User login
Secukinumab Emerges as a Rapidly Effective Therapy for Pityriasis Rubra Pilaris
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
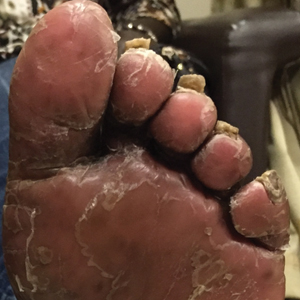
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
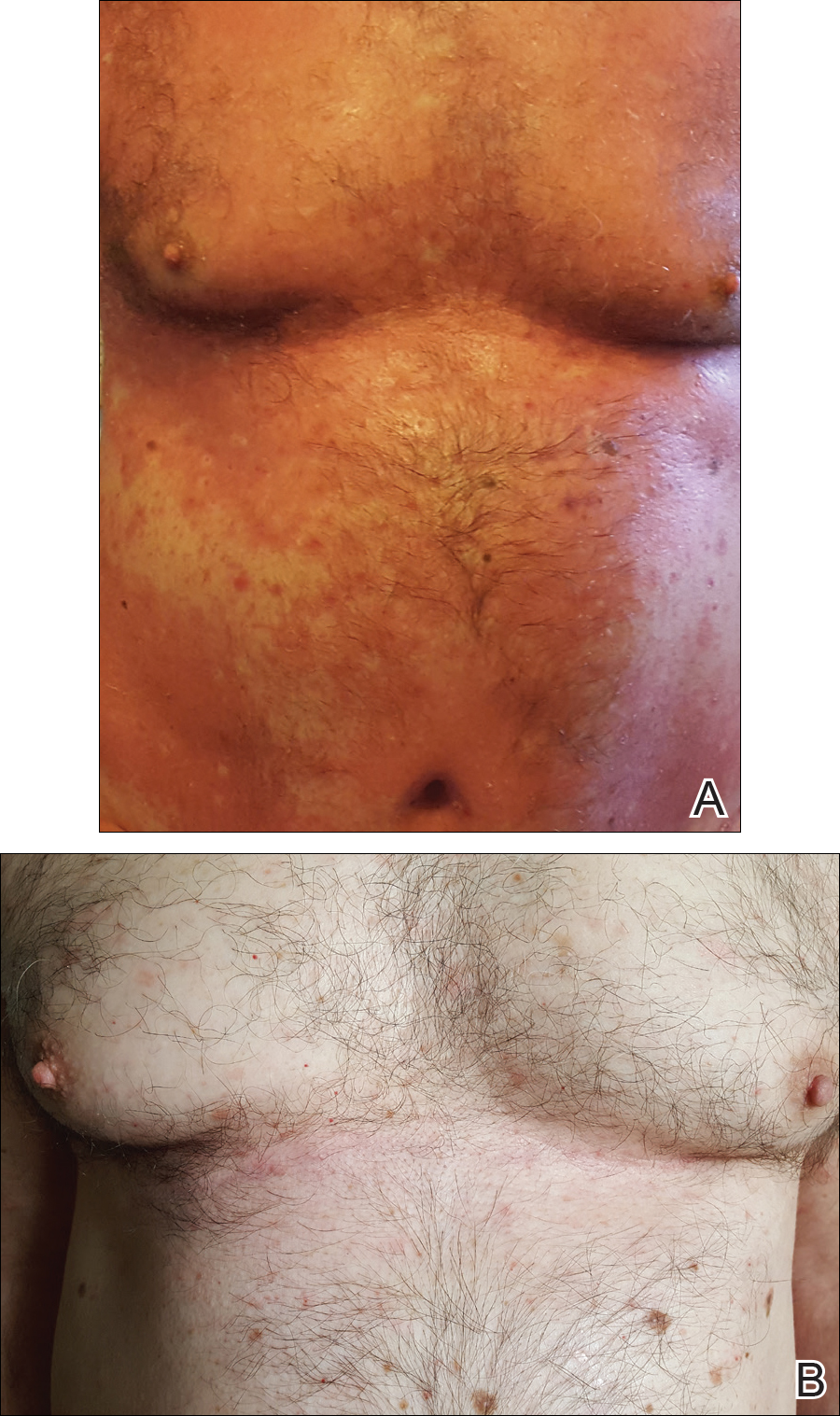
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.
Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.
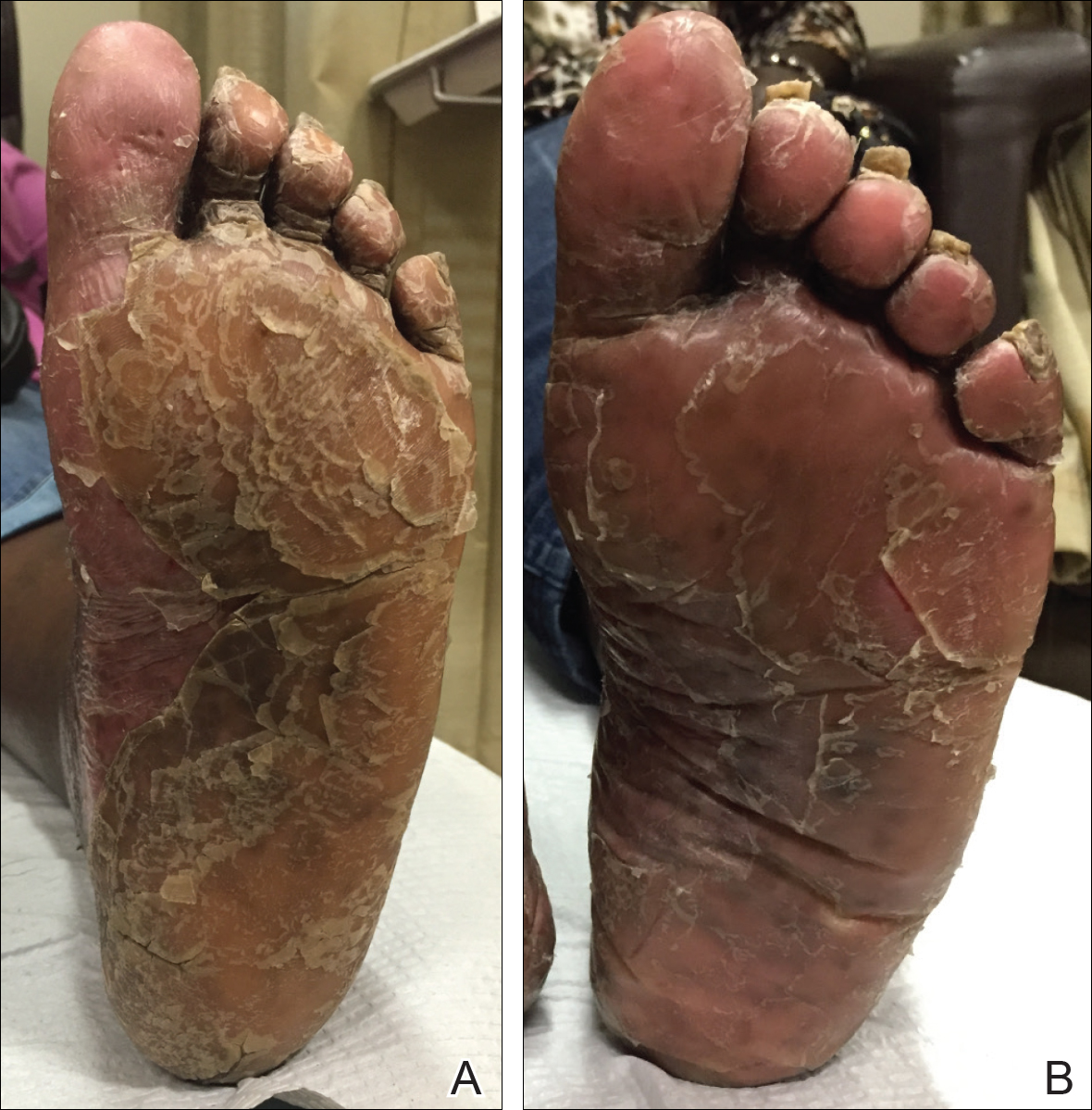
By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Practice Points
- In patients with pityriasis rubra pilaris (PRP) who have not responded to topical treatments, off-label treatment with systemic therapies approved for plaque psoriasis can be considered.
- Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP.
Transient epileptic amnesia: Rare, treatable, and easy to miss
LOS ANGELES – Transient epileptic amnesia is a rare but a treatable memory condition that usually occurs in late life and can be mistaken for neurodegenerative disease among patients presenting to a neurology or memory clinic.
Transient epileptic amnesia (TEA) is thought to be a focal epilepsy whose major clinical feature is the presence of recurrent spells of anterograde or retrograde amnesia lasting under an hour. The spells tend to occur on waking from sleep.
At the annual meeting of the American Academy of Neurology, Vijay Ramanan, MD, PhD, of the Mayo Clinic in Rochester, Minn., presented a retrospective series of 31 TEA cases from a study attempting to characterize the disorder in more demographic, clinical, and neuroimaging detail than has been done in the literature to date.
The cases were seen over a 20-year period (1998-2017) at the Mayo Clinic. All had at least one EEG and at least one MRI result reviewed by a neuroradiologist. Half also underwent fluorodeoxyglucose (FDG)-positron emission tomography (PET). All cases were classed as TEA if they included recurrent amnesia and an epileptic trait (lip smacking, for example), recurrent amnesiac spells and memory complaints between spells, or memory complaints and an epileptic trait.
Of the 31 cases, two-thirds were male, and the mean age was 70. Neuropsychological testing found mild nonspecific abnormalities in 10 individuals and mild cognitive impairment in 2.
The investigators found 20 patients had abnormalities on EEG, usually in the temporal epileptogenic region. On MRI, abnormalities were found in only 6 patients.
FDG-PET, however, revealed focal abnormalities in 11 of the 16 cases that underwent scanning. “Most of them had focal areas of hypometabolism; none of those metabolic patterns fit those of known neurodegenerative disorders, and more rarely they were entirely normal,” Dr. Ramanan said during a presentation of his findings.
The results suggest that FDG-PET “may be a more useful tool than EEG” in distinguishing TEA from other disorders, he said. “I think the fascinating question going forward is whether TEA has an underlying biomarker and if there’s a neuroimaging biomarker for this. From these data, I think FDG-PET could be a very promising avenue for that,” he said.
In most of these cases where there was an abnormality detected on EEG, he noted that the patient “had multiple or prolonged EEGs, so it’s not always an easy thing to catch.”
Dr. Ramanan stressed that it’s important for clinicians “to have your antennae up for this diagnosis, particularly as these patients will come in with chronic memory trouble, because this is something we can fix.” In his study, all of the 22 individuals followed up after treatment with antiepileptic drugs, most commonly lamotrigine or levetiracetam, improved on follow-up.
Dr. Ramanan and his colleagues disclosed no conflicts of interest related to their findings.
SOURCE: Ramanan V et al. Neurology. 2018 Apr 90(15 Suppl.):P3.035.
LOS ANGELES – Transient epileptic amnesia is a rare but a treatable memory condition that usually occurs in late life and can be mistaken for neurodegenerative disease among patients presenting to a neurology or memory clinic.
Transient epileptic amnesia (TEA) is thought to be a focal epilepsy whose major clinical feature is the presence of recurrent spells of anterograde or retrograde amnesia lasting under an hour. The spells tend to occur on waking from sleep.
At the annual meeting of the American Academy of Neurology, Vijay Ramanan, MD, PhD, of the Mayo Clinic in Rochester, Minn., presented a retrospective series of 31 TEA cases from a study attempting to characterize the disorder in more demographic, clinical, and neuroimaging detail than has been done in the literature to date.
The cases were seen over a 20-year period (1998-2017) at the Mayo Clinic. All had at least one EEG and at least one MRI result reviewed by a neuroradiologist. Half also underwent fluorodeoxyglucose (FDG)-positron emission tomography (PET). All cases were classed as TEA if they included recurrent amnesia and an epileptic trait (lip smacking, for example), recurrent amnesiac spells and memory complaints between spells, or memory complaints and an epileptic trait.
Of the 31 cases, two-thirds were male, and the mean age was 70. Neuropsychological testing found mild nonspecific abnormalities in 10 individuals and mild cognitive impairment in 2.
The investigators found 20 patients had abnormalities on EEG, usually in the temporal epileptogenic region. On MRI, abnormalities were found in only 6 patients.
FDG-PET, however, revealed focal abnormalities in 11 of the 16 cases that underwent scanning. “Most of them had focal areas of hypometabolism; none of those metabolic patterns fit those of known neurodegenerative disorders, and more rarely they were entirely normal,” Dr. Ramanan said during a presentation of his findings.
The results suggest that FDG-PET “may be a more useful tool than EEG” in distinguishing TEA from other disorders, he said. “I think the fascinating question going forward is whether TEA has an underlying biomarker and if there’s a neuroimaging biomarker for this. From these data, I think FDG-PET could be a very promising avenue for that,” he said.
In most of these cases where there was an abnormality detected on EEG, he noted that the patient “had multiple or prolonged EEGs, so it’s not always an easy thing to catch.”
Dr. Ramanan stressed that it’s important for clinicians “to have your antennae up for this diagnosis, particularly as these patients will come in with chronic memory trouble, because this is something we can fix.” In his study, all of the 22 individuals followed up after treatment with antiepileptic drugs, most commonly lamotrigine or levetiracetam, improved on follow-up.
Dr. Ramanan and his colleagues disclosed no conflicts of interest related to their findings.
SOURCE: Ramanan V et al. Neurology. 2018 Apr 90(15 Suppl.):P3.035.
LOS ANGELES – Transient epileptic amnesia is a rare but a treatable memory condition that usually occurs in late life and can be mistaken for neurodegenerative disease among patients presenting to a neurology or memory clinic.
Transient epileptic amnesia (TEA) is thought to be a focal epilepsy whose major clinical feature is the presence of recurrent spells of anterograde or retrograde amnesia lasting under an hour. The spells tend to occur on waking from sleep.
At the annual meeting of the American Academy of Neurology, Vijay Ramanan, MD, PhD, of the Mayo Clinic in Rochester, Minn., presented a retrospective series of 31 TEA cases from a study attempting to characterize the disorder in more demographic, clinical, and neuroimaging detail than has been done in the literature to date.
The cases were seen over a 20-year period (1998-2017) at the Mayo Clinic. All had at least one EEG and at least one MRI result reviewed by a neuroradiologist. Half also underwent fluorodeoxyglucose (FDG)-positron emission tomography (PET). All cases were classed as TEA if they included recurrent amnesia and an epileptic trait (lip smacking, for example), recurrent amnesiac spells and memory complaints between spells, or memory complaints and an epileptic trait.
Of the 31 cases, two-thirds were male, and the mean age was 70. Neuropsychological testing found mild nonspecific abnormalities in 10 individuals and mild cognitive impairment in 2.
The investigators found 20 patients had abnormalities on EEG, usually in the temporal epileptogenic region. On MRI, abnormalities were found in only 6 patients.
FDG-PET, however, revealed focal abnormalities in 11 of the 16 cases that underwent scanning. “Most of them had focal areas of hypometabolism; none of those metabolic patterns fit those of known neurodegenerative disorders, and more rarely they were entirely normal,” Dr. Ramanan said during a presentation of his findings.
The results suggest that FDG-PET “may be a more useful tool than EEG” in distinguishing TEA from other disorders, he said. “I think the fascinating question going forward is whether TEA has an underlying biomarker and if there’s a neuroimaging biomarker for this. From these data, I think FDG-PET could be a very promising avenue for that,” he said.
In most of these cases where there was an abnormality detected on EEG, he noted that the patient “had multiple or prolonged EEGs, so it’s not always an easy thing to catch.”
Dr. Ramanan stressed that it’s important for clinicians “to have your antennae up for this diagnosis, particularly as these patients will come in with chronic memory trouble, because this is something we can fix.” In his study, all of the 22 individuals followed up after treatment with antiepileptic drugs, most commonly lamotrigine or levetiracetam, improved on follow-up.
Dr. Ramanan and his colleagues disclosed no conflicts of interest related to their findings.
SOURCE: Ramanan V et al. Neurology. 2018 Apr 90(15 Suppl.):P3.035.
Key clinical point:
Major finding: Brain FDG-PET revealed focal abnormalities in 69% of subjects with suspected TEA.
Study details: A retrospective analysis of 31 suspected TEA cases treated from 1998-2017 at one clinic.
Disclosures: Dr. Ramanan and his colleagues disclosed no conflicts of interest.
Source: Ramanan V et al. Neurology. 2018 Apr 90(15 Suppl.):P3.035.
Perianal Basal Cell Carcinoma Treated With Mohs Micrographic Surgery
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
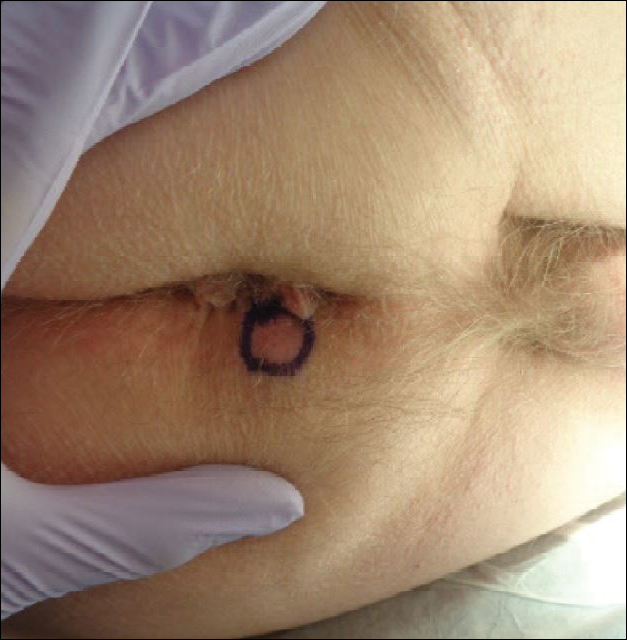
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.
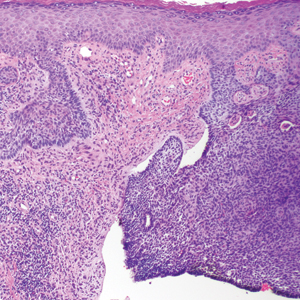
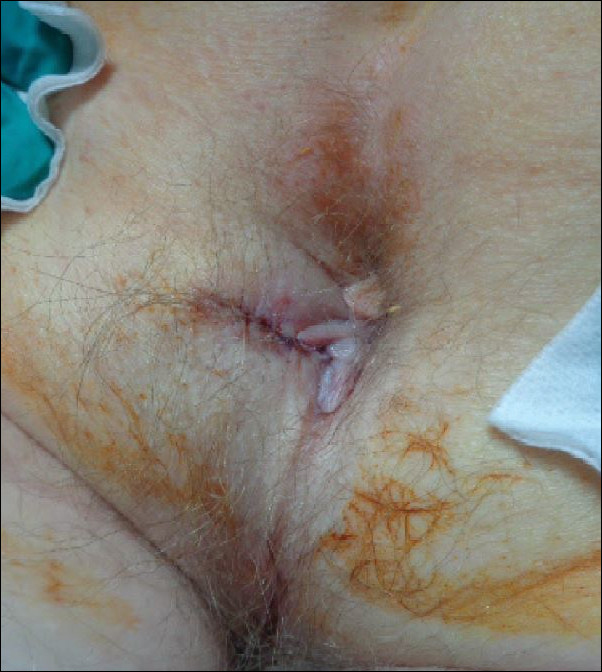
A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).

Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.

A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).


Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.

A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).


Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
Practice Points
- Basal cell carcinoma is less common in non–sun-exposed areas of the body and is exceptionally rare in the perineal and perianal regions.
- Thorough total-body skin examinations may lead to early detection of asymptomatic skin lesions, allowing for earlier and less invasive treatment.
- Appointment attendance and patient compliance are common challenges that dermatologists face. Patient reminders via their preferred method of communication may help reduce missed dermatology appointments.
MDedge Daily News: How to handle opioid constipation
Bath emollients are a washout for childhood eczema. Does warfarin cause acute kidney injury? And there may be a new option for postpartum depression.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Bath emollients are a washout for childhood eczema. Does warfarin cause acute kidney injury? And there may be a new option for postpartum depression.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Bath emollients are a washout for childhood eczema. Does warfarin cause acute kidney injury? And there may be a new option for postpartum depression.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
SAMHSA Helps Translate Science Into Real-Life Practice
The Substance Abuse and Mental Health Services Administration (SAMHSA) has launched a new Resource Center, aiming to give communities, clinicians, policy makers, and others the tools they need to put evidence-based information into practice.
The Evidence-Based Resource Center (www.samhsa.gov/ebp-resource-center) provides new or updated Treatment Improvement Protocols, tool kits, resource guides, clinical practice guidelines , and other science-based resources. The website has an easy-to-use point-and-click system. Users can search by topic, resource, target population, and target audience. The site also includes an opioid-specific resources section.
The center is part of a new comprehensive approach that allows rapid development and dissemination of the latest expert consensus on prevention, treatment, and recovery, SAMHSA says. It also provides communities and practitioners with tools to facilitate comprehensive needs assessment, match interventions to those needs, support implementation, and evaluate and incorporate continuous quality improvement as they translate science into action.
The Substance Abuse and Mental Health Services Administration (SAMHSA) has launched a new Resource Center, aiming to give communities, clinicians, policy makers, and others the tools they need to put evidence-based information into practice.
The Evidence-Based Resource Center (www.samhsa.gov/ebp-resource-center) provides new or updated Treatment Improvement Protocols, tool kits, resource guides, clinical practice guidelines , and other science-based resources. The website has an easy-to-use point-and-click system. Users can search by topic, resource, target population, and target audience. The site also includes an opioid-specific resources section.
The center is part of a new comprehensive approach that allows rapid development and dissemination of the latest expert consensus on prevention, treatment, and recovery, SAMHSA says. It also provides communities and practitioners with tools to facilitate comprehensive needs assessment, match interventions to those needs, support implementation, and evaluate and incorporate continuous quality improvement as they translate science into action.
The Substance Abuse and Mental Health Services Administration (SAMHSA) has launched a new Resource Center, aiming to give communities, clinicians, policy makers, and others the tools they need to put evidence-based information into practice.
The Evidence-Based Resource Center (www.samhsa.gov/ebp-resource-center) provides new or updated Treatment Improvement Protocols, tool kits, resource guides, clinical practice guidelines , and other science-based resources. The website has an easy-to-use point-and-click system. Users can search by topic, resource, target population, and target audience. The site also includes an opioid-specific resources section.
The center is part of a new comprehensive approach that allows rapid development and dissemination of the latest expert consensus on prevention, treatment, and recovery, SAMHSA says. It also provides communities and practitioners with tools to facilitate comprehensive needs assessment, match interventions to those needs, support implementation, and evaluate and incorporate continuous quality improvement as they translate science into action.
‘Essential’ genes could be targeted to treat malaria
More than 2000 genes are “essential” for the malaria parasite Plasmodium falciparum, according to research published in Science.
Researchers identified 2680 genes that appear necessary for growth and survival during P falciparum’s asexual blood stage.
The researchers therefore believe these genes could be viable therapeutic targets for P falciparum malaria.
“Malaria parasites are extremely technically difficult to manipulate and sequence, and, until this study, only a few of P falciparum’s essential genes had been determined,” said study author Iraad Bronner, of the Wellcome Sanger Institute in Hinxton, UK.
“Our technological advances enabled us to identify all the essential genes in P falciparum—the first time this has been possible for a human malaria parasite.”
To determine which genes P falciparum needs to survive and thrive, the researchers disrupted the parasite’s genes.
The team used piggyBac-transposon insertional mutagenesis to inactivate genes at random and then used DNA sequencing technology to identify which genes were affected.
The researchers made more than 38,000 mutations, then looked for genes that hadn’t been changed, implying they were essential for P falciparum to survive and grow.
This revealed 2680 non-mutable genes, about 1000 of which are conserved in all Plasmodium species and have unknown functions.
“What our team has done is develop a way to analyze every gene in this parasite’s genome,” said study author John H. Adams, PhD, of the University of South Florida in Tampa.
“Using our genetic analysis tools, we’re able to determine the relative importance of each gene for parasite survival. This understanding will help guide future drug development efforts targeting those essential genes.”
The researchers noted that the proteasome pathway had a “high ratio of essential to dispensable genes,” and recent research has linked this pathway to resistance to artemisinin combination therapy.
“We need new drug targets against malaria now more than ever, since our current antimalarial drugs are failing,” said study author Julian C. Rayner, PhD, of Wellcome Sanger Institute.
“This is the first large-scale genetic study in the major human malaria parasite P falciparum and gives a list of 2680 essential genes that researchers can prioritize as promising possible drug targets. We hope this functional genomics approach will help to speed up the pipeline to develop new treatments for this devastating disease.”
More than 2000 genes are “essential” for the malaria parasite Plasmodium falciparum, according to research published in Science.
Researchers identified 2680 genes that appear necessary for growth and survival during P falciparum’s asexual blood stage.
The researchers therefore believe these genes could be viable therapeutic targets for P falciparum malaria.
“Malaria parasites are extremely technically difficult to manipulate and sequence, and, until this study, only a few of P falciparum’s essential genes had been determined,” said study author Iraad Bronner, of the Wellcome Sanger Institute in Hinxton, UK.
“Our technological advances enabled us to identify all the essential genes in P falciparum—the first time this has been possible for a human malaria parasite.”
To determine which genes P falciparum needs to survive and thrive, the researchers disrupted the parasite’s genes.
The team used piggyBac-transposon insertional mutagenesis to inactivate genes at random and then used DNA sequencing technology to identify which genes were affected.
The researchers made more than 38,000 mutations, then looked for genes that hadn’t been changed, implying they were essential for P falciparum to survive and grow.
This revealed 2680 non-mutable genes, about 1000 of which are conserved in all Plasmodium species and have unknown functions.
“What our team has done is develop a way to analyze every gene in this parasite’s genome,” said study author John H. Adams, PhD, of the University of South Florida in Tampa.
“Using our genetic analysis tools, we’re able to determine the relative importance of each gene for parasite survival. This understanding will help guide future drug development efforts targeting those essential genes.”
The researchers noted that the proteasome pathway had a “high ratio of essential to dispensable genes,” and recent research has linked this pathway to resistance to artemisinin combination therapy.
“We need new drug targets against malaria now more than ever, since our current antimalarial drugs are failing,” said study author Julian C. Rayner, PhD, of Wellcome Sanger Institute.
“This is the first large-scale genetic study in the major human malaria parasite P falciparum and gives a list of 2680 essential genes that researchers can prioritize as promising possible drug targets. We hope this functional genomics approach will help to speed up the pipeline to develop new treatments for this devastating disease.”
More than 2000 genes are “essential” for the malaria parasite Plasmodium falciparum, according to research published in Science.
Researchers identified 2680 genes that appear necessary for growth and survival during P falciparum’s asexual blood stage.
The researchers therefore believe these genes could be viable therapeutic targets for P falciparum malaria.
“Malaria parasites are extremely technically difficult to manipulate and sequence, and, until this study, only a few of P falciparum’s essential genes had been determined,” said study author Iraad Bronner, of the Wellcome Sanger Institute in Hinxton, UK.
“Our technological advances enabled us to identify all the essential genes in P falciparum—the first time this has been possible for a human malaria parasite.”
To determine which genes P falciparum needs to survive and thrive, the researchers disrupted the parasite’s genes.
The team used piggyBac-transposon insertional mutagenesis to inactivate genes at random and then used DNA sequencing technology to identify which genes were affected.
The researchers made more than 38,000 mutations, then looked for genes that hadn’t been changed, implying they were essential for P falciparum to survive and grow.
This revealed 2680 non-mutable genes, about 1000 of which are conserved in all Plasmodium species and have unknown functions.
“What our team has done is develop a way to analyze every gene in this parasite’s genome,” said study author John H. Adams, PhD, of the University of South Florida in Tampa.
“Using our genetic analysis tools, we’re able to determine the relative importance of each gene for parasite survival. This understanding will help guide future drug development efforts targeting those essential genes.”
The researchers noted that the proteasome pathway had a “high ratio of essential to dispensable genes,” and recent research has linked this pathway to resistance to artemisinin combination therapy.
“We need new drug targets against malaria now more than ever, since our current antimalarial drugs are failing,” said study author Julian C. Rayner, PhD, of Wellcome Sanger Institute.
“This is the first large-scale genetic study in the major human malaria parasite P falciparum and gives a list of 2680 essential genes that researchers can prioritize as promising possible drug targets. We hope this functional genomics approach will help to speed up the pipeline to develop new treatments for this devastating disease.”
Study shows increased risk of VTE among earthquake evacuees
New research has revealed a link between earthquake evacuation and venous thromboembolism (VTE).
The study showed that people who spent the night in their cars after the Kumamoto earthquakes had an increased risk of VTE.
Researchers have therefore called for education about the risk of VTE among people who remain seated and immobile in vehicles for prolonged periods.
“Preventive awareness activities by professional medical teams, supported by education in the media about the risk of VTEs after spending the night in a vehicle, and raising awareness of evacuation centers could lead to a reduced number of victims of VTE,” said Seiji Hokimoto, MD, PhD, of Kumamoto University in Kumamoto, Japan.
Dr Hokimoto and colleagues made this point in a letter published in the Canadian Journal of Cardiology.
The researchers studied the aftermath of the Kumamoto earthquakes that occurred in April 2016.
The team noted that a high number of aftershocks at night prompted many people to evacuate their homes. Although some individuals reached a public evacuation shelter, many were forced to stay in their vehicles overnight.
To assess the impact of remaining seated in cars for extended periods of time, the researchers gathered data from 21 local medical institutions.
They found that 51 patients were hospitalized for VTE after the earthquakes, including 35 who developed pulmonary embolism (PE).
Most of the patients who developed VTE had spent the night in a vehicle (82.4%, n=42).
The researchers found that VTE patients who spent the night in a vehicle were significantly younger than patients who did not, with mean ages of 64.6 ± 13.3 and 79.8 ± 12.1, respectively (P=0.001).
The mean time to onset of VTE after the earthquakes was significantly shorter in patients who spent the night in a vehicle—7.3 ± 5.3 days vs 20.1 ± 25.6 days (P=0.003).
And the incidence of PE was significantly higher in patients who spent the night in a vehicle—83% vs 33% (P=0.001).
“This is a dramatic example of the risks inherent in spending prolonged periods immobilized in a cramped position,” said Stanley Nattel, MD, editor-in-chief of the Canadian Journal of Cardiology.
“It is an important reminder of a public health point and reinforces the need to get up and walk around regularly when on an airplane or when forced to stay in a car for a long time.”
New research has revealed a link between earthquake evacuation and venous thromboembolism (VTE).
The study showed that people who spent the night in their cars after the Kumamoto earthquakes had an increased risk of VTE.
Researchers have therefore called for education about the risk of VTE among people who remain seated and immobile in vehicles for prolonged periods.
“Preventive awareness activities by professional medical teams, supported by education in the media about the risk of VTEs after spending the night in a vehicle, and raising awareness of evacuation centers could lead to a reduced number of victims of VTE,” said Seiji Hokimoto, MD, PhD, of Kumamoto University in Kumamoto, Japan.
Dr Hokimoto and colleagues made this point in a letter published in the Canadian Journal of Cardiology.
The researchers studied the aftermath of the Kumamoto earthquakes that occurred in April 2016.
The team noted that a high number of aftershocks at night prompted many people to evacuate their homes. Although some individuals reached a public evacuation shelter, many were forced to stay in their vehicles overnight.
To assess the impact of remaining seated in cars for extended periods of time, the researchers gathered data from 21 local medical institutions.
They found that 51 patients were hospitalized for VTE after the earthquakes, including 35 who developed pulmonary embolism (PE).
Most of the patients who developed VTE had spent the night in a vehicle (82.4%, n=42).
The researchers found that VTE patients who spent the night in a vehicle were significantly younger than patients who did not, with mean ages of 64.6 ± 13.3 and 79.8 ± 12.1, respectively (P=0.001).
The mean time to onset of VTE after the earthquakes was significantly shorter in patients who spent the night in a vehicle—7.3 ± 5.3 days vs 20.1 ± 25.6 days (P=0.003).
And the incidence of PE was significantly higher in patients who spent the night in a vehicle—83% vs 33% (P=0.001).
“This is a dramatic example of the risks inherent in spending prolonged periods immobilized in a cramped position,” said Stanley Nattel, MD, editor-in-chief of the Canadian Journal of Cardiology.
“It is an important reminder of a public health point and reinforces the need to get up and walk around regularly when on an airplane or when forced to stay in a car for a long time.”
New research has revealed a link between earthquake evacuation and venous thromboembolism (VTE).
The study showed that people who spent the night in their cars after the Kumamoto earthquakes had an increased risk of VTE.
Researchers have therefore called for education about the risk of VTE among people who remain seated and immobile in vehicles for prolonged periods.
“Preventive awareness activities by professional medical teams, supported by education in the media about the risk of VTEs after spending the night in a vehicle, and raising awareness of evacuation centers could lead to a reduced number of victims of VTE,” said Seiji Hokimoto, MD, PhD, of Kumamoto University in Kumamoto, Japan.
Dr Hokimoto and colleagues made this point in a letter published in the Canadian Journal of Cardiology.
The researchers studied the aftermath of the Kumamoto earthquakes that occurred in April 2016.
The team noted that a high number of aftershocks at night prompted many people to evacuate their homes. Although some individuals reached a public evacuation shelter, many were forced to stay in their vehicles overnight.
To assess the impact of remaining seated in cars for extended periods of time, the researchers gathered data from 21 local medical institutions.
They found that 51 patients were hospitalized for VTE after the earthquakes, including 35 who developed pulmonary embolism (PE).
Most of the patients who developed VTE had spent the night in a vehicle (82.4%, n=42).
The researchers found that VTE patients who spent the night in a vehicle were significantly younger than patients who did not, with mean ages of 64.6 ± 13.3 and 79.8 ± 12.1, respectively (P=0.001).
The mean time to onset of VTE after the earthquakes was significantly shorter in patients who spent the night in a vehicle—7.3 ± 5.3 days vs 20.1 ± 25.6 days (P=0.003).
And the incidence of PE was significantly higher in patients who spent the night in a vehicle—83% vs 33% (P=0.001).
“This is a dramatic example of the risks inherent in spending prolonged periods immobilized in a cramped position,” said Stanley Nattel, MD, editor-in-chief of the Canadian Journal of Cardiology.
“It is an important reminder of a public health point and reinforces the need to get up and walk around regularly when on an airplane or when forced to stay in a car for a long time.”
Blood type linked to death risk after trauma
Having type O blood is associated with high death rates in severe trauma patients, according to a study published in Critical Care.
Researchers found that severe trauma patients with type O blood had a death rate of 28%, compared to a rate of 11% in patients with other blood types.
“Loss of blood is the leading cause of death in patients with severe trauma, but studies on the association between different blood types and the risk of trauma death have been scarce,” said study author Wataru Takayama, of Tokyo Medical and Dental University Hospital of Medicine in Japan.
“We wanted to test the hypothesis that trauma survival is affected by differences in blood types.”
To do this, the researchers evaluated the medical records of 901 patients with severe trauma who had been transported to either of 2 tertiary emergency critical care medical centers in Japan from 2013 to 2016.
Most patients had type O (n=284, 32%) or type A blood (n=285, 32%), followed by type B (n=209, 23%) and type AB (n=123, 13%).
The mortality rate was significantly higher in patients with type O blood than in patients with the other blood types—28% and 11%, respectively (P<0.001).
In a multivariate analysis, mortality was significantly higher for patients with type O blood. The adjusted odds ratio was 2.86 (P<0.001).
Patients with type O blood have been shown to have lower levels of von Willebrand factor than patients with other blood types. The researchers suggested that a lower level of von Willebrand factor is a possible explanation for the higher death rate in trauma patients with blood type O.
“Our results also raise questions about how emergency transfusion of O type red blood cells to a severe trauma patient could affect homeostasis . . . and if this is different from other blood types,” Dr Takayama said.
“Further research is necessary to investigate the results of our study and develop the best treatment strategy for severe trauma patients.”
In particular, further research is needed to determine if the findings from this study apply to other ethnic groups, as all the patients in this study were Japanese.
In addition, the researchers didn’t evaluate the impact of the individual blood types A, AB, or B on severe trauma death rates. They only compared type O to non-O blood types, which may have diluted the effect of individual blood types on patient survival.
Having type O blood is associated with high death rates in severe trauma patients, according to a study published in Critical Care.
Researchers found that severe trauma patients with type O blood had a death rate of 28%, compared to a rate of 11% in patients with other blood types.
“Loss of blood is the leading cause of death in patients with severe trauma, but studies on the association between different blood types and the risk of trauma death have been scarce,” said study author Wataru Takayama, of Tokyo Medical and Dental University Hospital of Medicine in Japan.
“We wanted to test the hypothesis that trauma survival is affected by differences in blood types.”
To do this, the researchers evaluated the medical records of 901 patients with severe trauma who had been transported to either of 2 tertiary emergency critical care medical centers in Japan from 2013 to 2016.
Most patients had type O (n=284, 32%) or type A blood (n=285, 32%), followed by type B (n=209, 23%) and type AB (n=123, 13%).
The mortality rate was significantly higher in patients with type O blood than in patients with the other blood types—28% and 11%, respectively (P<0.001).
In a multivariate analysis, mortality was significantly higher for patients with type O blood. The adjusted odds ratio was 2.86 (P<0.001).
Patients with type O blood have been shown to have lower levels of von Willebrand factor than patients with other blood types. The researchers suggested that a lower level of von Willebrand factor is a possible explanation for the higher death rate in trauma patients with blood type O.
“Our results also raise questions about how emergency transfusion of O type red blood cells to a severe trauma patient could affect homeostasis . . . and if this is different from other blood types,” Dr Takayama said.
“Further research is necessary to investigate the results of our study and develop the best treatment strategy for severe trauma patients.”
In particular, further research is needed to determine if the findings from this study apply to other ethnic groups, as all the patients in this study were Japanese.
In addition, the researchers didn’t evaluate the impact of the individual blood types A, AB, or B on severe trauma death rates. They only compared type O to non-O blood types, which may have diluted the effect of individual blood types on patient survival.
Having type O blood is associated with high death rates in severe trauma patients, according to a study published in Critical Care.
Researchers found that severe trauma patients with type O blood had a death rate of 28%, compared to a rate of 11% in patients with other blood types.
“Loss of blood is the leading cause of death in patients with severe trauma, but studies on the association between different blood types and the risk of trauma death have been scarce,” said study author Wataru Takayama, of Tokyo Medical and Dental University Hospital of Medicine in Japan.
“We wanted to test the hypothesis that trauma survival is affected by differences in blood types.”
To do this, the researchers evaluated the medical records of 901 patients with severe trauma who had been transported to either of 2 tertiary emergency critical care medical centers in Japan from 2013 to 2016.
Most patients had type O (n=284, 32%) or type A blood (n=285, 32%), followed by type B (n=209, 23%) and type AB (n=123, 13%).
The mortality rate was significantly higher in patients with type O blood than in patients with the other blood types—28% and 11%, respectively (P<0.001).
In a multivariate analysis, mortality was significantly higher for patients with type O blood. The adjusted odds ratio was 2.86 (P<0.001).
Patients with type O blood have been shown to have lower levels of von Willebrand factor than patients with other blood types. The researchers suggested that a lower level of von Willebrand factor is a possible explanation for the higher death rate in trauma patients with blood type O.
“Our results also raise questions about how emergency transfusion of O type red blood cells to a severe trauma patient could affect homeostasis . . . and if this is different from other blood types,” Dr Takayama said.
“Further research is necessary to investigate the results of our study and develop the best treatment strategy for severe trauma patients.”
In particular, further research is needed to determine if the findings from this study apply to other ethnic groups, as all the patients in this study were Japanese.
In addition, the researchers didn’t evaluate the impact of the individual blood types A, AB, or B on severe trauma death rates. They only compared type O to non-O blood types, which may have diluted the effect of individual blood types on patient survival.
Interferon-gamma release assay trumps tuberculin skin test in school-aged children
The interferon-gamma release assay (IGRA) was significantly more sensitive than a tuberculin skin test as an adjunct tuberculosis diagnosis of children aged 5 years and older, according to data from a population-based study of 778 cases.
IGRAs have shown greater specificity than tuberculin skin tests (TSTs), but data on their sensitivity to TB in children are limited, wrote Alexander W. Kay, MD, of the California Department of Public Health and his colleagues in a study published in Pediatrics.
Children younger than 1 year of age and those with CNS disease were significantly more likely to have indeterminate IGRA results, the researchers noted.
The study results were limited by the use of mainly enzyme-linked immunosorbent assay–based IGRA, which limited the data on enzyme-linked immunospot tests, the researchers said. The findings also were limited by the small number of children younger than 5 years.
However, the study is the largest North American analysis of IGRA in children, and based on the findings, “we argue that an IGRA should be considered the test of choice when evaluating children 5-18 years old for TB disease in high-resource, low-TB burden settings,” Dr. Kay and his associates wrote.
The study was funded by the Centers for Disease Control and Prevention. Coauthor Shamim Islam, MD, disclosed financial support from Qiagen, maker of the QuantiFERON test. Dr. Kay and the other investigators had no financial conflicts to disclose.
SOURCE: Kay A et al. Pediatrics. 2018 May 4. doi: 10.1542/peds.2017-3918.
The interferon-gamma release assay (IGRA) was significantly more sensitive than a tuberculin skin test as an adjunct tuberculosis diagnosis of children aged 5 years and older, according to data from a population-based study of 778 cases.
IGRAs have shown greater specificity than tuberculin skin tests (TSTs), but data on their sensitivity to TB in children are limited, wrote Alexander W. Kay, MD, of the California Department of Public Health and his colleagues in a study published in Pediatrics.
Children younger than 1 year of age and those with CNS disease were significantly more likely to have indeterminate IGRA results, the researchers noted.
The study results were limited by the use of mainly enzyme-linked immunosorbent assay–based IGRA, which limited the data on enzyme-linked immunospot tests, the researchers said. The findings also were limited by the small number of children younger than 5 years.
However, the study is the largest North American analysis of IGRA in children, and based on the findings, “we argue that an IGRA should be considered the test of choice when evaluating children 5-18 years old for TB disease in high-resource, low-TB burden settings,” Dr. Kay and his associates wrote.
The study was funded by the Centers for Disease Control and Prevention. Coauthor Shamim Islam, MD, disclosed financial support from Qiagen, maker of the QuantiFERON test. Dr. Kay and the other investigators had no financial conflicts to disclose.
SOURCE: Kay A et al. Pediatrics. 2018 May 4. doi: 10.1542/peds.2017-3918.
The interferon-gamma release assay (IGRA) was significantly more sensitive than a tuberculin skin test as an adjunct tuberculosis diagnosis of children aged 5 years and older, according to data from a population-based study of 778 cases.
IGRAs have shown greater specificity than tuberculin skin tests (TSTs), but data on their sensitivity to TB in children are limited, wrote Alexander W. Kay, MD, of the California Department of Public Health and his colleagues in a study published in Pediatrics.
Children younger than 1 year of age and those with CNS disease were significantly more likely to have indeterminate IGRA results, the researchers noted.
The study results were limited by the use of mainly enzyme-linked immunosorbent assay–based IGRA, which limited the data on enzyme-linked immunospot tests, the researchers said. The findings also were limited by the small number of children younger than 5 years.
However, the study is the largest North American analysis of IGRA in children, and based on the findings, “we argue that an IGRA should be considered the test of choice when evaluating children 5-18 years old for TB disease in high-resource, low-TB burden settings,” Dr. Kay and his associates wrote.
The study was funded by the Centers for Disease Control and Prevention. Coauthor Shamim Islam, MD, disclosed financial support from Qiagen, maker of the QuantiFERON test. Dr. Kay and the other investigators had no financial conflicts to disclose.
SOURCE: Kay A et al. Pediatrics. 2018 May 4. doi: 10.1542/peds.2017-3918.
FROM PEDIATRICS
Key clinical point:
Major finding: Sensitivity was 96% for IGRA versus 83% for TST among children aged 5-18 years.
Study details: The data come from TB patients aged 18 years and younger enrolled in the California TB registry during 2010-2015.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. Coauthor Shamim Islam, MD, disclosed financial support from Qiagen, maker of the QuantiFERON test; Dr. Kay and the other investigators had no financial conflicts to disclose.
Source: Kay A et al. Pediatrics. 2018 May 4. doi: 10. 1542/ peds. 2017- 3918.
Abstract: Risk of colorectal cancer after a negative colonoscopy in low-to-moderate risk individuals: impact of a 10-year colonoscopy
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Murthy, S.K., et al, Endoscopy 49(12):1228, December 2017
BACKGROUND: Repeat colonoscopy is recommended ten years after a negative screening for colorectal cancer (CRC) in low-risk persons, but the real-world benefit of this recommendation is uncertain.
METHODS: These Canadian authors, coordinated at the University of Ottawa, performed a retrospective cohort study to determine the utility of ten-year repeat screening colonoscopy using population-level data from Ontario adults aged 50-74 years with a low to moderate risk of CRC (no relevant gastrointestinal disorders) who had a negative colonoscopy in 1996-2001 and a repeat negative screening within eight to twelve years, excluding those with intervening events (CRC detection, colectomy, or lower endoscopy). The primary outcome was early incident CRC in the group having repeat screening within twelve years compared with an unexposed control group matched by age, sex and year of baseline colonoscopy.
RESULTS: A total of 13,350 matched pairs (median age 68 years; 56% female) were analyzed for CRC incidence over a median follow-up of 4.5 years. The cumulative probability of CRC over three, five and eight years following a negative baseline colonoscopy was 0.16%, 0.30%, and 0.54%, respectively. Among patients having repeat colonoscopy, 46 developed CRC, compared with 52 in unexposed controls, for cumulative probabilities of 0.70% and 0.77%, respectively, and a hazard ratio of 0.91 (95% CI 0.68-1.22) after adjusting for competing risks and comorbidity burden. CRC-related mortality was also similar between groups (8 and 9 patients). Short follow-up was a study limitation.
CONCLUSIONS: Repeat colonoscopy within eight to twelve years of a negative screen was not associated with subsequent CRC incidence, which raises questions about the utility of ten-year repeat screening. 21 references (smurthy@toh.on.ca – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Murthy, S.K., et al, Endoscopy 49(12):1228, December 2017
BACKGROUND: Repeat colonoscopy is recommended ten years after a negative screening for colorectal cancer (CRC) in low-risk persons, but the real-world benefit of this recommendation is uncertain.
METHODS: These Canadian authors, coordinated at the University of Ottawa, performed a retrospective cohort study to determine the utility of ten-year repeat screening colonoscopy using population-level data from Ontario adults aged 50-74 years with a low to moderate risk of CRC (no relevant gastrointestinal disorders) who had a negative colonoscopy in 1996-2001 and a repeat negative screening within eight to twelve years, excluding those with intervening events (CRC detection, colectomy, or lower endoscopy). The primary outcome was early incident CRC in the group having repeat screening within twelve years compared with an unexposed control group matched by age, sex and year of baseline colonoscopy.
RESULTS: A total of 13,350 matched pairs (median age 68 years; 56% female) were analyzed for CRC incidence over a median follow-up of 4.5 years. The cumulative probability of CRC over three, five and eight years following a negative baseline colonoscopy was 0.16%, 0.30%, and 0.54%, respectively. Among patients having repeat colonoscopy, 46 developed CRC, compared with 52 in unexposed controls, for cumulative probabilities of 0.70% and 0.77%, respectively, and a hazard ratio of 0.91 (95% CI 0.68-1.22) after adjusting for competing risks and comorbidity burden. CRC-related mortality was also similar between groups (8 and 9 patients). Short follow-up was a study limitation.
CONCLUSIONS: Repeat colonoscopy within eight to twelve years of a negative screen was not associated with subsequent CRC incidence, which raises questions about the utility of ten-year repeat screening. 21 references (smurthy@toh.on.ca – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Murthy, S.K., et al, Endoscopy 49(12):1228, December 2017
BACKGROUND: Repeat colonoscopy is recommended ten years after a negative screening for colorectal cancer (CRC) in low-risk persons, but the real-world benefit of this recommendation is uncertain.
METHODS: These Canadian authors, coordinated at the University of Ottawa, performed a retrospective cohort study to determine the utility of ten-year repeat screening colonoscopy using population-level data from Ontario adults aged 50-74 years with a low to moderate risk of CRC (no relevant gastrointestinal disorders) who had a negative colonoscopy in 1996-2001 and a repeat negative screening within eight to twelve years, excluding those with intervening events (CRC detection, colectomy, or lower endoscopy). The primary outcome was early incident CRC in the group having repeat screening within twelve years compared with an unexposed control group matched by age, sex and year of baseline colonoscopy.
RESULTS: A total of 13,350 matched pairs (median age 68 years; 56% female) were analyzed for CRC incidence over a median follow-up of 4.5 years. The cumulative probability of CRC over three, five and eight years following a negative baseline colonoscopy was 0.16%, 0.30%, and 0.54%, respectively. Among patients having repeat colonoscopy, 46 developed CRC, compared with 52 in unexposed controls, for cumulative probabilities of 0.70% and 0.77%, respectively, and a hazard ratio of 0.91 (95% CI 0.68-1.22) after adjusting for competing risks and comorbidity burden. CRC-related mortality was also similar between groups (8 and 9 patients). Short follow-up was a study limitation.
CONCLUSIONS: Repeat colonoscopy within eight to twelve years of a negative screen was not associated with subsequent CRC incidence, which raises questions about the utility of ten-year repeat screening. 21 references (smurthy@toh.on.ca – no reprints)
Learn more about the Primary Care Medical Abstracts and podcasts, for which you can earn up to 9 CME credits per month.
Copyright © The Center for Medical Education