User login
Cartilage Sutures for a Large Nasal Defect
Practice Gap
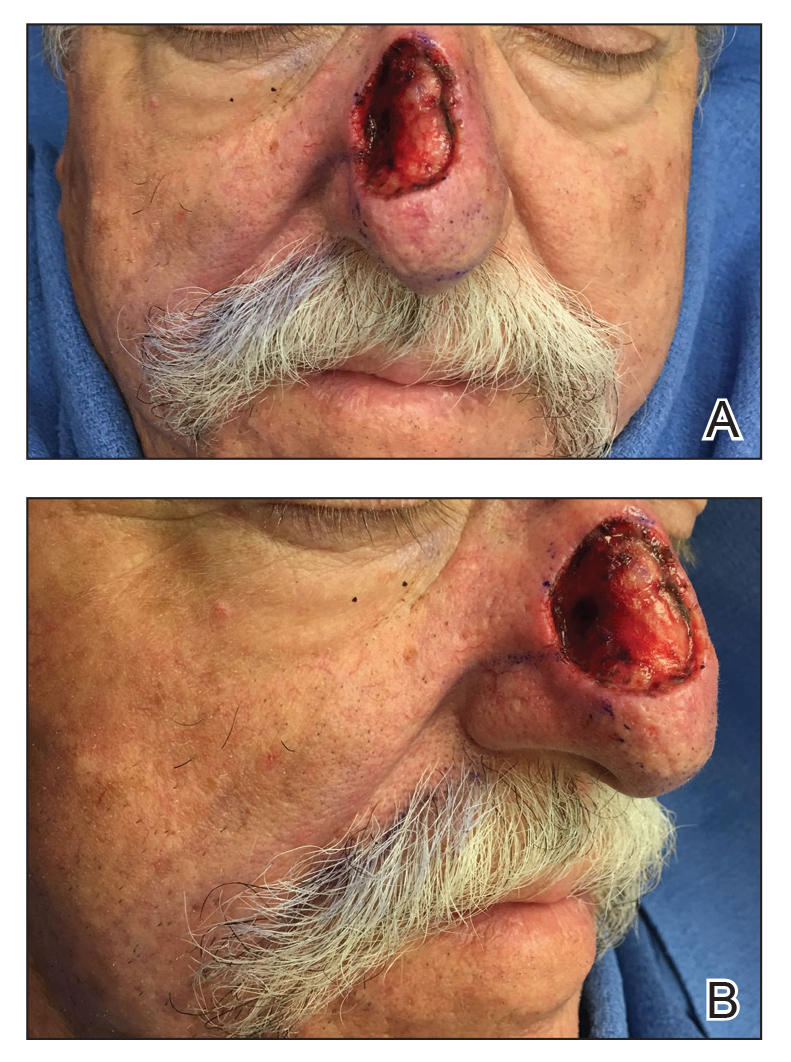
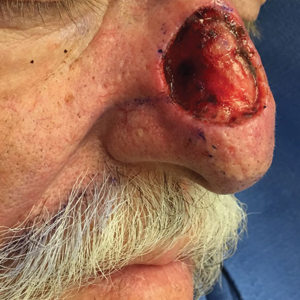
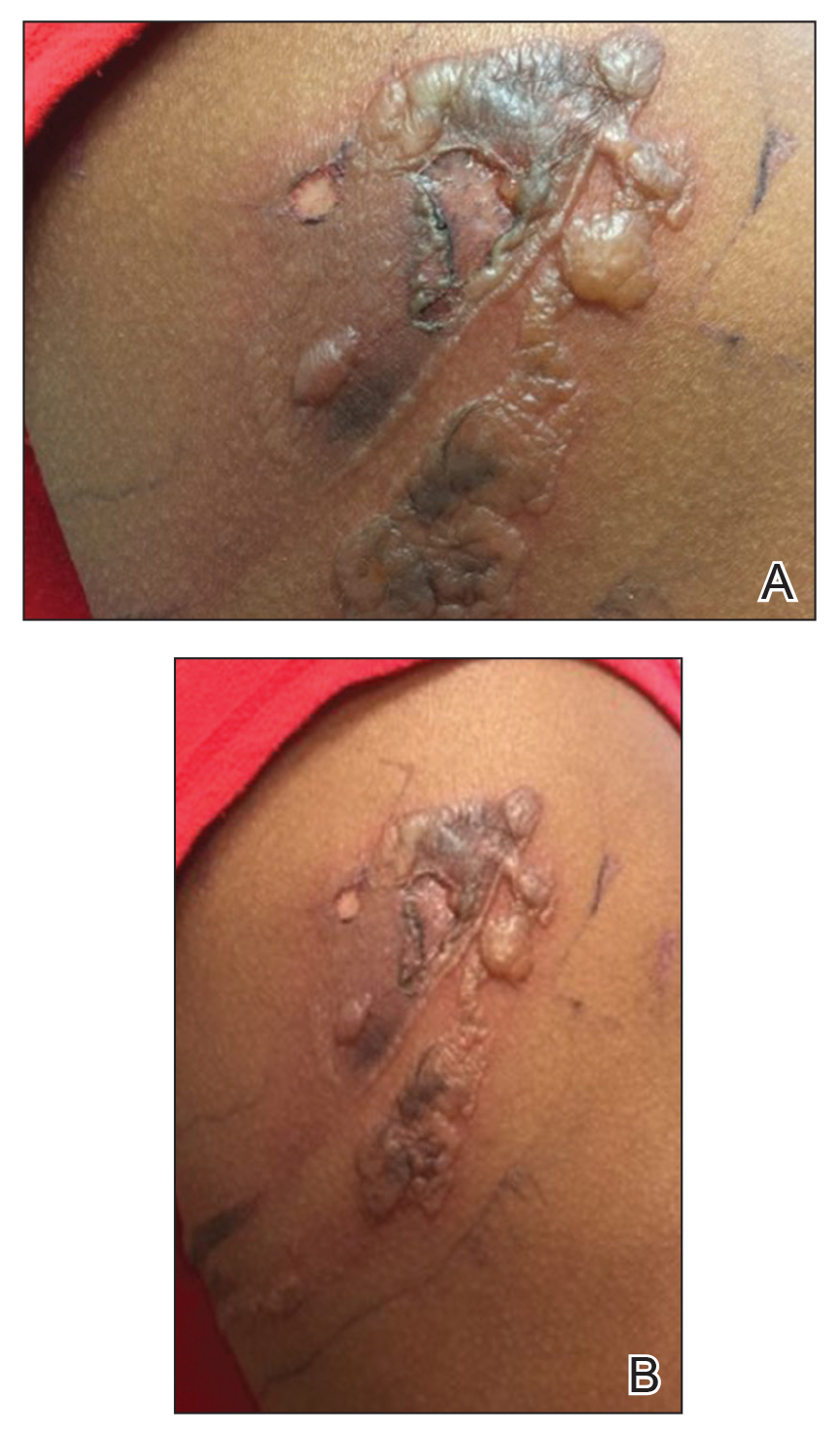
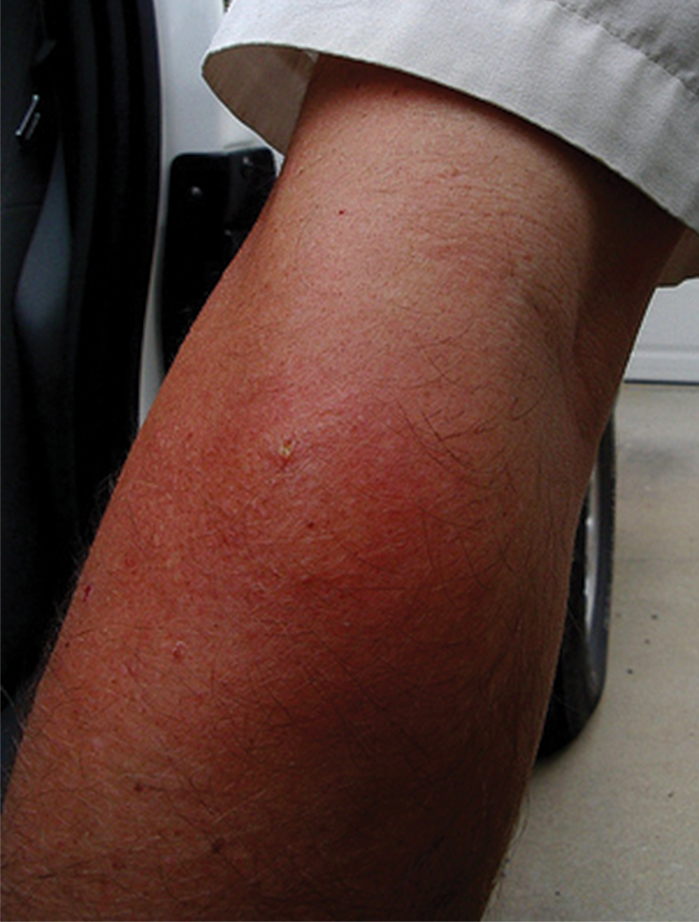
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
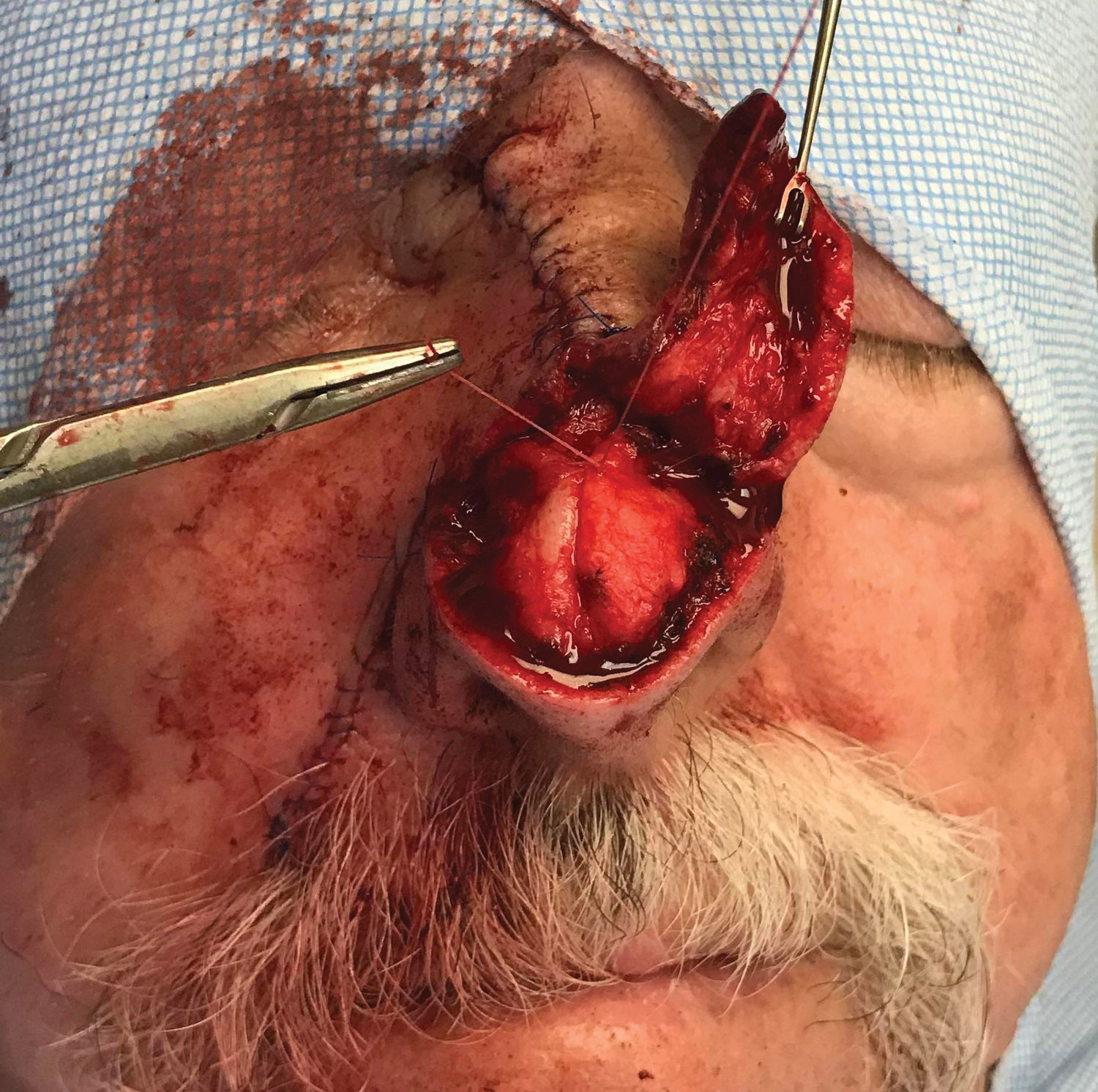
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
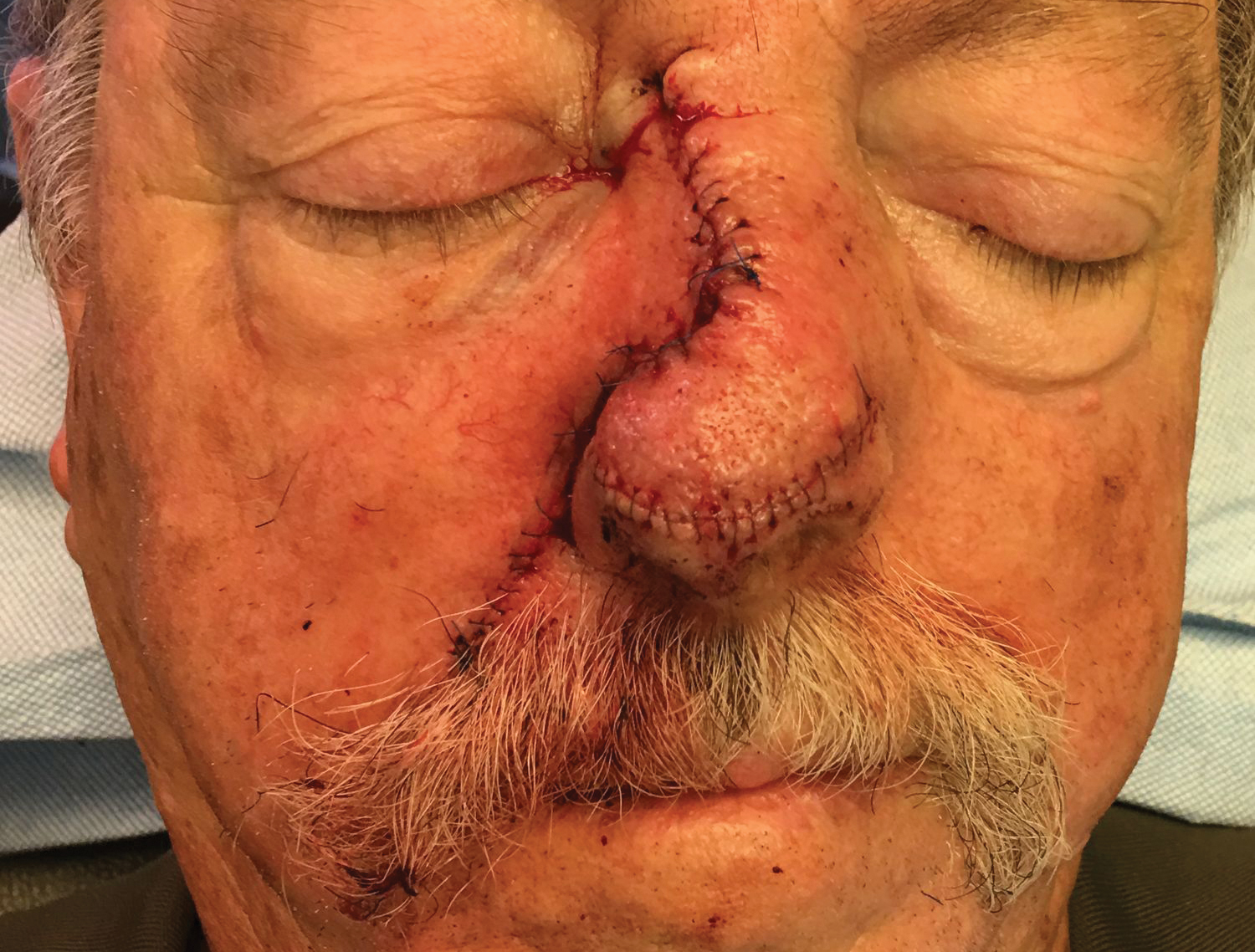
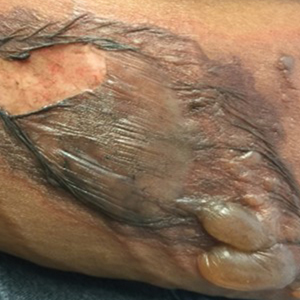
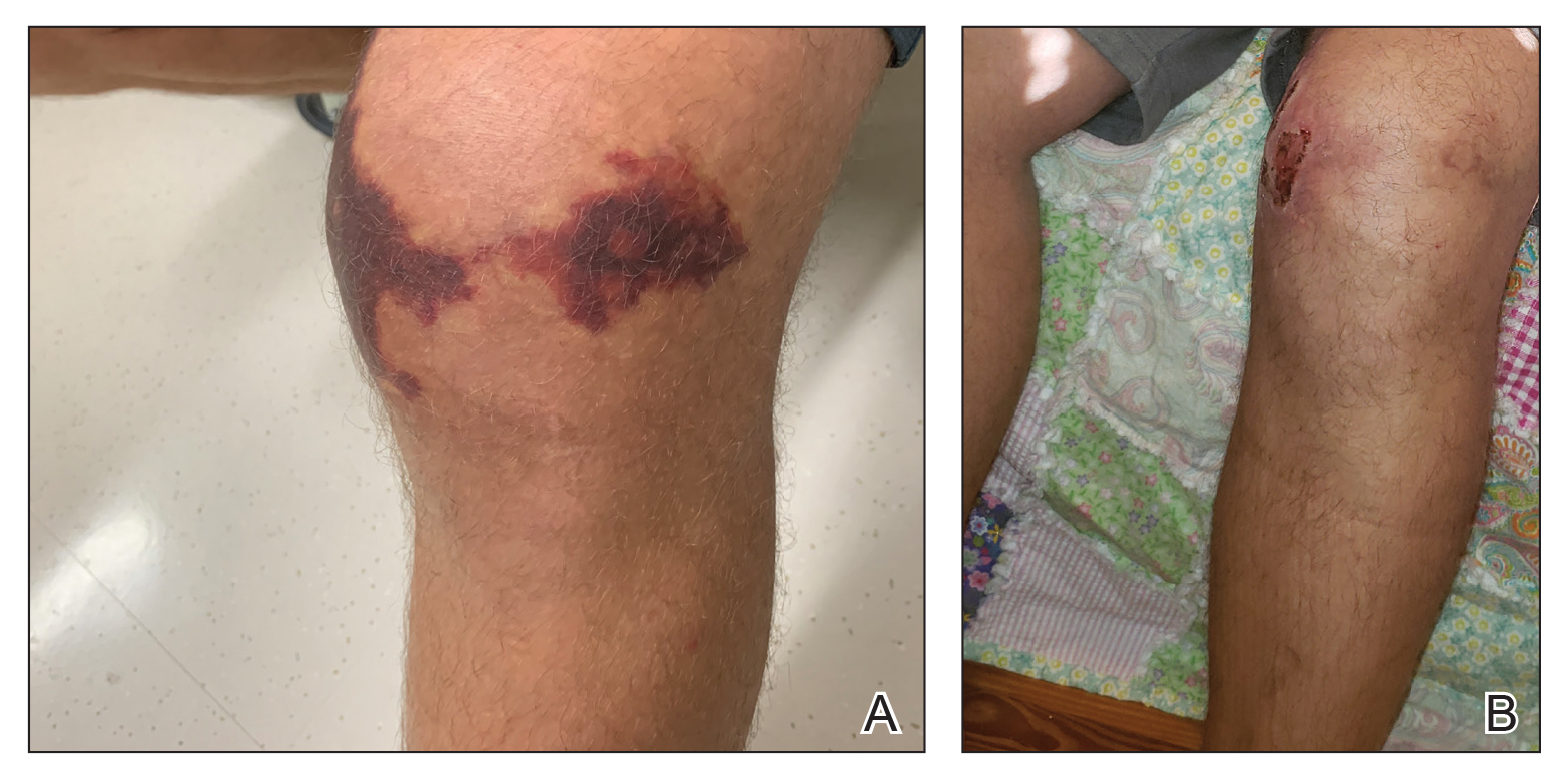
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
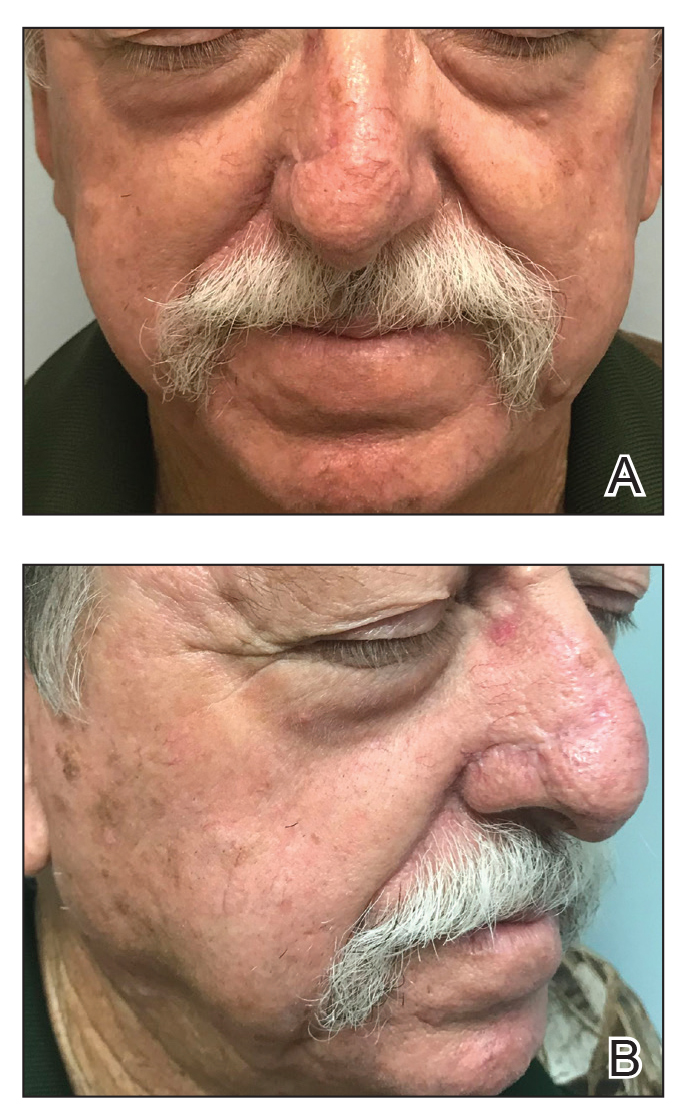
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
Practice Gap
A 69-year-old man underwent staged excision for an invasive melanoma (0.4-mm Breslow depth; stage Ia) of the right dorsal nose. Two stages were required to achieve clear margins, leaving a 3.0×2.5-cm defect involving the nasal dorsum, right nasal sidewall, and nasal supratip (Figure 1). He declined any multistage repair and preferred a full-thickness skin graft (FTSG) over any interpolation flap.
Given the size of our patient’s defect, primary repair was not possible and second intention healing may have resulted in a suboptimal cosmetic outcome, potential alar distortion, and prolonged healing. No single local flap, such as the dorsal nasal rotation flap, crescentic advancement flap, bilobed flap, and Rintala flap, would have provided adequate coverage. A FTSG of the entire defect would not have been an ideal tissue match, and given the limited surrounding laxity, a Burow FTSG would have required the linear repair to extend well into the forehead with a questionable cosmetic outcome.
The Technique
We opted to repair the defect using a combination of local flaps for a single-stage repair. Using the right cheek reservoir, a crescentic advancement flap was performed to restore the right nasal sidewall as best as possible with a standing cone taken superiorly. To execute this flap, an incision was made extending from the alar sulcus into the nasolabial fold while preserving the apical triangle of the upper cutaneous lip. The flap was elevated submuscularly on the nose, and broad undermining was performed in the subcutaneous plane of the medial cheek. A crescentic redundancy above the alar sulcus was excised, and periosteal tacking sutures were placed to both help advance the flap and to recreate the nasofacial sulcus.1
Next, a nasal tip spiral/rotation flap was designed to restore the remaining nasal defect.2 An incision was made at the right inferiormost aspect of the defect and extended along the inferior border of the nasal tip as it crossed the midline to the left side of the nose. After incising and elevating the flap in the submuscular plane, there was not enough of a tissue reservoir to cover the entire remaining nasal defect.
To resolve this intraoperative conundrum, simple interrupted sutures were placed into the nasal cartilage at midline to narrow the structure of the nose (Figure 2). Three 4-0 polyglactin 910 sutures were placed beginning with the upper lateral cartilages and extending inferiorly to the lower lateral cartilages. Narrowing the nasal cartilages allowed for a smaller residual defect. The nasal tip rotation flap was then spiraled into place with adequate coverage. Some of the flap tip was trimmed after the superior aspect of the rotation flap was sutured to the inferior edge of the crescentic advancement flap. The immediate postoperative appearance is shown in Figure 3.
At 4-month follow-up, intralesional triamcinolone was injected into the slight induration at the right nasal tip. At 7-month follow-up, the patient was pleased with the cosmetic and functional result (Figure 4).
Practice Implications
Cartilage sutures highlight an underutilized technique in nasal reconstruction, with few cases reported
A combination of local flaps may be used to repair large nasal defects involving multiple subunits, especially in patients who decline multistage reconstruction. A nasal tip rotation/spiral flap can be considered for the appropriate nasal tip defect. Suturing the nasal cartilage with either permanent or long-lasting suture can narrow the cartilage and facilitate flap coverage for nasal defects while also improving the appearance of patients with wide prominent lower noses.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
- Smith JM, Orseth ML, Nijhawan RI. Reconstruction of large nasal dorsum defects. Dermatol Surg. 2018;44:1607-1610.
- Snow SN. Rotation flaps to reconstruct nasal tip defects following Mohs surgery. Dermatol Surg. 1997;23:916-919.
- Malone CH, Hays JP, Tausend WE, et al. Interdomal sutures for nasal tip refinement and reduced wound size. J Am Acad Dermatol. 2017;77:E107-E108.
- Pelster MW, Behshad R, Maher IA. Large nasal tip defects-utilization of interdomal sutures before Burow’s graft for optimization of nasal contour. Dermatol Surg. 2019;45:743-746.
- Gruber RP, Chang E, Buchanan E. Suture techniques in rhinoplasty. Clin Plast Surg. 2010;37:231-243.
The Ketogenic Diet and Dermatology: A Primer on Current Literature
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
Practice Points
- The ketogenic diet has been employed since antiquity for varying ailments and has a good safety and efficacy profile if administered by a knowledgeable provider.
- New literature is showing promising potential roles for the ketogenic diet as an adjunctive therapy, particularly in the realm of inflammatory disorders, metabolic diseases, and malignancy.
- The dermatologist should be aware of this diet because it is gaining popularity with physicians and patients alike. Dermatologists also should know how it can potentially benefit a number of patients with dermatologic diseases based on small clinical trials, population studies, and basic science research.
A Comparison of Knowledge Acquisition and Perceived Efficacy of a Traditional vs Flipped Classroom–Based Dermatology Residency Curriculum
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
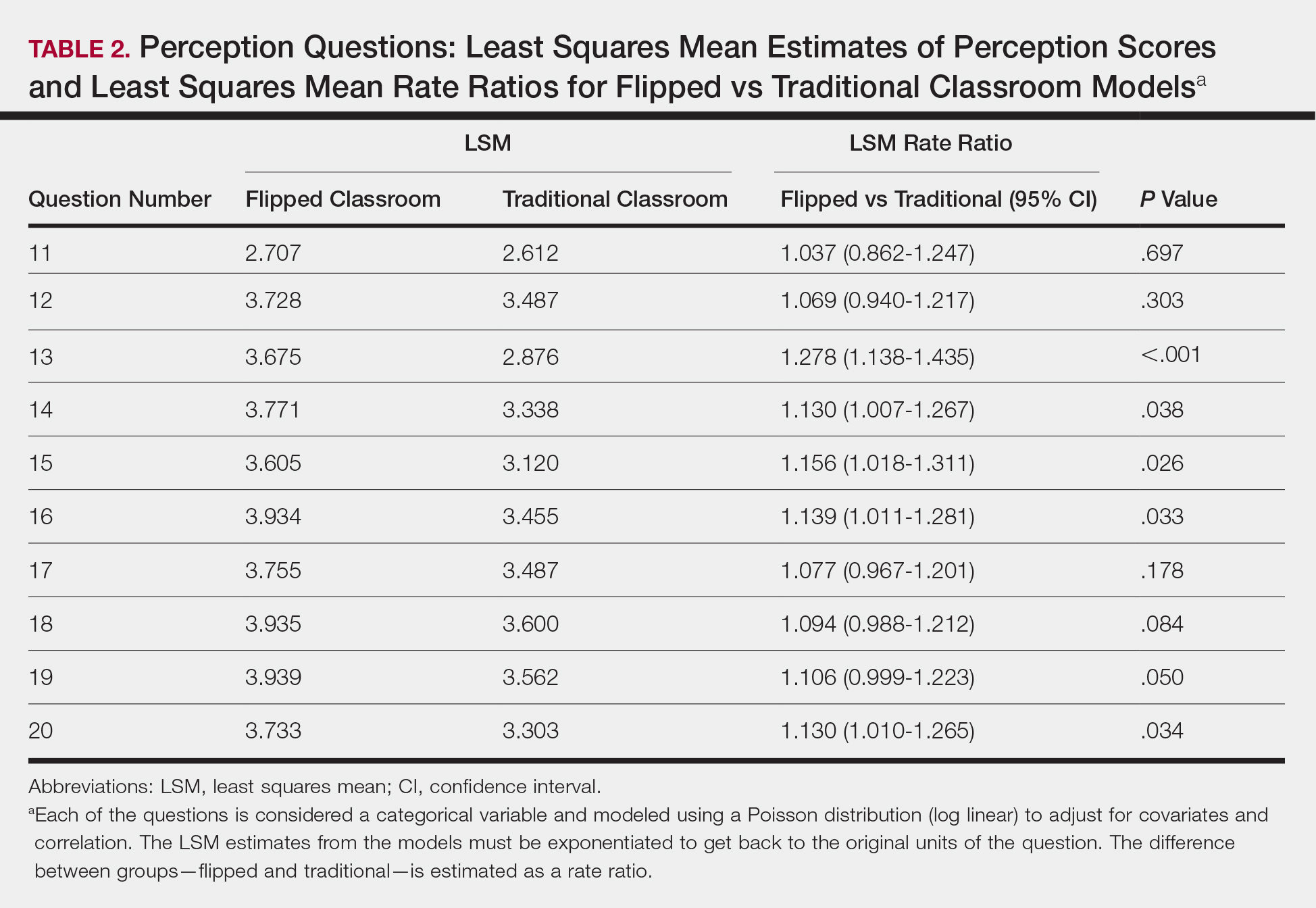
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).
Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).

Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).

Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
Practice Points
- There was not a significant difference in dermatology resident factual quiz scores when comparing flipped vs traditional classroom teaching sessions.
- There was a significant difference between the flipped vs traditional teaching models, with dermatology residents favoring the flipped classroom, for co-resident lecture participation and individual and co-resident enjoyment of the lecture.
- Residents also perceived that the flipped classroom sessions were more efficient, better prepared them for boards, and better prepared them for clinical practice.
Mystery Burns and Nocturnal Seizure Safety
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
Patients with seizures are placed at an increased risk for sustaining burn injuries, which may occur during common daily activities such as cooking, showering, and using heaters.1 Although patients are warned of the risks of injury at the time of their epilepsy diagnosis, patients still experience injuries that commonly occur during the seizure or the postictal phase. In a study of 134 patients with epilepsy, only 38% recalled being burned during a seizure, with approximately 9% being burned multiple times.2 Another study investigated the circumstances resulting in burns in this patient population and found that cooking on a stove was the most common cause, followed by hot water while showering and exposed room heaters.1 Another study found that the majority of burns in seizure patients were from spilled hot drinks.3
We report 2 patients who presented to the dermatology clinic with second-degree burns following nocturnal seizures. In both cases, the patients were sleeping next to exposed heaters, which led to burn injuries from seizures that occurred in the night.
Case Reports
Patient 1
A 30-year-old woman with a history of a seizure disorder presented with painful second-degree blistering burns along the left arm and flank (Figure 1). One day prior to presentation, she had woken up to find these lesions and visited the emergency department where she was prescribed silver sulfadiazine cream to prevent infection of the wound site and was referred to our dermatology clinic. Initially, the patient had difficulty pinpointing the source of the burn lesions and thought that it may have been due to sleeping with her cell phone, but she later realized that they were due to the space heater placed next to her bed. Because of the unclear etiology at the initial presentation, a skin biopsy of a lesion was taken while she was at the clinic.
Biopsy of the lesions exhibited separation of the epidermal and dermal layers (Figure 2). Thermal damage was seen extending into the dermal layers with notable edema present. A few inflammatory cells, neutrophils, and monocytes were noted in the biopsy. The initial pathology results showed the epidermis was necrotic with edema, spongiform vesicles, and few neutrophils. The histologic findings aligned with the timeline of the injury occurring 2 days prior to the biopsy. She was treated supportively using mupirocin ointment to prevent secondary infection.
Case 2
A 27-year-old woman with a history of epilepsy presented to the dermatology clinic with painful blistering lesions along the right upper arm (Figure 3). She was found to have notable second-degree burns along the right arm. She reported placing her bed near a baseboard heater to stay warm overnight. She noticed the painful lesions after waking up next to the heater following a suspected seizure. She was treated supportively using mupirocin ointment to prevent secondary infection.
Comment
Classification of Burns and Damage
According to the World Health Organization, nonfatal burn injuries are a leading cause of morbidity and occur mainly in the home and workplace.4 There are many types of burns: radiation, electrical, chemical, friction, and thermal. The most common type of burns are thermal burns,4 which can be further subdivided into wet and dry. Both of our patients experienced dry thermal burns.
Based on the skin tissue layers involved in the thermal damage, burn wounds are further divided into first-degree burns, superficial second-degree burns, deep second-degree burns, and third-degree burns.5 These classifications each have characteristic gross features. Based on these criteria, our patients both presented with blistering and ruptured bullae and no eschar formation, which is classified as second-degree superficial burns.
Following thermal insult to the skin, 3 zones are formed. The central zone consists of irreparable damage referred to as the zone of coagulation. The zone of stasis lies between the completely damaged central region and the outermost regions of the burn lesion, and it receives slightly less blood flow. This area can fully recover after complete perfusion is returned early in the healing process. The outermost zone of hyperemia can fully recover and is an area marked by intense vasodilation from inflammatory reactions.5
Wound Healing
During the healing process, metabolic activity is remarkably increased, which leads to formation of
Burns in Patients With Seizure Disorders
Burns pose a serious risk to patients with seizure disorders that often is underappreciated by patients and health care providers. Although many burns are first-degree burns, up to 10% of burns require medical attention.1 In the initial phase following a thermal insult, the skin’s microflora is killed off, but within a week the sterile skin can become infected.5 The most common microbial invasions seen in blistering wounds are due to Pseudomonas aeruginosa and Staphylococcus aureus.8 With larger burns associated with immunocompromising factors such as diabetes mellitus or older age, patients are at an increased risk for becoming septic. Prior to the period of infection, the damage caused by the heat leads to vasodilation of the microvasculature surrounding the injured area. In addition, release of cytokines leads to migration of inflammatory cells. With the vasodilation of vasculature, proteinaceous fluids from the intravascular space can collect between the dead epidermal and dermal layers to form blisters.5 In larger burns, the fluid shifts will lead to severe oncotic pressure decreases intravascularly and can lead to hypotensive shock.6 When burns have a more severe global effect, aggressive resuscitation and vasopressors are required to maintain perfusion of vital organs.
Both of our patients experienced painful lesions, but they were fortunate to have factors of youth, superficial damage, and low total body surface area burns for a smaller risk for infection, fluid loss, and severely disfiguring scars.8 Because the duration of the postictal phase can vary, there is potential for more severe burns that can leave a lifelong reminder of the event. Depending on the skin type and the depth of the thermal insult, evidence of injury may last many years in the form of hypertrophic scars, contractures, and changes in skin pigmentation.5 At distances 30 cm or less from the standard blow-dryer, it takes 2 minutes to cause cell death.9 In comparison to a heat source that is meant to provide warmth to a room, there is a notable difference in potential for severe burns with the standard heater vs the standard blow-dryer.
Along with the physical pain, the visual reminders of the injurious event can have notable psychological effects. Scars can decrease self-esteem and lead to depression, anxiety, body image problems, and sexuality issues.10
Given the immense risks associated with burn injuries and the many unfortunate outcomes, emphasis should be placed on patient education regarding safety precautions with seizure disorders. In one study, it was found that only 5% of patients recall receiving a warning about the risk for burn injuries with seizures.2 It is important for patients and physicians to develop a written comprehensive safety plan that addresses the risks for daily activities during the day and night. Although patients may not remember being told about the risks, a written safety plan likely will increase patient awareness and reduce avoidable injuries. In addition to written safety plans, prior recommendations for reducing burn injuries in seizure patients include the use of fire and heater guards as well as flame-retardant clothing and blankets.11
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
- Spitz MC, Towbin JA, Shantz D, et al. Risk factors for burns as a consequence of seizures in persons with epilepsy. Epilepsia. 1994;35:764-767.
- Hampton KK, Peatfield RC, Pullar T, et al. Burns because of epilepsy. Br Med J (Clin Res Ed). 1988;296:1659-1660.
- Kinton L, Duncan JS. Frequency, causes, and consequences of burns in patients with epilepsy. J Neurol Neurosurg Psychiatry. 1998;65:404-405.
- World Health Organization. Burns. http://www.who.int/news-room/fact-sheets/detail/burns. Published March 6, 2018. Accessed December 13, 2019.
- Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg. 2012;45:364-373.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:E469-E481.
- Travers JB, Murphy RC, Johnson CA, et al. Identification and pharmacological characterization of platelet-activating factor and related 1-palmitoyl species found in human inflammatory blistering diseases. Prostaglandins Other Lipid Mediat. 1998;5:305-324.
- Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434.
- Aslam A, Khoo CT. No sense; no sensibility—a tale of two adult hair-drier burns. Burns. 1997;23:454-457.
- Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars: epidemiology and management. Am J Clin Dermatol. 2003;4:245-272.
- Josty IC, Narayanan V, Dickson WA. Burns in patients with epilepsy: changes in epidemiology and implications for burn treatment and prevention. Epilepsia. 2000;41:453-456.
Practice Points
- Burns and scars from burns can lead to both life-threatening consequences and lifelong psychological effects.
- Many epileptic patients who present with thermal burn injuries do not remember getting burned.
- Clinicians should be aware of all the potential dangers that patients with epilepsy may encounter both during the day and night.
Is Artificial Intelligence Going to Replace Dermatologists?
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
Practice Points
- The use of computer-assisted diagnosis in medicine dates back to the 1960s in radiology.
- New techniques in machine learning, also known as deep learning, were introduced around 2010. Compared to the predecessor forms of computing, these new methods are dynamically changing systems that improve with continuous data exposure and therefore performance is dependent on the quality and generalizability of the training data sets.
- Standardized large data sets and prospective real-life clinical trials are lacking in radiology and subsequently dermatology for diagnosis.
- Artificial intelligence is helpful with triaging and is improving workflow efficiency for radiologists by helping prioritize tasks, which is the current direction for dermatology.
Hyperbaric Oxygen Therapy in Dermatology
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
Hyperbaric oxygen therapy (HOT) is a treatment modality dating to 1861 in the United States.1 Today, there are 14 indications2 for HOT (Table), issued by the Undersea & Hyperbaric Medical Society, which also administers an accreditation program for facilities providing HOT.3 The 14 indications also are relevant because it is unlikely that HOT will be covered by insurance for unapproved indications.4
Although HOT is not commonly seen as a first-line intervention in dermatology, there are scenarios in which it can be used to good effect: compromised grafts and flaps; poorly healing ulceration related to vasculitis and autoimmune disorders; and possibly for vascular compromise, including cutaneous ischemia caused by fillers. We review its indications, dermatologic applications, and potential complications.
Overview of HOT
Hyperbaric oxygen therapy involves sitting or lying in a special chamber that allows for controlled levels of oxygen (O2) at increased atmospheric pressure, which specifically involves breathing near 100% O2 while inside a monoplace or multiplace chamber5 that is pressurized to greater than sea level pressure (≥1.4 atmosphere absolute).2
A monoplace chamber is designed to treat a single person (Figure 1); a multiplace chamber (Figure 2) accommodates as many as 5 to 25 patients.5,6 The chambers also accommodate hospital beds and medical attendants, if needed. Hyperbaric O2 is inhaled through a mask, a tight-fitting hood, or an endotracheal tube, depending on the patient’s status.7 Treatment ranges from only 1 or 2 iterations for acute conditions to 30 sessions or more for chronic conditions. Individual sessions last 45 minutes to 5 hours; 120 minutes is considered a safe maximum duration.7 A television often is provided to help the patient pass the time.8
Long-standing Use in Decompression Sickness
Hyperbaric oxygen therapy is best known for its effectiveness in treating decompression sickness (DCS) and carbon monoxide poisoning. Decompression sickness involves liberation of free gas from tissue, in the form of bubbles, when a person experiences a relative decrease in atmospheric pressure, which results in an imbalance in the sum of gas tensions in tissue compared to ambient pressure.
Decompression sickness has special military significance because it can affect divers and pilots, particularly those flying at high altitude. Over the course of 12 years, approximately 50 pilot trainees at an Air Force training site in Colorado required HOT when ground-level O2 failed to resolve their DCS symptoms.10
Symptoms of DCS range from musculoskeletal pain to severe neurologic and pulmonary complications. First-line therapy for DCS is 100% O2 at ground level. When symptoms are severe or persistent, HOT is the treatment of choice. It works by decreasing the volume of air bubbles (as predicted by Boyle’s Law), providing oxygenation to hypoxic tissue and mitigating inflammatory responses implicated in tissue injury9; HOT can be considered salvage treatment for rare, severe, or unresponsive complications of DCS during common activities such as diving and flying.
The emergent nature of DCS often necessitates an on-call, on-site HOT facility or contracted community services. Although DCS is a rare complication, it can be devastating, as was the case for a military pilot flying an ultrahigh altitude reconnaissance aircraft.11 He developed a near fatal case of neurologic DCS during a military mission and required treatment with emergent HOT. Although his symptoms were reduced with therapy, he has persistent cognitive deficits.11
Other Indications
Dermatologic Flaps and Grafts
Although less commonly discussed in dermatologic literature, the use of HOT in compromised grafts and flaps has been addressed in the plastic surgery literature. In a large multicenter study, researchers evaluated 20,821 Mohs micrographic surgery procedures and reported 149 adverse events, of which 20.1% were dehiscence and partial or full necrosis.12 These complications, though rare, are potentially devastating, particularly in cosmetically sensitive locations such as the face. Traditional care for compromised grafts and flaps includes local wound care, surgical debridement, and additional reconstructive procedures. These interventions can be expensive and uncomfortable for patients and carry risk for further morbidity.13
Grafts become compromised when their metabolic demand outpaces the ability of the recipient bed due to characteristics of the graft or the recipient bed or both. Flaps carry their own blood supply, which can be compromised if the flap is too long or too large for the pedicle, there is notable tension on the wound, or blood flow is mechanically obstructed by kinking or twisting. Under these conditions, HOT can be beneficial, as O2 dissolves in plasma, thus improving the O2 tissue cellular diffusion gradient.7 An increased level of systemic O2 promotes wound healing and graft or flap survival by improving fibroblast function, blood flow, and vascularity, and by mitigating ischemia-reperfusion injury.13
Radiation-Induced Ulceration
Radionecrosis, a complication of radiotherapy, is caused by progressive obliterating endarteritis with resultant vascular stenosis and fibroatrophy, which eventually cause stromal fibrosis.15 In a study that looked at 1267 nonmelanoma skin cancers that had been treated with radiotherapy, the ulceration rate was 6.3%. Most of the ulcerated lesions were treatable conservatively, but some were more treatment resistant.16 Hampson et al17 reported on 58 patients with cutaneous wounds due to soft-tissue radionecrosis who were treated with HOT as part of a larger observational case series in which investigators looked at multiple types of radionecrosis. They found that 76% of these patients improved: 26% showed complete resolution and the remaining 50% had 50% to 90% improvement.17
Vasculitis or Autoimmune Ulceration
Vasculitis and vasculopathy can occur independent of, or in association with, connective tissue disease and can result in chronic ulceration. At our institution, a patient with antimelanoma differentiation-associated protein 5 dermatomyositis who had refractory digital ulcerations despite intensive systemic therapy had an excellent response to HOT; ulcerations resolved after 37 treatments.18
Efrati et al19 reported on 35 patients who had chronic nonhealing vasculitic ulcerations despite immunosuppression medication who were treated with HOT. Twenty-eight patients completely healed, 4 had partial healing, and 3 had no improvement.
Mirasoglu et al20 reported on a case series of 6 systemic sclerosis patients who had ulcerations that persisted despite other treatments. After initiation of HOT, 4 patients experienced complete response and 2 experienced partial response, which is notable because such ulcerations are often extremely difficult to treat and have usually failed multiple therapies before being addressed with HOT.
Cutaneous Vascular Compromise
At our institution, a 36-year-old man was referred to the dermatology clinic 2 days after undergoing embolization of a symptomatic arteriovenous malformation in the right knee (Figure 3A). The procedure was complicated by cutaneous purpura concerning for necrosis, a known complication of this procedure. We referred the patient for evaluation to consider HOT. Although he was outside the ideal window for starting treatment, HOT was initiated. With a late start in treatment, areas of skin had already progressed to full necrosis, which did not respond to treatment; however, contiguous areas that initially looked very similar clinically did respond to treatment (Figure 3B). This case suggests a penumbralike effect in which vulnerable tissue that would most likely have been lost was salvaged by HOT.
Ischemia
Hyperbaric oxygen therapy has been used to treat ischemia caused by injection of cosmetic filler. Henderson et al21 described a 37-year-old woman who experienced occlusion of the left superficial temporal artery while self-injecting a hyaluronic acid filler around the temples. The problem was complicated by left-sided hearing loss, cutaneous blanching of the left face, and pain. She was treated with enoxaparin, aspirin, dexamethasone, antibiotics, and intradermal lidocaine. Additionally, she was urgently referred to a HOT facility and was treated with 6 HOT treatments in 3 days, with the first treatment provided 15 hours after the initial insult. The patient showed a decrease in ischemic discoloration over the course of the treatment. Eventually, her hearing returned to baseline and she achieved an acceptable cosmetic outcome.21
Uittenbogaard et al22 reported the treatment of a patient who experienced dermal ischemia after receiving calcium hydroxylapatite at an aesthetic clinic. She did not improve with standard treatment but subsequently experienced resolution of symptoms after treatment with HOT. She had an excellent cosmetic outcome at 6-month follow-up.22
Complications and a Contraindication
Hyperbaric oxygen therapy generally is safe, but there is potential for complications.
Fire
This rare risk has a catastrophic outcome.23 Standards for fire prevention in hyperbaric facilities are issued by the National Fire Protection Association, covering construction and building materials, lighting, electrical wiring, exposure to flammable materials, and other possible ignition sources.24
Middle Ear Barotrauma
The incidence of the most common adverse effect of HOT is reported at 2% to 30%.7,25 Middle ear barotrauma occurs most commonly during the compression phase of treatment. It is more common in patients treated in a monoplace chamber because they are kept supine and are less able to regulate middle ear pressure.26 Symptoms of middle ear barotrauma can be relieved by teaching patients autoinflation technique, such as the Valsalva maneuver, or by placing tympanoplasty tubes.27
Reversible Myopia
Caused by direct O2 toxicity to the lens, this complication can last for weeks, though it eventually resolves spontaneously. Reversible myopia has been reported to be at least as common as middle ear barotrauma.27
Other Complications
Central nervous system complications, such as seizures, and pulmonary O2 toxicity are rare, more serious complications.27
Untreated Pneumothorax
The only absolute contraindication to HOT, pneumothorax can decompensate during HOT if left untreated. However, HOT can proceed once pneumothorax is addressed.7
Conclusion
Hyperbaric O2 therapy can make a positive contribution to the dermatologic therapeutic armamentarium, in specific patients, for impending graft or flap failure, chronic wounds and ulcerations, and cutaneous vascular compromise. Although HOT is not a commonly needed treatment in dermatology, it is important to be aware of its potential because delay in treatment can decrease its effectiveness. It is recommended that dermatologists locate the nearest HOT facility and become familiar with its capabilities.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
- Carney AY. Hyperbaric oxygen therapy: an introduction. Crit Care Nurs Q. 2013;36:274-279.
- Weaver LK, ed. Hyperbaric Oxygen Therapy Indications: The Hyperbaric Oxygen Therapy Committee Report. 13th ed. Undersea and Hyperbaric Medical Society. 2014.https://www.uhms.
org/images/indications/UHMS_HBO2_Indications
_13th_Ed._Front_Matter__References.pdf. Accessed December 18, 2019. - Undersea & Hyperbaric Medical Society. UHMS Hyperbaric Facility Accreditation Program. https://www.uhms.org/about/accreditation/accreditation-for-hyperbaric-medicine.html. Accessed December 18, 2019.
- Hyperbaric oxygen (HBO) therapy. US Centers for Medicare & Medicaid Services. https://www.medicare.gov/coverage/hyperbaric-oxygen-hbo-therapy. Accessed December 18, 2019.
- Gracia L, Perez-Vidal C, de Paco JM, et al. Identification and control of a multiplace hyperbaric chamber. PLoS One. 2018;13:e0200407.
- Monoplace vs multiplace hyperbaric chamber. CutisCare. https://cutiscareusa.com/hyperbaric-oxygen-therapy/monoplace-vs-multiplace-hyperbaric-chamber/. Published August 31, 2018. Accessed December 18, 2019.
- Leach RM, Rees PJ, Wilmshurst PP. Hyperbaric oxygen therapy. BMJ. 1998;317:1140-1143.
- Health Quality Ontario. Hyperbaric oxygen therapy for the treatment of diabetic foot ulcers: a health technology assessment. Ont Health Technol Assess Ser. 2017;17:1-142.
- Vann RD, Butler FK, Mitchell SJ, et al. Decompression illness. Lancet. 2011;377:153-164.
- Rhodes WC, Hertner G, Price R, et al. Treating decompression sickness: military flight simulation site-community hospital partnership. Mil Med. 2017;182:e1718-e1721.
- Jersey SL, Baril RT, McCarty RD, et al. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med. 2010;81:64-68.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Francis A, Baynosa RC. Hyperbaric oxygen therapy for the compromised graft or flap. Adv Wound Care (New Rochelle). 2017;6:23-32.
- Bowersox JC, Strauss MB, Hart GB. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperb Med. 1986;1:141-149.
- Fernández Canedo I, Padilla España L, Francisco Millán Cayetano J, et al. Hyperbaric oxygen therapy: an alternative treatment for radiation-induced cutaneous ulcers. Australas J Dermatol. 2018;59:e203-e207.
- Schulte KW, Lippold A, Auras C, et al. Soft x-ray therapy for cutaneous basal cell and squamous cell carcinomas. J Am Acad Dermatol. 2005;53:993-1001.
- Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
- Jeter J, Wolf EG, Richards M, et al. Successful treatment of anti-MDA5 dermatomyositis associated cutaneous digital pulp ulcerations with hyperbaric oxygen therapy [published online August 21, 2019]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001114.
- Efrati S, Bergan J, Fishlev G, et al. Hyperbaric oxygen therapy for nonhealing vasculitic ulcers. Clin Exp Dermatol. 2007;32:12-17.
- Mirasoglu B, Bagli BS, Aktas S. Hyperbaric oxygen therapy for chronic ulcers in systemic sclerosis—case series. Int J Dermatol. 2017;56:636-640.
- Henderson R, Reilly DA, Cooper JS. Hyperbaric oxygen for ischemia due to injection of cosmetic fillers: case report and issues. Plast Reconstr Surg Glob Open. 2018;6:e1618.
- Uittenbogaard D, Lansdorp CA, Bauland CG, et al. Hyperbaric oxygen therapy for dermal ischemia after dermal filler injection with calcium hydroxylapatite: a case report. Undersea Hyperb Med. 2019;46:207-210.
- Schorow S. The air in there. NFPA Journal. January 3, 2017. https://www.nfpa.org/News-and-Research/Publications-and-media/NFPA-Journal/2017/January-February-2017/Features/Hyperbaric-chambers. Accessed December 18, 2019.
- National Fire Protection Association. NFPA 99: Health Care Facilities Code 2018. https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=99. Accessed December 18, 2019.
- Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol. 1996;21:400-403.
- Lima MA, Farage L, Cury MC, et al. Update on middle ear barotrauma after hyperbaric oxygen therapy—insights on pathophysiology. Int Arch Otorhinolaryngol. 2014;18:204-209.
- Heyboer M, Sharma D, Santiago W, et al. Hyperbaric oxygen therapy: side effects defined and quantified. Adv Wound Care (New Rochelle). 2017;6:210-224.
Practice Points
- Hyperbaric oxygen therapy can be considered for the treatment of failing cutaneous grafts and flaps, chronic ulcerations caused by vasculitis or autoimmune disorders, and vascular compromise, including cutaneous ischemia caused by fillers.
- Hyperbaric oxygen therapy involves 1- to 2-hour treatments, 5 days a week, for as long as 1 month.
- Hyperbaric oxygen therapy is safe and well-tolerated, with few contraindications. The sooner therapy is started, the greater the potential for benefit.
What’s Eating You? Vespids Revisited
Identification
The Hymenoptera order of insects includes Apidae (bees), Vespidae (wasps, yellow jackets, hornets), and Formicidae (fire ants). All 3 of these families of insects inject venom into their prey or as a defense mechanism via ovipositors in their abdomen. Vespids are the most aggressive and are found in each of the United States.1 They have membranous wings, broad antennae, and a nonbarbed stinger (Figure 1).2 The nonbarbed stinger of Vespidae differentiates them from Apidae and allows these insects to sting their prey multiple times. Vespids can build nests in the ground (yellow jackets), trees (hornets), or areas of cover such as window shutters (mud wasps). Because only the queens survive winter, larger populations do not develop until late summer when the most stings take place. Stings most often take place near the nest of the vespid or while the victim is eating outdoors.3
Envenomation
When vespids sting their prey they inject venom via their ovipositors.1 The venom is composed of a mixture of low-molecular-weight proteins, kinins, proteolytic enzymes, lipids, carbohydrates, and high-molecular-weight proteins that act as allergens.1,4,5 The proteolytic enzymes degrade the surrounding tissue, basophils become activated, and histamine is released secondary to mast cell degranulation, which results in vasodilation and an inflammatory response characterized by edema, erythema, warmth, and pain.1 The pain of the sting is immediate and can be intense; almost all victims are acutely aware of the discomforting sensation.4
Management of Reactions
Three types of reactions can be seen after a vespid sting: uncomplicated local reactions, large local reactions, and systemic reactions (SRs). The most common reaction is the self-limiting uncomplicated local reaction that includes a focal area of warmth, edema, erythema, induration, and tenderness.1 Treatment of this kind of reaction is supportive, with ice, nonsteroidal anti-inflammatory drugs, and H1 and H2 blockers being commonly used methods. Large local reactions (Figure 2) are similar to uncomplicated local reactions but are greater than 10 cm in diameter and last longer. The same symptomatic treatment may be used along with possible short (3–5 days) oral glucocorticoid (40–60 mg prednisone) or potent topical steroid administration if symptoms persist. Systemic reactions involve IgE-mediated generalized urticaria, angioedema, face swelling, stridor, bronchospasm, nausea, vomiting, flushing, and respiratory distress.1 Emergency management includes maintenance of airway, breathing, and circulation. Epinephrine injection commonly is employed and should be given via intramuscular injection into the anterolateral thigh; a dose of 0.3 to 0.5 mg can be repeatedly injected every 5 to 15 minutes, as needed.1
If an individual has an SR, it is recommended to go to an emergency department after stabilization for monitoring. Referral to an allergist for desensitization is appropriate. A radioallergosorbent test to measure allergen-specific IgE can be helpful to confirm an allergy.4 This test also should be done weeks after the incident because during the first few days IgE may be too low to measure. Once the allergy is confirmed, the desensitization with venom immunotherapy (VIT) can begin. Venom immunotherapy is effective and reduces a patient’s risk for recurrent SRs to less than 5% to 20%.6 A 2015 study recommended longer duration of VIT therapy due to risk for repeat SRs after discontinuing therapy. This study concluded that VIT is to be administered for 5 years, unless the patient is at high risk for SRs after VIT therapy—risk factors include older age, cardiopulmonary disease, SR during VIT treatment, mast cell disorders, and elevated serum tryptase—in which case VIT may have to be continued indefinitely. It is recommended that all patients with history of SR carry an epinephrine autoinjector in case of emergency.6
Epidemiologic data show a prevalence of 0.3% to 7.5% for self-reported SRs due to stings, with lower prevalence in children (0.15%–0.3%).4,7 An additional study looking at data from an allergy practice determined 24% of all cases of anaphylaxis were due to insect stings.5
Conclusion
Although many vespid stings can be managed symptomatically, it is imperative for patients and providers to be aware of the possible severe reactions that can take place. It is essential for providers to be aware of how to care for and treat large local reactions and SRs, as symptom recognition and timely treatment can improve patient safety and result in better outcomes.
- Arif F, Williams M. Hymenoptera Stings (Bee, Vespids and Ants). Treasure Island, FL: StatPearls Publishing LLC; 2019. https://www.ncbi.nlm.nih.gov/books/NBK518972/. Updated April 20, 2019. Accessed December 11, 2019.
- Elston, DM. Life-threatening stings, bites, infestations, and parasitic diseases. Clin Dermatol. 2005;23:164-170.
- Ulrich RM, Gabrielle H, Arthur H. Allergic reactions to stinging and biting insects. In: Rich RR, Fleisher T, Shearer W, et al, eds. Clinical Immunology: Principles and Practice. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2008:657-666.
- Biló BM, Rueff F, Mosbech H, et al. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
- Schafer T, Przybilla B. IgE antibodies to hymenoptera venoms in the serum are common in the general population and are related to indication of atopy. Allergy. 1996;51:372-377.
- Ulrich MR, Johannes R. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol. 2015;3:324-328.
- Abrishami MH, Boyd GK, Settipane GA. Prevalence of bee sting allergy in 2010 girl scouts. Acta Allergol. 1971;26:117-120.
Identification
The Hymenoptera order of insects includes Apidae (bees), Vespidae (wasps, yellow jackets, hornets), and Formicidae (fire ants). All 3 of these families of insects inject venom into their prey or as a defense mechanism via ovipositors in their abdomen. Vespids are the most aggressive and are found in each of the United States.1 They have membranous wings, broad antennae, and a nonbarbed stinger (Figure 1).2 The nonbarbed stinger of Vespidae differentiates them from Apidae and allows these insects to sting their prey multiple times. Vespids can build nests in the ground (yellow jackets), trees (hornets), or areas of cover such as window shutters (mud wasps). Because only the queens survive winter, larger populations do not develop until late summer when the most stings take place. Stings most often take place near the nest of the vespid or while the victim is eating outdoors.3
Envenomation
When vespids sting their prey they inject venom via their ovipositors.1 The venom is composed of a mixture of low-molecular-weight proteins, kinins, proteolytic enzymes, lipids, carbohydrates, and high-molecular-weight proteins that act as allergens.1,4,5 The proteolytic enzymes degrade the surrounding tissue, basophils become activated, and histamine is released secondary to mast cell degranulation, which results in vasodilation and an inflammatory response characterized by edema, erythema, warmth, and pain.1 The pain of the sting is immediate and can be intense; almost all victims are acutely aware of the discomforting sensation.4
Management of Reactions
Three types of reactions can be seen after a vespid sting: uncomplicated local reactions, large local reactions, and systemic reactions (SRs). The most common reaction is the self-limiting uncomplicated local reaction that includes a focal area of warmth, edema, erythema, induration, and tenderness.1 Treatment of this kind of reaction is supportive, with ice, nonsteroidal anti-inflammatory drugs, and H1 and H2 blockers being commonly used methods. Large local reactions (Figure 2) are similar to uncomplicated local reactions but are greater than 10 cm in diameter and last longer. The same symptomatic treatment may be used along with possible short (3–5 days) oral glucocorticoid (40–60 mg prednisone) or potent topical steroid administration if symptoms persist. Systemic reactions involve IgE-mediated generalized urticaria, angioedema, face swelling, stridor, bronchospasm, nausea, vomiting, flushing, and respiratory distress.1 Emergency management includes maintenance of airway, breathing, and circulation. Epinephrine injection commonly is employed and should be given via intramuscular injection into the anterolateral thigh; a dose of 0.3 to 0.5 mg can be repeatedly injected every 5 to 15 minutes, as needed.1
If an individual has an SR, it is recommended to go to an emergency department after stabilization for monitoring. Referral to an allergist for desensitization is appropriate. A radioallergosorbent test to measure allergen-specific IgE can be helpful to confirm an allergy.4 This test also should be done weeks after the incident because during the first few days IgE may be too low to measure. Once the allergy is confirmed, the desensitization with venom immunotherapy (VIT) can begin. Venom immunotherapy is effective and reduces a patient’s risk for recurrent SRs to less than 5% to 20%.6 A 2015 study recommended longer duration of VIT therapy due to risk for repeat SRs after discontinuing therapy. This study concluded that VIT is to be administered for 5 years, unless the patient is at high risk for SRs after VIT therapy—risk factors include older age, cardiopulmonary disease, SR during VIT treatment, mast cell disorders, and elevated serum tryptase—in which case VIT may have to be continued indefinitely. It is recommended that all patients with history of SR carry an epinephrine autoinjector in case of emergency.6
Epidemiologic data show a prevalence of 0.3% to 7.5% for self-reported SRs due to stings, with lower prevalence in children (0.15%–0.3%).4,7 An additional study looking at data from an allergy practice determined 24% of all cases of anaphylaxis were due to insect stings.5
Conclusion
Although many vespid stings can be managed symptomatically, it is imperative for patients and providers to be aware of the possible severe reactions that can take place. It is essential for providers to be aware of how to care for and treat large local reactions and SRs, as symptom recognition and timely treatment can improve patient safety and result in better outcomes.
Identification
The Hymenoptera order of insects includes Apidae (bees), Vespidae (wasps, yellow jackets, hornets), and Formicidae (fire ants). All 3 of these families of insects inject venom into their prey or as a defense mechanism via ovipositors in their abdomen. Vespids are the most aggressive and are found in each of the United States.1 They have membranous wings, broad antennae, and a nonbarbed stinger (Figure 1).2 The nonbarbed stinger of Vespidae differentiates them from Apidae and allows these insects to sting their prey multiple times. Vespids can build nests in the ground (yellow jackets), trees (hornets), or areas of cover such as window shutters (mud wasps). Because only the queens survive winter, larger populations do not develop until late summer when the most stings take place. Stings most often take place near the nest of the vespid or while the victim is eating outdoors.3
Envenomation
When vespids sting their prey they inject venom via their ovipositors.1 The venom is composed of a mixture of low-molecular-weight proteins, kinins, proteolytic enzymes, lipids, carbohydrates, and high-molecular-weight proteins that act as allergens.1,4,5 The proteolytic enzymes degrade the surrounding tissue, basophils become activated, and histamine is released secondary to mast cell degranulation, which results in vasodilation and an inflammatory response characterized by edema, erythema, warmth, and pain.1 The pain of the sting is immediate and can be intense; almost all victims are acutely aware of the discomforting sensation.4
Management of Reactions
Three types of reactions can be seen after a vespid sting: uncomplicated local reactions, large local reactions, and systemic reactions (SRs). The most common reaction is the self-limiting uncomplicated local reaction that includes a focal area of warmth, edema, erythema, induration, and tenderness.1 Treatment of this kind of reaction is supportive, with ice, nonsteroidal anti-inflammatory drugs, and H1 and H2 blockers being commonly used methods. Large local reactions (Figure 2) are similar to uncomplicated local reactions but are greater than 10 cm in diameter and last longer. The same symptomatic treatment may be used along with possible short (3–5 days) oral glucocorticoid (40–60 mg prednisone) or potent topical steroid administration if symptoms persist. Systemic reactions involve IgE-mediated generalized urticaria, angioedema, face swelling, stridor, bronchospasm, nausea, vomiting, flushing, and respiratory distress.1 Emergency management includes maintenance of airway, breathing, and circulation. Epinephrine injection commonly is employed and should be given via intramuscular injection into the anterolateral thigh; a dose of 0.3 to 0.5 mg can be repeatedly injected every 5 to 15 minutes, as needed.1
If an individual has an SR, it is recommended to go to an emergency department after stabilization for monitoring. Referral to an allergist for desensitization is appropriate. A radioallergosorbent test to measure allergen-specific IgE can be helpful to confirm an allergy.4 This test also should be done weeks after the incident because during the first few days IgE may be too low to measure. Once the allergy is confirmed, the desensitization with venom immunotherapy (VIT) can begin. Venom immunotherapy is effective and reduces a patient’s risk for recurrent SRs to less than 5% to 20%.6 A 2015 study recommended longer duration of VIT therapy due to risk for repeat SRs after discontinuing therapy. This study concluded that VIT is to be administered for 5 years, unless the patient is at high risk for SRs after VIT therapy—risk factors include older age, cardiopulmonary disease, SR during VIT treatment, mast cell disorders, and elevated serum tryptase—in which case VIT may have to be continued indefinitely. It is recommended that all patients with history of SR carry an epinephrine autoinjector in case of emergency.6
Epidemiologic data show a prevalence of 0.3% to 7.5% for self-reported SRs due to stings, with lower prevalence in children (0.15%–0.3%).4,7 An additional study looking at data from an allergy practice determined 24% of all cases of anaphylaxis were due to insect stings.5
Conclusion
Although many vespid stings can be managed symptomatically, it is imperative for patients and providers to be aware of the possible severe reactions that can take place. It is essential for providers to be aware of how to care for and treat large local reactions and SRs, as symptom recognition and timely treatment can improve patient safety and result in better outcomes.
- Arif F, Williams M. Hymenoptera Stings (Bee, Vespids and Ants). Treasure Island, FL: StatPearls Publishing LLC; 2019. https://www.ncbi.nlm.nih.gov/books/NBK518972/. Updated April 20, 2019. Accessed December 11, 2019.
- Elston, DM. Life-threatening stings, bites, infestations, and parasitic diseases. Clin Dermatol. 2005;23:164-170.
- Ulrich RM, Gabrielle H, Arthur H. Allergic reactions to stinging and biting insects. In: Rich RR, Fleisher T, Shearer W, et al, eds. Clinical Immunology: Principles and Practice. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2008:657-666.
- Biló BM, Rueff F, Mosbech H, et al. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
- Schafer T, Przybilla B. IgE antibodies to hymenoptera venoms in the serum are common in the general population and are related to indication of atopy. Allergy. 1996;51:372-377.
- Ulrich MR, Johannes R. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol. 2015;3:324-328.
- Abrishami MH, Boyd GK, Settipane GA. Prevalence of bee sting allergy in 2010 girl scouts. Acta Allergol. 1971;26:117-120.
- Arif F, Williams M. Hymenoptera Stings (Bee, Vespids and Ants). Treasure Island, FL: StatPearls Publishing LLC; 2019. https://www.ncbi.nlm.nih.gov/books/NBK518972/. Updated April 20, 2019. Accessed December 11, 2019.
- Elston, DM. Life-threatening stings, bites, infestations, and parasitic diseases. Clin Dermatol. 2005;23:164-170.
- Ulrich RM, Gabrielle H, Arthur H. Allergic reactions to stinging and biting insects. In: Rich RR, Fleisher T, Shearer W, et al, eds. Clinical Immunology: Principles and Practice. 3rd ed. St. Louis, MO: Mosby/Elsevier; 2008:657-666.
- Biló BM, Rueff F, Mosbech H, et al. Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339-1349.
- Schafer T, Przybilla B. IgE antibodies to hymenoptera venoms in the serum are common in the general population and are related to indication of atopy. Allergy. 1996;51:372-377.
- Ulrich MR, Johannes R. When can immunotherapy for insect sting allergy be stopped? J Allergy Clin Immunol. 2015;3:324-328.
- Abrishami MH, Boyd GK, Settipane GA. Prevalence of bee sting allergy in 2010 girl scouts. Acta Allergol. 1971;26:117-120.
Practice Points
- Most vespid stings can be managed with nonsteroidal anti-inflammatory drugs, ice, and antihistamines.
- For systemic reactions, prompt recognition and initiation of intramuscular epinephrine is recommended.
- In patients with confirmed allergy, recent data now suggest at least 5 years of venom immunotherapy and potentially lifelong for specific patients.
Dermatology Continuing Certification Changes for the Better
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
Major changes in continuing board certification are occurring across medical specialties. On January 6, 2020, the American Board of Dermatology (ABD) launches its new web-based longitudinal assessment program called CertLink (https://abderm.mycertlink.org/).1 This new platform is designed to eventually replace the sit-down, high-stakes, once-every-10-year medical knowledge examination that dermatologists take to remain board certified. With this alternative, every participating dermatologist will receive a batch of 13 web-based questions every quarter that he/she may answer at a convenient time and place. Questions are answered one at a time or in batches, depending on the test taker’s preference, and can be completed on home or office computers (and eventually on smartphones). Participating in this type of testing does not require shutting down practice, traveling to a test center, or paying for expensive board review courses. CertLink is designed to be convenient, affordable, and relevant to an individual’s practice.
How did the ABD arrive at CertLink?
The ABD launched its original Maintenance of Certification (MOC) program in 2006. Since then, newly board-certified dermatologists, recertifying dermatologists with time-limited certificates, and time-unlimited dermatologists who volunteered to participate in MOC have experienced the dermatology MOC program. In its first 10 years, the program was met with very mixed reviews. The program was designed to assess and promote competence in a 4-part framework, including professionalism; commitment to lifelong learning and self-assessment; demonstration of knowledge, judgment, and skills; and improvement in medical practice. All 4 are areas of rational pursuit for medical professionals seeking to perform and maintain the highest quality patient care possible. But there were problems. First iterations are rarely perfect, and dermatology MOC was no exception.
At the onset, the ABD chose to oversee the MOC requirements and remained hands off in the delivery of education, relying instead on other organizations to fulfill the ABD’s requirements. Unfortunately, with limited educational offerings available, many diplomates paid notable registration fees for each qualifying MOC activity. Quality improvement activities were a relatively new experience for dermatologists and were time consuming. Required medical record reviews were onerous, often requiring more than 35 data points to be collected per medical record reviewed. The limited number and limited diversity of educational offerings also created circumstances in which the material covered was not maximally relevant to many participants. When paying to answer questions about patient populations or procedure types never encountered by the dermatologist who purchased the particular MOC activity, many asked the question “How does this make me a better doctor?” They were right to ask.
Cost, time commitment to participate in MOC, and relevance to practice were 3 key areas of concern for many dermatologists. In response to internal and external MOC feedback, in 2015 the ABD took a hard look at its 10-year experience with MOC. While contemplating its next strategies, the ABD temporarily put its component 4—practice improvement—requirements on hold. After much review, the ABD decided to take over a notable portion of the education delivery. Its goal was to provide education that would fulfill MOC requirements in a more affordable, relevant, quicker, and easier manner.
First, the ABD made the decision to assume a more notable role as educator, in part to offer qualifying activities at no additional cost to diplomates. By taking on the role as educator, 3 major changes resulted: the way ABD approached quality improvement activities, partnership to initiate a question-of-the-week self-assessment program, and initiation of a longitudinal assessment strategy that resulted in this month’s launch of CertLink.
The ABD revolutionized its quality improvement requirements with the launch of its practice improvement modules made available through its website.2 These modules utilize recently published clinical practice gaps in 5 dermatology subspecialty domains to fulfill the practice improvement requirements. Participants read a brief synopsis of the supporting literature explaining practice improvement recommendations found in the module. Next, they find 5 patients in their practice with the condition, medication, or process in question and review whether they provided the care supported by the best available evidence. No module requires more than 5 medical records to review, and no more than 3 questions are answered per medical record review. If review confirms that the care was appropriate, no further action is needed. If a care gap is identified, then participants implement changes and later remeasure practice to detect any change. This certification activity was incredibly popular with the thousands of diplomates who have participated thus far; more than 97% stated the modules were relevant to practice, 98% stated they would recommend the modules to fellow dermatologists, and nearly 25% reported the module helped to change their practice for the better (unpublished data, July 2019). Relevance had been restored.
The ABD worked closely with the American Academy of Dermatology (AAD) to develop new education for weekly self-assessment. The ABD created the content and delivered to the AAD the first year of material for what would become the most successful and popular dermatology CME activity in history: the AAD Question of the Week (QOW). Thousands of dermatologists are registered to receive the QOW, with very active weekly participation. Participants receive 1 self-assessment point and 0.25 CME credits for each attempted question, right or wrong. This quizzing tool also was educational, with explanation of right answers and wrong choices included. The average amount of time spent answering each question was approximately 40 seconds. American Academy of Dermatology members can participate in its QOW as a member benefit. Self-assessment is no longer a time- consuming or costly process.
The third major change was the ABD initiation of the longitudinal assessment strategy called CertLink, a web-based testing platform operated by the American Board of Medical Specialties. Longitudinal assessment differs from traditional certification and recertification assessment. It allows the test taker to answer the certification test questions over time instead of all at once. Longitudinal assessment not only provides a greater level of convenience to the test taker but also allows boards a more continuous set of touch points in the assessment of diplomates over the course of the continuing certification period.
What will be part of CertLink?
In addition to standard multiple-choice questions, there are many interesting elements to the CertLink program, such as article-based questions. At the beginning of each year, dermatologists select 8 articles from a list of those hosted by CertLink. These are recently published articles, chosen for their meaningfulness to practicing dermatologists. Each subsequent quarter, 2 of these articles are issued to the diplomate to read at his/her leisure. Once ready, participants launch and answer 2 questions about the key points of each article. The article-based questions were designed to help the practicing dermatologist stay up-to-date and relevant in personally chosen areas.
Diplomates are offered a chance to learn from any question that was missed, with explanations or resources provided to help them understand why the correct answer is correct. In this new learn-to-competence model, diplomates are not penalized the first time they answer a particular question incorrectly. Each is provided an opportunity to learn through the explanations given, and then in a future quarter, the dermatologist is given a second chance to answer a similarly themed question, with only that second chance counting toward his/her overall score.
Another unique aspect of CertLink is the allowance of time off from assessment. The ABD recognizes that life happens, and that intermittent time off from career-long assessment will be necessary to accommodate life events, including but not limited to maternity leave, other medical leave, or mental health breaks. Diplomates may take off up to 1 quarter of testing each year to accommodate such life events. Those who need extra time (beyond 1 quarter per year) would need to communicate directly with ABD to request. Those who continue to answer questions throughout the year will have their lowest-performing quarter dropped, to maximize fairness to all. Only the top 3 quarters of CertLink test performance will be counted each year when making certification status decisions. Those who take 1 quarter off will have their other 3 quarters counted toward their scoring.
How will CertLink measure performance?
At the onset of CertLink, there is no predetermined passing score. It will take a few years for the ABD psychometricians to determine an acceptable performance. Questions are written not to be tricky but rather to assess patient issues the dermatologist is likely to encounter in practice. Article-based questions are designed to assess the key points of important recent articles to advance the dermatologist’s practice.
Final Thoughts
In the end, the ABD approach to the new area of continuing certification centers on strategies to be relevant, inexpensive, and minimally disruptive to practice, and to teach to competence and advance practice by bringing forward articles that address key recent literature. We think it is a much better approach to dermatology continuing certification.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
- ABD announces CertLink launch in 2020 [news release]. Newton, MA: American Board of Dermatology; 2019. https://www.abderm.org/public/announcements/certlink-2020.aspx. Accessed December 17, 2019.
- American Board of Dermatology. Focused practice improvement modules. https://www.abderm.org/diplomates/fulfilling-moc-requirements/abd-focused-pi-modules-for-moc.aspx. Accessed December 18, 2019.
Research on statin for preeclampsia prevention advances
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
EXPERT ANALYSIS FROM THE DPSG-NA 2019
SimLEARN Musculoskeletal Training for VHA Primary Care Providers and Health Professions Educators
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.