User login
Big pharma sues to block Minnesota insulin affordability law
PhRMA filed the complaint in the U.S. District Court in Minnesota on July 1, the day the Alec Smith Insulin Affordability Act went into effect. The law created the Minnesota Insulin Safety Net Program, which is continuing to operate in the meantime.
Advocates said they were appalled by the PhRMA action.
PhRMA says law is unconstitutional
In the filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
“A state cannot simply commandeer private property to achieve its public policy goals,” the PhRMA lawyers argued.
The suit said the three leading insulin makers already provide discounts, copayment assistance, and free insulin to “a great number of patients.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“No one living with diabetes should be forced to ration or go without their life-saving insulin because they can’t afford it,” said PhRMA executive vice president and general counsel James C. Stansel in a statement.
The law, said Mr. Stansel, “overlooks common sense solutions to help patients afford their insulin and, despite its claims, still allows for patients to be charged at the pharmacy for the insulin that manufacturers are required to provide for free.”
Advocates decry suit
Advocates had worked for several years to secure passage of the legislation, named in honor of a young man who died in 2017 after rationing his insulin. Minnesota Gov. Tim Walz of the Democratic-Farmer-Labor Party signed the bill into law on April 15.
It requires manufacturers to make at least a 30-day supply of insulin available to those who are in urgent need and cannot afford the medication. Manufacturers can be fined $200,000 per month for not complying.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, who called for action on the cost of insulin in an article published in the January 2020 issue of the Mayo Clinic Proceedings, as reported by Medscape Medical News, said the lawsuit was a “bad move.”
Dr. Rajkumar, the Edward W. and Betty Knight Scripps professor of medicine at the Mayo Clinic, noted that it has strict limits and is aimed to help patients in emergent need.
“There is nothing in the US constitution that prevents states from saving the lives of its citizens who are in imminent danger,” Dr. Rajkumar said. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.”
Alec Smith’s mother, Nicole Smith-Holt, who is active with T1International’s #insulin4all campaign, took to Twitter to express her anger.
“Throwing up road blocks to securing affordable insulin for the people of MN, haven’t they taken enough innocent lives? How many more bodies are they looking for?” she tweeted. “When are they going to realize we are not going to stop fighting?”
T1International said in a statement: “It is clear that the pharmaceutical industry can see only one thing – their bottom line,” and promised that patients would not give up.
“We will not stop showing them the real price we pay for their greed,” said the organization.
Abigail Hansmeyer, a Minnesota-based #insulin4all advocate, also talked about her frustration at what appeared to be disingenuous behavior by the insulin makers.
“I guess the endless opportunities insulin manufacturer reps had as stakeholders during numerous discussions and negotiations in the making of this law, wasn’t what they wanted,” she tweeted. “They were buying time to protect their profits. Yeah, we’re not done here.”
A version of this article originally appeared on Medscape.com.
PhRMA filed the complaint in the U.S. District Court in Minnesota on July 1, the day the Alec Smith Insulin Affordability Act went into effect. The law created the Minnesota Insulin Safety Net Program, which is continuing to operate in the meantime.
Advocates said they were appalled by the PhRMA action.
PhRMA says law is unconstitutional
In the filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
“A state cannot simply commandeer private property to achieve its public policy goals,” the PhRMA lawyers argued.
The suit said the three leading insulin makers already provide discounts, copayment assistance, and free insulin to “a great number of patients.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“No one living with diabetes should be forced to ration or go without their life-saving insulin because they can’t afford it,” said PhRMA executive vice president and general counsel James C. Stansel in a statement.
The law, said Mr. Stansel, “overlooks common sense solutions to help patients afford their insulin and, despite its claims, still allows for patients to be charged at the pharmacy for the insulin that manufacturers are required to provide for free.”
Advocates decry suit
Advocates had worked for several years to secure passage of the legislation, named in honor of a young man who died in 2017 after rationing his insulin. Minnesota Gov. Tim Walz of the Democratic-Farmer-Labor Party signed the bill into law on April 15.
It requires manufacturers to make at least a 30-day supply of insulin available to those who are in urgent need and cannot afford the medication. Manufacturers can be fined $200,000 per month for not complying.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, who called for action on the cost of insulin in an article published in the January 2020 issue of the Mayo Clinic Proceedings, as reported by Medscape Medical News, said the lawsuit was a “bad move.”
Dr. Rajkumar, the Edward W. and Betty Knight Scripps professor of medicine at the Mayo Clinic, noted that it has strict limits and is aimed to help patients in emergent need.
“There is nothing in the US constitution that prevents states from saving the lives of its citizens who are in imminent danger,” Dr. Rajkumar said. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.”
Alec Smith’s mother, Nicole Smith-Holt, who is active with T1International’s #insulin4all campaign, took to Twitter to express her anger.
“Throwing up road blocks to securing affordable insulin for the people of MN, haven’t they taken enough innocent lives? How many more bodies are they looking for?” she tweeted. “When are they going to realize we are not going to stop fighting?”
T1International said in a statement: “It is clear that the pharmaceutical industry can see only one thing – their bottom line,” and promised that patients would not give up.
“We will not stop showing them the real price we pay for their greed,” said the organization.
Abigail Hansmeyer, a Minnesota-based #insulin4all advocate, also talked about her frustration at what appeared to be disingenuous behavior by the insulin makers.
“I guess the endless opportunities insulin manufacturer reps had as stakeholders during numerous discussions and negotiations in the making of this law, wasn’t what they wanted,” she tweeted. “They were buying time to protect their profits. Yeah, we’re not done here.”
A version of this article originally appeared on Medscape.com.
PhRMA filed the complaint in the U.S. District Court in Minnesota on July 1, the day the Alec Smith Insulin Affordability Act went into effect. The law created the Minnesota Insulin Safety Net Program, which is continuing to operate in the meantime.
Advocates said they were appalled by the PhRMA action.
PhRMA says law is unconstitutional
In the filing, PhRMA’s attorneys said the law is unconstitutional. It “order[s] pharmaceutical manufacturers to give insulin to state residents, on the state’s prescribed terms, at no charge to the recipients and without compensating the manufacturers in any way.”
“A state cannot simply commandeer private property to achieve its public policy goals,” the PhRMA lawyers argued.
The suit said the three leading insulin makers already provide discounts, copayment assistance, and free insulin to “a great number of patients.”
The state has estimated that as many as 30,000 Minnesotans would be eligible for free insulin in the first year of the program. The drugmakers strenuously objected, noting that would mean they would “be compelled to provide 173,800 monthly supplies of free insulin” just in the first year.
“No one living with diabetes should be forced to ration or go without their life-saving insulin because they can’t afford it,” said PhRMA executive vice president and general counsel James C. Stansel in a statement.
The law, said Mr. Stansel, “overlooks common sense solutions to help patients afford their insulin and, despite its claims, still allows for patients to be charged at the pharmacy for the insulin that manufacturers are required to provide for free.”
Advocates decry suit
Advocates had worked for several years to secure passage of the legislation, named in honor of a young man who died in 2017 after rationing his insulin. Minnesota Gov. Tim Walz of the Democratic-Farmer-Labor Party signed the bill into law on April 15.
It requires manufacturers to make at least a 30-day supply of insulin available to those who are in urgent need and cannot afford the medication. Manufacturers can be fined $200,000 per month for not complying.
Mayo Clinic hematologist S. Vincent Rajkumar, MD, who called for action on the cost of insulin in an article published in the January 2020 issue of the Mayo Clinic Proceedings, as reported by Medscape Medical News, said the lawsuit was a “bad move.”
Dr. Rajkumar, the Edward W. and Betty Knight Scripps professor of medicine at the Mayo Clinic, noted that it has strict limits and is aimed to help patients in emergent need.
“There is nothing in the US constitution that prevents states from saving the lives of its citizens who are in imminent danger,” Dr. Rajkumar said. “The only motives for this lawsuit in my opinion are greed and the worry that other states may also choose to put lives of patients ahead of pharma profits.”
Alec Smith’s mother, Nicole Smith-Holt, who is active with T1International’s #insulin4all campaign, took to Twitter to express her anger.
“Throwing up road blocks to securing affordable insulin for the people of MN, haven’t they taken enough innocent lives? How many more bodies are they looking for?” she tweeted. “When are they going to realize we are not going to stop fighting?”
T1International said in a statement: “It is clear that the pharmaceutical industry can see only one thing – their bottom line,” and promised that patients would not give up.
“We will not stop showing them the real price we pay for their greed,” said the organization.
Abigail Hansmeyer, a Minnesota-based #insulin4all advocate, also talked about her frustration at what appeared to be disingenuous behavior by the insulin makers.
“I guess the endless opportunities insulin manufacturer reps had as stakeholders during numerous discussions and negotiations in the making of this law, wasn’t what they wanted,” she tweeted. “They were buying time to protect their profits. Yeah, we’re not done here.”
A version of this article originally appeared on Medscape.com.
Primary care practices may lose about $68k per physician this year
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
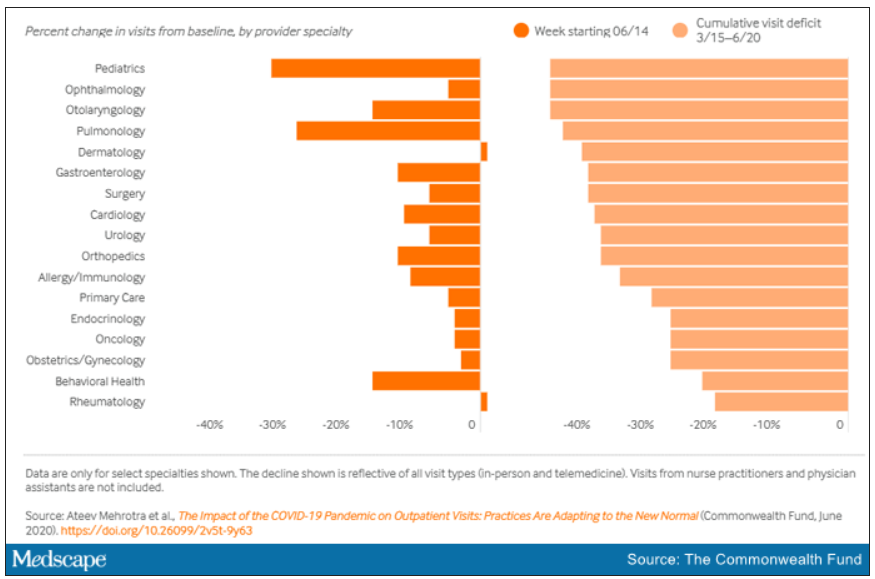
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Primary care practices stand to lose almost $68,000 per full-time physician this year as COVID-19 causes care delays and cancellations, researchers estimate. And while some outpatient care has started to rebound to near baseline appointment levels, other ambulatory specialties remain dramatically down from prepandemic rates.
For primary care practices, Sanjay Basu, MD, and colleagues calculated the losses at $67,774 in gross revenue per physician (interquartile range, $80,577-$54,990), with a national toll of $15.1 billion this year.
That’s without a potential second wave of COVID-19, noted Dr. Basu, director of research and population health at Collective Health in San Francisco, and colleagues.
When they added a theoretical stay-at-home order for November and December, the estimated loss climbed to $85,666 in gross revenue per full-time physician, with a loss of $19.1 billion nationally. The findings were published online in Health Affairs.
Meanwhile, clinical losses from canceled outpatient care are piling up as well, according to a study by Ateev Mehrotra, MD, associate professor of health care policy and medicine at Harvard Medical School in Boston, and colleagues, which calculated the clinical losses in outpatient care.
“The ‘cumulative deficit’ in visits over the last 3 months (March 15 to June 20) is nearly 40%,” the authors wrote. They reported their findings in an article published online June 25 by the Commonwealth Fund.
When examined by specialty, Dr. Mehrotra and colleagues found that appointment rebound rates have been uneven. Whereas dermatology and rheumatology visits have already recovered, a couple of specialties have cumulative deficits that are particularly concerning. For example, pediatric visits were down by 47% in the 3 months since March 15 and pulmonology visits were down 45% in that time.
Much depends on the future of telehealth
Closing the financial and care gaps will depend largely on changing payment models for outpatient care and assuring adequate and enduring reimbursement for telehealth, according to experts.
COVID-19 has put a spotlight on the fragility of a fee-for-service system that depends on in-person visits for stability, Daniel Horn, MD, director of population health and quality at Massachusetts General Hospital in Boston, said in an interview.
Several things need to happen to change the outlook for outpatient care, he said.
A need mentioned in both studies is that the COVID-19 waivers that make it possible for telehealth visits to be reimbursed like other visits must continue after the pandemic. Those assurances are critical as practices decide whether to invest in telemedicine.
If U.S. practices revert as of Oct. 1, 2020, to the pre–COVID-19 payment system for telehealth, national losses for the year would be more than double the current estimates.
“Given the number of active primary care physicians (n = 223,125), we estimated that the cost would be $38.7 billion (IQR, $31.1 billion-$48.3 billion) at a national level to neutralize the gross revenue losses caused by COVID-19 among primary care practices, without subjecting staff to furloughs,” Dr. Basu and colleagues wrote.
In addition to stabilizing telehealth payment models, another need to improve the outlook for outpatient care is more effective communication that in-person care is safe again in regions with protocols in place, Dr. Horn said.
However, the most important change, Dr. Horn said, is a switch to prospective lump-sum payments – payments made in advance to physicians to treat each patient in the way they and the patient deem best with the most appropriate appointment type – whether by in-person visit, phone call, text reminders, or video session.
Prospective payments would take multipayer coalitions working in conjunction with leadership on the federal level from the Centers for Medicare & Medicaid Services, Dr. Horn said. Commercial payers and states (through Medicaid funds) should already have that money available with the cancellations of nonessential procedures, he said.
“We expect ongoing turbulent times, so having a prospective payment could unleash the capacity for primary care practices to be creative in the way they care for their patients,” Dr. Horn said.
Visit trends still down
Calculations by Dr. Basu, who is also on the faculty at Harvard Medical School’s Center for Primary Care, and colleagues were partially informed by Dr. Mehrotra’s data on how many visits have been lost because of COVID-19.
Dr. Mehrotra said a clear message in their study is that “visit trends are not back to baseline.”
They found that the number of visits to ambulatory practices had dropped nearly 60% by early April. Since then, numbers have rebounded substantially. As of the week of June 14, overall visits, compared with baseline were down 11%. But the drops varied widely across specialties.
Dr. Mehrotra said he found particularly disturbing the drop in pediatric visits and the sharp contrast between those rates and the higher number of visits for adults. While visits for patients aged 75 and older had climbed back to just 3% below baseline, the drop seen among kids aged 3-5 years remains 43% below baseline.
“Even kids 0-2 years old are still down 30% from baseline,” he pointed out.
It’s possible that kids are getting care from other sources or perhaps are not sick as often because they are not in school. However, he added, “I do think there’s a concern that some kids are not getting the care they need for chronic illnesses such as attention deficit hyperactivity disorder, asthma, eczema, and psoriasis, and vaccination rates have fallen.”
Telemedicine rates dropping
Telemedicine was “supposed to have its shining moment,” Dr. Mehrotra said, but trends show it cannot make up the gaps of in-person care. His team’s data show a decline in telemedicine as a percentage of all visits from a high of 13.8% in mid-April to 7.4% the week of June 14.
He attributes that partially to physicians’ mixed success in getting reimbursed. “While Medicare has done a good job reimbursing, commercial payers and Medicaid plans have been mixed in their coverage.”
Some physicians who don’t get reimbursed or receive delayed or reduced payments are going back to in-person visits, Dr. Mehrotra said.
He said it’s important to remember that, before the pandemic, “telemedicine was making up 0.1% of all visits. Even if now it declines (from the April high of 13.8%) to 5% or 3%, that’s still a 30-fold increase within the course of a couple of months.”
Prospective payments would help expand the possibilities for telemedicine, he said, and could include apps and wearables and texts in addition to or instead of traditional video sessions.
Dr. Mehrotra said change won’t come fast enough for some and many practices won’t survive. “People are worried about their livelihood. This is nothing we’ve ever – at least in my career as a physician – had to focus on. Now we’re really having practices ask whether they can financially sustain themselves.”
For many, he said, the damage will be long term. “That cumulative deficit in visits – I’m not sure if it’s ever coming back. If you’re a primary care practice, you can only work so hard.”
Dr. Basu reported receiving a salary for clinical duties from HealthRIGHT360, a Federally Qualified Health Center, and Collective Health, a care management organization. Dr. Horn and Dr. Mehrotra reported no relevant financial relationships.
A version of this article originally on Medscape.com.
Bariatric embolotherapy helps shed pounds in obese patients
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
Transcatheter bariatric embolotherapy (TBE) provides sustained weight loss without serious adverse effects among obese patients, results of a pilot sham-controlled study suggest.
At 6-month follow-up, the patients receiving the intervention had lost 7.4 kg (16.3 lbs), compared with 3.0 kg (6.6 lbs) in those randomized to a sham procedure in an intention-to-treat analysis (P = .034).
Results were similar in a per-protocol analysis (9.4 kg/20.7 lbs vs. 1.9 kg/4.1 lbs; P = .0002).
Weight loss after embolotherapy was sustained over 12 months, falling 7.8 kg (17.1 lbs) from baseline in the intention-to-treat population (P = .0011) and 9.3 kg (20.5 lbs) in the per-protocol population (P = .0005).
Safety events after TBE were mild nausea or vomiting, reported Vivek Reddy, MD, Mount Sinai Hospital, New York City. Five participants had minor, asymptomatic ulcers that required no additional treatment.
“In this randomized pilot trial, we established the proof of principle that transcatheter bariatric embolotherapy of the left gastric artery is safe and it promotes clinically significant weight loss,” he concluded at PCR e-Course, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions 2020.
Although bariatric surgery is highly effective, he noted that the associated morbidity and mortality limit its use to the severely obese with a body mass index (BMI) typically over 40 kg/m2.
TBE is a minimally invasive approach that uses a custom occlusion balloon microcatheter and robotic manifold to inject 300- to 500-mcm beads to the left gastric artery. Preclinical and case studies suggest it promotes weight loss by reducing ghrelin, an appetite-stimulating hormone secreted from the gastric fundus, Dr. Reddy said.
The study enrolled 44 patients (aged 21-60 years) with a BMI of 35-55, excluding those with prior bariatric surgery and a history of ulcers, type 2 diabetes, chronic aspirin or nonsteroidal inflammatory use, and active Helicobacter pylori infection.
A total of 40 patients were randomly assigned to TBE or a sham procedure, in which lidocaine was applied to the femoral area and propofol infused for 1 hour. The two groups were well matched, with a mean age of 45 vs. 46 years, weight of 110 kg vs. 119 kg, and BMI of 39 vs. 40, Dr. Reddy noted.
Embolotherapy was performed at a single center in Prague, and, on average, took 82.3 minutes and used 127 mL of contrast, 163 Gy/cm2 radiation, and 4.2 mL of microspheres. A single vessel was injected in 80% of cases.
The intention-to-treat population comprised 19 TBE and 18 control subjects, and the per-protocol population comprised 15 TBE and 16 control subjects, after the exclusion of patients in whom embolotherapy was unsuccessful or incomplete or who withdrew consent.
All patients received endoscopy at baseline and 1 week, as well as an intensive 19-session lifestyle and dietary education intervention out to 6 months.
Patients who underwent TBE had significant improvement in hunger scores at 6 and 12 months, compared with baseline. Similarly, quality of life improved across all six domains, including significant gains in physical function, self-esteem, and overall quality of life at both time points, Dr. Reddy reported.
Dr. Reddy disclosed receiving research support from Endobar Solutions.
This article first appeared on Medscape.com.
One-year mortality after dialysis initiation nearly double prior estimates
Background: The United States Renal Data System registry estimates that approximately 30% of patients die within 1 year of initiating hemodialysis.
Study design: Retrospective, observational analysis.
Setting: The Health and Retirement Study is a nationally representative survey of Medicare beneficiaries during 1998-2014. Medicare claims were linked to mortality data from the National Death Index.
Synopsis: Among 391 patients who initiated dialysis, 22.5%, 44.2%, and 54.5% died within 30 days, 6 months, and 1 year, respectively. After multivariate adjustment, 1-year mortality was higher among those who initiated dialysis while inpatients (hazard ratio, 2.17; 62.2%), had any activity of daily living dependence prior to dialysis (HR, 1.88; 73.0%), or had more than four comorbidities (HR, 1.5; 59.9%).
Bottom line: Medicare beneficiaries may have significantly higher mortality after initiating dialysis than prior data suggest.
Citation: Wachterman MW et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2019.0125.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The United States Renal Data System registry estimates that approximately 30% of patients die within 1 year of initiating hemodialysis.
Study design: Retrospective, observational analysis.
Setting: The Health and Retirement Study is a nationally representative survey of Medicare beneficiaries during 1998-2014. Medicare claims were linked to mortality data from the National Death Index.
Synopsis: Among 391 patients who initiated dialysis, 22.5%, 44.2%, and 54.5% died within 30 days, 6 months, and 1 year, respectively. After multivariate adjustment, 1-year mortality was higher among those who initiated dialysis while inpatients (hazard ratio, 2.17; 62.2%), had any activity of daily living dependence prior to dialysis (HR, 1.88; 73.0%), or had more than four comorbidities (HR, 1.5; 59.9%).
Bottom line: Medicare beneficiaries may have significantly higher mortality after initiating dialysis than prior data suggest.
Citation: Wachterman MW et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2019.0125.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Background: The United States Renal Data System registry estimates that approximately 30% of patients die within 1 year of initiating hemodialysis.
Study design: Retrospective, observational analysis.
Setting: The Health and Retirement Study is a nationally representative survey of Medicare beneficiaries during 1998-2014. Medicare claims were linked to mortality data from the National Death Index.
Synopsis: Among 391 patients who initiated dialysis, 22.5%, 44.2%, and 54.5% died within 30 days, 6 months, and 1 year, respectively. After multivariate adjustment, 1-year mortality was higher among those who initiated dialysis while inpatients (hazard ratio, 2.17; 62.2%), had any activity of daily living dependence prior to dialysis (HR, 1.88; 73.0%), or had more than four comorbidities (HR, 1.5; 59.9%).
Bottom line: Medicare beneficiaries may have significantly higher mortality after initiating dialysis than prior data suggest.
Citation: Wachterman MW et al. One-year mortality after dialysis initiation among older adults. JAMA Intern Med. 2019 Apr 22. doi: 10.1001/jamainternmed.2019.0125.
Dr. Lublin is a hospitalist at the University of Colorado at Denver, Aurora.
Physician shortage grows in latest projections
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fifteen-year projections for the shortage of primary care and specialty physicians in the United States grew to between 54,000 and 139,000 in the latest annual report by the Association of American Medical Colleges.

Those estimates are up from last year’s projections of a shortfall of 46,900-121,900 by 2032.
The Complexities of Physician Supply and Demand: Projections from 2018 to 2033, was the sixth annual study conducted for the AAMC by the Life Science division of global analytics firm IHS Markit.
This analysis, conducted in 2019, includes supply and demand scenarios but predates the COVID-19 pandemic.
In a telephone press briefing this morning, David J. Skorton, MD, AAMC’s president and CEO, told reporters that the pandemic has highlighted the acute effects of physician shortages.
“We’ve seen in stark detail how fragile and quickly overwhelmed America’s health care system truly is, and we’re nowhere near out of the woods with this public health emergency yet,” he said.
The persistent shortages mean people “will have ongoing difficulty accessing the care that they need, especially as we all age.”
Some of the biggest shortages will be seen in non–primary care specialists. Dr. Skorton notes that, during the pandemic, shortages of specialists in hospital settings, including critical care, emergency medicine, pulmonology, and infectious disease, are an urgent concern.
Population trends continue to be the biggest drivers of the shortage. Report authors found that by 2033, the U.S. population is expected to grow by 10.4% from 327 million to 361 million, with wide differences by age.
The under-18 population is expected to grow by 3.9%, whereas the numbers of those aged 65 and older is expected to balloon by 45.1% in that time, thus stoking demand for specialties focused on care for older Americans.
Physician age is also a large factor in the projections. More than two in five currently active physicians will be 65 or older in the next 10 years, according to the report. A wave of retirements will have a large impact on the supply of physicians.
The report explains that the projected shortages remain under predictable scenarios: an increase in the use of advanced practice nurses (APRNs) and physician assistants (PAs), more care in alternate settings such as retail clinics, and changes in payment and delivery.
According to the report, the supply of APRNs and PAs is on track to double over the next 15 years (with growth rates varying by APRN and PA specialty).
“At current rates of production, by 2033 APRN supply will grow by 276,000 [full-time equivalents (FTEs)] and PA supply by nearly 138,000 FTEs,” the report states.
However, authors acknowledge there is scant evidence on what effect these numbers will have on demand for physicians.
The report points out that if underserved communities were able to access health care in numbers similar to those without barriers imposed by where they live or what insurance they have, demand could rise beyond the projections in this report by an additional 74,000 to 145,000 physicians.
Stemming the shortages
The first step in addressing the shortage, Dr. Skorton said, is assuring a healthy physician pipeline to meet the demand for generations.
“One essential step that we believe Congress must take is to end the freeze that has been in place since 1997 that limits federal support for residency training of new physicians,” Skorton said.
He noted that AAMC supports the bipartisan Resident Physician Shortage Reduction Act, introduced to Congress in 2019, which calls for an increase in Medicare support for 3000 new residency positions each year over the next 5 years.
However, additional steps are needed, including enabling advanced practice providers to play a greater role in increasing the health care workforce, Dr. Skorton said.
Pointing out some of the effects of physician shortages, Janis M. Orlowski, MD, chief health care officer for the AAMC, noted that high rates of maternal morbidity are partially linked to lack of adequate numbers of physicians in the United States, and a lack of behavioral health specialists has exacerbated effects of the opioid epidemic.
Shortages are already evident in the current pandemic, she added, saying, “Today we see governors calling for retired physicians or physicians from other states to come and help battle the pandemic within their states.”
The report explains that long-term effects on physician numbers from the pandemic likely will include workforce exits because of COVID-19 deaths, early retirements from burnout, or a shift in interest in certain specialties.
Karen Fisher, JD, chief public policy officer for AAMC, said telehealth will also play an important role in bridging gaps in access to care, and its importance has already been seen in this first wave of the pandemic.
She noted that temporary federal waivers have made it easier for those enrolled in Medicare, Medicaid, and the Children’s Health Insurance Program to receive telehealth services during the pandemic.
Expanding the access to telehealth permanently will be important in helping to fill gaps, Ms. Fisher said.
Dr. Skorton, Dr. Orlowski, and Ms. Fisher have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Colonoscopy over age 75 should be ‘carefully considered’
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
Older individuals had a higher risk of complications 30 days after undergoing a colonoscopy as an outpatient procedure compared with a younger group of colorectal cancer screening–eligible individuals, according to recent research published in JAMA Network Open.
Natalia Causada-Calo, MD, MSc, division of gastroenterology, at St. Michael’s Hospital, University of Toronto, and colleagues performed a retrospective cohort study of 38,069 patients in Ontario administrative databases who underwent colonoscopy between April 2008 and September 2017. The patients included were older than 50 years (mean age, 65.2 years) with a majority (73.1%) undergoing their first colonoscopy. Those with inflammatory bowel disease and hereditary colorectal cancer syndromes were excluded. Researchers divided patients into groups based on age, placing patients aged 50-74 years into a colorectal cancer–screening eligible group (30,443 patients), and individuals 75 years or older into an “older cohort” (7,627 patients). Dr. Causada-Calo and colleagues analyzed 30-day admission to hospital or emergency department, and also examined 30-day all-cause mortality and incidence of colorectal cancer.
Among individuals in the older cohort, 515 of 7,627 patients (6.8%) experienced complications after colonoscopy compared with 795 of 30,443 patients (2.6%) in the screening-eligible cohort (P less than .001). Older age was an independent risk factor for postcolonoscopy complications, with individuals older than 75 years having twofold greater odds of complications after colonoscopy (odds ratio, 2.3; 95% confidence interval, 2.0-2.6) compared with individuals aged 50-74 years.
Other independent risk factors for complications included liver disease (OR, 4.7; 95% CI, 3.5-6.5), heart failure (OR, 3.4; 95% CI, 2.5-4.6), smoking history (OR, 3.2; 95% CI, 2.4-4.3), obesity (OR, 2.3; 95% CI, 1.2-4.2), chronic kidney disease (OR, 1.8; 95% CI, 1.1-3.0), cardiac arrhythmia (OR, 1.7; 95% CI, 1.2-2.2), anemia (OR, 1.4; 95% CI, 1.2-1.7), and hypertension (OR, 1.2; 95% CI, 1.0-1.5). Individuals who had previously undergone colonoscopy had a lower risk of complications after the procedure (OR, 0.9; 95% CI, 0.7-1.0).
There was a significantly higher incidence of colorectal cancer treated with surgery in the older group (119 of 7,626; 1.6%) compared with the younger (144 of 30,443; 0.5%) group (P less than .001). Mortality from any cause was also significantly higher in the older group (20 patients; 0.2%) compared with the younger (39 patients; 0.1%) group (P less than .001).
“In accordance with our findings, the decision to perform colonoscopy should be considered carefully in older patients, particularly in the presence of comorbidities,” Dr. Causada-Calo and colleagues wrote.
Aasma Shaukat, MD, MPH, GI section chief at Minneapolis VA Health Care System and professor of medicine at University of Minnesota, said in an interview that screening colonoscopy in a population older than 75 years should be an individualized discussion with a patient who has minimal comorbidities, and the decision to move forward with a colonoscopy should be considered only if a patient’s life expectancy is at least 10 years.
“This study shows that diagnostic colonoscopy is associated with high risk of complications and quantifies the risk, to frame the discussion with the patient about going forward,” she said. “Colorectal cancers are slow growing. In individuals age 75 and older, competing health risks and risk of the colonoscopy often outweigh the small benefit they may derive. Older individuals should thus focus on other health priorities.”
Physicians should make their older patients aware that there is a risk for serious adverse events, including death, which increases after age 75. “[The] risk-benefit ratio for performing colonoscopy needs to carefully weighed,” Dr. Shaukat said. “[T]he patient should be presented with options, including the option of no screening.”
The American Cancer Society advocates “for individualized decision-making regarding screening for individuals after 75 but [does] not give any firm recommendations,” while the U.S. Preventive Services Task Force noted in its recommendations on colorectal cancer screening that the “harms are large and benefits are small” after 75 years of age, and choice to screen for colorectal cancer in that age group is an individual one, Dr. Shaukat said.
Robert A. Smith, PhD, senior vice president of cancer screening at the American Cancer Society, said in an interview that while colonoscopy is the dominant screening test for colorectal cancer, it is not known how often physicians and their older patients discuss noninvasive colorectal cancer screening methods. Noninvasive screening methods such as a high-sensitivity stool test should be a consideration even for older adults with mild chronic conditions, “especially if they have a history of screening with negative results,” he said. “[A] history of regular screening with normal test results should be a basis for considering cessation of screening after age 75, or at least transition to a test with lower risks of complications.”
Future research in this area could use a hybrid model of screening, such as using different tests among various age groups or risk groups, to see whether an invasive or noninvasive method would lower a complication rate, Dr. Smith said. “Further, we need to have a greater understanding of when individuals can confidently stop getting screened, based on their underlying risk and history of prior screening results,” he noted.
Dr. Shaukat said future studies should focus on randomized trials for individuals 75 years and older to assess the benefits and harms of screening, “[d]eveloping risk stratification tools that factor in an individual’s risk of colon cancer, their life expectancy, and guide individualized decision making to undergo screening.”
Dr. Armstrong is the chair of the National Colon Cancer Screening Network for the Canadian Partnership Against Cancer and the past president of the Canadian Association of Gastroenterology. Dr. Albashir has received honoraria and speaker fees from Janssen, and grants from AbbVie and ATGen. Dr. Shaukat and Dr. Smith report no relevant conflicts of interest.
SOURCE: Causada-Calo N et al. JAMA Netw Open. 2020;3(6):e208958.
FROM JAMA NETWORK OPEN
Diagnostic criteria may miss some MIS-C cases, experts say
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
How many hormones make an ideal ‘artificial pancreas?’
Some automated insulin delivery systems currently in development add glucagon and/or pramlintide to insulin, but whether the extra hormones are worth the additional cost and effort is a subject of debate.
Also called closed-loop or artificial pancreas systems, they are comprised of an insulin pump and a continuous glucose monitor (CGM) that communicate via a built-in algorithm to deliver insulin based on glucose levels. Currently available systems are called hybrid closed loops because they still require user input for meals, exercise, illness, and other special circumstances.
Two hybrid closed-loop systems available in the United States, the Medtronic Minimed 670G and the Tandem Control-IQ, as well as the Medtronic Minimed 780G that was just approved in Europe, use insulin only.
Of all ongoing active closed-loop clinical studies, 44 involve insulin-only systems, as of May 2020.
However, two such systems in development add a glucagon analogue to insulin in the same pump (in separate cartridges), with the aim of minimizing the risk of hypoglycemia. And four investigational systems combine insulin with pramlintide (Symlin, AstraZeneca), an amylin analogue that reduces postmeal glucose spikes. Three systems in development combine all three hormones.
In a debate during the virtual American Diabetes Association 80th Scientific Sessions, Roman Hovorka, PhD, of the University of Cambridge (England) argued in favor of insulin-only systems on the basis of efficacy, less burden and complexity, and lower cost.
But Steven J. Russell, MD, PhD, of Massachusetts General Hospital, Boston, countered that glucagon adds safety and value to the system by allowing for more aggressive insulin dosing with lower hypoglycemia risk, benefits which he said would overcome any downsides.
Insulin-only systems are good enough
Dr. Hovorka began by defining a “good” artificial pancreas as one that produces consensus time-in-range targets of at least 70% of glucose values between 3.9 to 10 mmol/L (70-180 mg/dL) and less than 3% below 3.9 mmol/L (70 mg/dL). At the same time, he said, the burden should be low, which he suggested means no more than 10-20 minutes a day spent managing the system, low “alarm burden,” and minimal technical issues.
“We need to balance glucose control and the burden. For some people, reducing the burden is sometimes even more important than the glucose control,” Dr. Hovorka commented.
He pointed out that, in addition to Medtronic’s and Tandem’s systems, two other insulin-only hybrid closed-loop systems are marketed outside the United States. These are the CamDiab system, available in the United Kingdom, which uses his group’s Cambridge control algorithm in a Dana pump with the Dexcom G6 sensor, and the Diabeloop algorithm, available in Europe, that combines a patch pump with the Dexcom G6.
“Lots of energy and resources are going to taking [insulin-only] systems into clinic use,” Dr. Hovorka observed.
He reviewed recently published data for both the Tandem Control-IQ and the Cambridge control algorithm showing similar results meeting the “good artificial pancreas” definition.
In his current clinic population of 160 patients aged 2-80 years using the Cambridge algorithm, 69% of users have achieved 70% or greater time in range and 28% have achieved 80% or greater time in range.
“So, the insulin-only system can achieve acceptable, and in some instances very good, glucose control,” Dr. Hovorka said.
He acknowledged that there are still challenges with insulin-only systems, including exercise-related dysglycemia and postprandial hyperglycemia related to slow insulin absorption, missed or incorrect boluses, or large meals.
But, Dr. Hovorka said, downsides of dual-hormone systems include the need for room-temperature stable glucagon and for dual-chamber pumps with two cannulas and two infusion sites (in addition to the sensor site), and the unknown long-term biological risks of chronic subcutaneous glucagon or pramlintide delivery.
Moreover, he said, costs are expected to be higher for a two-chamber versus single-chamber pump, as well as for the second hormone, reservoir, and infusion set.
Data thus far from short-term studies suggest that insulin-only systems are sufficient in eliminating nocturnal hypoglycemia, while the addition of glucagon potentially reduces daytime hypoglycemia, especially during exercise.
However, longer-term head-to-head studies will be needed, Dr. Hovorka said, noting, “Comparative benefits of the single- and dual-hormone system for improving hemoglobin A1c and preventing severe hypoglycemia remain unknown.”
He suggested that glucagon dual-hormone closed-loop systems might be suitable for patients who are particularly prone to hypoglycemia, whereas pramlintide dual-hormone systems have the potential to more fully close the loop when used with ultra rapid-acting insulin analogues.
Nonetheless, he said, “Many, if not most, users may achieve acceptable control with insulin-only systems.”
Dual-hormone systems: Extra benefit worth it?
Dr. Russell, who is an investigator in multicenter pivotal studies of both insulin-only and bihormonal configurations of the Beta Bionics iLet bionic pancreas, began his debate presentation by endorsing the effectiveness of insulin-only hybrid systems and stating that he encourages his patients with type 1 diabetes to use them.
But, he said, adding glucagon can allow for better automation of hypoglycemia prevention and treatment in situations such as exercise.
“A bihormonal system achieves lower glucose, higher time-in-range, and less hypoglycemia than a well-functioning insulin-only system.”
Moreover, Dr. Russell said, “Glucagon reduces the need for medicinal carbohydrates, promotes satiety, and increases energy expenditure. ... Combined, these three factors may oppose weight gain or encourage weight loss as opposed to a system that uses insulin only.”
He pointed to a 2017 meta-analysis that showed improved time-in-range and greater reductions in hypoglycemia with dual- versus single-hormone systems.
And, in unpublished data from a randomized random-order crossover study of 23 patients with type 1 diabetes who each spent a week with usual care (insulin pump with or without CGM), insulin-only iLet, and bihormonal iLet, mean glucose levels were 165, 148, and 139 mg/dL, respectively. Time-in-range was 60%, 72%, and 79%, and median time with glucose below 54 mg/dL was 0.6%, 0.6%, and 0.2%, respectively.
Dr. Russell also addressed each of the arguments made by Dr. Hovorka and others against glucagon use.
Regarding the need for a stable glucagon formulation, he said that the analogue being developed for the iLet, dasiglucagon (Zealand pharma), is stable for more than a month at 40º C, with higher bioavailability and slightly slower absorption than glucagon.
And while he acknowledged the need for two separate hormone cartridges, Dr. Russell said that the Gen4 version of the iLet is fairly simple and intuitive, and the device itself is about the same size as the Tandem t:slim.
Use of glucagon didn’t increase insulin use in iLet trials, nor was it associated with increased reported nausea or liver glycogen depletion.
And users universally reported preferring the bihormonal system.
Long-term safety of chronic glucagon exposure has yet to be addressed, but animal data are reassuring, Dr. Russell said.
Regarding increased cost, he pointed to 2018 data showing that the incremental improvement in glycemic control from no automation to single-hormone automation is the same as from single to dual (mean glucose reductions of 7.4 and 13.6 mg/dL, respectively, and decreases in time spent in hypoglycemia of 1.28% vs. 2.95%).
“I would argue that, if one can justify adding automation, one could justify some additional expense to add the cost of glucagon.” And, he said, the cost would likely be based on a negotiation around the extra value offered by the dual-hormone system.
“The addition of glucagon, I believe, will be justified by the improved outcomes and improved quality of life,” he concluded.
Dr. Hovorka has reported receiving research support from MiniMed Medtronic, Abbott Diabetes Care, and Dexcom; being a speaker for Novo Nordisk, Eli Lilly, and Dexcom; holding license fees from B. Braun and Medtronic; and being director of CamDiab. Dr. Russell has reported holding patents on aspects of the bionic pancreas; receiving honoraria, travel expenses, and/or research support from Dexcom, Eli Lilly, Tandem Diabetes, Sanofi, Novo Nordisk, Roche, Ascensia, Zealand Pharma, and Beta Bionics; being a consultant for Flexion Therapeutics, Senseonics, and Beta Bionics; and participating in scientific advisory boards for Companion Medical, Tandem Diabetes, and Unomedical.
A version of this article originally appeared on Medscape.com.
Some automated insulin delivery systems currently in development add glucagon and/or pramlintide to insulin, but whether the extra hormones are worth the additional cost and effort is a subject of debate.
Also called closed-loop or artificial pancreas systems, they are comprised of an insulin pump and a continuous glucose monitor (CGM) that communicate via a built-in algorithm to deliver insulin based on glucose levels. Currently available systems are called hybrid closed loops because they still require user input for meals, exercise, illness, and other special circumstances.
Two hybrid closed-loop systems available in the United States, the Medtronic Minimed 670G and the Tandem Control-IQ, as well as the Medtronic Minimed 780G that was just approved in Europe, use insulin only.
Of all ongoing active closed-loop clinical studies, 44 involve insulin-only systems, as of May 2020.
However, two such systems in development add a glucagon analogue to insulin in the same pump (in separate cartridges), with the aim of minimizing the risk of hypoglycemia. And four investigational systems combine insulin with pramlintide (Symlin, AstraZeneca), an amylin analogue that reduces postmeal glucose spikes. Three systems in development combine all three hormones.
In a debate during the virtual American Diabetes Association 80th Scientific Sessions, Roman Hovorka, PhD, of the University of Cambridge (England) argued in favor of insulin-only systems on the basis of efficacy, less burden and complexity, and lower cost.
But Steven J. Russell, MD, PhD, of Massachusetts General Hospital, Boston, countered that glucagon adds safety and value to the system by allowing for more aggressive insulin dosing with lower hypoglycemia risk, benefits which he said would overcome any downsides.
Insulin-only systems are good enough
Dr. Hovorka began by defining a “good” artificial pancreas as one that produces consensus time-in-range targets of at least 70% of glucose values between 3.9 to 10 mmol/L (70-180 mg/dL) and less than 3% below 3.9 mmol/L (70 mg/dL). At the same time, he said, the burden should be low, which he suggested means no more than 10-20 minutes a day spent managing the system, low “alarm burden,” and minimal technical issues.
“We need to balance glucose control and the burden. For some people, reducing the burden is sometimes even more important than the glucose control,” Dr. Hovorka commented.
He pointed out that, in addition to Medtronic’s and Tandem’s systems, two other insulin-only hybrid closed-loop systems are marketed outside the United States. These are the CamDiab system, available in the United Kingdom, which uses his group’s Cambridge control algorithm in a Dana pump with the Dexcom G6 sensor, and the Diabeloop algorithm, available in Europe, that combines a patch pump with the Dexcom G6.
“Lots of energy and resources are going to taking [insulin-only] systems into clinic use,” Dr. Hovorka observed.
He reviewed recently published data for both the Tandem Control-IQ and the Cambridge control algorithm showing similar results meeting the “good artificial pancreas” definition.
In his current clinic population of 160 patients aged 2-80 years using the Cambridge algorithm, 69% of users have achieved 70% or greater time in range and 28% have achieved 80% or greater time in range.
“So, the insulin-only system can achieve acceptable, and in some instances very good, glucose control,” Dr. Hovorka said.
He acknowledged that there are still challenges with insulin-only systems, including exercise-related dysglycemia and postprandial hyperglycemia related to slow insulin absorption, missed or incorrect boluses, or large meals.
But, Dr. Hovorka said, downsides of dual-hormone systems include the need for room-temperature stable glucagon and for dual-chamber pumps with two cannulas and two infusion sites (in addition to the sensor site), and the unknown long-term biological risks of chronic subcutaneous glucagon or pramlintide delivery.
Moreover, he said, costs are expected to be higher for a two-chamber versus single-chamber pump, as well as for the second hormone, reservoir, and infusion set.
Data thus far from short-term studies suggest that insulin-only systems are sufficient in eliminating nocturnal hypoglycemia, while the addition of glucagon potentially reduces daytime hypoglycemia, especially during exercise.
However, longer-term head-to-head studies will be needed, Dr. Hovorka said, noting, “Comparative benefits of the single- and dual-hormone system for improving hemoglobin A1c and preventing severe hypoglycemia remain unknown.”
He suggested that glucagon dual-hormone closed-loop systems might be suitable for patients who are particularly prone to hypoglycemia, whereas pramlintide dual-hormone systems have the potential to more fully close the loop when used with ultra rapid-acting insulin analogues.
Nonetheless, he said, “Many, if not most, users may achieve acceptable control with insulin-only systems.”
Dual-hormone systems: Extra benefit worth it?
Dr. Russell, who is an investigator in multicenter pivotal studies of both insulin-only and bihormonal configurations of the Beta Bionics iLet bionic pancreas, began his debate presentation by endorsing the effectiveness of insulin-only hybrid systems and stating that he encourages his patients with type 1 diabetes to use them.
But, he said, adding glucagon can allow for better automation of hypoglycemia prevention and treatment in situations such as exercise.
“A bihormonal system achieves lower glucose, higher time-in-range, and less hypoglycemia than a well-functioning insulin-only system.”
Moreover, Dr. Russell said, “Glucagon reduces the need for medicinal carbohydrates, promotes satiety, and increases energy expenditure. ... Combined, these three factors may oppose weight gain or encourage weight loss as opposed to a system that uses insulin only.”
He pointed to a 2017 meta-analysis that showed improved time-in-range and greater reductions in hypoglycemia with dual- versus single-hormone systems.
And, in unpublished data from a randomized random-order crossover study of 23 patients with type 1 diabetes who each spent a week with usual care (insulin pump with or without CGM), insulin-only iLet, and bihormonal iLet, mean glucose levels were 165, 148, and 139 mg/dL, respectively. Time-in-range was 60%, 72%, and 79%, and median time with glucose below 54 mg/dL was 0.6%, 0.6%, and 0.2%, respectively.
Dr. Russell also addressed each of the arguments made by Dr. Hovorka and others against glucagon use.
Regarding the need for a stable glucagon formulation, he said that the analogue being developed for the iLet, dasiglucagon (Zealand pharma), is stable for more than a month at 40º C, with higher bioavailability and slightly slower absorption than glucagon.
And while he acknowledged the need for two separate hormone cartridges, Dr. Russell said that the Gen4 version of the iLet is fairly simple and intuitive, and the device itself is about the same size as the Tandem t:slim.
Use of glucagon didn’t increase insulin use in iLet trials, nor was it associated with increased reported nausea or liver glycogen depletion.
And users universally reported preferring the bihormonal system.
Long-term safety of chronic glucagon exposure has yet to be addressed, but animal data are reassuring, Dr. Russell said.
Regarding increased cost, he pointed to 2018 data showing that the incremental improvement in glycemic control from no automation to single-hormone automation is the same as from single to dual (mean glucose reductions of 7.4 and 13.6 mg/dL, respectively, and decreases in time spent in hypoglycemia of 1.28% vs. 2.95%).
“I would argue that, if one can justify adding automation, one could justify some additional expense to add the cost of glucagon.” And, he said, the cost would likely be based on a negotiation around the extra value offered by the dual-hormone system.
“The addition of glucagon, I believe, will be justified by the improved outcomes and improved quality of life,” he concluded.
Dr. Hovorka has reported receiving research support from MiniMed Medtronic, Abbott Diabetes Care, and Dexcom; being a speaker for Novo Nordisk, Eli Lilly, and Dexcom; holding license fees from B. Braun and Medtronic; and being director of CamDiab. Dr. Russell has reported holding patents on aspects of the bionic pancreas; receiving honoraria, travel expenses, and/or research support from Dexcom, Eli Lilly, Tandem Diabetes, Sanofi, Novo Nordisk, Roche, Ascensia, Zealand Pharma, and Beta Bionics; being a consultant for Flexion Therapeutics, Senseonics, and Beta Bionics; and participating in scientific advisory boards for Companion Medical, Tandem Diabetes, and Unomedical.
A version of this article originally appeared on Medscape.com.
Some automated insulin delivery systems currently in development add glucagon and/or pramlintide to insulin, but whether the extra hormones are worth the additional cost and effort is a subject of debate.
Also called closed-loop or artificial pancreas systems, they are comprised of an insulin pump and a continuous glucose monitor (CGM) that communicate via a built-in algorithm to deliver insulin based on glucose levels. Currently available systems are called hybrid closed loops because they still require user input for meals, exercise, illness, and other special circumstances.
Two hybrid closed-loop systems available in the United States, the Medtronic Minimed 670G and the Tandem Control-IQ, as well as the Medtronic Minimed 780G that was just approved in Europe, use insulin only.
Of all ongoing active closed-loop clinical studies, 44 involve insulin-only systems, as of May 2020.
However, two such systems in development add a glucagon analogue to insulin in the same pump (in separate cartridges), with the aim of minimizing the risk of hypoglycemia. And four investigational systems combine insulin with pramlintide (Symlin, AstraZeneca), an amylin analogue that reduces postmeal glucose spikes. Three systems in development combine all three hormones.
In a debate during the virtual American Diabetes Association 80th Scientific Sessions, Roman Hovorka, PhD, of the University of Cambridge (England) argued in favor of insulin-only systems on the basis of efficacy, less burden and complexity, and lower cost.
But Steven J. Russell, MD, PhD, of Massachusetts General Hospital, Boston, countered that glucagon adds safety and value to the system by allowing for more aggressive insulin dosing with lower hypoglycemia risk, benefits which he said would overcome any downsides.
Insulin-only systems are good enough
Dr. Hovorka began by defining a “good” artificial pancreas as one that produces consensus time-in-range targets of at least 70% of glucose values between 3.9 to 10 mmol/L (70-180 mg/dL) and less than 3% below 3.9 mmol/L (70 mg/dL). At the same time, he said, the burden should be low, which he suggested means no more than 10-20 minutes a day spent managing the system, low “alarm burden,” and minimal technical issues.
“We need to balance glucose control and the burden. For some people, reducing the burden is sometimes even more important than the glucose control,” Dr. Hovorka commented.
He pointed out that, in addition to Medtronic’s and Tandem’s systems, two other insulin-only hybrid closed-loop systems are marketed outside the United States. These are the CamDiab system, available in the United Kingdom, which uses his group’s Cambridge control algorithm in a Dana pump with the Dexcom G6 sensor, and the Diabeloop algorithm, available in Europe, that combines a patch pump with the Dexcom G6.
“Lots of energy and resources are going to taking [insulin-only] systems into clinic use,” Dr. Hovorka observed.
He reviewed recently published data for both the Tandem Control-IQ and the Cambridge control algorithm showing similar results meeting the “good artificial pancreas” definition.
In his current clinic population of 160 patients aged 2-80 years using the Cambridge algorithm, 69% of users have achieved 70% or greater time in range and 28% have achieved 80% or greater time in range.
“So, the insulin-only system can achieve acceptable, and in some instances very good, glucose control,” Dr. Hovorka said.
He acknowledged that there are still challenges with insulin-only systems, including exercise-related dysglycemia and postprandial hyperglycemia related to slow insulin absorption, missed or incorrect boluses, or large meals.
But, Dr. Hovorka said, downsides of dual-hormone systems include the need for room-temperature stable glucagon and for dual-chamber pumps with two cannulas and two infusion sites (in addition to the sensor site), and the unknown long-term biological risks of chronic subcutaneous glucagon or pramlintide delivery.
Moreover, he said, costs are expected to be higher for a two-chamber versus single-chamber pump, as well as for the second hormone, reservoir, and infusion set.
Data thus far from short-term studies suggest that insulin-only systems are sufficient in eliminating nocturnal hypoglycemia, while the addition of glucagon potentially reduces daytime hypoglycemia, especially during exercise.
However, longer-term head-to-head studies will be needed, Dr. Hovorka said, noting, “Comparative benefits of the single- and dual-hormone system for improving hemoglobin A1c and preventing severe hypoglycemia remain unknown.”
He suggested that glucagon dual-hormone closed-loop systems might be suitable for patients who are particularly prone to hypoglycemia, whereas pramlintide dual-hormone systems have the potential to more fully close the loop when used with ultra rapid-acting insulin analogues.
Nonetheless, he said, “Many, if not most, users may achieve acceptable control with insulin-only systems.”
Dual-hormone systems: Extra benefit worth it?
Dr. Russell, who is an investigator in multicenter pivotal studies of both insulin-only and bihormonal configurations of the Beta Bionics iLet bionic pancreas, began his debate presentation by endorsing the effectiveness of insulin-only hybrid systems and stating that he encourages his patients with type 1 diabetes to use them.
But, he said, adding glucagon can allow for better automation of hypoglycemia prevention and treatment in situations such as exercise.
“A bihormonal system achieves lower glucose, higher time-in-range, and less hypoglycemia than a well-functioning insulin-only system.”
Moreover, Dr. Russell said, “Glucagon reduces the need for medicinal carbohydrates, promotes satiety, and increases energy expenditure. ... Combined, these three factors may oppose weight gain or encourage weight loss as opposed to a system that uses insulin only.”
He pointed to a 2017 meta-analysis that showed improved time-in-range and greater reductions in hypoglycemia with dual- versus single-hormone systems.
And, in unpublished data from a randomized random-order crossover study of 23 patients with type 1 diabetes who each spent a week with usual care (insulin pump with or without CGM), insulin-only iLet, and bihormonal iLet, mean glucose levels were 165, 148, and 139 mg/dL, respectively. Time-in-range was 60%, 72%, and 79%, and median time with glucose below 54 mg/dL was 0.6%, 0.6%, and 0.2%, respectively.
Dr. Russell also addressed each of the arguments made by Dr. Hovorka and others against glucagon use.
Regarding the need for a stable glucagon formulation, he said that the analogue being developed for the iLet, dasiglucagon (Zealand pharma), is stable for more than a month at 40º C, with higher bioavailability and slightly slower absorption than glucagon.
And while he acknowledged the need for two separate hormone cartridges, Dr. Russell said that the Gen4 version of the iLet is fairly simple and intuitive, and the device itself is about the same size as the Tandem t:slim.
Use of glucagon didn’t increase insulin use in iLet trials, nor was it associated with increased reported nausea or liver glycogen depletion.
And users universally reported preferring the bihormonal system.
Long-term safety of chronic glucagon exposure has yet to be addressed, but animal data are reassuring, Dr. Russell said.
Regarding increased cost, he pointed to 2018 data showing that the incremental improvement in glycemic control from no automation to single-hormone automation is the same as from single to dual (mean glucose reductions of 7.4 and 13.6 mg/dL, respectively, and decreases in time spent in hypoglycemia of 1.28% vs. 2.95%).
“I would argue that, if one can justify adding automation, one could justify some additional expense to add the cost of glucagon.” And, he said, the cost would likely be based on a negotiation around the extra value offered by the dual-hormone system.
“The addition of glucagon, I believe, will be justified by the improved outcomes and improved quality of life,” he concluded.
Dr. Hovorka has reported receiving research support from MiniMed Medtronic, Abbott Diabetes Care, and Dexcom; being a speaker for Novo Nordisk, Eli Lilly, and Dexcom; holding license fees from B. Braun and Medtronic; and being director of CamDiab. Dr. Russell has reported holding patents on aspects of the bionic pancreas; receiving honoraria, travel expenses, and/or research support from Dexcom, Eli Lilly, Tandem Diabetes, Sanofi, Novo Nordisk, Roche, Ascensia, Zealand Pharma, and Beta Bionics; being a consultant for Flexion Therapeutics, Senseonics, and Beta Bionics; and participating in scientific advisory boards for Companion Medical, Tandem Diabetes, and Unomedical.
A version of this article originally appeared on Medscape.com.
Captopril questioned for diabetes patients in COVID-19 setting
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Daily Recap: Migraine affects pregnancy planning; FDA okays urothelial carcinoma therapy
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Migraine is often a deciding factor in pregnancy planning
Migraine can significantly influence a woman’s decision to have children, new research shows.
Results from a multicenter study of more than 600 women showed that, among participants with migraine, those who were younger, had menstrual migraine, or had chronic migraine were more likely to decide to not become pregnant.
“Women who avoided pregnancy due to migraine were most concerned that migraine would make raising a child difficult, that the migraine medications they take would have a negative impact on their child’s development, and that their migraine pattern would worsen during or just after pregnancy,” said study investigator Ryotaro Ishii, MD, PhD, a visiting scientist at Mayo Clinic in Phoenix.
The findings were presented at the virtual annual meeting of the American Headache Society. Read more.
FDA approves avelumab as maintenance for urothelial carcinoma
The Food and Drug Administration has approved avelumab (Bavencio) as a maintenance treatment for patients with locally advanced or metastatic urothelial carcinoma (UC) that has not progressed after first-line platinum-containing chemotherapy.
The new maintenance therapy indication for avelumab is based on efficacy demonstrated in the JAVELIN Bladder 100 trial. Results from this trial were presented as part of the American Society of Clinical Oncology virtual scientific program.
The new indication adds to avelumab use in other patient populations, including people with locally advanced or metastatic UC who experience disease progression during or following platinum-containing chemotherapy. The FDA also previously approved avelumab for patients who experienced UC progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. The FDA first approved marketing of avelumab in 2017. Other uses include treatment of metastatic Merkel cell carcinoma and first-line treatment of advanced renal cell carcinoma in combination with axitinib. Read more.
Lifestyle changes may explain skin lesions in pandemic-era patients
Two European prospective case series found no direct association between skin lesions on the hands and feet and SARS-CoV-2 in young people, which raises questions about other contributing factors, such as lockdown conditions, which may be clarified with additional research. The study appeared in JAMA Dermatology.
Meanwhile, data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients.
“It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” explained Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study. Read more.
Take-home test strips allow drug users to detect fentanyl
Illicit drug users seem to overwhelmingly appreciate being able to use take-home test strips to detect the presence of dangerous fentanyl in opioids and other drugs, a new study finds.
More than 95% said they’d use the inexpensive strips again.
“These tests accurately detect fentanyl in the drug supply, and they can be a valuable addition to other drug prevention strategies,” the study’s lead author and addiction medicine specialist Sukhpreet Klaire, MD, of the British Columbia Center on Substance Use in Vancouver, said in an interview.
Dr. Klaire presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, just 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.