User login
Marketing perks increased opioid prescriptions
, according to a study published in JAMA Internal Medicine.
With 40% of opioid-related deaths still coming from prescription opioids, understanding how marketing influences prescriber habits could lead to the creation of specific policies to lower the number of prescription drugs exchanging hands and save lives.
Dr. Hadland and his colleagues conducted a comparative analysis of opioid prescriptions from 2014 and 2015, retrieved from the Medicare Part D Opioid Prescriber Summary File and cross referenced that information with all recorded transactions from companies to physicians in 2014 from the Open Payments database.
In 2015, a total of 369,139 physicians were recorded prescribing opioids to Medicare patients. About 25,767 (7%) received a combined total of 105,368 “nonresearch opioid-related payments” with a sum total of $9,071,976 in 2014.
While Medicare opioid claims went down in 2015, physicians who received these payments, on average, prescribed 9.3% more opioids than those who did not, according to investigators.
INSYS Therapeutics, Teva Pharmaceuticals USA, and Janssen Pharmaceuticals were the three highest-paying companies, contributing $4.5 million, $869,155, and $854,251, respectively.
Payments included speaking fees ($6.2 million), meals ($1.8 million), travel ($730,824), consulting fees ($290,395), and education ($79,660). Investigators estimated that, with each meal a physician received, there was an associated 0.7% increase in opioid claims.
Dr. Hadland and fellow investigators do acknowledge the possibility of reverse causality, with physicians who already prescribe more opioids being more likely to receive industry payments.
Dr. Hadland and his team report no relevant financial disclosures.
SOURCE: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
, according to a study published in JAMA Internal Medicine.
With 40% of opioid-related deaths still coming from prescription opioids, understanding how marketing influences prescriber habits could lead to the creation of specific policies to lower the number of prescription drugs exchanging hands and save lives.
Dr. Hadland and his colleagues conducted a comparative analysis of opioid prescriptions from 2014 and 2015, retrieved from the Medicare Part D Opioid Prescriber Summary File and cross referenced that information with all recorded transactions from companies to physicians in 2014 from the Open Payments database.
In 2015, a total of 369,139 physicians were recorded prescribing opioids to Medicare patients. About 25,767 (7%) received a combined total of 105,368 “nonresearch opioid-related payments” with a sum total of $9,071,976 in 2014.
While Medicare opioid claims went down in 2015, physicians who received these payments, on average, prescribed 9.3% more opioids than those who did not, according to investigators.
INSYS Therapeutics, Teva Pharmaceuticals USA, and Janssen Pharmaceuticals were the three highest-paying companies, contributing $4.5 million, $869,155, and $854,251, respectively.
Payments included speaking fees ($6.2 million), meals ($1.8 million), travel ($730,824), consulting fees ($290,395), and education ($79,660). Investigators estimated that, with each meal a physician received, there was an associated 0.7% increase in opioid claims.
Dr. Hadland and fellow investigators do acknowledge the possibility of reverse causality, with physicians who already prescribe more opioids being more likely to receive industry payments.
Dr. Hadland and his team report no relevant financial disclosures.
SOURCE: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
, according to a study published in JAMA Internal Medicine.
With 40% of opioid-related deaths still coming from prescription opioids, understanding how marketing influences prescriber habits could lead to the creation of specific policies to lower the number of prescription drugs exchanging hands and save lives.
Dr. Hadland and his colleagues conducted a comparative analysis of opioid prescriptions from 2014 and 2015, retrieved from the Medicare Part D Opioid Prescriber Summary File and cross referenced that information with all recorded transactions from companies to physicians in 2014 from the Open Payments database.
In 2015, a total of 369,139 physicians were recorded prescribing opioids to Medicare patients. About 25,767 (7%) received a combined total of 105,368 “nonresearch opioid-related payments” with a sum total of $9,071,976 in 2014.
While Medicare opioid claims went down in 2015, physicians who received these payments, on average, prescribed 9.3% more opioids than those who did not, according to investigators.
INSYS Therapeutics, Teva Pharmaceuticals USA, and Janssen Pharmaceuticals were the three highest-paying companies, contributing $4.5 million, $869,155, and $854,251, respectively.
Payments included speaking fees ($6.2 million), meals ($1.8 million), travel ($730,824), consulting fees ($290,395), and education ($79,660). Investigators estimated that, with each meal a physician received, there was an associated 0.7% increase in opioid claims.
Dr. Hadland and fellow investigators do acknowledge the possibility of reverse causality, with physicians who already prescribe more opioids being more likely to receive industry payments.
Dr. Hadland and his team report no relevant financial disclosures.
SOURCE: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Doctors who received nonresearch payments on average had a higher number of opioid prescriptions than those who did not.
Major finding: Receiving a nonresearch payment from a pharmaceutical company was associated with about 9.3% more opioid claims.
Study details: Record analysis of 369,139 physicians collected from the Open Payments database and the Medicare Part D Opioid Prescriber Summary File for the years 2014 and 2015.
Disclosures: Dr. Hadland and his team report no relevant financial disclosures.
Source: S Hadland et al. JAMA Intern Med. 2018 May 14. doi: 10.1001/jamainternmed.2018.1999.
MDedge Daily News: Many moms with postpartum depression go untreated
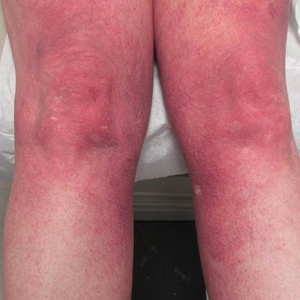
Progressive Widespread Telangiectasias
The Diagnosis: Cutaneous Collagenous Vasculopathy
Histopathologic examination revealed ectatic blood vessels lined with unremarkable endothelial cells and thickened, hyalinized vessel walls scattered within the papillary dermis (Figure 1). The epidermis was unremarkable. There was minimal associated inflammation and no extravasation of erythrocytes. The hyalinized material was weakly positive on periodic acid-Schiff staining (Figure 2) and negative on Congo red staining, which supported of a diagnosis of cutaneous collagenous vasculopathy (CCV).
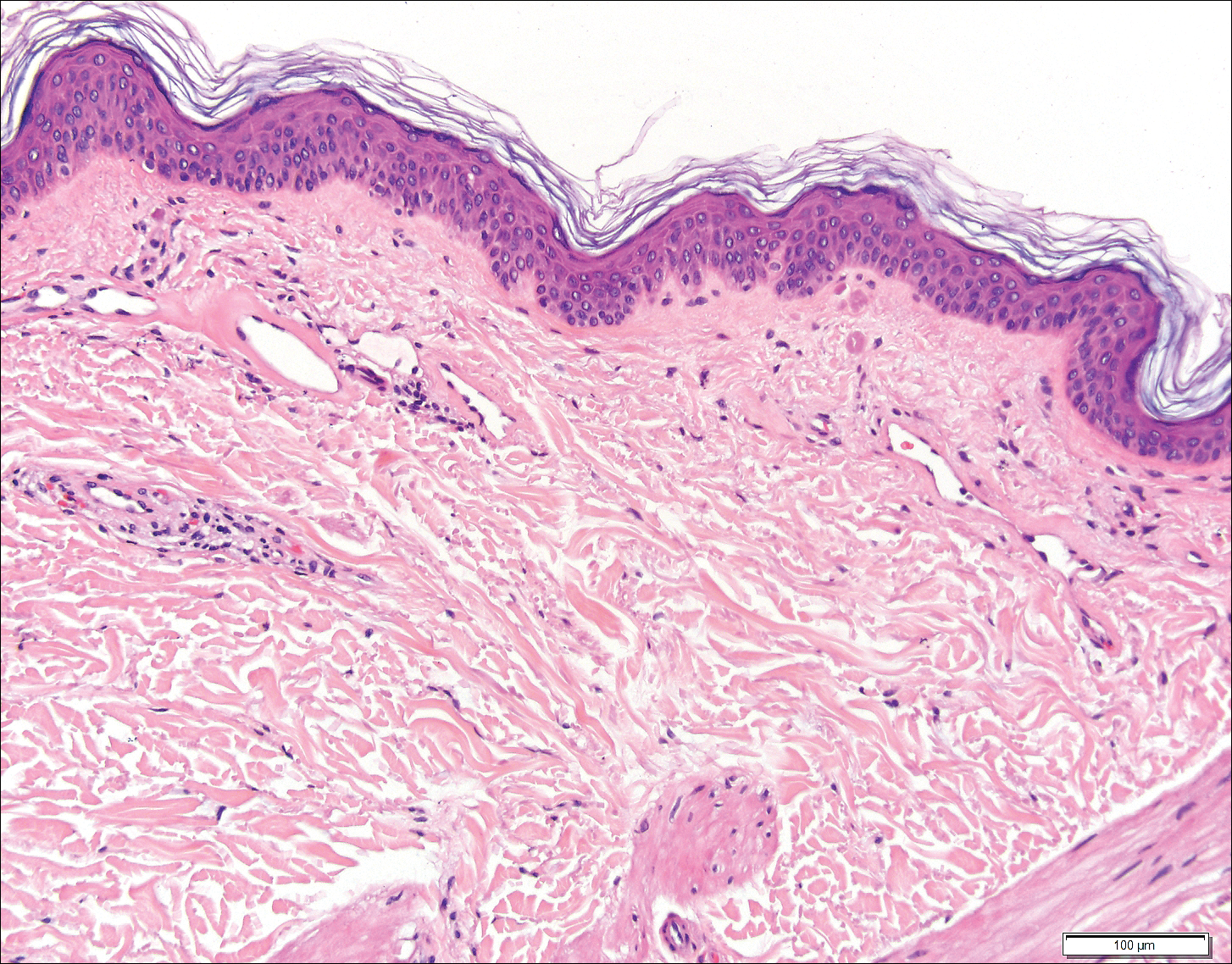
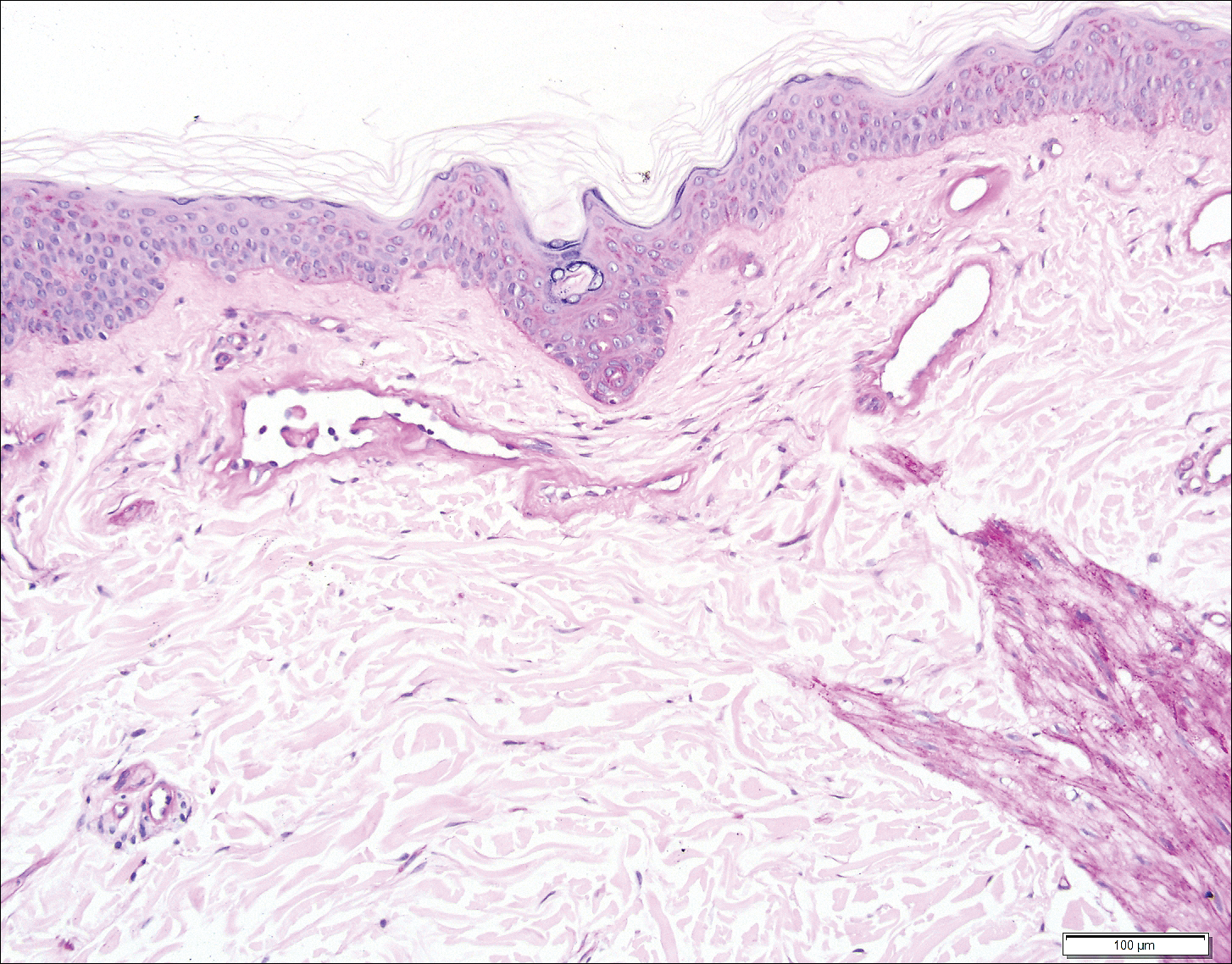
The patient previously had been given a suspected diagnosis of generalized essential telangiectasia by an outside dermatologist several years prior to the current presentation, as CCV had yet to be recognized as its own entity and therefore few cases had been described in the literature. She had a known history of obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus, which are associated with the condition. Multiple specialists concluded that the disease was too extensive for laser treatment. A review of PubMed articles indexed for MEDLINE yielded no established treatment options.
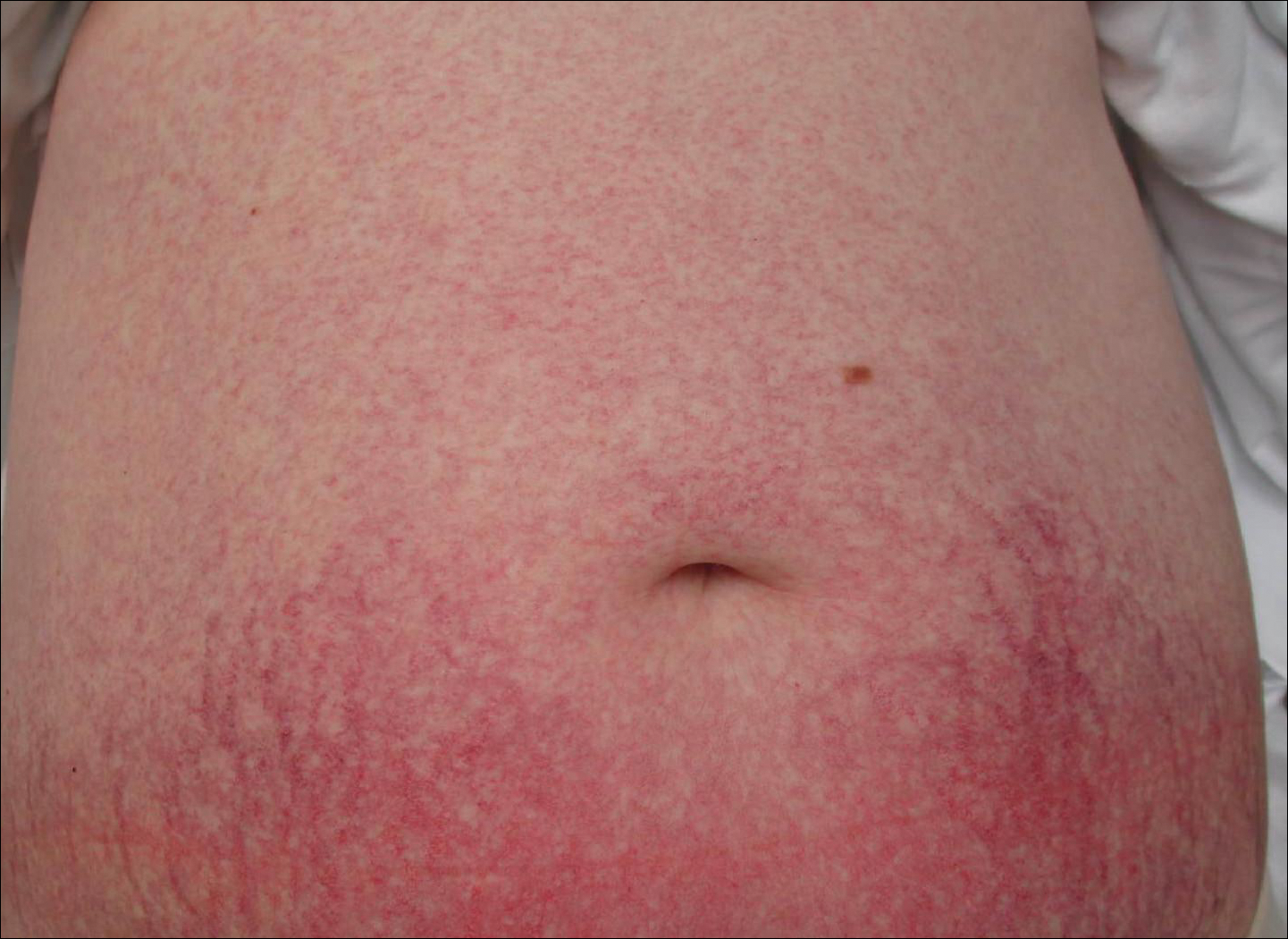
Cutaneous collagenous vasculopathy is a rare acquired microangiopathy involving the small vessels of the skin. Its clinical presentation is indistinguishable from that of generalized essential telangiectasia (GET). Patients generally present with asymptomatic, widespread, blanching, symmetric telangiectasias that classically begin on the legs and steadily progress upward with classic sparing of the face (Figure 3). Whereas GET has been reported to involve the oral and conjunctival mucosa, mucosal involvement is not typically observed in CCV and is considered to be a distinguishing factor between the 2 conditions.1,2 However, our patient reported oral symptoms, and oral erosions were seen on multiple physical examinations; therefore, ours is a rare case of mucosal involvement in conjunction with CCV. Given this finding, it is possible that more cases of CCV with mucosal involvement may exist but have been clinically misdiagnosed as GET.
First described by Salama and Rosenthal3 in 2000, CCV remains a rarely reported entity, with approximately 33 reported cases in the worldwide literature.2,4-7 The condition typically arises in adults with an equal predilection for males and females.2 The true incidence of CCV is unknown and likely is underreported given its close similarities to GET, which often is diagnosed clinically. The unique histopathologic finding of superficial ectatic vascular spaces with eosinophilic hyalinized vessel walls in CCV is key to distinguishing these similar entities, and even this finding can be subtle and is easily overlooked. Inflammation is sparse to absent. Deposited material is positive on periodic acid-Schiff and cytokeratin IV staining (representing reduplicated basement membrane-type collagen) and is diastase resistant. Smooth muscle actin staining is diminished or absent. Ultrastructural examination reveals reduplicated, laminated basement membrane; Luse bodies (abnormally long, widely spaced collagen fibers); and a decrease in or loss of pericytes. Of note, Luse bodies are nonspecific and their absence does not exclude a diagnosis of CCV.1
The etiology of CCV is unclear, and multiple pathogenetic mechanisms have been proposed. Ultimately, this entity is thought to arise from repeated endothelial cell damage, although the trigger for the endothelial cell injury is not completely understood. Diabetes mellitus sometimes is associated with microangiopathy and may be a confounding but not causative factor in some cases.1 Some investigators believe CCV is caused by a genetic defect that alters collagen production in the small vessels of the skin.5 Others have hypothesized that it is a secondary manifestation of an underlying disease or is associated with a medication; however, no disease or drug has been convincingly implicated in CCV.8
Cutaneous collagenous vasculopathy is limited to the skin, with no known reports of systemic involvement in the literature.7 There are no recommended laboratory studies to aid in diagnosis.1 It is critical to exclude hereditary hemorrhagic telangiectasia (HHT), as these patients can have life-threatening systemic involvement. Patients with CCV generally have no history of a bleeding diathesis, patients with HHT classically report recurrent epistaxis and gastrointestinal bleeding.7 A family history of HHT also is helpful for diagnosis, as the condition is autosomal dominant.1 Neither HHT or telangiectasia macularis eruptiva perstans, which also can be included in the differential diagnosis, demonstrate vessel wall hyalinization.
Treatment options for CCV are limited. Basso et al6 reported notable improvement in a patient with CCV treated with a combined 595-nm pulsed dye laser and 1064-nm Nd:YAG laser and optimized pulsed light. In one patient, treatment with a 585-nm pulsed dye laser produced a blanching response, suggesting that this may be a potential treatment option.7 Treatment with sclerotherapy has been ineffective.2
It is critical for both dermatologists and dermatopathologists to recognize and report this newly described entity, as the unique finding of vessel wall hyalinization in CCV may be indicative of a certain pathogenetic mechanism and effective treatment avenue that has yet to be established due to the relatively few number of reports that currently exist in the literature.
- Burdick LM, Lohser S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and structural study. J Cutan Pathol. 2000;27:40-48.
- Toda-Brito H, Resende C, Catorze G, et al. Cutaneous collagenous vasculopathy: a rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210635.
- Ma DL, Vano-Galvan S. Images in clinical medicine: cutaneous collagenous vasculopathy. N Engl J Med. 2015;373:E14.
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111.
- Moteqi SI, Yasuda M, Yamanaka M, et al. Cutaneous collagenous vasculopathy: report of first Japanese case and review of the literature. Australas J Dermatol. 2017;58:145-149.
- González Fernández D, Gómez Bernal S, Vivanco Allende B, et al. Cutaneous collagenous vasculopathy: description of two new cases in elderly women and review of the literature. Dermatology. 2012;225:1-8.
The Diagnosis: Cutaneous Collagenous Vasculopathy
Histopathologic examination revealed ectatic blood vessels lined with unremarkable endothelial cells and thickened, hyalinized vessel walls scattered within the papillary dermis (Figure 1). The epidermis was unremarkable. There was minimal associated inflammation and no extravasation of erythrocytes. The hyalinized material was weakly positive on periodic acid-Schiff staining (Figure 2) and negative on Congo red staining, which supported of a diagnosis of cutaneous collagenous vasculopathy (CCV).


The patient previously had been given a suspected diagnosis of generalized essential telangiectasia by an outside dermatologist several years prior to the current presentation, as CCV had yet to be recognized as its own entity and therefore few cases had been described in the literature. She had a known history of obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus, which are associated with the condition. Multiple specialists concluded that the disease was too extensive for laser treatment. A review of PubMed articles indexed for MEDLINE yielded no established treatment options.
Cutaneous collagenous vasculopathy is a rare acquired microangiopathy involving the small vessels of the skin. Its clinical presentation is indistinguishable from that of generalized essential telangiectasia (GET). Patients generally present with asymptomatic, widespread, blanching, symmetric telangiectasias that classically begin on the legs and steadily progress upward with classic sparing of the face (Figure 3). Whereas GET has been reported to involve the oral and conjunctival mucosa, mucosal involvement is not typically observed in CCV and is considered to be a distinguishing factor between the 2 conditions.1,2 However, our patient reported oral symptoms, and oral erosions were seen on multiple physical examinations; therefore, ours is a rare case of mucosal involvement in conjunction with CCV. Given this finding, it is possible that more cases of CCV with mucosal involvement may exist but have been clinically misdiagnosed as GET.

First described by Salama and Rosenthal3 in 2000, CCV remains a rarely reported entity, with approximately 33 reported cases in the worldwide literature.2,4-7 The condition typically arises in adults with an equal predilection for males and females.2 The true incidence of CCV is unknown and likely is underreported given its close similarities to GET, which often is diagnosed clinically. The unique histopathologic finding of superficial ectatic vascular spaces with eosinophilic hyalinized vessel walls in CCV is key to distinguishing these similar entities, and even this finding can be subtle and is easily overlooked. Inflammation is sparse to absent. Deposited material is positive on periodic acid-Schiff and cytokeratin IV staining (representing reduplicated basement membrane-type collagen) and is diastase resistant. Smooth muscle actin staining is diminished or absent. Ultrastructural examination reveals reduplicated, laminated basement membrane; Luse bodies (abnormally long, widely spaced collagen fibers); and a decrease in or loss of pericytes. Of note, Luse bodies are nonspecific and their absence does not exclude a diagnosis of CCV.1
The etiology of CCV is unclear, and multiple pathogenetic mechanisms have been proposed. Ultimately, this entity is thought to arise from repeated endothelial cell damage, although the trigger for the endothelial cell injury is not completely understood. Diabetes mellitus sometimes is associated with microangiopathy and may be a confounding but not causative factor in some cases.1 Some investigators believe CCV is caused by a genetic defect that alters collagen production in the small vessels of the skin.5 Others have hypothesized that it is a secondary manifestation of an underlying disease or is associated with a medication; however, no disease or drug has been convincingly implicated in CCV.8
Cutaneous collagenous vasculopathy is limited to the skin, with no known reports of systemic involvement in the literature.7 There are no recommended laboratory studies to aid in diagnosis.1 It is critical to exclude hereditary hemorrhagic telangiectasia (HHT), as these patients can have life-threatening systemic involvement. Patients with CCV generally have no history of a bleeding diathesis, patients with HHT classically report recurrent epistaxis and gastrointestinal bleeding.7 A family history of HHT also is helpful for diagnosis, as the condition is autosomal dominant.1 Neither HHT or telangiectasia macularis eruptiva perstans, which also can be included in the differential diagnosis, demonstrate vessel wall hyalinization.
Treatment options for CCV are limited. Basso et al6 reported notable improvement in a patient with CCV treated with a combined 595-nm pulsed dye laser and 1064-nm Nd:YAG laser and optimized pulsed light. In one patient, treatment with a 585-nm pulsed dye laser produced a blanching response, suggesting that this may be a potential treatment option.7 Treatment with sclerotherapy has been ineffective.2
It is critical for both dermatologists and dermatopathologists to recognize and report this newly described entity, as the unique finding of vessel wall hyalinization in CCV may be indicative of a certain pathogenetic mechanism and effective treatment avenue that has yet to be established due to the relatively few number of reports that currently exist in the literature.
The Diagnosis: Cutaneous Collagenous Vasculopathy
Histopathologic examination revealed ectatic blood vessels lined with unremarkable endothelial cells and thickened, hyalinized vessel walls scattered within the papillary dermis (Figure 1). The epidermis was unremarkable. There was minimal associated inflammation and no extravasation of erythrocytes. The hyalinized material was weakly positive on periodic acid-Schiff staining (Figure 2) and negative on Congo red staining, which supported of a diagnosis of cutaneous collagenous vasculopathy (CCV).


The patient previously had been given a suspected diagnosis of generalized essential telangiectasia by an outside dermatologist several years prior to the current presentation, as CCV had yet to be recognized as its own entity and therefore few cases had been described in the literature. She had a known history of obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus, which are associated with the condition. Multiple specialists concluded that the disease was too extensive for laser treatment. A review of PubMed articles indexed for MEDLINE yielded no established treatment options.
Cutaneous collagenous vasculopathy is a rare acquired microangiopathy involving the small vessels of the skin. Its clinical presentation is indistinguishable from that of generalized essential telangiectasia (GET). Patients generally present with asymptomatic, widespread, blanching, symmetric telangiectasias that classically begin on the legs and steadily progress upward with classic sparing of the face (Figure 3). Whereas GET has been reported to involve the oral and conjunctival mucosa, mucosal involvement is not typically observed in CCV and is considered to be a distinguishing factor between the 2 conditions.1,2 However, our patient reported oral symptoms, and oral erosions were seen on multiple physical examinations; therefore, ours is a rare case of mucosal involvement in conjunction with CCV. Given this finding, it is possible that more cases of CCV with mucosal involvement may exist but have been clinically misdiagnosed as GET.

First described by Salama and Rosenthal3 in 2000, CCV remains a rarely reported entity, with approximately 33 reported cases in the worldwide literature.2,4-7 The condition typically arises in adults with an equal predilection for males and females.2 The true incidence of CCV is unknown and likely is underreported given its close similarities to GET, which often is diagnosed clinically. The unique histopathologic finding of superficial ectatic vascular spaces with eosinophilic hyalinized vessel walls in CCV is key to distinguishing these similar entities, and even this finding can be subtle and is easily overlooked. Inflammation is sparse to absent. Deposited material is positive on periodic acid-Schiff and cytokeratin IV staining (representing reduplicated basement membrane-type collagen) and is diastase resistant. Smooth muscle actin staining is diminished or absent. Ultrastructural examination reveals reduplicated, laminated basement membrane; Luse bodies (abnormally long, widely spaced collagen fibers); and a decrease in or loss of pericytes. Of note, Luse bodies are nonspecific and their absence does not exclude a diagnosis of CCV.1
The etiology of CCV is unclear, and multiple pathogenetic mechanisms have been proposed. Ultimately, this entity is thought to arise from repeated endothelial cell damage, although the trigger for the endothelial cell injury is not completely understood. Diabetes mellitus sometimes is associated with microangiopathy and may be a confounding but not causative factor in some cases.1 Some investigators believe CCV is caused by a genetic defect that alters collagen production in the small vessels of the skin.5 Others have hypothesized that it is a secondary manifestation of an underlying disease or is associated with a medication; however, no disease or drug has been convincingly implicated in CCV.8
Cutaneous collagenous vasculopathy is limited to the skin, with no known reports of systemic involvement in the literature.7 There are no recommended laboratory studies to aid in diagnosis.1 It is critical to exclude hereditary hemorrhagic telangiectasia (HHT), as these patients can have life-threatening systemic involvement. Patients with CCV generally have no history of a bleeding diathesis, patients with HHT classically report recurrent epistaxis and gastrointestinal bleeding.7 A family history of HHT also is helpful for diagnosis, as the condition is autosomal dominant.1 Neither HHT or telangiectasia macularis eruptiva perstans, which also can be included in the differential diagnosis, demonstrate vessel wall hyalinization.
Treatment options for CCV are limited. Basso et al6 reported notable improvement in a patient with CCV treated with a combined 595-nm pulsed dye laser and 1064-nm Nd:YAG laser and optimized pulsed light. In one patient, treatment with a 585-nm pulsed dye laser produced a blanching response, suggesting that this may be a potential treatment option.7 Treatment with sclerotherapy has been ineffective.2
It is critical for both dermatologists and dermatopathologists to recognize and report this newly described entity, as the unique finding of vessel wall hyalinization in CCV may be indicative of a certain pathogenetic mechanism and effective treatment avenue that has yet to be established due to the relatively few number of reports that currently exist in the literature.
- Burdick LM, Lohser S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and structural study. J Cutan Pathol. 2000;27:40-48.
- Toda-Brito H, Resende C, Catorze G, et al. Cutaneous collagenous vasculopathy: a rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210635.
- Ma DL, Vano-Galvan S. Images in clinical medicine: cutaneous collagenous vasculopathy. N Engl J Med. 2015;373:E14.
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111.
- Moteqi SI, Yasuda M, Yamanaka M, et al. Cutaneous collagenous vasculopathy: report of first Japanese case and review of the literature. Australas J Dermatol. 2017;58:145-149.
- González Fernández D, Gómez Bernal S, Vivanco Allende B, et al. Cutaneous collagenous vasculopathy: description of two new cases in elderly women and review of the literature. Dermatology. 2012;225:1-8.
- Burdick LM, Lohser S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and structural study. J Cutan Pathol. 2000;27:40-48.
- Toda-Brito H, Resende C, Catorze G, et al. Cutaneous collagenous vasculopathy: a rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210635.
- Ma DL, Vano-Galvan S. Images in clinical medicine: cutaneous collagenous vasculopathy. N Engl J Med. 2015;373:E14.
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111.
- Moteqi SI, Yasuda M, Yamanaka M, et al. Cutaneous collagenous vasculopathy: report of first Japanese case and review of the literature. Australas J Dermatol. 2017;58:145-149.
- González Fernández D, Gómez Bernal S, Vivanco Allende B, et al. Cutaneous collagenous vasculopathy: description of two new cases in elderly women and review of the literature. Dermatology. 2012;225:1-8.
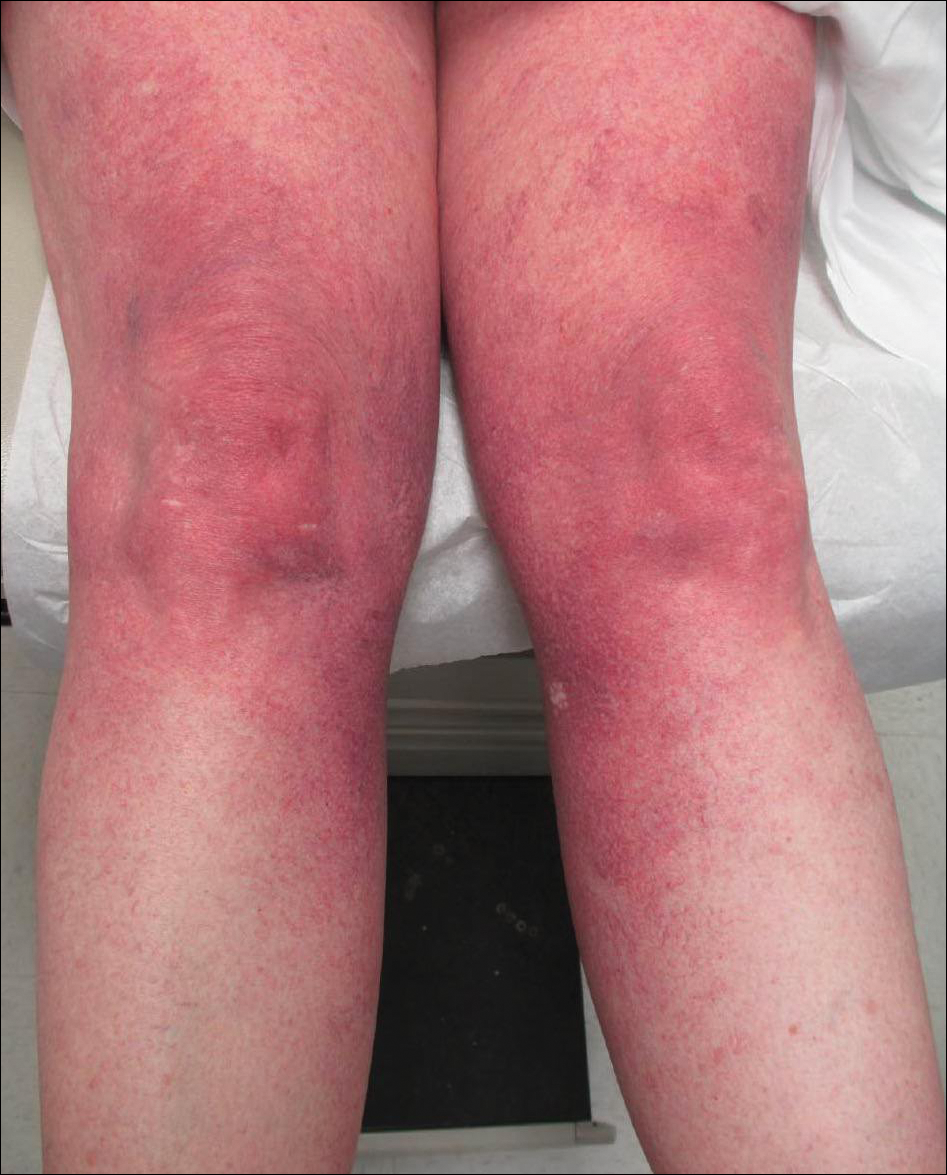
A 55-year-old woman presented for evaluation of widespread asymptomatic telangiectasias of several years' duration that first appeared on the legs and steadily progressed to involve the trunk and arms. A review of systems was remarkable for episodic glossitis and oral erosions that developed at the same time as the eruption. The patient had no history of bleeding diasthesis, and her family history was unremarkable. A laboratory workup (including autoimmune screening) and a malignancy workup were negative. Physical examination revealed confluent sheets of erythematous and purple blanching telangiectasias scattered symmetrically on the trunk, bilateral arms and legs, buttocks, and dorsal aspects of the feet with sparing of the palms, soles, and head and neck regions. A small, shallow erosion was present on the lateral aspect of the tongue. A 4-mm punch biopsy of a thigh lesion revealed ectatic blood vessels with hyalinized walls.
Keep pushing the envelope
By the time this column is published, we will have wrapped up Hospital Medicine 2018 in Orlando, it will be well into spring, and I will have completed my year as past president as well as my 6-year tenure on the Society of Hospital Medicine board of directors.
I can imagine that will feel like a relief and a milestone, and it also will feel like a loss to no longer be part of something that I have contributed my time, energy, passion, and emotion to for so long. I will retire at the ripe age of 48 – a pretty typical age for ending SHM board tenure, and it’s terribly important for SHM that I do so.
If you attended HM18, I hope you appreciated, as I do every year, the energy, enthusiasm, and youth – if not in years, then in spirit – of the event and of hospitalists. As a society and a profession, we take risks. We have set standards for excellence in hospital medicine programs. We have recognized a unique set of competencies and then not only attempted to expand them with education but also defined a specialty around them. We have welcomed practitioners and administrators as equals into our fold. These and many other accomplishments are the work of a board, committees, chapter leaders, and members who look for opportunity to expand our work into new and necessary domains, and not be limited by precedent.
On the SHM board and committees, we tackle issues of governance and strategy. For most of us, the SHM board is our first exposure to nonprofit oversight. And, to be sure, there is a steep learning curve as new members discover the issues and substance of the work of the society. I recall that I barely spoke the first year on the board, uncertain that I understood items fully, and I also was burned once or twice by making suggestions that reflected my lack of knowledge. While ignorance slowly gave way to experience, we also matured as a group as we found ways to debate and resolve tough, sometimes ambiguous, issues.
I came to appreciate that the strength of the board – and of SHM – is that we join the board naive to much of the past. After 6 years, while I may have come to understand issues with greater depth, I also see that the newer members bring fresher thinking, more creative energy, and even thoughts about how the group could function differently and perhaps better. Over the last few years, I realized that we veterans had developed a cadence and predictability to our work, and every year’s new members disrupt that rhythm. This disruption forces us to challenge each other and to be a better board – and hopefully – represent and advocate for you, our membership, better.
So, it’s time for me to move on. Even though I certainly feel like I still could contribute, it’s time to retire my own way of thinking from the leadership of SHM. The fact that we term-limit out at a (relatively) young age is, I believe, an extraordinary aspect of our organization, which is reflected in the work that our staff, our committees, and our members do.
SHM is an organization that, from the top down, embraces change in ways that few other organizations do. I believe we owe it to you to keep pushing the envelope of creativity – of what our goals are, of what a society can accomplish, of what an annual meeting can consist of. My ask of all of you is that you continue to challenge the leadership of SHM to be disruptive, to push the profession to better places, and to always strive to be more diverse, more inclusive, more communicative, more visible – and to stay young. In spirit and attitude if not in age. Thank you for giving me the opportunity to work on your behalf. It has been the greatest privilege of my career.
Dr. Harte is a past president of SHM and president of Cleveland Clinic Akron General and Southern Region.
By the time this column is published, we will have wrapped up Hospital Medicine 2018 in Orlando, it will be well into spring, and I will have completed my year as past president as well as my 6-year tenure on the Society of Hospital Medicine board of directors.
I can imagine that will feel like a relief and a milestone, and it also will feel like a loss to no longer be part of something that I have contributed my time, energy, passion, and emotion to for so long. I will retire at the ripe age of 48 – a pretty typical age for ending SHM board tenure, and it’s terribly important for SHM that I do so.
If you attended HM18, I hope you appreciated, as I do every year, the energy, enthusiasm, and youth – if not in years, then in spirit – of the event and of hospitalists. As a society and a profession, we take risks. We have set standards for excellence in hospital medicine programs. We have recognized a unique set of competencies and then not only attempted to expand them with education but also defined a specialty around them. We have welcomed practitioners and administrators as equals into our fold. These and many other accomplishments are the work of a board, committees, chapter leaders, and members who look for opportunity to expand our work into new and necessary domains, and not be limited by precedent.
On the SHM board and committees, we tackle issues of governance and strategy. For most of us, the SHM board is our first exposure to nonprofit oversight. And, to be sure, there is a steep learning curve as new members discover the issues and substance of the work of the society. I recall that I barely spoke the first year on the board, uncertain that I understood items fully, and I also was burned once or twice by making suggestions that reflected my lack of knowledge. While ignorance slowly gave way to experience, we also matured as a group as we found ways to debate and resolve tough, sometimes ambiguous, issues.
I came to appreciate that the strength of the board – and of SHM – is that we join the board naive to much of the past. After 6 years, while I may have come to understand issues with greater depth, I also see that the newer members bring fresher thinking, more creative energy, and even thoughts about how the group could function differently and perhaps better. Over the last few years, I realized that we veterans had developed a cadence and predictability to our work, and every year’s new members disrupt that rhythm. This disruption forces us to challenge each other and to be a better board – and hopefully – represent and advocate for you, our membership, better.
So, it’s time for me to move on. Even though I certainly feel like I still could contribute, it’s time to retire my own way of thinking from the leadership of SHM. The fact that we term-limit out at a (relatively) young age is, I believe, an extraordinary aspect of our organization, which is reflected in the work that our staff, our committees, and our members do.
SHM is an organization that, from the top down, embraces change in ways that few other organizations do. I believe we owe it to you to keep pushing the envelope of creativity – of what our goals are, of what a society can accomplish, of what an annual meeting can consist of. My ask of all of you is that you continue to challenge the leadership of SHM to be disruptive, to push the profession to better places, and to always strive to be more diverse, more inclusive, more communicative, more visible – and to stay young. In spirit and attitude if not in age. Thank you for giving me the opportunity to work on your behalf. It has been the greatest privilege of my career.
Dr. Harte is a past president of SHM and president of Cleveland Clinic Akron General and Southern Region.
By the time this column is published, we will have wrapped up Hospital Medicine 2018 in Orlando, it will be well into spring, and I will have completed my year as past president as well as my 6-year tenure on the Society of Hospital Medicine board of directors.
I can imagine that will feel like a relief and a milestone, and it also will feel like a loss to no longer be part of something that I have contributed my time, energy, passion, and emotion to for so long. I will retire at the ripe age of 48 – a pretty typical age for ending SHM board tenure, and it’s terribly important for SHM that I do so.
If you attended HM18, I hope you appreciated, as I do every year, the energy, enthusiasm, and youth – if not in years, then in spirit – of the event and of hospitalists. As a society and a profession, we take risks. We have set standards for excellence in hospital medicine programs. We have recognized a unique set of competencies and then not only attempted to expand them with education but also defined a specialty around them. We have welcomed practitioners and administrators as equals into our fold. These and many other accomplishments are the work of a board, committees, chapter leaders, and members who look for opportunity to expand our work into new and necessary domains, and not be limited by precedent.
On the SHM board and committees, we tackle issues of governance and strategy. For most of us, the SHM board is our first exposure to nonprofit oversight. And, to be sure, there is a steep learning curve as new members discover the issues and substance of the work of the society. I recall that I barely spoke the first year on the board, uncertain that I understood items fully, and I also was burned once or twice by making suggestions that reflected my lack of knowledge. While ignorance slowly gave way to experience, we also matured as a group as we found ways to debate and resolve tough, sometimes ambiguous, issues.
I came to appreciate that the strength of the board – and of SHM – is that we join the board naive to much of the past. After 6 years, while I may have come to understand issues with greater depth, I also see that the newer members bring fresher thinking, more creative energy, and even thoughts about how the group could function differently and perhaps better. Over the last few years, I realized that we veterans had developed a cadence and predictability to our work, and every year’s new members disrupt that rhythm. This disruption forces us to challenge each other and to be a better board – and hopefully – represent and advocate for you, our membership, better.
So, it’s time for me to move on. Even though I certainly feel like I still could contribute, it’s time to retire my own way of thinking from the leadership of SHM. The fact that we term-limit out at a (relatively) young age is, I believe, an extraordinary aspect of our organization, which is reflected in the work that our staff, our committees, and our members do.
SHM is an organization that, from the top down, embraces change in ways that few other organizations do. I believe we owe it to you to keep pushing the envelope of creativity – of what our goals are, of what a society can accomplish, of what an annual meeting can consist of. My ask of all of you is that you continue to challenge the leadership of SHM to be disruptive, to push the profession to better places, and to always strive to be more diverse, more inclusive, more communicative, more visible – and to stay young. In spirit and attitude if not in age. Thank you for giving me the opportunity to work on your behalf. It has been the greatest privilege of my career.
Dr. Harte is a past president of SHM and president of Cleveland Clinic Akron General and Southern Region.
Check SVS Website for New Research Opportunities
Looking for a research opportunity? Check our updated website for current programs in your area. If your institution has an opportunity to promote, let us know at communications@vascularsociety.org.
Looking for a research opportunity? Check our updated website for current programs in your area. If your institution has an opportunity to promote, let us know at communications@vascularsociety.org.
Looking for a research opportunity? Check our updated website for current programs in your area. If your institution has an opportunity to promote, let us know at communications@vascularsociety.org.
Submit a case to VAM's “Ask the Experts"
Help build the Vascular Annual Meeting educational program for the new “Ask the Experts” sessions, to be held daily, Wednesday through Saturday. Topics are coding, aortic care for occlusive disease, hemodialysis and PAD. Learn more about case submission here.
And if you haven’t registered for VAM yet, do so! The premier meeting for vascular surgeons is just five weeks (and one day) away. Learn more and register here. And obtain a hotel room here.
Help build the Vascular Annual Meeting educational program for the new “Ask the Experts” sessions, to be held daily, Wednesday through Saturday. Topics are coding, aortic care for occlusive disease, hemodialysis and PAD. Learn more about case submission here.
And if you haven’t registered for VAM yet, do so! The premier meeting for vascular surgeons is just five weeks (and one day) away. Learn more and register here. And obtain a hotel room here.
Help build the Vascular Annual Meeting educational program for the new “Ask the Experts” sessions, to be held daily, Wednesday through Saturday. Topics are coding, aortic care for occlusive disease, hemodialysis and PAD. Learn more about case submission here.
And if you haven’t registered for VAM yet, do so! The premier meeting for vascular surgeons is just five weeks (and one day) away. Learn more and register here. And obtain a hotel room here.
For Members Only: View “Negotiations” Webinar Materials
Did you miss the April 30 webinar on "Negotiating Physician Employment Contracts," presented by the SVS and the SVS Community Practice Committee? Materials -- available only to SVS members -- can be viewed here. Topics include benefits, call pay, termination rights, non-compete clauses, tenure opportunities and more.
Did you miss the April 30 webinar on "Negotiating Physician Employment Contracts," presented by the SVS and the SVS Community Practice Committee? Materials -- available only to SVS members -- can be viewed here. Topics include benefits, call pay, termination rights, non-compete clauses, tenure opportunities and more.
Did you miss the April 30 webinar on "Negotiating Physician Employment Contracts," presented by the SVS and the SVS Community Practice Committee? Materials -- available only to SVS members -- can be viewed here. Topics include benefits, call pay, termination rights, non-compete clauses, tenure opportunities and more.
Device-related thrombus associated with ischemic events
BOSTON – Device-related thrombus (DRT) does not occur often after left atrial appendage closure with the Watchman device. When it does, however, it is associated with a significantly higher rate of stroke and systemic embolism compared with that of patients with no DRT, according to a recent analysis presented at the annual scientific sessions of the Heart Rhythm Society.
Given the negative implications of DRT, a judicious surveillance strategy should be considered, especially when DRT risk factors are present, investigators said in a report on the study, which was published simultaneously in Circulation.
“Certainly, DRT is associated with an increased risk of stroke, and therapeutic anticoagulation should be resumed when discovered with rigorous transesophageal echocardiography follow-up to ensure resolution,” noted senior investigator Vivek Y. Reddy, MD.
Despite the higher rates of all strokes, ischemic strokes, and hemorrhagic strokes linked with DRT, the complication did not link with a higher rate of all-cause mortality compared with patients who never had a DRT. The results also suggested a causal link between DRT and subsequent stroke in about half the patients with DRT because their strokes occurred within a month following DRT diagnosis.
Despite these findings, a majority – 74% – of patients with an identified DRT did not have a stroke, and 87% of the strokes that occurred in the patients who received the Watchman device occurred in the absence of a DRT, reported Dr. Reddy, who presented the findings at the meeting.
The most immediate implication of the findings is the strong case they make for rethinking the timing of planned follow-up transesophageal echocardiography (TEE) examinations of patients after they receive a Watchman device. The current, standard protocol schedules a TEE at 45 days after Watchman placement, when routine anticoagulation usually stops, and then a second TEE 12 months after placement. A better schedule might be to perform the first TEE 3-4 months after Watchman placement to give a potential DRT time to form once oral anticoagulant therapy stops, suggested Dr. Reddy, professor and director of the cardiac arrhythmia service at Mount Sinai Hospital and Health System in New York.
“Surveillance is very important. I don’t think DRT usually occurs unless anticoagulation is suboptimal or stops.” Dr. Reddy noted that he and his associates are analyzing the best time for TEE surveillance in other large databases of patients treated with left atrial appendage (LAA) closure. Newer models of LAA closure devices structurally modified to reduce thrombus formation are nearing clinical use, he added.
The analysis, believed to be the largest to date of DRT following left atrial appendage closure, was based on prospective data from four clinical trials. That included two randomized controlled trials, PROTECT AF and PREVAIL, as well as the CAP and CAP2 prospective registries.
Among 1,739 patients in those studies receiving an implant, 65 (3.74%) had DRT, the investigators found.
Over 1 year of follow-up, 25% of patients with DRT had an ischemic stroke or systemic embolism, versus 6.8% of patients without DRTs (P less than .001), they reported. That worked out to an event rate of 6.28 and 1.65 events per 100 patient years, respectively.
The strongest predictors of DRT in multivariable analysis included vascular disease, history of stroke or transient ischemic attack, permanent atrial fibrillation, and left atrial appendage diameter, according to the report. Conversely, increasing left ventricular ejection fraction was protective against DRT.
Taken together, these data support reevaluating the transesophageal echocardiography strategy, according to Dr. Reddy and his coauthors. Those approaches might include targeting patients with DRT risk factors, routine additional surveillance at 6 months, or delaying the first transesophageal echocardiography to 4 months.
“Importantly, none of these strategies have been rigorously compared, so these suggestions are subject to future studies,” the researchers wrote.
“DRT remains a problem despite increased operator experience with LAA occlusion and improved occluding devices,” commented David B. De Lurgio, MD, a cardiac electrophysiologist at Emory Healthcare in Atlanta. “What is a little alarming is that the risk for DRT extends beyond the period of prescribed anticoagulation. Although the risk from DRT mitigates the benefit of LAA closure compared with warfarin, it does not mitigate the benefit from occlusion compared with no treatment,” said Dr. De Lurgio, designated discussant for the report. “Prevention and management of DRT may require that each patient receive a tailored regimen of anticoagulation and surveillance.”
The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Updated, 5/17/18: This article has been updated with reporting from Mitchel L. Zoler at the meeting, and has been revised for clarity and to reflect that the results were presented by Dr. Reddy.
SOURCE: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
BOSTON – Device-related thrombus (DRT) does not occur often after left atrial appendage closure with the Watchman device. When it does, however, it is associated with a significantly higher rate of stroke and systemic embolism compared with that of patients with no DRT, according to a recent analysis presented at the annual scientific sessions of the Heart Rhythm Society.
Given the negative implications of DRT, a judicious surveillance strategy should be considered, especially when DRT risk factors are present, investigators said in a report on the study, which was published simultaneously in Circulation.
“Certainly, DRT is associated with an increased risk of stroke, and therapeutic anticoagulation should be resumed when discovered with rigorous transesophageal echocardiography follow-up to ensure resolution,” noted senior investigator Vivek Y. Reddy, MD.
Despite the higher rates of all strokes, ischemic strokes, and hemorrhagic strokes linked with DRT, the complication did not link with a higher rate of all-cause mortality compared with patients who never had a DRT. The results also suggested a causal link between DRT and subsequent stroke in about half the patients with DRT because their strokes occurred within a month following DRT diagnosis.
Despite these findings, a majority – 74% – of patients with an identified DRT did not have a stroke, and 87% of the strokes that occurred in the patients who received the Watchman device occurred in the absence of a DRT, reported Dr. Reddy, who presented the findings at the meeting.
The most immediate implication of the findings is the strong case they make for rethinking the timing of planned follow-up transesophageal echocardiography (TEE) examinations of patients after they receive a Watchman device. The current, standard protocol schedules a TEE at 45 days after Watchman placement, when routine anticoagulation usually stops, and then a second TEE 12 months after placement. A better schedule might be to perform the first TEE 3-4 months after Watchman placement to give a potential DRT time to form once oral anticoagulant therapy stops, suggested Dr. Reddy, professor and director of the cardiac arrhythmia service at Mount Sinai Hospital and Health System in New York.
“Surveillance is very important. I don’t think DRT usually occurs unless anticoagulation is suboptimal or stops.” Dr. Reddy noted that he and his associates are analyzing the best time for TEE surveillance in other large databases of patients treated with left atrial appendage (LAA) closure. Newer models of LAA closure devices structurally modified to reduce thrombus formation are nearing clinical use, he added.
The analysis, believed to be the largest to date of DRT following left atrial appendage closure, was based on prospective data from four clinical trials. That included two randomized controlled trials, PROTECT AF and PREVAIL, as well as the CAP and CAP2 prospective registries.
Among 1,739 patients in those studies receiving an implant, 65 (3.74%) had DRT, the investigators found.
Over 1 year of follow-up, 25% of patients with DRT had an ischemic stroke or systemic embolism, versus 6.8% of patients without DRTs (P less than .001), they reported. That worked out to an event rate of 6.28 and 1.65 events per 100 patient years, respectively.
The strongest predictors of DRT in multivariable analysis included vascular disease, history of stroke or transient ischemic attack, permanent atrial fibrillation, and left atrial appendage diameter, according to the report. Conversely, increasing left ventricular ejection fraction was protective against DRT.
Taken together, these data support reevaluating the transesophageal echocardiography strategy, according to Dr. Reddy and his coauthors. Those approaches might include targeting patients with DRT risk factors, routine additional surveillance at 6 months, or delaying the first transesophageal echocardiography to 4 months.
“Importantly, none of these strategies have been rigorously compared, so these suggestions are subject to future studies,” the researchers wrote.
“DRT remains a problem despite increased operator experience with LAA occlusion and improved occluding devices,” commented David B. De Lurgio, MD, a cardiac electrophysiologist at Emory Healthcare in Atlanta. “What is a little alarming is that the risk for DRT extends beyond the period of prescribed anticoagulation. Although the risk from DRT mitigates the benefit of LAA closure compared with warfarin, it does not mitigate the benefit from occlusion compared with no treatment,” said Dr. De Lurgio, designated discussant for the report. “Prevention and management of DRT may require that each patient receive a tailored regimen of anticoagulation and surveillance.”
The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Updated, 5/17/18: This article has been updated with reporting from Mitchel L. Zoler at the meeting, and has been revised for clarity and to reflect that the results were presented by Dr. Reddy.
SOURCE: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
BOSTON – Device-related thrombus (DRT) does not occur often after left atrial appendage closure with the Watchman device. When it does, however, it is associated with a significantly higher rate of stroke and systemic embolism compared with that of patients with no DRT, according to a recent analysis presented at the annual scientific sessions of the Heart Rhythm Society.
Given the negative implications of DRT, a judicious surveillance strategy should be considered, especially when DRT risk factors are present, investigators said in a report on the study, which was published simultaneously in Circulation.
“Certainly, DRT is associated with an increased risk of stroke, and therapeutic anticoagulation should be resumed when discovered with rigorous transesophageal echocardiography follow-up to ensure resolution,” noted senior investigator Vivek Y. Reddy, MD.
Despite the higher rates of all strokes, ischemic strokes, and hemorrhagic strokes linked with DRT, the complication did not link with a higher rate of all-cause mortality compared with patients who never had a DRT. The results also suggested a causal link between DRT and subsequent stroke in about half the patients with DRT because their strokes occurred within a month following DRT diagnosis.
Despite these findings, a majority – 74% – of patients with an identified DRT did not have a stroke, and 87% of the strokes that occurred in the patients who received the Watchman device occurred in the absence of a DRT, reported Dr. Reddy, who presented the findings at the meeting.
The most immediate implication of the findings is the strong case they make for rethinking the timing of planned follow-up transesophageal echocardiography (TEE) examinations of patients after they receive a Watchman device. The current, standard protocol schedules a TEE at 45 days after Watchman placement, when routine anticoagulation usually stops, and then a second TEE 12 months after placement. A better schedule might be to perform the first TEE 3-4 months after Watchman placement to give a potential DRT time to form once oral anticoagulant therapy stops, suggested Dr. Reddy, professor and director of the cardiac arrhythmia service at Mount Sinai Hospital and Health System in New York.
“Surveillance is very important. I don’t think DRT usually occurs unless anticoagulation is suboptimal or stops.” Dr. Reddy noted that he and his associates are analyzing the best time for TEE surveillance in other large databases of patients treated with left atrial appendage (LAA) closure. Newer models of LAA closure devices structurally modified to reduce thrombus formation are nearing clinical use, he added.
The analysis, believed to be the largest to date of DRT following left atrial appendage closure, was based on prospective data from four clinical trials. That included two randomized controlled trials, PROTECT AF and PREVAIL, as well as the CAP and CAP2 prospective registries.
Among 1,739 patients in those studies receiving an implant, 65 (3.74%) had DRT, the investigators found.
Over 1 year of follow-up, 25% of patients with DRT had an ischemic stroke or systemic embolism, versus 6.8% of patients without DRTs (P less than .001), they reported. That worked out to an event rate of 6.28 and 1.65 events per 100 patient years, respectively.
The strongest predictors of DRT in multivariable analysis included vascular disease, history of stroke or transient ischemic attack, permanent atrial fibrillation, and left atrial appendage diameter, according to the report. Conversely, increasing left ventricular ejection fraction was protective against DRT.
Taken together, these data support reevaluating the transesophageal echocardiography strategy, according to Dr. Reddy and his coauthors. Those approaches might include targeting patients with DRT risk factors, routine additional surveillance at 6 months, or delaying the first transesophageal echocardiography to 4 months.
“Importantly, none of these strategies have been rigorously compared, so these suggestions are subject to future studies,” the researchers wrote.
“DRT remains a problem despite increased operator experience with LAA occlusion and improved occluding devices,” commented David B. De Lurgio, MD, a cardiac electrophysiologist at Emory Healthcare in Atlanta. “What is a little alarming is that the risk for DRT extends beyond the period of prescribed anticoagulation. Although the risk from DRT mitigates the benefit of LAA closure compared with warfarin, it does not mitigate the benefit from occlusion compared with no treatment,” said Dr. De Lurgio, designated discussant for the report. “Prevention and management of DRT may require that each patient receive a tailored regimen of anticoagulation and surveillance.”
The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Updated, 5/17/18: This article has been updated with reporting from Mitchel L. Zoler at the meeting, and has been revised for clarity and to reflect that the results were presented by Dr. Reddy.
SOURCE: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
REPORTING FROM HEART RHYTHM 2018
Key clinical point: Device-related thrombus (DRT) following left atrial appendage closure occurs in less than 4% of patients but is associated with a higher rate of stroke and systemic embolism vs. no DRT.
Major finding: The rate of ischemic stroke and systemic embolism was 6.28 and 1.65 per 100 patient years for patients with DRT and with no DRT, respectively (P less than .001).
Study details: Analysis of data from the device arms of four prospective clinical trials.
Disclosures: The Watchman studies were funded by Boston Scientific, the company that markets the device. Dr. Reddy has been a consultant to and has received research funding from Boston Scientific and from Abbott and Biosense-Webster, and he reported having an equity interest in Javelin and Surecor. Coauthors reported disclosures related to Boston Scientific, Johnson & Johnson, Abbott, and other medical device companies. Dr. De Lurgio has been a consultant to Boston Scientific.
Source: Dukkipati SR et al. Circulation. 2018 May 11. doi: 10.1161/CIRCULATIONAHA.118.035090.
Air pollution linked to childhood hypertension
Third-trimester exposure to airborne particulate matter (PM) smaller than 2.5 mcm in size (PM2.5) has been linked to higher levels of systolic blood pressure during childhood.
Mingyu Zhang of Johns Hopkins Bloomberg School of Public Health, Baltimore, and his associates found that the highest tertile exposure was tied to an increased likelihood of childhood elevated BP, defined as systolic blood pressure higher than the 90th percentile, compared with those in the lowest tertile (relative risk, 1.60; 95% confidence interval, 1.12-2.27).
Previous studies have shown a relationship between exposure to PM2.5and elevated BP in children and adults. Mouse models suggest that PM2.5 may interfere with in utero development of the cardiovascular system. One previous study found evidence that third-trimester exposure to PM2.5was linked to heightened BP in newborns, while a retrospective analysis showed no association with BP in adolescents.
They identified PM2.5 exposure by matching the mother’s residential address to the U.S. Environmental Protection Agency’s local air quality monitors, from which they extracted daily PM2.5 values.
For every 5 mcg/m3 increment in PM2.5 exposure, the relative risk for heightened BP in a child at a single visit was 1.46 (95% CI, 1.17-1.83). The relative risk increased after independent additions to the confounder model of birth weight (RR, 3.39; 95% CI, 0.63-6.15), gestational age (RR, 3.08; 95% CI, 0.33-5.82), and child body mass index (BMI) z score (RR, 2.75; 95% CI, 0.01-5.50).
A multivariable-adjusted cubic spline model revealed a significant increase in risk of elevated BP at a cutoff threshold of 13 mcg/m3 (RR, 1.80; 95% CI, 1.33-2.44).
Further analysis suggested that 35% of the association between exposure and elevated BP risk was mediated by birth weight and BMI z score during childhood. When these factors were added to the models, the association between PM exposure and BP risk was no longer significant.
Although the findings are intriguing, they cannot prove causation, according to the researchers.
The study was funded by the National Institutes of Health and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
SOURCE: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
A paper recently published by the same group outlines a possible mechanism by which PM2.5 might cause harm – specifically, an increase in placental intrauterine inflammation. The study adds evidence to the case that air pollution is linked to hypertension as it includes more economically disadvantaged subjects, children of color, preterm births, and small-for-gestational-age births. Overall, the study imaginatively made use of pediatric data in a disadvantaged urban setting to better understand how air pollution affects an important physiological outcome.
Diane Gold, MD, and Antonella Zanobetti, PhD, are at the Harvard T.H. Chan School of Public Health, Boston.
A paper recently published by the same group outlines a possible mechanism by which PM2.5 might cause harm – specifically, an increase in placental intrauterine inflammation. The study adds evidence to the case that air pollution is linked to hypertension as it includes more economically disadvantaged subjects, children of color, preterm births, and small-for-gestational-age births. Overall, the study imaginatively made use of pediatric data in a disadvantaged urban setting to better understand how air pollution affects an important physiological outcome.
Diane Gold, MD, and Antonella Zanobetti, PhD, are at the Harvard T.H. Chan School of Public Health, Boston.
A paper recently published by the same group outlines a possible mechanism by which PM2.5 might cause harm – specifically, an increase in placental intrauterine inflammation. The study adds evidence to the case that air pollution is linked to hypertension as it includes more economically disadvantaged subjects, children of color, preterm births, and small-for-gestational-age births. Overall, the study imaginatively made use of pediatric data in a disadvantaged urban setting to better understand how air pollution affects an important physiological outcome.
Diane Gold, MD, and Antonella Zanobetti, PhD, are at the Harvard T.H. Chan School of Public Health, Boston.
Third-trimester exposure to airborne particulate matter (PM) smaller than 2.5 mcm in size (PM2.5) has been linked to higher levels of systolic blood pressure during childhood.
Mingyu Zhang of Johns Hopkins Bloomberg School of Public Health, Baltimore, and his associates found that the highest tertile exposure was tied to an increased likelihood of childhood elevated BP, defined as systolic blood pressure higher than the 90th percentile, compared with those in the lowest tertile (relative risk, 1.60; 95% confidence interval, 1.12-2.27).
Previous studies have shown a relationship between exposure to PM2.5and elevated BP in children and adults. Mouse models suggest that PM2.5 may interfere with in utero development of the cardiovascular system. One previous study found evidence that third-trimester exposure to PM2.5was linked to heightened BP in newborns, while a retrospective analysis showed no association with BP in adolescents.
They identified PM2.5 exposure by matching the mother’s residential address to the U.S. Environmental Protection Agency’s local air quality monitors, from which they extracted daily PM2.5 values.
For every 5 mcg/m3 increment in PM2.5 exposure, the relative risk for heightened BP in a child at a single visit was 1.46 (95% CI, 1.17-1.83). The relative risk increased after independent additions to the confounder model of birth weight (RR, 3.39; 95% CI, 0.63-6.15), gestational age (RR, 3.08; 95% CI, 0.33-5.82), and child body mass index (BMI) z score (RR, 2.75; 95% CI, 0.01-5.50).
A multivariable-adjusted cubic spline model revealed a significant increase in risk of elevated BP at a cutoff threshold of 13 mcg/m3 (RR, 1.80; 95% CI, 1.33-2.44).
Further analysis suggested that 35% of the association between exposure and elevated BP risk was mediated by birth weight and BMI z score during childhood. When these factors were added to the models, the association between PM exposure and BP risk was no longer significant.
Although the findings are intriguing, they cannot prove causation, according to the researchers.
The study was funded by the National Institutes of Health and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
SOURCE: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
Third-trimester exposure to airborne particulate matter (PM) smaller than 2.5 mcm in size (PM2.5) has been linked to higher levels of systolic blood pressure during childhood.
Mingyu Zhang of Johns Hopkins Bloomberg School of Public Health, Baltimore, and his associates found that the highest tertile exposure was tied to an increased likelihood of childhood elevated BP, defined as systolic blood pressure higher than the 90th percentile, compared with those in the lowest tertile (relative risk, 1.60; 95% confidence interval, 1.12-2.27).
Previous studies have shown a relationship between exposure to PM2.5and elevated BP in children and adults. Mouse models suggest that PM2.5 may interfere with in utero development of the cardiovascular system. One previous study found evidence that third-trimester exposure to PM2.5was linked to heightened BP in newborns, while a retrospective analysis showed no association with BP in adolescents.
They identified PM2.5 exposure by matching the mother’s residential address to the U.S. Environmental Protection Agency’s local air quality monitors, from which they extracted daily PM2.5 values.
For every 5 mcg/m3 increment in PM2.5 exposure, the relative risk for heightened BP in a child at a single visit was 1.46 (95% CI, 1.17-1.83). The relative risk increased after independent additions to the confounder model of birth weight (RR, 3.39; 95% CI, 0.63-6.15), gestational age (RR, 3.08; 95% CI, 0.33-5.82), and child body mass index (BMI) z score (RR, 2.75; 95% CI, 0.01-5.50).
A multivariable-adjusted cubic spline model revealed a significant increase in risk of elevated BP at a cutoff threshold of 13 mcg/m3 (RR, 1.80; 95% CI, 1.33-2.44).
Further analysis suggested that 35% of the association between exposure and elevated BP risk was mediated by birth weight and BMI z score during childhood. When these factors were added to the models, the association between PM exposure and BP risk was no longer significant.
Although the findings are intriguing, they cannot prove causation, according to the researchers.
The study was funded by the National Institutes of Health and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
SOURCE: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
FROM HYPERTENSION
Key clinical point: Maternal air pollution exposure could be useful in screening and prevention of childhood hypertension.
Major finding: Each 5 µg/m3 exposure increment was associated with 46% increased odds of elevated BP.
Study details: Prospective study of 1,293 mothers.
Disclosures: The study was funded by the NIH and the Maternal and Child Health Bureau. Dr. Gold and Dr. Zanobetti have received funding from NIH.
Source: Zhang et al. 2018 Jul. doi: 10.1161/hypertensionaha.117.10944.
Rapid Deterioration and Death Caused by Bilateral Phlegmasia Cerulea Dolens
Phlegmasia cerulea dolens (PCD), a life-threatening complication of deep venous thrombosis (DVT), is characterized by massive iliofemoral thrombus that extends to the collateral veins, leading to fluid sequestration and elevated compartment pressures that ultimately compromise arterial flow. Phlegmasia cerulea dolens can rapidly progress to compartment syndrome and gangrene.1,2 The affected limbs of patients with PCD can be hypoxic and appear purple in color due to substantial lack of blood flow, with diminished or absent pulses. Risk factors for PCD include malignancy, hypercoagulable states, venous stasis, contraceptive agents, inferior vena cava (IVC) filter, aneurysm, history of DVT, trauma, heparin-induced thrombocytopenia, femoral vein catheterization, antiphospholipid syndrome, or pregnancy.3-6 Failure to treat PCD early and aggressively carries an amputation rate of up to 50% and a mortality rate of up to 40%.4
We present the case of a patient with PCD, whose condition rapidly deteriorated despite prompt diagnosis and treatment.
Case
A 58-year-old woman presented to the ED with a 1-day history of back and leg pain and difficulty walking. When asked about the severity of her pain, she rated her leg pain at 10 on a scale of 0 to 10. The patient’s history was significant for DVT and pulmonary embolism (PE), for which a Greenfield IVC had been placed and for which she was on prophylactic warfarin therapy. The patient stated that she had been taken off warfarin several weeks prior to presentation in preparation for an elective colonoscopy and dental procedure, but had restarted the warfarin therapy 2 days prior to presentation. She had no history of diabetes mellitus or renal disease.
Initial vital signs at presentation were: blood pressure, 120/91 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min; and temperature, 96.6°F. Oxygen saturation was 100% on a nonrebreather mask.
On examination, the patient was alert and oriented to person, time, and place, but appeared dyspneic. An electrocardiogram revealed sinus tachycardia. On physical examination, lung sounds were clear to auscultation bilaterally with good air movement, and the abdomen was soft and nontender with normal bowel sounds. The dorsalis pedis and posterior tibial pulses were absent bilaterally, lower extremity capillary refill was 3 seconds, and the legs appeared mildly erythematous and cool to touch. No speech or neurological deficits were present.
Laboratory evaluation was remarkable for metabolic acidosis, venous pH, 7.11; bicarbonate, 11.7; partial pressure of carbon dioxide, 37.6; lactic acid, 8.8 mEq/L leukocytosis, 24,900 u/L; glucose, 296 mg/dL; creatinine, 2.41 mg/dL; and international normalized ratio, 1.36.
Before additional laboratory studies and imaging could be obtained, the patient developed altered mental status, hypotension, and paralysis of the lower extremities. She was orally intubated for airway protection and was given a total of 4 L of normal saline intravenously (IV) for hypotension and acidosis; sodium bicarbonate for metabolic acidosis; norepinephrine for hypotension; fentanyl for pain; and ondansetron for nausea. A central line and arterial line were placed for administering medication and hemodynamic monitoring.
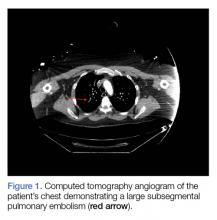
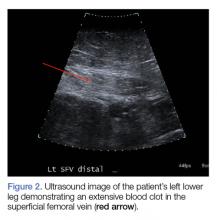
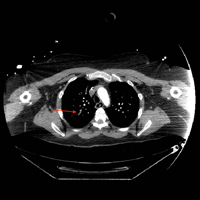
Computed tomography (CT) angiography of the chest, abdomen, and pelvis demonstrated multiple subsegmental bilateral PE with no arterial pathology (Figure 1). Beside ultrasound revealed extensive bilateral DVTs involving the superficial and common femoral veins (Figure 2). The patient’s bilateral DVTs, arterial compromise, and leg cyanosis led to the diagnosis of PCD.
Critical care and vascular surgery services were consulted, and the patient was admitted to the intensive care unit. Since the patient was too unstable to undergo thrombectomy, she was given IV tissue plasminogen activator. Despite aggressive pharmacological treatment, the patient’s condition continued to deteriorate. On hospital day 2, the patient’s family changed the patient’s code status to do-not-resuscitate/comfort-care only; she died shortly thereafter.
Discussion
This case illustrates the severity and complications of PCD and the rapidity with which this condition can deteriorate. At the time of ED presentation, the patient had already developed bilateral PCD, metabolic acidosis, and bilateral PE. Unfortunately, due to decreased venous return, decreased cardiac output, and severe shock, she quickly became unstable and progressed rapidly to multisystem organ failure leading to death.
Risk Factors
A prior patient history DVT and an IVC filter are both significant risk factors for the progression of DVT to PCD;3,6 however, in this case, IVC filter failed to prevent emboli from reaching the lungs. Extensive thrombi led to severely decreased venous return and cardiac output, causing life-threatening shock, ischemia, and metabolic acidosis. A lactic acid level taken on hospital day 2 was elevated at 19 mEq/L, demonstrating the severity, morbidity, and progression of PCD.
Signs and Symptoms
The three cardinal signs that lead to a clinical diagnosis of PCD are edema, pain, and violaceous discoloration or skin mottling.3 Although most commonly found in the lower extremity, PCD can occur in any limb due to occlusion of venous outflow.7 Unfortunately, a clinical diagnosis of PCD is not often made until the venous occlusion becomes severe enough to impair arterial flow and cause venous gangrene, tissue ischemia, shock, and death.8
Although IVC filters are designed to prevent life-threatening PE, there are risk factors associated with their use. Whether placed recently or decades prior, urgent investigation, such as immediate CT scan, should be undertaken in patients presenting with DVT-like symptoms who have a history of an IVC filter, to ensure the filter has not shifted from its original placement and is not occluding the IVC.
Conclusion
Phlegmasia cerulea dolens is an uncommon vascular emergency, but one that has a high-morbidity and high-mortality rate. This case demonstrates the importance of early diagnosis, aggressive treatment, and the severe complications that can develop in PCD.
There are cases in the literature where patients diagnosed with PCD had a successful outcome with pharmacological or surgical intervention such as thrombectomy. Treatment for PCD is most effective when instituted early in onset. As seen in our patient, the tendency for rapid deterioration in PCD can limit potentially lifesaving therapeutic options, decreasing the chances of a successful outcome. Emergency physicians, therefore, must be aware of the high-mortality rate associated with this disorder and the possibility of rapid progression from stable to critical condition.
1. Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59-69. doi:10.2147/JBM.S19009.
2. Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Ultrasound. 2007;35(7):401-404. doi:10.1002/jcu.20317.
3. Maiti A, Das A, Smith DT. Phlegmasia cerulean dolens. Postgrad Med J. 2016;pii: postgradmedj-2016-134185. doi:10.1136/postgradmedj-2016-134185.
4. Abdul W, Hickey B, Wilson C. Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency. BMJ Case Rep. 2016;2016:pii:bcr2016215078. doi:10.1136/bcr-2016-215078.
5. Onuoha CU. Phlegmasia cerulea dolens: A rare clinical presentation. Am J Med. 2015;128(9):e27-e28. doi:10.1016/j.amjmed.2015.04.009.
6. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5-14. doi:10.1177/1538574410388309.
7. Bagenal JD, Nasralla D. Bilateral phlegmasia cerulea dolens in an occluded inferior vena cava filter. BMJ Case Rep. 2013;pii: bcr2013009302. doi:10.1136/bcr-2013-009302.
8. Kiefer CS, Colletti JE. Phlegmasia cerulea dolens in a patient with an inferior vena cava filter. J Emerg Med. 2013;44(1):e95-e97. doi:10.1016/j.jemermed.2012.01.018.
Phlegmasia cerulea dolens (PCD), a life-threatening complication of deep venous thrombosis (DVT), is characterized by massive iliofemoral thrombus that extends to the collateral veins, leading to fluid sequestration and elevated compartment pressures that ultimately compromise arterial flow. Phlegmasia cerulea dolens can rapidly progress to compartment syndrome and gangrene.1,2 The affected limbs of patients with PCD can be hypoxic and appear purple in color due to substantial lack of blood flow, with diminished or absent pulses. Risk factors for PCD include malignancy, hypercoagulable states, venous stasis, contraceptive agents, inferior vena cava (IVC) filter, aneurysm, history of DVT, trauma, heparin-induced thrombocytopenia, femoral vein catheterization, antiphospholipid syndrome, or pregnancy.3-6 Failure to treat PCD early and aggressively carries an amputation rate of up to 50% and a mortality rate of up to 40%.4
We present the case of a patient with PCD, whose condition rapidly deteriorated despite prompt diagnosis and treatment.
Case
A 58-year-old woman presented to the ED with a 1-day history of back and leg pain and difficulty walking. When asked about the severity of her pain, she rated her leg pain at 10 on a scale of 0 to 10. The patient’s history was significant for DVT and pulmonary embolism (PE), for which a Greenfield IVC had been placed and for which she was on prophylactic warfarin therapy. The patient stated that she had been taken off warfarin several weeks prior to presentation in preparation for an elective colonoscopy and dental procedure, but had restarted the warfarin therapy 2 days prior to presentation. She had no history of diabetes mellitus or renal disease.
Initial vital signs at presentation were: blood pressure, 120/91 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min; and temperature, 96.6°F. Oxygen saturation was 100% on a nonrebreather mask.
On examination, the patient was alert and oriented to person, time, and place, but appeared dyspneic. An electrocardiogram revealed sinus tachycardia. On physical examination, lung sounds were clear to auscultation bilaterally with good air movement, and the abdomen was soft and nontender with normal bowel sounds. The dorsalis pedis and posterior tibial pulses were absent bilaterally, lower extremity capillary refill was 3 seconds, and the legs appeared mildly erythematous and cool to touch. No speech or neurological deficits were present.
Laboratory evaluation was remarkable for metabolic acidosis, venous pH, 7.11; bicarbonate, 11.7; partial pressure of carbon dioxide, 37.6; lactic acid, 8.8 mEq/L leukocytosis, 24,900 u/L; glucose, 296 mg/dL; creatinine, 2.41 mg/dL; and international normalized ratio, 1.36.
Before additional laboratory studies and imaging could be obtained, the patient developed altered mental status, hypotension, and paralysis of the lower extremities. She was orally intubated for airway protection and was given a total of 4 L of normal saline intravenously (IV) for hypotension and acidosis; sodium bicarbonate for metabolic acidosis; norepinephrine for hypotension; fentanyl for pain; and ondansetron for nausea. A central line and arterial line were placed for administering medication and hemodynamic monitoring.
Computed tomography (CT) angiography of the chest, abdomen, and pelvis demonstrated multiple subsegmental bilateral PE with no arterial pathology (Figure 1). Beside ultrasound revealed extensive bilateral DVTs involving the superficial and common femoral veins (Figure 2). The patient’s bilateral DVTs, arterial compromise, and leg cyanosis led to the diagnosis of PCD.
Critical care and vascular surgery services were consulted, and the patient was admitted to the intensive care unit. Since the patient was too unstable to undergo thrombectomy, she was given IV tissue plasminogen activator. Despite aggressive pharmacological treatment, the patient’s condition continued to deteriorate. On hospital day 2, the patient’s family changed the patient’s code status to do-not-resuscitate/comfort-care only; she died shortly thereafter.
Discussion
This case illustrates the severity and complications of PCD and the rapidity with which this condition can deteriorate. At the time of ED presentation, the patient had already developed bilateral PCD, metabolic acidosis, and bilateral PE. Unfortunately, due to decreased venous return, decreased cardiac output, and severe shock, she quickly became unstable and progressed rapidly to multisystem organ failure leading to death.
Risk Factors
A prior patient history DVT and an IVC filter are both significant risk factors for the progression of DVT to PCD;3,6 however, in this case, IVC filter failed to prevent emboli from reaching the lungs. Extensive thrombi led to severely decreased venous return and cardiac output, causing life-threatening shock, ischemia, and metabolic acidosis. A lactic acid level taken on hospital day 2 was elevated at 19 mEq/L, demonstrating the severity, morbidity, and progression of PCD.
Signs and Symptoms
The three cardinal signs that lead to a clinical diagnosis of PCD are edema, pain, and violaceous discoloration or skin mottling.3 Although most commonly found in the lower extremity, PCD can occur in any limb due to occlusion of venous outflow.7 Unfortunately, a clinical diagnosis of PCD is not often made until the venous occlusion becomes severe enough to impair arterial flow and cause venous gangrene, tissue ischemia, shock, and death.8
Although IVC filters are designed to prevent life-threatening PE, there are risk factors associated with their use. Whether placed recently or decades prior, urgent investigation, such as immediate CT scan, should be undertaken in patients presenting with DVT-like symptoms who have a history of an IVC filter, to ensure the filter has not shifted from its original placement and is not occluding the IVC.
Conclusion
Phlegmasia cerulea dolens is an uncommon vascular emergency, but one that has a high-morbidity and high-mortality rate. This case demonstrates the importance of early diagnosis, aggressive treatment, and the severe complications that can develop in PCD.
There are cases in the literature where patients diagnosed with PCD had a successful outcome with pharmacological or surgical intervention such as thrombectomy. Treatment for PCD is most effective when instituted early in onset. As seen in our patient, the tendency for rapid deterioration in PCD can limit potentially lifesaving therapeutic options, decreasing the chances of a successful outcome. Emergency physicians, therefore, must be aware of the high-mortality rate associated with this disorder and the possibility of rapid progression from stable to critical condition.
Phlegmasia cerulea dolens (PCD), a life-threatening complication of deep venous thrombosis (DVT), is characterized by massive iliofemoral thrombus that extends to the collateral veins, leading to fluid sequestration and elevated compartment pressures that ultimately compromise arterial flow. Phlegmasia cerulea dolens can rapidly progress to compartment syndrome and gangrene.1,2 The affected limbs of patients with PCD can be hypoxic and appear purple in color due to substantial lack of blood flow, with diminished or absent pulses. Risk factors for PCD include malignancy, hypercoagulable states, venous stasis, contraceptive agents, inferior vena cava (IVC) filter, aneurysm, history of DVT, trauma, heparin-induced thrombocytopenia, femoral vein catheterization, antiphospholipid syndrome, or pregnancy.3-6 Failure to treat PCD early and aggressively carries an amputation rate of up to 50% and a mortality rate of up to 40%.4
We present the case of a patient with PCD, whose condition rapidly deteriorated despite prompt diagnosis and treatment.
Case
A 58-year-old woman presented to the ED with a 1-day history of back and leg pain and difficulty walking. When asked about the severity of her pain, she rated her leg pain at 10 on a scale of 0 to 10. The patient’s history was significant for DVT and pulmonary embolism (PE), for which a Greenfield IVC had been placed and for which she was on prophylactic warfarin therapy. The patient stated that she had been taken off warfarin several weeks prior to presentation in preparation for an elective colonoscopy and dental procedure, but had restarted the warfarin therapy 2 days prior to presentation. She had no history of diabetes mellitus or renal disease.
Initial vital signs at presentation were: blood pressure, 120/91 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min; and temperature, 96.6°F. Oxygen saturation was 100% on a nonrebreather mask.
On examination, the patient was alert and oriented to person, time, and place, but appeared dyspneic. An electrocardiogram revealed sinus tachycardia. On physical examination, lung sounds were clear to auscultation bilaterally with good air movement, and the abdomen was soft and nontender with normal bowel sounds. The dorsalis pedis and posterior tibial pulses were absent bilaterally, lower extremity capillary refill was 3 seconds, and the legs appeared mildly erythematous and cool to touch. No speech or neurological deficits were present.
Laboratory evaluation was remarkable for metabolic acidosis, venous pH, 7.11; bicarbonate, 11.7; partial pressure of carbon dioxide, 37.6; lactic acid, 8.8 mEq/L leukocytosis, 24,900 u/L; glucose, 296 mg/dL; creatinine, 2.41 mg/dL; and international normalized ratio, 1.36.
Before additional laboratory studies and imaging could be obtained, the patient developed altered mental status, hypotension, and paralysis of the lower extremities. She was orally intubated for airway protection and was given a total of 4 L of normal saline intravenously (IV) for hypotension and acidosis; sodium bicarbonate for metabolic acidosis; norepinephrine for hypotension; fentanyl for pain; and ondansetron for nausea. A central line and arterial line were placed for administering medication and hemodynamic monitoring.
Computed tomography (CT) angiography of the chest, abdomen, and pelvis demonstrated multiple subsegmental bilateral PE with no arterial pathology (Figure 1). Beside ultrasound revealed extensive bilateral DVTs involving the superficial and common femoral veins (Figure 2). The patient’s bilateral DVTs, arterial compromise, and leg cyanosis led to the diagnosis of PCD.
Critical care and vascular surgery services were consulted, and the patient was admitted to the intensive care unit. Since the patient was too unstable to undergo thrombectomy, she was given IV tissue plasminogen activator. Despite aggressive pharmacological treatment, the patient’s condition continued to deteriorate. On hospital day 2, the patient’s family changed the patient’s code status to do-not-resuscitate/comfort-care only; she died shortly thereafter.
Discussion
This case illustrates the severity and complications of PCD and the rapidity with which this condition can deteriorate. At the time of ED presentation, the patient had already developed bilateral PCD, metabolic acidosis, and bilateral PE. Unfortunately, due to decreased venous return, decreased cardiac output, and severe shock, she quickly became unstable and progressed rapidly to multisystem organ failure leading to death.
Risk Factors
A prior patient history DVT and an IVC filter are both significant risk factors for the progression of DVT to PCD;3,6 however, in this case, IVC filter failed to prevent emboli from reaching the lungs. Extensive thrombi led to severely decreased venous return and cardiac output, causing life-threatening shock, ischemia, and metabolic acidosis. A lactic acid level taken on hospital day 2 was elevated at 19 mEq/L, demonstrating the severity, morbidity, and progression of PCD.
Signs and Symptoms
The three cardinal signs that lead to a clinical diagnosis of PCD are edema, pain, and violaceous discoloration or skin mottling.3 Although most commonly found in the lower extremity, PCD can occur in any limb due to occlusion of venous outflow.7 Unfortunately, a clinical diagnosis of PCD is not often made until the venous occlusion becomes severe enough to impair arterial flow and cause venous gangrene, tissue ischemia, shock, and death.8
Although IVC filters are designed to prevent life-threatening PE, there are risk factors associated with their use. Whether placed recently or decades prior, urgent investigation, such as immediate CT scan, should be undertaken in patients presenting with DVT-like symptoms who have a history of an IVC filter, to ensure the filter has not shifted from its original placement and is not occluding the IVC.
Conclusion
Phlegmasia cerulea dolens is an uncommon vascular emergency, but one that has a high-morbidity and high-mortality rate. This case demonstrates the importance of early diagnosis, aggressive treatment, and the severe complications that can develop in PCD.
There are cases in the literature where patients diagnosed with PCD had a successful outcome with pharmacological or surgical intervention such as thrombectomy. Treatment for PCD is most effective when instituted early in onset. As seen in our patient, the tendency for rapid deterioration in PCD can limit potentially lifesaving therapeutic options, decreasing the chances of a successful outcome. Emergency physicians, therefore, must be aware of the high-mortality rate associated with this disorder and the possibility of rapid progression from stable to critical condition.
1. Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59-69. doi:10.2147/JBM.S19009.
2. Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Ultrasound. 2007;35(7):401-404. doi:10.1002/jcu.20317.
3. Maiti A, Das A, Smith DT. Phlegmasia cerulean dolens. Postgrad Med J. 2016;pii: postgradmedj-2016-134185. doi:10.1136/postgradmedj-2016-134185.
4. Abdul W, Hickey B, Wilson C. Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency. BMJ Case Rep. 2016;2016:pii:bcr2016215078. doi:10.1136/bcr-2016-215078.
5. Onuoha CU. Phlegmasia cerulea dolens: A rare clinical presentation. Am J Med. 2015;128(9):e27-e28. doi:10.1016/j.amjmed.2015.04.009.
6. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5-14. doi:10.1177/1538574410388309.
7. Bagenal JD, Nasralla D. Bilateral phlegmasia cerulea dolens in an occluded inferior vena cava filter. BMJ Case Rep. 2013;pii: bcr2013009302. doi:10.1136/bcr-2013-009302.
8. Kiefer CS, Colletti JE. Phlegmasia cerulea dolens in a patient with an inferior vena cava filter. J Emerg Med. 2013;44(1):e95-e97. doi:10.1016/j.jemermed.2012.01.018.
1. Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59-69. doi:10.2147/JBM.S19009.
2. Bhatt S, Wehbe C, Dogra VS. Phlegmasia cerulea dolens. J Clin Ultrasound. 2007;35(7):401-404. doi:10.1002/jcu.20317.
3. Maiti A, Das A, Smith DT. Phlegmasia cerulean dolens. Postgrad Med J. 2016;pii: postgradmedj-2016-134185. doi:10.1136/postgradmedj-2016-134185.
4. Abdul W, Hickey B, Wilson C. Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency. BMJ Case Rep. 2016;2016:pii:bcr2016215078. doi:10.1136/bcr-2016-215078.
5. Onuoha CU. Phlegmasia cerulea dolens: A rare clinical presentation. Am J Med. 2015;128(9):e27-e28. doi:10.1016/j.amjmed.2015.04.009.
6. Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg. 2011;45(1):5-14. doi:10.1177/1538574410388309.
7. Bagenal JD, Nasralla D. Bilateral phlegmasia cerulea dolens in an occluded inferior vena cava filter. BMJ Case Rep. 2013;pii: bcr2013009302. doi:10.1136/bcr-2013-009302.
8. Kiefer CS, Colletti JE. Phlegmasia cerulea dolens in a patient with an inferior vena cava filter. J Emerg Med. 2013;44(1):e95-e97. doi:10.1016/j.jemermed.2012.01.018.