User login
Vaccination rates up in U.S. kindergartners in 2015, steady in 19- to 35-month-olds
Vaccination coverage for MMR and DTaP increased for children in kindergarten during the 2015-2016 school year, but remained steady for children aged 19-35 months in 2015, according to reports from the Centers for Disease Control and Prevention.
The median MMR vaccination rate for kindergartners in 2015 was 94.6%, up significantly from 92.6% in 2014. DTaP coverage also increased, rising from 92.4% to 94.2%. A total of 32 states saw an increase in MMR coverage in 2015, with 22 states reporting greater than 95% coverage. Only 3 states and the District of Columbia reported less than 90% coverage, down from 7 states and D.C. in 2014.
While the median vaccination rates were increased in 2015, the median exemption rate also increased by nearly 11% to 1.9% overall. This was caused in part by the addition of reports from Texas and Wyoming, neither of which reported the number of exemptions in 2014, the CDC investigators remarked.
In a second CDC report from Dr. Holly Hill and her associates based on data collected from the National Immunization Survey, the vaccination rate for children aged 19-35 months in 2015 did not increase significantly from the previous year (MMWR. 2016 Oct 6. doi: 10.15585/mmwr.mm639a4). The rate of children who received four or more doses of DTaP and at least one dose of MMR increased by 0.4 percentage points each from 84.2% to 84.6% and from 91.5% to 91.9%, respectively. The largest increase was seen in hepatitis A vaccine, where the rate of vaccination increased from 57.5% to 59.6%.
Healthy People 2020 goals for greater than 90% coverage for children aged 19-35 months were met for four vaccines in 2015: three or more doses of poliovirus vaccine, one or more doses of MMR, three or more doses of hepatitis B vaccine, and one or more doses of varicella vaccine. Vaccine coverage was lower in almost all cases for children living below the poverty level. The largest discrepancies were seen in rotavirus and varicella vaccines. The combined seven-vaccine series rate for children at or above the poverty line was 74.7%, and was 68.7% for children below the poverty line.
“Continued surveillance is needed to monitor coverage, locate pockets of susceptibility, and evaluate the impact of interventions designed to ensure that all children remain adequately protected against vaccine-preventable diseases,” Dr. Hill and her associates noted.
The CDC investigators had no relevant financial disclosures to report.
Vaccination coverage for MMR and DTaP increased for children in kindergarten during the 2015-2016 school year, but remained steady for children aged 19-35 months in 2015, according to reports from the Centers for Disease Control and Prevention.
The median MMR vaccination rate for kindergartners in 2015 was 94.6%, up significantly from 92.6% in 2014. DTaP coverage also increased, rising from 92.4% to 94.2%. A total of 32 states saw an increase in MMR coverage in 2015, with 22 states reporting greater than 95% coverage. Only 3 states and the District of Columbia reported less than 90% coverage, down from 7 states and D.C. in 2014.
While the median vaccination rates were increased in 2015, the median exemption rate also increased by nearly 11% to 1.9% overall. This was caused in part by the addition of reports from Texas and Wyoming, neither of which reported the number of exemptions in 2014, the CDC investigators remarked.
In a second CDC report from Dr. Holly Hill and her associates based on data collected from the National Immunization Survey, the vaccination rate for children aged 19-35 months in 2015 did not increase significantly from the previous year (MMWR. 2016 Oct 6. doi: 10.15585/mmwr.mm639a4). The rate of children who received four or more doses of DTaP and at least one dose of MMR increased by 0.4 percentage points each from 84.2% to 84.6% and from 91.5% to 91.9%, respectively. The largest increase was seen in hepatitis A vaccine, where the rate of vaccination increased from 57.5% to 59.6%.
Healthy People 2020 goals for greater than 90% coverage for children aged 19-35 months were met for four vaccines in 2015: three or more doses of poliovirus vaccine, one or more doses of MMR, three or more doses of hepatitis B vaccine, and one or more doses of varicella vaccine. Vaccine coverage was lower in almost all cases for children living below the poverty level. The largest discrepancies were seen in rotavirus and varicella vaccines. The combined seven-vaccine series rate for children at or above the poverty line was 74.7%, and was 68.7% for children below the poverty line.
“Continued surveillance is needed to monitor coverage, locate pockets of susceptibility, and evaluate the impact of interventions designed to ensure that all children remain adequately protected against vaccine-preventable diseases,” Dr. Hill and her associates noted.
The CDC investigators had no relevant financial disclosures to report.
Vaccination coverage for MMR and DTaP increased for children in kindergarten during the 2015-2016 school year, but remained steady for children aged 19-35 months in 2015, according to reports from the Centers for Disease Control and Prevention.
The median MMR vaccination rate for kindergartners in 2015 was 94.6%, up significantly from 92.6% in 2014. DTaP coverage also increased, rising from 92.4% to 94.2%. A total of 32 states saw an increase in MMR coverage in 2015, with 22 states reporting greater than 95% coverage. Only 3 states and the District of Columbia reported less than 90% coverage, down from 7 states and D.C. in 2014.
While the median vaccination rates were increased in 2015, the median exemption rate also increased by nearly 11% to 1.9% overall. This was caused in part by the addition of reports from Texas and Wyoming, neither of which reported the number of exemptions in 2014, the CDC investigators remarked.
In a second CDC report from Dr. Holly Hill and her associates based on data collected from the National Immunization Survey, the vaccination rate for children aged 19-35 months in 2015 did not increase significantly from the previous year (MMWR. 2016 Oct 6. doi: 10.15585/mmwr.mm639a4). The rate of children who received four or more doses of DTaP and at least one dose of MMR increased by 0.4 percentage points each from 84.2% to 84.6% and from 91.5% to 91.9%, respectively. The largest increase was seen in hepatitis A vaccine, where the rate of vaccination increased from 57.5% to 59.6%.
Healthy People 2020 goals for greater than 90% coverage for children aged 19-35 months were met for four vaccines in 2015: three or more doses of poliovirus vaccine, one or more doses of MMR, three or more doses of hepatitis B vaccine, and one or more doses of varicella vaccine. Vaccine coverage was lower in almost all cases for children living below the poverty level. The largest discrepancies were seen in rotavirus and varicella vaccines. The combined seven-vaccine series rate for children at or above the poverty line was 74.7%, and was 68.7% for children below the poverty line.
“Continued surveillance is needed to monitor coverage, locate pockets of susceptibility, and evaluate the impact of interventions designed to ensure that all children remain adequately protected against vaccine-preventable diseases,” Dr. Hill and her associates noted.
The CDC investigators had no relevant financial disclosures to report.
Silicone joint arthroplasty for RA shows sustained improvements at 7 years
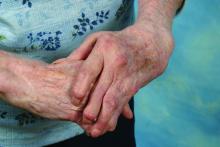
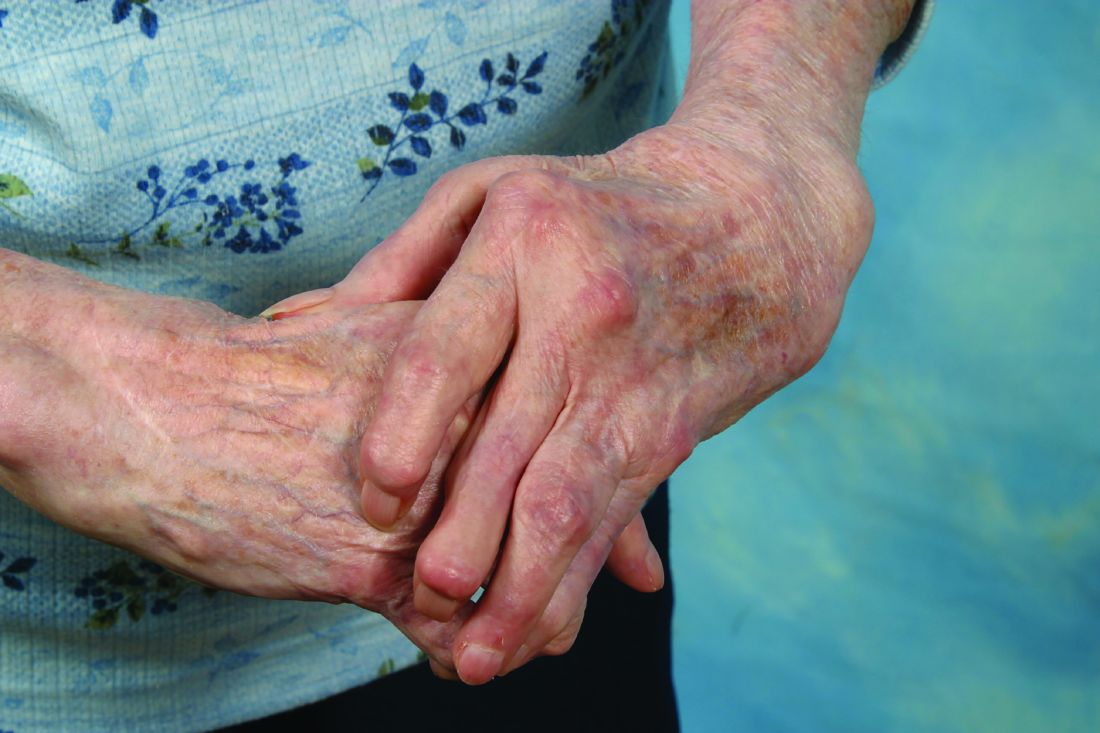
Rheumatoid arthritis patients who underwent silicone metacarpophalangeal joint replacement maintained significant improvement in ulnar drift and extensor lag after 7 years of follow-up in the prospective, multicenter Silicone Arthroplasty in Rheumatoid Arthritis (SARA) study.
The silicone metacarpophalangeal joint arthroplasty (SMPA) group also showed significantly better metacarpophalangeal (MCP) joint arc of motion, as well as function, aesthetics, and satisfaction scores than did patients in the cohort who chose nonsurgical management.
The practice of replacing deformed MCP joints with a hinged silicone implant has been around for almost half a century, the investigators noted, but despite widespread use, there is a lack of high-quality surgical outcomes data for the procedure. The authors suggested that the scarcity of data may create a barrier for surgical referrals from rheumatologists and could account for the declining rate of surgical intervention for RA joint deformities.
The significant improvement in ulnar drift that occurred in patients who underwent SMPA versus no surgery remained even after adjustment for baseline severity, age, sex, and use of biologics. The adjustment was especially important because the nonsurgical group had better function at baseline, with significantly stronger grip and pinch strength, better Michigan Hand Questionnaire (MHQ) scores, and significantly better ulnar drift, extensor lag, and arc of motion than in the surgical group (Arthritis Care Res. 2016 Oct 1. doi: 10.1002/acr.23105).
The nonsurgical group largely maintained its baseline functional state during the 7-year follow-up period except for a decline in pinch strength.
“When the SMPA cohort was compared with the non-SMPA cohort, the covariate-adjusted difference showed significant benefits associated with SMPA hand outcome as measured by the MHQ function, aesthetics, and satisfaction scores, with no measures showing significantly better outcomes for the non-SMPA group,” the researchers wrote. “Although the average treatment effect estimate (ATT estimate) showed a decline over time for the average benefit from SMPA in those treated, the function score benefit as shown by the ATT estimate remained significant at year 5.”
Patients in the nonsurgical group were also given the option to crossover and receive surgery after 1 year, which two did. Eleven patients in the surgical group also decided to undergo surgery on their other hand.
Researchers saw one mild and three moderate implant-related adverse events during the study, including one patient who experienced ulnar deviation and needed a new joint replacement, one who dislocated the implant of the little finger a few weeks after insertion, and one who experienced sepsis in the joints 6 years after insertion and needed replacements for two implants. The fracture incidence of about 10% fits into the mid-range of previously reported fracture rates with this implant.
The study experienced significant losses to follow-up, particularly in the surgical group in which 7-year data were only available for 43% of participants in this group. The authors suggested this could have been because the surgical patients achieved their goals and were less inclined to the follow-up visits.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. No conflicts of interest were declared.
Rheumatoid arthritis patients who underwent silicone metacarpophalangeal joint replacement maintained significant improvement in ulnar drift and extensor lag after 7 years of follow-up in the prospective, multicenter Silicone Arthroplasty in Rheumatoid Arthritis (SARA) study.
The silicone metacarpophalangeal joint arthroplasty (SMPA) group also showed significantly better metacarpophalangeal (MCP) joint arc of motion, as well as function, aesthetics, and satisfaction scores than did patients in the cohort who chose nonsurgical management.
The practice of replacing deformed MCP joints with a hinged silicone implant has been around for almost half a century, the investigators noted, but despite widespread use, there is a lack of high-quality surgical outcomes data for the procedure. The authors suggested that the scarcity of data may create a barrier for surgical referrals from rheumatologists and could account for the declining rate of surgical intervention for RA joint deformities.
The significant improvement in ulnar drift that occurred in patients who underwent SMPA versus no surgery remained even after adjustment for baseline severity, age, sex, and use of biologics. The adjustment was especially important because the nonsurgical group had better function at baseline, with significantly stronger grip and pinch strength, better Michigan Hand Questionnaire (MHQ) scores, and significantly better ulnar drift, extensor lag, and arc of motion than in the surgical group (Arthritis Care Res. 2016 Oct 1. doi: 10.1002/acr.23105).
The nonsurgical group largely maintained its baseline functional state during the 7-year follow-up period except for a decline in pinch strength.
“When the SMPA cohort was compared with the non-SMPA cohort, the covariate-adjusted difference showed significant benefits associated with SMPA hand outcome as measured by the MHQ function, aesthetics, and satisfaction scores, with no measures showing significantly better outcomes for the non-SMPA group,” the researchers wrote. “Although the average treatment effect estimate (ATT estimate) showed a decline over time for the average benefit from SMPA in those treated, the function score benefit as shown by the ATT estimate remained significant at year 5.”
Patients in the nonsurgical group were also given the option to crossover and receive surgery after 1 year, which two did. Eleven patients in the surgical group also decided to undergo surgery on their other hand.
Researchers saw one mild and three moderate implant-related adverse events during the study, including one patient who experienced ulnar deviation and needed a new joint replacement, one who dislocated the implant of the little finger a few weeks after insertion, and one who experienced sepsis in the joints 6 years after insertion and needed replacements for two implants. The fracture incidence of about 10% fits into the mid-range of previously reported fracture rates with this implant.
The study experienced significant losses to follow-up, particularly in the surgical group in which 7-year data were only available for 43% of participants in this group. The authors suggested this could have been because the surgical patients achieved their goals and were less inclined to the follow-up visits.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. No conflicts of interest were declared.
Rheumatoid arthritis patients who underwent silicone metacarpophalangeal joint replacement maintained significant improvement in ulnar drift and extensor lag after 7 years of follow-up in the prospective, multicenter Silicone Arthroplasty in Rheumatoid Arthritis (SARA) study.
The silicone metacarpophalangeal joint arthroplasty (SMPA) group also showed significantly better metacarpophalangeal (MCP) joint arc of motion, as well as function, aesthetics, and satisfaction scores than did patients in the cohort who chose nonsurgical management.
The practice of replacing deformed MCP joints with a hinged silicone implant has been around for almost half a century, the investigators noted, but despite widespread use, there is a lack of high-quality surgical outcomes data for the procedure. The authors suggested that the scarcity of data may create a barrier for surgical referrals from rheumatologists and could account for the declining rate of surgical intervention for RA joint deformities.
The significant improvement in ulnar drift that occurred in patients who underwent SMPA versus no surgery remained even after adjustment for baseline severity, age, sex, and use of biologics. The adjustment was especially important because the nonsurgical group had better function at baseline, with significantly stronger grip and pinch strength, better Michigan Hand Questionnaire (MHQ) scores, and significantly better ulnar drift, extensor lag, and arc of motion than in the surgical group (Arthritis Care Res. 2016 Oct 1. doi: 10.1002/acr.23105).
The nonsurgical group largely maintained its baseline functional state during the 7-year follow-up period except for a decline in pinch strength.
“When the SMPA cohort was compared with the non-SMPA cohort, the covariate-adjusted difference showed significant benefits associated with SMPA hand outcome as measured by the MHQ function, aesthetics, and satisfaction scores, with no measures showing significantly better outcomes for the non-SMPA group,” the researchers wrote. “Although the average treatment effect estimate (ATT estimate) showed a decline over time for the average benefit from SMPA in those treated, the function score benefit as shown by the ATT estimate remained significant at year 5.”
Patients in the nonsurgical group were also given the option to crossover and receive surgery after 1 year, which two did. Eleven patients in the surgical group also decided to undergo surgery on their other hand.
Researchers saw one mild and three moderate implant-related adverse events during the study, including one patient who experienced ulnar deviation and needed a new joint replacement, one who dislocated the implant of the little finger a few weeks after insertion, and one who experienced sepsis in the joints 6 years after insertion and needed replacements for two implants. The fracture incidence of about 10% fits into the mid-range of previously reported fracture rates with this implant.
The study experienced significant losses to follow-up, particularly in the surgical group in which 7-year data were only available for 43% of participants in this group. The authors suggested this could have been because the surgical patients achieved their goals and were less inclined to the follow-up visits.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. No conflicts of interest were declared.
Key clinical point:
Major finding: Patients who elected to undergo silicone MCP joint replacement showed significant improvements in ulnar drift and extensor lag, as well as in function, aesthetics, and satisfaction scores at 7 years after the procedure.
Data source: Cohort study of 170 patients with rheumatoid arthritis–related severe deformity at the metacarpophalangeal joints.
Disclosures: The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. No conflicts of interest were declared.
Study finds picosecond laser, with diffractive lens array, effective wrinkle treatment
An industry-funded study suggests that picosecond lasers, touted for their effectiveness at tattoo removal, can safely and effectively treat perioral and periocular wrinkles.
Six months after treatment with picosecond 755-nm alexandrite laser with a diffractive lens array, subjects reported high levels of satisfaction and blinded physicians rated the treated wrinkles as improved or much improved over the same time period (Lasers Surg Med. 2016 Sep 29. doi: 10.1002/lsm.22577).
The laser “is very useful for treating fine lines and other visible signs of premature photoaging,” said study coauthor David H. McDaniel, MD, a dermatologist in Virginia Beach, Va., and codirector of the Hampton University Skin of Color Research Institute. “These types of lasers have great potential benefit for patients and minimal risk of any significant adverse events.”
According to Dr. McDaniel, picosecond 755-nm alexandrite lasers are commonly used to treat wrinkles, acne scars, and pigment dyschromia, while picosecond 532- and 1,064-nm lasers are used to remove tattoos and treat some pigment dyschromia.
He and his coinvestigators sought to “better define the parameters that are uniquely contributing to wrinkle reduction and dermal matrix remodeling and also define the actual clinical benefits and treatment protocol,” Dr. McDaniel said in an interview.
At four 1-month intervals, they used a picosecond 755 nm alexandrite laser with diffractive lens array to treat the full faces of 40 women with wrinkles caused by photodamage. The subjects were all healthy white nonsmokers, whose average age was 58 years.
At 6 months following treatment, the average Fitzpatrick wrinkle score improved, dropping from 5.48 to 3.47 (P less than .05). Also at 6 months, blinded physician evaluators rated the average degree of improvement from baseline as “moderate” (for fine lines) “less than mild” (for erythema), “high moderate” (for dyschromia) and “mid moderate” (for global improvement).
The evaluators successfully identified posttreatment photos in 82% of cases. As for patients, 36.8% said they were extremely satisfied and 57.9% said they were satisfied at 6 months. Adverse effects were reported as mild: One patient reported 2 days of erythema, another reported 4 days of edema, and one experienced bruising. Serial punch biopsies obtained at 6 months after the last treatment in six patients revealed increases in dermal collagen and thicker, denser elastin fibers.
The picosecond 755- nm alexandrite laser produces “very little thermal effect, which reduces both treatment discomfort as well as the risk of adverse events,” Dr. McDaniel said in an interview. “It typically leads to shorter recovery time socially, as erythema is very mild and quite transient.”
He noted that the laser can be used in conjunction with other lasers. “Some of the other gold standard fractional lasers are still used in our practice, and they still deliver good results when properly indicated,” he said. “For example, we may use – or even combine with the picosecond laser – a fractional erbium laser to reach deeper into the dermis for severe acne scarring or for deep wrinkles. Or we may use a fractional thulium laser for people with early actinic keratosis or also combine it with the picosecond laser.
Cynosure, a maker of picosecond lasers, funded the study and provided a discounted price for the laser. Dr. McDaniel and another author report serving as consultants, researchers and speakers for Cynosure.
An industry-funded study suggests that picosecond lasers, touted for their effectiveness at tattoo removal, can safely and effectively treat perioral and periocular wrinkles.
Six months after treatment with picosecond 755-nm alexandrite laser with a diffractive lens array, subjects reported high levels of satisfaction and blinded physicians rated the treated wrinkles as improved or much improved over the same time period (Lasers Surg Med. 2016 Sep 29. doi: 10.1002/lsm.22577).
The laser “is very useful for treating fine lines and other visible signs of premature photoaging,” said study coauthor David H. McDaniel, MD, a dermatologist in Virginia Beach, Va., and codirector of the Hampton University Skin of Color Research Institute. “These types of lasers have great potential benefit for patients and minimal risk of any significant adverse events.”
According to Dr. McDaniel, picosecond 755-nm alexandrite lasers are commonly used to treat wrinkles, acne scars, and pigment dyschromia, while picosecond 532- and 1,064-nm lasers are used to remove tattoos and treat some pigment dyschromia.
He and his coinvestigators sought to “better define the parameters that are uniquely contributing to wrinkle reduction and dermal matrix remodeling and also define the actual clinical benefits and treatment protocol,” Dr. McDaniel said in an interview.
At four 1-month intervals, they used a picosecond 755 nm alexandrite laser with diffractive lens array to treat the full faces of 40 women with wrinkles caused by photodamage. The subjects were all healthy white nonsmokers, whose average age was 58 years.
At 6 months following treatment, the average Fitzpatrick wrinkle score improved, dropping from 5.48 to 3.47 (P less than .05). Also at 6 months, blinded physician evaluators rated the average degree of improvement from baseline as “moderate” (for fine lines) “less than mild” (for erythema), “high moderate” (for dyschromia) and “mid moderate” (for global improvement).
The evaluators successfully identified posttreatment photos in 82% of cases. As for patients, 36.8% said they were extremely satisfied and 57.9% said they were satisfied at 6 months. Adverse effects were reported as mild: One patient reported 2 days of erythema, another reported 4 days of edema, and one experienced bruising. Serial punch biopsies obtained at 6 months after the last treatment in six patients revealed increases in dermal collagen and thicker, denser elastin fibers.
The picosecond 755- nm alexandrite laser produces “very little thermal effect, which reduces both treatment discomfort as well as the risk of adverse events,” Dr. McDaniel said in an interview. “It typically leads to shorter recovery time socially, as erythema is very mild and quite transient.”
He noted that the laser can be used in conjunction with other lasers. “Some of the other gold standard fractional lasers are still used in our practice, and they still deliver good results when properly indicated,” he said. “For example, we may use – or even combine with the picosecond laser – a fractional erbium laser to reach deeper into the dermis for severe acne scarring or for deep wrinkles. Or we may use a fractional thulium laser for people with early actinic keratosis or also combine it with the picosecond laser.
Cynosure, a maker of picosecond lasers, funded the study and provided a discounted price for the laser. Dr. McDaniel and another author report serving as consultants, researchers and speakers for Cynosure.
An industry-funded study suggests that picosecond lasers, touted for their effectiveness at tattoo removal, can safely and effectively treat perioral and periocular wrinkles.
Six months after treatment with picosecond 755-nm alexandrite laser with a diffractive lens array, subjects reported high levels of satisfaction and blinded physicians rated the treated wrinkles as improved or much improved over the same time period (Lasers Surg Med. 2016 Sep 29. doi: 10.1002/lsm.22577).
The laser “is very useful for treating fine lines and other visible signs of premature photoaging,” said study coauthor David H. McDaniel, MD, a dermatologist in Virginia Beach, Va., and codirector of the Hampton University Skin of Color Research Institute. “These types of lasers have great potential benefit for patients and minimal risk of any significant adverse events.”
According to Dr. McDaniel, picosecond 755-nm alexandrite lasers are commonly used to treat wrinkles, acne scars, and pigment dyschromia, while picosecond 532- and 1,064-nm lasers are used to remove tattoos and treat some pigment dyschromia.
He and his coinvestigators sought to “better define the parameters that are uniquely contributing to wrinkle reduction and dermal matrix remodeling and also define the actual clinical benefits and treatment protocol,” Dr. McDaniel said in an interview.
At four 1-month intervals, they used a picosecond 755 nm alexandrite laser with diffractive lens array to treat the full faces of 40 women with wrinkles caused by photodamage. The subjects were all healthy white nonsmokers, whose average age was 58 years.
At 6 months following treatment, the average Fitzpatrick wrinkle score improved, dropping from 5.48 to 3.47 (P less than .05). Also at 6 months, blinded physician evaluators rated the average degree of improvement from baseline as “moderate” (for fine lines) “less than mild” (for erythema), “high moderate” (for dyschromia) and “mid moderate” (for global improvement).
The evaluators successfully identified posttreatment photos in 82% of cases. As for patients, 36.8% said they were extremely satisfied and 57.9% said they were satisfied at 6 months. Adverse effects were reported as mild: One patient reported 2 days of erythema, another reported 4 days of edema, and one experienced bruising. Serial punch biopsies obtained at 6 months after the last treatment in six patients revealed increases in dermal collagen and thicker, denser elastin fibers.
The picosecond 755- nm alexandrite laser produces “very little thermal effect, which reduces both treatment discomfort as well as the risk of adverse events,” Dr. McDaniel said in an interview. “It typically leads to shorter recovery time socially, as erythema is very mild and quite transient.”
He noted that the laser can be used in conjunction with other lasers. “Some of the other gold standard fractional lasers are still used in our practice, and they still deliver good results when properly indicated,” he said. “For example, we may use – or even combine with the picosecond laser – a fractional erbium laser to reach deeper into the dermis for severe acne scarring or for deep wrinkles. Or we may use a fractional thulium laser for people with early actinic keratosis or also combine it with the picosecond laser.
Cynosure, a maker of picosecond lasers, funded the study and provided a discounted price for the laser. Dr. McDaniel and another author report serving as consultants, researchers and speakers for Cynosure.
FROM LASERS IN SURGERY AND MEDICINE
Key clinical point: Wrinkle treatment via picosecond 755-nm alexandrite laser with a diffractive lens array appears to be safe and effective.
Major finding: Six months after the last treatment, 36.8% of patients were extremely satisfied and 57.9% were satisfied with the results, with minor, transient adverse effects. Blinded physician evaluators reported “mid moderate” global improvement.
Data source: A prospective, blinded study of 40 healthy white women, nonsmokers, average age 58 (range: 47-64), who underwent four full-face treatments via laser at 1-month intervals.
Disclosures: Cynosure, a maker of picosecond lasers, funded the study and provided a discounted price for the laser. Dr. McDaniel and another author report serving as consultants, researchers, and speakers for Cynosure.
More restrictive hemoglobin threshold recommended for transfusion
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
FROM JAMA
Key clinical point: A restrictive threshold for red blood cell transfusion, in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL, is now recommended for most patients.
Major finding: A more restrictive threshold for red blood cell transfusion is not associated with an increased risk of mortality or other adverse outcomes from transfusion.
Data source: Updated guidelines from the AABB (formerly known as the American Association of Blood Banks).
Disclosures: Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies including CSL and Fresenius Kabi, but no other conflicts of interest were declared.
Conservative oxygen therapy in the ICU reduces mortality
A strategy of conservatively controlling oxygen delivery to patients in the intensive care unit results in lower mortality than the conventional, more liberal approach whereby patients are often kept in a hyperoxemic state, finds a randomized controlled trial.
The trial, known as Oxygen-ICU, enrolled more than 400 adult ICU patients from an Italian center. Initially planned to last 2 years, it was terminated early because of slow enrollment after an earthquake reduced ICU capacity, with the decision supported by positive results of an interim analysis.
Patients had an absolute nearly 9% lower risk of dying in the ICU with use of the conservative oxygen strategy as compared with the conventional one, according to data reported at the annual congress of the European Society of Intensive Care Medicine and simultaneously published (JAMA. 2016 Oct 5. doi: 10.1001/jama.2016.11993).
“To our knowledge, this is the first randomized clinical trial to evaluate the effect of a conservative oxygen therapy on mortality compared with a standard, more liberal approach in a medical-surgical population of adult critically ill patients,” write the investigators, who were led by Massimo Girardis, MD, of the Intensive Care Unit, Department of Anesthesiology and Intensive Care, University Hospital of Modena (Italy).
Among critically ill patients with an ICU length of stay of 72 hours or longer, a conservative protocol for oxygen therapy compared with conventional therapy resulted in a lower ICU mortality,” they conclude. “However, these preliminary findings were based on unplanned early termination of the trial, and a larger multicenter trial is needed to evaluate the potential benefit of such conservative oxygen therapy in critically ill patients.”
In the trial, consecutive patients were randomized evenly to receive conservative oxygen therapy (maintenance of PaO2 between 70 and 100 mm Hg or arterial oxyhemoglobin saturation [SpO2] between 94% and 98%) or conventional oxygen therapy (allowance of PaO2 values up to 150 mm Hg or SpO2 values between 97% and 100%) on an open-label basis.
The originally targeted enrollment was 660 patients, but the study was stopped early after only 480 patients had been enrolled.
Results of modified intent-to-treat analyses showed that daily time-weighted PaO2 averages during patients’ ICU stays were higher in the conventional group than in the conservative group (median PaO2, 102 vs. 87 mm Hg; P less than .001).
The rate of ICU mortality, the trial’s primary endpoint, was 11.6% with conservative therapy, about half of the 20.2% seen with conventional therapy (absolute mean difference, 0.086; P = .01).
The conservative group also had lower rates of shock (3.7% vs. 10.6%, P = .006), liver failure (1.9% vs. 6.4%, P = .02), and bacteremia (5.1% vs. 10.1%, P = .049). And they spent a day less on the ventilator (median mechanical ventilation–free hours, 72 vs. 48; P = .02).
Lengths of ICU stay and hospital stay did not differ between the two groups.
One of the study authors reports serving as the data monitoring chair for a phase II study sponsored by InflaRx, on the antibiotic advisory board for Bayer, and on sepsis advisory boards for Biotest and Merck. The study was supported by the National Fund for Scientific Research of the University of Modena and Reggio Emilia.
The reduction in mortality seen with conservative oxygen therapy in the Oxygen-ICU trial was “striking,” according to editorialist Dr. Niall D. Ferguson. However, “it is likely that to some extent, this trial has overestimated the true treatment effect of conservative oxygen therapy,” he cautions, given baseline imbalances between groups, early stopping based in part on an unplanned interim analysis, and the small number of deaths. The editorialist noted that the study was underpowered and criticized its use of a modified intent-to-treat analysis.
The trial’s findings contrast with those of a pilot study conducted by the ANZICS clinical trials group that did not find better outcomes with use of lower oxygen targets, according to Dr. Ferguson. However, in that trial, both arms had lower target and actual PaO2 levels. Thus, the optimal clinical approach remains uncertain.
“Until the results of further trials addressing this issue are available, there appears to be little downside in the careful titration and monitoring of supplemental oxygen in the ICU to achieve physiologically normal levels of PaO2 while avoiding potentially dangerous hyperoxia,” he concludes.
Dr. Ferguson disclosed that he has no relevant conflicts of interest.
Niall D. Ferguson, MD, MSc, is with the Interdepartmental Division of Critical Care Medicine and Departments of Medicine and Physiology, University of Toronto; the Institute of Health Policy, Management, & Evaluation, University of Toronto; the Division of Respirology, Department of Medicine, University Health Network and Mount Sinai Hospital; and the Toronto General Research Institute.
The reduction in mortality seen with conservative oxygen therapy in the Oxygen-ICU trial was “striking,” according to editorialist Dr. Niall D. Ferguson. However, “it is likely that to some extent, this trial has overestimated the true treatment effect of conservative oxygen therapy,” he cautions, given baseline imbalances between groups, early stopping based in part on an unplanned interim analysis, and the small number of deaths. The editorialist noted that the study was underpowered and criticized its use of a modified intent-to-treat analysis.
The trial’s findings contrast with those of a pilot study conducted by the ANZICS clinical trials group that did not find better outcomes with use of lower oxygen targets, according to Dr. Ferguson. However, in that trial, both arms had lower target and actual PaO2 levels. Thus, the optimal clinical approach remains uncertain.
“Until the results of further trials addressing this issue are available, there appears to be little downside in the careful titration and monitoring of supplemental oxygen in the ICU to achieve physiologically normal levels of PaO2 while avoiding potentially dangerous hyperoxia,” he concludes.
Dr. Ferguson disclosed that he has no relevant conflicts of interest.
Niall D. Ferguson, MD, MSc, is with the Interdepartmental Division of Critical Care Medicine and Departments of Medicine and Physiology, University of Toronto; the Institute of Health Policy, Management, & Evaluation, University of Toronto; the Division of Respirology, Department of Medicine, University Health Network and Mount Sinai Hospital; and the Toronto General Research Institute.
The reduction in mortality seen with conservative oxygen therapy in the Oxygen-ICU trial was “striking,” according to editorialist Dr. Niall D. Ferguson. However, “it is likely that to some extent, this trial has overestimated the true treatment effect of conservative oxygen therapy,” he cautions, given baseline imbalances between groups, early stopping based in part on an unplanned interim analysis, and the small number of deaths. The editorialist noted that the study was underpowered and criticized its use of a modified intent-to-treat analysis.
The trial’s findings contrast with those of a pilot study conducted by the ANZICS clinical trials group that did not find better outcomes with use of lower oxygen targets, according to Dr. Ferguson. However, in that trial, both arms had lower target and actual PaO2 levels. Thus, the optimal clinical approach remains uncertain.
“Until the results of further trials addressing this issue are available, there appears to be little downside in the careful titration and monitoring of supplemental oxygen in the ICU to achieve physiologically normal levels of PaO2 while avoiding potentially dangerous hyperoxia,” he concludes.
Dr. Ferguson disclosed that he has no relevant conflicts of interest.
Niall D. Ferguson, MD, MSc, is with the Interdepartmental Division of Critical Care Medicine and Departments of Medicine and Physiology, University of Toronto; the Institute of Health Policy, Management, & Evaluation, University of Toronto; the Division of Respirology, Department of Medicine, University Health Network and Mount Sinai Hospital; and the Toronto General Research Institute.
A strategy of conservatively controlling oxygen delivery to patients in the intensive care unit results in lower mortality than the conventional, more liberal approach whereby patients are often kept in a hyperoxemic state, finds a randomized controlled trial.
The trial, known as Oxygen-ICU, enrolled more than 400 adult ICU patients from an Italian center. Initially planned to last 2 years, it was terminated early because of slow enrollment after an earthquake reduced ICU capacity, with the decision supported by positive results of an interim analysis.
Patients had an absolute nearly 9% lower risk of dying in the ICU with use of the conservative oxygen strategy as compared with the conventional one, according to data reported at the annual congress of the European Society of Intensive Care Medicine and simultaneously published (JAMA. 2016 Oct 5. doi: 10.1001/jama.2016.11993).
“To our knowledge, this is the first randomized clinical trial to evaluate the effect of a conservative oxygen therapy on mortality compared with a standard, more liberal approach in a medical-surgical population of adult critically ill patients,” write the investigators, who were led by Massimo Girardis, MD, of the Intensive Care Unit, Department of Anesthesiology and Intensive Care, University Hospital of Modena (Italy).
Among critically ill patients with an ICU length of stay of 72 hours or longer, a conservative protocol for oxygen therapy compared with conventional therapy resulted in a lower ICU mortality,” they conclude. “However, these preliminary findings were based on unplanned early termination of the trial, and a larger multicenter trial is needed to evaluate the potential benefit of such conservative oxygen therapy in critically ill patients.”
In the trial, consecutive patients were randomized evenly to receive conservative oxygen therapy (maintenance of PaO2 between 70 and 100 mm Hg or arterial oxyhemoglobin saturation [SpO2] between 94% and 98%) or conventional oxygen therapy (allowance of PaO2 values up to 150 mm Hg or SpO2 values between 97% and 100%) on an open-label basis.
The originally targeted enrollment was 660 patients, but the study was stopped early after only 480 patients had been enrolled.
Results of modified intent-to-treat analyses showed that daily time-weighted PaO2 averages during patients’ ICU stays were higher in the conventional group than in the conservative group (median PaO2, 102 vs. 87 mm Hg; P less than .001).
The rate of ICU mortality, the trial’s primary endpoint, was 11.6% with conservative therapy, about half of the 20.2% seen with conventional therapy (absolute mean difference, 0.086; P = .01).
The conservative group also had lower rates of shock (3.7% vs. 10.6%, P = .006), liver failure (1.9% vs. 6.4%, P = .02), and bacteremia (5.1% vs. 10.1%, P = .049). And they spent a day less on the ventilator (median mechanical ventilation–free hours, 72 vs. 48; P = .02).
Lengths of ICU stay and hospital stay did not differ between the two groups.
One of the study authors reports serving as the data monitoring chair for a phase II study sponsored by InflaRx, on the antibiotic advisory board for Bayer, and on sepsis advisory boards for Biotest and Merck. The study was supported by the National Fund for Scientific Research of the University of Modena and Reggio Emilia.
A strategy of conservatively controlling oxygen delivery to patients in the intensive care unit results in lower mortality than the conventional, more liberal approach whereby patients are often kept in a hyperoxemic state, finds a randomized controlled trial.
The trial, known as Oxygen-ICU, enrolled more than 400 adult ICU patients from an Italian center. Initially planned to last 2 years, it was terminated early because of slow enrollment after an earthquake reduced ICU capacity, with the decision supported by positive results of an interim analysis.
Patients had an absolute nearly 9% lower risk of dying in the ICU with use of the conservative oxygen strategy as compared with the conventional one, according to data reported at the annual congress of the European Society of Intensive Care Medicine and simultaneously published (JAMA. 2016 Oct 5. doi: 10.1001/jama.2016.11993).
“To our knowledge, this is the first randomized clinical trial to evaluate the effect of a conservative oxygen therapy on mortality compared with a standard, more liberal approach in a medical-surgical population of adult critically ill patients,” write the investigators, who were led by Massimo Girardis, MD, of the Intensive Care Unit, Department of Anesthesiology and Intensive Care, University Hospital of Modena (Italy).
Among critically ill patients with an ICU length of stay of 72 hours or longer, a conservative protocol for oxygen therapy compared with conventional therapy resulted in a lower ICU mortality,” they conclude. “However, these preliminary findings were based on unplanned early termination of the trial, and a larger multicenter trial is needed to evaluate the potential benefit of such conservative oxygen therapy in critically ill patients.”
In the trial, consecutive patients were randomized evenly to receive conservative oxygen therapy (maintenance of PaO2 between 70 and 100 mm Hg or arterial oxyhemoglobin saturation [SpO2] between 94% and 98%) or conventional oxygen therapy (allowance of PaO2 values up to 150 mm Hg or SpO2 values between 97% and 100%) on an open-label basis.
The originally targeted enrollment was 660 patients, but the study was stopped early after only 480 patients had been enrolled.
Results of modified intent-to-treat analyses showed that daily time-weighted PaO2 averages during patients’ ICU stays were higher in the conventional group than in the conservative group (median PaO2, 102 vs. 87 mm Hg; P less than .001).
The rate of ICU mortality, the trial’s primary endpoint, was 11.6% with conservative therapy, about half of the 20.2% seen with conventional therapy (absolute mean difference, 0.086; P = .01).
The conservative group also had lower rates of shock (3.7% vs. 10.6%, P = .006), liver failure (1.9% vs. 6.4%, P = .02), and bacteremia (5.1% vs. 10.1%, P = .049). And they spent a day less on the ventilator (median mechanical ventilation–free hours, 72 vs. 48; P = .02).
Lengths of ICU stay and hospital stay did not differ between the two groups.
One of the study authors reports serving as the data monitoring chair for a phase II study sponsored by InflaRx, on the antibiotic advisory board for Bayer, and on sepsis advisory boards for Biotest and Merck. The study was supported by the National Fund for Scientific Research of the University of Modena and Reggio Emilia.
FROM ESICM CONGRESS 2016
Key clinical point:
Major finding: Relative to conventional therapy, conservative therapy was associated with a lower ICU mortality (absolute risk reduction, 0.086; P = .01).
Data source: A randomized controlled trial among 434 patients admitted to a medical-surgical ICU and expected to stay at least 72 hours (Oxygen-ICU trial).
Disclosures: One of the study authors reports serving as the data monitoring chair for a phase II study sponsored by InflaRx, on the antibiotic advisory board for Bayer, and on sepsis advisory boards for Biotest and Merck. The study was supported by the National Fund for Scientific Research of the University of Modena and Reggio Emilia. Dr. Ferguson disclosed that he has no relevant conflicts of interest.
Guselkumab achieves highest-ever response rates in psoriasis
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
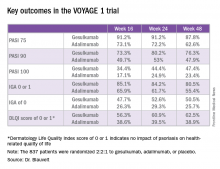
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
VIENNA – The investigational interleukin-23 inhibitor guselkumab decisively outperformed adalimumab in a head-to-head comparison for treatment of moderate or severe plaque psoriasis in the pivotal VOYAGE 1 study, Andrew Blauvelt, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
VOYAGE 1 was a 48-week, multicenter, international phase III trial in which 837 patients were randomized 2:2:1 to guselkumab, adalimumab (Humira), or placebo, with the placebo group switched to guselkumab at 16 weeks. Roughly three-quarters of patients had moderate psoriasis, the rest had severe disease. One in five had previously been treated with biologic agents; the only biologic disallowed was adalimumab.
The primary endpoints required by regulatory agencies involved efficacy comparisons between guselkumab and placebo at 16 weeks. Those results were a foregone conclusion. Far more arresting were the prespecified secondary endpoints comparing guselkumab to adalimumab at 24 and 48 weeks.
“These are very exciting results. We’re seeing efficacy in this trial that has not ever been seen before in a phase III study,” said Dr. Blauvelt, president of the Oregon Medical Research Center in Portland.
Take, for example, an efficacy yardstick dermatologists are quite familiar with: the PASI 75 response, defined as at least a 75% improvement from baseline in the Psoriasis Area Severity Index score, which averaged 22 at baseline in this trial. The PASI 75 rate in guselkumab-treated patients was 91.2% at 16 weeks, remained at 91.2% at 24 weeks, and was 87.8% at week 48.
“To my knowledge this is the highest PASI 75 response rate that’s been seen in a phase III study of any biologic in psoriasis,” the dermatologist said.
The PASI 75 rates with adalimumab, a tumor necrosis factor–alpha blocker widely prescribed for psoriasis, were markedly lower, although just a few years ago they would have been considered stratospheric: 73.1% at 16 weeks, 72.2% at 24 weeks, and 62.6% at 48 weeks.
The same pattern held for PASI 90, PASI 100, Investigator’s Global Assessment (IGA), and quality-of-life measures.
“There is a clear early separation of guselkumab from adalimumab, sustained over time, curves staying flat, responses not dropping off,” Dr. Blauvelt said in summary.
Guselkumab was dosed at 100 mg subcutaneously at weeks 0 and 4, then every 8 weeks thereafter. Adalimumab was dosed subcutaneously at 80 mg at week 0, 40 mg at week 1, and then 40 mg every other week.
The two coprimary outcomes at week 16 in VOYAGE 1 were the guselkumab and placebo groups’ rates of clear or almost clear skin as defined by an IGA score of 0 or 1, and their PASI 90 response rates. An IGA of 0 or 1 was achieved by 85.1% of the guselkumab group compared with 6.9% on placebo. The week-16 PASI 90 rates – a “high bar” Dr. Blauvelt noted – were 73.3% and 2.9%, respectively.
“Clearly we’re now in an era where PASI 90 is the new PASI 75,” said session cochair Lajos Kemény, MD, professor and chairman of the department of dermatology and allergology at the University of Szeged, Hungary.
Guselkumab is a human monoclonal antibody directed at the p-19 subunit of interleukin-23, thereby preventing the inflammatory cytokine from binding to its receptor. In contrast, ustekinumab (Stelara) blocks both IL-23 and IL-12. Given that ustekinumab has established an excellent long-term safety record in PSOLAR, the Psoriasis Longitudinal Assessment and Registry, it stands to reason that guselkumab should have a favorable safety profile, too, since it targets only one of the two cytokines (J Drugs Dermatol. 2015 Jul;14[7]:706-14). And this indeed proved to be the case through 48 weeks in VOYAGE 1, according to Dr. Blauvelt.
Infections treated with antibiotics occurred in 6.1% of the guselkumab group, 7.2% of patients on adalimumab, and 7.5% on placebo. Mild to moderate injection site reactions occurred in 2.4% of patients on guselkumab and 7.5% on adalimumab. One patient on each of the biologics experienced an acute MI. Two malignancies occurred, both in the guselkumab group. One was prostate cancer, the other was a case of male breast cancer in a patient with a breast mass present at enrollment.
Results of two additional pivotal phase III trials, VOYAGE 2 and NAVIGATE, will be presented at future meetings. NAVIGATE is looking specifically at guselkumab’s performance in psoriasis patients with an inadequate response to ustekinumab.
“Those results look promising. It appears that patients who didn’t clear adequately on ustekinumab do well on guselkumab,” Dr. Blauvelt said in response to an audience question.
A phase II study of guselkumab in treating moderate to severe psoriatic arthritis is ongoing.
VOYAGE 1 was funded by Janssen, which is developing guselkumab. Dr. Blauvelt reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Key clinical point:
Major finding: The PASI 90 response rate at 24 weeks was 80% in psoriasis patients on guselkumab compared with 53% in those on adalimumab.
Data source: A randomized, multinational, 48-week, pivotal phase III clinical trial involving 837 psoriasis patients assigned to guselkumab, adalimumab, or placebo.
Disclosures: The VOYAGE 1 trial was funded by Janssen, which is developing guselkumab. The study presenter reported receiving research grants from and serving as a scientific consultant to Janssen and numerous other pharmaceutical companies.
Facing a medical board investigation
(This column is the third of a three-part series.)
Question: The state medical board is requesting the medical records of an angry patient who has filed a formal complaint alleging incompetent care. Your immediate impulse is to rush off a letter defending your treatment – and to include a sarcastic remark about ingratitude.
However, you decided not to respond, choosing to await the scheduled interview with the board investigator. To your surprise, he turned out to be warm and friendly, and his relative youth reminded you of your son. Letting your guard down, you spoke freely during the meeting and showed him the medical records. Later, you appeared at the formal board hearing without legal counsel, as the complainant had not suffered any injury while under your care. Under this scenario, which of the following statements is best?
A. You must never respond in writing to any board inquiry.
B. You should have called your insurance carrier immediately.
C. You trusted the investigator because what you told him could not be introduced as evidence.
D. You correctly assumed that, without injuries, there is no liability.
E. You did not insist on having an attorney, because this is not part of your due process rights.
Answer: B. Who gets into trouble with medical disciplinary boards? In a California study,1 the authors concluded that male physicians were nearly three times as likely to as women physicians, and those who were non–board certified twice as likely to as their board-certified counterparts. Obstetricians, gynecologists, family physicians, general practitioners, and psychiatrists were more likely than internists, whereas pediatricians and radiologists were the least likely. Age had a smaller influence (elevated risk with increasing age), and foreign medical graduates also had an increased risk.
It is true that many board complaints are dismissed and never investigated, but the number of state licensure actions in 2013 was almost four times greater than the number of malpractice payouts. Moreover, board actions against a doctor, unlike a malpractice lawsuit, are premised on substandard or unethical conduct, and do not require a showing of actual patient harm.
A recent news article bemoans the travails of a medical board encounter.2 According to a 2009 report, one of every eight physicians in California was being reported to the board each year, with a quarter of complaints leading to an investigation. In turn, a quarter of investigated complaints led to disciplinary proceedings against the physician.
A medical board investigation begins with a complaint lodged by a patient or some other party such as a pharmacist, nurse, or hospital peer review committee. Some states – e.g., California, Georgia, and Maryland – allow complaints to be anonymous, and many states now allow online submissions, whose ease may be expected to increase the number of filings. Oklahoma, for example, saw its board complaints rise by 40% in the 2 years after permitting this option.
Some complaints such as rudeness and disputes over fees may seem like a minor nuisance, but they can mushroom into new and more serious charges. The medical records may reveal other potential misconduct, such as delinquent record keeping or failure to obtain informed consent.
It is therefore prudent to treat all complaints seriously. A written response is necessary, but it must be cowritten with your attorney. Immediately contact your malpractice insurance carrier once an investigator approaches you, or upon notification by the medical board. Most malpractice policies include coverage for medical board investigations.
The simple rule is not to discuss with anyone – including (especially) the complainant – and await a call from the attorney assigned to you. Better yet, ask for an attorney whose skills you are aware of. Make sure he/she is experienced with board proceedings and not just malpractice litigation. There are legal technicalities that may be of importance, such as statutes of limitations barring board actions or the timely filing of an appeal. Everything should flow from here. The lawyer’s advice will most likely be: “Don’t speak to anyone, including your colleagues; don’t contact the patient or family; and don’t release any records without first consulting me.”
You should together draft a response letter. Whereas the attorney will be attentive to the legal ramifications, only you as the doctor can provide the clinical knowledge, context, and empathy. In formulating any written response where patient care is at issue, heed the following principles:
1. Be honest. Truth always surfaces in due course. Candor and trustworthiness are virtues expected of all doctors.
2. Be accurate. Review carefully all relevant medical records. Pay compulsive attention to factual accuracy.
3. Be focused. Do not ramble on and on regarding unrelated or tangential issues. Focus on what the complaint is about. No one is interested in your views regarding your philosophy of medical practice or the health care system – they do not belong in a complaint response letter.
4. Be humble. Arrogance at this stage may prove disastrous. Adopt a contrite and humble tone. Blame no one, especially the patient.
5. Be a patient advocate. Show that patient well-being is always your first and last concern.
In the preliminary stages, a board investigator may call the doctor, typically taking a friendly and casual approach, and this may lull the physician into saying more than is necessary or releasing the medical records. The shared information can come back to haunt the doctor when it is disclosed in a subsequent hearing.
In some situations, the board may offer an informal settlement conference to resolve the issue and obtain a “consent agreement” from the doctor. Depending on the facts – and the proposed settlement terms – your attorney may instead advise proceeding to a formal hearing to present exculpatory evidence and to confront the complainant.
Physicians are guaranteed the right of due process during an investigation. Timely notice, the right to a hearing, to confront the evidence, and to have legal representation are the basic due process rights. And boards must treat the physician fairly and reasonably.
However, where public harm is an imminent risk, boards have the power to immediately institute a temporary suspension of the doctor’s right to practice. Examples warranting such summary suspensions may include sexual misconduct, inappropriate opioid prescriptions, egregious negligent conduct, or impairment from alcohol or drug abuse.
Boards face a dilemma over these cases, because they need to balance depriving a doctor of due process rights, albeit temporarily, against allowing a bad doctor to pose a clear and present threat to the public. In Texas, 32 summary suspensions took place in 2011, a figure that fell to 13 in 2014.
The aggrieved physician typically has the right to appeal an adverse board decision to the courts, although “courts have no inherent appellate jurisdiction over official acts of administrative agencies except where the legislature has made some statutory provision for judicial review.”3
For example, under California’s Code of Civil Procedure section 1094.5, one has the right to have a Superior Court judge review the board’s decision to determine if there has been an abuse of discretion.
Despite the fact that board administrative proceedings are quasi criminal in nature, most states require only a “preponderance of evidence” to find the doctor guilty. This is the evidentiary standard used in civil cases where only monetary damages are at stake, yet it is used in determining whether a doctor would lose the liberty to practice his or her profession.
However, some jurisdictions – such as California, Florida, and Illinois – call for a higher threshold of guilt, requiring proof with “clear and convincing evidence.”
Finally, in the United Kingdom and countries such as Singapore, a guilty decision requires even more evidence – i.e., proof “beyond reasonable doubt” – which is the threshold required in criminal prosecutions.
References
1. Arch Intern Med. 2004 Mar 22;164(6):653-8.
2. Leigh Page. The Black Cloud of a Medical Board Investigation. Medscape, Dec 23, 2015.
3. Crane v. Cont’l Tel. Co. of Cal., 775 P.2d 705 (Nev. 1989).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at siang@hawaii.edu.
(This column is the third of a three-part series.)
Question: The state medical board is requesting the medical records of an angry patient who has filed a formal complaint alleging incompetent care. Your immediate impulse is to rush off a letter defending your treatment – and to include a sarcastic remark about ingratitude.
However, you decided not to respond, choosing to await the scheduled interview with the board investigator. To your surprise, he turned out to be warm and friendly, and his relative youth reminded you of your son. Letting your guard down, you spoke freely during the meeting and showed him the medical records. Later, you appeared at the formal board hearing without legal counsel, as the complainant had not suffered any injury while under your care. Under this scenario, which of the following statements is best?
A. You must never respond in writing to any board inquiry.
B. You should have called your insurance carrier immediately.
C. You trusted the investigator because what you told him could not be introduced as evidence.
D. You correctly assumed that, without injuries, there is no liability.
E. You did not insist on having an attorney, because this is not part of your due process rights.
Answer: B. Who gets into trouble with medical disciplinary boards? In a California study,1 the authors concluded that male physicians were nearly three times as likely to as women physicians, and those who were non–board certified twice as likely to as their board-certified counterparts. Obstetricians, gynecologists, family physicians, general practitioners, and psychiatrists were more likely than internists, whereas pediatricians and radiologists were the least likely. Age had a smaller influence (elevated risk with increasing age), and foreign medical graduates also had an increased risk.
It is true that many board complaints are dismissed and never investigated, but the number of state licensure actions in 2013 was almost four times greater than the number of malpractice payouts. Moreover, board actions against a doctor, unlike a malpractice lawsuit, are premised on substandard or unethical conduct, and do not require a showing of actual patient harm.
A recent news article bemoans the travails of a medical board encounter.2 According to a 2009 report, one of every eight physicians in California was being reported to the board each year, with a quarter of complaints leading to an investigation. In turn, a quarter of investigated complaints led to disciplinary proceedings against the physician.
A medical board investigation begins with a complaint lodged by a patient or some other party such as a pharmacist, nurse, or hospital peer review committee. Some states – e.g., California, Georgia, and Maryland – allow complaints to be anonymous, and many states now allow online submissions, whose ease may be expected to increase the number of filings. Oklahoma, for example, saw its board complaints rise by 40% in the 2 years after permitting this option.
Some complaints such as rudeness and disputes over fees may seem like a minor nuisance, but they can mushroom into new and more serious charges. The medical records may reveal other potential misconduct, such as delinquent record keeping or failure to obtain informed consent.
It is therefore prudent to treat all complaints seriously. A written response is necessary, but it must be cowritten with your attorney. Immediately contact your malpractice insurance carrier once an investigator approaches you, or upon notification by the medical board. Most malpractice policies include coverage for medical board investigations.
The simple rule is not to discuss with anyone – including (especially) the complainant – and await a call from the attorney assigned to you. Better yet, ask for an attorney whose skills you are aware of. Make sure he/she is experienced with board proceedings and not just malpractice litigation. There are legal technicalities that may be of importance, such as statutes of limitations barring board actions or the timely filing of an appeal. Everything should flow from here. The lawyer’s advice will most likely be: “Don’t speak to anyone, including your colleagues; don’t contact the patient or family; and don’t release any records without first consulting me.”
You should together draft a response letter. Whereas the attorney will be attentive to the legal ramifications, only you as the doctor can provide the clinical knowledge, context, and empathy. In formulating any written response where patient care is at issue, heed the following principles:
1. Be honest. Truth always surfaces in due course. Candor and trustworthiness are virtues expected of all doctors.
2. Be accurate. Review carefully all relevant medical records. Pay compulsive attention to factual accuracy.
3. Be focused. Do not ramble on and on regarding unrelated or tangential issues. Focus on what the complaint is about. No one is interested in your views regarding your philosophy of medical practice or the health care system – they do not belong in a complaint response letter.
4. Be humble. Arrogance at this stage may prove disastrous. Adopt a contrite and humble tone. Blame no one, especially the patient.
5. Be a patient advocate. Show that patient well-being is always your first and last concern.
In the preliminary stages, a board investigator may call the doctor, typically taking a friendly and casual approach, and this may lull the physician into saying more than is necessary or releasing the medical records. The shared information can come back to haunt the doctor when it is disclosed in a subsequent hearing.
In some situations, the board may offer an informal settlement conference to resolve the issue and obtain a “consent agreement” from the doctor. Depending on the facts – and the proposed settlement terms – your attorney may instead advise proceeding to a formal hearing to present exculpatory evidence and to confront the complainant.
Physicians are guaranteed the right of due process during an investigation. Timely notice, the right to a hearing, to confront the evidence, and to have legal representation are the basic due process rights. And boards must treat the physician fairly and reasonably.
However, where public harm is an imminent risk, boards have the power to immediately institute a temporary suspension of the doctor’s right to practice. Examples warranting such summary suspensions may include sexual misconduct, inappropriate opioid prescriptions, egregious negligent conduct, or impairment from alcohol or drug abuse.
Boards face a dilemma over these cases, because they need to balance depriving a doctor of due process rights, albeit temporarily, against allowing a bad doctor to pose a clear and present threat to the public. In Texas, 32 summary suspensions took place in 2011, a figure that fell to 13 in 2014.
The aggrieved physician typically has the right to appeal an adverse board decision to the courts, although “courts have no inherent appellate jurisdiction over official acts of administrative agencies except where the legislature has made some statutory provision for judicial review.”3
For example, under California’s Code of Civil Procedure section 1094.5, one has the right to have a Superior Court judge review the board’s decision to determine if there has been an abuse of discretion.
Despite the fact that board administrative proceedings are quasi criminal in nature, most states require only a “preponderance of evidence” to find the doctor guilty. This is the evidentiary standard used in civil cases where only monetary damages are at stake, yet it is used in determining whether a doctor would lose the liberty to practice his or her profession.
However, some jurisdictions – such as California, Florida, and Illinois – call for a higher threshold of guilt, requiring proof with “clear and convincing evidence.”
Finally, in the United Kingdom and countries such as Singapore, a guilty decision requires even more evidence – i.e., proof “beyond reasonable doubt” – which is the threshold required in criminal prosecutions.
References
1. Arch Intern Med. 2004 Mar 22;164(6):653-8.
2. Leigh Page. The Black Cloud of a Medical Board Investigation. Medscape, Dec 23, 2015.
3. Crane v. Cont’l Tel. Co. of Cal., 775 P.2d 705 (Nev. 1989).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at siang@hawaii.edu.
(This column is the third of a three-part series.)
Question: The state medical board is requesting the medical records of an angry patient who has filed a formal complaint alleging incompetent care. Your immediate impulse is to rush off a letter defending your treatment – and to include a sarcastic remark about ingratitude.
However, you decided not to respond, choosing to await the scheduled interview with the board investigator. To your surprise, he turned out to be warm and friendly, and his relative youth reminded you of your son. Letting your guard down, you spoke freely during the meeting and showed him the medical records. Later, you appeared at the formal board hearing without legal counsel, as the complainant had not suffered any injury while under your care. Under this scenario, which of the following statements is best?
A. You must never respond in writing to any board inquiry.
B. You should have called your insurance carrier immediately.
C. You trusted the investigator because what you told him could not be introduced as evidence.
D. You correctly assumed that, without injuries, there is no liability.
E. You did not insist on having an attorney, because this is not part of your due process rights.
Answer: B. Who gets into trouble with medical disciplinary boards? In a California study,1 the authors concluded that male physicians were nearly three times as likely to as women physicians, and those who were non–board certified twice as likely to as their board-certified counterparts. Obstetricians, gynecologists, family physicians, general practitioners, and psychiatrists were more likely than internists, whereas pediatricians and radiologists were the least likely. Age had a smaller influence (elevated risk with increasing age), and foreign medical graduates also had an increased risk.
It is true that many board complaints are dismissed and never investigated, but the number of state licensure actions in 2013 was almost four times greater than the number of malpractice payouts. Moreover, board actions against a doctor, unlike a malpractice lawsuit, are premised on substandard or unethical conduct, and do not require a showing of actual patient harm.
A recent news article bemoans the travails of a medical board encounter.2 According to a 2009 report, one of every eight physicians in California was being reported to the board each year, with a quarter of complaints leading to an investigation. In turn, a quarter of investigated complaints led to disciplinary proceedings against the physician.
A medical board investigation begins with a complaint lodged by a patient or some other party such as a pharmacist, nurse, or hospital peer review committee. Some states – e.g., California, Georgia, and Maryland – allow complaints to be anonymous, and many states now allow online submissions, whose ease may be expected to increase the number of filings. Oklahoma, for example, saw its board complaints rise by 40% in the 2 years after permitting this option.
Some complaints such as rudeness and disputes over fees may seem like a minor nuisance, but they can mushroom into new and more serious charges. The medical records may reveal other potential misconduct, such as delinquent record keeping or failure to obtain informed consent.
It is therefore prudent to treat all complaints seriously. A written response is necessary, but it must be cowritten with your attorney. Immediately contact your malpractice insurance carrier once an investigator approaches you, or upon notification by the medical board. Most malpractice policies include coverage for medical board investigations.
The simple rule is not to discuss with anyone – including (especially) the complainant – and await a call from the attorney assigned to you. Better yet, ask for an attorney whose skills you are aware of. Make sure he/she is experienced with board proceedings and not just malpractice litigation. There are legal technicalities that may be of importance, such as statutes of limitations barring board actions or the timely filing of an appeal. Everything should flow from here. The lawyer’s advice will most likely be: “Don’t speak to anyone, including your colleagues; don’t contact the patient or family; and don’t release any records without first consulting me.”
You should together draft a response letter. Whereas the attorney will be attentive to the legal ramifications, only you as the doctor can provide the clinical knowledge, context, and empathy. In formulating any written response where patient care is at issue, heed the following principles:
1. Be honest. Truth always surfaces in due course. Candor and trustworthiness are virtues expected of all doctors.
2. Be accurate. Review carefully all relevant medical records. Pay compulsive attention to factual accuracy.
3. Be focused. Do not ramble on and on regarding unrelated or tangential issues. Focus on what the complaint is about. No one is interested in your views regarding your philosophy of medical practice or the health care system – they do not belong in a complaint response letter.
4. Be humble. Arrogance at this stage may prove disastrous. Adopt a contrite and humble tone. Blame no one, especially the patient.
5. Be a patient advocate. Show that patient well-being is always your first and last concern.
In the preliminary stages, a board investigator may call the doctor, typically taking a friendly and casual approach, and this may lull the physician into saying more than is necessary or releasing the medical records. The shared information can come back to haunt the doctor when it is disclosed in a subsequent hearing.
In some situations, the board may offer an informal settlement conference to resolve the issue and obtain a “consent agreement” from the doctor. Depending on the facts – and the proposed settlement terms – your attorney may instead advise proceeding to a formal hearing to present exculpatory evidence and to confront the complainant.
Physicians are guaranteed the right of due process during an investigation. Timely notice, the right to a hearing, to confront the evidence, and to have legal representation are the basic due process rights. And boards must treat the physician fairly and reasonably.
However, where public harm is an imminent risk, boards have the power to immediately institute a temporary suspension of the doctor’s right to practice. Examples warranting such summary suspensions may include sexual misconduct, inappropriate opioid prescriptions, egregious negligent conduct, or impairment from alcohol or drug abuse.
Boards face a dilemma over these cases, because they need to balance depriving a doctor of due process rights, albeit temporarily, against allowing a bad doctor to pose a clear and present threat to the public. In Texas, 32 summary suspensions took place in 2011, a figure that fell to 13 in 2014.
The aggrieved physician typically has the right to appeal an adverse board decision to the courts, although “courts have no inherent appellate jurisdiction over official acts of administrative agencies except where the legislature has made some statutory provision for judicial review.”3
For example, under California’s Code of Civil Procedure section 1094.5, one has the right to have a Superior Court judge review the board’s decision to determine if there has been an abuse of discretion.
Despite the fact that board administrative proceedings are quasi criminal in nature, most states require only a “preponderance of evidence” to find the doctor guilty. This is the evidentiary standard used in civil cases where only monetary damages are at stake, yet it is used in determining whether a doctor would lose the liberty to practice his or her profession.
However, some jurisdictions – such as California, Florida, and Illinois – call for a higher threshold of guilt, requiring proof with “clear and convincing evidence.”
Finally, in the United Kingdom and countries such as Singapore, a guilty decision requires even more evidence – i.e., proof “beyond reasonable doubt” – which is the threshold required in criminal prosecutions.
References
1. Arch Intern Med. 2004 Mar 22;164(6):653-8.
2. Leigh Page. The Black Cloud of a Medical Board Investigation. Medscape, Dec 23, 2015.
3. Crane v. Cont’l Tel. Co. of Cal., 775 P.2d 705 (Nev. 1989).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at siang@hawaii.edu.
October 2016 Quiz 2
Q2: Answer: C
Critique
Isolated gastric varices can be a complication of splenic vein thrombosis. The splenic vein courses superior to the pancreas from the splenic hilum to the main portal vein. Recurrent (or severe acute) inflammation of the pancreas can lead to splenic vein thrombosis, which can cause gastric varices due to backup of blood flow into the short gastric veins. The incidence of gastric varices in those with splenic vein thrombosis ranges from 15% to 55%.
Diagnosis can be made with a CT of the abdomen with contrast, and definitive treatment is splenectomy. Bleeding from a splenic artery pseudoaneurysm can also occur after pancreatitis (hemosuccus pancreaticus), and causes hemodynanically significant bleeding into the peritoneum or the bowel lumen via the pancreatic duct. However, it does not result in gastric varices.
Reference
- Henry Z., Uppal D., Caldwell S., et al. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-88.
Q2: Answer: C
Critique
Isolated gastric varices can be a complication of splenic vein thrombosis. The splenic vein courses superior to the pancreas from the splenic hilum to the main portal vein. Recurrent (or severe acute) inflammation of the pancreas can lead to splenic vein thrombosis, which can cause gastric varices due to backup of blood flow into the short gastric veins. The incidence of gastric varices in those with splenic vein thrombosis ranges from 15% to 55%.
Diagnosis can be made with a CT of the abdomen with contrast, and definitive treatment is splenectomy. Bleeding from a splenic artery pseudoaneurysm can also occur after pancreatitis (hemosuccus pancreaticus), and causes hemodynanically significant bleeding into the peritoneum or the bowel lumen via the pancreatic duct. However, it does not result in gastric varices.
Q2: Answer: C
Critique
Isolated gastric varices can be a complication of splenic vein thrombosis. The splenic vein courses superior to the pancreas from the splenic hilum to the main portal vein. Recurrent (or severe acute) inflammation of the pancreas can lead to splenic vein thrombosis, which can cause gastric varices due to backup of blood flow into the short gastric veins. The incidence of gastric varices in those with splenic vein thrombosis ranges from 15% to 55%.
Diagnosis can be made with a CT of the abdomen with contrast, and definitive treatment is splenectomy. Bleeding from a splenic artery pseudoaneurysm can also occur after pancreatitis (hemosuccus pancreaticus), and causes hemodynanically significant bleeding into the peritoneum or the bowel lumen via the pancreatic duct. However, it does not result in gastric varices.
Reference
- Henry Z., Uppal D., Caldwell S., et al. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-88.
Reference
- Henry Z., Uppal D., Caldwell S., et al. Gastric and ectopic varices. Clin Liver Dis. 2014;18:371-88.
A 52-year-old woman with a history of both alcohol and IV drug abuse presents to the emergency department with hematemesis and melena. She was recently discharged from the hospital after an admission for severe acute pancreatitis and has been hospitalized multiple times with alcoholic pancreatitis. After resuscitation, she undergoes urgent endoscopy and is found to have a large gastric varix with a red wale sign, but no active bleeding. There are no esophageal varices.
October 2016 Quiz 1
Q1: Answer: A
Rationale
Vibrio cholerae is a cause of secretory diarrhea. Fecal osmotic gap calculation can be useful in the evaluation of a patient with chronic diarrhea to differentiate between osmotic and secretory diarrhea. It is calculated by subtracting measured fecal electrolytes from normal lumen osmolality (290 – 2(fecal Na+ fecal K)). Secretory diarrhea is characterized by a fecal osmotic gap of less than 50 mOsm/kg, therefore answer A is the correct answer. Osmotic diarrhea is characterized by a fecal osmotic gap greater than 50 mOsm/kg or more specifically, more than 100 mOsm/kg, therefore answer B is incorrect. Accurate fecal osmotic gap calculation depends on normal stool osmolality, which is 290 mOsm/kg. Hypertonic (answer D) or dilute (answer C) stool samples are not appropriate for the calculation of the fecal osmotic gap, therefore answer C and D are incorrect.
Reference
- Eherer A.J., Fordtran J.S. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
Q1: Answer: A
Rationale
Vibrio cholerae is a cause of secretory diarrhea. Fecal osmotic gap calculation can be useful in the evaluation of a patient with chronic diarrhea to differentiate between osmotic and secretory diarrhea. It is calculated by subtracting measured fecal electrolytes from normal lumen osmolality (290 – 2(fecal Na+ fecal K)). Secretory diarrhea is characterized by a fecal osmotic gap of less than 50 mOsm/kg, therefore answer A is the correct answer. Osmotic diarrhea is characterized by a fecal osmotic gap greater than 50 mOsm/kg or more specifically, more than 100 mOsm/kg, therefore answer B is incorrect. Accurate fecal osmotic gap calculation depends on normal stool osmolality, which is 290 mOsm/kg. Hypertonic (answer D) or dilute (answer C) stool samples are not appropriate for the calculation of the fecal osmotic gap, therefore answer C and D are incorrect.
Q1: Answer: A
Rationale
Vibrio cholerae is a cause of secretory diarrhea. Fecal osmotic gap calculation can be useful in the evaluation of a patient with chronic diarrhea to differentiate between osmotic and secretory diarrhea. It is calculated by subtracting measured fecal electrolytes from normal lumen osmolality (290 – 2(fecal Na+ fecal K)). Secretory diarrhea is characterized by a fecal osmotic gap of less than 50 mOsm/kg, therefore answer A is the correct answer. Osmotic diarrhea is characterized by a fecal osmotic gap greater than 50 mOsm/kg or more specifically, more than 100 mOsm/kg, therefore answer B is incorrect. Accurate fecal osmotic gap calculation depends on normal stool osmolality, which is 290 mOsm/kg. Hypertonic (answer D) or dilute (answer C) stool samples are not appropriate for the calculation of the fecal osmotic gap, therefore answer C and D are incorrect.
Reference
- Eherer A.J., Fordtran J.S. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
Reference
- Eherer A.J., Fordtran J.S. Fecal osmotic gap and pH in experimental diarrhea of various causes. Gastroenterology. 1992;103:545-51.
A 37-year-old man presents with acute nausea, vomiting, and diarrhea consistent with an acute Vibrio cholerae infection.
Ribociclib/letrozole combo a ‘game changer’ in advanced breast cancer
COPENHAGEN – Progression-free survival for postmenopausal women with hormone receptor–positive advanced breast cancer was significantly improved when the cyclin-dependent kinase inhibitor ribociclib was added to standard therapy with the aromatase inhibitor letrozole, interim analysis from a phase III randomized trial shows.
After 18 months of follow-up, the risk of progression-free survival (PFS) among patients assigned to receive letrozole (Femara) and ribociclib was 44% lower than for patients assigned to receive letrozole and placebo, reported Gabriel N. Hortobagyi, MD, of the University of Texas MD Anderson Cancer Center, Houston, on behalf of coinvestigators in the MONALEESA-2 trial.
“We concluded that patients who received ribociclib with letrozole had a statistically significant and a clinically meaningful increase in progress-free survival compared to letrozole plus placebo, or letrozole alone,” he said at the European Society for Medical Oncology Congress.
“Is this a game changer? I think it probably is,” said invited discussant Stephen R.D. Johnston, MD, PhD, professor of breast cancer medicine and consultant oncologist at Royal Marsden Hospital in London.
Results of the first interim analysis of MONALEESA-2 (Mammary Oncology Assessment of LEE011’s Efficacy and Safety) were published simultaneously in the New England Journal of Medicine.
CDK 4 and 6 are frequently overexpressed in hormone receptor–positive breast cancer, and are key mediators of endocrine resistance, Dr. Hortobagyi explained.
Ribociclib is an oral small-molecule inhibitor of the pathway that includes CDK4/6 and the retinoblastoma protein, and has been shown to have efficacy in previously untreated HR-positive advanced breast cancer, and in patients with progressive disease on other therapies.
The MONALEESA-2 study is a phase III, double-blind, placebo-controlled trial of 668 postmenopausal women with HR-positive, HER2-negative advanced breast cancer who had not received prior therapy for advanced disease. Patients were stratified by the presence or absence of liver and/or lung metastases, and then assigned to receive letrozole 2.5 mg/day plus either ribociclib 600 mg/day for 3 weeks followed by 1 week off, or placebo on the same schedule.
At a preplanned interim analysis, after 243 events (disease progression or death) had occurred, the investigators found that the trial met its primary endpoint of locally assessed PFS, with a hazard ratio for ribociclib of 0.556 (P = .0000329 for superiority).
At the time of the analysis, median PFS had not been reached in the combination arm, compared with 14.7 months for the letrozole/placebo arm.
At 18 months’ follow-up (median 15.3 months), the PFS rate for ribociclib and letrozole was 63%, compared with 42.2% in the placebo group.
Respective response rates for patients with measurable disease at baseline were 52.7% vs. 37.1% (P less than .001).
Grade 3 or 4 adverse events occurring in more than 10% of patients included neutropenia in nearly two-thirds (59.3%) of the women who received ribociclib vs. 0.9% of those who received placebo, and leukopenia in 21% vs. 0.6%, respectively. In all, 7.5% of patients in the ribociclib group discontinued therapy because of adverse events, compared with 2.1% in the placebo group.
The challenge, Dr. Johnston said, will be to figure out how to integrate the findings from this trial and similar results with the CDK4/6 inhibitor palbociclib and letrozole seen in the PALOMA-2 trial into clinical practice.
Clinicians will be faced with deciding how to select various patient groups – endocrine sensitive, endocrine resistant, and treatment-naive – for targeted combinations or perhaps, for some patients, an aromatase inhibitor alone.
It also remains to be seen whether the CDK4/6 inhibitor/AI combination will be cost effective or affordable in many countries, and the pattern of resistance to CDK 4/6 inhibition is sill unknown, Dr. Johnston said.
The study was funded by Novartis. Dr. Hortobagyi disclosed grants and personal fees from the company during the conduct of the study, and personal fees from Eli Lilly and Pfizer outside the submitted work. Dr. Johnston disclosed consulting and/or research funding with AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, Eli Lilly, Roche/Genentech and Puma
COPENHAGEN – Progression-free survival for postmenopausal women with hormone receptor–positive advanced breast cancer was significantly improved when the cyclin-dependent kinase inhibitor ribociclib was added to standard therapy with the aromatase inhibitor letrozole, interim analysis from a phase III randomized trial shows.
After 18 months of follow-up, the risk of progression-free survival (PFS) among patients assigned to receive letrozole (Femara) and ribociclib was 44% lower than for patients assigned to receive letrozole and placebo, reported Gabriel N. Hortobagyi, MD, of the University of Texas MD Anderson Cancer Center, Houston, on behalf of coinvestigators in the MONALEESA-2 trial.
“We concluded that patients who received ribociclib with letrozole had a statistically significant and a clinically meaningful increase in progress-free survival compared to letrozole plus placebo, or letrozole alone,” he said at the European Society for Medical Oncology Congress.
“Is this a game changer? I think it probably is,” said invited discussant Stephen R.D. Johnston, MD, PhD, professor of breast cancer medicine and consultant oncologist at Royal Marsden Hospital in London.
Results of the first interim analysis of MONALEESA-2 (Mammary Oncology Assessment of LEE011’s Efficacy and Safety) were published simultaneously in the New England Journal of Medicine.
CDK 4 and 6 are frequently overexpressed in hormone receptor–positive breast cancer, and are key mediators of endocrine resistance, Dr. Hortobagyi explained.
Ribociclib is an oral small-molecule inhibitor of the pathway that includes CDK4/6 and the retinoblastoma protein, and has been shown to have efficacy in previously untreated HR-positive advanced breast cancer, and in patients with progressive disease on other therapies.
The MONALEESA-2 study is a phase III, double-blind, placebo-controlled trial of 668 postmenopausal women with HR-positive, HER2-negative advanced breast cancer who had not received prior therapy for advanced disease. Patients were stratified by the presence or absence of liver and/or lung metastases, and then assigned to receive letrozole 2.5 mg/day plus either ribociclib 600 mg/day for 3 weeks followed by 1 week off, or placebo on the same schedule.
At a preplanned interim analysis, after 243 events (disease progression or death) had occurred, the investigators found that the trial met its primary endpoint of locally assessed PFS, with a hazard ratio for ribociclib of 0.556 (P = .0000329 for superiority).
At the time of the analysis, median PFS had not been reached in the combination arm, compared with 14.7 months for the letrozole/placebo arm.
At 18 months’ follow-up (median 15.3 months), the PFS rate for ribociclib and letrozole was 63%, compared with 42.2% in the placebo group.
Respective response rates for patients with measurable disease at baseline were 52.7% vs. 37.1% (P less than .001).
Grade 3 or 4 adverse events occurring in more than 10% of patients included neutropenia in nearly two-thirds (59.3%) of the women who received ribociclib vs. 0.9% of those who received placebo, and leukopenia in 21% vs. 0.6%, respectively. In all, 7.5% of patients in the ribociclib group discontinued therapy because of adverse events, compared with 2.1% in the placebo group.
The challenge, Dr. Johnston said, will be to figure out how to integrate the findings from this trial and similar results with the CDK4/6 inhibitor palbociclib and letrozole seen in the PALOMA-2 trial into clinical practice.
Clinicians will be faced with deciding how to select various patient groups – endocrine sensitive, endocrine resistant, and treatment-naive – for targeted combinations or perhaps, for some patients, an aromatase inhibitor alone.
It also remains to be seen whether the CDK4/6 inhibitor/AI combination will be cost effective or affordable in many countries, and the pattern of resistance to CDK 4/6 inhibition is sill unknown, Dr. Johnston said.
The study was funded by Novartis. Dr. Hortobagyi disclosed grants and personal fees from the company during the conduct of the study, and personal fees from Eli Lilly and Pfizer outside the submitted work. Dr. Johnston disclosed consulting and/or research funding with AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, Eli Lilly, Roche/Genentech and Puma
COPENHAGEN – Progression-free survival for postmenopausal women with hormone receptor–positive advanced breast cancer was significantly improved when the cyclin-dependent kinase inhibitor ribociclib was added to standard therapy with the aromatase inhibitor letrozole, interim analysis from a phase III randomized trial shows.
After 18 months of follow-up, the risk of progression-free survival (PFS) among patients assigned to receive letrozole (Femara) and ribociclib was 44% lower than for patients assigned to receive letrozole and placebo, reported Gabriel N. Hortobagyi, MD, of the University of Texas MD Anderson Cancer Center, Houston, on behalf of coinvestigators in the MONALEESA-2 trial.
“We concluded that patients who received ribociclib with letrozole had a statistically significant and a clinically meaningful increase in progress-free survival compared to letrozole plus placebo, or letrozole alone,” he said at the European Society for Medical Oncology Congress.
“Is this a game changer? I think it probably is,” said invited discussant Stephen R.D. Johnston, MD, PhD, professor of breast cancer medicine and consultant oncologist at Royal Marsden Hospital in London.
Results of the first interim analysis of MONALEESA-2 (Mammary Oncology Assessment of LEE011’s Efficacy and Safety) were published simultaneously in the New England Journal of Medicine.
CDK 4 and 6 are frequently overexpressed in hormone receptor–positive breast cancer, and are key mediators of endocrine resistance, Dr. Hortobagyi explained.
Ribociclib is an oral small-molecule inhibitor of the pathway that includes CDK4/6 and the retinoblastoma protein, and has been shown to have efficacy in previously untreated HR-positive advanced breast cancer, and in patients with progressive disease on other therapies.
The MONALEESA-2 study is a phase III, double-blind, placebo-controlled trial of 668 postmenopausal women with HR-positive, HER2-negative advanced breast cancer who had not received prior therapy for advanced disease. Patients were stratified by the presence or absence of liver and/or lung metastases, and then assigned to receive letrozole 2.5 mg/day plus either ribociclib 600 mg/day for 3 weeks followed by 1 week off, or placebo on the same schedule.
At a preplanned interim analysis, after 243 events (disease progression or death) had occurred, the investigators found that the trial met its primary endpoint of locally assessed PFS, with a hazard ratio for ribociclib of 0.556 (P = .0000329 for superiority).
At the time of the analysis, median PFS had not been reached in the combination arm, compared with 14.7 months for the letrozole/placebo arm.
At 18 months’ follow-up (median 15.3 months), the PFS rate for ribociclib and letrozole was 63%, compared with 42.2% in the placebo group.
Respective response rates for patients with measurable disease at baseline were 52.7% vs. 37.1% (P less than .001).
Grade 3 or 4 adverse events occurring in more than 10% of patients included neutropenia in nearly two-thirds (59.3%) of the women who received ribociclib vs. 0.9% of those who received placebo, and leukopenia in 21% vs. 0.6%, respectively. In all, 7.5% of patients in the ribociclib group discontinued therapy because of adverse events, compared with 2.1% in the placebo group.
The challenge, Dr. Johnston said, will be to figure out how to integrate the findings from this trial and similar results with the CDK4/6 inhibitor palbociclib and letrozole seen in the PALOMA-2 trial into clinical practice.
Clinicians will be faced with deciding how to select various patient groups – endocrine sensitive, endocrine resistant, and treatment-naive – for targeted combinations or perhaps, for some patients, an aromatase inhibitor alone.
It also remains to be seen whether the CDK4/6 inhibitor/AI combination will be cost effective or affordable in many countries, and the pattern of resistance to CDK 4/6 inhibition is sill unknown, Dr. Johnston said.
The study was funded by Novartis. Dr. Hortobagyi disclosed grants and personal fees from the company during the conduct of the study, and personal fees from Eli Lilly and Pfizer outside the submitted work. Dr. Johnston disclosed consulting and/or research funding with AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, Eli Lilly, Roche/Genentech and Puma
Key clinical point: Adding the CDK 4/6 inhibitor ribociclib to an aromatase inhibitor significantly improved PFS in postmenopausal women with HR+/HER2- advanced breast cancer.
Major finding: The hazard ratio for progression with the combination of ribociclib and letrozole was 0.556 compared with letrozole/placebo.
Data source: Randomized phase III, placebo controlled trial in 668 postmenopausal women with untreated HR-positive/HER2- advanced breast cancer.
Disclosures: The study was funded by Novartis. Dr. Hortobagyi disclosed grants and personal fees from the company during the conduct of the study, and personal fees from Eli Lilly and Pfizer outside the submitted work. Dr. Johnston disclosed consulting and/or research funding with AstraZeneca, GlaxoSmithKline, Novartis, Pfizer, Eli Lilly, Roche/Genentech and Puma.