User login
Bone-bashing effects of air pollution becoming clearer
We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer.
It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.
Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.
More recently, researchers have started examining the impact of air pollution on bone health.
As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.
Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. Particulate pollutants include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.
Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.
Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.
Why the impact on bones?
The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.
Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.
Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.
However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.
When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.
As an example of the latter, the city of London extended its ultralow emission zone (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.
We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.
For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.
Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.
A version of this article first appeared on Medscape.com.
We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer.
It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.
Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.
More recently, researchers have started examining the impact of air pollution on bone health.
As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.
Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. Particulate pollutants include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.
Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.
Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.
Why the impact on bones?
The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.
Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.
Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.
However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.
When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.
As an example of the latter, the city of London extended its ultralow emission zone (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.
We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.
For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.
Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.
A version of this article first appeared on Medscape.com.
We have long recognized that our environment has a significant impact on our general health. Air pollution is known to contribute to respiratory conditions, poor cardiovascular outcomes, and certain kinds of cancer.
It’s increasingly important to identify factors that might contribute to suboptimal bone density and associated fracture risk in the population as a whole, and particularly in older adults. Aging is associated with a higher risk for osteoporosis and fractures, with their attendant morbidity, but individuals differ in their extent of bone loss and risk for fractures.
Known factors affecting bone health include genetics, age, sex, nutrition, physical activity, and hormonal factors. Certain medications, diseases, and lifestyle choices – such as smoking and alcohol intake – can also have deleterious effects on bone.
More recently, researchers have started examining the impact of air pollution on bone health.
As we know, the degree of pollution varies greatly from one region to another and can potentially significantly affect life in many parts of the world. In fact, the World Health Organization indicates that 99% of the world’s population breathes air exceeding the WHO guideline limits for pollutants.
Air pollutants include particulate matter (PM) as well as gases, such as nitric oxide, nitrogen dioxide, ammonia, carbon monoxide, sulfur dioxide, ozone, and certain volatile organic compounds. Particulate pollutants include a variety of substances produced from mostly human activities (such as vehicle emissions, biofuel combustion, mining, agriculture, and manufacturing, and also forest fires). They are classified not by their composition, but by their size (for example, PM1.0, PM2.5, and PM10 indicate PM with a diameter < 1.0, 2.5, and 10 microns, respectively). The finer the particle, the more likely it is to cross into the systemic circulation from the respiratory tract, with the potential to induce oxidative, inflammatory, and other changes in the body.
Many studies report that air pollution is a risk factor for osteoporosis. Some have found associations of lower bone density, osteoporosis, and fracture risk with higher concentrations of PM1.0, PM2.5, or PM10, even after controlling for other factors that could affect bone health. Some researchers have reported that although they didn’t find a significant association between PM and bone health, they did find an association between distance from the freeway and bone health – thus, exposure to polycyclic aromatic hydrocarbons and black carbon from vehicle emissions needs to be studied as a contributor to fracture risk.
Importantly, a prospective, observational study from the Women’s Health Initiative (which included more than 9,000 ethnically diverse women from three sites in the United States) reported a significant negative impact of PM10, nitric oxide, nitrogen dioxide, and sulfur dioxide over 1, 3, and 5 years on bone density at multiple sites, and particularly at the lumbar spine, in both cross-sectional and longitudinal analyses after controlling for demographic and socioeconomic factors. This study reported that nitrogen dioxide exposure may be a key determinant of bone density at the lumbar spine and in the whole body. Similarly, other studies have reported associations between atmospheric nitrogen dioxide or sulfur dioxide and risk for osteoporotic fractures.
Why the impact on bones?
The potential negative impact of pollution on bone has been attributed to many factors. PM induces systemic inflammation and an increase in cytokines that stimulate bone cells (osteoclasts) that cause bone loss. Other pollutants (gases and metal compounds) can cause oxidative damage to bone cells, whereas others act as endocrine disrupters and affect the functioning of these cells.
Pollution might also affect the synthesis and metabolism of vitamin D, which is necessary for absorption of calcium from the gut. High rates of pollution can reduce the amount of ultraviolet radiation reaching the earth which is important because certain wavelengths of ultraviolet radiation are necessary for vitamin D synthesis in our skin. Reduced vitamin D synthesis in skin can lead to poorly mineralized bone unless there is sufficient intake of vitamin D in diet or as supplements. Also, the conversion of vitamin D to its active form happens in the kidneys, and PM can be harmful to renal function. PM is also believed to cause increased breakdown of vitamin D into its inactive form.
Conversely, some studies have reported no association between pollution and bone density or osteoporosis risk, and two meta-analyses indicated that the association between the two is inconsistent. Some factors explaining variances in results include the number of individuals included in the study (larger studies are generally considered to be more reproducible), the fact that most studies are cross-sectional and not prospective, many do not control for other factors that might be deleterious to bone, and prediction models for the extent of PM or other exposure may not be completely accurate.
However, another recent meta-analysis reported an increased risk for lower total-body bone density and hip fracture after exposure to air pollution, particularly PM2.5 and nitrogen dioxide, but not to PM10, nitric oxide, or ozone. More studies are needed to confirm, or refute, the association between air pollution and impaired bone health. But accumulating evidence suggests that air pollution very likely has a deleterious effect on bone.
When feasible, it’s important to avoid living or working in areas with poor air quality and high pollution rates. However, this isn’t always possible based on one’s occupation, geography, circumstances, or economic status. Therefore, attention to a cleaner environment is critical at both the individual and the macro level.
As an example of the latter, the city of London extended its ultralow emission zone (ULEZ) farther out of the city in October 2021, and a further expansion is planned to include all of the city’s boroughs in August 2023.
We can do our bit by driving less and walking, biking, or using public transportation more often. We can also turn off the car engine when it’s not running, maintain our vehicles, switch to electric or hand-powered yard equipment, and not burn household garbage and limit backyard fires. We can also switch from gas to solar energy or wind, use efficient appliances and heating, and avoid unnecessary energy use. And we can choose sustainable products when possible.
For optimal bone health, we should remind patients to eat a healthy diet with the requisite amount of protein, calcium, and vitamin D. Vitamin D and calcium supplementation may be necessary for people whose intake of dairy and dairy products is low. Other important strategies to optimize bone health include engaging in healthy physical activity; avoiding smoking or excessive alcohol intake; and treating underlying gastrointestinal, endocrine, or other conditions that can reduce bone density.
Madhusmita Misra, MD, MPH, is the chief of the division of pediatric endocrinology, Mass General for Children; the associate director of the Harvard Catalyst Translation and Clinical Research Center; and the director of the Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital, Boston.
A version of this article first appeared on Medscape.com.
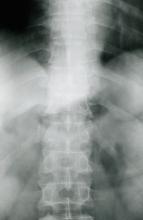
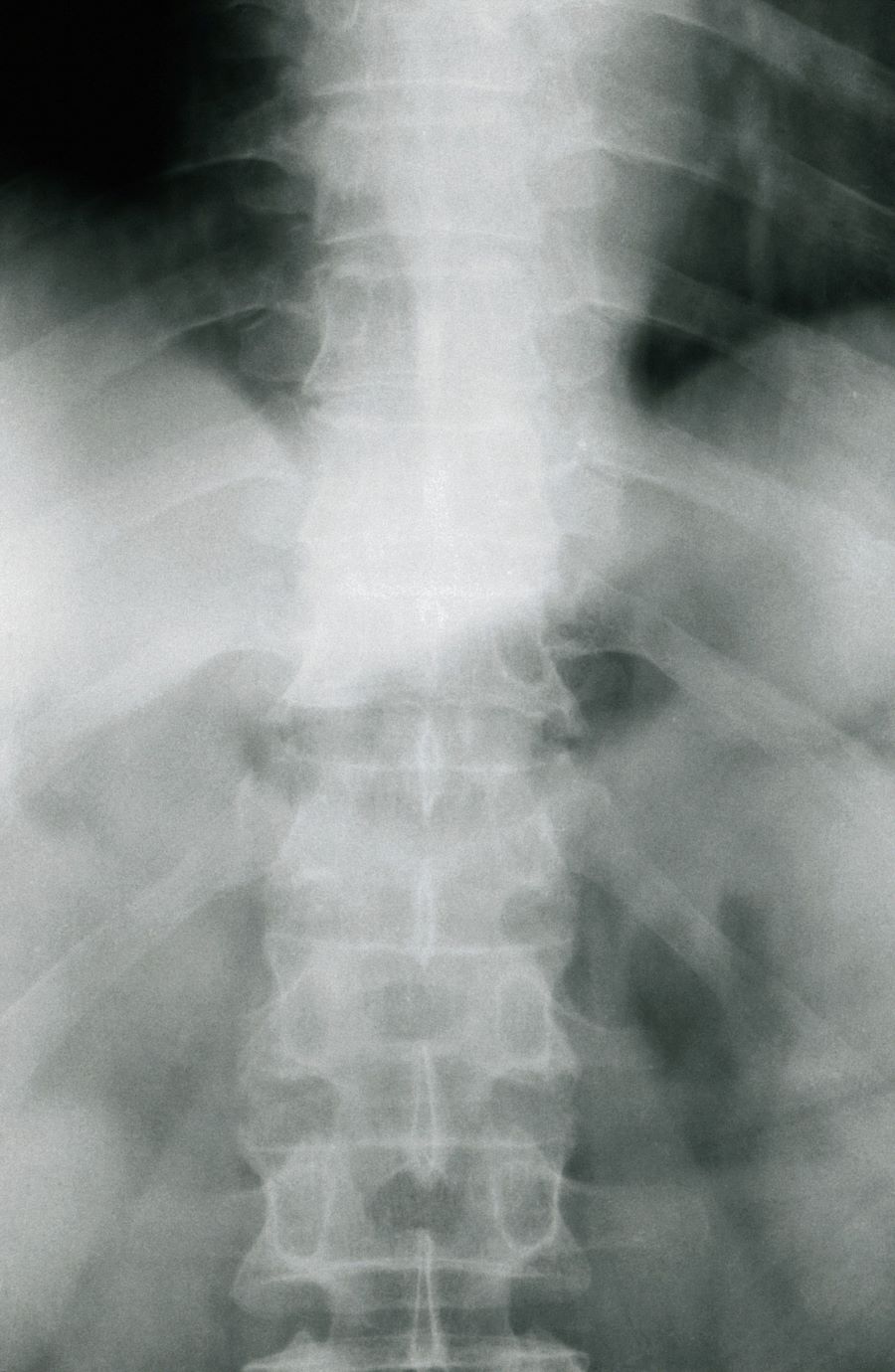
Moderate to severe back pain
The history and findings in this case are suggestive of axial psoriatic arthritis (PsA).
Psoriasis is a complex, chronic, inflammatory, immune-mediated disease that is associated with significant morbidity, reduced quality of life, and increased mortality. Approximately 7.4 million adults in the United States have psoriasis; worldwide, approximately 2%-3% of the population is affected. Patients with psoriasis frequently have comorbidities; PsA, an inflammatory, seronegative musculoskeletal disease, is among the most common. It is estimated that 25%-30% of patients with psoriasis develop PsA.
PsA is a heterogeneous disease. Patients may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Men and women are equally affected by PsA, which typically develops when patients are age 30-50 years. Like psoriasis, PsA is associated with numerous comorbidities, including cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
PsA is a potentially erosive disease. Structural damage and functional impairment occurs within 2 years of initial assessment in approximately 50% of patients; as the disease progresses, patients may experience irreversible joint damage and disability. Axial involvement occurs in 25%-70% of patients with PsA; exclusive axial involvement is uncommon, occurring in 5% of patients. Common symptoms of axial PsA include inflammatory back pain (eg, pain that improves with activity but worsens with rest, morning stiffness lasting longer than 30 minutes). Some patients with axial involvement may be asymptomatic. If untreated, cervical spinal mobility and lateral flexion significantly decline within 5 years in patients with axial PsA. In addition, sacroiliitis worsens over time; 37% and 52% of patients develop grade 2 or higher sacroiliitis within 5 and 10 years, respectively. This highlights the importance of early identification and treatment of patients with axial PsA.
The diagnosis of axial PsA is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosis). PsA can be differentiated from ankylosing spondylitis by the asymmetric and frequently unilateral presentation of sacroiliitis and syndesmophytes, which frequently presents as nonmarginal, bulky, asymmetric, and discontinuous skipping vertebral levels.
Plain radiography, CT, ultrasound, and MRI are all useful tools for evaluating patients with PsA. MRI and ultrasound may be more sensitive than plain radiography is for detecting early joint inflammation and damage as well as axial changes, including sacroiliitis; however, they are not required for a diagnosis of PsA.
The treatment of axial PsA is based on international guidelines developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Assessment of SpondyloArthritis International Society–European League Against Rheumatism. Treatment focuses on minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; enhancing functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Medications for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; however, long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or if erosive disease or other indications of high disease activity is observed, guidelines recommend initiation of a TNF inhibitor. Disease-modifying antirheumatic drugs, such as methotrexate, are not routinely prescribed for patients with axial disease because they have not been shown to be effective.
If symptoms of axial PsA are not controlled by NSAIDs, tumor necrosis factor (TNF) inhibitors are recommended. However, interleukin 17A inhibitors may be used in preference to TNF inhibitors in patients with significant skin involvement. In the United States, adalimumab, certolizumab pegol, golimumab, and infliximab are recommended over etanercept for patients with axial SpA in the presence of concomitant inflammatory bowel disease (IBD) or recurrent uveitis (although there is no evidence for golimumab) because etanercept has contradictory results for uveitis and has not been shown to have efficacy in IBD.
If patients fail to respond to a first trial of a TNF inhibitor, trying a second TNF inhibitor before switching to a different class of biologic is recommended by US guidelines. A Janus kinase inhibitor (tofacitinib) may be considered for patients who do not respond to TNF inhibitors.
Nonpharmacologic therapies (ie, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of axial psoriatic arthritis (PsA).
Psoriasis is a complex, chronic, inflammatory, immune-mediated disease that is associated with significant morbidity, reduced quality of life, and increased mortality. Approximately 7.4 million adults in the United States have psoriasis; worldwide, approximately 2%-3% of the population is affected. Patients with psoriasis frequently have comorbidities; PsA, an inflammatory, seronegative musculoskeletal disease, is among the most common. It is estimated that 25%-30% of patients with psoriasis develop PsA.
PsA is a heterogeneous disease. Patients may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Men and women are equally affected by PsA, which typically develops when patients are age 30-50 years. Like psoriasis, PsA is associated with numerous comorbidities, including cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
PsA is a potentially erosive disease. Structural damage and functional impairment occurs within 2 years of initial assessment in approximately 50% of patients; as the disease progresses, patients may experience irreversible joint damage and disability. Axial involvement occurs in 25%-70% of patients with PsA; exclusive axial involvement is uncommon, occurring in 5% of patients. Common symptoms of axial PsA include inflammatory back pain (eg, pain that improves with activity but worsens with rest, morning stiffness lasting longer than 30 minutes). Some patients with axial involvement may be asymptomatic. If untreated, cervical spinal mobility and lateral flexion significantly decline within 5 years in patients with axial PsA. In addition, sacroiliitis worsens over time; 37% and 52% of patients develop grade 2 or higher sacroiliitis within 5 and 10 years, respectively. This highlights the importance of early identification and treatment of patients with axial PsA.
The diagnosis of axial PsA is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosis). PsA can be differentiated from ankylosing spondylitis by the asymmetric and frequently unilateral presentation of sacroiliitis and syndesmophytes, which frequently presents as nonmarginal, bulky, asymmetric, and discontinuous skipping vertebral levels.
Plain radiography, CT, ultrasound, and MRI are all useful tools for evaluating patients with PsA. MRI and ultrasound may be more sensitive than plain radiography is for detecting early joint inflammation and damage as well as axial changes, including sacroiliitis; however, they are not required for a diagnosis of PsA.
The treatment of axial PsA is based on international guidelines developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Assessment of SpondyloArthritis International Society–European League Against Rheumatism. Treatment focuses on minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; enhancing functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Medications for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; however, long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or if erosive disease or other indications of high disease activity is observed, guidelines recommend initiation of a TNF inhibitor. Disease-modifying antirheumatic drugs, such as methotrexate, are not routinely prescribed for patients with axial disease because they have not been shown to be effective.
If symptoms of axial PsA are not controlled by NSAIDs, tumor necrosis factor (TNF) inhibitors are recommended. However, interleukin 17A inhibitors may be used in preference to TNF inhibitors in patients with significant skin involvement. In the United States, adalimumab, certolizumab pegol, golimumab, and infliximab are recommended over etanercept for patients with axial SpA in the presence of concomitant inflammatory bowel disease (IBD) or recurrent uveitis (although there is no evidence for golimumab) because etanercept has contradictory results for uveitis and has not been shown to have efficacy in IBD.
If patients fail to respond to a first trial of a TNF inhibitor, trying a second TNF inhibitor before switching to a different class of biologic is recommended by US guidelines. A Janus kinase inhibitor (tofacitinib) may be considered for patients who do not respond to TNF inhibitors.
Nonpharmacologic therapies (ie, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of axial psoriatic arthritis (PsA).
Psoriasis is a complex, chronic, inflammatory, immune-mediated disease that is associated with significant morbidity, reduced quality of life, and increased mortality. Approximately 7.4 million adults in the United States have psoriasis; worldwide, approximately 2%-3% of the population is affected. Patients with psoriasis frequently have comorbidities; PsA, an inflammatory, seronegative musculoskeletal disease, is among the most common. It is estimated that 25%-30% of patients with psoriasis develop PsA.
PsA is a heterogeneous disease. Patients may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Men and women are equally affected by PsA, which typically develops when patients are age 30-50 years. Like psoriasis, PsA is associated with numerous comorbidities, including cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
PsA is a potentially erosive disease. Structural damage and functional impairment occurs within 2 years of initial assessment in approximately 50% of patients; as the disease progresses, patients may experience irreversible joint damage and disability. Axial involvement occurs in 25%-70% of patients with PsA; exclusive axial involvement is uncommon, occurring in 5% of patients. Common symptoms of axial PsA include inflammatory back pain (eg, pain that improves with activity but worsens with rest, morning stiffness lasting longer than 30 minutes). Some patients with axial involvement may be asymptomatic. If untreated, cervical spinal mobility and lateral flexion significantly decline within 5 years in patients with axial PsA. In addition, sacroiliitis worsens over time; 37% and 52% of patients develop grade 2 or higher sacroiliitis within 5 and 10 years, respectively. This highlights the importance of early identification and treatment of patients with axial PsA.
The diagnosis of axial PsA is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosis). PsA can be differentiated from ankylosing spondylitis by the asymmetric and frequently unilateral presentation of sacroiliitis and syndesmophytes, which frequently presents as nonmarginal, bulky, asymmetric, and discontinuous skipping vertebral levels.
Plain radiography, CT, ultrasound, and MRI are all useful tools for evaluating patients with PsA. MRI and ultrasound may be more sensitive than plain radiography is for detecting early joint inflammation and damage as well as axial changes, including sacroiliitis; however, they are not required for a diagnosis of PsA.
The treatment of axial PsA is based on international guidelines developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Assessment of SpondyloArthritis International Society–European League Against Rheumatism. Treatment focuses on minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; enhancing functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Medications for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; however, long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or if erosive disease or other indications of high disease activity is observed, guidelines recommend initiation of a TNF inhibitor. Disease-modifying antirheumatic drugs, such as methotrexate, are not routinely prescribed for patients with axial disease because they have not been shown to be effective.
If symptoms of axial PsA are not controlled by NSAIDs, tumor necrosis factor (TNF) inhibitors are recommended. However, interleukin 17A inhibitors may be used in preference to TNF inhibitors in patients with significant skin involvement. In the United States, adalimumab, certolizumab pegol, golimumab, and infliximab are recommended over etanercept for patients with axial SpA in the presence of concomitant inflammatory bowel disease (IBD) or recurrent uveitis (although there is no evidence for golimumab) because etanercept has contradictory results for uveitis and has not been shown to have efficacy in IBD.
If patients fail to respond to a first trial of a TNF inhibitor, trying a second TNF inhibitor before switching to a different class of biologic is recommended by US guidelines. A Janus kinase inhibitor (tofacitinib) may be considered for patients who do not respond to TNF inhibitors.
Nonpharmacologic therapies (ie, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 38-year-old nonsmoking woman presents with complaints of moderate to severe back pain of approximately 6 months' duration. She also reports morning back/neck stiffness that lasts for approximately 45 minutes and pain/stiffness in her wrists and fingers. The patient states that her back pain improves with exercise (walking and stretching) and worsens in the evening and during long periods of rest. On occasion, she is awakened during the early morning hours because of her back pain. The patient has a 15-year history of moderate to severe psoriasis and a history of irritable bowel disease (IBD). Current medications include cyclosporine 3 mg/d, topical roflumilast 0.3%/d, and loperamide 3 mg as needed. The patient is 5 ft 5 in and weighs 183 lb (BMI of 30.4).
Physical examination reveals psoriatic plaques on the hands, elbows, and knees and nail dystrophy (onycholysis and pitting). Vital signs are within normal ranges. Pertinent laboratory findings include white blood count of 12,000 mcL (> 50% polymorphonuclear leukocytes), erythrocyte sedimentation rate of 19 mm/h, and c-reactive protein of 3 mg/dL. Rheumatoid factor, antinuclear antibody, and anti-citrullinated protein antibody antibody were negative.
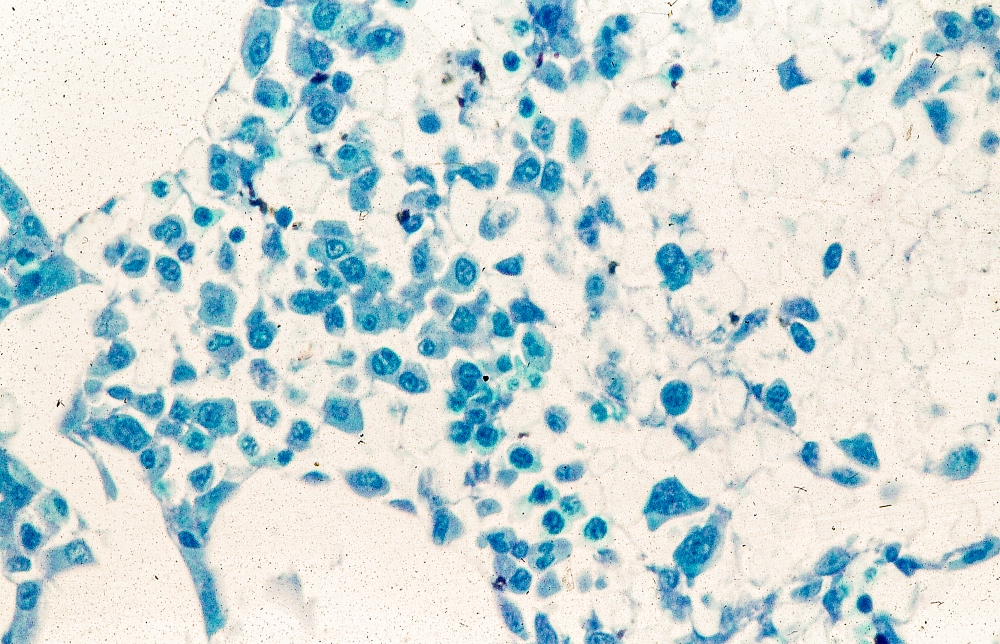
AI predicts endometrial cancer recurrence
Endometrial cancer is the most frequently occurring uterine cancer. Early-stage patients have about a 95% 5-year survival, but distant recurrence is associated with very poor survival, according to Sarah Fremond, MSc, an author of the research (Abstract 5695), which she presented at the annual meeting of the American Association for Cancer Research.
“Most patients with endometrial cancer have a good prognosis and would not require any adjuvant treatment, but there is a proportion that will develop distant recurrence. For those you want to recommend adjuvant chemotherapy, because currently in the adjuvant setting, that’s the only treatment that is known to lower the risk of distant recurrence. But that also causes morbidity. Therefore, our clinical question was how to accurately identify patients at low and high risk of distant recurrence to reduce under- and overtreatment,” said Ms. Fremond, a PhD candidate at Leiden (the Netherlands) University Medical Center.
Pathologists can attempt such predictions, but Ms. Fremond noted that there are challenges. “There is a lot of variability between pathologists, and we don’t even use the entire visual information present in the H&E [hematoxylin and eosin] tumor slide. When it comes to molecular testing, it is hampered by cost, turnaround time, and sometimes interpretation. It’s quite complex to combine those data to specifically target risk of distant recurrence for patients with endometrial cancer.”
In her presentation, Ms. Fremond described how she and her colleagues used digitized histopathological slides in their research. She and her coauthors developed the AI model as part of a collaboration that included the AIRMEC Consortium, Leiden University Medical Center, the TransPORTEC Consortium, and the University of Zürich.
The researchers used long-term follow-up data from 1,408 patients drawn from three clinical cohorts and participants in the PORTEC-1, PORTEC-2, and PORTEC-3 studies, which tested radiotherapy and adjuvant therapy outcomes in endometrial cancer. Patients who had received prior adjuvant chemotherapy were excluded. In the model development phase, the system analyzed a single representative histopathological slide image from each patient and compared it with the known time to distant recurrence to identify patterns.
Once the system had been trained, the researchers applied it to a novel group of 353 patients. It ranked 89 patients as having a low risk of recurrence, 175 at intermediate risk, and 89 at high risk of recurrence. The system performed well: 3.37% of low-risk patients experienced a distant recurrence, as did 15.43% of the intermediate-risk group and 36% of the high-risk group.
The researchers also employed an external validation group with 152 patients and three slides per patient, with a 2.8-year follow-up. The model performed with a C index of 0.805 (±0.0136) when a random slide was selected for each patient, and the median predicted risk score per patient was associated with differences in distant recurrence-free survival between the three risk groups with a C index of 0.816 (P < .0001).
Questions about research and their answers
Session moderator Kristin Swanson, PhD, asked if the AI could be used with the pathology slide’s visible features to learn more about the underlying biology and pathophysiology of tumors.
“Overlying the HECTOR on to the tissue seems like a logical opportunity to go and then explore the biology and what’s attributed as a high-risk region,” said Dr. Swanson, who is director of the Mathematical NeuroOncology Lab and codirector of the Precision NeuroTherapeutics Innovation Program at Mayo Clinic Arizona, Phoenix.
Ms. Fremond agreed that the AI has the potential to be used that way.”
During the Q&A, an audience member asked how likely the model is to perform in populations that differ significantly from the populations used in her study.
Ms. Fremond responded that the populations used to develop and test the models were in or close to the Netherlands, and little information was available regarding patient ethnicity. “There is a possibility that perhaps we would have a different performance on a population that includes more minorities. That needs to be checked,” said Ms. Fremond.
The study is limited by its retrospective nature.
Ms. Fremond and Dr. Swanson have no relevant financial disclosures.
Endometrial cancer is the most frequently occurring uterine cancer. Early-stage patients have about a 95% 5-year survival, but distant recurrence is associated with very poor survival, according to Sarah Fremond, MSc, an author of the research (Abstract 5695), which she presented at the annual meeting of the American Association for Cancer Research.
“Most patients with endometrial cancer have a good prognosis and would not require any adjuvant treatment, but there is a proportion that will develop distant recurrence. For those you want to recommend adjuvant chemotherapy, because currently in the adjuvant setting, that’s the only treatment that is known to lower the risk of distant recurrence. But that also causes morbidity. Therefore, our clinical question was how to accurately identify patients at low and high risk of distant recurrence to reduce under- and overtreatment,” said Ms. Fremond, a PhD candidate at Leiden (the Netherlands) University Medical Center.
Pathologists can attempt such predictions, but Ms. Fremond noted that there are challenges. “There is a lot of variability between pathologists, and we don’t even use the entire visual information present in the H&E [hematoxylin and eosin] tumor slide. When it comes to molecular testing, it is hampered by cost, turnaround time, and sometimes interpretation. It’s quite complex to combine those data to specifically target risk of distant recurrence for patients with endometrial cancer.”
In her presentation, Ms. Fremond described how she and her colleagues used digitized histopathological slides in their research. She and her coauthors developed the AI model as part of a collaboration that included the AIRMEC Consortium, Leiden University Medical Center, the TransPORTEC Consortium, and the University of Zürich.
The researchers used long-term follow-up data from 1,408 patients drawn from three clinical cohorts and participants in the PORTEC-1, PORTEC-2, and PORTEC-3 studies, which tested radiotherapy and adjuvant therapy outcomes in endometrial cancer. Patients who had received prior adjuvant chemotherapy were excluded. In the model development phase, the system analyzed a single representative histopathological slide image from each patient and compared it with the known time to distant recurrence to identify patterns.
Once the system had been trained, the researchers applied it to a novel group of 353 patients. It ranked 89 patients as having a low risk of recurrence, 175 at intermediate risk, and 89 at high risk of recurrence. The system performed well: 3.37% of low-risk patients experienced a distant recurrence, as did 15.43% of the intermediate-risk group and 36% of the high-risk group.
The researchers also employed an external validation group with 152 patients and three slides per patient, with a 2.8-year follow-up. The model performed with a C index of 0.805 (±0.0136) when a random slide was selected for each patient, and the median predicted risk score per patient was associated with differences in distant recurrence-free survival between the three risk groups with a C index of 0.816 (P < .0001).
Questions about research and their answers
Session moderator Kristin Swanson, PhD, asked if the AI could be used with the pathology slide’s visible features to learn more about the underlying biology and pathophysiology of tumors.
“Overlying the HECTOR on to the tissue seems like a logical opportunity to go and then explore the biology and what’s attributed as a high-risk region,” said Dr. Swanson, who is director of the Mathematical NeuroOncology Lab and codirector of the Precision NeuroTherapeutics Innovation Program at Mayo Clinic Arizona, Phoenix.
Ms. Fremond agreed that the AI has the potential to be used that way.”
During the Q&A, an audience member asked how likely the model is to perform in populations that differ significantly from the populations used in her study.
Ms. Fremond responded that the populations used to develop and test the models were in or close to the Netherlands, and little information was available regarding patient ethnicity. “There is a possibility that perhaps we would have a different performance on a population that includes more minorities. That needs to be checked,” said Ms. Fremond.
The study is limited by its retrospective nature.
Ms. Fremond and Dr. Swanson have no relevant financial disclosures.
Endometrial cancer is the most frequently occurring uterine cancer. Early-stage patients have about a 95% 5-year survival, but distant recurrence is associated with very poor survival, according to Sarah Fremond, MSc, an author of the research (Abstract 5695), which she presented at the annual meeting of the American Association for Cancer Research.
“Most patients with endometrial cancer have a good prognosis and would not require any adjuvant treatment, but there is a proportion that will develop distant recurrence. For those you want to recommend adjuvant chemotherapy, because currently in the adjuvant setting, that’s the only treatment that is known to lower the risk of distant recurrence. But that also causes morbidity. Therefore, our clinical question was how to accurately identify patients at low and high risk of distant recurrence to reduce under- and overtreatment,” said Ms. Fremond, a PhD candidate at Leiden (the Netherlands) University Medical Center.
Pathologists can attempt such predictions, but Ms. Fremond noted that there are challenges. “There is a lot of variability between pathologists, and we don’t even use the entire visual information present in the H&E [hematoxylin and eosin] tumor slide. When it comes to molecular testing, it is hampered by cost, turnaround time, and sometimes interpretation. It’s quite complex to combine those data to specifically target risk of distant recurrence for patients with endometrial cancer.”
In her presentation, Ms. Fremond described how she and her colleagues used digitized histopathological slides in their research. She and her coauthors developed the AI model as part of a collaboration that included the AIRMEC Consortium, Leiden University Medical Center, the TransPORTEC Consortium, and the University of Zürich.
The researchers used long-term follow-up data from 1,408 patients drawn from three clinical cohorts and participants in the PORTEC-1, PORTEC-2, and PORTEC-3 studies, which tested radiotherapy and adjuvant therapy outcomes in endometrial cancer. Patients who had received prior adjuvant chemotherapy were excluded. In the model development phase, the system analyzed a single representative histopathological slide image from each patient and compared it with the known time to distant recurrence to identify patterns.
Once the system had been trained, the researchers applied it to a novel group of 353 patients. It ranked 89 patients as having a low risk of recurrence, 175 at intermediate risk, and 89 at high risk of recurrence. The system performed well: 3.37% of low-risk patients experienced a distant recurrence, as did 15.43% of the intermediate-risk group and 36% of the high-risk group.
The researchers also employed an external validation group with 152 patients and three slides per patient, with a 2.8-year follow-up. The model performed with a C index of 0.805 (±0.0136) when a random slide was selected for each patient, and the median predicted risk score per patient was associated with differences in distant recurrence-free survival between the three risk groups with a C index of 0.816 (P < .0001).
Questions about research and their answers
Session moderator Kristin Swanson, PhD, asked if the AI could be used with the pathology slide’s visible features to learn more about the underlying biology and pathophysiology of tumors.
“Overlying the HECTOR on to the tissue seems like a logical opportunity to go and then explore the biology and what’s attributed as a high-risk region,” said Dr. Swanson, who is director of the Mathematical NeuroOncology Lab and codirector of the Precision NeuroTherapeutics Innovation Program at Mayo Clinic Arizona, Phoenix.
Ms. Fremond agreed that the AI has the potential to be used that way.”
During the Q&A, an audience member asked how likely the model is to perform in populations that differ significantly from the populations used in her study.
Ms. Fremond responded that the populations used to develop and test the models were in or close to the Netherlands, and little information was available regarding patient ethnicity. “There is a possibility that perhaps we would have a different performance on a population that includes more minorities. That needs to be checked,” said Ms. Fremond.
The study is limited by its retrospective nature.
Ms. Fremond and Dr. Swanson have no relevant financial disclosures.
FROM AACR 2023
CDC backs FDA’s call for second COVID booster for those at high risk
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
This backs the Food and Drug Administration’s authorization April 18 of the additional shot.
“Following FDA regulatory action, CDC has taken steps to simplify COVID-19 vaccine recommendations and allow more flexibility for people at higher risk who want the option of added protection from additional COVID-19 vaccine doses,” the CDC said in a statement.
The agency is following the recommendations made by its Advisory Committee on Immunization Practices (ACIP). While there was no vote, the group reaffirmed its commitment to boosters overall, proposing that all Americans over age 6 who have not had a bivalent mRNA COVID-19 booster vaccine go ahead and get one.
But most others who’ve already had the bivalent shot – which targets the original COVID strain and the two Omicron variants BA.4 and BA.5 – should wait until the fall to get whatever updated vaccine is available.
The panel did carve out exceptions for people over age 65 and those who are immunocompromised because they are at higher risk for severe COVID-19 complications, Evelyn Twentyman, MD, MPH, the lead official in the CDC’s COVID-19 Vaccine Policy Unit, said during the meeting.
People over 65 can now choose to get a second bivalent mRNA booster shot as long as it has been at least 4 months since the last one, she said, and people who are immunocompromised also should have the flexibility to receive one or more additional bivalent boosters at least 2 months after an initial dose.
Regardless of whether someone is unvaccinated, and regardless of how many single-strain COVID vaccines an individual has previously received, they should get a mRNA bivalent shot, Dr. Twentyman said.
If an individual has already received a bivalent mRNA booster – made by either Pfizer/BioNTech or Moderna – “your vaccination is complete,” she said. “No doses indicated at this time, come back and see us in autumn of 2023.”
The CDC is trying to encourage more people to get the updated COVID shot, as just 17% of Americans of any age have received a bivalent booster and only 43% of those age 65 and over.
The CDC followed the FDA’s lead in its statement, phasing out the original single-strain COVID vaccine, saying it will no longer be recommended for use in the United States.
‘Unnecessary drama’ over children’s recs
The CDC panel mostly followed the FDA’s guidance on who should get a booster, but many ACIP members expressed consternation and confusion about what was being recommended for children.
For children aged 6 months to 4 years, the CDC will offer tables to help physicians determine how many bivalent doses to give, depending on the child’s vaccination history.
All children those ages should get at least two vaccine doses, one of which is bivalent, Dr. Twentyman said. For children in that age group who have already received a monovalent series and a bivalent dose, “their vaccination is complete,” she said.
For 5-year-olds, the recommendations will be similar if they received a Pfizer monovalent series, but the shot regimen will have to be customized if they had previously received a Moderna shot, because of differences in the dosages.
ACIP member Sarah S. Long, MD, professor of pediatrics, Drexel University, Philadelphia, said that it was unclear why a set age couldn’t be established for COVID-19 vaccination as it had been for other immunizations.
“We picked 60 months for most immunizations in children,” Dr. Long said. “Immunologically there is not a difference between a 4-, a 5- and a 6-year-old.
“There isn’t a reason to have all this unnecessary drama around those ages,” she said, adding that having the different ages would make it harder for pediatricians to appropriately stock vaccines.
Dr. Twentyman said that the CDC would be providing more detailed guidance on its COVID-19 website soon and would be holding a call with health care professionals to discuss the updated recommendations on May 11.
New vaccine by fall
CDC and ACIP members both said they hoped to have an even simpler vaccine schedule by the fall, when it is anticipated that the FDA may have authorized a new, updated bivalent vaccine that targets other COVID variants.
“We all recognize this is a work in progress,” said ACIP Chair Grace M. Lee, MD, MPH, acknowledging that there is continued confusion over COVID-19 vaccination.
“The goal really is to try to simplify things over time to be able to help communicate with our provider community, and our patients and families what vaccine is right for them, when do they need it, and how often should they get it,” said Dr. Lee, professor of pediatrics, Stanford (Calif.) University.
A version of this article originally appeared on Medscape.com .
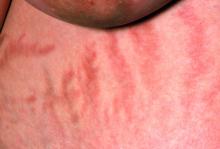
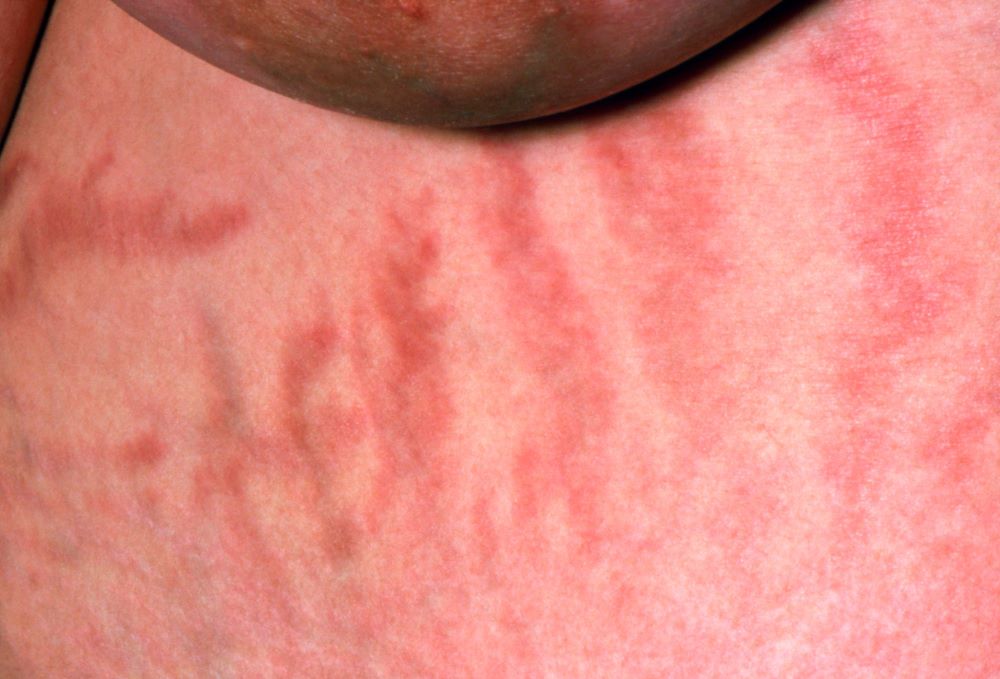
Weight gain and excessive fatigue
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of Cushing syndrome (CS).
CS is a rare endocrine disease caused by prolonged exposure to high circulating cortisol levels. Exogenous hypercortisolism is the most common cause of CS. It is largely iatrogenic and results from the prolonged use of glucocorticoids. Less frequently, endogenous CS may occur as the result of excessive production of cortisol by adrenal glands. Endogenous CS can be ACTH-dependent or ACTH-independent. ACTH-dependent CS results from ACTH-secreting pituitary adenomas (Cushing disease) and ectopic ACTH secretion by neoplasms, whereas adrenal hyperplasia, adenoma, and carcinoma are the primary causes of ACTH-independent CS.
The annual incidence and prevalence of CS are unknown; the reported incidence of newly diagnosed cases has ranged from 1.2 to 2.4 per million people per year. Women are affected more often than are men, with a peak of incidence in the third to fourth decade of life. CS is associated with various metabolic, psychiatric, musculoskeletal, and cardiovascular comorbidities. Untreated, it is associated with increased mortality, typically as the result of cardiovascular and infectious complications; however, even in appropriately treated patients, mortality is elevated.
The chronic elevations of glucocorticoid concentrations in CS result in its characteristic phenotype, which includes weight gain, moon-shaped face, buffalo hump, muscle weakness, increased bruising, skin atrophy, red abdominal striae, menstrual irregularities, hirsutism, and acne. It is also associated with numerous comorbidities including diabetes, hypertension, hypercholesterolemia, and osteoporosis. Patients often experience mental health complications, such as depression, emotional lability, and cognitive dysfunction.
Given the rarity of CS and the fact that these symptoms overlap with other conditions, delayed diagnosis is common. The current obesity epidemic also poses diagnostic challenges because true CS can be difficult to differentiate from metabolic syndrome. The duration of hypercortisolism appears to be the most significant factor associated with the degree of morbidity and preterm mortality in CS; thus, an accurate diagnosis as early as possible is important.
Screening and diagnostic tests for CS evaluate cortisol secretion. Available options include late-night salivary cortisol (LNSC), impaired glucocorticoid feedback with overnight 1-mg DST or low-dose 2-day dexamethasone test (LDDT) and increased bioavailable cortisol with 24-hour UFC.
A 2021 consensus statement by Fleseriu and colleagues provides recommendations for the diagnosis of CS. If CS is suspected: begin with UFC, LNSC, or both; DST is an option if LNSC not feasible. If CS because of adrenal tumor is suspected: begin with DST because LNSC has lower specificity in these patients. To confirm CS, any of these tests can be used.
An individualized approach is recommended for the treatment of CS. The optimal approach for iatrogenic CS is to slowly taper exogenous steroids. Chronic exposure to steroids can suppress adrenal functioning; as such, recovery may take several months. Surgical resection is the first-line option for hypercortisolism because of Cushing disease, adrenal tumor, or ectopic tumor. Patients should be closely monitored after surgery to evaluate for possible recurrence. Radiotherapy may be recommended after failed transsphenoidal surgery or in Cushing disease with mass effect or invasion of surrounding structures. Medical therapy, such as pasireotide, cabergoline, and mifepristone, are also sometimes used. In addition, the treatment of comorbidities, such as obesity and type 2 diabetes, hypertension, osteoporosis, psychiatric issues, and electrolyte disorders, is critical.
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of Cushing syndrome (CS).
CS is a rare endocrine disease caused by prolonged exposure to high circulating cortisol levels. Exogenous hypercortisolism is the most common cause of CS. It is largely iatrogenic and results from the prolonged use of glucocorticoids. Less frequently, endogenous CS may occur as the result of excessive production of cortisol by adrenal glands. Endogenous CS can be ACTH-dependent or ACTH-independent. ACTH-dependent CS results from ACTH-secreting pituitary adenomas (Cushing disease) and ectopic ACTH secretion by neoplasms, whereas adrenal hyperplasia, adenoma, and carcinoma are the primary causes of ACTH-independent CS.
The annual incidence and prevalence of CS are unknown; the reported incidence of newly diagnosed cases has ranged from 1.2 to 2.4 per million people per year. Women are affected more often than are men, with a peak of incidence in the third to fourth decade of life. CS is associated with various metabolic, psychiatric, musculoskeletal, and cardiovascular comorbidities. Untreated, it is associated with increased mortality, typically as the result of cardiovascular and infectious complications; however, even in appropriately treated patients, mortality is elevated.
The chronic elevations of glucocorticoid concentrations in CS result in its characteristic phenotype, which includes weight gain, moon-shaped face, buffalo hump, muscle weakness, increased bruising, skin atrophy, red abdominal striae, menstrual irregularities, hirsutism, and acne. It is also associated with numerous comorbidities including diabetes, hypertension, hypercholesterolemia, and osteoporosis. Patients often experience mental health complications, such as depression, emotional lability, and cognitive dysfunction.
Given the rarity of CS and the fact that these symptoms overlap with other conditions, delayed diagnosis is common. The current obesity epidemic also poses diagnostic challenges because true CS can be difficult to differentiate from metabolic syndrome. The duration of hypercortisolism appears to be the most significant factor associated with the degree of morbidity and preterm mortality in CS; thus, an accurate diagnosis as early as possible is important.
Screening and diagnostic tests for CS evaluate cortisol secretion. Available options include late-night salivary cortisol (LNSC), impaired glucocorticoid feedback with overnight 1-mg DST or low-dose 2-day dexamethasone test (LDDT) and increased bioavailable cortisol with 24-hour UFC.
A 2021 consensus statement by Fleseriu and colleagues provides recommendations for the diagnosis of CS. If CS is suspected: begin with UFC, LNSC, or both; DST is an option if LNSC not feasible. If CS because of adrenal tumor is suspected: begin with DST because LNSC has lower specificity in these patients. To confirm CS, any of these tests can be used.
An individualized approach is recommended for the treatment of CS. The optimal approach for iatrogenic CS is to slowly taper exogenous steroids. Chronic exposure to steroids can suppress adrenal functioning; as such, recovery may take several months. Surgical resection is the first-line option for hypercortisolism because of Cushing disease, adrenal tumor, or ectopic tumor. Patients should be closely monitored after surgery to evaluate for possible recurrence. Radiotherapy may be recommended after failed transsphenoidal surgery or in Cushing disease with mass effect or invasion of surrounding structures. Medical therapy, such as pasireotide, cabergoline, and mifepristone, are also sometimes used. In addition, the treatment of comorbidities, such as obesity and type 2 diabetes, hypertension, osteoporosis, psychiatric issues, and electrolyte disorders, is critical.
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of Cushing syndrome (CS).
CS is a rare endocrine disease caused by prolonged exposure to high circulating cortisol levels. Exogenous hypercortisolism is the most common cause of CS. It is largely iatrogenic and results from the prolonged use of glucocorticoids. Less frequently, endogenous CS may occur as the result of excessive production of cortisol by adrenal glands. Endogenous CS can be ACTH-dependent or ACTH-independent. ACTH-dependent CS results from ACTH-secreting pituitary adenomas (Cushing disease) and ectopic ACTH secretion by neoplasms, whereas adrenal hyperplasia, adenoma, and carcinoma are the primary causes of ACTH-independent CS.
The annual incidence and prevalence of CS are unknown; the reported incidence of newly diagnosed cases has ranged from 1.2 to 2.4 per million people per year. Women are affected more often than are men, with a peak of incidence in the third to fourth decade of life. CS is associated with various metabolic, psychiatric, musculoskeletal, and cardiovascular comorbidities. Untreated, it is associated with increased mortality, typically as the result of cardiovascular and infectious complications; however, even in appropriately treated patients, mortality is elevated.
The chronic elevations of glucocorticoid concentrations in CS result in its characteristic phenotype, which includes weight gain, moon-shaped face, buffalo hump, muscle weakness, increased bruising, skin atrophy, red abdominal striae, menstrual irregularities, hirsutism, and acne. It is also associated with numerous comorbidities including diabetes, hypertension, hypercholesterolemia, and osteoporosis. Patients often experience mental health complications, such as depression, emotional lability, and cognitive dysfunction.
Given the rarity of CS and the fact that these symptoms overlap with other conditions, delayed diagnosis is common. The current obesity epidemic also poses diagnostic challenges because true CS can be difficult to differentiate from metabolic syndrome. The duration of hypercortisolism appears to be the most significant factor associated with the degree of morbidity and preterm mortality in CS; thus, an accurate diagnosis as early as possible is important.
Screening and diagnostic tests for CS evaluate cortisol secretion. Available options include late-night salivary cortisol (LNSC), impaired glucocorticoid feedback with overnight 1-mg DST or low-dose 2-day dexamethasone test (LDDT) and increased bioavailable cortisol with 24-hour UFC.
A 2021 consensus statement by Fleseriu and colleagues provides recommendations for the diagnosis of CS. If CS is suspected: begin with UFC, LNSC, or both; DST is an option if LNSC not feasible. If CS because of adrenal tumor is suspected: begin with DST because LNSC has lower specificity in these patients. To confirm CS, any of these tests can be used.
An individualized approach is recommended for the treatment of CS. The optimal approach for iatrogenic CS is to slowly taper exogenous steroids. Chronic exposure to steroids can suppress adrenal functioning; as such, recovery may take several months. Surgical resection is the first-line option for hypercortisolism because of Cushing disease, adrenal tumor, or ectopic tumor. Patients should be closely monitored after surgery to evaluate for possible recurrence. Radiotherapy may be recommended after failed transsphenoidal surgery or in Cushing disease with mass effect or invasion of surrounding structures. Medical therapy, such as pasireotide, cabergoline, and mifepristone, are also sometimes used. In addition, the treatment of comorbidities, such as obesity and type 2 diabetes, hypertension, osteoporosis, psychiatric issues, and electrolyte disorders, is critical.
Courtney Whittle, MD, MSW, Diplomate of ABOM, Pediatric Lead, Obesity Champion, TSPMG, Weight A Minute Clinic, Atlanta, Georgia.
Courtney Whittle, MD, MSW, Diplomate of ABOM, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 37-year-old woman presents with reports of insomnia, weight gain (approximately 12 lb over the last 9 months), and excessive fatigue. The patient's medical history is significant for hypertension (diagnosed 4 years earlier) and depression (diagnosed 7 years earlier). Her current medications include lisinopril 10 mg/d, bupropion 75 mg/d, and venlafaxine 75 mg/d. There is no history of alcohol or drug abuse; family history is unremarkable. The patient's height and weight are 5 ft 5 in and 182 lb (body mass index of 30.3).
During physical examination, facial hirsutism is observed along with increased adipose tissue in the face (moon-shaped face), upper back at the base of the neck (buffalo hump), and abdomen. Vertical red abdominal striae are present. Several bruises are observed on the patient's thighs and arms; when questioned, she reports noting an increased tendency to bruise in recent months.
Pertinent laboratory findings include urinary free cortisol excretion (UFC) 324 mcg/24 h, 1-mg dexamethasone suppression test (DST) with a cortisol value of 3.64 mcg/dL (100.42 nmol/L), and adrenocorticotropic hormone (ACTH) level of 84.9 pg/mL.
What are the healthiest drinks for patients with type 2 diabetes?
The researchers examined data on almost 15,500 participants with type 2 diabetes from two major studies, finding that the highest level of consumption of SSBs was associated with a 20% increased risk of all-cause mortality and a 25% raised risk of cardiovascular disease, compared with consumption of the least amounts of these products.
The research, published in BMJ, also showed that drinking coffee, tea, plain water, and low-fat milk reduced the risk of all-cause death and that switching from SSBs to the other beverages was linked to lower mortality.
“Overall, these results provide additional evidence that emphasizes the importance of beverage choices in maintaining overall health among adults with diabetes,” say senior author Le Ma, PhD, department of nutrition, Harvard School of Public Health, Boston, and colleagues.
“Collectively, these findings all point in the same direction. Lower consumption of SSBs and higher consumption of coffee, tea, plain water, or low-fat milk are optimal for better health outcomes in adults with type 2 diabetes,” Nita G. Forouhi, MD, PhD, emphasizes in an accompanying editorial.
Choice of drink matters
Dr. Forouhi, from the University of Cambridge (England), warned, however, that the findings “cannot be considered cause and effect,” despite the large-scale analysis.
Moreover, “questions remain,” such as the impact of beverage consumption on coronary heart disease and stroke risk, and cancer mortality, with the current study providing “inconclusive” data on the latter.
There was also no data on the addition of sugar to tea or coffee, “so the comparative health effects of unsweetened and sweetened hot beverages remain unclear,” Dr. Forouhi points out. Also unknown is whether the type of tea consumed has a differential effect.
Despite these and other reservations, she says that overall, “Choice of beverage clearly matters.”
“The case for avoiding sugar-sweetened beverages is compelling, and it is supported by various fiscal measures in more than 45 countries. It is reasonable to shift the focus to drinks that are most likely to have positive health impacts: coffee, tea, plain water, and low-fat milk,” she notes.
Dr. Forouhi ends by underlining that the current findings tally with those seen in the general population, so “one important message is that having diabetes does not have to be especially restrictive.”
Expanding the evidence
It was estimated that 537 million adults worldwide had type 2 diabetes in 2021, a figure set to increase to 783 million by 2045, say the authors.
Individuals with type 2 diabetes have an increased risk of cardiovascular disease, among many other comorbidities, as well as premature death. Dietary interventions can play an important role in managing these risks.
Recommendations on the healthiest beverages to drink are largely based on evidence from the general population, and data are limited on the best options for adults with type 2 diabetes, who have altered metabolism, the researchers note.
To expand on this, they examined data from the Nurses’ Health Study, which enrolled female registered nurses aged 30-55 years and was initiated in 1976, and the Health Professionals Follow-Up Study, which included male health professionals aged 40-75 years and was initiated in 1996.
For the current analysis, 11,399 women and 4,087 men with type 2 diabetes were included from the two studies, of whom 2,715 were diagnosed before study entry.
Participants’ average daily beverage intake was assessed using a validated food frequency questionnaire administered every 2-4 years. SSBs included caffeinated and caffeine-free colas, other carbonated SSBs, and noncarbonated SSBs, such as fruit punches, lemonades, or other fruit drinks.
During 285,967 person-years of follow-up, there were 7,638 (49.3%) deaths, and 3,447 (22.3%) cases of incident cardiovascular disease were documented during 248,447 person-years of follow-up.
Fully adjusted multivariate analysis comparing the lowest and highest beverage intake indicated that SSBs were associated with a significant increase in all-cause mortality, at a pooled hazard ratio of 1.20, or 1.08 for each additional serving per day (P = .01).
In contrast, the associations between all-cause mortality and consumption of artificially sweetened beverages, fruit juice, and full-fat milk were not significant, whereas coffee (HR, 0.74), tea (HR, 0.79), plain water (HR, 0.77), and low-fat milk (HR, 0.88) were linked to a reduced risk.
The team reported that there were similar associations between beverage intake and cardiovascular disease incidence, at an HR of 1.25 for SSBs, as well as for cardiovascular disease mortality, at an HR of 1.29.
Participants who increased their tea, coffee, and low-fat milk consumption during the course of the study had lower all-cause mortality than those who did not. Switching from SSBs to other beverages was also associated with lower mortality.
The researchers note, however, that there are “several potential limitations” to their study, including that “individual beverage consumption may be correlated with other dietary and lifestyle risk factors for cardiovascular disease incidence and mortality among adults with [type 2] diabetes.”
The study was sponsored by the National Institutes of Health. Dr. Ma has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. Forouhi has declared receiving support from the U.K. Medical Research Council Epidemiology Unit and U.K. National Institute for Health and Care Research Biomedical Research Centre Cambridge.
A version of this article first appeared on Medscape.com.
The researchers examined data on almost 15,500 participants with type 2 diabetes from two major studies, finding that the highest level of consumption of SSBs was associated with a 20% increased risk of all-cause mortality and a 25% raised risk of cardiovascular disease, compared with consumption of the least amounts of these products.
The research, published in BMJ, also showed that drinking coffee, tea, plain water, and low-fat milk reduced the risk of all-cause death and that switching from SSBs to the other beverages was linked to lower mortality.
“Overall, these results provide additional evidence that emphasizes the importance of beverage choices in maintaining overall health among adults with diabetes,” say senior author Le Ma, PhD, department of nutrition, Harvard School of Public Health, Boston, and colleagues.
“Collectively, these findings all point in the same direction. Lower consumption of SSBs and higher consumption of coffee, tea, plain water, or low-fat milk are optimal for better health outcomes in adults with type 2 diabetes,” Nita G. Forouhi, MD, PhD, emphasizes in an accompanying editorial.
Choice of drink matters
Dr. Forouhi, from the University of Cambridge (England), warned, however, that the findings “cannot be considered cause and effect,” despite the large-scale analysis.
Moreover, “questions remain,” such as the impact of beverage consumption on coronary heart disease and stroke risk, and cancer mortality, with the current study providing “inconclusive” data on the latter.
There was also no data on the addition of sugar to tea or coffee, “so the comparative health effects of unsweetened and sweetened hot beverages remain unclear,” Dr. Forouhi points out. Also unknown is whether the type of tea consumed has a differential effect.
Despite these and other reservations, she says that overall, “Choice of beverage clearly matters.”
“The case for avoiding sugar-sweetened beverages is compelling, and it is supported by various fiscal measures in more than 45 countries. It is reasonable to shift the focus to drinks that are most likely to have positive health impacts: coffee, tea, plain water, and low-fat milk,” she notes.
Dr. Forouhi ends by underlining that the current findings tally with those seen in the general population, so “one important message is that having diabetes does not have to be especially restrictive.”
Expanding the evidence
It was estimated that 537 million adults worldwide had type 2 diabetes in 2021, a figure set to increase to 783 million by 2045, say the authors.
Individuals with type 2 diabetes have an increased risk of cardiovascular disease, among many other comorbidities, as well as premature death. Dietary interventions can play an important role in managing these risks.
Recommendations on the healthiest beverages to drink are largely based on evidence from the general population, and data are limited on the best options for adults with type 2 diabetes, who have altered metabolism, the researchers note.
To expand on this, they examined data from the Nurses’ Health Study, which enrolled female registered nurses aged 30-55 years and was initiated in 1976, and the Health Professionals Follow-Up Study, which included male health professionals aged 40-75 years and was initiated in 1996.
For the current analysis, 11,399 women and 4,087 men with type 2 diabetes were included from the two studies, of whom 2,715 were diagnosed before study entry.
Participants’ average daily beverage intake was assessed using a validated food frequency questionnaire administered every 2-4 years. SSBs included caffeinated and caffeine-free colas, other carbonated SSBs, and noncarbonated SSBs, such as fruit punches, lemonades, or other fruit drinks.
During 285,967 person-years of follow-up, there were 7,638 (49.3%) deaths, and 3,447 (22.3%) cases of incident cardiovascular disease were documented during 248,447 person-years of follow-up.
Fully adjusted multivariate analysis comparing the lowest and highest beverage intake indicated that SSBs were associated with a significant increase in all-cause mortality, at a pooled hazard ratio of 1.20, or 1.08 for each additional serving per day (P = .01).
In contrast, the associations between all-cause mortality and consumption of artificially sweetened beverages, fruit juice, and full-fat milk were not significant, whereas coffee (HR, 0.74), tea (HR, 0.79), plain water (HR, 0.77), and low-fat milk (HR, 0.88) were linked to a reduced risk.
The team reported that there were similar associations between beverage intake and cardiovascular disease incidence, at an HR of 1.25 for SSBs, as well as for cardiovascular disease mortality, at an HR of 1.29.
Participants who increased their tea, coffee, and low-fat milk consumption during the course of the study had lower all-cause mortality than those who did not. Switching from SSBs to other beverages was also associated with lower mortality.
The researchers note, however, that there are “several potential limitations” to their study, including that “individual beverage consumption may be correlated with other dietary and lifestyle risk factors for cardiovascular disease incidence and mortality among adults with [type 2] diabetes.”
The study was sponsored by the National Institutes of Health. Dr. Ma has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. Forouhi has declared receiving support from the U.K. Medical Research Council Epidemiology Unit and U.K. National Institute for Health and Care Research Biomedical Research Centre Cambridge.
A version of this article first appeared on Medscape.com.
The researchers examined data on almost 15,500 participants with type 2 diabetes from two major studies, finding that the highest level of consumption of SSBs was associated with a 20% increased risk of all-cause mortality and a 25% raised risk of cardiovascular disease, compared with consumption of the least amounts of these products.
The research, published in BMJ, also showed that drinking coffee, tea, plain water, and low-fat milk reduced the risk of all-cause death and that switching from SSBs to the other beverages was linked to lower mortality.
“Overall, these results provide additional evidence that emphasizes the importance of beverage choices in maintaining overall health among adults with diabetes,” say senior author Le Ma, PhD, department of nutrition, Harvard School of Public Health, Boston, and colleagues.
“Collectively, these findings all point in the same direction. Lower consumption of SSBs and higher consumption of coffee, tea, plain water, or low-fat milk are optimal for better health outcomes in adults with type 2 diabetes,” Nita G. Forouhi, MD, PhD, emphasizes in an accompanying editorial.
Choice of drink matters
Dr. Forouhi, from the University of Cambridge (England), warned, however, that the findings “cannot be considered cause and effect,” despite the large-scale analysis.
Moreover, “questions remain,” such as the impact of beverage consumption on coronary heart disease and stroke risk, and cancer mortality, with the current study providing “inconclusive” data on the latter.
There was also no data on the addition of sugar to tea or coffee, “so the comparative health effects of unsweetened and sweetened hot beverages remain unclear,” Dr. Forouhi points out. Also unknown is whether the type of tea consumed has a differential effect.
Despite these and other reservations, she says that overall, “Choice of beverage clearly matters.”
“The case for avoiding sugar-sweetened beverages is compelling, and it is supported by various fiscal measures in more than 45 countries. It is reasonable to shift the focus to drinks that are most likely to have positive health impacts: coffee, tea, plain water, and low-fat milk,” she notes.
Dr. Forouhi ends by underlining that the current findings tally with those seen in the general population, so “one important message is that having diabetes does not have to be especially restrictive.”
Expanding the evidence
It was estimated that 537 million adults worldwide had type 2 diabetes in 2021, a figure set to increase to 783 million by 2045, say the authors.
Individuals with type 2 diabetes have an increased risk of cardiovascular disease, among many other comorbidities, as well as premature death. Dietary interventions can play an important role in managing these risks.
Recommendations on the healthiest beverages to drink are largely based on evidence from the general population, and data are limited on the best options for adults with type 2 diabetes, who have altered metabolism, the researchers note.
To expand on this, they examined data from the Nurses’ Health Study, which enrolled female registered nurses aged 30-55 years and was initiated in 1976, and the Health Professionals Follow-Up Study, which included male health professionals aged 40-75 years and was initiated in 1996.
For the current analysis, 11,399 women and 4,087 men with type 2 diabetes were included from the two studies, of whom 2,715 were diagnosed before study entry.
Participants’ average daily beverage intake was assessed using a validated food frequency questionnaire administered every 2-4 years. SSBs included caffeinated and caffeine-free colas, other carbonated SSBs, and noncarbonated SSBs, such as fruit punches, lemonades, or other fruit drinks.
During 285,967 person-years of follow-up, there were 7,638 (49.3%) deaths, and 3,447 (22.3%) cases of incident cardiovascular disease were documented during 248,447 person-years of follow-up.
Fully adjusted multivariate analysis comparing the lowest and highest beverage intake indicated that SSBs were associated with a significant increase in all-cause mortality, at a pooled hazard ratio of 1.20, or 1.08 for each additional serving per day (P = .01).
In contrast, the associations between all-cause mortality and consumption of artificially sweetened beverages, fruit juice, and full-fat milk were not significant, whereas coffee (HR, 0.74), tea (HR, 0.79), plain water (HR, 0.77), and low-fat milk (HR, 0.88) were linked to a reduced risk.
The team reported that there were similar associations between beverage intake and cardiovascular disease incidence, at an HR of 1.25 for SSBs, as well as for cardiovascular disease mortality, at an HR of 1.29.
Participants who increased their tea, coffee, and low-fat milk consumption during the course of the study had lower all-cause mortality than those who did not. Switching from SSBs to other beverages was also associated with lower mortality.
The researchers note, however, that there are “several potential limitations” to their study, including that “individual beverage consumption may be correlated with other dietary and lifestyle risk factors for cardiovascular disease incidence and mortality among adults with [type 2] diabetes.”
The study was sponsored by the National Institutes of Health. Dr. Ma has reported no relevant financial relationships. Disclosures for the other authors are listed with the article. Dr. Forouhi has declared receiving support from the U.K. Medical Research Council Epidemiology Unit and U.K. National Institute for Health and Care Research Biomedical Research Centre Cambridge.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Infographic: Is your compensation rising as fast as your peers?
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.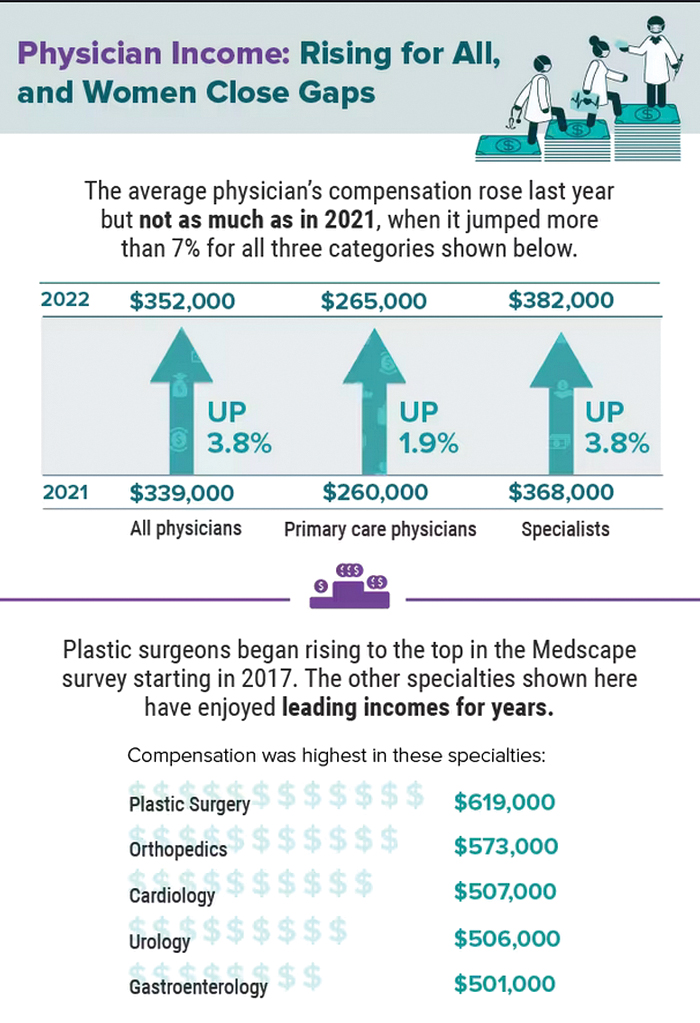
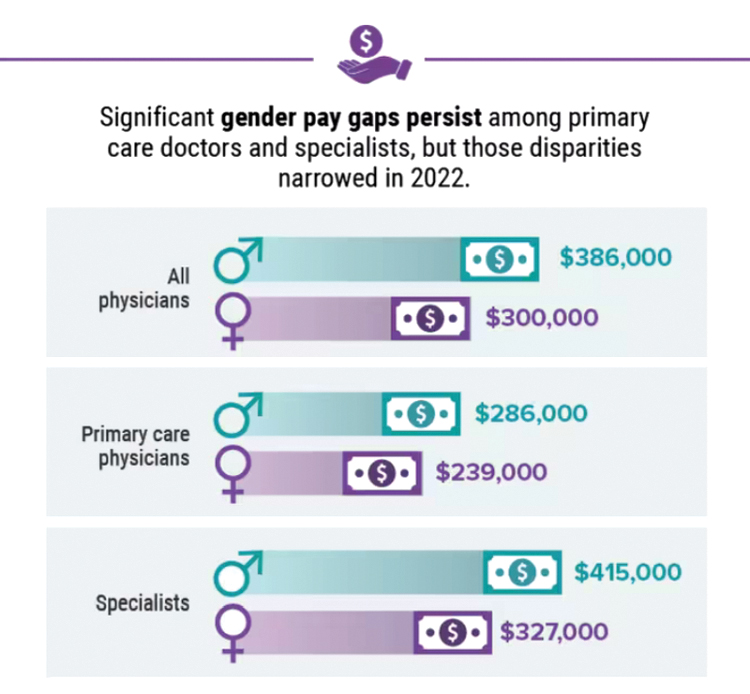
A version of this article first appeared on Medscape.com.
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.

A version of this article first appeared on Medscape.com.
Did doctors’ salaries continue their zesty postpandemic rise in 2022? Are female physicians making pay gains versus their male counterparts that spark optimism for the future?
reveals which medical specialties pay better than others, and evaluates the current gender pay gap in medicine. If you’re interested in delving deeper into the data, check out Your Income vs. Your Peers’: Physician Compensation Report 2023.

A version of this article first appeared on Medscape.com.
Five chronic mistakes that can sabotage your medical practice
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A baby stops breathing at a grocery store – An ICU nurse steps in
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
Three-month history of fever
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of pleomorphic mantle cell lymphoma (MCL).
MCL is a rare, clinically and biologically heterogeneous B-cell non-Hodgkin lymphoma. It accounts for approximately 5%-7% of all lymphomas. In North America and Europe, its incidence is akin to that of noncutaneous, peripheral T-cell lymphomas. The typical age at diagnosis is between 60 and 70 years. Approximately 70% of all cases are seen in men.
Little is known about risk factors for the development of MCL. Factors that have been associated with the development of other lymphomas (eg, familial risk, immunosuppression, other immune disorders, chemical and occupational exposures, and infectious agents) have not been convincingly identified as predisposing factors for MCL, with the possible exception of family history.
MCL is usually associated with reciprocal chromosomal translocation between chromosomes 11 and 14, t(11;14)(q13:q32), resulting in overexpression of cyclin D1, which plays a key role in tumor cell proliferation through cell-cycle dysregulation, chromosomal instability, and epigenetic regulation. Tumor cells (monoclonal B cells) express surface immunoglobulin, immunoglobulin M, or immunoglobulin D. Cells are usually CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) with no expression of CD10 and CD23. Histologic features include small-to-medium lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Cytologic subtypes include classic MCL, the blastoid variant (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic variant (cells of varying size, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli). Blastoid and pleomorphic MCL typically have a more aggressive natural history and are associated with inferior clinical outcomes.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), an accurate pathologic diagnosis of the subtype is the most important initial step in the management of B-cell lymphomas, including pleomorphic MCL. The basic pathologic exam is the same for all subtypes, although additional testing may be needed in certain cases. An incisional or excisional lymph node biopsy is recommended. Fine-needle aspiration biopsy alone is typically not sufficient for the initial diagnosis of lymphoma; however, its diagnostic accuracy is significantly improved when it is used in combination with immunohistochemistry and flow cytometry. Immunohistochemistry is essential to differentiate MCL subtypes.
Essential workup procedures include a complete physical exam, with particular attention to node-bearing areas, including the Waldeyer ring, as well as the size of the liver and spleen, and assessment of performance status and B symptoms (fever, night sweats, unintentional weight loss). Laboratory studies should include complete blood count with differential, measurement of serum lactate dehydrogenase, hepatitis B virus testing, and a comprehensive metabolic panel. Required imaging studies include PET/CT (or chest/abdominal/pelvic CT with oral and intravenous contrast if PET/CT is not available) and multigated acquisition scanning or echocardiography when anthracyclines and anthracenedione-containing regimens are indicated.
A watch-and-wait approach may be appropriate for some patients with indolent MCL; however, patients with aggressive MCL, such as pleomorphic histology, require chemoimmunotherapy at diagnosis. For patients who relapse or achieve an incomplete response to first-line therapy, the NCCN guidelines recommend second-line treatment with a Bruton tyrosine kinase (BTK) inhibitor–containing regimen. Available BTK inhibitors include acalabrutinib, ibrutinib ± rituximab, zanubrutinib, and pirtobrutinib. Chemoimmunotherapy with lenalidomide + rituximab is another second-line option and may be particularly helpful for patients in whom a BTK inhibitor is contraindicated. Anti-CD19 CAR T-cell therapy is a recommended option for the third line and beyond.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of pleomorphic mantle cell lymphoma (MCL).
MCL is a rare, clinically and biologically heterogeneous B-cell non-Hodgkin lymphoma. It accounts for approximately 5%-7% of all lymphomas. In North America and Europe, its incidence is akin to that of noncutaneous, peripheral T-cell lymphomas. The typical age at diagnosis is between 60 and 70 years. Approximately 70% of all cases are seen in men.
Little is known about risk factors for the development of MCL. Factors that have been associated with the development of other lymphomas (eg, familial risk, immunosuppression, other immune disorders, chemical and occupational exposures, and infectious agents) have not been convincingly identified as predisposing factors for MCL, with the possible exception of family history.
MCL is usually associated with reciprocal chromosomal translocation between chromosomes 11 and 14, t(11;14)(q13:q32), resulting in overexpression of cyclin D1, which plays a key role in tumor cell proliferation through cell-cycle dysregulation, chromosomal instability, and epigenetic regulation. Tumor cells (monoclonal B cells) express surface immunoglobulin, immunoglobulin M, or immunoglobulin D. Cells are usually CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) with no expression of CD10 and CD23. Histologic features include small-to-medium lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Cytologic subtypes include classic MCL, the blastoid variant (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic variant (cells of varying size, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli). Blastoid and pleomorphic MCL typically have a more aggressive natural history and are associated with inferior clinical outcomes.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), an accurate pathologic diagnosis of the subtype is the most important initial step in the management of B-cell lymphomas, including pleomorphic MCL. The basic pathologic exam is the same for all subtypes, although additional testing may be needed in certain cases. An incisional or excisional lymph node biopsy is recommended. Fine-needle aspiration biopsy alone is typically not sufficient for the initial diagnosis of lymphoma; however, its diagnostic accuracy is significantly improved when it is used in combination with immunohistochemistry and flow cytometry. Immunohistochemistry is essential to differentiate MCL subtypes.
Essential workup procedures include a complete physical exam, with particular attention to node-bearing areas, including the Waldeyer ring, as well as the size of the liver and spleen, and assessment of performance status and B symptoms (fever, night sweats, unintentional weight loss). Laboratory studies should include complete blood count with differential, measurement of serum lactate dehydrogenase, hepatitis B virus testing, and a comprehensive metabolic panel. Required imaging studies include PET/CT (or chest/abdominal/pelvic CT with oral and intravenous contrast if PET/CT is not available) and multigated acquisition scanning or echocardiography when anthracyclines and anthracenedione-containing regimens are indicated.
A watch-and-wait approach may be appropriate for some patients with indolent MCL; however, patients with aggressive MCL, such as pleomorphic histology, require chemoimmunotherapy at diagnosis. For patients who relapse or achieve an incomplete response to first-line therapy, the NCCN guidelines recommend second-line treatment with a Bruton tyrosine kinase (BTK) inhibitor–containing regimen. Available BTK inhibitors include acalabrutinib, ibrutinib ± rituximab, zanubrutinib, and pirtobrutinib. Chemoimmunotherapy with lenalidomide + rituximab is another second-line option and may be particularly helpful for patients in whom a BTK inhibitor is contraindicated. Anti-CD19 CAR T-cell therapy is a recommended option for the third line and beyond.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of pleomorphic mantle cell lymphoma (MCL).
MCL is a rare, clinically and biologically heterogeneous B-cell non-Hodgkin lymphoma. It accounts for approximately 5%-7% of all lymphomas. In North America and Europe, its incidence is akin to that of noncutaneous, peripheral T-cell lymphomas. The typical age at diagnosis is between 60 and 70 years. Approximately 70% of all cases are seen in men.
Little is known about risk factors for the development of MCL. Factors that have been associated with the development of other lymphomas (eg, familial risk, immunosuppression, other immune disorders, chemical and occupational exposures, and infectious agents) have not been convincingly identified as predisposing factors for MCL, with the possible exception of family history.
MCL is usually associated with reciprocal chromosomal translocation between chromosomes 11 and 14, t(11;14)(q13:q32), resulting in overexpression of cyclin D1, which plays a key role in tumor cell proliferation through cell-cycle dysregulation, chromosomal instability, and epigenetic regulation. Tumor cells (monoclonal B cells) express surface immunoglobulin, immunoglobulin M, or immunoglobulin D. Cells are usually CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) with no expression of CD10 and CD23. Histologic features include small-to-medium lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Cytologic subtypes include classic MCL, the blastoid variant (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic variant (cells of varying size, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli). Blastoid and pleomorphic MCL typically have a more aggressive natural history and are associated with inferior clinical outcomes.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), an accurate pathologic diagnosis of the subtype is the most important initial step in the management of B-cell lymphomas, including pleomorphic MCL. The basic pathologic exam is the same for all subtypes, although additional testing may be needed in certain cases. An incisional or excisional lymph node biopsy is recommended. Fine-needle aspiration biopsy alone is typically not sufficient for the initial diagnosis of lymphoma; however, its diagnostic accuracy is significantly improved when it is used in combination with immunohistochemistry and flow cytometry. Immunohistochemistry is essential to differentiate MCL subtypes.
Essential workup procedures include a complete physical exam, with particular attention to node-bearing areas, including the Waldeyer ring, as well as the size of the liver and spleen, and assessment of performance status and B symptoms (fever, night sweats, unintentional weight loss). Laboratory studies should include complete blood count with differential, measurement of serum lactate dehydrogenase, hepatitis B virus testing, and a comprehensive metabolic panel. Required imaging studies include PET/CT (or chest/abdominal/pelvic CT with oral and intravenous contrast if PET/CT is not available) and multigated acquisition scanning or echocardiography when anthracyclines and anthracenedione-containing regimens are indicated.
A watch-and-wait approach may be appropriate for some patients with indolent MCL; however, patients with aggressive MCL, such as pleomorphic histology, require chemoimmunotherapy at diagnosis. For patients who relapse or achieve an incomplete response to first-line therapy, the NCCN guidelines recommend second-line treatment with a Bruton tyrosine kinase (BTK) inhibitor–containing regimen. Available BTK inhibitors include acalabrutinib, ibrutinib ± rituximab, zanubrutinib, and pirtobrutinib. Chemoimmunotherapy with lenalidomide + rituximab is another second-line option and may be particularly helpful for patients in whom a BTK inhibitor is contraindicated. Anti-CD19 CAR T-cell therapy is a recommended option for the third line and beyond.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 62-year-old man with no significant past medical history presents with a 3-month history of fever, night sweats, upper abdominal pain and bloating, and unintentional weight loss. He does not currently take any medications. His height and weight are 6 ft 2 in and 171 lb (BMI 22).
Physical examination reveals generalized lymphadenopathy and splenomegaly. Subsequently, an excisional lymph node biopsy is performed. Histologic examination of the specimen reveals sheets of mostly large cells of varying sizes, with nuclear overlap and extensive necrosis. Cytology findings include large lymphocytes with pale cytoplasm, clumped chromatin, oval irregular nuclei, and prominent nucleoli. Pertinent findings from immunohistochemical staining include the presence of t(11:14), Ki67 > 30%, CD5 and CD20 positivity, and CD10 and CD23 negativity. Centroblasts are absent.