User login
ASCO issues guideline for early detection, management of colorectal cancer
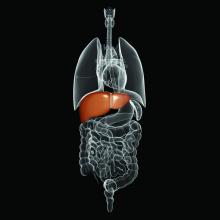
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flxible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding abdominal pain and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
Review AGA and U.S. Multi-society Task Force on Colorectal Cancer guidelines and recommendations on colorectal cancer screening, evaluation of Lynch Syndrome, colonoscopy and bowel cleansing at https://www.gastro.org/guidelines/colorectal-cancer.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
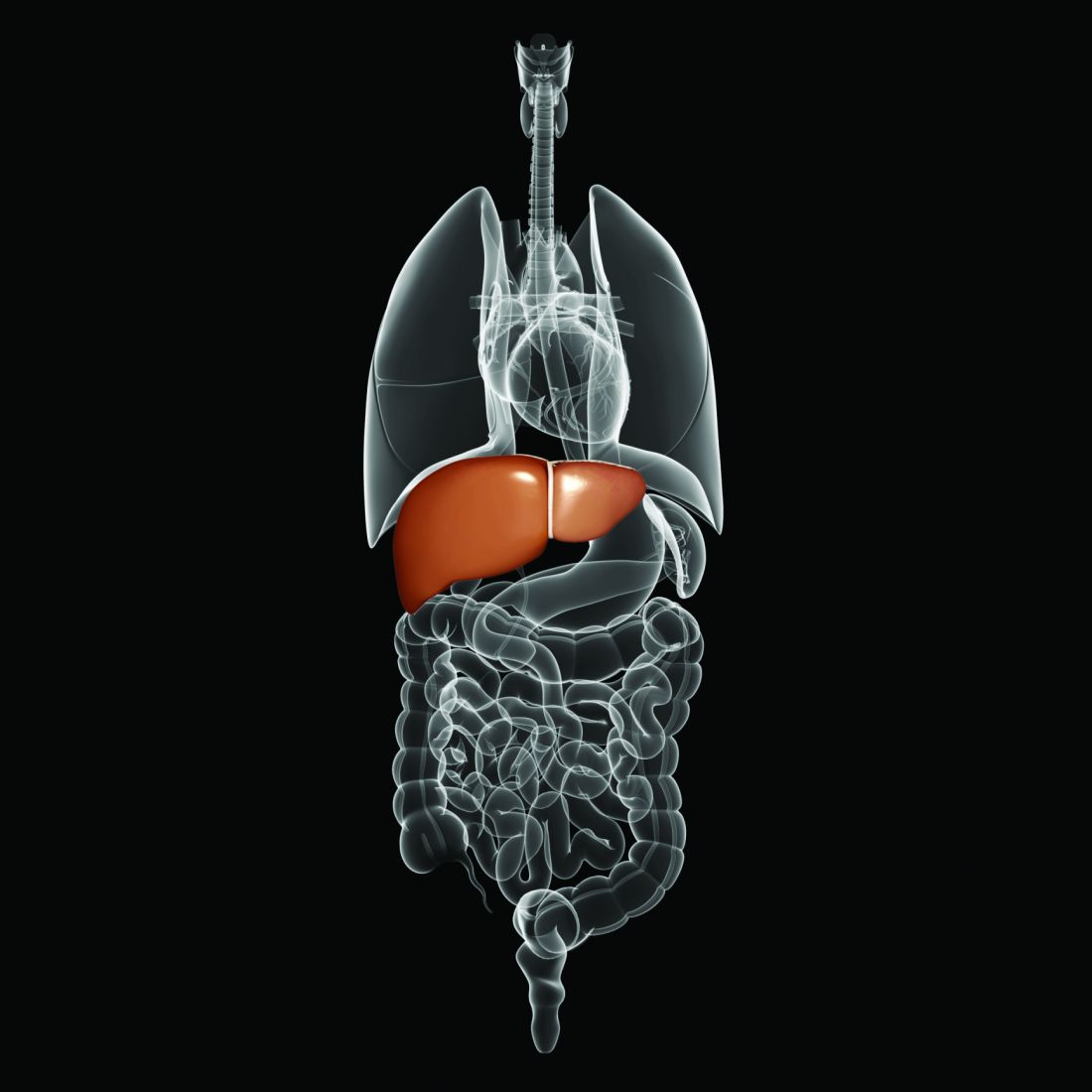
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flxible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding abdominal pain and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
Review AGA and U.S. Multi-society Task Force on Colorectal Cancer guidelines and recommendations on colorectal cancer screening, evaluation of Lynch Syndrome, colonoscopy and bowel cleansing at https://www.gastro.org/guidelines/colorectal-cancer.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
The American Society of Clinical Oncology has issued a new guideline on the early detection and management of colorectal cancer in people at average risk for colorectal cancer, which was written by Gilberto Lopes, MD, of the University of Miami and his associates on an ASCO expert panel.
The panel assembled by ASCO to write the guideline consisted of medical oncology, surgical oncology, surgery, gastroenterology, health technology assessment, cancer epidemiology, pathology, radiology, radiation oncology, and patient advocacy experts. Guidelines from eight different developers were examined, and recommendations from those guidelines were adapted to form the new ASCO guideline. The guideline was published in the Journal of Global Oncology.
In people who are asymptomatic, are aged 50-75 years, have no family history of colorectal cancer, are at average risk, and are in settings with high incidences of colorectal cancer, the expert panel recommends guaiac fecal occult blood test or fecal immunochemical testing every 1-2 years, flexible sigmoidoscopy every 5 years, a combination of flexible sigmoidoscopy every 10 years and annual stool-based testing, or colonoscopy every 10 years, depending on available resources. The testing strategy for those with positive stool-based testing or flxible sigmoidoscopy is colonoscopy or a double-contrast barium enema if colonoscopy is unavailable.
For patients who have polyps, polypectomy at the time of colonoscopy is recommended, with the option of referral for surgical resection if not suitable for endoscopic resection. When symptoms (iron-deficiency anemia, bleeding abdominal pain and/or change in bowel habits) are present, a colonoscopy should be performed if available. If colonoscopy is contraindicated, a double-contrast barium enema can be performed; if endoscopy is contraindicated, CT colonography can be performed.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, the guideline noted.
Several members of the expert panel reported conflicts of interest.
Review AGA and U.S. Multi-society Task Force on Colorectal Cancer guidelines and recommendations on colorectal cancer screening, evaluation of Lynch Syndrome, colonoscopy and bowel cleansing at https://www.gastro.org/guidelines/colorectal-cancer.
SOURCE: Lopes G et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00213.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
ASCO publishes new guideline for treatment, follow-up of early-stage colorectal cancer
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
Help your patients better understand colorectal cancer risk factors and screening by sharing AGA patient education materials written by specialists, for patients at http://ow.ly/s7XS30nVPI4.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
Help your patients better understand colorectal cancer risk factors and screening by sharing AGA patient education materials written by specialists, for patients at http://ow.ly/s7XS30nVPI4.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
Help your patients better understand colorectal cancer risk factors and screening by sharing AGA patient education materials written by specialists, for patients at http://ow.ly/s7XS30nVPI4.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
What’s the price of rude behavior in the hospital?
SAN DIEGO – Does rudeness from a colleague prevent physicians from noticing a diagnostic error and challenging it? A new study suggests it might not, at least in the context of hand-offs from dismissive and insulting fellow doctors.
Instead, a simulation found that experience seems to be the key factor in giving physicians the guts – or the awareness – to change course. Still, the findings hint that rudeness may still have a negative effect on one group – resident physicians.
“It appears that we are building resilience somewhere in training,” said study lead author Michael Avesar, MD, a pediatric critical care medicine fellow at Children’s Hospital Los Angeles.
Dr. Avesar spoke in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The initial motivation of the study wasn’t to gain more understanding of rudeness in medicine. Instead, Dr. Avesar said, “We started off with trying to find ways to understand how physicians think during high-stakes decisions in stressful or time-limited situations. We wanted to see if people were able to challenge the momentum of diagnostic error. That’s when we learned more about the rudeness literature.”
Yes, it’s true: Researchers have devoted time to studying rudeness in medicine. After all, it’s quite common. A 2017 Israeli study in Pediatrics declared it’s “routinely experienced by medical teams.” That study, also based on simulations, determined that “rudeness has robust, deleterious effects on the performance of medical teams. Moreover, exposure to rudeness debilitated the very collaborative mechanisms recognized as essential for patient care and safety” (Pediatrics. 2017 Feb. doi: 10.1542/peds.2016-2305).
For the new study, Dr. Avesar and his colleagues ultimately decided to explore possible links between rudeness and diagnostic error. To explore the issue, they created a simulation of a hand-off of a pediatric patient from the operating team to the ICU.
In the simulation, the “physician” handing off the “patient” incorrectly noted a diagnosis of sepsis. In fact, the patient had cardiac tamponade.
The physician, played by an actor, was instructed to either act in a neutral fashion during the hand-off or be rude. But rudeness, it turns out, isn’t easy to define, even if we all think we know it when we see it.
“There’s a lot of debate as to what is ‘rude,’ ” Dr. Avesar said. The researchers settled on a level of rudeness that wasn’t “too mean” but was still inappropriate: It featured frequent interruptions during the hand-off, lack of eye contact, and abrupt departures. In some simulations, the actor insulted the colleagues of the recipient of the hand-off.
In other words, Dr. Avesar said, the actor was a jerk.
The researchers tested the “neutral” and “rude” hand-off scenarios in 41 simulations. The physicians who played the recipients of the hand-offs included 11 attendings, 14 fellows, and 16 residents.
Eighty-two percent of the attendings (9/11) challenged the diagnosis, as did 86% (12/14) of the fellows. Only 31% (5/16) of residents challenged the diagnosis; this difference from the other groups was statistically significant.
Half of the eight residents exposed to a “neutral” handoff challenged the correct diagnosis, while only 13% (1/8) of those who were treated rudely did. “While the P value was not significant, previous literature focused on residents supports this trend,” Dr. Avesar said.
It’s possible that certain residents gain the knowledge and experience to overcome rudeness over time, he said. That, he said, leads to an intriguing question: “Could we find out how resilience is learned and how to replicate it?”
Moving forward, he said, the team will try to figure out whether there’s a link between personality types and reactions to rudeness.
Eventually, he said, the team may test ways to reduce the effects of rudeness and boost critical thinking. “We see this as a long-term strategy to enhance medical education and patient safety,” he said.
No study funding is reported. Dr. Avesar reports no relevant disclosures.
SOURCE: Avesar M et al. Crit Care Med. 2019 Jan;47(1):682, Abstract 1412.
SAN DIEGO – Does rudeness from a colleague prevent physicians from noticing a diagnostic error and challenging it? A new study suggests it might not, at least in the context of hand-offs from dismissive and insulting fellow doctors.
Instead, a simulation found that experience seems to be the key factor in giving physicians the guts – or the awareness – to change course. Still, the findings hint that rudeness may still have a negative effect on one group – resident physicians.
“It appears that we are building resilience somewhere in training,” said study lead author Michael Avesar, MD, a pediatric critical care medicine fellow at Children’s Hospital Los Angeles.
Dr. Avesar spoke in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The initial motivation of the study wasn’t to gain more understanding of rudeness in medicine. Instead, Dr. Avesar said, “We started off with trying to find ways to understand how physicians think during high-stakes decisions in stressful or time-limited situations. We wanted to see if people were able to challenge the momentum of diagnostic error. That’s when we learned more about the rudeness literature.”
Yes, it’s true: Researchers have devoted time to studying rudeness in medicine. After all, it’s quite common. A 2017 Israeli study in Pediatrics declared it’s “routinely experienced by medical teams.” That study, also based on simulations, determined that “rudeness has robust, deleterious effects on the performance of medical teams. Moreover, exposure to rudeness debilitated the very collaborative mechanisms recognized as essential for patient care and safety” (Pediatrics. 2017 Feb. doi: 10.1542/peds.2016-2305).
For the new study, Dr. Avesar and his colleagues ultimately decided to explore possible links between rudeness and diagnostic error. To explore the issue, they created a simulation of a hand-off of a pediatric patient from the operating team to the ICU.
In the simulation, the “physician” handing off the “patient” incorrectly noted a diagnosis of sepsis. In fact, the patient had cardiac tamponade.
The physician, played by an actor, was instructed to either act in a neutral fashion during the hand-off or be rude. But rudeness, it turns out, isn’t easy to define, even if we all think we know it when we see it.
“There’s a lot of debate as to what is ‘rude,’ ” Dr. Avesar said. The researchers settled on a level of rudeness that wasn’t “too mean” but was still inappropriate: It featured frequent interruptions during the hand-off, lack of eye contact, and abrupt departures. In some simulations, the actor insulted the colleagues of the recipient of the hand-off.
In other words, Dr. Avesar said, the actor was a jerk.
The researchers tested the “neutral” and “rude” hand-off scenarios in 41 simulations. The physicians who played the recipients of the hand-offs included 11 attendings, 14 fellows, and 16 residents.
Eighty-two percent of the attendings (9/11) challenged the diagnosis, as did 86% (12/14) of the fellows. Only 31% (5/16) of residents challenged the diagnosis; this difference from the other groups was statistically significant.
Half of the eight residents exposed to a “neutral” handoff challenged the correct diagnosis, while only 13% (1/8) of those who were treated rudely did. “While the P value was not significant, previous literature focused on residents supports this trend,” Dr. Avesar said.
It’s possible that certain residents gain the knowledge and experience to overcome rudeness over time, he said. That, he said, leads to an intriguing question: “Could we find out how resilience is learned and how to replicate it?”
Moving forward, he said, the team will try to figure out whether there’s a link between personality types and reactions to rudeness.
Eventually, he said, the team may test ways to reduce the effects of rudeness and boost critical thinking. “We see this as a long-term strategy to enhance medical education and patient safety,” he said.
No study funding is reported. Dr. Avesar reports no relevant disclosures.
SOURCE: Avesar M et al. Crit Care Med. 2019 Jan;47(1):682, Abstract 1412.
SAN DIEGO – Does rudeness from a colleague prevent physicians from noticing a diagnostic error and challenging it? A new study suggests it might not, at least in the context of hand-offs from dismissive and insulting fellow doctors.
Instead, a simulation found that experience seems to be the key factor in giving physicians the guts – or the awareness – to change course. Still, the findings hint that rudeness may still have a negative effect on one group – resident physicians.
“It appears that we are building resilience somewhere in training,” said study lead author Michael Avesar, MD, a pediatric critical care medicine fellow at Children’s Hospital Los Angeles.
Dr. Avesar spoke in an interview following the presentation of the study findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The initial motivation of the study wasn’t to gain more understanding of rudeness in medicine. Instead, Dr. Avesar said, “We started off with trying to find ways to understand how physicians think during high-stakes decisions in stressful or time-limited situations. We wanted to see if people were able to challenge the momentum of diagnostic error. That’s when we learned more about the rudeness literature.”
Yes, it’s true: Researchers have devoted time to studying rudeness in medicine. After all, it’s quite common. A 2017 Israeli study in Pediatrics declared it’s “routinely experienced by medical teams.” That study, also based on simulations, determined that “rudeness has robust, deleterious effects on the performance of medical teams. Moreover, exposure to rudeness debilitated the very collaborative mechanisms recognized as essential for patient care and safety” (Pediatrics. 2017 Feb. doi: 10.1542/peds.2016-2305).
For the new study, Dr. Avesar and his colleagues ultimately decided to explore possible links between rudeness and diagnostic error. To explore the issue, they created a simulation of a hand-off of a pediatric patient from the operating team to the ICU.
In the simulation, the “physician” handing off the “patient” incorrectly noted a diagnosis of sepsis. In fact, the patient had cardiac tamponade.
The physician, played by an actor, was instructed to either act in a neutral fashion during the hand-off or be rude. But rudeness, it turns out, isn’t easy to define, even if we all think we know it when we see it.
“There’s a lot of debate as to what is ‘rude,’ ” Dr. Avesar said. The researchers settled on a level of rudeness that wasn’t “too mean” but was still inappropriate: It featured frequent interruptions during the hand-off, lack of eye contact, and abrupt departures. In some simulations, the actor insulted the colleagues of the recipient of the hand-off.
In other words, Dr. Avesar said, the actor was a jerk.
The researchers tested the “neutral” and “rude” hand-off scenarios in 41 simulations. The physicians who played the recipients of the hand-offs included 11 attendings, 14 fellows, and 16 residents.
Eighty-two percent of the attendings (9/11) challenged the diagnosis, as did 86% (12/14) of the fellows. Only 31% (5/16) of residents challenged the diagnosis; this difference from the other groups was statistically significant.
Half of the eight residents exposed to a “neutral” handoff challenged the correct diagnosis, while only 13% (1/8) of those who were treated rudely did. “While the P value was not significant, previous literature focused on residents supports this trend,” Dr. Avesar said.
It’s possible that certain residents gain the knowledge and experience to overcome rudeness over time, he said. That, he said, leads to an intriguing question: “Could we find out how resilience is learned and how to replicate it?”
Moving forward, he said, the team will try to figure out whether there’s a link between personality types and reactions to rudeness.
Eventually, he said, the team may test ways to reduce the effects of rudeness and boost critical thinking. “We see this as a long-term strategy to enhance medical education and patient safety,” he said.
No study funding is reported. Dr. Avesar reports no relevant disclosures.
SOURCE: Avesar M et al. Crit Care Med. 2019 Jan;47(1):682, Abstract 1412.
REPORTING FROM CCC48
March 2019 Highlights
Developing an HCV vaccine faces significant challenges
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
The development of a prophylactic hepatitis C vaccine faces significant challenges, according to a Justin R. Bailey, MD, of Johns Hopkins University, Baltimore, and his colleagues.
Barriers to developing a prophylactic HCV vaccine include the great diversity of the virus, the limited models that are available for vaccine testing, and the currently incomplete understanding of protective immune responses, according to their review published in Gastroenterology.
Functionally, the inability to culture HCV, until recently, and continuing limitations of HCV culture systems pose challenges to standard production of a live-attenuated or inactivated whole HCV vaccine. In addition, there is the risk of causing HCV infection with live-attenuated vaccines.
On a practical level for all forms of vaccine development, a principal challenge “is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1,” according to the authors (Gastroenterology 2019;156[2]:418-30).
With regard to vaccine testing, there are also significant difficulties: There is a lack of in vitro systems and immunocompetent small-animal models useful for determining whether vaccination induces protective immunity. Although a use of an HCV-like virus, the rat Hepacivirus, provides a new small-animal model for vaccine testing, this virus has limited sequence analogy to HCV.
The development of immunity to HCV in humans is complex and under broad investigation. However, decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection, according to the authors, and vaccine strategies to induce all three adaptive immune responses are in development.
“A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge,” the authors concluded.
Dr. Bailey and his colleagues reported that they had no conflicts.
SOURCE: Bailey JR et al. Gastroenterology 2019(2);156:418-30.
FROM GASTROENTEROLOGY
Possible biomarkers found for progression to liver cancer in chronic HCV infection
according to the results of a biochemical analysis of human blood samples performed by PhD student Paywast J. Jalal of the University of Sulaimani (Iraq) and colleagues.
Archived HCV-positive serum samples, including those from 31 patients who had developed HCC, were retrieved from the Trent HCV clinical cohort. They were compared with each other over time and against samples from HCV-infected individuals in the cohort who did not develop HCC. In addition, HCV-negative serum samples were obtained commercially and assessed identically. Circulating liver-expressed lectins, ficolin-2, ficolin-3, and MBL were all examined as potential biomarkers for the development of HCC, the authors wrote in Virology.
Binding of ficolin-3 to reference ligands was greater in chronic HCV infection, while ficolin-2 and MBL were significantly elevated in individuals who develop HCC, compared with HCV-infected individuals without HCC. Ficolin-2 and MBL were found to be elevated at 1 and 3 years prior to HCC diagnosis, respectively, suggesting they could be used as prognostic serum markers for the development of HCC.
“The strong evidence for an association between elevated MBL binding activity and the development of HCC is supportive for a larger prospective study of these biomarkers in HCV-induced liver cancer,” the researchers concluded.
This study was funded by a split-site PhD scholarship between the University of Sulaimani and the University of Nottingham (England). The authors reported they had no conflicts.
SOURCE: Jalal PJ et al. Virology. 2019;530:99-106.
according to the results of a biochemical analysis of human blood samples performed by PhD student Paywast J. Jalal of the University of Sulaimani (Iraq) and colleagues.
Archived HCV-positive serum samples, including those from 31 patients who had developed HCC, were retrieved from the Trent HCV clinical cohort. They were compared with each other over time and against samples from HCV-infected individuals in the cohort who did not develop HCC. In addition, HCV-negative serum samples were obtained commercially and assessed identically. Circulating liver-expressed lectins, ficolin-2, ficolin-3, and MBL were all examined as potential biomarkers for the development of HCC, the authors wrote in Virology.
Binding of ficolin-3 to reference ligands was greater in chronic HCV infection, while ficolin-2 and MBL were significantly elevated in individuals who develop HCC, compared with HCV-infected individuals without HCC. Ficolin-2 and MBL were found to be elevated at 1 and 3 years prior to HCC diagnosis, respectively, suggesting they could be used as prognostic serum markers for the development of HCC.
“The strong evidence for an association between elevated MBL binding activity and the development of HCC is supportive for a larger prospective study of these biomarkers in HCV-induced liver cancer,” the researchers concluded.
This study was funded by a split-site PhD scholarship between the University of Sulaimani and the University of Nottingham (England). The authors reported they had no conflicts.
SOURCE: Jalal PJ et al. Virology. 2019;530:99-106.
according to the results of a biochemical analysis of human blood samples performed by PhD student Paywast J. Jalal of the University of Sulaimani (Iraq) and colleagues.
Archived HCV-positive serum samples, including those from 31 patients who had developed HCC, were retrieved from the Trent HCV clinical cohort. They were compared with each other over time and against samples from HCV-infected individuals in the cohort who did not develop HCC. In addition, HCV-negative serum samples were obtained commercially and assessed identically. Circulating liver-expressed lectins, ficolin-2, ficolin-3, and MBL were all examined as potential biomarkers for the development of HCC, the authors wrote in Virology.
Binding of ficolin-3 to reference ligands was greater in chronic HCV infection, while ficolin-2 and MBL were significantly elevated in individuals who develop HCC, compared with HCV-infected individuals without HCC. Ficolin-2 and MBL were found to be elevated at 1 and 3 years prior to HCC diagnosis, respectively, suggesting they could be used as prognostic serum markers for the development of HCC.
“The strong evidence for an association between elevated MBL binding activity and the development of HCC is supportive for a larger prospective study of these biomarkers in HCV-induced liver cancer,” the researchers concluded.
This study was funded by a split-site PhD scholarship between the University of Sulaimani and the University of Nottingham (England). The authors reported they had no conflicts.
SOURCE: Jalal PJ et al. Virology. 2019;530:99-106.
FROM VIROLOGY
Umbilical cord allograft may boost diabetic foot ulcer healing
Dehydrated human umbilical cord allograft may have benefit over alginate wound dressings as a treatment for chronic, nonhealing diabetic foot ulcers (DFU), findings from an industry-funded, randomized controlled study suggest.
The findings “provide additional evidence of the safety and efficacy of dehydrated placental tissues,” wrote William Tettelbach, MD, and his colleagues. Their report is in International Wound Journal.
The burden of diabetic foot disease in the United States is immense. A 2014 study estimated that treatment of DFUs alone cost public and private insurers as much as $13 billion per year (Diabetes Care. 2014;37(3):651-8).
MiMedx, which funded the new study, has developed a product called EpiCord to protect the DFU wound site. The product’s website describes it as a “unique, thick membrane derived from umbilical cord” that’s “minimally manipulated, dehydrated, [and] non-viable” (www.mimedx.com/epicord). The study authors noted that “immunogenicity of placental tissue lends credence to its use as an allograft material for difficult-to-heal wounds.”
For the new study, which was conducted from 2016 to 2018 and led by Dr. Tettelbach, an infectious disease specialist who is now an employee of MiMedx, the researchers enlisted 155 adult patients with stubborn DFUs at 11 centers in the United States.
All the ulcers had 30% or less wound area reduction after 14 days of standard care. The majority of patients (81%) were male; 63% were obese, 43% were smokers, and 17% had a prior amputation.
The patients were randomly assigned to receive a weekly application of EpiCord (n = 101) or treatment with an alginate wound dressing (n = 54) in addition to standard care. The percentage of patients whose wounds healed completely by 12 weeks later was higher in the study group than in those who were treated with alginate dressings (70% vs. 48%, respectively; P = .0089), per an intent-to-treat analysis.
The researchers also focused purely on patients who had received adequate debridement (107/155 ulcers, 69%). Of those ulcers, 64/67 (96%), in the study group healed completely at 12 weeks, compared with 26/40 (65%) of the alginate group (P less than .0001.)
The researchers did not notice any adverse effects related to either dressing.
According to the study, the findings regarding EpiCord are comparable with a sister study of a similar product by the same company that was tested in diabetic lower-extremity ulcers. That study, of a product called EpiFix, was published in the same issue of the journal (Int Wound J. 2019 Feb;16[1]:19-29).
“A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired,” the authors wrote of the EpiCord study.
MiMedx provided research funding to all of the authors.
SOURCE: Tettelbach W et al. Int Wound J. 2019;16(1):122-130. doi: 10.1111/iwj.12976.
Dehydrated human umbilical cord allograft may have benefit over alginate wound dressings as a treatment for chronic, nonhealing diabetic foot ulcers (DFU), findings from an industry-funded, randomized controlled study suggest.
The findings “provide additional evidence of the safety and efficacy of dehydrated placental tissues,” wrote William Tettelbach, MD, and his colleagues. Their report is in International Wound Journal.
The burden of diabetic foot disease in the United States is immense. A 2014 study estimated that treatment of DFUs alone cost public and private insurers as much as $13 billion per year (Diabetes Care. 2014;37(3):651-8).
MiMedx, which funded the new study, has developed a product called EpiCord to protect the DFU wound site. The product’s website describes it as a “unique, thick membrane derived from umbilical cord” that’s “minimally manipulated, dehydrated, [and] non-viable” (www.mimedx.com/epicord). The study authors noted that “immunogenicity of placental tissue lends credence to its use as an allograft material for difficult-to-heal wounds.”
For the new study, which was conducted from 2016 to 2018 and led by Dr. Tettelbach, an infectious disease specialist who is now an employee of MiMedx, the researchers enlisted 155 adult patients with stubborn DFUs at 11 centers in the United States.
All the ulcers had 30% or less wound area reduction after 14 days of standard care. The majority of patients (81%) were male; 63% were obese, 43% were smokers, and 17% had a prior amputation.
The patients were randomly assigned to receive a weekly application of EpiCord (n = 101) or treatment with an alginate wound dressing (n = 54) in addition to standard care. The percentage of patients whose wounds healed completely by 12 weeks later was higher in the study group than in those who were treated with alginate dressings (70% vs. 48%, respectively; P = .0089), per an intent-to-treat analysis.
The researchers also focused purely on patients who had received adequate debridement (107/155 ulcers, 69%). Of those ulcers, 64/67 (96%), in the study group healed completely at 12 weeks, compared with 26/40 (65%) of the alginate group (P less than .0001.)
The researchers did not notice any adverse effects related to either dressing.
According to the study, the findings regarding EpiCord are comparable with a sister study of a similar product by the same company that was tested in diabetic lower-extremity ulcers. That study, of a product called EpiFix, was published in the same issue of the journal (Int Wound J. 2019 Feb;16[1]:19-29).
“A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired,” the authors wrote of the EpiCord study.
MiMedx provided research funding to all of the authors.
SOURCE: Tettelbach W et al. Int Wound J. 2019;16(1):122-130. doi: 10.1111/iwj.12976.
Dehydrated human umbilical cord allograft may have benefit over alginate wound dressings as a treatment for chronic, nonhealing diabetic foot ulcers (DFU), findings from an industry-funded, randomized controlled study suggest.
The findings “provide additional evidence of the safety and efficacy of dehydrated placental tissues,” wrote William Tettelbach, MD, and his colleagues. Their report is in International Wound Journal.
The burden of diabetic foot disease in the United States is immense. A 2014 study estimated that treatment of DFUs alone cost public and private insurers as much as $13 billion per year (Diabetes Care. 2014;37(3):651-8).
MiMedx, which funded the new study, has developed a product called EpiCord to protect the DFU wound site. The product’s website describes it as a “unique, thick membrane derived from umbilical cord” that’s “minimally manipulated, dehydrated, [and] non-viable” (www.mimedx.com/epicord). The study authors noted that “immunogenicity of placental tissue lends credence to its use as an allograft material for difficult-to-heal wounds.”
For the new study, which was conducted from 2016 to 2018 and led by Dr. Tettelbach, an infectious disease specialist who is now an employee of MiMedx, the researchers enlisted 155 adult patients with stubborn DFUs at 11 centers in the United States.
All the ulcers had 30% or less wound area reduction after 14 days of standard care. The majority of patients (81%) were male; 63% were obese, 43% were smokers, and 17% had a prior amputation.
The patients were randomly assigned to receive a weekly application of EpiCord (n = 101) or treatment with an alginate wound dressing (n = 54) in addition to standard care. The percentage of patients whose wounds healed completely by 12 weeks later was higher in the study group than in those who were treated with alginate dressings (70% vs. 48%, respectively; P = .0089), per an intent-to-treat analysis.
The researchers also focused purely on patients who had received adequate debridement (107/155 ulcers, 69%). Of those ulcers, 64/67 (96%), in the study group healed completely at 12 weeks, compared with 26/40 (65%) of the alginate group (P less than .0001.)
The researchers did not notice any adverse effects related to either dressing.
According to the study, the findings regarding EpiCord are comparable with a sister study of a similar product by the same company that was tested in diabetic lower-extremity ulcers. That study, of a product called EpiFix, was published in the same issue of the journal (Int Wound J. 2019 Feb;16[1]:19-29).
“A thicker and more durable allograft such as EpiCord may be a good choice for implantation into deeper wounds and in situations where suturing the allograft in place is desired,” the authors wrote of the EpiCord study.
MiMedx provided research funding to all of the authors.
SOURCE: Tettelbach W et al. Int Wound J. 2019;16(1):122-130. doi: 10.1111/iwj.12976.
FROM INTERNATIONAL WOUND JOURNAL
Vagus nerve stimulation for rheumatology? Maybe
The work is being led by SetPoint Medical, a small company in Valencia, Calif., just north of Los Angeles. Its vagus nerve stimulation (VNS) device, dubbed the microregulator, has been implanted in 14 patients with refractory rheumatoid arthritis (RA) in the company’s initial safety study.
The microregulator is a small lithium ion battery encased in an inert silastic pod; it’s surgically implanted to sit atop the vagus nerve in the left side of the neck, and delivers an electrical pulse at set intervals. Data from the 12-week, sham-controlled safety study is set to be unblinded in coming weeks. A pivotal trial also is in the works, perhaps to start in late 2019, according to rheumatologist and SetPoint’s Chief Medical Officer David Chernoff, MD.
Although SetPoint is ahead of the pack, it’s not alone. ElectroCore, a biotech company in Basking Ridge, N.J., has expressed interest in pursuing rheumatoid arthritis and Sjögren’s syndrome indications for its gammaCore device, a vagus nerve stimulator patients apply to the neck. It’s already on the market for migraines and cluster headaches.
Researchers recently reported a small decrease in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) results after 16 RA patients with flares used the device for 4 days (Ann Rheum Dis. 2018;77:1401. Abstract AB0481). In another recent open-label study, 15 women with Sjögren’s reported less fatigue while using the device for a month (Arthritis Rheumatol. 2017;69[suppl 10]: Abstract 563).
Meanwhile, The Feinstein Institute for Medical Research, based in Manhasset, N.Y., on Long Island, recently reported positive outcomes in 18 patients with systemic lupus erythematosus, using its own novel device, which stimulates the vagus nerve through the ear lobe. VNS was delivered for 5 minutes per day for 4 days (Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2652).
On day 5, patients who received VNS, versus sham patients in whom the device was not turned on, had a significant decrease in pain, fatigue, and joint scores. The investigators concluded that “additional studies evaluating this promising intervention and its potential mechanisms are warranted.”
“We are clearly ahead of everybody because we’ve already implanted people, but I think it’s good for the field if more people are chasing this. The more resources that are put into it, the more we can show that this approach actually works,” said SetPoint’s Dr. Chernoff.
The hope
In general, interest in VNS for rheumatology is being driven by the possibility that it may reduce proinflammatory cytokines, which opens the door for VNS as an alternative to biologics. The hope is that instead of going after tumor necrosis factor and other cytokines one at a time, VNS could be used to target a range of cytokines all at once, without the cost and side effects of biologics.
“It seems so dramatically different” from what rheumatologists have done in the past, “that our first instinct is to say ‘oh, that’s ridiculous,’ but the science behind it is actually not bad. There may indeed be something to this,” said rheumatologist Joel Kremer, MD, Pfaff Family Professor of Medicine at Albany (N.Y.) Medical College.
Dr. Kremer reviewed SetPoint’s early scientific data after being asked by the company to participate in the safety study; he declined for logistical reasons.
He noted that “there are some strange interactions between the CNS and inflammatory disease.” When RA patients have a stroke, for instance, RA goes into remission on the side of their body affected by the stroke. “That’s been known for decades, but we really don’t understand what’s going on there,” Dr. Kremer said.
The evidence
Perhaps the strongest evidence to date for VNS as a cytokine blocker in rheumatology comes from an open-label, 12-week study, also conducted by SetPoint, in 17 patients with active RA despite methotrexate treatment; some had failed biologics (Proc Natl Acad Sci U S A. 2016 Jul 19;113[29]:8284-9. doi: 10.1073/pnas.160563511).
The microregulator wasn’t ready yet, so investigators implanted a VNS system commercially available for epilepsy and reprogrammed it to deliver a 60-second pulse once a day to the left cervical vagus nerve, which was increased after a month to four 60-second stimulations a day in nonresponders.
The investigators “observed that TNF production in cultured peripheral blood obtained ... on day 42 was significantly reduced from” 21 days before the study was started (TNF 2,900 pg/mL on day –21, versus 1,776 pg/mL on day 42; P less than .05).
When VNS was shut off, TNF production increased; when it was turned back on, it dropped. Interleukin 6 also fell significantly among responders. Overall, DAS28-CRP scores fell about 1.5 points on the 10-point scale from baseline to week 12.
Two-year outcomes were recently reported (Ann Rheum Dis. 2018;77:981-2. Abstract SAT0240). All 17 patients elected to continue treatment after the initial 12 weeks. Biologics were added in nine subjects (53%), because of no or limited response to VNS. Investigators were free to change the VNS dosing regimen, which varied during the study extension up to eight 60-second bursts a day. The roughly 1.5-point improvement in DAS28-CRP was maintained at 2 years.
“These long-term data suggest that bioelectronic therapy may be used as an alternative to, or in combination with, biological[s],” concluded Dr. Chernoff and other study team members.
Awaiting more data
When asked for comment, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, said “there certainly are neurotropic factors” at play in rheumatology, “so there’s sort of a potential reason why” VNS might work, “but we need to understand far more about its mechanism, and [remember] that open-label studies are not to be believed until” large, randomized, blinded, placebo-controlled studies are done.
Dr. Furst also is an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). He is in part-time practice in Los Angeles and Seattle.
If everything works out, however, “the vagus nerve may give us a much wider opportunity to block a host of cytokines; it may change the whole paradigm of how we manage rheumatoid arthritis. I think this is possibly a groundbreaking new therapeutic area, much in the way the biologics were” 20 years ago, said rheumatologist Norman B. Gaylis, MD.
Several of the 14 patients in SetPoint’s safety study were enrolled at Dr. Gaylis’s practice in Aventura, Fla., just north of Miami; he said he is eagerly awaiting for the results to be unblinded. If clinical response in that study and others correlates with a cytokine response, “that’s going to be big, and very significant” in the rheumatology community, he said.
SetPoint’s microregulator is charged wirelessly through a collar patients wear for a few minutes once a week. Dosing can also be adjusted through the collar with the help of a computer application.
The device wasn’t turned on in 4 of the 14 patients in the safety study, as a sham control, but shamming was problematic because patients can potentially feel VNS as a buzz or a change in their voice. To get around that potential confounder, both sham and treated patients were told they might or might not feel something during the study.
Implantation takes about an hour, and is much less complex than implanting currently available epilepsy VNS systems, which require implantation of both a power source on the chest wall and wire coils on the vagus nerve.
Cardiac concerns are the main safety issue with VNS, beyond the surgery itself. Cardiac monitoring was done in the safety study to “ensure that we did not cause things like bradycardia, heart block, syncope, etc.” Dr. Chernoff said. So far, they haven’t turned out to be a problem.
Dr. Furst and Dr. Kremer had no relevant disclosures. Dr. Gaylis was compensated by SetPoint for participating in the safety study; he is a consultant and investigator for Electrocore. Dr. Furst and Dr. Gaylis are members of the editorial advisory board for MDedge Rheumatology/Rheumatology News.
The work is being led by SetPoint Medical, a small company in Valencia, Calif., just north of Los Angeles. Its vagus nerve stimulation (VNS) device, dubbed the microregulator, has been implanted in 14 patients with refractory rheumatoid arthritis (RA) in the company’s initial safety study.
The microregulator is a small lithium ion battery encased in an inert silastic pod; it’s surgically implanted to sit atop the vagus nerve in the left side of the neck, and delivers an electrical pulse at set intervals. Data from the 12-week, sham-controlled safety study is set to be unblinded in coming weeks. A pivotal trial also is in the works, perhaps to start in late 2019, according to rheumatologist and SetPoint’s Chief Medical Officer David Chernoff, MD.
Although SetPoint is ahead of the pack, it’s not alone. ElectroCore, a biotech company in Basking Ridge, N.J., has expressed interest in pursuing rheumatoid arthritis and Sjögren’s syndrome indications for its gammaCore device, a vagus nerve stimulator patients apply to the neck. It’s already on the market for migraines and cluster headaches.
Researchers recently reported a small decrease in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) results after 16 RA patients with flares used the device for 4 days (Ann Rheum Dis. 2018;77:1401. Abstract AB0481). In another recent open-label study, 15 women with Sjögren’s reported less fatigue while using the device for a month (Arthritis Rheumatol. 2017;69[suppl 10]: Abstract 563).
Meanwhile, The Feinstein Institute for Medical Research, based in Manhasset, N.Y., on Long Island, recently reported positive outcomes in 18 patients with systemic lupus erythematosus, using its own novel device, which stimulates the vagus nerve through the ear lobe. VNS was delivered for 5 minutes per day for 4 days (Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2652).
On day 5, patients who received VNS, versus sham patients in whom the device was not turned on, had a significant decrease in pain, fatigue, and joint scores. The investigators concluded that “additional studies evaluating this promising intervention and its potential mechanisms are warranted.”
“We are clearly ahead of everybody because we’ve already implanted people, but I think it’s good for the field if more people are chasing this. The more resources that are put into it, the more we can show that this approach actually works,” said SetPoint’s Dr. Chernoff.
The hope
In general, interest in VNS for rheumatology is being driven by the possibility that it may reduce proinflammatory cytokines, which opens the door for VNS as an alternative to biologics. The hope is that instead of going after tumor necrosis factor and other cytokines one at a time, VNS could be used to target a range of cytokines all at once, without the cost and side effects of biologics.
“It seems so dramatically different” from what rheumatologists have done in the past, “that our first instinct is to say ‘oh, that’s ridiculous,’ but the science behind it is actually not bad. There may indeed be something to this,” said rheumatologist Joel Kremer, MD, Pfaff Family Professor of Medicine at Albany (N.Y.) Medical College.
Dr. Kremer reviewed SetPoint’s early scientific data after being asked by the company to participate in the safety study; he declined for logistical reasons.
He noted that “there are some strange interactions between the CNS and inflammatory disease.” When RA patients have a stroke, for instance, RA goes into remission on the side of their body affected by the stroke. “That’s been known for decades, but we really don’t understand what’s going on there,” Dr. Kremer said.
The evidence
Perhaps the strongest evidence to date for VNS as a cytokine blocker in rheumatology comes from an open-label, 12-week study, also conducted by SetPoint, in 17 patients with active RA despite methotrexate treatment; some had failed biologics (Proc Natl Acad Sci U S A. 2016 Jul 19;113[29]:8284-9. doi: 10.1073/pnas.160563511).
The microregulator wasn’t ready yet, so investigators implanted a VNS system commercially available for epilepsy and reprogrammed it to deliver a 60-second pulse once a day to the left cervical vagus nerve, which was increased after a month to four 60-second stimulations a day in nonresponders.
The investigators “observed that TNF production in cultured peripheral blood obtained ... on day 42 was significantly reduced from” 21 days before the study was started (TNF 2,900 pg/mL on day –21, versus 1,776 pg/mL on day 42; P less than .05).
When VNS was shut off, TNF production increased; when it was turned back on, it dropped. Interleukin 6 also fell significantly among responders. Overall, DAS28-CRP scores fell about 1.5 points on the 10-point scale from baseline to week 12.
Two-year outcomes were recently reported (Ann Rheum Dis. 2018;77:981-2. Abstract SAT0240). All 17 patients elected to continue treatment after the initial 12 weeks. Biologics were added in nine subjects (53%), because of no or limited response to VNS. Investigators were free to change the VNS dosing regimen, which varied during the study extension up to eight 60-second bursts a day. The roughly 1.5-point improvement in DAS28-CRP was maintained at 2 years.
“These long-term data suggest that bioelectronic therapy may be used as an alternative to, or in combination with, biological[s],” concluded Dr. Chernoff and other study team members.
Awaiting more data
When asked for comment, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, said “there certainly are neurotropic factors” at play in rheumatology, “so there’s sort of a potential reason why” VNS might work, “but we need to understand far more about its mechanism, and [remember] that open-label studies are not to be believed until” large, randomized, blinded, placebo-controlled studies are done.
Dr. Furst also is an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). He is in part-time practice in Los Angeles and Seattle.
If everything works out, however, “the vagus nerve may give us a much wider opportunity to block a host of cytokines; it may change the whole paradigm of how we manage rheumatoid arthritis. I think this is possibly a groundbreaking new therapeutic area, much in the way the biologics were” 20 years ago, said rheumatologist Norman B. Gaylis, MD.
Several of the 14 patients in SetPoint’s safety study were enrolled at Dr. Gaylis’s practice in Aventura, Fla., just north of Miami; he said he is eagerly awaiting for the results to be unblinded. If clinical response in that study and others correlates with a cytokine response, “that’s going to be big, and very significant” in the rheumatology community, he said.
SetPoint’s microregulator is charged wirelessly through a collar patients wear for a few minutes once a week. Dosing can also be adjusted through the collar with the help of a computer application.
The device wasn’t turned on in 4 of the 14 patients in the safety study, as a sham control, but shamming was problematic because patients can potentially feel VNS as a buzz or a change in their voice. To get around that potential confounder, both sham and treated patients were told they might or might not feel something during the study.
Implantation takes about an hour, and is much less complex than implanting currently available epilepsy VNS systems, which require implantation of both a power source on the chest wall and wire coils on the vagus nerve.
Cardiac concerns are the main safety issue with VNS, beyond the surgery itself. Cardiac monitoring was done in the safety study to “ensure that we did not cause things like bradycardia, heart block, syncope, etc.” Dr. Chernoff said. So far, they haven’t turned out to be a problem.
Dr. Furst and Dr. Kremer had no relevant disclosures. Dr. Gaylis was compensated by SetPoint for participating in the safety study; he is a consultant and investigator for Electrocore. Dr. Furst and Dr. Gaylis are members of the editorial advisory board for MDedge Rheumatology/Rheumatology News.
The work is being led by SetPoint Medical, a small company in Valencia, Calif., just north of Los Angeles. Its vagus nerve stimulation (VNS) device, dubbed the microregulator, has been implanted in 14 patients with refractory rheumatoid arthritis (RA) in the company’s initial safety study.
The microregulator is a small lithium ion battery encased in an inert silastic pod; it’s surgically implanted to sit atop the vagus nerve in the left side of the neck, and delivers an electrical pulse at set intervals. Data from the 12-week, sham-controlled safety study is set to be unblinded in coming weeks. A pivotal trial also is in the works, perhaps to start in late 2019, according to rheumatologist and SetPoint’s Chief Medical Officer David Chernoff, MD.
Although SetPoint is ahead of the pack, it’s not alone. ElectroCore, a biotech company in Basking Ridge, N.J., has expressed interest in pursuing rheumatoid arthritis and Sjögren’s syndrome indications for its gammaCore device, a vagus nerve stimulator patients apply to the neck. It’s already on the market for migraines and cluster headaches.
Researchers recently reported a small decrease in 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) results after 16 RA patients with flares used the device for 4 days (Ann Rheum Dis. 2018;77:1401. Abstract AB0481). In another recent open-label study, 15 women with Sjögren’s reported less fatigue while using the device for a month (Arthritis Rheumatol. 2017;69[suppl 10]: Abstract 563).
Meanwhile, The Feinstein Institute for Medical Research, based in Manhasset, N.Y., on Long Island, recently reported positive outcomes in 18 patients with systemic lupus erythematosus, using its own novel device, which stimulates the vagus nerve through the ear lobe. VNS was delivered for 5 minutes per day for 4 days (Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2652).
On day 5, patients who received VNS, versus sham patients in whom the device was not turned on, had a significant decrease in pain, fatigue, and joint scores. The investigators concluded that “additional studies evaluating this promising intervention and its potential mechanisms are warranted.”
“We are clearly ahead of everybody because we’ve already implanted people, but I think it’s good for the field if more people are chasing this. The more resources that are put into it, the more we can show that this approach actually works,” said SetPoint’s Dr. Chernoff.
The hope
In general, interest in VNS for rheumatology is being driven by the possibility that it may reduce proinflammatory cytokines, which opens the door for VNS as an alternative to biologics. The hope is that instead of going after tumor necrosis factor and other cytokines one at a time, VNS could be used to target a range of cytokines all at once, without the cost and side effects of biologics.
“It seems so dramatically different” from what rheumatologists have done in the past, “that our first instinct is to say ‘oh, that’s ridiculous,’ but the science behind it is actually not bad. There may indeed be something to this,” said rheumatologist Joel Kremer, MD, Pfaff Family Professor of Medicine at Albany (N.Y.) Medical College.
Dr. Kremer reviewed SetPoint’s early scientific data after being asked by the company to participate in the safety study; he declined for logistical reasons.
He noted that “there are some strange interactions between the CNS and inflammatory disease.” When RA patients have a stroke, for instance, RA goes into remission on the side of their body affected by the stroke. “That’s been known for decades, but we really don’t understand what’s going on there,” Dr. Kremer said.
The evidence
Perhaps the strongest evidence to date for VNS as a cytokine blocker in rheumatology comes from an open-label, 12-week study, also conducted by SetPoint, in 17 patients with active RA despite methotrexate treatment; some had failed biologics (Proc Natl Acad Sci U S A. 2016 Jul 19;113[29]:8284-9. doi: 10.1073/pnas.160563511).
The microregulator wasn’t ready yet, so investigators implanted a VNS system commercially available for epilepsy and reprogrammed it to deliver a 60-second pulse once a day to the left cervical vagus nerve, which was increased after a month to four 60-second stimulations a day in nonresponders.
The investigators “observed that TNF production in cultured peripheral blood obtained ... on day 42 was significantly reduced from” 21 days before the study was started (TNF 2,900 pg/mL on day –21, versus 1,776 pg/mL on day 42; P less than .05).
When VNS was shut off, TNF production increased; when it was turned back on, it dropped. Interleukin 6 also fell significantly among responders. Overall, DAS28-CRP scores fell about 1.5 points on the 10-point scale from baseline to week 12.
Two-year outcomes were recently reported (Ann Rheum Dis. 2018;77:981-2. Abstract SAT0240). All 17 patients elected to continue treatment after the initial 12 weeks. Biologics were added in nine subjects (53%), because of no or limited response to VNS. Investigators were free to change the VNS dosing regimen, which varied during the study extension up to eight 60-second bursts a day. The roughly 1.5-point improvement in DAS28-CRP was maintained at 2 years.
“These long-term data suggest that bioelectronic therapy may be used as an alternative to, or in combination with, biological[s],” concluded Dr. Chernoff and other study team members.
Awaiting more data
When asked for comment, Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, said “there certainly are neurotropic factors” at play in rheumatology, “so there’s sort of a potential reason why” VNS might work, “but we need to understand far more about its mechanism, and [remember] that open-label studies are not to be believed until” large, randomized, blinded, placebo-controlled studies are done.
Dr. Furst also is an adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy). He is in part-time practice in Los Angeles and Seattle.
If everything works out, however, “the vagus nerve may give us a much wider opportunity to block a host of cytokines; it may change the whole paradigm of how we manage rheumatoid arthritis. I think this is possibly a groundbreaking new therapeutic area, much in the way the biologics were” 20 years ago, said rheumatologist Norman B. Gaylis, MD.
Several of the 14 patients in SetPoint’s safety study were enrolled at Dr. Gaylis’s practice in Aventura, Fla., just north of Miami; he said he is eagerly awaiting for the results to be unblinded. If clinical response in that study and others correlates with a cytokine response, “that’s going to be big, and very significant” in the rheumatology community, he said.
SetPoint’s microregulator is charged wirelessly through a collar patients wear for a few minutes once a week. Dosing can also be adjusted through the collar with the help of a computer application.
The device wasn’t turned on in 4 of the 14 patients in the safety study, as a sham control, but shamming was problematic because patients can potentially feel VNS as a buzz or a change in their voice. To get around that potential confounder, both sham and treated patients were told they might or might not feel something during the study.
Implantation takes about an hour, and is much less complex than implanting currently available epilepsy VNS systems, which require implantation of both a power source on the chest wall and wire coils on the vagus nerve.
Cardiac concerns are the main safety issue with VNS, beyond the surgery itself. Cardiac monitoring was done in the safety study to “ensure that we did not cause things like bradycardia, heart block, syncope, etc.” Dr. Chernoff said. So far, they haven’t turned out to be a problem.
Dr. Furst and Dr. Kremer had no relevant disclosures. Dr. Gaylis was compensated by SetPoint for participating in the safety study; he is a consultant and investigator for Electrocore. Dr. Furst and Dr. Gaylis are members of the editorial advisory board for MDedge Rheumatology/Rheumatology News.
IV ketamine, intranasal esketamine likely to ‘happily coexist’
Dr. Steven Levine offers perspective on how the FDA approval will affect patients, practice
Q: Why is this a “banner day” for psychiatry?
A: This is truly the first new option for depression in 60 years. The selective serotonin reuptake inhibitors (SSRIs) developed in the mid-’80s were not truly new, not much different from the monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants. In fact, they work much like watered-down MAOIs. Esketamine works by a truly novel mechanism.
Even though it constitutes a relatively new treatment, ketamine is a very old medicine, and we probably know more about the pharmacology and mechanisms in depression than for the SSRIs.
The idea of SSRIs working by increasing levels of neurotransmitters like serotonin has never held water. We never really believed that, but for people who respond to them – and many are helped – what is really happening weeks to months down the line is that these drugs increase the plasticity of the brain. Depression, like other mental health conditions, disrupts connections between important brain regions, reducing the number, function, and quality of the connections, and we believe SSRIs improve these.
Ketamine does these same things by a different route and much, much more quickly.
Q: What is esketamine’s method of action, and how long will a dose last?
A: Ketamine and esketamine bind to and block glutamate N-methyl-D-aspartate (NMDA) receptors. This leads to the release of several chemical messengers, the result of which increases the production of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF), that play a key role in healing damaged connections in the brain. A single dose of esketamine would only be expected to relieve depression symptoms for days, up to a week or 2. Multiple doses over the first few weeks can extend the durability of response to several weeks and sometimes months.
It is not true for every patient, but some do have improvement within 2-4 hours that correlates with physiologic changes. Others can be later responders and require up to 6 exposures.
Q: The FDA approval requires those who administer the drug to complete special training and meet licensure requirements. Is this realistic for small practices?
A: Initially, not every psychiatrist will be able to offer esketamine, and I think it might be beyond the reach of small practices, and that’s probably okay. Enough people and enough centers will be able to offer it to meet the initial demand.
Q: Is esketamine “better” than ketamine infusions? With the approved drug available, will ketamine infusion clinics still have a place?
A: There are major pros with this. The FDA approval takes out of the gray area of off-label administration. It will most likely be covered by insurance now – a huge advantage that will put this in the reach of so many patients who haven’t been able to access this treatment.
I think that, because there are advantages and disadvantages for both IV ketamine and nasal esketamine, they will happily coexist for years to come. However, because nasal esketamine will likely be restricted to prescription by psychiatrists, it may have more impact on non-psychiatrist-led practices. In this way,
Dr. Steven Levine is the founder of Actify Neurotherapies, which operates nine clinics providing ketamine treatment for depression.
Dr. Steven Levine offers perspective on how the FDA approval will affect patients, practice
Dr. Steven Levine offers perspective on how the FDA approval will affect patients, practice
Q: Why is this a “banner day” for psychiatry?
A: This is truly the first new option for depression in 60 years. The selective serotonin reuptake inhibitors (SSRIs) developed in the mid-’80s were not truly new, not much different from the monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants. In fact, they work much like watered-down MAOIs. Esketamine works by a truly novel mechanism.
Even though it constitutes a relatively new treatment, ketamine is a very old medicine, and we probably know more about the pharmacology and mechanisms in depression than for the SSRIs.
The idea of SSRIs working by increasing levels of neurotransmitters like serotonin has never held water. We never really believed that, but for people who respond to them – and many are helped – what is really happening weeks to months down the line is that these drugs increase the plasticity of the brain. Depression, like other mental health conditions, disrupts connections between important brain regions, reducing the number, function, and quality of the connections, and we believe SSRIs improve these.
Ketamine does these same things by a different route and much, much more quickly.
Q: What is esketamine’s method of action, and how long will a dose last?
A: Ketamine and esketamine bind to and block glutamate N-methyl-D-aspartate (NMDA) receptors. This leads to the release of several chemical messengers, the result of which increases the production of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF), that play a key role in healing damaged connections in the brain. A single dose of esketamine would only be expected to relieve depression symptoms for days, up to a week or 2. Multiple doses over the first few weeks can extend the durability of response to several weeks and sometimes months.
It is not true for every patient, but some do have improvement within 2-4 hours that correlates with physiologic changes. Others can be later responders and require up to 6 exposures.
Q: The FDA approval requires those who administer the drug to complete special training and meet licensure requirements. Is this realistic for small practices?
A: Initially, not every psychiatrist will be able to offer esketamine, and I think it might be beyond the reach of small practices, and that’s probably okay. Enough people and enough centers will be able to offer it to meet the initial demand.
Q: Is esketamine “better” than ketamine infusions? With the approved drug available, will ketamine infusion clinics still have a place?
A: There are major pros with this. The FDA approval takes out of the gray area of off-label administration. It will most likely be covered by insurance now – a huge advantage that will put this in the reach of so many patients who haven’t been able to access this treatment.
I think that, because there are advantages and disadvantages for both IV ketamine and nasal esketamine, they will happily coexist for years to come. However, because nasal esketamine will likely be restricted to prescription by psychiatrists, it may have more impact on non-psychiatrist-led practices. In this way,
Dr. Steven Levine is the founder of Actify Neurotherapies, which operates nine clinics providing ketamine treatment for depression.
Q: Why is this a “banner day” for psychiatry?
A: This is truly the first new option for depression in 60 years. The selective serotonin reuptake inhibitors (SSRIs) developed in the mid-’80s were not truly new, not much different from the monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants. In fact, they work much like watered-down MAOIs. Esketamine works by a truly novel mechanism.
Even though it constitutes a relatively new treatment, ketamine is a very old medicine, and we probably know more about the pharmacology and mechanisms in depression than for the SSRIs.
The idea of SSRIs working by increasing levels of neurotransmitters like serotonin has never held water. We never really believed that, but for people who respond to them – and many are helped – what is really happening weeks to months down the line is that these drugs increase the plasticity of the brain. Depression, like other mental health conditions, disrupts connections between important brain regions, reducing the number, function, and quality of the connections, and we believe SSRIs improve these.
Ketamine does these same things by a different route and much, much more quickly.
Q: What is esketamine’s method of action, and how long will a dose last?
A: Ketamine and esketamine bind to and block glutamate N-methyl-D-aspartate (NMDA) receptors. This leads to the release of several chemical messengers, the result of which increases the production of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF), that play a key role in healing damaged connections in the brain. A single dose of esketamine would only be expected to relieve depression symptoms for days, up to a week or 2. Multiple doses over the first few weeks can extend the durability of response to several weeks and sometimes months.
It is not true for every patient, but some do have improvement within 2-4 hours that correlates with physiologic changes. Others can be later responders and require up to 6 exposures.
Q: The FDA approval requires those who administer the drug to complete special training and meet licensure requirements. Is this realistic for small practices?
A: Initially, not every psychiatrist will be able to offer esketamine, and I think it might be beyond the reach of small practices, and that’s probably okay. Enough people and enough centers will be able to offer it to meet the initial demand.
Q: Is esketamine “better” than ketamine infusions? With the approved drug available, will ketamine infusion clinics still have a place?
A: There are major pros with this. The FDA approval takes out of the gray area of off-label administration. It will most likely be covered by insurance now – a huge advantage that will put this in the reach of so many patients who haven’t been able to access this treatment.
I think that, because there are advantages and disadvantages for both IV ketamine and nasal esketamine, they will happily coexist for years to come. However, because nasal esketamine will likely be restricted to prescription by psychiatrists, it may have more impact on non-psychiatrist-led practices. In this way,
Dr. Steven Levine is the founder of Actify Neurotherapies, which operates nine clinics providing ketamine treatment for depression.
The Oscars of contact allergens
Multiple sclerosis prevalence estimates reach their highest point to date. ASCO issues a new guideline for early detection and management of colorectal cancer in average-risk patients. And food allergies at age 1 often disappear by age 6.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Multiple sclerosis prevalence estimates reach their highest point to date. ASCO issues a new guideline for early detection and management of colorectal cancer in average-risk patients. And food allergies at age 1 often disappear by age 6.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Multiple sclerosis prevalence estimates reach their highest point to date. ASCO issues a new guideline for early detection and management of colorectal cancer in average-risk patients. And food allergies at age 1 often disappear by age 6.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify