User login
Prompting during rounds decreases lab utilization in patients nearing discharge
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Medicare hospital deaths decline, hospice usage increases
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
Since 2000, Medicare beneficiaries have become less likely to die in hospitals, and more likely to die in their homes or in community health care facilities.
A review of Medicare records also determined that there was a decline in health care transitions in the final 3 days of life for these patients, Joan M. Teno, MD, and her colleagues wrote in JAMA.
It is not possible to identify a specific reason for the shift, wrote Dr. Teno, professor of medicine at the Oregon Health & Science University, Portland. Between the study years of 2000 and 2015, there were several large efforts to improve care at the end of life.
“Since 2009, programs ranging from ensuring informed patient decision making to enhanced care coordination have had the goal of improving care at the end of life. Specific interventions have included promoting conversations about the goals of care, continued growth of hospice services and palliative care, and the debate and passage of the Affordable Care Act … It is difficult to disentangle efforts such as public education, promotion of advance directives through the Patient Self- Determination Act, increased access to hospice and palliative care services, financial incentives of payment policies, and other secular changes.”
The study mined data from the Centers for Medicare & Medicaid Services, and examined end-of-life outcomes among two Medicare groups: Medicare fee-for-service recipients (1,361,870) during 2009-2015, and Medicare Advantage recipients (871,845), comparing 2011 and 2015. The mean age of both cohorts was 82 years.
Outcomes included site of death and “potentially burdensome transitions,” during the last days of life. These were defined as three or more hospitalizations in the previous 3 months, or two or more hospitalizations for pneumonia, urinary tract infection, dehydration, or sepsis during the last 120 days of life. Prolonged mechanical ventilation also was deemed potentially burdensome.
Among fee-for-service recipients, deaths in acute care hospitals declined from 32.6% to19.8%. Deaths in nursing homes remained steady, at 27.2% and 24.9%. Many of these deaths (42.9%) were preceded by a stay in an ICU. There was a transient increase in end-of-life ICU use, around 2009, but by 2015, the percentage was down to 29%, compared with 65.2% in 2000.
Transitions between a nursing home and hospital in the last 90 days of life were 0.49/person in 2000 and 0.33/person in 2015. Hospitalizations for infection or dehydration fell from 14.6% to12.2%. Hospitalization with prolonged ventilation fell from 3.1% to 2.5%.
Dying in hospice care increased from 21.6% to 50.4%, and people were taking advantage of hospice services longer: the proportion using short-term services (3 days or less) fell from 9.8% to 7.7%.
Among Medicare Advantage recipients, the numbers were somewhat different. More than 50% of recipients entered hospice care in both 2011 and 2015; in both years, 8% had services for more than 3 days. About 27% had ICU care in the last days of life, in both years. Compared to fee-for-service recipients, fewer Medicare Advantage patients were in nursing homes at the time of death, and that number declined from 2011 to 2015 (37.7% to 33.2%).
In each year, about 10% of these patients had a hospitalization for dehydration or infection, and 3% had a stay requiring prolonged mechanical ventilation in each year. The mean number of health care transitions remained steady, at 0.23 and 0.21 per person each year.
Dr. Teno had no financial disclosures.
SOURCE: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
FROM JAMA
Key clinical point: During 2000-2015, Medicare recipients became less likely to die in hospitals.
Major finding:
Study details: The retrospective study comprised more than 2.3 million Medicare recipients.
Disclosures: Dr. Teno had no financial disclosures.
Source: Teno JM et al. JAMA. 2018 Jun 25. doi: 10.1001/jama.2018.8981.
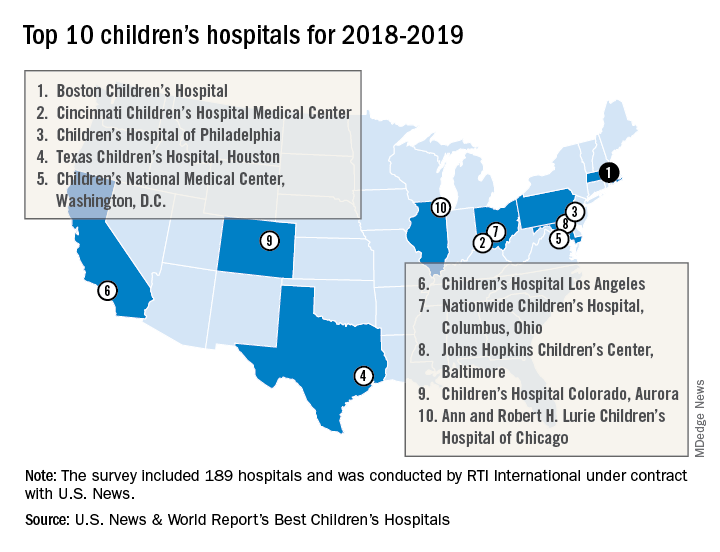
Boston Children’s Hospital named nation’s best
For the fourth consecutive year, Boston Children’s Hospital has been named the top children’s hospital by U.S. News & World Report.
The hospital finished among the top five in all 10 pediatric specialties included in the rankings: cancer (third), cardiology and heart surgery (second), diabetes and endocrinology (second), gastroenterology and GI surgery (second), neonatology (third), nephrology (first), neurology and neurosurgery (first), orthopedics (first), pulmonology (fourth), and urology (third), according to the 2018-2019 Best Children’s Hospitals rankings.
Of the 189 facilities that qualified for inclusion this year, 118 submitted sufficient data to be considered in at least 1 of the 10 specialties and 86 were ranked among the top 50 in at least 1 specialty. In addition, a survey of individuals conducted to establish the hospitals’ reputations – generally worth about 15% of a hospital’s score in each specialty – was completed by 4,165 physicians.
RTI International, a research and consulting firm, conducted the physician survey and produced the methodology and national rankings under contract with U.S. News.
For the fourth consecutive year, Boston Children’s Hospital has been named the top children’s hospital by U.S. News & World Report.
The hospital finished among the top five in all 10 pediatric specialties included in the rankings: cancer (third), cardiology and heart surgery (second), diabetes and endocrinology (second), gastroenterology and GI surgery (second), neonatology (third), nephrology (first), neurology and neurosurgery (first), orthopedics (first), pulmonology (fourth), and urology (third), according to the 2018-2019 Best Children’s Hospitals rankings.
Of the 189 facilities that qualified for inclusion this year, 118 submitted sufficient data to be considered in at least 1 of the 10 specialties and 86 were ranked among the top 50 in at least 1 specialty. In addition, a survey of individuals conducted to establish the hospitals’ reputations – generally worth about 15% of a hospital’s score in each specialty – was completed by 4,165 physicians.
RTI International, a research and consulting firm, conducted the physician survey and produced the methodology and national rankings under contract with U.S. News.
For the fourth consecutive year, Boston Children’s Hospital has been named the top children’s hospital by U.S. News & World Report.
The hospital finished among the top five in all 10 pediatric specialties included in the rankings: cancer (third), cardiology and heart surgery (second), diabetes and endocrinology (second), gastroenterology and GI surgery (second), neonatology (third), nephrology (first), neurology and neurosurgery (first), orthopedics (first), pulmonology (fourth), and urology (third), according to the 2018-2019 Best Children’s Hospitals rankings.
Of the 189 facilities that qualified for inclusion this year, 118 submitted sufficient data to be considered in at least 1 of the 10 specialties and 86 were ranked among the top 50 in at least 1 specialty. In addition, a survey of individuals conducted to establish the hospitals’ reputations – generally worth about 15% of a hospital’s score in each specialty – was completed by 4,165 physicians.
RTI International, a research and consulting firm, conducted the physician survey and produced the methodology and national rankings under contract with U.S. News.
Data suggest harm outweighs benefit of opioids for musculoskeletal pain
AMSTERDAM – Opioids cannot be justified for the routine treatment for musculoskeletal pain because risks outweigh benefits, according to a detailed review of published studies presented at the European Congress of Rheumatology.
“There is very little evidence of benefit for the long-term management of nonmalignant pain, but very good evidence for harm,” reported Blair Smith, MD, head of the population health sciences division, University of Dundee (Scotland).
In the treatment of musculoskeletal pain, the goals are increased function and quality of life, rather than complete relief of pain, according to Dr. Smith. On this basis, opioids are not an appropriate routine therapy. He reported that pain relief is not well documented, while side effects such as sedation, dizziness, and constipation, are likely to be counterproductive to improved outcomes.
There is no absolute contraindication for opioids in the control of chronic musculoskeletal pain, but Dr. Smith’s summary of the data led him to conclude that they should be used judiciously and “only for carefully selected patients.”
Of the many studies he reviewed to draw this conclusion, one of the most recent was identified as the most persuasive. Published earlier this year, the SPACE study is “the first good-quality study of long-term opioid use” in patients with musculoskeletal complaints. It was negative.
“The pain intensity at the end of 12 months of treatment was slightly but significantly worse among those randomized to opioids,” reported Dr. Smith. “There was no difference in patient function, but there was an increased risk of adverse events.”
In the SPACE study, 240 patients with moderate to severe chronic back pain or hip or knee osteoarthritis were randomized to opioid or nonopioid pain management. In the nonopioid group, the first therapeutic step was acetaminophen, but medications could be changed, added, or adjusted within both groups to improve patient response.
At the end of 12 months, a lack of benefit on both pain control and functional improvement from opioids relative to nonopioid treatment was accompanied by a higher rate of adverse effects. This led the authors to conclude that opioids are not supported for musculoskeletal pain.
Not all the evidence argues against opioids for noncancer pain management, according to Dr. Smith, but he emphasized that those who support use of opioids do so for pain control only. They do not confirm an advantage for function and quality of life, which he suggested are the key endpoints. For example, a 2010 Cochrane review concluded from a systematic literature review that there is “weak evidence” for pain relief but inconclusive evidence of an improvement in functioning and quality of life.
Other investigators have drawn the same conclusion, according to Dr. Smith. He cited a statement from the International Association for Study of Pain that advises, “Caution should be used for prescribing opioids for chronic pain.” Although this statement was not specific to musculoskeletal pain, the IASP does specify that pain medications should be employed “to promote increased function and improved quality of life rather than complete relief of pain,” according to Dr. Smith.
Opioid prescriptions for chronic pain have been increasing in Europe as they have in the United States, but Dr. Smith indicated that opioids, if used at all, should be prescribed for very short periods and for very specific goals, particularly improvement in function.
“Probably most important for my [primary care] colleagues, patients prescribed opioids should be evaluated early and frequently to gauge benefit,” Dr. Smith said. Although he believes pain control is an important and worthwhile goal, it must be approached within the context of improved well-being rather than as an isolated endpoint.
SOURCE: Smith B et al. EULAR 2018, Abstract No. SP0073.
AMSTERDAM – Opioids cannot be justified for the routine treatment for musculoskeletal pain because risks outweigh benefits, according to a detailed review of published studies presented at the European Congress of Rheumatology.
“There is very little evidence of benefit for the long-term management of nonmalignant pain, but very good evidence for harm,” reported Blair Smith, MD, head of the population health sciences division, University of Dundee (Scotland).
In the treatment of musculoskeletal pain, the goals are increased function and quality of life, rather than complete relief of pain, according to Dr. Smith. On this basis, opioids are not an appropriate routine therapy. He reported that pain relief is not well documented, while side effects such as sedation, dizziness, and constipation, are likely to be counterproductive to improved outcomes.
There is no absolute contraindication for opioids in the control of chronic musculoskeletal pain, but Dr. Smith’s summary of the data led him to conclude that they should be used judiciously and “only for carefully selected patients.”
Of the many studies he reviewed to draw this conclusion, one of the most recent was identified as the most persuasive. Published earlier this year, the SPACE study is “the first good-quality study of long-term opioid use” in patients with musculoskeletal complaints. It was negative.
“The pain intensity at the end of 12 months of treatment was slightly but significantly worse among those randomized to opioids,” reported Dr. Smith. “There was no difference in patient function, but there was an increased risk of adverse events.”
In the SPACE study, 240 patients with moderate to severe chronic back pain or hip or knee osteoarthritis were randomized to opioid or nonopioid pain management. In the nonopioid group, the first therapeutic step was acetaminophen, but medications could be changed, added, or adjusted within both groups to improve patient response.
At the end of 12 months, a lack of benefit on both pain control and functional improvement from opioids relative to nonopioid treatment was accompanied by a higher rate of adverse effects. This led the authors to conclude that opioids are not supported for musculoskeletal pain.
Not all the evidence argues against opioids for noncancer pain management, according to Dr. Smith, but he emphasized that those who support use of opioids do so for pain control only. They do not confirm an advantage for function and quality of life, which he suggested are the key endpoints. For example, a 2010 Cochrane review concluded from a systematic literature review that there is “weak evidence” for pain relief but inconclusive evidence of an improvement in functioning and quality of life.
Other investigators have drawn the same conclusion, according to Dr. Smith. He cited a statement from the International Association for Study of Pain that advises, “Caution should be used for prescribing opioids for chronic pain.” Although this statement was not specific to musculoskeletal pain, the IASP does specify that pain medications should be employed “to promote increased function and improved quality of life rather than complete relief of pain,” according to Dr. Smith.
Opioid prescriptions for chronic pain have been increasing in Europe as they have in the United States, but Dr. Smith indicated that opioids, if used at all, should be prescribed for very short periods and for very specific goals, particularly improvement in function.
“Probably most important for my [primary care] colleagues, patients prescribed opioids should be evaluated early and frequently to gauge benefit,” Dr. Smith said. Although he believes pain control is an important and worthwhile goal, it must be approached within the context of improved well-being rather than as an isolated endpoint.
SOURCE: Smith B et al. EULAR 2018, Abstract No. SP0073.
AMSTERDAM – Opioids cannot be justified for the routine treatment for musculoskeletal pain because risks outweigh benefits, according to a detailed review of published studies presented at the European Congress of Rheumatology.
“There is very little evidence of benefit for the long-term management of nonmalignant pain, but very good evidence for harm,” reported Blair Smith, MD, head of the population health sciences division, University of Dundee (Scotland).
In the treatment of musculoskeletal pain, the goals are increased function and quality of life, rather than complete relief of pain, according to Dr. Smith. On this basis, opioids are not an appropriate routine therapy. He reported that pain relief is not well documented, while side effects such as sedation, dizziness, and constipation, are likely to be counterproductive to improved outcomes.
There is no absolute contraindication for opioids in the control of chronic musculoskeletal pain, but Dr. Smith’s summary of the data led him to conclude that they should be used judiciously and “only for carefully selected patients.”
Of the many studies he reviewed to draw this conclusion, one of the most recent was identified as the most persuasive. Published earlier this year, the SPACE study is “the first good-quality study of long-term opioid use” in patients with musculoskeletal complaints. It was negative.
“The pain intensity at the end of 12 months of treatment was slightly but significantly worse among those randomized to opioids,” reported Dr. Smith. “There was no difference in patient function, but there was an increased risk of adverse events.”
In the SPACE study, 240 patients with moderate to severe chronic back pain or hip or knee osteoarthritis were randomized to opioid or nonopioid pain management. In the nonopioid group, the first therapeutic step was acetaminophen, but medications could be changed, added, or adjusted within both groups to improve patient response.
At the end of 12 months, a lack of benefit on both pain control and functional improvement from opioids relative to nonopioid treatment was accompanied by a higher rate of adverse effects. This led the authors to conclude that opioids are not supported for musculoskeletal pain.
Not all the evidence argues against opioids for noncancer pain management, according to Dr. Smith, but he emphasized that those who support use of opioids do so for pain control only. They do not confirm an advantage for function and quality of life, which he suggested are the key endpoints. For example, a 2010 Cochrane review concluded from a systematic literature review that there is “weak evidence” for pain relief but inconclusive evidence of an improvement in functioning and quality of life.
Other investigators have drawn the same conclusion, according to Dr. Smith. He cited a statement from the International Association for Study of Pain that advises, “Caution should be used for prescribing opioids for chronic pain.” Although this statement was not specific to musculoskeletal pain, the IASP does specify that pain medications should be employed “to promote increased function and improved quality of life rather than complete relief of pain,” according to Dr. Smith.
Opioid prescriptions for chronic pain have been increasing in Europe as they have in the United States, but Dr. Smith indicated that opioids, if used at all, should be prescribed for very short periods and for very specific goals, particularly improvement in function.
“Probably most important for my [primary care] colleagues, patients prescribed opioids should be evaluated early and frequently to gauge benefit,” Dr. Smith said. Although he believes pain control is an important and worthwhile goal, it must be approached within the context of improved well-being rather than as an isolated endpoint.
SOURCE: Smith B et al. EULAR 2018, Abstract No. SP0073.
REPORTING FROM THE EULAR 2018 CONGRESS
Buprenorphine endangers lives and health of children
Eleven children died from exposure to buprenorphine – a drug used to treat opioid exposure – from 2007 to 2016, mostly very young children who accidentally ingested the drug.
Four deaths, however, were teens who took buprenorphine recreationally or used it in a suicide attempt, according to a new database review by Sara Post, MS, of the Research Institute at Nationwide Children’s Hospital, Columbus, Ohio, and her associates.
“In 2016, the American Academy of Pediatrics issued a statement advocating for increased access to buprenorphine for opioid-addicted adolescents in primary care settings,” the authors noted. “This recommendation is warranted because of the high and increasing prevalence of opioid dependence among adolescents. However, caution should be used, because increased prescriptions among adolescents could lead to increased diversion and abuse and increased access to younger children in the home. Therefore, patient education for adolescents should include information about the dangers of misusing and/or abusing prescription drugs and the proper storage of medications.”
The deaths comprise a small fraction of the 11,275 children aged 19 years and younger whose buprenorphine ingestions were reported to a poison control center during that time, the investigators said. Nevertheless, almost half (45%) of the exposed children were admitted to a health care facility – with 22% needing treatment in a critical care unit.
The rate of exposures was highest during the years when only tablet formulations were available and fell after film was introduced, wrote Ms. Post, a medical student at the Northeast Ohio Medical University in Rootstown, and her colleagues. But after 2013, the rate held steady, at about 38 exposures per 1 million children per year.
Childproof packaging for all buprenorphine formulations could help protect younger children, and education could help protect older ones, she and her coinvestigators said. Manufacturers should use unit-dose packaging for all buprenorphine products to help prevent unintentional exposure among young children. Health care providers should inform caregivers of young children about the dangers of buprenorphine exposure and provide instructions on proper storage and disposal of medications. Adolescents should receive information regarding the risks of substance abuse and misuse.”
Ms. Post and her colleagues analyzed calls to poison control centers affiliated with the National Poison Data System from 2007 to 2016. During that time, the centers received 11,275 calls about buprenorphine exposure among children and adolescents 19 years and younger.
The mean age of exposure in children was about 4 years; children younger than 6 years comprised 86% of the exposures (9,709).
The investigators noted temporal trends in exposure rates in this group. From 2007 to 2013, the rate increased by 215%, peaking at 20 per 1 million in 2010. A decline followed, with exposure dropping to 12 per 1 million in 2013, before rising again to 13 in 2016.
The increase “was likely attributable to the increasing number of buprenorphine prescriptions dispensed since the Food and Drug Administration approved its use as a treatment of opioid dependence in 2002,” Ms. Post and her colleagues wrote.
The transient decrease may have been related to a shift in adult prescribing patterns, as the drug was prescribed less often to those in their 20s and gradually given more often to people aged 40-59 years.
The decrease also was probably related to the packaging shift from tablet to film. “In 2013, the leading brand-name tablets were voluntarily withdrawn from the U.S. market because of potential risk of unintentional pediatric exposures,” the team wrote. Unfortunately, the film packaging didn’t completely deter some children; from 2013 to 2016, there was a 30% increase in the frequency of exposures to buprenorphine film.
The bulk of exposures were unintentional (98%) and involved ingestion of a single buprenorphine product. However, the authors noted, even a single adult therapeutic dose can be extremely dangerous to a small child.
“Therapeutic doses of buprenorphine-naloxone for pediatric patients are 2 to 6 mcg/kg, so ingestion of a single 2-mg sublingual tablet in a 10-kg child can result in more than a 30-fold overdose. This is particularly dangerous, because children exposed to buprenorphine do not display the ‘ceiling effect’ reported in adults, in which escalating doses do not lead to additional increases in respiratory depression,” Ms. Post and her coauthors said.
This was reflected in the serious clinical effects experienced: respiratory depression, bradycardia, coma, cyanosis, respiratory arrest, seizure, and cardiac arrest. These youngest children experienced the most serious outcomes, with half requiring a hospital admittance and 21% experiencing a serious medical outcome. Seven died, six of whom were 2 years or younger.
There were 315 (3%) exposures in children aged 6-12 years; most of these (83%) were either unintentional or therapeutic errors (18%). About 30% of the group required hospital admission and about 12% experienced a serious medical outcome. There were no fatalities among this group, the investigators noted.
Among adolescents aged 13-19 years, there were 1,251 (11%) exposures and four deaths. The bulk of these (77%) was intentional, with suspected suicide accounting for 12%, and 30% involving more than one substance. The exposure rate followed the same general trends, rising to a peak of about 6 per 1 million in 2010 and the falling and leveling off at about 3 per 1 million in 2016, they said.
About 22% of teen exposures required hospital admission, with 11% needing treatment in a critical care unit. The four deaths, one of which was a suicide, all involved multiple substances (benzodiazepines, alcohol, and marijuana).
Ms. Post received a research stipend from the National Student Injury Research Training Program while she worked on the study. The coauthors had no relevant financial disclosures.
SOURCE: Post et al. Pediatrics. 2018;142:e20173652.
Eleven children died from exposure to buprenorphine – a drug used to treat opioid exposure – from 2007 to 2016, mostly very young children who accidentally ingested the drug.
Four deaths, however, were teens who took buprenorphine recreationally or used it in a suicide attempt, according to a new database review by Sara Post, MS, of the Research Institute at Nationwide Children’s Hospital, Columbus, Ohio, and her associates.
“In 2016, the American Academy of Pediatrics issued a statement advocating for increased access to buprenorphine for opioid-addicted adolescents in primary care settings,” the authors noted. “This recommendation is warranted because of the high and increasing prevalence of opioid dependence among adolescents. However, caution should be used, because increased prescriptions among adolescents could lead to increased diversion and abuse and increased access to younger children in the home. Therefore, patient education for adolescents should include information about the dangers of misusing and/or abusing prescription drugs and the proper storage of medications.”
The deaths comprise a small fraction of the 11,275 children aged 19 years and younger whose buprenorphine ingestions were reported to a poison control center during that time, the investigators said. Nevertheless, almost half (45%) of the exposed children were admitted to a health care facility – with 22% needing treatment in a critical care unit.
The rate of exposures was highest during the years when only tablet formulations were available and fell after film was introduced, wrote Ms. Post, a medical student at the Northeast Ohio Medical University in Rootstown, and her colleagues. But after 2013, the rate held steady, at about 38 exposures per 1 million children per year.
Childproof packaging for all buprenorphine formulations could help protect younger children, and education could help protect older ones, she and her coinvestigators said. Manufacturers should use unit-dose packaging for all buprenorphine products to help prevent unintentional exposure among young children. Health care providers should inform caregivers of young children about the dangers of buprenorphine exposure and provide instructions on proper storage and disposal of medications. Adolescents should receive information regarding the risks of substance abuse and misuse.”
Ms. Post and her colleagues analyzed calls to poison control centers affiliated with the National Poison Data System from 2007 to 2016. During that time, the centers received 11,275 calls about buprenorphine exposure among children and adolescents 19 years and younger.
The mean age of exposure in children was about 4 years; children younger than 6 years comprised 86% of the exposures (9,709).
The investigators noted temporal trends in exposure rates in this group. From 2007 to 2013, the rate increased by 215%, peaking at 20 per 1 million in 2010. A decline followed, with exposure dropping to 12 per 1 million in 2013, before rising again to 13 in 2016.
The increase “was likely attributable to the increasing number of buprenorphine prescriptions dispensed since the Food and Drug Administration approved its use as a treatment of opioid dependence in 2002,” Ms. Post and her colleagues wrote.
The transient decrease may have been related to a shift in adult prescribing patterns, as the drug was prescribed less often to those in their 20s and gradually given more often to people aged 40-59 years.
The decrease also was probably related to the packaging shift from tablet to film. “In 2013, the leading brand-name tablets were voluntarily withdrawn from the U.S. market because of potential risk of unintentional pediatric exposures,” the team wrote. Unfortunately, the film packaging didn’t completely deter some children; from 2013 to 2016, there was a 30% increase in the frequency of exposures to buprenorphine film.
The bulk of exposures were unintentional (98%) and involved ingestion of a single buprenorphine product. However, the authors noted, even a single adult therapeutic dose can be extremely dangerous to a small child.
“Therapeutic doses of buprenorphine-naloxone for pediatric patients are 2 to 6 mcg/kg, so ingestion of a single 2-mg sublingual tablet in a 10-kg child can result in more than a 30-fold overdose. This is particularly dangerous, because children exposed to buprenorphine do not display the ‘ceiling effect’ reported in adults, in which escalating doses do not lead to additional increases in respiratory depression,” Ms. Post and her coauthors said.
This was reflected in the serious clinical effects experienced: respiratory depression, bradycardia, coma, cyanosis, respiratory arrest, seizure, and cardiac arrest. These youngest children experienced the most serious outcomes, with half requiring a hospital admittance and 21% experiencing a serious medical outcome. Seven died, six of whom were 2 years or younger.
There were 315 (3%) exposures in children aged 6-12 years; most of these (83%) were either unintentional or therapeutic errors (18%). About 30% of the group required hospital admission and about 12% experienced a serious medical outcome. There were no fatalities among this group, the investigators noted.
Among adolescents aged 13-19 years, there were 1,251 (11%) exposures and four deaths. The bulk of these (77%) was intentional, with suspected suicide accounting for 12%, and 30% involving more than one substance. The exposure rate followed the same general trends, rising to a peak of about 6 per 1 million in 2010 and the falling and leveling off at about 3 per 1 million in 2016, they said.
About 22% of teen exposures required hospital admission, with 11% needing treatment in a critical care unit. The four deaths, one of which was a suicide, all involved multiple substances (benzodiazepines, alcohol, and marijuana).
Ms. Post received a research stipend from the National Student Injury Research Training Program while she worked on the study. The coauthors had no relevant financial disclosures.
SOURCE: Post et al. Pediatrics. 2018;142:e20173652.
Eleven children died from exposure to buprenorphine – a drug used to treat opioid exposure – from 2007 to 2016, mostly very young children who accidentally ingested the drug.
Four deaths, however, were teens who took buprenorphine recreationally or used it in a suicide attempt, according to a new database review by Sara Post, MS, of the Research Institute at Nationwide Children’s Hospital, Columbus, Ohio, and her associates.
“In 2016, the American Academy of Pediatrics issued a statement advocating for increased access to buprenorphine for opioid-addicted adolescents in primary care settings,” the authors noted. “This recommendation is warranted because of the high and increasing prevalence of opioid dependence among adolescents. However, caution should be used, because increased prescriptions among adolescents could lead to increased diversion and abuse and increased access to younger children in the home. Therefore, patient education for adolescents should include information about the dangers of misusing and/or abusing prescription drugs and the proper storage of medications.”
The deaths comprise a small fraction of the 11,275 children aged 19 years and younger whose buprenorphine ingestions were reported to a poison control center during that time, the investigators said. Nevertheless, almost half (45%) of the exposed children were admitted to a health care facility – with 22% needing treatment in a critical care unit.
The rate of exposures was highest during the years when only tablet formulations were available and fell after film was introduced, wrote Ms. Post, a medical student at the Northeast Ohio Medical University in Rootstown, and her colleagues. But after 2013, the rate held steady, at about 38 exposures per 1 million children per year.
Childproof packaging for all buprenorphine formulations could help protect younger children, and education could help protect older ones, she and her coinvestigators said. Manufacturers should use unit-dose packaging for all buprenorphine products to help prevent unintentional exposure among young children. Health care providers should inform caregivers of young children about the dangers of buprenorphine exposure and provide instructions on proper storage and disposal of medications. Adolescents should receive information regarding the risks of substance abuse and misuse.”
Ms. Post and her colleagues analyzed calls to poison control centers affiliated with the National Poison Data System from 2007 to 2016. During that time, the centers received 11,275 calls about buprenorphine exposure among children and adolescents 19 years and younger.
The mean age of exposure in children was about 4 years; children younger than 6 years comprised 86% of the exposures (9,709).
The investigators noted temporal trends in exposure rates in this group. From 2007 to 2013, the rate increased by 215%, peaking at 20 per 1 million in 2010. A decline followed, with exposure dropping to 12 per 1 million in 2013, before rising again to 13 in 2016.
The increase “was likely attributable to the increasing number of buprenorphine prescriptions dispensed since the Food and Drug Administration approved its use as a treatment of opioid dependence in 2002,” Ms. Post and her colleagues wrote.
The transient decrease may have been related to a shift in adult prescribing patterns, as the drug was prescribed less often to those in their 20s and gradually given more often to people aged 40-59 years.
The decrease also was probably related to the packaging shift from tablet to film. “In 2013, the leading brand-name tablets were voluntarily withdrawn from the U.S. market because of potential risk of unintentional pediatric exposures,” the team wrote. Unfortunately, the film packaging didn’t completely deter some children; from 2013 to 2016, there was a 30% increase in the frequency of exposures to buprenorphine film.
The bulk of exposures were unintentional (98%) and involved ingestion of a single buprenorphine product. However, the authors noted, even a single adult therapeutic dose can be extremely dangerous to a small child.
“Therapeutic doses of buprenorphine-naloxone for pediatric patients are 2 to 6 mcg/kg, so ingestion of a single 2-mg sublingual tablet in a 10-kg child can result in more than a 30-fold overdose. This is particularly dangerous, because children exposed to buprenorphine do not display the ‘ceiling effect’ reported in adults, in which escalating doses do not lead to additional increases in respiratory depression,” Ms. Post and her coauthors said.
This was reflected in the serious clinical effects experienced: respiratory depression, bradycardia, coma, cyanosis, respiratory arrest, seizure, and cardiac arrest. These youngest children experienced the most serious outcomes, with half requiring a hospital admittance and 21% experiencing a serious medical outcome. Seven died, six of whom were 2 years or younger.
There were 315 (3%) exposures in children aged 6-12 years; most of these (83%) were either unintentional or therapeutic errors (18%). About 30% of the group required hospital admission and about 12% experienced a serious medical outcome. There were no fatalities among this group, the investigators noted.
Among adolescents aged 13-19 years, there were 1,251 (11%) exposures and four deaths. The bulk of these (77%) was intentional, with suspected suicide accounting for 12%, and 30% involving more than one substance. The exposure rate followed the same general trends, rising to a peak of about 6 per 1 million in 2010 and the falling and leveling off at about 3 per 1 million in 2016, they said.
About 22% of teen exposures required hospital admission, with 11% needing treatment in a critical care unit. The four deaths, one of which was a suicide, all involved multiple substances (benzodiazepines, alcohol, and marijuana).
Ms. Post received a research stipend from the National Student Injury Research Training Program while she worked on the study. The coauthors had no relevant financial disclosures.
SOURCE: Post et al. Pediatrics. 2018;142:e20173652.
FROM PEDIATRICS
Key clinical point: Buprenorphine ingestion remains a threat to children, especially those younger than 6 years.
Major finding: From 2007 to 2016, 11,275 exposures were reported; 11 children died.
Study details: The database review looked at records from the National Poison Data System.
Disclosures: Ms. Post received a research stipend from the National Student Injury Research Training Program while she worked on the study. The coauthors had no relevant financial disclosures.
Source: Post et al. Pediatrics. 2018;142:e20173652.
Quick Byte: PrEP advances
There are recent advances in preexposure prophylaxis, or PrEP, as a promising prevention option for HIV, according to a recent study.1
Reference
1. Desai M, Field N, Grant R, McCormack S. “Recent advances in pre-exposure prophylaxis for HIV.” BMJ. 2017;359:j5011.
There are recent advances in preexposure prophylaxis, or PrEP, as a promising prevention option for HIV, according to a recent study.1
Reference
1. Desai M, Field N, Grant R, McCormack S. “Recent advances in pre-exposure prophylaxis for HIV.” BMJ. 2017;359:j5011.
There are recent advances in preexposure prophylaxis, or PrEP, as a promising prevention option for HIV, according to a recent study.1
Reference
1. Desai M, Field N, Grant R, McCormack S. “Recent advances in pre-exposure prophylaxis for HIV.” BMJ. 2017;359:j5011.
Biomarker duo rapidly identifies serious bacterial infections
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.
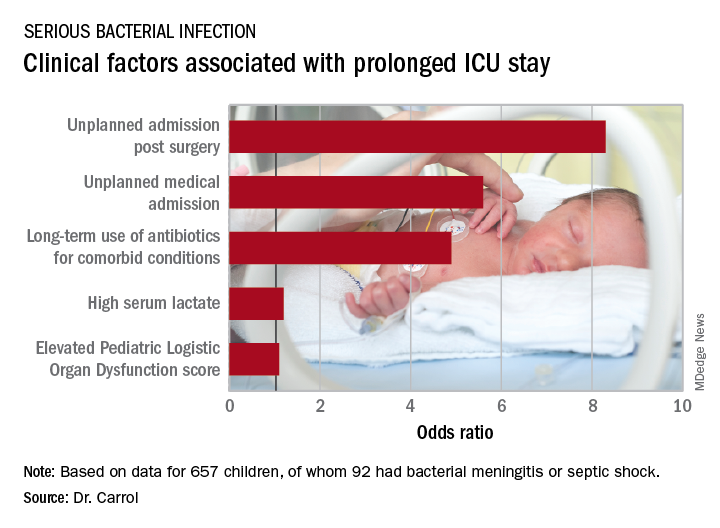
In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.

In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MALMO, SWEDEN – The combination of serum procalcitonin and C-reactive protein levels upon admission to a pediatric ICU displayed high utility for early diagnosis of serious bacterial infection in critically ill children in a large prospective observational study presented at the annual meeting of the European Society for Paediatric Infectious Diseases.
This winning combination significantly outperformed neutrophil gelatinase-associated lipocalin, activated partial thromboplastin time, and resistin, both individually and in various combinations, for the vital task of making a rapid distinction between infectious and noninfectious causes of pediatric systemic inflammatory response syndrome, reported Enitan D. Carrol, MD, professor of pediatric infection at the University of Liverpool (England).
“One of the clinical dilemmas we face in intensive care is being able to differentiate between infectious and noninfectious causes of systemic inflammatory response syndrome. This is important because we need to identify which children have life-threatening infections so that we can promptly initiate antimicrobial therapy,” she explained.
One in four deaths in pediatric ICUs are infection related, Dr. Carrol noted.
“There is an urgent need for infection markers which, firstly, change early in the course of bacterial infection, secondly, correlate with real-time clinical progression, and thirdly, have a rapid turn-around time to allow effective clinical decision making,” she observed.
The combination of procalcitonin and C-reactive protein (CRP) levels measured at admission fits the bill, Dr. Carrol continued. Of the five biomarkers evaluated in her study – all backed by some supporting evidence of efficacy in earlier studies – the top two individual performers in terms of negative predictive value (NPV) were a CRP less than 4.2 mg/dL with a negative NPV of 99%, and a procalcitonin less than 1.52 ng/mL with an NPV of 96%. The positive predictive value of each of the biomarkers was 37%. The sensitivity and specificity of procalcitonin for diagnosis of serious bacterial infection were 78% and 80%, respectively. For CRP, the figures were 93% and 76%.
The combination of procalcitonin and CRP outperformed a multitude of other two-, three-, and four-biomarker combinations tested, with an area under the curve of 93% for combined sensitivity and specificity.
The study included 657 children admitted to the pediatric ICU at Alder Hey Children’s Hospital in Liverpool with systemic inflammatory response syndrome. All had blood samples measured for the five biomarkers on days 1-7. Clinicians were blinded as to the biomarker results. Ninety-two (14%) patients were ultimately found to have a serious bacterial infection – essentially, bacterial meningitis or septic shock – and 565 (86%) had a nonbacterial etiology.
The 28-day mortality rate was 9% in the group with serious bacterial infection, significantly higher than the 2% rate in the group with other causes of their systemic inflammatory response syndrome.
Longitudinal trends in procalcitonin and CRP as evidenced in the study can be used in clinical decision making, according to Dr. Carrol. Mean values of procalcitonin plummeted by 80% from day 1 to day 5 in response to antimicrobial therapy in the group with serious bacterial infections. In contrast, CRP levels rose sharply from day 1 to a peak on day 2, then fell, although the 50% drop from day 2 to day 5 in response to antimicrobial therapy wasn’t as pronounced as the change in procalcitonin.
“There is an additive benefit for both biomarkers compared with CRP alone. The problem with CRP on admission, as I’ve demonstrated in this study, is it often hasn’t risen yet early after admission. So although it gave the best area under the curve of any of the biomarkers, I think that combined with procalcitonin you get a much better descriminator,” Dr. Carrol said.
The median duration of ICU stay in the patients with serious bacterial infection at admission was 5 days, compared with 3 days when the cause of systemic inflammatory response syndrome lay elsewhere. Their median duration of ventilation was significantly longer, too: 4 days versus 2 in children without a serious bacterial infection.
Stepwise logistic regression analysis pinpointed several clinical variables as being associated with prolonged ICU stay.

In addition, initiation of antibiotic therapy prior to admission to the pediatric ICU was associated with a 50% reduction in the likelihood of a prolonged ICU stay. “This reflects the fact that early antibiotics give you a better prognosis if you have sepsis,” according to Dr. Carrol.
She and her coinvestigators now have embarked on a multicenter U.K. study looking at the impact of procalcitonin to guide duration of antimicrobial therapy in critically ill children.
The Alder Hey study was funded by the U.K. National Institute for Health Research. Dr. Carrol reported having no financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: The area under the curve combining sensitivity and specificity was 93%.
Study details: This was a prospective, observational, single-center, clinician-blinded study of 657 patients admitted to a pediatric ICU with symptoms of systemic inflammatory response syndrome.
Disclosures: The study was funded by the U.K. National Institute for Health Research. The presenter reported having no relevant financial conflicts. Although she serves as a consultant to several health care companies, all remuneration goes directly to the University of Liverpool.
MI risk prediction after noncardiac surgery simplified
ORLANDO – The risk of perioperative MI or death associated with noncardiac surgery is vanishingly low in patients free of diabetes, hypertension, and smoking, Tanya Wilcox, MD, reported at the annual meeting of the American College of Cardiology.
How small is the risk? A mere 1 in 1,000, according to her analysis of more than 3.8 million major noncardiac surgeries in the American College of Surgeons National Surgical Quality Improvement Program database for 2009-2015, according to Dr. Wilcox of New York University.
Physicians are frequently asked by surgeons to clear patients for noncardiac surgery in terms of cardiovascular risk. Because current risk scores are complex, aren’t amenable to rapid bedside calculations, and may entail cardiac stress testing, Dr. Wilcox decided it was worth assessing the impact of three straightforward cardiovascular risk factors – current smoking and treatment for hypertension or diabetes – on 30-day postoperative MI-free survival. For this purpose she turned to the National Surgical Quality Improvement Program database, a validated, risk-adjusted, outcomes-based program to measure and improve the quality of surgical care utilizing data from 250 U.S. surgical centers.
Of the 3,817,113 patients who underwent major noncardiac surgery, 1,586,020 (42%) of them had none of the three cardiovascular risk factors of interest, 1,541,846 (40%) had one, 643,424 (17%) had two, and 45,823, or 1.2%, had all three. The patients’ mean age was 57, 75% were white, and 57% were women. About half of all patients underwent various operations within the realm of general surgery; next most frequent were orthopedic procedures, accounting for 18% of total noncardiac surgery. Of note, only 23% of patients with zero risk factors were American Society of Anesthesiologists Class 3-5, compared with 51% of those with one cardiovascular risk factor, 76% with two, and 71% with all three.
The incidence of acute MI or death within 30 days of noncardiac surgery climbed in stepwise fashion according to a patient’s risk factor burden. In a multivariate analysis adjusted for age, race, and gender, patients with any one of the cardiovascular risk factors had a 30-day risk of acute MI or death that was 1.52 times greater than those with no risk factors, patients with two risk factors were at 2.4-fold increased risk, and those with all three were at 3.63-fold greater risk than those with none. The degree of increased risk associated with any single risk factor ranged from 1.47-fold for hypertension to 1.94-fold for smoking.
“Further study is needed to determine whether aggressive risk factor modifications in the form of blood pressure control, glycemic control, and smoking cessation could reduce the incidence of postoperative MI,” Dr. Wilcox observed.
She reported having no financial conflicts regarding her study.
ORLANDO – The risk of perioperative MI or death associated with noncardiac surgery is vanishingly low in patients free of diabetes, hypertension, and smoking, Tanya Wilcox, MD, reported at the annual meeting of the American College of Cardiology.
How small is the risk? A mere 1 in 1,000, according to her analysis of more than 3.8 million major noncardiac surgeries in the American College of Surgeons National Surgical Quality Improvement Program database for 2009-2015, according to Dr. Wilcox of New York University.
Physicians are frequently asked by surgeons to clear patients for noncardiac surgery in terms of cardiovascular risk. Because current risk scores are complex, aren’t amenable to rapid bedside calculations, and may entail cardiac stress testing, Dr. Wilcox decided it was worth assessing the impact of three straightforward cardiovascular risk factors – current smoking and treatment for hypertension or diabetes – on 30-day postoperative MI-free survival. For this purpose she turned to the National Surgical Quality Improvement Program database, a validated, risk-adjusted, outcomes-based program to measure and improve the quality of surgical care utilizing data from 250 U.S. surgical centers.
Of the 3,817,113 patients who underwent major noncardiac surgery, 1,586,020 (42%) of them had none of the three cardiovascular risk factors of interest, 1,541,846 (40%) had one, 643,424 (17%) had two, and 45,823, or 1.2%, had all three. The patients’ mean age was 57, 75% were white, and 57% were women. About half of all patients underwent various operations within the realm of general surgery; next most frequent were orthopedic procedures, accounting for 18% of total noncardiac surgery. Of note, only 23% of patients with zero risk factors were American Society of Anesthesiologists Class 3-5, compared with 51% of those with one cardiovascular risk factor, 76% with two, and 71% with all three.
The incidence of acute MI or death within 30 days of noncardiac surgery climbed in stepwise fashion according to a patient’s risk factor burden. In a multivariate analysis adjusted for age, race, and gender, patients with any one of the cardiovascular risk factors had a 30-day risk of acute MI or death that was 1.52 times greater than those with no risk factors, patients with two risk factors were at 2.4-fold increased risk, and those with all three were at 3.63-fold greater risk than those with none. The degree of increased risk associated with any single risk factor ranged from 1.47-fold for hypertension to 1.94-fold for smoking.
“Further study is needed to determine whether aggressive risk factor modifications in the form of blood pressure control, glycemic control, and smoking cessation could reduce the incidence of postoperative MI,” Dr. Wilcox observed.
She reported having no financial conflicts regarding her study.
ORLANDO – The risk of perioperative MI or death associated with noncardiac surgery is vanishingly low in patients free of diabetes, hypertension, and smoking, Tanya Wilcox, MD, reported at the annual meeting of the American College of Cardiology.
How small is the risk? A mere 1 in 1,000, according to her analysis of more than 3.8 million major noncardiac surgeries in the American College of Surgeons National Surgical Quality Improvement Program database for 2009-2015, according to Dr. Wilcox of New York University.
Physicians are frequently asked by surgeons to clear patients for noncardiac surgery in terms of cardiovascular risk. Because current risk scores are complex, aren’t amenable to rapid bedside calculations, and may entail cardiac stress testing, Dr. Wilcox decided it was worth assessing the impact of three straightforward cardiovascular risk factors – current smoking and treatment for hypertension or diabetes – on 30-day postoperative MI-free survival. For this purpose she turned to the National Surgical Quality Improvement Program database, a validated, risk-adjusted, outcomes-based program to measure and improve the quality of surgical care utilizing data from 250 U.S. surgical centers.
Of the 3,817,113 patients who underwent major noncardiac surgery, 1,586,020 (42%) of them had none of the three cardiovascular risk factors of interest, 1,541,846 (40%) had one, 643,424 (17%) had two, and 45,823, or 1.2%, had all three. The patients’ mean age was 57, 75% were white, and 57% were women. About half of all patients underwent various operations within the realm of general surgery; next most frequent were orthopedic procedures, accounting for 18% of total noncardiac surgery. Of note, only 23% of patients with zero risk factors were American Society of Anesthesiologists Class 3-5, compared with 51% of those with one cardiovascular risk factor, 76% with two, and 71% with all three.
The incidence of acute MI or death within 30 days of noncardiac surgery climbed in stepwise fashion according to a patient’s risk factor burden. In a multivariate analysis adjusted for age, race, and gender, patients with any one of the cardiovascular risk factors had a 30-day risk of acute MI or death that was 1.52 times greater than those with no risk factors, patients with two risk factors were at 2.4-fold increased risk, and those with all three were at 3.63-fold greater risk than those with none. The degree of increased risk associated with any single risk factor ranged from 1.47-fold for hypertension to 1.94-fold for smoking.
“Further study is needed to determine whether aggressive risk factor modifications in the form of blood pressure control, glycemic control, and smoking cessation could reduce the incidence of postoperative MI,” Dr. Wilcox observed.
She reported having no financial conflicts regarding her study.
REPORTING FROM ACC 2018
Key clinical point: Noncardiac surgery patients can breathe easier regarding perioperative cardiovascular risk provided they don’t smoke and aren’t hypertensive or diabetic.
Major finding: .
Study details: This was a retrospective analysis of more than 3.8 million noncardiac surgeries contained in the American College of Surgeons National Surgical Quality Improvement Program database for 2009-2015.
Disclosures: The study presenter reported having no financial conflicts.
Creating a digital pill
Technology battles medication noncompliance
Hospitalists and other physicians have long struggled with medication noncompliance, which can lead to sicker patients and higher rates of readmittance, and costs some $100-$289 billion a year.
There is a growing field of digital devices being developed to address this problem. The Food and Drug Administration has just approved the newest one: a medication with a sensor embedded that can tell doctors if, and when, patients take their medicine, according to an article in the New York Times.1 It’s expected to become available in 2018.
The digital medication is a version of the antipsychotic Abilify. Patients who agree to take it will sign consent forms allowing their doctors (and up to four other people) to receive electronic data showing the date and time pills are ingested.
The sensor, created by Proteus Digital Health, contains copper, magnesium, and silicon, all said to be safe ingredients found in foods. The electrical signal is created when stomach fluids contact the sensor; a patch worn on the rib cage detects that signal and sends the message.
Other companies are joining the race to create digital medication technologies; these are being tested in medications for patients with conditions including heart disease, diabetes, and HIV infection. Some researchers predict the technology might have applications for monitoring the opioid intake of postsurgical patients or patients in medication clinical trials.
Reference
1. Belluck P. “First Digital Pill Approved to Worries About Biomedical ‘Big Brother.’ ” New York Times. Nov 13, 2017.
Technology battles medication noncompliance
Technology battles medication noncompliance
Hospitalists and other physicians have long struggled with medication noncompliance, which can lead to sicker patients and higher rates of readmittance, and costs some $100-$289 billion a year.
There is a growing field of digital devices being developed to address this problem. The Food and Drug Administration has just approved the newest one: a medication with a sensor embedded that can tell doctors if, and when, patients take their medicine, according to an article in the New York Times.1 It’s expected to become available in 2018.
The digital medication is a version of the antipsychotic Abilify. Patients who agree to take it will sign consent forms allowing their doctors (and up to four other people) to receive electronic data showing the date and time pills are ingested.
The sensor, created by Proteus Digital Health, contains copper, magnesium, and silicon, all said to be safe ingredients found in foods. The electrical signal is created when stomach fluids contact the sensor; a patch worn on the rib cage detects that signal and sends the message.
Other companies are joining the race to create digital medication technologies; these are being tested in medications for patients with conditions including heart disease, diabetes, and HIV infection. Some researchers predict the technology might have applications for monitoring the opioid intake of postsurgical patients or patients in medication clinical trials.
Reference
1. Belluck P. “First Digital Pill Approved to Worries About Biomedical ‘Big Brother.’ ” New York Times. Nov 13, 2017.
Hospitalists and other physicians have long struggled with medication noncompliance, which can lead to sicker patients and higher rates of readmittance, and costs some $100-$289 billion a year.
There is a growing field of digital devices being developed to address this problem. The Food and Drug Administration has just approved the newest one: a medication with a sensor embedded that can tell doctors if, and when, patients take their medicine, according to an article in the New York Times.1 It’s expected to become available in 2018.
The digital medication is a version of the antipsychotic Abilify. Patients who agree to take it will sign consent forms allowing their doctors (and up to four other people) to receive electronic data showing the date and time pills are ingested.
The sensor, created by Proteus Digital Health, contains copper, magnesium, and silicon, all said to be safe ingredients found in foods. The electrical signal is created when stomach fluids contact the sensor; a patch worn on the rib cage detects that signal and sends the message.
Other companies are joining the race to create digital medication technologies; these are being tested in medications for patients with conditions including heart disease, diabetes, and HIV infection. Some researchers predict the technology might have applications for monitoring the opioid intake of postsurgical patients or patients in medication clinical trials.
Reference
1. Belluck P. “First Digital Pill Approved to Worries About Biomedical ‘Big Brother.’ ” New York Times. Nov 13, 2017.
Galectin-3: A new post-MI prognostic biomarker?
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
ORLANDO – An elevated circulating galactin-3 level after an acute MI is a potent long-term predictor of both heart failure and mortality, independent of known prognostic markers, Rabea Asleh, MD, PhD, reported at the annual meeting of the American College of Cardiology.
“These findings suggest that galectin-3 measurement may have a role in the risk stratification of patients presenting with MI,” according to Dr. Asleh, an Israeli cardiologist doing a fellowship in advanced heart failure and transplant cardiology at the Mayo Clinic in Rochester, Minn.
“The changing clinical presentation of MI necessitates evolution in our approach to risk stratification,” he explained. “Over the last 2 decades we’ve observed a change in the epidemiology of MI, with more patients developing non-ST-elevation MI compared to STEMI. They present at an older age and develop heart failure with preserved ejection fraction more than heart failure with reduced ejection fraction.”
He presented a prospective population-based community cohort study of 1,401 Olmsted County, Minn., residents who had a validated MI during 2002-2012. Their mean age was 67 years, 61% were men, and 79% presented with non-STEMI. During a mean follow-up of 5.3 years, 389 of the participants developed heart failure and 512 patients died.
Galectin-3 was measured a median of 2 days post MI. The median level was 18.4 ng/mL. Patients were divided into tertiles based upon their galactin-3 measurement: Tertile 1 required a post-MI galectin-3 level below 15.2 ng/mL; tertile 2 had a level of 15.2-22.6 ng/mL; and the top tertile was for individuals with a galectin-3 above 22.6 ng/mL.
Of note, patients with a higher galectin-3 level were older, had a higher prevalence of diabetes, hypertension, hyperlipidemia, anterior MI, a higher Killip class, a higher Charlson comorbidity score, and a lower peak troponin T level. They also had a lower estimated glomerular filtration rate; indeed, the median eGFR in the top tertile for galactin-3 was 48 mL/min per 1.73 m2, compared with 68 mL/min in the lowest galectin-3 tertile. Women accounted for 27% of patients in tertile 1, 41% in tertile 2, and fully half of those in tertile 3.
In an unadjusted analysis, the risk of mortality during follow-up was sixfold greater for patients in galectin-3 tertile 3 than in tertile 1; the risk of heart failure was increased 5.5-fold.
More meaningfully, in a Cox multivariate analysis extensively adjusted for age, gender, comorbidities, malignancy, standard cardiovascular risk factors, MI characteristics, eGFR, Killip class, cardiac troponin T, and other potential confounders, patients in galectin-3 tertile 2 had a 1.6-fold increased risk of death and a 1.62-fold increased likelihood of heart failure during follow-up, compared with subjects in tertile 1, Dr. Asleh noted.
Patients in tertile 3 had a 2.4-fold increased risk of death and were at 2.1 times greater risk of heart failure than those in tertile 1. The degree of risk for heart failure associated with elevated galactin-3 was virtually identical for heart failure with preserved as compared with reduced ejection fraction, he added.
Session cochair L. Kristin Newby, MD, of Duke University, Durham, N.C., noted that the Mayo study did not adjust for brain natriuretic peptide (BNP) or N-terminal pro hormone BNP (NT-proBNP), both of which are known to be strong predictors of both heart failure and mortality after acute MI. Doesn’t their absence weaken the strength of galactin-3’s prognostic power as demonstrated in the study? she asked.
Dr. Asleh replied that those biomarkers weren’t collected in this study, which began in 2002.
“What I can tell you is, other studies show there is only a weak correlation between galactin-3 and NT-proBNP post MI. Some studies have even shown an inverse correlation,” he said. “The pathophysiological explanation is that galactin-3 is more implicated in fibrosis before the stage of development of left ventricular loading and stretching of the myocardium. So galactin-3 may be implicated in LV fibrosis leading to heart failure before the NT-proBNP comes into play.”
Also, he cited a study by other investigators conducted in patients with a left ventricular assist device for advanced heart failure. Upon device-induced left ventricular unloading the patients’ NT-proBNP levels dropped significantly while their galactin-3 remained high and unchanged. This suggests the two biomarkers are implicated in different disease pathways.
Both animal and human studies indicate galactin-3 is involved specifically in fibrosis, as opposed to, say, C-reactive protein, a well established marker of systemic inflammation, the cardiologist added.
Dr. Asleh reported having no financial conflicts of interest regarding his study, which was supported by the National Institutes of Health.
REPORTING FROM ACC 18
Key clinical point: Galectin-3 level post-MI is a potent long-term predictor of both heart failure and mortality independent of known prognostic markers.
Major finding: Post-MI patients in the top tertile of circulating galectin-3 were at an adjusted 2.4-fold increased mortality risk and a 2.05-fold greater risk of developing heart failure compared with those in the lowest tertile.
Study details: This prospective population-based cohort study included 1,401 MI patients followed for a mean of 5.3 years.
Disclosures: The National Institutes of Health supported the study. The presenter reported having no financial conflicts of interest.