User login
Most daratumumab infusion reactions occur in first infusion
MADRID – The high rate of infusion-related reactions at first daratumumab infusion may be related to treatment duration, based on data from the CASTOR and POLLUX studies presented at the European Society for Medical Oncology Congress.
Infusion-related reactions occur in half of relapsed or refractory multiple myeloma patients who receive daratumumab, but nearly all reactions are grade 2 or less and rarely lead to treatment discontinuation, reported Philippe Moreau, MD, of University Hospital, Nantes, France.
“In the two phase 3 trials, CASTOR and POLLUX, infusion-related reactions occurred in 45% and 48% of patients, respectively. Of these, 98% and 96%, respectively, occurred during the first infusion,” he said. Treatment duration was 7 hours for first infusion vs. 4 hours and 3 hours for the second and third infusions, respectively. Grade 3 infusion-related reactions occurred in 5.3% and 8.6% of patients in CASTOR and POLLUX, respectively. No grade 4 infusion-related reactions were observed in either trial.
(In CASTOR [NCT02136134], daratumumab was combined with bortezomib and dexamethasone. In POLLUX [NCT02076009], it was combined with lenalidomide and dexamethasone. Based on improvements in progression-free survival relative to the background drugs alone, daratumumab was approved for relapsed or refractory multiple myeloma.)
All patients in the trials received some form of preinfusion medications. These included 650-1,000 mg of paracetamol by intravenous or oral administration, 25-50 mg of diphenhydramine, 10 mg of montelukast, and 20 mg of dexamethasone. Patients thought to be at high risk of respiratory complications were candidates for postinfusion medications such as diphenhydramine or a short-acting beta agonist. However, only about 10% of high-risk patients received these therapies, so the impact of this potentially preventive approach is not clear, Dr. Moreau said.
In grade 1 reactions, Dr. Moreau recommended that infusions be paused at the first sign of an infusion-related reaction and then restarted at half the infusion rate when the condition is considered stable. Daratumumab treatment should be withdrawn in grade 2 or higher infusion-related reactions associated with laryngeal edema or grade 2 or higher bronchospasm that does not respond to systemic therapy and resolves within 6 hours of onset.
In grade 3 infusion-related reactions, the recommendation is to stop the daratumumab infusion and closely observe the patient. The infusion should be restarted only if the severity drops to grade 1. Again, the rate of infusion after the interruption should be half the rate provided prior to the infusion-related reaction. Therapy should be withdrawn if the infusion-related reaction recurs for a second time, according to Dr. Moreau.
Infusion-related reactions involving the upper respiratory tract – such as dyspnea, cough, bronchospasm, or throat irritation – may be related to the physiologic function of CD38, Dr. Moreau said. For this reason, grade 3 upper respiratory-related events deserve close attention and persisting symptoms warrant halting treatment.
The evidence is “reassuring” that the majority of infusion-related reactions are confined to the first infusion, said the ESMO-invited discussant, Evangelos Terpos, MD, PhD, of the University of Athens. He noted that the specific treatment recommendations outlined by Dr. Moreau could be helpful for minimizing nuisance infusion-related reactions as well as reducing the risk of more serious infusion-related reactions, particularly those involving respiratory events.
Prophylactic strategies for infusion-related reactions are particularly important in patients with risk factors for respiratory complications, Dr. Terpos added.
MADRID – The high rate of infusion-related reactions at first daratumumab infusion may be related to treatment duration, based on data from the CASTOR and POLLUX studies presented at the European Society for Medical Oncology Congress.
Infusion-related reactions occur in half of relapsed or refractory multiple myeloma patients who receive daratumumab, but nearly all reactions are grade 2 or less and rarely lead to treatment discontinuation, reported Philippe Moreau, MD, of University Hospital, Nantes, France.
“In the two phase 3 trials, CASTOR and POLLUX, infusion-related reactions occurred in 45% and 48% of patients, respectively. Of these, 98% and 96%, respectively, occurred during the first infusion,” he said. Treatment duration was 7 hours for first infusion vs. 4 hours and 3 hours for the second and third infusions, respectively. Grade 3 infusion-related reactions occurred in 5.3% and 8.6% of patients in CASTOR and POLLUX, respectively. No grade 4 infusion-related reactions were observed in either trial.
(In CASTOR [NCT02136134], daratumumab was combined with bortezomib and dexamethasone. In POLLUX [NCT02076009], it was combined with lenalidomide and dexamethasone. Based on improvements in progression-free survival relative to the background drugs alone, daratumumab was approved for relapsed or refractory multiple myeloma.)
All patients in the trials received some form of preinfusion medications. These included 650-1,000 mg of paracetamol by intravenous or oral administration, 25-50 mg of diphenhydramine, 10 mg of montelukast, and 20 mg of dexamethasone. Patients thought to be at high risk of respiratory complications were candidates for postinfusion medications such as diphenhydramine or a short-acting beta agonist. However, only about 10% of high-risk patients received these therapies, so the impact of this potentially preventive approach is not clear, Dr. Moreau said.
In grade 1 reactions, Dr. Moreau recommended that infusions be paused at the first sign of an infusion-related reaction and then restarted at half the infusion rate when the condition is considered stable. Daratumumab treatment should be withdrawn in grade 2 or higher infusion-related reactions associated with laryngeal edema or grade 2 or higher bronchospasm that does not respond to systemic therapy and resolves within 6 hours of onset.
In grade 3 infusion-related reactions, the recommendation is to stop the daratumumab infusion and closely observe the patient. The infusion should be restarted only if the severity drops to grade 1. Again, the rate of infusion after the interruption should be half the rate provided prior to the infusion-related reaction. Therapy should be withdrawn if the infusion-related reaction recurs for a second time, according to Dr. Moreau.
Infusion-related reactions involving the upper respiratory tract – such as dyspnea, cough, bronchospasm, or throat irritation – may be related to the physiologic function of CD38, Dr. Moreau said. For this reason, grade 3 upper respiratory-related events deserve close attention and persisting symptoms warrant halting treatment.
The evidence is “reassuring” that the majority of infusion-related reactions are confined to the first infusion, said the ESMO-invited discussant, Evangelos Terpos, MD, PhD, of the University of Athens. He noted that the specific treatment recommendations outlined by Dr. Moreau could be helpful for minimizing nuisance infusion-related reactions as well as reducing the risk of more serious infusion-related reactions, particularly those involving respiratory events.
Prophylactic strategies for infusion-related reactions are particularly important in patients with risk factors for respiratory complications, Dr. Terpos added.
MADRID – The high rate of infusion-related reactions at first daratumumab infusion may be related to treatment duration, based on data from the CASTOR and POLLUX studies presented at the European Society for Medical Oncology Congress.
Infusion-related reactions occur in half of relapsed or refractory multiple myeloma patients who receive daratumumab, but nearly all reactions are grade 2 or less and rarely lead to treatment discontinuation, reported Philippe Moreau, MD, of University Hospital, Nantes, France.
“In the two phase 3 trials, CASTOR and POLLUX, infusion-related reactions occurred in 45% and 48% of patients, respectively. Of these, 98% and 96%, respectively, occurred during the first infusion,” he said. Treatment duration was 7 hours for first infusion vs. 4 hours and 3 hours for the second and third infusions, respectively. Grade 3 infusion-related reactions occurred in 5.3% and 8.6% of patients in CASTOR and POLLUX, respectively. No grade 4 infusion-related reactions were observed in either trial.
(In CASTOR [NCT02136134], daratumumab was combined with bortezomib and dexamethasone. In POLLUX [NCT02076009], it was combined with lenalidomide and dexamethasone. Based on improvements in progression-free survival relative to the background drugs alone, daratumumab was approved for relapsed or refractory multiple myeloma.)
All patients in the trials received some form of preinfusion medications. These included 650-1,000 mg of paracetamol by intravenous or oral administration, 25-50 mg of diphenhydramine, 10 mg of montelukast, and 20 mg of dexamethasone. Patients thought to be at high risk of respiratory complications were candidates for postinfusion medications such as diphenhydramine or a short-acting beta agonist. However, only about 10% of high-risk patients received these therapies, so the impact of this potentially preventive approach is not clear, Dr. Moreau said.
In grade 1 reactions, Dr. Moreau recommended that infusions be paused at the first sign of an infusion-related reaction and then restarted at half the infusion rate when the condition is considered stable. Daratumumab treatment should be withdrawn in grade 2 or higher infusion-related reactions associated with laryngeal edema or grade 2 or higher bronchospasm that does not respond to systemic therapy and resolves within 6 hours of onset.
In grade 3 infusion-related reactions, the recommendation is to stop the daratumumab infusion and closely observe the patient. The infusion should be restarted only if the severity drops to grade 1. Again, the rate of infusion after the interruption should be half the rate provided prior to the infusion-related reaction. Therapy should be withdrawn if the infusion-related reaction recurs for a second time, according to Dr. Moreau.
Infusion-related reactions involving the upper respiratory tract – such as dyspnea, cough, bronchospasm, or throat irritation – may be related to the physiologic function of CD38, Dr. Moreau said. For this reason, grade 3 upper respiratory-related events deserve close attention and persisting symptoms warrant halting treatment.
The evidence is “reassuring” that the majority of infusion-related reactions are confined to the first infusion, said the ESMO-invited discussant, Evangelos Terpos, MD, PhD, of the University of Athens. He noted that the specific treatment recommendations outlined by Dr. Moreau could be helpful for minimizing nuisance infusion-related reactions as well as reducing the risk of more serious infusion-related reactions, particularly those involving respiratory events.
Prophylactic strategies for infusion-related reactions are particularly important in patients with risk factors for respiratory complications, Dr. Terpos added.
AT ESMO 2017
Key clinical point: In grade 1 infusion-related reactions, daratumumab infusion should be paused at the first sign of a reaction and then restarted at half the infusion rate when the condition is considered stable.
Major finding:
Data source: Post hoc analysis of the phase 3 trials, CASTOR and POLLUX.
Disclosures: Dr. Moreau reported financial relationships with Amgen, Celgene, Janssen, Novartis, and Takeda.
VIDEO: Alopecia areata patients seek emotional support
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
AT AN FDA PUBLIC MEETING
Suicide attempts on the rise among young U.S. adults
Suicide attempts continue to increase in the United States, particularly among young adults with lower education levels and greater economic challenges, according to an analysis published Sept. 13.
These conclusions are based on data gleaned from two studies – the 2004-2005 wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-II) and the 2012-2013 NESARC-III. Of the 69,341 people surveyed, 57.2% were women, and the mean age was 48.1 years.
However, the demographic groups that had the most notable increases were young adults aged 21-34 (ARD, 0.48%; 95% CI, 0.09%-0.87%; P = .02) and those with no more than a high school education (ADR, 0.49%; 95% CI, 0.18%-0.80%; P less than .002).
Dr. Olfson and his coauthors also found an increase in suicide attempts among adults with certain psychiatric disorders. Specifically, the risk for suicide attempts was higher for adults with antisocial personality disorder; among that group, the risk increased from 0.07% (95% CI, –0.09% to 0.23%) in 2004-2005 to 2.16% (95% CI, 0.61%-3.71%) in 2012-2013. “Other high-risk groups included persons with ... schizotypal ... personality disorders and those with anxiety and depressive disorders,” according to Dr. Olfson and his coauthors. “These findings highlight an increasing prevalence of suicide attempts and underscore the prominent role of mental disorders ... in risks for suicide attempts at the population level.”
Almost two-thirds of the adults who had recent suicide attempts in both NESARC survey groups had borderline personality disorder, the investigators reported. However, a finding the coauthors called “encouraging” is that “although most of the adults in the 2012-2013 survey who had recent suicide attempts had borderline personality disorder, the risk of attempted suicide among adults with borderline personality disorder significantly decreased during the study period,” Dr. Olfson and his coauthors wrote. They speculated that this decrease could be tied to findings showing that 40.8% of U.S. psychiatry residency programs offer training in dialectical behavior therapy for borderline personality disorder (Acad Psychiatry. 2013 Jul 1:37[4]:287-8). Clinician training programs are needed to help frontline clinicians manage self-harm among patients with borderline personality disorder, the investigators said.
In addition to many other risk factors, the investigators emphasized the correlation between recent suicide attempts and prior suicide attempts. About one-half of adults who reported a recent attempt also reported a prior attempt (95% CI, 16.46-33.67). “Because 15% to 25% of adults who die by suicide have received treatment for a suicide attempt within the past year, a substantial proportion of suicide deaths” could be subject to prior intervention that could be associated with an attempt, they said.
The data were limited in that adults who are homeless or incarcerated or who have schizophrenia were not surveyed. In addition, the coauthors cited the retrospective nature of NESARC self-reports as a limitation. Given the nature of the study, no data were collected from individuals who died of suicide. “This lack may have led to an underestimation of suicide attempts in each survey,” they wrote.
The study was supported by grants from the National Institutes of Health and the New York State Psychiatric Institute. The surveys were funded in part by the NIH Intramural Research Program. The authors did not report any financial disclosures.
The findings by Mark Olfson, MD, MPH, and his associates “strongly echo” the discussions of Anne Case, PhD, and Angus Deaton, PhD, that underscore “the mortality rate effect of lower education levels, fundamental economic and vocational challenges, and social dislocation occurring in many communities across the United States.” But the work of Dr. Case and Dr. Deaton emphasizes that the biggest impact on deaths, including those caused by suicides, occurred in the middle years.
Meanwhile, findings from the National Violent Death Reporting System on suicide attempts in middle life show that more than two-thirds of men and more than 80% of women report having a disorder related to substance use or mental health, but only 25% of those men and 44% of those women ever received treatment for such conditions. The characteristics of these people, as recorded by the NVDRS, “fit into the populations discerned” by Dr. Case and Dr. Deaton. “While there are suggestions that the surveys used by Olfson et al. may not have been tuned to pick up the ‘signal’ that was associated with the rise in fatal suicide attempts during the first 15 years of the new century, they provided a clear warning that the coming generation of people aging into the ‘middle years’ may see a further rise in suicide rates.”
The National Epidemiologic Survey on Alcohol and Related Conditions surveys were not conducted in clinical settings. Therefore, clinicians need to “look beyond the walls” of their health facilities to engage potentially vulnerable individuals and their families before they become suicidal.
Eric D. Caine, MD, is the chair of the department of psychiatry and codirector of the Center for Study and Prevention of Suicide at the University of Rochester (N.Y.). These remarks have been adapted from an editorial accompanying the article by Dr. Olfson and his associates (JAMA Psychiatry. 2017 Sep 13. doi: 10.1001/jamapsychiatry.2017.2524 ). He reported no financial disclosures.
The findings by Mark Olfson, MD, MPH, and his associates “strongly echo” the discussions of Anne Case, PhD, and Angus Deaton, PhD, that underscore “the mortality rate effect of lower education levels, fundamental economic and vocational challenges, and social dislocation occurring in many communities across the United States.” But the work of Dr. Case and Dr. Deaton emphasizes that the biggest impact on deaths, including those caused by suicides, occurred in the middle years.
Meanwhile, findings from the National Violent Death Reporting System on suicide attempts in middle life show that more than two-thirds of men and more than 80% of women report having a disorder related to substance use or mental health, but only 25% of those men and 44% of those women ever received treatment for such conditions. The characteristics of these people, as recorded by the NVDRS, “fit into the populations discerned” by Dr. Case and Dr. Deaton. “While there are suggestions that the surveys used by Olfson et al. may not have been tuned to pick up the ‘signal’ that was associated with the rise in fatal suicide attempts during the first 15 years of the new century, they provided a clear warning that the coming generation of people aging into the ‘middle years’ may see a further rise in suicide rates.”
The National Epidemiologic Survey on Alcohol and Related Conditions surveys were not conducted in clinical settings. Therefore, clinicians need to “look beyond the walls” of their health facilities to engage potentially vulnerable individuals and their families before they become suicidal.
Eric D. Caine, MD, is the chair of the department of psychiatry and codirector of the Center for Study and Prevention of Suicide at the University of Rochester (N.Y.). These remarks have been adapted from an editorial accompanying the article by Dr. Olfson and his associates (JAMA Psychiatry. 2017 Sep 13. doi: 10.1001/jamapsychiatry.2017.2524 ). He reported no financial disclosures.
The findings by Mark Olfson, MD, MPH, and his associates “strongly echo” the discussions of Anne Case, PhD, and Angus Deaton, PhD, that underscore “the mortality rate effect of lower education levels, fundamental economic and vocational challenges, and social dislocation occurring in many communities across the United States.” But the work of Dr. Case and Dr. Deaton emphasizes that the biggest impact on deaths, including those caused by suicides, occurred in the middle years.
Meanwhile, findings from the National Violent Death Reporting System on suicide attempts in middle life show that more than two-thirds of men and more than 80% of women report having a disorder related to substance use or mental health, but only 25% of those men and 44% of those women ever received treatment for such conditions. The characteristics of these people, as recorded by the NVDRS, “fit into the populations discerned” by Dr. Case and Dr. Deaton. “While there are suggestions that the surveys used by Olfson et al. may not have been tuned to pick up the ‘signal’ that was associated with the rise in fatal suicide attempts during the first 15 years of the new century, they provided a clear warning that the coming generation of people aging into the ‘middle years’ may see a further rise in suicide rates.”
The National Epidemiologic Survey on Alcohol and Related Conditions surveys were not conducted in clinical settings. Therefore, clinicians need to “look beyond the walls” of their health facilities to engage potentially vulnerable individuals and their families before they become suicidal.
Eric D. Caine, MD, is the chair of the department of psychiatry and codirector of the Center for Study and Prevention of Suicide at the University of Rochester (N.Y.). These remarks have been adapted from an editorial accompanying the article by Dr. Olfson and his associates (JAMA Psychiatry. 2017 Sep 13. doi: 10.1001/jamapsychiatry.2017.2524 ). He reported no financial disclosures.
Suicide attempts continue to increase in the United States, particularly among young adults with lower education levels and greater economic challenges, according to an analysis published Sept. 13.
These conclusions are based on data gleaned from two studies – the 2004-2005 wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-II) and the 2012-2013 NESARC-III. Of the 69,341 people surveyed, 57.2% were women, and the mean age was 48.1 years.
However, the demographic groups that had the most notable increases were young adults aged 21-34 (ARD, 0.48%; 95% CI, 0.09%-0.87%; P = .02) and those with no more than a high school education (ADR, 0.49%; 95% CI, 0.18%-0.80%; P less than .002).
Dr. Olfson and his coauthors also found an increase in suicide attempts among adults with certain psychiatric disorders. Specifically, the risk for suicide attempts was higher for adults with antisocial personality disorder; among that group, the risk increased from 0.07% (95% CI, –0.09% to 0.23%) in 2004-2005 to 2.16% (95% CI, 0.61%-3.71%) in 2012-2013. “Other high-risk groups included persons with ... schizotypal ... personality disorders and those with anxiety and depressive disorders,” according to Dr. Olfson and his coauthors. “These findings highlight an increasing prevalence of suicide attempts and underscore the prominent role of mental disorders ... in risks for suicide attempts at the population level.”
Almost two-thirds of the adults who had recent suicide attempts in both NESARC survey groups had borderline personality disorder, the investigators reported. However, a finding the coauthors called “encouraging” is that “although most of the adults in the 2012-2013 survey who had recent suicide attempts had borderline personality disorder, the risk of attempted suicide among adults with borderline personality disorder significantly decreased during the study period,” Dr. Olfson and his coauthors wrote. They speculated that this decrease could be tied to findings showing that 40.8% of U.S. psychiatry residency programs offer training in dialectical behavior therapy for borderline personality disorder (Acad Psychiatry. 2013 Jul 1:37[4]:287-8). Clinician training programs are needed to help frontline clinicians manage self-harm among patients with borderline personality disorder, the investigators said.
In addition to many other risk factors, the investigators emphasized the correlation between recent suicide attempts and prior suicide attempts. About one-half of adults who reported a recent attempt also reported a prior attempt (95% CI, 16.46-33.67). “Because 15% to 25% of adults who die by suicide have received treatment for a suicide attempt within the past year, a substantial proportion of suicide deaths” could be subject to prior intervention that could be associated with an attempt, they said.
The data were limited in that adults who are homeless or incarcerated or who have schizophrenia were not surveyed. In addition, the coauthors cited the retrospective nature of NESARC self-reports as a limitation. Given the nature of the study, no data were collected from individuals who died of suicide. “This lack may have led to an underestimation of suicide attempts in each survey,” they wrote.
The study was supported by grants from the National Institutes of Health and the New York State Psychiatric Institute. The surveys were funded in part by the NIH Intramural Research Program. The authors did not report any financial disclosures.
Suicide attempts continue to increase in the United States, particularly among young adults with lower education levels and greater economic challenges, according to an analysis published Sept. 13.
These conclusions are based on data gleaned from two studies – the 2004-2005 wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-II) and the 2012-2013 NESARC-III. Of the 69,341 people surveyed, 57.2% were women, and the mean age was 48.1 years.
However, the demographic groups that had the most notable increases were young adults aged 21-34 (ARD, 0.48%; 95% CI, 0.09%-0.87%; P = .02) and those with no more than a high school education (ADR, 0.49%; 95% CI, 0.18%-0.80%; P less than .002).
Dr. Olfson and his coauthors also found an increase in suicide attempts among adults with certain psychiatric disorders. Specifically, the risk for suicide attempts was higher for adults with antisocial personality disorder; among that group, the risk increased from 0.07% (95% CI, –0.09% to 0.23%) in 2004-2005 to 2.16% (95% CI, 0.61%-3.71%) in 2012-2013. “Other high-risk groups included persons with ... schizotypal ... personality disorders and those with anxiety and depressive disorders,” according to Dr. Olfson and his coauthors. “These findings highlight an increasing prevalence of suicide attempts and underscore the prominent role of mental disorders ... in risks for suicide attempts at the population level.”
Almost two-thirds of the adults who had recent suicide attempts in both NESARC survey groups had borderline personality disorder, the investigators reported. However, a finding the coauthors called “encouraging” is that “although most of the adults in the 2012-2013 survey who had recent suicide attempts had borderline personality disorder, the risk of attempted suicide among adults with borderline personality disorder significantly decreased during the study period,” Dr. Olfson and his coauthors wrote. They speculated that this decrease could be tied to findings showing that 40.8% of U.S. psychiatry residency programs offer training in dialectical behavior therapy for borderline personality disorder (Acad Psychiatry. 2013 Jul 1:37[4]:287-8). Clinician training programs are needed to help frontline clinicians manage self-harm among patients with borderline personality disorder, the investigators said.
In addition to many other risk factors, the investigators emphasized the correlation between recent suicide attempts and prior suicide attempts. About one-half of adults who reported a recent attempt also reported a prior attempt (95% CI, 16.46-33.67). “Because 15% to 25% of adults who die by suicide have received treatment for a suicide attempt within the past year, a substantial proportion of suicide deaths” could be subject to prior intervention that could be associated with an attempt, they said.
The data were limited in that adults who are homeless or incarcerated or who have schizophrenia were not surveyed. In addition, the coauthors cited the retrospective nature of NESARC self-reports as a limitation. Given the nature of the study, no data were collected from individuals who died of suicide. “This lack may have led to an underestimation of suicide attempts in each survey,” they wrote.
The study was supported by grants from the National Institutes of Health and the New York State Psychiatric Institute. The surveys were funded in part by the NIH Intramural Research Program. The authors did not report any financial disclosures.
FROM JAMA PSYCHIATRY
Key clinical point:
Major finding: The percentage of adults over the age of 21 years who attempted suicide during the study period increased from 0.62% to 0.79% (adjusted risk difference, 0.17%; 95% confidence interval, 0.01%-0.33%; P = .04).
Data source: An analysis of the 2004-2005 wave 2 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC-II) and the 2012-2013 NESARC-III.
Disclosures: This study was supported by grants from the National Institutes of Health and the New York State Psychiatric Institute. The surveys were funded in part by the NIH Intramural Research Program. The authors did not report any financial disclosures.
Patent foramen ovale closure reduces risk of stroke in three trials
Three separate trials examining the impact of closing a patent foramen ovale on the risk of stroke all point to endovascular closure offering a greater reduction in risk than with anticoagulant or antiplatelet therapy alone.
However, this benefit may be evident only in patients at higher risk of stroke associated with patent foramen ovale (PFO), and comes at the cost of an increased risk of atrial fibrillation and procedure-related adverse events, according to papers published in the Sept. 14 issue of the New England Journal of Medicine.
Closure plus anticoagulation vs. anticoagulation or antiplatelets alone
In the CLOSE trial, 663 patients aged 16-60 years who had experienced a recent stroke attributed to PFO and who had an associated atrial septal aneurysm or large interatrial shunt were randomized to transcatheter closure plus long-term antiplatelet therapy, antiplatelets alone, or oral anticoagulation alone.
After a mean follow-up of 5.3 years, Jean‑Louis Mas, MD, of Sainte-Anne Hospital, Paris, and his colleagues reported that there were no recurrent strokes in the closure group, but 14 of the 235 patients in the antiplatelets-only group experienced a stroke, representing a 97% reduction in the risk of stroke with endovascular closure (P less than .001). Patients in the antiplatelets-only group had a 4.9% overall probability of stroke, and no explanation other than PFO could be found for their recurrent stroke (N Engl J Med. 2017;377:1011-21), reported .
The study was not adequately powered to compare the outcomes of anticoagulant therapy with antiplatelet therapy alone.
The closure group also had a 61% lower risk of the secondary composite outcome of stroke, transient ischemic attack, or systemic embolism, compared with the antiplatelet-only group (P = .01).
However, closure of the PFO was associated with a higher rate of new-onset atrial fibrillation or flutter than antiplatelet therapy alone (4.6% vs. 0.9%, P = .02), and major procedural complications occurred in 5.9% of patients.
Closure plus antiplatelets vs. antiplatelets alone
In the second study – REDUCE – 664 patients with PFO who had experienced an ischemic stroke with no other obvious cause were randomized either to closure plus antiplatelet therapy or antiplatelet therapy alone (N Engl J Med. 2017;377:1033-42).
Over the median follow-up of 3.2 years, there were ischemic strokes in 1.4% of patients in the closure group, compared with 5.4% in the antiplatelet-only group; this was a 77% reduction in risk (P = .002), Lars Søndergaard, MD, of the University of Copenhagen, and his coauthors reported.
The closure group also had a 49% lower incidence of new brain infarctions, compared with the antiplatelet-only group, although the incidence of silent brain infarction was similar between the two groups.
While the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism and serious adverse events were similar between the two groups, the closure group had a 2.5% rate of procedure-related serious adverse events and 1.4% rate of device-related serious adverse events. The closure group also had a significantly higher incidence of atrial fibrillation when compared with the control group (6.6% vs. 0.4%; P less than .001).
Closure vs. medical therapy alone
Finally, in the third paper, Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates reported the long-term outcomes of the RESPECT trial of PFO closure versus medical therapy alone in 980 patients with PFO who had experienced a cryptogenic ischemic stroke (N Engl J Med. 2017; 377:1022-32).
After a median follow-up of 5.9 years, there was a 45% lower risk of recurrent ischemic stroke in the closure group, compared with the medical therapy alone group. The overall incidence of recurrent ischemic stroke was 0.58 events per 100 patient-years after closure, compared against 1.07 events with medical therapy, which included aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole.
However, the rate of pulmonary embolism was more than threefold higher, and the rate of deep vein thrombosis was more than fourfold higher in the closure group, compared with the medical therapy group, although the latter was not statistically significant.
FDA perspective
In an accompanying perspective on the three studies, Andrew Farb, MD, and his colleagues from the Center for Devices and Radiological Health at the Food and Drug Administration noted that the clinical benefit of closing a PFO has been an ongoing question for several decades, but the data on the Amplatzer PFO Occluder – a device for PFO closure – had met the agency’s approval threshold.
“The FDA concluded that although there were few recurrent strokes in both groups and some uncertainty regarding the reduction in stroke risk attributable to the device, preventing recurrent stroke is of high value,” the authors wrote (N Engl J Med. 2017;377:1006-9).
However, they stressed that because of the high prevalence of PFO, patients being considered for surgical closure should undergo comprehensive clinical assessment by a neurologist and cardiologist to ensure the ischemic stroke did not have any other possible cause.
The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies, and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry. The authors of the accompanying perspective had no conflicts of interest to declare.
The evidence for causation of embolic stroke in any given person is, of course, circumstantial (for example, atrial fibrillation or carotid stenosis), and it seems reasonable that the presence of a PFO and a sizable interatrial shunt should similarly no longer result in the categorization of a stroke as cryptogenic.
One conclusion from previous trials of closure of patent foramen ovale is that the potential benefit from closure is determined on the basis of the positive characteristics of the PFO rather than on the basis of exclusionary factors that make a stroke cryptogenic. Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.
Dr. Allan H. Ropper is deputy editor of the New England Journal of Medicine. These comments are taken from an accompanying editorial (N Engl J Med. 2017; 377:1093-5). He had no conflicts of interest to declare.
The evidence for causation of embolic stroke in any given person is, of course, circumstantial (for example, atrial fibrillation or carotid stenosis), and it seems reasonable that the presence of a PFO and a sizable interatrial shunt should similarly no longer result in the categorization of a stroke as cryptogenic.
One conclusion from previous trials of closure of patent foramen ovale is that the potential benefit from closure is determined on the basis of the positive characteristics of the PFO rather than on the basis of exclusionary factors that make a stroke cryptogenic. Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.
Dr. Allan H. Ropper is deputy editor of the New England Journal of Medicine. These comments are taken from an accompanying editorial (N Engl J Med. 2017; 377:1093-5). He had no conflicts of interest to declare.
The evidence for causation of embolic stroke in any given person is, of course, circumstantial (for example, atrial fibrillation or carotid stenosis), and it seems reasonable that the presence of a PFO and a sizable interatrial shunt should similarly no longer result in the categorization of a stroke as cryptogenic.
One conclusion from previous trials of closure of patent foramen ovale is that the potential benefit from closure is determined on the basis of the positive characteristics of the PFO rather than on the basis of exclusionary factors that make a stroke cryptogenic. Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.
Dr. Allan H. Ropper is deputy editor of the New England Journal of Medicine. These comments are taken from an accompanying editorial (N Engl J Med. 2017; 377:1093-5). He had no conflicts of interest to declare.
Three separate trials examining the impact of closing a patent foramen ovale on the risk of stroke all point to endovascular closure offering a greater reduction in risk than with anticoagulant or antiplatelet therapy alone.
However, this benefit may be evident only in patients at higher risk of stroke associated with patent foramen ovale (PFO), and comes at the cost of an increased risk of atrial fibrillation and procedure-related adverse events, according to papers published in the Sept. 14 issue of the New England Journal of Medicine.
Closure plus anticoagulation vs. anticoagulation or antiplatelets alone
In the CLOSE trial, 663 patients aged 16-60 years who had experienced a recent stroke attributed to PFO and who had an associated atrial septal aneurysm or large interatrial shunt were randomized to transcatheter closure plus long-term antiplatelet therapy, antiplatelets alone, or oral anticoagulation alone.
After a mean follow-up of 5.3 years, Jean‑Louis Mas, MD, of Sainte-Anne Hospital, Paris, and his colleagues reported that there were no recurrent strokes in the closure group, but 14 of the 235 patients in the antiplatelets-only group experienced a stroke, representing a 97% reduction in the risk of stroke with endovascular closure (P less than .001). Patients in the antiplatelets-only group had a 4.9% overall probability of stroke, and no explanation other than PFO could be found for their recurrent stroke (N Engl J Med. 2017;377:1011-21), reported .
The study was not adequately powered to compare the outcomes of anticoagulant therapy with antiplatelet therapy alone.
The closure group also had a 61% lower risk of the secondary composite outcome of stroke, transient ischemic attack, or systemic embolism, compared with the antiplatelet-only group (P = .01).
However, closure of the PFO was associated with a higher rate of new-onset atrial fibrillation or flutter than antiplatelet therapy alone (4.6% vs. 0.9%, P = .02), and major procedural complications occurred in 5.9% of patients.
Closure plus antiplatelets vs. antiplatelets alone
In the second study – REDUCE – 664 patients with PFO who had experienced an ischemic stroke with no other obvious cause were randomized either to closure plus antiplatelet therapy or antiplatelet therapy alone (N Engl J Med. 2017;377:1033-42).
Over the median follow-up of 3.2 years, there were ischemic strokes in 1.4% of patients in the closure group, compared with 5.4% in the antiplatelet-only group; this was a 77% reduction in risk (P = .002), Lars Søndergaard, MD, of the University of Copenhagen, and his coauthors reported.
The closure group also had a 49% lower incidence of new brain infarctions, compared with the antiplatelet-only group, although the incidence of silent brain infarction was similar between the two groups.
While the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism and serious adverse events were similar between the two groups, the closure group had a 2.5% rate of procedure-related serious adverse events and 1.4% rate of device-related serious adverse events. The closure group also had a significantly higher incidence of atrial fibrillation when compared with the control group (6.6% vs. 0.4%; P less than .001).
Closure vs. medical therapy alone
Finally, in the third paper, Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates reported the long-term outcomes of the RESPECT trial of PFO closure versus medical therapy alone in 980 patients with PFO who had experienced a cryptogenic ischemic stroke (N Engl J Med. 2017; 377:1022-32).
After a median follow-up of 5.9 years, there was a 45% lower risk of recurrent ischemic stroke in the closure group, compared with the medical therapy alone group. The overall incidence of recurrent ischemic stroke was 0.58 events per 100 patient-years after closure, compared against 1.07 events with medical therapy, which included aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole.
However, the rate of pulmonary embolism was more than threefold higher, and the rate of deep vein thrombosis was more than fourfold higher in the closure group, compared with the medical therapy group, although the latter was not statistically significant.
FDA perspective
In an accompanying perspective on the three studies, Andrew Farb, MD, and his colleagues from the Center for Devices and Radiological Health at the Food and Drug Administration noted that the clinical benefit of closing a PFO has been an ongoing question for several decades, but the data on the Amplatzer PFO Occluder – a device for PFO closure – had met the agency’s approval threshold.
“The FDA concluded that although there were few recurrent strokes in both groups and some uncertainty regarding the reduction in stroke risk attributable to the device, preventing recurrent stroke is of high value,” the authors wrote (N Engl J Med. 2017;377:1006-9).
However, they stressed that because of the high prevalence of PFO, patients being considered for surgical closure should undergo comprehensive clinical assessment by a neurologist and cardiologist to ensure the ischemic stroke did not have any other possible cause.
The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies, and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry. The authors of the accompanying perspective had no conflicts of interest to declare.
Three separate trials examining the impact of closing a patent foramen ovale on the risk of stroke all point to endovascular closure offering a greater reduction in risk than with anticoagulant or antiplatelet therapy alone.
However, this benefit may be evident only in patients at higher risk of stroke associated with patent foramen ovale (PFO), and comes at the cost of an increased risk of atrial fibrillation and procedure-related adverse events, according to papers published in the Sept. 14 issue of the New England Journal of Medicine.
Closure plus anticoagulation vs. anticoagulation or antiplatelets alone
In the CLOSE trial, 663 patients aged 16-60 years who had experienced a recent stroke attributed to PFO and who had an associated atrial septal aneurysm or large interatrial shunt were randomized to transcatheter closure plus long-term antiplatelet therapy, antiplatelets alone, or oral anticoagulation alone.
After a mean follow-up of 5.3 years, Jean‑Louis Mas, MD, of Sainte-Anne Hospital, Paris, and his colleagues reported that there were no recurrent strokes in the closure group, but 14 of the 235 patients in the antiplatelets-only group experienced a stroke, representing a 97% reduction in the risk of stroke with endovascular closure (P less than .001). Patients in the antiplatelets-only group had a 4.9% overall probability of stroke, and no explanation other than PFO could be found for their recurrent stroke (N Engl J Med. 2017;377:1011-21), reported .
The study was not adequately powered to compare the outcomes of anticoagulant therapy with antiplatelet therapy alone.
The closure group also had a 61% lower risk of the secondary composite outcome of stroke, transient ischemic attack, or systemic embolism, compared with the antiplatelet-only group (P = .01).
However, closure of the PFO was associated with a higher rate of new-onset atrial fibrillation or flutter than antiplatelet therapy alone (4.6% vs. 0.9%, P = .02), and major procedural complications occurred in 5.9% of patients.
Closure plus antiplatelets vs. antiplatelets alone
In the second study – REDUCE – 664 patients with PFO who had experienced an ischemic stroke with no other obvious cause were randomized either to closure plus antiplatelet therapy or antiplatelet therapy alone (N Engl J Med. 2017;377:1033-42).
Over the median follow-up of 3.2 years, there were ischemic strokes in 1.4% of patients in the closure group, compared with 5.4% in the antiplatelet-only group; this was a 77% reduction in risk (P = .002), Lars Søndergaard, MD, of the University of Copenhagen, and his coauthors reported.
The closure group also had a 49% lower incidence of new brain infarctions, compared with the antiplatelet-only group, although the incidence of silent brain infarction was similar between the two groups.
While the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism and serious adverse events were similar between the two groups, the closure group had a 2.5% rate of procedure-related serious adverse events and 1.4% rate of device-related serious adverse events. The closure group also had a significantly higher incidence of atrial fibrillation when compared with the control group (6.6% vs. 0.4%; P less than .001).
Closure vs. medical therapy alone
Finally, in the third paper, Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates reported the long-term outcomes of the RESPECT trial of PFO closure versus medical therapy alone in 980 patients with PFO who had experienced a cryptogenic ischemic stroke (N Engl J Med. 2017; 377:1022-32).
After a median follow-up of 5.9 years, there was a 45% lower risk of recurrent ischemic stroke in the closure group, compared with the medical therapy alone group. The overall incidence of recurrent ischemic stroke was 0.58 events per 100 patient-years after closure, compared against 1.07 events with medical therapy, which included aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole.
However, the rate of pulmonary embolism was more than threefold higher, and the rate of deep vein thrombosis was more than fourfold higher in the closure group, compared with the medical therapy group, although the latter was not statistically significant.
FDA perspective
In an accompanying perspective on the three studies, Andrew Farb, MD, and his colleagues from the Center for Devices and Radiological Health at the Food and Drug Administration noted that the clinical benefit of closing a PFO has been an ongoing question for several decades, but the data on the Amplatzer PFO Occluder – a device for PFO closure – had met the agency’s approval threshold.
“The FDA concluded that although there were few recurrent strokes in both groups and some uncertainty regarding the reduction in stroke risk attributable to the device, preventing recurrent stroke is of high value,” the authors wrote (N Engl J Med. 2017;377:1006-9).
However, they stressed that because of the high prevalence of PFO, patients being considered for surgical closure should undergo comprehensive clinical assessment by a neurologist and cardiologist to ensure the ischemic stroke did not have any other possible cause.
The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies, and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry. The authors of the accompanying perspective had no conflicts of interest to declare.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Patent foramen ovale closure was associated with a 45%-97% reduction in the incidence of recurrent ischemic stroke, compared with treatment with antiplatelets or anticoagulants alone.
Data source: The CLOSE, REDUCE, and RESPECT randomized, controlled trials in patients with patent foramen ovale who had experienced a cryptogenic ischemic stroke.
Disclosures: The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry.
Tezepelumab reduces exacerbations in persistent, treatment-resistant asthma
Patients whose asthma remains uncontrolled despite treatment may benefit from a new monoclonal antibody that targets an inflammatory cytokine known to be promoted in asthmatic airways, according to data presented at the annual congress of the European Respiratory Society.
Writing in the Sept. 7 issue of the New England Journal of Medicine, researchers reported on a phase 2, randomized placebo-controlled trial of three dosing regimens of subcutaneous tezepelumab, which targets the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP). The trial involved 584 patients with uncontrolled asthma, despite treatment with long-acting beta-agonists and medium to high doses of inhaled glucocorticoids.
At 70 mg every 4 weeks, exacerbation rates were 61% lower than in the placebo group; at 210 mg every 4 weeks, they were 71% lower; and at 280 mg every 2 weeks, they were 66% lower (P was less than 001 in comparisons between each group and the placebo).
The overall annualized exacerbation rates by week 52 were 0.26 for the 70-mg group, 0.19 for the 210-mg group, and 0.22 for the 280-mg group, compared with 0.67 in the placebo group, regardless of a patient’s baseline eosinophil count. Patients treated with tezepelumab had a longer time to first asthma exacerbation. They also experienced a significantly higher change from baseline in their prebronchodilator forced expiratory volume in 1 second at week 52, when compared with patients on the placebo.
“The observed improvements in disease control in patients who received tezepelumab highlight the potential pathogenic role of TSLP across different asthma phenotypes,” reported Jonathan Corren, MD, of the University of California, Los Angeles, and his coauthors. “... Although TSLP is central to the regulation of type 2 immunity, many cell types that are activated by or respond to TSLP, such as mast cells, basophils, natural killer T cells, innate lymphoid cells, and neutrophils, may play a role in inflammation in asthma beyond type 2 inflammation.”
The incidences of adverse events and serious adverse events were similar across all groups in the study. Three serious adverse events – pneumonia and stroke in the same patient and one case of Guillain-Barré syndrome – in patients taking tezepelumab, were deemed to be related to the treatment.
The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
Tezepelumab is the first biologic that has a substantial positive effect on two important markers of the inflammation of asthma – namely, blood eosinophil counts and the fraction of exhaled nitric oxide. It appears to be the broadest and most promising biologic for the treatment of persistent uncontrolled asthma to date.
The observation that tezepelumab reduces the level of both inflammatory markers shows that it hits a more upstream target and that it blocks at least two relevant inflammatory pathways in asthma. This is likely to be clinically relevant, since simultaneously increased exhaled nitric oxide levels and blood eosinophil counts are related to increased morbidity due to asthma.
Elisabeth H. Bel, MD, PhD, is with the department of respiratory medicine, Academic Medical Center, the University of Amsterdam. These comments were taken from an accompanying editorial (N Engl J Med. 2017;377:989-91). Dr. Bel declared consultancies and grants from pharmaceutical companies including AstraZeneca.
Tezepelumab is the first biologic that has a substantial positive effect on two important markers of the inflammation of asthma – namely, blood eosinophil counts and the fraction of exhaled nitric oxide. It appears to be the broadest and most promising biologic for the treatment of persistent uncontrolled asthma to date.
The observation that tezepelumab reduces the level of both inflammatory markers shows that it hits a more upstream target and that it blocks at least two relevant inflammatory pathways in asthma. This is likely to be clinically relevant, since simultaneously increased exhaled nitric oxide levels and blood eosinophil counts are related to increased morbidity due to asthma.
Elisabeth H. Bel, MD, PhD, is with the department of respiratory medicine, Academic Medical Center, the University of Amsterdam. These comments were taken from an accompanying editorial (N Engl J Med. 2017;377:989-91). Dr. Bel declared consultancies and grants from pharmaceutical companies including AstraZeneca.
Tezepelumab is the first biologic that has a substantial positive effect on two important markers of the inflammation of asthma – namely, blood eosinophil counts and the fraction of exhaled nitric oxide. It appears to be the broadest and most promising biologic for the treatment of persistent uncontrolled asthma to date.
The observation that tezepelumab reduces the level of both inflammatory markers shows that it hits a more upstream target and that it blocks at least two relevant inflammatory pathways in asthma. This is likely to be clinically relevant, since simultaneously increased exhaled nitric oxide levels and blood eosinophil counts are related to increased morbidity due to asthma.
Elisabeth H. Bel, MD, PhD, is with the department of respiratory medicine, Academic Medical Center, the University of Amsterdam. These comments were taken from an accompanying editorial (N Engl J Med. 2017;377:989-91). Dr. Bel declared consultancies and grants from pharmaceutical companies including AstraZeneca.
Patients whose asthma remains uncontrolled despite treatment may benefit from a new monoclonal antibody that targets an inflammatory cytokine known to be promoted in asthmatic airways, according to data presented at the annual congress of the European Respiratory Society.
Writing in the Sept. 7 issue of the New England Journal of Medicine, researchers reported on a phase 2, randomized placebo-controlled trial of three dosing regimens of subcutaneous tezepelumab, which targets the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP). The trial involved 584 patients with uncontrolled asthma, despite treatment with long-acting beta-agonists and medium to high doses of inhaled glucocorticoids.
At 70 mg every 4 weeks, exacerbation rates were 61% lower than in the placebo group; at 210 mg every 4 weeks, they were 71% lower; and at 280 mg every 2 weeks, they were 66% lower (P was less than 001 in comparisons between each group and the placebo).
The overall annualized exacerbation rates by week 52 were 0.26 for the 70-mg group, 0.19 for the 210-mg group, and 0.22 for the 280-mg group, compared with 0.67 in the placebo group, regardless of a patient’s baseline eosinophil count. Patients treated with tezepelumab had a longer time to first asthma exacerbation. They also experienced a significantly higher change from baseline in their prebronchodilator forced expiratory volume in 1 second at week 52, when compared with patients on the placebo.
“The observed improvements in disease control in patients who received tezepelumab highlight the potential pathogenic role of TSLP across different asthma phenotypes,” reported Jonathan Corren, MD, of the University of California, Los Angeles, and his coauthors. “... Although TSLP is central to the regulation of type 2 immunity, many cell types that are activated by or respond to TSLP, such as mast cells, basophils, natural killer T cells, innate lymphoid cells, and neutrophils, may play a role in inflammation in asthma beyond type 2 inflammation.”
The incidences of adverse events and serious adverse events were similar across all groups in the study. Three serious adverse events – pneumonia and stroke in the same patient and one case of Guillain-Barré syndrome – in patients taking tezepelumab, were deemed to be related to the treatment.
The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
Patients whose asthma remains uncontrolled despite treatment may benefit from a new monoclonal antibody that targets an inflammatory cytokine known to be promoted in asthmatic airways, according to data presented at the annual congress of the European Respiratory Society.
Writing in the Sept. 7 issue of the New England Journal of Medicine, researchers reported on a phase 2, randomized placebo-controlled trial of three dosing regimens of subcutaneous tezepelumab, which targets the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP). The trial involved 584 patients with uncontrolled asthma, despite treatment with long-acting beta-agonists and medium to high doses of inhaled glucocorticoids.
At 70 mg every 4 weeks, exacerbation rates were 61% lower than in the placebo group; at 210 mg every 4 weeks, they were 71% lower; and at 280 mg every 2 weeks, they were 66% lower (P was less than 001 in comparisons between each group and the placebo).
The overall annualized exacerbation rates by week 52 were 0.26 for the 70-mg group, 0.19 for the 210-mg group, and 0.22 for the 280-mg group, compared with 0.67 in the placebo group, regardless of a patient’s baseline eosinophil count. Patients treated with tezepelumab had a longer time to first asthma exacerbation. They also experienced a significantly higher change from baseline in their prebronchodilator forced expiratory volume in 1 second at week 52, when compared with patients on the placebo.
“The observed improvements in disease control in patients who received tezepelumab highlight the potential pathogenic role of TSLP across different asthma phenotypes,” reported Jonathan Corren, MD, of the University of California, Los Angeles, and his coauthors. “... Although TSLP is central to the regulation of type 2 immunity, many cell types that are activated by or respond to TSLP, such as mast cells, basophils, natural killer T cells, innate lymphoid cells, and neutrophils, may play a role in inflammation in asthma beyond type 2 inflammation.”
The incidences of adverse events and serious adverse events were similar across all groups in the study. Three serious adverse events – pneumonia and stroke in the same patient and one case of Guillain-Barré syndrome – in patients taking tezepelumab, were deemed to be related to the treatment.
The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
FROM THE ERS CONGRESS 2017
Key clinical point: The monoclonal antibody tezepelumab is associated with a significant reduction in asthma exacerbations in patients with treatment-resistant and persistent disease.
Major finding: Patients treated with tezepelumab had a 34% reduction in the risk of asthma exacerbations, compared with those on placebo.
Data source: A phase 2, randomized placebo-controlled trial in 584 patients with persistent asthma.
Disclosures: The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
Parents of very preterm and VLBW infants have resilient quality of life decades later
A prospective cohort study of parents whose children were born very preterm (VP) or very low birth weight (VLBW) showed that their quality of life once their children reached adulthood was comparable to that of parents of term children. What did predict a poorer quality of life for these parents was whether their children had mental health problems or worse peer relationships in childhood.
The questionnaire used was the World Health Organization Quality of Life instrument, Short Edition, which assesses physical health, psychological health, social relationships, and environment in 26 items. Parents also answered the five-item Satisfaction with Life Scale.
“The overall Quality of Life factor score of parents of VP and VLBW and term offspring was not different (VP and VLBW: mean standardized factor score −0.02; 95% confidence interval, −0.13 to 0.08; term controls: mean standardized factor score 0.02; 95% CI, −0.07 to 0.12; P greater than .05),” wrote Dieter Wolke, PhD, of the University of Warwick (England) and his coauthors.
VP and VLBW, disabilities, poorer academic achievement, and poorer parent-child relationships did not predict a lower quality of life for the parents. However, “in offspring, better mental health and peer relationships in childhood (independently of whether they were born VP or VLBW or term) still predicted parents’ quality of life more than a decade later,” they said.
Thus, taking measures to improve children’s mental health and peer relationships likely would aid both the children and their parents, they observed.
One thing that the authors do not remark on is that a significant number of the VP and VLBW infants in the Bavarian study’s initial sample died in the hospital (172 of 682; 25%), with another 12 deaths between the infants’ discharge and adulthood; the cohort study’s focus, therefore, is limited to the quality of life of parents whose children are still alive. Some other eligible parents dropped out, although the authors noted that they tried to control for factors differing between the dropouts and the participants.
Read more at Pediatrics (2017 Sep;140[3]:e20171263)
A prospective cohort study of parents whose children were born very preterm (VP) or very low birth weight (VLBW) showed that their quality of life once their children reached adulthood was comparable to that of parents of term children. What did predict a poorer quality of life for these parents was whether their children had mental health problems or worse peer relationships in childhood.
The questionnaire used was the World Health Organization Quality of Life instrument, Short Edition, which assesses physical health, psychological health, social relationships, and environment in 26 items. Parents also answered the five-item Satisfaction with Life Scale.
“The overall Quality of Life factor score of parents of VP and VLBW and term offspring was not different (VP and VLBW: mean standardized factor score −0.02; 95% confidence interval, −0.13 to 0.08; term controls: mean standardized factor score 0.02; 95% CI, −0.07 to 0.12; P greater than .05),” wrote Dieter Wolke, PhD, of the University of Warwick (England) and his coauthors.
VP and VLBW, disabilities, poorer academic achievement, and poorer parent-child relationships did not predict a lower quality of life for the parents. However, “in offspring, better mental health and peer relationships in childhood (independently of whether they were born VP or VLBW or term) still predicted parents’ quality of life more than a decade later,” they said.
Thus, taking measures to improve children’s mental health and peer relationships likely would aid both the children and their parents, they observed.
One thing that the authors do not remark on is that a significant number of the VP and VLBW infants in the Bavarian study’s initial sample died in the hospital (172 of 682; 25%), with another 12 deaths between the infants’ discharge and adulthood; the cohort study’s focus, therefore, is limited to the quality of life of parents whose children are still alive. Some other eligible parents dropped out, although the authors noted that they tried to control for factors differing between the dropouts and the participants.
Read more at Pediatrics (2017 Sep;140[3]:e20171263)
A prospective cohort study of parents whose children were born very preterm (VP) or very low birth weight (VLBW) showed that their quality of life once their children reached adulthood was comparable to that of parents of term children. What did predict a poorer quality of life for these parents was whether their children had mental health problems or worse peer relationships in childhood.
The questionnaire used was the World Health Organization Quality of Life instrument, Short Edition, which assesses physical health, psychological health, social relationships, and environment in 26 items. Parents also answered the five-item Satisfaction with Life Scale.
“The overall Quality of Life factor score of parents of VP and VLBW and term offspring was not different (VP and VLBW: mean standardized factor score −0.02; 95% confidence interval, −0.13 to 0.08; term controls: mean standardized factor score 0.02; 95% CI, −0.07 to 0.12; P greater than .05),” wrote Dieter Wolke, PhD, of the University of Warwick (England) and his coauthors.
VP and VLBW, disabilities, poorer academic achievement, and poorer parent-child relationships did not predict a lower quality of life for the parents. However, “in offspring, better mental health and peer relationships in childhood (independently of whether they were born VP or VLBW or term) still predicted parents’ quality of life more than a decade later,” they said.
Thus, taking measures to improve children’s mental health and peer relationships likely would aid both the children and their parents, they observed.
One thing that the authors do not remark on is that a significant number of the VP and VLBW infants in the Bavarian study’s initial sample died in the hospital (172 of 682; 25%), with another 12 deaths between the infants’ discharge and adulthood; the cohort study’s focus, therefore, is limited to the quality of life of parents whose children are still alive. Some other eligible parents dropped out, although the authors noted that they tried to control for factors differing between the dropouts and the participants.
Read more at Pediatrics (2017 Sep;140[3]:e20171263)
FROM PEDIATRICS
Advances in ablative and non-ablative lasers in gynecology: A clinician’s guide

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Adding T-vec might help surmount PD-1 resistance in melanoma
Almost two-thirds of patients with advanced melanoma responded to combination therapy with pembrolizumab and talimogene laherparepvec (T-vec) in a small phase 1b trial, investigators reported.
A third of patients achieved a complete response and median progression-free and overall survival were not reached after typically 18.6 (range, 17.7 to 20.8) months of follow-up, said Antoni Ribas, MD, of the University of California, Los Angeles, and his coinvestigators. In contrast to single-agent pembrolizumab therapy, responders to the combination regimen included patients with very low levels of CD8+ T cell infiltrates or negative interferon-gamma (IFN-gamma) gene signatures in baseline tumor biopsies, suggesting that oncolytic virotherapy might make anti-PD-1 therapy more effective by altering the tumor microenvironment, the researchers concluded. Serious adverse events were uncommon in this study, and there were no dose-limiting toxicities, they wrote (Cell. 2017 Sept. 7 doi: 10.1016/j.cell.2017.08.027).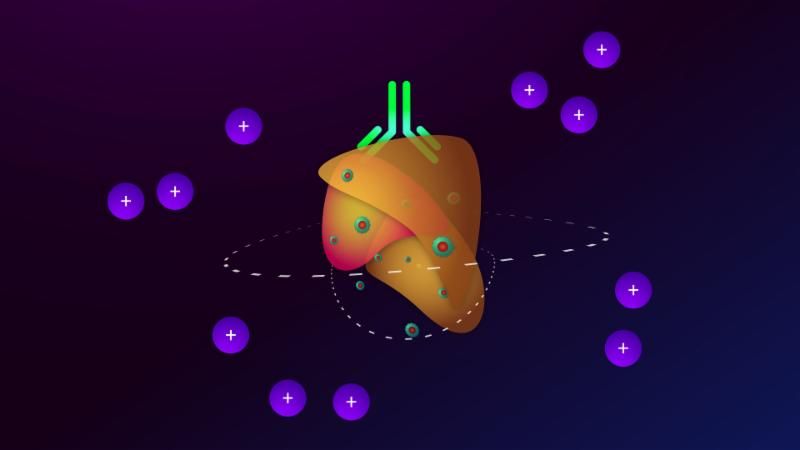
To see if attracting CD8+ T cells into tumors helped surmount this obstacle, the researchers treated 21 patients with advanced melanoma with pembrolizumab and T-vec, an intratumorally administered, genetically modified clinical herpes simplex virus-1 strain approved for treating melanoma. Patients first received up to 4 mL T-vec (106 plaque-forming units [pfu] per mL) to induce a protective immune response. Three weeks later, they started receiving to 4 mL (108 pfu/mL) T-vec plus 200 mg intravenous pembrolizumab every 2 weeks.
Thirteen patients (62%) showed at least a partial response, and seven (33%) had a complete response based on immune criteria. Notably, 9 of 13 (69%) patients with baseline tumor CD8+ densities below 1,000 cells/mm2 responded to combination treatment, as did three of five patients with low baseline IFN-gamma signatures.
“There was only one baseline biopsy that was scored as PD-L1 negative, but that patient went on to have a complete response to the combined therapy,” the researchers wrote. “Patients who responded to combination therapy had increased CD8+ T cells, elevated PD-L1 protein expression, [and] IFN-gamma gene expression on several cell subsets in tumors after [T-vec] treatment. Response to combination therapy did not appear to be associated with baseline CD8+ T cell infiltration or baseline IFN-gamma signature.” Increased levels of circulating immune cells and shrinkage of untreated tumors both suggested that intratumoral T-vec injections led to systemic effects, they added.
Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
Almost two-thirds of patients with advanced melanoma responded to combination therapy with pembrolizumab and talimogene laherparepvec (T-vec) in a small phase 1b trial, investigators reported.
A third of patients achieved a complete response and median progression-free and overall survival were not reached after typically 18.6 (range, 17.7 to 20.8) months of follow-up, said Antoni Ribas, MD, of the University of California, Los Angeles, and his coinvestigators. In contrast to single-agent pembrolizumab therapy, responders to the combination regimen included patients with very low levels of CD8+ T cell infiltrates or negative interferon-gamma (IFN-gamma) gene signatures in baseline tumor biopsies, suggesting that oncolytic virotherapy might make anti-PD-1 therapy more effective by altering the tumor microenvironment, the researchers concluded. Serious adverse events were uncommon in this study, and there were no dose-limiting toxicities, they wrote (Cell. 2017 Sept. 7 doi: 10.1016/j.cell.2017.08.027).
To see if attracting CD8+ T cells into tumors helped surmount this obstacle, the researchers treated 21 patients with advanced melanoma with pembrolizumab and T-vec, an intratumorally administered, genetically modified clinical herpes simplex virus-1 strain approved for treating melanoma. Patients first received up to 4 mL T-vec (106 plaque-forming units [pfu] per mL) to induce a protective immune response. Three weeks later, they started receiving to 4 mL (108 pfu/mL) T-vec plus 200 mg intravenous pembrolizumab every 2 weeks.
Thirteen patients (62%) showed at least a partial response, and seven (33%) had a complete response based on immune criteria. Notably, 9 of 13 (69%) patients with baseline tumor CD8+ densities below 1,000 cells/mm2 responded to combination treatment, as did three of five patients with low baseline IFN-gamma signatures.
“There was only one baseline biopsy that was scored as PD-L1 negative, but that patient went on to have a complete response to the combined therapy,” the researchers wrote. “Patients who responded to combination therapy had increased CD8+ T cells, elevated PD-L1 protein expression, [and] IFN-gamma gene expression on several cell subsets in tumors after [T-vec] treatment. Response to combination therapy did not appear to be associated with baseline CD8+ T cell infiltration or baseline IFN-gamma signature.” Increased levels of circulating immune cells and shrinkage of untreated tumors both suggested that intratumoral T-vec injections led to systemic effects, they added.
Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
Almost two-thirds of patients with advanced melanoma responded to combination therapy with pembrolizumab and talimogene laherparepvec (T-vec) in a small phase 1b trial, investigators reported.
A third of patients achieved a complete response and median progression-free and overall survival were not reached after typically 18.6 (range, 17.7 to 20.8) months of follow-up, said Antoni Ribas, MD, of the University of California, Los Angeles, and his coinvestigators. In contrast to single-agent pembrolizumab therapy, responders to the combination regimen included patients with very low levels of CD8+ T cell infiltrates or negative interferon-gamma (IFN-gamma) gene signatures in baseline tumor biopsies, suggesting that oncolytic virotherapy might make anti-PD-1 therapy more effective by altering the tumor microenvironment, the researchers concluded. Serious adverse events were uncommon in this study, and there were no dose-limiting toxicities, they wrote (Cell. 2017 Sept. 7 doi: 10.1016/j.cell.2017.08.027).
To see if attracting CD8+ T cells into tumors helped surmount this obstacle, the researchers treated 21 patients with advanced melanoma with pembrolizumab and T-vec, an intratumorally administered, genetically modified clinical herpes simplex virus-1 strain approved for treating melanoma. Patients first received up to 4 mL T-vec (106 plaque-forming units [pfu] per mL) to induce a protective immune response. Three weeks later, they started receiving to 4 mL (108 pfu/mL) T-vec plus 200 mg intravenous pembrolizumab every 2 weeks.
Thirteen patients (62%) showed at least a partial response, and seven (33%) had a complete response based on immune criteria. Notably, 9 of 13 (69%) patients with baseline tumor CD8+ densities below 1,000 cells/mm2 responded to combination treatment, as did three of five patients with low baseline IFN-gamma signatures.
“There was only one baseline biopsy that was scored as PD-L1 negative, but that patient went on to have a complete response to the combined therapy,” the researchers wrote. “Patients who responded to combination therapy had increased CD8+ T cells, elevated PD-L1 protein expression, [and] IFN-gamma gene expression on several cell subsets in tumors after [T-vec] treatment. Response to combination therapy did not appear to be associated with baseline CD8+ T cell infiltration or baseline IFN-gamma signature.” Increased levels of circulating immune cells and shrinkage of untreated tumors both suggested that intratumoral T-vec injections led to systemic effects, they added.
Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
FROM CELL
Key clinical point: Adding talimogene laherparepvec (T-vec) might help overcome resistance to anti-PD-1 antibodies in patients with advanced melanoma.
Major finding: In all, 62% of patients had at least a partial response and 33% had a complete response. Median progression-free and overall survival were not reached after a median of 18.6 weeks of follow-up.
Data source: A phase 1b clinical trial of 21 adults with advanced melanoma who received T-vec and pembrolizumab.
Disclosures: Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
FDA approves biosimilar to bevacizumab
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
New data prompt update to ACC guidance on nonstatin LDL lowering
The American College of Cardiology Task Force on Expert Consensus Decision Pathways has released a “focused update” for the 2016 ACC Expert Consensus Decision Pathway (ECDP) on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease (ASCVD) risk.
The update was deemed by the ECDP writing committee to be desirable given the additional evidence and perspectives that have emerged since the publication of the 2016 version, particularly with respect to the efficacy and safety of proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors for the secondary prevention of ASCVD, as well as the best use of ezetimibe in addition to statin therapy after acute coronary syndrome.
The ECDP algorithms endorse the four evidence-based statin benefit groups identified in the 2013 guidelines (adults aged 21 and older with clinical ASCVD, adults aged 21 and older with LDL-C of 190 mg/dL or greater, adults aged 40-75 years without ASCVD but with diabetes and with LDL-C of 70-189 mg/dL, and adults aged 40-75 without ASCVD or diabetes but with LDL-C of 70-189 mg/dL and an estimated 10-year risk for ASCVD of 7.5% or greater) and assume that the patient is currently taking or has attempted to take a statin, they noted.
Among the changes in the 2017 focused update are:
- Consideration of new randomized clinical trial data for the PCSK9 inhibitors evolocumab and bococizumab. Namely, they included results from the cardiovascular outcomes trials FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) and SPIRE-1 and SPIRE-2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events), which were published in early 2017.
- An adjustment in the ECDP algorithms with respect to thresholds for consideration of net ASCVD risk reduction. The 2016 ECDP thresholds for risk reduction benefit were percent reduction in LDL-C with consideration of absolute LDL-C level in patients with clinical ASCVD, baseline LDL-C of 190 mg/dL or greater, and primary prevention. In patients with diabetes with or without clinical ASCVD, clinicians were allowed to consider absolute LDL-C and/or non-HDL-cholesterol levels. In the 2017 ECDP update, the thresholds are percent reduction in LDL-C with consideration of absolute LDL-C or non-HDL-C levels for patients in each of the four statin benefit groups. This change was based on the inclusion criteria of the FOURIER trial, the ongoing ODYSSEY Outcomes trial (Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab), and the SPIRE-2 trial, all of which included non-HDL-C thresholds. “In alignment with these inclusion criteria, the 2017 Focused Update includes both LDL-C and non-HDL-C thresholds for evaluation of net ASCVD risk-reduction benefit when considering the addition of nonstatin therapies for patients in each of the four statin benefit groups” the update explained.
- An expansion of the threshold for consideration of net ASCVD risk-reduction benefit from a reduction of LDL C of at least 50%, as well as consideration of LDL-C less than 70 mg/dL or non-HDL-C less than 100 mg/dL for all patients (that is, both those with and those without comorbidities) who have clinical ASCVD and baseline LDL-C of 70-189 mg/dL. The 2016 ECDP had different thresholds for those with versus those without comorbidities. This change was based on findings from the FOURIER trial and the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).“Based on consideration of all available evidence, the consensus of the writing committee members is that lower LDL-C levels are safe and optimal in patients with clinical ASCVD due to [their] increased risk of recurrent events,” they said.
- An expanded recommendation on the use of ezetimibe and PCSK9 inhibition. The 2016 ECDP stated that “if a decision is made to proceed with the addition of nonstatin therapy to maximally tolerated statin therapy, it is reasonable to consider the addition of ezetimibe as the initial agent and a PCSK9 inhibitor as the second agent.” However, based on the FOURIER findings, the ongoing ODYSSEY Outcomes trial, and the IMPROVE-IT trial, the 2017 Focused Update states that, if such a decision is made in patients with clinical ASCVD with comorbidities and baseline LDL-C of 70-189 mg/dL, it is reasonable to weigh the addition of either ezetimibe or a PCSK9 inhibitor in light of “considerations of the additional percent LDL-C reduction desired, patient preferences, costs, route of administration, and other factors.” The update also spells out considerations that may favor the initial choice of ezetimibe versus a PCSK9 inhibitor (such as requiring less than 25% additional lowering of LDL-C, an age of over 75 years, cost, and other patient factors and preferences) .
- Additional factors, based on the FOURIER trial results and inclusion criteria, that may be considered for the identification of higher-risk patients with clinical ASCVD. The 2016 ECDP included on this list diabetes, a recent ASCVD event, an ASCVD event while already taking a statin, poorly controlled other major ASCVD risk factors, elevated lipoprotein, chronic kidney disease, symptomatic heart failure, maintenance hemodialysis, and baseline LDL-C of at least 190 mg/dL not due to secondary causes. The 2017 update added being 65 years or older, prior MI or nonhemorrhagic stroke, current daily cigarette smoking, symptomatic peripheral artery disease with prior MI or stroke, history of non-MI related coronary revascularization, residual coronary artery disease with at least 40% stenosis in at least two large vessels, HDL-C less than 40 mg/dL for men and less than 50 mg/dL for women, high-sensitivity C-reactive protein greater than 2 mg/L, and metabolic syndrome.
The content of the full ECDP has been changed in accordance with these updates and now includes more extensive and detailed guidance for decision making – both in the text and in treatment algorithms.
Aspects that remain unchanged include the decision pathways and algorithms for the use of ezetimibe or PCSK9 inhibitors in primary prevention patients with LDL-C less than 190 mg/dL or in those without ASCVD and LDL-C of 190 mg/dL or greater unattributable to secondary causes.
In addition to other changes made for the purpose of clarification and consistency, recommendations regarding bile acid–sequestrant use were downgraded; these are now only recommended as optional secondary agents for consideration in patients who cannot tolerate ezetimibe.
“[These] recommendations attempt to provide practical guidance for clinicians and patients regarding the use of nonstatin therapies to further reduce ASCVD risk in situations not covered by the guideline until such time as the scientific evidence base expands and cardiovascular outcomes trials are completed with new agents for ASCVD risk reduction,” the committee concluded.
Dr. Lloyd-Jones reported having no disclosures.
The American College of Cardiology Task Force on Expert Consensus Decision Pathways has released a “focused update” for the 2016 ACC Expert Consensus Decision Pathway (ECDP) on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease (ASCVD) risk.
The update was deemed by the ECDP writing committee to be desirable given the additional evidence and perspectives that have emerged since the publication of the 2016 version, particularly with respect to the efficacy and safety of proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors for the secondary prevention of ASCVD, as well as the best use of ezetimibe in addition to statin therapy after acute coronary syndrome.
The ECDP algorithms endorse the four evidence-based statin benefit groups identified in the 2013 guidelines (adults aged 21 and older with clinical ASCVD, adults aged 21 and older with LDL-C of 190 mg/dL or greater, adults aged 40-75 years without ASCVD but with diabetes and with LDL-C of 70-189 mg/dL, and adults aged 40-75 without ASCVD or diabetes but with LDL-C of 70-189 mg/dL and an estimated 10-year risk for ASCVD of 7.5% or greater) and assume that the patient is currently taking or has attempted to take a statin, they noted.
Among the changes in the 2017 focused update are:
- Consideration of new randomized clinical trial data for the PCSK9 inhibitors evolocumab and bococizumab. Namely, they included results from the cardiovascular outcomes trials FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) and SPIRE-1 and SPIRE-2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events), which were published in early 2017.
- An adjustment in the ECDP algorithms with respect to thresholds for consideration of net ASCVD risk reduction. The 2016 ECDP thresholds for risk reduction benefit were percent reduction in LDL-C with consideration of absolute LDL-C level in patients with clinical ASCVD, baseline LDL-C of 190 mg/dL or greater, and primary prevention. In patients with diabetes with or without clinical ASCVD, clinicians were allowed to consider absolute LDL-C and/or non-HDL-cholesterol levels. In the 2017 ECDP update, the thresholds are percent reduction in LDL-C with consideration of absolute LDL-C or non-HDL-C levels for patients in each of the four statin benefit groups. This change was based on the inclusion criteria of the FOURIER trial, the ongoing ODYSSEY Outcomes trial (Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab), and the SPIRE-2 trial, all of which included non-HDL-C thresholds. “In alignment with these inclusion criteria, the 2017 Focused Update includes both LDL-C and non-HDL-C thresholds for evaluation of net ASCVD risk-reduction benefit when considering the addition of nonstatin therapies for patients in each of the four statin benefit groups” the update explained.
- An expansion of the threshold for consideration of net ASCVD risk-reduction benefit from a reduction of LDL C of at least 50%, as well as consideration of LDL-C less than 70 mg/dL or non-HDL-C less than 100 mg/dL for all patients (that is, both those with and those without comorbidities) who have clinical ASCVD and baseline LDL-C of 70-189 mg/dL. The 2016 ECDP had different thresholds for those with versus those without comorbidities. This change was based on findings from the FOURIER trial and the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).“Based on consideration of all available evidence, the consensus of the writing committee members is that lower LDL-C levels are safe and optimal in patients with clinical ASCVD due to [their] increased risk of recurrent events,” they said.
- An expanded recommendation on the use of ezetimibe and PCSK9 inhibition. The 2016 ECDP stated that “if a decision is made to proceed with the addition of nonstatin therapy to maximally tolerated statin therapy, it is reasonable to consider the addition of ezetimibe as the initial agent and a PCSK9 inhibitor as the second agent.” However, based on the FOURIER findings, the ongoing ODYSSEY Outcomes trial, and the IMPROVE-IT trial, the 2017 Focused Update states that, if such a decision is made in patients with clinical ASCVD with comorbidities and baseline LDL-C of 70-189 mg/dL, it is reasonable to weigh the addition of either ezetimibe or a PCSK9 inhibitor in light of “considerations of the additional percent LDL-C reduction desired, patient preferences, costs, route of administration, and other factors.” The update also spells out considerations that may favor the initial choice of ezetimibe versus a PCSK9 inhibitor (such as requiring less than 25% additional lowering of LDL-C, an age of over 75 years, cost, and other patient factors and preferences) .
- Additional factors, based on the FOURIER trial results and inclusion criteria, that may be considered for the identification of higher-risk patients with clinical ASCVD. The 2016 ECDP included on this list diabetes, a recent ASCVD event, an ASCVD event while already taking a statin, poorly controlled other major ASCVD risk factors, elevated lipoprotein, chronic kidney disease, symptomatic heart failure, maintenance hemodialysis, and baseline LDL-C of at least 190 mg/dL not due to secondary causes. The 2017 update added being 65 years or older, prior MI or nonhemorrhagic stroke, current daily cigarette smoking, symptomatic peripheral artery disease with prior MI or stroke, history of non-MI related coronary revascularization, residual coronary artery disease with at least 40% stenosis in at least two large vessels, HDL-C less than 40 mg/dL for men and less than 50 mg/dL for women, high-sensitivity C-reactive protein greater than 2 mg/L, and metabolic syndrome.
The content of the full ECDP has been changed in accordance with these updates and now includes more extensive and detailed guidance for decision making – both in the text and in treatment algorithms.
Aspects that remain unchanged include the decision pathways and algorithms for the use of ezetimibe or PCSK9 inhibitors in primary prevention patients with LDL-C less than 190 mg/dL or in those without ASCVD and LDL-C of 190 mg/dL or greater unattributable to secondary causes.
In addition to other changes made for the purpose of clarification and consistency, recommendations regarding bile acid–sequestrant use were downgraded; these are now only recommended as optional secondary agents for consideration in patients who cannot tolerate ezetimibe.
“[These] recommendations attempt to provide practical guidance for clinicians and patients regarding the use of nonstatin therapies to further reduce ASCVD risk in situations not covered by the guideline until such time as the scientific evidence base expands and cardiovascular outcomes trials are completed with new agents for ASCVD risk reduction,” the committee concluded.
Dr. Lloyd-Jones reported having no disclosures.
The American College of Cardiology Task Force on Expert Consensus Decision Pathways has released a “focused update” for the 2016 ACC Expert Consensus Decision Pathway (ECDP) on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease (ASCVD) risk.
The update was deemed by the ECDP writing committee to be desirable given the additional evidence and perspectives that have emerged since the publication of the 2016 version, particularly with respect to the efficacy and safety of proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors for the secondary prevention of ASCVD, as well as the best use of ezetimibe in addition to statin therapy after acute coronary syndrome.
The ECDP algorithms endorse the four evidence-based statin benefit groups identified in the 2013 guidelines (adults aged 21 and older with clinical ASCVD, adults aged 21 and older with LDL-C of 190 mg/dL or greater, adults aged 40-75 years without ASCVD but with diabetes and with LDL-C of 70-189 mg/dL, and adults aged 40-75 without ASCVD or diabetes but with LDL-C of 70-189 mg/dL and an estimated 10-year risk for ASCVD of 7.5% or greater) and assume that the patient is currently taking or has attempted to take a statin, they noted.
Among the changes in the 2017 focused update are:
- Consideration of new randomized clinical trial data for the PCSK9 inhibitors evolocumab and bococizumab. Namely, they included results from the cardiovascular outcomes trials FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) and SPIRE-1 and SPIRE-2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events), which were published in early 2017.
- An adjustment in the ECDP algorithms with respect to thresholds for consideration of net ASCVD risk reduction. The 2016 ECDP thresholds for risk reduction benefit were percent reduction in LDL-C with consideration of absolute LDL-C level in patients with clinical ASCVD, baseline LDL-C of 190 mg/dL or greater, and primary prevention. In patients with diabetes with or without clinical ASCVD, clinicians were allowed to consider absolute LDL-C and/or non-HDL-cholesterol levels. In the 2017 ECDP update, the thresholds are percent reduction in LDL-C with consideration of absolute LDL-C or non-HDL-C levels for patients in each of the four statin benefit groups. This change was based on the inclusion criteria of the FOURIER trial, the ongoing ODYSSEY Outcomes trial (Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab), and the SPIRE-2 trial, all of which included non-HDL-C thresholds. “In alignment with these inclusion criteria, the 2017 Focused Update includes both LDL-C and non-HDL-C thresholds for evaluation of net ASCVD risk-reduction benefit when considering the addition of nonstatin therapies for patients in each of the four statin benefit groups” the update explained.
- An expansion of the threshold for consideration of net ASCVD risk-reduction benefit from a reduction of LDL C of at least 50%, as well as consideration of LDL-C less than 70 mg/dL or non-HDL-C less than 100 mg/dL for all patients (that is, both those with and those without comorbidities) who have clinical ASCVD and baseline LDL-C of 70-189 mg/dL. The 2016 ECDP had different thresholds for those with versus those without comorbidities. This change was based on findings from the FOURIER trial and the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).“Based on consideration of all available evidence, the consensus of the writing committee members is that lower LDL-C levels are safe and optimal in patients with clinical ASCVD due to [their] increased risk of recurrent events,” they said.
- An expanded recommendation on the use of ezetimibe and PCSK9 inhibition. The 2016 ECDP stated that “if a decision is made to proceed with the addition of nonstatin therapy to maximally tolerated statin therapy, it is reasonable to consider the addition of ezetimibe as the initial agent and a PCSK9 inhibitor as the second agent.” However, based on the FOURIER findings, the ongoing ODYSSEY Outcomes trial, and the IMPROVE-IT trial, the 2017 Focused Update states that, if such a decision is made in patients with clinical ASCVD with comorbidities and baseline LDL-C of 70-189 mg/dL, it is reasonable to weigh the addition of either ezetimibe or a PCSK9 inhibitor in light of “considerations of the additional percent LDL-C reduction desired, patient preferences, costs, route of administration, and other factors.” The update also spells out considerations that may favor the initial choice of ezetimibe versus a PCSK9 inhibitor (such as requiring less than 25% additional lowering of LDL-C, an age of over 75 years, cost, and other patient factors and preferences) .
- Additional factors, based on the FOURIER trial results and inclusion criteria, that may be considered for the identification of higher-risk patients with clinical ASCVD. The 2016 ECDP included on this list diabetes, a recent ASCVD event, an ASCVD event while already taking a statin, poorly controlled other major ASCVD risk factors, elevated lipoprotein, chronic kidney disease, symptomatic heart failure, maintenance hemodialysis, and baseline LDL-C of at least 190 mg/dL not due to secondary causes. The 2017 update added being 65 years or older, prior MI or nonhemorrhagic stroke, current daily cigarette smoking, symptomatic peripheral artery disease with prior MI or stroke, history of non-MI related coronary revascularization, residual coronary artery disease with at least 40% stenosis in at least two large vessels, HDL-C less than 40 mg/dL for men and less than 50 mg/dL for women, high-sensitivity C-reactive protein greater than 2 mg/L, and metabolic syndrome.
The content of the full ECDP has been changed in accordance with these updates and now includes more extensive and detailed guidance for decision making – both in the text and in treatment algorithms.
Aspects that remain unchanged include the decision pathways and algorithms for the use of ezetimibe or PCSK9 inhibitors in primary prevention patients with LDL-C less than 190 mg/dL or in those without ASCVD and LDL-C of 190 mg/dL or greater unattributable to secondary causes.
In addition to other changes made for the purpose of clarification and consistency, recommendations regarding bile acid–sequestrant use were downgraded; these are now only recommended as optional secondary agents for consideration in patients who cannot tolerate ezetimibe.
“[These] recommendations attempt to provide practical guidance for clinicians and patients regarding the use of nonstatin therapies to further reduce ASCVD risk in situations not covered by the guideline until such time as the scientific evidence base expands and cardiovascular outcomes trials are completed with new agents for ASCVD risk reduction,” the committee concluded.
Dr. Lloyd-Jones reported having no disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY