User login
Ringing the alarm about black youth suicide
A “growing and disturbing” increase in suicidal behavior among black youth has quietly been underway in the United States during the past several decades, even while rates in white and Latino youth have declined, Michael A. Lindsey, PhD, MSW, MPH, declared at the virtual annual meeting of the American Association of Suicidology.
Until recently this trend remained below the radar of public awareness. That’s changing. Dr. Lindsey was coauthor of a December 2019 report to Congress prepared in collaboration with the Congressional Black Caucus entitled, “Ring the Alarm: The Crisis of Black Youth Suicide In America.” Release of the report was accompanied by submission of an omnibus bill aimed at addressing the issue comprehensively, including what Dr. Lindsey considers to be the single most important policy imperative: providing federal resources to support more and better school mental health services proportionate to student needs.
“Black youth, relative to white youth, do not receive treatment for depression, which may be a precursor issue. They’re often disconnected from mental health therapy. This is perhaps a reason why we’re seeing this uptick in suicide expression among black youth,” according to Dr. Lindsey, executive director of the McSilver Institute for Poverty Policy and Research and professor of poverty studies at New York University.
Investigators at Ohio State University analyzed youth suicide data for the years 2001-2015 obtained from the Centers for Disease Control and Prevention. They determined that black children aged 5-12 years had an 82% higher incidence of completed suicide than white children (JAMA Pediatr. 2018 Jul 1;172[7]:697-9).
This report was followed by a study of trends in suicidal behaviors among U.S. high school students during 1991-2017. The study, led by Dr. Lindsey, used data from the Youth Risk Behavior Survey covering the years 1991-2017 to document an overall 19% prevalence of thoughts about suicide, while 15% of high school students had a suicide plan. During the study years there was a 73% increase in suicide attempts among black adolescents, while rates in white and Latino teens fell by 7.5% and 11.4%, respectively (Pediatrics. 2019 Nov;144[5]:e20191187).
Dr. Lindsey cited multiple reasons for undertreatment of depression in black youth. The lack of adequate mental health services in many schools figures prominently. As a result of this situation, mental health problems in black youth are often misinterpreted as conduct problems, leading to well-documented overuse of school suspensions and expulsions.
“We tend to oversuspend and expel black kids from school for problems that are treatable. This becomes a major, major issue in the pathway from schools to prisons,” he said.
Another factor in underutilization of mental health services by black youth is the stigma involved. Many black families see mental health therapy as irrelevant. Dr. Lindsey has received grant support from the National Institute of Mental Health for development of engagement interventions that focus on stigma reduction and enhancing family support for mental health therapy in black youth. He has found that, once those barriers are lowered, therapies seem to be as effective in black youth as in other populations, despite the cultural differences.
Yet another potential explanation for the racial disparity in pediatric suicide might be that suicide may, in some cases, be more of an impulsive behavior in black youth. Dr. Lindsey presented data from a soon-to-be-published analysis of Youth Risk Behavior Survey data on nearly 5,000 adolescents with suicidal thoughts, plans, and/or attempts within the previous 12 months. About 23% had suicidal thoughts only, 37% had suicidal thoughts and a plan, another 37% had thoughts, plans, and suicide attempts, and 3% had attempts without thoughts or a plan.
Black youth were 3.7 times more likely than white youth to have attempted suicide in the absence of background suicidal thoughts and 3.3 times more likely to have attempted suicide without having suicidal thoughts and plans.
He and his coinvestigators identified a similar pattern of suicide as an impulsive behavior in youths of all races with a history of sexual assault. They were 4.2 times more likely to have attempted suicide without prior suicidal thoughts than individuals without such a history and 3.9 times more likely to have attempted suicide without thinking about it or having a plan.
“This has implications for screening and prevention; warning signs may not be present,” he said.
Dr. Lindsey reported having no financial conflicts regarding his presentation.
A “growing and disturbing” increase in suicidal behavior among black youth has quietly been underway in the United States during the past several decades, even while rates in white and Latino youth have declined, Michael A. Lindsey, PhD, MSW, MPH, declared at the virtual annual meeting of the American Association of Suicidology.
Until recently this trend remained below the radar of public awareness. That’s changing. Dr. Lindsey was coauthor of a December 2019 report to Congress prepared in collaboration with the Congressional Black Caucus entitled, “Ring the Alarm: The Crisis of Black Youth Suicide In America.” Release of the report was accompanied by submission of an omnibus bill aimed at addressing the issue comprehensively, including what Dr. Lindsey considers to be the single most important policy imperative: providing federal resources to support more and better school mental health services proportionate to student needs.
“Black youth, relative to white youth, do not receive treatment for depression, which may be a precursor issue. They’re often disconnected from mental health therapy. This is perhaps a reason why we’re seeing this uptick in suicide expression among black youth,” according to Dr. Lindsey, executive director of the McSilver Institute for Poverty Policy and Research and professor of poverty studies at New York University.
Investigators at Ohio State University analyzed youth suicide data for the years 2001-2015 obtained from the Centers for Disease Control and Prevention. They determined that black children aged 5-12 years had an 82% higher incidence of completed suicide than white children (JAMA Pediatr. 2018 Jul 1;172[7]:697-9).
This report was followed by a study of trends in suicidal behaviors among U.S. high school students during 1991-2017. The study, led by Dr. Lindsey, used data from the Youth Risk Behavior Survey covering the years 1991-2017 to document an overall 19% prevalence of thoughts about suicide, while 15% of high school students had a suicide plan. During the study years there was a 73% increase in suicide attempts among black adolescents, while rates in white and Latino teens fell by 7.5% and 11.4%, respectively (Pediatrics. 2019 Nov;144[5]:e20191187).
Dr. Lindsey cited multiple reasons for undertreatment of depression in black youth. The lack of adequate mental health services in many schools figures prominently. As a result of this situation, mental health problems in black youth are often misinterpreted as conduct problems, leading to well-documented overuse of school suspensions and expulsions.
“We tend to oversuspend and expel black kids from school for problems that are treatable. This becomes a major, major issue in the pathway from schools to prisons,” he said.
Another factor in underutilization of mental health services by black youth is the stigma involved. Many black families see mental health therapy as irrelevant. Dr. Lindsey has received grant support from the National Institute of Mental Health for development of engagement interventions that focus on stigma reduction and enhancing family support for mental health therapy in black youth. He has found that, once those barriers are lowered, therapies seem to be as effective in black youth as in other populations, despite the cultural differences.
Yet another potential explanation for the racial disparity in pediatric suicide might be that suicide may, in some cases, be more of an impulsive behavior in black youth. Dr. Lindsey presented data from a soon-to-be-published analysis of Youth Risk Behavior Survey data on nearly 5,000 adolescents with suicidal thoughts, plans, and/or attempts within the previous 12 months. About 23% had suicidal thoughts only, 37% had suicidal thoughts and a plan, another 37% had thoughts, plans, and suicide attempts, and 3% had attempts without thoughts or a plan.
Black youth were 3.7 times more likely than white youth to have attempted suicide in the absence of background suicidal thoughts and 3.3 times more likely to have attempted suicide without having suicidal thoughts and plans.
He and his coinvestigators identified a similar pattern of suicide as an impulsive behavior in youths of all races with a history of sexual assault. They were 4.2 times more likely to have attempted suicide without prior suicidal thoughts than individuals without such a history and 3.9 times more likely to have attempted suicide without thinking about it or having a plan.
“This has implications for screening and prevention; warning signs may not be present,” he said.
Dr. Lindsey reported having no financial conflicts regarding his presentation.
A “growing and disturbing” increase in suicidal behavior among black youth has quietly been underway in the United States during the past several decades, even while rates in white and Latino youth have declined, Michael A. Lindsey, PhD, MSW, MPH, declared at the virtual annual meeting of the American Association of Suicidology.
Until recently this trend remained below the radar of public awareness. That’s changing. Dr. Lindsey was coauthor of a December 2019 report to Congress prepared in collaboration with the Congressional Black Caucus entitled, “Ring the Alarm: The Crisis of Black Youth Suicide In America.” Release of the report was accompanied by submission of an omnibus bill aimed at addressing the issue comprehensively, including what Dr. Lindsey considers to be the single most important policy imperative: providing federal resources to support more and better school mental health services proportionate to student needs.
“Black youth, relative to white youth, do not receive treatment for depression, which may be a precursor issue. They’re often disconnected from mental health therapy. This is perhaps a reason why we’re seeing this uptick in suicide expression among black youth,” according to Dr. Lindsey, executive director of the McSilver Institute for Poverty Policy and Research and professor of poverty studies at New York University.
Investigators at Ohio State University analyzed youth suicide data for the years 2001-2015 obtained from the Centers for Disease Control and Prevention. They determined that black children aged 5-12 years had an 82% higher incidence of completed suicide than white children (JAMA Pediatr. 2018 Jul 1;172[7]:697-9).
This report was followed by a study of trends in suicidal behaviors among U.S. high school students during 1991-2017. The study, led by Dr. Lindsey, used data from the Youth Risk Behavior Survey covering the years 1991-2017 to document an overall 19% prevalence of thoughts about suicide, while 15% of high school students had a suicide plan. During the study years there was a 73% increase in suicide attempts among black adolescents, while rates in white and Latino teens fell by 7.5% and 11.4%, respectively (Pediatrics. 2019 Nov;144[5]:e20191187).
Dr. Lindsey cited multiple reasons for undertreatment of depression in black youth. The lack of adequate mental health services in many schools figures prominently. As a result of this situation, mental health problems in black youth are often misinterpreted as conduct problems, leading to well-documented overuse of school suspensions and expulsions.
“We tend to oversuspend and expel black kids from school for problems that are treatable. This becomes a major, major issue in the pathway from schools to prisons,” he said.
Another factor in underutilization of mental health services by black youth is the stigma involved. Many black families see mental health therapy as irrelevant. Dr. Lindsey has received grant support from the National Institute of Mental Health for development of engagement interventions that focus on stigma reduction and enhancing family support for mental health therapy in black youth. He has found that, once those barriers are lowered, therapies seem to be as effective in black youth as in other populations, despite the cultural differences.
Yet another potential explanation for the racial disparity in pediatric suicide might be that suicide may, in some cases, be more of an impulsive behavior in black youth. Dr. Lindsey presented data from a soon-to-be-published analysis of Youth Risk Behavior Survey data on nearly 5,000 adolescents with suicidal thoughts, plans, and/or attempts within the previous 12 months. About 23% had suicidal thoughts only, 37% had suicidal thoughts and a plan, another 37% had thoughts, plans, and suicide attempts, and 3% had attempts without thoughts or a plan.
Black youth were 3.7 times more likely than white youth to have attempted suicide in the absence of background suicidal thoughts and 3.3 times more likely to have attempted suicide without having suicidal thoughts and plans.
He and his coinvestigators identified a similar pattern of suicide as an impulsive behavior in youths of all races with a history of sexual assault. They were 4.2 times more likely to have attempted suicide without prior suicidal thoughts than individuals without such a history and 3.9 times more likely to have attempted suicide without thinking about it or having a plan.
“This has implications for screening and prevention; warning signs may not be present,” he said.
Dr. Lindsey reported having no financial conflicts regarding his presentation.
FROM AAS 2020
Consistent effects for galcanezumab in cluster headache
new research suggests. A post hoc analysis of patients from the phase 3 CGAL study who also entered the open-label CGAR extension study was conducted. Results showed that the majority of participants whose scores on the Patient Global Impression of Improvement (PGI-I) showed improvement 1 month after the initial dose of galcanezumab in the CGAL study also showed improvement after treatment for subsequent cluster bouts during the CGAR study.
“There was good agreement between PGI-I between the two [cluster headache] periods,” noted the investigators, led by Brian Plato, DO, a neurologist at Norton Neuroscience Institute in Louisville, Ky.
The findings were presented at the virtual annual meeting of the American Headache Society.
Two cluster periods
Galcanezumab was approved by the Food and Drug Administration in 2019 for the treatment of episodic cluster headache in adults.
In cluster headache, attacks of recurrent, unilateral headaches with cranial autonomic symptoms last for weeks or months and are followed by periods of remission. Most studies of therapies for cluster headache examine only one cluster period. Few data about the consistency of treatment response throughout consecutive cluster periods are available, the investigators noted.
The current analysis was undertaken to examine the consistency of galcanezumab’s effect in episodic cluster headache during two cluster periods. Patients eligible for inclusion in the analysis had completed the double-blind phase of the CGAL study and had entered the open-label CGAR study.
CGAL was a phase 3, multicenter, randomized, double-blind study in which patients with episodic cluster headache were assigned to receive galcanezumab 300 mg per month or placebo. Patients who completed the double-blind and washout phases of this study were eligible for enrollment into CGAR, a phase 3b, single-arm safety study. The investigators determined the dose of galcanezumab in accordance with each patient’s symptoms and clinical response.
Response agreement
In both studies, the PGI-I was administered 1 month after the initial dose of galcanezumab. Only patients who were in an active cluster bout on entry into CGAR and who had valid PGI-I results 1 month after the first dose in CGAL and CGAR were included in the analysis.
PGI-I responses ranged from 1, signifying very much better, to 7, signifying very much worse. The investigators summarized the proportions of patients who reported each level of PGI-I score in CGAR and analyzed the results by dichotomizing PGI-I scores at both time points in two ways.
Fifty patients entered CGAR (78% men; mean age, 46.8 years). Of this group, Dr. Plato and colleagues included 39 in their analysis. Of the 17 patients who had a PGI-I score of 1 or 2 in CGAL, 12 (70.6%) had a score in the same range in CGAR. All four participants who had a score of 3 or higher in CGAL had a score in the same range in CGAR. Eighteen participants had a PGI-I score of 1, 2, or 3 in CGAL. Of this group, 15 patients (83.3%) had a score in the same range in CGAR. Of the three patients who had a score above 3 in CGAL, two (66.7%) had a score in the same range in CGAR.
The results indicate that most patients whose PGI-I score improved in one cluster bout, such as in CGAL, also improved in a subsequent bout, such as in CGAR, the investigators noted.
‘Encouraging’ results
Commenting on the study, Brian E. McGeeney, MD, a neurologist at the John R. Graham Headache Center, Brigham and Women’s Faulkner Hospital, Boston, noted that the PGI-I is an “easy-to-understand” outcome that has been widely used in headache medicine.
“Patient-assessed outcomes have become increasingly important and are an important complement to other outcomes,” said Dr. McGeeney, who was not involved in the research. However, a disadvantage is that “it is entirely subjective and may or may not reflect a change on other outcome measures that reflect the disorder itself,” he said.
“It can be difficult to demonstrate how much usefulness a treatment has with the helpful but simple outcome measures that are seen in CGAL and CGAR,” Dr. McGeeney added. “This is due to the nature of cluster headache and not to any methodological shortcomings of those studies.”
He said this is a core problem in general with cluster headache studies, “of which there are very few.”
In addition, CGAR only included episodic cluster headache, and the study period was relatively short; and CGAL only explored one cluster period per patient, Dr. McGeeney noted.
The current research attempts to provide insight that was previously unavailable, he said. “Many headache medicine clinical trial results reflect only one episode, and in general, we infer repeated usefulness – although it is not demonstrated in clinical trials,” said Dr. McGeeney.
“In this recent presentation, the authors attempt to go further and demonstrate some consistency across multiple cluster periods. The results are encouraging and what one might expect,” he said. However, “the small numbers and ad hoc nature preclude much inference from this study alone.”
Dr. Plato has received honoraria for speaking from Allergan, Amgen/Novartis, and Eli Lilly. He has also received research grants and support from Electrocore and Teva. Dr. McGeeney has consulted for Upsher-Smith and Theranica.
A version of this article originally appeared on Medscape.com.
new research suggests. A post hoc analysis of patients from the phase 3 CGAL study who also entered the open-label CGAR extension study was conducted. Results showed that the majority of participants whose scores on the Patient Global Impression of Improvement (PGI-I) showed improvement 1 month after the initial dose of galcanezumab in the CGAL study also showed improvement after treatment for subsequent cluster bouts during the CGAR study.
“There was good agreement between PGI-I between the two [cluster headache] periods,” noted the investigators, led by Brian Plato, DO, a neurologist at Norton Neuroscience Institute in Louisville, Ky.
The findings were presented at the virtual annual meeting of the American Headache Society.
Two cluster periods
Galcanezumab was approved by the Food and Drug Administration in 2019 for the treatment of episodic cluster headache in adults.
In cluster headache, attacks of recurrent, unilateral headaches with cranial autonomic symptoms last for weeks or months and are followed by periods of remission. Most studies of therapies for cluster headache examine only one cluster period. Few data about the consistency of treatment response throughout consecutive cluster periods are available, the investigators noted.
The current analysis was undertaken to examine the consistency of galcanezumab’s effect in episodic cluster headache during two cluster periods. Patients eligible for inclusion in the analysis had completed the double-blind phase of the CGAL study and had entered the open-label CGAR study.
CGAL was a phase 3, multicenter, randomized, double-blind study in which patients with episodic cluster headache were assigned to receive galcanezumab 300 mg per month or placebo. Patients who completed the double-blind and washout phases of this study were eligible for enrollment into CGAR, a phase 3b, single-arm safety study. The investigators determined the dose of galcanezumab in accordance with each patient’s symptoms and clinical response.
Response agreement
In both studies, the PGI-I was administered 1 month after the initial dose of galcanezumab. Only patients who were in an active cluster bout on entry into CGAR and who had valid PGI-I results 1 month after the first dose in CGAL and CGAR were included in the analysis.
PGI-I responses ranged from 1, signifying very much better, to 7, signifying very much worse. The investigators summarized the proportions of patients who reported each level of PGI-I score in CGAR and analyzed the results by dichotomizing PGI-I scores at both time points in two ways.
Fifty patients entered CGAR (78% men; mean age, 46.8 years). Of this group, Dr. Plato and colleagues included 39 in their analysis. Of the 17 patients who had a PGI-I score of 1 or 2 in CGAL, 12 (70.6%) had a score in the same range in CGAR. All four participants who had a score of 3 or higher in CGAL had a score in the same range in CGAR. Eighteen participants had a PGI-I score of 1, 2, or 3 in CGAL. Of this group, 15 patients (83.3%) had a score in the same range in CGAR. Of the three patients who had a score above 3 in CGAL, two (66.7%) had a score in the same range in CGAR.
The results indicate that most patients whose PGI-I score improved in one cluster bout, such as in CGAL, also improved in a subsequent bout, such as in CGAR, the investigators noted.
‘Encouraging’ results
Commenting on the study, Brian E. McGeeney, MD, a neurologist at the John R. Graham Headache Center, Brigham and Women’s Faulkner Hospital, Boston, noted that the PGI-I is an “easy-to-understand” outcome that has been widely used in headache medicine.
“Patient-assessed outcomes have become increasingly important and are an important complement to other outcomes,” said Dr. McGeeney, who was not involved in the research. However, a disadvantage is that “it is entirely subjective and may or may not reflect a change on other outcome measures that reflect the disorder itself,” he said.
“It can be difficult to demonstrate how much usefulness a treatment has with the helpful but simple outcome measures that are seen in CGAL and CGAR,” Dr. McGeeney added. “This is due to the nature of cluster headache and not to any methodological shortcomings of those studies.”
He said this is a core problem in general with cluster headache studies, “of which there are very few.”
In addition, CGAR only included episodic cluster headache, and the study period was relatively short; and CGAL only explored one cluster period per patient, Dr. McGeeney noted.
The current research attempts to provide insight that was previously unavailable, he said. “Many headache medicine clinical trial results reflect only one episode, and in general, we infer repeated usefulness – although it is not demonstrated in clinical trials,” said Dr. McGeeney.
“In this recent presentation, the authors attempt to go further and demonstrate some consistency across multiple cluster periods. The results are encouraging and what one might expect,” he said. However, “the small numbers and ad hoc nature preclude much inference from this study alone.”
Dr. Plato has received honoraria for speaking from Allergan, Amgen/Novartis, and Eli Lilly. He has also received research grants and support from Electrocore and Teva. Dr. McGeeney has consulted for Upsher-Smith and Theranica.
A version of this article originally appeared on Medscape.com.
new research suggests. A post hoc analysis of patients from the phase 3 CGAL study who also entered the open-label CGAR extension study was conducted. Results showed that the majority of participants whose scores on the Patient Global Impression of Improvement (PGI-I) showed improvement 1 month after the initial dose of galcanezumab in the CGAL study also showed improvement after treatment for subsequent cluster bouts during the CGAR study.
“There was good agreement between PGI-I between the two [cluster headache] periods,” noted the investigators, led by Brian Plato, DO, a neurologist at Norton Neuroscience Institute in Louisville, Ky.
The findings were presented at the virtual annual meeting of the American Headache Society.
Two cluster periods
Galcanezumab was approved by the Food and Drug Administration in 2019 for the treatment of episodic cluster headache in adults.
In cluster headache, attacks of recurrent, unilateral headaches with cranial autonomic symptoms last for weeks or months and are followed by periods of remission. Most studies of therapies for cluster headache examine only one cluster period. Few data about the consistency of treatment response throughout consecutive cluster periods are available, the investigators noted.
The current analysis was undertaken to examine the consistency of galcanezumab’s effect in episodic cluster headache during two cluster periods. Patients eligible for inclusion in the analysis had completed the double-blind phase of the CGAL study and had entered the open-label CGAR study.
CGAL was a phase 3, multicenter, randomized, double-blind study in which patients with episodic cluster headache were assigned to receive galcanezumab 300 mg per month or placebo. Patients who completed the double-blind and washout phases of this study were eligible for enrollment into CGAR, a phase 3b, single-arm safety study. The investigators determined the dose of galcanezumab in accordance with each patient’s symptoms and clinical response.
Response agreement
In both studies, the PGI-I was administered 1 month after the initial dose of galcanezumab. Only patients who were in an active cluster bout on entry into CGAR and who had valid PGI-I results 1 month after the first dose in CGAL and CGAR were included in the analysis.
PGI-I responses ranged from 1, signifying very much better, to 7, signifying very much worse. The investigators summarized the proportions of patients who reported each level of PGI-I score in CGAR and analyzed the results by dichotomizing PGI-I scores at both time points in two ways.
Fifty patients entered CGAR (78% men; mean age, 46.8 years). Of this group, Dr. Plato and colleagues included 39 in their analysis. Of the 17 patients who had a PGI-I score of 1 or 2 in CGAL, 12 (70.6%) had a score in the same range in CGAR. All four participants who had a score of 3 or higher in CGAL had a score in the same range in CGAR. Eighteen participants had a PGI-I score of 1, 2, or 3 in CGAL. Of this group, 15 patients (83.3%) had a score in the same range in CGAR. Of the three patients who had a score above 3 in CGAL, two (66.7%) had a score in the same range in CGAR.
The results indicate that most patients whose PGI-I score improved in one cluster bout, such as in CGAL, also improved in a subsequent bout, such as in CGAR, the investigators noted.
‘Encouraging’ results
Commenting on the study, Brian E. McGeeney, MD, a neurologist at the John R. Graham Headache Center, Brigham and Women’s Faulkner Hospital, Boston, noted that the PGI-I is an “easy-to-understand” outcome that has been widely used in headache medicine.
“Patient-assessed outcomes have become increasingly important and are an important complement to other outcomes,” said Dr. McGeeney, who was not involved in the research. However, a disadvantage is that “it is entirely subjective and may or may not reflect a change on other outcome measures that reflect the disorder itself,” he said.
“It can be difficult to demonstrate how much usefulness a treatment has with the helpful but simple outcome measures that are seen in CGAL and CGAR,” Dr. McGeeney added. “This is due to the nature of cluster headache and not to any methodological shortcomings of those studies.”
He said this is a core problem in general with cluster headache studies, “of which there are very few.”
In addition, CGAR only included episodic cluster headache, and the study period was relatively short; and CGAL only explored one cluster period per patient, Dr. McGeeney noted.
The current research attempts to provide insight that was previously unavailable, he said. “Many headache medicine clinical trial results reflect only one episode, and in general, we infer repeated usefulness – although it is not demonstrated in clinical trials,” said Dr. McGeeney.
“In this recent presentation, the authors attempt to go further and demonstrate some consistency across multiple cluster periods. The results are encouraging and what one might expect,” he said. However, “the small numbers and ad hoc nature preclude much inference from this study alone.”
Dr. Plato has received honoraria for speaking from Allergan, Amgen/Novartis, and Eli Lilly. He has also received research grants and support from Electrocore and Teva. Dr. McGeeney has consulted for Upsher-Smith and Theranica.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Truth in Tension: Reflections on Racism in Medicine
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
© 2020 Society of Hospital Medicine
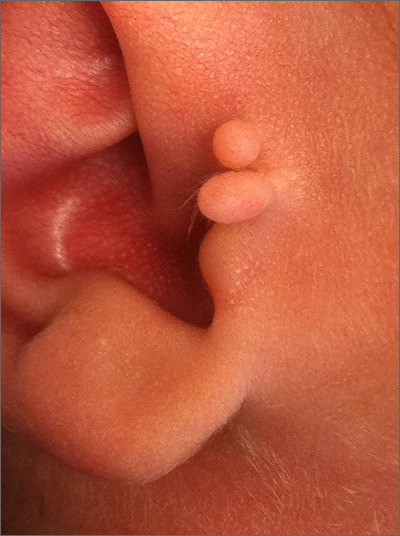
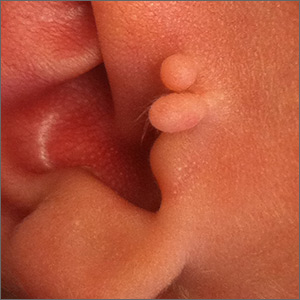
Newborn ear lesion
This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This lesion was diagnosed as an accessory tragus. Although sometimes called a preauricular skin tag, it is more aptly named accessory tragus since it arises embryonically along with the tragus from the first branchial arch. It usually is slightly anterior to the ear and can be seen unilaterally or bilaterally. The lesions usually feel firm to palpation due to an underlying cartilaginous component.
While this finding can be seen in congenital syndromes—most commonly oculoauriculovertebral syndrome (also known as Goldenhar syndrome)—it usually occurs in isolation. If there are no other abnormalities on exam to suggest a congenital syndrome, no additional work-up is necessary.
Concerns have been raised about a possible association with decreased hearing. Routine hearing screens are performed in the United States and this child’s screen was normal, so no further investigation for inner ear abnormalities was warranted.
These lesions usually are asymptomatic (unless traumatized). If removal is desired, it is important to remove the cartilaginous component, which can be deep and require additional dissection rather than simple transection at the base. Incomplete removal of the cartilage can cause chondritis, impede skin healing, and lead to infection.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
Bahrani B, Khachemoune A. Review of accessory tragus with highlights of its associated syndromes. Int J Dermatol. 2014;53:1442-1446.
New quadrivalent meningococcal vaccine joins VFC arsenal
No changes to the current meningococcal vaccination recommendations were made. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted 14-0 to include MenACWY-TT as an option for vaccination against meningococcal serogroups A, C, W, and Y in the VFC program. The vote took place in a virtual meeting held on June 24.
The currently available MenACWY vaccines in the United States are MenACWY-D (Menactra), MenACWY-CRW (Menveo), and MenACWY-TT (MedQuadfi), with MenACWY-TT approved by the Food and Drug Administration in April 2020.
Meningococcal vaccination is currently recommended for adolescents, with one dose at age 11 or 12 years and a booster at age 16 years, as well as individuals aged 2 months and older at increased risk for meningococcal disease, according to Lucy McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases.
Dr. McNamara presented considerations from the Meningococcal Work Group, which determined that the inclusion of MenACWY-TT “is of public health importance given recent vaccine licensure and to support security of vaccine supply.”
The Work Group reviewed 10 studies (phase 2 or 3) of MenACWY-TT that included data on short-term immune response, persistence of immune response, immune interference because of coadministration with other routine adolescent vaccines, and incidence of serious adverse events. Overall, the data showed noninferiority of MenACWY-TT, compared with other available products, in terms of response rates, as well as higher levels of immune response in some studies. Serious adverse events were similar, and none determined to be associated with the vaccines.
ACIP member Paul Hunter, MD, of the University of Milwaukee, Wisc., expressed some concerns about pain or side effects for the new vaccine and Tdap when given together. However, a study of coadministration of MedACWY-TT and Tdap, compared with Tdap alone, showed no impact on geometric mean titer ratios.
Overall, the Work Group concluded that “desirable effects outweigh undesirable effects” and that the data favor the inclusion of MenACWY-TT as an option for meningococcal vaccination.
The committee members and Dr. McNamara had no relevant financial conflicts to disclose.
No changes to the current meningococcal vaccination recommendations were made. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted 14-0 to include MenACWY-TT as an option for vaccination against meningococcal serogroups A, C, W, and Y in the VFC program. The vote took place in a virtual meeting held on June 24.
The currently available MenACWY vaccines in the United States are MenACWY-D (Menactra), MenACWY-CRW (Menveo), and MenACWY-TT (MedQuadfi), with MenACWY-TT approved by the Food and Drug Administration in April 2020.
Meningococcal vaccination is currently recommended for adolescents, with one dose at age 11 or 12 years and a booster at age 16 years, as well as individuals aged 2 months and older at increased risk for meningococcal disease, according to Lucy McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases.
Dr. McNamara presented considerations from the Meningococcal Work Group, which determined that the inclusion of MenACWY-TT “is of public health importance given recent vaccine licensure and to support security of vaccine supply.”
The Work Group reviewed 10 studies (phase 2 or 3) of MenACWY-TT that included data on short-term immune response, persistence of immune response, immune interference because of coadministration with other routine adolescent vaccines, and incidence of serious adverse events. Overall, the data showed noninferiority of MenACWY-TT, compared with other available products, in terms of response rates, as well as higher levels of immune response in some studies. Serious adverse events were similar, and none determined to be associated with the vaccines.
ACIP member Paul Hunter, MD, of the University of Milwaukee, Wisc., expressed some concerns about pain or side effects for the new vaccine and Tdap when given together. However, a study of coadministration of MedACWY-TT and Tdap, compared with Tdap alone, showed no impact on geometric mean titer ratios.
Overall, the Work Group concluded that “desirable effects outweigh undesirable effects” and that the data favor the inclusion of MenACWY-TT as an option for meningococcal vaccination.
The committee members and Dr. McNamara had no relevant financial conflicts to disclose.
No changes to the current meningococcal vaccination recommendations were made. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted 14-0 to include MenACWY-TT as an option for vaccination against meningococcal serogroups A, C, W, and Y in the VFC program. The vote took place in a virtual meeting held on June 24.
The currently available MenACWY vaccines in the United States are MenACWY-D (Menactra), MenACWY-CRW (Menveo), and MenACWY-TT (MedQuadfi), with MenACWY-TT approved by the Food and Drug Administration in April 2020.
Meningococcal vaccination is currently recommended for adolescents, with one dose at age 11 or 12 years and a booster at age 16 years, as well as individuals aged 2 months and older at increased risk for meningococcal disease, according to Lucy McNamara, PhD, of the CDC’s National Center for Immunization and Respiratory Diseases.
Dr. McNamara presented considerations from the Meningococcal Work Group, which determined that the inclusion of MenACWY-TT “is of public health importance given recent vaccine licensure and to support security of vaccine supply.”
The Work Group reviewed 10 studies (phase 2 or 3) of MenACWY-TT that included data on short-term immune response, persistence of immune response, immune interference because of coadministration with other routine adolescent vaccines, and incidence of serious adverse events. Overall, the data showed noninferiority of MenACWY-TT, compared with other available products, in terms of response rates, as well as higher levels of immune response in some studies. Serious adverse events were similar, and none determined to be associated with the vaccines.
ACIP member Paul Hunter, MD, of the University of Milwaukee, Wisc., expressed some concerns about pain or side effects for the new vaccine and Tdap when given together. However, a study of coadministration of MedACWY-TT and Tdap, compared with Tdap alone, showed no impact on geometric mean titer ratios.
Overall, the Work Group concluded that “desirable effects outweigh undesirable effects” and that the data favor the inclusion of MenACWY-TT as an option for meningococcal vaccination.
The committee members and Dr. McNamara had no relevant financial conflicts to disclose.
Daily Recap: Healthy lifestyle may stave off dementia; Tentative evidence on marijuana for migraine
Here are the stories our MDedge editors across specialties think you need to know about today:
First reported U.S. case of COVID-19 linked to Guillain-Barré syndrome
The first official U.S. case of Guillain-Barré syndrome (GBS) associated with COVID-19 has been reported by neurologists from Allegheny General Hospital in Pittsburgh, further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case that initially presented as acute GBS. Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
“This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted. Read more.
Five healthy lifestyle choices tied to dramatic cut in dementia risk
Combining four of five healthy lifestyle choices has been linked to up to a 60% reduced risk for Alzheimer’s dementia in new research that strengthens ties between healthy behaviors and lower dementia risk. “I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, said in an interview.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise; light to moderate alcohol consumption; consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Luca Giliberto, MD, PhD. Read more.
Marijuana for migraine? Some tentative evidence
Medical marijuana may have promise for managing headache pain, according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
“A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Jefferson headache fellow Claire Ceriani, MD, in an interview. Read more.
Inside Mercy’s mission to care for non-COVID patients in Los Angeles
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable. “We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission.
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals.
Care aboard the ship ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support. Special Feature.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
First reported U.S. case of COVID-19 linked to Guillain-Barré syndrome
The first official U.S. case of Guillain-Barré syndrome (GBS) associated with COVID-19 has been reported by neurologists from Allegheny General Hospital in Pittsburgh, further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case that initially presented as acute GBS. Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
“This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted. Read more.
Five healthy lifestyle choices tied to dramatic cut in dementia risk
Combining four of five healthy lifestyle choices has been linked to up to a 60% reduced risk for Alzheimer’s dementia in new research that strengthens ties between healthy behaviors and lower dementia risk. “I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, said in an interview.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise; light to moderate alcohol consumption; consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Luca Giliberto, MD, PhD. Read more.
Marijuana for migraine? Some tentative evidence
Medical marijuana may have promise for managing headache pain, according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
“A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Jefferson headache fellow Claire Ceriani, MD, in an interview. Read more.
Inside Mercy’s mission to care for non-COVID patients in Los Angeles
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable. “We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission.
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals.
Care aboard the ship ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support. Special Feature.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
First reported U.S. case of COVID-19 linked to Guillain-Barré syndrome
The first official U.S. case of Guillain-Barré syndrome (GBS) associated with COVID-19 has been reported by neurologists from Allegheny General Hospital in Pittsburgh, further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case that initially presented as acute GBS. Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
“This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted. Read more.
Five healthy lifestyle choices tied to dramatic cut in dementia risk
Combining four of five healthy lifestyle choices has been linked to up to a 60% reduced risk for Alzheimer’s dementia in new research that strengthens ties between healthy behaviors and lower dementia risk. “I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, said in an interview.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise; light to moderate alcohol consumption; consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Luca Giliberto, MD, PhD. Read more.
Marijuana for migraine? Some tentative evidence
Medical marijuana may have promise for managing headache pain, according to results from a small study conducted at the Jefferson Headache Center at Thomas Jefferson University. The researchers found general satisfaction with medical marijuana, more frequent use as an abortive medication rather than a preventative, and more than two-thirds using the inhaled form rather than oral.
“A lot of patients are interested in medical marijuana but don’t know how to integrate it into the therapy plan they already have – whether it should be just to treat bad headaches when they happen, or is it meant to be a preventive medicine they use every day? We have some data out there that it can be helpful, but not a lot of specific information to guide your recommendations,” said Jefferson headache fellow Claire Ceriani, MD, in an interview. Read more.
Inside Mercy’s mission to care for non-COVID patients in Los Angeles
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable. “We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission.
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals.
Care aboard the ship ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support. Special Feature.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
How can we better engage black men as patients?
I’m a black man, husband, father, son, brother, and a board-certified psychiatrist, child and adolescent psychiatry fellow, and addiction medicine fellow. I write this article as the latter, a colleague, from the former’s perspective, which you would not need to verify via Google, social media, or a badge upon meeting me.
July is Minority Mental Health Awareness Month, established to bring awareness to the unique struggles that marginalized groups face concerning mental illness in the United States.
Given the events of the last few months, including a global pandemic and videotaped killings of Ahmaud Arbery and George Floyd, two unarmed black men, America’s structural racism and inequality are being challenged in historic ways. Black people are suffering. In fact, I was not surprised to learn1 that some black families with sons have expanded the “talk” – which traditionally has focused on dealing with police officers – to include vigilantes.
Because of my extensive work with and treatment of men of color, I would like to answer a key question: “How do psychiatrists and other mental health clinicians better engage men of color? Before the “how,” let’s review the state of black men’s mental health.
According to Healthy People 2020, mental disorders are the leading cause of disability in the United States.2 Among those with diagnosable mental disorders, black people are more likely than are their white counterparts to experience severe symptoms and protracted diseases. Roughly 7% of black men meet the criteria for a lifetime prevalence of major depressive disorder.3 Applying that figure to recent national population estimates means that there are 1.4 million black men currently suffering from major depression. Suicide has been on a continued uptrend among black male youth for more than 2 decades. Moreover, given the high rates of stigma and unmet need in this population, it is likely that these figures are even more dire.
Compared with other groups, black men in the United States face a disproportionate burden of preventable morbidity and mortality rates. Of all the health concerns faced by black men, mental health challenges may be among the most stigmatized.4 Evidence suggests that black men have more adverse life experiences than do men of other racial/ethnic groups, and consequently, experience poorer mental health.5 Black men experience high rates of poverty, unemployment, and underemployment, and are incarcerated at much higher rates than those of men of other racial/ethnic groups.6 It is notable that black male youth are often perceived as older by law enforcement, beginning as early as 10 years old, often resulting in negative interactions.7
Despite those challenges, black men are often expected to project strength, they are expected to minimize displays of emotion when off the field or court (i.e., “Just shut up and dribble”), and they are expected to be true versions of folk hero John Henry. This caricature of black males is used at times to validate shootings of unarmed black males (adults and youth).
Black men’s mental health should be a priority for those in the mental health field. This is particularly the case light of our field’s historical involvement in and promotion of stereotyped clinical descriptions of black men and contributing to health disparities that persist. Black men are nearly six times as likely to be diagnosed with schizophrenia as are white men. To read about holdovers from the days of targeted advertising against black protesters of the 1960s and 1970s, check out “The Protest Psychosis” (Beacon Press, 2010) by psychiatrist and anthropologist Jonathan Metzl, MD, PhD. If you go further back in psychiatric history, the late 1800s, you can learn about the devious diagnosis of drapetomania attributed to enslaved people who were seeking freedom.
Those on the front lines providing mental health services should understand black men’s mental health from an ecological perspective. Beyond the emotional burden that mental illness imposes on the individual, there are more considerable interpersonal and societal implications for the state of black men’s mental health. As such, in our full capacity like other men, black men play an essential role within families, churches, neighborhoods, and organizations.
Given our brief review, we can reconsider our question, “How do psychiatrists and mental health clinicians better engage men of color?”
I will suggest a few fundamental principles that honestly can be applied to any patient but should be strongly considered with your black male patients – given they are likely not accustomed to engaging with the health care system, let alone with a mental health clinician:
1. Create a comfortable environment. Because of stigma, persistent myths, and lack of normalcy with talking to a mental health professional, many patients, including black men, do not have a framework for a psychiatric/psychological evaluation or treatment. It would be essential to set the frame of your encounter. Evidence suggests this can improve engagement and follow-up care among black men.8 In addition, keep in mind that “fictive kin”9 tend to play a major role in the transmission of culture, health promotion, and decision-making in the black community. This helps explain why barbershop initiatives10 are effective. If clinicians are able to allow black male patients to feel comfortable, the clinician, too, might become part of that fictive community and enhance the patient-provider relationship.
2. Allow for storytelling. In the age of the checklist, it can be relatively easy to lose sight that our patients, including black men, have their own narratives. Evidence suggests that physicians interrupt patients early and often. Challenge yourself to allow the patient to tell his story. In consideration of an initial evaluation, it may help to begin by first gathering sociodemographic information (i.e. housing, education, employment, family, etc.); doing so will allow the patient time to get comfortable before you assess possible psychiatric symptoms.
3. Confidentiality assurance. Many black men have a distrust for the health care profession; as such, it is vital that clinicians emphasize that their patients’ information and history will be used only to help the patient. It will be important to inform black male patients of their rights, because often in the greater society, their rights seem to be negated.
4. Be aware of nonverbal language. Given black men’s stereotyped roles in society and recognition that they are regularly perceived as threats, many black men have become adept at reading nonverbal cues (i.e., purse clutched, side comment, etc.). In doing so, clinicians must be attuned to their own nonverbal language. For example, a glance at one’s watch might be interpreted as you’re not listening. It would be better to be upfront and candid by saying something like, “I need to check the time,” rather than attempting to be stealth. Being transparent in that way will let the patient know that you will be upfront with him.
5. Be respectful. During an encounter, and in particular when discussing treatment plans, clinicians must allow the patient space to process and be involved in his care. Allowing the patient time to think through how he would want to proceed provides him a sense of personal agency and lets him know that he is capable of improving his mental wellness.
Black male patients need to feel comfortable, safe, able to trust the clinician. They must feel listened to, understood, and respected. This information might help some clinicians better understand what needs to happen between a black male patient and a nonblack clinician so the patient can feel good about his mental health engagement. To some, these recommendations might seem obvious or too simple, yet if we consider the countless reports of poor patient treatment engagement, adherence, and retention, we cannot deny the need for change. Having black male patients disclose important information during encounters could prevent poor clinical interactions that leave them feeling uncomfortable, uncertain, skeptical, disrespected, and further cynical about mental health care.
Dr. Simon practices at Boston Children’s Hospital. He has no disclosures.
References
1. Bunn C. After Arbery shooting, black parents are rethinking “the talk” to explain white vigilantes. NBC News. 2020 May 19.
2. U.S. Department of Health and Human Services. Office of Disease Prevention and Promotion. Healthy People 2020.
3. Ward E and Mangesha M. Am J Orthopsychiatry. 2013 Apr-Jul;83(2 0 3):386-97.
4. Holden KB et al. J Mens health. 2012 Jun 1;9(2):63-9.
5. Brown TH et al. Fam Community Health. 2015 Oct-Dec;38(4):307-18.
6. Jäggi et al. Soc Ment Health. 2016 Nov;6(3):187-296.
7. Goff PA et al. J Pers Soc Psychol. 2014;106(4):526-45.
8. Alsan M et al. National Bureau of Economic Research. NBER Working Paper No. 24787. 2018 Jun. Revised 2019 Aug.
9. Spruill IJ. J Nat Black Nurses Assoc. 2014 Dec;25(2):23-30.
10. Graham LF et al. Am J Mens Health. 2018 Sep;12(5):1307-16.
I’m a black man, husband, father, son, brother, and a board-certified psychiatrist, child and adolescent psychiatry fellow, and addiction medicine fellow. I write this article as the latter, a colleague, from the former’s perspective, which you would not need to verify via Google, social media, or a badge upon meeting me.
July is Minority Mental Health Awareness Month, established to bring awareness to the unique struggles that marginalized groups face concerning mental illness in the United States.
Given the events of the last few months, including a global pandemic and videotaped killings of Ahmaud Arbery and George Floyd, two unarmed black men, America’s structural racism and inequality are being challenged in historic ways. Black people are suffering. In fact, I was not surprised to learn1 that some black families with sons have expanded the “talk” – which traditionally has focused on dealing with police officers – to include vigilantes.
Because of my extensive work with and treatment of men of color, I would like to answer a key question: “How do psychiatrists and other mental health clinicians better engage men of color? Before the “how,” let’s review the state of black men’s mental health.
According to Healthy People 2020, mental disorders are the leading cause of disability in the United States.2 Among those with diagnosable mental disorders, black people are more likely than are their white counterparts to experience severe symptoms and protracted diseases. Roughly 7% of black men meet the criteria for a lifetime prevalence of major depressive disorder.3 Applying that figure to recent national population estimates means that there are 1.4 million black men currently suffering from major depression. Suicide has been on a continued uptrend among black male youth for more than 2 decades. Moreover, given the high rates of stigma and unmet need in this population, it is likely that these figures are even more dire.
Compared with other groups, black men in the United States face a disproportionate burden of preventable morbidity and mortality rates. Of all the health concerns faced by black men, mental health challenges may be among the most stigmatized.4 Evidence suggests that black men have more adverse life experiences than do men of other racial/ethnic groups, and consequently, experience poorer mental health.5 Black men experience high rates of poverty, unemployment, and underemployment, and are incarcerated at much higher rates than those of men of other racial/ethnic groups.6 It is notable that black male youth are often perceived as older by law enforcement, beginning as early as 10 years old, often resulting in negative interactions.7
Despite those challenges, black men are often expected to project strength, they are expected to minimize displays of emotion when off the field or court (i.e., “Just shut up and dribble”), and they are expected to be true versions of folk hero John Henry. This caricature of black males is used at times to validate shootings of unarmed black males (adults and youth).
Black men’s mental health should be a priority for those in the mental health field. This is particularly the case light of our field’s historical involvement in and promotion of stereotyped clinical descriptions of black men and contributing to health disparities that persist. Black men are nearly six times as likely to be diagnosed with schizophrenia as are white men. To read about holdovers from the days of targeted advertising against black protesters of the 1960s and 1970s, check out “The Protest Psychosis” (Beacon Press, 2010) by psychiatrist and anthropologist Jonathan Metzl, MD, PhD. If you go further back in psychiatric history, the late 1800s, you can learn about the devious diagnosis of drapetomania attributed to enslaved people who were seeking freedom.
Those on the front lines providing mental health services should understand black men’s mental health from an ecological perspective. Beyond the emotional burden that mental illness imposes on the individual, there are more considerable interpersonal and societal implications for the state of black men’s mental health. As such, in our full capacity like other men, black men play an essential role within families, churches, neighborhoods, and organizations.
Given our brief review, we can reconsider our question, “How do psychiatrists and mental health clinicians better engage men of color?”
I will suggest a few fundamental principles that honestly can be applied to any patient but should be strongly considered with your black male patients – given they are likely not accustomed to engaging with the health care system, let alone with a mental health clinician:
1. Create a comfortable environment. Because of stigma, persistent myths, and lack of normalcy with talking to a mental health professional, many patients, including black men, do not have a framework for a psychiatric/psychological evaluation or treatment. It would be essential to set the frame of your encounter. Evidence suggests this can improve engagement and follow-up care among black men.8 In addition, keep in mind that “fictive kin”9 tend to play a major role in the transmission of culture, health promotion, and decision-making in the black community. This helps explain why barbershop initiatives10 are effective. If clinicians are able to allow black male patients to feel comfortable, the clinician, too, might become part of that fictive community and enhance the patient-provider relationship.
2. Allow for storytelling. In the age of the checklist, it can be relatively easy to lose sight that our patients, including black men, have their own narratives. Evidence suggests that physicians interrupt patients early and often. Challenge yourself to allow the patient to tell his story. In consideration of an initial evaluation, it may help to begin by first gathering sociodemographic information (i.e. housing, education, employment, family, etc.); doing so will allow the patient time to get comfortable before you assess possible psychiatric symptoms.
3. Confidentiality assurance. Many black men have a distrust for the health care profession; as such, it is vital that clinicians emphasize that their patients’ information and history will be used only to help the patient. It will be important to inform black male patients of their rights, because often in the greater society, their rights seem to be negated.
4. Be aware of nonverbal language. Given black men’s stereotyped roles in society and recognition that they are regularly perceived as threats, many black men have become adept at reading nonverbal cues (i.e., purse clutched, side comment, etc.). In doing so, clinicians must be attuned to their own nonverbal language. For example, a glance at one’s watch might be interpreted as you’re not listening. It would be better to be upfront and candid by saying something like, “I need to check the time,” rather than attempting to be stealth. Being transparent in that way will let the patient know that you will be upfront with him.
5. Be respectful. During an encounter, and in particular when discussing treatment plans, clinicians must allow the patient space to process and be involved in his care. Allowing the patient time to think through how he would want to proceed provides him a sense of personal agency and lets him know that he is capable of improving his mental wellness.
Black male patients need to feel comfortable, safe, able to trust the clinician. They must feel listened to, understood, and respected. This information might help some clinicians better understand what needs to happen between a black male patient and a nonblack clinician so the patient can feel good about his mental health engagement. To some, these recommendations might seem obvious or too simple, yet if we consider the countless reports of poor patient treatment engagement, adherence, and retention, we cannot deny the need for change. Having black male patients disclose important information during encounters could prevent poor clinical interactions that leave them feeling uncomfortable, uncertain, skeptical, disrespected, and further cynical about mental health care.
Dr. Simon practices at Boston Children’s Hospital. He has no disclosures.
References
1. Bunn C. After Arbery shooting, black parents are rethinking “the talk” to explain white vigilantes. NBC News. 2020 May 19.
2. U.S. Department of Health and Human Services. Office of Disease Prevention and Promotion. Healthy People 2020.
3. Ward E and Mangesha M. Am J Orthopsychiatry. 2013 Apr-Jul;83(2 0 3):386-97.
4. Holden KB et al. J Mens health. 2012 Jun 1;9(2):63-9.
5. Brown TH et al. Fam Community Health. 2015 Oct-Dec;38(4):307-18.
6. Jäggi et al. Soc Ment Health. 2016 Nov;6(3):187-296.
7. Goff PA et al. J Pers Soc Psychol. 2014;106(4):526-45.
8. Alsan M et al. National Bureau of Economic Research. NBER Working Paper No. 24787. 2018 Jun. Revised 2019 Aug.
9. Spruill IJ. J Nat Black Nurses Assoc. 2014 Dec;25(2):23-30.
10. Graham LF et al. Am J Mens Health. 2018 Sep;12(5):1307-16.
I’m a black man, husband, father, son, brother, and a board-certified psychiatrist, child and adolescent psychiatry fellow, and addiction medicine fellow. I write this article as the latter, a colleague, from the former’s perspective, which you would not need to verify via Google, social media, or a badge upon meeting me.
July is Minority Mental Health Awareness Month, established to bring awareness to the unique struggles that marginalized groups face concerning mental illness in the United States.
Given the events of the last few months, including a global pandemic and videotaped killings of Ahmaud Arbery and George Floyd, two unarmed black men, America’s structural racism and inequality are being challenged in historic ways. Black people are suffering. In fact, I was not surprised to learn1 that some black families with sons have expanded the “talk” – which traditionally has focused on dealing with police officers – to include vigilantes.
Because of my extensive work with and treatment of men of color, I would like to answer a key question: “How do psychiatrists and other mental health clinicians better engage men of color? Before the “how,” let’s review the state of black men’s mental health.
According to Healthy People 2020, mental disorders are the leading cause of disability in the United States.2 Among those with diagnosable mental disorders, black people are more likely than are their white counterparts to experience severe symptoms and protracted diseases. Roughly 7% of black men meet the criteria for a lifetime prevalence of major depressive disorder.3 Applying that figure to recent national population estimates means that there are 1.4 million black men currently suffering from major depression. Suicide has been on a continued uptrend among black male youth for more than 2 decades. Moreover, given the high rates of stigma and unmet need in this population, it is likely that these figures are even more dire.
Compared with other groups, black men in the United States face a disproportionate burden of preventable morbidity and mortality rates. Of all the health concerns faced by black men, mental health challenges may be among the most stigmatized.4 Evidence suggests that black men have more adverse life experiences than do men of other racial/ethnic groups, and consequently, experience poorer mental health.5 Black men experience high rates of poverty, unemployment, and underemployment, and are incarcerated at much higher rates than those of men of other racial/ethnic groups.6 It is notable that black male youth are often perceived as older by law enforcement, beginning as early as 10 years old, often resulting in negative interactions.7
Despite those challenges, black men are often expected to project strength, they are expected to minimize displays of emotion when off the field or court (i.e., “Just shut up and dribble”), and they are expected to be true versions of folk hero John Henry. This caricature of black males is used at times to validate shootings of unarmed black males (adults and youth).
Black men’s mental health should be a priority for those in the mental health field. This is particularly the case light of our field’s historical involvement in and promotion of stereotyped clinical descriptions of black men and contributing to health disparities that persist. Black men are nearly six times as likely to be diagnosed with schizophrenia as are white men. To read about holdovers from the days of targeted advertising against black protesters of the 1960s and 1970s, check out “The Protest Psychosis” (Beacon Press, 2010) by psychiatrist and anthropologist Jonathan Metzl, MD, PhD. If you go further back in psychiatric history, the late 1800s, you can learn about the devious diagnosis of drapetomania attributed to enslaved people who were seeking freedom.
Those on the front lines providing mental health services should understand black men’s mental health from an ecological perspective. Beyond the emotional burden that mental illness imposes on the individual, there are more considerable interpersonal and societal implications for the state of black men’s mental health. As such, in our full capacity like other men, black men play an essential role within families, churches, neighborhoods, and organizations.
Given our brief review, we can reconsider our question, “How do psychiatrists and mental health clinicians better engage men of color?”
I will suggest a few fundamental principles that honestly can be applied to any patient but should be strongly considered with your black male patients – given they are likely not accustomed to engaging with the health care system, let alone with a mental health clinician:
1. Create a comfortable environment. Because of stigma, persistent myths, and lack of normalcy with talking to a mental health professional, many patients, including black men, do not have a framework for a psychiatric/psychological evaluation or treatment. It would be essential to set the frame of your encounter. Evidence suggests this can improve engagement and follow-up care among black men.8 In addition, keep in mind that “fictive kin”9 tend to play a major role in the transmission of culture, health promotion, and decision-making in the black community. This helps explain why barbershop initiatives10 are effective. If clinicians are able to allow black male patients to feel comfortable, the clinician, too, might become part of that fictive community and enhance the patient-provider relationship.
2. Allow for storytelling. In the age of the checklist, it can be relatively easy to lose sight that our patients, including black men, have their own narratives. Evidence suggests that physicians interrupt patients early and often. Challenge yourself to allow the patient to tell his story. In consideration of an initial evaluation, it may help to begin by first gathering sociodemographic information (i.e. housing, education, employment, family, etc.); doing so will allow the patient time to get comfortable before you assess possible psychiatric symptoms.
3. Confidentiality assurance. Many black men have a distrust for the health care profession; as such, it is vital that clinicians emphasize that their patients’ information and history will be used only to help the patient. It will be important to inform black male patients of their rights, because often in the greater society, their rights seem to be negated.
4. Be aware of nonverbal language. Given black men’s stereotyped roles in society and recognition that they are regularly perceived as threats, many black men have become adept at reading nonverbal cues (i.e., purse clutched, side comment, etc.). In doing so, clinicians must be attuned to their own nonverbal language. For example, a glance at one’s watch might be interpreted as you’re not listening. It would be better to be upfront and candid by saying something like, “I need to check the time,” rather than attempting to be stealth. Being transparent in that way will let the patient know that you will be upfront with him.
5. Be respectful. During an encounter, and in particular when discussing treatment plans, clinicians must allow the patient space to process and be involved in his care. Allowing the patient time to think through how he would want to proceed provides him a sense of personal agency and lets him know that he is capable of improving his mental wellness.
Black male patients need to feel comfortable, safe, able to trust the clinician. They must feel listened to, understood, and respected. This information might help some clinicians better understand what needs to happen between a black male patient and a nonblack clinician so the patient can feel good about his mental health engagement. To some, these recommendations might seem obvious or too simple, yet if we consider the countless reports of poor patient treatment engagement, adherence, and retention, we cannot deny the need for change. Having black male patients disclose important information during encounters could prevent poor clinical interactions that leave them feeling uncomfortable, uncertain, skeptical, disrespected, and further cynical about mental health care.
Dr. Simon practices at Boston Children’s Hospital. He has no disclosures.
References
1. Bunn C. After Arbery shooting, black parents are rethinking “the talk” to explain white vigilantes. NBC News. 2020 May 19.
2. U.S. Department of Health and Human Services. Office of Disease Prevention and Promotion. Healthy People 2020.
3. Ward E and Mangesha M. Am J Orthopsychiatry. 2013 Apr-Jul;83(2 0 3):386-97.
4. Holden KB et al. J Mens health. 2012 Jun 1;9(2):63-9.
5. Brown TH et al. Fam Community Health. 2015 Oct-Dec;38(4):307-18.
6. Jäggi et al. Soc Ment Health. 2016 Nov;6(3):187-296.
7. Goff PA et al. J Pers Soc Psychol. 2014;106(4):526-45.
8. Alsan M et al. National Bureau of Economic Research. NBER Working Paper No. 24787. 2018 Jun. Revised 2019 Aug.
9. Spruill IJ. J Nat Black Nurses Assoc. 2014 Dec;25(2):23-30.
10. Graham LF et al. Am J Mens Health. 2018 Sep;12(5):1307-16.
COVID-associated pancreatitis may disproportionately affect young, overweight men
Patients with COVID-19 develop a distinct subset of pancreatitis hallmarked by duodenal and periduodenal inflammation, according to a recent case series.
Although all five patients presented with multiple predictive markers of severe pancreatitis, the subsequent clinical pathway “was much more benign than anticipated,” reported lead author Peter Szatmary, MB, BChir, PhD, of the University of Liverpool (England) and colleagues. Still, they noted prolonged hospital stays because of persistent inflammation and poor diabetic control.
“As the global pandemic of SARS-CoV-2 continues, nuances of the disease it precipitates in humans continue to emerge,” the investigators wrote in Gastroenterology. “[A] group from Wuhan reported a series of 9 patients with purported pancreatic injury in the context of SARS-CoV-2 infection, but did not provide robust evidence for pancreatitis relying on mild hyperamylasemia alone.”
For the present series, Dr. Szatmary and colleagues restricted diagnosis of pancreatitis to international consensus guidelines, which require “abdominal pain consistent with pancreatitis, serum amylase/lipase greater than three times the upper limit of normal, and characteristic findings on cross-sectional imaging.”
From middle of March to late April, the investigators identified 35 patients with acute pancreatitis at Royal Liverpool (England) University Hospital, 25 of whom tested negative for SARS-CoV-2, which resulted in study exclusion. An additional five patients were excluded from the series as another etiology for pancreatitis was clearly present, such as gallstones.
“The remaining 5 patients, all with SARS-CoV-2, presented atypically yet homogenously with a distinct metabolic-pancreatitis phenotype,” the investigators wrote.
All five patients were obese or overweight young men with a median body mass index of 30 kg/m2 and age of 42 years. On presentation, all patients had elevated, but nondiagnostic, levels of amylase (median, 149 U/L). Contrast-enhanced abdominal CT revealed moderate to severe hepatic steatosis (less than 104 HU), which rapidly regressed within a week among patients who underwent repeat imaging.
“The pattern of pancreatic inflammation was similarly unusual in these patients,” the investigators wrote, going on to describe “mild pancreatic edema without significant pancreatic or peripancreatic necrosis, with distinct duodenal/periduodenal inflammation involving the second and third part of the duodenum.”
According to Dr. Szatmary and colleagues, these findings were “accompanied by a profound systemic inflammatory response,” including 1-2 criteria for systemic inflammatory response syndrome that increased to 2-4 criteria within 48 hours. During hospitalization, patients also exhibited a “dramatic elevation” of C-reactive protein, from a median of 31 mg/L upon admission to 485 mg/L within 48 hours.
Although these markers predicted severe disease, all cases followed a clinical course similar to “a typical attack of moderate pancreatitis,” the investigators wrote.
All patients were treated with IV fluids, four out of five received broad-spectrum IV antibiotics for pneumonitis, three out of five received fibrate and/or insulin therapy, and two out of five received pancreatic enzyme replacement therapy. No patients required corticosteroids, organ support, or respiratory support beyond low-flow oxygen. Median hospital stay was 14 days.
“We ... propose the combination of male sex, abdominal pain, metabolic stress, and CT-findings of predominantly pancreatico-duodenal inflammation with steatosis represent a distinct subset of pancreatitis in patients infected with SARS-CoV-2,” the investigators wrote.
They suggested that the endocrine pancreas may be “particularly vulnerable to this infection,” citing prolonged hospital stays because of poor diabetic control.
“[T]ransient dyslipidemias and impaired glucose tolerance may be common in SARS-CoV-2 patients and warrant further investigation,” they concluded.
Oscar J. Hines, MD, chief of the division of general surgery at UCLA Medical Center and leader in the field of pancreatitis management, said that the case series has a limited impact.
“The findings are unlikely to change practice and only call attention for physicians to the possibility of pancreatitis in COVID-positive patients,” Dr. Hines said.
The investigators reported grants from NIHR, Wellcome Trust, Mylan, and others.
SOURCE: Szatmary P et al. Gastroenterology. 2020 Jun 1. doi: 10.1053/j.gastro.2020.05.069.
Patients with COVID-19 develop a distinct subset of pancreatitis hallmarked by duodenal and periduodenal inflammation, according to a recent case series.
Although all five patients presented with multiple predictive markers of severe pancreatitis, the subsequent clinical pathway “was much more benign than anticipated,” reported lead author Peter Szatmary, MB, BChir, PhD, of the University of Liverpool (England) and colleagues. Still, they noted prolonged hospital stays because of persistent inflammation and poor diabetic control.
“As the global pandemic of SARS-CoV-2 continues, nuances of the disease it precipitates in humans continue to emerge,” the investigators wrote in Gastroenterology. “[A] group from Wuhan reported a series of 9 patients with purported pancreatic injury in the context of SARS-CoV-2 infection, but did not provide robust evidence for pancreatitis relying on mild hyperamylasemia alone.”
For the present series, Dr. Szatmary and colleagues restricted diagnosis of pancreatitis to international consensus guidelines, which require “abdominal pain consistent with pancreatitis, serum amylase/lipase greater than three times the upper limit of normal, and characteristic findings on cross-sectional imaging.”
From middle of March to late April, the investigators identified 35 patients with acute pancreatitis at Royal Liverpool (England) University Hospital, 25 of whom tested negative for SARS-CoV-2, which resulted in study exclusion. An additional five patients were excluded from the series as another etiology for pancreatitis was clearly present, such as gallstones.
“The remaining 5 patients, all with SARS-CoV-2, presented atypically yet homogenously with a distinct metabolic-pancreatitis phenotype,” the investigators wrote.
All five patients were obese or overweight young men with a median body mass index of 30 kg/m2 and age of 42 years. On presentation, all patients had elevated, but nondiagnostic, levels of amylase (median, 149 U/L). Contrast-enhanced abdominal CT revealed moderate to severe hepatic steatosis (less than 104 HU), which rapidly regressed within a week among patients who underwent repeat imaging.
“The pattern of pancreatic inflammation was similarly unusual in these patients,” the investigators wrote, going on to describe “mild pancreatic edema without significant pancreatic or peripancreatic necrosis, with distinct duodenal/periduodenal inflammation involving the second and third part of the duodenum.”
According to Dr. Szatmary and colleagues, these findings were “accompanied by a profound systemic inflammatory response,” including 1-2 criteria for systemic inflammatory response syndrome that increased to 2-4 criteria within 48 hours. During hospitalization, patients also exhibited a “dramatic elevation” of C-reactive protein, from a median of 31 mg/L upon admission to 485 mg/L within 48 hours.
Although these markers predicted severe disease, all cases followed a clinical course similar to “a typical attack of moderate pancreatitis,” the investigators wrote.
All patients were treated with IV fluids, four out of five received broad-spectrum IV antibiotics for pneumonitis, three out of five received fibrate and/or insulin therapy, and two out of five received pancreatic enzyme replacement therapy. No patients required corticosteroids, organ support, or respiratory support beyond low-flow oxygen. Median hospital stay was 14 days.
“We ... propose the combination of male sex, abdominal pain, metabolic stress, and CT-findings of predominantly pancreatico-duodenal inflammation with steatosis represent a distinct subset of pancreatitis in patients infected with SARS-CoV-2,” the investigators wrote.
They suggested that the endocrine pancreas may be “particularly vulnerable to this infection,” citing prolonged hospital stays because of poor diabetic control.
“[T]ransient dyslipidemias and impaired glucose tolerance may be common in SARS-CoV-2 patients and warrant further investigation,” they concluded.
Oscar J. Hines, MD, chief of the division of general surgery at UCLA Medical Center and leader in the field of pancreatitis management, said that the case series has a limited impact.
“The findings are unlikely to change practice and only call attention for physicians to the possibility of pancreatitis in COVID-positive patients,” Dr. Hines said.
The investigators reported grants from NIHR, Wellcome Trust, Mylan, and others.
SOURCE: Szatmary P et al. Gastroenterology. 2020 Jun 1. doi: 10.1053/j.gastro.2020.05.069.
Patients with COVID-19 develop a distinct subset of pancreatitis hallmarked by duodenal and periduodenal inflammation, according to a recent case series.
Although all five patients presented with multiple predictive markers of severe pancreatitis, the subsequent clinical pathway “was much more benign than anticipated,” reported lead author Peter Szatmary, MB, BChir, PhD, of the University of Liverpool (England) and colleagues. Still, they noted prolonged hospital stays because of persistent inflammation and poor diabetic control.
“As the global pandemic of SARS-CoV-2 continues, nuances of the disease it precipitates in humans continue to emerge,” the investigators wrote in Gastroenterology. “[A] group from Wuhan reported a series of 9 patients with purported pancreatic injury in the context of SARS-CoV-2 infection, but did not provide robust evidence for pancreatitis relying on mild hyperamylasemia alone.”
For the present series, Dr. Szatmary and colleagues restricted diagnosis of pancreatitis to international consensus guidelines, which require “abdominal pain consistent with pancreatitis, serum amylase/lipase greater than three times the upper limit of normal, and characteristic findings on cross-sectional imaging.”
From middle of March to late April, the investigators identified 35 patients with acute pancreatitis at Royal Liverpool (England) University Hospital, 25 of whom tested negative for SARS-CoV-2, which resulted in study exclusion. An additional five patients were excluded from the series as another etiology for pancreatitis was clearly present, such as gallstones.
“The remaining 5 patients, all with SARS-CoV-2, presented atypically yet homogenously with a distinct metabolic-pancreatitis phenotype,” the investigators wrote.
All five patients were obese or overweight young men with a median body mass index of 30 kg/m2 and age of 42 years. On presentation, all patients had elevated, but nondiagnostic, levels of amylase (median, 149 U/L). Contrast-enhanced abdominal CT revealed moderate to severe hepatic steatosis (less than 104 HU), which rapidly regressed within a week among patients who underwent repeat imaging.
“The pattern of pancreatic inflammation was similarly unusual in these patients,” the investigators wrote, going on to describe “mild pancreatic edema without significant pancreatic or peripancreatic necrosis, with distinct duodenal/periduodenal inflammation involving the second and third part of the duodenum.”
According to Dr. Szatmary and colleagues, these findings were “accompanied by a profound systemic inflammatory response,” including 1-2 criteria for systemic inflammatory response syndrome that increased to 2-4 criteria within 48 hours. During hospitalization, patients also exhibited a “dramatic elevation” of C-reactive protein, from a median of 31 mg/L upon admission to 485 mg/L within 48 hours.
Although these markers predicted severe disease, all cases followed a clinical course similar to “a typical attack of moderate pancreatitis,” the investigators wrote.
All patients were treated with IV fluids, four out of five received broad-spectrum IV antibiotics for pneumonitis, three out of five received fibrate and/or insulin therapy, and two out of five received pancreatic enzyme replacement therapy. No patients required corticosteroids, organ support, or respiratory support beyond low-flow oxygen. Median hospital stay was 14 days.
“We ... propose the combination of male sex, abdominal pain, metabolic stress, and CT-findings of predominantly pancreatico-duodenal inflammation with steatosis represent a distinct subset of pancreatitis in patients infected with SARS-CoV-2,” the investigators wrote.
They suggested that the endocrine pancreas may be “particularly vulnerable to this infection,” citing prolonged hospital stays because of poor diabetic control.
“[T]ransient dyslipidemias and impaired glucose tolerance may be common in SARS-CoV-2 patients and warrant further investigation,” they concluded.
Oscar J. Hines, MD, chief of the division of general surgery at UCLA Medical Center and leader in the field of pancreatitis management, said that the case series has a limited impact.
“The findings are unlikely to change practice and only call attention for physicians to the possibility of pancreatitis in COVID-positive patients,” Dr. Hines said.
The investigators reported grants from NIHR, Wellcome Trust, Mylan, and others.
SOURCE: Szatmary P et al. Gastroenterology. 2020 Jun 1. doi: 10.1053/j.gastro.2020.05.069.
FROM GASTROENTEROLOGY
First reported U.S. case of COVID-19 linked to Guillain-Barré syndrome
further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case of COVID-19 that initially presented as acute GBS. The patient was a 61-year-old woman returning home from Wuhan during the pandemic.
Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
The first U.S. case is described in the June issue of the Journal of Clinical Neuromuscular Disease.
Like cases from China and Italy, the U.S. patient’s symptoms of GBS reportedly occurred within days of being infected with SARS-CoV-2. “This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted.
The 54-year-old man was transferred to Allegheny General Hospital after developing ascending limb weakness and numbness that followed symptoms of a respiratory infection. Two weeks earlier, he initially developed rhinorrhea, odynophagia, fevers, chills, and night sweats. The man reported that his wife had tested positive for COVID-19 and that his symptoms started soon after her illness. The man also tested positive for COVID-19.
His deficits were characterized by quadriparesis and areflexia, burning dysesthesias, mild ophthalmoparesis, and dysautonomia. He did not have the loss of smell and taste documented in other COVID-19 patients. He briefly required mechanical ventilation and was successfully weaned after receiving a course of intravenous immunoglobulin.
Compared with other cases reported in the literature, the unique clinical features in the U.S. case are urinary retention secondary to dysautonomia and ocular symptoms of diplopia. These highlight the variability in the clinical presentation of GBS associated with COVID-19, the researchers noted.
They added that, with the Pittsburgh patient, electrophysiological findings were typical of demyelinating polyneuropathy seen in patients with GBS. The case series from Italy suggests that axonal variants could be as common in COVID-19–associated GBS.
“Although the number of documented cases internationally is notably small to date, it’s not completely surprising that a COVID-19 diagnosis may lead to a patient developing GBS. The increase of inflammation and inflammatory cells caused by the infection may trigger an irregular immune response that leads to the hallmark symptoms of this neurological disorder,” Dr. Rana said in a news release.
“Since GBS can significantly affect the respiratory system and other vital organs being pushed into overdrive during a COVID-19 immune response, it will be critically important to further investigate and understand this potential connection,” he added.
A version of this article originally appeared on Medscape.com.
further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case of COVID-19 that initially presented as acute GBS. The patient was a 61-year-old woman returning home from Wuhan during the pandemic.
Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
The first U.S. case is described in the June issue of the Journal of Clinical Neuromuscular Disease.
Like cases from China and Italy, the U.S. patient’s symptoms of GBS reportedly occurred within days of being infected with SARS-CoV-2. “This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted.
The 54-year-old man was transferred to Allegheny General Hospital after developing ascending limb weakness and numbness that followed symptoms of a respiratory infection. Two weeks earlier, he initially developed rhinorrhea, odynophagia, fevers, chills, and night sweats. The man reported that his wife had tested positive for COVID-19 and that his symptoms started soon after her illness. The man also tested positive for COVID-19.
His deficits were characterized by quadriparesis and areflexia, burning dysesthesias, mild ophthalmoparesis, and dysautonomia. He did not have the loss of smell and taste documented in other COVID-19 patients. He briefly required mechanical ventilation and was successfully weaned after receiving a course of intravenous immunoglobulin.
Compared with other cases reported in the literature, the unique clinical features in the U.S. case are urinary retention secondary to dysautonomia and ocular symptoms of diplopia. These highlight the variability in the clinical presentation of GBS associated with COVID-19, the researchers noted.
They added that, with the Pittsburgh patient, electrophysiological findings were typical of demyelinating polyneuropathy seen in patients with GBS. The case series from Italy suggests that axonal variants could be as common in COVID-19–associated GBS.
“Although the number of documented cases internationally is notably small to date, it’s not completely surprising that a COVID-19 diagnosis may lead to a patient developing GBS. The increase of inflammation and inflammatory cells caused by the infection may trigger an irregular immune response that leads to the hallmark symptoms of this neurological disorder,” Dr. Rana said in a news release.
“Since GBS can significantly affect the respiratory system and other vital organs being pushed into overdrive during a COVID-19 immune response, it will be critically important to further investigate and understand this potential connection,” he added.
A version of this article originally appeared on Medscape.com.
further supporting a link between the virus and neurologic complications, including GBS.
Physicians in China reported the first case of COVID-19 that initially presented as acute GBS. The patient was a 61-year-old woman returning home from Wuhan during the pandemic.
Subsequently, physicians in Italy reported five cases of GBS in association with COVID-19.
The first U.S. case is described in the June issue of the Journal of Clinical Neuromuscular Disease.
Like cases from China and Italy, the U.S. patient’s symptoms of GBS reportedly occurred within days of being infected with SARS-CoV-2. “This onset is similar to a case report of acute Zika virus infection with concurrent GBS suggesting a parainfectious complication,” first author Sandeep Rana, MD, and colleagues noted.
The 54-year-old man was transferred to Allegheny General Hospital after developing ascending limb weakness and numbness that followed symptoms of a respiratory infection. Two weeks earlier, he initially developed rhinorrhea, odynophagia, fevers, chills, and night sweats. The man reported that his wife had tested positive for COVID-19 and that his symptoms started soon after her illness. The man also tested positive for COVID-19.
His deficits were characterized by quadriparesis and areflexia, burning dysesthesias, mild ophthalmoparesis, and dysautonomia. He did not have the loss of smell and taste documented in other COVID-19 patients. He briefly required mechanical ventilation and was successfully weaned after receiving a course of intravenous immunoglobulin.
Compared with other cases reported in the literature, the unique clinical features in the U.S. case are urinary retention secondary to dysautonomia and ocular symptoms of diplopia. These highlight the variability in the clinical presentation of GBS associated with COVID-19, the researchers noted.
They added that, with the Pittsburgh patient, electrophysiological findings were typical of demyelinating polyneuropathy seen in patients with GBS. The case series from Italy suggests that axonal variants could be as common in COVID-19–associated GBS.
“Although the number of documented cases internationally is notably small to date, it’s not completely surprising that a COVID-19 diagnosis may lead to a patient developing GBS. The increase of inflammation and inflammatory cells caused by the infection may trigger an irregular immune response that leads to the hallmark symptoms of this neurological disorder,” Dr. Rana said in a news release.
“Since GBS can significantly affect the respiratory system and other vital organs being pushed into overdrive during a COVID-19 immune response, it will be critically important to further investigate and understand this potential connection,” he added.
A version of this article originally appeared on Medscape.com.
Eczema may increase lymphoma risk, cohort studies suggest
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
FROM JAMA DERMATOLOGY