User login
A baby stops breathing at a grocery store – An ICU nurse steps in
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
Three-month history of fever
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of pleomorphic mantle cell lymphoma (MCL).
MCL is a rare, clinically and biologically heterogeneous B-cell non-Hodgkin lymphoma. It accounts for approximately 5%-7% of all lymphomas. In North America and Europe, its incidence is akin to that of noncutaneous, peripheral T-cell lymphomas. The typical age at diagnosis is between 60 and 70 years. Approximately 70% of all cases are seen in men.
Little is known about risk factors for the development of MCL. Factors that have been associated with the development of other lymphomas (eg, familial risk, immunosuppression, other immune disorders, chemical and occupational exposures, and infectious agents) have not been convincingly identified as predisposing factors for MCL, with the possible exception of family history.
MCL is usually associated with reciprocal chromosomal translocation between chromosomes 11 and 14, t(11;14)(q13:q32), resulting in overexpression of cyclin D1, which plays a key role in tumor cell proliferation through cell-cycle dysregulation, chromosomal instability, and epigenetic regulation. Tumor cells (monoclonal B cells) express surface immunoglobulin, immunoglobulin M, or immunoglobulin D. Cells are usually CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) with no expression of CD10 and CD23. Histologic features include small-to-medium lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Cytologic subtypes include classic MCL, the blastoid variant (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic variant (cells of varying size, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli). Blastoid and pleomorphic MCL typically have a more aggressive natural history and are associated with inferior clinical outcomes.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), an accurate pathologic diagnosis of the subtype is the most important initial step in the management of B-cell lymphomas, including pleomorphic MCL. The basic pathologic exam is the same for all subtypes, although additional testing may be needed in certain cases. An incisional or excisional lymph node biopsy is recommended. Fine-needle aspiration biopsy alone is typically not sufficient for the initial diagnosis of lymphoma; however, its diagnostic accuracy is significantly improved when it is used in combination with immunohistochemistry and flow cytometry. Immunohistochemistry is essential to differentiate MCL subtypes.
Essential workup procedures include a complete physical exam, with particular attention to node-bearing areas, including the Waldeyer ring, as well as the size of the liver and spleen, and assessment of performance status and B symptoms (fever, night sweats, unintentional weight loss). Laboratory studies should include complete blood count with differential, measurement of serum lactate dehydrogenase, hepatitis B virus testing, and a comprehensive metabolic panel. Required imaging studies include PET/CT (or chest/abdominal/pelvic CT with oral and intravenous contrast if PET/CT is not available) and multigated acquisition scanning or echocardiography when anthracyclines and anthracenedione-containing regimens are indicated.
A watch-and-wait approach may be appropriate for some patients with indolent MCL; however, patients with aggressive MCL, such as pleomorphic histology, require chemoimmunotherapy at diagnosis. For patients who relapse or achieve an incomplete response to first-line therapy, the NCCN guidelines recommend second-line treatment with a Bruton tyrosine kinase (BTK) inhibitor–containing regimen. Available BTK inhibitors include acalabrutinib, ibrutinib ± rituximab, zanubrutinib, and pirtobrutinib. Chemoimmunotherapy with lenalidomide + rituximab is another second-line option and may be particularly helpful for patients in whom a BTK inhibitor is contraindicated. Anti-CD19 CAR T-cell therapy is a recommended option for the third line and beyond.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of pleomorphic mantle cell lymphoma (MCL).
MCL is a rare, clinically and biologically heterogeneous B-cell non-Hodgkin lymphoma. It accounts for approximately 5%-7% of all lymphomas. In North America and Europe, its incidence is akin to that of noncutaneous, peripheral T-cell lymphomas. The typical age at diagnosis is between 60 and 70 years. Approximately 70% of all cases are seen in men.
Little is known about risk factors for the development of MCL. Factors that have been associated with the development of other lymphomas (eg, familial risk, immunosuppression, other immune disorders, chemical and occupational exposures, and infectious agents) have not been convincingly identified as predisposing factors for MCL, with the possible exception of family history.
MCL is usually associated with reciprocal chromosomal translocation between chromosomes 11 and 14, t(11;14)(q13:q32), resulting in overexpression of cyclin D1, which plays a key role in tumor cell proliferation through cell-cycle dysregulation, chromosomal instability, and epigenetic regulation. Tumor cells (monoclonal B cells) express surface immunoglobulin, immunoglobulin M, or immunoglobulin D. Cells are usually CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) with no expression of CD10 and CD23. Histologic features include small-to-medium lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Cytologic subtypes include classic MCL, the blastoid variant (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic variant (cells of varying size, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli). Blastoid and pleomorphic MCL typically have a more aggressive natural history and are associated with inferior clinical outcomes.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), an accurate pathologic diagnosis of the subtype is the most important initial step in the management of B-cell lymphomas, including pleomorphic MCL. The basic pathologic exam is the same for all subtypes, although additional testing may be needed in certain cases. An incisional or excisional lymph node biopsy is recommended. Fine-needle aspiration biopsy alone is typically not sufficient for the initial diagnosis of lymphoma; however, its diagnostic accuracy is significantly improved when it is used in combination with immunohistochemistry and flow cytometry. Immunohistochemistry is essential to differentiate MCL subtypes.
Essential workup procedures include a complete physical exam, with particular attention to node-bearing areas, including the Waldeyer ring, as well as the size of the liver and spleen, and assessment of performance status and B symptoms (fever, night sweats, unintentional weight loss). Laboratory studies should include complete blood count with differential, measurement of serum lactate dehydrogenase, hepatitis B virus testing, and a comprehensive metabolic panel. Required imaging studies include PET/CT (or chest/abdominal/pelvic CT with oral and intravenous contrast if PET/CT is not available) and multigated acquisition scanning or echocardiography when anthracyclines and anthracenedione-containing regimens are indicated.
A watch-and-wait approach may be appropriate for some patients with indolent MCL; however, patients with aggressive MCL, such as pleomorphic histology, require chemoimmunotherapy at diagnosis. For patients who relapse or achieve an incomplete response to first-line therapy, the NCCN guidelines recommend second-line treatment with a Bruton tyrosine kinase (BTK) inhibitor–containing regimen. Available BTK inhibitors include acalabrutinib, ibrutinib ± rituximab, zanubrutinib, and pirtobrutinib. Chemoimmunotherapy with lenalidomide + rituximab is another second-line option and may be particularly helpful for patients in whom a BTK inhibitor is contraindicated. Anti-CD19 CAR T-cell therapy is a recommended option for the third line and beyond.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and laboratory findings are consistent with a diagnosis of pleomorphic mantle cell lymphoma (MCL).
MCL is a rare, clinically and biologically heterogeneous B-cell non-Hodgkin lymphoma. It accounts for approximately 5%-7% of all lymphomas. In North America and Europe, its incidence is akin to that of noncutaneous, peripheral T-cell lymphomas. The typical age at diagnosis is between 60 and 70 years. Approximately 70% of all cases are seen in men.
Little is known about risk factors for the development of MCL. Factors that have been associated with the development of other lymphomas (eg, familial risk, immunosuppression, other immune disorders, chemical and occupational exposures, and infectious agents) have not been convincingly identified as predisposing factors for MCL, with the possible exception of family history.
MCL is usually associated with reciprocal chromosomal translocation between chromosomes 11 and 14, t(11;14)(q13:q32), resulting in overexpression of cyclin D1, which plays a key role in tumor cell proliferation through cell-cycle dysregulation, chromosomal instability, and epigenetic regulation. Tumor cells (monoclonal B cells) express surface immunoglobulin, immunoglobulin M, or immunoglobulin D. Cells are usually CD5+ and pan B-cell antigen positive (eg, CD19, CD20, CD22) with no expression of CD10 and CD23. Histologic features include small-to-medium lymphocytes with scant cytoplasm, clumped chromatin, inconspicuous nucleoli, and prominent nuclear clefts. Cytologic subtypes include classic MCL, the blastoid variant (large cells, dispersed chromatin, and a high mitotic rate), and the pleomorphic variant (cells of varying size, although many are large, with pale cytoplasm, oval irregular nuclei, and prominent nucleoli). Blastoid and pleomorphic MCL typically have a more aggressive natural history and are associated with inferior clinical outcomes.
According to 2023 guidelines from the National Comprehensive Cancer Network (NCCN), an accurate pathologic diagnosis of the subtype is the most important initial step in the management of B-cell lymphomas, including pleomorphic MCL. The basic pathologic exam is the same for all subtypes, although additional testing may be needed in certain cases. An incisional or excisional lymph node biopsy is recommended. Fine-needle aspiration biopsy alone is typically not sufficient for the initial diagnosis of lymphoma; however, its diagnostic accuracy is significantly improved when it is used in combination with immunohistochemistry and flow cytometry. Immunohistochemistry is essential to differentiate MCL subtypes.
Essential workup procedures include a complete physical exam, with particular attention to node-bearing areas, including the Waldeyer ring, as well as the size of the liver and spleen, and assessment of performance status and B symptoms (fever, night sweats, unintentional weight loss). Laboratory studies should include complete blood count with differential, measurement of serum lactate dehydrogenase, hepatitis B virus testing, and a comprehensive metabolic panel. Required imaging studies include PET/CT (or chest/abdominal/pelvic CT with oral and intravenous contrast if PET/CT is not available) and multigated acquisition scanning or echocardiography when anthracyclines and anthracenedione-containing regimens are indicated.
A watch-and-wait approach may be appropriate for some patients with indolent MCL; however, patients with aggressive MCL, such as pleomorphic histology, require chemoimmunotherapy at diagnosis. For patients who relapse or achieve an incomplete response to first-line therapy, the NCCN guidelines recommend second-line treatment with a Bruton tyrosine kinase (BTK) inhibitor–containing regimen. Available BTK inhibitors include acalabrutinib, ibrutinib ± rituximab, zanubrutinib, and pirtobrutinib. Chemoimmunotherapy with lenalidomide + rituximab is another second-line option and may be particularly helpful for patients in whom a BTK inhibitor is contraindicated. Anti-CD19 CAR T-cell therapy is a recommended option for the third line and beyond.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
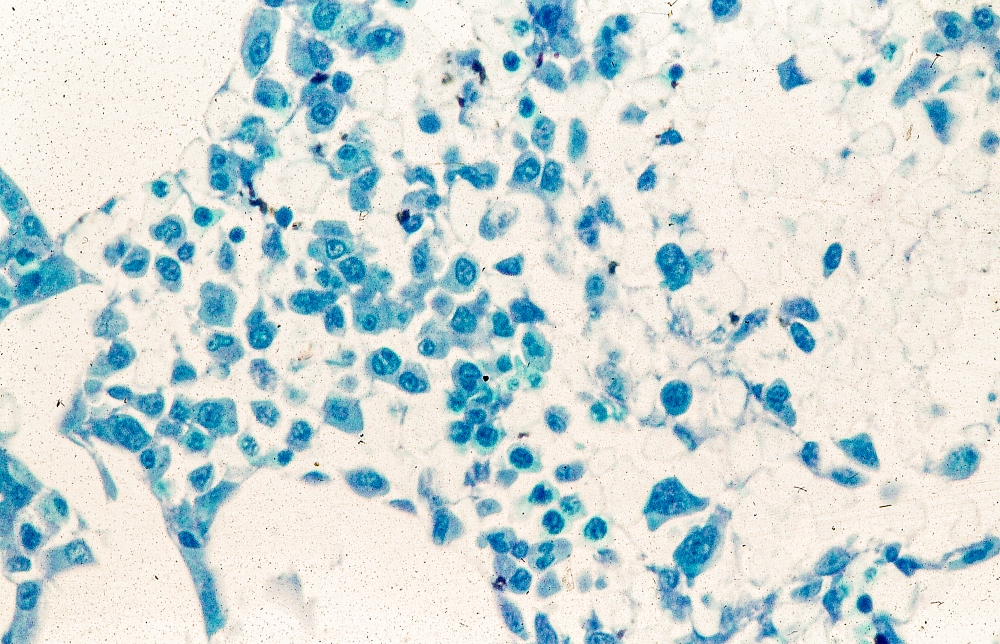
A 62-year-old man with no significant past medical history presents with a 3-month history of fever, night sweats, upper abdominal pain and bloating, and unintentional weight loss. He does not currently take any medications. His height and weight are 6 ft 2 in and 171 lb (BMI 22).
Physical examination reveals generalized lymphadenopathy and splenomegaly. Subsequently, an excisional lymph node biopsy is performed. Histologic examination of the specimen reveals sheets of mostly large cells of varying sizes, with nuclear overlap and extensive necrosis. Cytology findings include large lymphocytes with pale cytoplasm, clumped chromatin, oval irregular nuclei, and prominent nucleoli. Pertinent findings from immunohistochemical staining include the presence of t(11:14), Ki67 > 30%, CD5 and CD20 positivity, and CD10 and CD23 negativity. Centroblasts are absent.
From Beirut to frontline hematology research
“If we start treatment earlier, in the smoldering phase, maybe there is a chance of actually curing the disease and completely getting rid of it,” said Dr. Mouhieddine, 31, a research fellow at the Icahn School of Medicine at Mount Sinai, in New York. “We haven’t proven that yet, and it’s going to take years before we’re able to prove it. I’m hoping to be one of those spearheading the initiative.”
As he develops clinical trials, the young physician scientist has another focus: A deeply personal connection to the very disease he’s trying to cure. Last year Dr. Mouhieddine diagnosed his aunt back in Lebanon with multiple myeloma. “I have always been close to her, and I’m like her son,” he said, and her situation is especially scary because she lives in a country where treatment options are limited.
Dr. Mouhieddine was born and raised in Beirut, the son of a sports journalist father and a mother who worked in a bank. Lebanon’s civil war ended in 1990, shortly before his birth, but political instability returned when he was a child.
“Everything was a disaster,” he recalled. “There was a period of time when there were bombs throughout the city because certain politicians were being targeted. I remember when groups of people would have gunfights in the street.”
Dr. Mouhieddine attended the American University of Beirut, then after college and medical school there, he headed to the United States.
“I wanted to make a difference in medicine. And I knew that if I stayed back home, I wouldn’t be able to,” he said. Fortunately, “everybody has made me feel that I really belong here, and I’ve never felt like I’m an outsider.”
Early on, as he went through fellowships and residency, he developed an interest in multiple myeloma.
Ajai Chari, MD, a colleague of Dr. Mouhieddine’s at Icahn School of Medicine, said in an interview, “I remember meeting him at a conference before he had even started an internal medicine residency, let alone a hematology oncology fellowship. He was already certain he wanted to work in multiple myeloma, due to his work at Dana-Farber Cancer Institute.”
Myeloma was especially intriguing to Dr. Mouhieddine because of the rapid rate of progress in treating the disease. “Over the past 10 years, the myeloma field has advanced at such an extremely fast pace, more than any other cancer,” he said. “Maybe 15 years ago, you would tell someone with newly diagnosed myeloma that they had a chance for an average of another 2 years. Now, we tell patients they have 10 years to live on average, which means you could live 15 or 20 years. That alone was astounding to me and piqued my interest in myeloma.”
At the same time, smoldering myeloma – which can be discovered during routine blood work – remains little understood. As the National Cancer Institute explains, “smoldering myeloma is a precancerous condition that alters certain proteins in blood and/or increases plasma cells in bone marrow, but it does not cause symptoms of disease. About half of those diagnosed with the condition, however, will develop multiple myeloma within 5 years.”
“If we understand what drives smoldering myeloma, we may be able to prevent it from progressing to its active form,” said hematologist oncologist Samir Parekh, MD, who works with Dr. Mouhieddine at Icahn School of Medicine. “Or at the minimum, we could better predict who will progress so we can tailor therapy for high-risk patients and minimize toxicity by not overtreating patients who may not need therapy.”
Dr. Mouhieddine’s current work is focusing on developing clinical trials to test whether immunotherapy can snuff out myeloma when it’s at the smoldering stage, “before anything bad happens.
“If a myeloma patient comes in with renal failure, and we treat the myeloma at that stage, it doesn’t mean that the patient’s kidneys are gonna go back to normal. A lot of the damage can be permanent,” he said. “Even when you treat multiple myeloma, and it goes into remission, it ends up coming back. And you just have to go from one therapy to the other.”
In contrast, a successful treatment for smoldering myeloma would prevent progression to the full disease. In other words, it would be a cure – which is now elusive.
Specifically, Dr. Mouhieddine hopes to test whether bispecific antibodies, a type of immunotherapy that enlists the body’s T cells to kill myeloma cells, will be effective in the smoldering phase. Bispecific antibodies are now being explored as treatments for full multiple myeloma when the immune system is weaker, he said, and they may be even more effective earlier, when the body is better equipped to fight off the disease.
Dr. Mouhieddine hopes better treatments for multiple myeloma itself will help save his 64-year-old aunt Hassana, back in Beirut. He diagnosed her in 2022 after she told him that she felt tired all the time and underwent various tests. The woman he calls his “second mom” is doing well, despite struggles to buy medication due to the lack of access to bank funds in Lebanon.
“I’m always going to be afraid that the disease is going to progress or come back at some point,” he said. “Lebanon doesn’t have as many options as people in the U.S. do. Once you exhaust your first option, and maybe your second option, then you don’t have any other options. Here, we have outpatients who exhaust option number 15 and go to option number 16. That’s definitely not the case over there.”
For now, Dr. Mouhieddine is treating patients and working to launch clinical trials into smoldering myeloma. “His work ethic is incredible,” said his colleague, Dr. Chari. “He has seen multiple projects to publication, and he develops deep connections with his patients and follows up on their care whether or not he is in clinic on a particular day.”
Dr. Parekh, another colleague, said Dr. Mouhieddine can even be a role model. “Other trainees may benefit from thinking about their career early on and exploring both lab and clinical research projects, so that they can develop the necessary experience to be competitive in academia later on.”
His workload can a burden for Dr. Mouhieddine, who is Muslim. He expressed regret that his busy schedule does not always permit him to fast during Ramadan. On a nonmedical front, his recent efforts have paid off. In March 2023 Dr. Mouhieddine became a U.S. citizen.
“It’s surreal,” he said, “but also a dream come true. I feel very grateful, like it’s like an appreciation of who I am, what I’ve done, and what I can do for this country.”
“If we start treatment earlier, in the smoldering phase, maybe there is a chance of actually curing the disease and completely getting rid of it,” said Dr. Mouhieddine, 31, a research fellow at the Icahn School of Medicine at Mount Sinai, in New York. “We haven’t proven that yet, and it’s going to take years before we’re able to prove it. I’m hoping to be one of those spearheading the initiative.”
As he develops clinical trials, the young physician scientist has another focus: A deeply personal connection to the very disease he’s trying to cure. Last year Dr. Mouhieddine diagnosed his aunt back in Lebanon with multiple myeloma. “I have always been close to her, and I’m like her son,” he said, and her situation is especially scary because she lives in a country where treatment options are limited.
Dr. Mouhieddine was born and raised in Beirut, the son of a sports journalist father and a mother who worked in a bank. Lebanon’s civil war ended in 1990, shortly before his birth, but political instability returned when he was a child.
“Everything was a disaster,” he recalled. “There was a period of time when there were bombs throughout the city because certain politicians were being targeted. I remember when groups of people would have gunfights in the street.”
Dr. Mouhieddine attended the American University of Beirut, then after college and medical school there, he headed to the United States.
“I wanted to make a difference in medicine. And I knew that if I stayed back home, I wouldn’t be able to,” he said. Fortunately, “everybody has made me feel that I really belong here, and I’ve never felt like I’m an outsider.”
Early on, as he went through fellowships and residency, he developed an interest in multiple myeloma.
Ajai Chari, MD, a colleague of Dr. Mouhieddine’s at Icahn School of Medicine, said in an interview, “I remember meeting him at a conference before he had even started an internal medicine residency, let alone a hematology oncology fellowship. He was already certain he wanted to work in multiple myeloma, due to his work at Dana-Farber Cancer Institute.”
Myeloma was especially intriguing to Dr. Mouhieddine because of the rapid rate of progress in treating the disease. “Over the past 10 years, the myeloma field has advanced at such an extremely fast pace, more than any other cancer,” he said. “Maybe 15 years ago, you would tell someone with newly diagnosed myeloma that they had a chance for an average of another 2 years. Now, we tell patients they have 10 years to live on average, which means you could live 15 or 20 years. That alone was astounding to me and piqued my interest in myeloma.”
At the same time, smoldering myeloma – which can be discovered during routine blood work – remains little understood. As the National Cancer Institute explains, “smoldering myeloma is a precancerous condition that alters certain proteins in blood and/or increases plasma cells in bone marrow, but it does not cause symptoms of disease. About half of those diagnosed with the condition, however, will develop multiple myeloma within 5 years.”
“If we understand what drives smoldering myeloma, we may be able to prevent it from progressing to its active form,” said hematologist oncologist Samir Parekh, MD, who works with Dr. Mouhieddine at Icahn School of Medicine. “Or at the minimum, we could better predict who will progress so we can tailor therapy for high-risk patients and minimize toxicity by not overtreating patients who may not need therapy.”
Dr. Mouhieddine’s current work is focusing on developing clinical trials to test whether immunotherapy can snuff out myeloma when it’s at the smoldering stage, “before anything bad happens.
“If a myeloma patient comes in with renal failure, and we treat the myeloma at that stage, it doesn’t mean that the patient’s kidneys are gonna go back to normal. A lot of the damage can be permanent,” he said. “Even when you treat multiple myeloma, and it goes into remission, it ends up coming back. And you just have to go from one therapy to the other.”
In contrast, a successful treatment for smoldering myeloma would prevent progression to the full disease. In other words, it would be a cure – which is now elusive.
Specifically, Dr. Mouhieddine hopes to test whether bispecific antibodies, a type of immunotherapy that enlists the body’s T cells to kill myeloma cells, will be effective in the smoldering phase. Bispecific antibodies are now being explored as treatments for full multiple myeloma when the immune system is weaker, he said, and they may be even more effective earlier, when the body is better equipped to fight off the disease.
Dr. Mouhieddine hopes better treatments for multiple myeloma itself will help save his 64-year-old aunt Hassana, back in Beirut. He diagnosed her in 2022 after she told him that she felt tired all the time and underwent various tests. The woman he calls his “second mom” is doing well, despite struggles to buy medication due to the lack of access to bank funds in Lebanon.
“I’m always going to be afraid that the disease is going to progress or come back at some point,” he said. “Lebanon doesn’t have as many options as people in the U.S. do. Once you exhaust your first option, and maybe your second option, then you don’t have any other options. Here, we have outpatients who exhaust option number 15 and go to option number 16. That’s definitely not the case over there.”
For now, Dr. Mouhieddine is treating patients and working to launch clinical trials into smoldering myeloma. “His work ethic is incredible,” said his colleague, Dr. Chari. “He has seen multiple projects to publication, and he develops deep connections with his patients and follows up on their care whether or not he is in clinic on a particular day.”
Dr. Parekh, another colleague, said Dr. Mouhieddine can even be a role model. “Other trainees may benefit from thinking about their career early on and exploring both lab and clinical research projects, so that they can develop the necessary experience to be competitive in academia later on.”
His workload can a burden for Dr. Mouhieddine, who is Muslim. He expressed regret that his busy schedule does not always permit him to fast during Ramadan. On a nonmedical front, his recent efforts have paid off. In March 2023 Dr. Mouhieddine became a U.S. citizen.
“It’s surreal,” he said, “but also a dream come true. I feel very grateful, like it’s like an appreciation of who I am, what I’ve done, and what I can do for this country.”
“If we start treatment earlier, in the smoldering phase, maybe there is a chance of actually curing the disease and completely getting rid of it,” said Dr. Mouhieddine, 31, a research fellow at the Icahn School of Medicine at Mount Sinai, in New York. “We haven’t proven that yet, and it’s going to take years before we’re able to prove it. I’m hoping to be one of those spearheading the initiative.”
As he develops clinical trials, the young physician scientist has another focus: A deeply personal connection to the very disease he’s trying to cure. Last year Dr. Mouhieddine diagnosed his aunt back in Lebanon with multiple myeloma. “I have always been close to her, and I’m like her son,” he said, and her situation is especially scary because she lives in a country where treatment options are limited.
Dr. Mouhieddine was born and raised in Beirut, the son of a sports journalist father and a mother who worked in a bank. Lebanon’s civil war ended in 1990, shortly before his birth, but political instability returned when he was a child.
“Everything was a disaster,” he recalled. “There was a period of time when there were bombs throughout the city because certain politicians were being targeted. I remember when groups of people would have gunfights in the street.”
Dr. Mouhieddine attended the American University of Beirut, then after college and medical school there, he headed to the United States.
“I wanted to make a difference in medicine. And I knew that if I stayed back home, I wouldn’t be able to,” he said. Fortunately, “everybody has made me feel that I really belong here, and I’ve never felt like I’m an outsider.”
Early on, as he went through fellowships and residency, he developed an interest in multiple myeloma.
Ajai Chari, MD, a colleague of Dr. Mouhieddine’s at Icahn School of Medicine, said in an interview, “I remember meeting him at a conference before he had even started an internal medicine residency, let alone a hematology oncology fellowship. He was already certain he wanted to work in multiple myeloma, due to his work at Dana-Farber Cancer Institute.”
Myeloma was especially intriguing to Dr. Mouhieddine because of the rapid rate of progress in treating the disease. “Over the past 10 years, the myeloma field has advanced at such an extremely fast pace, more than any other cancer,” he said. “Maybe 15 years ago, you would tell someone with newly diagnosed myeloma that they had a chance for an average of another 2 years. Now, we tell patients they have 10 years to live on average, which means you could live 15 or 20 years. That alone was astounding to me and piqued my interest in myeloma.”
At the same time, smoldering myeloma – which can be discovered during routine blood work – remains little understood. As the National Cancer Institute explains, “smoldering myeloma is a precancerous condition that alters certain proteins in blood and/or increases plasma cells in bone marrow, but it does not cause symptoms of disease. About half of those diagnosed with the condition, however, will develop multiple myeloma within 5 years.”
“If we understand what drives smoldering myeloma, we may be able to prevent it from progressing to its active form,” said hematologist oncologist Samir Parekh, MD, who works with Dr. Mouhieddine at Icahn School of Medicine. “Or at the minimum, we could better predict who will progress so we can tailor therapy for high-risk patients and minimize toxicity by not overtreating patients who may not need therapy.”
Dr. Mouhieddine’s current work is focusing on developing clinical trials to test whether immunotherapy can snuff out myeloma when it’s at the smoldering stage, “before anything bad happens.
“If a myeloma patient comes in with renal failure, and we treat the myeloma at that stage, it doesn’t mean that the patient’s kidneys are gonna go back to normal. A lot of the damage can be permanent,” he said. “Even when you treat multiple myeloma, and it goes into remission, it ends up coming back. And you just have to go from one therapy to the other.”
In contrast, a successful treatment for smoldering myeloma would prevent progression to the full disease. In other words, it would be a cure – which is now elusive.
Specifically, Dr. Mouhieddine hopes to test whether bispecific antibodies, a type of immunotherapy that enlists the body’s T cells to kill myeloma cells, will be effective in the smoldering phase. Bispecific antibodies are now being explored as treatments for full multiple myeloma when the immune system is weaker, he said, and they may be even more effective earlier, when the body is better equipped to fight off the disease.
Dr. Mouhieddine hopes better treatments for multiple myeloma itself will help save his 64-year-old aunt Hassana, back in Beirut. He diagnosed her in 2022 after she told him that she felt tired all the time and underwent various tests. The woman he calls his “second mom” is doing well, despite struggles to buy medication due to the lack of access to bank funds in Lebanon.
“I’m always going to be afraid that the disease is going to progress or come back at some point,” he said. “Lebanon doesn’t have as many options as people in the U.S. do. Once you exhaust your first option, and maybe your second option, then you don’t have any other options. Here, we have outpatients who exhaust option number 15 and go to option number 16. That’s definitely not the case over there.”
For now, Dr. Mouhieddine is treating patients and working to launch clinical trials into smoldering myeloma. “His work ethic is incredible,” said his colleague, Dr. Chari. “He has seen multiple projects to publication, and he develops deep connections with his patients and follows up on their care whether or not he is in clinic on a particular day.”
Dr. Parekh, another colleague, said Dr. Mouhieddine can even be a role model. “Other trainees may benefit from thinking about their career early on and exploring both lab and clinical research projects, so that they can develop the necessary experience to be competitive in academia later on.”
His workload can a burden for Dr. Mouhieddine, who is Muslim. He expressed regret that his busy schedule does not always permit him to fast during Ramadan. On a nonmedical front, his recent efforts have paid off. In March 2023 Dr. Mouhieddine became a U.S. citizen.
“It’s surreal,” he said, “but also a dream come true. I feel very grateful, like it’s like an appreciation of who I am, what I’ve done, and what I can do for this country.”
Managing Type 2 Diabetes in Pediatric Patients
Type 2 diabetes (T2D) is associated with obesity and is increasing at an alarming rate in youth. Pediatric T2D disease progresses more rapidly than adult T2D and is harder to treat.
Dr Maria Redondo, a pediatric endocrinologist at Baylor College of Medicine in Houston, Texas, explains that insulin resistance is the major trigger of T2D, and beta-cell dysfunction is key to its development. In children, beta-cell dysfunction occurs more rapidly compared with adults. Children also have higher rates of complications and associated conditions, such as renal disease, cardiovascular disease, and nonalcoholic fatty liver disease.
Dr Redondo references evidence from the TODAY study, which indicates that treatment failure with first-line metformin is common in the pediatric population and affords minimal weight loss.
She then discusses GLP-1 receptor agonists as second-line therapy for children aged 10 years or older. There are currently two FDA-approved options: once-daily liraglutide and once-weekly exenatide. Both are given subcutaneously.
Finally, Dr Redondo highlights DPP-4 inhibitors, such as linagliptin, saxagliptin, and sitagliptin, as well as SGLT-2 inhibitors, such as dapagliflozin and canagliflozin, as emerging therapies currently in clinical trials.
--
Maria J. Redondo, MD, PhD, MPH, Professor, Department of Pediatrics, Baylor College of Medicine; Staff Physician, Texas Children's Hospital, Houston, Texas
Maria J. Redondo, MD, PhD, MPH, has disclosed the following relevant financial relationships:
Received research grant from: NIH; NIDDK
Type 2 diabetes (T2D) is associated with obesity and is increasing at an alarming rate in youth. Pediatric T2D disease progresses more rapidly than adult T2D and is harder to treat.
Dr Maria Redondo, a pediatric endocrinologist at Baylor College of Medicine in Houston, Texas, explains that insulin resistance is the major trigger of T2D, and beta-cell dysfunction is key to its development. In children, beta-cell dysfunction occurs more rapidly compared with adults. Children also have higher rates of complications and associated conditions, such as renal disease, cardiovascular disease, and nonalcoholic fatty liver disease.
Dr Redondo references evidence from the TODAY study, which indicates that treatment failure with first-line metformin is common in the pediatric population and affords minimal weight loss.
She then discusses GLP-1 receptor agonists as second-line therapy for children aged 10 years or older. There are currently two FDA-approved options: once-daily liraglutide and once-weekly exenatide. Both are given subcutaneously.
Finally, Dr Redondo highlights DPP-4 inhibitors, such as linagliptin, saxagliptin, and sitagliptin, as well as SGLT-2 inhibitors, such as dapagliflozin and canagliflozin, as emerging therapies currently in clinical trials.
--
Maria J. Redondo, MD, PhD, MPH, Professor, Department of Pediatrics, Baylor College of Medicine; Staff Physician, Texas Children's Hospital, Houston, Texas
Maria J. Redondo, MD, PhD, MPH, has disclosed the following relevant financial relationships:
Received research grant from: NIH; NIDDK
Type 2 diabetes (T2D) is associated with obesity and is increasing at an alarming rate in youth. Pediatric T2D disease progresses more rapidly than adult T2D and is harder to treat.
Dr Maria Redondo, a pediatric endocrinologist at Baylor College of Medicine in Houston, Texas, explains that insulin resistance is the major trigger of T2D, and beta-cell dysfunction is key to its development. In children, beta-cell dysfunction occurs more rapidly compared with adults. Children also have higher rates of complications and associated conditions, such as renal disease, cardiovascular disease, and nonalcoholic fatty liver disease.
Dr Redondo references evidence from the TODAY study, which indicates that treatment failure with first-line metformin is common in the pediatric population and affords minimal weight loss.
She then discusses GLP-1 receptor agonists as second-line therapy for children aged 10 years or older. There are currently two FDA-approved options: once-daily liraglutide and once-weekly exenatide. Both are given subcutaneously.
Finally, Dr Redondo highlights DPP-4 inhibitors, such as linagliptin, saxagliptin, and sitagliptin, as well as SGLT-2 inhibitors, such as dapagliflozin and canagliflozin, as emerging therapies currently in clinical trials.
--
Maria J. Redondo, MD, PhD, MPH, Professor, Department of Pediatrics, Baylor College of Medicine; Staff Physician, Texas Children's Hospital, Houston, Texas
Maria J. Redondo, MD, PhD, MPH, has disclosed the following relevant financial relationships:
Received research grant from: NIH; NIDDK

Living the introvert’s dream: Alone for 500 days, but never lonely
Beating the allegory of the cave
When Beatriz Flamini spoke with reporters on April 14, she knew nothing of the previous 18 months. The Russian invasion of Ukraine? Nope. The death of Queen Elizabeth? Also no. But before you make fun of her, she has an excuse. She’s been living under a rock.
As part of an experiment to test how social isolation and disorientation affect a person’s mind, sense of time, and sleeping patterns, Ms. Flamini lived in a 70-meter-deep cave in southern Spain for 500 days, starting in November 2021. Alone. No outside communication with the outside world in any way, though she was constantly monitored by a team of researchers. She also had multiple cameras filming her for an upcoming documentary.
This is a massive step up from the previous record for time spent underground for science: A team of 15 spent 50 days underground in 2021 to similar study of isolation and how it affected circadian rhythms. It’s also almost certainly a world record for time spent underground.
All that time alone certainly sounds like some sort of medieval torture, but Ms. Flamini had access to food, water, and a library of books. Which she made liberal use of, reading at least 60 books during her stay. She also had a panic button in case the isolation became too much or an emergency developed, but she never considered using it.
She lost track of time after 2 months, flies invaded the cave on occasion, and maintaining coherence was occasionally a struggle, but she kept things together very well. In fact, she didn’t even want to leave when her team came for her. She wasn’t even finished with her 61st book.
When she spoke with gathered reporters after the ordeal, words were obviously difficult to come by for her, having not spoken in nearly 18 months, but her mind was clearly still sharp and she had a very important question for everyone gathered around her.
Who’s buying the beer?
We approve of this request.
Staphylococcus and the speed of evolution
Bacteria, we know, are tough little buggers that are hard to see and even harder to get rid of. So hard, actually, that human bodies eventually gave up on the task and decided to just incorporate them into our organ systems. But why are bacteria so hard to eliminate?
Two words: rapid evolution. How rapid? For the first time, scientists have directly observed adaptive evolution by Staphylococcus aureus in a single person’s skin microbiome. That’s how rapid.
For their study, the researchers collected samples from the nostrils, backs of knees, insides of elbows, and forearms of 23 children with eczema. They eventually cultured almost 1,500 unique colonies of S. aureus cells from those samples and sequenced the cells’ genomes.
All that sampling and culturing and sequencing showed that it was rare for a new S. aureus strain to come in and replace the existing strain. “Despite the stability at the lineage level, we see a lot of dynamics at the whole genome level, where new mutations are constantly arising in these bacteria and then spreading throughout the entire body,” Tami D. Lieberman, PhD, of the Massachusetts Institute of Technology, Cambridge, said in a written statement from MIT.
One frequent mutation involved a gene called capD, which encodes an enzyme necessary for synthesizing the capsular polysaccharide – a coating that protects S. aureus from recognition by immune cells. In one patient, four different mutations of capD arose independently in different samples before one variant became dominant and spread over the entire microbiome, MIT reported.
The mutation, which actually results in the loss of the polysaccharide capsule, may allow cells to grow faster than those without the mutation because they have more fuel to power their own growth, the researchers suggested. It’s also possible that loss of the capsule allows S. aureus cells to stick to the skin better because proteins that allow them to adhere to the skin are more exposed.
Dr. Lieberman and her associates hope that these variant-containing cells could be a new target for eczema treatments, but we’re never optimistic when it comes to bacteria. That’s because some of us are old enough to remember evolutionary biologist Stephen Jay Gould, who wrote in his book “Full House”: “Our planet has always been in the ‘Age of Bacteria,’ ever since the first fossils – bacteria, of course – were entombed in rocks more than 3 billion years ago. On any possible, reasonable or fair criterion, bacteria are – and always have been – the dominant forms of life on Earth.”
In the distant future, long after humans have left the scene, the bacteria will be laughing at the last rats and cockroaches scurrying across the landscape. Wanna bet?
The height of genetic prediction
Genetics are practically a DNA Scrabble bag. Traits like eye color and hair texture are chosen in the same fashion, based on what gets pulled from our own genetic bag of letters, but what about height? Researchers may now have a way to predict adult height and make it more than just an educated guess.
How? By looking at the genes in our growth plates. The cartilage on the ends of our bones hardens as we age, eventually deciding an individual’s stature. In a recently published study, a research team looked at 600 million cartilage cells linked to maturation and cell growth in mice. Because everything starts with rodents.
After that search identified 145 genes linked to growth plate maturation and formation of the bones, they compared the mouse genes with data from genome-wide association studies (GWAS) of human height to look for hotspots where the height genes exist in human DNA.
The results showed which genes play a role in deciding height, and the GWAS data also suggested that genetic changes affecting cartilage cell maturation may strongly influence adult height, said the investigators, who hope that earlier interventions can improve outcomes in patients with conditions such as skeletal dysplasia.
So, yeah, you may want to be a little taller or shorter, but the outcome of that particular Scrabble game was determined when your parents, you know, dropped the letters in the bag.
Beating the allegory of the cave
When Beatriz Flamini spoke with reporters on April 14, she knew nothing of the previous 18 months. The Russian invasion of Ukraine? Nope. The death of Queen Elizabeth? Also no. But before you make fun of her, she has an excuse. She’s been living under a rock.
As part of an experiment to test how social isolation and disorientation affect a person’s mind, sense of time, and sleeping patterns, Ms. Flamini lived in a 70-meter-deep cave in southern Spain for 500 days, starting in November 2021. Alone. No outside communication with the outside world in any way, though she was constantly monitored by a team of researchers. She also had multiple cameras filming her for an upcoming documentary.
This is a massive step up from the previous record for time spent underground for science: A team of 15 spent 50 days underground in 2021 to similar study of isolation and how it affected circadian rhythms. It’s also almost certainly a world record for time spent underground.
All that time alone certainly sounds like some sort of medieval torture, but Ms. Flamini had access to food, water, and a library of books. Which she made liberal use of, reading at least 60 books during her stay. She also had a panic button in case the isolation became too much or an emergency developed, but she never considered using it.
She lost track of time after 2 months, flies invaded the cave on occasion, and maintaining coherence was occasionally a struggle, but she kept things together very well. In fact, she didn’t even want to leave when her team came for her. She wasn’t even finished with her 61st book.
When she spoke with gathered reporters after the ordeal, words were obviously difficult to come by for her, having not spoken in nearly 18 months, but her mind was clearly still sharp and she had a very important question for everyone gathered around her.
Who’s buying the beer?
We approve of this request.
Staphylococcus and the speed of evolution
Bacteria, we know, are tough little buggers that are hard to see and even harder to get rid of. So hard, actually, that human bodies eventually gave up on the task and decided to just incorporate them into our organ systems. But why are bacteria so hard to eliminate?
Two words: rapid evolution. How rapid? For the first time, scientists have directly observed adaptive evolution by Staphylococcus aureus in a single person’s skin microbiome. That’s how rapid.
For their study, the researchers collected samples from the nostrils, backs of knees, insides of elbows, and forearms of 23 children with eczema. They eventually cultured almost 1,500 unique colonies of S. aureus cells from those samples and sequenced the cells’ genomes.
All that sampling and culturing and sequencing showed that it was rare for a new S. aureus strain to come in and replace the existing strain. “Despite the stability at the lineage level, we see a lot of dynamics at the whole genome level, where new mutations are constantly arising in these bacteria and then spreading throughout the entire body,” Tami D. Lieberman, PhD, of the Massachusetts Institute of Technology, Cambridge, said in a written statement from MIT.
One frequent mutation involved a gene called capD, which encodes an enzyme necessary for synthesizing the capsular polysaccharide – a coating that protects S. aureus from recognition by immune cells. In one patient, four different mutations of capD arose independently in different samples before one variant became dominant and spread over the entire microbiome, MIT reported.
The mutation, which actually results in the loss of the polysaccharide capsule, may allow cells to grow faster than those without the mutation because they have more fuel to power their own growth, the researchers suggested. It’s also possible that loss of the capsule allows S. aureus cells to stick to the skin better because proteins that allow them to adhere to the skin are more exposed.
Dr. Lieberman and her associates hope that these variant-containing cells could be a new target for eczema treatments, but we’re never optimistic when it comes to bacteria. That’s because some of us are old enough to remember evolutionary biologist Stephen Jay Gould, who wrote in his book “Full House”: “Our planet has always been in the ‘Age of Bacteria,’ ever since the first fossils – bacteria, of course – were entombed in rocks more than 3 billion years ago. On any possible, reasonable or fair criterion, bacteria are – and always have been – the dominant forms of life on Earth.”
In the distant future, long after humans have left the scene, the bacteria will be laughing at the last rats and cockroaches scurrying across the landscape. Wanna bet?
The height of genetic prediction
Genetics are practically a DNA Scrabble bag. Traits like eye color and hair texture are chosen in the same fashion, based on what gets pulled from our own genetic bag of letters, but what about height? Researchers may now have a way to predict adult height and make it more than just an educated guess.
How? By looking at the genes in our growth plates. The cartilage on the ends of our bones hardens as we age, eventually deciding an individual’s stature. In a recently published study, a research team looked at 600 million cartilage cells linked to maturation and cell growth in mice. Because everything starts with rodents.
After that search identified 145 genes linked to growth plate maturation and formation of the bones, they compared the mouse genes with data from genome-wide association studies (GWAS) of human height to look for hotspots where the height genes exist in human DNA.
The results showed which genes play a role in deciding height, and the GWAS data also suggested that genetic changes affecting cartilage cell maturation may strongly influence adult height, said the investigators, who hope that earlier interventions can improve outcomes in patients with conditions such as skeletal dysplasia.
So, yeah, you may want to be a little taller or shorter, but the outcome of that particular Scrabble game was determined when your parents, you know, dropped the letters in the bag.
Beating the allegory of the cave
When Beatriz Flamini spoke with reporters on April 14, she knew nothing of the previous 18 months. The Russian invasion of Ukraine? Nope. The death of Queen Elizabeth? Also no. But before you make fun of her, she has an excuse. She’s been living under a rock.
As part of an experiment to test how social isolation and disorientation affect a person’s mind, sense of time, and sleeping patterns, Ms. Flamini lived in a 70-meter-deep cave in southern Spain for 500 days, starting in November 2021. Alone. No outside communication with the outside world in any way, though she was constantly monitored by a team of researchers. She also had multiple cameras filming her for an upcoming documentary.
This is a massive step up from the previous record for time spent underground for science: A team of 15 spent 50 days underground in 2021 to similar study of isolation and how it affected circadian rhythms. It’s also almost certainly a world record for time spent underground.
All that time alone certainly sounds like some sort of medieval torture, but Ms. Flamini had access to food, water, and a library of books. Which she made liberal use of, reading at least 60 books during her stay. She also had a panic button in case the isolation became too much or an emergency developed, but she never considered using it.
She lost track of time after 2 months, flies invaded the cave on occasion, and maintaining coherence was occasionally a struggle, but she kept things together very well. In fact, she didn’t even want to leave when her team came for her. She wasn’t even finished with her 61st book.
When she spoke with gathered reporters after the ordeal, words were obviously difficult to come by for her, having not spoken in nearly 18 months, but her mind was clearly still sharp and she had a very important question for everyone gathered around her.
Who’s buying the beer?
We approve of this request.
Staphylococcus and the speed of evolution
Bacteria, we know, are tough little buggers that are hard to see and even harder to get rid of. So hard, actually, that human bodies eventually gave up on the task and decided to just incorporate them into our organ systems. But why are bacteria so hard to eliminate?
Two words: rapid evolution. How rapid? For the first time, scientists have directly observed adaptive evolution by Staphylococcus aureus in a single person’s skin microbiome. That’s how rapid.
For their study, the researchers collected samples from the nostrils, backs of knees, insides of elbows, and forearms of 23 children with eczema. They eventually cultured almost 1,500 unique colonies of S. aureus cells from those samples and sequenced the cells’ genomes.
All that sampling and culturing and sequencing showed that it was rare for a new S. aureus strain to come in and replace the existing strain. “Despite the stability at the lineage level, we see a lot of dynamics at the whole genome level, where new mutations are constantly arising in these bacteria and then spreading throughout the entire body,” Tami D. Lieberman, PhD, of the Massachusetts Institute of Technology, Cambridge, said in a written statement from MIT.
One frequent mutation involved a gene called capD, which encodes an enzyme necessary for synthesizing the capsular polysaccharide – a coating that protects S. aureus from recognition by immune cells. In one patient, four different mutations of capD arose independently in different samples before one variant became dominant and spread over the entire microbiome, MIT reported.
The mutation, which actually results in the loss of the polysaccharide capsule, may allow cells to grow faster than those without the mutation because they have more fuel to power their own growth, the researchers suggested. It’s also possible that loss of the capsule allows S. aureus cells to stick to the skin better because proteins that allow them to adhere to the skin are more exposed.
Dr. Lieberman and her associates hope that these variant-containing cells could be a new target for eczema treatments, but we’re never optimistic when it comes to bacteria. That’s because some of us are old enough to remember evolutionary biologist Stephen Jay Gould, who wrote in his book “Full House”: “Our planet has always been in the ‘Age of Bacteria,’ ever since the first fossils – bacteria, of course – were entombed in rocks more than 3 billion years ago. On any possible, reasonable or fair criterion, bacteria are – and always have been – the dominant forms of life on Earth.”
In the distant future, long after humans have left the scene, the bacteria will be laughing at the last rats and cockroaches scurrying across the landscape. Wanna bet?
The height of genetic prediction
Genetics are practically a DNA Scrabble bag. Traits like eye color and hair texture are chosen in the same fashion, based on what gets pulled from our own genetic bag of letters, but what about height? Researchers may now have a way to predict adult height and make it more than just an educated guess.
How? By looking at the genes in our growth plates. The cartilage on the ends of our bones hardens as we age, eventually deciding an individual’s stature. In a recently published study, a research team looked at 600 million cartilage cells linked to maturation and cell growth in mice. Because everything starts with rodents.
After that search identified 145 genes linked to growth plate maturation and formation of the bones, they compared the mouse genes with data from genome-wide association studies (GWAS) of human height to look for hotspots where the height genes exist in human DNA.
The results showed which genes play a role in deciding height, and the GWAS data also suggested that genetic changes affecting cartilage cell maturation may strongly influence adult height, said the investigators, who hope that earlier interventions can improve outcomes in patients with conditions such as skeletal dysplasia.
So, yeah, you may want to be a little taller or shorter, but the outcome of that particular Scrabble game was determined when your parents, you know, dropped the letters in the bag.
PARP/ATR inhibitor combo shows hints of promise in children with tumors
The small phase 1 trial also identified some molecular signatures in responders that may inform future clinical trials.
The results, presented at the annual meeting of the American Association of Cancer Research, came from a single arm of the European Proof-of-Concept Therapeutic Stratification Trial of Molecular Anomalies in Relapsed or Refractory Tumors (ESMART) trial. This trial matches pediatric, adolescent, and young adult cancer patients with treatment regimens based on the molecular profile of their tumors.
In over 220 children to date, the trial has investigated 15 different treatment regimens, most of which are combination therapies.
In adults, PARP) inhibitors have been shown to be effective in tumors with deficiencies in homologous repair, which is a DNA repair mechanism, with notable successes in patients carrying the BRCA1 and BRCA2 mutations. But BRCA1 and BRCA2 mutations are rare in pediatric cancer, and there is a belief that there may be primary resistance to PARP inhibitors in pediatric tumors, according to Susanne Gatz, MD, PhD, who presented the research at the meeting.
Previous research identified alterations in pediatric tumors that are candidates for patient selection. “These tumors have alterations which could potentially cause this resistance effect [against PARP inhibitors] and [also cause] sensitivity to ataxia telangiectasia–mutated Rad3-related inhibitors. This is how this arm [of the ESMART trial] was born,” said Dr. Gatz.
The phase 1 portion of the study included 18 pediatric and young adult patients with relapsed or treatment-refractory tumors. There were eight sarcomas, five central nervous system tumors, four neuroblastomas, and one carcinoma. Each had mutations thought to lead to HR deficiency or replication stress. The study included three dose levels of twice-daily oral olaparib that was given continuously, and ceralasertib, which was given day 1-14 of each 28-day cycle.
Patients underwent a median of 3.5 cycles of treatment. There were dose-limiting adverse events of thrombocytopenia and neutropenia in five patients, two of which occurred at the dose that was recommended for phase 2.
There were some positive clinical signs, including one partial response in a pineoblastoma patient who received treatment for 11 cycles. A neuroblastoma patient had stable disease until cycle 9 of treatment, and then converted to a partial response and is currently in cycle 12. Two other patients remain in treatment at cycle 8 and one is in treatment at cycle 15. None of the patients who experienced clinical benefit had BRCA mutations.
An important goal of the study was to understand molecular signature that might predict response to the drug combination. Although no firm conclusions could be drawn, there were some interesting patterns. In particular, five of the six worst responders had TP53 mutations. “It is striking ... so we need to learn what TP53 in this setting means if it’s mutated, and if it could be a resistance factor,” said Dr. Gatz, an associate clinical professor in pediatric oncology at the Institute of Cancer and Genomic Sciences of the University of Birmingham, during her talk.
Although the study is too small and included too many tumor types to identify tumor-based patterns of response, it did provide some hints as to biomarkers that could inform future studies, according to Julia Glade Bender, MD, who served as a discussant following the presentation and is a pediatric oncologist at Memorial Sloan Kettering Cancer Center, New York.
“The pediatric frequency of the common DNA damage repair biomarkers that have been [identified in] the adult literature – that is to say, BRCA1 and 2 and [ataxia-telangiectasia mutation] – are exceedingly rare in pediatrics,” said Dr. Bender during the session while serving as a discussant. She highlighted the following findings: Loss of the 11q region on chromosome 11 is common among the patients and that region contains three genes involved in the DNA damage response, along with a gene involved in homologous recombination, telomere maintenance, and double strand break repair.
She added that 11q deletion is also found in up to 40% of neuroblastomas, and is associated with poor prognosis, and the patients have multiple segmental chromosomal abnormalities. “That begs the question [of] whether chromosomal instability is another biomarker for pediatric cancer,” said Dr. Bender.
“The research highlights the complexity of pediatric cancers, whose distinct biology could make them more vulnerable to ATR [kinase], [checkpoint kinase 1], and WEE1 pathway inhibition with a PARP inhibitor used to induce replication stress and be the sensitizer. The biomarker profiles are going to be complex, context-dependent, and likely to reflect a constellation of findings that would be signatures or algorithms, rather than single gene alterations. The post hoc iterative analysis of responders and nonresponders is going to be absolutely critical to understanding those biomarkers and the role of DNA damage response inhibitors in pediatrics. Given the rarity of these diagnoses, and then the molecular subclasses, I think collaboration across ages and geography is absolutely critical, and I really congratulate the ESMART consortium for doing just that in Europe,” said Dr. Bender.
The study is limited by its small sample size and the fact that it was not randomized.
The study received funding from French Institut National de Cancer, Imagine for Margo, Fondation ARC, AstraZeneca France, AstraZeneca Global R&D, AstraZeneca UK, Cancer Research UK, Fondation Gustave Roussy, and Little Princess Trust/Children’s Cancer and Leukaemia Group. Dr. Gatz has no relevant financial disclosures. Dr. Bender has done paid consulting for Jazz Pharmaceuticals and has done unpaid work for Bristol-Myers Squibb, Eisai, Springworks Therapeutics, Merck Sharp & Dohme, and Pfizer. She has received research support from Eli Lilly, Loxo-oncology, Eisai, Cellectar, Bayer, Amgen, and Jazz Pharmaceuticals.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Abstract CT019. Presented Tuesday, April 18.
The small phase 1 trial also identified some molecular signatures in responders that may inform future clinical trials.
The results, presented at the annual meeting of the American Association of Cancer Research, came from a single arm of the European Proof-of-Concept Therapeutic Stratification Trial of Molecular Anomalies in Relapsed or Refractory Tumors (ESMART) trial. This trial matches pediatric, adolescent, and young adult cancer patients with treatment regimens based on the molecular profile of their tumors.
In over 220 children to date, the trial has investigated 15 different treatment regimens, most of which are combination therapies.
In adults, PARP) inhibitors have been shown to be effective in tumors with deficiencies in homologous repair, which is a DNA repair mechanism, with notable successes in patients carrying the BRCA1 and BRCA2 mutations. But BRCA1 and BRCA2 mutations are rare in pediatric cancer, and there is a belief that there may be primary resistance to PARP inhibitors in pediatric tumors, according to Susanne Gatz, MD, PhD, who presented the research at the meeting.
Previous research identified alterations in pediatric tumors that are candidates for patient selection. “These tumors have alterations which could potentially cause this resistance effect [against PARP inhibitors] and [also cause] sensitivity to ataxia telangiectasia–mutated Rad3-related inhibitors. This is how this arm [of the ESMART trial] was born,” said Dr. Gatz.
The phase 1 portion of the study included 18 pediatric and young adult patients with relapsed or treatment-refractory tumors. There were eight sarcomas, five central nervous system tumors, four neuroblastomas, and one carcinoma. Each had mutations thought to lead to HR deficiency or replication stress. The study included three dose levels of twice-daily oral olaparib that was given continuously, and ceralasertib, which was given day 1-14 of each 28-day cycle.
Patients underwent a median of 3.5 cycles of treatment. There were dose-limiting adverse events of thrombocytopenia and neutropenia in five patients, two of which occurred at the dose that was recommended for phase 2.
There were some positive clinical signs, including one partial response in a pineoblastoma patient who received treatment for 11 cycles. A neuroblastoma patient had stable disease until cycle 9 of treatment, and then converted to a partial response and is currently in cycle 12. Two other patients remain in treatment at cycle 8 and one is in treatment at cycle 15. None of the patients who experienced clinical benefit had BRCA mutations.
An important goal of the study was to understand molecular signature that might predict response to the drug combination. Although no firm conclusions could be drawn, there were some interesting patterns. In particular, five of the six worst responders had TP53 mutations. “It is striking ... so we need to learn what TP53 in this setting means if it’s mutated, and if it could be a resistance factor,” said Dr. Gatz, an associate clinical professor in pediatric oncology at the Institute of Cancer and Genomic Sciences of the University of Birmingham, during her talk.
Although the study is too small and included too many tumor types to identify tumor-based patterns of response, it did provide some hints as to biomarkers that could inform future studies, according to Julia Glade Bender, MD, who served as a discussant following the presentation and is a pediatric oncologist at Memorial Sloan Kettering Cancer Center, New York.
“The pediatric frequency of the common DNA damage repair biomarkers that have been [identified in] the adult literature – that is to say, BRCA1 and 2 and [ataxia-telangiectasia mutation] – are exceedingly rare in pediatrics,” said Dr. Bender during the session while serving as a discussant. She highlighted the following findings: Loss of the 11q region on chromosome 11 is common among the patients and that region contains three genes involved in the DNA damage response, along with a gene involved in homologous recombination, telomere maintenance, and double strand break repair.
She added that 11q deletion is also found in up to 40% of neuroblastomas, and is associated with poor prognosis, and the patients have multiple segmental chromosomal abnormalities. “That begs the question [of] whether chromosomal instability is another biomarker for pediatric cancer,” said Dr. Bender.
“The research highlights the complexity of pediatric cancers, whose distinct biology could make them more vulnerable to ATR [kinase], [checkpoint kinase 1], and WEE1 pathway inhibition with a PARP inhibitor used to induce replication stress and be the sensitizer. The biomarker profiles are going to be complex, context-dependent, and likely to reflect a constellation of findings that would be signatures or algorithms, rather than single gene alterations. The post hoc iterative analysis of responders and nonresponders is going to be absolutely critical to understanding those biomarkers and the role of DNA damage response inhibitors in pediatrics. Given the rarity of these diagnoses, and then the molecular subclasses, I think collaboration across ages and geography is absolutely critical, and I really congratulate the ESMART consortium for doing just that in Europe,” said Dr. Bender.
The study is limited by its small sample size and the fact that it was not randomized.
The study received funding from French Institut National de Cancer, Imagine for Margo, Fondation ARC, AstraZeneca France, AstraZeneca Global R&D, AstraZeneca UK, Cancer Research UK, Fondation Gustave Roussy, and Little Princess Trust/Children’s Cancer and Leukaemia Group. Dr. Gatz has no relevant financial disclosures. Dr. Bender has done paid consulting for Jazz Pharmaceuticals and has done unpaid work for Bristol-Myers Squibb, Eisai, Springworks Therapeutics, Merck Sharp & Dohme, and Pfizer. She has received research support from Eli Lilly, Loxo-oncology, Eisai, Cellectar, Bayer, Amgen, and Jazz Pharmaceuticals.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Abstract CT019. Presented Tuesday, April 18.
The small phase 1 trial also identified some molecular signatures in responders that may inform future clinical trials.
The results, presented at the annual meeting of the American Association of Cancer Research, came from a single arm of the European Proof-of-Concept Therapeutic Stratification Trial of Molecular Anomalies in Relapsed or Refractory Tumors (ESMART) trial. This trial matches pediatric, adolescent, and young adult cancer patients with treatment regimens based on the molecular profile of their tumors.
In over 220 children to date, the trial has investigated 15 different treatment regimens, most of which are combination therapies.
In adults, PARP) inhibitors have been shown to be effective in tumors with deficiencies in homologous repair, which is a DNA repair mechanism, with notable successes in patients carrying the BRCA1 and BRCA2 mutations. But BRCA1 and BRCA2 mutations are rare in pediatric cancer, and there is a belief that there may be primary resistance to PARP inhibitors in pediatric tumors, according to Susanne Gatz, MD, PhD, who presented the research at the meeting.
Previous research identified alterations in pediatric tumors that are candidates for patient selection. “These tumors have alterations which could potentially cause this resistance effect [against PARP inhibitors] and [also cause] sensitivity to ataxia telangiectasia–mutated Rad3-related inhibitors. This is how this arm [of the ESMART trial] was born,” said Dr. Gatz.
The phase 1 portion of the study included 18 pediatric and young adult patients with relapsed or treatment-refractory tumors. There were eight sarcomas, five central nervous system tumors, four neuroblastomas, and one carcinoma. Each had mutations thought to lead to HR deficiency or replication stress. The study included three dose levels of twice-daily oral olaparib that was given continuously, and ceralasertib, which was given day 1-14 of each 28-day cycle.
Patients underwent a median of 3.5 cycles of treatment. There were dose-limiting adverse events of thrombocytopenia and neutropenia in five patients, two of which occurred at the dose that was recommended for phase 2.
There were some positive clinical signs, including one partial response in a pineoblastoma patient who received treatment for 11 cycles. A neuroblastoma patient had stable disease until cycle 9 of treatment, and then converted to a partial response and is currently in cycle 12. Two other patients remain in treatment at cycle 8 and one is in treatment at cycle 15. None of the patients who experienced clinical benefit had BRCA mutations.
An important goal of the study was to understand molecular signature that might predict response to the drug combination. Although no firm conclusions could be drawn, there were some interesting patterns. In particular, five of the six worst responders had TP53 mutations. “It is striking ... so we need to learn what TP53 in this setting means if it’s mutated, and if it could be a resistance factor,” said Dr. Gatz, an associate clinical professor in pediatric oncology at the Institute of Cancer and Genomic Sciences of the University of Birmingham, during her talk.
Although the study is too small and included too many tumor types to identify tumor-based patterns of response, it did provide some hints as to biomarkers that could inform future studies, according to Julia Glade Bender, MD, who served as a discussant following the presentation and is a pediatric oncologist at Memorial Sloan Kettering Cancer Center, New York.
“The pediatric frequency of the common DNA damage repair biomarkers that have been [identified in] the adult literature – that is to say, BRCA1 and 2 and [ataxia-telangiectasia mutation] – are exceedingly rare in pediatrics,” said Dr. Bender during the session while serving as a discussant. She highlighted the following findings: Loss of the 11q region on chromosome 11 is common among the patients and that region contains three genes involved in the DNA damage response, along with a gene involved in homologous recombination, telomere maintenance, and double strand break repair.
She added that 11q deletion is also found in up to 40% of neuroblastomas, and is associated with poor prognosis, and the patients have multiple segmental chromosomal abnormalities. “That begs the question [of] whether chromosomal instability is another biomarker for pediatric cancer,” said Dr. Bender.
“The research highlights the complexity of pediatric cancers, whose distinct biology could make them more vulnerable to ATR [kinase], [checkpoint kinase 1], and WEE1 pathway inhibition with a PARP inhibitor used to induce replication stress and be the sensitizer. The biomarker profiles are going to be complex, context-dependent, and likely to reflect a constellation of findings that would be signatures or algorithms, rather than single gene alterations. The post hoc iterative analysis of responders and nonresponders is going to be absolutely critical to understanding those biomarkers and the role of DNA damage response inhibitors in pediatrics. Given the rarity of these diagnoses, and then the molecular subclasses, I think collaboration across ages and geography is absolutely critical, and I really congratulate the ESMART consortium for doing just that in Europe,” said Dr. Bender.
The study is limited by its small sample size and the fact that it was not randomized.
The study received funding from French Institut National de Cancer, Imagine for Margo, Fondation ARC, AstraZeneca France, AstraZeneca Global R&D, AstraZeneca UK, Cancer Research UK, Fondation Gustave Roussy, and Little Princess Trust/Children’s Cancer and Leukaemia Group. Dr. Gatz has no relevant financial disclosures. Dr. Bender has done paid consulting for Jazz Pharmaceuticals and has done unpaid work for Bristol-Myers Squibb, Eisai, Springworks Therapeutics, Merck Sharp & Dohme, and Pfizer. She has received research support from Eli Lilly, Loxo-oncology, Eisai, Cellectar, Bayer, Amgen, and Jazz Pharmaceuticals.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Abstract CT019. Presented Tuesday, April 18.
FROM AACR 2023
Circulating DNA has promise for cancer detection, but faces challenges
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
Cancer screening remains challenging. There are screens available for a handful of solid tumors, but uptake is low caused in part by health care access barriers as well as the potential for unnecessary follow-up procedures, according to Phillip Febbo, MD.
These issues could threaten efforts like that of President Joe Biden’s Cancer Moonshot initiative, which aims to reduce cancer mortality by 50%. Advances in circulating tumor (ct) DNA analysis could help address these problems, but a lack of diversity among study participants needs to be addressed to ensure it has broad utility, continued Dr. Febbo, during his presentation at the annual meeting of the American Association for Cancer Research.
The problem is particularly acute among African American, Hispanic, and other underserved populations who often face health care barriers that can exacerbate the issue, said Dr. Febbo, who is chief medical officer for Illumina. The lack of access is compounded by the fact that there are only currently screens for lung, breast, colorectal, cervical, and prostate cancer, leaving a vast unmet need.
“We still do not have screening tests for 70% of the deaths that are due to cancer,” he said.
ctDNA released from dying cancer cells has the potential to reveal a wide range of cancer types and reduce barriers to access, because it is based on a blood test. It can be analyzed for various factors, including mutations, chromosomal rearrangements, methylation patterns, and other characteristics that hint at the presence of cancer. However, it can’t be successful without sufficient inclusion in research studies, Dr. Febbo explained.
“We have to ensure we have the right representation [of] populations when we develop these tests, when we go through the clinical trials, and as we bring these into communities,” he said.
During his presentation, Dr. Febbo shared a slide showing that about 78% of participants in published gene-association studies were White.
ctDNA showed promise in at least on recent study. Dr. Febbo discussed the ECLIPSE trial, which used the Guardant Health SHIELD assay for colorectal cancer (CRC). About 13% of its approximately 20,000 participants were Black or African American, 15% were Hispanic, and 7% were Asian Americans. It also included both urban and rural individuals. In results announced in December 2022, the researchers found a sensitivity of 83%, which was lower than the 92.3% found in standard CRC screening, but above the 74% threshold set by the Food and Drug Administration. The specificity was 90%.
One approach that could dramatically change the landscape of cancer screening is a multicancer early detection (MCED) test, according to Dr. Febbo. The CancerSeek MCED test, developed by Johns Hopkins Kimmel Cancer Center researchers, uses a series of genetic and protein biomarkers to detect all cancers, with the exception of leukemia, skin cancer, and central nervous system tumors. Among 10,006 women aged between 65 and 75 years with no history of cancer, it had a sensitivity of 27.1% and a specificity of 98.9%, with a positive predictive value of 19.4%. The study’s population was 95% non-Hispanic White.
He also discussed the Pathfinder study, sponsored by the Illumina subsidiary Grail, which included 6,662 individuals age 50 and over from seven sites in the United States, and grouped them into normal and increased risk; 92% were non-Hispanic White. It used the Galleri MCED test, which performed with a sensitivity of 29%, specificity of 99.1%, and a positive predictive value of 38.0%. False positives produced to limited burden, with 93% undergoing imaging, 28% nonsurgical invasive procedures, and 2% undergoing fruitless invasive surgical procedures.
Dr. Febbo touted the potential for such tests to greatly reduce cancer mortality, but only if there is adequate uptake of screening procedures, particularly in underserved groups. He put up a slide of a model showing that MCED has the potential to reduce cancer mortality by 20%, but only if the screen is widely accepted among all groups. “I’ve had my team model this. If we accept the current use of screening tests, and we don’t address disparities, and we don’t ensure everybody feels included and participates actively – not only in the research, but also in the testing and adoption, you would cut that potential benefit in half. That would be hundreds of thousands of lives lost because we didn’t address disparities.”
Successful recruiting of African Americans for research
Following Dr. Febbo’s talk, Karriem Watson, MS, spoke about some potential solutions to the issue, including his own experiences and success stories in recruiting African Americans to play active roles in research. He is chief engagement officer for the National Institute of Health’s All of Us Research Program, which aims to gather health data on at least 1 million residents of the United States. Mr. Watson has spent time reaching out to people living in communities in the Chicago area to encourage participation in breast cancer screening. An event at his church inspired his own sister to get a mammogram, and she was diagnosed with early-stage breast cancer.
“I’m a living witness that early engagement can lead to early detection,” said Mr. Watson during his talk.
He reported that the All of Us research program has succeeded in creating diversity within its data collection, as 46.7% of participants identify as racial and ethnic minorities.
Mr. Watson took issue with the common perception that underrepresented communities may be hard to reach.
“I want to challenge us to think outside the box and really ask ourselves: Are populations hard to reach, or are there opportunities for us to do better and more intentional engagement?” He went on to describe a program to recruit African American men as citizen scientists. He and his colleagues developed a network that included barbers, faith leaders, and fraternity and civic organization members to help recruit participants for a prostate cancer screening project. They exceeded their initial recruitment goal.
They went on to develop a network of barbers in the south and west sides of Chicago to recruit individuals to participate in studies of protein methylation and lung cancer screening, as well as a project that investigated associations between neighborhood of residence and lung cancer. The results of those efforts have also informed other projects, including a smoking cessation study. “We’ve not only included African American men in our research, but we’ve included them as part of our research team,” said Mr. Watson.
Dr. Febbo is also a stockholder of Illumina. Mr. Watson has no relevant financial disclosures.
From American Association for Cancer Research (AACR) Annual Meeting 2023: Improving cancer outcomes through equitable access to cfDNA tests. Presented Monday, April 17, 2023.
FROM AACR 2023
Endometriosis and Abnormal Uterine Bleeding
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
The Prediabetes Debate: Should the Diabetes Diagnostic Threshold Be Lowered, Allowing Clinicians to Intervene Earlier?
I believe that the diagnosis of type 2 diabetes mellitus (T2DM) should be broadened to match the glycemic thresholds currently used for prediabetes. This would eliminate the need for a separate category, the “prediabetes” nomenclature, and allow for earlier therapeutic intervention in patients. Current diabetes diagnostic thresholds do not reflect the latest advancements in T2DM understanding. The latest in T2DM research suggests that intervening and treating prediabetes earlier could potentially offer better clinical outcomes and enhance patients’ quality of life, as cell and tissue damage occurs early and leads to dysfunction prior to a diabetes diagnosis.1 T2DM is a highly complex disease with multifactorial causes beyond hyperglycemia that should be considered, such as hyperlipidemia, insulin resistance, hyperinsulinemia, and autoimmune inflammatory mechanisms that lead to β-cell dysfunction or failure, which results in hyperglycemia.
Prediabetes is associated with micro- and macrovascular complications that can occur early in the progression to frank disease state.1,2 This phase of diabetes also includes insulin resistance, impaired incretin action, insulin hypersecretion, increased lipolysis, and ectopic lipid storage—all of which damage β cells. These dysfunctions are also present in the frank diabetic disease state.3,4 Furthermore, diabetic retinopathy occurs in 8% to 12% of patients with prediabetes, and retinopathy begins earlier than previously thought, with neuroinflammation occurring even before vascular damage.5,6 Unfortunately, these neuro-inflammatory lesions cannot be detected with the typical instruments used in an ophthalmologist’s office.
It is believed that, through the principle of metabolic memory, even a moderate increase or episodic spikes in blood glucose can lead to negative effects in prediabetic patients who are susceptible to T2DM.1,5 Therefore, a lower diabetes diagnostic threshold could allow for earlier, more precise, and personalized therapies based on each patient’s individual risk factors and biomarkers. With a diagnosis of T2DM at the current prediabetes threshold, patients could receive treatment covered by health insurance while in the “prediabetic” state—treatment that would not have been previously approved. Patients should be treated earlier and on an individual basis with counseling on diet and lifestyle changes and antidiabetic agents to reduce glycemic levels, preserve β cells, and reduce cardiovascular [CV] or renal risk, among other complications.6,7
Moreover, the newer agents for treating diabetes such as glucagon-like pepetide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors, which are also associated with reduction in adverse CV and/or renal outcomes, could be beneficial if administered early in the prediabetic stage of disease.8,9
Early lifestyle and pharmacologic interventions can reduce the rate of progression from prediabetes to diabetes as well as complications and associated conditions, and even potentially result in remission or a full reversal of diabetes. These “side benefits” of lowering the diabetes diagnostic threshold (over and above glycemic control) make any cost-effectiveness calculations all the more advantageous to individual patients as well as to society.
Given the numerous benefits of earlier intervention for diabetes treatment, I believe a call to re-evaluate the current diabetes diagnostic threshold is in order, as it will do a great service for all patients who are currently at risk for developing T2DM.
Armato JP, DeFronzo RA, Abdul-Ghani M, Ruby RJ. Successful treatment of prediabetes in clinical practice using physiological assessment (STOP DIABETES). Lancet Diabetes Endocrinol. 2018;6:781-789.
Schwartz SS, Epstein S, Corkey BE, et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol Metab. 2017;28:645-655.
American Diabetes Association. Standards of Medical Care in Diabetes – 2021. Diabetes Care. 2021;44(S15–S39):S111-S124.
Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood). 2016;241:1323-1331.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
Sinclair SH, Schwartz SS. Diabetic retinopathy–an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front Endocrinol (Lausanne). 2019;10:843.
Edwards CM, Cusi K. Prediabetes: a worldwide epidemic. Endocrinol Metab Clin N Am. 2016;45:751-764.
Kanat M, DeFronzo RA, Abdul-Ghani MA. Treatment of prediabetes. World J Diabetes. 2015;6:1207-1222.
Dankner R, Roth J. The personalized approach for detecting prediabetes and diabetes. Curr Diabetes Rev. 2016;12:58-65.
I believe that the diagnosis of type 2 diabetes mellitus (T2DM) should be broadened to match the glycemic thresholds currently used for prediabetes. This would eliminate the need for a separate category, the “prediabetes” nomenclature, and allow for earlier therapeutic intervention in patients. Current diabetes diagnostic thresholds do not reflect the latest advancements in T2DM understanding. The latest in T2DM research suggests that intervening and treating prediabetes earlier could potentially offer better clinical outcomes and enhance patients’ quality of life, as cell and tissue damage occurs early and leads to dysfunction prior to a diabetes diagnosis.1 T2DM is a highly complex disease with multifactorial causes beyond hyperglycemia that should be considered, such as hyperlipidemia, insulin resistance, hyperinsulinemia, and autoimmune inflammatory mechanisms that lead to β-cell dysfunction or failure, which results in hyperglycemia.
Prediabetes is associated with micro- and macrovascular complications that can occur early in the progression to frank disease state.1,2 This phase of diabetes also includes insulin resistance, impaired incretin action, insulin hypersecretion, increased lipolysis, and ectopic lipid storage—all of which damage β cells. These dysfunctions are also present in the frank diabetic disease state.3,4 Furthermore, diabetic retinopathy occurs in 8% to 12% of patients with prediabetes, and retinopathy begins earlier than previously thought, with neuroinflammation occurring even before vascular damage.5,6 Unfortunately, these neuro-inflammatory lesions cannot be detected with the typical instruments used in an ophthalmologist’s office.
It is believed that, through the principle of metabolic memory, even a moderate increase or episodic spikes in blood glucose can lead to negative effects in prediabetic patients who are susceptible to T2DM.1,5 Therefore, a lower diabetes diagnostic threshold could allow for earlier, more precise, and personalized therapies based on each patient’s individual risk factors and biomarkers. With a diagnosis of T2DM at the current prediabetes threshold, patients could receive treatment covered by health insurance while in the “prediabetic” state—treatment that would not have been previously approved. Patients should be treated earlier and on an individual basis with counseling on diet and lifestyle changes and antidiabetic agents to reduce glycemic levels, preserve β cells, and reduce cardiovascular [CV] or renal risk, among other complications.6,7
Moreover, the newer agents for treating diabetes such as glucagon-like pepetide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors, which are also associated with reduction in adverse CV and/or renal outcomes, could be beneficial if administered early in the prediabetic stage of disease.8,9
Early lifestyle and pharmacologic interventions can reduce the rate of progression from prediabetes to diabetes as well as complications and associated conditions, and even potentially result in remission or a full reversal of diabetes. These “side benefits” of lowering the diabetes diagnostic threshold (over and above glycemic control) make any cost-effectiveness calculations all the more advantageous to individual patients as well as to society.
Given the numerous benefits of earlier intervention for diabetes treatment, I believe a call to re-evaluate the current diabetes diagnostic threshold is in order, as it will do a great service for all patients who are currently at risk for developing T2DM.
I believe that the diagnosis of type 2 diabetes mellitus (T2DM) should be broadened to match the glycemic thresholds currently used for prediabetes. This would eliminate the need for a separate category, the “prediabetes” nomenclature, and allow for earlier therapeutic intervention in patients. Current diabetes diagnostic thresholds do not reflect the latest advancements in T2DM understanding. The latest in T2DM research suggests that intervening and treating prediabetes earlier could potentially offer better clinical outcomes and enhance patients’ quality of life, as cell and tissue damage occurs early and leads to dysfunction prior to a diabetes diagnosis.1 T2DM is a highly complex disease with multifactorial causes beyond hyperglycemia that should be considered, such as hyperlipidemia, insulin resistance, hyperinsulinemia, and autoimmune inflammatory mechanisms that lead to β-cell dysfunction or failure, which results in hyperglycemia.
Prediabetes is associated with micro- and macrovascular complications that can occur early in the progression to frank disease state.1,2 This phase of diabetes also includes insulin resistance, impaired incretin action, insulin hypersecretion, increased lipolysis, and ectopic lipid storage—all of which damage β cells. These dysfunctions are also present in the frank diabetic disease state.3,4 Furthermore, diabetic retinopathy occurs in 8% to 12% of patients with prediabetes, and retinopathy begins earlier than previously thought, with neuroinflammation occurring even before vascular damage.5,6 Unfortunately, these neuro-inflammatory lesions cannot be detected with the typical instruments used in an ophthalmologist’s office.
It is believed that, through the principle of metabolic memory, even a moderate increase or episodic spikes in blood glucose can lead to negative effects in prediabetic patients who are susceptible to T2DM.1,5 Therefore, a lower diabetes diagnostic threshold could allow for earlier, more precise, and personalized therapies based on each patient’s individual risk factors and biomarkers. With a diagnosis of T2DM at the current prediabetes threshold, patients could receive treatment covered by health insurance while in the “prediabetic” state—treatment that would not have been previously approved. Patients should be treated earlier and on an individual basis with counseling on diet and lifestyle changes and antidiabetic agents to reduce glycemic levels, preserve β cells, and reduce cardiovascular [CV] or renal risk, among other complications.6,7
Moreover, the newer agents for treating diabetes such as glucagon-like pepetide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors, which are also associated with reduction in adverse CV and/or renal outcomes, could be beneficial if administered early in the prediabetic stage of disease.8,9
Early lifestyle and pharmacologic interventions can reduce the rate of progression from prediabetes to diabetes as well as complications and associated conditions, and even potentially result in remission or a full reversal of diabetes. These “side benefits” of lowering the diabetes diagnostic threshold (over and above glycemic control) make any cost-effectiveness calculations all the more advantageous to individual patients as well as to society.
Given the numerous benefits of earlier intervention for diabetes treatment, I believe a call to re-evaluate the current diabetes diagnostic threshold is in order, as it will do a great service for all patients who are currently at risk for developing T2DM.
Armato JP, DeFronzo RA, Abdul-Ghani M, Ruby RJ. Successful treatment of prediabetes in clinical practice using physiological assessment (STOP DIABETES). Lancet Diabetes Endocrinol. 2018;6:781-789.
Schwartz SS, Epstein S, Corkey BE, et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol Metab. 2017;28:645-655.
American Diabetes Association. Standards of Medical Care in Diabetes – 2021. Diabetes Care. 2021;44(S15–S39):S111-S124.
Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood). 2016;241:1323-1331.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
Sinclair SH, Schwartz SS. Diabetic retinopathy–an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front Endocrinol (Lausanne). 2019;10:843.
Edwards CM, Cusi K. Prediabetes: a worldwide epidemic. Endocrinol Metab Clin N Am. 2016;45:751-764.
Kanat M, DeFronzo RA, Abdul-Ghani MA. Treatment of prediabetes. World J Diabetes. 2015;6:1207-1222.
Dankner R, Roth J. The personalized approach for detecting prediabetes and diabetes. Curr Diabetes Rev. 2016;12:58-65.
Armato JP, DeFronzo RA, Abdul-Ghani M, Ruby RJ. Successful treatment of prediabetes in clinical practice using physiological assessment (STOP DIABETES). Lancet Diabetes Endocrinol. 2018;6:781-789.
Schwartz SS, Epstein S, Corkey BE, et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol Metab. 2017;28:645-655.
American Diabetes Association. Standards of Medical Care in Diabetes – 2021. Diabetes Care. 2021;44(S15–S39):S111-S124.
Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med (Maywood). 2016;241:1323-1331.
Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
Sinclair SH, Schwartz SS. Diabetic retinopathy–an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front Endocrinol (Lausanne). 2019;10:843.
Edwards CM, Cusi K. Prediabetes: a worldwide epidemic. Endocrinol Metab Clin N Am. 2016;45:751-764.
Kanat M, DeFronzo RA, Abdul-Ghani MA. Treatment of prediabetes. World J Diabetes. 2015;6:1207-1222.
Dankner R, Roth J. The personalized approach for detecting prediabetes and diabetes. Curr Diabetes Rev. 2016;12:58-65.
Green Mediterranean diet may relieve aortic stiffness
A green adaptation to the traditional Mediterranean diet improves proximal aortic stiffness (PAS), a distinct marker of vascular aging and increased cardiovascular risk, according to an exploratory post hoc analysis of the DIRECT-PLUS randomized clinical trial.
The green Mediterranean diet is distinct from the traditional Mediterranean diet because of its more abundant dietary polyphenols, from green tea and a Wolffia globosa (Mankai) plant green shake, and lower intake of red or processed meat.
Independent of weight loss, the modified green Mediterranean diet regressed PAS by 15%, the traditional Mediterranean diet by 7.3%, and the healthy dietary guideline–based diet by 4.8%, the study team observed.
“The DIRECT-PLUS trial research team was the first to introduce the concept of the green-Mediterranean/high polyphenols diet,” lead researcher Iris Shai, RD, PhD, with Ben-Gurion University of the Negev, Be’er-Sheva, Israel, told this news organization.
This diet promoted “dramatic proximal aortic de-stiffening” as assessed by MRI over 18 months in roughly 300 participants with abdominal obesity/dyslipidemia. “To date, no dietary strategies have been shown to impact vascular aging physiology,” Dr. Shai said.
The analysis was published online in the Journal of the American College of Cardiology.
Not all healthy diets are equal
Of the 294 participants, 281 had valid PAS measurements at baseline. The baseline PAS (6.1 m/s) was similar across intervention groups (P = .20). Increased PAS was associated with aging, hypertension, dyslipidemia, diabetes, and visceral adiposity (P < .05).
After 18 months’ intervention (retention rate 89.8%), all diet groups showed significant PAS reductions: –0.05 m/s with the standard healthy diet (4.8%), –0.08 m/s with the traditional Mediterranean diet (7.3%) and –0.15 the green Mediterranean diet (15%).
In the multivariable model, the green Mediterranean dieters had greater PAS reduction than did the healthy-diet and Mediterranean dieters (P = .003 and P = .032, respectively).
The researchers caution that DIRECT-PLUS had multiple endpoints and this exploratory post hoc analysis might be sensitive to type I statistical error and should be considered “hypothesis-generating.”
High-quality study, believable results
Reached for comment on the study, Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart in New York, said, “There is not a lot of high-quality research on diet, and I would call this high-quality research in as much as they used randomization which most dietary studies don’t do.
“The greener Mediterranean diet seemed to be the best one on the surrogate marker of MRI-defined aortic stiffness,” Dr. Bhatt, professor of cardiovascular medicine, Icahn School of Medicine at Mount Sinai, who wasn’t involved in the study, told this news organization.
“It makes sense that a diet that has more green in it, more polyphenols, would be healthier. This has been shown in some other studies, that these plant-based polyphenols might have various cardiovascular protective aspects to them,” Dr. Bhatt said.
Overall, he said the results are “quite believable, with the caveat that it would be nice to see the results reproduced in a more diverse and larger sample.”
“There is emerging evidence that diets that are higher in fresh fruits and vegetables and whole grains and lower in overall caloric intake, in general, seem to be good diets to reduce cardiovascular risk factors and maybe even reduce actual cardiovascular risk,” Dr. Bhatt added.
The study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Rosetrees Trust, Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. Dr. Shai and Dr. Bhatt have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A green adaptation to the traditional Mediterranean diet improves proximal aortic stiffness (PAS), a distinct marker of vascular aging and increased cardiovascular risk, according to an exploratory post hoc analysis of the DIRECT-PLUS randomized clinical trial.
The green Mediterranean diet is distinct from the traditional Mediterranean diet because of its more abundant dietary polyphenols, from green tea and a Wolffia globosa (Mankai) plant green shake, and lower intake of red or processed meat.
Independent of weight loss, the modified green Mediterranean diet regressed PAS by 15%, the traditional Mediterranean diet by 7.3%, and the healthy dietary guideline–based diet by 4.8%, the study team observed.
“The DIRECT-PLUS trial research team was the first to introduce the concept of the green-Mediterranean/high polyphenols diet,” lead researcher Iris Shai, RD, PhD, with Ben-Gurion University of the Negev, Be’er-Sheva, Israel, told this news organization.
This diet promoted “dramatic proximal aortic de-stiffening” as assessed by MRI over 18 months in roughly 300 participants with abdominal obesity/dyslipidemia. “To date, no dietary strategies have been shown to impact vascular aging physiology,” Dr. Shai said.
The analysis was published online in the Journal of the American College of Cardiology.
Not all healthy diets are equal
Of the 294 participants, 281 had valid PAS measurements at baseline. The baseline PAS (6.1 m/s) was similar across intervention groups (P = .20). Increased PAS was associated with aging, hypertension, dyslipidemia, diabetes, and visceral adiposity (P < .05).
After 18 months’ intervention (retention rate 89.8%), all diet groups showed significant PAS reductions: –0.05 m/s with the standard healthy diet (4.8%), –0.08 m/s with the traditional Mediterranean diet (7.3%) and –0.15 the green Mediterranean diet (15%).
In the multivariable model, the green Mediterranean dieters had greater PAS reduction than did the healthy-diet and Mediterranean dieters (P = .003 and P = .032, respectively).
The researchers caution that DIRECT-PLUS had multiple endpoints and this exploratory post hoc analysis might be sensitive to type I statistical error and should be considered “hypothesis-generating.”
High-quality study, believable results
Reached for comment on the study, Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart in New York, said, “There is not a lot of high-quality research on diet, and I would call this high-quality research in as much as they used randomization which most dietary studies don’t do.
“The greener Mediterranean diet seemed to be the best one on the surrogate marker of MRI-defined aortic stiffness,” Dr. Bhatt, professor of cardiovascular medicine, Icahn School of Medicine at Mount Sinai, who wasn’t involved in the study, told this news organization.
“It makes sense that a diet that has more green in it, more polyphenols, would be healthier. This has been shown in some other studies, that these plant-based polyphenols might have various cardiovascular protective aspects to them,” Dr. Bhatt said.
Overall, he said the results are “quite believable, with the caveat that it would be nice to see the results reproduced in a more diverse and larger sample.”
“There is emerging evidence that diets that are higher in fresh fruits and vegetables and whole grains and lower in overall caloric intake, in general, seem to be good diets to reduce cardiovascular risk factors and maybe even reduce actual cardiovascular risk,” Dr. Bhatt added.
The study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Rosetrees Trust, Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. Dr. Shai and Dr. Bhatt have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A green adaptation to the traditional Mediterranean diet improves proximal aortic stiffness (PAS), a distinct marker of vascular aging and increased cardiovascular risk, according to an exploratory post hoc analysis of the DIRECT-PLUS randomized clinical trial.
The green Mediterranean diet is distinct from the traditional Mediterranean diet because of its more abundant dietary polyphenols, from green tea and a Wolffia globosa (Mankai) plant green shake, and lower intake of red or processed meat.
Independent of weight loss, the modified green Mediterranean diet regressed PAS by 15%, the traditional Mediterranean diet by 7.3%, and the healthy dietary guideline–based diet by 4.8%, the study team observed.
“The DIRECT-PLUS trial research team was the first to introduce the concept of the green-Mediterranean/high polyphenols diet,” lead researcher Iris Shai, RD, PhD, with Ben-Gurion University of the Negev, Be’er-Sheva, Israel, told this news organization.
This diet promoted “dramatic proximal aortic de-stiffening” as assessed by MRI over 18 months in roughly 300 participants with abdominal obesity/dyslipidemia. “To date, no dietary strategies have been shown to impact vascular aging physiology,” Dr. Shai said.
The analysis was published online in the Journal of the American College of Cardiology.
Not all healthy diets are equal
Of the 294 participants, 281 had valid PAS measurements at baseline. The baseline PAS (6.1 m/s) was similar across intervention groups (P = .20). Increased PAS was associated with aging, hypertension, dyslipidemia, diabetes, and visceral adiposity (P < .05).
After 18 months’ intervention (retention rate 89.8%), all diet groups showed significant PAS reductions: –0.05 m/s with the standard healthy diet (4.8%), –0.08 m/s with the traditional Mediterranean diet (7.3%) and –0.15 the green Mediterranean diet (15%).
In the multivariable model, the green Mediterranean dieters had greater PAS reduction than did the healthy-diet and Mediterranean dieters (P = .003 and P = .032, respectively).
The researchers caution that DIRECT-PLUS had multiple endpoints and this exploratory post hoc analysis might be sensitive to type I statistical error and should be considered “hypothesis-generating.”
High-quality study, believable results
Reached for comment on the study, Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart in New York, said, “There is not a lot of high-quality research on diet, and I would call this high-quality research in as much as they used randomization which most dietary studies don’t do.
“The greener Mediterranean diet seemed to be the best one on the surrogate marker of MRI-defined aortic stiffness,” Dr. Bhatt, professor of cardiovascular medicine, Icahn School of Medicine at Mount Sinai, who wasn’t involved in the study, told this news organization.
“It makes sense that a diet that has more green in it, more polyphenols, would be healthier. This has been shown in some other studies, that these plant-based polyphenols might have various cardiovascular protective aspects to them,” Dr. Bhatt said.
Overall, he said the results are “quite believable, with the caveat that it would be nice to see the results reproduced in a more diverse and larger sample.”
“There is emerging evidence that diets that are higher in fresh fruits and vegetables and whole grains and lower in overall caloric intake, in general, seem to be good diets to reduce cardiovascular risk factors and maybe even reduce actual cardiovascular risk,” Dr. Bhatt added.
The study was funded by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), the Rosetrees Trust, Israel Ministry of Health, Israel Ministry of Science and Technology, and the California Walnuts Commission. Dr. Shai and Dr. Bhatt have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.