User login
Does weight loss surgery up the risk for bone fractures?
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Currently, the two most common types of weight loss surgery performed include sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB). Sleeve gastrectomy involves removing a large portion of the stomach so that its capacity is significantly decreased (to about 20%), reducing the ability to consume large quantities of food. Also, the procedure leads to marked reductions in ghrelin (an appetite-stimulating hormone), and some studies have reported increases in glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), hormones that induce satiety. Gastric bypass involves creating a small stomach pouch and rerouting the small intestine so that it bypasses much of the stomach and also the upper portion of the small intestine. This reduces the amount of food that can be consumed at any time, increases levels of GLP-1 and PYY, and reduces absorption of nutrients with resultant weight loss. Less common bariatric surgeries include gastric banding and biliopancreatic diversion with duodenal switch (BPD-DS). Gastric banding involves placing a ring in the upper portion of the stomach, and the size of the pouch created can be altered by injecting more or less saline through a port inserted under the skin. BPD-DS includes sleeve gastrectomy, resection of a large section of the small intestine, and diversion of the pancreatic and biliary duct to a point below the junction of the ends of the resected gut.
Weight loss surgery is currently recommended for people who have a body mass index greater than or equal to 35 regardless of obesity-related complication and may be considered for those with a BMI greater than or equal to 30. BMI is calculated by dividing the weight (in kilograms) by the height (in meters). In children and adolescents, weight loss surgery should be considered in those with a BMI greater than 120% of the 95th percentile and with a major comorbidity or in those with a BMI greater than 140% of the 95th percentile.
What impact does weight loss surgery have on bone?
Multiple studies in both adults and teenagers have demonstrated that sleeve gastrectomy, RYGB, and BPD-DS (but not gastric banding) are associated with a decrease in bone density, impaired bone structure, and reduced strength estimates over time (Beavers et al; Gagnon, Schafer; Misra, Bredella). The relative risk for fracture after RYGB and BPD-DS is reported to be 1.2-2.3 (that is, 20%-130% more than normal), whereas fracture risk after sleeve gastrectomy is still under study with some conflicting results. Fracture risk starts to increase 2-3 years after surgery and peaks at 5-plus years after surgery. Most of the data for fractures come from studies in adults. With the rising use of weight loss surgery, particularly sleeve gastrectomy, in teenagers, studies are needed to determine fracture risk in this younger age group, who also seem to experience marked reductions in bone density, altered bone structure, and reduced bone strength after bariatric surgery.
What contributes to impaired bone health after weight loss surgery?
The deleterious effect of weight loss surgery on bone appears to be caused by various factors, including the massive and rapid weight loss that occurs after surgery, because body weight has a mechanical loading effect on bone and otherwise promotes bone formation. Weight loss results in mechanical unloading and thus a decrease in bone density. Further, when weight loss occurs, there is loss of both muscle and fat mass, and the reduction in muscle mass is deleterious to bone.
Other possible causes of bone density reduction include reduced absorption of certain nutrients, such as calcium and vitamin D critical for bone mineralization, and alterations in certain hormones that impact bone health. These include increases in parathyroid hormone, which increases bone loss when secreted in excess; increases in PYY (a hormone that reduces bone formation); decreases in ghrelin (a hormone that typically increases bone formation), particularly after sleeve gastrectomy; and decreases in estrone (a kind of estrogen that like other estrogens prevents bone loss). Further, age and gender may modify the bone consequences of surgery as outcomes in postmenopausal women appear to be worse than in younger women and men.
Preventing bone density loss
Given the many benefits of weight loss surgery, what can we do to prevent this decrease in bone density after surgery? It’s important for people undergoing weight loss surgery to be cognizant of this potentially negative outcome and to take appropriate precautions to mitigate this concern.
We should monitor bone density after surgery with the help of dual energy x-ray absorptiometry, starting a few years after surgery, particularly in those who are at greatest risk for fracture, so that we can be proactive about addressing any severe bone loss that warrants pharmacologic intervention.
More general recommendations include optimizing intake of calcium (1,200-1,500 mg/d), vitamin D (2,000-3,000 IUs/d), and protein (60-75 g/d) via diet and/or as supplements and engaging in weight-bearing physical activity because this exerts mechanical loading effects on the skeleton leading to increased bone formation and also increases muscle mass over time, which is beneficial to bone. A progressive resistance training program has been demonstrated to have beneficial effects on bone, and measures should be taken to reduce the risk for falls, which increases after certain kinds of weight loss surgery, such as gastric bypass.
Meeting with a dietitian can help determine any other nutrients that need to be optimized.
Though many hormonal changes after surgery have been linked to reductions in bone density, there are still no recommended hormonal therapies at this time, and more work is required to determine whether specific pharmacologic therapies might help improve bone outcomes after surgery.
Dr. Misra is chief of the division of pediatric endocrinology, Mass General for Children; associate director, Harvard Catalyst Translation and Clinical Research Center; director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; and professor, department of pediatrics, Harvard Medical School, Boston.
A version of this article originally appeared on Medscape.com.
Fibroid characteristics can help us anticipate postpartum hemorrhage
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at yyaghoubian@northwell.edu.
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at yyaghoubian@northwell.edu.
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at yyaghoubian@northwell.edu.
Why Is There a Lack of Representation of Skin of Color in the COVID-19 Literature?
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
Throughout the COVID-19 pandemic, there has been a striking paucity of representations of patients with skin of color (SOC) in the dermatology literature. Was COVID-19 underdiagnosed in this patient population due to a lack of patient-centered resources and inadequate dermatology training; reduced access to care, resulting from social determinants of health and reduced skin-color concordance; or the absence of population-based prevalence studies?
Tan et al1 reviewed 51 articles describing skin findings secondary to COVID-19. Patients were stratified by country of origin, which yielded an increased prevalence of cutaneous manifestations among Americans and Europeans compared to Asians, but patients were not stratified by race.1 However, in one case series of 318 predominantly American patients, 89% were White and 0.7% were Black.2 This systematic review by Tan et al1 suggested that skin manifestations of COVID-19 were present in patients with SOC but less frequently than in White patients. However, case series are not a strong proxy for population-level prevalence.
More broadly, patients with SOC are underrepresented in Google image search results, as the medical resource websites (eg, DermNet [https://dermnetnz.org], MedicalNewsToday [www.medicalnewstoday.com], and Healthline [www.healthline.com]) are lacking these images.3 As a result, it is difficult for patients with SOC to recognize diseases presenting in darker skin types. This same tendency may exist for COVID-19 skin manifestations. A systematic review found that articles describing cutaneous manifestations of COVID-19 almost exclusively presented images of lighter skin and completely omitted darker skin.4 If images of patients with SOC are absent from online resources, it is increasingly unlikely for these patients to recognize if their skin lesions are associated with COVID-19, which may result in a decrease in the number of patients with SOC presenting with skin lesions secondary to COVID-19, thereby influencing the representation of patients with SOC in case studies.
The lack of representation of SOC in online resources mirrors the paucity of images in dermatology textbooks. According to a search of 7170 images in major dermatology textbooks, most images depicted light or white skin (80.6%), followed by medium or brown skin in 15.5% of images and dark or black skin in only 3.9%.5 Physicians rely on online and print resources for making diagnoses; inadequate resources highlight a component of a larger issue: inadequate training of dermatologists in SOC. In a survey of American dermatologists and dermatology residents (N=262), 47% thought that their medical education had not adequately trained them on skin conditions in Black patients.6
A lack of adequate training for dermatologists may decrease the rate of correct diagnosis of skin lesions secondary to COVID-19 in patients with SOC. A lack of trust in the health care system and social determinants of health may hinder patients with SOC from seeking medical help. Dermatology is the second least diverse of medical specialties; only 3% of dermatologists are Black.7 This is impactful: First, because minority physicians are increasingly likely to provide care for patients of the same race or background, and second, because race-concordant physician visits are associated with greater patient-reported positive affect.7 A lack of availability of race-concordant physicians or physicians with perceived cultural competence may deter patients with SOC from seeking help, which may be further prevalent in dermatologic practice.
Barriers at all levels of social determinants of health hinder access to health care. Patients with SOC experience greater housing insecurity, increased reliance on public transportation, more issues with health literacy, and limited English-language fluency.8 Combined, these factors equate to decreased access to health care resources and subsequently a lack of inclusion in case studies.
COVID-19 infection disproportionately affects patients with SOC,8 but there is a clear lack of representation of SOC in the COVID-19 dermatology literature. It is imperative to investigate factors that may contribute to this inequity. Recognizing skin manifestations can play a role in diagnosing COVID-19; increased awareness of its presentation in darker skin types may help bridge existing racial inequities. It is vital that physicians receive adequate resources and training to be able to recognize cutaneous manifestations of COVID-19 in all skin types. Finally, it is important to recognize that the lack of representation of SOC in the COVID-19 literature represents a larger trend that exists in dermatologic research that warrants further investigation and advocacy for inclusivity.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133. doi:10.1016/j.jdin.2020.12.003
- Freeman EE, McMahon DE, Lipoff JB, et al; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dematol. 2020;83:486-492. doi:10.1016/j.jaad.2020.05.109
- Fathy R, Lipoff JB. Lack of skin of color in Google image searches may reflect under-representation in all educational resources. J Am Acad Dermatol. 2022;86:E113-E114. doi:10.1016/j.jaad.2021.04.097
- Lester JC, Jia JL, Zhang L, et al. Absence of images of skin of colour in publications of COVID-19 skin manifestations. Br J Dermatol. 2020;183:593-595. doi:10.1111/bjd.19258
- Kamath P, Sundaram N, Morillo-Hernandez C, et al. Visual racism in internet searches and dermatology textbooks. J Am Acad Dermatol. 2021;85:1348-1349. doi:10.1016/j.jaad.2020.10.072
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59,viii. doi:10.1016/j.det.2011.08.002
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Tai DBG, Shah A, Doubeni CA, et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703-706. doi:10.1093/cid/ciaa815
A Joint Effort to Save the Joints: What Dermatologists Need to Know About Psoriatic Arthritis
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
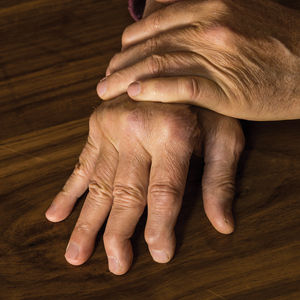
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Finding a home in psychiatry: A medical student’s story
Perched on a meditation cushion with the day’s first light creeping up the Himalayan foothills around me, I felt more at ease within myself than I could ever recall over my previous 19 years.
My immersion in daily conversations within the Tibetan monastic community on achieving a more harmonious relationship to our thoughts and feelings awoke a consideration of myself and my inner life in a way that I’d never truly contemplated before. These reflections gave me a vocabulary and a toolkit for navigating my own internal landscape that I have used ever since.
However, upon returning home, I was forced to acknowledge how fortunate I had been, and that these tools and the underlying spirit of inquiry are not commonplace in our society. Despite great strides in shifting views toward mental illness over the past few decades, our public discourse rarely captures the nuances of the mental health crisis that our culture has faced well before COVID-19 catalyzed even greater distress. We all pay the price of this cultural deficit to varying degrees, and I became captivated by the notion that things could be different.
I followed that thread of inquiry through the practices of Buddhist studies, massage therapy, yoga instruction, and refugee aid before coming to psychiatry as the unlikely yet ideal crucible for integrating my experiences in these spaces. Since arriving at medical school, however, my vision of myself as a psychiatrist has changed dramatically as my aspirations have collided with the realities of clinical experience and been tempered by the wisdom of mentors, colleagues, and patients, opening up a space for a deeper appreciation of what psychiatry might offer.
Clinical experience changes perspective
Short on clinical experience, I had previously imagined my future practice primarily as one of mindful listening and finding presence with each patient as a kind ear, supplemented by the ability to prescribe medication. Since then, working with patients has offered me insight into the ways in which my personality, perception, and potential access to a range of affective stances can serve as tools for skillfully developing the therapeutic encounter.
Moreover, “challenging” patients have taught me that my role is not always to offer unbounded empathetic support, but to potentially initiate compassionately tactful confrontation, shifting my sense of my role in the therapeutic relationship.
This is a lofty goal, which might entail modeling the successful navigation of potential ruptures and the subsequent repair of relationships so that they can live more adaptably in the world.
However, while I can support their envisioning of a realistic future for themselves and facilitate their acquisition of the tools needed to get there, my role is significant yet limited. This has been a hard truth to reckon with, but one that’s opened up pathways to greater empathy and a deeper understanding of each patient’s struggles. As a result, my view of pathology as a state has shifted to one of a dynamic process that emerges through the interaction of their genes, environment, life history, pharmacological supplements, psychodynamic tendencies, diet, and more.
Yet, while holding this reality of the complexities of mental illness, clinical decision-making often hinges on making binary choices regarding diagnoses, medications, and criteria for legal determinations. Developing this capacity to simultaneously practice different ways of knowing and sit with uncertainty excites me tremendously, not only equipping me to balance clinical practice with the demands of the modern health care system, but also nourishing the roots of a rich and ethical life.
Psychiatry calls to me for this expectation of sustaining an appropriate tension between uncertainty and decisiveness. It also inspires a deeper dive into the history of the field in order to learn the roots of its theories and perspectives so I can better understand how those inform contemporary practice in ways that are both helpful and harmful.
From individual to community
In tandem with this outer work of learning to appropriately position myself within individual patient relationships, the broader health care system, and the legacy of the field, I’ve also sought to develop a better understanding of how my own history, beliefs, and motivations shape my collaborative efforts.
Through my mindfulness practice and participation in exploratory psychoanalysis, I’ve caught glimpses of my own countertransference investments and opened up space for seeing how patients might experience me as a clinician. This has allowed for tuning in to my own response to them, identifying where in the typology of personality structures our reciprocal experiences might exist, and learning to manage those feelings to ultimately foster empathy through the interaction.
This has shifted my sense of the work from solely mindfully listening and thoughtfully responding to honing deliberate ways of both listening and responding in a way that is directly informed by the person sitting in front of me so I can best support them in creating change.
Given the responsibility inherent to this work, I have treated my medical education as an opportunity to build a foundation for stepping into this role. This has involved going beyond exploring these dynamics within individual clinician-patient relationships and carried over into my experiences with community-based research and program development. It has asked me to recognize the perceptual frames and prioritization of values that I bring to any given project.
This process has sharpened my aim of discovering each community’s understanding of their mental health needs so that I’m not implicitly imposing my own notions of psychological wholeness and “wellness” on others.
Working with San Diego’s Somali and Spanish-speaking populations has helped me to better understand each community’s own conceptualization of their strengths and needs, teaching me how to engage in reciprocal partnerships that honor each of our areas of expertise. Investing myself in medical school curricular reform represents the flip side of this coin, serving as an attempt to better understand my own medical community, how we think about health, and how we can best care for ourselves.
These experiences have offered opportunities to refine my skills in appreciative inquiry, coalition building, navigating institutional dynamics, and initiating and sustaining change within complex systems to carry the lessons of psychiatry beyond explicitly clinical spaces.
Toward integrative care
Ultimately, I view my community-based research and academic program development as outgrowths of my commitment to clinical psychiatry and my desire to learn how to provide people with the tools for changing their relationship to themselves, others, and their communities.
Equipped with formal medical training as the bedrock of this skill set, I have actively sought out opportunities to draw from practices that are outside the scope of the formal curriculum. These range from psychoanalysis and narrative medicine to cultural psychiatry and psychological anthropology, as well as my background in bodywork and mindfulness education. I’m eager to dive more fully into psychiatric practice as I work to integrate these disparate knowledge bases with the biomedical and psychodynamic views of the mind to develop a strengths-based practice that tends to patients’ bodies, minds, and spirits by bringing forth their own knowledge of themselves and their lives as they imagine what could be.
These realizations bring me back to that Himalayan sunrise more than a decade ago. They affirm that my heart lies with traversing disciplines to provide integrative psychiatric care in the community and developing infrastructure that supports these efforts. I’m filled with enthusiasm by the breadth of what psychiatry training offers as I continue expanding my capacity to support patients in this lifelong healing journey.
Alec Terrana is a rising fourth-year medical student at the University of California, San Diego, who intends to apply into psychiatry residency programs. He’s invested in exploring how we can more effectively conceptualize and measure mental health outcomes within San Diego’s Somali and Spanish-speaking communities, as well as advancing mindfulness and compassion training in undergraduate medical education. His professional interests also include implementation science, cultural psychiatry, psychodynamics, and strengthening public mental health infrastructure.
Perched on a meditation cushion with the day’s first light creeping up the Himalayan foothills around me, I felt more at ease within myself than I could ever recall over my previous 19 years.
My immersion in daily conversations within the Tibetan monastic community on achieving a more harmonious relationship to our thoughts and feelings awoke a consideration of myself and my inner life in a way that I’d never truly contemplated before. These reflections gave me a vocabulary and a toolkit for navigating my own internal landscape that I have used ever since.
However, upon returning home, I was forced to acknowledge how fortunate I had been, and that these tools and the underlying spirit of inquiry are not commonplace in our society. Despite great strides in shifting views toward mental illness over the past few decades, our public discourse rarely captures the nuances of the mental health crisis that our culture has faced well before COVID-19 catalyzed even greater distress. We all pay the price of this cultural deficit to varying degrees, and I became captivated by the notion that things could be different.
I followed that thread of inquiry through the practices of Buddhist studies, massage therapy, yoga instruction, and refugee aid before coming to psychiatry as the unlikely yet ideal crucible for integrating my experiences in these spaces. Since arriving at medical school, however, my vision of myself as a psychiatrist has changed dramatically as my aspirations have collided with the realities of clinical experience and been tempered by the wisdom of mentors, colleagues, and patients, opening up a space for a deeper appreciation of what psychiatry might offer.
Clinical experience changes perspective
Short on clinical experience, I had previously imagined my future practice primarily as one of mindful listening and finding presence with each patient as a kind ear, supplemented by the ability to prescribe medication. Since then, working with patients has offered me insight into the ways in which my personality, perception, and potential access to a range of affective stances can serve as tools for skillfully developing the therapeutic encounter.
Moreover, “challenging” patients have taught me that my role is not always to offer unbounded empathetic support, but to potentially initiate compassionately tactful confrontation, shifting my sense of my role in the therapeutic relationship.
This is a lofty goal, which might entail modeling the successful navigation of potential ruptures and the subsequent repair of relationships so that they can live more adaptably in the world.
However, while I can support their envisioning of a realistic future for themselves and facilitate their acquisition of the tools needed to get there, my role is significant yet limited. This has been a hard truth to reckon with, but one that’s opened up pathways to greater empathy and a deeper understanding of each patient’s struggles. As a result, my view of pathology as a state has shifted to one of a dynamic process that emerges through the interaction of their genes, environment, life history, pharmacological supplements, psychodynamic tendencies, diet, and more.
Yet, while holding this reality of the complexities of mental illness, clinical decision-making often hinges on making binary choices regarding diagnoses, medications, and criteria for legal determinations. Developing this capacity to simultaneously practice different ways of knowing and sit with uncertainty excites me tremendously, not only equipping me to balance clinical practice with the demands of the modern health care system, but also nourishing the roots of a rich and ethical life.
Psychiatry calls to me for this expectation of sustaining an appropriate tension between uncertainty and decisiveness. It also inspires a deeper dive into the history of the field in order to learn the roots of its theories and perspectives so I can better understand how those inform contemporary practice in ways that are both helpful and harmful.
From individual to community
In tandem with this outer work of learning to appropriately position myself within individual patient relationships, the broader health care system, and the legacy of the field, I’ve also sought to develop a better understanding of how my own history, beliefs, and motivations shape my collaborative efforts.
Through my mindfulness practice and participation in exploratory psychoanalysis, I’ve caught glimpses of my own countertransference investments and opened up space for seeing how patients might experience me as a clinician. This has allowed for tuning in to my own response to them, identifying where in the typology of personality structures our reciprocal experiences might exist, and learning to manage those feelings to ultimately foster empathy through the interaction.
This has shifted my sense of the work from solely mindfully listening and thoughtfully responding to honing deliberate ways of both listening and responding in a way that is directly informed by the person sitting in front of me so I can best support them in creating change.
Given the responsibility inherent to this work, I have treated my medical education as an opportunity to build a foundation for stepping into this role. This has involved going beyond exploring these dynamics within individual clinician-patient relationships and carried over into my experiences with community-based research and program development. It has asked me to recognize the perceptual frames and prioritization of values that I bring to any given project.
This process has sharpened my aim of discovering each community’s understanding of their mental health needs so that I’m not implicitly imposing my own notions of psychological wholeness and “wellness” on others.
Working with San Diego’s Somali and Spanish-speaking populations has helped me to better understand each community’s own conceptualization of their strengths and needs, teaching me how to engage in reciprocal partnerships that honor each of our areas of expertise. Investing myself in medical school curricular reform represents the flip side of this coin, serving as an attempt to better understand my own medical community, how we think about health, and how we can best care for ourselves.
These experiences have offered opportunities to refine my skills in appreciative inquiry, coalition building, navigating institutional dynamics, and initiating and sustaining change within complex systems to carry the lessons of psychiatry beyond explicitly clinical spaces.
Toward integrative care
Ultimately, I view my community-based research and academic program development as outgrowths of my commitment to clinical psychiatry and my desire to learn how to provide people with the tools for changing their relationship to themselves, others, and their communities.
Equipped with formal medical training as the bedrock of this skill set, I have actively sought out opportunities to draw from practices that are outside the scope of the formal curriculum. These range from psychoanalysis and narrative medicine to cultural psychiatry and psychological anthropology, as well as my background in bodywork and mindfulness education. I’m eager to dive more fully into psychiatric practice as I work to integrate these disparate knowledge bases with the biomedical and psychodynamic views of the mind to develop a strengths-based practice that tends to patients’ bodies, minds, and spirits by bringing forth their own knowledge of themselves and their lives as they imagine what could be.
These realizations bring me back to that Himalayan sunrise more than a decade ago. They affirm that my heart lies with traversing disciplines to provide integrative psychiatric care in the community and developing infrastructure that supports these efforts. I’m filled with enthusiasm by the breadth of what psychiatry training offers as I continue expanding my capacity to support patients in this lifelong healing journey.
Alec Terrana is a rising fourth-year medical student at the University of California, San Diego, who intends to apply into psychiatry residency programs. He’s invested in exploring how we can more effectively conceptualize and measure mental health outcomes within San Diego’s Somali and Spanish-speaking communities, as well as advancing mindfulness and compassion training in undergraduate medical education. His professional interests also include implementation science, cultural psychiatry, psychodynamics, and strengthening public mental health infrastructure.
Perched on a meditation cushion with the day’s first light creeping up the Himalayan foothills around me, I felt more at ease within myself than I could ever recall over my previous 19 years.
My immersion in daily conversations within the Tibetan monastic community on achieving a more harmonious relationship to our thoughts and feelings awoke a consideration of myself and my inner life in a way that I’d never truly contemplated before. These reflections gave me a vocabulary and a toolkit for navigating my own internal landscape that I have used ever since.
However, upon returning home, I was forced to acknowledge how fortunate I had been, and that these tools and the underlying spirit of inquiry are not commonplace in our society. Despite great strides in shifting views toward mental illness over the past few decades, our public discourse rarely captures the nuances of the mental health crisis that our culture has faced well before COVID-19 catalyzed even greater distress. We all pay the price of this cultural deficit to varying degrees, and I became captivated by the notion that things could be different.
I followed that thread of inquiry through the practices of Buddhist studies, massage therapy, yoga instruction, and refugee aid before coming to psychiatry as the unlikely yet ideal crucible for integrating my experiences in these spaces. Since arriving at medical school, however, my vision of myself as a psychiatrist has changed dramatically as my aspirations have collided with the realities of clinical experience and been tempered by the wisdom of mentors, colleagues, and patients, opening up a space for a deeper appreciation of what psychiatry might offer.
Clinical experience changes perspective
Short on clinical experience, I had previously imagined my future practice primarily as one of mindful listening and finding presence with each patient as a kind ear, supplemented by the ability to prescribe medication. Since then, working with patients has offered me insight into the ways in which my personality, perception, and potential access to a range of affective stances can serve as tools for skillfully developing the therapeutic encounter.
Moreover, “challenging” patients have taught me that my role is not always to offer unbounded empathetic support, but to potentially initiate compassionately tactful confrontation, shifting my sense of my role in the therapeutic relationship.
This is a lofty goal, which might entail modeling the successful navigation of potential ruptures and the subsequent repair of relationships so that they can live more adaptably in the world.
However, while I can support their envisioning of a realistic future for themselves and facilitate their acquisition of the tools needed to get there, my role is significant yet limited. This has been a hard truth to reckon with, but one that’s opened up pathways to greater empathy and a deeper understanding of each patient’s struggles. As a result, my view of pathology as a state has shifted to one of a dynamic process that emerges through the interaction of their genes, environment, life history, pharmacological supplements, psychodynamic tendencies, diet, and more.
Yet, while holding this reality of the complexities of mental illness, clinical decision-making often hinges on making binary choices regarding diagnoses, medications, and criteria for legal determinations. Developing this capacity to simultaneously practice different ways of knowing and sit with uncertainty excites me tremendously, not only equipping me to balance clinical practice with the demands of the modern health care system, but also nourishing the roots of a rich and ethical life.
Psychiatry calls to me for this expectation of sustaining an appropriate tension between uncertainty and decisiveness. It also inspires a deeper dive into the history of the field in order to learn the roots of its theories and perspectives so I can better understand how those inform contemporary practice in ways that are both helpful and harmful.
From individual to community
In tandem with this outer work of learning to appropriately position myself within individual patient relationships, the broader health care system, and the legacy of the field, I’ve also sought to develop a better understanding of how my own history, beliefs, and motivations shape my collaborative efforts.
Through my mindfulness practice and participation in exploratory psychoanalysis, I’ve caught glimpses of my own countertransference investments and opened up space for seeing how patients might experience me as a clinician. This has allowed for tuning in to my own response to them, identifying where in the typology of personality structures our reciprocal experiences might exist, and learning to manage those feelings to ultimately foster empathy through the interaction.
This has shifted my sense of the work from solely mindfully listening and thoughtfully responding to honing deliberate ways of both listening and responding in a way that is directly informed by the person sitting in front of me so I can best support them in creating change.
Given the responsibility inherent to this work, I have treated my medical education as an opportunity to build a foundation for stepping into this role. This has involved going beyond exploring these dynamics within individual clinician-patient relationships and carried over into my experiences with community-based research and program development. It has asked me to recognize the perceptual frames and prioritization of values that I bring to any given project.
This process has sharpened my aim of discovering each community’s understanding of their mental health needs so that I’m not implicitly imposing my own notions of psychological wholeness and “wellness” on others.
Working with San Diego’s Somali and Spanish-speaking populations has helped me to better understand each community’s own conceptualization of their strengths and needs, teaching me how to engage in reciprocal partnerships that honor each of our areas of expertise. Investing myself in medical school curricular reform represents the flip side of this coin, serving as an attempt to better understand my own medical community, how we think about health, and how we can best care for ourselves.
These experiences have offered opportunities to refine my skills in appreciative inquiry, coalition building, navigating institutional dynamics, and initiating and sustaining change within complex systems to carry the lessons of psychiatry beyond explicitly clinical spaces.
Toward integrative care
Ultimately, I view my community-based research and academic program development as outgrowths of my commitment to clinical psychiatry and my desire to learn how to provide people with the tools for changing their relationship to themselves, others, and their communities.
Equipped with formal medical training as the bedrock of this skill set, I have actively sought out opportunities to draw from practices that are outside the scope of the formal curriculum. These range from psychoanalysis and narrative medicine to cultural psychiatry and psychological anthropology, as well as my background in bodywork and mindfulness education. I’m eager to dive more fully into psychiatric practice as I work to integrate these disparate knowledge bases with the biomedical and psychodynamic views of the mind to develop a strengths-based practice that tends to patients’ bodies, minds, and spirits by bringing forth their own knowledge of themselves and their lives as they imagine what could be.
These realizations bring me back to that Himalayan sunrise more than a decade ago. They affirm that my heart lies with traversing disciplines to provide integrative psychiatric care in the community and developing infrastructure that supports these efforts. I’m filled with enthusiasm by the breadth of what psychiatry training offers as I continue expanding my capacity to support patients in this lifelong healing journey.
Alec Terrana is a rising fourth-year medical student at the University of California, San Diego, who intends to apply into psychiatry residency programs. He’s invested in exploring how we can more effectively conceptualize and measure mental health outcomes within San Diego’s Somali and Spanish-speaking communities, as well as advancing mindfulness and compassion training in undergraduate medical education. His professional interests also include implementation science, cultural psychiatry, psychodynamics, and strengthening public mental health infrastructure.
What’s in a drug name?
My use of drug names is a mixed bag of terms.
In medical school we learn drugs by their generic names, but it doesn’t take long before we realize that each has both a generic name and one (or more) brand names. I suppose there’s also the chemical names, but no one outside the lab uses those. They’re waaaaay too long.
There is, for better or worse, a lot of variability in this. The purists (almost always academics, or cardiologists, or academic cardiologists) insist on generic names only. In their notes, conversations, presentations, whatever. If you’re a medical student or resident under them, you learn fast not to use the brand name.
After 30 years of doing this ... I don’t care. My notes are a mishmash of both.
Let’s face it, brand names are generally shorter and easier to type, spell, and pronounce than the generic names. I still need to know both, but when I’m writing up a note Keppra is far easier than levetiracetam. And most patients find the brand names a lot easier to say and remember.
An even weirder point, which is my own, is that one of my teaching attendings insisted that we capitalize both generic and brand names while on his rotation. Why? He never explained that, but he was pretty insistent. Now, for whatever reason, the habit has stuck with me. I’m sure the cardiologist down the hall would love to send my notes back, heavily marked up with red ink.
There’s even a weird subdivisions in this: Aspirin is a brand name by Bayer. Shouldn’t it be capitalized in our notes? But it isn’t, and to make things more confusing that varies by country. Why? (if you’re curious, it’s a strange combination of 100-year-old patent claims, generic trademark rulings, and also what country you’re in, whether it was involved in World War I, and, if so, which side. Really).
So the medical lists in my notes are certainly understandable, though aren’t going to score me any points for academic correctness. Not that I care. As a medical Shakespeare might have written, Imitrex, Onzetra, Zembrace, Tosymra, Sumavel, Alsuma, Imigran, Migraitan, and Zecuity ... are still sumatriptan by any other name.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My use of drug names is a mixed bag of terms.
In medical school we learn drugs by their generic names, but it doesn’t take long before we realize that each has both a generic name and one (or more) brand names. I suppose there’s also the chemical names, but no one outside the lab uses those. They’re waaaaay too long.
There is, for better or worse, a lot of variability in this. The purists (almost always academics, or cardiologists, or academic cardiologists) insist on generic names only. In their notes, conversations, presentations, whatever. If you’re a medical student or resident under them, you learn fast not to use the brand name.
After 30 years of doing this ... I don’t care. My notes are a mishmash of both.
Let’s face it, brand names are generally shorter and easier to type, spell, and pronounce than the generic names. I still need to know both, but when I’m writing up a note Keppra is far easier than levetiracetam. And most patients find the brand names a lot easier to say and remember.
An even weirder point, which is my own, is that one of my teaching attendings insisted that we capitalize both generic and brand names while on his rotation. Why? He never explained that, but he was pretty insistent. Now, for whatever reason, the habit has stuck with me. I’m sure the cardiologist down the hall would love to send my notes back, heavily marked up with red ink.
There’s even a weird subdivisions in this: Aspirin is a brand name by Bayer. Shouldn’t it be capitalized in our notes? But it isn’t, and to make things more confusing that varies by country. Why? (if you’re curious, it’s a strange combination of 100-year-old patent claims, generic trademark rulings, and also what country you’re in, whether it was involved in World War I, and, if so, which side. Really).
So the medical lists in my notes are certainly understandable, though aren’t going to score me any points for academic correctness. Not that I care. As a medical Shakespeare might have written, Imitrex, Onzetra, Zembrace, Tosymra, Sumavel, Alsuma, Imigran, Migraitan, and Zecuity ... are still sumatriptan by any other name.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My use of drug names is a mixed bag of terms.
In medical school we learn drugs by their generic names, but it doesn’t take long before we realize that each has both a generic name and one (or more) brand names. I suppose there’s also the chemical names, but no one outside the lab uses those. They’re waaaaay too long.
There is, for better or worse, a lot of variability in this. The purists (almost always academics, or cardiologists, or academic cardiologists) insist on generic names only. In their notes, conversations, presentations, whatever. If you’re a medical student or resident under them, you learn fast not to use the brand name.
After 30 years of doing this ... I don’t care. My notes are a mishmash of both.
Let’s face it, brand names are generally shorter and easier to type, spell, and pronounce than the generic names. I still need to know both, but when I’m writing up a note Keppra is far easier than levetiracetam. And most patients find the brand names a lot easier to say and remember.
An even weirder point, which is my own, is that one of my teaching attendings insisted that we capitalize both generic and brand names while on his rotation. Why? He never explained that, but he was pretty insistent. Now, for whatever reason, the habit has stuck with me. I’m sure the cardiologist down the hall would love to send my notes back, heavily marked up with red ink.
There’s even a weird subdivisions in this: Aspirin is a brand name by Bayer. Shouldn’t it be capitalized in our notes? But it isn’t, and to make things more confusing that varies by country. Why? (if you’re curious, it’s a strange combination of 100-year-old patent claims, generic trademark rulings, and also what country you’re in, whether it was involved in World War I, and, if so, which side. Really).
So the medical lists in my notes are certainly understandable, though aren’t going to score me any points for academic correctness. Not that I care. As a medical Shakespeare might have written, Imitrex, Onzetra, Zembrace, Tosymra, Sumavel, Alsuma, Imigran, Migraitan, and Zecuity ... are still sumatriptan by any other name.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The new vaccine your patients may not want
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Applications of office hysteroscopy for the infertility patient
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.
If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
What role does diagnostic office hysteroscopy play in an infertility evaluation?
.1
More specifically, hysteroscopy is the gold standard for assessing the uterine cavity. The sensitivity, specificity, and positive predictive and negative predictive values of hysterosalpingography (HSG) in evaluating uterine cavity abnormalities were 44.83%; 86.67%; 56.52%; and 80.25%, respectively.2 Given the poor sensitivity of HSG, a diagnosis of endometrial polyps and/or chronic endometritis is more likely to be missed.
Our crossover trial comparing HSG to office hysteroscopy for tubal patency showed that women were 110 times more likely to have the maximum level of pain with HSG than diagnostic hysteroscopy when using a 2.8-mm flexible hysteroscope.3 Further, infection rates and vasovagal events were far lower with hysteroscopy.1
Finally, compared with HSG, we showed 98%-100% sensitivity and 84% specificity for tubal occlusion with hysteroscopy by air-infused saline. Conversely, HSG typically is associated with 76%-96% sensitivity and 67%-100% specificity.4 Additionally, we can often perform diagnostic hysteroscopies for approximately $35 per procedure for total fixed and disposable equipment costs.
How should physicians perform office hysteroscopy to minimize patient discomfort?
The classic paradigm has been to focus on paracervical blocks, anxiolytics, and a supportive environment (such as mood music). However, those are far more important when your hysteroscope is larger than the natural cervical lumen. If you can use small hysteroscopes (< 3 mm for the nulliparous cervix, < 4 mm for the parous cervix), most women will not require cervical dilation, which further enhances the patient experience.
Using a flexible hysteroscope for suspected pathology, making sure not to overdistend the uterus (particularly in high-risk patients such as those with tubal occlusion and cervical stenosis), and vaginoscopy can all minimize patient discomfort. We have published data showing that by using a 2.8-mm flexible diagnostic hysteroscope in a group of mostly nulliparous women, greater than 50% have no discomfort, and more than 90% will have mild to no discomfort.3
What operative hysteroscopy procedures can be performed safely in a physician’s office, and what equipment is required?
Though highly dependent on experience and resources, reproductive endocrinology and infertility specialists (REIs) arguably have the easiest transition to operative office hysteroscopy by utilizing the analgesia and procedure room that is standard for oocyte retrieval and simply adding hysteroscopic procedures. The accompanying table stratifies general hysteroscopic procedures by difficulty.

If one can use propofol or a similar level of sedation (which is routinely utilized for oocyte aspiration), there are few hysteroscopies that cannot be accomplished in the office. However, the less sedation and analgesia, the more judicious one must be in patient selection. Moreover, there are trade-offs between visualization, comfort, and instrumentation.
The greater the uterine distention and diameter of the hysteroscope, the more patients experience pain. One-third of patients (especially nulliparous) will discontinue a procedure with a 5-mm hysteroscope because of discomfort.5 However, as one drops to 4.5 mm and smaller operative hysteroscopes, instruments often occupy the inflow channel, limiting distention and visualization, which also can affect completion rates and safety.
When is operative hysteroscopy best suited for the OR?
In addition to physician experience and clinical resources, the critical factors guiding our choices for selecting the OR rather than the office, include:
- Loss of landmarks. Though Dr. Parry now does most severe intrauterine adhesion cases in the office with ultrasound guidance, when neither ostia can be visualized there is meaningful risk for perforation. Preoperative estrogen, development of planes with the diagnostic hysteroscope prior, and preparing the patient for a possible multistage procedure are all important.
- Use of energy. There are many excellent hysteroscopic surgeons who use the resectoscope well in the office. However, with possible patient movement and potential perforation with energy leading to a bowel injury, there can be greater risk when using energy relative to other methods (such as forceps, scissors, and mechanical morcellation).
- Deeper fibroids. Fibroids displace rather than invade the myometrium, and one can sonographically visualize the myometrium reapproximate over a fibroid as it herniates more into the uterine cavity. Nevertheless, the closer a fibroid comes to the serosa, the more mindful one should be of risks and balances for hysteroscopic removal.
In a patient with a severely stenotic cervix or tortuous endocervical canal, what preprocedure methods do you find helpful, and do you utilize abdominal ultrasound guidance?
If using a 2.8-mm flexible diagnostic hysteroscope, we find 99.8%-99.9% of cervices can be successfully cannulated in the office, with rare exception, that is, following cryotherapy or chlamydia cervicitis. This is the equivalent of your dilator having a camera on the tip and fully articulating to adjust to the cervical path.
Transvaginal sonography prior to hysteroscopy where one maps the cervical lumen helps anticipate problems (along with being familiar with the patient’s history). For the rare dilation under anesthesia, concurrent sonography with a 2.8-mm flexible hysteroscope and intermittent dilator use has been sufficient for our exceptions without the need for lacrimal dilators, vasopressin, misoprostol, and other adjuncts. Of note, we use a 1080p flexible endoscope, as lower resolution would make this more challenging.
In patients with recurrent implantation failure following IVF, is hysteroscopy superior to 3D saline infusion sonogram?
At an American Society of Reproductive Medicine 2021 session, Ilan Tur-Kaspa, MD, and Dr. Parry debated the topic of 2D ultrasound combined with hysteroscopy vs. 3D saline infusion sonography. Core areas of agreement were that expert hands for any approach are better than nonexpert, and high-resolution technology is better than lower resolution. There was also agreement that extrauterine and myometrial disease, such as intramural fibroids and adenomyosis, are contributory factors.
So, sonography will always have a role. However, existing and forthcoming data show hysteroscopy to improve live birth rates for patients with recurrent implantation failure after IVF. Dr. Parry finds diagnostic hysteroscopy easier for identifying endometritis, sessile and cornual polyps, retained products of conception (which are often isoechogenic with the endometrium) and lateral adhesions.
The reality is that there is variability among physicians and midlevel providers in both sonographic and diagnostic hysteroscopic skill. If one wants to verify findings with another team member, acknowledging that there can be nuances to identifying these pathologies by sonography, it is easier to share and discuss findings through hysteroscopic video than sonographic records.
When is endometrial biopsy indicated during office hysteroscopy?
The patients of an REI are very unlikely to have endometrial cancer (or even hyperplasia) outside of polyps (or arguably hypervascular areas of overgrowth), so the focus is on resecting visualized pathology relative to random biopsy.
However, the threshold for biopsy should be adjusted to the patient population, as well as to individual findings and risk. RVUs are greatly increased (11.1 > 41.57) with biopsy, helping sustainability. Additionally, if one places the hysteroscope on endometrium and applies suction through the inflow channel, one can obtain a sample with small-caliber diagnostic hysteroscopes and without having to use forceps.
What is your threshold for fluid deficit in hysteroscopy?
We follow AAGL guidelines, which for operative hysteroscopy are 2,500 mL of isotonic fluids or 1,000 mL of hypotonic fluids in low-risk patients. This should be further reduced to 500 mL of isotonic fluids in the elderly and even 300 mL in those with cardiovascular compromise.6
For patients who request sedation for office hysteroscopy, which option do you recommend – paracervical block alone, nitrous oxide, or the combination?
For diagnostic, greater than 95% of our patients do not require even over-the-counter analgesic medications. For operative, we consider all permissible resources that allow for a safe combination that is appropriate to the pathology and clinical setting, such as paracervical blocks, nitrous oxide, NSAIDs such as ketorolac, anxiolytics, and more.
The goal is to optimize the patient experience. However, the top three criteria that influence successful operative office hysteroscopy for a conscious patient are a parous cervix, judicious patient selection, and pre- and intraoperative verbal analgesia. Informed consent and engagement improve the experience of both the patient and physician.
Dr. Parry is the founder of Positive Steps Fertility in Madison, Miss. Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Parry JP et al. J Minim Invasive Gynecol. 2017 May-Jun. doi: 10.1016/j.jmig.2017.02.010.
2. Wadhwa L et al. 2017 Apr-Jun. doi: 10.4103/jhrs.JHRS_123_16.
3. Parry JP et al. Fertil Steril. 2017 Oct. doi: 10.1016/j.fertnstert.2017.07.1159.
4. Penzias A et al. Fertil Steril. 2021 Nov. doi: 10.1016/j.fertnstert.2021.08.038.
5. Campo R et al. Hum Reprod. 2005 Jan;20(1):258-63. doi: 10.1093/humrep/deh559.
6. AAGL AAGL practice report: Practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013 Mar-Apr. doi: 10.1016/j.jmig.2012.12.002.
Is the contemporary mental health crisis among youth due to DMN disruption?
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
The advent of unprecedented technologies drastically altering the behavior of children and adolescents, compounded by prolonged isolation from a once-in-a-century pandemic, may have negatively impacted the normal connectivity of the human brain among youth, leading to the current alarming increase of depression, anxiety, and suicidality among this population.
The human brain is comprised of multiple large-scale networks that are functionally connected and control feelings, thoughts, and behaviors. As clinical neuroscientists, psychiatrists must consider the profound impact of a massive societal shift in human behavior on the functional connectivity of brain networks in health and disease. The advent of smartphones, social media, and video game addiction may have disrupted the developing brain networks in children and adolescents, leading to the current escalating epidemic of mental disorders in youth.
The major networks in the human brain include the default mode network (DMN), the salience network, the limbic system, the dorsal attention network, the central executive network, and the visual system.1 Each network connects several brain regions. Researchers can use functional MRI to detect the connectivity of those networks. When blood flow increases concurrently across 2 or 3 networks, this indicates those networks are functionally connected.
There was an old “dogma” that brain regions use energy only when activated and being used. Hans Berger, who developed the EEG in 1929, noticed electrical activity at rest and proposed that the brain is constantly busy, but his neurology peers did not take him seriously.2 In the 1950s, Louis Sokoloff noticed that brain metabolism was the same whether a person is at rest or doing math. In the 1970s, David Ingvar discovered that the highest blood flow in the frontal lobe occurred when a person was at rest.3 Finally, in 2007, Raichle et al4 used positron emission tomography scans to confirm that the frontal lobe is most active when a person is not doing anything. He labeled this phenomenon the DMN, comprising the medial fronto-parietal cortex, the posterior cingulate gyrus, the precuneus, and the angular gyrus. Interestingly, the number of publications about the DMN has skyrocketed since 2007.
The many roles of the DMN
Ongoing research has revealed that the DMN is most active at rest, and its anatomical hubs mediate several key functions5:
- Posterior cingulate gyrus (the central core of the DMN): remembering the past and thinking about the future
- Medial prefrontal cortex: autobiographical memories, future goals and events, reflecting on one’s emotional self, and considering decisions about family members
- Dorsal medial subsystem: thinking about others, determining and inferring the purpose of other people’s actions
- Temporo-parietal junction: reflecting on the beliefs and emotions of others (known as “theory of mind”6)
- Lateral parietal junction: retrieval of social and conceptual knowledge
- Hippocampus: forming new memories, remembering the past, imagining the future
- Posterior-inferior parietal lobe: junction of auditory, visual, and somatic sensory information and attention
- Precuneus: Visual, sensory-motor, and attention.
Many terms have been used to describe the function of the DMN, including “daydreaming,” “auto-pilot,” “mind-wondering,” “reminiscing,” “contemplating,” “self-reflection,” “the neurological basis of the self,” and “seat of literary creativity.”
Psychiatric consequences of DMN deactivation
When another brain network, the attention network (which is also referred to as the task-positive network), is activated consciously and volitionally to perform a task that demands focus (such as text messaging, playing video games, or continuously interacting with social media sites), DMN activity declines.
Continue to: The DMN does not exist...
The DMN does not exist in infants, but starts to develop in childhood.7 It is enhanced by exercise, daydreaming, and sleep, activities that are common in childhood but have declined drastically with the widespread use of smartphones, video games, and social media, which for many youth occupy the bulk of their waking hours. Those tasks, which require continuous attention, deactivate the DMN. In fact, research has shown that addictive behavior decreases the connectivity of the DMN and suppresses its activity.8 Most children and adolescents can be regarded as essentially addicted to social media, text messaging, and video games. Unsurprisingly, serious psychiatric consequences follow.9
DMN dysfunction has been reported in several psychiatric conditions, including depression, posttraumatic stress disorder, autism, schizophrenia, anxiety, obsessive-compulsive disorder, and substance use.10-12 Impaired social interactions and communications, negative ruminations, suicidal ideas, and impaired encoding of long-term memories are some of the adverse effects of DMN dysfunction. The good news is that the DMN’s connectivity and functioning can be modulated and restored by meditation, mentalizing, exercise, psychotherapy, antidepressants, and psychedelics.13,14
The lockdown and stress of the COVID-19 pandemic added insult to injury and exacerbated mental illness in children by isolating them from each other and intensifying their technological addiction to fill the void of isolation. This crisis in youth mental health continues unabated, and calls for action to prevent grim outcomes. DMN dysfunction in youth can be reversed with treatment, but access to mental health care has become more challenging due to workforce shortages and insurance restrictions. Psychiatrists and parents must work diligently to treat psychiatrically affected youth, which has become a DaMN serious problem…
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
1. Yao Z, Hu B, Xie Y, et al. A review of structural and functional brain networks: small world and atlas. Brain Inform. 2015;2(1):45-52. doi:10.1007/s40708-015-0009-z
2. Raichle ME. The brain’s dark energy. Sci Am. 2010;302(3):44-49. doi:10.1038/scientific american0310-44
3. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. doi:10.1196/annals.1440.011
4. Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083-1090; discussion 1097-1099. doi:10.1016/j.neuroimage.2007.02.041
5. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251-270. doi:10.1177/1073858411403316
6. Tsoukalas I. Theory of mind: towards an evolutionary theory. Evolutionary Psychological Science. 2018;4(1):38-66. https://doi.org/10.1007/s40806-017-0112-x
7. Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33(3):279-296. doi:10.1016/j.neubiorev.2008.09.002
8. Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage. 2019;200:313-331. doi:10.1016/j.neuroimage.2019.06.036
9. Bommersbach TJ, McKean AJ, Olfson M, et al. National trends in mental health-related emergency department visits among youth, 2011-2020. JAMA. 2023;329(17):1469-1477. doi:10.1001/jama.2023.4809
10. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49-76. doi:10.1146/annurev-clinpsy-032511-143049
11. Akiki TJ, Averill CL, Wrocklage KM, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489-498. doi:10.1016/j.neuroimage.2018.05.005
12. Nagata JM, Chu J, Zamora G, et al. Screen time and obsessive-compulsive disorder among children 9-10 years old: a prospective cohort study. J Adolesc Health. 2023;72(3):390-396. doi:10.1016/j.jadohealth.2022.10.023
13. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48-73. doi:10.1016/j.neubiorev.2014.03.016
14. Gattuso JJ, Perkins D, Ruffell S, et al. Default mode network modulation by psychedelics: a systematic review. Int J Neuropsychopharmacol. 2023;26(3):155-188. doi:10.1093/ijnp/pyac074
Dysphagia in a patient with schizophrenia: Is the antipsychotic the culprit?
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. N, age 58, has a history of schizophrenia, tobacco use disorder, and alcohol use disorder. For many years, Mr. N has been receiving IM olanzapine 2.5 mg/d to treat his schizophrenia. He lives in a psychiatric hospital but was sent to our hospital after being found to have severe oropharyngeal dysphasia on a modified barium swallow study. There was concern for aspiration due to a history of choking episodes, which had been occurring for almost 1 month. During the modified barium swallow study, Mr. N was noted to have aspiration with deep laryngeal penetration during the pharyngeal stages of swallowing to all consistencies; this did not improve with the chin-tuck maneuver. In addition, during a CT scan of the cervical spine, an osteophyte was noted at the C5-C6 level, with possible impingement of the cervical esophagus and decreased upper esophageal sphincter opening.
Due to these findings, Mr. N was sent to our emergency department (ED) for further evaluation. In the ED, his vital signs were stable. He endorsed having a cough after eating, a sensation of having food stuck in his throat, and some hoarseness. His physical examination was notable for poor dentition. Results of a standard laboratory workup were all within normal limits. X-ray was notable for hazy opacities in the right upper to mid lung zones. Mr. N was admitted to the medical unit for further evaluation and management.
Narrowing the diagnosis
Because Mr. N was aspirating both liquids and solids, it was imperative that we identify the cause as soon as possible. The consultations that followed slowly guided the treatment team toward a diagnosis of antipsychotic-induced dysphagia. Otolaryngology identified insensate larynx during a flexible fiberoptic laryngoscopy exam, which was highly suggestive of a neurological dysfunction such as dystonia. Furthermore, an esophagogastroduodenoscopy found no structural abnormalities to explain Mr. N’s dysphagia, which ruled out impingement of the cervical esophagus by the osteophyte. An MRI of the brain ruled out structural abnormalities or evidence of stroke. Finally, a speech and language pathologist confirmed decreased laryngeal closure and airway protection with a repeat modified barium swallow, which led to aspiration during swallowing. Psychiatry recommended starting diphenhydramine to treat Mr. N’s extrapyramidal symptoms (EPS). A 6-day trial was initiated, with a single 50 mg IV dose on the first day followed by 25 mL oral twice daily for the remaining 5 days. In addition, olanzapine was discontinued.
Switching to a different diet and antipsychotic
Two days after starting diphenhydramine, Mr. N was switched to a puree diet. His ability to swallow improved, and he no longer coughed. However, on repeat modified barium swallow, aspiration was still noted for all types of liquids and solids. No structural improvements were seen.
Mr. N was discharged back to his psychiatric hospital, and his antipsychotic was changed from olanzapine to oral aripiprazole 2 mg/d. The aripiprazole dose was kept low to prevent the recurrence of dystonia and because at the time, his schizophrenia was asymptomatic. Mr. N was also prescribed oral diphenhydramine 25 mL twice daily.
At a 2-week follow-up appointment, Mr. N continued to show clinical improvement on the puree diet with thin liquids and continued the prescribed medication regimen.
Dysphagia as a manifestation of EPS
All antipsychotics, and particularly first-generation agents, are associated with EPS.1 These symptoms may be the result of antagonistic binding of dopaminergic D2 receptors within mesolimbic and mesocortical pathways of the brain, as well as parts of basal ganglia such as the caudate nucleus.2
In addition to the examples listed in the Table,2 EPS can present as dysphagia, esophageal dysmotility, or aspiration, none of which may be recognized as EPS. Research has found haloperidol, loxapine, trifluoperazine, olanzapine, risperidone, quetiapine, clozapine, and aripiprazole are associated with dysphagia.3-6 Strategies to treat antipsychotic-induced dysphagia include discontinuing the antipsychotic, lowering the dose, and changing to another medication.7
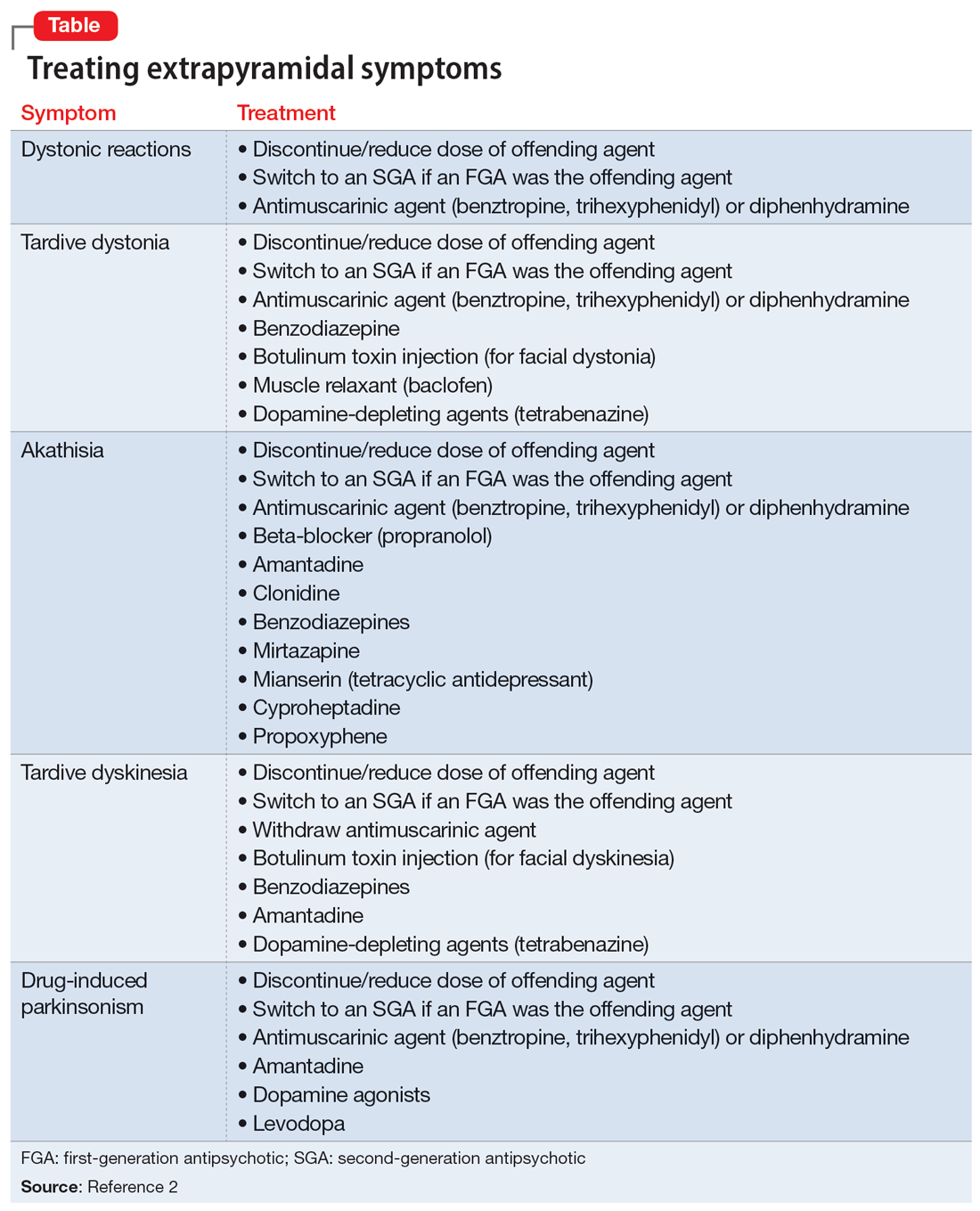
1. Crouse EL, Alastanos JN, Bozymski KM, et al. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56-64. doi:10.9740/mhc.2017.03.056
2. D’Souza RS, Hooten WM. Extrapyramidal symptoms. StatPearls Publishing; 2022. Updated January 8, 2023. Accessed April 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK534115/
3. Dziewas R, Warnecke T, Schnabel M, et al. Neuroleptic-induced dysphagia: case report and literature review. Dysphagia. 2007;22(1):63-67. doi:10.1007/s00455-006-9032-9
4. Kalf JG, de Swart BJ, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-315. doi:10.1016/j.parkreldis.2011.11.006
5. Lin TW, Lee BS, Liao YC, et al. High dosage of aripiprazole-induced dysphagia. Int J Eat Disord. 2012;45(2):305-306. doi:10.1002/eat.20934
6. Stewart JT. Dysphagia associated with risperidone therapy. Dysphagia. 2003;18(4):274-275. doi:10.1007/s00455-003-0006-x
7. Lee JC, Takeshita J. Antipsychotic-induced dysphagia: a case report. Prim Care Companion CNS Disord. 2015;17(5):10.4088/PCC.15I01792. doi:10.4088/PCC.15I01792
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. N, age 58, has a history of schizophrenia, tobacco use disorder, and alcohol use disorder. For many years, Mr. N has been receiving IM olanzapine 2.5 mg/d to treat his schizophrenia. He lives in a psychiatric hospital but was sent to our hospital after being found to have severe oropharyngeal dysphasia on a modified barium swallow study. There was concern for aspiration due to a history of choking episodes, which had been occurring for almost 1 month. During the modified barium swallow study, Mr. N was noted to have aspiration with deep laryngeal penetration during the pharyngeal stages of swallowing to all consistencies; this did not improve with the chin-tuck maneuver. In addition, during a CT scan of the cervical spine, an osteophyte was noted at the C5-C6 level, with possible impingement of the cervical esophagus and decreased upper esophageal sphincter opening.
Due to these findings, Mr. N was sent to our emergency department (ED) for further evaluation. In the ED, his vital signs were stable. He endorsed having a cough after eating, a sensation of having food stuck in his throat, and some hoarseness. His physical examination was notable for poor dentition. Results of a standard laboratory workup were all within normal limits. X-ray was notable for hazy opacities in the right upper to mid lung zones. Mr. N was admitted to the medical unit for further evaluation and management.
Narrowing the diagnosis
Because Mr. N was aspirating both liquids and solids, it was imperative that we identify the cause as soon as possible. The consultations that followed slowly guided the treatment team toward a diagnosis of antipsychotic-induced dysphagia. Otolaryngology identified insensate larynx during a flexible fiberoptic laryngoscopy exam, which was highly suggestive of a neurological dysfunction such as dystonia. Furthermore, an esophagogastroduodenoscopy found no structural abnormalities to explain Mr. N’s dysphagia, which ruled out impingement of the cervical esophagus by the osteophyte. An MRI of the brain ruled out structural abnormalities or evidence of stroke. Finally, a speech and language pathologist confirmed decreased laryngeal closure and airway protection with a repeat modified barium swallow, which led to aspiration during swallowing. Psychiatry recommended starting diphenhydramine to treat Mr. N’s extrapyramidal symptoms (EPS). A 6-day trial was initiated, with a single 50 mg IV dose on the first day followed by 25 mL oral twice daily for the remaining 5 days. In addition, olanzapine was discontinued.
Switching to a different diet and antipsychotic
Two days after starting diphenhydramine, Mr. N was switched to a puree diet. His ability to swallow improved, and he no longer coughed. However, on repeat modified barium swallow, aspiration was still noted for all types of liquids and solids. No structural improvements were seen.
Mr. N was discharged back to his psychiatric hospital, and his antipsychotic was changed from olanzapine to oral aripiprazole 2 mg/d. The aripiprazole dose was kept low to prevent the recurrence of dystonia and because at the time, his schizophrenia was asymptomatic. Mr. N was also prescribed oral diphenhydramine 25 mL twice daily.
At a 2-week follow-up appointment, Mr. N continued to show clinical improvement on the puree diet with thin liquids and continued the prescribed medication regimen.
Dysphagia as a manifestation of EPS
All antipsychotics, and particularly first-generation agents, are associated with EPS.1 These symptoms may be the result of antagonistic binding of dopaminergic D2 receptors within mesolimbic and mesocortical pathways of the brain, as well as parts of basal ganglia such as the caudate nucleus.2
In addition to the examples listed in the Table,2 EPS can present as dysphagia, esophageal dysmotility, or aspiration, none of which may be recognized as EPS. Research has found haloperidol, loxapine, trifluoperazine, olanzapine, risperidone, quetiapine, clozapine, and aripiprazole are associated with dysphagia.3-6 Strategies to treat antipsychotic-induced dysphagia include discontinuing the antipsychotic, lowering the dose, and changing to another medication.7

Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Mr. N, age 58, has a history of schizophrenia, tobacco use disorder, and alcohol use disorder. For many years, Mr. N has been receiving IM olanzapine 2.5 mg/d to treat his schizophrenia. He lives in a psychiatric hospital but was sent to our hospital after being found to have severe oropharyngeal dysphasia on a modified barium swallow study. There was concern for aspiration due to a history of choking episodes, which had been occurring for almost 1 month. During the modified barium swallow study, Mr. N was noted to have aspiration with deep laryngeal penetration during the pharyngeal stages of swallowing to all consistencies; this did not improve with the chin-tuck maneuver. In addition, during a CT scan of the cervical spine, an osteophyte was noted at the C5-C6 level, with possible impingement of the cervical esophagus and decreased upper esophageal sphincter opening.
Due to these findings, Mr. N was sent to our emergency department (ED) for further evaluation. In the ED, his vital signs were stable. He endorsed having a cough after eating, a sensation of having food stuck in his throat, and some hoarseness. His physical examination was notable for poor dentition. Results of a standard laboratory workup were all within normal limits. X-ray was notable for hazy opacities in the right upper to mid lung zones. Mr. N was admitted to the medical unit for further evaluation and management.
Narrowing the diagnosis
Because Mr. N was aspirating both liquids and solids, it was imperative that we identify the cause as soon as possible. The consultations that followed slowly guided the treatment team toward a diagnosis of antipsychotic-induced dysphagia. Otolaryngology identified insensate larynx during a flexible fiberoptic laryngoscopy exam, which was highly suggestive of a neurological dysfunction such as dystonia. Furthermore, an esophagogastroduodenoscopy found no structural abnormalities to explain Mr. N’s dysphagia, which ruled out impingement of the cervical esophagus by the osteophyte. An MRI of the brain ruled out structural abnormalities or evidence of stroke. Finally, a speech and language pathologist confirmed decreased laryngeal closure and airway protection with a repeat modified barium swallow, which led to aspiration during swallowing. Psychiatry recommended starting diphenhydramine to treat Mr. N’s extrapyramidal symptoms (EPS). A 6-day trial was initiated, with a single 50 mg IV dose on the first day followed by 25 mL oral twice daily for the remaining 5 days. In addition, olanzapine was discontinued.
Switching to a different diet and antipsychotic
Two days after starting diphenhydramine, Mr. N was switched to a puree diet. His ability to swallow improved, and he no longer coughed. However, on repeat modified barium swallow, aspiration was still noted for all types of liquids and solids. No structural improvements were seen.
Mr. N was discharged back to his psychiatric hospital, and his antipsychotic was changed from olanzapine to oral aripiprazole 2 mg/d. The aripiprazole dose was kept low to prevent the recurrence of dystonia and because at the time, his schizophrenia was asymptomatic. Mr. N was also prescribed oral diphenhydramine 25 mL twice daily.
At a 2-week follow-up appointment, Mr. N continued to show clinical improvement on the puree diet with thin liquids and continued the prescribed medication regimen.
Dysphagia as a manifestation of EPS
All antipsychotics, and particularly first-generation agents, are associated with EPS.1 These symptoms may be the result of antagonistic binding of dopaminergic D2 receptors within mesolimbic and mesocortical pathways of the brain, as well as parts of basal ganglia such as the caudate nucleus.2
In addition to the examples listed in the Table,2 EPS can present as dysphagia, esophageal dysmotility, or aspiration, none of which may be recognized as EPS. Research has found haloperidol, loxapine, trifluoperazine, olanzapine, risperidone, quetiapine, clozapine, and aripiprazole are associated with dysphagia.3-6 Strategies to treat antipsychotic-induced dysphagia include discontinuing the antipsychotic, lowering the dose, and changing to another medication.7

1. Crouse EL, Alastanos JN, Bozymski KM, et al. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56-64. doi:10.9740/mhc.2017.03.056
2. D’Souza RS, Hooten WM. Extrapyramidal symptoms. StatPearls Publishing; 2022. Updated January 8, 2023. Accessed April 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK534115/
3. Dziewas R, Warnecke T, Schnabel M, et al. Neuroleptic-induced dysphagia: case report and literature review. Dysphagia. 2007;22(1):63-67. doi:10.1007/s00455-006-9032-9
4. Kalf JG, de Swart BJ, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-315. doi:10.1016/j.parkreldis.2011.11.006
5. Lin TW, Lee BS, Liao YC, et al. High dosage of aripiprazole-induced dysphagia. Int J Eat Disord. 2012;45(2):305-306. doi:10.1002/eat.20934
6. Stewart JT. Dysphagia associated with risperidone therapy. Dysphagia. 2003;18(4):274-275. doi:10.1007/s00455-003-0006-x
7. Lee JC, Takeshita J. Antipsychotic-induced dysphagia: a case report. Prim Care Companion CNS Disord. 2015;17(5):10.4088/PCC.15I01792. doi:10.4088/PCC.15I01792
1. Crouse EL, Alastanos JN, Bozymski KM, et al. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56-64. doi:10.9740/mhc.2017.03.056
2. D’Souza RS, Hooten WM. Extrapyramidal symptoms. StatPearls Publishing; 2022. Updated January 8, 2023. Accessed April 28, 2023. https://www.ncbi.nlm.nih.gov/books/NBK534115/
3. Dziewas R, Warnecke T, Schnabel M, et al. Neuroleptic-induced dysphagia: case report and literature review. Dysphagia. 2007;22(1):63-67. doi:10.1007/s00455-006-9032-9
4. Kalf JG, de Swart BJ, Bloem BR, et al. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311-315. doi:10.1016/j.parkreldis.2011.11.006
5. Lin TW, Lee BS, Liao YC, et al. High dosage of aripiprazole-induced dysphagia. Int J Eat Disord. 2012;45(2):305-306. doi:10.1002/eat.20934
6. Stewart JT. Dysphagia associated with risperidone therapy. Dysphagia. 2003;18(4):274-275. doi:10.1007/s00455-003-0006-x
7. Lee JC, Takeshita J. Antipsychotic-induced dysphagia: a case report. Prim Care Companion CNS Disord. 2015;17(5):10.4088/PCC.15I01792. doi:10.4088/PCC.15I01792