User login
Latest report adds almost 44,000 child COVID-19 cases in 1 week
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
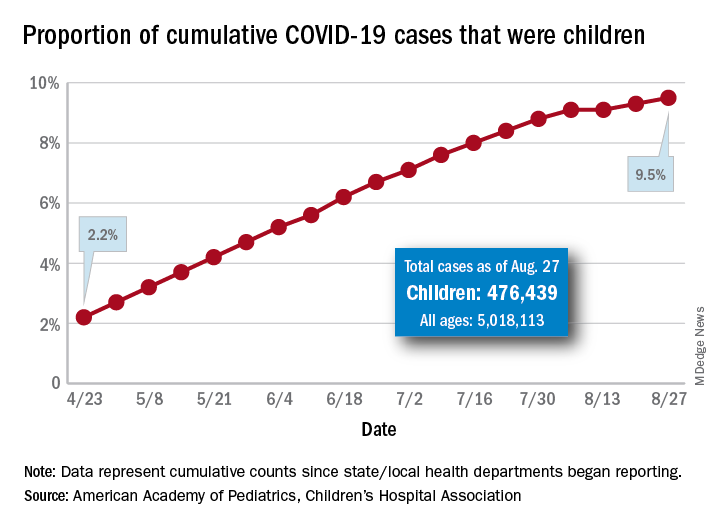
The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19: In-hospital mortality data miss bigger picture of racial inequality
A recent study that reported no association between race and in-hospital mortality among patients with COVID-19 failed to capture broader health care inequities, according to a leading expert.
During an AGA FORWARD Program webinar, Darrell Gray II, MD, deputy director of the Center for Cancer Health Equity at Ohio State University in Columbus, noted that the study by Baligh R. Yehia, MD, and colleagues had several important limitations: specifically, a lack of data from before or after hospitalization, flawed neighborhood deprivation indices, and poorly characterized comorbidities.
While Dr. Yehia and colleagues described these limitations in their publication, Dr. Gray suggested that future studies evaluating race and health outcomes need to be “deliberate and intentional with collecting data.”
According to Dr. Gray, statistics from the Centers for Disease Control and Prevention and the APM Research Lab paint a more accurate picture of health care inequities. The CDC, for instance, reports that people who are Black are nearly five times as likely to be hospitalized for COVID-19, and approximately twice as likely to die from the disease, compared with those who are White. The APM Research Lab reports an even more striking relative mortality rate for Black Americans – almost four times higher than that of White Americans.
“People of color have been disproportionately impacted by COVID-19, whether it be by cases, hospitalizations, or deaths,” Dr. Gray said. “We have to think about why that is, and what has led to this.”
Dr. Gray emphasized that poorer outcomes among people of color are “not necessarily biological.”
“It’s the environment and social constructs that contribute to why there’s a disproportionate burden of chronic disease and why there’s a disproportionate burden of COVID-19,” he said.
According to Dr. Gray, disparate health care outcomes can be traced back to social determinants of health, which he and his colleagues highlighted in a June comment published in Nature Reviews Gastroenterology & Hepatology.
“Although much attention has focused on the high burden of chronic disease among [people of color], which predisposes them to poor outcomes if they acquire COVID-19, there is less recognition of the nonmedical health-related social needs and social determinants of health that represent the root causes of such health disparities,” they wrote.
Social determinants of health include an array of population factors, including economic stability, social and community context, neighborhood and environment, education, and access to health care.
For each, Dr. Gray encouraged comprehensive and nuanced assessment.
“Is there access to health care?” Dr. Gray asked. “Not just access in the sense of having insurance – certainly that’s a benefit – but if someone has insurance, can they get to where the health center is? Or is that something they might have to catch three buses and a cab to get to?”
Dr. Gray said that such obstacles are not outside the scope of the medical community.
“This is not beyond our responsibility ... to address social determinants of health,” Dr. Gray said.
When asked by a webinar attendee how the medical community can tackle racism, Dr. Gray offered several practical steps to move forward.
First, he suggested that clinicians and researchers listen to affected patient populations.
“Many of us, including clinicians, have been privileged to have their blinders on, if you will, to issues of racism that have been affecting our patients for a long time,” he said.
Second, Dr. Gray encouraged those who have learned to teach others.
“You need to start teaching your peers, your colleagues, your family, and friends about how racism affects patient outcomes.”
Third, he recommended that clinicians incorporate these lessons into routine practice, whether in a private or an academic setting.
“Are there ways in which you can refer patients to address social determinants of health? Are you capturing that information in your check-in materials?” Dr. Gray asked. “If you’re an investigator, when you’re doing research – whether it’s health disparities research or other – are you looking at your research through a health equity lens? Are you asking questions about social determinants of health?”
Finally, Dr. Gray called for stronger community engagement during design and conduction of clinical trials.
“People don’t care how much you know until they know how much you care,” he said. “And they won’t know how much you care unless you’re visible, and unless you’re there, and these are sustainable relationships.”
The FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
A recent study that reported no association between race and in-hospital mortality among patients with COVID-19 failed to capture broader health care inequities, according to a leading expert.
During an AGA FORWARD Program webinar, Darrell Gray II, MD, deputy director of the Center for Cancer Health Equity at Ohio State University in Columbus, noted that the study by Baligh R. Yehia, MD, and colleagues had several important limitations: specifically, a lack of data from before or after hospitalization, flawed neighborhood deprivation indices, and poorly characterized comorbidities.
While Dr. Yehia and colleagues described these limitations in their publication, Dr. Gray suggested that future studies evaluating race and health outcomes need to be “deliberate and intentional with collecting data.”
According to Dr. Gray, statistics from the Centers for Disease Control and Prevention and the APM Research Lab paint a more accurate picture of health care inequities. The CDC, for instance, reports that people who are Black are nearly five times as likely to be hospitalized for COVID-19, and approximately twice as likely to die from the disease, compared with those who are White. The APM Research Lab reports an even more striking relative mortality rate for Black Americans – almost four times higher than that of White Americans.
“People of color have been disproportionately impacted by COVID-19, whether it be by cases, hospitalizations, or deaths,” Dr. Gray said. “We have to think about why that is, and what has led to this.”
Dr. Gray emphasized that poorer outcomes among people of color are “not necessarily biological.”
“It’s the environment and social constructs that contribute to why there’s a disproportionate burden of chronic disease and why there’s a disproportionate burden of COVID-19,” he said.
According to Dr. Gray, disparate health care outcomes can be traced back to social determinants of health, which he and his colleagues highlighted in a June comment published in Nature Reviews Gastroenterology & Hepatology.
“Although much attention has focused on the high burden of chronic disease among [people of color], which predisposes them to poor outcomes if they acquire COVID-19, there is less recognition of the nonmedical health-related social needs and social determinants of health that represent the root causes of such health disparities,” they wrote.
Social determinants of health include an array of population factors, including economic stability, social and community context, neighborhood and environment, education, and access to health care.
For each, Dr. Gray encouraged comprehensive and nuanced assessment.
“Is there access to health care?” Dr. Gray asked. “Not just access in the sense of having insurance – certainly that’s a benefit – but if someone has insurance, can they get to where the health center is? Or is that something they might have to catch three buses and a cab to get to?”
Dr. Gray said that such obstacles are not outside the scope of the medical community.
“This is not beyond our responsibility ... to address social determinants of health,” Dr. Gray said.
When asked by a webinar attendee how the medical community can tackle racism, Dr. Gray offered several practical steps to move forward.
First, he suggested that clinicians and researchers listen to affected patient populations.
“Many of us, including clinicians, have been privileged to have their blinders on, if you will, to issues of racism that have been affecting our patients for a long time,” he said.
Second, Dr. Gray encouraged those who have learned to teach others.
“You need to start teaching your peers, your colleagues, your family, and friends about how racism affects patient outcomes.”
Third, he recommended that clinicians incorporate these lessons into routine practice, whether in a private or an academic setting.
“Are there ways in which you can refer patients to address social determinants of health? Are you capturing that information in your check-in materials?” Dr. Gray asked. “If you’re an investigator, when you’re doing research – whether it’s health disparities research or other – are you looking at your research through a health equity lens? Are you asking questions about social determinants of health?”
Finally, Dr. Gray called for stronger community engagement during design and conduction of clinical trials.
“People don’t care how much you know until they know how much you care,” he said. “And they won’t know how much you care unless you’re visible, and unless you’re there, and these are sustainable relationships.”
The FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
A recent study that reported no association between race and in-hospital mortality among patients with COVID-19 failed to capture broader health care inequities, according to a leading expert.
During an AGA FORWARD Program webinar, Darrell Gray II, MD, deputy director of the Center for Cancer Health Equity at Ohio State University in Columbus, noted that the study by Baligh R. Yehia, MD, and colleagues had several important limitations: specifically, a lack of data from before or after hospitalization, flawed neighborhood deprivation indices, and poorly characterized comorbidities.
While Dr. Yehia and colleagues described these limitations in their publication, Dr. Gray suggested that future studies evaluating race and health outcomes need to be “deliberate and intentional with collecting data.”
According to Dr. Gray, statistics from the Centers for Disease Control and Prevention and the APM Research Lab paint a more accurate picture of health care inequities. The CDC, for instance, reports that people who are Black are nearly five times as likely to be hospitalized for COVID-19, and approximately twice as likely to die from the disease, compared with those who are White. The APM Research Lab reports an even more striking relative mortality rate for Black Americans – almost four times higher than that of White Americans.
“People of color have been disproportionately impacted by COVID-19, whether it be by cases, hospitalizations, or deaths,” Dr. Gray said. “We have to think about why that is, and what has led to this.”
Dr. Gray emphasized that poorer outcomes among people of color are “not necessarily biological.”
“It’s the environment and social constructs that contribute to why there’s a disproportionate burden of chronic disease and why there’s a disproportionate burden of COVID-19,” he said.
According to Dr. Gray, disparate health care outcomes can be traced back to social determinants of health, which he and his colleagues highlighted in a June comment published in Nature Reviews Gastroenterology & Hepatology.
“Although much attention has focused on the high burden of chronic disease among [people of color], which predisposes them to poor outcomes if they acquire COVID-19, there is less recognition of the nonmedical health-related social needs and social determinants of health that represent the root causes of such health disparities,” they wrote.
Social determinants of health include an array of population factors, including economic stability, social and community context, neighborhood and environment, education, and access to health care.
For each, Dr. Gray encouraged comprehensive and nuanced assessment.
“Is there access to health care?” Dr. Gray asked. “Not just access in the sense of having insurance – certainly that’s a benefit – but if someone has insurance, can they get to where the health center is? Or is that something they might have to catch three buses and a cab to get to?”
Dr. Gray said that such obstacles are not outside the scope of the medical community.
“This is not beyond our responsibility ... to address social determinants of health,” Dr. Gray said.
When asked by a webinar attendee how the medical community can tackle racism, Dr. Gray offered several practical steps to move forward.
First, he suggested that clinicians and researchers listen to affected patient populations.
“Many of us, including clinicians, have been privileged to have their blinders on, if you will, to issues of racism that have been affecting our patients for a long time,” he said.
Second, Dr. Gray encouraged those who have learned to teach others.
“You need to start teaching your peers, your colleagues, your family, and friends about how racism affects patient outcomes.”
Third, he recommended that clinicians incorporate these lessons into routine practice, whether in a private or an academic setting.
“Are there ways in which you can refer patients to address social determinants of health? Are you capturing that information in your check-in materials?” Dr. Gray asked. “If you’re an investigator, when you’re doing research – whether it’s health disparities research or other – are you looking at your research through a health equity lens? Are you asking questions about social determinants of health?”
Finally, Dr. Gray called for stronger community engagement during design and conduction of clinical trials.
“People don’t care how much you know until they know how much you care,” he said. “And they won’t know how much you care unless you’re visible, and unless you’re there, and these are sustainable relationships.”
The FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
FROM THE AGA FORWARD PROGRAM
Molecular developments in treatment of UPSC
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Uterine papillary serous carcinoma (UPSC) is an infrequent but deadly form of endometrial cancer comprising 10% of cases but contributing 40% of deaths from the disease. Recurrence rates are high for this disease. Five-year survival is 55% for all patients and only 70% for stage I disease.1 Patterns of recurrence tend to be distant (extrapelvic and extraabdominal) as frequently as they are localized to the pelvis, and metastases and recurrences are unrelated to the extent of uterine disease (such as myometrial invasion). It is for these reasons that the recommended course of adjuvant therapy for this disease is systemic therapy (typically six doses of carboplatin and paclitaxel chemotherapy) with consideration for radiation to the vagina or pelvis to consolidate pelvic and vaginal control.2 This differs from early-stage high/intermediate–risk endometrioid adenocarcinomas, for which adjuvant chemotherapy has not been found to be helpful.
Because of the lower incidence of UPSC, it frequently has been studied alongside endometrioid cell types in clinical trials which explore novel adjuvant therapies. However, UPSC is biologically distinct from endometrioid endometrial cancers, which likely results in inferior clinical responses to conventional interventions. Fortunately we are beginning to better understand UPSC at a molecular level, and advancements are being made in the targeted therapies for these patients that are unique, compared with those applied to other cancer subtypes.
As discussed above, UPSC is a particularly aggressive form of uterine cancer. Histologically it is characterized by a precursor lesion of endometrial glandular dysplasia progressing to endometrial intraepithelial neoplasia (EIC). Histologically it presents with a highly atypical slit-like glandular configuration, which appears similar to serous carcinomas of the fallopian tube and ovary. Molecularly these tumors commonly manifest mutations in tumor protein p53 (TP53) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), which are both genes associated with oncogenic potential.1 While most UPSC tumors have loss of expression in hormone receptors such as estrogen and progesterone, 25%-30% of cases overexpress the tyrosine kinase receptor human epidermal growth factor receptor 2 (HER2).3-5 This has proven to provide an exciting target for therapeutic interventions.
A target for therapeutic intervention
HER2 is a transmembrane receptor which, when activated, signals complex downstream pathways responsible for cellular proliferation, dedifferentiation, and metastasis. In a recent multi-institutional analysis of early-stage UPSC, HER2 overexpression was identified among 25% of cases.4 Approximately 30% of cases of advanced disease manifest overexpression of this biomarker.5 HER2 overexpression (HER2-positive status) is significantly associated with higher rates of recurrence and mortality, even among patients treated with conventional therapies.3 Thus HER2-positive status is obviously an indicator of particularly aggressive disease.
Fortunately this particular biomarker is one for which we have established and developing therapeutics. The humanized monoclonal antibody, trastuzumab, has been highly effective in improving survival for HER2-positive breast cancer.6 More recently, it was studied in a phase 2 trial with carboplatin and paclitaxel chemotherapy for advanced or recurrent HER2-positive UPSC.5 This trial showed that the addition of this targeted therapy to conventional chemotherapy improved recurrence-free survival from 8 months to 12 months, and improved overall survival from 24.4 months to 29.6 months.5
One discovery leads to another treatment
This discovery led to the approval of trastuzumab to be used in addition to chemotherapy for advanced or recurrent disease.2 The most significant effects appear to be among those who have not received prior therapies, with a doubling of progression-free survival among these patients, and a more modest response among patients treated for recurrent, mostly pretreated disease.
Work currently is underway to explore an array of antibody or small-molecule blockades of HER2 in addition to vaccines against the protein or treatment with conjugate compounds in which an antibody to HER2 is paired with a cytotoxic drug able to be internalized into HER2-expressing cells.7 This represents a form of personalized medicine referred to as biomarker-driven targeted therapy, in which therapies are prescribed based on the expression of specific molecular markers (such as HER2 expression) typically in combination with other clinical markers such as surgical staging results, race, age, etc. These approaches can be very effective strategies in rare tumor subtypes with distinct molecular and clinical behaviors.
As previously mentioned, the targeting of HER2 overexpression with trastuzumab has been shown to be highly effective in the treatment of HER2-positive breast cancers where even patients with early-stage disease receive a multimodal therapy approach including antibody, chemotherapy, surgical, and often radiation treatments.6 We are moving towards a similar multimodal comprehensive treatment strategy for UPSC. If it is as successful as it is in breast cancer, it will be long overdue, and desperately necessary given the poor prognosis of this disease for all stages because of the inadequacies of current treatments strategies.
Routine testing of UPSC for HER2 expression is now a part of routine molecular substaging of uterine cancers in the same way we have embraced testing for microsatellite instability and hormone-receptor status. While a diagnosis of HER2 overexpression in UPSC portends a poor prognosis, patients can be reassured that treatment strategies exist that can target this malignant mechanism in advanced disease and more are under further development for early-stage disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Curr Opin Obstet Gynecol. 2010 Feb. doi: 10.1097/GCO.0b013e328334d8a3.
2. National Comprehensive Cancer Network. Uterine Neoplasms (version 2.2020).
3. Cancer 2005 Oct 1. doi: 10.1002/cncr.21308.
4. Gynecol Oncol 2020 doi: 10.1016/j.ygyno.2020.07.016.
5. J Clin Oncol 2018. doi: 10.1200/JCO.2017.76.5966.
6. N Engl J Med 2011. doi: 10.1056/NEJMoa0910383.
7. Discov Med. 2016 Apr;21(116):293-303.
Three malpractice risks of video visits
During a telemedicine visit with his physician, a 62-year-old obese patient with an ankle injury reported new swelling of his leg. Three weeks had passed since the man visited an emergency department, where he underwent surgery and had a cast applied to the wound. The physician, during the telemedicine visit, advised the patient to elevate his leg and see an orthopedist within 24 hours. A Doppler ultrasound was ordered for 12:30 p.m. that same day.
The patient never made it to the appointment. He became unresponsive and went into full arrest hours later. His death fueled a lawsuit by his family that claimed failure to diagnose and treat deep venous thrombosis. The family contended the providers involved should have referred the patient to care immediately during the video visit.
The case, which comes from the claims database of national medical liability insurer The Doctors Company, illustrates the legal risks that can stem from video visits with patients, says Richard Cahill, JD, vice president and associate general counsel for The Doctors Company.
“By evaluating the patient remotely, the physician failed to appreciate the often subtle nuances of the clinical presentation, which undoubtedly could have been more accurately assessed in the office setting, and would probably have led to more urgent evaluation and intervention, thereby likely preventing the unfortunate and otherwise avoidable result,” said Mr. Cahill.
According to a Harris poll, 42% of Americans reported using video visits during the pandemic, a trend that is likely to continue as practices reopen and virtual care becomes the norm. But as physicians conduct more video visits, so grows their risk for lawsuits associated with the technology.
“We probably will see more malpractice suits filed the more telehealth is used,” said Mei Wa Kwong, JD, executive director of the Center for Connected Health Policy. “It’s a numbers game. The more it’s used, the higher likelihood that lawsuits occur.”
Three problems in not being able to touch the patient
1. The primary challenge with video visits “is the inability to directly observe and lay hands on the patient,” says Jonathan Einbinder, MD, assistant vice president of analytics for CRICO, a medical liability insurer based in Boston.
said Dr. Einbinder, a practicing internist.
Such incomplete pictures can lead to diagnostic errors and the potential for lawsuits, as demonstrated by a recent CRICO analysis. Of 106 telemedicine-related claims from 2014 to 2018, 66% were diagnosis related, according to the analysis of claims from CRICO’s national database. Twelve percent of the telemedicine-related claims were associated with surgical treatment, 11% were related to medical treatment, and 5% were associated with medication issues. A smaller number of claims resulted from patient monitoring, ob.gyn. care, and safety and security.
Another analysis by The Doctors Company similarly determined that diagnostic errors are the most common allegation in telemedicine-related claims. In the study of 28 telemedicine-related claims from The Doctors’ database, 71% were diagnosis related, 11% were associated with mismanagement of treatment, and 7% were related to improper management of a surgical patient. Other allegations included improper performance of treatment or procedure and improper performance of surgery.
“Because a ‘typical’ exam can’t be done, there is the potential to miss things,” said David L. Feldman, MD, chief medical officer for The Doctors Company Group. “A subtlety, perhaps a lump that can’t be seen but only felt, and only by an experienced examiner, for example, may be missed.”
2. Documentation dangers also loom, said William Sullivan, DO, JD, an emergency physician and an attorney who specializes in health care. The legal risk lies in documenting a video visit in the same way the doctor would document an in-person visit, he explained.
“Investigation into a potential lawsuit begins when there is some type of bad outcome related to medical care,” Dr. Sullivan explained. “To determine whether the lawsuit has merit, patients/attorneys review the medical records to retrospectively determine the potential cause of the bad outcome. If the documentation reflects an examination that could not have been performed, a lawyer might be more likely to pursue a case, and it would be more difficult to defend the care provided.”
Dr. Sullivan provided this example: During a video visit, a patient complains of acute onset weakness. The physician documents that the patient’s heart has a “regular rate and rhythm,” and “muscle strength is equal bilaterally.” The following day, the patient’s weakness continues, and the patient goes to the emergency department where he is diagnosed with stroke. An EKG in the ED shows that the patient is in atrial fibrillation.
“The telehealth provider would have a difficult time explaining how it was determined that the patient had normal muscle strength and a normal heart rhythm over a video visit the day before,” Dr. Sullivan said. “A lawyer in a subsequent malpractice case would present the provider as careless and would argue that if the provider had only sent the patient to the emergency department after the telehealth visit instead of documenting exam findings that couldn’t have been performed, the patient could have been successfully treated for the stroke.”
3. Poorly executed informed consent can also give rise to a lawsuit. This includes informed consent regarding the use of telehealth as the accepted modality for the visit rather than traditional on-site evaluations, as well as preprocedure informed consent.
“Inadequate and/or poorly documented informed consent can result in a claim for medical battery,” Mr. Cahill said.
A medical battery allegation refers to the alleged treatment or touching of a patient’s body without that person’s consent. As the AMA Journal of Ethics explains, a patient’s consent must be given, either expressly or implicitly, before a physician may legally “interfere” with the physical body of the patient.
Ideally, the informed consent process is undertaken during a first in-person visit, before virtual visits begin, Dr. Feldman said.
“There is a lot that a patient has to understand when a visit is done virtually, which is part of the informed consent process,” Dr. Feldman said. “The pandemic has forced some physicians to do their first visit virtually, and this makes the process of informing patients more onerous. It is not a simple matter of converting an in-person office practice to a remote office practice. The work flows are different, so there are definitely legal concerns as it relates to privacy and cybersecurity to name a few.”
Waivers may be weak protection
Since the pandemic started, a number of states have enacted emergency malpractice protections to shield health professionals from lawsuits. Some protections, such as those in Massachusetts, offer immunity to health professionals who provide general care to patients during the COVID-19 emergency, in addition to treatment of COVID-19 patients. Other protections, like those in Connecticut, apply specifically to care provided in support of the state’s pandemic response.
Whether that immunity applies both to in-person visits and video visits during the pandemic is not certain, said J. Richard Moore, JD, a medical liability defense attorney based in Indianapolis. Indiana’s immunity statute for example, does not make a specific provision for telehealth, he said.
“My best prediction is that if considered by the courts, the immunity would be applied to telehealth services, so long as they are being provided ‘in response to the emergency,’ which is the scope of the immunity,” he said. “I would not consider telehealth physicians to be either more or less protected than in-person providers.”
Regulatory scrutiny for telehealth providers has also been relaxed in response to COVID-19, but experts warn not to rely on the temporary shields for ultimate protection.
In March, the U.S. Department of Health and Human Service’s Office of Civil Rights (OCR) eased enforcement actions for noncompliance with Health Insurance Portability and Accountability Act requirements in connection with the good faith provision of telehealth during the COVID-19 health crisis. Under the notice, health providers can use popular applications such as Apple FaceTime, Facebook Messenger, Zoom, or Google Hangouts, to offer telehealth care without risk that OCR will impose fines or penalties for HIPAA violations.
But once the current health care emergency is mitigated, the waivers will likely be withdrawn, and enforcement actions will probably resume, Mr. Cahill said.
“It is recommended that, to avoid potential problems going forward, practitioners use due diligence and undertake best efforts to obey existing privacy and security requirements, including the use of technology that satisfies compliance regulations, despite the waiver by OCR,” he said.
In addition, a majority of states have relaxed state-specific rules for practicing telehealth and loosened licensure requirements during the pandemic. At least 47 states have issued waivers to alter in-state licensure requirements for telemedicine in response to COVID-19, according to the Federation of State Medical Boards. Most of the waivers allow physicians licensed in other states to provide care in states where they do not hold licenses, and some enable doctors to treat patients without first having had an in-person evaluation.
But at least for now, these are temporary changes, reminds Amy Lerman, JD, a health care attorney based in Washington, who specializes in telehealth and corporate compliance. Given the current pandemic environment, a significant concern is that physicians new to the telemedicine space are reacting only to the most recent rules established in the context of the pandemic, Ms. Lerman said.
“As previously noted, the recent developments are temporary in nature – states and various federal agencies have been pretty clear in setting this temporal boundary,” she said. “It is not advisable for providers to build telepractice models around temporary sets of rules.
“Furthermore, the recent developments are not necessarily comprehensive relative to all of the state-specific and other requirements that telemedicine providers are otherwise expected to follow, so relying only on the most recent guidance may cause providers to create telepractice models that have key gaps with respect to regulatory compliance.”
How you can avoid a lawsuit
As businesses reopen and practices resume treatments, physicians should weigh the choice between in-person care and video visits very carefully, said Joseph Kvedar, MD, president of the American Telemedicine Association and a dermatology professor at Harvard Medical School, Boston.
“We have to be very thoughtful about quality in this current phase, where we are doing what I call a hybrid model,” he said. “Some services are offered by telehealth and some require patients to come into the doctor’s office. We have to be very thoughtful about what types of care we determine to be appropriate for telehealth, and that has to be based on clinical quality. And if it is, it should follow that we’ll have low incidence of liability claims.”
Data should be at the center of that conclusion, Dr. Kvedar advises.
“Think about what data is needed to make a therapeutic or diagnostic decision,” he said. “If a health care provider can gather the information needed without touching the patient, then the provider is probably on safe, solid ground making that decision via a telehealth interaction. If the patient can come into the doctor’s office, and the provider deems it necessary to see the patient in person and touch the patient in order to make that clinical decision, then the patient should come in.”
An important step to preventing liability is also having strong telehealth systems and protocols in place and the necessary support to carry them out, said Dr. Einbinder of medical liability insurer CRICO.
For example, Dr. Einbinder, who practices in a 12-doctor internal medicine group, said when he finishes a virtual visit, he enters any orders into the electronic health record. Some of the orders will result in notifications to Dr. Einbinder if they are not executed, such as a referral appointment or a procedure that was not completed.
“I also can forward my orders to a front desk pool that is responsible for making sure things get done,” he said. “And, in our hospital system, we have good case management for complex patients and population management for a variety of chronic conditions. These represent additional safety nets.”
Another liability safeguard is sending patients a “visit summary” after each virtual visit, Dr. Sullivan said. This could be in the form of an email or a text that includes a brief template including items such as diagnosis, recommendations, follow-up, and a reminder to contact the doctor or go to the emergency department if symptoms worsen or new problems develop.
“Patients tend to remember about half of what physicians tell them and half of the information patients do remember is incorrect,” he said. “Consider a few sentences in an e-mail or text message as a substitute for the after-visit instructions from an office visit to enhance patient understanding. There are several inexpensive programs/services that allow text messages to be sent from a computer using a separate dedicated phone number and pretty much every patient has a cell phone to receive the instructions.”
Dr. Sullivan suggests having a documentation template specifically for telehealth visits. He also recommends the inclusion an “informed refusal of care” in the record when necessary. Dr. Sullivan’s wife, a family physician, has encountered several patients who fear contracting COVID-19 and who have refused her recommendations for in-person visits, he said. In such cases, he said it’s a good idea to document that the patient decided to forgo the recommendations given.
“If a patient suffers a bad outcome because of a failure to seek an in-person exam, a short note in the patient’s chart would help to establish that the lack of a follow-up physical exam was the patient’s informed decision, not due to some alleged negligence of the medical provider,” he said.
Concerning informed consent, Dr. Feldman says at a minimum physicians should discuss the following with patients:
- Names and credentials of staff participating.
- The right to stop or refuse treatment by telemedicine.
- Technology that will be used.
- Privacy and security risks.
- Technology-specific risks and permission to bill.
- Alternative care in case of an emergency or technology malfunction.
- Any state-specific requirements.
“Physicians can ensure they have a strong informed consent process during video visits by taking the time to cover these points at the beginning of the first visit, and being sure the patient understands and agrees to these,” Dr. Sullivan explained. “Ideally, this conversation can be recorded for future reference if necessary or at a minimum documented in the medical record.”
Consider these extra precautions
Mr. Cahill advises that practitioners be especially mindful of their “web-side manner” and the setting in which they are communicating with virtual patients to promote confidentiality, professionalism, and uninterrupted interactions.
“Use of a headset in a quiet home office is advisable,” he said. “Physicians must also be cognizant of their physical appearance and the background behind them when the visit includes both audio and visual capability. For ‘face-to-face’ telehealth encounters, it is recommended that a white lab jacket be worn as the appropriate attire; coat and tie are unnecessary.”
Some patients may need to be reminded of the need for confidentiality during a video visit, Mr. Moore added. Physicians are typically in a position to ensure confidentiality, but some patients may not understand how to protect their privacy on their end.
“If the physician sees on the screen or hears from an audio connection that there are other people around who may be able to overhear what is communicated, the physician probably has some responsibility to remind the patient that she or he may want to go to a more private place, close the door, etc.,” he said. “While I think a claim against a physician on this basis would be pretty weak, it is still a good practice for the physician to be cognizant of those kinds of concerns even if the patient is not.”
Finally, for physicians who set up telehealth operability during the pandemic – possibly in a hurry – consider using your actual case data to take a look backward, said Ms. Lerman, the Washington-based health care attorney. Reviewing the data can help determine whether you’re in compliance with relevant state laws, she said.
“If, for example, a provider set up telehealth operations during the pandemic and can see that most of [the] patients are based in a single state, or a small group of states, it is worthwhile to take [the] time and become familiar with the telemedicine laws in those states,” she said. “If there are modifications that need to be made, it may be easier to make them incrementally before the telehealth operations grow any larger in scope.”
A version of this article originally appeared on Medscape.com.
During a telemedicine visit with his physician, a 62-year-old obese patient with an ankle injury reported new swelling of his leg. Three weeks had passed since the man visited an emergency department, where he underwent surgery and had a cast applied to the wound. The physician, during the telemedicine visit, advised the patient to elevate his leg and see an orthopedist within 24 hours. A Doppler ultrasound was ordered for 12:30 p.m. that same day.
The patient never made it to the appointment. He became unresponsive and went into full arrest hours later. His death fueled a lawsuit by his family that claimed failure to diagnose and treat deep venous thrombosis. The family contended the providers involved should have referred the patient to care immediately during the video visit.
The case, which comes from the claims database of national medical liability insurer The Doctors Company, illustrates the legal risks that can stem from video visits with patients, says Richard Cahill, JD, vice president and associate general counsel for The Doctors Company.
“By evaluating the patient remotely, the physician failed to appreciate the often subtle nuances of the clinical presentation, which undoubtedly could have been more accurately assessed in the office setting, and would probably have led to more urgent evaluation and intervention, thereby likely preventing the unfortunate and otherwise avoidable result,” said Mr. Cahill.
According to a Harris poll, 42% of Americans reported using video visits during the pandemic, a trend that is likely to continue as practices reopen and virtual care becomes the norm. But as physicians conduct more video visits, so grows their risk for lawsuits associated with the technology.
“We probably will see more malpractice suits filed the more telehealth is used,” said Mei Wa Kwong, JD, executive director of the Center for Connected Health Policy. “It’s a numbers game. The more it’s used, the higher likelihood that lawsuits occur.”
Three problems in not being able to touch the patient
1. The primary challenge with video visits “is the inability to directly observe and lay hands on the patient,” says Jonathan Einbinder, MD, assistant vice president of analytics for CRICO, a medical liability insurer based in Boston.
said Dr. Einbinder, a practicing internist.
Such incomplete pictures can lead to diagnostic errors and the potential for lawsuits, as demonstrated by a recent CRICO analysis. Of 106 telemedicine-related claims from 2014 to 2018, 66% were diagnosis related, according to the analysis of claims from CRICO’s national database. Twelve percent of the telemedicine-related claims were associated with surgical treatment, 11% were related to medical treatment, and 5% were associated with medication issues. A smaller number of claims resulted from patient monitoring, ob.gyn. care, and safety and security.
Another analysis by The Doctors Company similarly determined that diagnostic errors are the most common allegation in telemedicine-related claims. In the study of 28 telemedicine-related claims from The Doctors’ database, 71% were diagnosis related, 11% were associated with mismanagement of treatment, and 7% were related to improper management of a surgical patient. Other allegations included improper performance of treatment or procedure and improper performance of surgery.
“Because a ‘typical’ exam can’t be done, there is the potential to miss things,” said David L. Feldman, MD, chief medical officer for The Doctors Company Group. “A subtlety, perhaps a lump that can’t be seen but only felt, and only by an experienced examiner, for example, may be missed.”
2. Documentation dangers also loom, said William Sullivan, DO, JD, an emergency physician and an attorney who specializes in health care. The legal risk lies in documenting a video visit in the same way the doctor would document an in-person visit, he explained.
“Investigation into a potential lawsuit begins when there is some type of bad outcome related to medical care,” Dr. Sullivan explained. “To determine whether the lawsuit has merit, patients/attorneys review the medical records to retrospectively determine the potential cause of the bad outcome. If the documentation reflects an examination that could not have been performed, a lawyer might be more likely to pursue a case, and it would be more difficult to defend the care provided.”
Dr. Sullivan provided this example: During a video visit, a patient complains of acute onset weakness. The physician documents that the patient’s heart has a “regular rate and rhythm,” and “muscle strength is equal bilaterally.” The following day, the patient’s weakness continues, and the patient goes to the emergency department where he is diagnosed with stroke. An EKG in the ED shows that the patient is in atrial fibrillation.
“The telehealth provider would have a difficult time explaining how it was determined that the patient had normal muscle strength and a normal heart rhythm over a video visit the day before,” Dr. Sullivan said. “A lawyer in a subsequent malpractice case would present the provider as careless and would argue that if the provider had only sent the patient to the emergency department after the telehealth visit instead of documenting exam findings that couldn’t have been performed, the patient could have been successfully treated for the stroke.”
3. Poorly executed informed consent can also give rise to a lawsuit. This includes informed consent regarding the use of telehealth as the accepted modality for the visit rather than traditional on-site evaluations, as well as preprocedure informed consent.
“Inadequate and/or poorly documented informed consent can result in a claim for medical battery,” Mr. Cahill said.
A medical battery allegation refers to the alleged treatment or touching of a patient’s body without that person’s consent. As the AMA Journal of Ethics explains, a patient’s consent must be given, either expressly or implicitly, before a physician may legally “interfere” with the physical body of the patient.
Ideally, the informed consent process is undertaken during a first in-person visit, before virtual visits begin, Dr. Feldman said.
“There is a lot that a patient has to understand when a visit is done virtually, which is part of the informed consent process,” Dr. Feldman said. “The pandemic has forced some physicians to do their first visit virtually, and this makes the process of informing patients more onerous. It is not a simple matter of converting an in-person office practice to a remote office practice. The work flows are different, so there are definitely legal concerns as it relates to privacy and cybersecurity to name a few.”
Waivers may be weak protection
Since the pandemic started, a number of states have enacted emergency malpractice protections to shield health professionals from lawsuits. Some protections, such as those in Massachusetts, offer immunity to health professionals who provide general care to patients during the COVID-19 emergency, in addition to treatment of COVID-19 patients. Other protections, like those in Connecticut, apply specifically to care provided in support of the state’s pandemic response.
Whether that immunity applies both to in-person visits and video visits during the pandemic is not certain, said J. Richard Moore, JD, a medical liability defense attorney based in Indianapolis. Indiana’s immunity statute for example, does not make a specific provision for telehealth, he said.
“My best prediction is that if considered by the courts, the immunity would be applied to telehealth services, so long as they are being provided ‘in response to the emergency,’ which is the scope of the immunity,” he said. “I would not consider telehealth physicians to be either more or less protected than in-person providers.”
Regulatory scrutiny for telehealth providers has also been relaxed in response to COVID-19, but experts warn not to rely on the temporary shields for ultimate protection.
In March, the U.S. Department of Health and Human Service’s Office of Civil Rights (OCR) eased enforcement actions for noncompliance with Health Insurance Portability and Accountability Act requirements in connection with the good faith provision of telehealth during the COVID-19 health crisis. Under the notice, health providers can use popular applications such as Apple FaceTime, Facebook Messenger, Zoom, or Google Hangouts, to offer telehealth care without risk that OCR will impose fines or penalties for HIPAA violations.
But once the current health care emergency is mitigated, the waivers will likely be withdrawn, and enforcement actions will probably resume, Mr. Cahill said.
“It is recommended that, to avoid potential problems going forward, practitioners use due diligence and undertake best efforts to obey existing privacy and security requirements, including the use of technology that satisfies compliance regulations, despite the waiver by OCR,” he said.
In addition, a majority of states have relaxed state-specific rules for practicing telehealth and loosened licensure requirements during the pandemic. At least 47 states have issued waivers to alter in-state licensure requirements for telemedicine in response to COVID-19, according to the Federation of State Medical Boards. Most of the waivers allow physicians licensed in other states to provide care in states where they do not hold licenses, and some enable doctors to treat patients without first having had an in-person evaluation.
But at least for now, these are temporary changes, reminds Amy Lerman, JD, a health care attorney based in Washington, who specializes in telehealth and corporate compliance. Given the current pandemic environment, a significant concern is that physicians new to the telemedicine space are reacting only to the most recent rules established in the context of the pandemic, Ms. Lerman said.
“As previously noted, the recent developments are temporary in nature – states and various federal agencies have been pretty clear in setting this temporal boundary,” she said. “It is not advisable for providers to build telepractice models around temporary sets of rules.
“Furthermore, the recent developments are not necessarily comprehensive relative to all of the state-specific and other requirements that telemedicine providers are otherwise expected to follow, so relying only on the most recent guidance may cause providers to create telepractice models that have key gaps with respect to regulatory compliance.”
How you can avoid a lawsuit
As businesses reopen and practices resume treatments, physicians should weigh the choice between in-person care and video visits very carefully, said Joseph Kvedar, MD, president of the American Telemedicine Association and a dermatology professor at Harvard Medical School, Boston.
“We have to be very thoughtful about quality in this current phase, where we are doing what I call a hybrid model,” he said. “Some services are offered by telehealth and some require patients to come into the doctor’s office. We have to be very thoughtful about what types of care we determine to be appropriate for telehealth, and that has to be based on clinical quality. And if it is, it should follow that we’ll have low incidence of liability claims.”
Data should be at the center of that conclusion, Dr. Kvedar advises.
“Think about what data is needed to make a therapeutic or diagnostic decision,” he said. “If a health care provider can gather the information needed without touching the patient, then the provider is probably on safe, solid ground making that decision via a telehealth interaction. If the patient can come into the doctor’s office, and the provider deems it necessary to see the patient in person and touch the patient in order to make that clinical decision, then the patient should come in.”
An important step to preventing liability is also having strong telehealth systems and protocols in place and the necessary support to carry them out, said Dr. Einbinder of medical liability insurer CRICO.
For example, Dr. Einbinder, who practices in a 12-doctor internal medicine group, said when he finishes a virtual visit, he enters any orders into the electronic health record. Some of the orders will result in notifications to Dr. Einbinder if they are not executed, such as a referral appointment or a procedure that was not completed.
“I also can forward my orders to a front desk pool that is responsible for making sure things get done,” he said. “And, in our hospital system, we have good case management for complex patients and population management for a variety of chronic conditions. These represent additional safety nets.”
Another liability safeguard is sending patients a “visit summary” after each virtual visit, Dr. Sullivan said. This could be in the form of an email or a text that includes a brief template including items such as diagnosis, recommendations, follow-up, and a reminder to contact the doctor or go to the emergency department if symptoms worsen or new problems develop.
“Patients tend to remember about half of what physicians tell them and half of the information patients do remember is incorrect,” he said. “Consider a few sentences in an e-mail or text message as a substitute for the after-visit instructions from an office visit to enhance patient understanding. There are several inexpensive programs/services that allow text messages to be sent from a computer using a separate dedicated phone number and pretty much every patient has a cell phone to receive the instructions.”
Dr. Sullivan suggests having a documentation template specifically for telehealth visits. He also recommends the inclusion an “informed refusal of care” in the record when necessary. Dr. Sullivan’s wife, a family physician, has encountered several patients who fear contracting COVID-19 and who have refused her recommendations for in-person visits, he said. In such cases, he said it’s a good idea to document that the patient decided to forgo the recommendations given.
“If a patient suffers a bad outcome because of a failure to seek an in-person exam, a short note in the patient’s chart would help to establish that the lack of a follow-up physical exam was the patient’s informed decision, not due to some alleged negligence of the medical provider,” he said.
Concerning informed consent, Dr. Feldman says at a minimum physicians should discuss the following with patients:
- Names and credentials of staff participating.
- The right to stop or refuse treatment by telemedicine.
- Technology that will be used.
- Privacy and security risks.
- Technology-specific risks and permission to bill.
- Alternative care in case of an emergency or technology malfunction.
- Any state-specific requirements.
“Physicians can ensure they have a strong informed consent process during video visits by taking the time to cover these points at the beginning of the first visit, and being sure the patient understands and agrees to these,” Dr. Sullivan explained. “Ideally, this conversation can be recorded for future reference if necessary or at a minimum documented in the medical record.”
Consider these extra precautions
Mr. Cahill advises that practitioners be especially mindful of their “web-side manner” and the setting in which they are communicating with virtual patients to promote confidentiality, professionalism, and uninterrupted interactions.
“Use of a headset in a quiet home office is advisable,” he said. “Physicians must also be cognizant of their physical appearance and the background behind them when the visit includes both audio and visual capability. For ‘face-to-face’ telehealth encounters, it is recommended that a white lab jacket be worn as the appropriate attire; coat and tie are unnecessary.”
Some patients may need to be reminded of the need for confidentiality during a video visit, Mr. Moore added. Physicians are typically in a position to ensure confidentiality, but some patients may not understand how to protect their privacy on their end.
“If the physician sees on the screen or hears from an audio connection that there are other people around who may be able to overhear what is communicated, the physician probably has some responsibility to remind the patient that she or he may want to go to a more private place, close the door, etc.,” he said. “While I think a claim against a physician on this basis would be pretty weak, it is still a good practice for the physician to be cognizant of those kinds of concerns even if the patient is not.”
Finally, for physicians who set up telehealth operability during the pandemic – possibly in a hurry – consider using your actual case data to take a look backward, said Ms. Lerman, the Washington-based health care attorney. Reviewing the data can help determine whether you’re in compliance with relevant state laws, she said.
“If, for example, a provider set up telehealth operations during the pandemic and can see that most of [the] patients are based in a single state, or a small group of states, it is worthwhile to take [the] time and become familiar with the telemedicine laws in those states,” she said. “If there are modifications that need to be made, it may be easier to make them incrementally before the telehealth operations grow any larger in scope.”
A version of this article originally appeared on Medscape.com.
During a telemedicine visit with his physician, a 62-year-old obese patient with an ankle injury reported new swelling of his leg. Three weeks had passed since the man visited an emergency department, where he underwent surgery and had a cast applied to the wound. The physician, during the telemedicine visit, advised the patient to elevate his leg and see an orthopedist within 24 hours. A Doppler ultrasound was ordered for 12:30 p.m. that same day.
The patient never made it to the appointment. He became unresponsive and went into full arrest hours later. His death fueled a lawsuit by his family that claimed failure to diagnose and treat deep venous thrombosis. The family contended the providers involved should have referred the patient to care immediately during the video visit.
The case, which comes from the claims database of national medical liability insurer The Doctors Company, illustrates the legal risks that can stem from video visits with patients, says Richard Cahill, JD, vice president and associate general counsel for The Doctors Company.
“By evaluating the patient remotely, the physician failed to appreciate the often subtle nuances of the clinical presentation, which undoubtedly could have been more accurately assessed in the office setting, and would probably have led to more urgent evaluation and intervention, thereby likely preventing the unfortunate and otherwise avoidable result,” said Mr. Cahill.
According to a Harris poll, 42% of Americans reported using video visits during the pandemic, a trend that is likely to continue as practices reopen and virtual care becomes the norm. But as physicians conduct more video visits, so grows their risk for lawsuits associated with the technology.
“We probably will see more malpractice suits filed the more telehealth is used,” said Mei Wa Kwong, JD, executive director of the Center for Connected Health Policy. “It’s a numbers game. The more it’s used, the higher likelihood that lawsuits occur.”
Three problems in not being able to touch the patient
1. The primary challenge with video visits “is the inability to directly observe and lay hands on the patient,” says Jonathan Einbinder, MD, assistant vice president of analytics for CRICO, a medical liability insurer based in Boston.
said Dr. Einbinder, a practicing internist.
Such incomplete pictures can lead to diagnostic errors and the potential for lawsuits, as demonstrated by a recent CRICO analysis. Of 106 telemedicine-related claims from 2014 to 2018, 66% were diagnosis related, according to the analysis of claims from CRICO’s national database. Twelve percent of the telemedicine-related claims were associated with surgical treatment, 11% were related to medical treatment, and 5% were associated with medication issues. A smaller number of claims resulted from patient monitoring, ob.gyn. care, and safety and security.
Another analysis by The Doctors Company similarly determined that diagnostic errors are the most common allegation in telemedicine-related claims. In the study of 28 telemedicine-related claims from The Doctors’ database, 71% were diagnosis related, 11% were associated with mismanagement of treatment, and 7% were related to improper management of a surgical patient. Other allegations included improper performance of treatment or procedure and improper performance of surgery.
“Because a ‘typical’ exam can’t be done, there is the potential to miss things,” said David L. Feldman, MD, chief medical officer for The Doctors Company Group. “A subtlety, perhaps a lump that can’t be seen but only felt, and only by an experienced examiner, for example, may be missed.”
2. Documentation dangers also loom, said William Sullivan, DO, JD, an emergency physician and an attorney who specializes in health care. The legal risk lies in documenting a video visit in the same way the doctor would document an in-person visit, he explained.
“Investigation into a potential lawsuit begins when there is some type of bad outcome related to medical care,” Dr. Sullivan explained. “To determine whether the lawsuit has merit, patients/attorneys review the medical records to retrospectively determine the potential cause of the bad outcome. If the documentation reflects an examination that could not have been performed, a lawyer might be more likely to pursue a case, and it would be more difficult to defend the care provided.”
Dr. Sullivan provided this example: During a video visit, a patient complains of acute onset weakness. The physician documents that the patient’s heart has a “regular rate and rhythm,” and “muscle strength is equal bilaterally.” The following day, the patient’s weakness continues, and the patient goes to the emergency department where he is diagnosed with stroke. An EKG in the ED shows that the patient is in atrial fibrillation.
“The telehealth provider would have a difficult time explaining how it was determined that the patient had normal muscle strength and a normal heart rhythm over a video visit the day before,” Dr. Sullivan said. “A lawyer in a subsequent malpractice case would present the provider as careless and would argue that if the provider had only sent the patient to the emergency department after the telehealth visit instead of documenting exam findings that couldn’t have been performed, the patient could have been successfully treated for the stroke.”
3. Poorly executed informed consent can also give rise to a lawsuit. This includes informed consent regarding the use of telehealth as the accepted modality for the visit rather than traditional on-site evaluations, as well as preprocedure informed consent.
“Inadequate and/or poorly documented informed consent can result in a claim for medical battery,” Mr. Cahill said.
A medical battery allegation refers to the alleged treatment or touching of a patient’s body without that person’s consent. As the AMA Journal of Ethics explains, a patient’s consent must be given, either expressly or implicitly, before a physician may legally “interfere” with the physical body of the patient.
Ideally, the informed consent process is undertaken during a first in-person visit, before virtual visits begin, Dr. Feldman said.
“There is a lot that a patient has to understand when a visit is done virtually, which is part of the informed consent process,” Dr. Feldman said. “The pandemic has forced some physicians to do their first visit virtually, and this makes the process of informing patients more onerous. It is not a simple matter of converting an in-person office practice to a remote office practice. The work flows are different, so there are definitely legal concerns as it relates to privacy and cybersecurity to name a few.”
Waivers may be weak protection
Since the pandemic started, a number of states have enacted emergency malpractice protections to shield health professionals from lawsuits. Some protections, such as those in Massachusetts, offer immunity to health professionals who provide general care to patients during the COVID-19 emergency, in addition to treatment of COVID-19 patients. Other protections, like those in Connecticut, apply specifically to care provided in support of the state’s pandemic response.
Whether that immunity applies both to in-person visits and video visits during the pandemic is not certain, said J. Richard Moore, JD, a medical liability defense attorney based in Indianapolis. Indiana’s immunity statute for example, does not make a specific provision for telehealth, he said.
“My best prediction is that if considered by the courts, the immunity would be applied to telehealth services, so long as they are being provided ‘in response to the emergency,’ which is the scope of the immunity,” he said. “I would not consider telehealth physicians to be either more or less protected than in-person providers.”
Regulatory scrutiny for telehealth providers has also been relaxed in response to COVID-19, but experts warn not to rely on the temporary shields for ultimate protection.
In March, the U.S. Department of Health and Human Service’s Office of Civil Rights (OCR) eased enforcement actions for noncompliance with Health Insurance Portability and Accountability Act requirements in connection with the good faith provision of telehealth during the COVID-19 health crisis. Under the notice, health providers can use popular applications such as Apple FaceTime, Facebook Messenger, Zoom, or Google Hangouts, to offer telehealth care without risk that OCR will impose fines or penalties for HIPAA violations.
But once the current health care emergency is mitigated, the waivers will likely be withdrawn, and enforcement actions will probably resume, Mr. Cahill said.
“It is recommended that, to avoid potential problems going forward, practitioners use due diligence and undertake best efforts to obey existing privacy and security requirements, including the use of technology that satisfies compliance regulations, despite the waiver by OCR,” he said.
In addition, a majority of states have relaxed state-specific rules for practicing telehealth and loosened licensure requirements during the pandemic. At least 47 states have issued waivers to alter in-state licensure requirements for telemedicine in response to COVID-19, according to the Federation of State Medical Boards. Most of the waivers allow physicians licensed in other states to provide care in states where they do not hold licenses, and some enable doctors to treat patients without first having had an in-person evaluation.
But at least for now, these are temporary changes, reminds Amy Lerman, JD, a health care attorney based in Washington, who specializes in telehealth and corporate compliance. Given the current pandemic environment, a significant concern is that physicians new to the telemedicine space are reacting only to the most recent rules established in the context of the pandemic, Ms. Lerman said.
“As previously noted, the recent developments are temporary in nature – states and various federal agencies have been pretty clear in setting this temporal boundary,” she said. “It is not advisable for providers to build telepractice models around temporary sets of rules.
“Furthermore, the recent developments are not necessarily comprehensive relative to all of the state-specific and other requirements that telemedicine providers are otherwise expected to follow, so relying only on the most recent guidance may cause providers to create telepractice models that have key gaps with respect to regulatory compliance.”
How you can avoid a lawsuit
As businesses reopen and practices resume treatments, physicians should weigh the choice between in-person care and video visits very carefully, said Joseph Kvedar, MD, president of the American Telemedicine Association and a dermatology professor at Harvard Medical School, Boston.
“We have to be very thoughtful about quality in this current phase, where we are doing what I call a hybrid model,” he said. “Some services are offered by telehealth and some require patients to come into the doctor’s office. We have to be very thoughtful about what types of care we determine to be appropriate for telehealth, and that has to be based on clinical quality. And if it is, it should follow that we’ll have low incidence of liability claims.”
Data should be at the center of that conclusion, Dr. Kvedar advises.
“Think about what data is needed to make a therapeutic or diagnostic decision,” he said. “If a health care provider can gather the information needed without touching the patient, then the provider is probably on safe, solid ground making that decision via a telehealth interaction. If the patient can come into the doctor’s office, and the provider deems it necessary to see the patient in person and touch the patient in order to make that clinical decision, then the patient should come in.”
An important step to preventing liability is also having strong telehealth systems and protocols in place and the necessary support to carry them out, said Dr. Einbinder of medical liability insurer CRICO.
For example, Dr. Einbinder, who practices in a 12-doctor internal medicine group, said when he finishes a virtual visit, he enters any orders into the electronic health record. Some of the orders will result in notifications to Dr. Einbinder if they are not executed, such as a referral appointment or a procedure that was not completed.
“I also can forward my orders to a front desk pool that is responsible for making sure things get done,” he said. “And, in our hospital system, we have good case management for complex patients and population management for a variety of chronic conditions. These represent additional safety nets.”
Another liability safeguard is sending patients a “visit summary” after each virtual visit, Dr. Sullivan said. This could be in the form of an email or a text that includes a brief template including items such as diagnosis, recommendations, follow-up, and a reminder to contact the doctor or go to the emergency department if symptoms worsen or new problems develop.
“Patients tend to remember about half of what physicians tell them and half of the information patients do remember is incorrect,” he said. “Consider a few sentences in an e-mail or text message as a substitute for the after-visit instructions from an office visit to enhance patient understanding. There are several inexpensive programs/services that allow text messages to be sent from a computer using a separate dedicated phone number and pretty much every patient has a cell phone to receive the instructions.”
Dr. Sullivan suggests having a documentation template specifically for telehealth visits. He also recommends the inclusion an “informed refusal of care” in the record when necessary. Dr. Sullivan’s wife, a family physician, has encountered several patients who fear contracting COVID-19 and who have refused her recommendations for in-person visits, he said. In such cases, he said it’s a good idea to document that the patient decided to forgo the recommendations given.
“If a patient suffers a bad outcome because of a failure to seek an in-person exam, a short note in the patient’s chart would help to establish that the lack of a follow-up physical exam was the patient’s informed decision, not due to some alleged negligence of the medical provider,” he said.
Concerning informed consent, Dr. Feldman says at a minimum physicians should discuss the following with patients:
- Names and credentials of staff participating.
- The right to stop or refuse treatment by telemedicine.
- Technology that will be used.
- Privacy and security risks.
- Technology-specific risks and permission to bill.
- Alternative care in case of an emergency or technology malfunction.
- Any state-specific requirements.
“Physicians can ensure they have a strong informed consent process during video visits by taking the time to cover these points at the beginning of the first visit, and being sure the patient understands and agrees to these,” Dr. Sullivan explained. “Ideally, this conversation can be recorded for future reference if necessary or at a minimum documented in the medical record.”
Consider these extra precautions
Mr. Cahill advises that practitioners be especially mindful of their “web-side manner” and the setting in which they are communicating with virtual patients to promote confidentiality, professionalism, and uninterrupted interactions.
“Use of a headset in a quiet home office is advisable,” he said. “Physicians must also be cognizant of their physical appearance and the background behind them when the visit includes both audio and visual capability. For ‘face-to-face’ telehealth encounters, it is recommended that a white lab jacket be worn as the appropriate attire; coat and tie are unnecessary.”
Some patients may need to be reminded of the need for confidentiality during a video visit, Mr. Moore added. Physicians are typically in a position to ensure confidentiality, but some patients may not understand how to protect their privacy on their end.
“If the physician sees on the screen or hears from an audio connection that there are other people around who may be able to overhear what is communicated, the physician probably has some responsibility to remind the patient that she or he may want to go to a more private place, close the door, etc.,” he said. “While I think a claim against a physician on this basis would be pretty weak, it is still a good practice for the physician to be cognizant of those kinds of concerns even if the patient is not.”
Finally, for physicians who set up telehealth operability during the pandemic – possibly in a hurry – consider using your actual case data to take a look backward, said Ms. Lerman, the Washington-based health care attorney. Reviewing the data can help determine whether you’re in compliance with relevant state laws, she said.
“If, for example, a provider set up telehealth operations during the pandemic and can see that most of [the] patients are based in a single state, or a small group of states, it is worthwhile to take [the] time and become familiar with the telemedicine laws in those states,” she said. “If there are modifications that need to be made, it may be easier to make them incrementally before the telehealth operations grow any larger in scope.”
A version of this article originally appeared on Medscape.com.
The long road to a PsA prevention trial
About one-third of all patients with psoriasis will develop psoriatic arthritis (PsA), a condition that comes with a host of vague symptoms and no definitive blood test for diagnosis. Prevention trials could help to identify higher-risk groups for PsA, with a goal to catch disease early and improve outcomes. The challenge is finding enough participants in a disease that lacks a clear clinical profile, then tracking them for long periods of time to generate any significant data.
Researchers have been taking several approaches to improve outcomes in PsA, Christopher Ritchlin, MD, MPH, chief of the allergy/immunology and rheumatology division at the University of Rochester (N.Y.), said in an interview. “We are in the process of identifying biomarkers and imaging findings that characterize psoriasis patients at high risk to develop PsA.”
The next step would be to design an interventional trial to treat high-risk patients before they develop musculoskeletal inflammation, with a goal to delay onset, attenuate severity, or completely prevent arthritis. The issue now is “we don’t know which agents would be most effective in this prevention effort,” Dr. Ritchlin said. Biologics that target specific pathways significant in PsA pathogenesis are an appealing prospect. However, “it may be that alternative therapies such as methotrexate or ultraviolet A radiation therapy, for example, may help arrest the development of joint inflammation.”
Underdiagnosis impedes research
Several factors may undermine this important research.
For one, psoriasis patients are often unaware that they have PsA. “Many times they are diagnosed incorrectly by nonspecialists. As a consequence, they do not get access to appropriate medications,” said Lihi Eder, MD, PhD, staff rheumatologist and director of the psoriatic arthritis research program at the University of Toronto’s Women’s College Research Institute.
The condition also lacks a good diagnostic tool, Dr. Eder said. There’s no blood test that identifies this condition in the same manner as RA and lupus, for example. For these conditions, a general practitioner such as a family physician may conduct a blood test, and if positive, refer them to a rheumatologist. Such a system doesn’t exist for PsA. “Instead, nonspecialists are ordering tests and when they’re negative, they assume wrongly that these patients don’t have a rheumatic condition,” she explained.
Many clinicians aren’t that well versed in PsA, although dermatology has taken steps to become better educated. As a result, more dermatologists are referring patients to rheumatologists for PsA. Despite this small step forward, the heterogeneous clinical presentation of this condition makes diagnosis especially difficult. Unlike RA, which presents with inflammation in the joints, PsA can present as back or joint pain, “which makes our life as rheumatologists much more complex,” Dr. Eder said.
Defining a risk group
Most experts agree that the presence of psoriasis isn’t sufficient to conduct a prevention trial. Ideally, the goal of a prevention study would be to identify a critical subgroup of psoriasis patients at high risk of developing PsA.
However, this presents a challenging task, Dr. Eder said. Psoriasis is a risk factor for PsA, but most patients with psoriasis won’t actually develop it. Given that there’s an incidence rate of 2.7% a year, “you would need to recruit many hundreds of psoriasis patients and follow them for a long period of time until you have enough events.”
Moving forward with prevention studies calls for a better definition of the PsA risk group, according to Georg Schett, MD, chair of internal medicine in the department of internal medicine, rheumatology, and immunology at Friedrich‐Alexander University, Erlangen, Germany. “That’s very important, because you need to define such a group to make a prevention trial feasible. The whole benefit of such an approach is to catch the disease early, to say that psoriasis is a biomarker that’s linked to psoriatic arthritis.”
Indicators of risk other than psoriasis, such as pain, inflammation seen in ultrasound or MRI, and other specificities of psoriasis, could be used to define a population where interception can take place, Dr. Schett added. Although it’s not always clinically recognized, the combination of pain and structural lesions can be an indicator for developing PsA.
One prospective study he and his colleagues conducted in 114 psoriasis patients cited structural entheseal lesions and low cortical volumetric bone mineral density as risk factors in developing PsA. Keeping these factors in mind, Dr. Schett expects to see more studies in biointervention in these populations, “with the idea to prevent the onset of PsA and also decrease pain and subcutaneous inflammation.”
Researchers are currently working to identify those high-risk patients to include in an interventional trial, Dr. Ritchlin said.
That said, there’s been a great deal of “clinical trial angst” among investigators, Dr. Ritchlin noted. Outcomes in clinical trials for a wide range of biologic agents have not demonstrated significant advances in outcomes, compared with initial studies with anti–tumor necrosis factor–alpha (TNF-alpha) agents 20 years ago.
Combination biologics
One approach that’s generated some interest is the use of combination biologics medications. Sequential inhibition of cytokines such as interleukin-17A and TNF-alpha is of interest given their central contribution in joint inflammation and damage. “The challenge here of course is toxicity,” Dr. Ritchlin said. Trials that combined blockade of IL-1 and TNF-alpha in a RA trial years ago resulted in significant adverse events without improving outcomes.
Comparatively, a recent study in The Lancet Rheumatology reported success in using the IL-17A inhibitor secukinumab (Cosentyx) to reduce PsA symptoms. Tested on patients in the FUTURE 2 trial, investigators demonstrated that secukinumab in 300- and 150-mg doses safely reduced PsA signs and symptoms over a period of 5 years. Secukinumab also outperformed the TNF-alpha inhibitor adalimumab in 853 PsA patients in the 52-week, randomized, head-to-head, phase 3b EXCEED study, which was recently reported in The Lancet. Articular outcomes were similar between the two therapies, yet the secukinumab group did markedly better in Psoriasis Area and Severity Index scores, compared with the adalimumab group.
Based on these findings, “I suspect that studies examining the efficacy of combination biologics for treatment of PsA will surface in the near future,” Dr. Ritchlin said.
Yet another approach encompasses the spirit of personalized medicine. Clinicians often treat PsA patients empirically because they lack biomarkers that indicate which drug may be most effective for an individual patient, Dr. Ritchlin said. However, the technologies for investigating specific cell subsets in both the blood and tissues have advanced greatly over the last decade. “I am confident that a more precision medicine–based approach to the diagnosis and treatment of PsA is on the near horizon.”
Diet as an intervention
Other research has looked at the strong link between metabolic abnormalities and psoriasis and PsA. Some diets, such as the Mediterranean diet, show promise in improving the metabolic profile of these patients, making it a candidate as a potential intervention to reduce PsA risk. Another strategy would be to focus on limiting calories and promoting weight reduction.
One study in the British Journal of Dermatology looked at the associations between PsA and smoking, alcohol, and body mass index, identifying obesity as an important risk factor. Analyzing more than 90,000 psoriasis cases from the U.K. Clinical Practice Research Datalink between 1998 and 2014, researchers identified 1,409 PsA diagnoses. Among this cohort, researchers found an association between PsA and increased body mass index and moderate drinking. This finding underscores the need to support weight-reduction programs to reduce risk, Dr. Eder and Alexis Ogdie, MD, of the University of Pennsylvania, Philadelphia, wrote in a related editorial.
While observational studies such as this one provide further guidance for interventional trials, confounders can affect results. “Patients who lost weight could have made a positive lifestyle change (e.g., a dietary change) that was associated with the decreased risk for PsA rather than weight loss specifically, or they could have lost weight for unhealthy reasons,” Dr. Eder and Dr. Ogdie explained. Future research could address whether weight loss or other interventional factors may reduce PsA progression.
To make this work, “we would need to select patients that would benefit from diet. Secondly, we’d need to identify what kind of diet would be good for preventing PsA. And we don’t know that yet,” Dr. Eder further elaborated.
As with any prevention trial, the challenge is to follow patients over a long period of time, making sure they comply with the restrictions of the prescribed diet, Dr. Eder noted. “I do think it’s a really exciting type of intervention because it’s something that people are very interested in. There’s little risk of side effects, and it’s not very expensive.”
In other research on weight-loss methods, an observational study from Denmark found that bariatric surgery, especially gastric bypass, reduced the risk of developing PsA. This suggests that weight reduction by itself is important, “although we don’t know that yet,” Dr. Eder said.
A risk model for PsA
Dr. Eder and colleagues have been working on an algorithm that will incorporate clinical information (for example, the presence of nail lesions and the severity of psoriasis) to provide an estimated risk of developing PsA over the next 5 years. Subsequently, this information could be used to identify high-risk psoriasis patients as candidates for a prevention trial.
Other groups are looking at laboratory or imaging biomarkers to help develop PsA prediction models, she said. “Once we have these tools, we can move to next steps of prevention trials. What kinds of interventions should we apply? Are we talking biologic medications or other lifestyle interventions like diet? We are still at the early stages. However, with an improved understanding of the underlying mechanisms and risk factors we are expected to see prevention trials for PsA in the future.”
About one-third of all patients with psoriasis will develop psoriatic arthritis (PsA), a condition that comes with a host of vague symptoms and no definitive blood test for diagnosis. Prevention trials could help to identify higher-risk groups for PsA, with a goal to catch disease early and improve outcomes. The challenge is finding enough participants in a disease that lacks a clear clinical profile, then tracking them for long periods of time to generate any significant data.
Researchers have been taking several approaches to improve outcomes in PsA, Christopher Ritchlin, MD, MPH, chief of the allergy/immunology and rheumatology division at the University of Rochester (N.Y.), said in an interview. “We are in the process of identifying biomarkers and imaging findings that characterize psoriasis patients at high risk to develop PsA.”
The next step would be to design an interventional trial to treat high-risk patients before they develop musculoskeletal inflammation, with a goal to delay onset, attenuate severity, or completely prevent arthritis. The issue now is “we don’t know which agents would be most effective in this prevention effort,” Dr. Ritchlin said. Biologics that target specific pathways significant in PsA pathogenesis are an appealing prospect. However, “it may be that alternative therapies such as methotrexate or ultraviolet A radiation therapy, for example, may help arrest the development of joint inflammation.”
Underdiagnosis impedes research
Several factors may undermine this important research.
For one, psoriasis patients are often unaware that they have PsA. “Many times they are diagnosed incorrectly by nonspecialists. As a consequence, they do not get access to appropriate medications,” said Lihi Eder, MD, PhD, staff rheumatologist and director of the psoriatic arthritis research program at the University of Toronto’s Women’s College Research Institute.
The condition also lacks a good diagnostic tool, Dr. Eder said. There’s no blood test that identifies this condition in the same manner as RA and lupus, for example. For these conditions, a general practitioner such as a family physician may conduct a blood test, and if positive, refer them to a rheumatologist. Such a system doesn’t exist for PsA. “Instead, nonspecialists are ordering tests and when they’re negative, they assume wrongly that these patients don’t have a rheumatic condition,” she explained.
Many clinicians aren’t that well versed in PsA, although dermatology has taken steps to become better educated. As a result, more dermatologists are referring patients to rheumatologists for PsA. Despite this small step forward, the heterogeneous clinical presentation of this condition makes diagnosis especially difficult. Unlike RA, which presents with inflammation in the joints, PsA can present as back or joint pain, “which makes our life as rheumatologists much more complex,” Dr. Eder said.
Defining a risk group
Most experts agree that the presence of psoriasis isn’t sufficient to conduct a prevention trial. Ideally, the goal of a prevention study would be to identify a critical subgroup of psoriasis patients at high risk of developing PsA.
However, this presents a challenging task, Dr. Eder said. Psoriasis is a risk factor for PsA, but most patients with psoriasis won’t actually develop it. Given that there’s an incidence rate of 2.7% a year, “you would need to recruit many hundreds of psoriasis patients and follow them for a long period of time until you have enough events.”
Moving forward with prevention studies calls for a better definition of the PsA risk group, according to Georg Schett, MD, chair of internal medicine in the department of internal medicine, rheumatology, and immunology at Friedrich‐Alexander University, Erlangen, Germany. “That’s very important, because you need to define such a group to make a prevention trial feasible. The whole benefit of such an approach is to catch the disease early, to say that psoriasis is a biomarker that’s linked to psoriatic arthritis.”
Indicators of risk other than psoriasis, such as pain, inflammation seen in ultrasound or MRI, and other specificities of psoriasis, could be used to define a population where interception can take place, Dr. Schett added. Although it’s not always clinically recognized, the combination of pain and structural lesions can be an indicator for developing PsA.
One prospective study he and his colleagues conducted in 114 psoriasis patients cited structural entheseal lesions and low cortical volumetric bone mineral density as risk factors in developing PsA. Keeping these factors in mind, Dr. Schett expects to see more studies in biointervention in these populations, “with the idea to prevent the onset of PsA and also decrease pain and subcutaneous inflammation.”
Researchers are currently working to identify those high-risk patients to include in an interventional trial, Dr. Ritchlin said.
That said, there’s been a great deal of “clinical trial angst” among investigators, Dr. Ritchlin noted. Outcomes in clinical trials for a wide range of biologic agents have not demonstrated significant advances in outcomes, compared with initial studies with anti–tumor necrosis factor–alpha (TNF-alpha) agents 20 years ago.
Combination biologics
One approach that’s generated some interest is the use of combination biologics medications. Sequential inhibition of cytokines such as interleukin-17A and TNF-alpha is of interest given their central contribution in joint inflammation and damage. “The challenge here of course is toxicity,” Dr. Ritchlin said. Trials that combined blockade of IL-1 and TNF-alpha in a RA trial years ago resulted in significant adverse events without improving outcomes.
Comparatively, a recent study in The Lancet Rheumatology reported success in using the IL-17A inhibitor secukinumab (Cosentyx) to reduce PsA symptoms. Tested on patients in the FUTURE 2 trial, investigators demonstrated that secukinumab in 300- and 150-mg doses safely reduced PsA signs and symptoms over a period of 5 years. Secukinumab also outperformed the TNF-alpha inhibitor adalimumab in 853 PsA patients in the 52-week, randomized, head-to-head, phase 3b EXCEED study, which was recently reported in The Lancet. Articular outcomes were similar between the two therapies, yet the secukinumab group did markedly better in Psoriasis Area and Severity Index scores, compared with the adalimumab group.
Based on these findings, “I suspect that studies examining the efficacy of combination biologics for treatment of PsA will surface in the near future,” Dr. Ritchlin said.
Yet another approach encompasses the spirit of personalized medicine. Clinicians often treat PsA patients empirically because they lack biomarkers that indicate which drug may be most effective for an individual patient, Dr. Ritchlin said. However, the technologies for investigating specific cell subsets in both the blood and tissues have advanced greatly over the last decade. “I am confident that a more precision medicine–based approach to the diagnosis and treatment of PsA is on the near horizon.”
Diet as an intervention
Other research has looked at the strong link between metabolic abnormalities and psoriasis and PsA. Some diets, such as the Mediterranean diet, show promise in improving the metabolic profile of these patients, making it a candidate as a potential intervention to reduce PsA risk. Another strategy would be to focus on limiting calories and promoting weight reduction.
One study in the British Journal of Dermatology looked at the associations between PsA and smoking, alcohol, and body mass index, identifying obesity as an important risk factor. Analyzing more than 90,000 psoriasis cases from the U.K. Clinical Practice Research Datalink between 1998 and 2014, researchers identified 1,409 PsA diagnoses. Among this cohort, researchers found an association between PsA and increased body mass index and moderate drinking. This finding underscores the need to support weight-reduction programs to reduce risk, Dr. Eder and Alexis Ogdie, MD, of the University of Pennsylvania, Philadelphia, wrote in a related editorial.
While observational studies such as this one provide further guidance for interventional trials, confounders can affect results. “Patients who lost weight could have made a positive lifestyle change (e.g., a dietary change) that was associated with the decreased risk for PsA rather than weight loss specifically, or they could have lost weight for unhealthy reasons,” Dr. Eder and Dr. Ogdie explained. Future research could address whether weight loss or other interventional factors may reduce PsA progression.
To make this work, “we would need to select patients that would benefit from diet. Secondly, we’d need to identify what kind of diet would be good for preventing PsA. And we don’t know that yet,” Dr. Eder further elaborated.
As with any prevention trial, the challenge is to follow patients over a long period of time, making sure they comply with the restrictions of the prescribed diet, Dr. Eder noted. “I do think it’s a really exciting type of intervention because it’s something that people are very interested in. There’s little risk of side effects, and it’s not very expensive.”
In other research on weight-loss methods, an observational study from Denmark found that bariatric surgery, especially gastric bypass, reduced the risk of developing PsA. This suggests that weight reduction by itself is important, “although we don’t know that yet,” Dr. Eder said.
A risk model for PsA
Dr. Eder and colleagues have been working on an algorithm that will incorporate clinical information (for example, the presence of nail lesions and the severity of psoriasis) to provide an estimated risk of developing PsA over the next 5 years. Subsequently, this information could be used to identify high-risk psoriasis patients as candidates for a prevention trial.
Other groups are looking at laboratory or imaging biomarkers to help develop PsA prediction models, she said. “Once we have these tools, we can move to next steps of prevention trials. What kinds of interventions should we apply? Are we talking biologic medications or other lifestyle interventions like diet? We are still at the early stages. However, with an improved understanding of the underlying mechanisms and risk factors we are expected to see prevention trials for PsA in the future.”
About one-third of all patients with psoriasis will develop psoriatic arthritis (PsA), a condition that comes with a host of vague symptoms and no definitive blood test for diagnosis. Prevention trials could help to identify higher-risk groups for PsA, with a goal to catch disease early and improve outcomes. The challenge is finding enough participants in a disease that lacks a clear clinical profile, then tracking them for long periods of time to generate any significant data.
Researchers have been taking several approaches to improve outcomes in PsA, Christopher Ritchlin, MD, MPH, chief of the allergy/immunology and rheumatology division at the University of Rochester (N.Y.), said in an interview. “We are in the process of identifying biomarkers and imaging findings that characterize psoriasis patients at high risk to develop PsA.”
The next step would be to design an interventional trial to treat high-risk patients before they develop musculoskeletal inflammation, with a goal to delay onset, attenuate severity, or completely prevent arthritis. The issue now is “we don’t know which agents would be most effective in this prevention effort,” Dr. Ritchlin said. Biologics that target specific pathways significant in PsA pathogenesis are an appealing prospect. However, “it may be that alternative therapies such as methotrexate or ultraviolet A radiation therapy, for example, may help arrest the development of joint inflammation.”
Underdiagnosis impedes research
Several factors may undermine this important research.
For one, psoriasis patients are often unaware that they have PsA. “Many times they are diagnosed incorrectly by nonspecialists. As a consequence, they do not get access to appropriate medications,” said Lihi Eder, MD, PhD, staff rheumatologist and director of the psoriatic arthritis research program at the University of Toronto’s Women’s College Research Institute.
The condition also lacks a good diagnostic tool, Dr. Eder said. There’s no blood test that identifies this condition in the same manner as RA and lupus, for example. For these conditions, a general practitioner such as a family physician may conduct a blood test, and if positive, refer them to a rheumatologist. Such a system doesn’t exist for PsA. “Instead, nonspecialists are ordering tests and when they’re negative, they assume wrongly that these patients don’t have a rheumatic condition,” she explained.
Many clinicians aren’t that well versed in PsA, although dermatology has taken steps to become better educated. As a result, more dermatologists are referring patients to rheumatologists for PsA. Despite this small step forward, the heterogeneous clinical presentation of this condition makes diagnosis especially difficult. Unlike RA, which presents with inflammation in the joints, PsA can present as back or joint pain, “which makes our life as rheumatologists much more complex,” Dr. Eder said.
Defining a risk group
Most experts agree that the presence of psoriasis isn’t sufficient to conduct a prevention trial. Ideally, the goal of a prevention study would be to identify a critical subgroup of psoriasis patients at high risk of developing PsA.
However, this presents a challenging task, Dr. Eder said. Psoriasis is a risk factor for PsA, but most patients with psoriasis won’t actually develop it. Given that there’s an incidence rate of 2.7% a year, “you would need to recruit many hundreds of psoriasis patients and follow them for a long period of time until you have enough events.”
Moving forward with prevention studies calls for a better definition of the PsA risk group, according to Georg Schett, MD, chair of internal medicine in the department of internal medicine, rheumatology, and immunology at Friedrich‐Alexander University, Erlangen, Germany. “That’s very important, because you need to define such a group to make a prevention trial feasible. The whole benefit of such an approach is to catch the disease early, to say that psoriasis is a biomarker that’s linked to psoriatic arthritis.”
Indicators of risk other than psoriasis, such as pain, inflammation seen in ultrasound or MRI, and other specificities of psoriasis, could be used to define a population where interception can take place, Dr. Schett added. Although it’s not always clinically recognized, the combination of pain and structural lesions can be an indicator for developing PsA.
One prospective study he and his colleagues conducted in 114 psoriasis patients cited structural entheseal lesions and low cortical volumetric bone mineral density as risk factors in developing PsA. Keeping these factors in mind, Dr. Schett expects to see more studies in biointervention in these populations, “with the idea to prevent the onset of PsA and also decrease pain and subcutaneous inflammation.”
Researchers are currently working to identify those high-risk patients to include in an interventional trial, Dr. Ritchlin said.
That said, there’s been a great deal of “clinical trial angst” among investigators, Dr. Ritchlin noted. Outcomes in clinical trials for a wide range of biologic agents have not demonstrated significant advances in outcomes, compared with initial studies with anti–tumor necrosis factor–alpha (TNF-alpha) agents 20 years ago.
Combination biologics
One approach that’s generated some interest is the use of combination biologics medications. Sequential inhibition of cytokines such as interleukin-17A and TNF-alpha is of interest given their central contribution in joint inflammation and damage. “The challenge here of course is toxicity,” Dr. Ritchlin said. Trials that combined blockade of IL-1 and TNF-alpha in a RA trial years ago resulted in significant adverse events without improving outcomes.
Comparatively, a recent study in The Lancet Rheumatology reported success in using the IL-17A inhibitor secukinumab (Cosentyx) to reduce PsA symptoms. Tested on patients in the FUTURE 2 trial, investigators demonstrated that secukinumab in 300- and 150-mg doses safely reduced PsA signs and symptoms over a period of 5 years. Secukinumab also outperformed the TNF-alpha inhibitor adalimumab in 853 PsA patients in the 52-week, randomized, head-to-head, phase 3b EXCEED study, which was recently reported in The Lancet. Articular outcomes were similar between the two therapies, yet the secukinumab group did markedly better in Psoriasis Area and Severity Index scores, compared with the adalimumab group.
Based on these findings, “I suspect that studies examining the efficacy of combination biologics for treatment of PsA will surface in the near future,” Dr. Ritchlin said.
Yet another approach encompasses the spirit of personalized medicine. Clinicians often treat PsA patients empirically because they lack biomarkers that indicate which drug may be most effective for an individual patient, Dr. Ritchlin said. However, the technologies for investigating specific cell subsets in both the blood and tissues have advanced greatly over the last decade. “I am confident that a more precision medicine–based approach to the diagnosis and treatment of PsA is on the near horizon.”
Diet as an intervention
Other research has looked at the strong link between metabolic abnormalities and psoriasis and PsA. Some diets, such as the Mediterranean diet, show promise in improving the metabolic profile of these patients, making it a candidate as a potential intervention to reduce PsA risk. Another strategy would be to focus on limiting calories and promoting weight reduction.
One study in the British Journal of Dermatology looked at the associations between PsA and smoking, alcohol, and body mass index, identifying obesity as an important risk factor. Analyzing more than 90,000 psoriasis cases from the U.K. Clinical Practice Research Datalink between 1998 and 2014, researchers identified 1,409 PsA diagnoses. Among this cohort, researchers found an association between PsA and increased body mass index and moderate drinking. This finding underscores the need to support weight-reduction programs to reduce risk, Dr. Eder and Alexis Ogdie, MD, of the University of Pennsylvania, Philadelphia, wrote in a related editorial.
While observational studies such as this one provide further guidance for interventional trials, confounders can affect results. “Patients who lost weight could have made a positive lifestyle change (e.g., a dietary change) that was associated with the decreased risk for PsA rather than weight loss specifically, or they could have lost weight for unhealthy reasons,” Dr. Eder and Dr. Ogdie explained. Future research could address whether weight loss or other interventional factors may reduce PsA progression.
To make this work, “we would need to select patients that would benefit from diet. Secondly, we’d need to identify what kind of diet would be good for preventing PsA. And we don’t know that yet,” Dr. Eder further elaborated.
As with any prevention trial, the challenge is to follow patients over a long period of time, making sure they comply with the restrictions of the prescribed diet, Dr. Eder noted. “I do think it’s a really exciting type of intervention because it’s something that people are very interested in. There’s little risk of side effects, and it’s not very expensive.”
In other research on weight-loss methods, an observational study from Denmark found that bariatric surgery, especially gastric bypass, reduced the risk of developing PsA. This suggests that weight reduction by itself is important, “although we don’t know that yet,” Dr. Eder said.
A risk model for PsA
Dr. Eder and colleagues have been working on an algorithm that will incorporate clinical information (for example, the presence of nail lesions and the severity of psoriasis) to provide an estimated risk of developing PsA over the next 5 years. Subsequently, this information could be used to identify high-risk psoriasis patients as candidates for a prevention trial.
Other groups are looking at laboratory or imaging biomarkers to help develop PsA prediction models, she said. “Once we have these tools, we can move to next steps of prevention trials. What kinds of interventions should we apply? Are we talking biologic medications or other lifestyle interventions like diet? We are still at the early stages. However, with an improved understanding of the underlying mechanisms and risk factors we are expected to see prevention trials for PsA in the future.”
Hot Topics in Primary Care 2020


Click here to read Hot Topics in Primary Care.
This supplement includes 7 CME credits (scroll down for more information).
Topics include:
- Nutritional Gaps
- Irritable Bowel Syndrome
- Asthma Management
- Hyperlipidemia
- Heart Failure
- Efficacy and Safety of Naproxen
- Dementia-Related Delusions and Hallucinations
- Insomnia
- Autosomal Dominant Polycystic Kidney Disease
- Hypoglycemia Management
- Chronic Obstructive Pulmonary Disease
- Diabetic Kidney Disease
- Burden of Overweight
This supplement offers the opportunity to earn a total of 7 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below. These links may also be found within the supplement on the first page of each article.
- Addressing Nutritional Gaps: Simple Steps for the Primary Care Provider
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/nutrition to complete the online post-test and receive your certificate of credit.
- An Individualized, Case-Based Approach to the Management of Irritable Bowel Syndrome
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/ibs to complete the online post-test and receive your certificate of credit.
- Managing the Burden of Dementia-Related Delusions and Hallucinations
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/dementia to complete the online post-test and receive your certificate of credit.
- Overcoming Barriers to the Diagnosis and Treatment of Insomnia
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/insomnia2 to complete the online post-test and receive your certificate of credit.
- Recognition and Management of a Less Common Cause of Chronic Kidney Disease: Autosomal Dominant Polycystic Kidney Disease
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/ADPKD to complete the online post-test and receive your certificate of credit.
- Review of LDL-C-Lowering with Focus on New and Emerging Agents
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/hyper to complete the online post-test and receive your certificate of credit.
- Stemming the Progression of Diabetic Kidney Disease: The Role of the Primary Care Clinician
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/DKD2 to complete the online post-test and receive your certificate of credit.


Click here to read Hot Topics in Primary Care.
This supplement includes 7 CME credits (scroll down for more information).
Topics include:
- Nutritional Gaps
- Irritable Bowel Syndrome
- Asthma Management
- Hyperlipidemia
- Heart Failure
- Efficacy and Safety of Naproxen
- Dementia-Related Delusions and Hallucinations
- Insomnia
- Autosomal Dominant Polycystic Kidney Disease
- Hypoglycemia Management
- Chronic Obstructive Pulmonary Disease
- Diabetic Kidney Disease
- Burden of Overweight
This supplement offers the opportunity to earn a total of 7 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below. These links may also be found within the supplement on the first page of each article.
- Addressing Nutritional Gaps: Simple Steps for the Primary Care Provider
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/nutrition to complete the online post-test and receive your certificate of credit.
- An Individualized, Case-Based Approach to the Management of Irritable Bowel Syndrome
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/ibs to complete the online post-test and receive your certificate of credit.
- Managing the Burden of Dementia-Related Delusions and Hallucinations
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/dementia to complete the online post-test and receive your certificate of credit.
- Overcoming Barriers to the Diagnosis and Treatment of Insomnia
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/insomnia2 to complete the online post-test and receive your certificate of credit.
- Recognition and Management of a Less Common Cause of Chronic Kidney Disease: Autosomal Dominant Polycystic Kidney Disease
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/ADPKD to complete the online post-test and receive your certificate of credit.
- Review of LDL-C-Lowering with Focus on New and Emerging Agents
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/hyper to complete the online post-test and receive your certificate of credit.
- Stemming the Progression of Diabetic Kidney Disease: The Role of the Primary Care Clinician
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/DKD2 to complete the online post-test and receive your certificate of credit.


Click here to read Hot Topics in Primary Care.
This supplement includes 7 CME credits (scroll down for more information).
Topics include:
- Nutritional Gaps
- Irritable Bowel Syndrome
- Asthma Management
- Hyperlipidemia
- Heart Failure
- Efficacy and Safety of Naproxen
- Dementia-Related Delusions and Hallucinations
- Insomnia
- Autosomal Dominant Polycystic Kidney Disease
- Hypoglycemia Management
- Chronic Obstructive Pulmonary Disease
- Diabetic Kidney Disease
- Burden of Overweight
This supplement offers the opportunity to earn a total of 7 CME credits.
Credit is awarded for successful completion of the online evaluations at the links below. These links may also be found within the supplement on the first page of each article.
- Addressing Nutritional Gaps: Simple Steps for the Primary Care Provider
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/nutrition to complete the online post-test and receive your certificate of credit.
- An Individualized, Case-Based Approach to the Management of Irritable Bowel Syndrome
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/ibs to complete the online post-test and receive your certificate of credit.
- Managing the Burden of Dementia-Related Delusions and Hallucinations
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/dementia to complete the online post-test and receive your certificate of credit.
- Overcoming Barriers to the Diagnosis and Treatment of Insomnia
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/insomnia2 to complete the online post-test and receive your certificate of credit.
- Recognition and Management of a Less Common Cause of Chronic Kidney Disease: Autosomal Dominant Polycystic Kidney Disease
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/ADPKD to complete the online post-test and receive your certificate of credit.
- Review of LDL-C-Lowering with Focus on New and Emerging Agents
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/hyper to complete the online post-test and receive your certificate of credit.
- Stemming the Progression of Diabetic Kidney Disease: The Role of the Primary Care Clinician
- To receive CME credit, please read the journal article and, upon completion, go to www.pceconsortium.org/DKD2 to complete the online post-test and receive your certificate of credit.
Pandemic worsens disparities in GI and liver disease
Suspension of disease screening and nonurgent procedures because of the COVID-19 pandemic will negatively impact long-term outcomes of GI and liver disease, and people of color will be disproportionately affected, according to a leading expert.
Novel, multipronged approaches are needed to overcome widening disparities in gastroenterology and hepatology, said Rachel Issaka, MD, of Fred Hutchinson Cancer Research Center in Seattle.
“The COVID-19 pandemic has led to unprecedented drops in breast, colorectal, and cervical cancer screenings,” Dr. Issaka said during an AGA FORWARD Program webinar. Screening rates for these diseases are down 83%-90%, she said.
“Certainly this creates a backlog of cancer screenings that need to occur, which poses very significant challenges for health systems as they’re adapting to this new state of health care that we have to provide,” Dr. Issaka said.
During her presentation, Dr. Issaka first addressed pandemic-related issues in colorectal cancer (CRC).
The sudden decrease in colonoscopies has already affected diagnoses, she said, as 32% fewer cases of CRC were diagnosed in April 2020 compared with April 2019, a finding that is “obviously very concerning.” All downstream effects remain to be seen; however, one estimate suggests that over the next decade, delayed screening may lead to an additional 4,500 deaths from CRC.
“These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest,” Dr. Issaka wrote, as coauthor of a study published in Gastrointestinal Endoscopy.
Dr. Issaka and colleagues predict that the pandemic will likely worsen “persistent CRC disparities” in African-American and Hispanic communities, including relatively decreased screening participation, delayed follow-up of abnormal stool results, limited community-based research and partnerships, and limited community engagement and advocacy.
“COVID-19 related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations,” wrote Dr. Issaka and colleagues.
Dr. Issaka described similar issues in hepatology. She referred to a recent opinion article by Tapper and colleagues, which predicted that the COVID-19 pandemic will impact patients with liver disease in three waves: first, by delaying liver transplants, elective procedures, imaging, and routine patient follow-up; second, by increasing emergent decompensations, transplant wait-list dropouts, and care deferrals; and third, by losing patients to follow-up, resulting in missed diagnoses, incomplete cancer screening, and progressive disease.
“This could disproportionately impact Black, Hispanic, and Native-American populations, who may have already had difficulty accessing [liver care],” Dr. Issaka said.
To mitigate growing disparities, Dr. Issaka proposed a variety of strategies for CRC and liver disease.
For CRC screening, Dr. Issaka suggested noninvasive modalities, including mailed fecal immunochemical tests (FIT), with focused follow-up on patients with highest FIT values. For those conducting CRC research, Dr. Issaka recommended using accessible technology, engaging with community partners, providing incentives where appropriate, and other methods. For cirrhosis care, Dr. Issaka suggested that practitioners turn to telehealth and remote care, including weight monitoring, cognitive function testing, home medication delivery, and online education.
More broadly, Dr. Issaka called for universal health insurance not associated with employment, research funding for health disparities, sustainable employment wages, climate justice, desegregation of housing, and universal broadband Internet.
“The solutions to these problems are multipronged,” Dr. Issaka said. “Some will happen locally; for instance, well-executed planning around telehealth. Some will happen at the state level through opportunities like advocacy or even just reaching out to your own [congressional representative]. And then some will also happen programmatically – How can we as a health system begin to leverage something like mailed FIT?”
Finally, Dr. Issaka suggested that tools from another branch of science can help improve screening rates.
“We don’t, in medicine, tap into the benefits of behavioral psychology enough,” she said. “That’s a great discipline with really great tools that we can all use.”
Dr. Issaka described the power of community, in that people are more likely to undergo screening if they know how many others in their community are also being screened.
“I think as much as we can gather those kinds of data and share those with individuals to provide reassurance about the safety and importance of screening, I think [that] will help,” she said.
The AGA FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK118761). Dr. Issaka has no conflicts of interest.
SOURCES: Issaka. AGA FORWARD Program Webinar. 2020 Aug 27; Balzora et al. Gastrointestinal Endoscopy. 2020 June 20. doi: 10.1016/j.gie.2020.06.042; Tapper et al. Journal of Hepatology. 2020 Apr 13. doi: 10.1016/j.jhep.2020.04.005.
Suspension of disease screening and nonurgent procedures because of the COVID-19 pandemic will negatively impact long-term outcomes of GI and liver disease, and people of color will be disproportionately affected, according to a leading expert.
Novel, multipronged approaches are needed to overcome widening disparities in gastroenterology and hepatology, said Rachel Issaka, MD, of Fred Hutchinson Cancer Research Center in Seattle.
“The COVID-19 pandemic has led to unprecedented drops in breast, colorectal, and cervical cancer screenings,” Dr. Issaka said during an AGA FORWARD Program webinar. Screening rates for these diseases are down 83%-90%, she said.
“Certainly this creates a backlog of cancer screenings that need to occur, which poses very significant challenges for health systems as they’re adapting to this new state of health care that we have to provide,” Dr. Issaka said.
During her presentation, Dr. Issaka first addressed pandemic-related issues in colorectal cancer (CRC).
The sudden decrease in colonoscopies has already affected diagnoses, she said, as 32% fewer cases of CRC were diagnosed in April 2020 compared with April 2019, a finding that is “obviously very concerning.” All downstream effects remain to be seen; however, one estimate suggests that over the next decade, delayed screening may lead to an additional 4,500 deaths from CRC.
“These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest,” Dr. Issaka wrote, as coauthor of a study published in Gastrointestinal Endoscopy.
Dr. Issaka and colleagues predict that the pandemic will likely worsen “persistent CRC disparities” in African-American and Hispanic communities, including relatively decreased screening participation, delayed follow-up of abnormal stool results, limited community-based research and partnerships, and limited community engagement and advocacy.
“COVID-19 related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations,” wrote Dr. Issaka and colleagues.
Dr. Issaka described similar issues in hepatology. She referred to a recent opinion article by Tapper and colleagues, which predicted that the COVID-19 pandemic will impact patients with liver disease in three waves: first, by delaying liver transplants, elective procedures, imaging, and routine patient follow-up; second, by increasing emergent decompensations, transplant wait-list dropouts, and care deferrals; and third, by losing patients to follow-up, resulting in missed diagnoses, incomplete cancer screening, and progressive disease.
“This could disproportionately impact Black, Hispanic, and Native-American populations, who may have already had difficulty accessing [liver care],” Dr. Issaka said.
To mitigate growing disparities, Dr. Issaka proposed a variety of strategies for CRC and liver disease.
For CRC screening, Dr. Issaka suggested noninvasive modalities, including mailed fecal immunochemical tests (FIT), with focused follow-up on patients with highest FIT values. For those conducting CRC research, Dr. Issaka recommended using accessible technology, engaging with community partners, providing incentives where appropriate, and other methods. For cirrhosis care, Dr. Issaka suggested that practitioners turn to telehealth and remote care, including weight monitoring, cognitive function testing, home medication delivery, and online education.
More broadly, Dr. Issaka called for universal health insurance not associated with employment, research funding for health disparities, sustainable employment wages, climate justice, desegregation of housing, and universal broadband Internet.
“The solutions to these problems are multipronged,” Dr. Issaka said. “Some will happen locally; for instance, well-executed planning around telehealth. Some will happen at the state level through opportunities like advocacy or even just reaching out to your own [congressional representative]. And then some will also happen programmatically – How can we as a health system begin to leverage something like mailed FIT?”
Finally, Dr. Issaka suggested that tools from another branch of science can help improve screening rates.
“We don’t, in medicine, tap into the benefits of behavioral psychology enough,” she said. “That’s a great discipline with really great tools that we can all use.”
Dr. Issaka described the power of community, in that people are more likely to undergo screening if they know how many others in their community are also being screened.
“I think as much as we can gather those kinds of data and share those with individuals to provide reassurance about the safety and importance of screening, I think [that] will help,” she said.
The AGA FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK118761). Dr. Issaka has no conflicts of interest.
SOURCES: Issaka. AGA FORWARD Program Webinar. 2020 Aug 27; Balzora et al. Gastrointestinal Endoscopy. 2020 June 20. doi: 10.1016/j.gie.2020.06.042; Tapper et al. Journal of Hepatology. 2020 Apr 13. doi: 10.1016/j.jhep.2020.04.005.
Suspension of disease screening and nonurgent procedures because of the COVID-19 pandemic will negatively impact long-term outcomes of GI and liver disease, and people of color will be disproportionately affected, according to a leading expert.
Novel, multipronged approaches are needed to overcome widening disparities in gastroenterology and hepatology, said Rachel Issaka, MD, of Fred Hutchinson Cancer Research Center in Seattle.
“The COVID-19 pandemic has led to unprecedented drops in breast, colorectal, and cervical cancer screenings,” Dr. Issaka said during an AGA FORWARD Program webinar. Screening rates for these diseases are down 83%-90%, she said.
“Certainly this creates a backlog of cancer screenings that need to occur, which poses very significant challenges for health systems as they’re adapting to this new state of health care that we have to provide,” Dr. Issaka said.
During her presentation, Dr. Issaka first addressed pandemic-related issues in colorectal cancer (CRC).
The sudden decrease in colonoscopies has already affected diagnoses, she said, as 32% fewer cases of CRC were diagnosed in April 2020 compared with April 2019, a finding that is “obviously very concerning.” All downstream effects remain to be seen; however, one estimate suggests that over the next decade, delayed screening may lead to an additional 4,500 deaths from CRC.
“These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest,” Dr. Issaka wrote, as coauthor of a study published in Gastrointestinal Endoscopy.
Dr. Issaka and colleagues predict that the pandemic will likely worsen “persistent CRC disparities” in African-American and Hispanic communities, including relatively decreased screening participation, delayed follow-up of abnormal stool results, limited community-based research and partnerships, and limited community engagement and advocacy.
“COVID-19 related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations,” wrote Dr. Issaka and colleagues.
Dr. Issaka described similar issues in hepatology. She referred to a recent opinion article by Tapper and colleagues, which predicted that the COVID-19 pandemic will impact patients with liver disease in three waves: first, by delaying liver transplants, elective procedures, imaging, and routine patient follow-up; second, by increasing emergent decompensations, transplant wait-list dropouts, and care deferrals; and third, by losing patients to follow-up, resulting in missed diagnoses, incomplete cancer screening, and progressive disease.
“This could disproportionately impact Black, Hispanic, and Native-American populations, who may have already had difficulty accessing [liver care],” Dr. Issaka said.
To mitigate growing disparities, Dr. Issaka proposed a variety of strategies for CRC and liver disease.
For CRC screening, Dr. Issaka suggested noninvasive modalities, including mailed fecal immunochemical tests (FIT), with focused follow-up on patients with highest FIT values. For those conducting CRC research, Dr. Issaka recommended using accessible technology, engaging with community partners, providing incentives where appropriate, and other methods. For cirrhosis care, Dr. Issaka suggested that practitioners turn to telehealth and remote care, including weight monitoring, cognitive function testing, home medication delivery, and online education.
More broadly, Dr. Issaka called for universal health insurance not associated with employment, research funding for health disparities, sustainable employment wages, climate justice, desegregation of housing, and universal broadband Internet.
“The solutions to these problems are multipronged,” Dr. Issaka said. “Some will happen locally; for instance, well-executed planning around telehealth. Some will happen at the state level through opportunities like advocacy or even just reaching out to your own [congressional representative]. And then some will also happen programmatically – How can we as a health system begin to leverage something like mailed FIT?”
Finally, Dr. Issaka suggested that tools from another branch of science can help improve screening rates.
“We don’t, in medicine, tap into the benefits of behavioral psychology enough,” she said. “That’s a great discipline with really great tools that we can all use.”
Dr. Issaka described the power of community, in that people are more likely to undergo screening if they know how many others in their community are also being screened.
“I think as much as we can gather those kinds of data and share those with individuals to provide reassurance about the safety and importance of screening, I think [that] will help,” she said.
The AGA FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK118761). Dr. Issaka has no conflicts of interest.
SOURCES: Issaka. AGA FORWARD Program Webinar. 2020 Aug 27; Balzora et al. Gastrointestinal Endoscopy. 2020 June 20. doi: 10.1016/j.gie.2020.06.042; Tapper et al. Journal of Hepatology. 2020 Apr 13. doi: 10.1016/j.jhep.2020.04.005.
FROM THE AGA FORWARD PROGRAM
Study highlights role of structural racism in delayed autism diagnoses
An analysis of a large cohort of African American children with autism spectrum disorder (ASD) reveals a more than 3-year time lag between parental recognition of a development delay and an ASD diagnosis, reported John N. Constantino, MD, of the department of psychiatry at Washington University, St. Louis, and coauthors.
The analysis also highlights the need to further understand – and act on – the disproportionate burden of intellectual disability comorbidity among these children, the investigators say.
Their analysis, published in Pediatrics, builds on national community surveillance data from the Centers for Disease Control and Prevention published in 2018 showing near-equal rates of identification of ASD among African American (and other minorities) and non-Hispanic white children, but greater delays in diagnosis for African American children as well as twice the rate of comorbid intellectual disability, compared with non-Hispanic white children (44% vs. 22%).
To explore potential drivers of both diagnostic delays and the intellectual disability disparity, the investigators tapped into a repository of data on 584 African American children with ASD and their first-degree relatives who were consecutively enrolled in the National Institutes of Health–funded Autism Genetic Resource Exchange across four academic sites. The program aims to improve the representation of families of self-reported African ancestry in ASD genetics research.
The mean age of diagnosis was 65 months, which came at an average of more than 3 years after parents reported having concerns about their child’s language, behavior or development – and “the period when early developmental therapies (typically delivered through part C interventions in the United States) are initiated to ameliorate the disability associated with ASD,” Dr. Constantino and associates said.
Approximately 42% reported seeing multiple professionals before receiving an ASD diagnosis (14% saw six or more), and 31% said that a lack of available professionals had contributed to the delay. Almost all families – 98% – reported having some type of health insurance at the time of their first concerns (49% private, 46% public, and 5% others).
The data also show that excess intellectual disability comorbidity seen in African American children cannot be explained by familial or sociodemographic factors that have traditionally been associated with variation in IQ in the general population. Nor can it be explained by overclassification of intellectual disability, the authors said.
they wrote.
The NIH-funded program involved parental completion of “Diagnostic Odyssey” interviews (characterizing service-seeking experiences and obstacles to diagnosis, treatment, and care) and several in-person cognitive assessments and measurements of adaptive functioning. “These were in-depth [highly validated] intellectual assessments based on IQ measures that were tailored to the level of functioning of the children and backed by extensive assessments of adaptive functioning,” Dr. Constantino said in an interview.
Historically, autism has been underdiagnosed among minority children. Now that this is no longer the case, as demonstrated in the CDC’s 2018 report from its Autism and Developmental Disabilities Monitoring Network, the thought that higher rates of intellectual disability comorbidity among minority children are due to ascertainment bias – to “our only diagnosing the most serious cases” – “is no longer true,” he said.
And now, with use of data from the largest-available repository of diagnostic and phenotypic information on African American children with ASD, “we’ve dispelled other misperceptions, other factors that might be used to dismiss or explain away why Black children might have a high rate of intellectual disability,” Dr. Constantino said in the interview, referring to the absence of association between the IQ scores of children with ASD and those of their first-degree relatives as well as the lack of significant associations between the IQ of children with ASD and factors such as family income (above versus below the median income of the cohort), the mother having or not having a college degree, and the estimated gestational age at birth (before versus after 37 weeks).
“This kind of complication of autism lasts for the life of a child and profoundly impairs their functioning,” he said. “If anyone thinks there isn’t an opportunity to improve the timing of diagnosis and to improve access to services and appropriate developmental therapies, that’s wrong. There is opportunity – parents are raising the alarm, and we are not coming to these children’s rescue.”
In an accompanying editorial, Sarabeth Broder-Fingert, MD, MPH, of Boston University and Boston Medical Center, and associates wrote that the “confluence of findings [in this study] are suggestive of structural racism leading to inequity.”
For example, they said, “although the causes of delays from first parental concern to ASD diagnosis are complex, they can result from a number of issues, including racial bias and discrimination experienced by families, a lack of diversity in health professionals (impacting patient-provider relationships), and/or concentration of specialists in geographic areas with fewer minority residents.” Each of these issues, they wrote, can be linked to racism, both structural and individual.
Combined with previous research on inequity in ASD, the new data “necessitate an immediate evaluation of how issues of structural racism have impacted ASD diagnosis and, more importantly, an action plan to address inequity,” they said.
Issues of workforce capacity for ASD diagnosis need to be addressed by evaluating Medicaid reimbursement rates for ASD diagnosis, for instance, and removing restrictions on who is qualified to make a diagnosis, increasing adherence to evidence-based practices, “increasing workforce cultural humility, and recruiting a workforce that reflects the diverse communities they serve,” Dr. Broder-Fingert and associates said.
Jeremiah Dickerson, MD, a child and adolescent psychiatrist at the University of Vermont, Burlington, said he is hopeful that the inequities and structural obstacles that he sees in his patients are “being informed more and more by science” and that “we’re taking steps in exploring our own cultural humility and focusing on problems ... that have been under the surface for decades.”
Years of diagnostic delay and missed intervention for ASD can lead to increasing lifelong functional difficulties, which in turn can be compounded by enduring inequities and stigma, he said when asked to comment on the study.
“Diagnosing and teasing apart ID [intellectual disability] versus ASD, and ID co-occurring with ASD, can be very complicated, particularly in young children with significant language impairments,” he added. “If indeed there is a higher proportion of African American children with co-occurring ASD and ID ... we have to do a much better job with identifying those children earlier and providing longitudinal follow-up/assessment that can help provide diagnostic clarity and inform recommendations.”
Understanding better why this occurs “without repeating the history of assigning cause based upon racist thinking” is also important, said Dr. Dickerson, who directs the autism assessment clinic at the university’s Vermont Center for Children, Youth & Families.
Dr. Constantino, who has been involved in the CDC’s 11-site ASD surveillance network, said it is unclear to what extent broad implementation of early and high-quality interventions can reduce the proportion of African American children with autism and comorbid intellectual disability. “We may be only half-successful in closing the gap, or there may be other factors involved that we don’t understand yet,” he said. “But we haven’t even attempted to close the gap [through] reasonable application of an intervention strategy that is available and that we know can improve cognitive outcomes.”
“Some primary care doctors are reluctant to make the diagnosis in the first place because they have nothing to offer the families,” Dr. Constantino noted.
Continued research on the interactions between genetic background and specific biological susceptibilities to autism is an ongoing objective of the Autism Genetic Resource Exchange.
The CDC recently published its latest report on the prevalence of ASD in children aged 8 years, concluding again that continued efforts are needed for “early and equitable identification of ASD and timely enrollment in services.
The study was supported by grants from the National Institutes of Health and from the Intellectual and Developmental Disabilities Research Center at Washington University. Dr. Constantino is author of the Social Responsiveness Scale-2, from which he receives royalties. A coauthor helped author the Vineland-3 and receives royalties, and this coauthor and another associate also have ties to pharmaceutical companies or other organizations; the remaining authors of the study said they had no relevant financial disclosures. Dr. Broder-Fingert and associates said they have no relevant financial relationships; the editorial was funded by the National Institutes of Health. Dr. Dickerson said he had no relevant financial disclosures.
SOURCE: Constantino JN et al. Pediatrics. 2020. doi: 10.1542/peds.2019-3629.
An analysis of a large cohort of African American children with autism spectrum disorder (ASD) reveals a more than 3-year time lag between parental recognition of a development delay and an ASD diagnosis, reported John N. Constantino, MD, of the department of psychiatry at Washington University, St. Louis, and coauthors.
The analysis also highlights the need to further understand – and act on – the disproportionate burden of intellectual disability comorbidity among these children, the investigators say.
Their analysis, published in Pediatrics, builds on national community surveillance data from the Centers for Disease Control and Prevention published in 2018 showing near-equal rates of identification of ASD among African American (and other minorities) and non-Hispanic white children, but greater delays in diagnosis for African American children as well as twice the rate of comorbid intellectual disability, compared with non-Hispanic white children (44% vs. 22%).
To explore potential drivers of both diagnostic delays and the intellectual disability disparity, the investigators tapped into a repository of data on 584 African American children with ASD and their first-degree relatives who were consecutively enrolled in the National Institutes of Health–funded Autism Genetic Resource Exchange across four academic sites. The program aims to improve the representation of families of self-reported African ancestry in ASD genetics research.
The mean age of diagnosis was 65 months, which came at an average of more than 3 years after parents reported having concerns about their child’s language, behavior or development – and “the period when early developmental therapies (typically delivered through part C interventions in the United States) are initiated to ameliorate the disability associated with ASD,” Dr. Constantino and associates said.
Approximately 42% reported seeing multiple professionals before receiving an ASD diagnosis (14% saw six or more), and 31% said that a lack of available professionals had contributed to the delay. Almost all families – 98% – reported having some type of health insurance at the time of their first concerns (49% private, 46% public, and 5% others).
The data also show that excess intellectual disability comorbidity seen in African American children cannot be explained by familial or sociodemographic factors that have traditionally been associated with variation in IQ in the general population. Nor can it be explained by overclassification of intellectual disability, the authors said.
they wrote.
The NIH-funded program involved parental completion of “Diagnostic Odyssey” interviews (characterizing service-seeking experiences and obstacles to diagnosis, treatment, and care) and several in-person cognitive assessments and measurements of adaptive functioning. “These were in-depth [highly validated] intellectual assessments based on IQ measures that were tailored to the level of functioning of the children and backed by extensive assessments of adaptive functioning,” Dr. Constantino said in an interview.
Historically, autism has been underdiagnosed among minority children. Now that this is no longer the case, as demonstrated in the CDC’s 2018 report from its Autism and Developmental Disabilities Monitoring Network, the thought that higher rates of intellectual disability comorbidity among minority children are due to ascertainment bias – to “our only diagnosing the most serious cases” – “is no longer true,” he said.
And now, with use of data from the largest-available repository of diagnostic and phenotypic information on African American children with ASD, “we’ve dispelled other misperceptions, other factors that might be used to dismiss or explain away why Black children might have a high rate of intellectual disability,” Dr. Constantino said in the interview, referring to the absence of association between the IQ scores of children with ASD and those of their first-degree relatives as well as the lack of significant associations between the IQ of children with ASD and factors such as family income (above versus below the median income of the cohort), the mother having or not having a college degree, and the estimated gestational age at birth (before versus after 37 weeks).
“This kind of complication of autism lasts for the life of a child and profoundly impairs their functioning,” he said. “If anyone thinks there isn’t an opportunity to improve the timing of diagnosis and to improve access to services and appropriate developmental therapies, that’s wrong. There is opportunity – parents are raising the alarm, and we are not coming to these children’s rescue.”
In an accompanying editorial, Sarabeth Broder-Fingert, MD, MPH, of Boston University and Boston Medical Center, and associates wrote that the “confluence of findings [in this study] are suggestive of structural racism leading to inequity.”
For example, they said, “although the causes of delays from first parental concern to ASD diagnosis are complex, they can result from a number of issues, including racial bias and discrimination experienced by families, a lack of diversity in health professionals (impacting patient-provider relationships), and/or concentration of specialists in geographic areas with fewer minority residents.” Each of these issues, they wrote, can be linked to racism, both structural and individual.
Combined with previous research on inequity in ASD, the new data “necessitate an immediate evaluation of how issues of structural racism have impacted ASD diagnosis and, more importantly, an action plan to address inequity,” they said.
Issues of workforce capacity for ASD diagnosis need to be addressed by evaluating Medicaid reimbursement rates for ASD diagnosis, for instance, and removing restrictions on who is qualified to make a diagnosis, increasing adherence to evidence-based practices, “increasing workforce cultural humility, and recruiting a workforce that reflects the diverse communities they serve,” Dr. Broder-Fingert and associates said.
Jeremiah Dickerson, MD, a child and adolescent psychiatrist at the University of Vermont, Burlington, said he is hopeful that the inequities and structural obstacles that he sees in his patients are “being informed more and more by science” and that “we’re taking steps in exploring our own cultural humility and focusing on problems ... that have been under the surface for decades.”
Years of diagnostic delay and missed intervention for ASD can lead to increasing lifelong functional difficulties, which in turn can be compounded by enduring inequities and stigma, he said when asked to comment on the study.
“Diagnosing and teasing apart ID [intellectual disability] versus ASD, and ID co-occurring with ASD, can be very complicated, particularly in young children with significant language impairments,” he added. “If indeed there is a higher proportion of African American children with co-occurring ASD and ID ... we have to do a much better job with identifying those children earlier and providing longitudinal follow-up/assessment that can help provide diagnostic clarity and inform recommendations.”
Understanding better why this occurs “without repeating the history of assigning cause based upon racist thinking” is also important, said Dr. Dickerson, who directs the autism assessment clinic at the university’s Vermont Center for Children, Youth & Families.
Dr. Constantino, who has been involved in the CDC’s 11-site ASD surveillance network, said it is unclear to what extent broad implementation of early and high-quality interventions can reduce the proportion of African American children with autism and comorbid intellectual disability. “We may be only half-successful in closing the gap, or there may be other factors involved that we don’t understand yet,” he said. “But we haven’t even attempted to close the gap [through] reasonable application of an intervention strategy that is available and that we know can improve cognitive outcomes.”
“Some primary care doctors are reluctant to make the diagnosis in the first place because they have nothing to offer the families,” Dr. Constantino noted.
Continued research on the interactions between genetic background and specific biological susceptibilities to autism is an ongoing objective of the Autism Genetic Resource Exchange.
The CDC recently published its latest report on the prevalence of ASD in children aged 8 years, concluding again that continued efforts are needed for “early and equitable identification of ASD and timely enrollment in services.
The study was supported by grants from the National Institutes of Health and from the Intellectual and Developmental Disabilities Research Center at Washington University. Dr. Constantino is author of the Social Responsiveness Scale-2, from which he receives royalties. A coauthor helped author the Vineland-3 and receives royalties, and this coauthor and another associate also have ties to pharmaceutical companies or other organizations; the remaining authors of the study said they had no relevant financial disclosures. Dr. Broder-Fingert and associates said they have no relevant financial relationships; the editorial was funded by the National Institutes of Health. Dr. Dickerson said he had no relevant financial disclosures.
SOURCE: Constantino JN et al. Pediatrics. 2020. doi: 10.1542/peds.2019-3629.
An analysis of a large cohort of African American children with autism spectrum disorder (ASD) reveals a more than 3-year time lag between parental recognition of a development delay and an ASD diagnosis, reported John N. Constantino, MD, of the department of psychiatry at Washington University, St. Louis, and coauthors.
The analysis also highlights the need to further understand – and act on – the disproportionate burden of intellectual disability comorbidity among these children, the investigators say.
Their analysis, published in Pediatrics, builds on national community surveillance data from the Centers for Disease Control and Prevention published in 2018 showing near-equal rates of identification of ASD among African American (and other minorities) and non-Hispanic white children, but greater delays in diagnosis for African American children as well as twice the rate of comorbid intellectual disability, compared with non-Hispanic white children (44% vs. 22%).
To explore potential drivers of both diagnostic delays and the intellectual disability disparity, the investigators tapped into a repository of data on 584 African American children with ASD and their first-degree relatives who were consecutively enrolled in the National Institutes of Health–funded Autism Genetic Resource Exchange across four academic sites. The program aims to improve the representation of families of self-reported African ancestry in ASD genetics research.
The mean age of diagnosis was 65 months, which came at an average of more than 3 years after parents reported having concerns about their child’s language, behavior or development – and “the period when early developmental therapies (typically delivered through part C interventions in the United States) are initiated to ameliorate the disability associated with ASD,” Dr. Constantino and associates said.
Approximately 42% reported seeing multiple professionals before receiving an ASD diagnosis (14% saw six or more), and 31% said that a lack of available professionals had contributed to the delay. Almost all families – 98% – reported having some type of health insurance at the time of their first concerns (49% private, 46% public, and 5% others).
The data also show that excess intellectual disability comorbidity seen in African American children cannot be explained by familial or sociodemographic factors that have traditionally been associated with variation in IQ in the general population. Nor can it be explained by overclassification of intellectual disability, the authors said.
they wrote.
The NIH-funded program involved parental completion of “Diagnostic Odyssey” interviews (characterizing service-seeking experiences and obstacles to diagnosis, treatment, and care) and several in-person cognitive assessments and measurements of adaptive functioning. “These were in-depth [highly validated] intellectual assessments based on IQ measures that were tailored to the level of functioning of the children and backed by extensive assessments of adaptive functioning,” Dr. Constantino said in an interview.
Historically, autism has been underdiagnosed among minority children. Now that this is no longer the case, as demonstrated in the CDC’s 2018 report from its Autism and Developmental Disabilities Monitoring Network, the thought that higher rates of intellectual disability comorbidity among minority children are due to ascertainment bias – to “our only diagnosing the most serious cases” – “is no longer true,” he said.
And now, with use of data from the largest-available repository of diagnostic and phenotypic information on African American children with ASD, “we’ve dispelled other misperceptions, other factors that might be used to dismiss or explain away why Black children might have a high rate of intellectual disability,” Dr. Constantino said in the interview, referring to the absence of association between the IQ scores of children with ASD and those of their first-degree relatives as well as the lack of significant associations between the IQ of children with ASD and factors such as family income (above versus below the median income of the cohort), the mother having or not having a college degree, and the estimated gestational age at birth (before versus after 37 weeks).
“This kind of complication of autism lasts for the life of a child and profoundly impairs their functioning,” he said. “If anyone thinks there isn’t an opportunity to improve the timing of diagnosis and to improve access to services and appropriate developmental therapies, that’s wrong. There is opportunity – parents are raising the alarm, and we are not coming to these children’s rescue.”
In an accompanying editorial, Sarabeth Broder-Fingert, MD, MPH, of Boston University and Boston Medical Center, and associates wrote that the “confluence of findings [in this study] are suggestive of structural racism leading to inequity.”
For example, they said, “although the causes of delays from first parental concern to ASD diagnosis are complex, they can result from a number of issues, including racial bias and discrimination experienced by families, a lack of diversity in health professionals (impacting patient-provider relationships), and/or concentration of specialists in geographic areas with fewer minority residents.” Each of these issues, they wrote, can be linked to racism, both structural and individual.
Combined with previous research on inequity in ASD, the new data “necessitate an immediate evaluation of how issues of structural racism have impacted ASD diagnosis and, more importantly, an action plan to address inequity,” they said.
Issues of workforce capacity for ASD diagnosis need to be addressed by evaluating Medicaid reimbursement rates for ASD diagnosis, for instance, and removing restrictions on who is qualified to make a diagnosis, increasing adherence to evidence-based practices, “increasing workforce cultural humility, and recruiting a workforce that reflects the diverse communities they serve,” Dr. Broder-Fingert and associates said.
Jeremiah Dickerson, MD, a child and adolescent psychiatrist at the University of Vermont, Burlington, said he is hopeful that the inequities and structural obstacles that he sees in his patients are “being informed more and more by science” and that “we’re taking steps in exploring our own cultural humility and focusing on problems ... that have been under the surface for decades.”
Years of diagnostic delay and missed intervention for ASD can lead to increasing lifelong functional difficulties, which in turn can be compounded by enduring inequities and stigma, he said when asked to comment on the study.
“Diagnosing and teasing apart ID [intellectual disability] versus ASD, and ID co-occurring with ASD, can be very complicated, particularly in young children with significant language impairments,” he added. “If indeed there is a higher proportion of African American children with co-occurring ASD and ID ... we have to do a much better job with identifying those children earlier and providing longitudinal follow-up/assessment that can help provide diagnostic clarity and inform recommendations.”
Understanding better why this occurs “without repeating the history of assigning cause based upon racist thinking” is also important, said Dr. Dickerson, who directs the autism assessment clinic at the university’s Vermont Center for Children, Youth & Families.
Dr. Constantino, who has been involved in the CDC’s 11-site ASD surveillance network, said it is unclear to what extent broad implementation of early and high-quality interventions can reduce the proportion of African American children with autism and comorbid intellectual disability. “We may be only half-successful in closing the gap, or there may be other factors involved that we don’t understand yet,” he said. “But we haven’t even attempted to close the gap [through] reasonable application of an intervention strategy that is available and that we know can improve cognitive outcomes.”
“Some primary care doctors are reluctant to make the diagnosis in the first place because they have nothing to offer the families,” Dr. Constantino noted.
Continued research on the interactions between genetic background and specific biological susceptibilities to autism is an ongoing objective of the Autism Genetic Resource Exchange.
The CDC recently published its latest report on the prevalence of ASD in children aged 8 years, concluding again that continued efforts are needed for “early and equitable identification of ASD and timely enrollment in services.
The study was supported by grants from the National Institutes of Health and from the Intellectual and Developmental Disabilities Research Center at Washington University. Dr. Constantino is author of the Social Responsiveness Scale-2, from which he receives royalties. A coauthor helped author the Vineland-3 and receives royalties, and this coauthor and another associate also have ties to pharmaceutical companies or other organizations; the remaining authors of the study said they had no relevant financial disclosures. Dr. Broder-Fingert and associates said they have no relevant financial relationships; the editorial was funded by the National Institutes of Health. Dr. Dickerson said he had no relevant financial disclosures.
SOURCE: Constantino JN et al. Pediatrics. 2020. doi: 10.1542/peds.2019-3629.
FROM PEDIATRICS
FDA OKs new ‘artificial pancreas’ Medtronic 770G
The Food and Drug Administration has approved the MiniMed 770G (Medtronic) automated insulin delivery system for children aged 2-6 years.
The 770G system adds Bluetooth smartphone connectivity to the SmartGuard technology that is present in the hybrid closed-loop MiniMed 670G system, which has been available in the United States since 2016 for individuals aged 14 years and older who have type 1 diabetes. It has been available since 2018 for children aged 7 years.
The 770G will also be available to older children and adults once it has been launched.
As with other so-called artificial pancreas systems, the 770G is made up of an insulin pump and continuous glucose monitor that are connected via software that allows the pump to deliver or withhold insulin on the basis of glucose readings.
It is a “hybrid closed-loop” system in that users or caregivers must still manually signal carbohydrate consumption.
The 770G includes a “share” feature that allows health care providers, users, and caregivers to follow the user’s glucose levels remotely via smartphones. In-app notices indicate when glucose levels are out of range. The data can be uploaded prior to telehealth visits.
The approval was based on a 3-month study of 151 children aged 2-6 years who showed improvement in outcomes comparable with those seen in 124 older adolescents and adults with the 770G system as compared to patients who used manual (nonlooped) mode over a 2-week period. There were no episodes of severe hypoglycemia or diabetic ketoacidosis and no serious device-related adverse events while in hybrid closed-loop mode.
The FDA will require Medtronic to conduct a postmarketing study to evaluate the 770G in real-world settings. It is not approved for use in children younger than 2 years nor in any patient who requires less than 8 units of insulin per day.
The next-generation Medtronic closed-loop system, the 780G, has already been approved in Europe. It improves on the technology by delivering automated bolus correction doses in addition to basal insulin every 5 minutes. The company is preparing to submit the 780G for approval in the United States.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved the MiniMed 770G (Medtronic) automated insulin delivery system for children aged 2-6 years.
The 770G system adds Bluetooth smartphone connectivity to the SmartGuard technology that is present in the hybrid closed-loop MiniMed 670G system, which has been available in the United States since 2016 for individuals aged 14 years and older who have type 1 diabetes. It has been available since 2018 for children aged 7 years.
The 770G will also be available to older children and adults once it has been launched.
As with other so-called artificial pancreas systems, the 770G is made up of an insulin pump and continuous glucose monitor that are connected via software that allows the pump to deliver or withhold insulin on the basis of glucose readings.
It is a “hybrid closed-loop” system in that users or caregivers must still manually signal carbohydrate consumption.
The 770G includes a “share” feature that allows health care providers, users, and caregivers to follow the user’s glucose levels remotely via smartphones. In-app notices indicate when glucose levels are out of range. The data can be uploaded prior to telehealth visits.
The approval was based on a 3-month study of 151 children aged 2-6 years who showed improvement in outcomes comparable with those seen in 124 older adolescents and adults with the 770G system as compared to patients who used manual (nonlooped) mode over a 2-week period. There were no episodes of severe hypoglycemia or diabetic ketoacidosis and no serious device-related adverse events while in hybrid closed-loop mode.
The FDA will require Medtronic to conduct a postmarketing study to evaluate the 770G in real-world settings. It is not approved for use in children younger than 2 years nor in any patient who requires less than 8 units of insulin per day.
The next-generation Medtronic closed-loop system, the 780G, has already been approved in Europe. It improves on the technology by delivering automated bolus correction doses in addition to basal insulin every 5 minutes. The company is preparing to submit the 780G for approval in the United States.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved the MiniMed 770G (Medtronic) automated insulin delivery system for children aged 2-6 years.
The 770G system adds Bluetooth smartphone connectivity to the SmartGuard technology that is present in the hybrid closed-loop MiniMed 670G system, which has been available in the United States since 2016 for individuals aged 14 years and older who have type 1 diabetes. It has been available since 2018 for children aged 7 years.
The 770G will also be available to older children and adults once it has been launched.
As with other so-called artificial pancreas systems, the 770G is made up of an insulin pump and continuous glucose monitor that are connected via software that allows the pump to deliver or withhold insulin on the basis of glucose readings.
It is a “hybrid closed-loop” system in that users or caregivers must still manually signal carbohydrate consumption.
The 770G includes a “share” feature that allows health care providers, users, and caregivers to follow the user’s glucose levels remotely via smartphones. In-app notices indicate when glucose levels are out of range. The data can be uploaded prior to telehealth visits.
The approval was based on a 3-month study of 151 children aged 2-6 years who showed improvement in outcomes comparable with those seen in 124 older adolescents and adults with the 770G system as compared to patients who used manual (nonlooped) mode over a 2-week period. There were no episodes of severe hypoglycemia or diabetic ketoacidosis and no serious device-related adverse events while in hybrid closed-loop mode.
The FDA will require Medtronic to conduct a postmarketing study to evaluate the 770G in real-world settings. It is not approved for use in children younger than 2 years nor in any patient who requires less than 8 units of insulin per day.
The next-generation Medtronic closed-loop system, the 780G, has already been approved in Europe. It improves on the technology by delivering automated bolus correction doses in addition to basal insulin every 5 minutes. The company is preparing to submit the 780G for approval in the United States.
A version of this article originally appeared on Medscape.com.