User login
Inadequate antibiotic use persists in gonorrhea
Inappropriate treatment of gonorrhea persists despite growing antibiotic resistance, investigators reported in the Morbidity and Mortality Weekly Report.
In 2016, about 19% of gonorrhea cases diagnosed in the United States were not treated according to recommendations from the Centers for Disease Control and Prevention, wrote Emily J. Weston, MPH, and her associates. They recommended additional training and education on the need for providers to follow treatment recommendations.
To assess adherence to this recommendation, Ms. Weston and her associates analyzed data for 3,213 gonorrhea cases with complete treatment data reported in the United States in 2016. The cases spanned seven CDC sentinel surveillance jurisdictions, including California (excluding San Francisco), Florida, Massachusetts, Minnesota, Philadelphia (Pa.), Baltimore (Md.), and Multnomah County (Oregon).
In all, 18.7% of patients did not receive CDC-recommended treatment, the investigators reported. Inappropriate treatment most often consisted of ceftriaxone (250 mg) alone (5.9%), ceftriaxone with doxycycline (4.4%), azithromycin only (3.1%), ceftriaxone with azithromycin of other or unknown dosages (2.1%), or doxycycline only (1.2%).
Rates of appropriate treatment varied from 79% to 83% among individual jurisdictions and were unrelated to patient race, ethnicity, sex, or age, the researchers found. Men who had sex with men were more likely to receive recommended treatment (85%) than were heterosexual men and women (79%). Patients also were more likely to receive appropriate treatment at family planning, reproductive health, or sexually transmitted disease clinics than in other health care settings.
The results highlight the need for state and local departments to identify and educate providers who are inadequately treating gonorrhea, the researchers concluded. “State and local health departments should continue to work with providers and patients to assure timely detection and treatment of gonorrhea according to current CDC treatment recommendations.”
The study received no external funding. The investigators reported having no conflicts of interest.
SOURCE: Weston EJ et al. MMWR. 2018 Apr 27. doi: 10.15585/mmwr.mm6716a4
Inappropriate treatment of gonorrhea persists despite growing antibiotic resistance, investigators reported in the Morbidity and Mortality Weekly Report.
In 2016, about 19% of gonorrhea cases diagnosed in the United States were not treated according to recommendations from the Centers for Disease Control and Prevention, wrote Emily J. Weston, MPH, and her associates. They recommended additional training and education on the need for providers to follow treatment recommendations.
To assess adherence to this recommendation, Ms. Weston and her associates analyzed data for 3,213 gonorrhea cases with complete treatment data reported in the United States in 2016. The cases spanned seven CDC sentinel surveillance jurisdictions, including California (excluding San Francisco), Florida, Massachusetts, Minnesota, Philadelphia (Pa.), Baltimore (Md.), and Multnomah County (Oregon).
In all, 18.7% of patients did not receive CDC-recommended treatment, the investigators reported. Inappropriate treatment most often consisted of ceftriaxone (250 mg) alone (5.9%), ceftriaxone with doxycycline (4.4%), azithromycin only (3.1%), ceftriaxone with azithromycin of other or unknown dosages (2.1%), or doxycycline only (1.2%).
Rates of appropriate treatment varied from 79% to 83% among individual jurisdictions and were unrelated to patient race, ethnicity, sex, or age, the researchers found. Men who had sex with men were more likely to receive recommended treatment (85%) than were heterosexual men and women (79%). Patients also were more likely to receive appropriate treatment at family planning, reproductive health, or sexually transmitted disease clinics than in other health care settings.
The results highlight the need for state and local departments to identify and educate providers who are inadequately treating gonorrhea, the researchers concluded. “State and local health departments should continue to work with providers and patients to assure timely detection and treatment of gonorrhea according to current CDC treatment recommendations.”
The study received no external funding. The investigators reported having no conflicts of interest.
SOURCE: Weston EJ et al. MMWR. 2018 Apr 27. doi: 10.15585/mmwr.mm6716a4
Inappropriate treatment of gonorrhea persists despite growing antibiotic resistance, investigators reported in the Morbidity and Mortality Weekly Report.
In 2016, about 19% of gonorrhea cases diagnosed in the United States were not treated according to recommendations from the Centers for Disease Control and Prevention, wrote Emily J. Weston, MPH, and her associates. They recommended additional training and education on the need for providers to follow treatment recommendations.
To assess adherence to this recommendation, Ms. Weston and her associates analyzed data for 3,213 gonorrhea cases with complete treatment data reported in the United States in 2016. The cases spanned seven CDC sentinel surveillance jurisdictions, including California (excluding San Francisco), Florida, Massachusetts, Minnesota, Philadelphia (Pa.), Baltimore (Md.), and Multnomah County (Oregon).
In all, 18.7% of patients did not receive CDC-recommended treatment, the investigators reported. Inappropriate treatment most often consisted of ceftriaxone (250 mg) alone (5.9%), ceftriaxone with doxycycline (4.4%), azithromycin only (3.1%), ceftriaxone with azithromycin of other or unknown dosages (2.1%), or doxycycline only (1.2%).
Rates of appropriate treatment varied from 79% to 83% among individual jurisdictions and were unrelated to patient race, ethnicity, sex, or age, the researchers found. Men who had sex with men were more likely to receive recommended treatment (85%) than were heterosexual men and women (79%). Patients also were more likely to receive appropriate treatment at family planning, reproductive health, or sexually transmitted disease clinics than in other health care settings.
The results highlight the need for state and local departments to identify and educate providers who are inadequately treating gonorrhea, the researchers concluded. “State and local health departments should continue to work with providers and patients to assure timely detection and treatment of gonorrhea according to current CDC treatment recommendations.”
The study received no external funding. The investigators reported having no conflicts of interest.
SOURCE: Weston EJ et al. MMWR. 2018 Apr 27. doi: 10.15585/mmwr.mm6716a4
Key clinical point: Inadequate treatment of gonorrhea persists despite its growing antibiotic resistance.
Major finding: In all, 18.7% patients did not receive CDC-recommended treatment.
Study details: Analyses of 3,213 cases from seven U.S. jurisdictions.
Disclosures: The investigators reported no external funding sources. They reported having no conflicts of interest.
Source: Weston EJ et al. MMWR. 2018 Apr 27. doi: 10.15585/mmwr.mm6716a4
Endovascular interventions associated with large benefits in peripheral artery disease
WASHINGTON – An all-comer observational study associated endovascular treatment of lower limb peripheral artery disease (PAD) with low event rates and substantial improvements in quality of life at 18 months, even in Rutherford stage 6 patients.
Although the proportion of patients with Rutherford stage 6 PAD was relatively small, the study results showed that peripheral vascular intervention “can be successful in this patient population as evidenced by a high freedom from major amputation,” reported William Gray, MD, system chief of the division of cardiovascular disease at the Lankenau Heart Group, Wynnewood, Pa., at CRT 2018, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“Many of the patients in this trial, particularly the Rutherford 6 patients, would never be included in the pivotal trial for endovascular devices,” Dr. Gray said. He called this “a unique study” in that it had almost no exclusions.
The study enrolled 1,204 patients with peripheral artery disease at 51 participating sites. After 18 months, follow-up data were available on 793 patients. These were divided by Rutherford classifications into three groups: 374 patients in the combined Rutherford 2 and 3 classifications (R2/3); 371 in the combined Rutherford 4 and 5 classifications (R4/5); and 48 in the Rutherford 6 classification (R6). Patients treated with any Food and Drug Administration–approved technology for treatment of claudication and critical limb ischemia for PAD were eligible.
The endpoints considered at 18 months included procedural and lesion success, major adverse events, and quality of life. Four core laboratories were responsible for an independent analysis of outcomes. A follow-up of 5 years is planned and will include an economic analysis.
The procedural success rates for were 84.4% for the R2/3 group, 76.9% for the R4/5 group, and 70.2% for the R6 group. Almost all of those in the R2/3 and R4/5 groups were discharged immediately after treatment. In the R6 group, approximately 25% of patients were held for complications or additional care.
At 18 months of follow-up, freedom from major adverse events, defined as death, major amputation, or a target vessel revascularization, was achieved by 76.9% of those in the R2/3 group, 68.2% of those in the R4/5 group, and 52.8% of those in the R6 group. The analysis also looked at specific events: The rates for freedom from amputation were 99.3%, 95.3%, and 81.7% in the R2/3, R4/5, and R6 groups, respectively; the freedom from death was 93.9%, 85.5%, and 76.2%; and the freedom from target vessel revascularization was 77.5%, 70.6%, ad 65.7%.
Those in R2/3 maintained the improvement in Rutherford classification observed at 30 days for the subsequent 12 months. Those in R2/3 and R4/5 showed continued improvement in Rutherford classification. For example, R4 represented approximately 50% of the patients in R3/4 classification at baseline but less than 20% of this group at 18 months.
The change in Rutherford classification was reflected in quality of life (QOL) analyses. As far as total QOL scores, the R6 group, which had lower scores at baseline, was no longer significantly different at 18 months from the R4/5 group. On the pain subdomain QOL score, which was incrementally worse at baseline for increased PAD severity, there were no differences at 18 months after improvements in all groups.
Overall, the LIBERTY 360 study “supports aggressive management” with endovascular procedures in symptomatic patients with PAD. This is important because PAD often is inadequately treated or left untreated, according to Dr. Gray. He cited data suggesting that up to 50% of patients who undergo amputation because of lower limb claudication never even undergo a vascular evaluation.
Although there was no control group to evaluate outcomes in patients not treated or treated with another intervention, such as surgery, Dr. Gray suggested that there are encouraging results in a study that was conducted to enroll patients “with as many confounders as possible.”
Dr. Gray reported financial relationships with Abbott Vascular, Cordis, Medtronic, WL Gore, and a number of other device manufacturers.
WASHINGTON – An all-comer observational study associated endovascular treatment of lower limb peripheral artery disease (PAD) with low event rates and substantial improvements in quality of life at 18 months, even in Rutherford stage 6 patients.
Although the proportion of patients with Rutherford stage 6 PAD was relatively small, the study results showed that peripheral vascular intervention “can be successful in this patient population as evidenced by a high freedom from major amputation,” reported William Gray, MD, system chief of the division of cardiovascular disease at the Lankenau Heart Group, Wynnewood, Pa., at CRT 2018, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“Many of the patients in this trial, particularly the Rutherford 6 patients, would never be included in the pivotal trial for endovascular devices,” Dr. Gray said. He called this “a unique study” in that it had almost no exclusions.
The study enrolled 1,204 patients with peripheral artery disease at 51 participating sites. After 18 months, follow-up data were available on 793 patients. These were divided by Rutherford classifications into three groups: 374 patients in the combined Rutherford 2 and 3 classifications (R2/3); 371 in the combined Rutherford 4 and 5 classifications (R4/5); and 48 in the Rutherford 6 classification (R6). Patients treated with any Food and Drug Administration–approved technology for treatment of claudication and critical limb ischemia for PAD were eligible.
The endpoints considered at 18 months included procedural and lesion success, major adverse events, and quality of life. Four core laboratories were responsible for an independent analysis of outcomes. A follow-up of 5 years is planned and will include an economic analysis.
The procedural success rates for were 84.4% for the R2/3 group, 76.9% for the R4/5 group, and 70.2% for the R6 group. Almost all of those in the R2/3 and R4/5 groups were discharged immediately after treatment. In the R6 group, approximately 25% of patients were held for complications or additional care.
At 18 months of follow-up, freedom from major adverse events, defined as death, major amputation, or a target vessel revascularization, was achieved by 76.9% of those in the R2/3 group, 68.2% of those in the R4/5 group, and 52.8% of those in the R6 group. The analysis also looked at specific events: The rates for freedom from amputation were 99.3%, 95.3%, and 81.7% in the R2/3, R4/5, and R6 groups, respectively; the freedom from death was 93.9%, 85.5%, and 76.2%; and the freedom from target vessel revascularization was 77.5%, 70.6%, ad 65.7%.
Those in R2/3 maintained the improvement in Rutherford classification observed at 30 days for the subsequent 12 months. Those in R2/3 and R4/5 showed continued improvement in Rutherford classification. For example, R4 represented approximately 50% of the patients in R3/4 classification at baseline but less than 20% of this group at 18 months.
The change in Rutherford classification was reflected in quality of life (QOL) analyses. As far as total QOL scores, the R6 group, which had lower scores at baseline, was no longer significantly different at 18 months from the R4/5 group. On the pain subdomain QOL score, which was incrementally worse at baseline for increased PAD severity, there were no differences at 18 months after improvements in all groups.
Overall, the LIBERTY 360 study “supports aggressive management” with endovascular procedures in symptomatic patients with PAD. This is important because PAD often is inadequately treated or left untreated, according to Dr. Gray. He cited data suggesting that up to 50% of patients who undergo amputation because of lower limb claudication never even undergo a vascular evaluation.
Although there was no control group to evaluate outcomes in patients not treated or treated with another intervention, such as surgery, Dr. Gray suggested that there are encouraging results in a study that was conducted to enroll patients “with as many confounders as possible.”
Dr. Gray reported financial relationships with Abbott Vascular, Cordis, Medtronic, WL Gore, and a number of other device manufacturers.
WASHINGTON – An all-comer observational study associated endovascular treatment of lower limb peripheral artery disease (PAD) with low event rates and substantial improvements in quality of life at 18 months, even in Rutherford stage 6 patients.
Although the proportion of patients with Rutherford stage 6 PAD was relatively small, the study results showed that peripheral vascular intervention “can be successful in this patient population as evidenced by a high freedom from major amputation,” reported William Gray, MD, system chief of the division of cardiovascular disease at the Lankenau Heart Group, Wynnewood, Pa., at CRT 2018, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“Many of the patients in this trial, particularly the Rutherford 6 patients, would never be included in the pivotal trial for endovascular devices,” Dr. Gray said. He called this “a unique study” in that it had almost no exclusions.
The study enrolled 1,204 patients with peripheral artery disease at 51 participating sites. After 18 months, follow-up data were available on 793 patients. These were divided by Rutherford classifications into three groups: 374 patients in the combined Rutherford 2 and 3 classifications (R2/3); 371 in the combined Rutherford 4 and 5 classifications (R4/5); and 48 in the Rutherford 6 classification (R6). Patients treated with any Food and Drug Administration–approved technology for treatment of claudication and critical limb ischemia for PAD were eligible.
The endpoints considered at 18 months included procedural and lesion success, major adverse events, and quality of life. Four core laboratories were responsible for an independent analysis of outcomes. A follow-up of 5 years is planned and will include an economic analysis.
The procedural success rates for were 84.4% for the R2/3 group, 76.9% for the R4/5 group, and 70.2% for the R6 group. Almost all of those in the R2/3 and R4/5 groups were discharged immediately after treatment. In the R6 group, approximately 25% of patients were held for complications or additional care.
At 18 months of follow-up, freedom from major adverse events, defined as death, major amputation, or a target vessel revascularization, was achieved by 76.9% of those in the R2/3 group, 68.2% of those in the R4/5 group, and 52.8% of those in the R6 group. The analysis also looked at specific events: The rates for freedom from amputation were 99.3%, 95.3%, and 81.7% in the R2/3, R4/5, and R6 groups, respectively; the freedom from death was 93.9%, 85.5%, and 76.2%; and the freedom from target vessel revascularization was 77.5%, 70.6%, ad 65.7%.
Those in R2/3 maintained the improvement in Rutherford classification observed at 30 days for the subsequent 12 months. Those in R2/3 and R4/5 showed continued improvement in Rutherford classification. For example, R4 represented approximately 50% of the patients in R3/4 classification at baseline but less than 20% of this group at 18 months.
The change in Rutherford classification was reflected in quality of life (QOL) analyses. As far as total QOL scores, the R6 group, which had lower scores at baseline, was no longer significantly different at 18 months from the R4/5 group. On the pain subdomain QOL score, which was incrementally worse at baseline for increased PAD severity, there were no differences at 18 months after improvements in all groups.
Overall, the LIBERTY 360 study “supports aggressive management” with endovascular procedures in symptomatic patients with PAD. This is important because PAD often is inadequately treated or left untreated, according to Dr. Gray. He cited data suggesting that up to 50% of patients who undergo amputation because of lower limb claudication never even undergo a vascular evaluation.
Although there was no control group to evaluate outcomes in patients not treated or treated with another intervention, such as surgery, Dr. Gray suggested that there are encouraging results in a study that was conducted to enroll patients “with as many confounders as possible.”
Dr. Gray reported financial relationships with Abbott Vascular, Cordis, Medtronic, WL Gore, and a number of other device manufacturers.
Key clinical point: An observational study supports endovascular strategies even in the most advanced cases of peripheral artery disease (PAD).
Major finding: In Rutherford stage 6 patients, low amputation rates and large improvements in quality of life were observed at 18 months of follow-up.
Data source: Prospective, observational multicenter study.
Disclosures: Dr. Gray reports financial relationships with Abbott Vascular, BioCardia, Boston Scientific, Cardiovascular Systems, Coherex Medical, Contego Cook, Cordis, Medtronic, Shockwave Medical, Silk Road Medical, and WL Gore.
Safety and support experiences of transgender and gender nonconforming youth explored
TORONTO – Youth with nonbinary identities – those that are beyond or outside of the categories of male/man and female/woman – feel significantly safer and more supported at school, compared with their transgender and gender nonconforming peers with binary identities, results from a survey of more than 300 youth showed.
“There has been little research specifically about the experiences of transgender and gender nonconforming youth that have nonbinary identities,” lead study author Brittany Allen, MD, said in an interview in advance of the Pediatric Academic Societies meeting.
Dr. Allen, a pediatrician at the University of Wisconsin–Madison, and her associates conducted an online survey of 311 transgender, nonbinary, and gender nonconforming youth in the state who ranged aged 12-22 years. Study participants were asked about their school safety and support experiences, and the researchers used Wilcoxon rank-sum tests to compare Likert scale responses among youth who reported nonbinary identities with those who reported binary identities. On the 1-5 scale, 1 meant “strongly agree” while 5 meant “strongly disagree.”
Dr. Allen, who is also comedical director of the Pediatric and Adolescent Transgender Health Clinic at American Family Children’s Hospital, Madison, reported that 311 young people completed more than 70% of the survey. Of those, 287 identified as having either binary (164; 57%) or nonbinary (123; 43%) gender identities. That percentage of those reporting nonbinary identities “is striking,” she said, and is “a much higher percentage than seen in adult studies of transgender and gender nonconforming people.”
Compared with respondents with binary identities, those with nonbinary identities were more often Caucasian/White (81% vs. 65% for those with binary identities; P = .003) and less likely to qualify for free lunch (28% vs. 55%; P = .001). Both binary and nonbinary groups reported similar school attendance and belonging. However, compared with the binary group, the nonbinary group reported significantly higher ratings of school safety (Likert score of 2.62 vs. 2.96, respectively; P = .0078) and peer support (Likert score of 2.54 vs. 2.87; P = .0139) and also were more likely to report being able to access adult support at school if needed (Likert score of 2.31 vs. 2.66; P = .0085).
“The primary message is that many transgender or gender nonconforming youth have identities outside of a gender binary and that transgender and gender nonconforming youth with different gender identities may have different strengths and challenges in different settings,” Dr. Allen said.
“Our work shows that nonbinary youth have relative safety and support at school compared to their transgender and gender nonconforming peers with binary identities – though it’s important to note that transgender and gender nonconforming youth overall are still at high risk of school harassment and violence. Interventions to promote school safety for youth of all gender identities should consider that transgender and gender nonconforming youth with different gender identities have different risks related to school safety and support.”
She acknowledged certain limitations of the study, including the fact that the researchers used a convenience sample to recruit participants, “which means that we may not have reached transgender and gender nonconforming youth that were less connected to support services or transgender and gender nonconforming peers,” Dr. Allen said. “This study also specifically assessed transgender and gender nonconforming youth; we did not have a comparison group of cisgender participants for comparison due to our study design.”
The study was funded by the National Institutes of Health, the Baldwin Wisconsin Ideas Endowment, the University of Wisconsin Advancing Health Equity and Diversity initiative, and the Wisconsin Partnership Program. Dr. Allen reported having no financial disclosures.
TORONTO – Youth with nonbinary identities – those that are beyond or outside of the categories of male/man and female/woman – feel significantly safer and more supported at school, compared with their transgender and gender nonconforming peers with binary identities, results from a survey of more than 300 youth showed.
“There has been little research specifically about the experiences of transgender and gender nonconforming youth that have nonbinary identities,” lead study author Brittany Allen, MD, said in an interview in advance of the Pediatric Academic Societies meeting.
Dr. Allen, a pediatrician at the University of Wisconsin–Madison, and her associates conducted an online survey of 311 transgender, nonbinary, and gender nonconforming youth in the state who ranged aged 12-22 years. Study participants were asked about their school safety and support experiences, and the researchers used Wilcoxon rank-sum tests to compare Likert scale responses among youth who reported nonbinary identities with those who reported binary identities. On the 1-5 scale, 1 meant “strongly agree” while 5 meant “strongly disagree.”
Dr. Allen, who is also comedical director of the Pediatric and Adolescent Transgender Health Clinic at American Family Children’s Hospital, Madison, reported that 311 young people completed more than 70% of the survey. Of those, 287 identified as having either binary (164; 57%) or nonbinary (123; 43%) gender identities. That percentage of those reporting nonbinary identities “is striking,” she said, and is “a much higher percentage than seen in adult studies of transgender and gender nonconforming people.”
Compared with respondents with binary identities, those with nonbinary identities were more often Caucasian/White (81% vs. 65% for those with binary identities; P = .003) and less likely to qualify for free lunch (28% vs. 55%; P = .001). Both binary and nonbinary groups reported similar school attendance and belonging. However, compared with the binary group, the nonbinary group reported significantly higher ratings of school safety (Likert score of 2.62 vs. 2.96, respectively; P = .0078) and peer support (Likert score of 2.54 vs. 2.87; P = .0139) and also were more likely to report being able to access adult support at school if needed (Likert score of 2.31 vs. 2.66; P = .0085).
“The primary message is that many transgender or gender nonconforming youth have identities outside of a gender binary and that transgender and gender nonconforming youth with different gender identities may have different strengths and challenges in different settings,” Dr. Allen said.
“Our work shows that nonbinary youth have relative safety and support at school compared to their transgender and gender nonconforming peers with binary identities – though it’s important to note that transgender and gender nonconforming youth overall are still at high risk of school harassment and violence. Interventions to promote school safety for youth of all gender identities should consider that transgender and gender nonconforming youth with different gender identities have different risks related to school safety and support.”
She acknowledged certain limitations of the study, including the fact that the researchers used a convenience sample to recruit participants, “which means that we may not have reached transgender and gender nonconforming youth that were less connected to support services or transgender and gender nonconforming peers,” Dr. Allen said. “This study also specifically assessed transgender and gender nonconforming youth; we did not have a comparison group of cisgender participants for comparison due to our study design.”
The study was funded by the National Institutes of Health, the Baldwin Wisconsin Ideas Endowment, the University of Wisconsin Advancing Health Equity and Diversity initiative, and the Wisconsin Partnership Program. Dr. Allen reported having no financial disclosures.
TORONTO – Youth with nonbinary identities – those that are beyond or outside of the categories of male/man and female/woman – feel significantly safer and more supported at school, compared with their transgender and gender nonconforming peers with binary identities, results from a survey of more than 300 youth showed.
“There has been little research specifically about the experiences of transgender and gender nonconforming youth that have nonbinary identities,” lead study author Brittany Allen, MD, said in an interview in advance of the Pediatric Academic Societies meeting.
Dr. Allen, a pediatrician at the University of Wisconsin–Madison, and her associates conducted an online survey of 311 transgender, nonbinary, and gender nonconforming youth in the state who ranged aged 12-22 years. Study participants were asked about their school safety and support experiences, and the researchers used Wilcoxon rank-sum tests to compare Likert scale responses among youth who reported nonbinary identities with those who reported binary identities. On the 1-5 scale, 1 meant “strongly agree” while 5 meant “strongly disagree.”
Dr. Allen, who is also comedical director of the Pediatric and Adolescent Transgender Health Clinic at American Family Children’s Hospital, Madison, reported that 311 young people completed more than 70% of the survey. Of those, 287 identified as having either binary (164; 57%) or nonbinary (123; 43%) gender identities. That percentage of those reporting nonbinary identities “is striking,” she said, and is “a much higher percentage than seen in adult studies of transgender and gender nonconforming people.”
Compared with respondents with binary identities, those with nonbinary identities were more often Caucasian/White (81% vs. 65% for those with binary identities; P = .003) and less likely to qualify for free lunch (28% vs. 55%; P = .001). Both binary and nonbinary groups reported similar school attendance and belonging. However, compared with the binary group, the nonbinary group reported significantly higher ratings of school safety (Likert score of 2.62 vs. 2.96, respectively; P = .0078) and peer support (Likert score of 2.54 vs. 2.87; P = .0139) and also were more likely to report being able to access adult support at school if needed (Likert score of 2.31 vs. 2.66; P = .0085).
“The primary message is that many transgender or gender nonconforming youth have identities outside of a gender binary and that transgender and gender nonconforming youth with different gender identities may have different strengths and challenges in different settings,” Dr. Allen said.
“Our work shows that nonbinary youth have relative safety and support at school compared to their transgender and gender nonconforming peers with binary identities – though it’s important to note that transgender and gender nonconforming youth overall are still at high risk of school harassment and violence. Interventions to promote school safety for youth of all gender identities should consider that transgender and gender nonconforming youth with different gender identities have different risks related to school safety and support.”
She acknowledged certain limitations of the study, including the fact that the researchers used a convenience sample to recruit participants, “which means that we may not have reached transgender and gender nonconforming youth that were less connected to support services or transgender and gender nonconforming peers,” Dr. Allen said. “This study also specifically assessed transgender and gender nonconforming youth; we did not have a comparison group of cisgender participants for comparison due to our study design.”
The study was funded by the National Institutes of Health, the Baldwin Wisconsin Ideas Endowment, the University of Wisconsin Advancing Health Equity and Diversity initiative, and the Wisconsin Partnership Program. Dr. Allen reported having no financial disclosures.
Key clinical point: Nonbinary youth felt significantly safer and more supported at school, compared with transgender peers with binary identities.
Major finding: Compared with the binary group, the nonbinary group reported significantly higher ratings of school safety (Likert score of 2.62 vs. 2.96, respectively) and peer support (Likert score of 2.54 vs. 2.87).
Study details: An online survey of 311 transgender, nonbinary, and gender nonconforming youth in Wisconsin who aged 12-22 years.
Disclosures: The study was funded by the National Institutes of Health, the Baldwin Wisconsin Ideas Endowment, the University of Wisconsin Advancing Health Equity and Diversity initiative, and the Wisconsin Partnership Program. Dr. Allen reported having no financial disclosures.
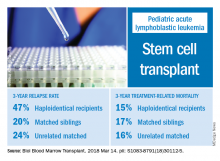
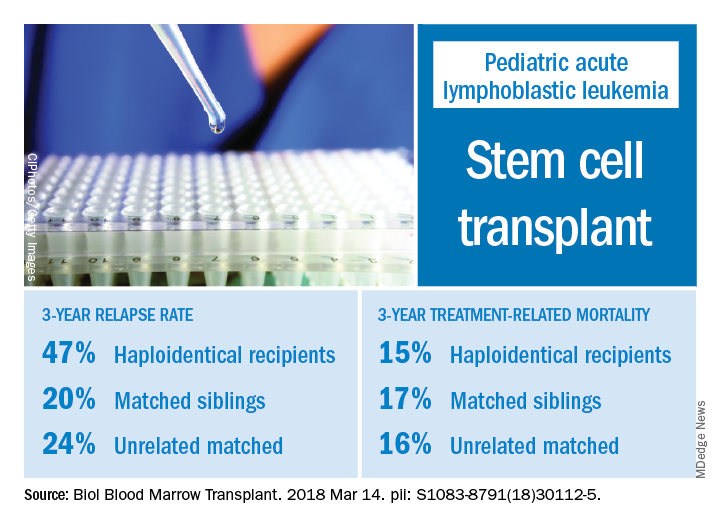
Relapse rate drives stem cell transplant failure in pediatric ALL patients
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
Relapse was the main impediment to successful hematopoietic stem cell transplant (HSCT) in high-risk pediatric acute lymphocytic leukemia (ALL), according to one of the largest single-center experiences reported to date.
The effects of relapse were especially evident for patients with haploidentical donors; these patients had a 3-year cumulative relapse incidence of 47% and event-free survival rate of 35%, both significantly higher than what was seen in other transplant recipients treated at the same center.
The findings, recently published in the journal Biology of Blood and Marrow Transplantation, suggest a substantial unmet need in the treatment of high-risk patients in first remission.
“Newer methods to improve graft-versus-leukemia effect are being tested and will need to be incorporated into the management of high-risk patients,” Asaf D. Yanir, MD, and coauthors at Baylor College of Medicine in Houston said in the report.
Dr. Yanir and colleagues reported recent outcomes for 124 patients who had undergone HSCT for ALL at their center during 2008-2016. That group included 20 haploidentical transplant recipients, 48 patients with matched sibling donors, and 56 with unrelated matched donors.
The 3-year cumulative incidence of relapse was 47% for haploidentical recipients, compared with 20% for matched sibling donors recipients and 24% for unrelated matched donors recipients (P = .02), according to their findings.
The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Those findings are in line with other studies showing inferior outcomes following haploidentical donor HSCT. However, in contrast to those studies, Dr. Yanir and colleagues did find a rate of treatment-related mortality comparable with other transplant approaches. The 3-year incidence of treatment-related mortality was 15% for the haploidentical group and, similarly, 17% in the matched sibling donor group and 16% in the unrelated matched donor group.
That lower rate of treatment-related mortality in the haploidentical group may be caused by improvements in procedures and supportive care. “Unfortunately, the benefits gained by reducing treatment-related mortality were offset by the high rate of relapse, which remains the main obstacle to successful haploidentical donor HSCT,” Dr. Yanir and coauthors wrote in their report.
New strategies are being studied to retain the graft-versus-leukemia effect or enhance it in patients who’ve undergone haploidentical HSCT, such as selectively depleting alloreactive T cells while sparing other immune effectors, investigators wrote.
“Given evolving practices, it is important to continually evaluate the projected event-free survival for pediatric ALL following HSCT, based on the donor type used,” they wrote.
Dr. Yanir and coauthors had no financial disclosures or conflicts of interest to report.
SOURCE: Yanir AD et al. Biol Blood Marrow Transplant. 2018 Mar 14. doi: 10.1016/j.bbmt.2018.03.001.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point: In pediatric acute lymphocytic leukemia (ALL) patients, relapse is the main barrier to successful hematopoietic stem cell transplant (HSCT), especially for those who receive haploidentical donor grafts.
Major finding: The main cause of HSCT failure was relapse, occurring in 47% of haploidentical transplant recipients, compared with 20% for those with matched sibling donors and 24% for those with unrelated matched donors (P = .02).
Study details: A retrospective analysis of 124 transplants performed at a single center during 2008-2016.
Disclosures: Authors had no financial disclosures or conflicts of interest to report.
Source: Yanir AD et al. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2018.03.001.
Meta-analyses clarify roles for gabapentin, naltrexone, and psychotherapy in alcohol use disorder
NEW YORK – A meta-analysis of 10 studies provides at least preliminary support for the use of gabapentin for the treatment of alcohol cravings and withdrawal.
In this video interview, Ali Mahmood Khan, MD, of Kings County Hospital, New York, discusses the findings – presented in a poster at the annual meeting of the American Psychiatric Association – which show that patients treated with gabapentin have significantly reduced alcohol craving and withdrawal. While the findings require further study, he said.
Gapapentin also can be used in combination with naltrexone, which was shown in a separate poster presented by Dr. Khan and his colleagues to be useful for treating alcohol use disorders – but mainly through reducing consumption rather than cravings.
In that meta-analysis of 30 studies, no significant added benefit was seen when psychotherapy was combined with naltrexone, he said, noting, however, that additional study is warranted given that various psychotherapies were used across the studies.
Dr. Khan reported having no disclosures.
NEW YORK – A meta-analysis of 10 studies provides at least preliminary support for the use of gabapentin for the treatment of alcohol cravings and withdrawal.
In this video interview, Ali Mahmood Khan, MD, of Kings County Hospital, New York, discusses the findings – presented in a poster at the annual meeting of the American Psychiatric Association – which show that patients treated with gabapentin have significantly reduced alcohol craving and withdrawal. While the findings require further study, he said.
Gapapentin also can be used in combination with naltrexone, which was shown in a separate poster presented by Dr. Khan and his colleagues to be useful for treating alcohol use disorders – but mainly through reducing consumption rather than cravings.
In that meta-analysis of 30 studies, no significant added benefit was seen when psychotherapy was combined with naltrexone, he said, noting, however, that additional study is warranted given that various psychotherapies were used across the studies.
Dr. Khan reported having no disclosures.
NEW YORK – A meta-analysis of 10 studies provides at least preliminary support for the use of gabapentin for the treatment of alcohol cravings and withdrawal.
In this video interview, Ali Mahmood Khan, MD, of Kings County Hospital, New York, discusses the findings – presented in a poster at the annual meeting of the American Psychiatric Association – which show that patients treated with gabapentin have significantly reduced alcohol craving and withdrawal. While the findings require further study, he said.
Gapapentin also can be used in combination with naltrexone, which was shown in a separate poster presented by Dr. Khan and his colleagues to be useful for treating alcohol use disorders – but mainly through reducing consumption rather than cravings.
In that meta-analysis of 30 studies, no significant added benefit was seen when psychotherapy was combined with naltrexone, he said, noting, however, that additional study is warranted given that various psychotherapies were used across the studies.
Dr. Khan reported having no disclosures.
REPORTING FROM APA
Class III obesity increases risk of acute on chronic liver failure in cirrhotic patients
Class III obesity was significantly, independently associated with acute on chronic liver failure (ACLF) in patients with decompensated cirrhosis, and patients with both class III obesity and acute on chronic liver failure also had a significant risk of renal failure, according to a recent retrospective analysis of two databases publised in the Journal of Hepatology.
Vinay Sundaram, MD, from Cedars-Sinai Medical Center in Los Angeles, and his colleagues evaluated 387,884 patients who were in the United Network for Organ Sharing (UNOS) during 2005-2016; were class I or II obese (body mass index 30-39 kg/m2), class III obese (BMI greater than or equal to 40), or not obese (BMI less than 30); and were on a wait list for liver transplantation.
They used the definition of ACLF outlined in the CANONIC (Consortium Acute on Chronic Liver Failure in Cirrhosis) study, which defined it as having “a single hepatic decompensation, such as ascites, hepatic encephalopathy, variceal bleed, or bacterial infection, and one of the following organ failures: single renal failure, single nonrenal organ failure with renal dysfunction or hepatic encephalopathy, or two nonrenal organ failures,” and confirmed the results in the Nationwide Inpatient Sample (NIS) databases by using diagnostic coding algorithms to identify factors such as hepatic decompensation, obesity, and ACLF in that study population.
Dr. Sundarem and his colleagues identified 116,704 patients (30.1%) with acute on chronic liver failure in both the UNOS and NIS databases. At the time of liver transplantation, there was a significant association between ACLF and class I and class II obesity (hazard ratio, 1.12; 95% confidence interval, 1.05-1.19; P less than .001) and class III obesity (HR, 1.24; 95% CI, 1.09-1.41; P less than .001). Other predictors of ACLF in this population were increased age (HR, 1.01 per year; 95% CI, 1.00-1.01; P = .037), hepatitis C virus (HR, 1.25; 95% CI, 1.16-1.35; P less than .001) and hepatitis C combined with alcoholic liver disease (HR, 1.18; 95% CI, 1.06-1.30; P = .002). Regarding organ failure, “renal insufficiency was similar among the three groups,” with increasing obesity class associated with a greater prevalence of renal failure.
“Given the heightened risk of renal failure among obese patients with cirrhosis, we suggest particularly careful management of this fragile population regarding diuretic usage, avoidance of nephrotoxic agents, and administration of an adequate albumin challenge in the setting of acute kidney injury,” the researchers wrote.
The researchers encouraged “an even greater emphasis on weight reduction” for class III obese patients. They noted the association between class III obesity and ACLF is likely caused by an “obesity-related chronic inflammatory state” and said future prospective studies should seek to describe the inflammatory pathways for each condition to predict risk of ACLF in these patients.
The authors reported having no financial disclosures.
SOURCE: Sundarem V et al. J Hepatol. 2018 April 27. doi: 10.1016/j.jhep.2018.04.016.
Class III obesity was significantly, independently associated with acute on chronic liver failure (ACLF) in patients with decompensated cirrhosis, and patients with both class III obesity and acute on chronic liver failure also had a significant risk of renal failure, according to a recent retrospective analysis of two databases publised in the Journal of Hepatology.
Vinay Sundaram, MD, from Cedars-Sinai Medical Center in Los Angeles, and his colleagues evaluated 387,884 patients who were in the United Network for Organ Sharing (UNOS) during 2005-2016; were class I or II obese (body mass index 30-39 kg/m2), class III obese (BMI greater than or equal to 40), or not obese (BMI less than 30); and were on a wait list for liver transplantation.
They used the definition of ACLF outlined in the CANONIC (Consortium Acute on Chronic Liver Failure in Cirrhosis) study, which defined it as having “a single hepatic decompensation, such as ascites, hepatic encephalopathy, variceal bleed, or bacterial infection, and one of the following organ failures: single renal failure, single nonrenal organ failure with renal dysfunction or hepatic encephalopathy, or two nonrenal organ failures,” and confirmed the results in the Nationwide Inpatient Sample (NIS) databases by using diagnostic coding algorithms to identify factors such as hepatic decompensation, obesity, and ACLF in that study population.
Dr. Sundarem and his colleagues identified 116,704 patients (30.1%) with acute on chronic liver failure in both the UNOS and NIS databases. At the time of liver transplantation, there was a significant association between ACLF and class I and class II obesity (hazard ratio, 1.12; 95% confidence interval, 1.05-1.19; P less than .001) and class III obesity (HR, 1.24; 95% CI, 1.09-1.41; P less than .001). Other predictors of ACLF in this population were increased age (HR, 1.01 per year; 95% CI, 1.00-1.01; P = .037), hepatitis C virus (HR, 1.25; 95% CI, 1.16-1.35; P less than .001) and hepatitis C combined with alcoholic liver disease (HR, 1.18; 95% CI, 1.06-1.30; P = .002). Regarding organ failure, “renal insufficiency was similar among the three groups,” with increasing obesity class associated with a greater prevalence of renal failure.
“Given the heightened risk of renal failure among obese patients with cirrhosis, we suggest particularly careful management of this fragile population regarding diuretic usage, avoidance of nephrotoxic agents, and administration of an adequate albumin challenge in the setting of acute kidney injury,” the researchers wrote.
The researchers encouraged “an even greater emphasis on weight reduction” for class III obese patients. They noted the association between class III obesity and ACLF is likely caused by an “obesity-related chronic inflammatory state” and said future prospective studies should seek to describe the inflammatory pathways for each condition to predict risk of ACLF in these patients.
The authors reported having no financial disclosures.
SOURCE: Sundarem V et al. J Hepatol. 2018 April 27. doi: 10.1016/j.jhep.2018.04.016.
Class III obesity was significantly, independently associated with acute on chronic liver failure (ACLF) in patients with decompensated cirrhosis, and patients with both class III obesity and acute on chronic liver failure also had a significant risk of renal failure, according to a recent retrospective analysis of two databases publised in the Journal of Hepatology.
Vinay Sundaram, MD, from Cedars-Sinai Medical Center in Los Angeles, and his colleagues evaluated 387,884 patients who were in the United Network for Organ Sharing (UNOS) during 2005-2016; were class I or II obese (body mass index 30-39 kg/m2), class III obese (BMI greater than or equal to 40), or not obese (BMI less than 30); and were on a wait list for liver transplantation.
They used the definition of ACLF outlined in the CANONIC (Consortium Acute on Chronic Liver Failure in Cirrhosis) study, which defined it as having “a single hepatic decompensation, such as ascites, hepatic encephalopathy, variceal bleed, or bacterial infection, and one of the following organ failures: single renal failure, single nonrenal organ failure with renal dysfunction or hepatic encephalopathy, or two nonrenal organ failures,” and confirmed the results in the Nationwide Inpatient Sample (NIS) databases by using diagnostic coding algorithms to identify factors such as hepatic decompensation, obesity, and ACLF in that study population.
Dr. Sundarem and his colleagues identified 116,704 patients (30.1%) with acute on chronic liver failure in both the UNOS and NIS databases. At the time of liver transplantation, there was a significant association between ACLF and class I and class II obesity (hazard ratio, 1.12; 95% confidence interval, 1.05-1.19; P less than .001) and class III obesity (HR, 1.24; 95% CI, 1.09-1.41; P less than .001). Other predictors of ACLF in this population were increased age (HR, 1.01 per year; 95% CI, 1.00-1.01; P = .037), hepatitis C virus (HR, 1.25; 95% CI, 1.16-1.35; P less than .001) and hepatitis C combined with alcoholic liver disease (HR, 1.18; 95% CI, 1.06-1.30; P = .002). Regarding organ failure, “renal insufficiency was similar among the three groups,” with increasing obesity class associated with a greater prevalence of renal failure.
“Given the heightened risk of renal failure among obese patients with cirrhosis, we suggest particularly careful management of this fragile population regarding diuretic usage, avoidance of nephrotoxic agents, and administration of an adequate albumin challenge in the setting of acute kidney injury,” the researchers wrote.
The researchers encouraged “an even greater emphasis on weight reduction” for class III obese patients. They noted the association between class III obesity and ACLF is likely caused by an “obesity-related chronic inflammatory state” and said future prospective studies should seek to describe the inflammatory pathways for each condition to predict risk of ACLF in these patients.
The authors reported having no financial disclosures.
SOURCE: Sundarem V et al. J Hepatol. 2018 April 27. doi: 10.1016/j.jhep.2018.04.016.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: Patients with a BMI greater than or equal to 40 kg/m2 with decompensated cirrhosis are at greater risk of developing acute on chronic liver failure.
Major finding: Class III obesity carried a hazard ratio of 1.24 in the UNOS database and an odds ratio of 1.30 in the NIS database at the time of liver transplantation.
Data source: A retrospective cohort database study of 116,704 patients with acute on chronic liver failure listed during 2005-2016.
Disclosures: The authors reported having no financial disclosures.
Source: Sundaram V et al. J Hepatol. 2018 Apr 27. doi: 10.1016/j.jhep.2018.04.016.
Very few infants born to HCV-infected mothers receive testing
Despite the increasing prevalence of hepatitis C virus (HCV) infection in pregnant women, infants exposed to the disease are screened at a very low rate, Catherine A. Chappell, MD, and her associates wrote in Pediatrics.
During 2006-2014, 87,924 women gave birth at the Magee-Womens Hospital at the University of Pittsburgh Medical Center, of whom 1,043 had HCV. Over this time, the HCV prevalence rate increased 60%, from 1,026 cases per 100,000 women to 1,637 cases per 100,000 women. Women with HCV were more likely to be white, have Medicaid, have opiate use disorder, have other substance use disorders, and be under the age of 30 years.
Infants born to HCV-infected women are significantly more likely to be preterm and of low birth weight.
An additional 32 infants who did not receive well child care did receive HCV testing. A total of nine infants, seven in the well child group and two in the non-well child group, tested positive for HCV.
“Of the infants tested with conclusive results, the HCV transmission rate was 8.4%, with 7.2% having chronic HCV infection,” which is in line with previous reports, according to the researchers.
“Because of the poor rates of pediatric HCV screening described, future researchers should focus on interventions to increase screening in infants who are at risk for perinatal HCV acquisition by including technology to improve the transfer of maternal HCV status to the pediatric record and increase pediatric provider awareness regarding HCV screening guidelines,” the investigators concluded.
SOURCE: Chappell CA et al. Pediatrics. 2018 May 2. doi: 10.1542/peds.2017-3273.
Despite the increasing prevalence of hepatitis C virus (HCV) infection in pregnant women, infants exposed to the disease are screened at a very low rate, Catherine A. Chappell, MD, and her associates wrote in Pediatrics.
During 2006-2014, 87,924 women gave birth at the Magee-Womens Hospital at the University of Pittsburgh Medical Center, of whom 1,043 had HCV. Over this time, the HCV prevalence rate increased 60%, from 1,026 cases per 100,000 women to 1,637 cases per 100,000 women. Women with HCV were more likely to be white, have Medicaid, have opiate use disorder, have other substance use disorders, and be under the age of 30 years.
Infants born to HCV-infected women are significantly more likely to be preterm and of low birth weight.
An additional 32 infants who did not receive well child care did receive HCV testing. A total of nine infants, seven in the well child group and two in the non-well child group, tested positive for HCV.
“Of the infants tested with conclusive results, the HCV transmission rate was 8.4%, with 7.2% having chronic HCV infection,” which is in line with previous reports, according to the researchers.
“Because of the poor rates of pediatric HCV screening described, future researchers should focus on interventions to increase screening in infants who are at risk for perinatal HCV acquisition by including technology to improve the transfer of maternal HCV status to the pediatric record and increase pediatric provider awareness regarding HCV screening guidelines,” the investigators concluded.
SOURCE: Chappell CA et al. Pediatrics. 2018 May 2. doi: 10.1542/peds.2017-3273.
Despite the increasing prevalence of hepatitis C virus (HCV) infection in pregnant women, infants exposed to the disease are screened at a very low rate, Catherine A. Chappell, MD, and her associates wrote in Pediatrics.
During 2006-2014, 87,924 women gave birth at the Magee-Womens Hospital at the University of Pittsburgh Medical Center, of whom 1,043 had HCV. Over this time, the HCV prevalence rate increased 60%, from 1,026 cases per 100,000 women to 1,637 cases per 100,000 women. Women with HCV were more likely to be white, have Medicaid, have opiate use disorder, have other substance use disorders, and be under the age of 30 years.
Infants born to HCV-infected women are significantly more likely to be preterm and of low birth weight.
An additional 32 infants who did not receive well child care did receive HCV testing. A total of nine infants, seven in the well child group and two in the non-well child group, tested positive for HCV.
“Of the infants tested with conclusive results, the HCV transmission rate was 8.4%, with 7.2% having chronic HCV infection,” which is in line with previous reports, according to the researchers.
“Because of the poor rates of pediatric HCV screening described, future researchers should focus on interventions to increase screening in infants who are at risk for perinatal HCV acquisition by including technology to improve the transfer of maternal HCV status to the pediatric record and increase pediatric provider awareness regarding HCV screening guidelines,” the investigators concluded.
SOURCE: Chappell CA et al. Pediatrics. 2018 May 2. doi: 10.1542/peds.2017-3273.
FROM PEDIATRICS
Slime is not sublime: It may cause hand dermatitis
A young, otherwise healthy 9-year-old girl was evaluated for pruritic hand dermatitis which lasted 5 months after exposure to homemade slime. Physical exam revealed erythematous, scaly plaques on the palmar surfaces of her hands; her fingernails had onychomadesis and longitudinal ridging. Despite frequent emolliation, her dermatitis persisted. She was then treated empirically for scabies and for culture-positive Staphylococcus aureus infection, which required a full round of cephalexin and mupirocin ointment. This also did not alleviate the dermatitis. A combination of homemade borax-containing slime avoidance, brief course of high-dose corticosteroids, and frequent bland emollients was prescribed because the dermatitis was assumed to be caused by an irritant.
After review of this case and evaluation of other children with hand dermatitis, Julia K. Gittler, MD, of Columbia University, New York, and her colleagues have made a case that “slime” and new-onset hand dermatitis may be linked.
SOURCE: Gittler JK et al. J Pediatr. 2018 May 3. doi: 10.1016/j.jpeds.2018.03.064 .
A young, otherwise healthy 9-year-old girl was evaluated for pruritic hand dermatitis which lasted 5 months after exposure to homemade slime. Physical exam revealed erythematous, scaly plaques on the palmar surfaces of her hands; her fingernails had onychomadesis and longitudinal ridging. Despite frequent emolliation, her dermatitis persisted. She was then treated empirically for scabies and for culture-positive Staphylococcus aureus infection, which required a full round of cephalexin and mupirocin ointment. This also did not alleviate the dermatitis. A combination of homemade borax-containing slime avoidance, brief course of high-dose corticosteroids, and frequent bland emollients was prescribed because the dermatitis was assumed to be caused by an irritant.
After review of this case and evaluation of other children with hand dermatitis, Julia K. Gittler, MD, of Columbia University, New York, and her colleagues have made a case that “slime” and new-onset hand dermatitis may be linked.
SOURCE: Gittler JK et al. J Pediatr. 2018 May 3. doi: 10.1016/j.jpeds.2018.03.064 .
A young, otherwise healthy 9-year-old girl was evaluated for pruritic hand dermatitis which lasted 5 months after exposure to homemade slime. Physical exam revealed erythematous, scaly plaques on the palmar surfaces of her hands; her fingernails had onychomadesis and longitudinal ridging. Despite frequent emolliation, her dermatitis persisted. She was then treated empirically for scabies and for culture-positive Staphylococcus aureus infection, which required a full round of cephalexin and mupirocin ointment. This also did not alleviate the dermatitis. A combination of homemade borax-containing slime avoidance, brief course of high-dose corticosteroids, and frequent bland emollients was prescribed because the dermatitis was assumed to be caused by an irritant.
After review of this case and evaluation of other children with hand dermatitis, Julia K. Gittler, MD, of Columbia University, New York, and her colleagues have made a case that “slime” and new-onset hand dermatitis may be linked.
SOURCE: Gittler JK et al. J Pediatr. 2018 May 3. doi: 10.1016/j.jpeds.2018.03.064 .
FROM THE JOURNAL OF PEDIATRICS
Do black women pay a price for hair care regimens?
A new analysis of 18 hair products used by black women finds that they contain 45 endocrine-disrupting or asthma-associated chemicals, a finding that could help explain why this population suffers from higher rates of chemical exposure and hormone-related health conditions.
“We found multiples of our targeted chemicals in all of our products,” said study lead author Jessica S. Helm, PhD, of the Silent Spring Institute, Newton, Mass., in an interview. “We’re concerned about the additive effect of multiple products being used together.”
According to the study, previous research has found that, compared with white women, U.S. black women have higher urinary levels of chemicals like phthalates and parabens. Black women also have higher rates of asthma and hormone-related health conditions like uterine fibroids and infertility, Dr. Helms said.
The researchers launched their study to better understand the possible role of hair care products in raising chemical levels in black women, Dr. Helm said.
The researchers tested 18 types of hair care products shown by a 2004-2005 survey to be popular among black women: hot oil treatments, anti-frizz products and polishes, leave-in conditioners, root stimulators, hair lotions, and relaxers. Researchers had purchased the products in 2008.
The researchers detected 45 of 66 target chemicals in the samples, including some that are banned in the European Union or regulated in California based on health concerns, according to Dr. Helms.
Most of the products contained parabens and phthalates (both 78%), UV filters (72%), and cyclosiloxanes (67%).
All products contained at least 1 of 19 targeted fragrances, while “hair lotions, root stimulators, and hair relaxers contained multiple fragrance chemicals per product, with an average of five to eight targeted fragrance chemicals detected per product versus an average of two in the anti-frizz products.”
How do the findings compare with previous research? “They’re roughly consistent with what’s been found before, but potentially on the higher end,” Dr. Helms said. “For some of these chemicals, there’s not a lot of data from the past.”
Most of the chemicals aren’t listed on product labels, Dr. Helm said. “It’s possible that some of the ingredients were unintentionally added as part of manufacturing or other processes.”
Dr. Helm urged physicians to consider the connections between hair care products and chemical exposure. “Maybe there’s an opportunity to use fewer products,” she said.
Dr. Helm acknowledged that it is difficult to find hair care products that don’t include fragrance. She recommends the use of products made from plants or organic ingredients, and she pointed to a Silver Spring Institute–affiliated app called DetoxMe that offers suggestions about reducing chemical exposure from consumer products.
The study was funded by the Rose Foundation, the Goldman Fund, and Hurricane Voices Breast Cancer Foundation. The authors report no relevant disclosures.
cenews@mdedge.com
SOURCE: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
A new analysis of 18 hair products used by black women finds that they contain 45 endocrine-disrupting or asthma-associated chemicals, a finding that could help explain why this population suffers from higher rates of chemical exposure and hormone-related health conditions.
“We found multiples of our targeted chemicals in all of our products,” said study lead author Jessica S. Helm, PhD, of the Silent Spring Institute, Newton, Mass., in an interview. “We’re concerned about the additive effect of multiple products being used together.”
According to the study, previous research has found that, compared with white women, U.S. black women have higher urinary levels of chemicals like phthalates and parabens. Black women also have higher rates of asthma and hormone-related health conditions like uterine fibroids and infertility, Dr. Helms said.
The researchers launched their study to better understand the possible role of hair care products in raising chemical levels in black women, Dr. Helm said.
The researchers tested 18 types of hair care products shown by a 2004-2005 survey to be popular among black women: hot oil treatments, anti-frizz products and polishes, leave-in conditioners, root stimulators, hair lotions, and relaxers. Researchers had purchased the products in 2008.
The researchers detected 45 of 66 target chemicals in the samples, including some that are banned in the European Union or regulated in California based on health concerns, according to Dr. Helms.
Most of the products contained parabens and phthalates (both 78%), UV filters (72%), and cyclosiloxanes (67%).
All products contained at least 1 of 19 targeted fragrances, while “hair lotions, root stimulators, and hair relaxers contained multiple fragrance chemicals per product, with an average of five to eight targeted fragrance chemicals detected per product versus an average of two in the anti-frizz products.”
How do the findings compare with previous research? “They’re roughly consistent with what’s been found before, but potentially on the higher end,” Dr. Helms said. “For some of these chemicals, there’s not a lot of data from the past.”
Most of the chemicals aren’t listed on product labels, Dr. Helm said. “It’s possible that some of the ingredients were unintentionally added as part of manufacturing or other processes.”
Dr. Helm urged physicians to consider the connections between hair care products and chemical exposure. “Maybe there’s an opportunity to use fewer products,” she said.
Dr. Helm acknowledged that it is difficult to find hair care products that don’t include fragrance. She recommends the use of products made from plants or organic ingredients, and she pointed to a Silver Spring Institute–affiliated app called DetoxMe that offers suggestions about reducing chemical exposure from consumer products.
The study was funded by the Rose Foundation, the Goldman Fund, and Hurricane Voices Breast Cancer Foundation. The authors report no relevant disclosures.
cenews@mdedge.com
SOURCE: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
A new analysis of 18 hair products used by black women finds that they contain 45 endocrine-disrupting or asthma-associated chemicals, a finding that could help explain why this population suffers from higher rates of chemical exposure and hormone-related health conditions.
“We found multiples of our targeted chemicals in all of our products,” said study lead author Jessica S. Helm, PhD, of the Silent Spring Institute, Newton, Mass., in an interview. “We’re concerned about the additive effect of multiple products being used together.”
According to the study, previous research has found that, compared with white women, U.S. black women have higher urinary levels of chemicals like phthalates and parabens. Black women also have higher rates of asthma and hormone-related health conditions like uterine fibroids and infertility, Dr. Helms said.
The researchers launched their study to better understand the possible role of hair care products in raising chemical levels in black women, Dr. Helm said.
The researchers tested 18 types of hair care products shown by a 2004-2005 survey to be popular among black women: hot oil treatments, anti-frizz products and polishes, leave-in conditioners, root stimulators, hair lotions, and relaxers. Researchers had purchased the products in 2008.
The researchers detected 45 of 66 target chemicals in the samples, including some that are banned in the European Union or regulated in California based on health concerns, according to Dr. Helms.
Most of the products contained parabens and phthalates (both 78%), UV filters (72%), and cyclosiloxanes (67%).
All products contained at least 1 of 19 targeted fragrances, while “hair lotions, root stimulators, and hair relaxers contained multiple fragrance chemicals per product, with an average of five to eight targeted fragrance chemicals detected per product versus an average of two in the anti-frizz products.”
How do the findings compare with previous research? “They’re roughly consistent with what’s been found before, but potentially on the higher end,” Dr. Helms said. “For some of these chemicals, there’s not a lot of data from the past.”
Most of the chemicals aren’t listed on product labels, Dr. Helm said. “It’s possible that some of the ingredients were unintentionally added as part of manufacturing or other processes.”
Dr. Helm urged physicians to consider the connections between hair care products and chemical exposure. “Maybe there’s an opportunity to use fewer products,” she said.
Dr. Helm acknowledged that it is difficult to find hair care products that don’t include fragrance. She recommends the use of products made from plants or organic ingredients, and she pointed to a Silver Spring Institute–affiliated app called DetoxMe that offers suggestions about reducing chemical exposure from consumer products.
The study was funded by the Rose Foundation, the Goldman Fund, and Hurricane Voices Breast Cancer Foundation. The authors report no relevant disclosures.
cenews@mdedge.com
SOURCE: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
Key clinical point: Endocrine-disrupting and asthma-associated chemicals are commonly found in hair care products used by black women.
Major finding: Of the 66 target chemicals, 45 were found in the 18 products tested.
Study details: Analysis of 18 hair care products purchased in 2008.
Disclosures: The study was funded by the Goldman Fund, Hurricane Voices Breast Cancer Foundation, and the Rose Foundation. The authors report no relevant disclosures.
Source: Helm JS et al. Environ Res. 2018 Apr 25. doi: 10.1016/j.envres.2018.03.030.
Most HIV patients need treatment for acute HCV
BOSTON – Fewer than 12% of HIV infected men will clear new hepatitis C infections on their own.
If they haven’t had a 2-log drop in their hepatitis C virus (HCV) RNA loads after a month, they aren’t going to clear the infection, and need direct-acting antiretrovirals (DAAs), according to an observational European study of 465 HIV patients with newly acquired HCV infections, almost all of them men who have sex with men (MSM).
The findings spoke to a hot topic at the Conference on Retroviruses & Opportunistic Infections (CROI): DAAs for acute HCV infection in patients with HIV. It’s a pressing problem; the incidence of sexually transmitted HCV among MSM with HIV is rising worldwide.
That’s a problem with HIV coinfection, and not just because far fewer patients will rid themselves of the virus. HCV is most likely to be spread by sexual contact in the acute phase; allowing patients destined to become chronic carriers to linger without treatment means that the infection will probably spread to new partners, according to researchers at CROI,
Proactive measures could help. Swiss investigators reported a 50% drop in new HCV infections when HIV-positive MSM were screened for the infection then treated immediately with DAAs. Some at the meeting argued that HCV screening – and treatment – should be automatic for patients taking pre-exposure HIV prophylaxis, as well as for newly diagnosed HIV.
Several said that, on a population level, treatment in the acute phase would save money by preventing new infections. It’s also cheaper to treat in the acute phase because coinfected patients only need 8 weeks of DAA treatment rather than the usual 12-16 for chronic infection, according to another European study at the meeting.
“The idea of targeting acute HCV makes a lot of sense on all levels. If treatment” among patients with HIV “is two-thirds as long and more than two-thirds have persistent infection, there is no reason to hold” off just because of cost, said David Thomas, MD, director of the division of infectious diseases at Johns Hopkins University, Baltimore, who moderated the study presentations.
Eight weeks is enough
The efficacy of an 8-week treatment course was established in a study of 63 MSM in the Netherlands and Belgium. They were 47 years old, on average, and all but three were HIV positive; two-thirds had HCV genotype 1, and the rest had genotype 4, some with extremely high viral loads.
Subjects received grazoprevir/elbasvir (Zepatier) once daily for 8 weeks, beginning at a mean of 4.5 months after their estimated infection date, and all within 6 months. Twelve weeks after the end of therapy, 59 (94%) were negative for HCV RNA on blood tests. Three of the patients who were still positive for HCV had new infections; the remaining patient had relapsed, and was the only true treatment failure.
The 2-log cutoff
“We are withholding effective treatment from people just because we’re concerned about the money,” said Christoph Boesecke, MD, of Bonn (Germany) University Hospital, who was the lead investigator in the study that found low spontaneous clearance rates with HIV coinfection.
Almost all the 465 subjects were on combination antiretroviral HIV therapy. Most had HCV genotype 1, but there were also type 3 and 4 cases. The investigators followed their subjects for at least a year after HCV infection. Just 55 (11.8%) cleared the infection on their own.
“Almost 90% of acutely infected patients face a chronic course. ... DAA drug labels, as well as clinical guidelines, need to be amended to allow usage of DAA during the acute phase of HCV infection in a high-risk population,” Dr. Boesecke and his team concluded.
Clearance was harbingered by a 2-log decline in HCV RNA by week 4. The 2-log drop cutoff is “the best predictor [we have] ... to identify patients” for early treatment. “There may be some patients you overtreat, but we have to keep in mind that we are not causing any harms with DAAs; maybe harms in terms of money, but not in terms of toxicity,” he said.
The message is beginning to get out. New European AIDS Clinical Society guidelines recommend DAA treatment for patients with HIV who don’t have a 2-log drop after a month.
‘Treatment as prevention’ works
The Swiss study added evidence to the frequent assertion at CROI that early treatment stops the spread of HCV.
The investigators screened 3,722 MSM from the Swiss HIV Cohort Study and found 178 (4.8%) men with replicating genotype 1 or 4 HCV infections. Of these cases, 31 were deemed incident and 147 chronic. Almost all the men agreed to treatment with standard-of-care DAAs, usually grazoprevir/elbasvir plus or minus ribavirin. Just about everyone had a sustained virologic response 12 weeks later.
The team rescreened the men after about a year, and found 28 infections; 16 were newly acquired, almost a 50% drop from baseline. The remaining 12 infections were chronic cases not treated earlier.
“Systemic screening followed by prompt DAA treatment can serve as a model to eliminate HCV in coinfected MSM,” said lead investigator Dominique Braun, MD, of the University of Zurich.
Dr. Boesecke is a consultant and/or speaker for AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Dr. Thomas is a Merck consultant. Dr. Boerekamps and Dr. Braun’s studies were both funded by Merck, maker of elbasvir/grazoprevir.
SOURCE: Anne Boerekamps. 2018 CROI, Abstract 128. Christoph Boesecke. 2018 CROI, Abstract 129. Dominique Braun. 2018 CROI, Abstract 81LB.
BOSTON – Fewer than 12% of HIV infected men will clear new hepatitis C infections on their own.
If they haven’t had a 2-log drop in their hepatitis C virus (HCV) RNA loads after a month, they aren’t going to clear the infection, and need direct-acting antiretrovirals (DAAs), according to an observational European study of 465 HIV patients with newly acquired HCV infections, almost all of them men who have sex with men (MSM).
The findings spoke to a hot topic at the Conference on Retroviruses & Opportunistic Infections (CROI): DAAs for acute HCV infection in patients with HIV. It’s a pressing problem; the incidence of sexually transmitted HCV among MSM with HIV is rising worldwide.
That’s a problem with HIV coinfection, and not just because far fewer patients will rid themselves of the virus. HCV is most likely to be spread by sexual contact in the acute phase; allowing patients destined to become chronic carriers to linger without treatment means that the infection will probably spread to new partners, according to researchers at CROI,
Proactive measures could help. Swiss investigators reported a 50% drop in new HCV infections when HIV-positive MSM were screened for the infection then treated immediately with DAAs. Some at the meeting argued that HCV screening – and treatment – should be automatic for patients taking pre-exposure HIV prophylaxis, as well as for newly diagnosed HIV.
Several said that, on a population level, treatment in the acute phase would save money by preventing new infections. It’s also cheaper to treat in the acute phase because coinfected patients only need 8 weeks of DAA treatment rather than the usual 12-16 for chronic infection, according to another European study at the meeting.
“The idea of targeting acute HCV makes a lot of sense on all levels. If treatment” among patients with HIV “is two-thirds as long and more than two-thirds have persistent infection, there is no reason to hold” off just because of cost, said David Thomas, MD, director of the division of infectious diseases at Johns Hopkins University, Baltimore, who moderated the study presentations.
Eight weeks is enough
The efficacy of an 8-week treatment course was established in a study of 63 MSM in the Netherlands and Belgium. They were 47 years old, on average, and all but three were HIV positive; two-thirds had HCV genotype 1, and the rest had genotype 4, some with extremely high viral loads.
Subjects received grazoprevir/elbasvir (Zepatier) once daily for 8 weeks, beginning at a mean of 4.5 months after their estimated infection date, and all within 6 months. Twelve weeks after the end of therapy, 59 (94%) were negative for HCV RNA on blood tests. Three of the patients who were still positive for HCV had new infections; the remaining patient had relapsed, and was the only true treatment failure.
The 2-log cutoff
“We are withholding effective treatment from people just because we’re concerned about the money,” said Christoph Boesecke, MD, of Bonn (Germany) University Hospital, who was the lead investigator in the study that found low spontaneous clearance rates with HIV coinfection.
Almost all the 465 subjects were on combination antiretroviral HIV therapy. Most had HCV genotype 1, but there were also type 3 and 4 cases. The investigators followed their subjects for at least a year after HCV infection. Just 55 (11.8%) cleared the infection on their own.
“Almost 90% of acutely infected patients face a chronic course. ... DAA drug labels, as well as clinical guidelines, need to be amended to allow usage of DAA during the acute phase of HCV infection in a high-risk population,” Dr. Boesecke and his team concluded.
Clearance was harbingered by a 2-log decline in HCV RNA by week 4. The 2-log drop cutoff is “the best predictor [we have] ... to identify patients” for early treatment. “There may be some patients you overtreat, but we have to keep in mind that we are not causing any harms with DAAs; maybe harms in terms of money, but not in terms of toxicity,” he said.
The message is beginning to get out. New European AIDS Clinical Society guidelines recommend DAA treatment for patients with HIV who don’t have a 2-log drop after a month.
‘Treatment as prevention’ works
The Swiss study added evidence to the frequent assertion at CROI that early treatment stops the spread of HCV.
The investigators screened 3,722 MSM from the Swiss HIV Cohort Study and found 178 (4.8%) men with replicating genotype 1 or 4 HCV infections. Of these cases, 31 were deemed incident and 147 chronic. Almost all the men agreed to treatment with standard-of-care DAAs, usually grazoprevir/elbasvir plus or minus ribavirin. Just about everyone had a sustained virologic response 12 weeks later.
The team rescreened the men after about a year, and found 28 infections; 16 were newly acquired, almost a 50% drop from baseline. The remaining 12 infections were chronic cases not treated earlier.
“Systemic screening followed by prompt DAA treatment can serve as a model to eliminate HCV in coinfected MSM,” said lead investigator Dominique Braun, MD, of the University of Zurich.
Dr. Boesecke is a consultant and/or speaker for AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Dr. Thomas is a Merck consultant. Dr. Boerekamps and Dr. Braun’s studies were both funded by Merck, maker of elbasvir/grazoprevir.
SOURCE: Anne Boerekamps. 2018 CROI, Abstract 128. Christoph Boesecke. 2018 CROI, Abstract 129. Dominique Braun. 2018 CROI, Abstract 81LB.
BOSTON – Fewer than 12% of HIV infected men will clear new hepatitis C infections on their own.
If they haven’t had a 2-log drop in their hepatitis C virus (HCV) RNA loads after a month, they aren’t going to clear the infection, and need direct-acting antiretrovirals (DAAs), according to an observational European study of 465 HIV patients with newly acquired HCV infections, almost all of them men who have sex with men (MSM).
The findings spoke to a hot topic at the Conference on Retroviruses & Opportunistic Infections (CROI): DAAs for acute HCV infection in patients with HIV. It’s a pressing problem; the incidence of sexually transmitted HCV among MSM with HIV is rising worldwide.
That’s a problem with HIV coinfection, and not just because far fewer patients will rid themselves of the virus. HCV is most likely to be spread by sexual contact in the acute phase; allowing patients destined to become chronic carriers to linger without treatment means that the infection will probably spread to new partners, according to researchers at CROI,
Proactive measures could help. Swiss investigators reported a 50% drop in new HCV infections when HIV-positive MSM were screened for the infection then treated immediately with DAAs. Some at the meeting argued that HCV screening – and treatment – should be automatic for patients taking pre-exposure HIV prophylaxis, as well as for newly diagnosed HIV.
Several said that, on a population level, treatment in the acute phase would save money by preventing new infections. It’s also cheaper to treat in the acute phase because coinfected patients only need 8 weeks of DAA treatment rather than the usual 12-16 for chronic infection, according to another European study at the meeting.
“The idea of targeting acute HCV makes a lot of sense on all levels. If treatment” among patients with HIV “is two-thirds as long and more than two-thirds have persistent infection, there is no reason to hold” off just because of cost, said David Thomas, MD, director of the division of infectious diseases at Johns Hopkins University, Baltimore, who moderated the study presentations.
Eight weeks is enough
The efficacy of an 8-week treatment course was established in a study of 63 MSM in the Netherlands and Belgium. They were 47 years old, on average, and all but three were HIV positive; two-thirds had HCV genotype 1, and the rest had genotype 4, some with extremely high viral loads.
Subjects received grazoprevir/elbasvir (Zepatier) once daily for 8 weeks, beginning at a mean of 4.5 months after their estimated infection date, and all within 6 months. Twelve weeks after the end of therapy, 59 (94%) were negative for HCV RNA on blood tests. Three of the patients who were still positive for HCV had new infections; the remaining patient had relapsed, and was the only true treatment failure.
The 2-log cutoff
“We are withholding effective treatment from people just because we’re concerned about the money,” said Christoph Boesecke, MD, of Bonn (Germany) University Hospital, who was the lead investigator in the study that found low spontaneous clearance rates with HIV coinfection.
Almost all the 465 subjects were on combination antiretroviral HIV therapy. Most had HCV genotype 1, but there were also type 3 and 4 cases. The investigators followed their subjects for at least a year after HCV infection. Just 55 (11.8%) cleared the infection on their own.
“Almost 90% of acutely infected patients face a chronic course. ... DAA drug labels, as well as clinical guidelines, need to be amended to allow usage of DAA during the acute phase of HCV infection in a high-risk population,” Dr. Boesecke and his team concluded.
Clearance was harbingered by a 2-log decline in HCV RNA by week 4. The 2-log drop cutoff is “the best predictor [we have] ... to identify patients” for early treatment. “There may be some patients you overtreat, but we have to keep in mind that we are not causing any harms with DAAs; maybe harms in terms of money, but not in terms of toxicity,” he said.
The message is beginning to get out. New European AIDS Clinical Society guidelines recommend DAA treatment for patients with HIV who don’t have a 2-log drop after a month.
‘Treatment as prevention’ works
The Swiss study added evidence to the frequent assertion at CROI that early treatment stops the spread of HCV.
The investigators screened 3,722 MSM from the Swiss HIV Cohort Study and found 178 (4.8%) men with replicating genotype 1 or 4 HCV infections. Of these cases, 31 were deemed incident and 147 chronic. Almost all the men agreed to treatment with standard-of-care DAAs, usually grazoprevir/elbasvir plus or minus ribavirin. Just about everyone had a sustained virologic response 12 weeks later.
The team rescreened the men after about a year, and found 28 infections; 16 were newly acquired, almost a 50% drop from baseline. The remaining 12 infections were chronic cases not treated earlier.
“Systemic screening followed by prompt DAA treatment can serve as a model to eliminate HCV in coinfected MSM,” said lead investigator Dominique Braun, MD, of the University of Zurich.
Dr. Boesecke is a consultant and/or speaker for AbbVie, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. Dr. Thomas is a Merck consultant. Dr. Boerekamps and Dr. Braun’s studies were both funded by Merck, maker of elbasvir/grazoprevir.
SOURCE: Anne Boerekamps. 2018 CROI, Abstract 128. Christoph Boesecke. 2018 CROI, Abstract 129. Dominique Braun. 2018 CROI, Abstract 81LB.
REPORTING FROM CROI