User login
Postpartum sepsis risk persists after 6 weeks
PARK CITY, UTAH – The risk of sepsis after delivery persists beyond 6 weeks, the traditional point at which women are thought to be in the clear, according to investigators from Stanford (Calif.) University.
The team analyzed 506 sepsis hospitalizations following delivery, culled from almost 2 million live births in California from 2008-2012; 199 (39%) were at or before 6 weeks postpartum, and 310 (61%) were after 6 weeks, out to a year.
“Going into this, our view was that sepsis cases before 6 weeks would be due to obstetrical causes, and cases after 6 weeks would be due to [nonobstetrical causes],” said senior investigator Ronald Gibbs, MD, clinical professor of obstetrics and gynecology at Stanford. But that’s not what the team found.
In both the early and late admission groups, early preterm delivery was one of the leading risks for postpartum sepsis and other risk factors were largely the same. Pyelonephritis and pneumonia were by far the most common diagnoses in both groups, accounting for more than 70% of cases. The rank order of causative organisms was the same whether women presented before 6 weeks or after: gram-negative bacteria, staphylococcus, and streptococcus.
“In view of this, we think the risk for sepsis goes beyond 6 weeks,” Dr. Gibbs said at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology. Although women were admitted largely for nonuterine infections, “the reservoir of infection could be in the uterus,” with later seeding to the urinary tract or lungs. “I think there’s a chronic intrauterine infection that sets women up for” both early preterm birth and later sepsis, he added.
“These late admissions would probably go to a nonobstetrical service, but we are thinking that there may be a pelvic origin related to something that went on at delivery. I can’t really say that we ought to change our practice, but it sets the stage for looking at that. Finding that late [admissions for sepsis] have a lot in common with the early admissions and are probably related to the pregnancy really reorders the thinking,” Dr. Gibbs said.
The team found that among women who were delivered at 24-28 weeks, the adjusted odds ratio was 8.6 (95% confidence interval[CI], 4.4-17.1) for early and 4.2 (95% CI, 1.9-9.0) for late postpartum sepsis admission, even after delivery mode, maternal comorbidities, maternal age, “and everything else we could think of” were controlled for, said lead investigator Megan Foeller, MD, a maternal-fetal medicine fellow at Stanford.
A body mass index above 35 kg/m2 also increased the risk for sepsis admission, as did government-provided insurance, primary cesarean delivery, a failed trial of labor after a previous cesarean, and four or more previous deliveries.
Postoperative infection, acute hepatic failure, acute renal failure, acute respiratory failure, and heart failure during the delivery hospitalization greatly increased the risk of subsequent sepsis, as well.
The findings help define a group of women who likely need especially close follow-up after delivery to prevent sepsis, Dr. Foeller said.
Sepsis was defined in the study by ICD-9 codes for septicemia plus acute organ dysfunction.
There was no industry funding for the work and the investigators reported having no relevant financial disclosures.
PARK CITY, UTAH – The risk of sepsis after delivery persists beyond 6 weeks, the traditional point at which women are thought to be in the clear, according to investigators from Stanford (Calif.) University.
The team analyzed 506 sepsis hospitalizations following delivery, culled from almost 2 million live births in California from 2008-2012; 199 (39%) were at or before 6 weeks postpartum, and 310 (61%) were after 6 weeks, out to a year.
“Going into this, our view was that sepsis cases before 6 weeks would be due to obstetrical causes, and cases after 6 weeks would be due to [nonobstetrical causes],” said senior investigator Ronald Gibbs, MD, clinical professor of obstetrics and gynecology at Stanford. But that’s not what the team found.
In both the early and late admission groups, early preterm delivery was one of the leading risks for postpartum sepsis and other risk factors were largely the same. Pyelonephritis and pneumonia were by far the most common diagnoses in both groups, accounting for more than 70% of cases. The rank order of causative organisms was the same whether women presented before 6 weeks or after: gram-negative bacteria, staphylococcus, and streptococcus.
“In view of this, we think the risk for sepsis goes beyond 6 weeks,” Dr. Gibbs said at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology. Although women were admitted largely for nonuterine infections, “the reservoir of infection could be in the uterus,” with later seeding to the urinary tract or lungs. “I think there’s a chronic intrauterine infection that sets women up for” both early preterm birth and later sepsis, he added.
“These late admissions would probably go to a nonobstetrical service, but we are thinking that there may be a pelvic origin related to something that went on at delivery. I can’t really say that we ought to change our practice, but it sets the stage for looking at that. Finding that late [admissions for sepsis] have a lot in common with the early admissions and are probably related to the pregnancy really reorders the thinking,” Dr. Gibbs said.
The team found that among women who were delivered at 24-28 weeks, the adjusted odds ratio was 8.6 (95% confidence interval[CI], 4.4-17.1) for early and 4.2 (95% CI, 1.9-9.0) for late postpartum sepsis admission, even after delivery mode, maternal comorbidities, maternal age, “and everything else we could think of” were controlled for, said lead investigator Megan Foeller, MD, a maternal-fetal medicine fellow at Stanford.
A body mass index above 35 kg/m2 also increased the risk for sepsis admission, as did government-provided insurance, primary cesarean delivery, a failed trial of labor after a previous cesarean, and four or more previous deliveries.
Postoperative infection, acute hepatic failure, acute renal failure, acute respiratory failure, and heart failure during the delivery hospitalization greatly increased the risk of subsequent sepsis, as well.
The findings help define a group of women who likely need especially close follow-up after delivery to prevent sepsis, Dr. Foeller said.
Sepsis was defined in the study by ICD-9 codes for septicemia plus acute organ dysfunction.
There was no industry funding for the work and the investigators reported having no relevant financial disclosures.
PARK CITY, UTAH – The risk of sepsis after delivery persists beyond 6 weeks, the traditional point at which women are thought to be in the clear, according to investigators from Stanford (Calif.) University.
The team analyzed 506 sepsis hospitalizations following delivery, culled from almost 2 million live births in California from 2008-2012; 199 (39%) were at or before 6 weeks postpartum, and 310 (61%) were after 6 weeks, out to a year.
“Going into this, our view was that sepsis cases before 6 weeks would be due to obstetrical causes, and cases after 6 weeks would be due to [nonobstetrical causes],” said senior investigator Ronald Gibbs, MD, clinical professor of obstetrics and gynecology at Stanford. But that’s not what the team found.
In both the early and late admission groups, early preterm delivery was one of the leading risks for postpartum sepsis and other risk factors were largely the same. Pyelonephritis and pneumonia were by far the most common diagnoses in both groups, accounting for more than 70% of cases. The rank order of causative organisms was the same whether women presented before 6 weeks or after: gram-negative bacteria, staphylococcus, and streptococcus.
“In view of this, we think the risk for sepsis goes beyond 6 weeks,” Dr. Gibbs said at the annual scientific meeting of the Infectious Diseases Society for Obstetrics and Gynecology. Although women were admitted largely for nonuterine infections, “the reservoir of infection could be in the uterus,” with later seeding to the urinary tract or lungs. “I think there’s a chronic intrauterine infection that sets women up for” both early preterm birth and later sepsis, he added.
“These late admissions would probably go to a nonobstetrical service, but we are thinking that there may be a pelvic origin related to something that went on at delivery. I can’t really say that we ought to change our practice, but it sets the stage for looking at that. Finding that late [admissions for sepsis] have a lot in common with the early admissions and are probably related to the pregnancy really reorders the thinking,” Dr. Gibbs said.
The team found that among women who were delivered at 24-28 weeks, the adjusted odds ratio was 8.6 (95% confidence interval[CI], 4.4-17.1) for early and 4.2 (95% CI, 1.9-9.0) for late postpartum sepsis admission, even after delivery mode, maternal comorbidities, maternal age, “and everything else we could think of” were controlled for, said lead investigator Megan Foeller, MD, a maternal-fetal medicine fellow at Stanford.
A body mass index above 35 kg/m2 also increased the risk for sepsis admission, as did government-provided insurance, primary cesarean delivery, a failed trial of labor after a previous cesarean, and four or more previous deliveries.
Postoperative infection, acute hepatic failure, acute renal failure, acute respiratory failure, and heart failure during the delivery hospitalization greatly increased the risk of subsequent sepsis, as well.
The findings help define a group of women who likely need especially close follow-up after delivery to prevent sepsis, Dr. Foeller said.
Sepsis was defined in the study by ICD-9 codes for septicemia plus acute organ dysfunction.
There was no industry funding for the work and the investigators reported having no relevant financial disclosures.
AT IDSOG
Key clinical point:
Major finding: Of 506 cases of sepsis hospitalization following delivery, 199 (39%) cases were at or before postpartum week 6, and 310 (61%) were after week 6.
Data source: A database review of 506 sepsis hospitalizations following delivery, culled from almost 2 million live births in California from the period of 2008-2012.
Disclosures: There was no industry funding for the work and the investigators reported having no relevant financial disclosures.
PROTECT trial: No DFS benefit with adjuvant pazopanib for high-risk RCC
Adjuvant pazopanib provided no disease-free survival benefit compared with placebo in the randomized phase 3 PROTECT trial of patients with locally advanced renal cell carcinoma at high risk for relapse after nephrectomy.
In the primary analysis for disease-free survival among 571 patients treated for 1 year with 600 mg of pazopanib and 564 who received placebo, no significant improvement was seen with pazopanib (hazard ratio, 0.86). In a follow-up analysis 12 months later, the hazard ratio was 0.94. Secondary analysis in 403 additional patients who were treated with 800 mg of pazopanib before the dose was lowered to 600 mg due to intolerance and toxicity attrition showed a benefit with pazopanib (HR, 0.69), but this group represented only a third of the study population, reported Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. The study results were published online in the Journal of Clinical Oncology.
Common adverse events leading to treatment discontinuation included increased alanine transaminase and aspartate transaminase levels, which occurred in 16% and 5% of patients treated with 600 mg, respectively, and in 18% and 7% of patients treated with 800 mg, respectively. Four grade 5 adverse events occurred in the pazopanib groups (vs. 2 in the placebo group), and one of the deaths (in a patient who received 800 mg dosing) involved cardiomyopathy that was considered to be related to treatment, the investigators said (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.73.5324).
The study comprised adults with resected pT2 or pT3 and greater disease, including N1, clear cell renal cell carcinoma, who were enrolled between Dec. 9, 2010, and Sept. 10, 2013, from 263 centers in 26 countries. Primary analysis was done after 350 disease-free events occurred in the intent-to-treat population receiving 600 mg.
The difference in treatment effect between those receiving 600 mg and 800 mg of pazopanib could be explained by the different starting dose, or by better performance of the placebo arm in the 600 mg group, the investigators noted.
As for overall survival, the results are inconclusive, because the data are not mature, they said.
Novartis supported the study. Dr. Mercer reported consulting or advisory roles with Pfizer, Novartis, Eisai, and Exelixis, and research funding to his institution from Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Eisai, Novartis, and Genentech. Numerous coauthors also reported financial relationships with pharmaceutical companies.
Adjuvant pazopanib provided no disease-free survival benefit compared with placebo in the randomized phase 3 PROTECT trial of patients with locally advanced renal cell carcinoma at high risk for relapse after nephrectomy.
In the primary analysis for disease-free survival among 571 patients treated for 1 year with 600 mg of pazopanib and 564 who received placebo, no significant improvement was seen with pazopanib (hazard ratio, 0.86). In a follow-up analysis 12 months later, the hazard ratio was 0.94. Secondary analysis in 403 additional patients who were treated with 800 mg of pazopanib before the dose was lowered to 600 mg due to intolerance and toxicity attrition showed a benefit with pazopanib (HR, 0.69), but this group represented only a third of the study population, reported Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. The study results were published online in the Journal of Clinical Oncology.
Common adverse events leading to treatment discontinuation included increased alanine transaminase and aspartate transaminase levels, which occurred in 16% and 5% of patients treated with 600 mg, respectively, and in 18% and 7% of patients treated with 800 mg, respectively. Four grade 5 adverse events occurred in the pazopanib groups (vs. 2 in the placebo group), and one of the deaths (in a patient who received 800 mg dosing) involved cardiomyopathy that was considered to be related to treatment, the investigators said (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.73.5324).
The study comprised adults with resected pT2 or pT3 and greater disease, including N1, clear cell renal cell carcinoma, who were enrolled between Dec. 9, 2010, and Sept. 10, 2013, from 263 centers in 26 countries. Primary analysis was done after 350 disease-free events occurred in the intent-to-treat population receiving 600 mg.
The difference in treatment effect between those receiving 600 mg and 800 mg of pazopanib could be explained by the different starting dose, or by better performance of the placebo arm in the 600 mg group, the investigators noted.
As for overall survival, the results are inconclusive, because the data are not mature, they said.
Novartis supported the study. Dr. Mercer reported consulting or advisory roles with Pfizer, Novartis, Eisai, and Exelixis, and research funding to his institution from Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Eisai, Novartis, and Genentech. Numerous coauthors also reported financial relationships with pharmaceutical companies.
Adjuvant pazopanib provided no disease-free survival benefit compared with placebo in the randomized phase 3 PROTECT trial of patients with locally advanced renal cell carcinoma at high risk for relapse after nephrectomy.
In the primary analysis for disease-free survival among 571 patients treated for 1 year with 600 mg of pazopanib and 564 who received placebo, no significant improvement was seen with pazopanib (hazard ratio, 0.86). In a follow-up analysis 12 months later, the hazard ratio was 0.94. Secondary analysis in 403 additional patients who were treated with 800 mg of pazopanib before the dose was lowered to 600 mg due to intolerance and toxicity attrition showed a benefit with pazopanib (HR, 0.69), but this group represented only a third of the study population, reported Robert J. Motzer, MD, of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. The study results were published online in the Journal of Clinical Oncology.
Common adverse events leading to treatment discontinuation included increased alanine transaminase and aspartate transaminase levels, which occurred in 16% and 5% of patients treated with 600 mg, respectively, and in 18% and 7% of patients treated with 800 mg, respectively. Four grade 5 adverse events occurred in the pazopanib groups (vs. 2 in the placebo group), and one of the deaths (in a patient who received 800 mg dosing) involved cardiomyopathy that was considered to be related to treatment, the investigators said (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.73.5324).
The study comprised adults with resected pT2 or pT3 and greater disease, including N1, clear cell renal cell carcinoma, who were enrolled between Dec. 9, 2010, and Sept. 10, 2013, from 263 centers in 26 countries. Primary analysis was done after 350 disease-free events occurred in the intent-to-treat population receiving 600 mg.
The difference in treatment effect between those receiving 600 mg and 800 mg of pazopanib could be explained by the different starting dose, or by better performance of the placebo arm in the 600 mg group, the investigators noted.
As for overall survival, the results are inconclusive, because the data are not mature, they said.
Novartis supported the study. Dr. Mercer reported consulting or advisory roles with Pfizer, Novartis, Eisai, and Exelixis, and research funding to his institution from Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Eisai, Novartis, and Genentech. Numerous coauthors also reported financial relationships with pharmaceutical companies.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: In the primary analysis for disease-free survival, no significant improvement was seen with pazopanib vs. placebo (hazard ratio, 0.86).
Data source: The phase 3 PROTECT study of 1,538 patients.
Disclosures: Novartis supported the study. Dr. Mercer reported consulting or advisory roles with Pfizer, Novartis, Eisai, and Exelixis, and research funding to his institution from Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Eisai, Novartis, and Genentech. Numerous coauthors also reported financial relationships with pharmaceutical companies.
No increased overall cardiovascular risk seen with exenatide use
“We did not observe any specific safety issues during our trial; there was no adverse signal with respect to heart failure, despite the higher mean heart rate in the exenatide group than in the placebo group, and events of acute pancreatitis and pancreatic cancer were rare, with similar rates in the two groups,” according to researchers led by Rury R. Holman, F.Med.Sci. Their study was published online Sept. 14 in the New England Journal of Medicine, and was presented at the annual meeting of the European Association for the Study of Diabetes.
For the trial, which was funded by Amylin Pharmaceuticals and known as the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), the researchers randomly assigned 14,752 patients with type 2 diabetes at 687 sites in 35 countries to receive subcutaneous injections of extended-released exenatide at a dose of 2 mg, or matching placebo once per week, from June 18, 2010, through Sept. 16, 2015. The patients were followed for a median of 3.2 years and the main outcome of interest was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or stroke.
In the meantime, the intention-to-treat analysis revealed that exenatide was noninferior to placebo with respect to safety (P less than .001) but was not superior to placebo with respect to safety (P = .06). The researchers observed no significant differences between the two groups in the rates of death from cardiovascular causes, fatal or nonfatal MI, or stroke, hospitalization for heart failure or for acute coronary syndrome, or in the incidence of cute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events.
“The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria,” the researchers wrote. “For example, patients with any degree of cardiovascular risk who were at least 18 years of age (with no upper age limit) were eligible. To further augment the potential generalizability of any findings, we evaluated the cardiovascular effect of once-weekly extended-release exenatide in the usual-care setting by maintaining the focus of management of diabetes and cardiovascular risk with the usual-care provider.”
The researchers acknowledged certain limitations of the study, including the rate of premature discontinuation of the trial regimen, “which was driven by patient decision. We speculate that probable factors for discontinuation were the complexity of the first-generation injection device that was used and the fact that our trial had no run-in period.”
The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
PRIMARY SOURCE: N Engl J Med 2017 Sept. 14. doi: 10.1056/NEJMoa1612917
“We did not observe any specific safety issues during our trial; there was no adverse signal with respect to heart failure, despite the higher mean heart rate in the exenatide group than in the placebo group, and events of acute pancreatitis and pancreatic cancer were rare, with similar rates in the two groups,” according to researchers led by Rury R. Holman, F.Med.Sci. Their study was published online Sept. 14 in the New England Journal of Medicine, and was presented at the annual meeting of the European Association for the Study of Diabetes.
For the trial, which was funded by Amylin Pharmaceuticals and known as the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), the researchers randomly assigned 14,752 patients with type 2 diabetes at 687 sites in 35 countries to receive subcutaneous injections of extended-released exenatide at a dose of 2 mg, or matching placebo once per week, from June 18, 2010, through Sept. 16, 2015. The patients were followed for a median of 3.2 years and the main outcome of interest was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or stroke.
In the meantime, the intention-to-treat analysis revealed that exenatide was noninferior to placebo with respect to safety (P less than .001) but was not superior to placebo with respect to safety (P = .06). The researchers observed no significant differences between the two groups in the rates of death from cardiovascular causes, fatal or nonfatal MI, or stroke, hospitalization for heart failure or for acute coronary syndrome, or in the incidence of cute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events.
“The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria,” the researchers wrote. “For example, patients with any degree of cardiovascular risk who were at least 18 years of age (with no upper age limit) were eligible. To further augment the potential generalizability of any findings, we evaluated the cardiovascular effect of once-weekly extended-release exenatide in the usual-care setting by maintaining the focus of management of diabetes and cardiovascular risk with the usual-care provider.”
The researchers acknowledged certain limitations of the study, including the rate of premature discontinuation of the trial regimen, “which was driven by patient decision. We speculate that probable factors for discontinuation were the complexity of the first-generation injection device that was used and the fact that our trial had no run-in period.”
The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
PRIMARY SOURCE: N Engl J Med 2017 Sept. 14. doi: 10.1056/NEJMoa1612917
“We did not observe any specific safety issues during our trial; there was no adverse signal with respect to heart failure, despite the higher mean heart rate in the exenatide group than in the placebo group, and events of acute pancreatitis and pancreatic cancer were rare, with similar rates in the two groups,” according to researchers led by Rury R. Holman, F.Med.Sci. Their study was published online Sept. 14 in the New England Journal of Medicine, and was presented at the annual meeting of the European Association for the Study of Diabetes.
For the trial, which was funded by Amylin Pharmaceuticals and known as the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), the researchers randomly assigned 14,752 patients with type 2 diabetes at 687 sites in 35 countries to receive subcutaneous injections of extended-released exenatide at a dose of 2 mg, or matching placebo once per week, from June 18, 2010, through Sept. 16, 2015. The patients were followed for a median of 3.2 years and the main outcome of interest was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or stroke.
In the meantime, the intention-to-treat analysis revealed that exenatide was noninferior to placebo with respect to safety (P less than .001) but was not superior to placebo with respect to safety (P = .06). The researchers observed no significant differences between the two groups in the rates of death from cardiovascular causes, fatal or nonfatal MI, or stroke, hospitalization for heart failure or for acute coronary syndrome, or in the incidence of cute pancreatitis, pancreatic cancer, medullary thyroid carcinoma, and serious adverse events.
“The pragmatic design of the trial included integration with usual care and wide-ranging eligibility criteria,” the researchers wrote. “For example, patients with any degree of cardiovascular risk who were at least 18 years of age (with no upper age limit) were eligible. To further augment the potential generalizability of any findings, we evaluated the cardiovascular effect of once-weekly extended-release exenatide in the usual-care setting by maintaining the focus of management of diabetes and cardiovascular risk with the usual-care provider.”
The researchers acknowledged certain limitations of the study, including the rate of premature discontinuation of the trial regimen, “which was driven by patient decision. We speculate that probable factors for discontinuation were the complexity of the first-generation injection device that was used and the fact that our trial had no run-in period.”
The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
PRIMARY SOURCE: N Engl J Med 2017 Sept. 14. doi: 10.1056/NEJMoa1612917
FROM EASD 2017
Key clinical point: Exenatide was noninferior to placebo with respect to cardiovascular safety but was not superior with respect to efficacy.
Major finding: Among patients with type 2 diabetes with and without previous cardiovascular disease, once-weekly administration of exenatide does not appear to cause an increase in their overall cardiovascular risk.
Study details: A randomized, placebo-controlled trial of 14,752 diabetic patients with or without previous cardiovascular disease.
Disclosures: The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trial Unit, in collaboration with Amylin Pharmaceuticals. Dr. Holman and his coauthors reported having numerous financial ties to the pharmaceutical industry.
Source: Rury R. Holman, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017 Sept 14. doi: 10.1056/NEJMoa1612917.
Here’s what’s trending at SHM - Sept. 2017
Early decision for Fellows applications is Sept. 15. Apply now!
SHM’s Fellows designation is a prestigious way to differentiate yourself among your peers in hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating core values of leadership, teamwork, and quality improvement.
“I was encouraged to consider SHM’s Fellow designation by other members of an SHM committee that I belonged to. Although reluctant at first, I realized that this was an opportunity to really confirm that I was a career hospitalist. The application process itself allowed me to objectively evaluate the work I had done to date and how it fit into my overall career plan. I believe that this FHM designation has fostered connections in the HM community and within my own institution that may not have been open to me before.” – Dr. Patricia Seymour, MD, FAAFP, FHM
Got research? Get noticed at HM18!
Don’t miss your chance to present your research to a national audience. SHM’s scientific abstract and poster competition, known as Research, Innovations, and Clinical Vignettes (RIV), is one of the most popular events at SHM’s annual meeting, enabling hospitalists from across the country to discuss emerging scientific and clinical cases, share feedback, and make valuable professional connections.
Hospital Medicine 2018 (HM18) will be held April 8-11, 2018 at the Orlando World Center Marriott. Many cutting-edge abstracts first presented at SHM’s RIV sessions go on to be published in respected medical journals. Yours could be next.
For more details, visit hospitalmedicine2018.org.
Know someone with exceptional achievements in Hospital Medicine?
SHM’s prestigious Awards of Excellence recognize exceptional achievements in the field of hospital medicine in the following categories:
• Excellence in Research.
• Management Excellence in Hospital Medicine.
• Outstanding Service in Hospital Medicine.
• Excellence in Teaching.
• Clinical Excellence for Physicians.
• Clinical Excellence for Nurse Practitioners and Physician Assistants.
• Excellence in Humanitarian Services.
• Excellence in Teamwork.
Awards of Excellence nominations are due on Oct. 2, 2017. Nominate yourself or a colleague today at hospitalmedicine.org/awards.
Invest in your career with SPARK ONE
SPARK ONE, SHM’s premier online self-assessment created specifically for hospital medicine professionals, is the perfect tool to help you reach your goals. The activity contains 450+ vignette-style multiple-choice questions covering 100% of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine (FPHM) exam blueprint.
This online tool is your complete resource for successfully preparing for the FPHM exam or assessing your general knowledge in hospital medicine. Used as a self-paced study guide, it engages learners through an open-book format, allowing users to review detailed learning objectives and discussion points, and define individual areas of strengths and weaknesses. Earn up to 23 AMA PRA Category 1 Credit™ and 23 MOC points.
Learn more at hospitalmedicine.org/sparkone.
Strengthen your knowledge & skills in practice administration
Get involved in the SHM Practice Administrators’ Committee 2018 Mentor/Mentee Program.
This program helps you create relationships and serves as an outlet for you to pose questions or ideas to a seasoned hospital medicine group administrator. There are two different ways you can participate: as a less experienced administrator looking for a mentor or as a more experienced administrator looking to be paired with a peer. This program is free to members only. Not a member? Join today at hospitalmedicine.org/join.
Learn more about the program and submit your application at hospitalmedicine.org/pamentor.
Obtain an extensive insight into Hospital Medicine groups configuration and operation
SHM’s State of Hospital Medicine Report includes data collected from 600 hospital medicine groups (HMGs) representing 9,000 providers to keep you current on hospitalist compensation and production, in addition to cutting-edge knowledge covering practice demographics, staffing levels, turnover, staff growth, compensation methods, and financial support for solid, evidence-based management decisions.
The 2016 State of Hospital Medicine Report is not only in print but also available in an enhanced, fully searchable digital version. Order your copy at hospitalmedicine.org/sohm.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Early decision for Fellows applications is Sept. 15. Apply now!
SHM’s Fellows designation is a prestigious way to differentiate yourself among your peers in hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating core values of leadership, teamwork, and quality improvement.
“I was encouraged to consider SHM’s Fellow designation by other members of an SHM committee that I belonged to. Although reluctant at first, I realized that this was an opportunity to really confirm that I was a career hospitalist. The application process itself allowed me to objectively evaluate the work I had done to date and how it fit into my overall career plan. I believe that this FHM designation has fostered connections in the HM community and within my own institution that may not have been open to me before.” – Dr. Patricia Seymour, MD, FAAFP, FHM
Got research? Get noticed at HM18!
Don’t miss your chance to present your research to a national audience. SHM’s scientific abstract and poster competition, known as Research, Innovations, and Clinical Vignettes (RIV), is one of the most popular events at SHM’s annual meeting, enabling hospitalists from across the country to discuss emerging scientific and clinical cases, share feedback, and make valuable professional connections.
Hospital Medicine 2018 (HM18) will be held April 8-11, 2018 at the Orlando World Center Marriott. Many cutting-edge abstracts first presented at SHM’s RIV sessions go on to be published in respected medical journals. Yours could be next.
For more details, visit hospitalmedicine2018.org.
Know someone with exceptional achievements in Hospital Medicine?
SHM’s prestigious Awards of Excellence recognize exceptional achievements in the field of hospital medicine in the following categories:
• Excellence in Research.
• Management Excellence in Hospital Medicine.
• Outstanding Service in Hospital Medicine.
• Excellence in Teaching.
• Clinical Excellence for Physicians.
• Clinical Excellence for Nurse Practitioners and Physician Assistants.
• Excellence in Humanitarian Services.
• Excellence in Teamwork.
Awards of Excellence nominations are due on Oct. 2, 2017. Nominate yourself or a colleague today at hospitalmedicine.org/awards.
Invest in your career with SPARK ONE
SPARK ONE, SHM’s premier online self-assessment created specifically for hospital medicine professionals, is the perfect tool to help you reach your goals. The activity contains 450+ vignette-style multiple-choice questions covering 100% of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine (FPHM) exam blueprint.
This online tool is your complete resource for successfully preparing for the FPHM exam or assessing your general knowledge in hospital medicine. Used as a self-paced study guide, it engages learners through an open-book format, allowing users to review detailed learning objectives and discussion points, and define individual areas of strengths and weaknesses. Earn up to 23 AMA PRA Category 1 Credit™ and 23 MOC points.
Learn more at hospitalmedicine.org/sparkone.
Strengthen your knowledge & skills in practice administration
Get involved in the SHM Practice Administrators’ Committee 2018 Mentor/Mentee Program.
This program helps you create relationships and serves as an outlet for you to pose questions or ideas to a seasoned hospital medicine group administrator. There are two different ways you can participate: as a less experienced administrator looking for a mentor or as a more experienced administrator looking to be paired with a peer. This program is free to members only. Not a member? Join today at hospitalmedicine.org/join.
Learn more about the program and submit your application at hospitalmedicine.org/pamentor.
Obtain an extensive insight into Hospital Medicine groups configuration and operation
SHM’s State of Hospital Medicine Report includes data collected from 600 hospital medicine groups (HMGs) representing 9,000 providers to keep you current on hospitalist compensation and production, in addition to cutting-edge knowledge covering practice demographics, staffing levels, turnover, staff growth, compensation methods, and financial support for solid, evidence-based management decisions.
The 2016 State of Hospital Medicine Report is not only in print but also available in an enhanced, fully searchable digital version. Order your copy at hospitalmedicine.org/sohm.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Early decision for Fellows applications is Sept. 15. Apply now!
SHM’s Fellows designation is a prestigious way to differentiate yourself among your peers in hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating core values of leadership, teamwork, and quality improvement.
“I was encouraged to consider SHM’s Fellow designation by other members of an SHM committee that I belonged to. Although reluctant at first, I realized that this was an opportunity to really confirm that I was a career hospitalist. The application process itself allowed me to objectively evaluate the work I had done to date and how it fit into my overall career plan. I believe that this FHM designation has fostered connections in the HM community and within my own institution that may not have been open to me before.” – Dr. Patricia Seymour, MD, FAAFP, FHM
Got research? Get noticed at HM18!
Don’t miss your chance to present your research to a national audience. SHM’s scientific abstract and poster competition, known as Research, Innovations, and Clinical Vignettes (RIV), is one of the most popular events at SHM’s annual meeting, enabling hospitalists from across the country to discuss emerging scientific and clinical cases, share feedback, and make valuable professional connections.
Hospital Medicine 2018 (HM18) will be held April 8-11, 2018 at the Orlando World Center Marriott. Many cutting-edge abstracts first presented at SHM’s RIV sessions go on to be published in respected medical journals. Yours could be next.
For more details, visit hospitalmedicine2018.org.
Know someone with exceptional achievements in Hospital Medicine?
SHM’s prestigious Awards of Excellence recognize exceptional achievements in the field of hospital medicine in the following categories:
• Excellence in Research.
• Management Excellence in Hospital Medicine.
• Outstanding Service in Hospital Medicine.
• Excellence in Teaching.
• Clinical Excellence for Physicians.
• Clinical Excellence for Nurse Practitioners and Physician Assistants.
• Excellence in Humanitarian Services.
• Excellence in Teamwork.
Awards of Excellence nominations are due on Oct. 2, 2017. Nominate yourself or a colleague today at hospitalmedicine.org/awards.
Invest in your career with SPARK ONE
SPARK ONE, SHM’s premier online self-assessment created specifically for hospital medicine professionals, is the perfect tool to help you reach your goals. The activity contains 450+ vignette-style multiple-choice questions covering 100% of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine (FPHM) exam blueprint.
This online tool is your complete resource for successfully preparing for the FPHM exam or assessing your general knowledge in hospital medicine. Used as a self-paced study guide, it engages learners through an open-book format, allowing users to review detailed learning objectives and discussion points, and define individual areas of strengths and weaknesses. Earn up to 23 AMA PRA Category 1 Credit™ and 23 MOC points.
Learn more at hospitalmedicine.org/sparkone.
Strengthen your knowledge & skills in practice administration
Get involved in the SHM Practice Administrators’ Committee 2018 Mentor/Mentee Program.
This program helps you create relationships and serves as an outlet for you to pose questions or ideas to a seasoned hospital medicine group administrator. There are two different ways you can participate: as a less experienced administrator looking for a mentor or as a more experienced administrator looking to be paired with a peer. This program is free to members only. Not a member? Join today at hospitalmedicine.org/join.
Learn more about the program and submit your application at hospitalmedicine.org/pamentor.
Obtain an extensive insight into Hospital Medicine groups configuration and operation
SHM’s State of Hospital Medicine Report includes data collected from 600 hospital medicine groups (HMGs) representing 9,000 providers to keep you current on hospitalist compensation and production, in addition to cutting-edge knowledge covering practice demographics, staffing levels, turnover, staff growth, compensation methods, and financial support for solid, evidence-based management decisions.
The 2016 State of Hospital Medicine Report is not only in print but also available in an enhanced, fully searchable digital version. Order your copy at hospitalmedicine.org/sohm.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Pityriasis Rubra Pilaris and Severe Hypereosinophilia
To the Editor:
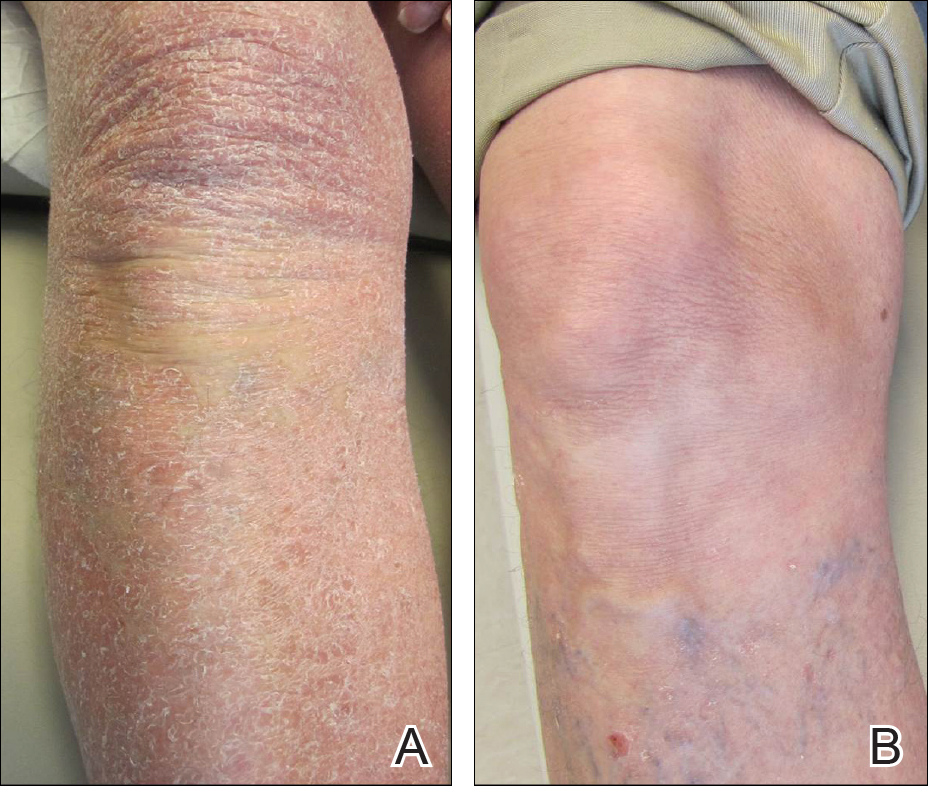
A 63-year-old man presented with a prior diagnosis of severe psoriasis affecting the extremities, neck, face, and scalp of 1 year’s duration. He reported pain, itching, and swelling in the affected areas. He felt the rash was worst on the hands and feet, and pain made performing activities of daily living difficult. His treatment regimen at presentation included triamcinolone cream 0.1% and azathioprine 150 mg daily as prescribed by an outside dermatologist without any response. Physical examination revealed diffuse erythema with lichenification and thick, white, flaking scale on the arms and legs (Figure 1A), face, neck, palms, and soles with islands of sparing. Multiple salmon-colored, follicular-based papules topped with central hyperkeratosis were scattered on these same areas. The palms and soles had severe confluent keratoderma (Figure 2A). Histologic examination of a follicular-based papule showed foci of parakeratosis and hypergranulosis consistent with the patient’s clinical picture of pityriasis rubra pilaris (PRP).
Baseline laboratory tests at the time of PRP diagnosis revealed 20.8% eosinophils (reference range, 0%–7%) and an absolute eosinophil count of 2.17×109/L (reference range, 0–0.7×109/L). Laboratory test results from an outside dermatologist conducted 10 to 12 months prior to the current presentation showed 12% eosinophils with a white blood cell count of 8.9×109/L (reference range, 4.5–11.0×109/L) around the time of rash onset and before treatment with azathioprine, making a drug reaction an unlikely cause of the eosinophilia.
After consulting with the hematology department, a hypereosinophilia workup including erythrocyte sedimentation rate, lactate dehydrogenase, serum protein electrophoresis, urine protein electrophoresis, tryptase, double-stranded DNA antibody, human T-lymphotrophic virus I/II, stool ova, and parasites, as well as a Strongyloides antibody titer, were performed; all were within reference range. His antinuclear antibody level was mildly elevated at 1:160, but the patient had no clinical manifestations of lupus. Given this negative workup, the most likely explanation for the hypereosinophilia was a reactive process secondary to the extreme inflammatory state.

The patient was started on isotretinoin 40 mg daily in addition to urea cream 40% mixed with clobetasol ointment at least once daily to the extremities. Hydrocortisone ointment 2.5% and petrolatum-based ointment were applied to the face, and hydroxyzine was used as needed for pruritus. One month after initiating isotretinoin, erythema had decreased and a repeat complete blood cell count with differential showed a decrease of eosinophils to 14.7% and an absolute eosinophil count of 1.56×109/L. After 2 months of therapy, the patient showed remarkable improvement. After 3.5 months of therapy, the keratoderma on the palms and soles was almost completely resolved, the follicular-based papules disappeared, and the patient had no areas of lichenification (Figures 1B and 2B). After 5 months of therapy, the patient experienced resolution of the PRP, except for residual facial erythema. His eosinophil count continued to trend downward during these 5 months, reaching 7.6% with an absolute eosinophil count of 0.93×109/L. Three years after the initial onset of the rash and 2 years after completing isotretinoin, his eosinophil level was normal at 5.3% with an absolute eosinophil count of 0.7×109/L.
We present a case of PRP and severe eosinophilia. We initially considered a second disease process to explain the extremely elevated eosinophil count; however, a negative eosinophilia workup and simultaneous resolution of these problems suggest that the eosinophilia was related to the severity of the PRP.
To the Editor:
A 63-year-old man presented with a prior diagnosis of severe psoriasis affecting the extremities, neck, face, and scalp of 1 year’s duration. He reported pain, itching, and swelling in the affected areas. He felt the rash was worst on the hands and feet, and pain made performing activities of daily living difficult. His treatment regimen at presentation included triamcinolone cream 0.1% and azathioprine 150 mg daily as prescribed by an outside dermatologist without any response. Physical examination revealed diffuse erythema with lichenification and thick, white, flaking scale on the arms and legs (Figure 1A), face, neck, palms, and soles with islands of sparing. Multiple salmon-colored, follicular-based papules topped with central hyperkeratosis were scattered on these same areas. The palms and soles had severe confluent keratoderma (Figure 2A). Histologic examination of a follicular-based papule showed foci of parakeratosis and hypergranulosis consistent with the patient’s clinical picture of pityriasis rubra pilaris (PRP).

Baseline laboratory tests at the time of PRP diagnosis revealed 20.8% eosinophils (reference range, 0%–7%) and an absolute eosinophil count of 2.17×109/L (reference range, 0–0.7×109/L). Laboratory test results from an outside dermatologist conducted 10 to 12 months prior to the current presentation showed 12% eosinophils with a white blood cell count of 8.9×109/L (reference range, 4.5–11.0×109/L) around the time of rash onset and before treatment with azathioprine, making a drug reaction an unlikely cause of the eosinophilia.
After consulting with the hematology department, a hypereosinophilia workup including erythrocyte sedimentation rate, lactate dehydrogenase, serum protein electrophoresis, urine protein electrophoresis, tryptase, double-stranded DNA antibody, human T-lymphotrophic virus I/II, stool ova, and parasites, as well as a Strongyloides antibody titer, were performed; all were within reference range. His antinuclear antibody level was mildly elevated at 1:160, but the patient had no clinical manifestations of lupus. Given this negative workup, the most likely explanation for the hypereosinophilia was a reactive process secondary to the extreme inflammatory state.

The patient was started on isotretinoin 40 mg daily in addition to urea cream 40% mixed with clobetasol ointment at least once daily to the extremities. Hydrocortisone ointment 2.5% and petrolatum-based ointment were applied to the face, and hydroxyzine was used as needed for pruritus. One month after initiating isotretinoin, erythema had decreased and a repeat complete blood cell count with differential showed a decrease of eosinophils to 14.7% and an absolute eosinophil count of 1.56×109/L. After 2 months of therapy, the patient showed remarkable improvement. After 3.5 months of therapy, the keratoderma on the palms and soles was almost completely resolved, the follicular-based papules disappeared, and the patient had no areas of lichenification (Figures 1B and 2B). After 5 months of therapy, the patient experienced resolution of the PRP, except for residual facial erythema. His eosinophil count continued to trend downward during these 5 months, reaching 7.6% with an absolute eosinophil count of 0.93×109/L. Three years after the initial onset of the rash and 2 years after completing isotretinoin, his eosinophil level was normal at 5.3% with an absolute eosinophil count of 0.7×109/L.
We present a case of PRP and severe eosinophilia. We initially considered a second disease process to explain the extremely elevated eosinophil count; however, a negative eosinophilia workup and simultaneous resolution of these problems suggest that the eosinophilia was related to the severity of the PRP.
To the Editor:
A 63-year-old man presented with a prior diagnosis of severe psoriasis affecting the extremities, neck, face, and scalp of 1 year’s duration. He reported pain, itching, and swelling in the affected areas. He felt the rash was worst on the hands and feet, and pain made performing activities of daily living difficult. His treatment regimen at presentation included triamcinolone cream 0.1% and azathioprine 150 mg daily as prescribed by an outside dermatologist without any response. Physical examination revealed diffuse erythema with lichenification and thick, white, flaking scale on the arms and legs (Figure 1A), face, neck, palms, and soles with islands of sparing. Multiple salmon-colored, follicular-based papules topped with central hyperkeratosis were scattered on these same areas. The palms and soles had severe confluent keratoderma (Figure 2A). Histologic examination of a follicular-based papule showed foci of parakeratosis and hypergranulosis consistent with the patient’s clinical picture of pityriasis rubra pilaris (PRP).

Baseline laboratory tests at the time of PRP diagnosis revealed 20.8% eosinophils (reference range, 0%–7%) and an absolute eosinophil count of 2.17×109/L (reference range, 0–0.7×109/L). Laboratory test results from an outside dermatologist conducted 10 to 12 months prior to the current presentation showed 12% eosinophils with a white blood cell count of 8.9×109/L (reference range, 4.5–11.0×109/L) around the time of rash onset and before treatment with azathioprine, making a drug reaction an unlikely cause of the eosinophilia.
After consulting with the hematology department, a hypereosinophilia workup including erythrocyte sedimentation rate, lactate dehydrogenase, serum protein electrophoresis, urine protein electrophoresis, tryptase, double-stranded DNA antibody, human T-lymphotrophic virus I/II, stool ova, and parasites, as well as a Strongyloides antibody titer, were performed; all were within reference range. His antinuclear antibody level was mildly elevated at 1:160, but the patient had no clinical manifestations of lupus. Given this negative workup, the most likely explanation for the hypereosinophilia was a reactive process secondary to the extreme inflammatory state.

The patient was started on isotretinoin 40 mg daily in addition to urea cream 40% mixed with clobetasol ointment at least once daily to the extremities. Hydrocortisone ointment 2.5% and petrolatum-based ointment were applied to the face, and hydroxyzine was used as needed for pruritus. One month after initiating isotretinoin, erythema had decreased and a repeat complete blood cell count with differential showed a decrease of eosinophils to 14.7% and an absolute eosinophil count of 1.56×109/L. After 2 months of therapy, the patient showed remarkable improvement. After 3.5 months of therapy, the keratoderma on the palms and soles was almost completely resolved, the follicular-based papules disappeared, and the patient had no areas of lichenification (Figures 1B and 2B). After 5 months of therapy, the patient experienced resolution of the PRP, except for residual facial erythema. His eosinophil count continued to trend downward during these 5 months, reaching 7.6% with an absolute eosinophil count of 0.93×109/L. Three years after the initial onset of the rash and 2 years after completing isotretinoin, his eosinophil level was normal at 5.3% with an absolute eosinophil count of 0.7×109/L.
We present a case of PRP and severe eosinophilia. We initially considered a second disease process to explain the extremely elevated eosinophil count; however, a negative eosinophilia workup and simultaneous resolution of these problems suggest that the eosinophilia was related to the severity of the PRP.
Practice Points
- Pityriasis rubra pilaris (PRP) can clinically mimic psoriasis. Look for islands of sparing and palmar and plantar hyperkeratosis to help diagnose PRP. A biopsy may be useful to help with this differentiation.
- Pityriasis rubra pilaris may be associated with eosinophilia, but one should rule out other causes of eosinophilia first.
Sporotrichoid Pattern of Mycobacterium chelonae-abscessus Infection
To the Editor:
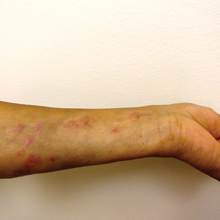
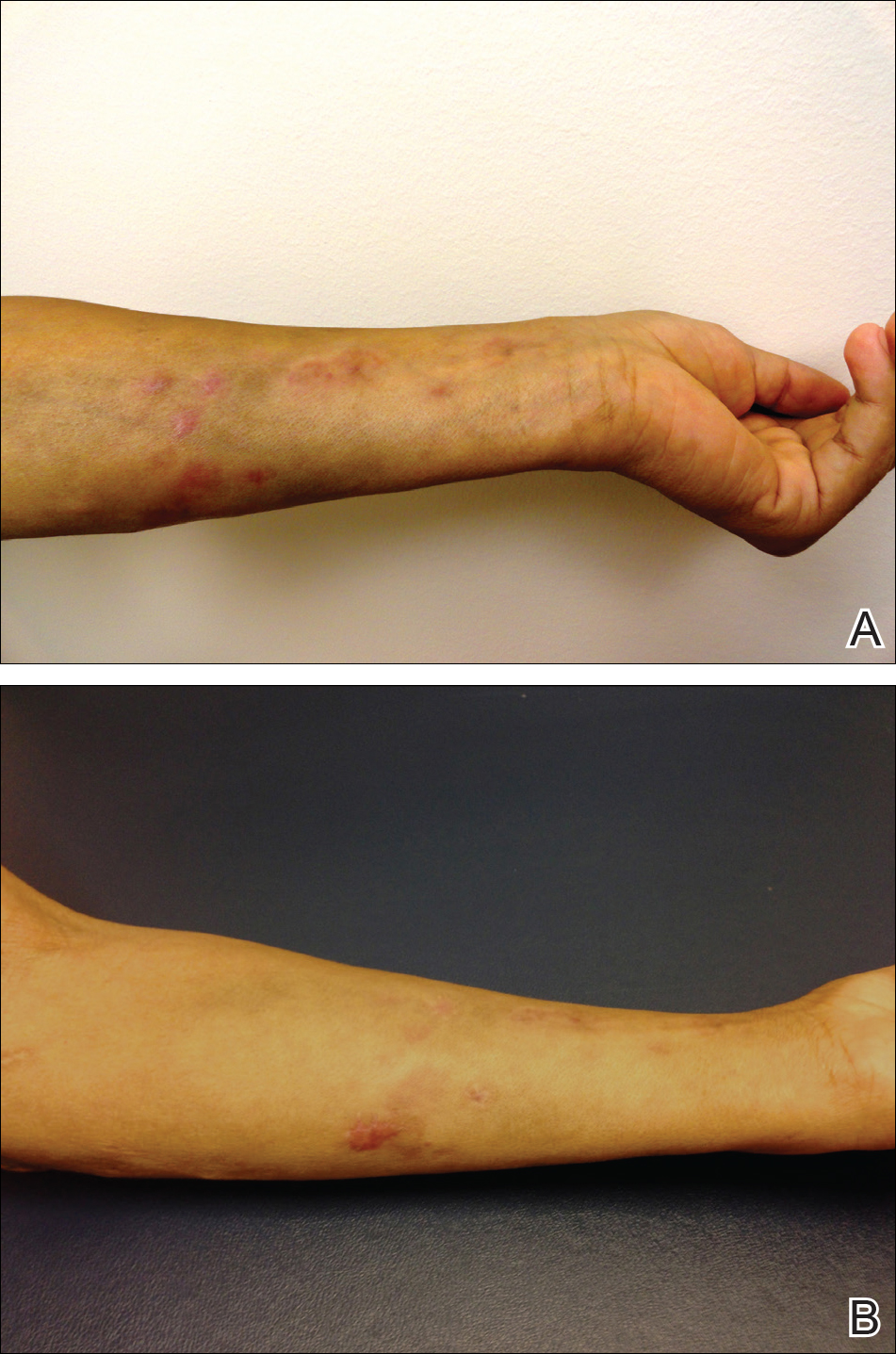
We present a case of Mycobacterium chelonae-abscessus cutaneous infection in a sporotrichoid pattern, a rare presentation most often found in immunocompromised patients. A 34-year-old man with lupus nephritis who was taking oral prednisone, mycophenolate mofetil, and hydroxychloroquine presented with multiple erythematous fluctuant nodules and plaques on the left volar forearm in a sporotrichoid pattern of 3 months’ duration (Figure, A). He denied recent travel, exposure to fish or fish tanks, and penetrating wounds. Punch biopsy showed granulomatous inflammation and scarring with negative tissue cultures. Repeat biopsies and cultures were obtained when the lesions increased in number over 2 months.
Final biopsy showed upper dermal granulomatous inflammation with karyorrhectic debris, suggesting infection, and acid-fast bacilli. Culture grew M chelonae-abscessus on Löwenstein-Jensen agar at 37°C and blood culture media from which the complex was identified using high-performance liquid chromatography. Empiric therapy with renal dosing based on the Infectious Diseases Society of America statement of susceptibilities1 was initiated with clarithromycin, doxycycline, and ciprofloxacin for 4 months. Furthermore, the prednisone dose was tapered to 7.5 mg daily. Two months later, the lesions regressed and ciprofloxacin was discontinued (Figure, B).
The sporotrichoid spread of nodules suggests infection with mycobacteria, Sporothrix schenckii, Leishmania, Francisella tularensis, or Nocardia. Most cultures for nontuberculous mycobacteria will grow on Löwenstein-Jensen agar between 28°C and 37°C. Runyon rapidly growing (group IV) mycobacteria are defined by their ubiquitous presence in the environment and ability to develop colonies in 7 days.2 Cutaneous infections are increasing in prevalence, as reported in a retrospective study spanning nearly 30 years.3 The presentation is variable but often includes the distal extremities and usually is a nodule, ulcer, or abscess at a single site; a sporotrichoid pattern is more rare. Preceding skin trauma is the major risk factor for immunocompetent hosts, and the infection can spontaneously resolve in 8 to 12 months.1 In contrast, immunosuppressed patients may have no known source of infection and often have a progressive course with an increasing number of lesions and increased time until clearance.4
It is difficult to differentiate M chelonae and M abscessus based on growth characteristics, and they share the same 16S ribosomal RNA sequence commonly used to differentiate other mycobacterial species.2Mycobacterium abscessus can be more difficult to treat, thus distinction via polymerase chain reaction of the heat-shock protein 65 gene, hsp65, can be valuable in cases recalcitrant to initial therapy.1
The likelihood of M chelonae and M abscessus isolates to be initially sensitive to clarithromycin is 100%,1 and this antibiotic remains the cornerstone of therapy. A clinical trial of treatments for M chelonae-abscessus found that clarithromycin monotherapy can be successful or complicated by resistance5; therefore, multidrug therapy is recommended. The antibiotic regimen for our patient was chosen to limit renal toxicity.
In summary, we report a case of M chelonae-abscessus cutaneous infection in a sporotrichoid pattern in a patient with lupus nephritis on immunosuppressive drugs. As the incidence of rapidly growing mycobacterial cutaneous infections rises, dermatologists must be aware of this pattern of infection.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- De Groote MA, Huitt G. Infections due to rapidly-growing Mycobacteria. Clin Infect Dis. 2006;42:1756-1763.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010:37:965-972.
- Wallace RJ, Tanner D, Brennan PJ, et al. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482-486.
To the Editor:
We present a case of Mycobacterium chelonae-abscessus cutaneous infection in a sporotrichoid pattern, a rare presentation most often found in immunocompromised patients. A 34-year-old man with lupus nephritis who was taking oral prednisone, mycophenolate mofetil, and hydroxychloroquine presented with multiple erythematous fluctuant nodules and plaques on the left volar forearm in a sporotrichoid pattern of 3 months’ duration (Figure, A). He denied recent travel, exposure to fish or fish tanks, and penetrating wounds. Punch biopsy showed granulomatous inflammation and scarring with negative tissue cultures. Repeat biopsies and cultures were obtained when the lesions increased in number over 2 months.
Final biopsy showed upper dermal granulomatous inflammation with karyorrhectic debris, suggesting infection, and acid-fast bacilli. Culture grew M chelonae-abscessus on Löwenstein-Jensen agar at 37°C and blood culture media from which the complex was identified using high-performance liquid chromatography. Empiric therapy with renal dosing based on the Infectious Diseases Society of America statement of susceptibilities1 was initiated with clarithromycin, doxycycline, and ciprofloxacin for 4 months. Furthermore, the prednisone dose was tapered to 7.5 mg daily. Two months later, the lesions regressed and ciprofloxacin was discontinued (Figure, B).

The sporotrichoid spread of nodules suggests infection with mycobacteria, Sporothrix schenckii, Leishmania, Francisella tularensis, or Nocardia. Most cultures for nontuberculous mycobacteria will grow on Löwenstein-Jensen agar between 28°C and 37°C. Runyon rapidly growing (group IV) mycobacteria are defined by their ubiquitous presence in the environment and ability to develop colonies in 7 days.2 Cutaneous infections are increasing in prevalence, as reported in a retrospective study spanning nearly 30 years.3 The presentation is variable but often includes the distal extremities and usually is a nodule, ulcer, or abscess at a single site; a sporotrichoid pattern is more rare. Preceding skin trauma is the major risk factor for immunocompetent hosts, and the infection can spontaneously resolve in 8 to 12 months.1 In contrast, immunosuppressed patients may have no known source of infection and often have a progressive course with an increasing number of lesions and increased time until clearance.4
It is difficult to differentiate M chelonae and M abscessus based on growth characteristics, and they share the same 16S ribosomal RNA sequence commonly used to differentiate other mycobacterial species.2Mycobacterium abscessus can be more difficult to treat, thus distinction via polymerase chain reaction of the heat-shock protein 65 gene, hsp65, can be valuable in cases recalcitrant to initial therapy.1
The likelihood of M chelonae and M abscessus isolates to be initially sensitive to clarithromycin is 100%,1 and this antibiotic remains the cornerstone of therapy. A clinical trial of treatments for M chelonae-abscessus found that clarithromycin monotherapy can be successful or complicated by resistance5; therefore, multidrug therapy is recommended. The antibiotic regimen for our patient was chosen to limit renal toxicity.
In summary, we report a case of M chelonae-abscessus cutaneous infection in a sporotrichoid pattern in a patient with lupus nephritis on immunosuppressive drugs. As the incidence of rapidly growing mycobacterial cutaneous infections rises, dermatologists must be aware of this pattern of infection.
To the Editor:
We present a case of Mycobacterium chelonae-abscessus cutaneous infection in a sporotrichoid pattern, a rare presentation most often found in immunocompromised patients. A 34-year-old man with lupus nephritis who was taking oral prednisone, mycophenolate mofetil, and hydroxychloroquine presented with multiple erythematous fluctuant nodules and plaques on the left volar forearm in a sporotrichoid pattern of 3 months’ duration (Figure, A). He denied recent travel, exposure to fish or fish tanks, and penetrating wounds. Punch biopsy showed granulomatous inflammation and scarring with negative tissue cultures. Repeat biopsies and cultures were obtained when the lesions increased in number over 2 months.
Final biopsy showed upper dermal granulomatous inflammation with karyorrhectic debris, suggesting infection, and acid-fast bacilli. Culture grew M chelonae-abscessus on Löwenstein-Jensen agar at 37°C and blood culture media from which the complex was identified using high-performance liquid chromatography. Empiric therapy with renal dosing based on the Infectious Diseases Society of America statement of susceptibilities1 was initiated with clarithromycin, doxycycline, and ciprofloxacin for 4 months. Furthermore, the prednisone dose was tapered to 7.5 mg daily. Two months later, the lesions regressed and ciprofloxacin was discontinued (Figure, B).

The sporotrichoid spread of nodules suggests infection with mycobacteria, Sporothrix schenckii, Leishmania, Francisella tularensis, or Nocardia. Most cultures for nontuberculous mycobacteria will grow on Löwenstein-Jensen agar between 28°C and 37°C. Runyon rapidly growing (group IV) mycobacteria are defined by their ubiquitous presence in the environment and ability to develop colonies in 7 days.2 Cutaneous infections are increasing in prevalence, as reported in a retrospective study spanning nearly 30 years.3 The presentation is variable but often includes the distal extremities and usually is a nodule, ulcer, or abscess at a single site; a sporotrichoid pattern is more rare. Preceding skin trauma is the major risk factor for immunocompetent hosts, and the infection can spontaneously resolve in 8 to 12 months.1 In contrast, immunosuppressed patients may have no known source of infection and often have a progressive course with an increasing number of lesions and increased time until clearance.4
It is difficult to differentiate M chelonae and M abscessus based on growth characteristics, and they share the same 16S ribosomal RNA sequence commonly used to differentiate other mycobacterial species.2Mycobacterium abscessus can be more difficult to treat, thus distinction via polymerase chain reaction of the heat-shock protein 65 gene, hsp65, can be valuable in cases recalcitrant to initial therapy.1
The likelihood of M chelonae and M abscessus isolates to be initially sensitive to clarithromycin is 100%,1 and this antibiotic remains the cornerstone of therapy. A clinical trial of treatments for M chelonae-abscessus found that clarithromycin monotherapy can be successful or complicated by resistance5; therefore, multidrug therapy is recommended. The antibiotic regimen for our patient was chosen to limit renal toxicity.
In summary, we report a case of M chelonae-abscessus cutaneous infection in a sporotrichoid pattern in a patient with lupus nephritis on immunosuppressive drugs. As the incidence of rapidly growing mycobacterial cutaneous infections rises, dermatologists must be aware of this pattern of infection.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- De Groote MA, Huitt G. Infections due to rapidly-growing Mycobacteria. Clin Infect Dis. 2006;42:1756-1763.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010:37:965-972.
- Wallace RJ, Tanner D, Brennan PJ, et al. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482-486.
- Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- De Groote MA, Huitt G. Infections due to rapidly-growing Mycobacteria. Clin Infect Dis. 2006;42:1756-1763.
- Wentworth AB, Drage LA, Wengenack NL, et al. Increased incidence of cutaneous nontuberculous mycobacterial infection, 1980 to 2009: a population-based study. Mayo Clin Proc. 2013;88:38-45.
- Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010:37:965-972.
- Wallace RJ, Tanner D, Brennan PJ, et al. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482-486.
Practice Points
- Dermatologists should consider atypical mycobacterial infections, including rapidly growing mycobacteria, in the differential diagnosis for lesions with sporotrichoid-pattern spread.
- Multidrug therapy often is required for treatment of infection caused by Mycobacteria chelonae-abscessus complex.
FDA moves to guard against abuse of ‘orphan drug’ program
The Food and Drug Administration is changing the way it approves orphan drugs after revelations that drugmakers may be abusing a law intended to help patients with rare diseases.
In a blog post Sept. 12, FDA Commissioner Scott Gottlieb, MD, said he wants to ensure financial incentives are granted “in a way that’s consistent with the manner Congress intended” when the Orphan Drug Act was passed in 1983. That legislation gave drugmakers a package of incentives, including tax credits, user-fee waivers and 7 years of market exclusivity if they developed medicines for rare diseases.
A Kaiser Health News investigation published in January 2017 found many drugs that now have orphan status aren’t entirely new. Of about 450 drugs that have won orphan approval since 1983, more than 70 were drugs first approved by the FDA for mass-market use. Those include rosuvastatin (Crestor), aripiprazole (Abilify), and adalimumab (Humira), the world’s best-selling drug.
Dr. Gottlieb announced plans to close a loophole that allows manufacturers to skip pediatric testing requirements when developing a common-disease drug for orphan use in children. He also signaled that bigger changes are being considered, announcing a public meeting to explore issues raised by scientific advances, such as the increase in precision medicine and biologics.
“We need to make sure our policies take notice of all of these new challenges and opportunities,” he wrote. Dr. Gottlieb, through his agency, declined multiple requests for interviews.
Over the years, drugmakers have fueled a boom in orphan drugs, which often carry six-figure price tags. Nearly half of the new drugs approved by the FDA are now for rare diseases – even though many of them also treat and are marketed for common diseases.
Dr. Gottlieb became commissioner in May, a few months after three key Republican senators called for a federal investigation into potential abuses of the Orphan Drug Act, and the Government Accountability Office agreed to investigate.
The GAO has yet to begin its investigation, saying it doesn’t expect to start work until late this year, when staff is available. Regardless, in late June, Dr. Gottlieb announced what would be the first in a series of updates that shift the way the FDA handles orphan drugs.
Those include:
- Eliminating a backlog in drug applications for orphan designation or status. Getting a designation is a critical first step if a company wants to win orphan incentives once the drug is approved for treatment use. And, much like the rise in approvals, the requests by companies to get drugs designated with orphan status has also skyrocketed. Dr. Gottlieb said in June that he wanted to get rid of the backlog; his blog post noted the effort was complete. About half of the 200 applications from drugmakers won orphan status.
- Mandating that drugmakers prove their medicine is clinically superior before getting the market exclusivity that comes with orphan drug status. The agency had lost a lawsuit in which a company said it was owed the exclusivity period regardless of whether its medicine was better. And two more lawsuits had been filed by Eagle Pharmaceuticals and United Therapeutics. The FDA Reauthorization Act, which passed in August, made it law that a drug has to be clinically superior to get the incentives.
- Closing the loophole for pediatric orphan drugs by requiring all drugs approved for common adult diseases, like inflammatory bowel disease, undergo pediatric testing when getting approval as a pediatric orphan drug. Pediatric testing is not required for orphan drugs, and last month Congress mandated that orphan drugs for cancer be tested for children. Still, the American Academy of Pediatrics celebrated the proposed change but warned it was only a first step. Bridgette Jones, MD, chair of American Academy of Pediatrics Committee on Drugs, said Sept. 12 that orphan drugs are “still mostly exempt from pediatric study requirements … children deserve access to safe, effective medications.”
Martin Makary, MD, who wrote a critical 2015 paper on orphan approvals, said the changes at the agency indicate that Dr. Gottlieb seems “concerned about all the right things. The government does a lot of lip service in general. This is not lip service.”
The restructuring has been swift in some ways.
Sandra Heibel, PhD, a senior consultant at Haffner Associates, a firm that helps companies submit orphan drug applications, noted that the approval process for designations definitely sped up over the summer, and “we are absolutely getting responses from the FDA back in 90 days. That has come through.”
Other changes to the agency, though, will evolve slowly. For example, the orphan drug office has begun reaching across the FDA’s divisions for help in reviewing drugs. In May, the FDA’s orphan reviews began to work with the office of pediatric therapeutics to review pediatric applications – ideally increasing the expertise applied when considering a company’s request for orphan drug use in children.
In an interview, FDA confirmed that Dr. Gottlieb’s orphan modernization plan is part of a larger effort to increase competition and decrease drug prices. One focus is on targeted drugs – especially those that affect rare diseases or diseases for which there is no effective therapy, the agency said.
“Such drugs present some of the biggest opportunities in medicine to treat and cure debilitating and very costly diseases,” the agency stated.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
The Food and Drug Administration is changing the way it approves orphan drugs after revelations that drugmakers may be abusing a law intended to help patients with rare diseases.
In a blog post Sept. 12, FDA Commissioner Scott Gottlieb, MD, said he wants to ensure financial incentives are granted “in a way that’s consistent with the manner Congress intended” when the Orphan Drug Act was passed in 1983. That legislation gave drugmakers a package of incentives, including tax credits, user-fee waivers and 7 years of market exclusivity if they developed medicines for rare diseases.
A Kaiser Health News investigation published in January 2017 found many drugs that now have orphan status aren’t entirely new. Of about 450 drugs that have won orphan approval since 1983, more than 70 were drugs first approved by the FDA for mass-market use. Those include rosuvastatin (Crestor), aripiprazole (Abilify), and adalimumab (Humira), the world’s best-selling drug.
Dr. Gottlieb announced plans to close a loophole that allows manufacturers to skip pediatric testing requirements when developing a common-disease drug for orphan use in children. He also signaled that bigger changes are being considered, announcing a public meeting to explore issues raised by scientific advances, such as the increase in precision medicine and biologics.
“We need to make sure our policies take notice of all of these new challenges and opportunities,” he wrote. Dr. Gottlieb, through his agency, declined multiple requests for interviews.
Over the years, drugmakers have fueled a boom in orphan drugs, which often carry six-figure price tags. Nearly half of the new drugs approved by the FDA are now for rare diseases – even though many of them also treat and are marketed for common diseases.
Dr. Gottlieb became commissioner in May, a few months after three key Republican senators called for a federal investigation into potential abuses of the Orphan Drug Act, and the Government Accountability Office agreed to investigate.
The GAO has yet to begin its investigation, saying it doesn’t expect to start work until late this year, when staff is available. Regardless, in late June, Dr. Gottlieb announced what would be the first in a series of updates that shift the way the FDA handles orphan drugs.
Those include:
- Eliminating a backlog in drug applications for orphan designation or status. Getting a designation is a critical first step if a company wants to win orphan incentives once the drug is approved for treatment use. And, much like the rise in approvals, the requests by companies to get drugs designated with orphan status has also skyrocketed. Dr. Gottlieb said in June that he wanted to get rid of the backlog; his blog post noted the effort was complete. About half of the 200 applications from drugmakers won orphan status.
- Mandating that drugmakers prove their medicine is clinically superior before getting the market exclusivity that comes with orphan drug status. The agency had lost a lawsuit in which a company said it was owed the exclusivity period regardless of whether its medicine was better. And two more lawsuits had been filed by Eagle Pharmaceuticals and United Therapeutics. The FDA Reauthorization Act, which passed in August, made it law that a drug has to be clinically superior to get the incentives.
- Closing the loophole for pediatric orphan drugs by requiring all drugs approved for common adult diseases, like inflammatory bowel disease, undergo pediatric testing when getting approval as a pediatric orphan drug. Pediatric testing is not required for orphan drugs, and last month Congress mandated that orphan drugs for cancer be tested for children. Still, the American Academy of Pediatrics celebrated the proposed change but warned it was only a first step. Bridgette Jones, MD, chair of American Academy of Pediatrics Committee on Drugs, said Sept. 12 that orphan drugs are “still mostly exempt from pediatric study requirements … children deserve access to safe, effective medications.”
Martin Makary, MD, who wrote a critical 2015 paper on orphan approvals, said the changes at the agency indicate that Dr. Gottlieb seems “concerned about all the right things. The government does a lot of lip service in general. This is not lip service.”
The restructuring has been swift in some ways.
Sandra Heibel, PhD, a senior consultant at Haffner Associates, a firm that helps companies submit orphan drug applications, noted that the approval process for designations definitely sped up over the summer, and “we are absolutely getting responses from the FDA back in 90 days. That has come through.”
Other changes to the agency, though, will evolve slowly. For example, the orphan drug office has begun reaching across the FDA’s divisions for help in reviewing drugs. In May, the FDA’s orphan reviews began to work with the office of pediatric therapeutics to review pediatric applications – ideally increasing the expertise applied when considering a company’s request for orphan drug use in children.
In an interview, FDA confirmed that Dr. Gottlieb’s orphan modernization plan is part of a larger effort to increase competition and decrease drug prices. One focus is on targeted drugs – especially those that affect rare diseases or diseases for which there is no effective therapy, the agency said.
“Such drugs present some of the biggest opportunities in medicine to treat and cure debilitating and very costly diseases,” the agency stated.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
The Food and Drug Administration is changing the way it approves orphan drugs after revelations that drugmakers may be abusing a law intended to help patients with rare diseases.
In a blog post Sept. 12, FDA Commissioner Scott Gottlieb, MD, said he wants to ensure financial incentives are granted “in a way that’s consistent with the manner Congress intended” when the Orphan Drug Act was passed in 1983. That legislation gave drugmakers a package of incentives, including tax credits, user-fee waivers and 7 years of market exclusivity if they developed medicines for rare diseases.
A Kaiser Health News investigation published in January 2017 found many drugs that now have orphan status aren’t entirely new. Of about 450 drugs that have won orphan approval since 1983, more than 70 were drugs first approved by the FDA for mass-market use. Those include rosuvastatin (Crestor), aripiprazole (Abilify), and adalimumab (Humira), the world’s best-selling drug.
Dr. Gottlieb announced plans to close a loophole that allows manufacturers to skip pediatric testing requirements when developing a common-disease drug for orphan use in children. He also signaled that bigger changes are being considered, announcing a public meeting to explore issues raised by scientific advances, such as the increase in precision medicine and biologics.
“We need to make sure our policies take notice of all of these new challenges and opportunities,” he wrote. Dr. Gottlieb, through his agency, declined multiple requests for interviews.
Over the years, drugmakers have fueled a boom in orphan drugs, which often carry six-figure price tags. Nearly half of the new drugs approved by the FDA are now for rare diseases – even though many of them also treat and are marketed for common diseases.
Dr. Gottlieb became commissioner in May, a few months after three key Republican senators called for a federal investigation into potential abuses of the Orphan Drug Act, and the Government Accountability Office agreed to investigate.
The GAO has yet to begin its investigation, saying it doesn’t expect to start work until late this year, when staff is available. Regardless, in late June, Dr. Gottlieb announced what would be the first in a series of updates that shift the way the FDA handles orphan drugs.
Those include:
- Eliminating a backlog in drug applications for orphan designation or status. Getting a designation is a critical first step if a company wants to win orphan incentives once the drug is approved for treatment use. And, much like the rise in approvals, the requests by companies to get drugs designated with orphan status has also skyrocketed. Dr. Gottlieb said in June that he wanted to get rid of the backlog; his blog post noted the effort was complete. About half of the 200 applications from drugmakers won orphan status.
- Mandating that drugmakers prove their medicine is clinically superior before getting the market exclusivity that comes with orphan drug status. The agency had lost a lawsuit in which a company said it was owed the exclusivity period regardless of whether its medicine was better. And two more lawsuits had been filed by Eagle Pharmaceuticals and United Therapeutics. The FDA Reauthorization Act, which passed in August, made it law that a drug has to be clinically superior to get the incentives.
- Closing the loophole for pediatric orphan drugs by requiring all drugs approved for common adult diseases, like inflammatory bowel disease, undergo pediatric testing when getting approval as a pediatric orphan drug. Pediatric testing is not required for orphan drugs, and last month Congress mandated that orphan drugs for cancer be tested for children. Still, the American Academy of Pediatrics celebrated the proposed change but warned it was only a first step. Bridgette Jones, MD, chair of American Academy of Pediatrics Committee on Drugs, said Sept. 12 that orphan drugs are “still mostly exempt from pediatric study requirements … children deserve access to safe, effective medications.”
Martin Makary, MD, who wrote a critical 2015 paper on orphan approvals, said the changes at the agency indicate that Dr. Gottlieb seems “concerned about all the right things. The government does a lot of lip service in general. This is not lip service.”
The restructuring has been swift in some ways.
Sandra Heibel, PhD, a senior consultant at Haffner Associates, a firm that helps companies submit orphan drug applications, noted that the approval process for designations definitely sped up over the summer, and “we are absolutely getting responses from the FDA back in 90 days. That has come through.”
Other changes to the agency, though, will evolve slowly. For example, the orphan drug office has begun reaching across the FDA’s divisions for help in reviewing drugs. In May, the FDA’s orphan reviews began to work with the office of pediatric therapeutics to review pediatric applications – ideally increasing the expertise applied when considering a company’s request for orphan drug use in children.
In an interview, FDA confirmed that Dr. Gottlieb’s orphan modernization plan is part of a larger effort to increase competition and decrease drug prices. One focus is on targeted drugs – especially those that affect rare diseases or diseases for which there is no effective therapy, the agency said.
“Such drugs present some of the biggest opportunities in medicine to treat and cure debilitating and very costly diseases,” the agency stated.
Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
Predicting Efficacy of AEDs in Children: Extrapolation of Data From Adult Clinical Trials
Click here to Read Supplement.
Topics include:
- The Pediatric Epilepsy Academic Consortium for Extrapolation (PEACE) initiative
- Ramifications for future pediatric AED approvals by the FDA
- Future PEACE initiatives
Authors:
O’NEILL D’CRUZ, MD, MBA, FAAN
Pediatric Epileptologist and Neurologist
OD Consulting and Neurological Services, PLLC
Chapel Hill, North Carolina
JOHN B. BODENSTEINER, MD
Consultant in Child and Adolescent Neurology and
Professor of Neurology and Pediatrics
Mayo Clinic and Mayo Medical School
Rochester, Minnesota
Click here to Read Supplement.
Topics include:
- The Pediatric Epilepsy Academic Consortium for Extrapolation (PEACE) initiative
- Ramifications for future pediatric AED approvals by the FDA
- Future PEACE initiatives
Authors:
O’NEILL D’CRUZ, MD, MBA, FAAN
Pediatric Epileptologist and Neurologist
OD Consulting and Neurological Services, PLLC
Chapel Hill, North Carolina
JOHN B. BODENSTEINER, MD
Consultant in Child and Adolescent Neurology and
Professor of Neurology and Pediatrics
Mayo Clinic and Mayo Medical School
Rochester, Minnesota
Click here to Read Supplement.
Topics include:
- The Pediatric Epilepsy Academic Consortium for Extrapolation (PEACE) initiative
- Ramifications for future pediatric AED approvals by the FDA
- Future PEACE initiatives
Authors:
O’NEILL D’CRUZ, MD, MBA, FAAN
Pediatric Epileptologist and Neurologist
OD Consulting and Neurological Services, PLLC
Chapel Hill, North Carolina
JOHN B. BODENSTEINER, MD
Consultant in Child and Adolescent Neurology and
Professor of Neurology and Pediatrics
Mayo Clinic and Mayo Medical School
Rochester, Minnesota
13th Annual AVAHO Meeting Set to Begin
This year’s annual Assocaition of VA Hematology and Oncology (AVAHO) conference offers nearly 50 sessions over the course of 3 days that focus on providing evidence-based hematology and oncology practice. According to AVAHO President Mary Thomas, MS, “I am hopeful that people come away from this meeting with a different conception of evidence-based practice and how they might apply it.”
John P.A. Ioannidis, MD, DSc, the C. F. Rehnborg Professor in Disease Prevention in the School of Medicine and Professor of Health Research and Policy (Epidemiology) at Stanford University will offer the opening keynote address on integrating evidence into patient care. According to Ms. Thomas, Dr. Ioannidis is a “provocative speaker” who may challenge conceptions and execution of evidence-based practice. Other keynote addresses will be presented by Steve Harvey, vice president of IBM Watson Health, to discuss the use of artificial intelligence in health care and specifically cancer care and playwright Maggie Edson, winner of a Pulitzer Prize for the play “Wit.” Ms. Edson’s presentation should provide a unique look at the patient experience and a new consideration of patients’ perspectives and values.
The full agenda can be found here: http://www.avaho.org/2017-annual-meeting.html
This year’s annual Assocaition of VA Hematology and Oncology (AVAHO) conference offers nearly 50 sessions over the course of 3 days that focus on providing evidence-based hematology and oncology practice. According to AVAHO President Mary Thomas, MS, “I am hopeful that people come away from this meeting with a different conception of evidence-based practice and how they might apply it.”
John P.A. Ioannidis, MD, DSc, the C. F. Rehnborg Professor in Disease Prevention in the School of Medicine and Professor of Health Research and Policy (Epidemiology) at Stanford University will offer the opening keynote address on integrating evidence into patient care. According to Ms. Thomas, Dr. Ioannidis is a “provocative speaker” who may challenge conceptions and execution of evidence-based practice. Other keynote addresses will be presented by Steve Harvey, vice president of IBM Watson Health, to discuss the use of artificial intelligence in health care and specifically cancer care and playwright Maggie Edson, winner of a Pulitzer Prize for the play “Wit.” Ms. Edson’s presentation should provide a unique look at the patient experience and a new consideration of patients’ perspectives and values.
The full agenda can be found here: http://www.avaho.org/2017-annual-meeting.html
This year’s annual Assocaition of VA Hematology and Oncology (AVAHO) conference offers nearly 50 sessions over the course of 3 days that focus on providing evidence-based hematology and oncology practice. According to AVAHO President Mary Thomas, MS, “I am hopeful that people come away from this meeting with a different conception of evidence-based practice and how they might apply it.”
John P.A. Ioannidis, MD, DSc, the C. F. Rehnborg Professor in Disease Prevention in the School of Medicine and Professor of Health Research and Policy (Epidemiology) at Stanford University will offer the opening keynote address on integrating evidence into patient care. According to Ms. Thomas, Dr. Ioannidis is a “provocative speaker” who may challenge conceptions and execution of evidence-based practice. Other keynote addresses will be presented by Steve Harvey, vice president of IBM Watson Health, to discuss the use of artificial intelligence in health care and specifically cancer care and playwright Maggie Edson, winner of a Pulitzer Prize for the play “Wit.” Ms. Edson’s presentation should provide a unique look at the patient experience and a new consideration of patients’ perspectives and values.
The full agenda can be found here: http://www.avaho.org/2017-annual-meeting.html
How to Have a Good Time in Denver
From Friday September 15 through Sunday September 17, the Association of VA Hematology and Oncology (AVAHO) will be in Denver, Colorado for their 13th Annual Meeting. If you are looking for something to do in the Mile High City outside of the convention center, here are 4 ways to get the most of a little time to spare.
1. Know the best way to get around
Transportation in a new city can be a stressor, especially when you’re on a compressed schedule. In Denver, there are multiple modes of public transportation with inexpensive price tags. The Regional Transportation District (RTD) has bus and light rail options that take you around Denver and the surrounding areas. For only $5.20, you can get a 1-day pass that allows you to hop on and off as you please. Activity duty U.S. military members ride for free on all RTD services. If you want a more open air option to see the city, try Denver B-cycle. The city-wide bike rental allows you to rent a bike and drop it off at any of the racks around town. Just buy an Access Pass for $9 and you’re good to go!
For more info on RTD, visit http://www.rtd-denver.com/index.shtml
For info in Denver B-cycle, visit https://www.denverbcycle.com/
2. Immerse yourself in culture
Denver has a rich culture and history worth exploring. All around the city there are public art exhibitions for viewing, from the 40-foot tall Blue Bear at the Colorado Convention Center to vibrant murals like the “Love This City” series in 3 different art districts. Information on walking tours is available here. Every 2 years, a week long arts and culture festival called The Biennial of the Americas is held in Denver. Fortunately, the biennial coincides with the AVAHO meeting and offers the opportunity to experience symposiums and concerts by artists, leaders, innovators, and experts from North, South, and Central America and the Caribbean.
For more about Denver Arts and culture visit https://www.denver.org/things-to-do/denver-arts-culture/
3. Eat well
Sometimes the best way to enjoy a city is by eating your way around it. In Denver you can get a taste of the old west when trying Rocky Mountain oysters (bull, sheep, or pig testicles) and locally brewed beers or experience a “smooth as silk, tough as nails” master sommelier like Bobby Stuckey. There are 4 thriving foodie neighborhoods; RiNo where the hipsters get sushi and live jazz, LoHi that continues to be a haven for group outings, Uptown for a mix of old and new school Americana eats, and LoDo as a drinking and dining hub perfect for lunch or dinner. All are easy to access from the meeting via public transportation, taxi, or a short walk.
For more about eating around Denver visit https://denver.eater.com/2017/8/29/16180854/best-food-denver-restaurants-city-guide or https://www.thrillist.com/eat/denver
4. Enjoy the scenery
If nature and fresh air is where you find solace, Denver has no shortage of places for you to experience and enjoy. The Denver Parks and Recreation was founded in 1868 and has about 20,00 acres of mountain parkland and urban parks to visit and more than 80 miles of trails within the city. Just steps from the meeting there are paths for walking or jogging that wind along the South Platte River and Cherry Creek. The architecture in Denver is also a great way take in the scenery and a little bit of history too. There’s a healthy mix of old styles like Beaux Arts at Union Station and French Gothic at Cathedral Basilica of the Immaculate Conception, with new modern designs in the Denver Art Museum: Frederic C. Hamilton Building and the Boettcher Memorial Tropical Conservatory. Whether marveling at nature or buildings, there’s plenty to see.
For more on Denver Parks and Recreation, visit https://www.denvergov.org/content/denvergov/en/denver-parks-and-recreation.html
From Friday September 15 through Sunday September 17, the Association of VA Hematology and Oncology (AVAHO) will be in Denver, Colorado for their 13th Annual Meeting. If you are looking for something to do in the Mile High City outside of the convention center, here are 4 ways to get the most of a little time to spare.
1. Know the best way to get around
Transportation in a new city can be a stressor, especially when you’re on a compressed schedule. In Denver, there are multiple modes of public transportation with inexpensive price tags. The Regional Transportation District (RTD) has bus and light rail options that take you around Denver and the surrounding areas. For only $5.20, you can get a 1-day pass that allows you to hop on and off as you please. Activity duty U.S. military members ride for free on all RTD services. If you want a more open air option to see the city, try Denver B-cycle. The city-wide bike rental allows you to rent a bike and drop it off at any of the racks around town. Just buy an Access Pass for $9 and you’re good to go!
For more info on RTD, visit http://www.rtd-denver.com/index.shtml
For info in Denver B-cycle, visit https://www.denverbcycle.com/
2. Immerse yourself in culture
Denver has a rich culture and history worth exploring. All around the city there are public art exhibitions for viewing, from the 40-foot tall Blue Bear at the Colorado Convention Center to vibrant murals like the “Love This City” series in 3 different art districts. Information on walking tours is available here. Every 2 years, a week long arts and culture festival called The Biennial of the Americas is held in Denver. Fortunately, the biennial coincides with the AVAHO meeting and offers the opportunity to experience symposiums and concerts by artists, leaders, innovators, and experts from North, South, and Central America and the Caribbean.
For more about Denver Arts and culture visit https://www.denver.org/things-to-do/denver-arts-culture/
3. Eat well
Sometimes the best way to enjoy a city is by eating your way around it. In Denver you can get a taste of the old west when trying Rocky Mountain oysters (bull, sheep, or pig testicles) and locally brewed beers or experience a “smooth as silk, tough as nails” master sommelier like Bobby Stuckey. There are 4 thriving foodie neighborhoods; RiNo where the hipsters get sushi and live jazz, LoHi that continues to be a haven for group outings, Uptown for a mix of old and new school Americana eats, and LoDo as a drinking and dining hub perfect for lunch or dinner. All are easy to access from the meeting via public transportation, taxi, or a short walk.
For more about eating around Denver visit https://denver.eater.com/2017/8/29/16180854/best-food-denver-restaurants-city-guide or https://www.thrillist.com/eat/denver
4. Enjoy the scenery
If nature and fresh air is where you find solace, Denver has no shortage of places for you to experience and enjoy. The Denver Parks and Recreation was founded in 1868 and has about 20,00 acres of mountain parkland and urban parks to visit and more than 80 miles of trails within the city. Just steps from the meeting there are paths for walking or jogging that wind along the South Platte River and Cherry Creek. The architecture in Denver is also a great way take in the scenery and a little bit of history too. There’s a healthy mix of old styles like Beaux Arts at Union Station and French Gothic at Cathedral Basilica of the Immaculate Conception, with new modern designs in the Denver Art Museum: Frederic C. Hamilton Building and the Boettcher Memorial Tropical Conservatory. Whether marveling at nature or buildings, there’s plenty to see.
For more on Denver Parks and Recreation, visit https://www.denvergov.org/content/denvergov/en/denver-parks-and-recreation.html
From Friday September 15 through Sunday September 17, the Association of VA Hematology and Oncology (AVAHO) will be in Denver, Colorado for their 13th Annual Meeting. If you are looking for something to do in the Mile High City outside of the convention center, here are 4 ways to get the most of a little time to spare.
1. Know the best way to get around
Transportation in a new city can be a stressor, especially when you’re on a compressed schedule. In Denver, there are multiple modes of public transportation with inexpensive price tags. The Regional Transportation District (RTD) has bus and light rail options that take you around Denver and the surrounding areas. For only $5.20, you can get a 1-day pass that allows you to hop on and off as you please. Activity duty U.S. military members ride for free on all RTD services. If you want a more open air option to see the city, try Denver B-cycle. The city-wide bike rental allows you to rent a bike and drop it off at any of the racks around town. Just buy an Access Pass for $9 and you’re good to go!
For more info on RTD, visit http://www.rtd-denver.com/index.shtml
For info in Denver B-cycle, visit https://www.denverbcycle.com/
2. Immerse yourself in culture
Denver has a rich culture and history worth exploring. All around the city there are public art exhibitions for viewing, from the 40-foot tall Blue Bear at the Colorado Convention Center to vibrant murals like the “Love This City” series in 3 different art districts. Information on walking tours is available here. Every 2 years, a week long arts and culture festival called The Biennial of the Americas is held in Denver. Fortunately, the biennial coincides with the AVAHO meeting and offers the opportunity to experience symposiums and concerts by artists, leaders, innovators, and experts from North, South, and Central America and the Caribbean.
For more about Denver Arts and culture visit https://www.denver.org/things-to-do/denver-arts-culture/
3. Eat well
Sometimes the best way to enjoy a city is by eating your way around it. In Denver you can get a taste of the old west when trying Rocky Mountain oysters (bull, sheep, or pig testicles) and locally brewed beers or experience a “smooth as silk, tough as nails” master sommelier like Bobby Stuckey. There are 4 thriving foodie neighborhoods; RiNo where the hipsters get sushi and live jazz, LoHi that continues to be a haven for group outings, Uptown for a mix of old and new school Americana eats, and LoDo as a drinking and dining hub perfect for lunch or dinner. All are easy to access from the meeting via public transportation, taxi, or a short walk.
For more about eating around Denver visit https://denver.eater.com/2017/8/29/16180854/best-food-denver-restaurants-city-guide or https://www.thrillist.com/eat/denver
4. Enjoy the scenery
If nature and fresh air is where you find solace, Denver has no shortage of places for you to experience and enjoy. The Denver Parks and Recreation was founded in 1868 and has about 20,00 acres of mountain parkland and urban parks to visit and more than 80 miles of trails within the city. Just steps from the meeting there are paths for walking or jogging that wind along the South Platte River and Cherry Creek. The architecture in Denver is also a great way take in the scenery and a little bit of history too. There’s a healthy mix of old styles like Beaux Arts at Union Station and French Gothic at Cathedral Basilica of the Immaculate Conception, with new modern designs in the Denver Art Museum: Frederic C. Hamilton Building and the Boettcher Memorial Tropical Conservatory. Whether marveling at nature or buildings, there’s plenty to see.
For more on Denver Parks and Recreation, visit https://www.denvergov.org/content/denvergov/en/denver-parks-and-recreation.html