User login
‘Collateral damage’: COVID-19 threatens patients with COPD
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
FROM CHEST
Headache may predict clinical evolution of COVID-19
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. An observational study of more than 100 patients showed that headache onset could occur during the presymptomatic or symptomatic phase of COVID-19 and could resemble tension-type or migraine headache.
Headache itself was associated with a shorter symptomatic period, while headache and anosmia were associated with a shorter hospitalization period. In a subgroup of participants, headache persisted even after the symptoms of COVID-19 had been resolved.
Investigators noted that understanding the pathophysiology of headache in COVID-19 could improve understanding of migraine and other headache disorders. “It seems that those patients who start early on, during the asymptomatic or early symptomatic period of COVID-19, with headache have a more localized inflammatory response that may reflect the ability of the body to better control and respond to the infection by SARS-CoV-2,” lead investigator Patricia Pozo-Rosich, MD, PhD, head of the headache and craniofacial pain unit at Vall d’Hebron University Hospital, Barcelona, said in an interview.
She presented the findings at the virtual annual meeting of the American Headache Society.
Systemic inflammation
Headache is one of the main symptoms of COVID-19. A recent study of 214 patients with COVID-19 showed that approximately 13% of the participants had headache and 5% had anosmia.
SARS-CoV-2 penetrates the cells through the ACE2 receptor, which is present throughout the body. “SARS-CoV-2 enters the body through the nasal cavity and it probably penetrates the nervous system in the periphery through afferent branches of the olfactory and trigeminal nerve,” Dr. Pozo-Rosich said. It travels to the lungs and, later, the bloodstream. This generates systemic inflammation that may turn into a cytokine storm. Evidence has identified cortical hyperintensities and olfactory bulb hyperintensities in patients with COVID-19, suggesting that the virus directly infects the CNS.
Interleukin-6, one of the main inflammatory molecules, has been proven to be related to COVID-19 and has become a therapeutic target. Levels of IL-6 may be lower and tend to be more stable in patients with both COVID-19 and headache than in patients with COVID-19 only.
The researchers observed 130 patients (51% women; mean age, 54 years) with COVID-19 who were attended by neurologists at Vall d’Hebron. In this group, 74.4% had headache. Patients with headache tended to be younger than those without headache (mean age, 50 years vs. 63 years, respectively) and tended to be women (58.6% vs. 29.4%).
Approximately one-third of patients with headache had a history of migraine. Most reported mild to moderate pain that resembled tension-type headache. In participants with severe pain and migraine-like features, headache more often began during the asymptomatic phase of COVID-19.
Disease evolution predictor?
The investigators followed up on 100 of the 130 patients with COVID-19, of whom 74 had headache. About 38% of these patients had ongoing headache after 6 weeks, which suggests that some patients may develop a new daily persistent headache once a 3-month period has elapsed. Half of this group had no previous headache history. Headache had been the prodromal symptom of COVID-19 for 21.4% of these patients.
Results showed that headache predicted the clinical evolution of COVID-19. The symptomatic phase of COVID-19 was 7 days shorter for patients with headache than for those without headache. In addition, the period of hospitalization was 7 days shorter for patients with headache and anosmia, compared with patients who had neither headache nor anosmia.
Most therapies, including ibuprofen, candesartan, and anti–calcitonin gene–related peptide (CGRP) monoclonal antibodies, are safe for treating headache in COVID-19, the investigators noted. “We should just try to initially avoid steroids to avoid interference with the body’s reaction to SARS-CoV-2,” Dr. Pozo-Rosich said.
Researchers at Sidney Kimmel Medical College, Philadelphia, are currently studying intranasal vazegepant, an anti-CGRP therapy, as a way to potentially blunt the severe inflammatory response in the lungs of patients with COVID-19, she noted, adding that this peptide may have a future role not only in headache, but also in COVID-19.
Historical link to viral infections
Commenting on the study, Matthew S. Robbins, MD, associate professor of neurology at Weill Cornell Medicine, New York, said the findings associating headache with a shorter symptomatic phase of COVID-19 were “interesting.”
“Headache is common with mild viral infections. More severe viral infections may simply feature more overwhelming respiratory symptoms and fever that lead to underreporting or underascertainment of headache,” said Dr. Robbins, who was not involved with the research.
He noted that the finding showing an association of headache and COVID-19 with a younger age and in women “may be related to a higher prevalence of migraine biology in such patients, and being triggered by the virus or the psychological stress associated with it.”
Dr. Robbins added that viral illnesses have long been associated with new daily persistent headache, “dating back to the early 1980s,” when it was first described in association with Epstein-Barr virus. These infections have also been implicated in the progression of migraine to chronic migraine in adolescents.
“In my view, treatment should be aimed at the symptomatic headache type for which new daily persistent headache resembles, regardless of the potential inciting factor,” Dr. Robbins said.
Dr. Pozo-Rosich has received consulting fees from Allergan, Amgen, Almirall, Biohaven, Chiesi, Eli Lilly, Medscape, Novartis, and Teva Pharmaceuticals. Dr. Robbins has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AHS 2020
Where does dexamethasone fit in with diabetic ketoacidosis in COVID-19?
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
After the ICU: A ‘fraternity of people who are struggling’
By the time she was discharged from a suburban New Jersey hospital on April 10, Kathleen Ronan thought the worst was behind her. For a week before her husband rushed her to the emergency department (ED), incoherent and struggling to breathe, the novel coronavirus had ravaged her body. She tried to treat her fevers with acetaminophen and ice packs. Despite taking enough Tylenol to risk liver damage and packing herself on ice like the catch of the day, Ronan’s fever continued to rise. By the time her temperature reached 104.5° F, Ronan knew the time had come for more drastic measures.
A team of masked and gowned nurses greeted her at a triage tent outside the ED, and from there, everything becomes hazy for Ronan. She was immediately rushed to the hospital’s special COVID-19 intensive care unit (ICU), where she spent 5 days. But she has few distinct memories from this time. What she does remember is the exhaustion, the pain, the loneliness, and the fear. Her family couldn’t visit, and though Ronan works as a home health nurse, her brain was so addled with fever that she couldn’t make sense of what was happening. After a week in the hospital, 5 days of which were spent in the ICU, 51-year-old Ronan was discharged.
Her years of working as a home health nurse told her that the return home wouldn’t be easy, but nothing prepared her for just how much she would struggle. The once-active Ronan, who had supplemented long days on her feet caring for others as a nurse with regular trips to the gym, now needed a walker to traverse the few steps from her bed to the toilet, an effort that left her gasping for air. Her brain couldn’t even focus on an audiobook, let alone a short magazine article.
“It just completely knocked the stuffing out of me,” Ronan said.
Ronan’s lingering symptoms aren’t unique to COVID-19 patients. In as many as 80% of patients leaving the ICU, . Although underlying illness plays a role in these symptoms, the amount of time spent in critical care is a major factor.
Nor is PICS simply a set of side effects that will go away on their own. It includes ongoing cognitive difficulties and physical weakness, both of which can lead to employment problems. Beyond that, depression and anxiety can exacerbate – and be exacerbated by – these challenges. Psychologist Jim Jackson, PsyD, assistant director of the ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tennessee, recently spoke with a former ICU patient who has struggled since her discharge 30 years ago.
“Her life essentially stopped with her critical care stay. She hasn’t been able to move forward,” he said. “She’s part of a whole fraternity of people who are struggling.”
The good news is that over the past decade, researchers have made important strides in understanding what makes PICS symptoms worse and how critical care physicians can tweak ICU protocols to reduce PICS severity. Practitioners will need to draw on this knowledge to help Ronan and the thousands of COVID-19 ICU patients like her.
Surviving the ICU
Although the new coronavirus has pushed the world’s critical care system to its limits, it was an outbreak in 1952 that inspired the creation of intensive care units. That summer, a wave of paralytic polio swept over Copenhagen, Denmark, and anesthesiologist Bjørn Ibsen, MD, PhD, used mechanical ventilation — physically operated by medical and dental students – to help 316 children breathe for weeks at a time while their small bodies worked to fight off the virus. The effort halved the mortality rate from polio that affected breathing, from 80% to 40%.
In these wards, dedicated to the very sickest, each patient was assigned his or her own nurse. Over the next decade, hospitals in the United Kingdom and the United States established their own ICUs to treat patients with a variety of conditions. Although it helped improve survival, mortality rates in critical care units remained stubbornly high, owing to the patients’ severe underlying illnesses.
“We thought we were doing a good job if the patient survived, but we had no idea what happened after discharge,” said Carla Sevin, MD, medical director of Vanderbilt’s ICU Recovery Center. Nor did their efforts to find out always bring answers. “We struggled to get people to come in for support — they were debilitated, physically burdened, and weak.”
Through further advances in life support, by the early 2000s, the average mortality rates in American ICUs had dropped to 8% to 19%. As the number of critical care survivors began to climb, clinical researchers noticed that the lives of these patients and their families were profoundly altered by their severe illness.
As Dale Needham, MD, PhD, began his pulmonology and critical care residency in Toronto, Canada, in 2005, a group of physicians there began a 5-year longitudinal study to assess long-term outcomes of patients who developed acute respiratory distress syndrome (ARDS). Although ARDS is an acute condition, the investigators found that patients felt effects for years. Younger patients recovered better than older ones, but none of the patients› physical functioning was equivalent to that of age-matched control persons. Even 5 years later, former ICU patients only reached 76% of expected physical functioning, according to results published in the New England Journal of Medicine. The study was a wake-up call.
At a meeting in Chicago in 2010, Needham, now an intensivist at Johns Hopkins Hospital in Baltimore, Maryland, gathered an interdisciplinary group of colleagues, including patients and caregivers, to clarify the phenomena they were seeing. What emerged from that meeting, published in 2012 in Critical Care Medicine, were the diagnostic criteria for PICS: According to the new definition, PICS is characterized by new or worsening physical and neuropsychiatric deficits that range from forgetfulness and loss of motivation to physical weakness and insomnia.
The issue, Needham says, is that although the trouble starts in the ICU, it only becomes clear once patients leave. “ICU doctors aren’t the ones dealing with this,” Needham said. “We need to build stronger bridges between critical care and other professions.” That’s where PICS comes in, a definition that exists explicitly to alert healthcare providers about the constellation of challenges many of these individuals face as they try to reenter “normal” life.
Defining the problem
As an ICU nurse at the Mayo Clinic in Rochester, Minnesota, Annie Johnson, ACNP-BC, knew lots about helping hospitalized patients, but she says she didn’t know anything about what to do after discharge – at least not until her own mother became a patient.
On the first day of retirement in October 2014, Johnson’s mother flatlined. Quick-thinking paramedics resuscitated her, and after several days in critical care, she was discharged. Since then, her heart has remained healthy. Johnson’s sister, who spent time worrying over her mother at the hospital, also had lingering effects. Both have since struggled, plagued by nightmares, flashbacks, and insomnia.
Johnson initially believed her mom’s and sister’s neuropsychiatric, post-ICU struggles were unique to her family. It was only a year later, at a seminar she was attending, that she first heard the words “post–intensive care syndrome.” Suddenly, Johnson had a name for her family’s experiences, and she began to create support groups and resources to help other families like hers.
“I thought of all the patients I had treated over the years who had been on ventilators for days and days and days. And if this happened to my mom after 48 hours, what must they be going through?” she asked.
Once physicians formally defined PICS, the Society for Critical Care Medicine helped create programs to educate ICU staff, patients, and families about potential post-discharge challenges. Researchers also began to investigate factors affecting post-ICU functioning. Follow-up studies of patients with delirium (ranging from general confusion about time and place to extreme agitation and violence) showed they had striking cognitive deficits. Problems with short-term memory, flexible thinking, and motivation plagued patients for years after their critical illness, similar to the physical deficiencies seen after ARDS. Delirium was one of the strongest risk factors for neuropsychiatric problems.
“Delirium is basically a stress test for the brain,” said Babar Khan, MD, a critical care specialist at Indiana University’s Regenstrief Institute, in Bloomington. But whether delirium accentuates preexisting cognitive difficulties or creates them afresh isn’t yet clear.
Sophia Wang, MD, a geriatric psychiatrist at Indiana University who works with many critical care patients, says patients who had experienced delirium in the ICU showed significant defects in memory and executive functioning long after their hospital stay. She points to a 2015 study that followed 47 ICU patients for a year post discharge. Among those who experienced delirium, brain volumes, as measured by MRI, were smaller at 3 months, something associated with cognitive problems at 1 year. Many struggled at work, and unemployment was common. Depression and posttraumatic stress compounded these difficulties. Among those with acute respiratory distress, ICU patients who are young, female, and unemployed are most likely to suffer from posttraumatic stress disorder after they are discharge.
Critical care medicine may have given these patients a second chance at life, Wang says, but the life they return to often looks nothing like the one they had before their illness.
Prolonged mechanical ventilation and the heavy sedation that often accompanies it are predictors of PICS severity. Some of these links could be explained by the gravity of the illness that landed someone in critical care, but others are more likely to be iatrogenic, says Gerald Weinhouse, MD, a pulmonology and critical care physician and co-director of the Critical Illness Recovery Program at the Brigham and Women’s Hospital in Boston. The involvement of loved ones at the patient’s bedside, however, improved the entire family’s outcome.
When Weinhouse saw those data, he and his colleagues founded a peer support program for ICU survivors. In a study published in 2019 in Critical Care Medicine, they identified six different models for peer support for those with PICS and their families, including both online and in-person approaches. An ongoing challenge for physicians, Weinhouse says, is getting patients to engage with these programs, given that their calendars are crowded with medical appointments and that they suffer from increased physical and mental disability.
Studies such as these led critical care physicians to form the ICU Liberation Collaborative to rethink critical care medicine. At Vanderbilt, Sevin and Jackson headed up one of the world’s first post-ICU clinics, which uses an interdisciplinary team to help patients maximize their functioning. They redesigned their critical care unit in a way that allows families to spend the night and that encourages patient mobility. Both Needham and Weinhouse continue tracking patient outcomes.
Even before the novel coronavirus struck, the United States — and the world — had begun to realize that graduating from the ICU was only the start of what was often an extensive recovery.
The long road back
When COVID-19 patients began flooding intensive care wards around the world, physicians scrambled to meet their complex and desperate acute medical needs. Over the past few months, physicians have focused on keeping these patients alive. “We’ve never seen anything like it ― not even during polio — with the sheer number of patients, all with respiratory distress,” Needham said.
But he and his colleagues know this is only the beginning.
“We’re aware that survivorship issues are coming. There’s going to be a wave of sick people who survived the coronavirus but are going to need more help,” Weinhouse said.
Intensivists have been drawing on PICS research in their fight to help COVID-19 patients. Work from the past few years has shown that although sedation is required during intubation itself, not everyone needs it while on a ventilator. Titrating down sedating medication helps reduce delirium, Wang says. Such medication has been shown to contribute to later cognitive problems. Needham’s studies showing that prolonged bedrest by ICU patients causes muscular atrophy has led him to encourage patients to move as much as possible. With the help of physical therapists, many patients on ventilators can be awake, alert, and moving around the ward.
One of the biggest challenges critical-care coronavirus patients face is prolonged isolation. The constant presence of a familiar face helps orient confused and delirious patients and provides emotional support during a frightening time. But because the immediate need for infection control outweighs these benefits, few hospitals allow visitors, especially for COVID-19 patients.
To address this, some units have been using video technology to allow loved ones to call in. At Johns Hopkins, physicians have also been relying on the expertise of occupational therapists (OTs). Needham says that one OT found that rubbing the hand and back of an agitated, delirious patient helped soothe and calm him better than many medications.
Ronan, who spent 5 days in intensive care, echoes that problem. She says she found the relative lack of human contact to be one of the most challenging parts of being in a bed on a COVID-19 ward. Separated from her husband and daughter, suffering from high fever and severe illness, she lost all track of time.
Her return home was difficult, too. Although her job as a home health nurse had prepared her on some level for the challenges she would face after discharge, Ronan says the hospital provided little practical help.
“Everything is so much harder at home, even little things like going to the bathroom,” she said. “I feel like I’m trying to bail out a sinking ship with a teacup.”
Khan and other physicians, aware of the challenges Ronan and others face once home, aim to create post-ICU clinics specifically for COVID-19 patients. They want to build what Khan calls a “one-stop shop” for all the support patients need to recover. Some of that can be provided via telehealth, which may also help ease the physical burden.
Because there’s so much physicians don’t know about the coronavirus, Johnson says, such clinics are not only a chance to help the sickest COVID-19 patients, they will also help researchers learn more about the virus and improve critical care for other illnesses.
Today, nearly 2 months after discharge, Ronan is back on the job but struggles with a persistent cough — likely due to the lung damage she sustained while ill. She has constant fatigue, as well as ongoing upset stomach from all the medications she took to reduce fever and body aches. When she dons a mask for work, the tangible reminder of her hospital stay sends her into a panic attack. Physically, she’s weaker than before.
Researchers are still trying to understand everything that Ronan and other COVID-19 patients need to move on with their lives after being in the ICU. Mysteries abound, but the ground laid by Sevin, Needham, Weinhouse, and others has provided a solid foundation on which to build.
This article first appeared on Medscape.com.
By the time she was discharged from a suburban New Jersey hospital on April 10, Kathleen Ronan thought the worst was behind her. For a week before her husband rushed her to the emergency department (ED), incoherent and struggling to breathe, the novel coronavirus had ravaged her body. She tried to treat her fevers with acetaminophen and ice packs. Despite taking enough Tylenol to risk liver damage and packing herself on ice like the catch of the day, Ronan’s fever continued to rise. By the time her temperature reached 104.5° F, Ronan knew the time had come for more drastic measures.
A team of masked and gowned nurses greeted her at a triage tent outside the ED, and from there, everything becomes hazy for Ronan. She was immediately rushed to the hospital’s special COVID-19 intensive care unit (ICU), where she spent 5 days. But she has few distinct memories from this time. What she does remember is the exhaustion, the pain, the loneliness, and the fear. Her family couldn’t visit, and though Ronan works as a home health nurse, her brain was so addled with fever that she couldn’t make sense of what was happening. After a week in the hospital, 5 days of which were spent in the ICU, 51-year-old Ronan was discharged.
Her years of working as a home health nurse told her that the return home wouldn’t be easy, but nothing prepared her for just how much she would struggle. The once-active Ronan, who had supplemented long days on her feet caring for others as a nurse with regular trips to the gym, now needed a walker to traverse the few steps from her bed to the toilet, an effort that left her gasping for air. Her brain couldn’t even focus on an audiobook, let alone a short magazine article.
“It just completely knocked the stuffing out of me,” Ronan said.
Ronan’s lingering symptoms aren’t unique to COVID-19 patients. In as many as 80% of patients leaving the ICU, . Although underlying illness plays a role in these symptoms, the amount of time spent in critical care is a major factor.
Nor is PICS simply a set of side effects that will go away on their own. It includes ongoing cognitive difficulties and physical weakness, both of which can lead to employment problems. Beyond that, depression and anxiety can exacerbate – and be exacerbated by – these challenges. Psychologist Jim Jackson, PsyD, assistant director of the ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tennessee, recently spoke with a former ICU patient who has struggled since her discharge 30 years ago.
“Her life essentially stopped with her critical care stay. She hasn’t been able to move forward,” he said. “She’s part of a whole fraternity of people who are struggling.”
The good news is that over the past decade, researchers have made important strides in understanding what makes PICS symptoms worse and how critical care physicians can tweak ICU protocols to reduce PICS severity. Practitioners will need to draw on this knowledge to help Ronan and the thousands of COVID-19 ICU patients like her.
Surviving the ICU
Although the new coronavirus has pushed the world’s critical care system to its limits, it was an outbreak in 1952 that inspired the creation of intensive care units. That summer, a wave of paralytic polio swept over Copenhagen, Denmark, and anesthesiologist Bjørn Ibsen, MD, PhD, used mechanical ventilation — physically operated by medical and dental students – to help 316 children breathe for weeks at a time while their small bodies worked to fight off the virus. The effort halved the mortality rate from polio that affected breathing, from 80% to 40%.
In these wards, dedicated to the very sickest, each patient was assigned his or her own nurse. Over the next decade, hospitals in the United Kingdom and the United States established their own ICUs to treat patients with a variety of conditions. Although it helped improve survival, mortality rates in critical care units remained stubbornly high, owing to the patients’ severe underlying illnesses.
“We thought we were doing a good job if the patient survived, but we had no idea what happened after discharge,” said Carla Sevin, MD, medical director of Vanderbilt’s ICU Recovery Center. Nor did their efforts to find out always bring answers. “We struggled to get people to come in for support — they were debilitated, physically burdened, and weak.”
Through further advances in life support, by the early 2000s, the average mortality rates in American ICUs had dropped to 8% to 19%. As the number of critical care survivors began to climb, clinical researchers noticed that the lives of these patients and their families were profoundly altered by their severe illness.
As Dale Needham, MD, PhD, began his pulmonology and critical care residency in Toronto, Canada, in 2005, a group of physicians there began a 5-year longitudinal study to assess long-term outcomes of patients who developed acute respiratory distress syndrome (ARDS). Although ARDS is an acute condition, the investigators found that patients felt effects for years. Younger patients recovered better than older ones, but none of the patients› physical functioning was equivalent to that of age-matched control persons. Even 5 years later, former ICU patients only reached 76% of expected physical functioning, according to results published in the New England Journal of Medicine. The study was a wake-up call.
At a meeting in Chicago in 2010, Needham, now an intensivist at Johns Hopkins Hospital in Baltimore, Maryland, gathered an interdisciplinary group of colleagues, including patients and caregivers, to clarify the phenomena they were seeing. What emerged from that meeting, published in 2012 in Critical Care Medicine, were the diagnostic criteria for PICS: According to the new definition, PICS is characterized by new or worsening physical and neuropsychiatric deficits that range from forgetfulness and loss of motivation to physical weakness and insomnia.
The issue, Needham says, is that although the trouble starts in the ICU, it only becomes clear once patients leave. “ICU doctors aren’t the ones dealing with this,” Needham said. “We need to build stronger bridges between critical care and other professions.” That’s where PICS comes in, a definition that exists explicitly to alert healthcare providers about the constellation of challenges many of these individuals face as they try to reenter “normal” life.
Defining the problem
As an ICU nurse at the Mayo Clinic in Rochester, Minnesota, Annie Johnson, ACNP-BC, knew lots about helping hospitalized patients, but she says she didn’t know anything about what to do after discharge – at least not until her own mother became a patient.
On the first day of retirement in October 2014, Johnson’s mother flatlined. Quick-thinking paramedics resuscitated her, and after several days in critical care, she was discharged. Since then, her heart has remained healthy. Johnson’s sister, who spent time worrying over her mother at the hospital, also had lingering effects. Both have since struggled, plagued by nightmares, flashbacks, and insomnia.
Johnson initially believed her mom’s and sister’s neuropsychiatric, post-ICU struggles were unique to her family. It was only a year later, at a seminar she was attending, that she first heard the words “post–intensive care syndrome.” Suddenly, Johnson had a name for her family’s experiences, and she began to create support groups and resources to help other families like hers.
“I thought of all the patients I had treated over the years who had been on ventilators for days and days and days. And if this happened to my mom after 48 hours, what must they be going through?” she asked.
Once physicians formally defined PICS, the Society for Critical Care Medicine helped create programs to educate ICU staff, patients, and families about potential post-discharge challenges. Researchers also began to investigate factors affecting post-ICU functioning. Follow-up studies of patients with delirium (ranging from general confusion about time and place to extreme agitation and violence) showed they had striking cognitive deficits. Problems with short-term memory, flexible thinking, and motivation plagued patients for years after their critical illness, similar to the physical deficiencies seen after ARDS. Delirium was one of the strongest risk factors for neuropsychiatric problems.
“Delirium is basically a stress test for the brain,” said Babar Khan, MD, a critical care specialist at Indiana University’s Regenstrief Institute, in Bloomington. But whether delirium accentuates preexisting cognitive difficulties or creates them afresh isn’t yet clear.
Sophia Wang, MD, a geriatric psychiatrist at Indiana University who works with many critical care patients, says patients who had experienced delirium in the ICU showed significant defects in memory and executive functioning long after their hospital stay. She points to a 2015 study that followed 47 ICU patients for a year post discharge. Among those who experienced delirium, brain volumes, as measured by MRI, were smaller at 3 months, something associated with cognitive problems at 1 year. Many struggled at work, and unemployment was common. Depression and posttraumatic stress compounded these difficulties. Among those with acute respiratory distress, ICU patients who are young, female, and unemployed are most likely to suffer from posttraumatic stress disorder after they are discharge.
Critical care medicine may have given these patients a second chance at life, Wang says, but the life they return to often looks nothing like the one they had before their illness.
Prolonged mechanical ventilation and the heavy sedation that often accompanies it are predictors of PICS severity. Some of these links could be explained by the gravity of the illness that landed someone in critical care, but others are more likely to be iatrogenic, says Gerald Weinhouse, MD, a pulmonology and critical care physician and co-director of the Critical Illness Recovery Program at the Brigham and Women’s Hospital in Boston. The involvement of loved ones at the patient’s bedside, however, improved the entire family’s outcome.
When Weinhouse saw those data, he and his colleagues founded a peer support program for ICU survivors. In a study published in 2019 in Critical Care Medicine, they identified six different models for peer support for those with PICS and their families, including both online and in-person approaches. An ongoing challenge for physicians, Weinhouse says, is getting patients to engage with these programs, given that their calendars are crowded with medical appointments and that they suffer from increased physical and mental disability.
Studies such as these led critical care physicians to form the ICU Liberation Collaborative to rethink critical care medicine. At Vanderbilt, Sevin and Jackson headed up one of the world’s first post-ICU clinics, which uses an interdisciplinary team to help patients maximize their functioning. They redesigned their critical care unit in a way that allows families to spend the night and that encourages patient mobility. Both Needham and Weinhouse continue tracking patient outcomes.
Even before the novel coronavirus struck, the United States — and the world — had begun to realize that graduating from the ICU was only the start of what was often an extensive recovery.
The long road back
When COVID-19 patients began flooding intensive care wards around the world, physicians scrambled to meet their complex and desperate acute medical needs. Over the past few months, physicians have focused on keeping these patients alive. “We’ve never seen anything like it ― not even during polio — with the sheer number of patients, all with respiratory distress,” Needham said.
But he and his colleagues know this is only the beginning.
“We’re aware that survivorship issues are coming. There’s going to be a wave of sick people who survived the coronavirus but are going to need more help,” Weinhouse said.
Intensivists have been drawing on PICS research in their fight to help COVID-19 patients. Work from the past few years has shown that although sedation is required during intubation itself, not everyone needs it while on a ventilator. Titrating down sedating medication helps reduce delirium, Wang says. Such medication has been shown to contribute to later cognitive problems. Needham’s studies showing that prolonged bedrest by ICU patients causes muscular atrophy has led him to encourage patients to move as much as possible. With the help of physical therapists, many patients on ventilators can be awake, alert, and moving around the ward.
One of the biggest challenges critical-care coronavirus patients face is prolonged isolation. The constant presence of a familiar face helps orient confused and delirious patients and provides emotional support during a frightening time. But because the immediate need for infection control outweighs these benefits, few hospitals allow visitors, especially for COVID-19 patients.
To address this, some units have been using video technology to allow loved ones to call in. At Johns Hopkins, physicians have also been relying on the expertise of occupational therapists (OTs). Needham says that one OT found that rubbing the hand and back of an agitated, delirious patient helped soothe and calm him better than many medications.
Ronan, who spent 5 days in intensive care, echoes that problem. She says she found the relative lack of human contact to be one of the most challenging parts of being in a bed on a COVID-19 ward. Separated from her husband and daughter, suffering from high fever and severe illness, she lost all track of time.
Her return home was difficult, too. Although her job as a home health nurse had prepared her on some level for the challenges she would face after discharge, Ronan says the hospital provided little practical help.
“Everything is so much harder at home, even little things like going to the bathroom,” she said. “I feel like I’m trying to bail out a sinking ship with a teacup.”
Khan and other physicians, aware of the challenges Ronan and others face once home, aim to create post-ICU clinics specifically for COVID-19 patients. They want to build what Khan calls a “one-stop shop” for all the support patients need to recover. Some of that can be provided via telehealth, which may also help ease the physical burden.
Because there’s so much physicians don’t know about the coronavirus, Johnson says, such clinics are not only a chance to help the sickest COVID-19 patients, they will also help researchers learn more about the virus and improve critical care for other illnesses.
Today, nearly 2 months after discharge, Ronan is back on the job but struggles with a persistent cough — likely due to the lung damage she sustained while ill. She has constant fatigue, as well as ongoing upset stomach from all the medications she took to reduce fever and body aches. When she dons a mask for work, the tangible reminder of her hospital stay sends her into a panic attack. Physically, she’s weaker than before.
Researchers are still trying to understand everything that Ronan and other COVID-19 patients need to move on with their lives after being in the ICU. Mysteries abound, but the ground laid by Sevin, Needham, Weinhouse, and others has provided a solid foundation on which to build.
This article first appeared on Medscape.com.
By the time she was discharged from a suburban New Jersey hospital on April 10, Kathleen Ronan thought the worst was behind her. For a week before her husband rushed her to the emergency department (ED), incoherent and struggling to breathe, the novel coronavirus had ravaged her body. She tried to treat her fevers with acetaminophen and ice packs. Despite taking enough Tylenol to risk liver damage and packing herself on ice like the catch of the day, Ronan’s fever continued to rise. By the time her temperature reached 104.5° F, Ronan knew the time had come for more drastic measures.
A team of masked and gowned nurses greeted her at a triage tent outside the ED, and from there, everything becomes hazy for Ronan. She was immediately rushed to the hospital’s special COVID-19 intensive care unit (ICU), where she spent 5 days. But she has few distinct memories from this time. What she does remember is the exhaustion, the pain, the loneliness, and the fear. Her family couldn’t visit, and though Ronan works as a home health nurse, her brain was so addled with fever that she couldn’t make sense of what was happening. After a week in the hospital, 5 days of which were spent in the ICU, 51-year-old Ronan was discharged.
Her years of working as a home health nurse told her that the return home wouldn’t be easy, but nothing prepared her for just how much she would struggle. The once-active Ronan, who had supplemented long days on her feet caring for others as a nurse with regular trips to the gym, now needed a walker to traverse the few steps from her bed to the toilet, an effort that left her gasping for air. Her brain couldn’t even focus on an audiobook, let alone a short magazine article.
“It just completely knocked the stuffing out of me,” Ronan said.
Ronan’s lingering symptoms aren’t unique to COVID-19 patients. In as many as 80% of patients leaving the ICU, . Although underlying illness plays a role in these symptoms, the amount of time spent in critical care is a major factor.
Nor is PICS simply a set of side effects that will go away on their own. It includes ongoing cognitive difficulties and physical weakness, both of which can lead to employment problems. Beyond that, depression and anxiety can exacerbate – and be exacerbated by – these challenges. Psychologist Jim Jackson, PsyD, assistant director of the ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tennessee, recently spoke with a former ICU patient who has struggled since her discharge 30 years ago.
“Her life essentially stopped with her critical care stay. She hasn’t been able to move forward,” he said. “She’s part of a whole fraternity of people who are struggling.”
The good news is that over the past decade, researchers have made important strides in understanding what makes PICS symptoms worse and how critical care physicians can tweak ICU protocols to reduce PICS severity. Practitioners will need to draw on this knowledge to help Ronan and the thousands of COVID-19 ICU patients like her.
Surviving the ICU
Although the new coronavirus has pushed the world’s critical care system to its limits, it was an outbreak in 1952 that inspired the creation of intensive care units. That summer, a wave of paralytic polio swept over Copenhagen, Denmark, and anesthesiologist Bjørn Ibsen, MD, PhD, used mechanical ventilation — physically operated by medical and dental students – to help 316 children breathe for weeks at a time while their small bodies worked to fight off the virus. The effort halved the mortality rate from polio that affected breathing, from 80% to 40%.
In these wards, dedicated to the very sickest, each patient was assigned his or her own nurse. Over the next decade, hospitals in the United Kingdom and the United States established their own ICUs to treat patients with a variety of conditions. Although it helped improve survival, mortality rates in critical care units remained stubbornly high, owing to the patients’ severe underlying illnesses.
“We thought we were doing a good job if the patient survived, but we had no idea what happened after discharge,” said Carla Sevin, MD, medical director of Vanderbilt’s ICU Recovery Center. Nor did their efforts to find out always bring answers. “We struggled to get people to come in for support — they were debilitated, physically burdened, and weak.”
Through further advances in life support, by the early 2000s, the average mortality rates in American ICUs had dropped to 8% to 19%. As the number of critical care survivors began to climb, clinical researchers noticed that the lives of these patients and their families were profoundly altered by their severe illness.
As Dale Needham, MD, PhD, began his pulmonology and critical care residency in Toronto, Canada, in 2005, a group of physicians there began a 5-year longitudinal study to assess long-term outcomes of patients who developed acute respiratory distress syndrome (ARDS). Although ARDS is an acute condition, the investigators found that patients felt effects for years. Younger patients recovered better than older ones, but none of the patients› physical functioning was equivalent to that of age-matched control persons. Even 5 years later, former ICU patients only reached 76% of expected physical functioning, according to results published in the New England Journal of Medicine. The study was a wake-up call.
At a meeting in Chicago in 2010, Needham, now an intensivist at Johns Hopkins Hospital in Baltimore, Maryland, gathered an interdisciplinary group of colleagues, including patients and caregivers, to clarify the phenomena they were seeing. What emerged from that meeting, published in 2012 in Critical Care Medicine, were the diagnostic criteria for PICS: According to the new definition, PICS is characterized by new or worsening physical and neuropsychiatric deficits that range from forgetfulness and loss of motivation to physical weakness and insomnia.
The issue, Needham says, is that although the trouble starts in the ICU, it only becomes clear once patients leave. “ICU doctors aren’t the ones dealing with this,” Needham said. “We need to build stronger bridges between critical care and other professions.” That’s where PICS comes in, a definition that exists explicitly to alert healthcare providers about the constellation of challenges many of these individuals face as they try to reenter “normal” life.
Defining the problem
As an ICU nurse at the Mayo Clinic in Rochester, Minnesota, Annie Johnson, ACNP-BC, knew lots about helping hospitalized patients, but she says she didn’t know anything about what to do after discharge – at least not until her own mother became a patient.
On the first day of retirement in October 2014, Johnson’s mother flatlined. Quick-thinking paramedics resuscitated her, and after several days in critical care, she was discharged. Since then, her heart has remained healthy. Johnson’s sister, who spent time worrying over her mother at the hospital, also had lingering effects. Both have since struggled, plagued by nightmares, flashbacks, and insomnia.
Johnson initially believed her mom’s and sister’s neuropsychiatric, post-ICU struggles were unique to her family. It was only a year later, at a seminar she was attending, that she first heard the words “post–intensive care syndrome.” Suddenly, Johnson had a name for her family’s experiences, and she began to create support groups and resources to help other families like hers.
“I thought of all the patients I had treated over the years who had been on ventilators for days and days and days. And if this happened to my mom after 48 hours, what must they be going through?” she asked.
Once physicians formally defined PICS, the Society for Critical Care Medicine helped create programs to educate ICU staff, patients, and families about potential post-discharge challenges. Researchers also began to investigate factors affecting post-ICU functioning. Follow-up studies of patients with delirium (ranging from general confusion about time and place to extreme agitation and violence) showed they had striking cognitive deficits. Problems with short-term memory, flexible thinking, and motivation plagued patients for years after their critical illness, similar to the physical deficiencies seen after ARDS. Delirium was one of the strongest risk factors for neuropsychiatric problems.
“Delirium is basically a stress test for the brain,” said Babar Khan, MD, a critical care specialist at Indiana University’s Regenstrief Institute, in Bloomington. But whether delirium accentuates preexisting cognitive difficulties or creates them afresh isn’t yet clear.
Sophia Wang, MD, a geriatric psychiatrist at Indiana University who works with many critical care patients, says patients who had experienced delirium in the ICU showed significant defects in memory and executive functioning long after their hospital stay. She points to a 2015 study that followed 47 ICU patients for a year post discharge. Among those who experienced delirium, brain volumes, as measured by MRI, were smaller at 3 months, something associated with cognitive problems at 1 year. Many struggled at work, and unemployment was common. Depression and posttraumatic stress compounded these difficulties. Among those with acute respiratory distress, ICU patients who are young, female, and unemployed are most likely to suffer from posttraumatic stress disorder after they are discharge.
Critical care medicine may have given these patients a second chance at life, Wang says, but the life they return to often looks nothing like the one they had before their illness.
Prolonged mechanical ventilation and the heavy sedation that often accompanies it are predictors of PICS severity. Some of these links could be explained by the gravity of the illness that landed someone in critical care, but others are more likely to be iatrogenic, says Gerald Weinhouse, MD, a pulmonology and critical care physician and co-director of the Critical Illness Recovery Program at the Brigham and Women’s Hospital in Boston. The involvement of loved ones at the patient’s bedside, however, improved the entire family’s outcome.
When Weinhouse saw those data, he and his colleagues founded a peer support program for ICU survivors. In a study published in 2019 in Critical Care Medicine, they identified six different models for peer support for those with PICS and their families, including both online and in-person approaches. An ongoing challenge for physicians, Weinhouse says, is getting patients to engage with these programs, given that their calendars are crowded with medical appointments and that they suffer from increased physical and mental disability.
Studies such as these led critical care physicians to form the ICU Liberation Collaborative to rethink critical care medicine. At Vanderbilt, Sevin and Jackson headed up one of the world’s first post-ICU clinics, which uses an interdisciplinary team to help patients maximize their functioning. They redesigned their critical care unit in a way that allows families to spend the night and that encourages patient mobility. Both Needham and Weinhouse continue tracking patient outcomes.
Even before the novel coronavirus struck, the United States — and the world — had begun to realize that graduating from the ICU was only the start of what was often an extensive recovery.
The long road back
When COVID-19 patients began flooding intensive care wards around the world, physicians scrambled to meet their complex and desperate acute medical needs. Over the past few months, physicians have focused on keeping these patients alive. “We’ve never seen anything like it ― not even during polio — with the sheer number of patients, all with respiratory distress,” Needham said.
But he and his colleagues know this is only the beginning.
“We’re aware that survivorship issues are coming. There’s going to be a wave of sick people who survived the coronavirus but are going to need more help,” Weinhouse said.
Intensivists have been drawing on PICS research in their fight to help COVID-19 patients. Work from the past few years has shown that although sedation is required during intubation itself, not everyone needs it while on a ventilator. Titrating down sedating medication helps reduce delirium, Wang says. Such medication has been shown to contribute to later cognitive problems. Needham’s studies showing that prolonged bedrest by ICU patients causes muscular atrophy has led him to encourage patients to move as much as possible. With the help of physical therapists, many patients on ventilators can be awake, alert, and moving around the ward.
One of the biggest challenges critical-care coronavirus patients face is prolonged isolation. The constant presence of a familiar face helps orient confused and delirious patients and provides emotional support during a frightening time. But because the immediate need for infection control outweighs these benefits, few hospitals allow visitors, especially for COVID-19 patients.
To address this, some units have been using video technology to allow loved ones to call in. At Johns Hopkins, physicians have also been relying on the expertise of occupational therapists (OTs). Needham says that one OT found that rubbing the hand and back of an agitated, delirious patient helped soothe and calm him better than many medications.
Ronan, who spent 5 days in intensive care, echoes that problem. She says she found the relative lack of human contact to be one of the most challenging parts of being in a bed on a COVID-19 ward. Separated from her husband and daughter, suffering from high fever and severe illness, she lost all track of time.
Her return home was difficult, too. Although her job as a home health nurse had prepared her on some level for the challenges she would face after discharge, Ronan says the hospital provided little practical help.
“Everything is so much harder at home, even little things like going to the bathroom,” she said. “I feel like I’m trying to bail out a sinking ship with a teacup.”
Khan and other physicians, aware of the challenges Ronan and others face once home, aim to create post-ICU clinics specifically for COVID-19 patients. They want to build what Khan calls a “one-stop shop” for all the support patients need to recover. Some of that can be provided via telehealth, which may also help ease the physical burden.
Because there’s so much physicians don’t know about the coronavirus, Johnson says, such clinics are not only a chance to help the sickest COVID-19 patients, they will also help researchers learn more about the virus and improve critical care for other illnesses.
Today, nearly 2 months after discharge, Ronan is back on the job but struggles with a persistent cough — likely due to the lung damage she sustained while ill. She has constant fatigue, as well as ongoing upset stomach from all the medications she took to reduce fever and body aches. When she dons a mask for work, the tangible reminder of her hospital stay sends her into a panic attack. Physically, she’s weaker than before.
Researchers are still trying to understand everything that Ronan and other COVID-19 patients need to move on with their lives after being in the ICU. Mysteries abound, but the ground laid by Sevin, Needham, Weinhouse, and others has provided a solid foundation on which to build.
This article first appeared on Medscape.com.
CVD risk continues to fall down to systolic BP of 90 mm HG
The study analyzed data from a cohort of 1,457 participants (mean age, 58 years) who did not have any traditional cardiovascular risk factors and had a systolic blood pressure level between 90 and 129 mm Hg at baseline. Results showed that, during a mean follow-up of 14.5 years, there was an increase in traditional cardiovascular risk factors, coronary artery calcium, and incident cardiovascular events with increasing systolic blood pressure levels.
“We modeled systolic blood pressure on a continuous scale and saw the risk increasing in a linear fashion as blood pressure increased and this occurred right down to 90 mm Hg. We didn’t see any nadir or J-point where there may be an increased risk at lower pressures,” said lead author Seamus Whelton, MD.
Dr. Whelton is assistant professor of medicine at the division of cardiology at Johns Hopkins Medicine, Baltimore. He is the son of Paul Whelton, MD, chair of the 2017 American College of Cardiology/American Heart Association hypertension guideline writing committee.
“From an individual level we can now say that in healthy individuals, a systolic pressure in the 90s is not too low. It is a positive thing. And it is recommended to try and keep systolic pressure at these levels if possible by maintaining a healthy lifestyle,” Dr. Whelton said in an interview. “At a population level this finding could lead to stronger recommendations on interventions to prevent increasing blood pressure such as healthier diets, reducing sodium intake, and increasing exercise. Small changes in blood pressure on a population level will lead to large changes in cardiovascular risk on a population a level.”
The study was published online in JAMA Cardiology on June 10.
The researchers noted that populations in nonindustrialized countries have little to no increase in systolic blood pressure levels with age, while systolic blood pressure levels typically increase with age in countries with industrialized diets and lifestyles. This has important implications, because atherosclerosis is a slowly progressive disease and the lower an individual’s lifetime exposure to cardiovascular risk factors, such as increased systolic blood pressure, the lower their probable risk for a future cardiovascular event, they wrote.
While the association between systolic blood pressure level, coronary artery calcium, and atherosclerotic cardiovascular disease is well established at higher blood pressure levels, optimal systolic pressure levels for a healthy adult and whether there is a J-shaped relationship or lower limit of systolic pressure necessary to maintain adequate organ perfusion has been uncertain, they explained.
In addition, prior studies have typically used a reference systolic pressure of less than 115-120 mm Hg to define a normal level, and it is uncertain whether there is a lower level at which the risk for incident cardiovascular disease plateaus or increases.
To investigate this, they analyzed data from the Multi-Ethnic Study of Atherosclerosis, a community-based, multiethnic cohort free from known cardiovascular disease at enrollment. The current analysis included individuals with a systolic blood pressure between 90 and 129 mm Hg without other traditional cardiovascular risk factors including dyslipidemia (LDL cholesterol >160 mg/dL or HDL cholesterol <40 mg/dL), diabetes, or current tobacco use.
Results showed an adjusted hazard ratio for atherosclerotic cardiovascular disease was 1.53 for every 10 mm Hg increase in systolic blood pressure levels.
Compared with people with systolic pressures of 90-99 mm Hg, the adjusted hazard ratio for atherosclerotic cardiovascular disease risk was 3.00 for those with 100-109 mm Hg, 3.10 for those with 110-119 mm Hg, and 4.58 for those with 120-129 mm Hg.
There was also a graded increase in the prevalence of coronary artery calcium starting from systolic blood pressure levels as low as 90 mm Hg.
“Previous research on the J-shaped curve for blood pressure has primarily focused on diastolic pressure. We did control for diastolic pressure in this analysis but that was not the focus,” Dr. Whelton said. “Obviously, there will be a minimum optimum value for both diastolic and systolic pressure. But from this study we can say that for systolic pressure, that minimum recommended value is below 90 mm Hg.”
In terms of implications, the researchers wrote: “Among individuals at low or intermediate atherosclerotic cardiovascular risk, it may be more efficacious to focus on a life-course approach for preventing an increase in systolic blood pressure levels rather than treatment of established hypertension to lower systolic blood pressure levels.”
What is a normal blood pressure?
In an accompanying commentary, Daniel Jones, MD, of the University of Mississippi Medical Center, Jackson, said these new findings support the position that risk imposed by blood pressure level begins well below the current 130/80 mm Hg definition of hypertension and guideline-recommended goal.
The study is “a reminder that even a good execution of treatment of hypertension is far from an ideal way to prevent atherosclerotic cardiovascular disease,” he said.
“A systolic of 130 is not the number we should focus on for patients who are not yet hypertensive, as 130 is not a normal blood pressure,” Dr. Jones added in an audio interview on the JAMA website.
“The findings also suggest that the disease process for atherosclerotic cardiovascular disease begins early in life and support the importance of primordial prevention through a healthy lifestyle, including a healthy diet and levels of physical activity. In addition, the findings highlight the need for a population-based strategy focusing on primordial prevention to reduce the age-related increase in BP reported in all industrialized societies,” Dr. Jones wrote.
He recommended that clinicians encourage a healthy lifestyle in patients and families of patients with cardiovascular disease. “This intervention requires no sophisticated genetic testing or clinical trials to credibly inform a family that the children and grandchildren of a patient with atherosclerotic cardiovascular disease or risk factors will benefit from a healthy lifestyle beginning at the earliest age.
“Clinicians often lose sight of the big picture with regard to blood pressure because they have the patient in front of them. But that patient has children and grandchildren who may share the risk and may be in a better position with regard to prevention of future [coronary artery disease], stroke, and kidney disease,” he said.
Conducting the JAMA audio interview, Clyde Yancy, MD, chief of cardiology at Northwestern University, Chicago, said that “this is very stimulating research. It is not asking the question of what is the target blood pressure for patients with hypertension, but rather: What is the goal blood pressure if you actually want to avoid atherosclerotic cardiovascular disease risk altogether?
“These data have made us understand that there is a difference between the goal blood pressure reduction and treatment thresholds that we respect, the normative blood pressure values we see in a clinical setting, and what is truly normal blood pressure,” Dr. Yancy concluded. “That is a very important nuance, especially when we’re talking about population health. Families and communities need to understand what the true normal is.”
A version of this article originally appeared on Medscape.com.
The study analyzed data from a cohort of 1,457 participants (mean age, 58 years) who did not have any traditional cardiovascular risk factors and had a systolic blood pressure level between 90 and 129 mm Hg at baseline. Results showed that, during a mean follow-up of 14.5 years, there was an increase in traditional cardiovascular risk factors, coronary artery calcium, and incident cardiovascular events with increasing systolic blood pressure levels.
“We modeled systolic blood pressure on a continuous scale and saw the risk increasing in a linear fashion as blood pressure increased and this occurred right down to 90 mm Hg. We didn’t see any nadir or J-point where there may be an increased risk at lower pressures,” said lead author Seamus Whelton, MD.
Dr. Whelton is assistant professor of medicine at the division of cardiology at Johns Hopkins Medicine, Baltimore. He is the son of Paul Whelton, MD, chair of the 2017 American College of Cardiology/American Heart Association hypertension guideline writing committee.
“From an individual level we can now say that in healthy individuals, a systolic pressure in the 90s is not too low. It is a positive thing. And it is recommended to try and keep systolic pressure at these levels if possible by maintaining a healthy lifestyle,” Dr. Whelton said in an interview. “At a population level this finding could lead to stronger recommendations on interventions to prevent increasing blood pressure such as healthier diets, reducing sodium intake, and increasing exercise. Small changes in blood pressure on a population level will lead to large changes in cardiovascular risk on a population a level.”
The study was published online in JAMA Cardiology on June 10.
The researchers noted that populations in nonindustrialized countries have little to no increase in systolic blood pressure levels with age, while systolic blood pressure levels typically increase with age in countries with industrialized diets and lifestyles. This has important implications, because atherosclerosis is a slowly progressive disease and the lower an individual’s lifetime exposure to cardiovascular risk factors, such as increased systolic blood pressure, the lower their probable risk for a future cardiovascular event, they wrote.
While the association between systolic blood pressure level, coronary artery calcium, and atherosclerotic cardiovascular disease is well established at higher blood pressure levels, optimal systolic pressure levels for a healthy adult and whether there is a J-shaped relationship or lower limit of systolic pressure necessary to maintain adequate organ perfusion has been uncertain, they explained.
In addition, prior studies have typically used a reference systolic pressure of less than 115-120 mm Hg to define a normal level, and it is uncertain whether there is a lower level at which the risk for incident cardiovascular disease plateaus or increases.
To investigate this, they analyzed data from the Multi-Ethnic Study of Atherosclerosis, a community-based, multiethnic cohort free from known cardiovascular disease at enrollment. The current analysis included individuals with a systolic blood pressure between 90 and 129 mm Hg without other traditional cardiovascular risk factors including dyslipidemia (LDL cholesterol >160 mg/dL or HDL cholesterol <40 mg/dL), diabetes, or current tobacco use.
Results showed an adjusted hazard ratio for atherosclerotic cardiovascular disease was 1.53 for every 10 mm Hg increase in systolic blood pressure levels.
Compared with people with systolic pressures of 90-99 mm Hg, the adjusted hazard ratio for atherosclerotic cardiovascular disease risk was 3.00 for those with 100-109 mm Hg, 3.10 for those with 110-119 mm Hg, and 4.58 for those with 120-129 mm Hg.
There was also a graded increase in the prevalence of coronary artery calcium starting from systolic blood pressure levels as low as 90 mm Hg.
“Previous research on the J-shaped curve for blood pressure has primarily focused on diastolic pressure. We did control for diastolic pressure in this analysis but that was not the focus,” Dr. Whelton said. “Obviously, there will be a minimum optimum value for both diastolic and systolic pressure. But from this study we can say that for systolic pressure, that minimum recommended value is below 90 mm Hg.”
In terms of implications, the researchers wrote: “Among individuals at low or intermediate atherosclerotic cardiovascular risk, it may be more efficacious to focus on a life-course approach for preventing an increase in systolic blood pressure levels rather than treatment of established hypertension to lower systolic blood pressure levels.”
What is a normal blood pressure?
In an accompanying commentary, Daniel Jones, MD, of the University of Mississippi Medical Center, Jackson, said these new findings support the position that risk imposed by blood pressure level begins well below the current 130/80 mm Hg definition of hypertension and guideline-recommended goal.
The study is “a reminder that even a good execution of treatment of hypertension is far from an ideal way to prevent atherosclerotic cardiovascular disease,” he said.
“A systolic of 130 is not the number we should focus on for patients who are not yet hypertensive, as 130 is not a normal blood pressure,” Dr. Jones added in an audio interview on the JAMA website.
“The findings also suggest that the disease process for atherosclerotic cardiovascular disease begins early in life and support the importance of primordial prevention through a healthy lifestyle, including a healthy diet and levels of physical activity. In addition, the findings highlight the need for a population-based strategy focusing on primordial prevention to reduce the age-related increase in BP reported in all industrialized societies,” Dr. Jones wrote.
He recommended that clinicians encourage a healthy lifestyle in patients and families of patients with cardiovascular disease. “This intervention requires no sophisticated genetic testing or clinical trials to credibly inform a family that the children and grandchildren of a patient with atherosclerotic cardiovascular disease or risk factors will benefit from a healthy lifestyle beginning at the earliest age.
“Clinicians often lose sight of the big picture with regard to blood pressure because they have the patient in front of them. But that patient has children and grandchildren who may share the risk and may be in a better position with regard to prevention of future [coronary artery disease], stroke, and kidney disease,” he said.
Conducting the JAMA audio interview, Clyde Yancy, MD, chief of cardiology at Northwestern University, Chicago, said that “this is very stimulating research. It is not asking the question of what is the target blood pressure for patients with hypertension, but rather: What is the goal blood pressure if you actually want to avoid atherosclerotic cardiovascular disease risk altogether?
“These data have made us understand that there is a difference between the goal blood pressure reduction and treatment thresholds that we respect, the normative blood pressure values we see in a clinical setting, and what is truly normal blood pressure,” Dr. Yancy concluded. “That is a very important nuance, especially when we’re talking about population health. Families and communities need to understand what the true normal is.”
A version of this article originally appeared on Medscape.com.
The study analyzed data from a cohort of 1,457 participants (mean age, 58 years) who did not have any traditional cardiovascular risk factors and had a systolic blood pressure level between 90 and 129 mm Hg at baseline. Results showed that, during a mean follow-up of 14.5 years, there was an increase in traditional cardiovascular risk factors, coronary artery calcium, and incident cardiovascular events with increasing systolic blood pressure levels.
“We modeled systolic blood pressure on a continuous scale and saw the risk increasing in a linear fashion as blood pressure increased and this occurred right down to 90 mm Hg. We didn’t see any nadir or J-point where there may be an increased risk at lower pressures,” said lead author Seamus Whelton, MD.
Dr. Whelton is assistant professor of medicine at the division of cardiology at Johns Hopkins Medicine, Baltimore. He is the son of Paul Whelton, MD, chair of the 2017 American College of Cardiology/American Heart Association hypertension guideline writing committee.
“From an individual level we can now say that in healthy individuals, a systolic pressure in the 90s is not too low. It is a positive thing. And it is recommended to try and keep systolic pressure at these levels if possible by maintaining a healthy lifestyle,” Dr. Whelton said in an interview. “At a population level this finding could lead to stronger recommendations on interventions to prevent increasing blood pressure such as healthier diets, reducing sodium intake, and increasing exercise. Small changes in blood pressure on a population level will lead to large changes in cardiovascular risk on a population a level.”
The study was published online in JAMA Cardiology on June 10.
The researchers noted that populations in nonindustrialized countries have little to no increase in systolic blood pressure levels with age, while systolic blood pressure levels typically increase with age in countries with industrialized diets and lifestyles. This has important implications, because atherosclerosis is a slowly progressive disease and the lower an individual’s lifetime exposure to cardiovascular risk factors, such as increased systolic blood pressure, the lower their probable risk for a future cardiovascular event, they wrote.
While the association between systolic blood pressure level, coronary artery calcium, and atherosclerotic cardiovascular disease is well established at higher blood pressure levels, optimal systolic pressure levels for a healthy adult and whether there is a J-shaped relationship or lower limit of systolic pressure necessary to maintain adequate organ perfusion has been uncertain, they explained.
In addition, prior studies have typically used a reference systolic pressure of less than 115-120 mm Hg to define a normal level, and it is uncertain whether there is a lower level at which the risk for incident cardiovascular disease plateaus or increases.
To investigate this, they analyzed data from the Multi-Ethnic Study of Atherosclerosis, a community-based, multiethnic cohort free from known cardiovascular disease at enrollment. The current analysis included individuals with a systolic blood pressure between 90 and 129 mm Hg without other traditional cardiovascular risk factors including dyslipidemia (LDL cholesterol >160 mg/dL or HDL cholesterol <40 mg/dL), diabetes, or current tobacco use.
Results showed an adjusted hazard ratio for atherosclerotic cardiovascular disease was 1.53 for every 10 mm Hg increase in systolic blood pressure levels.
Compared with people with systolic pressures of 90-99 mm Hg, the adjusted hazard ratio for atherosclerotic cardiovascular disease risk was 3.00 for those with 100-109 mm Hg, 3.10 for those with 110-119 mm Hg, and 4.58 for those with 120-129 mm Hg.
There was also a graded increase in the prevalence of coronary artery calcium starting from systolic blood pressure levels as low as 90 mm Hg.
“Previous research on the J-shaped curve for blood pressure has primarily focused on diastolic pressure. We did control for diastolic pressure in this analysis but that was not the focus,” Dr. Whelton said. “Obviously, there will be a minimum optimum value for both diastolic and systolic pressure. But from this study we can say that for systolic pressure, that minimum recommended value is below 90 mm Hg.”
In terms of implications, the researchers wrote: “Among individuals at low or intermediate atherosclerotic cardiovascular risk, it may be more efficacious to focus on a life-course approach for preventing an increase in systolic blood pressure levels rather than treatment of established hypertension to lower systolic blood pressure levels.”
What is a normal blood pressure?
In an accompanying commentary, Daniel Jones, MD, of the University of Mississippi Medical Center, Jackson, said these new findings support the position that risk imposed by blood pressure level begins well below the current 130/80 mm Hg definition of hypertension and guideline-recommended goal.
The study is “a reminder that even a good execution of treatment of hypertension is far from an ideal way to prevent atherosclerotic cardiovascular disease,” he said.
“A systolic of 130 is not the number we should focus on for patients who are not yet hypertensive, as 130 is not a normal blood pressure,” Dr. Jones added in an audio interview on the JAMA website.
“The findings also suggest that the disease process for atherosclerotic cardiovascular disease begins early in life and support the importance of primordial prevention through a healthy lifestyle, including a healthy diet and levels of physical activity. In addition, the findings highlight the need for a population-based strategy focusing on primordial prevention to reduce the age-related increase in BP reported in all industrialized societies,” Dr. Jones wrote.
He recommended that clinicians encourage a healthy lifestyle in patients and families of patients with cardiovascular disease. “This intervention requires no sophisticated genetic testing or clinical trials to credibly inform a family that the children and grandchildren of a patient with atherosclerotic cardiovascular disease or risk factors will benefit from a healthy lifestyle beginning at the earliest age.
“Clinicians often lose sight of the big picture with regard to blood pressure because they have the patient in front of them. But that patient has children and grandchildren who may share the risk and may be in a better position with regard to prevention of future [coronary artery disease], stroke, and kidney disease,” he said.
Conducting the JAMA audio interview, Clyde Yancy, MD, chief of cardiology at Northwestern University, Chicago, said that “this is very stimulating research. It is not asking the question of what is the target blood pressure for patients with hypertension, but rather: What is the goal blood pressure if you actually want to avoid atherosclerotic cardiovascular disease risk altogether?
“These data have made us understand that there is a difference between the goal blood pressure reduction and treatment thresholds that we respect, the normative blood pressure values we see in a clinical setting, and what is truly normal blood pressure,” Dr. Yancy concluded. “That is a very important nuance, especially when we’re talking about population health. Families and communities need to understand what the true normal is.”
A version of this article originally appeared on Medscape.com.
Asthma leads spending on avoidable pediatric inpatient stays
according to the Agency for Healthcare Research and Quality.
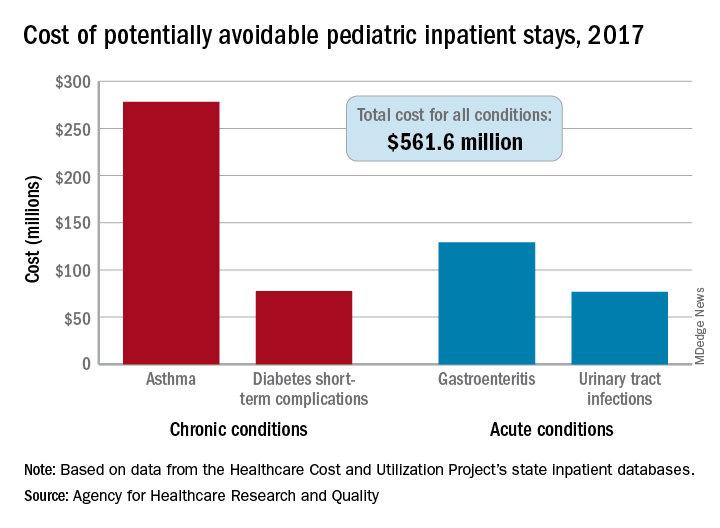
The cost of potentially avoidable visits for asthma that year was $278 million, versus $284 million combined for the other three conditions “that evidence suggests may be avoidable, in part, through timely and quality primary and preventive care,” Kimberly W. McDermott, PhD, and H. Joanna Jiang, PhD, said in an AHRQ statistical brief.
Those three other conditions are diabetes short-term complications, gastroenteritis, and urinary tract infections (UTIs). Neonatal stays were excluded from the analysis, Dr. McDermott of IBM Watson Health and Dr. Jiang of the AHRQ noted.
The state inpatient databases of the AHRQ’s Healthcare Cost and Utilization Project included 1.4 million inpatient stays among children aged 3 months to 17 years in 2017, of which 8% (108,300) were deemed potentially preventable. Hospital charges for the preventable stays came to $561.6 million, or 3% of the $20 billion in total costs for all nonneonatal stays, they said.
Rates of potentially avoidable stays for asthma (159 per 100,000 population), gastroenteritis (90 per 100,000), and UTIs (41 per 100,000) were highest for children aged 0-4 years and generally decreased with age, but diabetes stays increased with age, rising from 12 per 100,000 in children aged 5-9 years to 38 per 100,000 for those 15-17 years old, the researchers said.
Black children had a much higher rate of potentially avoidable stays for asthma (218 per 100,000) than did Hispanic children (74), Asian/Pacific Islander children (46), or white children (43), but children classified as other race/ethnicity were higher still: 380 per 100,000. Rates for children classified as other race/ethnicity were highest for the other three conditions as well, they reported.
Comparisons by sex for the four conditions ended up in a 2-2 tie: Girls had higher rates for diabetes (28 vs. 23) and UTIs (35 vs. 8), and boys had higher rates for asthma (96 vs. 67) and gastroenteritis (38 vs. 35), Dr. McDermott and Dr. Jiang reported.
SOURCE: McDermott KW, Jiang HJ. HCUP Statistical Brief #259. June 2020.
according to the Agency for Healthcare Research and Quality.

The cost of potentially avoidable visits for asthma that year was $278 million, versus $284 million combined for the other three conditions “that evidence suggests may be avoidable, in part, through timely and quality primary and preventive care,” Kimberly W. McDermott, PhD, and H. Joanna Jiang, PhD, said in an AHRQ statistical brief.
Those three other conditions are diabetes short-term complications, gastroenteritis, and urinary tract infections (UTIs). Neonatal stays were excluded from the analysis, Dr. McDermott of IBM Watson Health and Dr. Jiang of the AHRQ noted.
The state inpatient databases of the AHRQ’s Healthcare Cost and Utilization Project included 1.4 million inpatient stays among children aged 3 months to 17 years in 2017, of which 8% (108,300) were deemed potentially preventable. Hospital charges for the preventable stays came to $561.6 million, or 3% of the $20 billion in total costs for all nonneonatal stays, they said.
Rates of potentially avoidable stays for asthma (159 per 100,000 population), gastroenteritis (90 per 100,000), and UTIs (41 per 100,000) were highest for children aged 0-4 years and generally decreased with age, but diabetes stays increased with age, rising from 12 per 100,000 in children aged 5-9 years to 38 per 100,000 for those 15-17 years old, the researchers said.
Black children had a much higher rate of potentially avoidable stays for asthma (218 per 100,000) than did Hispanic children (74), Asian/Pacific Islander children (46), or white children (43), but children classified as other race/ethnicity were higher still: 380 per 100,000. Rates for children classified as other race/ethnicity were highest for the other three conditions as well, they reported.
Comparisons by sex for the four conditions ended up in a 2-2 tie: Girls had higher rates for diabetes (28 vs. 23) and UTIs (35 vs. 8), and boys had higher rates for asthma (96 vs. 67) and gastroenteritis (38 vs. 35), Dr. McDermott and Dr. Jiang reported.
SOURCE: McDermott KW, Jiang HJ. HCUP Statistical Brief #259. June 2020.
according to the Agency for Healthcare Research and Quality.

The cost of potentially avoidable visits for asthma that year was $278 million, versus $284 million combined for the other three conditions “that evidence suggests may be avoidable, in part, through timely and quality primary and preventive care,” Kimberly W. McDermott, PhD, and H. Joanna Jiang, PhD, said in an AHRQ statistical brief.
Those three other conditions are diabetes short-term complications, gastroenteritis, and urinary tract infections (UTIs). Neonatal stays were excluded from the analysis, Dr. McDermott of IBM Watson Health and Dr. Jiang of the AHRQ noted.
The state inpatient databases of the AHRQ’s Healthcare Cost and Utilization Project included 1.4 million inpatient stays among children aged 3 months to 17 years in 2017, of which 8% (108,300) were deemed potentially preventable. Hospital charges for the preventable stays came to $561.6 million, or 3% of the $20 billion in total costs for all nonneonatal stays, they said.
Rates of potentially avoidable stays for asthma (159 per 100,000 population), gastroenteritis (90 per 100,000), and UTIs (41 per 100,000) were highest for children aged 0-4 years and generally decreased with age, but diabetes stays increased with age, rising from 12 per 100,000 in children aged 5-9 years to 38 per 100,000 for those 15-17 years old, the researchers said.
Black children had a much higher rate of potentially avoidable stays for asthma (218 per 100,000) than did Hispanic children (74), Asian/Pacific Islander children (46), or white children (43), but children classified as other race/ethnicity were higher still: 380 per 100,000. Rates for children classified as other race/ethnicity were highest for the other three conditions as well, they reported.
Comparisons by sex for the four conditions ended up in a 2-2 tie: Girls had higher rates for diabetes (28 vs. 23) and UTIs (35 vs. 8), and boys had higher rates for asthma (96 vs. 67) and gastroenteritis (38 vs. 35), Dr. McDermott and Dr. Jiang reported.
SOURCE: McDermott KW, Jiang HJ. HCUP Statistical Brief #259. June 2020.
The evolution of “COVIDists”
Adapting to the demands placed on hospital resources by COVID-19
The challenges posed by COVID-19 have crippled health care systems around the globe. By February 2020, the first outbreak in the United States had been set off in Washington State. We quickly became the world’s epicenter of the epidemic, with over 1.8 million patients and over 110,000 deaths.1 The rapidity of spread and the severity of the disease created a tremendous strain on resources. It blindsided policymakers and hospital administrators, which left little time to react to the challenges placed on hospital operations all over the country.
The necessity of a new care model
Although health systems in the United States are adept in managing complications of common seasonal viral respiratory illnesses, COVID-19 presented an entirely different challenge with its significantly higher mortality rate. A respiratory disease turning into a multiorgan disease that causes debilitating cardiac, renal, neurological, hematological, and psychosocial complications2 was not something we had experience managing effectively. Additional challenges included a massive surge of COVID-19 patients, a limited supply of personal protective equipment (PPE), an inadequate number of intensivists for managing the anticipated ventilated patients, and most importantly, the potential of losing some of our workforce if they became infected.
Based on the experiences in China and Italy, and various predictive models, the division of hospital medicine at Baystate Health quickly realized the necessity of a new model of care for COVID-19 patients. We came up with an elaborate plan to manage the disease burden and the strain on resources effectively. The measures we put in place could be broadly divided into three categories following the timeline of the disease: the preparatory phase, the execution phase, and the maintenance phase.
The preparatory phase: From “Hospitalists” to “COVIDists”
As in most hospitals around the country, hospitalists are the backbone of inpatient clinical operations at our health system. A focused group of 10 hospitalists who volunteered to take care of COVID-19 patients with a particular interest in the pandemic and experience in critical care were selected, and the term “COVIDists” was coined to refer to them.
COVIDists were trained in various treatment protocols and ongoing clinical trials. They were given refresher training in Advanced Cardiac Life Support (ACLS) and Fundamental Critical Care Support (FCCS) courses and were taught in critical care/ventilator management by the intensivists through rapid indoctrination in the ICU. All of them had their N-95 mask fitting updated and were trained in the safe donning and doffing of all kinds of PPE by PPE coaches. The palliative care team trained them in conducting end-of-life/code status discussions with a focus on being unable to speak with family members at the bedside. COVIDists were also assigned as Code Blue leaders for any “COVID code blue” in the hospital.
In addition to the rapid training course, COVID-related updates were disseminated daily using three different modalities: brief huddles at the start of the day with the COVIDists; a COVID-19 newsletter summarizing daily updates, new treatments, strategies, and policies; and a WhatsApp group for instantly broadcasting information to the COVIDists (Table 1).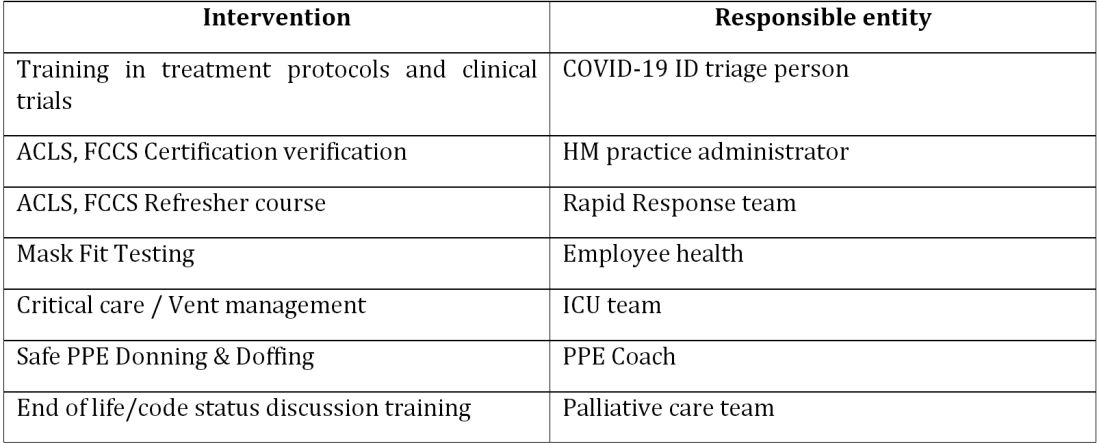
The execution phase
All the hospitalized COVID-19 patients were grouped together to COVID units, and the COVIDists were deployed to those units geographically. COVIDists were given lighter than usual patient loads to deal with the extra time needed for donning and doffing of PPE and for coordination with specialists. COVIDists were almost the only clinicians physically visiting the patients in most cases, and they became the “eyes and ears” of specialists since the specialists were advised to minimize exposure and pursue telemedicine consults. The COVIDists were also undertaking the most challenging part of the care – talking to families about end-of-life issues and the futility of aggressive care in certain patients with preexisting conditions.
Some COVIDists were deployed to the ICU to work alongside the intensivists and became an invaluable resource in ICU management when the ICU census skyrocketed during the initial phase of the outbreak. This helped in tiding the health system over during the initial crisis. Within a short time, we shifted away from an early intubation strategy, and most of the ICU patients were managed in the intermediate care units on high flow oxygen along with the awake-proning protocol. The COVIDists exclusively managed these units. They led multidisciplinary rounds two times a day with the ICU, rapid response team (RRT), the palliative care team, and the nursing team. This step drastically decreased the number of intubations, RRT activations, reduced ICU census,3 and helped with hospital capacity and patient flow (Tables 2 and 3).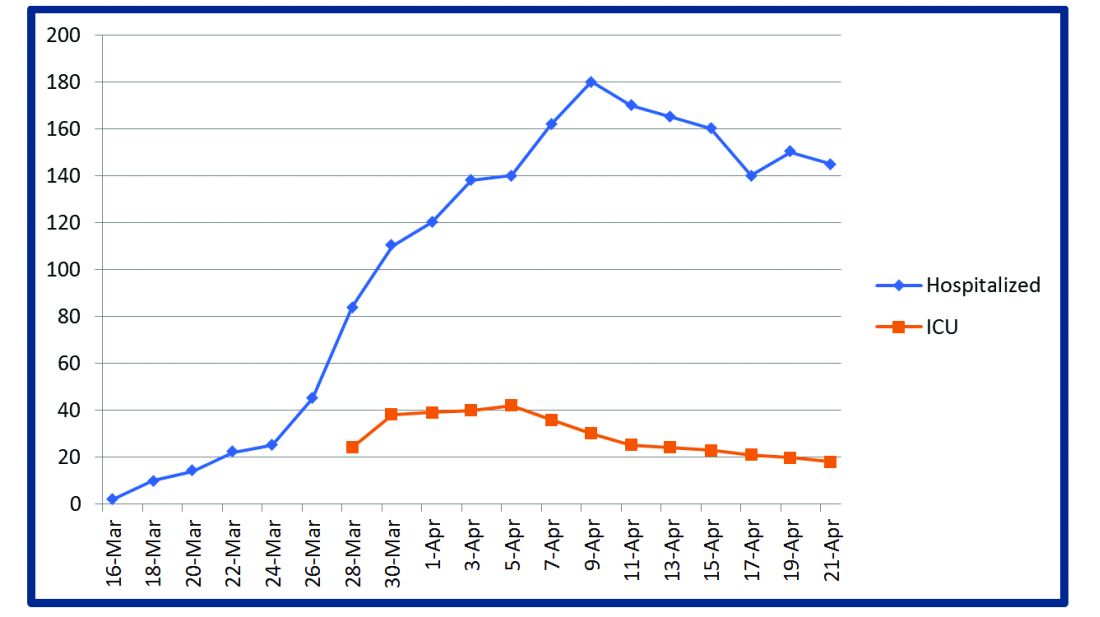
This strategy also helped build solidarity and camaraderie between all these groups, making the COVIDists feel that they were never alone and that the whole hospital supported them. We are currently evaluating clinical outcomes and attempting to identify effects on mortality, length of stay, days on the ventilator, and days in ICU. 
The maintenance phase
It is already 2 months since the first devising COVIDists. There is no difference in sick callouts between COVIDists and non-COVIDists. One COVIDist and one non-COVIDist contracted the disease, but none of them required hospitalization. Although we initially thought that COVIDists would be needed for only a short period of time, the evolution of the disease is showing signs that it might be prolonged over the next several months. Hence, we are planning to continue COVIDist service for at least the next 6 months and reevaluate the need.
Hospital medicine leadership checked on COVIDists daily in regard to their physical health and, more importantly, their mental well-being. They were offered the chance to be taken off the schedule if they felt burned out, but no one wanted to come off their scheduled service before finishing their shifts. BlueCross MA recognized one of the COVIDists, Raghuveer Rakasi, MD, as a “hero on the front line.”4 In Dr. Rakasi’s words, “We took a nosedive into something without knowing its depth, and aware that we could have fatalities among ourselves. We took up new roles, faced new challenges, learned new things every day, evolving every step of the way. We had to change the way we practice medicine, finding new ways to treat patients, and protecting the workforce by limiting patient exposure, prioritizing investigations.” He added that “we have to adapt to a new normal; we should be prepared for this to come in waves. Putting aside our political views, we should stand united 6 feet apart, with a mask covering our brave faces, frequently washing our helping hands to overcome these uncertain times.”
Conclusion
The creation of a focused group of hospitalists called COVIDists and providing them with structured and rapid training (in various aspects of clinical care of COVID-19 patients, critical care/ventilator management, efficient and safe use of PPE) and daily information dissemination allowed our health system to prepare for the large volume of COVID-19 patients. It also helped in preserving the larger hospital workforce for a possible future surge.
The rapid development and implementation of the COVIDist strategy succeeded because of the intrinsic motivation of the providers to improve the outcomes of this high-risk patient population and the close collaboration of the stakeholders. Our institution remains successful in managing the pandemic in Western Massachusetts, with reserve capacity remaining even during the peak of the epidemic. A large part of this was because of creating and training a pool of COVIDists.
Dr. Medarametla is medical director, clinical operations, in the division of hospital medicine at Baystate Health, and assistant professor at University of Massachusetts, Worcester. Readers can contact him at Venkatrao.MedarametlaMD@Baystatehealth.org. Dr. Prabhakaran is unit medical director, geriatrics unit, in the division of hospital medicine at Baystate Health and assistant professor at University of Massachusetts. Dr. Bryson is associate program director of the Internal Medicine Residency at Baystate Health and assistant professor at University of Massachusetts. Dr. Umar is medical director, clinical operations, in the division of hospital medicine at Baystate Health. Dr. Natanasabapathy is division chief of hospital medicine at Baystate Health and assistant professor at University of Massachusetts.
References
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Updated Jun 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
3. Westafer LM et al. A transdisciplinary COVID-19 early respiratory intervention protocol: An implementation story. J Hosp Med. 2020 May 21;15(6):372-374.
4. Miller J. “Heroes on the front line: Dr. Raghuveer Rakasi.” Coverage. May 18, 2020. https://coverage.bluecrossma.com/article/heroes-front-line-dr-raghuveer-rakasi
Adapting to the demands placed on hospital resources by COVID-19
Adapting to the demands placed on hospital resources by COVID-19
The challenges posed by COVID-19 have crippled health care systems around the globe. By February 2020, the first outbreak in the United States had been set off in Washington State. We quickly became the world’s epicenter of the epidemic, with over 1.8 million patients and over 110,000 deaths.1 The rapidity of spread and the severity of the disease created a tremendous strain on resources. It blindsided policymakers and hospital administrators, which left little time to react to the challenges placed on hospital operations all over the country.
The necessity of a new care model
Although health systems in the United States are adept in managing complications of common seasonal viral respiratory illnesses, COVID-19 presented an entirely different challenge with its significantly higher mortality rate. A respiratory disease turning into a multiorgan disease that causes debilitating cardiac, renal, neurological, hematological, and psychosocial complications2 was not something we had experience managing effectively. Additional challenges included a massive surge of COVID-19 patients, a limited supply of personal protective equipment (PPE), an inadequate number of intensivists for managing the anticipated ventilated patients, and most importantly, the potential of losing some of our workforce if they became infected.
Based on the experiences in China and Italy, and various predictive models, the division of hospital medicine at Baystate Health quickly realized the necessity of a new model of care for COVID-19 patients. We came up with an elaborate plan to manage the disease burden and the strain on resources effectively. The measures we put in place could be broadly divided into three categories following the timeline of the disease: the preparatory phase, the execution phase, and the maintenance phase.
The preparatory phase: From “Hospitalists” to “COVIDists”
As in most hospitals around the country, hospitalists are the backbone of inpatient clinical operations at our health system. A focused group of 10 hospitalists who volunteered to take care of COVID-19 patients with a particular interest in the pandemic and experience in critical care were selected, and the term “COVIDists” was coined to refer to them.
COVIDists were trained in various treatment protocols and ongoing clinical trials. They were given refresher training in Advanced Cardiac Life Support (ACLS) and Fundamental Critical Care Support (FCCS) courses and were taught in critical care/ventilator management by the intensivists through rapid indoctrination in the ICU. All of them had their N-95 mask fitting updated and were trained in the safe donning and doffing of all kinds of PPE by PPE coaches. The palliative care team trained them in conducting end-of-life/code status discussions with a focus on being unable to speak with family members at the bedside. COVIDists were also assigned as Code Blue leaders for any “COVID code blue” in the hospital.
In addition to the rapid training course, COVID-related updates were disseminated daily using three different modalities: brief huddles at the start of the day with the COVIDists; a COVID-19 newsletter summarizing daily updates, new treatments, strategies, and policies; and a WhatsApp group for instantly broadcasting information to the COVIDists (Table 1).
The execution phase
All the hospitalized COVID-19 patients were grouped together to COVID units, and the COVIDists were deployed to those units geographically. COVIDists were given lighter than usual patient loads to deal with the extra time needed for donning and doffing of PPE and for coordination with specialists. COVIDists were almost the only clinicians physically visiting the patients in most cases, and they became the “eyes and ears” of specialists since the specialists were advised to minimize exposure and pursue telemedicine consults. The COVIDists were also undertaking the most challenging part of the care – talking to families about end-of-life issues and the futility of aggressive care in certain patients with preexisting conditions.
Some COVIDists were deployed to the ICU to work alongside the intensivists and became an invaluable resource in ICU management when the ICU census skyrocketed during the initial phase of the outbreak. This helped in tiding the health system over during the initial crisis. Within a short time, we shifted away from an early intubation strategy, and most of the ICU patients were managed in the intermediate care units on high flow oxygen along with the awake-proning protocol. The COVIDists exclusively managed these units. They led multidisciplinary rounds two times a day with the ICU, rapid response team (RRT), the palliative care team, and the nursing team. This step drastically decreased the number of intubations, RRT activations, reduced ICU census,3 and helped with hospital capacity and patient flow (Tables 2 and 3).
This strategy also helped build solidarity and camaraderie between all these groups, making the COVIDists feel that they were never alone and that the whole hospital supported them. We are currently evaluating clinical outcomes and attempting to identify effects on mortality, length of stay, days on the ventilator, and days in ICU. 
The maintenance phase
It is already 2 months since the first devising COVIDists. There is no difference in sick callouts between COVIDists and non-COVIDists. One COVIDist and one non-COVIDist contracted the disease, but none of them required hospitalization. Although we initially thought that COVIDists would be needed for only a short period of time, the evolution of the disease is showing signs that it might be prolonged over the next several months. Hence, we are planning to continue COVIDist service for at least the next 6 months and reevaluate the need.
Hospital medicine leadership checked on COVIDists daily in regard to their physical health and, more importantly, their mental well-being. They were offered the chance to be taken off the schedule if they felt burned out, but no one wanted to come off their scheduled service before finishing their shifts. BlueCross MA recognized one of the COVIDists, Raghuveer Rakasi, MD, as a “hero on the front line.”4 In Dr. Rakasi’s words, “We took a nosedive into something without knowing its depth, and aware that we could have fatalities among ourselves. We took up new roles, faced new challenges, learned new things every day, evolving every step of the way. We had to change the way we practice medicine, finding new ways to treat patients, and protecting the workforce by limiting patient exposure, prioritizing investigations.” He added that “we have to adapt to a new normal; we should be prepared for this to come in waves. Putting aside our political views, we should stand united 6 feet apart, with a mask covering our brave faces, frequently washing our helping hands to overcome these uncertain times.”
Conclusion
The creation of a focused group of hospitalists called COVIDists and providing them with structured and rapid training (in various aspects of clinical care of COVID-19 patients, critical care/ventilator management, efficient and safe use of PPE) and daily information dissemination allowed our health system to prepare for the large volume of COVID-19 patients. It also helped in preserving the larger hospital workforce for a possible future surge.
The rapid development and implementation of the COVIDist strategy succeeded because of the intrinsic motivation of the providers to improve the outcomes of this high-risk patient population and the close collaboration of the stakeholders. Our institution remains successful in managing the pandemic in Western Massachusetts, with reserve capacity remaining even during the peak of the epidemic. A large part of this was because of creating and training a pool of COVIDists.
Dr. Medarametla is medical director, clinical operations, in the division of hospital medicine at Baystate Health, and assistant professor at University of Massachusetts, Worcester. Readers can contact him at Venkatrao.MedarametlaMD@Baystatehealth.org. Dr. Prabhakaran is unit medical director, geriatrics unit, in the division of hospital medicine at Baystate Health and assistant professor at University of Massachusetts. Dr. Bryson is associate program director of the Internal Medicine Residency at Baystate Health and assistant professor at University of Massachusetts. Dr. Umar is medical director, clinical operations, in the division of hospital medicine at Baystate Health. Dr. Natanasabapathy is division chief of hospital medicine at Baystate Health and assistant professor at University of Massachusetts.
References
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Updated Jun 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
3. Westafer LM et al. A transdisciplinary COVID-19 early respiratory intervention protocol: An implementation story. J Hosp Med. 2020 May 21;15(6):372-374.
4. Miller J. “Heroes on the front line: Dr. Raghuveer Rakasi.” Coverage. May 18, 2020. https://coverage.bluecrossma.com/article/heroes-front-line-dr-raghuveer-rakasi
The challenges posed by COVID-19 have crippled health care systems around the globe. By February 2020, the first outbreak in the United States had been set off in Washington State. We quickly became the world’s epicenter of the epidemic, with over 1.8 million patients and over 110,000 deaths.1 The rapidity of spread and the severity of the disease created a tremendous strain on resources. It blindsided policymakers and hospital administrators, which left little time to react to the challenges placed on hospital operations all over the country.
The necessity of a new care model
Although health systems in the United States are adept in managing complications of common seasonal viral respiratory illnesses, COVID-19 presented an entirely different challenge with its significantly higher mortality rate. A respiratory disease turning into a multiorgan disease that causes debilitating cardiac, renal, neurological, hematological, and psychosocial complications2 was not something we had experience managing effectively. Additional challenges included a massive surge of COVID-19 patients, a limited supply of personal protective equipment (PPE), an inadequate number of intensivists for managing the anticipated ventilated patients, and most importantly, the potential of losing some of our workforce if they became infected.
Based on the experiences in China and Italy, and various predictive models, the division of hospital medicine at Baystate Health quickly realized the necessity of a new model of care for COVID-19 patients. We came up with an elaborate plan to manage the disease burden and the strain on resources effectively. The measures we put in place could be broadly divided into three categories following the timeline of the disease: the preparatory phase, the execution phase, and the maintenance phase.
The preparatory phase: From “Hospitalists” to “COVIDists”
As in most hospitals around the country, hospitalists are the backbone of inpatient clinical operations at our health system. A focused group of 10 hospitalists who volunteered to take care of COVID-19 patients with a particular interest in the pandemic and experience in critical care were selected, and the term “COVIDists” was coined to refer to them.
COVIDists were trained in various treatment protocols and ongoing clinical trials. They were given refresher training in Advanced Cardiac Life Support (ACLS) and Fundamental Critical Care Support (FCCS) courses and were taught in critical care/ventilator management by the intensivists through rapid indoctrination in the ICU. All of them had their N-95 mask fitting updated and were trained in the safe donning and doffing of all kinds of PPE by PPE coaches. The palliative care team trained them in conducting end-of-life/code status discussions with a focus on being unable to speak with family members at the bedside. COVIDists were also assigned as Code Blue leaders for any “COVID code blue” in the hospital.
In addition to the rapid training course, COVID-related updates were disseminated daily using three different modalities: brief huddles at the start of the day with the COVIDists; a COVID-19 newsletter summarizing daily updates, new treatments, strategies, and policies; and a WhatsApp group for instantly broadcasting information to the COVIDists (Table 1).
The execution phase
All the hospitalized COVID-19 patients were grouped together to COVID units, and the COVIDists were deployed to those units geographically. COVIDists were given lighter than usual patient loads to deal with the extra time needed for donning and doffing of PPE and for coordination with specialists. COVIDists were almost the only clinicians physically visiting the patients in most cases, and they became the “eyes and ears” of specialists since the specialists were advised to minimize exposure and pursue telemedicine consults. The COVIDists were also undertaking the most challenging part of the care – talking to families about end-of-life issues and the futility of aggressive care in certain patients with preexisting conditions.
Some COVIDists were deployed to the ICU to work alongside the intensivists and became an invaluable resource in ICU management when the ICU census skyrocketed during the initial phase of the outbreak. This helped in tiding the health system over during the initial crisis. Within a short time, we shifted away from an early intubation strategy, and most of the ICU patients were managed in the intermediate care units on high flow oxygen along with the awake-proning protocol. The COVIDists exclusively managed these units. They led multidisciplinary rounds two times a day with the ICU, rapid response team (RRT), the palliative care team, and the nursing team. This step drastically decreased the number of intubations, RRT activations, reduced ICU census,3 and helped with hospital capacity and patient flow (Tables 2 and 3).
This strategy also helped build solidarity and camaraderie between all these groups, making the COVIDists feel that they were never alone and that the whole hospital supported them. We are currently evaluating clinical outcomes and attempting to identify effects on mortality, length of stay, days on the ventilator, and days in ICU. 
The maintenance phase
It is already 2 months since the first devising COVIDists. There is no difference in sick callouts between COVIDists and non-COVIDists. One COVIDist and one non-COVIDist contracted the disease, but none of them required hospitalization. Although we initially thought that COVIDists would be needed for only a short period of time, the evolution of the disease is showing signs that it might be prolonged over the next several months. Hence, we are planning to continue COVIDist service for at least the next 6 months and reevaluate the need.
Hospital medicine leadership checked on COVIDists daily in regard to their physical health and, more importantly, their mental well-being. They were offered the chance to be taken off the schedule if they felt burned out, but no one wanted to come off their scheduled service before finishing their shifts. BlueCross MA recognized one of the COVIDists, Raghuveer Rakasi, MD, as a “hero on the front line.”4 In Dr. Rakasi’s words, “We took a nosedive into something without knowing its depth, and aware that we could have fatalities among ourselves. We took up new roles, faced new challenges, learned new things every day, evolving every step of the way. We had to change the way we practice medicine, finding new ways to treat patients, and protecting the workforce by limiting patient exposure, prioritizing investigations.” He added that “we have to adapt to a new normal; we should be prepared for this to come in waves. Putting aside our political views, we should stand united 6 feet apart, with a mask covering our brave faces, frequently washing our helping hands to overcome these uncertain times.”
Conclusion
The creation of a focused group of hospitalists called COVIDists and providing them with structured and rapid training (in various aspects of clinical care of COVID-19 patients, critical care/ventilator management, efficient and safe use of PPE) and daily information dissemination allowed our health system to prepare for the large volume of COVID-19 patients. It also helped in preserving the larger hospital workforce for a possible future surge.
The rapid development and implementation of the COVIDist strategy succeeded because of the intrinsic motivation of the providers to improve the outcomes of this high-risk patient population and the close collaboration of the stakeholders. Our institution remains successful in managing the pandemic in Western Massachusetts, with reserve capacity remaining even during the peak of the epidemic. A large part of this was because of creating and training a pool of COVIDists.
Dr. Medarametla is medical director, clinical operations, in the division of hospital medicine at Baystate Health, and assistant professor at University of Massachusetts, Worcester. Readers can contact him at Venkatrao.MedarametlaMD@Baystatehealth.org. Dr. Prabhakaran is unit medical director, geriatrics unit, in the division of hospital medicine at Baystate Health and assistant professor at University of Massachusetts. Dr. Bryson is associate program director of the Internal Medicine Residency at Baystate Health and assistant professor at University of Massachusetts. Dr. Umar is medical director, clinical operations, in the division of hospital medicine at Baystate Health. Dr. Natanasabapathy is division chief of hospital medicine at Baystate Health and assistant professor at University of Massachusetts.
References
1. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Updated Jun 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
2. Zhou F et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-62.
3. Westafer LM et al. A transdisciplinary COVID-19 early respiratory intervention protocol: An implementation story. J Hosp Med. 2020 May 21;15(6):372-374.
4. Miller J. “Heroes on the front line: Dr. Raghuveer Rakasi.” Coverage. May 18, 2020. https://coverage.bluecrossma.com/article/heroes-front-line-dr-raghuveer-rakasi
Lung ultrasound works well in children with COVID-19
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
FROM PEDIATRICS
Face mask type matters when sterilizing, study finds
according to researchers. The greatest reduction in filtration efficiency after sterilization occurred with surgical face masks.
With plasma vapor hydrogen peroxide (H2O2) sterilization, filtration efficiency of N95 and KN95 masks was maintained at more than 95%, but for surgical face masks, filtration efficiency was reduced to less than 95%. With chlorine dioxide (ClO2) sterilization, on the other hand, filtration efficiency was maintained at above 95% for N95 masks, but for KN95 and surgical face masks, filtration efficiency was reduced to less than 80%.
In a research letter published online June 15 in JAMA Network Open, researchers from the University of Oklahoma Health Sciences Center, Oklahoma City, report the results of a study of the two sterilization techniques on the pressure drop and filtration efficiency of N95, KN95, and surgical face masks.
“The H2O2 treatment showed a small effect on the overall filtration efficiency of the tested masks, but the ClO2 treatment showed marked reduction in the overall filtration efficiency of the KN95s and surgical face masks. All pressure drop changes were within the acceptable range,” the researchers write.
The study did not evaluate the effect of repeated sterilizations on face masks.
Five masks of each type were sterilized with either H2O2 or ClO2. Masks were then placed in a test chamber, and a salt aerosol was nebulized to assess both upstream and downstream filtration as well as pressure drop. The researchers used a mobility particle sizer to measure particle number concentration from 16.8 nm to 514 nm. An acceptable pressure drop was defined as a drop of less than 1.38 inches of water (35 mm) for inhalation.
Although pressure drop changes were within the acceptable range for all three mask types following sterilization with either method, H2O2 sterilization yielded the least reduction in filtration efficacy in all cases. After sterilization with H2O2, filtration efficiencies were 96.6%, 97.1%, and 91.6% for the N95s, KN95s, and the surgical face masks, respectively. In contrast, filtration efficiencies after ClO2 sterilization were 95.1%, 76.2%, and 77.9%, respectively.
The researchers note that, although overall filtration efficiency was maintained with ClO2 sterilization, there was a significant drop in efficiency with respect to particles of approximately 300 nm (0.3 microns) in size. For particles of that size, mean filtration efficiency decreased to 86.2% for N95s, 40.8% for KN95s, and 47.1% for surgical face masks.
The testing described in the report is “quite affordable at $350 per mask type, so it is hard to imagine any health care provider cannot set aside a small budget to conduct such an important test,” author Evan Floyd, PhD, told Medscape Medical News.
Given the high demand for effective face masks and the current risk for counterfeit products, Floyd suggested that individual facilities test all masks intended for use by healthcare workers before and after sterilization procedures.
“However, if for some reason testing is not an option, we would recommend sticking to established brands and suppliers, perhaps reach out to your state health department or a local representative of the strategic stockpile of PPE,” he noted.
The authors acknowledge that further studies using a larger sample size and a greater variety of masks, as well as studies to evaluate different sterilization techniques, are required. Further, “measuring the respirator’s filtration efficiency by aerosol size instead of only measuring the overall filtration efficiency” should also be considered. Such an approach would enable researchers to evaluate the degree to which masks protect against specific infectious agents.
This article first appeared on Medscape.com.
according to researchers. The greatest reduction in filtration efficiency after sterilization occurred with surgical face masks.
With plasma vapor hydrogen peroxide (H2O2) sterilization, filtration efficiency of N95 and KN95 masks was maintained at more than 95%, but for surgical face masks, filtration efficiency was reduced to less than 95%. With chlorine dioxide (ClO2) sterilization, on the other hand, filtration efficiency was maintained at above 95% for N95 masks, but for KN95 and surgical face masks, filtration efficiency was reduced to less than 80%.
In a research letter published online June 15 in JAMA Network Open, researchers from the University of Oklahoma Health Sciences Center, Oklahoma City, report the results of a study of the two sterilization techniques on the pressure drop and filtration efficiency of N95, KN95, and surgical face masks.
“The H2O2 treatment showed a small effect on the overall filtration efficiency of the tested masks, but the ClO2 treatment showed marked reduction in the overall filtration efficiency of the KN95s and surgical face masks. All pressure drop changes were within the acceptable range,” the researchers write.
The study did not evaluate the effect of repeated sterilizations on face masks.
Five masks of each type were sterilized with either H2O2 or ClO2. Masks were then placed in a test chamber, and a salt aerosol was nebulized to assess both upstream and downstream filtration as well as pressure drop. The researchers used a mobility particle sizer to measure particle number concentration from 16.8 nm to 514 nm. An acceptable pressure drop was defined as a drop of less than 1.38 inches of water (35 mm) for inhalation.
Although pressure drop changes were within the acceptable range for all three mask types following sterilization with either method, H2O2 sterilization yielded the least reduction in filtration efficacy in all cases. After sterilization with H2O2, filtration efficiencies were 96.6%, 97.1%, and 91.6% for the N95s, KN95s, and the surgical face masks, respectively. In contrast, filtration efficiencies after ClO2 sterilization were 95.1%, 76.2%, and 77.9%, respectively.
The researchers note that, although overall filtration efficiency was maintained with ClO2 sterilization, there was a significant drop in efficiency with respect to particles of approximately 300 nm (0.3 microns) in size. For particles of that size, mean filtration efficiency decreased to 86.2% for N95s, 40.8% for KN95s, and 47.1% for surgical face masks.
The testing described in the report is “quite affordable at $350 per mask type, so it is hard to imagine any health care provider cannot set aside a small budget to conduct such an important test,” author Evan Floyd, PhD, told Medscape Medical News.
Given the high demand for effective face masks and the current risk for counterfeit products, Floyd suggested that individual facilities test all masks intended for use by healthcare workers before and after sterilization procedures.
“However, if for some reason testing is not an option, we would recommend sticking to established brands and suppliers, perhaps reach out to your state health department or a local representative of the strategic stockpile of PPE,” he noted.
The authors acknowledge that further studies using a larger sample size and a greater variety of masks, as well as studies to evaluate different sterilization techniques, are required. Further, “measuring the respirator’s filtration efficiency by aerosol size instead of only measuring the overall filtration efficiency” should also be considered. Such an approach would enable researchers to evaluate the degree to which masks protect against specific infectious agents.
This article first appeared on Medscape.com.
according to researchers. The greatest reduction in filtration efficiency after sterilization occurred with surgical face masks.
With plasma vapor hydrogen peroxide (H2O2) sterilization, filtration efficiency of N95 and KN95 masks was maintained at more than 95%, but for surgical face masks, filtration efficiency was reduced to less than 95%. With chlorine dioxide (ClO2) sterilization, on the other hand, filtration efficiency was maintained at above 95% for N95 masks, but for KN95 and surgical face masks, filtration efficiency was reduced to less than 80%.
In a research letter published online June 15 in JAMA Network Open, researchers from the University of Oklahoma Health Sciences Center, Oklahoma City, report the results of a study of the two sterilization techniques on the pressure drop and filtration efficiency of N95, KN95, and surgical face masks.
“The H2O2 treatment showed a small effect on the overall filtration efficiency of the tested masks, but the ClO2 treatment showed marked reduction in the overall filtration efficiency of the KN95s and surgical face masks. All pressure drop changes were within the acceptable range,” the researchers write.
The study did not evaluate the effect of repeated sterilizations on face masks.
Five masks of each type were sterilized with either H2O2 or ClO2. Masks were then placed in a test chamber, and a salt aerosol was nebulized to assess both upstream and downstream filtration as well as pressure drop. The researchers used a mobility particle sizer to measure particle number concentration from 16.8 nm to 514 nm. An acceptable pressure drop was defined as a drop of less than 1.38 inches of water (35 mm) for inhalation.
Although pressure drop changes were within the acceptable range for all three mask types following sterilization with either method, H2O2 sterilization yielded the least reduction in filtration efficacy in all cases. After sterilization with H2O2, filtration efficiencies were 96.6%, 97.1%, and 91.6% for the N95s, KN95s, and the surgical face masks, respectively. In contrast, filtration efficiencies after ClO2 sterilization were 95.1%, 76.2%, and 77.9%, respectively.
The researchers note that, although overall filtration efficiency was maintained with ClO2 sterilization, there was a significant drop in efficiency with respect to particles of approximately 300 nm (0.3 microns) in size. For particles of that size, mean filtration efficiency decreased to 86.2% for N95s, 40.8% for KN95s, and 47.1% for surgical face masks.
The testing described in the report is “quite affordable at $350 per mask type, so it is hard to imagine any health care provider cannot set aside a small budget to conduct such an important test,” author Evan Floyd, PhD, told Medscape Medical News.
Given the high demand for effective face masks and the current risk for counterfeit products, Floyd suggested that individual facilities test all masks intended for use by healthcare workers before and after sterilization procedures.
“However, if for some reason testing is not an option, we would recommend sticking to established brands and suppliers, perhaps reach out to your state health department or a local representative of the strategic stockpile of PPE,” he noted.
The authors acknowledge that further studies using a larger sample size and a greater variety of masks, as well as studies to evaluate different sterilization techniques, are required. Further, “measuring the respirator’s filtration efficiency by aerosol size instead of only measuring the overall filtration efficiency” should also be considered. Such an approach would enable researchers to evaluate the degree to which masks protect against specific infectious agents.
This article first appeared on Medscape.com.
DAPA-HF: Dapagliflozin slows T2D onset in heart failure patients
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
Dapagliflozin treatment of patients with heart failure but without diabetes in the DAPA-HF trial led to a one-third cut in the relative incidence of new-onset diabetes over a median follow-up of 18 months in a prespecified analysis from the multicenter trial that included 2,605 heart failure patients without diabetes at baseline.
The findings represented the first evidence that a drug from dapagliflozin’s class, the sodium-glucose cotransporter 2 (SGLT2) inhibitors, could prevent or slow the onset of type 2 diabetes. It represents “an additional benefit” that dapagliflozin (Farxiga) offers to patients with heart failure with reduced ejection fraction (HFrEF) like those enrolled in the DAPA-HF trial, Silvio E. Inzucchi, MD, said at the virtual annual scientific sessions of the American Diabetes Association. DAPA-HF had previously proved that treatment with this drug significantly reduced the study’s primary endpoint of cardiovascular death or heart failure worsening.
During 18 months of follow-up, 7.1% of patients in the placebo arm developed type 2 diabetes, compared with 4.9% in those who received dapagliflozin, a 2.2% absolute difference and a 32% relative risk reduction that was statistically significant for this prespecified but “exploratory” endpoint, reported Dr. Inzucchi, an endocrinologist and professor of medicine at Yale University, New Haven, Conn.
For this analysis, a hemoglobin A1c level of at least 6.5% measured in two consecutive assessments was the criterion for diagnosing incident diabetes. The 2,605 enrolled patients without diabetes in the DAPA-HF trial represented 55% of the entire trial cohort of 4,744 patients with HFrEF.
The 32% relative risk reduction for incident diabetes was primarily relevant to enrolled patients with prediabetes at entry, who constituted 67% of the enrolled cohort based on the usual definition of prediabetes, an A1c of 5.7%-6.4%.
Among all 157 (6%) of the DAPA-HF patients who developed diabetes during the trial, 150 (96%) occurred in patients with prediabetes by the usual definition; 136 of the incident cases (87%) had prediabetes by a more stringent criterion of an A1c of 6.0%-6.4%.
To put the preventive efficacy of dapagliflozin into more context, Dr. Inzucchi cited the 31% relative protection rate exerted by metformin in the Diabetes Prevention Program study (N Engl J Med. 2002 Feb 7;346[6]:393-403).
The findings showed that “dapagliflozin is the first medication demonstrated to reduce both incident type 2 diabetes and mortality in a single trial,” as well as the first agent from the SGLT2 inhibitor class to show a diabetes prevention effect, Dr. Inzucchi noted. Patients with both heart failure and diabetes are known to have a substantially increased mortality risk, compared with patients with just one of these diseases, and the potent risk posed by the confluence of both was confirmed in the results Dr. Inzucchi reported.
The 157 HFrEF patients in the trial who developed diabetes had a statistically significant 70% increased incidence of all-cause mortality during the trial’s follow-up, compared with similar HFrEF patients who remained free from a diabetes diagnosis, and they also had a significant 77% relative increase in their incidence of cardiovascular death. This analysis failed to show that incident diabetes had a significant impact on hospitalizations for heart failure coupled with cardiovascular death, another endpoint of the trial.
“This is a tremendously important analysis. We recognize that diabetes is an important factor that can forecast heart failure risk, even over relatively short follow-up. A drug that targets both diseases can be quite beneficial,” commented Muthiah Vaduganathan, MD, a cardiologist at Brigham and Women’s Hospital in Boston.
The impact of dapagliflozin on average A1c levels during the DAPA-HF trial was minimal, reducing levels by an average of 0.04% among those who entered with prediabetes and by 0.05% among the other patients. This suggests that the mechanisms by which dapagliflozin reduced incident diabetes was by routes that did not involve simply reducing hyperglycemia, and the observed decrease in incident diabetes was not apparently caused by “masking” of hyperglycemia by dapagliflozin, said Dr. Inzucchi.
One possibility is that dapagliflozin, which also improved quality of life and reduced hospitalizations in the DAPA-HF trial, led to improved function and mobility among patients that had beneficial effects on their insulin sensitivity, Dr. Vaduganathan speculated in an interview.
The new finding of dapagliflozin’s benefit “is great news,” commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif. “It’s an impressive and important result, and another reason to use dapagliflozin in patients with HFrEF, a group of patients whom you want to prevent from having worse outcomes” by developing diabetes.
The DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial enrolled HFrEF patients at 410 centers in 20 countries during February 2017–August 2018. The study’s primary endpoint was the composite incidence of cardiovascular death or worsening heart failure, which occurred in 16.3% of patients randomized to receive dapagliflozin and in 21.2% of control patients on standard care but on placebo instead of the study drug, a statistically significant relative risk reduction of 26% (N Engl J Med. 2019 Nov 21;381[21]:1995-2008). In the 2,605-patient subgroup without type 2 diabetes at baseline the primary endpoint fell by a statistically significant 27% with dapagliflozin treatment, the first time an SGLT2 inhibitor drug was shown effective for reducing this endpoint in patients with HFrEF but without diabetes. DAPA-HF did not enroll any patients with type 1 diabetes.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Inzucchi has been a consultant to AstraZeneca and to Abbott, Boehringer Ingelheim, Merck, Novo Nordisk, Sanofi/Lexicon, and vTv Therapeutics. Dr. Vaduganathan has been an adviser to AstraZeneca and to Amgen, Baxter, Bayer, Boehringer Ingelheim, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including AstraZeneca.
SOURCE: Inzucchi SE et al. ADA 2020, abstract 271-OR.
FROM ADA 2020








