 for patients without HIV (P < .001). They also had a higher prevalence of obesity, moderate to severe liver disease, higher lymphocyte counts and C-reactive protein, as well as more systemic symptoms.
There were no differences in respiratory rate, need for oxygen, or prevalence of chest infiltrates.
The cumulative incidence of mortality at day 28 was 25.2% in HIV-positive patients compared with 32.1% in HIV-negative patients (P = .12).
But when the researchers looked more closely, they noticed that the mortality rate was actually higher in younger HIV+ patients compared with HIV-negative patients.
Stratified by age, 28-day mortality was significantly higher in HIV+ patients aged <50 years (P =.004); and those aged 50 to 59 years (P = .05).
“So below the age of 70, the risk of mortality was double in people with HIV. The people with HIV who died often had diabetes with complications and also more frequent obesity, but this was not the only explanation,” Dr. Geretti said. “There is something to do with the HIV status per se.”
Next steps will be to expand the data set and repeat the analysis with an additional 100 patients “at least” she said.
The researchers also hope to zero in on what about being HIV+ is increasing the mortality risk from COVID-19.
“Right now we need greater numbers and we hope that the research community will be stimulated to take a closer look at this information, and merge other data so that we can strengthen confidence in the data and tease out what factors are causing this increased risk for mortality,” Dr. Geretti said.
She also emphasized that all patients admitted to hospital with COVID-19 should be asked about their HIV status.
“It is important that the HIV status be recorded if we want to increase our ability to understand how HIV impacts survival,” she stressed. “In our experience we found that most of the hospital records were not doing that. Since HIV+ patients seem to be at increased risk, HIV status should be factored into the clinical management. Ask patients if they are HIV+, and if it is not known, then do a test. That would be good practice.”
Dr. Geretti reported no relevant financial relationships. The work was supported by grants from the National Institute of Health Research, the Medical Research Council, the Wellcome Trust, the Department for International Development, and the Bill and Melinda Gates Foundation.
A version of this article originally appeared on Medscape.com.
Switch to integrase inhibitor regimen safe and effective
data from a randomized trial indicate.
Among 212 women with successful HIV virologic suppression following 48 weeks of treatment with ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate (ATV/r +TDF), among those who were switched to continued therapy with an integrase inhibitor–based regimen of elvitegravir/cobicistat/emtricitabine and tenofovir alafenamide (E/C/F/TAF), mean increases in lumbar spine bone mineral density (BMD) were greater and renal function was improved compared with patients who were maintained with ATV/r + TDF, reported Monica Thormann, MD, from Salvador B. Gautier Hospital in Santo Domingo, Dominican Republic, and colleagues at the HIV Glasgow drug therapy meeting, which was held online in 2020.
Although the E/C/F/TAF regimen was associated with a significantly greater increase in lipids, there was no significant change in the total cholesterol to high-density lipoprotein (HDL) cholesterol ratio.
The patients in the study had previously participated in a blinded randomized trial comparing the integrase inhibitor combination plus TDF with ATV/r + TDF in treatment-naive women.
In the current study, patients were randomly assigned in a 3:1 ratio to maintenance with either E/C/F/TAF (159 patients) or ATV/r + TDF (53 patients).
Forty-eight weeks after the switch, virologic suppression (to fewer than 50 copies/mL) was maintained among 94.3% of those on the integrase inhibitor–based regimen, compared with 86.8% of those on the protease inhibitor–based regimen. Virologic failure was seen in 1.9% of those on the integrase inhibitor–based regimen and in 3.8% of those on the protease inhibitor–based regimen.
In addition, virologic suppression below 20 c/mL at week 48 was more common among women maintained on E/C/F/TAF, at 84.9% vs 71.7% (P = .041). No treatment-emergent resistance was seen with either regimen.
As noted, there were higher mean percentage increases in BMD in the E/C/F/TAF group for both total hip and lumbar spine, but only the latter measure improved significantly in comparison with patients treated with ATV/r + TDF (2.82% vs 0%, P < .001).
Markers of renal tubule damage, including the beta-2 microglobulin to creatinine ratio and the rentinol-binding protein to creatinine ratio, were significantly improved with the integrase inhibitor regimen.
Increases in total cholesterol, LDL cholesterol, and HDL cholesterol were 27 vs 5 mg/dL, 16 vs 8 mg/dL, and 5 vs 0 mg/dL in each case comparing the integrase inhibitor–based regimen to the protease inhibitor–based regimen. All of those comparisons were statistically significant.
As noted, however, the total cholesterol to HDL cholesterol ratio was not significantly different between the treatment arms. The rate or initiation of lipid-modifying agents was 1.3% in the E/C/F/TAF group vs 0 in the ATV/r + TDF group, but this difference was not statistically significant.
“These data demonstrate that women who switch to an integrase inhibitor + TAF‐based regimen maintain high levels of virologic suppression with improvement in BMD and renal function biomarkers, as compared with those remaining on their ritonavir boosted atazanavir + TDF‐based regimen,” the authors wrote.
This article first appeared on Medscape.com.
data from a randomized trial indicate.
Among 212 women with successful HIV virologic suppression following 48 weeks of treatment with ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate (ATV/r +TDF), among those who were switched to continued therapy with an integrase inhibitor–based regimen of elvitegravir/cobicistat/emtricitabine and tenofovir alafenamide (E/C/F/TAF), mean increases in lumbar spine bone mineral density (BMD) were greater and renal function was improved compared with patients who were maintained with ATV/r + TDF, reported Monica Thormann, MD, from Salvador B. Gautier Hospital in Santo Domingo, Dominican Republic, and colleagues at the HIV Glasgow drug therapy meeting, which was held online in 2020.
Although the E/C/F/TAF regimen was associated with a significantly greater increase in lipids, there was no significant change in the total cholesterol to high-density lipoprotein (HDL) cholesterol ratio.
The patients in the study had previously participated in a blinded randomized trial comparing the integrase inhibitor combination plus TDF with ATV/r + TDF in treatment-naive women.
In the current study, patients were randomly assigned in a 3:1 ratio to maintenance with either E/C/F/TAF (159 patients) or ATV/r + TDF (53 patients).
Forty-eight weeks after the switch, virologic suppression (to fewer than 50 copies/mL) was maintained among 94.3% of those on the integrase inhibitor–based regimen, compared with 86.8% of those on the protease inhibitor–based regimen. Virologic failure was seen in 1.9% of those on the integrase inhibitor–based regimen and in 3.8% of those on the protease inhibitor–based regimen.
In addition, virologic suppression below 20 c/mL at week 48 was more common among women maintained on E/C/F/TAF, at 84.9% vs 71.7% (P = .041). No treatment-emergent resistance was seen with either regimen.
As noted, there were higher mean percentage increases in BMD in the E/C/F/TAF group for both total hip and lumbar spine, but only the latter measure improved significantly in comparison with patients treated with ATV/r + TDF (2.82% vs 0%, P < .001).
Markers of renal tubule damage, including the beta-2 microglobulin to creatinine ratio and the rentinol-binding protein to creatinine ratio, were significantly improved with the integrase inhibitor regimen.
Increases in total cholesterol, LDL cholesterol, and HDL cholesterol were 27 vs 5 mg/dL, 16 vs 8 mg/dL, and 5 vs 0 mg/dL in each case comparing the integrase inhibitor–based regimen to the protease inhibitor–based regimen. All of those comparisons were statistically significant.
As noted, however, the total cholesterol to HDL cholesterol ratio was not significantly different between the treatment arms. The rate or initiation of lipid-modifying agents was 1.3% in the E/C/F/TAF group vs 0 in the ATV/r + TDF group, but this difference was not statistically significant.
“These data demonstrate that women who switch to an integrase inhibitor + TAF‐based regimen maintain high levels of virologic suppression with improvement in BMD and renal function biomarkers, as compared with those remaining on their ritonavir boosted atazanavir + TDF‐based regimen,” the authors wrote.
This article first appeared on Medscape.com.
data from a randomized trial indicate.
Among 212 women with successful HIV virologic suppression following 48 weeks of treatment with ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate (ATV/r +TDF), among those who were switched to continued therapy with an integrase inhibitor–based regimen of elvitegravir/cobicistat/emtricitabine and tenofovir alafenamide (E/C/F/TAF), mean increases in lumbar spine bone mineral density (BMD) were greater and renal function was improved compared with patients who were maintained with ATV/r + TDF, reported Monica Thormann, MD, from Salvador B. Gautier Hospital in Santo Domingo, Dominican Republic, and colleagues at the HIV Glasgow drug therapy meeting, which was held online in 2020.
Although the E/C/F/TAF regimen was associated with a significantly greater increase in lipids, there was no significant change in the total cholesterol to high-density lipoprotein (HDL) cholesterol ratio.
The patients in the study had previously participated in a blinded randomized trial comparing the integrase inhibitor combination plus TDF with ATV/r + TDF in treatment-naive women.
In the current study, patients were randomly assigned in a 3:1 ratio to maintenance with either E/C/F/TAF (159 patients) or ATV/r + TDF (53 patients).
Forty-eight weeks after the switch, virologic suppression (to fewer than 50 copies/mL) was maintained among 94.3% of those on the integrase inhibitor–based regimen, compared with 86.8% of those on the protease inhibitor–based regimen. Virologic failure was seen in 1.9% of those on the integrase inhibitor–based regimen and in 3.8% of those on the protease inhibitor–based regimen.
In addition, virologic suppression below 20 c/mL at week 48 was more common among women maintained on E/C/F/TAF, at 84.9% vs 71.7% (P = .041). No treatment-emergent resistance was seen with either regimen.
As noted, there were higher mean percentage increases in BMD in the E/C/F/TAF group for both total hip and lumbar spine, but only the latter measure improved significantly in comparison with patients treated with ATV/r + TDF (2.82% vs 0%, P < .001).
Markers of renal tubule damage, including the beta-2 microglobulin to creatinine ratio and the rentinol-binding protein to creatinine ratio, were significantly improved with the integrase inhibitor regimen.
Increases in total cholesterol, LDL cholesterol, and HDL cholesterol were 27 vs 5 mg/dL, 16 vs 8 mg/dL, and 5 vs 0 mg/dL in each case comparing the integrase inhibitor–based regimen to the protease inhibitor–based regimen. All of those comparisons were statistically significant.
As noted, however, the total cholesterol to HDL cholesterol ratio was not significantly different between the treatment arms. The rate or initiation of lipid-modifying agents was 1.3% in the E/C/F/TAF group vs 0 in the ATV/r + TDF group, but this difference was not statistically significant.
“These data demonstrate that women who switch to an integrase inhibitor + TAF‐based regimen maintain high levels of virologic suppression with improvement in BMD and renal function biomarkers, as compared with those remaining on their ritonavir boosted atazanavir + TDF‐based regimen,” the authors wrote.
This article first appeared on Medscape.com.
Delayed cancer screening could cause increase in deaths, study says
Delays in colorectal cancer screening due to the COVID-19 pandemic could lead to higher rates of advanced-stage cancer and death, according to a new study.
When compared with a delay of less than three months, the longer delay seen this year may result in an 11.9% increase in death rates.
“Across the globe, health care systems are facing serious difficulties while dealing with COVID-19, and it is imperative that support is given to the public and patients throughout the crisis, including for high-impact diseases such as colorectal cancer,” Luigi Ricciardiello, the lead study author and a professor at the University of Bologna in Italy, said in a statement.
Ricciardiello and colleagues presented their research on Monday at UEG Week Virtual 2020, an international conference for gastroenterologists. The study will be published in the UEG Journal .
The researchers created a model to forecast the effects of delayed cancer screening during 2020. A “moderate” delay of 7-12 months caused a 3% increase in advanced-stage colon cancer, and a long delay of more than 12 months caused a 7% increase in advanced cancer.
Based on a survival rate of 5 years for stage 3 or stage 4 colorectal cancer, the death rate would increase nearly 12% when screening is delayed for more than a year, as compared with less than three months of delay.
The research team found similar results when forecasting advanced-stage cancer and deaths earlier this year. In a paper published in Clinical Gastroenterology and Hepatology in early September, they projected that deaths could increase 12% if screening is delayed for more than a year.
Throughout the pandemic, screening programs have been delayed in many countries, particularly across Europe.
“Healthcare authorities need to act urgently on how they reorganise activities during COVID-19, without compromising the diagnosis of other high-impact diseases like this research shows,” Ricciardiello said.
United European Gastroenterology, a professional medical organization for digestive health specialists, has called for policymakers to implement colon cancer screening programs across the European Union. Annually, more than 375,000 new cases are diagnosed across the EU, and more than 170,000 people die from colorectal cancer, according to a UEG report.
“Early-stage diagnosis of colorectal cancer is crucial — it’s far easier to treat and enhances optimal patient outcomes,” Ricciardiello said. “It is therefore essential that vital diagnosis tools, like screening programmes, continue and help to prevent mortality rates from rising even further.”
This article first appeared on Medscape.com.
Delays in colorectal cancer screening due to the COVID-19 pandemic could lead to higher rates of advanced-stage cancer and death, according to a new study.
When compared with a delay of less than three months, the longer delay seen this year may result in an 11.9% increase in death rates.
“Across the globe, health care systems are facing serious difficulties while dealing with COVID-19, and it is imperative that support is given to the public and patients throughout the crisis, including for high-impact diseases such as colorectal cancer,” Luigi Ricciardiello, the lead study author and a professor at the University of Bologna in Italy, said in a statement.
Ricciardiello and colleagues presented their research on Monday at UEG Week Virtual 2020, an international conference for gastroenterologists. The study will be published in the UEG Journal .
The researchers created a model to forecast the effects of delayed cancer screening during 2020. A “moderate” delay of 7-12 months caused a 3% increase in advanced-stage colon cancer, and a long delay of more than 12 months caused a 7% increase in advanced cancer.
Based on a survival rate of 5 years for stage 3 or stage 4 colorectal cancer, the death rate would increase nearly 12% when screening is delayed for more than a year, as compared with less than three months of delay.
The research team found similar results when forecasting advanced-stage cancer and deaths earlier this year. In a paper published in Clinical Gastroenterology and Hepatology in early September, they projected that deaths could increase 12% if screening is delayed for more than a year.
Throughout the pandemic, screening programs have been delayed in many countries, particularly across Europe.
“Healthcare authorities need to act urgently on how they reorganise activities during COVID-19, without compromising the diagnosis of other high-impact diseases like this research shows,” Ricciardiello said.
United European Gastroenterology, a professional medical organization for digestive health specialists, has called for policymakers to implement colon cancer screening programs across the European Union. Annually, more than 375,000 new cases are diagnosed across the EU, and more than 170,000 people die from colorectal cancer, according to a UEG report.
“Early-stage diagnosis of colorectal cancer is crucial — it’s far easier to treat and enhances optimal patient outcomes,” Ricciardiello said. “It is therefore essential that vital diagnosis tools, like screening programmes, continue and help to prevent mortality rates from rising even further.”
This article first appeared on Medscape.com.
Delays in colorectal cancer screening due to the COVID-19 pandemic could lead to higher rates of advanced-stage cancer and death, according to a new study.
When compared with a delay of less than three months, the longer delay seen this year may result in an 11.9% increase in death rates.
“Across the globe, health care systems are facing serious difficulties while dealing with COVID-19, and it is imperative that support is given to the public and patients throughout the crisis, including for high-impact diseases such as colorectal cancer,” Luigi Ricciardiello, the lead study author and a professor at the University of Bologna in Italy, said in a statement.
Ricciardiello and colleagues presented their research on Monday at UEG Week Virtual 2020, an international conference for gastroenterologists. The study will be published in the UEG Journal .
The researchers created a model to forecast the effects of delayed cancer screening during 2020. A “moderate” delay of 7-12 months caused a 3% increase in advanced-stage colon cancer, and a long delay of more than 12 months caused a 7% increase in advanced cancer.
Based on a survival rate of 5 years for stage 3 or stage 4 colorectal cancer, the death rate would increase nearly 12% when screening is delayed for more than a year, as compared with less than three months of delay.
The research team found similar results when forecasting advanced-stage cancer and deaths earlier this year. In a paper published in Clinical Gastroenterology and Hepatology in early September, they projected that deaths could increase 12% if screening is delayed for more than a year.
Throughout the pandemic, screening programs have been delayed in many countries, particularly across Europe.
“Healthcare authorities need to act urgently on how they reorganise activities during COVID-19, without compromising the diagnosis of other high-impact diseases like this research shows,” Ricciardiello said.
United European Gastroenterology, a professional medical organization for digestive health specialists, has called for policymakers to implement colon cancer screening programs across the European Union. Annually, more than 375,000 new cases are diagnosed across the EU, and more than 170,000 people die from colorectal cancer, according to a UEG report.
“Early-stage diagnosis of colorectal cancer is crucial — it’s far easier to treat and enhances optimal patient outcomes,” Ricciardiello said. “It is therefore essential that vital diagnosis tools, like screening programmes, continue and help to prevent mortality rates from rising even further.”
This article first appeared on Medscape.com.
Low back pain in youth: Recognizing red flags
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30
History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10
Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; sphillips6@pennstatehealth.psu.edu.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30
History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10
Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; sphillips6@pennstatehealth.psu.edu.
Low back pain in not uncommon in children and adolescents.1-3 Although the prevalence of low back pain in children < 7 years is low, it increases with age, with studies reporting lifetime prevalence at age 12 years between 16% and 18% and rates as high as 66% by 16 years of age.4,5 Although children and adolescents usually have pain that is transient and benign without a defined cause, structural causes of low back pain should be considered in school-aged children with pain that persists for > 3 to 6 weeks. 4 The most common structural causes of adolescent low back pain are reviewed here.
Etiology: A mixed bag
Back pain in school-aged children is most commonly due to muscular strain, overuse, or poor posture. The pain is often transient in nature and responds to rest and postural education.4,6 A herniated disc is an uncommon finding in younger school-aged children, but incidence increases slightly among older adolescents, particularly those who are active in collision sports and/or weight-lifting.7,8 Pain caused by a herniated disc often radiates along the distribution of the sciatic nerve and worsens during lumbar flexion.
Spondylolysis and spondylolisthesis are important causes of back pain in children. Spondylolysis is defined as a defect or abnormality of the pars interarticularis and surrounding lamina and pedicle. Spondylolisthesis, which is less common, is defined as the translation or “slippage” of one vertebral segment in relation to the next caudal segment. These conditions commonly occur as a result of repetitive stress.
In a prospective study of adolescents < 19 years with low back pain for > 2 weeks, the prevalence of spondylolysis was 39.7%.9 Adolescent athletes with symptomatic low back pain are more likely to have spondylolysis than nonathletes (32% vs 2%, respectively).2,10 Pain is often made worse by extension of the spine. Spondylolysis and spondylolisthesis can be congenital or acquired, and both can be asymptomatic. Children and teens who are athletes are at higher risk for symptomatic spondylolysis and spondylolisthesis.10-12 This is especially true for those involved in gymnastics, dance, football, and/or volleyball, where a repetitive load is placed onto an extended spine.
Idiopathic scoliosis is an abnormal lateral curvature of the spine that usually develops during adolescence and worsens with growth. Historically, painful scoliosis was considered rare, but more recently researchers determined that children with scoliosis have a higher rate of pain compared to their peers.13,14 School-aged children with scoliosis were found to be at 2 times the risk of low back pain compared to those without scoliosis.13 It is important to identify scoliosis in adolescents so that progression can be monitored.
Screening for scoliosis in primary care is somewhat controversial. The US Preventive Services Task Force (USPSTF) finds insufficient evidence for screening asymptomatic adolescents for scoliosis.15 This recommendation is based on the fact that there is little evidence on the effect of screening on long-term outcomes. Screening may also lead to unnecessary radiation. Conversely, a position statement released by the Scoliosis Research Society, the Pediatric Orthopedic Society of North America, the American Association of Orthopedic Surgeons, and the American Academy of Pediatrics recommends scoliosis screening during routine pediatric office visits.16 Screening for girls is recommended at ages 10 and 12 years, and for boys, once between ages 13 and 14 years. The statement highlights evidence showing that focused screening by appropriate personnel has value in detecting a clinically significant curve (> 20°).
Scheuermann disease is a rare cause of back pain in children that usually develops during adolescence and results in increasing thoracic kyphosis. An autosomal dominant mutation plays a role in this disease of the growth cartilage endplate; repetitive strain on the growth cartilage is also a contributing factor.17,18 An atypical variant manifests with kyphosis in the thoracolumbar region.17
Continue to: Other causes of low back pain
Other causes of low back pain—including inflammatory arthritis, infection (eg, discitis), and tumor—are rare in children but must always be considered, especially in the setting of persistent symptoms.4,19-21 More on the features of these conditions is listed in TABLE 1.1-7,13-15,17-30
History: Focus on onset, timing, and duration of symptoms
As with adults, obtaining a history that includes the onset, timing, and duration of symptoms is key in the evaluation of low back pain in children, as is obtaining a history of the patient’s activities; sports that repetitively load the lumbar spine in an extended position increase the risk of injury.10
Specific risk factors for low back pain in children and adolescents are poorly understood.4,9,31 Pain can be associated with trauma, or it can have a more progressive or insidious onset. Generally, pain that is present for up to 6 weeks and is intermittent or improving has a self-limited course. Pain that persists beyond 3 to 6 weeks or is worsening is more likely to have an anatomical cause that needs further evaluation.2,3,10,21
Identifying exacerbating and alleviating factors can provide useful information. Pain that is worse with lumbar flexion is more likely to come from muscular strain or disc pathology. Pain with extension is more likely due to a structural cause such as spondylolysis/spondylolisthesis, scoliosis, or Scheuermann disease.2,4,10,17,18,21 See TABLE 2 for red flag symptoms that indicate the need for imaging and further work-up.
The physical exam: Visualize, assess range of motion, and reproduce pain
The physical examination of any patient with low back pain should include direct visualization and inspection of the back, spine, and pelvis; palpation of the spine and paraspinal regions; assessment of lumbar range of motion and of the lumbar nerve roots, including tests of sensation, strength, and deep tendon reflexes; and an evaluation of the patient’s posture, which can provide clues to underlying causes of pain.
Continue to: Increased thoracic kyphosis...
Increased thoracic kyphosis that is not reversible is concerning for Scheuermann disease.9,17,18 A significant elevation in one shoulder or side of the pelvis can be indicative of scoliosis. Increased lumbar lordosis may predispose a patient to spondylolysis.
In patients with spondylolysis, lumbar extension will usually reproduce pain, which is often unilateral. Hyperextension in a single-leg stance, commonly known as the Stork test, is positive for unilateral spondylolysis when it reproduces pain on the ipsilateral side. The sensitivity of the Stork test for unilateral spondylolysis is approximately 50%.32 (For more information on the Stork test, see www.physio-pedia.com/Stork_test.)
Pain reproduced with lumbar flexion is less concerning for bony pathology and is most often related to soft-tissue strain. Lumbar flexion with concomitant radicular pain is associated with disc pathology.8 Pain with a straight-leg raise is also associated with disk pathology, especially if raising the contralateral leg increases pain.8
Using a scoliometer. Evaluate the flexed spine for the presence of asymmetry, which can indicate scoliosis.33 If asymmetry is present, use a scoliometer to determine the degree of asymmetry. Zero to 5° is considered clinically insignificant; monitor and reevaluate these patients at subsequent visits.34,35 Ten degrees or more of asymmetry with a scoliometer should prompt you to order radiographs.35,36 A smartphone-based scoliometer for iPhones was evaluated in 1 study and was shown to have reasonable reliability and validity for clinical use.37
Deformity of the lower extremities. Because low back pain may be caused by biomechanical or structural deformity of the lower extremities, examine the flexibility of the hip flexors, gluteal musculature, hamstrings, and the iliotibial band.38 In addition, evaluate for leg-length discrepancy and lower-extremity malalignment, such as femoral anteversion, tibial torsion, or pes planus.
Continue to: Imaging
Imaging: Know when it’s needed
Although imaging of the lumbar spine is often unnecessary in the presence of acute low back pain in children, always consider imaging in the setting of bony tenderness, pain that wakes a patient from sleep, and in the setting of other red flag symptoms (see TABLE 2). Low back pain in children that is reproducible with lumbar extension is concerning for spondylolysis or spondylolisthesis. If the pain with extension persists beyond 3 to 6 weeks, order imaging starting with radiographs.2,39
Traditionally, 4 views of the spine—anteroposterior (AP), lateral, and oblique (one right and one left)—were obtained, but recent evidence indicates that 2 views (AP and lateral) have similar sensitivity and specificity to 4 views with significantly reduced radiation exposure.2,39 Because the sensitivity of plain films is relatively low, consider more advanced imaging if spondylolysis or spondylolisthesis is strongly suspected. Recent studies indicate that magnetic resonance imaging (MRI) may be as effective as computed tomography (CT) or bone scan and has the advantage of lower radiation (FIGURE 1).2,22
Similarly, order radiographs if there is > 10° of asymmetry noted on physical exam using a scoliometer.15,23 Calculate the Cobb angle to determine the severity of scoliosis. Refer patients with angles ≥ 20° to a pediatric orthopedist for monitoring of progression and consideration of bracing (FIGURE 2).23,34 For patients with curvatures between 10° and 19°, repeat imaging every 6 to 12 months. Because scoliosis is a risk factor for spondylolysis, evaluate radiographs in the setting of painful scoliosis for the presence of a spondylolysis.34,35
If excessive kyphosis is noted on exam, order radiographs to evaluate for Scheuermann disease. Classic imaging findings include Schmorl nodes, vertebral endplate changes, and anterior wedging (FIGURE 3).17,18
In the absence of the above concerns, defer imaging of the lumbar spine until after adequate rest and rehabilitation have been attempted.
Continue to: Treatment typically involves restor physical therapy
Treatment typically involves restor physical therapy
Most cases of low back pain in children and adolescents are benign and self-limited. Many children with low back pain can be treated with relative rest from the offending activity. For children with more persistent pain, physical therapy (PT) is often indicated. Similar to that for adults, there is little evidence for specific PT programs to help children with low back pain. Rehabilitation should be individualized based on the condition being treated.
Medications. There have been no high-quality studies on the benefit of medications to treat low back pain in children. Studies have shown nonsteroidal anti-inflammatory drugs (NSAIDs) have value in adults, and they are likely safe for use in children,40 but the risk of opiate abuse is significantly increased in adolescents who have been prescribed opiate pain medication prior to 12th grade.41
Lumbar disc herniation. Although still relatively rare, lumbar disc herniation is more common in older children and adolescents than in younger children and is treated similarly to that in adults.8 Range-of-motion exercise to restore lumbar motion is often first-line treatment. Research has shown that exercises that strengthen the abdominal or “core” musculature help prevent the return of low back pain.24,25
In the case of spondylolysis or spondylolisthesis, rest from activity is generally required for a minimum of 4 to 6 weeks. Rehabilitation in the form of range of motion, especially into the lumbar extension, and spinal stabilization exercises are effective for both reducing pain and restoring range-of-motion and strength.42 Have patients avoid heavy backpacks, which can reproduce pain. Children often benefit from leaving a second set of schoolbooks at home. For most patients with spondylolysis, conservative treatment with rehabilitation is equal to or better than surgical intervention in returning the patient to his/her pre-injury activity level.26,43,44 When returning athletes to their sport, aggressive PT, defined as rest for < 10 weeks prior to initiating PT, is superior to delaying PT beyond 10 weeks of rest.27
Idiopathic scoliosis. Much of the literature on the treatment of scoliosis is focused on limiting progression of the scoliotic curvature. Researchers thought that more severe curves were associated with more severe pain, but a recent systematic review showed that back pain can occur in patients with even small curvatures.28 Treatment for patients with smaller degrees of curvature is similar to that for mechanical low back pain. PT may have a role in the treatment of scoliosis, but there is little evidence in the literature of its effectiveness.
Continue to: A Cochrane review showed...
A Cochrane review showed that PT and exercise-based treatments had no effect on back pain or disability in patients with scoliosis.29 And outpatient PT alone, in the absence of bracing, does not arrest progression of the scoliotic curvature.35 One trial did demonstrate that an intensive inpatient treatment program of 4 to 6 weeks for patients with curvature of at least 40° reduced progression of curvature compared to an untreated control group at 1 year.34 The outcomes of functional mobility and pain were not measured. Follow-up data on curve progression beyond 1 year are not available. Unfortunately, intensive inpatient treatment is not readily available or cost-effective for most patients with scoliosis.
Scheuermann disease. The mainstay of treatment for mild Scheuermann disease is advising the patient to avoid repetitive loading of the spine. Patients should avoid sports such as competitive weight-lifting, gymnastics, and football. Lower impact athletics are encouraged. Refer patients with pain to PT to address posture and core stabilization. Patients with severe kyphosis may require surgery.17,18
Bracing: Rarely helpful for low back pain
The use of lumbar braces or corsets is rarely helpful for low back pain in children. Bracing in the setting of spondylolysis is controversial.One study indicated that bracing in combination with activity restriction and lumbar extension exercise is superior to activity restriction and lumbar flexion exercises alone.43 But a meta-analysis did not demonstrate a significant difference in recovery when bracing was added.44 Bracing may help to reduce pain initially in patients with spondylolysis who have pain at rest. Bracing is not recommended for patients with pain that abates with activity modification.
Scoliosis and Scheuermann kyphosis. Treatment of adolescent idiopathic scoliosis usually consists of observation and periodic reevaluation. Bracing is a mainstay of the nonsurgical management of scoliosis and is appropriate for curves of 20° to 40°; studies have reported successful control of curve progression in > 70% of patients.36 According to 1 study, the number of cases of scoliosis needed to treat with bracing to prevent 1 surgery is 3.30 Surgery is often indicated for patients with curvatures > 40°, although this is also debated.33
Bracing is used rarely for Scheuermann kyphosis but may be helpful in more severe or painful cases.17
CORRESPONDENCE
Shawn F. Phillips, MD, MSPT, 500 University Drive H154, Hershey, PA, 17033; sphillips6@pennstatehealth.psu.edu.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
1. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children: a review. JAMA Pediatr. 2017;171:280-287.
2. Tofte JN CarlLee TL, Holte AJ, et al. Imaging pediatric spondylolysis: a systematic review. Spine. 2017;42:777-782.
3. Sakai T, Sairyo K, Suzue N, et al. Incidence and etiology of lumbar spondylolysis: review of the literature. J Orthop Sci. 2010;15:281-288.
4. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatrics. 2013;13:14.
5. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76:1669-1676.
6. Taxter AJ, Chauvin NA, Weiss PF. Diagnosis and treatment of low back pain in the pediatric population. Phys Sportsmed. 2014;42:94-104.
7. Haus BM, Micheli LJ. Back pain in the pediatric and adolescent athlete. Clin Sports Med. 2012;31:423-440.
8. Lavelle WF, Bianco A, Mason R, et al. Pediatric disk herniation. J Am Acad Orthop Surg. 2011;19:649-656.
9. Taimela S, Kujala UM, Salminen JJ, et al. The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine. 1997;22:1132-1136.
10. Schroeder GD, LaBella CR, Mendoza M, et al. The role of intense athletic activity on structural lumbar abnormalities in adolescent patients with symptomatic low back pain. Eur Spine J. 2016;25:2842-2848.
11. Waicus KM, Smith BW. Back injuries in the pediatric athlete. Curr Sports Med Rep. 2002;1:52-58.
12. Daniels JM, Pontius G, El-Amin S, et al. Evaluation of low back pain in athletes. Sports Health. 2011;3:336-345.
13. Sato T, Hirano T, Ito T, et al. Back pain in adolescents with idiopathic scoliosis: epidemiological study for 43,630 pupils in Niigata City, Japan. Eur Spine J. 2011;20:274-279.
14. Smorgick Y, Mirovsky Y, Baker KC, et al. Predictors of back pain in adolescent idiopathic scoliosis surgical candidates. J Pediatr Orthop. 2013;33:289-292.
15. US Preventive Services Task Force. Screening for Adolescent Idiopathic Scoliosis. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:165-172.
16. Hresko MT, Talwalkar VR, Schwend RM. Position statement–Screening for the early detection of idiopathic scoliosis in adolescents. SRS/POSNA/AAOS/AAP Position Statement. 2015. www.srs.org/about-srs/news-and-announcements/position-statement---screening-for-the-early-detection-for-idiopathic-scoliosis-in-adolescents. Accessed September 30, 2020.
17. Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214.
18. Ali RM, Green DW, Patel TC. Scheuermann’s kyphosis. Curr Opin Pediatr. 1999;11:70-75.
19. de Moraes Barros Fucs PM, Meves R, Yamada HH, et al. Spinal infections in children: a review. Int Orthop. 2012;36:387-395.
20. Joaquim AF, Ghizoni E, Valadares MG, et al. Spinal tumors in children. Revista da Associação Médica Brasileira. 2017;63:459-465.
21. Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am. 2018;65:675-690.
22. Rush JK, Astur N, Scott S, et al. Use of magnetic resonance imaging in the evaluation of spondylolysis. J Pediatr Orthop. 2015;35:271-275.
23. Janicki JA, Alman B. Scoliosis: review of diagnosis and treatment. Pediatr Child Health. 2007;12:771-776.
24. O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine.1997;22:2959-2967.
25. Inani SB, Selkar SP. Effect of core stabilization exercises versus conventional exercises on pain and functional status in patients with non-specific low back pain: a randomized clinical trial. J Back Musculoskelet Rehabil. 2013;26:37-43.
26. Garet M, Reiman MP, Mathers J, et al. Nonoperative treatment in lumbar spondylolysis and spondylolisthesis: a systematic review. Sports Health. 2013;5:225-232.
27. Selhorst M, Fischer A, Graft K, et al. Timing of physical therapy referral in adolescent athletes with acute spondylolysis: a retrospective chart review. Clin J Sport Med. 2017;27:296-301.
28. Théroux J, Stomski N, Hodgetts CJ, et al. Prevalence of low back pain in adolescents with idiopathic scoliosis: a systematic review. Chiropr Man Ther. 2017;25:10.
29. Romano M, Minozzi S, Zaina F, et al. Exercises for adolescent idiopathic scoliosis: a Cochrane systematic review. Spine (Phila Pa 1976). 2013;38:E883-E893.
30. Sanders JO, Newton PO, Browne RH, et al. Bracing for idiopathic scoliosis: how many patients require treatment to prevent one surgery? J Bone Joint Surg Am. 2014;96:649-653.
31. Hill JJ, Keating JL. Risk factors for the first episode of low back pain in children are infrequently validated across samples and conditions: a systematic review. J Physiother. 2010;56:237-244.
32. Grødahl LHJ, Fawcett L, Nazareth M, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: a systematic review. Man Ther. 2016;24:7-17.
33. Asher MA, Burton DC. Adolescent idiopathic scoliosis: natural history and long term treatment effects. Scoliosis. 2006;1:2.
34. Weiss HR, Weiss G, Petermann F. Incidence of curvature progression in idiopathic scoliosis patients treated with scoliosis inpatient rehabilitation (SIR): an age- and sex-matched controlled study. Pediatr Rehabil. 2003;6:23-30.
35. Gomez JA, Hresko MT, Glotzbecker MP. Nonsurgical management of adolescent idiopathic scoliosis. J Am Acad Orthop Surg. 2016;24:555-564.
36. Weinstein SL, Dolan LA, Wright JG, et al. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512-1521.
37. Balg F, Juteau M, Theoret C, et al. Validity and reliability of the iPhone to measure rib hump in scoliosis. J Pediatr Orthop. 2014;34:774-779.
38. Auerbach JD, Ahn J, Zgonis MH, et al. Streamlining the evaluation of low back pain in children. Clin Orthop Relatl Res. 2008;466:1971-1977.
39. Beck NA, Miller R, Baldwin K, et al. Do oblique views add value in the diagnosis of spondylolysis in adolescents? J Bone Joint Surg Am. 2013;95:e65.
40. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain: an updated Cochrane review. Spine (Phila Pa 1976). 2008;33:1766-1774.
41. Miech R, Johnston L, O’Malley PM, et al. Prescription opioids in adolescence and future opioid misuse. Pediatrics. 2015;136:e1169-e1177.
42. Hu S, Tribus C, Diab M, et al. Spondylolysis and spondylolisthesis. J Bone Joint Surg. 2008;90:655-671.
43. Panteliadis P, Nagra NS, Edwards KL, et al. Athletic population with spondylolysis: review of outcomes following surgical repair or conservative management. Global Spine J. 2016;6:615-625.
44. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29:146-156.
PRACTICE RECOMMENDATIONS
› Be aware that low back pain is rare in children < 7 years but increases in incidence as children near adolescence. A
› Consider imaging in the setting of bony tenderness, pain that awakens the patient from sleep, or in the presence of other “red flag” symptoms. A
› Consider spondylolysis and spondylolisthesis in adolescent athletes with low back pain lasting longer than 3 to 6 weeks. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Burosumab is a ‘game changer,’ effective in all subgroups of XLH
A recently approved agent, burosumab (Crysvita), was better than placebo across a range of efficacy outcomes for 14 predefined subgroups of adults with X-linked hypophosphatemia (XLH), new research shows.
The authors analyzed data from the initial 24-week randomized blinded phase of the pivotal phase 3 trial that led to regulatory approval of this drug in the United States in 2018 for XLH, a rare form of rickets characterized by low serum phosphorus levels, skeletal defects, pain, and stiffness.
As in the main analysis, in the subgroups, among patients who received burosumab, serum phosphorus levels were improved, and outcomes were better on the following measures: Western Ontario and McMaster Universities Arthritis Index (WOMAC) stiffness scale, the WOMAC physical function measure, and the Brief Pain Inventory (BPI), which were the main efficacy outcomes. Improvements were seen for many other outcomes as well.
Maria-Luisa Brandi, MD, Careggi University Hospital, Florence, Italy, presented the new subanalysis during the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting.
The subgroup results were consistent with the overall trial findings, “showing a favorable direction of effect of burosumab relative to placebo” except for results in patients recruited in Asia and non-White patients; those results were considered inconclusive because there were too few participants in those categories, she told Medscape Medical News,.
Lorenz Hofbauer, MD, scientific chair of the ASBMR meeting, said that the take-away message is that the drug “works to reduce pain and disability” in adults with XLH with more severe/less severe symptoms, and “it provides new hope for many patients suffering from this disease,” he told Medscape Medical News.
Burosemab also appears superior to what has previously been considered standard therapy for XLH, phosphate/calcitriol, the experts say.
‘Rare is relative,’ burosumab is a ‘transformative therapy’
“The disease prevalence is 1 to 9 in a million,” Brandi said. “Undiagnosed adults are treated by the doctor that makes the diagnosis, usually a nephrologist or a rheumatologist or a bone doctor; this depends on the prevalent complications in a given patient. The endocrinologist who treats this patient is the one expert in bone disorders.”
Hofbauer noted, however, that “[r]are is relative. If you run a bone clinic, you will see four to five patients with XLH; if you are a regional center, 20 to 30 patients. People with rare disease travel more than 1000 miles to see experts.”
The US Food and Drug Administration approved burosumab for use in children and adults with XLH 2 years ago. The European Medicines Agency (EMA) approved it for use in children.
The drug is expected to be approved by the EMA for adults with XLH some time this year, said Hofbauer, who is from Dresden Technical University, Dresden, Germany.
Burosumab is a “game changer” with respect to previous treatments, he stressed.
This study is one of the top five clinical abstracts of the ASBMR meeting, which are selected on the basis of “scientific content/novelty, making a difference in clinical practice,” Hofbauer explained. He noted that “new drugs that work are always in the top ranks.”
Craig Munns, PhD, who was senior author of a recent review about burosumab, agrees.
“Burosumab is transformative, as it is a paradigm shift in the way we manage XLH,” he told Medscape Medical News.
“Standard therapy for children is with oral phosphate and calcitriol, and many adults do not receive any therapy,” said Munns, from the University of Sydney, Sydney, Australia.
“Phosphate and calcitriol need to be taken multiple times per day, is an incomplete therapy, and has many complications. Burosumab offers a 2-weekly (children) or 4-weekly (adult) dosing regime with superior outcomes compared to no treatment or phosphate/calcitriol,” he emphasized.
Efficacy in 14 predefined subgroups
“Burosumab is an anti-FGF-23 [anti–fibroblast growth factor-23] antibody for a rare genetic disease, XLH, in which the gene for PHEX is defective,” Hofbauer explained.
“PHEX is an enzyme that clears FGF-23; if it does not work, then FGF-23 accumulates in the body and causes phosphate wasting with wide consequences for bone, muscle, and joints. Burosumab is a smart approach, since it blocks these excessive FGF-23 effects.”
Children with XLH have rickets, deformities in the lower skeleton, and short stature, Brandi noted, whereas adults have fractures, pseudofractures, enthesopathy (calcification of joint capsule, tendon insertions, and ligaments), pain, stiffness, and impaired physical function.
However, “treatment with oral phosphate and vitamin D is associated with nephrocalcinosis and hyperparathyroidism,” she said.
In the phase 3 trial, 134 adults (aged 18 to 65 years) with XLH were randomly assigned in a double-blind manner to receive either burosumab or placebo for 24 weeks, followed by 24 weeks of open-label burosumab. The patients’ serum phosphorus levels were <2.5 mg/dL, and they were experiencing measurable bone/joint pain.
Baseline characteristics were similar for the patients who received placebo (66) and those who received burosumab (68). The mean age of the patients was 40 years; 65% were women; and 81% were White.
The current exploratory analysis examined efficacy outcomes in patients grouped according to the following factors and characteristics: sex; age (≤41 years or >41 years); race (non-White, White); region (Asia, North America/Europe); baseline WOMAC pain score; WOMAC total pain; WOMAC stiffness; WOMAC physical function; BPI worst pain; BPI average pain; opioid use; pain medication use; active fractures and pseudofractures; and 6-minute walking test distance.
The efficacy outcomes were as follows: serum phosphorus level (primary outcome), BPI worst pain, WOMAC stiffness, and WOMAC physical function (key secondary outcomes); and WOMAC pain, WOMAC total score, BPI average pain, BPI pain interference, BPI worst fatigue, BPI global score, patient global impression (PGI), and 6-minute walking distance.
In the overall cohort, at 24 weeks, in comparison with patients who received placebo, patients who received burosumab had favorable responses with respect to serum phosphorus level, WOMAC stiffness (P =. 012),WOMAC physical function (P = .048), and BPI worst pain (P = .092, not significant), as well as significant improvements in WOMAC total score and the 6-minute walk test. There were nonsignificant improvements in WOMAC pain and BPI average pain.
In the subgroup analysis, burosumab was superior to placebo for the primary outcome (serum phosphorus) in all subgroups. It was also superior to placebo for the key secondary outcomes (worst pain, stiffness, and physical function) across all subgroups except for patients from Asia (18 patients) and non-White patients (26).
The study was funded by Kyowa Kirin in partnership with Ultragenyx. Brandi receives consultancy and speaker fees as well as research grants from Kyowa Kirin and other pharmaceutical companies. Munns has received research funding from Kyowa Kirin.
This article first appeared on Medscape.com.
A recently approved agent, burosumab (Crysvita), was better than placebo across a range of efficacy outcomes for 14 predefined subgroups of adults with X-linked hypophosphatemia (XLH), new research shows.
The authors analyzed data from the initial 24-week randomized blinded phase of the pivotal phase 3 trial that led to regulatory approval of this drug in the United States in 2018 for XLH, a rare form of rickets characterized by low serum phosphorus levels, skeletal defects, pain, and stiffness.
As in the main analysis, in the subgroups, among patients who received burosumab, serum phosphorus levels were improved, and outcomes were better on the following measures: Western Ontario and McMaster Universities Arthritis Index (WOMAC) stiffness scale, the WOMAC physical function measure, and the Brief Pain Inventory (BPI), which were the main efficacy outcomes. Improvements were seen for many other outcomes as well.
Maria-Luisa Brandi, MD, Careggi University Hospital, Florence, Italy, presented the new subanalysis during the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting.
The subgroup results were consistent with the overall trial findings, “showing a favorable direction of effect of burosumab relative to placebo” except for results in patients recruited in Asia and non-White patients; those results were considered inconclusive because there were too few participants in those categories, she told Medscape Medical News,.
Lorenz Hofbauer, MD, scientific chair of the ASBMR meeting, said that the take-away message is that the drug “works to reduce pain and disability” in adults with XLH with more severe/less severe symptoms, and “it provides new hope for many patients suffering from this disease,” he told Medscape Medical News.
Burosemab also appears superior to what has previously been considered standard therapy for XLH, phosphate/calcitriol, the experts say.
‘Rare is relative,’ burosumab is a ‘transformative therapy’
“The disease prevalence is 1 to 9 in a million,” Brandi said. “Undiagnosed adults are treated by the doctor that makes the diagnosis, usually a nephrologist or a rheumatologist or a bone doctor; this depends on the prevalent complications in a given patient. The endocrinologist who treats this patient is the one expert in bone disorders.”
Hofbauer noted, however, that “[r]are is relative. If you run a bone clinic, you will see four to five patients with XLH; if you are a regional center, 20 to 30 patients. People with rare disease travel more than 1000 miles to see experts.”
The US Food and Drug Administration approved burosumab for use in children and adults with XLH 2 years ago. The European Medicines Agency (EMA) approved it for use in children.
The drug is expected to be approved by the EMA for adults with XLH some time this year, said Hofbauer, who is from Dresden Technical University, Dresden, Germany.
Burosumab is a “game changer” with respect to previous treatments, he stressed.
This study is one of the top five clinical abstracts of the ASBMR meeting, which are selected on the basis of “scientific content/novelty, making a difference in clinical practice,” Hofbauer explained. He noted that “new drugs that work are always in the top ranks.”
Craig Munns, PhD, who was senior author of a recent review about burosumab, agrees.
“Burosumab is transformative, as it is a paradigm shift in the way we manage XLH,” he told Medscape Medical News.
“Standard therapy for children is with oral phosphate and calcitriol, and many adults do not receive any therapy,” said Munns, from the University of Sydney, Sydney, Australia.
“Phosphate and calcitriol need to be taken multiple times per day, is an incomplete therapy, and has many complications. Burosumab offers a 2-weekly (children) or 4-weekly (adult) dosing regime with superior outcomes compared to no treatment or phosphate/calcitriol,” he emphasized.
Efficacy in 14 predefined subgroups
“Burosumab is an anti-FGF-23 [anti–fibroblast growth factor-23] antibody for a rare genetic disease, XLH, in which the gene for PHEX is defective,” Hofbauer explained.
“PHEX is an enzyme that clears FGF-23; if it does not work, then FGF-23 accumulates in the body and causes phosphate wasting with wide consequences for bone, muscle, and joints. Burosumab is a smart approach, since it blocks these excessive FGF-23 effects.”
Children with XLH have rickets, deformities in the lower skeleton, and short stature, Brandi noted, whereas adults have fractures, pseudofractures, enthesopathy (calcification of joint capsule, tendon insertions, and ligaments), pain, stiffness, and impaired physical function.
However, “treatment with oral phosphate and vitamin D is associated with nephrocalcinosis and hyperparathyroidism,” she said.
In the phase 3 trial, 134 adults (aged 18 to 65 years) with XLH were randomly assigned in a double-blind manner to receive either burosumab or placebo for 24 weeks, followed by 24 weeks of open-label burosumab. The patients’ serum phosphorus levels were <2.5 mg/dL, and they were experiencing measurable bone/joint pain.
Baseline characteristics were similar for the patients who received placebo (66) and those who received burosumab (68). The mean age of the patients was 40 years; 65% were women; and 81% were White.
The current exploratory analysis examined efficacy outcomes in patients grouped according to the following factors and characteristics: sex; age (≤41 years or >41 years); race (non-White, White); region (Asia, North America/Europe); baseline WOMAC pain score; WOMAC total pain; WOMAC stiffness; WOMAC physical function; BPI worst pain; BPI average pain; opioid use; pain medication use; active fractures and pseudofractures; and 6-minute walking test distance.
The efficacy outcomes were as follows: serum phosphorus level (primary outcome), BPI worst pain, WOMAC stiffness, and WOMAC physical function (key secondary outcomes); and WOMAC pain, WOMAC total score, BPI average pain, BPI pain interference, BPI worst fatigue, BPI global score, patient global impression (PGI), and 6-minute walking distance.
In the overall cohort, at 24 weeks, in comparison with patients who received placebo, patients who received burosumab had favorable responses with respect to serum phosphorus level, WOMAC stiffness (P =. 012),WOMAC physical function (P = .048), and BPI worst pain (P = .092, not significant), as well as significant improvements in WOMAC total score and the 6-minute walk test. There were nonsignificant improvements in WOMAC pain and BPI average pain.
In the subgroup analysis, burosumab was superior to placebo for the primary outcome (serum phosphorus) in all subgroups. It was also superior to placebo for the key secondary outcomes (worst pain, stiffness, and physical function) across all subgroups except for patients from Asia (18 patients) and non-White patients (26).
The study was funded by Kyowa Kirin in partnership with Ultragenyx. Brandi receives consultancy and speaker fees as well as research grants from Kyowa Kirin and other pharmaceutical companies. Munns has received research funding from Kyowa Kirin.
This article first appeared on Medscape.com.
A recently approved agent, burosumab (Crysvita), was better than placebo across a range of efficacy outcomes for 14 predefined subgroups of adults with X-linked hypophosphatemia (XLH), new research shows.
The authors analyzed data from the initial 24-week randomized blinded phase of the pivotal phase 3 trial that led to regulatory approval of this drug in the United States in 2018 for XLH, a rare form of rickets characterized by low serum phosphorus levels, skeletal defects, pain, and stiffness.
As in the main analysis, in the subgroups, among patients who received burosumab, serum phosphorus levels were improved, and outcomes were better on the following measures: Western Ontario and McMaster Universities Arthritis Index (WOMAC) stiffness scale, the WOMAC physical function measure, and the Brief Pain Inventory (BPI), which were the main efficacy outcomes. Improvements were seen for many other outcomes as well.
Maria-Luisa Brandi, MD, Careggi University Hospital, Florence, Italy, presented the new subanalysis during the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting.
The subgroup results were consistent with the overall trial findings, “showing a favorable direction of effect of burosumab relative to placebo” except for results in patients recruited in Asia and non-White patients; those results were considered inconclusive because there were too few participants in those categories, she told Medscape Medical News,.
Lorenz Hofbauer, MD, scientific chair of the ASBMR meeting, said that the take-away message is that the drug “works to reduce pain and disability” in adults with XLH with more severe/less severe symptoms, and “it provides new hope for many patients suffering from this disease,” he told Medscape Medical News.
Burosemab also appears superior to what has previously been considered standard therapy for XLH, phosphate/calcitriol, the experts say.
‘Rare is relative,’ burosumab is a ‘transformative therapy’
“The disease prevalence is 1 to 9 in a million,” Brandi said. “Undiagnosed adults are treated by the doctor that makes the diagnosis, usually a nephrologist or a rheumatologist or a bone doctor; this depends on the prevalent complications in a given patient. The endocrinologist who treats this patient is the one expert in bone disorders.”
Hofbauer noted, however, that “[r]are is relative. If you run a bone clinic, you will see four to five patients with XLH; if you are a regional center, 20 to 30 patients. People with rare disease travel more than 1000 miles to see experts.”
The US Food and Drug Administration approved burosumab for use in children and adults with XLH 2 years ago. The European Medicines Agency (EMA) approved it for use in children.
The drug is expected to be approved by the EMA for adults with XLH some time this year, said Hofbauer, who is from Dresden Technical University, Dresden, Germany.
Burosumab is a “game changer” with respect to previous treatments, he stressed.
This study is one of the top five clinical abstracts of the ASBMR meeting, which are selected on the basis of “scientific content/novelty, making a difference in clinical practice,” Hofbauer explained. He noted that “new drugs that work are always in the top ranks.”
Craig Munns, PhD, who was senior author of a recent review about burosumab, agrees.
“Burosumab is transformative, as it is a paradigm shift in the way we manage XLH,” he told Medscape Medical News.
“Standard therapy for children is with oral phosphate and calcitriol, and many adults do not receive any therapy,” said Munns, from the University of Sydney, Sydney, Australia.
“Phosphate and calcitriol need to be taken multiple times per day, is an incomplete therapy, and has many complications. Burosumab offers a 2-weekly (children) or 4-weekly (adult) dosing regime with superior outcomes compared to no treatment or phosphate/calcitriol,” he emphasized.
Efficacy in 14 predefined subgroups
“Burosumab is an anti-FGF-23 [anti–fibroblast growth factor-23] antibody for a rare genetic disease, XLH, in which the gene for PHEX is defective,” Hofbauer explained.
“PHEX is an enzyme that clears FGF-23; if it does not work, then FGF-23 accumulates in the body and causes phosphate wasting with wide consequences for bone, muscle, and joints. Burosumab is a smart approach, since it blocks these excessive FGF-23 effects.”
Children with XLH have rickets, deformities in the lower skeleton, and short stature, Brandi noted, whereas adults have fractures, pseudofractures, enthesopathy (calcification of joint capsule, tendon insertions, and ligaments), pain, stiffness, and impaired physical function.
However, “treatment with oral phosphate and vitamin D is associated with nephrocalcinosis and hyperparathyroidism,” she said.
In the phase 3 trial, 134 adults (aged 18 to 65 years) with XLH were randomly assigned in a double-blind manner to receive either burosumab or placebo for 24 weeks, followed by 24 weeks of open-label burosumab. The patients’ serum phosphorus levels were <2.5 mg/dL, and they were experiencing measurable bone/joint pain.
Baseline characteristics were similar for the patients who received placebo (66) and those who received burosumab (68). The mean age of the patients was 40 years; 65% were women; and 81% were White.
The current exploratory analysis examined efficacy outcomes in patients grouped according to the following factors and characteristics: sex; age (≤41 years or >41 years); race (non-White, White); region (Asia, North America/Europe); baseline WOMAC pain score; WOMAC total pain; WOMAC stiffness; WOMAC physical function; BPI worst pain; BPI average pain; opioid use; pain medication use; active fractures and pseudofractures; and 6-minute walking test distance.
The efficacy outcomes were as follows: serum phosphorus level (primary outcome), BPI worst pain, WOMAC stiffness, and WOMAC physical function (key secondary outcomes); and WOMAC pain, WOMAC total score, BPI average pain, BPI pain interference, BPI worst fatigue, BPI global score, patient global impression (PGI), and 6-minute walking distance.
In the overall cohort, at 24 weeks, in comparison with patients who received placebo, patients who received burosumab had favorable responses with respect to serum phosphorus level, WOMAC stiffness (P =. 012),WOMAC physical function (P = .048), and BPI worst pain (P = .092, not significant), as well as significant improvements in WOMAC total score and the 6-minute walk test. There were nonsignificant improvements in WOMAC pain and BPI average pain.
In the subgroup analysis, burosumab was superior to placebo for the primary outcome (serum phosphorus) in all subgroups. It was also superior to placebo for the key secondary outcomes (worst pain, stiffness, and physical function) across all subgroups except for patients from Asia (18 patients) and non-White patients (26).
The study was funded by Kyowa Kirin in partnership with Ultragenyx. Brandi receives consultancy and speaker fees as well as research grants from Kyowa Kirin and other pharmaceutical companies. Munns has received research funding from Kyowa Kirin.
This article first appeared on Medscape.com.