User login
Cardiology groups push back on hydroxychloroquine, azithromycin for COVID-19
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
COVID-19: Are acute stroke patients avoiding emergency care?
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
Comorbidities the rule in New York’s COVID-19 deaths
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.
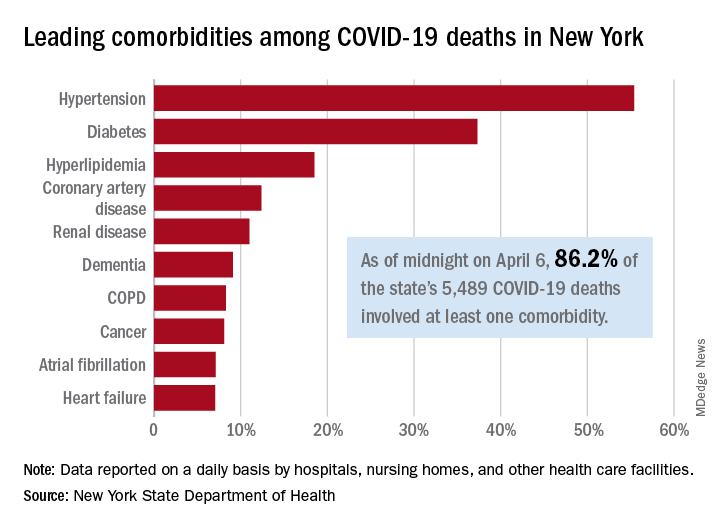
As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
SARS-CoV-2 escapes cotton, surgical masks of infected
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
FROM ANNALS OF INTERNAL MEDICINE
Treatment for RA, SpA may not affect COVID-19 severity
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
FROM ANNALS OF THE RHEUMATIC DISEASES
COVID-19 linked to multiple cardiovascular presentations
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact qualityresearch@heart.org.
This article first appeared on Medscape.com.
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact qualityresearch@heart.org.
This article first appeared on Medscape.com.
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact qualityresearch@heart.org.
This article first appeared on Medscape.com.
AMA president calls for greater reliance on science in COVID-19 fight
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
Nearly 24 tests for the novel coronavirus are available
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
Cytokine release syndrome in severe COVID-19: Is tocilizumab effective?
A large amount of data suggest that mild or severe cytokine storms, accompanied by high expression of interleukin-6 (IL-6), occur in patients with severe coronavirus disease and can be an important cause of death. Blocking the signal transduction pathway of IL-6 is expected to become a new method for the treatment of patients with severe COVID-19, with the IL-6 inhibitor, tocilizumab (Actemra), poised to become an effective drug for these patients, according to the authors of a review published online in the International Journal of Antimicrobial Agents.
The reviewers from China detailed the metabolic pathways and regulation of cytokine release syndrome, especially with respect to what is known about severe COVID-19, and discussed the results of recent trials with tocilizumab, which is currently used for treatment of CRS in a variety of cancers and other metabolic disorders.
Tocilizumab is a recombinant humanized monoclonal antibody against human IL-6 receptor of immunoglobulin IgG1 subtype and has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. The antibody specifically binds soluble- and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R– and mIL-6R–mediated signal transduction. It has been shown to be effective in the treatment of severe CRS patients. In 2017, the U.S. Food and Drug Administration approved tocilizumab for the treatment of CRS caused by CAR-T (chimeric antigen receptor T-cell immunotherapy) therapy.
A small clinical trial in China examined the effectiveness of tocilizumab in 21 patients who met the criteria for severe or critical COVID-19, including respiratory failure requiring mechanical ventilation, shock, or admission to the ICU with other organ failure. After a few days of tocilizumab treatment, the body temperatures returned to normal (initially, all 21 patients had fevers), and all other symptoms were significantly improved, according to the authors. A total of 75% (15/20) of the patients reduced their oxygen intake, and 1 patient did not need oxygen. CT scanning showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions, and lab tests showed that the proportion of peripheral blood lymphocytes and C-reactive protein in the patients returned to normal.
The main deficiency of the study was that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value, 132.38 ± 278.54 pg/mL), but the level of IL-6 following treatment was not given, according to the reviewers. Serum levels of IL-6 in normal patients are undetectable or very low.
Based upon their analysis of COVID-19’s possible mechanism and the small samples of clinical data available, tocilizumab appeared effective, and “we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6,” the authors stated.
“CRS occurs in a large number of patients with severe COVID-19, which is also an important cause of death. IL-6 is the key molecule of CRS, so IL-6R antagonist tocilizumab may be an important drug to save patients’ lives,” the researchers concluded.
This study was supported by China Mega-Project for Infectious Diseases and the China Mega-Project for Innovative Drugs. The authors reported that they had no conflicts.
SOURCE: Zhang C et al. Int J Antimicrobial Agents. 2020. doi. org/10.1016/j.ijantimicag.2020.105954.
A large amount of data suggest that mild or severe cytokine storms, accompanied by high expression of interleukin-6 (IL-6), occur in patients with severe coronavirus disease and can be an important cause of death. Blocking the signal transduction pathway of IL-6 is expected to become a new method for the treatment of patients with severe COVID-19, with the IL-6 inhibitor, tocilizumab (Actemra), poised to become an effective drug for these patients, according to the authors of a review published online in the International Journal of Antimicrobial Agents.
The reviewers from China detailed the metabolic pathways and regulation of cytokine release syndrome, especially with respect to what is known about severe COVID-19, and discussed the results of recent trials with tocilizumab, which is currently used for treatment of CRS in a variety of cancers and other metabolic disorders.
Tocilizumab is a recombinant humanized monoclonal antibody against human IL-6 receptor of immunoglobulin IgG1 subtype and has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. The antibody specifically binds soluble- and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R– and mIL-6R–mediated signal transduction. It has been shown to be effective in the treatment of severe CRS patients. In 2017, the U.S. Food and Drug Administration approved tocilizumab for the treatment of CRS caused by CAR-T (chimeric antigen receptor T-cell immunotherapy) therapy.
A small clinical trial in China examined the effectiveness of tocilizumab in 21 patients who met the criteria for severe or critical COVID-19, including respiratory failure requiring mechanical ventilation, shock, or admission to the ICU with other organ failure. After a few days of tocilizumab treatment, the body temperatures returned to normal (initially, all 21 patients had fevers), and all other symptoms were significantly improved, according to the authors. A total of 75% (15/20) of the patients reduced their oxygen intake, and 1 patient did not need oxygen. CT scanning showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions, and lab tests showed that the proportion of peripheral blood lymphocytes and C-reactive protein in the patients returned to normal.
The main deficiency of the study was that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value, 132.38 ± 278.54 pg/mL), but the level of IL-6 following treatment was not given, according to the reviewers. Serum levels of IL-6 in normal patients are undetectable or very low.
Based upon their analysis of COVID-19’s possible mechanism and the small samples of clinical data available, tocilizumab appeared effective, and “we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6,” the authors stated.
“CRS occurs in a large number of patients with severe COVID-19, which is also an important cause of death. IL-6 is the key molecule of CRS, so IL-6R antagonist tocilizumab may be an important drug to save patients’ lives,” the researchers concluded.
This study was supported by China Mega-Project for Infectious Diseases and the China Mega-Project for Innovative Drugs. The authors reported that they had no conflicts.
SOURCE: Zhang C et al. Int J Antimicrobial Agents. 2020. doi. org/10.1016/j.ijantimicag.2020.105954.
A large amount of data suggest that mild or severe cytokine storms, accompanied by high expression of interleukin-6 (IL-6), occur in patients with severe coronavirus disease and can be an important cause of death. Blocking the signal transduction pathway of IL-6 is expected to become a new method for the treatment of patients with severe COVID-19, with the IL-6 inhibitor, tocilizumab (Actemra), poised to become an effective drug for these patients, according to the authors of a review published online in the International Journal of Antimicrobial Agents.
The reviewers from China detailed the metabolic pathways and regulation of cytokine release syndrome, especially with respect to what is known about severe COVID-19, and discussed the results of recent trials with tocilizumab, which is currently used for treatment of CRS in a variety of cancers and other metabolic disorders.
Tocilizumab is a recombinant humanized monoclonal antibody against human IL-6 receptor of immunoglobulin IgG1 subtype and has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis. The antibody specifically binds soluble- and membrane-bound IL-6 receptors (sIL-6R and mIL-6R) and inhibits sIL-6R– and mIL-6R–mediated signal transduction. It has been shown to be effective in the treatment of severe CRS patients. In 2017, the U.S. Food and Drug Administration approved tocilizumab for the treatment of CRS caused by CAR-T (chimeric antigen receptor T-cell immunotherapy) therapy.
A small clinical trial in China examined the effectiveness of tocilizumab in 21 patients who met the criteria for severe or critical COVID-19, including respiratory failure requiring mechanical ventilation, shock, or admission to the ICU with other organ failure. After a few days of tocilizumab treatment, the body temperatures returned to normal (initially, all 21 patients had fevers), and all other symptoms were significantly improved, according to the authors. A total of 75% (15/20) of the patients reduced their oxygen intake, and 1 patient did not need oxygen. CT scanning showed that 90.5% (19/21) of the patients had absorption of pulmonary lesions, and lab tests showed that the proportion of peripheral blood lymphocytes and C-reactive protein in the patients returned to normal.
The main deficiency of the study was that only the level of IL-6 in peripheral blood before treatment with tocilizumab was reported (mean value, 132.38 ± 278.54 pg/mL), but the level of IL-6 following treatment was not given, according to the reviewers. Serum levels of IL-6 in normal patients are undetectable or very low.
Based upon their analysis of COVID-19’s possible mechanism and the small samples of clinical data available, tocilizumab appeared effective, and “we suggest that it should be used in critically ill COVID-19 patients with significantly elevated IL-6,” the authors stated.
“CRS occurs in a large number of patients with severe COVID-19, which is also an important cause of death. IL-6 is the key molecule of CRS, so IL-6R antagonist tocilizumab may be an important drug to save patients’ lives,” the researchers concluded.
This study was supported by China Mega-Project for Infectious Diseases and the China Mega-Project for Innovative Drugs. The authors reported that they had no conflicts.
SOURCE: Zhang C et al. Int J Antimicrobial Agents. 2020. doi. org/10.1016/j.ijantimicag.2020.105954.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
U.S. hospitals facing severe challenges from COVID-19, HHS report says
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.





