User login
REALITY trial supports restrictive transfusion in anemic MI
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
in the landmark REALITY trial.
Randomized trial data already support a restrictive transfusion strategy in patients undergoing cardiac and noncardiac surgery, as well as in other settings. Those trials deliberately excluded patients with acute myocardial ischemia.
Cardiologists have been loath to adopt a restrictive strategy in the absence of persuasive supporting evidence because of a theoretic concern that low hemoglobin might be particularly harmful to ischemic myocardium. Anemia occurs in 5%-10% patients with MI, and clinicians have been eager for evidence-based guidance on how to best manage it.
“Blood is a precious resource and transfusion is costly, logistically cumbersome, and has side effects,” Philippe Gabriel Steg, MD, chair of the REALITY trial, noted in presenting the study results at the virtual annual congress of the European Society of Cardiology.
REALITY was the first-ever large randomized trial of a restrictive versus liberal transfusion strategy in acute MI. The study, which featured a noninferiority design, included 668 stable patients with acute MI and anemia with a hemoglobin of 7-10 g/dL at 35 hospitals in France and Spain. Participants were randomized to a restrictive strategy in which transfusion was withheld unless the hemoglobin dropped to 8 g/dL or less, or to a conventional liberal strategy triggered by a hemoglobin of 10 g/dL or lower. The transfusion target was a hemoglobin level of 8-10 g/dL in the restrictive strategy group and greater than 11 g/dL in the liberal transfusion group. In the restrictive transfusion group, 36% received at least one RBC transfusion, as did 87% in the liberal transfusion study arm. The restrictive strategy group used 414 fewer units of blood.
The two coprimary endpoints were 30-day major adverse cardiovascular events and cost-effectiveness. The 30-day composite of all-cause mortality, reinfarction, stroke, and emergency percutaneous coronary intervention for myocardial ischemia occurred in 11% of the restrictive transfusion group and 14% of the liberal transfusion group. The resultant 21% relative risk reduction established that the restrictive strategy was noninferior. Of note, all of the individual components of the composite endpoint numerically favored the restrictive approach.
In terms of safety, patients in the restrictive transfusion group were significantly less likely to develop an infection, by a margin of 0% versus 1.5%. The rate of acute lung injury was also significantly lower in the restrictive group: 0.3%, compared with 2.2%. The median hospital length of stay was identical at 7 days in both groups.
The cost-effectiveness analysis concluded that the restrictive transfusion strategy had an 84% probability of being both less expensive and more effective.
Patients were enrolled in REALITY regardless of whether they had active bleeding, as long as the bleeding wasn’t deemed massive and life-threatening. Notably, there was no difference in the results of restrictive versus liberal transfusion regardless of whether active bleeding was present, nor did baseline hemoglobin or the presence or absence of preexisting anemia affect the results.
Dr. Steg noted that a much larger randomized trial of restrictive versus liberal transfusion in the setting of acute MI with anemia is underway in the United States and Canada. The 3,000-patient MINT trial, sponsored by the National Institutes of Health, is testing the superiority of restrictive transfusion, rather than its noninferiority, as in REALITY. Results are a couple of years away.
“I think that will be an important piece of additional evidence,” he said.
Discussant Marco Roffi, MD, didn’t mince words.
“I really love the REALITY trial,” declared Dr. Roffi, professor and vice chairman of the cardiology department and director of the interventional cardiology unit at University Hospital of Geneva.
He ticked off a series of reasons: The trial addressed a common clinical dilemma about which there has been essentially no prior high-quality evidence, it provided convincing results, and it carried important implications for responsible stewardship of the blood supply.
“REALITY allows clinicians to comfortably refrain from transfusing anemic patients presenting with myocardial infarction, and this should lead to a reduction in the consumption of blood products,” Dr. Roffi said.
He applauded the investigators for their success in obtaining public funding for a study lacking a commercial hook. And as a clinical investigator, he was particularly impressed by one of the technical details about the REALITY trial: “I was amazed by the fact that the observed event rates virtually corresponded to the estimated ones used for the power calculations. This is rarely the case in such a trial.”
Dr. Roffi said the REALITY findings should have an immediate impact on clinical practice, as well as on the brand new 2020 ESC guidelines on the management of non–ST-elevation ACS issued during the ESC virtual congress.
The freshly inked guidelines state: “Based on inconsistent study results and the lack of adequately powered randomized, controlled trials, a restrictive policy of transfusion in anemic patients with MI may be considered.” As of today, Dr. Roffi argued, the phrase “may be considered” ought to be replaced by the stronger phrase “should be considered.”
During the discussion period, he was asked if it’s appropriate to extrapolate the REALITY results to patients undergoing transcatheter aortic valve replacement, among whom anemia is highly prevalent.
“I think this is a different patient population. Nevertheless, the concept of being restrictive is one that in my opinion now remains until proven otherwise. So we are being very restrictive in these patients,” he replied.
Asked about possible mechanisms by which liberal transfusion might have detrimental effects in acute MI patients, Dr. Steg cited several, including evidence that transfusion may not improve oxygen delivery to as great an extent as traditionally thought. There is also the risk of volume overload, increased blood viscosity, and enhanced platelet aggregation and activation, which could promote myocardial ischemia.
The REALITY trial was funded by the French Ministry of Health and the Spanish Ministry of Economy and Competitiveness with no commercial support. Outside the scope of the trial, Dr. Steg reported receiving research grants from Bayer, Merck, Servier, and Sanofi as well as serving as a consultant to numerous pharmaceutical companies.
REPORTING FROM ESC CONGRESS 2020
Latest report adds almost 44,000 child COVID-19 cases in 1 week
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
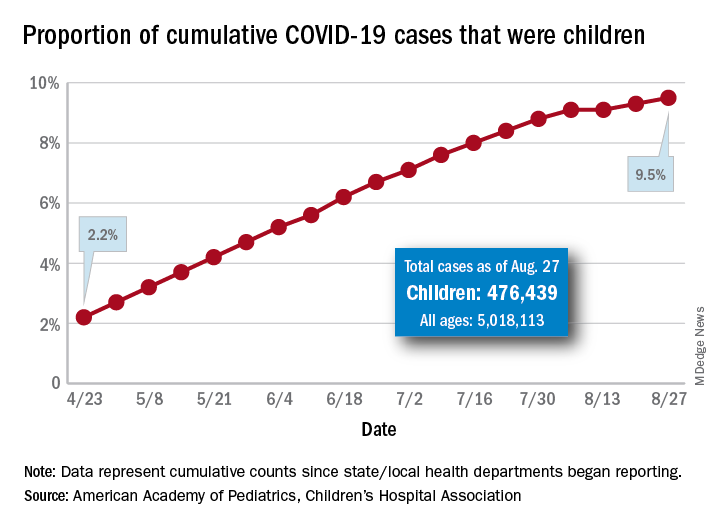
The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The new cases bring the cumulative number of infected children to over 476,000, and that figure represents 9.5% of the over 5 million COVID-19 cases reported among all ages, the AAP and the CHA said in their weekly report. The cumulative number of children covers 49 states (New York is not reporting age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
From lowest to highest, the states occupying opposite ends of the cumulative proportion spectrum are New Jersey at 3.4% – New York City was lower with a 3.2% figure but is not a state – and Wyoming at 18.3%, the report showed.
Children represent more than 15% of all reported COVID-19 cases in five other states: Tennessee (17.1%), North Dakota (16.0%), Alaska (15.9%), New Mexico (15.7%), and Minnesota (15.1%). The states just above New Jersey are Florida (5.8%), Connecticut (5.9%), and Massachusetts (6.7%). Texas has a rate of 5.6% but has reported age for only 8% of confirmed cases, the AAP and CHA noted.
Children make up a much lower share of COVID-19 hospitalizations – 1.7% of the cumulative number for all ages – although that figure has been slowly rising over the course of the pandemic: it was 1.2% on July 9 and 0.9% on May 8. Arizona (4.1%) is the highest of the 22 states reporting age for hospitalizations and Hawaii (0.6%) is the lowest, based on the AAP/CHA data.
Mortality figures for children continue to be even lower. Nationwide, 0.07% of all COVID-19 deaths occurred in children, and 19 of the 43 states reporting age distributions have had no deaths yet. Pediatric deaths totaled 101 as of Aug. 27, the two groups reported.
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pandemic worsens disparities in GI and liver disease
Suspension of disease screening and nonurgent procedures because of the COVID-19 pandemic will negatively impact long-term outcomes of GI and liver disease, and people of color will be disproportionately affected, according to a leading expert.
Novel, multipronged approaches are needed to overcome widening disparities in gastroenterology and hepatology, said Rachel Issaka, MD, of Fred Hutchinson Cancer Research Center in Seattle.
“The COVID-19 pandemic has led to unprecedented drops in breast, colorectal, and cervical cancer screenings,” Dr. Issaka said during an AGA FORWARD Program webinar. Screening rates for these diseases are down 83%-90%, she said.
“Certainly this creates a backlog of cancer screenings that need to occur, which poses very significant challenges for health systems as they’re adapting to this new state of health care that we have to provide,” Dr. Issaka said.
During her presentation, Dr. Issaka first addressed pandemic-related issues in colorectal cancer (CRC).
The sudden decrease in colonoscopies has already affected diagnoses, she said, as 32% fewer cases of CRC were diagnosed in April 2020 compared with April 2019, a finding that is “obviously very concerning.” All downstream effects remain to be seen; however, one estimate suggests that over the next decade, delayed screening may lead to an additional 4,500 deaths from CRC.
“These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest,” Dr. Issaka wrote, as coauthor of a study published in Gastrointestinal Endoscopy.
Dr. Issaka and colleagues predict that the pandemic will likely worsen “persistent CRC disparities” in African-American and Hispanic communities, including relatively decreased screening participation, delayed follow-up of abnormal stool results, limited community-based research and partnerships, and limited community engagement and advocacy.
“COVID-19 related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations,” wrote Dr. Issaka and colleagues.
Dr. Issaka described similar issues in hepatology. She referred to a recent opinion article by Tapper and colleagues, which predicted that the COVID-19 pandemic will impact patients with liver disease in three waves: first, by delaying liver transplants, elective procedures, imaging, and routine patient follow-up; second, by increasing emergent decompensations, transplant wait-list dropouts, and care deferrals; and third, by losing patients to follow-up, resulting in missed diagnoses, incomplete cancer screening, and progressive disease.
“This could disproportionately impact Black, Hispanic, and Native-American populations, who may have already had difficulty accessing [liver care],” Dr. Issaka said.
To mitigate growing disparities, Dr. Issaka proposed a variety of strategies for CRC and liver disease.
For CRC screening, Dr. Issaka suggested noninvasive modalities, including mailed fecal immunochemical tests (FIT), with focused follow-up on patients with highest FIT values. For those conducting CRC research, Dr. Issaka recommended using accessible technology, engaging with community partners, providing incentives where appropriate, and other methods. For cirrhosis care, Dr. Issaka suggested that practitioners turn to telehealth and remote care, including weight monitoring, cognitive function testing, home medication delivery, and online education.
More broadly, Dr. Issaka called for universal health insurance not associated with employment, research funding for health disparities, sustainable employment wages, climate justice, desegregation of housing, and universal broadband Internet.
“The solutions to these problems are multipronged,” Dr. Issaka said. “Some will happen locally; for instance, well-executed planning around telehealth. Some will happen at the state level through opportunities like advocacy or even just reaching out to your own [congressional representative]. And then some will also happen programmatically – How can we as a health system begin to leverage something like mailed FIT?”
Finally, Dr. Issaka suggested that tools from another branch of science can help improve screening rates.
“We don’t, in medicine, tap into the benefits of behavioral psychology enough,” she said. “That’s a great discipline with really great tools that we can all use.”
Dr. Issaka described the power of community, in that people are more likely to undergo screening if they know how many others in their community are also being screened.
“I think as much as we can gather those kinds of data and share those with individuals to provide reassurance about the safety and importance of screening, I think [that] will help,” she said.
The AGA FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK118761). Dr. Issaka has no conflicts of interest.
SOURCES: Issaka. AGA FORWARD Program Webinar. 2020 Aug 27; Balzora et al. Gastrointestinal Endoscopy. 2020 June 20. doi: 10.1016/j.gie.2020.06.042; Tapper et al. Journal of Hepatology. 2020 Apr 13. doi: 10.1016/j.jhep.2020.04.005.
Suspension of disease screening and nonurgent procedures because of the COVID-19 pandemic will negatively impact long-term outcomes of GI and liver disease, and people of color will be disproportionately affected, according to a leading expert.
Novel, multipronged approaches are needed to overcome widening disparities in gastroenterology and hepatology, said Rachel Issaka, MD, of Fred Hutchinson Cancer Research Center in Seattle.
“The COVID-19 pandemic has led to unprecedented drops in breast, colorectal, and cervical cancer screenings,” Dr. Issaka said during an AGA FORWARD Program webinar. Screening rates for these diseases are down 83%-90%, she said.
“Certainly this creates a backlog of cancer screenings that need to occur, which poses very significant challenges for health systems as they’re adapting to this new state of health care that we have to provide,” Dr. Issaka said.
During her presentation, Dr. Issaka first addressed pandemic-related issues in colorectal cancer (CRC).
The sudden decrease in colonoscopies has already affected diagnoses, she said, as 32% fewer cases of CRC were diagnosed in April 2020 compared with April 2019, a finding that is “obviously very concerning.” All downstream effects remain to be seen; however, one estimate suggests that over the next decade, delayed screening may lead to an additional 4,500 deaths from CRC.
“These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest,” Dr. Issaka wrote, as coauthor of a study published in Gastrointestinal Endoscopy.
Dr. Issaka and colleagues predict that the pandemic will likely worsen “persistent CRC disparities” in African-American and Hispanic communities, including relatively decreased screening participation, delayed follow-up of abnormal stool results, limited community-based research and partnerships, and limited community engagement and advocacy.
“COVID-19 related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations,” wrote Dr. Issaka and colleagues.
Dr. Issaka described similar issues in hepatology. She referred to a recent opinion article by Tapper and colleagues, which predicted that the COVID-19 pandemic will impact patients with liver disease in three waves: first, by delaying liver transplants, elective procedures, imaging, and routine patient follow-up; second, by increasing emergent decompensations, transplant wait-list dropouts, and care deferrals; and third, by losing patients to follow-up, resulting in missed diagnoses, incomplete cancer screening, and progressive disease.
“This could disproportionately impact Black, Hispanic, and Native-American populations, who may have already had difficulty accessing [liver care],” Dr. Issaka said.
To mitigate growing disparities, Dr. Issaka proposed a variety of strategies for CRC and liver disease.
For CRC screening, Dr. Issaka suggested noninvasive modalities, including mailed fecal immunochemical tests (FIT), with focused follow-up on patients with highest FIT values. For those conducting CRC research, Dr. Issaka recommended using accessible technology, engaging with community partners, providing incentives where appropriate, and other methods. For cirrhosis care, Dr. Issaka suggested that practitioners turn to telehealth and remote care, including weight monitoring, cognitive function testing, home medication delivery, and online education.
More broadly, Dr. Issaka called for universal health insurance not associated with employment, research funding for health disparities, sustainable employment wages, climate justice, desegregation of housing, and universal broadband Internet.
“The solutions to these problems are multipronged,” Dr. Issaka said. “Some will happen locally; for instance, well-executed planning around telehealth. Some will happen at the state level through opportunities like advocacy or even just reaching out to your own [congressional representative]. And then some will also happen programmatically – How can we as a health system begin to leverage something like mailed FIT?”
Finally, Dr. Issaka suggested that tools from another branch of science can help improve screening rates.
“We don’t, in medicine, tap into the benefits of behavioral psychology enough,” she said. “That’s a great discipline with really great tools that we can all use.”
Dr. Issaka described the power of community, in that people are more likely to undergo screening if they know how many others in their community are also being screened.
“I think as much as we can gather those kinds of data and share those with individuals to provide reassurance about the safety and importance of screening, I think [that] will help,” she said.
The AGA FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK118761). Dr. Issaka has no conflicts of interest.
SOURCES: Issaka. AGA FORWARD Program Webinar. 2020 Aug 27; Balzora et al. Gastrointestinal Endoscopy. 2020 June 20. doi: 10.1016/j.gie.2020.06.042; Tapper et al. Journal of Hepatology. 2020 Apr 13. doi: 10.1016/j.jhep.2020.04.005.
Suspension of disease screening and nonurgent procedures because of the COVID-19 pandemic will negatively impact long-term outcomes of GI and liver disease, and people of color will be disproportionately affected, according to a leading expert.
Novel, multipronged approaches are needed to overcome widening disparities in gastroenterology and hepatology, said Rachel Issaka, MD, of Fred Hutchinson Cancer Research Center in Seattle.
“The COVID-19 pandemic has led to unprecedented drops in breast, colorectal, and cervical cancer screenings,” Dr. Issaka said during an AGA FORWARD Program webinar. Screening rates for these diseases are down 83%-90%, she said.
“Certainly this creates a backlog of cancer screenings that need to occur, which poses very significant challenges for health systems as they’re adapting to this new state of health care that we have to provide,” Dr. Issaka said.
During her presentation, Dr. Issaka first addressed pandemic-related issues in colorectal cancer (CRC).
The sudden decrease in colonoscopies has already affected diagnoses, she said, as 32% fewer cases of CRC were diagnosed in April 2020 compared with April 2019, a finding that is “obviously very concerning.” All downstream effects remain to be seen; however, one estimate suggests that over the next decade, delayed screening may lead to an additional 4,500 deaths from CRC.
“These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest,” Dr. Issaka wrote, as coauthor of a study published in Gastrointestinal Endoscopy.
Dr. Issaka and colleagues predict that the pandemic will likely worsen “persistent CRC disparities” in African-American and Hispanic communities, including relatively decreased screening participation, delayed follow-up of abnormal stool results, limited community-based research and partnerships, and limited community engagement and advocacy.
“COVID-19 related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations,” wrote Dr. Issaka and colleagues.
Dr. Issaka described similar issues in hepatology. She referred to a recent opinion article by Tapper and colleagues, which predicted that the COVID-19 pandemic will impact patients with liver disease in three waves: first, by delaying liver transplants, elective procedures, imaging, and routine patient follow-up; second, by increasing emergent decompensations, transplant wait-list dropouts, and care deferrals; and third, by losing patients to follow-up, resulting in missed diagnoses, incomplete cancer screening, and progressive disease.
“This could disproportionately impact Black, Hispanic, and Native-American populations, who may have already had difficulty accessing [liver care],” Dr. Issaka said.
To mitigate growing disparities, Dr. Issaka proposed a variety of strategies for CRC and liver disease.
For CRC screening, Dr. Issaka suggested noninvasive modalities, including mailed fecal immunochemical tests (FIT), with focused follow-up on patients with highest FIT values. For those conducting CRC research, Dr. Issaka recommended using accessible technology, engaging with community partners, providing incentives where appropriate, and other methods. For cirrhosis care, Dr. Issaka suggested that practitioners turn to telehealth and remote care, including weight monitoring, cognitive function testing, home medication delivery, and online education.
More broadly, Dr. Issaka called for universal health insurance not associated with employment, research funding for health disparities, sustainable employment wages, climate justice, desegregation of housing, and universal broadband Internet.
“The solutions to these problems are multipronged,” Dr. Issaka said. “Some will happen locally; for instance, well-executed planning around telehealth. Some will happen at the state level through opportunities like advocacy or even just reaching out to your own [congressional representative]. And then some will also happen programmatically – How can we as a health system begin to leverage something like mailed FIT?”
Finally, Dr. Issaka suggested that tools from another branch of science can help improve screening rates.
“We don’t, in medicine, tap into the benefits of behavioral psychology enough,” she said. “That’s a great discipline with really great tools that we can all use.”
Dr. Issaka described the power of community, in that people are more likely to undergo screening if they know how many others in their community are also being screened.
“I think as much as we can gather those kinds of data and share those with individuals to provide reassurance about the safety and importance of screening, I think [that] will help,” she said.
The AGA FORWARD program is funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DK118761). Dr. Issaka has no conflicts of interest.
SOURCES: Issaka. AGA FORWARD Program Webinar. 2020 Aug 27; Balzora et al. Gastrointestinal Endoscopy. 2020 June 20. doi: 10.1016/j.gie.2020.06.042; Tapper et al. Journal of Hepatology. 2020 Apr 13. doi: 10.1016/j.jhep.2020.04.005.
FROM THE AGA FORWARD PROGRAM
Obesity boosts risks in COVID-19 from diagnosis to death
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
FROM OBESITY REVIEWS
HOME-PE trial clarifies which pulmonary embolism patients to treat at home
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
REPORTING FROM ESC CONGRESS 2020
ESC’s revised NSTE-ACS guidelines embrace hsT, personalized anti-ischemia treatments
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
FROM ESC CONGRESS 2020
FDA expands remdesivir use for all COVID-19 hospitalized patients
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
Gut bacteria linked to cardiovascular, other health conditions
Microorganisms in the human digestive tract are linked to 29 specific health conditions, including chronic obstructive pulmonary disease, high blood pressure, and type 2 diabetes, according to a genome analysis in more than 400,000 individuals.
Although previous studies have suggested a link between gut microbiota and diseases in humans, “the extent to which the human gut microbiome can be considered a determinant of disease and healthy aging remains unknown,” Hilde E. Groot, MD, of the University of Groningen (The Netherlands), said in a presentation at the virtual annual congress of the European Society of Cardiology.
To identify the spectrum of diseases linked to the gut microbiome, the researchers identified 422,417 unrelated adults of White British ancestry with genotype and matching genetic data. The average age of the participants was 57 years and 46% were male.
The researchers conducted a phenomewide association study including 35 distinct single-nucleotide polymorphisms (SNPs) that are known to influence the microbiome of the human gut.
Overall, seven SNPs were significantly associated with 29 disease outcomes including hypertension, type 2 diabetes, hypercholesterolemia, heart failure, renal failure, and osteoarthritis.
In addition, after a further sensitivity analysis using a Mendelian randomization (MR) approach, associations between Ruminococcus flavefaciens and hypertension and between Clostridium and platelet count might point to a causal link, the researchers said.
“Over the past few years, the amount of research concerning the human gut microbiome and the associations with health and disease has tremendously increased. However, most studies investigated one or a few traits. The strength of our study is the possibility to cover a wide range of traits simultaneously within one population,” Dr. Groot said in an interview.
“Our data support the hypothesis that the human gut microbiome is a complex system, involved in many pathophysiological mechanisms in the human body. So, our results are additional to earlier research and strengthen this hypothesis,” Dr. Groot added.
“Microbiota and their metabolites might be of importance in the interplay between overlapping pathophysiological processes, and could serve as potential therapeutic targets for the maintenance of health and prevention and treatment of cardiovascular diseases. However, before it is possible to give advice for the public and medical practice, further research is needed to study causality,” she emphasized.
“Currently, it is too soon to advise patients concerning their microbiome,” Dr. Groot noted. “However, genetic studies like ours might help other researchers to study causality between the gut microbiome and particular traits, which might potentially lead to new therapeutic targets. Next to genetic variants as a proxy, we’re currently studying the gut microbiome composition in myocardial infarction patients and healthy controls in a longitudinal setting.”
“Previous studies have suggested a potential link between the gut microbiome and the development of cardiovascular disease, type 2 diabetes mellitus, and other chronic disorders,” Carol Ann Remme, MD, of the Amsterdam University Medical Center, said in an interview. “However, it is challenging to study the effect of gut microbiome composition in large patient cohorts. As an alternative approach, the study authors showed in a very large population that genetic variants previously shown to influence gut microbiome composition were significantly associated with conditions such as hypertension, type 2 diabetes, hypercholesterolemia, and heart failure.”
The study is unique in that it employed a very large cohort of more than 400,000 individuals, which is typically required to be able to draw clear conclusions, Dr. Remme continued. “The authors were able to further refine their findings by linking genetic variants known to influence specific gut bacteria to some particular disorders,” she noted.
“It is becoming increasingly clear that an individual’s gut microbiome composition, which is defined by both genetic and environmental factors such as diet, may affect his/her susceptibility to certain diseases – including cardiovascular – in addition to disease progression and outcome,” said Dr. Remme. “This may ultimately lead to development of novel, personalized strategies for risk stratification in addition to potential preventive measures targeting the gut microbiome. I expect this area of research will become increasingly important in the coming years.”
The study received no outside funding. Dr. Groot and colleagues had no financial conflicts to disclose. Dr. Remme had no financial conflicts to disclose.
Microorganisms in the human digestive tract are linked to 29 specific health conditions, including chronic obstructive pulmonary disease, high blood pressure, and type 2 diabetes, according to a genome analysis in more than 400,000 individuals.
Although previous studies have suggested a link between gut microbiota and diseases in humans, “the extent to which the human gut microbiome can be considered a determinant of disease and healthy aging remains unknown,” Hilde E. Groot, MD, of the University of Groningen (The Netherlands), said in a presentation at the virtual annual congress of the European Society of Cardiology.
To identify the spectrum of diseases linked to the gut microbiome, the researchers identified 422,417 unrelated adults of White British ancestry with genotype and matching genetic data. The average age of the participants was 57 years and 46% were male.
The researchers conducted a phenomewide association study including 35 distinct single-nucleotide polymorphisms (SNPs) that are known to influence the microbiome of the human gut.
Overall, seven SNPs were significantly associated with 29 disease outcomes including hypertension, type 2 diabetes, hypercholesterolemia, heart failure, renal failure, and osteoarthritis.
In addition, after a further sensitivity analysis using a Mendelian randomization (MR) approach, associations between Ruminococcus flavefaciens and hypertension and between Clostridium and platelet count might point to a causal link, the researchers said.
“Over the past few years, the amount of research concerning the human gut microbiome and the associations with health and disease has tremendously increased. However, most studies investigated one or a few traits. The strength of our study is the possibility to cover a wide range of traits simultaneously within one population,” Dr. Groot said in an interview.
“Our data support the hypothesis that the human gut microbiome is a complex system, involved in many pathophysiological mechanisms in the human body. So, our results are additional to earlier research and strengthen this hypothesis,” Dr. Groot added.
“Microbiota and their metabolites might be of importance in the interplay between overlapping pathophysiological processes, and could serve as potential therapeutic targets for the maintenance of health and prevention and treatment of cardiovascular diseases. However, before it is possible to give advice for the public and medical practice, further research is needed to study causality,” she emphasized.
“Currently, it is too soon to advise patients concerning their microbiome,” Dr. Groot noted. “However, genetic studies like ours might help other researchers to study causality between the gut microbiome and particular traits, which might potentially lead to new therapeutic targets. Next to genetic variants as a proxy, we’re currently studying the gut microbiome composition in myocardial infarction patients and healthy controls in a longitudinal setting.”
“Previous studies have suggested a potential link between the gut microbiome and the development of cardiovascular disease, type 2 diabetes mellitus, and other chronic disorders,” Carol Ann Remme, MD, of the Amsterdam University Medical Center, said in an interview. “However, it is challenging to study the effect of gut microbiome composition in large patient cohorts. As an alternative approach, the study authors showed in a very large population that genetic variants previously shown to influence gut microbiome composition were significantly associated with conditions such as hypertension, type 2 diabetes, hypercholesterolemia, and heart failure.”
The study is unique in that it employed a very large cohort of more than 400,000 individuals, which is typically required to be able to draw clear conclusions, Dr. Remme continued. “The authors were able to further refine their findings by linking genetic variants known to influence specific gut bacteria to some particular disorders,” she noted.
“It is becoming increasingly clear that an individual’s gut microbiome composition, which is defined by both genetic and environmental factors such as diet, may affect his/her susceptibility to certain diseases – including cardiovascular – in addition to disease progression and outcome,” said Dr. Remme. “This may ultimately lead to development of novel, personalized strategies for risk stratification in addition to potential preventive measures targeting the gut microbiome. I expect this area of research will become increasingly important in the coming years.”
The study received no outside funding. Dr. Groot and colleagues had no financial conflicts to disclose. Dr. Remme had no financial conflicts to disclose.
Microorganisms in the human digestive tract are linked to 29 specific health conditions, including chronic obstructive pulmonary disease, high blood pressure, and type 2 diabetes, according to a genome analysis in more than 400,000 individuals.
Although previous studies have suggested a link between gut microbiota and diseases in humans, “the extent to which the human gut microbiome can be considered a determinant of disease and healthy aging remains unknown,” Hilde E. Groot, MD, of the University of Groningen (The Netherlands), said in a presentation at the virtual annual congress of the European Society of Cardiology.
To identify the spectrum of diseases linked to the gut microbiome, the researchers identified 422,417 unrelated adults of White British ancestry with genotype and matching genetic data. The average age of the participants was 57 years and 46% were male.
The researchers conducted a phenomewide association study including 35 distinct single-nucleotide polymorphisms (SNPs) that are known to influence the microbiome of the human gut.
Overall, seven SNPs were significantly associated with 29 disease outcomes including hypertension, type 2 diabetes, hypercholesterolemia, heart failure, renal failure, and osteoarthritis.
In addition, after a further sensitivity analysis using a Mendelian randomization (MR) approach, associations between Ruminococcus flavefaciens and hypertension and between Clostridium and platelet count might point to a causal link, the researchers said.
“Over the past few years, the amount of research concerning the human gut microbiome and the associations with health and disease has tremendously increased. However, most studies investigated one or a few traits. The strength of our study is the possibility to cover a wide range of traits simultaneously within one population,” Dr. Groot said in an interview.
“Our data support the hypothesis that the human gut microbiome is a complex system, involved in many pathophysiological mechanisms in the human body. So, our results are additional to earlier research and strengthen this hypothesis,” Dr. Groot added.
“Microbiota and their metabolites might be of importance in the interplay between overlapping pathophysiological processes, and could serve as potential therapeutic targets for the maintenance of health and prevention and treatment of cardiovascular diseases. However, before it is possible to give advice for the public and medical practice, further research is needed to study causality,” she emphasized.
“Currently, it is too soon to advise patients concerning their microbiome,” Dr. Groot noted. “However, genetic studies like ours might help other researchers to study causality between the gut microbiome and particular traits, which might potentially lead to new therapeutic targets. Next to genetic variants as a proxy, we’re currently studying the gut microbiome composition in myocardial infarction patients and healthy controls in a longitudinal setting.”
“Previous studies have suggested a potential link between the gut microbiome and the development of cardiovascular disease, type 2 diabetes mellitus, and other chronic disorders,” Carol Ann Remme, MD, of the Amsterdam University Medical Center, said in an interview. “However, it is challenging to study the effect of gut microbiome composition in large patient cohorts. As an alternative approach, the study authors showed in a very large population that genetic variants previously shown to influence gut microbiome composition were significantly associated with conditions such as hypertension, type 2 diabetes, hypercholesterolemia, and heart failure.”
The study is unique in that it employed a very large cohort of more than 400,000 individuals, which is typically required to be able to draw clear conclusions, Dr. Remme continued. “The authors were able to further refine their findings by linking genetic variants known to influence specific gut bacteria to some particular disorders,” she noted.
“It is becoming increasingly clear that an individual’s gut microbiome composition, which is defined by both genetic and environmental factors such as diet, may affect his/her susceptibility to certain diseases – including cardiovascular – in addition to disease progression and outcome,” said Dr. Remme. “This may ultimately lead to development of novel, personalized strategies for risk stratification in addition to potential preventive measures targeting the gut microbiome. I expect this area of research will become increasingly important in the coming years.”
The study received no outside funding. Dr. Groot and colleagues had no financial conflicts to disclose. Dr. Remme had no financial conflicts to disclose.
FROM ESC CONGRESS 2020
NYC public hospitals rose to the demands of the COVID-19 crisis
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
Hospitalists at the center of the storm
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.