User login
COPD and asthma: Diagnostic accuracy requires spirometry
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
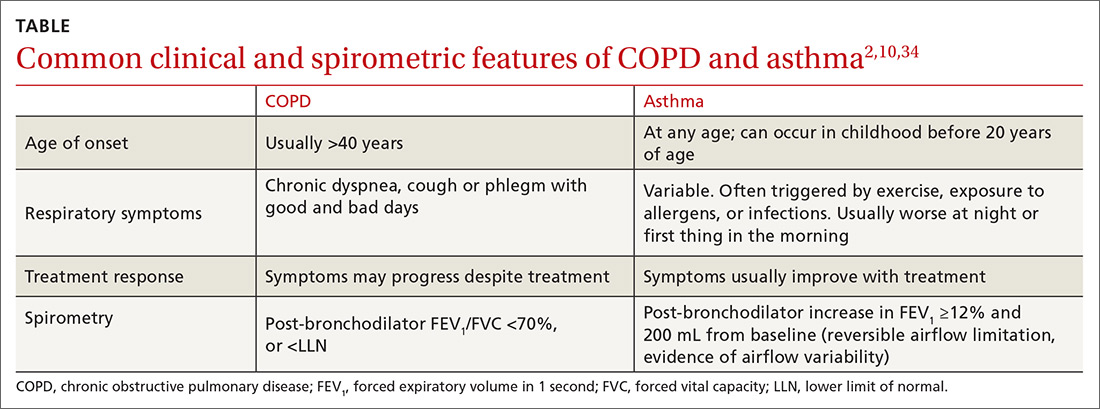
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; cwells2@uic.edu.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; cwells2@uic.edu.
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; cwells2@uic.edu.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
PRACTICE RECOMMENDATIONS
› Perform spirometry in all patients with symptoms and risk factors suggestive of chronic obstructive pulmonary disease (COPD) or asthma. B
› Consider having a patient use a peak flow meter to support a diagnosis of asthma if spirometry is unavailable. B
› Consider the possibility of a diagnostic error if COPD or asthma is unresponsive to treatment and the initial diagnosis was made without spirometry. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
KD025 produces durable responses in patients with cGVHD
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
REPORTING FROM TCT 2019
Anticoagulation in cancer
In this episode, Alok Khorana, MD, of the Cleveland Clinic, joins David H. Henry, MD, to discuss results from the CASSINI trial and what it means for anticoagulation in cancer patients.
And Ilana Yurkiewicz, MD, begins part 1 of her look at informed consent. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple Podcasts
In this episode, Alok Khorana, MD, of the Cleveland Clinic, joins David H. Henry, MD, to discuss results from the CASSINI trial and what it means for anticoagulation in cancer patients.
And Ilana Yurkiewicz, MD, begins part 1 of her look at informed consent. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple Podcasts
In this episode, Alok Khorana, MD, of the Cleveland Clinic, joins David H. Henry, MD, to discuss results from the CASSINI trial and what it means for anticoagulation in cancer patients.
And Ilana Yurkiewicz, MD, begins part 1 of her look at informed consent. Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University and is also a columnist for Hematology News. More from Dr. Yurkiewicz here.
Subscribe to Blood & Cancer here:
Apple Podcasts
Esketamine gets the green light for depression
A behavioral intervention improves physical activity in patients with diabetes. Groups of physicians produce more accurate diagnoses than individuals. And there’s a new target for reducing sodium consumption.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A behavioral intervention improves physical activity in patients with diabetes. Groups of physicians produce more accurate diagnoses than individuals. And there’s a new target for reducing sodium consumption.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A behavioral intervention improves physical activity in patients with diabetes. Groups of physicians produce more accurate diagnoses than individuals. And there’s a new target for reducing sodium consumption.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Histoplasmosis Manifests After Decades
Immunocompromised patients can be at risk for complications long after the original health issue was resolved—a problem illustrated by a patient who had a heart transplant in 1986 but developed acute progressive disseminated histoplasmosis decades later.
The patient presented with altered mental status; a Mini-Mental State Exam showed confusion. A computed tomography scan of the patient’s head revealed lesions, raising the suspicion of metastatic malignancy, which was ruled out after biopsy of a medial right temporal brain lesion. MRIs of his chest, abdomen, and pelvis revealed bilateral masses on his adrenal glands. Guided adrenal biopsy showed necrotizing granulomas consistent with a diagnosis of disseminated histoplasmosis.
However, that diagnosis was questioned—the patient had lived in Arizona for years, not, for instance, the Midwest, where histoplasmosis is more common. Nor did he have a history of spelunking, prior exposure to bird or bat droppings. He did report a short visit to North Carolina 30 years earlier. And he had been on immunosuppressive drugs for years.
The patient was started on liposomal amphotericin B, which was discontinued when his renal function deteriorated. He was switched to itraconazole, then restarted on amphotericin B with close monitoring after the diagnosis was confirmed. His doses of immunosuppressive drugs were reduced.
The clinicians note that HIV/AIDS and use of immunosuppressive drugs are among the risk factors for disseminated infection. They cite 1 study that found immunosuppression was the single most common risk factor. In another study, the risk of histoplasmosis increased as CD4+ T cells dropped below 300/µL.
The patient’s case was complicated by the fact that it was > 30 years after his heart transplant, and he had made only a short visit to an endemic area. He also had no history of histoplasmosis—the clinicians say a database search turned up the fact that most reported cases were preceded by symptomatic infection.
When charting patient history, they advise placing emphasis on a history of travel to endemic areas and considering histoplasmosis in immunocompromised patients in nonendemic areas.
Immunocompromised patients can be at risk for complications long after the original health issue was resolved—a problem illustrated by a patient who had a heart transplant in 1986 but developed acute progressive disseminated histoplasmosis decades later.
The patient presented with altered mental status; a Mini-Mental State Exam showed confusion. A computed tomography scan of the patient’s head revealed lesions, raising the suspicion of metastatic malignancy, which was ruled out after biopsy of a medial right temporal brain lesion. MRIs of his chest, abdomen, and pelvis revealed bilateral masses on his adrenal glands. Guided adrenal biopsy showed necrotizing granulomas consistent with a diagnosis of disseminated histoplasmosis.
However, that diagnosis was questioned—the patient had lived in Arizona for years, not, for instance, the Midwest, where histoplasmosis is more common. Nor did he have a history of spelunking, prior exposure to bird or bat droppings. He did report a short visit to North Carolina 30 years earlier. And he had been on immunosuppressive drugs for years.
The patient was started on liposomal amphotericin B, which was discontinued when his renal function deteriorated. He was switched to itraconazole, then restarted on amphotericin B with close monitoring after the diagnosis was confirmed. His doses of immunosuppressive drugs were reduced.
The clinicians note that HIV/AIDS and use of immunosuppressive drugs are among the risk factors for disseminated infection. They cite 1 study that found immunosuppression was the single most common risk factor. In another study, the risk of histoplasmosis increased as CD4+ T cells dropped below 300/µL.
The patient’s case was complicated by the fact that it was > 30 years after his heart transplant, and he had made only a short visit to an endemic area. He also had no history of histoplasmosis—the clinicians say a database search turned up the fact that most reported cases were preceded by symptomatic infection.
When charting patient history, they advise placing emphasis on a history of travel to endemic areas and considering histoplasmosis in immunocompromised patients in nonendemic areas.
Immunocompromised patients can be at risk for complications long after the original health issue was resolved—a problem illustrated by a patient who had a heart transplant in 1986 but developed acute progressive disseminated histoplasmosis decades later.
The patient presented with altered mental status; a Mini-Mental State Exam showed confusion. A computed tomography scan of the patient’s head revealed lesions, raising the suspicion of metastatic malignancy, which was ruled out after biopsy of a medial right temporal brain lesion. MRIs of his chest, abdomen, and pelvis revealed bilateral masses on his adrenal glands. Guided adrenal biopsy showed necrotizing granulomas consistent with a diagnosis of disseminated histoplasmosis.
However, that diagnosis was questioned—the patient had lived in Arizona for years, not, for instance, the Midwest, where histoplasmosis is more common. Nor did he have a history of spelunking, prior exposure to bird or bat droppings. He did report a short visit to North Carolina 30 years earlier. And he had been on immunosuppressive drugs for years.
The patient was started on liposomal amphotericin B, which was discontinued when his renal function deteriorated. He was switched to itraconazole, then restarted on amphotericin B with close monitoring after the diagnosis was confirmed. His doses of immunosuppressive drugs were reduced.
The clinicians note that HIV/AIDS and use of immunosuppressive drugs are among the risk factors for disseminated infection. They cite 1 study that found immunosuppression was the single most common risk factor. In another study, the risk of histoplasmosis increased as CD4+ T cells dropped below 300/µL.
The patient’s case was complicated by the fact that it was > 30 years after his heart transplant, and he had made only a short visit to an endemic area. He also had no history of histoplasmosis—the clinicians say a database search turned up the fact that most reported cases were preceded by symptomatic infection.
When charting patient history, they advise placing emphasis on a history of travel to endemic areas and considering histoplasmosis in immunocompromised patients in nonendemic areas.
Individual pediatric hypertension trials support personalized care
The preferred medication in children with hypertension varied among three common medications in n-of-1 studies with repeated ambulatory blood pressure monitoring that allowed for individualized treatment plans.
“In usual care, the choice of specific antihypertensive regimen is based on physician preference with little or no systematic assessment of treatment benefits and hazards,” wrote Joyce P. Samuel, MD, of the University of Texas Health Science Center, Houston, and her colleagues.
In a study published in Pediatrics, the researchers assessed 32 hypertensive children who participated in n-of-1 studies at a single center. The children underwent repeated ambulatory blood pressure monitoring (APBM) to compare the effects of three medications: lisinopril, amlodipine, and hydrochlorothiazide. The children were at least 9 years old, and their primary referring physician had recommended antihypertensive treatment.
The preferred medication was defined as the one that yielded both a normal ambulatory blood pressure and the greatest reduction in average systolic blood pressure when awake for two treatment periods with no unacceptable side effects. If more than one medication met these criteria, the one with the least side effects was chosen.
Overall, the preferred medication was lisinopril for 16 patients (49%), amlodipine for 8 patients (24%), and hydrochlorothiazide for 4 patients (12%); 4 patients remained uncontrolled on monotherapy.
Each of the three medications was taken for 2 weeks, and patients were assessed with a 24-hour ABPM and a side-effect questionnaire during the final 24 hours of each treatment. A total of 27 patients reported at least one side effect during the study. Unacceptable side effects were most frequent on hydrochlorothiazide (25%), compared with 16% for lisinopril and 13% for amlodipine. None of the patients experienced hypertensive crisis, hypotension, hyperkalemia, or an increase in serum creatinine levels of more than 20% from baseline.
No single medication was preferred for at least 80% of the patients. “Within-patient variation in BP (blood pressure) response was considerable because the best-performing medication decreased the BP by about 12 mm Hg more than the worst-performing medication,” the researchers wrote.
The findings were limited by the possibility that a 2-week trial might be insufficient to evaluate medication effects, and more research is needed to refine the methods of the trials and the generalizability of the n-of-1 approach, the researchers noted. However, “this individualized approach to antihypertensive medication selection holds potential value by involving patients in their own care and facilitating informed treatment decisions,” they said.
“A randomized trial in which researchers compare usual care to routine use of n-of-1 trials with ABPM is needed to assess effects on treatment adherence, patient satisfaction, long-term BP control assessed with ABPM, and hypertensive target organ damage,” Dr. Samuel and her associates advised.
The study was supported in part by the National Center for Advancing Translational Sciences and the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Samuel JP et al. Pediatrics. 2019 Mar 6. doi: 10.1542/peds.2018-1818.
The preferred medication in children with hypertension varied among three common medications in n-of-1 studies with repeated ambulatory blood pressure monitoring that allowed for individualized treatment plans.
“In usual care, the choice of specific antihypertensive regimen is based on physician preference with little or no systematic assessment of treatment benefits and hazards,” wrote Joyce P. Samuel, MD, of the University of Texas Health Science Center, Houston, and her colleagues.
In a study published in Pediatrics, the researchers assessed 32 hypertensive children who participated in n-of-1 studies at a single center. The children underwent repeated ambulatory blood pressure monitoring (APBM) to compare the effects of three medications: lisinopril, amlodipine, and hydrochlorothiazide. The children were at least 9 years old, and their primary referring physician had recommended antihypertensive treatment.
The preferred medication was defined as the one that yielded both a normal ambulatory blood pressure and the greatest reduction in average systolic blood pressure when awake for two treatment periods with no unacceptable side effects. If more than one medication met these criteria, the one with the least side effects was chosen.
Overall, the preferred medication was lisinopril for 16 patients (49%), amlodipine for 8 patients (24%), and hydrochlorothiazide for 4 patients (12%); 4 patients remained uncontrolled on monotherapy.
Each of the three medications was taken for 2 weeks, and patients were assessed with a 24-hour ABPM and a side-effect questionnaire during the final 24 hours of each treatment. A total of 27 patients reported at least one side effect during the study. Unacceptable side effects were most frequent on hydrochlorothiazide (25%), compared with 16% for lisinopril and 13% for amlodipine. None of the patients experienced hypertensive crisis, hypotension, hyperkalemia, or an increase in serum creatinine levels of more than 20% from baseline.
No single medication was preferred for at least 80% of the patients. “Within-patient variation in BP (blood pressure) response was considerable because the best-performing medication decreased the BP by about 12 mm Hg more than the worst-performing medication,” the researchers wrote.
The findings were limited by the possibility that a 2-week trial might be insufficient to evaluate medication effects, and more research is needed to refine the methods of the trials and the generalizability of the n-of-1 approach, the researchers noted. However, “this individualized approach to antihypertensive medication selection holds potential value by involving patients in their own care and facilitating informed treatment decisions,” they said.
“A randomized trial in which researchers compare usual care to routine use of n-of-1 trials with ABPM is needed to assess effects on treatment adherence, patient satisfaction, long-term BP control assessed with ABPM, and hypertensive target organ damage,” Dr. Samuel and her associates advised.
The study was supported in part by the National Center for Advancing Translational Sciences and the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Samuel JP et al. Pediatrics. 2019 Mar 6. doi: 10.1542/peds.2018-1818.
The preferred medication in children with hypertension varied among three common medications in n-of-1 studies with repeated ambulatory blood pressure monitoring that allowed for individualized treatment plans.
“In usual care, the choice of specific antihypertensive regimen is based on physician preference with little or no systematic assessment of treatment benefits and hazards,” wrote Joyce P. Samuel, MD, of the University of Texas Health Science Center, Houston, and her colleagues.
In a study published in Pediatrics, the researchers assessed 32 hypertensive children who participated in n-of-1 studies at a single center. The children underwent repeated ambulatory blood pressure monitoring (APBM) to compare the effects of three medications: lisinopril, amlodipine, and hydrochlorothiazide. The children were at least 9 years old, and their primary referring physician had recommended antihypertensive treatment.
The preferred medication was defined as the one that yielded both a normal ambulatory blood pressure and the greatest reduction in average systolic blood pressure when awake for two treatment periods with no unacceptable side effects. If more than one medication met these criteria, the one with the least side effects was chosen.
Overall, the preferred medication was lisinopril for 16 patients (49%), amlodipine for 8 patients (24%), and hydrochlorothiazide for 4 patients (12%); 4 patients remained uncontrolled on monotherapy.
Each of the three medications was taken for 2 weeks, and patients were assessed with a 24-hour ABPM and a side-effect questionnaire during the final 24 hours of each treatment. A total of 27 patients reported at least one side effect during the study. Unacceptable side effects were most frequent on hydrochlorothiazide (25%), compared with 16% for lisinopril and 13% for amlodipine. None of the patients experienced hypertensive crisis, hypotension, hyperkalemia, or an increase in serum creatinine levels of more than 20% from baseline.
No single medication was preferred for at least 80% of the patients. “Within-patient variation in BP (blood pressure) response was considerable because the best-performing medication decreased the BP by about 12 mm Hg more than the worst-performing medication,” the researchers wrote.
The findings were limited by the possibility that a 2-week trial might be insufficient to evaluate medication effects, and more research is needed to refine the methods of the trials and the generalizability of the n-of-1 approach, the researchers noted. However, “this individualized approach to antihypertensive medication selection holds potential value by involving patients in their own care and facilitating informed treatment decisions,” they said.
“A randomized trial in which researchers compare usual care to routine use of n-of-1 trials with ABPM is needed to assess effects on treatment adherence, patient satisfaction, long-term BP control assessed with ABPM, and hypertensive target organ damage,” Dr. Samuel and her associates advised.
The study was supported in part by the National Center for Advancing Translational Sciences and the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Samuel JP et al. Pediatrics. 2019 Mar 6. doi: 10.1542/peds.2018-1818.
FROM PEDIATRICS
Poppy-seeking parrots, harmonious mice, and feline-fueled hospital bills
Polly want another hit?
Once again, the animals are getting high.
Parrots in India are giving opium farmers a huge and expensive headache. The birds have become addicted to the poppy crop and are taking a huge bite out of the seasonal yields. According to an opium specialist, the birds get a jolt of instant energy from the poppy flower, similar to drinking a cup of coffee. No wonder the birds keep coming back for more.
This roving (flying?) gang of parrot menaces are feeding 30-40 times a day on the plants. Farmers have tried in vain to use firecrackers to scare off the birds, but nothing will stop Polly from getting her next fix. Opium dens might be long gone, but opium nests might be the next big thing.
Sing your little mouse heart out
In addition to being a big star on American Idol: Rodent Edition, the Alston’s singing mouse could also be a key player in understanding how mammalian brains control conversations.
These musical mice, native to Central America, do something unique: They take turns singing, rather than all belting it out at once. Researchers at New York University are using these mouse powerhouses as a model to study how conversation is regulated.
Unsurprisingly, this superstar is reportedly the mouse version of Mariah Carey, requiring a very specific environment: a palatial terrarium, a highly specialized diet, a microphone-shaped swimming pool, no brown M&Ms … you get the picture.
Researchers believe the demands are worth it, however. They’ve discovered that the mice time their songs very precisely to avoid any overlap with a singing neighbor. This could lead to insight on how humans delay conversation in order to not talk over one another (except for that one annoying coworker – you know who you are).
Reason No. 48,512 to hate cats
It really is true that no good deed goes unpunished. Jeannette Parker was nice to a cat, and she ended up with a hospital bill of $48,512.
We will elaborate. Ms. Parker, a wildlife biologist in Florida, offered a stray kitten some tuna and got bitten in the process. (Reason No. 48,513: Cats will bite the hand that feeds them.) There had been rabies warnings recently and the bite did break the skin, so she decided to go to the emergency department at Mariners Hospital in Tavernier, Fla., according to Kaiser Health News. She was there 2 hours and never spoke with a physician, but she did get the first in a series of rabies shots and 12 mL of rabies immune globulin.
The next thing she received from the hospital was a bill for – you guessed it – $48,512, of which $46,422 was for the immune globulin. “My funeral would have been cheaper,” she told Kaiser.
Her husband’s insurance covered most of the bill, but Ms. Parker ended up paying the rest of her deductible and 10% of the charges accepted by her insurer, almost $4,200.
All because she tried to help a cat. Way to go, cat.
And the best doctor award goes to ...
Normally, it requires a lot of time and money to become a respected physician. Medical school, residencies – it’s tough just becoming a doctor, let alone reaching the top of your field.
However, if you’re a reporter at ProPublica who specializes in health care, you can receive the prestigious Top Doctor award for just $289 – or only $99 if you act right now. For your money, you get a customized plaque made of either cherry wood with gold trim or black with chrome trim. That’s an offer too good to pass up.
A reasonable question to ask at this point would probably be, Why is a reporter receiving an award presumably meant for an actual doctor? Well, according to the company selling the award, the reporter’s peers had nominated him and his patients had given him stellar reviews. He was without a doubt one of America’s Top Doctors. His lack of medical degree was absolutely not a problem.
Okay, the award is probably a scam. But we’re also health care reporters here at Livin’ on the MDedge, so if the Top Doctor people are reading, we’ll have the cherry wood with gold trim.

Polly want another hit?
Once again, the animals are getting high.
Parrots in India are giving opium farmers a huge and expensive headache. The birds have become addicted to the poppy crop and are taking a huge bite out of the seasonal yields. According to an opium specialist, the birds get a jolt of instant energy from the poppy flower, similar to drinking a cup of coffee. No wonder the birds keep coming back for more.
This roving (flying?) gang of parrot menaces are feeding 30-40 times a day on the plants. Farmers have tried in vain to use firecrackers to scare off the birds, but nothing will stop Polly from getting her next fix. Opium dens might be long gone, but opium nests might be the next big thing.
Sing your little mouse heart out
In addition to being a big star on American Idol: Rodent Edition, the Alston’s singing mouse could also be a key player in understanding how mammalian brains control conversations.
These musical mice, native to Central America, do something unique: They take turns singing, rather than all belting it out at once. Researchers at New York University are using these mouse powerhouses as a model to study how conversation is regulated.
Unsurprisingly, this superstar is reportedly the mouse version of Mariah Carey, requiring a very specific environment: a palatial terrarium, a highly specialized diet, a microphone-shaped swimming pool, no brown M&Ms … you get the picture.
Researchers believe the demands are worth it, however. They’ve discovered that the mice time their songs very precisely to avoid any overlap with a singing neighbor. This could lead to insight on how humans delay conversation in order to not talk over one another (except for that one annoying coworker – you know who you are).
Reason No. 48,512 to hate cats
It really is true that no good deed goes unpunished. Jeannette Parker was nice to a cat, and she ended up with a hospital bill of $48,512.
We will elaborate. Ms. Parker, a wildlife biologist in Florida, offered a stray kitten some tuna and got bitten in the process. (Reason No. 48,513: Cats will bite the hand that feeds them.) There had been rabies warnings recently and the bite did break the skin, so she decided to go to the emergency department at Mariners Hospital in Tavernier, Fla., according to Kaiser Health News. She was there 2 hours and never spoke with a physician, but she did get the first in a series of rabies shots and 12 mL of rabies immune globulin.
The next thing she received from the hospital was a bill for – you guessed it – $48,512, of which $46,422 was for the immune globulin. “My funeral would have been cheaper,” she told Kaiser.
Her husband’s insurance covered most of the bill, but Ms. Parker ended up paying the rest of her deductible and 10% of the charges accepted by her insurer, almost $4,200.
All because she tried to help a cat. Way to go, cat.
And the best doctor award goes to ...
Normally, it requires a lot of time and money to become a respected physician. Medical school, residencies – it’s tough just becoming a doctor, let alone reaching the top of your field.
However, if you’re a reporter at ProPublica who specializes in health care, you can receive the prestigious Top Doctor award for just $289 – or only $99 if you act right now. For your money, you get a customized plaque made of either cherry wood with gold trim or black with chrome trim. That’s an offer too good to pass up.
A reasonable question to ask at this point would probably be, Why is a reporter receiving an award presumably meant for an actual doctor? Well, according to the company selling the award, the reporter’s peers had nominated him and his patients had given him stellar reviews. He was without a doubt one of America’s Top Doctors. His lack of medical degree was absolutely not a problem.
Okay, the award is probably a scam. But we’re also health care reporters here at Livin’ on the MDedge, so if the Top Doctor people are reading, we’ll have the cherry wood with gold trim.

Polly want another hit?
Once again, the animals are getting high.
Parrots in India are giving opium farmers a huge and expensive headache. The birds have become addicted to the poppy crop and are taking a huge bite out of the seasonal yields. According to an opium specialist, the birds get a jolt of instant energy from the poppy flower, similar to drinking a cup of coffee. No wonder the birds keep coming back for more.
This roving (flying?) gang of parrot menaces are feeding 30-40 times a day on the plants. Farmers have tried in vain to use firecrackers to scare off the birds, but nothing will stop Polly from getting her next fix. Opium dens might be long gone, but opium nests might be the next big thing.
Sing your little mouse heart out
In addition to being a big star on American Idol: Rodent Edition, the Alston’s singing mouse could also be a key player in understanding how mammalian brains control conversations.
These musical mice, native to Central America, do something unique: They take turns singing, rather than all belting it out at once. Researchers at New York University are using these mouse powerhouses as a model to study how conversation is regulated.
Unsurprisingly, this superstar is reportedly the mouse version of Mariah Carey, requiring a very specific environment: a palatial terrarium, a highly specialized diet, a microphone-shaped swimming pool, no brown M&Ms … you get the picture.
Researchers believe the demands are worth it, however. They’ve discovered that the mice time their songs very precisely to avoid any overlap with a singing neighbor. This could lead to insight on how humans delay conversation in order to not talk over one another (except for that one annoying coworker – you know who you are).
Reason No. 48,512 to hate cats
It really is true that no good deed goes unpunished. Jeannette Parker was nice to a cat, and she ended up with a hospital bill of $48,512.
We will elaborate. Ms. Parker, a wildlife biologist in Florida, offered a stray kitten some tuna and got bitten in the process. (Reason No. 48,513: Cats will bite the hand that feeds them.) There had been rabies warnings recently and the bite did break the skin, so she decided to go to the emergency department at Mariners Hospital in Tavernier, Fla., according to Kaiser Health News. She was there 2 hours and never spoke with a physician, but she did get the first in a series of rabies shots and 12 mL of rabies immune globulin.
The next thing she received from the hospital was a bill for – you guessed it – $48,512, of which $46,422 was for the immune globulin. “My funeral would have been cheaper,” she told Kaiser.
Her husband’s insurance covered most of the bill, but Ms. Parker ended up paying the rest of her deductible and 10% of the charges accepted by her insurer, almost $4,200.
All because she tried to help a cat. Way to go, cat.
And the best doctor award goes to ...
Normally, it requires a lot of time and money to become a respected physician. Medical school, residencies – it’s tough just becoming a doctor, let alone reaching the top of your field.
However, if you’re a reporter at ProPublica who specializes in health care, you can receive the prestigious Top Doctor award for just $289 – or only $99 if you act right now. For your money, you get a customized plaque made of either cherry wood with gold trim or black with chrome trim. That’s an offer too good to pass up.
A reasonable question to ask at this point would probably be, Why is a reporter receiving an award presumably meant for an actual doctor? Well, according to the company selling the award, the reporter’s peers had nominated him and his patients had given him stellar reviews. He was without a doubt one of America’s Top Doctors. His lack of medical degree was absolutely not a problem.
Okay, the award is probably a scam. But we’re also health care reporters here at Livin’ on the MDedge, so if the Top Doctor people are reading, we’ll have the cherry wood with gold trim.

Lentiviral gene therapy appears effective in X-CGD
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
REPORTING FROM TCT 2019
Concurrent Keratoacanthomas and Nonsarcoidal Granulomatous Reactions in New and Preexisting Tattoos
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.
contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).
The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.
contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).
The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.
contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).
The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
Practice Points
- Keratoacanthomas (KAs) are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution.
- The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, scarring disorders, and trauma (including tattoos).
- Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Our case adds additional weight to the idea that some KAs are primarily reactive phenomena sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
Infective endocarditis isn’t what it used to be
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
REPORTING FROM ACC SNOWMASS 2019