User login
Ultrasound Monitoring of IBD May Prompt Faster Treatment Change
, according to a small retrospective analysis.
“Current disease monitoring tools have significant limitations,” said Noa Krugliak Cleveland, MD, director of the intestinal ultrasound program at the University of Chicago. “Intestinal ultrasound is an innovative technology that enables point-of-care assessment.”
Dr. Cleveland presented the findings at the October 2023 American College of Gastroenterology’s annual scientific meeting in Vancouver, Canada.
The analysis was based on 30 patients with IBD in an ongoing real-world prospective study of upadacitinib (Rinvoq, Abbvie) who were not in clinical remission at week 8. For 11 patients, routine clinical care included IUS; the other 19 patients were monitored using a conventional approach.
In the study, both groups were almost evenly split in terms of diagnosis. In the IUS group, four patients had Crohn’s disease and five had ulcerative colitis. In the conventional management group, six had Crohn’s disease and five had ulcerative colitis.
The primary endpoint was time to treatment change.
For the secondary endpoint, the researchers defined clinical remission as a Simple Clinical Colitis Activity Index ≤ 2, or Harvey-Bradshaw Index ≤ 4, and by IUS as bowel wall thickness ≤ 3 mm in the colon or terminal ileum and no hyperemia by color Doppler signal.
The average time to treatment change in the IUS group was 1.1 days, compared with 16.6 days for the conventional management group, Dr. Cleveland reported.
The average time to clinical remission was 26.8 days for the IUS group, compared with 55.3 days for the conventional management group.
The delays in treatment change in the conventional management group were attributed to awaiting test results and endoscopy procedures, as well as communications among clinical team members.
Strength of this research project included its prospective data collection and the experienced sonographers who participated, Dr. Cleveland and colleagues said. Limitations included retrospective analysis, a small number of patients on a single therapy, and the potential for bias in patient selection. Studies of other therapies and a prospective trial are underway.
During the presentation, Dr. Cleveland commented about what kinds of treatment changes were made for patients in the study. They commonly involved extending the induction time, and, in some cases, patients were switched to another treatment, she said.
In an interview, Michael Dolinger, MD, of the Icahn School of Medicine at Mount Sinai in New York, said more research needs to be done to show whether IUS will improve outcomes.
“They’re showing that they make more changes sooner,” he said. “Does that actually affect and improve outcomes? That’s the big question.”
Dr. Dolinger said the concept for using IUS is that it helps physicians catch disease flares earlier and respond faster with changes to the treatment plan, thus preventing the buildup of chronic bowel damage.
“That’s the concept, but that concept is actually not so proven in reality” yet, he said. “But I do believe that they’re on the right path.”
In Dr. Dolinger’s view, adding ultrasound provides a more patient-centric approach to care of people with IBD. With more traditional approaches, patients often are waiting for results of tests done outside of the visit, such as MRI.
“With ultrasound, I am walking them through the results as it’s happening in real time during the clinic visit,” Dr. Dolinger said. ”I am showing them on the screen, allowing them to ask questions. They’re telling me about their symptoms, as I’m putting the probe on where it may hurt, as I’m showing them inflammation or healing. And that changes the whole conversation.”
The study received support from the Mutchnik Family Foundation. Dr. Cleveland reported financial relationships with Bristol Myers Squibb, Neurologica, and Takeda. Her coauthors reported financial relationships with multiple drug and device makers. Dr. Dolinger said he is a consultant for Samsung’s Neurologica Corp., which makes ultrasound equipment.
, according to a small retrospective analysis.
“Current disease monitoring tools have significant limitations,” said Noa Krugliak Cleveland, MD, director of the intestinal ultrasound program at the University of Chicago. “Intestinal ultrasound is an innovative technology that enables point-of-care assessment.”
Dr. Cleveland presented the findings at the October 2023 American College of Gastroenterology’s annual scientific meeting in Vancouver, Canada.
The analysis was based on 30 patients with IBD in an ongoing real-world prospective study of upadacitinib (Rinvoq, Abbvie) who were not in clinical remission at week 8. For 11 patients, routine clinical care included IUS; the other 19 patients were monitored using a conventional approach.
In the study, both groups were almost evenly split in terms of diagnosis. In the IUS group, four patients had Crohn’s disease and five had ulcerative colitis. In the conventional management group, six had Crohn’s disease and five had ulcerative colitis.
The primary endpoint was time to treatment change.
For the secondary endpoint, the researchers defined clinical remission as a Simple Clinical Colitis Activity Index ≤ 2, or Harvey-Bradshaw Index ≤ 4, and by IUS as bowel wall thickness ≤ 3 mm in the colon or terminal ileum and no hyperemia by color Doppler signal.
The average time to treatment change in the IUS group was 1.1 days, compared with 16.6 days for the conventional management group, Dr. Cleveland reported.
The average time to clinical remission was 26.8 days for the IUS group, compared with 55.3 days for the conventional management group.
The delays in treatment change in the conventional management group were attributed to awaiting test results and endoscopy procedures, as well as communications among clinical team members.
Strength of this research project included its prospective data collection and the experienced sonographers who participated, Dr. Cleveland and colleagues said. Limitations included retrospective analysis, a small number of patients on a single therapy, and the potential for bias in patient selection. Studies of other therapies and a prospective trial are underway.
During the presentation, Dr. Cleveland commented about what kinds of treatment changes were made for patients in the study. They commonly involved extending the induction time, and, in some cases, patients were switched to another treatment, she said.
In an interview, Michael Dolinger, MD, of the Icahn School of Medicine at Mount Sinai in New York, said more research needs to be done to show whether IUS will improve outcomes.
“They’re showing that they make more changes sooner,” he said. “Does that actually affect and improve outcomes? That’s the big question.”
Dr. Dolinger said the concept for using IUS is that it helps physicians catch disease flares earlier and respond faster with changes to the treatment plan, thus preventing the buildup of chronic bowel damage.
“That’s the concept, but that concept is actually not so proven in reality” yet, he said. “But I do believe that they’re on the right path.”
In Dr. Dolinger’s view, adding ultrasound provides a more patient-centric approach to care of people with IBD. With more traditional approaches, patients often are waiting for results of tests done outside of the visit, such as MRI.
“With ultrasound, I am walking them through the results as it’s happening in real time during the clinic visit,” Dr. Dolinger said. ”I am showing them on the screen, allowing them to ask questions. They’re telling me about their symptoms, as I’m putting the probe on where it may hurt, as I’m showing them inflammation or healing. And that changes the whole conversation.”
The study received support from the Mutchnik Family Foundation. Dr. Cleveland reported financial relationships with Bristol Myers Squibb, Neurologica, and Takeda. Her coauthors reported financial relationships with multiple drug and device makers. Dr. Dolinger said he is a consultant for Samsung’s Neurologica Corp., which makes ultrasound equipment.
, according to a small retrospective analysis.
“Current disease monitoring tools have significant limitations,” said Noa Krugliak Cleveland, MD, director of the intestinal ultrasound program at the University of Chicago. “Intestinal ultrasound is an innovative technology that enables point-of-care assessment.”
Dr. Cleveland presented the findings at the October 2023 American College of Gastroenterology’s annual scientific meeting in Vancouver, Canada.
The analysis was based on 30 patients with IBD in an ongoing real-world prospective study of upadacitinib (Rinvoq, Abbvie) who were not in clinical remission at week 8. For 11 patients, routine clinical care included IUS; the other 19 patients were monitored using a conventional approach.
In the study, both groups were almost evenly split in terms of diagnosis. In the IUS group, four patients had Crohn’s disease and five had ulcerative colitis. In the conventional management group, six had Crohn’s disease and five had ulcerative colitis.
The primary endpoint was time to treatment change.
For the secondary endpoint, the researchers defined clinical remission as a Simple Clinical Colitis Activity Index ≤ 2, or Harvey-Bradshaw Index ≤ 4, and by IUS as bowel wall thickness ≤ 3 mm in the colon or terminal ileum and no hyperemia by color Doppler signal.
The average time to treatment change in the IUS group was 1.1 days, compared with 16.6 days for the conventional management group, Dr. Cleveland reported.
The average time to clinical remission was 26.8 days for the IUS group, compared with 55.3 days for the conventional management group.
The delays in treatment change in the conventional management group were attributed to awaiting test results and endoscopy procedures, as well as communications among clinical team members.
Strength of this research project included its prospective data collection and the experienced sonographers who participated, Dr. Cleveland and colleagues said. Limitations included retrospective analysis, a small number of patients on a single therapy, and the potential for bias in patient selection. Studies of other therapies and a prospective trial are underway.
During the presentation, Dr. Cleveland commented about what kinds of treatment changes were made for patients in the study. They commonly involved extending the induction time, and, in some cases, patients were switched to another treatment, she said.
In an interview, Michael Dolinger, MD, of the Icahn School of Medicine at Mount Sinai in New York, said more research needs to be done to show whether IUS will improve outcomes.
“They’re showing that they make more changes sooner,” he said. “Does that actually affect and improve outcomes? That’s the big question.”
Dr. Dolinger said the concept for using IUS is that it helps physicians catch disease flares earlier and respond faster with changes to the treatment plan, thus preventing the buildup of chronic bowel damage.
“That’s the concept, but that concept is actually not so proven in reality” yet, he said. “But I do believe that they’re on the right path.”
In Dr. Dolinger’s view, adding ultrasound provides a more patient-centric approach to care of people with IBD. With more traditional approaches, patients often are waiting for results of tests done outside of the visit, such as MRI.
“With ultrasound, I am walking them through the results as it’s happening in real time during the clinic visit,” Dr. Dolinger said. ”I am showing them on the screen, allowing them to ask questions. They’re telling me about their symptoms, as I’m putting the probe on where it may hurt, as I’m showing them inflammation or healing. And that changes the whole conversation.”
The study received support from the Mutchnik Family Foundation. Dr. Cleveland reported financial relationships with Bristol Myers Squibb, Neurologica, and Takeda. Her coauthors reported financial relationships with multiple drug and device makers. Dr. Dolinger said he is a consultant for Samsung’s Neurologica Corp., which makes ultrasound equipment.
FROM ACG 2023
Association Between LDL-C and Androgenetic Alopecia Among Female Patients in a Specialty Alopecia Clinic
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).
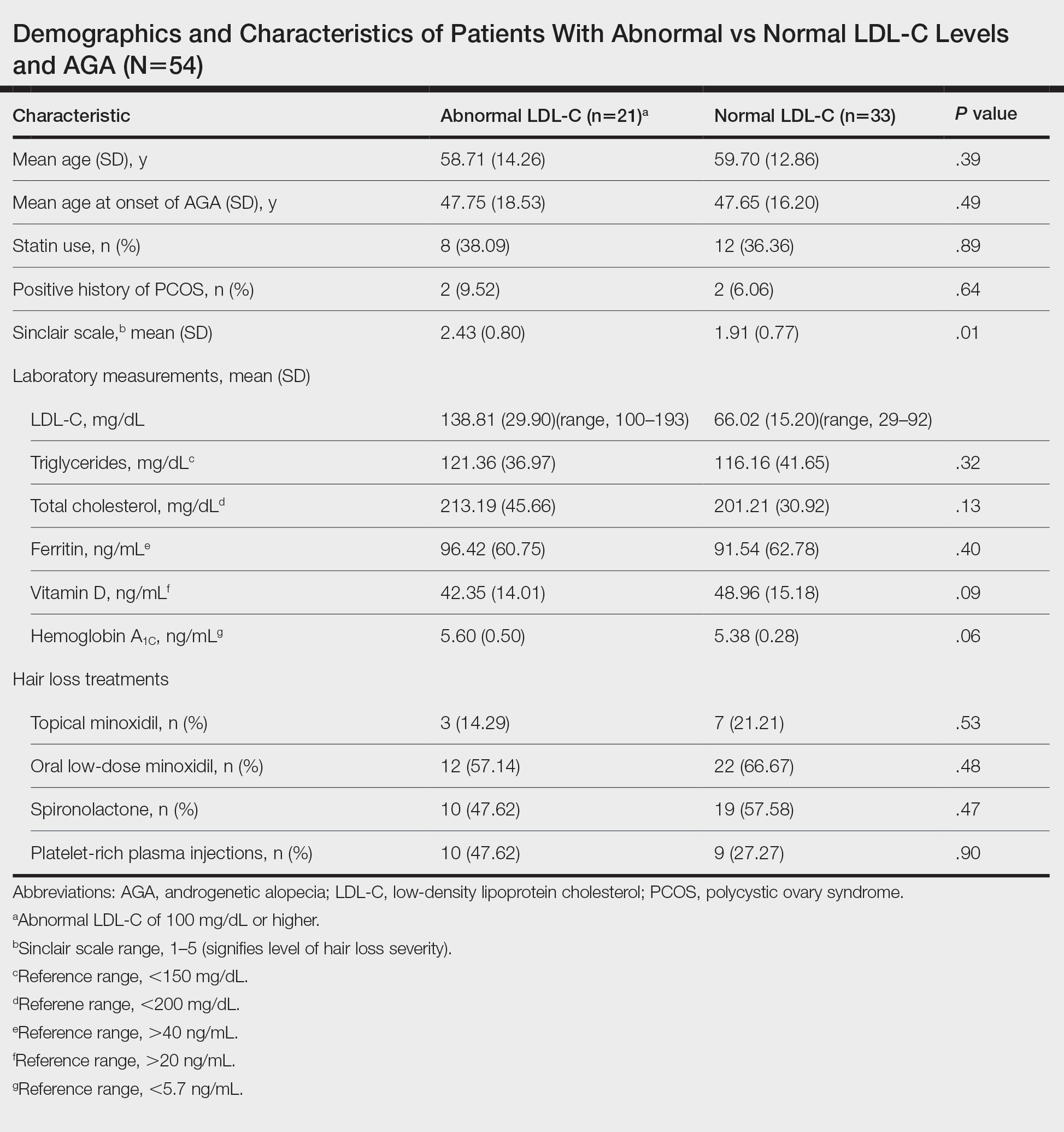
We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
Practice Points
- Associations have been shown between hair loss and markers of bad health such as insulin resistance and high cholesterol. Research has not yet shown the relationship between hair loss severity and these markers, particularly cholesterol.
Acne and Pregnancy: A Clinical Review and Practice Pearls
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.
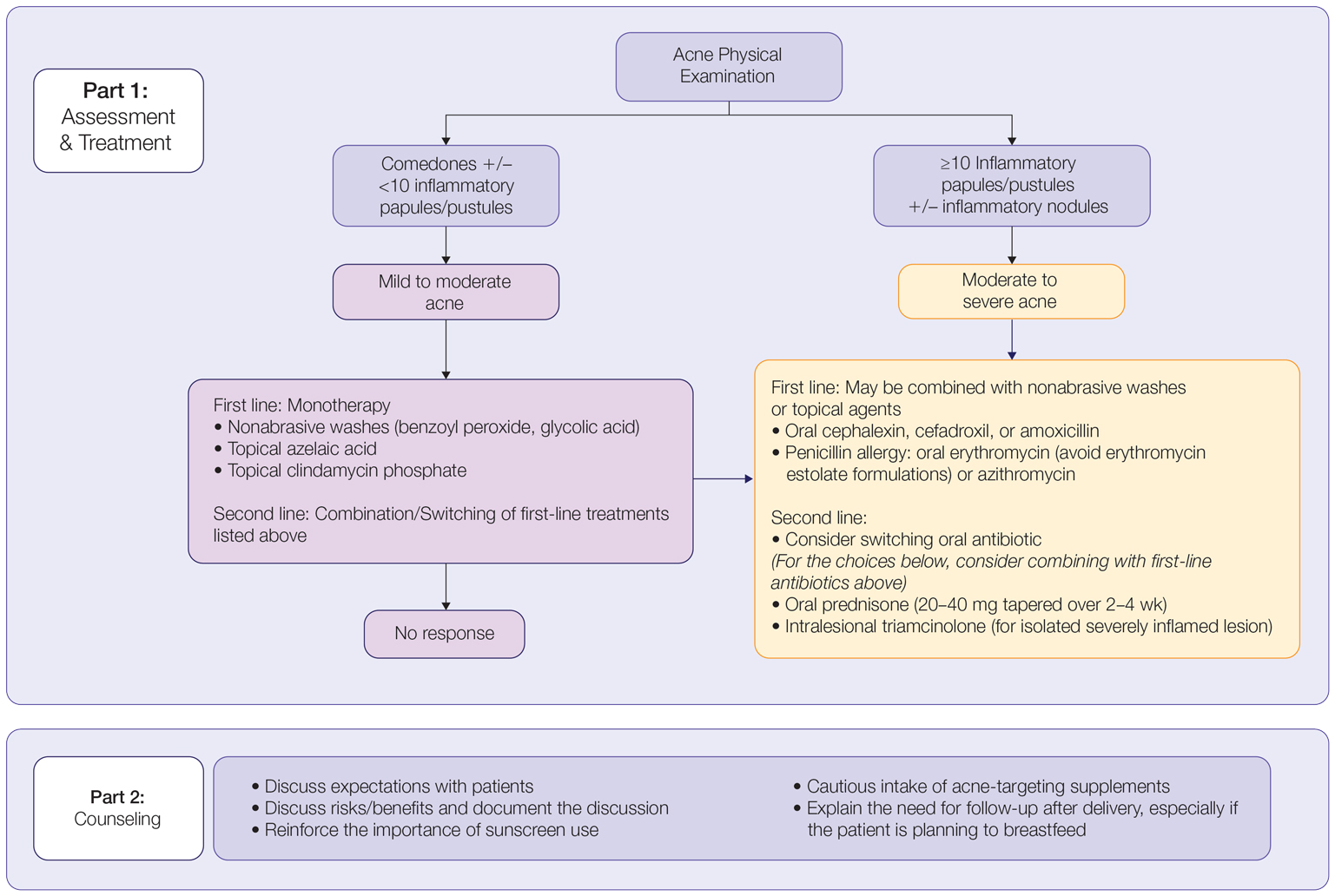
In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Practice Points
- The management of acne in pregnancy requires careful consideration of therapeutic choices to guarantee the safety of both the mother and the developing fetus.
- The use of topicals should be observed as first-line therapy, but consideration for systemic therapy in cases of treatment failure or more severe disease is warranted.
- Discussion of patient expectations and involving them in decision-making for therapeutic choice is crucial.
A New Treatment Target for PTSD?
Adults with posttraumatic stress disorder (PTSD) have smaller cerebellums than unaffected adults, suggesting that this part of the brain may be a potential therapeutic target.
According to recent research on more than 4000 adults, cerebellum volume was significantly smaller (by about 2%) in those with PTSD than in trauma-exposed and trauma-naive controls without PTSD.
“The differences were largely within the posterior lobe, where a lot of the more cognitive functions attributed to the cerebellum seem to localize, as well as the vermis, which is linked to a lot of emotional processing functions,” lead author Ashley Huggins, PhD, said in a news release.
“If we know what areas are implicated, then we can start to focus interventions like brain stimulation on the cerebellum and potentially improve treatment outcomes,” said Dr. Huggins, who worked on the study while a postdoctoral researcher in the lab of Rajendra A. Morey, MD, at Duke University, Durham, North Carolina, and is now at the University of Arizona, Tucson.
While the cerebellum is known for its role in coordinating movement and balance, it also plays a key role in emotions and memory, which are affected by PTSD.
Smaller cerebellar volume has been observed in some adult and pediatric populations with PTSD.
However, those studies have been limited by either small sample sizes, the failure to consider key neuroanatomical subdivisions of the cerebellum, or a focus on certain populations such as veterans of sexual assault victims with PTSD.
To overcome these limitations, the researchers conducted a mega-analysis of total and subregional cerebellar volumes in a large, multicohort dataset from the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA)-Psychiatric Genomics Consortium PTSD workgroup that was published online on January 10, 2024, in Molecular Psychiatry.
They employed a novel, standardized ENIGMA cerebellum parcellation protocol to quantify cerebellar lobule volumes using structural MRI data from 1642 adults with PTSD and 2573 healthy controls without PTSD (88% trauma-exposed and 12% trauma-naive).
After adjustment for age, gender, and total intracranial volume, PTSD was associated with significant gray and white matter reductions of the cerebellum.
People with PTSD demonstrated smaller total cerebellum volume as well as reduced volume in subregions primarily within the posterior cerebellum, vermis, and flocculonodular cerebellum than controls.
In general, PTSD severity was more robustly associated with cerebellar volume differences than PTSD diagnosis.
Focusing purely on a “yes-or-no” categorical diagnosis didn’t always provide the clearest picture. “When we looked at PTSD severity, people who had more severe forms of the disorder had an even smaller cerebellar volume,” Dr. Huggins explained in the news release.
Novel Treatment Target
These findings add to “an emerging literature that underscores the relevance of cerebellar structure in the pathophysiology of PTSD,” the researchers noted.
They caution that despite the significant findings suggesting associations between PTSD and smaller cerebellar volumes, effect sizes were small. “As such, it is unlikely that structural cerebellar volumes alone will provide a clinically useful biomarker (eg, for individual-level prediction).”
Nonetheless, the study highlights the cerebellum as a “novel treatment target that may be leveraged to improve treatment outcomes for PTSD,” they wrote.
They noted that prior work has shown that the cerebellum is sensitive to external modulation. For example, noninvasive brain stimulation of the cerebellum has been shown to modulate cognitive, emotional, and social processes commonly disrupted in PTSD.
Commenting on this research, Cyrus A. Raji, MD, PhD, associate professor of radiology and neurology at Washington University in St. Louis, noted that this “large neuroimaging study links PTSD to cerebellar volume loss.”
“However, PTSD and traumatic brain injury frequently co-occur, and PTSD also frequently arises after TBI. Additionally, TBI is strongly linked to cerebellar volume loss,” Dr. Raji pointed out.
“Future studies need to better delineate volume loss from these conditions, especially when they are comorbid, though the expectation is these effects would be additive with TBI being the initial and most severe driving force,” Dr. Raji added.
The research had no commercial funding. Author disclosures are listed with the original article. Dr. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution Medicine LLC.
A version of this article appears on Medscape.com.
Adults with posttraumatic stress disorder (PTSD) have smaller cerebellums than unaffected adults, suggesting that this part of the brain may be a potential therapeutic target.
According to recent research on more than 4000 adults, cerebellum volume was significantly smaller (by about 2%) in those with PTSD than in trauma-exposed and trauma-naive controls without PTSD.
“The differences were largely within the posterior lobe, where a lot of the more cognitive functions attributed to the cerebellum seem to localize, as well as the vermis, which is linked to a lot of emotional processing functions,” lead author Ashley Huggins, PhD, said in a news release.
“If we know what areas are implicated, then we can start to focus interventions like brain stimulation on the cerebellum and potentially improve treatment outcomes,” said Dr. Huggins, who worked on the study while a postdoctoral researcher in the lab of Rajendra A. Morey, MD, at Duke University, Durham, North Carolina, and is now at the University of Arizona, Tucson.
While the cerebellum is known for its role in coordinating movement and balance, it also plays a key role in emotions and memory, which are affected by PTSD.
Smaller cerebellar volume has been observed in some adult and pediatric populations with PTSD.
However, those studies have been limited by either small sample sizes, the failure to consider key neuroanatomical subdivisions of the cerebellum, or a focus on certain populations such as veterans of sexual assault victims with PTSD.
To overcome these limitations, the researchers conducted a mega-analysis of total and subregional cerebellar volumes in a large, multicohort dataset from the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA)-Psychiatric Genomics Consortium PTSD workgroup that was published online on January 10, 2024, in Molecular Psychiatry.
They employed a novel, standardized ENIGMA cerebellum parcellation protocol to quantify cerebellar lobule volumes using structural MRI data from 1642 adults with PTSD and 2573 healthy controls without PTSD (88% trauma-exposed and 12% trauma-naive).
After adjustment for age, gender, and total intracranial volume, PTSD was associated with significant gray and white matter reductions of the cerebellum.
People with PTSD demonstrated smaller total cerebellum volume as well as reduced volume in subregions primarily within the posterior cerebellum, vermis, and flocculonodular cerebellum than controls.
In general, PTSD severity was more robustly associated with cerebellar volume differences than PTSD diagnosis.
Focusing purely on a “yes-or-no” categorical diagnosis didn’t always provide the clearest picture. “When we looked at PTSD severity, people who had more severe forms of the disorder had an even smaller cerebellar volume,” Dr. Huggins explained in the news release.
Novel Treatment Target
These findings add to “an emerging literature that underscores the relevance of cerebellar structure in the pathophysiology of PTSD,” the researchers noted.
They caution that despite the significant findings suggesting associations between PTSD and smaller cerebellar volumes, effect sizes were small. “As such, it is unlikely that structural cerebellar volumes alone will provide a clinically useful biomarker (eg, for individual-level prediction).”
Nonetheless, the study highlights the cerebellum as a “novel treatment target that may be leveraged to improve treatment outcomes for PTSD,” they wrote.
They noted that prior work has shown that the cerebellum is sensitive to external modulation. For example, noninvasive brain stimulation of the cerebellum has been shown to modulate cognitive, emotional, and social processes commonly disrupted in PTSD.
Commenting on this research, Cyrus A. Raji, MD, PhD, associate professor of radiology and neurology at Washington University in St. Louis, noted that this “large neuroimaging study links PTSD to cerebellar volume loss.”
“However, PTSD and traumatic brain injury frequently co-occur, and PTSD also frequently arises after TBI. Additionally, TBI is strongly linked to cerebellar volume loss,” Dr. Raji pointed out.
“Future studies need to better delineate volume loss from these conditions, especially when they are comorbid, though the expectation is these effects would be additive with TBI being the initial and most severe driving force,” Dr. Raji added.
The research had no commercial funding. Author disclosures are listed with the original article. Dr. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution Medicine LLC.
A version of this article appears on Medscape.com.
Adults with posttraumatic stress disorder (PTSD) have smaller cerebellums than unaffected adults, suggesting that this part of the brain may be a potential therapeutic target.
According to recent research on more than 4000 adults, cerebellum volume was significantly smaller (by about 2%) in those with PTSD than in trauma-exposed and trauma-naive controls without PTSD.
“The differences were largely within the posterior lobe, where a lot of the more cognitive functions attributed to the cerebellum seem to localize, as well as the vermis, which is linked to a lot of emotional processing functions,” lead author Ashley Huggins, PhD, said in a news release.
“If we know what areas are implicated, then we can start to focus interventions like brain stimulation on the cerebellum and potentially improve treatment outcomes,” said Dr. Huggins, who worked on the study while a postdoctoral researcher in the lab of Rajendra A. Morey, MD, at Duke University, Durham, North Carolina, and is now at the University of Arizona, Tucson.
While the cerebellum is known for its role in coordinating movement and balance, it also plays a key role in emotions and memory, which are affected by PTSD.
Smaller cerebellar volume has been observed in some adult and pediatric populations with PTSD.
However, those studies have been limited by either small sample sizes, the failure to consider key neuroanatomical subdivisions of the cerebellum, or a focus on certain populations such as veterans of sexual assault victims with PTSD.
To overcome these limitations, the researchers conducted a mega-analysis of total and subregional cerebellar volumes in a large, multicohort dataset from the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA)-Psychiatric Genomics Consortium PTSD workgroup that was published online on January 10, 2024, in Molecular Psychiatry.
They employed a novel, standardized ENIGMA cerebellum parcellation protocol to quantify cerebellar lobule volumes using structural MRI data from 1642 adults with PTSD and 2573 healthy controls without PTSD (88% trauma-exposed and 12% trauma-naive).
After adjustment for age, gender, and total intracranial volume, PTSD was associated with significant gray and white matter reductions of the cerebellum.
People with PTSD demonstrated smaller total cerebellum volume as well as reduced volume in subregions primarily within the posterior cerebellum, vermis, and flocculonodular cerebellum than controls.
In general, PTSD severity was more robustly associated with cerebellar volume differences than PTSD diagnosis.
Focusing purely on a “yes-or-no” categorical diagnosis didn’t always provide the clearest picture. “When we looked at PTSD severity, people who had more severe forms of the disorder had an even smaller cerebellar volume,” Dr. Huggins explained in the news release.
Novel Treatment Target
These findings add to “an emerging literature that underscores the relevance of cerebellar structure in the pathophysiology of PTSD,” the researchers noted.
They caution that despite the significant findings suggesting associations between PTSD and smaller cerebellar volumes, effect sizes were small. “As such, it is unlikely that structural cerebellar volumes alone will provide a clinically useful biomarker (eg, for individual-level prediction).”
Nonetheless, the study highlights the cerebellum as a “novel treatment target that may be leveraged to improve treatment outcomes for PTSD,” they wrote.
They noted that prior work has shown that the cerebellum is sensitive to external modulation. For example, noninvasive brain stimulation of the cerebellum has been shown to modulate cognitive, emotional, and social processes commonly disrupted in PTSD.
Commenting on this research, Cyrus A. Raji, MD, PhD, associate professor of radiology and neurology at Washington University in St. Louis, noted that this “large neuroimaging study links PTSD to cerebellar volume loss.”
“However, PTSD and traumatic brain injury frequently co-occur, and PTSD also frequently arises after TBI. Additionally, TBI is strongly linked to cerebellar volume loss,” Dr. Raji pointed out.
“Future studies need to better delineate volume loss from these conditions, especially when they are comorbid, though the expectation is these effects would be additive with TBI being the initial and most severe driving force,” Dr. Raji added.
The research had no commercial funding. Author disclosures are listed with the original article. Dr. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution Medicine LLC.
A version of this article appears on Medscape.com.
Weight Loss Not Enough to Sustain Type 2 Diabetes Remission
Very few patients with type 2 diabetes (T2D) achieve and sustain diabetes remission via weight loss alone, new research suggests.
Among more than 37,000 people with T2D in Hong Kong, only 6% had achieved and sustained diabetes remission solely through weight loss up to 8 years after diagnosis. Among those who initially achieved remission, 67% had hyperglycemia at 3 years.
People who lost the most weight (10% of their body weight or more) in the first year after diagnosis were most likely to have sustained remission.
The study “helped to confirm the low rate of diabetes remission and high rate of returning to hyperglycemia in real-world practice,” Andrea Luk, MD, of the Chinese University of Hong Kong, told this news organization. “Over 80% of diabetes remission occurred within the first 5 years of a diabetes diagnosis. This is in line with our understanding that beta cell function will gradually decline over time, making diabetes remission increasingly difficult even with weight reduction.”
The study was published in PLOS Medicine.
Early Weight Management Works
Recent clinical trials have demonstrated that T2D remission can be achieved following sustained weight loss through bariatric surgery or lifestyle interventions, the authors noted. In this study, they investigated the association of weight change at 1 year after a diabetes diagnosis with the long-term incidence and sustainability of T2D remission in real-world settings, using data from the territory-wide Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM).
A total of 37,326 people with newly diagnosed T2D who were enrolled in the RAMP-DM between 2000 and 2017 were included and followed until 2019.
At baseline, participants’ mean age was 56.6 years, mean body mass index (BMI) was 26.4 kg/m2, and mean A1c was 7.7%, and 65% were using glucose-lowering drugs (GLDs).
T2D remission was defined as two consecutive A1c < 6.5% measurements at least 6 months apart without GLDs currently or in the previous 3 months.
During a median follow-up of 7.9 years, 6.1% of people achieved remission, with an incidence rate of 7.8 per 1000 person-years. The proportion was higher among those with greater weight loss: 14.4% of people who lost 10% of their body weight or more achieved remission compared with 9.9% of those with 5%-9.9% weight loss, 6.5% of those with 0%-4.9% weight loss, and 4.5% of those who gained weight.
After adjustment for age at diagnosis, sex, assessment year, BMI, other metabolic indices, smoking, alcohol drinking, and medication use, the hazard ratio (HR) for diabetes remission was 3.28 for those with 10% or greater weight loss within 1 year of diagnosis, 2.29 for 5%-9.9% weight loss, and 1.34 for 0%-4.9% weight loss compared to weight gain.
The incidence of diabetes remission in the study was significantly lower than that in clinical trials, possibly because trial participants were in structured programs that included intensive lifestyle interventions, regular monitoring and feedback, and reinforcement of a holistic approach to managing diabetes, the authors noted. Real-world settings may or may not include such interventions.
Further analyses showed that within a median follow-up of 3.1 years, 67.2% of people who had achieved diabetes remission returned to hyperglycemia — an incidence rate of 184.8 per 1000 person-years.
The adjusted HR for returning to hyperglycemia was 0.52 for people with 10% or greater weight loss, 0.78 for those with 5%-9.9% weight loss, and 0.90 for those with 0%-4.9% weight loss compared to people with weight gain.
In addition, diabetes remission was associated with a 31% (HR, 0.69) decreased risk for all-cause mortality.
The study “provides evidence for policymakers to design and implement early weight management interventions” for people diagnosed with T2D, the authors concluded.
Clinicians also have a role to play, Dr. Luk said. “At the first encounter with an individual with newly diagnosed T2D, clinicians should emphasize the importance of weight reduction and guide the individual on how this can be achieved through making healthy lifestyle choices. Pharmacotherapy and metabolic surgery for weight management can be considered in appropriate individuals.”
Overall, she added, “clinicians should be informed that the likelihood of achieving and maintaining diabetes remission is low, and patients should be counseled accordingly.”
Similar to US Experience
Mona Mshayekhi, MD, PhD, an assistant professor of medicine in the division of Diabetes, Endocrinology and Metabolism at Vanderbilt University Medical Center, Nashville, Tennessee, commented on the study for this news organization.
“These findings mirror clinical experience in the US very well,” she said. “We know that sustained weight loss without the use of medications or surgery is extremely difficult in the real-world setting due to the hormonal drivers of obesity, in combination with socioeconomic challenges.”
The study was done before newer weight-management strategies such as glucagon-like peptide 1 receptor agonists were widely available, she noted. “This actually strengthens the finding that weight loss without the routine use of medications has a multitude of benefits, including diabetes remission and reduction of all-cause mortality.”
That said, she added, “I suspect that future studies with more modern cohorts will reveal much higher rates of diabetes remission with the use of newer medications.”
“Our ability to help our patients lose meaningful weight has been limited until recently,” she said. “With new tools in our armamentarium, clinicians need to take the lead in helping patients address and treat obesity and fight the stigma that prevents many from even discussing it with their providers.”
The study did not receive funding. Dr. Luk has received research grants or contracts from Amgen, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Eli Lilly, Junshi, Lee Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Shanghai Junshi Biosciences, Sugardown, and Takeda and received travel grants and honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MSD. Dr. Mshayekhi reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Very few patients with type 2 diabetes (T2D) achieve and sustain diabetes remission via weight loss alone, new research suggests.
Among more than 37,000 people with T2D in Hong Kong, only 6% had achieved and sustained diabetes remission solely through weight loss up to 8 years after diagnosis. Among those who initially achieved remission, 67% had hyperglycemia at 3 years.
People who lost the most weight (10% of their body weight or more) in the first year after diagnosis were most likely to have sustained remission.
The study “helped to confirm the low rate of diabetes remission and high rate of returning to hyperglycemia in real-world practice,” Andrea Luk, MD, of the Chinese University of Hong Kong, told this news organization. “Over 80% of diabetes remission occurred within the first 5 years of a diabetes diagnosis. This is in line with our understanding that beta cell function will gradually decline over time, making diabetes remission increasingly difficult even with weight reduction.”
The study was published in PLOS Medicine.
Early Weight Management Works
Recent clinical trials have demonstrated that T2D remission can be achieved following sustained weight loss through bariatric surgery or lifestyle interventions, the authors noted. In this study, they investigated the association of weight change at 1 year after a diabetes diagnosis with the long-term incidence and sustainability of T2D remission in real-world settings, using data from the territory-wide Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM).
A total of 37,326 people with newly diagnosed T2D who were enrolled in the RAMP-DM between 2000 and 2017 were included and followed until 2019.
At baseline, participants’ mean age was 56.6 years, mean body mass index (BMI) was 26.4 kg/m2, and mean A1c was 7.7%, and 65% were using glucose-lowering drugs (GLDs).
T2D remission was defined as two consecutive A1c < 6.5% measurements at least 6 months apart without GLDs currently or in the previous 3 months.
During a median follow-up of 7.9 years, 6.1% of people achieved remission, with an incidence rate of 7.8 per 1000 person-years. The proportion was higher among those with greater weight loss: 14.4% of people who lost 10% of their body weight or more achieved remission compared with 9.9% of those with 5%-9.9% weight loss, 6.5% of those with 0%-4.9% weight loss, and 4.5% of those who gained weight.
After adjustment for age at diagnosis, sex, assessment year, BMI, other metabolic indices, smoking, alcohol drinking, and medication use, the hazard ratio (HR) for diabetes remission was 3.28 for those with 10% or greater weight loss within 1 year of diagnosis, 2.29 for 5%-9.9% weight loss, and 1.34 for 0%-4.9% weight loss compared to weight gain.
The incidence of diabetes remission in the study was significantly lower than that in clinical trials, possibly because trial participants were in structured programs that included intensive lifestyle interventions, regular monitoring and feedback, and reinforcement of a holistic approach to managing diabetes, the authors noted. Real-world settings may or may not include such interventions.
Further analyses showed that within a median follow-up of 3.1 years, 67.2% of people who had achieved diabetes remission returned to hyperglycemia — an incidence rate of 184.8 per 1000 person-years.
The adjusted HR for returning to hyperglycemia was 0.52 for people with 10% or greater weight loss, 0.78 for those with 5%-9.9% weight loss, and 0.90 for those with 0%-4.9% weight loss compared to people with weight gain.
In addition, diabetes remission was associated with a 31% (HR, 0.69) decreased risk for all-cause mortality.
The study “provides evidence for policymakers to design and implement early weight management interventions” for people diagnosed with T2D, the authors concluded.
Clinicians also have a role to play, Dr. Luk said. “At the first encounter with an individual with newly diagnosed T2D, clinicians should emphasize the importance of weight reduction and guide the individual on how this can be achieved through making healthy lifestyle choices. Pharmacotherapy and metabolic surgery for weight management can be considered in appropriate individuals.”
Overall, she added, “clinicians should be informed that the likelihood of achieving and maintaining diabetes remission is low, and patients should be counseled accordingly.”
Similar to US Experience
Mona Mshayekhi, MD, PhD, an assistant professor of medicine in the division of Diabetes, Endocrinology and Metabolism at Vanderbilt University Medical Center, Nashville, Tennessee, commented on the study for this news organization.
“These findings mirror clinical experience in the US very well,” she said. “We know that sustained weight loss without the use of medications or surgery is extremely difficult in the real-world setting due to the hormonal drivers of obesity, in combination with socioeconomic challenges.”
The study was done before newer weight-management strategies such as glucagon-like peptide 1 receptor agonists were widely available, she noted. “This actually strengthens the finding that weight loss without the routine use of medications has a multitude of benefits, including diabetes remission and reduction of all-cause mortality.”
That said, she added, “I suspect that future studies with more modern cohorts will reveal much higher rates of diabetes remission with the use of newer medications.”
“Our ability to help our patients lose meaningful weight has been limited until recently,” she said. “With new tools in our armamentarium, clinicians need to take the lead in helping patients address and treat obesity and fight the stigma that prevents many from even discussing it with their providers.”
The study did not receive funding. Dr. Luk has received research grants or contracts from Amgen, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Eli Lilly, Junshi, Lee Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Shanghai Junshi Biosciences, Sugardown, and Takeda and received travel grants and honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MSD. Dr. Mshayekhi reported no conflicts of interest.
A version of this article appeared on Medscape.com.
Very few patients with type 2 diabetes (T2D) achieve and sustain diabetes remission via weight loss alone, new research suggests.
Among more than 37,000 people with T2D in Hong Kong, only 6% had achieved and sustained diabetes remission solely through weight loss up to 8 years after diagnosis. Among those who initially achieved remission, 67% had hyperglycemia at 3 years.
People who lost the most weight (10% of their body weight or more) in the first year after diagnosis were most likely to have sustained remission.
The study “helped to confirm the low rate of diabetes remission and high rate of returning to hyperglycemia in real-world practice,” Andrea Luk, MD, of the Chinese University of Hong Kong, told this news organization. “Over 80% of diabetes remission occurred within the first 5 years of a diabetes diagnosis. This is in line with our understanding that beta cell function will gradually decline over time, making diabetes remission increasingly difficult even with weight reduction.”
The study was published in PLOS Medicine.
Early Weight Management Works
Recent clinical trials have demonstrated that T2D remission can be achieved following sustained weight loss through bariatric surgery or lifestyle interventions, the authors noted. In this study, they investigated the association of weight change at 1 year after a diabetes diagnosis with the long-term incidence and sustainability of T2D remission in real-world settings, using data from the territory-wide Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM).
A total of 37,326 people with newly diagnosed T2D who were enrolled in the RAMP-DM between 2000 and 2017 were included and followed until 2019.
At baseline, participants’ mean age was 56.6 years, mean body mass index (BMI) was 26.4 kg/m2, and mean A1c was 7.7%, and 65% were using glucose-lowering drugs (GLDs).
T2D remission was defined as two consecutive A1c < 6.5% measurements at least 6 months apart without GLDs currently or in the previous 3 months.
During a median follow-up of 7.9 years, 6.1% of people achieved remission, with an incidence rate of 7.8 per 1000 person-years. The proportion was higher among those with greater weight loss: 14.4% of people who lost 10% of their body weight or more achieved remission compared with 9.9% of those with 5%-9.9% weight loss, 6.5% of those with 0%-4.9% weight loss, and 4.5% of those who gained weight.
After adjustment for age at diagnosis, sex, assessment year, BMI, other metabolic indices, smoking, alcohol drinking, and medication use, the hazard ratio (HR) for diabetes remission was 3.28 for those with 10% or greater weight loss within 1 year of diagnosis, 2.29 for 5%-9.9% weight loss, and 1.34 for 0%-4.9% weight loss compared to weight gain.
The incidence of diabetes remission in the study was significantly lower than that in clinical trials, possibly because trial participants were in structured programs that included intensive lifestyle interventions, regular monitoring and feedback, and reinforcement of a holistic approach to managing diabetes, the authors noted. Real-world settings may or may not include such interventions.
Further analyses showed that within a median follow-up of 3.1 years, 67.2% of people who had achieved diabetes remission returned to hyperglycemia — an incidence rate of 184.8 per 1000 person-years.
The adjusted HR for returning to hyperglycemia was 0.52 for people with 10% or greater weight loss, 0.78 for those with 5%-9.9% weight loss, and 0.90 for those with 0%-4.9% weight loss compared to people with weight gain.
In addition, diabetes remission was associated with a 31% (HR, 0.69) decreased risk for all-cause mortality.
The study “provides evidence for policymakers to design and implement early weight management interventions” for people diagnosed with T2D, the authors concluded.
Clinicians also have a role to play, Dr. Luk said. “At the first encounter with an individual with newly diagnosed T2D, clinicians should emphasize the importance of weight reduction and guide the individual on how this can be achieved through making healthy lifestyle choices. Pharmacotherapy and metabolic surgery for weight management can be considered in appropriate individuals.”
Overall, she added, “clinicians should be informed that the likelihood of achieving and maintaining diabetes remission is low, and patients should be counseled accordingly.”
Similar to US Experience
Mona Mshayekhi, MD, PhD, an assistant professor of medicine in the division of Diabetes, Endocrinology and Metabolism at Vanderbilt University Medical Center, Nashville, Tennessee, commented on the study for this news organization.
“These findings mirror clinical experience in the US very well,” she said. “We know that sustained weight loss without the use of medications or surgery is extremely difficult in the real-world setting due to the hormonal drivers of obesity, in combination with socioeconomic challenges.”
The study was done before newer weight-management strategies such as glucagon-like peptide 1 receptor agonists were widely available, she noted. “This actually strengthens the finding that weight loss without the routine use of medications has a multitude of benefits, including diabetes remission and reduction of all-cause mortality.”
That said, she added, “I suspect that future studies with more modern cohorts will reveal much higher rates of diabetes remission with the use of newer medications.”
“Our ability to help our patients lose meaningful weight has been limited until recently,” she said. “With new tools in our armamentarium, clinicians need to take the lead in helping patients address and treat obesity and fight the stigma that prevents many from even discussing it with their providers.”
The study did not receive funding. Dr. Luk has received research grants or contracts from Amgen, AstraZeneca, Bayer, Biogen, Boehringer Ingelheim, Eli Lilly, Junshi, Lee Pharmaceutical, MSD, Novo Nordisk, Roche, Sanofi, Shanghai Junshi Biosciences, Sugardown, and Takeda and received travel grants and honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MSD. Dr. Mshayekhi reported no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM PLOS MEDICINE
Young Myeloma Specialist Forges Ahead, Gives Back
Ahead of the conference held in San Diego in December, Dr. Mohyuddin, a blood cancer specialist with a focus on multiple myeloma and medical education, put out a heartfelt appeal on X (formerly Twitter): “If you’re a trainee and interested in meeting me at #ASH23, please reach out … (especially if [international medical graduate]) I’d love to meet and offer support in whatever capacity I can! I can’t have a research project for each one of you, but happy to help/mentor in any other way possible,” he posted on X back in late November.
An international medical graduate himself, Dr. Mohyuddin recalls how overwhelmed he felt when he first attended an annual ASH conference as a trainee, so he aims to reassure others that they “don’t have to know everything.”
“It’s about networking and broadening horizons,” he said in an interview that took place between ASH sessions, his own research presentations, and meetings with the many trainees who took him up on the offer he made via X. “I’ve spent most of this ASH meeting trainees — it’s the most rewarding thing for me at these meetings.
“Reassurance is a lot of what we do in oncology,” he continued, drawing a connection between his affinity for helping trainees and providing compassionate care to patients. “For an oncologist, the single most important thing is having excellent communication skills and being able to express support and empathy. The ability to connect deeply with your patients during their time of need is profoundly important.
“You can compensate for lack of knowledge, because we have so many other sources of support for knowledge, but you simply cannot compensate for poor communication skills, and your patient suffers as a result,” he said.
Relationship Building
In addition to the guidance he received from mentors, Dr. Mohyuddin noted that it was the chance to build supportive, empathetic relationships that drew him to specialize in blood cancer and, in particular, to caring for patients with multiple myeloma and conducting research focused on improving the patient experience.
Dr. Mohyuddin attended medical school at the Aga Khan University in Pakistan, then completed his internal medicine residency and fellowship at the University of Kansas in Kansas City. As a chief resident there, he focused on novel approaches to education delivery and improving access to research for trainees. As a fellow, he developed clinical and research interests in multiple myeloma, which he describes as an “incredibly rewarding field” marked by “truly spectacular advances over the last two decades.”
“There are some cancers you can cure, which means you don’t get to see patients often, and there are some you can’t cure, where patients die early, and there’s not a lot of time to build a relationship,” he said. “But there are some where patients can do well even though they aren’t currently cured, and you get to form really amazing and meaningful relationships over a long period of time.
“Multiple myeloma occupies that space, and that’s why I’m drawn to it,” Dr. Mohyuddin added, noting that he doesn’t shy away from forging emotional connections with patients. “I recognize that makes me vulnerable, but I think that is essentially what your patients deserve from you — to be invested at an emotional level with them through their suffering.”
Improving value and the patient experience
“One thing, philosophically, that I research is value in multiple myeloma care: identifying areas where we are overtreating patients and where we can do less and get away with it,” he said.
Despite the major advances in multiple myeloma in recent years, which “represent a lot of what is going right with oncology,” this blood cancer still “also represents a lot of what is wrong with oncology,” he noted. As an example, he cited “the approval of low-value drugs, the sequencing of drugs, adding more and more drugs without responsibly addressing quality-of-life questions, and identifying more responsible ways to provide high-value efficacious care without bankrupting the economy.
“So my research and policy work apply to that,” he explained. “What can we do better? What sort of trials should we be doing? What populations do we enroll? Are we asking the right questions or looking at trivialities? Are we serving patients foremost?”
Sometimes, this means comparing multiple myeloma staging systems in a real-world cohort, or assessing whether a widely available, cheap, and safe drug like budesonide can help patients avoid diarrhea during chemotherapy, whether control arms in myeloma randomized trials are fair, whether drugs ever get approved in low- or middle-income countries after their approval in the United States, and whether smoldering myeloma, a multiple myeloma precursor, really requires treatment, as current guidelines suggest, or if patients would do just as well — or perhaps better — with a close surveillance protocol.
“Pharma won’t do those studies and many key opinion leaders feel the question [about whether smoldering myeloma needs to be treated] has already been answered, so we are launching a prospective study that will define the natural history of smoldering myeloma and allow for patients to stay off therapy while undergoing rigorous surveillance with imaging,” he said.
Another study Dr. Mohyuddin hopes to launch soon will look at a “start low, go slow” treatment approach for the frailest patients with newly diagnosed myeloma.
His upbringing in Pakistan, where there are “mind-boggling” differences in health care access, affordability, and outcomes when compared with the United States, provided a foundation for both his “enthusiasm for cost-effective care” and his desire to give back, he said.
Another aspect of life in Pakistan — an across-the-board sense of closeness and solidarity in families and communities that is sometimes lacking in the United States — contributed to his desire to build relationships.
“That is something I dearly miss,” he said. “I am very privileged and so thankful to be here in the US, but that is one thing I do deeply miss.”
Connecting and Making a Difference
Dr. Mohyuddin seeks connection through his relationships with patients, trainees, and his many followers on social media platforms like X, where he frequently shares his thoughts on research quality and findings, heme/onc trends, and treatment-related insight.
“How to treat myeloma after #ASH23,” he posted on X as the conference came to a close. His takeaways: Don’t treat smoldering myeloma, do quadruple therapy for transplant-eligible patients (but no cd38 maintenance therapy afterward), don’t do quads for carfilzomib in newly diagnosed frail or older patients, and don’t do a salvage autologous transplant, no matter how good the first transplant was.
Dr. Mohyuddin also works to make a difference through his research and involvement in helping to launch initiatives like Common Sense Oncology, an ambitious global effort to reform cancer clinical trials and care, and through a current project with colleagues in India and Pakistan to create a consortium for pooling data on hematologic malignancies from South Asian countries. The hope is that such a collaborative effort will lead to good prospective research relevant to the needs of participating countries, he explained.
“Those are things where I want to make a difference. Taking care of patients is number one, but more than research, the number two thing for me is teaching and hopefully inspiring trainees and others to think differently, to look at data differently,” he said, noting that despite the major advances in myeloma, the reality is that “a lot of what we offer in oncology is very marginal.”
The effect sizes of interventions are often very small, and outcomes can still be really bad, he explained, adding that “[i]t really hits you when you see a lot of death and suffering. It’s a huge wake-up call … we have so many advances, but the reality is very, very sobering.
“Critically understanding and interpreting data is something where education really fails us. I’m incredibly passionate about it. I’ve found great resources to help me interpret data better, and I want to make them more accessible and inspire others to understand better,” he said. “We need to know how to defend ourselves from the hype.”
His efforts have not gone unnoticed. Dr. Mohyuddin was the recipient of the 2023 Hematology and Medical Oncology Fellowship Faculty Teaching Award at the University of Utah, Salt Lake City, where he is currently a faculty member.
“The recognition means more than any publication or grant award,” he said. “It’s great to know that medical education is appreciated, because so often we are in a rat race of getting more papers and grants out, but teaching and inspiring people is what is really, really important to me.”
Ahead of the conference held in San Diego in December, Dr. Mohyuddin, a blood cancer specialist with a focus on multiple myeloma and medical education, put out a heartfelt appeal on X (formerly Twitter): “If you’re a trainee and interested in meeting me at #ASH23, please reach out … (especially if [international medical graduate]) I’d love to meet and offer support in whatever capacity I can! I can’t have a research project for each one of you, but happy to help/mentor in any other way possible,” he posted on X back in late November.
An international medical graduate himself, Dr. Mohyuddin recalls how overwhelmed he felt when he first attended an annual ASH conference as a trainee, so he aims to reassure others that they “don’t have to know everything.”
“It’s about networking and broadening horizons,” he said in an interview that took place between ASH sessions, his own research presentations, and meetings with the many trainees who took him up on the offer he made via X. “I’ve spent most of this ASH meeting trainees — it’s the most rewarding thing for me at these meetings.
“Reassurance is a lot of what we do in oncology,” he continued, drawing a connection between his affinity for helping trainees and providing compassionate care to patients. “For an oncologist, the single most important thing is having excellent communication skills and being able to express support and empathy. The ability to connect deeply with your patients during their time of need is profoundly important.
“You can compensate for lack of knowledge, because we have so many other sources of support for knowledge, but you simply cannot compensate for poor communication skills, and your patient suffers as a result,” he said.
Relationship Building
In addition to the guidance he received from mentors, Dr. Mohyuddin noted that it was the chance to build supportive, empathetic relationships that drew him to specialize in blood cancer and, in particular, to caring for patients with multiple myeloma and conducting research focused on improving the patient experience.
Dr. Mohyuddin attended medical school at the Aga Khan University in Pakistan, then completed his internal medicine residency and fellowship at the University of Kansas in Kansas City. As a chief resident there, he focused on novel approaches to education delivery and improving access to research for trainees. As a fellow, he developed clinical and research interests in multiple myeloma, which he describes as an “incredibly rewarding field” marked by “truly spectacular advances over the last two decades.”
“There are some cancers you can cure, which means you don’t get to see patients often, and there are some you can’t cure, where patients die early, and there’s not a lot of time to build a relationship,” he said. “But there are some where patients can do well even though they aren’t currently cured, and you get to form really amazing and meaningful relationships over a long period of time.
“Multiple myeloma occupies that space, and that’s why I’m drawn to it,” Dr. Mohyuddin added, noting that he doesn’t shy away from forging emotional connections with patients. “I recognize that makes me vulnerable, but I think that is essentially what your patients deserve from you — to be invested at an emotional level with them through their suffering.”
Improving value and the patient experience
“One thing, philosophically, that I research is value in multiple myeloma care: identifying areas where we are overtreating patients and where we can do less and get away with it,” he said.
Despite the major advances in multiple myeloma in recent years, which “represent a lot of what is going right with oncology,” this blood cancer still “also represents a lot of what is wrong with oncology,” he noted. As an example, he cited “the approval of low-value drugs, the sequencing of drugs, adding more and more drugs without responsibly addressing quality-of-life questions, and identifying more responsible ways to provide high-value efficacious care without bankrupting the economy.
“So my research and policy work apply to that,” he explained. “What can we do better? What sort of trials should we be doing? What populations do we enroll? Are we asking the right questions or looking at trivialities? Are we serving patients foremost?”
Sometimes, this means comparing multiple myeloma staging systems in a real-world cohort, or assessing whether a widely available, cheap, and safe drug like budesonide can help patients avoid diarrhea during chemotherapy, whether control arms in myeloma randomized trials are fair, whether drugs ever get approved in low- or middle-income countries after their approval in the United States, and whether smoldering myeloma, a multiple myeloma precursor, really requires treatment, as current guidelines suggest, or if patients would do just as well — or perhaps better — with a close surveillance protocol.
“Pharma won’t do those studies and many key opinion leaders feel the question [about whether smoldering myeloma needs to be treated] has already been answered, so we are launching a prospective study that will define the natural history of smoldering myeloma and allow for patients to stay off therapy while undergoing rigorous surveillance with imaging,” he said.
Another study Dr. Mohyuddin hopes to launch soon will look at a “start low, go slow” treatment approach for the frailest patients with newly diagnosed myeloma.
His upbringing in Pakistan, where there are “mind-boggling” differences in health care access, affordability, and outcomes when compared with the United States, provided a foundation for both his “enthusiasm for cost-effective care” and his desire to give back, he said.
Another aspect of life in Pakistan — an across-the-board sense of closeness and solidarity in families and communities that is sometimes lacking in the United States — contributed to his desire to build relationships.
“That is something I dearly miss,” he said. “I am very privileged and so thankful to be here in the US, but that is one thing I do deeply miss.”
Connecting and Making a Difference
Dr. Mohyuddin seeks connection through his relationships with patients, trainees, and his many followers on social media platforms like X, where he frequently shares his thoughts on research quality and findings, heme/onc trends, and treatment-related insight.
“How to treat myeloma after #ASH23,” he posted on X as the conference came to a close. His takeaways: Don’t treat smoldering myeloma, do quadruple therapy for transplant-eligible patients (but no cd38 maintenance therapy afterward), don’t do quads for carfilzomib in newly diagnosed frail or older patients, and don’t do a salvage autologous transplant, no matter how good the first transplant was.
Dr. Mohyuddin also works to make a difference through his research and involvement in helping to launch initiatives like Common Sense Oncology, an ambitious global effort to reform cancer clinical trials and care, and through a current project with colleagues in India and Pakistan to create a consortium for pooling data on hematologic malignancies from South Asian countries. The hope is that such a collaborative effort will lead to good prospective research relevant to the needs of participating countries, he explained.
“Those are things where I want to make a difference. Taking care of patients is number one, but more than research, the number two thing for me is teaching and hopefully inspiring trainees and others to think differently, to look at data differently,” he said, noting that despite the major advances in myeloma, the reality is that “a lot of what we offer in oncology is very marginal.”
The effect sizes of interventions are often very small, and outcomes can still be really bad, he explained, adding that “[i]t really hits you when you see a lot of death and suffering. It’s a huge wake-up call … we have so many advances, but the reality is very, very sobering.
“Critically understanding and interpreting data is something where education really fails us. I’m incredibly passionate about it. I’ve found great resources to help me interpret data better, and I want to make them more accessible and inspire others to understand better,” he said. “We need to know how to defend ourselves from the hype.”
His efforts have not gone unnoticed. Dr. Mohyuddin was the recipient of the 2023 Hematology and Medical Oncology Fellowship Faculty Teaching Award at the University of Utah, Salt Lake City, where he is currently a faculty member.
“The recognition means more than any publication or grant award,” he said. “It’s great to know that medical education is appreciated, because so often we are in a rat race of getting more papers and grants out, but teaching and inspiring people is what is really, really important to me.”
Ahead of the conference held in San Diego in December, Dr. Mohyuddin, a blood cancer specialist with a focus on multiple myeloma and medical education, put out a heartfelt appeal on X (formerly Twitter): “If you’re a trainee and interested in meeting me at #ASH23, please reach out … (especially if [international medical graduate]) I’d love to meet and offer support in whatever capacity I can! I can’t have a research project for each one of you, but happy to help/mentor in any other way possible,” he posted on X back in late November.
An international medical graduate himself, Dr. Mohyuddin recalls how overwhelmed he felt when he first attended an annual ASH conference as a trainee, so he aims to reassure others that they “don’t have to know everything.”
“It’s about networking and broadening horizons,” he said in an interview that took place between ASH sessions, his own research presentations, and meetings with the many trainees who took him up on the offer he made via X. “I’ve spent most of this ASH meeting trainees — it’s the most rewarding thing for me at these meetings.
“Reassurance is a lot of what we do in oncology,” he continued, drawing a connection between his affinity for helping trainees and providing compassionate care to patients. “For an oncologist, the single most important thing is having excellent communication skills and being able to express support and empathy. The ability to connect deeply with your patients during their time of need is profoundly important.
“You can compensate for lack of knowledge, because we have so many other sources of support for knowledge, but you simply cannot compensate for poor communication skills, and your patient suffers as a result,” he said.
Relationship Building
In addition to the guidance he received from mentors, Dr. Mohyuddin noted that it was the chance to build supportive, empathetic relationships that drew him to specialize in blood cancer and, in particular, to caring for patients with multiple myeloma and conducting research focused on improving the patient experience.
Dr. Mohyuddin attended medical school at the Aga Khan University in Pakistan, then completed his internal medicine residency and fellowship at the University of Kansas in Kansas City. As a chief resident there, he focused on novel approaches to education delivery and improving access to research for trainees. As a fellow, he developed clinical and research interests in multiple myeloma, which he describes as an “incredibly rewarding field” marked by “truly spectacular advances over the last two decades.”
“There are some cancers you can cure, which means you don’t get to see patients often, and there are some you can’t cure, where patients die early, and there’s not a lot of time to build a relationship,” he said. “But there are some where patients can do well even though they aren’t currently cured, and you get to form really amazing and meaningful relationships over a long period of time.
“Multiple myeloma occupies that space, and that’s why I’m drawn to it,” Dr. Mohyuddin added, noting that he doesn’t shy away from forging emotional connections with patients. “I recognize that makes me vulnerable, but I think that is essentially what your patients deserve from you — to be invested at an emotional level with them through their suffering.”
Improving value and the patient experience
“One thing, philosophically, that I research is value in multiple myeloma care: identifying areas where we are overtreating patients and where we can do less and get away with it,” he said.
Despite the major advances in multiple myeloma in recent years, which “represent a lot of what is going right with oncology,” this blood cancer still “also represents a lot of what is wrong with oncology,” he noted. As an example, he cited “the approval of low-value drugs, the sequencing of drugs, adding more and more drugs without responsibly addressing quality-of-life questions, and identifying more responsible ways to provide high-value efficacious care without bankrupting the economy.
“So my research and policy work apply to that,” he explained. “What can we do better? What sort of trials should we be doing? What populations do we enroll? Are we asking the right questions or looking at trivialities? Are we serving patients foremost?”
Sometimes, this means comparing multiple myeloma staging systems in a real-world cohort, or assessing whether a widely available, cheap, and safe drug like budesonide can help patients avoid diarrhea during chemotherapy, whether control arms in myeloma randomized trials are fair, whether drugs ever get approved in low- or middle-income countries after their approval in the United States, and whether smoldering myeloma, a multiple myeloma precursor, really requires treatment, as current guidelines suggest, or if patients would do just as well — or perhaps better — with a close surveillance protocol.
“Pharma won’t do those studies and many key opinion leaders feel the question [about whether smoldering myeloma needs to be treated] has already been answered, so we are launching a prospective study that will define the natural history of smoldering myeloma and allow for patients to stay off therapy while undergoing rigorous surveillance with imaging,” he said.
Another study Dr. Mohyuddin hopes to launch soon will look at a “start low, go slow” treatment approach for the frailest patients with newly diagnosed myeloma.
His upbringing in Pakistan, where there are “mind-boggling” differences in health care access, affordability, and outcomes when compared with the United States, provided a foundation for both his “enthusiasm for cost-effective care” and his desire to give back, he said.
Another aspect of life in Pakistan — an across-the-board sense of closeness and solidarity in families and communities that is sometimes lacking in the United States — contributed to his desire to build relationships.
“That is something I dearly miss,” he said. “I am very privileged and so thankful to be here in the US, but that is one thing I do deeply miss.”
Connecting and Making a Difference
Dr. Mohyuddin seeks connection through his relationships with patients, trainees, and his many followers on social media platforms like X, where he frequently shares his thoughts on research quality and findings, heme/onc trends, and treatment-related insight.
“How to treat myeloma after #ASH23,” he posted on X as the conference came to a close. His takeaways: Don’t treat smoldering myeloma, do quadruple therapy for transplant-eligible patients (but no cd38 maintenance therapy afterward), don’t do quads for carfilzomib in newly diagnosed frail or older patients, and don’t do a salvage autologous transplant, no matter how good the first transplant was.
Dr. Mohyuddin also works to make a difference through his research and involvement in helping to launch initiatives like Common Sense Oncology, an ambitious global effort to reform cancer clinical trials and care, and through a current project with colleagues in India and Pakistan to create a consortium for pooling data on hematologic malignancies from South Asian countries. The hope is that such a collaborative effort will lead to good prospective research relevant to the needs of participating countries, he explained.
“Those are things where I want to make a difference. Taking care of patients is number one, but more than research, the number two thing for me is teaching and hopefully inspiring trainees and others to think differently, to look at data differently,” he said, noting that despite the major advances in myeloma, the reality is that “a lot of what we offer in oncology is very marginal.”
The effect sizes of interventions are often very small, and outcomes can still be really bad, he explained, adding that “[i]t really hits you when you see a lot of death and suffering. It’s a huge wake-up call … we have so many advances, but the reality is very, very sobering.
“Critically understanding and interpreting data is something where education really fails us. I’m incredibly passionate about it. I’ve found great resources to help me interpret data better, and I want to make them more accessible and inspire others to understand better,” he said. “We need to know how to defend ourselves from the hype.”
His efforts have not gone unnoticed. Dr. Mohyuddin was the recipient of the 2023 Hematology and Medical Oncology Fellowship Faculty Teaching Award at the University of Utah, Salt Lake City, where he is currently a faculty member.
“The recognition means more than any publication or grant award,” he said. “It’s great to know that medical education is appreciated, because so often we are in a rat race of getting more papers and grants out, but teaching and inspiring people is what is really, really important to me.”
Bladder Cancer: Is Active Surveillance the Way Forward?
PARIS — Should clinicians promote active surveillance for non–muscle-invasive bladder tumors (NMIBT) and establish it as a comprehensive management approach, as with prostate and kidney cancers?
During the 117th congress of the French Association of Urology (AFU), Benjamin Pradère, MD, urologic surgeon at Croix du Sud Clinic in Quint-Fonsegrives, France, advocated for this approach, suggesting that the use of biomarkers could enhance its effectiveness.
However, it requires careful patient selection, proper information, and relevant follow-up,” said Dr. Pradère, who is a member of the AFU Cancer Committee (CCAFU).
Low-Grade Tumors
NMIBTs are precancerous lesions and constitute 70%-80% of diagnosed bladder tumors. The remaining tumors are more severe invasive tumors that infiltrate deep tissues. NMIBTs, however, entail a high risk for recurrence (reaching 80% after endoscopic resection), as well as a high risk for progression.
As a result, the diagnosis of NMIBT involves follow-up that significantly impacts patients’ quality of life due to repeated cystoscopies and endovesical treatments. “Tumors with the most impact are low-grade Ta tumors”, with longer-term monitoring required for these low-risk tumors.
Hematuria is the most frequent clinical sign. NMIBT diagnosis occurs after endoscopic tumor resection via transurethral resection, followed by an anatomopathological analysis to determine cell grade and tumor stage. Treatment depends on the risk of recurrence and progression, as well as the risk of therapeutic failure after the initial resection.
Risk stratification distinguishes the following four levels:
- Low-risk tumors: Low-grade pTa urothelial tumors, unifocal, < 3 cm, no history of bladder tumors. Low risk of recurrence and progression.
- Intermediate-risk tumors: Other low-grade pTa urothelial tumors with no high-risk criteria. Low risk of progression but high risk of recurrence.
- High-risk tumors: Tumors with at least one risk factor: Stage pT1, high grade, presence of carcinoma in situ. High risks of progression and recurrence.
- Very high-risk tumors: Tumors combining all risk factors (pT1 grade with carcinoma in situ). Very high and early risk of progression.
“We know that low-grade NMIBTs have no impact on survival,” said Dr. Pradère. For these tumors, which represent 60% of diagnosed NMIBTs, or approximately 250,000 new cases annually in France, specific survival is > 99%, meaning that most diagnosed patients will not die of bladder cancer.
The recurrence rate for low-grade tumors is 50%, but recurrences are “almost always low-grade and rarely invade the basement membrane,” said Pradère. Implementing active surveillance to limit surgical intervention to more advanced forms seems to be relevant for these tumors.
Cystoscopy Every 3 Months
According to CCAFU recommendations, “active surveillance is a therapeutic alternative that can be proposed for patients with recurrent low-risk NMIBT after the initial diagnosis.” Criteria include low-grade pTa, fewer than five tumors, size ≤ 15 mm, negative urinary cytology, asymptomatic nature, and the patient’s acceptance of closer monitoring.
While active surveillance has become the standard treatment for low-risk prostate cancer, this therapeutic option remains marginal in bladder cancer, as in kidney cancer. The goal is to defer or avoid surgical treatment by closely monitoring the natural progression of the disease.
For NMIBTs, follow-up modalities are not yet specifically recommended because of a lack of data, said Dr. Pradère. According to a consensus, cystoscopy should be repeated every 3 months for a year and then every 6 months. Unlike standard follow-up, it includes cytology “to not miss the transition to high grade.”
CCAFU recommends discontinuing active surveillance if any of the following criteria are present:
- More than 10 lesions
- Size > 30 mm
- Positive cytology
- Symptoms (hematuria, micturition disorders, and recurring infections).
Literature on the benefits of active surveillance in bladder tumors is still limited. Only seven studies are available. Overall, for nearly 600 included patients, tumors progressed in about 12% of cases. Progression to invasive tumors occurred in 0.8% of patients (n = 5).
13 Months’ Surveillance
According to a long-term study (median follow-up of 38 months), patients mostly exit active surveillance in the first year. The median duration of active surveillance is 13 months. Active surveillance is discontinued to surgically treat tumors that turn out to be low-grade Ta tumors in 70% of cases.
The following factors predicting recurrence and progression of tumors have been identified: Multiple tumors, early recurrence (within a year of initial diagnosis), frequent recurrence (more than one recurrence per year), tumors > 3 cm, and failure of previous endovesical treatment.
Recent studies have shown that with at least three of these recurrence and progression factors, the median duration under active surveillance is 15 months compared with 28 months in the absence of such factors. “Considering these factors, it is possible to assess the benefit of active surveillance for the patient,” said Dr. Pradère.
If active surveillance for bladder tumors is still not widely practiced, then the contribution of imaging (MRI and ultrasound) and biomarkers could promote its adoption. “The use of biomarkers should change the game and encourage active surveillance in patients with small polyps,” said Dr. Pradère.
ADXBladder Test Utility
A study highlighted the importance of evaluating minichromosome maintenance protein 5 expression during active surveillance using the ADXBladder ELISA test on a urine sample. This test is usually used in bladder cancer diagnosis.
“This study showed that a negative result in two consecutive tests during active surveillance is associated with an almost zero recurrence risk. After two negative tests, most patients do not exit active surveillance,” said Dr. Pradère. But the positive predictive value of biomarkers remains low for low-grade tumors.
The future of active surveillance in bladder cancer should involve better patient selection that relies on risk factors, enhanced modalities through imaging and biomarkers, and the advent of artificial intelligence to analyze cystoscopy results, concluded Dr. Pradère.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
PARIS — Should clinicians promote active surveillance for non–muscle-invasive bladder tumors (NMIBT) and establish it as a comprehensive management approach, as with prostate and kidney cancers?
During the 117th congress of the French Association of Urology (AFU), Benjamin Pradère, MD, urologic surgeon at Croix du Sud Clinic in Quint-Fonsegrives, France, advocated for this approach, suggesting that the use of biomarkers could enhance its effectiveness.
However, it requires careful patient selection, proper information, and relevant follow-up,” said Dr. Pradère, who is a member of the AFU Cancer Committee (CCAFU).
Low-Grade Tumors
NMIBTs are precancerous lesions and constitute 70%-80% of diagnosed bladder tumors. The remaining tumors are more severe invasive tumors that infiltrate deep tissues. NMIBTs, however, entail a high risk for recurrence (reaching 80% after endoscopic resection), as well as a high risk for progression.
As a result, the diagnosis of NMIBT involves follow-up that significantly impacts patients’ quality of life due to repeated cystoscopies and endovesical treatments. “Tumors with the most impact are low-grade Ta tumors”, with longer-term monitoring required for these low-risk tumors.
Hematuria is the most frequent clinical sign. NMIBT diagnosis occurs after endoscopic tumor resection via transurethral resection, followed by an anatomopathological analysis to determine cell grade and tumor stage. Treatment depends on the risk of recurrence and progression, as well as the risk of therapeutic failure after the initial resection.
Risk stratification distinguishes the following four levels:
- Low-risk tumors: Low-grade pTa urothelial tumors, unifocal, < 3 cm, no history of bladder tumors. Low risk of recurrence and progression.
- Intermediate-risk tumors: Other low-grade pTa urothelial tumors with no high-risk criteria. Low risk of progression but high risk of recurrence.
- High-risk tumors: Tumors with at least one risk factor: Stage pT1, high grade, presence of carcinoma in situ. High risks of progression and recurrence.
- Very high-risk tumors: Tumors combining all risk factors (pT1 grade with carcinoma in situ). Very high and early risk of progression.
“We know that low-grade NMIBTs have no impact on survival,” said Dr. Pradère. For these tumors, which represent 60% of diagnosed NMIBTs, or approximately 250,000 new cases annually in France, specific survival is > 99%, meaning that most diagnosed patients will not die of bladder cancer.
The recurrence rate for low-grade tumors is 50%, but recurrences are “almost always low-grade and rarely invade the basement membrane,” said Pradère. Implementing active surveillance to limit surgical intervention to more advanced forms seems to be relevant for these tumors.
Cystoscopy Every 3 Months
According to CCAFU recommendations, “active surveillance is a therapeutic alternative that can be proposed for patients with recurrent low-risk NMIBT after the initial diagnosis.” Criteria include low-grade pTa, fewer than five tumors, size ≤ 15 mm, negative urinary cytology, asymptomatic nature, and the patient’s acceptance of closer monitoring.
While active surveillance has become the standard treatment for low-risk prostate cancer, this therapeutic option remains marginal in bladder cancer, as in kidney cancer. The goal is to defer or avoid surgical treatment by closely monitoring the natural progression of the disease.
For NMIBTs, follow-up modalities are not yet specifically recommended because of a lack of data, said Dr. Pradère. According to a consensus, cystoscopy should be repeated every 3 months for a year and then every 6 months. Unlike standard follow-up, it includes cytology “to not miss the transition to high grade.”
CCAFU recommends discontinuing active surveillance if any of the following criteria are present:
- More than 10 lesions
- Size > 30 mm
- Positive cytology
- Symptoms (hematuria, micturition disorders, and recurring infections).
Literature on the benefits of active surveillance in bladder tumors is still limited. Only seven studies are available. Overall, for nearly 600 included patients, tumors progressed in about 12% of cases. Progression to invasive tumors occurred in 0.8% of patients (n = 5).
13 Months’ Surveillance
According to a long-term study (median follow-up of 38 months), patients mostly exit active surveillance in the first year. The median duration of active surveillance is 13 months. Active surveillance is discontinued to surgically treat tumors that turn out to be low-grade Ta tumors in 70% of cases.
The following factors predicting recurrence and progression of tumors have been identified: Multiple tumors, early recurrence (within a year of initial diagnosis), frequent recurrence (more than one recurrence per year), tumors > 3 cm, and failure of previous endovesical treatment.
Recent studies have shown that with at least three of these recurrence and progression factors, the median duration under active surveillance is 15 months compared with 28 months in the absence of such factors. “Considering these factors, it is possible to assess the benefit of active surveillance for the patient,” said Dr. Pradère.
If active surveillance for bladder tumors is still not widely practiced, then the contribution of imaging (MRI and ultrasound) and biomarkers could promote its adoption. “The use of biomarkers should change the game and encourage active surveillance in patients with small polyps,” said Dr. Pradère.
ADXBladder Test Utility
A study highlighted the importance of evaluating minichromosome maintenance protein 5 expression during active surveillance using the ADXBladder ELISA test on a urine sample. This test is usually used in bladder cancer diagnosis.
“This study showed that a negative result in two consecutive tests during active surveillance is associated with an almost zero recurrence risk. After two negative tests, most patients do not exit active surveillance,” said Dr. Pradère. But the positive predictive value of biomarkers remains low for low-grade tumors.
The future of active surveillance in bladder cancer should involve better patient selection that relies on risk factors, enhanced modalities through imaging and biomarkers, and the advent of artificial intelligence to analyze cystoscopy results, concluded Dr. Pradère.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
PARIS — Should clinicians promote active surveillance for non–muscle-invasive bladder tumors (NMIBT) and establish it as a comprehensive management approach, as with prostate and kidney cancers?
During the 117th congress of the French Association of Urology (AFU), Benjamin Pradère, MD, urologic surgeon at Croix du Sud Clinic in Quint-Fonsegrives, France, advocated for this approach, suggesting that the use of biomarkers could enhance its effectiveness.
However, it requires careful patient selection, proper information, and relevant follow-up,” said Dr. Pradère, who is a member of the AFU Cancer Committee (CCAFU).
Low-Grade Tumors
NMIBTs are precancerous lesions and constitute 70%-80% of diagnosed bladder tumors. The remaining tumors are more severe invasive tumors that infiltrate deep tissues. NMIBTs, however, entail a high risk for recurrence (reaching 80% after endoscopic resection), as well as a high risk for progression.
As a result, the diagnosis of NMIBT involves follow-up that significantly impacts patients’ quality of life due to repeated cystoscopies and endovesical treatments. “Tumors with the most impact are low-grade Ta tumors”, with longer-term monitoring required for these low-risk tumors.
Hematuria is the most frequent clinical sign. NMIBT diagnosis occurs after endoscopic tumor resection via transurethral resection, followed by an anatomopathological analysis to determine cell grade and tumor stage. Treatment depends on the risk of recurrence and progression, as well as the risk of therapeutic failure after the initial resection.
Risk stratification distinguishes the following four levels:
- Low-risk tumors: Low-grade pTa urothelial tumors, unifocal, < 3 cm, no history of bladder tumors. Low risk of recurrence and progression.
- Intermediate-risk tumors: Other low-grade pTa urothelial tumors with no high-risk criteria. Low risk of progression but high risk of recurrence.
- High-risk tumors: Tumors with at least one risk factor: Stage pT1, high grade, presence of carcinoma in situ. High risks of progression and recurrence.
- Very high-risk tumors: Tumors combining all risk factors (pT1 grade with carcinoma in situ). Very high and early risk of progression.
“We know that low-grade NMIBTs have no impact on survival,” said Dr. Pradère. For these tumors, which represent 60% of diagnosed NMIBTs, or approximately 250,000 new cases annually in France, specific survival is > 99%, meaning that most diagnosed patients will not die of bladder cancer.
The recurrence rate for low-grade tumors is 50%, but recurrences are “almost always low-grade and rarely invade the basement membrane,” said Pradère. Implementing active surveillance to limit surgical intervention to more advanced forms seems to be relevant for these tumors.
Cystoscopy Every 3 Months
According to CCAFU recommendations, “active surveillance is a therapeutic alternative that can be proposed for patients with recurrent low-risk NMIBT after the initial diagnosis.” Criteria include low-grade pTa, fewer than five tumors, size ≤ 15 mm, negative urinary cytology, asymptomatic nature, and the patient’s acceptance of closer monitoring.
While active surveillance has become the standard treatment for low-risk prostate cancer, this therapeutic option remains marginal in bladder cancer, as in kidney cancer. The goal is to defer or avoid surgical treatment by closely monitoring the natural progression of the disease.
For NMIBTs, follow-up modalities are not yet specifically recommended because of a lack of data, said Dr. Pradère. According to a consensus, cystoscopy should be repeated every 3 months for a year and then every 6 months. Unlike standard follow-up, it includes cytology “to not miss the transition to high grade.”
CCAFU recommends discontinuing active surveillance if any of the following criteria are present:
- More than 10 lesions
- Size > 30 mm
- Positive cytology
- Symptoms (hematuria, micturition disorders, and recurring infections).
Literature on the benefits of active surveillance in bladder tumors is still limited. Only seven studies are available. Overall, for nearly 600 included patients, tumors progressed in about 12% of cases. Progression to invasive tumors occurred in 0.8% of patients (n = 5).
13 Months’ Surveillance
According to a long-term study (median follow-up of 38 months), patients mostly exit active surveillance in the first year. The median duration of active surveillance is 13 months. Active surveillance is discontinued to surgically treat tumors that turn out to be low-grade Ta tumors in 70% of cases.
The following factors predicting recurrence and progression of tumors have been identified: Multiple tumors, early recurrence (within a year of initial diagnosis), frequent recurrence (more than one recurrence per year), tumors > 3 cm, and failure of previous endovesical treatment.
Recent studies have shown that with at least three of these recurrence and progression factors, the median duration under active surveillance is 15 months compared with 28 months in the absence of such factors. “Considering these factors, it is possible to assess the benefit of active surveillance for the patient,” said Dr. Pradère.
If active surveillance for bladder tumors is still not widely practiced, then the contribution of imaging (MRI and ultrasound) and biomarkers could promote its adoption. “The use of biomarkers should change the game and encourage active surveillance in patients with small polyps,” said Dr. Pradère.
ADXBladder Test Utility
A study highlighted the importance of evaluating minichromosome maintenance protein 5 expression during active surveillance using the ADXBladder ELISA test on a urine sample. This test is usually used in bladder cancer diagnosis.
“This study showed that a negative result in two consecutive tests during active surveillance is associated with an almost zero recurrence risk. After two negative tests, most patients do not exit active surveillance,” said Dr. Pradère. But the positive predictive value of biomarkers remains low for low-grade tumors.
The future of active surveillance in bladder cancer should involve better patient selection that relies on risk factors, enhanced modalities through imaging and biomarkers, and the advent of artificial intelligence to analyze cystoscopy results, concluded Dr. Pradère.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
DoxyPEP: A New Option to Prevent Sexually Transmitted Infections
Sexually transmitted infections (STIs) continue to have a significant impact on the lives of adolescents and young adults. According to the Centers for Disease Control and Prevention’s (CDC) 2021 surveillance report, rates of gonorrhea and syphilis in the United States were at their highest since the early 1990s. Chlamydia, one of the most common STIs, had a peak rate in 2019, but more recent rates may have been impacted by the COVID-19 pandemic. In 2021, those 15-24 years of age accounted for 58.5% of all chlamydia infections, 40.4% of all gonorrhea infections, and 18.3% of all syphilis infections in the US.
While pediatricians should discuss sexual health and STI screening with all their adolescent and young adult patients, LGBTQ+ youth are disproportionately impacted by STIs. For example, cisgender men who have sex with men have significantly higher rates of HIV, gonorrhea, and syphilis. These disparities are likely related to unequal access to care, systemic homophobia/transphobia, stigma, and differences in sexual networks and are even more pronounced for those with intersectional minoritized identities such as LGBTQ+ youth of color.1
In the past 12 years, there have been significant advances in HIV prevention methods, including the approval and use of pre-exposure prophylaxis or “PrEP” (a medication regimen that is taken on an ongoing basis to provide highly effective protection against HIV infection) and more widespread use of post-exposure prophylaxis or “PEP” (a medication regimen taken for 1 month after a potential exposure to HIV to prevent HIV from establishing an ongoing infection) outside of the medical setting. While these interventions have the potential to decrease rates of HIV infection, they do not prevent any other STIs.2-3
The current strategies to prevent bacterial STIs include discussions of sexual practices, counseling on risk reduction strategies such as decreased number of partners and condom use, routine screening in those at risk every 3-12 months, and timely diagnosis and treatment of infections in patients and their partners to avoid further transmission.4 Given the increasing rates of bacterial STIs (gonorrhea, chlamydia, and syphilis), additional biomedical prevention methods are greatly needed.
Doxycycline as post-exposure prophylaxis
Doxycycline is a tetracycline antibiotic effective against a wide range of bacteria and is commonly used in the treatment of acne, skin infections, Lyme disease, and STIs, and can also be used in the treatment and prevention of malaria.5 For STIs, doxycycline is currently the recommended treatment for chlamydia and is also an alternate therapy for syphilis in nonpregnant patients when penicillin is not accessible or in those with severe penicillin allergy.4 This medication is often well tolerated with the most common side effects including gastrointestinal irritation and photosensitivity. Doxycycline is contraindicated in pregnancy due to its potential impact on tooth and bone development.5
Given its general tolerability and activity against bacterial STIs, new and emerging studies have examined doxycycline as post-exposure prophylaxis (DoxyPEP: one dose of doxycycline 200 mg PO within 72 hours after unprotected sex) in an attempt to decrease the incidence of new bacterial STIs in populations at risk. Three of the studies cited in the preliminary CDC DoxyPEP guidelines were conducted in cisgender men who have sex with men and transgender women either living with HIV or taking PrEP for HIV prevention. All three studies demonstrated significantly lower risk of chlamydia and syphilis, while two of the studies also showed a significantly lower risk of gonorrhea. One additional study was conducted in cisgender women in Kenya. This study did not show any statistically significant difference in the risk of chlamydia or gonorrhea in the intervention group, but may have been limited by low adherence to the DoxyPEP regimen. There were no serious adverse events reported in any of the studies attributed to doxycycline.6
Avoiding antibiotic resistance
With the increased use of antibiotics, attention must always be paid to the potential for increasing antibiotic resistance. The preliminary CDC DoxyPEP guidelines outline mixed results in the DoxyPEP studies that had limited follow-up timeframes, making it difficult to draw conclusions: “Current data suggest overall benefit of the use of doxycycline PEP, but potential risks related to the development of resistance and impacts on the microbiome will need to be closely monitored after implementation of these guidelines.” Official guideline recommendations from the CDC regarding DoxyPEP are currently pending after a period of public comment on the preliminary drafted guidelines.6 However, the New York State Department of Health AIDS Institute released guidelines for DoxyPEP in September 2023 and several large urban public health departments have also issued their own guidance that largely align with the preliminary CDC guidelines.7
Recommendations currently emphasize that the goal is to allow for implementation of DoxyPEP in those who would benefit the most from the intervention (i.e., cisgender men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months with ongoing risk of infection), while also minimizing antibiotic use. It should also be considered for those without a recent infection who have increased likelihood of exposure to bacterial STIs. DoxyPEP is likely to be effective in other populations (e.g., cisgender women, cisgender men who have sex with women, transgender men), but data are currently limited, and the risk/benefit ratio may be different in these populations. The recommended dose for DoxyPEP is doxycycline 200 mg once as soon as possible (within 72 hours) of unprotected oral, vaginal, or anal sex with a maximum of one dose every 24 hours. For those being prescribed DoxyPEP, gonorrhea and chlamydia screening of all anatomic sites of exposure (urine sample or frontal swab, throat swab, and/or rectal swab) should be conducted at baseline and then every 3-6 months in addition to blood testing for syphilis and HIV if indicated.6-7
Another option in our toolkit
DoxyPEP should be viewed as one more option in our toolkit of sexual health services alongside risk reduction strategies (e.g., open discussions with partners, decreased number of partners, and condom use), routine STI screening and treatment, PrEP and PEP for HIV prevention, and pregnancy prevention. Not all of these tools will be relevant to each individual and discussions around sexual health should be patient-centered and focused on their own personal goals. As pediatricians, we should provide guidance to all adolescents and young adults on options to improve their sexual health and empower them to embrace their bodily autonomy.
Dr. Warus is in the division of adolescent and young adult medicine at Children’s Hospital of Los Angeles, where he specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth. He is an assistant professor of pediatrics at USC.
Resources
CDC – Sexually Transmitted Infection Treatment Guidelines, 2021
CDC – [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention
New York State Department of Health AIDS Institute – Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections
Los Angeles County Department of Public Health – DoxyPEP for STI Prevention
References
1. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021: National Overview of STDs, 2021. Last Reviewed May 16, 2023.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021.
3. Centers for Disease Control and Prevention: US Department of Health and Human Services: Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. Published 2016. .
4. Workowski KA et al. Sexually Transmitted Infection Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):2-10.
5. Patel RS and Parmar M. Doxycycline Hyclate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Last Updated May 22, 2023.
6. Centers for Disease Control and Prevention. [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention. Published Oct 1, 2023.
7. DiMarco DE, et al. Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections. New York State Department of Health AIDS Institute. Published September 25, 2023.
Sexually transmitted infections (STIs) continue to have a significant impact on the lives of adolescents and young adults. According to the Centers for Disease Control and Prevention’s (CDC) 2021 surveillance report, rates of gonorrhea and syphilis in the United States were at their highest since the early 1990s. Chlamydia, one of the most common STIs, had a peak rate in 2019, but more recent rates may have been impacted by the COVID-19 pandemic. In 2021, those 15-24 years of age accounted for 58.5% of all chlamydia infections, 40.4% of all gonorrhea infections, and 18.3% of all syphilis infections in the US.
While pediatricians should discuss sexual health and STI screening with all their adolescent and young adult patients, LGBTQ+ youth are disproportionately impacted by STIs. For example, cisgender men who have sex with men have significantly higher rates of HIV, gonorrhea, and syphilis. These disparities are likely related to unequal access to care, systemic homophobia/transphobia, stigma, and differences in sexual networks and are even more pronounced for those with intersectional minoritized identities such as LGBTQ+ youth of color.1
In the past 12 years, there have been significant advances in HIV prevention methods, including the approval and use of pre-exposure prophylaxis or “PrEP” (a medication regimen that is taken on an ongoing basis to provide highly effective protection against HIV infection) and more widespread use of post-exposure prophylaxis or “PEP” (a medication regimen taken for 1 month after a potential exposure to HIV to prevent HIV from establishing an ongoing infection) outside of the medical setting. While these interventions have the potential to decrease rates of HIV infection, they do not prevent any other STIs.2-3
The current strategies to prevent bacterial STIs include discussions of sexual practices, counseling on risk reduction strategies such as decreased number of partners and condom use, routine screening in those at risk every 3-12 months, and timely diagnosis and treatment of infections in patients and their partners to avoid further transmission.4 Given the increasing rates of bacterial STIs (gonorrhea, chlamydia, and syphilis), additional biomedical prevention methods are greatly needed.
Doxycycline as post-exposure prophylaxis
Doxycycline is a tetracycline antibiotic effective against a wide range of bacteria and is commonly used in the treatment of acne, skin infections, Lyme disease, and STIs, and can also be used in the treatment and prevention of malaria.5 For STIs, doxycycline is currently the recommended treatment for chlamydia and is also an alternate therapy for syphilis in nonpregnant patients when penicillin is not accessible or in those with severe penicillin allergy.4 This medication is often well tolerated with the most common side effects including gastrointestinal irritation and photosensitivity. Doxycycline is contraindicated in pregnancy due to its potential impact on tooth and bone development.5
Given its general tolerability and activity against bacterial STIs, new and emerging studies have examined doxycycline as post-exposure prophylaxis (DoxyPEP: one dose of doxycycline 200 mg PO within 72 hours after unprotected sex) in an attempt to decrease the incidence of new bacterial STIs in populations at risk. Three of the studies cited in the preliminary CDC DoxyPEP guidelines were conducted in cisgender men who have sex with men and transgender women either living with HIV or taking PrEP for HIV prevention. All three studies demonstrated significantly lower risk of chlamydia and syphilis, while two of the studies also showed a significantly lower risk of gonorrhea. One additional study was conducted in cisgender women in Kenya. This study did not show any statistically significant difference in the risk of chlamydia or gonorrhea in the intervention group, but may have been limited by low adherence to the DoxyPEP regimen. There were no serious adverse events reported in any of the studies attributed to doxycycline.6
Avoiding antibiotic resistance
With the increased use of antibiotics, attention must always be paid to the potential for increasing antibiotic resistance. The preliminary CDC DoxyPEP guidelines outline mixed results in the DoxyPEP studies that had limited follow-up timeframes, making it difficult to draw conclusions: “Current data suggest overall benefit of the use of doxycycline PEP, but potential risks related to the development of resistance and impacts on the microbiome will need to be closely monitored after implementation of these guidelines.” Official guideline recommendations from the CDC regarding DoxyPEP are currently pending after a period of public comment on the preliminary drafted guidelines.6 However, the New York State Department of Health AIDS Institute released guidelines for DoxyPEP in September 2023 and several large urban public health departments have also issued their own guidance that largely align with the preliminary CDC guidelines.7
Recommendations currently emphasize that the goal is to allow for implementation of DoxyPEP in those who would benefit the most from the intervention (i.e., cisgender men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months with ongoing risk of infection), while also minimizing antibiotic use. It should also be considered for those without a recent infection who have increased likelihood of exposure to bacterial STIs. DoxyPEP is likely to be effective in other populations (e.g., cisgender women, cisgender men who have sex with women, transgender men), but data are currently limited, and the risk/benefit ratio may be different in these populations. The recommended dose for DoxyPEP is doxycycline 200 mg once as soon as possible (within 72 hours) of unprotected oral, vaginal, or anal sex with a maximum of one dose every 24 hours. For those being prescribed DoxyPEP, gonorrhea and chlamydia screening of all anatomic sites of exposure (urine sample or frontal swab, throat swab, and/or rectal swab) should be conducted at baseline and then every 3-6 months in addition to blood testing for syphilis and HIV if indicated.6-7
Another option in our toolkit
DoxyPEP should be viewed as one more option in our toolkit of sexual health services alongside risk reduction strategies (e.g., open discussions with partners, decreased number of partners, and condom use), routine STI screening and treatment, PrEP and PEP for HIV prevention, and pregnancy prevention. Not all of these tools will be relevant to each individual and discussions around sexual health should be patient-centered and focused on their own personal goals. As pediatricians, we should provide guidance to all adolescents and young adults on options to improve their sexual health and empower them to embrace their bodily autonomy.
Dr. Warus is in the division of adolescent and young adult medicine at Children’s Hospital of Los Angeles, where he specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth. He is an assistant professor of pediatrics at USC.
Resources
CDC – Sexually Transmitted Infection Treatment Guidelines, 2021
CDC – [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention
New York State Department of Health AIDS Institute – Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections
Los Angeles County Department of Public Health – DoxyPEP for STI Prevention
References
1. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021: National Overview of STDs, 2021. Last Reviewed May 16, 2023.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021.
3. Centers for Disease Control and Prevention: US Department of Health and Human Services: Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. Published 2016. .
4. Workowski KA et al. Sexually Transmitted Infection Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):2-10.
5. Patel RS and Parmar M. Doxycycline Hyclate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Last Updated May 22, 2023.
6. Centers for Disease Control and Prevention. [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention. Published Oct 1, 2023.
7. DiMarco DE, et al. Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections. New York State Department of Health AIDS Institute. Published September 25, 2023.
Sexually transmitted infections (STIs) continue to have a significant impact on the lives of adolescents and young adults. According to the Centers for Disease Control and Prevention’s (CDC) 2021 surveillance report, rates of gonorrhea and syphilis in the United States were at their highest since the early 1990s. Chlamydia, one of the most common STIs, had a peak rate in 2019, but more recent rates may have been impacted by the COVID-19 pandemic. In 2021, those 15-24 years of age accounted for 58.5% of all chlamydia infections, 40.4% of all gonorrhea infections, and 18.3% of all syphilis infections in the US.
While pediatricians should discuss sexual health and STI screening with all their adolescent and young adult patients, LGBTQ+ youth are disproportionately impacted by STIs. For example, cisgender men who have sex with men have significantly higher rates of HIV, gonorrhea, and syphilis. These disparities are likely related to unequal access to care, systemic homophobia/transphobia, stigma, and differences in sexual networks and are even more pronounced for those with intersectional minoritized identities such as LGBTQ+ youth of color.1
In the past 12 years, there have been significant advances in HIV prevention methods, including the approval and use of pre-exposure prophylaxis or “PrEP” (a medication regimen that is taken on an ongoing basis to provide highly effective protection against HIV infection) and more widespread use of post-exposure prophylaxis or “PEP” (a medication regimen taken for 1 month after a potential exposure to HIV to prevent HIV from establishing an ongoing infection) outside of the medical setting. While these interventions have the potential to decrease rates of HIV infection, they do not prevent any other STIs.2-3
The current strategies to prevent bacterial STIs include discussions of sexual practices, counseling on risk reduction strategies such as decreased number of partners and condom use, routine screening in those at risk every 3-12 months, and timely diagnosis and treatment of infections in patients and their partners to avoid further transmission.4 Given the increasing rates of bacterial STIs (gonorrhea, chlamydia, and syphilis), additional biomedical prevention methods are greatly needed.
Doxycycline as post-exposure prophylaxis
Doxycycline is a tetracycline antibiotic effective against a wide range of bacteria and is commonly used in the treatment of acne, skin infections, Lyme disease, and STIs, and can also be used in the treatment and prevention of malaria.5 For STIs, doxycycline is currently the recommended treatment for chlamydia and is also an alternate therapy for syphilis in nonpregnant patients when penicillin is not accessible or in those with severe penicillin allergy.4 This medication is often well tolerated with the most common side effects including gastrointestinal irritation and photosensitivity. Doxycycline is contraindicated in pregnancy due to its potential impact on tooth and bone development.5
Given its general tolerability and activity against bacterial STIs, new and emerging studies have examined doxycycline as post-exposure prophylaxis (DoxyPEP: one dose of doxycycline 200 mg PO within 72 hours after unprotected sex) in an attempt to decrease the incidence of new bacterial STIs in populations at risk. Three of the studies cited in the preliminary CDC DoxyPEP guidelines were conducted in cisgender men who have sex with men and transgender women either living with HIV or taking PrEP for HIV prevention. All three studies demonstrated significantly lower risk of chlamydia and syphilis, while two of the studies also showed a significantly lower risk of gonorrhea. One additional study was conducted in cisgender women in Kenya. This study did not show any statistically significant difference in the risk of chlamydia or gonorrhea in the intervention group, but may have been limited by low adherence to the DoxyPEP regimen. There were no serious adverse events reported in any of the studies attributed to doxycycline.6
Avoiding antibiotic resistance
With the increased use of antibiotics, attention must always be paid to the potential for increasing antibiotic resistance. The preliminary CDC DoxyPEP guidelines outline mixed results in the DoxyPEP studies that had limited follow-up timeframes, making it difficult to draw conclusions: “Current data suggest overall benefit of the use of doxycycline PEP, but potential risks related to the development of resistance and impacts on the microbiome will need to be closely monitored after implementation of these guidelines.” Official guideline recommendations from the CDC regarding DoxyPEP are currently pending after a period of public comment on the preliminary drafted guidelines.6 However, the New York State Department of Health AIDS Institute released guidelines for DoxyPEP in September 2023 and several large urban public health departments have also issued their own guidance that largely align with the preliminary CDC guidelines.7
Recommendations currently emphasize that the goal is to allow for implementation of DoxyPEP in those who would benefit the most from the intervention (i.e., cisgender men who have sex with men and transgender women with a history of at least one bacterial STI in the last 12 months with ongoing risk of infection), while also minimizing antibiotic use. It should also be considered for those without a recent infection who have increased likelihood of exposure to bacterial STIs. DoxyPEP is likely to be effective in other populations (e.g., cisgender women, cisgender men who have sex with women, transgender men), but data are currently limited, and the risk/benefit ratio may be different in these populations. The recommended dose for DoxyPEP is doxycycline 200 mg once as soon as possible (within 72 hours) of unprotected oral, vaginal, or anal sex with a maximum of one dose every 24 hours. For those being prescribed DoxyPEP, gonorrhea and chlamydia screening of all anatomic sites of exposure (urine sample or frontal swab, throat swab, and/or rectal swab) should be conducted at baseline and then every 3-6 months in addition to blood testing for syphilis and HIV if indicated.6-7
Another option in our toolkit
DoxyPEP should be viewed as one more option in our toolkit of sexual health services alongside risk reduction strategies (e.g., open discussions with partners, decreased number of partners, and condom use), routine STI screening and treatment, PrEP and PEP for HIV prevention, and pregnancy prevention. Not all of these tools will be relevant to each individual and discussions around sexual health should be patient-centered and focused on their own personal goals. As pediatricians, we should provide guidance to all adolescents and young adults on options to improve their sexual health and empower them to embrace their bodily autonomy.
Dr. Warus is in the division of adolescent and young adult medicine at Children’s Hospital of Los Angeles, where he specializes in care for transgender and gender-nonconforming youth, and LGBTQ health for youth. He is an assistant professor of pediatrics at USC.
Resources
CDC – Sexually Transmitted Infection Treatment Guidelines, 2021
CDC – [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention
New York State Department of Health AIDS Institute – Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections
Los Angeles County Department of Public Health – DoxyPEP for STI Prevention
References
1. Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2021: National Overview of STDs, 2021. Last Reviewed May 16, 2023.
2. Centers for Disease Control and Prevention: US Public Health Service: Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update: A Clinical Practice Guideline. Published 2021.
3. Centers for Disease Control and Prevention: US Department of Health and Human Services: Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV – United States, 2016. Published 2016. .
4. Workowski KA et al. Sexually Transmitted Infection Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):2-10.
5. Patel RS and Parmar M. Doxycycline Hyclate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Last Updated May 22, 2023.
6. Centers for Disease Control and Prevention. [Preliminary] Guidelines for the Use of Doxycycline Post-Exposure Prophylaxis for Bacterial Sexually Transmitted Infection (STI) Prevention. Published Oct 1, 2023.
7. DiMarco DE, et al. Doxycycline Post-Exposure Prophylaxis to Prevent Bacterial Sexually Transmitted Infections. New York State Department of Health AIDS Institute. Published September 25, 2023.
Commentary: Drug Comparisons and Contact Allergy in AD, February 2024
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
A Military Nurse Saves a Life After a Brutal Rollover Crash
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.





