User login
Longer poly-JIA inactivity not necessarily better before stopping anti-TNF therapy
Recent data suggest the longer a patient with polyarticular forms of juvenile idiopathic arthritis stays on anti–tumor necrosis factor therapy to maintain a clinical inactive disease state, the higher the likelihood of experiencing disease flare after discontinuing anti-TNF therapy.
Daniel J. Lovell, MD, MPH, of the Cincinnati Children’s Hospital Medical Center, and his coauthors prospectively evaluated 137 patients with clinical inactive PF-JIA who were receiving anti-TNF therapy at 16 academic pediatric centers. Of these, 7 patients dropped from the study and 24 patients did not maintain clinical inactive disease (CID) for 6 months.
Among the 106 patients who continued anti-TNF therapy for 6 months and maintained CID, the investigators then stopped anti-TNF therapy and examined patients for disease flare at 1-month, 2-month, 3-month, 4-month, 6-month, and 8-month follow-up. A total of 42% of these patients were also taking background medication such as methotrexate. Investigators found 39 patients (37%) who showed signs of disease flare within 8 months of discontinuing anti-TNF therapy. A number of factors proved to be significantly associated with disease flare, including age at disease onset (hazard ratio, 0.92; 95% confidence interval, 0.85-0.99; P = .03), age at disease diagnosis (HR, 0.91; 95% CI, 0.84-0.99; P = .02), disease duration at enrollment (HR, 1.12; 95% CI, 1.04-1.21; P less than .01) and time from onset until first CID (HR, 1.10; 95% CI, 1.01-1.20; P = .04). Flare occurred at a mean 7.01 months (standard error of the mean, 0.32) and median 8.26 months (95% CI, 7.80-8.66).
“These data certainly do not support the existence of a protective effect of longer duration of CID before considering stopping anti-TNF therapy,” the authors wrote in their study. “In fact, the data suggest that CID, even in those who did demonstrate CID consistently for the first 6 months of the study, continued to be an unstable clinical state and prolonged observation of CID resulted in a significantly greater risk for flare.”
Dr. Lovell and his colleagues noted their results suggest a “window of opportunity” where treating JIA early with “aggressive therapy” to reach CID sooner will help improve outcomes and long-term control of the disease.
The study was sponsored by a grant from the National Institutes of Health.
SOURCE: Lovell D et al. Arthritis Rheumatol. 2018 Mar 31. doi: 10.1002/art.40509.
Recent data suggest the longer a patient with polyarticular forms of juvenile idiopathic arthritis stays on anti–tumor necrosis factor therapy to maintain a clinical inactive disease state, the higher the likelihood of experiencing disease flare after discontinuing anti-TNF therapy.
Daniel J. Lovell, MD, MPH, of the Cincinnati Children’s Hospital Medical Center, and his coauthors prospectively evaluated 137 patients with clinical inactive PF-JIA who were receiving anti-TNF therapy at 16 academic pediatric centers. Of these, 7 patients dropped from the study and 24 patients did not maintain clinical inactive disease (CID) for 6 months.
Among the 106 patients who continued anti-TNF therapy for 6 months and maintained CID, the investigators then stopped anti-TNF therapy and examined patients for disease flare at 1-month, 2-month, 3-month, 4-month, 6-month, and 8-month follow-up. A total of 42% of these patients were also taking background medication such as methotrexate. Investigators found 39 patients (37%) who showed signs of disease flare within 8 months of discontinuing anti-TNF therapy. A number of factors proved to be significantly associated with disease flare, including age at disease onset (hazard ratio, 0.92; 95% confidence interval, 0.85-0.99; P = .03), age at disease diagnosis (HR, 0.91; 95% CI, 0.84-0.99; P = .02), disease duration at enrollment (HR, 1.12; 95% CI, 1.04-1.21; P less than .01) and time from onset until first CID (HR, 1.10; 95% CI, 1.01-1.20; P = .04). Flare occurred at a mean 7.01 months (standard error of the mean, 0.32) and median 8.26 months (95% CI, 7.80-8.66).
“These data certainly do not support the existence of a protective effect of longer duration of CID before considering stopping anti-TNF therapy,” the authors wrote in their study. “In fact, the data suggest that CID, even in those who did demonstrate CID consistently for the first 6 months of the study, continued to be an unstable clinical state and prolonged observation of CID resulted in a significantly greater risk for flare.”
Dr. Lovell and his colleagues noted their results suggest a “window of opportunity” where treating JIA early with “aggressive therapy” to reach CID sooner will help improve outcomes and long-term control of the disease.
The study was sponsored by a grant from the National Institutes of Health.
SOURCE: Lovell D et al. Arthritis Rheumatol. 2018 Mar 31. doi: 10.1002/art.40509.
Recent data suggest the longer a patient with polyarticular forms of juvenile idiopathic arthritis stays on anti–tumor necrosis factor therapy to maintain a clinical inactive disease state, the higher the likelihood of experiencing disease flare after discontinuing anti-TNF therapy.
Daniel J. Lovell, MD, MPH, of the Cincinnati Children’s Hospital Medical Center, and his coauthors prospectively evaluated 137 patients with clinical inactive PF-JIA who were receiving anti-TNF therapy at 16 academic pediatric centers. Of these, 7 patients dropped from the study and 24 patients did not maintain clinical inactive disease (CID) for 6 months.
Among the 106 patients who continued anti-TNF therapy for 6 months and maintained CID, the investigators then stopped anti-TNF therapy and examined patients for disease flare at 1-month, 2-month, 3-month, 4-month, 6-month, and 8-month follow-up. A total of 42% of these patients were also taking background medication such as methotrexate. Investigators found 39 patients (37%) who showed signs of disease flare within 8 months of discontinuing anti-TNF therapy. A number of factors proved to be significantly associated with disease flare, including age at disease onset (hazard ratio, 0.92; 95% confidence interval, 0.85-0.99; P = .03), age at disease diagnosis (HR, 0.91; 95% CI, 0.84-0.99; P = .02), disease duration at enrollment (HR, 1.12; 95% CI, 1.04-1.21; P less than .01) and time from onset until first CID (HR, 1.10; 95% CI, 1.01-1.20; P = .04). Flare occurred at a mean 7.01 months (standard error of the mean, 0.32) and median 8.26 months (95% CI, 7.80-8.66).
“These data certainly do not support the existence of a protective effect of longer duration of CID before considering stopping anti-TNF therapy,” the authors wrote in their study. “In fact, the data suggest that CID, even in those who did demonstrate CID consistently for the first 6 months of the study, continued to be an unstable clinical state and prolonged observation of CID resulted in a significantly greater risk for flare.”
Dr. Lovell and his colleagues noted their results suggest a “window of opportunity” where treating JIA early with “aggressive therapy” to reach CID sooner will help improve outcomes and long-term control of the disease.
The study was sponsored by a grant from the National Institutes of Health.
SOURCE: Lovell D et al. Arthritis Rheumatol. 2018 Mar 31. doi: 10.1002/art.40509.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Discontinuing anti–tumor necrosis factor therapy caused disease flare within 8 months in over one-third of children with clinically inactive polyarticular forms of juvenile idiopathic arthritis.
Major finding:
Story details: A two-phase prospective study of 137 patients with PF-JIA in CID across 16 centers over a 16-month period.
Disclosures: This study was sponsored by a grant from the National Institutes of Health.
Source: Lovell D et al. Arthritis Rheumatol. 2018 Mar 31. doi: 10.1002/art.40509.
Patients presenting with saddle pulmonary emboli (PE) versus nonsaddle PE have no mortality difference but have an increased risk for decompensation
Background: Patients with saddle PEs can differ in terms of their clinical presentation and may present as hemodynamically stable or unstable. There have been few studies to quantify the presentation, management, and outcome of patients who present with saddle PEs.
Study design: Retrospective cohort study.
Setting: Quaternary care hospital in Minnesota.
Synopsis: Using a localized database, 187 consecutive patients with saddle PEs were matched with 187 nonsaddle PEs using age and the simplified Pulmonary Embolism Severity Index (sPESI). Saddle PE patients had no significant in-hospital mortality differences versus nonsaddle PEs. However, they were more likely to present with massive and submassive hemodynamics (80% vs. 52%; P less than .05), RV dilatation (84% vs. 67%; P less than .001), and troponin elevation (71% vs. 40%; P less than .001). Patients with saddle PEs were more likely to receive thrombolytics, inferior vena cava filter placement, and require mechanical ventilation. They also had increased risk of decompensation after 6 hours (12 patients vs. 6 patients). Limitations of this study include some selection bias and use of a single-center study. Regardless, hospitalists should consider close monitoring for patients with saddle PEs upon admission, independent of hemodynamics given risk of decompensation regardless of presenting vitals.
Bottom line: Saddle PEs have an increased risk of late decompensation, clot burden, and presentation with massive and submassive hemodynamics but not an increased risk of mortality when compared with nonsaddle PEs.
Citation: Alkinj B et al. Saddle vs. nonsaddle pulmonary embolism: Clinical presentation, hemodynamics, management, and outcomes. Mayo Clin Proc. 2017 Oct;92(10):1511-8.
Dr. O’Donnell is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Background: Patients with saddle PEs can differ in terms of their clinical presentation and may present as hemodynamically stable or unstable. There have been few studies to quantify the presentation, management, and outcome of patients who present with saddle PEs.
Study design: Retrospective cohort study.
Setting: Quaternary care hospital in Minnesota.
Synopsis: Using a localized database, 187 consecutive patients with saddle PEs were matched with 187 nonsaddle PEs using age and the simplified Pulmonary Embolism Severity Index (sPESI). Saddle PE patients had no significant in-hospital mortality differences versus nonsaddle PEs. However, they were more likely to present with massive and submassive hemodynamics (80% vs. 52%; P less than .05), RV dilatation (84% vs. 67%; P less than .001), and troponin elevation (71% vs. 40%; P less than .001). Patients with saddle PEs were more likely to receive thrombolytics, inferior vena cava filter placement, and require mechanical ventilation. They also had increased risk of decompensation after 6 hours (12 patients vs. 6 patients). Limitations of this study include some selection bias and use of a single-center study. Regardless, hospitalists should consider close monitoring for patients with saddle PEs upon admission, independent of hemodynamics given risk of decompensation regardless of presenting vitals.
Bottom line: Saddle PEs have an increased risk of late decompensation, clot burden, and presentation with massive and submassive hemodynamics but not an increased risk of mortality when compared with nonsaddle PEs.
Citation: Alkinj B et al. Saddle vs. nonsaddle pulmonary embolism: Clinical presentation, hemodynamics, management, and outcomes. Mayo Clin Proc. 2017 Oct;92(10):1511-8.
Dr. O’Donnell is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Background: Patients with saddle PEs can differ in terms of their clinical presentation and may present as hemodynamically stable or unstable. There have been few studies to quantify the presentation, management, and outcome of patients who present with saddle PEs.
Study design: Retrospective cohort study.
Setting: Quaternary care hospital in Minnesota.
Synopsis: Using a localized database, 187 consecutive patients with saddle PEs were matched with 187 nonsaddle PEs using age and the simplified Pulmonary Embolism Severity Index (sPESI). Saddle PE patients had no significant in-hospital mortality differences versus nonsaddle PEs. However, they were more likely to present with massive and submassive hemodynamics (80% vs. 52%; P less than .05), RV dilatation (84% vs. 67%; P less than .001), and troponin elevation (71% vs. 40%; P less than .001). Patients with saddle PEs were more likely to receive thrombolytics, inferior vena cava filter placement, and require mechanical ventilation. They also had increased risk of decompensation after 6 hours (12 patients vs. 6 patients). Limitations of this study include some selection bias and use of a single-center study. Regardless, hospitalists should consider close monitoring for patients with saddle PEs upon admission, independent of hemodynamics given risk of decompensation regardless of presenting vitals.
Bottom line: Saddle PEs have an increased risk of late decompensation, clot burden, and presentation with massive and submassive hemodynamics but not an increased risk of mortality when compared with nonsaddle PEs.
Citation: Alkinj B et al. Saddle vs. nonsaddle pulmonary embolism: Clinical presentation, hemodynamics, management, and outcomes. Mayo Clin Proc. 2017 Oct;92(10):1511-8.
Dr. O’Donnell is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
FDA grants priority review of follicular lymphoma drug
by the Food and Drug Administration.
The biopharmaceutical company Verastem is seeking full approval for duvelisib for the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for the treatment of relapsed/refractory follicular lymphoma. The FDA has set Oct. 5, 2018, as the target action date, according to Verastem.
by the Food and Drug Administration.
The biopharmaceutical company Verastem is seeking full approval for duvelisib for the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for the treatment of relapsed/refractory follicular lymphoma. The FDA has set Oct. 5, 2018, as the target action date, according to Verastem.
by the Food and Drug Administration.
The biopharmaceutical company Verastem is seeking full approval for duvelisib for the treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and accelerated approval for the treatment of relapsed/refractory follicular lymphoma. The FDA has set Oct. 5, 2018, as the target action date, according to Verastem.
Product News: 04 2018
Avène Mineral Light Mattifying Sunscreen Lotion
Pierre Fabre Dermo-Cosmetique introduces the Avène Mineral Light Mattifying Sunscreen Lotion with SPF 50+. This sunscreen offers broad-spectrum sun protection without irritation while delivering oil control and providing a natural mattifying finish for oily and acne-prone skin. This product absorbs quickly into the skin and can be worn under makeup. Avène Mineral Light Mattifying Sunscreen Lotion should be applied to the face 15 minutes prior to sun exposure and reapplied after 80 minutes of swimming or sweating, immediately after towel drying, or every 2 hours. For more information, visit www.aveneusa.com.
Ducray Anacaps Activ+ Dietary Supplement
Pierre Fabre Dermo-Cosmetique introduces Ducray Anacaps Activ+ Dietary Supplement, a once-daily capsule that contains zinc, molybdenum, iron, and selenium. This supplement targets factors that trigger sudden hair loss, including seasonal changes, stress, and diet. It also targets chronic hair loss with genetic, hormonal, and vascular causes. This formula provides essential nutrients needed to promote healthy hair growth from within, preserve hair density, and maintain the strength and vitality of hair. This supplement also is used for weak, devitalized nails and has a vegan formula with good digestive tolerance. For more information, visit www.ducray.com/en-us/.
Luzu
Ortho Dermatologics receives US Food and Drug Administration approval of the Supplemental New Drug Application to expand the use of Luzu (luliconazole) Cream 1% to pediatric patients. This new indication is for the topical treatment of interdigital tinea pedis and tinea cruris in patients 12 years of age and older and for tinea corporis in patients 2 years of age and older. Luzu is a topical azole antifungal agent with a 1-week, once-daily treatment regimen with results available 3 weeks post-treatment. Luzu previously was approved for use in adult patients. For more information, visit www.luzurx.com/HCP.
Xeljanz and Xeljanz XR
Pfizer Inc announces US Food and Drug Administration approval of twice-daily Xeljanz 5 mg and once-daily Xeljanz XR extended release 11 mg (tofacitinib) for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs. Xeljanz and Xeljanz XR are Janus kinase inhibitors that previously were approved for the treatment of rheumatoid arthritis. For more information, visit www.xeljanz.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Avène Mineral Light Mattifying Sunscreen Lotion
Pierre Fabre Dermo-Cosmetique introduces the Avène Mineral Light Mattifying Sunscreen Lotion with SPF 50+. This sunscreen offers broad-spectrum sun protection without irritation while delivering oil control and providing a natural mattifying finish for oily and acne-prone skin. This product absorbs quickly into the skin and can be worn under makeup. Avène Mineral Light Mattifying Sunscreen Lotion should be applied to the face 15 minutes prior to sun exposure and reapplied after 80 minutes of swimming or sweating, immediately after towel drying, or every 2 hours. For more information, visit www.aveneusa.com.
Ducray Anacaps Activ+ Dietary Supplement
Pierre Fabre Dermo-Cosmetique introduces Ducray Anacaps Activ+ Dietary Supplement, a once-daily capsule that contains zinc, molybdenum, iron, and selenium. This supplement targets factors that trigger sudden hair loss, including seasonal changes, stress, and diet. It also targets chronic hair loss with genetic, hormonal, and vascular causes. This formula provides essential nutrients needed to promote healthy hair growth from within, preserve hair density, and maintain the strength and vitality of hair. This supplement also is used for weak, devitalized nails and has a vegan formula with good digestive tolerance. For more information, visit www.ducray.com/en-us/.
Luzu
Ortho Dermatologics receives US Food and Drug Administration approval of the Supplemental New Drug Application to expand the use of Luzu (luliconazole) Cream 1% to pediatric patients. This new indication is for the topical treatment of interdigital tinea pedis and tinea cruris in patients 12 years of age and older and for tinea corporis in patients 2 years of age and older. Luzu is a topical azole antifungal agent with a 1-week, once-daily treatment regimen with results available 3 weeks post-treatment. Luzu previously was approved for use in adult patients. For more information, visit www.luzurx.com/HCP.
Xeljanz and Xeljanz XR
Pfizer Inc announces US Food and Drug Administration approval of twice-daily Xeljanz 5 mg and once-daily Xeljanz XR extended release 11 mg (tofacitinib) for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs. Xeljanz and Xeljanz XR are Janus kinase inhibitors that previously were approved for the treatment of rheumatoid arthritis. For more information, visit www.xeljanz.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Avène Mineral Light Mattifying Sunscreen Lotion
Pierre Fabre Dermo-Cosmetique introduces the Avène Mineral Light Mattifying Sunscreen Lotion with SPF 50+. This sunscreen offers broad-spectrum sun protection without irritation while delivering oil control and providing a natural mattifying finish for oily and acne-prone skin. This product absorbs quickly into the skin and can be worn under makeup. Avène Mineral Light Mattifying Sunscreen Lotion should be applied to the face 15 minutes prior to sun exposure and reapplied after 80 minutes of swimming or sweating, immediately after towel drying, or every 2 hours. For more information, visit www.aveneusa.com.
Ducray Anacaps Activ+ Dietary Supplement
Pierre Fabre Dermo-Cosmetique introduces Ducray Anacaps Activ+ Dietary Supplement, a once-daily capsule that contains zinc, molybdenum, iron, and selenium. This supplement targets factors that trigger sudden hair loss, including seasonal changes, stress, and diet. It also targets chronic hair loss with genetic, hormonal, and vascular causes. This formula provides essential nutrients needed to promote healthy hair growth from within, preserve hair density, and maintain the strength and vitality of hair. This supplement also is used for weak, devitalized nails and has a vegan formula with good digestive tolerance. For more information, visit www.ducray.com/en-us/.
Luzu
Ortho Dermatologics receives US Food and Drug Administration approval of the Supplemental New Drug Application to expand the use of Luzu (luliconazole) Cream 1% to pediatric patients. This new indication is for the topical treatment of interdigital tinea pedis and tinea cruris in patients 12 years of age and older and for tinea corporis in patients 2 years of age and older. Luzu is a topical azole antifungal agent with a 1-week, once-daily treatment regimen with results available 3 weeks post-treatment. Luzu previously was approved for use in adult patients. For more information, visit www.luzurx.com/HCP.
Xeljanz and Xeljanz XR
Pfizer Inc announces US Food and Drug Administration approval of twice-daily Xeljanz 5 mg and once-daily Xeljanz XR extended release 11 mg (tofacitinib) for the treatment of adult patients with active psoriatic arthritis who have had an inadequate response or intolerance to methotrexate or other disease-modifying antirheumatic drugs. Xeljanz and Xeljanz XR are Janus kinase inhibitors that previously were approved for the treatment of rheumatoid arthritis. For more information, visit www.xeljanz.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Genetic Screening May Reduce Carbamazepine-Induced Cutaneous Adverse Reactions
Preemptive HLA-A*31:01 genetic screening may significantly decrease the incidence of carbamazepine-induced cutaneous adverse reactions in Japanese patients, according to a report published online ahead of print April 2 in JAMA Neurology. According to the researchers, their finding suggests that such screening may be warranted in routine clinical practice.
Carbamazepine, which is widely used to treat seizures, neuropathic pain, and other disorders, is a common cause of cutaneous adverse drug reactions worldwide. The allele HLA-A*31:01 has been associated with
Genetic Screening Informed Treatment
To assess the use of HLA-A*31:01 genetic screening to identify individuals at risk of carbamazepine-induced cutaneous drug reactions, researchers from the Genotype-Based Carbamazepine Therapy Study Group conducted a cohort study in 36 hospitals in Japan from January 2012 to November 2014. The investigators enrolled 1,202 patients who were eligible to receive carbamazepine during the study period. Preemptive HLA-A*31:01 screening was performed for 1,187 study participants. Patients who did not start treatment with carbamazepine or alternative drugs were excluded. Participants were interviewed once weekly for eight weeks to monitor the development of
Neuropsychiatrists were asked to prescribe carbamazepine for patients who tested negative for HLA-A*31:01 and alternative drugs for those who tested positive for the allele. The study’s main outcome was the incidence of carbamazepine-induced cutaneous adverse drug reactions.
Of the 1,130 patients who were prescribed carbamazepine or alternative drugs, the mean age was 37.4; 614 (54.3%) were men, and 198 (17.5%) were positive for HLA-A*31:01. Dermatologists identified 23 patients (2.0%) who had carbamazepine-induced cutaneous adverse drug reactions, of whom four patients required hospitalization. Drug-induced hypersensitivity syndrome was observed for three patients, maculopapular eruption for nine patients, erythema multiforme for five patients, and an undetermined type of cutaneous adverse drug reaction for six patients. No patient developed Stevens-Johnson syndrome or toxic epidermal necrolysis.
Comparison with a historical control indicated that the preemptive use of HLA-A*31:01 screening was associated with a 40% reduction in the incidence of carbamazepine-induced cutaneous adverse drug reactions.
Two Alleles
The researchers noted that the clinical utility of the HLA-B*15:02 genetic test for reducing carbamazepine-induced cutaneous drug reactions has already been established by preemptive screening. However, the frequency of the HLA-B*15:02 allele is low in Korean, Japanese, African, and European populations.
In contrast, the frequency of the HLA-A*31:01 allele is 7% to 9% in Japanese, 5% in Korean, 2% in Chinese, 2% to 3% in European, and 1% in African populations. Moreover, HLA-A*31:01 has been associated with a full spectrum of carbamazepine-induced
A First Step in Precision Neurology?
The present study “provides initial data that preemptive strategies may allow for a more consistent approach to safely administer this commonly used medication,” said Yijing He, MD, PhD; Lucia Seminario-Vidal, MD, PhD; and Howard L. McLeod, PharmD, in an accompanying editorial. A substantial decrease in the incidence and severity of carbamazepine-associated
“However, 23 patients experienced a carbamazepine-associated cutaneous adverse drug reaction,” and four patients required hospitalization, the editorialists noted. “Therefore, the use of HLA-A*31:01 testing will not eliminate the risk of carbamazepine-associated events, nor will it decrease the need for neurologists to provide skin-focused monitoring as part of their management of cases involving carbamazepine-treated patients.”
This research, the editorialists said, indicates that there is still more to discover. “To date, a small number of heroes has relentlessly pursued mechanistic and preventive research on drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis. However, the field needs to build on these efforts with a more systematic approach that will aid more rapid progress and provide more consistent influence across the globe.” Much still needs to be learned about the basic mechanisms, clinical confounders, biomarkers, and epidemiologic aspects of the various drug reactions that become Stevens-Johnson syndrome and toxic epidermal necrolysis. Attributing this clinical, immunologic, and therapeutic problem to various idiosyncratic reactions does not serve patients well, said the editorialists.
A key first step, the editorialists said, is to “apply existing preemptive strategies, including genomic analysis. Whether this is initially implemented broadly or in regions with higher probability of the risk alleles is less important than learning how to change a culture that tolerates adverse drug events and inventing informatics tools to make proactive approaches less of a burden. Although we will not get it completely right the first time, we need to start the iterative process to eradicate severe cutaneous adverse drug reactions.”
—Glenn S. Williams
Suggested Reading
Mushiroda T, Takahashi Y, Onuma T, et al. Association of HLA-A*31:01 screening with the incidence of carbamazepine-induced cutaneous adverse reactions in a Japanese population. JAMA Neurol. 2018 Apr 2 [Epub ahead of print].
He Y, Seminario-Vidal S, McLeod HL. Avoidance of severe cutaneous adverse drug events as a first step in precision neurology. JAMA Neurol. Apr 2 [Epub ahead of print].
Preemptive HLA-A*31:01 genetic screening may significantly decrease the incidence of carbamazepine-induced cutaneous adverse reactions in Japanese patients, according to a report published online ahead of print April 2 in JAMA Neurology. According to the researchers, their finding suggests that such screening may be warranted in routine clinical practice.
Carbamazepine, which is widely used to treat seizures, neuropathic pain, and other disorders, is a common cause of cutaneous adverse drug reactions worldwide. The allele HLA-A*31:01 has been associated with
Genetic Screening Informed Treatment
To assess the use of HLA-A*31:01 genetic screening to identify individuals at risk of carbamazepine-induced cutaneous drug reactions, researchers from the Genotype-Based Carbamazepine Therapy Study Group conducted a cohort study in 36 hospitals in Japan from January 2012 to November 2014. The investigators enrolled 1,202 patients who were eligible to receive carbamazepine during the study period. Preemptive HLA-A*31:01 screening was performed for 1,187 study participants. Patients who did not start treatment with carbamazepine or alternative drugs were excluded. Participants were interviewed once weekly for eight weeks to monitor the development of
Neuropsychiatrists were asked to prescribe carbamazepine for patients who tested negative for HLA-A*31:01 and alternative drugs for those who tested positive for the allele. The study’s main outcome was the incidence of carbamazepine-induced cutaneous adverse drug reactions.
Of the 1,130 patients who were prescribed carbamazepine or alternative drugs, the mean age was 37.4; 614 (54.3%) were men, and 198 (17.5%) were positive for HLA-A*31:01. Dermatologists identified 23 patients (2.0%) who had carbamazepine-induced cutaneous adverse drug reactions, of whom four patients required hospitalization. Drug-induced hypersensitivity syndrome was observed for three patients, maculopapular eruption for nine patients, erythema multiforme for five patients, and an undetermined type of cutaneous adverse drug reaction for six patients. No patient developed Stevens-Johnson syndrome or toxic epidermal necrolysis.
Comparison with a historical control indicated that the preemptive use of HLA-A*31:01 screening was associated with a 40% reduction in the incidence of carbamazepine-induced cutaneous adverse drug reactions.
Two Alleles
The researchers noted that the clinical utility of the HLA-B*15:02 genetic test for reducing carbamazepine-induced cutaneous drug reactions has already been established by preemptive screening. However, the frequency of the HLA-B*15:02 allele is low in Korean, Japanese, African, and European populations.
In contrast, the frequency of the HLA-A*31:01 allele is 7% to 9% in Japanese, 5% in Korean, 2% in Chinese, 2% to 3% in European, and 1% in African populations. Moreover, HLA-A*31:01 has been associated with a full spectrum of carbamazepine-induced
A First Step in Precision Neurology?
The present study “provides initial data that preemptive strategies may allow for a more consistent approach to safely administer this commonly used medication,” said Yijing He, MD, PhD; Lucia Seminario-Vidal, MD, PhD; and Howard L. McLeod, PharmD, in an accompanying editorial. A substantial decrease in the incidence and severity of carbamazepine-associated
“However, 23 patients experienced a carbamazepine-associated cutaneous adverse drug reaction,” and four patients required hospitalization, the editorialists noted. “Therefore, the use of HLA-A*31:01 testing will not eliminate the risk of carbamazepine-associated events, nor will it decrease the need for neurologists to provide skin-focused monitoring as part of their management of cases involving carbamazepine-treated patients.”
This research, the editorialists said, indicates that there is still more to discover. “To date, a small number of heroes has relentlessly pursued mechanistic and preventive research on drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis. However, the field needs to build on these efforts with a more systematic approach that will aid more rapid progress and provide more consistent influence across the globe.” Much still needs to be learned about the basic mechanisms, clinical confounders, biomarkers, and epidemiologic aspects of the various drug reactions that become Stevens-Johnson syndrome and toxic epidermal necrolysis. Attributing this clinical, immunologic, and therapeutic problem to various idiosyncratic reactions does not serve patients well, said the editorialists.
A key first step, the editorialists said, is to “apply existing preemptive strategies, including genomic analysis. Whether this is initially implemented broadly or in regions with higher probability of the risk alleles is less important than learning how to change a culture that tolerates adverse drug events and inventing informatics tools to make proactive approaches less of a burden. Although we will not get it completely right the first time, we need to start the iterative process to eradicate severe cutaneous adverse drug reactions.”
—Glenn S. Williams
Suggested Reading
Mushiroda T, Takahashi Y, Onuma T, et al. Association of HLA-A*31:01 screening with the incidence of carbamazepine-induced cutaneous adverse reactions in a Japanese population. JAMA Neurol. 2018 Apr 2 [Epub ahead of print].
He Y, Seminario-Vidal S, McLeod HL. Avoidance of severe cutaneous adverse drug events as a first step in precision neurology. JAMA Neurol. Apr 2 [Epub ahead of print].
Preemptive HLA-A*31:01 genetic screening may significantly decrease the incidence of carbamazepine-induced cutaneous adverse reactions in Japanese patients, according to a report published online ahead of print April 2 in JAMA Neurology. According to the researchers, their finding suggests that such screening may be warranted in routine clinical practice.
Carbamazepine, which is widely used to treat seizures, neuropathic pain, and other disorders, is a common cause of cutaneous adverse drug reactions worldwide. The allele HLA-A*31:01 has been associated with
Genetic Screening Informed Treatment
To assess the use of HLA-A*31:01 genetic screening to identify individuals at risk of carbamazepine-induced cutaneous drug reactions, researchers from the Genotype-Based Carbamazepine Therapy Study Group conducted a cohort study in 36 hospitals in Japan from January 2012 to November 2014. The investigators enrolled 1,202 patients who were eligible to receive carbamazepine during the study period. Preemptive HLA-A*31:01 screening was performed for 1,187 study participants. Patients who did not start treatment with carbamazepine or alternative drugs were excluded. Participants were interviewed once weekly for eight weeks to monitor the development of
Neuropsychiatrists were asked to prescribe carbamazepine for patients who tested negative for HLA-A*31:01 and alternative drugs for those who tested positive for the allele. The study’s main outcome was the incidence of carbamazepine-induced cutaneous adverse drug reactions.
Of the 1,130 patients who were prescribed carbamazepine or alternative drugs, the mean age was 37.4; 614 (54.3%) were men, and 198 (17.5%) were positive for HLA-A*31:01. Dermatologists identified 23 patients (2.0%) who had carbamazepine-induced cutaneous adverse drug reactions, of whom four patients required hospitalization. Drug-induced hypersensitivity syndrome was observed for three patients, maculopapular eruption for nine patients, erythema multiforme for five patients, and an undetermined type of cutaneous adverse drug reaction for six patients. No patient developed Stevens-Johnson syndrome or toxic epidermal necrolysis.
Comparison with a historical control indicated that the preemptive use of HLA-A*31:01 screening was associated with a 40% reduction in the incidence of carbamazepine-induced cutaneous adverse drug reactions.
Two Alleles
The researchers noted that the clinical utility of the HLA-B*15:02 genetic test for reducing carbamazepine-induced cutaneous drug reactions has already been established by preemptive screening. However, the frequency of the HLA-B*15:02 allele is low in Korean, Japanese, African, and European populations.
In contrast, the frequency of the HLA-A*31:01 allele is 7% to 9% in Japanese, 5% in Korean, 2% in Chinese, 2% to 3% in European, and 1% in African populations. Moreover, HLA-A*31:01 has been associated with a full spectrum of carbamazepine-induced
A First Step in Precision Neurology?
The present study “provides initial data that preemptive strategies may allow for a more consistent approach to safely administer this commonly used medication,” said Yijing He, MD, PhD; Lucia Seminario-Vidal, MD, PhD; and Howard L. McLeod, PharmD, in an accompanying editorial. A substantial decrease in the incidence and severity of carbamazepine-associated
“However, 23 patients experienced a carbamazepine-associated cutaneous adverse drug reaction,” and four patients required hospitalization, the editorialists noted. “Therefore, the use of HLA-A*31:01 testing will not eliminate the risk of carbamazepine-associated events, nor will it decrease the need for neurologists to provide skin-focused monitoring as part of their management of cases involving carbamazepine-treated patients.”
This research, the editorialists said, indicates that there is still more to discover. “To date, a small number of heroes has relentlessly pursued mechanistic and preventive research on drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis. However, the field needs to build on these efforts with a more systematic approach that will aid more rapid progress and provide more consistent influence across the globe.” Much still needs to be learned about the basic mechanisms, clinical confounders, biomarkers, and epidemiologic aspects of the various drug reactions that become Stevens-Johnson syndrome and toxic epidermal necrolysis. Attributing this clinical, immunologic, and therapeutic problem to various idiosyncratic reactions does not serve patients well, said the editorialists.
A key first step, the editorialists said, is to “apply existing preemptive strategies, including genomic analysis. Whether this is initially implemented broadly or in regions with higher probability of the risk alleles is less important than learning how to change a culture that tolerates adverse drug events and inventing informatics tools to make proactive approaches less of a burden. Although we will not get it completely right the first time, we need to start the iterative process to eradicate severe cutaneous adverse drug reactions.”
—Glenn S. Williams
Suggested Reading
Mushiroda T, Takahashi Y, Onuma T, et al. Association of HLA-A*31:01 screening with the incidence of carbamazepine-induced cutaneous adverse reactions in a Japanese population. JAMA Neurol. 2018 Apr 2 [Epub ahead of print].
He Y, Seminario-Vidal S, McLeod HL. Avoidance of severe cutaneous adverse drug events as a first step in precision neurology. JAMA Neurol. Apr 2 [Epub ahead of print].
Life and health are not even across the U.S.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.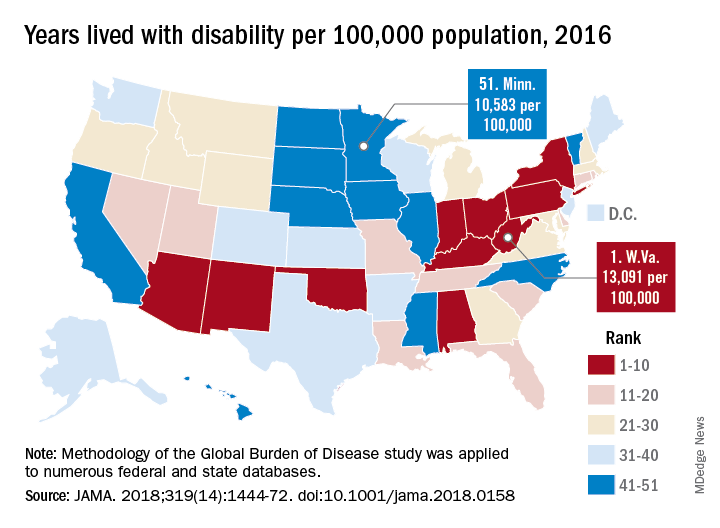
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
This report on Global Burden of Disease (GBD) study data profoundly and powerfully illuminates U.S. health trends over time and by geography. There is much unfinished business for us, nationally and at the state level.
Clinicians and policy makers can use the rankings to evaluate why many individuals are still experiencing injury, disease, and deaths that are preventable; in doing so, the entire nation could move closely resemble a United States of health.
Clinicians could use the results to help guide patients through evidence-based disease prevention and early intervention, a strategy that has led to decreases in death due to cancer and cardiovascular disease over the past few decades.
At the same time, policy makers could use GBD 2016 results to reevaluate current national attitudes toward disease prevention.
Howard K. Koh, MD, MPH, is with the Harvard T.H. Chan School of Public Health, Boston. Anand K. Parekh, MD, MPH, is with the Bipartisan Policy Center in Washington. The comments above are derived from an editorial accompanying the report from the US Burden of Disease Collaborators ( JAMA. 2018;319[14]:1438-40 ). Dr. Koh and Dr. Parekh reported no conflicts of interest related to the editorial.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors, according to a comprehensive analysis.
Life expectancy varies substantially, for example, ranging from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, according to results from the analysis of data from the Global Burden of Disease (GBD) study (JAMA. 2018;319[14]:1444-72).
Previously decreasing death rates for adults have reversed in 19 states, according to the analysis, which covers the years 1990 to 2016.
Hardest hit were Kentucky, New Mexico, Oklahoma, West Virginia, and Wyoming, which had mortality increases of more than 10% among adults aged 20-55 years. Those increases were largely due to causes such as substance use disorders, self-harm, and cirrhosis, according to the US Burden of Disease Collaborators, who authored the report.
“These findings should be used to examine the causes of health variations and to plan, develop, and implement programs and policies to improve health overall and eliminate disparities in the United States,” the authors wrote.
Overall, U.S. death rates have declined from 745.2 per 100,000 persons in 1990 to 578.0 per 100,000 persons in 2016, according to the report.
Likewise, health outcomes throughout the United States have improved over time for some conditions, such as ischemic heart disease, lung cancer, and neonatal preterm complications, the report says.
However, those gains are offset by rising death rates due to drug-use disorders, chronic kidney disease, cirrhosis, chronic obstructive pulmonary disease, hypertension, and self-harm.

The three most important risk factors in the United States are high body mass index, smoking, and high fasting plasma glucose, the analysis showed. Of those risk factors, only smoking is decreasing, authors noted.
Many risk factors contributing to disparities in burden among states are amenable to medical treatment that emphasizes supportive behavioral and lifestyle changes, according to the authors.
“Expanding health coverage for certain conditions and medications should be considered and adopted to reduce burden,” they said.
Substance abuse disorders, cirrhosis, and self-harm, the causes of the mortality reversal in Kentucky, New Mexico, and other states, could be addressed via a wide range of interventions, according to the investigators.
Prevention programs could address the root causes of substance use and causes of relapse, while physicians can play a “major role” in addiction control through counseling of patients on pain control medication, they said.
Interventions to treat hepatitis C and decrease excessive alcohol consumption could help address cirrhosis, while for self-harm, the most promising approaches focus on restricting access to lethal means, they said, noting that a large proportion of U.S. suicides are due to firearms.
“While multiple strategies are available for dealing with these problems, they have not until very recently garnered attention,” investigators wrote.
The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Some individual study collaborators reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
SOURCE: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-72.
FROM JAMA
Key clinical point: While U.S. death rates have declined overall, marked geographic disparities exist at the state level in burden of disease, injuries, and risk factors.
Major finding: Life expectancy ranged from a high of 81.3 years in Hawaii to a low of 74.7 years in Mississippi, and previously decreasing death rates for adults have reversed in 19 states.
Study details: A U.S. state-level analysis of results from the Global Burden of Disease (GBD) study illustrating trends in diseases, injuries, risk factors, and deaths from 1990 to 2016.
Disclosures: The study was supported in part by the National Institute of Environmental Health Sciences and the Bill and Melinda Gates Foundation. Study authors reported disclosures related to Savient, Takeda, Crealta/Horizon, Regeneron, Allergan, and others.
Source: The US Burden of Disease Collaborators. JAMA 2018;319(14):1444-1472.
Multidisciplinary teams improve diagnoses in ILD
New research provides strong statistical support for the use of dynamic multidisciplinary discussion in the diagnosis of patients who may have interstitial lung diseases (ILD).
and it changed the diagnosis in 41% of the other cases.
The American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association adopted joint guidelines for the treatment of idiopathic pulmonary fibrosis in 2015, and the ATS and ERS updated guidelines for the classification and terminology for idiopathic interstitial pneumonias in 2013. The Lancet Respiratory Medicine published what some consider to be a landmark evaluation of multidisciplinary team agreement on diagnosis of interstitial lung disease following the adoption of these guidelines (Walsh SLF et al. 2016;4[7]:557-65). This study showed that in idiopathic pulmonary fibrosis, multidisciplinary team meetings “have a higher level of agreement on diagnoses, assign diagnoses with higher confidence more frequently, and provide diagnoses that have nonsignificant greater prognostic separation than do clinicians or radiologists in most cases,” the researchers wrote.
In the new study, MDD failed to produce a diagnosis or suggestions about a way forward in only 3.5% of patients, according to the study, which appeared March 30 in CHEST®.
According to Dr. Antin-Ozerkis, accurate diagnosis of ILD is crucial to treatment, but it can be challenging to achieve. The MDD approach has been recommended since 2002 by the ATS and ERS, she said.
The study authors, led by Laurens J. De Sadeleer, MD, of Belgium’s University Hospitals Leuven, define the MDD approach as one “in which expert ILD clinicians, radiologists, and pathologists integrate all available clinical data, laboratory results, high-resolution computed tomography [HRCT] findings, and lung biopsy [when performed].”
For the study, the researchers tracked pre-MDD and MDD diagnoses of 938 consecutive patients with possible ILD who were discussed during 2005-2015. Of these patients, referring physicians made preliminary diagnoses in 49% of cases; in the rest, physicians either failed to develop a diagnosis or offered multiple possible diagnoses.
MDD teams produced a change in diagnosis in 191 – 42% – of patients with a pre-MDD diagnosis. Another condition was diagnosed in 118 of these patients, and the MDD teams declined to classify the other 73 patients pending further investigation.
The MDD teams also were able to produce diagnoses in 80% of cases when referring physicians could not come up with diagnoses.
“Discrepancy between pre-MDD diagnosis before work-up and discussion was remarkable,” the study authors wrote, estimating that MDD added value for 70% of referred patients.
“We believe MDD should be a common practice in the diagnosis of every patient with suspected ILD,” the researchers said.
The study doesn’t examine the challenges of putting MDD into practice, but Dr. Antin-Ozerkis provided some perspective. “It may be difficult for physicians to take the time from a busy practice to meet with a multidisciplinary team. It can require resources to gather the data necessary to comprehensively assess each patient case. Additionally, maintaining staff with experienced pulmonologists, radiologists and pathologists may be costly.”
She added that “there are various ways in which MDD may occur,” and that the pros and cons of different methods have not been well studied. “This practice will likely evolve with the development of new biomarkers and other diagnostic strategies in IPF [idiopathic pulmonary fibrosis].”
Still, she said, “this joint undertaking is clearly vital in helping to guide clinical practice, including therapeutic decisions and discussion of prognosis. For now, any discussion between clinician, radiologist, and pathologist is of benefit.”
Research Foundation-Flanders and University Hospitals Leuven funded the study. Some study authors reported various disclosures. Dr. Antin-Ozerkis disclosed serving as an investigator on several clinical trials for IPF and other ILDs by Boehringer, Promedior, Fibrogen and Roche. She noted that payments go directly to the university with no direct payments to the investigator.
SOURCE: De Sadeleer LJ et al. Chest. 2018 Mar 30. doi: 10.1016/j.chest.2018.03.026.
MDD strategy is crucial for accurate ILD diagnoses
The field of interstitial lung diseases (ILD) is challenging, with more than 200 disorders as possible diagnoses for patients who present to clinicians with similar symptoms and chest x-ray findings. The multidisciplinary discussion (MDD) strategy is very important for attaining an accurate ILD diagnosis.
We have had routine, formal, multidisciplinary discussions at our center since 2008. My guesstimate is that at least a third of patients referred as having idiopathic pulmonary fibrosis or another form of ILD by pulmonologists had been given the wrong diagnosis. Frequently, this was because of incorrect impressions provided by local radiologists and/or pathologists along with the clinician’s own limited knowledge of ILD.
In my experience, some patients described their pulmonologists as becoming irate with them when they asked for a second opinion, and I have had to try to avoid confrontations with referring physicians when trying to explain why the referral diagnosis was inaccurate.
Challenges to instituting the multidisciplinary discussion approach include coverage by health plans for a second-opinion evaluation, the willingness of physicians (for example, pulmonologists) outside of academic referral centers to refer patients to a center capable of adequately conducting an MDD, and patients’ desire to undergo an evaluation at centers of excellence where an MDD can be performed.
One must have also adequate resources to perform a proper MDD. But even in centers that refer patients, pulmonologists should confer with their colleague radiologists – and pathologists when appropriate – to try to make the most accurate diagnosis. And they should continue to question their diagnosis at follow-up appointments, as new symptoms and findings may arise or additional crucial information can become available over time that can point to an alternative diagnosis.
Kenneth C. Meyer, MD, MS, served as medical director of the lung transplant program and head of ILD at the University of Wisconsin–Madison. He reported no relevant disclosures.
Second MDD may be helpful for CTD-related ILD
Accumulating evidence suggests that multidisciplinary committees play a central role in improving the diagnostic accuracy of complex medical conditions. Interstitial lung disease (ILD) encompasses a number of clinical entities and no single diagnostic test alone can discriminate among the various causes of ILD. Instead, these diagnoses are based on a constellation of signs and symptoms, and radiographic, pathologic, and laboratory studies.
However, unanswered questions remain. First, it is unclear whether a single MDD is sufficient. The present study found that 20% of cases were unclassifiable after the MDD. A second MDD may be helpful, especially in patients with ILDs related to connective tissue disease (CTD). The rheumatic diseases most commonly associated with ILD (for example, systemic sclerosis, rheumatoid arthritis, myositis) often evolve at different rates, and not all of the signs and symptoms of these conditions may be present or apparent at the time of the ILD presentation. A second MDD discussion may be particularly helpful in patients presenting with a specific CTD-related autoantibody in the absence of clinical signs and symptoms of a CTD. Another unanswered question is whether MDDs actually improve clinically meaningful outcomes for patients, such as survival and quality of life. At our CTD-ILD Program at the University of California, Los Angeles, we have found that our MDD has augmented patient satisfaction with their care, and it has also improved our ability to identify patients who are eligible for specific clinical studies. Future research is needed to determine to assess the impact of MDD on a variety of patient-centered and practice/research-focused outcomes.
Elizabeth Volkmann, MD, is founder and codirector of the CTD-ILD Program at the University of California, Los Angeles. She disclosed serving as a consultant or as a member of an advisory board for Boehringer Ingelheim and Astellas Pharma. She has received grants from Boehringer Ingelheim, Merck Serono, and the Rheumatology Research Foundation.
MDD strategy is crucial for accurate ILD diagnoses
The field of interstitial lung diseases (ILD) is challenging, with more than 200 disorders as possible diagnoses for patients who present to clinicians with similar symptoms and chest x-ray findings. The multidisciplinary discussion (MDD) strategy is very important for attaining an accurate ILD diagnosis.
We have had routine, formal, multidisciplinary discussions at our center since 2008. My guesstimate is that at least a third of patients referred as having idiopathic pulmonary fibrosis or another form of ILD by pulmonologists had been given the wrong diagnosis. Frequently, this was because of incorrect impressions provided by local radiologists and/or pathologists along with the clinician’s own limited knowledge of ILD.
In my experience, some patients described their pulmonologists as becoming irate with them when they asked for a second opinion, and I have had to try to avoid confrontations with referring physicians when trying to explain why the referral diagnosis was inaccurate.
Challenges to instituting the multidisciplinary discussion approach include coverage by health plans for a second-opinion evaluation, the willingness of physicians (for example, pulmonologists) outside of academic referral centers to refer patients to a center capable of adequately conducting an MDD, and patients’ desire to undergo an evaluation at centers of excellence where an MDD can be performed.
One must have also adequate resources to perform a proper MDD. But even in centers that refer patients, pulmonologists should confer with their colleague radiologists – and pathologists when appropriate – to try to make the most accurate diagnosis. And they should continue to question their diagnosis at follow-up appointments, as new symptoms and findings may arise or additional crucial information can become available over time that can point to an alternative diagnosis.
Kenneth C. Meyer, MD, MS, served as medical director of the lung transplant program and head of ILD at the University of Wisconsin–Madison. He reported no relevant disclosures.
Second MDD may be helpful for CTD-related ILD
Accumulating evidence suggests that multidisciplinary committees play a central role in improving the diagnostic accuracy of complex medical conditions. Interstitial lung disease (ILD) encompasses a number of clinical entities and no single diagnostic test alone can discriminate among the various causes of ILD. Instead, these diagnoses are based on a constellation of signs and symptoms, and radiographic, pathologic, and laboratory studies.
However, unanswered questions remain. First, it is unclear whether a single MDD is sufficient. The present study found that 20% of cases were unclassifiable after the MDD. A second MDD may be helpful, especially in patients with ILDs related to connective tissue disease (CTD). The rheumatic diseases most commonly associated with ILD (for example, systemic sclerosis, rheumatoid arthritis, myositis) often evolve at different rates, and not all of the signs and symptoms of these conditions may be present or apparent at the time of the ILD presentation. A second MDD discussion may be particularly helpful in patients presenting with a specific CTD-related autoantibody in the absence of clinical signs and symptoms of a CTD. Another unanswered question is whether MDDs actually improve clinically meaningful outcomes for patients, such as survival and quality of life. At our CTD-ILD Program at the University of California, Los Angeles, we have found that our MDD has augmented patient satisfaction with their care, and it has also improved our ability to identify patients who are eligible for specific clinical studies. Future research is needed to determine to assess the impact of MDD on a variety of patient-centered and practice/research-focused outcomes.
Elizabeth Volkmann, MD, is founder and codirector of the CTD-ILD Program at the University of California, Los Angeles. She disclosed serving as a consultant or as a member of an advisory board for Boehringer Ingelheim and Astellas Pharma. She has received grants from Boehringer Ingelheim, Merck Serono, and the Rheumatology Research Foundation.
MDD strategy is crucial for accurate ILD diagnoses
The field of interstitial lung diseases (ILD) is challenging, with more than 200 disorders as possible diagnoses for patients who present to clinicians with similar symptoms and chest x-ray findings. The multidisciplinary discussion (MDD) strategy is very important for attaining an accurate ILD diagnosis.
We have had routine, formal, multidisciplinary discussions at our center since 2008. My guesstimate is that at least a third of patients referred as having idiopathic pulmonary fibrosis or another form of ILD by pulmonologists had been given the wrong diagnosis. Frequently, this was because of incorrect impressions provided by local radiologists and/or pathologists along with the clinician’s own limited knowledge of ILD.
In my experience, some patients described their pulmonologists as becoming irate with them when they asked for a second opinion, and I have had to try to avoid confrontations with referring physicians when trying to explain why the referral diagnosis was inaccurate.
Challenges to instituting the multidisciplinary discussion approach include coverage by health plans for a second-opinion evaluation, the willingness of physicians (for example, pulmonologists) outside of academic referral centers to refer patients to a center capable of adequately conducting an MDD, and patients’ desire to undergo an evaluation at centers of excellence where an MDD can be performed.
One must have also adequate resources to perform a proper MDD. But even in centers that refer patients, pulmonologists should confer with their colleague radiologists – and pathologists when appropriate – to try to make the most accurate diagnosis. And they should continue to question their diagnosis at follow-up appointments, as new symptoms and findings may arise or additional crucial information can become available over time that can point to an alternative diagnosis.
Kenneth C. Meyer, MD, MS, served as medical director of the lung transplant program and head of ILD at the University of Wisconsin–Madison. He reported no relevant disclosures.
Second MDD may be helpful for CTD-related ILD
Accumulating evidence suggests that multidisciplinary committees play a central role in improving the diagnostic accuracy of complex medical conditions. Interstitial lung disease (ILD) encompasses a number of clinical entities and no single diagnostic test alone can discriminate among the various causes of ILD. Instead, these diagnoses are based on a constellation of signs and symptoms, and radiographic, pathologic, and laboratory studies.
However, unanswered questions remain. First, it is unclear whether a single MDD is sufficient. The present study found that 20% of cases were unclassifiable after the MDD. A second MDD may be helpful, especially in patients with ILDs related to connective tissue disease (CTD). The rheumatic diseases most commonly associated with ILD (for example, systemic sclerosis, rheumatoid arthritis, myositis) often evolve at different rates, and not all of the signs and symptoms of these conditions may be present or apparent at the time of the ILD presentation. A second MDD discussion may be particularly helpful in patients presenting with a specific CTD-related autoantibody in the absence of clinical signs and symptoms of a CTD. Another unanswered question is whether MDDs actually improve clinically meaningful outcomes for patients, such as survival and quality of life. At our CTD-ILD Program at the University of California, Los Angeles, we have found that our MDD has augmented patient satisfaction with their care, and it has also improved our ability to identify patients who are eligible for specific clinical studies. Future research is needed to determine to assess the impact of MDD on a variety of patient-centered and practice/research-focused outcomes.
Elizabeth Volkmann, MD, is founder and codirector of the CTD-ILD Program at the University of California, Los Angeles. She disclosed serving as a consultant or as a member of an advisory board for Boehringer Ingelheim and Astellas Pharma. She has received grants from Boehringer Ingelheim, Merck Serono, and the Rheumatology Research Foundation.
New research provides strong statistical support for the use of dynamic multidisciplinary discussion in the diagnosis of patients who may have interstitial lung diseases (ILD).
and it changed the diagnosis in 41% of the other cases.
The American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association adopted joint guidelines for the treatment of idiopathic pulmonary fibrosis in 2015, and the ATS and ERS updated guidelines for the classification and terminology for idiopathic interstitial pneumonias in 2013. The Lancet Respiratory Medicine published what some consider to be a landmark evaluation of multidisciplinary team agreement on diagnosis of interstitial lung disease following the adoption of these guidelines (Walsh SLF et al. 2016;4[7]:557-65). This study showed that in idiopathic pulmonary fibrosis, multidisciplinary team meetings “have a higher level of agreement on diagnoses, assign diagnoses with higher confidence more frequently, and provide diagnoses that have nonsignificant greater prognostic separation than do clinicians or radiologists in most cases,” the researchers wrote.
In the new study, MDD failed to produce a diagnosis or suggestions about a way forward in only 3.5% of patients, according to the study, which appeared March 30 in CHEST®.
According to Dr. Antin-Ozerkis, accurate diagnosis of ILD is crucial to treatment, but it can be challenging to achieve. The MDD approach has been recommended since 2002 by the ATS and ERS, she said.
The study authors, led by Laurens J. De Sadeleer, MD, of Belgium’s University Hospitals Leuven, define the MDD approach as one “in which expert ILD clinicians, radiologists, and pathologists integrate all available clinical data, laboratory results, high-resolution computed tomography [HRCT] findings, and lung biopsy [when performed].”
For the study, the researchers tracked pre-MDD and MDD diagnoses of 938 consecutive patients with possible ILD who were discussed during 2005-2015. Of these patients, referring physicians made preliminary diagnoses in 49% of cases; in the rest, physicians either failed to develop a diagnosis or offered multiple possible diagnoses.
MDD teams produced a change in diagnosis in 191 – 42% – of patients with a pre-MDD diagnosis. Another condition was diagnosed in 118 of these patients, and the MDD teams declined to classify the other 73 patients pending further investigation.
The MDD teams also were able to produce diagnoses in 80% of cases when referring physicians could not come up with diagnoses.
“Discrepancy between pre-MDD diagnosis before work-up and discussion was remarkable,” the study authors wrote, estimating that MDD added value for 70% of referred patients.
“We believe MDD should be a common practice in the diagnosis of every patient with suspected ILD,” the researchers said.
The study doesn’t examine the challenges of putting MDD into practice, but Dr. Antin-Ozerkis provided some perspective. “It may be difficult for physicians to take the time from a busy practice to meet with a multidisciplinary team. It can require resources to gather the data necessary to comprehensively assess each patient case. Additionally, maintaining staff with experienced pulmonologists, radiologists and pathologists may be costly.”
She added that “there are various ways in which MDD may occur,” and that the pros and cons of different methods have not been well studied. “This practice will likely evolve with the development of new biomarkers and other diagnostic strategies in IPF [idiopathic pulmonary fibrosis].”
Still, she said, “this joint undertaking is clearly vital in helping to guide clinical practice, including therapeutic decisions and discussion of prognosis. For now, any discussion between clinician, radiologist, and pathologist is of benefit.”
Research Foundation-Flanders and University Hospitals Leuven funded the study. Some study authors reported various disclosures. Dr. Antin-Ozerkis disclosed serving as an investigator on several clinical trials for IPF and other ILDs by Boehringer, Promedior, Fibrogen and Roche. She noted that payments go directly to the university with no direct payments to the investigator.
SOURCE: De Sadeleer LJ et al. Chest. 2018 Mar 30. doi: 10.1016/j.chest.2018.03.026.
New research provides strong statistical support for the use of dynamic multidisciplinary discussion in the diagnosis of patients who may have interstitial lung diseases (ILD).
and it changed the diagnosis in 41% of the other cases.
The American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association adopted joint guidelines for the treatment of idiopathic pulmonary fibrosis in 2015, and the ATS and ERS updated guidelines for the classification and terminology for idiopathic interstitial pneumonias in 2013. The Lancet Respiratory Medicine published what some consider to be a landmark evaluation of multidisciplinary team agreement on diagnosis of interstitial lung disease following the adoption of these guidelines (Walsh SLF et al. 2016;4[7]:557-65). This study showed that in idiopathic pulmonary fibrosis, multidisciplinary team meetings “have a higher level of agreement on diagnoses, assign diagnoses with higher confidence more frequently, and provide diagnoses that have nonsignificant greater prognostic separation than do clinicians or radiologists in most cases,” the researchers wrote.
In the new study, MDD failed to produce a diagnosis or suggestions about a way forward in only 3.5% of patients, according to the study, which appeared March 30 in CHEST®.
According to Dr. Antin-Ozerkis, accurate diagnosis of ILD is crucial to treatment, but it can be challenging to achieve. The MDD approach has been recommended since 2002 by the ATS and ERS, she said.
The study authors, led by Laurens J. De Sadeleer, MD, of Belgium’s University Hospitals Leuven, define the MDD approach as one “in which expert ILD clinicians, radiologists, and pathologists integrate all available clinical data, laboratory results, high-resolution computed tomography [HRCT] findings, and lung biopsy [when performed].”
For the study, the researchers tracked pre-MDD and MDD diagnoses of 938 consecutive patients with possible ILD who were discussed during 2005-2015. Of these patients, referring physicians made preliminary diagnoses in 49% of cases; in the rest, physicians either failed to develop a diagnosis or offered multiple possible diagnoses.
MDD teams produced a change in diagnosis in 191 – 42% – of patients with a pre-MDD diagnosis. Another condition was diagnosed in 118 of these patients, and the MDD teams declined to classify the other 73 patients pending further investigation.
The MDD teams also were able to produce diagnoses in 80% of cases when referring physicians could not come up with diagnoses.
“Discrepancy between pre-MDD diagnosis before work-up and discussion was remarkable,” the study authors wrote, estimating that MDD added value for 70% of referred patients.
“We believe MDD should be a common practice in the diagnosis of every patient with suspected ILD,” the researchers said.
The study doesn’t examine the challenges of putting MDD into practice, but Dr. Antin-Ozerkis provided some perspective. “It may be difficult for physicians to take the time from a busy practice to meet with a multidisciplinary team. It can require resources to gather the data necessary to comprehensively assess each patient case. Additionally, maintaining staff with experienced pulmonologists, radiologists and pathologists may be costly.”
She added that “there are various ways in which MDD may occur,” and that the pros and cons of different methods have not been well studied. “This practice will likely evolve with the development of new biomarkers and other diagnostic strategies in IPF [idiopathic pulmonary fibrosis].”
Still, she said, “this joint undertaking is clearly vital in helping to guide clinical practice, including therapeutic decisions and discussion of prognosis. For now, any discussion between clinician, radiologist, and pathologist is of benefit.”
Research Foundation-Flanders and University Hospitals Leuven funded the study. Some study authors reported various disclosures. Dr. Antin-Ozerkis disclosed serving as an investigator on several clinical trials for IPF and other ILDs by Boehringer, Promedior, Fibrogen and Roche. She noted that payments go directly to the university with no direct payments to the investigator.
SOURCE: De Sadeleer LJ et al. Chest. 2018 Mar 30. doi: 10.1016/j.chest.2018.03.026.
FROM CHEST
Key clinical point: Multidisciplinary discussion (MDD) in cases of suspected interstitial lung disease frequently produces adjustments of previous diagnoses and new diagnoses when none existed previously.
Major finding: MDD teams produced a change in diagnosis in 42% of patients with a pre-MDD diagnosis and in 80% of those without one.
Study details: 938 consecutive patients at University Hospitals Leuven, Belgium, with possible ILD who underwent MDD diagnostics during 2005-2015.
Disclosures: Research Foundation–Flanders and University Hospitals Leuven funded the study. Some study authors reported various disclosures. Dr. Antin-Ozerkis disclosed serving as an investigator on several clinical trials for idiopathic pulmonary fibrosis and other ILDs by Boehringer, Promedior, FibroGen, and Roche. She noted that payments go directly to the university, with no direct payments to the investigator.
Source: De Sadeleer LJ et al. Chest 2018. 2018 Mar 30. doi: 10.1016/j.chest.2018.03.026.
MDedge Daily News: Diabetes patients ignore a deadly risk
Biomarkers rewrite the definition of Alzheimer’s disease. America has a fecal incontinence problem. And a three-in-one pill could change hypertension treatment.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Biomarkers rewrite the definition of Alzheimer’s disease. America has a fecal incontinence problem. And a three-in-one pill could change hypertension treatment.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Biomarkers rewrite the definition of Alzheimer’s disease. America has a fecal incontinence problem. And a three-in-one pill could change hypertension treatment.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Presenting the 2018 SHM Awards of Excellence winners
SHM’s Award of Excellence in Outstanding Service in Hospital Medicine
Dr. Flora Kisuule, MD, SFHM, is an assistant professor at Johns Hopkins School of Medicine and the vice chair for clinical operations for the department of medicine at Johns Hopkins Bayview Medical Center. While at Johns Hopkins University, both in Baltimore, she codeveloped a hospitalist fellowship program that she now directs, as well as a fellowship program specifically for nurse practitioners and physician assistants. Under her leadership as the associate director of the division of hospital medicine, she helped to bring Johns Hopkins Bayview hospitalists’ quality and mortality indicators into the top 5% nationwide and reduced hospital-acquired conditions at Hopkins Bayview to the best of the four regional Hopkins Health System hospitals.
Nationally, she has served on several SHM committees, facilitated at multiple SHM leadership courses, served as vice president of SHM’s Baltimore Chapter, and consulted on hospitalist programs around the country. Internationally, Dr. Kisuule has developed and mentored hospitalist programs in Saudi Arabia, the United Arab Emirates, and Central America. She is currently developing a training program in hospital medicine in Panama and is a Senior Fellow in Hospital Medicine.
SHM’s Award of Excellence in Teamwork in Quality Improvement
Since 2011, the Johns Hopkins Health System hospitals have conducted quality improvement projects to increase the value of care for their patients. Their amazing work catalyzed a grass-roots initiative involving faculty and residents at both Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center.
With a unification of the EHR across the health system, RedondaMiller, MD, MBA, president of Johns Hopkins Hospital, and Renee Demski, MSW, MBA, vice president of quality for Johns Hopkins Health System, created the Johns Hopkins Health System High-Value Care Committee to operationalize initiatives across all six hospitals and Johns Hopkins community physicians. The committee is under the leadership of Trushar Dungarani, DO, FHM, from Howard County General Hospital and Lenny Feldman, MD, SFHM, Amit Pahwa, MD, and Pamela Johnson, MD, from Johns Hopkins Hospital, and comprises provider representatives from each institution and other important contributors from various specialties, including Mike Borowitz, MD, PhD; Dr. Ken Lee, DrPH; Emily Pherson, PharmD; Amy Knight, MD; Tim Niessen, MD, MPH; Keisha Perrin, and Clare Rock.
Initiatives directed by the committee have included reducing inappropriate testing for Clostridium difficile, folate testing for anemia, and duplicative imaging exams, among others. In the 18 months since inception, the committee reduced charges to patients and payers by nearly $4 million and hospital costs by more than $200,000.
Members of this committee joined forces with like-minded institutions to create the High Value Practice Academic Alliance in 2016. Faculty leaders from more than 80 academic centers are now collaborating to increase health care value on a national scale through quality improvement projects, education, and dissemination.
SHM’s Award of Excellence in Teaching
Jennifer O’Toole, MD, MEd, is a med-peds hospitalist at the Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. She serves as the director of the combined internal medicine and pediatrics residency program and is the director of education for the Children’s Hospital division of hospital medicine. In these roles, Dr. O’Toole has established an acute care track for her med-peds residents interested in careers in hospital medicine and a career development boot camp for early-career faculty members and fellows in the division of hospital medicine.
Perhaps Dr. O’Toole’s most instrumental role as an educator has been her involvement in influential educational programs nationally, including the I-PASS Handoff program. Implementation of I-PASS decreased medical errors by 23% and preventable adverse events by 30% in more than 10,000 patient admissions across nine North American hospitals. She has authored more than 30 peer-reviewed publications related to medical education and has presented more than 50 workshops at national meetings. After serving as a member of the planning committee for the Pediatric Hospital Medicine meeting, she is cochair for the 2018 meeting.
SHM’s Award of Clinical Excellence for Physicians
Rick Hilger, MD, SFHM, has been a hospitalist with HealthPartners Regions Hospital in St. Paul, Minnesota for 16 years. He is a national expert in the areas of readmission prevention and care delivery for high-utilizer patients. His work in this area was named Best Clinical Innovation at Hospital Medicine 2012. His collaborative approach to solving complex problems is demonstrated by the amount of time he has volunteered to help other organizations improve their care of high utilizers. Over 90 organizations and hospitals have reached out for assistance in starting their own committees, and Dr. Hilger has shared his time and care plan templates with each one. He was one of the first hospitalists asked to participate on a National Quality Forum committee and has improved hospital reimbursement by over 4 million dollars by developing an internal physician advisor program.
Dr. Hilger also has served on the SHM Annual Conference Committee and the Public Policy Committee, where he has worked with other committee members to advocate for policy changes related to observation status and readmission penalties.
SHM’s Award for Excellence in Humanitarian Services
Michelle Morse, MD, MPH, is a hospitalist and the assistant program director for the internal medicine residency at Brigham and Women’s Hospital in Boston. She is also the founding codirector of EqualHealth, an organization that aims to inspire and support the development of Haiti’s next generation of health care leaders. In 2015, Dr. Morse helped to found the Social Medicine Consortium, a global coalition of more than 450 people representing over 50 universities and organizations in 12 countries, which seeks to address the miseducation of health professionals.
In the aftermath of the 2010 earthquake in Haiti, Dr. Morse was compelled to collaborate with colleagues and friends to help build capacity of health care providers committed to social medicine, medical and nursing education, and social justice. She helped to open and operate a new 300-bed teaching hospital in rural Haiti and founded the first three residency training programs at the hospital. Since that time, the hospital has expanded to serve an area of 3 million people with an annual budget of $12 million.
SHM’s Award of Excellence in Research
Teryl Nuckols, MD, FHM, is a hospitalist and the director of general internal medicine at Cedars-Sinai Medical Center in Los Angeles. She also serves as associate professor of medicine at the University of California, Los Angeles, and a health services researcher at the RAND Corporation in Santa Monica.
Currently, she is principal investigator and coprincipal investigator on two projects funded by the Agency for Healthcare Research and Quality that are evaluating effects of the Medicare Hospital Readmissions Reduction Program. Dr. Nuckols was previously the principal investigator on two R01 research grants from the Agency for Healthcare Research and Quality and the recipient of a K08 Career Development Award. She has evaluated clinical practice guidelines on behalf of SHM as well as policy makers in California and Australia, and her work has led to more than 30 peer-reviewed publications in NEJM, Journal of Hospital Medicine, and JAMA Internal Medicine among others.
SHM’s Award of Clinical Excellence for NPs/PAs
Meredith K. Wold, PA-C, is the supervisor of advanced practice clinicians at HealthPartners Medical Group, which includes 20 nurse practitioners and physician assistants, at Regions Hospital in St. Paul, Minn. She is also the cofounder and co-curriculum director for the HealthPartners Hospital Medicine Physician Assistant Fellowship Program. Under her leadership, the fellowship program offers a clear curriculum that exposes key clinical scenarios and specialties crucial to hospital medicine, preparing new PAs to contribute immediately to team-based care upon completion of the fellowship.
To prevent burnout in a 7-on/7-off schedule, Ms. Wold thought creatively and developed a pooling and “draft” system to give some flexibility and breaks in the block schedule – almost like a fantasy football draft but with patient care shifts for noncontinuity services.
Excellence in Management in Hospital Medicine
Maria Lourdes Novelero, MA, MPA, is the associate chair for administration of the department of medicine at the University of California, San Francisco, and has also served as the administrator of the UCSF division of hospital medicine and its associated medical service from 2005 to 2016. In this role, she managed the department’s expansion from a 20-physician division to an 80-physician division. She also led the department’s pioneering efforts in quality, safety, and value.
Ms. Novelero created a structure to build and manage 10 different hospitalist-run services, including cancer, cardiology, neurosurgery, and liver transplant comanagement services; a procedure service; a palliative care service; and a large non–housestaff medicine service. She co-led a multidisciplinary effort to transform the discharge process, and established and cochaired the department of hospital medicine’s high value care committee, which catalyzed value improvement activities throughout UCSF. This committee’s work led to tangible value improvements, such as a 14% reduction in direct costs and a first-ever positive net margin for the medical service.
Ms. Novelero has more than 25 years of experience in management positions in the United States and Japan.
Certificate of Leadership in Hospital Medicine
Benji K. Mathews, MD, SFHM, CLHM, graduated from the Institute of Technology at the University of Minnesota and the University of Minnesota Medical School. He completed his internal medicine residency and chief residency through the University of Minnesota, Minneapolis. He joined HealthPartners as an academic hospitalist and holds the titles of section head of hospital medicine at Regions Hospital and director of point of care ultrasound (POCUS) for Hospital Medicine at HealthPartners. He is the president for the Minnesota Chapter of the Society of Hospital Medicine (SHM).
Dr. Mathews has a passion for medical education, care delivery, and quality. He is an assistant professor of medicine at the University of Minnesota. As such, he is core faculty for the internal medicine residency program and chair of the Clinical Competency Committee. Dr. Mathews is an active member of SHM as a member of SHM's annual meeting planning committee, organizing and setting the agenda for SHM's record breaking meetings for the last 4 years. Dr. Mathews also serves on SHM's Quality Improvement and Patient Safety Committee and is the chair of the Diagnostic Error Subcommittee. He has completed the Certificate of Leadership in Hospital Medicine (CLHM) with his area of focus on ultrasound in hospital medicine. His work in diagnostic error and POCUS has been well recognized with multiple workshops and presentations given locally and nationally. He is a Fellow in Diagnostic Safety through the Society to Improve Diagnosis in Medicine.
Dr. Mathews also has a passion for global health, rooted in his commitment to reducing health care disparities both locally and globally. He has worked with medical missions, NGOs, and orphanages in Nepal, India, Bolivia, Honduras, and Costa Rica. This led him to complete the global health course at the University of Minnesota.
SHM’s Junior Investigator Award
Christine D. Jones, MD, MS, is an assistant professor of medicine at the University of Colorado's Anschutz medical campus, Aurora, where she is the director of care transitions and director of scholarship for the division of hospital medicine.
Dr. Jones’ research is focused on improving care coordination between clinicians in different settings to improve outcomes for patients discharged with home health care. Her research is supported by a K08 career development award from the Agency for Healthcare Research and Quality. Her overall goal is to improve the quality of care transitions for hospitalized patients discharged to post-acute care settings, including home health care. Dr. Jones has presented at every SHM annual conference since she joined in 2013.
Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize outstanding SHM chapters at HM18 for the fifth annual Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes at SHM’s annual conference.
Platinum Chapters: Gulf States, Iowa, Maryland, Michigan, Minnesota, New Mexico, North Carolina Triangle, St. Louis.
Gold Chapters: Hampton Roads, Kentucky, North Jersey, Pacific Northwest.
Silver Chapters: Boston/Eastern Massachusetts, Houston, Los Angeles, Maine, NYC/Westchester, Rocky Mountain, San Francisco Bay Area, South Central PA, Southwest Florida, Wiregrass.
Outstanding Chapter of the Year
New Mexico. SHM’s New Mexico Chapter has been nominated to receive the Outstanding Chapter of the Year Award for 2017. Consistently, leadership and members go above and beyond the basic requirements to maintain status by creating and cultivating multiple innovative and dynamic opportunities for attendees to educate and network. The chapter participated in SHM’s pilot program to offer CME for chapters, bringing a CME-accredited educational presentation on the care of diverse patients. Their annual meeting this year featured a poster competition for residents, students, and early-career hospitalists; a didactic on antimicrobial stewardship; and a business meeting with elections, member benefits, and highlights of the chapter’s year, and chapter awards. The chapter initiated breakfast meetings with didactics on DACA and on burnout and wellness, and collaborated with the University of New Mexico, Albuquerque on a grand rounds series on physician wellness that was available via telecast to numerous hospitals and providers off site. The chapter hosted a book club, a networking session at HM17, and an informational meeting on SHM membership and the Fellows Program. They have supported the future of hospital medicine by having a table at the UNM School of Medicine’s student activities fair, a Resident of the Year Award, and a position on our council for a resident representative. They have collaborated with the sole internal medicine residency program in New Mexico so that residents in the hospitalist training track have membership in SHM, and they have expanded our juried poster competition to include students. The chapter’s level of originality is not only a benefit to the chapter, but SHM’s chapter program as a whole.
Rising Star Chapter
Kentucky. SHM’s Kentucky Chapter has been nominated to receive the Rising Star Chapter Award for 2017 for their innovation and growth. The chapter’s leadership was involved with the establishment of the Heartland Hospital Medicine Conference and hosted a hospital medicine career panel and bustling Research, Innovations, and Clinical Vignettes abstract and poster competition. Nearly 50 abstracts were submitted, and the top 40 were invited to present posters at the competition. These posters included a mix of attendings, advanced practice providers, residents, and students interested in participating in the local hospital medicine community. The chapter sponsored the first prize winner’s travel to attend Hospital Medicine 2018 and gave awards to best resident and best student posters. The Kentucky Chapter directly recruited 27 hospitalists to join SHM membership in 2017. The Kentucky Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Student Hospitalist Scholarship Recipients
The Society of Hospital Medicine’s Physician in Training Committee launched a Student Hospitalist Scholar Grant program in 2015 for medical students to conduct mentored scholarly projects related to quality improvement and patient safety.
The program was expanded in 2017 and now includes both a summer and longitudinal program for students.
The committee is happy to announce the 4th year of scholar grant recipients to six students based on their ability and interest in hospital medicine, general qualifications, prior educational training, and promise for scholarly activity.
Summer Program
Ilana Scandariato
Cornell University, New York
Project: Understanding the experience of the long-term hospitalized patient with provider fragmentation: A qualitative study
Mentor: Ernie Esquivel, MD, FHM
Maximilian Hemmrich
University of Chicago
Project: Derivation and validation of a COPD readmission risk prediction tool
Mentor: Valerie Press, MD, MPH, SFHM
Sandeep Bala
University of Central Florida, Orlando
Project: The impact of plain language open medical notes on patient activation
Mentor: Marisha Burden, MD, SFHM
Longitudinal Program
Erin Rainosek
University of Texas, San Antonio
Project Title: Design thinking to improve patient experience
Mentor: Luci Leykum, MD, SFHM
Matthew Fallon
Creighton University, Omaha, Neb.
Project: Reducing the hospital readmission rate of congestive heart failure (CHF)
Mentor: Venkata Andukuri, MD, MPH, FHM
Philip Huang
University of Iowa, Iowa City
Project: Expanded patient regionalization to improve care efficiency
Mentor: Ethan Kuperman, MD, FHM
Resident Travel Grant Recipients
The Society of Hospital Medicine’s Physician in Training Committee launched a Resident Travel Grant Program in 2017 for residents to receive funding to attend SHM’s Annual Conference and be recognized for their scholarly work.
The Committee is pleased to announce the first year of Resident Travel Grant Recipients to 10 Residents based on their ability and interest in hospital medicine, research originality, and teaching value, and general qualifications.
Ashley M. Jenkins, MD
Cincinnati Children’s Hospital Medical Center
Poster 41 - Aren’t adults just big kids?: Standardizing care of adults in pediatric hospitals
Brian A. MacDonald Jr., MD, PhD
State University of New York at Buffalo
Poster 135 – Phosphatidylethanol level as a predictor of acute alcohol withdrawal
Christopher S. Bartlett, MD, MPH
University of New Mexico Health Sciences Center, Albuquerque
Poster 47 - Lessons learned from a resident-created experiential quality improvement and patient safety curriculum for medical and nursing students at the University of New Mexico
Christopher T. Su, MD, MPH
Montefiore Medical Center/Albert Einstein College of Medicine, New York
Poster 173 - Concurrent NSAID and warfarin use is associated with increased blood transfusions in hospitalized patients
Madeleine Ivrit Matthiesen, MD
Harvard Medical School/Massachusetts General Hospital Medicine–Pediatrics, Boston
Poster 69 - Resident perceptions of feedback and teaching
Neil Keshvani, MD
University of Texas Southwestern Medical Center, Dallas
Poster 237 - Improving respiratory rate measurement accuracy in the hospital: A quality improvement initiative
Peter N. Barish, MD
University of California, San Francisco
Poster 344 - Costs Related to potential overuse of respiratory viral panel PCRs in general medicine patients
Rachna Rawal, MD
Saint Louis University
Poster 259 - Empowering medicine residents to think before ordering daily labs: A quality improvement study
Yihan Chen, MD
University of California, Los Angeles, Medical Center
Poster 12 - Hospitalist-directed transfers improve emergency room length of stay
Zachary G. Jacobs, MD
University of California, San Francisco
Poster 233 - The prevalence of treating asymptomatic elevated blood pressure with intravenous antihypertensives on the general medicine wards: A potential target for a quality improvement inter-vention
Poster 96 - Factors Impacting time to antibiotic administration in patients with sepsis: A single-center study of electronic health record data
SHM’s Award of Excellence in Outstanding Service in Hospital Medicine
Dr. Flora Kisuule, MD, SFHM, is an assistant professor at Johns Hopkins School of Medicine and the vice chair for clinical operations for the department of medicine at Johns Hopkins Bayview Medical Center. While at Johns Hopkins University, both in Baltimore, she codeveloped a hospitalist fellowship program that she now directs, as well as a fellowship program specifically for nurse practitioners and physician assistants. Under her leadership as the associate director of the division of hospital medicine, she helped to bring Johns Hopkins Bayview hospitalists’ quality and mortality indicators into the top 5% nationwide and reduced hospital-acquired conditions at Hopkins Bayview to the best of the four regional Hopkins Health System hospitals.
Nationally, she has served on several SHM committees, facilitated at multiple SHM leadership courses, served as vice president of SHM’s Baltimore Chapter, and consulted on hospitalist programs around the country. Internationally, Dr. Kisuule has developed and mentored hospitalist programs in Saudi Arabia, the United Arab Emirates, and Central America. She is currently developing a training program in hospital medicine in Panama and is a Senior Fellow in Hospital Medicine.
SHM’s Award of Excellence in Teamwork in Quality Improvement
Since 2011, the Johns Hopkins Health System hospitals have conducted quality improvement projects to increase the value of care for their patients. Their amazing work catalyzed a grass-roots initiative involving faculty and residents at both Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center.
With a unification of the EHR across the health system, RedondaMiller, MD, MBA, president of Johns Hopkins Hospital, and Renee Demski, MSW, MBA, vice president of quality for Johns Hopkins Health System, created the Johns Hopkins Health System High-Value Care Committee to operationalize initiatives across all six hospitals and Johns Hopkins community physicians. The committee is under the leadership of Trushar Dungarani, DO, FHM, from Howard County General Hospital and Lenny Feldman, MD, SFHM, Amit Pahwa, MD, and Pamela Johnson, MD, from Johns Hopkins Hospital, and comprises provider representatives from each institution and other important contributors from various specialties, including Mike Borowitz, MD, PhD; Dr. Ken Lee, DrPH; Emily Pherson, PharmD; Amy Knight, MD; Tim Niessen, MD, MPH; Keisha Perrin, and Clare Rock.
Initiatives directed by the committee have included reducing inappropriate testing for Clostridium difficile, folate testing for anemia, and duplicative imaging exams, among others. In the 18 months since inception, the committee reduced charges to patients and payers by nearly $4 million and hospital costs by more than $200,000.
Members of this committee joined forces with like-minded institutions to create the High Value Practice Academic Alliance in 2016. Faculty leaders from more than 80 academic centers are now collaborating to increase health care value on a national scale through quality improvement projects, education, and dissemination.
SHM’s Award of Excellence in Teaching
Jennifer O’Toole, MD, MEd, is a med-peds hospitalist at the Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. She serves as the director of the combined internal medicine and pediatrics residency program and is the director of education for the Children’s Hospital division of hospital medicine. In these roles, Dr. O’Toole has established an acute care track for her med-peds residents interested in careers in hospital medicine and a career development boot camp for early-career faculty members and fellows in the division of hospital medicine.
Perhaps Dr. O’Toole’s most instrumental role as an educator has been her involvement in influential educational programs nationally, including the I-PASS Handoff program. Implementation of I-PASS decreased medical errors by 23% and preventable adverse events by 30% in more than 10,000 patient admissions across nine North American hospitals. She has authored more than 30 peer-reviewed publications related to medical education and has presented more than 50 workshops at national meetings. After serving as a member of the planning committee for the Pediatric Hospital Medicine meeting, she is cochair for the 2018 meeting.
SHM’s Award of Clinical Excellence for Physicians
Rick Hilger, MD, SFHM, has been a hospitalist with HealthPartners Regions Hospital in St. Paul, Minnesota for 16 years. He is a national expert in the areas of readmission prevention and care delivery for high-utilizer patients. His work in this area was named Best Clinical Innovation at Hospital Medicine 2012. His collaborative approach to solving complex problems is demonstrated by the amount of time he has volunteered to help other organizations improve their care of high utilizers. Over 90 organizations and hospitals have reached out for assistance in starting their own committees, and Dr. Hilger has shared his time and care plan templates with each one. He was one of the first hospitalists asked to participate on a National Quality Forum committee and has improved hospital reimbursement by over 4 million dollars by developing an internal physician advisor program.
Dr. Hilger also has served on the SHM Annual Conference Committee and the Public Policy Committee, where he has worked with other committee members to advocate for policy changes related to observation status and readmission penalties.
SHM’s Award for Excellence in Humanitarian Services
Michelle Morse, MD, MPH, is a hospitalist and the assistant program director for the internal medicine residency at Brigham and Women’s Hospital in Boston. She is also the founding codirector of EqualHealth, an organization that aims to inspire and support the development of Haiti’s next generation of health care leaders. In 2015, Dr. Morse helped to found the Social Medicine Consortium, a global coalition of more than 450 people representing over 50 universities and organizations in 12 countries, which seeks to address the miseducation of health professionals.
In the aftermath of the 2010 earthquake in Haiti, Dr. Morse was compelled to collaborate with colleagues and friends to help build capacity of health care providers committed to social medicine, medical and nursing education, and social justice. She helped to open and operate a new 300-bed teaching hospital in rural Haiti and founded the first three residency training programs at the hospital. Since that time, the hospital has expanded to serve an area of 3 million people with an annual budget of $12 million.
SHM’s Award of Excellence in Research
Teryl Nuckols, MD, FHM, is a hospitalist and the director of general internal medicine at Cedars-Sinai Medical Center in Los Angeles. She also serves as associate professor of medicine at the University of California, Los Angeles, and a health services researcher at the RAND Corporation in Santa Monica.
Currently, she is principal investigator and coprincipal investigator on two projects funded by the Agency for Healthcare Research and Quality that are evaluating effects of the Medicare Hospital Readmissions Reduction Program. Dr. Nuckols was previously the principal investigator on two R01 research grants from the Agency for Healthcare Research and Quality and the recipient of a K08 Career Development Award. She has evaluated clinical practice guidelines on behalf of SHM as well as policy makers in California and Australia, and her work has led to more than 30 peer-reviewed publications in NEJM, Journal of Hospital Medicine, and JAMA Internal Medicine among others.
SHM’s Award of Clinical Excellence for NPs/PAs
Meredith K. Wold, PA-C, is the supervisor of advanced practice clinicians at HealthPartners Medical Group, which includes 20 nurse practitioners and physician assistants, at Regions Hospital in St. Paul, Minn. She is also the cofounder and co-curriculum director for the HealthPartners Hospital Medicine Physician Assistant Fellowship Program. Under her leadership, the fellowship program offers a clear curriculum that exposes key clinical scenarios and specialties crucial to hospital medicine, preparing new PAs to contribute immediately to team-based care upon completion of the fellowship.
To prevent burnout in a 7-on/7-off schedule, Ms. Wold thought creatively and developed a pooling and “draft” system to give some flexibility and breaks in the block schedule – almost like a fantasy football draft but with patient care shifts for noncontinuity services.
Excellence in Management in Hospital Medicine
Maria Lourdes Novelero, MA, MPA, is the associate chair for administration of the department of medicine at the University of California, San Francisco, and has also served as the administrator of the UCSF division of hospital medicine and its associated medical service from 2005 to 2016. In this role, she managed the department’s expansion from a 20-physician division to an 80-physician division. She also led the department’s pioneering efforts in quality, safety, and value.
Ms. Novelero created a structure to build and manage 10 different hospitalist-run services, including cancer, cardiology, neurosurgery, and liver transplant comanagement services; a procedure service; a palliative care service; and a large non–housestaff medicine service. She co-led a multidisciplinary effort to transform the discharge process, and established and cochaired the department of hospital medicine’s high value care committee, which catalyzed value improvement activities throughout UCSF. This committee’s work led to tangible value improvements, such as a 14% reduction in direct costs and a first-ever positive net margin for the medical service.
Ms. Novelero has more than 25 years of experience in management positions in the United States and Japan.
Certificate of Leadership in Hospital Medicine
Benji K. Mathews, MD, SFHM, CLHM, graduated from the Institute of Technology at the University of Minnesota and the University of Minnesota Medical School. He completed his internal medicine residency and chief residency through the University of Minnesota, Minneapolis. He joined HealthPartners as an academic hospitalist and holds the titles of section head of hospital medicine at Regions Hospital and director of point of care ultrasound (POCUS) for Hospital Medicine at HealthPartners. He is the president for the Minnesota Chapter of the Society of Hospital Medicine (SHM).
Dr. Mathews has a passion for medical education, care delivery, and quality. He is an assistant professor of medicine at the University of Minnesota. As such, he is core faculty for the internal medicine residency program and chair of the Clinical Competency Committee. Dr. Mathews is an active member of SHM as a member of SHM's annual meeting planning committee, organizing and setting the agenda for SHM's record breaking meetings for the last 4 years. Dr. Mathews also serves on SHM's Quality Improvement and Patient Safety Committee and is the chair of the Diagnostic Error Subcommittee. He has completed the Certificate of Leadership in Hospital Medicine (CLHM) with his area of focus on ultrasound in hospital medicine. His work in diagnostic error and POCUS has been well recognized with multiple workshops and presentations given locally and nationally. He is a Fellow in Diagnostic Safety through the Society to Improve Diagnosis in Medicine.
Dr. Mathews also has a passion for global health, rooted in his commitment to reducing health care disparities both locally and globally. He has worked with medical missions, NGOs, and orphanages in Nepal, India, Bolivia, Honduras, and Costa Rica. This led him to complete the global health course at the University of Minnesota.
SHM’s Junior Investigator Award
Christine D. Jones, MD, MS, is an assistant professor of medicine at the University of Colorado's Anschutz medical campus, Aurora, where she is the director of care transitions and director of scholarship for the division of hospital medicine.
Dr. Jones’ research is focused on improving care coordination between clinicians in different settings to improve outcomes for patients discharged with home health care. Her research is supported by a K08 career development award from the Agency for Healthcare Research and Quality. Her overall goal is to improve the quality of care transitions for hospitalized patients discharged to post-acute care settings, including home health care. Dr. Jones has presented at every SHM annual conference since she joined in 2013.
Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize outstanding SHM chapters at HM18 for the fifth annual Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes at SHM’s annual conference.
Platinum Chapters: Gulf States, Iowa, Maryland, Michigan, Minnesota, New Mexico, North Carolina Triangle, St. Louis.
Gold Chapters: Hampton Roads, Kentucky, North Jersey, Pacific Northwest.
Silver Chapters: Boston/Eastern Massachusetts, Houston, Los Angeles, Maine, NYC/Westchester, Rocky Mountain, San Francisco Bay Area, South Central PA, Southwest Florida, Wiregrass.
Outstanding Chapter of the Year
New Mexico. SHM’s New Mexico Chapter has been nominated to receive the Outstanding Chapter of the Year Award for 2017. Consistently, leadership and members go above and beyond the basic requirements to maintain status by creating and cultivating multiple innovative and dynamic opportunities for attendees to educate and network. The chapter participated in SHM’s pilot program to offer CME for chapters, bringing a CME-accredited educational presentation on the care of diverse patients. Their annual meeting this year featured a poster competition for residents, students, and early-career hospitalists; a didactic on antimicrobial stewardship; and a business meeting with elections, member benefits, and highlights of the chapter’s year, and chapter awards. The chapter initiated breakfast meetings with didactics on DACA and on burnout and wellness, and collaborated with the University of New Mexico, Albuquerque on a grand rounds series on physician wellness that was available via telecast to numerous hospitals and providers off site. The chapter hosted a book club, a networking session at HM17, and an informational meeting on SHM membership and the Fellows Program. They have supported the future of hospital medicine by having a table at the UNM School of Medicine’s student activities fair, a Resident of the Year Award, and a position on our council for a resident representative. They have collaborated with the sole internal medicine residency program in New Mexico so that residents in the hospitalist training track have membership in SHM, and they have expanded our juried poster competition to include students. The chapter’s level of originality is not only a benefit to the chapter, but SHM’s chapter program as a whole.
Rising Star Chapter
Kentucky. SHM’s Kentucky Chapter has been nominated to receive the Rising Star Chapter Award for 2017 for their innovation and growth. The chapter’s leadership was involved with the establishment of the Heartland Hospital Medicine Conference and hosted a hospital medicine career panel and bustling Research, Innovations, and Clinical Vignettes abstract and poster competition. Nearly 50 abstracts were submitted, and the top 40 were invited to present posters at the competition. These posters included a mix of attendings, advanced practice providers, residents, and students interested in participating in the local hospital medicine community. The chapter sponsored the first prize winner’s travel to attend Hospital Medicine 2018 and gave awards to best resident and best student posters. The Kentucky Chapter directly recruited 27 hospitalists to join SHM membership in 2017. The Kentucky Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Student Hospitalist Scholarship Recipients
The Society of Hospital Medicine’s Physician in Training Committee launched a Student Hospitalist Scholar Grant program in 2015 for medical students to conduct mentored scholarly projects related to quality improvement and patient safety.
The program was expanded in 2017 and now includes both a summer and longitudinal program for students.
The committee is happy to announce the 4th year of scholar grant recipients to six students based on their ability and interest in hospital medicine, general qualifications, prior educational training, and promise for scholarly activity.
Summer Program
Ilana Scandariato
Cornell University, New York
Project: Understanding the experience of the long-term hospitalized patient with provider fragmentation: A qualitative study
Mentor: Ernie Esquivel, MD, FHM
Maximilian Hemmrich
University of Chicago
Project: Derivation and validation of a COPD readmission risk prediction tool
Mentor: Valerie Press, MD, MPH, SFHM
Sandeep Bala
University of Central Florida, Orlando
Project: The impact of plain language open medical notes on patient activation
Mentor: Marisha Burden, MD, SFHM
Longitudinal Program
Erin Rainosek
University of Texas, San Antonio
Project Title: Design thinking to improve patient experience
Mentor: Luci Leykum, MD, SFHM
Matthew Fallon
Creighton University, Omaha, Neb.
Project: Reducing the hospital readmission rate of congestive heart failure (CHF)
Mentor: Venkata Andukuri, MD, MPH, FHM
Philip Huang
University of Iowa, Iowa City
Project: Expanded patient regionalization to improve care efficiency
Mentor: Ethan Kuperman, MD, FHM
Resident Travel Grant Recipients
The Society of Hospital Medicine’s Physician in Training Committee launched a Resident Travel Grant Program in 2017 for residents to receive funding to attend SHM’s Annual Conference and be recognized for their scholarly work.
The Committee is pleased to announce the first year of Resident Travel Grant Recipients to 10 Residents based on their ability and interest in hospital medicine, research originality, and teaching value, and general qualifications.
Ashley M. Jenkins, MD
Cincinnati Children’s Hospital Medical Center
Poster 41 - Aren’t adults just big kids?: Standardizing care of adults in pediatric hospitals
Brian A. MacDonald Jr., MD, PhD
State University of New York at Buffalo
Poster 135 – Phosphatidylethanol level as a predictor of acute alcohol withdrawal
Christopher S. Bartlett, MD, MPH
University of New Mexico Health Sciences Center, Albuquerque
Poster 47 - Lessons learned from a resident-created experiential quality improvement and patient safety curriculum for medical and nursing students at the University of New Mexico
Christopher T. Su, MD, MPH
Montefiore Medical Center/Albert Einstein College of Medicine, New York
Poster 173 - Concurrent NSAID and warfarin use is associated with increased blood transfusions in hospitalized patients
Madeleine Ivrit Matthiesen, MD
Harvard Medical School/Massachusetts General Hospital Medicine–Pediatrics, Boston
Poster 69 - Resident perceptions of feedback and teaching
Neil Keshvani, MD
University of Texas Southwestern Medical Center, Dallas
Poster 237 - Improving respiratory rate measurement accuracy in the hospital: A quality improvement initiative
Peter N. Barish, MD
University of California, San Francisco
Poster 344 - Costs Related to potential overuse of respiratory viral panel PCRs in general medicine patients
Rachna Rawal, MD
Saint Louis University
Poster 259 - Empowering medicine residents to think before ordering daily labs: A quality improvement study
Yihan Chen, MD
University of California, Los Angeles, Medical Center
Poster 12 - Hospitalist-directed transfers improve emergency room length of stay
Zachary G. Jacobs, MD
University of California, San Francisco
Poster 233 - The prevalence of treating asymptomatic elevated blood pressure with intravenous antihypertensives on the general medicine wards: A potential target for a quality improvement inter-vention
Poster 96 - Factors Impacting time to antibiotic administration in patients with sepsis: A single-center study of electronic health record data
SHM’s Award of Excellence in Outstanding Service in Hospital Medicine
Dr. Flora Kisuule, MD, SFHM, is an assistant professor at Johns Hopkins School of Medicine and the vice chair for clinical operations for the department of medicine at Johns Hopkins Bayview Medical Center. While at Johns Hopkins University, both in Baltimore, she codeveloped a hospitalist fellowship program that she now directs, as well as a fellowship program specifically for nurse practitioners and physician assistants. Under her leadership as the associate director of the division of hospital medicine, she helped to bring Johns Hopkins Bayview hospitalists’ quality and mortality indicators into the top 5% nationwide and reduced hospital-acquired conditions at Hopkins Bayview to the best of the four regional Hopkins Health System hospitals.
Nationally, she has served on several SHM committees, facilitated at multiple SHM leadership courses, served as vice president of SHM’s Baltimore Chapter, and consulted on hospitalist programs around the country. Internationally, Dr. Kisuule has developed and mentored hospitalist programs in Saudi Arabia, the United Arab Emirates, and Central America. She is currently developing a training program in hospital medicine in Panama and is a Senior Fellow in Hospital Medicine.
SHM’s Award of Excellence in Teamwork in Quality Improvement
Since 2011, the Johns Hopkins Health System hospitals have conducted quality improvement projects to increase the value of care for their patients. Their amazing work catalyzed a grass-roots initiative involving faculty and residents at both Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center.
With a unification of the EHR across the health system, RedondaMiller, MD, MBA, president of Johns Hopkins Hospital, and Renee Demski, MSW, MBA, vice president of quality for Johns Hopkins Health System, created the Johns Hopkins Health System High-Value Care Committee to operationalize initiatives across all six hospitals and Johns Hopkins community physicians. The committee is under the leadership of Trushar Dungarani, DO, FHM, from Howard County General Hospital and Lenny Feldman, MD, SFHM, Amit Pahwa, MD, and Pamela Johnson, MD, from Johns Hopkins Hospital, and comprises provider representatives from each institution and other important contributors from various specialties, including Mike Borowitz, MD, PhD; Dr. Ken Lee, DrPH; Emily Pherson, PharmD; Amy Knight, MD; Tim Niessen, MD, MPH; Keisha Perrin, and Clare Rock.
Initiatives directed by the committee have included reducing inappropriate testing for Clostridium difficile, folate testing for anemia, and duplicative imaging exams, among others. In the 18 months since inception, the committee reduced charges to patients and payers by nearly $4 million and hospital costs by more than $200,000.
Members of this committee joined forces with like-minded institutions to create the High Value Practice Academic Alliance in 2016. Faculty leaders from more than 80 academic centers are now collaborating to increase health care value on a national scale through quality improvement projects, education, and dissemination.
SHM’s Award of Excellence in Teaching
Jennifer O’Toole, MD, MEd, is a med-peds hospitalist at the Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. She serves as the director of the combined internal medicine and pediatrics residency program and is the director of education for the Children’s Hospital division of hospital medicine. In these roles, Dr. O’Toole has established an acute care track for her med-peds residents interested in careers in hospital medicine and a career development boot camp for early-career faculty members and fellows in the division of hospital medicine.
Perhaps Dr. O’Toole’s most instrumental role as an educator has been her involvement in influential educational programs nationally, including the I-PASS Handoff program. Implementation of I-PASS decreased medical errors by 23% and preventable adverse events by 30% in more than 10,000 patient admissions across nine North American hospitals. She has authored more than 30 peer-reviewed publications related to medical education and has presented more than 50 workshops at national meetings. After serving as a member of the planning committee for the Pediatric Hospital Medicine meeting, she is cochair for the 2018 meeting.
SHM’s Award of Clinical Excellence for Physicians
Rick Hilger, MD, SFHM, has been a hospitalist with HealthPartners Regions Hospital in St. Paul, Minnesota for 16 years. He is a national expert in the areas of readmission prevention and care delivery for high-utilizer patients. His work in this area was named Best Clinical Innovation at Hospital Medicine 2012. His collaborative approach to solving complex problems is demonstrated by the amount of time he has volunteered to help other organizations improve their care of high utilizers. Over 90 organizations and hospitals have reached out for assistance in starting their own committees, and Dr. Hilger has shared his time and care plan templates with each one. He was one of the first hospitalists asked to participate on a National Quality Forum committee and has improved hospital reimbursement by over 4 million dollars by developing an internal physician advisor program.
Dr. Hilger also has served on the SHM Annual Conference Committee and the Public Policy Committee, where he has worked with other committee members to advocate for policy changes related to observation status and readmission penalties.
SHM’s Award for Excellence in Humanitarian Services
Michelle Morse, MD, MPH, is a hospitalist and the assistant program director for the internal medicine residency at Brigham and Women’s Hospital in Boston. She is also the founding codirector of EqualHealth, an organization that aims to inspire and support the development of Haiti’s next generation of health care leaders. In 2015, Dr. Morse helped to found the Social Medicine Consortium, a global coalition of more than 450 people representing over 50 universities and organizations in 12 countries, which seeks to address the miseducation of health professionals.
In the aftermath of the 2010 earthquake in Haiti, Dr. Morse was compelled to collaborate with colleagues and friends to help build capacity of health care providers committed to social medicine, medical and nursing education, and social justice. She helped to open and operate a new 300-bed teaching hospital in rural Haiti and founded the first three residency training programs at the hospital. Since that time, the hospital has expanded to serve an area of 3 million people with an annual budget of $12 million.
SHM’s Award of Excellence in Research
Teryl Nuckols, MD, FHM, is a hospitalist and the director of general internal medicine at Cedars-Sinai Medical Center in Los Angeles. She also serves as associate professor of medicine at the University of California, Los Angeles, and a health services researcher at the RAND Corporation in Santa Monica.
Currently, she is principal investigator and coprincipal investigator on two projects funded by the Agency for Healthcare Research and Quality that are evaluating effects of the Medicare Hospital Readmissions Reduction Program. Dr. Nuckols was previously the principal investigator on two R01 research grants from the Agency for Healthcare Research and Quality and the recipient of a K08 Career Development Award. She has evaluated clinical practice guidelines on behalf of SHM as well as policy makers in California and Australia, and her work has led to more than 30 peer-reviewed publications in NEJM, Journal of Hospital Medicine, and JAMA Internal Medicine among others.
SHM’s Award of Clinical Excellence for NPs/PAs
Meredith K. Wold, PA-C, is the supervisor of advanced practice clinicians at HealthPartners Medical Group, which includes 20 nurse practitioners and physician assistants, at Regions Hospital in St. Paul, Minn. She is also the cofounder and co-curriculum director for the HealthPartners Hospital Medicine Physician Assistant Fellowship Program. Under her leadership, the fellowship program offers a clear curriculum that exposes key clinical scenarios and specialties crucial to hospital medicine, preparing new PAs to contribute immediately to team-based care upon completion of the fellowship.
To prevent burnout in a 7-on/7-off schedule, Ms. Wold thought creatively and developed a pooling and “draft” system to give some flexibility and breaks in the block schedule – almost like a fantasy football draft but with patient care shifts for noncontinuity services.
Excellence in Management in Hospital Medicine
Maria Lourdes Novelero, MA, MPA, is the associate chair for administration of the department of medicine at the University of California, San Francisco, and has also served as the administrator of the UCSF division of hospital medicine and its associated medical service from 2005 to 2016. In this role, she managed the department’s expansion from a 20-physician division to an 80-physician division. She also led the department’s pioneering efforts in quality, safety, and value.
Ms. Novelero created a structure to build and manage 10 different hospitalist-run services, including cancer, cardiology, neurosurgery, and liver transplant comanagement services; a procedure service; a palliative care service; and a large non–housestaff medicine service. She co-led a multidisciplinary effort to transform the discharge process, and established and cochaired the department of hospital medicine’s high value care committee, which catalyzed value improvement activities throughout UCSF. This committee’s work led to tangible value improvements, such as a 14% reduction in direct costs and a first-ever positive net margin for the medical service.
Ms. Novelero has more than 25 years of experience in management positions in the United States and Japan.
Certificate of Leadership in Hospital Medicine
Benji K. Mathews, MD, SFHM, CLHM, graduated from the Institute of Technology at the University of Minnesota and the University of Minnesota Medical School. He completed his internal medicine residency and chief residency through the University of Minnesota, Minneapolis. He joined HealthPartners as an academic hospitalist and holds the titles of section head of hospital medicine at Regions Hospital and director of point of care ultrasound (POCUS) for Hospital Medicine at HealthPartners. He is the president for the Minnesota Chapter of the Society of Hospital Medicine (SHM).
Dr. Mathews has a passion for medical education, care delivery, and quality. He is an assistant professor of medicine at the University of Minnesota. As such, he is core faculty for the internal medicine residency program and chair of the Clinical Competency Committee. Dr. Mathews is an active member of SHM as a member of SHM's annual meeting planning committee, organizing and setting the agenda for SHM's record breaking meetings for the last 4 years. Dr. Mathews also serves on SHM's Quality Improvement and Patient Safety Committee and is the chair of the Diagnostic Error Subcommittee. He has completed the Certificate of Leadership in Hospital Medicine (CLHM) with his area of focus on ultrasound in hospital medicine. His work in diagnostic error and POCUS has been well recognized with multiple workshops and presentations given locally and nationally. He is a Fellow in Diagnostic Safety through the Society to Improve Diagnosis in Medicine.
Dr. Mathews also has a passion for global health, rooted in his commitment to reducing health care disparities both locally and globally. He has worked with medical missions, NGOs, and orphanages in Nepal, India, Bolivia, Honduras, and Costa Rica. This led him to complete the global health course at the University of Minnesota.
SHM’s Junior Investigator Award
Christine D. Jones, MD, MS, is an assistant professor of medicine at the University of Colorado's Anschutz medical campus, Aurora, where she is the director of care transitions and director of scholarship for the division of hospital medicine.
Dr. Jones’ research is focused on improving care coordination between clinicians in different settings to improve outcomes for patients discharged with home health care. Her research is supported by a K08 career development award from the Agency for Healthcare Research and Quality. Her overall goal is to improve the quality of care transitions for hospitalized patients discharged to post-acute care settings, including home health care. Dr. Jones has presented at every SHM annual conference since she joined in 2013.
Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize outstanding SHM chapters at HM18 for the fifth annual Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes at SHM’s annual conference.
Platinum Chapters: Gulf States, Iowa, Maryland, Michigan, Minnesota, New Mexico, North Carolina Triangle, St. Louis.
Gold Chapters: Hampton Roads, Kentucky, North Jersey, Pacific Northwest.
Silver Chapters: Boston/Eastern Massachusetts, Houston, Los Angeles, Maine, NYC/Westchester, Rocky Mountain, San Francisco Bay Area, South Central PA, Southwest Florida, Wiregrass.
Outstanding Chapter of the Year
New Mexico. SHM’s New Mexico Chapter has been nominated to receive the Outstanding Chapter of the Year Award for 2017. Consistently, leadership and members go above and beyond the basic requirements to maintain status by creating and cultivating multiple innovative and dynamic opportunities for attendees to educate and network. The chapter participated in SHM’s pilot program to offer CME for chapters, bringing a CME-accredited educational presentation on the care of diverse patients. Their annual meeting this year featured a poster competition for residents, students, and early-career hospitalists; a didactic on antimicrobial stewardship; and a business meeting with elections, member benefits, and highlights of the chapter’s year, and chapter awards. The chapter initiated breakfast meetings with didactics on DACA and on burnout and wellness, and collaborated with the University of New Mexico, Albuquerque on a grand rounds series on physician wellness that was available via telecast to numerous hospitals and providers off site. The chapter hosted a book club, a networking session at HM17, and an informational meeting on SHM membership and the Fellows Program. They have supported the future of hospital medicine by having a table at the UNM School of Medicine’s student activities fair, a Resident of the Year Award, and a position on our council for a resident representative. They have collaborated with the sole internal medicine residency program in New Mexico so that residents in the hospitalist training track have membership in SHM, and they have expanded our juried poster competition to include students. The chapter’s level of originality is not only a benefit to the chapter, but SHM’s chapter program as a whole.
Rising Star Chapter
Kentucky. SHM’s Kentucky Chapter has been nominated to receive the Rising Star Chapter Award for 2017 for their innovation and growth. The chapter’s leadership was involved with the establishment of the Heartland Hospital Medicine Conference and hosted a hospital medicine career panel and bustling Research, Innovations, and Clinical Vignettes abstract and poster competition. Nearly 50 abstracts were submitted, and the top 40 were invited to present posters at the competition. These posters included a mix of attendings, advanced practice providers, residents, and students interested in participating in the local hospital medicine community. The chapter sponsored the first prize winner’s travel to attend Hospital Medicine 2018 and gave awards to best resident and best student posters. The Kentucky Chapter directly recruited 27 hospitalists to join SHM membership in 2017. The Kentucky Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Student Hospitalist Scholarship Recipients
The Society of Hospital Medicine’s Physician in Training Committee launched a Student Hospitalist Scholar Grant program in 2015 for medical students to conduct mentored scholarly projects related to quality improvement and patient safety.
The program was expanded in 2017 and now includes both a summer and longitudinal program for students.
The committee is happy to announce the 4th year of scholar grant recipients to six students based on their ability and interest in hospital medicine, general qualifications, prior educational training, and promise for scholarly activity.
Summer Program
Ilana Scandariato
Cornell University, New York
Project: Understanding the experience of the long-term hospitalized patient with provider fragmentation: A qualitative study
Mentor: Ernie Esquivel, MD, FHM
Maximilian Hemmrich
University of Chicago
Project: Derivation and validation of a COPD readmission risk prediction tool
Mentor: Valerie Press, MD, MPH, SFHM
Sandeep Bala
University of Central Florida, Orlando
Project: The impact of plain language open medical notes on patient activation
Mentor: Marisha Burden, MD, SFHM
Longitudinal Program
Erin Rainosek
University of Texas, San Antonio
Project Title: Design thinking to improve patient experience
Mentor: Luci Leykum, MD, SFHM
Matthew Fallon
Creighton University, Omaha, Neb.
Project: Reducing the hospital readmission rate of congestive heart failure (CHF)
Mentor: Venkata Andukuri, MD, MPH, FHM
Philip Huang
University of Iowa, Iowa City
Project: Expanded patient regionalization to improve care efficiency
Mentor: Ethan Kuperman, MD, FHM
Resident Travel Grant Recipients
The Society of Hospital Medicine’s Physician in Training Committee launched a Resident Travel Grant Program in 2017 for residents to receive funding to attend SHM’s Annual Conference and be recognized for their scholarly work.
The Committee is pleased to announce the first year of Resident Travel Grant Recipients to 10 Residents based on their ability and interest in hospital medicine, research originality, and teaching value, and general qualifications.
Ashley M. Jenkins, MD
Cincinnati Children’s Hospital Medical Center
Poster 41 - Aren’t adults just big kids?: Standardizing care of adults in pediatric hospitals
Brian A. MacDonald Jr., MD, PhD
State University of New York at Buffalo
Poster 135 – Phosphatidylethanol level as a predictor of acute alcohol withdrawal
Christopher S. Bartlett, MD, MPH
University of New Mexico Health Sciences Center, Albuquerque
Poster 47 - Lessons learned from a resident-created experiential quality improvement and patient safety curriculum for medical and nursing students at the University of New Mexico
Christopher T. Su, MD, MPH
Montefiore Medical Center/Albert Einstein College of Medicine, New York
Poster 173 - Concurrent NSAID and warfarin use is associated with increased blood transfusions in hospitalized patients
Madeleine Ivrit Matthiesen, MD
Harvard Medical School/Massachusetts General Hospital Medicine–Pediatrics, Boston
Poster 69 - Resident perceptions of feedback and teaching
Neil Keshvani, MD
University of Texas Southwestern Medical Center, Dallas
Poster 237 - Improving respiratory rate measurement accuracy in the hospital: A quality improvement initiative
Peter N. Barish, MD
University of California, San Francisco
Poster 344 - Costs Related to potential overuse of respiratory viral panel PCRs in general medicine patients
Rachna Rawal, MD
Saint Louis University
Poster 259 - Empowering medicine residents to think before ordering daily labs: A quality improvement study
Yihan Chen, MD
University of California, Los Angeles, Medical Center
Poster 12 - Hospitalist-directed transfers improve emergency room length of stay
Zachary G. Jacobs, MD
University of California, San Francisco
Poster 233 - The prevalence of treating asymptomatic elevated blood pressure with intravenous antihypertensives on the general medicine wards: A potential target for a quality improvement inter-vention
Poster 96 - Factors Impacting time to antibiotic administration in patients with sepsis: A single-center study of electronic health record data


















