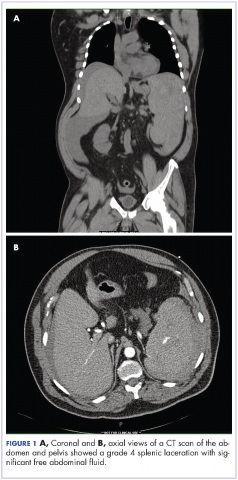
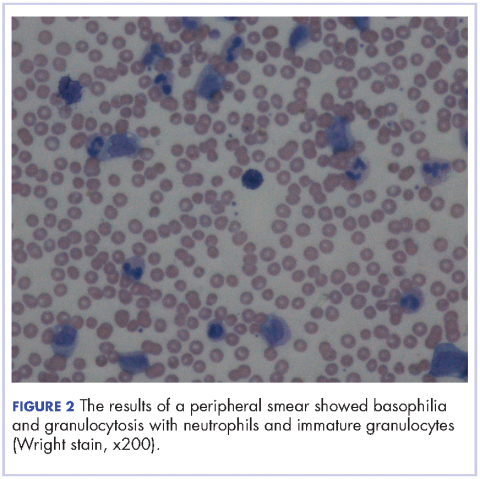
User login
Three regular meals a day is best in T2DM
CHICAGO – Spreading calories out over three regular meals a day, instead of six small ones, helps patients with type 2 diabetes lose weight and better control their blood glucose, especially when breakfast is the main meal of the day, according to Israeli investigators.
Too often, obese type 2 patients end up on a treadmill of ever-increasing insulin doses that lead, in turn, to more weight gain and still more insulin, plus greater risk of diabetic complications. “To break this vicious cycle, we need to better control diabetes with less insulin,” said lead investigator Daniela Jakubowicz, MD, an endocrinologist at the Wolfson Medical Center at Tel Aviv University.
The team had a hunch that meal-timing would help, so they randomized 19 obese, uncontrolled type 2 patients to three meals a day, and 18 to six meals. The overall calories were the same in each arm: 1,500-1,800 per day.
Those in the three-meal (3m) group took 50% of their calories at breakfast, mostly from carbohydrates and protein, and consumed by 9:30 a.m. One-third of their calories were consumed at lunch, and the final 17% at dinner, where carbohydrates were not allowed. Between-meal snacking on vegetables was allowed. The six-meal (6m) group took 20%, 25%, and 25% of their calories at breakfast, lunch, and dinner, plus three snacks each consisting of 10% of their daily caloric intake. The study included a few more men than women, and insulin was titrated biweekly in the two groups as needed.
The groups were statistically identical at baseline, with a mean age of about 70 years, a 20-year or so history of type 2 diabetes, and a mean body mass index (BMI) of just under 33 kg/m2. However, at the end of 3 months, the 3m group was doing much better.They had lost a mean of 5.4 kg at that point and reduced their BMI by a mean of 1.9 kg/m2 and their HbA1c 1.2%. The 6m group, meanwhile, gained a mean of 0.26 kg, increased their BMI 0.1 kg/m2, and dropped their HbA1c only 0.2%. Both groups started with a mean HbA1c of around 8%.
Hunger and craving scores improved in the 3m group, as well, and their total daily insulin dose dropped 27 units from a baseline of 74 units; 6m subjects went up 5 units/day, from a baseline of 71 units. The findings were all statistically significant.
In short, the 3m is “more effective than the traditional diet with six small meals evenly distributed along the day, for weight loss, overall glycemia, HbA1c, appetite, and for the reduction of insulin requirements. Therefore, the three-meal schedule with a high-energy breakfast “should be a strategy to improve diabetes control and outcome,” Dr. Jakubowicz said at the annual meeting of the Endocrine Society.
The findings caught the attention of her audience. One endocrinologist said that “patients always ask me about doing six small meals a day.”
Most of the mean blood glucose reduction found in the 3m group happened after only 14 days on the diet, suggesting an improvement in hepatic insulin sensitivity. There was no correlation between glucose levels and weight; the beneficial effects of the 3m diet appeared independent of weight loss.
Those benefits instead might attributable to effects on “clock genes,” which control the circadian oscillation of gene expression. The investigators are looking into the matter further. .
The funding source was not reported. Dr. Jakubowicz, author of “The Big Breakfast Diet” (Workman Publishing Co., 2009), had no other disclosures. The other investigators had no disclosures.
aotto@frontlinemedcom.com
SOURCE: Jakubowicz D et al. ENDO 2018, Abstract OR05-2.
CHICAGO – Spreading calories out over three regular meals a day, instead of six small ones, helps patients with type 2 diabetes lose weight and better control their blood glucose, especially when breakfast is the main meal of the day, according to Israeli investigators.
Too often, obese type 2 patients end up on a treadmill of ever-increasing insulin doses that lead, in turn, to more weight gain and still more insulin, plus greater risk of diabetic complications. “To break this vicious cycle, we need to better control diabetes with less insulin,” said lead investigator Daniela Jakubowicz, MD, an endocrinologist at the Wolfson Medical Center at Tel Aviv University.
The team had a hunch that meal-timing would help, so they randomized 19 obese, uncontrolled type 2 patients to three meals a day, and 18 to six meals. The overall calories were the same in each arm: 1,500-1,800 per day.
Those in the three-meal (3m) group took 50% of their calories at breakfast, mostly from carbohydrates and protein, and consumed by 9:30 a.m. One-third of their calories were consumed at lunch, and the final 17% at dinner, where carbohydrates were not allowed. Between-meal snacking on vegetables was allowed. The six-meal (6m) group took 20%, 25%, and 25% of their calories at breakfast, lunch, and dinner, plus three snacks each consisting of 10% of their daily caloric intake. The study included a few more men than women, and insulin was titrated biweekly in the two groups as needed.
The groups were statistically identical at baseline, with a mean age of about 70 years, a 20-year or so history of type 2 diabetes, and a mean body mass index (BMI) of just under 33 kg/m2. However, at the end of 3 months, the 3m group was doing much better.They had lost a mean of 5.4 kg at that point and reduced their BMI by a mean of 1.9 kg/m2 and their HbA1c 1.2%. The 6m group, meanwhile, gained a mean of 0.26 kg, increased their BMI 0.1 kg/m2, and dropped their HbA1c only 0.2%. Both groups started with a mean HbA1c of around 8%.
Hunger and craving scores improved in the 3m group, as well, and their total daily insulin dose dropped 27 units from a baseline of 74 units; 6m subjects went up 5 units/day, from a baseline of 71 units. The findings were all statistically significant.
In short, the 3m is “more effective than the traditional diet with six small meals evenly distributed along the day, for weight loss, overall glycemia, HbA1c, appetite, and for the reduction of insulin requirements. Therefore, the three-meal schedule with a high-energy breakfast “should be a strategy to improve diabetes control and outcome,” Dr. Jakubowicz said at the annual meeting of the Endocrine Society.
The findings caught the attention of her audience. One endocrinologist said that “patients always ask me about doing six small meals a day.”
Most of the mean blood glucose reduction found in the 3m group happened after only 14 days on the diet, suggesting an improvement in hepatic insulin sensitivity. There was no correlation between glucose levels and weight; the beneficial effects of the 3m diet appeared independent of weight loss.
Those benefits instead might attributable to effects on “clock genes,” which control the circadian oscillation of gene expression. The investigators are looking into the matter further. .
The funding source was not reported. Dr. Jakubowicz, author of “The Big Breakfast Diet” (Workman Publishing Co., 2009), had no other disclosures. The other investigators had no disclosures.
aotto@frontlinemedcom.com
SOURCE: Jakubowicz D et al. ENDO 2018, Abstract OR05-2.
CHICAGO – Spreading calories out over three regular meals a day, instead of six small ones, helps patients with type 2 diabetes lose weight and better control their blood glucose, especially when breakfast is the main meal of the day, according to Israeli investigators.
Too often, obese type 2 patients end up on a treadmill of ever-increasing insulin doses that lead, in turn, to more weight gain and still more insulin, plus greater risk of diabetic complications. “To break this vicious cycle, we need to better control diabetes with less insulin,” said lead investigator Daniela Jakubowicz, MD, an endocrinologist at the Wolfson Medical Center at Tel Aviv University.
The team had a hunch that meal-timing would help, so they randomized 19 obese, uncontrolled type 2 patients to three meals a day, and 18 to six meals. The overall calories were the same in each arm: 1,500-1,800 per day.
Those in the three-meal (3m) group took 50% of their calories at breakfast, mostly from carbohydrates and protein, and consumed by 9:30 a.m. One-third of their calories were consumed at lunch, and the final 17% at dinner, where carbohydrates were not allowed. Between-meal snacking on vegetables was allowed. The six-meal (6m) group took 20%, 25%, and 25% of their calories at breakfast, lunch, and dinner, plus three snacks each consisting of 10% of their daily caloric intake. The study included a few more men than women, and insulin was titrated biweekly in the two groups as needed.
The groups were statistically identical at baseline, with a mean age of about 70 years, a 20-year or so history of type 2 diabetes, and a mean body mass index (BMI) of just under 33 kg/m2. However, at the end of 3 months, the 3m group was doing much better.They had lost a mean of 5.4 kg at that point and reduced their BMI by a mean of 1.9 kg/m2 and their HbA1c 1.2%. The 6m group, meanwhile, gained a mean of 0.26 kg, increased their BMI 0.1 kg/m2, and dropped their HbA1c only 0.2%. Both groups started with a mean HbA1c of around 8%.
Hunger and craving scores improved in the 3m group, as well, and their total daily insulin dose dropped 27 units from a baseline of 74 units; 6m subjects went up 5 units/day, from a baseline of 71 units. The findings were all statistically significant.
In short, the 3m is “more effective than the traditional diet with six small meals evenly distributed along the day, for weight loss, overall glycemia, HbA1c, appetite, and for the reduction of insulin requirements. Therefore, the three-meal schedule with a high-energy breakfast “should be a strategy to improve diabetes control and outcome,” Dr. Jakubowicz said at the annual meeting of the Endocrine Society.
The findings caught the attention of her audience. One endocrinologist said that “patients always ask me about doing six small meals a day.”
Most of the mean blood glucose reduction found in the 3m group happened after only 14 days on the diet, suggesting an improvement in hepatic insulin sensitivity. There was no correlation between glucose levels and weight; the beneficial effects of the 3m diet appeared independent of weight loss.
Those benefits instead might attributable to effects on “clock genes,” which control the circadian oscillation of gene expression. The investigators are looking into the matter further. .
The funding source was not reported. Dr. Jakubowicz, author of “The Big Breakfast Diet” (Workman Publishing Co., 2009), had no other disclosures. The other investigators had no disclosures.
aotto@frontlinemedcom.com
SOURCE: Jakubowicz D et al. ENDO 2018, Abstract OR05-2.
REPORTING FROM ENDO 2018
Key clinical point: Spreading calories out over three regular meals a day, instead of six small ones, helps patients with type 2 diabetes lose weight and better control their blood glucose, especially when breakfast is the main meal of the day.
Major finding: The total daily insulin dose dropped 27 units from a baseline of 74 units among patients assigned to get their calories at three regular meals; among subjects who had them spread out over six meals, the daily insulin dose increased 5 units/day, from a baseline of 71 units.
Study details: Randomized trial with 37 people with type 2 diabetes.
Disclosures: The funding source was not reported. Dr. Jakubowicz, author of “The Big Breakfast Diet” (Workman Publishing Co., 2009), had no other disclosures. The other investigators had no disclosures.
Source: Jakubowicz D et al. ENDO 2018, Abstract OR05-2.
Dose-escalated radiation therapy brings mixed results in prostate cancer
Dose-escalated radiation therapy reduced the need for subsequent therapy in patients with intermediate-risk prostate cancer but did not improve overall survival, according to results of a large, randomized clinical trial.
The absence of a survival benefit compared with standard radiation therapy was seen despite a reduction in rates of both biochemical failure and distant metastases, Jeff M. Michalski, MD, and his associates reported in JAMA Oncology.
Negative overall survival results may be attributable to the growing availability of systemic salvage therapies that have prolonged the natural history of this disease, said Dr. Michalski, MD, of the department of radiation oncology at Washington University in St. Louis.
“Patients experiencing a biochemical or clinical failure may go on to receive several life-prolonging systemic agents, which may negate any clinical advantage from a more effective primary local therapy,” Dr. Michalski and associates wrote.
Results of the study support that hypothesis. Patients who received the standard dose radiotherapy were significantly more likely to go on to salvage therapy, compared with patients in the dose-escalation arm.
Dr. Michalski and his associated reported on the randomized NRG Oncology/RTOG 0126 clinical trial, which included 1,532 patients with intermediate-risk cancer enrolled between March 2002 and August 2008 at one of 104 North American sites.
In the trial, patients with intermediate-risk prostate cancer were randomized to receive three-dimensional conformal radiation therapy or intensity-modulated radiation therapy to 79.2 Gy in 44 fractions or 70.2 Gy in 39 fractions, the study said.
With a median follow-up of 8.4 years for 1,499 patients, there was no difference in overall survival between arms, study results show. The 8-year rates of overall survival were 76% for dose-escalated radiotherapy and 75% for standard radiotherapy (hazard ratio, 1.00; 95% confidence interval, 0.83-1.20; P = .98).
Otherwise, patients in the dose-escalated radiotherapy arm had significantly lower rates of distant metastases (4% vs. 6%; P = 0.05), and lower rates of biochemical failure rates at both 5 and 8 years, investigators said.
Patients in the high-dose arm less often went on to salvage therapy; however, investigators noted that they also had more toxic effects. compared with patients in the standard radiotherapy arm.
“,” Dr. Michalski and his associates wrote.
Dr. Michalski reported no conflicts of interest. The co-authors reported several conflicts of interest, including ties to ViewRay, Augmenix, Sanofi, AstraZeneca, AbbVie, and other companies.
op@frontlinemedcom.com
SOURCE: Michalsky Jeff M et al. JAMA Oncol. 2018 Mar 15. doi: 10.1001/jamaoncol.2018.0039.
Dose-escalated radiation therapy reduced the need for subsequent therapy in patients with intermediate-risk prostate cancer but did not improve overall survival, according to results of a large, randomized clinical trial.
The absence of a survival benefit compared with standard radiation therapy was seen despite a reduction in rates of both biochemical failure and distant metastases, Jeff M. Michalski, MD, and his associates reported in JAMA Oncology.
Negative overall survival results may be attributable to the growing availability of systemic salvage therapies that have prolonged the natural history of this disease, said Dr. Michalski, MD, of the department of radiation oncology at Washington University in St. Louis.
“Patients experiencing a biochemical or clinical failure may go on to receive several life-prolonging systemic agents, which may negate any clinical advantage from a more effective primary local therapy,” Dr. Michalski and associates wrote.
Results of the study support that hypothesis. Patients who received the standard dose radiotherapy were significantly more likely to go on to salvage therapy, compared with patients in the dose-escalation arm.
Dr. Michalski and his associated reported on the randomized NRG Oncology/RTOG 0126 clinical trial, which included 1,532 patients with intermediate-risk cancer enrolled between March 2002 and August 2008 at one of 104 North American sites.
In the trial, patients with intermediate-risk prostate cancer were randomized to receive three-dimensional conformal radiation therapy or intensity-modulated radiation therapy to 79.2 Gy in 44 fractions or 70.2 Gy in 39 fractions, the study said.
With a median follow-up of 8.4 years for 1,499 patients, there was no difference in overall survival between arms, study results show. The 8-year rates of overall survival were 76% for dose-escalated radiotherapy and 75% for standard radiotherapy (hazard ratio, 1.00; 95% confidence interval, 0.83-1.20; P = .98).
Otherwise, patients in the dose-escalated radiotherapy arm had significantly lower rates of distant metastases (4% vs. 6%; P = 0.05), and lower rates of biochemical failure rates at both 5 and 8 years, investigators said.
Patients in the high-dose arm less often went on to salvage therapy; however, investigators noted that they also had more toxic effects. compared with patients in the standard radiotherapy arm.
“,” Dr. Michalski and his associates wrote.
Dr. Michalski reported no conflicts of interest. The co-authors reported several conflicts of interest, including ties to ViewRay, Augmenix, Sanofi, AstraZeneca, AbbVie, and other companies.
op@frontlinemedcom.com
SOURCE: Michalsky Jeff M et al. JAMA Oncol. 2018 Mar 15. doi: 10.1001/jamaoncol.2018.0039.
Dose-escalated radiation therapy reduced the need for subsequent therapy in patients with intermediate-risk prostate cancer but did not improve overall survival, according to results of a large, randomized clinical trial.
The absence of a survival benefit compared with standard radiation therapy was seen despite a reduction in rates of both biochemical failure and distant metastases, Jeff M. Michalski, MD, and his associates reported in JAMA Oncology.
Negative overall survival results may be attributable to the growing availability of systemic salvage therapies that have prolonged the natural history of this disease, said Dr. Michalski, MD, of the department of radiation oncology at Washington University in St. Louis.
“Patients experiencing a biochemical or clinical failure may go on to receive several life-prolonging systemic agents, which may negate any clinical advantage from a more effective primary local therapy,” Dr. Michalski and associates wrote.
Results of the study support that hypothesis. Patients who received the standard dose radiotherapy were significantly more likely to go on to salvage therapy, compared with patients in the dose-escalation arm.
Dr. Michalski and his associated reported on the randomized NRG Oncology/RTOG 0126 clinical trial, which included 1,532 patients with intermediate-risk cancer enrolled between March 2002 and August 2008 at one of 104 North American sites.
In the trial, patients with intermediate-risk prostate cancer were randomized to receive three-dimensional conformal radiation therapy or intensity-modulated radiation therapy to 79.2 Gy in 44 fractions or 70.2 Gy in 39 fractions, the study said.
With a median follow-up of 8.4 years for 1,499 patients, there was no difference in overall survival between arms, study results show. The 8-year rates of overall survival were 76% for dose-escalated radiotherapy and 75% for standard radiotherapy (hazard ratio, 1.00; 95% confidence interval, 0.83-1.20; P = .98).
Otherwise, patients in the dose-escalated radiotherapy arm had significantly lower rates of distant metastases (4% vs. 6%; P = 0.05), and lower rates of biochemical failure rates at both 5 and 8 years, investigators said.
Patients in the high-dose arm less often went on to salvage therapy; however, investigators noted that they also had more toxic effects. compared with patients in the standard radiotherapy arm.
“,” Dr. Michalski and his associates wrote.
Dr. Michalski reported no conflicts of interest. The co-authors reported several conflicts of interest, including ties to ViewRay, Augmenix, Sanofi, AstraZeneca, AbbVie, and other companies.
op@frontlinemedcom.com
SOURCE: Michalsky Jeff M et al. JAMA Oncol. 2018 Mar 15. doi: 10.1001/jamaoncol.2018.0039.
FROM JAMA ONCOLOGY
Key clinical point: Despite reducing the need for subsequent therapy, dose-escalated radiation therapy for intermediate-risk prostate cancer did not improve overall survival.
Major finding: Eight-year overall survival rates were 76% for dose-escalated radiation therapy, compared with 75% for standard radiation therapy (P = 0.98).
Study details: The randomized NRG Oncology/RTOG 0126 clinical trial including 1,532 patients enrolled between March 2002 and August 2008 at one of 104 North American sites.
Disclosures: Dr. Michalsky reported no conflicts of interest. The study authors disclosed conflicts tied to several companies, including ViewRay, Augmenix, Sanofi, AstraZeneca, and AbbVie.
Source: Michalsky Jeff M et al. JAMA Oncol. 2018 Mar 15. doi: 10.1001/jamaoncol.2018.0039.
Surgery indicates higher survival with adrenal cortical carcinoma
CHICAGO –
These findings, uncovered using the largest cancer registry in the United States, will help give physicians insight into what the best course of action is for treating their patients, according to presenters.
“Surgical resection of the primary tumor improved survival in all stages of disease, whereas adjuvant therapy with chemotherapy or radiation improved overall survival only in stage IVpatients,” said Sri Harsha Tella, MD, endocrinologist at the University of South Carolina, Columbia. “These results may help the prognostication of patients in treatment decision making.”
Investigators conducted a retrospective study of 3,185 pathologically confirmed cases of ACC, registered into the National Cancer Database between 2004 and 2015.
Patients were mostly women with an average age of 55 years old and private insurance, with a nearly even split of patients with stage I-III (26%) and stage IV ACC (24%). Nearly three-quarters of those studied chose to have surgery, of which 31% chose open resection.
Patients with stage I-III ACC had a significant median survival rate of 63 months, compared with those who did not have surgery who had an average survival of 8 months.
In patients with stage IV ACC, surgery lengthened overall survival to 19 months, compared with 6 months for those without surgery, according to Dr. Tella and fellow investigators.
While surgery did have a greater positive effect on patients’ live spans across all stages, the impact of chemotherapy and radiation was significant only among stage IV patients who had complete surgery.
Those in the stage IV group who were given post-surgery adjuvant chemotherapy were likely to live an average of nearly 9 more months than did those who did not have chemotherapy after radiation (22 vs. 13), while those given radiation therapy saw an increase in survival by 19 months (29 vs. 10). These increases did not affect stage I-III patients, who had a similar rate of survival regardless of additional therapies after their surgery (24 vs. 25 months).
One possible explanation for why additional therapy made little difference in survival for stage I-III patients is that, given that the tumors did not spread as widely, the surgical procedures were likely to be more effective at removing most of the disease, according to Dr. Tella.
“One of the possibilities is that surgeons were able to get the whole mass out,” Dr. Tella hypothesized in response to a question from attendees. “On the other hand, patients with stage IV ACC may be more likely to have more presence of metastases and so would benefit more greatly from the removal of the primary tumor and then also additional therapy.”
Investigators noted that because of the structure of the registry, they were unable to determine the initiation and duration of chemotherapy, as well as doses of radiation therapy received by the patient.
A more robust database and future stage-specific, prospective clinical trials are needed in order to better understand these findings, Dr. Tella said.
The investigators reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
SOURCE: Tella SH et al. Endo 2018.
CHICAGO –
These findings, uncovered using the largest cancer registry in the United States, will help give physicians insight into what the best course of action is for treating their patients, according to presenters.
“Surgical resection of the primary tumor improved survival in all stages of disease, whereas adjuvant therapy with chemotherapy or radiation improved overall survival only in stage IVpatients,” said Sri Harsha Tella, MD, endocrinologist at the University of South Carolina, Columbia. “These results may help the prognostication of patients in treatment decision making.”
Investigators conducted a retrospective study of 3,185 pathologically confirmed cases of ACC, registered into the National Cancer Database between 2004 and 2015.
Patients were mostly women with an average age of 55 years old and private insurance, with a nearly even split of patients with stage I-III (26%) and stage IV ACC (24%). Nearly three-quarters of those studied chose to have surgery, of which 31% chose open resection.
Patients with stage I-III ACC had a significant median survival rate of 63 months, compared with those who did not have surgery who had an average survival of 8 months.
In patients with stage IV ACC, surgery lengthened overall survival to 19 months, compared with 6 months for those without surgery, according to Dr. Tella and fellow investigators.
While surgery did have a greater positive effect on patients’ live spans across all stages, the impact of chemotherapy and radiation was significant only among stage IV patients who had complete surgery.
Those in the stage IV group who were given post-surgery adjuvant chemotherapy were likely to live an average of nearly 9 more months than did those who did not have chemotherapy after radiation (22 vs. 13), while those given radiation therapy saw an increase in survival by 19 months (29 vs. 10). These increases did not affect stage I-III patients, who had a similar rate of survival regardless of additional therapies after their surgery (24 vs. 25 months).
One possible explanation for why additional therapy made little difference in survival for stage I-III patients is that, given that the tumors did not spread as widely, the surgical procedures were likely to be more effective at removing most of the disease, according to Dr. Tella.
“One of the possibilities is that surgeons were able to get the whole mass out,” Dr. Tella hypothesized in response to a question from attendees. “On the other hand, patients with stage IV ACC may be more likely to have more presence of metastases and so would benefit more greatly from the removal of the primary tumor and then also additional therapy.”
Investigators noted that because of the structure of the registry, they were unable to determine the initiation and duration of chemotherapy, as well as doses of radiation therapy received by the patient.
A more robust database and future stage-specific, prospective clinical trials are needed in order to better understand these findings, Dr. Tella said.
The investigators reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
SOURCE: Tella SH et al. Endo 2018.
CHICAGO –
These findings, uncovered using the largest cancer registry in the United States, will help give physicians insight into what the best course of action is for treating their patients, according to presenters.
“Surgical resection of the primary tumor improved survival in all stages of disease, whereas adjuvant therapy with chemotherapy or radiation improved overall survival only in stage IVpatients,” said Sri Harsha Tella, MD, endocrinologist at the University of South Carolina, Columbia. “These results may help the prognostication of patients in treatment decision making.”
Investigators conducted a retrospective study of 3,185 pathologically confirmed cases of ACC, registered into the National Cancer Database between 2004 and 2015.
Patients were mostly women with an average age of 55 years old and private insurance, with a nearly even split of patients with stage I-III (26%) and stage IV ACC (24%). Nearly three-quarters of those studied chose to have surgery, of which 31% chose open resection.
Patients with stage I-III ACC had a significant median survival rate of 63 months, compared with those who did not have surgery who had an average survival of 8 months.
In patients with stage IV ACC, surgery lengthened overall survival to 19 months, compared with 6 months for those without surgery, according to Dr. Tella and fellow investigators.
While surgery did have a greater positive effect on patients’ live spans across all stages, the impact of chemotherapy and radiation was significant only among stage IV patients who had complete surgery.
Those in the stage IV group who were given post-surgery adjuvant chemotherapy were likely to live an average of nearly 9 more months than did those who did not have chemotherapy after radiation (22 vs. 13), while those given radiation therapy saw an increase in survival by 19 months (29 vs. 10). These increases did not affect stage I-III patients, who had a similar rate of survival regardless of additional therapies after their surgery (24 vs. 25 months).
One possible explanation for why additional therapy made little difference in survival for stage I-III patients is that, given that the tumors did not spread as widely, the surgical procedures were likely to be more effective at removing most of the disease, according to Dr. Tella.
“One of the possibilities is that surgeons were able to get the whole mass out,” Dr. Tella hypothesized in response to a question from attendees. “On the other hand, patients with stage IV ACC may be more likely to have more presence of metastases and so would benefit more greatly from the removal of the primary tumor and then also additional therapy.”
Investigators noted that because of the structure of the registry, they were unable to determine the initiation and duration of chemotherapy, as well as doses of radiation therapy received by the patient.
A more robust database and future stage-specific, prospective clinical trials are needed in order to better understand these findings, Dr. Tella said.
The investigators reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
SOURCE: Tella SH et al. Endo 2018.
REPORTING FROM ENDO 2018
Key clinical point: Patients who undergo surgical resection at all stages are more likely to survive.
Major finding: Patients stage I-III who underwent surgery survived over nearly 8 times longer than non-surgery patients (63 vs. 8 months [P less than .001]).
Study details: Retrospective study of 3,185 adrenal cortical carcinoma cases entered into the National Cancer Database between 2004 and 2015.
Disclosures: The presenter reported no relevant financial disclosures.
Source: Tella SH et al. Endo 2018.
Without reliability, testosterone testing may fall short
CHICAGO – is one addition to the evaluation of men with suspected hypogonadism that is included in the revised clinical practice guideline on testosterone therapy in men with hypogonadism, issued March 17 by the Endocrine Society. The previous guideline was issued in 2010.
“Since 2010, the CDC has provided an accuracy-based standardization program for T (CDC Hormone Standardization Program for Testosterone). Although several commercial laboratories, some assay manufacturers, and some academic laboratories are now CDC certified, most T immunoassay kit manufacturers and local hospital-based laboratories have not been certified. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays for TT generally offer higher concentrations of specificity, sensitivity, and precision (especially in the low range) than do most immunoassays. Clinicians should ideally measure TT using a CDC-certified assay or an assay verified by an accuracy-based external quality control program,” according to the guideline.
Diagnosis, treatment, and follow-up of men with suspected hypogonadism often involve nuanced decisions, according to guideline panel chair Shalender Bhasin, MB, an endocrinologist who is professor of medicine at both Harvard Medical School and Brigham and Women’s Hospital, in Boston. The guideline will be published in its entirety in the May 2018 print issue of The Journal of Clinical Endocrinology & Metabolism.
Evaluation for suspected hypogonadism is indicated in men with symptoms and signs of testosterone deficiency – decreased libido, unexplained anemia, and/or osteopenia – and unequivocally and consistently low serum total testosterone and/or free testosterone concentrations, according to the guideline, which is co-sponsored by European Society of Endocrinology and endorsed by the European Academy of Andrology.
The guideline illuminates one area of diagnostic uncertainty that has dogged clinicians: Should one use age-specific testosterone levels? All the guideline authors speaking at the panel, in addition to Dr. Bhasin – Glenn R. Cunningham, MD, Baylor College of Medicine in Houston; Alvin M. Matsumoto, MD, VA Puget Sound Health Care System, Seattle; and Maria A. Yialamas, MD, Brigham and Women’s Hospital, Boston – agreed that age-specific testosterone levels do not improve diagnosis.
“The harmonized Reference range for TT in healthy, nonobese young men (aged 19 to 39 years) was 264 to 916 ng/dL (9.2 to 31.8 nmol/L) using the 2.5th and 97.5th percentile, and 303 to 852 ng/dL (10.5 to 29.5 nmol/L) using the 5th and 95th percentile (31). Clinicians can use this range for all CDC-certified TT assays,” according to the guideline.
Asking about history to explain low testosterone levels is an often overlooked part of patient evaluation. Two known causes of low testosterone in men often are not part of history taking but should be. One is use of opioids. The other is withdrawal from anabolic steroids. The best way to root these out in a history is to ask about any recent surgery and use of anabolic steroids, said Dr. Bhasin, speaking at the annual meeting of the Endocrine Society.
When it works, testosterone can make some men feel better: Testosterone replacement was associated with increased libido, erectile function, and sexual activity. However, testosterone replacement had no effect on energy and/or mood.
Not every man over the age of 65 with low testosterone is a candidate for testosterone replacement. There are some risks associated with its use; PSA may rise within 6 months of starting testosterone replacement, leaving physicians and men facing the issue of whether to have a prostate biopsy. The guideline recommends evaluating the patients with PSA the first time within 3-12 months after the start of therapy. When the test shows that PSA has risen, the decision of whether to have a prostate biopsy is one that should be shared by the patient and physician.
Dr. Bhasin declared financing from the National Institute on Aging, the National Institute of Nursing Research, the Patient-Centered Outcomes Research Institute, AbbVie, Metro International Biotechnology, Alivegen, Abbott, Transition Therapeutics, and Function Promoting Therapies.
skubetin@frontlinemedcom.com
SOURCE: ENDO 2018
CHICAGO – is one addition to the evaluation of men with suspected hypogonadism that is included in the revised clinical practice guideline on testosterone therapy in men with hypogonadism, issued March 17 by the Endocrine Society. The previous guideline was issued in 2010.
“Since 2010, the CDC has provided an accuracy-based standardization program for T (CDC Hormone Standardization Program for Testosterone). Although several commercial laboratories, some assay manufacturers, and some academic laboratories are now CDC certified, most T immunoassay kit manufacturers and local hospital-based laboratories have not been certified. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays for TT generally offer higher concentrations of specificity, sensitivity, and precision (especially in the low range) than do most immunoassays. Clinicians should ideally measure TT using a CDC-certified assay or an assay verified by an accuracy-based external quality control program,” according to the guideline.
Diagnosis, treatment, and follow-up of men with suspected hypogonadism often involve nuanced decisions, according to guideline panel chair Shalender Bhasin, MB, an endocrinologist who is professor of medicine at both Harvard Medical School and Brigham and Women’s Hospital, in Boston. The guideline will be published in its entirety in the May 2018 print issue of The Journal of Clinical Endocrinology & Metabolism.
Evaluation for suspected hypogonadism is indicated in men with symptoms and signs of testosterone deficiency – decreased libido, unexplained anemia, and/or osteopenia – and unequivocally and consistently low serum total testosterone and/or free testosterone concentrations, according to the guideline, which is co-sponsored by European Society of Endocrinology and endorsed by the European Academy of Andrology.
The guideline illuminates one area of diagnostic uncertainty that has dogged clinicians: Should one use age-specific testosterone levels? All the guideline authors speaking at the panel, in addition to Dr. Bhasin – Glenn R. Cunningham, MD, Baylor College of Medicine in Houston; Alvin M. Matsumoto, MD, VA Puget Sound Health Care System, Seattle; and Maria A. Yialamas, MD, Brigham and Women’s Hospital, Boston – agreed that age-specific testosterone levels do not improve diagnosis.
“The harmonized Reference range for TT in healthy, nonobese young men (aged 19 to 39 years) was 264 to 916 ng/dL (9.2 to 31.8 nmol/L) using the 2.5th and 97.5th percentile, and 303 to 852 ng/dL (10.5 to 29.5 nmol/L) using the 5th and 95th percentile (31). Clinicians can use this range for all CDC-certified TT assays,” according to the guideline.
Asking about history to explain low testosterone levels is an often overlooked part of patient evaluation. Two known causes of low testosterone in men often are not part of history taking but should be. One is use of opioids. The other is withdrawal from anabolic steroids. The best way to root these out in a history is to ask about any recent surgery and use of anabolic steroids, said Dr. Bhasin, speaking at the annual meeting of the Endocrine Society.
When it works, testosterone can make some men feel better: Testosterone replacement was associated with increased libido, erectile function, and sexual activity. However, testosterone replacement had no effect on energy and/or mood.
Not every man over the age of 65 with low testosterone is a candidate for testosterone replacement. There are some risks associated with its use; PSA may rise within 6 months of starting testosterone replacement, leaving physicians and men facing the issue of whether to have a prostate biopsy. The guideline recommends evaluating the patients with PSA the first time within 3-12 months after the start of therapy. When the test shows that PSA has risen, the decision of whether to have a prostate biopsy is one that should be shared by the patient and physician.
Dr. Bhasin declared financing from the National Institute on Aging, the National Institute of Nursing Research, the Patient-Centered Outcomes Research Institute, AbbVie, Metro International Biotechnology, Alivegen, Abbott, Transition Therapeutics, and Function Promoting Therapies.
skubetin@frontlinemedcom.com
SOURCE: ENDO 2018
CHICAGO – is one addition to the evaluation of men with suspected hypogonadism that is included in the revised clinical practice guideline on testosterone therapy in men with hypogonadism, issued March 17 by the Endocrine Society. The previous guideline was issued in 2010.
“Since 2010, the CDC has provided an accuracy-based standardization program for T (CDC Hormone Standardization Program for Testosterone). Although several commercial laboratories, some assay manufacturers, and some academic laboratories are now CDC certified, most T immunoassay kit manufacturers and local hospital-based laboratories have not been certified. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays for TT generally offer higher concentrations of specificity, sensitivity, and precision (especially in the low range) than do most immunoassays. Clinicians should ideally measure TT using a CDC-certified assay or an assay verified by an accuracy-based external quality control program,” according to the guideline.
Diagnosis, treatment, and follow-up of men with suspected hypogonadism often involve nuanced decisions, according to guideline panel chair Shalender Bhasin, MB, an endocrinologist who is professor of medicine at both Harvard Medical School and Brigham and Women’s Hospital, in Boston. The guideline will be published in its entirety in the May 2018 print issue of The Journal of Clinical Endocrinology & Metabolism.
Evaluation for suspected hypogonadism is indicated in men with symptoms and signs of testosterone deficiency – decreased libido, unexplained anemia, and/or osteopenia – and unequivocally and consistently low serum total testosterone and/or free testosterone concentrations, according to the guideline, which is co-sponsored by European Society of Endocrinology and endorsed by the European Academy of Andrology.
The guideline illuminates one area of diagnostic uncertainty that has dogged clinicians: Should one use age-specific testosterone levels? All the guideline authors speaking at the panel, in addition to Dr. Bhasin – Glenn R. Cunningham, MD, Baylor College of Medicine in Houston; Alvin M. Matsumoto, MD, VA Puget Sound Health Care System, Seattle; and Maria A. Yialamas, MD, Brigham and Women’s Hospital, Boston – agreed that age-specific testosterone levels do not improve diagnosis.
“The harmonized Reference range for TT in healthy, nonobese young men (aged 19 to 39 years) was 264 to 916 ng/dL (9.2 to 31.8 nmol/L) using the 2.5th and 97.5th percentile, and 303 to 852 ng/dL (10.5 to 29.5 nmol/L) using the 5th and 95th percentile (31). Clinicians can use this range for all CDC-certified TT assays,” according to the guideline.
Asking about history to explain low testosterone levels is an often overlooked part of patient evaluation. Two known causes of low testosterone in men often are not part of history taking but should be. One is use of opioids. The other is withdrawal from anabolic steroids. The best way to root these out in a history is to ask about any recent surgery and use of anabolic steroids, said Dr. Bhasin, speaking at the annual meeting of the Endocrine Society.
When it works, testosterone can make some men feel better: Testosterone replacement was associated with increased libido, erectile function, and sexual activity. However, testosterone replacement had no effect on energy and/or mood.
Not every man over the age of 65 with low testosterone is a candidate for testosterone replacement. There are some risks associated with its use; PSA may rise within 6 months of starting testosterone replacement, leaving physicians and men facing the issue of whether to have a prostate biopsy. The guideline recommends evaluating the patients with PSA the first time within 3-12 months after the start of therapy. When the test shows that PSA has risen, the decision of whether to have a prostate biopsy is one that should be shared by the patient and physician.
Dr. Bhasin declared financing from the National Institute on Aging, the National Institute of Nursing Research, the Patient-Centered Outcomes Research Institute, AbbVie, Metro International Biotechnology, Alivegen, Abbott, Transition Therapeutics, and Function Promoting Therapies.
skubetin@frontlinemedcom.com
SOURCE: ENDO 2018
REPORTING FROM ENDO 2018
Axitinib/avelumab combo shows preliminary efficacy in RCC
The combination of axitinib and avelumab had manageable toxicity and demonstrated preliminary efficacy as a front-line treatment of advanced renal cell carcinoma (RCC), according to results of a recent study.
More than half of patients had a response on the combination of axitinib (Inlyta), a receptor inhibitor of vascular endothelial growth factor (VEGF), and avelumab (Bavencio), an anti-PD-L1 monoclonal antibody, investigators reported in The Lancet Oncology.
The most common treatment-related adverse event was hypertension, wrote lead author Toni K. Choueiri, MD, of the Lank Center for Genitourinary Oncology at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, and his colleagues.
“The combination of avelumab and axitinib in treatment-naive patients with advanced RCC had a manageable safety profile consistent with the profiles of the individual agents when administered as monotherapy, and antitumor activity was encouraging,” said Dr. Choueiri, and his colleagues.
The study, known as JAVELIN Renal 100, was a phase 1b investigation that included 55 patients with advanced clear-cell RCC. A total of 6 patients were enrolled in a smaller dose-finding cohort, and 49 were enrolled in a dose-expansion cohort.
In the dose-finding phase, patients received oral axitinib 5 mg twice a day for 7 days, at which point they started intravenous avelumab 10 mg/kg every 2 weeks, according to the study description. In the dose-expansion phase, patients were directly started on the combination.
Out of 55 patients enrolled, 26 (53%) had a response on the axitinib-avelumab combination, including 3 (6%) who had complete responses, investigators reported.
Grade 3 or greater treatment-related adverse events were reported in 32 patients (58%). The most common of those was hypertension, occurring in 16 patients (29%), followed by ALT increase and palmar-plantar erythrodysesthesia syndrome in 4 patients each (7%), Dr. Choueiri and colleagues wrote.
In the dose-finding phase, investigators reported one dose-limiting toxicity, which was grade 3 proteinuria due to axitinib.
. In that randomized trial, the axitinib-avelumab combination is being compared to sunitinib as a first-line approach in patients with advanced RCC.
“The combination of an antibody that inhibits PD-L1 and PD-1 interactions with a targeted antiangiogenic agent might take advantage of complementary mechanisms of action to provide clinical benefit in patients with advanced renal-cell carcinoma that exceeds the effects of the respective drugs alone without increasing associated toxicity,” Dr. Choueiri and colleagues wrote.
Pfizer and Merck funded the study. Dr. Choueiri declared interests related to several companies, including AstraZeneca, Bristol-Myers Squibb, Eisai, Exelixis, GlaxoSmithKline, Merck, Novartis, Pfizer, Peloton, and Roche/Genentech.
SOURCE: Choueiri TK et al. Lancet Oncol. 2018 Mar 9. doi: 10.1016/S1470-2045(18)30107-4.
The combination of axitinib and avelumab had manageable toxicity and demonstrated preliminary efficacy as a front-line treatment of advanced renal cell carcinoma (RCC), according to results of a recent study.
More than half of patients had a response on the combination of axitinib (Inlyta), a receptor inhibitor of vascular endothelial growth factor (VEGF), and avelumab (Bavencio), an anti-PD-L1 monoclonal antibody, investigators reported in The Lancet Oncology.
The most common treatment-related adverse event was hypertension, wrote lead author Toni K. Choueiri, MD, of the Lank Center for Genitourinary Oncology at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, and his colleagues.
“The combination of avelumab and axitinib in treatment-naive patients with advanced RCC had a manageable safety profile consistent with the profiles of the individual agents when administered as monotherapy, and antitumor activity was encouraging,” said Dr. Choueiri, and his colleagues.
The study, known as JAVELIN Renal 100, was a phase 1b investigation that included 55 patients with advanced clear-cell RCC. A total of 6 patients were enrolled in a smaller dose-finding cohort, and 49 were enrolled in a dose-expansion cohort.
In the dose-finding phase, patients received oral axitinib 5 mg twice a day for 7 days, at which point they started intravenous avelumab 10 mg/kg every 2 weeks, according to the study description. In the dose-expansion phase, patients were directly started on the combination.
Out of 55 patients enrolled, 26 (53%) had a response on the axitinib-avelumab combination, including 3 (6%) who had complete responses, investigators reported.
Grade 3 or greater treatment-related adverse events were reported in 32 patients (58%). The most common of those was hypertension, occurring in 16 patients (29%), followed by ALT increase and palmar-plantar erythrodysesthesia syndrome in 4 patients each (7%), Dr. Choueiri and colleagues wrote.
In the dose-finding phase, investigators reported one dose-limiting toxicity, which was grade 3 proteinuria due to axitinib.
. In that randomized trial, the axitinib-avelumab combination is being compared to sunitinib as a first-line approach in patients with advanced RCC.
“The combination of an antibody that inhibits PD-L1 and PD-1 interactions with a targeted antiangiogenic agent might take advantage of complementary mechanisms of action to provide clinical benefit in patients with advanced renal-cell carcinoma that exceeds the effects of the respective drugs alone without increasing associated toxicity,” Dr. Choueiri and colleagues wrote.
Pfizer and Merck funded the study. Dr. Choueiri declared interests related to several companies, including AstraZeneca, Bristol-Myers Squibb, Eisai, Exelixis, GlaxoSmithKline, Merck, Novartis, Pfizer, Peloton, and Roche/Genentech.
SOURCE: Choueiri TK et al. Lancet Oncol. 2018 Mar 9. doi: 10.1016/S1470-2045(18)30107-4.
The combination of axitinib and avelumab had manageable toxicity and demonstrated preliminary efficacy as a front-line treatment of advanced renal cell carcinoma (RCC), according to results of a recent study.
More than half of patients had a response on the combination of axitinib (Inlyta), a receptor inhibitor of vascular endothelial growth factor (VEGF), and avelumab (Bavencio), an anti-PD-L1 monoclonal antibody, investigators reported in The Lancet Oncology.
The most common treatment-related adverse event was hypertension, wrote lead author Toni K. Choueiri, MD, of the Lank Center for Genitourinary Oncology at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, and his colleagues.
“The combination of avelumab and axitinib in treatment-naive patients with advanced RCC had a manageable safety profile consistent with the profiles of the individual agents when administered as monotherapy, and antitumor activity was encouraging,” said Dr. Choueiri, and his colleagues.
The study, known as JAVELIN Renal 100, was a phase 1b investigation that included 55 patients with advanced clear-cell RCC. A total of 6 patients were enrolled in a smaller dose-finding cohort, and 49 were enrolled in a dose-expansion cohort.
In the dose-finding phase, patients received oral axitinib 5 mg twice a day for 7 days, at which point they started intravenous avelumab 10 mg/kg every 2 weeks, according to the study description. In the dose-expansion phase, patients were directly started on the combination.
Out of 55 patients enrolled, 26 (53%) had a response on the axitinib-avelumab combination, including 3 (6%) who had complete responses, investigators reported.
Grade 3 or greater treatment-related adverse events were reported in 32 patients (58%). The most common of those was hypertension, occurring in 16 patients (29%), followed by ALT increase and palmar-plantar erythrodysesthesia syndrome in 4 patients each (7%), Dr. Choueiri and colleagues wrote.
In the dose-finding phase, investigators reported one dose-limiting toxicity, which was grade 3 proteinuria due to axitinib.
. In that randomized trial, the axitinib-avelumab combination is being compared to sunitinib as a first-line approach in patients with advanced RCC.
“The combination of an antibody that inhibits PD-L1 and PD-1 interactions with a targeted antiangiogenic agent might take advantage of complementary mechanisms of action to provide clinical benefit in patients with advanced renal-cell carcinoma that exceeds the effects of the respective drugs alone without increasing associated toxicity,” Dr. Choueiri and colleagues wrote.
Pfizer and Merck funded the study. Dr. Choueiri declared interests related to several companies, including AstraZeneca, Bristol-Myers Squibb, Eisai, Exelixis, GlaxoSmithKline, Merck, Novartis, Pfizer, Peloton, and Roche/Genentech.
SOURCE: Choueiri TK et al. Lancet Oncol. 2018 Mar 9. doi: 10.1016/S1470-2045(18)30107-4.
FROM THE LANCET ONCOLOGY
Key clinical point: The targeted-immune combination of axitinib and avelumab had manageable toxicity and encouraging preliminary efficacy as a front-line treatment of advanced renal cell carcinoma (RCC).
Major finding: Out of 55 patients enrolled, 26 (53%) had a response, including 3 (6%) who had complete responses.
Study details: A dose-expansion and dose-finding phase 1b study including 55 patients with advanced clear-cell RCC.
Disclosures: Funding for the study came from Pfizer and Merck. Study authors declared interests related to several companies, including Pfizer, Merck, Bristol-Myers Squibb, Novartis, Roche/Genentech.
Source: Choueiri TK et al. Lancet Oncol. 2018 Mar 9. doi: 10.1016/S1470-2045(18)30107-4.
views
The antitumor activity of axitinib and avelumab in this study indicate the potential clinical benefit of targeted-immune combinations for advanced renal cell carcinoma (RCC), according to Viktor Grünwald, MD, PhD.
“Further studies are warranted to explore whether a first-line targeted-immune combination might overcome the standard of sequential targeted and immune therapies,” Dr. Grünwald said in an editorial.
The current absence of long-term safety data for the combination approach is limiting in terms of making definitive conclusions about the toxicity of axitinib and avelumab as a first line combination therapy for advanced clear-cell RCC, Dr. Grünwald wrote.
Even so, axitinib has a “low” incidence of hepatic toxicity, making it a “preferable” agent to evaluate in targeted-immune combination trials such as JAVELIN Renal 100, he added.
Objective responses in JAVELIN Renal 100 were seen in 32 out of 55 patients (58%), of which 3 patients (6%) were complete responses, according to reported data.
Similar findings previously reported for the immune-immune combination of ipilimumab and nivolumab versus sunitinib. In that study, patients receiving the immune-immune combination had an overall response rate of 42% and complete response rate of 9%, while the group patients receiving sunitinib had overall and complete response rates of 27% and 1%, respectively, he said.
“With the dawn of immune-immune and targeted-immune combinations, for the first time in a decade, major progress towards improving the median overall survival and possibly delivering cure to some of our patients with metastatic renal-cell carcinoma seems to have been made,” Dr. Grünwald said.
Viktor Grünwald, MD, PhD, is with the department of hematology, haemostasis, oncology, and stem cell transplantation at Hannover Medical School, Germany. These comments are based on an editorial accompanying the report (Lancet Oncol. 2018 Mar 9. doi: 10.1016/S1470-2045[18]30126-8). Dr. Grünwald disclosed ties to Bristol-Myers Squibb, Merck Sharp and Dohme, Ipsen, Novartis, Roche, AstraZeneca, Pfizer, Cerulean, Eisai, and EUSA Pharma.
Court: State cannot sue over religious exemption expansion
Massachusetts cannot sue the Trump administration for broadening exemptions to the Affordable Care Act’s contraceptive mandate, a district court has ruled.
In a March decision, the U.S. District Court for the District of Massachusetts concluded that the state lacks standing to sue the federal government and that it could not prove that its residents would be harmed by the expanded exemption.
Under two regulations, issued in October 2017 in the Federal Register, the Trump administration allowed that an expanded group of employers and insurers can object to paying for contraception coverage on religious or moral grounds. The new policy “better balances the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions,” according to the rule issued by the departments of Health & Human Services, Labor, and Treasury.
Several state attorneys general sued over the rules, including Massachusetts Attorney General Maura Healey (D). Ms. Healey argued that the rules violates women’s equal protection rights by restricting their ability to access contraception. The lawsuit also claimed the narrowing of the mandate allowed employers to unconstitutionally impose their religious and moral beliefs on employees.
“The [rules] jeopardize the health care of women in Massachusetts and nationwide, promote the religious freedom of corporations over the autonomy and health of women, and leave the states to bear additional health care costs both with regard to contraceptive and prenatal care as well as other services associated with unintended pregnancies and related negative health outcomes for both women and their children,” the lawsuit stated.
In his March 12 opinion however, U.S. District Judge Nathaniel M. Gorton dismissed the state’s claims, ruling that Massachusetts failed to prove its citizens will suffer future injury from the exemption expansion. The judge noted a 2017 law passed by Massachusetts called the ACCESS Act that requires certain employer-sponsored health plans to cover contraceptives without imposing out-of-pocket costs on employees. This law and other state regulations make Massachusetts less likely than women in other states to be affected negatively by the expanded exemption, said Judge Gorton, who was appointed to the bench by President George H.W. Bush.
In a statement, Ms. Healey said the state was disappointed in the decision, but remained steadfast in its commitment to “ensuring affordable and reliable reproductive health care for women.”
“Access to contraceptive coverage is a critical issue for the health, equality, and economic well-being of women and their families, and we will continue to fight for these protections.” Ms. Healey said.
The legal fight over the ACA’s contraceptive mandate exemption is far from over. The Massachusetts decision comes after federal judges in California and Pennsylvania halted the administration’s exemption rules. In December 2017, U.S. District Judge Haywood Gilliam Jr. temporarily blocked the exemption extension from going forward in favor of California and four other states that joined the lawsuit – New York, Delaware, Maryland, and Virginia. Earlier that month, U.S. District Judge Wendy Beetlestone ruled similarly in a decision for the U.S. District Court for the Eastern District of Pennsylvania. Both Mr. Gilliam and Ms. Beetlestone were appointed to the bench by President Obama.
The Trump administration has indicated that it plans to appeal the rulings. In a summary of the rules, the administration said the new policies will “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.”
Massachusetts cannot sue the Trump administration for broadening exemptions to the Affordable Care Act’s contraceptive mandate, a district court has ruled.
In a March decision, the U.S. District Court for the District of Massachusetts concluded that the state lacks standing to sue the federal government and that it could not prove that its residents would be harmed by the expanded exemption.
Under two regulations, issued in October 2017 in the Federal Register, the Trump administration allowed that an expanded group of employers and insurers can object to paying for contraception coverage on religious or moral grounds. The new policy “better balances the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions,” according to the rule issued by the departments of Health & Human Services, Labor, and Treasury.
Several state attorneys general sued over the rules, including Massachusetts Attorney General Maura Healey (D). Ms. Healey argued that the rules violates women’s equal protection rights by restricting their ability to access contraception. The lawsuit also claimed the narrowing of the mandate allowed employers to unconstitutionally impose their religious and moral beliefs on employees.
“The [rules] jeopardize the health care of women in Massachusetts and nationwide, promote the religious freedom of corporations over the autonomy and health of women, and leave the states to bear additional health care costs both with regard to contraceptive and prenatal care as well as other services associated with unintended pregnancies and related negative health outcomes for both women and their children,” the lawsuit stated.
In his March 12 opinion however, U.S. District Judge Nathaniel M. Gorton dismissed the state’s claims, ruling that Massachusetts failed to prove its citizens will suffer future injury from the exemption expansion. The judge noted a 2017 law passed by Massachusetts called the ACCESS Act that requires certain employer-sponsored health plans to cover contraceptives without imposing out-of-pocket costs on employees. This law and other state regulations make Massachusetts less likely than women in other states to be affected negatively by the expanded exemption, said Judge Gorton, who was appointed to the bench by President George H.W. Bush.
In a statement, Ms. Healey said the state was disappointed in the decision, but remained steadfast in its commitment to “ensuring affordable and reliable reproductive health care for women.”
“Access to contraceptive coverage is a critical issue for the health, equality, and economic well-being of women and their families, and we will continue to fight for these protections.” Ms. Healey said.
The legal fight over the ACA’s contraceptive mandate exemption is far from over. The Massachusetts decision comes after federal judges in California and Pennsylvania halted the administration’s exemption rules. In December 2017, U.S. District Judge Haywood Gilliam Jr. temporarily blocked the exemption extension from going forward in favor of California and four other states that joined the lawsuit – New York, Delaware, Maryland, and Virginia. Earlier that month, U.S. District Judge Wendy Beetlestone ruled similarly in a decision for the U.S. District Court for the Eastern District of Pennsylvania. Both Mr. Gilliam and Ms. Beetlestone were appointed to the bench by President Obama.
The Trump administration has indicated that it plans to appeal the rulings. In a summary of the rules, the administration said the new policies will “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.”
Massachusetts cannot sue the Trump administration for broadening exemptions to the Affordable Care Act’s contraceptive mandate, a district court has ruled.
In a March decision, the U.S. District Court for the District of Massachusetts concluded that the state lacks standing to sue the federal government and that it could not prove that its residents would be harmed by the expanded exemption.
Under two regulations, issued in October 2017 in the Federal Register, the Trump administration allowed that an expanded group of employers and insurers can object to paying for contraception coverage on religious or moral grounds. The new policy “better balances the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions,” according to the rule issued by the departments of Health & Human Services, Labor, and Treasury.
Several state attorneys general sued over the rules, including Massachusetts Attorney General Maura Healey (D). Ms. Healey argued that the rules violates women’s equal protection rights by restricting their ability to access contraception. The lawsuit also claimed the narrowing of the mandate allowed employers to unconstitutionally impose their religious and moral beliefs on employees.
“The [rules] jeopardize the health care of women in Massachusetts and nationwide, promote the religious freedom of corporations over the autonomy and health of women, and leave the states to bear additional health care costs both with regard to contraceptive and prenatal care as well as other services associated with unintended pregnancies and related negative health outcomes for both women and their children,” the lawsuit stated.
In his March 12 opinion however, U.S. District Judge Nathaniel M. Gorton dismissed the state’s claims, ruling that Massachusetts failed to prove its citizens will suffer future injury from the exemption expansion. The judge noted a 2017 law passed by Massachusetts called the ACCESS Act that requires certain employer-sponsored health plans to cover contraceptives without imposing out-of-pocket costs on employees. This law and other state regulations make Massachusetts less likely than women in other states to be affected negatively by the expanded exemption, said Judge Gorton, who was appointed to the bench by President George H.W. Bush.
In a statement, Ms. Healey said the state was disappointed in the decision, but remained steadfast in its commitment to “ensuring affordable and reliable reproductive health care for women.”
“Access to contraceptive coverage is a critical issue for the health, equality, and economic well-being of women and their families, and we will continue to fight for these protections.” Ms. Healey said.
The legal fight over the ACA’s contraceptive mandate exemption is far from over. The Massachusetts decision comes after federal judges in California and Pennsylvania halted the administration’s exemption rules. In December 2017, U.S. District Judge Haywood Gilliam Jr. temporarily blocked the exemption extension from going forward in favor of California and four other states that joined the lawsuit – New York, Delaware, Maryland, and Virginia. Earlier that month, U.S. District Judge Wendy Beetlestone ruled similarly in a decision for the U.S. District Court for the Eastern District of Pennsylvania. Both Mr. Gilliam and Ms. Beetlestone were appointed to the bench by President Obama.
The Trump administration has indicated that it plans to appeal the rulings. In a summary of the rules, the administration said the new policies will “better balance the government’s interest in promoting coverage for contraceptive and sterilization services with the government’s interests in providing conscience protections for entities with sincerely held moral convictions.”
DOACs may be beneficial in post-op atrial fib after CABG
SAN DIEGO – The use of direct oral anticoagulants were not significantly different from warfarin for safety and efficacy in cases of postoperative atrial fibrillation following coronary artery bypass grafting, according to results from a single-center study.
In fact, , which suggests a potential for reduced hospital stay-related costs. “There are several different niche populations for which the DOACs have not been studied,” one of the study authors, Ammie J. Patel, PharmD, said in an interview at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “This provides the opportunity for institutions and hospitals to study the use of these drugs in non-traditional ways.”
Dr. Patel, of Temple University, Philadelphia, said that postoperative atrial fibrillation occurs in about 30% of patients following CABG and that new-onset postoperative atrial fibrillation after CABG has been linked to a 21% increase in relative mortality.
Noting that patients with planned major surgery were excluded from RE-LY, ROCKET AF, ARISTOTLE, and other pivotal clinical trials of DOACs, she and her research mentor, Rachael Durie, PharmD, retrospectively evaluated the safety and efficacy of DOACs in 285 cases of new-onset postoperative atrial fibrillation following CABG performed at Jersey Shore University Medical Center, Neptune, N.J., between July 1, 2014, and June 30, 2016. They hypothesized that using DOACs in this patient population might offer advantages over warfarin, including rapid onset and earlier hospital discharge, fewer drug interactions, and no coagulation monitoring.
Of the 146 patients on anticoagulants, 79 were discharged on warfarin, 43 on apixaban, 20 on rivaroxaban, and 4 on dabigatran. The other 139 patients were not anticoagulated for various reasons, one of which included normal sinus rhythm at discharge.
The researchers found that the DOACs were not significantly different from warfarin for efficacy endpoints in stroke (P = 0.23) or systemic embolism (P = 0.68). Safety endpoints also were similar among all groups for major bleeding (P = 0.57) or minor bleeding (P = 0.63). Median post-CABG length of stay was significantly longer in the warfarin group (8 days, P = 0.005), compared with dabigatran (7.5 days), rivaroxaban (6.5 days), and apixaban (6 days). In addition, the median total hospital length of stay was significantly longer with warfarin (11 days, P = 0.004), compared with rivaroxaban (8.5 days) and apixaban (9 days), but not compared with dabigatran (12 days).
Dr. Patel acknowledged that the study’s retrospective design and small sample size are limitations and said that larger prospective trials are warranted to confirm these results. She reported having no financial disclosures.
dbrunk@frontlinemedcom.com
SOURCE: Patel AJ et al. THSNA 2018. Poster 64.
SAN DIEGO – The use of direct oral anticoagulants were not significantly different from warfarin for safety and efficacy in cases of postoperative atrial fibrillation following coronary artery bypass grafting, according to results from a single-center study.
In fact, , which suggests a potential for reduced hospital stay-related costs. “There are several different niche populations for which the DOACs have not been studied,” one of the study authors, Ammie J. Patel, PharmD, said in an interview at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “This provides the opportunity for institutions and hospitals to study the use of these drugs in non-traditional ways.”
Dr. Patel, of Temple University, Philadelphia, said that postoperative atrial fibrillation occurs in about 30% of patients following CABG and that new-onset postoperative atrial fibrillation after CABG has been linked to a 21% increase in relative mortality.
Noting that patients with planned major surgery were excluded from RE-LY, ROCKET AF, ARISTOTLE, and other pivotal clinical trials of DOACs, she and her research mentor, Rachael Durie, PharmD, retrospectively evaluated the safety and efficacy of DOACs in 285 cases of new-onset postoperative atrial fibrillation following CABG performed at Jersey Shore University Medical Center, Neptune, N.J., between July 1, 2014, and June 30, 2016. They hypothesized that using DOACs in this patient population might offer advantages over warfarin, including rapid onset and earlier hospital discharge, fewer drug interactions, and no coagulation monitoring.
Of the 146 patients on anticoagulants, 79 were discharged on warfarin, 43 on apixaban, 20 on rivaroxaban, and 4 on dabigatran. The other 139 patients were not anticoagulated for various reasons, one of which included normal sinus rhythm at discharge.
The researchers found that the DOACs were not significantly different from warfarin for efficacy endpoints in stroke (P = 0.23) or systemic embolism (P = 0.68). Safety endpoints also were similar among all groups for major bleeding (P = 0.57) or minor bleeding (P = 0.63). Median post-CABG length of stay was significantly longer in the warfarin group (8 days, P = 0.005), compared with dabigatran (7.5 days), rivaroxaban (6.5 days), and apixaban (6 days). In addition, the median total hospital length of stay was significantly longer with warfarin (11 days, P = 0.004), compared with rivaroxaban (8.5 days) and apixaban (9 days), but not compared with dabigatran (12 days).
Dr. Patel acknowledged that the study’s retrospective design and small sample size are limitations and said that larger prospective trials are warranted to confirm these results. She reported having no financial disclosures.
dbrunk@frontlinemedcom.com
SOURCE: Patel AJ et al. THSNA 2018. Poster 64.
SAN DIEGO – The use of direct oral anticoagulants were not significantly different from warfarin for safety and efficacy in cases of postoperative atrial fibrillation following coronary artery bypass grafting, according to results from a single-center study.
In fact, , which suggests a potential for reduced hospital stay-related costs. “There are several different niche populations for which the DOACs have not been studied,” one of the study authors, Ammie J. Patel, PharmD, said in an interview at the biennial summit of the Thrombosis & Hemostasis Societies of North America. “This provides the opportunity for institutions and hospitals to study the use of these drugs in non-traditional ways.”
Dr. Patel, of Temple University, Philadelphia, said that postoperative atrial fibrillation occurs in about 30% of patients following CABG and that new-onset postoperative atrial fibrillation after CABG has been linked to a 21% increase in relative mortality.
Noting that patients with planned major surgery were excluded from RE-LY, ROCKET AF, ARISTOTLE, and other pivotal clinical trials of DOACs, she and her research mentor, Rachael Durie, PharmD, retrospectively evaluated the safety and efficacy of DOACs in 285 cases of new-onset postoperative atrial fibrillation following CABG performed at Jersey Shore University Medical Center, Neptune, N.J., between July 1, 2014, and June 30, 2016. They hypothesized that using DOACs in this patient population might offer advantages over warfarin, including rapid onset and earlier hospital discharge, fewer drug interactions, and no coagulation monitoring.
Of the 146 patients on anticoagulants, 79 were discharged on warfarin, 43 on apixaban, 20 on rivaroxaban, and 4 on dabigatran. The other 139 patients were not anticoagulated for various reasons, one of which included normal sinus rhythm at discharge.
The researchers found that the DOACs were not significantly different from warfarin for efficacy endpoints in stroke (P = 0.23) or systemic embolism (P = 0.68). Safety endpoints also were similar among all groups for major bleeding (P = 0.57) or minor bleeding (P = 0.63). Median post-CABG length of stay was significantly longer in the warfarin group (8 days, P = 0.005), compared with dabigatran (7.5 days), rivaroxaban (6.5 days), and apixaban (6 days). In addition, the median total hospital length of stay was significantly longer with warfarin (11 days, P = 0.004), compared with rivaroxaban (8.5 days) and apixaban (9 days), but not compared with dabigatran (12 days).
Dr. Patel acknowledged that the study’s retrospective design and small sample size are limitations and said that larger prospective trials are warranted to confirm these results. She reported having no financial disclosures.
dbrunk@frontlinemedcom.com
SOURCE: Patel AJ et al. THSNA 2018. Poster 64.
REPORTING FROM THSNA 2018
Key clinical point: DOACs may be safe and effective alternatives to warfarin in postoperative atrial fibrillation after CABG.
Major finding: DOACs were not significantly different from warfarin for efficacy endpoints in stroke (P = 0.23) or systemic embolism (P = 0.68).
Study details: A retrospective, single-center study of DOACs in 285 cases of new-onset postoperative atrial fibrillation following CABG.
Disclosures: Dr. Patel reported having no financial disclosures.
Source: Patel AJ et al. THSNA 2018. Poster 64.
More evidence links increased BMI to higher multiple myeloma risk
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
FROM BRITISH JOURNAL OF CANCER
Key clinical point: Moderate increases in body mass index (BMI) can dramatically increase the risk of developing multiple myeloma (MM).
Major finding: Each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17%.
Study details: Prospective analysis of 49,374 men and 153,260 women from three databases.
Disclosures: None of the researchers had competing financial interests to disclose.
Source: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
Atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Open Clinical Trials for Patients With Multiple Sclerosis (FULL)
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. More than 51,000 open trials are listed on the ClinicalTrials.gov website. Many explicitly recruit patients from the VA (212 studies), the military (132 studies), and IHS and native health organizations (4 studies). The VA Health Services Research and Development department alone sponsors > 250 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of February 22, 2017, and are focused on the treatment of multiple sclerosis (MS) and other neurologic disorders. For additional information and full inclusion/exclusion criteria, please consult https://clinicaltrials.gov.
Integrating Caregiver Support Into MS Care (MS Caregiver)
With loss of mobility in MS comes an increase in amount and types of caregiver assistance, with a concomitant increase in burden for the caregiver. This feasibility study will test integration of a successful behavioral caregiving intervention into clinical practice to improve functioning of veterans with MS and their caregivers. Caregivers of veterans with MS will receive a behavioral caregiver intervention designed to address caregiver coping and management of patient concerns, with special focus on patient mobility and walking. A pre-post intervention design will compare outcomes for veterans and caregivers.
ID: NCT02835677
Sponsor: Memphis VA Medical Center
Contact: Linda O. Nichols, PhD, linda.nichols@va.gov, Jennifer L. Martindale-Adams, jennifer.martindale-adams@va.gov
Study and Treatment of Visual Dysfunction and Motor Fatigue in Multiple Sclerosis
Primary fatigue represents a major cause of disability in patients with MS, being reported in about 90% of cases. The investigators propose a characteristic eye movement abnormality (internuclear ophthalmoparesis), commonly encountered in MS, as a simple model for primary motor fatigue. The investigators propose a medical treatment to improve ocular performance/fatigue in internuclear ophthalmoparesis, which can reduce visual disability and improve quality of life in veterans with MS.
ID: NCT02391961
Sponsor: VA Office of Research and Development
Location (contact): Louis Stokes VA Medical Center, Cleveland, Ohio (Holly B. Henry)
Dysport Treatment of Urinary Incontinence in Adults Subjects With Neurogenic Detrusor Overactivity Due to Spinal Cord Injury or Multiple Sclerosis
The purpose of this study is to provide confirmatory evidence of the safety and efficacy of 2 Dysport (AbobotulinumtoxinA) doses (600 units [U] and 800 U), compared to placebo in reducing urinary incontinence in adult subjects treated for neurogenic detrusor overactivity due to spinal cord injury or MS.
ID: NCT02660138
Sponsor: Ipsen
Location (contact Ipsen Recruitment Enquiries, clinical.trials@ ipsen.com): VA Long Beach Healthcare System, California, VA Palo Alto Health Care System, California, Raymond G. Murphy VAMC Albuquerque, New Mexico, Louis Stokes Cleveland Veterans Affairs Medical Center, Ohio.
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. More than 51,000 open trials are listed on the ClinicalTrials.gov website. Many explicitly recruit patients from the VA (212 studies), the military (132 studies), and IHS and native health organizations (4 studies). The VA Health Services Research and Development department alone sponsors > 250 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of February 22, 2017, and are focused on the treatment of multiple sclerosis (MS) and other neurologic disorders. For additional information and full inclusion/exclusion criteria, please consult https://clinicaltrials.gov.
Integrating Caregiver Support Into MS Care (MS Caregiver)
With loss of mobility in MS comes an increase in amount and types of caregiver assistance, with a concomitant increase in burden for the caregiver. This feasibility study will test integration of a successful behavioral caregiving intervention into clinical practice to improve functioning of veterans with MS and their caregivers. Caregivers of veterans with MS will receive a behavioral caregiver intervention designed to address caregiver coping and management of patient concerns, with special focus on patient mobility and walking. A pre-post intervention design will compare outcomes for veterans and caregivers.
ID: NCT02835677
Sponsor: Memphis VA Medical Center
Contact: Linda O. Nichols, PhD, linda.nichols@va.gov, Jennifer L. Martindale-Adams, jennifer.martindale-adams@va.gov
Study and Treatment of Visual Dysfunction and Motor Fatigue in Multiple Sclerosis
Primary fatigue represents a major cause of disability in patients with MS, being reported in about 90% of cases. The investigators propose a characteristic eye movement abnormality (internuclear ophthalmoparesis), commonly encountered in MS, as a simple model for primary motor fatigue. The investigators propose a medical treatment to improve ocular performance/fatigue in internuclear ophthalmoparesis, which can reduce visual disability and improve quality of life in veterans with MS.
ID: NCT02391961
Sponsor: VA Office of Research and Development
Location (contact): Louis Stokes VA Medical Center, Cleveland, Ohio (Holly B. Henry)
Dysport Treatment of Urinary Incontinence in Adults Subjects With Neurogenic Detrusor Overactivity Due to Spinal Cord Injury or Multiple Sclerosis
The purpose of this study is to provide confirmatory evidence of the safety and efficacy of 2 Dysport (AbobotulinumtoxinA) doses (600 units [U] and 800 U), compared to placebo in reducing urinary incontinence in adult subjects treated for neurogenic detrusor overactivity due to spinal cord injury or MS.
ID: NCT02660138
Sponsor: Ipsen
Location (contact Ipsen Recruitment Enquiries, clinical.trials@ ipsen.com): VA Long Beach Healthcare System, California, VA Palo Alto Health Care System, California, Raymond G. Murphy VAMC Albuquerque, New Mexico, Louis Stokes Cleveland Veterans Affairs Medical Center, Ohio.
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. More than 51,000 open trials are listed on the ClinicalTrials.gov website. Many explicitly recruit patients from the VA (212 studies), the military (132 studies), and IHS and native health organizations (4 studies). The VA Health Services Research and Development department alone sponsors > 250 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of February 22, 2017, and are focused on the treatment of multiple sclerosis (MS) and other neurologic disorders. For additional information and full inclusion/exclusion criteria, please consult https://clinicaltrials.gov.
Integrating Caregiver Support Into MS Care (MS Caregiver)
With loss of mobility in MS comes an increase in amount and types of caregiver assistance, with a concomitant increase in burden for the caregiver. This feasibility study will test integration of a successful behavioral caregiving intervention into clinical practice to improve functioning of veterans with MS and their caregivers. Caregivers of veterans with MS will receive a behavioral caregiver intervention designed to address caregiver coping and management of patient concerns, with special focus on patient mobility and walking. A pre-post intervention design will compare outcomes for veterans and caregivers.
ID: NCT02835677
Sponsor: Memphis VA Medical Center
Contact: Linda O. Nichols, PhD, linda.nichols@va.gov, Jennifer L. Martindale-Adams, jennifer.martindale-adams@va.gov
Study and Treatment of Visual Dysfunction and Motor Fatigue in Multiple Sclerosis
Primary fatigue represents a major cause of disability in patients with MS, being reported in about 90% of cases. The investigators propose a characteristic eye movement abnormality (internuclear ophthalmoparesis), commonly encountered in MS, as a simple model for primary motor fatigue. The investigators propose a medical treatment to improve ocular performance/fatigue in internuclear ophthalmoparesis, which can reduce visual disability and improve quality of life in veterans with MS.
ID: NCT02391961
Sponsor: VA Office of Research and Development
Location (contact): Louis Stokes VA Medical Center, Cleveland, Ohio (Holly B. Henry)
Dysport Treatment of Urinary Incontinence in Adults Subjects With Neurogenic Detrusor Overactivity Due to Spinal Cord Injury or Multiple Sclerosis
The purpose of this study is to provide confirmatory evidence of the safety and efficacy of 2 Dysport (AbobotulinumtoxinA) doses (600 units [U] and 800 U), compared to placebo in reducing urinary incontinence in adult subjects treated for neurogenic detrusor overactivity due to spinal cord injury or MS.
ID: NCT02660138
Sponsor: Ipsen
Location (contact Ipsen Recruitment Enquiries, clinical.trials@ ipsen.com): VA Long Beach Healthcare System, California, VA Palo Alto Health Care System, California, Raymond G. Murphy VAMC Albuquerque, New Mexico, Louis Stokes Cleveland Veterans Affairs Medical Center, Ohio.