User login
I am not your burnout expert
Look, I am not a burnout expert. And neither are you (presumably). None of us know much, but that won’t stop the regulations from coming. Program directors are already being asked to provide “wellness plans.” Through the SVS, experts have been enlisted to help, but it is now clear that what works for others won’t necessarily work for vascular surgeons. The next step is up to us. We are the only ones with detailed knowledge of our lives. I believe we are moving closer to answers but still face a few significant hurdles. Don’t worry, there are solutions. Hear me out …
Previously, I shared three studies with you, which found that vascular surgeons had the highest rates of suicidal ideation and career dissatisfaction among surgeons while spending more hours in the hospital than any other specialty. So what has been done to address these horrific numbers? Very little. We need answers now, but most of the data are over 10 years old. Much has changed in our specialty. The endovascular revolution created an entirely new working paradigm. A busy vascular surgeon used to perform 300 cases annually; now this number approaches 1,000. More procedures means more clerical work. Lead aprons and radiation exposure have added new ergonomic and medical concerns. Reimbursement dynamics now favor shorter, more frequent patient interactions over longer, more complex cases. We are benchmarked against old work standards while CPT bundling continuously lowers current RVU designations. EMR was supposed to make our lives better; it has done the opposite. Patient-centered health care has become a mantra, but the measures taken often backfire. Practicing medicine where the desired outcome is a high score on patient satisfaction surveys will likely lead to unnecessary tests, poor cost allocation, and low physician fulfillment. Quality of care is now measured scrupulously while the quality of our lives remains undocumented.
In the absence of organized reform, burnout appears to be increasing. A recent Mayo Clinic–AMA study found the current prevalence to be 54% among physicians. All of this has not happened overnight. I believe practicing vascular surgeons are resilient by default. The majority of us trained prior to the enforcement of duty hour restrictions. Out of high school, I enrolled in a 6-year BA/MD program (skipping 2 years of college seemed like a great idea in high school, less so when I got there). Half of my class never finished. In my intern year, six of the eight categorical residents dropped out. My odds of reaching PGY 2 were 12.5%. Fuzzy math aside, all of your stories are similar. We have proved our resilience over and over again. What is happening here is different.
Burnout is described as emotional exhaustion, low self-esteem, and depersonalization/cynicism. It develops slowly, progressively as stressors increase. A common thread seems to be the feeling that you alone are not enough. Examine your daily life. What are your most common stressors? For me, they relate to time management, clinical documentation, and whatever fresh hell my kids’ teachers have cooked up for “school projects.”
*****Scene*****
Wife: Can you help Luke (kindergarten) finish his diorama? It needs to be a scale depiction of his 3 favorite scenes from Wagner’s Ring cycle.
Me: Sure, I just need to complete the wind tunnel testing on Jack’s (3rd grade) carbon-neutral peanut-free alternative fuel source rocket booster.
Off stage – 7th Grade Son: The genetically modified spiders got loose again!
*****End Scene*****
We want to do a good job, but more hurdles are placed in our way. A recent AMA/Dartmouth Hitchcock study found that 50% of physicians’ time is spent performing data entry and other administrative work. Only 27% of time was spent on patient care. Every hour of face-to-face patient time requires 2 hours of EMR/clerical work. We are trapped in a bureaucratic prison. For years, every quality initiative was solved with a new form. To enter a simple note today, we must first “establish our relationship” with the patient, then ably click through a minefield of “warning boxes” signifying impending DVT prophylaxis catastrophes and antibiotic crimes and misdemeanors, next we scroll through a pre-populated postapocalyptic hellscape of minute- by-minute vital sign entries and lab values dating back to inception. Then, and only then, finally, ON PAGE 11, we can meagerly type: Patient at wound care, will come back on evening rounds.
Another important component of the burnout syndrome is dehumanization. Recently I spoke with Donald Zimmerman, PhD, author of the textbook “Person-Focused Health Care Management.” His thoughts on health care were dramatically altered after spending 43 days in an ICU following abdominal aortic aneurysm repair. He describes the experience as “my worst nightmare that then got worse and then never ended.” While we can learn from his experience, how many of us were trained to face this horror? Dehumanization is a natural protective response, especially when we have so little time for patient interactions. Compassion fatigue sets in when we don’t have the time and resources to care for our patients.While poor outcomes have been cited as a result of burnout, this appears to be an end-stage result. The Minimizing Error, Maximizing Outcome (MEMO) study funded by the AHRQ found that physicians often served as a buffer between their patients and poor medical environments. The organizational flaws that led to burnout also independently resulted in substandard patient care. The burnout physicians experienced was a symptom of the defective health care system and not causative of the poor care. Doctors were literally sacrificing their well-being to care for their patients.
Not surprisingly, attitudes regarding burnout vary significantly between health care executives and physicians. A New England Journal of Medicine survey of their Insights Council found that 96% of respondents agreed that burnout is a moderate or serious problem, although physicians were significantly more likely than executives to rate the problem as “serious.” Opinions on solutions varied as well, with executives more likely to support redesign of EMR, while physicians favored reduction of documentation and clerical work. Obviously the physicians’ solution would be more costly to the corporation as the executives deflected the problem back to the EMR designers. Neither group favored the use of resilience/wellness programs as a primary solution.
Of all the remedies proposed, I find resilience training to be especially egregious. Studies consistently show a 40%-50% prevalence of burnout among physicians. How can this be an individual problem? Why train doctors to endure a broken system? This type of problem solving is why burnout continues to flourish. Doctors are not suffering from a disease but rather exhibiting a symptom.
To arrive at possible solutions, let’s look at the elite athlete analogy. What are you trained to do? What are your exceptional skills? For me it is clearly EMR documentation (just checking to see if any of my residents have read this far). How many of us would describe ourselves as expert at billing? Paperwork? Medication reconciliation? Discharge summaries? Should LeBron James hawk 16-ounce Miller Lites in the nosebleeds during halftime? This may sound like I am expressing a cocky attitude that these tasks are beneath us, but we now have concrete evidence that forcing physicians to perform these duties hurts patient care and literally kills us. Full stop. Physician burnout can lead to suicide in the absence of clinical depression.
While hopelessness is part and parcel of the burnout syndrome, there are now potential solutions within our grasp. Clearly a reduction in clerical duties will be a key component of any realistic plan. Our time must be proportioned. Few of us are asking to work less. Reducing patient interactions while increasing the average time of these encounters has been shown to reduce burnout without decreasing work hours. We want to do a good job. It is time to remove these barriers.
Our next steps have already been taken, and for me it represents the best example of the potential of Vascular Specialist and the SVS. Under the leadership of SVS President Clem Darling, MD, and Executive Director Ken Slaw, PhD, a task force was created to address this issue. Ably chaired by Dawn Coleman, MD, and including Sam Money, MD, from the SVS Executive Council and Past SVS President Julie Freischlag, MD, the task force has collaborated with actual burnout experts Tait Shanafelt, MD, and Susan Hallbeck, PhD, to create a survey designed to identify the causes, prevalence, and potential solutions to the burnout problem in vascular surgery.
The first survey has been completed and will be issued to all SVS members this month to coincide with the SCVS annual symposium. The second, which will focus more on physical issues, will be released during the VAM in June.
Look, no one hates surveys more than I do. We simply have to get this information. Each survey is designed to only take 10 minutes. Things are going to change one way or another. Let’s lead, not wait to follow. With your help this will be the last time I write this ignorantly on this crisis. Vascular surgeons are few in number but this gives us the potential to deliver the most comprehensive self-assessment any specialty has ever performed. Lend your voice to the coming change.
Finally, there are now innovations in use which have proved beneficial in mitigating burnout. A Stanford University School of Medicine program allows physicians to “bank” time spent on committees, teaching, or other administrative duties and exchange these credits for home delivery meals, cleaning services, or even work tasks such as grant applications and paper writing. While the physicians could certainly afford to pay for these assistances, the success of the program demonstrates it is the time saved in arranging the services that the doctors truly valued. Our happiness seems to excel when we spend our time performing the tasks for which we are best suited.
It is time to change. When a system reaches this point, something breaks. Let’s stop being the thing that breaks. Fill out the survey. Get involved. There is time to act before we all burn out on burnout.
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, Division of Vascular and Endovascular Surgery, Louisiana State University Health Sciences Center, New Orleans.
Look, I am not a burnout expert. And neither are you (presumably). None of us know much, but that won’t stop the regulations from coming. Program directors are already being asked to provide “wellness plans.” Through the SVS, experts have been enlisted to help, but it is now clear that what works for others won’t necessarily work for vascular surgeons. The next step is up to us. We are the only ones with detailed knowledge of our lives. I believe we are moving closer to answers but still face a few significant hurdles. Don’t worry, there are solutions. Hear me out …
Previously, I shared three studies with you, which found that vascular surgeons had the highest rates of suicidal ideation and career dissatisfaction among surgeons while spending more hours in the hospital than any other specialty. So what has been done to address these horrific numbers? Very little. We need answers now, but most of the data are over 10 years old. Much has changed in our specialty. The endovascular revolution created an entirely new working paradigm. A busy vascular surgeon used to perform 300 cases annually; now this number approaches 1,000. More procedures means more clerical work. Lead aprons and radiation exposure have added new ergonomic and medical concerns. Reimbursement dynamics now favor shorter, more frequent patient interactions over longer, more complex cases. We are benchmarked against old work standards while CPT bundling continuously lowers current RVU designations. EMR was supposed to make our lives better; it has done the opposite. Patient-centered health care has become a mantra, but the measures taken often backfire. Practicing medicine where the desired outcome is a high score on patient satisfaction surveys will likely lead to unnecessary tests, poor cost allocation, and low physician fulfillment. Quality of care is now measured scrupulously while the quality of our lives remains undocumented.
In the absence of organized reform, burnout appears to be increasing. A recent Mayo Clinic–AMA study found the current prevalence to be 54% among physicians. All of this has not happened overnight. I believe practicing vascular surgeons are resilient by default. The majority of us trained prior to the enforcement of duty hour restrictions. Out of high school, I enrolled in a 6-year BA/MD program (skipping 2 years of college seemed like a great idea in high school, less so when I got there). Half of my class never finished. In my intern year, six of the eight categorical residents dropped out. My odds of reaching PGY 2 were 12.5%. Fuzzy math aside, all of your stories are similar. We have proved our resilience over and over again. What is happening here is different.
Burnout is described as emotional exhaustion, low self-esteem, and depersonalization/cynicism. It develops slowly, progressively as stressors increase. A common thread seems to be the feeling that you alone are not enough. Examine your daily life. What are your most common stressors? For me, they relate to time management, clinical documentation, and whatever fresh hell my kids’ teachers have cooked up for “school projects.”
*****Scene*****
Wife: Can you help Luke (kindergarten) finish his diorama? It needs to be a scale depiction of his 3 favorite scenes from Wagner’s Ring cycle.
Me: Sure, I just need to complete the wind tunnel testing on Jack’s (3rd grade) carbon-neutral peanut-free alternative fuel source rocket booster.
Off stage – 7th Grade Son: The genetically modified spiders got loose again!
*****End Scene*****
We want to do a good job, but more hurdles are placed in our way. A recent AMA/Dartmouth Hitchcock study found that 50% of physicians’ time is spent performing data entry and other administrative work. Only 27% of time was spent on patient care. Every hour of face-to-face patient time requires 2 hours of EMR/clerical work. We are trapped in a bureaucratic prison. For years, every quality initiative was solved with a new form. To enter a simple note today, we must first “establish our relationship” with the patient, then ably click through a minefield of “warning boxes” signifying impending DVT prophylaxis catastrophes and antibiotic crimes and misdemeanors, next we scroll through a pre-populated postapocalyptic hellscape of minute- by-minute vital sign entries and lab values dating back to inception. Then, and only then, finally, ON PAGE 11, we can meagerly type: Patient at wound care, will come back on evening rounds.
Another important component of the burnout syndrome is dehumanization. Recently I spoke with Donald Zimmerman, PhD, author of the textbook “Person-Focused Health Care Management.” His thoughts on health care were dramatically altered after spending 43 days in an ICU following abdominal aortic aneurysm repair. He describes the experience as “my worst nightmare that then got worse and then never ended.” While we can learn from his experience, how many of us were trained to face this horror? Dehumanization is a natural protective response, especially when we have so little time for patient interactions. Compassion fatigue sets in when we don’t have the time and resources to care for our patients.While poor outcomes have been cited as a result of burnout, this appears to be an end-stage result. The Minimizing Error, Maximizing Outcome (MEMO) study funded by the AHRQ found that physicians often served as a buffer between their patients and poor medical environments. The organizational flaws that led to burnout also independently resulted in substandard patient care. The burnout physicians experienced was a symptom of the defective health care system and not causative of the poor care. Doctors were literally sacrificing their well-being to care for their patients.
Not surprisingly, attitudes regarding burnout vary significantly between health care executives and physicians. A New England Journal of Medicine survey of their Insights Council found that 96% of respondents agreed that burnout is a moderate or serious problem, although physicians were significantly more likely than executives to rate the problem as “serious.” Opinions on solutions varied as well, with executives more likely to support redesign of EMR, while physicians favored reduction of documentation and clerical work. Obviously the physicians’ solution would be more costly to the corporation as the executives deflected the problem back to the EMR designers. Neither group favored the use of resilience/wellness programs as a primary solution.
Of all the remedies proposed, I find resilience training to be especially egregious. Studies consistently show a 40%-50% prevalence of burnout among physicians. How can this be an individual problem? Why train doctors to endure a broken system? This type of problem solving is why burnout continues to flourish. Doctors are not suffering from a disease but rather exhibiting a symptom.
To arrive at possible solutions, let’s look at the elite athlete analogy. What are you trained to do? What are your exceptional skills? For me it is clearly EMR documentation (just checking to see if any of my residents have read this far). How many of us would describe ourselves as expert at billing? Paperwork? Medication reconciliation? Discharge summaries? Should LeBron James hawk 16-ounce Miller Lites in the nosebleeds during halftime? This may sound like I am expressing a cocky attitude that these tasks are beneath us, but we now have concrete evidence that forcing physicians to perform these duties hurts patient care and literally kills us. Full stop. Physician burnout can lead to suicide in the absence of clinical depression.
While hopelessness is part and parcel of the burnout syndrome, there are now potential solutions within our grasp. Clearly a reduction in clerical duties will be a key component of any realistic plan. Our time must be proportioned. Few of us are asking to work less. Reducing patient interactions while increasing the average time of these encounters has been shown to reduce burnout without decreasing work hours. We want to do a good job. It is time to remove these barriers.
Our next steps have already been taken, and for me it represents the best example of the potential of Vascular Specialist and the SVS. Under the leadership of SVS President Clem Darling, MD, and Executive Director Ken Slaw, PhD, a task force was created to address this issue. Ably chaired by Dawn Coleman, MD, and including Sam Money, MD, from the SVS Executive Council and Past SVS President Julie Freischlag, MD, the task force has collaborated with actual burnout experts Tait Shanafelt, MD, and Susan Hallbeck, PhD, to create a survey designed to identify the causes, prevalence, and potential solutions to the burnout problem in vascular surgery.
The first survey has been completed and will be issued to all SVS members this month to coincide with the SCVS annual symposium. The second, which will focus more on physical issues, will be released during the VAM in June.
Look, no one hates surveys more than I do. We simply have to get this information. Each survey is designed to only take 10 minutes. Things are going to change one way or another. Let’s lead, not wait to follow. With your help this will be the last time I write this ignorantly on this crisis. Vascular surgeons are few in number but this gives us the potential to deliver the most comprehensive self-assessment any specialty has ever performed. Lend your voice to the coming change.
Finally, there are now innovations in use which have proved beneficial in mitigating burnout. A Stanford University School of Medicine program allows physicians to “bank” time spent on committees, teaching, or other administrative duties and exchange these credits for home delivery meals, cleaning services, or even work tasks such as grant applications and paper writing. While the physicians could certainly afford to pay for these assistances, the success of the program demonstrates it is the time saved in arranging the services that the doctors truly valued. Our happiness seems to excel when we spend our time performing the tasks for which we are best suited.
It is time to change. When a system reaches this point, something breaks. Let’s stop being the thing that breaks. Fill out the survey. Get involved. There is time to act before we all burn out on burnout.
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, Division of Vascular and Endovascular Surgery, Louisiana State University Health Sciences Center, New Orleans.
Look, I am not a burnout expert. And neither are you (presumably). None of us know much, but that won’t stop the regulations from coming. Program directors are already being asked to provide “wellness plans.” Through the SVS, experts have been enlisted to help, but it is now clear that what works for others won’t necessarily work for vascular surgeons. The next step is up to us. We are the only ones with detailed knowledge of our lives. I believe we are moving closer to answers but still face a few significant hurdles. Don’t worry, there are solutions. Hear me out …
Previously, I shared three studies with you, which found that vascular surgeons had the highest rates of suicidal ideation and career dissatisfaction among surgeons while spending more hours in the hospital than any other specialty. So what has been done to address these horrific numbers? Very little. We need answers now, but most of the data are over 10 years old. Much has changed in our specialty. The endovascular revolution created an entirely new working paradigm. A busy vascular surgeon used to perform 300 cases annually; now this number approaches 1,000. More procedures means more clerical work. Lead aprons and radiation exposure have added new ergonomic and medical concerns. Reimbursement dynamics now favor shorter, more frequent patient interactions over longer, more complex cases. We are benchmarked against old work standards while CPT bundling continuously lowers current RVU designations. EMR was supposed to make our lives better; it has done the opposite. Patient-centered health care has become a mantra, but the measures taken often backfire. Practicing medicine where the desired outcome is a high score on patient satisfaction surveys will likely lead to unnecessary tests, poor cost allocation, and low physician fulfillment. Quality of care is now measured scrupulously while the quality of our lives remains undocumented.
In the absence of organized reform, burnout appears to be increasing. A recent Mayo Clinic–AMA study found the current prevalence to be 54% among physicians. All of this has not happened overnight. I believe practicing vascular surgeons are resilient by default. The majority of us trained prior to the enforcement of duty hour restrictions. Out of high school, I enrolled in a 6-year BA/MD program (skipping 2 years of college seemed like a great idea in high school, less so when I got there). Half of my class never finished. In my intern year, six of the eight categorical residents dropped out. My odds of reaching PGY 2 were 12.5%. Fuzzy math aside, all of your stories are similar. We have proved our resilience over and over again. What is happening here is different.
Burnout is described as emotional exhaustion, low self-esteem, and depersonalization/cynicism. It develops slowly, progressively as stressors increase. A common thread seems to be the feeling that you alone are not enough. Examine your daily life. What are your most common stressors? For me, they relate to time management, clinical documentation, and whatever fresh hell my kids’ teachers have cooked up for “school projects.”
*****Scene*****
Wife: Can you help Luke (kindergarten) finish his diorama? It needs to be a scale depiction of his 3 favorite scenes from Wagner’s Ring cycle.
Me: Sure, I just need to complete the wind tunnel testing on Jack’s (3rd grade) carbon-neutral peanut-free alternative fuel source rocket booster.
Off stage – 7th Grade Son: The genetically modified spiders got loose again!
*****End Scene*****
We want to do a good job, but more hurdles are placed in our way. A recent AMA/Dartmouth Hitchcock study found that 50% of physicians’ time is spent performing data entry and other administrative work. Only 27% of time was spent on patient care. Every hour of face-to-face patient time requires 2 hours of EMR/clerical work. We are trapped in a bureaucratic prison. For years, every quality initiative was solved with a new form. To enter a simple note today, we must first “establish our relationship” with the patient, then ably click through a minefield of “warning boxes” signifying impending DVT prophylaxis catastrophes and antibiotic crimes and misdemeanors, next we scroll through a pre-populated postapocalyptic hellscape of minute- by-minute vital sign entries and lab values dating back to inception. Then, and only then, finally, ON PAGE 11, we can meagerly type: Patient at wound care, will come back on evening rounds.
Another important component of the burnout syndrome is dehumanization. Recently I spoke with Donald Zimmerman, PhD, author of the textbook “Person-Focused Health Care Management.” His thoughts on health care were dramatically altered after spending 43 days in an ICU following abdominal aortic aneurysm repair. He describes the experience as “my worst nightmare that then got worse and then never ended.” While we can learn from his experience, how many of us were trained to face this horror? Dehumanization is a natural protective response, especially when we have so little time for patient interactions. Compassion fatigue sets in when we don’t have the time and resources to care for our patients.While poor outcomes have been cited as a result of burnout, this appears to be an end-stage result. The Minimizing Error, Maximizing Outcome (MEMO) study funded by the AHRQ found that physicians often served as a buffer between their patients and poor medical environments. The organizational flaws that led to burnout also independently resulted in substandard patient care. The burnout physicians experienced was a symptom of the defective health care system and not causative of the poor care. Doctors were literally sacrificing their well-being to care for their patients.
Not surprisingly, attitudes regarding burnout vary significantly between health care executives and physicians. A New England Journal of Medicine survey of their Insights Council found that 96% of respondents agreed that burnout is a moderate or serious problem, although physicians were significantly more likely than executives to rate the problem as “serious.” Opinions on solutions varied as well, with executives more likely to support redesign of EMR, while physicians favored reduction of documentation and clerical work. Obviously the physicians’ solution would be more costly to the corporation as the executives deflected the problem back to the EMR designers. Neither group favored the use of resilience/wellness programs as a primary solution.
Of all the remedies proposed, I find resilience training to be especially egregious. Studies consistently show a 40%-50% prevalence of burnout among physicians. How can this be an individual problem? Why train doctors to endure a broken system? This type of problem solving is why burnout continues to flourish. Doctors are not suffering from a disease but rather exhibiting a symptom.
To arrive at possible solutions, let’s look at the elite athlete analogy. What are you trained to do? What are your exceptional skills? For me it is clearly EMR documentation (just checking to see if any of my residents have read this far). How many of us would describe ourselves as expert at billing? Paperwork? Medication reconciliation? Discharge summaries? Should LeBron James hawk 16-ounce Miller Lites in the nosebleeds during halftime? This may sound like I am expressing a cocky attitude that these tasks are beneath us, but we now have concrete evidence that forcing physicians to perform these duties hurts patient care and literally kills us. Full stop. Physician burnout can lead to suicide in the absence of clinical depression.
While hopelessness is part and parcel of the burnout syndrome, there are now potential solutions within our grasp. Clearly a reduction in clerical duties will be a key component of any realistic plan. Our time must be proportioned. Few of us are asking to work less. Reducing patient interactions while increasing the average time of these encounters has been shown to reduce burnout without decreasing work hours. We want to do a good job. It is time to remove these barriers.
Our next steps have already been taken, and for me it represents the best example of the potential of Vascular Specialist and the SVS. Under the leadership of SVS President Clem Darling, MD, and Executive Director Ken Slaw, PhD, a task force was created to address this issue. Ably chaired by Dawn Coleman, MD, and including Sam Money, MD, from the SVS Executive Council and Past SVS President Julie Freischlag, MD, the task force has collaborated with actual burnout experts Tait Shanafelt, MD, and Susan Hallbeck, PhD, to create a survey designed to identify the causes, prevalence, and potential solutions to the burnout problem in vascular surgery.
The first survey has been completed and will be issued to all SVS members this month to coincide with the SCVS annual symposium. The second, which will focus more on physical issues, will be released during the VAM in June.
Look, no one hates surveys more than I do. We simply have to get this information. Each survey is designed to only take 10 minutes. Things are going to change one way or another. Let’s lead, not wait to follow. With your help this will be the last time I write this ignorantly on this crisis. Vascular surgeons are few in number but this gives us the potential to deliver the most comprehensive self-assessment any specialty has ever performed. Lend your voice to the coming change.
Finally, there are now innovations in use which have proved beneficial in mitigating burnout. A Stanford University School of Medicine program allows physicians to “bank” time spent on committees, teaching, or other administrative duties and exchange these credits for home delivery meals, cleaning services, or even work tasks such as grant applications and paper writing. While the physicians could certainly afford to pay for these assistances, the success of the program demonstrates it is the time saved in arranging the services that the doctors truly valued. Our happiness seems to excel when we spend our time performing the tasks for which we are best suited.
It is time to change. When a system reaches this point, something breaks. Let’s stop being the thing that breaks. Fill out the survey. Get involved. There is time to act before we all burn out on burnout.
Dr. Sheahan is the Claude C. Craighead Jr. Professor and Chair, Division of Vascular and Endovascular Surgery, Louisiana State University Health Sciences Center, New Orleans.
Closure of High-Risk PFOs Reduces Risk of Recurrent Stroke
Closure of a patent foramen ovale (PFO) with an atrial septal aneurysm, hypermobility, or size of 2 mm or greater reduces the risk of stroke recurrence in patients with cryptogenic stroke, according to research published online ahead of print March 12 in the Journal of the American College of Cardiology.
“Considering the high prevalence of PFO in the general population and cryptogenic stroke patients, the key to appropriate use of this medical device is determining how to select optimal candidates for the procedure,” said Jae-Kwan Song, MD, PhD, a Professor in the Department of Medicine at Asan Medical Center, University of Ulsan College of Medicine, in Seoul, South Korea. “With our study and other recent trials, the criteria for selecting patients for the procedure are becoming clearer; in particular, the results suggest that closure is beneficial for those with high-risk PFO.”
A Multisite Superiority Trial
Previous research has not offered a definitive answer to the question of whether physicians can determine the potential benefit of PFO closure according to the PFO’s morphologic characteristics. In an earlier study, Dr. Song and colleagues found that high-risk PFO, as defined by transesophageal echocardiography (TEE), helped to predict stroke recurrence. The investigators then initiated the DEFENSE-PFO trial to evaluate whether restricting treatment to patients with cryptogenic stroke and PFO morphology associated with a higher rate of recurrent stroke would enhance the benefits of PFO closure.
Dr. Song and colleagues conducted DEFENSE-PFO, an open-label superiority trial, at two sites in South Korea from June 2011 through October 2017. Eligible patients had an ischemic stroke within the previous six months with no identifiable cause other than a high-risk PFO with left-to-right shunting. The researchers performed a standardized evaluation to rule out other identifiable mechanisms of stroke. The exclusion criteria were at least 50% stenosis of a major vessel, occlusion of a major vessel, and stroke resulting from small-vessel occlusive disease. Dr. Song and colleagues performed Holter monitoring or prolonged monitoring of cardiac rhythm to rule out paroxysmal atrial fibrillation.
A high-risk PFO was defined as one with an atrial septal aneurysm (ie, protrusion of the dilated segment of the septum at least 15 mm beyond the level surface of the atrial septum), hypermobility (ie, phasic septal excursion of 10 mm or more into either atrium), or size (ie, maximum separation of the septum primum from the secundum during the Valsalva maneuver) of 2 mm or greater on TEE.
Patients were randomized in equal groups to transcatheter PFO closure with the Amplatzer PFO Occluder plus medical therapy or medical therapy alone. All participants received antiplatelet therapy or anticoagulation chosen by the local investigator. During follow-up visits at one, three, six, 12, and 24 months, investigators recorded clinical data.
The primary end point was a composite of stroke, vascular death, or Thrombolysis in Myocardial Infarction (TIMI)-defined major bleeding during two years of follow-up. The secondary end point was asymptomatic ischemic stroke on follow-up MRI.
No End-Point Events After PFO Closure
Dr. Song and colleagues identified 450 patients with cryptogenic stoke and PFO, of whom 175 had high-risk PFO. They randomized 60 patients to each study arm. Participants’ mean age was 51.8. The groups were well balanced in terms of age, sex, medical history, qualifying event, modified Rankin scale score at discharge, and the anatomic characteristics of the PFO and atrial septum.
Seven patients randomized to PFO closure declined the treatment. Dual antiplatelet therapy was the most common medication in both groups at 30 days after randomization. This trend continued for as long as 12 months in the medication-only group, but single antiplatelet therapy became the most common strategy after six months in the PFO-closure group. About 17% of patients in the PFO-closure group stopped medication after the intervention. The median duration of follow-up was 2.8 years.
In the intention-to-treat analysis, no patient in the PFO-closure group had a primary end point event, compared with six patients in the medication-only group. Events recorded in the latter group included five ischemic strokes, one cerebral hemorrhage, two TIMI-defined major bleeding events, and one transient ischemic attack. The Kaplan-Meier two-year cumulative estimate of the probability of stroke was 10.5% in the medication-only group. The number of patients needed to treat with PFO closure to avoid one stroke at two years thus was 10.
Implications for Selection Criteria
The DEFENSE-PFO study differs from two previous trials that found benefits of PFO closure, but did not consider the anatomic features of the atrial septum or PFO. “The only trial with stringent entry criteria similar to ours is the CLOSE trial, which required that patients have a large interatrial right-to-left shunt (more than 30 microbubbles in the left atrium within three cardiac cycles after opacification of the right atrium) or an atrial septal aneurysm (a septum primum excursion greater than 10 mm),” said Dr. Song and colleagues. “Both the CLOSE trial and our trial showed no occurrence of stroke in patients who underwent PFO closure, suggesting that the beneficial effect of percutaneous device closure of PFO can be maximized by adding the morphologic characteristics of PFO, as evaluated by TEE, to the selection criteria for the procedure.”
Because the DEFENSE-PFO study was terminated early for patient safety, it was underpowered to provide a hazard ratio for its primary end point. In addition, selection bias may have affected the study, since it was conducted at a small number of centers.
—Erik Greb
Suggested Reading
Lee PH, Song J-K, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: The DEFENSE-PFO trial. J Am Coll Cardiol. 2018 Mar 12 [Epub ahead of print].
Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021.
Closure of a patent foramen ovale (PFO) with an atrial septal aneurysm, hypermobility, or size of 2 mm or greater reduces the risk of stroke recurrence in patients with cryptogenic stroke, according to research published online ahead of print March 12 in the Journal of the American College of Cardiology.
“Considering the high prevalence of PFO in the general population and cryptogenic stroke patients, the key to appropriate use of this medical device is determining how to select optimal candidates for the procedure,” said Jae-Kwan Song, MD, PhD, a Professor in the Department of Medicine at Asan Medical Center, University of Ulsan College of Medicine, in Seoul, South Korea. “With our study and other recent trials, the criteria for selecting patients for the procedure are becoming clearer; in particular, the results suggest that closure is beneficial for those with high-risk PFO.”
A Multisite Superiority Trial
Previous research has not offered a definitive answer to the question of whether physicians can determine the potential benefit of PFO closure according to the PFO’s morphologic characteristics. In an earlier study, Dr. Song and colleagues found that high-risk PFO, as defined by transesophageal echocardiography (TEE), helped to predict stroke recurrence. The investigators then initiated the DEFENSE-PFO trial to evaluate whether restricting treatment to patients with cryptogenic stroke and PFO morphology associated with a higher rate of recurrent stroke would enhance the benefits of PFO closure.
Dr. Song and colleagues conducted DEFENSE-PFO, an open-label superiority trial, at two sites in South Korea from June 2011 through October 2017. Eligible patients had an ischemic stroke within the previous six months with no identifiable cause other than a high-risk PFO with left-to-right shunting. The researchers performed a standardized evaluation to rule out other identifiable mechanisms of stroke. The exclusion criteria were at least 50% stenosis of a major vessel, occlusion of a major vessel, and stroke resulting from small-vessel occlusive disease. Dr. Song and colleagues performed Holter monitoring or prolonged monitoring of cardiac rhythm to rule out paroxysmal atrial fibrillation.
A high-risk PFO was defined as one with an atrial septal aneurysm (ie, protrusion of the dilated segment of the septum at least 15 mm beyond the level surface of the atrial septum), hypermobility (ie, phasic septal excursion of 10 mm or more into either atrium), or size (ie, maximum separation of the septum primum from the secundum during the Valsalva maneuver) of 2 mm or greater on TEE.
Patients were randomized in equal groups to transcatheter PFO closure with the Amplatzer PFO Occluder plus medical therapy or medical therapy alone. All participants received antiplatelet therapy or anticoagulation chosen by the local investigator. During follow-up visits at one, three, six, 12, and 24 months, investigators recorded clinical data.
The primary end point was a composite of stroke, vascular death, or Thrombolysis in Myocardial Infarction (TIMI)-defined major bleeding during two years of follow-up. The secondary end point was asymptomatic ischemic stroke on follow-up MRI.
No End-Point Events After PFO Closure
Dr. Song and colleagues identified 450 patients with cryptogenic stoke and PFO, of whom 175 had high-risk PFO. They randomized 60 patients to each study arm. Participants’ mean age was 51.8. The groups were well balanced in terms of age, sex, medical history, qualifying event, modified Rankin scale score at discharge, and the anatomic characteristics of the PFO and atrial septum.
Seven patients randomized to PFO closure declined the treatment. Dual antiplatelet therapy was the most common medication in both groups at 30 days after randomization. This trend continued for as long as 12 months in the medication-only group, but single antiplatelet therapy became the most common strategy after six months in the PFO-closure group. About 17% of patients in the PFO-closure group stopped medication after the intervention. The median duration of follow-up was 2.8 years.
In the intention-to-treat analysis, no patient in the PFO-closure group had a primary end point event, compared with six patients in the medication-only group. Events recorded in the latter group included five ischemic strokes, one cerebral hemorrhage, two TIMI-defined major bleeding events, and one transient ischemic attack. The Kaplan-Meier two-year cumulative estimate of the probability of stroke was 10.5% in the medication-only group. The number of patients needed to treat with PFO closure to avoid one stroke at two years thus was 10.
Implications for Selection Criteria
The DEFENSE-PFO study differs from two previous trials that found benefits of PFO closure, but did not consider the anatomic features of the atrial septum or PFO. “The only trial with stringent entry criteria similar to ours is the CLOSE trial, which required that patients have a large interatrial right-to-left shunt (more than 30 microbubbles in the left atrium within three cardiac cycles after opacification of the right atrium) or an atrial septal aneurysm (a septum primum excursion greater than 10 mm),” said Dr. Song and colleagues. “Both the CLOSE trial and our trial showed no occurrence of stroke in patients who underwent PFO closure, suggesting that the beneficial effect of percutaneous device closure of PFO can be maximized by adding the morphologic characteristics of PFO, as evaluated by TEE, to the selection criteria for the procedure.”
Because the DEFENSE-PFO study was terminated early for patient safety, it was underpowered to provide a hazard ratio for its primary end point. In addition, selection bias may have affected the study, since it was conducted at a small number of centers.
—Erik Greb
Suggested Reading
Lee PH, Song J-K, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: The DEFENSE-PFO trial. J Am Coll Cardiol. 2018 Mar 12 [Epub ahead of print].
Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021.
Closure of a patent foramen ovale (PFO) with an atrial septal aneurysm, hypermobility, or size of 2 mm or greater reduces the risk of stroke recurrence in patients with cryptogenic stroke, according to research published online ahead of print March 12 in the Journal of the American College of Cardiology.
“Considering the high prevalence of PFO in the general population and cryptogenic stroke patients, the key to appropriate use of this medical device is determining how to select optimal candidates for the procedure,” said Jae-Kwan Song, MD, PhD, a Professor in the Department of Medicine at Asan Medical Center, University of Ulsan College of Medicine, in Seoul, South Korea. “With our study and other recent trials, the criteria for selecting patients for the procedure are becoming clearer; in particular, the results suggest that closure is beneficial for those with high-risk PFO.”
A Multisite Superiority Trial
Previous research has not offered a definitive answer to the question of whether physicians can determine the potential benefit of PFO closure according to the PFO’s morphologic characteristics. In an earlier study, Dr. Song and colleagues found that high-risk PFO, as defined by transesophageal echocardiography (TEE), helped to predict stroke recurrence. The investigators then initiated the DEFENSE-PFO trial to evaluate whether restricting treatment to patients with cryptogenic stroke and PFO morphology associated with a higher rate of recurrent stroke would enhance the benefits of PFO closure.
Dr. Song and colleagues conducted DEFENSE-PFO, an open-label superiority trial, at two sites in South Korea from June 2011 through October 2017. Eligible patients had an ischemic stroke within the previous six months with no identifiable cause other than a high-risk PFO with left-to-right shunting. The researchers performed a standardized evaluation to rule out other identifiable mechanisms of stroke. The exclusion criteria were at least 50% stenosis of a major vessel, occlusion of a major vessel, and stroke resulting from small-vessel occlusive disease. Dr. Song and colleagues performed Holter monitoring or prolonged monitoring of cardiac rhythm to rule out paroxysmal atrial fibrillation.
A high-risk PFO was defined as one with an atrial septal aneurysm (ie, protrusion of the dilated segment of the septum at least 15 mm beyond the level surface of the atrial septum), hypermobility (ie, phasic septal excursion of 10 mm or more into either atrium), or size (ie, maximum separation of the septum primum from the secundum during the Valsalva maneuver) of 2 mm or greater on TEE.
Patients were randomized in equal groups to transcatheter PFO closure with the Amplatzer PFO Occluder plus medical therapy or medical therapy alone. All participants received antiplatelet therapy or anticoagulation chosen by the local investigator. During follow-up visits at one, three, six, 12, and 24 months, investigators recorded clinical data.
The primary end point was a composite of stroke, vascular death, or Thrombolysis in Myocardial Infarction (TIMI)-defined major bleeding during two years of follow-up. The secondary end point was asymptomatic ischemic stroke on follow-up MRI.
No End-Point Events After PFO Closure
Dr. Song and colleagues identified 450 patients with cryptogenic stoke and PFO, of whom 175 had high-risk PFO. They randomized 60 patients to each study arm. Participants’ mean age was 51.8. The groups were well balanced in terms of age, sex, medical history, qualifying event, modified Rankin scale score at discharge, and the anatomic characteristics of the PFO and atrial septum.
Seven patients randomized to PFO closure declined the treatment. Dual antiplatelet therapy was the most common medication in both groups at 30 days after randomization. This trend continued for as long as 12 months in the medication-only group, but single antiplatelet therapy became the most common strategy after six months in the PFO-closure group. About 17% of patients in the PFO-closure group stopped medication after the intervention. The median duration of follow-up was 2.8 years.
In the intention-to-treat analysis, no patient in the PFO-closure group had a primary end point event, compared with six patients in the medication-only group. Events recorded in the latter group included five ischemic strokes, one cerebral hemorrhage, two TIMI-defined major bleeding events, and one transient ischemic attack. The Kaplan-Meier two-year cumulative estimate of the probability of stroke was 10.5% in the medication-only group. The number of patients needed to treat with PFO closure to avoid one stroke at two years thus was 10.
Implications for Selection Criteria
The DEFENSE-PFO study differs from two previous trials that found benefits of PFO closure, but did not consider the anatomic features of the atrial septum or PFO. “The only trial with stringent entry criteria similar to ours is the CLOSE trial, which required that patients have a large interatrial right-to-left shunt (more than 30 microbubbles in the left atrium within three cardiac cycles after opacification of the right atrium) or an atrial septal aneurysm (a septum primum excursion greater than 10 mm),” said Dr. Song and colleagues. “Both the CLOSE trial and our trial showed no occurrence of stroke in patients who underwent PFO closure, suggesting that the beneficial effect of percutaneous device closure of PFO can be maximized by adding the morphologic characteristics of PFO, as evaluated by TEE, to the selection criteria for the procedure.”
Because the DEFENSE-PFO study was terminated early for patient safety, it was underpowered to provide a hazard ratio for its primary end point. In addition, selection bias may have affected the study, since it was conducted at a small number of centers.
—Erik Greb
Suggested Reading
Lee PH, Song J-K, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: The DEFENSE-PFO trial. J Am Coll Cardiol. 2018 Mar 12 [Epub ahead of print].
Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377(11):1011-1021.
Analytics, board support are quality improvement keys
Jeffrey Glasheen, MD, had not considered focusing on quality improvement (QI) while studying at the University of Wisconsin, Madison. It was not until a medical error led to the death of a family member that his eyes were opened to the potential consequences of a system not invested in care quality.
“I couldn’t square with it because I had spent the last two to three years of my life working with some of the most dedicated, passionate, hard working people who all were trying to improve lives, and the fact that what I was seeing could result in a family member dying just didn’t make sense,” said Dr. Glasheen. “At the time I thought ‘This must be one of those unfortunate things that happens once in a lifetime,’ and I put it on the back burner.”
As more research on medical errors emerged, however, Dr. Glasheen realized his family’s experience was not as unique as he had thought.
It was after reading the now famous Institute of Medicine report, “To err is human,” which found that medical errors were responsible for 44,000-98,000 deaths a year, that Dr. Glasheen resolved to pursue a career in quality improvement.
Because it was early in his medical career, he began on a small level, teaching his residents about the importance of patient safety and giving lessons on core competencies involved in quality care and higher liability. But he quickly expanded his efforts.
“I started with what I had control over,” Dr. Glasheen explained. “From there, I moved to teaching more medical students, which lead to teaching in front of classrooms, which opened the door to the idea of starting a hospitalist training program.”
In 2003, Dr. Glasheen pitched the program to the University of Colorado at Denver, Aurora, where he completed his residency; this pitch led to the development of a hospitalist training program that focused on improving safety outcomes.
He served as the director of the University of Colorado Hospital Medicine Group from 2003 to 2015, during which time he was approached by the dean to assist in creating and leading the hospitalist training program for internal medicine residents.
The first of its kind, the rigorous University of Colorado program was designed to give residents tools useful beyond the clinical setting to become successful health system leaders.
In 2013, Dr. Glasheen and his colleagues founded the Institute for Healthcare Quality, Safety & Efficiency, which is guided by the mission to improve the quality of care provided on the local level. He has since become the chief quality officer for UCHealth and the University of Colorado Hospital Authority and an associate dean for clinical affairs in quality and safety education, as well as continuing to be a professor of medicine.
For those hoping to pursue quality improvement, Dr. Glasheen stressed the importance of a strong basis in data analytics.
“One of the most common things I see with data is people start to chase what’s called common cause variation, which means they’ll look at a run chart over the course of 12 months and react to every up and down when those are essentially random,” Dr. Glasheen said. “Being able to understand when something is particularly significant and when your interventions are actually making an impact is a skill set I think people who are new to quality improvement don’t often have.”
Having support from board members is also critical to success, although starting without such support should not deter future QI leaders.
“There needs to be a vision from the leadership that this work is important, and not just through words but through deeds, because no board in the country will say that quality is not important,” Dr. Glasheen said. “I would say start with small projects you can control, that tie back not only to patient lives but financial performance as well. If you can tell a board you saved the lives of 40 patients who would have died during the year and saved $1-$2 million in the process, the question will shift from whether the board should invest in QI resources to how much should be invested.”
Looking ahead, Dr. Glasheen highlighted the growing importance of hospital-acquired infections, such as surgical-site infections, catheter-associated urinary tract infections, and ventilator-associated pneumonia, as areas that need to be focused on in the QI sphere.
Jeffrey Glasheen, MD, had not considered focusing on quality improvement (QI) while studying at the University of Wisconsin, Madison. It was not until a medical error led to the death of a family member that his eyes were opened to the potential consequences of a system not invested in care quality.
“I couldn’t square with it because I had spent the last two to three years of my life working with some of the most dedicated, passionate, hard working people who all were trying to improve lives, and the fact that what I was seeing could result in a family member dying just didn’t make sense,” said Dr. Glasheen. “At the time I thought ‘This must be one of those unfortunate things that happens once in a lifetime,’ and I put it on the back burner.”
As more research on medical errors emerged, however, Dr. Glasheen realized his family’s experience was not as unique as he had thought.
It was after reading the now famous Institute of Medicine report, “To err is human,” which found that medical errors were responsible for 44,000-98,000 deaths a year, that Dr. Glasheen resolved to pursue a career in quality improvement.
Because it was early in his medical career, he began on a small level, teaching his residents about the importance of patient safety and giving lessons on core competencies involved in quality care and higher liability. But he quickly expanded his efforts.
“I started with what I had control over,” Dr. Glasheen explained. “From there, I moved to teaching more medical students, which lead to teaching in front of classrooms, which opened the door to the idea of starting a hospitalist training program.”
In 2003, Dr. Glasheen pitched the program to the University of Colorado at Denver, Aurora, where he completed his residency; this pitch led to the development of a hospitalist training program that focused on improving safety outcomes.
He served as the director of the University of Colorado Hospital Medicine Group from 2003 to 2015, during which time he was approached by the dean to assist in creating and leading the hospitalist training program for internal medicine residents.
The first of its kind, the rigorous University of Colorado program was designed to give residents tools useful beyond the clinical setting to become successful health system leaders.
In 2013, Dr. Glasheen and his colleagues founded the Institute for Healthcare Quality, Safety & Efficiency, which is guided by the mission to improve the quality of care provided on the local level. He has since become the chief quality officer for UCHealth and the University of Colorado Hospital Authority and an associate dean for clinical affairs in quality and safety education, as well as continuing to be a professor of medicine.
For those hoping to pursue quality improvement, Dr. Glasheen stressed the importance of a strong basis in data analytics.
“One of the most common things I see with data is people start to chase what’s called common cause variation, which means they’ll look at a run chart over the course of 12 months and react to every up and down when those are essentially random,” Dr. Glasheen said. “Being able to understand when something is particularly significant and when your interventions are actually making an impact is a skill set I think people who are new to quality improvement don’t often have.”
Having support from board members is also critical to success, although starting without such support should not deter future QI leaders.
“There needs to be a vision from the leadership that this work is important, and not just through words but through deeds, because no board in the country will say that quality is not important,” Dr. Glasheen said. “I would say start with small projects you can control, that tie back not only to patient lives but financial performance as well. If you can tell a board you saved the lives of 40 patients who would have died during the year and saved $1-$2 million in the process, the question will shift from whether the board should invest in QI resources to how much should be invested.”
Looking ahead, Dr. Glasheen highlighted the growing importance of hospital-acquired infections, such as surgical-site infections, catheter-associated urinary tract infections, and ventilator-associated pneumonia, as areas that need to be focused on in the QI sphere.
Jeffrey Glasheen, MD, had not considered focusing on quality improvement (QI) while studying at the University of Wisconsin, Madison. It was not until a medical error led to the death of a family member that his eyes were opened to the potential consequences of a system not invested in care quality.
“I couldn’t square with it because I had spent the last two to three years of my life working with some of the most dedicated, passionate, hard working people who all were trying to improve lives, and the fact that what I was seeing could result in a family member dying just didn’t make sense,” said Dr. Glasheen. “At the time I thought ‘This must be one of those unfortunate things that happens once in a lifetime,’ and I put it on the back burner.”
As more research on medical errors emerged, however, Dr. Glasheen realized his family’s experience was not as unique as he had thought.
It was after reading the now famous Institute of Medicine report, “To err is human,” which found that medical errors were responsible for 44,000-98,000 deaths a year, that Dr. Glasheen resolved to pursue a career in quality improvement.
Because it was early in his medical career, he began on a small level, teaching his residents about the importance of patient safety and giving lessons on core competencies involved in quality care and higher liability. But he quickly expanded his efforts.
“I started with what I had control over,” Dr. Glasheen explained. “From there, I moved to teaching more medical students, which lead to teaching in front of classrooms, which opened the door to the idea of starting a hospitalist training program.”
In 2003, Dr. Glasheen pitched the program to the University of Colorado at Denver, Aurora, where he completed his residency; this pitch led to the development of a hospitalist training program that focused on improving safety outcomes.
He served as the director of the University of Colorado Hospital Medicine Group from 2003 to 2015, during which time he was approached by the dean to assist in creating and leading the hospitalist training program for internal medicine residents.
The first of its kind, the rigorous University of Colorado program was designed to give residents tools useful beyond the clinical setting to become successful health system leaders.
In 2013, Dr. Glasheen and his colleagues founded the Institute for Healthcare Quality, Safety & Efficiency, which is guided by the mission to improve the quality of care provided on the local level. He has since become the chief quality officer for UCHealth and the University of Colorado Hospital Authority and an associate dean for clinical affairs in quality and safety education, as well as continuing to be a professor of medicine.
For those hoping to pursue quality improvement, Dr. Glasheen stressed the importance of a strong basis in data analytics.
“One of the most common things I see with data is people start to chase what’s called common cause variation, which means they’ll look at a run chart over the course of 12 months and react to every up and down when those are essentially random,” Dr. Glasheen said. “Being able to understand when something is particularly significant and when your interventions are actually making an impact is a skill set I think people who are new to quality improvement don’t often have.”
Having support from board members is also critical to success, although starting without such support should not deter future QI leaders.
“There needs to be a vision from the leadership that this work is important, and not just through words but through deeds, because no board in the country will say that quality is not important,” Dr. Glasheen said. “I would say start with small projects you can control, that tie back not only to patient lives but financial performance as well. If you can tell a board you saved the lives of 40 patients who would have died during the year and saved $1-$2 million in the process, the question will shift from whether the board should invest in QI resources to how much should be invested.”
Looking ahead, Dr. Glasheen highlighted the growing importance of hospital-acquired infections, such as surgical-site infections, catheter-associated urinary tract infections, and ventilator-associated pneumonia, as areas that need to be focused on in the QI sphere.
Make The Diagnosis - April 2018
Once an individual has been exposed to varicella-zoster virus, either from primary infection (chickenpox) or vaccination, the virus remains dormant in dorsal root ganglion cells. It may become reactivated at a later time, which results in herpes zoster. Typically, immunosuppression (hematologic malignancy and HIV infection) and age are factors that play a role in reactivation, although young people may develop shingles as well. Older age increases the incidence of herpes zoster.
More than 90% of patients will experience a prodrome of pain, burning, or tingling in the dermatome prior to the development of cutaneous lesions. Occasionally, there will be no symptoms prior. Papules and plaques begin to form, which quickly develop into vesicles and blisters. After a few days, lesions become crusted. Bullae or necrosis may occur in more severe cases. Typically, the condition resolves in 2-3 weeks, but can take 6 weeks or longer in elderly patients. In zoster sine herpete, patients have pain but no skin lesions.
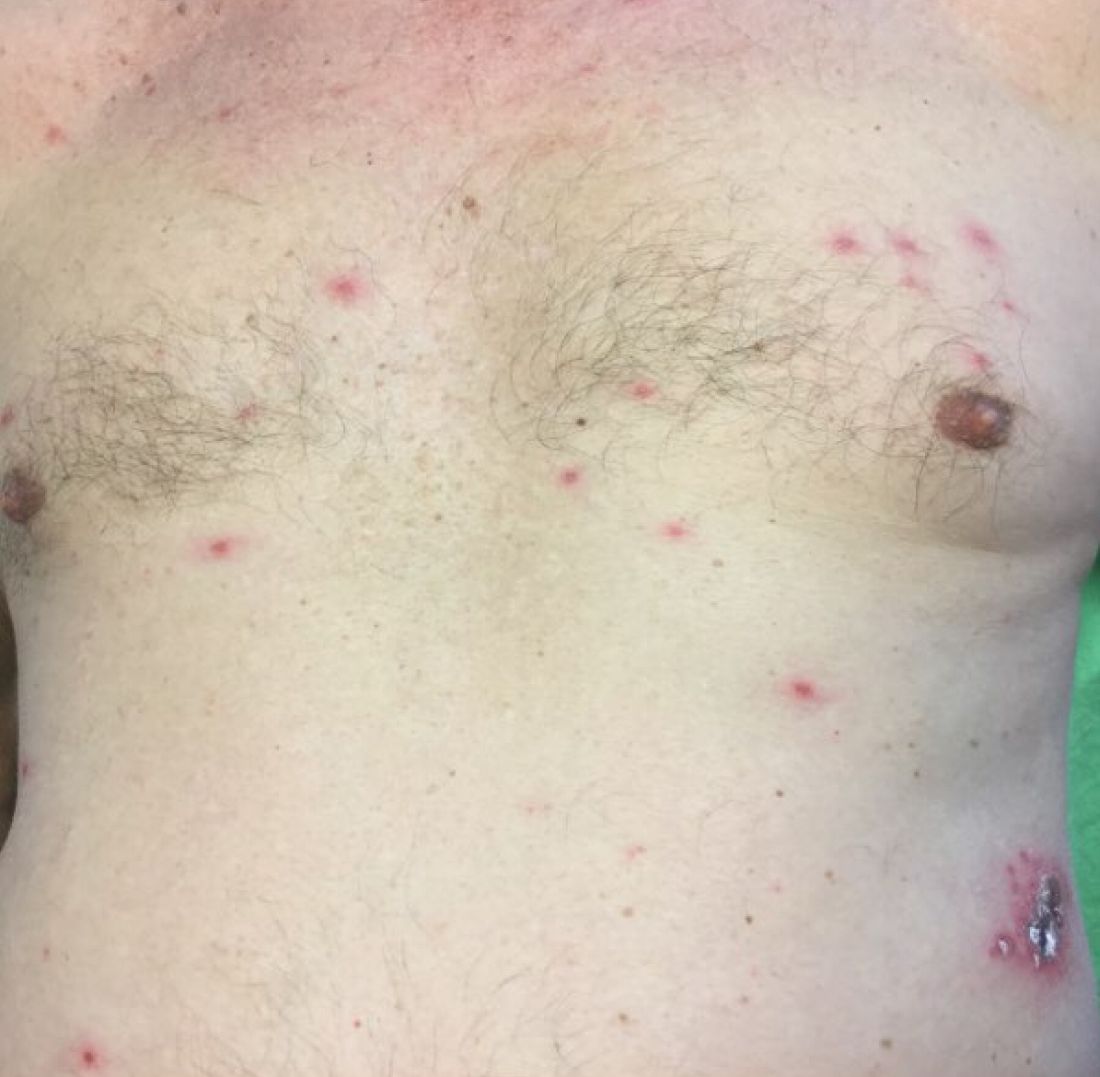
In typical herpes zoster, lesions can be scattered outside the dermatome as well. When more than 20 lesions are scattered outside the area of primary or adjacent dermatomes, this is defined as disseminated herpes zoster. This occurs more commonly in debilitated or immune-compromised individuals. The outlying vesicles are often singular, not grouped, and resemble the “dew drop on a rose petal” look of varicella-zoster lesions. Dissemination necessitates systemic antiviral therapy, preferably intravenous followed by oral treatment once stable. Central nervous system and pulmonary involvement can occur.
Complications of zoster can occur. Postherpetic neuralgia and pain is more common in patients over the age of 50 and may become chronic. Ramsay Hunt syndrome may result in facial paralysis and hearing loss when there is involvement of the facial or auditory nerve. Occasionally, inflammatory lesions can occur within the affected area after the infection has resolved. Secondary bacterial infection, scarring, and motor paralysis can occur.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com. This case and photo were submitted by Dr. Bilu Martin.
Once an individual has been exposed to varicella-zoster virus, either from primary infection (chickenpox) or vaccination, the virus remains dormant in dorsal root ganglion cells. It may become reactivated at a later time, which results in herpes zoster. Typically, immunosuppression (hematologic malignancy and HIV infection) and age are factors that play a role in reactivation, although young people may develop shingles as well. Older age increases the incidence of herpes zoster.
More than 90% of patients will experience a prodrome of pain, burning, or tingling in the dermatome prior to the development of cutaneous lesions. Occasionally, there will be no symptoms prior. Papules and plaques begin to form, which quickly develop into vesicles and blisters. After a few days, lesions become crusted. Bullae or necrosis may occur in more severe cases. Typically, the condition resolves in 2-3 weeks, but can take 6 weeks or longer in elderly patients. In zoster sine herpete, patients have pain but no skin lesions.
In typical herpes zoster, lesions can be scattered outside the dermatome as well. When more than 20 lesions are scattered outside the area of primary or adjacent dermatomes, this is defined as disseminated herpes zoster. This occurs more commonly in debilitated or immune-compromised individuals. The outlying vesicles are often singular, not grouped, and resemble the “dew drop on a rose petal” look of varicella-zoster lesions. Dissemination necessitates systemic antiviral therapy, preferably intravenous followed by oral treatment once stable. Central nervous system and pulmonary involvement can occur.
Complications of zoster can occur. Postherpetic neuralgia and pain is more common in patients over the age of 50 and may become chronic. Ramsay Hunt syndrome may result in facial paralysis and hearing loss when there is involvement of the facial or auditory nerve. Occasionally, inflammatory lesions can occur within the affected area after the infection has resolved. Secondary bacterial infection, scarring, and motor paralysis can occur.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com. This case and photo were submitted by Dr. Bilu Martin.
Once an individual has been exposed to varicella-zoster virus, either from primary infection (chickenpox) or vaccination, the virus remains dormant in dorsal root ganglion cells. It may become reactivated at a later time, which results in herpes zoster. Typically, immunosuppression (hematologic malignancy and HIV infection) and age are factors that play a role in reactivation, although young people may develop shingles as well. Older age increases the incidence of herpes zoster.
More than 90% of patients will experience a prodrome of pain, burning, or tingling in the dermatome prior to the development of cutaneous lesions. Occasionally, there will be no symptoms prior. Papules and plaques begin to form, which quickly develop into vesicles and blisters. After a few days, lesions become crusted. Bullae or necrosis may occur in more severe cases. Typically, the condition resolves in 2-3 weeks, but can take 6 weeks or longer in elderly patients. In zoster sine herpete, patients have pain but no skin lesions.
In typical herpes zoster, lesions can be scattered outside the dermatome as well. When more than 20 lesions are scattered outside the area of primary or adjacent dermatomes, this is defined as disseminated herpes zoster. This occurs more commonly in debilitated or immune-compromised individuals. The outlying vesicles are often singular, not grouped, and resemble the “dew drop on a rose petal” look of varicella-zoster lesions. Dissemination necessitates systemic antiviral therapy, preferably intravenous followed by oral treatment once stable. Central nervous system and pulmonary involvement can occur.
Complications of zoster can occur. Postherpetic neuralgia and pain is more common in patients over the age of 50 and may become chronic. Ramsay Hunt syndrome may result in facial paralysis and hearing loss when there is involvement of the facial or auditory nerve. Occasionally, inflammatory lesions can occur within the affected area after the infection has resolved. Secondary bacterial infection, scarring, and motor paralysis can occur.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com. This case and photo were submitted by Dr. Bilu Martin.
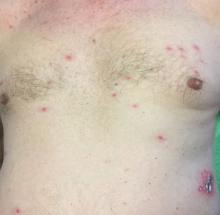
A healthy 70-year-old white male presented with an 8-day history of fatigue and a tingling, erythematous plaque with crusting on the left flank. Four days after the flank lesions appeared, he developed vesicles with an erythematous base on the right abdomen and back. There were more than 20 vesicles present on the abdomen and back, but there were no lesions on other parts of the body.
Tanning addiction associated with multiple behavioral comorbidities
said Kimberly A. Miller, PhD, of the University of Southern California, Los Angeles, and her associates.
Of a multiethnic sample of 2,637 high school students aged 16-17 years from Los Angeles, 7% met the modified CAGE criteria for tanning addiction, a compulsive drive to use indoor tanning beds frequently. The rate was similar in Hispanic teens and non-Hispanic white adolescents (7.6% versus 7.9%, respectively). Asian and Asian American teens had the lowest prevalence of tanning addiction (4.3%), while Native Hawaiian/Pacific Islanders had the highest (10.5%). Slightly more females than males met the criteria (9% vs. 5%), Dr. Miller and her associates reported in the Journal of Investigative Dermatology.
Past 30-day tobacco and marijuana use was significantly associated with tanning addiction, and teens with problem drinking were 3.4 times as likely to meet tanning addiction criteria as were those without problem drinking. Adolescents with panic disorder symptoms were two times more likely to meet tanning addiction criteria than were those without symptoms, and those with obsessive-compulsive disorder symptoms were three times more likely, Dr. Miller and associates said.
“ for each additional psychological symptom, this figure was 30%,” the researchers said.
Dr. Miller and her associates cited several limitations. One was the cross-sectional design of the study. Another was the study’s focus on adolescents from Los Angeles, which limits the generalizability of the findings.
The authors declared no conflicts of interest.
SOURCE: Miller KA et al. J Investig Dermatol. 2018. doi: 10.1016/j.jid.2018.02.018.
said Kimberly A. Miller, PhD, of the University of Southern California, Los Angeles, and her associates.
Of a multiethnic sample of 2,637 high school students aged 16-17 years from Los Angeles, 7% met the modified CAGE criteria for tanning addiction, a compulsive drive to use indoor tanning beds frequently. The rate was similar in Hispanic teens and non-Hispanic white adolescents (7.6% versus 7.9%, respectively). Asian and Asian American teens had the lowest prevalence of tanning addiction (4.3%), while Native Hawaiian/Pacific Islanders had the highest (10.5%). Slightly more females than males met the criteria (9% vs. 5%), Dr. Miller and her associates reported in the Journal of Investigative Dermatology.
Past 30-day tobacco and marijuana use was significantly associated with tanning addiction, and teens with problem drinking were 3.4 times as likely to meet tanning addiction criteria as were those without problem drinking. Adolescents with panic disorder symptoms were two times more likely to meet tanning addiction criteria than were those without symptoms, and those with obsessive-compulsive disorder symptoms were three times more likely, Dr. Miller and associates said.
“ for each additional psychological symptom, this figure was 30%,” the researchers said.
Dr. Miller and her associates cited several limitations. One was the cross-sectional design of the study. Another was the study’s focus on adolescents from Los Angeles, which limits the generalizability of the findings.
The authors declared no conflicts of interest.
SOURCE: Miller KA et al. J Investig Dermatol. 2018. doi: 10.1016/j.jid.2018.02.018.
said Kimberly A. Miller, PhD, of the University of Southern California, Los Angeles, and her associates.
Of a multiethnic sample of 2,637 high school students aged 16-17 years from Los Angeles, 7% met the modified CAGE criteria for tanning addiction, a compulsive drive to use indoor tanning beds frequently. The rate was similar in Hispanic teens and non-Hispanic white adolescents (7.6% versus 7.9%, respectively). Asian and Asian American teens had the lowest prevalence of tanning addiction (4.3%), while Native Hawaiian/Pacific Islanders had the highest (10.5%). Slightly more females than males met the criteria (9% vs. 5%), Dr. Miller and her associates reported in the Journal of Investigative Dermatology.
Past 30-day tobacco and marijuana use was significantly associated with tanning addiction, and teens with problem drinking were 3.4 times as likely to meet tanning addiction criteria as were those without problem drinking. Adolescents with panic disorder symptoms were two times more likely to meet tanning addiction criteria than were those without symptoms, and those with obsessive-compulsive disorder symptoms were three times more likely, Dr. Miller and associates said.
“ for each additional psychological symptom, this figure was 30%,” the researchers said.
Dr. Miller and her associates cited several limitations. One was the cross-sectional design of the study. Another was the study’s focus on adolescents from Los Angeles, which limits the generalizability of the findings.
The authors declared no conflicts of interest.
SOURCE: Miller KA et al. J Investig Dermatol. 2018. doi: 10.1016/j.jid.2018.02.018.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
MDedge Daily News: Alcohol dependence ages your brain
Artificial intelligence comes to glucose monitoring. There’s a promising combo treatment for allergic rhinitis. And evidence mounts for the importance of thrombectomy in stroke.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Artificial intelligence comes to glucose monitoring. There’s a promising combo treatment for allergic rhinitis. And evidence mounts for the importance of thrombectomy in stroke.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Artificial intelligence comes to glucose monitoring. There’s a promising combo treatment for allergic rhinitis. And evidence mounts for the importance of thrombectomy in stroke.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
AML patients may fare better at NCI centers
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
Abstract: Electrocardiograms in Low-Risk Patients Undergoing an Annual Health Examination
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Bhatia, R.S., et al, JAMA Intern Med 177(9):1326, September 1, 2017
BACKGROUND: Both the USPSTF and the Choosing Wisely campaign recommend against routine ECG screening in low-risk patients. A routine ECG at the time of an annual physical in this population is considered to be an example of low-value care.
METHODS: The authors, coordinated at Women’s College Hospital in Toronto, examined the frequency of ECGs after annual health examinations in low-risk adults seen in primary care. Scrutiny of provincial databases identified 3,629,859 patients who had an annual exam in 2010-2015, excluding those with a history of cardiac disease or high-risk criteria. The primary outcomes were receipt of an ECG within 30 days after the annual exam and downstream cardiac care (cardiac testing or consultations) within 90 days.
RESULTS: Just over one-fifth of the patients (21.5%) had an ECG following the annual exam. Rates of ECG ordering varied widely among regions (from 0.7% to 24.4%), among the 679 primary care practices (1.8% to 76.1% of patients), and among the 8036 primary care physicians (1.1% to 94.9%). Receipt of an ECG was significantly more likely for older patients with certain comorbidities (cancer, rheumatologic disease) and less likely for rural residents. Physician traits associated with ECG ordering included male sex, medical school in an international program, and practicing for 30 years or more; in fact, practice-level variation explained 22% of the variation in ECG use. Patients having (versus not having) ECGs had significantly higher rates of cardiac consultations (odds ratio [OR] 5.38; 95% CI 5.24- 5.52) and cardiac tests (transthoracic echocardiogram, OR 7.1; stress test, OR 6.5; and nuclear stress test, OR 4.2). Despite this, one-year follow-up was consistent with low rates of cardiac morbidity and mortality in both groups.
CONCLUSIONS: Routine performance of an ECG appears to be relatively common in low-risk patients and increases the likelihood of unnecessary downstream testing. 37 references (sacha.bhatia@wchospital.ca – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Bhatia, R.S., et al, JAMA Intern Med 177(9):1326, September 1, 2017
BACKGROUND: Both the USPSTF and the Choosing Wisely campaign recommend against routine ECG screening in low-risk patients. A routine ECG at the time of an annual physical in this population is considered to be an example of low-value care.
METHODS: The authors, coordinated at Women’s College Hospital in Toronto, examined the frequency of ECGs after annual health examinations in low-risk adults seen in primary care. Scrutiny of provincial databases identified 3,629,859 patients who had an annual exam in 2010-2015, excluding those with a history of cardiac disease or high-risk criteria. The primary outcomes were receipt of an ECG within 30 days after the annual exam and downstream cardiac care (cardiac testing or consultations) within 90 days.
RESULTS: Just over one-fifth of the patients (21.5%) had an ECG following the annual exam. Rates of ECG ordering varied widely among regions (from 0.7% to 24.4%), among the 679 primary care practices (1.8% to 76.1% of patients), and among the 8036 primary care physicians (1.1% to 94.9%). Receipt of an ECG was significantly more likely for older patients with certain comorbidities (cancer, rheumatologic disease) and less likely for rural residents. Physician traits associated with ECG ordering included male sex, medical school in an international program, and practicing for 30 years or more; in fact, practice-level variation explained 22% of the variation in ECG use. Patients having (versus not having) ECGs had significantly higher rates of cardiac consultations (odds ratio [OR] 5.38; 95% CI 5.24- 5.52) and cardiac tests (transthoracic echocardiogram, OR 7.1; stress test, OR 6.5; and nuclear stress test, OR 4.2). Despite this, one-year follow-up was consistent with low rates of cardiac morbidity and mortality in both groups.
CONCLUSIONS: Routine performance of an ECG appears to be relatively common in low-risk patients and increases the likelihood of unnecessary downstream testing. 37 references (sacha.bhatia@wchospital.ca – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Bhatia, R.S., et al, JAMA Intern Med 177(9):1326, September 1, 2017
BACKGROUND: Both the USPSTF and the Choosing Wisely campaign recommend against routine ECG screening in low-risk patients. A routine ECG at the time of an annual physical in this population is considered to be an example of low-value care.
METHODS: The authors, coordinated at Women’s College Hospital in Toronto, examined the frequency of ECGs after annual health examinations in low-risk adults seen in primary care. Scrutiny of provincial databases identified 3,629,859 patients who had an annual exam in 2010-2015, excluding those with a history of cardiac disease or high-risk criteria. The primary outcomes were receipt of an ECG within 30 days after the annual exam and downstream cardiac care (cardiac testing or consultations) within 90 days.
RESULTS: Just over one-fifth of the patients (21.5%) had an ECG following the annual exam. Rates of ECG ordering varied widely among regions (from 0.7% to 24.4%), among the 679 primary care practices (1.8% to 76.1% of patients), and among the 8036 primary care physicians (1.1% to 94.9%). Receipt of an ECG was significantly more likely for older patients with certain comorbidities (cancer, rheumatologic disease) and less likely for rural residents. Physician traits associated with ECG ordering included male sex, medical school in an international program, and practicing for 30 years or more; in fact, practice-level variation explained 22% of the variation in ECG use. Patients having (versus not having) ECGs had significantly higher rates of cardiac consultations (odds ratio [OR] 5.38; 95% CI 5.24- 5.52) and cardiac tests (transthoracic echocardiogram, OR 7.1; stress test, OR 6.5; and nuclear stress test, OR 4.2). Despite this, one-year follow-up was consistent with low rates of cardiac morbidity and mortality in both groups.
CONCLUSIONS: Routine performance of an ECG appears to be relatively common in low-risk patients and increases the likelihood of unnecessary downstream testing. 37 references (sacha.bhatia@wchospital.ca – no reprints)
Learn more about the Primary Care Medical Abstracts and podcasts, for which you can earn up to 9 CME credits per month.
Copyright © The Center for Medical Education
Minimally invasive colon surgery: Managing conversions
LAS VEGAS – Minimally invasive colon surgery has many advantages over an open procedure with respect to complications and patient recovery, but as surgeons are well aware, sometimes conversion cannot and should not be avoided. “It’s going to happen, and if you’re not converting any of your patients, then you’re probably not being aggressive enough,” said Bradley R. Davis, MD, FACS, at a talk he gave on the topic at the Annual Minimally Invasive Surgery Symposium (MISS) 2018 by Global Academy for Medical Education.
Dr. Davis discussed some of the most common reasons for conversion to open surgery and strategies to try to reduce the incidence. He is the chief of general surgery and the chief of rectal and rectal surgery at Carolinas Medical Center, Charlotte, N.C.
A 2017 survey of 41,417 left hemicolectomy and sigmoidectomy procedures revealed that 63.4% were attempted laparoscopically, and the rate of conversion to an open procedure was 13.4% (JSLS. 2017 Jul-Sep;21[3]:e2017.00036). “I think that if your conversation rate is between 5% and 15%, [it’s] perfectly acceptable,” said Dr. Davis.
He suggested that surgeons should be willing to consider an increasing number of cases for minimally invasive surgery, despite the risk of conversion. By taking some precautions and being aware of which cases are most likely to lead to conversion, surgeons can potentially reduce the conversion rate – or at least lessen the effects it can have on patients and on costs.
Dr. Davis started with a discussion of the surgeon factors that can affect conversion rates. Medial and lateral approaches seem to have similar learning curves. “You’ve got to just stick to one approach. There’s not going to be any difference in terms of [frequency of] conversions,” said Dr. Davis.
Vascular pedicle ligation is the easiest approach, he said. Flexure mobilizations can be challenging, but they aren’t necessarily easier in open surgery. “If you’re struggling to mobilize the flexure, that may be the time to keep struggling because often when we go to open surgery [it doesn’t] get any easier,” said Dr. Davis.
The transverse colon mesentery is most difficult. “If you’re early in your learning curve, that’s something that’s going to be a little more difficult. The learning curve is between 50 and 60 cases,” said Dr. Davis.
Adhesions are the most common cause of conversions, but Dr. Davis said he generally starts with an attempt at laparoscopy. When he has a questionable case, he notifies the operating room staff that it should be prepared for a conversion so they don’t open a lot of disposables.
Other causes of conversion include pedicle or solid organ bleeding, hollow viscus injuries, and anastomotic complications. “As you get more up on your learning curve, you’ll be more comfortable in managing a hole in the bowel laparoscopically. ... Often you can manage those through your extraction site, so you can temporize that with a stitch and then bring it out and look at it,” said Dr. Davis.
Air leaks while doing an anastomosis on the sigmoid can also lead to conversion. “If you have a Pfannenstiel incision, you can do it through the Pfannenstiel, but if you have no incision, you are probably going to want to do some kind of incision to take a peek at that,” said Dr. Davis.
In neoplasms, conversions are common to ensure negative margins, which can’t always be accomplished laparoscopically.
Severe diverticulitis is another case that can mean a conversion, but hand-assisted techniques can be employed to avoid conversion. In severe diverticulitis, ureteral catheters can be helpful. “We identified a lower incidence of ureteral injury [with the use of ureteral catheters] in diverticulitis and T4 cancers. If you have a big phlegmon or a big cancer, I would definitely consider ureteral catheters,” said Dr. Davis. He pointed out that an inability to pinpoint the ureter is daunting in these types of cases. “That’s another thing to plan on if you know you’re going into these tough cases – trying to maximize your chances of not having a conversion by giving yourself the best possible tools to and the best visualization possible,” he added.
Obesity and inflammatory bowel disease are other conversion risk factors, as is performing a left hemicolectomy versus a sigmoidectomy. “As you plan your surgery, if you know you’ve got an obese patient with bad diverticulitis, this might be someone you would schedule as a laparoscopic versus open, with minimum disposable equipment in the room, knowing that, if it’s just not going to happen, then you need to open,” said Dr. Davis.
Technical factors that can contribute to conversion include failures of staplers, clips, and energy devices. When bleeding occurs as a result of an energy device, he doesn’t repeat its use. “If the energy has failed, I go right to an endoloop,” said Dr. Davis.
Bleeding in general needs to be controlled quickly or converted to open. “If you can’t get control of bleeding, that’s when you want to make a quick decision to open. You don’t want to lose two liters of blood trying to be fancy,” said Dr. Davis.
“Cautery injuries will happen, and it doesn’t take much to cause a full-thickness injury. It’s important to address it immediately, rather than move on, since it can be difficult to find after you’ve moved on to something else. Serosal injuries should also be dealt with right away,” he said.
A staple misfire can sometimes be repaired laparoscopically, but if it can’t, the patient should be opened up. “It’s just not worth the leak to prevent an incision,” said Dr. Davis.
Finally, body mass index is a strong predictor of conversion because of the difficulties it presents. “These aren’t cases that are fun to do open, either, but it’s going to be something that we’ll have to get better and better at as we see more of these patients,” said Dr. Davis.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Minimally invasive colon surgery has many advantages over an open procedure with respect to complications and patient recovery, but as surgeons are well aware, sometimes conversion cannot and should not be avoided. “It’s going to happen, and if you’re not converting any of your patients, then you’re probably not being aggressive enough,” said Bradley R. Davis, MD, FACS, at a talk he gave on the topic at the Annual Minimally Invasive Surgery Symposium (MISS) 2018 by Global Academy for Medical Education.
Dr. Davis discussed some of the most common reasons for conversion to open surgery and strategies to try to reduce the incidence. He is the chief of general surgery and the chief of rectal and rectal surgery at Carolinas Medical Center, Charlotte, N.C.
A 2017 survey of 41,417 left hemicolectomy and sigmoidectomy procedures revealed that 63.4% were attempted laparoscopically, and the rate of conversion to an open procedure was 13.4% (JSLS. 2017 Jul-Sep;21[3]:e2017.00036). “I think that if your conversation rate is between 5% and 15%, [it’s] perfectly acceptable,” said Dr. Davis.
He suggested that surgeons should be willing to consider an increasing number of cases for minimally invasive surgery, despite the risk of conversion. By taking some precautions and being aware of which cases are most likely to lead to conversion, surgeons can potentially reduce the conversion rate – or at least lessen the effects it can have on patients and on costs.
Dr. Davis started with a discussion of the surgeon factors that can affect conversion rates. Medial and lateral approaches seem to have similar learning curves. “You’ve got to just stick to one approach. There’s not going to be any difference in terms of [frequency of] conversions,” said Dr. Davis.
Vascular pedicle ligation is the easiest approach, he said. Flexure mobilizations can be challenging, but they aren’t necessarily easier in open surgery. “If you’re struggling to mobilize the flexure, that may be the time to keep struggling because often when we go to open surgery [it doesn’t] get any easier,” said Dr. Davis.
The transverse colon mesentery is most difficult. “If you’re early in your learning curve, that’s something that’s going to be a little more difficult. The learning curve is between 50 and 60 cases,” said Dr. Davis.
Adhesions are the most common cause of conversions, but Dr. Davis said he generally starts with an attempt at laparoscopy. When he has a questionable case, he notifies the operating room staff that it should be prepared for a conversion so they don’t open a lot of disposables.
Other causes of conversion include pedicle or solid organ bleeding, hollow viscus injuries, and anastomotic complications. “As you get more up on your learning curve, you’ll be more comfortable in managing a hole in the bowel laparoscopically. ... Often you can manage those through your extraction site, so you can temporize that with a stitch and then bring it out and look at it,” said Dr. Davis.
Air leaks while doing an anastomosis on the sigmoid can also lead to conversion. “If you have a Pfannenstiel incision, you can do it through the Pfannenstiel, but if you have no incision, you are probably going to want to do some kind of incision to take a peek at that,” said Dr. Davis.
In neoplasms, conversions are common to ensure negative margins, which can’t always be accomplished laparoscopically.
Severe diverticulitis is another case that can mean a conversion, but hand-assisted techniques can be employed to avoid conversion. In severe diverticulitis, ureteral catheters can be helpful. “We identified a lower incidence of ureteral injury [with the use of ureteral catheters] in diverticulitis and T4 cancers. If you have a big phlegmon or a big cancer, I would definitely consider ureteral catheters,” said Dr. Davis. He pointed out that an inability to pinpoint the ureter is daunting in these types of cases. “That’s another thing to plan on if you know you’re going into these tough cases – trying to maximize your chances of not having a conversion by giving yourself the best possible tools to and the best visualization possible,” he added.
Obesity and inflammatory bowel disease are other conversion risk factors, as is performing a left hemicolectomy versus a sigmoidectomy. “As you plan your surgery, if you know you’ve got an obese patient with bad diverticulitis, this might be someone you would schedule as a laparoscopic versus open, with minimum disposable equipment in the room, knowing that, if it’s just not going to happen, then you need to open,” said Dr. Davis.
Technical factors that can contribute to conversion include failures of staplers, clips, and energy devices. When bleeding occurs as a result of an energy device, he doesn’t repeat its use. “If the energy has failed, I go right to an endoloop,” said Dr. Davis.
Bleeding in general needs to be controlled quickly or converted to open. “If you can’t get control of bleeding, that’s when you want to make a quick decision to open. You don’t want to lose two liters of blood trying to be fancy,” said Dr. Davis.
“Cautery injuries will happen, and it doesn’t take much to cause a full-thickness injury. It’s important to address it immediately, rather than move on, since it can be difficult to find after you’ve moved on to something else. Serosal injuries should also be dealt with right away,” he said.
A staple misfire can sometimes be repaired laparoscopically, but if it can’t, the patient should be opened up. “It’s just not worth the leak to prevent an incision,” said Dr. Davis.
Finally, body mass index is a strong predictor of conversion because of the difficulties it presents. “These aren’t cases that are fun to do open, either, but it’s going to be something that we’ll have to get better and better at as we see more of these patients,” said Dr. Davis.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Minimally invasive colon surgery has many advantages over an open procedure with respect to complications and patient recovery, but as surgeons are well aware, sometimes conversion cannot and should not be avoided. “It’s going to happen, and if you’re not converting any of your patients, then you’re probably not being aggressive enough,” said Bradley R. Davis, MD, FACS, at a talk he gave on the topic at the Annual Minimally Invasive Surgery Symposium (MISS) 2018 by Global Academy for Medical Education.
Dr. Davis discussed some of the most common reasons for conversion to open surgery and strategies to try to reduce the incidence. He is the chief of general surgery and the chief of rectal and rectal surgery at Carolinas Medical Center, Charlotte, N.C.
A 2017 survey of 41,417 left hemicolectomy and sigmoidectomy procedures revealed that 63.4% were attempted laparoscopically, and the rate of conversion to an open procedure was 13.4% (JSLS. 2017 Jul-Sep;21[3]:e2017.00036). “I think that if your conversation rate is between 5% and 15%, [it’s] perfectly acceptable,” said Dr. Davis.
He suggested that surgeons should be willing to consider an increasing number of cases for minimally invasive surgery, despite the risk of conversion. By taking some precautions and being aware of which cases are most likely to lead to conversion, surgeons can potentially reduce the conversion rate – or at least lessen the effects it can have on patients and on costs.
Dr. Davis started with a discussion of the surgeon factors that can affect conversion rates. Medial and lateral approaches seem to have similar learning curves. “You’ve got to just stick to one approach. There’s not going to be any difference in terms of [frequency of] conversions,” said Dr. Davis.
Vascular pedicle ligation is the easiest approach, he said. Flexure mobilizations can be challenging, but they aren’t necessarily easier in open surgery. “If you’re struggling to mobilize the flexure, that may be the time to keep struggling because often when we go to open surgery [it doesn’t] get any easier,” said Dr. Davis.
The transverse colon mesentery is most difficult. “If you’re early in your learning curve, that’s something that’s going to be a little more difficult. The learning curve is between 50 and 60 cases,” said Dr. Davis.
Adhesions are the most common cause of conversions, but Dr. Davis said he generally starts with an attempt at laparoscopy. When he has a questionable case, he notifies the operating room staff that it should be prepared for a conversion so they don’t open a lot of disposables.
Other causes of conversion include pedicle or solid organ bleeding, hollow viscus injuries, and anastomotic complications. “As you get more up on your learning curve, you’ll be more comfortable in managing a hole in the bowel laparoscopically. ... Often you can manage those through your extraction site, so you can temporize that with a stitch and then bring it out and look at it,” said Dr. Davis.
Air leaks while doing an anastomosis on the sigmoid can also lead to conversion. “If you have a Pfannenstiel incision, you can do it through the Pfannenstiel, but if you have no incision, you are probably going to want to do some kind of incision to take a peek at that,” said Dr. Davis.
In neoplasms, conversions are common to ensure negative margins, which can’t always be accomplished laparoscopically.
Severe diverticulitis is another case that can mean a conversion, but hand-assisted techniques can be employed to avoid conversion. In severe diverticulitis, ureteral catheters can be helpful. “We identified a lower incidence of ureteral injury [with the use of ureteral catheters] in diverticulitis and T4 cancers. If you have a big phlegmon or a big cancer, I would definitely consider ureteral catheters,” said Dr. Davis. He pointed out that an inability to pinpoint the ureter is daunting in these types of cases. “That’s another thing to plan on if you know you’re going into these tough cases – trying to maximize your chances of not having a conversion by giving yourself the best possible tools to and the best visualization possible,” he added.
Obesity and inflammatory bowel disease are other conversion risk factors, as is performing a left hemicolectomy versus a sigmoidectomy. “As you plan your surgery, if you know you’ve got an obese patient with bad diverticulitis, this might be someone you would schedule as a laparoscopic versus open, with minimum disposable equipment in the room, knowing that, if it’s just not going to happen, then you need to open,” said Dr. Davis.
Technical factors that can contribute to conversion include failures of staplers, clips, and energy devices. When bleeding occurs as a result of an energy device, he doesn’t repeat its use. “If the energy has failed, I go right to an endoloop,” said Dr. Davis.
Bleeding in general needs to be controlled quickly or converted to open. “If you can’t get control of bleeding, that’s when you want to make a quick decision to open. You don’t want to lose two liters of blood trying to be fancy,” said Dr. Davis.
“Cautery injuries will happen, and it doesn’t take much to cause a full-thickness injury. It’s important to address it immediately, rather than move on, since it can be difficult to find after you’ve moved on to something else. Serosal injuries should also be dealt with right away,” he said.
A staple misfire can sometimes be repaired laparoscopically, but if it can’t, the patient should be opened up. “It’s just not worth the leak to prevent an incision,” said Dr. Davis.
Finally, body mass index is a strong predictor of conversion because of the difficulties it presents. “These aren’t cases that are fun to do open, either, but it’s going to be something that we’ll have to get better and better at as we see more of these patients,” said Dr. Davis.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM MISS
The Rest of the Story: Anthem rescinds modifier 25 reductions
When I was a kid, there used to be a radio show by Paul Harvey called “The Rest of the Story.” I loved this show because there was always a big back story behind the happy ending. And that’s how I feel now, it is indeed a happy day, and glad we should be, because the modifier 25 reductions have been rescinded by Anthem. But there is much more to the story, so here is the back story.
The OIG report kicked several things into motion. Insurers started auditing physicians for using modifier 25. The American Academy of Dermatology (AAD) rapidly educated membership on documentation requirements for billing modifier 25. That is, in many cases, separate evaluation and management services should be billed on the same day as a minor procedure, but the chart wasn’t showing the needed details. In an audit, if it is not in the chart, it wasn’t done.
The American Medical Association’s RVS Update Committee (RUC) took note of the OIG report and started to reduce overlapping time and payments on codes being reviewed, which were performed more than 50% of the time on the same day as an evaluation and management code.
The situation was stable for a number of years. Insurers would audit and reclaim some funds and physicians would try and document more. Then, one of the few good things about an electronic medical record became apparent. The documentation became overwhelming. You want to audit me? Here, you can have a BIG “chaw” of records in a PDF. No hassle for staff, just click a few buttons, and no lost records, which mean an automatic loss for doctors. No more settlements by doctors because it just wasn’t worth the time.
In 2012, Harvard Pilgrim HealthCare in Boston started routinely reducing the evaluation and management payment by 50% when filed with modifier 25. No audit, no review, just whack! The Massachusetts Academy of Dermatology vigorously opposed this, as did the AAD. In 2014, Tufts Health Plan followed suit, followed by Blue Cross & Blue Shield of Rhode Island in 2016. Then, in 2017, Anthem started rolling this policy out across the United States. Dermatologists felt like we were howling alone in the wilderness.
The AAD has a committee that deserves special mention here. The Patient Access and Payer Relations (PAPR) Committee, chaired by Howard Rogers, MD, was established to develop relationships with insurers so problems like this could be corrected expeditiously. Dr. Howard and the committee worked tirelessly on the modifier 25 problem. They pointed out that the RUC already had taken value out of the minor procedure codes to account for any overlap. State dermatology, state medical, and specialty societies all were alerted and protested. The PAPR committee had numerous calls with insurers, and Dr. Howard traveled all over the country to meet with Anthem representatives.
Perhaps what Anthem didn’t realize was that, in 2015, two new CPT codes for advanced care planning by primary care physicians (think written advanced directives) were approved for payment by Medicare in 2016, in addition to the regular visit, with a modifier 25 – as well they should have been. That 50% reduction would affect primary care as well.
The howling took on a new timbre.
Last year, the AAD-AMA delegation, led by Cyndi Yag-Howard, MD, took the lead on getting the AMA to adopt a tough stance on modifier 25 reductions. The AMA backed our position, and AMA trustee and chair elect of the AMA Board of Trustees, dermatologist Jack Resneck Jr., MD – with the help of dermatology RUC team lead Scott Collins, MD – outlined in succinct detail, proof that reductions by insurers were inappropriate. The AMA president and chair of the board of trustees took special interest in this issue, and Dr. Resneck personally assured the dermatology delegation that this was at the top of their priority list.
This, my colleagues, is why it is so important for you to join and support your academy, your state dermatology, state medical, and national medical societies. A lifetime of membership fees has just been credited to you.
Anthem then took the 50% reduction down to 25%. Not good enough, so PAPR agreed to call in the cavalry. A consultant who knows all the large employers who buy insurance from Anthem was contacted. Phone calls were made, the tipping point was reached, and Anthem finally rescinded its policy, announcing in late February that the company had decided not to proceed with the policy.
And that, my friends, is the rest of the story.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
When I was a kid, there used to be a radio show by Paul Harvey called “The Rest of the Story.” I loved this show because there was always a big back story behind the happy ending. And that’s how I feel now, it is indeed a happy day, and glad we should be, because the modifier 25 reductions have been rescinded by Anthem. But there is much more to the story, so here is the back story.
The OIG report kicked several things into motion. Insurers started auditing physicians for using modifier 25. The American Academy of Dermatology (AAD) rapidly educated membership on documentation requirements for billing modifier 25. That is, in many cases, separate evaluation and management services should be billed on the same day as a minor procedure, but the chart wasn’t showing the needed details. In an audit, if it is not in the chart, it wasn’t done.
The American Medical Association’s RVS Update Committee (RUC) took note of the OIG report and started to reduce overlapping time and payments on codes being reviewed, which were performed more than 50% of the time on the same day as an evaluation and management code.
The situation was stable for a number of years. Insurers would audit and reclaim some funds and physicians would try and document more. Then, one of the few good things about an electronic medical record became apparent. The documentation became overwhelming. You want to audit me? Here, you can have a BIG “chaw” of records in a PDF. No hassle for staff, just click a few buttons, and no lost records, which mean an automatic loss for doctors. No more settlements by doctors because it just wasn’t worth the time.
In 2012, Harvard Pilgrim HealthCare in Boston started routinely reducing the evaluation and management payment by 50% when filed with modifier 25. No audit, no review, just whack! The Massachusetts Academy of Dermatology vigorously opposed this, as did the AAD. In 2014, Tufts Health Plan followed suit, followed by Blue Cross & Blue Shield of Rhode Island in 2016. Then, in 2017, Anthem started rolling this policy out across the United States. Dermatologists felt like we were howling alone in the wilderness.
The AAD has a committee that deserves special mention here. The Patient Access and Payer Relations (PAPR) Committee, chaired by Howard Rogers, MD, was established to develop relationships with insurers so problems like this could be corrected expeditiously. Dr. Howard and the committee worked tirelessly on the modifier 25 problem. They pointed out that the RUC already had taken value out of the minor procedure codes to account for any overlap. State dermatology, state medical, and specialty societies all were alerted and protested. The PAPR committee had numerous calls with insurers, and Dr. Howard traveled all over the country to meet with Anthem representatives.
Perhaps what Anthem didn’t realize was that, in 2015, two new CPT codes for advanced care planning by primary care physicians (think written advanced directives) were approved for payment by Medicare in 2016, in addition to the regular visit, with a modifier 25 – as well they should have been. That 50% reduction would affect primary care as well.
The howling took on a new timbre.
Last year, the AAD-AMA delegation, led by Cyndi Yag-Howard, MD, took the lead on getting the AMA to adopt a tough stance on modifier 25 reductions. The AMA backed our position, and AMA trustee and chair elect of the AMA Board of Trustees, dermatologist Jack Resneck Jr., MD – with the help of dermatology RUC team lead Scott Collins, MD – outlined in succinct detail, proof that reductions by insurers were inappropriate. The AMA president and chair of the board of trustees took special interest in this issue, and Dr. Resneck personally assured the dermatology delegation that this was at the top of their priority list.
This, my colleagues, is why it is so important for you to join and support your academy, your state dermatology, state medical, and national medical societies. A lifetime of membership fees has just been credited to you.
Anthem then took the 50% reduction down to 25%. Not good enough, so PAPR agreed to call in the cavalry. A consultant who knows all the large employers who buy insurance from Anthem was contacted. Phone calls were made, the tipping point was reached, and Anthem finally rescinded its policy, announcing in late February that the company had decided not to proceed with the policy.
And that, my friends, is the rest of the story.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
When I was a kid, there used to be a radio show by Paul Harvey called “The Rest of the Story.” I loved this show because there was always a big back story behind the happy ending. And that’s how I feel now, it is indeed a happy day, and glad we should be, because the modifier 25 reductions have been rescinded by Anthem. But there is much more to the story, so here is the back story.
The OIG report kicked several things into motion. Insurers started auditing physicians for using modifier 25. The American Academy of Dermatology (AAD) rapidly educated membership on documentation requirements for billing modifier 25. That is, in many cases, separate evaluation and management services should be billed on the same day as a minor procedure, but the chart wasn’t showing the needed details. In an audit, if it is not in the chart, it wasn’t done.
The American Medical Association’s RVS Update Committee (RUC) took note of the OIG report and started to reduce overlapping time and payments on codes being reviewed, which were performed more than 50% of the time on the same day as an evaluation and management code.
The situation was stable for a number of years. Insurers would audit and reclaim some funds and physicians would try and document more. Then, one of the few good things about an electronic medical record became apparent. The documentation became overwhelming. You want to audit me? Here, you can have a BIG “chaw” of records in a PDF. No hassle for staff, just click a few buttons, and no lost records, which mean an automatic loss for doctors. No more settlements by doctors because it just wasn’t worth the time.
In 2012, Harvard Pilgrim HealthCare in Boston started routinely reducing the evaluation and management payment by 50% when filed with modifier 25. No audit, no review, just whack! The Massachusetts Academy of Dermatology vigorously opposed this, as did the AAD. In 2014, Tufts Health Plan followed suit, followed by Blue Cross & Blue Shield of Rhode Island in 2016. Then, in 2017, Anthem started rolling this policy out across the United States. Dermatologists felt like we were howling alone in the wilderness.
The AAD has a committee that deserves special mention here. The Patient Access and Payer Relations (PAPR) Committee, chaired by Howard Rogers, MD, was established to develop relationships with insurers so problems like this could be corrected expeditiously. Dr. Howard and the committee worked tirelessly on the modifier 25 problem. They pointed out that the RUC already had taken value out of the minor procedure codes to account for any overlap. State dermatology, state medical, and specialty societies all were alerted and protested. The PAPR committee had numerous calls with insurers, and Dr. Howard traveled all over the country to meet with Anthem representatives.
Perhaps what Anthem didn’t realize was that, in 2015, two new CPT codes for advanced care planning by primary care physicians (think written advanced directives) were approved for payment by Medicare in 2016, in addition to the regular visit, with a modifier 25 – as well they should have been. That 50% reduction would affect primary care as well.
The howling took on a new timbre.
Last year, the AAD-AMA delegation, led by Cyndi Yag-Howard, MD, took the lead on getting the AMA to adopt a tough stance on modifier 25 reductions. The AMA backed our position, and AMA trustee and chair elect of the AMA Board of Trustees, dermatologist Jack Resneck Jr., MD – with the help of dermatology RUC team lead Scott Collins, MD – outlined in succinct detail, proof that reductions by insurers were inappropriate. The AMA president and chair of the board of trustees took special interest in this issue, and Dr. Resneck personally assured the dermatology delegation that this was at the top of their priority list.
This, my colleagues, is why it is so important for you to join and support your academy, your state dermatology, state medical, and national medical societies. A lifetime of membership fees has just been credited to you.
Anthem then took the 50% reduction down to 25%. Not good enough, so PAPR agreed to call in the cavalry. A consultant who knows all the large employers who buy insurance from Anthem was contacted. Phone calls were made, the tipping point was reached, and Anthem finally rescinded its policy, announcing in late February that the company had decided not to proceed with the policy.
And that, my friends, is the rest of the story.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.