User login
An Interdisciplinary Approach to Educating Medical Students About Dementia Assessment and Treatment Planning
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
The global burden of dementia is increasing at an alarming pace and is estimated to soon affect 81 million individuals worldwide.1 The World Health Organization and the Institute of Medicine have recommended greater dementia awareness and education.2,3 Despite this emphasis on dementia education, many general practitioners consider dementia care beyond their clinical domain and feel that specialists, such as geriatricians, geriatric psychiatrists, or neurologists should address dementia assessment and treatment. 4 Unfortunately, the geriatric health care workforce has been shrinking. The American Geriatrics Society estimates the need for 30,000 geriatricians by 2030, although there are only 7,300 board-certified geriatricians currently in the US.5 There is an urgent need for educating all medical trainees in dementia care regardless of their specialization interest. As the largest underwriter of graduate medical education in the US, the US Department of Veterans Affairs (VA) is well placed for rolling out focused dementia education. Training needs to be practical, brief, and responsive to knowledge gaps to reach the most trainees.
Despite growing emphasis on geriatric training, many medical students have limited experience with patients with dementia or their caregivers, lack exposure to interdisciplinary teams, have a poor attitude toward geriatric patients, and display specific knowledge gaps in dementia assessment and management. 6-9 Other knowledge gaps noted in medical students included assessing behavioral problems, function, safety, and caregiver burden. Medical students also had limited exposure to interdisciplinary team dementia assessment and management.
Our goal was to develop a multicomponent, experiential, brief curriculum using team-based learning to expose senior medical students to interdisciplinary assessment of dementia. The curriculum was developed with input from the interdisciplinary team to address dementia knowledge gaps while providing an opportunity to interact with caregivers. The curriculum targeted all medical students regardless of their interest in geriatrics. Particular emphasis was placed on systems-based learning and the importance of teamwork in managing complex conditions such as dementia. Students were taught that incorporating interdisciplinary input would be more effective during dementia care planning rather than developing specialized knowledge.
Methods
Our team developed a curriculum for fourthyear medical students who rotated through the VA Memory Disorders Clinic as a part of their geriatric medicine clerkship at the University of Arkansas for Medical Sciences in Little Rock. The Memory Disorders Clinic is a consultation practice at the Central Arkansas Veterans Healthcare System (CAVHS) where patients with memory problems are evaluated by a team consisting of a geriatric psychiatrist, a geriatrician, a social worker, and a neuropsychologist. Each specialist addresses specific areas of dementia assessment and management. The curriculum included didactics, clinical experience, and team-based learning.
Didactics
An hour-long didactic session lead by the team geriatrician provided a general overview of interdisciplinary assessment of dementia to groups of 2 to 3 students at a time. The geriatrician presented an overview of dementia types, comorbidities, medications that affect memory, details of the physical examination, and laboratory, cognitive, and behavioral assessments along with treatment plan development. Students also learned about the roles of the social worker, geriatrician, neuropsychologist, and geriatric psychiatrist in the clinic. Pictographs and pie charts highlighted the role of disciplines in assessing and managing aspects of dementia.
The social work evaluation included advance care planning, functional assessment, safety assessment (driving, guns, wandering behaviors, etc), home safety evaluation, support system, and financial evaluation. Each medical student received a binder with local resources to become familiar with the depth and breadth of agencies involved in dementia care. Each medical student learned how to administer the Zarit Burden Scale to assess caregiver burden.10 The details of the geriatrician assessment included reviewing medical comorbidities and medications contributing to dementia, a physical examination, including a focused neurologic examination, laboratory assessment, and judicious use of neuroimaging.
The neuropsychology assessment education included a battery of tests and assessments. The global screening instruments included the Modified Mini-Mental State examination (3MS), Montreal Cognitive Assessment (MoCA), and Saint Louis University Mental Status examination (SLUMS).11-13 Executive function is evaluated using the Trails Making Test A and Trails Making Test B, Controlled Oral Word Association Test, Semantic Fluency Test, and Repeatable Battery for the Assessment of Neuropsychological Status test. Cognitive tests were compared and age- , education-, and race-adjusted norms for rating scales were listed if available. Each student was expected to show proficiency in ≥ 2 cognitive screening instruments (3MS, MoCA, or SLUMS). The geriatric psychiatry assessment included clinical history, onset, and course of memory problems from patient and caregiver perspectives, the Neuropsychiatric Inventory for assessing behavioral problems, employing the clinical dementia rating scale, integrating the team data, summarizing assessment, and formulating a treatment plan.14
Clinical
Students had a single clinical exposure. Students followed 1 patient and his or her caregiver through the team assessment and observed each provider’s assessment to learn interview techniques to adapt to the patient’s sensory or cognitive impairment and become familiar with different tools and devices used in the dementia clinic, such as hearing amplifiers. Each specialist provided hands-on experience. This encounter helped the students connect with caregivers and appreciate their role in patient care.
Systems learning was an important component integrated throughout the clinical experience. Examples include using video teleconferences to communicate findings among team members and electronic health records to seamlessly obtain and integrate data. Students learned how to create worksheets to graph laboratory data such as B12, thyroid-stimulating hormone, and rapid plasma regain levels. Student gained experience in using applications to retrieve neuroimaging data, results of sleep studies, and other data. Many patients had not received the results of their sleep study, and students had the responsibility to share these reports, including the number of apneic episodes. Students used the VA Computerized Patient Record System for reviewing patient records. One particularly useful tool was Joint Legacy Viewer, a remote access tool used to retrieve data on veterans from anywhere within the US. Students were also trained on medication and consult order menus in the system.
Team-Based
Learning The objectives of the team-based learning section were to teach students basic concepts of integrating the interdisciplinary assessment and formulating a treatment plan, to provide an opportunity to present their case in a group format, to discuss the differential diagnosis, management and treatment plan with a geriatrician in the team-based learning format, and to answer questions from other students. The instructors developed a set of prepared take-home points (Table 1). The team-based learning sessions were structured so that all take-home points were covered.
Evaluations
Evaluations were performed before and immediately after the clinical experience. In preevaluation, students reported the frequency of their participation in an interdisciplinary team assessment of any condition and specifically for dementia. In pre- and postevaluation, students rated their perception of the role of interdisciplinary team members in assessing and managing dementia, their personal abilities to assess cognition, behavioral problems, caregiver burden, and their perception of the impact of behavioral problems on dementia care. A Likert scale (poor = 1; fair = 2; good = 3; very good = 4; and excellent = 5) was employed (eApendices 1 and 2 can be found at doi:10.12788/fp.0052). The only demographic information collected was the student’s gender. Semistructured interviews were conducted to assess students’ current knowledge, experience, and needs. These interviews lasted about 20 minutes and collected information regarding the students’ knowledge about cognitive and behavioral problems in general and those occurring in dementia, their experience with screening, and any problems they encountered.
Statistical Analysis
Student baseline characteristics were assessed. Pre- and postassessments were analyzed with the McNemar test for paired data, and associations with experience were evaluated using χ2 tests. Ratings were dichotomized as very good/excellent vs poor/fair/ good because our educational goal was “very good” to “excellent” experience in dementia care and to avoid expected small cell counts. Two-sided P < .05 indicated statistical significance. Data were analyzed using SAS Enterprise Guide v5.1.
Results
One hundred fourth-year medical students participated, including 54 women. Thirtysix percent reported they had not previously attended an interdisciplinary team assessment for dementia, while 18% stated that they had attended only 1 interdisciplinary team assessment for dementia.
Before the education, students rated their dementia ability as poor. Only 2% (1 of 54), of those with 0 to 1 assessment experience rated their ability for assessing dementia with an interdisciplinary team format as very good/excellent compared with 20% (9/46) of those previously attending ≥ 2 assessments (P = .03); other ratings of ability were not associated with prior experience.
There was a significant change in the students’ self-efficacy ratings pre- to postassessment (P < .05) (Table 2). Only 10% rated their ability to assess for dementia as very good/excellent in before the intervention compared with 96% in postassessment (P < .01). Students’ perception of the impact of behavioral problems on dementia care improved significantly (45% to 98%, P < .01). Similarly, student’s perception of their ability to assess behavioral problems, caregiver burden, and cognition improved significantly from 7 to 88%; 7 to 78%, and 18 to 92%, respectively (P < .01). Students perception of the role of social worker, neuropsychologist, geriatrician, and geriatric psychiatrist also improved significantly for most measures from 81 to 98% (P = .02), 87 to 98% (P = .05), 94 to 99% (P = .06), and 88 to 100% (P = .01), respectively.
The semistructured interviews revealed that awareness of behavioral problems associated with dementia varied for different behavioral problems. Although many students showed familiarity with depression, agitation, and psychosis, they were not comfortable assessing them in a patient with dementia. These students were less aware of other behavioral problems such as disinhibition, apathy, and movement disorders. Deficits were noted in the skill of administering commonly used global cognitive screens, such as the Mini-Mental State Examination (MMSE).15
In semistructured interviews, only 7% of senior medical students were comfortable assessing behavioral problems associated with dementia. Most were not aware of any validated rating scale to assess neuropsychiatric symptoms. Similarly, only 7% of students were comfortable assessing caregiver burden, and most were not aware of any validated rating scale to assess caregiver burden. Only 1 in 5 students were comfortable using 2 cognitive screens to assess cognitive deficits. Many students stated that they were not routinely expected to perform common cognitive screens, such as the MMSE during their medical training except students who had expressed an interest in psychiatry and were expected to be proficient in the MMSE. Most students were making common mistakes, such as converting the 3-command task to 3 individual single commands, helping too much with serial 7s, and giving too much positive feedback throughout the test.
Discussion
Significant knowledge gaps regarding dementia were found in our study, which is in keeping with other studies in the area. Dementia knowledge deficits among medical trainees have been identified in the United Kingdom, Australia, and the US.6-9
In our study, a brief multicomponent experiential curriculum improved senior medical students’ perception and self-efficacy in diagnosing dementia. This is in keeping with other studies, such as the PAIRS Program.7 Findings from another study indicated that education for geriatric- oriented physicians should focus on experiential learning components through observation and interaction with older adults.16
A background of direct experience with older adults is associated with more positive attitudes toward older adults and increased interest in geriatric medicine.16 In our study, the exposure was brief; therefore, the results could not be compared with other long-term exposure studies. However, even with this brief intervention most students reported being comfortable with assessing caregiver burden (78%), behavioral problems of dementia (88%), and using ≥ 2 cognitive screens (92%). Comfortable in dementia assessment increased after the intervention from 10% to 96%. This finding is encouraging because brief multicomponent dementia education can be devised easily. This finding needs to be taken with caution because we did not conduct a formal skills evaluation.
A unique component of our experience was to learn medical students’ perception about the impact of neuropsychiatric symptoms on the trajectory, outcomes, and management of dementia. These symptoms included delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, and appetite and eating. Less than half the students thought that neuropsychiatric symptoms had a significant impact on dementia before the experience. Through didactics, systematic assessment of neuropsychiatric symptoms and interaction with caregivers, > 98% of students learned that these symptoms have a significant impact on dementia management.
This experience also emphasized the role of several disciplines in dementia assessment and management. Students’ experience positively influenced appreciation of the role of the memory clinic team. Our hope is that students will seek input from social workers, neuropsychologists, and other team members when working with patients with dementia or their caregivers. The common reason why primary care physicians focus on an exclusive medical model is the time commitment for communicating with an interdisciplinary team. Students experienced the feasibility of the interdisciplinary team involvement and how technology could be used for synchronous and asynchronous communication among team members. Medical students also were introduced to complex billing codes used when ≥ 3 disciplines assess/manage a geriatric patient.
Limitations
This study is limited by the lack of long-term follow-up evaluations, no metrics for practice changes clinical outcomes, and implementation in a single medical school. The postexperience evaluation in this study was performed immediately after the intervention. Long-term follow-up would inform whether the changes noted are durable. Because of the brief nature of our intervention, we do not believe that it would change practice in clinical care. It will be informative to follow this cohort of students to study whether their clinical approach to dementia care changes. The intervention needs to be replicated in other medical schools and in more heterogeneous groups to generalize the results of the study.
Conclusions
Senior medical students are not routinely exposed to interdisciplinary team assessments. Dementia knowledge gaps were prevalent in this cohort of senior medical students. Providing interdisciplinary geriatric educational experience improved their perception of their ability to assess for dementia and their recognition of the roles of interdisciplinary team members. Plans are in place to continue and expand the program to other complex geriatric syndromes.
Acknowledgments
Poster presented at the 2019 annual meeting of the American Geriatrics Society. Oral presentation at the same meeting as part of the select Geriatric Education Methods and Materials Swap workshop.
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. doi:10.1016/S0140-6736(05)67889-0
2. Janca A, Aarli JA, Prilipko L, Dua T, Saxena S, Saraceno B. WHO/WFN survey of neurological services: a worldwide perspective. J Neurol Sci. 2006;247(1):29-34. doi:10.1016/j.jns.2006.03.003
3. Wilkins KM, Blazek MC, Brooks WB, Lehmann SW, Popeo D, Wagenaar D. Six things all medical students need to know about geriatric psychiatry (and how to teach them). Acad Psychiatry. 2017;41(5):693-700. doi:10.1007/s40596-017-0691-7
4. Turner S, Iliffe S, Downs M, et al. General practitioners’ knowledge, confidence and attitudes in the diagnosis and management of dementia. Age Ageing. 2004;33(5):461- 467. doi:10.1093/ageing/afh140
5. Lester PE, Dharmarajan TS, Weinstein E. The looming geriatrician shortage: ramifications and solutions. J Aging Health. 2019:898264319879325. doi:10.1177/0898264319879325
6. Struck BD, Bernard MA, Teasdale TA; Oklahoma University Geriatric Education G. Effect of a mandatory geriatric medicine clerkship on third-year students. J Am Geriatr Soc. 2005;53(11):2007-2011. doi:10.1111/j.1532-5415.2005.00473.x
7. Jefferson AL, Cantwell NG, Byerly LK, Morhardt D. Medical student education program in Alzheimer’s disease: the PAIRS Program. BMC Med Educ. 2012;12:80. doi:10.1186/1472-6920-12-80
8. Nagle BJ, Usita PM, Edland SD. United States medical students’ knowledge of Alzheimer disease. J Educ Eval Health Prof. 2013;10:4. doi:10.3352/jeehp.2013.10.4
9. Scott TL, Kugelman M, Tulloch K. How medical professional students view older people with dementia: Implications for education and practice. PLoS One. 2019;14(11):e0225329. doi:10.1371/journal.pone.0225329.
10. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655. doi:10.1093/geront/20.6.649
11. McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377-383. doi:10.1016/s0895-4356(97)00060-7
12. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Ger iatr Soc. 2005;53(4):695-699. doi:10.1111/j.1532-5415.2005.53221.x
13. Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910. doi:10.1097/01.JGP.0000221510.33817.86
14. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi:10.1212/wnl.44.12.2308
15. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
16. Fitzgerald JT, Wray LA, Halter JB, Williams BC, Supiano MA. Relating medical students’ knowledge, attitudes, and experience to an interest in geriatric medicine. Gerontologist. 2003;43(6):849-855. doi:10.1093/geront/43.6.849
Highlights on Treatment of Progressive MS From ECTRIMS 2020
Promising phase 3 trial results from French researchers indicate that the first-in-class oral TKI masitinib may provide a new treatment option for patients with primary progressive multiple sclerosis (PPMS) or nonactive secondary progressive MS (SPMS).
The masitinib study was noted by Dr Mark Freedman, professor of neurology at the University of Ottawa, as among the key findings on PPMS presented at ACTRIMS-ECTRIMS 2020. The French study reported that patients receiving masitinib over 96 weeks experienced significant delay in disability progression.
Dr Freedman explains how an analysis done by Mellon Center researchers may change how clinicians counsel patients about the risk for progressive multifocal leukoencephalopathy (PML) related to fingolimod treatment. Their research shows the incidence rate of PML among patients receiving fingolimod to be very low — in fact, fewer than 40 times that of patients receiving natalizumab.
Finally, Dr Freedman discuses an ad hoc analysis presented by leading MS researchers from University Hospital in Basel, Switzerland, which points to plasma glial fibrillary acidic protein (GFAP) levels as a prognostic biomarker of increased risk for worsening disability. Using data from the EXPAND trial, researchers found significant risk for increased disability among patients with nonactive SPMS who had elevated baseline GFAP.
Professor, Department of Neurology, University of Ottawa and The Ottawa Hospital Research Institute; Director, Multiple Sclerosis Research Unit, The Ottawa Hospital – General Campus, Ottawa, Ontario, Canada.
Mark S. Freedman, MSc, MD, has disclosed the following relevant financial relationships: Serve(d) on the advisory board, board of directors, or other similar groups for: Actelion (Janssen/Johnson & Johnson); Alexion; Atara Biotherapeutics; BayerHealthcare; BiogenIdec; Celgene; Clene Nanomedicine; GRI Bio; Hoffman La-Roche; Magenta Therapeutics; Merck Serono; MedDay; Novartis; Sanofi-Genzyme; Teva Canada Innovation. Serve(d) as a member of a speakers bureau for: Sanofi-Genzyme; EMD Serono. Received honoraria or consultation fees for: Actelion (Janssen/Johnson & Johnson); Alexion; BiogenIdec; Celgene (BMS); EMD Inc; Sanofi-Genzyme; Hoffman La-Roche; Merck Serono; Novartis; Teva Canada Innovation. Received research or educational grants from: Sanofi-Genzyme Canada; Hoffman-La Roche; EMD Inc.
Promising phase 3 trial results from French researchers indicate that the first-in-class oral TKI masitinib may provide a new treatment option for patients with primary progressive multiple sclerosis (PPMS) or nonactive secondary progressive MS (SPMS).
The masitinib study was noted by Dr Mark Freedman, professor of neurology at the University of Ottawa, as among the key findings on PPMS presented at ACTRIMS-ECTRIMS 2020. The French study reported that patients receiving masitinib over 96 weeks experienced significant delay in disability progression.
Dr Freedman explains how an analysis done by Mellon Center researchers may change how clinicians counsel patients about the risk for progressive multifocal leukoencephalopathy (PML) related to fingolimod treatment. Their research shows the incidence rate of PML among patients receiving fingolimod to be very low — in fact, fewer than 40 times that of patients receiving natalizumab.
Finally, Dr Freedman discuses an ad hoc analysis presented by leading MS researchers from University Hospital in Basel, Switzerland, which points to plasma glial fibrillary acidic protein (GFAP) levels as a prognostic biomarker of increased risk for worsening disability. Using data from the EXPAND trial, researchers found significant risk for increased disability among patients with nonactive SPMS who had elevated baseline GFAP.
Professor, Department of Neurology, University of Ottawa and The Ottawa Hospital Research Institute; Director, Multiple Sclerosis Research Unit, The Ottawa Hospital – General Campus, Ottawa, Ontario, Canada.
Mark S. Freedman, MSc, MD, has disclosed the following relevant financial relationships: Serve(d) on the advisory board, board of directors, or other similar groups for: Actelion (Janssen/Johnson & Johnson); Alexion; Atara Biotherapeutics; BayerHealthcare; BiogenIdec; Celgene; Clene Nanomedicine; GRI Bio; Hoffman La-Roche; Magenta Therapeutics; Merck Serono; MedDay; Novartis; Sanofi-Genzyme; Teva Canada Innovation. Serve(d) as a member of a speakers bureau for: Sanofi-Genzyme; EMD Serono. Received honoraria or consultation fees for: Actelion (Janssen/Johnson & Johnson); Alexion; BiogenIdec; Celgene (BMS); EMD Inc; Sanofi-Genzyme; Hoffman La-Roche; Merck Serono; Novartis; Teva Canada Innovation. Received research or educational grants from: Sanofi-Genzyme Canada; Hoffman-La Roche; EMD Inc.
Promising phase 3 trial results from French researchers indicate that the first-in-class oral TKI masitinib may provide a new treatment option for patients with primary progressive multiple sclerosis (PPMS) or nonactive secondary progressive MS (SPMS).
The masitinib study was noted by Dr Mark Freedman, professor of neurology at the University of Ottawa, as among the key findings on PPMS presented at ACTRIMS-ECTRIMS 2020. The French study reported that patients receiving masitinib over 96 weeks experienced significant delay in disability progression.
Dr Freedman explains how an analysis done by Mellon Center researchers may change how clinicians counsel patients about the risk for progressive multifocal leukoencephalopathy (PML) related to fingolimod treatment. Their research shows the incidence rate of PML among patients receiving fingolimod to be very low — in fact, fewer than 40 times that of patients receiving natalizumab.
Finally, Dr Freedman discuses an ad hoc analysis presented by leading MS researchers from University Hospital in Basel, Switzerland, which points to plasma glial fibrillary acidic protein (GFAP) levels as a prognostic biomarker of increased risk for worsening disability. Using data from the EXPAND trial, researchers found significant risk for increased disability among patients with nonactive SPMS who had elevated baseline GFAP.
Professor, Department of Neurology, University of Ottawa and The Ottawa Hospital Research Institute; Director, Multiple Sclerosis Research Unit, The Ottawa Hospital – General Campus, Ottawa, Ontario, Canada.
Mark S. Freedman, MSc, MD, has disclosed the following relevant financial relationships: Serve(d) on the advisory board, board of directors, or other similar groups for: Actelion (Janssen/Johnson & Johnson); Alexion; Atara Biotherapeutics; BayerHealthcare; BiogenIdec; Celgene; Clene Nanomedicine; GRI Bio; Hoffman La-Roche; Magenta Therapeutics; Merck Serono; MedDay; Novartis; Sanofi-Genzyme; Teva Canada Innovation. Serve(d) as a member of a speakers bureau for: Sanofi-Genzyme; EMD Serono. Received honoraria or consultation fees for: Actelion (Janssen/Johnson & Johnson); Alexion; BiogenIdec; Celgene (BMS); EMD Inc; Sanofi-Genzyme; Hoffman La-Roche; Merck Serono; Novartis; Teva Canada Innovation. Received research or educational grants from: Sanofi-Genzyme Canada; Hoffman-La Roche; EMD Inc.

Survey explores mental health, services use in police officers
New research shows that about a quarter of police officers in one large force report past or present mental health problems.
Responding to a survey, 26% of police officers on the Dallas police department screened positive for depression, anxiety, PTSD, or symptoms of suicide ideation or self-harm.
Mental illness rates were particularly high among female officers, those who were divorced, widowed, or separated, and those with military experience.
The study also showed that concerns over confidentiality and stigma may prevent officers with mental illness from seeking treatment.
The results underscored the need to identify police officers with psychiatric problems and to connect them to the most appropriate individualized care, author Katelyn K. Jetelina, PhD, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“This is a very hard-to-reach population, and because of that, we need to be innovative in reaching them for services,” she said.
The study was published online Oct. 7 in JAMA Network Open.
Dr. Jetelina and colleagues are investigating various aspects of police officers’ well-being, including their nutritional needs and their occupational, physical, and mental health.
The current study included 434 members of the Dallas police department, the ninth largest in the United States. The mean age of the participants was 37 years, 82% were men, and about half were White. The 434 officers represented 97% of those invited to participate (n = 446) and 31% of the total patrol population of the Dallas police department (n = 1,413).
These officers completed a short survey on their smartphone that asked about lifetime diagnoses of depression, anxiety, and PTSD. They were also asked whether they experienced suicidal ideation or self-harm during the previous 2 weeks.
Overall, 12% of survey respondents reported having been diagnosed with a mental illness. This, said Jetelina, is slightly lower than the rate reported in the general population.
Study participants who had not currently been diagnosed with a mental illness completed the Patient Health Questionnaire–2 (PHQ-2), the Generalized Anxiety Disorder–2 (GAD-2), and the Primary Care–Posttraumatic Stress Disorder (PC-PTSD).
Officers were considered to have a positive result if they had a score of 3 or more (PHQ-2, sensitivity of 83% and specificity of 92%; PC-PTSD-5, sensitivity of 93% and specificity of 85%; GAD-2, sensitivity of 86% and specificity of 83%).
About 26% of respondents had a positive screening for mental illness symptoms, mainly PTSD and depression, which Dr. Jetelina noted is a higher percentage than in the general population.
This rate of mental health symptoms is “high and concerning,” but not surprising because of the work of police officers, which could include attending to sometimes violent car crashes, domestic abuse situations, and armed conflicts, said Dr. Jetelina.
“They’re constantly exposed to traumatic calls for service; they see people on their worst day, 8 hours a day, 5 days a week. That stress and exposure will have a detrimental effect on mental health, and we have to pay more attention to that,” she said.
Dr. Jetelina pointed out that the surveys were completed in January and February 2020, before COVID-19 had become a cause of stress for everyone and before the increase in calls for defunding police amid a resurgence of Black Lives Matter demonstrations.
However, she stressed that racial biases and occupational stress among police officers are “nothing new for them.” For example, in 2016, five Dallas police officers were killed during Black Lives Matter protests because of their race/ethnicity.
More at risk
The study showed that certain subgroups of officers were more at risk for mental illness. After adjustment for confounders, including demographic characteristics, marital status, and educational level, the odds of being diagnosed with a mental illness during the course of one’s life were significantly higher among female officers than male officers (adjusted odds ratio, 3.20; 95% confidence interval, 1.18-8.68).
Officers who were divorced, widowed, or separated and those who had more experience and held a higher rank were also at greater risk for mental illness.
As well, (aOR, 3.25; 95% CI, 1.38-7.67).
The study also asked participants about use of mental health care services over the past 12 months. About 35% of those who had a current mental health diagnosis and 17% of those who screened positive for mental health symptoms reported using such services.
The study also asked those who screened positive about their interest in seeking such services. After adjustments, officers with suicidal ideation or self-harm were significantly more likely to be interested in getting help, compared with officers who did not report suicidal ideation or self-harm (aOR, 7.66; 95% CI, 1.70-34.48).
Dr. Jetelina was impressed that so many officers were keen to seek help, which “is a big positive,” she said. “It’s just a matter of better detecting who needs the help and better connecting them to medical services that meet their needs.”
Mindfulness exercise
Dr. Jetelina and colleagues are conducting a pilot test of the use by police officers of smartwatches that monitor heart rate and oxygen levels. If measurements with these devices reach a predetermined threshold, the officers are “pinged” and are instructed to perform a mindfulness exercise in the field, she said.
Results so far “are really exciting,” said Dr. Jetelina. “Officers have found this extremely helpful and feasible, and so the next step is to test if this truly impacts mental illness over time.”
Routine mental health screening of officers might be beneficial, but only if it’s conducted in a manner “respectful of the officers’ needs and wants,” said Dr. Jetelina.
She pointed out that although psychological assessments are routinely carried out following an extreme traumatic call, such as one involving an officer-involved shooting, the “in-between” calls could have a more severe cumulative impact on mental health.
It’s important to provide officers with easy-to-access services tailored for their individual needs, said Dr. Jetelina.
‘Numb to it’
Eighteen patrol officers also participated in a focus group, during which several themes regarding the use of mental health care services emerged. One theme was the inability of officers to identify when they’re personally experiencing a mental health problem.
Participants said they had become “numb” to the traumatic events on the job, which is “concerning,” Dr. Jetelina said. “They think that having nightmares every week is completely normal, but it’s not, and this needs to be addressed.”
Other themes that emerged from focus groups included the belief that psychologists can’t relate to police stressors; concerns about confidentiality (one sentiment that was expressed was “you’re an idiot” if you “trust this department”); and stigma for officers who seek mental health care (participants talked about “reprisal” from seeing “a shrink,” including being labeled as “a nutter” and losing their job).
Dr. Jetelina noted that some “champion” officers revealed their mental health journey during focus groups, which tended to “open a Pandora’s box” for others to discuss their experience. She said these champions could be leveraged throughout the police department to help reduce stigma.
The study included participants from only one police department, although rigorous data collection allows for generalizability to the entire patrol department, say the authors. Although the study included only brief screens of mental illness symptoms, these short versions of screening tests have high sensitivity and specificity for mental illness in primary care, they noted.
The next step for the researchers is to study how mental illness and symptoms affect job performance, said Dr. Jetelina. “Does this impact excessive use of force? Does this impact workers’ compensation? Does this impact dispatch times, the time it takes for a police officer to respond to [a] 911 call?”
Possible underrepresentation
Anthony T. Ng, MD, regional medical director, East Region Hartford HealthCare Behavioral Health Network in Mansfield, Conn., and member of the American Psychiatric Association’s Council on Communications, found the study “helpful.”
However, the 26% who tested positive for mental illness may be an “underrepresentation” of the true picture, inasmuch as police officers might minimize or be less than truthful about their mental health status, said Dr. Ng.
Law enforcement has “never been easy,” but stressors may have escalated recently as police forces deal with shortages of staff and jails, said Dr. Ng.
He also noted that officers might face stressors at home. “Evidence shows that domestic violence is quite high – or higher than average – among law enforcement,” he said. “All these things add up.”
Psychiatrists and other mental health professionals should be “aware of the unique challenges” that police officers face and be “proactively involved” in providing guidance and education on mitigating stress, said Dr. Ng.
“You have police officers wearing body armor, so why can’t you give them some training to learn how to have psychiatric or psychological body armor?” he said. But it’s a two-way street; police forces should be open to outreach from mental health professionals. “We have to meet halfway.”
Compassion fatigue
In an accompanying commentary, John M. Violanti, PhD, of the department of epidemiology and environmental health at the State University of New York at Buffalo, said the article helps bring “to the forefront” the issue of the psychological dangers of police work.
There is conjecture as to why police experience mental distress, said Dr. Violanti, who pointed to a study of New York City police suicides during the 1930s that suggested that police have a “social license” for aggressive behavior but are restrained as part of public trust, placing them in a position of “psychological strain.”
“This situation may be reflective of the same situation police find themselves today,” said Dr. Violanti.
“Compassion fatigue,” a feeling of mental exhaustion caused by the inability to care for all persons in trouble, may also be a factor, as could the constant stress that leaves police officers feeling “cynical and isolated from others,” he wrote.
“The socialization process of becoming a police officer is associated with constrictive reasoning, viewing the world as either right or wrong, which leaves no middle ground for alternatives to deal with mental distress,” Dr. Violanti said.
He noted that police officers may abuse alcohol because of stress, peer pressure, isolation, and a culture that approves of alcohol use. “Officers tend to drink together and reinforce their own values.”.
Although no prospective studies have linked police mental health problems with childhood abuse or neglect, some mental health professionals estimate that about 25% of their police clients have a history of childhood abuse or neglect, said Dr. Violanti.
He agreed that mindfulness may help manage stress and increase cognitive flexibility in dealing with trauma and crises.
A possible way to ensure confidentiality is a peer support program that allows distressed officers to first talk privately with a trained and trusted peer officer and to then seek professional help if necessary, said Dr. Violanti.
The study was funded by a grant from the National Institute of Occupational Health and Safety. Dr. Jetelina, Dr. Ng, and Dr. Violanti disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New research shows that about a quarter of police officers in one large force report past or present mental health problems.
Responding to a survey, 26% of police officers on the Dallas police department screened positive for depression, anxiety, PTSD, or symptoms of suicide ideation or self-harm.
Mental illness rates were particularly high among female officers, those who were divorced, widowed, or separated, and those with military experience.
The study also showed that concerns over confidentiality and stigma may prevent officers with mental illness from seeking treatment.
The results underscored the need to identify police officers with psychiatric problems and to connect them to the most appropriate individualized care, author Katelyn K. Jetelina, PhD, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“This is a very hard-to-reach population, and because of that, we need to be innovative in reaching them for services,” she said.
The study was published online Oct. 7 in JAMA Network Open.
Dr. Jetelina and colleagues are investigating various aspects of police officers’ well-being, including their nutritional needs and their occupational, physical, and mental health.
The current study included 434 members of the Dallas police department, the ninth largest in the United States. The mean age of the participants was 37 years, 82% were men, and about half were White. The 434 officers represented 97% of those invited to participate (n = 446) and 31% of the total patrol population of the Dallas police department (n = 1,413).
These officers completed a short survey on their smartphone that asked about lifetime diagnoses of depression, anxiety, and PTSD. They were also asked whether they experienced suicidal ideation or self-harm during the previous 2 weeks.
Overall, 12% of survey respondents reported having been diagnosed with a mental illness. This, said Jetelina, is slightly lower than the rate reported in the general population.
Study participants who had not currently been diagnosed with a mental illness completed the Patient Health Questionnaire–2 (PHQ-2), the Generalized Anxiety Disorder–2 (GAD-2), and the Primary Care–Posttraumatic Stress Disorder (PC-PTSD).
Officers were considered to have a positive result if they had a score of 3 or more (PHQ-2, sensitivity of 83% and specificity of 92%; PC-PTSD-5, sensitivity of 93% and specificity of 85%; GAD-2, sensitivity of 86% and specificity of 83%).
About 26% of respondents had a positive screening for mental illness symptoms, mainly PTSD and depression, which Dr. Jetelina noted is a higher percentage than in the general population.
This rate of mental health symptoms is “high and concerning,” but not surprising because of the work of police officers, which could include attending to sometimes violent car crashes, domestic abuse situations, and armed conflicts, said Dr. Jetelina.
“They’re constantly exposed to traumatic calls for service; they see people on their worst day, 8 hours a day, 5 days a week. That stress and exposure will have a detrimental effect on mental health, and we have to pay more attention to that,” she said.
Dr. Jetelina pointed out that the surveys were completed in January and February 2020, before COVID-19 had become a cause of stress for everyone and before the increase in calls for defunding police amid a resurgence of Black Lives Matter demonstrations.
However, she stressed that racial biases and occupational stress among police officers are “nothing new for them.” For example, in 2016, five Dallas police officers were killed during Black Lives Matter protests because of their race/ethnicity.
More at risk
The study showed that certain subgroups of officers were more at risk for mental illness. After adjustment for confounders, including demographic characteristics, marital status, and educational level, the odds of being diagnosed with a mental illness during the course of one’s life were significantly higher among female officers than male officers (adjusted odds ratio, 3.20; 95% confidence interval, 1.18-8.68).
Officers who were divorced, widowed, or separated and those who had more experience and held a higher rank were also at greater risk for mental illness.
As well, (aOR, 3.25; 95% CI, 1.38-7.67).
The study also asked participants about use of mental health care services over the past 12 months. About 35% of those who had a current mental health diagnosis and 17% of those who screened positive for mental health symptoms reported using such services.
The study also asked those who screened positive about their interest in seeking such services. After adjustments, officers with suicidal ideation or self-harm were significantly more likely to be interested in getting help, compared with officers who did not report suicidal ideation or self-harm (aOR, 7.66; 95% CI, 1.70-34.48).
Dr. Jetelina was impressed that so many officers were keen to seek help, which “is a big positive,” she said. “It’s just a matter of better detecting who needs the help and better connecting them to medical services that meet their needs.”
Mindfulness exercise
Dr. Jetelina and colleagues are conducting a pilot test of the use by police officers of smartwatches that monitor heart rate and oxygen levels. If measurements with these devices reach a predetermined threshold, the officers are “pinged” and are instructed to perform a mindfulness exercise in the field, she said.
Results so far “are really exciting,” said Dr. Jetelina. “Officers have found this extremely helpful and feasible, and so the next step is to test if this truly impacts mental illness over time.”
Routine mental health screening of officers might be beneficial, but only if it’s conducted in a manner “respectful of the officers’ needs and wants,” said Dr. Jetelina.
She pointed out that although psychological assessments are routinely carried out following an extreme traumatic call, such as one involving an officer-involved shooting, the “in-between” calls could have a more severe cumulative impact on mental health.
It’s important to provide officers with easy-to-access services tailored for their individual needs, said Dr. Jetelina.
‘Numb to it’
Eighteen patrol officers also participated in a focus group, during which several themes regarding the use of mental health care services emerged. One theme was the inability of officers to identify when they’re personally experiencing a mental health problem.
Participants said they had become “numb” to the traumatic events on the job, which is “concerning,” Dr. Jetelina said. “They think that having nightmares every week is completely normal, but it’s not, and this needs to be addressed.”
Other themes that emerged from focus groups included the belief that psychologists can’t relate to police stressors; concerns about confidentiality (one sentiment that was expressed was “you’re an idiot” if you “trust this department”); and stigma for officers who seek mental health care (participants talked about “reprisal” from seeing “a shrink,” including being labeled as “a nutter” and losing their job).
Dr. Jetelina noted that some “champion” officers revealed their mental health journey during focus groups, which tended to “open a Pandora’s box” for others to discuss their experience. She said these champions could be leveraged throughout the police department to help reduce stigma.
The study included participants from only one police department, although rigorous data collection allows for generalizability to the entire patrol department, say the authors. Although the study included only brief screens of mental illness symptoms, these short versions of screening tests have high sensitivity and specificity for mental illness in primary care, they noted.
The next step for the researchers is to study how mental illness and symptoms affect job performance, said Dr. Jetelina. “Does this impact excessive use of force? Does this impact workers’ compensation? Does this impact dispatch times, the time it takes for a police officer to respond to [a] 911 call?”
Possible underrepresentation
Anthony T. Ng, MD, regional medical director, East Region Hartford HealthCare Behavioral Health Network in Mansfield, Conn., and member of the American Psychiatric Association’s Council on Communications, found the study “helpful.”
However, the 26% who tested positive for mental illness may be an “underrepresentation” of the true picture, inasmuch as police officers might minimize or be less than truthful about their mental health status, said Dr. Ng.
Law enforcement has “never been easy,” but stressors may have escalated recently as police forces deal with shortages of staff and jails, said Dr. Ng.
He also noted that officers might face stressors at home. “Evidence shows that domestic violence is quite high – or higher than average – among law enforcement,” he said. “All these things add up.”
Psychiatrists and other mental health professionals should be “aware of the unique challenges” that police officers face and be “proactively involved” in providing guidance and education on mitigating stress, said Dr. Ng.
“You have police officers wearing body armor, so why can’t you give them some training to learn how to have psychiatric or psychological body armor?” he said. But it’s a two-way street; police forces should be open to outreach from mental health professionals. “We have to meet halfway.”
Compassion fatigue
In an accompanying commentary, John M. Violanti, PhD, of the department of epidemiology and environmental health at the State University of New York at Buffalo, said the article helps bring “to the forefront” the issue of the psychological dangers of police work.
There is conjecture as to why police experience mental distress, said Dr. Violanti, who pointed to a study of New York City police suicides during the 1930s that suggested that police have a “social license” for aggressive behavior but are restrained as part of public trust, placing them in a position of “psychological strain.”
“This situation may be reflective of the same situation police find themselves today,” said Dr. Violanti.
“Compassion fatigue,” a feeling of mental exhaustion caused by the inability to care for all persons in trouble, may also be a factor, as could the constant stress that leaves police officers feeling “cynical and isolated from others,” he wrote.
“The socialization process of becoming a police officer is associated with constrictive reasoning, viewing the world as either right or wrong, which leaves no middle ground for alternatives to deal with mental distress,” Dr. Violanti said.
He noted that police officers may abuse alcohol because of stress, peer pressure, isolation, and a culture that approves of alcohol use. “Officers tend to drink together and reinforce their own values.”.
Although no prospective studies have linked police mental health problems with childhood abuse or neglect, some mental health professionals estimate that about 25% of their police clients have a history of childhood abuse or neglect, said Dr. Violanti.
He agreed that mindfulness may help manage stress and increase cognitive flexibility in dealing with trauma and crises.
A possible way to ensure confidentiality is a peer support program that allows distressed officers to first talk privately with a trained and trusted peer officer and to then seek professional help if necessary, said Dr. Violanti.
The study was funded by a grant from the National Institute of Occupational Health and Safety. Dr. Jetelina, Dr. Ng, and Dr. Violanti disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New research shows that about a quarter of police officers in one large force report past or present mental health problems.
Responding to a survey, 26% of police officers on the Dallas police department screened positive for depression, anxiety, PTSD, or symptoms of suicide ideation or self-harm.
Mental illness rates were particularly high among female officers, those who were divorced, widowed, or separated, and those with military experience.
The study also showed that concerns over confidentiality and stigma may prevent officers with mental illness from seeking treatment.
The results underscored the need to identify police officers with psychiatric problems and to connect them to the most appropriate individualized care, author Katelyn K. Jetelina, PhD, assistant professor in the department of epidemiology, human genetics, and environmental sciences, University of Texas Health Science Center, Dallas, said in an interview.
“This is a very hard-to-reach population, and because of that, we need to be innovative in reaching them for services,” she said.
The study was published online Oct. 7 in JAMA Network Open.
Dr. Jetelina and colleagues are investigating various aspects of police officers’ well-being, including their nutritional needs and their occupational, physical, and mental health.
The current study included 434 members of the Dallas police department, the ninth largest in the United States. The mean age of the participants was 37 years, 82% were men, and about half were White. The 434 officers represented 97% of those invited to participate (n = 446) and 31% of the total patrol population of the Dallas police department (n = 1,413).
These officers completed a short survey on their smartphone that asked about lifetime diagnoses of depression, anxiety, and PTSD. They were also asked whether they experienced suicidal ideation or self-harm during the previous 2 weeks.
Overall, 12% of survey respondents reported having been diagnosed with a mental illness. This, said Jetelina, is slightly lower than the rate reported in the general population.
Study participants who had not currently been diagnosed with a mental illness completed the Patient Health Questionnaire–2 (PHQ-2), the Generalized Anxiety Disorder–2 (GAD-2), and the Primary Care–Posttraumatic Stress Disorder (PC-PTSD).
Officers were considered to have a positive result if they had a score of 3 or more (PHQ-2, sensitivity of 83% and specificity of 92%; PC-PTSD-5, sensitivity of 93% and specificity of 85%; GAD-2, sensitivity of 86% and specificity of 83%).
About 26% of respondents had a positive screening for mental illness symptoms, mainly PTSD and depression, which Dr. Jetelina noted is a higher percentage than in the general population.
This rate of mental health symptoms is “high and concerning,” but not surprising because of the work of police officers, which could include attending to sometimes violent car crashes, domestic abuse situations, and armed conflicts, said Dr. Jetelina.
“They’re constantly exposed to traumatic calls for service; they see people on their worst day, 8 hours a day, 5 days a week. That stress and exposure will have a detrimental effect on mental health, and we have to pay more attention to that,” she said.
Dr. Jetelina pointed out that the surveys were completed in January and February 2020, before COVID-19 had become a cause of stress for everyone and before the increase in calls for defunding police amid a resurgence of Black Lives Matter demonstrations.
However, she stressed that racial biases and occupational stress among police officers are “nothing new for them.” For example, in 2016, five Dallas police officers were killed during Black Lives Matter protests because of their race/ethnicity.
More at risk
The study showed that certain subgroups of officers were more at risk for mental illness. After adjustment for confounders, including demographic characteristics, marital status, and educational level, the odds of being diagnosed with a mental illness during the course of one’s life were significantly higher among female officers than male officers (adjusted odds ratio, 3.20; 95% confidence interval, 1.18-8.68).
Officers who were divorced, widowed, or separated and those who had more experience and held a higher rank were also at greater risk for mental illness.
As well, (aOR, 3.25; 95% CI, 1.38-7.67).
The study also asked participants about use of mental health care services over the past 12 months. About 35% of those who had a current mental health diagnosis and 17% of those who screened positive for mental health symptoms reported using such services.
The study also asked those who screened positive about their interest in seeking such services. After adjustments, officers with suicidal ideation or self-harm were significantly more likely to be interested in getting help, compared with officers who did not report suicidal ideation or self-harm (aOR, 7.66; 95% CI, 1.70-34.48).
Dr. Jetelina was impressed that so many officers were keen to seek help, which “is a big positive,” she said. “It’s just a matter of better detecting who needs the help and better connecting them to medical services that meet their needs.”
Mindfulness exercise
Dr. Jetelina and colleagues are conducting a pilot test of the use by police officers of smartwatches that monitor heart rate and oxygen levels. If measurements with these devices reach a predetermined threshold, the officers are “pinged” and are instructed to perform a mindfulness exercise in the field, she said.
Results so far “are really exciting,” said Dr. Jetelina. “Officers have found this extremely helpful and feasible, and so the next step is to test if this truly impacts mental illness over time.”
Routine mental health screening of officers might be beneficial, but only if it’s conducted in a manner “respectful of the officers’ needs and wants,” said Dr. Jetelina.
She pointed out that although psychological assessments are routinely carried out following an extreme traumatic call, such as one involving an officer-involved shooting, the “in-between” calls could have a more severe cumulative impact on mental health.
It’s important to provide officers with easy-to-access services tailored for their individual needs, said Dr. Jetelina.
‘Numb to it’
Eighteen patrol officers also participated in a focus group, during which several themes regarding the use of mental health care services emerged. One theme was the inability of officers to identify when they’re personally experiencing a mental health problem.
Participants said they had become “numb” to the traumatic events on the job, which is “concerning,” Dr. Jetelina said. “They think that having nightmares every week is completely normal, but it’s not, and this needs to be addressed.”
Other themes that emerged from focus groups included the belief that psychologists can’t relate to police stressors; concerns about confidentiality (one sentiment that was expressed was “you’re an idiot” if you “trust this department”); and stigma for officers who seek mental health care (participants talked about “reprisal” from seeing “a shrink,” including being labeled as “a nutter” and losing their job).
Dr. Jetelina noted that some “champion” officers revealed their mental health journey during focus groups, which tended to “open a Pandora’s box” for others to discuss their experience. She said these champions could be leveraged throughout the police department to help reduce stigma.
The study included participants from only one police department, although rigorous data collection allows for generalizability to the entire patrol department, say the authors. Although the study included only brief screens of mental illness symptoms, these short versions of screening tests have high sensitivity and specificity for mental illness in primary care, they noted.
The next step for the researchers is to study how mental illness and symptoms affect job performance, said Dr. Jetelina. “Does this impact excessive use of force? Does this impact workers’ compensation? Does this impact dispatch times, the time it takes for a police officer to respond to [a] 911 call?”
Possible underrepresentation
Anthony T. Ng, MD, regional medical director, East Region Hartford HealthCare Behavioral Health Network in Mansfield, Conn., and member of the American Psychiatric Association’s Council on Communications, found the study “helpful.”
However, the 26% who tested positive for mental illness may be an “underrepresentation” of the true picture, inasmuch as police officers might minimize or be less than truthful about their mental health status, said Dr. Ng.
Law enforcement has “never been easy,” but stressors may have escalated recently as police forces deal with shortages of staff and jails, said Dr. Ng.
He also noted that officers might face stressors at home. “Evidence shows that domestic violence is quite high – or higher than average – among law enforcement,” he said. “All these things add up.”
Psychiatrists and other mental health professionals should be “aware of the unique challenges” that police officers face and be “proactively involved” in providing guidance and education on mitigating stress, said Dr. Ng.
“You have police officers wearing body armor, so why can’t you give them some training to learn how to have psychiatric or psychological body armor?” he said. But it’s a two-way street; police forces should be open to outreach from mental health professionals. “We have to meet halfway.”
Compassion fatigue
In an accompanying commentary, John M. Violanti, PhD, of the department of epidemiology and environmental health at the State University of New York at Buffalo, said the article helps bring “to the forefront” the issue of the psychological dangers of police work.
There is conjecture as to why police experience mental distress, said Dr. Violanti, who pointed to a study of New York City police suicides during the 1930s that suggested that police have a “social license” for aggressive behavior but are restrained as part of public trust, placing them in a position of “psychological strain.”
“This situation may be reflective of the same situation police find themselves today,” said Dr. Violanti.
“Compassion fatigue,” a feeling of mental exhaustion caused by the inability to care for all persons in trouble, may also be a factor, as could the constant stress that leaves police officers feeling “cynical and isolated from others,” he wrote.
“The socialization process of becoming a police officer is associated with constrictive reasoning, viewing the world as either right or wrong, which leaves no middle ground for alternatives to deal with mental distress,” Dr. Violanti said.
He noted that police officers may abuse alcohol because of stress, peer pressure, isolation, and a culture that approves of alcohol use. “Officers tend to drink together and reinforce their own values.”.
Although no prospective studies have linked police mental health problems with childhood abuse or neglect, some mental health professionals estimate that about 25% of their police clients have a history of childhood abuse or neglect, said Dr. Violanti.
He agreed that mindfulness may help manage stress and increase cognitive flexibility in dealing with trauma and crises.
A possible way to ensure confidentiality is a peer support program that allows distressed officers to first talk privately with a trained and trusted peer officer and to then seek professional help if necessary, said Dr. Violanti.
The study was funded by a grant from the National Institute of Occupational Health and Safety. Dr. Jetelina, Dr. Ng, and Dr. Violanti disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hidradenitis Suppurativa in the Military
Case Report
A 19-year-old female marine with a 10-year history of hidradenitis suppurativa (HS) presented with hyperpigmented nodules in the inguinal folds and a recurrent cyst in the right groin area of 2 to 3 weeks’ duration. She denied axillary or inframammary involvement. She underwent several incision and drainage procedures 1 year prior to her enlistment in the US Marine Corps at 18 years of age. She previously had been treated by dermatology with doxycycline 100-mg tablets twice daily, benzoyl peroxide wash 5% applied to affected areas and rinsed daily, and clindamycin solution 1% with minimal improvement. She denied smoking or alcohol intake and said she typically wore a loose-fitting uniform to work. As a marine, she was expected to participate in daily physical training and exercises with her military unit, during which she wore a standardized physical training uniform, including nylon shorts and a cotton T-shirt. She requested light duty—military duty status with physical limitations or restrictions—to avoid physical training that would cause further friction and irritation to the inguinal region.
Physical examination demonstrated a woman with Fitzpatrick skin type III and normal body mass index. There were hyperpigmented nodules and scarring in the inguinal folds, most consistent with Hurley stage 2. A single, 0.5-cm, draining lesion was visualized. No hyperhidrosis was noted. The patient was placed on light duty for 7 days, with physical training only at her own pace and discretion. Moreover, she was restricted from field training, rifle range training, and other situations where she may excessively sweat or not be able to adequately maintain personal hygiene. She was encouraged to continue clindamycin solution 1% to the affected area twice daily and was prescribed chlorhexidine solution 4% to use when washing affected areas in the shower. The patient also was referred to the dermatology department at the Naval Hospital Camp Pendleton (Oceanside, California), where she was treated with laser hair removal in the inguinal region, thus avoiding waxing and further aggravation of HS flares. Due to the combination of topical therapies along with laser hair removal and duty restrictions, the patient had a dramatic decrease in development of severe nodular lesions.
Comment
Presentation
Historically, the incidence of HS is estimated at 0.5% to 4% of the general population with female predominance.1 Predisposing factors include obesity, smoking, genetic predisposition to acne, apocrine duct obstruction, and secondary bacterial infection.2 During acute flares, patients generally present with tender subcutaneous nodules that drain malodorous purulent material.3,4 Acute flares are unpredictable, and patients deal with chronic, recurrent, draining wounds, leading to a poor quality of life with resulting physical, psychological, financial, social, and emotional distress.3-5 The negative impact of HS on a patient’s quality of life has been reported to be greater than other dermatologic conditions.6 Lesions can be particularly painful and can cause disfiguration to the surface of the skin.7 Lesion severity is described using the Hurley staging system. Patient quality of life is directly correlated with disease severity and Hurley stage. In stage 1, abscesses develop, but no sinus tracts or cicatrization is present. In stage 2, recurrent abscesses will form tracts and cicatrization. In stage 3, the abscesses become diffuse or near diffuse, with multiple interconnected tracts and abscesses across the entire area of the body.8,9
Severe or refractory HS within the physically active military population may require consideration of light or limited duty or even separation from service. Similarly, severe HS may pose challenges with other physically demanding occupations, such as the police force and firefighters.
Prevention Focus
Prevention of flares is key for patients with HS; secondary prevention aims to reduce impact of the disease or injury that has already occurred,10,11 which includes prevention of the infundibulofolliculitis from becoming a deep folliculitis, nodule, or fistula, as well as Hurley stage progression. Prompt diagnosis with appropriate treatment can decrease the severity of lesions, pain, and scarring. Globally, HS patients continue to experience considerable diagnostic delays of 8 to 12 years after onset of initial symptoms.11,12 Earlier accurate diagnosis and initiation of treatment from the primary care provider or general medical officer is imperative. Initial accurate management may help keep symptoms from progressing to more severe painful lesions. Similarly, patients should be educated on how to prevent HS flares. Patients should avoid known triggers, including smoking, obesity, sweating, mechanical irritation, stress, and poor hygiene.11
Shaving for hair reduction creates ingrown hair shafts, which may lead to folliculitis in mechanically stressed areas in skin folds, thus initiating the inflammatory cascade of HS.11,13 Therefore, shaving along with any other mechanical stress should be avoided in patients with HS. Laser hair removal has been shown to be quite helpful in both the prevention and treatment of HS. In one study, 22 patients with Hurley stage 2 to 3 disease were treated with an Nd:YAG laser once monthly. Results demonstrated a 65% decrease in disease severity after 3 monthly treatments.11 Similarly, other lasers have been used with success in several small case series; an 800-nm diode laser, intense pulsed light therapy, and a ruby laser have each demonstrated efficacy.14 Given these results, hair removal should be recommended to patients with HS. Military servicemembers (SMs) with certain conditions, such as polycystic ovary syndrome, pseudofolliculitis barbae, and HS, are eligible for laser hair removal when available at local military treatment facilities. Primary care providers for military SMs must have a working understanding of the disease process of HS and awareness of what resources are available for treatment, which allows for more streamlined care and improved outcomes.
Treatment Options
Treatment options are diverse and depend on the severity of HS. Typically, treatment begins with medical therapy followed by escalation to surgical intervention. Medical therapies often include antibiotics, acne treatments, antiandrogen therapy, immunosuppressive agents, and biologic therapy.15,16 If first-line medical interventions fail to control HS, surgical interventions should be considered. Surgical intervention in conjunction with medical therapy decreases the chance for recurrence.3,15,16
Although HS is internationally recognized as an inflammatory disease and not an infectious process, topical antibiotics can help to prevent and improve formation of abscesses, nodules, and pustules.11 Agents such as clindamycin and chlorhexidine wash have proven effective in preventing flares.11,16 Other antibiotics used alone or in combination also are efficacious. Tetracyclines are recommended as monotherapy for mild stages of HS.17-19 Doxycycline is the most commonly used tetracycline in HS patients and has been demonstrated to penetrate Staphylococcus aureus biofilm in high enough concentrations to maintain its antibacterial activity.20 Moreover, doxycycline, as with other tetracyclines, has a multitude of anti-inflammatory and immunomodulatory properties21 and can reduce the production of IL-1, IL-6, tumor necrosis factor α, and IL-8; downregulate chemotaxis; and promote lipo-oxygenase, matrix metalloproteinase, and nuclear factor κB (NF-κB) signaling inhibition.17
Clindamycin is the only known agent that has been studied for topical treatment and utilization in milder cases of HS.17,22 Systemic combination of clindamycin and rifampicin is the most studied, with well-established efficacy in managing HS.17,23,24 Clindamycin has bacteriostatic activity toward both aerobic and anaerobic gram-positive bacteria by binding irreversibly to the 50S ribosomal subunit, thereby inhibiting bacterial protein synthesis. Rifampicin binds to the beta subunit of DNA-dependent RNA polymerase, inhibiting bacterial DNA-dependent RNA synthesis. Rifampicin has broad-spectrum activity, mostly against gram-positive as well as some gram-negative bacteria. Moreover, rifampicin has anti-inflammatory and immunomodulatory properties, including evidence that it inhibits excessive helper T cell (TH17) responses by reducing inducible nitric oxide synthase transcription and NF-κB activity.25,26
Metronidazole, moxifloxacin, and rifampicin as triple combination therapy has been shown to be effective in reducing HS activity in moderate to severe cases that were refractory to other treatments.27 Research suggests that moxifloxacin has anti-inflammatory properties, mainly by reducing IL-1β, IL-8, and tumor necrosis factor α; stabilizing IXb protein; suppressing NF-κB signaling; and reducing IL-17A.28,29
Ertapenem can be utilized as a single 6-week antibiotic course during surgical planning or rescue therapy.18 Moreover, ertapenem can be used to treat complicated skin and soft tissue infections and has been shown to substantially improve clinical aspects of severe HS.17,27
Disease-modifying antirheumatic drugs are effective in the treatment of moderate to severe HS.17-19 In 2 phase 3 trials (PIONEER I and II), adalimumab was used as monotherapy or in conjunction with antibiotics in patients with moderate to severe HS compared to placebo.30 Results demonstrated a disease burden reduction of greater than 50%. Antibiotic dual therapy was not noted to significantly affect disease burden.30 Of note, use of immunosuppressants in the military affects an SM’s availability for worldwide deployment and duty station assignment.
Antiandrogen therapies have demonstrated some reduction in HS flares. Although recommendations for use in HS is based on limited evidence, one randomized controlled trial compared ethinyl estradiol–norgestrel to ethinyl estradiol and cyproterone acetate. Both therapies resulted in similar efficacy, with 12 of 24 (50%) patients reporting HS symptoms improving or completely resolved.31 In another retrospective study of women treated with antiandrogen therapies, including ethinyl estriol, cyproterone acetate, and spironolactone, 16 of 29 (55%) patients reported improvement.32 In another study, daily doses of 100 to 150 mg of spironolactone resulted in improvement in 17 of 20 (85%) patients, including complete remission in 11 of 20 (55%) patients. Of the 3 patients with severe HS, none had complete clearing of disease burden.33 Patients with polycystic ovary syndrome or HS flares that occur around menstruation are more likely to benefit from treatment with spironolactone.18,32,34
Retinoids frequently have been utilized in the management of HS. In some retrospective studies and other prospective studies with 5 or more patients, isotretinoin monotherapy was utilized for a 4- to 10-month period.18,35-38 In the Alikhan et al18 study, 85 of 207 patients demonstrated improvement of HS symptoms, with more remarkable improvements in milder cases. Isotretinoin for management of patients with HS who have concomitant nodulocystic acne would have two-fold benefits.18
Wound Care
Given the purulent nodular formation in HS, adequate wound care management is vital. There is an abundance of HS wound care management strategies utilized by clinicians and patients. When selecting the appropriate dressing, consideration for the type of HS wound, cost, ease of application, patient comfort, absorbency, and odor management is important.3 However, living arrangements for military SMs can create difficulties applying and maintaining HS dressings, especially if deployed or in a field setting. Active-duty SMs often find themselves in austere living conditions in the field, aboard ships, or in other scenarios where they may or may not have running water or showers. Maintaining adequate hygiene may be difficult, and additional education about how to keep wounds clean must be imparted. Ideal dressings for HS should be highly absorbent, comfortable when applied to the anatomic locations of the HS lesions, and easily self-applied. Ideally, dressings would have atraumatic adhesion and antimicrobial properties.3 Cost-effective dressing options that have good absorption capability include sanitary napkins, adult briefs, infant diapers, and gauze.3 These dressings help to wick moisture, thus protecting the wound from maceration, which is a common patient concern. Although gauze dressings are easier to obtain, they are not as absorbent. Abdominal pads can be utilized, but they are moderately absorbent, bulky, and more challenging to obtain over-the-counter. Hydrofiber and calcium alginate dressings with silver are not accessible to the common consumer and are more expensive than the aforementioned dressings, but they do have some antimicrobial activity. Silver-impregnated foam dressings are moldable to intertriginous areas, easy to self-apply, and have moderate-heavy absorption abilities.
Final Thoughts
Hidradenitis suppurativa poses cumbersome and uncomfortable symptoms for all patients and may pose additional hardships for military SMs or those with physically demanding occupations who work in austere environments. Severe HS can restrict a military SM from certain duty stations, positions, or deployments. Early identification of HS can help reduce HS flares, disfigurement, and placement on limited duty status, therefore rendering the SM more able to engage in his/her operational responsibilities. Hidradenitis suppurativa should be discussed with the patient, with the goal to prevent flares for SMs that will be in the field, placed in austere environments, or be deployed. Use of immunosuppressants in active-duty SMs may affect their deployability, duty assignment, and retention.
For a military SM with HS, all aspects of prevention and treatment need to be balanced with his/her ability to remain deployable and complete his/her daily duties. Military SMs are not guaranteed the ideal scenario for treatment and prevention of HS. Unsanitary environments and occlusive uniforms undoubtedly contribute to disease process and make treatment more challenging. If a military SM is in a field setting or deployed, frequent daily dressing changes should still be attempted.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221.
- Beshara MA. Hidradenitis suppurativa: a clinician’s tool for early diagnosis and treatment. Nurse Pract. 2010;35:24-28.
- Kazemi A, Carnaggio K, Clark M, et al. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat. 2017;29:165-167.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003:49:96-98.
- Blattner C, Polley DC, Ferrito F, et al. Central centrifugal cicatricial alopecia. Indian Dermatol Online J. 2013:4:50.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623.
- Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444.
- Alavi A, Anooshirvani N, Kim WB, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61-65.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed. New York, NY: Marcel Dekker; 1996:623-645.
- Kligman AM. Welcome letter. 2nd International Conference on the Sebaceous Gland, Acne, Rosacea and Related Disorders; September 13-16, 2008; Rome Italy.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Reports. 2019;11:8243.
- Sabat R, Tsaousi A, Rossbacher J, et al. Acne inversa/hidradenitis suppurativa: an update [in German]. Hautarzt. 2017;68:999-1006.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43.
- Hamzavi IH, Griffith JL, Riyaz F, et al. Laser and light-based treatment options for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S78-S81.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Michel C, DiBianco JM, Sabarwal V, et al. The treatment of genitoperineal hidradenitis suppurativa: a review of the literature. Urology. 2019;124:1-5.
- Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both [published online December 30, 2019]? Ther Adv Musculoskelet Dis. doi:10.1177/1759720x19895488.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Zouboulis CC, Desai N, Emtestam, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Mandell JB, Orr S, Koch J, et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604-1609.
- Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397-404.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154.
- Griffiths CEM. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154:977-978.
- Ma K, Chen X, Chen J-C, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem. 2016;139:1151-1162.
- Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396-398.
- Join-Lambert O, Coignard H, Jais J-P, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49-58.
- Choi J-H, Song M-J, Kim S-H, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704-3707.
- Weiss T, Shalit I, Blau H, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.” Antimicrob Agents Chemother. 2004;48:1974-1982.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131.
- Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
- Dicken CH, Powell ST, Spear KL. Evaluation of isotretinoin treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1984;11:500-502.
- Huang CM, Kirchof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125.
- Norris JF, Cunliffe WJ. Failure of treatment of familial widespread hidradenitis suppurativa with isotretinoin. Clin Exp Dermatol. 1986;11:579-583.
- Soria A, Canoui-Poitrine F, Wolkenstein P, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134-135.
Case Report
A 19-year-old female marine with a 10-year history of hidradenitis suppurativa (HS) presented with hyperpigmented nodules in the inguinal folds and a recurrent cyst in the right groin area of 2 to 3 weeks’ duration. She denied axillary or inframammary involvement. She underwent several incision and drainage procedures 1 year prior to her enlistment in the US Marine Corps at 18 years of age. She previously had been treated by dermatology with doxycycline 100-mg tablets twice daily, benzoyl peroxide wash 5% applied to affected areas and rinsed daily, and clindamycin solution 1% with minimal improvement. She denied smoking or alcohol intake and said she typically wore a loose-fitting uniform to work. As a marine, she was expected to participate in daily physical training and exercises with her military unit, during which she wore a standardized physical training uniform, including nylon shorts and a cotton T-shirt. She requested light duty—military duty status with physical limitations or restrictions—to avoid physical training that would cause further friction and irritation to the inguinal region.
Physical examination demonstrated a woman with Fitzpatrick skin type III and normal body mass index. There were hyperpigmented nodules and scarring in the inguinal folds, most consistent with Hurley stage 2. A single, 0.5-cm, draining lesion was visualized. No hyperhidrosis was noted. The patient was placed on light duty for 7 days, with physical training only at her own pace and discretion. Moreover, she was restricted from field training, rifle range training, and other situations where she may excessively sweat or not be able to adequately maintain personal hygiene. She was encouraged to continue clindamycin solution 1% to the affected area twice daily and was prescribed chlorhexidine solution 4% to use when washing affected areas in the shower. The patient also was referred to the dermatology department at the Naval Hospital Camp Pendleton (Oceanside, California), where she was treated with laser hair removal in the inguinal region, thus avoiding waxing and further aggravation of HS flares. Due to the combination of topical therapies along with laser hair removal and duty restrictions, the patient had a dramatic decrease in development of severe nodular lesions.
Comment
Presentation
Historically, the incidence of HS is estimated at 0.5% to 4% of the general population with female predominance.1 Predisposing factors include obesity, smoking, genetic predisposition to acne, apocrine duct obstruction, and secondary bacterial infection.2 During acute flares, patients generally present with tender subcutaneous nodules that drain malodorous purulent material.3,4 Acute flares are unpredictable, and patients deal with chronic, recurrent, draining wounds, leading to a poor quality of life with resulting physical, psychological, financial, social, and emotional distress.3-5 The negative impact of HS on a patient’s quality of life has been reported to be greater than other dermatologic conditions.6 Lesions can be particularly painful and can cause disfiguration to the surface of the skin.7 Lesion severity is described using the Hurley staging system. Patient quality of life is directly correlated with disease severity and Hurley stage. In stage 1, abscesses develop, but no sinus tracts or cicatrization is present. In stage 2, recurrent abscesses will form tracts and cicatrization. In stage 3, the abscesses become diffuse or near diffuse, with multiple interconnected tracts and abscesses across the entire area of the body.8,9
Severe or refractory HS within the physically active military population may require consideration of light or limited duty or even separation from service. Similarly, severe HS may pose challenges with other physically demanding occupations, such as the police force and firefighters.
Prevention Focus
Prevention of flares is key for patients with HS; secondary prevention aims to reduce impact of the disease or injury that has already occurred,10,11 which includes prevention of the infundibulofolliculitis from becoming a deep folliculitis, nodule, or fistula, as well as Hurley stage progression. Prompt diagnosis with appropriate treatment can decrease the severity of lesions, pain, and scarring. Globally, HS patients continue to experience considerable diagnostic delays of 8 to 12 years after onset of initial symptoms.11,12 Earlier accurate diagnosis and initiation of treatment from the primary care provider or general medical officer is imperative. Initial accurate management may help keep symptoms from progressing to more severe painful lesions. Similarly, patients should be educated on how to prevent HS flares. Patients should avoid known triggers, including smoking, obesity, sweating, mechanical irritation, stress, and poor hygiene.11
Shaving for hair reduction creates ingrown hair shafts, which may lead to folliculitis in mechanically stressed areas in skin folds, thus initiating the inflammatory cascade of HS.11,13 Therefore, shaving along with any other mechanical stress should be avoided in patients with HS. Laser hair removal has been shown to be quite helpful in both the prevention and treatment of HS. In one study, 22 patients with Hurley stage 2 to 3 disease were treated with an Nd:YAG laser once monthly. Results demonstrated a 65% decrease in disease severity after 3 monthly treatments.11 Similarly, other lasers have been used with success in several small case series; an 800-nm diode laser, intense pulsed light therapy, and a ruby laser have each demonstrated efficacy.14 Given these results, hair removal should be recommended to patients with HS. Military servicemembers (SMs) with certain conditions, such as polycystic ovary syndrome, pseudofolliculitis barbae, and HS, are eligible for laser hair removal when available at local military treatment facilities. Primary care providers for military SMs must have a working understanding of the disease process of HS and awareness of what resources are available for treatment, which allows for more streamlined care and improved outcomes.
Treatment Options
Treatment options are diverse and depend on the severity of HS. Typically, treatment begins with medical therapy followed by escalation to surgical intervention. Medical therapies often include antibiotics, acne treatments, antiandrogen therapy, immunosuppressive agents, and biologic therapy.15,16 If first-line medical interventions fail to control HS, surgical interventions should be considered. Surgical intervention in conjunction with medical therapy decreases the chance for recurrence.3,15,16
Although HS is internationally recognized as an inflammatory disease and not an infectious process, topical antibiotics can help to prevent and improve formation of abscesses, nodules, and pustules.11 Agents such as clindamycin and chlorhexidine wash have proven effective in preventing flares.11,16 Other antibiotics used alone or in combination also are efficacious. Tetracyclines are recommended as monotherapy for mild stages of HS.17-19 Doxycycline is the most commonly used tetracycline in HS patients and has been demonstrated to penetrate Staphylococcus aureus biofilm in high enough concentrations to maintain its antibacterial activity.20 Moreover, doxycycline, as with other tetracyclines, has a multitude of anti-inflammatory and immunomodulatory properties21 and can reduce the production of IL-1, IL-6, tumor necrosis factor α, and IL-8; downregulate chemotaxis; and promote lipo-oxygenase, matrix metalloproteinase, and nuclear factor κB (NF-κB) signaling inhibition.17
Clindamycin is the only known agent that has been studied for topical treatment and utilization in milder cases of HS.17,22 Systemic combination of clindamycin and rifampicin is the most studied, with well-established efficacy in managing HS.17,23,24 Clindamycin has bacteriostatic activity toward both aerobic and anaerobic gram-positive bacteria by binding irreversibly to the 50S ribosomal subunit, thereby inhibiting bacterial protein synthesis. Rifampicin binds to the beta subunit of DNA-dependent RNA polymerase, inhibiting bacterial DNA-dependent RNA synthesis. Rifampicin has broad-spectrum activity, mostly against gram-positive as well as some gram-negative bacteria. Moreover, rifampicin has anti-inflammatory and immunomodulatory properties, including evidence that it inhibits excessive helper T cell (TH17) responses by reducing inducible nitric oxide synthase transcription and NF-κB activity.25,26
Metronidazole, moxifloxacin, and rifampicin as triple combination therapy has been shown to be effective in reducing HS activity in moderate to severe cases that were refractory to other treatments.27 Research suggests that moxifloxacin has anti-inflammatory properties, mainly by reducing IL-1β, IL-8, and tumor necrosis factor α; stabilizing IXb protein; suppressing NF-κB signaling; and reducing IL-17A.28,29
Ertapenem can be utilized as a single 6-week antibiotic course during surgical planning or rescue therapy.18 Moreover, ertapenem can be used to treat complicated skin and soft tissue infections and has been shown to substantially improve clinical aspects of severe HS.17,27
Disease-modifying antirheumatic drugs are effective in the treatment of moderate to severe HS.17-19 In 2 phase 3 trials (PIONEER I and II), adalimumab was used as monotherapy or in conjunction with antibiotics in patients with moderate to severe HS compared to placebo.30 Results demonstrated a disease burden reduction of greater than 50%. Antibiotic dual therapy was not noted to significantly affect disease burden.30 Of note, use of immunosuppressants in the military affects an SM’s availability for worldwide deployment and duty station assignment.
Antiandrogen therapies have demonstrated some reduction in HS flares. Although recommendations for use in HS is based on limited evidence, one randomized controlled trial compared ethinyl estradiol–norgestrel to ethinyl estradiol and cyproterone acetate. Both therapies resulted in similar efficacy, with 12 of 24 (50%) patients reporting HS symptoms improving or completely resolved.31 In another retrospective study of women treated with antiandrogen therapies, including ethinyl estriol, cyproterone acetate, and spironolactone, 16 of 29 (55%) patients reported improvement.32 In another study, daily doses of 100 to 150 mg of spironolactone resulted in improvement in 17 of 20 (85%) patients, including complete remission in 11 of 20 (55%) patients. Of the 3 patients with severe HS, none had complete clearing of disease burden.33 Patients with polycystic ovary syndrome or HS flares that occur around menstruation are more likely to benefit from treatment with spironolactone.18,32,34
Retinoids frequently have been utilized in the management of HS. In some retrospective studies and other prospective studies with 5 or more patients, isotretinoin monotherapy was utilized for a 4- to 10-month period.18,35-38 In the Alikhan et al18 study, 85 of 207 patients demonstrated improvement of HS symptoms, with more remarkable improvements in milder cases. Isotretinoin for management of patients with HS who have concomitant nodulocystic acne would have two-fold benefits.18
Wound Care
Given the purulent nodular formation in HS, adequate wound care management is vital. There is an abundance of HS wound care management strategies utilized by clinicians and patients. When selecting the appropriate dressing, consideration for the type of HS wound, cost, ease of application, patient comfort, absorbency, and odor management is important.3 However, living arrangements for military SMs can create difficulties applying and maintaining HS dressings, especially if deployed or in a field setting. Active-duty SMs often find themselves in austere living conditions in the field, aboard ships, or in other scenarios where they may or may not have running water or showers. Maintaining adequate hygiene may be difficult, and additional education about how to keep wounds clean must be imparted. Ideal dressings for HS should be highly absorbent, comfortable when applied to the anatomic locations of the HS lesions, and easily self-applied. Ideally, dressings would have atraumatic adhesion and antimicrobial properties.3 Cost-effective dressing options that have good absorption capability include sanitary napkins, adult briefs, infant diapers, and gauze.3 These dressings help to wick moisture, thus protecting the wound from maceration, which is a common patient concern. Although gauze dressings are easier to obtain, they are not as absorbent. Abdominal pads can be utilized, but they are moderately absorbent, bulky, and more challenging to obtain over-the-counter. Hydrofiber and calcium alginate dressings with silver are not accessible to the common consumer and are more expensive than the aforementioned dressings, but they do have some antimicrobial activity. Silver-impregnated foam dressings are moldable to intertriginous areas, easy to self-apply, and have moderate-heavy absorption abilities.
Final Thoughts
Hidradenitis suppurativa poses cumbersome and uncomfortable symptoms for all patients and may pose additional hardships for military SMs or those with physically demanding occupations who work in austere environments. Severe HS can restrict a military SM from certain duty stations, positions, or deployments. Early identification of HS can help reduce HS flares, disfigurement, and placement on limited duty status, therefore rendering the SM more able to engage in his/her operational responsibilities. Hidradenitis suppurativa should be discussed with the patient, with the goal to prevent flares for SMs that will be in the field, placed in austere environments, or be deployed. Use of immunosuppressants in active-duty SMs may affect their deployability, duty assignment, and retention.
For a military SM with HS, all aspects of prevention and treatment need to be balanced with his/her ability to remain deployable and complete his/her daily duties. Military SMs are not guaranteed the ideal scenario for treatment and prevention of HS. Unsanitary environments and occlusive uniforms undoubtedly contribute to disease process and make treatment more challenging. If a military SM is in a field setting or deployed, frequent daily dressing changes should still be attempted.
Case Report
A 19-year-old female marine with a 10-year history of hidradenitis suppurativa (HS) presented with hyperpigmented nodules in the inguinal folds and a recurrent cyst in the right groin area of 2 to 3 weeks’ duration. She denied axillary or inframammary involvement. She underwent several incision and drainage procedures 1 year prior to her enlistment in the US Marine Corps at 18 years of age. She previously had been treated by dermatology with doxycycline 100-mg tablets twice daily, benzoyl peroxide wash 5% applied to affected areas and rinsed daily, and clindamycin solution 1% with minimal improvement. She denied smoking or alcohol intake and said she typically wore a loose-fitting uniform to work. As a marine, she was expected to participate in daily physical training and exercises with her military unit, during which she wore a standardized physical training uniform, including nylon shorts and a cotton T-shirt. She requested light duty—military duty status with physical limitations or restrictions—to avoid physical training that would cause further friction and irritation to the inguinal region.
Physical examination demonstrated a woman with Fitzpatrick skin type III and normal body mass index. There were hyperpigmented nodules and scarring in the inguinal folds, most consistent with Hurley stage 2. A single, 0.5-cm, draining lesion was visualized. No hyperhidrosis was noted. The patient was placed on light duty for 7 days, with physical training only at her own pace and discretion. Moreover, she was restricted from field training, rifle range training, and other situations where she may excessively sweat or not be able to adequately maintain personal hygiene. She was encouraged to continue clindamycin solution 1% to the affected area twice daily and was prescribed chlorhexidine solution 4% to use when washing affected areas in the shower. The patient also was referred to the dermatology department at the Naval Hospital Camp Pendleton (Oceanside, California), where she was treated with laser hair removal in the inguinal region, thus avoiding waxing and further aggravation of HS flares. Due to the combination of topical therapies along with laser hair removal and duty restrictions, the patient had a dramatic decrease in development of severe nodular lesions.
Comment
Presentation
Historically, the incidence of HS is estimated at 0.5% to 4% of the general population with female predominance.1 Predisposing factors include obesity, smoking, genetic predisposition to acne, apocrine duct obstruction, and secondary bacterial infection.2 During acute flares, patients generally present with tender subcutaneous nodules that drain malodorous purulent material.3,4 Acute flares are unpredictable, and patients deal with chronic, recurrent, draining wounds, leading to a poor quality of life with resulting physical, psychological, financial, social, and emotional distress.3-5 The negative impact of HS on a patient’s quality of life has been reported to be greater than other dermatologic conditions.6 Lesions can be particularly painful and can cause disfiguration to the surface of the skin.7 Lesion severity is described using the Hurley staging system. Patient quality of life is directly correlated with disease severity and Hurley stage. In stage 1, abscesses develop, but no sinus tracts or cicatrization is present. In stage 2, recurrent abscesses will form tracts and cicatrization. In stage 3, the abscesses become diffuse or near diffuse, with multiple interconnected tracts and abscesses across the entire area of the body.8,9
Severe or refractory HS within the physically active military population may require consideration of light or limited duty or even separation from service. Similarly, severe HS may pose challenges with other physically demanding occupations, such as the police force and firefighters.
Prevention Focus
Prevention of flares is key for patients with HS; secondary prevention aims to reduce impact of the disease or injury that has already occurred,10,11 which includes prevention of the infundibulofolliculitis from becoming a deep folliculitis, nodule, or fistula, as well as Hurley stage progression. Prompt diagnosis with appropriate treatment can decrease the severity of lesions, pain, and scarring. Globally, HS patients continue to experience considerable diagnostic delays of 8 to 12 years after onset of initial symptoms.11,12 Earlier accurate diagnosis and initiation of treatment from the primary care provider or general medical officer is imperative. Initial accurate management may help keep symptoms from progressing to more severe painful lesions. Similarly, patients should be educated on how to prevent HS flares. Patients should avoid known triggers, including smoking, obesity, sweating, mechanical irritation, stress, and poor hygiene.11
Shaving for hair reduction creates ingrown hair shafts, which may lead to folliculitis in mechanically stressed areas in skin folds, thus initiating the inflammatory cascade of HS.11,13 Therefore, shaving along with any other mechanical stress should be avoided in patients with HS. Laser hair removal has been shown to be quite helpful in both the prevention and treatment of HS. In one study, 22 patients with Hurley stage 2 to 3 disease were treated with an Nd:YAG laser once monthly. Results demonstrated a 65% decrease in disease severity after 3 monthly treatments.11 Similarly, other lasers have been used with success in several small case series; an 800-nm diode laser, intense pulsed light therapy, and a ruby laser have each demonstrated efficacy.14 Given these results, hair removal should be recommended to patients with HS. Military servicemembers (SMs) with certain conditions, such as polycystic ovary syndrome, pseudofolliculitis barbae, and HS, are eligible for laser hair removal when available at local military treatment facilities. Primary care providers for military SMs must have a working understanding of the disease process of HS and awareness of what resources are available for treatment, which allows for more streamlined care and improved outcomes.
Treatment Options
Treatment options are diverse and depend on the severity of HS. Typically, treatment begins with medical therapy followed by escalation to surgical intervention. Medical therapies often include antibiotics, acne treatments, antiandrogen therapy, immunosuppressive agents, and biologic therapy.15,16 If first-line medical interventions fail to control HS, surgical interventions should be considered. Surgical intervention in conjunction with medical therapy decreases the chance for recurrence.3,15,16
Although HS is internationally recognized as an inflammatory disease and not an infectious process, topical antibiotics can help to prevent and improve formation of abscesses, nodules, and pustules.11 Agents such as clindamycin and chlorhexidine wash have proven effective in preventing flares.11,16 Other antibiotics used alone or in combination also are efficacious. Tetracyclines are recommended as monotherapy for mild stages of HS.17-19 Doxycycline is the most commonly used tetracycline in HS patients and has been demonstrated to penetrate Staphylococcus aureus biofilm in high enough concentrations to maintain its antibacterial activity.20 Moreover, doxycycline, as with other tetracyclines, has a multitude of anti-inflammatory and immunomodulatory properties21 and can reduce the production of IL-1, IL-6, tumor necrosis factor α, and IL-8; downregulate chemotaxis; and promote lipo-oxygenase, matrix metalloproteinase, and nuclear factor κB (NF-κB) signaling inhibition.17
Clindamycin is the only known agent that has been studied for topical treatment and utilization in milder cases of HS.17,22 Systemic combination of clindamycin and rifampicin is the most studied, with well-established efficacy in managing HS.17,23,24 Clindamycin has bacteriostatic activity toward both aerobic and anaerobic gram-positive bacteria by binding irreversibly to the 50S ribosomal subunit, thereby inhibiting bacterial protein synthesis. Rifampicin binds to the beta subunit of DNA-dependent RNA polymerase, inhibiting bacterial DNA-dependent RNA synthesis. Rifampicin has broad-spectrum activity, mostly against gram-positive as well as some gram-negative bacteria. Moreover, rifampicin has anti-inflammatory and immunomodulatory properties, including evidence that it inhibits excessive helper T cell (TH17) responses by reducing inducible nitric oxide synthase transcription and NF-κB activity.25,26
Metronidazole, moxifloxacin, and rifampicin as triple combination therapy has been shown to be effective in reducing HS activity in moderate to severe cases that were refractory to other treatments.27 Research suggests that moxifloxacin has anti-inflammatory properties, mainly by reducing IL-1β, IL-8, and tumor necrosis factor α; stabilizing IXb protein; suppressing NF-κB signaling; and reducing IL-17A.28,29
Ertapenem can be utilized as a single 6-week antibiotic course during surgical planning or rescue therapy.18 Moreover, ertapenem can be used to treat complicated skin and soft tissue infections and has been shown to substantially improve clinical aspects of severe HS.17,27
Disease-modifying antirheumatic drugs are effective in the treatment of moderate to severe HS.17-19 In 2 phase 3 trials (PIONEER I and II), adalimumab was used as monotherapy or in conjunction with antibiotics in patients with moderate to severe HS compared to placebo.30 Results demonstrated a disease burden reduction of greater than 50%. Antibiotic dual therapy was not noted to significantly affect disease burden.30 Of note, use of immunosuppressants in the military affects an SM’s availability for worldwide deployment and duty station assignment.
Antiandrogen therapies have demonstrated some reduction in HS flares. Although recommendations for use in HS is based on limited evidence, one randomized controlled trial compared ethinyl estradiol–norgestrel to ethinyl estradiol and cyproterone acetate. Both therapies resulted in similar efficacy, with 12 of 24 (50%) patients reporting HS symptoms improving or completely resolved.31 In another retrospective study of women treated with antiandrogen therapies, including ethinyl estriol, cyproterone acetate, and spironolactone, 16 of 29 (55%) patients reported improvement.32 In another study, daily doses of 100 to 150 mg of spironolactone resulted in improvement in 17 of 20 (85%) patients, including complete remission in 11 of 20 (55%) patients. Of the 3 patients with severe HS, none had complete clearing of disease burden.33 Patients with polycystic ovary syndrome or HS flares that occur around menstruation are more likely to benefit from treatment with spironolactone.18,32,34
Retinoids frequently have been utilized in the management of HS. In some retrospective studies and other prospective studies with 5 or more patients, isotretinoin monotherapy was utilized for a 4- to 10-month period.18,35-38 In the Alikhan et al18 study, 85 of 207 patients demonstrated improvement of HS symptoms, with more remarkable improvements in milder cases. Isotretinoin for management of patients with HS who have concomitant nodulocystic acne would have two-fold benefits.18
Wound Care
Given the purulent nodular formation in HS, adequate wound care management is vital. There is an abundance of HS wound care management strategies utilized by clinicians and patients. When selecting the appropriate dressing, consideration for the type of HS wound, cost, ease of application, patient comfort, absorbency, and odor management is important.3 However, living arrangements for military SMs can create difficulties applying and maintaining HS dressings, especially if deployed or in a field setting. Active-duty SMs often find themselves in austere living conditions in the field, aboard ships, or in other scenarios where they may or may not have running water or showers. Maintaining adequate hygiene may be difficult, and additional education about how to keep wounds clean must be imparted. Ideal dressings for HS should be highly absorbent, comfortable when applied to the anatomic locations of the HS lesions, and easily self-applied. Ideally, dressings would have atraumatic adhesion and antimicrobial properties.3 Cost-effective dressing options that have good absorption capability include sanitary napkins, adult briefs, infant diapers, and gauze.3 These dressings help to wick moisture, thus protecting the wound from maceration, which is a common patient concern. Although gauze dressings are easier to obtain, they are not as absorbent. Abdominal pads can be utilized, but they are moderately absorbent, bulky, and more challenging to obtain over-the-counter. Hydrofiber and calcium alginate dressings with silver are not accessible to the common consumer and are more expensive than the aforementioned dressings, but they do have some antimicrobial activity. Silver-impregnated foam dressings are moldable to intertriginous areas, easy to self-apply, and have moderate-heavy absorption abilities.
Final Thoughts
Hidradenitis suppurativa poses cumbersome and uncomfortable symptoms for all patients and may pose additional hardships for military SMs or those with physically demanding occupations who work in austere environments. Severe HS can restrict a military SM from certain duty stations, positions, or deployments. Early identification of HS can help reduce HS flares, disfigurement, and placement on limited duty status, therefore rendering the SM more able to engage in his/her operational responsibilities. Hidradenitis suppurativa should be discussed with the patient, with the goal to prevent flares for SMs that will be in the field, placed in austere environments, or be deployed. Use of immunosuppressants in active-duty SMs may affect their deployability, duty assignment, and retention.
For a military SM with HS, all aspects of prevention and treatment need to be balanced with his/her ability to remain deployable and complete his/her daily duties. Military SMs are not guaranteed the ideal scenario for treatment and prevention of HS. Unsanitary environments and occlusive uniforms undoubtedly contribute to disease process and make treatment more challenging. If a military SM is in a field setting or deployed, frequent daily dressing changes should still be attempted.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221.
- Beshara MA. Hidradenitis suppurativa: a clinician’s tool for early diagnosis and treatment. Nurse Pract. 2010;35:24-28.
- Kazemi A, Carnaggio K, Clark M, et al. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat. 2017;29:165-167.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003:49:96-98.
- Blattner C, Polley DC, Ferrito F, et al. Central centrifugal cicatricial alopecia. Indian Dermatol Online J. 2013:4:50.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623.
- Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444.
- Alavi A, Anooshirvani N, Kim WB, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61-65.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed. New York, NY: Marcel Dekker; 1996:623-645.
- Kligman AM. Welcome letter. 2nd International Conference on the Sebaceous Gland, Acne, Rosacea and Related Disorders; September 13-16, 2008; Rome Italy.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Reports. 2019;11:8243.
- Sabat R, Tsaousi A, Rossbacher J, et al. Acne inversa/hidradenitis suppurativa: an update [in German]. Hautarzt. 2017;68:999-1006.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43.
- Hamzavi IH, Griffith JL, Riyaz F, et al. Laser and light-based treatment options for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S78-S81.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Michel C, DiBianco JM, Sabarwal V, et al. The treatment of genitoperineal hidradenitis suppurativa: a review of the literature. Urology. 2019;124:1-5.
- Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both [published online December 30, 2019]? Ther Adv Musculoskelet Dis. doi:10.1177/1759720x19895488.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Zouboulis CC, Desai N, Emtestam, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Mandell JB, Orr S, Koch J, et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604-1609.
- Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397-404.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154.
- Griffiths CEM. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154:977-978.
- Ma K, Chen X, Chen J-C, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem. 2016;139:1151-1162.
- Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396-398.
- Join-Lambert O, Coignard H, Jais J-P, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49-58.
- Choi J-H, Song M-J, Kim S-H, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704-3707.
- Weiss T, Shalit I, Blau H, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.” Antimicrob Agents Chemother. 2004;48:1974-1982.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131.
- Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
- Dicken CH, Powell ST, Spear KL. Evaluation of isotretinoin treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1984;11:500-502.
- Huang CM, Kirchof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125.
- Norris JF, Cunliffe WJ. Failure of treatment of familial widespread hidradenitis suppurativa with isotretinoin. Clin Exp Dermatol. 1986;11:579-583.
- Soria A, Canoui-Poitrine F, Wolkenstein P, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134-135.
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216-221.
- Beshara MA. Hidradenitis suppurativa: a clinician’s tool for early diagnosis and treatment. Nurse Pract. 2010;35:24-28.
- Kazemi A, Carnaggio K, Clark M, et al. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat. 2017;29:165-167.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003:49:96-98.
- Blattner C, Polley DC, Ferrito F, et al. Central centrifugal cicatricial alopecia. Indian Dermatol Online J. 2013:4:50.
- Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623.
- Smith HS, Chao JD, Teitelbaum J. Painful hidradenitis suppurativa. Clin J Pain. 2010;26:435-444.
- Alavi A, Anooshirvani N, Kim WB, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61-65.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed. New York, NY: Marcel Dekker; 1996:623-645.
- Kligman AM. Welcome letter. 2nd International Conference on the Sebaceous Gland, Acne, Rosacea and Related Disorders; September 13-16, 2008; Rome Italy.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Reports. 2019;11:8243.
- Sabat R, Tsaousi A, Rossbacher J, et al. Acne inversa/hidradenitis suppurativa: an update [in German]. Hautarzt. 2017;68:999-1006.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin. 2016;34:37-43.
- Hamzavi IH, Griffith JL, Riyaz F, et al. Laser and light-based treatment options for hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 suppl 1):S78-S81.
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032.
- Michel C, DiBianco JM, Sabarwal V, et al. The treatment of genitoperineal hidradenitis suppurativa: a review of the literature. Urology. 2019;124:1-5.
- Constantinou CA, Fragoulis GE, Nikiphorou E. Hidradenitis suppurativa: infection, autoimmunity, or both [published online December 30, 2019]? Ther Adv Musculoskelet Dis. doi:10.1177/1759720x19895488.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Zouboulis CC, Desai N, Emtestam, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619-644.
- Mandell JB, Orr S, Koch J, et al. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res. 2019;37:1604-1609.
- Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-induced cytokines and chemokines in THP-1 cells via ERK, p38, and nuclear factor-κB signaling pathways. Biochem Biophys Rep. 2015;4:397-404.
- Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325-328.
- Gener G, Canoui-Poitrine F, Revuz JE, et al. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatology. 2009;219:148-154.
- Griffiths CEM. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br J Dermatol. 2006;154:977-978.
- Ma K, Chen X, Chen J-C, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem. 2016;139:1151-1162.
- Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother. 2006;50:396-398.
- Join-Lambert O, Coignard H, Jais J-P, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49-58.
- Choi J-H, Song M-J, Kim S-H, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2003;47:3704-3707.
- Weiss T, Shalit I, Blau H, et al. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.” Antimicrob Agents Chemother. 2004;48:1974-1982.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Mortimer PS, Dawber RP, Gales MA, et al. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol. 1986;115:263-268.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg. 2007;11:125-131.
- Lee A, Fischer G. A case series of 20 women with hidradenitis suppurativa treated with spironolactone. Australas J Dermatol. 2015;56:192-196.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a “male” therapy for a predominantly “female” disease. J Clin Aesthet Dermatol. 2016;9:44-50.
- Dicken CH, Powell ST, Spear KL. Evaluation of isotretinoin treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1984;11:500-502.
- Huang CM, Kirchof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology. 2017;233:120-125.
- Norris JF, Cunliffe WJ. Failure of treatment of familial widespread hidradenitis suppurativa with isotretinoin. Clin Exp Dermatol. 1986;11:579-583.
- Soria A, Canoui-Poitrine F, Wolkenstein P, et al. Absence of efficacy of oral isotretinoin in hidradenitis suppurativa: a retrospective study based on patients’ outcome assessment. Dermatology. 2009;218:134-135.
Practice Points
- Hidradenitis suppurativa (HS) can be more difficult to treat in physically active military servicemembers (SMs).
- Patient education and primary care physician awareness of HS is critical to initial diagnosis and long-term management.
- Primary care physician knowledge of HS as well as an understanding of the capabilities at local military medical facilities is important for optimal treatment of HS in military SMs.
Human Papillomavirus Vaccination in LGBTQ Patients: The Need for Dermatologists on the Front Lines
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States. It is the causative agent of genital warts, as well as cervical, anal, penile, vulvar, vaginal, and some head and neck cancers.1 Development of the HPV vaccine and its introduction into the scheduled vaccine series recommended by the Centers for Disease Control and Prevention (CDC) represented a major public health milestone. The CDC recommends the HPV vaccine for all children beginning at 11 or 12 years of age, even as early as 9 years, regardless of gender identity or sexuality. As of late 2016, the 9-valent formulation (Gardasil 9 [Merck]) is the only HPV vaccine distributed in the United States, and the vaccination schedule depends specifically on age. The Advisory Committee on Immunization Practices (ACIP) of the CDC revised its recommendations in 2019 to include “shared clinical decision-making regarding HPV vaccination . . . for some adults aged 27 through 45 years.”2 This change in policy has notable implications for sexual and gender minority populations, such as lesbian, gay, bisexual, transgender, and queer or questioning (LGBTQ) patients, especially in the context of dermatologic care. Herein, we discuss HPV-related conditions for LGBTQ patients, barriers to vaccine administration, and the role of dermatologists in promoting an increased vaccination rate in the LGBTQ community.
HPV-Related Conditions
A 2019 review of dermatologic care for LGBTQ patients identified many specific health disparities of HPV.3 Specifically, men who have sex with men (MSM) are more likely than heterosexual men to have oral, anal, and penile HPV infections, including high-risk HPV types.3 From 2011 to 2014, 18% and 13% of MSM had oral HPV infection and high-risk oral HPV infection, respectively, compared to only 11% and 7%, respectively, of men who reported never having had a same-sex sexual partner.4
Similarly, despite the CDC’s position that patients with perianal warts might benefit from digital anal examination or referral for standard or high-resolution anoscopy to detect intra-anal warts, improvements in morbidity have not yet been realized. In 2017, anal cancer incidence was 45.9 cases for every 100,000 person-years among human immunodeficiency (HIV)–positive MSM and 5.1 cases for every 100,000 person-years among HIV-negative MSM vs only 1.5 cases for every 100,000 person-years among men in the United States overall.3 Yet the CDC states that there is insufficient evidence to recommend routine anal cancer screening among MSM, even when a patient is HIV positive. Therefore, current screening practices and treatments are insufficient as MSM continue to have a disproportionately higher rate of HPV-associated disease compared to other populations.
Barriers to HPV Vaccine Administration
The HPV vaccination rate among MSM in adolescent populations varies across reports.5-7 Interestingly, a 2016 survey study found that MSM had approximately 2-times greater odds of initiating the HPV vaccine than heterosexual men.8 However, a study specifically sampling young gay and bisexual men (N=428) found that only 13% had received any doses of the HPV vaccine.6
Regardless, HPV vaccination is much less common among all males than it is among all females, and the low rate of vaccination among sexual minority men has a disproportionate impact, given their higher risk for HPV infection.4 Although the HPV vaccination rate increased from 2014 to 2017, the HPV vaccination rate in MSM overall is less than half of the Healthy People 2020 goal of 80%.9 A 2018 review determined that HPV vaccination is a cost-effective strategy for preventing anal cancer in MSM10; yet male patients might still view the HPV vaccine as a “women’s issue” and are less likely to be vaccinated if they are not prompted by health care providers. Additionally, HPV vaccination is remarkably less likely in MSM when patients are older, uninsured, of lower socioeconomic status, or have not disclosed their sexual identity to their health care provider.9 Dermatologists should be mindful of these barriers to promote HPV vaccination in MSM before, or soon after, sexual debut.
Other members of the LGBTQ community, such as women who have sex with women, face notable HPV-related health disparities and would benefit from increased vaccination efforts by dermatologists. Adolescent and young adult women who have sex with women are less likely than heterosexual adolescent and young adult women to receive routine Papanicolaou tests and initiate HPV vaccination, despite having a higher number of lifetime sexual partners and a higher risk for HPV exposure.11 A 2015 survey study (N=3253) found that after adjusting for covariates, only 8.5% of lesbians and 33.2% of bisexual women and girls who had heard of the HPV vaccine had initiated vaccination compared to 28.4% of their heterosexual counterparts.11 The HPV vaccine is an effective public health tool for the prevention of cervical cancer in these populations. A study of women aged 15 to 19 years in the HPV vaccination era (2007-2014) found significant (P<.05) observed population-level decreases in cervical intraepithelial neoplasia incidence across all grades.12
Transgender women also face a high rate of HPV infection, HIV infection, and other structural and financial disparities, such as low insurance coverage, that can limit their access to vaccination. Transgender men have a higher rate of HPV infection than cisgender men, and those with female internal reproductive organs are less likely to receive routine Papanicolaou tests. A 2018 survey study found that approximately one-third of transgender men and women reported initiating the HPV vaccination series,13 but further investigation is required to make balanced comparisons to cisgender patients.
The Role of the Dermatologist
Collectively, these disparities emphasize the need for increased involvement by dermatologists in HPV vaccination efforts for all LGBTQ patients. Adult patients may have concerns about ties of the HPV vaccine to drug manufacturers and the general safety of vaccination. For pediatric patients, parents/guardians also may be concerned about an assumed but not evidence-based increase in sexual promiscuity following HPV vaccination.14 These topics can be challenging to discuss, but dermatologists have the duty to be proactive and initiate conversation about HPV vaccination, as opposed to waiting for patients to express interest. Dermatologists should stress the safety of the vaccine as well as its potential to protect against multiple, even life-threatening diseases. Providers also can explain that the ACIP recommends catch-up vaccination for all individuals through 26 years of age, regardless of sexual orientation or gender identity.
With the ACIP having recently expanded the appropriate age range for HPV vaccination, we encourage dermatologists to engage in education and shared decision-making to ensure that adult patients with specific risk factors receive the HPV vaccine. Because the expanded ACIP recommendations are aimed at vaccination before HPV exposure, vaccination might not be appropriate for all LGBTQ patients. However, eliciting a sexual history with routine patient intake forms or during the clinical encounter ensures equal access to the HPV vaccine.
Greater awareness of HPV-related disparities and barriers to vaccination in LGBTQ populations has the potential to notably decrease HPV-associated mortality and morbidity. Increased involvement by dermatologists contributes to the efforts of other specialties in universal HPV vaccination, regardless of sexual orientation or gender identity—ideally in younger age groups, such that patients receive the vaccine prior to coitarche.
There are many ways that dermatologists can advocate for HPV vaccination. Those in a multispecialty or academic practice can readily refer patients to an associated internist, primary care physician, or vaccination clinic in the same building or institution. Dermatologists in private practice might be able to administer the HPV vaccine themselves or can advocate for patients to receive the vaccine at a local facility of the Department of Health or at a nonprofit organization, such as a Planned Parenthood center. Although pediatricians and family physicians remain front-line providers of these services, dermatologists represent an additional member of a patient’s care team, capable of advocating for this important intervention.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80-85.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714-724.
- Kosche C, Mansh M, Luskus M, et al. Dermatologic care of sexual and gender minority/LGBTQIA youth, part 2: recognition and management of the unique dermatologic needs of SGM adolescents. Pediatr Dermatol. 2019;35:587-593.
- Reiter PL, McRee A-L, Katz ML, et al. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96-102.
- Charlton BM, Reisner SL, Ag
énor M, et al. Sexual orientation disparities in human papillomavirus vaccination in a longitudinal cohort of U.S. males and females. LGBT Health. 2017;4:202-209. - Agénor M, Peitzmeier SM, Gordon AR, et al. Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control. 2016;27:1187-1196.
- Loretan C, Chamberlain AT, Sanchez T, et al. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465-473.
- Setiawan D, Wondimu A, Ong K, et al. Cost effectiveness of human papillomavirus vaccination for men who have sex with men; reviewing the available evidence. Pharmacoeconomics. 2018;36:929-939.
- Agénor M, Peitzmeier S, Gordon AR, et al. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med. 2015;163:99-106.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3:833-837.
- McRee A-L, Gower AL, Reiter PL. Preventive healthcare services use among transgender young adults. Int J Transgend. 2018;19:417-423.
- Trinidad J. Policy focus: promoting human papilloma virus vaccine to prevent genital warts and cancer. Boston, MA: The Fenway Institute; 2012. https://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_HPV_v4_10.09.12.pdf. Accessed September 15, 2020.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States. It is the causative agent of genital warts, as well as cervical, anal, penile, vulvar, vaginal, and some head and neck cancers.1 Development of the HPV vaccine and its introduction into the scheduled vaccine series recommended by the Centers for Disease Control and Prevention (CDC) represented a major public health milestone. The CDC recommends the HPV vaccine for all children beginning at 11 or 12 years of age, even as early as 9 years, regardless of gender identity or sexuality. As of late 2016, the 9-valent formulation (Gardasil 9 [Merck]) is the only HPV vaccine distributed in the United States, and the vaccination schedule depends specifically on age. The Advisory Committee on Immunization Practices (ACIP) of the CDC revised its recommendations in 2019 to include “shared clinical decision-making regarding HPV vaccination . . . for some adults aged 27 through 45 years.”2 This change in policy has notable implications for sexual and gender minority populations, such as lesbian, gay, bisexual, transgender, and queer or questioning (LGBTQ) patients, especially in the context of dermatologic care. Herein, we discuss HPV-related conditions for LGBTQ patients, barriers to vaccine administration, and the role of dermatologists in promoting an increased vaccination rate in the LGBTQ community.
HPV-Related Conditions
A 2019 review of dermatologic care for LGBTQ patients identified many specific health disparities of HPV.3 Specifically, men who have sex with men (MSM) are more likely than heterosexual men to have oral, anal, and penile HPV infections, including high-risk HPV types.3 From 2011 to 2014, 18% and 13% of MSM had oral HPV infection and high-risk oral HPV infection, respectively, compared to only 11% and 7%, respectively, of men who reported never having had a same-sex sexual partner.4
Similarly, despite the CDC’s position that patients with perianal warts might benefit from digital anal examination or referral for standard or high-resolution anoscopy to detect intra-anal warts, improvements in morbidity have not yet been realized. In 2017, anal cancer incidence was 45.9 cases for every 100,000 person-years among human immunodeficiency (HIV)–positive MSM and 5.1 cases for every 100,000 person-years among HIV-negative MSM vs only 1.5 cases for every 100,000 person-years among men in the United States overall.3 Yet the CDC states that there is insufficient evidence to recommend routine anal cancer screening among MSM, even when a patient is HIV positive. Therefore, current screening practices and treatments are insufficient as MSM continue to have a disproportionately higher rate of HPV-associated disease compared to other populations.
Barriers to HPV Vaccine Administration
The HPV vaccination rate among MSM in adolescent populations varies across reports.5-7 Interestingly, a 2016 survey study found that MSM had approximately 2-times greater odds of initiating the HPV vaccine than heterosexual men.8 However, a study specifically sampling young gay and bisexual men (N=428) found that only 13% had received any doses of the HPV vaccine.6
Regardless, HPV vaccination is much less common among all males than it is among all females, and the low rate of vaccination among sexual minority men has a disproportionate impact, given their higher risk for HPV infection.4 Although the HPV vaccination rate increased from 2014 to 2017, the HPV vaccination rate in MSM overall is less than half of the Healthy People 2020 goal of 80%.9 A 2018 review determined that HPV vaccination is a cost-effective strategy for preventing anal cancer in MSM10; yet male patients might still view the HPV vaccine as a “women’s issue” and are less likely to be vaccinated if they are not prompted by health care providers. Additionally, HPV vaccination is remarkably less likely in MSM when patients are older, uninsured, of lower socioeconomic status, or have not disclosed their sexual identity to their health care provider.9 Dermatologists should be mindful of these barriers to promote HPV vaccination in MSM before, or soon after, sexual debut.
Other members of the LGBTQ community, such as women who have sex with women, face notable HPV-related health disparities and would benefit from increased vaccination efforts by dermatologists. Adolescent and young adult women who have sex with women are less likely than heterosexual adolescent and young adult women to receive routine Papanicolaou tests and initiate HPV vaccination, despite having a higher number of lifetime sexual partners and a higher risk for HPV exposure.11 A 2015 survey study (N=3253) found that after adjusting for covariates, only 8.5% of lesbians and 33.2% of bisexual women and girls who had heard of the HPV vaccine had initiated vaccination compared to 28.4% of their heterosexual counterparts.11 The HPV vaccine is an effective public health tool for the prevention of cervical cancer in these populations. A study of women aged 15 to 19 years in the HPV vaccination era (2007-2014) found significant (P<.05) observed population-level decreases in cervical intraepithelial neoplasia incidence across all grades.12
Transgender women also face a high rate of HPV infection, HIV infection, and other structural and financial disparities, such as low insurance coverage, that can limit their access to vaccination. Transgender men have a higher rate of HPV infection than cisgender men, and those with female internal reproductive organs are less likely to receive routine Papanicolaou tests. A 2018 survey study found that approximately one-third of transgender men and women reported initiating the HPV vaccination series,13 but further investigation is required to make balanced comparisons to cisgender patients.
The Role of the Dermatologist
Collectively, these disparities emphasize the need for increased involvement by dermatologists in HPV vaccination efforts for all LGBTQ patients. Adult patients may have concerns about ties of the HPV vaccine to drug manufacturers and the general safety of vaccination. For pediatric patients, parents/guardians also may be concerned about an assumed but not evidence-based increase in sexual promiscuity following HPV vaccination.14 These topics can be challenging to discuss, but dermatologists have the duty to be proactive and initiate conversation about HPV vaccination, as opposed to waiting for patients to express interest. Dermatologists should stress the safety of the vaccine as well as its potential to protect against multiple, even life-threatening diseases. Providers also can explain that the ACIP recommends catch-up vaccination for all individuals through 26 years of age, regardless of sexual orientation or gender identity.
With the ACIP having recently expanded the appropriate age range for HPV vaccination, we encourage dermatologists to engage in education and shared decision-making to ensure that adult patients with specific risk factors receive the HPV vaccine. Because the expanded ACIP recommendations are aimed at vaccination before HPV exposure, vaccination might not be appropriate for all LGBTQ patients. However, eliciting a sexual history with routine patient intake forms or during the clinical encounter ensures equal access to the HPV vaccine.
Greater awareness of HPV-related disparities and barriers to vaccination in LGBTQ populations has the potential to notably decrease HPV-associated mortality and morbidity. Increased involvement by dermatologists contributes to the efforts of other specialties in universal HPV vaccination, regardless of sexual orientation or gender identity—ideally in younger age groups, such that patients receive the vaccine prior to coitarche.
There are many ways that dermatologists can advocate for HPV vaccination. Those in a multispecialty or academic practice can readily refer patients to an associated internist, primary care physician, or vaccination clinic in the same building or institution. Dermatologists in private practice might be able to administer the HPV vaccine themselves or can advocate for patients to receive the vaccine at a local facility of the Department of Health or at a nonprofit organization, such as a Planned Parenthood center. Although pediatricians and family physicians remain front-line providers of these services, dermatologists represent an additional member of a patient’s care team, capable of advocating for this important intervention.
Human papillomavirus (HPV) is one of the most common sexually transmitted infections in the United States. It is the causative agent of genital warts, as well as cervical, anal, penile, vulvar, vaginal, and some head and neck cancers.1 Development of the HPV vaccine and its introduction into the scheduled vaccine series recommended by the Centers for Disease Control and Prevention (CDC) represented a major public health milestone. The CDC recommends the HPV vaccine for all children beginning at 11 or 12 years of age, even as early as 9 years, regardless of gender identity or sexuality. As of late 2016, the 9-valent formulation (Gardasil 9 [Merck]) is the only HPV vaccine distributed in the United States, and the vaccination schedule depends specifically on age. The Advisory Committee on Immunization Practices (ACIP) of the CDC revised its recommendations in 2019 to include “shared clinical decision-making regarding HPV vaccination . . . for some adults aged 27 through 45 years.”2 This change in policy has notable implications for sexual and gender minority populations, such as lesbian, gay, bisexual, transgender, and queer or questioning (LGBTQ) patients, especially in the context of dermatologic care. Herein, we discuss HPV-related conditions for LGBTQ patients, barriers to vaccine administration, and the role of dermatologists in promoting an increased vaccination rate in the LGBTQ community.
HPV-Related Conditions
A 2019 review of dermatologic care for LGBTQ patients identified many specific health disparities of HPV.3 Specifically, men who have sex with men (MSM) are more likely than heterosexual men to have oral, anal, and penile HPV infections, including high-risk HPV types.3 From 2011 to 2014, 18% and 13% of MSM had oral HPV infection and high-risk oral HPV infection, respectively, compared to only 11% and 7%, respectively, of men who reported never having had a same-sex sexual partner.4
Similarly, despite the CDC’s position that patients with perianal warts might benefit from digital anal examination or referral for standard or high-resolution anoscopy to detect intra-anal warts, improvements in morbidity have not yet been realized. In 2017, anal cancer incidence was 45.9 cases for every 100,000 person-years among human immunodeficiency (HIV)–positive MSM and 5.1 cases for every 100,000 person-years among HIV-negative MSM vs only 1.5 cases for every 100,000 person-years among men in the United States overall.3 Yet the CDC states that there is insufficient evidence to recommend routine anal cancer screening among MSM, even when a patient is HIV positive. Therefore, current screening practices and treatments are insufficient as MSM continue to have a disproportionately higher rate of HPV-associated disease compared to other populations.
Barriers to HPV Vaccine Administration
The HPV vaccination rate among MSM in adolescent populations varies across reports.5-7 Interestingly, a 2016 survey study found that MSM had approximately 2-times greater odds of initiating the HPV vaccine than heterosexual men.8 However, a study specifically sampling young gay and bisexual men (N=428) found that only 13% had received any doses of the HPV vaccine.6
Regardless, HPV vaccination is much less common among all males than it is among all females, and the low rate of vaccination among sexual minority men has a disproportionate impact, given their higher risk for HPV infection.4 Although the HPV vaccination rate increased from 2014 to 2017, the HPV vaccination rate in MSM overall is less than half of the Healthy People 2020 goal of 80%.9 A 2018 review determined that HPV vaccination is a cost-effective strategy for preventing anal cancer in MSM10; yet male patients might still view the HPV vaccine as a “women’s issue” and are less likely to be vaccinated if they are not prompted by health care providers. Additionally, HPV vaccination is remarkably less likely in MSM when patients are older, uninsured, of lower socioeconomic status, or have not disclosed their sexual identity to their health care provider.9 Dermatologists should be mindful of these barriers to promote HPV vaccination in MSM before, or soon after, sexual debut.
Other members of the LGBTQ community, such as women who have sex with women, face notable HPV-related health disparities and would benefit from increased vaccination efforts by dermatologists. Adolescent and young adult women who have sex with women are less likely than heterosexual adolescent and young adult women to receive routine Papanicolaou tests and initiate HPV vaccination, despite having a higher number of lifetime sexual partners and a higher risk for HPV exposure.11 A 2015 survey study (N=3253) found that after adjusting for covariates, only 8.5% of lesbians and 33.2% of bisexual women and girls who had heard of the HPV vaccine had initiated vaccination compared to 28.4% of their heterosexual counterparts.11 The HPV vaccine is an effective public health tool for the prevention of cervical cancer in these populations. A study of women aged 15 to 19 years in the HPV vaccination era (2007-2014) found significant (P<.05) observed population-level decreases in cervical intraepithelial neoplasia incidence across all grades.12
Transgender women also face a high rate of HPV infection, HIV infection, and other structural and financial disparities, such as low insurance coverage, that can limit their access to vaccination. Transgender men have a higher rate of HPV infection than cisgender men, and those with female internal reproductive organs are less likely to receive routine Papanicolaou tests. A 2018 survey study found that approximately one-third of transgender men and women reported initiating the HPV vaccination series,13 but further investigation is required to make balanced comparisons to cisgender patients.
The Role of the Dermatologist
Collectively, these disparities emphasize the need for increased involvement by dermatologists in HPV vaccination efforts for all LGBTQ patients. Adult patients may have concerns about ties of the HPV vaccine to drug manufacturers and the general safety of vaccination. For pediatric patients, parents/guardians also may be concerned about an assumed but not evidence-based increase in sexual promiscuity following HPV vaccination.14 These topics can be challenging to discuss, but dermatologists have the duty to be proactive and initiate conversation about HPV vaccination, as opposed to waiting for patients to express interest. Dermatologists should stress the safety of the vaccine as well as its potential to protect against multiple, even life-threatening diseases. Providers also can explain that the ACIP recommends catch-up vaccination for all individuals through 26 years of age, regardless of sexual orientation or gender identity.
With the ACIP having recently expanded the appropriate age range for HPV vaccination, we encourage dermatologists to engage in education and shared decision-making to ensure that adult patients with specific risk factors receive the HPV vaccine. Because the expanded ACIP recommendations are aimed at vaccination before HPV exposure, vaccination might not be appropriate for all LGBTQ patients. However, eliciting a sexual history with routine patient intake forms or during the clinical encounter ensures equal access to the HPV vaccine.
Greater awareness of HPV-related disparities and barriers to vaccination in LGBTQ populations has the potential to notably decrease HPV-associated mortality and morbidity. Increased involvement by dermatologists contributes to the efforts of other specialties in universal HPV vaccination, regardless of sexual orientation or gender identity—ideally in younger age groups, such that patients receive the vaccine prior to coitarche.
There are many ways that dermatologists can advocate for HPV vaccination. Those in a multispecialty or academic practice can readily refer patients to an associated internist, primary care physician, or vaccination clinic in the same building or institution. Dermatologists in private practice might be able to administer the HPV vaccine themselves or can advocate for patients to receive the vaccine at a local facility of the Department of Health or at a nonprofit organization, such as a Planned Parenthood center. Although pediatricians and family physicians remain front-line providers of these services, dermatologists represent an additional member of a patient’s care team, capable of advocating for this important intervention.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80-85.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714-724.
- Kosche C, Mansh M, Luskus M, et al. Dermatologic care of sexual and gender minority/LGBTQIA youth, part 2: recognition and management of the unique dermatologic needs of SGM adolescents. Pediatr Dermatol. 2019;35:587-593.
- Reiter PL, McRee A-L, Katz ML, et al. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96-102.
- Charlton BM, Reisner SL, Ag
énor M, et al. Sexual orientation disparities in human papillomavirus vaccination in a longitudinal cohort of U.S. males and females. LGBT Health. 2017;4:202-209. - Agénor M, Peitzmeier SM, Gordon AR, et al. Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control. 2016;27:1187-1196.
- Loretan C, Chamberlain AT, Sanchez T, et al. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465-473.
- Setiawan D, Wondimu A, Ong K, et al. Cost effectiveness of human papillomavirus vaccination for men who have sex with men; reviewing the available evidence. Pharmacoeconomics. 2018;36:929-939.
- Agénor M, Peitzmeier S, Gordon AR, et al. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med. 2015;163:99-106.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3:833-837.
- McRee A-L, Gower AL, Reiter PL. Preventive healthcare services use among transgender young adults. Int J Transgend. 2018;19:417-423.
- Trinidad J. Policy focus: promoting human papilloma virus vaccine to prevent genital warts and cancer. Boston, MA: The Fenway Institute; 2012. https://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_HPV_v4_10.09.12.pdf. Accessed September 15, 2020.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80-85.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Sonawane K, Suk R, Chiao EY, et al. Oral human papillomavirus infection: differences in prevalence between sexes and concordance with genital human papillomavirus infection, NHANES 2011 to 2014. Ann Intern Med. 2017;167:714-724.
- Kosche C, Mansh M, Luskus M, et al. Dermatologic care of sexual and gender minority/LGBTQIA youth, part 2: recognition and management of the unique dermatologic needs of SGM adolescents. Pediatr Dermatol. 2019;35:587-593.
- Reiter PL, McRee A-L, Katz ML, et al. Human papillomavirus vaccination among young adult gay and bisexual men in the United States. Am J Public Health. 2015;105:96-102.
- Charlton BM, Reisner SL, Ag
énor M, et al. Sexual orientation disparities in human papillomavirus vaccination in a longitudinal cohort of U.S. males and females. LGBT Health. 2017;4:202-209. - Agénor M, Peitzmeier SM, Gordon AR, et al. Sexual orientation identity disparities in human papillomavirus vaccination initiation and completion among young adult US women and men. Cancer Causes Control. 2016;27:1187-1196.
- Loretan C, Chamberlain AT, Sanchez T, et al. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014-2017. Sex Transm Dis. 2019;46:465-473.
- Setiawan D, Wondimu A, Ong K, et al. Cost effectiveness of human papillomavirus vaccination for men who have sex with men; reviewing the available evidence. Pharmacoeconomics. 2018;36:929-939.
- Agénor M, Peitzmeier S, Gordon AR, et al. Sexual orientation identity disparities in awareness and initiation of the human papillomavirus vaccine among U.S. women and girls: a national survey. Ann Intern Med. 2015;163:99-106.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3:833-837.
- McRee A-L, Gower AL, Reiter PL. Preventive healthcare services use among transgender young adults. Int J Transgend. 2018;19:417-423.
- Trinidad J. Policy focus: promoting human papilloma virus vaccine to prevent genital warts and cancer. Boston, MA: The Fenway Institute; 2012. https://fenwayhealth.org/documents/the-fenway-institute/policy-briefs/PolicyFocus_HPV_v4_10.09.12.pdf. Accessed September 15, 2020.
An Unusual Skin Infection With Achromobacter xylosoxidans
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.
The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).

The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.

The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).


The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.

The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).


The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
Practice Points
- Achromobacter xylosoxidans is an emerging pathogen primarily in the immunocompromised patient.
- Achromobacter xylosoxidans can form biofilms on plastics treated with disinfectant solution, including medical products.
- Strains of A xylosoxidans have shown multiantibiotic resistance.
Cutaneous Leishmaniasis Successfully Treated With Miltefosine
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.
Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.
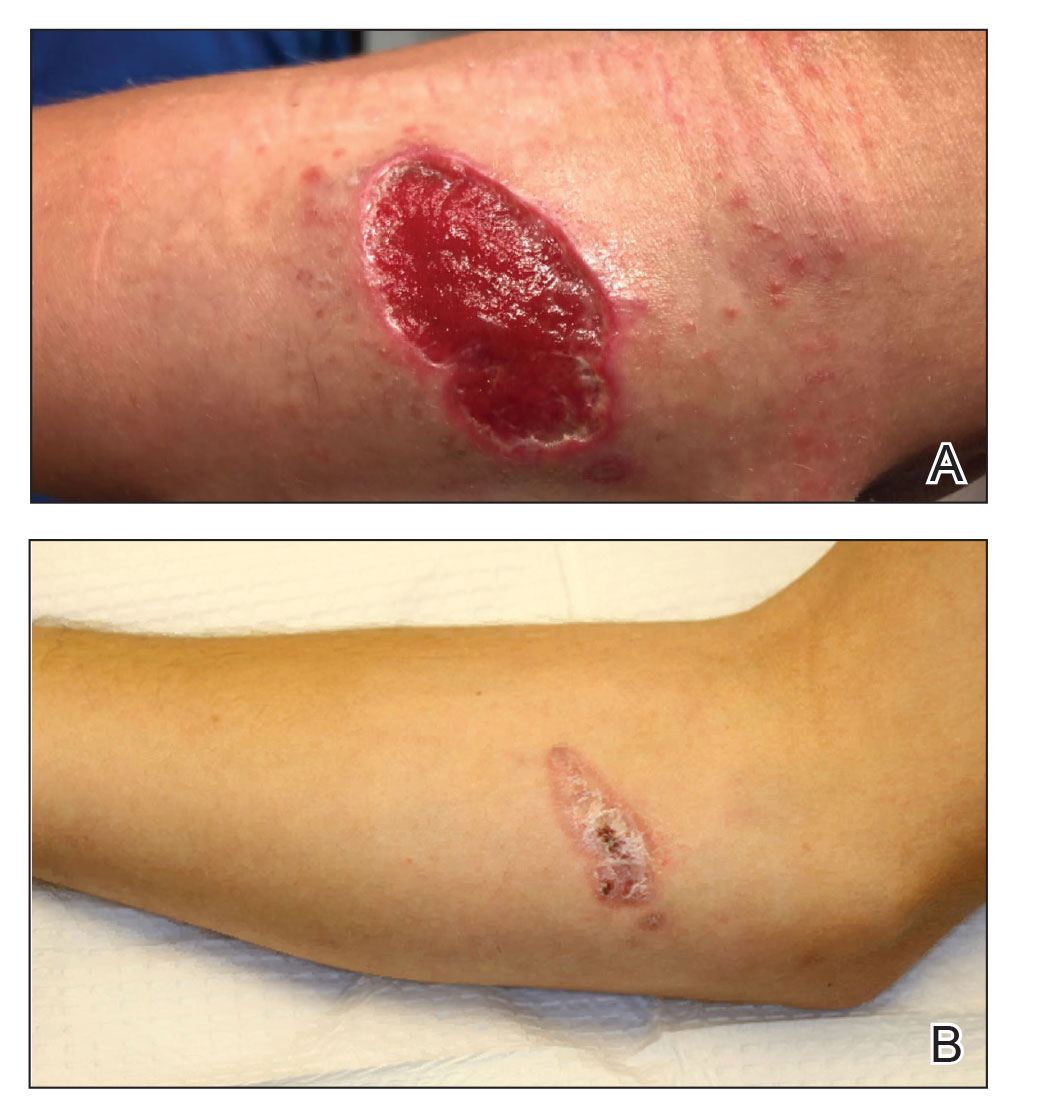
Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Practice Points
- Avoiding phlebotomine sand fly vector bites is the most effective way to prevent leishmaniasis.
- Prompt diagnosis and treatment of cutaneous leishmaniasis caused by Leishmania species that have potential for mucocutaneous spread are key to limiting morbidity and mortality.
- Partnering with the Centers for Disease Control and Prevention is critical for timely diagnosis.
- Miltefosine should be considered as a first-line agent for cutaneous leishmaniasis given its efficacy, tolerability, and ease of administration.
ECTRIMS 2020 Highlights: Managing RRMS, Symptoms in the Time of COVID-19
A shift in managing symptoms for patients with relapsing-remitting multiple sclerosis (RRMS) may be in order as new research questions the efficacy of three commonly used drugs for MS-related fatigue. Results of a study from Johns Hopkins University show that amantadine, modafinil, and methylphenidate were not superior to placebo. As Dr Mark Freedman reports in this ReCAP, the study suggests that clinicians consider focusing more on patient sleep quality rather than tiredness in their evaluation of fatigue.
This study was presented during the 8th Joint Meeting of ACTRIMS-ECTRIMS, this year branded MSVirtual2020. Dr Freedman, a recognized neurologist from the University of Ottawa, shares key highlights from the online conference.
He explains the significance of new evidence that points to the potential for a selective retinoid X receptor agonist to promote remyelination in relapsing disease. He also discusses a study by researchers at the University of Melbourne that looked at data from the largest cohort of MS patients with COVID-19 and drew troubling conclusions.
Professor, Department of Neurology, University of Ottawa and The Ottawa Hospital Research Institute; Director, Multiple Sclerosis Research Unit, The Ottawa Hospital – General Campus, Ottawa, Ontario, Canada.
Mark S. Freedman, MSc, MD, has disclosed the following relevant financial relationships: Serve(d) on the advisory board, board of directors, or other similar groups for: Actelion (Janssen/Johnson & Johnson); Alexion; Atara Biotherapeutics; BayerHealthcare; BiogenIdec; Celgene; Clene Nanomedicine; GRI Bio; Hoffman La-Roche; Magenta Therapeutics; Merck Serono; MedDay; Novartis; Sanofi-Genzyme; Teva Canada Innovation. Serve(d) as a member of a speakers bureau for: Sanofi-Genzyme; EMD Serono. Received honoraria or consultation fees for: Actelion (Janssen/Johnson & Johnson); Alexion; BiogenIdec; Celgene (BMS); EMD Inc; Sanofi-Genzyme; Hoffman La-Roche; Merck Serono; Novartis; Teva Canada Innovation. Received research or educational grants from: Sanofi-Genzyme Canada; Hoffman-La Roche; EMD Inc.
A shift in managing symptoms for patients with relapsing-remitting multiple sclerosis (RRMS) may be in order as new research questions the efficacy of three commonly used drugs for MS-related fatigue. Results of a study from Johns Hopkins University show that amantadine, modafinil, and methylphenidate were not superior to placebo. As Dr Mark Freedman reports in this ReCAP, the study suggests that clinicians consider focusing more on patient sleep quality rather than tiredness in their evaluation of fatigue.
This study was presented during the 8th Joint Meeting of ACTRIMS-ECTRIMS, this year branded MSVirtual2020. Dr Freedman, a recognized neurologist from the University of Ottawa, shares key highlights from the online conference.
He explains the significance of new evidence that points to the potential for a selective retinoid X receptor agonist to promote remyelination in relapsing disease. He also discusses a study by researchers at the University of Melbourne that looked at data from the largest cohort of MS patients with COVID-19 and drew troubling conclusions.
Professor, Department of Neurology, University of Ottawa and The Ottawa Hospital Research Institute; Director, Multiple Sclerosis Research Unit, The Ottawa Hospital – General Campus, Ottawa, Ontario, Canada.
Mark S. Freedman, MSc, MD, has disclosed the following relevant financial relationships: Serve(d) on the advisory board, board of directors, or other similar groups for: Actelion (Janssen/Johnson & Johnson); Alexion; Atara Biotherapeutics; BayerHealthcare; BiogenIdec; Celgene; Clene Nanomedicine; GRI Bio; Hoffman La-Roche; Magenta Therapeutics; Merck Serono; MedDay; Novartis; Sanofi-Genzyme; Teva Canada Innovation. Serve(d) as a member of a speakers bureau for: Sanofi-Genzyme; EMD Serono. Received honoraria or consultation fees for: Actelion (Janssen/Johnson & Johnson); Alexion; BiogenIdec; Celgene (BMS); EMD Inc; Sanofi-Genzyme; Hoffman La-Roche; Merck Serono; Novartis; Teva Canada Innovation. Received research or educational grants from: Sanofi-Genzyme Canada; Hoffman-La Roche; EMD Inc.
A shift in managing symptoms for patients with relapsing-remitting multiple sclerosis (RRMS) may be in order as new research questions the efficacy of three commonly used drugs for MS-related fatigue. Results of a study from Johns Hopkins University show that amantadine, modafinil, and methylphenidate were not superior to placebo. As Dr Mark Freedman reports in this ReCAP, the study suggests that clinicians consider focusing more on patient sleep quality rather than tiredness in their evaluation of fatigue.
This study was presented during the 8th Joint Meeting of ACTRIMS-ECTRIMS, this year branded MSVirtual2020. Dr Freedman, a recognized neurologist from the University of Ottawa, shares key highlights from the online conference.
He explains the significance of new evidence that points to the potential for a selective retinoid X receptor agonist to promote remyelination in relapsing disease. He also discusses a study by researchers at the University of Melbourne that looked at data from the largest cohort of MS patients with COVID-19 and drew troubling conclusions.
Professor, Department of Neurology, University of Ottawa and The Ottawa Hospital Research Institute; Director, Multiple Sclerosis Research Unit, The Ottawa Hospital – General Campus, Ottawa, Ontario, Canada.
Mark S. Freedman, MSc, MD, has disclosed the following relevant financial relationships: Serve(d) on the advisory board, board of directors, or other similar groups for: Actelion (Janssen/Johnson & Johnson); Alexion; Atara Biotherapeutics; BayerHealthcare; BiogenIdec; Celgene; Clene Nanomedicine; GRI Bio; Hoffman La-Roche; Magenta Therapeutics; Merck Serono; MedDay; Novartis; Sanofi-Genzyme; Teva Canada Innovation. Serve(d) as a member of a speakers bureau for: Sanofi-Genzyme; EMD Serono. Received honoraria or consultation fees for: Actelion (Janssen/Johnson & Johnson); Alexion; BiogenIdec; Celgene (BMS); EMD Inc; Sanofi-Genzyme; Hoffman La-Roche; Merck Serono; Novartis; Teva Canada Innovation. Received research or educational grants from: Sanofi-Genzyme Canada; Hoffman-La Roche; EMD Inc.

Risk for Deep Fungal Infections During IL-17 and IL-23 Inhibitor Therapy for Psoriasis
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.
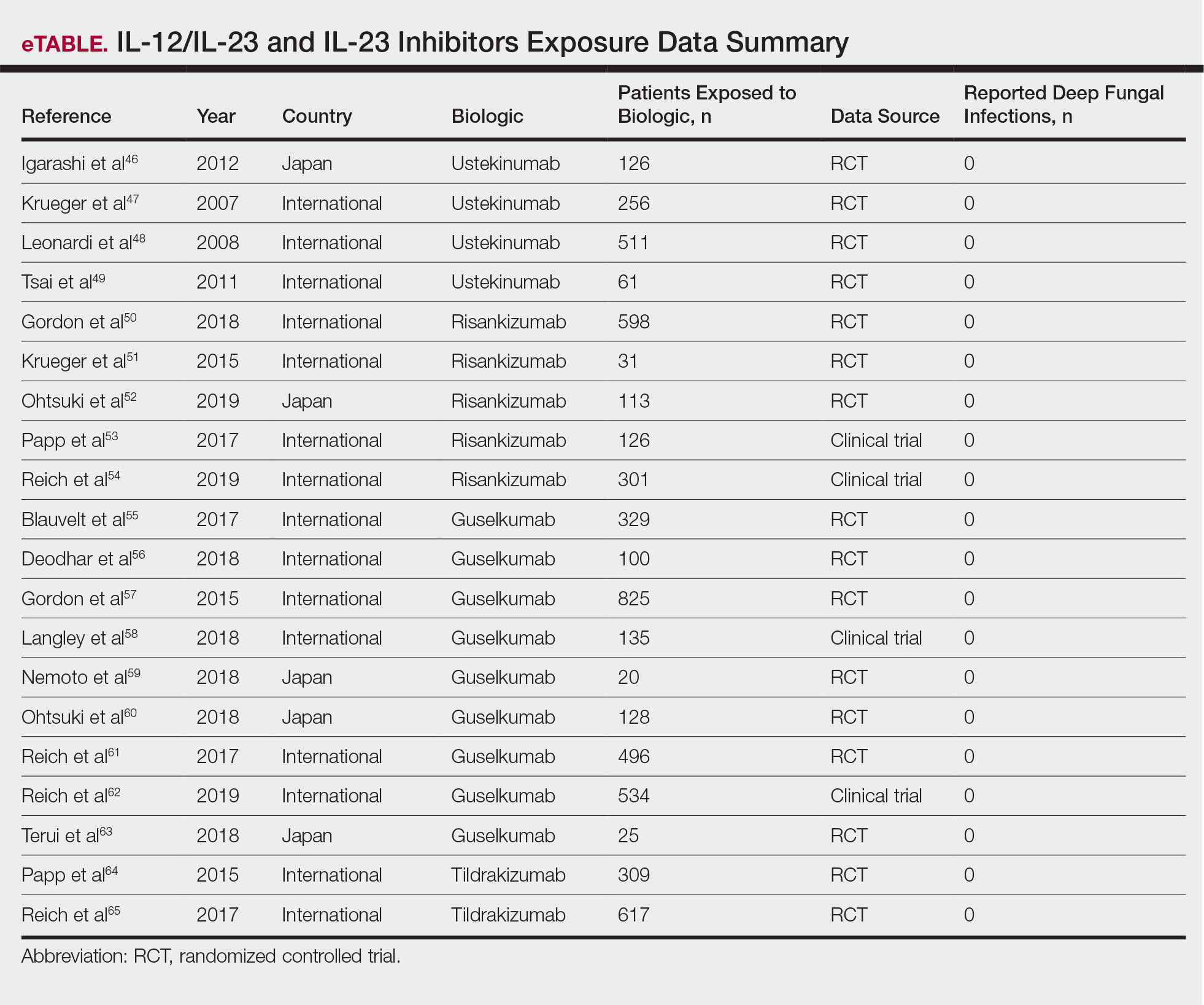
IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Practice Points
- The use of IL-17, IL-12/IL-23, and IL-23 inhibitors for psoriasis and other inflammatory conditions does not appear to increase the risk for deep fungal infections.
- Physicians should still be cautiously optimistic in prescribing these medications, as IL-17 and IL-23 play a central role in immunologic defenses, particularly against fungi.
- A high index of suspicion should be maintained for patients from endemic areas who are being treated with biologics.
Fourteen-day sports hiatus recommended for children after COVID-19
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.