User login
Ofatumumab shows high elimination of disease activity in MS
, a new study shows.
The drug, which is already approved for the treatment of chronic lymphocytic leukemia, is currently under review for relapsing MS as a once-per-month self-injected therapy that could offer a convenient alternative to DMTs that require in-office infusion.
The new findings are from a pooled analysis from the phase 3 ASCLEPIOS I/II trials of the use of ofatumumab for patients with relapsing MS. There were 927 patients in the ASCLEPIOS I trial and 955 in the ASCLEPIOS II trial. The trials were conducted in 37 countries and involved patients aged 18-55 years.
The late-breaking research was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The studies compared patients who were treated with subcutaneous ofatumumab 20 mg with patients treated with oral teriflunomide 14 mg once daily for up to 30 months. The average duration of follow-up was 18 months.
NEDA-3, commonly used to determine treatment outcomes for patients with relapsing MS, was defined as a composite of having no worsening of disability over a 6-month period (6mCDW), no confirmed MS relapse, no new/enlarging T2 lesions, and no gadolinium-enhancing T1 lesions.
The pooled results showed that the odds of achieving NEDA-3 during the first 12 months were three times greater with ofatumumab than with teriflunomide (47.0% vs. 24.5%; odds ratio [OR], 3.36; P < .001) and were more than eight times greater from months 12 to 24 (87.8% vs. 48.2%; OR, 8.09; P < .001).
In addition, compared with patients who received teriflunomide, a higher proportion of patients who received ofatumumab were free from 6mCDW over 2 years (91.9% vs. 88.9%), as well as from relapses (82.3% vs 69.2%) and lesion activity (54.1% vs. 27.5%).
There was a significantly greater reduction in annualized relapse rate with ofatumumab compared with teriflunomide at all cumulative time intervals, including months 0 to 3 (P = .011), and at all subsequent time intervals from month 0 to 27 (P < .001).
The pooled findings further showed that ofatumumab reduced the mean number of gadolinium-enhancing T1 lesions per scan by 95.9% compared with teriflunomide (P < .001).
“Ofatumumab increased the probability of achieving NEDA-3 and demonstrated superior efficacy vs teriflunomide in patients with relapsing MS,” said the authors, led by Stephen L. Hauser, MD, of the department of neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco.
Ofatumumab superior in primary, secondary outcomes
As previously reported, subcutaneous ofatumumab also demonstrated superior efficacy over oral teriflunomide in the primary and secondary endpoints in the ASCLEPIOS I/II trials. The annualized relapse rate was reduced by 0.22 in the teriflunomide group, vs 0.11 in the ofatumumab group (50.5% relative reduction; P < .001) in the ASCLEPIOS I trial, and by 0.25 vs. 0.10 (58.5% relative reduction P < .001) in ASCLEPIOS II.
Ofatumumab also reduced the number of gadolinium-enhancing T1 lesions and new or enlarging T2 lesions compared with teriflunomide (all P < .001). It reduced the risk for disability progression by 34.4% over 3 months and by 32.5% over 6 months.
In the studies, the rate of serious infection with ofatumumab was 2.5%, compared with 1.8% with teriflunomide. Rates of malignancies were 0.5% and 0.3%, respectively.
“Ofatumumab demonstrated superior efficacy versus teriflunomide, with an acceptable safety profile, in patients with relapsing MS,” the authors reported.
Adherence rates with self-injection encouraging
An additional analysis from the two trials presented virtually in a separate abstract at the CMSC showed greater adherence to the self-administered regimen.
The analysis shows that in the ASCLEPIOS I study, 86.0% patients who were randomly assigned to receive ofatumumab and 77.7% who received teriflunomide completed the study on the assigned study drug. The proportion of patients who received ofatumumab and who discontinued treatment was 14.0%, versus 21.2% for those in the teriflunomide group. The most common reasons for discontinuation were patient/guardian decision (ofatumumab, 4.9%; teriflunomide, 8.2%), adverse event (ofatumumab, 5.2%; teriflunomide, 5.0%), and physician decision (ofatumumab, 2.2%; teriflunomide, 6.5%).
In the ASCLEPIOS II study, the rates were similar in all measures.
“In ASCLEPIOS trials, compliance with home-administered subcutaneous ofatumumab was high, and fewer patients discontinued ofatumumab as compared to teriflunomide,” the authors concluded.
Comparator drug a weak choice?
In commenting on the research, Stephen Kamin, MD, professor, vice chair, and chief of service, department of neurology, New Jersey Medical School, in Newark, noted that a limitation of the ASCLEPIOS trials is the comparison with teriflunomide.
“The comparator drug, teriflunomide, is one of the least effective DMTs, and one that some clinicians, including myself, don’t use,” he said.
Previously, when asked in an interview about the choice of teriflunomide as the comparator, Dr. Hauser noted that considerable discussion had gone into the decision. “The rationale was that we wanted to have a comparator that would be present not only against focal disease activity but also potentially against progression, and we were also able to blind the study successfully,” he said at the time.
Dr. Kamin said that ofatumumab will nevertheless likely represent a welcome addition to the tool kit of treatment options for MS. “Any new drug is helpful in adding to our choices as a general rule,” he said. “Subcutaneous injection does have increased convenience.”
It is not likely that the drug will be a game changer, he added, although the treatment’s efficacy compared with other drugs remains to be seen. “It all depends upon the relative efficacy of ofatumumab versus ocrelizumab or siponimod,” Dr. Kamin said.
“There has been another subcutaneous monoclonal for MS, daclizumab, although this was withdrawn from the market due to severe adverse effects not related to route of administration,” he added.
Dr. Hauser has relationships with Alector, Annexon, Bionure, Molecular Stethoscope, Symbiotix, and F. Hoffmann-La Roche. Dr. Kamin has received research support from Biogen, Novartis and CMSC.
A version of this article originally appeared on Medscape.com.
, a new study shows.
The drug, which is already approved for the treatment of chronic lymphocytic leukemia, is currently under review for relapsing MS as a once-per-month self-injected therapy that could offer a convenient alternative to DMTs that require in-office infusion.
The new findings are from a pooled analysis from the phase 3 ASCLEPIOS I/II trials of the use of ofatumumab for patients with relapsing MS. There were 927 patients in the ASCLEPIOS I trial and 955 in the ASCLEPIOS II trial. The trials were conducted in 37 countries and involved patients aged 18-55 years.
The late-breaking research was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The studies compared patients who were treated with subcutaneous ofatumumab 20 mg with patients treated with oral teriflunomide 14 mg once daily for up to 30 months. The average duration of follow-up was 18 months.
NEDA-3, commonly used to determine treatment outcomes for patients with relapsing MS, was defined as a composite of having no worsening of disability over a 6-month period (6mCDW), no confirmed MS relapse, no new/enlarging T2 lesions, and no gadolinium-enhancing T1 lesions.
The pooled results showed that the odds of achieving NEDA-3 during the first 12 months were three times greater with ofatumumab than with teriflunomide (47.0% vs. 24.5%; odds ratio [OR], 3.36; P < .001) and were more than eight times greater from months 12 to 24 (87.8% vs. 48.2%; OR, 8.09; P < .001).
In addition, compared with patients who received teriflunomide, a higher proportion of patients who received ofatumumab were free from 6mCDW over 2 years (91.9% vs. 88.9%), as well as from relapses (82.3% vs 69.2%) and lesion activity (54.1% vs. 27.5%).
There was a significantly greater reduction in annualized relapse rate with ofatumumab compared with teriflunomide at all cumulative time intervals, including months 0 to 3 (P = .011), and at all subsequent time intervals from month 0 to 27 (P < .001).
The pooled findings further showed that ofatumumab reduced the mean number of gadolinium-enhancing T1 lesions per scan by 95.9% compared with teriflunomide (P < .001).
“Ofatumumab increased the probability of achieving NEDA-3 and demonstrated superior efficacy vs teriflunomide in patients with relapsing MS,” said the authors, led by Stephen L. Hauser, MD, of the department of neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco.
Ofatumumab superior in primary, secondary outcomes
As previously reported, subcutaneous ofatumumab also demonstrated superior efficacy over oral teriflunomide in the primary and secondary endpoints in the ASCLEPIOS I/II trials. The annualized relapse rate was reduced by 0.22 in the teriflunomide group, vs 0.11 in the ofatumumab group (50.5% relative reduction; P < .001) in the ASCLEPIOS I trial, and by 0.25 vs. 0.10 (58.5% relative reduction P < .001) in ASCLEPIOS II.
Ofatumumab also reduced the number of gadolinium-enhancing T1 lesions and new or enlarging T2 lesions compared with teriflunomide (all P < .001). It reduced the risk for disability progression by 34.4% over 3 months and by 32.5% over 6 months.
In the studies, the rate of serious infection with ofatumumab was 2.5%, compared with 1.8% with teriflunomide. Rates of malignancies were 0.5% and 0.3%, respectively.
“Ofatumumab demonstrated superior efficacy versus teriflunomide, with an acceptable safety profile, in patients with relapsing MS,” the authors reported.
Adherence rates with self-injection encouraging
An additional analysis from the two trials presented virtually in a separate abstract at the CMSC showed greater adherence to the self-administered regimen.
The analysis shows that in the ASCLEPIOS I study, 86.0% patients who were randomly assigned to receive ofatumumab and 77.7% who received teriflunomide completed the study on the assigned study drug. The proportion of patients who received ofatumumab and who discontinued treatment was 14.0%, versus 21.2% for those in the teriflunomide group. The most common reasons for discontinuation were patient/guardian decision (ofatumumab, 4.9%; teriflunomide, 8.2%), adverse event (ofatumumab, 5.2%; teriflunomide, 5.0%), and physician decision (ofatumumab, 2.2%; teriflunomide, 6.5%).
In the ASCLEPIOS II study, the rates were similar in all measures.
“In ASCLEPIOS trials, compliance with home-administered subcutaneous ofatumumab was high, and fewer patients discontinued ofatumumab as compared to teriflunomide,” the authors concluded.
Comparator drug a weak choice?
In commenting on the research, Stephen Kamin, MD, professor, vice chair, and chief of service, department of neurology, New Jersey Medical School, in Newark, noted that a limitation of the ASCLEPIOS trials is the comparison with teriflunomide.
“The comparator drug, teriflunomide, is one of the least effective DMTs, and one that some clinicians, including myself, don’t use,” he said.
Previously, when asked in an interview about the choice of teriflunomide as the comparator, Dr. Hauser noted that considerable discussion had gone into the decision. “The rationale was that we wanted to have a comparator that would be present not only against focal disease activity but also potentially against progression, and we were also able to blind the study successfully,” he said at the time.
Dr. Kamin said that ofatumumab will nevertheless likely represent a welcome addition to the tool kit of treatment options for MS. “Any new drug is helpful in adding to our choices as a general rule,” he said. “Subcutaneous injection does have increased convenience.”
It is not likely that the drug will be a game changer, he added, although the treatment’s efficacy compared with other drugs remains to be seen. “It all depends upon the relative efficacy of ofatumumab versus ocrelizumab or siponimod,” Dr. Kamin said.
“There has been another subcutaneous monoclonal for MS, daclizumab, although this was withdrawn from the market due to severe adverse effects not related to route of administration,” he added.
Dr. Hauser has relationships with Alector, Annexon, Bionure, Molecular Stethoscope, Symbiotix, and F. Hoffmann-La Roche. Dr. Kamin has received research support from Biogen, Novartis and CMSC.
A version of this article originally appeared on Medscape.com.
, a new study shows.
The drug, which is already approved for the treatment of chronic lymphocytic leukemia, is currently under review for relapsing MS as a once-per-month self-injected therapy that could offer a convenient alternative to DMTs that require in-office infusion.
The new findings are from a pooled analysis from the phase 3 ASCLEPIOS I/II trials of the use of ofatumumab for patients with relapsing MS. There were 927 patients in the ASCLEPIOS I trial and 955 in the ASCLEPIOS II trial. The trials were conducted in 37 countries and involved patients aged 18-55 years.
The late-breaking research was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The studies compared patients who were treated with subcutaneous ofatumumab 20 mg with patients treated with oral teriflunomide 14 mg once daily for up to 30 months. The average duration of follow-up was 18 months.
NEDA-3, commonly used to determine treatment outcomes for patients with relapsing MS, was defined as a composite of having no worsening of disability over a 6-month period (6mCDW), no confirmed MS relapse, no new/enlarging T2 lesions, and no gadolinium-enhancing T1 lesions.
The pooled results showed that the odds of achieving NEDA-3 during the first 12 months were three times greater with ofatumumab than with teriflunomide (47.0% vs. 24.5%; odds ratio [OR], 3.36; P < .001) and were more than eight times greater from months 12 to 24 (87.8% vs. 48.2%; OR, 8.09; P < .001).
In addition, compared with patients who received teriflunomide, a higher proportion of patients who received ofatumumab were free from 6mCDW over 2 years (91.9% vs. 88.9%), as well as from relapses (82.3% vs 69.2%) and lesion activity (54.1% vs. 27.5%).
There was a significantly greater reduction in annualized relapse rate with ofatumumab compared with teriflunomide at all cumulative time intervals, including months 0 to 3 (P = .011), and at all subsequent time intervals from month 0 to 27 (P < .001).
The pooled findings further showed that ofatumumab reduced the mean number of gadolinium-enhancing T1 lesions per scan by 95.9% compared with teriflunomide (P < .001).
“Ofatumumab increased the probability of achieving NEDA-3 and demonstrated superior efficacy vs teriflunomide in patients with relapsing MS,” said the authors, led by Stephen L. Hauser, MD, of the department of neurology, UCSF Weill Institute for Neurosciences, University of California, San Francisco.
Ofatumumab superior in primary, secondary outcomes
As previously reported, subcutaneous ofatumumab also demonstrated superior efficacy over oral teriflunomide in the primary and secondary endpoints in the ASCLEPIOS I/II trials. The annualized relapse rate was reduced by 0.22 in the teriflunomide group, vs 0.11 in the ofatumumab group (50.5% relative reduction; P < .001) in the ASCLEPIOS I trial, and by 0.25 vs. 0.10 (58.5% relative reduction P < .001) in ASCLEPIOS II.
Ofatumumab also reduced the number of gadolinium-enhancing T1 lesions and new or enlarging T2 lesions compared with teriflunomide (all P < .001). It reduced the risk for disability progression by 34.4% over 3 months and by 32.5% over 6 months.
In the studies, the rate of serious infection with ofatumumab was 2.5%, compared with 1.8% with teriflunomide. Rates of malignancies were 0.5% and 0.3%, respectively.
“Ofatumumab demonstrated superior efficacy versus teriflunomide, with an acceptable safety profile, in patients with relapsing MS,” the authors reported.
Adherence rates with self-injection encouraging
An additional analysis from the two trials presented virtually in a separate abstract at the CMSC showed greater adherence to the self-administered regimen.
The analysis shows that in the ASCLEPIOS I study, 86.0% patients who were randomly assigned to receive ofatumumab and 77.7% who received teriflunomide completed the study on the assigned study drug. The proportion of patients who received ofatumumab and who discontinued treatment was 14.0%, versus 21.2% for those in the teriflunomide group. The most common reasons for discontinuation were patient/guardian decision (ofatumumab, 4.9%; teriflunomide, 8.2%), adverse event (ofatumumab, 5.2%; teriflunomide, 5.0%), and physician decision (ofatumumab, 2.2%; teriflunomide, 6.5%).
In the ASCLEPIOS II study, the rates were similar in all measures.
“In ASCLEPIOS trials, compliance with home-administered subcutaneous ofatumumab was high, and fewer patients discontinued ofatumumab as compared to teriflunomide,” the authors concluded.
Comparator drug a weak choice?
In commenting on the research, Stephen Kamin, MD, professor, vice chair, and chief of service, department of neurology, New Jersey Medical School, in Newark, noted that a limitation of the ASCLEPIOS trials is the comparison with teriflunomide.
“The comparator drug, teriflunomide, is one of the least effective DMTs, and one that some clinicians, including myself, don’t use,” he said.
Previously, when asked in an interview about the choice of teriflunomide as the comparator, Dr. Hauser noted that considerable discussion had gone into the decision. “The rationale was that we wanted to have a comparator that would be present not only against focal disease activity but also potentially against progression, and we were also able to blind the study successfully,” he said at the time.
Dr. Kamin said that ofatumumab will nevertheless likely represent a welcome addition to the tool kit of treatment options for MS. “Any new drug is helpful in adding to our choices as a general rule,” he said. “Subcutaneous injection does have increased convenience.”
It is not likely that the drug will be a game changer, he added, although the treatment’s efficacy compared with other drugs remains to be seen. “It all depends upon the relative efficacy of ofatumumab versus ocrelizumab or siponimod,” Dr. Kamin said.
“There has been another subcutaneous monoclonal for MS, daclizumab, although this was withdrawn from the market due to severe adverse effects not related to route of administration,” he added.
Dr. Hauser has relationships with Alector, Annexon, Bionure, Molecular Stethoscope, Symbiotix, and F. Hoffmann-La Roche. Dr. Kamin has received research support from Biogen, Novartis and CMSC.
A version of this article originally appeared on Medscape.com.
From CMSC 2020
Moving on up: Maintenance therapy extends OS in bladder cancer
Is maintenance therapy with an immune checkpoint inhibitor a good idea for patients with advanced bladder cancer who do not progress after initial chemotherapy?
Yes, and furthermore this approach offers “a new first-line standard of care for advanced urothelial cancer,” said Thomas Powles, MD, professor of genitourinary oncology and director of the Barts Cancer Centre in London.
Dr. Powles was discussing “first-line maintenance therapy” with avelumab (Bavencio, EMD Serono and Pfizer) from the JAVELIN Bladder 100 trial.
Results from this trial will be presented at the plenary session of the 2020 annual meeting of the American Society of Clinical Oncology, held virtually because the coronavirus pandemic. ASCO chief medical officer Richard Schilsky, MD, PhD, highlighted this abstract as one of three from the plenary session that were “practice changing.”
Dr. Powles provided a glimpse of the results at a premeeting press briefing.
The trial involved 700 patients who had not progressed after at least four cycles of first-line, platinum-based chemotherapy. Maintenance therapy with avelumab improved overall survival by 7.1 months when compared with best supportive care (BSC) alone.
The median OS was 21.4 months for avelumab plus BSC versus 14.3 months for BSC alone (hazard ratio, 0.69; P = .0005).
An expert not involved with the study was impressed with the outcome.
“The data are encouraging and we look forward to FDA review, and hopefully approval [in this setting],” said Padmanee Sharma, MD, PhD, a genitourinary medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
Avelumab is already approved for use in advanced urothelial cancer, but in a second-line setting, like a number of other immune checkpoint inhibitors.
“Instead of waiting for cancer to return”
Dr. Powles commented that about 65%-75% of patients with advanced urothelial cancer have disease control with first-line chemotherapy, but that progression-free survival (PFS) and OS are “short” because of chemoresistance.
Many patients do not receive second-line treatment with immunotherapy and only a “minority” achieve durable clinical benefit, he added.
“Instead of waiting for the cancer to return,” which it will do “quickly,” Dr. Powles suggested that maintenance with immunotherapy should become the standard of care.
“Our findings should give hope to many patients with advanced urothelial cancer who face a very challenging and difficult condition,” coauthor Petros Grivas, MD, PhD, clinical director of the Genitourinary Cancers Program at the Seattle Cancer Care Alliance, said in a statement. He was the global coprincipal investigator of the JAVELIN Bladder 100 trial.
“People with advanced urothelial cancer generally have a poor prognosis, and most experience cancer progression (growth) within 8 months after initiation of first-line chemotherapy,” he said.
“We are very excited with these results, which indicate that immunotherapy with avelumab first-line maintenance could offer a new treatment option that helps patients live longer. Even if this is likely not a complete cure and may cause potential side effects in some patients, the significant prolongation of overall survival is clearly a remarkable improvement, while many treated patients may not experience significant side effects from this approach,” he added.
The safety profile was “manageable” and consistent with other studies of avelumab, Dr. Powles reported.
All-causality adverse events (AEs) were reported at any grade in 98% versus 77.7% in the avelumab plus BSC versus BSC-alone groups; AEs of grade 3 or higher were 47.4% vs 25.2%. The most frequent grade ≥3 AEs were urinary tract infection (4.4% vs. 2.6%), anemia (3.8% vs. 2.9%), hematuria (1.7% vs. 1.4%), fatigue (1.7% vs. 0.6%), and back pain (1.2% vs. 2.3%).
The results from JAVELIN with avelumab show the “largest survival benefit” seen so far in advanced urothelial cancer in the maintenance setting, according to ASCO press materials.
Has there ever been a survival benefit found with maintenance therapy?
No, according to a 2019 review in Future Oncology. Three prospective, randomized, controlled trials (of vinflunine, sunitinib, and lapatinib, respectively) did not reveal any significant oncologic benefit vs placebo.
But in a phase 2, randomized, controlled trial involving 107 patients, maintenance pembrolizumab provided longer PFS, compared with placebo (5.4 vs 3.2 months, HR, 0.64; 95% confidence interval, 0.41-0.98).
This pembrolizumab trial showed a “similar PFS hazard ratio” to that seen with avelumab in JAVELIN, Dr. Powles commented, noting however that the pembrolizumab trial was not designed to look at survival.
Even better response among PD-L1-positive patients
JAVELIN patients had unresectable locally advanced or metastatic urothelial carcinoma and were treated with gemcitabine with either cisplatin or carboplatin.
Just over half (51%) of these patients had tumors that were PD-L1 positive.
The maintenance therapy strategy was even more effective in these patients. Avelumab plus BSC significantly prolonged OS versus BSC alone in patients with PD-L1-positive tumors (HR, 0.56; 1-sided P = .0003). Median OS was not reached versus 17.1 months, respectively.
An OS benefit was also observed across all prespecified subgroups, including those patients with visceral metastases.
Commenting on the study, Dr. Sharma said she would like to see more detailed outcome data related to the number of chemotherapy cycles administered (the range was 4 to 6) and information on the amount of time between the end of chemo to the start of avelumab. Dr. Powles commented that his international team has not looked at number of cycles and outcome, nor the time from completion of chemotherapy and randomization. “They are both valid questions for the future,” he said.
The study was funded by Pfizer. Dr. Powles and many of the coauthors have financial relationships with Pfizer and other pharmaceuticals. Dr. Sharma has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Is maintenance therapy with an immune checkpoint inhibitor a good idea for patients with advanced bladder cancer who do not progress after initial chemotherapy?
Yes, and furthermore this approach offers “a new first-line standard of care for advanced urothelial cancer,” said Thomas Powles, MD, professor of genitourinary oncology and director of the Barts Cancer Centre in London.
Dr. Powles was discussing “first-line maintenance therapy” with avelumab (Bavencio, EMD Serono and Pfizer) from the JAVELIN Bladder 100 trial.
Results from this trial will be presented at the plenary session of the 2020 annual meeting of the American Society of Clinical Oncology, held virtually because the coronavirus pandemic. ASCO chief medical officer Richard Schilsky, MD, PhD, highlighted this abstract as one of three from the plenary session that were “practice changing.”
Dr. Powles provided a glimpse of the results at a premeeting press briefing.
The trial involved 700 patients who had not progressed after at least four cycles of first-line, platinum-based chemotherapy. Maintenance therapy with avelumab improved overall survival by 7.1 months when compared with best supportive care (BSC) alone.
The median OS was 21.4 months for avelumab plus BSC versus 14.3 months for BSC alone (hazard ratio, 0.69; P = .0005).
An expert not involved with the study was impressed with the outcome.
“The data are encouraging and we look forward to FDA review, and hopefully approval [in this setting],” said Padmanee Sharma, MD, PhD, a genitourinary medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
Avelumab is already approved for use in advanced urothelial cancer, but in a second-line setting, like a number of other immune checkpoint inhibitors.
“Instead of waiting for cancer to return”
Dr. Powles commented that about 65%-75% of patients with advanced urothelial cancer have disease control with first-line chemotherapy, but that progression-free survival (PFS) and OS are “short” because of chemoresistance.
Many patients do not receive second-line treatment with immunotherapy and only a “minority” achieve durable clinical benefit, he added.
“Instead of waiting for the cancer to return,” which it will do “quickly,” Dr. Powles suggested that maintenance with immunotherapy should become the standard of care.
“Our findings should give hope to many patients with advanced urothelial cancer who face a very challenging and difficult condition,” coauthor Petros Grivas, MD, PhD, clinical director of the Genitourinary Cancers Program at the Seattle Cancer Care Alliance, said in a statement. He was the global coprincipal investigator of the JAVELIN Bladder 100 trial.
“People with advanced urothelial cancer generally have a poor prognosis, and most experience cancer progression (growth) within 8 months after initiation of first-line chemotherapy,” he said.
“We are very excited with these results, which indicate that immunotherapy with avelumab first-line maintenance could offer a new treatment option that helps patients live longer. Even if this is likely not a complete cure and may cause potential side effects in some patients, the significant prolongation of overall survival is clearly a remarkable improvement, while many treated patients may not experience significant side effects from this approach,” he added.
The safety profile was “manageable” and consistent with other studies of avelumab, Dr. Powles reported.
All-causality adverse events (AEs) were reported at any grade in 98% versus 77.7% in the avelumab plus BSC versus BSC-alone groups; AEs of grade 3 or higher were 47.4% vs 25.2%. The most frequent grade ≥3 AEs were urinary tract infection (4.4% vs. 2.6%), anemia (3.8% vs. 2.9%), hematuria (1.7% vs. 1.4%), fatigue (1.7% vs. 0.6%), and back pain (1.2% vs. 2.3%).
The results from JAVELIN with avelumab show the “largest survival benefit” seen so far in advanced urothelial cancer in the maintenance setting, according to ASCO press materials.
Has there ever been a survival benefit found with maintenance therapy?
No, according to a 2019 review in Future Oncology. Three prospective, randomized, controlled trials (of vinflunine, sunitinib, and lapatinib, respectively) did not reveal any significant oncologic benefit vs placebo.
But in a phase 2, randomized, controlled trial involving 107 patients, maintenance pembrolizumab provided longer PFS, compared with placebo (5.4 vs 3.2 months, HR, 0.64; 95% confidence interval, 0.41-0.98).
This pembrolizumab trial showed a “similar PFS hazard ratio” to that seen with avelumab in JAVELIN, Dr. Powles commented, noting however that the pembrolizumab trial was not designed to look at survival.
Even better response among PD-L1-positive patients
JAVELIN patients had unresectable locally advanced or metastatic urothelial carcinoma and were treated with gemcitabine with either cisplatin or carboplatin.
Just over half (51%) of these patients had tumors that were PD-L1 positive.
The maintenance therapy strategy was even more effective in these patients. Avelumab plus BSC significantly prolonged OS versus BSC alone in patients with PD-L1-positive tumors (HR, 0.56; 1-sided P = .0003). Median OS was not reached versus 17.1 months, respectively.
An OS benefit was also observed across all prespecified subgroups, including those patients with visceral metastases.
Commenting on the study, Dr. Sharma said she would like to see more detailed outcome data related to the number of chemotherapy cycles administered (the range was 4 to 6) and information on the amount of time between the end of chemo to the start of avelumab. Dr. Powles commented that his international team has not looked at number of cycles and outcome, nor the time from completion of chemotherapy and randomization. “They are both valid questions for the future,” he said.
The study was funded by Pfizer. Dr. Powles and many of the coauthors have financial relationships with Pfizer and other pharmaceuticals. Dr. Sharma has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Is maintenance therapy with an immune checkpoint inhibitor a good idea for patients with advanced bladder cancer who do not progress after initial chemotherapy?
Yes, and furthermore this approach offers “a new first-line standard of care for advanced urothelial cancer,” said Thomas Powles, MD, professor of genitourinary oncology and director of the Barts Cancer Centre in London.
Dr. Powles was discussing “first-line maintenance therapy” with avelumab (Bavencio, EMD Serono and Pfizer) from the JAVELIN Bladder 100 trial.
Results from this trial will be presented at the plenary session of the 2020 annual meeting of the American Society of Clinical Oncology, held virtually because the coronavirus pandemic. ASCO chief medical officer Richard Schilsky, MD, PhD, highlighted this abstract as one of three from the plenary session that were “practice changing.”
Dr. Powles provided a glimpse of the results at a premeeting press briefing.
The trial involved 700 patients who had not progressed after at least four cycles of first-line, platinum-based chemotherapy. Maintenance therapy with avelumab improved overall survival by 7.1 months when compared with best supportive care (BSC) alone.
The median OS was 21.4 months for avelumab plus BSC versus 14.3 months for BSC alone (hazard ratio, 0.69; P = .0005).
An expert not involved with the study was impressed with the outcome.
“The data are encouraging and we look forward to FDA review, and hopefully approval [in this setting],” said Padmanee Sharma, MD, PhD, a genitourinary medical oncologist at the University of Texas MD Anderson Cancer Center, Houston.
Avelumab is already approved for use in advanced urothelial cancer, but in a second-line setting, like a number of other immune checkpoint inhibitors.
“Instead of waiting for cancer to return”
Dr. Powles commented that about 65%-75% of patients with advanced urothelial cancer have disease control with first-line chemotherapy, but that progression-free survival (PFS) and OS are “short” because of chemoresistance.
Many patients do not receive second-line treatment with immunotherapy and only a “minority” achieve durable clinical benefit, he added.
“Instead of waiting for the cancer to return,” which it will do “quickly,” Dr. Powles suggested that maintenance with immunotherapy should become the standard of care.
“Our findings should give hope to many patients with advanced urothelial cancer who face a very challenging and difficult condition,” coauthor Petros Grivas, MD, PhD, clinical director of the Genitourinary Cancers Program at the Seattle Cancer Care Alliance, said in a statement. He was the global coprincipal investigator of the JAVELIN Bladder 100 trial.
“People with advanced urothelial cancer generally have a poor prognosis, and most experience cancer progression (growth) within 8 months after initiation of first-line chemotherapy,” he said.
“We are very excited with these results, which indicate that immunotherapy with avelumab first-line maintenance could offer a new treatment option that helps patients live longer. Even if this is likely not a complete cure and may cause potential side effects in some patients, the significant prolongation of overall survival is clearly a remarkable improvement, while many treated patients may not experience significant side effects from this approach,” he added.
The safety profile was “manageable” and consistent with other studies of avelumab, Dr. Powles reported.
All-causality adverse events (AEs) were reported at any grade in 98% versus 77.7% in the avelumab plus BSC versus BSC-alone groups; AEs of grade 3 or higher were 47.4% vs 25.2%. The most frequent grade ≥3 AEs were urinary tract infection (4.4% vs. 2.6%), anemia (3.8% vs. 2.9%), hematuria (1.7% vs. 1.4%), fatigue (1.7% vs. 0.6%), and back pain (1.2% vs. 2.3%).
The results from JAVELIN with avelumab show the “largest survival benefit” seen so far in advanced urothelial cancer in the maintenance setting, according to ASCO press materials.
Has there ever been a survival benefit found with maintenance therapy?
No, according to a 2019 review in Future Oncology. Three prospective, randomized, controlled trials (of vinflunine, sunitinib, and lapatinib, respectively) did not reveal any significant oncologic benefit vs placebo.
But in a phase 2, randomized, controlled trial involving 107 patients, maintenance pembrolizumab provided longer PFS, compared with placebo (5.4 vs 3.2 months, HR, 0.64; 95% confidence interval, 0.41-0.98).
This pembrolizumab trial showed a “similar PFS hazard ratio” to that seen with avelumab in JAVELIN, Dr. Powles commented, noting however that the pembrolizumab trial was not designed to look at survival.
Even better response among PD-L1-positive patients
JAVELIN patients had unresectable locally advanced or metastatic urothelial carcinoma and were treated with gemcitabine with either cisplatin or carboplatin.
Just over half (51%) of these patients had tumors that were PD-L1 positive.
The maintenance therapy strategy was even more effective in these patients. Avelumab plus BSC significantly prolonged OS versus BSC alone in patients with PD-L1-positive tumors (HR, 0.56; 1-sided P = .0003). Median OS was not reached versus 17.1 months, respectively.
An OS benefit was also observed across all prespecified subgroups, including those patients with visceral metastases.
Commenting on the study, Dr. Sharma said she would like to see more detailed outcome data related to the number of chemotherapy cycles administered (the range was 4 to 6) and information on the amount of time between the end of chemo to the start of avelumab. Dr. Powles commented that his international team has not looked at number of cycles and outcome, nor the time from completion of chemotherapy and randomization. “They are both valid questions for the future,” he said.
The study was funded by Pfizer. Dr. Powles and many of the coauthors have financial relationships with Pfizer and other pharmaceuticals. Dr. Sharma has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FROM ASCO 2020
COVID-19: Putting distance between projection and reality
When it comes to COVID-19, studies show that social distancing flattened the curve.
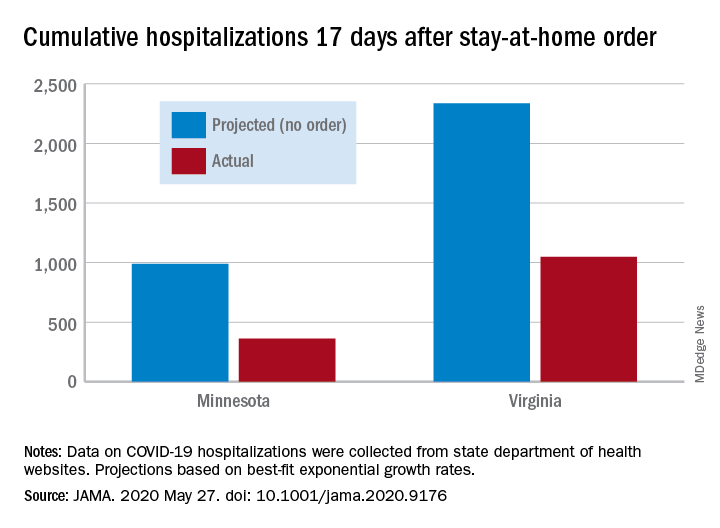
Cumulative hospitalizations in four states with stay-at-home orders were well short of the projected exponential growth curves, Soumya Sen, PhD, of the University of Minnesota, Minneapolis, and associates reported May 27 in a research letter in JAMA. All states were observed through April 28.
The deviations between observed cases and worst-case projections in the four states – Colorado, Minnesota, Ohio, and Virginia – all began within 8-10 days of the stay-at-home orders. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In Virginia, the corresponding numbers were 1,048 observed and 2,335 projected, they reported.
“Observed hospitalizations consistently fell outside of the 95% prediction bands of the projected exponential growth curve,” Dr. Sen and associates noted.
In a separate Canadian study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention, Ashleigh R. Tuite, PhD, MPH, of the University of Toronto and associates wrote May 27 in a letter in Annals of Internal Medicine.
Their model, based on a 70% reduction in physical contacts for March 19–May 3, projected 2.0 cases per 100,000 population with physical distancing and 37.4 per 100,000 without. Deaths among those ICU patients were projected at 2.5 per 100,000 with distancing and 12.7 per 100,000 without intervention, they reported.
“Our modeling also shows the challenges associated with relaxation of physical distancing measures without a concomitant increase in other public health measures. Specifically, when the number of contacts between persons returns to more than 50% of normal, we expect disease activity to resurge rapidly and ICUs to quickly reach capacity,” they wrote.
The study published in JAMA used publicly available data from the University of Minnesota COVID-19 Hospitalization Project, which is partially funded by the University of Minnesota Office of Academic Clinical Affairs and United Health Foundation.
SOURCES: Sen S et al. JAMA. 2020 May 27. doi: 10.1001/jama.2020.9176; Tuite AR et al. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2945.
When it comes to COVID-19, studies show that social distancing flattened the curve.

Cumulative hospitalizations in four states with stay-at-home orders were well short of the projected exponential growth curves, Soumya Sen, PhD, of the University of Minnesota, Minneapolis, and associates reported May 27 in a research letter in JAMA. All states were observed through April 28.
The deviations between observed cases and worst-case projections in the four states – Colorado, Minnesota, Ohio, and Virginia – all began within 8-10 days of the stay-at-home orders. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In Virginia, the corresponding numbers were 1,048 observed and 2,335 projected, they reported.
“Observed hospitalizations consistently fell outside of the 95% prediction bands of the projected exponential growth curve,” Dr. Sen and associates noted.
In a separate Canadian study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention, Ashleigh R. Tuite, PhD, MPH, of the University of Toronto and associates wrote May 27 in a letter in Annals of Internal Medicine.
Their model, based on a 70% reduction in physical contacts for March 19–May 3, projected 2.0 cases per 100,000 population with physical distancing and 37.4 per 100,000 without. Deaths among those ICU patients were projected at 2.5 per 100,000 with distancing and 12.7 per 100,000 without intervention, they reported.
“Our modeling also shows the challenges associated with relaxation of physical distancing measures without a concomitant increase in other public health measures. Specifically, when the number of contacts between persons returns to more than 50% of normal, we expect disease activity to resurge rapidly and ICUs to quickly reach capacity,” they wrote.
The study published in JAMA used publicly available data from the University of Minnesota COVID-19 Hospitalization Project, which is partially funded by the University of Minnesota Office of Academic Clinical Affairs and United Health Foundation.
SOURCES: Sen S et al. JAMA. 2020 May 27. doi: 10.1001/jama.2020.9176; Tuite AR et al. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2945.
When it comes to COVID-19, studies show that social distancing flattened the curve.

Cumulative hospitalizations in four states with stay-at-home orders were well short of the projected exponential growth curves, Soumya Sen, PhD, of the University of Minnesota, Minneapolis, and associates reported May 27 in a research letter in JAMA. All states were observed through April 28.
The deviations between observed cases and worst-case projections in the four states – Colorado, Minnesota, Ohio, and Virginia – all began within 8-10 days of the stay-at-home orders. In Minnesota, 17 days after the order, there were 361 cumulative hospitalizations, compared with a projection of 988 had no such action been taken. In Virginia, the corresponding numbers were 1,048 observed and 2,335 projected, they reported.
“Observed hospitalizations consistently fell outside of the 95% prediction bands of the projected exponential growth curve,” Dr. Sen and associates noted.
In a separate Canadian study measuring COVID-19 patients occupying ICU beds in Ontario and deaths among those cases, hospitals “would have rapidly exceeded ICU capacity and observed substantially higher mortality” without any physical distancing intervention, Ashleigh R. Tuite, PhD, MPH, of the University of Toronto and associates wrote May 27 in a letter in Annals of Internal Medicine.
Their model, based on a 70% reduction in physical contacts for March 19–May 3, projected 2.0 cases per 100,000 population with physical distancing and 37.4 per 100,000 without. Deaths among those ICU patients were projected at 2.5 per 100,000 with distancing and 12.7 per 100,000 without intervention, they reported.
“Our modeling also shows the challenges associated with relaxation of physical distancing measures without a concomitant increase in other public health measures. Specifically, when the number of contacts between persons returns to more than 50% of normal, we expect disease activity to resurge rapidly and ICUs to quickly reach capacity,” they wrote.
The study published in JAMA used publicly available data from the University of Minnesota COVID-19 Hospitalization Project, which is partially funded by the University of Minnesota Office of Academic Clinical Affairs and United Health Foundation.
SOURCES: Sen S et al. JAMA. 2020 May 27. doi: 10.1001/jama.2020.9176; Tuite AR et al. Ann Intern Med. 2020 May 27. doi: 10.7326/M20-2945.
Patients find CAC more persuasive than ASCVD risk score for statin decisions
Patients who received a protocol-driven recommendation to initiate statin therapy for primary prevention of cardiovascular disease based upon their CT angiography coronary artery calcium score were twice as likely to actually start on the drug than those whose recommendation was guided by the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator, according to the results of the randomized CorCal Vanguard study.
These results suggest that patients – and their primary care physicians – find the conventional method of screening for cardiovascular risk using the Pooled Cohort Equations to estimate the 10-year risk of MI or stroke, as recommended in ACC/AHA guidelines, to be less persuasive than screening for the presence or absence of actual disease as captured by CT angiography images and the associated coronary artery calcium (CAC) score, Joseph B. Muhlestein, MD, said at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The CorCal Vanguard study included 601 patients with an average baseline LDL cholesterol of 120 mg/dL, an average age of 60 years, and no history of cardiovascular disease, diabetes, or prior statin therapy. They were randomized to decision-making regarding statin therapy based on either the ACC/AHA guideline–endorsed Pooled Cohort Equations, which use an estimated 10-year risk of 7.5% or more as the threshold for statin initiation, or their CAC score.
If a patient’s CAC score was 0, the recommendation was against starting a statin. Everyone with a CAC greater than 100 received a recommendation for high-intensity statin therapy. And for those with a CAC of 1-100, the decision defaulted to the results of the Pooled Cohort Equations. The screening results were provided to a patient’s primary physician so they could engage in joint decision-making regarding initiation of statin therapy. Adherence to a screening-based recommendation to start on a statin was assessed at 3 and 12 months of follow-up, explained Dr. Muhlestein, a cardiologist at the Intermountain Medical Center Heart Institute in Salt Lake City.
He noted that CorCal Vanguard was merely a feasibility study. Based on the study results he presented at ACC 2020, the full 9,000-patient CorCal primary prevention trial is now enrolling participants. CorCal is the first randomized trial to pit the Pooled Cohort Equations against the CAC score in a large study looking for differences in downstream clinical outcomes.
The rationale for this line of clinical research lies in the known limitations of the ACC/AHA risk calculator. “It may overestimate risk in some populations, patients aren’t always adherent to Pooled Cohort Equations Risk Calculator recommendations, and it doesn’t include novel risk markers such as C-reactive protein that some consider important for risk assessment. And the big question: Should we continue risk screening to determine potential benefit from drug therapy, or should we switch to disease screening?” the cardiologist commented.
The CorCal Vanguard results
A recommendation to start statin therapy was made in 48% of patients in the Pooled Cohort Equations group, versus 36% of the group randomized to CAC. However, only 17% of patients in the Pooled Cohort Equations group actually initiated a statin, a significantly lower rate than the 26% figure in the CAC arm. Fully 70% of patients who received a recommendation to start taking a statin on the basis of their CAC score actually did so, compared to just 36% of those whose recommendation was based upon their Pooled Cohort Equations Risk Calculator.
At 3 months of follow-up, 61% of patients who received an initial recommendation to start statin therapy based upon their CAC screening were actually taking a statin, compared with 41% of those whose recommendation was based upon the Pooled Cohort Equations. At 12 months, the figures were 64% and 49%.
In both groups, at 12 months of follow-up, the No. 1 reason patients weren’t taking a statin as recommended was that their personal physician had advised against it or never prescribed it. That accounted for roughly half of the nonadherence. Another quarter was because of a preference to try lifestyle change first. Fear of drug side effects was a less common reason.
Putting the CorCal Vanguard study results in perspective, Dr. Muhlestein observed that, prior to the screening study, none of the participants had ever been on a statin, yet 37% of them were found by one screening method or the other to be at high cardiovascular risk. Of those high-risk patients, 51% actually initiated statin therapy and the majority of them were still taking their medication 12 months later.
“That has to be a good thing. It emphasizes what can be done when proactive primary prevention is practiced,” the cardiologist said.
He reported having no financial conflicts regarding the CorCal study, which was funded by a grant from the Dell Loy Hansen Cardiovascular Research Fund.
SOURCE: Muhlestein JB et al. ACC 2020, Abstract 909-12.
Patients who received a protocol-driven recommendation to initiate statin therapy for primary prevention of cardiovascular disease based upon their CT angiography coronary artery calcium score were twice as likely to actually start on the drug than those whose recommendation was guided by the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator, according to the results of the randomized CorCal Vanguard study.
These results suggest that patients – and their primary care physicians – find the conventional method of screening for cardiovascular risk using the Pooled Cohort Equations to estimate the 10-year risk of MI or stroke, as recommended in ACC/AHA guidelines, to be less persuasive than screening for the presence or absence of actual disease as captured by CT angiography images and the associated coronary artery calcium (CAC) score, Joseph B. Muhlestein, MD, said at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The CorCal Vanguard study included 601 patients with an average baseline LDL cholesterol of 120 mg/dL, an average age of 60 years, and no history of cardiovascular disease, diabetes, or prior statin therapy. They were randomized to decision-making regarding statin therapy based on either the ACC/AHA guideline–endorsed Pooled Cohort Equations, which use an estimated 10-year risk of 7.5% or more as the threshold for statin initiation, or their CAC score.
If a patient’s CAC score was 0, the recommendation was against starting a statin. Everyone with a CAC greater than 100 received a recommendation for high-intensity statin therapy. And for those with a CAC of 1-100, the decision defaulted to the results of the Pooled Cohort Equations. The screening results were provided to a patient’s primary physician so they could engage in joint decision-making regarding initiation of statin therapy. Adherence to a screening-based recommendation to start on a statin was assessed at 3 and 12 months of follow-up, explained Dr. Muhlestein, a cardiologist at the Intermountain Medical Center Heart Institute in Salt Lake City.
He noted that CorCal Vanguard was merely a feasibility study. Based on the study results he presented at ACC 2020, the full 9,000-patient CorCal primary prevention trial is now enrolling participants. CorCal is the first randomized trial to pit the Pooled Cohort Equations against the CAC score in a large study looking for differences in downstream clinical outcomes.
The rationale for this line of clinical research lies in the known limitations of the ACC/AHA risk calculator. “It may overestimate risk in some populations, patients aren’t always adherent to Pooled Cohort Equations Risk Calculator recommendations, and it doesn’t include novel risk markers such as C-reactive protein that some consider important for risk assessment. And the big question: Should we continue risk screening to determine potential benefit from drug therapy, or should we switch to disease screening?” the cardiologist commented.
The CorCal Vanguard results
A recommendation to start statin therapy was made in 48% of patients in the Pooled Cohort Equations group, versus 36% of the group randomized to CAC. However, only 17% of patients in the Pooled Cohort Equations group actually initiated a statin, a significantly lower rate than the 26% figure in the CAC arm. Fully 70% of patients who received a recommendation to start taking a statin on the basis of their CAC score actually did so, compared to just 36% of those whose recommendation was based upon their Pooled Cohort Equations Risk Calculator.
At 3 months of follow-up, 61% of patients who received an initial recommendation to start statin therapy based upon their CAC screening were actually taking a statin, compared with 41% of those whose recommendation was based upon the Pooled Cohort Equations. At 12 months, the figures were 64% and 49%.
In both groups, at 12 months of follow-up, the No. 1 reason patients weren’t taking a statin as recommended was that their personal physician had advised against it or never prescribed it. That accounted for roughly half of the nonadherence. Another quarter was because of a preference to try lifestyle change first. Fear of drug side effects was a less common reason.
Putting the CorCal Vanguard study results in perspective, Dr. Muhlestein observed that, prior to the screening study, none of the participants had ever been on a statin, yet 37% of them were found by one screening method or the other to be at high cardiovascular risk. Of those high-risk patients, 51% actually initiated statin therapy and the majority of them were still taking their medication 12 months later.
“That has to be a good thing. It emphasizes what can be done when proactive primary prevention is practiced,” the cardiologist said.
He reported having no financial conflicts regarding the CorCal study, which was funded by a grant from the Dell Loy Hansen Cardiovascular Research Fund.
SOURCE: Muhlestein JB et al. ACC 2020, Abstract 909-12.
Patients who received a protocol-driven recommendation to initiate statin therapy for primary prevention of cardiovascular disease based upon their CT angiography coronary artery calcium score were twice as likely to actually start on the drug than those whose recommendation was guided by the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk Calculator, according to the results of the randomized CorCal Vanguard study.
These results suggest that patients – and their primary care physicians – find the conventional method of screening for cardiovascular risk using the Pooled Cohort Equations to estimate the 10-year risk of MI or stroke, as recommended in ACC/AHA guidelines, to be less persuasive than screening for the presence or absence of actual disease as captured by CT angiography images and the associated coronary artery calcium (CAC) score, Joseph B. Muhlestein, MD, said at the joint scientific sessions of the ACC and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The CorCal Vanguard study included 601 patients with an average baseline LDL cholesterol of 120 mg/dL, an average age of 60 years, and no history of cardiovascular disease, diabetes, or prior statin therapy. They were randomized to decision-making regarding statin therapy based on either the ACC/AHA guideline–endorsed Pooled Cohort Equations, which use an estimated 10-year risk of 7.5% or more as the threshold for statin initiation, or their CAC score.
If a patient’s CAC score was 0, the recommendation was against starting a statin. Everyone with a CAC greater than 100 received a recommendation for high-intensity statin therapy. And for those with a CAC of 1-100, the decision defaulted to the results of the Pooled Cohort Equations. The screening results were provided to a patient’s primary physician so they could engage in joint decision-making regarding initiation of statin therapy. Adherence to a screening-based recommendation to start on a statin was assessed at 3 and 12 months of follow-up, explained Dr. Muhlestein, a cardiologist at the Intermountain Medical Center Heart Institute in Salt Lake City.
He noted that CorCal Vanguard was merely a feasibility study. Based on the study results he presented at ACC 2020, the full 9,000-patient CorCal primary prevention trial is now enrolling participants. CorCal is the first randomized trial to pit the Pooled Cohort Equations against the CAC score in a large study looking for differences in downstream clinical outcomes.
The rationale for this line of clinical research lies in the known limitations of the ACC/AHA risk calculator. “It may overestimate risk in some populations, patients aren’t always adherent to Pooled Cohort Equations Risk Calculator recommendations, and it doesn’t include novel risk markers such as C-reactive protein that some consider important for risk assessment. And the big question: Should we continue risk screening to determine potential benefit from drug therapy, or should we switch to disease screening?” the cardiologist commented.
The CorCal Vanguard results
A recommendation to start statin therapy was made in 48% of patients in the Pooled Cohort Equations group, versus 36% of the group randomized to CAC. However, only 17% of patients in the Pooled Cohort Equations group actually initiated a statin, a significantly lower rate than the 26% figure in the CAC arm. Fully 70% of patients who received a recommendation to start taking a statin on the basis of their CAC score actually did so, compared to just 36% of those whose recommendation was based upon their Pooled Cohort Equations Risk Calculator.
At 3 months of follow-up, 61% of patients who received an initial recommendation to start statin therapy based upon their CAC screening were actually taking a statin, compared with 41% of those whose recommendation was based upon the Pooled Cohort Equations. At 12 months, the figures were 64% and 49%.
In both groups, at 12 months of follow-up, the No. 1 reason patients weren’t taking a statin as recommended was that their personal physician had advised against it or never prescribed it. That accounted for roughly half of the nonadherence. Another quarter was because of a preference to try lifestyle change first. Fear of drug side effects was a less common reason.
Putting the CorCal Vanguard study results in perspective, Dr. Muhlestein observed that, prior to the screening study, none of the participants had ever been on a statin, yet 37% of them were found by one screening method or the other to be at high cardiovascular risk. Of those high-risk patients, 51% actually initiated statin therapy and the majority of them were still taking their medication 12 months later.
“That has to be a good thing. It emphasizes what can be done when proactive primary prevention is practiced,” the cardiologist said.
He reported having no financial conflicts regarding the CorCal study, which was funded by a grant from the Dell Loy Hansen Cardiovascular Research Fund.
SOURCE: Muhlestein JB et al. ACC 2020, Abstract 909-12.
FROM ACC 2020
Cardiologists’ pay increases; most satisfied with profession
Cardiologists remain among the top earners in medicine in 2020 and their annual pay has increased over 2019, although female cardiologists continue to earn less than their male peers, according to the 2020 Medscape Cardiologist Compensation Report.
However, an important caveat is that the data for this year’s report were collected prior to Feb. 10 and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% drop in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily. With this in mind, the Medscape 2020 report shows that annual compensation for cardiologists increased to $438,000 in 2020, up from $430,000 in 2019.
Cardiologist pay is the fourth highest of all specialties in the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole and more than 17,000 physicians in more than 30 specialties.
Nearly two-thirds of cardiologists (61%) report that they feel fairly compensated, somewhat higher than last year’s percentage (54%).
On average, cardiologists are eligible for an average incentive bonus of $63,000. Average incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
More than half of cardiologists (55%) say they receive three-quarters of their potential annual incentive bonus.
But COVID-19 may change that. Experts interviewed recently by this news organization noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Most cardiologists happy at work
On average, male cardiologists spend 42.6 hours per week seeing patients, somewhat higher than female cardiologists (36.9 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, cardiologists spend 16.9 hours per week on paperwork and administration, similar to physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a cardiologist? Relationships with and gratitude from patients (31%) tops the list, followed by being good at what they do/finding answers, diagnoses (26%), making the world a better place (18%), and making good money at a job they like (12%). A few cited pride in their profession (6%) and teaching (3%). These figures are in line with last year’s responses.
The most challenging part of practicing cardiology is having so many rules and regulations (30%), respondents report. Other challenges include having to work long hours (21%), working with electronic health records (17%), dealing with difficult patients (8%), and trouble getting fair reimbursement (7%).
Despite the challenges, 82% of cardiologists said they would choose medicine again, and 92% would choose cardiology again.
Other key findings from the latest report regarding cardiologists include the following:
- At 15%, cardiologists rank at the lower end of physicians potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but health care professionals spend about $118 per claim on appeals.
- 41% of cardiologists say they use physician assistants to treat patients in their practices, while two-thirds use nurse practitioners; 26% use neither for patient care. Half of cardiologists who work with physician assistants and nurse practitioners in their offices say these employees have helped boost profitability.
- 84% of cardiologists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients, and 13% are undecided, roughly the same as last year.
- The large majority of cardiologists rely on payers; 44% rely on fee-for-service arrangements and 29% on accountable care organizations for patient-based income.
- 42% of cardiologists expect to participate in merit-based incentive payment system, but only 9% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
Cardiologists remain among the top earners in medicine in 2020 and their annual pay has increased over 2019, although female cardiologists continue to earn less than their male peers, according to the 2020 Medscape Cardiologist Compensation Report.
However, an important caveat is that the data for this year’s report were collected prior to Feb. 10 and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% drop in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily. With this in mind, the Medscape 2020 report shows that annual compensation for cardiologists increased to $438,000 in 2020, up from $430,000 in 2019.
Cardiologist pay is the fourth highest of all specialties in the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole and more than 17,000 physicians in more than 30 specialties.
Nearly two-thirds of cardiologists (61%) report that they feel fairly compensated, somewhat higher than last year’s percentage (54%).
On average, cardiologists are eligible for an average incentive bonus of $63,000. Average incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
More than half of cardiologists (55%) say they receive three-quarters of their potential annual incentive bonus.
But COVID-19 may change that. Experts interviewed recently by this news organization noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Most cardiologists happy at work
On average, male cardiologists spend 42.6 hours per week seeing patients, somewhat higher than female cardiologists (36.9 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, cardiologists spend 16.9 hours per week on paperwork and administration, similar to physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a cardiologist? Relationships with and gratitude from patients (31%) tops the list, followed by being good at what they do/finding answers, diagnoses (26%), making the world a better place (18%), and making good money at a job they like (12%). A few cited pride in their profession (6%) and teaching (3%). These figures are in line with last year’s responses.
The most challenging part of practicing cardiology is having so many rules and regulations (30%), respondents report. Other challenges include having to work long hours (21%), working with electronic health records (17%), dealing with difficult patients (8%), and trouble getting fair reimbursement (7%).
Despite the challenges, 82% of cardiologists said they would choose medicine again, and 92% would choose cardiology again.
Other key findings from the latest report regarding cardiologists include the following:
- At 15%, cardiologists rank at the lower end of physicians potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but health care professionals spend about $118 per claim on appeals.
- 41% of cardiologists say they use physician assistants to treat patients in their practices, while two-thirds use nurse practitioners; 26% use neither for patient care. Half of cardiologists who work with physician assistants and nurse practitioners in their offices say these employees have helped boost profitability.
- 84% of cardiologists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients, and 13% are undecided, roughly the same as last year.
- The large majority of cardiologists rely on payers; 44% rely on fee-for-service arrangements and 29% on accountable care organizations for patient-based income.
- 42% of cardiologists expect to participate in merit-based incentive payment system, but only 9% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
Cardiologists remain among the top earners in medicine in 2020 and their annual pay has increased over 2019, although female cardiologists continue to earn less than their male peers, according to the 2020 Medscape Cardiologist Compensation Report.
However, an important caveat is that the data for this year’s report were collected prior to Feb. 10 and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% drop in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily. With this in mind, the Medscape 2020 report shows that annual compensation for cardiologists increased to $438,000 in 2020, up from $430,000 in 2019.
Cardiologist pay is the fourth highest of all specialties in the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole and more than 17,000 physicians in more than 30 specialties.
Nearly two-thirds of cardiologists (61%) report that they feel fairly compensated, somewhat higher than last year’s percentage (54%).
On average, cardiologists are eligible for an average incentive bonus of $63,000. Average incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
More than half of cardiologists (55%) say they receive three-quarters of their potential annual incentive bonus.
But COVID-19 may change that. Experts interviewed recently by this news organization noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Most cardiologists happy at work
On average, male cardiologists spend 42.6 hours per week seeing patients, somewhat higher than female cardiologists (36.9 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, cardiologists spend 16.9 hours per week on paperwork and administration, similar to physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a cardiologist? Relationships with and gratitude from patients (31%) tops the list, followed by being good at what they do/finding answers, diagnoses (26%), making the world a better place (18%), and making good money at a job they like (12%). A few cited pride in their profession (6%) and teaching (3%). These figures are in line with last year’s responses.
The most challenging part of practicing cardiology is having so many rules and regulations (30%), respondents report. Other challenges include having to work long hours (21%), working with electronic health records (17%), dealing with difficult patients (8%), and trouble getting fair reimbursement (7%).
Despite the challenges, 82% of cardiologists said they would choose medicine again, and 92% would choose cardiology again.
Other key findings from the latest report regarding cardiologists include the following:
- At 15%, cardiologists rank at the lower end of physicians potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but health care professionals spend about $118 per claim on appeals.
- 41% of cardiologists say they use physician assistants to treat patients in their practices, while two-thirds use nurse practitioners; 26% use neither for patient care. Half of cardiologists who work with physician assistants and nurse practitioners in their offices say these employees have helped boost profitability.
- 84% of cardiologists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients, and 13% are undecided, roughly the same as last year.
- The large majority of cardiologists rely on payers; 44% rely on fee-for-service arrangements and 29% on accountable care organizations for patient-based income.
- 42% of cardiologists expect to participate in merit-based incentive payment system, but only 9% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
The Pediatric Hospital Medicine Core Competencies: 2020 Revision. Introduction and Methodology
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
The Pediatric Hospital Medicine Core Competencies were first published in 2010 to help define a specific body of knowledge and measurable skills needed to practice high quality care for hospitalized pediatric patients across all practice settings.1 Since then, the number of practicing pediatric hospitalists has grown to a conservative estimate of 3,000 physicians and the scope of practice among pediatric hospitalists has matured.2 Pediatric hospitalists are increasingly leading or participating in organizational and national efforts that emphasize interprofessional collaboration and the delivery of high value care to hospitalized children and their caregivers—including innovative and family-centered care models, patient safety and quality improvement initiatives, and research and educational enterprises.3-8 In response to these changes, the American Board of Medical Specialties designated Pediatric Hospital Medicine (PHM) as a pediatric subspecialty in 2016.
The field of PHM in the United States continues to be supported by three core societies—Society of Hospital Medicine (SHM), American Academy of Pediatrics (AAP), and Academic Pediatric Association (APA). Together, these societies serve as tri-sponsors of the annual Pediatric Hospital Medicine national conference, which now welcomes over 1,200 attendees from the United States and abroad.9 Each society also individually sponsors a variety of professional development and continuing medical education activities specific to PHM.
In addition, pediatric hospitalists often serve a pivotal role in teaching learners (medical students, residents, and other health profession students), physician colleagues, and other healthcare professionals on the hospital wards and via institutional educational programs. Nearly 50 institutions in the United States offer graduate medical education training in PHM.10 The PHM Fellowship Directors Council has developed a standardized curricular framework and entrustable professional activities, which reflect the tenets of competency-based medical education, for use in PHM training programs.11-13
These changes in the practice environment of pediatric hospitalists, as well as the changing landscape of graduate and continuing medical education in PHM, have informed this revision of The PHM Core Competencies. The purpose of this article is to describe the methodology of the review and revision process.
OVERVIEW OF THE PHM CORECOMPETENCIES: 2020
Revision
The PHM Core Competencies: 2020 Revision provide a framework for graduate and continuing medical education that reflects the current roles and expectations for all pediatric hospitalists in the United States. The acuity and complexity of hospitalized children, the availability of pediatric subspecialty care and other resources, and the institutional orientation towards pediatric populations vary across community, tertiary, and children’s hospital settings. In order to unify the practice of PHM across these environments, The PHM Core Competencies: 2020 Revision address the fundamental and most common components of PHM which are encountered by the majority of practicing pediatric hospitalists, as opposed to an extensive review of all aspects of the field.
The compendium includes 66 chapters on both clinical and nonclinical topics, divided into four sections—Common Clinical Diagnoses and Conditions, Core Skills, Specialized Services, and Healthcare Systems: Supporting and Advancing Child Health (Table 1). Within each chapter is an introductory paragraph and learning objectives in three domains of educational outcomes—cognitive (knowledge), psychomotor (skills), and affective (attitudes)—as well as systems organization and improvement, to reflect the emphasis of PHM practice on improving healthcare systems. The objectives encompass a range of observable behaviors and other attributes, from foundational skills such as taking a history and performing a physical exam to more advanced actions such as participating in the development of care models to support the health of complex patient populations. Implicit in these objectives is the expectation that pediatric hospitalists build on experiences in medical school and residency training to attain a level of competency at the advanced levels of a developmental continuum, such as proficient, expert, or master.14
The objectives also balance specificity to the topic with a timeless quality, allowing for flexibility both as new information emerges and when applied to various educational activities and learner groups. Each chapter can stand alone, and thus themes recur if one reads the compendium in its entirety. However, in order to reflect related content among the chapters, the appendix contains a list of associated chapters (Chapter Links) for further exploration. In addition, a short reference list is provided in each chapter to reflect the literature and best practices at the time of publication.
Finally, The PHM Core Competencies: 2020 Revision reflect the status of children as a vulnerable population. Care for hospitalized children requires attention to many elements unique to the pediatric population. These include age-based differences in development, behavior, physiology, and prevalence of clinical conditions, the impact of acute and chronic disease states on child development, the use of medications and other medical interventions with limited investigative guidance, and the role of caregivers in decision-making and care delivery. Heightened awareness of these factors is required in the hospital setting, where diagnoses and interventions often include the use of high-risk modalities and require coordination of care across multiple providers.
METHODS
Project Initiation
Revision of The PHM Core Competencies: 2020 Revision began in early 2017 following SHM’s work on The Core Competencies in Hospital Medicine 2017 Revision.15 The Executive Committee of the SHM Pediatrics Special Interest Group (SIG) supported the initiation of the revision. The 3 editors from the original compendium created an initial plan for the project that included a proposed timeline, processes for engagement of previously involved experts and new talent, and performance of a needs assessment to guide content selection. The Figure highlights these and other important steps in the revision process.
Editor and Associate Editor Selection
The above editors reviewed best practice examples of roles and responsibilities for editor and associate editor positions from relevant, leading societies and journals. From this review, the editors created an editorial structure specifically for The PHM Core Competencies: 2020 Revision. A new position of Contributing Editor was created to address the need for dedicated attention to the community site perspective and ensure review of all content, within and across chapters, by a pediatric hospitalist who is dedicated to this environment. Solicitation for additional editors and associate editors occurred via the SHM Pediatrics SIG to the wider SHM membership. The criteria for selection included active engagement in regional or national activities related to the growth and operations of PHM, strong organizational and leadership skills, including the ability to manage tasks and foster creativity, among others. In addition, a deliberate effort was made to recruit a diverse editorial cohort, considering geographic location, primary work environment, organizational affiliations, content expertise, time in practice, gender, and other factors.
Chapter Topic Selection
The editors conducted a two-pronged needs assessment related to optimal content for inclusion in The PHM Core Competencies: 2020 Revision. First, the editors reviewed content from conferences, textbooks, and handbooks specific to the field of PHM, including the conference programs for the most recent 5 years of both the annual PHM national conference and annual meetings of PHM’s 3 core societies in the United States—SHM, AAP, and APA. Second, the editors conducted a needs assessment survey with several stakeholder groups, including SHM’s Pediatrics and Medicine-Pediatrics SIGs, AAP Section on Hospital Medicine and its subcommittees, APA Hospital Medicine SIG, PHM Fellowship Directors Council, and PHM Division Directors, with encouragement to pass the survey link to others in the PHM community interested in providing input (Appendix Figure). The solicitation asked for comment on existing chapters and suggestions for new chapters. For any new chapter, respondents were asked to note the intended purpose of the chapter and the anticipated value that chapter would bring to our profession and the children and the caregivers served by pediatric hospitalists.
The entire editorial board then reviewed all of the needs assessment data and considered potential changes (additions or deletions) based on emerging trends in pediatric healthcare, the frequency, relevance, and value of the item across all environments in which pediatric hospitalists function, and the value to or impact on hospitalized children and caregivers. Almost all survey ratings and comments were either incorporated into an existing chapter or used to create a new chapter. There was a paucity of comments related to the deletion of chapters, and thus no chapters were entirely excluded. However, there were several comments supporting the exclusion of the suprapubic bladder tap procedure, and thus related content was eliminated from the relevant section in Core Skills. Of the 66 chapters in this revision, the needs assessment data directly informed the creation of 12 new chapters, as well as adjustments and/or additions to the titles of 7 chapters and the content of 29 chapters. In addition, the title of the Specialized Clinical Services section was changed to Specialized Services to represent that both clinical and nonclinical competencies reside in this section devoted to comprehensive management of these unique patient populations commonly encountered by pediatric hospitalists. Many of these changes are highlighted in Table 2.
Author selection
Authors from the initial work were invited to participate again as author of their given chapter. Subsequently, authors were identified for new chapters and chapters for which previous authors were no longer able to be engaged. Authors with content expertise were found by reviewing content from conferences, textbooks, and handbooks specific to the field of PHM. Any content expert who was not identified as a pediatric hospitalist was paired with a pediatric hospitalist as coauthor. In addition, as with the editorial board, a deliberate effort was made to recruit a diverse author cohort, considering geographic location, primary work environment, time in practice, gender, and other factors.
The editorial board held numerous conference calls to review potential authors, and the SHM Pediatrics SIG was directly engaged to ensure authorship opportunities were extended broadly. This vetting process resulted in a robust author list and included members of all three of PHM’s sponsoring societies in the United States. Once participation was confirmed, authors received an “author packet” detailing the process with the proposed timeline, resources related to writing learning objectives, the past chapter (if applicable), assigned associate editor, and other helpful resources.
Internal and External Review Process
After all chapters were drafted, the editorial board conducted a rigorous, internal review process. Each chapter was reviewed by at least one associate editor and two editors, with a focus on content, scope, and a standard approach to phrasing and formatting. In addition, the contributing editor reviewed all the chapters to ensure the community hospitalist perspective was adequately represented.
Thirty-two agencies and societies were solicited for external review, including both those involved in review of the previous edition and new stakeholder groups. External reviewers were first contacted to ascertain their interest in participating in the review process, and if interested, were provided with information on the review process. Robust feedback was received from the APA Hospital Medicine SIG, SHM Pediatrics and Medicine-Pediatrics SIGs, Association of Pediatric Program Directors Curriculum Committee, and 20 AAP committees, councils, and sections.
The feedback from the external reviewers and subsequent edits for each chapter were reviewed by at least one associate editor, two editors, and the contributing editor. Authors were engaged to address any salient changes recommended. As the final steps in the review process, the SHM Board of Directors approved the compendium and the APA provided their endorsement.
SUMMARY AND FUTURE DIRECTIONS
This second edition of The PHM Core Competencies: 2020 Revision addresses the knowledge, skills, attitudes, and systems organization and improvement objectives that define the field of pediatric hospital medicine and the leadership roles of pediatric hospitalists. This compendium reflects the recent changes in the practice and educational environments of pediatric hospitalists and can inform education, training, and career development for pediatric hospitalists across all environments in which comprehensive care is rendered for the hospitalized child. Future work at the local and national level can lead to development of associated curricula, conference content, and other training materials.
Acknowledgments
We wish to humbly and respectfully acknowledge the work of the authors, editors, and reviewers involved in the creation of the first edition, as well as this revision, of The PHM Core Competencies. In addition, we are grateful for the input of all pediatric hospitalists and other stakeholders who informed this compendium via contributions to the needs assessment survey, conference proceedings, publications, and other works. Finally, we acknowledge the support and work of SHM project coordinator, Nyla Nicholson, the SHM Pediatrics SIG, and the SHM Board of Directors.
Disclosures
SHM provided administrative support for project coordination (N. Nicholson). No author, editor, or other involved member received any compensation for efforts related to this work. There are no reported conflicts of interest.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
1. Pediatric hospital medicine core competencies. Stucky ER, Ottolini MC, Maniscalco J, editors. J Hosp Med April 2010; Vol 5 No 2 (Supplement), 86 pages. Available at: https://www.journalofhospitalmedicine.com/jhospmed/issue/128018/journal-hospital-medicine-52. Accessed August 7, 2019.
2. Association of American Medical Colleges: Analysis in Brief. Estimating the Number and Characteristics of Hospitalist Physicians in the United States and Their Possible Workforce Implications. August 2012 Edition. https://www.aamc.org/download/300620/data/aibvol12_no3-hospitalist.pdf. Accessed August 19, 2019.
3. White CM, Thomson JE, Statile AM, et al. Development of a new care model for hospitalized children with medical complexity. Hosp Pediatr. 2017;7(7):410-414. https://doi.org/10.1542/hpeds.2016-0149.
4. Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatr. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
5. Pediatric Research in Inpatient Setting. https://www.prisnetwork.org/. Accessed August 27, 2019.
6. American Academy of Pediatrics. Value in Inpatient Pediatric Network. 2019 Edition. https://www.aap.org/en-us/professional-resources/quality-improvement/Pages/Value-in-Inpatient-Pediatrics.aspx. Accessed August 27, 2019.
7. American Academy of Pediatrics. Advancing Pediatric Educator Excellence Teaching Program. 2019 Edition. https://www.aap.org/en-us/continuing-medical-education/APEX/Pages/APEX.aspx. Accessed August 27, 2019.
8. O’Toole JK, Starmer AJ, Calaman S, et al. I-PASS mentored implementation handoff curriculum: Champion training materials. MedEdPORTAL. 2019;15:10794. https://doi.org/10.15766/mep_2374-8265.10794.
9. Academic Pediatric Association. Pediatric Hospital Medicine 2018 Recap. 2018 Edition. http://2018.phmmeeting.org/. Accessed July 20, 2019.
10. PHM Fellowship Programs. 2019 Edition. http://phmfellows.org/phm-programs/. Accessed July 20, 2019.
11. Shah NH, Rhim HJH, Maniscalco J, et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11:324–328.21. https://doi.org/10.1002/jhm.2571.
12. Jerardi K, Fisher E, Rassbach C, et al. Development of a curricular framework for pediatric hospital medicine fellowships. Pediatr. 2017;140(1): e20170698.22. https://doi.org/10.1542/peds.2017-0698.
13. Blankenburg R, Chase L, Maniscalco J, Ottolini M. Hospital Medicine Entrustable Professional Activities, American Board of Pediatrics, 2018. https://www.abp.org/subspecialty-epas#Hospitalist%20Medicine. Accessed July 20, 2019.
14. Carraccio CL, Benson BJ, Nixon LJ, Derstine PL. From the educational bench to the clinical bedside: translating the Dreyfus Developmental Model to the learning of clinical skills. Accad Med. 2008;83(8):761-767. https://doi.org/10.1097/ACM.0b013e31817eb632.
15. Nichani S, Crocker J, Fetterman N, Lukela M. Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med. 2017;4;283-287. https://doi.org/10.12788/jhm.2715.
© 2020 Society of Hospital Medicine
Top AGA Community patient cases
The AGA Community (https://community.gastro.org) received a makeover – the upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field. In case you missed it, here are the most popular clinical discussions happening in the newsfeed:
- UC patient with new diagnosis of breast cancer (https://community.gastro.org/posts/20142)
- COVID testing before elective procedures (https://community.gastro.org/posts/21106)
- Remdesivir and hepatic failure (https://community.gastro.org/posts/21130)
- Doses of antibiotics for IBS-D patient (https://community.gastro.org/posts/19749)
- Vedolizumab and sinus migraines (https://community.gastro.org/posts/20204)
Follow and ask experts your questions in Roundtable:
- Resumption of elective endoscopy during COVID-19
- COVID-19 and GI: Caring for IBD
- Q&A with EoE guideline authors
- Q&A with the U.S. Multi-Society Task Force on Colorectal Cancer: follow-up after normal colonoscopy and polypectomy
View all upcoming Roundtables in the community at https://community.gastro.org/discussions.
The AGA Community (https://community.gastro.org) received a makeover – the upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field. In case you missed it, here are the most popular clinical discussions happening in the newsfeed:
- UC patient with new diagnosis of breast cancer (https://community.gastro.org/posts/20142)
- COVID testing before elective procedures (https://community.gastro.org/posts/21106)
- Remdesivir and hepatic failure (https://community.gastro.org/posts/21130)
- Doses of antibiotics for IBS-D patient (https://community.gastro.org/posts/19749)
- Vedolizumab and sinus migraines (https://community.gastro.org/posts/20204)
Follow and ask experts your questions in Roundtable:
- Resumption of elective endoscopy during COVID-19
- COVID-19 and GI: Caring for IBD
- Q&A with EoE guideline authors
- Q&A with the U.S. Multi-Society Task Force on Colorectal Cancer: follow-up after normal colonoscopy and polypectomy
View all upcoming Roundtables in the community at https://community.gastro.org/discussions.
The AGA Community (https://community.gastro.org) received a makeover – the upgraded networking platform now features a newsfeed for difficult patient scenarios and regularly scheduled Roundtable discussions with experts in the field. In case you missed it, here are the most popular clinical discussions happening in the newsfeed:
- UC patient with new diagnosis of breast cancer (https://community.gastro.org/posts/20142)
- COVID testing before elective procedures (https://community.gastro.org/posts/21106)
- Remdesivir and hepatic failure (https://community.gastro.org/posts/21130)
- Doses of antibiotics for IBS-D patient (https://community.gastro.org/posts/19749)
- Vedolizumab and sinus migraines (https://community.gastro.org/posts/20204)
Follow and ask experts your questions in Roundtable:
- Resumption of elective endoscopy during COVID-19
- COVID-19 and GI: Caring for IBD
- Q&A with EoE guideline authors
- Q&A with the U.S. Multi-Society Task Force on Colorectal Cancer: follow-up after normal colonoscopy and polypectomy
View all upcoming Roundtables in the community at https://community.gastro.org/discussions.
Meet Congressman Roger Marshall, MD, R-KS
This article is brought you by AGA PAC, a voluntary, non-partisan political organization affiliated with and supported by AGA and the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions.
The 116th Congress is well represented by the physician community, featuring a total of 17 physicians: 3 in the U.S. Senate and 14 in the House of Representatives. One of the physicians in the House, Rep. Roger Marshall, MD, R-KS, is an OBGYN by trade who is currently serving his second term in Congress. First elected in 2016, he arrived in Washington as one of only two physicians in his freshman class. He actively engaged in health care policy from the very beginning, working across party lines on a range of health care issues facing Capitol Hill. Upon entering Congress, he proactively reached out to AGA as well as other specialty physician organizations to learn our priority issues and expressed his desire to serve as a champion of the physician community.
In addition to the two committees he sits on, Dr. Marshall also serves as the chairman of the health task force for the Republican Study Committee. Additionally, Dr. Marshall is a member of the GOP Doctors Caucus, a coalition of 21 Republican medical providers with a mission statement “to utilize medical expertise to develop patient-centered health care reforms focused on quality, access, affordability, portability, and choice.” The GOP Doctors Caucus was instrumental in pushing for a permanent repeal of the sustainable growth rate (SGR) and helped to coalesce bipartisan, bicameral support for repeal legislation in the 113th Congress. The GOP Doctors Caucus continues to be active in the current Congress, advocating for policies that strengthen both the patient and provider communities.
As a member of the GOP Doctors Caucus and as a physician held in high regard by his House colleagues, Dr. Marshall is uniquely situated to advance agendas and legislative priorities that promote sound health care policy. He willingly works across the aisle with his Democratic counterparts on legislation of importance to the physician and patient community. Dr. Marshall recently worked with one of his Democratic, physician colleagues, Rep. Ami Bera, MD, D-CA, on the Improving Seniors Timely Access to Care Act, legislation addressing prior authorization burdens in Medicare Advantage plans. Dr. Marshall has vocalized the importance of physicians getting involved in the political process and to that effect, spoke to AGA members at AGA’s annual Advocacy Day about his experience as a physician running for Congress and the importance of physician advocacy.
Dr. Marshall is running for the open Senate seat in Kansas. Given that Dr. Marshall has reiterated his desire to continue to work with the physician community to ensure access to care for our patients, AGA looks forward to supporting Dr. Marshall’s Senate candidacy and continuing to work with him and his office on issues and initiatives to advance the science and practice of gastroenterology.
This article is brought you by AGA PAC, a voluntary, non-partisan political organization affiliated with and supported by AGA and the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions.
The 116th Congress is well represented by the physician community, featuring a total of 17 physicians: 3 in the U.S. Senate and 14 in the House of Representatives. One of the physicians in the House, Rep. Roger Marshall, MD, R-KS, is an OBGYN by trade who is currently serving his second term in Congress. First elected in 2016, he arrived in Washington as one of only two physicians in his freshman class. He actively engaged in health care policy from the very beginning, working across party lines on a range of health care issues facing Capitol Hill. Upon entering Congress, he proactively reached out to AGA as well as other specialty physician organizations to learn our priority issues and expressed his desire to serve as a champion of the physician community.
In addition to the two committees he sits on, Dr. Marshall also serves as the chairman of the health task force for the Republican Study Committee. Additionally, Dr. Marshall is a member of the GOP Doctors Caucus, a coalition of 21 Republican medical providers with a mission statement “to utilize medical expertise to develop patient-centered health care reforms focused on quality, access, affordability, portability, and choice.” The GOP Doctors Caucus was instrumental in pushing for a permanent repeal of the sustainable growth rate (SGR) and helped to coalesce bipartisan, bicameral support for repeal legislation in the 113th Congress. The GOP Doctors Caucus continues to be active in the current Congress, advocating for policies that strengthen both the patient and provider communities.
As a member of the GOP Doctors Caucus and as a physician held in high regard by his House colleagues, Dr. Marshall is uniquely situated to advance agendas and legislative priorities that promote sound health care policy. He willingly works across the aisle with his Democratic counterparts on legislation of importance to the physician and patient community. Dr. Marshall recently worked with one of his Democratic, physician colleagues, Rep. Ami Bera, MD, D-CA, on the Improving Seniors Timely Access to Care Act, legislation addressing prior authorization burdens in Medicare Advantage plans. Dr. Marshall has vocalized the importance of physicians getting involved in the political process and to that effect, spoke to AGA members at AGA’s annual Advocacy Day about his experience as a physician running for Congress and the importance of physician advocacy.
Dr. Marshall is running for the open Senate seat in Kansas. Given that Dr. Marshall has reiterated his desire to continue to work with the physician community to ensure access to care for our patients, AGA looks forward to supporting Dr. Marshall’s Senate candidacy and continuing to work with him and his office on issues and initiatives to advance the science and practice of gastroenterology.
This article is brought you by AGA PAC, a voluntary, non-partisan political organization affiliated with and supported by AGA and the only political action committee supported by a national gastroenterology society. Its mission is to give gastroenterologists a greater presence on Capitol Hill and a more effective voice in policy discussions.
The 116th Congress is well represented by the physician community, featuring a total of 17 physicians: 3 in the U.S. Senate and 14 in the House of Representatives. One of the physicians in the House, Rep. Roger Marshall, MD, R-KS, is an OBGYN by trade who is currently serving his second term in Congress. First elected in 2016, he arrived in Washington as one of only two physicians in his freshman class. He actively engaged in health care policy from the very beginning, working across party lines on a range of health care issues facing Capitol Hill. Upon entering Congress, he proactively reached out to AGA as well as other specialty physician organizations to learn our priority issues and expressed his desire to serve as a champion of the physician community.
In addition to the two committees he sits on, Dr. Marshall also serves as the chairman of the health task force for the Republican Study Committee. Additionally, Dr. Marshall is a member of the GOP Doctors Caucus, a coalition of 21 Republican medical providers with a mission statement “to utilize medical expertise to develop patient-centered health care reforms focused on quality, access, affordability, portability, and choice.” The GOP Doctors Caucus was instrumental in pushing for a permanent repeal of the sustainable growth rate (SGR) and helped to coalesce bipartisan, bicameral support for repeal legislation in the 113th Congress. The GOP Doctors Caucus continues to be active in the current Congress, advocating for policies that strengthen both the patient and provider communities.
As a member of the GOP Doctors Caucus and as a physician held in high regard by his House colleagues, Dr. Marshall is uniquely situated to advance agendas and legislative priorities that promote sound health care policy. He willingly works across the aisle with his Democratic counterparts on legislation of importance to the physician and patient community. Dr. Marshall recently worked with one of his Democratic, physician colleagues, Rep. Ami Bera, MD, D-CA, on the Improving Seniors Timely Access to Care Act, legislation addressing prior authorization burdens in Medicare Advantage plans. Dr. Marshall has vocalized the importance of physicians getting involved in the political process and to that effect, spoke to AGA members at AGA’s annual Advocacy Day about his experience as a physician running for Congress and the importance of physician advocacy.
Dr. Marshall is running for the open Senate seat in Kansas. Given that Dr. Marshall has reiterated his desire to continue to work with the physician community to ensure access to care for our patients, AGA looks forward to supporting Dr. Marshall’s Senate candidacy and continuing to work with him and his office on issues and initiatives to advance the science and practice of gastroenterology.
Active cancer increases death risk in patients with COVID-19
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
FROM ASCO 2020
Key clinical point: Patients with progressing cancer and COVID-19 are at an especially high risk of 30-day mortality.
Major finding: Patients with COVID-19 whose cancers were progressing had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients in remission or with no evidence of cancer.
Study details: Analysis of data on 928 patients enrolled in the COVID-19 and Cancer Consortium (CCC19) registry.
Disclosures: The research was supported, in part, by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed relationships with HemOnc.org, IBM, and Westat.
Source: Warner J L et al. ASCO 2020, Abstract LBA110.
Adjuvant osimertinib extends DFS in localized NSCLC
, results of the ADAURA trial showed.
The randomized, phase 3 trial was a comparison of osimertinib treatment with placebo following complete resection of localized or locally advanced NSCLC with negative margins. The trial was unblinded early and halted on the recommendation of the independent data-monitoring committee, due to the efficacy of osimertinib.
“If I were on the committee, I would have done the same thing. These are extraordinary results,” said study investigator Roy S. Herbst, MD, PhD, chief of medical oncology at the Yale Cancer Center and Smilow Cancer Center at Yale University in New Haven, Conn.
Dr. Herbst is scheduled to present results from ADAURA as part of the American Society of Clinical Oncology virtual scientific program.
In an online briefing prior to the meeting, Dr. Herbst said the impressive results reminded him of a lesson imparted by his mentor, the late Isaiah Fidler, DVM, PhD.
“He taught me, he taught all of us, that metastasis is a spread of tumor that kills patients,” Dr. Herbst said. “Drugs such as this, based on biology, given to patients at the earliest possible time, prevent those metastases and allow patients to live longer and with a better quality of life.”
Results from the ADAURA trial provide compelling evidence of the benefit of adjuvant osimertinib for a select group of patients, according to Tina Cascone, MD, PhD, assistant professor in the department of thoracic head and neck medical oncology at The University of Texas MD Anderson Cancer Center in Houston. She was not involved in the study.
“These are unprecedented results for a potentially curable, resected population of patients,” Dr. Cascone said in an interview. “This definitely has the potential to shift the paradigm in the treatments that we have available for patients with resected disease. It’s very important to emphasize how much we’ve learned from the metastatic setting and how we’re bringing what we’ve learned into early stage disease.”
High recurrence rates
An estimated 30% of patients with NSCLC present with resectable disease at diagnosis, but 5-year recurrence rates following surgery and cisplatin-based adjuvant chemotherapy remain high, ranging from 45% among patients with stage IB disease to 62% for patients with stage II NSCLC and 76% for patients with stage III disease, Dr. Herbst noted.
Osimertinib is a third-generation tyrosine kinase inhibitor (TKI) targeted to EGFR. It has been shown to offer improvements in both progression-free survival and overall survival compared with the EGFR-TKIs erlotinib and gefitinib for patients with advanced EGFR-mutated NSCLC, as well as in patients with central nervous system metastases.
Osimertinib’s efficacy and safety profile against advanced disease suggests it may also be effective against early stage disease, a hypothesis the ADAURA trial was designed to test.
Study details
The phase 3, randomized, double-blind trial was conducted at centers in the United States, Europe, Asia, and Australia. A total of 682 patients with completely resected stage IB, II, or IIIA NSCLC, with or without planned adjuvant chemotherapy, were enrolled.
After stratification by stage, EGFR mutation, and race (Asian vs. non-Asian), patients were randomized on a 1:1 basis to receive either osimertinib at 80 mg once daily or placebo. The planned treatment duration was a maximum of 3 years.
Members of the independent data-monitoring committee held a meeting in April 2020. Although they had not planned an efficacy analysis at that time, they decided the results were clearly in favor of osimertinib. So they recommended unblinding and halting of the trial.
At the time of unblinding, the study had completed enrollment, and all patients had been followed for at least 1 year.
Efficacy and safety
For the primary endpoint of disease-free survival (DFS) in patients with stage II to IIIA disease, the median DFS was not reached for patients assigned to osimertinib, but it was 20.4 months for patients assigned to placebo (hazard ratio, 0.17; P < .0001).
The numbers were similar for the secondary endpoint of DFS in the overall population, including patients with stage IB disease. The median DFS was not reached for patients on osimertinib but was 28.1 months for patients on placebo (HR, 0.21; P < .0001).
DFS was significantly superior with osimertinib across all subgroups in the overall population, including sex, age, smoking status, race, stage, EGFR mutation, and adjuvant chemotherapy (yes or no).
Dr. Herbst said patients tolerated osimertinib well, and the drug’s safety profile was consistent with that already known. There were no adverse events leading to death in the osimertinib arm, and the incidence of grade 3 or 4 adverse events of any kind was low.
In all, 10 patients (3%) in the osimertinib arm were reported to have interstitial lung disease. Prolongation of the QT interval was reported in 22 patients (7%) on osimertinib and 4 patients (1%) in the placebo arm.
The results show that “adjuvant osimertinib provides a highly effective, practice-changing treatment for patients with stage IB, II, IIIA, EGFR mutation-positive non–small cell lung cancer after complete tumor resection,” Dr. Herbst said.
Dr. Herbst disclosed relationships with AstraZeneca, which funded the study, as well as Jun Shi Pharmaceuticals and other companies. Dr. Cascone is the international principal investigator of the NeoCOAST trial evaluating durvalumab, an AstraZeneca product.
SOURCE: Herbst RS et al. ASCO 2020, Abstract LBA5.
, results of the ADAURA trial showed.
The randomized, phase 3 trial was a comparison of osimertinib treatment with placebo following complete resection of localized or locally advanced NSCLC with negative margins. The trial was unblinded early and halted on the recommendation of the independent data-monitoring committee, due to the efficacy of osimertinib.
“If I were on the committee, I would have done the same thing. These are extraordinary results,” said study investigator Roy S. Herbst, MD, PhD, chief of medical oncology at the Yale Cancer Center and Smilow Cancer Center at Yale University in New Haven, Conn.
Dr. Herbst is scheduled to present results from ADAURA as part of the American Society of Clinical Oncology virtual scientific program.
In an online briefing prior to the meeting, Dr. Herbst said the impressive results reminded him of a lesson imparted by his mentor, the late Isaiah Fidler, DVM, PhD.
“He taught me, he taught all of us, that metastasis is a spread of tumor that kills patients,” Dr. Herbst said. “Drugs such as this, based on biology, given to patients at the earliest possible time, prevent those metastases and allow patients to live longer and with a better quality of life.”
Results from the ADAURA trial provide compelling evidence of the benefit of adjuvant osimertinib for a select group of patients, according to Tina Cascone, MD, PhD, assistant professor in the department of thoracic head and neck medical oncology at The University of Texas MD Anderson Cancer Center in Houston. She was not involved in the study.
“These are unprecedented results for a potentially curable, resected population of patients,” Dr. Cascone said in an interview. “This definitely has the potential to shift the paradigm in the treatments that we have available for patients with resected disease. It’s very important to emphasize how much we’ve learned from the metastatic setting and how we’re bringing what we’ve learned into early stage disease.”
High recurrence rates
An estimated 30% of patients with NSCLC present with resectable disease at diagnosis, but 5-year recurrence rates following surgery and cisplatin-based adjuvant chemotherapy remain high, ranging from 45% among patients with stage IB disease to 62% for patients with stage II NSCLC and 76% for patients with stage III disease, Dr. Herbst noted.
Osimertinib is a third-generation tyrosine kinase inhibitor (TKI) targeted to EGFR. It has been shown to offer improvements in both progression-free survival and overall survival compared with the EGFR-TKIs erlotinib and gefitinib for patients with advanced EGFR-mutated NSCLC, as well as in patients with central nervous system metastases.
Osimertinib’s efficacy and safety profile against advanced disease suggests it may also be effective against early stage disease, a hypothesis the ADAURA trial was designed to test.
Study details
The phase 3, randomized, double-blind trial was conducted at centers in the United States, Europe, Asia, and Australia. A total of 682 patients with completely resected stage IB, II, or IIIA NSCLC, with or without planned adjuvant chemotherapy, were enrolled.
After stratification by stage, EGFR mutation, and race (Asian vs. non-Asian), patients were randomized on a 1:1 basis to receive either osimertinib at 80 mg once daily or placebo. The planned treatment duration was a maximum of 3 years.
Members of the independent data-monitoring committee held a meeting in April 2020. Although they had not planned an efficacy analysis at that time, they decided the results were clearly in favor of osimertinib. So they recommended unblinding and halting of the trial.
At the time of unblinding, the study had completed enrollment, and all patients had been followed for at least 1 year.
Efficacy and safety
For the primary endpoint of disease-free survival (DFS) in patients with stage II to IIIA disease, the median DFS was not reached for patients assigned to osimertinib, but it was 20.4 months for patients assigned to placebo (hazard ratio, 0.17; P < .0001).
The numbers were similar for the secondary endpoint of DFS in the overall population, including patients with stage IB disease. The median DFS was not reached for patients on osimertinib but was 28.1 months for patients on placebo (HR, 0.21; P < .0001).
DFS was significantly superior with osimertinib across all subgroups in the overall population, including sex, age, smoking status, race, stage, EGFR mutation, and adjuvant chemotherapy (yes or no).
Dr. Herbst said patients tolerated osimertinib well, and the drug’s safety profile was consistent with that already known. There were no adverse events leading to death in the osimertinib arm, and the incidence of grade 3 or 4 adverse events of any kind was low.
In all, 10 patients (3%) in the osimertinib arm were reported to have interstitial lung disease. Prolongation of the QT interval was reported in 22 patients (7%) on osimertinib and 4 patients (1%) in the placebo arm.
The results show that “adjuvant osimertinib provides a highly effective, practice-changing treatment for patients with stage IB, II, IIIA, EGFR mutation-positive non–small cell lung cancer after complete tumor resection,” Dr. Herbst said.
Dr. Herbst disclosed relationships with AstraZeneca, which funded the study, as well as Jun Shi Pharmaceuticals and other companies. Dr. Cascone is the international principal investigator of the NeoCOAST trial evaluating durvalumab, an AstraZeneca product.
SOURCE: Herbst RS et al. ASCO 2020, Abstract LBA5.
, results of the ADAURA trial showed.
The randomized, phase 3 trial was a comparison of osimertinib treatment with placebo following complete resection of localized or locally advanced NSCLC with negative margins. The trial was unblinded early and halted on the recommendation of the independent data-monitoring committee, due to the efficacy of osimertinib.
“If I were on the committee, I would have done the same thing. These are extraordinary results,” said study investigator Roy S. Herbst, MD, PhD, chief of medical oncology at the Yale Cancer Center and Smilow Cancer Center at Yale University in New Haven, Conn.
Dr. Herbst is scheduled to present results from ADAURA as part of the American Society of Clinical Oncology virtual scientific program.
In an online briefing prior to the meeting, Dr. Herbst said the impressive results reminded him of a lesson imparted by his mentor, the late Isaiah Fidler, DVM, PhD.
“He taught me, he taught all of us, that metastasis is a spread of tumor that kills patients,” Dr. Herbst said. “Drugs such as this, based on biology, given to patients at the earliest possible time, prevent those metastases and allow patients to live longer and with a better quality of life.”
Results from the ADAURA trial provide compelling evidence of the benefit of adjuvant osimertinib for a select group of patients, according to Tina Cascone, MD, PhD, assistant professor in the department of thoracic head and neck medical oncology at The University of Texas MD Anderson Cancer Center in Houston. She was not involved in the study.
“These are unprecedented results for a potentially curable, resected population of patients,” Dr. Cascone said in an interview. “This definitely has the potential to shift the paradigm in the treatments that we have available for patients with resected disease. It’s very important to emphasize how much we’ve learned from the metastatic setting and how we’re bringing what we’ve learned into early stage disease.”
High recurrence rates
An estimated 30% of patients with NSCLC present with resectable disease at diagnosis, but 5-year recurrence rates following surgery and cisplatin-based adjuvant chemotherapy remain high, ranging from 45% among patients with stage IB disease to 62% for patients with stage II NSCLC and 76% for patients with stage III disease, Dr. Herbst noted.
Osimertinib is a third-generation tyrosine kinase inhibitor (TKI) targeted to EGFR. It has been shown to offer improvements in both progression-free survival and overall survival compared with the EGFR-TKIs erlotinib and gefitinib for patients with advanced EGFR-mutated NSCLC, as well as in patients with central nervous system metastases.
Osimertinib’s efficacy and safety profile against advanced disease suggests it may also be effective against early stage disease, a hypothesis the ADAURA trial was designed to test.
Study details
The phase 3, randomized, double-blind trial was conducted at centers in the United States, Europe, Asia, and Australia. A total of 682 patients with completely resected stage IB, II, or IIIA NSCLC, with or without planned adjuvant chemotherapy, were enrolled.
After stratification by stage, EGFR mutation, and race (Asian vs. non-Asian), patients were randomized on a 1:1 basis to receive either osimertinib at 80 mg once daily or placebo. The planned treatment duration was a maximum of 3 years.
Members of the independent data-monitoring committee held a meeting in April 2020. Although they had not planned an efficacy analysis at that time, they decided the results were clearly in favor of osimertinib. So they recommended unblinding and halting of the trial.
At the time of unblinding, the study had completed enrollment, and all patients had been followed for at least 1 year.
Efficacy and safety
For the primary endpoint of disease-free survival (DFS) in patients with stage II to IIIA disease, the median DFS was not reached for patients assigned to osimertinib, but it was 20.4 months for patients assigned to placebo (hazard ratio, 0.17; P < .0001).
The numbers were similar for the secondary endpoint of DFS in the overall population, including patients with stage IB disease. The median DFS was not reached for patients on osimertinib but was 28.1 months for patients on placebo (HR, 0.21; P < .0001).
DFS was significantly superior with osimertinib across all subgroups in the overall population, including sex, age, smoking status, race, stage, EGFR mutation, and adjuvant chemotherapy (yes or no).
Dr. Herbst said patients tolerated osimertinib well, and the drug’s safety profile was consistent with that already known. There were no adverse events leading to death in the osimertinib arm, and the incidence of grade 3 or 4 adverse events of any kind was low.
In all, 10 patients (3%) in the osimertinib arm were reported to have interstitial lung disease. Prolongation of the QT interval was reported in 22 patients (7%) on osimertinib and 4 patients (1%) in the placebo arm.
The results show that “adjuvant osimertinib provides a highly effective, practice-changing treatment for patients with stage IB, II, IIIA, EGFR mutation-positive non–small cell lung cancer after complete tumor resection,” Dr. Herbst said.
Dr. Herbst disclosed relationships with AstraZeneca, which funded the study, as well as Jun Shi Pharmaceuticals and other companies. Dr. Cascone is the international principal investigator of the NeoCOAST trial evaluating durvalumab, an AstraZeneca product.
SOURCE: Herbst RS et al. ASCO 2020, Abstract LBA5.
FROM ASCO 2020
Key clinical point: Adjuvant osimertinib extended disease-free survival, compared with placebo, in patients with EGFR-mutated non–small cell lung cancer.
Major finding: In the overall population, the median disease-free survival was not reached for patients on osimertinib and was 28.1 months for patients on placebo (hazard ratio, 0.21, P < .0001).
Study details: Randomized, double-blind, phase 3 trial of 682 patients with stage IB-IIIA non–small cell lung cancer bearing EGFR mutations.
Disclosures: Dr. Herbst disclosed relationships with AstraZeneca, which funded the study, as well as Jun Shi Pharmaceuticals and other companies.
Source: Herbst RS et al. ASCO 2020, Abstract LBA5.