User login
Sports: An underutilized tool for patients with disabilities
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6
However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; kjones15@usf.edu
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; kjones15@usf.edu
Approximately 6.5 million people in the United States have an intellectual disability, the most common type of developmental disability.1 People with disabilities are 3 times more likely to have heart disease, stroke, or diabetes than adults without disabilities.2
Sports as a treatment modality are not used to full advantage to combat these conditions in people with intellectual/developmental disabilities (IDDs). Participation in sport activities can lead to weight loss, reduce risk for cardiovascular disease, and optimize physical health. Sports also can help enhance social and communication skills and improve quality of life for this patient population (TABLE).3-6

However, a 2014 report found that while inactive adults with disabilities (hearing, vision, cognition, mobility) were 50% more likely to report 1 or more chronic diseases than those who were physically active, only 44% of adults with disabilities who visited a health professional in the previous 12 months received a physical activity recommendation.7 In addition, more than 50% of adults with disabilities are not meeting US recommended exercise guidelines.7-9
Family physicians may not feel they have adequate training to counsel patients with IDDs. Additional limiting factors include dependence on caregivers for exercise participation, expense, transportation difficulties, a lack of choice in sporting activities, and the patient’s level of motivation.10The guidance reviewed here details how to modify the pre-participation sports physical exam specifically for patients with IDDs. It also provides sport and exercise recommendations for patients with 3 disabilities: Down syndrome, cerebral palsy, and autism spectrum disorder.
Worth noting: As is true for adults without disabilities, those with IDDs should participate in at least 150 minutes of moderate-intensity, or 75 minutes of vigorous intensity, aerobic physical activity each week.9 Recommend muscle-strengthening activities be performed at least 2 days each week.9
Exercise recommendations for patients with Down syndrome
One in every 700 babies receives a diagnosis of Down syndrome.11 Among its many possible manifestations—which include intellectual disability, heart disease, and diabetes—Down syndrome is associated with an increased risk for obesity, which makes exercise an extremely important lifestyle modification for these patients. Obesity can lead to obstructive sleep apnea causing cor pulmonale and even premature death. Continuous positive airway pressure intervention can be difficult in terms of patient compliance. However, weight loss through exercise and sports is an effective intervention to mitigate these obesity-related health comorbidities.
Pre-participation exam. A focused history and physical exam are often conducted before a patient engages in organized competitive or recreational sports. The pre-participation sports physical exam typically focuses on cardiac, neurologic, hereditary, and musculoskeletal disorders. While we recommend including these baseline elements as part of the exam for patients with disabilities, we also recommend modifying the exam to include disability-specific screening for associated comorbidities.
Continue to: For patients with Down syndrome...
For patients with Down syndrome, a complete pre-participation sports physical exam is warranted. Inquire specifically about neck pain or dislocations, heart murmur, cardiac surgery, seizures, sleep issues, history of congenital abdominal defect, hematologic malignancy, and bone pain as part of the focused physical exam.
Look for evidence of patellofemoral instability, pes planus, scoliosis, hallux deformities, decreased muscle tone, and muscular weakness. Check for cataracts and perform a thorough cardiovascular exam to assess for murmur or signs of chronic hypoxia, such as cyanosis. If a heart murmur is detected, refer the patient to a cardiologist.
Patients with Down syndrome are also at increased risk for atlantoaxial instability. A thorough neurologic evaluation to screen for this condition is indicated; however, routine radiologic screening is not needed.12
An annual complete blood cell count and thyroid-stimulating hormone test are recommended for all children with Down syndrome.13 For patients with Down syndrome who are 13 to 21 years of age, an echocardiogram also is recommended for concerning symptoms.13 Ferritin levels also should be assessed annually for patients who are younger than 13 years of age to check for iron-deficiency anemia.13Consider high-risk screening strategies for patients with diabetes and metabolic syndrome.
Special considerations. Patients with Down syndrome were found to be injured more frequently than individuals with other disabilities during the Special Olympics.14 These patients may be hypersensitive to pain with prolonged pain responses, or unable to verbally communicate their pain or injury.15
Continue to: The complexity of pain assessment...
The complexity of pain assessment in patients with Down syndrome may increase the difficulty of accurately diagnosing an injury, leading to underdiagnosis or overdiagnosis. To increase accuracy of pain assessment in this setting, we recommend using the Wong-Baker FACES Pain Rating Scale or a numeric pain rating scale in verbal patients.15 In nonverbal patients, facial expressions are reliable indicators of pain.
Which exercise? Healthy patients with Down syndrome can participate in any sport. Aerobic exercise can help lower body fat, reduce oxidative stress, and improve blood flow.6 Muscle-strengthening exercises can lead to improved daily functioning and balance. Strength training and aerobic exercise benefit aging patients with Down syndrome who are struggling with obesity. Such exercise also helps increase bone mineral density and improve cardiovascular fitness, especially when initiated at a young age. Consistent exercise promotes positive health outcomes throughout the lifespan.16
Exercise recommendations for patients with cerebral palsy
Cerebral palsy, the most common motor disability in children, is associated with intellectual disability, seizures, respiratory insufficiency, scoliosis, osteoporosis, mood disorders, dysphagia, and speech and hearing impairment.17 The increasing survival of premature babies born with cerebral palsy and the growing prevalence of adults with the condition point to the importance of expanding one’s knowledge of how best to care for this population.18
Pre-participation exam. In addition to a complete sport physical exam, it’s important to further evaluate patients with cerebral palsy for epilepsy, joint contractures, muscle weakness, spinal deformities, and respiratory insufficiency. The Gross Motor Function Classification system, commonly used for patients with cerebral palsy, scores functional ability in 5 levels.18 Patients at Level I are the most mobile; patients at Level V need wheelchair transport in all settings.
Further evaluation of spinal deformities can be initiated with x-ray screening. Consider ordering dual x-ray absorptiometry scans to evaluate bone mass.17
Continue to: Special considerations
Special considerations. Patients with cerebral palsy have a heightened risk for depression and anxiety.19 Mental health can be assessed via the General Anxiety Disorder-7, the Patient Health Questionnaire-9, and the Ask Suicide-Screening Questions tools, among others. Mental health screening may need to be adjusted depending on the patient’s level of cognition and ability to communicate. The patient’s caregiver also can provide supplemental information.
Consider screening vitamin D levels in patients with cerebral palsy. Approximately 50% of adults with cerebral palsy are vitamin D–deficient secondary to sedentary behavior and lack of sun exposure.20-22
Optimal medical management has been shown to decrease muscle spasticity and may be beneficial before initiating an exercise program. For patients with moderate-to-severe symptoms, referral for physical therapy to further improve gross motor function and spasticity may be required before initiating an exercise program.
Which exercise? Individuals with cerebral palsy spend 76% to 99% of their waking hours being sedentary.5 Consequently, they typically have decreased cardiorespiratory endurance and decreased muscle strength. Strength training may improve muscle spasticity, gross motor function, joint health, and respiratory insufficiency.5 Even in those who function at Level IV-V of the Gross Motor Function Classification system, exercise reduces vertebral fractures and improves time spent standing.23 By improving endurance, spasticity, and strength with exercise, deconditioning can be mitigated.
Involvement in sports promotes peer interactions, personal interests, and positive self-identity. It can give a newfound passion for life. Additionally, families of children with disabilities who engage in leisure activities together have less caregiver burden.24,25 Sporting activities offer a way to optimize psychosocial well-being for the patient and the entire family.
Continue to: Dance promotes functionality...
Dance promotes functionality and psychosocial adjustment.26 Hippotherapy, defined as therapy and rehabilitation during which the patient interacts with horses, can diminish muscle spasticity.27 Aquatic therapy also may increase muscle strength.28
Sports for patients with autism spectrum disorder
Autism spectrum disorder is defined as persistent deficits in social communication and social interaction that are usually evident in the first 3 years of life.29 Autism can manifest with or without intellectual or language impairment. Patients with autism commonly have difficulty processing sensory stimuli and can experience “sensory overload.” More than half have a coexisting mental health disorder, such as attention-deficit/hyperactivity disorder, anxiety, depression, schizophrenia, or bipolar disorder.30
Aversions to foods and food selectivity, as well as adverse effects from medical treatment of autism-related agitation, result in a higher incidence of obesity in patients with autism.31,32
Pre-participation exam. In addition to a comprehensive pre-participation exam, the Autism Spectrum Syndrome Questionnaire (ASSQ) and Modified Checklist for Autism in Toddlers are tools to screen school-age children with normal cognition to mild intellectual disability.33 These questionnaires have limitations, however. For example, ASSQ has limited ability to identify the female autistic phenotype.34 As such, these are solely screening tools. Final diagnosis is based on clinical judgment.
Special considerations. Include screening for constipation or diarrhea, fiber intake, food aversions, and common mental health comorbidities using Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition criteria.29 Psychiatric referral may be necessary if certain previously undiagnosed condition(s) become apparent. The patient’s caregiver can provide supplemental information
Continue to: During the physical exam...
During the physical exam, limit sensory stimuli as much as possible, including lights and sounds. Verbalize components of the exam before touching a patient with autism who is sensitive to physical touch.
Which exercise? Participation in sports is an effective therapy for autism and can help patients develop communication skills and promote socialization. Vigorous exercise is associated with a reduction in stereotypic behaviors, hyperactivity, aggression, and self-injury.3 Sports also can offer an alternative channel for social interaction. Children with autism may have impaired or delayed motor skills, and exercise can improve motor skill proficiency.4
The prevalence of feeding problems in children with autism spectrum disorder is estimated to be as high as 90%, and close to 70% are selective eaters.31,35,36 For those with gastrointestinal disorders, exercise can exert positive effects on the microbiome-gut-brain axis.37 Additionally, patients with autism are much more likely to be overweight or obese.32 Physical activity offers those with autism health benefits similar to those for the general population.32
Children with autism spectrum disorder have similar odds of injury, including serious injury, relative to population controls.38 Karate and swimming are among the most researched sports therapy options for patients with autism.38-40 Both are shown to improve motor ability and reduce communication deficits.
Summing up
The literature, although limited, demonstrates that exercise and sports improve the health and well-being of people with IDDs throughout the lifespan, especially if childhood exercise/sports involvement is maintained.
Encourage your patients to participate in sports, but be aware of factors that can limit (or facilitate) participation.41 Exercise participation increases based on, among other things, the individual’s desire to be fit and active, skills practice, peer involvement, family support, accessible facilities, and skilled staff.10
Additional resources that can help people with IDDs access sports and recreational activities include the Special Olympics; Paralympics; YMCA; after-school programs; The American College of Sports Medicine; The National Center on Health, Physical Activity, and Disability; and disability-certified inclusive fitness trainers.
CORRESPONDENCE
Kristina Jones, BS, 12901 Bruce B. Downs Boulevard, Tampa, FL 33612; kjones15@usf.edu
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
1. CDC. Addressing gaps in healthcare for individuals with intellectual disabilities. Updated October 15, 2019. Accessed January 21, 2023. www.cdc.gov/grand-rounds/pp/2019/20191015-intellectual-disabilities.html
2. CDC. Vital signs: adults with disabilities. Physical activity is for everybody. Updated November 16, 2018. Accessed January 21, 2023. www.cdc.gov/vitalsigns/disabilities/index.html
3. Di Palma D, Molisso V. Sport for autism. J Humanities Soc Pol. 2017;3:42-49.
4. Pan CY, Chu CH, Tsai CL, et al. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21:190-202. doi: 10.1177/1362361316633562
5. Verschuren O, Peterson MD, Balemans AC, et al. Exercise and physical activity recommendations for people with cerebral palsy. Dev Med Child Neurol. 2016;58:798-808. doi: 10.1111/dmcn.13053
6. Paul Y, Ellapen TJ, Barnard M, et al. The health benefits of exercise therapy for patients with Down syndrome: a systematic review. Afr J Disabil. 2019;8:576. doi: 10.4102/ajod.v8i0.576
7. Carroll DD, Courtney-Long EA, Stevens AC, et al. Vital signs: disability and physical activity—United States, 2009-2012. MMWR Morb Mortal Wkly Rep. 2014;63:407-413.
8. Rimmer JH. Physical activity for people with disabilities: how do we reach those with the greatest need? NAM Perspectives. Published April 6, 2015. Accessed March 23, 2023. https://nam.edu/perspectives-2015-physical-activity-for-people-with-disabilities-how-do-we-reach-those-with-the-greatest-need/
9. Department of Health and Human Services. Physical Activity Guidelines For Americans. 2nd edition. Published 2018. Accessed March 23, 2023. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
10. Darcy S, Dowse L. In search of a level playing field—the constraints and benefits of sport participation for people with intellectual disability. Disabil Soc. 2013;28:393-407. doi: 10.1080/ 09687599.2012.714258
11. Mai CT, Isenburg JL, Canfield MA, et al. National population‐based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
12. MyŚliwiec A, Posłuszny A, Saulicz E, et al. Atlanto-axial instability in people with Down’s syndrome and its impact on the ability to perform sports activities—a review. J Hum Kinet. 2015;48:17-24. doi: 10.1515/hukin-2015-0087
13. Bunt CW, Bunt SK. Role of the family physician in the care of children with Down syndrome. Am Fam Physician. 2014;90:851-858.
14. McCormick DP, Niebuhr VN, Risser WL. Injury and illness surveillance at local Special Olympic Games. Br J Sports Med. 1990; 24:221-224. doi: 10.1136/bjsm.24.4.221
15. McGuire BE, Defrin R. Pain perception in people with Down syndrome: a synthesis of clinical and experimental research. Front Behav Neurosci. 2015;9. doi: 10.3389/fnbeh.2015.00194
16. Barnhart RC, Connolly B. Aging and Down syndrome: implications for physical therapy. Phys Ther. 2007;87:1399-1406. doi: 10.2522/ptj.20060334
17. Vitrikas K, Dalton H, Breish D. Cerebral palsy: an overview. Am Fam Physician. 2020;101:213-220.
18. Maenner MJ, Blumberg SJ, Kogan MD, et al. Prevalence of cerebral palsy and intellectual disability among children identified in two US national surveys, 2011-2013. Ann Epidemiol. 2016;26:222-226. doi: 10.1016/j.annepidem.2016.01.001
19. Smith KJ, Peterson MD, O’Connell NE, et al. Risk of depression and anxiety in adults with cerebral palsy. JAMA Neurol. 2019;76;294-300. doi: 10.1001/jamaneurol.2018.4147
20. Peterson MD, Haapala HJ, Chaddha A, et al. Abdominal obesity is an independent predictor of serum 25-hydroxyvitamin D deficiency in adults with cerebral palsy. Nutr Metab (Lond). 2014;11:22. doi: 10.1186/1743-7075-11-22
21. Yi YG, Jung SH, Bang MS. Emerging issues in cerebral palsy associated with aging: a physiatrist perspective. Ann Rehabil Med. 2019;43:241-249. doi: 10.5535/arm.2019.43.3.241
22. Sarathy K, Doshi C, Aroojis A. Clinical examination of children with cerebral palsy. Indian J Orthop. 2019;53:35-44. doi: 10.4103/ortho.IJOrtho_409_17
23. Caulton JM, Ward KA, Alsop CW, et al. A randomised controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131-135. doi: 10.1136/adc.2002.009316
24. Clutterbuck G, Auld M, Johnston L. Active exercise interventions improve gross motor function of ambulant/semi-ambulant children with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:1131-1151. doi: 10.1080/09638288.2017.1422035
25. Shikako-Thomas K, Majnemer A, Law M, et al. Determinants of participation in leisure activities in children and youth with cerebral palsy: systematic review. Phys Occup Ther Pedi. 2008;28:155-169. doi: 10.1080/01942630802031834
26. Teixeira-Machado L, Azevedo-Santos I, DeSantana JM. Dance improves functionality and psychosocial adjustment in cerebral palsy: a randomized controlled clinical trial. Am J Phys Med Rehabil. 2017;96:424-429. doi: 10.1097/PHM.0000000000000646
27. Lucena-Antón D, Rosety-Rodríguez I, Moral-Munoz JA. Effects of a hippotherapy intervention on muscle spasticity in children with cerebral palsy: a randomized controlled trial. Complement Ther Clin Pract. 2018;31:188-192. doi: 10.1016/j.ctcp.2018.02.013
28. Roostaei M, Baharlouei H, Azadi H, et al. Effects of aquatic intervention on gross motor skills in children with cerebral palsy: a systematic review. Phys Occup Ther Pediatr. 2017;37:496-515. doi: 10.1080/01942638.2016.1247938
29. American Psychiatric Association. Autism spectrum disorder, section II. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013:50-56.
30. Romero M, Aguilar JM, Del-Rey-Mejías Á, et al. Psychiatric comorbidities in autism spectrum disorder: a comparative study between DSM-IV-TR and DSM-5 diagnosis. Int J Clin Health Psychol. 2016;16:266-275. doi: 10.1016/j.ijchp.2016.03.001
31. Volkert VM, Vaz PC. Recent studies on feeding problems in children with autism. J Appl Behav Anal. 2015;43:155-159. doi: 10.1901/jaba.2010.43-155
32. Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr. 2014;14:408-414. doi: 10.1016/j.acap.2014.04.004. PMID: 24976353
33. Adachi M, Takahashi M, Takayanagi N, et al. Adaptation of the Autism Spectrum Screening Questionnaire (ASSQ) to preschool children. PLoS One. 2018;10;13:e0199590. doi: 10.1371/journal.pone.0199590
34. Kopp S. Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Develop Disabil. 2011:32: 2875-2888.
35. Kotak T. Piazza CC. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adol Psych Clin North Am. 2008;17:887-905. doi: 10.1016/j.chc.2008.06.005
36. Twachtman-Reilly J, Amaral SC, Zebrowski PP. Addressing feeding behaviors in children on the autism spectrum in school-based settings: physiological and behavioral issues. Lang Speech Hear Serv Sch. 2008:39:261-272. doi: 10.1044/0161-1461(2008/025)
37. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10:555-568. doi: 10.1080/19490976.2018.1562268
38. Iliadis I, Apteslis N. The role of physical education and exercise for children with autism spectrum disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dial Clin Neurosc Mental Health. 2020;3:71-78. doi: 10.26386/obrela.v3i1.178
39. Phung JN, Goldberg WA. Promoting executive functioning in children with autism spectrum disorder through mixed martial arts training. J Autism Dev Dis. 2019;49:3660-3684. doi: 10.1007/s10803-019-04072-3
40. Bahrami F, Movahedi A, Marandi SM, et al. The effect of karate techniques training on communication deficit of children with autism spectrum disorder. J Autism Dev Disord. 2016;46: 978-986. doi: 10.1007/s10803-015-2643-y
41. Shields N, Synnot A. Perceived barriers and facilitators to participation in physical activity for children with disability: a qualitative study. BMC Pediatr. 2016;16:9. doi: 10.1186/s12887-016-0544-7
PRACTICE RECOMMENDATIONS
› Recommend physical activity as an adjunct to traditional medical management to maximize physical and psychosocial benefits in patients with intellectual/developmental disabilities. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Conversion disorder: An integrated care approach
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; kalston@umc.edu
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; kalston@umc.edu
THE CASE
Janice M* presented to the emergency department (ED) with worsening slurred speech. The 55-year-old patient’s history was significant for diabetes; hypertension; depression; sleep apnea; multiple transient ischemic attacks (TIAs) thought to be stress related; and left lower-extremity weakness secondary to prior infarct. Ms. M had been to the hospital multiple times in the previous 2 to 3 years for similar symptoms. Her most recent visit to the ED had been 2 months earlier.
In the ED, the patient’s NIH stroke score was 1 for the presence of dysarthria, and a code for emergency stroke management was initiated. Ms. M was alert and oriented x 3, with no focal motor or sensory deficits noted. Computed tomography (CT) and CT angiography were negative for any acute abnormality. Throughout the course of the ED visit, her NIH score improved to 0. Ms. M exhibited staccato/stuttering speech, but it was believed that this would likely improve over the next few days.
According to the hospital neurologist, the ED work-up suggested either a TIA, stress-induced psychiatric speech disorder, or conversion disorder. The patient was discharged home in stable condition and was asked to follow up with the outpatient neurologist in 1 week.
Ms. M was seen approximately 2 weeks later in the outpatient neurology stroke clinic. Her symptoms had resolved, and she did not report any new or worsening symptoms. An outpatient stroke work-up was initiated, including magnetic resonance imaging (MRI) of the brain, echocardiography, and measurement of low-density lipoprotein and hemoglobin A1C; all results were unremarkable. Given the timeline for symptom improvement and results of the work-up, the patient was given a diagnosis of conversion disorder. Ms. M was encouraged to follow up with her primary care physician (PCP) for further medical management.
●
* The patient’s name has been changed to protect her identity.
What is conversion disorder, and how common is it?
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, text revision, conversion disorder (also known as functional neurological symptom disorder) is characterized as a somatic symptom and related disorder.1 The prominent feature shared among disorders in this category is the presence of somatic symptoms that are associated with distress and impairment.
In conversion disorder, the focus is on symptoms that are neurologic in nature but are not due to underlying neurologic disease and are incongruent with typical patterns of presentation for any neurologic condition. Patients with conversion disorder may present with motor symptoms (eg, weakness, paralysis, tremor, dystonia), altered sensory or cognitive function, seizure-like symptoms, alterations in speech, or changes in swallowing.1,2
For a diagnosis of conversion disorder, the following criteria must be met1:
- The patient has 1 or more symptoms of altered voluntary motor or sensory function.
- Symptom presentation is incongruent with recognized neurologic or medical disease or conditions.
S ymptoms are not better explained by another medical or mental health condition.- There is significant distress or impairment in functioning due to symptoms or the deficit.
The etiology of conversion disorder has not been firmly established. While the literature suggests that psychological stressors play a role,3,4 an effort also has been made to better understand the underlying neural and biological basis. Specifically, studies have utilized brain imaging to explore brain pathways and mechanisms that could account for symptom presentation.5,6
Prevalence rates for conversion disorder vary depending on the population studied. While it is estimated that 5% of patients in a general hospital setting meet full criteria for conversion disorder,7 higher rates may exist in specialty settings; 1 study found that 30% of patients in a neurology specialty clinic exhibited symptoms that were medically unexplained.8
Continue to: In primary care...
In primary care, prevalence of conversion disorder can be difficult to pinpoint; however, 1 study indicated that physicians identified medically unexplained symptoms as the main presenting problem for nearly 20% of patients in a primary care setting.9 Therefore, it is important for family physicians (FPs) to be familiar with the assessment and treatment of conversion disorder (and other disorders in which medically unexplained symptoms may be at the core of the patient presentation).
The differential: Neurologic and psychiatric conditions
Patients with conversion disorder may present with a variety of neurologic symptoms that can mimic those of organic disease. This can pose a diagnostic challenge, increase the chance of misdiagnosis, and delay treatment.
Motor symptoms may include paralysis, gait disturbance, dysphagia, or aphasia. Patients also may have sensory symptoms, such as blindness, deafness, or anesthesia.10,11 As a result, it is important to rule out both urgent neurologic presentations, such as TIA, acute stroke, and brain tumor, and other chronic neurologic conditions, including multiple sclerosis, myasthenia gravis, and epilepsy.11,12
Multiple sclerosis will demonstrate characteristic lesions on MRI that differentiate it from conversion disorder.
Myasthenia gravis is distinguished by positive findings on autoantibodies testing and on electrophysiologic studies.
Continue to: Epilepsy
Epilepsy. Patients with conversion disorder may present with unresponsiveness and abnormal movements, such as generalized limb shaking and hip thrusting, that mimic an epileptic seizure. In contrast to epileptic seizures, psychogenic nonepileptic seizures may last longer, symptoms may wax and wane, and patients generally do not have bowel or bladder incontinence or sustain injury as they would during an actual seizure.12
There are several psychiatric/psychosocial conditions that also should be considered in the differential diagnosis of conversion disorder.
Somatic symptom disorder, like conversion disorder, produces somatic symptoms that can cause significant distress for patients. The difference in the 2 conditions is that symptoms of somatic symptom disorder may be compatible with a recognized neurologic or general medical condition, whereas in conversion disorder, the symptoms are not consistent with a recognized disease.1,12
Factitious disorder, similar to conversion disorder, can involve neurologic symptoms that are not attributed to disease. However, patients with factitious disorder deliberately simulate symptoms to receive medical care. A thorough clinical interview and physical exam can help to distinguish conversion disorder from factitious disorder.
Malingering is not a psychiatric condition but a behavior that involves intentionally feigning symptoms for the purpose of personal or financial gain. There is no evidence that patients with conversion disorder simulate their symptoms.12,13
Continue to: Negative results and positive signs point to the Dx
Negative results and positive signs point to the Dx
Conversion disorder is not a diagnosis of exclusion. Diagnosis requires detailed history taking and a thorough neurologic exam. Laboratory testing and neuroimaging are also important, and results will have to be negative to support the diagnosis.
Neurologic deficits with conversion disorder do not follow a known neurologic insult.14 There are many tests that can be used to distinguish functional symptoms vs organic symptoms. Two of the most well-known tests are the Hoover sign and the abductor sign, which will be positive in conversion disorder. Both can be performed easily in an outpatient setting.
The Hoover sign is considered positive when there is weakness of voluntary hip extension in the presence of normal involuntary hip extension during contralateral hip flexion against resistance. According to a meta-analysis of multiple studies of patients with conversion disorder, the overall estimated sensitivity of this test is 94% and the specificity, 99%.15
The abductor sign follows the same principle as the Hoover sign: When the patient abducts the nonparetic leg, both the nonparetic and “paretic” leg are strong. When the patient abducts just the “paretic” leg, both legs become weak.16
Other symptom evaluations. For patients who have functional seizures, video electroencephalography is helpful to distinguish functional seizures from “true” seizures.17,18 In conversion disorder, functional dysarthria normally resembles a stutter or speech that is extremely slow with long hesitations that are hard to interrupt.18 Dysphonia and functional dysphagia are also very common functional symptoms. Usually after extensive work-up, no organic cause of the patient’s symptoms is ever found.18
Continue to: Treatment requires an integrated team approach
Treatment requires an integrated team approach
Treatment for conversion disorder can be difficult due to the complex and not fully understood etiology of the condition. Due to its multifaceted nature, an integrated team approach can be beneficial at each stage, including assessment and intervention.
Explain the diagnosis clearly. An essential initial step in the treatment of conversion disorder is careful explanation of the diagnosis. Clear explanation of the terminology and presentation of conversion disorder may prevent the patient from misinterpreting their diagnosis as a suggestion that they are feigning or malingering symptoms or feeling that their symptoms or concerns are being dismissed.2 Understanding the condition can help improve the likelihood of the patient accepting the treatment plan and help decrease the likelihood of unnecessary testing, health care visits, and consultations. Developing a strong rapport with the patient is key when explaining the diagnosis.
Recommend cognitive behavioral therapy (CBT). In a meta-analysis of 15 randomized controlled trials, CBT significantly reduced somatic, anxious, and depressive symptoms and improved physical functioning in patients with somatoform disorders and medically unexplained symptoms.19 Another study, utilizing a case series, demonstrated significant improvement in social, emotional, and behavioral functioning in children and adolescents with functional neurologic symptoms (conversion disorder) post–CBT intervention.20
Given that research supports CBT’s effectiveness in the management of conversion disorder, it is beneficial to engage a behavioral health professional as a part of the treatment team to focus on factors such as stress management, development of coping skills, and treatment of underlying psychiatric conditions.
Consider these other options. The addition of medication management can be considered for patients with comorbid psychiatric disorders. Evidence suggests that physical therapy is helpful in the treatment of motor and gait dysfunction seen in conversion disorder.21,22 The role of hypnosis in the management of conversion disorder has also been studied, but more randomized clinical trials are needed to further explore this treatment.2,23,24
Continue to: The FP's role in coordination of care
The FP’s role in coordination of care
Conversion disorder can be challenging to diagnose and often involves a multidisciplinary approach. Patients with conversion disorder may see multiple clinicians as they undergo evaluation for their symptoms, but they usually are referred back to their PCP for management and coordination of care. Thus, the FP’s understanding of how the condition is diagnosed and appropriately managed is beneficial.
Open and effective communication among all members of the health care team can ensure consistency in treatment, a strong patient–provider relationship, favorable prognosis, and prevention of symptom relapse. FPs, by establishing a good rapport with patients, can help them understand the condition and the mind-body connection. Once other diagnoses have been ruled out, the FP can provide reassurance to patients and minimize further diagnostic testing.
The prognosis of conversion disorder is associated with symptom duration25; thus, consultation between FPs and mental health providers is essential. The FP also can be integral in the recognition of psychiatric comorbidities, such as anxiety and depression, helping to ensure that these conditions also are treated appropriately.25,26
THE CASE
Ms. M was referred to a neuropsychologist for further assessment, and the diagnosis of conversion disorder was confirmed. She was then referred to a family medicine behavioral health psychologist for CBT. The initial consult indicated that psychological stressors were contributing to symptoms, and Ms. M was diagnosed with depression and anxiety as well as conversion disorder.
Treatment started with patient education. The treatment framework was carefully explained to Ms. M, with a focus on identifying possible symptom triggers, helping her build a more effective stress response, increasing skills to more effectively manage stressors, and managing underlying psychiatric disorders (ie, depression, anxiety).
Ms. M continued regular visits with the family medicine behavioral health psychologist for CBT and followed up with her PCP as needed to manage chronic health conditions and stroke risk factors. The patient was able to implement skills discussed in treatment sessions, including identifying triggers and implementing coping skills (eg, managing negative thoughts that contribute to symptoms, setting boundaries) to manage stressors.
Her depressive and anxious symptoms improved, as indicated by symptom measurement tools and self-report. The frequency and severity of episodes of slurred speech and muscle weakness decreased, and the patient reported only 1 ED visit related to speech difficulties in the 2 years while following up with the behavioral health psychologist.
CORRESPONDENCE
Kristen J. Alston, PhD, University of Mississippi Medical Center, 2400 North State Street, Jackson, MS 39216; kalston@umc.edu
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
1. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and Statistical Manual of Mental Disorders. 5th edition, text revision. American Psychiatric Association Publishing; 2022. doi: 10.1176/appi.books.9780890425787.x09_Somatic_Symptom_and_Related_Disorders
2. O’Neal MA, Baslet G. Treatment for patients with a functional neurological disorder (conversion disorder): an integrated approach. Am J Psychiatry. 2018;175:307-314. doi: 10.1176/appi.ajp.2017.17040450
3. Roelofs K, Spinhoven P, Sandijck P, et al. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. 2005;193:508-514. doi: 10.1097/01.nmd.0000172472.60197.4d
4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med. 2016;46:2617-2626. doi: 10.1017/S0033291716000714
5. Ejareh Dar M, Kanaan RA. Uncovering the etiology of conversion disorder: insights from functional neuroimaging. Neuropsychiatr Dis Treat. 2016;12:143-153. doi: 10.2147/NDT.S65880
6. Aybek S, Vuilleumier P. Imaging studies of functional neurologic disorders. Handb Clin Neurol. 2016;139:73-84. doi: 10.1016/B978-0-12-801772-2.00007-2
7. Folks DG, Ford CV, Regan WM. Conversion symptoms in a general hospital. Psychosomatics. 1984;25:285-295. doi: 10.1016/S0033-3182(84)73046-5
8. Carson AJ, Best S, Postma K, et al. The outcome of neurology outpatients with medically unexplained symptoms: a prospective cohort study. J Neurol Neurosurg Psychiatry. 2003;74:897-900. doi: 10.1136/jnnp.74.7.897
9. Peveler R, Kilkenny L, Kinmonth AL. Medically unexplained physical symptoms in primary care: a comparison of self-report screening questionnaires and clinical opinion. J Psychosom Res. 1997;42:245-252. doi: 10.1016/s0022-3999(96)00292-9
10. Tobiano PS, Wang HE, McCausland JB, et al. A case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286. doi: 10.1016/j.jemermed.2005.05.024
11. Chou HY, Weng MC, Huang MH, et al. Conversion disorder in stroke: a case report. Kaohsiung J Med Sci. 2006;22:586-589. doi: 10.1016/S1607-551X(09)70357-2
12. Peeling JL, Muzio MR. Conversion disorder. StatPearls [Internet]. Updated May 19, 2021. Accessed March 14, 2023. www.ncbi.nlm.nih.gov/books/NBK551567/
13. Ali S, Jabeen S, Pate RJ, et al. Conversion disorder—mind versus body: a review. Innov Clin Neurosci. 2015;12:27-33.
14. Hurwitz TA. Somatization and conversion disorder. Can J Psychiatry. 2004;49:172-178. doi: 10.1177/070674370404900304
15. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190. doi: 10.1136/jnnp-2012-304607
16. Sonoo M. Abductor sign: a reliable new sign to detect unilateral non-organic paresis of the lower limb. J Neurol Neurosurg Psychiatry. 2004;75:121-125.
17. Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep. 2017;21:29. doi: 10.1007/s11916-017-0627-7
18. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12. doi: 10.1136/jnnp.2004.061655
19. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
20. McFarlane FA, Allcott-Watson H, Hadji-Michael M, et al. Cognitive-behavioural treatment of functional neurological symptoms (conversion disorder) in children and adolescents: a case series. Eur J Paediatr Neurol. 2019;23:317-328. doi: 10.1016/j.ejpn.2018.12.002
21. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31:30-39. doi: 10.1097/01.npt.0000260571.77487.14
22. Nielsen G, Ricciardi L, Demartini B, et al. Outcomes of a 5-day physiotherapy programme for functional (psychogenic) motor disorders. J Neurol. 2015;262:674-681. doi: 10.1007/s00415-014-7631-1
23. Sanyal R, Raseta M, Natarajan I, et al. The use of hypnotherapy as treatment for functional stroke: a case series from a single center in the UK. Int J Stroke. 2022;17:59-66. doi: 10.1177/1747493021995590
24. Moene FC, Spinhoven P, Hoogduin KA, et al. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn. 2003;51:29-50. doi: 10.1076/iceh.51.1.29.14067
25. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183:915-920. doi: 10.1503/cmaj.110490
26. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93:49-54.
Is combination pharmacotherapy effective for patients with acute depression?
ILLUSTRATIVE CASE
A healthy 33-year-old woman presents to your office with a 3-month history of depressed mood. She reports difficulty concentrating, insomnia, decreased appetite, and generalized fatigue. She denies suicidal or homicidal ideation, substance misuse, or history consistent with manic episodes. Her vital signs are normal and overall her physical examination is unremarkable, although the patient is tearful when discussing her mood. Using shared decision-making, you and the patient determine it is appropriate to initiate pharmacotherapy. Is there a role for combination pharmacotherapy to treat this patient’s acute depression?
Unipolar depression is a highly prevalent condition, estimated to affect 21% of US adults at some point in their lifetime.2 It is the second leading cause of disability in the United States, with an estimated economic impact of more than $200 billion annually.3
The diagnosis of unipolar depression is based on the criteria set forth in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and commonly includes depressed mood, anhedonia, sleep disturbance, appetite changes, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate, and psychomotor symptoms occurring over at least a 2-week period.4 Symptoms represent a decrease in functioning from previous levels that are not attributable to another medical condition or substance, and must not include a history of past manic or hypomanic episodes. Thoughts of death and suicidal ideation are common.
Several systematic reviews and meta-analyses have shown that a combination of psychotherapy and pharmacotherapy is more efficacious for treatment of unipolar depression than either therapy alone.5-7 As for which medication is most effective and tolerable, multiple systematic reviews and meta-analyses have not demonstrated superiority of 1 second-generation antidepressant (eg, SSRIs, SNRIs) over another.7,8
General practice guidelines support titration of the dose or a switch in monotherapy medications until treatment response is achieved, prior to initiation of a second agent. When an adjunctive medication is considered, there are several options: a second-generation antipsychotic, a second antidepressant from a different class, thyroid hormone, and lithium. Special consideration is given to the adverse effect profile and potential tolerability; higher adverse effect profiles are observed with second-generation antipsychotics and lithium.9
It is not common practice to initiate 2 antidepressants for a new diagnosis of acute depression. The systematic review and meta-analysis conducted by Henssler et al1 attempted to provide evidence to support the efficacy and tolerability of specific antidepressants when used in combination for initial treatment of acute depression. Of note, a 2008 national survey showed that a majority of psychotropic medications in the United States are prescribed by primary care physicians (73.6%) rather than psychiatrists, making this analysis relevant to family physicians.10
STUDY SUMMARY
Combination pharmacotherapy yields superior efficacy in acute depression
This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression.1 The study also aimed to address which specific combination therapies were superior.
Continue to: Selected RCTs included...
Selected RCTs included an intervention group using a combination of 2 antidepressants, regardless of dosage, and a control group of patients taking antidepressant monotherapy. Studies evaluated both patients being treated for the first time and those with a previously inadequate response to medical treatment. All participants were ages 18 years or older (mean age not reported) and had received a diagnosis of depressive disorder according to standard operationalized criteria; patients with multiple psychiatric comorbidities were not excluded.
Studies used various standardized questionnaires—most frequently, the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Åsberg Depression Rating Scale (MADRS)—to determine the severity of depression at baseline and following treatment. The HDRS is a 17-item depression scale and the MADRS is a 10-item depression scale; for both, higher scores indicate worsening depression. Follow-up time ranged from 2 to 12 weeks.
The primary outcome was treatment efficacy measured as the standardized mean difference (SMD). Secondary outcomes included remission (normal-range scores) and response to treatment (eg, ≥ 50% reduction in scores), as defined by the study authors.
Combination therapy was determined to have superior efficacy relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44; P < .001). Combinations with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone, or mianserin [the last of which is not approved by the US Food and Drug Administration for use in the United States]) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or TCA) were superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Combinations that included bupropion were not superior to monotherapy (SMD = 0.10; 95% CI, –0.07 to 0.27).
Secondary outcomes revealed combination therapy to be superior to monotherapy with respect to remission (odds ratio [OR] = 1.52; 95% CI, 1.20-1.92) and response (OR = 1.40; 95% CI, 1.15-1.69). Subgroup analyses showed that combinations with presynaptic α2-autoreceptor antagonists led to improved remission (OR = 1.42; 95% CI, 1.01-2.01) and response (OR = 1.49; 95% CI, 1.18-1.87) compared with monotherapy, whereas combinations that included bupropion were not superior to monotherapy. For patients who dropped out of treatment for any reason, including adverse drug events, results for combination pharmacotherapy and monotherapy were similar.
Continue to: WHAT'S NEW
WHAT’S NEW
One combination proved more effective than others
Current clinical guidelines indicate the suitability of trialing pharmacologic monotherapy during the acute phase of depression treatment prior to initiating an adjunctive medication.9 All classes of medication investigated in this meta-analysis are generally regarded as first-line therapies, although they are rarely started in combination. This study’s findings suggest that combination pharmacotherapy, especially with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or a TCA), is superior to monotherapy, both at the time of treatment initiation and in patients with previous inadequate pharmacologic response.
CAVEATS
Potential limitations due to publication bias
Concerns about publication bias and significant study heterogeneity may limit the generalizability of these findings. However, conclusions were robust in a subgroup analysis that was restricted to publications with low risk for bias.
CHALLENGES TO IMPLEMENTATION
None to report
There are no major challenges to implementing this combination treatment. Importantly, there were no differences in tolerability between monotherapy and combination treatment.
1. Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336-346. doi: 10.1001/jamapsychiatry.2017.4602
3. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. doi: 10.4088/JCP.14m09298
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Cuijpers P, Reynolds CF III, Donker T, et al. Personalized treatment of adult depression: medication, psychotherapy, or both? A systematic review. Depress Anxiety. 2012;29:855-864. doi: 10.1002/da.21985
6. Cuijpers P, van Straten A, Hollon SD, et al. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121:415-423. doi: 10.1111/j.1600-0447.2009.01513.x
7. Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54: 1009-1015. doi: 10.1001/archpsyc.1997.01830230043006
8. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:722-785. doi: 10.7326/0003-4819-155-11-201112060-00009
9. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010. Accessed February 27, 2023. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
10. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69:1064-1074. doi: 10.4088/jcp.v69n0704
ILLUSTRATIVE CASE
A healthy 33-year-old woman presents to your office with a 3-month history of depressed mood. She reports difficulty concentrating, insomnia, decreased appetite, and generalized fatigue. She denies suicidal or homicidal ideation, substance misuse, or history consistent with manic episodes. Her vital signs are normal and overall her physical examination is unremarkable, although the patient is tearful when discussing her mood. Using shared decision-making, you and the patient determine it is appropriate to initiate pharmacotherapy. Is there a role for combination pharmacotherapy to treat this patient’s acute depression?
Unipolar depression is a highly prevalent condition, estimated to affect 21% of US adults at some point in their lifetime.2 It is the second leading cause of disability in the United States, with an estimated economic impact of more than $200 billion annually.3
The diagnosis of unipolar depression is based on the criteria set forth in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and commonly includes depressed mood, anhedonia, sleep disturbance, appetite changes, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate, and psychomotor symptoms occurring over at least a 2-week period.4 Symptoms represent a decrease in functioning from previous levels that are not attributable to another medical condition or substance, and must not include a history of past manic or hypomanic episodes. Thoughts of death and suicidal ideation are common.
Several systematic reviews and meta-analyses have shown that a combination of psychotherapy and pharmacotherapy is more efficacious for treatment of unipolar depression than either therapy alone.5-7 As for which medication is most effective and tolerable, multiple systematic reviews and meta-analyses have not demonstrated superiority of 1 second-generation antidepressant (eg, SSRIs, SNRIs) over another.7,8
General practice guidelines support titration of the dose or a switch in monotherapy medications until treatment response is achieved, prior to initiation of a second agent. When an adjunctive medication is considered, there are several options: a second-generation antipsychotic, a second antidepressant from a different class, thyroid hormone, and lithium. Special consideration is given to the adverse effect profile and potential tolerability; higher adverse effect profiles are observed with second-generation antipsychotics and lithium.9
It is not common practice to initiate 2 antidepressants for a new diagnosis of acute depression. The systematic review and meta-analysis conducted by Henssler et al1 attempted to provide evidence to support the efficacy and tolerability of specific antidepressants when used in combination for initial treatment of acute depression. Of note, a 2008 national survey showed that a majority of psychotropic medications in the United States are prescribed by primary care physicians (73.6%) rather than psychiatrists, making this analysis relevant to family physicians.10
STUDY SUMMARY
Combination pharmacotherapy yields superior efficacy in acute depression
This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression.1 The study also aimed to address which specific combination therapies were superior.
Continue to: Selected RCTs included...
Selected RCTs included an intervention group using a combination of 2 antidepressants, regardless of dosage, and a control group of patients taking antidepressant monotherapy. Studies evaluated both patients being treated for the first time and those with a previously inadequate response to medical treatment. All participants were ages 18 years or older (mean age not reported) and had received a diagnosis of depressive disorder according to standard operationalized criteria; patients with multiple psychiatric comorbidities were not excluded.
Studies used various standardized questionnaires—most frequently, the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Åsberg Depression Rating Scale (MADRS)—to determine the severity of depression at baseline and following treatment. The HDRS is a 17-item depression scale and the MADRS is a 10-item depression scale; for both, higher scores indicate worsening depression. Follow-up time ranged from 2 to 12 weeks.
The primary outcome was treatment efficacy measured as the standardized mean difference (SMD). Secondary outcomes included remission (normal-range scores) and response to treatment (eg, ≥ 50% reduction in scores), as defined by the study authors.
Combination therapy was determined to have superior efficacy relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44; P < .001). Combinations with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone, or mianserin [the last of which is not approved by the US Food and Drug Administration for use in the United States]) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or TCA) were superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Combinations that included bupropion were not superior to monotherapy (SMD = 0.10; 95% CI, –0.07 to 0.27).
Secondary outcomes revealed combination therapy to be superior to monotherapy with respect to remission (odds ratio [OR] = 1.52; 95% CI, 1.20-1.92) and response (OR = 1.40; 95% CI, 1.15-1.69). Subgroup analyses showed that combinations with presynaptic α2-autoreceptor antagonists led to improved remission (OR = 1.42; 95% CI, 1.01-2.01) and response (OR = 1.49; 95% CI, 1.18-1.87) compared with monotherapy, whereas combinations that included bupropion were not superior to monotherapy. For patients who dropped out of treatment for any reason, including adverse drug events, results for combination pharmacotherapy and monotherapy were similar.
Continue to: WHAT'S NEW
WHAT’S NEW
One combination proved more effective than others
Current clinical guidelines indicate the suitability of trialing pharmacologic monotherapy during the acute phase of depression treatment prior to initiating an adjunctive medication.9 All classes of medication investigated in this meta-analysis are generally regarded as first-line therapies, although they are rarely started in combination. This study’s findings suggest that combination pharmacotherapy, especially with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or a TCA), is superior to monotherapy, both at the time of treatment initiation and in patients with previous inadequate pharmacologic response.
CAVEATS
Potential limitations due to publication bias
Concerns about publication bias and significant study heterogeneity may limit the generalizability of these findings. However, conclusions were robust in a subgroup analysis that was restricted to publications with low risk for bias.
CHALLENGES TO IMPLEMENTATION
None to report
There are no major challenges to implementing this combination treatment. Importantly, there were no differences in tolerability between monotherapy and combination treatment.
ILLUSTRATIVE CASE
A healthy 33-year-old woman presents to your office with a 3-month history of depressed mood. She reports difficulty concentrating, insomnia, decreased appetite, and generalized fatigue. She denies suicidal or homicidal ideation, substance misuse, or history consistent with manic episodes. Her vital signs are normal and overall her physical examination is unremarkable, although the patient is tearful when discussing her mood. Using shared decision-making, you and the patient determine it is appropriate to initiate pharmacotherapy. Is there a role for combination pharmacotherapy to treat this patient’s acute depression?
Unipolar depression is a highly prevalent condition, estimated to affect 21% of US adults at some point in their lifetime.2 It is the second leading cause of disability in the United States, with an estimated economic impact of more than $200 billion annually.3
The diagnosis of unipolar depression is based on the criteria set forth in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) and commonly includes depressed mood, anhedonia, sleep disturbance, appetite changes, fatigue, feelings of worthlessness or guilt, decreased ability to concentrate, and psychomotor symptoms occurring over at least a 2-week period.4 Symptoms represent a decrease in functioning from previous levels that are not attributable to another medical condition or substance, and must not include a history of past manic or hypomanic episodes. Thoughts of death and suicidal ideation are common.
Several systematic reviews and meta-analyses have shown that a combination of psychotherapy and pharmacotherapy is more efficacious for treatment of unipolar depression than either therapy alone.5-7 As for which medication is most effective and tolerable, multiple systematic reviews and meta-analyses have not demonstrated superiority of 1 second-generation antidepressant (eg, SSRIs, SNRIs) over another.7,8
General practice guidelines support titration of the dose or a switch in monotherapy medications until treatment response is achieved, prior to initiation of a second agent. When an adjunctive medication is considered, there are several options: a second-generation antipsychotic, a second antidepressant from a different class, thyroid hormone, and lithium. Special consideration is given to the adverse effect profile and potential tolerability; higher adverse effect profiles are observed with second-generation antipsychotics and lithium.9
It is not common practice to initiate 2 antidepressants for a new diagnosis of acute depression. The systematic review and meta-analysis conducted by Henssler et al1 attempted to provide evidence to support the efficacy and tolerability of specific antidepressants when used in combination for initial treatment of acute depression. Of note, a 2008 national survey showed that a majority of psychotropic medications in the United States are prescribed by primary care physicians (73.6%) rather than psychiatrists, making this analysis relevant to family physicians.10
STUDY SUMMARY
Combination pharmacotherapy yields superior efficacy in acute depression
This 2022 systematic review and meta-analysis (39 randomized clinical trials [RCTs]; N = 6751) compared the efficacy and tolerability of monotherapy to combination therapy in the treatment of patients with acute depression.1 The study also aimed to address which specific combination therapies were superior.
Continue to: Selected RCTs included...
Selected RCTs included an intervention group using a combination of 2 antidepressants, regardless of dosage, and a control group of patients taking antidepressant monotherapy. Studies evaluated both patients being treated for the first time and those with a previously inadequate response to medical treatment. All participants were ages 18 years or older (mean age not reported) and had received a diagnosis of depressive disorder according to standard operationalized criteria; patients with multiple psychiatric comorbidities were not excluded.
Studies used various standardized questionnaires—most frequently, the Hamilton Depression Rating Scale (HDRS) and the Montgomery-Åsberg Depression Rating Scale (MADRS)—to determine the severity of depression at baseline and following treatment. The HDRS is a 17-item depression scale and the MADRS is a 10-item depression scale; for both, higher scores indicate worsening depression. Follow-up time ranged from 2 to 12 weeks.
The primary outcome was treatment efficacy measured as the standardized mean difference (SMD). Secondary outcomes included remission (normal-range scores) and response to treatment (eg, ≥ 50% reduction in scores), as defined by the study authors.
Combination therapy was determined to have superior efficacy relative to monotherapy (SMD = 0.31; 95% CI, 0.19-0.44; P < .001). Combinations with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone, or mianserin [the last of which is not approved by the US Food and Drug Administration for use in the United States]) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or TCA) were superior to other combinations (SMD = 0.37; 95% CI, 0.19-0.55). Combinations that included bupropion were not superior to monotherapy (SMD = 0.10; 95% CI, –0.07 to 0.27).
Secondary outcomes revealed combination therapy to be superior to monotherapy with respect to remission (odds ratio [OR] = 1.52; 95% CI, 1.20-1.92) and response (OR = 1.40; 95% CI, 1.15-1.69). Subgroup analyses showed that combinations with presynaptic α2-autoreceptor antagonists led to improved remission (OR = 1.42; 95% CI, 1.01-2.01) and response (OR = 1.49; 95% CI, 1.18-1.87) compared with monotherapy, whereas combinations that included bupropion were not superior to monotherapy. For patients who dropped out of treatment for any reason, including adverse drug events, results for combination pharmacotherapy and monotherapy were similar.
Continue to: WHAT'S NEW
WHAT’S NEW
One combination proved more effective than others
Current clinical guidelines indicate the suitability of trialing pharmacologic monotherapy during the acute phase of depression treatment prior to initiating an adjunctive medication.9 All classes of medication investigated in this meta-analysis are generally regarded as first-line therapies, although they are rarely started in combination. This study’s findings suggest that combination pharmacotherapy, especially with a presynaptic α2-autoreceptor antagonist (eg, mirtazapine, trazodone) and a monoamine reuptake inhibitor (eg, an SSRI, SNRI, or a TCA), is superior to monotherapy, both at the time of treatment initiation and in patients with previous inadequate pharmacologic response.
CAVEATS
Potential limitations due to publication bias
Concerns about publication bias and significant study heterogeneity may limit the generalizability of these findings. However, conclusions were robust in a subgroup analysis that was restricted to publications with low risk for bias.
CHALLENGES TO IMPLEMENTATION
None to report
There are no major challenges to implementing this combination treatment. Importantly, there were no differences in tolerability between monotherapy and combination treatment.
1. Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336-346. doi: 10.1001/jamapsychiatry.2017.4602
3. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. doi: 10.4088/JCP.14m09298
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Cuijpers P, Reynolds CF III, Donker T, et al. Personalized treatment of adult depression: medication, psychotherapy, or both? A systematic review. Depress Anxiety. 2012;29:855-864. doi: 10.1002/da.21985
6. Cuijpers P, van Straten A, Hollon SD, et al. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121:415-423. doi: 10.1111/j.1600-0447.2009.01513.x
7. Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54: 1009-1015. doi: 10.1001/archpsyc.1997.01830230043006
8. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:722-785. doi: 10.7326/0003-4819-155-11-201112060-00009
9. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010. Accessed February 27, 2023. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
10. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69:1064-1074. doi: 10.4088/jcp.v69n0704
1. Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
2. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336-346. doi: 10.1001/jamapsychiatry.2017.4602
3. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155-162. doi: 10.4088/JCP.14m09298
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
5. Cuijpers P, Reynolds CF III, Donker T, et al. Personalized treatment of adult depression: medication, psychotherapy, or both? A systematic review. Depress Anxiety. 2012;29:855-864. doi: 10.1002/da.21985
6. Cuijpers P, van Straten A, Hollon SD, et al. The contribution of active medication to combined treatments of psychotherapy and pharmacotherapy for adult depression: a meta-analysis. Acta Psychiatr Scand. 2010;121:415-423. doi: 10.1111/j.1600-0447.2009.01513.x
7. Thase ME, Greenhouse JB, Frank E, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54: 1009-1015. doi: 10.1001/archpsyc.1997.01830230043006
8. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155:722-785. doi: 10.7326/0003-4819-155-11-201112060-00009
9. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3rd ed. American Psychiatric Association; 2010. Accessed February 27, 2023. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
10. Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69:1064-1074. doi: 10.4088/jcp.v69n0704
PRACTICE CHANGER
Use a combination of a presynaptic α2-autoreceptor antagonist (eg, mirtazapine or trazodone) and a monoamine reuptake inhibitor (eg, selective serotonin reuptake inhibitor [SSRI], serotonin-norepinephrine reuptake inhibitor [SNRI], or tricyclic antidepressant [TCA]) to treat acute depression in adult patients.
STRENGTH OF RECOMMENDATION
A: Based on a single systematic review with meta-analysis.1
Henssler J, Alexander D, Schwarzer G, et al. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79:300-312. doi: 10.1001/jamapsychiatry.2021.4313
AFib risk with cancer drugs underestimated
Atrial fibrillation (AFib) is a known and serious side effect of some cancer treatments, but it is underreported in cancer drug trials, French investigators said in a new report.
As a result, oncologists likely underestimate the risk of atrial fibrillation when new cancer drugs come to market, they said.
The team came to these conclusions after conducting a meta-analysis of 191 phase 2 or 3 clinical trials that included 26,604 patients. The trials investigated 15 anticancer drugs used as monotherapy.
The meta-analysis showed that the annualized incidence rate of AFib ranged from 0.26 cases per 100 person-years – about the same as placebo – to 4.92 cases, a nearly 20 times’ higher risk.
Rates were the highest for ibrutinib, clofarabine, and ponatinib.
The study was published in JACC: CardioOncology, a journal of the American College of Cardiology.
Actual rates of AFib are probably higher than what they found in this meta-analysis, the authors suspect, because most oncology trials only identify and report severe cases of AFib that require immediate medical attention. Less severe cases can also lead to serious complications, including strokes, but they go unreported, said the investigators, led by Joachim Alexandre, MD, PhD, a member of the cardio-oncology program at the University of Caen Normandie Hospital Center, France.
“These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants,” and AFib reporting is “particularly affected,” they said.
Call for routine monitoring
The root of the problem is the lack of routine rhythm monitoring in cancer trials. This in turn “leads to a significant underestimation of AFib incidence” and rates “markedly lower than those observed among real-life” patients, the authors pointed out.
To address the issue, Dr. Alexandre and his team called for routine cardiac monitoring in trials to capture the true incidence of AFib and to “clearly define which anticancer drugs are significantly associated” with the condition.
Approached for comment, Michael G. Fradley, MD, medical director of cardio-oncology at the University of Pennsylvania, Philadelphia, agreed.
“It’s incredibly important” to “identify the drugs most likely to cause arrhythmias and determine the best prevention and treatment strategies. Unfortunately, systematic evaluation of arrhythmias in cancer clinical trials has often been lacking,” Dr. Fradley told this news organization.
The investigators said the issue is particularly pressing for drugs known to be associated with AFib. For Bruton’s tyrosine kinase inhibitors such as ibrutinib, for instance, they call for standardize AFib detection in trials “not only on 12-lead ECGs” for symptomatic AFib but also with “longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AFib.”
Dr. Fradley said there might also be a role for newer wearable technologies that can detect arrhythmias through a skin patch or by other means.
Details of the meta-analysis
The investigators pulled the 191 studies they used in their meta-analysis from the ClinicalTrials.gov database.
The trials covered anticancer drugs used as monotherapy up to Sept. 18, 2020. Almost half were randomized trials, but only seven had placebo arms. Trials involving hematologic cancers outnumbered those involving solid tumors.
The 15 drugs examined were dacarbazine, abiraterone, clofarabine, azacitidine, ibrutinib, nilotinib, ponatinib, midostaurin, ipilimumab, aldesleukin, lenalidomide, pomalidomide, rituximab, bortezomib, and docetaxel.
The annualized incidence AFib rates per 100 person-years were 4.92 cases for ibrutinib, 2.38 cases for clofarabine, and 2.35 cases for ponatinib.
The lowest AFib rates were for ipilimumab (0.26 cases), rituximab (0.27), and nilotinib (0.29).
For placebo, the annualized rate was 0.25 cases per 100 person-years.
The team said caution is warranted regarding their estimations for clofarabine and midostaurin (0.65 cases) because no trials were registered after September 2009, when adverse event reporting became mandatory. As a result, estimates may be artificially low.
One of the limits of the study is that it focused on monotherapy in an age when combination treatment is generally the rule for cancer, the authors noted.
No external funding was reported for the study. Dr. Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity.
A version of this article first appeared on Medscape.com.
Atrial fibrillation (AFib) is a known and serious side effect of some cancer treatments, but it is underreported in cancer drug trials, French investigators said in a new report.
As a result, oncologists likely underestimate the risk of atrial fibrillation when new cancer drugs come to market, they said.
The team came to these conclusions after conducting a meta-analysis of 191 phase 2 or 3 clinical trials that included 26,604 patients. The trials investigated 15 anticancer drugs used as monotherapy.
The meta-analysis showed that the annualized incidence rate of AFib ranged from 0.26 cases per 100 person-years – about the same as placebo – to 4.92 cases, a nearly 20 times’ higher risk.
Rates were the highest for ibrutinib, clofarabine, and ponatinib.
The study was published in JACC: CardioOncology, a journal of the American College of Cardiology.
Actual rates of AFib are probably higher than what they found in this meta-analysis, the authors suspect, because most oncology trials only identify and report severe cases of AFib that require immediate medical attention. Less severe cases can also lead to serious complications, including strokes, but they go unreported, said the investigators, led by Joachim Alexandre, MD, PhD, a member of the cardio-oncology program at the University of Caen Normandie Hospital Center, France.
“These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants,” and AFib reporting is “particularly affected,” they said.
Call for routine monitoring
The root of the problem is the lack of routine rhythm monitoring in cancer trials. This in turn “leads to a significant underestimation of AFib incidence” and rates “markedly lower than those observed among real-life” patients, the authors pointed out.
To address the issue, Dr. Alexandre and his team called for routine cardiac monitoring in trials to capture the true incidence of AFib and to “clearly define which anticancer drugs are significantly associated” with the condition.
Approached for comment, Michael G. Fradley, MD, medical director of cardio-oncology at the University of Pennsylvania, Philadelphia, agreed.
“It’s incredibly important” to “identify the drugs most likely to cause arrhythmias and determine the best prevention and treatment strategies. Unfortunately, systematic evaluation of arrhythmias in cancer clinical trials has often been lacking,” Dr. Fradley told this news organization.
The investigators said the issue is particularly pressing for drugs known to be associated with AFib. For Bruton’s tyrosine kinase inhibitors such as ibrutinib, for instance, they call for standardize AFib detection in trials “not only on 12-lead ECGs” for symptomatic AFib but also with “longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AFib.”
Dr. Fradley said there might also be a role for newer wearable technologies that can detect arrhythmias through a skin patch or by other means.
Details of the meta-analysis
The investigators pulled the 191 studies they used in their meta-analysis from the ClinicalTrials.gov database.
The trials covered anticancer drugs used as monotherapy up to Sept. 18, 2020. Almost half were randomized trials, but only seven had placebo arms. Trials involving hematologic cancers outnumbered those involving solid tumors.
The 15 drugs examined were dacarbazine, abiraterone, clofarabine, azacitidine, ibrutinib, nilotinib, ponatinib, midostaurin, ipilimumab, aldesleukin, lenalidomide, pomalidomide, rituximab, bortezomib, and docetaxel.
The annualized incidence AFib rates per 100 person-years were 4.92 cases for ibrutinib, 2.38 cases for clofarabine, and 2.35 cases for ponatinib.
The lowest AFib rates were for ipilimumab (0.26 cases), rituximab (0.27), and nilotinib (0.29).
For placebo, the annualized rate was 0.25 cases per 100 person-years.
The team said caution is warranted regarding their estimations for clofarabine and midostaurin (0.65 cases) because no trials were registered after September 2009, when adverse event reporting became mandatory. As a result, estimates may be artificially low.
One of the limits of the study is that it focused on monotherapy in an age when combination treatment is generally the rule for cancer, the authors noted.
No external funding was reported for the study. Dr. Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity.
A version of this article first appeared on Medscape.com.
Atrial fibrillation (AFib) is a known and serious side effect of some cancer treatments, but it is underreported in cancer drug trials, French investigators said in a new report.
As a result, oncologists likely underestimate the risk of atrial fibrillation when new cancer drugs come to market, they said.
The team came to these conclusions after conducting a meta-analysis of 191 phase 2 or 3 clinical trials that included 26,604 patients. The trials investigated 15 anticancer drugs used as monotherapy.
The meta-analysis showed that the annualized incidence rate of AFib ranged from 0.26 cases per 100 person-years – about the same as placebo – to 4.92 cases, a nearly 20 times’ higher risk.
Rates were the highest for ibrutinib, clofarabine, and ponatinib.
The study was published in JACC: CardioOncology, a journal of the American College of Cardiology.
Actual rates of AFib are probably higher than what they found in this meta-analysis, the authors suspect, because most oncology trials only identify and report severe cases of AFib that require immediate medical attention. Less severe cases can also lead to serious complications, including strokes, but they go unreported, said the investigators, led by Joachim Alexandre, MD, PhD, a member of the cardio-oncology program at the University of Caen Normandie Hospital Center, France.
“These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants,” and AFib reporting is “particularly affected,” they said.
Call for routine monitoring
The root of the problem is the lack of routine rhythm monitoring in cancer trials. This in turn “leads to a significant underestimation of AFib incidence” and rates “markedly lower than those observed among real-life” patients, the authors pointed out.
To address the issue, Dr. Alexandre and his team called for routine cardiac monitoring in trials to capture the true incidence of AFib and to “clearly define which anticancer drugs are significantly associated” with the condition.
Approached for comment, Michael G. Fradley, MD, medical director of cardio-oncology at the University of Pennsylvania, Philadelphia, agreed.
“It’s incredibly important” to “identify the drugs most likely to cause arrhythmias and determine the best prevention and treatment strategies. Unfortunately, systematic evaluation of arrhythmias in cancer clinical trials has often been lacking,” Dr. Fradley told this news organization.
The investigators said the issue is particularly pressing for drugs known to be associated with AFib. For Bruton’s tyrosine kinase inhibitors such as ibrutinib, for instance, they call for standardize AFib detection in trials “not only on 12-lead ECGs” for symptomatic AFib but also with “longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AFib.”
Dr. Fradley said there might also be a role for newer wearable technologies that can detect arrhythmias through a skin patch or by other means.
Details of the meta-analysis
The investigators pulled the 191 studies they used in their meta-analysis from the ClinicalTrials.gov database.
The trials covered anticancer drugs used as monotherapy up to Sept. 18, 2020. Almost half were randomized trials, but only seven had placebo arms. Trials involving hematologic cancers outnumbered those involving solid tumors.
The 15 drugs examined were dacarbazine, abiraterone, clofarabine, azacitidine, ibrutinib, nilotinib, ponatinib, midostaurin, ipilimumab, aldesleukin, lenalidomide, pomalidomide, rituximab, bortezomib, and docetaxel.
The annualized incidence AFib rates per 100 person-years were 4.92 cases for ibrutinib, 2.38 cases for clofarabine, and 2.35 cases for ponatinib.
The lowest AFib rates were for ipilimumab (0.26 cases), rituximab (0.27), and nilotinib (0.29).
For placebo, the annualized rate was 0.25 cases per 100 person-years.
The team said caution is warranted regarding their estimations for clofarabine and midostaurin (0.65 cases) because no trials were registered after September 2009, when adverse event reporting became mandatory. As a result, estimates may be artificially low.
One of the limits of the study is that it focused on monotherapy in an age when combination treatment is generally the rule for cancer, the authors noted.
No external funding was reported for the study. Dr. Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity.
A version of this article first appeared on Medscape.com.
Retiform Purpura on the Lower Legs
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.
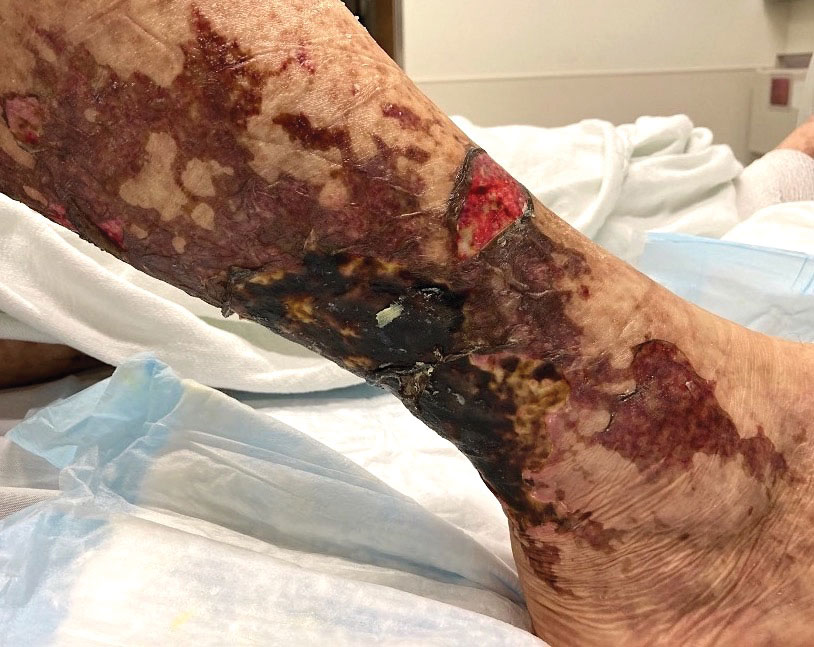
Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.
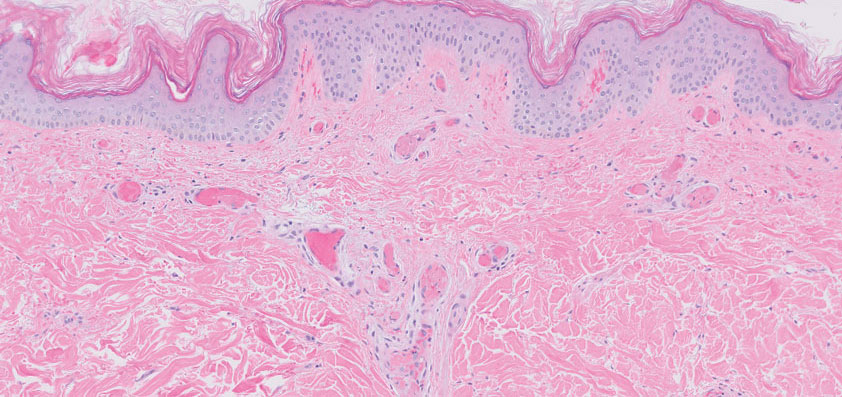
Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.

Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.

Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
The Diagnosis: Type I Cryoglobulinemia
Retiform purpura with overlying necrosis subsequently developed over the course of a week following presentation (Figure 1). A skin biopsy showed fibrin thrombi and congestion of small- and medium-sized blood vessels, consistent with vasculopathy (Figure 2). Urinalysis revealed hematuria and proteinuria. A renal biopsy performed due to a continually elevated serum creatinine level revealed glomerulonephritis with numerous IgG1 lambda–restricted glomerular capillary hyaline thrombi, compatible with a lymphoproliferative disorder–associated type I cryoglobulinemia. A serum cryoglobulin immunofixation test confirmed type I cryoglobulinemia involving monoclonal IgG lambda. The combination of cutaneous, renal, and hematologic findings was consistent with type I cryoglobulinemia. A subsequent bone marrow biopsy demonstrated a CD20+ lambda–restricted plasma cell neoplasm. Initial treatment with high-dose corticosteroids followed by targeted treatment of the underlying hematologic condition with bortezomib, rituximab, and dexamethasone improved the skin disease.

Cryoglobulins are abnormal immunoglobulins that precipitate at temperatures below 37 °C. The persistent presence of cryoglobulins in the serum is termed cryoglobulinemia.1 Type I cryoglobulinemia is distinguished from mixed cryoglobulinemia—types II and III—by the presence of a single monoclonal immunoglobulin, typically IgM or IgG. It is associated with lymphoproliferative disorders, most commonly monoclonal gammopathy of undetermined significance and B-cell malignancies such as Waldenström macroglobulinemia, multiple myeloma, or chronic lymphocytic leukemia. Histopathology shows occlusion of small vessel lumina with homogenous eosinophilic material containing the monoclonal cryoprecipitate.2 Disease manifestations are caused by small vessel occlusion, which leads to ischemia and tissue damage.

Retiform purpura, livedo reticularis/racemosa, and necrosis leading to ulcers are the most common cutaneous clinical findings. Extracutaneous signs include peripheral neuropathy, arthralgia, Raynaud phenomenon, and acrocyanosis. Renal involvement, most commonly glomerulonephritis with associated proteinuria, is noted in 14% to 20% of cases.3,4 An elevated cryocrit can lead to symptoms of hyperviscosity syndrome.2
Treatment is difficult and primarily is focused on addressing the underlying hematologic condition, which is responsible for synthesis of the cryoglobulin. Decreasing cryoglobulin production leads to decreased occlusion of blood vessels, thus alleviating the ischemia and skin damage. Monoclonal gammopathy of undetermined significance–related type I cryoglobulinemia initially is treated with corticosteroids followed by rituximab if a CD20+ B-cell clone is identified.2 Bortezomib is recommended for cases associated with Waldenström macroglobulinemia and cases associated with multiple myeloma with concurrent renal failure. In patients with neuropathy, a lenalidomide-based treatment can be employed. Patients should be instructed to keep extremities warm.2 Diabetic foot care guidelines should be followed to prevent wound complications. The differential diagnosis for type I cryoglobulinemia includes other causes of retiform purpura–like angioinvasive fungal infection, antiphospholipid antibody syndrome, calciphylaxis, and livedoid vasculopathy.5 Angioinvasive fungal infections are caused by Candida, Aspergillus, and Mucorales species, as well as other hyaline molds. They typically occur in immunocompromised patients and invade the blood vessels via direct inoculation or dissemination.6 Patients present with retiform purpura but typically will be acutely ill with fevers and vital sign abnormalities. Histopathology with special stains often will identify the fungal organisms in the dermis or inside blood vessel walls with vessel wall destruction and hemorrhage.7 Accurate diagnosis is essential to selecting appropriate antifungal agents. If angioinvasive fungal infection is clinically suspected, treatment should begin before culture and histopathologic data are available.7
Antiphospholipid antibody syndrome is an autoimmune thrombophilia that can occur as primary disease or in association with other autoimmune conditions, most commonly systemic lupus erythematosus. Diagnosis requires the presence of antiphospholipid antibodies, such as lupus anticoagulant, anticardiolipin antibody, anti–β2-glycoprotein-1 antibody, with arterial or venous thrombosis and/or recurrent pregnancy loss. Paraproteinemia is not seen. The most common cutaneous finding is livedo reticularis, with livedo racemosa being a more distinctive finding.8 Small vessel thrombosis is seen histopathologically. Treatment includes antiplatelet and anticoagulant medications. Patients with refractory disease may benefit from additional therapy with hydroxychloroquine or intravenous immunoglobulins.8
Calciphylaxis is a rare depositional vasculopathy that often occurs in patients with end-stage renal disease on dialysis. Patients present with painful and poor-healing skin lesions including indurated nodules, violaceous plaques, and retiform purpura that typically affect areas of high adiposity such as the thighs, abdomen, and buttocks.9 Ulceration and superimposed infections are common complications. Histopathologically, small dermal and subcutaneous vessels demonstrate calcification, microthrombosis, and fibrointimal hyperplasia.9 Wound management is critically important in patients with calciphylaxis. Treatment with intravenous sodium thiosulfate is typical, but prognosis remains poor. Although livedoid vasculopathy may present with retiform purpura in the ankles, paraproteinemia is not seen and patients frequently present with punched-out ulcerations that tend to heal into atrophie blanche.10 Livedoid vasculopathy has been associated with underlying hypercoagulable states, connective tissue diseases, and chronic venous hypertension. Hypercoagulability and endothelial cell damage contribute to the formation of fibrin thrombi in the superficial dermal blood vessels. Histopathology demonstrates thickening of vessel walls and intraluminal hyaline thrombi. Successful treatment in most cases is achieved with anticoagulation therapy, typically rivaroxaban, especially in patients with underlying hypercoagulability. Antiplatelet therapy also may be considered, while anabolic agents have been shown to be helpful in patients with connective tissue disease.10
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017;129:289-298. doi:10.1182/blood-2016-09-719773
- Sidana S, Rajkumar SV, Dispenzieri A, et al. Clinical presentation and outcomes of patients with type 1 monoclonal cryoglobulinemia. Am J Hematol. 2017;92:668-673. doi:10.1002/ajh.24745
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. Br J Haematol. 2015;168:671-678. doi:10.1111/bjh.13196
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796. doi:10.1016/j.jaad.2019.07.112
- Shields BE, Rosenbach M, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: background, epidemiology, and clinical presentation. J Am Acad Dermatol. 2019;80:869-880.e5. doi:10.1016/j.jaad.2018.04.059
- Berger AP, Ford BA, Brown-Joel Z, et al. Angioinvasive fungal infections impacting the skin: diagnosis, management, and complications. J Am Acad Dermatol. 2019;80:883-898.e2. doi:10.1016/j.jaad.2018.04.058
- Negrini S, Pappalardo F, Murdaca G, et al. The antiphospholipid syndrome: from pathophysiology to treatment. Clin Exp Med. 2017;17:257-267. doi:10.1007/s10238-016-0430-5
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Georgesen C, Fox LP, Harp J. Retiform purpura: workup and therapeutic considerations in select conditions. J Am Acad Dermatol. 2020;82:799-816. doi:10.1016/j.jaad.2019.07.113
A 58-year-old man presented with a petechial and purpuric rash limited to the lower extremities. He reported that the rash had been present for months but worsened acutely over the last 3 days with new-onset dark urine, joint pain, and edema limiting his ability to walk. Physical examination showed areas of violaceous macules and papules on the legs and dorsal feet in a reticular distribution. Laboratory findings were remarkable for an elevated serum creatinine level of 2.75 mg/dL (reference range, 0.70–1.30 mg/dL), and serum immunofixation revealed the presence of markedly elevated IgG lambda monoclonal proteins. He was afebrile and his vital signs were stable. Dermatology, nephrology, and rheumatology services were consulted.
Single-dose psilocybin promising for resistant depression
PARIS –
Known as COMP360, the synthetic agent, a proprietary, purified form of psilocybin, improved symptoms related to mood and anhedonia while leaving aspects such as appetite and weight changes unaffected, reported investigators led by Guy M. Goodwin, PhD, emeritus professor of psychiatry, University of Oxford, England, and chief medical officer, COMPASS Pathways.
The study was presented at the European Psychiatric Association (EPA) 2023 Congress.
100 million affected
Affecting up to 100 million people globally, TRD is “not an official diagnosis,” although it is often defined as the failure to elicit a response with at least two antidepressant treatments, said Dr. Goodwin.
Compared to their counterparts with non-TRD, those with TRD experience higher relapse rates, higher rates of suicidal behavior, and more residual symptoms even when they do respond to treatment.
Previous results from the study known as P-TRD indicated that a single 25-mg dose of COMP360 significantly reduced depression scores for up to 12 weeks when given along with psychological support, although a later analysis suggested the effect subsequently dropped off.
The vast majority of the patients in the trial were naive to psychedelics, and so, Dr. Goodwin explained, they undergo a preparation phase during which they receive psychoeducation and have at least two visits with a therapist, who then stays with them during administration of the drug to offer support if they experience psychological distress.
Following the psilocybin session, participants go through a process known as integration, which involves two sessions with a therapist within 2 weeks.
“That, in our view, is essentially about safety, and about identifying problems that have arisen as a result of taking the drug,” said Dr. Goodwin.
The phase 2b trial examined changes in specific depression symptoms after psilocybin treatment in 233 patients with TRD. Participants were a mean age of 39.8 years and 59% were women. They were randomized to receive one of three doses of the drug: a 1-mg dose (n = 79), a 10-mg dose (n = 75), or a 25-mg dose (n = 79).
The primary outcome was changes in individual items on the Montgomery-Åsberg Depression Rating Scale (MADRS) and 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16) scale.
While the effect on overall depression scores is important, said Dr. Goodwin, many of the items included in the depression assessment scales are “uninformative.”
Reduction in ‘core’ symptoms
Participants were assessed by a blinded rater at baseline, day 1, day 2, and at 1, 2, 3, 6, 9, and 12 weeks after administration of COMP360. The primary endpoint was a reduction in individual items on MADRS and scores from baseline to 3 weeks. Individual items on the QIDS-SR-16 were rated by participants at the same time points.
Investigators found the largest mean changes from baseline were on reported and apparent sadness, lassitude, inability to feel, and concentration difficulties, with “very nice and clear dose-related differences,” Dr. Goodwin said.
The results indicate that the significant benefit with the largest dose at 3 weeks versus baseline was confined to items such as inability to feel and reported and apparent sadness on the MADRS and feeling sad and general interest on the QIDS-SR-16 (Table 1).
The results suggest the effect of COMP360 is “on the core symptoms of depression,” said Dr. Goodwin.
Results were similar for individual items on the QIDS-SR-16, with the greatest changes in items including feeling sad, general interest, energy level, falling asleep, view of myself, concentration/decision-making, and feeling down.
Other scale items, such as decreased appetite, feel restless, and weight changes, showed negligible changes in response to COMP360 therapy and were described by Dr. Goodwin as “inconsequential.”
“Essentially, these items are contributing nothing but noise to the signal,” he said.
He added the results of the study need to be replicated and that plans for phase 3 trials are underway. These studies, he said, are designed to convince the Food and Drug Administration that “this is not just a recreational drug, it’s a medicine.”
Enthusiasm running ahead of the data
Commenting on the findings, Bertha K. Madras, PhD, professor of psychobiology, department of psychiatry, Harvard Medical School, Boston, who was not involved in the study, said “hallucinogens are an intriguing class of drugs and I support ongoing high-quality research in this area.”
However, she told this news organization that the “breathtaking endorsement of this drug is far ahead of scientific data.”
She cited concerns such as the “narrow demographics” of participants, their previous experience with and expectations of hallucinogens, the “potential for symptom fluidity of enrollees,” such as depression evolving into psychosis, and the “undefined role” of the therapist during a hallucinogenic session.
“Finally, I am concerned that enthusiasm for therapeutic potential has been, and will continue to be, preempted and directed towards legalization and widespread access for vulnerable populations,” Dr. Madras said.
This, she said, “is occurring at breakneck speed in the U.S., with scant resistance or skepticism from the investigators engaged in therapeutic assessment.”
The study was funded by COMPASS Pathways. Dr. Goodwin has reported relationships with COMPASS Pathways, Buckley Psytech, Boehringer Ingelheim, Clerkenwell Health, EVA Pharma, Lundbeck, Janssen Global Services, Novartis, Ocean Neurosciences, P1vital, Sage Therapeutics, Servier, Takeda, and WebMD.
A version of this article first appeared on Medscape.com.
PARIS –
Known as COMP360, the synthetic agent, a proprietary, purified form of psilocybin, improved symptoms related to mood and anhedonia while leaving aspects such as appetite and weight changes unaffected, reported investigators led by Guy M. Goodwin, PhD, emeritus professor of psychiatry, University of Oxford, England, and chief medical officer, COMPASS Pathways.
The study was presented at the European Psychiatric Association (EPA) 2023 Congress.
100 million affected
Affecting up to 100 million people globally, TRD is “not an official diagnosis,” although it is often defined as the failure to elicit a response with at least two antidepressant treatments, said Dr. Goodwin.
Compared to their counterparts with non-TRD, those with TRD experience higher relapse rates, higher rates of suicidal behavior, and more residual symptoms even when they do respond to treatment.
Previous results from the study known as P-TRD indicated that a single 25-mg dose of COMP360 significantly reduced depression scores for up to 12 weeks when given along with psychological support, although a later analysis suggested the effect subsequently dropped off.
The vast majority of the patients in the trial were naive to psychedelics, and so, Dr. Goodwin explained, they undergo a preparation phase during which they receive psychoeducation and have at least two visits with a therapist, who then stays with them during administration of the drug to offer support if they experience psychological distress.
Following the psilocybin session, participants go through a process known as integration, which involves two sessions with a therapist within 2 weeks.
“That, in our view, is essentially about safety, and about identifying problems that have arisen as a result of taking the drug,” said Dr. Goodwin.
The phase 2b trial examined changes in specific depression symptoms after psilocybin treatment in 233 patients with TRD. Participants were a mean age of 39.8 years and 59% were women. They were randomized to receive one of three doses of the drug: a 1-mg dose (n = 79), a 10-mg dose (n = 75), or a 25-mg dose (n = 79).
The primary outcome was changes in individual items on the Montgomery-Åsberg Depression Rating Scale (MADRS) and 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16) scale.
While the effect on overall depression scores is important, said Dr. Goodwin, many of the items included in the depression assessment scales are “uninformative.”
Reduction in ‘core’ symptoms
Participants were assessed by a blinded rater at baseline, day 1, day 2, and at 1, 2, 3, 6, 9, and 12 weeks after administration of COMP360. The primary endpoint was a reduction in individual items on MADRS and scores from baseline to 3 weeks. Individual items on the QIDS-SR-16 were rated by participants at the same time points.
Investigators found the largest mean changes from baseline were on reported and apparent sadness, lassitude, inability to feel, and concentration difficulties, with “very nice and clear dose-related differences,” Dr. Goodwin said.
The results indicate that the significant benefit with the largest dose at 3 weeks versus baseline was confined to items such as inability to feel and reported and apparent sadness on the MADRS and feeling sad and general interest on the QIDS-SR-16 (Table 1).
The results suggest the effect of COMP360 is “on the core symptoms of depression,” said Dr. Goodwin.
Results were similar for individual items on the QIDS-SR-16, with the greatest changes in items including feeling sad, general interest, energy level, falling asleep, view of myself, concentration/decision-making, and feeling down.
Other scale items, such as decreased appetite, feel restless, and weight changes, showed negligible changes in response to COMP360 therapy and were described by Dr. Goodwin as “inconsequential.”
“Essentially, these items are contributing nothing but noise to the signal,” he said.
He added the results of the study need to be replicated and that plans for phase 3 trials are underway. These studies, he said, are designed to convince the Food and Drug Administration that “this is not just a recreational drug, it’s a medicine.”
Enthusiasm running ahead of the data
Commenting on the findings, Bertha K. Madras, PhD, professor of psychobiology, department of psychiatry, Harvard Medical School, Boston, who was not involved in the study, said “hallucinogens are an intriguing class of drugs and I support ongoing high-quality research in this area.”
However, she told this news organization that the “breathtaking endorsement of this drug is far ahead of scientific data.”
She cited concerns such as the “narrow demographics” of participants, their previous experience with and expectations of hallucinogens, the “potential for symptom fluidity of enrollees,” such as depression evolving into psychosis, and the “undefined role” of the therapist during a hallucinogenic session.
“Finally, I am concerned that enthusiasm for therapeutic potential has been, and will continue to be, preempted and directed towards legalization and widespread access for vulnerable populations,” Dr. Madras said.
This, she said, “is occurring at breakneck speed in the U.S., with scant resistance or skepticism from the investigators engaged in therapeutic assessment.”
The study was funded by COMPASS Pathways. Dr. Goodwin has reported relationships with COMPASS Pathways, Buckley Psytech, Boehringer Ingelheim, Clerkenwell Health, EVA Pharma, Lundbeck, Janssen Global Services, Novartis, Ocean Neurosciences, P1vital, Sage Therapeutics, Servier, Takeda, and WebMD.
A version of this article first appeared on Medscape.com.
PARIS –
Known as COMP360, the synthetic agent, a proprietary, purified form of psilocybin, improved symptoms related to mood and anhedonia while leaving aspects such as appetite and weight changes unaffected, reported investigators led by Guy M. Goodwin, PhD, emeritus professor of psychiatry, University of Oxford, England, and chief medical officer, COMPASS Pathways.
The study was presented at the European Psychiatric Association (EPA) 2023 Congress.
100 million affected
Affecting up to 100 million people globally, TRD is “not an official diagnosis,” although it is often defined as the failure to elicit a response with at least two antidepressant treatments, said Dr. Goodwin.
Compared to their counterparts with non-TRD, those with TRD experience higher relapse rates, higher rates of suicidal behavior, and more residual symptoms even when they do respond to treatment.
Previous results from the study known as P-TRD indicated that a single 25-mg dose of COMP360 significantly reduced depression scores for up to 12 weeks when given along with psychological support, although a later analysis suggested the effect subsequently dropped off.
The vast majority of the patients in the trial were naive to psychedelics, and so, Dr. Goodwin explained, they undergo a preparation phase during which they receive psychoeducation and have at least two visits with a therapist, who then stays with them during administration of the drug to offer support if they experience psychological distress.
Following the psilocybin session, participants go through a process known as integration, which involves two sessions with a therapist within 2 weeks.
“That, in our view, is essentially about safety, and about identifying problems that have arisen as a result of taking the drug,” said Dr. Goodwin.
The phase 2b trial examined changes in specific depression symptoms after psilocybin treatment in 233 patients with TRD. Participants were a mean age of 39.8 years and 59% were women. They were randomized to receive one of three doses of the drug: a 1-mg dose (n = 79), a 10-mg dose (n = 75), or a 25-mg dose (n = 79).
The primary outcome was changes in individual items on the Montgomery-Åsberg Depression Rating Scale (MADRS) and 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16) scale.
While the effect on overall depression scores is important, said Dr. Goodwin, many of the items included in the depression assessment scales are “uninformative.”
Reduction in ‘core’ symptoms
Participants were assessed by a blinded rater at baseline, day 1, day 2, and at 1, 2, 3, 6, 9, and 12 weeks after administration of COMP360. The primary endpoint was a reduction in individual items on MADRS and scores from baseline to 3 weeks. Individual items on the QIDS-SR-16 were rated by participants at the same time points.
Investigators found the largest mean changes from baseline were on reported and apparent sadness, lassitude, inability to feel, and concentration difficulties, with “very nice and clear dose-related differences,” Dr. Goodwin said.
The results indicate that the significant benefit with the largest dose at 3 weeks versus baseline was confined to items such as inability to feel and reported and apparent sadness on the MADRS and feeling sad and general interest on the QIDS-SR-16 (Table 1).
The results suggest the effect of COMP360 is “on the core symptoms of depression,” said Dr. Goodwin.
Results were similar for individual items on the QIDS-SR-16, with the greatest changes in items including feeling sad, general interest, energy level, falling asleep, view of myself, concentration/decision-making, and feeling down.
Other scale items, such as decreased appetite, feel restless, and weight changes, showed negligible changes in response to COMP360 therapy and were described by Dr. Goodwin as “inconsequential.”
“Essentially, these items are contributing nothing but noise to the signal,” he said.
He added the results of the study need to be replicated and that plans for phase 3 trials are underway. These studies, he said, are designed to convince the Food and Drug Administration that “this is not just a recreational drug, it’s a medicine.”
Enthusiasm running ahead of the data
Commenting on the findings, Bertha K. Madras, PhD, professor of psychobiology, department of psychiatry, Harvard Medical School, Boston, who was not involved in the study, said “hallucinogens are an intriguing class of drugs and I support ongoing high-quality research in this area.”
However, she told this news organization that the “breathtaking endorsement of this drug is far ahead of scientific data.”
She cited concerns such as the “narrow demographics” of participants, their previous experience with and expectations of hallucinogens, the “potential for symptom fluidity of enrollees,” such as depression evolving into psychosis, and the “undefined role” of the therapist during a hallucinogenic session.
“Finally, I am concerned that enthusiasm for therapeutic potential has been, and will continue to be, preempted and directed towards legalization and widespread access for vulnerable populations,” Dr. Madras said.
This, she said, “is occurring at breakneck speed in the U.S., with scant resistance or skepticism from the investigators engaged in therapeutic assessment.”
The study was funded by COMPASS Pathways. Dr. Goodwin has reported relationships with COMPASS Pathways, Buckley Psytech, Boehringer Ingelheim, Clerkenwell Health, EVA Pharma, Lundbeck, Janssen Global Services, Novartis, Ocean Neurosciences, P1vital, Sage Therapeutics, Servier, Takeda, and WebMD.
A version of this article first appeared on Medscape.com.
AT EPA 2023
Parents of patients with rheumatic disease, MIS-C strongly hesitant of COVID vaccination
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
The earlier baricitinib for severe alopecia areata is started, the better
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
AT AAD 2023
Study offers dozens of reasons to cut sugar
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
A new compilation of nearly all research to date on the health impacts of sugar offers dozens of reasons to cut back.
The studies accounted for decades of research on the topic, stretching back to the beginning of the largest electronic databases for scientific papers.
The result is a list that cites the world’s most common health problems like heart disease, diabetes, obesity, high blood pressure, heart attack, high cholesterol, cancer, and depression. The findings were published in the BMJ. Researchers looked at studies that evaluated the impacts of consuming free sugars, which means any food that contains processed or naturally occurring sugars like table sugar, honey, or maple syrup. Sugar found in whole fruits and vegetables and in milk is not free sugar.
U.S. dietary guidelines recommend getting no more than 10% of daily calories from added sugars. For a typical 2,000-calorie-per-day diet, that equals no more than 200 calories, or about 12 teaspoons. The CDC reports that the average person consumes 17 teaspoons per day, with the largest sources being sugar-sweetened beverages, desserts, and snacks. (For context: one 12-ounce can of soda contains the equivalent of 9 teaspoons of sugar, according to beverage maker Coca-Cola.)
The new analysis also found links between sugary beverage consumption and other diet and lifestyle characteristics that may contribute to health problems.
“People who consumed sugar-sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium, and less fruit, fiber, dairy products, and whole grain foods,” the authors wrote. “This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television. Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.”
Recommendations for limiting sugar consumption are in place worldwide, the authors noted. They concluded that more needs to be done given the known health dangers of sugar.
“To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed,” they said.
A version of this article first appeared on WebMD.com.
FROM THE BMJ
Antiphospholipid antibodies linked to future CV events
The presence of antiphospholipid antibodies is associated with an increased risk for future cardiovascular events, according to a new study.
The findings point to possible new approaches to risk stratification and the potential for new therapeutic targets in heart disease.
“In this study of the general population, we found that two antiphospholipid antibodies were associated with an increased risk of having a serious cardiovascular event over a follow-up of 8 years,” coauthor Jason Knight, MD, University of Michigan, Ann Arbor, said in an interview.
“If confirmed in further studies, these findings could be used to identify a subgroup of patients who need more careful monitoring and more aggressive risk-factor modification, and if the increased risk linked to these antibodies is high enough, it may also justify preemptive treatments such as the anticoagulants that are routinely used in antiphospholipid syndrome,” Dr. Knight said.
“The long-term vision is that we may identify some people in the general population who would benefit from treating the immune system for the prevention and treatment of cardiovascular disease instead of, or in addition to, using typical cardiovascular medications,” he added.
The study was published online in JAMA Network Open.
Individuals with autoimmune and inflammatory diseases have a greater risk for cardiovascular events than expected based on traditional cardiovascular risk factors, with mechanisms proposed to explain this risk including inflammation-mediated disruption of vascular integrity and activation of platelets and coagulation pathways, the authors explained. However, the role of autoantibodies remains unclear.
They noted that antiphospholipid antibodies can activate endothelial cells, platelets, and neutrophils, and some patients with persistently circulating antiphospholipid antibodies can develop antiphospholipid syndrome – an acquired thromboinflammatory disease characterized by arterial, venous, and microvascular thrombotic events and obstetric complications.
Cross-sectional studies have shown that antiphospholipid antibodies are acutely present in up to 17.4% of patients with stroke or transient ischemic attack, and small cohort studies have suggested that such antibodies may be present in 1%-12% of seemingly healthy individuals. However, the impact of sex, race, and ethnicity on the prevalence of antiphospholipid antibodies and their association with atherosclerotic cardiovascular disease is not known.
The researchers conducted the current study to look at the association between antiphospholipid antibodies and future risk for atherosclerotic cardiovascular events.
They analyzed data from 2,427 participants in the population-based Dallas Heart Study who had no history of atherosclerotic cardiovascular disease or autoimmune diseases requiring immunosuppressive medications at the time of blood sampling at study entry in 2007-2009.
Eight different types of antiphospholipid antibodies were measured, and data on cardiovascular events over the next 8 years was recorded.
Results showed that 14.5% of the cohort tested positive for one of these antiphospholipid antibodies at the start of the study, with approximately one-third of those detected at a moderate or high titer.
The researchers also found that the IgA isotypes of two antiphospholipid antibodies – anticardiolipin and anti-beta-2 glycoprotein – were associated with future atherosclerotic cardiovascular events.
After adjustment for other known risk factors, individuals testing positive for the IgA isotype of anticardiolipin had an almost five times increased risk (hazard ratio, 4.92) of the primary endpoint (myocardial infarction, stroke, coronary revascularization, or cardiovascular death); while those testing positive for anti–beta2-glycoprotein had an almost three times increased risk (HR, 2.91).
Furthermore, there was what appeared to be a dose effect. People with the highest levels of these antibodies also had the highest risk for cardiovascular events, with up to an almost 10-fold increased risk with the higher level of anticardiolipin.
Dr. Knight said that more research into the IgA isotypes of these antiphospholipid antibodies is needed.
“Most of the mechanistic work in the antiphospholipid syndrome field has focused on IgG antiphospholipid antibodies. While we commonly find these IgA antibodies in patients with APS, the extent to which they contribute to disease has not been firmly established,” he said. “The fact that IgA was the primary hit in our unbiased screen suggests that there is more to the story and we need to better understand the implications of having these antibodies in circulation, and what specific problems they may be causing.”
Noting that antiphospholipid antibodies can form transiently after certain situations, such as infections, Dr. Knight said that further studies were needed with repeat blood testing to detect the chronic presence of the antibodies.
He added that information of venous thromboses was not available in this study and “perhaps some of the other antibodies might have stood out if we were able to analyze for different outcomes.”
This study was supported by a Pfizer Aspire Award. Dr. Knight reported receiving research funding and consulting fees from Jazz Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
The presence of antiphospholipid antibodies is associated with an increased risk for future cardiovascular events, according to a new study.
The findings point to possible new approaches to risk stratification and the potential for new therapeutic targets in heart disease.
“In this study of the general population, we found that two antiphospholipid antibodies were associated with an increased risk of having a serious cardiovascular event over a follow-up of 8 years,” coauthor Jason Knight, MD, University of Michigan, Ann Arbor, said in an interview.
“If confirmed in further studies, these findings could be used to identify a subgroup of patients who need more careful monitoring and more aggressive risk-factor modification, and if the increased risk linked to these antibodies is high enough, it may also justify preemptive treatments such as the anticoagulants that are routinely used in antiphospholipid syndrome,” Dr. Knight said.
“The long-term vision is that we may identify some people in the general population who would benefit from treating the immune system for the prevention and treatment of cardiovascular disease instead of, or in addition to, using typical cardiovascular medications,” he added.
The study was published online in JAMA Network Open.
Individuals with autoimmune and inflammatory diseases have a greater risk for cardiovascular events than expected based on traditional cardiovascular risk factors, with mechanisms proposed to explain this risk including inflammation-mediated disruption of vascular integrity and activation of platelets and coagulation pathways, the authors explained. However, the role of autoantibodies remains unclear.
They noted that antiphospholipid antibodies can activate endothelial cells, platelets, and neutrophils, and some patients with persistently circulating antiphospholipid antibodies can develop antiphospholipid syndrome – an acquired thromboinflammatory disease characterized by arterial, venous, and microvascular thrombotic events and obstetric complications.
Cross-sectional studies have shown that antiphospholipid antibodies are acutely present in up to 17.4% of patients with stroke or transient ischemic attack, and small cohort studies have suggested that such antibodies may be present in 1%-12% of seemingly healthy individuals. However, the impact of sex, race, and ethnicity on the prevalence of antiphospholipid antibodies and their association with atherosclerotic cardiovascular disease is not known.
The researchers conducted the current study to look at the association between antiphospholipid antibodies and future risk for atherosclerotic cardiovascular events.
They analyzed data from 2,427 participants in the population-based Dallas Heart Study who had no history of atherosclerotic cardiovascular disease or autoimmune diseases requiring immunosuppressive medications at the time of blood sampling at study entry in 2007-2009.
Eight different types of antiphospholipid antibodies were measured, and data on cardiovascular events over the next 8 years was recorded.
Results showed that 14.5% of the cohort tested positive for one of these antiphospholipid antibodies at the start of the study, with approximately one-third of those detected at a moderate or high titer.
The researchers also found that the IgA isotypes of two antiphospholipid antibodies – anticardiolipin and anti-beta-2 glycoprotein – were associated with future atherosclerotic cardiovascular events.
After adjustment for other known risk factors, individuals testing positive for the IgA isotype of anticardiolipin had an almost five times increased risk (hazard ratio, 4.92) of the primary endpoint (myocardial infarction, stroke, coronary revascularization, or cardiovascular death); while those testing positive for anti–beta2-glycoprotein had an almost three times increased risk (HR, 2.91).
Furthermore, there was what appeared to be a dose effect. People with the highest levels of these antibodies also had the highest risk for cardiovascular events, with up to an almost 10-fold increased risk with the higher level of anticardiolipin.
Dr. Knight said that more research into the IgA isotypes of these antiphospholipid antibodies is needed.
“Most of the mechanistic work in the antiphospholipid syndrome field has focused on IgG antiphospholipid antibodies. While we commonly find these IgA antibodies in patients with APS, the extent to which they contribute to disease has not been firmly established,” he said. “The fact that IgA was the primary hit in our unbiased screen suggests that there is more to the story and we need to better understand the implications of having these antibodies in circulation, and what specific problems they may be causing.”
Noting that antiphospholipid antibodies can form transiently after certain situations, such as infections, Dr. Knight said that further studies were needed with repeat blood testing to detect the chronic presence of the antibodies.
He added that information of venous thromboses was not available in this study and “perhaps some of the other antibodies might have stood out if we were able to analyze for different outcomes.”
This study was supported by a Pfizer Aspire Award. Dr. Knight reported receiving research funding and consulting fees from Jazz Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
The presence of antiphospholipid antibodies is associated with an increased risk for future cardiovascular events, according to a new study.
The findings point to possible new approaches to risk stratification and the potential for new therapeutic targets in heart disease.
“In this study of the general population, we found that two antiphospholipid antibodies were associated with an increased risk of having a serious cardiovascular event over a follow-up of 8 years,” coauthor Jason Knight, MD, University of Michigan, Ann Arbor, said in an interview.
“If confirmed in further studies, these findings could be used to identify a subgroup of patients who need more careful monitoring and more aggressive risk-factor modification, and if the increased risk linked to these antibodies is high enough, it may also justify preemptive treatments such as the anticoagulants that are routinely used in antiphospholipid syndrome,” Dr. Knight said.
“The long-term vision is that we may identify some people in the general population who would benefit from treating the immune system for the prevention and treatment of cardiovascular disease instead of, or in addition to, using typical cardiovascular medications,” he added.
The study was published online in JAMA Network Open.
Individuals with autoimmune and inflammatory diseases have a greater risk for cardiovascular events than expected based on traditional cardiovascular risk factors, with mechanisms proposed to explain this risk including inflammation-mediated disruption of vascular integrity and activation of platelets and coagulation pathways, the authors explained. However, the role of autoantibodies remains unclear.
They noted that antiphospholipid antibodies can activate endothelial cells, platelets, and neutrophils, and some patients with persistently circulating antiphospholipid antibodies can develop antiphospholipid syndrome – an acquired thromboinflammatory disease characterized by arterial, venous, and microvascular thrombotic events and obstetric complications.
Cross-sectional studies have shown that antiphospholipid antibodies are acutely present in up to 17.4% of patients with stroke or transient ischemic attack, and small cohort studies have suggested that such antibodies may be present in 1%-12% of seemingly healthy individuals. However, the impact of sex, race, and ethnicity on the prevalence of antiphospholipid antibodies and their association with atherosclerotic cardiovascular disease is not known.
The researchers conducted the current study to look at the association between antiphospholipid antibodies and future risk for atherosclerotic cardiovascular events.
They analyzed data from 2,427 participants in the population-based Dallas Heart Study who had no history of atherosclerotic cardiovascular disease or autoimmune diseases requiring immunosuppressive medications at the time of blood sampling at study entry in 2007-2009.
Eight different types of antiphospholipid antibodies were measured, and data on cardiovascular events over the next 8 years was recorded.
Results showed that 14.5% of the cohort tested positive for one of these antiphospholipid antibodies at the start of the study, with approximately one-third of those detected at a moderate or high titer.
The researchers also found that the IgA isotypes of two antiphospholipid antibodies – anticardiolipin and anti-beta-2 glycoprotein – were associated with future atherosclerotic cardiovascular events.
After adjustment for other known risk factors, individuals testing positive for the IgA isotype of anticardiolipin had an almost five times increased risk (hazard ratio, 4.92) of the primary endpoint (myocardial infarction, stroke, coronary revascularization, or cardiovascular death); while those testing positive for anti–beta2-glycoprotein had an almost three times increased risk (HR, 2.91).
Furthermore, there was what appeared to be a dose effect. People with the highest levels of these antibodies also had the highest risk for cardiovascular events, with up to an almost 10-fold increased risk with the higher level of anticardiolipin.
Dr. Knight said that more research into the IgA isotypes of these antiphospholipid antibodies is needed.
“Most of the mechanistic work in the antiphospholipid syndrome field has focused on IgG antiphospholipid antibodies. While we commonly find these IgA antibodies in patients with APS, the extent to which they contribute to disease has not been firmly established,” he said. “The fact that IgA was the primary hit in our unbiased screen suggests that there is more to the story and we need to better understand the implications of having these antibodies in circulation, and what specific problems they may be causing.”
Noting that antiphospholipid antibodies can form transiently after certain situations, such as infections, Dr. Knight said that further studies were needed with repeat blood testing to detect the chronic presence of the antibodies.
He added that information of venous thromboses was not available in this study and “perhaps some of the other antibodies might have stood out if we were able to analyze for different outcomes.”
This study was supported by a Pfizer Aspire Award. Dr. Knight reported receiving research funding and consulting fees from Jazz Pharmaceuticals outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN