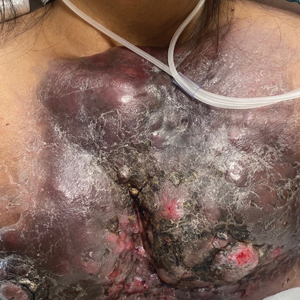
User login
What is the link between cellphones and male fertility?
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
Infertility affects approximately one in six couples worldwide. More than half the time, it is the man’s low sperm quality that is to blame. Over the last three decades, sperm quality seems to have declined for no clearly identifiable reason. Theories are running rampant without anyone having the proof to back them up.
Potential Causes
The environment, lifestyle, excess weight or obesity, smoking, alcohol consumption, and psychological stress have all been alternately offered up as potential causes, following low-quality epidemiological studies. Cellphones are not exempt from this list, due to their emission of high-frequency (800-2200 MHz) electromagnetic waves that can be absorbed by the body.
Clinical trials conducted in rats or mice suggest that these waves can affect sperm quality and lead to histological changes to the testicles, bearing in mind that the conditions met in these trials are very far from our day-to-day exposure to electromagnetic waves, mostly via our cellphones.
The same observation can be made about experiments conducted on human sperm in vitro, but changes to the latter caused by electromagnetic waves leave doubts. Observational studies are rare, carried out in small cohorts, and marred by largely conflicting results. Publication bias plays a major role, just as much as the abundance of potential confounding factors does.
Swiss Observational Study
An observational study carried out in Switzerland had the benefit of involving a large cohort of 2886 young men who were representative of the general population. The participants completed an online questionnaire describing their relationship with their cellphone in detail and in qualitative and quantitative terms.
The study was launched in 2005, before cellphone use became so widespread, and this timeline was considered when looking for a link between cellphone exposure and sperm quality. In addition, multiple adjustments were made in the multivariate analyses to account for as many potential confounding factors as possible.
The participants, aged between 18 and 22 years, were recruited during a 3-day period to assess their suitability for military service. Each year, this cohort makes up 97% of the male population in Switzerland in this age range, with the remaining 3% being excluded from the selection process due to disability or chronic illness.
Regardless of the review board’s decision, subjects wishing to take part in the study were given a detailed description of what it involved, a consent form, and two questionnaires. The first focused on the individual directly, asking questions about his health and lifestyle. The second, intended for his parents, dealt with the period before conception.
This recruitment, which took place between September 2005 and November 2018, involved the researchers contacting 106,924 men. Ultimately, only 5.3% of subjects contacted returned the completed documentation. In the end, the study involved 2886 participants (3.1%) who provided all the necessary information, especially the laboratory testing (including a sperm analysis) needed to meet the study objectives. The number of hours spent on a smartphone and how it was used were routinely considered, as was sperm quality (volume, concentration, and total sperm count, as well as sperm mobility and morphology).
Significant Associations
A data analysis using an adjusted linear model revealed a significant association between frequent phone use (> 20 times per day) and lower sperm concentration (in mL) (adjusted β: -0.152, 95% CI -0.316 to 0.011). The same was found for their total concentration in ejaculate (adjusted β: -0.271, 95% CI -0.515 to -0.027).
An adjusted logistic regression analysis estimated that the risk for subnormal male fertility levels, as determined by the World Health Organization (WHO), was increased by at most 30%, when referring to the concentration of sperm per mL (21% in terms of total concentration). This inverse link was shown to be more pronounced during the first phase of the study (2005-2007), compared with the other two phases (2008-2011 and 2012-2018). Yet no links involving sperm mobility or morphology were found, and carrying a cellphone in a trouser pocket had no impact on the results.
This study certainly involves a large cohort of nearly 3000 young men. It is, nonetheless, retrospective, and its methodology, despite being better than that of previous studies, is still open to criticism. Its results can only fuel hypotheses, nothing more. Only prospective cohort studies will allow conclusions to be drawn and, in the meantime,
This article was translated from JIM, which is part of the Medscape professional network. A version of this article appeared on Medscape.com.
1 in 3 women have lasting health problems after giving birth: Study
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
Those problems include pain during sexual intercourse (35%), low back pain (32%), urinary incontinence (8% to 31%), anxiety (9% to 24%), anal incontinence (19%), depression (11% to 17%), fear of childbirth (6% to 15%), perineal pain (11%), and secondary infertility (11%).
Other problems included pelvic organ prolapse, posttraumatic stress disorder, thyroid dysfunction, mastitis, HIV seroconversion (when the body begins to produce detectable levels of HIV antibodies), nerve injury, and psychosis.
The study says most women see a doctor 6 to 12 weeks after birth and then rarely talk to doctors about these nagging health problems. Many of the problems don’t show up until 6 or more weeks after birth.
“To comprehensively address these conditions, broader and more comprehensive health service opportunities are needed, which should extend beyond 6 weeks postpartum and embrace multidisciplinary models of care,” the study says. “This approach can ensure that these conditions are promptly identified and given the attention that they deserve.”
The study is part of a series organized by the United Nation’s Special Program on Human Reproduction, the World Health Organization, and the U.S. Agency for International Development. The authors said most of the data came from high-income nations. There was little data from low-income and middle-income countries except for postpartum depression, anxiety, and psychosis.
“Many postpartum conditions cause considerable suffering in women’s daily life long after birth, both emotionally and physically, and yet they are largely underappreciated, underrecognized, and underreported,” Pascale Allotey, MD, director of Sexual and Reproductive Health and Research at WHO, said in a statement.
“Throughout their lives, and beyond motherhood, women need access to a range of services from health-care providers who listen to their concerns and meet their needs — so they not only survive childbirth but can enjoy good health and quality of life.”
A version of this article appeared on WebMD.com.
FROM THE LANCET GLOBAL HEALTH
MRD status predicts transplant benefit in NPM1-mutated AML
.
This survival benefit did not extend to patients who were MRD-negative after their second induction therapy, Jad Othman, MBBS, reported at the American Society of Hematology annual meeting.
The findings confirm the value of assessing MRD after induction chemotherapy to help identify patients with NPM1-mutated AML in first complete remission who are more likely to benefit from allogeneic transplant, said Dr. Othman, of King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, London, and the University of Sydney, Australia.
Recently, updated European LeukemiaNet recommendations, which stratify patients with AML by favorable, intermediate, and adverse prognoses, now include a revised genetic-risk classification. This classification generally considers NPM1-mutated AML favorable risk. However, having a co-mutation with FLT3-ITD raises the risk to intermediate.
Despite this increased granularity in risk stratification, “it’s still not really clear who should have transplant in first remission with NPM1-mutated AML,” Dr. Othman said. “And there is still significant variation in practice, not just worldwide but even center to center.”
Although accumulating evidence suggests that MRD-negative patients with intermediate-risk AML are unlikely to benefit from allogeneic transplant in first complete remission, the presence of a FLT3-ITD mutation is often considered an indication for transplant, Othman explained. However, most studies supporting this view occurred before the development of sensitive molecular MRD measurement techniques.
The latest findings, from two sequential prospective randomized trials of intensive chemotherapy in adults aged 18-60 years with newly diagnosed AML may help clarify who will probably benefit from transplant and who won’t based on MRD status and relevant molecular features.
The first study (AML17), conducted from 2009 to 2014, selected patients for transplant in first complete remission using a validated risk score that incorporated features including age, sex, and response after therapy. The other (AML19), conducted from 2015 to 2020, selected patients with NPM1-mutated AML for transplant only if they tested positive for MRD in peripheral blood after their second course of treatment, regardless of FLT3-ITD status or other baseline risk factors.
Overall, the current analysis included the 737 patients with NPM1-mutated AML, 348 from AML17 and 389 from AML19, who were in complete remission after two courses of treatment and had an MRD sample at that point.
In AML17, 27% of MRD-positive patients (16 of 60) and 18% of MRD-negative patients (52 of 288) underwent transplant in first complete remission compared with 60% (50 of 83) and 16% (49 of 306), respectively, in AML19.
Among all 737 patients, Dr. Othman and colleagues did not observe an overall survival benefit among those who underwent transplant vs those who did not (hazard ratio [HR], 1.01) or among patients who were MRD-negative (HR, 0.82).
However, patients who were MRD-positive did have a significant survival advantage after transplant (HR, 0.39). In these patients, 3-year overall survival was 61% among those who underwent transplant vs 24% among those who did not.
In MRD-negative patients, transplant in first complete remission did not improve overall survival despite improved relapse-free survival (HR, 0.50). This outcome, Othman explained, probably occurred because most patients who did not undergo transplant and who relapsed were salvaged, with about two thirds undergoing a transplant during their second complete response.
Results in patients with NPM1 FLT3-ITD co-mutation mirrored those in the overall population: MRD-positive patients in first complete remission who underwent transplant demonstrated improved overall survival compared with those without transplant (HR, 0.52), but the overall survival benefit did not extend to MRD-negative patients (HR, 0.80).
The findings show that molecular MRD after induction chemotherapy can identify patients with NPM1-mutated AML who are more likely to benefit from transplant in first remission, Dr. Othman concluded. However, he noted, because only 16% of patients overall were older than 60 years, the results may not be generalizable to older patients.
A version of this article appeared on Medscape.com.
.
This survival benefit did not extend to patients who were MRD-negative after their second induction therapy, Jad Othman, MBBS, reported at the American Society of Hematology annual meeting.
The findings confirm the value of assessing MRD after induction chemotherapy to help identify patients with NPM1-mutated AML in first complete remission who are more likely to benefit from allogeneic transplant, said Dr. Othman, of King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, London, and the University of Sydney, Australia.
Recently, updated European LeukemiaNet recommendations, which stratify patients with AML by favorable, intermediate, and adverse prognoses, now include a revised genetic-risk classification. This classification generally considers NPM1-mutated AML favorable risk. However, having a co-mutation with FLT3-ITD raises the risk to intermediate.
Despite this increased granularity in risk stratification, “it’s still not really clear who should have transplant in first remission with NPM1-mutated AML,” Dr. Othman said. “And there is still significant variation in practice, not just worldwide but even center to center.”
Although accumulating evidence suggests that MRD-negative patients with intermediate-risk AML are unlikely to benefit from allogeneic transplant in first complete remission, the presence of a FLT3-ITD mutation is often considered an indication for transplant, Othman explained. However, most studies supporting this view occurred before the development of sensitive molecular MRD measurement techniques.
The latest findings, from two sequential prospective randomized trials of intensive chemotherapy in adults aged 18-60 years with newly diagnosed AML may help clarify who will probably benefit from transplant and who won’t based on MRD status and relevant molecular features.
The first study (AML17), conducted from 2009 to 2014, selected patients for transplant in first complete remission using a validated risk score that incorporated features including age, sex, and response after therapy. The other (AML19), conducted from 2015 to 2020, selected patients with NPM1-mutated AML for transplant only if they tested positive for MRD in peripheral blood after their second course of treatment, regardless of FLT3-ITD status or other baseline risk factors.
Overall, the current analysis included the 737 patients with NPM1-mutated AML, 348 from AML17 and 389 from AML19, who were in complete remission after two courses of treatment and had an MRD sample at that point.
In AML17, 27% of MRD-positive patients (16 of 60) and 18% of MRD-negative patients (52 of 288) underwent transplant in first complete remission compared with 60% (50 of 83) and 16% (49 of 306), respectively, in AML19.
Among all 737 patients, Dr. Othman and colleagues did not observe an overall survival benefit among those who underwent transplant vs those who did not (hazard ratio [HR], 1.01) or among patients who were MRD-negative (HR, 0.82).
However, patients who were MRD-positive did have a significant survival advantage after transplant (HR, 0.39). In these patients, 3-year overall survival was 61% among those who underwent transplant vs 24% among those who did not.
In MRD-negative patients, transplant in first complete remission did not improve overall survival despite improved relapse-free survival (HR, 0.50). This outcome, Othman explained, probably occurred because most patients who did not undergo transplant and who relapsed were salvaged, with about two thirds undergoing a transplant during their second complete response.
Results in patients with NPM1 FLT3-ITD co-mutation mirrored those in the overall population: MRD-positive patients in first complete remission who underwent transplant demonstrated improved overall survival compared with those without transplant (HR, 0.52), but the overall survival benefit did not extend to MRD-negative patients (HR, 0.80).
The findings show that molecular MRD after induction chemotherapy can identify patients with NPM1-mutated AML who are more likely to benefit from transplant in first remission, Dr. Othman concluded. However, he noted, because only 16% of patients overall were older than 60 years, the results may not be generalizable to older patients.
A version of this article appeared on Medscape.com.
.
This survival benefit did not extend to patients who were MRD-negative after their second induction therapy, Jad Othman, MBBS, reported at the American Society of Hematology annual meeting.
The findings confirm the value of assessing MRD after induction chemotherapy to help identify patients with NPM1-mutated AML in first complete remission who are more likely to benefit from allogeneic transplant, said Dr. Othman, of King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, London, and the University of Sydney, Australia.
Recently, updated European LeukemiaNet recommendations, which stratify patients with AML by favorable, intermediate, and adverse prognoses, now include a revised genetic-risk classification. This classification generally considers NPM1-mutated AML favorable risk. However, having a co-mutation with FLT3-ITD raises the risk to intermediate.
Despite this increased granularity in risk stratification, “it’s still not really clear who should have transplant in first remission with NPM1-mutated AML,” Dr. Othman said. “And there is still significant variation in practice, not just worldwide but even center to center.”
Although accumulating evidence suggests that MRD-negative patients with intermediate-risk AML are unlikely to benefit from allogeneic transplant in first complete remission, the presence of a FLT3-ITD mutation is often considered an indication for transplant, Othman explained. However, most studies supporting this view occurred before the development of sensitive molecular MRD measurement techniques.
The latest findings, from two sequential prospective randomized trials of intensive chemotherapy in adults aged 18-60 years with newly diagnosed AML may help clarify who will probably benefit from transplant and who won’t based on MRD status and relevant molecular features.
The first study (AML17), conducted from 2009 to 2014, selected patients for transplant in first complete remission using a validated risk score that incorporated features including age, sex, and response after therapy. The other (AML19), conducted from 2015 to 2020, selected patients with NPM1-mutated AML for transplant only if they tested positive for MRD in peripheral blood after their second course of treatment, regardless of FLT3-ITD status or other baseline risk factors.
Overall, the current analysis included the 737 patients with NPM1-mutated AML, 348 from AML17 and 389 from AML19, who were in complete remission after two courses of treatment and had an MRD sample at that point.
In AML17, 27% of MRD-positive patients (16 of 60) and 18% of MRD-negative patients (52 of 288) underwent transplant in first complete remission compared with 60% (50 of 83) and 16% (49 of 306), respectively, in AML19.
Among all 737 patients, Dr. Othman and colleagues did not observe an overall survival benefit among those who underwent transplant vs those who did not (hazard ratio [HR], 1.01) or among patients who were MRD-negative (HR, 0.82).
However, patients who were MRD-positive did have a significant survival advantage after transplant (HR, 0.39). In these patients, 3-year overall survival was 61% among those who underwent transplant vs 24% among those who did not.
In MRD-negative patients, transplant in first complete remission did not improve overall survival despite improved relapse-free survival (HR, 0.50). This outcome, Othman explained, probably occurred because most patients who did not undergo transplant and who relapsed were salvaged, with about two thirds undergoing a transplant during their second complete response.
Results in patients with NPM1 FLT3-ITD co-mutation mirrored those in the overall population: MRD-positive patients in first complete remission who underwent transplant demonstrated improved overall survival compared with those without transplant (HR, 0.52), but the overall survival benefit did not extend to MRD-negative patients (HR, 0.80).
The findings show that molecular MRD after induction chemotherapy can identify patients with NPM1-mutated AML who are more likely to benefit from transplant in first remission, Dr. Othman concluded. However, he noted, because only 16% of patients overall were older than 60 years, the results may not be generalizable to older patients.
A version of this article appeared on Medscape.com.
FROM ASH 2023
Federal program offers free COVID, flu at-home tests, treatments
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
Children who are overweight at risk for chronic kidney disease
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE
, with the association, though weaker, still significant among those who do not develop type 2 diabetes or hypertension, in a large cohort study.
METHODOLOGY
- The study included data on 593,660 adolescents aged 16-20, born after January 1, 1975, who had medical assessments as part of mandatory military service in Israel.
- The mean age at study entry was 17.2 and 54.5% were male.
- Early CKD was defined as stage 1 to 2 CKD with moderately or severely increased albuminuria, with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or higher.
- The study excluded those with kidney pathology, albuminuria, hypertension, dysglycemia, or missing blood pressure or BMI data.
- Participants were followed up until early CKD onset, death, the last day insured, or August 23, 2020.
TAKEAWAY
- With a mean follow-up of 13.4 years, 1963 adolescents (0.3%) overall developed early chronic kidney disease. Among males, an increased risk of developing CKD was observed with a high-normal BMI in adolescence (hazard ratio [HR], 1.8); with overweight BMI (HR, 4.0); with mild obesity (HR, 6.7); and severe obesity (HR, 9.4).
- Among females, the increased risk was also observed with high-normal BMI (HR 1.4); overweight (HR, 2.3); mild obesity (HR, 2.7); and severe obesity (HR, 4.3).
- In excluding those who developed diabetes or hypertension, the overall rate of early CKD in the cohort was 0.2%.
- For males without diabetes or hypertension, the adjusted HR for early CKD with high-normal weight was 1.2; for overweight, HR 1.6; for mild obesity, HR 2.2; and for severe obesity, HR 2.7.
- For females without diabetes or hypertension, the corresponding increased risk for early CKD was HR 1.2 for high-normal BMI; HR 1.8 for overweight; 1.5 for mild obesity and 2.3 for severe obesity.
IN PRACTICE
“These findings suggest that adolescent obesity is a major risk factor for early CKD in young adulthood; this underscores the importance of mitigating adolescent obesity rates and managing risk factors for kidney disease in adolescents with high BMI,” the authors report.
“The association was evident even in persons with high-normal BMI in adolescence, was more pronounced in men, and appeared before the age of 30 years,” they say.
“Given the increasing obesity rates among adolescents, our findings are a harbinger of the potentially preventable increasing burden of CKD and subsequent cardiovascular disease.”
SOURCE
The study was conducted by first author Avishai M. Tsur, MD, of the Israel Defense Forces, Medical Corps, Tel Hashomer, Ramat Gan, Israel and Department of Military Medicine, Hebrew University of Jerusalem Faculty of Medicine, Jerusalem, Israel, and colleagues. The study was published online in JAMA Pediatrics.
LIMITATIONS
The study lacked longitudinal data on clinical and lifestyle factors, including stress, diet and physical activity. While adolescents were screened using urine dipstick, a lack of serum creatinine measurements could have missed some adolescents with reduced eGFR at the study entry. The generalizability of the results is limited by the lack of people from West Africa and East Asia in the study population.
DISCLOSURES
Coauthor Josef Coresh, MD, reported receiving grants from the National Institutes of Health outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
Researchers making strides to better understand RA-associated interstitial lung disease
SAN DIEGO — Clinically significant interstitial lung disease (ILD) is believed to occur in 5%-10% of patients with rheumatoid arthritis (RA), but robust data are lacking on how to best predict which patients face the highest risk for RA-associated ILD. However, the results of several studies presented at the American College of Rheumatology annual meeting indicate that researchers are making strides in this field of rheumatologic care.
Adding Genetic Factors Improves ILD Risk Prediction
In the realm of risk stratification, Austin M. Wheeler, MD, a rheumatology fellow at the University of Nebraska Medical Center, Omaha, discussed the development and validation of a combined clinical and genetic risk score for ILD. “There is clear and well documented phenotypic and genetic overlap of ILD with idiopathic pulmonary fibrosis (IPF),” Dr. Wheeler said. “A number of clinical risk factors have been described for RA-ILD, including older age, male sex, smoking history, higher disease activity, and seropositivity. There are also well-documented genetic risk factors for RA-ILD. The MUC5B genetic variant is the strongest risk factor for IPF, and it’s been described in RA-ILD as well.”
A recently published study indicated that a genetic risk score without the MUC5B variant improved predictive ability for IPF and interstitial lung abnormalities better than using the MUC5B variant alone, “but no prior attempts have been made at developing a composite genetic risk score in RA-ILD” using both genetic and clinical risk factors, he said.
For the current study, Dr. Wheeler and colleagues drew from 2,386 participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter, prospective cohort of US veterans with rheumatologist-diagnosed RA and who fulfilled the 1987 ACR classification criteria. The researchers validated ILD through a systematic review of medical records, including clinical diagnosis of ILD plus either imaging or lung biopsy findings, and collected whole genome data that included 12 single nucleotide polymorphisms (SNPs) previously identified to be associated with risk for RA-ILD. They then used a meta-analytic approach to create pooled associations for each of those respective SNPs using data from the VARA registry participants as well as participants from the past study where the SNPs were first identified. “Those pooled associations were what we used for our effects size within the genetic risk score,” which ended up using five of the SNPs, Dr. Wheeler explained. Next, he and his colleagues combined the genetic risk score with clinical risk factors including age, sex, smoking history, disease activity, and rheumatoid factor (RF) positivity to create their combined risk score.
The mean age of the cohort was 70 years, 89% were male, 78% had a smoking history, and 78% were anti–cyclic citrullinated peptide (CCP) antibody positive. Of the 2,386 participants, 224 (9.4%) had RA-ILD. The full composite risk score had the highest area under the receiver operating curve (AUC) of 0.67, compared with an AUC of 0.623 using the clinical factors alone, 0.651 using the clinical factors plus only the MUC5B variant, and 0.654 using the composite score minus only the MUC5B variant. These AUCs show that “the combined risk score performs better than clinical factors even without the inclusion of the MUC5B variant in the score, which is notable because it supports the importance of further investigation into polygenic risk scores in RA-ILD as there is clearly more at play in a patient’s overall genetic risk,” Dr. Wheeler said.
As an example of the composite score’s ability to discriminate between people with and without RA-ILD, a cutpoint of 0.05 gave a sensitivity of 90.2% and would have eliminated about 25% of the cohort from unnecessary high-resolution CT scans and pulmonary function tests, he said.
“This study demonstrates the potential utility of genetic risk scores in RA-ILD identification and supports further investigation into individual risk stratification and screening,” he concluded. “This isn’t ready for clinical applicability by any means, but I think it serves as a proof of concept of the idea of a genetic risk score in RA-ILD.”
Biomarker Score Investigated
In a separate abstract, Brent Luedders, MD, assistant professor of rheumatology and immunology at the University of Nebraska Medical Center, and colleagues set out to determine if a previously derived biomarker score is associated with prevalent and incident ILD in the same VARA Registry cohort. An abstract presented at the ACR 2022 annual meeting found that a panel derived from IPF peripheral biomarkers was significantly associated with RA-ILD, including matrix metalloproteinase (MMP)-2, -7, and -9, eotaxin, macrophage-derived chemokine (MDC), monocyte chemoattractant protein-1 (MCP-1), fms-like tyrosine kinase 3 ligand (Flt3L) and interleukin-8 (IL-8). For the current analysis, Dr. Luedders and colleagues measured the concentrations of seven biomarkers (MMP-7, MMP-9, eotaxin, MDC, MCP-1, Flt3L, IL-8) from serum/plasma samples collected from VARA’s participants at enrollment to develop a score based on the concentrations of each biomarker.
Baseline characteristics were similar between the groups, although those with prevalent RA-ILD were slightly older than those without ILD, and those who developed incident ILD during follow-up had slightly higher RA disease activity at the time of enrollment. When the researchers examined the association of the biomarker score with prevalent RA-ILD as a continuous measure, they found an adjusted OR of 1.08 for prevalent RA-ILD for each 1-point increase in the biomarker score. “When this was divided into quartiles, we found that the highest quartile of the biomarker score was associated with an adjusted odds ratio of 2.31 for prevalent RA-ILD,” Dr. Luedders said. “We saw a significant P for trend of < .001, suggesting a dose-response relationship, in which higher scores had higher risk.” Similar associations were observed for incident RA-ILD, in which participants with the highest quartile had an adjusted hazard ratio of 2.26 for incident RA-ILD.
The AUC of 0.653 that was obtained with clinical factors did not significantly improve with inclusion of the biomarker score, rising to only 0.669. “In receiver operating characteristic analysis, the addition of the biomarker score to clinical variables (age, sex, race, smoking status, anti-CCP positivity, and RA disease activity by DAS28) did not lead to a significant increase in the area under the curve. Therefore, further work is needed to identify combinations of clinical, biomarker, and other factors to accurately predict which people with RA will develop ILD,” he said.
Dr. Luedders acknowledged certain limitations of the results, including the fact that MMP-2 was not measured in this cohort and thus not included in the score. “This was an observational study with usual care; therefore, the absence of systemic evaluation for ILD may miss early or mild RA-ILD cases,” he added. “Similarly, a male predominance may limit the generalizability, and we have limited information on the RA-ILD pattern.” He concluded that the study results “support the shared pathogenesis of IPF and RA-ILD. However, we found that this score has limited discriminative performance, compared to clinical risk factors alone.”
Drilling Down on ILD Subtypes
In a poster abstract presentation at the meeting, Gregory Campbell McDermott, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, highlighted results from a study that investigated differences in demographic, serologic, and lifestyle factors for RA-ILD and the major subtypes of RA-ILD: usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). “Historically, RA-ILD has been studied as a single entity, even though we increasingly recognized that there are lots of different subtypes that fall under the umbrella of RA-ILD,” Dr. McDermott said in an interview. “We are also learning that the different subtypes probably have both prognostic and potentially therapeutic implications. For example, the UIP subtype, which is the most fibrotic subtype, has the worst prognosis but also may be a potential target for antifibrotic therapies. We’ve been trying to see if we can identify factors that are associated with specific subtypes, in particular the UIP subtype which has the worst prognosis.”
He and his colleagues examined 208 patients with RA-ILD with a mean age of 51 years and 547 patients with RA but no ILD with a mean age of 49 years from two RA cohorts comprising 3,328 patients: the Mass General Brigham Biobank RA Cohort and the Brigham RA Sequential Study (BRASS). Of the 208 RA-ILD cases, nearly half (48%) were RA-UIP, 18% were RA-NSIP, 8% were organizing pneumonia, 3% were respiratory bronchiolitis-ILD, and 23% were other/indeterminate. After conducting multivariable adjusted analyses, the researchers found that RA-ILD was associated with male sex (OR, 1.58; 95% CI, 1.09-2.23), seropositivity for RF and/or anti-CCP (OR, 2.22; 95% CI, 1.51-3.24) and being an ever smoker (OR, 1.70; 95% CI, 1.13-2.54). Having all three of these risk factors was strongly associated with RA-ILD (OR, 6.04; 95% CI, 2.92-12.47) and with RA-UIP in particular (OR, 7.1). “We found that a lot of the traditional RA-ILD risk factors like male sex, history of smoking, and seropositive status were most strongly associated with a UIP pattern,” Dr. McDermott said. “We think this is a first step in trying to understand how these different ILD subtypes may have different risk factors, pathogenesis, and potentially different treatments, prevention, and screening strategies.”
While clinicians wait for guidelines on systemic autoimmune rheumatic disease-associated ILD that are expected to be published by the ACR in 2024, he added that “we probably shouldn’t screen every single person with RA for ILD, but we need to identify people who have symptoms or findings on clinical exam. This study wasn’t designed to look specifically at who is at high risk, but I think we are moving toward that question: Who is high risk, and who’s asymptomatic [but] may need more screening?”
He pointed out limitations of the study, including its retrospective design and the fact that imaging was done for clinical purposes, “so it’s probably a higher risk group to begin with than the whole RA population,” he said. “We also didn’t have data on RA disease activity or erosions, some of these other measures that we think are important for understanding the full RA disease phenotype in these patients.”
Dr. Wheeler reported having no disclosures. Dr. Luedders reported that his study was supported by the VA, the Rheumatology Research Foundation, and the University of Nebraska Medical Center Mentored Scholars Program. Dr. McDermott reported that his study was supported by the Rheumatology Research Foundation.
SAN DIEGO — Clinically significant interstitial lung disease (ILD) is believed to occur in 5%-10% of patients with rheumatoid arthritis (RA), but robust data are lacking on how to best predict which patients face the highest risk for RA-associated ILD. However, the results of several studies presented at the American College of Rheumatology annual meeting indicate that researchers are making strides in this field of rheumatologic care.
Adding Genetic Factors Improves ILD Risk Prediction
In the realm of risk stratification, Austin M. Wheeler, MD, a rheumatology fellow at the University of Nebraska Medical Center, Omaha, discussed the development and validation of a combined clinical and genetic risk score for ILD. “There is clear and well documented phenotypic and genetic overlap of ILD with idiopathic pulmonary fibrosis (IPF),” Dr. Wheeler said. “A number of clinical risk factors have been described for RA-ILD, including older age, male sex, smoking history, higher disease activity, and seropositivity. There are also well-documented genetic risk factors for RA-ILD. The MUC5B genetic variant is the strongest risk factor for IPF, and it’s been described in RA-ILD as well.”
A recently published study indicated that a genetic risk score without the MUC5B variant improved predictive ability for IPF and interstitial lung abnormalities better than using the MUC5B variant alone, “but no prior attempts have been made at developing a composite genetic risk score in RA-ILD” using both genetic and clinical risk factors, he said.
For the current study, Dr. Wheeler and colleagues drew from 2,386 participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter, prospective cohort of US veterans with rheumatologist-diagnosed RA and who fulfilled the 1987 ACR classification criteria. The researchers validated ILD through a systematic review of medical records, including clinical diagnosis of ILD plus either imaging or lung biopsy findings, and collected whole genome data that included 12 single nucleotide polymorphisms (SNPs) previously identified to be associated with risk for RA-ILD. They then used a meta-analytic approach to create pooled associations for each of those respective SNPs using data from the VARA registry participants as well as participants from the past study where the SNPs were first identified. “Those pooled associations were what we used for our effects size within the genetic risk score,” which ended up using five of the SNPs, Dr. Wheeler explained. Next, he and his colleagues combined the genetic risk score with clinical risk factors including age, sex, smoking history, disease activity, and rheumatoid factor (RF) positivity to create their combined risk score.
The mean age of the cohort was 70 years, 89% were male, 78% had a smoking history, and 78% were anti–cyclic citrullinated peptide (CCP) antibody positive. Of the 2,386 participants, 224 (9.4%) had RA-ILD. The full composite risk score had the highest area under the receiver operating curve (AUC) of 0.67, compared with an AUC of 0.623 using the clinical factors alone, 0.651 using the clinical factors plus only the MUC5B variant, and 0.654 using the composite score minus only the MUC5B variant. These AUCs show that “the combined risk score performs better than clinical factors even without the inclusion of the MUC5B variant in the score, which is notable because it supports the importance of further investigation into polygenic risk scores in RA-ILD as there is clearly more at play in a patient’s overall genetic risk,” Dr. Wheeler said.
As an example of the composite score’s ability to discriminate between people with and without RA-ILD, a cutpoint of 0.05 gave a sensitivity of 90.2% and would have eliminated about 25% of the cohort from unnecessary high-resolution CT scans and pulmonary function tests, he said.
“This study demonstrates the potential utility of genetic risk scores in RA-ILD identification and supports further investigation into individual risk stratification and screening,” he concluded. “This isn’t ready for clinical applicability by any means, but I think it serves as a proof of concept of the idea of a genetic risk score in RA-ILD.”
Biomarker Score Investigated
In a separate abstract, Brent Luedders, MD, assistant professor of rheumatology and immunology at the University of Nebraska Medical Center, and colleagues set out to determine if a previously derived biomarker score is associated with prevalent and incident ILD in the same VARA Registry cohort. An abstract presented at the ACR 2022 annual meeting found that a panel derived from IPF peripheral biomarkers was significantly associated with RA-ILD, including matrix metalloproteinase (MMP)-2, -7, and -9, eotaxin, macrophage-derived chemokine (MDC), monocyte chemoattractant protein-1 (MCP-1), fms-like tyrosine kinase 3 ligand (Flt3L) and interleukin-8 (IL-8). For the current analysis, Dr. Luedders and colleagues measured the concentrations of seven biomarkers (MMP-7, MMP-9, eotaxin, MDC, MCP-1, Flt3L, IL-8) from serum/plasma samples collected from VARA’s participants at enrollment to develop a score based on the concentrations of each biomarker.
Baseline characteristics were similar between the groups, although those with prevalent RA-ILD were slightly older than those without ILD, and those who developed incident ILD during follow-up had slightly higher RA disease activity at the time of enrollment. When the researchers examined the association of the biomarker score with prevalent RA-ILD as a continuous measure, they found an adjusted OR of 1.08 for prevalent RA-ILD for each 1-point increase in the biomarker score. “When this was divided into quartiles, we found that the highest quartile of the biomarker score was associated with an adjusted odds ratio of 2.31 for prevalent RA-ILD,” Dr. Luedders said. “We saw a significant P for trend of < .001, suggesting a dose-response relationship, in which higher scores had higher risk.” Similar associations were observed for incident RA-ILD, in which participants with the highest quartile had an adjusted hazard ratio of 2.26 for incident RA-ILD.
The AUC of 0.653 that was obtained with clinical factors did not significantly improve with inclusion of the biomarker score, rising to only 0.669. “In receiver operating characteristic analysis, the addition of the biomarker score to clinical variables (age, sex, race, smoking status, anti-CCP positivity, and RA disease activity by DAS28) did not lead to a significant increase in the area under the curve. Therefore, further work is needed to identify combinations of clinical, biomarker, and other factors to accurately predict which people with RA will develop ILD,” he said.
Dr. Luedders acknowledged certain limitations of the results, including the fact that MMP-2 was not measured in this cohort and thus not included in the score. “This was an observational study with usual care; therefore, the absence of systemic evaluation for ILD may miss early or mild RA-ILD cases,” he added. “Similarly, a male predominance may limit the generalizability, and we have limited information on the RA-ILD pattern.” He concluded that the study results “support the shared pathogenesis of IPF and RA-ILD. However, we found that this score has limited discriminative performance, compared to clinical risk factors alone.”
Drilling Down on ILD Subtypes
In a poster abstract presentation at the meeting, Gregory Campbell McDermott, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, highlighted results from a study that investigated differences in demographic, serologic, and lifestyle factors for RA-ILD and the major subtypes of RA-ILD: usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). “Historically, RA-ILD has been studied as a single entity, even though we increasingly recognized that there are lots of different subtypes that fall under the umbrella of RA-ILD,” Dr. McDermott said in an interview. “We are also learning that the different subtypes probably have both prognostic and potentially therapeutic implications. For example, the UIP subtype, which is the most fibrotic subtype, has the worst prognosis but also may be a potential target for antifibrotic therapies. We’ve been trying to see if we can identify factors that are associated with specific subtypes, in particular the UIP subtype which has the worst prognosis.”
He and his colleagues examined 208 patients with RA-ILD with a mean age of 51 years and 547 patients with RA but no ILD with a mean age of 49 years from two RA cohorts comprising 3,328 patients: the Mass General Brigham Biobank RA Cohort and the Brigham RA Sequential Study (BRASS). Of the 208 RA-ILD cases, nearly half (48%) were RA-UIP, 18% were RA-NSIP, 8% were organizing pneumonia, 3% were respiratory bronchiolitis-ILD, and 23% were other/indeterminate. After conducting multivariable adjusted analyses, the researchers found that RA-ILD was associated with male sex (OR, 1.58; 95% CI, 1.09-2.23), seropositivity for RF and/or anti-CCP (OR, 2.22; 95% CI, 1.51-3.24) and being an ever smoker (OR, 1.70; 95% CI, 1.13-2.54). Having all three of these risk factors was strongly associated with RA-ILD (OR, 6.04; 95% CI, 2.92-12.47) and with RA-UIP in particular (OR, 7.1). “We found that a lot of the traditional RA-ILD risk factors like male sex, history of smoking, and seropositive status were most strongly associated with a UIP pattern,” Dr. McDermott said. “We think this is a first step in trying to understand how these different ILD subtypes may have different risk factors, pathogenesis, and potentially different treatments, prevention, and screening strategies.”
While clinicians wait for guidelines on systemic autoimmune rheumatic disease-associated ILD that are expected to be published by the ACR in 2024, he added that “we probably shouldn’t screen every single person with RA for ILD, but we need to identify people who have symptoms or findings on clinical exam. This study wasn’t designed to look specifically at who is at high risk, but I think we are moving toward that question: Who is high risk, and who’s asymptomatic [but] may need more screening?”
He pointed out limitations of the study, including its retrospective design and the fact that imaging was done for clinical purposes, “so it’s probably a higher risk group to begin with than the whole RA population,” he said. “We also didn’t have data on RA disease activity or erosions, some of these other measures that we think are important for understanding the full RA disease phenotype in these patients.”
Dr. Wheeler reported having no disclosures. Dr. Luedders reported that his study was supported by the VA, the Rheumatology Research Foundation, and the University of Nebraska Medical Center Mentored Scholars Program. Dr. McDermott reported that his study was supported by the Rheumatology Research Foundation.
SAN DIEGO — Clinically significant interstitial lung disease (ILD) is believed to occur in 5%-10% of patients with rheumatoid arthritis (RA), but robust data are lacking on how to best predict which patients face the highest risk for RA-associated ILD. However, the results of several studies presented at the American College of Rheumatology annual meeting indicate that researchers are making strides in this field of rheumatologic care.
Adding Genetic Factors Improves ILD Risk Prediction
In the realm of risk stratification, Austin M. Wheeler, MD, a rheumatology fellow at the University of Nebraska Medical Center, Omaha, discussed the development and validation of a combined clinical and genetic risk score for ILD. “There is clear and well documented phenotypic and genetic overlap of ILD with idiopathic pulmonary fibrosis (IPF),” Dr. Wheeler said. “A number of clinical risk factors have been described for RA-ILD, including older age, male sex, smoking history, higher disease activity, and seropositivity. There are also well-documented genetic risk factors for RA-ILD. The MUC5B genetic variant is the strongest risk factor for IPF, and it’s been described in RA-ILD as well.”
A recently published study indicated that a genetic risk score without the MUC5B variant improved predictive ability for IPF and interstitial lung abnormalities better than using the MUC5B variant alone, “but no prior attempts have been made at developing a composite genetic risk score in RA-ILD” using both genetic and clinical risk factors, he said.
For the current study, Dr. Wheeler and colleagues drew from 2,386 participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter, prospective cohort of US veterans with rheumatologist-diagnosed RA and who fulfilled the 1987 ACR classification criteria. The researchers validated ILD through a systematic review of medical records, including clinical diagnosis of ILD plus either imaging or lung biopsy findings, and collected whole genome data that included 12 single nucleotide polymorphisms (SNPs) previously identified to be associated with risk for RA-ILD. They then used a meta-analytic approach to create pooled associations for each of those respective SNPs using data from the VARA registry participants as well as participants from the past study where the SNPs were first identified. “Those pooled associations were what we used for our effects size within the genetic risk score,” which ended up using five of the SNPs, Dr. Wheeler explained. Next, he and his colleagues combined the genetic risk score with clinical risk factors including age, sex, smoking history, disease activity, and rheumatoid factor (RF) positivity to create their combined risk score.
The mean age of the cohort was 70 years, 89% were male, 78% had a smoking history, and 78% were anti–cyclic citrullinated peptide (CCP) antibody positive. Of the 2,386 participants, 224 (9.4%) had RA-ILD. The full composite risk score had the highest area under the receiver operating curve (AUC) of 0.67, compared with an AUC of 0.623 using the clinical factors alone, 0.651 using the clinical factors plus only the MUC5B variant, and 0.654 using the composite score minus only the MUC5B variant. These AUCs show that “the combined risk score performs better than clinical factors even without the inclusion of the MUC5B variant in the score, which is notable because it supports the importance of further investigation into polygenic risk scores in RA-ILD as there is clearly more at play in a patient’s overall genetic risk,” Dr. Wheeler said.
As an example of the composite score’s ability to discriminate between people with and without RA-ILD, a cutpoint of 0.05 gave a sensitivity of 90.2% and would have eliminated about 25% of the cohort from unnecessary high-resolution CT scans and pulmonary function tests, he said.
“This study demonstrates the potential utility of genetic risk scores in RA-ILD identification and supports further investigation into individual risk stratification and screening,” he concluded. “This isn’t ready for clinical applicability by any means, but I think it serves as a proof of concept of the idea of a genetic risk score in RA-ILD.”
Biomarker Score Investigated
In a separate abstract, Brent Luedders, MD, assistant professor of rheumatology and immunology at the University of Nebraska Medical Center, and colleagues set out to determine if a previously derived biomarker score is associated with prevalent and incident ILD in the same VARA Registry cohort. An abstract presented at the ACR 2022 annual meeting found that a panel derived from IPF peripheral biomarkers was significantly associated with RA-ILD, including matrix metalloproteinase (MMP)-2, -7, and -9, eotaxin, macrophage-derived chemokine (MDC), monocyte chemoattractant protein-1 (MCP-1), fms-like tyrosine kinase 3 ligand (Flt3L) and interleukin-8 (IL-8). For the current analysis, Dr. Luedders and colleagues measured the concentrations of seven biomarkers (MMP-7, MMP-9, eotaxin, MDC, MCP-1, Flt3L, IL-8) from serum/plasma samples collected from VARA’s participants at enrollment to develop a score based on the concentrations of each biomarker.
Baseline characteristics were similar between the groups, although those with prevalent RA-ILD were slightly older than those without ILD, and those who developed incident ILD during follow-up had slightly higher RA disease activity at the time of enrollment. When the researchers examined the association of the biomarker score with prevalent RA-ILD as a continuous measure, they found an adjusted OR of 1.08 for prevalent RA-ILD for each 1-point increase in the biomarker score. “When this was divided into quartiles, we found that the highest quartile of the biomarker score was associated with an adjusted odds ratio of 2.31 for prevalent RA-ILD,” Dr. Luedders said. “We saw a significant P for trend of < .001, suggesting a dose-response relationship, in which higher scores had higher risk.” Similar associations were observed for incident RA-ILD, in which participants with the highest quartile had an adjusted hazard ratio of 2.26 for incident RA-ILD.
The AUC of 0.653 that was obtained with clinical factors did not significantly improve with inclusion of the biomarker score, rising to only 0.669. “In receiver operating characteristic analysis, the addition of the biomarker score to clinical variables (age, sex, race, smoking status, anti-CCP positivity, and RA disease activity by DAS28) did not lead to a significant increase in the area under the curve. Therefore, further work is needed to identify combinations of clinical, biomarker, and other factors to accurately predict which people with RA will develop ILD,” he said.
Dr. Luedders acknowledged certain limitations of the results, including the fact that MMP-2 was not measured in this cohort and thus not included in the score. “This was an observational study with usual care; therefore, the absence of systemic evaluation for ILD may miss early or mild RA-ILD cases,” he added. “Similarly, a male predominance may limit the generalizability, and we have limited information on the RA-ILD pattern.” He concluded that the study results “support the shared pathogenesis of IPF and RA-ILD. However, we found that this score has limited discriminative performance, compared to clinical risk factors alone.”
Drilling Down on ILD Subtypes
In a poster abstract presentation at the meeting, Gregory Campbell McDermott, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, highlighted results from a study that investigated differences in demographic, serologic, and lifestyle factors for RA-ILD and the major subtypes of RA-ILD: usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). “Historically, RA-ILD has been studied as a single entity, even though we increasingly recognized that there are lots of different subtypes that fall under the umbrella of RA-ILD,” Dr. McDermott said in an interview. “We are also learning that the different subtypes probably have both prognostic and potentially therapeutic implications. For example, the UIP subtype, which is the most fibrotic subtype, has the worst prognosis but also may be a potential target for antifibrotic therapies. We’ve been trying to see if we can identify factors that are associated with specific subtypes, in particular the UIP subtype which has the worst prognosis.”
He and his colleagues examined 208 patients with RA-ILD with a mean age of 51 years and 547 patients with RA but no ILD with a mean age of 49 years from two RA cohorts comprising 3,328 patients: the Mass General Brigham Biobank RA Cohort and the Brigham RA Sequential Study (BRASS). Of the 208 RA-ILD cases, nearly half (48%) were RA-UIP, 18% were RA-NSIP, 8% were organizing pneumonia, 3% were respiratory bronchiolitis-ILD, and 23% were other/indeterminate. After conducting multivariable adjusted analyses, the researchers found that RA-ILD was associated with male sex (OR, 1.58; 95% CI, 1.09-2.23), seropositivity for RF and/or anti-CCP (OR, 2.22; 95% CI, 1.51-3.24) and being an ever smoker (OR, 1.70; 95% CI, 1.13-2.54). Having all three of these risk factors was strongly associated with RA-ILD (OR, 6.04; 95% CI, 2.92-12.47) and with RA-UIP in particular (OR, 7.1). “We found that a lot of the traditional RA-ILD risk factors like male sex, history of smoking, and seropositive status were most strongly associated with a UIP pattern,” Dr. McDermott said. “We think this is a first step in trying to understand how these different ILD subtypes may have different risk factors, pathogenesis, and potentially different treatments, prevention, and screening strategies.”
While clinicians wait for guidelines on systemic autoimmune rheumatic disease-associated ILD that are expected to be published by the ACR in 2024, he added that “we probably shouldn’t screen every single person with RA for ILD, but we need to identify people who have symptoms or findings on clinical exam. This study wasn’t designed to look specifically at who is at high risk, but I think we are moving toward that question: Who is high risk, and who’s asymptomatic [but] may need more screening?”
He pointed out limitations of the study, including its retrospective design and the fact that imaging was done for clinical purposes, “so it’s probably a higher risk group to begin with than the whole RA population,” he said. “We also didn’t have data on RA disease activity or erosions, some of these other measures that we think are important for understanding the full RA disease phenotype in these patients.”
Dr. Wheeler reported having no disclosures. Dr. Luedders reported that his study was supported by the VA, the Rheumatology Research Foundation, and the University of Nebraska Medical Center Mentored Scholars Program. Dr. McDermott reported that his study was supported by the Rheumatology Research Foundation.
FROM ACR 2023
New KDIGO guideline encourages use of HCV-positive kidneys for HCV-negative recipients
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
The Kidney Disease: Improving Global Outcomes (KDIGO) Work Group has updated its guideline concerning the prevention, diagnosis, evaluation, and treatment of hepatitis C virus (HCV) infection in patients with chronic kidney disease (CKD).
Of note, KDIGO now supports transplant of HCV-positive kidneys to HCV-negative recipients.
The guidance document, authored by Ahmed Arslan Yousuf Awan, MD, of Baylor College of Medicine, Houston, and colleagues, was written in light of new evidence that has emerged since the 2018 guideline was published.
“The focused update was triggered by new data on antiviral treatment in patients with advanced stages of CKD (G4, G5, or G5D), transplant of HCV-infected kidneys into uninfected recipients, and evolution of the viewpoint on the role of kidney biopsy in managing kidney disease caused by HCV,” the guideline panelists wrote in Annals of Internal Medicine. “This update is intended to assist clinicians in the care of patients with HCV infection and CKD, including patients receiving dialysis (CKD G5D) and patients with a kidney transplant (CKD G1T-G5T).”
Anjay Rastogi, MD, PhD, professor and clinical chief of nephrology at the David Geffen School of Medicine at UCLA, said the update is both “timely and relevant,” and “will really have an impact on the organ shortage that we have for kidney transplant”
The updates are outlined below.
Expanded Access to HCV-Positive Kidneys
While the 2018 guideline recommended that HCV-positive kidneys be directed to HCV-positive recipients, the new guideline suggests that these kidneys are appropriate for all patients regardless of HCV status.
In support, the panelists cited a follow-up of THINKER-1 trial, which showed that eGFR and quality of life were not negatively affected when HCV-negative patients received an HCV-positive kidney, compared with an HCV-negative kidney. Data from 525 unmatched recipients in 16 other studies support this conclusion, the panelists noted.
Jose Debes, MD, PhD, associate professor at the University of Minnesota, Minneapolis, suggested that this is the most important update to the KDIGO guidelines.
“That [change] would be the main impact of these recommendations,” Dr. Debes said in an interview. “Several centers were already doing this, since some data [were] out there, but I think the fact that they’re making this into a guideline is quite important.”
Dr. Rastogi agreed that this recommendation is the most impactful update.
“That’s a big move,” Dr. Rastogi said in an interview. He predicted that the change will “definitely increase the donor pool, which is very, very important.”
For this new recommendation to have the greatest positive effect, however, Dr. Rastogi suggested that health care providers and treatment centers need to prepare an effective implementation strategy. He emphasized the importance of early communication with patients concerning the safety of HCV-positive kidneys, which depends on early initiation of direct-acting antiviral (DAA) therapy.
In the guideline, Dr. Awan and colleagues reported three documented cases of fibrosing cholestatic hepatitis occurred in patients who did not begin DAA therapy until 30 days after transplant.
“[Patients] should start [DAA treatment] right away,” Dr. Rastogi said, “and sometimes even before the transplant.”
This will require institutional support, he noted, as centers need to ensure that patients are covered for DAA therapy and medication is readily available.
Sofosbuvir Given the Green Light
Compared with the 2018 guideline, which recommended against sofosbuvir in patients with CKD G4 and G5, including those on dialysis, because of concerns about metabolization via the kidneys, the new guideline suggests that sofosbuvir-based DAA regimens are appropriate in patients with glomerular filtration rate (GFR) less than 30 mL/min per 1.73 m2, including those receiving dialysis.
This recommendation was based on a systematic review of 106 studies including both sofosbuvir-based and non-sofosbuvir-based DAA regimens that showed high safety and efficacy for all DAA regimen types across a broad variety of patient types.
“DAAs are highly effective and well tolerated treatments for hepatitis C in patients across all stages of CKD, including those undergoing dialysis and kidney transplant recipients, with no need for dose adjustment,” Dr. Awan and colleagues wrote.
Loosened Biopsy Requirements
Unlike the 2018 guideline, which advised kidney biopsy in HCV-positive patients with clinical evidence of glomerular disease prior to initiating DAA treatment, the new guideline suggests that HCV-infected patients with a typical presentation of immune-complex proliferative glomerulonephritis do not require confirmatory kidney biopsy.
“Because almost all patients with chronic hepatitis C (with or without glomerulonephritis) should be treated with DAAs, a kidney biopsy is unlikely to change management in most patients with hepatitis C and clinical glomerulonephritis,” the panelists wrote.
If kidney disease does not stabilize or improve with achievement of sustained virologic response, or if there is evidence of rapidly progressive glomerulonephritis, then a kidney biopsy should be considered before beginning immunosuppressive therapy, according to the guideline, which includes a flow chart to guide clinicians through this decision-making process.
Individualizing Immunosuppressive Therapy
Consistent with the old guideline, the new guideline recommends DAA treatment with concurrent immunosuppressive therapy for patients with cryoglobulinemic flare or rapidly progressive kidney failure. But in contrast, the new guideline calls for an individualized approach to immunosuppression in patients with nephrotic syndrome.
Dr. Awan and colleagues suggested that “nephrotic-range proteinuria (greater than 3.5 g/d) alone does not warrant use of immunosuppressive treatment because such patients can achieve remission of proteinuria after treatment with DAAs.” Still, if other associated complications — such as anasarca, thromboembolic disease, or severe hypoalbuminemia — are present, then immunosuppressive therapy may be warranted, with rituximab remaining the preferred first-line agent.
More Work Is Needed
Dr. Awan and colleagues concluded the guideline by highlighting areas of unmet need, and how filling these knowledge gaps could lead to additional guideline updates.
“Future studies of kidney donations from HCV-positive donors to HCV-negative recipients are needed to refine and clarify the timing of initiation and duration of DAA therapy and to assess long-term outcomes associated with this practice,” they wrote. “Also, randomized controlled trials are needed to determine which patients with HCV-associated kidney disease can be treated with DAA therapy alone versus in combination with immunosuppression and plasma exchange. KDIGO will assess the currency of its recommendations and the need to update them in the next 3 years.”
The guideline was funded by KDIGO. The investigators disclosed relationships with GSK, Gilead, Intercept, Novo Nordisk, and others. Dr. Rastogi and Dr. Debes had no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Technology for primary care — terrific, terrifying, or both?
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
Large Indurated Plaque on the Chest With Ulceration and Necrosis
The Diagnosis: Carcinoma en Cuirasse
Histopathology demonstrated a cellular infiltrate filling the dermis with sparing of the papillary and superficial reticular dermis (Figure 1A). The cells were arranged in strands and cords that infiltrated between sclerotic collagen bundles. Cytomorphologically, the cells ranged from epithelioid with large vesicular nuclei and prominent nucleoli to cuboidal with hyperchromatic nuclei with irregular contours and a high nuclear to cytoplasmic ratio (Figure 1B). Occasional mitotic figures were identified, and cells demonstrated diffuse nuclear positivity for GATA-3 (Figure 1C); 55% of the cells demonstrated estrogen receptor positivity, and immunohistochemistry of progesterone receptors was negative. These findings confirmed our patient’s diagnosis of breast carcinoma en cuirasse (CeC) as the primary manifestation of metastatic invasive ductal carcinoma. Our patient was treated with intravenous chemotherapy and tamoxifen.
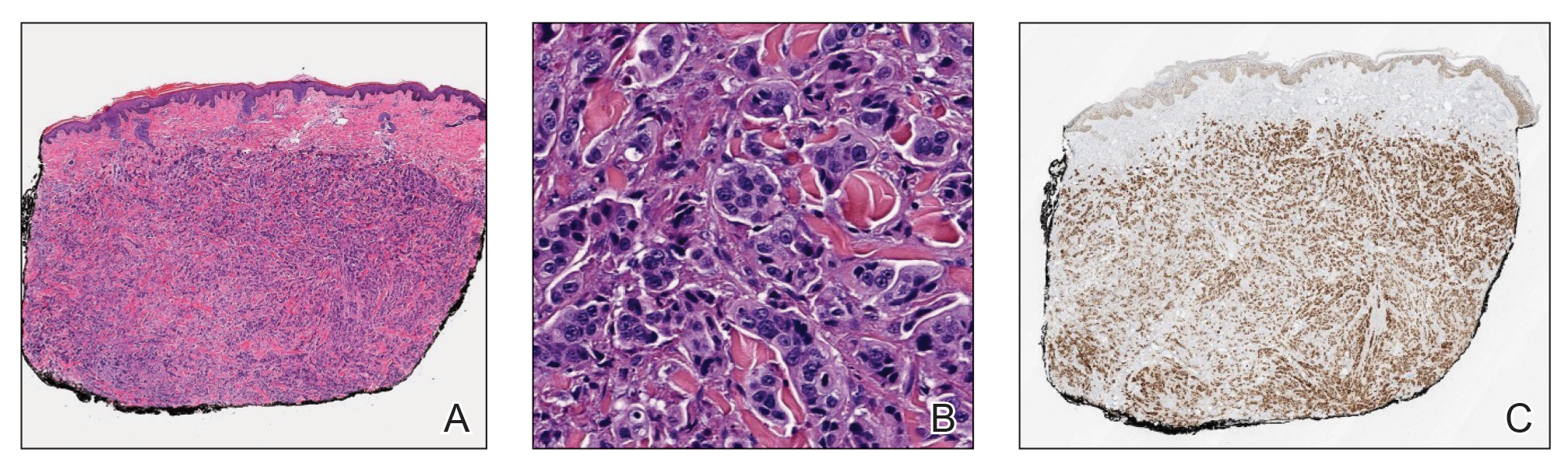
Histopathologic findings of morphea include thickened hyalinized collagen bundles and loss of adventitial fat.1 A diagnosis of chronic radiation dermatitis was inconsistent with our patient’s medical history and biopsy results, as pathology should reveal hyalinized collagen or stellate radiation fibroblasts.2,3 Nests of squamous epithelial cells with abundant eosinophilic cytoplasm and large vesicular nuclei were not seen, excluding squamous cell carcinoma as a possible diagnosis.4 Although sclerosing sweat duct carcinoma is characterized by infiltrating cords in sclerotic dermis, the cells were not arranged in ductlike structures 1– to 2–cell layers thick, excluding this diagnosis.5
Carcinoma en cuirasse—named for skin involvement that appears similar to the metal breastplate of a cuirassier—is a rare form of cutaneous metastasis that typically presents with extensive infiltrative plaques resulting in fibrosis of the skin and subcutaneous tissue.6,7 Carcinoma en cuirasse most commonly metastasizes from the breast but also may represent metastases from the lungs, gastrointestinal tract, or genitourinary systems.8 In the setting of a primary breast malignancy, metastatic plaques of CeC tend to represent tumor recurrence following a mastectomy procedure; however, in rare cases CeC can present as the primary manifestation of breast cancer or as a result of untreated malignancy.6,9 In our patient, CeC was the primary manifestation of metastatic invasive ductal carcinoma with additional paraneoplastic ichthyosis (Figure 2).
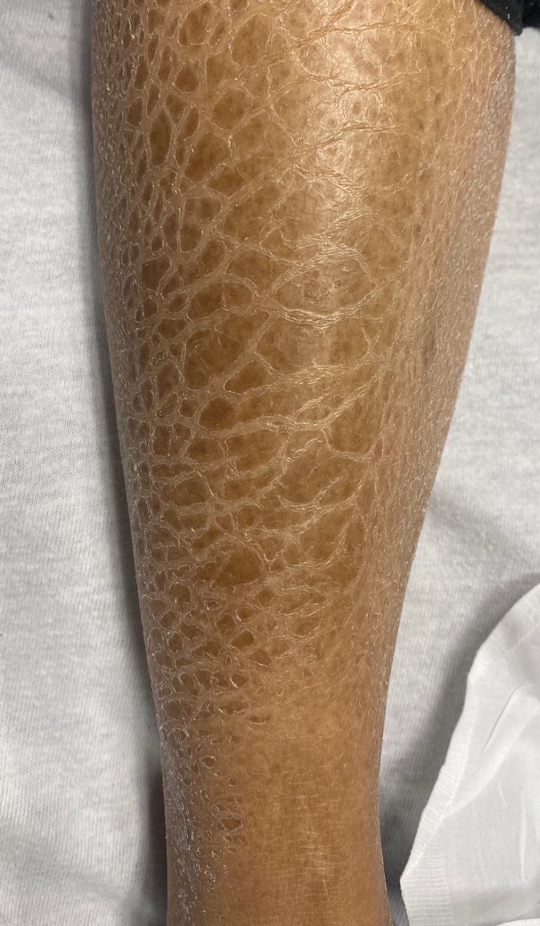
Carcinoma en cuirasse comprises 3% to 6% of cutaneous metastases originating from the breast.10,11 Breast cancer is the most common primary neoplasm displaying extracutaneous metastasis, comprising 70% of all cutaneous metastases in females.11 Cutaneous metastasis often indicates late stage of disease, portending a poor prognosis. In our patient, the cutaneous nodules were present for approximately 3 years prior to the diagnosis of stage IV invasive ductal cell carcinoma with metastasis to the skin and lungs. Prior to admission, she had not been diagnosed with breast cancer, thus no treatments had been administered. It is uncommon for CeC to present as the initial finding and without prior treatment of the underlying malignancy. The median length of survival after diagnosis of cutaneous metastasis from breast cancer is 13.8 months, with a 10-year survival rate of 3.1%.12
In addition to cutaneous metastasis, breast cancer also may present with paraneoplastic dermatoses such as ichthyosis.13 Ichthyosis is characterized by extreme dryness, flaking, thickening, and mild pruritus.14 It most commonly is an inherited condition, but it may be acquired due to malignancy. Acquired ichthyosis may manifest in systemic diseases including systemic lupus erythematosus, sarcoidosis, and hypothyroidism.15 Although acquired ichthyosis is rare, it has been reported in cases of internal malignancy, most commonly lymphoproliferative malignancies and less frequently carcinoma of the breasts, cervix, and lungs. Patients who acquire ichthyosis in association with malignancy usually present with late-stage disease.15 Our patient acquired ichthyosis 3 months prior to admission and had never experienced it previously. Although the exact mechanism for acquiring ichthyosis remains unknown, it is uncertain if ichthyosis associated with malignancy is paraneoplastic or a result of chemotherapy.14,16 In this case, the patient had not yet started chemotherapy at the time of the ichthyosis diagnosis, suggesting a paraneoplastic etiology.
Carcinoma en cuirasse and paraneoplastic ichthyosis individually are extremely rare manifestations of breast cancer. Thus, it is even rarer for these conditions to present concurrently. Treatment options for CeC include chemotherapy, radiotherapy, hormonal antagonists, and snake venom.11 Systemic chemotherapy targeting the histopathologic type of the primary tumor is the treatment of choice. Other treatment methods usually are chosen for late stages of disease progression.10 Paraneoplastic ichthyosis has been reported to show improvement with treatment of the underlying primary malignancy by surgical removal or chemotherapy.14,17 Tamoxifen less commonly is used for systemic treatment of CeC, but one case in the literature reported favorable outcomes.18
We describe 2 rare cutaneous manifestations of breast cancer occurring concomitantly: CeC and paraneoplastic ichthyosis. The combination of clinical and pathologic findings presented in this case solidified the diagnosis of metastatic invasive ductal carcinoma. We aim to improve recognition of paraneoplastic skin findings to accelerate the process of effective and efficient treatment.
- Walker D, Susa JS, Currimbhoy S, et al. Histopathological changes in morphea and their clinical correlates: results from the Morphea in Adults and Children Cohort V. J Am Acad Dermatol. 2017;76:1124-1130. https://doi.org/10.1016/j.jaad.2016.12.020
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4 suppl 1):S59-S64. https://doi.org/10.1097/SAP.0000000000002098
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. https://doi.org/10.1111/j .1600-0560.2011.01754.x
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-206. https://doi.org/10.1111 /j.0303-6987.2006.00516_1.x
- Harvey DT, Hu J, Long JA, et al. Sclerosing sweat duct carcinoma of the lower extremity treated with Mohs micrographic surgery. JAAD Case Rep. 2016;2:284-286. https://doi.org/10.1016/j.jdcr.2016.05.017
- Sharma V, Kumar A. Carcinoma en cuirasse. N Engl J Med. 2021;385:2562. doi:10.1056/NEJMicm2111669
- Oliveira GM, Zachetti DB, Barros HR, et al. Breast carcinoma en cuirasse—case report. An Bras Dermatol. 2013;88:608-610. doi:10.1590/abd1806-4841.20131926
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. doi:10.1097 /DAD.0b013e31823069cf
- Glazebrook AJ, Tomaszewski W. Ichthyosiform atrophy of the skin in Hodgkin’s disease: report of a case, with reference to vitamin A metabolism. Arch Derm Syphilol. 1944;50:85-89. doi:10.1001 /archderm.1944.01510140008002
- Mordenti C, Concetta F, Cerroni M, et al. Cutaneous metastatic breast carcinoma: a study of 164 patients. Acta Dermatovenerol Alp Pannonica Adriat. 2000;9:143-148.
- Culver AL, Metter DM, Pippen JE Jr. Carcinoma en cuirasse. Proc (Bayl Univ Med Cent). 2019;32:263-265. doi:10.1080/08998280.2018.1564966
- Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases [in French]. Ann Dermatol Venereol. 2001;128:1310-1315.
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334. doi:10.1053/j.seminoncol.2016.02.030
- Song Y, Wu Y, Fan T. Dermatosis as the initial manifestation of malignant breast tumors: retrospective analysis of 4 cases. Breast Care. 2010;5:174-176. doi:10.1159/000314265
- Polisky RB, Bronson DM. Acquired ichthyosis in a patient with adenocarcinoma of the breast. Cutis. 1986;38:359-360.
- Haste AR. Acquired ichthyosis from breast cancer. Br Med J. 1967;4:96-98.
- Riesco Martínez MC, Muñoz Martín AJ, Zamberk Majlis P, et al. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin Transl Oncol. 2009;11:552-553. doi:10.1007/s12094-009-0402-2
- Siddiqui MA, Zaman MN. Primary carcinoma en cuirasse. J Am Geriatr Soc. 1996;44:221-222. doi:10.1111/j.1532-5415.1996.tb02455.xssss
The Diagnosis: Carcinoma en Cuirasse
Histopathology demonstrated a cellular infiltrate filling the dermis with sparing of the papillary and superficial reticular dermis (Figure 1A). The cells were arranged in strands and cords that infiltrated between sclerotic collagen bundles. Cytomorphologically, the cells ranged from epithelioid with large vesicular nuclei and prominent nucleoli to cuboidal with hyperchromatic nuclei with irregular contours and a high nuclear to cytoplasmic ratio (Figure 1B). Occasional mitotic figures were identified, and cells demonstrated diffuse nuclear positivity for GATA-3 (Figure 1C); 55% of the cells demonstrated estrogen receptor positivity, and immunohistochemistry of progesterone receptors was negative. These findings confirmed our patient’s diagnosis of breast carcinoma en cuirasse (CeC) as the primary manifestation of metastatic invasive ductal carcinoma. Our patient was treated with intravenous chemotherapy and tamoxifen.

Histopathologic findings of morphea include thickened hyalinized collagen bundles and loss of adventitial fat.1 A diagnosis of chronic radiation dermatitis was inconsistent with our patient’s medical history and biopsy results, as pathology should reveal hyalinized collagen or stellate radiation fibroblasts.2,3 Nests of squamous epithelial cells with abundant eosinophilic cytoplasm and large vesicular nuclei were not seen, excluding squamous cell carcinoma as a possible diagnosis.4 Although sclerosing sweat duct carcinoma is characterized by infiltrating cords in sclerotic dermis, the cells were not arranged in ductlike structures 1– to 2–cell layers thick, excluding this diagnosis.5
Carcinoma en cuirasse—named for skin involvement that appears similar to the metal breastplate of a cuirassier—is a rare form of cutaneous metastasis that typically presents with extensive infiltrative plaques resulting in fibrosis of the skin and subcutaneous tissue.6,7 Carcinoma en cuirasse most commonly metastasizes from the breast but also may represent metastases from the lungs, gastrointestinal tract, or genitourinary systems.8 In the setting of a primary breast malignancy, metastatic plaques of CeC tend to represent tumor recurrence following a mastectomy procedure; however, in rare cases CeC can present as the primary manifestation of breast cancer or as a result of untreated malignancy.6,9 In our patient, CeC was the primary manifestation of metastatic invasive ductal carcinoma with additional paraneoplastic ichthyosis (Figure 2).

Carcinoma en cuirasse comprises 3% to 6% of cutaneous metastases originating from the breast.10,11 Breast cancer is the most common primary neoplasm displaying extracutaneous metastasis, comprising 70% of all cutaneous metastases in females.11 Cutaneous metastasis often indicates late stage of disease, portending a poor prognosis. In our patient, the cutaneous nodules were present for approximately 3 years prior to the diagnosis of stage IV invasive ductal cell carcinoma with metastasis to the skin and lungs. Prior to admission, she had not been diagnosed with breast cancer, thus no treatments had been administered. It is uncommon for CeC to present as the initial finding and without prior treatment of the underlying malignancy. The median length of survival after diagnosis of cutaneous metastasis from breast cancer is 13.8 months, with a 10-year survival rate of 3.1%.12
In addition to cutaneous metastasis, breast cancer also may present with paraneoplastic dermatoses such as ichthyosis.13 Ichthyosis is characterized by extreme dryness, flaking, thickening, and mild pruritus.14 It most commonly is an inherited condition, but it may be acquired due to malignancy. Acquired ichthyosis may manifest in systemic diseases including systemic lupus erythematosus, sarcoidosis, and hypothyroidism.15 Although acquired ichthyosis is rare, it has been reported in cases of internal malignancy, most commonly lymphoproliferative malignancies and less frequently carcinoma of the breasts, cervix, and lungs. Patients who acquire ichthyosis in association with malignancy usually present with late-stage disease.15 Our patient acquired ichthyosis 3 months prior to admission and had never experienced it previously. Although the exact mechanism for acquiring ichthyosis remains unknown, it is uncertain if ichthyosis associated with malignancy is paraneoplastic or a result of chemotherapy.14,16 In this case, the patient had not yet started chemotherapy at the time of the ichthyosis diagnosis, suggesting a paraneoplastic etiology.
Carcinoma en cuirasse and paraneoplastic ichthyosis individually are extremely rare manifestations of breast cancer. Thus, it is even rarer for these conditions to present concurrently. Treatment options for CeC include chemotherapy, radiotherapy, hormonal antagonists, and snake venom.11 Systemic chemotherapy targeting the histopathologic type of the primary tumor is the treatment of choice. Other treatment methods usually are chosen for late stages of disease progression.10 Paraneoplastic ichthyosis has been reported to show improvement with treatment of the underlying primary malignancy by surgical removal or chemotherapy.14,17 Tamoxifen less commonly is used for systemic treatment of CeC, but one case in the literature reported favorable outcomes.18
We describe 2 rare cutaneous manifestations of breast cancer occurring concomitantly: CeC and paraneoplastic ichthyosis. The combination of clinical and pathologic findings presented in this case solidified the diagnosis of metastatic invasive ductal carcinoma. We aim to improve recognition of paraneoplastic skin findings to accelerate the process of effective and efficient treatment.
The Diagnosis: Carcinoma en Cuirasse
Histopathology demonstrated a cellular infiltrate filling the dermis with sparing of the papillary and superficial reticular dermis (Figure 1A). The cells were arranged in strands and cords that infiltrated between sclerotic collagen bundles. Cytomorphologically, the cells ranged from epithelioid with large vesicular nuclei and prominent nucleoli to cuboidal with hyperchromatic nuclei with irregular contours and a high nuclear to cytoplasmic ratio (Figure 1B). Occasional mitotic figures were identified, and cells demonstrated diffuse nuclear positivity for GATA-3 (Figure 1C); 55% of the cells demonstrated estrogen receptor positivity, and immunohistochemistry of progesterone receptors was negative. These findings confirmed our patient’s diagnosis of breast carcinoma en cuirasse (CeC) as the primary manifestation of metastatic invasive ductal carcinoma. Our patient was treated with intravenous chemotherapy and tamoxifen.

Histopathologic findings of morphea include thickened hyalinized collagen bundles and loss of adventitial fat.1 A diagnosis of chronic radiation dermatitis was inconsistent with our patient’s medical history and biopsy results, as pathology should reveal hyalinized collagen or stellate radiation fibroblasts.2,3 Nests of squamous epithelial cells with abundant eosinophilic cytoplasm and large vesicular nuclei were not seen, excluding squamous cell carcinoma as a possible diagnosis.4 Although sclerosing sweat duct carcinoma is characterized by infiltrating cords in sclerotic dermis, the cells were not arranged in ductlike structures 1– to 2–cell layers thick, excluding this diagnosis.5
Carcinoma en cuirasse—named for skin involvement that appears similar to the metal breastplate of a cuirassier—is a rare form of cutaneous metastasis that typically presents with extensive infiltrative plaques resulting in fibrosis of the skin and subcutaneous tissue.6,7 Carcinoma en cuirasse most commonly metastasizes from the breast but also may represent metastases from the lungs, gastrointestinal tract, or genitourinary systems.8 In the setting of a primary breast malignancy, metastatic plaques of CeC tend to represent tumor recurrence following a mastectomy procedure; however, in rare cases CeC can present as the primary manifestation of breast cancer or as a result of untreated malignancy.6,9 In our patient, CeC was the primary manifestation of metastatic invasive ductal carcinoma with additional paraneoplastic ichthyosis (Figure 2).

Carcinoma en cuirasse comprises 3% to 6% of cutaneous metastases originating from the breast.10,11 Breast cancer is the most common primary neoplasm displaying extracutaneous metastasis, comprising 70% of all cutaneous metastases in females.11 Cutaneous metastasis often indicates late stage of disease, portending a poor prognosis. In our patient, the cutaneous nodules were present for approximately 3 years prior to the diagnosis of stage IV invasive ductal cell carcinoma with metastasis to the skin and lungs. Prior to admission, she had not been diagnosed with breast cancer, thus no treatments had been administered. It is uncommon for CeC to present as the initial finding and without prior treatment of the underlying malignancy. The median length of survival after diagnosis of cutaneous metastasis from breast cancer is 13.8 months, with a 10-year survival rate of 3.1%.12
In addition to cutaneous metastasis, breast cancer also may present with paraneoplastic dermatoses such as ichthyosis.13 Ichthyosis is characterized by extreme dryness, flaking, thickening, and mild pruritus.14 It most commonly is an inherited condition, but it may be acquired due to malignancy. Acquired ichthyosis may manifest in systemic diseases including systemic lupus erythematosus, sarcoidosis, and hypothyroidism.15 Although acquired ichthyosis is rare, it has been reported in cases of internal malignancy, most commonly lymphoproliferative malignancies and less frequently carcinoma of the breasts, cervix, and lungs. Patients who acquire ichthyosis in association with malignancy usually present with late-stage disease.15 Our patient acquired ichthyosis 3 months prior to admission and had never experienced it previously. Although the exact mechanism for acquiring ichthyosis remains unknown, it is uncertain if ichthyosis associated with malignancy is paraneoplastic or a result of chemotherapy.14,16 In this case, the patient had not yet started chemotherapy at the time of the ichthyosis diagnosis, suggesting a paraneoplastic etiology.
Carcinoma en cuirasse and paraneoplastic ichthyosis individually are extremely rare manifestations of breast cancer. Thus, it is even rarer for these conditions to present concurrently. Treatment options for CeC include chemotherapy, radiotherapy, hormonal antagonists, and snake venom.11 Systemic chemotherapy targeting the histopathologic type of the primary tumor is the treatment of choice. Other treatment methods usually are chosen for late stages of disease progression.10 Paraneoplastic ichthyosis has been reported to show improvement with treatment of the underlying primary malignancy by surgical removal or chemotherapy.14,17 Tamoxifen less commonly is used for systemic treatment of CeC, but one case in the literature reported favorable outcomes.18
We describe 2 rare cutaneous manifestations of breast cancer occurring concomitantly: CeC and paraneoplastic ichthyosis. The combination of clinical and pathologic findings presented in this case solidified the diagnosis of metastatic invasive ductal carcinoma. We aim to improve recognition of paraneoplastic skin findings to accelerate the process of effective and efficient treatment.
- Walker D, Susa JS, Currimbhoy S, et al. Histopathological changes in morphea and their clinical correlates: results from the Morphea in Adults and Children Cohort V. J Am Acad Dermatol. 2017;76:1124-1130. https://doi.org/10.1016/j.jaad.2016.12.020
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4 suppl 1):S59-S64. https://doi.org/10.1097/SAP.0000000000002098
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. https://doi.org/10.1111/j .1600-0560.2011.01754.x
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-206. https://doi.org/10.1111 /j.0303-6987.2006.00516_1.x
- Harvey DT, Hu J, Long JA, et al. Sclerosing sweat duct carcinoma of the lower extremity treated with Mohs micrographic surgery. JAAD Case Rep. 2016;2:284-286. https://doi.org/10.1016/j.jdcr.2016.05.017
- Sharma V, Kumar A. Carcinoma en cuirasse. N Engl J Med. 2021;385:2562. doi:10.1056/NEJMicm2111669
- Oliveira GM, Zachetti DB, Barros HR, et al. Breast carcinoma en cuirasse—case report. An Bras Dermatol. 2013;88:608-610. doi:10.1590/abd1806-4841.20131926
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. doi:10.1097 /DAD.0b013e31823069cf
- Glazebrook AJ, Tomaszewski W. Ichthyosiform atrophy of the skin in Hodgkin’s disease: report of a case, with reference to vitamin A metabolism. Arch Derm Syphilol. 1944;50:85-89. doi:10.1001 /archderm.1944.01510140008002
- Mordenti C, Concetta F, Cerroni M, et al. Cutaneous metastatic breast carcinoma: a study of 164 patients. Acta Dermatovenerol Alp Pannonica Adriat. 2000;9:143-148.
- Culver AL, Metter DM, Pippen JE Jr. Carcinoma en cuirasse. Proc (Bayl Univ Med Cent). 2019;32:263-265. doi:10.1080/08998280.2018.1564966
- Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases [in French]. Ann Dermatol Venereol. 2001;128:1310-1315.
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334. doi:10.1053/j.seminoncol.2016.02.030
- Song Y, Wu Y, Fan T. Dermatosis as the initial manifestation of malignant breast tumors: retrospective analysis of 4 cases. Breast Care. 2010;5:174-176. doi:10.1159/000314265
- Polisky RB, Bronson DM. Acquired ichthyosis in a patient with adenocarcinoma of the breast. Cutis. 1986;38:359-360.
- Haste AR. Acquired ichthyosis from breast cancer. Br Med J. 1967;4:96-98.
- Riesco Martínez MC, Muñoz Martín AJ, Zamberk Majlis P, et al. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin Transl Oncol. 2009;11:552-553. doi:10.1007/s12094-009-0402-2
- Siddiqui MA, Zaman MN. Primary carcinoma en cuirasse. J Am Geriatr Soc. 1996;44:221-222. doi:10.1111/j.1532-5415.1996.tb02455.xssss
- Walker D, Susa JS, Currimbhoy S, et al. Histopathological changes in morphea and their clinical correlates: results from the Morphea in Adults and Children Cohort V. J Am Acad Dermatol. 2017;76:1124-1130. https://doi.org/10.1016/j.jaad.2016.12.020
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4 suppl 1):S59-S64. https://doi.org/10.1097/SAP.0000000000002098
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. https://doi.org/10.1111/j .1600-0560.2011.01754.x
- Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-206. https://doi.org/10.1111 /j.0303-6987.2006.00516_1.x
- Harvey DT, Hu J, Long JA, et al. Sclerosing sweat duct carcinoma of the lower extremity treated with Mohs micrographic surgery. JAAD Case Rep. 2016;2:284-286. https://doi.org/10.1016/j.jdcr.2016.05.017
- Sharma V, Kumar A. Carcinoma en cuirasse. N Engl J Med. 2021;385:2562. doi:10.1056/NEJMicm2111669
- Oliveira GM, Zachetti DB, Barros HR, et al. Breast carcinoma en cuirasse—case report. An Bras Dermatol. 2013;88:608-610. doi:10.1590/abd1806-4841.20131926
- Alcaraz I, Cerroni L, Rütten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393. doi:10.1097 /DAD.0b013e31823069cf
- Glazebrook AJ, Tomaszewski W. Ichthyosiform atrophy of the skin in Hodgkin’s disease: report of a case, with reference to vitamin A metabolism. Arch Derm Syphilol. 1944;50:85-89. doi:10.1001 /archderm.1944.01510140008002
- Mordenti C, Concetta F, Cerroni M, et al. Cutaneous metastatic breast carcinoma: a study of 164 patients. Acta Dermatovenerol Alp Pannonica Adriat. 2000;9:143-148.
- Culver AL, Metter DM, Pippen JE Jr. Carcinoma en cuirasse. Proc (Bayl Univ Med Cent). 2019;32:263-265. doi:10.1080/08998280.2018.1564966
- Schoenlaub P, Sarraux A, Grosshans E, et al. Survival after cutaneous metastasis: a study of 200 cases [in French]. Ann Dermatol Venereol. 2001;128:1310-1315.
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334. doi:10.1053/j.seminoncol.2016.02.030
- Song Y, Wu Y, Fan T. Dermatosis as the initial manifestation of malignant breast tumors: retrospective analysis of 4 cases. Breast Care. 2010;5:174-176. doi:10.1159/000314265
- Polisky RB, Bronson DM. Acquired ichthyosis in a patient with adenocarcinoma of the breast. Cutis. 1986;38:359-360.
- Haste AR. Acquired ichthyosis from breast cancer. Br Med J. 1967;4:96-98.
- Riesco Martínez MC, Muñoz Martín AJ, Zamberk Majlis P, et al. Acquired ichthyosis as a paraneoplastic syndrome in Hodgkin’s disease. Clin Transl Oncol. 2009;11:552-553. doi:10.1007/s12094-009-0402-2
- Siddiqui MA, Zaman MN. Primary carcinoma en cuirasse. J Am Geriatr Soc. 1996;44:221-222. doi:10.1111/j.1532-5415.1996.tb02455.xssss
A 47-year-old woman with no notable medical history presented to the emergency department with shortness of breath on simple exertion as well as a large lesion on the chest that had slowly increased in size over the last 3 years. The lesion was not painful or pruritic, and she had been treating it with topical emollients without substantial improvement. Physical examination revealed a large indurated plaque with areas of ulceration and necrosis spanning the mid to lateral chest. Additionally, ichthyotic brown scaling was present on the arms and legs. Upon further questioning, the patient reported that the scales on the extremities appeared in the last 3 months and were not previously noted. She had no recent routine cancer screenings, and her family history was notable for a brother with brain cancer. A punch biopsy of the chest plaque was performed.
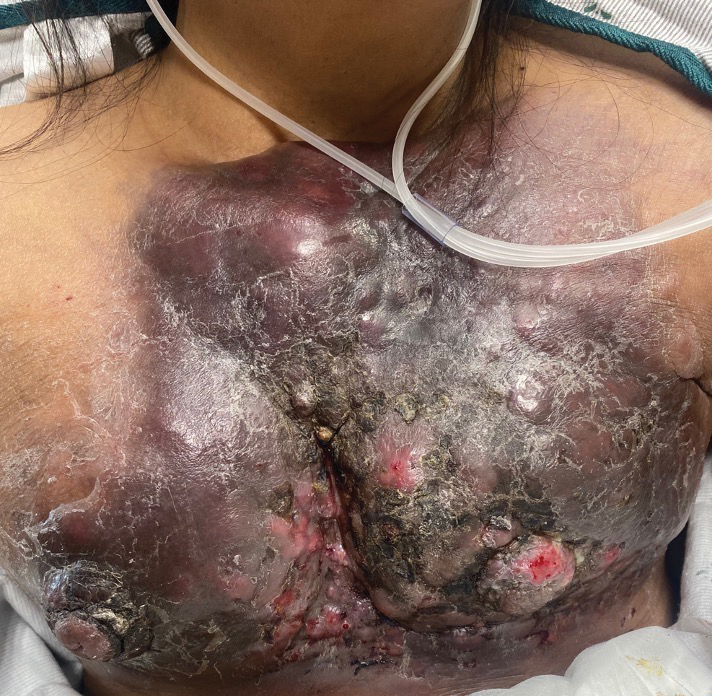
Fellowships in Complex Medical Dermatology
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.
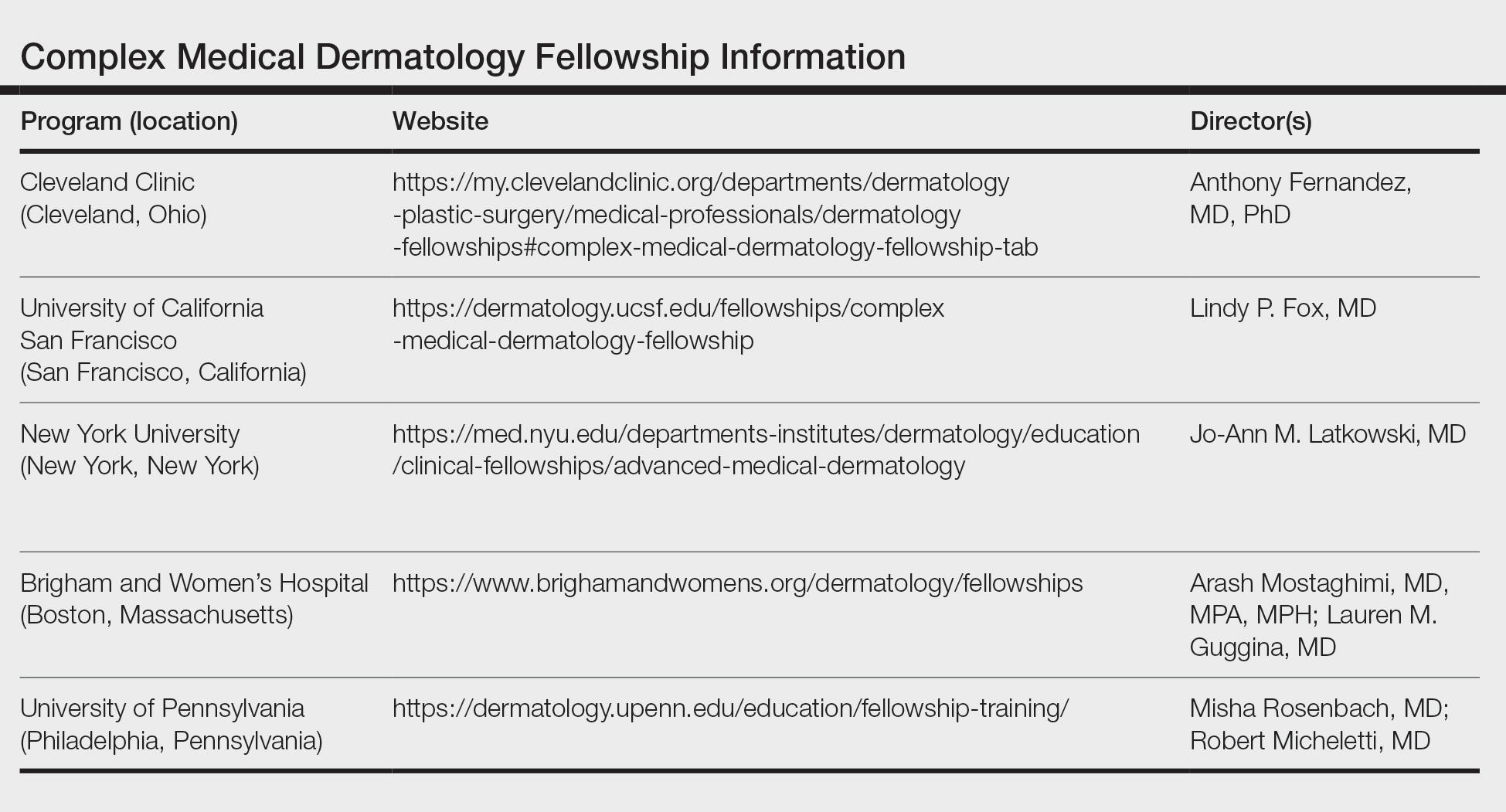
Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.

Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
Complex medical dermatology has become an emerging field in dermatology. Although a rather protean and broad term, complex medical dermatology encompasses patients with autoimmune conditions, bullous disease, connective tissue disease, vasculitis, severe dermatoses requiring immunomodulation, and inpatient consultations. Importantly, dermatology inpatient consultations aid in lowering health care costs due to accurate diagnoses, correct treatment, and decreased hospital stays.1 A fellowship is not required for holding an inpatient role in the hospital system as a dermatologist but can be beneficial. There are combined internal medicine–dermatology programs available for medical students applying to dermatology residency, but a complex medical dermatology fellowship is an option after residency for those who are interested. I believe that a focused complex medical dermatology fellowship differs from the training offered in combined internal medicine–dermatology residency. My fellow colleagues in combined internal medicine–dermatology programs are exposed to systemic manifestations of cutaneous disease and are experts in the interplay between the skin and other organ systems. However, the focus of their programs is with the intention of becoming double boarded in internal medicine and dermatology with comprehensive exposure to both fields. In my fellowship, I am able to tailor my schedule to focus on any dermatologic disease such as connective tissue disease, pruritus, graft vs host disease, and Merkel cell carcinoma. I ultimately can determine a niche in dermatology and hone my skills for a year under supervision.
Available Fellowships
Fellowship Locations—Importantly, the complex medical dermatology fellowship is not accredited by the Accreditation Council for Graduate Medical Education, which can make it difficult to identify and apply to programs. The complex medical dermatology fellowship is different than a rheumatology-dermatology fellowship, cutaneous oncology fellowship, pediatric dermatology fellowship, or other subspecialty fellowships such as those in itch or autoimmune blistering diseases. The fellowship often encompasses gaining clinical expertise in many of these conditions. I performed a thorough search online and spoke with complex medical dermatologists to compile a list of programs that offer a complex medical dermatology fellowship: Brigham and Women’s Hospital (Boston, Massachusetts); University of California San Francisco (San Francisco, California); University of Pennsylvania (Philadelphia, Pennsylvania); Cleveland Clinic (Cleveland, Ohio); and New York University (New York, New York)(Table). Only 1 spot is offered at each of these programs.

Reason to Pursue the Fellowship—There are many reasons to pursue a fellowship in complex medical dermatology such as a desire to enhance exposure to the field, to practice in an academic center and develop a niche within dermatology, to practice dermatology in an inpatient setting, to improve delivery of health care to medically challenging populations in a community setting, and to become an expert on cutaneous manifestations of internal and systemic disease.
Application—There is no standardized application or deadline for this fellowship; however, there is a concerted attempt from some of the programs to offer interviews and decisions at a similar time. Deadlines and contact information are listed on the program websites, along with more details (Table).
Recommendations—I would recommend reaching out at the beginning of postgraduate year (PGY) 4 to these programs and voicing your interest in the fellowship. It is possible to set up an away rotation at some of the programs, and if your program offers elective time, pursuing an away rotation during PGY-3 or early in PGY-4 can prove to be advantageous. Furthermore, during my application cycle I toured the University of California San Francisco, University of Pennsylvania, and Brigham and Women’s Hospital to gain further insight into each program.
Brigham and Women’s Complex Medical Dermatology Fellowship
I am currently the complex medical dermatology fellow at Brigham and Women’s Hospital, and it has been an outstanding experience thus far. The program offers numerous subspecialty clinics focusing solely on cutaneous-oncodermatology, psoriasis, rheumatology-dermatology, skin of color, mole mapping backed by artificial intelligence, cosmetics, high-risk skin cancer, neutrophilic dermatoses, patch testing, phototherapy, psychodermatology, and transplant dermatology. In addition to a wide variety of subspecialty clinics, fellows have the opportunity to participate in inpatient dermatology rounds and act as a junior attending. I appreciate the flexibility of this program combined with the ability to work alongside worldwide experts. There are numerous teaching opportunities, and all of the faculty are amiable and intelligent and emphasize wellness, education, and autonomy. Overall, my experience and decision to pursue a complex medical dermatology fellowship has been extremely rewarding and invaluable. I am gaining additional skills to aid medically challenging patients while pursuing my true passion in dermatology.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
1. Sahni DR. Inpatient dermatology consultation services in hospital institutions. Cutis. 2023;111:E11-E12. doi:10.12788/cutis.0776.
RESIDENT PEARL
- Complex medical dermatology is a rewarding and fascinating subspecialty of dermatology, and additional training can be accomplished through a fellowship at a variety of prestigious institutions.